- Changchun University of Chinese Medicine, Changchun, China
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder. It is characterised by the following: amyloid-β (Aβ) deposition, tau hyperphosphorylation, neuroinflammation and oxidative stress. Unfortunately, there is no curative treatment available. Recently, natural products have attracted growing interest as potential therapeutic agents for AD, thanks to their multi-target actions and favourable safety profiles. This review highlights recent advances in the use of various natural compounds, including flavonoids, phenolic compounds, saponins, terpenoids, alkaloids and coumarins, with a particular focus on how they modulate the mitogen-activated protein kinase (MAPK) signaling pathway. Representative agents such as myricetin, nobiletin, resveratrol, gallic acid, paeoniflorin, ganoderic acid A, huperzine A, triptolide, berberine, crocin, and ginsenosides have been shown to regulate MAPK subpathways (ERK, JNK, p38), thereby attenuating oxidative stress, neuroinflammation, synaptic dysfunction, and neuronal apoptosis. Preclinical studies suggest that these compounds improve cognitive function and ameliorate AD-related pathology, thereby supporting the idea that MAPK signaling is a critical therapeutic target. Nevertheless, current evidence is limited by short-term animal experiments, insufficient toxicological evaluations, and challenges related to bioavailability and blood–brain barrier penetration. Future studies should emphasize long-term efficacy, safety assessments, optimized drug delivery systems, and high-quality clinical trials. Overall, natural products represent a valuable source for AD drug discovery, and targeting MAPK signaling offers promising opportunities for novel therapeutic development.
1 Introduction
Since Alois Alzheimer first described it in 1907 (Alzheimer, 1907), Alzheimer’s disease (AD) has become the most prevalent form of dementia, accounting for 60 to 80 percent of cases. The World Health Organization has recognized AD as a key disease for global public health. It currently affects approximately 47.5 million people (Vos et al., 2017), predominantly those aged 65 years and older. The latest epidemiological data shows that there are currently approximately 44 million people with dementia worldwide. Due to the accelerating global ageing trend, the number of dementia patients is expected to continue increasing, doubling approximately every 5 years (D'Cruz and Banerjee, 2021), and potentially reaching 152 million by 2050. This upward trend exhibits marked regional disparities, with the most substantial increases expected in low- and middle-income countries (Patterson, 2018). When adopting the biological definition of AD, the actual prevalence may be up to three times higher than that based on clinical diagnosis, thereby further compounding the associated social and economic burden (Prince et al., 2014; Ganguli et al., 2005).
Despite over a century of research since its discovery, the underlying pathogenesis of AD remains poorly understood. Although some treatments can temporarily alleviate symptoms (Yiannopoulou and Papageorgiou, 2020; Livingston et al., 2017), no curative therapy is currently available. The pathological hallmarks of AD include extracellular amyloid-β (Aβ) plaques (Haass and Selkoe, 2007) and intracellular neurofibrillary tangles (NFTs) (Guan et al., 2021; Sebastián-Serrano et al., 2018). Additionally, neuroinflammation (Leng and Edison, 2021), oxidative stress (Bai et al., 2022), cholinergic dysfunction (Francis et al., 1999), genetic predispositions (Latimer et al., 2021), mitochondrial impairment (Song et al., 2021), gut microbiota dysbiosis (Cryan et al., 2019; Angelucci et al., 2019), and compromised blood–brain barrier (BBB) integrity (Sweeney et al., 2018) have also been implicated in the neurodegenerative processes of AD. Against this backdrop, elucidating the pathological mechanisms of AD and developing effective therapeutic strategies have become urgent priorities in geriatric research.
In recent years, significant progress has been made in the investigation of natural compounds for the treatment of AD. A plethora of studies have reported the potential therapeutic effects of individual herbal medicines or their extracts, such as baicalein (Siddiqui et al., 2024a), punicalagin (Siddiqui et al., 2024b), ginsenosides (She et al., 2024), quercetin (Khan et al., 2019), salidroside (Zhang N. et al., 2023), naringin (Singh and Kumar Singh, 2024), and astragalosides (Ding et al., 2022), in AD management. These studies primarily focus on the regulation of multiple signaling pathways by natural compounds, including PI3K/Akt (Fakhri et al., 2021; Long et al., 2021), autophagy (Zhang Z. et al., 2021), Nrf2 (George et al., 2022), the cholinergic system (Hampel et al., 2018), the gut–brain axis (Zhang T. et al., 2023), glutamate signaling (Puranik and Song, 2024), and STAT3 (Wen and Hu, 2024). These compounds interfere with the core pathological mechanisms of AD, thereby demonstrating multi-target and integrative therapeutic potential. However, despite the critical role of the MAPK inflammatory signaling pathway in the pathogenesis and progression of AD, systematic reviews addressing its regulation by natural compounds remain scarce. Therefore, a comprehensive summary of the mechanisms by which natural compounds modulate the MAPK pathway in the treatment of AD is of substantial research significance.
2 Neuroinflammation and AD
Neuroinflammation, a significant mechanism underlying NDDs, has become a major focus of AD research in recent years. In AD, neuroinflammation plays a critical role in disease initiation, pathological progression, and clinical deterioration (Kinney et al., 2018; Kwon and Koh, 2020; Newcombe et al., 2018), primarily characterized by excessive activation of microglia and astrocytes, along with the involvement of multiple pro-inflammatory mediators (Uddin et al., 2020). In the early stages of AD, glial cells recognize pathological Aβ and tau proteins via pattern recognition receptors (e.g., TLRs, TREM2), which then mediate their clearance and exert neuroprotective effects (Temviriyanukul et al., 2023; Liu J. et al., 2020). However, as the disease progresses, sustained abnormal glial activation triggers the overactivation of signaling pathways such as NF-κB and p38 MAPK, leading to the excessive release of pro-inflammatory cytokines (e.g., IL-1β, TNF-α, IL-6) (Newcombe et al., 2018; Liao et al., 2021) and reactive oxygen/nitrogen species (ROS/RNS) (Temviriyanukul et al., 2023), thereby establishing a chronic neuroinflammatory microenvironment (Walters et al., 2016).
This persistent inflammatory state interacts with Aβ deposition and tau hyperphosphorylation, forming a vicious cycle. On one hand, inflammatory mediators promote the pathological changes in tau protein by activating kinases such as GSK-3β (Laurent et al., 2018). Furthermore, aggravation of these pathological changes in tau protein has been demonstrated to further amplify glial activation (Leng and Edison, 2021; Song et al., 2021; Laurent et al., 2018; Hickman et al., 2008). Notably, neuroinflammation exhibits dual regulatory roles: acute inflammation has a certain neuroprotective effect, while chronic inflammation can accelerate the decline of cognitive function by inducing synaptic damage and neuronal death.
The process is influenced by a variety of endogenous and exogenous factors. The endogenous factors encompass sex (e.g., estrogen deficiency) (Moser and Pike, 2016; Maioli et al., 2021; Cui et al., 2013; Lee et al., 2014; Chakrabarti et al., 2014; Tecalco-Cruz et al., 2021; Yun et al., 2018), aging (Hoozemans et al., 2011), and genetic mutations such as TREM2 R47H (Fuller et al., 2010) and ApoE4 (Kloske and Wilcock, 2020). In addition, exogenous factors include chronic stress-induced activation of the hypothalamic–pituitary–adrenal (HPA) axis (Justice, 2018; Lesuis et al., 2018; Carroll et al., 2011), heavy metal exposure (Teleanu et al., 2022; Bondy, 2021; Harischandra et al., 2019), metabolic disorders (e.g., obesity and diabetes) (Nuzzo et al., 2015), and Western diet-induced gut microbiota dysbiosis (Cavaliere et al., 2019; McGrattan et al., 2019; Chen et al., 2022; Wang X. et al., 2019; Colombo et al., 2021; Lin et al., 2022; Vogt et al., 2017). Gut dysbiosis has been demonstrated to promote central neuroinflammation and Aβ accumulation through mechanisms such as abnormal short-chain fatty acid metabolism, inflammatory signaling activation, and disruption of BBB integrity.
In summary, neuroinflammation serves not only as a key bridge between the two pathological mechanisms of Aβ and tau, but also as an important target for the early diagnosis and therapeutic intervention of AD.
3 MAPK signaling pathway and AD
Microglia and astrocytes in the central nervous system (CNS) are activated via multiple molecular signaling pathways, leading to the release of various inflammatory mediators, including nuclear factor-κB (NF-κB), p38 MAPK, mammalian target of rapamycin (mTOR), cyclooxygenase (COX), peroxisome proliferator-activated receptor-γ (PPAR-γ), and the NLRP3 inflammasome. This activation process has been shown to trigger neuronal and synaptic damage as well as neuronal apoptosis, thereby accelerating the pathological progression of AD.
The MAPK signaling pathway family plays a pivotal regulatory role in AD pathogenesis. The three primary subpathways are all significantly activated in the damaged neurons of AD patients, which are named extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK. This activation indicates the involvement of the MAPK pathway in the pathophysiological processes and pathogenesis of AD. The MAPK pathway is extensively implicated in key pathological processes of AD, including neuroinflammation, tau hyperphosphorylation, synaptic dysfunction, neuronal apoptosis, and oxidative stress.
ERK is predominantly activated by growth factors, which play critical roles in cell differentiation, proliferation, and development. Conversely, JNK and p38 MAPK are known to be activated by mitogens, cytokines, cell death receptors, and various stress stimuli, including oxidative stress, heat shock, hypoxia, and ultraviolet radiation.
The pathogenesis of AD is intricate, and in recent years, the p38 MAPK signaling pathway has emerged as a research hotspot (Jia et al., 2012). p38 MAPK, a key protein that is abundantly expressed in multiple brain regions associated with cognitive function, can be activated by various inflammatory mediators, including cytokines, chemokines, and bacterial lipopolysaccharides (LPS).
In the process of glial cell-mediated neuroinflammation, activated microglia generate substantial amounts of neurotoxic mediators, including IL-1β, TNF-α, COX-2, and inducible nitric oxide synthase (iNOS), via the p38 MAPK signaling pathway. These inflammatory factors further activate the p38 MAPK pathway in astrocytes, thereby promoting the formation of an inflammatory cycle that is difficult to halt.
Mechanistic studies suggest that p38 MAPK exerts a detrimental effect on neurons by inducing abnormal tau phosphorylation, mitochondrial dysfunction, and apoptosis but also disrupts glutamate homeostasis and synaptic plasticity through activation of the NF-κB signaling pathway (Singh, 2022). Furthermore, evidence suggests a direct correlation between aberrant microglia activation during the initial stages of AD and the subsequent development of synaptic dysfunction and neuronal death (Leng and Edison, 2021).
Clinical studies have demonstrated that p38 MAPK activity in the brain tissue of AD patients is significantly elevated compared to healthy controls (Lee and Kim, 2017; Kheiri et al., 2018). These findings provide a critical theoretical basis for targeted regulation of the MAPK pathway in the treatment of AD.
Research has demonstrated that the inhibition of Aβ toxicity and tau protein hyperphosphorylation, with the objective of protecting neurons, as well as the reduction of neuroinflammation by inhibiting the p38 MAPK pathway, are the key mechanisms by which this signaling pathway exerts therapeutic effects. Excessive Aβ deposition has been demonstrated to result in neuronal damage, induce a cytotoxic reaction, activates inflammatory signaling pathways, induces neuroinflammation and oxidative stress responses, and simultaneously damages the long-term enhancement (LTP) function of the synapses in the hippocampal region. Furthermore, excessive deposition of Aβ induces cellular stress, leading to the occurrence of neuroinflammation, which stimulates astrocytes to release inflammatory cytokines (e.g., TNF-α, IL-1β), thereby activating the p38 MAPK signaling pathway. Consequently, the activity of its downstream nuclear factor -κB (NF-κB) also increases accordingly, further promoting the release of pro-inflammatory factors and thereby exacerbating the neuroinflammatory response.
In addition, the inhibition of this pathway has been shown to reduce levels of reactive oxygen species (ROS) and superoxide (O2−), and downregulates the expression of nsy-1, sek-1, and pmk-1 mRNA (Li et al., 2018). This, in turn, has been demonstrated to mitigate oxidative stress and reduce Aβ plaque formation, ultimately exerting anti-AD effects. Another hallmark of AD pathology is the formation of neurofibrillary tangles, primarily composed of hyperphosphorylated tau protein. Under normal conditions, tau proteins are predominantly localized in neuronal axons, and participate in maintaining the stability of microtubules. The process of hyperphosphorylation of tau results in the impairment of its microtubule-binding capacity. This, in turn, leads to the destabilization of the cytoskeleton and the disruption of axonal transport. These phenomena contribute to the manifestation of synaptic dysfunction.
Synapses serve as the fundamental structures that regulate neural functions and directly participate in the transmission of neural signals. Among them, LTP plays a key role in the formation of learning and memory and is an important physiological basis for both. Studies have revealed that activation of p38 MAPK can inhibit LTP and reduce synaptic plasticity in the hippocampus, thereby directly affecting the process of memory formation. Therefore, inhibiting the activation of the p38 MAPK signaling pathway helps improve synaptic dysfunction and restore synaptic plasticity, which is a potentially effective strategy for intervening in AD (Yu et al., 2018; Figure 1).
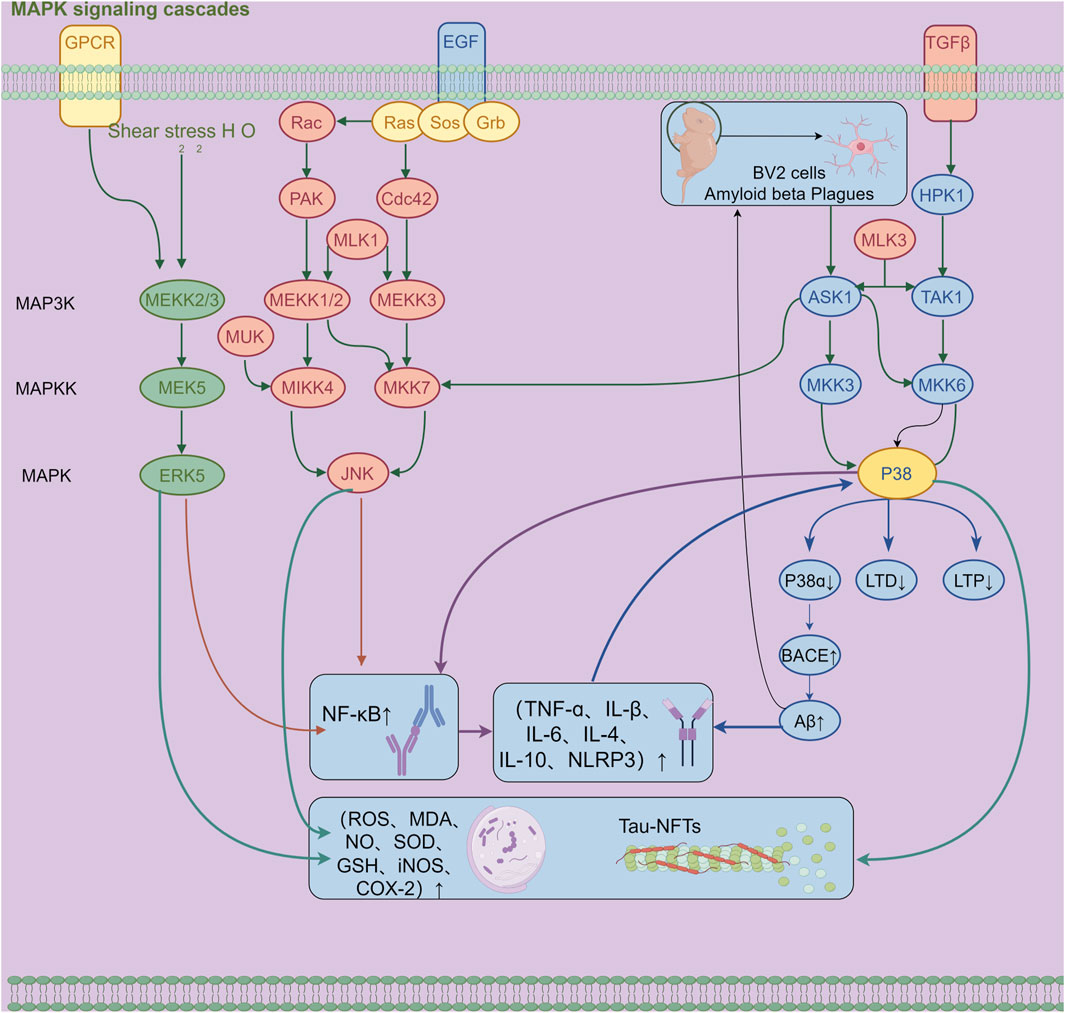
Figure 1. Mechanism diagram of β-amyloid-induced activation of the MAPK signaling pathway and neuroinflammatory response in BV2 microglia (300 dpi.Thanks for Figdraw).
4 Traditional Chinese medicine (TCM) compounds treat AD through the MAPK signaling pathway
A substantial body of research has demonstrated that plant-derived natural compounds—such as flavonoids, alkaloids, saponins, and phenolic acids—modulate neurotransmitter levels through multiple signaling pathways and cascade reactions, thereby improving behavioral performance and memory function, reducing Aβ protein deposition, inhibiting acetylcholinesterase (AChE) activity, preventing neuronal apoptosis, and enhancing cerebral antioxidant capacity. Moreover, accumulating evidence indicates that these compounds can specifically modulate the MAPK signaling pathway, demonstrating considerable therapeutic potential in the treatment of AD (Table 1, 2).
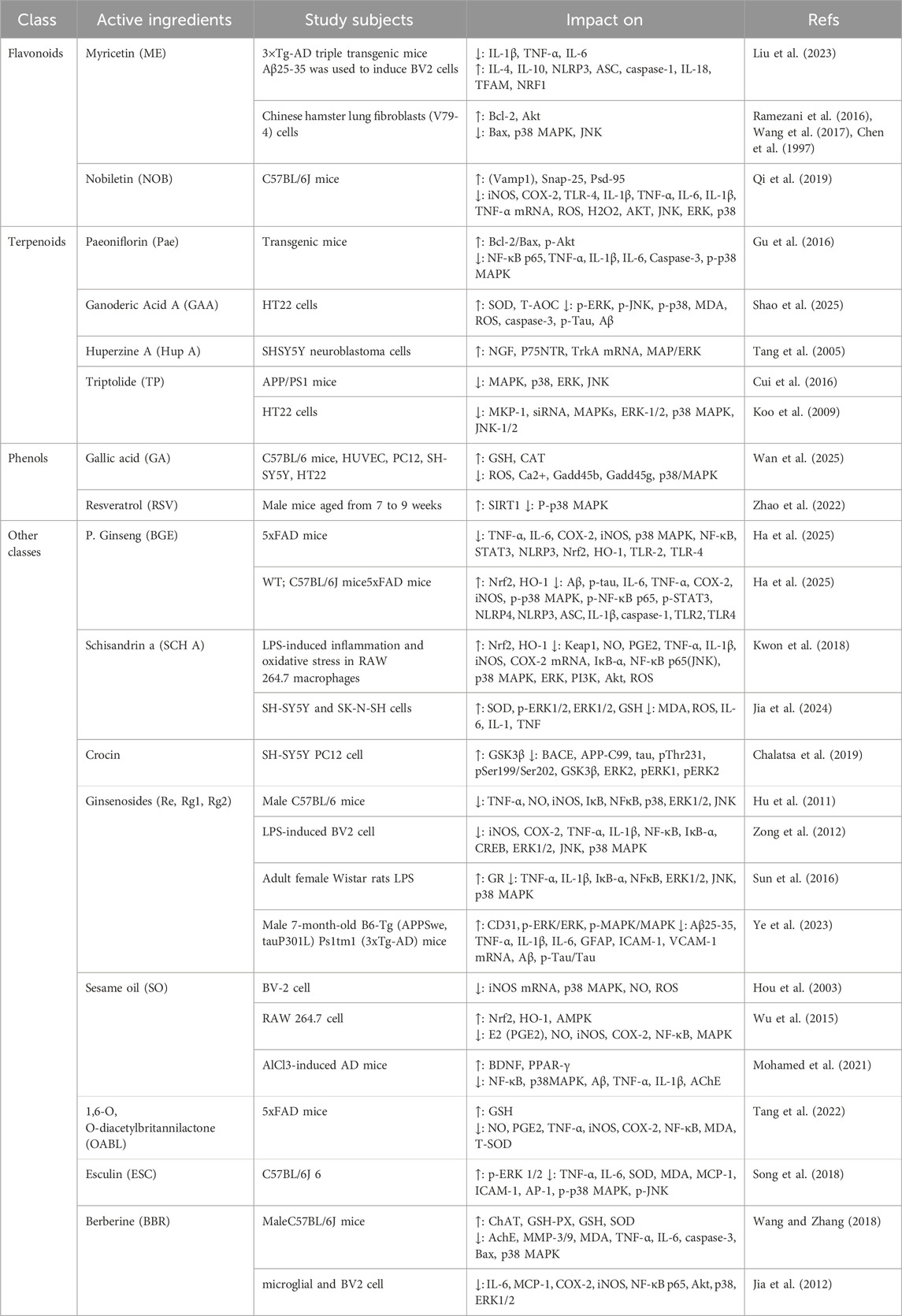
Table 1. Plant-derived natural compounds modulating the MAPK signaling pathway in clinical studies for the prevention and treatment of Alzheimer’s disease.
4.1 Flavonoids
4.1.1 Myricetin (ME)
ME is a naturally occurring flavonoid with diverse biological activities. It is predominantly found in plant species belonging to the Myricaceae and Euphorbiaceae families, and the main sources include plant extracts such as berries and tea leaves (Taheri et al., 2020). Studies have demonstrated that ME exerts a broad range of pharmacological effects, including anti-inflammatory activity (by modulating the NLRP3 inflammasome, the NF-κB pathway, and various pro-inflammatory cytokines), antioxidant properties, improvement of mitochondrial dysfunction, and regulation of autophagy.
Liu. et al. (Liu et al., 2023) employed the 3×Tg-AD mouse model and integrated network pharmacology with molecular docking analysis to predict that ME can regulate the MAPK signaling pathway through multiple targets. The experimental findings revealed that ME significantly inhibited activation of the p38 MAPK pathway, thereby alleviating Aβ25-35-induced mitochondrial dysfunction, suppressing overactivation of the NLRP3 inflammasome, and improving cognitive and memory deficits in AD model mice.
Furthermore, Kyoung et al. (Kang et al., 2010; Ramezani et al., 2016; Wang et al., 2017) also found that ME has a neuroprotective effect on oxidative stress-induced mitochondrial-dependent and caspase-dependent apoptosis processes by regulating the p38 MAPK and JNK signaling pathways, suggesting that it may exert an anti-AD effect by regulating the MAPK signaling pathway.
4.1.2 Nobiletin (NOB)
NOB is a naturally occurring flavonoid that is predominantly found in the peel of citrus fruits belonging to the Rutaceae family (Chen et al., 1997; Nogata et al., 2006). Studies have demonstrated that NOB exhibits multiple pharmacological activities, including anti-inflammatory, antioxidant, anticancer, antidiabetic, antiatherosclerotic, neuroprotective, and anti-obesity effects (Tanaka et al., 2004; Choi et al., 2007; Hirata et al., 2008; Cui et al., 2010; Lee et al., 2010; Yoshim et al., 2004; Miyamoto et al., 2008; Lam et al., 2011; Lee et al., 2011; Mulvihill et al., 2011; Choi et al., 2011; Kanda et al., 2012). According to extant research, NOB has been demonstrated to ameliorate AD-related cognitive impairments, such as decline in learning and memory, by inhibiting apoptosis, alleviating oxidative stress, and reducing cerebral Aβ protein levels.
Neuroinflammation has been identified as a significant mechanism contributing to the progression of NDDs. In the mouse microglial BV-2 cell line, NOB exhibits potent anti-neuroinflammatory effects, significantly inhibiting the production and release of LPS-induced pro-inflammatory mediators, including NO, TNF-α, IL-1β, and IL-6 (Cui et al., 2010; Ho and Kuo, 2014; Wang Y. et al., 2019). A recent study demonstrated that mice administered 100 mg/kg of nobiletin daily for 6 weeks exhibited effective alleviation of LPS-induced memory impairment. The study also found that nobiletin inhibited the activation of microglia and the secretion of related pro-inflammatory cytokines.
Qi et al. (2019) further demonstrated that NOB treatment significantly decreased serum levels of iNOS, COX-2, TLR4, IL-1β, and TNF-α, inhibited NF-κB nuclear translocation, and enhanced phosphorylation and activation of key signaling proteins including AKT, JNK, ERK, and p38 MAPK.
Further experiments demonstrated that under NOB treatment, inhibitors of ERK (U0126), p38 (SB203580), and JNK (SP600125) can synergistically alleviate the inflammatory response induced by LPS, confirming that NOB may alleviate the inflammatory state of BV-2 microglia by regulating the MAPK signaling pathway.
In summary, NOB effectively alleviates inflammation-induced cognitive deficits and neuroinflammation by reducing neuronal damage, inhibiting microglial activation, suppressing inflammatory factor release, and restoring mitochondrial function, thereby highlighting its potential therapeutic value in the prevention and treatment of AD.
4.2 Phenols
4.2.1 Gallic acid (GA)
GA is a polyphenolic organic compound that is found in plants such as tea leaves, oranges, papayas, pomegranates, and cardamom (Latha and Daisy, 2011). It has been demonstrated that GA exhibits a variety of pharmacological activities, including anticancer and antioxidant effects, and has been extensively applied in medical research (Gu et al., 2025). Studies have demonstrated that GA reduces brain injury by decreasing infarct size in rat models of cerebral ischemia (Kumar et al., 2021). In neurological disorder models, GA significantly ameliorates cognitive impairment in rotenone-induced Parkinson’s disease (PD) rat models (Sheikhpour et al., 2023). Related studies indicate that GA improves learning and memory performance and enhances motor function in AD mice. As demonstrated in the experimental data from Wan et al. (2025), the application of GA has been shown to attenuate the damage induced by glutamate (Glu) in PC12, SH-SY5Y, and HT22 neural cells, exhibiting a dose- and time-dependent response. The protective effects of GA are more pronounced in PC12 and SH-SY5Y cells. Mechanistic studies suggest that GA mitigates AD pathological progression by inhibiting the expression of Gadd45g and Gadd45b and their downstream P38/MAPK signaling pathway, thereby attenuating oxidative stress responses. Transcriptomic analyses further reveal that the P38/MAPK signaling pathway plays a critical role in mediating GA’s neuroprotective effects against AD. In summary, GA attenuates the progression of AD by inhibiting the P38/MAPK signaling pathway. It has potential value as a natural candidate drug for the prevention of AD.
4.2.2 Resveratrol (RSV)
Resveratrol, a naturally occurring phenolic compound, functions as a plant antitoxin. It is produced in response to mechanical damage or attack by pathogens, including bacteria and fungi (Vestergaard and Ingmer, 2019). It exhibits diverse pharmacological activities, including anti-angiogenic, immunomodulatory, antibacterial, neuroprotective, anticancer, antidiabetic, and cardiovascular disease (CVD) preventive effects (Breuss et al., 2019). Resveratrol has been demonstrated to effectively ameliorate mitochondrial dysfunction, mitigate oxidative stress, modulate inflammatory responses, and inhibit apoptosis. Moreover, preliminary studies suggest that resveratrol may also have an improving effect on NDDs (Fantacuzzi et al., 2022). In aged C57BL/6 mice, oral administration of resveratrol at 200 mg/kg for 10 consecutive days restored brain microvascular endothelial function and suppressed ROS production, thereby improving the coupling response of cortical neurovascular and promoting neuronal activity and functional recovery (Toth et al., 2014). In a C57BL/6 mouse model, intraperitoneal injection of 100 mg/kg resveratrol for seven consecutive days alleviated hippocampus-dependent cognitive deficits via anti-inflammatory and anti-apoptotic mechanisms (Li et al., 2014). In male F344 rats, intraperitoneal administration of resveratrol at 40 mg/kg for 4 weeks significantly improved memory and emotional functions, facilitated hippocampal neurogenesis and microvascular remodeling, and suppressed glial cell activation (Kodali et al., 2015). Zhao et al. (2022) demonstrated that resveratrol ameliorates post-traumatic cognitive dysfunction in mice by activating the deacetylase Sirtuin one and inhibiting phosphorylation of p38 MAPK. Studies suggest that p38 MAPK is activated following traumatic brain injury (TBI), and resveratrol exerts regulatory effects on this pathway.
4.3 Terpene
4.3.1 Paeoniflorin (Pae)
Pae is a water-soluble monoterpene glucoside predominantly extracted from the dried roots of Paeonia lactiflora Pall., a species within the Paeoniaceae family. It constitutes the major active component of total paeony glycosides (TGP), comprising over 40% of the total glycoside content. Pae exhibits diverse pharmacological effects, such as anti-inflammatory, antioxidant, antithrombotic, anticonvulsant, antidepressant, sedative, analgesic, antispasmodic, and immunomodulatory activities (Chen et al., 2011; Ye et al., 2016; Hino et al., 2012; Qiu et al., 2013; Zhang et al., 2009). Pae modulates multiple signaling pathways, including G protein-coupled receptors (GPCRs), MAPKs/NF-κB, PI3K/Akt/mTOR, JAK2/STAT3, and TGF-β/Smads pathways. Pae has been shown to regulate calcium ion (Ca2+) and reactive oxygen species (ROS) homeostasis, thereby exerting therapeutic effects against NDDs.
Gu et al. (2016) established an AD model using transgenic mice and demonstrated that Pae exerts significant neuroprotective effects, markedly improving cognitive functions in AD mice, as evidenced by enhanced escape distance and latency performance. The study revealed that Pae inhibits apoptosis by elevating the Bcl-2/Bax ratio and p-Akt expression in brain tissue of AD mice, concurrently downregulating p-P38 MAPK expression. This results in the attenuation of inflammatory responses and caspase-3 activity. Further investigations suggest that prolonged Pae treatment suppresses JNK and P38 MAPK activation while enhancing ERK activation. Pae effectively reverses ischemia-induced activation of the NF-κB signaling pathway and exerts marked neuroprotective effects in rats with cerebral ischemic injury by mitigating inflammatory responses within brain tissue.
4.3.2 Ganoderic acid a (GAA)
GAA is a triterpenoid compound isolated from Ganoderma lucidum (reishi mushroom) has been shown to possess inherent natural neuroprotective properties. GAA exhibits diverse pharmacological activities, including anti-inflammatory, antioxidant, antitumor, neuropsychopharmacological, hepatoprotective, cardioprotective, nephroprotective, and pulmonary protective effects by modulating various signal transduction pathways (Jiang et al., 2018; Meng et al., 2020; Wan et al., 2019; Lixin et al., 2019; Yang et al., 2018; Zhang et al., 2020; Zhang L. et al., 2021; Zheng et al., 2022), underscoring its substantial clinical application potential. In a Caenorhabditis elegans model, GAA treatment significantly delayed cellular senescence and extended healthspan (Chen et al., 2025). Studies have reported that in an Aβ42-induced AD mouse model, GAA activates the Axl receptor tyrosine kinase (Axl)/CDC42-associated kinase 1 (Pak1) signaling pathway, stimulates autophagy in BV2 microglial cells, enhances Aβ42 clearance, and subsequently ameliorates cognitive deficits (Qi et al., 2021). Furthermore, GAA dose-dependently increased the viability of HT22 cells injured by Aβ25-35, while concurrently suppressing the expression of MAPK pathway-related proteins. GAA markedly downregulates cleaved caspase-3 levels, decreases apoptosis, and suppresses Aβ and phosphorylated tau (p-Tau) expression via inhibition of the ERK signaling pathway (Shao et al., 2025). Given its ability to inhibit apoptosis via the ERK/MAPK signaling pathway, GAA shows broad prospects as a potential candidate drug for the treatment of AD.
4.3.3 Huperzine A (Hup A)
Elsholtzia ciliata, more commonly referred to as the Thousand-layer Tower, is a traditional Chinese medicinal herb that belongs to the Huperziaceae family. Modern studies have identified alkaloids and triterpenoids as the main bioactive constituents, among which Hup A is the important active compound. Hup A has been successfully utilized in the treatment of AD, dementia, and myasthenia gravis (College, 1985). It has been demonstrated that this agent functions by impeding the phosphorylation of p38 MAPK and ERK within the MAPK signaling pathway, leading to decreased expression of iNOS and cyclooxygenase-2 (COX-2), thereby suppressing the release of pro-inflammatory mediators. Hup A exhibits neurotrophic effects against oxidative stress by promoting nerve growth factor (NGF) synthesis in SH-SY5Y cells, a process contingent upon activation of the MAPK/ERK signaling pathway (Tang et al., 2005). Furthermore, the MAPK/ERK signaling pathway has been implicated in mediating the neuroprotective effects of Hup A in transient cerebral ischemia-reperfusion animal models (Wang et al., 2006). The MAPK/ERK signaling pathway plays a pivotal role in regulating various biological processes, including proliferation, differentiation, and the expression of multiple transcription factors.
4.3.4 Triptolide (TP)
The genus Tripterygium, which belongs to the family Celastraceae, contains Tripterygium lactone, a natural diterpenoid compound that is one of its principal bioactive constituents (Chen et al., 2018). This compound has been demonstrated to possess a wide range of pharmacological activities, including anti-inflammatory, immunomodulatory, antitumor, and anti-aging effects (Gao et al., 2021; Tong et al., 2021). Tripterygium glycoside has been identified as a regulator of β-amyloid (Aβ) levels, with the capacity to mitigate synaptic dysfunction and memory impairments associated with AD. Owing to its high lipophilicity and low molecular weight, Tripterygium glycoside has been observed to cross the blood-brain barrier (BBB), thereby demonstrating potential therapeutic efficacy in treating neurological disorders (Zhang et al., 2012). Reduction of oxidative stress is regarded as a key protective mechanism of Tripterygium wilfordii heterophyllum against AD; however, its potential preventive effects on AD pathology via anti-inflammatory pathways require further elucidation. TP inhibits the expression of MKP-1, which primarily deactivates ERK1/2, p38 MAPK, and JNK1/2 signaling pathways, thereby exerting anti-proliferative and pro-apoptotic effects (Koo et al., 2009). Cui et al. (2016) demonstrated that Tripterygium wilfordii significantly suppresses microglial activation in the cerebral cortex and hippocampus of APP/PS1 transgenic mice. Recent molecular biology studies have identified the MAPK signaling pathway is one of the core mechanisms for regulating inflammatory responses. Treatment with Tripterygium wilfordii lactone markedly reduced phosphorylation levels of p38, ERK, and JNK in the brain tissue of APP/PS1 mice, indicating inhibition of MAPK pathway activation. Furthermore, Tripterygium wilfordii lactone has been shown to suppress the expression of pro-inflammatory cytokines TNF-α and IL-1β, effects that are likely linked to its inhibitory action on the MAPK signaling pathway.
4.4 Other categories
4.4.1 1,6-O, O-diacetylbritannilactone (OABL)
OABL is a natural 1,10-bislactone-type sesquiterpene lactone compound that has been isolated from Impatiens grandiflorum (Shi et al., 2022). It exhibits a broad spectrum of pharmacological activities and has been applied in the treatment of bronchitis, diabetes, intestinal ulcers, gastrointestinal disorders, and various inflammatory conditions (Zhao et al., 2006). In addition, OABL has demonstrated encouraging efficacy in the treatment of AD. Its anti-inflammatory mechanisms mainly involve the inhibition of inflammatory mediator production (including NO, PGE2, TNF-α, iNOS, and COX-2) and the suppression of nuclear translocation of the transcription factor NF-κB (Chen et al., 2017). Furthermore, OABL has been shown to possess antioxidant properties that protect neurons against oxidative damage (Wang et al., 2022). In AD animal models, OABL has been shown to significantly improve cognitive performance, restore neuronal morphology in the hippocampus, reduce Aβ amyloid protein deposition, and inhibit excessive phosphorylation of the Tau protein. Research has shown that its structural analog, ABL, also suppresses the expression of COX-2 and NF-κB and alleviates Aβ23-35-induced learning and memory deficits in rats (Wang et al., 2008).
In the 5xFAD transgenic AD mouse model, OABL significantly reduced the immunofluorescence signal intensity of the NF-κB p-p65 subunit in both the cortex and hippocampus.This reduction occurred through modulation of the TLR4/NF-κB and p38 MAPK signaling pathways, and decreased the mRNA expression of pro-inflammatory cytokines such as TNF-α and IL-1β. It has also been demonstrated to promote the M1/M2 transformation of microglia, enhance the expression of arginase-1 (Arg-1) and IL-10, and suppress the production of TNF-α, PGE2, iNOS, and COX-2, thereby reducing the inflammatory response of the CNS and exerting potential neuroprotective effects (Tang et al., 2022).
4.4.2 Berberine (BBR)
BBR is a naturally occurring isoquinoline alkaloid primarily derived from the roots, bark, and stems of various medicinal plants, such as the rhizomes of Coptis chinensis (Ye et al., 2009). It exhibits a wide range of pharmacological activities, including anti-inflammatory, cardioprotective, neuroprotective, antitumor, and antimalarial properties (Ma et al., 1999; Küpeli et al., 2002; Le Tran et al., 2003; Zheng et al., 2003; Kettmann et al., 2004; Račková et al., 2004; Letašiová et al., 2006). In the domain of AD research, BBR has demonstrated a variety of mechanisms of action, indicating its potential for therapeutic use.Recent studies have demonstrated that BBR can inhibit the production of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and C-C motif chemokine ligand 2 (CCL2), in Aβ-stimulated primary microglia and BV-2 cell lines. Furthermore, it has been observed to downregulate the expression of COX-2 and iNOS. While the precise mechanisms through which BBR exerts its anti-inflammatory effects remain to be fully elucidated, current evidence suggests that it may do so primarily through the modulation of signaling pathways, including NF-κB, phosphoinositide 3-kinase (PI3K), and MAPK.
Wang and Zhang’s research in 2018 revealed that BBR demonstrated neuroprotective effects in preventing learning and memory deficits induced by traumatic brain injury. These effects were potentially attributable to the reduction of inflammation, oxidative stress, and neuronal apoptosis, as well as the modulation of the Sirt1/p38 MAPK signaling pathway (Wang and Zhang, 2018). In addition, BBR has demonstrated protective effects in rat models of heavy metal-induced neurotoxicity and AD-like pathology. Jia et al. (2012) utilized network pharmacology to identify cross-targets of BBR in AD and pinpointed the JNK–p38 MAPK signaling pathway as a critical regulatory pathway. Subsequent in vitro and in vivo experiments confirmed that BBR exerts its therapeutic effects in AD by activating autophagy, modulating the JNK–p38 MAPK signaling pathway to clear Aβ deposits, suppressing neuroinflammation, and promoting neuronal repair.
4.4.3 Sesame oil (SO)
The Chinese herbal medicine Sesame is rich in sesame oil, which is the main natural source of sesamin. Sesamin has been demonstrated to possess a variety of pharmacological activities, including anti-inflammatory, antioxidant, antitumor, and cardiovascular protective effects (Jayaraj et al., 2020). SO has also been demonstrated to reduce monoamine oxidase (MAO) activity by inhibiting the production of nitric oxide (NO) and hydrogen peroxide (H2O2) in astrocytes. Given that MAO plays a critical role in the pathogenesis of NDDs, sesamol is considered to have significant potential in the prevention and treatment of CNS diseases.
Hou et al. (2003) demonstrated that SO significantly reduces NO production as well as iNOS mRNA and protein expression in LPS-stimulated BV-2 microglial cells. Furthermore, SO markedly inhibited the activation of p38 MAPK. The specific p38 MAPK inhibitor SB203580 also exhibited dose-dependent inhibition of NO production, further supporting the hypothesis that polyphenolic compounds capable of suppressing NO generation may exert neuroprotective effects.
In a related study, Wu et al. (2015) treated RAW 264.7 macrophages with sesamol followed by LPS stimulation to induce an inflammatory response. Their findings indicated that sesamol exhibited the capacity to impede NF-κB nuclear translocation and MAPK pathway activation, while concomitantly promoting the activation of AMP-activated protein kinase (AMPK). These findings suggest that sesamol improves inflammatory responses and oxidative stress damage by activating the AMPK and Nrf2 signaling pathways while inhibiting the NF-κB and MAPK pathways.
Mohamed et al. (2021) reported that SO significantly ameliorated AlCl3-induced learning and memory deficits in mice. It reduced AChE activity and Aβ levels, downregulated the expression of pro-inflammatory cytokines TNF-α and IL-1β, suppressed NF-κB and p38 MAPK signaling, and upregulated the expression of brain-derived neurotrophic factor (BDNF) and peroxisome proliferator-activated receptor gamma (PPAR-γ). These results suggest that SO alleviates neuroinflammation and oxidative stress damage by modulating the NF-κB/p38MAPK/BDNF/PPAR-γ signaling pathway, thereby contributing to the recovery of cognitive function and showing its potential value in the treatment of AD.
4.4.4 Schisandrin A (SCH A)
SCH A is a bioactive lignan compound that has been isolated from Schisandra chinensis, a traditional Chinese medicinal herb. In recent years, SCH A has attracted growing scientific interest owing to its broad spectrum of pharmacological activities. It has been demonstrated to exert diverse biological effects, including anti-inflammatory, anticancer, hepatoprotective, antioxidant, neuroprotective, antidiabetic, and musculoskeletal protective properties (Choi, 2018; Cui et al., 2020; Kong et al., 2018; Meng et al., 2019; Jeong et al., 2019; Wang et al., 2014; Zhang et al., 2010). Notably, Schisandra and its active constituents have shown promising potential in the prevention and treatment of AD.
A series of experimental studies have demonstrated SCH A (10, 20, and 50 μM) suppresses the expression of NO, tumor necrosis factor-α (TNF-α), and IL-6 in LPS-stimulated BV-2 microglia and primary microglial cells, thus exerting anti-inflammatory properties. It mitigates microglia-mediated neuroinflammation by inhibiting key signaling pathways, such as TRAF6–IKKβ–NF-κB and JAK2–STAT3, thereby exerting neuroprotective effects (Song et al., 2016).
Furthermore, schisandrin has been shown to enhance neuronal viability in Aβ1–42-induced SH-SY5Y cell models of AD through activation of the PI3K/Akt signaling pathway, thus exerting protective effects (Zhao et al., 2019). Jia et al. (2024) reported that SCH A significantly reduces oxidative stress response and downregulates inflammatory cytokine expression in cells induced by Aβ25-35, while also increasing the p-ERK1/2 to ERK1/2 ratio, indicating that its underlying mechanism may involve activation of the ERK/MAPK pathway.
Further research by Kwon et al. (2018) using an in vitro RAW 264.7 macrophage model demonstrated that SCH A attenuates LPS-induced inflammation and oxidative stress by activating the Nrf2/HO-1 signaling pathway, while concurrently suppressing the NF-κB, MAPK, and PI3K/Akt pathways. Among these, SCH A pretreatment markedly inhibited the phosphorylation of ERK, JNK, and p38 MAPK, providing further evidence of its multi-target anti-inflammatory and antioxidant effects.
4.4.5 Crocin (CRO)
Crocin (CRO) is a natural carotenoid that is found in high concentrations in the stigmas of saffron (Crocus sativus) and the fruits of gardenia (Gardenia jasminoides) (Liu T. et al., 2020). Extensive in vitro, in vivo, and clinical studies have demonstrated that CRO exerts beneficial effects across multiple organ systems, including the nervous, immune, cardiovascular, gastrointestinal, reproductive, and endocrine systems (Khorasany and Hosseinzadeh, 2016).
Research indicates that CRO exerts significant memory-enhancing effects, which are partly attributed to its anti-inflammatory properties and modulation of the ERK/MAPK signaling pathway. In a D-galactose-induced aging model, CRO improves cognitive function via its anti-glycation and antioxidant activities, thereby suppressing the expression of neuroinflammatory mediators (e.g., IL-1β, TNF-α, and NF-κB) and activating the PI3K/Akt and ERK/MAPK signaling pathways (Adabizadeh et al., 2019; Heidari et al., 2017; Looti Bashiyan et al., 2021).
Furthermore, the use of CRO has been shown to markedly decrease total tau protein levels and phosphorylation, suppresses β- and γ-secretase activities, and reduces the deposition of Aβ precursor protein (AβPP) accumulation in AD models by inhibiting ERK1/2 kinase activity (Chalatsa et al., 2019). Another study shows that CRO mitigates acrolein-induced neurotoxicity, potentially through the attenuation of oxidative stress via the ERK/MAPK pathway, thus delaying the progression of NDDs (Rashedinia et al., 2015).
4.4.6 Ginsenosides Re, Rg1, and Rg2
Ginsenosides Re, Rg1, and Rg2 are the major triol-type natural saponins in ginseng and represent the principal active constituents of this traditional Chinese medicinal herb. These compounds have been demonstrated to exert a variety of pharmacological effects, including the enhancement of cognitive function, the inhibition of apoptosis, and the exertion of neuroprotective activities (Li et al., 2020). Among them, ginsenoside Re is a pivotal component (Shi et al., 2019) and remains the most extensively investigated ginsenoside to date. It has been demonstrated to possess antioxidant and anti-inflammatory properties, suppressing the production of IL-6, tumor necrosis TNF-α, and NO in microglial cells without impairing cellular viability (Lee et al., 2020; Lee I-A. et al., 2012). The reduction in the release of pro-inflammatory and neurotoxic mediators from microglia has been demonstrated to provide a protective effect on hippocampal neurons (Madhi et al., 2021). Furthermore, ginsenoside Re has been shown to attenuate neuroinflammation progression by inhibiting LPS-induced MAPK phosphorylation (Lee K-W. et al., 2012).
Among the diverse ginsenosides, Rg1 demonstrates notable neuroprotective benefits, especially in NDDs such as AD and PD. Hu et al. (2011) demonstrated that ginsenoside Rg1 suppresses LPS-induced microglial activation via downregulation of Iba-1 and iNOS expression. Furthermore, Rg1 effectively inhibits the phosphorylation of p38 MAPK, ERK1/2, and JNK, and prevents the degradation of IκB as well as the nuclear translocation of the NF-κB p65 subunit. Rg1 attenuates LPS-induced inflammatory responses by activating the phospholipase C-γ1 signaling pathway in mouse BV-2 microglia. It inhibits the phosphorylation of p38 MAPK, IκB-α, CREB, and ERK1/2, significantly reduces NF-κB expression, and decreases the production of pro-inflammatory cytokines, including TNF-α, IL-1β, iNOS, and COX-2 (Zong et al., 2012). The neuroprotective effect of ginsenoside Rg1 against LPS-induced neuronal degeneration in rats is mediated via the glucocorticoid receptor, involving inhibition of the p38 MAPK signaling pathway to suppress LPS-induced inflammation in midbrain dopaminergic neuronal microglia (Sun et al., 2016).
Treatment with ginsenoside Rg2 significantly elevates the ratios of phosphorylated ERK to total ERK (p-ERK/ERK) and phosphorylated MAPK to total MAPK (p-MAPK/MAPK) in the brain tissue of 3xTg-AD mice, thereby mitigating neurovascular damage in this AD model (Ye et al., 2023).
4.4.7 Black ginseng extract (BGE)
Panax ginseng, which is more commonly referred to as Korean ginseng, contains primary active components including ginsenosides (-Rg3, -Rg5, and -Rk1), polysaccharides, and phenolic compounds, with particularly high concentrations in Korean BGE (Metwaly et al., 2019). Research has demonstrated that extracts of Korean BGE, when administered as an ethanol solution, have been shown to attenuate neuroinflammation by inhibiting the NF-κB and MAPK signaling pathways in LPS-stimulated BV2 microglia. This attenuation is achieved via a Toll-like receptor 4 (TLR4)-MyD88-dependent mechanism (Kim et al., 2023). Furthermore, BGE has been demonstrated to significantly enhance cognitive function in the 5xFAD AD mouse model, concomitant with reduced Aβ accumulation in the frontal cortex and hippocampus (Ha et al., 2025). BGE has been demonstrated to suppress the activation of microglia and astrocytes, as well as to downregulate pro-inflammatory cytokines, including IL-6 and tumor necrosis factor-alpha (TNF-α), along with the expression of enzymes such as COX-2 and iNOS. Further studies have demonstrated that BGE reduces Aβ plaque deposition via activation of the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway and suppresses p38 MAPK, NF-κB, and STAT3 signaling pathways, as well as NLRP3 inflammasome activation, thus protecting cognitive function in 5xFAD mice and highlighting its therapeutic potential in AD.
4.4.8 Esculetin (ESC)
Esculetin is a natural dihydroxy coumarin; it is mainly extracted from twig skin and the trunk bark of the Chinese herbal medicine Fraxinus rhynchophylla Hance.Natural coumarin derivatives have demonstrated anti-inflammatory effects through various inflammatory signaling pathways, including TLRs, JAK/STAT, inflammasomes, MAPK, NF-κB, and TGF-β/SMAD. Possesses antioxidant (Wang et al., 2011), antiinflammatory (Kirsch et al., 2016), antiapoptotic (Kim et al., 2015), anticancer (Pinto and Silva, 2017), antidiabetic (Li et al., 2017), neuroprotective (Delogu and Matos, 2017), and cardiovascular protective activities (Najmanová et al., 2015). Pruccoli et al. (2020) demonstrated the ability of ESC to prevent and counteract ROS formation in neuronal SH-SY5Y cells, suggesting its profile as a bifunctional antioxidant. In particular, ESC increased the resistance of the SH-SY5Y cells against OS through the activation of Nrf2 and increase of GSH. In similar experimental conditions, ESC could also protect the SH-SY5Y cells from the OS and neuronal death evoked by oligomers of Aβ1-42 peptides. Further, the use of the inhibitors PD98059 and LY294002 also showed that Erk1/2 and Akt signaling pathways were involved in the neuroprotection mediated by ESC.
ESC, a common coumarin derivative, was reported by Song et al. (2018) to exhibit protective potential against diabetic nephropathy (DN). In this study, a diabetic mouse model was established in 6-week-old male C57BL/6J mice by a single intravenous injection of streptozotocin (STZ, 30 mg/kg). Two weeks after STZ injection, the mice received intravenous administration of ESC at doses of 5, 10, or 20 mg/kg for an additional 2 weeks. The results demonstrated that ESC markedly suppressed STZ-induced renal expression of AP-1, p-p38 MAPK, and p-JNK, while upregulating p-ERK1/2. These findings suggest that ESC may alleviate experimental DN-associated cognitive impairment through modulation of the MAPK signaling pathway, exerting both antioxidative and anti-inflammatory effects.
5 Discussion
Alzheimer’s disease (AD) is a complex chronic neurodegenerative disorder characterized by multiple pathological processes, including β-amyloid (Aβ) deposition, tau hyperphosphorylation, neuroinflammation, and oxidative stress. Given the limited efficacy and adverse effects of current therapies, natural products, owing to their multi-target actions and relative safety, have attracted increasing attention as promising candidates for AD prevention and treatment.
This review systematically summarizes recent progress on various classes of natural compounds in AD research, including flavonoids, phenolics, saponins, terpenoids, and alkaloids. Representative compounds such as 1,6-O,O-diacetylbritannilactone, berberine, sesamol, schisandrin A, crocin, ginsenosides, and coumarins have demonstrated potential neuroprotective effects by improving cognitive performance, alleviating neuroinflammation, reducing oxidative stress, and inhibiting neuronal apoptosis. Accumulating evidence suggests that these compounds exert their beneficial effects mainly through the modulation of signaling pathways such as NF-κB, MAPK, PI3K/Akt, and Nrf2/HO-1, thereby interfering with key pathological events of AD. Moreover, both in vitro and in vivo studies have shown that natural products significantly suppress neuroinflammatory responses and ameliorate cognitive impairments in AD animal models.
Despite these advances, current research models remain limited. Most animal studies rely on short-term acute dosing and lack long-term administration protocols, whereas the chronic and progressive nature of AD suggests that therapeutic efficacy may depend on sustained exposure. In addition, systematic toxicological evaluations of candidate compounds are insufficient, particularly concerning blood-brain barrier (BBB) permeability, organ-specific toxicity, and long-term safety. Furthermore, the intrinsic issues of low bioavailability and complex in vivo metabolism substantially restrict their clinical translation. The potential of combining natural products with existing drugs also remains largely unexplored.
To facilitate the effective translation of natural compounds from bench to bedside, it is essential to establish experimental systems that align with translational medicine standards. These include long-term pharmacodynamic evaluations in chronic disease models and comprehensive preclinical safety assessments in accordance with ICH guidelines. More importantly, high-quality clinical studies that comply with international standards—such as multicenter, randomized, double-blind trials and biomarker-based validation studies—are urgently required to confirm the clinical efficacy of natural bioactive compounds and meet regulatory approval requirements. Currently, several promising natural compounds (e.g., huperzine A derivatives, ginkgolide-related components) are at different stages of development, and systematic investigations are expected to accelerate the clinical translation of more AD candidate drugs with therapeutic potential.
In conclusion, natural products, by virtue of their multi-target mechanisms and relatively low toxicity, represent a promising avenue for AD therapy. Future studies should focus on systematic evaluations of long-term efficacy and safety, optimization of drug delivery strategies, and implementation of high-quality clinical trials, thereby laying a solid foundation for their eventual clinical translation.
Author contributions
XZ: Resources, Supervision, Writing – original draft. SH: Data curation, Writing – review and editing. HX: Validation, Writing – review and editing. YH: Project administration, Supervision, Validation, Writing – review and editing. LG: Funding acquisition, Methodology, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is funded by Natural Science Foundation of Jilin Province of China—General Project for Free Exploration (grant No. YDZJ202201ZYTS273) and Research Project of the Education Department of Jilin Province (grant No. JJKH20241049KJ).
Acknowledgments
We thank the Changchun University of Chinese Medicine for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1710820.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adabizadeh, M., Mehri, S., Rajabpour, M., Abnous, K., Rashedinia, M., and Hosseinzadeh, H. (2019). The effects of crocin on spatial memory impairment induced by hyoscine: role of NMDA, AMPA, ERK, and CaMKII proteins in rat hippocampus. Iran. J. basic Med. Sci. 22 (6), 601–609. doi:10.22038/ijbms.2019.30138.7266
Alzheimer, A. (1907). Uber eigenartige Erkrankung der Hirnrinde. All Z Psychiatr. 64, 146–148. doi:10.1002/ca.980080612
Angelucci, F., Cechova, K., Amlerova, J., and Hort, J. (2019). Antibiotics, gut microbiota, and Alzheimer’s disease. J. neuroinflammation 16, 108–110. doi:10.1186/s12974-019-1494-4
Bai, R., Guo, J., Ye, X.-Y., Xie, Y., and Xie, T. (2022). Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 77, 101619. doi:10.1016/j.arr.2022.101619
Bhattarai, N., Kumbhar, A. A., Pokharel, Y. R., and Yadav, P. N. (2021). Anticancer potential of coumarin and its derivatives. Mini Rev. Med. Chem. 21 (19), 2996–3029. doi:10.2174/1389557521666210405160323
Bondy, S. C. (2021). Metal toxicity and neuroinflammation. Curr. Opin. Toxicol. 26, 8–13. doi:10.1016/j.cotox.2021.03.008
Breuss, J. M., Atanasov, A. G., and Uhrin, P. (2019). Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 20 (7), 1523. doi:10.3390/ijms20071523
Carroll, J. C., Iba, M., Bangasser, D. A., Valentino, R. J., James, M. J., Brunden, K. R., et al. (2011). Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci. 31 (40), 14436–14449. doi:10.1523/JNEUROSCI.3836-11.2011
Cavaliere, G., Trinchese, G., Penna, E., Cimmino, F., Pirozzi, C., Lama, A., et al. (2019). High-fat diet induces neuroinflammation and mitochondrial impairment in mice cerebral cortex and synaptic fraction. Front. Cell. Neurosci. 13, 509. doi:10.3389/fncel.2019.00509
Chakrabarti, M., Haque, A., Banik, N. L., Nagarkatti, P., Nagarkatti, M., and Ray, S. K. (2014). Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res. Bull. 109, 22–31. doi:10.1016/j.brainresbull.2014.09.004
Chalatsa, I., Arvanitis, D. A., Koulakiotis, N. S., Giagini, A., Skaltsounis, A. L., Papadopoulou-Daifoti, Z., et al. (2019). The crocus sativus compounds trans-crocin 4 and trans-crocetin modulate the amyloidogenic pathway and tau misprocessing in alzheimer disease neuronal cell culture models. Front. Neurosci. 13, 249. doi:10.3389/fnins.2019.00249
Chen, J., Montanari, A. M., and Widmer, W. W. (1997). Two new polymethoxylated flavones, a class of compounds with potential anticancer activity, isolated from cold pressed dancy tangerine peel oil solids. J. Agric. food Chem. 45 (2), 364–368. doi:10.1021/jf960110i
Chen, T., Guo, Z.-p., Jiao, X.-y., Zhang, Y.-h., Li, J.-y., and Liu, H.-j. (2011). Protective effects of peoniflorin against hydrogen peroxide-induced oxidative stress in human umbilical vein endothelial cells. Can. J. physiology Pharmacol. 89 (6), 445–453. doi:10.1139/y11-034
Chen, X., Ji, N., Qin, N., Tang, S.-a., Wang, R., Qiu, Y., et al. (2017). 1, 6-O, O-Diacetylbritannilactone inhibits eotaxin-1 and ALOX15 expression through inactivation of STAT6 in A549 cells. Inflammation 40 (6), 1967–1974. doi:10.1007/s10753-017-0637-y
Chen, S.-R., Dai, Y., Zhao, J., Lin, L., Wang, Y., and Wang, Y. (2018). A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii hook F. Front. Pharmacol. 9, 104. doi:10.3389/fphar.2018.00104
Chen, C., Liao, J., Xia, Y., Liu, X., Jones, R., Haran, J., et al. (2022). Gut microbiota regulate Alzheimer’s disease pathologies and cognitive disorders via PUFA-Associated neuroinflammation. Gut 71 (11), 2233–2252. doi:10.1136/gutjnl-2021-326269
Chen, L., Wu, B., Mo, L., Chen, H., Yin, X., Zhao, Y., et al. (2025). High-content screening identifies ganoderic acid A as a senotherapeutic to prevent cellular senescence and extend healthspan in preclinical models. Nat. Commun. 16 (1), 2878. doi:10.1038/s41467-025-58188-5
Choi, Y. H. (2018). Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomed. and Pharmacother. 106, 902–909. doi:10.1016/j.biopha.2018.07.035
Choi, S.-Y., Hwang, J.-H., Ko, H.-C., Park, J.-G., and Kim, S.-J. (2007). Nobiletin from citrus fruit peel inhibits the DNA-Binding activity of NF-kappaB and ROS production in LPS-Activated RAW 264.7 cells. J. Ethnopharmacol. 113 (1), 149–155. doi:10.1016/j.jep.2007.05.021
Choi, Y., Kim, Y., Ham, H., Park, Y., Jeong, H.-S., and Lee, J. (2011). Nobiletin suppresses adipogenesis by regulating the expression of adipogenic transcription factors and the activation of AMP-Activated protein kinase (AMPK). J. Agric. food Chem. 59 (24), 12843–12849. doi:10.1021/jf2033208
Colombo, A. V., Sadler, R. K., Llovera, G., Singh, V., Roth, S., Heindl, S., et al. (2021). Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. elife 10, e59826. doi:10.7554/eLife.59826
Cryan, J. F., O'Riordan, K. J., Cowan, C. S., Sandhu, K. V., Bastiaanssen, T. F., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013. doi:10.1152/physrev.00018.2018
Cui, Y., Wu, J., Jung, S.-C., Park, D.-B., Maeng, Y.-H., Hong, J. Y., et al. (2010). Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 33 (11), 1814–1821. doi:10.1248/bpb.33.1814
Cui, J., Shen, Y., and Li, R. (2013). Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol. Med. 19 (3), 197–209. doi:10.1016/j.molmed.2012.12.007
Cui, Y.-Q., Wang, Q., Zhang, D.-M., Wang, J.-Y., Xiao, B., Zheng, Y., et al. (2016). Triptolide rescues spatial memory deficits and amyloid-β aggregation accompanied by inhibition of inflammatory responses and MAPKs activity in APP/PS1 transgenic mice. Curr. Alzheimer Res. 13 (3), 288–296. doi:10.2174/156720501303160217122803
Cui, L., Zhu, W., Yang, Z., Song, X., Xu, C., Cui, Z., et al. (2020). Evidence of anti-inflammatory activity of schizandrin A in animal models of acute inflammation. Naunyn-Schmiedeberg's archives Pharmacol. 393, 2221–2229. doi:10.1007/s00210-020-01837-x
D'Cruz, M. M., and Banerjee, D. (2021). The person is not the disease–revisiting Alzheimer's dementia after 120 years. J. Geriatric Ment. Health 8 (2), 136–137. doi:10.4103/jgmh.jgmh_39_21
Delogu, G. L., and Matos, M. J. (2017). Coumarins as promising scaffold for the treatment of age-related diseases - an overview of the last five years. Curr. Top. Med. Chem. 17 (29), 3173–3189. doi:10.2174/1568026618666171215094029
Ding, M. R., Qu, Y. J., Hu, B., and An, H. M. (2022). Signal pathways in the treatment of alzheimer's disease with traditional Chinese medicine. Biomed. Pharmacother. 152, 113208. doi:10.1016/j.biopha.2022.113208
Fakhri, S., Iranpanah, A., Gravandi, M. M., Moradi, S. Z., Ranjbari, M., Majnooni, M. B., et al. (2021). Natural products attenuate PI3K/Akt/mTOR signaling pathway: a promising strategy in regulating neurodegeneration. Phytomedicine 91, 153664. doi:10.1016/j.phymed.2021.153664
Fantacuzzi, M., Amoroso, R., Carradori, S., and De Filippis, B. (2022). Resveratrol-based compounds and neurodegeneration: recent insight in multitarget therapy. Eur. J. Med. Chem. 233, 114242. doi:10.1016/j.ejmech.2022.114242
Francis, P. T., Palmer, A. M., Snape, M., and Wilcock, G. K. (1999). The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurology, Neurosurg. and Psychiatry 66 (2), 137–147. doi:10.1136/jnnp.66.2.137
Fuller, S., Steele, M., and Münch, G. (2010). Activated astroglia during chronic inflammation in Alzheimer's Disease—Do they neglect their neurosupportive roles? Mutat. research/fundamental Mol. Mech. Mutagen. 690 (1-2), 40–49. doi:10.1016/j.mrfmmm.2009.08.016
Ganguli, M., Dodge, H. H., Shen, C., Pandav, R. S., and DeKosky, S. T. (2005). Alzheimer disease and mortality: a 15-year epidemiological study. Archives neurology 62 (5), 779–784. doi:10.1001/archneur.62.5.779
Gao, J., Zhang, Y., Liu, X., Wu, X., Huang, L., and Gao, W. (2021). Triptolide: pharmacological spectrum, biosynthesis, chemical synthesis and derivatives. Theranostics 11 (15), 7199–7221. doi:10.7150/thno.57745
George, M., Tharakan, M., Culberson, J., Reddy, A. P., and Reddy, P. H. (2022). Role of Nrf2 in aging, Alzheimer's and other neurodegenerative diseases. Ageing Res. Rev. 82, 101756. doi:10.1016/j.arr.2022.101756
Gu, X., Cai, Z., Cai, M., Liu, K., Liu, D., Zhang, Q., et al. (2016). Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of alzheimer's disease. Mol. Med. Rep. 13 (3), 2247–2252. doi:10.3892/mmr.2016.4805
Gu, L., Li, X., Chen, G., Yang, H., Qian, H., Pan, J., et al. (2025). A glutathione-activated bismuth-gallic acid metal-organic framework nano-prodrug for enhanced sonodynamic therapy of breast tumor. J. Colloid Interface Sci. 679, 214–223. doi:10.1016/j.jcis.2024.09.233
Guan, P.-P., Cao, L.-L., and Wang, P. (2021). Elevating the levels of calcium ions exacerbate Alzheimer’s disease via inducing the production and aggregation of β-amyloid protein and phosphorylated tau. Int. J. Mol. Sci. 22 (11), 5900. doi:10.3390/ijms22115900
Ha, Y., Jo, H.-S., Kwon, T. W., Jeon, S. H., Moon, S.-K., Jung, J. H., et al. (2025). Korean Black ginseng extract alleviates Alzheimer's disease-related cognitive impairment by activating the Nrf2/HO-1 pathway and suppressing the p38 MAPK/NF-κB/STAT3 pathways and NLRP3 inflammasome via TLR2 and TLR4 modulation. J. Ginseng Res. 49 (3), 294–305. doi:10.1016/j.jgr.2025.02.002
Haass, C., and Selkoe, D. J. (2007). Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat. Rev. Mol. cell Biol. 8 (2), 101–112. doi:10.1038/nrm2101
Hampel, H., Mesulam, M. M., Cuello, A. C., Farlow, M. R., Giacobini, E., Grossberg, G. T., et al. (2018). The cholinergic system in the pathophysiology and treatment of alzheimer's disease. Brain 141 (7), 1917–1933. doi:10.1093/brain/awy132
Harischandra, D. S., Ghaisas, S., Zenitsky, G., Jin, H., Kanthasamy, A., Anantharam, V., et al. (2019). Manganese-induced neurotoxicity: new insights into the triad of protein misfolding, mitochondrial impairment, and neuroinflammation. Front. Neurosci. 13, 654. doi:10.3389/fnins.2019.00654
Heidari, S., Mehri, S., and Hosseinzadeh, H. (2017). Memory enhancement and protective effects of crocin against D-galactose aging model in the hippocampus of wistar rats. Iran. J. Basic Med. Sci. 20 (11), 1250–1259. doi:10.22038/IJBMS.2017.9541
Hickman, S. E., Allison, E. K., and El Khoury, J. (2008). Microglial dysfunction and defective β-amyloid clearance pathways in aging alzheimer's disease mice. J. Neurosci. 28 (33), 8354–8360. doi:10.1523/JNEUROSCI.0616-08.2008
Hino, H., Takahashi, H., Suzuki, Y., Tanaka, J., Ishii, E., and Fukuda, M. (2012). Anticonvulsive effect of paeoniflorin on experimental febrile seizures in immature rats: possible application for febrile seizures in children. PLoS One 7, e42920. doi:10.1371/journal.pone.0042920
Hirata, Y., Masuda, Y., Kakutani, H., Higuchi, T., Takada, K., Ito, A., et al. (2008). Sp1 is an essential transcription factor for LPS-Induced tissue factor expression in THP-1 monocytic cells, and nobiletin represses the expression through inhibition of NF-kappaB, AP-1, and Sp1 activation. Biochem. Pharmacol. 75 (7), 1504–1514. doi:10.1016/j.bcp.2007.12.019
Ho, S.-C., and Kuo, C.-T. (2014). Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (citri reticulatae pericarpium). Food Chem. Toxicol. 71, 176–182. doi:10.1016/j.fct.2014.06.014
Hoozemans, J. J., Rozemuller, A. J., van Haastert, E. S., Eikelenboom, P., and van Gool, W. A. (2011). Neuroinflammation in alzheimer's disease wanes with age. J. neuroinflammation 8, 171–178. doi:10.1186/1742-2094-8-171
Hou, R. C.-W., Chen, H.-L., Tzen, J. T., and Jeng, K.-C. G. (2003). Effect of sesame antioxidants on LPS-Induced NO production by BV2 microglial cells. Neuroreport 14 (14), 1815–1819. doi:10.1097/00001756-200310060-00011
Hu, J.-F., Song, X.-Y., Chu, S.-F., Chen, J., Ji, H.-J., Chen, X.-Y., et al. (2011). Inhibitory effect of ginsenoside Rg1 on lipopolysaccharide-induced microglial activation in mice. Brain Res. 1374, 8–14. doi:10.1016/j.brainres.2010.11.069
Jayaraj, P., Narasimhulu, C. A., Rajagopalan, S., Parthasarathy, S., and Desikan, R. (2020). Sesamol: a powerful functional food ingredient from sesame oil for cardioprotection. Food and Funct. 11 (2), 1198–1210. doi:10.1039/c9fo01873e
Jeong, M. J., Kim, S. R., and Jung, U. J. (2019). Schizandrin A supplementation improves nonalcoholic fatty liver disease in mice fed a high-fat and high-cholesterol diet. Nutr. Res. 64, 64–71. doi:10.1016/j.nutres.2019.01.001
Jia, L., Liu, J., Song, Z., Pan, X., Chen, L., Cui, X., et al. (2012). Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 64 (10), 1510–1521. doi:10.1111/j.2042-7158.2012.01529.x
Jia, S., Guan, H., Zhang, S., and Li, Q. (2024). Schisandrin A alleviates inflammation and oxidative stress in Aβ25-35-Induced alzheimer’s disease in vitro model. Actas Españolas Psiquiatr. 52 (5), 724–732. doi:10.62641/aep.v52i5.1680
Jiang, Z., Qiu, H., Wang, S., Guo, J., Yang, Z., and Zhou, S. (2018). Ganoderic acid A potentiates the antioxidant effect and protection of mitochondrial membranes and reduces the apoptosis rate in primary hippocampal neurons in magnesium free medium. Die Pharmazie-An Int. J. Pharm. Sci. 73 (2), 87–91. doi:10.1691/ph.2018.7108
Justice, N. J. (2018). The relationship between stress and alzheimer's disease. Neurobiol. stress 8, 127–133. doi:10.1016/j.ynstr.2018.04.002
Kanda, K., Nishi, K., Kadota, A., Nishimoto, S., Liu, M.-C., and Sugahara, T. (2012). Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochimica Biophysica Acta (BBA)-General Subj. 1820 (4), 461–468. doi:10.1016/j.bbagen.2011.11.015
Kang, K. A., Wang, Z. H., Zhang, R., Piao, M. J., Kim, K. C., Kang, S. S., et al. (2010). Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int. J. Mol. Sci. 11 (11), 4348–4360. doi:10.3390/ijms11114348
Kettmann, V., Košťálová, D., Jantova, S., and Čerňáková, M. (2004). In vitro cytotoxicity of berberine against HeLa and L1210 cancer cell lines. Die Pharmazie-An Int. J. Pharm. Sci. 59 (7), 548–551. Available online at: https://pubmed.ncbi.nlm.nih.gov/15296093/.
Khan, H., Ullah, H., Aschner, M., Cheang, W. S., and Akkol, E. K. (2019). Neuroprotective effects of quercetin in alzheimer's disease. Biomolecules 10 (1), 59. doi:10.3390/biom10010059
Kheiri, G., Dolatshahi, M., Rahmani, F., and Rezaei, N. (2018). Role of p38/MAPKs in Alzheimer’s disease: implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 30 (1), 9–30. doi:10.1515/revneuro-2018-0008
Khorasany, A. R., and Hosseinzadeh, H. (2016). Therapeutic effects of saffron (crocus sativus L.) in digestive disorders: a review. Iran. J. basic Med. Sci. 19 (5), 455–469. Available online at: https://pubmed.ncbi.nlm.nih.gov/27403251/.
Kim, J. H., Jeong, M. S., Kim, D. Y., Her, S., and Wie, M. B. (2015). Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 90, 204–214. doi:10.1016/j.neuint.2015.09.002
Kim, K.-W., Lee, Y.-S., Choi, B.-R., Yoon, D., and Lee, D. Y. (2023). Anti-neuroinflammatory effect of the ethanolic extract of Black ginseng through TLR4-MyD88-regulated inhibition of NF-κB and MAPK signaling pathways in LPS-Induced BV2 microglial cells. Int. J. Mol. Sci. 24 (20), 15320. doi:10.3390/ijms242015320
Kinney, J. W., Bemiller, S. M., Murtishaw, A. S., Leisgang, A. M., Salazar, A. M., and Lamb, B. T. (2018). Inflammation as a central mechanism in alzheimer's disease. Alzheimer's and Dementia Transl. Res. and Clin. Interventions 4, 575–590. doi:10.1016/j.trci.2018.06.014
Kirsch, G., Abdelwahab, A. B., and Chaimbault, P. (2016). Natural and synthetic coumarins with effects on inflammation. Molecules 21 (10), 1322. doi:10.3390/molecules21101322
Kloske, C. M., and Wilcock, D. M. (2020). The important interface between apolipoprotein E and neuroinflammation in Alzheimer’s disease. Front. Immunol. 11, 754. doi:10.3389/fimmu.2020.00754
Kodali, M., Parihar, V. K., Hattiangady, B., Mishra, V., Shuai, B., and Shetty, A. K. (2015). Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature and reduced glial activation. Sci. Rep. 5 (1), 8075. doi:10.1038/srep08075
Kong, D., Zhang, D., Chu, X., and Wang, J. (2018). RETRACTED: schizandrin A enhances chemosensitivity of Colon carcinoma cells to 5-fluorouracil through up-regulation of miR-195. Elsevier.
Koo, H. S., Kang, S. D., Lee, J. H., Kim, N.-H., Chung, H.-T., and Pae, H.-O. (2009). Triptolide inhibits the proliferation of immortalized HT22 hippocampal cells via persistent activation of extracellular signal-regulated kinase-1/2 by down-regulating mitogen-activated protein kinase phosphatase-1 expression. J. Korean Neurosurg. Soc. 46 (4), 389–396. doi:10.3340/jkns.2009.46.4.389
Kumar, P. P., Madhuri, D., Reddy, L. S. S., Reddy, Y. D., Somasekhar, G., Sirisha, N., et al. (2021). A new cerebral ischemic injury model in rats, preventive effect of gallic acid and in silico approaches. Saudi J. Biol. Sci. 28 (9), 5204–5213. doi:10.1016/j.sjbs.2021.05.044
Küpeli, E., Koşar, M., Yeşilada, E., Başer, K. H. C., and Başer, C. (2002). A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish berberis species. Life Sci. 72 (6), 645–657. doi:10.1016/s0024-3205(02)02200-2
Kwon, H. S., and Koh, S.-H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 9 (1), 42. doi:10.1186/s40035-020-00221-2
Kwon, D. H., Cha, H.-J., Choi, E. O., Leem, S.-H., Kim, G.-Y., Moon, S.-K., et al. (2018). Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 41 (1), 264–274. doi:10.3892/ijmm.2017.3209
Lam, K. H., Alex, D., Lam, I. K., Tsui, S. K. W., Yang, Z. F., and Lee, S. M. Y. (2011). Nobiletin, a polymethoxylated flavonoid from citrus, shows anti-angiogenic activity in a zebrafish in vivo model and HUVEC in vitro model. J. Cell. Biochem. 112 (11), 3313–3321. doi:10.1002/jcb.23257
Latha, R. C. R., and Daisy, P. (2011). Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from terminalia bellerica roxb. In streptozotocin-induced diabetic rats. Chemico-biological Interact. 189 (1-2), 112–118. doi:10.1016/j.cbi.2010.11.005
Latimer, C. S., Lucot, K. L., Keene, C. D., Cholerton, B., and Montine, T. J. (2021). Genetic insights into alzheimer's disease. Annu. Rev. Pathology Mech. Dis. 16 (1), 351–376. doi:10.1146/annurev-pathmechdis-012419-032551
Laurent, C., Buée, L., and Blum, D. (2018). Tau and neuroinflammation: what impact for alzheimer's disease and tauopathies? Biomed. J. 41 (1), 21–33. doi:10.1016/j.bj.2018.01.003
Le Tran, Q., Tezuka, Y., Ueda, J.-y., Nguyen, N. T., Maruyama, Y., Begum, K., et al. (2003). In vitro antiplasmodial activity of antimalarial medicinal plants used in Vietnamese traditional medicine. J. Ethnopharmacol. 86 (2-3), 249–252. doi:10.1016/s0378-8741(03)00045-x
Lee, J. K., and Kim, N.-J. (2017). Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 22 (8), 1287. doi:10.3390/molecules22081287
Lee, Y.-S., Cha, B.-Y., Saito, K., Yamakawa, H., Choi, S.-S., Yamaguchi, K., et al. (2010). Nobiletin improves hyperglycemia and insulin resistance in Obese diabetic Ob/Ob mice. Biochem. Pharmacol. 79 (11), 1674–1683. doi:10.1016/j.bcp.2010.01.034
Lee, Y.-C., Cheng, T.-H., Lee, J.-S., Chen, J.-H., Liao, Y.-C., Fong, Y., et al. (2011). Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol. Cell. Biochem. 347, 103–115. doi:10.1007/s11010-010-0618-z
Lee, I.-A., Hyam, S. R., Jang, S.-E., Han, M. J., and Kim, D.-H. (2012a). Ginsenoside re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J. Agric. food Chem. 60 (38), 9595–9602. doi:10.1021/jf301372g
Lee, K.-W., Jung, S. Y., Choi, S.-M., and Yang, E. J. (2012b). Effects of ginsenoside re on LPS-Induced inflammatory mediators in BV2 microglial cells. BMC complementary Altern. Med. 12, 196–198. doi:10.1186/1472-6882-12-196
Lee, J. H., Jiang, Y., Han, D. H., Shin, S. K., Choi, W. H., and Lee, M. J. (2014). Targeting estrogen receptors for the treatment of Alzheimer’s disease. Mol. Neurobiol. 49, 39–49. doi:10.1007/s12035-013-8484-9
Lee, G. H., Lee, W. J., Hur, J., Kim, E., Lee, H. G., and Seo, H. G. (2020). Ginsenoside re mitigates 6-hydroxydopamine-induced oxidative stress through upregulation of GPX4. Molecules 25 (1), 188. doi:10.3390/molecules25010188
Leng, F., and Edison, P. (2021). Neuroinflammation and microglial activation in alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17 (3), 157–172. doi:10.1038/s41582-020-00435-y
Lesuis, S. L., Weggen, S., Baches, S., Lucassen, P. J., and Krugers, H. J. (2018). Targeting glucocorticoid receptors prevents the effects of early life stress on amyloid pathology and cognitive performance in APP/PS1 mice. Transl. psychiatry 8 (1), 53. doi:10.1038/s41398-018-0101-2
Letašiová, S., Jantová, S., Lu, Č., and Múčková, M. (2006). Berberine—Antiproliferative activity in vitro and induction of apoptosis/necrosis of the U937 and B16 cells. Cancer Lett. 239 (2), 254–262. doi:10.1016/j.canlet.2005.08.024
Li, X.-m., Zhou, M.-t., Wang, X.-m., Ji, M.-h., Zhou, Z.-q., and Yang, J.-j. (2014). Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti-inflammation and-apoptosis actions in aged mice. J. Mol. Neurosci. 52, 286–293. doi:10.1007/s12031-013-0141-2
Li, H., Yao, Y., and Li, L. (2017). Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 69 (10), 1253–1264. doi:10.1111/jphp.12774
Li, W., Wang, D., and Wang, D. (2018). Regulation of the response of Caenorhabditis elegans to simulated microgravity by p38 mitogen-activated protein kinase signaling. Sci. Rep. 8 (1), 857. doi:10.1038/s41598-018-19377-z
Li, Y., Wang, L., Wang, P., Fan, C., Zhang, P., Shen, J., et al. (2020). Ginsenoside-Rg1 rescues stress-induced depression-like behaviors via suppression of oxidative stress and neural inflammation in rats. Oxidative Med. Cell. Longev. (2020) 2020 (1), 2325391. doi:10.1155/2020/2325391
Liao, H., Ye, J., Gao, L., and Liu, Y. (2021). The main bioactive compounds of Scutellaria baicalensis georgi. For alleviation of inflammatory cytokines: a comprehensive review. Biomed. and Pharmacother. 133, 110917. doi:10.1016/j.biopha.2020.110917
Lin, Y.-T., Shi, Q.-Q., Zhang, L., Yue, C.-P., He, Z.-J., Li, X.-X., et al. (2022). Hydrogen-rich water ameliorates neuropathological impairments in a mouse model of alzheimer's disease through reducing neuroinflammation and modulating intestinal microbiota. Neural Regen. Res. 17 (2), 409–417. doi:10.4103/1673-5374.317992
Liu, J., Li, H., Gong, T., Chen, W., Mao, S., Kong, Y., et al. (2020a). Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer’s disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 68 (27), 7152–7161. doi:10.1021/acs.jafc.0c02807
Liu, T., Yu, S., Xu, Z., Tan, J., Wang, B., Liu, Y.-G., et al. (2020b). Prospects and progress on crocin biosynthetic pathway and metabolic engineering. Comput. Struct. Biotechnol. J. 18, 3278–3286. doi:10.1016/j.csbj.2020.10.019
Liu, P., Zhou, Y., Shi, J., Wang, F., Yang, X., Zheng, X., et al. (2023). Myricetin improves pathological changes in 3× Tg-AD mice by regulating the mitochondria-NLRP3 inflammasome-microglia channel by targeting P38 MAPK signaling pathway. Phytomedicine 115, 154801. doi:10.1016/j.phymed.2023.154801
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. lancet 390 (10113), 2673–2734. doi:10.1016/S0140-6736(17)31363-6
Lixin, X., Lijun, Y., and Songping, H. (2019). Ganoderic acid A against cyclophosphamide-induced hepatic toxicity in mice. J. Biochem. Mol. Toxicol. 33 (4), e22271. doi:10.1002/jbt.22271
Long, H. Z., Cheng, Y., Zhou, Z. W., Luo, H. Y., Wen, D. D., and Gao, L. C. (2021). PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of alzheimer's disease and parkinson's disease. Front. Pharmacol. 12, 648636. doi:10.3389/fphar.2021.648636
Looti Bashiyan, M., Nasehi, M., Vaseghi, S., and Khalifeh, S. (2021). Investigating the effect of crocin on memory deficits induced by total sleep deprivation (TSD) with respect to the BDNF, TrkB and ERK levels in the hippocampus of Male wistar rats. J. Psychopharmacol. 35 (6), 744–754. doi:10.1177/02698811211000762
Ma, L., Xiao, P., Guo, B., Wu, J., Liang, F., and Dong, S. (1999). Cerebral protective effects of some compounds isolated from traditional Chinese herbs. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China J. Chin. materia medica 24 (4), 238–239. Available online at: https://pubmed.ncbi.nlm.nih.gov/12205950/.
Madhi, I., Kim, J.-H., Shin, J. E., and Kim, Y. (2021). Ginsenoside re exhibits neuroprotective effects by inhibiting neuroinflammation via CAMK/MAPK/NF-κB signaling in microglia. Mol. Med. Rep. 24 (4), 698. doi:10.3892/mmr.2021.12337
Maioli, S., Leander, K., Nilsson, P., and Nalvarte, I. (2021). Estrogen receptors and the aging brain. Essays Biochem. 65 (6), 913–925. doi:10.1042/EBC20200162
McGrattan, A. M., McGuinness, B., McKinley, M. C., Kee, F., Passmore, P., Woodside, J. V., et al. (2019). Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 8, 53–65. doi:10.1007/s13668-019-0271-4
Meng, X.-L., Zhu, Z.-X., Lu, R.-H., Li, S., Hu, W.-P., Qin, C.-B., et al. (2019). Regulation of growth performance and lipid metabolism in juvenile grass carp (Ctenopharyngodon idella) with honeysuckle (Lonicera japonica) extract. Fish Physiology Biochem. 45, 1563–1573. doi:10.1007/s10695-019-00644-3
Meng, J., Wang, S.-Z., He, J.-z., Zhu, S., Huang, B.-y., Wang, S.-y., et al. (2020). Ganoderic acid A is the effective ingredient of ganoderma triterpenes in retarding renal cyst development in polycystic kidney disease. Acta Pharmacol. Sin. 41 (6), 782–790. doi:10.1038/s41401-019-0329-2
Metwaly, A. M., Lianlian, Z., Luqi, H., and Deqiang, D. (2019). Black ginseng and its saponins: preparation, phytochemistry and pharmacological effects. Molecules 24 (10), 1856. doi:10.3390/molecules24101856
Miyamoto, S., Yasui, Y., Tanaka, T., Ohigashi, H., and Murakami, A. (2008). Suppressive effects of nobiletin on hyperleptinemia and colitis-related Colon carcinogenesis in Male ICR mice. Carcinogenesis 29 (5), 1057–1063. doi:10.1093/carcin/bgn080
Mohamed, E. A., Ahmed, H. I., Zaky, H. S., and Badr, A. M. (2021). Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of alzheimer's disease. A pivotal role of NF-κB/p38MAPK/BDNF/PPAR-γ pathways. J. Ethnopharmacol. 267, 113468. doi:10.1016/j.jep.2020.113468
Moser, V. A., and Pike, C. J. (2016). Obesity and sex interact in the regulation of alzheimer's disease. Neurosci. and Biobehav. Rev. 67, 102–118. doi:10.1016/j.neubiorev.2015.08.021
Mulvihill, E. E., Assini, J. M., Lee, J. K., Allister, E. M., Sutherland, B. G., Koppes, J. B., et al. (2011). Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 60 (5), 1446–1457. doi:10.2337/db10-0589
Najmanová, I., Doseděl, M., Hrdina, R., Anzenbacher, P., Filipský, T., Říha, M., et al. (2015). Cardiovascular effects of coumarins besides their antioxidant activity. Curr. Top. Med. Chem. 15 (9), 830–849. doi:10.2174/1568026615666150220112437
Newcombe, E. A., Camats-Perna, J., Silva, M. L., Valmas, N., Huat, T. J., and Medeiros, R. (2018). Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J. neuroinflammation 15, 276–26. doi:10.1186/s12974-018-1313-3
Nogata, Y., Sakamoto, K., Shiratsuchi, H., Ishii, T., Yano, M., and Ohta, H. (2006). Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 70 (1), 178–192. doi:10.1271/bbb.70.178
Nuzzo, D., Picone, P., Baldassano, S., Caruana, L., Messina, E., Marino Gammazza, A., et al. (2015). Insulin resistance as common molecular denominator linking obesity to Alzheimer’s disease. Curr. Alzheimer Res. 12 (8), 723–735. doi:10.2174/1567205012666150710115506
Orioli, R., Belluti, F., Gobbi, S., Rampa, A., and Bisi, A. (2024). Naturally inspired coumarin derivatives in alzheimer's disease drug discovery: latest advances and current challenges. Molecules 29 (15), 3514. doi:10.3390/molecules29153514
Pinto, D., and Silva, A. M. S. (2017). Anticancer natural coumarins as lead compounds for the discovery of new drugs. Curr. Top. Med. Chem. 17 (29), 3190–3198. doi:10.2174/1568026618666171215095750
Pisani, L., Catto, M., Muncipinto, G., Nicolotti, O., Carrieri, A., Rullo, M., et al. (2022). A twenty-year journey exploring coumarin-based derivatives as bioactive molecules. Front. Chem. 10, 1002547. doi:10.3389/fchem.2022.1002547
Prince, M., Albanese, E., Guerchet, M., and Prina, M. (2014). Dementia and risk reduction: an analysis of protective and modifiable factors. World Alzheimer Rep., 66–83. Available online at: https://kclpure.kcl.ac.uk/portal/en/publications/world-alzheimer-report-2014-dementia-and-risk-reduction-an-analys#:∼:text=World%20Alzheimer%20Report%202014%3A%20Dementia%20and%20risk%20reduction%3A,and%20modifiable%20risk%20factors.%20Alzheimer%27s%20Disease%20International.%20http%3A%2F%2Fwww.alz.co.uk%2Fresearch%2Fworld-report-2014.
Pruccoli, L., Morroni, F., Sita, G., Hrelia, P., and Tarozzi, A. (2020). Esculetin as a bifunctional antioxidant prevents and counteracts the oxidative stress and neuronal death induced by amyloid protein in SH-SY5Y cells. Antioxidants (Basel) 9 (6), 551. doi:10.3390/antiox9060551
Puranik, N., and Song, M. (2024). Glutamate: molecular mechanisms and signaling pathway in alzheimer's disease, a potential therapeutic target. Molecules 29 (23), 5744. doi:10.3390/molecules29235744
Qi, G., Mi, Y., Fan, R., Li, R., Liu, Z., and Liu, X. (2019). Nobiletin protects against systemic inflammation-stimulated memory impairment via MAPK and NF-κB signaling pathways. J. Agric. food Chem. 67 (18), 5122–5134. doi:10.1021/acs.jafc.9b00133
Qi, L.-F.-R., Liu, S., Liu, Y.-C., Li, P., and Xu, X. (2021). Ganoderic acid a promotes amyloid-β clearance (in vitro) and ameliorates cognitive deficiency in Alzheimer’s disease (Mouse model) through autophagy induced by activating axl. Int. J. Mol. Sci. 22 (11), 5559. doi:10.3390/ijms22115559
Qiu, F.-M., Zhong, X.-M., Mao, Q.-Q., and Huang, Z. (2013). Antidepressant-like effects of paeoniflorin on the behavioural, biochemical, and neurochemical patterns of rats exposed to chronic unpredictable stress. Neurosci. Lett. 541, 209–213. doi:10.1016/j.neulet.2013.02.029
Račková, L., Májeková, M., Košt’álová, D., and Štefek, M. (2004). Antiradical and antioxidant activities of alkaloids isolated from mahonia aquifolium. Structural aspects. Bioorg. and Med. Chem. 12 (17), 4709–4715. doi:10.1016/j.bmc.2004.06.035
Ramezani, M., Darbandi, N., Khodagholi, F., and Hashemi, A. (2016). Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with alzheimer's disease. Neural Regen. Res. 11 (12), 1976–1980. doi:10.4103/1673-5374.197141
Rashedinia, M., Lari, P., Abnous, K., and Hosseinzadeh, H. (2015). Protective effect of crocin on acrolein-induced tau phosphorylation in the rat brain. Acta neurobiol. Exp. 75 (2), 208–219. doi:10.55782/ane-2015-2029
Sebastián-Serrano, Á., de Diego-García, L., and Díaz-Hernández, M. (2018). The neurotoxic role of extracellular tau protein. Int. J. Mol. Sci. 19 (4), 998. doi:10.3390/ijms19040998
Shao, N., Lu, Q., Ouyang, Z., Yang, P., Wei, T., Wang, J., et al. (2025). Ganoderic acid a alleviates Aβ25− 35-induced HT22 cell apoptosis through the ERK/MAPK pathway: a system pharmacology and in vitro experimental validation. Metab. Brain Dis. 40 (1), 51–16. doi:10.1007/s11011-024-01429-1
She, L., Sun, J., Xiong, L., Li, A., Li, L., Wu, H., et al. (2024). Ginsenoside RK1 improves cognitive impairments and pathological changes in alzheimer's disease via stimulation of the AMPK/Nrf2 signaling pathway. Phytomedicine 122, 155168. doi:10.1016/j.phymed.2023.155168
Sheikhpour, E., Mard, S. A., Farbood, Y., Bavarsad, K., and Sarkaki, A. (2023). The effects of gallic acid and vagotomy on motor function, intestinal transit, brain electrophysiology and oxidative stress alterations in a rat model of parkinson's disease induced by rotenone. Life Sci. 315, 121356. doi:10.1016/j.lfs.2022.121356
Shi, Z.-Y., Zeng, J.-Z., and Wong, A. S. T. (2019). Chemical structures and pharmacological profiles of ginseng saponins. Molecules 24 (13), 2443. doi:10.3390/molecules24132443
Shi, J., Zhu, T., Lin, H., Liu, Z., Zhou, M., Yu, Z., et al. (2022). Proteotranscriptomics of ocular adnexal B-cell lymphoma reveals an oncogenic role of alternative splicing and identifies a diagnostic marker. J. Exp. and Clin. Cancer Res. 41 (1), 234. doi:10.1186/s13046-022-02445-8
Siddiqui, N., Talib, M., Tripathi, P. N., Kumar, A., and Sharma, A. (2024a). Therapeutic potential of baicalein against neurodegenerative diseases: an updated review. Health Sci. Rev. 11, 100172. doi:10.1016/j.hsr.2024.100172
Siddiqui, N., Saifi, A., Chaudhary, A., Tripathi, P. N., Chaudhary, A., and Sharma, A. (2024b). Multifaceted neuroprotective role of punicalagin: a review. Neurochem. Res. 49 (6), 1427–1436. doi:10.1007/s11064-023-04081-w
Singh, D. (2022). Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. neuroinflammation 19 (1), 206. doi:10.1186/s12974-022-02565-0
Singh, A., and Kumar Singh, N. (2024). Pre-clinical evidence-based neuroprotective potential of naringin against alzheimer's disease-like pathology: a comprehensive review. Curr. Pharm. Biotechnol. 25 (9), 1112–1123. doi:10.2174/1389201024666230801095526
Song, F., Zeng, K., Liao, L., Yu, Q., Tu, P., and Wang, X. (2016). Schizandrin A inhibits microglia-mediated neuroninflammation through inhibiting TRAF6-NF-κB and Jak2-Stat3 signaling pathways. PLoS One 11 (2), e0149991. doi:10.1371/journal.pone.0149991
Song, Y., Wang, X., Qin, S., Zhou, S., Li, J., and Gao, Y. (2018). Esculin ameliorates cognitive impairment in experimental diabetic nephropathy and induces anti-oxidative stress and anti-inflammatory effects via the MAPK pathway. Mol. Med. Rep. 17 (5), 7395–7402. doi:10.3892/mmr.2018.8727
Song, T., Song, X., Zhu, C., Patrick, R., Skurla, M., Santangelo, I., et al. (2021). Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: a meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res. Rev. 72, 101503. doi:10.1016/j.arr.2021.101503
Sun, X.-C., Ren, X.-F., Chen, L., Gao, X.-Q., Xie, J.-X., and Chen, W.-F. (2016). Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J. Steroid Biochem. Mol. Biol. 155, 94–103. doi:10.1016/j.jsbmb.2015.09.040
Sweeney, M. D., Sagare, A. P., and Zlokovic, B. V. (2018). Blood–brain barrier breakdown in alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14 (3), 133–150. doi:10.1038/nrneurol.2017.188
Taheri, Y., Suleria, H. A. R., Martins, N., Sytar, O., Beyatli, A., Yeskaliyeva, B., et al. (2020). Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications. BMC complementary Med. Ther. 20, 241–14. doi:10.1186/s12906-020-03033-z
Tanaka, S., Sato, T., Akimoto, N., Yano, M., and Ito, A. (2004). Prevention of UVB-Induced photoinflammation and photoaging by a polymethoxy flavonoid, nobiletin, in human keratinocytes in vivo and in vitro. Biochem. Pharmacol. 68 (3), 433–439. doi:10.1016/j.bcp.2004.04.006
Tang, L.-L., Wang, R., and Tang, X.-C. (2005). Huperzine A protects SHSY5Y neuroblastoma cells against oxidative stress damage via nerve growth factor production. Eur. J. Pharmacol. 519 (1-2), 9–15. doi:10.1016/j.ejphar.2005.06.026
Tang, J.-J., Huang, L.-F., Deng, J.-L., Wang, Y.-M., Guo, C., Peng, X.-N., et al. (2022). Cognitive enhancement and neuroprotective effects of OABL, a sesquiterpene lactone in 5xFAD alzheimer's disease mice model. Redox Biol. 50, 102229. doi:10.1016/j.redox.2022.102229
Tecalco-Cruz, A. C., Zepeda–Cervantes, J., and Ortega-Domínguez, B. (2021). Estrogenic hormones receptors in Alzheimer’s disease. Mol. Biol. Rep. 48 (11), 7517–7526. doi:10.1007/s11033-021-06792-1
Teleanu, D. M., Niculescu, A.-G., Lungu, I. I., Radu, C. I., Vladâcenco, O., Roza, E., et al. (2022). An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 23 (11), 5938. doi:10.3390/ijms23115938
Temviriyanukul, P., Chansawhang, A., Karinchai, J., Phochantachinda, S., Buranasinsup, S., Inthachat, W., et al. (2023). Kaempferia parviflora extracts protect neural stem cells from amyloid peptide-mediated inflammation in co-culture model with microglia. Nutrients 15 (5), 1098. doi:10.3390/nu15051098
Tong, L., Zhao, Q., Datan, E., Lin, G.-Q., Minn, I., Pomper, M. G., et al. (2021). Triptolide: reflections on two decades of research and prospects for the future. Nat. Product. Rep. 38 (4), 843–860. doi:10.1039/d0np00054j
Toth, P., Tarantini, S., Tucsek, Z., Ashpole, N. M., Sosnowska, D., Gautam, T., et al. (2014). Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am. J. Physiology-Heart Circulatory Physiology 306 (3), H299–H308. doi:10.1152/ajpheart.00744.2013
Uddin, M. S., Kabir, M. T., Al Mamun, A., Barreto, G. E., Rashid, M., Perveen, A., et al. (2020). Pharmacological approaches to mitigate neuroinflammation in Alzheimer’s disease. Int. Immunopharmacol. 84, 106479. doi:10.1016/j.intimp.2020.106479
Vestergaard, M., and Ingmer, H. (2019). Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. agents 53 (6), 716–723. doi:10.1016/j.ijantimicag.2019.02.015
Vogt, N. M., Kerby, R. L., Dill-McFarland, K. A., Harding, S. J., Merluzzi, A. P., Johnson, S. C., et al. (2017). Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7 (1), 13537. doi:10.1038/s41598-017-13601-y
Vos, T., Allen, C., Arora, M., and Barber, R. (2017). A systematic analysis for the global burden of disease study 2016. Lancet 390 (2017), 1151–1210. doi:10.1016/S0140-6736(17)32154-2
Walters, A., Phillips, E., Zheng, R., Biju, M., and Kuruvilla, T. (2016). Evidence for neuroinflammation in alzheimer's disease. Prog. Neurology Psychiatry 20 (5), 25–31. doi:10.1002/pnp.444
Wan, B., Li, Y., Sun, S., Yang, Y., Lv, Y., Wang, L., et al. (2019). Ganoderic acid A attenuates lipopolysaccharide-induced lung injury in mice. Biosci. Rep. 39 (5), BSR20190301. doi:10.1042/BSR20190301
Wang, J., Wang, Q., Tu, X., Yang, R., Hu, J., Kang, J., et al. (2025). Gallic acid alleviates alzheimer's disease by inhibiting p38/MAPK signaling pathway. J. Funct. Foods 127, 106745. doi:10.1016/j.jff.2025.106745
Wang, J., and Zhang, Y. (2018). Neuroprotective effect of berberine agonist against impairment of learning and memory skills in severe traumatic brain injury via Sirt1/p38 MAPK expression. Mol. Med. Rep. 17 (5), 6881–6886. doi:10.3892/mmr.2018.8674
Wang, R., Yan, H., and Tang, Xc (2006). Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 27 (1), 1–26. doi:10.1111/j.1745-7254.2006.00255.x
Wang, Y., Chai, X., Han, M., and Wen, J. (2008). Inhibition of 1-O-acetylbritannilactone on expression of COX-2 and NF-kappa B in rats hippocampus with AD. Chin. Pharmacol. Bull. 24 (4), 437. Available online at: https://www.semanticscholar.org/paper/Inhibition-of-l-O-acetylbritannilactone-on-of-COX-2-Jin-kun/6751c68d2154fa438e7b4d9e2d7415fdd4f106a7.
Wang, K., Zhang, Y., Ekunwe, S. I. N., Yi, X. H., Liu, X. X., Wang, H. S., et al. (2011). Antioxidant activity and inhibition effect on the growth of human Colon carcinoma (HT-29) cells of esculetin from cortex fraxini. Med. Chem. Res. 20 (7), 968–974. doi:10.1007/s00044-010-9426-y
Wang, C.-P., Li, G.-C., Shi, Y.-W., Zhang, X.-C., Li, J.-L., Wang, Z.-W., et al. (2014). Neuroprotective effect of schizandrin A on oxygen and glucose deprivation/reperfusion-induced cell injury in primary culture of rat cortical neurons. J. Physiol. Biochem. 70, 735–747. doi:10.1007/s13105-014-0342-3
Wang, B., Zhong, Y., Gao, C., and Li, J. (2017). Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophysical Res. Commun. 490 (2), 336–342. doi:10.1016/j.bbrc.2017.06.045
Wang, X., Sun, G., Feng, T., Zhang, J., Huang, X., Wang, T., et al. (2019a). Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 29 (10), 787–803. doi:10.1038/s41422-019-0216-x
Wang, Y., Zang, W., Ji, S., Cao, J., and Sun, C. (2019b). Three polymethoxyflavones purified from ougan (citrus reticulata cv. Suavissima) inhibited LPS-Induced NO elevation in the neuroglia BV-2 cell line via the JAK2/STAT3 pathway. Nutrients 11 (4), 791. doi:10.3390/nu11040791
Wang, M.-R., Huang, L.-F., Guo, C., Yang, J., Dong, S., Tang, J.-J., et al. (2022). Identification of NLRP3 as a covalent target of 1, 6-O, O-diacetylbritannilactone against neuroinflammation by quantitative thiol reactivity profiling (QTRP). Bioorg. Chem. 119, 105536. doi:10.1016/j.bioorg.2021.105536
Wen, X., and Hu, J. (2024). Targeting STAT3 signaling pathway in the treatment of alzheimer's disease with compounds from natural products. Int. Immunopharmacol. 141, 112936. doi:10.1016/j.intimp.2024.112936
Wu, X.-L., Liou, C.-J., Li, Z.-Y., Lai, X.-Y., Fang, L.-W., and Huang, W.-C. (2015). Sesamol suppresses the inflammatory response by inhibiting NF-κB/MAPK activation and upregulating AMP kinase signaling in RAW 264.7 macrophages. Inflamm. Res. 64, 577–588. doi:10.1007/s00011-015-0836-7
Yang, Y., Zhou, H., Liu, W., Wu, J., Yue, X., Wang, J., et al. (2018). Ganoderic acid A exerts antitumor activity against MDA-MB-231 human breast cancer cells by inhibiting the janus kinase 2/signal transducer and activator of transcription 3 signaling pathway. Oncol. Lett. 16 (5), 6515–6521. doi:10.3892/ol.2018.9475
Ye, M., Fu, S., Pi, R., and He, F. (2009). Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. J. Pharm. Pharmacol. 61 (7), 831–837. doi:10.1211/jpp/61.07.0001
Ye, S., Mao, B., Yang, L., Fu, W., and Hou, J. (2016). Thrombosis recanalization by paeoniflorin through the upregulation of urokinase-type plasminogen activator via the MAPK signaling pathway. Mol. Med. Rep. 13 (6), 4593–4598. doi:10.3892/mmr.2016.5146
Ye, X., Shao, S., Wang, Y., and Su, W. (2023). Ginsenoside Rg2 alleviates neurovascular damage in 3xTg-AD mice with alzheimer's disease through the MAPK-ERK pathway. J. Chem. Neuroanat. 133, 102346. doi:10.1016/j.jchemneu.2023.102346
Yiannopoulou, K. G., and Papageorgiou, S. G. (2020). Current and future treatments in alzheimer disease: an update. J. central Nerv. Syst. Dis. 12, 1179573520907397. doi:10.1177/1179573520907397
Yoshimizu, N., Otani, Y., Saikawa, Y., Kubota, T., Yoshida, M., Furukawa, T., et al. (2004). Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: antiproliferative effects, induction of apoptosis and cell cycle deregulation. Alimentary Pharmacol. and Ther. 20, 95–101. doi:10.1111/j.1365-2036.2004.02082.x
Yu, Q., Wang, Y., Du, F., Yan, S., Hu, G., Origlia, N., et al. (2018). Overexpression of endophilin A1 exacerbates synaptic alterations in a mouse model of Alzheimer’s disease. Nat. Commun. 9 (1), 2968. doi:10.1038/s41467-018-04389-0
Yun, J., Yeo, I. J., Hwang, C. J., Choi, D.-Y., Im, H.-S., Kim, J. Y., et al. (2018). Estrogen deficiency exacerbates Aβ-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-ĸB activation in ovariectomized mice. Brain, Behav. Immun. 73, 282–293. doi:10.1016/j.bbi.2018.05.013
Zhang, X.-J., Chen, H.-L., Li, Z., Zhang, H.-Q., Xu, H.-X., Sung, J. J., et al. (2009). Analgesic effect of paeoniflorin in rats with neonatal maternal separation-induced visceral hyperalgesia is mediated through adenosine A1 receptor by inhibiting the extracellular signal-regulated protein kinase (ERK) pathway. Pharmacol. Biochem. Behav. 94 (1), 88–97. doi:10.1016/j.pbb.2009.07.013
Zhang, J., Shi, L. L., and Zheng, Y. N. (2010). Dibenzocyclooctadiene lignans from fructus schisandrae chinensis improve glucose uptake in vitro. Nat. Product. Commun. 5 (2), 1934578X1000500212–234. doi:10.1177/1934578x1000500212
Zhang, H., Zhu, W., Su, X., Wu, S., Lin, Y., Li, J., et al. (2012). Triptolide inhibits proliferation and invasion of malignant glioma cells. J. neuro-oncology 109, 53–62. doi:10.1007/s11060-012-0885-5
Zhang, Y., Shi, K., Lin, T., Xia, F., Cai, Y., Ye, Y., et al. (2020). Ganoderic acid A alleviates myocardial ischemia-reperfusion injury in rats by regulating JAK2/STAT3/NF-κB pathway. Int. Immunopharmacol. 84, 106543. doi:10.1016/j.intimp.2020.106543
Zhang, Z., Yang, X., Song, Y. Q., and Tu, J. (2021a). Autophagy in alzheimer's disease pathogenesis: therapeutic potential and future perspectives. Ageing Res. Rev. 72, 101464. doi:10.1016/j.arr.2021.101464
Zhang, L., Zhang, L., and Sui, R. (2021b). Ganoderic acid A-mediated modulation of microglial polarization is involved in depressive-like behaviors and neuroinflammation in a rat model of post-stroke depression. Neuropsychiatric Dis. Treat. 17, 2671–2681. doi:10.2147/NDT.S317207
Zhang, N., Nao, J., and Dong, X. (2023a). Neuroprotective mechanisms of salidroside in alzheimer's disease: a systematic review and meta-analysis of preclinical studies. J. Agric. Food Chem. 71 (46), 17597–17614. doi:10.1021/acs.jafc.3c06672
Zhang, T., Gao, G., Kwok, L. Y., and Sun, Z. (2023b). Gut microbiome-targeted therapies for alzheimer's disease. Gut Microbes 15 (2), 2271613. doi:10.1080/19490976.2023.2271613
Zhao, Y. M., Zhang, M. L., Shi, Q. W., and Kiyota, H. (2006). Chemical constituents of plants from the genus inula. Chem. Biodivers. 3 (4), 371–384. doi:10.1002/cbdv.200690041
Zhao, Z.-Y., Zhang, Y.-Q., Zhang, Y.-H., Wei, X.-Z., Wang, H., Zhang, M., et al. (2019). The protective underlying mechanisms of schisandrin on SH-SY5Y cell model of Alzheimer’s disease. J. Toxicol. Environ. Health, Part A 82 (19), 1019–1026. doi:10.1080/15287394.2019.1684007
Zhao, X.-Y., Yu, D., Shi, X., Hou, S., and Teng, D. (2022). Resveratrol reduces p38 mitogen-activated protein kinase phosphorylation by activating sirtuin 1 to alleviate cognitive dysfunction after traumatic brain injury in mice. Neuroreport 33 (11), 463–469. doi:10.1097/WNR.0000000000001805
Zheng, L., Zhou, Z., Tao, D., and Lan, T. (2003). Protective effect of berberine on cardiac myocyte injured by ischemia-reperfusion. Sichuan da xue xue bao. Yi xue ban= J. Sichuan Univ. Med. Sci. Ed. 34 (3), 452–454. Available online at: https://pubmed.ncbi.nlm.nih.gov/12910687/.
Zheng, S., Ma, J., Zhao, X., Yu, X., and Ma, Y. (2022). Ganoderic acid A attenuates IL-1β-induced inflammation in human nucleus pulposus cells through inhibiting the NF-κB pathway. Inflammation 45 (2), 851–862. doi:10.1007/s10753-021-01590-0
Keywords: Alzheimer’s disease, neurodegenerative disease, natural compound, mitogen-activatedprotein kinase, research progress
Citation: Zhang X, Huang S, Xu H, Hu Y and Gao L (2025) Research progress on plant-derived natural compounds regulating the MAPK signaling pathway for the prevention and therapy of Alzheimer’s disease. Front. Pharmacol. 16:1666082. doi: 10.3389/fphar.2025.1666082
Received: 15 July 2025; Accepted: 09 September 2025;
Published: 19 September 2025; Corrected: 30 September 2025.
Edited by:
Annalaura Sabatucci, University of Teramo, ItalyReviewed by:
Mohd Kamil Hussain, Govt. Raza Post Graduate College, Rampur, IndiaPrabhash Nath Tripathi, University of Arkansas for Medical Sciences, United States
Copyright © 2025 Zhang, Huang, Xu, Hu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanan Hu, MTc4MDQzMTUxMDVAMTYzLmNvbQ==; Ling Gao, Z2FvbGluZzA2OTVAMTYzLmNvbQ==
 Xinyue Zhang
Xinyue Zhang Shujia Huang
Shujia Huang