Abstract
Background:
Lung adenocarcinoma with HER2 exon 20 insertions represents a rare subset of non-small cell lung cancer (NSCLC) associated with aggressive behavior and limited treatment options.
Case description:
This case report highlights the first perioperative use of trastuzumab deruxtecan (T-DXd) in a 51-year-old male diagnosed with HER2 exon 20-mutant pulmonary adenocarcinoma (T1cN2M0). At diagnosis, a 2.2 × 2.8 × 1.5 cm lesion in the right upper lobe showed moderately elevated metabolic activity (SUVmax 4.20). An initial cycle of chemoimmunotherapy failed to achieve significant tumor reduction. Genetic testing revealed HER2 exon 20 and TP53 mutations, prompting a shift to two cycles of neoadjuvant T-DXd, which reduced the tumor size to 2.0 × 2.4 × 1.0 cm with substantially lowered metabolic activity (SUVmax 1.38). The lesion was successfully resected, confirming invasive adenocarcinoma with 30% viable tumor tissue and 70% stromal tissue, indicating partial tumor suppression. The patient received 14 cycles of adjuvant T-DXd, achieving 28 months of sustained remission. Molecular residual disease (MRD) monitoring through circulating tumor DNA (ctDNA) was conducted throughout adjuvant therapy, initially showing no detectable disease. However, ctDNA positivity with HER2 and TP53 mutations emerged later, prompting timely treatment adjustments.
Conclusion:
This case shows that perioperative treatment with T-DXd could induce tumor regression and sustain remission, while MRD monitoring enables early detection of molecular progression. These findings emphasize the potential of combining T-DXd and MRD monitoring to tailor treatment to improve outcomes in HER2-mutant NSCLC.
1 Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide, with lung adenocarcinoma being its most common subtype, with its treatments being continuously improved based on recent clinical trials (Bray et al., 2024). However, the management of patients with rare genetic mutations, such as human epidermal receptor 2 (HER2) mutations, remains challenging due to limited clinical evidence and the absence of standardized treatment guidelines. Currently, though chemotherapy in combination with immune therapy has become the standard of care for operable lung cancer, evidence for the efficacy of the combination therapy is lacking in HER2-mutated patients and it remains unknown whether the combination therapy has better efficacy than chemotherapy alone. Particularly, for rare mutations such as HER2 exon 20 insertions, available perioperative data remain limited (Rossi and Di Maio, 2016). Thus, there is a growing need to explore novel therapeutic approaches that may improve surgical and oncologic outcomes in this rare subset of lung adenocarcinoma.
HER2 mutations, including exon 20 insertions, are identified in approximately 2%–4% of non-small cell lung cancer (NSCLC) cases (Sentana-Lledo et al., 2023) and are often associated with aggressive tumor behavior and poor prognosis (Choudhary et al., 2024) as they confer insensitivity to traditional tyrosine kinase inhibitors (TKIs), highlighting the need for specific targeted therapies. However, lung cancer harboring G778_P780 variant seems to be sensitive to afatinib (Fang et al., 2020; Zhao et al., 2020) and amivantamab and zipalertinib are active drugs in this setting. Trastuzumab deruxtecan (T-DXd), a HER2-directed antibody-drug conjugate, has demonstrated promising efficacy in later-line treatment and has been approved for HER2-mutant advanced NSCLC (Goto et al., 2023; Cheng et al., 2024). Available evidence supports adjuvant or neoadjuvant targeted therapy for patients with lung cancer harboring EGFR-mutations or ALK fusions in the perioperative setting. Meanwhile, such evidence is lacking for surgically resectable NSCLC, which represents a major evidence gap in current lung cancer management, especially for patients carrying HER2 exon 20 insertion mutations that may respond differently to treatment. Therefore, addressing this gap is of particular importance, as effective perioperative therapy could enhance tumor downstaging, facilitate complete resection, and improve long-term outcomes in these patients.
T-DXd is an antibody-drug conjugate that combines trastuzumab, a monoclonal antibody targeting HER2, with a topoisomerase I inhibitor via a cleavable linker for precise delivery of cytotoxic agents to HER2-expressing cancer cells (Nakada et al., 2019). This unique mechanism has led to remarkable efficacy in reducing tumor burden and improved survival across various cancers. Herein, we present a case report that describes the first documented use of T-DXd in a perioperative regimen for a patient with HER2 exon 20 insertion-containing lung adenocarcinoma, which provides preliminary clinical evidence supporting the potential of T-DXd as a neoadjuvant and adjuvant therapy, where it provided notable tumor regression before surgery and contributed to durable postoperative remission.
2 Case presentation
In January 2022, a 51-year-old male (smoker, 3–5 cigarettes/day) with a history of untreated mild fatty liver, high cholesterol, and hyperuricemia underwent a routine physical examination. A chest positron emission tomography-computed tomography (PET-CT) scan revealed an irregular mass in the anterior segment of the right upper lobe and adhesions to the chest wall and the interlobar pleura, raising suspicion of lung cancer (Figure 1A). A18F-FDG PET-CT scan confirmed the lesion to be moderately elevated with uneven metabolic activity (Figure 1B), showing adherence to the pleura and chest wall with associated deformation, suggesting malignancy. Fibrobronchoscopic biopsy confirmed lung adenocarcinoma with lymph node metastasis (4R/11R), with peripheral blood genetic testing showing no EGFR mutations by amplification refractory mutation system (ARMS) method. Immunohistochemistry was positive for cytokeratin (CK) 7, napsin A, and thyroid transcription factor-1 (TTF-1), and negative for CK20, P40, and programmed death-1 (PD-1), with programmed death-ligand 1 (PD-L1) expression at 1%. The tumor tissue was negative for mucicarmine, indicating the absence of mucin production. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed at stations 4R and 11R, with histopathologic analysis confirming metastatic carcinoma. Based on these, the patient was staged as IIIA (post-neoadjuvant pathological tumor stage T1cN2M0).
FIGURE 1
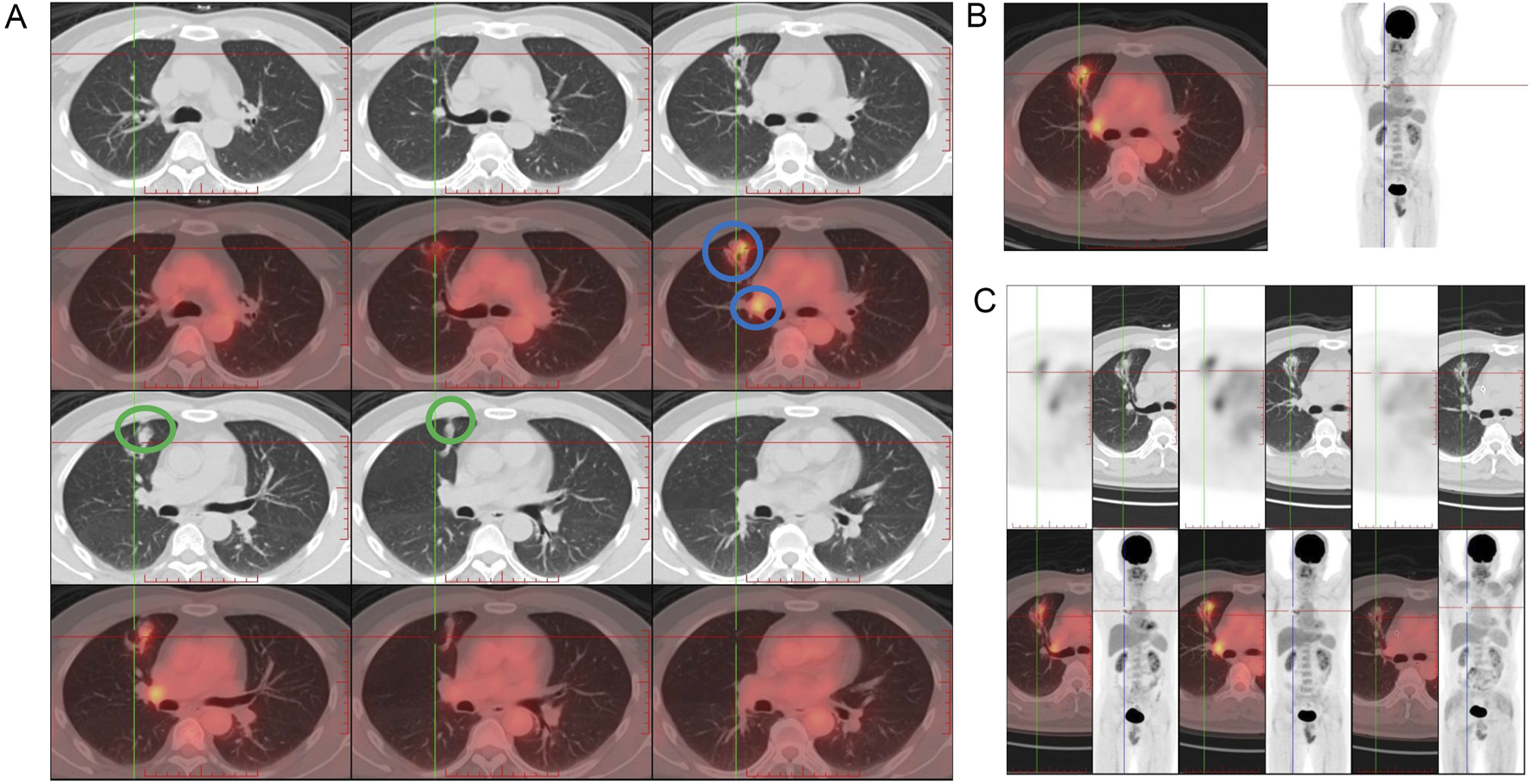
Initial imaging findings at diagnosis in January 2022. (A) Positron emission tomography-computed tomography (PET-CT) scan at the time of diagnosis in January 2022 revealed an irregular mass and involved lymph node (blue circles) in the anterior segment of the right upper lobe, raising suspicion of malignancy and adhesions to the chest wall and the interlobar pleura (green circle). (B) PET-CT scan showed moderately elevated and heterogeneous metabolic activity of the lesion and mildly hypermetabolic right hilar lymph nodes consistent with nodal involvement (left panel) and potential adhesion of the lesion to the anterior chest wall and to the interlobar pleura along the right horizontal fissure, with tractional deformation of the interlobar pleura. The coronal view is shown in the right panel. (C) After two cycles of T-DXd treatment, PET-CT scan in April 2022 showed a significant reduction in the size of the right upper lobe lesion.
In early February 2022, while waiting for the genetic test results of tissue samples, the patient was prescribed one cycle of pembrolizumab (200 mg, intravenous), bevacizumab (500 mg, intravenous), pemetrexed (0.9 g, intravenous), and carboplatin (600 mg, intravenous) following a multidisciplinary treatment board. After 2 weeks, the lesion size showed no significant decrease under a follow-up PET-CT. The right hilar nodes maintained their size but showed a reduced SUVmax of 3.72, while the mediastinal nodes (4R) remained 0.6 cm with a lower SUVmax of 2.32, and the 2R nodes measured 0.8 cm with an SUVmax of 3.10. Subsequently, next-generation sequencing (NGS) revealed the presence of a non-frameshift insertion mutation, p.G778-P780dup, in exon 20 of the ERBB2 gene, with a variant allele frequency of 8.6%. Additionally, a TP53 p.I195T missense mutation in exon 6 was identified, with an abundance of 0.9%. The ARMS method with tissue samples also detected a mutation in HER2 exon 20. Simultaneously conducted peripheral blood NGS analysis identified an ERBB2 p.G778-P780dup non-frameshift insertion mutation in exon 20, with a lower abundance of 0.4%, and a tet methylcytosine dioxygenase 2 (TET2) p.F1104Lfs*2 frameshift mutation in exon 3. Based on these findings, the treatment plan was modified, and at the end of February and mid -March 2022, he was treated with two cycles of T-DXd (400 mg, intravenous). After the initial two cycles of neoadjuvant therapy (including 1 cycle of T-Dxd), a chest CT scan indicated stability of the right upper lobe lesion compared to earlier scans, while identifying a small ground-glass nodule in the left upper lobe, raising suspicion of a preinvasive lesion and warranting close monitoring. In April 2022 (after two cycles of T-DXd), PET-CT scan revealed a significant reduction in the size of the right upper lobe lesion to 2.0 cm × 2.4 cm × 1.0 cm from 2.2 cm × 2.7 cm × 1.5 cm on previous scan, with markedly reduced metabolic activity (SUVmax: 1.38, previously 4.26) (Figure 1C). The right hilar nodes decreased to 0.6 cm (SUVmax: 2.13; Figure 2A), and the mediastinal nodes were reduced to 0.4 cm (SUVmax: 1.69) in the 4R region and 0.5 cm (SUVmax: 1.66) in the 2R region.
FIGURE 2
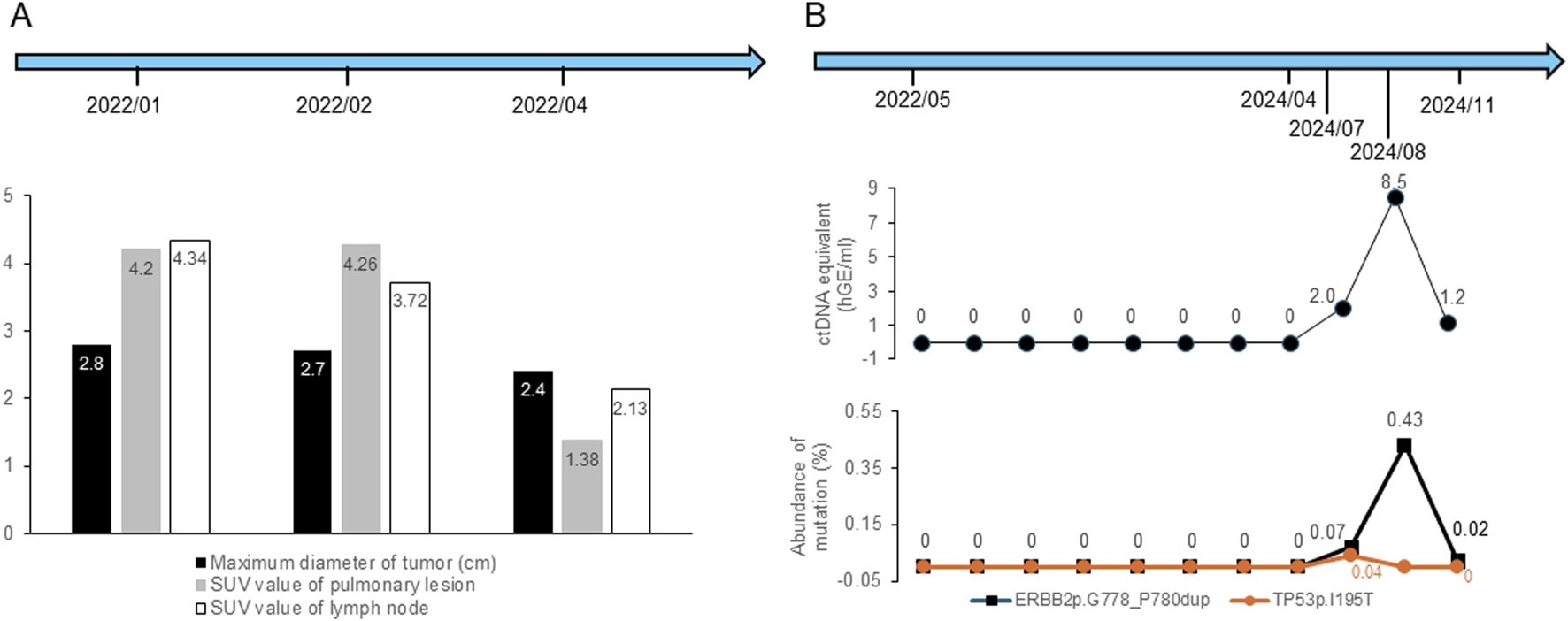
Changes in imaging parameters and ctDNA results during the treatments and follow-up periods. (A) Changes in maximum tumor diameter and SUV values of lung lesions and lymph nodes. (B) Changes in ctDNA concentration and mutation abundance. “ctDNA equivalent (hGE/mL)” refers to the total concentration of circulating tumor-derived DNA fragments quantified by digital PCR, expressed as haploid genome equivalents per milliliter of plasma.
Although multistation N2 involvement is generally considered a relative contraindication for surgical resection under most guidelines, the patient showed marked tumor and nodal regression on PET-CT and strongly requested surgical treatment after multidisciplinary discussions, and the patient underwent radical right upper lobectomy under general anesthesia via 3D single-port thoracoscopy in April 2022 at another center., and postoperative pathology showed that 30% of the tumor tissue remained viable, while 70% was stromal tissue without necrosis, indicating tumor suppression but no downstaging. In addition, it confirmed invasive adenocarcinoma with complex glandular structures. Overall, the tumor measured 2.5 cm in diameter and was classified as grade 3 (poorly differentiated) based on the World Health Organization (WHO) classification of invasive lung adenocarcinomas. Immunohistochemical analysis revealed that the tumor tissue was positive for CK7 and TTF-1 and negative for P40, with no mucin production, suggesting that the cancer had not penetrated beyond the elastic layer of the visceral pleura (PL0), as well as absence of vascular emboli and nervous involvement. Lymph node involvement was observed in the 2R, 4R, and 10R lymph nodes. From May 2022 to September 2023, the patient received 14 cycles of adjuvant T-DXd therapy. Concurrently, eight circulating tumor DNA (ctDNA) samples were obtained for monitoring molecular residual disease (MRD) between May 2022 and April 2024, all of which yielded no detectable recurrence. In addition, a chest CT scan in February 2024 revealed no recurrence, and a cranial magnetic resonance imaging (MRI) in March 2024 showed no signs of distant metastasis. However, in July 2024, MRD monitoring identified a ctDNA concentration of 2 hGE/mL, with detectable HER2 and TP53 mutations. Whole body PET-MRI and chest PET-CT scan on 18 July 2024 indicated lymph node involvement, and MRD monitoring in August 2024 revealed a ctDNA concentration of 8.5 hGE/mL. The patient underwent volumetric modulated arc therapy (VMAT) until October 2024 (GTV 66 Gy/30 F, CTV 54 Gy/30 F). and consequently, the patient was treated with two cycles of albumin-bound paclitaxel plus cisplatin during radiotherapy in August and September 2024. A subsequent MRD test in November 2024 showed a ctDNA concentration of 1.2 hGE/mL (Figure 2). Treatment was switched to T-DXd, which was administered for two cycles in December 2024, and the patient discontinued treatment in May 2025.
During treatment, he experienced grade 2 nausea, vomiting, and transient elevations in alanine amino transferase (ALT; peak: 81 U/L) and γ-glutamyl transferase (γ-GT; peak: 80 U/L), which resolved without medication or dose adjustments. γ-GT peaked in February 2022 and ALT peaked in December 2022. Blood tests and cardiac assessments, including ECG till April 2024, remained normal.
3 Discussion
This case demonstrates significant tumor regression with neoadjuvant and adjuvant T-DXd therapy. After the initial immunotherapy and chemotherapy could not significantly reduce the tumor size and metabolic activity, two cycles of neoadjuvant T-DXd were given, which resulted in a promising decrease in metabolic activity. Postoperative adjuvant T-DXd maintained these effects, with no recurrence over 28 months. Although T-DXd is currently approved for HER2-mutant lung cancer in the later-line setting (CSCO, 2024), this case highlights its potential in perioperative management for HER2 exon 20 insertion-mutant NSCLC, an aggressive subtype. The favorable tumor response contributed to successful, complication-free surgical resection.
Until now, no major oncology guidelines recommend using T-DXd in the neoadjuvant or adjuvant settings for HER2-mutant NSCLC (CSCO, 2024; NCCN, 2024), and its perioperative use remains investigational. T-DXd was approved in China in October 2024 for HER2-mutant NSCLC, marking a significant step forward in expanding treatment options for this challenging subgroup beyond traditional chemotherapy and non-targeted approaches. Before T-DXd approval, advanced HER2-mutant NSCLC was often treated using protocols similar to those for driver gene-negative cases, relying primarily on standard chemotherapy with/without immunotherapy or anti-angiogenic drugs due to the absence of targeted therapies specific to HER2 mutations (Tsuboi et al., 2023; Wu et al., 2024). The 2021 Chinese Society of Clinical Oncology (CSCO) guidelines provided a Grade III recommendation for pyrotinib, a small-molecule tyrosine kinase inhibitor, as a later-line treatment for HER2-mutant advanced NSCLC (Wang et al., 2022).
Historically, neoadjuvant chemotherapy for NSCLC has demonstrated only moderate efficacy, with limited long-term survival benefits (Forde et al., 2022) while the evidence for the efficacy of chemoimmunotherapy, the current standard of care, remains scant in HER2-mutated patients. In our case, the response observed suggests that T-DXd can achieve meaningful tumor regression, allowing complete resection. The ADAURA (Tsuboi et al., 2023) and ALINA (Wu et al., 2024) trials represent important references for the case discussed, as they highlighted the transformative potential of integrating targeted therapies into postoperative management of oncogenic-driven NSCLC. Although these trials focused on EGFR and ALK alterations, they underscore the importance of mutation-specific adjuvant treatment, a principle applicable to HER2-mutant disease as well.
T-DXd was prioritized over other HER2-targeted agents because its antibody-drug conjugate mechanism enables direct delivery of a topoisomerase I inhibitor to HER2-expressing tumor cells and exerts a bystander effect that enhances activity even in tumors with heterogeneous HER2 expression. Small-molecule TKIs or monoclonal antibodies have shown limited activities in lung cancer whereas evidence from the DESTINY-Lung02 and related studies demonstrated efficacy and sustained disease control with T-DXd (Goto et al., 2023), providing the rationale for selecting T-DXd. These mechanisms may also explain the marked stromal tissue replacement (70%) post-treatment, suggesting extensive tumor cell clearance and fibroinflammatory remodeling, which are consistent with antibody-drug conjugate activity.
For our case, T-DXd was chosen due to the presence of an ERBB2 p.G778-P780dup non-frameshift insertion mutation in exon 20, often resistant to classical EGFR TKIs (Zeng et al., 2021), thus, making targeted HER2 inhibitors more suitable. In addition, it was approved as a Grade III recommendation for HER2-mutant NSCLC treatment in China and was recently elevated to a Grade II recommendation for the same indication, though not in the adjuvant setting (Yingge et al., 2023; CSCO, 2024). With an abundance of 8.6%, the ERBB2 mutation represented a significant driver of the tumor’s behavior, highlighting the potential efficacy of T-DXd. Furthermore, the co-occurrence of a TP53 p.I195T missense mutation in exon 6, although present at a lower abundance (0.9%) and may lead to a more aggressive tumor phenotype and poorer prognosis (Kelemen et al., 2018), our patient demonstrated robust antitumor activity with T-DXd, possibly due to its mechanism of delivering a potent cytotoxic payload directly to HER2-expressing cells and its unique bystander effect that improves efficacy even in heterogeneous tumors (Yver et al., 2020). While the integration of targeted therapies like T-DXd in the adjuvant setting aligns with the broader trend of tailoring treatment to specific oncogenic drivers, these advances highlight the need for further research and large-scale clinical trials for validation.
The recurrence observed in this case may be attributed to the aggressive nature of HER2 exon 20-mutant NSCLC, characterized by tumor heterogeneity and therapy resistance, compounded by co-occurring TP53 mutations contributing to genomic instability. Moreover, preoperative regional lymph node metastasis and the residual viable tumor tissue (30%) post-surgery might have also be contributors for recurrence (Dickhoff et al., 2016), potentially due to inadequate neoadjuvant treatment. However, it should be noted that the optimal duration of adjuvant therapy remains an area of investigation as there is limited evidence on the appropriate length of anti-HER2 therapy in the perioperative setting. In addition, caution is advised in interpreting the results of this single case report, which need to be validated in multicenter cohort studies. In this case, 14 cycles of T-DXd were administered over 16 months, followed by a switch to salvage therapy upon recurrence. The ctDNA-based MRD monitoring successfully revealed potential micrometastatic disease (Xue et al., 2024), which was undetectable by imaging, thereby offering a significant advantage by detecting molecular progression earlier than imaging, enabling timely interventions and even allowing therapy de-escalation when MRD returned to negative, improving patient outcomes and reducing toxicity. However, further research is required to standardize the duration of adjuvant therapy and the optimal use of MRD status, which could help improve outcomes while minimizing unnecessary treatment, especially for aggressive cancer.
4 Conclusion
This case highlights T-DXd’s efficacy in treating HER2 exon 20-mutant NSCLC, showing that it achieved significant tumor regression that allowed for complete surgical resection and long-term remission. In addition, MRD monitoring was valuable for the early detection of molecular progression and facilitated timely therapeutic intervention. Further studies are warranted to validate the use of T-DXd in perioperative settings and to optimize MRD-guided management strategies for this aggressive subtype.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Guangdong Provincial Hospital of Traditional Chinese Medicine (NO: G2025-04). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WL: Data curation, Methodology, Writing – review and editing, Visualization, Investigation, Formal Analysis, Conceptualization, Writing – original draft, Project administration. ZX: Writing – review and editing, Data curation, Writing – original draft, Investigation, Methodology, Visualization, Conceptualization, Formal Analysis. JY: Investigation, Writing – original draft, Visualization, Formal Analysis. LL: Visualization, Investigation, Writing – original draft, Formal Analysis. XC: Project administration, Methodology, Data curation, Formal Analysis, Conceptualization, Writing – original draft, Writing – review and editing, Visualization, Investigation. HZ: Writing – review and editing, Investigation, Formal Analysis, Methodology, Data curation, Conceptualization, Project administration, Visualization, Writing – original draft.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALT, alanine aminotransferase; ARMs, amplification-refractory mutation system; CK, cytokeratin; CK7, cytokeratin 7; CK20, cytokeratin 20; CSCO, Chinese Society of Clinical Oncology; ctDNA: circulating tumor DNA; ECG, electrocardiogram; EGFR, epidermal growth factor receptor; ERBB2, erb-b2 receptor tyrosine kinase 2; GGT, gamma-glutamyl transferase; HER2, human epidermal growth factor receptor 2; MRD, minimal residual disease; MRI, magnetic resonance imaging; NGS, next-generation sequencing; NSCLC, non-small cell lung cancer; PET-CT, positron emission tomography-computed tomography; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PL0, pleural invasion status 0; T-DXd, trastuzumab deruxtecan; TET2, tet methylcytosine dioxygenase 2; TTF-1, thyroid transcription factor-1; WHO, World Health Organization
References
1
Bray F. Laversanne M. Sung H. Ferlay J. Siegel R. L. Soerjomataram I. et al (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74 (3), 229–263. 10.3322/caac.21834
2
Cheng Y. Wu L. Fang Y. Fan Y. Li X. Zhang M. et al (2024). Abstract CT248: trastuzumab deruxtecan (T-DXd) in Chinese patients (pts) with previously treated HER2 mutant non-small cell lung cancer (NSCLC): primary analysis from the phase 2 DESTINY-Lung05 (DL-05) trial. Cancer Res.84 (7_Suppl. ment), CT248. 10.1158/1538-7445.Am2024-ct248
3
Choudhary R. K. Koppaka D. Vaid A. K. Kulkarni S. S. Bose C. Morzaria D. D. et al (2024). Spectrum of HER2 alterations in NSCLC: comparative analysis of tissue and liquid biopsies in lung cancer. J. Clin. Oncol.42 (16_Suppl. l), e20602. 10.1200/JCO.2024.42.16_suppl.e20602
4
CSCO (2024). Guidelines of Chinese society of clinical oncology (CSCO): non-small cell lung cancer 2024. 1st ed. Beijing: People's Medical Publishing House.
5
Dickhoff C. Dahele M. Paul M. A. van de Ven P. M. de Langen A. J. Senan S. et al (2016). Salvage surgery for locoregional recurrence or persistent tumor after high dose chemoradiotherapy for locally advanced non-small cell lung cancer. Lung Cancer94, 108–113. 10.1016/j.lungcan.2016.02.005
6
Fang W. Zhao S. Liang Y. Yang Y. Yang L. Dong X. et al (2020). Mutation variants and co-mutations as genomic modifiers of response to afatinib in HER2-mutant lung adenocarcinoma. Oncologist25 (3), e545–e554. 10.1634/theoncologist.2019-0547
7
Forde P. M. Spicer J. Lu S. Provencio M. Mitsudomi T. Awad M. M. et al (2022). Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med.386 (21), 1973–1985. 10.1056/NEJMoa2202170
8
Goto K. Goto Y. Kubo T. Ninomiya K. Kim S.-W. Planchard D. et al (2023). Trastuzumab deruxtecan in patients with HER2-mutant metastatic non–small-cell lung cancer: primary results from the randomized, phase II DESTINY-Lung02 trial. J. Clin. Oncol.41 (31), 4852–4863. 10.1200/JCO.23.01361
9
Kelemen L. E. Brenton J. D. Bowtell D. D. Fridley B. L. (2018). Abstract A14: TP53 missense mutations associate with different metabolic pathways. Clin. Cancer Res.24 (15_Suppl.), A14. 10.1158/1557-3265.Ovca17-a14
10
Nakada T. Sugihara K. Jikoh T. Abe Y. Agatsuma T. (2019). The latest research and development into the antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a), for HER2 cancer therapy. Chem. Pharm. Bull. (Tokyo)67 (3), 173–185. 10.1248/cpb.c18-00744
11
NCCN (2024). Non-small cell lung cancer. NCCN Clinical Practice Guidelines in Oncology.
12
Rossi A. Di Maio M. (2016). Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev. Anticancer Ther.16 (6), 653–660. 10.1586/14737140.2016.1170596
13
Sentana-Lledo D. Academia E. Viray H. Rangachari D. Kobayashi S. S. VanderLaan P. A. et al (2023). EGFR exon 20 insertion mutations and ERBB2 mutations in lung cancer: a narrative review on approved targeted therapies from oral kinase inhibitors to antibody-drug conjugates. Transl. Lung Cancer Res.12 (7), 1590–1610. 10.21037/tlcr-23-98
14
Tsuboi M. Herbst R. S. John T. Kato T. Majem M. Grohe C. et al (2023). Overall survival with osimertinib in resected EGFR-mutated NSCLC. N. Engl. J. Med.389 (2), 137–147. 10.1056/NEJMoa2304594
15
Wang J. Zhang B. Cheng X. Li Q. Lv H. Nie C. et al (2022). Retrospective study on the efficacy and safety of pyrotinib-based therapy for HER2-positive nonbreast advanced solid tumors. J. Oncol.2022, 4233782. 10.1155/2022/4233782
16
Wu Y. L. Dziadziuszko R. Ahn J. S. Barlesi F. Nishio M. Lee D. H. et al (2024). Alectinib in resected ALK-positive non-small-cell lung cancer. N. Engl. J. Med.390 (14), 1265–1276. 10.1056/NEJMoa2310532
17
Xue W. Chen Q. Wang X. Kong W. Zhang J. Chen Y. et al (2024). A study on the value of ctDNA-based molecular residual disease (MRD) in dynamic monitoring in the clinical treatment of advanced/metastatic renal cell carcinoma. J. Clin. Oncol.42 (16_Suppl. l), e16522. 10.1200/JCO.2024.42.16_suppl.e16522
18
Yingge L. Yi H. Shuyang Y. Yingmei W. Qibin S. Yi Y. (2023). Diagnostic and therapeutic strategy updates of rare oncogenic mutations in Chinese society of clinical oncology guidelines on diagnosis and treatment of non-small cell lung cancer. Cancer Res. Prev. Treat.50 (12), 1232–1236.
19
Yver A. Agatsuma T. Soria J. C. (2020). The art of innovation: clinical development of trastuzumab deruxtecan and redefining how antibody-drug conjugates target HER2-positive cancers. Ann. Oncol.31 (3), 430–434. 10.1016/j.annonc.2019.11.019
20
Zeng J. Ma W. Young R. B. Li T. (2021). Targeting HER2 genomic alterations in non-small cell lung cancer. J. Natl. Cancer Cent.1 (2), 58–73. 10.1016/j.jncc.2021.04.001
21
Zhao S. Fang W. Pan H. Yang Y. Liang Y. Yang L. et al (2020). Conformational landscapes of HER2 exon 20 insertions explain their sensitivity to kinase inhibitors in lung adenocarcinoma. J. Thorac. Oncol.15 (6), 962–972. 10.1016/j.jtho.2020.01.020
Summary
Keywords
NSCLC, ADC, HER2 , exon 20 insertion, trastuzumab deruxtecan (T-DXd), perioperative, targeted therapy
Citation
Li W, Xiao Z, Yang J, Liu L, Chai X and Zhang H (2026) Case Report: Perioperative application of trastuzumab deruxtecan in HER2 exon 20 mutation-positive non-small cell lung cancer. Front. Pharmacol. 16:1629854. doi: 10.3389/fphar.2025.1629854
Received
16 May 2025
Revised
10 November 2025
Accepted
30 November 2025
Published
07 January 2026
Volume
16 - 2025
Edited by
Jinwei Zhang, University of Exeter, United Kingdom
Reviewed by
Nuno Gil, Champalimaud Foundation, Portugal
Yuanyuan Huang, Sun Yat-sen University Cancer Center (SYSUCC), China
Updates
Copyright
© 2026 Li, Xiao, Yang, Liu, Chai and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Zhang, haibozh@gzucm.edu.cn; Xiaoshu Chai, chaixiaoshu@126.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.