- 1School of Nursing, Shanxi University of Chinese Medicine, Yuci, China
- 2Department of Nursing, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
- 3Department of Central Operating Room, Shanxi Bethune Hospital Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, China
Background: As naturally occurring compounds in plant-based foods, phytosterols have attracted attention for their lipid-modulating potential and proposed role as adjunctive therapies in managing hyperlipidemia. Nevertheless, conflicting evidence persists regarding their dual impact on dyslipidemia and subclinical inflammation.
Objective: This systematic review aimed to assess the impact of phytosterol-rich foods on lipid metabolism and inflammatory responses in hyperlipidemic populations.
Methods: A thorough literature search was performed across nine databases (including China National Knowledge Infrastructure Wanfang Data, VIP, SinoMed, PubMed, Cochrane Library, Embase, Scopus, Web of Science) from their inception up to 15 February 2025. Studies included were randomized controlled trials evaluating phytosterol interventions in adults with hyperlipidemia. The quality of the included studies was evaluated using the Cochrane Randomized Trial Risk Bias Tool, and Data analysis was performed using RevMan 5.4.
Results: This study included 14 randomized controlled trials with a total of 1,088 participants. The pooled results demonstrated statistically significant reductions in total cholesterol (TC) levels (mean difference (MD) = −0.65, 95% CI −0.83 to −0.47, P < 0.00001) and low-density lipoprotein cholesterol (LDL-C) levels (MD = −0.52, 95% CI −0.66 to −0.38, P < 0.00001), along with a modest increase in high-density lipoprotein cholesterol (HDL-C) levels (MD = 0.08, 95% CI 0.05 to 0.10, P < 0.00001). No significant change was observed for C-reactive protein (CRP) levels (MD = −0.00, 95% CI −0.01 to 0.00, P = 0.32). Although a borderline significant reduction in triglycerides (TG) levels was noted (MD = −0.24, 95% CI −0.47 to −0.01, P = 0.04), this finding displayed considerable heterogeneity.
Conclusion: Phytosterol intervention demonstrates significant efficacy in modulating atherogenic lipid profiles, such as TC and LDL-C, while also elevating HDL-C levels in individuals with hyperlipidemia. Yet, it fails to demonstrate anti-inflammatory activity as measured by CRP levels. The observed marginal TG-lowering effect should be interpreted with caution given substantial interstudy heterogeneity. Therefore, larger, metabolomics-inclusive studies are required for definitive conclusions and clinical guidance.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/#loginpage, identifier CRD420251002645.
Introduction
Hyperlipidemia is a chronic metabolic disorder marked by lipid metabolism abnormalities. It is characterized by elevated levels of serum Total Cholesterol (TC), Triglycerides (TG), and Low-Density Lipoprotein cholesterol (LDL-C), while High-Density Lipoprotein cholesterol (HDL-C) levels are typically reduced (Yang et al., 2019). It is not only an important risk factor for atherosclerotic cardiovascular disease, but also contributes significantly to the global public health burden. According to the Centers for Disease Control and Prevention, approximately 53% of American adults have abnormal LDL-C levels, yet only 35% achieve recommended lipid targets. Moreover, approximately 31 million adults have TC levels exceeding 6.24 mmol/L, and those with uncontrolled hyperlipidemia face a 200% higher risk of cardiovascular events compared to the general population (Writing Group Members et al., 2016; Karr, 2017). In China, the prevalence of adult hyperlipidemia has surged to 35.6%, with younger populations increasingly affected (Wang et al., 2023). This has become an important area of focus for the prevention and control of chronic diseases.
Dyslipidemia is an independent risk factor for cardiovascular disease, posing a significant threat to human health if uncontrolled (Alloubani et al., 2021). In terms of disease classification, primary hyperlipidemia is mainly caused by inherited lipid metabolism defects, while secondary types are closely related to multi-system dysfunction caused by metabolic syndrome (such as obesity, diabetes, and hypertension) (Pan, 2023). While statins remain the cornerstone of treatment, approximately 50% of patients with familial hypercholesterolemia fail to reach target lipid levels even with high-dose therapy. Moreover, long-term use of these medications may cause serious adverse reactions, such as myalgia and rhabdomyolysis (Liu and Zhiping, 2023; Stroes et al., 2015). In addition, for patients with secondary hyperlipidemia complicated with multiple metabolic disorders, the clinical management needs to take into account the multi-target regulation of blood glucose and blood pressure, which further increases the complexity of treatment. Therefore, it is of great practical significance to explore safe and cost-effective auxiliary lipid-lowering strategies.
Phytosterols are natural triterpene compounds widely distributed in plant cell membranes, primarily existing in three chemical forms: free, esterified, and glycosidically-bound (Valitova et al., 2016). Its main sources are vegetable oils (such as canola oil, corn oil), nuts, seeds and legumes. Among them, β-sitosterol, campesterol, and stigmasterol collectively account for over 70% of the total phytosterols. Other common forms include spinach sterols, oat sterols, and sitostanol (Moreau et al., 2018). Recent studies have highlighted the diverse physiological functions of phytosterols, including lipid metabolism regulation, anti-inflammatory effects, and immune modulation (Nechchadi et al., 2024; Gagliardi et al., 2010). However, the clinical evidence regarding their lipid-lowering and anti-inflammatory effects remains inconsistent (Demonty et al., 2013; Garoufi et al., 2014; Rideout et al., 2015; Li and Xing, 2016; Ras et al., 2015; Sun et al., 2014; Dewi et al., 2024; Jie et al., 2022). Based on this, This systematic review and meta-analysis aims to clarify the impact of phytosterol supplementation on serum lipid profiles (TC, LDL-C, HDL-C, TG) and inflammatory markers (CRP) in hyperlipidemic populations, integrating evidence from randomized controlled trials (RCTs) to inform evidence-based dietary interventions.
Materials and methods
This study was registered with PROSPERO (registration number: CRD420251002645) (https://www.crd.york.ac.uk/PROSPERO/#loginpage). During the preparation of this manuscript, it strictly abided by the guidelines outlined in the Primary Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021).
Inclusion criteria
Participants: Adults aged ≥18 years diagnosed with hyperlipidemia based on clinical criteria.
Interventions: Experimental groups received phytosterol-enriched food supplements (e.g., functional foods or nutritionally enhanced diets).
Control: The placebo group received inactive food matrices that were visually and indistinguishable in taste and appearance from the active interventions.
Outcome: The primary outcomes were lipid parameters, including Low-density lipoprotein cholesterol (LDL-C), Total cholesterol (TC), and the inflammatory marker C-reactive protein (CRP). The secondary outcomes included Triglycerides (TG) and High-density lipoprotein cholesterol (HDL-C). The Studies must provide data on at least one outcome parameter.
Study type: Randomized controlled trials (RCTs).
Exclusion criteria
1. Studies on phytosterols combined with other drugs/nutrients intervention; 2. Studies that are replications of published studies; 3. Studies for which the full text or incomplete data were unavailable; 4. Reviews, conference abstracts, animal experimental studies, etc.; and 5. Studies on phytosterol pharmaceutical supplements.
Search strategy
Two researchers independently searched nine databases (China National Knowledge Infrastructure (CNKI), Wanfang Data, VIP, SinoMed, PubMed, Cochrane Library, Embase, Scopus, Web of Science) from inception to 15 February 2025. A hybrid search strategy combining subject headings with free terms was employed. Search terms included: Phytosterols, phytosterol*, Plant sterol*, Phytostanol*, Sitosterol*, plant stanol*, sitostanol*, Campestanol*, Stigmasterol*, Stigmastanol*, brassicasterol*, Hypercholesterolemia, hyperlipoproteinemia, Hyperlipemia, dyslipidemias, randomized controlled trial, RCT, random, stud*. The language filter included both Chinese and English literature.
Literature screening and data extraction
Two researchers independently conducted systematic search and literature management using EndNote 20, ensuring duplicate removal. Subsequently, the titles and abstracts were carefully reviewed to exclude irrelevant studies, followed by a thorough examination of the full texts to select relevant articles based on predefined inclusion and exclusion criteria. Data extraction was performed by two researchers independently, encompassing information such as the first author’s name, publication year, publication country, participants involved, sample size, intervention measures employed, intervention duration, outcome indicators assessed, among others.
Literature quality assessment
The Cochrane Risk of Bias Tool was utilized to evaluate the methodological quality in seven domains: Randomization sequence generation; Allocation concealment; Blinding of participants and personnel; Blinding of outcome assessment; Incomplete outcome data; Selective reporting and Other bias. Studies were categorized into three quality levels (low, high, or unclear risk of bias) based on the risk-of-bias diagram. In case of discrepancies during the above process, a third researcher would act as an arbitrator to facilitate consensus-building.
Evidence quality assessment
The certainty of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. The quality of evidence for Randomized Controlled Trials (RCTs) was initially rated as high according to the GRADE methodology. This rating may be downgraded to moderate, low, or very low if limitations are identified in any of the five domains: Risk of bias, Inconsistency, Indirectness, Imprecision, or Publication bias. Conversely, the evidence quality may be upgraded if large effect sizes or dose-response gradients are observed. In the event of disagreement during this assessment, a third researcher served as an arbitrator to reach a consensus.
Data analysis methods
Meta-analysis was performed using Review Manager 5.4, adhering to the PRISMA guidelines for eligible studies. The results were presented as forest plots. Interstudy heterogeneity was assessed by the Cochrane heterogeneity test: a fixed-effect model was applied if P ≥ 0.1 and I2 ≤ 50%, otherwise a random-effects model was used for analysis. All outcome measures were standardized continuous variables, and effect sizes were reported as weighted mean differences (MD) with 95% confidence interval (CI). A P-value < 0.05 denoted statistical significance.
Results
Literature search results
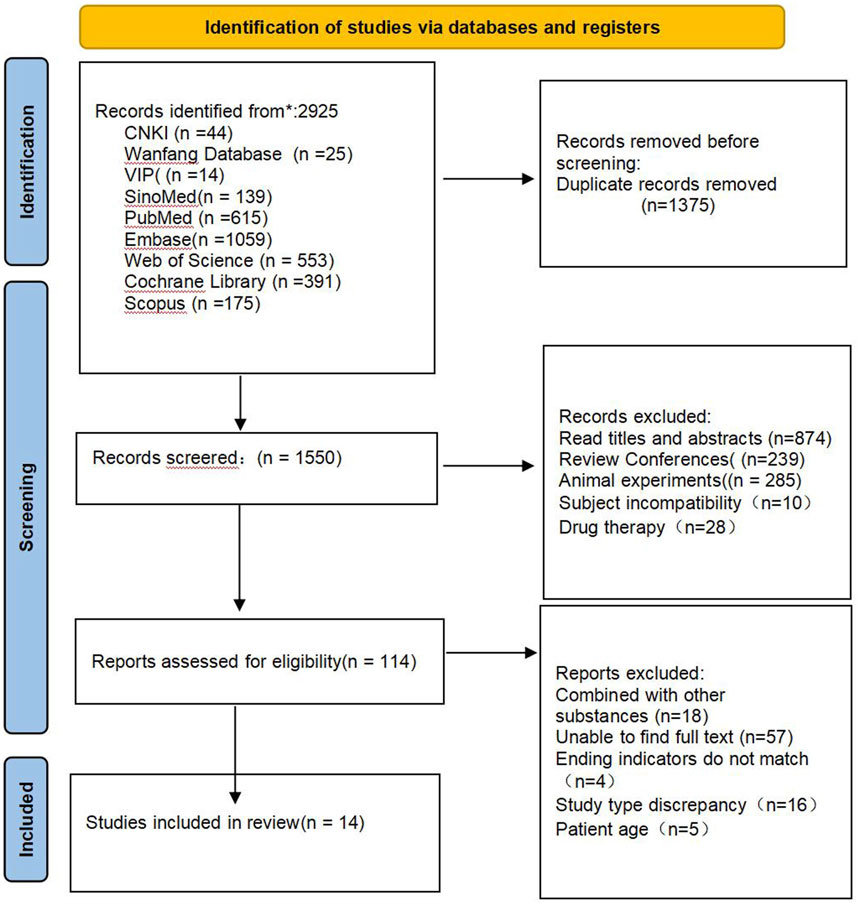
A total of 2,925 relevant literatures were obtained from the preliminary search database, and after excluding 1,375 duplicate literatures, 1,550 literatures remained. After preliminary screening by reading titles and abstracts, 1,436 articles that clearly did not meet the inclusion criteria were excluded, and 114 articles that might meet the inclusion criteria were obtained. After further reading the full text, 100 ineligible literatures were excluded, and 14 literatures were finally included (Dewi et al., 2024; Wang et al., 2015; Orem et al., 2017; Eady et al., 2011; Lestiani et al., 2018; Athyros et al., 2011; Vásquez-Trespalacios and Romero-Palacio, 2014; Hallikainen et al., 2013; Oliveira et al., 2020; Theuwissen et al., 2009; Kriengsinyos et al., 2015; Buyuktuncer et al., 2013; Cicero et al., 2023; Dong et al., 2016). The literature screening process and results are shown in Figure 1.
Basic characteristics of the included studies
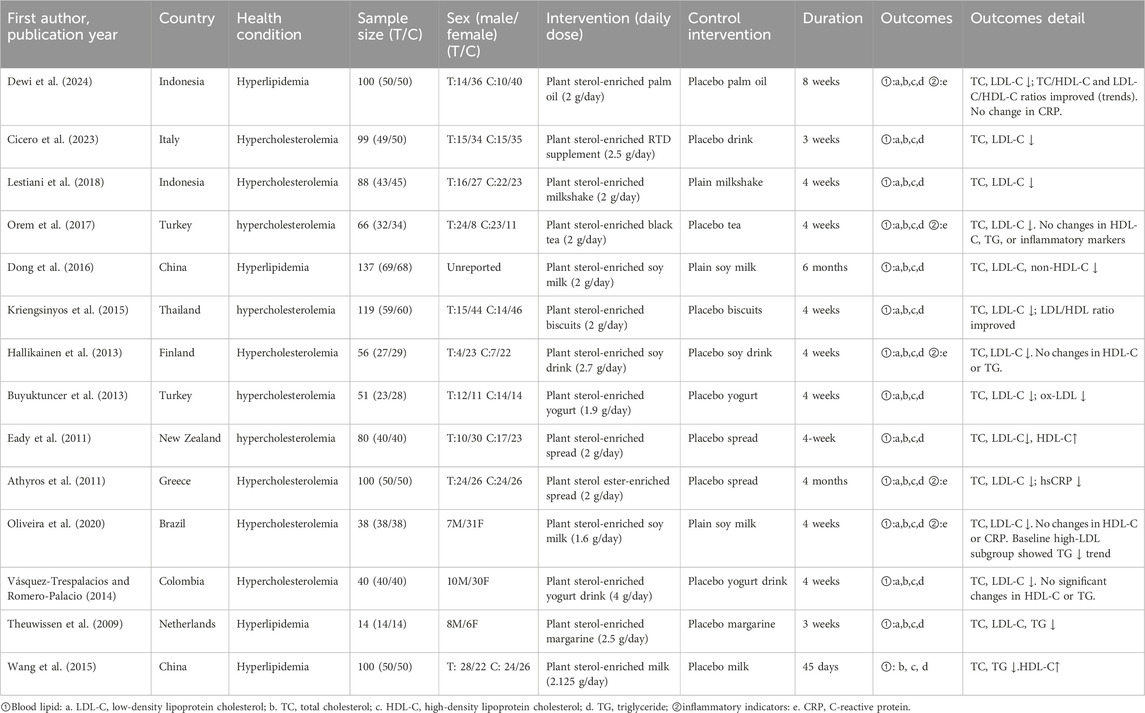
This study incorporated 14 randomized controlled trials involving 1,088 hyperlipidemic patients. All studies described the baseline characteristics of the two groups, and these groups were comparable. Furthermore, all studies reported the outcome measures. Detailed baseline information for the included studies is presented in Table 1.
Methodological quality of the included studies
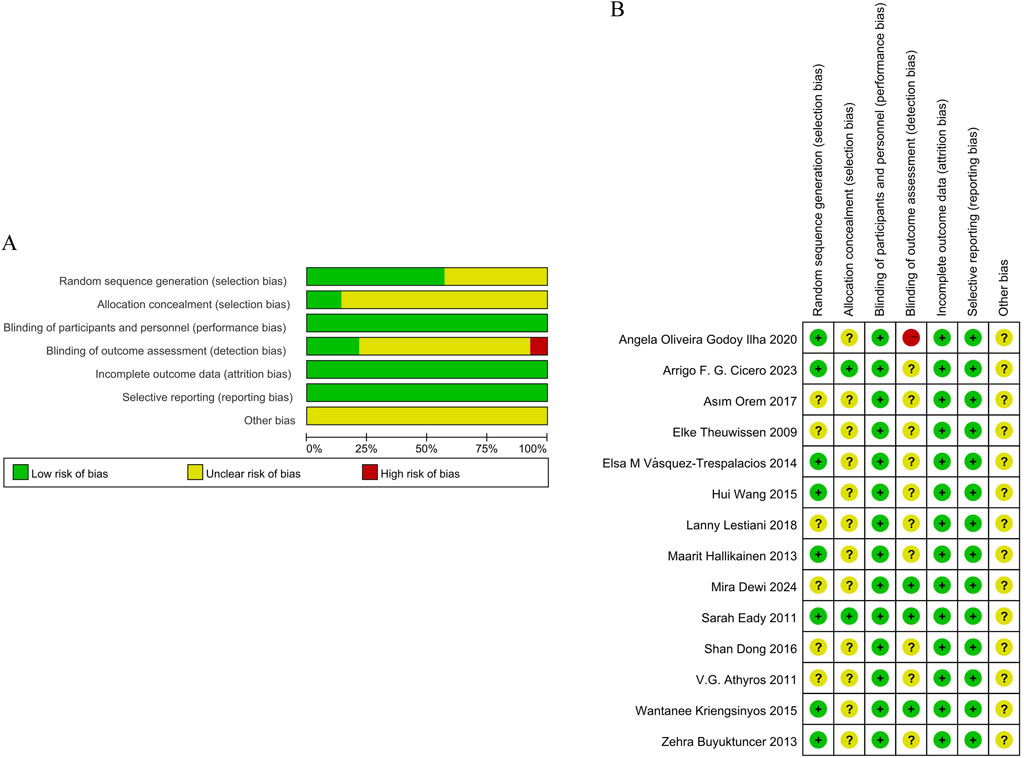
The methodological quality of the 14 included studies was systematically assessed using the Cochrane Risk of Bias tool, revealing heterogeneous risk profiles across evaluation domains. Eight studies (Wang et al., 2015; Eady et al., 2011; Vásquez-Trespalacios and Romero-Palacio, 2014; Hallikainen et al., 2013; Oliveira et al., 2020; Kriengsinyos et al., 2015; Buyuktuncer et al., 2013; Cicero et al., 2023) demonstrated low risk through explicit random number table or stratified randomization methods, while six studies lacked sufficient detail on randomization processes and were classified as unclear risk. Allocation concealment was adequately described in two studies (Eady et al., 2011; Cicero et al., 2023), earning them low risk ratings, but unclear in the rest. Regarding blinding, all studies reported participant blinding with low risk; three studies (Dewi et al., 2024; Eady et al., 2011; Kriengsinyos et al., 2015) further blinded outcome assessors, reinforcing their low risk classification. Conversely, 10 studies omitted details regarding blinding, resulting in unclear risk, and one study (Oliveira et al., 2020) failed to blind statisticians, resulting in a high-risk designation. Data integrity was generally robust, as all studies documented dropout rates and reasons, yielding low risk for bias. Reporting bias remained unclear due to insufficient evidence of selective outcome reporting, and no studies described quality control protocols. These findings are comprehensively presented in Figures 2A,B.
Quality of evidence
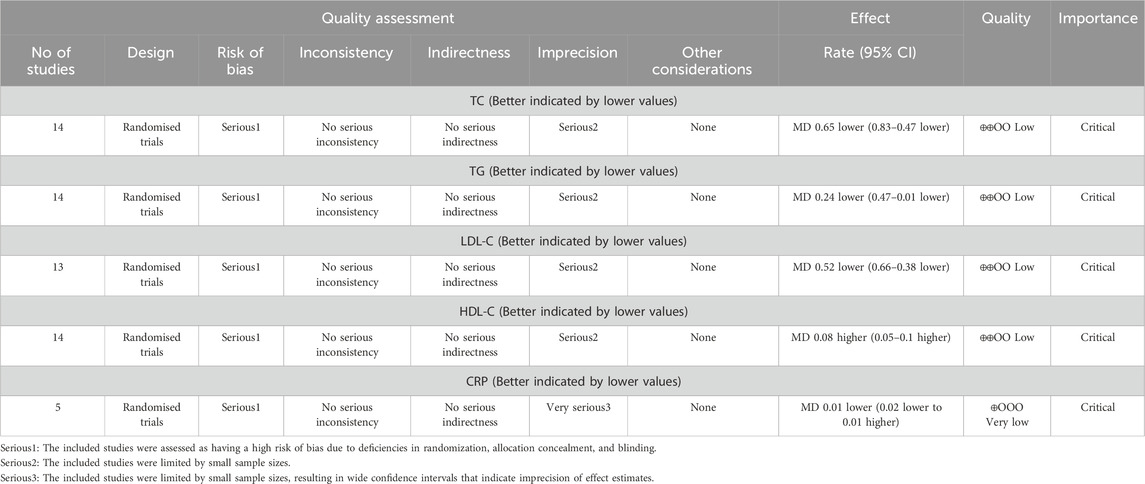
According to the GRADE assessment, the certainty of evidence was rated as low for TG, LDL-C, HDL-C, and TC outcomes. For CRP, the evidence was deemed very low certainty. These downgrades primarily stem from serious limitations in risk of bias (due to inadequate randomization, allocation concealment, and blinding) and imprecision (attributable to small sample sizes and wide confidence intervals). The evaluation details are in Table 2.
Effects of phytosterol-rich foods on patients with hyperlipidemia
Effects of phytosterol-rich foods on TC in patients with hyperlipidemia
14 studies (Dewi et al., 2024; Wang et al., 2015; Orem et al., 2017; Eady et al., 2011; Lestiani et al., 2018; Athyros et al., 2011; Vásquez-Trespalacios and Romero-Palacio, 2014; Hallikainen et al., 2013; Oliveira et al., 2020; Theuwissen et al., 2009; Kriengsinyos et al., 2015; Buyuktuncer et al., 2013; Cicero et al., 2023; Dong et al., 2016) demonstrated that phytosterol interventions significantly reduced TC levels (MD = −0.65, 95% CI −0.83 to −0.47, P < 0.00001). However, substantial heterogeneity (P < 0.00001, I2 = 86%) was observed. Subgroup analysis indicated that intervention duration exhibited a significant dose-independent effect on TC reduction (P < 0.05). But no meaningful interaction between phytosterol dosage and TC outcomes. The forest plots showed that although there was significant heterogeneity, the overall effect size remained stable. See Figures 3A,B.
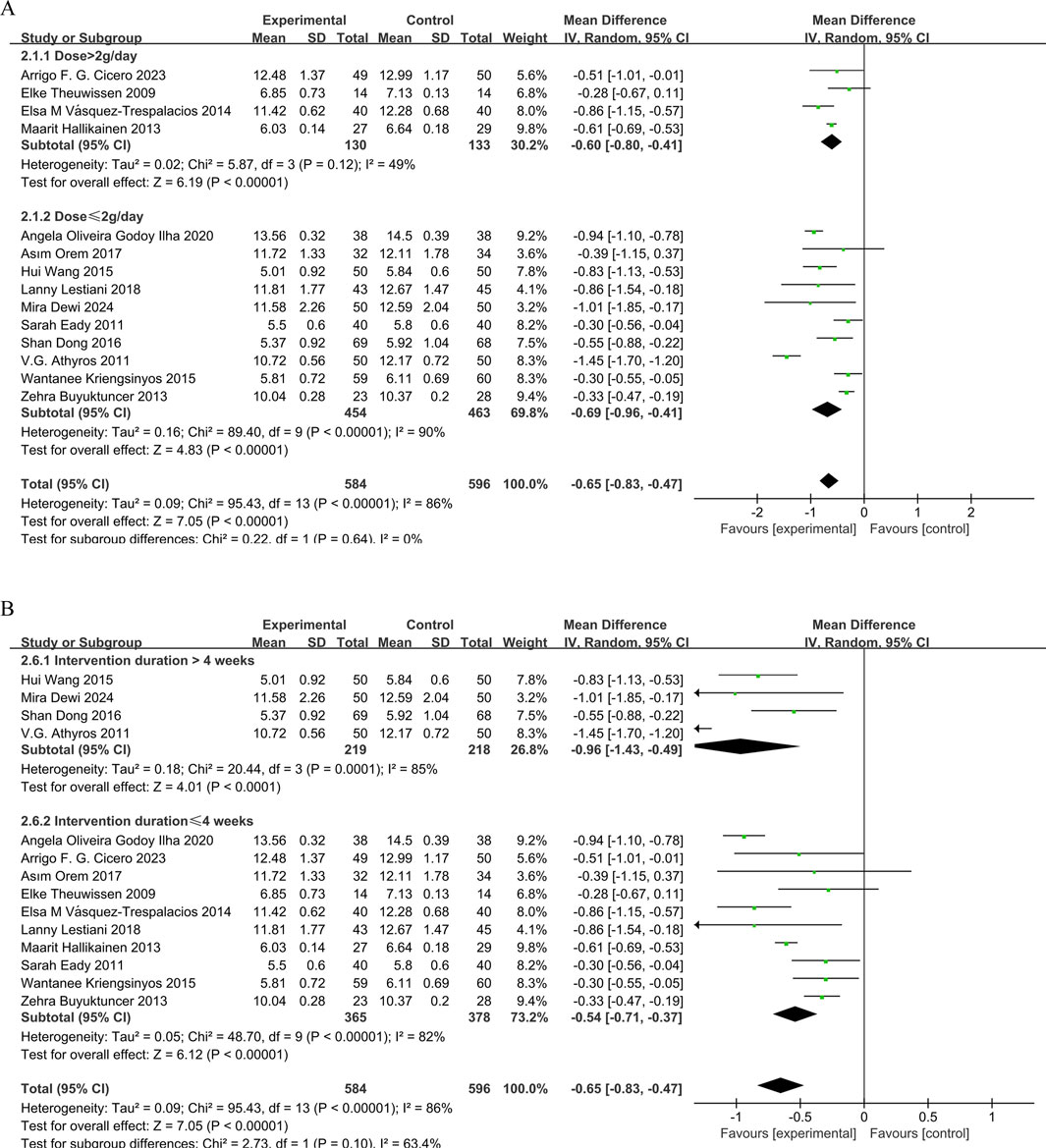
Figure 3. (A) Meta-analysis results of TC change in included trials, stratified by intervention dose and (B) Meta-analysis results of TC change in included trials, stratified by intervention duration.
Effects of phytosterol-rich foods on LDL-C in patients with hyperlipidemia
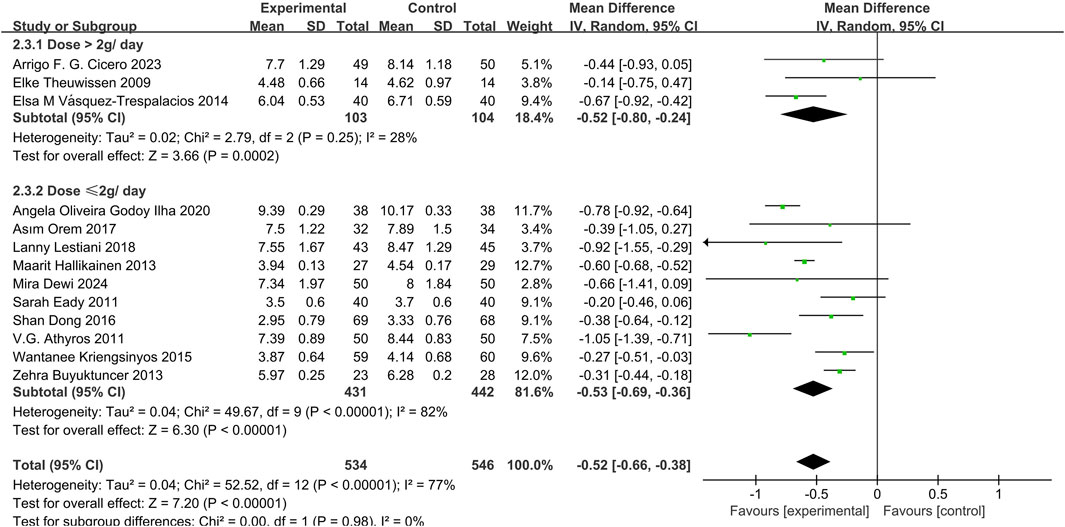
13 studies (Dewi et al., 2024; Orem et al., 2017; Eady et al., 2011; Lestiani et al., 2018; Athyros et al., 2011; Vásquez-Trespalacios and Romero-Palacio, 2014; Hallikainen et al., 2013; Oliveira et al., 2020; Theuwissen et al., 2009; Kriengsinyos et al., 2015; Buyuktuncer et al., 2013; Cicero et al., 2023; Dong et al., 2016) demonstrated that phytosterols could significantly reduce LDL-C levels (MD = −0.52, 95% CI −0.66 to −0.38, P < 0.00001), with high heterogeneity (P < 0.00001, I2 = 77%). Subgroup analysis showed no significant dose interaction and indicated stable overall effect sizes despite high heterogeneity. See Figure 4.
Effects of phytosterol-rich foods on HDL-C in patients with hyperlipidemia
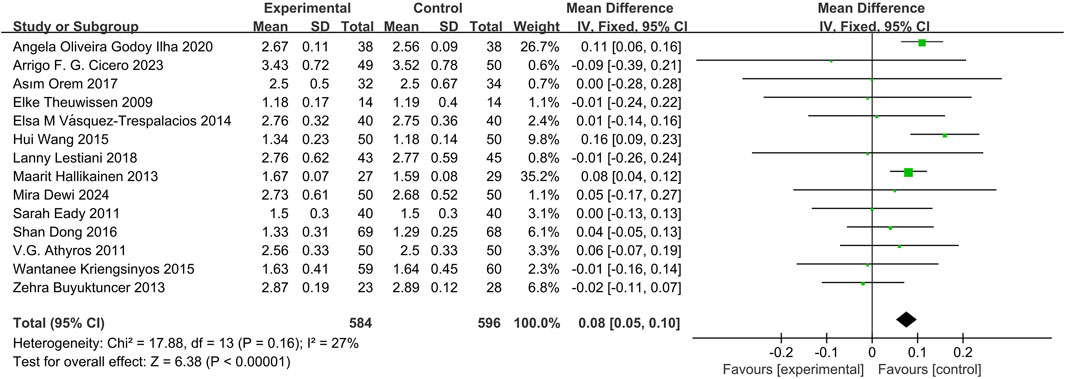
14 studies (Dewi et al., 2024; Wang et al., 2015; Orem et al., 2017; Eady et al., 2011; Lestiani et al., 2018; Athyros et al., 2011; Vásquez-Trespalacios and Romero-Palacio, 2014; Hallikainen et al., 2013; Oliveira et al., 2020; Theuwissen et al., 2009; Kriengsinyos et al., 2015; Buyuktuncer et al., 2013; Cicero et al., 2023; Dong et al., 2016) demonstrated that phytosterols could significantly reduce HDL-C levels, with a statistically significant difference (MD = 0.08, 95% CI 0.05 to 0.10, P < 0.00001). See Figure 5.
Effect of phytosterol-rich foods on TG in patients with hyperlipidemia
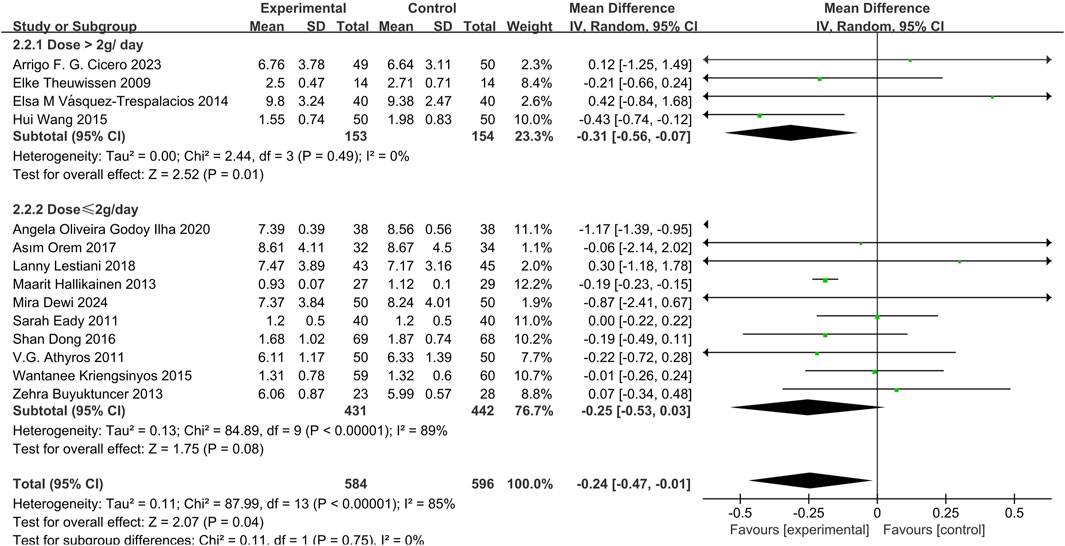
14 studies (Dewi et al., 2024; Orem et al., 2017; Eady et al., 2011; Lestiani et al., 2018; Athyros et al., 2011; Vásquez-Trespalacios and Romero-Palacio, 2014; Hallikainen et al., 2013; Oliveira et al., 2020; Theuwissen et al., 2009; Kriengsinyos et al., 2015; Buyuktuncer et al., 2013; Cicero et al., 2023; Dong et al., 2016) demonstrated that phytosterols reduced TG levels (MD = −0.24, 95% CI −0.47 to −0.01, P = 0.04). Heterogeneity analysis showed significant inter-study heterogeneity (P = 0.04, I2 = 85%). Subgroup analysis revealed a significant decrease in TG levels in the high-dose group (>2 g/day) (MD = −0.31, 95% CI −0.56 to −0.07, P = 0.01), but not in the low-dose group (≤2 g/day) (MD = −0.25, 95% CI −0.53 to 0.03, P = 0.08). The overall effect size was statistically significant. See Figure 6.
Effect of phytosterol-rich foods on CRP in patients with hyperlipidemia
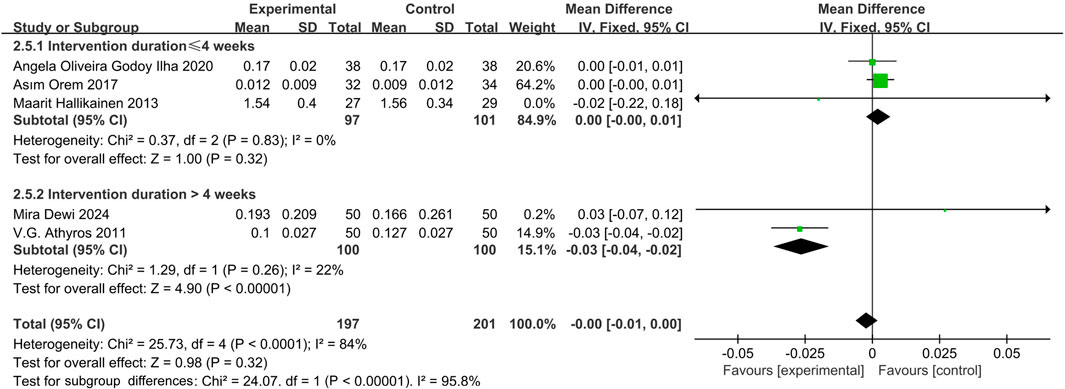
Five studies (Orem et al., 2017; Athyros et al., 2011; Hallikainen et al., 2013; Oliveira et al., 2020; Theuwissen et al., 2009) indicated that phytosterols had no significant effect on CRP levels (MD = −0.00, 95% CI −0.01 to 0.00, P = 0.32). Heterogeneity analysis showed significant inter-study heterogeneity (P = 0.06, I2 = 75%). Subgroup analysis showed a significant reduction in CRP levels when intervention duration >4 weeks (MD = −0.03, 95% CI −0.04 to −0.02, P < 0.00001), with no significant change in shorter interventions (MD = 0.00, 95% CI −0.00 to 0.01, P = 0.32). The forest plots suggested that although there was heterogeneity, the overall effect size was not significant. See Figure 7.
Adverse reactions
Only one of the included studies (Lestiani et al., 2018) on smoothie drinks reported phytosterol-related gastrointestinal adverse effects. There were no differences between the study groups in the symptoms of mild and transient changes in stool characteristics, upper abdominal discomfort, abdominal distension, increased flatulence and dyspepsia.
Sensitivity analysis and publication bias
The pooled effect sizes for TC, LDL-C, HDL-C, and CRP remained statistically significant (P < 0.05) and unchanged after excluding any single study. The confidence intervals consistently stayed within the clinical significance threshold, indicating robustness of the results. For TG, removing the study (Oliveira et al., 2020) substantially reduced heterogeneity (I2 from 85% to 0%) and yielded a more precise estimate (MD = −0.18, 95% CI −0.22 to −0.14, P < 0.00001). The results remained stable. This adjustment was attributed to the extreme effect size in the excluded study, which may reflect methodological differences or data distribution variations compared to other trials.
Funnel plot analysis was performed on TC, TG, LDL-C and HDL-C of ≥10 included studies: The funnel plot of TC and LDL-C was symmetrical, and the study points were distributed symmetrically on both sides of the axis, with a small publication bias. However, the funnel plots of TG and HDL-C showed funnel asymmetry, suggesting potential publication bias and heterogeneity. See Figure 8A–B.
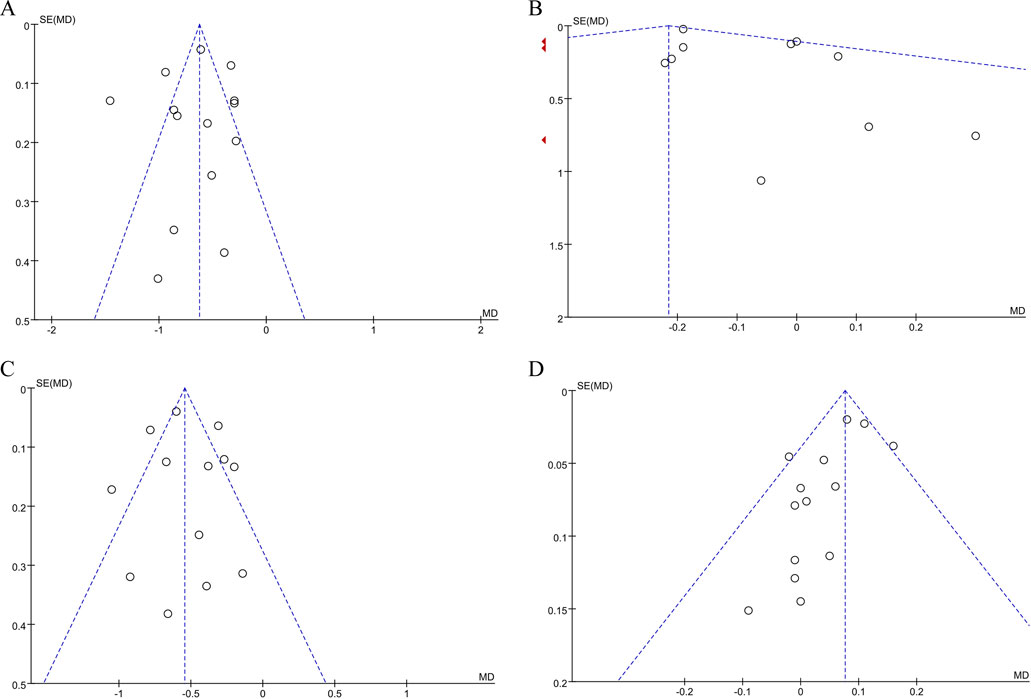
Figure 8. (A) Publication bias assessment: funnel plot for TC. (B) Publication bias assessment: funnel plot for TG. (C) Publication bias assessment: funnel plot for LDL-C. (D) Publication bias assessment: funnel plot for HDL-C.
Discussion
A total of 14 randomized controlled trials involving 1,088 patients were included in this study to evaluate the effects of phytosterols on blood lipids and inflammatory markers in patients with hyperlipidemia.
Effects of phytosterol-rich foods on blood lipids and inflammatory markers in patients with hyperlipidemia
This meta-analysis found phytosterol consumption significantly reduced TC and LDL-C while increasing HDL-C. Effects on TG and CRP, however, were inconsistent across studies (Amir Shaghaghi et al., 2013; Jie and Kang, 2017; Gao et al., 2023; Jm et al., 2009). While two studies reported no significant impact on TG and HDL-C (Jie and Kang, 2017; Gao et al., 2023), this study confirmed significant TG reduction and HDL-C elevation in hyperlipidemic patients.
A dose-dependent TG reduction was observed. Subgroup analysis indicated that high-dose phytosterol intake (>2 g/day) significantly lowered TG levels (MD = −0.31, 95% CI −0.56 to −0.07, P = 0.01), particularly in individuals with baseline TG > 150 mg/dL (Orem et al., 2017; Theuwissen et al., 2009). In contrast, the low-dose group (≤2 g/day) and overall combined analysis showed non-significant trends (MD = −0.25, 95% CI −0.53 to 0.03, P = 0.08). A dose-effect correlation was confirmed with phytosterol-fortified milk (Wang et al., 2015). However, the Egger test, conducted using Stata17 software to explore publication bias, suggested potential publication bias (P = 0.0154), and the effect size remained stable (Hedges’s g = −0.387, 95% CI −0.794 to 0.02) after trim-and-fill analysis, possibly due to small sample sizes and methodological heterogeneity. Thus, current evidence is insufficient to conclusively support the TG-lowering effect of phytosterols, and further large-scale studies are needed.
Regarding HDL-C, studies (Sun et al., 2014; Wang et al., 2015) reported significant increases, whereas another study (Ras et al., 2015) observed no significant changes. A meta-analysis (Jie and Kang, 2017) suggested that phytosterols had no effect on HDL-C in either healthy individuals or hyperlipidemic populations. Indicating that population-specific metabolic characteristics may influence the observed outcomes. The Egger test did not detect significant publication bias (P = 0.6054).
Several studies (Gagliardi et al., 2010; Athyros et al., 2011; Devaraj et al., 2006) have shown that phytosterols significantly reduce CRP levels, suggesting potential anti-inflammatory mechanisms via antioxidant pathways. However, current study found no significant overall effect of phytosterols on inflammation (MD = −0.00, 95% CI −0.01 to 0.00, P = 0.32). Subgroup analysis revealed that intervention durations >4 weeks were associated with significantly lower CRP levels (MD = −0.03, 95% CI −0.04 to −0.02, P < 0.00001), indicating duration-dependent modulation of inflammation. Further research on mechanisms and clinical relevance is needed.
Phytosterols feature a sterane ring system with a C-24 methyl or ethyl group, a C-3 hydroxyl group, and one to two double bonds in ring B (Khallouki et al., 2024). Resembling cholesterol structurally, they reduce cholesterol via a multi-tiered “gut-liver-fat” regulatory axis. Their effectiveness hinges on the C-24 substituent and ring saturation. Hydrophobic C-24 groups boost efficacy, like in 4-desmethyl sterols (sitosterol, stigmasterol) which activate liver X receptors (LXRα) and upregulate ABCG5/G8 (He et al., 2018). Sitostanol, owing to its saturated structure, exhibits minimal absorption and persists within intestinal micelles and emulsions, thereby continuously disrupting cholesterol solubilization to exert hypocholesterolemic effects (Ikeda and Sugano, 1983).
In the intestine, phytosterols branched hydroxyl groups enhance lipid solubility and displace dietary cholesterol from bile acid micelles, reducing cholesterol absorption by 30%–50% (Nechchadi et al., 2024; Dumolt and Rideout, 2017; Xue et al., 2019). Key mechanisms involve the Niemann-Pick C1-Like1 (NPC1L1) protein: phytosterols enter intestinal cells via NPC1L1 but inhibit its cholesterol uptake function. They also suppress the acyl-CoA: cholesterol acyltransferase (ACAT) enzyme, reducing chylomicron formation needed for cholesterol transport into the bloodstream (Paalvast et al., 2017; Liang et al., 2011; Alphonse and Jones, 2016). Furthermore, ABCG5/G8 transporters pump absorbed phytosterols back into the intestine, limiting their circulation and further reducing cholesterol absorption (Ghosh et al., 2021).
Within the liver, phytosterols trigger LXRα, a transcription factor controlling cholesterol levels. Activated LXRα increases the expression of transporters like ABCA1 and ABCG5/G8, promoting cholesterol excretion into bile It also stimulates bile acid synthesis via cytochrome P450 7A1 (CYP7A1), creating a feedback loop that lessens cholesterol reabsorption (He et al., 2013; Pannu et al., 2013). Furthermore, phytosterols interfere with the activation of SREBP2, a protein crucial for making cholesterol. This reduces the activity of HMG-CoA reductase, a key enzyme in cholesterol biosynthesis (Alphonse and Jones, 2016; Batta et al., 2006).
Regarding blood fats, phytosterols lower liver TG production by blocking fat-making enzymes and help break down TG by boosting an enzyme called lipoprotein lipase (Nechchadi et al., 2024). Phytosterols also reduce cholesterol absorption and synthesis, thereby prompting an upsurge in endogenous cholesterol production and augmenting the hepatic uptake of plasma LDL-C. enhancing its clearance and lowering plasma concentration (Poli et al., 2021).
Recent studies Indicated that non-nutrient bioactive compounds, like polyphenols and phytosterols, found in plant foods, have therapeutic potential for chronic diseases. These compounds possess antioxidant, anti-inflammatory, and other health-promoting properties, acting through mechanisms distinct from conventional nutrients. (Zhu et al., 2023). Researchers have analyzed common healthy dietary patterns such as the Mediterranean and Japanese diets, highlighting the significance of non-nutrients in disease prevention and health promotion. Based on this analysis, “theoretical model of family nurse diet therapy” has been proposed, highlighting the potential benefits of non-nutrients within dietary interventions (Han et al., 2023). This theory states that non-nutrients aid in the treatment of chronic diseases through their anti-inflammatory, antioxidant, and metabolic regulatory properties.
Study (Jia et al., 2024a) confirmed that polyphenol-rich non-nutrient foods can improve metabolic abnormalities in patients with hyperlipidemia. Furthermore, a systematic review (Jia et al., 2024b) on patients with coronary heart disease demonstrated that polyphenol-rich seed foods can significantly reduce blood lipid and inflammation levels in patients with coronary heart disease. It is worth noting that as an important non-nutrient, phytosterols exemplify this theory by integrating cholesterol-lowering, anti-inflammatory, and antioxidant effects. While humans lack endogenous synthesis pathways for these sterols, dietary supplementation confers measurable health benefits (Shi et al., 2023).
Current guidelines recommend ≥2 g/day of phytosterols to achieve LDL-C reductions (Yuen et al., 2019), with some trials suggesting enhanced efficacy at doses >2.5–3 g/day (Fontané et al., 2023). However, the efficacy of phytosterols is influenced by several factors, including the food matrix, dosage, and intervention duration (Abumweis et al., 2008). For instance, oil-based carriers such as palm oil and butter may enhance the bioavailability of phytosterols due to their solubility advantages, while water-soluble substrates like soy milk and yogurt show relatively limited effects (Dewi et al., 2024). Despite these differences, one study has reported that the lipid-lowering efficacy of phytosterols is independent of the food substrate (American Heart Association Nutrition Committee et al., 2006). Long-term intake of phytosterols has not been associated with serious adverse reactions, but the bioavailability differences among various substrates should be considered. Existing evidence indicates that phytosterols offer potential health benefits in dyslipidemia populations. However, the clinical translation of their anti-inflammatory and lipid-lowering mechanisms requires further verification through large-scale, high-quality studies.
Practical inspirations
Food is increasingly recognized as a frontline therapy for chronic disease management, offering advantages in safety, accessibility, and sustainability over pharmaceuticals (Moreno-Fernández et al., 2018). With the in-depth research on nutrition and chronic diseases, “Functional food” has attracted much attention as a new strategy for disease prevention and treatment, which has health-promoting and potential therapeutic use for chronic diseases (Malaguti et al., 2014). As natural, safe, and cost-effective dietary components, phytosterols exemplify this approach. Evidence indicates that dietary adjustments increasing plant sterol intake can effectively reduce chronic disease risk and improve health (Nattagh-Eshtivani et al., 2022).
In clinical practice, plant sterol intake strategies should be individualized to patients’ lipid profiles and dietary preferences. For patients with elevated baseline lipids, fortified foods (e.g., margarine, cereals) offer standardized dosing and effortless dietary integration. Conversely, those with lower lipid levels or seeking holistic nutrition benefit more from natural sources like nuts and vegetable oils, which simultaneously provide essential fatty acids and fiber while increasing sterol intake. Healthcare professionals must therefore tailor sterol source and dosage to each patient’s metabolic status and health objectives to optimize hyperlipidemia prevention and management.
Strengths and limitations of the study
The studies included in this study were all randomized controlled trials that had been assessed by Cochrane risk of bias, covering 11 countries, and the evidence level was high. As natural dietary components, plant sterols have a significantly lower incidence of adverse events than chemical drugs, which is in line with the concept of “homologous medicine and food” and has prominent clinical safety advantages. This study provides robust evidence that phytosterols not only lower lipid levels but also modulate TG levels in a dose-dependent manner in hyperlipidemic patients, with a clinically significant reduction in TG requiring an intake >2 g/day. Secondly, it reveals that the duration of intervention >4 weeks is crucial for its anti-inflammatory effects, significantly reducing CRP levels. Furthermore, within the framework of “Functional food” and non-nutrient therapy, phytosterols have been shown to be a safe, cost-effective, and health-promoting intervention, offering a new approach to the nutritional management of chronic diseases.
This study also has some limitations. The scope of study search is limited to Chinese and English studies, and high-quality studies in non-English/non-Chinese regions may be missed. Most of the included studies were short - and medium-term trials, and lack of long-term efficacy and safety tracking of phytosterols may affect the results of phytosterols on blood lipids and inflammatory indicators. Subgroup analysis of certain outcome indicators, such as TG is limited by small sample size, and the results may be biased. In the future, multi-language, multi-center, long-term randomized controlled trials should be carried out, and personalized dosing strategies should be explored.
Conclusion
In conclusion, phytosterols supplementation can improve the levels of LDL-C, TC, and HDL-C in patients with hyperlipidemia, but has no significant effect on CRP level. The underlying mechanisms may involve dual regulation of cholesterol absorption and anti-inflammatory pathways. In practice, researchers can design phytosterol-rich dietary plans tailored to patients’ energy needs and individual factors. Patients can choose food flexibly according to their own economic situation and dietary preferences. Future research should focus on large-scale, long-term studies to further elucidate the clinical significance and anti-inflammatory mechanisms of phytosterols. Thereby providing a robust scientific basis for clinical decision-making.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YZ: Investigation, Software, Writing – original draft, Conceptualization, Writing – review and editing. QZ: Methodology, Investigation, Supervision, Writing – review and editing, Formal Analysis. XW: Resources, Investigation, Supervision, Writing – review and editing. YJ: Writing – review and editing, Investigation, Formal Analysis, Conceptualization. QN: Writing – review and editing, Supervision, Conceptualization. SD: Supervision, Writing – review and editing. WL: Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project was supported by “2024 Annual The Nursing Research Fund Project of Shanxi Bethune Hospital +2024YH21.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1619922/full#supplementary-material
Abbreviations
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride; MD, Mean Difference; 95% CI, 95% Confidence Interval; CRP, C-reactive protein.
References
Abumweis, S. S., Barake, R., and Jones, P. J. (2008). Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr. Res. 52, 1811. doi:10.3402/fnr.v52i0.1811
Alloubani, A., Nimer, R., and Samara, R. (2021). Relationship between hyperlipidemia, cardiovascular disease and stroke: a systematic review. Curr. Cardiol. Rev. 17 (6), e051121189015. doi:10.2174/1573403X16999201210200342
Alphonse, P. A., and Jones, P. J. (2016). Revisiting human cholesterol synthesis and absorption: the reciprocity paradigm and its key regulators. Lipids 51 (5), 519–536. doi:10.1007/s11745-015-4096-7
American Heart Association Nutrition Committee, Lichtenstein, A. H., Appel, L. J., Brands, M., Carnethon, M., Daniels, S., et al. (2006). Diet and lifestyle recommendations revision 2006: a scientific statement from the American heart association nutrition committee. Circulation 114 (23), 82–96. doi:10.1161/CIRCULATIONAHA.106.176158
Amir Shaghaghi, M., Abumweis, S. S., and Jones, P. J. H. (2013). Cholesterol-lowering efficacy of plant sterols/stanols provided in capsule and tablet formats: results of a systematic review and meta-analysis. J. Acad. Nutr. Diet. 113 (11), 1494–1503. doi:10.1016/j.jand.2013.07.006
Athyros, V. G., Kakafika, A. I., Papageorgiou, A. A., Tziomalos, K., Peletidou, A., Vosikis, C., et al. (2011). Effect of a plant stanol ester-containing spread, placebo spread, or mediterranean diet on estimated cardiovascular risk and lipid, inflammatory and haemostatic factors. Nutr. Metab. Cardiovasc Dis. 21 (3), 213–221. doi:10.1016/j.numecd.2009.08.014
Batta, A. K., Xu, G., Honda, A., Miyazaki, T., and Salen, G. (2006). Stigmasterol reduces plasma cholesterol levels and inhibits hepatic synthesis and intestinal absorption in the rat. Metabolism 55 (3), 292–299. doi:10.1016/j.metabol.2005.08.024
Buyuktuncer, Z., Fisunoğlu, M., Guven, G. S., Unal, S., and Besler, H. T. (2013). The cholesterol lowering efficacy of plant stanol ester yoghurt in a Turkish population: a double-blind, placebo-controlled trial. Lipids Health Dis. 12, 91. doi:10.1186/1476-511X-12-91
Cicero, A. F. G., Fogacci, F., Giovannini, M., Rizzoli, E., Grandi, E., D'Addato, S., et al. (2023). The effect of dietary supplementation with plant sterols on total and LDL-cholesterol in plasma is affected by adherence to mediterranean diet: insights from the DESCO randomized clinical study. Nutrients 15 (21), 4555. doi:10.3390/nu15214555
Demonty, I., Ras, R. T., van der Knaap, H. C., Meijer, L., Zock, P. L., Geleijnse, J. M., et al. (2013). The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 52 (1), 153–160. doi:10.1007/s00394-011-0297-x
Devaraj, S., Autret, B. C., and Jialal, I. (2006). Reduced-calorie orange juice beverage with plant sterols lowers C-reactive protein concentrations and improves the lipid profile in human volunteers. Am. J. Clin. Nutr. 84 (4), 756–761. doi:10.1093/ajcn/84.4.756
Dewi, M., Martianto, D., Andarwulan, N., Kazimierczak, R., and Średnicka-Tober, D. (2024). Plant sterol-enriched palm oil intervention to improve lipid profile and inflammation status in hyperlipidemic individuals. Nutrients 16 (19), 3370. doi:10.3390/nu16193370
Dong, S., Zhang, R., Ji, Y. C., Hao, J. Y., Ma, W. W., Chen, X. D., et al. (2016). Soy milk powder supplemented with phytosterol esters reduced serum cholesterol level in hypercholesterolemia independently of lipoprotein E genotype: a random clinical placebo-controlled trial. Nutr. Res. 36 (8), 879–884. doi:10.1016/j.nutres.2016.05.006
Dumolt, J. H., and Rideout, T. C. (2017). The lipid-lowering effects and associated mechanisms of dietary phytosterol supplementation. Curr. Pharm. Des. 23 (34), 5077–5085. doi:10.2174/1381612823666170725142337
Eady, S., Wallace, A., Willis, J., Scott, R., and Frampton, C. (2011). Consumption of a plant sterol-based spread derived from rice bran oil is effective at reducing plasma lipid levels in mildly hypercholesterolaemic individuals. Br. J. Nutr. 105 (12), 1808–1818. doi:10.1017/S0007114510005519
Fontané, L., Pedro-Botet, J., Garcia-Ribera, S., Climent, E., Muns, M. D., Ballesta, S., et al. (2023). Use of phytosterol-fortified foods to improve LDL cholesterol levels: a systematic review and meta-analysis. Nutr. Metab. Cardiovasc Dis. 33 (8), 1472–1480. doi:10.1016/j.numecd.2023.04.014
Gagliardi, A. C., Maranhão, R. C., de Sousa, H. P., Schaefer, E. J., and Santos, R. D. (2010). Effects of margarines and butter consumption on lipid profiles, inflammation markers and lipid transfer to HDL particles in free-living subjects with the metabolic syndrome. Eur. J. Clin. Nutr. 64 (10), 1141–1149. doi:10.1038/ejcn.2010.122
Gao, Y., Xun, R., Xia, J., Xia, H., and Sun, G. (2023). Effects of phytosterol supplementation on lipid profiles in patients with hypercholesterolemia: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 14 (7), 2969–2997. doi:10.1039/d2fo03663k
Garoufi, A., Vorre, S., Soldatou, A., Tsentidis, C., Kossiva, L., Drakatos, A., et al. (2014). Plant sterols-enriched diet decreases small, dense LDL-cholesterol levels in children with hypercholesterolemia: a prospective study. Ital. J. Pediatr. 40, 42. doi:10.1186/1824-7288-40-42
Ghosh, S., Devereaux, M. W., Anderson, A. L., Gehrke, S., Reisz, J. A., D'Alessandro, A., et al. (2021). NF-κB regulation of LRH-1 and ABCG5/8 potentiates phytosterol role in the pathogenesis of parenteral nutrition-associated cholestasis. Hepatology 74 (6), 3284–3300. doi:10.1002/hep.32071
Hallikainen, M., Olsson, J., and Gylling, H. (2013). Low-fat nondairy minidrink containing plant stanol ester effectively reduces LDL cholesterol in subjects with mild to moderate hypercholesterolemia as part of a Western diet. Cholesterol 2013, 192325. doi:10.1155/2013/192325
Han, S. F., Feng, Y. Q., and Gao, W. Q. (2023). Theoretical model of non-nutrient diet therapy for prevention and treatment of chronic diseases. Chin. Nurs. Res. 37, 565–569. doi:10.12102/j.issn.1009-6493.2023.04.001
He, W. S., Wang, M. G., Pan, X. X., Li, J. J., Jia, C. S., Zhang, X. M., et al. (2013). Role of plant stanol derivatives in the modulation of cholesterol metabolism and liver gene expression in mice. Food Chem. 140 (1-2), 9–16. doi:10.1016/j.foodchem.2013.02.062
He, W. S., Zhu, H., and Chen, Z. Y. (2018). Plant sterols: Chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food Chem. 66 (12), 3047–3062. doi:10.1021/acs.jafc.8b00059
Ikeda, I., and Sugano, M. (1983). Some aspects of mechanism of inhibition of cholesterol absorption by beta-sitosterol. Biochimica Biophysica Acta 732 (3), 651–658. doi:10.1016/0005-2736(83)90243-2
Jia, Y., Wang, H., Fan, W., Lv, J., Niu, Q., Zhu, R., et al. (2024b). Effects of polyphenol-rich seed foods on lipid and inflammatory markers in patients with coronary heart disease: a systematic review. Front. Nutr. 11, 1493410. doi:10.3389/fnut.2024.1493410
Jia, Y., Zhang, Q., Zhang, Y., Wang, H., Niu, Q., Zhu, R., et al. (2024a). Effects of polyphenol-rich foods on lipids and oxidative stress status in patients with hyperlipidemia: a systematic review of randomized controlled trials. J. Multidiscip. Healthc. 17, 3167–3179. doi:10.2147/JMDH.S471372
Jie, F., Yang, X., Yang, B., Liu, Y., Wu, L., and Lu, B. (2022). Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. Biomed. Pharmacother. 153, 113317. doi:10.1016/j.biopha.2022.113317
Jie, Y., and Kang, Y. U. (2017). Mechanism and effect of phytosterols on lowering lipids: a systematic review and meta-analysis. Chin. J. Clin. Nutr. (6), 335–349. doi:10.3760/cma.j.issn.1674-635X.2017.06.002
Jm, S., Wl, B., R, T., and Coleman, C. I. (2009). The effect of adding plant sterols or stanols to statin therapy in hypercholesterolemic patients: systematic review and meta-analysis. J. Am. Coll. Nutr. 28 (5), 517–524. doi:10.1080/07315724.2009.10719784
Karr, S. (2017). Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 23 (9 Suppl. l), S139–S148.
Khallouki, F., Zennouhi, W., Hajji, L., Bourhia, M., Benbacer, L., El Bouhali, B., et al. (2024). Current advances in phytosterol free forms and esters: classification, biosynthesis, chemistry, and detection. Steroids 212, 109520. doi:10.1016/j.steroids.2024.109520
Kriengsinyos, W., Wangtong, A., and Komindr, S. (2015). Serum cholesterol reduction efficacy of biscuits with added plant stanol ester. Cholesterol 2015, 353164. doi:10.1155/2015/353164
Lestiani, L., Chandra, D. N., Laitinen, K., Ambarwati, F. D., Kuusisto, P., and Lukito, W. (2018). Double-blind randomized placebo controlled trial demonstrating serum cholesterol lowering efficacy of a smoothie drink with added plant stanol esters in an Indonesian population. Cholesterol 2018, 4857473. doi:10.1155/2018/4857473
Li, Q., and Xing, B. (2016). A phytosterol-enriched spread improves lipid profile and insulin resistance of women with gestational diabetes mellitus: a randomized, placebo-controlled double-blind clinical trial. Diabetes Technol. Ther. 18 (8), 499–504. doi:10.1089/dia.2016.0103
Liang, Y. T., Wong, W. T., Guan, L., Tian, X. Y., Ma, K. Y., Huang, Y., et al. (2011). Effect of phytosterols and their oxidation products on lipoprotein profiles and vascular function in hamster fed a high cholesterol diet. Atherosclerosis 219 (1), 124–133. doi:10.1016/j.atherosclerosis.2011.06.004
Liu, Y., and Zhiping, Q. (2023). Beware of the hepatotoxicity and myotoxicity of statins. Dr. Gan (5), 44–45.
Malaguti, M., Dinelli, G., Leoncini, E., Bregola, V., Bosi, S., Cicero, A. F. G., et al. (2014). Bioactive peptides in cereals and legumes: agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 15 (11), 21120–21135. doi:10.3390/ijms151121120
Moreau, R. A., Nyström, L., Whitaker, B. D., Winkler-Moser, J. K., Baer, D. J., Gebauer, S. K., et al. (2018). Phytosterols and their derivatives: structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 70, 35–61. doi:10.1016/j.plipres.2018.04.001
Moreno-Fernández, S., Garcés-Rimón, M., Uranga, J. A., Astier, J., Landrier, J. F., and Miguel, M. (2018). Expression enhancement in brown adipose tissue of genes related to thermogenesis and mitochondrial dynamics after administration of pepsin egg white hydrolysate. Food Funct. 9 (12), 6599–6607. doi:10.1039/c8fo01754a
Nattagh-Eshtivani, E., Barghchi, H., Pahlavani, N., Barati, M., Amiri, Y., Fadel, A., et al. (2022). Biological and pharmacological effects and nutritional impact of phytosterols: a comprehensive review. Phytotherapy Res. PTR 36 (1), 299–322. doi:10.1002/ptr.7312
Nechchadi, H., Nadir, Y., Benhssaine, K., Alem, C., Sellam, K., Boulbaroud, S., et al. (2024). Hypolipidemic activity of phytochemical combinations: a mechanistic review of preclinical and clinical studies. Food Chem. 459, 140264. doi:10.1016/j.foodchem.2024.140264
Oliveira, G. I. A., Sutti Nunes, V., Silva, A. M., Regina Nakandakare, E., da Silva Ferreira, G., de Paula Assis Bombo, R., et al. (2020). Phytosterols supplementation reduces Endothelin-1 plasma concentration in moderately hypercholesterolemic individuals independently of their cholesterol-lowering properties. Nutrients 12 (5), 1507. doi:10.3390/nu12051507
Orem, A., Alasalvar, C., Kural, B. V., Yaman, S., Orem, C., Karadag, A., et al. (2017). Cardio-protective effects of phytosterol-enriched functional black tea in mild hypercholesterolemia subjects. J. Funct. Foods 31, 311–319. doi:10.1016/j.jff.2017.01.048
Paalvast, Y., de Boer, J. F., and Groen, A. K. (2017). Developments in intestinal cholesterol transport and triglyceride absorption. Curr. Opin. Lipidol. 28 (3), 248–254. doi:10.1097/MOL.0000000000000415
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pan, Bo (2023). Research progress on clinical nursing of patients with hyperlipidemia. China Urban Rural Enterp. Health 38 (5), 45–47. doi:10.16286/j.1003-5052.2023.05.015
Pannu, P. S., Allahverdian, S., and Francis, G. A. (2013). Oxysterol generation and liver X receptor-dependent reverse cholesterol transport: not all roads lead to Rome. Mol. Cell Endocrinol. 368 (1-2), 99–107. doi:10.1016/j.mce.2012.07.013
Poli, A., Marangoni, F., Corsini, A., Manzato, E., Marrocco, W., Martini, D., et al. (2021). Phytosterols, cholesterol control, and cardiovascular disease. Nutrients 13 (8), 2810. doi:10.3390/nu13082810
Ras, R. T., Fuchs, D., Koppenol, W. P., Garczarek, U., Greyling, A., Keicher, C., et al. (2015). The effect of a low-fat spread with added plant sterols on vascular function markers: results of the investigating vascular function effects of plant sterols (INVEST) study. Am. J. Clin. Nutr. 101 (4), 733–741. doi:10.3945/ajcn.114.102053
Rideout, T. C., Marinangeli, C. P. F., and Harding, S. V. (2015). Triglyceride-lowering response to plant sterol and stanol consumption. J. AOAC Int. 98 (3), 707–715. doi:10.5740/jaoacint.SGERideout
Shi, Y., Han, S. F., Zhu, R. F., Feng, Y. Q., Cheng, J. X., and Kou, L. H. (2023). Research progress on the cardioprotective effect of legumes non-nutrients. J. Cardio-Cerebrovascular Dis. Integr. Traditional Chin. West. Med. 21 (10), 1795–1799.
Stroes, E. S., Thompson, P. D., Corsini, A., Vladutiu, G. D., Raal, F. J., Ray, K. K., et al. (2015). Statin-associated muscle symptoms: impact on statin therapy-European atherosclerosis society consensus panel statement on assessment, aetiology and management. Eur. Heart J. 36 (17), 1012–1022. doi:10.1093/eurheartj/ehv043
Sun, J., Xu, D., Xie, H., Wang, Y., Chen, M., Chang, X., et al. (2014). Low fat milk powder containing esterified plant sterols improves the blood lipid profile of adults with hypercholesterolemia. Zhonghua Xin Xue Guan Bing Za Zhi 42 (7), 588–592.
Theuwissen, E., Plat, J., van der Kallen, C. J., van Greevenbroek, M. M., and Mensink, R. P. (2009). Plant stanol supplementation decreases serum triacylglycerols in subjects with overt hypertriglyceridemia. Lipids 44 (12), 1131–1140. doi:10.1007/s11745-009-3367-6
Valitova, J. N., Sulkarnayeva, A. G., and Minibayeva, F. V. (2016). Plant sterols: diversity, biosynthesis, and physiological functions. Biochem. (Mosc). 81 (8), 819–834. doi:10.1134/S0006297916080046
Vásquez-Trespalacios, E. M., and Romero-Palacio, J. (2014). Efficacy of yogurt drink with added plant stanol esters (Benecol®, Colanta) in reducing total and LDL cholesterol in subjects with moderate hypercholesterolemia: a randomized placebo-controlled crossover trial NCT01461798. Lipids Health Dis. 13, 125. doi:10.1186/1476-511X-13-125
Wang, H., Yu, P., Ren, L., Miao, J. I., Cai, T., Xiao, Y., et al. (2015). Study on the effect of functional milk on lowering blood lipid in human diet. Food Ind. 36 (4), 214–217.
Wang, X. F., Lu, Y. L., Du, G. X., Sun, X. C., and Yi, F. L. (2023). Trend analysis of mortality and probability of premature death of cardiovascular disease in China from 2009 to 2019. Chin. J. Prev. Med. 24 (9), 901–905. doi:10.16506/j.1009-6639.2023.09.004
Writing Group Members, Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., et al. (2016). Heart disease and stroke Statistics-2016 update: a report from the American heart association. Circulation 133 (15), e599. doi:10.1161/CIR.0000000000000409
Xue, Y. T., Zhang, X. F., and Zhang, D. J. (2019). Research progress on the lipid-lowering effect of phytosterols. West China Pharm. J. 34 (1), 92–97. doi:10.13375/j.cnki.wcjps.2019.01.022
Yang, L., Li, Z., Song, Y., Liu, Y., Zhao, H., Liu, Y., et al. (2019). Study on urine metabolic profiling and pathogenesis of hyperlipidemia. Clin. Chim. Acta 495, 365–373. doi:10.1016/j.cca.2019.05.001
Yuen, K. C. J., Biller, B. M. K., Radovick, S., Carmichael, J. D., Jasim, S., Pantalone, K. M., et al. (2019). American association of clinical endocrinologists and American college of endocrinology guidelines for management of growth hormone deficiency in adults and patients transitioning from pediatric to adult care. Endocr. Pract. 25 (11), 1191–1232. doi:10.4158/GL-2019-0405
Keywords: phytosterols, hyperlipidemia, blood lipids, inflammatory markers, systematic review
Citation: Zhang Y, Zhang Q, Wang X, Jia Y, Niu Q, Ding S and Li W (2025) Effects of phytosterol-rich foods on lipid profile and inflammatory markers in patients with hyperlipidemia: a systematic review and meta-analysis. Front. Pharmacol. 16:1619922. doi: 10.3389/fphar.2025.1619922
Received: 30 April 2025; Accepted: 11 June 2025;
Published: 02 July 2025.
Edited by:
Aleksandra Sknepnek, University of Belgrade, SerbiaReviewed by:
Shooka Mohammadi, University of Malaya, MalaysiaMoumita Das, Swami Vivekananda University, India
Copyright © 2025 Zhang, Zhang, Wang, Jia, Niu, Ding and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Zhang, emhhbmdxaWFuQGQuc3htdS5lZHUuY24=
 Yihua Zhang
Yihua Zhang Qian Zhang2*
Qian Zhang2* Yatian Jia
Yatian Jia