- 1Department of Clinical Pharmacy, Key Laboratory of Chemical Biology (Ministry of Education), School of Pharmaceutical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Avanc Pharmaceutical Co., Ltd., Jinzhou, China
- 3Division of Clinical Pharmacology, Children’s National Hospital, Washington, DC, United States
- 4Departments of Pediatrics, Pharmacology & Physiology, Genomics & Precision Medicine, School of Medicine and Health Sciences, George Washington University, Washington, DC, United States
- 5Department of Paediatric Pharmacology and Pharmacometrics, University Children’s Hospital Basel, University of Basel, Basel, Switzerland
- 6Department of Anesthesiology, Laboratory of Anesthesia and Critical Care Medicine, National-Local Joint Engineering Research Centre of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China
Aim: To evaluate the pharmacokinetics, pharmacodynamics, and safety of the novel systemic intravenous anesthetic ET-26—an etomidate derivative designed to reduce adrenal suppression—in healthy elderly and non-elderly subjects.
Methods: In this Phase I, single-center, non-randomized, open-label trial, 16 volunteers were enrolled: eight elderly (≥65 years, including ≥75 years) and eight non-elderly (18–64 years), matched for gender and body weight. Each received a standardized IV infusion of ET-26. Plasma concentrations were measured for plasma protein binding, Cmax, and AUC; time to loss of consciousness (LOC) and safety were assessed.
Results: In 16 subjects (8 elderly/8 non-elderly), ET-26 showed higher exposure in the elderly (Cmax GMR 198.81%, 90% CI 126.51–312.45) and
Conclusion: Despite elevated systemic exposure in elderly subjects, ET-26 demonstrates comparable efficacy and retains a favorable tolerability profile across age groups, eliminating the need for dose adjustments in elderly populations.
Clinical Trial Registration: http://www.chinadrugtrials.org.cn/clinicaltrials.searchlist.dhtml?keywords=CTR20233784, identifier CTR20233784.
Background
Methoxyethyl etomidate hydrochloride (ET-26) is a novel intravenous general anesthetic developed analog of etomidate. By incorporating a methoxyethyl substituent, ET-26 is rapidly hydrolyzed by nonspecific esterases, resulting in a markedly shorter context-sensitive half-time (Wang et al., 2017b; Jiang et al., 2024). This structural modification enables ET-26 to retain the rapid-onset hypnotic action and hemodynamic stability of etomidate while significantly reducing its inhibition of adrenal 11β-hydroxylase, especially in elderly patients who often exhibit decreased hepatic and renal clearance (Wang et al., 2017a; Wang et al., 2017b). In preclinical evaluations, ET-26 demonstrated favorable pharmacokinetic (PK) characteristics and safety in aged rats, with its rapid metabolism profile suggesting potential clinical applicability in elderly populations (Chang et al., 2022). Phase I clinical studies employing both single ascending dose and multiple dosing regimens have demonstrated that ET-26 is safe and well tolerated in healthy subjects at doses up to 2.8 mg·kg−1. Moreover, compared with etomidate at 0.3 mg·kg−1, ET-26 exhibits superior preservation of adrenal cortical function (Yin et al., 2025). A Phase III clinical trial (NCT06203431, registered 11 October 2023.) is currently underway to evaluate the efficacy and safety of ET-26 for the induction of general anesthesia in elective surgical patients aged 18–70 years, with recruitment ongoing.
Currently, research on ET-26 is primarily focused on healthy adult subjects and animal models, while the pharmacokinetic characteristics in elderly patients remain unclear (Chang et al., 2022). With the increasing prominence of global aging issues and the inevitable physiological changes that accompany aging, including decreased organ function, altered body composition, and changes in receptor sensitivity, the processes of drug absorption, distribution, metabolism, and elimination can be significantly affected (Hammerlein et al., 1998; Shafer, 2000; Mangoni and Jackson, 2004; Curtis et al., 2005; Yizhak et al., 2013). For instance, reduced hepatic blood flow and decreased glomerular filtration rate in the elderly may lead to diminished drug clearance, a prolonged elimination half-life, and an increased risk of adverse drug reactions (Strom et al., 2016; Andres et al., 2019). Therefore, it is essential to establish the pharmacokinetic profile, efficacy, and safety of ET-26 in healthy elderly individuals and to provide precise dosing recommendations based on the specific pharmacological characteristics of this population.
Methods
Study design and population
This single-center, non-randomized, open-label Phase I clinical trial was designed to evaluate the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of ET-26 in healthy subjects, comprising both non-elderly and elderly populations. A total of 16 healthy subjects were enrolled, including 8 non-elderly subjects aged 18–64 years and 8 elderly subjects aged ≥65 years, with at least 3 subjects being ≥75 years old. To ensure group comparability, the non-elderly group was matched to the elderly group in terms of gender, and the mean body weight of non-elderly subjects was maintained within ±10 kg of the elderly group’s mean. All participants provided informed consent before participation in the trial. This study had appropriate inclusion/exclusion criteria and was approved by the Ethics Committee of the Shandong Provincial Qianfoshan Hospital before implementation (Supplementary Material S1).
Pharmacokinetic assessment
Plasma concentrations of ET-26 at various time points were measured using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. Pharmacokinetic samples were collected according to the planned schedule (Supplementary Table S1), and the bioanalytical methods are provided in the Supplementary Material S1.
Data from both the elderly and non-elderly groups were analyzed to generate individual as well as mean (Mean ± SD) plasma concentration-time (c-t) curves and semi-logarithmic c-t curves. The key PK parameters of ET-26 include Peak concentration (Cmax), Time to peak concentration (Tmax), Area under the concentration-time curve (AUC0-t,
After the log transformation of the primary PK parameters (Cmax, AUC0-t, and
Pharmacodynamic assessment
Following the Technical Guidelines for Clinical Evaluation of Intravenous General Anesthetics (NMPA, 2022), the pharmacodynamic evaluation in this study focused on two primary endpoints: the time to loss of consciousness and the area under the curve (AUC) of the Bispectral Index (BIS). Loss of consciousness was assessed by continuously monitoring the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) scale, with the endpoint defined as the interval from administration to the first instance when the MOAA/S score reached ≤1. Simultaneously, BIS values were recorded via electroencephalography to determine the minimum BIS level. The resulting BIS curves were subsequently fitted, and the AUC was calculated using WinNonlin or SAS 9.4 software. Descriptive statistics, including mean, median, standard deviation, maximum, and minimum values, were then used to comprehensively characterize the anesthetic effect.
Safety analysis
Descriptive statistical methods were employed to evaluate laboratory test results, vital signs, 12-lead electrocardiograms (ECG), physical examination findings, and additional safety parameters. Adverse events (AEs) were coded per the International Council for Harmonisation (ICH) Medical Dictionary for Regulatory Activities (MedDRA, version 25.1) and their severity was graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE, version 5.0) (see Supplementary Table S2). Treatment-emergent adverse events (TEAEs), serious adverse events (SAEs), and TEAEs leading to study discontinuation were systematically assessed. These events were further categorized based on participant subgroup (elderly vs. non-elderly), severity, and their causality relative to the investigational drug.
Results
Demographic characteristics
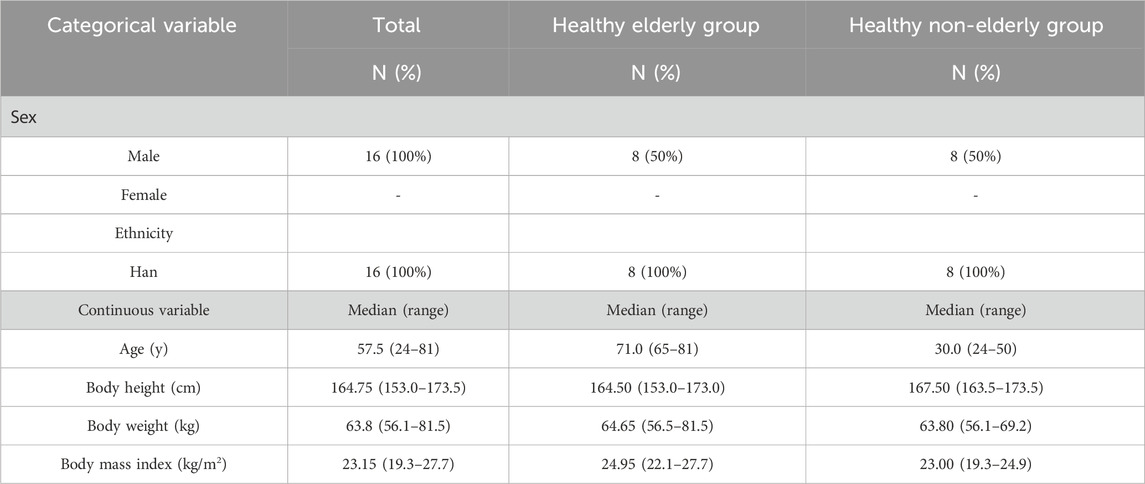
A total of 16 subjects were enrolled in the study, with 8 allocated to the healthy elderly group and 8 to the healthy non-elderly group. The median age was 57.5 years (range: 24–81 years). All subjects were male and of Han ethnicity. The median height was 164.75 cm (range: 153.0–173.5 cm), the median weight was 63.8 kg (range: 56.1–81.5 kg), and the median body mass index (BMI) was 23.15 kg/m2 (range: 19.3–27.7 kg/m2) (Table 1).
Plasma protein binding rate/free fraction
After intravenous administration of 0.8 mg/kg ET-26, plasma protein binding of ET-26 and the free fraction showed no significant time-dependent changes in healthy elderly subjects (n = 8) and healthy non-elderly subjects (n = 8). In the elderly group, plasma protein binding was 56.3% (free fraction 0.437) at 1 min post - dose, showing a slight increase to 60.3% (free fraction 0.397) after 4 h. In the non-elderly group, the binding rate was 57.3% (free fraction 0.427) at 1 min and 61.0% (free fraction 0.390) at 4 h. The binding rate fluctuated slightly within 4 h in both groups (4.0% in the elderly, 3.7% in the non-elderly), with no regular upward or downward trend. The inter-group comparison showed that the plasma protein binding difference was within 1.0% (1.0% at 1 min, 0.7% at 4 h), and the free fraction difference was less than 0.04 units at corresponding time points.
Pharmacokinetic analyses
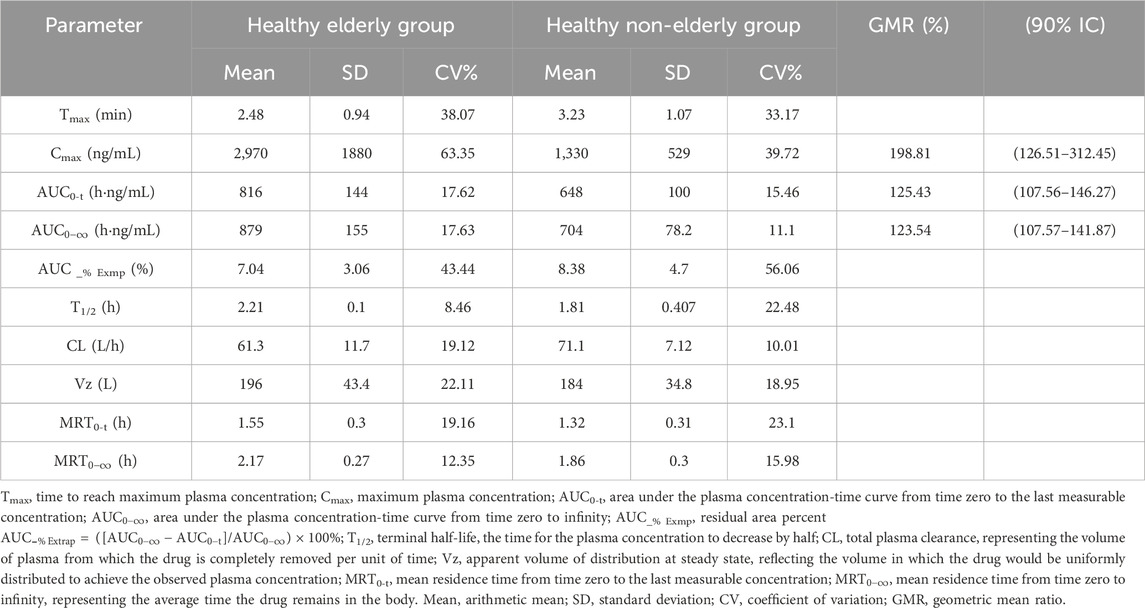
Eight subjects in the healthy elderly group received a single intravenous injection of ET-26 at a dose of 0.8 mg/kg. The median Tmax was 1.94 min. Geometric mean PK parameters were as follows: Cmax of 2,500 ng/mL, AUC0-t of 804 h·ng/mL,
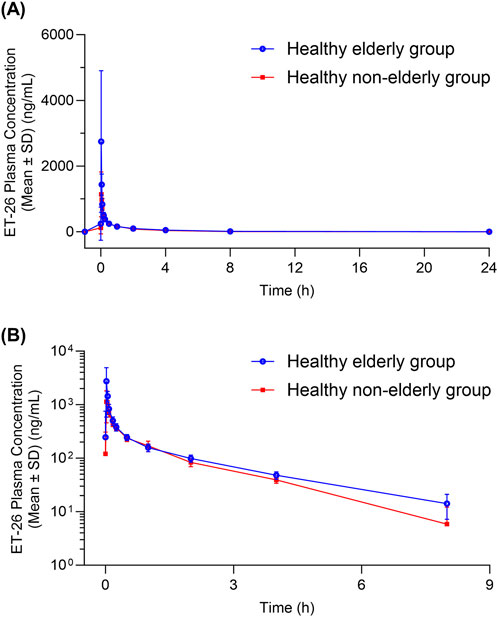
Figure 1. Plasma Concentration-Time curves of ET-26 in Healthy Elderly and Non-Elderly Subjects (A) Linear plot and (B) semi-logarithmic plot. The blue line with blue circles represents healthy elderly subjects, while the red line with red squares represents healthy non-elderly subjects.
Pharmacodynamic analysis
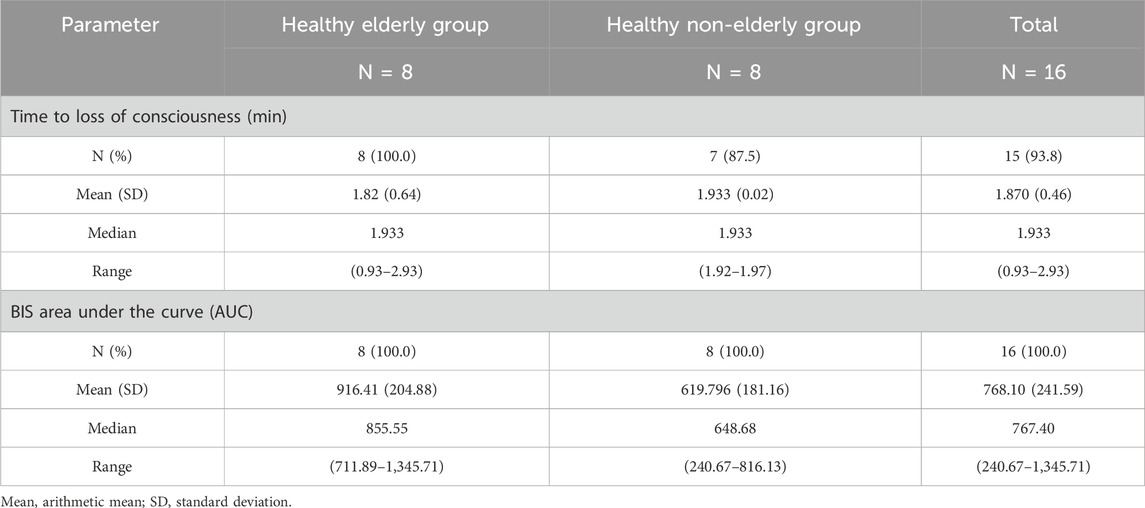
In the healthy elderly group, the median time to loss of consciousness post-dose was 1.933 min (range: 0.93–2.93 min). The area under the BIS curve was 855.55 (range: 711.89–1,345.71) (Table 3).
In the healthy non-elderly group, one subject exhibited a MOAA/S score >1 due to a response to repeated loud calls of his name. Excluding this subject, the remaining 7 subjects showed a median loss of consciousness time of 1.933 min (range: 1.92–1.97 min), with a BIS AUC of 648.68 (range: 240.67–816.13).
Safety outcomes
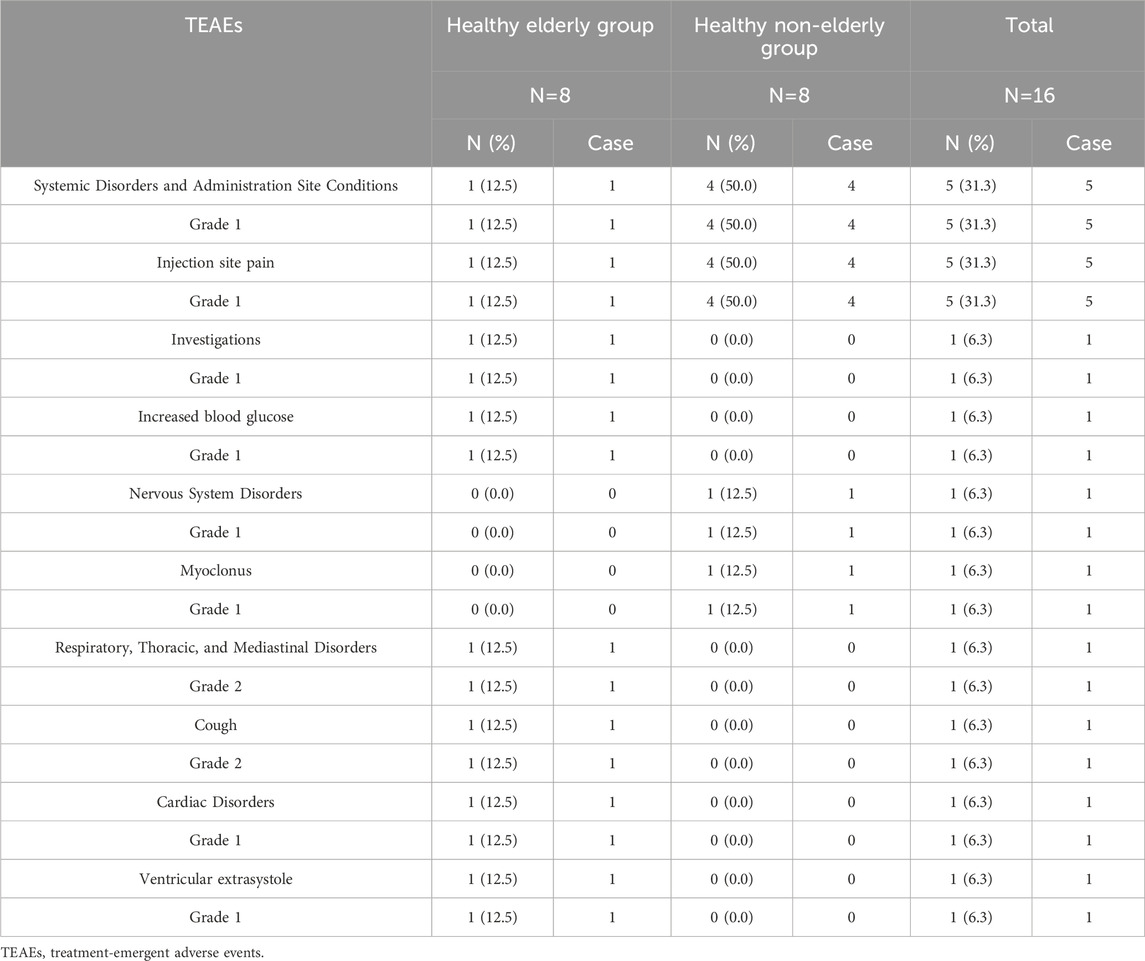
In this study, 9 TEAE events were reported in 6 subjects. Among these, 7 events were deemed related to the study drug, resulting in an overall incidence of 37.5% (6/16 subjects). In the healthy elderly group, drug-related TEAEs comprised Grade 1 injection site pain (12.5%, 1/8) and Grade 2 cough (12.5%, 1/8). The cough was managed with non-pharmacological treatment (suctioning), and no treatment was required for the other events; all recovered completely. In contrast, in the healthy non-elderly group, drug-related TEAEs included Grade 1 injection site pain (50.0%, 4/8) and myoclonus (12.5%, 1/8), none of which required any pharmacological or non-pharmacological intervention, and all recovered (Table 4).
Discussion
Elderly patients often present with evident physiological changes, including reduced hepatic and renal clearance and decreased plasma protein binding (PPB), which may affect the pharmacokinetics and pharmacodynamics of ET-26 (Sadean and Glass, 2003; Schlender et al., 2016; Thurmann, 2020). Considering the age-related challenges posed by the elderly, it is essential to investigate the PK, efficacy, and safety profile of ET-26 in this population. This study enrolled 16 subjects, with 8 in the healthy elderly group and 8 in the healthy non-elderly group. Demographic characteristics were consistent across groups in terms of gender and ethnicity.
In our study, ET-26 exhibited moderate plasma protein binding of 56.3%–60.3% in elderly subjects and 57.3%–61.0% in non-elderly subjects over a 4-h post-dose interval, with free fractions of approximately 0.397–0.437 and minimal time-dependent variation (<4.0% in elderly vs. 3.7% in non-elderly). These findings indicate that age has no appreciable effect on ET-26 PPB, despite well-documented age-related changes in plasma protein levels. Compared to etomidate (75% PPB) (Valk and Struys, 2021), ET-26 exhibited a high plasma protein binding rate, with minimal fluctuations in binding rates and free fractions observed in both groups throughout the monitoring period, indicating relatively stable plasma protein binding characteristics.
The pharmacokinetic analysis demonstrated that following a 1-min intravenous infusion of ET-26, healthy elderly subjects exhibited a geometric mean Cmax that was 198.81% (90% CI: 126.51%–312.45%) of that observed in healthy non-elderly subjects. In the elderly group (n = 8), five participants reached Tmax between 1.92 and 1.95 min, one at 2.15 min, and two at 4.00 min. By contrast, in the non-elderly group (n = 8), three subjects had a Tmax of approximately 1.93 min and five at 4.00 min. The shorter median Tmax in the elderly cohort likely underlies their higher peak concentration (2,970 ng/mL vs. 1,330 ng/mL in non-elderly). Additionally, the
The safety profile of ET-26 was evaluated in both healthy elderly and healthy non-elderly subjects. In the healthy elderly cohort, drug-related TEAEs included injection site pain (12.5%) and cough (12.5%). Notably, the cough was effectively controlled through non-pharmacological interventions such as suctioning, and both the cough and the injection site pain resolved completely without further treatment. In contrast, in the healthy non-elderly group, the most frequently observed adverse events were injection site pain and myoclonus. These adverse events may be attributable to age-related physiological differences. Specifically, degenerative changes in the skin and peripheral nervous system among the elderly, such as reduced sensitivity of nociceptive nerve endings and slower nerve conduction velocity may account for the lower incidence of injection site pain in this group (Junker et al., 2023; Bozzetto Ambrosi and Bozzetto Ambrosi, 2025). Although elderly subjects showed slightly higher pharmacokinetic exposure compared to non-elderly subjects, this did not translate to increased safety risks, with no significant differences in safety profiles observed between the two groups. Overall, these findings indicate that ET-26 was well tolerated across both populations.
Several limitations should be acknowledged. The relatively small sample size and the exclusive inclusion of male, Han ethnicity subjects limit the generalizability of the findings. Moreover, as a single-dose study, the data do not capture the potential cumulative effects associated with repeated dosing, which are important for long-term clinical applications. Future studies should consider a more diverse subject population and explore the PK/PD profile under multiple dosing conditions. To build on these findings, further research is recommended to conduct larger-scale studies including both genders and subjects from diverse ethnic backgrounds.
In summary, a single intravenous administration of ET-26 at 0.8 mg/kg was generally well tolerated in both healthy elderly and non-elderly subjects. Although pharmacokinetic parameters differed significantly between the two groups—with the elderly demonstrating higher drug exposure—the efficacy endpoint, as measured by the time to loss of consciousness, was similar across both populations. Overall, these findings suggest that ET-26 possesses a favorable safety profile and comparable efficacy in diverse age groups despite different systemic exposure.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Shandong Provincial Qianfoshan Hospital before implementation (Ethical approval number: 2023302). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FY: Visualization, Writing – original draft, Formal Analysis, Software. JS: Methodology, Writing – original draft. P-PY: Methodology, Writing – original draft. W-SL: Methodology, Writing – original draft, Investigation. L-ZL: Investigation, Project administration, Writing – review and editing. B-ZZ: Investigation, Project administration, Writing – review and editing. JV: Supervision, Writing – review and editing. YZ: Validation, Writing – review and editing. B-WK: Funding acquisition, Validation, Writing – review and editing. X-RY: Data curation, Resources, Supervision, Writing – review and editing. WZ: Funding acquisition, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by the National Key R&D Program of China (2023YFC2706100), Taishan Scholar Program of Shandong Province (NO. tstp20230660), National Natural Science Foundation of China (82425054 and 82273784). This study was sponsored by Jinzhou Avanc Pharmaceuticals.
Acknowledgments
We thank all participants in the study, along with the medical staff at the participating sites.
Conflict of interest
This study received funding from Jinzhou Avanc Pharmaceuticals. The funder had the following involvement in the study: study design, collection, analysis, interpretation of data, the writing of this article.
Authors L-ZL, B-ZZ, X-RY were employed by Avanc Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1665322/full#supplementary-material
References
Andres, T. M., McGrane, T., McEvoy, M. D., and Allen, B. F. S. (2019). Geriatric pharmacology: an update. Anesthesiol. Clin. 37 (3), 475–492. doi:10.1016/j.anclin.2019.04.007
Bozzetto Ambrosi, P., and Bozzetto Ambrosi, C. (2025). “Chronic pain in elderly: emerging challenges and updates about line of care and pathways,” in Neurological problems in the elderly - bridging current state and new outlooks. Editor P. Bozzetto Ambrosi (Rijeka: IntechOpen).
Chang, P., Su, Y., Gong, D., Kang, Y., Liu, J., Zhang, Y., et al. (2022). The preclinical pharmacological study of a novel intravenous anesthetic, ET-26 hydrochloride, in aged rats. PeerJ 10, e13995. doi:10.7717/peerj.13995
Curtis, R., Geesaman, B. J., and DiStefano, P. S. (2005). Ageing and metabolism: drug discovery opportunities. Nat. Rev. Drug Discov. 4 (7), 569–580. doi:10.1038/nrd1777
Hammerlein, A., Derendorf, H., and Lowenthal, D. T. (1998). Pharmacokinetic and pharmacodynamic changes in the elderly. Clinical implications. Clin. Pharmacokinet. 35 (1), 49–64. doi:10.2165/00003088-199835010-00004
Jiang, X., Yin, Q., Deng, X., Zhang, W., Zhang, W., and Liu, J. (2024). Advance of a new etomidate analogue — methoxyethyl etomidate hydrochloride (ET-26) for anesthesia induction in surgical patients. Anesthesiol. Perioper. Sci. 2 (3), 22. doi:10.1007/s44254-024-00062-6
Junker, S., Ebert, O., and Bartsch, R. (2023). A systematic literature review of injection site pain perception in adult patients treated with citrate-free and citrate-containing biologic agents. Curr. Rheumatol. Rev. 19 (3), 303–313. doi:10.2174/1573397118666220829123713
Mangoni, A. A., and Jackson, S. H. (2004). Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br. J. Clin. Pharmacol. 57 (1), 6–14. doi:10.1046/j.1365-2125.2003.02007.x
NMPA (2022). Technical guidelines for clinical evaluation of intravenous general anesthetics (CDE, 2022). Available online at: https://www.cde.org.cn/main/news/viewInfoCommon/8e75d4b87268d6e5e7e7bf03d8375ee6.
Sadean, M. R., and Glass, P. S. (2003). Pharmacokinetics in the elderly. Best. Pract. Res. Clin. Anaesthesiol. 17 (2), 191–205. doi:10.1016/s1521-6896(03)00002-8
Schlender, J. F., Meyer, M., Thelen, K., Krauss, M., Willmann, S., Eissing, T., et al. (2016). Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin. Pharmacokinet. 55 (12), 1573–1589. doi:10.1007/s40262-016-0422-3
Shafer, S. L. (2000). The pharmacology of anesthetic drugs in elderly patients. Anesthesiol. Clin. North Am. 18 (1), 1–29. doi:10.1016/s0889-8537(05)70146-2
Strom, C., Rasmussen, L. S., and Steinmetz, J. (2016). Practical management of anaesthesia in the elderly. Drugs Aging 33 (11), 765–777. doi:10.1007/s40266-016-0413-y
Thurmann, P. A. (2020). Pharmacodynamics and pharmacokinetics in older adults. Curr. Opin. Anaesthesiol. 33 (1), 109–113. doi:10.1097/ACO.0000000000000814
Valk, B. I., and Struys, M. (2021). Etomidate and its analogs: a review of pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 60 (10), 1253–1269. doi:10.1007/s40262-021-01038-6
Wang, B., Chen, S., Yang, J., Yang, L., Liu, J., and Zhang, W. (2017a). ET-26 hydrochloride (ET-26 HCl) has similar hemodynamic stability to that of etomidate in normal and uncontrolled hemorrhagic shock (UHS) rats. PLoS One 12 (8), e0183439. doi:10.1371/journal.pone.0183439
Wang, B., Yang, J., Chen, J., Kang, Y., Yang, L.-H., Liu, J., et al. (2017b). An etomidate analogue with less adrenocortical suppression, stable hemodynamics, and improved behavioral recovery in rats. Anesth. and Analgesia 125 (2), 442–450. doi:10.1213/ane.0000000000002063
Yin, Q., Yang, Y., Liu, J., Li, L., Yang, X., Diao, L., et al. (2025). Phase 1 single-centre placebo- and etomidate-controlled study in healthy volunteers to assess safety, tolerability, clinical effects, and pharmacokinetics of intravenous methoxyethyl etomidate hydrochloride (ET-26). Br. J. Anaesth. 134 (1), 80–88. doi:10.1016/j.bja.2024.09.009
Keywords: methoxyethyl etomidate hydrochloride, elderly, pharmacokinetics, safety, efficacy
Citation: Yang F, Sun J, Ye P-P, Lv W-S, Li L-Z, Zhao B-Z, Van Den Anker J, Zheng Y, Ke B-W, Yang X-R and Zhao W (2025) Comparative study of the pharmacokinetics, efficacy and safety of ET-26 in elderly and non-elderly subjects: the results of a phase I clinical trial. Front. Pharmacol. 16:1665322. doi: 10.3389/fphar.2025.1665322
Received: 14 July 2025; Accepted: 09 October 2025;
Published: 16 October 2025.
Edited by:
Xinning Yang, United States Food and Drug Administration, United StatesReviewed by:
André Leite Moreira, University of Porto, PortugalPing Du, Capital Medical University, China
Copyright © 2025 Yang, Sun, Ye, Lv, Li, Zhao, Van Den Anker, Zheng, Ke, Yang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zheng, eXpoZW5nMDgyMkAxNjMuY29t; Bo-Wen Ke, Ym93ZW5rZUBzY3UuZWR1LmNu; Xiao-Ran Yang, eWFuZ3hpYW9yYW5AYXZhbmNwaGFybWEuY29t; Wei Zhao, emhhbzR3ZWkyQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Fan Yang1†
Fan Yang1† John Van Den Anker
John Van Den Anker Yi Zheng
Yi Zheng Xiao-Ran Yang
Xiao-Ran Yang Wei Zhao
Wei Zhao