Abstract
Background:
Ferulic acid (FA) is a natural phenolic compound that has demonstrated effectiveness against Huntington’s disease (HD). However, its exact mechanism remains unclear. Therefore, the current study aims to investigate FA’s potential mechanism of action against 3-nitropropionic acid (3NP)-induced HD.
Methods:
Adult male Wistar albino rats were administered FA orally (100 mg/kg) for 3 weeks, and 3NP (10 mg/kg) was intraperitoneally administered during the last 2 weeks to induce HD. Behavioral performance was assessed using the open field and hanging wire tests. Striatal tissue was analyzed using ELISA, qRT-PCR, Western blotting, histopathology, and immunohistochemistry.
Results:
Administration of 3NP led to weight loss, neurobehavioral deficits, oxidative damage, apoptotic cell death, and neuroinflammation. FA treatment mitigated these pathological changes by activating Nrf2/HO-1 signaling, a critical player in cellular redox balance. This beneficial effect was mirrored in restoring TAC levels and suppressing MDA. Moreover, FA suppressed TLR4/NF-κB inflammatory signaling, thereby reducing TNF-α and IL-1β levels. In addition, the anti-apoptotic properties of FA were confirmed by modulating SIRT1/p53 signaling, leading to Bcl-2 enhancement and caspase-3 downsizing. Furthermore, FA enhanced neuronal survival and plasticity confirmed by neurotrophic BDNF elevation. Histopathological and immunohistochemical analyses confirmed improved neuronal survival and reduced gliosis following FA treatment.
Conclusion:
The current research demonstrates that FA exhibits potent neuroprotective effects in experimental HD by modifying Nrf2/HO-1, TLR4/NF-κB, and SIRT1/p53 signaling pathways. These findings provide new mechanistic insights into FA’s potential role in managing HD.
1 Introduction
Huntington’s disease (HD) was initially defined as a convulsive condition, while its formal description as hereditary chorea by George Huntington was provided in 1872 (Gonzalez-Alegre and Afifi, 2006; Túnez et al., 2010) HD is an autosomal dominant neurodegenerative disorder distinguished by cognitive impairment, involuntary movements, and psychiatric symptoms (Chen-Plotkin et al., 2006; Ayala-Peña, 2013). The condition is primarily characterized by progressive damage to the striatum within the basal ganglia (Ayala-Peña, 2013; Túnez et al., 2010). Although the exact mechanisms behind neuronal degeneration in HD remain uncertain, several pathological factors have been implicated, including oxidative stress, disrupted energy, persistent stimulation of astrocytes and microglia, and excessive pro-inflammatory cytokines contribute to the disease process (Palpagama et al., 2019; Paul and Snyder, 2019).
3-Nitropropionic acid (3NP) is a naturally occurring mycotoxin known for its ability to induce HD pathogenesis in experimental animals by inhibiting succinate dehydrogenase enzyme and disrupting mitochondrial energy production (Túnez et al., 2010). This disruption leads to a cascade of harmful events including reduced antioxidant defense mechanisms and the generation of reactive oxygen species generation (Bono-Yagüe et al., 2020). Additionally, 3NP triggers inflammatory responses as evidenced by elevated pro-inflammatory cytokines including intelukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) (Valadão et al., 2020). Moreover, it alters glial responses by enhancing glial fibrillary acidic protein (GFAP) expression and activating microglia (Mustafa et al., 2021; Shawki et al., 2021). Furthermore, it promotes caspase-mediated apoptotic initiation, contributing to the neurodegenerative process associated with HD (Goyal et al., 2024).
Silent information regulator 1 (SIRT1) plays a critical protective role in HD (Duan, 2013). SIRT1 activates nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) antioxidative signaling. Consequently, SIRT1 inhibition is associated with neuroinflammation, oxidative stress, and apoptosis (Huang et al., 2015; Sethi et al., 2025).
In addition, SIRT1 activation can suppress nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling and reduce its downstream effects. Moreover, SIRT1 is considered a promising therapeutic target due to its ability to inhibit p53 activity, which is implicated in the apoptotic progression of neurodegenerative diseases (Razick et al., 2023).
Taken together, the SIRT1/Nrf2/NF-κB/p53 axis represents an interconnected signaling network that simultaneously governs oxidative balance, inflammatory responses, and apoptosis in HD. Thus, targeting this axis provides a mechanistic rationale to investigate how ferulic acid (FA) orchestrates multi-faceted neuroprotective effects.
FA is a widely distributed natural bioactive phenolic acid that belongs to the hydroxycinnamic group. FA possesses various biological activities, particularly in vascular endothelial injury, apoptosis, oxidative stress, inflammation, and fibrosis (Li et al., 2021). Notably, previous research has documented the protective effect of FA against different neurological diseases including depression, Alzheimer’s disease, cerebral ischemia-reperfusion injury, epilepsy, and Parkinson’s disease (Thapliyal et al., 2021). Moreover, Denny Joseph (2014) documented the protective effect of FA against 3NP-induced neurotoxicity, however, the underlying mechanisms remain incompletely investigated, which was the goal of the present study. The present study advances this field by demonstrating for the first time that FA can potentially modulate the Nrf2/HO-1, TLR4/NF-κB, and SIRT1/p53 signaling pathways.
2 Materials and methods
2.1 Drugs and chemicals
The drug (FA, Y0001013) and the toxicant (3NP, N5636) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Both compounds were dissolved in normal saline daily (3NP pH was adjusted to 7.4 using NaOH).
2.2 Animals
Adult male Wistar albino rats (210–240 g) were procured from VACSERA (Helwan, Egypt) and acclimatized for 10 days in the animal facility of October 6 University’s Faculty of Pharmacy (O6U), under controlled conditions of ventilation, temperature, light, water, and diet. The experimental protocol adhered to NIH standards and was approved by the O6U Research Ethics Committee (Approval No: PRE-Ph-2411001).
2.3 Experimental design
The experiment lasts for 21 days (Danduga et al., 2018). Rats were randomly assigned to four groups (19 rats per group) using a computer-generated random number sequence as follows:
Normal group: Rats received normal saline (the vehicle) for 21 days.
FA group: Rats were given FA (100 mg/kg, p.o.) for 21 days.
3NP group: Rats were injected with 3NP (10 mg/kg, i.p.) from day 8 to day 21 of the experiment.
FA+3NP group: Rats received FA (100 mg/kg, p.o.) for 21 days (Zhang et al., 2023), administered 1 hour prior to 3NP injections, as previously described (Denny Joseph, 2014), with 3NP (10 mg/kg, i.p.) given only during the last 2 weeks (see Figure 1).
FIGURE 1
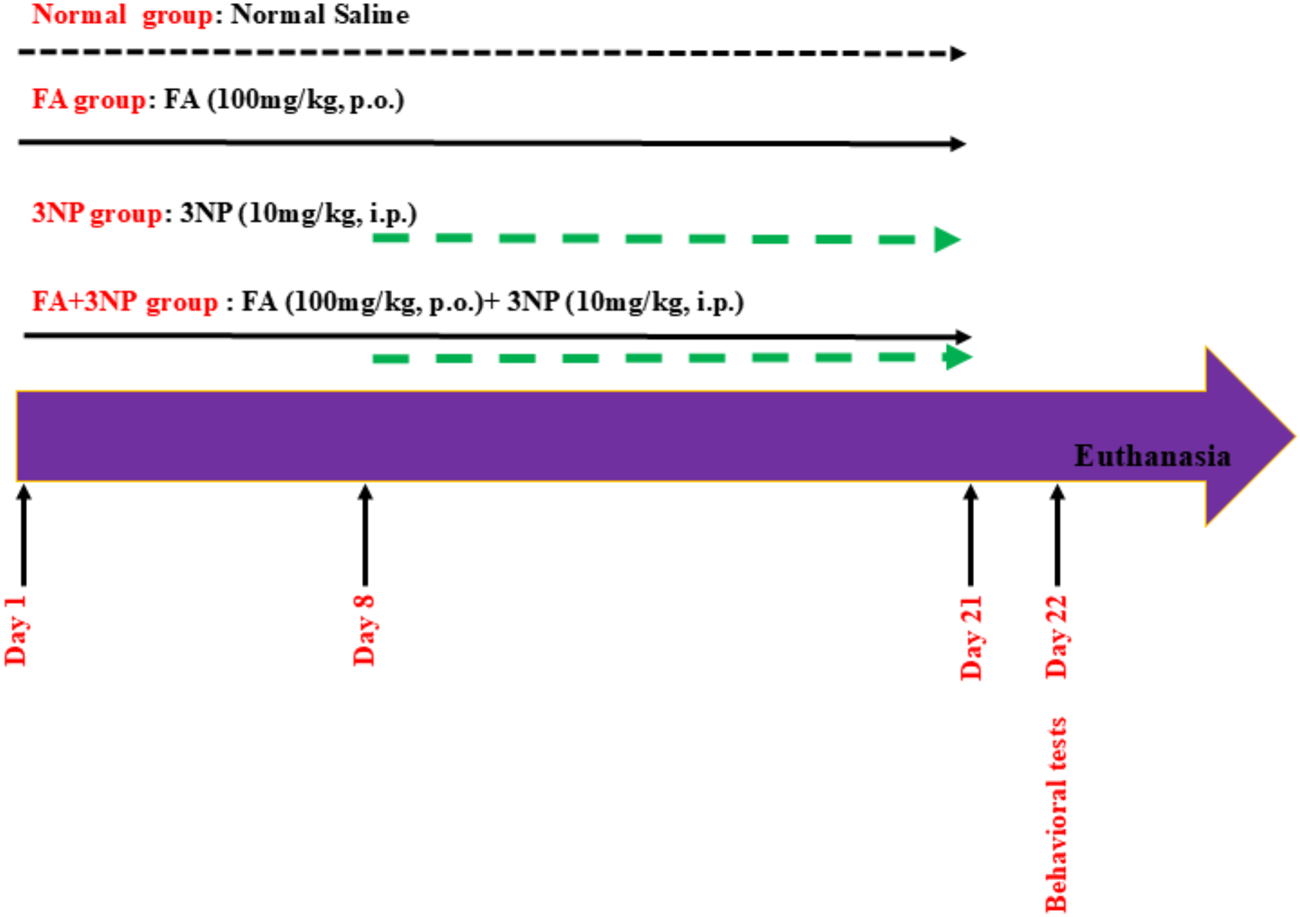
Schematic presentation of experimental design.
After completing the behavioral experiments on day 22, the animals’ weight was determined then the animals were euthanized by cervical dislocation under thiopental anesthesia (50 mg/kg; i.p.; Cat. No. T1019, Sigma-Aldrich, St. Louis, MO, United States) (Isik et al., 2025) and subdivided into four subsets. Three striatal subsets were stored at −80 °C for biochemical and ELISA assay (n = 6), qRT-PCR (quantitative Reverse Transcription Polymerase Chain Reaction, n = 6), and Western blot (n = 3). The remaining subset (n = 4) was fixed in 10% formalin for subsequent histopathological/immunohistochemical analyses. During the data collection/analysis, the investigators were unaware of sample identities and an independent experimenter handled all sample coding and decoding.
2.4 Behavioral assessments
All behavior experiments were conducted in a sound-isolated room. In each group, ten animals underwent the open field test, and the remaining animals were assessed using the hanging wire test.
2.4.1 Open field test
To assess the spontaneous locomotor activity, a wooden square box with a polished black floor (16 equal squares; 100 × 100 × 40 cm) was used (Ahmed et al., 2016). Each rat was individually allowed to explore freely for 10 min. The overhead camera recorded different parameters including the distance traveled, average velocity, and immobility duration. The videos were analyzed using ANY-maze video tracking software (Stoelting Co., United States). Locomotor activity parameters were quantified using automated thresholds set within the software (immobility was defined as movement below 2 cm/s for ≥1 s). All analyses were conducted by an assessor blinded to group allocation.
2.4.2 Hanging wire test
Rats were permitted to grip a steel wire with their forelimbs for 90 s. The wire was stretched horizontally at a height of 50 cm above a cushioned surface (Shalaby et al., 2018). The duration each rat held onto the wire was documented.
2.5 Biochemical and ELISA assay
All protocols were carried out according to the manufacturer’s pamphlets. Striatal total antioxidant capacity (TAC) and malondialdehyde (MDA) were assessed using commercial Biodiagnostic (Dokki, Egypt) colorimetric kit (TA 25 13 and MD 25 29, respectively). Striatal B-cell lymphoma-2 (Bcl-2; SL0108Ra), BDNF (SL0131Ra), and IL-1β (SL0402Ra) were assessed using Sunlong kits (Hangzhou, China). Striatal p53 (CSB-E08336r) and TNF-α (CSB- E11987r) were assessed using Cusabio kits (Wuhan, China).
2.6 Western blot
Briefly, striatal tissues were rinsed and homogenized in ice-cold lysis buffer containing protease and phosphatase inhibitor cocktails (Sigma, United States). The protein concentration was quantified colorimetrically. For immunoblotting, 30 µg of protein was incubated overnight at 4 °C with primary antibodies against: SIRT1 (1:1000; Cat. No. PA5-20964, Thermo Scientific, United States) and β-actin (1:1000; Cat. No. A5060, Sigma, United States). Membranes were washed and probed with horseradish peroxidase (HRP)-conjugated secondary antibodies (Dako, Denmark). Protein bands were detected using Western Lightning™ Plus ECL (Perkin Elmer, United States) and imaged with a ChemiDoc system (Bio-Rad, United States). Band intensities were normalized to β-actin and analyzed using Biorad ImageLab software. Means of bands optical densities were measured and their corresponding background subtracted, and then the subtracted intensities were divided on to their corresponding β-actin bands intensities (normalization), and the control group is set to “1.” (Burnette, 1981).
2.7 qRT-PCR
The expression levels of Nrf2, HO-1, and TLR4 were quantified by qRT-PCR following previously earlier reports (Sambrook et al., 1989; Burnette, 1981; Nasser et al., 2022). Briefly, total RNA was extracted using the SV Total RNA system (Cat. No. Z3100, Promega, Madison, WI, United States), and the purity was verified by the OD 260/280 nm absorbance ratios. Equal quantities of purified RNA were reverse transcribed to cDNA (Fermentas RT-PCR kit, Waltham, MA, United States). Quantitative PCR was performed using SYBR Green JumpStart Taq ReadyMix (Cat. No. S4438, Sigma–Aldrich, St. Louis, MO, United States). Primer specificity was confirmed by melt curve analysis showing a single sharp peak for each gene. Data were normalized to housekeeping genes and analyzed using the 2−ΔΔCT method. Primer sequences are listed in Table 1.
TABLE 1
| Gene | Primer sequence | Accession number |
|---|---|---|
| Nrf2 | F: 5′- GCCAGCTGAACTCCTTAGAC-3′ R: 5′- GATTCGTGCACAGCAGCA-3′ |
NM_031789.2 |
| HO-1 | F: 5′-CGACAGCATGTCCCAGGATT-3′ R: 5′-TCGCTCTATCTCCTCTTCCAGG-3.’ |
NM_012580.2 |
| TLR4 | F: 5′-CATGACATCCCTTATTCAACCAAG-3′ R: 5′-GCCATGCCTTGTCTTCAATTG-3′ |
NM_019178.2 |
| β-actin | F: 5′- AGGGAAATCGTGCGTGACAT-3′ R: 5′- GAACCGCTCATTGCCGATAG-3′ |
NM_031144.3 |
The sequence of all used primers.
2.8 Histopathology and Nissl staining for neuronal survival rate
Brain tissues were fixed in 10% neutral buffered formalin, dehydrated through a graded alcohol series, cleared in xylene, and embedded in paraffin wax. Five µm sections were cut and stained with hematoxylin and eosin (H&E) for histological examination by light microscopy. Nissl staining was used to evaluate the mean neuronal survival rate in each group (Gendy et al., 2023b).
2.9 Immunohistochemistry (IHC)
Brains tissue sections were mounted on adhesive slides, deparaffinized and re-hydrated to distilled water, subsequently a heat-induced epitope retrieval step was performed. Tissue sections were incubated for an hour at room temperature with primary anti-Caspase-3, anti-NF-қB (at a dilution of 1:200, Sanat Cruz, biotechnology, Inc.) and anti-GFAP (at a dilution of 1:300, Abbexa, United Kingdom). After washing, the HRP-labelled detection kit (BioSB, United States) was used to develop the color. Mayer’s hematoxylin was used as counter stain. Negative controls were processed without incubation with primary antibodies. Protein expression was quantified as mean area percentage in random non-overlapped five fields in each section using CellSens dimensions Olympus software (Olympus, Japan) (Gendy et al., 2023b).
2.10 Statistical analysis
Data were analyzed using GraphPad Prism 9.0.0 (United States) and expressed as mean ± standard deviation (SD). Data normality was assessed using the Shapiro–Wilk test and homogeneity of variance was evaluated using Levene’s test. Statistical comparisons were performed by one-way ANOVA with Tukey’s post hoc test. Statistical significance was set at p < 0.05.
3 Results
3.1 FA impact on the body weight
3NP administration induced significant body weight reduction (30% of normal group values; 186 ± 17.9 g vs. 268 ± 15.9 g in the normal group, p < 0.0001). Notably, the FA+3NP group showed significantly greater weight recovery (246 ± 15.5 g, p < 0.0001) compared to 3NP-treated animals (Figure 2).
FIGURE 2
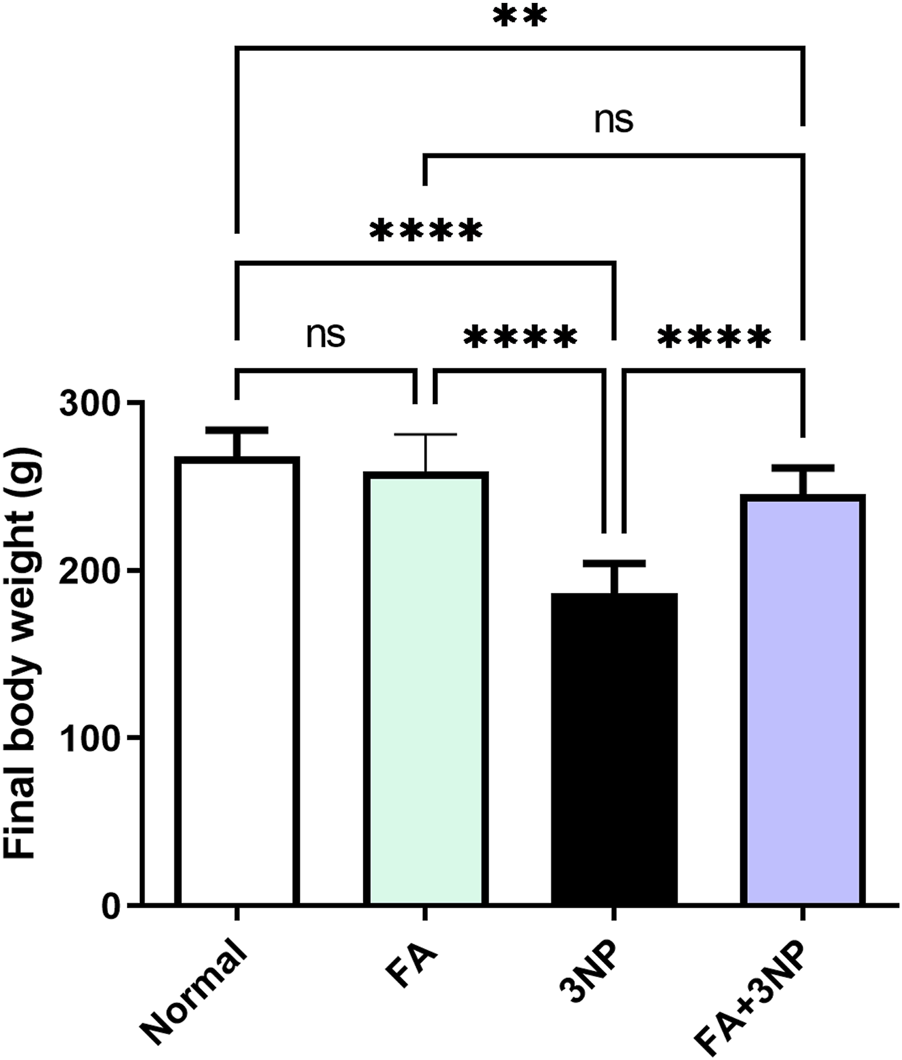
FA intake impacts on the final body weight in rats intoxicated with 3NP. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.2 FA impact on behavioral and motor studies
As presented in Figure 3, 3NP intoxication induces striatal damage, resulting in locomotor impairment. The 3NP group exhibited significant behavioral impairments in the open field test including decreased total distance traveled (3.86 ± 1.48 m vs. 9.66 ± 1.52 m, p < 0.0001), reduced mean speed (0.0138 ± 0.005 m/s vs. 0.0338 ± 0.0043 m/s, p < 0.0001), and prolonged immobility time (139 ± 51.1 s vs. 75.7 ± 26.1 s, p < 0.001). Similarly, fall-off latency in the hanging wire test decreased to 21.1 ± 8.24 s vs. 65.6 ± 24.6 s in the normal group (p < 0.001). FA treatment significantly mitigated these locomotor deficits, increasing total distance traveled (10.9 ± 1.74 m, p < 0.0001 vs. 3NP), mean speed (0.0387 ± 0.0104 m/s, p < 0.0001), and fall-off latency (49.8 ± 18.0 s, p < 0.05), while reducing immobility time (88.9 ± 30.4 s, p < 0.01 vs. 3NP) (Figure 3).
FIGURE 3
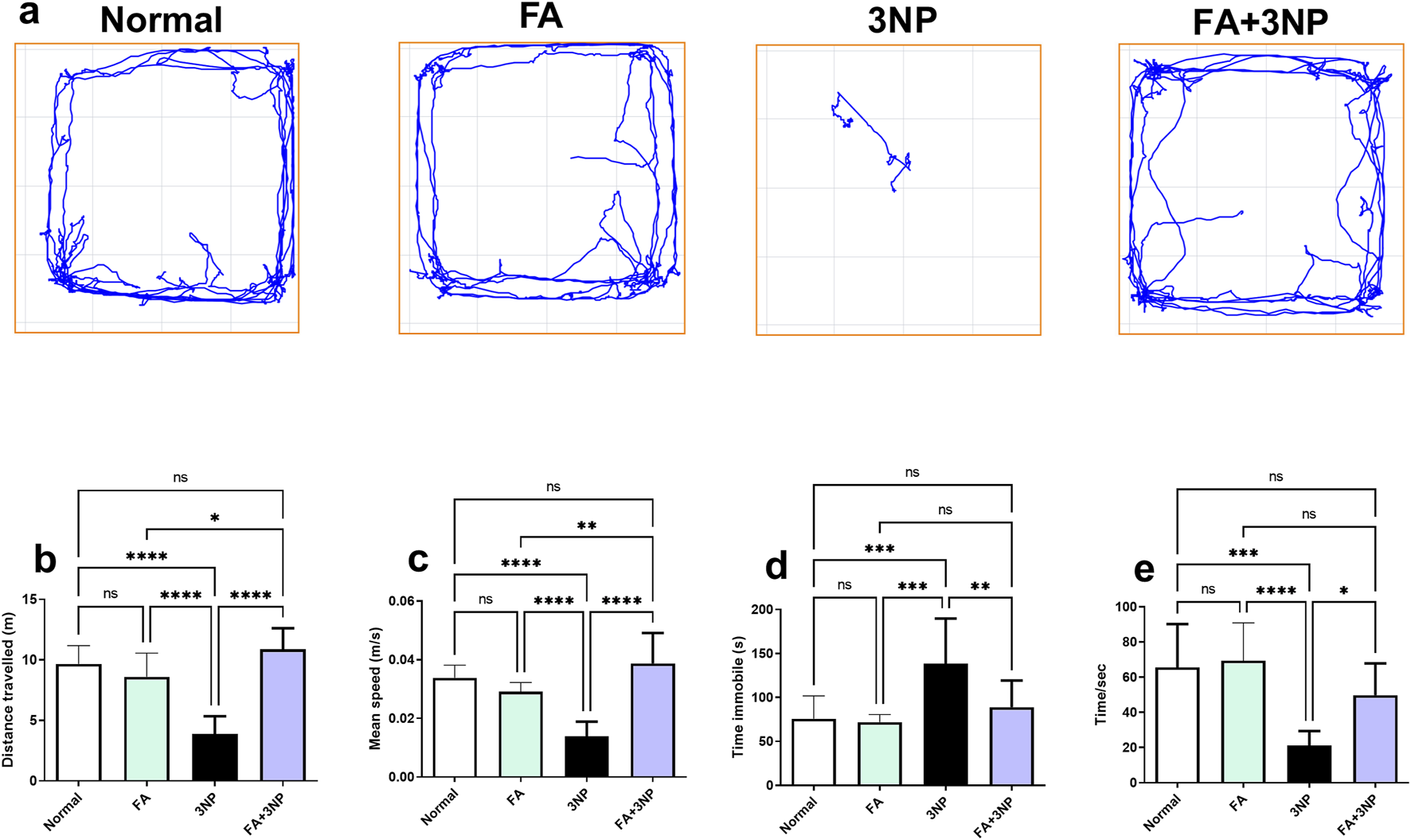
FA intake impacts on motor parameters (a), representative movement tracks; (b), distance traveled; (c), speed average; (d), immobility time and (e) fall of latency in rats intoxicated with 3NP. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.3 FA impact on Nrf2, HO-1, TAC, and MDA
3NP administration significantly downregulated striatal Nrf2 mRNA expression (0.38-fold ±0.08 vs. 1.02-fold ±0.18 in the normal group, p < 0.0001) and HO-1 mRNA expression (0.54-fold ±0.12 vs. 0.98-fold ±0.16, p < 0.0001). Likewise, TAC levels were markedly reduced (23.1 ± 2.8 vs. 59.9 ± 7.9 mmol/mg protein, p < 0.0001), while MDA content was significantly elevated (104 ± 13.7 vs. 44.4 ± 5.4 nmol/mg protein, p < 0.0001) compared to the normal group. FA treatment significantly restored Nrf2 (0.72-fold ±0.09, p < 0.001) and HO-1 (0.74-fold ±0.10, p < 0.05) expression, enhanced TAC levels (48.2 ± 7.5, p < 0.0001), and decreased MDA (71.5 ± 6.5, p < 0.0001) compared to the 3NP group (Figure 4).
FIGURE 4
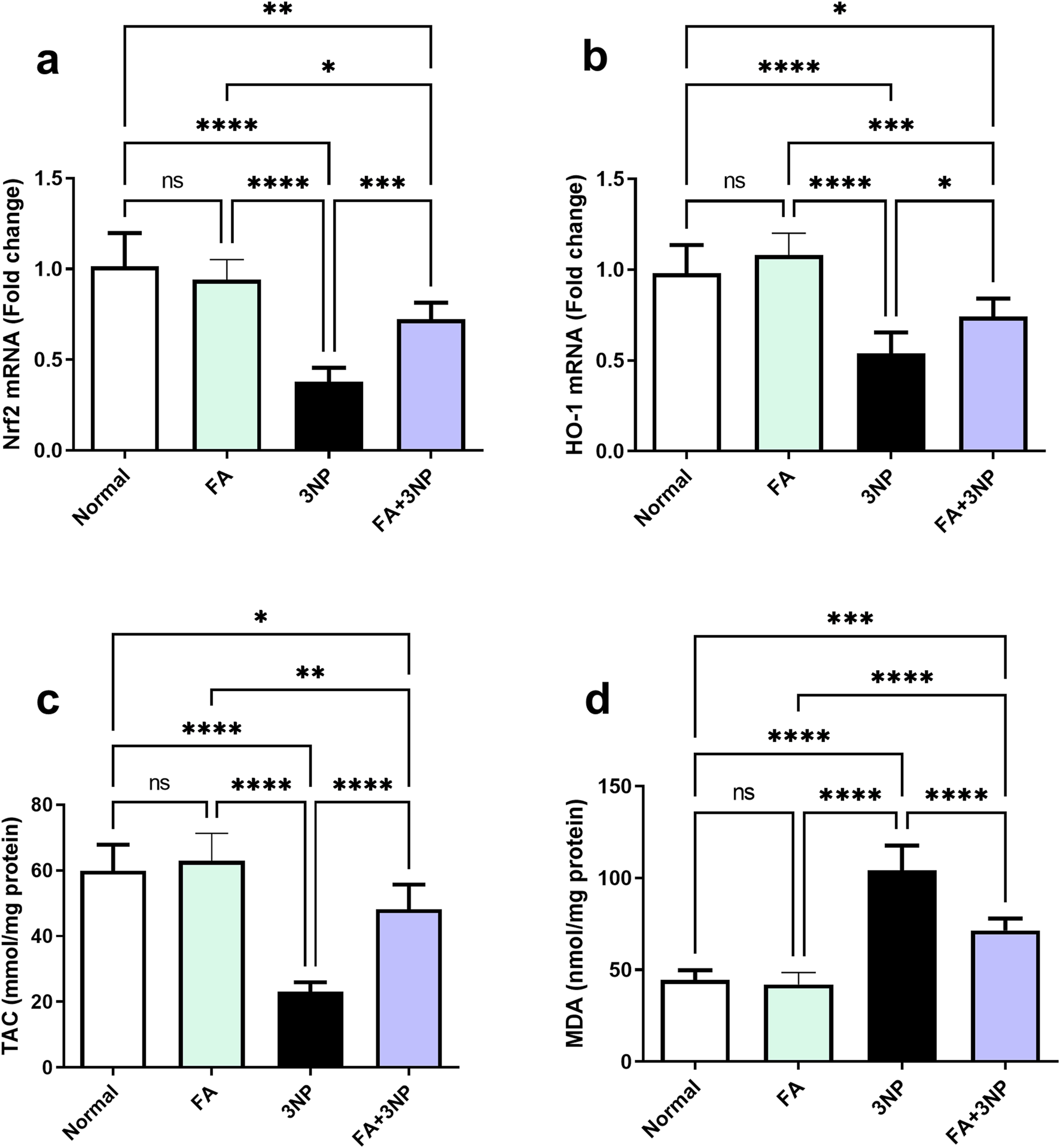
FA intake impacts on striatal (a) Nrf2 and (b) HO-1 mRNA expression, as well as (c) TAC and (d) MDA content in rats subjected to 3NP. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.4 FA impact on BDNF
As shown in Figure 5, intoxication by 3NP led to an approximate 50% reduction in striatal BDNF levels (Normal: 629 ± 32.2 pg/mg vs. 3NP: 312 ± 24.4 pg/mg, p < 0.0001). However, this decline was notably counteracted by FA treatment in the FA+3NP group (554 ± 47.1 pg/mg, p < 0.0001).
FIGURE 5
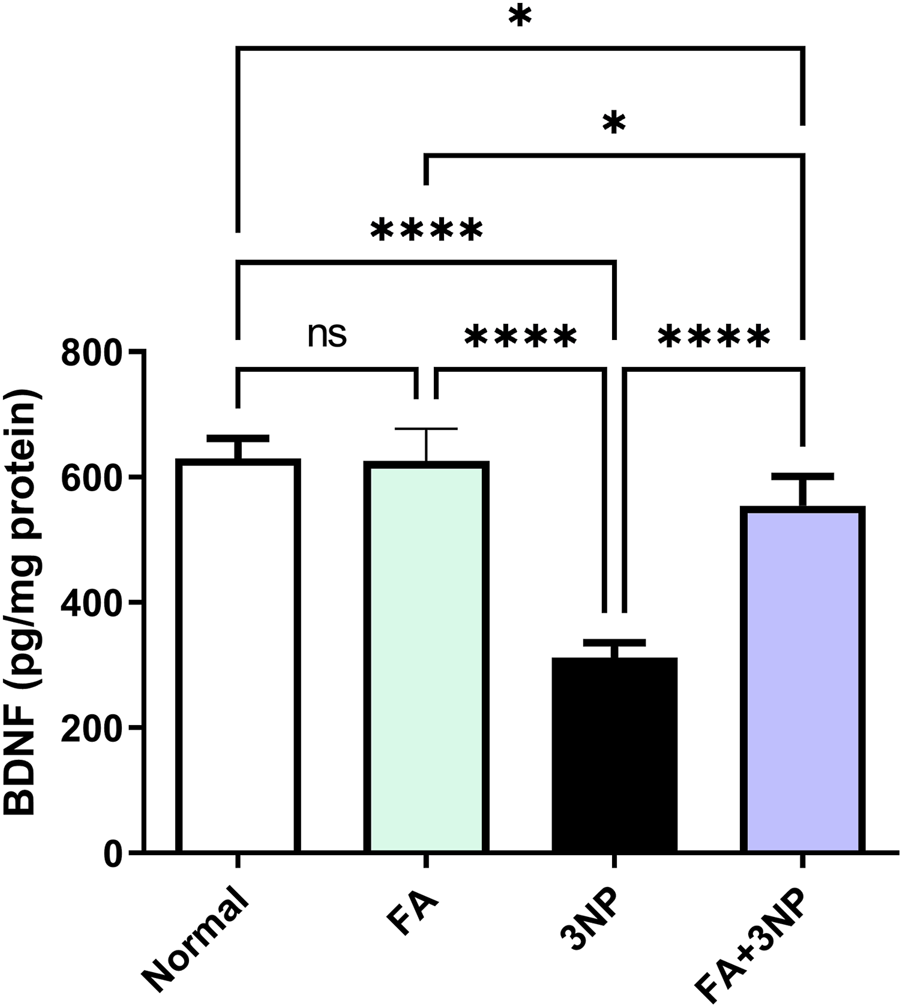
FA intake impacts on striatal neurotrophic BDNF content in rats subjected to 3NP. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.5 FA impact on TLR4, NF-κB p65, and inflammatory mediators
In contrast to the normal group (Figure 6), 3NP markedly enhanced TLR4 mRNA expression (5.5-fold ±0.9, p < 0.0001) and NF-κB p65 IHC protein content (7.7-fold ±0.5, p < 0.0001) leading to increment in TNF-α (Normal: 84.2 ± 5.74 pg/mg protein vs. 3NP: 272 ± 29.9 pg/mg protein, p < 0.0001) and IL-1β protein content (Normal: 128 ± 8.35 pg/mg protein vs. 3NP: 337 ± 16.9 pg/mg protein, p < 0.0001). Conversely, FA intake in 3NP-intoxicated rats counteracted these alterations certifying its anti-inflammatory effect (2.83-fold ±0.49, 2.16-fold ±0.43, 105 ± 10.3 pg/mg protein, and 195 ± 16.9 pg/mg protein, respectively).
FIGURE 6
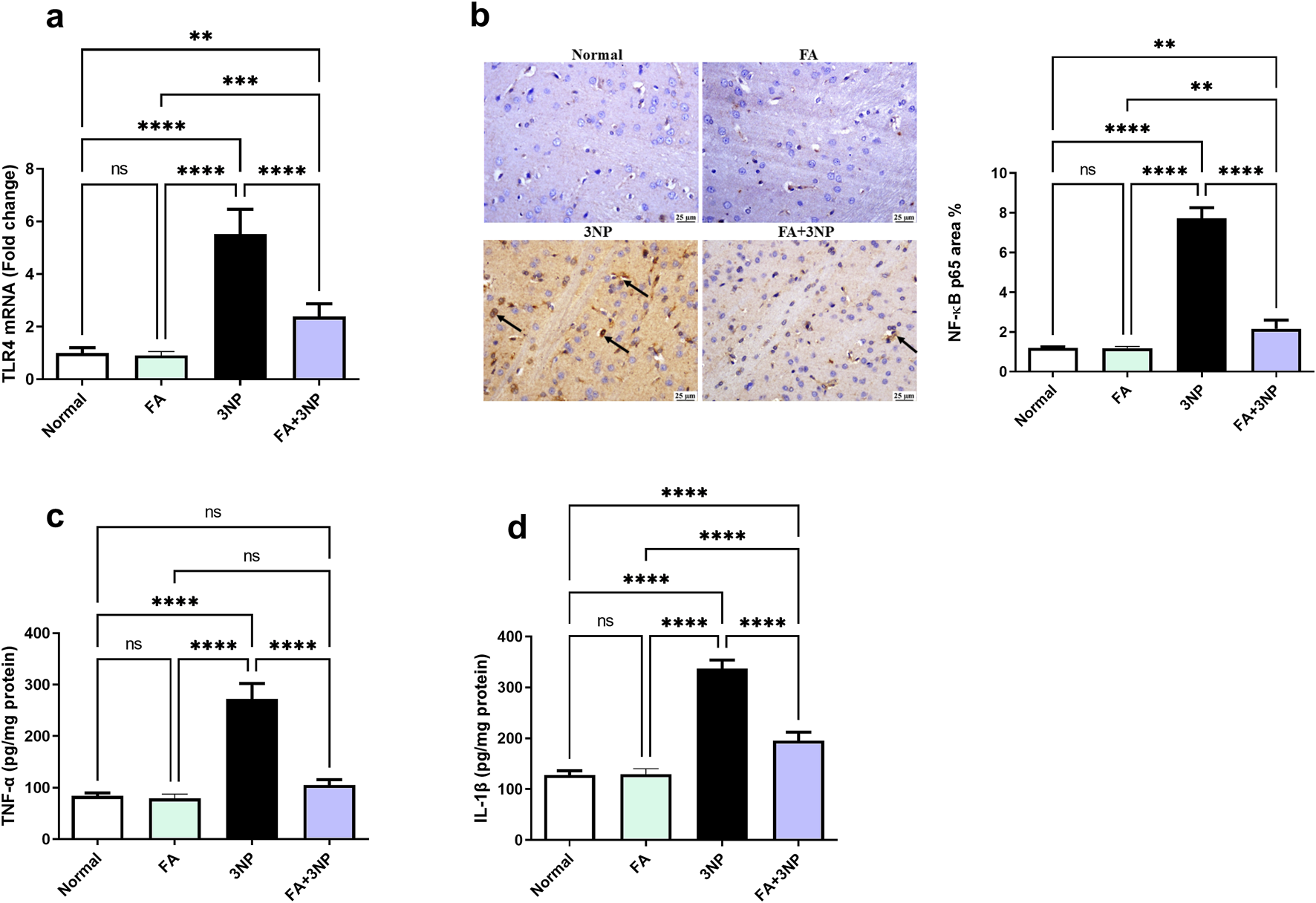
FA intake impacts on striatal (a) TLR4 mRNA expression, (b) NF-κB p65 IHC protein expression, as well as protein content of (c) TNF-α and (d) IL-1β in rats subjected to 3NP. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.6 FA impact on p53, Bcl-2, and caspase-3
The 3NP insult triggers apoptosis markers evidenced by a spike in both p53 content (34.5 ± 3.12 pg/mg protein, p < 0.0001) and caspase-3 IHC (8.91 ± 0.86-fold, p = < 0.0001) alongside reduction in Bcl-2 content (129 ± 8.68 pg/mg protein, p < 0.0001). Notably, FA demonstrated its anti-apoptotic potential by counteracting these changes (Figure 7).
FIGURE 7
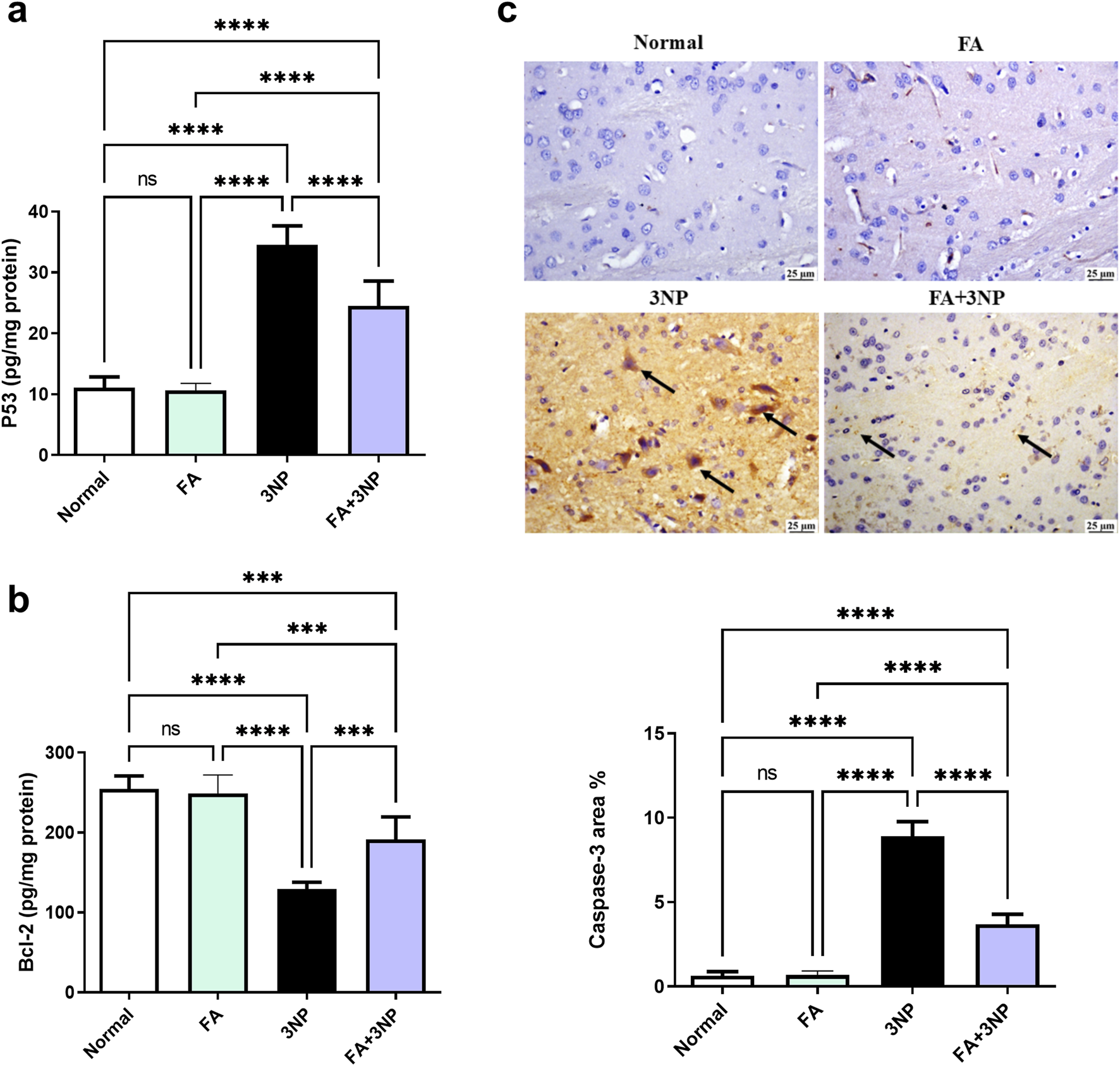
FA intake impacts on striatal (a) p53, (b) Bcl-2 content, and (c) caspase-3 IHC in rats subjected to 3NP. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.7 FA impact on SIRT1 expression
At the molecular level, 3NP disrupts cellular function by reducing SIRT1 protein expression by 21.7% (0.22-fold ±0.07, p < 0.0001). However, FA effectively counteracted this alteration (0.74-fold ±0.16, p < 0.001), demonstrating a protective regulatory role (Figure 8).
FIGURE 8
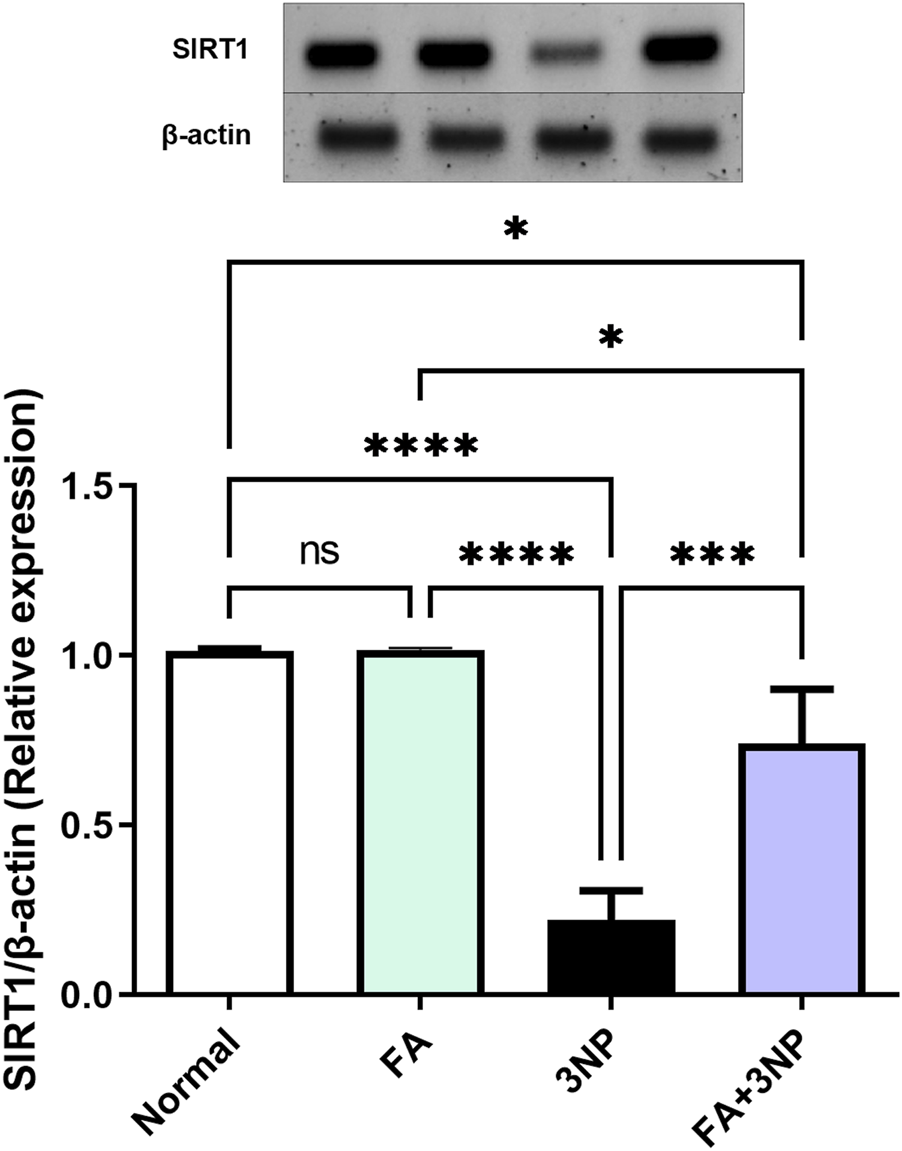
FA intake impacts on striatal protein expressions of SIRT1 in rats subjected to 3NP. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
3.8 FA impact on the histopathological findings
As shown in Figure 9, microscopic evaluation of brain sections from the normal group revealed normal structure of striatum. Likewise, no histopathological changes were detected in the sections examined from the FA group. On the contrary, 3NP group showed focal gliosis with marked edema as well as the existence of some dark degenerating neurons within the striatum. Marked improvement was detected in the examined sections from FA+3NP group as sporadic focal gliosis was detected meanwhile apparently normal stratum was detected in almost all examined sections.
FIGURE 9
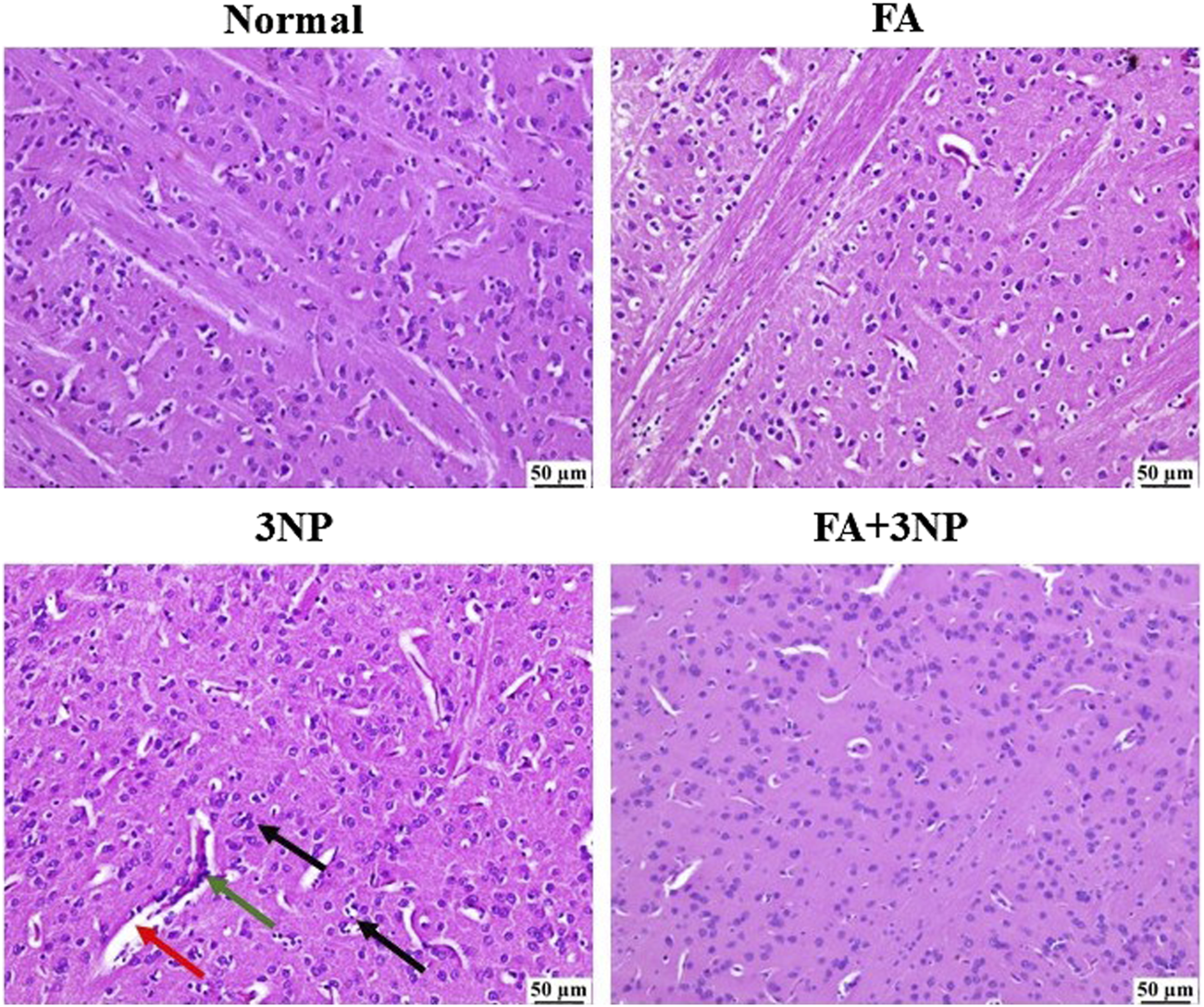
Representative photomicrographs of H & E stained stratia in the normal and FA groups showing intact neurons with normal histological architecture. 3NP group, showing gliosis (black arrow), distinct capillaries (green arrow), and edema (red arrow). FA+3NP group, showing apparently normal striatum.
Moreover, as illustrated in Figure 10, neuronal survival rate was dramatically lowered in 3NP group when compared to the normal control group. FA+3NP group showed meaningful elevation in neuronal survival rate when compared to the 3NP group. No notable difference was observed between the normal and the FA group.
FIGURE 10
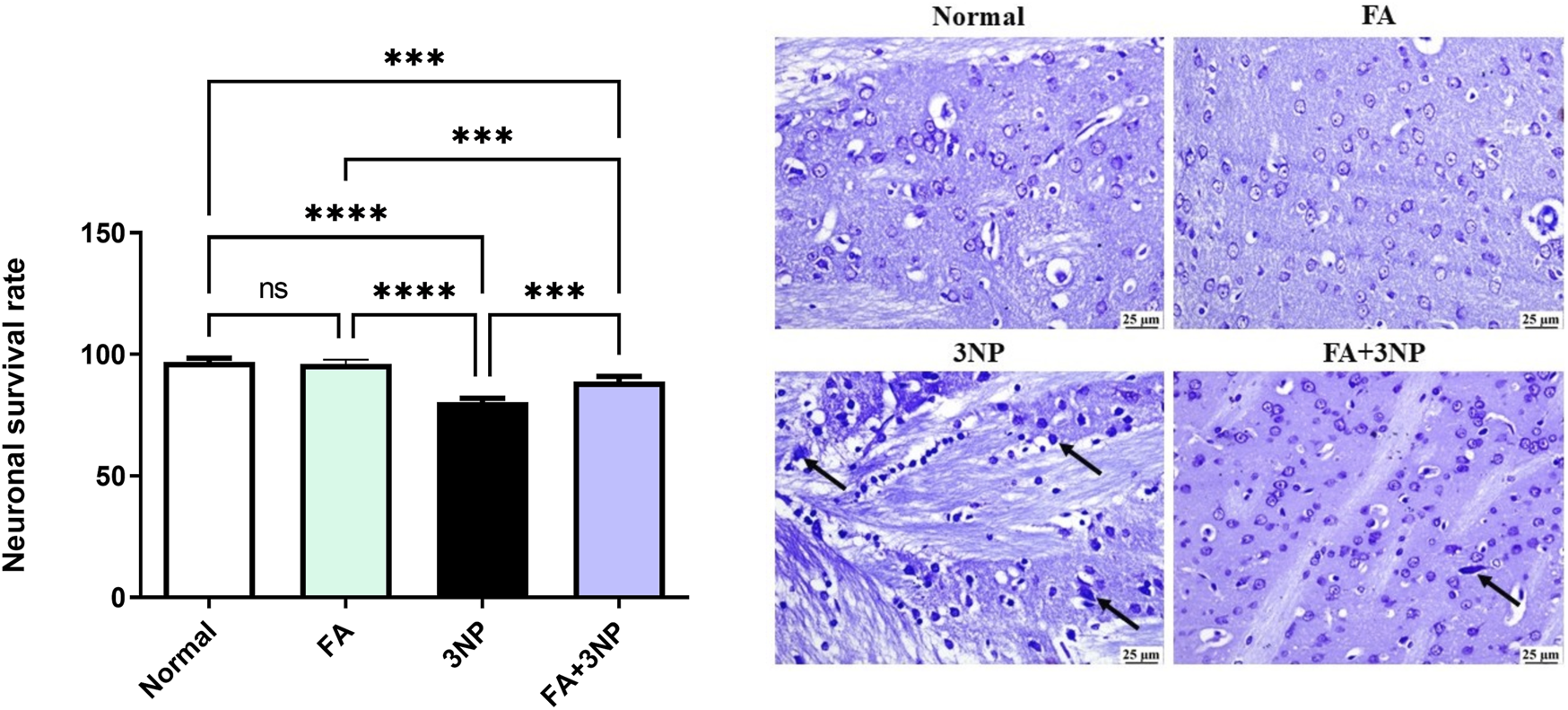
FA intake impacts on the neuronal survival rate in rats exposed to 3NP. Normal and FA groups, showing apparently normal lightly stained neurons within the striatum. 3NP group, showing numerous dark degenerating neurons (arrows) within the striatum. FA+3NP group, few dark degenerating neurons (arrows) within the striatum. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
In addition, the 3NP group disclosed a marked upsurge in GFAP expression compared to the normal group. However, the FA+3NP group exhibited noteworthy shrinkage in GFAP expression relative to the 3NP group (Figure 11).
FIGURE 11
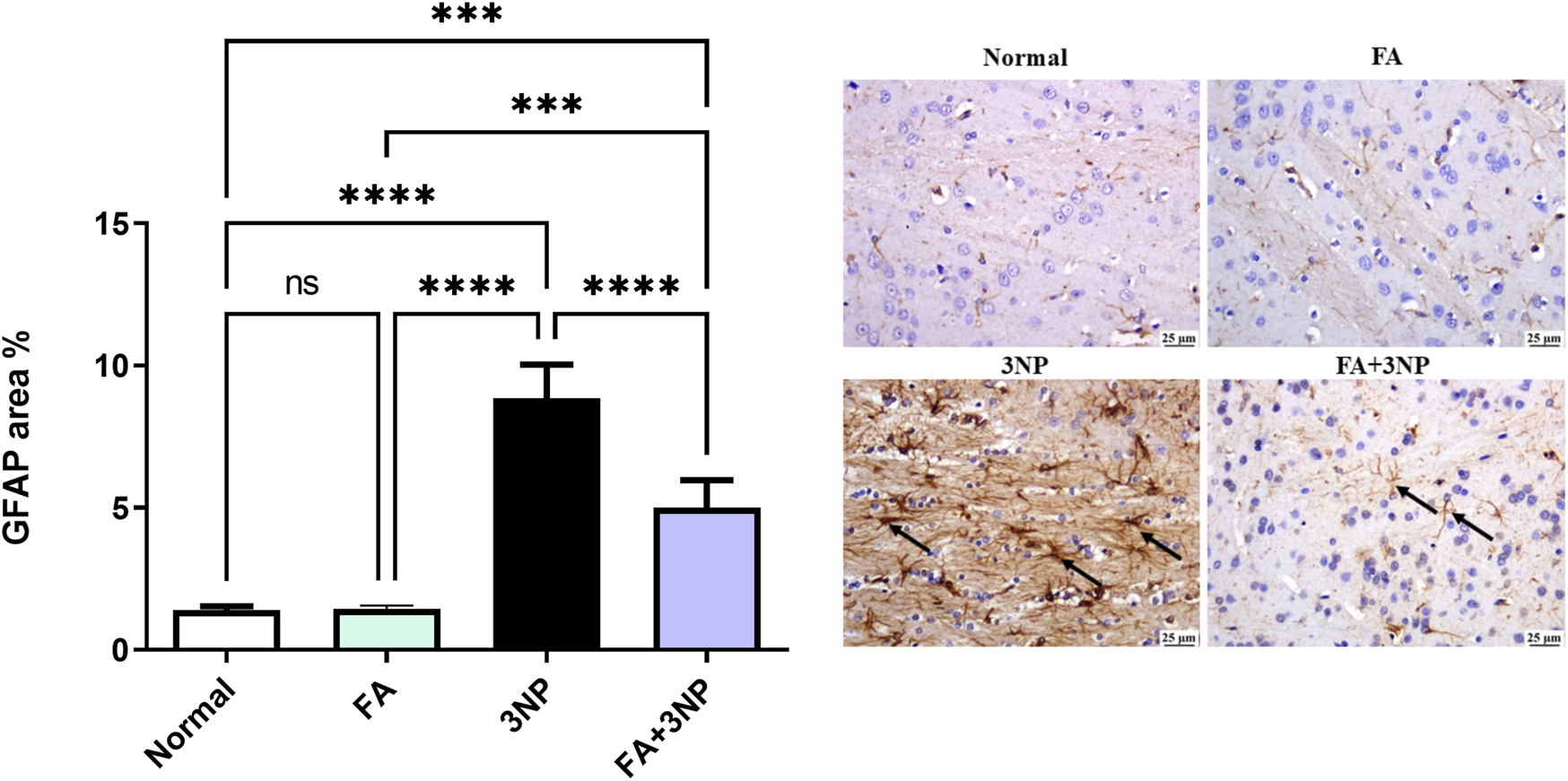
FA intake impacts on striatal GFAP in rats exposed to 3NP. Photomicrograph of brain, striatum, Normal and FA groups showing normal mild GFAP expression (Immune staining), 3NP group showing intense GFAP expression, and FA+3NP group showing moderate GFAP expression. Data are presented as mean ± SD; ns (non-significant, P > 0.05), *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
4 Discussion
In this study, we employed the established 3NP model to induce HD. This model allowed us to assess the potential neuroprotective effects of FA against multiple detrimental events associated with the disease. Our findings extend earlier reports of FA neuroprotection. Specifically, we provide possible mechanistic evidence that links FA’s behavioral and histological benefits to the simultaneous regulation of SIRT1, Nrf2, and NF-κB signaling in the 3NP-induced HD model. Unlike prior studies, which have not examined these pathways collectively in a single model, we demonstrate a new insight into the possible crosstalk that may underlie FA’s neuroprotective actions. These findings were further supported by results from qRT-PCR, Western blot, and immunohistochemistry. This data can position FA as a promising modulator of multiple pathogenic pathways in HD.
We investigated how this phenolic acid influenced body weight and motor behavioral parameters using open field and hanging wire tests. Additionally, we evaluated the antioxidant properties of FA by measuring levels of Nrf2, HO-1, TAC, and MDA. In terms of inflammation, we analyzed the cytokines TNF-α, and IL-1β. Additionally, the current study investigated the contribution of the TLR4/NF-κB p65 signaling in HD pathogenesis after FA treatment. Furthermore, we examined the apoptotic activity by assessing p53, Bcl-2, and caspase-3 levels. The expression of GFAP and BDNF was also evaluated alongside histopathological changes. Finally, the role of SIRT1 and its crosstalk with Nrf2, NF-κB, and p53 were discussed.
In the current research, the induction of HD by 3NP caused a decrease in the body weight of the animals, which aligns with findings from several previous studies (Elbaz et al., 2025; Mustafa et al., 2021). This weight loss may reflect the negative impact of 3NP on the animals’ energy metabolism, as well as motor deficit, anorexia, and the reduced food intake (Ahmed et al., 2016). Fortunately, FA intake was able to counteract these negative effects of 3NP, leading to a restoration of the rats’ body weight.
Alongside, significant changes in the neurobehavioral functions were observed during the open field tests. Following the administration of 3NP, animals exhibited a noticeable reduction in the total distance traveled and a decrease in mean speed, along with an increase in the time spent immobile. Conversely, treatment with FA effectively mitigated these issues, aligning with findings from earlier research (Zeni et al., 2012). The decline in muscle function, characterized by muscle weakness, is a primary indicator of HD and can be evaluated through grip strength tests, such as the hanging wire test. The test revealed significantly impaired limb strength following 3NP administration, as evidenced by reduced latency to fall. These findings are consistent with previous studies (Sayed et al., 2020; Elbaz et al., 2025). Conversely, administering FA led to an improvement in grip strength, as demonstrated by hanging duration enhancement. Overall, the neurobehavioral improvements associated with FA highlight its beneficial role in addressing the neurobehavioral abnormalities linked to HD.
Previous research has revealed that the neurotoxin (3NP) can induce oxidative stress in the striatum and other brain regions (Túnez et al., 2010; Sayed et al., 2020; Gao et al., 2015), where 3NP readily penetrates the blood-brain barrier and generate reactive oxygen species leads to pathological symptoms that resemble those associated with HD (Gonchar et al., 2021). Our study supports this finding as evidenced by the depletion of TAC following 3NP exposure, as well as increased levels of MDA which initiates lipid peroxidation. Similar outcomes have been documented in earlier studies (Mustafa et al., 2021; Gao et al., 2015; Sayed et al., 2020). The amplified oxidative state and neuronal damage resulting from 3NP injection may be partially due to the clear repression of the Nrf2/HO-1 antioxidant signaling as indicated herein. The Nrf2/HO-1 pathway is a well-known regulator of intracellular antioxidative processes. Its protective role in HD has been well documented (Sharma et al., 2025; Sethi et al., 2025). Nrf2 has been shown to promote the transcription of BDNF which is recognized for its critical functions in neuronal survival, neurogenesis, synaptic plasticity (Azman and Zakaria, 2025). On the other hand, BDNF is involved in the translocation and activation of Nrf2, which contributes to the restoration of redox homeostasis. It has been previously reported that levels of BDNF protein are diminished in animal models of HD. as documented herein and previously (Gendy et al., 2023b; Sayed et al., 2020). Moreover, HO-1 is known to provide neuroprotection against oxidative stress (Neis et al., 2018). In contrast, treatment with FA countered all markers of oxidative stress by restoring TAC levels while reducing MDA which can be attributed to the enhancement of Nrf2/HO-1 signaling (Mishra et al., 2022; Li et al., 2020b; Nouri et al., 2023). This indicates the antioxidant properties of FA in HD suggesting that antioxidants can modify or delay clinical manifestations including deficits in memory and motor skills associated with the disease (Sharma et al., 2021).
Another important signaling is TLR4-NF-κB p65 signaling where the current results indicated a rise in TLR4 content and NF-κB p65 immunoreactivity consistent with the findings of El-Abhar et al. (El-Abhar et al., 2018). TLR4 emerges as a significant molecular player in HD where its spike is known to contribute to the pathological status of multiple neurodegenerative disease (Dabi et al., 2023) as it plays an important role in the biochemical and neurological alterations (Dabi et al., 2023). Additionally, the pivotal role of NF-κB in the central nervous system via TLR4 overexpression has been well verified (Sharma et al., 2024), facts that coincide the current results. The high rate of TLR4 content can be linked to the increased cytokine levels and glial activation (Dabi et al., 2023). On the other hand, the documented reduction in Nrf2 levels following 3NP administration plays a role in enhancing NF-κB p65 levels, as Nrf2 is known to suppress NF-κB p65 subunit and its downstream inflammatory molecules (Ibrahim and Abdel Rasheed, 2022). FA depicted a decline in TLR4 content to concur with the results in LPS-induced neurotoxicity and sciatica models (Rehman et al., 2019; Zhang et al., 2023). Moreover, NF-κB p65 IHC observed attenuation was clear after FA intake which may be an outcome of decreased TLR4 levels. This hypothesis is evidenced by experimental data unveiling the role of FA-dependent downregulation in TLR4 for hindering NF-κB p65 and consequently serves as a key mechanism underlying FA anti-inflammatory effects. In this milieu, NF-κB inhibition may also result from Nrf2 activation by FA that has previously been documented to diminish NF-κB inflammatory character (Liu et al., 2021; Singh et al., 2022).
Inflammation also plays a significant role in HD pathogenesis (Valadão et al., 2020), as evidenced in the current study by the 3NP-induced increase in TNF-α and IL-1β levels, as well as the histopathological findings. These results align with a previous study investigating Dapagliflozin in the 3NP rat model of HD (El-Sahar et al., 2020). FA effectively mitigated inflammation by normalizing disrupted inflammatory parameters and enhancing histopathological outcomes. Similarly, FA has demonstrated anti-inflammatory properties in chronic unpredictable mild stress (Liu et al., 2017). Thus, attenuation of redox status and apoptosis via enhanced Nrf2 and reduced NF-κB p65 represents a potential mechanism underlying FA anti-inflammatory effects (Singh et al., 2022; Sharma et al., 2025; Gendy et al., 2023a).
The apoptotic death contributes a pivotal part in the neuronal degeneration in HD (Fan et al., 2017). In the present study, striatal exposure to 3NP elicited marked pro-apoptotic changes. This was evidenced by increased p53 content, upregulated caspase-3 immunoreactivity, and reduced levels of the anti-apoptotic protein Bcl-2. A result that match previous reports (Gendy et al., 2023a; Ibrahim and Abdel Rasheed, 2022; Ahmed et al., 2016). Importantly, our findings revealed that treatment with FA reversed these alterations by enhancing Nrf2 expression and reducing NF-κB p65 levels. Restoration of Nrf2 likely contributed to the reestablishment of redox homeostasis and upregulation of Bcl-2 (Singh et al., 2022), while suppression of NF-κB attenuated pro-apoptotic signals leading to reduced caspase-3 activation (Van Antwerp et al., 1998; Liu et al., 2021).
SIRT1 is an NAD-dependent enzyme plays a key role in the modulating of oxidative stress balance, inflammatory response, and apoptosis (Ren et al., 2019). Moreover, its role in HD is well documented (Naia and Rego, 2015; Jiang et al., 2012; Manjula et al., 2021). SIRT1 regulatory effect on apoptosis is well documented by inhibiting p53, leading to the downregulation of programmed cell death, as documented herein (Jazvinšćak Jembrek et al., 2021). Moreover, SIRT1 can restrain oxidative stress by activating Nrf2 nuclear translocation consequently amplifying transcriptional activation of antioxidant genes (Mosaoa et al., 2024; Sethi et al., 2025; Ibrahim and Abdel Rasheed, 2022). SIRT1 influences the interaction between Nrf2 and NF-κB. It promotes the expression of antioxidant genes expression while limiting pro-inflammatory cytokines production in response to oxidative stress (Jazvinšćak Jembrek et al., 2021). Additionally, SIRT1 plays a regulatory role in inflammatory factors transcription, including NF-κB, which is a crucial regulator of various pro-inflammatory cytokines (Peixoto et al., 2017; Song et al., 2022). Additionally, SIRT1 can influence neuroinflammation by decreasing the activation of astrocytes, which leads to a reduction in GFAP levels, a well-known marker associated with astrogliosis (Shaheen et al., 2021; Vaziri et al., 2001). Furthermore, SIRT1 has been shown to inhibit the activation of microglia and its detrimental inflammatory cascade (Li et al., 2020a). On the other hand, SIRT1 overexpression has been linked to BDNF expression (Harrison, 2012). Our findings verified the clear repression of SIRT1 after 3NP intoxication, while FA treatment reactivated this signaling (Chen et al., 2019; Wang et al., 2023). Based on these outcomes, it is reasonable to propose that the upregulation of SIRT1 by FA represents a potential mechanism underlying its therapeutic effects against 3NP-induced HD.
In the current research, the neurotoxicity induced by 3NP was associated with the activation of microglial cells, which was evidenced by the activation of astrocytes and reflected in the overexpression of GFAP. The administration of FA effectively protected the animals from astrocyte activation by lowering GFAP expression. This finding is consistent with previous study highlighting the defensive actions of FA in rats model of Alzheimer’s disease (Khalifa et al., 2025).
BDNF is recognized for its critical functions in stimulating neurogenesis, enhancing synaptic plasticity, promoting neuronal survival, and mitigating neuroinflammation caused by TNF-α (Patil et al., 2014). In HD, there is a notable decrease in BDNF expression (Pineda et al., 2005). Likewise, the 3NP model of HD has shown a significant reduction in BDNF levels, as previously reported (Sayed et al., 2020). One potential reason for this decline is the activation of NF-κB p65 induced by 3NP, which has been suggested in studies evaluating memory loss triggered by lipopolysaccharide (Gu et al., 2015). Fortunately, the administration of FA has been effective in preventing this reduction and reversing the neuronal damage caused by 3NP, a beneficial effect that has been documented previously in depressive-like behaviour model (Mallik et al., 2023). The beneficial effects of FA on BDNF restoration can also be linked to its modulation of SIRT1 and Nrf2 signaling. SIRT1 has been reported to enhance BDNF transcription and promote neuronal survival (Tang et al., 2020). Nrf2 activation can also stimulate BDNF expression as part of its neuroprotective effect (Cao et al., 2022). In our study, FA administration concurrently elevated SIRT1, Nrf2, and BDNF levels, indicating that the recovery of striatal BDNF may be mediated, at least in part, through these upstream regulators.
Neuronal damage was assessed using Nissl staining, which revealed a marked increase in the number of degenerated cells following the intraperitoneal injection of 3NP, as previously documented (Sayed et al., 2020; Ibrahim and Abdel Rasheed, 2022). Notably, FA intake demonstrated a neuroprotective effect by increasing neuronal survival. Finally, this study offers new insights into the neuroprotective effects of FA in the case of HD. The protective effect of FA is accompanied by changes in SIRT1/Nrf2/NF-кB/p53 signaling. These changes enhance the antioxidant capacity of striatal tissue while reducing both inflammatory and apoptotic responses.
Limitation of the current study: The use of different methodologies for apoptotic markers: ELISA for p53 and Bcl-2, and immunohistochemistry for caspase-3. This heterogeneity may affect direct comparability. The baseline behavioral performance was not assessed before 3NP administration. Future studies including baseline measurements will provide more assurance that the observed differences are due to treatment effects only. Moreover, the present study did not directly assess SIRT1 activity or p53 acetylation status. Likewise, we did not include pharmacological modulation of SIRT1 (e.g., inhibitor or activator) to establish causality. These experiments would allow confirmation that the observed molecular changes are specifically mediated via SIRT1 activation rather than parallel pathways. Future work will incorporate such approaches. Nonetheless, our findings are consistent with prior reports in neurodegenerative models showing that SIRT1 activation attenuates oxidative stress, inflammation, and apoptosis through these pathways, supporting the plausibility of our proposed mechanism. In addition, we acknowledge that a smaller sample size in the Western blot may limit sensitivity to detect more subtle protein changes. On the other hand, the observed effect sizes in our data were large enough to yield statistically significant difference. Future experiments will incorporate a priori power calculations to ensure that sample sizes are optimized prospectively for all endpoints.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by O6U Research Ethics Committee (PRE-Ph-2411001). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MA: Funding acquisition, Writing – review and editing. AG: Conceptualization, Methodology, Writing – review and editing. SZ: Formal Analysis, Investigation, Writing – original draft. WE: Formal Analysis, Investigation, Writing – original draft. MR: Formal Analysis, Investigation, Writing – original draft. MKE-S: Resources, Writing – review and editing. AE-H: Resources, Writing – review and editing. HM: Supervision, Writing – review and editing. IA: Funding acquisition, Writing – review and editing. ME: Conceptualization, Formal Analysis, Investigation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Deanship of Graduate Studies and Scientific Research at Jouf University under grant No. (DGSSR-2025-FC-01022).
Acknowledgments
The authors would like to thank Cardiff University for covering the publication fees. In addition, the authors gratefully acknowledge Asmaa Al-Mokaddem for her invaluable technical assistance and contributions to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahmed L. A. Darwish H. A. Abdelsalam R. M. Amin H. A. (2016). Role of rho kinase inhibition in the protective effect of fasudil and simvastatin against 3-nitropropionic acid-induced striatal neurodegeneration and mitochondrial dysfunction in rats. Mol. Neurobiol.53, 3927–3938. 10.1007/s12035-015-9303-2
2
Ayala-Peña S. (2013). Role of oxidative DNA damage in mitochondrial dysfunction and Huntington’s disease pathogenesis. Free Radic. Biol. Med.62, 102–110. 10.1016/j.freeradbiomed.2013.04.017
3
Azman K. F. Zakaria R. (2025). Brain-derived neurotrophic factor (BDNF) in Huntington’s disease: neurobiology and therapeutic potential. Curr. Neuropharmacol.23, 384–403. 10.2174/1570159X22666240530105516
4
Bono-Yagüe J. Gómez-Escribano A. P. Millán J. M. Vázquez-Manrique R. P. (2020). Reactive species in Huntington disease: are they really the radicals you want to catch?Antioxidants9, 577. 10.3390/antiox9070577
5
Burnette W. N. (1981). “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem.112, 195–203. 10.1016/0003-2697(81)90281-5
6
Cao Q. Zou Q. Zhao X. Zhang Y. Qu Y. Wang N. et al (2022). Regulation of BDNF transcription by Nrf2 and MeCP2 ameliorates MPTP-induced neurotoxicity. Cell. death Discov.8, 267. 10.1038/s41420-022-01063-9
7
Chen X. Guo Y. Jia G. Zhao H. Liu G. Huang Z. (2019). Ferulic acid regulates muscle fiber type formation through the Sirt1/AMPK signaling pathway. Food & Funct.10, 259–265. 10.1039/c8fo01902a
8
Chen-Plotkin A. S. Sadri-Vakili G. Yohrling G. J. Braveman M. W. Benn C. L. Glajch K. E. et al (2006). Decreased association of the transcription factor Sp1 with genes downregulated in Huntington's disease. Neurobiol. Dis.22, 233–241. 10.1016/j.nbd.2005.11.001
9
Dabi Y. T. Ajagbe A. O. Degechisa S. T. (2023). Toll‐like receptors in pathogenesis of neurodegenerative diseases and their therapeutic potential. Immun. Inflamm. Dis.11, e839. 10.1002/iid3.839
10
Danduga R. C. S. R. Dondapati S. R. Kola P. K. Grace L. Tadigiri R. V. B. Kanakaraju V. K. (2018). Neuroprotective activity of tetramethylpyrazine against 3-nitropropionic acid induced Huntington’s disease-like symptoms in rats. Biomed. & Pharmacother.105, 1254–1268. 10.1016/j.biopha.2018.06.079
11
Duan W. (2013). Targeting sirtuin-1 in Huntington’s disease: rationale and current status. CNS drugs27, 345–352. 10.1007/s40263-013-0055-0
12
El-Abhar H. Abd El Fattah M. A. Wadie W. El-Tanbouly D. M. (2018). Cilostazol disrupts TLR-4, Akt/GSK-3β/CREB, and IL-6/JAK-2/STAT-3/SOCS-3 crosstalk in a rat model of Huntington's disease. PLoS One13, e0203837. 10.1371/journal.pone.0203837
13
El-Sahar A. E. Rastanawi A. A. El-Yamany M. F. Saad M. A. (2020). Dapagliflozin improves behavioral dysfunction of Huntington's disease in rats via inhibiting apoptosis-related glycolysis. Life Sci.257, 118076. 10.1016/j.lfs.2020.118076
14
Elbaz E. M. Sayed R. H. Abdelkader A. A. Fahim A. T. (2025). Neuroprotective role of morin hydrate on 3-nitropropionic acid-elicited Huntington'S disease: in vivo investigation of RIPK1/RIPK3/MLKL necroptosis signaling pathway. Mol. Med.31, 135. 10.1186/s10020-025-01172-y
15
Fan J. Dawson T. M. Dawson V. L. (2017). Cell death mechanisms of neurodegeneration. Neurodegener. Dis. Pathology, Mech. potential Ther. targets15, 403–425. 10.1007/978-3-319-57193-5_16
16
Gao Y. Chu S.-F. Li J.-P. Zhang Z. Yan J.-Q. Wen Z.-L. et al (2015). Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington's disease. Acta Pharmacol. Sin.36, 311–322. 10.1038/aps.2014.107
17
Gendy A. M. El-Sadek H. M. Amin M. M. Ahmed K. A. El-Sayed M. K. El-Haddad A. E. et al (2023a). Glycyrrhizin prevents 3-nitropropionic acid-induced neurotoxicity by downregulating HMGB1/TLR4/NF-κB p65 signaling, and attenuating oxidative stress, inflammation, and apoptosis in rats. Life Sci.314, 121317. 10.1016/j.lfs.2022.121317
18
Gendy A. M. Soubh A. Elnagar M. R. Hamza E. Ahmed K. A. Aglan A. et al (2023b). New insights into the role of berberine against 3-nitropropionic acid-induced striatal neurotoxicity: possible role of BDNF–TrkB–PI3K/Akt and NF-κB signaling. Food Chem. Toxicol.175, 113721. 10.1016/j.fct.2023.113721
19
Gonchar O. O. Maznychenko A. V. Klyuchko O. M. Mankovska I. M. Butowska K. Borowik A. et al (2021). C60 fullerene reduces 3-nitropropionic acid-induced oxidative stress disorders and mitochondrial dysfunction in rats by modulation of p53, Bcl-2 and Nrf2 targeted proteins. Int. J. Mol. Sci.22, 5444. 10.3390/ijms22115444
20
Gonzalez-Alegre P. Afifi A. K. (2006). Clinical characteristics of childhood-onset (juvenile) Huntington disease: report of 12 patients and review of the literature. J. child neurology21, 223–229. 10.2310/7010.2006.00055
21
Goyal R. Wilson K. Saharan A. Gautam R. K. Chopra H. Gupta S. et al (2024). Insights on aspects of apoptosis in neurodegenerative disorders: a comprehensive review. Explor. Med.5, 89–100. 10.37349/emed.2024.00208
22
Gu S. M. Park M. H. Hwang C. J. Song H. S. Lee U. S. Han S. B. et al (2015). Bee venom ameliorates lipopolysaccharide-induced memory loss by preventing NF-kappaB pathway. J. neuroinflammation12, 124–15. 10.1186/s12974-015-0344-2
23
Harrison C. (2012). A neuroprotective role for sirtuin 1. Nat. Rev. Drug Discov.11, 108. 10.1038/nrd3672
24
Huang K. Chen C. Hao J. Huang J. Wang S. Liu P. et al (2015). Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol. Cell. Endocrinol.399, 178–189. 10.1016/j.mce.2014.08.014
25
Ibrahim W. W. Abdel Rasheed N. O. (2022). Diapocynin neuroprotective effects in 3-nitropropionic acid Huntington’s disease model in rats: emphasis on Sirt1/Nrf2 signaling pathway. Inflammopharmacology30, 1745–1758. 10.1007/s10787-022-01004-z
26
Isik B. Ates I. Yucel N. Suleyman B. Mendil A. S. Sezgin E. T. et al (2025). The protective effect of thiamine and thiamine pyrophosphate against linezolid-induced oxidative liver damage and lactic acidosis in rats. Antioxidants14, 920. 10.3390/antiox14080920
27
Jazvinšćak Jembrek M. Oršolić N. Mandić L. Sadžak A. Šegota S. (2021). Anti-oxidative, anti-inflammatory and anti-apoptotic effects of flavonols: targeting Nrf2, NF-κB and p53 pathways in neurodegeneration. Antioxidants10, 1628. 10.3390/antiox10101628
28
Jiang M. Wang J. Fu J. Du L. Jeong H. West T. et al (2012). Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat. Med.18, 153–158. 10.1038/nm.2558
29
Khalifa M. Fayed R. H. Ahmed Y. H. Abdelhameed M. F. Essa A. F. Khalil H. (2025). Ferulic acid ameliorates bisphenol A (BPA)-induced Alzheimer’s disease-like pathology through Akt-ERK crosstalk pathway in male rats. Psychopharmacology242, 461–480. 10.1007/s00213-024-06697-4
30
Denny Joseph K. M. (2014). Neuroprotective efficacy of a combination of fish oil and ferulic acid against 3-nitropropionic acid-induced oxidative stress and neurotoxicity in rats: behavioural and biochemical evidence. Appl. Physiology, Nutr. Metabolism39, 487–496. 10.1139/apnm-2013-0262
31
Li B. Liu J. Gu G. Han X. Zhang Q. Zhang W. (2020a). Impact of neural stem cell‐derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level, and synaptic deficits in Alzheimer’s disease. J. Neurochem.154, 502–518. 10.1111/jnc.15001
32
Li X. Zhang J. Rong H. Zhang X. Dong M. (2020b). Ferulic acid ameliorates MPP+/MPTP-induced oxidative stress via ERK1/2-dependent Nrf2 activation: translational implications for Parkinson disease treatment. Mol. Neurobiol.57, 2981–2995. 10.1007/s12035-020-01934-1
33
Li D. Rui Y.-X. Guo S.-D. Luan F. Liu R. Zeng N. (2021). Ferulic acid: a review of its pharmacology, pharmacokinetics and derivatives. Life Sci.284, 119921. 10.1016/j.lfs.2021.119921
34
Liu Y.-M. Shen J.-D. Xu L.-P. Li H.-B. Li Y.-C. Yi L.-T. (2017). Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol.45, 128–134. 10.1016/j.intimp.2017.02.007
35
Liu M. Zhang C. Xu X. Zhao X. Han Z. Liu D. et al (2021). Ferulic acid inhibits LPS-induced apoptosis in bovine mammary epithelial cells by regulating the NF-κB and Nrf2 signalling pathways to restore mitochondrial dynamics and ROS generation. Veterinary Res.52, 104–111. 10.1186/s13567-021-00973-3
36
Mallik S. B. Mudgal J. Kinra M. Hall S. Grant G. D. Anoopkumar-Dukie S. et al (2023). Involvement of indoleamine 2, 3-dioxygenase (IDO) and brain-derived neurotrophic factor (BDNF) in the neuroprotective mechanisms of ferulic acid against depressive-like behaviour. Metab. Brain Dis.38, 2243–2254. 10.1007/s11011-023-01267-7
37
Manjula R. Anuja K. Alcain F. J. (2021). SIRT1 and SIRT2 activity control in neurodegenerative diseases. Front. Pharmacol.11, 585821. 10.3389/fphar.2020.585821
38
Mishra T. Nagarajan K. Dixit P. K. Kumar V. (2022). Neuroprotective potential of ferulic acid against cyclophosphamide‐induced neuroinflammation and behavioral changes. J. Food Biochem.46, e14436. 10.1111/jfbc.14436
39
Mosaoa R. M. Al-Rabia M. W. Asfour H. Z. Alhakamy N. A. Mansouri R. A. El-Agamy D. S. et al (2024). Targeting SIRT1/AMPK/Nrf2/NF-кB by sitagliptin protects against oxidative stress-mediated ER stress and inflammation during ANIT-induced cholestatic liver injury. Toxicology507, 153889. 10.1016/j.tox.2024.153889
40
Mustafa A. M. Rabie M. A. Zaki H. F. Shaheen A. M. (2021). Inhibition of Brain GTP cyclohydrolase I attenuates 3-nitropropionic acid-induced striatal toxicity: involvement of Mas receptor/PI3k/Akt/CREB/BDNF axis. Front. Pharmacol.12, 740966. 10.3389/fphar.2021.740966
41
Naia L. Rego A. C. (2015). Sirtuins: double players in Huntington's disease. Biochimica Biophysica Acta (BBA)-Molecular Basis Dis.1852, 2183–2194. 10.1016/j.bbadis.2015.07.003
42
Nasser A. H. Gendy A. M. El-Yamany M. F. El-Tanbouly D. M. (2022). Upregulation of neuronal progranulin mediates the antinociceptive effect of trimetazidine in paclitaxel-induced peripheral neuropathy: Role of ERK1/2 signaling. Toxicol. Appl. Pharmacol.448, 116096. 10.1016/j.taap.2022.116096
43
Neis V. B. Rosa P. B. Moretti M. Rodrigues A. L. S. (2018). Involvement of heme oxygenase-1 in neuropsychiatric and neurodegenerative diseases. Curr. Pharm. Des.24, 2283–2302. 10.2174/1381612824666180717160623
44
Nouri A. Ghatreh‐Samani K. Amini‐Khoei H. Najafi M. Heidarian E. (2023). Ferulic acid exerts a protective effect against cyclosporine‐induced liver injury in rats via activation of the Nrf2/HO‐1 signaling, suppression of oxidative stress, inflammatory response, and halting the apoptotic cell death. J. Biochem. Mol. Toxicol.37, e23427. 10.1002/jbt.23427
45
Palpagama T. H. Waldvogel H. J. Faull R. L. Kwakowsky A. (2019). The role of microglia and astrocytes in Huntington’s disease. Front. Mol. Neurosci.12, 258. 10.3389/fnmol.2019.00258
46
Patil S. P. Liu C. Alban J. Yang N. Li X.-M. (2014). Glycyrrhiza uralensis flavonoids inhibit brain microglial cell TNF-α secretion, p-IκB expression, and increase brain-derived neurotropic factor (BDNF) secretion. J. Traditional Chin. Med. Sci.1, 28–37. 10.1016/j.jtcms.2014.11.004
47
Paul B. D. Snyder S. H. (2019). Impaired redox signaling in Huntington’s disease: therapeutic implications. Front. Mol. Neurosci.12, 68. 10.3389/fnmol.2019.00068
48
Peixoto C. A. De Oliveira W. H. Da Racho Araújo S. M. Nunes A. K. S. (2017). AMPK activation: role in the signaling pathways of neuroinflammation and neurodegeneration. Exp. Neurol.298, 31–41. 10.1016/j.expneurol.2017.08.013
49
Pineda J. R. Canals J. M. Bosch M. Adell A. Mengod G. Artigas F. et al (2005). Brain‐derived neurotrophic factor modulates dopaminergic deficits in a transgenic mouse model of Huntington's disease. J. Neurochem.93, 1057–1068. 10.1111/j.1471-4159.2005.03047.x
50
Razick D. I. Akhtar M. Wen J. Alam M. Dean N. Karabala M. et al (2023). The role of sirtuin 1 (SIRT1) in neurodegeneration. Cureus15. 10.7759/cureus.40463
51
Rehman S. U. Ali T. Alam S. I. Ullah R. Zeb A. Lee K. W. et al (2019). Ferulic acid rescues LPS-induced neurotoxicity via modulation of the TLR4 receptor in the mouse hippocampus. Mol. Neurobiol.56, 2774–2790. 10.1007/s12035-018-1280-9
52
Ren Z. He H. Zuo Z. Xu Z. Wei Z. Deng J. (2019). The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. & Mol. Biol. Lett.24, 36–10. 10.1186/s11658-019-0158-9
53
Sambrook J. Fritsch E. F. Maniatis T. (1989). Molecular cloning: a laboratory manual.
54
Sayed N. H. Fathy N. Kortam M. A. Rabie M. A. Mohamed A. F. Kamel A. S. (2020). Vildagliptin attenuates Huntington's disease through activation of GLP-1 receptor/PI3K/Akt/BDNF pathway in 3-nitropropionic acid rat model. Neurotherapeutics17, 252–268. 10.1007/s13311-019-00805-5
55
Sethi P. Mehan S. Khan Z. Maurya P. K. Kumar N. Kumar A. et al (2025). The SIRT-1/Nrf2/HO-1 axis: guardians of neuronal health in neurological disorders. Behav. Brain Res.476, 115280. 10.1016/j.bbr.2024.115280
56
Shaheen M. J. Bekdash A. M. Itani H. A. Borjac J. M. (2021). Saffron extract attenuates neuroinflammation in rmTBI mouse model by suppressing NLRP3 inflammasome activation via SIRT1. PLoS One16, e0257211. 10.1371/journal.pone.0257211
57
Shalaby H. N. El-Tanbouly D. M. Zaki H. F. (2018). Topiramate mitigates 3-nitropropionic acid-induced striatal neurotoxicity via modulation of AMPA receptors. Food Chem. Toxicol.118, 227–234. 10.1016/j.fct.2018.05.022
58
Sharma P. Kumar M. Bansal N. (2021). Ellagic acid prevents 3-nitropropionic acid induced symptoms of Huntington’s disease. Naunyn-Schmiedeberg's Archives Pharmacol.394, 1917–1928. 10.1007/s00210-021-02106-1
59
Sharma V. Sharma P. Singh T. G. (2024). Mechanistic insights on TLR-4 mediated inflammatory pathway in neurodegenerative diseases. Pharmacol. Rep.76, 679–692. 10.1007/s43440-024-00613-5
60
Sharma V. Sharma P. Singh T. G. (2025). Mechanistic insights on the role of Nrf-2 signalling in Huntington’s disease. Neurol. Sci.46, 593–604. 10.1007/s10072-024-07802-3
61
Shawki S. M. Saad M. A. Rahmo R. M. Wadie W. El-Abhar H. S. (2021). Liraglutide improves cognitive and neuronal function in 3-NP rat model of Huntington’s disease. Front. Pharmacol.12, 731483. 10.3389/fphar.2021.731483
62
Singh S. Arthur R. Upadhayay S. Kumar P. (2022). Ferulic acid ameliorates neurodegeneration via the Nrf2/ARE signalling pathway: a Review. Pharmacol. Research-Modern Chin. Med.5, 100190. 10.1016/j.prmcm.2022.100190
63
Song Y. Wu Z. Zhao P. (2022). The protective effects of activating Sirt1/NF-κB pathway for neurological disorders. Rev. Neurosci.33, 427–438. 10.1515/revneuro-2021-0118
64
Tang X. Zhao Y. Zhou Z. Yan J. Zhou B. Chi X. et al (2020). Resveratrol mitigates sevoflurane‐induced neurotoxicity by the SIRT1‐dependent regulation of BDNF expression in developing mice. Oxidative Med. Cell. Longev.2020, 9018624. 10.1155/2020/9018624
65
Thapliyal S. Singh T. Handu S. Bisht M. Kumari P. Arya P. et al (2021). A review on potential footprints of ferulic acid for treatment of neurological disorders. Neurochem. Res.46, 1043–1057. 10.1007/s11064-021-03257-6
66
Túnez I. Tasset I. Pérez-De La Cruz V. Santamaría A. (2010). 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: past, present and future. Molecules15, 878–916. 10.3390/molecules15020878
67
Valadão P. A. C. Santos K. B. S. E Vieira T. H. F. E Cordeiro T. M. Teixeira A. L. Guatimosim C. et al (2020). Inflammation in Huntington's disease: a few new twists on an old tale. J. Neuroimmunol.348, 577380. 10.1016/j.jneuroim.2020.577380
68
Van Antwerp D. J. Martin S. J. Verma I. M. Green D. R. (1998). Inhibition of TNF-induced apoptosis by NF-κB. Trends Cell. Biol.8, 107–111. 10.1016/s0962-8924(97)01215-4
69
Vaziri H. Dessain S. K. Eaton E. N. Imai S.-I. Frye R. A. Pandita T. K. et al (2001). hSIR2SIRT1 functions as an NAD-dependent p53 deacetylase. Cell.107, 149–159. 10.1016/s0092-8674(01)00527-x
70
Wang X. Shao N. Zhang X. Chen H. Chang Z. Xie D. et al (2023). Ferulic acid activates SIRT1-mediated ferroptosis signaling pathway to improve cognition dysfunction in Wilson’s disease. Neuropsychiatric Dis. Treat.19, 2681–2696. 10.2147/NDT.S443278
71
Zeni A. L. B. Zomkowski A. D. E. Maraschin M. Rodrigues A. L. S. Tasca C. I. (2012). Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: evidence for the involvement of the serotonergic system. Eur. J. Pharmacol.679, 68–74. 10.1016/j.ejphar.2011.12.041
72
Zhang D. Jing B. Chen Z. N. Li X. Shi H. M. Zheng Y. C. et al (2023). Ferulic acid alleviates sciatica by inhibiting neuroinflammation and promoting nerve repair via the TLR4/NF‐κB pathway. CNS Neurosci. & Ther.29, 1000–1011. 10.1111/cns.14060
Summary
Keywords
3-Nitropropionic acid, ferulic acid, SIRT1, Nrf2, neuroinflammation, TLR4
Citation
Abdelgawad MA, Gendy AM, Zaghlool SS, Elesawy WH, Ragab MF, Kotb El-Sayed MI, El-Haddad AE, Mohamed HS, Alsalahat I and Essa MA (2025) Ferulic acid mitigates 3-Nitropropionic acid-induced Huntington’s disease via modulation of Nrf2/HO-1, TLR4/NF-κB, and SIRT1/p53 signaling pathways. Front. Pharmacol. 16:1678724. doi: 10.3389/fphar.2025.1678724
Received
03 August 2025
Accepted
22 September 2025
Published
01 October 2025
Volume
16 - 2025
Edited by
Marcos Roberto De Oliveira, Federal University of Rio Grande do Sul, Brazil
Reviewed by
Syed Shams Ul Hassan, Shanghai Jiao Tong University, China
Hamiyet Eciroğlu-Sarban, Alanya Alaaddin Keykubat University, Türkiye
Updates
Copyright
© 2025 Abdelgawad, Gendy, Zaghlool, Elesawy, Ragab, Kotb El-Sayed, El-Haddad, Mohamed, Alsalahat and Essa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed A. Abdelgawad, mhmdgwd@ju.edu.sa; Izzeddin Alsalahat, alsalahati1@cardiff.ac.uk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.