Abstract
Introduction:
Staphylococcus aureus is a prominent pathogen capable of causing systemic infections and multi-organ damage, primarily driven by its high virulence and induction of oxidative stress. This study evaluated the therapeutic efficacy of tiamulin alone and in combination with bromhexine in a canine model of systemic S. aureus infection, focusing on oxidative stress biomarkers, bacterial burden, tissue histopathology, and the expression of cardiac and bacterial virulence-related genes.
Methods:
Experimental infection was induced in dogs, except for a healthy control group. Animals were assigned to five groups: uninfected control, infected untreated, tiamulin-treated, bromhexine-treated, and tiamulin plus bromhexine-treated. Oxidative stress was assessed through measurements of malondialdehyde (MDA) and total antioxidant capacity (TAC) in cardiac, hepatic, and renal tissues. Bacterial load was quantified, and minimum inhibitory concentrations (MICs) of the treatments were determined. Quantitative PCR was performed to evaluate the expression of S. aureus virulence genes including hla (alpha-hemolysin), ebpS (extracellular matrix-binding protein S), and icaA (intercellular adhesion A). Histopathological analyses of heart, liver, and kidney tissues were conducted, and hematological and biochemical parameters (total protein, albumin, and globulin) were measured. Cardiac injury markers, cytochrome P450 1B1 (CYP1B1) and interleukin-1 beta (IL-1β), were also assessed.
Results:
Infected untreated animals exhibited significantly elevated MDA, decreased TAC, high bacterial loads, severe histopathological alterations, and upregulated expression of IL-1β and CYP1B1. Tiamulin monotherapy produced moderate reductions in oxidative stress and bacterial burden. The combination of tiamulin and bromhexine resulted in a significant reduction in MDA, restoration of TAC, lower MIC values, suppressed expression of virulence genes (p < 0.05), and near-normal tissue architecture. Cardiac gene expression analysis showed substantial downregulation of IL-1β and CYP1B1 in the combination-treated group, indicating alleviation of inflammation and cardiac injury.
Discussion:
The combination therapy of tiamulin and bromhexine exhibited superior protective effects against S. aureus infection compared to monotherapy or untreated infection. These benefits appear to be mediated through synergistic antimicrobial, anti-virulence, and antioxidant mechanisms. The findings support the potential of this therapeutic approach for managing drug-resistant S. aureus infections and justify further clinical investigation.
Graphical Abstract

1 Introduction
The rapid emergence of multidrug-resistant (MDR) bacterial infections represents a significant threat to the efficacy of current antibiotic therapies and constitutes a major global public health challenge (Soliman et al., 2016). In response, the World Health Organization (WHO) has prioritized a group of critical MDR pathogens—including Staphylococcus aureus, Enterococcus faecium, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Enterobacter spp.—as leading causative agents of healthcare-associated infections. This alarming situation underscores the urgent need to develop innovative antimicrobial strategies to counteract MDR bacterial diseases (Ahmed et al., 2015).
Among these pathogens, S. aureus stands out as a significant zoonotic agent with the capacity to survive under diverse and extreme environmental conditions (Khalifa et al., 2024). It has developed resistance to multiple classes of antibiotics, notably methicillin, resulting in methicillin-resistant S. aureus (MRSA), which exhibits broad resistance to most β-lactam antibiotics (Khalifa et al., 2024; Oreiby et al., 2019). In addition to its antibiotic resistance, S. aureus readily forms biofilms, further enhancing its resilience against antimicrobial agents and complicating clinical treatment. This pathogen’s ability to infect various organs—including the heart, lungs, bones, brain, liver, and kidneys—contributes to its high virulence and adaptability, causing conditions that range from mild dermal infections to life-threatening systemic diseases (Mustafa et al., 2022; Touaitia et al., 2025).
Combining mucolytic agents with antibiotics has emerged as a promising approach to enhance therapeutic outcomes in MDR bacterial infections (Sui et al., 2025). Although studies on the concurrent use of bromhexine and tiamulin against S. aureus infections in dogs are limited, the pharmacodynamic properties of both drugs suggest potential synergistic effects. Bromhexine’s mucolytic action may improve antibiotic penetration into infected tissues, particularly in cases of respiratory tract infections, such as pulmonary pneumonia and septicemia caused by S. aureus (Parker and Prince, 2012).
Tiamulin is a semisynthetic pleuromutilin derivative, originating from the natural diterpene compound isolated from Pleurotus mutilus in 1951. Its unique tricyclic structure enables specific binding to the 50S ribosomal subunit of bacteria, thereby inhibiting protein synthesis. Tiamulin exhibits potent bacteriostatic activity against Mycoplasma spp., Gram-positive bacteria, and Leptospira spp., with possible bactericidal effects at higher concentrations. It is approved solely for veterinary use by the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) (Zhou et al., 2023; Sartini et al., 2023). Nonetheless, its administration must be carefully controlled due to the risk of adverse effects, including hepatotoxicity, nephrotoxicity, neurotoxicity, and cardiotoxicity (Pankowska et al., 2024).
Bromhexine, a widely used mucolytic agent, is a synthetic derivative of vasicine—an alkaloid extracted from the medicinal plant Adhatoda vasica (Ahmadi et al., 2024). It reduces mucus viscosity and enhances mucociliary clearance, thereby facilitating deeper tissue penetration of co-administered antibiotics and improving their pharmacological efficacy (Deretic and Timmins, 2019). Additionally, bromhexine exhibits anti-inflammatory properties, which may help alleviate respiratory tract inflammation and contribute indirectly to cardiovascular protection (Marinov et al., 2025).
This study aims to investigate the therapeutic efficacy of bromhexine and tiamulin, individually and in combination, in the treatment of S. aureus infections in dogs. It further seeks to determine whether bromhexine can potentiate the antimicrobial activity of tiamulin, particularly in infections associated with potential cardiotoxic effects.
2 Materials and methods
2.1 Chemicals and bacterial isolates
Tiamulin hydrogen fumarate (purity ≥98.0%) was obtained in powder form from Pharma-Swede (Cairo, Egypt). Bromhexine hydrochloride (98% purity) was also kindly provided by Pharma-Swede® as a white crystalline powder; its chemical name is 2-amino-3,5-dibromobenzyl methylamine hydrochloride. The Staphylococcus aureus strain ATCC 6538 used in this study was procured from the Animal Health Research Institute, Shibin Elkom branch, Egypt. This strain exhibited key virulence traits, including catalase positivity and the presence of virulence-associated genes. In addition to conventional biochemical identification (Supplementary Table 1), the identity of the S. aureus strain was molecularly confirmed by polymerase chain reaction (PCR) following the method described by Mohammed et al. (2025).
2.2 Determination of minimum inhibitory concentration (MIC) against S. aureus
The minimum inhibitory concentrations (MICs) of tiamulin, bromhexine hydrochloride, and their combination against Staphylococcus aureus strain ATCC 6538 were determined in vitro. A bacterial suspension of S. aureus at a concentration of 107 CFU/mL was prepared in its specific culture medium. Tiamulin was serially diluted two-fold across a concentration range of 0.7812–100 μg/mL using the culture medium as a 1:1 diluent. Similarly, bromhexine hydrochloride was subjected to two-fold serial dilutions ranging from 16 to 2048 μg/mL.
Combination assays were also conducted using fixed-ratio dilutions of the two agents. All experiments were performed in triplicate to ensure reproducibility and accuracy. The MIC was defined as the lowest concentration of tiamulin, bromhexine, or their combination that inhibited visible bacterial growth, as indicated by the absence of a color change in the culture medium. It should be noted that the fractional inhibitory concentration index (FICI) was not calculated in this study, as the combination assay was designed primarily to determine MIC changes rather than to quantify synergistic effects (Abonashey et al., 2024).
2.3 In vitro assessment of S. aureus virulence-related gene expression level using PCR and real-time PCR
2.3.1 Genotypic and gene expression analysis
Genotypic characterization of Staphylococcus aureus isolates was performed to detect key virulence-associated genes, including hla (alpha-hemolysin), ebpS (extracellular matrix-binding protein S), and icaA (intercellular adhesion gene A). The 16S small subunit ribosomal RNA gene (16S rRNA) was used as a housekeeping gene. Primers used in this study, supplied by Metabion (Germany), are listed in Supplementary Table 3. Previous research has demonstrated that exposure to sub-MIC levels of antibiotics, including tiamulin and bromhexine, can modulate the expression of virulence genes such as hla, ebpS, and icaA within 3 hours (Yang et al., 2020). Conventional PCR primers targeting these genes were validated for use in SYBR Green-based quantitative PCR (qPCR), as endpoint PCR primers have been shown to be effective for real-time PCR applications (Thornton and Basu, 2011).
2.3.2 DNA extraction and PCR
Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Germany, GmbH) with minor modifications to the manufacturer’s protocol. Briefly, 200 µL of bacterial suspension was incubated with 20 µL of proteinase K and 200 µL of lysis buffer at 56 °C for 10 min. Following incubation, 200 µL of absolute ethanol was added, and the sample was processed according to the standard washing and centrifugation steps. DNA was eluted in 100 µL of the elution buffer provided in the kit. Uniplex PCR was performed in 25 µL reaction volumes containing 12.5 µL of EmeraldAmp Max PCR Master Mix (Takara, Japan), 1 µL of each primer (20 pmol), 5.5 µL of nuclease-free water, and 5 µL of DNA template. Amplifications were conducted in an Applied Biosystems 2,720 thermal cycler. PCR products were resolved on 1.5% agarose gels (Applichem, Germany, GmbH) using 1× TBE buffer at room temperature (5 V/cm). For analysis, 20 µL of uniplex and 30 µL of multiplex PCR products were loaded per well. A GelPilot 100 bp Plus DNA Ladder (Qiagen, GmbH, Germany) was used to estimate fragment sizes. Gels were visualized using a gel documentation system (Alpha Innotech, Biometra), and band analysis was performed with dedicated software.
2.3.3 RNA extraction and quantitative real-time PCR (qPCR)
To prevent RNA degradation, 1 mL of RNAprotect Bacteria Reagent (Qiagen, Germany, GmbH) was added to 0.5 mL of harvested bacterial culture. The mixture was vortexed, incubated at room temperature for 5 min, and centrifuged at 8,000 rpm for 10 min. The resulting pellet was resuspended in 200 µL of Tris-EDTA buffer containing 1 mg/mL lysozyme (Biochemica, Applichem), followed by the addition of 700 µL of RLT buffer supplemented with 10 μL/mL β-mercaptoethanol. Subsequently, 500 µL of 100% ethanol was added, and total RNA was extracted following the Enzymatic Lysis of Bacteria protocol provided in the QIAamp RNeasy Mini Kit (Qiagen, Germany, GmbH).
The one-step SYBR Green RT-qPCR reaction mixture (25 µL total volume) included 12.5 µL of 2× QuantiTect SYBR Green PCR Master Mix (Qiagen, Germany, GmbH), 0.25 µL of RevertAid Reverse Transcriptase (200 U/µL, Thermo Fisher), 0.5 µL of each primer (20 pmol), 8.25 µL of nuclease-free water, and 3 µL of RNA template. Amplification was carried out in a Stratagene MX3005P real-time PCR system under the following thermal profile: reverse transcription at 50 °C for 30 min; initial denaturation at 94 °C for 15 min; followed by 40 cycles of 94 °C for 15 s, annealing at 49 °C–55 °C (depending on the primer) for 30 s, and extension at 72 °C for 30 s. The dissociation (melting) curve protocol included 95 °C for 1 min and a stepwise increase from 65 °C (+0.5 °C per cycle) for 61 cycles to confirm specificity of the amplified products. Amplification curves and threshold cycle (Ct) values were analyzed using the Stratagene MX3005P software. To evaluate changes in gene expression, Ct values for each gene in treated samples were compared to those in the positive control group using the 2–ΔΔCt method described by Yuan et al. (2006). Fold changes were calculated to determine upregulation or downregulation of hla, ebpS, and icaA in response to treatment with sub-MIC levels of tiamulin and bromhexine.
2.4 Animals and trial design
A total of twenty-five male dogs, between 3 and 6 months of age, were individually housed for a 2-week acclimation period, during which they had unrestricted access to food and water. The local Egyptian dog breed, commonly referred to as 'Baladi dogs', was used in this study. These dogs were obtained from a trusted local farm specializing in native breeds, El Monoufiya, Egypt. The owners' informed consent was obtained for the participation of their animals in this study. All experimental procedures complied with the Guidelines for Animal Experimentation and received approval from the Ethical Committee of the Faculty of Veterinary Medicine, University of Sadat City, Egypt. Animal care and handling were performed in line with the regulations and standards of the Animal Care House (Approval No. VUSC-007-1-25). The potential effect of bromhexine hydrochloride on the efficacy of tiamulin against Staphylococcus aureus infection in dogs was determined using a protocol similar to that previously described by Radi et al. (2020). Dogs were randomly allocated into five groups of five dogs each, as illustrated in Supplementary Table 2. After shaving each dog’s fur, the skin was cleaned with soap and sterile water. Dogs were given a 1 mL intradermal inoculation under local anesthesia with 105 S. aureus (strain ATCC 6538) colony-forming units, as previously reported by Kamr et al. (2020) and Arbaga et al. (2021), except the negative control group, which was injected with 1 mL of normal saline. The onset of cutaneous lesions occurred 3 days after injection. Skin infection was induced in the experimental animals according to standardized procedures. The successful establishment of the infection model was confirmed by observing characteristic clinical signs, including erythema (redness), edema (swelling), pus formation, and necrotic tissue at the site of inoculation within 48–72 h post-infection. Representative photographic images of the lesions were captured at different time points (pre-infection, post-infection, and post-treatment) to document the progression of infection and the therapeutic effects of the treatments, including the combination therapy. These images provide visual confirmation of model establishment and wound healing outcomes as in Figure 1. On day 3 post-inoculation, the skin lesions were swabbed using sterile swabs to verify their authenticity. To identify the developed colonies, samples were cultivated on Baird-Parker agar medium at 37 °C for 24 h after being subjected to Gram staining. Dogs were separated into groups as shown in Supplementary Table 2. The experiment lasted for 9 days.
FIGURE 1

Representative images showing the development and healing of skin lesions in Staphylococcus aureus–infected dogs. (a) Normal uninfected skin; (b,c) infected, untreated group displaying visible redness, swelling, and crust formation; (d) tiamulin-treated group showing partial recovery and reduced inflammation; (e) bromhexine-treated group showing moderate improvement with decreased erythema and crusting; (f) combination-treated group (tiamulin + bromhexine) exhibiting near-complete healing and hair regrowth, indicating enhanced therapeutic efficacy. Lesion severity was scored as follows: 0 = normal skin; 1 = mild erythema or minimal crusts; 2 = moderate erythema with visible crusts or partial hair loss; 3 = severe lesions with ulceration, thick crusts, or extensive inflammation. Representative scores: (a) 0, (b,c) 2, (d) 1, (e) 1–2, (f) 0–1.
Group 1, the negative control group, was intradermally injected with normal saline on day 1 and received 1 mL of distilled water orally for five consecutive days starting on day 3. In Group 2, the positive control group, dogs were injected intradermally with 1 mL of 105 CFU S. aureus on day 1 and received 1 mL of distilled water orally for five consecutive days starting on day 3. Group 3 dogs were injected with 1 mL of 105 CFU S. aureus on day 1 and, beginning on day 3, received tiamulin at a dose of 10 mg/kg body weight once daily (every 24 h) orally for 5 days (Laber, 1988; Garmyn et al., 2017). Group 4 dogs were injected with 1 mL of 105 CFU S. aureus on day 1 and, starting on day 3, received bromhexine hydrochloride at a dose of 1 mg/kg body weight twice daily (every 12 h) orally for 5 days (Radi et al., 2020). Group 5 dogs were injected with 1 mL of 105 CFU S. aureus on day 1 and, beginning on day 3, received tiamulin (10 mg/kg body weight, once daily) along with bromhexine hydrochloride (1 mg/kg body weight, twice daily) orally for five consecutive days.
2.5 Quantitative assessment of S. aureus in treated and control groups
Skin swab samples were aseptically collected from pyoderma lesions of dogs in each group 5 days after the initiation of treatment using sterile cotton swabs. The swabs were gently rotated over the infected area to ensure adequate sampling while avoiding contamination from surrounding healthy skin. Each swab was transferred into a sterile tube containing phosphate-buffered saline (PBS), vortexed, and subjected to serial tenfold dilutions. From the 103 and 105 dilutions, 100 µL aliquots were inoculated onto Baird-Parker Agar (Oxoid, United Kingdom) plates and incubated aerobically at 37 °C for 24–48 h. Presumptive S. aureus colonies appeared as black, shiny colonies with clear halos, measuring 1–3 mm in diameter with distinct margins. Colonies were confirmed through Gram staining, catalase, and coagulase tests. Bacterial counts were expressed as colony-forming units per milliliter (CFU/mL). Isolates were identified using conventional microbiological techniques, including Gram staining and a series of biochemical assays such as coagulase activity, catalase test, mannitol fermentation, urease activity, nitrate reduction, mannose and lactose fermentation, phosphatase, arginine dihydrolase, and indoxyl phosphatase activity, as outlined in Supplementary Table 1 (Koneman et al., 2006).
2.6 Blood and tissue sampling
On the ninth day, following an overnight fast, blood samples were collected from the jugular vein of each dog. Each sample was divided into two aliquots: one treated with EDTA for hematological analysis, and the other left untreated to allow clotting. The clotted samples were centrifuged at 3,000 rpm for 15 min to separate the serum, which was subsequently stored at −20 °C for biochemical assays. After blood collection, the animals were euthanized via intravenous administration of a lethal dose of pentobarbital. Tissue specimens from the heart, liver, and kidneys were harvested (Tao et al., 2022). A portion of each tissue was stored at −80 °C for subsequent biochemical and gene expression analyses, while the remaining portions were fixed in 10% neutral buffered formalin for histopathological examination.
2.7 Hematological analysis
Immediately after collection, blood samples were subjected to hematological analysis to determine total leukocyte count (TLC), differential leukocyte counts, hemoglobin concentration (Hb), platelet count (Plt), hematocrit percentage (PCV%), and red blood cell count (RBCs), using an automated hematology analyzer (Sysmex F-800, Tokyo, Japan) (El-Gendy et al., 2024a).
2.8 Biochemical assay
Biochemical markers related to cardiac, hepatic, and renal function were assessed in the collected serum samples, following the manufacturers' protocols. For cardiac evaluation, total creatine kinase (CK) and creatine kinase-MB (CK-MB), specific to myocardial tissue, were measured using an ELISA kit (CK-MB, ThermoFisher Scientific, Waltham, MA, USA), as described by Aujla and Patel (2022). Additionally, troponin I levels were quantified using a commercial ELISA kit (Troponin, ThermoFisher Scientific, Waltham, MA, USA), according to the method outlined by Jiang et al. (2017), in accordance with the manufacturer’s instructions. For hepatic evaluation, hepatic enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), were measured in serum samples using commercially available kits supplied by Biodiagnostic Company (Dokki, Giza, Egypt) (El-Gendy et al., 2022). For renal evaluation, renal biomarkers were evaluated in serum samples using commercial kits in accordance with the manufacturer’s instructions (Biomed Company, Cairo, Egypt). Renal function indicators, including creatinine, blood urea nitrogen (BUN), and urea, were quantified as previously described (Li et al., 2024). Additionally, serum total protein, albumin, and globulin concentrations were determined following the method of Henry et al. (1974).
2.9 Absolute and relative organ weights
On the day of sacrifice, the dogs were weighed using a precision scale. Following euthanasia, the heart, liver, and kidneys were carefully excised, cleared of surrounding tissues, and weighed. The relative organ weights (ROW) were calculated according to the method described by Mazukina et al. (2025), using the following formula: ROW = [Absolute organ weight (g)/Body weight of dog (g)] × 100.
2.10 Assessment of oxidative and antioxidant biomarkers in tissue homogenates
Portions of hepatic, renal, and cardiac tissues were homogenized in cold phosphate-buffered saline (PBS) using a glass homogenizer to prepare 25% (w/v) tissue homogenates. The homogenates were centrifuged at 10,000–12,000 rpm for 5 min, and the resulting supernatants were stored at −80 °C for subsequent analyses. Oxidative stress markers and antioxidant capacity were assessed colorimetrically using commercial assay kits (Biodiagnostic, Dokki, Giza, Egypt), following the manufacturer’s instructions. Specifically, malondialdehyde (MDA) levels were measured as described by Bahramibanan et al. (2025), and total antioxidant capacity (TAC) was evaluated according to Cordiano et al. (2023).
2.11 Assessment of cardiac gene expression level using quantitative RT-PCR
RNA was extracted from cardiac tissue samples using the Direct-zol™ RNA Miniprep Plus Kit (Zymo Research, Cat# R2072, USA), following the manufacturer’s protocol. This method allows direct purification of high-quality total RNA, including small RNAs, from TRIzol®-treated samples using Zymo-Spin™ IIICG columns. On-column DNase I treatment was performed to eliminate residual genomic DNA. RNA yield and purity were assessed spectrophotometrically (Beckman, USA) by measuring absorbance at 260 nm and calculating the A260/A280 ratio.
Subsequently, reverse transcription and real-time PCR (RT-qPCR) were performed using the SuperScript™ IV One-Step RT-PCR System (Thermo Fisher Scientific, Cat# 12594100, USA), which enables cDNA synthesis and amplification in a single tube using gene-specific primers. Reactions were conducted on a StepOne™ Real-Time PCR System (Applied Biosystems, USA). Specific primers for inflammation, mitochondrial dysfunction, and cardiac injury marker genes (Supplementary Table 3) were verified for specificity using NCBI BLAST.
The thermal cycling conditions were as follows: reverse transcription at 55 °C for 10 min; enzyme inactivation at 95 °C for 2 min; followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 10 s, and extension at 72 °C for 30 s.
Gene expression levels were evaluated using cycle threshold (Ct) values. The Ct values of target genes (IL-1β and CYP1B1) were normalized to the reference gene (GAPDH). A control group was used as a calibrator to assess relative expression. Relative quantification (RQ) was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001), allowing determination of fold changes in gene expression.
2.12 Histopathological examination
Liver, kidney, and heart tissue samples were collected and promptly fixed in 10% neutral buffered formalin. Following fixation, the tissues were washed thoroughly, dehydrated through a graded series of alcohol, cleared in xylene, and embedded in paraffin wax. Sections of 5 μm thickness were then prepared from the paraffin blocks and stained with hematoxylin and eosin (H&E) for histological examination, according to the standard method outlined by Bancroft and Gamble (2008). Histopathological alterations in liver, kidney, and heart were scored as: no changes (0), mild (1), moderate (2), and severe (3) changes. The grading was determined by percentage: <30% changes (mild change), 30%–50% (moderate change), and >50% (severe change) (Zaki et al., 2024).
2.13 Statistical analysis
The data were analyzed using one-way ANOVA (SPSS, version 21). Duncan’s multiple range test was applied to compare group means at a significance level of p < 0.05. The statistical procedures followed the guidelines of Sendecor and Cochran (1956). Results are expressed as means ± standard error (SE).
3 Results
3.1 Comparative analysis of bacterial load (CFU/mL) among study groups
Table 1 and Figure 2 present the quantitative evaluation of bacterial loads (CFU/mL) across the experimental groups. All treatment groups exhibited a notable and statistically significant reduction in bacterial counts compared to the positive control group (G2), at both 103 and 105 dilution levels. This consistent decrease underscores the potent antibacterial activity of the tested interventions.
TABLE 1
| Groups | G1 | G2 | G3 | G4 | G5 | P-value |
|---|---|---|---|---|---|---|
| 103 dilutions | 5.00 ± 0.73a | 65.00 ± 2.55b | 23.17 ± 2.38c | 39.66 ± 0.92d | 30.00 ± 3.58e | 0.002 |
| 105 dilutions | 1.50 ± 0.22c | 43.66 ± 2.23b | 10.00 ± 1.34e | 22.00 ± 0.45d | 13.33 ± 0.56e | 0.606 |
Comparison of bacterial Counts (CFU/mL) between tiamulin monotherapy and tiamulin–bromhexine combination therapy.
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
FIGURE 2

Bacterial count (CFU/mL) in different treatment groups at 103 and 105 dilutions.
Notably, the group treated with the combined therapy of bromhexine and tiamulin (Group 5) demonstrated substantial bacterial suppression. Although the reduction in CFU/ml at the 105 dilution level was not statistically significant compared to the tiamulin-only group, the values remained closely comparable, suggesting a similar therapeutic effect. These findings indicate that co-administration of bromhexine does not compromise the antibacterial efficacy of tiamulin and may potentially enhance it, possibly through improved drug delivery or mucolytic action. Overall, statistical analysis confirmed significant differences among the groups (P < 0.05), supporting the antibacterial effectiveness of the applied treatment protocols.
3.2 In vitro MIC values
The in vitro MICs of the isolated Staphylococcus aureus strain were determined using the broth microdilution method and are presented in Supplementary Table 4. The MIC values for tiamulin, bromhexine, and their combination (tiamulin + bromhexine) were 12.5, 128, and (6.25 + 64) μg/mL, respectively. These findings indicate that the tested treatments effectively reduced bacterial resistance in vitro. Notably, the combination therapy demonstrated an improved MIC compared to either agent alone, suggesting a potential synergistic or additive effect.
3.3 Molecular detection of virulence genes by PCR and RT- PCR
The presence of selected virulence-associated genes in the Staphylococcus aureus isolates was confirmed by conventional PCR. Amplification products corresponding to the hla, icaA, and ebpS genes were successfully detected by agarose gel electrophoresis, with distinct bands appearing at their respective expected sizes: hla (704 bp), icaA (1,315 bp), and ebpS (652 bp), as shown in Supplementary Figure 2. Additionally, the 16S rRNA gene (∼1,500 bp) was consistently amplified in all samples, confirming bacterial identity and serving as a positive internal control.
Furthermore, real-time PCR using SYBR Green fluorescence detection was employed to assess the expression levels of selected S. aureus virulence genes. Consistent amplification curves and specific melt peaks confirmed the reliability of this method, supporting the gel-based findings (Table 2; Supplementary Figure 3, p < 0.05). Relative gene expression analysis revealed a significant reduction in the expression of virulence genes in samples treated with the combination of bromhexine and tiamulin, followed by bromhexine alone and tiamulin alone, respectively.
TABLE 2
| Genes | 16S rRNA | Hla | ebpS | ica A | P-value |
|---|---|---|---|---|---|
| Control (S. aureus) | 1.00 ± 0.001 | 1.00 ± 0.001a | 1.00 ± 0.001a | 1.00 ± 0.001a | 0.0001 |
| Tiamulin + S. aureus | 1.00 ± 0.001 | 0.44 ± 0.006b | 0.61 ± 0.0005b | 0.48 ± 0.003b | 0.0001 |
| Bromhexine + S. aureus | 1.00 ± 0.001 | 0.25 ± 0.003c | 0.36 ± 0.0016c | 0.36 ± 0.003c | 0.0001 |
| Tiamulin + Bromhexine + S. aureus | 1.00 ± 0.001 | 0.07 ± 0.006d | 0.21 ± 0.0005d | 0.10 ± 0.0005d | 0.0001 |
In vitro quantitative detection of virulence targeted genes in S. aureus isolates.
16S rRNA, 16S small subunit ribosomal RNA, gene; hla, alpha-hemolysin gene; ebpS, extracellular matrix-binding protein S gene; ica A, intercellular adhesion gene A.
Means with no shared superscript in the same column vary significantly (p < 0.05).
Means with no shared superscript in the same column vary significantly (p < 0.05).
Means with no shared superscript in the same column vary significantly (p < 0.05).
Means with no shared superscript in the same column vary significantly (p < 0.05).
3.4 Absolute and relative organ weights
In our experiments, absolute organ weights varied considerably among the groups, reflecting corresponding differences in total body weight. Significant differences were observed in both absolute and relative weights of the liver, kidneys, and heart across the treatment groups, as presented in Table 3. Specifically, relative liver and kidney weights were significantly higher than those of the control group (p < 0.05). Additionally, the relative heart weight was significantly increased in Groups 3 and 5 compared to the other groups (p < 0.05).
TABLE 3
| Groups parameters | G1 | G2 | G3 | G4 | G5 | P-value |
|---|---|---|---|---|---|---|
| Absolute liver weight (g) | 156.67 ± 1.52a | 178.47 ± 0.18b | 165.03 ± 2.74c | 162.90 ± 0.95c | 171.83 ± 0.57d | 0.004 |
| Relative liver weight (%) | 9.55 ± 0.09c | 13.06 ± 0.11b | 10.11 ± 0.15d | 10.40 ± 0.12d | 9.61 ± 0.10c | 0.0001 |
| Absolute kidney weight (g) | 22.63 ± 0.23c | 23.46 ± 0.18c,d | 24.16 ± 0.33b,d | 25.03 ± 0.73b | 22.60 ± 0.22c | 0.001 |
| Relative kidney weight (%) | 1.38 ± 0.02a | 1.71 ± 0.02b | 1.48 ± 0.01c | 1.59 ± 0.05d | 1.26 ± 0.02e | 0.003 |
| Absolute heart weight (g) | 21.60 ± 0.20e | 25.36 ± 0.20a | 36.06 ± 0.40d | 27.66 ± 0.55c | 42.50 ± 0.76b | 0.0001 |
| Relative heart weight (%) | 1.31 ± 0.02a | 1.85 ± 0.02c | 2.21 ± 0.02d | 1.76 ± 0.04c | 2.37 ± 0.04b | 0.0001 |
Effect of tiamulin and bromhexine on absolute and relative organs weight of dogs infected with S. aureus.
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
3.5 Blood measurements
Table 4 presents the effects of tiamulin, bromhexine, and their combination on erythrogram parameters in dogs experimentally infected with S. aureus. Compared to the infected untreated group (G2), all treated groups (G3–G5) showed a significant restoration of packed cell volume (PCV) to near-normal levels (p < 0.05). Mean corpuscular volume (MCV) was highest in G3 (75.06 ± 0.02) and lowest in G1 (64.26 ± 0.22), with significant differences observed among groups bearing different superscript letters (p < 0.05). Regarding mean corpuscular hemoglobin (MCH), G3 exhibited the highest value (25.62 ± 0.04), which was significantly greater than all other groups (p < 0.05), while no significant differences were noted among the remaining groups. For mean corpuscular hemoglobin concentration (MCHC), significantly higher values were observed in G1, G3, G4, and G5 compared to G2 (p < 0.05). Overall, the infected untreated dogs (G2) demonstrated significantly lower erythrogram indices than the treated groups (p < 0.05). Platelet counts were significantly higher in G1 (459 ± 14.45) compared to all other groups, which showed marked reductions in platelet levels (p < 0.05). Moreover, G2 exhibited significantly elevated total leukocyte counts (TLC), neutrophil percentages, and neutrophil-to-lymphocyte (N/L) ratio, along with a significant reduction in lymphocyte percentages, when compared to the treated groups (p < 0.05).
TABLE 4
| Groups parameters | G1 | G2 | G3 | G4 | G5 | P-value |
|---|---|---|---|---|---|---|
| Hb (g/dL) | 9.22 ± 0.04a | 8.02 ± 0.14b | 9.06 ± 0.02a | 9.72 ± 0.17c | 8.66 ± 0.11d | 0.012 |
| RBCs (106/μL) | 3.78 ± 0.012c | 3.01 ± 0.14d | 3.45 ± 0.01a | 3.86 ± 0.02c | 3.48 ± 0.002a | 0.027 |
| PCV (%) | 24.24 ± 0.02a | 23.86 ± 0.11d | 25.76 ± 0.02a | 28.16 ± 1.61c | 25.76 ± 0.02a | 0.025 |
| MCV (fL) | 64.26 ± 0.22b | 71.52 ± 0.77a | 75.06 ± 0.02c | 68.40 ± 1.83d | 74.18 ± 0.23a,c | 0.003 |
| MCH (pg) | 22.66 ± 0.02a | 23.30 ± 0.78a | 25.62 ± 0.04c | 23.36 ± 0.26a | 23.89 ± 0.68a | 0.004 |
| MCHC (g/dL) | 35.02 ± 0.04c | 32.52 ± 0.77d | 34.22 ± 0.04a,c | 34.08 ± 0.41a,c | 33.42 ± 0.22a | 0.0001 |
| PLTs(×103/μL) | 459 ± 14.45c | 174 ± 0.83a,d | 165 ± 0.48a,d | 245 ± 7.89a | 131 ± 0.97d | 0.004 |
| TLC (103/μL) | 12.06 ± 0.02e | 18.50 ± 0.22c | 17.68 ± 0.07a | 15.18 ± 0.07b | 17.08 ± 0.18d | 0.0001 |
| Neutrophil (%) | 19.00 ± 0.002a,d | 33.60 ± 4.63c | 23.40 ± 0.24a | 11.00 ± 0.001b | 14.60 ± 0.24b,d | 0.0001 |
| Lymphocyte (%) | 65.40 ± 0.24a,c | 52.40 ± 4.69a | 61.60 ± 0.24a,d | 58.80 ± 0.20d | 70.60 ± 0.24c | 0.020 |
| Monocyte (%) | 10.60 ± 0.24a | 10.40 ± 0.24a | 10.00 ± 0.001a,d | 26.20 ± 0.20c | 9.40 ± 0.24d | 0.001 |
| Eosinophil (%) | 4.00 ± 0.0001c | 2.60 ± 0.24a | 4.00 ± 0.001c | 3.00 ± 0.002a | 4.40 ± 0.24c | 0.024 |
| Basophil (%) | 1.00 ± 0.001 | 1.00 ± 0.001 | 1.00 ± 0.001 | 1.00 ± 0.001 | 1.00 ± 0.001 | 0.06 |
| N/L ratio | 0.29 ± 0.001a | 0.69 ± 0.14c | 0.38 ± 0.005a | 0.18 ± 0.0006a | 0.20 ± 0.002a | 0.003 |
Effect of Tiamulin and bromhexine on blood profile of dogs.
RBCs, red blood cell count; Hb, hemoglobin; PCV, packed cell volume; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLTs, platelets; TLC, total leukocyte count; N/L ratio, neutrophil-to-lymphocyte ratio.
Values are expressed as mean ± standard error (SE), with a sample size of n = 5. Means within the same row that do not share a common superscript letter differ significantly (p < 0.05).
Values are expressed as mean ± standard error (SE), with a sample size of n = 5. Means within the same row that do not share a common superscript letter differ significantly (p < 0.05).
Values are expressed as mean ± standard error (SE), with a sample size of n = 5. Means within the same row that do not share a common superscript letter differ significantly (p < 0.05).
Values are expressed as mean ± standard error (SE), with a sample size of n = 5. Means within the same row that do not share a common superscript letter differ significantly (p < 0.05).
Values are expressed as mean ± standard error (SE), with a sample size of n = 5. Means within the same row that do not share a common superscript letter differ significantly (p < 0.05).
3.6 Serum biochemical markers in experimentally S. aureus -infected dogs
Cardiac, renal, and hepatic serum markers are presented in Table 5. A significant increase in CK and CK-MB levels was observed in the infected untreated group (G2) compared to the healthy control group (G1) and other treatment groups (P < 0.05). Troponin I levels showed no significant differences among the groups. Serum ALT and AST levels, indicators of hepatic enzyme activity, were significantly elevated in G2 compared to G1 (P < 0.05). Notably, G5 demonstrated a numerical reduction in both ALT and AST levels compared to G3 and G4. Additionally, G4 and G5 exhibited significantly reduced total protein and albumin levels relative to the infected untreated group (G2), while no significant differences were observed in globulin levels and A/G ratio among groups (P < 0.05). Renal function markers—including creatinine, urea, and blood urea nitrogen (BUN)—were also assessed. The infected untreated group (G2) showed a significant increase in all three parameters compared to G1 and the treatment groups (P < 0.05). The combination treatment group (G5) displayed the most pronounced improvements, with substantial reductions across most parameters.
TABLE 5
| Groups parameters | G1 | G2 | G3 | G4 | G5 | P-value |
|---|---|---|---|---|---|---|
| CK (U/L) | 1,669 ± 1.18a,b | 2,732 ± 28.00c | 1833 ± 6.36a | 1744 ± 0.001a | 1,539 ± 17.58b | 0.0001 |
| CK-MB (ng/mL) | 284 ± 12.26b | 449 ± 17.94c | 324 ± 4.16a | 389 ± 0.022a | 367 ± 14.13a | 0.011 |
| Troponin I (ng/mL) | 0.03 ± 0.005 | 0.05 ± 0.003 | 0.05 ± 0.002 | 0.06 ± 0.001 | 0.048 ± 0.004 | 0.06 |
| ALT (U/L) | 17.20 ± 0.73b | 39.80 ± 3.00c | 35.60 ± 0.24a | 33.40 ± 2.15a | 26.40 ± 1.77a,b | 0.028 |
| AST (U/L) | 37.60 ± 2.46b | 65.60 ± 2.24c | 55.00 ± 2.04a | 52.80 ± 0.86a | 47.40 ± 2.27a,b | 0.018 |
| Total protein (g/dL) | 4.17 ± 0.06c | 4.31 ± 0.03c | 4.25 ± 0.02a,c | 3.73 ± 0.07b | 3.98 ± 0.12a,b | 0.001 |
| Albumin (g/dL) | 2.44 ± 0.13c | 2.36 ± 4.50c | 2.24 ± 0.02a,c | 1.82 ± 0.08b | 1.97 ± 0.12a,b | 0.001 |
| Globulin (g/dL) | 1.73 ± 0.11 | 1.95 ± 4.53 | 2.01 ± 0.04 | 1.91 ± 0.12 | 2.01 ± 0.11 | 0.432 |
| A/G ratio | 1.45 ± 0.16 | 0.79 ± 0.50 | 1.11 ± 0.04 | 0.97 ± 0.09 | 0.99 ± 0.09 | 0.354 |
| Creatinine (mg/dL) | 0.44 ± 0.012a | 0.97 ± 0.23c | 0.50 ± 0.005a | 0.37 ± 0.012a | 0.43 ± 0.025a | 0.004 |
| BUN (mg/dL) | 7.26 ± 0.57a | 8.89 ± 0.52c | 7.89 ± 0.24a | 7.01 ± 0.004b | 7.57 ± 0.22a | 0.014 |
| Urea (mg/dL) | 16.80 ± 0.48a | 40.00 ± 5.18c | 20.60 ± 1.12a | 17.40 ± 0.81a | 17.20 ± 0.20a | 0.010 |
Effect of treatments on serum biochemical markers in experimentally S. aureus-infected dogs.
Values are represented as means ± SE, n = 5. CK, creatine kinase; CK-MB, creatine kinase-MB; ALT, alanine aminotransferase; AST, aspartate aminotransferase; A/G ratio, albumin/globulin ratio; BUN, blood urea nitrogen.
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
Means with no shared superscript in the same row vary significantly (p < 0.05).
3.7 Tissue oxidant and antioxidant parameters
The MDA levels in the heart, liver, and kidney were significantly elevated in the infected untreated group (G2) compared to the normal control group (G1) (P < 0.05), indicating increased oxidative stress. Treatment with either agent alone (G3 and G4) led to a significant, moderate reduction in MDA levels compared to G2. However, the combination treatment (G5) resulted in a marked and significant decrease in MDA levels, approaching near-normal values and outperforming both monotherapies (P < 0.05). Similarly, TAC was markedly reduced in the infected group (G2). Although both monotherapies modestly improved TAC levels, the combined treatment in G5 produced the highest TAC values, significantly exceeding the effects of either treatment alone (P < 0.05), suggesting enhanced antioxidant defense (Figure 3).
FIGURE 3

Tissue oxidant/antioxidant biomarkers, MDA; malondialdehyde, TAC; total antioxidant capacity. Values are represented as means ± SE.
3.8 Cardiac gene expression
Gene expression analysis demonstrated a significant upregulation of IL-1β in the infected untreated group (G2) compared to the control group (G1), indicating a pronounced inflammatory response (P < 0.001). Notably, all treatment groups exhibited a reduction in IL-1β expression, with the combination therapy group (G5) showing the most substantial decrease. Similarly, CYB1B1 expression was significantly elevated in the infected untreated group (G2), consistent with heightened oxidative stress. Treatment—particularly in groups G4 and G5—resulted in a marked downregulation of CYB1B1, approaching normal expression levels. This suggests a protective role of the treatments in mitigating oxidative stress and modulating harmful metabolic processes (Figure 4).
FIGURE 4

Relative expression levels of CYP1B1 and IL-1β genes across experimental groups. Data are presented as mean ± SEM, with statistical significance indicated by different letters (p < 0.001). CYP1B1: cytochrome P450 family 1 subfamily B member 1; IL-1β: interleukin-1 beta.
3.9 Histopathological findings
Histopathological examination revealed normal liver architecture in the control group (G1) (Figure 5a). In contrast, the infected untreated group (G2) showed severe, diffuse vacuolar degeneration, coagulative necrosis of hepatocytes (Figures 5b, c), and moderate fibrosis accompanied by mononuclear inflammatory cell infiltration in the portal areas (Figure 5d). Group G3 exhibited moderate vacuolar degeneration and hepatocellular necrosis (Figure 5e), while G4 showed only mild vacuolar changes in a few hepatocytes (Figure 5f). Notably, G5 presented minimal hepatic degeneration and necrosis (Figure 5g).
FIGURE 5
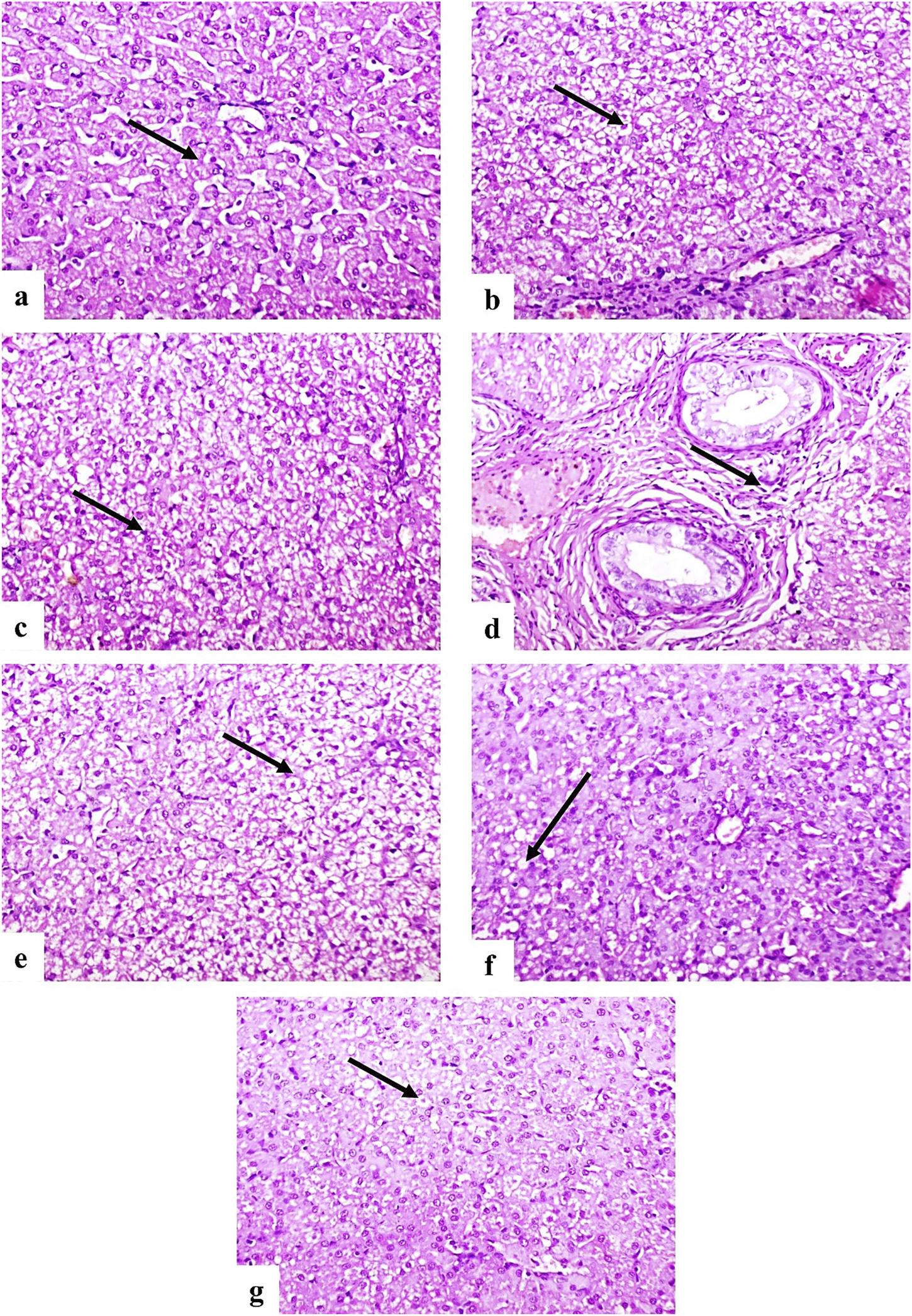
Photomicrographs of dog liver sections stained with H&E (×200). (a) Control group showing normal histological structure of hepatocytes (arrow). (b–d) G2 group showing diffuse vacuolar degeneration (b), coagulative necrosis (c), and portal fibrosis with mononuclear inflammatory cell infiltration (d) (arrows). (e) G3 showing moderate vacuolar degeneration and necrosis (arrow). (f) G4 showing mild vacuolar degeneration (arrow). (g) G5 showing few degenerated hepatocytes (arrow).
In the kidney, normal histological structures of renal tubules and glomeruli were observed in the control group (Figure 6a). G2 showed prominent vacuolar degeneration and coagulative necrosis of the renal tubular epithelium (Figures 6b, c). Similar pathological changes were observed in G3 (Figure 6d), whereas G4 and G5 displayed only mild and minimal degenerative changes, respectively (Figures 6e, f).
FIGURE 6

Photomicrographs of dog kidney sections stained with H&E (×200). (a) Control group showing normal renal tubules (long arrow) and corpuscles (short arrow). (b,c) G2 group showing vacuolar degeneration (b) and coagulative necrosis (c) of tubular epithelium (arrows). (d) G3 showing moderate vacuolar degeneration and necrosis (arrow). (e) G4 showing mild degeneration and necrosis (arrow). (f) G5 showing a few degenerated tubular epithelial cells (arrow).
Cardiac histology in the control group revealed normal myocardial fiber structure (Figure 7a). However, G2 showed Zenker’s necrosis and diffuse vacuolar degeneration (Figures 7b–d). G3 also exhibited vacuolar degeneration and Zenker’s necrosis (Figure 7e), while G4 displayed moderate vacuolation (Figure 7f), and G5 showed only mild degeneration and necrosis (Figure 7g). The severity of lesions in the liver, kidney, and heart was evaluated and scored, as presented in Table 6.
FIGURE 7

Photomicrographs of dog heart sections stained with H&E. (a) Control group showing normal myocyte structure (arrow) (×200). (b,c) G2 group showing Zenker’s necrosis (b) and diffuse vacuolation of myocytes (c) (arrows) (×200). (d) Higher magnification of (c) showing vacuolated myocytes (arrow) (×400). (e) G3 showing moderate vacuolar degeneration and Zenker’s necrosis (arrow) (×400). (f) G4 showing moderate vacuolation (arrow) (×400). (g) G5 showing mild degeneration and necrosis of myocytes (arrow) (×400).
TABLE 6
| Lesions | G1 | G2 | G3 | G4 | G5 |
|---|---|---|---|---|---|
| Liver | |||||
| Degeneration of hepatocytes | 0 | 3 | 2 | 1 | 1 |
| Necrosis of hepatocytes | 0 | 3 | 2 | 1 | 1 |
| Portal fibrosis | 0 | 2 | 1 | 0 | 0 |
| Portal mononuclear inflammatory cells infiltration | 0 | 2 | 1 | 0 | 0 |
| Kidney | |||||
| Degeneration of renal tubular lining epithelium | 0 | 3 | 2 | 1 | 1 |
| Necrosis of renal tubular lining epithelium | 0 | 3 | 2 | 1 | 0 |
| Heart | |||||
| Degeneration of myocytes | 0 | 3 | 2 | 1 | 1 |
| Necrosis of myocytes | 0 | 3 | 2 | 1 | 1 |
Histopathological lesion scoring in liver, kidney and heart of treated groups.
The score system was designed as: score 0 = absence of the lesion in all dogs of the group (n = 5), score 1= (<30%), score 2= (<30%–50%), score 3= (>50%).
4 Discussion
In the current study, we explored the therapeutic efficacy of tiamulin, bromhexine, and their combination in the management of experimentally induced Staphylococcus infections in dogs. Tiamulin, a pleuromutilin-class antimicrobial, exerts its bacteriostatic effect by selectively inhibiting protein synthesis through binding to the 50S ribosomal subunit, making it particularly effective against Gram-positive organisms, including S. aureus (Papich, 2016). However, the clinical success of antimicrobial therapy in staphylococcal infections often hinges not only on microbial susceptibility but also on adequate drug distribution to the site of infection. Bromhexine has been hypothesized to improve drug bioavailability and therapeutic outcomes (Matzembacker et al., 2024).
Our findings revealed that the combination of tiamulin and bromhexine led to a more pronounced clinical improvement and reduction in bacterial burden compared to either agent alone, suggesting a potential synergistic interaction. The dual therapy group (Tiamulin + Bromhexine) achieved the greatest bacterial reduction, indicating a synergistic or additive effect without evident antagonism. Recent veterinary research in bovines demonstrated that bromhexine hydrochloride can exert inherent antimicrobial activity and enhance the effect of other antibiotics, reinforcing its role as a viable adjunctive agent.
In preclinical studies, co-administration of bromhexine or its prodrug ambroxol with antibiotics has been shown to increase pulmonary antibiotic concentrations—most notably β-lactams and macrolides—though with variable clinical outcomes. One randomized clinical trial reported improved symptom resolution in respiratory infections treated with amoxicillin plus bromhexine versus amoxicillin alone. Nonetheless, concerns continue regarding inconsistencies in enhanced drug levels across antibiotic classes and infection sites (Deretic and Timmins, 2019). Our findings align with this positive trend, showing no diminution in tiamulin’s efficacy and instead suggest that bromhexine may enhance tissue penetration or distribution in canine Staphylococcus infections.
In vitro MIC testing in the present study showed that combining tiamulin with bromhexine led to a more pronounced reduction in bacterial growth than tiamulin alone, suggesting a potential enhancement in antibacterial activity. This observation may be explained by bromhexine’s known mucolytic properties, which can improve tissue diffusion and possibly alter bacterial membrane permeability. Earlier research demonstrated that bromhexine enhances antibiotic penetration into bronchial secretions (Matzembacker et al., 2024), and pharmacokinetic studies in poultry showed improved distribution of co-administered antimicrobials when combined with bromhexine (Ali et al., 2019). Although these effects were observed in vitro and may not fully translate to in vivo conditions, the reduced MIC values offer a rationale for further investigation into the potential synergistic role of bromhexine in antimicrobial protocols.
The observed superior efficacy of the dual therapy (tiamulin + bromhexine) extended beyond bacterial growth inhibition to a significant downregulation of key S. aureus virulence genes, such as hla, ebpS, and icaA. This downregulation implies that the combination therapy may impair pathogenic mechanisms such as α-hemolysin production (hla), fibronectin binding (ebpS), and PIA-mediated biofilm formation (icaA), thereby reducing bacterial virulence. The mechanism likely involves enhanced antibiotic penetration or disruption of bacterial regulatory pathways, possibly due to bromhexine’s mucolytic and membrane-modulating effects. The crucial roles of these genes are well established: hla encodes α-hemolysin, essential for host cell lysis (Lin et al., 2025); icaA is linked to biofilm matrix synthesis (Demir et al., 2020); and fibronectin-binding proteins like those encoded by ebpS are pivotal for tissue adhesion. Similar attenuation of virulence gene expression has been reported in studies involving antibiotic combinations targeting S. aureus and other Gram-positive pathogens. Thus, dual therapy not only reduces bacterial counts but also weakens pathogenic potential, making it a promising molecular-based strategy against Staphylococcus infections in dogs (Hammerschmidt et al., 2019).
In the present study, the blood parameters of the treated groups showed significant improvement compared to the S. aureus-infected control group. Infection with S. aureus is known to induce systemic inflammation, leading to hematological alterations such as leukocytosis, neutrophilia, lymphopenia, and anemia, as reported in previous studies (Kwiecinski and Horswill, 2020). These changes are attributed to the release of pro-inflammatory cytokines, oxidative stress, and bone marrow suppression caused by bacterial toxins. Our findings revealed that treated dogs exhibited restored levels of hemoglobin, red blood cells (RBCs), white blood cells (WBCs), and platelets compared to the infected untreated group. This suggests that the therapeutic interventions not only reduced bacterial burden but also alleviated the systemic effects of infection on hematopoiesis and immune response. Moreover, the recovery in leukocyte profiles in treated animals supports the hypothesis that the applied treatment protocols modulate the inflammatory response, reducing neutrophilic infiltration and restoring lymphocyte counts, consistent with findings from dog models (Farghali et al., 2019). Taken together, our data indicate that the tested therapeutic regimen provided effective control of the hematological alterations induced by S. aureus infection. Nevertheless, variations in response may depend on the treatment type, dosage, duration, and host factors. Further studies are warranted to elucidate the long-term impact of such treatments on hematological health and immune restoration.
In this study, dogs infected with S. aureus demonstrated a significant elevation in serum cardiac enzymes, including CK and CK-MB. These elevations suggest myocardial stress and possible subclinical cardiac involvement, likely resulting from systemic inflammatory responses and bacterial endotoxins (Janus et al., 2014). Following therapeutic intervention with tiamulin, bromhexine, or their combination, there was a marked reduction in the levels of these cardiac biomarkers. This improvement was most pronounced in the group receiving combined therapy, suggesting a synergistic cardioprotective effect. Tiamulin has been shown to effectively target Gram-positive organisms such as S. aureus, leading to reduced bacterial load and systemic inflammation (Xiang et al., 2022). In addition, bromhexine is known not only for its mucolytic action but also for enhancing tissue penetration of antibiotics and modulating immune responses, potentially reducing oxidative stress and protecting myocardial cells (Deretic and Timmins, 2019). The observed decrease in CK and CK-MB levels in the treated groups indicates a reduction in skeletal and myocardial muscle damage. Moreover, the superior outcomes in the group receiving both tiamulin and bromhexine may be attributed to improved drug delivery to inflamed tissues and enhanced host immune response. Although direct data on the cardiac effects of this combination are limited in canine models, similar therapeutic benefits have been reported in veterinary studies involving respiratory and systemic infections. To the best of our knowledge, there are currently no published studies in dogs evaluating the direct effects of bromhexine or tiamulin on serum cardiac troponin I levels. While absence of evidence is not evidence of absence, this notable gap highlights the originality of our findings.
In the present study, dogs infected with S. aureus exhibited a significant increase in liver enzymes ALT and AST. This elevation is consistent with previous reports indicating that systemic bacterial infections may lead to hepatic inflammation or dysfunction due to circulating endotoxins, oxidative stress, or immune-mediated hepatocellular damage (Murshed et al., 2025). The administration of tiamulin, bromhexine, or their combination showed a modulatory effect on these liver enzyme levels. Notably, the group treated with both agents demonstrated a more substantial decline in ALT and AST, suggesting an improved hepatic response. While tiamulin is generally regarded as safe for veterinary use, it has been reported to exhibit dose-dependent hepatotoxicity (Pankowska et al., 2024). Bromhexine, on the other hand, has shown hepatoprotective and antioxidant properties in various models, potentially by enhancing lysosomal enzyme release and reducing cellular inflammation (Radi et al., 2020). This may explain the more favorable liver enzyme profile in the group treated with bromhexine, particularly in combination with tiamulin. Overall, the reduction of liver enzyme levels in the treated groups may indicate not only clearance of infection but also a potential protective or stabilizing effect of the treatment protocols on hepatic tissues.
In this study, serum markers of renal function—urea, creatinine, and BUN—were significantly elevated in the S. aureus-infected group compared to the other groups. This suggests that the infection induced systemic effects extending to renal tissues, possibly through mechanisms such as sepsis-induced hypoperfusion, immune-mediated glomerular damage, or direct nephrotoxicity from bacterial toxins (Westgeest et al., 2022). According to Balkrishna et al. (2023), bacterial sepsis and endotoxemia are associated with impaired renal filtration and glomerular inflammation, leading to elevated serum urea and creatinine. The significant reduction of these renal markers in the treated groups, particularly in the combination therapy group, further supports the therapeutic benefit of bromhexine and tiamulin co-administration in mitigating renal damage during S. aureus infection.
Following therapeutic intervention with tiamulin, bromhexine, or their combination, there was a marked improvement in renal markers—especially in the combined treatment group—indicating a restoration of renal function and systemic stability. Although tiamulin is primarily excreted via the liver, its impact on kidney function has been shown to be minimal when used at therapeutic doses. Bromhexine, on the other hand, may indirectly support renal function through its anti-inflammatory and antioxidant actions, reducing systemic oxidative stress that can otherwise aggravate renal injury (Deretic and Timmins, 2019). The observed reduction in urea, creatinine, and BUN levels after treatment suggests that effective management of infection—especially using a combination of antimicrobial and supportive therapies—not only eradicates the pathogen but also contributes to the preservation or recovery of renal function.
The current findings revealed that infection with S. aureus led to a significant rise in MDA levels and a concurrent decline in TAC across the heart, liver, and kidney tissues of affected dogs. These alterations reflect the development of systemic oxidative stress and indicate that bacterial infection can compromise the oxidative balance in major organs. MDA is a key indicator of lipid peroxidation and is commonly used to assess cellular damage caused by oxidative stress. Its increased concentrations in these tissues suggest that S. aureus triggers organ-specific oxidative injury, likely through elevated reactive oxygen species (ROS) production, inflammatory responses, and toxin release from bacteria (Bastos et al., 2025). Simultaneously, the observed reduction in TAC highlights a weakened antioxidant defense, likely resulting from the rapid consumption of enzymatic and non-enzymatic antioxidants during the infection process. These patterns align with earlier research demonstrating similar oxidative stress responses in systemic bacterial conditions (Ponnampalam et al., 2022). After therapeutic intervention using tiamulin, bromhexine, or their combination, there was a noticeable improvement evidenced by reduced MDA levels and restored TAC. The most favorable outcomes were seen in the combined therapy group, indicating a potential synergistic effect. The role of bromhexine in this context may be attributed to its antioxidant and anti-inflammatory actions, including membrane stabilization and suppression of ROS generation (Bhagat, 2018). Meanwhile, tiamulin likely contributes indirectly by controlling bacterial growth and thereby minimizing oxidative burden.
Our findings showed that S. aureus infection significantly upregulated the expression of both CYP1B1 and IL-1β genes, reflecting an enhanced oxidative and inflammatory response in the affected tissues. In the heart, CYP1B1 is expressed in vascular endothelial cells, cardiomyocytes, and cardiac fibroblasts (Dubey et al., 2004) and is known to be highly inducible by oxidative stress. Its overexpression has been associated with inflammatory and immune responses. The observed upregulation of CYP1B1 in infected dogs suggests that S. aureus triggered ROS production, leading to enhanced CYP1B1 transcription. CYP1B1 has been identified as a key mediator in angiotensin II-induced activation of NADPH oxidase, leading to ROS generation, and promoting vascular smooth muscle cell migration, proliferation, and hypertrophy (Malik et al., 2012). Studies in rats have demonstrated that elevated CYP1B1 activity is linked to pathological conditions such as cardiac hypertrophy, vasoconstriction, endothelial dysfunction, and increased ROS production (Jennings et al., 2010; Jennings et al., 2014). CYP1B1 enzymes can metabolize arachidonic acid and ω-3 polyunsaturated fatty acids (PUFAs) into medium-chain hydroxyeicosatetraenoic acids (HETEs), which possess cardiotoxic properties contributing to these adverse effects (Westphal et al., 2015). Conversely, CYP1B1 inhibition has been shown to lower blood pressure and mitigate endothelial and renal dysfunction, as well as fibrosis, while simultaneously reducing ROS production (Gangadhariah et al., 2017; Jennings et al., 2014). Treatment with tiamulin and bromhexine, particularly in combination, resulted in a downregulation of CYP1B1 expression, suggesting that the treatment effectively reduced oxidative stress. This may be attributed to bromhexine’s known antioxidant activity (Bhagat, 2018), along with the antimicrobial effect of tiamulin which suppresses the source of ROS stimulation. The specific impact of tiamulin on CYP1B1 has not been extensively documented, but it is likely to be similar to its effects on other CYP isoforms, including inhibition (Pankowska et al., 2024).
IL-1β expression is a potent proinflammatory cytokine produced mainly by macrophages and neutrophils in response to microbial components, especially via Toll-like receptor (TLR) signaling. In our infected group, IL-1β expression was significantly elevated, consistent with activation of the innate immune response to bacterial invasion. Elevated IL-1β levels have been reported in numerous models of bacterial infection, including S. aureus, and are strongly linked to systemic inflammation and tissue damage (Rasquel-Oliveira et al., 2025). Therapeutic administration of tiamulin had an anti-inflammatory effect, which has been shown to suppress proinflammatory cytokine release (Xiang et al., 2022). In addition, therapeutic administration of bromhexine significantly downregulated IL-1β expression, particularly in the combined treatment group. This finding may reflect reduced bacterial load (Matzembacker et al., 2024) and the anti-inflammatory action of bromhexine, which has been shown to suppress proinflammatory cytokine release (Ansarin et al., 2020).
In our study, histopathological examination revealed significant structural alterations in the liver, kidney, and heart of the S. aureus-infected untreated group. The liver showed marked hepatocellular degeneration, congestion of central veins, and infiltration of inflammatory cells, indicating hepatic injury associated with infection. The kidneys displayed glomerular atrophy, tubular degeneration, and interstitial hemorrhage, reflecting renal damage. In the heart, myocardial fibers appeared disorganized with focal necrosis and leukocytic infiltration, suggesting cardiac involvement due to systemic infection (Onyebueke et al., 2018; Anton et al., 2024). In contrast, the tiamulin-treated group exhibited moderate improvement in tissue architecture. The hepatic and renal tissues showed reduced degeneration and inflammation compared to the untreated infected group, while the cardiac tissue displayed partial preservation of normal structure, although some pathological changes persisted. Bromhexine has mild effect on heart, liver, and kidney (El-Gendy et al., 2024b). This indicates a therapeutic effect of tiamulin in limiting bacterial-induced tissue damage. Remarkably, the group treated with the combination of tiamulin and bromhexine showed the most pronounced histological recovery. Nearly normal architecture was observed in the liver, kidney, and heart, with minimal inflammatory infiltration and absence of necrotic areas. The superior improvement may be attributed to the synergistic effect of tiamulin’s antibacterial action and bromhexine’s mucolytic and antioxidant properties, which may enhance drug absorption and tissue protection. These histological findings are consistent with the biochemical results, which demonstrated reduced oxidative stress markers (e.g., MDA) and improved antioxidant capacity (TAC), supporting the efficacy of the combined therapy in protecting vital organs against S. aureus-induced damage (Radi et al., 2020).
Overall, this study highlights that S. aureus infection leads to significant disruptions in the biochemical profile and histological integrity of critical organs such as the liver, kidneys, and heart. While tiamulin alone provided a moderate degree of protection, the co-administration of tiamulin and bromhexine offered superior therapeutic benefits. This was evident through improved antioxidant status and nearly normal tissue morphology. The enhanced efficacy of the combination suggests that bromhexine may serve as a valuable adjunct, boosting the performance of conventional antimicrobial therapy. Further investigations are recommended to explore the molecular basis of this synergism and assess the clinical relevance and long-term safety of such treatment strategies.
5 Conclusion
The present study confirms that S. aureus infection induces significant oxidative stress and multi-organ damage, as indicated by elevated MDA levels, reduced total TAC, and severe histopathological lesions in the liver, kidneys, and heart. Treatment with tiamulin alone resulted in moderate improvement in these parameters. However, the combined administration of tiamulin and bromhexine demonstrated superior therapeutic efficacy, as evidenced by a significant reduction in MDA levels, enhanced TAC activity, and notable restoration of normal tissue architecture. Additionally, the combination therapy achieved a lower MIC against S. aureus, significantly reduced bacterial loads in infected tissues, and markedly downregulated key virulence genes, including hla, ica, and ebps. These findings suggest that bromhexine enhances both the antibacterial and anti-virulence activities of tiamulin, providing a synergistic effect that may improve the management of S. aureus-induced infections. Further molecular and clinical investigations are warranted to validate these findings and assess the long-term safety and efficacy of this combinatorial approach.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the trial was permitted by the Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine, University of Sadat City, Egypt (Ethical approval number is VUSC-007-1-25). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review and editing. SM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – review and editing. NS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – review and editing. SE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – review and editing. DM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review and editing. RK: Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review and editing. HK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Validation, Visualization, Writing – review and editing. HE: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Supervision, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. H.O.K. is supported by the United Arab Emirates University (UAEU) Strategic Research Program 2024 grant (proposal number 3702; fund code 12R310) and UAEU Start-Up grant, (proposal number 3219; fund code 12FO58).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was used in the creation of this manuscript. English editing and paraphrasing of this manuscript were performed with the assistance of ChatGPT.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1679854/full#supplementary-material
References
1
Abonashey S. G. Fouad A. G. Hassan H. A. F. M. El-Banna A. H. Shalaby M. A. Mobarez E. et al (2024). Preparation and characterization of tiamulin-loaded niosomes for oral bioavailability enhancement in Mycoplasma-infected broilers. Micro4, 734–750. 10.3390/micro4040045
2
Ahmadi E. Afrooghe A. Soltani Z. E. Elahi M. Shayan M. Ohadi M. A. D. et al (2024). Beyond the lungs: exploring diverse applications of bromhexine and ambroxol. Life Sci.353, 122909. 10.1016/j.lfs.2024.122909
3
Ahmed A. M. Maruyama A. Khalifa H. O. Shimamoto T. Shimamoto T. (2015). Seafood as a reservoir of Gram-negative bacteria carrying integrons and antimicrobial resistance genes in Japan. Biomed. Environ. Sci.28 (12), 924–926. 10.3967/bes2015.128
4
Ali Q. Mafeng L. Anchun C. (2019). Use of bromhexine in the management of respiratory diseases in chickens. PSM Microbiol.4 (4), 83–87.
5
Aly L. A. A. El-Menoufy H. Ragae A. Rashed L. A. Sabry D. (2012). Adipose stem cells as alternatives for bone marrow mesenchymal stem cells in oral ulcer healing. Int. J. Stem Cells5 (2), 104–114. 10.15283/ijsc.2012.5.2.104
6
Ansarin K. Tolouian R. Ardalan M. Taghizadieh A. Varshochi M. Teimouri S. et al (2020). Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: a randomized clinical trial. Bioimpacts10 (4), 209–215. 10.34172/bi.2020.27
7
Anton C.-I. Ștefan I. Duțulescu S. Stăniceanu F. Buzilă C. A. Ștefan A.-T. et al (2024). Histological findings in infective endocarditis—A retrospective cohort study conducted at “Dr. Carol Davila” Central Military Emergency University Hospital in Bucharest. Life14, 1658. 10.3390/life14121658
8
Arbaga A. El-Bahrawy A. Elsify A. Khaled H. Hassan H. Y. Kamr A. (2021). Biochemical and histopathological changes related to the topical application of Aloe vera ointment for canine pyoderma. Veterinary World14 (5), 1354–1362. 10.14202/vetworld.2021.1354-1362
9
Aujla R. S. Patel R. (2022). “Creatine phosphokinase,” in StatPearls (StatPearls Publishing).Natl. Libr. Med.
10
Bahramibanan F. Rad M. V. Ranjbar A. Karbasi A. Abbasifard A. (2025). Comparison of oxidative stress status in the kidney tissue of male rats treated with paraquat and nanoparaquat. Sci. Rep.15 (389), 389. 10.1038/s41598-024-83156-2
11
Balkrishna A. Sinha S. Kumar A. Arya V. Gautam A. K. Valis M. et al (2023). Sepsis-mediated renal dysfunction: pathophysiology, biomarkers and role of phytoconstituents in its management. Biomed. Pharmacother.165, 115183. 10.1016/j.biopha.2023.115183
12
Bancroft J. D. Gamble M. (2008). Theory and practice of histological techniques. London: Churchill Livingstone.
13
Bastos M. L. C. Ferreira G. G. Kosmiscky I. d.O. Guedes I. M. L. Muniz J. A. P. C. Carneiro L. A. et al (2025). What do we know about Staphylococcus aureus and oxidative stress? Resistance, virulence, new targets, and therapeutic alternatives. Toxics13, 390. 10.3390/toxics13050390
14
Bhagat A. (2018). Bromhexine: a comprehensive review. International Journal of Biological and Medical Research.
15
Ciftci A. Findik A. Onuk A. Savasan S. (2009). Detection of methicillin resistance and slime factor production of Staphylococcus aureus in bovine mastitis. Braz. J. Microbiol.40, 254–261. 10.1590/S1517-83822009000200009
16
Cordiano R. Di Gioacchino M. Mangifesta R. Panzera C. Gangemi S. Minciullo P. L. (2023). Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: an update. Molecules28 (16), 5979. 10.3390/molecules28165979
17
Demir C. Demirci M. Yigin A. Tokman H. B. Cetik Yildiz S. (2020). Presence of biofilm and adhesin genes in Staphylococcus aureus strains taken from chronic wound infections and their genotypic and phenotypic antimicrobial sensitivity patterns. Photodiagnosis Photodyn. Ther.29, 101584. 10.1016/j.pdpdt.2019.101584
18
Deretic V. Timmins G. S. (2019). Enhancement of lung levels of antibiotics by ambroxol and bromhexine. Expert Opin. Drug Metab. Toxicol.15 (3), 213–218. 10.1080/17425255.2019.1578748
19
Dubey R. K. Tofovic S. P. Jackson E. K. (2004). Cardiovascular pharmacology of estradiol metabolites. J. Pharmacol. Exp. Ther.308, 403–409. 10.1124/jpet.103.058057
20
El-Gendy H. F. Tahoun E. A. Elfert A. Y. Mady R. (2022). Trial for decreasing ifosfamide-induced hematological toxicity, oxidative stress, inflammation, and hepatotoxicity by beetroot extract in male albino rats. Comp. Clin. Pathol.31, 699–712. 10.1007/s00580-022-03371-z
21
El-Gendy H. F. El-Bahrawy A. Mansour D. A. Sheraiba N. I. Abdel-Megeid N. S. Selim S. et al (2024a). Unraveling the potential of Saccharum officinarum and Chlorella vulgaris towards 5-fluorouracil-induced nephrotoxicity in rats. Pharmaceuticals17, 885. 10.3390/ph17070885
22
El-Gendy H. F. Masoud S. R. Elnahriry S. S. Attia T. Mansour A. Elhusseiny E. (2024b). Enhancement of the efficacy of Tylvalosin by administration with eucalyptus oil or bromhexine against Mycoplasma gallisepticum-infected broiler chickens. AJVS81, 74–87. 10.5455/ajvs.18F6051
23
Farghali H. A. AbdElKader N. A. AbuBakr H. O. Aljuaydi S. H. Khattab M. S. Elhelw R. et al (2019). Antimicrobial action of autologous platelet-rich plasma on MRSA-infected skin wounds in dogs. Sci. Rep.9, 12722. 10.1038/s41598-019-48657-5
24
Fei W. Hongjun Y. Hong-bin H. Changfa W. Yundong G. Qifeng Z. et al (2011). Study on the hemolysin phenotype and the genotype distribution of Staphylococcus aureus causing bovine mastitis in Shandong dairy farms. Intern J. Appl. Res. Vet. Med.9 (4).
25
Gamanie K. (2005). Toxicity studies in rats fed nature cure bitters. Afr. J. Biotechnol.4, 72–78.
26
Gangadhariah M. H. Dieckmann B. W. Lantier L. Kang L. Wasserman D. H. Chiusa M. et al (2017). Cytochrome P450 epoxygenase-derived epoxyeicosatrienoic acids contribute to insulin sensitivity in mice and in humans. Diabetologia60, 1066–1075. 10.1007/s00125-017-4260-0
27
Garmyn A. Vereecken M. Degussem K. Depondt W. Haesebrouck F. Martel A. (2017). Efficacy of tiamulin alone or in combination with chlortetracycline against experimental Mycoplasma gallisepticum infection in chickens. Poult. Sci.96 (9), 3367–3374. 10.3382/ps/pex105
28
Hammerschmidt S. Rohde M. Preissner K. T. (2019). Extracellular matrix interactions with Gram-positive pathogens. Microbiol. Spectr.7 (2), GPP3-0041–2018. 10.1128/microbiolspec.GPP3-0041-2018
29
Henry R. J. Canmon D. C. Winkelman J. W. (1967). “Determination of calcium by atomic absorption spectrophotometry,” in Clinical methods.
30
Henry R. J. Canmon D. C. Winkelman J. W. (1974). in Chemistry, principles and techniques. 2nd ed. (Harper and Row). Hagerstown.
31
Janus I. Noszczyk-Nowak A. Nowak M. Cepiel A. Ciaputa R. Pasławska U. et al (2014). Myocarditis in dogs: etiology, clinical and histopathological features (11 cases: 2007–2013). Ir. Vet. J.67 (1), 28. 10.1186/s13620-014-0028-8
32
Jennings B. L. Sahan-Firat S. Estes A. M. Das K. Farjana N. Fang X. R. et al (2010). Cytochrome P450 1B1 contributes to angiotensin II-induced hypertension and associated pathophysiology. Hypertension56, 667–674. 10.1161/HYPERTENSIONAHA.110.154518
33
Jennings B. L. Montanez D. E. May M. E. Jr. Estes A. M. Fang X. R. Yaghini F. A. et al (2014). Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc Drugs Ther.28, 145–161. 10.1007/s10557-014-6510-4
34
Jiang H. Zhu J. Liu W. Cao F. (2017). High-sensitivity cardiac troponins I sandwich assay by immunomagnetic microparticle and quantum dots. Front. Lab. Med.1 (3), 107–113. 10.1016/j.flm.2017.09.001
35
Kamr A. Arbaga A. El-Bahrawy A. Elsify A. Khaled H. Hassan H. (2020). The therapeutic efficacy of Aloe vera gel ointment on staphylococcal pyoderma in dogs. Vet. World13 (11), 2371–2380. 10.14202/vetworld.2020.2371-2380
36
Khalifa H. O. Abdelhamid M. A. Oreiby A. Mohamed M. Y. Ramadan H. Elfadadny A. et al (2024). Fire under the ashes: a descriptive review on the prevalence of methicillin-resistant Staphylococcus aureus in the food supply chain. J. Agric. Food Res.2024, 101606. 10.1016/j.jafr.2024.101606
37
Koneman E. W. Allen S. D. Janda W. M. Schreckenberger P. C. Winn W. C. (2006). Color Atlas and textbook of diagnostic microbiology. 6th ed. Lippincott Williams and Wilkins.
38
Kwiecinski J. M. Horswill A. R. (2020). Staphylococcus aureus bloodstream infections: pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol.53, 51–60. 10.1016/j.mib.2020.02.005
39
Laber G. (1988). Investigation of pharmacokinetic parameters of tiamulin after intramuscular and subcutaneous administration in normal dogs. J. Vet. Pharmacol. Ther.1988 (1), 45–49. 10.1111/j.1365-2885
40
Li B. Li J. Meng X. Yang S. Tian F. Song X. et al (2024). The association of blood urea nitrogen-to-creatinine ratio and in-hospital mortality in acute ischemic stroke patients with atrial fibrillation: data from the MIMIC-IV database. Front. Neurol.15, 1331626. 10.3389/fneur.2024.1331626
41
Lin Y. T. Bui N. N. Cheng Y. S. Lin C. W. Lee C. L. Lee T. F. et al (2025). High hemolytic activity in Staphylococcus aureus t1081/ST45 due to increased hla protein production and potential RNAIII-independent regulation. J. Microbiol. Immunol. Infect.58 (1), 70–76. 10.1016/j.jmii.2024.09.005
42
Livak K. J. Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods25 (4), 402–408. 10.1006/meth.2001.1262
43
Malik K. U. Jennings B. L. Yaghini F. A. Sahan-Firat S. Song C. Y. Estes A. M. et al (2012). Contribution of cytochrome P450 1B1 to hypertension and associated pathophysiology: a novel target for antihypertensive agents. Prostagl. Other Lipid Mediat98, 69–74. 10.1016/j.prostaglandins.2011.12.003
44
Marinov K. Mondeshki T. Georgiev H. Dimitrova V. S. Mitev V. (2025). Effects of long-term prophylaxis with bromhexine hydrochloride and treatment with high colchicine doses of COVID-19. Pharmacia72, 1–10. 10.3897/pharmacia.72.e141543
45
Mason W. J. Blevins J. S. Beenken K. Wibowo N. Ojha N. Smeltzer M. S. (2001). Multiplex PCR protocol for the diagnosis of Staphylococcal infection. J. Clin. Microbiol.39 (9), 3332–3338. 10.1128/JCM.39.9.3332-3338.2001
46
Matzembacker B. Fantinel D. D. S. Rodrigues C. M. da Silva S. P. Marin M. H. D. B. Rosa D. S. et al (2024). Antimicrobial efficiency of bromhexine hydrochloride against endometritis-causing Escherichia coli and Trueperella pyogenes in bovines. Braz. J. Microbiol.55 (2), 2013–2024. 10.1007/s42770-024-01320-2
47
Mazukina E. V. Izyurova E. A. Sultanova K. T. (2025). Use of absolute weights and mass coefficients of beagle dog internal organs relative to body and brain weights in preclinical studies. Russ. J. Veterinary Pathology24 (1), 7–14. 10.23947/2949-4826-2025-24-1-7-14
48
Mohammed M. M. Isa M. A. Abubakar M. B. Dikwa A. S. B. Kappo A. P. (2025). Molecular detection of mecA gene from methicillin-resistant Staphylococcus aureus isolated from clinical and environmental samples and its potential inhibition by phytochemicals using in vitro and in silico approach. Silico Pharmacol.13, 26. 10.1007/s40203-024-00297-y
49
Murshed S. Mustafa S. Dahham S. Maaroof M. (2025). Evaluating the Symphytum officinale effect against Staphylococcus aureus that induced liver pathochanges (histological study). J. Biosci. Appl. Res.11 (1), 322–330. 10.21608/jbaar.2025.420204
50
Mustafa M. S. Yasir J. O. A. Ali N. H. Ruqaya M. H. (2022). A review of animal diseases caused by Staphylococci. Rev. Latinoam. Hipertens.17 (1), 39–45. 10.5281/zenodo.6481584
51
Onyebueke E. A. Onyemelukwe N. F. Achukwu P. U. Onuba A. C. (2018). Pathological effects of staphylococcal species (S. aureus, S. xylosus and S. lentus) on some visceral organs (liver, kidney and bladder) of wistar rats following intraperitoneal inoculation. Afr. J. Microbiol. Res.12 (8), 192–199. 10.5897/AJMR2017.8679
52
Oreiby A. Khalifa H. Eid A. Ahmed A. Shimamoto T. (2019). Staphylococcus aureus and bovine mastitis: molecular typing of methicillin resistance and clinical description of infected quarters. J. Hell. Vet. Med. Soc.70, 1511–1516. 10.12681/jhvms.20826
53
Palaniappan S. Sadacharan C. M. Rostama B. (2022). Polystyrene and polyethylene microplastics decrease cell viability and dysregulate inflammatory and oxidative stress markers of MDCK and L929 cells in vitro. Expo. Health14 (1), 75–85. 10.1007/s12403-021-00419-3
54
Pankowska E. Kończak O. Żakowicz P. Wojciechowicz T. Gogulski M. Radko L. (2024). Protective action of cannabidiol on tiamulin toxicity in humans-in vitro study. Int. J. Mol. Sci.25 (24), 13542. 10.3390/ijms252413542
55
Papich M. G. (2016). Saunders handbook of veterinary drugs: small and large animal. Elsevier Health Sciences.
56
Parker D. Prince A. (2012). Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin. Immunopathol.34 (2), 281–297. 10.1007/s00281-011-0291-7
57
Perepechaeva M. L. Stefanova N. A. Grishanova A. Y. Kolosova N. G. (2024). The expression of genes CYP1A1, CYP1B1, and CYP2J3 in distinct regions of the heart and its possible contribution to the development of hypertension. Biomedicines12, 2374. 10.3390/biomedicines12102374
58
Ponnampalam E. N. Kiani A. Santhiravel S. Holman B. W. B. Lauridsen C. Dunshea F. R. (2022). The importance of dietary antioxidants on oxidative stress, meat and milk production, and their preservative aspects in farm animals. Animals12, 3279. 10.3390/ani12233279
59
Radi A. M. Shaban N. S. Abo El-Ela F. I. Ahmed Mobarez E. El-Gendy A. A. M. El-Banna H. A. (2020). The effect of bromhexine and thyme oil on enhancement of the efficacy of tilmicosin against pasteurellosis in broiler chickens. J. World Poult. Res.10 (2S), 151–164. 10.36380/jwpr.2020.20
60
Rasquel-Oliveira F. S. Ribeiro J. M. Martelossi-Cebinelli G. Costa F. B. Nakazato G. Casagrande R. et al (2025). Staphylococcus aureus in inflammation and pain: update on pathologic mechanisms. Pathogens14, 185. 10.3390/pathogens14020185
61
Sartini I. Vercelli C. Lebkowska-Wieruszewska B. Lisowski A. Fadel C. Poapolathep A. et al (2023). Pharmacokinetics and antibacterial activity of tiamulin after single and multiple oral administrations in geese. Vet. Anim. Sci.21, 100317. 10.1016/j.vas.2023.100317
62
Sendecor G. W. Cochran W. G. (1956). “The comparison of two samples,” in Statistical methods. 4th ed. (Ames: Iowa State University Press).
63
Soliman A. M. Khalifa H. O. Ahmed A. M. Shimamoto T. Shimamoto T. (2016). Emergence of an NDM-5-producing clinical Escherichia coli isolate in Egypt. Int. J. Infect. Dis.48, 46–48. 10.1016/j.ijid.2016.05.003
64
Sui B. Li X. Li N. Tao Y. Wang L. Xu Y. et al (2025). Synergistic action of mucoactive drugs and phages against Pseudomonas aeruginosa and Klebsiella pneumoniae. Microbiol. Spectr.0, e01601–e01624. 10.1128/spectrum.01601-24
65
Tao W. Yue X. Ye R. Nabi F. Shang Y. Zhu Z. et al (2022). Hepatoprotective effect of the Penthorum Chinense Pursh extract against the CCl4-induced acute liver injury via NF-κB and p38-MAPK pathways in dogs. Animals12 (5), 569. 10.3390/ani12050569
66
Thornton B. Basu C. (2011). Real-time PCR primer design using free online software. Biochem. Mol. Biol. Educ.39 (2), 145–154. 10.1002/bmb.20461
67
Touaitia R. Mairi A. Ibrahim N. A. Basher N. S. Idres T. Touati A. (2025). Staphylococcus aureus: a review of the pathogenesis and virulence mechanisms. Antibiotics14, 470. 10.3390/antibiotics14050470
68
Vancraeynest D. Hermans K. Haesebrouck F. (2004). Genotypic and phenotypic screening of high and low virulence Staphylococcus aureus isolates from rabbits for biofilm formation and MSCRAMMs. Vet. Microbiol.103, 241–247. 10.1016/j.vetmic.2004.09.002
69
Westgeest A. C. Schippers E. F. Delfos N. M. Visser L. G. de Fijter J. W. de Boer M. G. J. et al (2022). Acute kidney injury in Staphylococcus aureus bacteremia. Eur. J. Clin. Microbiol. Infect. Dis.41 (3), 431–437. 10.1007/s10096-021-04391-3
70
Westphal C. Konkel A. Schunck W. H. (2015). Cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids and their role in cardiovascular disease. Adv. Exp. Med. Biol.851, 151–187. 10.1007/978-3-319-16009-2_6
71
Xiang R. Hu L. Li S. Wei Z. Song Z. Chen Z. et al (2022). Tiamulin inhibits TNF-α and alleviates psoriasis-like dermatitis. J. Dermatol Sci.107 (1), 32–40. 10.1016/j.jdermsci.2022.05.006
72
Yang H. Xu S. Huang K. Xu X. Hu F. He C. et al (2020). Anti-staphylococcus antibiotics interfere with the transcription of leucocidin ED Gene in Staphylococcus aureus strain newman. Front. Microbiol.5 (11), 265. 10.3389/fmicb.2020.00265
73
Yuan J. S. Reed A. Chen F. Stewart C. N. (2006). Statistical analysis of real-time PCR data. BMC Bioinforma.7, 85. 10.1186/1471-2105-7-85
74
Zaki N. F. Orabi S. H. Abdel-Bar H. M. Elbaz H. T. Korany R. M. S. Ismail A. K. et al (2024). Zinc oxide resveratrol nanoparticles ameliorate testicular dysfunction due to levofloxacin-induced oxidative stress in rats. Sci. Rep.14, 2752. 10.1038/s41598-024-52830-w
75
Zhou Y. Yi Y. Yang J. Zhang H. Liu Q. Wang S. et al (2023). Anti-methicillin-resistant Staphylococcus aureus activity and safety evaluation of 14-O-[(5-ethoxycarbonyl-4,6-dimethylpyrimidine-2-yl) thioacetyl] mutilin (EDT). Sci. Rep.13, 15267. 10.1038/s41598-023-42621-0
Summary
Keywords
antioxidant, bromhexine, dogs, efficacy, inflammatory cytokine, Staphylococcus aureus , tiamulin, virulence
Citation
El-Gendy HF, Masoud SR, Sheraiba NI, Elnahriry SS, Madkour DA, Korany RMS, Khalifa HO and Elnagar HY (2025) Bromhexine hydrochloride enhances the therapeutic efficacy of tiamulin against experimental Staphylococcus aureus infection in dogs: targeting bacterial virulence, boosting antioxidant defense, and improving histopathology. Front. Pharmacol. 16:1679854. doi: 10.3389/fphar.2025.1679854
Received
05 August 2025
Revised
27 November 2025
Accepted
30 November 2025
Published
18 December 2025
Volume
16 - 2025
Edited by
Wangxue Chen, National Research Council Canada (NRC), Canada
Reviewed by
Engy Elekhnawy, Tanta University, Egypt
Zhihao Wang, Yangzhou University, China
Updates
Copyright
© 2025 El-Gendy, Masoud, Sheraiba, Elnahriry, Madkour, Korany, Khalifa and Elnagar.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hazim O. Khalifa, hazimkhalifa@uaeu.ac.ae
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.