- 1Queen Mary School, Jiangxi Medical College, Nanchang University, Nanchang, China
- 2Department of Neurology, The Second Affilliated Hospital of Nanchang University, Nanchang University, Nanchang, China
Neuroinflammation arises from the synergistic interplay of multiple inflammatory mediators and is pathologically associated with various neurological disorders. These conditions are complex, multifactorial diseases characterized by dynamic interactions between chronic neuroinflammation, oxidative stress, and progressive neuronal degeneration. Curcumin, a naturally occurring polyphenolic compound, exhibits significant pharmacological activity in anti-inflammatory processes and immune regulation. Within neuroinflammatory pathologies, microglial cells are crucial effector cells as they can secrete inflammatory mediators. Emerging evidence suggests that these resident immune cells are the primary site of the biological activity of curcumin in the central nervous system. The compound demonstrates multimodal regulatory effects, including modulation of key signaling pathways (NF-κB, NLRP3 inflammasome, and Nrf2) and upregulation of anti-inflammatory cytokines (TGF-β and interleukin-10), collectively contributing to the neuroinflammatory suppression effect of curcumin. This review comprehensively analyzed the therapeutic potential of curcumin in neuroinflammation and explored its clinical prospects for neurological disease intervention.
1 Introduction
Neuroinflammation is a sophisticated immunological response within the central nervous system that is pivotal in neuroprotective and neurodegenerative processes (Botella Lucena and Heneka, 2024; Lei et al., 2025; Pluta, 2025). This phenomenon is characterized by the activation of microglia and astrocytes, accompanied by the release of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), and reactive oxygen species (ROS) (Chen et al., 2024; Du et al., 2024; Kong et al., 2024b; Liu et al., 2024c). Acute neuroinflammation serves essential functions in tissue repair and pathogen clearance. However, chronic neuroinflammatory responses contribute significantly to the pathogenesis of various neurological disorders, such as Alzheimer’s disease (AD) (Chae et al., 2024), Parkinson’s disease (PD) (Qi et al., 2025), multiple sclerosis (MS) (Woo et al., 2024), and stroke (Guan et al., 2024). The underlying mechanisms involve persistent microglial activation mediated by pattern recognition receptors, including Toll-like receptors (TLRs) and the NLRP3 inflammasome, leading to neuronal damage through excessive cytokine production and oxidative stress (Zhang et al., 2021; Hou et al., 2024; Xu et al., 2025a). Furthermore, disruption of the blood-brain barrier facilitates the infiltration of peripheral immune cells, exacerbating neuroinflammatory conditions (Takata et al., 2021; Hou et al., 2023). Emerging therapeutic approaches targeting neuroinflammatory pathways, particularly the inhibition of NF-κB or NLRP3 inflammasome signaling, have demonstrated promising results in preclinical studies (Lei et al., 2023; Zhang et al., 2023a). Given the limitations of conventional anti-inflammatory medications, including adverse effects associated with non-steroidal anti-inflammatory drugs and corticosteroids, researchers are showing increasing interest in identifying natural compounds that exhibit potent anti-inflammatory properties while maintaining favorable safety profiles. This shift in therapeutic focus reflects the need for more targeted and tolerable interventions in neuroinflammatory disorders.
Curcumin, a naturally occurring polyphenolic compound derived from the rhizomes of Curcuma longa, has garnered significant attention in biomedical research owing to its diverse pharmacological properties encompassing anti-inflammatory, antioxidant, anticancer, and neuroprotective activities (Jabczyk et al., 2021; Ran et al., 2021; Liu et al., 2023a; Sadeghi et al., 2023; Wang et al., 2023). Curcumin, demethoxycurcumin, and bisdemethoxycurcumin are called curcuminoids (Figure 1). As the principal bioactive constituent of turmeric, curcumin has been extensively investigated for its capacity to modulate multiple signaling pathways, including NF-κB, mitogen-activated protein kinase, and phosphatidylinositol 3-kinase/Akt (PI3K/Akt) cascades, which play pivotal roles in regulating inflammatory responses, cellular proliferation, and apoptotic processes (Qiu et al., 2020; Ren et al., 2020; Ran et al., 2021; Shamsnia et al., 2023). Despite its considerable therapeutic potential, the clinical application of curcumin has been constrained by pharmacokinetic limitations, particularly its poor aqueous solubility, rapid metabolic degradation, and systemic elimination. Therefore, contemporary research has focused on developing advanced drug delivery platforms, including nanoparticle formulations, liposomal carriers, and phospholipid complexes, to enhance the bioavailability and pharmacokinetic profile of curcumin (Chen et al., 2020; Quispe et al., 2021; Hassanizadeh et al., 2023; Mohammadzadeh et al., 2024). Emerging scientific evidence further suggests that the therapeutic effects of curcumin are mediated through epigenetic regulatory mechanisms, including modulation of histone acetylation patterns and DNA methylation status, which potentially contribute to its chemopreventive and antineoplastic properties (Fabianowska-Majewska et al., 2021; Yang et al., 2021b; Ming et al., 2022; Sawesi et al., 2022). Current reviews on curcumin are focused on elucidating its therapeutic targets in neurological disorders (Zia et al., 2021; Sadeghi et al., 2023; Nunes et al., 2024; Yang et al., 2024a). Although its interactions with numerous molecular targets have been extensively documented, there remains a lack of comprehensive review addressing the specific mechanisms through which curcumin modulates inflammation in the treatment of neurological disorders (Menon and Sudheer, 2007; Peng et al., 2021; Dehzad et al., 2023; Sadeghi et al., 2023). A deeper understanding of anti-inflammatory mechanisms is crucial for the development of curcumin-based therapeutic strategies and their integration into routine clinical practice.
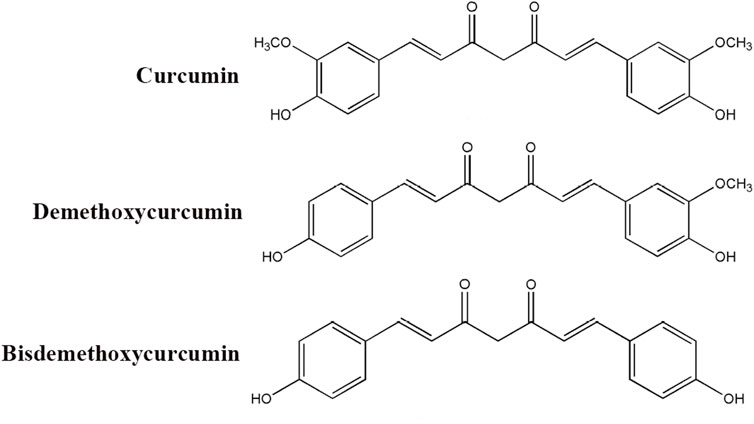
Figure 1. Chemical structure of curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin).
In summary, curcumin has remained a subject of significant scientific interest due to its long history in traditional medicine and its considerable potential in modern biomedical research. Its complex chemical structure, broad pharmacological activities, and challenges related to bioavailability offer compelling avenues for ongoing and future investigations. As research continues to elucidate its mechanisms of action at the molecular level and to improve its delivery methods, curcumin holds promise as a key agent in the management of various health conditions. This review provides a comprehensive exploration of the specific mechanisms by which curcumin treats neurological diseases through the regulation of inflammation. It systematically examines the role of curcumin in mitigating neuroinflammation via the gut–brain axis, outlining how it indirectly ameliorates neuroinflammatory processes by modulating the gut microbiota, maintaining intestinal barrier integrity, and subsequently reducing systemic inflammation. Furthermore, the review offers an in-depth analysis of novel materials (e.g., nanomaterials) developed to enhance its bioavailability—particularly within the context of neurological therapy—and evaluates innovative strategies currently under development aimed at optimizing its clinical efficacy.
2 Synthesis and metabolism of curcumin
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), is a naturally occurring polyphenolic compound that serves as the principal bioactive constituent extracted from the rhizomes of Curcuma longa, a perennial herbaceous plant belonging to the Zingiberaceae family (Liu et al., 2023b; Wang et al., 2023). For centuries, it has been traditionally employed for medicinal purposes, culinary applications, and as a coloring agent (Mohamadian et al., 2022; Kuzminska et al., 2024). The ubiquitous presence of curcumin across various plant taxa underscores its ecological significance and therapeutic potential, warranting further investigation into its biosynthetic pathways and health-promoting properties.
3 Curcumin inhibits the pro-inflammatory activation of immune cells in the central nervous system
3.1 Macrophages
Curcumin exhibits significant immunomodulatory effects on macrophages, which are pivotal innate immune cells involved in inflammatory responses and tissue homeostasis. Experimental studies demonstrate that curcumin preferentially suppresses pro-inflammatory M1 macrophage polarization while promoting anti-inflammatory M2 phenotype, primarily by inhibiting the signal transducer and activator of transcription 3 (STAT3) signaling pathway (Ran et al., 2021; Abdollahi et al., 2023; Deswal et al., 2024). At the molecular level, curcumin reduces lipopolysaccharide (LPS)-induced production of TNF-α, IL-6, and nitric oxide in macrophages by downregulating iNOS and COX-2 expression (Wang et al., 2019). In ischemic stroke models, curcumin administration attenuates stroke-induced white matter damage, improves functional outcomes, and reduces microglial pyroptosis, mediated at least partially through the suppression of NF-κB/NLRP3 signaling pathways (Ran et al., 2021). Similarly, in myocardial infarction, curcumin improves cardiac function and reduces post-MI fibrosis by inhibiting macrophage-fibroblast crosstalk during the acute phase and suppressing IL-18-TGF-β1-p-SMAD2/3 signaling in cardiac fibroblasts (Zhao et al., 2021). In addition, it modulates myocardial inflammation through AMPK-regulated M1/M2 macrophage polarization (Yan et al., 2021). Additional evidence indicates that curcumin protects against particulate matter-induced lung injury by suppressing oxidative stress and inflammatory activation in macrophages (Lee et al., 2023). In ulcerative colitis models characterized by dysregulated M1/M2 macrophage ratios and enhanced M1 activation, curcumin treatment normalizes aberrant macrophage activation, inhibits macrophage chemotaxis, and alleviates inflammatory responses (Chen et al., 2023). Collectively, these findings establish macrophages as crucial cellular mediators of the immunomodulatory actions of curcumin, playing a vital role in regulating immune responses and potentially ameliorating macrophage-associated systemic and neuroinflammatory conditions. The compound demonstrates therapeutic potential across multiple disease states by modulating macrophage polarization and function.
3.2 Microglia
Emerging research has elucidated the potent regulatory effects of curcumin on microglia, the resident immune cells of the central nervous system. Under neuroinflammatory conditions, curcumin (5-25 μM) can attenuate LPS-induced microglial activation, achieving approximately 60% reduction in pro-inflammatory cytokine release (TNF-α, IL-1β, IL-6) by suppressing the TLR4/MyD88/NF-κB signaling cascade (Gao et al., 2019; Zhang et al., 2019). The compound exhibits anti-inflammatory and antioxidant properties by inhibiting NOX2-mediated ROS production in activated microglia while upregulating the Nrf2/Heme Oxygenase-1 (HO-1) antioxidant pathway (Duan et al., 2022; Lin et al., 2022; Xu et al., 2025a). In models of traumatic brain injury, curcumin treatment mitigates brain damage, reduces IL-1β and IL-6 levels, promotes microglial polarization toward the M2 phenotype, and downregulates C1ql3 protein expression (Zhang et al., 2023b). Similarly, in subarachnoid hemorrhage, curcumin demonstrates neuroprotective effects by suppressing neuronal ferroptosis through the modulation of Nrf2/HO-1 signaling (Xu et al., 2025b). Notably, curcumin metabolites such as tetrahydrocurcumin retain biological activity in regulating microglial triggering receptor expressed on myeloid cells two signaling, potentially explaining the beneficial effects of the compound despite limited blood-brain barrier permeability (Jiang et al., 2023; Genchi et al., 2024). These findings collectively establish microglia as crucial cellular mediators of the immunomodulatory actions of curcumin, highlighting its therapeutic potential in alleviating microglia-associated neuroinflammatory pathologies through multifaceted mechanisms of action. The ability of the compound to modulate microglial activation states and signaling pathways positions it as a promising candidate for neuroinflammatory intervention.
3.3 T cell
Curcumin demonstrates significant immunomodulatory capacity in T cell-mediated diseases through pleiotropic mechanisms targeting cellular activation, differentiation, and effector functions. In autoimmune conditions, curcumin (10–25 μM) ameliorates disease severity by suppressing pathogenic Th1/Th17 responses while enhancing the activity of regulatory T cells (Tregs) (Fasihi et al., 2024; Ghoushi et al., 2024; Nosratabadi et al., 2024). Experimental autoimmune encephalomyelitis (EAE) studies reveal that the neuroprotective effects of curcumin are mediated, at least partially, through AMPK/SIRT1 activation, ultimately attenuating EAE-induced neuronal demyelination, oxidative stress, and neuroinflammation (ELBini-Dhouib et al., 2022; Sadek et al., 2024). Rheumatoid arthritis models demonstrate the ability of curcumin to inhibit Th17 differentiation via suppression of STAT3 phosphorylation, with additional evidence showing its regulation of the inc00052/miR-126-5p/PIAS2 axis through the JAK2/STAT3 signaling pathway (Xiao et al., 2022; Kou et al., 2023; Deng et al., 2024). In allergic disorders, curcumin modulates Th1/Th2 balance by downregulating GATA3 expression and reducing IL-4/IL-5 production in ovalbumin-sensitized models (Wang et al., 2022). Therapeutic applications in thyroid cancer reveal that curcumin can enhance anti-tumor immunity in anaplastic thyroid carcinoma by boosting CD8+ T-cell function and inactivating the AKT/mTORC1/STAT3/PD-L1 axis (Vaiss et al., 2024). Furthermore, curcumin reduces severity in acute lung injury/acute respiratory distress syndrome and uncontrolled inflammation by promoting naïve CD4+ T-cell differentiation into CD4+CD25+FOXP3+ Tregs (Chai et al., 2020). In summary, curcumin exhibits broad therapeutic potential across autoimmune diseases, allergic disorders, viral infections, and cancer immunotherapy through its multifaceted immunomodulatory effects on T-cell subsets, highlighting its value as a versatile immunotherapeutic agent (Figure 2).
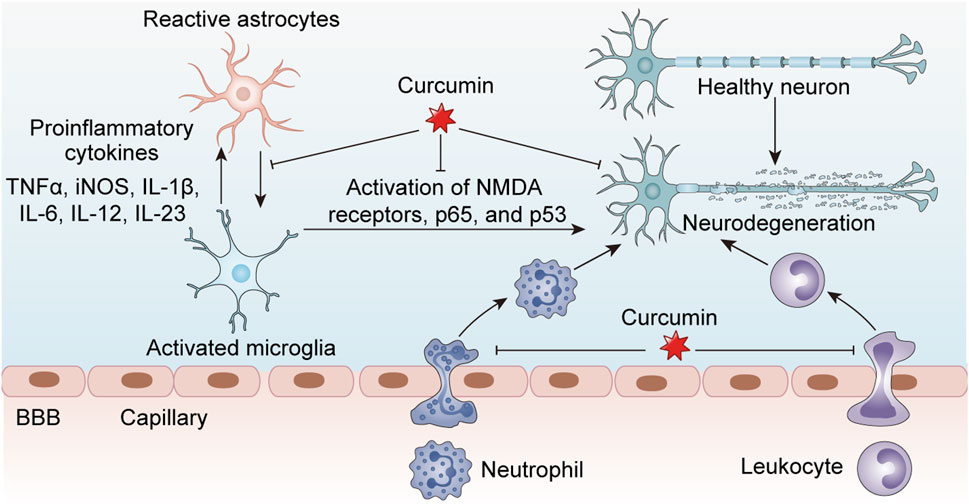
Figure 2. Curcumin exerts inhibitory effects on neuroinflammation associated with central nervous system disorders by modulating the activity of inflammatory cells, including macrophages, microglia, and astrocytes, thereby reducing the release of pro-inflammatory cytokines and subsequently protecting healthy neurons from damage.
4 Influence of curcumin on various neurological diseases
4.1 Alzheimer’s disease (AD)
AD is a progressive neurodegenerative disorder characterized by cognitive decline, memory impairment, and behavioral disturbances and is the most prevalent cause of dementia worldwide (Scheltens et al., 2021; Jucker and Walker, 2023). The neuropathological hallmarks of AD include the accumulation of extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein, which collectively contribute to synaptic dysfunction and neuronal degeneration (Graff-Radford et al., 2021; Serrano-Pozo et al., 2021; Ossenkoppele et al., 2022). Despite extensive research, the precise etiology of AD remains unclear, with proposed involvement of genetic predisposition, environmental factors, and metabolic disturbances. Owing to the growing global aging population and limited availability of disease-modifying therapies, elucidating AD pathogenesis is challenging in contemporary neuroscience research. Given the complex and multifactorial nature of AD pathogenesis, developing effective pharmacological interventions remains a formidable challenge in neurology and drug discovery. Current therapeutic approaches primarily focus on symptomatic management, with acetylcholinesterase inhibitors such as donepezil, rivastigmine, and galantamine enhancing cholinergic neurotransmission to alleviate cognitive deterioration (Se Thoe et al., 2021; Liu et al., 2024a). Furthermore, the N-methyl-D-aspartate receptor antagonist memantine provides partial neuroprotection by modulating glutamatergic excitotoxicity (Vaz and Silvestre, 2020; Pardo-Moreno et al., 2022; Varadharajan et al., 2023). However, these treatments offer merely transient symptomatic stabilization without altering disease progression, thus stimulating increasing interest in natural compounds with multimodal neuroprotective properties. Among these compounds, curcumin, the principal bioactive polyphenol derived from Curcuma longa (turmeric), has emerged as a promising candidate for AD intervention, owing to its diverse pharmacological activities targeting multiple pathological pathways implicated in AD progression.
Curcumin exhibits multiple pharmacological properties relevant to AD pathogenesis, including anti-amyloidogenic, anti-tau, antioxidant, and anti-inflammatory effects (Aggarwal and Harikumar, 2009; Azzini et al., 2024). Recent studies demonstrate that during AD progression, impaired adult neurogenesis in the dentate gyrus can be ameliorated by curcumin treatment through modulation of GSK3β/Wnt/β-catenin and CREB/BDNF pathways via PI3K/Akt regulation, reducing apoptosis and improving neurogenesis in AD mouse models (Lou et al., 2024). In AD transgenic mice, curcumin administration downregulates hippocampal expression of HMGB1, RAGE, TLR4, and NF-κB, improving cognitive function by suppressing the HMGB1-RAGE/TLR4-NF-κB inflammatory signaling cascade (Han et al., 2021). As a dietary supplement, curcumin benefits patients with insulin resistance, type 2 diabetes (T2D), and AD by reducing circulating levels of IAPP and GSK-3β while alleviating insulin resistance-related markers, consequently lowering the risk of T2D and AD (Thota et al., 2020). Notably, prophylactic administration of curcumin prior to Aβ deposition demonstrates preventive potential for AD, possibly through facilitating Aβ42 clearance from the brain to peripheral circulation (Mei et al., 2020). Additionally, curcumin enhances BDNF-ERK signaling to mitigate AD-associated cognitive deficits (Zhang et al., 2015). Emerging evidence reveals that curcumin treatment significantly alters the composition of gut microbiota in AD mice. The compound undergoes biotransformation by gut microbiota through reduction, demethoxylation, demethylation, and hydroxylation, yielding neuroprotective metabolites (Sun et al., 2020; Leblhuber et al., 2021). These findings suggest a gut-liver-brain axis mediating metabolic and cognitive functions, possibly by reducing fatty acid synthesis, altering cholesterol metabolism, inhibiting hepatic lipogenesis-related pathways, and modulating synaptic plasticity-related pathways in the brain, ultimately suppressing weight gain and improving behavioral and cognitive functions (Pluta et al., 2022; Lamichhane et al., 2024).
The therapeutic efficacy of curcumin in AD is considerably constrained by its limited ability to cross the blood-brain barrier. To address this limitation, researchers have explored combination therapy with ginkgo biloba extract and curcumin, demonstrating the suppression of acetylcholinesterase, caspase-3, hippocampal amyloid-β (Aβ1-42), and phosphorylated tau levels and reduced expression of pro-inflammatory cytokines TNF-α and IL-1β in rat models. This combined treatment also significantly decreased malondialdehyde and modulated reduced glutathione levels (Assi et al., 2023). To enhance the neuroprotective effects of curcumin in AD, advanced delivery systems have been developed. Notably, carrier-free curcumin nanoparticles (CNPs) formed through molecular self-assembly exhibit multivalent binding to Aβ, resulting in superior inhibition of Aβ aggregation. Following intranasal administration, these lipid-based formulations release CNPs and cardiolipin in response to the oxidative microenvironment characteristic of AD. The CNPs modulate microglial polarization (M1→M2) via TLR4/NF-κB pathway inhibition, while simultaneously suppressing Aβ aggregation and enhancing microglial phagocytic clearance of Aβ, thereby overcoming barriers to microglial repolarization (Feng et al., 2024). Intravenous administration of novel brain-targeted nanoparticles (Ce/Zr-MOF@Cur-Lf) facilitates rapid brain entry and ameliorates multiple AD pathological features, including neuronal damage, Aβ deposition, cholinergic system dysfunction, oxidative stress, and neuroinflammation (Yang et al., 2024c). Furthermore, localized delivery of curcumin using human hair keratin/chitosan (C/K) hydrogels may enhance neural regeneration and repair nerve damage, representing an innovative approach for targeted therapy.
Collectively, the existing preclinical evidence from in vitro and in vivo studies substantiates the neuroprotective efficacy of curcumin in AD pathogenesis, primarily mediated through its anti-neuroinflammatory properties and cognitive-enhancing effects. Nevertheless, substantial research efforts are still warranted to bridge the gap between these promising experimental findings and clinical applications, particularly concerning bioavailability optimization and therapeutic regimen standardization (Sun et al., 2024).
4.2 Parkinson’s disease (PD)
PD is a progressive neurodegenerative disorder pathologically characterized by selective degeneration of dopaminergic neurons in the substantia nigra pars compacta and the presence of intraneuronal proteinaceous inclusions known as Lewy bodies, predominantly composed of α-synuclein aggregates (Kalia and Lang, 2015; Bloem et al., 2021). Clinically, PD manifests with cardinal motor symptoms including bradykinesia, resting tremor, rigidity, and postural instability, accompanied by various non-motor features such as cognitive impairment, sleep disturbances, and autonomic dysfunction (Hayes, 2019; Morris et al., 2024). With a global prevalence exceeding six million cases, PD ranks as the second most common neurodegenerative disorder after AD, imposing substantial socioeconomic burdens (Ascherio and Schwarzschild, 2016; Weintraub et al., 2022). The etiology of PD remains multifactorial, involving complex interactions between genetic predisposition, environmental exposures, and aging-related cellular dysfunction (Lotankar et al., 2017; Tysnes and Storstein, 2017). Mutations in genes encoding α-synuclein, leucine-rich repeat kinase 2, and PARKIN have provided crucial insights into pathogenic mechanisms, including mitochondrial dysfunction, proteostasis failure, and neuroinflammation (Jankovic and Tan, 2020; Brooker and Gonzalez-Latapi, 2025; Xiao et al., 2025). Current therapeutic approaches primarily focus on dopamine replacement using levodopa, which provides symptomatic relief but fails to halt disease progression. Emerging disease-modifying strategies targeting α-synuclein pathology or employing gene-based interventions show promise but require further validation (Du et al., 2020; Chen et al., 2022). Curcumin, a natural polyphenol, demonstrates significant potential in PD and other neurodegenerative conditions. Experimental evidence indicates that curcumin exerts neuroprotective effects by modulating the BDNF/PI3k/Akt signaling pathway, which plays a critical role in neuroregeneration and anti-apoptotic processes (Jin et al., 2022). Furthermore, curcumin prevents rotenone-induced PD in murine models by inhibiting microglial NLRP3 inflammasome activation and mitigating mitochondrial dysfunction (Xu et al., 2023). In rotenone-exposed mice, curcumin administration activates the p62-Keap1-Nrf2 pathway, enhances autophagy, and improves antioxidant capacity (Rathore et al., 2023). Additional mechanisms include neuroprotection through HDAC6-NLRP3 pathway modulation and amelioration of MPTP-induced gastrointestinal dysfunction, gut microbiota dysbiosis, and short-chain fatty acid profile alterations (Cai et al., 2025). To enhance therapeutic efficacy, advanced delivery systems have been developed, including mitochondria-targeting biomimetic nanoparticles functionalized with curcumin (Zhu et al., 2022; Cai et al., 2023). These nanoparticles localize to damaged neuronal mitochondria in inflammatory environments and modulate the NAD+/SIRT1/PGC-1α/PPARγ/NRF1/TFAM signaling cascade, alleviating MPP + -induced neuronal toxicity and mitochondrial dysfunction (Zheng et al., 2023). Moreover, curcumin-loaded nanoemulsions have demonstrated superior efficacy in preventing motor deficits and inhibiting complex I dysfunction, representing promising nanomedicine applications for PD (Ramires Junior et al., 2021). While preclinical studies consistently demonstrate the ability of curcumin to mitigate PD symptoms and attenuate neuroinflammation in vivo, rigorous clinical trials remain necessary to substantiate its therapeutic potential for patients with PD and establish optimal treatment protocols.
4.3 Multiple sclerosis (MS)
MS is a chronic autoimmune-mediated demyelinating disease of the central nervous system, characterized by multifocal inflammatory lesions, axonal damage, and progressive neurological dysfunction (Kuhlmann et al., 2023). As the most prevalent non-traumatic cause of neurological impairment in young adults, MS affects approximately 2.8 million individuals worldwide, with a higher prevalence among females and in temperate geographical regions. The clinical manifestations of MS exhibit significant heterogeneity, ranging from relapsing-remitting episodes to progressive neurological decline, reflecting a complex interplay between genetic susceptibility and environmental triggers (Travers et al., 2022). The pathogenesis of MS involves the infiltration of autoreactive T cells across the blood-brain barrier, initiating an inflammatory cascade targeting myelin, followed by oligodendrocyte loss and impaired axonal conduction (Perez et al., 2023). Although the precise etiology remains elusive, genome-wide association studies have identified over 200 risk loci, particularly within the major histocompatibility complex region, underscoring the importance of immune dysregulation (Hauser and Cree, 2020). Current therapeutic options for the disease, such as anti-CD20 monoclonal antibodies and sphingosine-1-phosphate receptor modulators, demonstrate limited efficacy in progressive forms of MS (de Seze et al., 2023; Klotz et al., 2023). Th17 cells are critical immune participants in the pathophysiology of MS (Qureshi et al., 2018). Curcumin inhibits the proliferation of Th17 cells and reduces the production of pro-inflammatory cytokines, including TNF-α, IL-22, and IL-17, offering therapeutic potential for MS (Ghoushi et al., 2024). In addition, curcumin exerts neuroprotective effects by downregulating AXL-mediated astrocyte-driven inflammation in cuprizone-induced mouse models, targeting MS treatment (Zhang et al., 2025). In EAE mouse models and LPS-stimulated BV-2 microglial cells, curcumin may ameliorate microglial-mediated inflammatory responses by inhibiting the AXL/JAK2/STAT3 signaling pathway (Sun et al., 2022). Reports indicate that curcumin and its semi-synthetic derivative F-curcumin suppress the expression of IL-1β, IL-4, IL-10, IL-17, interferon-γ, and TGF-β during EAE induction, mitigating MS-associated inflammation (Khosropour et al., 2023). To enhance the bioavailability of curcumin in the treatment of MS, researchers have designed a prodrug, curcumin monoglucuronide, which, when administered intravenously or intraperitoneally, alters the overall gut microbiome composition and modifies the abundance of specific bacterial populations to suppress neuroinflammation and improve MS outcomes (Khadka et al., 2021). In materials science, investigators have utilized high-density lipoprotein-mimicking peptide-phospholipid scaffolds (HPPS) as carriers to improve the bioavailability of curcumin, effectively reducing inflammatory monocyte infiltration across the blood-brain barrier, inhibiting microglial proliferation, and limiting the infiltration of other effector immune cells, thereby decreasing the incidence of EAE in mice (Lu et al., 2020). Similarly, polymeric forms of nano-curcumin correct the balance of pro-inflammatory and anti-inflammatory gene expression in EAE models, reduce oxidative stress, enhance myelin regeneration, and increase progenitor cell markers (Mohajeri et al., 2015).
In summary, curcumin demonstrates the potential to inhibit neuroinflammation in EAE models, improving outcomes in MS.
4.4 Stroke
Stroke represents a significant global health burden, ranking as the second leading cause of death and the third leading cause of disability worldwide. This cerebrovascular event occurs when arterial occlusion or vessel rupture interrupts blood flow to the brain, resulting in the rapid onset of neurological deficits (Kong et al., 2022; Hilkens et al., 2024). The pathophysiological cascade involves excitotoxicity, oxidative stress, and neuroinflammation, ultimately leading to neuronal death within minutes to hours following ischemic injury (Feigin et al., 2025). Recent advancements in the management of acute stroke, particularly the expansion of thrombolytic time windows and the widespread adoption of endovascular thrombectomy, have fundamentally altered the treatment landscape for ischemic stroke (Bushnell et al., 2024). However, significant challenges persist, including narrow therapeutic time windows, the risk of hemorrhagic transformation, and limited neuroprotective strategies (Caso et al., 2024). Moreover, recovery post-stroke remains suboptimal for many patients, with approximately 50% of survivors experiencing long-term disabilities. Therefore, identifying more effective therapeutic targets is critical for enhancing stroke rehabilitation (Kong et al., 2024a; Wegener et al., 2024). Research indicates that curcumin partially mitigates stroke-induced white matter injury and improves neurological function by inhibiting the NF-κB/NLRP3 signaling pathway, providing neuroprotection following a stroke (Ran et al., 2021). Furthermore, curcumin pretreatment enhances ischemic stroke outcomes by preserving blood-brain barrier integrity, promoting synaptic remodeling, and downregulating the phosphorylation of NF-κB and MMP-9, thereby suppressing inflammatory responses (Wu et al., 2021). In models of intracerebral hemorrhage (ICH), curcumin treatment facilitates the inhibition of oxidative stress in microglia by activating the Nrf2/HO-1 pathway and promoting neurological recovery post-ICH, thereby alleviating neuronal damage (Duan et al., 2022). To enhance the therapeutic efficacy of curcumin in stroke, a combined therapy utilizing curcumin and human umbilical cord-derived mesenchymal stem cells exerts anti-inflammatory and antioxidant effects through the AKT/GSK-3β/β-TrCP/Nrf2 axis, improving neurological function following acute ischemic stroke (Li et al., 2023). Similarly, in materials science, researchers have encapsulated curcumin in mPEG-PCL nanoparticles, which are administered intranasally to deliver curcumin directly to the brain. This approach reprograms pro-inflammatory microglia to an anti-inflammatory phenotype, reducing neuronal inflammatory death and hematoma volume in mouse models of ICH (Duan et al., 2024). Additionally, encapsulating curcumin in polymer-based nanoparticles has shown superior therapeutic effects compared with curcumin alone in inhibiting erastin-induced ferroptosis in HT22 hippocampal cells (Yang et al., 2021a).
In summary, curcumin exhibits neuroprotective properties in ischemic and hemorrhagic stroke models by inhibiting neuroinflammation and mitigating neuronal damage, thereby promoting recovery from stroke.
4.5 Amyotrophic lateral sclerosis (ALS)
ALS is a devastating neurodegenerative disorder characterized by the progressive degeneration of upper and lower motor neurons, leading to muscle weakness, paralysis, and ultimately respiratory failure within 3–5 years of symptom onset (Feldman et al., 2022). The global prevalence of ALS is approximately 4–6 cases per 100,000 individuals, making it the most common motor neuron disease among adults and imposing significant physiological and psychological burdens on patients and caregivers (Goutman et al., 2022; Akcimen et al., 2023). The pathogenesis of ALS involves a complex interplay between genetic susceptibility, particularly mutations in C9ORF72, superoxide dismutase 1 (SOD1), TARDBP, and fused in sarcoma (FUS), and environmental factors, resulting in multiple pathological mechanisms, including protein misfolding, oxidative stress, mitochondrial dysfunction, and neuroinflammation. Despite extensive research efforts, current therapeutic options remain limited (Goutman et al., 2022; Ilieva et al., 2023).
Recent studies have identified solid lipid curcumin particles as a potential estrogen replacement therapy that may mitigate the progression and pathogenesis of TDP-43-related diseases (Majumder et al., 2025). In addition, curcumin treatment enhances ATP levels by attenuating the sequestration of pyruvate kinase mediated by FUS aggregation, thereby offering a promising avenue for ALS treatment (Shi et al., 2023). Furthermore, researchers have discovered a novel potent polyphenolic compound, ethoxycurcumin, an effective inhibitor in reducing the risk of fatal ALS by preventing the abnormal misfolding and aggregation of SOD1 into amyloid aggregates (Kouhi et al., 2024). This mechanism may be due to the stronger binding affinity of curcumin to SOD1 protofibrils, facilitated by greater van der Waals interactions (Sharma et al., 2023). Collectively, these findings indicate that curcumin significantly inhibits ALS symptoms in vitro and in vivo, providing new therapeutic directions for improving the prognosis of ALS.
4.6 Epilepsy and seizures
Epilepsy is a chronic neurological disorder characterized by recurrent, unprovoked seizures resulting from abnormal, synchronous neuronal activity in the brain. It is among the most prevalent neurological conditions, significantly impacting morbidity, mortality, and quality of life (Specchio et al., 2022). Seizures can manifest in various clinical phenotypes, ranging from brief focal awareness seizures to generalized convulsive events, reflecting underlying network dysfunction (Deng and Husari, 2024; Krishnamurthy, 2025). The mechanisms underlying epilepsy include ion channel dysfunction (such as SCN1A mutations in Dravet syndrome), heightened excitability of glutamatergic pathways, and impaired GABAergic inhibition. Despite the availability of antiepileptic drugs, approximately 30% of patients experience drug-resistant epilepsy, necessitating alternative interventions such as surgical resection, neurostimulation, or dietary therapies. Consequently, novel therapeutic approaches are essential (Kanner et al., 2024; Pellinen et al., 2024). Recent studies have demonstrated that curcumin can significantly reduce the frequency of seizures in the clinical treatment of pediatric refractory epilepsy (Erfani et al., 2022). In rat models of epilepsy, curcumin administration markedly decreased seizure-like activity, with reduced mRNA and protein levels of Na+, thereby diminishing seizure occurrences (Kumar et al., 2019). In a pentylenetetrazol (PTZ)-induced seizure model, curcumin exerted anticonvulsant effects by elevating serotonin levels in the brain, influencing receptors such as 5-HT1A, 5-HT2C, and 5-HT4, and potentially by downregulating 5-HT7 gene expression (Arbabi Jahan et al., 2018). Moreover, curcumin attenuates glial cell activation and ameliorates cognitive deficits in patients with chronic epilepsy (Kaur et al., 2015). In a lithium-pilocarpine rat model inducing status epilepticus, positron emission tomography revealed that curcumin treatment inhibited cerebral glucose metabolism, reduced body weight, mitigated hippocampal neuronal damage, and decreased neuroinflammation, ultimately reducing seizure frequency (Slowing et al., 2023). Furthermore, in the same model, curcumin conferred neuroprotection by inducing autophagy and inhibiting necroptotic apoptosis, safeguarding hippocampal neurons from status epilepticus-induced injury (Wang et al., 2017). To enhance the anticonvulsant properties of curcumin and improve its bioavailability, researchers have micronized the compound using supercritical carbon dioxide processing. In adult zebrafish models of PTZ-induced seizures, micronized curcumin exhibited effects comparable to those of classical antiepileptic drugs (Bertoncello et al., 2018).
In summary, curcumin demonstrates potential as an antiepileptic agent in various models by suppressing neuroinflammation, thereby reducing seizure frequency (Figure 3).
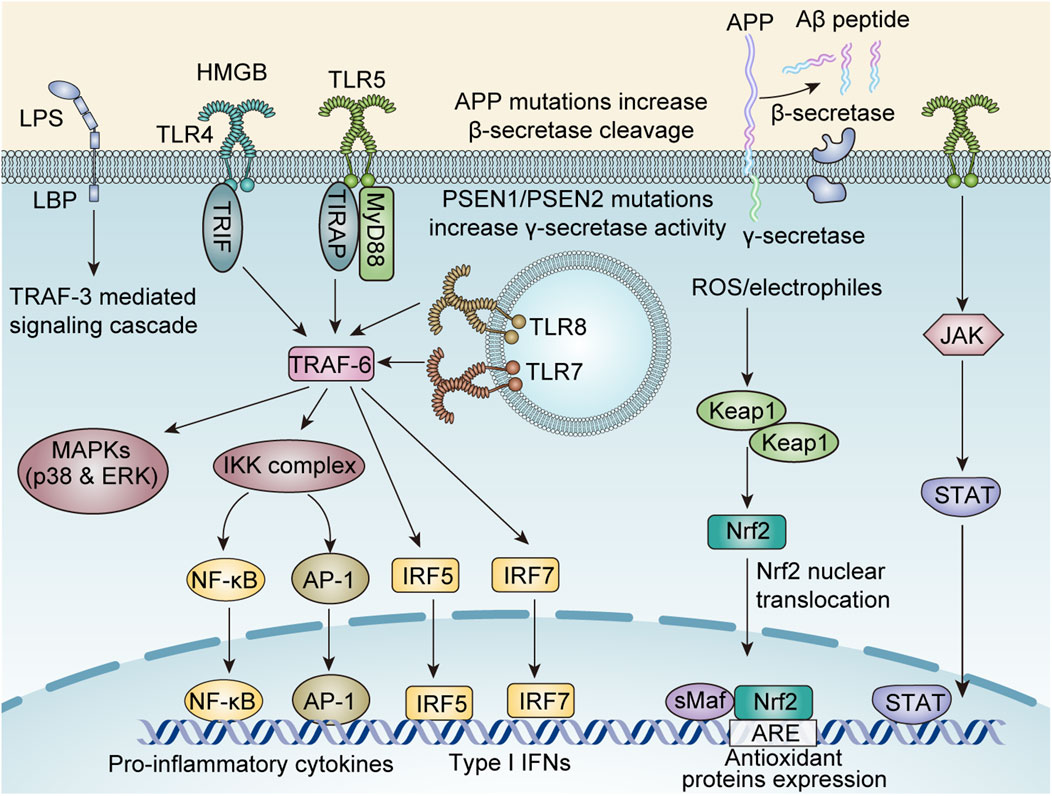
Figure 3. The molecular mechanisms underlying curcumin-mediated anti-neuroinflammatory effects involve multiple signaling pathways, including: the TNF receptor associated factors 6 (TRAF-6) -mediated nuclear factor kappa-B (NF-κB), activator protein-1 (AP-1), Interferon Regulatory Factor 5 (IRF5), IRF7, and mitogen-activated protein kinase (MAPK)/p38/Extracellular regulated protein kinases (ERK) axis; the Janus Kinase (JAK)/signal transducer and activator of transcription (STAT) axis; the reactive oxygen species (ROS)-Kelch-like ECH-associated protein 1 (KEAP1)/nuclear factor erythroid 2-related factor 2 (Nrf2) axis; the TRAF-3 axis; and amyloid-beta (Aβ) protein modulation.
5 Discussion
Neurodegenerative diseases (NDs), including stroke, AD, PD, ALS, and Huntington’s disease, are increasingly recognized as complex multifactorial disorders characterized by the interplay of chronic neuroinflammation, oxidative stress, and progressive neuronal degeneration (Polissidis et al., 2020; Gupta et al., 2023; Kong et al., 2023; Zhou et al., 2023; Goetzl, 2025). A growing body of evidence underscores the pivotal role of sustained neuroinflammatory processes in the onset and progression of these debilitating diseases. The neuroinflammatory cascade in NDs is primarily mediated by the persistent activation of resident immune cells in the central nervous system, namely microglia and astrocytes (Agnello and Ciaccio, 2022; Teleanu et al., 2022). This pathological activation initiates a self-perpetuating inflammatory cycle characterized by the excessive production of pro-inflammatory cytokines (such as TNF-α, IL-1β, and IL-6), ROS, and neurotoxic mediators (Youwakim and Girouard, 2021; Gao et al., 2023). Notably, the NLRP3 inflammasome has emerged as a critical molecular platform linking neuroinflammation and neurodegeneration, facilitating the maturation and secretion of IL-1β and IL-18 in response to pathological protein aggregation (Coll et al., 2022). Recent advancements in neuroimmunology indicate that the neuroinflammatory process exhibits neuroprotective and neurotoxic effects, depending on the disease stage and microenvironment (Nainu et al., 2023). While acute inflammation can promote tissue repair and pathogen clearance, chronic inflammation drives progressive neurodegeneration through feedforward loops involving damage-associated molecular patterns and sustained immune activation (Castro-Gomez and Heneka, 2024; Yu et al., 2025). Understanding these complex neuroimmune interactions provides crucial insights for developing targeted therapeutic strategies for modulating rather than suppressing neuroinflammatory responses (Kong et al., 2024c). Current research efforts are focused on identifying key regulatory nodes within the neuroinflammatory cascade that can serve as therapeutic targets, potentially disrupting the cycle of inflammation-mediated NDs (Figure 4).
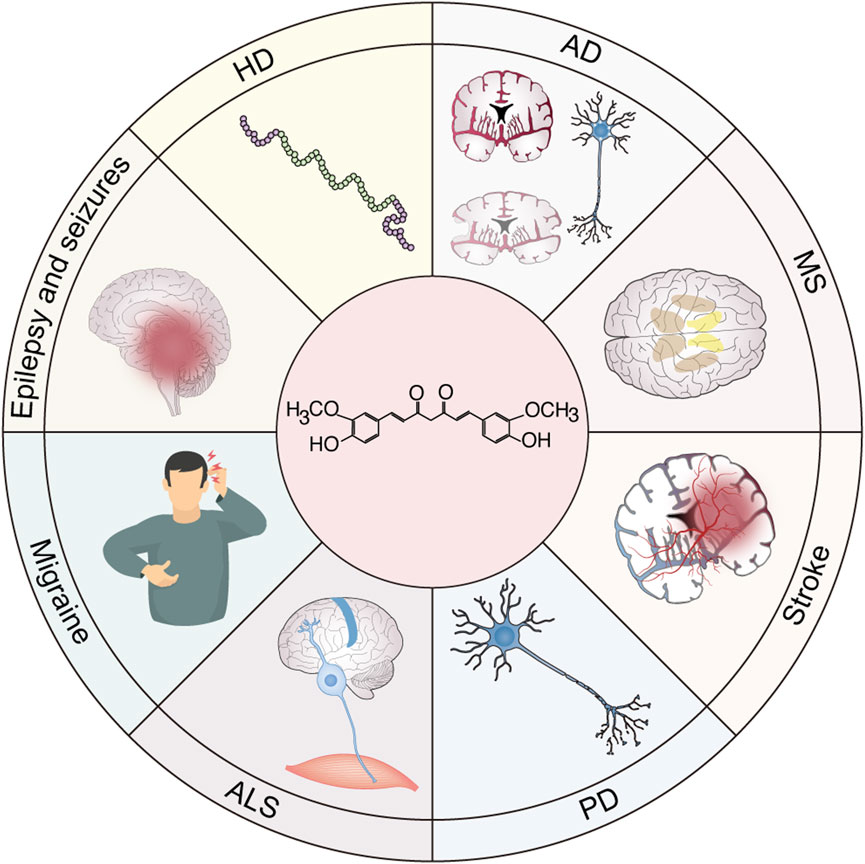
Figure 4. Potential application of curcumin in neuroinflammatory diseases. Neuroinflammation is a common pathogenic mechanism of various neurological diseases, including stroke, multiple sclerosis (MS), Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD), epilepsy and seizures, migraine, and amyotrophic lateral sclerosis (ALS). Therefore, curcumin shows great potential as a prodrug in the clinical translation of these inflammation-related neurological diseases.
The pharmacological treatment strategies for NDs approved by regulatory agencies such as the Food and Drug Administration and European Medicines Agency are primarily palliative, focusing on symptom management rather than addressing the underlying neuropathological mechanisms (Zhang et al., 2023c; Cantara et al., 2024). These conventional approaches, including acetylcholinesterase inhibitors for AD and dopaminergic replacement therapies for PD, are often associated with significant adverse effects and demonstrate limited efficacy in halting disease progression (Grayson, 2016; Beata et al., 2023). Furthermore, these therapies do not modulate the chronic neuroinflammatory processes that are increasingly recognized as key drivers of neurodegeneration (Zhang et al., 2023c). In contrast, extensive preclinical research has identified curcumin and its derivatives as multimodal neuroprotective agents that can target the fundamental inflammatory pathways associated with NDs (Lo Cascio et al., 2021; Genchi et al., 2024). Mechanistic studies indicate that curcumin exerts its therapeutic effects through the complex modulation of various neuroinflammatory signaling cascades, including the NF-κB pathway, NLRP3 inflammasome activation, and Nrf2-mediated antioxidant responses, while downregulating pro-inflammatory cytokines (such as IL-6 and TNF-α) and upregulating anti-inflammatory mediators (such as TGF-β and IL-10) (Zia et al., 2021; Erfani et al., 2022; Tripathi and Bhawana, 2024). Curcumin possesses the ability to simultaneously modulate multiple signaling pathways, conferring a comprehensive advantage compared to many single-target synthetic drugs or other flavonoids such as quercetin and resveratrol (Akaberi et al., 2021). Notably, curcumin can activate the BDNF/TrkB pathway, thereby promoting synaptic growth and hippocampal neurogenesis—a feature rarely observed in most synthetic drugs (e.g., acetylcholinesterase inhibitors) and superior to that of some flavonoids which only exhibit anti-inflammatory or antioxidant properties (Yang et al., 2020). Furthermore, curcumin is naturally low in toxicity, and long-term use is associated with significantly fewer side effects than synthetic drugs such as nonsteroidal anti-inflammatory drugs or immunosuppressants. While other flavonoids like resveratrol may impose hepatic or renal burden at high doses, curcumin has a well-established safety profile at appropriate dosages (Pourbagher-Shahri et al., 2021). Curcumin may offer a more favorable safety profile compared to the broad immunosuppressive effects associated with many conventional anti-inflammatory drugs.
Despite its compelling therapeutic potential, the clinical translation of curcumin is severely hampered by a constellation of pharmacokinetic limitations. Most prominent among these is its exceedingly low systemic bioavailability, which profoundly restricts its therapeutic efficacy. This deficiency arises from a combination of factors including poor aqueous solubility, inadequate absorption from the gastrointestinal tract, rapid metabolic inactivation, and swift systemic elimination. Consequently, even after oral administration of high doses, plasma and tissue concentrations of the native compound remain substantially below the levels required to elicit significant pharmacological effects within the central nervous system. Recent advancements in nanotechnology-based drug delivery systems have significantly transformed the therapeutic potential of curcumin in bioavailability pathways associated with NDs (Liu et al., 2024b; Mohammadzadeh et al., 2024). Nanostructured curcumin demonstrates considerable potential in reducing therapeutic doses. Through nanotechnology-based formulation, the particle size of curcumin can be effectively reduced, while its surface charge and specific surface area are optimized. Moreover, such processing facilitates the formation of a high-energy amorphous state stabilized by intermolecular hydrogen bonding (Mohammadzadeh et al., 2024). Compared to free curcumin, its nanoformulations exhibit not only significantly improved aqueous solubility and drug release profiles but also enhanced antioxidant and antitumor activities (Ratan et al., 2023). Furthermore, nano-carrier systems can effectively shield the drug from detrimental environmental factors, markedly improving its physicochemical stability. Owing to these multiple advantages—including superior stability, protection of the drug, controlled release properties, prolonged in vivo circulation time, and efficient drug loading without the need for chemical modification—both synthetic and natural polymers have been extensively employed to develop curcumin nano-delivery systems (Hajimirzaei et al., 2025). Commonly used polymeric carriers include poly (lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), poly (N-isopropylacrylamide) (PNIPAAm), chitosan, dextrin, polyethylene glycol (PEG), and polyvinyl alcohol (PVA). Various preparation techniques—such as nanoprecipitation, high-pressure homogenization, emulsion-solvent evaporation, and chemical crosslinking—have been utilized to fabricate these nanoparticles (Del Prado-Audelo et al., 2019). These methods enable efficient encapsulation of curcumin into polysaccharide-based nanoparticles, thereby significantly enhancing its stability and enabling controlled drug release. Sophisticated formulations, including nanoparticles, solid lipid nanoparticles, and liposomal carriers, have achieved notable success in enhancing the pharmacokinetic properties of curcumin through various mechanisms (Mahjoob and Stochaj, 2021). These enhancements include improved bioavailability, increased permeability across the blood-brain barrier, and targeted anti-inflammatory effects (Yang et al., 2024b; Hajimirzaei et al., 2025). Such innovative technologies have augmented the therapeutic potential of curcumin in neuroinflammation-related conditions, demonstrating the ability to reverse cognitive deficits, reduce oxidative stress markers, and maintain synaptic density (Attaluri et al., 2022; Ghaffari et al., 2024; Yang et al., 2024b).
However, the clinical translation of these findings remains constrained by the limitations of human studies and ethical considerations. Based on the current available evidence, curcumin demonstrates considerable potential in modulating neuroinflammation. Nevertheless, translating this promise into clinical applications requires addressing several critical challenges, including but not limited to: conducting large-scale, randomized controlled trials with rigorously defined endpoints, validating mechanistic pathways in human subjects, standardizing bioavailable formulations, and exploring adjunctive and combination therapies. Consequently, more extensive and well-designed trials are imperative to establish optimal dosing regimens and long-term safety profiles.
6 Conclusion
In conclusion, while curcumin exhibits promising therapeutic value in the context of neuroinflammation-related diseases, its efficacy and prognostic outcomes remain inconsistent. Consequently, it is imperative to develop and optimize curcumin treatment protocols—including administration routes and dosing strategies—to enhance its clinical effectiveness.
Author contributions
BZ: Writing – original draft, Data curation, Formal Analysis, Conceptualization. BH: Formal Analysis, Supervision, Project administration, Validation, Writing – review and editing, Software, Data curation, Resources, Funding acquisition, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahi, E., Johnston, T. P., Ghaneifar, Z., Vahedi, P., Goleij, P., Azhdari, S., et al. (2023). Immunomodulatory therapeutic effects of curcumin on M1/M2 macrophage polarization in inflammatory diseases. Curr. Mol. Pharmacol. 16, 2–14. doi:10.2174/1874467215666220324114624
Aggarwal, B. B., and Harikumar, K. B. (2009). Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 41, 40–59. doi:10.1016/j.biocel.2008.06.010
Agnello, L., and Ciaccio, M. (2022). Neurodegenerative diseases: from molecular basis to therapy. Int. J. Mol. Sci. 23, 12854. doi:10.3390/ijms232112854
Akaberi, M., Sahebkar, A., and Emami, S. A. (2021). Turmeric and curcumin: from traditional to modern medicine. Adv. Exp. Med. Biol. 1291, 15–39. doi:10.1007/978-3-030-56153-6_2
Akcimen, F., Lopez, E. R., Landers, J. E., Nath, A., Chio, A., Chia, R., et al. (2023). Amyotrophic lateral sclerosis: translating genetic discoveries into therapies. Nat. Rev. Genet. 24, 642–658. doi:10.1038/s41576-023-00592-y
Arbabi Jahan, A., Rad, A., Ghanbarabadi, M., Amin, B., and Mohammad-Zadeh, M. (2018). The role of serotonin and its receptors on the anticonvulsant effect of curcumin in pentylenetetrazol-induced seizures. Life Sci. 211, 252–260. doi:10.1016/j.lfs.2018.09.007
Ascherio, A., and Schwarzschild, M. A. (2016). The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272. doi:10.1016/S1474-4422(16)30230-7
Assi, A. A., Farrag, M. M. Y., Badary, D. M., Allam, E. a.H., and Nicola, M. A. (2023). Protective effects of curcumin and Ginkgo biloba extract combination on a new model of Alzheimer’s disease. Inflammopharmacology 31, 1449–1464. doi:10.1007/s10787-023-01164-6
Attaluri, S., Arora, M., Madhu, L. N., Kodali, M., Shuai, B., Melissari, L., et al. (2022). Oral nano-curcumin in a model of chronic gulf war illness alleviates brain dysfunction with modulation of oxidative stress, mitochondrial function, neuroinflammation, neurogenesis, and gene expression. Aging Dis. 13, 583–613. doi:10.14336/AD.2021.0829
Azzini, E., Pena-Corona, S. I., Hernandez-Parra, H., Chandran, D., Saleena, L. a.K., Sawikr, Y., et al. (2024). Neuroprotective and anti-inflammatory effects of curcumin in Alzheimer’s disease: targeting neuroinflammation strategies. Phytother. Res. 38, 3169–3189. doi:10.1002/ptr.8200
Beata, B. K., Wojciech, J., Johannes, K., Piotr, L., and Barbara, M. (2023). Alzheimer’s disease-biochemical and psychological background for diagnosis and treatment. Int. J. Mol. Sci. 24, 1059. doi:10.3390/ijms24021059
Bertoncello, K. T., Aguiar, G. P. S., Oliveira, J. V., and Siebel, A. M. (2018). Micronization potentiates curcumin’s anti-seizure effect and brings an important advance in epilepsy treatment. Sci. Rep. 8, 2645. doi:10.1038/s41598-018-20897-x
Bloem, B. R., Okun, M. S., and Klein, C. (2021). Parkinson’s disease. Lancet 397, 2284–2303. doi:10.1016/S0140-6736(21)00218-X
Botella Lucena, P., and Heneka, M. T. (2024). Inflammatory aspects of Alzheimer’s disease. Acta Neuropathol. 148, 31. doi:10.1007/s00401-024-02790-2
Brooker, S. M., and Gonzalez-Latapi, P. (2025). Biomarkers in Parkinson’s disease. Neurol. Clin. 43, 229–248. doi:10.1016/j.ncl.2024.12.005
Bushnell, C., Kernan, W. N., Sharrief, A. Z., Chaturvedi, S., Cole, J. W., Cornwell, W. K., et al. (2024). 2024 guideline for the primary prevention of stroke: a guideline from the American heart association/American stroke association. Stroke 55, e344–e424. doi:10.1161/STR.0000000000000475
Cai, B., Zhong, L., Wang, Q., Xu, W., Li, X., and Chen, T. (2023). Curcumin alleviates 1-methyl- 4-phenyl- 1,2,3,6-tetrahydropyridine- induced Parkinson’s disease in mice via modulating gut microbiota and short-chain fatty acids. Front. Pharmacol. 14, 1198335. doi:10.3389/fphar.2023.1198335
Cai, Z., Liang, C., Huang, K., Luo, J., Lu, R., Lai, Y., et al. (2025). Curcumin prevents neurodegeneration by blocking HDAC6-NLRP3 pathway-dependent neuroinflammation in Parkinson’s disease. Int. Immunopharmacol. 146, 113928. doi:10.1016/j.intimp.2024.113928
Cantara, S., Simoncelli, G., and Ricci, C. (2024). Antisense Oligonucleotides (ASOs) in motor neuron diseases: a road to cure in light and shade. Int. J. Mol. Sci. 25, 4809. doi:10.3390/ijms25094809
Caso, V., Turc, G., Abdul-Rahim, A. H., Castro, P., Hussain, S., Lal, A., et al. (2024). European Stroke Organisation (ESO) Guidelines on the diagnosis and management of patent foramen ovale (PFO) after stroke. Eur. Stroke J. 9, 800–834. doi:10.1177/23969873241247978
Castro-Gomez, S., and Heneka, M. T. (2024). Innate immune activation in neurodegenerative diseases. Immunity 57, 790–814. doi:10.1016/j.immuni.2024.03.010
Chae, S., Lee, H. J., Lee, H. E., Kim, J., Jeong, Y. J., Lin, Y., et al. (2024). The dopamine analogue CA140 alleviates AD pathology, neuroinflammation, and rescues synaptic/cognitive functions by modulating DRD1 signaling or directly binding to Abeta. J. Neuroinflammation 21, 200. doi:10.1186/s12974-024-03180-x
Chai, Y. S., Chen, Y. Q., Lin, S. H., Xie, K., Wang, C. J., Yang, Y. Z., et al. (2020). Curcumin regulates the differentiation of naive CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 125, 109946. doi:10.1016/j.biopha.2020.109946
Chen, Y., Lu, Y., Lee, R. J., and Xiang, G. (2020). Nano encapsulated curcumin: and its potential for biomedical applications. Int. J. Nanomedicine 15, 3099–3120. doi:10.2147/IJN.S210320
Chen, R., Gu, X., and Wang, X. (2022). α-Synuclein in Parkinson’s disease and advances in detection. Clin. Chim. Acta 529, 76–86. doi:10.1016/j.cca.2022.02.006
Chen, Y., Chen, D., Zhou, C., Cao, X., and He, J. (2023). Correlation of macrophages with inflammatory reaction in ulcerative colitis and influence of curcumin on macrophage chemotaxis. Altern. Ther. Health Med. 29, 97–103.
Chen, Z., Balachandran, Y. L., Chong, W. P., and Chan, K. W. Y. (2024). Roles of cytokines in Alzheimer’s disease. Int. J. Mol. Sci. 25, 5803. doi:10.3390/ijms25115803
Coll, R. C., Schroder, K., and Pelegrin, P. (2022). NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 43, 653–668. doi:10.1016/j.tips.2022.04.003
De Seze, J., Maillart, E., Gueguen, A., Laplaud, D. A., Michel, L., Thouvenot, E., et al. (2023). Anti-CD20 therapies in multiple sclerosis: from pathology to the clinic. Front. Immunol. 14, 1004795. doi:10.3389/fimmu.2023.1004795
Dehzad, M. J., Ghalandari, H., Nouri, M., and Askarpour, M. (2023). Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: a GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Cytokine 164, 156144. doi:10.1016/j.cyto.2023.156144
Del Prado-Audelo, M. L., Caballero-Floran, I. H., Meza-Toledo, J. A., Mendoza-Munoz, N., Gonzalez-Torres, M., Floran, B., et al. (2019). Formulations of curcumin nanoparticles for brain diseases. Biomolecules 9, 56. doi:10.3390/biom9020056
Deng, D. Z., and Husari, K. S. (2024). Approach to patients with seizures and epilepsy: a guide for primary care Physicians. Prim. Care 51, 211–232. doi:10.1016/j.pop.2024.02.008
Deng, T., Xu, J., Wang, Q., Wang, X., Jiao, Y., Cao, X., et al. (2024). Immunomodulatory effects of curcumin on macrophage polarization in rheumatoid arthritis. Front. Pharmacol. 15, 1369337. doi:10.3389/fphar.2024.1369337
Deswal, B., Bagchi, U., and Kapoor, S. (2024). Curcumin suppresses M2 macrophage-derived paclitaxel chemoresistance through inhibition of PI3K-AKT/STAT3 signaling. Anticancer Agents Med. Chem. 24, 146–156. doi:10.2174/0118715206275259231105184959
Du, X. Y., Xie, X. X., and Liu, R. T. (2020). The role of alpha-synuclein oligomers in Parkinson’s disease. Int. J. Mol. Sci. 21, 8645. doi:10.3390/ijms21228645
Du, G., Yang, Z., Wen, Y., Li, X., Zhong, W., Li, Z., et al. (2024). Heat stress induces IL-1β and IL-18 overproduction via ROS-activated NLRP3 inflammasome: implication in neuroinflammation in mice with heat stroke. Neuroreport 35, 558–567. doi:10.1097/WNR.0000000000002042
Duan, C., Wang, H., Jiao, D., Geng, Y., Wu, Q., Yan, H., et al. (2022). Curcumin restrains oxidative stress of after intracerebral hemorrhage in rat by activating the Nrf2/HO-1 pathway. Front. Pharmacol. 13, 889226. doi:10.3389/fphar.2022.889226
Duan, Z., Zhou, W., He, S., Wang, W., Huang, H., Yi, L., et al. (2024). Intranasal delivery of curcumin nanoparticles improves neuroinflammation and neurological deficits in mice with intracerebral hemorrhage. Small Methods 8, e2400304. doi:10.1002/smtd.202400304
ELBini-Dhouib, I., Manai, M., Neili, N. E., Marzouki, S., Sahraoui, G., Ben Achour, W., et al. (2022). Dual mechanism of action of curcumin in experimental models of multiple sclerosis. Int. J. Mol. Sci. 23, 8658. doi:10.3390/ijms23158658
Erfani, M., Ashrafzadeh, F., Rahimi, H. R., Ebrahimi, S. A., Kalali, K., Beiraghi Toosi, M., et al. (2022). Effect of curcumin on pediatric intractable epilepsy. Iran. J. Child. Neurol. 16, 35–45. doi:10.22037/ijcn.v15i4.28648
Fabianowska-Majewska, K., Kaufman-Szymczyk, A., Szymanska-Kolba, A., Jakubik, J., Majewski, G., and Lubecka, K. (2021). Curcumin from turmeric rhizome: a potential modulator of DNA methylation machinery in breast cancer inhibition. Nutrients 13, 332. doi:10.3390/nu13020332
Fasihi, M., Samimi-Badabi, M., Robat-Jazi, B., Bitarafan, S., Moghadasi, A. N., Mansouri, F., et al. (2024). Immunoregulatory effects of the active form of vitamin D (calcitriol), individually and in combination with curcumin, on peripheral blood mononuclear cells (PBMCs) of multiple sclerosis (MS) patients. Antiinflamm. Antiallergy Agents Med. Chem. 23, 138–147. doi:10.2174/0118715230293847240314073359
Feigin, V. L., Brainin, M., Norrving, B., Martins, S. O., Pandian, J., Lindsay, P., et al. (2025). World stroke organization: global stroke fact sheet 2025. Int. J. Stroke 20, 132–144. doi:10.1177/17474930241308142
Feldman, E. L., Goutman, S. A., Petri, S., Mazzini, L., Savelieff, M. G., Shaw, P. J., et al. (2022). Amyotrophic lateral sclerosis. Lancet 400, 1363–1380. doi:10.1016/S0140-6736(22)01272-7
Feng, Q., Zhang, X., Zhao, X., Liu, J., Wang, Q., Yao, Y., et al. (2024). Intranasal delivery of pure nanodrug loaded liposomes for Alzheimer’s disease treatment by efficiently regulating microglial polarization. Small 20, e2405781. doi:10.1002/smll.202405781
Gao, Y., Zhuang, Z., Lu, Y., Tao, T., Zhou, Y., Liu, G., et al. (2019). Curcumin mitigates neuro-inflammation by modulating microglia polarization through inhibiting TLR4 Axis signaling pathway following experimental subarachnoid hemorrhage. Front. Neurosci. 13, 1223. doi:10.3389/fnins.2019.01223
Gao, C., Jiang, J., Tan, Y., and Chen, S. (2023). Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct. Target Ther. 8, 359. doi:10.1038/s41392-023-01588-0
Genchi, G., Lauria, G., Catalano, A., Carocci, A., and Sinicropi, M. S. (2024). Neuroprotective effects of curcumin in neurodegenerative diseases. Foods 13, 1774. doi:10.3390/foods13111774
Ghaffari, N., Mokhtari, T., Adabi, M., Ebrahimi, B., Kamali, M., Gholaminejhad, M., et al. (2024). Neurological recovery and neurogenesis by curcumin sustained-release system cross-linked with an acellular spinal cord scaffold in rat spinal cord injury: targeting NLRP3 inflammasome pathway. Phytother. Res. 38, 2669–2686. doi:10.1002/ptr.8179
Ghoushi, E., Poudineh, M., Parsamanesh, N., Jamialahmadi, T., and Sahebkar, A. (2024). Curcumin as a regulator of Th17 cells: unveiling the mechanisms. Food Chem. (Oxf) 8, 100198. doi:10.1016/j.fochms.2024.100198
Goetzl, E. J. (2025). Current developments in Alzheimer’s disease. Am. J. Med. 138, 15–20. doi:10.1016/j.amjmed.2024.08.019
Goutman, S. A., Hardiman, O., Al-Chalabi, A., Chio, A., Savelieff, M. G., Kiernan, M. C., et al. (2022). Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 21, 480–493. doi:10.1016/S1474-4422(21)00465-8
Graff-Radford, J., Yong, K. X. X., Apostolova, L. G., Bouwman, F. H., Carrillo, M., Dickerson, B. C., et al. (2021). New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 20, 222–234. doi:10.1016/S1474-4422(20)30440-3
Guan, X., Zhu, S., Song, J., Liu, K., Liu, M., Xie, L., et al. (2024). Microglial CMPK2 promotes neuroinflammation and brain injury after ischemic stroke. Cell Rep. Med. 5, 101522. doi:10.1016/j.xcrm.2024.101522
Gupta, D., Vagha, S., Dhingra, H., and Shirsath, H. (2023). Advances in understanding and treating amyotrophic lateral sclerosis (ALS): a comprehensive review. Cureus 15, e48691. doi:10.7759/cureus.48691
Hajimirzaei, P., Eyni, H., Razmgir, M., Abolfazli, S., Pirzadeh, S., Ahmadi Tabatabaei, F. S., et al. (2025). The analgesic effect of curcumin and nano-curcumin in clinical and preclinical studies: a systematic review and meta-analysis. Naunyn Schmiedeb. Arch. Pharmacol. 398, 393–416. doi:10.1007/s00210-024-03369-0
Han, Y., Chen, R., Lin, Q., Liu, Y., Ge, W., Cao, H., et al. (2021). Curcumin improves memory deficits by inhibiting HMGB1-RAGE/TLR4-NF-κB signalling pathway in APPswe/PS1dE9 transgenic mice hippocampus. J. Cell Mol. Med. 25, 8947–8956. doi:10.1111/jcmm.16855
Hassanizadeh, S., Shojaei, M., Bagherniya, M., Orekhov, A. N., and Sahebkar, A. (2023). Effect of nano-curcumin on various diseases: a comprehensive review of clinical trials. Biofactors 49, 512–533. doi:10.1002/biof.1932
Hauser, S. L., and Cree, B. a.C. (2020). Treatment of multiple sclerosis: a review. Am. J. Med. 133, 1380–1390. doi:10.1016/j.amjmed.2020.05.049
Hayes, M. T. (2019). Parkinson’s disease and Parkinsonism. Am. J. Med. 132, 802–807. doi:10.1016/j.amjmed.2019.03.001
Hilkens, N. A., Casolla, B., Leung, T. W., and De Leeuw, F. E. (2024). Stroke. Lancet 403, 2820–2836. doi:10.1016/S0140-6736(24)00642-1
Hou, W., Yao, J., Liu, J., Lin, X., Wei, J., Yin, X., et al. (2023). USP14 inhibition promotes recovery by protecting BBB integrity and attenuating neuroinflammation in MCAO mice. CNS Neurosci. Ther. 29, 3612–3623. doi:10.1111/cns.14292
Hou, P., Yang, Y., Li, Z., Ye, D., Chen, L., Feng, T., et al. (2024). TAK-3 inhibits lipopolysaccharide-induced neuroinflammation in traumatic brain injury rats through the TLR-4/NF-κB pathway. J. Inflamm. Res. 17, 2147–2158. doi:10.2147/JIR.S454099
Ilieva, H., Vullaganti, M., and Kwan, J. (2023). Advances in molecular pathology, diagnosis, and treatment of amyotrophic lateral sclerosis. BMJ 383, e075037. doi:10.1136/bmj-2023-075037
Jabczyk, M., Nowak, J., Hudzik, B., and Zubelewicz-Szkodzinska, B. (2021). Curcumin in metabolic health and disease. Nutrients 13, 4440. doi:10.3390/nu13124440
Jankovic, J., and Tan, E. K. (2020). Parkinson’s disease: etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 91, 795–808. doi:10.1136/jnnp-2019-322338
Jiang, C., Chen, Z., Wang, X., Zhang, Y., Guo, X., Fan, H., et al. (2023). Curcumin-activated olfactory ensheathing cells improve functional recovery after spinal cord injury by modulating microglia polarization through APOE/TREM2/NF-κB signaling pathway. J. Neuroimmune Pharmacol. 18, 476–494. doi:10.1007/s11481-023-10081-y
Jin, T., Zhang, Y., Botchway, B. O. A., Zhang, J., Fan, R., Zhang, Y., et al. (2022). Curcumin can improve Parkinson’s disease via activating BDNF/PI3k/Akt signaling pathways. Food Chem. Toxicol. 164, 113091. doi:10.1016/j.fct.2022.113091
Jucker, M., and Walker, L. C. (2023). Alzheimer’s disease: from immunotherapy to immunoprevention. Cell 186, 4260–4270. doi:10.1016/j.cell.2023.08.021
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi:10.1016/S0140-6736(14)61393-3
Kanner, A. M., Carrazana, E., Munger Clary, H. M., Rabinowicz, A. L., and Faught, E. (2024). Anticipatory anxiety of seizures in epilepsy: a common, complex, and underrecognized phenomenon? Epileptic Disord. 26, 273–281. doi:10.1002/epd2.20224
Kaur, H., Patro, I., Tikoo, K., and Sandhir, R. (2015). Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 89, 40–50. doi:10.1016/j.neuint.2015.07.009
Khadka, S., Omura, S., Sato, F., Nishio, K., Kakeya, H., and Tsunoda, I. (2021). Curcumin beta-D-glucuronide modulates an autoimmune model of multiple sclerosis with altered gut microbiota in the ileum and feces. Front. Cell Infect. Microbiol. 11, 772962. doi:10.3389/fcimb.2021.772962
Khosropour, S., Shahvarooghi, E., Rezaeizadeh, H., and Esmaeelzadeh, M. (2023). Curcumin and its semisynthetic derivative F-curcumin ameliorate the expression of cytokines in autoimmune encephalomyelitis mouse models of multiple sclerosis. Iran. J. Allergy Asthma Immunol. 22, 575–587. doi:10.18502/ijaai.v22i6.14646
Klotz, L., Antel, J., and Kuhlmann, T. (2023). Inflammation in multiple sclerosis: consequences for remyelination and disease progression. Nat. Rev. Neurol. 19, 305–320. doi:10.1038/s41582-023-00801-6
Kong, X., Hu, W., Cui, Y., Gao, J., Yao, X., Ren, J., et al. (2022). Transcranial direct-current stimulation regulates MCT1-PPA-PTEN-LONP1 signaling to confer neuroprotection after rat cerebral ischemia-reperfusion injury. Mol. Neurobiol. 59, 7423–7438. doi:10.1007/s12035-022-03051-7
Kong, X., Yao, X., Ren, J., Gao, J., Cui, Y., Sun, J., et al. (2023). tDCS regulates ASBT-3-OxoLCA-PLOD2-PTEN signaling pathway to confer neuroprotection following rat cerebral ischemia-reperfusion injury. Mol. Neurobiol. 60, 6715–6730. doi:10.1007/s12035-023-03504-7
Kong, X., Lyu, W., Lin, X., Feng, H., Xu, L., Li, C., et al. (2024a). Transcranial direct current stimulation enhances the protective effect of isoflurane preconditioning on cerebral ischemia/reperfusion injury: a new mechanism associated with the nuclear protein Akirin2. CNS Neurosci. Ther. 30, e70033. doi:10.1111/cns.70033
Kong, X., Lyu, W., Lin, X., Lin, C., Feng, H., Xu, L., et al. (2024b). Itaconate alleviates anesthesia/surgery-induced cognitive impairment by activating a Nrf2-dependent anti-neuroinflammation and neurogenesis via gut-brain axis. J. Neuroinflammation 21, 104. doi:10.1186/s12974-024-03103-w
Kong, X., Xu, L., Mou, Z., Lyu, W., Shan, K., Wang, L., et al. (2024c). The anti-inflammatory effects of itaconate and its derivatives in neurological disorders. Cytokine Growth Factor Rev. 78, 37–49. doi:10.1016/j.cytogfr.2024.07.001
Kou, H., Huang, L., Jin, M., He, Q., Zhang, R., and Ma, J. (2023). Effect of curcumin on rheumatoid arthritis: a systematic review and meta-analysis. Front. Immunol. 14, 1121655. doi:10.3389/fimmu.2023.1121655
Kouhi, Z. H., Seyedalipour, B., Hosseinkhani, S., and Chaichi, M. J. (2024). Bisdemethoxycurcumin, a novel potent polyphenolic compound, effectively inhibits the formation of amyloid aggregates in ALS-associated hSOD1 mutant (L38R). Int. J. Biol. Macromol. 282, 136701. doi:10.1016/j.ijbiomac.2024.136701
Krishnamurthy, K. B. (2025). Epilepsy. Ann. Intern Med. 178, ITC49–ITC64. doi:10.7326/ANNALS-25-00494
Kuhlmann, T., Moccia, M., Coetzee, T., Cohen, J. A., Correale, J., Graves, J., et al. (2023). Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 22, 78–88. doi:10.1016/S1474-4422(22)00289-7
Kumar, V., Prakash, C., Singh, R., and Sharma, D. (2019). Curcumin’s antiepileptic effect, and alterations in Na(v)1.1 and Na(v)1.6 expression in iron-induced epilepsy. Epilepsy Res. 150, 7–16. doi:10.1016/j.eplepsyres.2018.12.007
Kuzminska, J., Szyk, P., Mlynarczyk, D. T., Bakun, P., Muszalska-Kolos, I., Dettlaff, K., et al. (2024). Curcumin derivatives in medicinal chemistry: potential applications in cancer treatment. Molecules 29, 5321. doi:10.3390/molecules29225321
Lamichhane, G., Liu, J., Lee, S. J., Lee, D. Y., Zhang, G., and Kim, Y. (2024). Curcumin mitigates the high-fat high-sugar diet-induced impairment of spatial memory, hepatic metabolism, and the alteration of the gut microbiome in Alzheimer’s disease-induced (3xTg-AD) mice. Nutrients 16, 240. doi:10.3390/nu16020240
Leblhuber, F., Ehrlich, D., Steiner, K., Geisler, S., Fuchs, D., Lanser, L., et al. (2021). The immunopathogenesis of Alzheimer’s disease is related to the composition of gut microbiota. Nutrients 13, 361. doi:10.3390/nu13020361
Lee, M. K., Kim, H. D., Lee, S. H., and Lee, J. H. (2023). Curcumin ameliorates particulate matter-induced pulmonary injury through bimodal regulation of macrophage inflammation via NF-κB and Nrf2. Int. J. Mol. Sci. 24, 1858. doi:10.3390/ijms24031858
Lei, P., Li, Z., Hua, Q., Song, P., Gao, L., Zhou, L., et al. (2023). Ursolic acid alleviates neuroinflammation after intracerebral hemorrhage by mediating microglial pyroptosis via the NF-κB/NLRP3/GSDMD pathway. Int. J. Mol. Sci. 24, 14771. doi:10.3390/ijms241914771
Lei, W., Zhuang, H., Huang, W., and Sun, J. (2025). Neuroinflammation and energy metabolism: a dual perspective on ischemic stroke. J. Transl. Med. 23, 413. doi:10.1186/s12967-025-06440-3
Li, Y., Huang, J., Wang, J., Xia, S., Ran, H., Gao, L., et al. (2023). Human umbilical cord-derived mesenchymal stem cell transplantation supplemented with curcumin improves the outcomes of ischemic stroke via AKT/GSK-3β/β-TrCP/Nrf2 axis. J. Neuroinflammation 20, 49. doi:10.1186/s12974-023-02738-5
Lin, H. W., Chen, T. C., Yeh, J. H., Tsou, S. C., Wang, I., Shen, T. J., et al. (2022). Suppressive effect of tetrahydrocurcumin on Pseudomonas aeruginosa lipopolysaccharide-induced inflammation by suppressing JAK/STAT and Nrf2/HO-1 pathways in microglial cells. Oxid. Med. Cell Longev. 2022, 4978556. doi:10.1155/2022/4978556
Liu, C., Rokavec, M., Huang, Z., and Hermeking, H. (2023a). Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 30, 1771–1785. doi:10.1038/s41418-023-01178-1
Liu, X., Qi, M., Li, X., Wang, J., and Wang, M. (2023b). Curcumin: a natural organic component that plays a multi-faceted role in ovarian cancer. J. Ovarian Res. 16, 47. doi:10.1186/s13048-023-01120-6
Liu, E., Zhang, Y., and Wang, J. Z. (2024a). Updates in Alzheimer’s disease: from basic research to diagnosis and therapies. Transl. Neurodegener. 13, 45. doi:10.1186/s40035-024-00432-x
Liu, Q., Wang, C., Guo, X., Du, Q., and Keshavarzi, M. (2024b). Curcumin and its nano-formulations combined with exercise: from molecular mechanisms to clinic. Cell Biochem. Funct. 42, e4061. doi:10.1002/cbf.4061
Liu, Y., Wang, W., Di, B., and Miao, J. (2024c). Curcumol ameliorates neuroinflammation after cerebral ischemia-reperfusion injury via affecting microglial polarization and Treg/Th17 balance through Nrf2/HO-1 and NF-κB signaling. Cell Death Discov. 10, 300. doi:10.1038/s41420-024-02067-3
Lo Cascio, F., Marzullo, P., Kayed, R., and Palumbo Piccionello, A. (2021). Curcumin as scaffold for drug discovery against neurodegenerative diseases. Biomedicines 9, 173. doi:10.3390/biomedicines9020173
Lotankar, S., Prabhavalkar, K. S., and Bhatt, L. K. (2017). Biomarkers for Parkinson’s disease: recent advancement. Neurosci. Bull. 33, 585–597. doi:10.1007/s12264-017-0183-5
Lou, S., Gong, D., Yang, M., Qiu, Q., Luo, J., and Chen, T. (2024). Curcumin improves neurogenesis in Alzheimer’s disease mice via the upregulation of Wnt/β-catenin and BDNF. Int. J. Mol. Sci. 25, 5123. doi:10.3390/ijms25105123
Lu, L., Qi, S., Chen, Y., Luo, H., Huang, S., Yu, X., et al. (2020). Targeted immunomodulation of inflammatory monocytes across the blood-brain barrier by curcumin-loaded nanoparticles delays the progression of experimental autoimmune encephalomyelitis. Biomaterials 245, 119987. doi:10.1016/j.biomaterials.2020.119987
Mahjoob, M., and Stochaj, U. (2021). Curcumin nanoformulations to combat aging-related diseases. Ageing Res. Rev. 69, 101364. doi:10.1016/j.arr.2021.101364
Majumder, P., Hsu, T. I., Hu, C. J., Huang, J. K., Lee, Y. C., Hsieh, Y. C., et al. (2025). Potential role of solid lipid curcumin particle (SLCP) as estrogen replacement therapy in mitigating TDP-43-related neuropathy in the mouse model of ALS disease. Exp. Neurol. 383, 114999. doi:10.1016/j.expneurol.2024.114999
Mei, X., Zhu, L., Zhou, Q., Li, X., and Chen, Z. (2020). Interplay of curcumin and its liver metabolism on the level of Aβ in the brain of APPswe/PS1dE9 mice before AD onset. Pharmacol. Rep. 72, 1604–1613. doi:10.1007/s43440-020-00116-z
Menon, V. P., and Sudheer, A. R. (2007). Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 595, 105–125. doi:10.1007/978-0-387-46401-5_3
Ming, T., Tao, Q., Tang, S., Zhao, H., Yang, H., Liu, M., et al. (2022). Curcumin: an epigenetic regulator and its application in cancer. Biomed. Pharmacother. 156, 113956. doi:10.1016/j.biopha.2022.113956
Mohajeri, M., Sadeghizadeh, M., Najafi, F., and Javan, M. (2015). Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair. Neuropharmacology 99, 156–167. doi:10.1016/j.neuropharm.2015.07.013
Mohamadian, M., Parsamanesh, N., Chiti, H., Sathyapalan, T., and Sahebkar, A. (2022). Protective effects of curcumin on ischemia/reperfusion injury. Phytother. Res. 36, 4299–4324. doi:10.1002/ptr.7620
Mohammadzadeh, R., Fathi, M., Pourseif, M. M., Omidi, Y., Farhang, S., Barzegar Jalali, M., et al. (2024). Curcumin and nano-curcumin applications in psychiatric disorders. Phytother. Res. 38, 4240–4260. doi:10.1002/ptr.8265
Morris, H. R., Spillantini, M. G., Sue, C. M., and Williams-Gray, C. H. (2024). The pathogenesis of Parkinson’s disease. Lancet 403, 293–304. doi:10.1016/S0140-6736(23)01478-2
Nainu, F., Mamada, S. S., Harapan, H., and Emran, T. B. (2023). Inflammation-mediated responses in the development of neurodegenerative diseases. Adv. Exp. Med. Biol. 1411, 39–70. doi:10.1007/978-981-19-7376-5_3
Nosratabadi, R., Ranjkesh, M., Safari, M., Ramezani, M., Zainodini, N., and Mahmoodi, M. (2024). In vitro effects of curcumin in free and phytosomal forms on the expression of T Helper1 and regulatory T cells’ transcription factors in collagen-induced arthritis. Adv. Biomed. Res. 13, 69. doi:10.4103/abr.abr_291_23
Nunes, Y. C., Mendes, N. M., Pereira De Lima, E., Chehadi, A. C., Lamas, C. B., Haber, J. F. S., et al. (2024). Curcumin: a golden approach to healthy aging: a systematic review of the evidence. Nutrients 16, 2721. doi:10.3390/nu16162721
Ossenkoppele, R., Van Der Kant, R., and Hansson, O. (2022). Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 21, 726–734. doi:10.1016/S1474-4422(22)00168-5
Pardo-Moreno, T., Gonzalez-Acedo, A., Rivas-Dominguez, A., Garcia-Morales, V., Garcia-Cozar, F. J., Ramos-Rodriguez, J. J., et al. (2022). Therapeutic approach to Alzheimer’s disease: current treatments and new perspectives. Pharmaceutics 14, 1117. doi:10.3390/pharmaceutics14061117
Pellinen, J., Foster, E. C., Wilmshurst, J. M., Zuberi, S. M., and French, J. (2024). Improving epilepsy diagnosis across the lifespan: approaches and innovations. Lancet Neurol. 23, 511–521. doi:10.1016/S1474-4422(24)00079-6
Peng, Y., Ao, M., Dong, B., Jiang, Y., Yu, L., Chen, Z., et al. (2021). Anti-inflammatory effects of curcumin in the inflammatory diseases: status, limitations and countermeasures. Drug Des. Devel Ther. 15, 4503–4525. doi:10.2147/DDDT.S327378
Perez, C. A., Cuascut, F. X., and Hutton, G. J. (2023). Immunopathogenesis, diagnosis, and treatment of multiple sclerosis: a clinical update. Neurol. Clin. 41, 87–106. doi:10.1016/j.ncl.2022.05.004
Pluta, R. (2025). Neuroinflammation in the post-ischemic brain in the presence of amyloid and tau protein. Discov. Med. 37, 1–18. doi:10.24976/Discov.Med.202537192.1
Pluta, R., Furmaga-Jablonska, W., Januszewski, S., and Czuczwar, S. J. (2022). Post-ischemic brain neurodegeneration in the form of Alzheimer’s disease proteinopathy: possible therapeutic role of curcumin. Nutrients 14, 248. doi:10.3390/nu14020248
Polissidis, A., Petropoulou-Vathi, L., Nakos-Bimpos, M., and Rideout, H. J. (2020). The future of targeted gene-based treatment strategies and biomarkers in Parkinson’s disease. Biomolecules 10, 912. doi:10.3390/biom10060912
Pourbagher-Shahri, A. M., Farkhondeh, T., Ashrafizadeh, M., Talebi, M., and Samargahndian, S. (2021). Curcumin and cardiovascular diseases: focus on cellular targets and cascades. Biomed. Pharmacother. 136, 111214. doi:10.1016/j.biopha.2020.111214
Qi, Y., Dong, Y., Chen, J., Xie, S., Ma, X., Yu, X., et al. (2025). Lactiplantibacillus plantarum SG5 inhibits neuroinflammation in MPTP-induced PD mice through GLP-1/PGC-1α pathway. Exp. Neurol. 383, 115001. doi:10.1016/j.expneurol.2024.115001
Qiu, B., Xu, X., Yi, P., and Hao, Y. (2020). Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell Mol. Med. 24, 10855–10865. doi:10.1111/jcmm.15714
Quispe, C., Cruz-Martins, N., Manca, M. L., Manconi, M., Sytar, O., Hudz, N., et al. (2021). Nano-derived therapeutic formulations with curcumin in inflammation-related diseases. Oxid. Med. Cell Longev. 2021, 3149223. doi:10.1155/2021/3149223
Qureshi, M., Al-Suhaimi, E. A., Wahid, F., Shehzad, O., and Shehzad, A. (2018). Therapeutic potential of curcumin for multiple sclerosis. Neurol. Sci. 39, 207–214. doi:10.1007/s10072-017-3149-5
Ramires Junior, O. V., Alves, B. D. S., Barros, P. a.B., Rodrigues, J. L., Ferreira, S. P., Monteiro, L. K. S., et al. (2021). Nanoemulsion improves the neuroprotective effects of curcumin in an experimental model of Parkinson’s disease. Neurotox. Res. 39, 787–799. doi:10.1007/s12640-021-00362-w
Ran, Y., Su, W., Gao, F., Ding, Z., Yang, S., Ye, L., et al. (2021). Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF-κB suppression and NLRP3 inflammasome inhibition. Oxid. Med. Cell Longev. 2021, 1552127. doi:10.1155/2021/1552127
Ratan, C., Arian, A. M., Rajendran, R., Jayakumar, R., Masson, M., and Mangalathillam, S. (2023). Nano-based formulations of curcumin: elucidating the potential benefits and future prospects in skin cancer. Biomed. Mater 18, 052008. doi:10.1088/1748-605X/acf0af
Rathore, A. S., Singh, S. S., Birla, H., Zahra, W., Keshri, P. K., Dilnashin, H., et al. (2023). Curcumin modulates p62-Keap1-Nrf2-mediated autophagy in rotenone-induced Parkinson’s disease mouse models. ACS Chem. Neurosci., acschemneuro.2c00706. doi:10.1021/acschemneuro.2c00706
Ren, B. C., Zhang, Y. F., Liu, S. S., Cheng, X. J., Yang, X., Cui, X. G., et al. (2020). Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell Mol. Med. 24, 12355–12367. doi:10.1111/jcmm.15725
Sadeghi, M., Dehnavi, S., Asadirad, A., Xu, S., Majeed, M., Jamialahmadi, T., et al. (2023). Curcumin and chemokines: mechanism of action and therapeutic potential in inflammatory diseases. Inflammopharmacology 31, 1069–1093. doi:10.1007/s10787-023-01136-w
Sadek, M. A., Rabie, M. A., El Sayed, N. S., Sayed, H. M., and Kandil, E. A. (2024). Neuroprotective effect of curcumin against experimental autoimmune encephalomyelitis-induced cognitive and physical impairments in mice: an insight into the role of the AMPK/SIRT1 pathway. Inflammopharmacology 32, 1499–1518. doi:10.1007/s10787-023-01399-3
Sawesi, S., Malkaram, S. A., Abd Elmageed, Z. Y., and Fandy, T. E. (2022). Modulation of the activity of histone lysine methyltransferases and demethylases by curcumin analog in leukaemia cells. J. Cell Mol. Med. 26, 5624–5633. doi:10.1111/jcmm.17589
Scheltens, P., De Strooper, B., Kivipelto, M., Holstege, H., Chetelat, G., Teunissen, C. E., et al. (2021). Alzheimer’s disease. Lancet 397, 1577–1590. doi:10.1016/S0140-6736(20)32205-4
Se Thoe, E., Fauzi, A., Tang, Y. Q., Chamyuang, S., and Chia, A. Y. Y. (2021). A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 276, 119129. doi:10.1016/j.lfs.2021.119129
Serrano-Pozo, A., Das, S., and Hyman, B. T. (2021). APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20, 68–80. doi:10.1016/S1474-4422(20)30412-9
Shamsnia, H. S., Roustaei, M., Ahmadvand, D., Butler, A. E., Amirlou, D., Soltani, S., et al. (2023). Impact of curcumin on p38 MAPK: therapeutic implications. Inflammopharmacology 31, 2201–2212. doi:10.1007/s10787-023-01265-2
Sharma, S., Tomar, V. R., and Deep, S. (2023). Mechanism of the interaction of toxic SOD1 fibrils with two potent polyphenols: curcumin and quercetin. Phys. Chem. Chem. Phys. 25, 23081–23091. doi:10.1039/d3cp02120c
Shi, W. P., Lin, W. J., Ge, W. Y., Chen, L. L., Zhang, T. D., Guo, W. H., et al. (2023). Curcumin inhibits liquid-liquid phase separation of fused in sarcoma and attenuates the sequestration of pyruvate kinase to restore cellular metabolism. Food Funct. 14, 4621–4631. doi:10.1039/d2fo03224d
Slowing, K., Gomez, F., Delgado, M., Fernandez De La Rosa, R., Hernandez-Martin, N., Pozo, M. A., et al. (2023). PET imaging and neurohistochemistry reveal that curcumin attenuates brain hypometabolism and hippocampal damage induced by status epilepticus in rats. Planta Med. 89, 364–376. doi:10.1055/a-1948-4378
Specchio, N., Wirrell, E. C., Scheffer, I. E., Nabbout, R., Riney, K., Samia, P., et al. (2022). International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ILAE task force on nosology and definitions. Epilepsia 63, 1398–1442. doi:10.1111/epi.17241
Sun, Z. Z., Li, X. Y., Wang, S., Shen, L., and Ji, H. F. (2020). Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 104, 3507–3515. doi:10.1007/s00253-020-10461-x
Sun, M., Liu, N., Sun, J., Li, X., Wang, H., Zhang, W., et al. (2022). Curcumin regulates anti-inflammatory responses by AXL/JAK2/STAT3 signaling pathway in experimental autoimmune encephalomyelitis. Neurosci. Lett. 787, 136821. doi:10.1016/j.neulet.2022.136821
Sun, X., Huang, X., Liang, Q., Wang, N., Zheng, X., Zhang, Q., et al. (2024). Curcumin-loaded keratin-chitosan hydrogels for enhanced peripheral nerve regeneration. Int. J. Biol. Macromol. 272, 132448. doi:10.1016/j.ijbiomac.2024.132448
Takata, F., Nakagawa, S., Matsumoto, J., and Dohgu, S. (2021). Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell Neurosci. 15, 661838. doi:10.3389/fncel.2021.661838
Teleanu, D. M., Niculescu, A. G., Lungu, Ii, Radu, C. I., Vladacenco, O., Roza, E., et al. (2022). Neurotransmitters-key factors in neurological and neurodegenerative disorders of the central nervous system. Int. J. Mol. Sci. 23, 5954. doi:10.3390/ijms23115954
Thota, R. N., Rosato, J. I., Dias, C. B., Burrows, T. L., Martins, R. N., and Garg, M. L. (2020). Dietary supplementation with curcumin reduce circulating levels of glycogen synthase kinase-3β and islet amyloid polypeptide in adults with high risk of type 2 diabetes and Alzheimer’s disease. Nutrients 12, 1032. doi:10.3390/nu12041032
Travers, B. S., Tsang, B. K., and Barton, J. L. (2022). Multiple sclerosis: diagnosis, disease-modifying therapy and prognosis. Aust. J. Gen. Pract. 51, 199–206. doi:10.31128/AJGP-07-21-6103
Tripathi, S.Bhawana (2024). Epigenetic orchestration of neurodegenerative disorders: a possible target for curcumin as a therapeutic. Neurochem. Res. 49, 2319–2335. doi:10.1007/s11064-024-04167-z
Tysnes, O. B., and Storstein, A. (2017). Epidemiology of Parkinson’s disease. J. Neural Transm. (Vienna) 124, 901–905. doi:10.1007/s00702-017-1686-y
Vaiss, D. P., Rodrigues, J. L., Yurgel, V. C., Do Carmo Guedes, F., Da Matta, L. L. M., Barros, P. a.B., et al. (2024). Curcumin and quercetin co-encapsulated in nanoemulsions for nasal administration: a promising therapeutic and prophylactic treatment for viral respiratory infections. Eur. J. Pharm. Sci. 197, 106766. doi:10.1016/j.ejps.2024.106766
Varadharajan, A., Davis, A. D., Ghosh, A., Jagtap, T., Xavier, A., Menon, A. J., et al. (2023). Guidelines for pharmacotherapy in Alzheimer’s disease - a primer on FDA-approved drugs. J. Neurosci. Rural. Pract. 14, 566–573. doi:10.25259/JNRP_356_2023
Vaz, M., and Silvestre, S. (2020). Alzheimer’s disease: recent treatment strategies. Eur. J. Pharmacol. 887, 173554. doi:10.1016/j.ejphar.2020.173554
Wang, J., Liu, Y., Li, X. H., Zeng, X. C., Li, J., Zhou, J., et al. (2017). Curcumin protects neuronal cells against status-epilepticus-induced hippocampal damage through induction of autophagy and inhibition of necroptosis. Can. J. Physiol. Pharmacol. 95, 501–509. doi:10.1139/cjpp-2016-0154
Wang, Q., Ye, C., Sun, S., Li, R., Shi, X., Wang, S., et al. (2019). Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int. Immunopharmacol. 72, 292–300. doi:10.1016/j.intimp.2019.04.027
Wang, Y., Zhang, P., Zhang, J., and Hong, T. (2022). Bisdemethoxycurcumin attenuates OVA-induced food allergy by inhibiting the MAPK and NF-κB signaling pathways. Exp. Ther. Med. 23, 401. doi:10.3892/etm.2022.11328
Wang, W., Li, M., Wang, L., Chen, L., and Goh, B. C. (2023). Curcumin in cancer therapy: exploring molecular mechanisms and overcoming clinical challenges. Cancer Lett. 570, 216332. doi:10.1016/j.canlet.2023.216332
Wegener, S., Baron, J. C., Derdeyn, C. P., Fierstra, J., Fromm, A., Klijn, C. J. M., et al. (2024). Hemodynamic stroke: emerging concepts, risk estimation, and treatment. Stroke 55, 1940–1950. doi:10.1161/STROKEAHA.123.044386
Weintraub, D., Aarsland, D., Chaudhuri, K. R., Dobkin, R. D., Leentjens, A. F., Rodriguez-Violante, M., et al. (2022). The neuropsychiatry of Parkinson’s disease: advances and challenges. Lancet Neurol. 21, 89–102. doi:10.1016/S1474-4422(21)00330-6
Woo, M. S., Mayer, C., Binkle-Ladisch, L., Sonner, J. K., Rosenkranz, S. C., Shaposhnykov, A., et al. (2024). STING orchestrates the neuronal inflammatory stress response in multiple sclerosis. Cell 187, 4043–4060.e30. doi:10.1016/j.cell.2024.05.031
Wu, S., Guo, T., Qi, W., Li, Y., Gu, J., Liu, C., et al. (2021). Curcumin ameliorates ischemic stroke injury in rats by protecting the integrity of the blood-brain barrier. Exp. Ther. Med. 22, 783. doi:10.3892/etm.2021.10215
Xiao, J., Cai, X., Zhou, W., Wang, R., and Ye, Z. (2022). Curcumin relieved the rheumatoid arthritis progression via modulating the linc00052/miR-126-5p/PIAS2 axis. Bioengineered 13, 10973–10983. doi:10.1080/21655979.2022.2066760
Xiao, B., Zhou, Z., Chao, Y., and Tan, E. K. (2025). Pathogenesis of Parkinson’s disease. Neurol. Clin. 43, 185–207. doi:10.1016/j.ncl.2024.12.003
Xu, L., Hao, L. P., Yu, J., Cheng, S. Y., Li, F., Ding, S. M., et al. (2023). Curcumin protects against rotenone-induced Parkinson’s disease in mice by inhibiting microglial NLRP3 inflammasome activation and alleviating mitochondrial dysfunction. Heliyon 9, e16195. doi:10.1016/j.heliyon.2023.e16195
Xu, W., Huang, Y., and Zhou, R. (2025a). NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol. Immunol. 22, 341–355. doi:10.1038/s41423-025-01275-w
Xu, Y., Liu, Y., Wu, Y., Sun, J., Lu, X., Dai, K., et al. (2025b). Curcumin alleviates microglia-mediated neuroinflammation and neuronal ferroptosis following experimental subarachnoid hemorrhage by modulating the Nrf2/HO-1 signaling pathway. Mol. Neurobiol. 62, 2995–3010. doi:10.1007/s12035-024-04443-7
Yan, S., Zhou, M., Zheng, X., Xing, Y., Dong, J., Yan, M., et al. (2021). Anti-inflammatory effect of curcumin on the mouse model of myocardial infarction through regulating macrophage polarization. Mediat. Inflamm. 2021, 9976912. doi:10.1155/2021/9976912
Yang, B., Luo, G., Zhang, C., Feng, L., Luo, X., and Gan, L. (2020). Curcumin protects rat hippocampal neurons against pseudorabies virus by regulating the BDNF/TrkB pathway. Sci. Rep. 10, 22204. doi:10.1038/s41598-020-78903-0
Yang, C., Han, M., Li, R., Zhou, L., Zhang, Y., Duan, L., et al. (2021a). Curcumin nanoparticles inhibiting ferroptosis for the enhanced treatment of intracerebral hemorrhage. Int. J. Nanomedicine 16, 8049–8065. doi:10.2147/IJN.S334965
Yang, L., Chen, X., Bi, Z., Liao, J., Zhao, W., and Huang, W. (2021b). Curcumin attenuates renal ischemia reperfusion injury via JNK pathway with the involvement of p300/CBP-mediated histone acetylation. Korean J. Physiol. Pharmacol. 25, 413–423. doi:10.4196/kjpp.2021.25.5.413
Yang, C., Zhu, Q., Chen, Y., Ji, K., Li, S., Wu, Q., et al. (2024a). Review of the protective mechanism of curcumin on cardiovascular disease. Drug Des. Devel Ther. 18, 165–192. doi:10.2147/DDDT.S445555
Yang, Q., Li, R., Hong, Y., Liu, H., Jian, C., and Zhao, S. (2024b). Curcumin-loaded gelatin nanoparticles cross the blood-brain barrier to treat ischemic stroke by attenuating oxidative stress and neuroinflammation. Int. J. Nanomedicine 19, 11633–11649. doi:10.2147/IJN.S487628
Yang, Y., Wang, Y., Jiang, X., Mi, J., Ge, D., Tong, Y., et al. (2024c). Modified Ce/Zr-MOF nanoparticles loaded with curcumin for Alzheimer’s disease via multifunctional modulation. Int. J. Nanomedicine 19, 9943–9959. doi:10.2147/IJN.S479242
Youwakim, J., and Girouard, H. (2021). Inflammation: a mediator between hypertension and neurodegenerative diseases. Am. J. Hypertens. 34, 1014–1030. doi:10.1093/ajh/hpab094
Yu, H., Ren, K., Jin, Y., Zhang, L., Liu, H., Huang, Z., et al. (2025). Mitochondrial DAMPs: key mediators in neuroinflammation and neurodegenerative disease pathogenesis. Neuropharmacology 264, 110217. doi:10.1016/j.neuropharm.2024.110217
Zhang, L., Fang, Y., Xu, Y., Lian, Y., Xie, N., Wu, T., et al. (2015). Curcumin improves amyloid beta-peptide (1-42) induced spatial memory deficits through BDNF-ERK signaling pathway. PLoS One 10, e0131525. doi:10.1371/journal.pone.0131525
Zhang, J., Zheng, Y., Luo, Y., Du, Y., Zhang, X., and Fu, J. (2019). Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol. Immunol. 116, 29–37. doi:10.1016/j.molimm.2019.09.020
Zhang, Y., Hou, B., Liang, P., Lu, X., Wu, Y., Zhang, X., et al. (2021). TRPV1 channel mediates NLRP3 inflammasome-dependent neuroinflammation in microglia. Cell Death Dis. 12, 1159. doi:10.1038/s41419-021-04450-9
Zhang, D., Jing, B., Chen, Z. N., Li, X., Shi, H. M., Zheng, Y. C., et al. (2023a). Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF-κB pathway. CNS Neurosci. Ther. 29, 1000–1011. doi:10.1111/cns.14060
Zhang, M., Hao, Z., Wu, J., Teng, Z., Qiu, W., and Cheng, J. (2023b). Curcumin ameliorates traumatic brain injury via C1ql3-mediated microglia M2 polarization. Tissue Cell 84, 102164. doi:10.1016/j.tice.2023.102164
Zhang, W., Xiao, D., Mao, Q., and Xia, H. (2023c). Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 8, 267. doi:10.1038/s41392-023-01486-5
Zhang, W., Sun, M., Liu, N., Li, X., Sun, J., and Wang, M. (2025). Curcumin ameliorates astrocyte inflammation through AXL in cuprizone-induced mice. Toxicol. Appl. Pharmacol. 494, 117170. doi:10.1016/j.taap.2024.117170
Zhao, J., Chen, Y., Chen, Q., Hong, T., Zhong, Z., He, J., et al. (2021). Curcumin ameliorates cardiac fibrosis by regulating macrophage-fibroblast crosstalk via IL18-P-SMAD2/3 signaling pathway inhibition. Front. Pharmacol. 12, 784041. doi:10.3389/fphar.2021.784041
Zheng, Q., Liu, H., Zhang, H., Han, Y., Yuan, J., Wang, T., et al. (2023). Ameliorating mitochondrial dysfunction of neurons by biomimetic targeting nanoparticles mediated mitochondrial biogenesis to boost the therapy of Parkinson’s disease. Adv. Sci. (Weinh) 10, e2300758. doi:10.1002/advs.202300758
Zhou, X., Zhao, R., Lv, M., Xu, X., Liu, W., Li, X., et al. (2023). ACSL4 promotes microglia-mediated neuroinflammation by regulating lipid metabolism and VGLL4 expression. Brain Behav. Immun. 109, 331–343. doi:10.1016/j.bbi.2023.02.012
Zhu, H., Zhang, H., Hou, B., Xu, B., Ji, L., and Wu, Y. (2022). Curcumin regulates gut microbiota and exerts a neuroprotective effect in the MPTP model of Parkinson’s disease. Evid. Based Complement. Altern. Med. 2022, 9110560. doi:10.1155/2022/9110560
Zia, A., Farkhondeh, T., Pourbagher-Shahri, A. M., and Samarghandian, S. (2021). The role of curcumin in aging and senescence: molecular mechanisms. Biomed. Pharmacother. 134, 111119. doi:10.1016/j.biopha.2020.111119
Glossary
NF-κB Nuclear factor kappa-B
NLRP3 NOD-like receptor thermal protein domain associated protein 3
Nrf2 Nuclear Factor Erythroid 2-related Factor 2
TGF-β Transforming growth factor β
IL-10 Interleukin 10
CNS Central nervous system
TNF-α Tumor necrosis factor-α
IL-1β Interleukin-1 β
IL-6 Interleukin- 6
ROS Reactive oxygen species
AD Alzheimer’s disease
PD Parkinson’s disease
MS Multiple sclerosis
TLRs Toll-like receptors
BBB Blood-brain barrier
NSAIDs Non-steroidal anti-inflammatory drugs
MAPK Mitogen-Activated Protein Kinase
PI3K/Akt Phosphatidylinositol 3-Kinase/Akt
HAT Hydrogen atom transfer
SET Single electron transfer
STAT3 Signal transducer and activator of transcription 3
LPS Lipopolysaccharide
NO Nitric oxide
HO-1 Heme Oxygenase-1
TBI Traumatic brain injury
SAH Subarachnoid hemorrhage
TREM2 Triggering receptor expressed on myeloid cells 2
EAE Experimental autoimmune encephalomyelitis
Aβ Amyloid-β
AChEIs Acetylcholinesterase inhibitors
DG Dentate gyrus
T2D Type 2 diabetes
GBE Ginkgo biloba extract
P-tau Phosphorylated tau
MDA Malondialdehyde
GSH Glutathione
CNPs Curcumin nanoparticles
SNCA α-synuclein
LRRK2 Leucine-rich repeat kinase 2
MHC Major histocompatibility complex
CPZ Cuprizone
IFN-γ Interferon-γ
HPPS High-density lipoprotein-mimicking peptide-phospholipid scaffold
PNC Polymeric forms of nano-curcumin
hUC-MSC Human umbilical cord-derived mesenchymal stem cells
ALS Amyotrophic lateral sclerosis
SLCP Solid lipid curcumin particles
FUS Fused in sarcoma
SOD1 Superoxide dismutase 1
PTZ Pentylenetetrazol
PET Positron emission tomography
NDs Neurodegenerative diseases
HD Huntington’s disease
DAMPs Damage-associated molecular patterns
NPs Nanoparticles
SLNs Solid lipid nanoparticles
Keywords: curcumin, neuroinflammation, neurological disorders, reactive oxygen species, neuroprotection
Citation: Zhou B and Hu B (2025) Anti-inflammatory effect of curcumin on neurological disorders: a narrative review. Front. Pharmacol. 16:1658115. doi: 10.3389/fphar.2025.1658115
Received: 02 July 2025; Accepted: 23 September 2025;
Published: 08 October 2025.
Edited by:
Ashish Mehta, Garvan Institute of Medical Research, AustraliaReviewed by:
Swaran J.S. Flora, National Institute of Pharmaceutical Education and Research, IndiaDanial Khayatan, Columbia University Irving Medical Center, United States
Copyright © 2025 Zhou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Binbin Hu, aHViaW5iaW4wOTI0QDE2My5jb20=
 Baoyin Zhou1
Baoyin Zhou1 Binbin Hu
Binbin Hu