Abstract
Dendrobium nobile Lindl. (D. nobile) has significant medicinal value. D. nobile is used in traditional Chinese medicine and is widely popular as a functional food and health supplement due to its nourishing properties and high safety. Among its key bioactive constituents, polysaccharides exhibit promising applications across medicine, personal care, food, and agriculture, owing to their anti-photoaging, improvement of complications of diabetes mellitus, ovarian protective, gastric protective, neuroprotective, anti-inflammatory and anti-viral effects. Despite these multifaceted benefits, research on D. nobile polysaccharides remains limited relative to more extensively studied components such as alkaloids and flavonoids. This review systematically summarizes current advances in extraction techniques, structural features, bioactivities, and structure–activity relationships of D. nobile polysaccharides, providing a theoretical framework for their future medical development and application.
1 Introduction
Nature represents an abundant reservoir of botanical resources, with diverse climatic conditions, soil compositions, topographies, and landforms fostering a wide array of plant species. Among these, medicinal plants possess significant therapeutic potential, offering remedies for various ailments and contributing to human health and wellbeing (Yang et al., 2023). Within the Orchidaceae family, Dendrobium ranks among the three principal genera, encompassing over 1,602 recognized species worldwide (Plants of the World Online) (Wang, 2021a). Distributed extensively across Asia, more than 80 Dendrobium species have been documented in China (Zhai et al., 2022). Dendrobium species, such as Dendrobium nobile Lindl. (D. nobile), have been included in the Chinese Pharmacopoeia (2020 edition). Among them, there is relatively more research on D. nobile. Epiphytically growing on shady, humid trees or rocks under natural conditions, D. nobile has a fleshy and jointed stem, leathery leaves, and violet-hued flowers (Zhu et al., 2025) (Figure 1). Due to its prolific blooms and extended flowering period, it commands a significant place in the global cut flower industry, highlighting its dual purpose as an ornament and medicinal plant (Martin and Madassery, 2006).
FIGURE 1
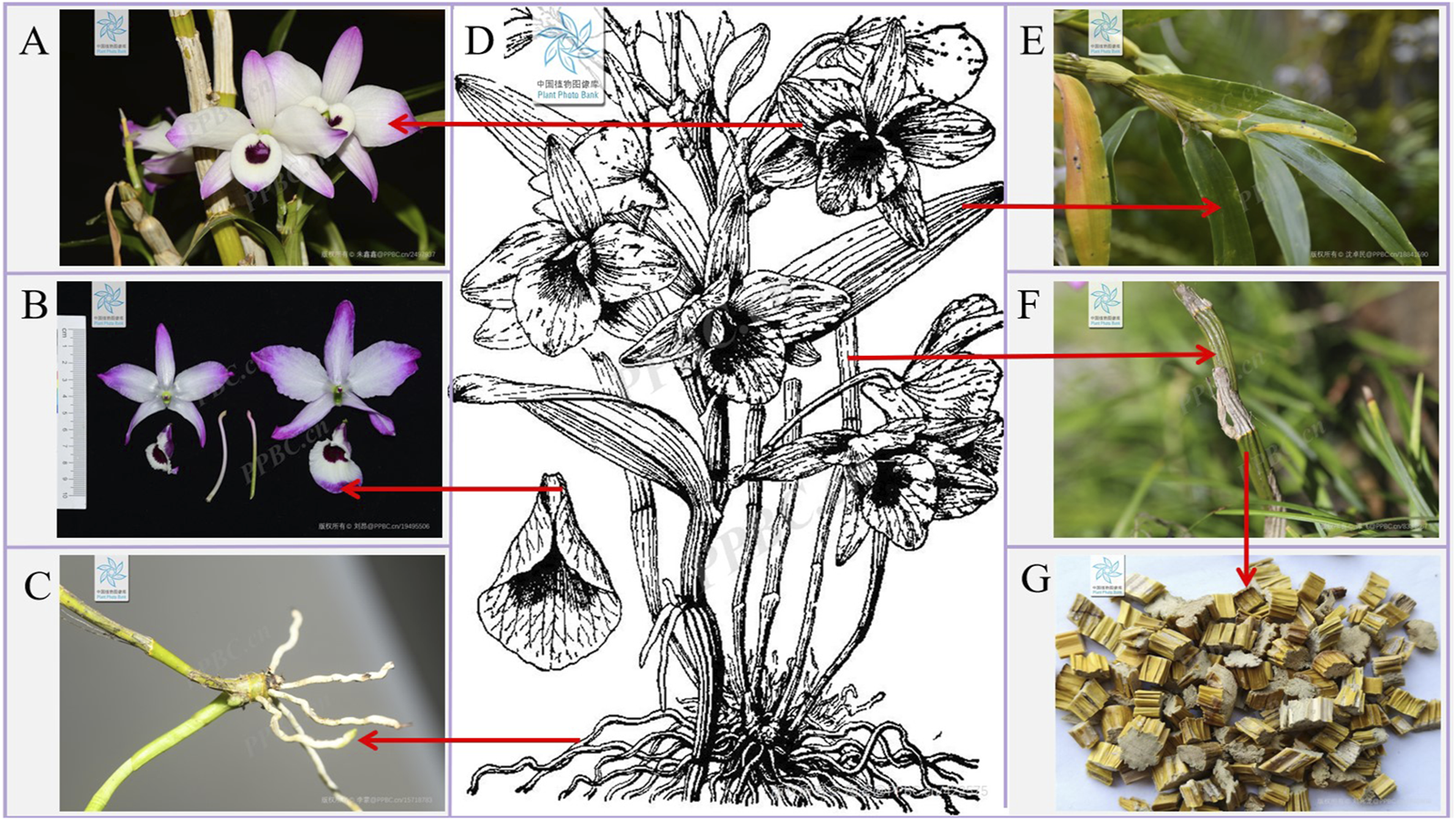
Plant morphology of D. nobile. (A,B) The flowers of D. nobile. (C) The roots of D. nobile. (D) The plant of D. nobile. (E) The leaves of D. nobile. (F) The rhizomes of D. nobile. (G) Dried rhizomes of D. nobile (The plant images in this article are from Plant Photo Bank of China, PPBC. https://ppbc.iplant.cn/).
With over two millennia of documented use in traditional Chinese medicine, D. nobile has been historically cited in classical Chinese texts (Hua et al., 2025). Clinically, it is commonly employed for the treatment or adjunct therapy of chronic pharyngitis, gastrointestinal disorders, ophthalmic conditions, diabetes, and cancer (Fan et al., 2023). In Bangladesh, it is also referred to as “Orchid” locally, and its pseudobulb extract is used for the treatment of eye infections and alleviate burns, and fresh leaf extract is used to put on fresh wounds for its healing properties (Hossain, 2009). Furthermore, D. nobile is also an important raw material in the development of various pharmaceuticals, such as Mailuoning injection, Shihuyeguang pills, and Shihumingmu pills (Wei et al., 2024). Aside from medicinal use, D. nobile is also a functional food; its juice, when consumed directly, is believed to quench thirst and provide cooling effects, particularly in summer. When soaked in water, it releases a pleasant aroma and offers long-term health benefits. Additionally, its extract possesses skin-whitening, moisturizing, and anti-aging activity, supporting its application in the cosmetics industry (Wang, 2021b).
Studying the chemical composition and structure of plants is a fundamental way to understand their biological activity. Extensive research has revealed that D. nobile contains a variety of bioactive compounds, including polysaccharides, alkaloids, phenols, and flavonoids (Liu et al., 2023; Zhang et al., 2023). D. nobile polysaccharides are an essential class of compounds that exhibit a broad spectrum of pharmacological activities such as anti-photoaging, improvement of complications of diabetes mellitus, ovarian protective, gastric protective, neuroprotective, anti-inflammatory and anti-viral effects (Lee et al., 2018; Pan et al., 2014; Wang H. et al., 2024). Advances in extraction and purification technologies have enabled the isolation of structurally diverse D. nobile polysaccharides, enriching the understanding of their structure–activity relationship relationships. The bioactivity of these polysaccharides is intricately linked to their structural parameters, including monosaccharide composition, molecular weight, glycosidic linkage patterns, and specific structural modifications. Unlike other small molecules, D. nobile polysaccharides have some unique advantages such as structural diversity, multi-function bioactivity, high chemical stability, and good biocompatibility, and therefore are receiving growing scientific interest.
Although D. nobile polysaccharides have been thoroughly studied, there are little reviews that summarize the current research progress. The systematic review of the article includes the extraction and purification methods, structural characteristics, biological activity, and structure-activity relationship of D. nobile polysaccharides, thus providing guidance to the future research directions and promoting the further usage of this polysaccharide.
2 Preparation strategies of D. nobile polysaccharides
Polysaccharides are normally extracted through a series of linked processes–extraction and purification–with neither of them being dispensable in attaining high-quality and purified products (Chen J. et al., 2024). The first important and crucial step is extraction; purification can determine the purity and functional integrity of the polysaccharides (Mohan et al., 2020; Xue et al., 2023). High-purity polysaccharides play a vital role in the research on molecular structure, bioactivity mechanisms, and potential applications.
2.1 Extraction of D. nobile polysaccharides
With continuous improvement of technology and ideas, it is becoming more intelligent, efficient, and environmentally friendly to obtain polysaccharides from plants. Selection of the appropriate technique is governed by the physicochemical properties of the target compounds, yield of polysaccharides, potential interfering substances, and overall process safety (Zhao et al., 2017). Comprehensively compare these parameters in different methods to determine the appropriate extraction method. Researchers also need to consider the impact on the environment, and efficient, green, economical, and environmentally friendly extraction methods are the most suitable.
There are several things that should be determined before the polysaccharide’s extraction process begins. These include the processing methods of the raw materials, the extraction methods and conditions, the selection of solvents, as well as the final purification methods for the polysaccharides. D. nobile polysaccharides are mainly located within the cell walls. To facilitate their release, mechanical damage is usually inflicted on plant tissues. Additionally, lipophilic substances included in plants should be removed to minimize impurities to ease purification. The most used solvents are petroleum ether and ethyl acetate (Zhang et al., 2020). These procedures are the foundation for the subsequent process of extraction. The choice of extraction method plays a key role in determining the reliability and yield of subsequent experiments. Currently employed methods for isolating D. nobile polysaccharides include hot water extraction (HWE), ultrasound-assisted extraction (UAE), and subcritical water extraction (SWE) (Figure 2).
FIGURE 2
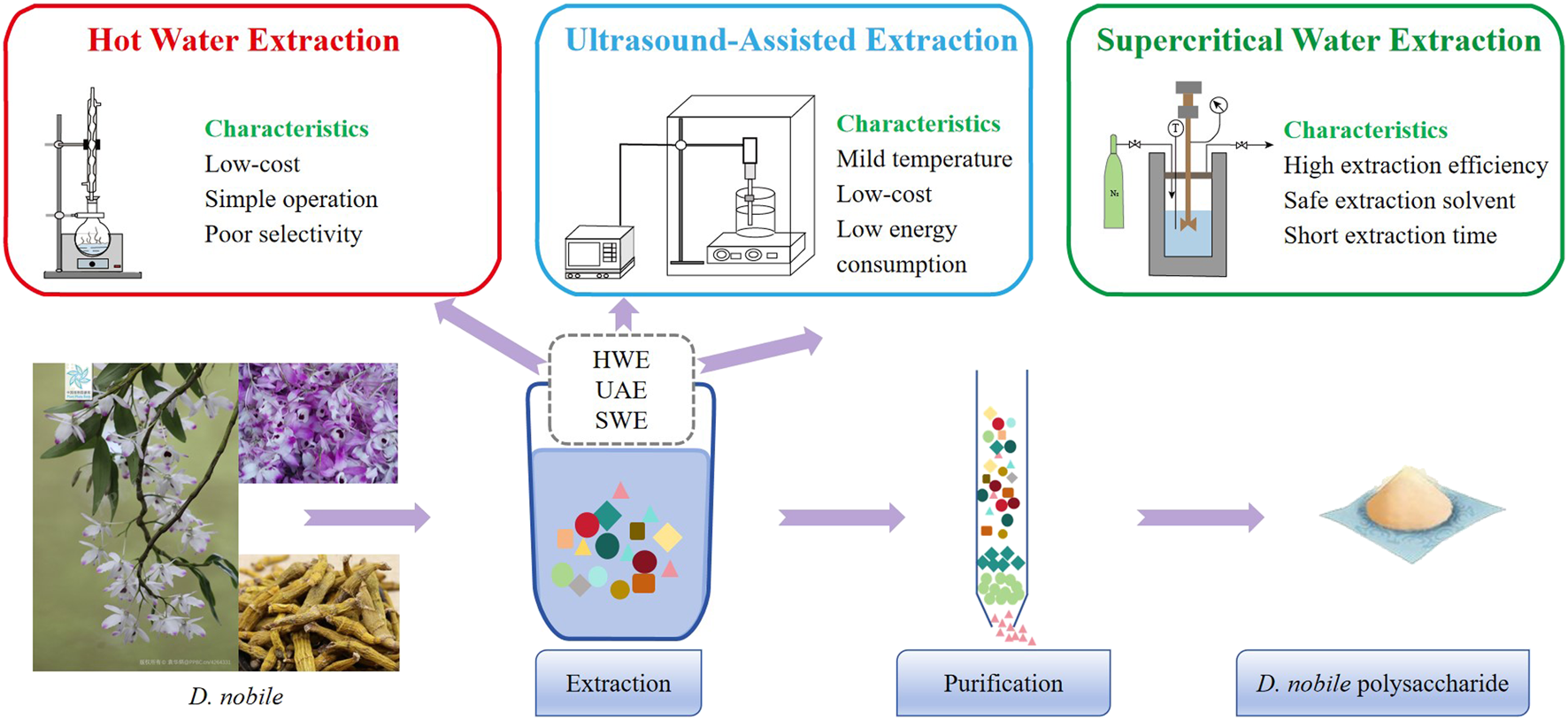
Regarding the extraction and purification of D. nobile polysaccharides (The plant images in this article are from Plant Photo Bank of China, PPBC. https://ppbc.iplant.cn/).
2.1.1 Hot water extraction method
Polysaccharides as high-polarity macromolecules exhibit excellent solubility in hot water. Following the principle of “like dissolves like”, HWE is commonly adopted for their extraction (Chen et al., 2016). Jin et al. (2017) extracted D. nobile polysaccharides (JCS) using HWE with a solid-to-liquid ratio of 1:20 and a 4 h extraction cycle, repeated seven times to maximize yield. However, the broad solvency of water often results in the co-extraction of non-polysaccharide constituents, thereby complicating the purification process. To enhance extraction specificity and minimize contaminants, Luo et al. (2009) adopted a continuous experimental procedure. The raw material was first defatted with petroleum ether (60°C–90°C) for 2 h to eliminate lipid-soluble compounds, followed by an 80% ethanol wash for 2 h to remove oligosaccharides, glycosides, proteins, and other soluble impurities. Subsequent aqueous extraction was performed three times, each for 2 h. This multistep approach significantly improved polysaccharide purity and extraction efficiency while reducing interference from non-essential constituents.
2.1.2 Ultrasound-assisted extraction method
UAE employs high-frequency sound waves (20–2,000 kHz) to induce cavitation, thereby disrupting plant cell matrices, enhancing solvent penetration, and accelerating mass transfer processes. The technique significantly facilitates intracellular polysaccharide release and is favored owing to its ease of operation, cost-effectiveness, and scalability in industrial settings (Cetinkaya et al., 2025) In the case of D. nobile polysaccharides (JCP) have been extracted via UAE using polyethylene glycol (PEG) as the solvent. Optimization through response surface methodology (RSM) identified optimal extraction conditions at 58.53°C, ultrasound power of 192.95 W, and PEG-200 concentration of 44.20% that yielded 15.57% of polysaccharide (Zhang et al., 2016). Essentially, the purpose of extraction is to get the maximum yield of polysaccharides without compromising their native structure and functional integrity.
2.1.3 Subcritical water extraction method
SWE has become a promising and green method for extracting polysaccharides. SWE essentially reducing solvent residues and minimizing environmental impact by using water that is kept at high temperatures and pressures—below its critical point (Basak and Annapure, 2022). Using RSM, Liu et al. (2019) adjusted the SWE conditions for D. nobile polysaccharides. A polysaccharide recovery rate of 21.88% was obtained with an extraction temperature of 129.83°C, an extraction time of 16.71 min, and a pressure of 1.12 MPa, which resulted in a significant improvement in extraction efficiency (Liu et al., 2019).
2.2 Purification of D. nobile polysaccharides
Crude polysaccharides usually contain impurities such as pigments, proteins and low-molecular-weight compounds, which require further purification (Xue et al., 2024). Traditional methods of decolorization are hydrogen peroxide (H2O2) oxidation, adsorption on macroporous resin, and adsorption on activated carbon. When it comes to D. nobile, the most common two methods are the oxidation decolorization of H2O2 and activated carbon adsorption. Protein removal is typically achieved using the Sevag method or trichloroacetic acid precipitation, both of which operate by denaturing or precipitating proteins to separate them from polysaccharides (Li et al., 2019; Wang K. et al., 2024). Li et al. (2020) purified DNP (D. nobile polysaccharide) by using Sevag reagent to remove proteins from crude polysaccharides and decolorizing with activated carbon (Li et al., 2020). Further purification processes like column chromatography, membrane filtration, and stepwise precipitation are normally used to achieve an even higher purity (Liu et al., 2023; Wang et al., 2017). Columns of DEAE-cellulose, Sephadex G-series or Sepharose CL-series resins are commonly used among the chromatographic methods (Table 1). These processes yield highly purified D. nobile polysaccharide fractions suitable for omprehensive structural elucidation and bioactivity assessment.
TABLE 1
| Part | Extraction | Purification | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Polysaccharide fraction | Extraction methods | Time | Temperature and other parameters | Solid–liquid ratio | Polysaccharide yield (%) | Polysaccharide fraction | Purification methods | ||
| Dried stems of D. nobile | DNP | HWE | 2 h × 3 | 100°C | N/A | 5% | N/A | N/A | Luo et al. (2009) |
| Dried stems of D. nobile | JCP | PEG-based UAE | N/A | 58.53°C 192.95 W PEG-200 concentration of 44.20% |
N/A | 15.57% | N/A | N/A | Zhang et al. (2016) |
| Dried stems of D. nobile | JCS | HWE | 4 h × 7 | 100°C | 1:20 | 1.3% | JCS1 | Q Sepharose Fast Flow column | Jin et al. (2017) |
| Dried stems of D. nobile | DNP-W | HWE | N/A | N/A | N/A | N/A | DNP-W4 | DEAE-cellulose anion exchange and Sephacryl S-200 gel filtration chromatography | Wang et al. (2017) |
| Dried stems of D. nobile | D. nobile Lindl. polysaccharide | SWE | 16.71 min | 129.83°C, 1.12 MPa | 1:25 | 21.88% | N/A | N/A | Liu et al. (2019) |
| D. nobile | DNP | HWE | 3 h × 3 | 100°C | 1:10 | 4.3% | DNPE6 (4), DNPE6 (11) | DEAE–Cellulose-52 anion-exchange column, Sephacryl S-200 and Sphadex G-100 columns | Li et al. (2019) |
| D. nobile | DNP | UAE | 30 min | Ultrasonic power: 40 W | 1:40 | 5.16% ± 0.41% | DNP1 and DNP2 | DEAE-QFF and Sephacryl S-300 HR chromatography | Chen et al. (2022) |
| Dried ground stems of D. nobile | DN-P | HWE | 6 h × 2 | 80°C | 1:100 | 10.84% | F1, F2, F3, F4 | Fractogel (BioSEC, Merck) column | Hsu et al. (2024) |
A summary of D. nobile polysaccharides extraction and purification methods.
Abbreviations: HWE, hot water extraction; PEG, polyethylene glycol; SWE, subcritical water extraction; UAE, ultrasound-assisted extraction; N/A, not available.
3 Structural characteristics of D. nobile polysaccharides
3.1 Molecular weight (Mw)
Mw as a physicochemical parameter has a determinant effect on the solubility, viscosity, and bioavailability of the polysaccharides (Wang et al., 2023). Gas-liquid permeation chromatography (GPC) and high-performance gel permeation chromatography (HPGPC) techniques are the two most frequently used to measure Mw with D. nobile polysaccharides (Li et al., 2024a; Zhang et al., 2020). In GPC, different elution times are attributed to polysaccharides with different Mw. A compound with known Mw is used as a reference standard to draw the calibration curve, and then the Mw of the unknown sample is determined by referring to the elution time. The current procedure has become the analytical method adopted in daily analyses. HPGPC is a combination of GPC with high-performance liquid chromatography (HPLC); shorter analysis time, better repeatability and ability to measure purity are achieved. Reported D. nobile polysaccharides Mw values are distributed within a broad range of 3.01–500 kDa, which can be explained by the sources of plants, method of extraction and purification, processing conditions (Li et al., 2020; Wang et al., 2017). Such Mw range is commonly construed to be the determinant behind such pronounced differences in pharmacological activity. Thus, Mw continues to be an essential parameter during both basic and applied research of polysaccharide.
3.2 Monosaccharide composition
Polysaccharides are polymers of monosaccharides with the exact composition significantly affecting functional properties. Examples of common monosaccharides are glucose (Glc), galactose (Gal), arabinose (Ara), and fucose (Fuc). The composition is usually identified by 1-phenyl-3-methyl-5-pyrazolone (PMP) derivatization technique or by acetylation-based technique (Liu et al., 2021). The polysaccharides of D. nobile are structurally diverse and often contain Ara, Gal, Glc, Man, Xyl, Rha and GalA (Hu et al., 2024; Li et al., 2022) (Table 2). The most commonly found monosaccharide are Gal, Glc and Man. In spite of the fact that certain polysaccharides contain identical monosaccharide, variation in molar proportion endows them with distinct structural and functional properties. As an example, both DNP and DNLP contain Man, Glc, and Gal, but the molar ratios of these components, 12.49%:65.2%:6.4% and 26.9%:66.2%:6.9%, indicate the differences in composition that can be the basis of the variations in bioactivity (Hu et al., 2024; Zhang et al., 2020).
TABLE 2
| Source | Compound name | Molecular weights | Monosaccharide composition | Structures | Analytical techniques | Ref |
|---|---|---|---|---|---|---|
| Dried stems of D. nobile | DNP | 87.6 kDa | Rha: Ara: Xyl: Man: Glc: Gal = 1:2.8:2.2:30.8:117.9:31.8 | The backbone of DNP was composed of (1→6)-linked-α-d-glucopyranosyl, and (1→6)-linked-α-d-galactopyranosyl residues, with branches of (1→4)-linked-α-d-glucopyranosyl and (1→4)-linked-α-d-mannopyranosyl residues | GC–MS, GPC, IR, NMR | Luo et al. (2009) |
| Dried stems of D. nobile | JCS1 | 23 kDa | Glc: Man: Xyl: Ara = 40.2:2.0:1.3:1.0 | The backbone of JCS1 might consist of repeated 1,4-linked β-Manp and 1,4-linked α-Glcp units with branches at the C-6 of 1,4-linked α-Glcp substituted by 1,4-linked α-Xylp and T-α-Araf linked at C-4 of 1,4-linked α-Xylp.The other branches might be linked by T-α-Glcp at C-6 of 1,4-linked α-Glcp | HPGPC, GC, IR, FT-IR, NMR | Jin et al. (2017) |
| Dried stems of D. nobile | DNP-W4 | 500 kDa | Man: Glc: Gal: Xyl: Rha: GalA = 1.0: 4.9: 2.5: 0.5: 1.0: 0.9 | DNP-W4 possesses a backbone of (1→4)-linked β-d-Glcp, (1→6)-linked β-d-Glcp, and (1→6)-linked β-d-Galp, with substitutes at O-4/6 of Glcp residues and O-3 of Galp. The side chains may be composed of terminal Manp, (1→6)-linked β-d-Manp, (1→3)-linked β-d-Glcp, β-d-Glcp, (1→4)-linked α-d-GalAp, (1→2)-linked α-l-Rhap, and Xylp | HPGPC, GC-MS, FT-IR, IR, NMR | Wang et al. (2017) |
| Dried stems of D. nobile | D. nobile Lindl. polysaccharide | 85.72 kDa | Ara: Gal: Glc: Man = 1.05: 1.6: 48.63: 48.73 | N/A | N/A | Liu et al. (2019) |
| D. nobile | DNPE6(4) | 99.2 kDa | Ara: Glc: Gal: Man = 2.5: 0.9: 0.3: 0.8 | The linkage type of DNP were →1)-l-Araf-(3→, →1)-d-Glcp-(4→, →1)-d-Galp-(3→, →1)-d-Galp-(6→, →1)-d-Manp-(3, 6→, and T-d-Manp | UV, FT-IR, HPGPC | Li et al. (2019) |
| D. nobile | DNPE6 (11) | 3.01 kDa | Man: Glc: Gal = 3: 11: 3 | The backbone of DNPE6(11) was composed of [→6)-d-Manp-(1→4)-d-Glcp-(1→] | HPGPC, UV and FTIR | Li et al. (2020) |
| D. nobile | DNLP | 1.2–11.2 kDa | Man: Glc: Gal = 26.9%: 66.2%: 6.9% | N/A | N/A | Zhang et al. (2020) |
| D. nobile | DNP1 | 67.72 kDa | Man: Glc = 75.86 ± 0.05%: 24.14% ± 0.05% | DNP1 is a straight-chain glucomannan composed mainly of β-1,4-D-Manp, β-1,4-day-Glcp residues, and some acetyl groups linked to C-2 or C-3 of the mannose residues | FT-IR, and NMR | Chen et al. (2022), Li et al. (2024) |
| D. nobile | DNP2 | 37.45 kDa | Man: Glc = 72.32 ± 0.03%: 27.68% ± 0.03% | N/A | N/A | Chen et al. (2022) |
| D. nobile | DNLP | N/A | Rha: Ara: Xyl: Man: Glc: Gal = 1.00: 1.65: 2.58: 1.08: 1.83: 1.15 | N/A | N/A | Li et al. (2022) |
| D. nobile | DNP | 106.6 kDa | Man: Glc: Gal = 23.2%: 68.5%: 3.5% | N/A | N/A | Luo et al. (2022) |
| D. nobile | DNP | 13.2 kDa | Man: Glc: Gal = 12.49%: 65.2%: 6.4% | N/A | N/A | Hu et al. (2024) |
Source, compound name, molecular weights, monosaccharide composition, structures of D. nobile polysaccharides, and analytical techniques.
Abbreviations: NA, not available.
3.3 Chemical structures
Numerous studies have elucidated the chemical structure of polysaccharides derived from D. nobile. A novel polysaccharide, DNP1, was isolated, with its core structure consisting of →4)-β-Manp-(→1 and →4)-β-Glcp-(→1 sugar residues forming the backbone. Nuclear magnetic resonance (NMR) spectroscopy revealed that the repeating units of DNP1 likely consist of [→4)-2-OAc-β-Manp-(1→]3→4)-β-Glcp-(1→) (Li et al., 2024b). Luo et al. (2009) isolated a heteropolysaccharide, DNP, from the dry stems of D. nobile. Structural analysis through gas chromatography-mass spectrometry (GC-MS), infrared spectroscopy (IR), and NMR confirmed that DNP comprises (1→6)-linked-α-d-glucopyranosyl and (1→6)-linked-α-d-galactopyranosyl units, along with (1→4)-linked-α-d-glucopyranosyl and (1→4)-linked-α-d-mannopyranosyl branches (Luo et al., 2009). The proposed structure of DNP is depicted in Figure 3. Additionally, a study suggests that the polysaccharide extracted from D. nobile is mannoglucan. Jin et al. (2017) characterized polysaccharide JCS1 using chemical and spectral methods. The structure of JCS1 is primarily composed of α- and β-glycosidic linkages, with 1,4-linked Man and Glc as the main chains. Branching structures, formed through specific linkages, are present along the chains. The backbone of JCS1 is hypothesized to consist of alternating 1,4-glycosidic bonds between β-Manp and α-Glcp units, with branching at the C-6 position of the 1,4-linked α-Glcp residues, where 1,4-linked α-Xylp units replace α-Glcp. A T-α-Araf is attached at the C-4 position of the 1,4-linked α-Xylp, and additional branches may be formed via T-α-Glcp linkages at C-6 of the 1,4-linked α-Glcp. These findings support the classification of JCS1 as a mannoglucan (Jin et al., 2017). Research of D. nobile polysaccharides is primarily focused on the linkage, branching, and methylation structure of the monosaccharide units. Chemical composition of the polysaccharides has been presented in Table 2. Simple structure of polysaccharides is made up of monosaccharides linked to each other by glycosidic linkages, differing in the proportion of each monosaccharide. Such structural diversity makes analysis of the chemical composition of D. nobile polysaccharides very inconvenient.
FIGURE 3
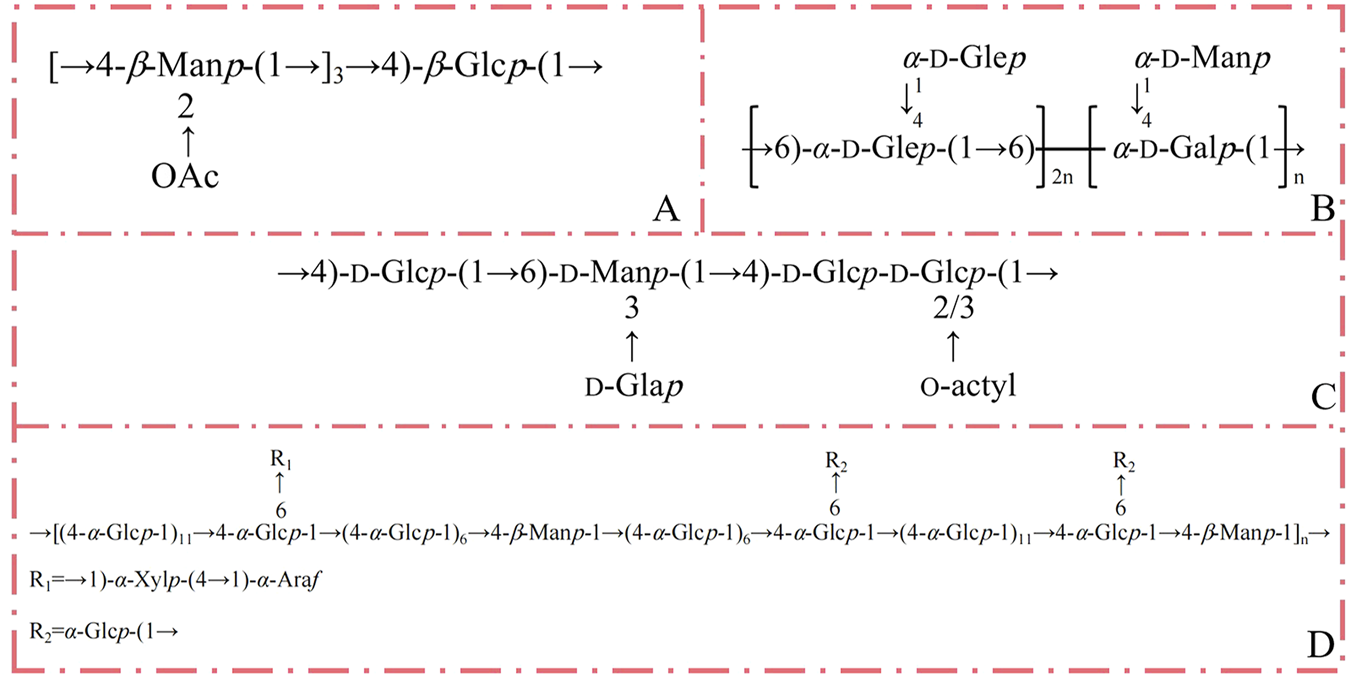
The hypothetical structure of D. nobile polysaccharides (A) DNP1. (B) DNP. (C) DNPE6 (11). (D) JCS1.
4 Biological activity of D. nobile polysaccharides
4.1 Anti-photoaging effect
4.1.1 Anti-UVA and UVB effect
The skin acts as a shield and protects the internal parts of the body against the external environmental factors. The skin, especially due to its large surface area and constant exposure to the environment, is especially vulnerable to the effects of ultraviolet (UV) radiation (Zhang M. et al., 2024). UV radiation is the most immediate physical environmental factor that comes in contact with the skin and is greatly linked to various skin disorders. As much as mild exposure to UV rays is beneficial, overexposure has adverse effects, which culminate to skin damages (Wang Z. et al., 2024). Chronic UV-induced exposure could be in the form of sunburn, immune suppression, malignancy and photoaging. Skin aging is categorized into the endogenous and exogenous with UV radiation as the main exogenous aging factor, also referred to as the photoaging (Dusabimana et al., 2024; Li et al., 2024). The UV spectrum (100–400 nm) is divided into three bands: UVA (320–400 nm), UVB (280–320 nm), and UVC (100–280 nm). While UVC has a potent cellular-killing effect, it is almost entirely absorbed by the ozone layer, posing minimal risk to human health. Therefore, UVA and UVB are the principal contributors to skin damage (Chen W. et al., 2024).
Polysaccharides derived from D. nobile exhibit anti-photoaging effects against both UVA and UVB radiation (Table 3). Long et al. (2023a) established a mouse model of UVB-induced acute photoaging and evaluated the anti-photoaging effects of varying doses of DNP (50, 200 mg/mL), with vitamin E serving as a positive control. UV radiation induces reactive oxygen species (ROS) that trigger oxidative stress, accelerating skin aging and wrinkle formation. DNP can reverse UVB-induced oxidative stress by enhancing the activity of antioxidant enzymes (SOD, CAT, GSH-Px) and reducing MDA levels. UVB irradiation also activates inflammatory cells, leading to inflammation. Excessive UVB irradiation can lead to a large accumulation of intracellular ROS, thereby promoting the activation of AP-1 and NF -κB, inducing abnormal secretion of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and stimulating the transcription of MMPs genes (Cui et al., 2022). TNF-α and IL-1β are the main cytokines in the inflammatory response, which can stimulate the production of secondary inflammatory mediators, induce the migration of inflammatory cells, and trigger the inflammatory response (Xiao et al., 2022). Compared to other pretreatment groups, the UVB + DNP-H group showed a significant reduction in IL-1β expression (P < 0.05). In the current study, DNP was found to have inhibitory action on expression of IL-6 and TNF-α. UV radiation activates matrix metalloproteinases (MMPs) which cleave collagen and elastin and increases skin photoaging. UVB + DNP-H and UVB + DNP-L groups inhibited the UVB-induced upregulation of the MMP, thus protecting extracellular matrix (ECM) components, and maintaining the integrity of collagen and elastin fibers. These findings were supported by Masson staining and quantification of hydroxyproline (HYP) contents. Such findings provide a scientific basis of DNP in the alleviation of UVB-induced skin photoaging (Long et al., 2023b; Wang et al., 2021b). Besides, the team of researchers evaluated the effect of DNP on UVB-induced oxidative stress and apoptosis in HaCaT cells. It was found out that DNP significantly inhibited the reduction of the viability and proliferation of the HaCaT cells induced by UVB treatment. DNP scavenged ROS increased endogenous antioxidant enzyme activity, reduced MDA levels, and partially alleviated cell cycle arrest, demonstrating its antioxidant and anti-apoptotic properties. The Western blot analysis revealed that DNP suppresses UVB-induced oxidative injury and apoptosis in HaCaT cells by regulating mitogen-activated protein kinase (MAPK) signaling pathways (Long et al., 2023a; Zhai et al., 2022). Li et al. (2022) also examined whether D. nobile polysaccharides (DNL) have any effect on photoaging caused by UVA in vitro in human foreskin fibroblasts (HFF-1). The results showed that DNL polysaccharides significantly reversed UVA-induced HFF-1 cell damage, improved oxidative stress, and regulated ROS levels, as well as SOD, CAT, and GSH-Px activity. Additionally, DNL polysaccharides reduced S-β-Gal expression and mitigated UVA-induced photoaging by inhibiting the secretion of MMP-1, MMP-2, MMP-3, and MMP-9 (Li et al., 2022; Yang et al., 2023). In conclusion, D. nobile polysaccharides effectively combat UVA and UVB-induced photoaging through their antioxidant, anti-inflammatory, and anti-apoptotic properties (Figure 4).
TABLE 3
| Biological activities | Polysaccharide name | Types | Testing subjects | Doses/duration | Effects/mechanisms | Ref |
|---|---|---|---|---|---|---|
| Anti-UVA and UVB effect | DNP | In vivo | Kunming mice | 50 mg/mL, 200 mg/mL | DNP can reverse UVB induced oxidative stress by increasing the activity of antioxidant enzymes and reducing MDA content. Compared with other pretreatment groups, the expression level of IL-1β, IL-6 and TNF-α were decreased in the UVB + DNP-H and UVB + DNP-L group. In addition, both groups were able to reduce the increase in MMPs expression induced by UVB radiation | Long et al. (2023b) |
| DNP | In vitro | HaCaT cells | 200 μg/mL, 800 μg/mL | DNP improves UVB induced oxidative damage and apoptosis in HaCaT cells by regulating MAPKs | Long et al. (2023a) | |
| DNLP | In vitro | HFF-1 cells | 0.06 mg/mL, 0.18 mg/mL, 0.54 mg/mL | DNLP could significantly reverse UVA induced HFF-1 cell damage, improve its oxidative stress state, regulate ROS content, and levels of SOD, CAT, and GSH-Px. In addition, DNLP improves UVA induced photoaging mediated collagen degradation by inhibiting the secretion of MMP-1, MMP-2, MMP-3, and MMP-9 protein expression, reducing the phosphorylation activation of the JNK/c-Fos/c-Jun pathway, and decreasing the expression of SA-β-Gal | Li et al. (2022) | |
| Anti-blue light effect | DN-P and DN-PP | In vitro and in vivo | ARPE-19 cells 661W cells, and Drosophila | DNP: 50, 100, 200 μg/mL. DN-PP: 25, 50, 100 μg/mL | Pretreatment with polysaccharides from D. nobile can reduce ROS and superoxide levels, as well as decrease the levels of antioxidant enzymes (SOD1 and CAT), inhibit the increase of IL-1β and IL-6, and alleviate eye damage in drosophila under blue light irradiation. It can also increase the expression levels of ninaE, norpA, Gαq, Gβ76C, Gγ30A, Trp,and Trpl | Hsu et al. (2024) |
| Ovarian protective effect | DNLP | In vivo | Female Sprague-Dawley rats | 200 mg/kg/day | Compared with the model group, the body weight of PCOS rats treated with DNLP was reduced (P < 0.01), the estrous cycle returned to normal, and serum testosterone levels and insulin resistance were also reduced. In addition, the volume of the corpus luteum increases, the number of antral follicles decreases, and the thickness of the granulosa cell layer increases. DNLP treatment also increased the expression levels of PCNA, and inhibited cell apoptosis in PCOS ovarian tissue by regulating apoptosis related proteins Bax, Bcl-2, and caspase-3 | Zhang et al. (2020) |
| DNP | In vivo | Female Sprague-Dawley rats | 200 mg/kg/day | Administration of DNP significantly reduced blood glucose, serum insulin, and HOMA-IR levels in PCOS rats, and restored the expression of IGF1 and IGF1R. Besides, DNP intervention can also promote GCs glycolysis and improve the mechanism of follicular dysplasia. Importantly, SIRT2 may be a key factor in regulating the glycolysis rate of granulosa cells by DNP. | Hu et al. (2024) | |
| Testicular protective effect | DNLP | In vivo | Male Sprague‐Dawley rats | 400 mg/kg/day | DNLP plays a protective role in DM induced reproductive damage by regulating the expression of SIRT1 in testicular tissue | Lei et al. (2022) |
| DNP | In vivo | Male C57BL/6J mice | 200 and 400 mg/kg | DNP can improve spermatogenesis in streptozotocin induced diabetes mice by regulating glycolysis pathway | Luo et al. (2022) | |
| Gastric protective effect | JCP | In vivo | Male Wistar rats | L-JCP: 100 mg/kg. H-JCP: 300 mg/kg | Compared with the vehicle group, JCP treatment showed a decrease in inflammatory cells, reduced bleeding, and effectively increased the expression level of SOD in rat gastric tissue, while reducing MDA content. In addition, oral administration of 300 mg/kg JCP to rats significantly increased EGF production. H-JCP can reduce the expression levels of gastric p-JNK, p-ERK, MMP-2, and MMP-9 | Zhang et al. (2018) |
| Neuroprotective effect | DNP | In vivo | Male Sprague-Dawley rats | 100 mg/kg/day | The mitochondrial membrane and cristae in the DNP group improved compared to the VD group. Compared with the model group, the expression of GSH, xCT, and GPx4 in the hippocampus of the DNP group was significantly upregulated (P < 0.01). TEM results showed that the DNP group had an increase in synaptic vehicles, a significant increase in synaptic active zone (SAZ) length and PSD thickness, and a significant upregulation of PSD-95 protein expression | Ming et al. (2023) |
| DNP | In vivo | Female Sprague-Dawley rats | 100 mg/kg/day | After DNP treatment, there was improvement and a decrease in iron content (24 and 48 h after injury). The HE staining after 28 days of treatment showed that the spinal cord tissue defect area in the DNP group was smaller compared to the model group. And compared with the sham operation group, the xCT, GSH, Gpx4, and GRSFI in the spinal cord tissue of the model group rats decreased (P < 0.05), while the expression of these indicators increased after DNP treatment. DNP treatment increased NeuN+ cells at 14 and 28 days after SCI. | Huang J. et al. (2024) | |
| Anti-inflammatory effect | DNP1 and DNP2 | In vitro | RAW264.7 macrophages | 12.5, 25, 50, 100, 200 μg/mL | DNP1 and DNP2 significantly reduce the production of inflammatory factors in a dose-dependent manner. DNP1 may exert immunomodulatory effects by binding to the TLR4-MD2 complex and inhibiting the TLR4-MD2 mediated signaling pathway | Chen et al. (2022), Li et al. (2024) |
| Anti-viral effect | DNPE6 (4) | In vivo | Nicotiana tabacum cv. K326 | 125 μg/mL | The protective activities of DNPE6 (4) against CMV and TMV were 40.4% and 69.9%, respectively. After DNPE6 (4) induction, NADPH oxidase and NAD(P)H increased. In addition, DNPE6 (4) can stimulate the activity of defensive enzymes (POD, PAL, SOD) in tobacco to resist plant virus infection. DNPE6 (4) could increase the expression level of SOD and the expression levels of ICSI, EDSI, and PRI up-stream and down-stream of the Salicylic acid (SA) pathway, while inhibiting the protein expression of CAT. | Li et al. (2019) |
| DNPE6 (11) | In vivo | Nicotiana tabacum cv. K326 | 500 μg/mL | DNPE6 (11) has significant therapeutic and inactivation activity against CMV, significant protective effect against PVY. In addition, preliminary mechanistic studies have found that DNPE6 (11) has a strong binding ability to the coat protein of cucumber mosaic virus | Li et al. (2020) |
Summary of biological activities of D. nobile polysaccharides.
FIGURE 4
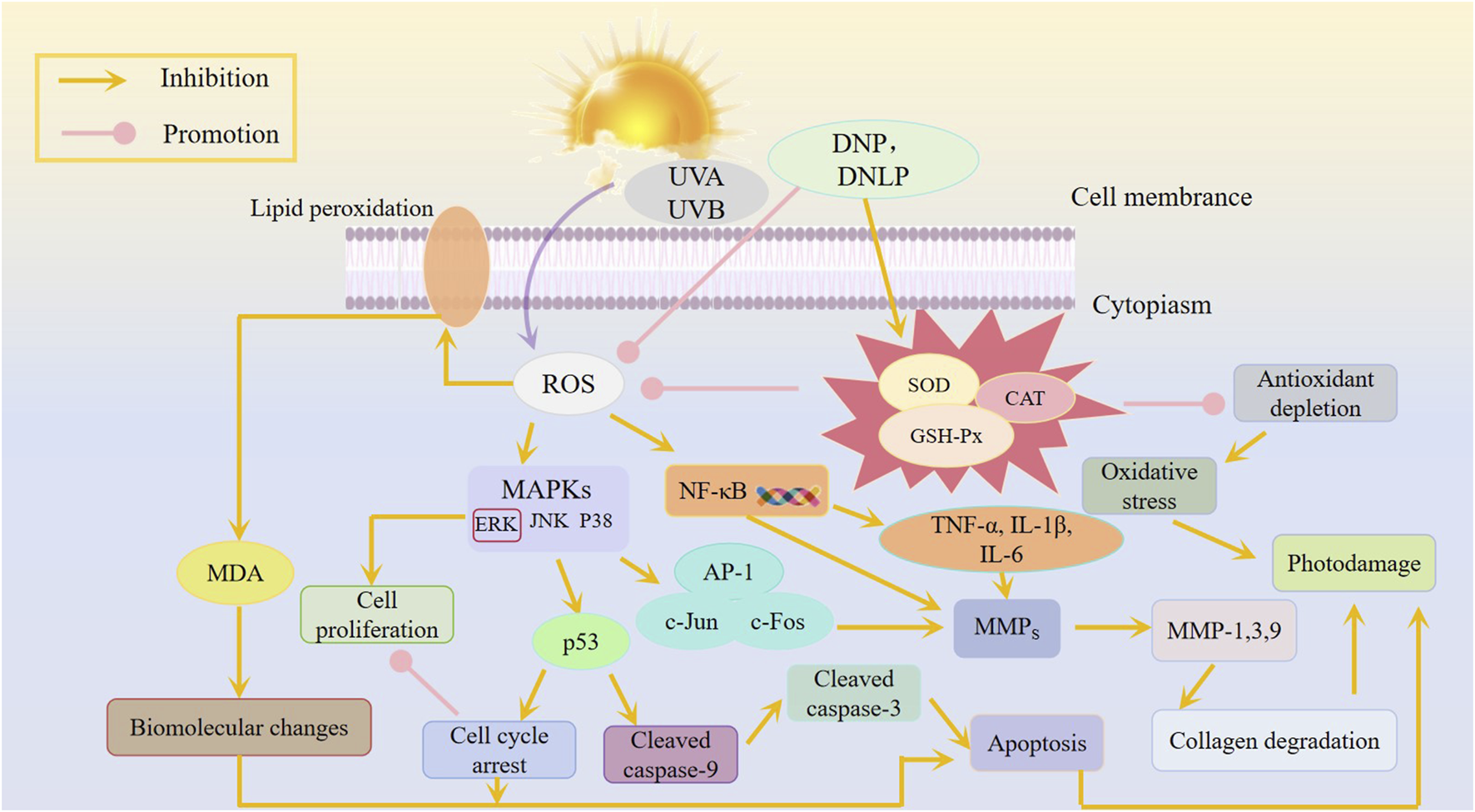
Potential mechanism of polysaccharides from D. nobile in anti-UVA and UVB.
4.1.2 Anti-blue light effect
Blue light, primarily emitted by the sun, offers various health benefits, including regulating human behavior and circadian rhythms, enhancing memory and cognitive functions, and stabilizing emotions. However, prolonged exposure to blue light can lead to eye damage due to its high energy (Chan et al., 2023). Consequently, excessive exposure to blue light should be avoided. With the increasing use of electronic devices, prolonged exposure to blue light has become inevitable. Despite the rising prevalence of protective measures, long-term research is essential in the development of more effective approaches to protect the eyes against the dangers of blue light (Li et al., 2024; Liu et al., 2024). D. nobile-derived polysaccharides, namely DNP and DN-PP, have significant protective effects against retinal damage by blue light. When cell population of ARPE-19 and 661W cells were pre-incubated with DNP and DN-PP in various concentrations, cellular viability increased significantly compared to that of cells exposed only to blue light in cell viability assays. Constant exposure to blue light often leads to the buildup of ROS and the development of an oxidative state in the retina (Ouyang et al., 2020; Yan et al., 2025). Blue light exposure notably increased ROS and superoxide levels in 661W cells, while ARPE-19 cells showed a lower, less sensitive response. A dose-dependent decrease in the ROS levels of 661W was noticed through pre-treatment with different doses of DNP, DN-PP, and the positive control of alpha-lipoic acid (ALA). In both 661W and ARPE-19 cells, the production of superoxide also reduced compared to blue light-treated cells. Moreover, the increased expression of IL-1 and IL-6 by the blue light was inhibited in a dose-dependent effect by pretreatment with DNP and DN-PP and reduced the increase in SOD1 and CAT antioxidant enzymes following blue light exposure. Opsins, G-protein-coupled receptors involved in photosensitivity, were significantly upregulated by blue light. In 661W cells, DN-P (400 μg/mL) and DN-PP (100 μg/mL) reversed the expression of opsin 3 to levels similar to the control group. In ARPE-19 cells, only DN-PP (100 μg/mL) showed comparable levels to the control group. Furthermore, the protective mechanism of DN-P and DN-PP against blue light damage in vivo was investigated using Drosophila. Electroretinography (ERG) analysis demonstrated that DN-P (12.5 mg/mL) exerted neuroprotective effects on blue light-induced retinal injury in Drosophila. q-PCR analysis revealed that DN-P (12.5 mg/mL) pretreatment restored the expression levels of key light-responsive genes (ninaE, norpA, Gαq, Gβ76C, Gγ30A, Trp, and Trpl) in Drosophila. Compared to the blue light-only group, the DN-P (12.5 mg/mL) group exhibited increased expression levels of these genes. In conclusion, pretreatment with DNP provides protection for retinal cells and photoreceptors against blue light-induced damage (Chen W. et al., 2024; Hsu et al., 2024).
4.2 Improvement of complications of diabetes mellitus
4.2.1 Testicular protective effect
Diabetes mellitus (DM) is a metabolic illness that is characterized by chronic hyperglycemia. Though DM is not necessarily lethal, persistent increases in blood sugar level can trigger malfunction or deterioration of several organs and systems leading to a variety of complications (Wang K. et al., 2024). One of these complications is the negative impact of DM on male reproductive system which may decrease sperm number and quality, testicular dysfunction and inhibit sperm production and maturation, and eventually influence male fertility (Xu et al., 2024). In this regard, DNP has been proven to provide a protective influence on the testicular tissue in DM. In the study by Lei et al. (2022), SD male rats were separated into four groups: nondiabetic, diabetic, model + metformin and model + DNLP. Diabetes was developed through a high-fat diet and intraperitoneal injection of 35 mg/kg streptozotocin (STZ). Major predictors of male reproductive health include testicular weight, testosterone concentration, sperm volume and sperm motility (Huang R. et al., 2024). Compared to the control group, rats in the model group exhibited significant reductions in testicular and epididymal quality (P < 0.05), as well as decreased sperm count and motility (P < 0.05). On the other hand, the DNLP improved significantly all four indicators in the rats (P < 0.05). The seminiferous tubules in the model group were highly atrophic with a sparse spermatogenic cells arrangement and had a very low sperm count. DNLP improved these pathological changes and promoted the quantity of spermatogonia and spermatocytes. Quantitative evaluation of apoptotic cells demonstrated that the apoptotic process in the testicular tissue in the DNLP group decreased significantly as compared to the model group (model + DNLP group: 43.13 ± 7.21; model group: 70.67 ± 3.16, P < 0.05). DNLP treatment also increased the expression of PCNA which is a basic protein of cell proliferation. Male reproductive health also relies on such a key regulator of glucose metabolism SIRT1, with reduced SIRT1 expression having been reported to contribute to germ-cell apoptosis in diabetic mice. DNLP promoted the level of mRNA and SIRT1 protein in DM rats (Lei et al., 2022; Nie et al., 2020). The present findings indicate that DNLP can reduce the DM-induced reproductive harm through the regulation of SIRT1 in testicular tissue expression, but the mechanisms underlying DM-induced tissue damage should be further investigated.
Thereafter, the investigators determined the protective role of another polysaccharides in D. nobile (DNP) on diabetic mellitus (DM)-induced reproductive dysfunction. In this experiment, the major concern was to assess the effect of DNP on glucose homeostasis in mice with DM focusing on the glycolysis pathway. The results showed that DNP could improve the spermatogenic dysfunction induced by DM by improving the abnormal structure of testis, inhibiting the apoptosis of spermatogenic cells and promoting proliferation. DNP also recovered the architecture and physiologic role of sertoli cells (SC) in DM mice, increased the expression of SC marker GATA4, WT1, and vimentin and the expression of major glycolysis-limiting enzymes LDHA, PKM2, and HK2. On the whole, these results indicate that DNP enhances spermatogenesis in streptozotocin-induced diabetic mice through the regulation of glycolysis pathway (Fan et al., 2023; Luo et al., 2022).
4.2.2 Improve retinal inflammation
Retinopathy is one of the most common complications in patients with DM (Cai et al., 2025). D. nobile polysaccharides can improve the inflammatory microenvironment in the retina of DM rat models. This study investigated the effects of DNP (D. nobile polysaccharides) at doses of 50, 100, and 200 mg/kg on the inflammatory microenvironment in DM rats, exploring the potential mechanisms through intestinal microbiology, metabonomic, and transcriptomics. Following DNP treatment, typical DM symptoms were alleviated, and the imbalance in the gut microbiota was reversed. DNP treatment increased the abundance of Parabacteroides and reduced the levels of Enterorhabdus, Enterococcus, Turicibacter, and Frisingicoccus. Dysbiosis of the gut microbiota affects the secretion of metabolites, and after DNP treatment, the types and levels of metabolites in DM rats closely resembled those in the control group, while untreated DM rats showed marked changes. This dysbiosis and the alterations in metabolite profiles can compromise intestinal barrier function (Chen et al., 2023). H&E staining of colon tissue from the DM rat model revealed that DNP treatment reduced mucosal degeneration and necrosis in the colon and partially restored the structure of intestinal glands. Additionally, the expression of ZO-1 and claudin-1 proteins was increased. DNP treatment also significantly reduced serum LPS levels, a marker of endotoxemia, and decreased the metabolism of intestinal and circulating branched-chain amino acids (BCAAs). Conversely, the metabolism of intestinal and circulating hippurate was increased, which inhibited the TLR4/NF-κB signaling pathway and ultimately improved the retinal inflammatory microenvironment. This multi-omics study is the first to systematically evaluate the therapeutic effect of DNP on type 2 DM and its associated retinal inflammation (Wang R. et al., 2025) (Figure 5). Based on these findings, DNP treatment presents a promising strategy for preventing or treating DM-related complications.
FIGURE 5

Potential mechanism of polysaccharides from D. nobile in improve retinal inflammation.
4.3 Ovarian protective effect
Polycystic ovary syndrome (PCOS) is the most common endocrine illness that affects women of reproductive age, and most often it is caused by abnormalities of ovarian follicles, particularly of the follicular membrane (Helvaci and Yildiz, 2025). Symptoms that characterize the condition include excessive hair growth, acne, and obesity (Mousa et al., 2024). In order to test the therapeutic value of D. nobile polysaccharides (DNLP) against PCOS, researchers performed animal studies. PCOS was induced by the daily intraperitoneal treatment of the rats with letrozole (1 mg/kg) in a solution of carboxymethyl cellulose (0.5%) and feeding them a high-fat diet (30 days). Therapeutic effect of the oral DNLP administration (200 mg/kg/day) was then evaluated for 21 days according to quantitative analysis of ovarian morphology, serum glucose, insulin, hormone levels, expression of proliferating cell nuclear antigen (PCNA), Bax, Bcl-2, caspase-3 mRNA in ovarian tissue, and ovarian granulosa cell apoptosis. As compared with the model group, DNLP treatment led to a statistically significant decrease in body weight (P < 0.01) and normalized the estrous cycle. Even though there was a reduction in serum testosterone levels and indicators of insulin resistance, there was no change in the level of luteinizing hormone. A histological examination showed a significant decrease in follicles and corpus luteum, an increase in antral follicles, a disorganized arrangement of granulosa cells, and a thinning of the granulosa cell layer in the model group. After DNLP treatment the volume of corpus luteum was enhanced, the amount of antral follicles was reduced, and the layer of granulosa cells thickened when compared with the model group. The overall result of these studies suggests that DNLP has positive therapeutic in the treatment of PCOS.
Insulin resistance is common in PCOS. D. nobile polysaccharides (DNP) can regulate IR in granulosa cells of PCOS. In the situation when DNP (200 mg/kg/day per day for 30 days) was administered to PCOS rats, the body weight and ovarian weight were reduced, normal estrous cycle was restored, and hormonal levels and ovarian morphology were returned to normal. Moreover, DNP significantly decreased the level of serum glucose, insulin, HOMA-IR, and restored the expression of IGF1 and IGF1R in PCOS rats. These results were substantiated histologically by immunohistochemistry (IHC). Comprehensively, these results suggest that DNP intervention has the potential to rectify IR in rats with PCOS. Also, DNP intervention can also promote granulosa cells (GCs) glycolysis and improve the mechanism of follicular dysplasia. The effect of DNP on insulin sensitivity and glycolysis process of KGN cells treated with insulin further confirms this result. Interestingly, SIRT2 might be the key for DNP to regulate the glycolysis rate of granulosa cells (Hu et al., 2024; Liang et al., 2023; Martin and Madassery, 2006) (Figure 6).
FIGURE 6
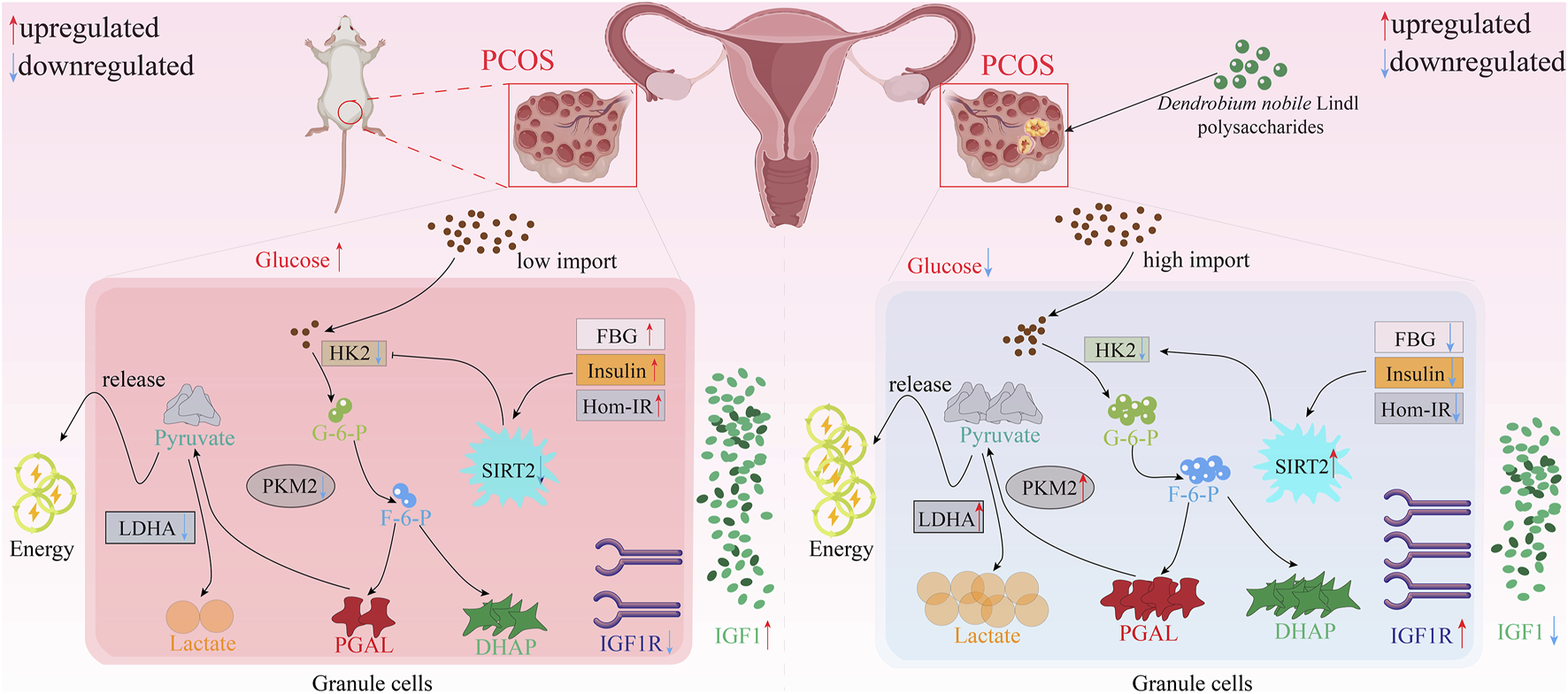
Potential mechanism of polysaccharides from D. nobile in ovarian protective effect.
4.4 Gastric protective effect
Drinking alcohol is a prevalent social activity, but it can also cause many diseases (Patel et al., 2022). Alcohol-mediated injury is particularly prone to occur in gastrointestinal tract. Alcohol passes out rapidly along the esophagus, into the stomach and the small intestine and finally into the bloodstream through capillaries. This conduct has a direct impact on the epithelial cells of the gastric mucosal, breaking the protective mucosal barrier and causing acute gastric congestion, erosion, and, in some cases, superficial ulceration (Sun et al., 2025). Zhang et al. (2018) assessed the therapeutic effect and mechanism of the D. nobile polysaccharides (JCP) on ethanol-induced gastric injury in rats. The forty male Wistar rats were provided randomly into five groups: the control group, vehicle group, omeprazole group, low-dose (L-JCP, 100 mg/kg) and high-dose (H-JCP, 300 mg/kg) JCP groups. After 28 days of continuous gavage administration, all groups except the control group were induced with gastric ulcers through gavage with 100% ethanol (4 mL/kg). Gastric ulcer evaluation followed by histological analysis revealed that the ulcer indices for omeprazole, L-JCP, and H-JCP pretreated rats were 0.17% ± 0.16%, 0.78% ± 0.26%, and 0.32% ± 0.19%, respectively (P < 0.05). Compared to the vehicle group, JCP pretreatment reduced gastric hemorrhage and the occurrence of inflammatory cells. Intragastric administration of ethanol decreased the expression of SOD and increased MDA content in normal rats, whereas the H-JCP group showed a significantly higher SOD expression and low MDA accumulation. EGF, one of the major cell-protective growth factors that promote mucosal blood flow and epithelial cell proliferation, was considerably upregulated in the rats that were given 300 mg/kg JCP. Western blot analysis indicated that H-JCP group inhibited the expression of p-JNK, p-ERK, MMP-2 and MMP-9 in gastric tissue (Zhang et al., 2018; Zhang et al., 2020).
4.5 Neuroprotective effect
Vascular dementia (VD) is a cerebrovascular disease characterized by cognitive impairment and is an also common form of dementia. Its incidence rate has continued to rise in recent years. Its pathophysiology involves a series of events triggered by blood-brain barrier (BBB) disruption, endothelial dysfunction, and microcirculation failure, leading to neuronal energy depletion and excitotoxicity (Wang Y. et al., 2025). However, currently there are limited treatment options for this disease, which is a major challenge for the medical community (Zhong et al., 2024). Ferroptosis, a form of cell death driven by iron, is characterized by iron overload and excessive lipid peroxidation. Iron deposition and lipid peroxidation are key pathological features in neurodegenerative diseases (Ali et al., 2025). The explanation of ferroptosis has created new prospects in the study of the pathophysiology and therapeutic potential of VD (Ji et al., 2022). D. nobile polysaccharides (DNP) have the capacity to mitigate ferroptosis and maintain cognitive ability in VD rats. The current research involved 72 males of SD rats that were randomly divided into a sham operation group, a VD group, and a DNP group (100 mg/kg/day). The model and DNP groups were subjected to permanent ligation of the bilateral common carotid arteries, and an equal procedure was done on the sham operation group without the carotid artery ligation. The Morris water maze (MWM) test was used to examine the escape latency and the number of platform crossings. The tissue of the hippocampus was dissected after the end of the MWM experiment to determine GSH, GPx4, xCT, and PSD-95 expression and and mitochondrial morphology and ultrastructure were observed using transmission electron microscopy (TEM). The DNP group had significantly shorter escape latency (P < 0.05) and increased performance of platform crossing (P < 0.05) compared with the model group. The TEM analysis demonstrated that there are increased structures of the mitochondrial membranes and cristae in the DNP group. The GSH, xCT, and GPx4 levels in the hippocampus were increased in this group (P < 0.01). Besides, there was an increase of the synaptic vesicles, synaptic active zone (SAZ) length, thicker postsynaptic densities (PSD) and PSD-95 protein expression in DNP-treated rats. These results suggest that DNP can improve cognitive function in VD by alleviating ferroptosis (Hossain, 2009; Ming et al., 2023).
It is worth mentioning that DNP can also improve behavioral disorders caused by spinal cord injury (SCI) by inhibiting ferroptosis. The Basso-Beattie-Bresnahan scores of motor function in the DNP group were lower than those in the sham operation group (P < 0.05) and higher than those in the model group (P < 0.05) after 7, 14, 21, and 28 days of treatment. TEM observed that the mitochondrial membrane of the model group was damaged, and the mitochondrial cristae almost disappeared. However, after DNP treatment, there was improvement and a decrease in iron content (24 and 48 h after injury). The HE stains after 28 days of treatment showed that the spinal cord tissue defect area in the DNP group was smaller compared to the model group. And compared with the sham operation group, the xCT, GSH, Gpx4, and GRSFI in the spinal cord tissue of the model group rats decreased (P < 0.05), while the expression of these indicators increased after DNP treatment. NeuN+ is a commonly used marker for mature neurons. DNP treatment increased NeuN+ cells at 14 and 28 days after SCI (Huang J. et al., 2024; Wei et al., 2024). DNP has neuroprotective effects and can improve some neurological diseases by alleviating or inhibiting ferroptosis.
4.6 Anti-inflammatory effect
Inflammation is an automatic defense response of the body. It is closely related to the occurrence and development of many diseases, including arthritis, inflammatory bowel disease, diabetes, nervous system diseases, etc (Han et al., 2024; Lee et al., 2024). Therefore, studying the anti-inflammatory effects of polysaccharides from D. nobile can provide reference and inspiration for the treatment of these diseases. The important regulatory cells in the inflammatory process are macrophages. Chen et al. (2022) investigated the anti-inflammatory effects of D. nobile polysaccharides (DNP1 and DNP2) using lipopolysaccharide (LPS) - induced RAW264.7 macrophages as a model. Firstly, treatment of RAW264.7 macrophages with DNP1 and DNP2 at concentrations of 12.5, 25, 50, 100, and 200 μg/mL showed no cytotoxicity and significant cell proliferation. LPS stimulation can excessively increase the levels of inflammatory factors such as NO, TNF-α, IL-1β, IL-6 in RAW264.7 macrophages, inducing inflammatory responses, while DNP1 and DNP2 significantly reduce the production of inflammatory factors in a dose-dependent manner (Chen et al., 2022; Pan et al., 2014). The study is used to provide a theoretical basis of producing and using new products containing DNP1 and DNP2 with the aim of relieving inflammation. The team then investigated the mechanism through which DNP1 exerts anti-inflammatory effects, using surface plasmon resonance (SPR) binding assay, molecular docking techniques, and macrophage receptor blockade experiments. From the binding kinetic parameters and sensor graph of SPR, the dissociation constant value of DNP1 is 0. Molecular-docking technology has revealed that the binding energy of the DNP1 and TLR4-MD2 is −7.9 kcal/mol. The results of macrophage receptor blockade experiments showed that after treatment with different concentrations of DNP1, there were no significant changes in the release of NO, TNF-α, IL-1β, IL-6 from RAW 264.7. It is speculated that DNP1 may exert immunomodulatory effects by binding to the TLR4-MD2 complex and inhibiting the TLR4-MD2 mediated signaling pathway (Li et al., 2024; Zhang et al., 2023).
4.7 Anti-viral effect
Plant viruses parasitize in plants and can affect their normal growth and development (Yuan et al., 2022). For crops, it also causes a decrease in their quality and yield. To explore more antiviral agents, researchers measured the anti-cucumber mosaic virus (CMV) and anti-tobacco mosaic virus (TMV) effects of 125 μg/mL−1 DNPE6 (4) in tobacco using chitosan oligosaccharides (COS) and lentinan as controls. The protective activities of DNPE6 (4) against CMV and TMV were 40.4% and 69.9%, respectively. Among them, DNPE6 (4) has better protective activity against TMV than COS (39.1%) and lentinan (52.3%). After treatment with DNPE6 (4), NADPH oxidase and NAD(P)H increased. In addition, DNPE6 (4) can enhance the activity of defensive enzymes (POD, PAL, SOD) in tobacco. The PALS enzyme activity of the CK + DNPE6 (4) group reached its peak on the first day, while SOD and POD enzyme activities reached their peak on the third day. Moreover, the enzyme activities that reached their peak in the CK + DNPE6 (4) group were higher than those of the CK group. The enzyme activities of PALS and POD in the TMV + DNPE6 (4) treatment group peaked on third day, while that of SOD enzyme peaked on fifth day. A similar tendency was noted in the current study: the activity of enzymes was the highest in the TMV + DNPE6 (4) group and were higher than those in the TMV group. The above implies that DNPE6 (4) can effectively induce the host to produce defensive enzymes to resist plant virus infection. The effect of DNPE6 (4) on the expression of defense related genes was detected by RT-PCR. The results showed that DNPE6 (4) could increase the expression level of SOD and the expression levels of ICSI, EDSI, and PRI up-stream and down-stream of the Salicylic acid (SA) pathway, while inhibiting the protein expression of CAT. This information shows that DNPE6 (4) can accumulate SA and induce systemic acquired resistance (SAR). In summary, DNPE6 (4) exhibits anti-CMV and anti-TMV effect (Li et al., 2019). Subsequently, the antiviral effect of DNPE6 (11) against CMV, TMV, and potato virus Y (PVY) were studied. DNPE6 (11) has significant therapeutic and inactivation activity against CMV, significant protective effect against PVY, and greater efficacy than that of the control Ningnanmycin. In addition, preliminary mechanistic studies have found that DNPE6 (11) has superior binding ability with cucumber mosaic virus coat protein (Li et al., 2020; Mohan et al., 2020). Taken together, DNPE6 (4) and DNPE6 (11) can be deemed as potential antiviral compounds.
5 Structure-activity relationships of D. nobile polysaccharides
Understanding the relationship between structure and activity not only deepens insights into the biological roles of polysaccharides but also provides a scientific foundation for their applications and offers valuable guidance for the study of other compounds (Yao et al., 2024; Zhang Y. et al., 2024). D. nobile polysaccharides consist of various monosaccharides, and the differences in their monosaccharide composition result in diverse pharmacological effects (Table 4). For instance, DNP1, which contains Man and Glc, exhibits anti-inflammatory effect (Pan et al., 2014). In contrast, DNP, consisting of Man, Glc, and Gal, shows protective effects on testicular tissue in DM (Fan et al., 2023; Luo et al., 2022). Even with the same monosaccharide composition, its pharmacological effects may vary due to differences in monosaccharide ratios. For example, DNLP and DNP both have Man: Glc: Gal in ratios of 26.9%: 66.2%: 6.9% and 12.49%: 65.2%: 6.4%, respectively. DNLP has a protective effect on polycystic ovary syndrome (Song et al., 2022; Zhang et al., 2020). DNP can regulate insulin resistance in granulosa cells of polycystic ovary syndrome (Hu et al., 2024; Martin and Madassery, 2006). Despite having the same monosaccharide components (Rha, Ara, Xyl, Man, Glc, Gal), DNLP exhibits anti-photoaging effects, whereas DNP acts as an anti-oxidant (Li et al., 2022; Luo et al., 2009). Moreover, the pharmacological effects of D. nobile polysaccharides depend on their Mw. For example, the Mw of JCS1 is 23 kDa, while that of its acetylated derivative YJCS1 is 18.8 kDa. Studies comparing the biological activity of these two polysaccharides in PC-12 cells show that YJCS1 induces neuron production, while JCS1 does not (Jin et al., 2017).
TABLE 4
| Polysaccharide name | Mw (kDa) | Ara (%) | Fuc (%) | Gal (%) | GalA (%) | Glc (%) | GclA (%) | Man (%) | Rha (%) | Xyl (%) | Structural related information | Biological activity | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNLP | N/A | 17.76 | 0 | 12.38 | 0 | 19.70 | 0 | 11.63 | 10.76 | 27.77 | N/A | Anti-photoaging effect | Li et al. (2022) |
| DNLP | 1.2–11.2 | 0 | 0 | 6.9 | 0 | 66.2 | 0 | 26.9 | 0 | 0 | N/A | Ovarian protective effect | Zhang et al. (2020) |
| DNP | 13.2 | 0 | 0 | 6.4 | 0 | 65.2 | 0 | 12.49 | 0 | 0 | N/A | Ovarian protective effect | Hu et al. (2024) |
| DNP | 106.6 | 0 | 0 | 3.5 | 0 | 68.5 | 0 | 23.2 | 0 | 0 | N/A | Testicular protective effect | Luo et al. (2022) |
| DNP1 | 67.72 | 0 | 0 | 0 | 0 | 24.14 | 0 | 75.86 | 0 | 0 | Straight-chain glucomannan β-(1 → 4) |
Anti-inflammatory effect | Li et al. (2024), Chen et al. (2022) |
| DNP2 | 37.45 | 0 | 0 | 0 | 0 | 27.68 | 0 | 72.32 | 0 | 0 | N/A | Anti-inflammatory effect | Chen et al. (2022) |
| DNPE6 (4) | 99.2 | 55.56 | 0 | 6.67 | 0 | 20 | 0 | 17.78 | 0 | 0 | Neutral homogeneous | Anti-viral effect | Li et al. (2019) |
| DNPE6 (11) | 3.01 | 0 | 0 | 17.65 | 0 | 64.71 | 0 | 17.65 | 0 | 0 | Acetylated polysaccharide | Anti-viral effect | Li et al. (2020) |
| DNP-W4 | 500 | 0 | 0 | 23.15 | 8.33 | 45.37 | 0 | 9.26 | 9.26 | 4.63 | Acidic polysaccharide β-(1 → 4), β-(1 → 6) |
Immunomodulatory effect | Wang et al. (2017) |
| JCS1 | 23 | 2.25 | 0 | 0 | 0 | 90.34 | 0 | 4.49 | 0 | 2.92 | Mannoglucan α-(1 → 4), β-(1 → 4) |
N/A | Jin et al. (2017) |
| JCS1S2 | N/A | Sulfated polysaccharide α-(1 → 4), β-(1 → 4) |
Anti-angiogenesis effect | Wang et al. (2019) | |||||||||
| YJCS1 | 18.8 | N/A | Acetylated polysaccharide | Neuritogenesis induced effect on PC-12 Cells | Jin et al. (2017) | ||||||||
| DNP | 87.6 | 1.50 | 0 | 17.05 | 0 | 63.22 | 0 | 16.51 | 0.54 | 1.18 | α-(1 → 4), α-(1 → 6) | Antioxidant effect | Luo et al. (2009) |
| D. nobile polysaccharide | 85.72 | 1.05 | 0 | 1.6 | 0 | 48.63 | 0 | 48.73 | 0 | 0 | N/A | Antioxidant effect | Liu et al. (2019) |
The structural characteristics and corresponding biological activities of D. nobile polysaccharides.
N/A, not available.
Polysaccharides with either excessively high or low Mw may fail to exert their biological activity effectively. High Mw polysaccharides struggle to enter cells via the cell membrane, while low Mw polysaccharides, despite being able to bind to active sites, may lack the complex spatial structures necessary for effective biological function (Wang et al., 2023). As Mw is a structural feature that can be regulated, further research on D. nobile polysaccharides with varying Mw will help identify the optimal Mw range for robust biological activity, contributing significantly to understanding the structure-activity relationship. Interestingly, D. nobile polysaccharides can exhibit similar pharmacological effects despite differences in Mw and monosaccharide composition. For example, both DNPE6 (4) and DNPE6 (11) demonstrate anti-viral activity, even though their Mw differ substantially, at 99.2 and 3.01 kDa, respectively. DNPE6 (4) is composed of Ara, Glc, Gal, and Man, while DNPE6 (11) consists of Man, Glc, and Gal. These similar pharmacological effects may be attributed to the presence of shared structural motifs, such as →4)-d-Glcp-(1→ and →1)-d-Manp-(3,6→) (Li et al., 2019; Li et al., 2020).
Modifying polysaccharides is a key strategy for enhancing their development and utilization. Fan et al. (2020) applied non-thermal plasma treatment to JCS1. The results demonstrated a significant increase in the hydrophilicity of the polysaccharides and a notable improvement in their phagocytic ability towards RAW264.7 cells. Additionally, the treatment stimulated the secretion of cytokines, including TNF-α, IL-6, and IL-1, thereby enhancing their immune activity in vitro. Furthermore, studies have indicated that the JCS1 does not promote the extension of neural processes in PC-12 cells. However, its acetylated derivative, YJCS1, was found to induce neuronal differentiation in these cells. The sulfated polysaccharide JCS1S2 has shown effects on angiogenesis by modulating the VEGF pathway. Specifically, it inhibits the expression of VEGF and its transcription factor AP-1, thereby suppressing angiogenesis (Fan et al., 2020; Jin et al., 2017; Wang et al., 2019).
Currently, research on D. nobile polysaccharides varies in focus and depth. Due to the complex and diverse structural characteristics of these polysaccharides, and the variability in extraction methods across different studies, challenges remain in establishing clear structure-activity relationships. These challenges make it difficult to carry out comprehensive and reliable analyses. To better understand the structure-activity relationship of D. nobile polysaccharides, it is recommended that an exchange platform for research data be established. This platform would integrate data from various research teams, enabling comprehensive analysis and improving research efficiency and result reliability. Such an initiative would also help bridge gaps and address differences in knowledge, methodologies, and experimental conditions across different research groups. In conclusion, while research on the structure-activity relationship of D. nobile polysaccharides is still in its infancy, further innovation in high-purity polysaccharide preparation techniques, as well as strengthened foundational research into their mechanisms of action, is essential to advancing polysaccharide research.
This platform would integrate data from numerous research groups, and this would facilitate careful analysis and increase the efficiency of research and accuracy of results. This endeavor would also further narrow differences and harmonize differences in knowledge, approaches, and conditions of experiments within and across different research groups. In all, while doing research on the structure-activity relationship of D. nobile polysaccharides is still in its infancy, further breakthrough in high-purity preparation methods of polysaccharides, and more thorough foundational research into their mechanisms of action is needed to further advance polysaccharide science.
6 Technological prospection of D. nobile polysaccharide
Search for patent applications related to D. nobile polysaccharides using the search terms “Dendrobium nobile Lindl.,” “Polysaccharides,” and “Dendrobium nobile Lindl. Polysaccharides.” Data reveals an upward trend in patent filings from 2010 to 2017, followed by a decline, with a slight fluctuation around 2020. Key countries involved in these applications include China, Japan, Korea, and Canada, with China accounting for approximately 74% of the applications, followed by Korea and Japan. Other countries or regions contribute to a smaller extent. As depicted in Figure 7C, the primary technical applications of D. nobile polysaccharides are classified under “medicine” and “preparation method”. The substantial proportion of patents related to “medicine” reflects the growing recognition of the medicinal value of D. nobile polysaccharides, which are increasingly being researched and developed. In addition, patents related to “preparation methods” highlight ongoing challenges such as low extraction rates, low purity, and high production costs. Optimizing these processes is crucial to improving the competitiveness of D. nobile polysaccharides products. Through technological advancements and process optimization, the market position of these products can be strengthened.
FIGURE 7
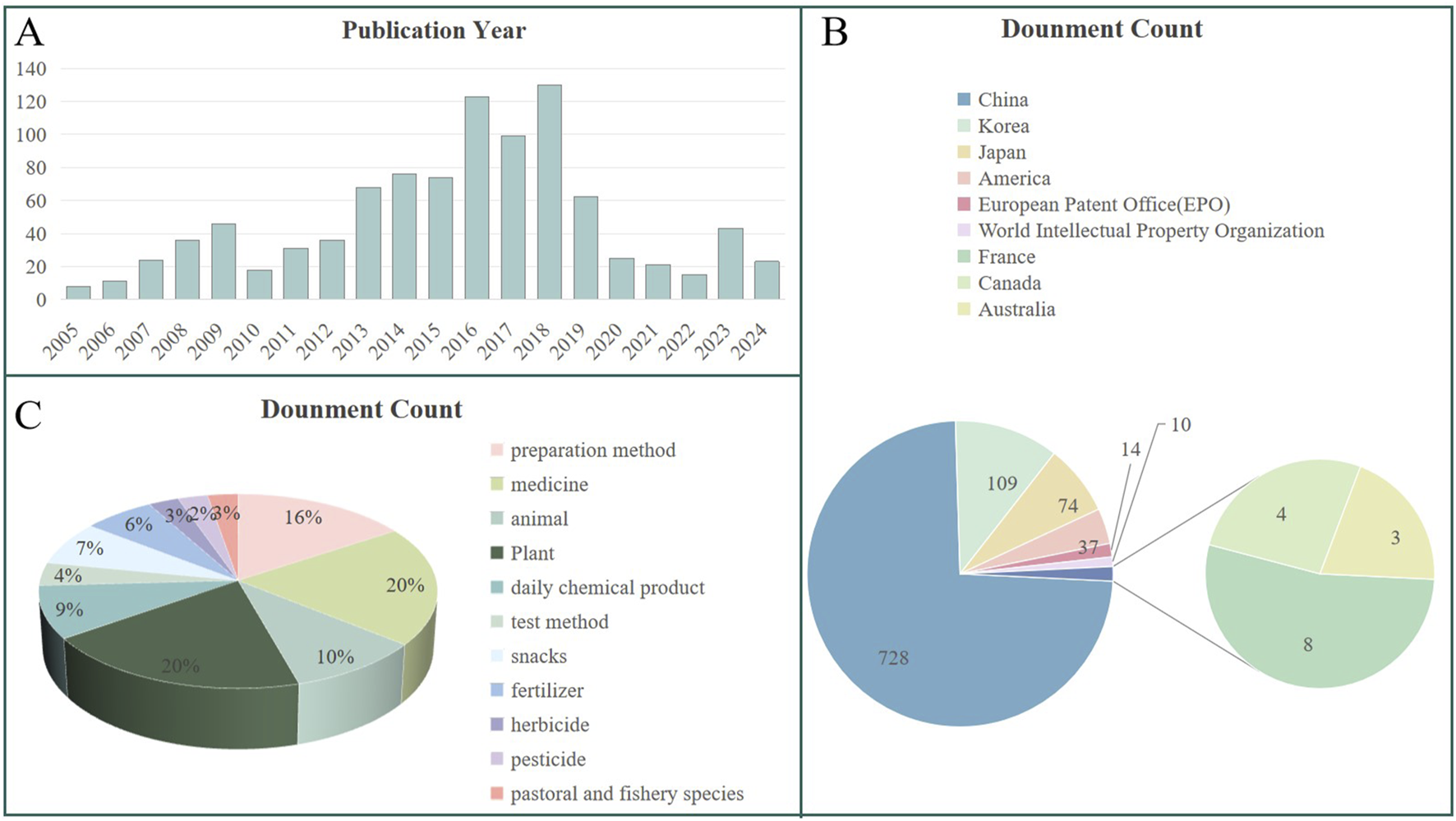
Analysis of D. nobile polysaccharides patents searched in https://t.incopat.com: (A) Number of patent applications per year. (B) Geographical ranking of patents. (C) Technical usage distribution.
To our knowledge, the commercialization of polysaccharides related to D. nobile is still in the exploratory stage. However, some application technology patents related to D. nobile polysaccharides have been made public. A patent (CN113413329A) shows that D. nobile polysaccharides can be used for making face cream. D. nobile polysaccharides have the effect of anti-oxidation and promoting the growth of fibroblasts. It is added with some other raw materials (such as Vaseline, glycerin monostearate, propylene glycol) in proportion to make face cream. Long term external applications can protect the skin and help remove harmful substances from the skin. In addition, a patent (CN115607564A) shows that polysaccharides from D. nobile can also be used in the preparation of drugs or daily chemical products for preventing and treating skin photoaging. Besides, D. nobile polysaccharides can also be used in the food industry. Patent CN110343191A shows that D. nobile polysaccharides can be used to make solid beverages. This beverage not only has the flavor and nutrition of D. nobile, but also has the effect of enhancing immunity, which meets the consumer demand under the influence of healthy diet.
7 Summary and prospect
D. nobile, a traditional Chinese medicine, is classified as a second-class protected plant in China due to its rarity, harvesting difficulty, and low yield. This status highlights the need for comprehensive research to fully exploit its potential value. The efficacy of traditional Chinese medicine is closely associated with its phytochemical composition (Yu et al., 2024). In the field of plant chemistry, both small molecule compounds and large molecule compounds are research objects. After investigation, it was found that most research has focused on small molecule compounds of D. nobile, until recent years when researchers began to be interested in polysaccharides (Fan et al., 2023). The development of extraction technology has led to variety of ways that are used to isolate these polysaccharides, and various processes produce polysaccharides with different structural features and biological activity. D. nobile polysaccharide has made notable progress in the research of its structure and biological activity, especially in the aspects of anti-photoaging, improvement of complications of diabetes mellitus, and ovarian protective effect. However, research on D. nobile polysaccharides still faces numerous challenges.
Isolation and purification of D. nobile polysaccharides is now a tedious and lengthy process. Modern studies focus on increasing the efficiency of extraction and purity of polysaccharide through the optimization of the existing protocol, or also by implementing additional methods (Chen M. et al., 2024; Ledesma-Escobar et al., 2015). Even though some of the emerging extraction technologies have been proposed in the recent past, their majority are still at the level of development, and the quality of D. nobile polysaccharides cannot be guaranteed without uniform protocols. Improving the existing separation techniques and legislatively unifying these procedures will therefore become a necessity in the following stage of D. nobile polysaccharide research. The pharmacological activity of D. nobile polysaccharides has been supported by cell and animal experimental data, but in order to comprehensively evaluate their biological processes and generate strong scientific data, deeper experiments need to be designed, including human experiments when necessary. The anti-photoaging potential of D. nobile polysaccharides has been already pointed out through experiments, making them potential natural resources with regard to skin photoaging prevention and treatment. This empirical background is the basis of creating new skincare products or pharmaceuticals. In addition, there is limited research on the absorption, distribution, and metabolism of D. nobile polysaccharides within the body. However, advancements in detection technologies such as immunoassays, fluorescence, and isotope labeling are making the in vivo pharmacokinetics of polysaccharides more apparent. Some studies indicate that polysaccharides exert beneficial pharmacological effects when administered orally. Conducting systematic studies on the absorption and metabolic pathways of D. nobile polysaccharides, coupled with comprehensive investigations, will be instrumental in promoting its industrialization. With technological advancements, D. nobile polysaccharides will present broader applications, bringing profound societal benefits.
Statements
Author contributions
XZ: Conceptualization, Writing – original draft. HB: Writing – review and editing. ZZ: Writing – review and editing. XW: Writing – review and editing. MZ: Writing – review and editing. MW: Conceptualization, Formal Analysis, Funding acquisition, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Heilongjiang Provincial University Basic Scientific Research Expenses Project (No. 2024KY-007).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Ara, Arabinose; DM, Diabetes mellitus; Fuc, Fucose; Gal, Galactose; GPC, Gel permeation chromography; Glc, Glucose; HPGPC, High-performance gel permeation chromography; HWE, Hot water extraction; MWM, Morris water maze; NMR, Nuclear Magnetic Resonance; PEG, Polyethylene glycol; PCOS, Polycystic ovary syndrome; ROS, Reactive oxygen species; RSM, Response surface methodology; SWE, Subcritical water extraction; UAE, Ultrasound assisted extraction; VD, Vascular dementia; UV, Ultraviolet.
References
1
Ali A. Babar Q. Saeed A. Sergi D. Naumovski N. Mohamed Ahmed I. A. et al (2025). Emerging links between ferroptosis and neurodegeneration: implications for disease mechanisms and nutraceutical interventions. Food Sci. Nutr.13 (6), e70385. 10.1002/fsn3.70385
2
Basak S. Annapure U. S. (2022). The potential of subcritical water as a “green” method for the extraction and modification of pectin: a critical review. Food Res. Int.161, 111849. 10.1016/j.foodres.2022.111849
3
Cai Y. Peng S. Duan B. Shao Y. Li X. Zou H. et al (2025). Isoquercetin alleviates diabetic retinopathy via inhibiting p53-mediated ferroptosis. Cell Biol. Int.49 (7), 852–864. 10.1002/cbin.70027
4
Cetinkaya A. Yayla S. Hurkul M. M. Ozkan S. A. (2025). The sample preparation techniques and their application in the extraction of bioactive compounds from medicinal plants. Crit. Rev. Anal. Chem., 1–36. 10.1080/10408347.2025.2503437
5
Chan Y. J. Hsiao G. Wan W. N. Yang T. M. Tsai C. H. Kang J. J. et al (2023). Blue light exposure collapses the inner blood-retinal barrier by accelerating endothelial CLDN5 degradation through the disturbance of GNAZ and the activation of ADAM17. Fluids Barriers CNS20 (1), 31. 10.1186/s12987-023-00430-7
6
Chen H. Shi X. Zhang L. Yao L. Cen L. Li L. et al (2022). Ultrasonic extraction process of polysaccharides from Dendrobium nobile Lindl.: optimization, physicochemical properties and anti-inflammatory activity. Foods (Basel, Switz.)11 (19), 2957. 10.3390/foods11192957
7
Chen J. Ge W. Wang P. Lv W. Wang H. (2024). PEG-based-ultrasound-microwave synergistic extraction of mucilage polysaccharides from chia seed: structural characterization and bioactivity. Int. J. Biol. Macromol.283 (Part 4), 138057. 10.1016/j.ijbiomac.2024.138057
8
Chen M. Li D. Zhang T. Sun Y. Liu R. Sun T. (2024). A mini-review of isolation, purification, structural characteristics and bioactivities of polysaccharides from Aralia elata (Miq.) Seem. Int. J. Biol. Macromol.277 (Part 4), 134572. 10.1016/j.ijbiomac.2024.134572
9
Chen W. Deng Q. Deng B. Li Y. Fan G. Yang F. et al (2024). Comprehensive analysis of Hibisci mutabilis Folium extract’s mechanisms in alleviating UV-induced skin photoaging through enhanced network pharmacology and experimental validation. Front. Pharmacol.15, 1431391. 10.3389/fphar.2024.1431391
10
Chen X. Chen C. Fu X. (2023). Dendrobium officinale polysaccharide alleviates type 2 diabetes mellitus by restoring gut microbiota and repairing intestinal barrier via the LPS/TLR4/TRIF/NF-kB axis. J. Agric. Food Chem.71 (31), 11929–11940. 10.1021/acs.jafc.3c02429
11
Chen Y. Yao F. Ming K. Wang D. Hu Y. Liu J. (2016). Polysaccharides from traditional Chinese medicines: extraction, purification, modification, and biological activity. Molecules21 (12), 1705. 10.3390/molecules21121705
12
Cui B. Wang Y. Jin J. Yang Z. Guo R. Li X. et al (2022). Resveratrol treats UVB-induced photoaging by anti-MMP expression, through anti-inflammatory, antioxidant, and antiapoptotic properties, and treats photoaging by upregulating VEGF-B expression. Oxidative Med. Cell. Longev.2022, 6037303. 10.1155/2022/6037303
13
Dusabimana T. Karekezi J. Nugroho T. A. Ndahigwa E. N. Choi Y. J. Kim H. et al (2024). Oyster hydrolysate ameliorates UVB-induced skin dehydration and barrier dysfunction. Life Sci.358, 123149. 10.1016/j.lfs.2024.123149
14
Fan C. Sun X. Wang X. Yu H. (2023). Therapeutic potential of the chemical composition of Dendrobium nobile Lindl. Front. Pharmacol.14, 1163830. 10.3389/fphar.2023.1163830
15
Fan Y. Yu Q. Wang G. Tan J. Liu S. Pu S. et al (2020). Effects of non-thermal plasma treatment on the polysaccharide from Dendrobium nobile Lindl. and its immune activities in vitro. Int. J. Biol. Macromol.153, 942–950. 10.1016/j.ijbiomac.2019.10.260
16
Han Z. Wang K. Ding S. Zhang M. (2024). Cross-talk of inflammation and cellular senescence: a new insight into the occurrence and progression of osteoarthritis. Bone Res.12 (1), 69. 10.1038/s41413-024-00375-z
17
Helvaci N. Yildiz B. O. (2025). Polycystic ovary syndrome as a metabolic disease. Nat. Rev. Endocrinol.21 (4), 230–244. 10.1038/s41574-024-01057-w
18
Hossain M. (2009). Traditional therapeutic uses of some indigenous orchids of Bangladesh. Med. Aromatic Plant Sci. Biotechnol.3, 100–106.
19
Hsu W. H. Sangkhathat C. Lu M. K. Lin W. Y. Liu H. P. Lin Y. L. (2024). Dendrobium nobile polysaccharide attenuates blue light-induced injury in retinal cells and in vivo in Drosophila. Antioxidants13 (5), 603. 10.3390/antiox13050603
20
Hu R. Nong W. Huo P. Hu L. Jiang W. Yang Z. et al (2024). Dendrobium nobile-derived polysaccharides stimulate the glycolytic pathway by activating SIRT2 to regulate insulin resistance in polycystic ovary syndrome granulosa cells. Int. J. Biol. Macromol.278 (Part 2), 134780. 10.1016/j.ijbiomac.2024.134780
21
Hua C. Wu M. Xiao Y. Zhang R. Yuan Y. Zhang L. et al (2025). Dendrobium nobile lindl extract modulates integrin αIIbβ3-mediated signaling pathways to inhibit platelet activation and thrombosis. J. Ethnopharmacol.347, 119728. 10.1016/j.jep.2025.119728
22
Huang J. Luo J. Huang Y. Wang L. Zhu H. Li Z. et al (2024). Mechanism of Dendrobium Nobile polysaccharide inhibition of ferroptosis in rats with spinal cord injury. J. Integr. Neurosci.23 (3), 65. 10.31083/j.jin2303065
23
Huang R. Chen J. Guo B. Jiang C. Sun W. (2024). Diabetes-induced male infertility: potential mechanisms and treatment options. Mol. Med.30 (1), 11. 10.1186/s10020-023-00771-x
24
Ji Y. Zheng K. Li S. Ren C. Shen Y. Tian L. et al (2022). Insight into the potential role of ferroptosis in neurodegenerative diseases. Front. Cell. Neurosci.16, 1005182. 10.3389/fncel.2022.1005182
25
Jin C. Du Z. Lin L. Zhou L. Li S. Liu Q. et al (2017). Structural characterization of mannoglucan from Dendrobium nobile Lindl and the neuritogenesis-induced effect of its acetylated derivative on PC-12 cells. Polymers9 (9), 399. 10.3390/polym9090399
26
Ledesma-Escobar C. A. Priego-Capote F. Luque de Castro M. D. (2015). Comparative study of the effect of auxiliary energies on the extraction of citrus fruit components. Talanta144, 522–528. 10.1016/j.talanta.2015.07.011
27
Lee C. T. Kuo H. C. Chen Y. H. Tsai M. Y. (2018). Current advances in the biological activity of polysaccharides in Dendrobium with intriguing therapeutic potential. Curr. Med. Chem.25 (14), 1663–1681. 10.2174/0929867324666170227114648
28
Lee S. O. Kuthati Y. Huang W. H. Wong C. S. (2024). Semaglutide ameliorates diabetic neuropathic pain by inhibiting neuroinflammation in the spinal cord. Cells13 (22), 1857. 10.3390/cells13221857
29
Lei X. Huo P. Xie Y. J. Wang Y. Liu G. Tu H. et al (2022). Dendrobium nobile Lindl polysaccharides improve testicular spermatogenic function in streptozotocin-induced diabetic rats. Mol. Reproduction Dev.89 (4), 202–213. 10.1002/mrd.23556
30
Li L. Chen H. Huang G. Lv Y. Yao L. Guo Z. et al (2024). Structure of polysaccharide from Dendrobium nobile Lindl. and its mode of action on TLR4 to exert immunomodulatory effects. Foods13 (9), 1356. 10.3390/foods13091356
31
Li M. Ge Y. Bai S. Xia J. Wang G. Zhang Y. et al (2024a). Atorvastatin calcium alleviates UVB-induced HaCat cell senescence and skin photoaging. Sci. Rep.14 (1), 30010. 10.1038/s41598-024-81573-x
32
Li P. Li Z. Sun Q. Zhang W. Huang X. Si M. et al (2024b). Protective effect and mechanism of Lycium ruthenicum Murray anthocyanins against retinal damage induced by blue light exposure. J. Food Sci.89 (8), 5113–5129. 10.1111/1750-3841.17184
33
Li W. Mu X. Wu X. He W. Liu Y. Liu Y. et al (2022). Dendrobium nobile Lindl. polysaccharides protect fibroblasts against UVA-induced photoaging via JNK/c-Jun/MMPs pathway. J. Ethnopharmacol.298, 115590. 10.1016/j.jep.2022.115590
34
Li Z. Shi J. Hu D. Song B. (2019). A polysaccharide found in Dendrobium nobile Lindl stimulates calcium signaling pathway and enhances tobacco defense against TMV. Int. J. Biol. Macromol.137, 1286–1297. 10.1016/j.ijbiomac.2019.06.179
35
Li Z. Xiang J. Hu D. Song B. (2020). Naturally potential antiviral agent polysaccharide from Dendrobium nobile Lindl. Pesticide Biochem. Physiology167, 104598. 10.1016/j.pestbp.2020.104598
36
Liang A. Zhang W. Wang Q. Huang L. Zhang J. Ma D. et al (2023). Resveratrol regulates insulin resistance to improve the glycolytic pathway by activating SIRT2 in PCOS granulosa cells. Front. Nutr.9, 1019562. 10.3389/fnut.2022.1019562
37
Liu D. Tang W. Yin J.-Yi. Nie S. P. Xie M. Y. (2021). Monosaccharide composition analysis of polysaccharides from natural sources: hydrolysis condition and detection method development. Food Hydrocoll.116, 106641. 10.1016/j.foodhyd.2021.106641
38
Liu J. Li Y. Liu W. Qi Q. Hu X. Li S. et al (2019). Extraction of polysaccharide from Dendrobium nobile Lindl. by subcritical water extraction. ACS Omega4 (24), 20586–20594. 10.1021/acsomega.9b02550
39
Liu K. Li Y. Li J. Yu X. Zhong X. Su W. et al (2024). Alleviation effect of lutein pickering emulsion formed by casein-dextran conjugates through maillard reaction against blue light retinal degeneration. Int. J. Biol. Macromol.282 (Part 2), 136878. 10.1016/j.ijbiomac.2024.136878
40
Liu X. Q. Qin C. F. Chen N. D. Hao J. W. Ma S. T. Zhang M. et al (2023). Simultaneous determination of phenols in the four main original plants of the famous traditional Chinese medicine Shihu by pressurized capillary electrochromatography. RSC Adv.13 (28), 19455–19463. 10.1039/d3ra00761h
41
Long Y. Wang W. Zhang Y. Du F. Zhang S. Li Z. et al (2023a). Photoprotective effects of Dendrobium nobile Lindl. polysaccharides against UVB-induced oxidative stress and apoptosis in HaCaT cells. Int. J. Biol. Macromol.24 (7), 6120. 10.3390/ijms24076120
42
Long Y. Wang W. Zhang Y. Zhang S. Li Z. Deng J. et al (2023b). Dendrobium nobile Lindl polysaccharides attenuate UVB-induced photodamage by regulating oxidative stress, inflammation and MMPs expression in mice model. Photochem. Photobiol.99 (5), 1269–1281. 10.1111/php.13780
43
Luo A. He X. Zhou S. Fan Y. He T. Chun Z. (2009). In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol.45 (4), 359–363. 10.1016/j.ijbiomac.2009.07.008
44
Luo M. Liao B. Ma D. Wang J. Wang J. Liu J. et al (2022). Dendrobium nobile-derived polysaccharides ameliorate spermatogenic disorders in mice with streptozotocin-induced diabetes through regulation of the glycolytic pathway. Int. J. Biol. Macromol.216, 203–212. 10.1016/j.ijbiomac.2022.06.193
45
Martin K. P. Madassery J. (2006). Rapid in vitro propagation of Dendrobium hybrids through direct shoot formation from foliar explants, and protocorm-like bodies. Sci. Hortic.108 (1), 95–99. 10.1016/j.scienta.2005.10.006
46
Ming M. Hu W. Xie G. Chen J. Huang Y. (2023). Dendrobium nobile polysaccharides attenuates ferroptosis and improves cognitive function in vascular dementia rats. Am. J. Alzheimer’s Dis.38, 15333175231185236. 10.1177/15333175231185236
47
Mohan K. Muralisankar T. Uthayakumar V. Chandirasekar R. Revathi N. Ramu Ganesan A. et al (2020). Trends in the extraction, purification, characterisation and biological activities of polysaccharides from tropical and sub-tropical fruits-A comprehensive review. Carbohydr. Polym.238, 116185. 10.1016/j.carbpol.2020.116185
48
Mousa A. Flanagan M. Tay C. T. Norman R. J. Costello M. Li W. et al (2024). Research integrity in guidelines and evidence synthesis (RIGID): a framework for assessing research integrity in guideline development and evidence synthesis. EClinicalMedicine74, 102717. 10.1016/j.eclinm.2024.102717
49
Nie X. Chen Y. Li W. Lu Y. (2020). Anti-aging properties of Dendrobium nobile Lindl.: from molecular mechanisms to potential treatments. J. Ethnopharmacol.257, 112839. 10.1016/j.jep.2020.112839
50
Ouyang X. Yang J. Hong Z. Wu Y. Xie Y. Wang G. (2020). Mechanisms of blue light-induced eye hazard and protective measures: a review. Biomed. Pharmacother.130, 110577. 10.1016/j.biopha.2020.110577
51
Pan L. H. Li X. F. Wang M. N. Zha X. Q. Yang X. F. Liu Z. J. et al (2014). Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol.64, 420–427. 10.1016/j.ijbiomac.2013.12.024
52
Patel D. Sharma D. Mandal P. (2022). Gut microbiota: target for modulation of gut-liver-adipose tissue axis in ethanol-induced liver disease. Mediat. Inflamm.2022, 4230599. 10.1155/2022/4230599
53
Song C. Ma J. Li G. Pan H. Zhu Y. Jin Q. et al (2022). Natural composition and biosynthetic pathways of alkaloids in medicinal Dendrobium species. Front. plant Sci.13, 850949. 10.3389/fpls.2022.850949
54
Sun C. Y. Li Y. T. Liu D. Chen C. W. Liao M. L. (2025). Gastroprotective potential of the aqueous extract of nine-steaming and nine-sun-drying processed polygonatum cyrtonema Hua against alcoholic gastric injury in mice. J. Ethnopharmacol.338 (Part 2), 119103. 10.1016/j.jep.2024.119103
55
Wang H. Wang Y. Liu Y. Xie J. Zhang Y. Jin H. et al (2024). Study on the structural features of eight Dendrobium polysaccharides and their protective effects on gastric mucosa. Foods13 (18), 3011. 10.3390/foods13183011
56
Wang J. H. Zuo S. R. Luo J. P. (2017). Structural analysis and immuno-stimulating activity of an acidic polysaccharide from the stems of Dendrobium nobile Lindl. Molecules22 (4), 611. 10.3390/molecules22040611
57
Wang K. Zhao H. Zhao X. Zhang X. Zhang W. Cheng Y. et al (2024). Photobiomodulation for diabetes and its complications: a review of general presentation, mechanisms and efficacy. Ann. Med.56 (1), 2433684. 10.1080/07853890.2024.2433684
58
Wang R. Bi Y. Xie Y. Chen X. Li H. Nie X. et al (2025). Dendrobium nobile Lindl. Polysaccharides ameliorate the inflammatory microenvironment in the retina of diabetic rats: a multi-omics study of the gut-blood-retina axis. Int. J. Biol. Macromol.316 (Part 1), 144732. 10.1016/j.ijbiomac.2025.144732
59
Wang Y. H. (2021a). Traditional uses and pharmacologically active constituents of Dendrobium plants for dermatological disorders: a review. Nat. Prod. bioprospecting11 (5), 465–487. 10.1007/s13659-021-00305-0
60
Wang Y. Wei X. Wang H. Zhang Y. Li P. Zhou Y. et al (2025). Ginsenoside Rg1 alleviates cognitive impairment in vascular dementia by modulating Adcy1/Kdr-mediated cholinergic synapse and PI3K-AKT pathway. Phytomedicine, 143, 156882. 10.1016/j.phymed.2025.156882
61
Wang Y. H. (2021b). Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: a review. J. Ethnopharmacol.270, 113851. 10.1016/j.jep.2021.113851
62
Wang Z. Chen H. Wang Y. Wu C. Ye T. Xia H. et al (2024). Recombinant filaggrin-2 improves skin barrier function and attenuates ultraviolet B (UVB) irradiation-induced epidermal barrier disruption. Int. J. Biol. Macromol.281 (Part 1), 136064. 10.1016/j.ijbiomac.2024.136064
63
Wang Z. Jin C. Li X. Ding K. (2019). Sulfated polysaccharide JCS1S2 inhibits angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Carbohydr. Polym.207, 502–509. 10.1016/j.carbpol.2018.11.091
64
Wang Z. Zhou X. Shu Z. Zheng Y. Hu X. Zhang P. et al (2023). Regulation strategy, bioactivity, and physical property of plant and microbial polysaccharides based on molecular weight. Int. J. Biol. Macromol.244, 125360. 10.1016/j.ijbiomac.2023.125360
65
Wei X. Wang D. Xu Z. Liu J. Zhu Q. Chen Q. et al (2024). Research progress on the regulatory and pharmacological mechanism of chemical components of Dendrobium. Heliyon10 (18), e37541. 10.1016/j.heliyon.2024.e37541
66
Xiao Z. Yang S. Liu Y. Zhou C. Hong P. Sun S. et al (2022). A novel glyceroglycolipid from brown algae Ishige okamurae improve photoaging and counteract inflammation in UVB-induced HaCaT cells. Chemico-biological Interact.351, 109737. 10.1016/j.cbi.2021.109737
67
Xu Y. Ren Y. Zou W. Ji S. Shen W. (2024). Neutrophil extracellular traps promote erectile dysfunction in rats with diabetes mellitus by enhancing NLRP3-mediated pyroptosis. Sci. Rep.14 (1), 16457. 10.1038/s41598-024-67281-6
68
Xue H. Gao Y. Wu L. Cai X. Liao J. Tan J. (2023). Research progress in extraction, purification, structure of fruit and vegetable polysaccharides and their interaction with anthocyanins/starch. Crit. Rev. food Sci. Nutr.65, 1235–1260. 10.1080/10408398.2023.2291187
69
Xue H. Zhang P. Zhang C. Gao Y. Tan J. (2024). Research progress in the preparation, structural characterization, and biological activities of polysaccharides from traditional Chinese medicine. Int. J. Biol. Macromol.262 (Part 1), 129923. 10.1016/j.ijbiomac.2024.129923
70
Yan Y. Wu Y. Zhao Y. Yang Y. An G. Liu Z. et al (2025). A review on eye diseases induced by blue light: pathology, model, active ingredients and mechanisms. Front. Pharmacol.16, 1513406. 10.3389/fphar.2025.1513406
71
Yang L. Yang Y. Huang L. Cui X. Liu Y. (2023). From single-to multi-omics: future research trends in medicinal plants. Briefings Bioinforma.24 (1), bbac485. 10.1093/bib/bbac485
72
Yao L. Zhu L. Chen C. Wang X. Zhang A. Gao S. et al (2024). A systematic review on polysaccharides from fermented Cordyceps sinensis: advances in the preparation, structural characterization, bioactivities, structure-activity relationships. Int. J. Biol. Macromol.282 (Pt 5), 137275. 10.1016/j.ijbiomac.2024.137275
73
Yu C. Wang P. Ding H. Hu Y. Wang F. Chen H. et al (2024). Effects of the epiphytic patterns on endophytes and metabolites of Dendrobium nobile Lindl. Front. plant Sci.15, 1326998. 10.3389/fpls.2024.1326998
74
Yuan M. Tian Z. Yin X. Yuan X. Gao J. Yuan W. et al (2022). Structural optimization of the natural product: discovery of Almazoles C-D and their derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. food Chem.70 (50), 15693–15702. 10.1021/acs.jafc.2c05898
75
Zhai D. Lv X. Chen J. Peng M. Cai J. (2022). Recent research progress on natural stilbenes in Dendrobium species. Molecules27 (21), 7233. 10.3390/molecules27217233
76
Zhang C. C. Kong Y. L. Zhang M. S. Wu Q. Shi J. S. (2023). Two new alkaloids from Dendrobium nobile Lindl. exhibited neuroprotective activity, and dendrobine alleviated Aβ1-42 -induced apoptosis by inhibiting CDK5 activation in PC12 cells. Drug Dev. Res.84 (2), 262–274. 10.1002/ddr.22030
77
Zhang M. Lin Y. Han Z. Huang X. Zhou S. Wang S. et al (2024). Exploring mechanisms of skin aging: insights for clinical treatment. Front. Immunol.15, 1421858. 10.3389/fimmu.2024.1421858
78
Zhang S. Tu H. Zhu J. Liang A. Huo P. Shan K. et al (2020). Dendrobium nobile Lindl. polysaccharides improve follicular development in PCOS rats. Int. J. Biol. Macromol.149, 826–834. 10.1016/j.ijbiomac.2020.01.196
79
Zhang Y. Chen B. Zhang H. Zhang J. Xue J. (2024). Extraction, purification, structural characterization, bioactivities, modifications and structure-activity relationship of polysaccharides from Ophiopogon japonicus: a review. Front. Nutr.11, 1484865. 10.3389/fnut.2024.1484865
80
Zhang Y. Wang C. Liu C. Wang X. Chen B. Yao L. et al (2020). Recent developments in stigma maydis polysaccharides: isolation, structural characteristics, biological activities and industrial application. Int. J. Biol. Macromol.150, 246–252. 10.1016/j.ijbiomac.2020.01.294
81
Zhang Y. Wang H. Mei N. Ma C. Lou Z. Lv W. et al (2018). Protective effects of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int. J. Biol. Macromol.107 (Part A), 230–235. 10.1016/j.ijbiomac.2017.08.175
82
Zhang Y. Wang H. Wang P. Ma C. He G. Rahman M. R. (2016). Optimization of PEG-based extraction of polysaccharides from Dendrobium nobile Lindl. and bioactivity study. Int. J. Biol. Macromol.92, 1057–1066. 10.1016/j.ijbiomac.2016.07.034
83
Zhao C. Li X. Miao J. Jing S. Li X. Huang L. et al (2017). The effect of different extraction techniques on property and bioactivity of polysaccharides from Dioscorea hemsleyi. Int. J. Biol. Macromol.102, 847–856. 10.1016/j.ijbiomac.2017.04.031
84
Zhong G. Wang X. Zhang Q. Zhang X. Fang X. Li S. et al (2024). Exploring the therapeutic implications of natural compounds modulating apoptosis in vascular dementia. Phytotherapy Res. PTR38 (11), 5270–5289. 10.1002/ptr.8316
85
Zhu H. Zhang H. W. Fan J. H. Jia S. S. Yi X. Han Z. W. et al (2025). Study on differences in structure and anti-inflammatory activity of polysaccharides in five species of Dendrobium. Polymers17 (9), 1164. 10.3390/polym17091164
Summary
Keywords
Dendrobium nobile Lindl., polysaccharides, extraction, purification, structural characteristics, biological activity
Citation
Zhou X, Bi H, Zhang Z, Wang X, Zhang M and Wang M (2025) Recent advances in polysaccharides derived from the Dendrobium nobile Lindl.: preparation strategies, structural characteristics, biological activity, and structure-activity relationships. Front. Pharmacol. 16:1631637. doi: 10.3389/fphar.2025.1631637
Received
20 May 2025
Accepted
07 July 2025
Published
24 July 2025
Volume
16 - 2025
Edited by
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Reviewed by
Arka Bhattacharya, National Agri-Food Biotechnology Institute, India
Zijun Yan, Panzhihua Central Hospital, China
Peilin Chen, Quanzhou Normal University, China
Reham Samra, Mansoura University, Egypt
Updates
Copyright
© 2025 Zhou, Bi, Zhang, Wang, Zhang and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Wang, wangmeng06tcm@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.