- 1Nursing Department, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Orthopedics, the First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Emergency, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Osteoarthritis (OA) is a progressive, whole-joint disorder driven by a convergence of biomechanical stress, inflammation, metabolic dysfunction, and cellular senescence. This review integrates recent advances in our understanding of the distinct yet interconnected pathological processes affecting articular cartilage, subchondral bone, synovium, infrapatellar fat pad, menisci, ligaments, and peri-articular musculature. Emerging mechanisms, such as chondrocyte ferroptosis, neurovascular remodeling, and synovial-mesenchymal reprogramming, are highlighted for their roles in disease propagation and chronic pain. We critically appraise current therapeutic modalities, including evidence-based non-pharmacological strategies, pharmacologic agents, intra-articular biologics, and surgical interventions. In parallel, we explore the promise of precision medicine, multi-omics profiling, advanced imaging biomarkers, regenerative therapies, and artificial intelligence in reshaping diagnostic and treatment paradigms. This comprehensive synthesis underscores the shift toward a mechanistic, individualized approach to OA management and identifies key translational opportunities for disease modification and early intervention.
1 Introduction
Osteoarthritis (OA) is a prevalent and debilitating musculoskeletal disorder that affects the structural and functional integrity of synovial joints. Globally, OA represents a substantial public health concern, impacting over 500 million individuals and contributing significantly to reduced quality of life, work loss, and healthcare expenditure (Ma W. et al., 2025; Leifer et al., 2022). No longer regarded merely as a consequence of mechanical wear and tear, OA is now conceptualized as a complex, whole-joint disease characterized by progressive degeneration of articular cartilage, subchondral bone remodeling, synovial inflammation, and alterations in surrounding peri-articular structures (Di Nicola, 2020; Coaccioli et al., 2022) (Figure 1). This multifactorial condition is driven by a convergence of biomechanical, inflammatory, metabolic, and post-traumatic processes, which collectively compromise joint homeostasis and lead to pain, stiffness, and functional disability. In addition, the disease disproportionately affects weight-bearing joints, particularly the knees and hips, and its incidence continues to rise in parallel with population aging and increasing prevalence of obesity. Notably, obesity and joint injury remain the most prominent modifiable risk factors, whereas age, sex, and genetic predisposition are considered non-modifiable contributors (Figure 2) (Nedunchezhiyan et al., 2022; Arden and Nevitt, 2006). Current management strategies emphasize symptom relief through non-pharmacological approaches such as exercise, education, and weight management. However, adherence to these interventions remains suboptimal, limiting their long-term effectiveness (Farinelli et al., 2024; Anandacoomarasamy and March, 2010). For individuals with advanced disease and persistent symptoms refractory to conservative treatment, joint replacement surgery is a viable option (Anandacoomarasamy and March, 2010). Despite ongoing advances in our understanding of OA pathogenesis, there remains an urgent need to develop disease-modifying therapies and improve early intervention strategies to mitigate the escalating global burden of OA.
In this review, we integrate recent advances in the understanding of osteoarthritis pathophysiology, spanning cartilage, subchondral bone, synovium, and peri-articular tissues, to highlight their interconnected roles in disease initiation and progression. We further examine emerging molecular mechanisms, including ferroptosis, synovial reprogramming, and neurovascular remodeling, and critically evaluate current and prospective therapeutic strategies. By bridging basic science with clinical application, this review aims to provide a comprehensive, multidisciplinary perspective on the evolving landscape of OA research, diagnosis, and management.
2 Pathological changes associated with osteoarthritis
2.1 Subchondral bone
Subchondral bone plays a critical role in the pathogenesis and progression of OA, undergoing a series of pathological alterations that contribute to joint degeneration and pain (Zhu et al., 2020). In the early stage of OA, the subchondral bone exhibits increased remodeling activity, characterized by elevated bone turnover, microstructural deterioration, and a shift from bone resorption to formation. These changes lead to sclerosis, trabecular thickening, and reduced mechanical shock absorption, which exacerbates cartilage degradation due to increased mechanical stress on the overlying articular surface (Zhu et al., 2020; Li et al., 2013). One hallmark feature of OA-related subchondral bone pathology in early stages is the disruption of its architecture, including the formation of bone marrow lesions (BMLs), microcracks, diffuse microdamage, and marginal osteophytes. These abnormalities are often associated with pathological angiogenesis and nerve invasion, contributing to inflammation and nociception (Li et al., 2013; Felson, 2004). Notably, subchondral nerve ingrowth, often occurring alongside neovascularization, introduces sensory and sympathetic nerve fibers into previously aneural regions of bone. This aberrant innervation sensitizes the subchondral microenvironment, contributing to the chronic pain experienced in OA (Morgan et al., 2022). Nerve growth factor (NGF), released by osteoblasts, endothelial cells, and infiltrating immune cells, plays a key role in promoting this ectopic innervation, establishing a direct link between structural bone changes and pain signaling (Grässel and Muschter, 2017). In addition, osteoclast promotes axonal growth by secreting axon-guiding molecules such as netrin-1. Netrin-1, acting via its receptor DCC, has been shown to drive the sprouting of calcitonin gene-related peptide (CGRP)+ nociceptive fibers in the subchondral bone, thereby contributing directly to pain hypersensitivity associated with OA (Zhu et al., 2019). Osteophytes, though anatomically distinct from subchondral bone, are closely associated with its pathological changes in OA. These osteocartilaginous outgrowths form at the joint margins at the interface of the synovium, periosteum, and articular cartilage, yet their development is tightly linked to the mechanical and molecular alterations occurring in the subchondral region (Li et al., 2013). They are formed through an endochondral-like process involving progenitor cells of the Gdf5 lineage (Wong et al., 2016). These include Sox9-expressing cells in the periosteum, which generate hybrid skeletal cells, and Prg4-expressing synovial cells that contribute chondrocytes to the cartilage cap of the osteophyte (Roelofs et al., 2020). While initially stabilizing the joint, osteophytes may disrupt biomechanics, impinge on adjacent tissues, and contribute to pain and dysfunction in OA. Histopathologically, several features distinguish OA subchondral bone from healthy bone. These include the presence of BMLs, fibrosis, microfractures, and abnormal vascularization, which strongly correlate with pain severity and structural disease progression (Aho et al., 2017). Microcracks and diffuse microdamage are frequently observed in calcified cartilage and adjacent trabecular bone, contributing to localized remodeling and osteocyte dysfunction (Seref-Ferlengez et al., 2015). At the cellular level, early OA subchondral bone remodeling is driven by an imbalance between bone-resorbing osteoclasts and bone-forming osteoblasts. Osteoclastogenesis is upregulated due to increased expression of receptor activator of nuclear factor kappa-B ligand (RANKL) and cathepsin K, resulting in enhanced bone resorption (Chen W. et al., 2024; Henrotin et al., 2012). Meanwhile, osteoblasts in OA exhibit a pathological phenotype, marked by increased secretion of RANKL, VEGF, and TGF-β1, and impaired mineralization due to altered collagen synthesis. The accumulation of osteoid tissue with poor mineral quality contributes to hypomineralization and bone fragility (Table 1) (Maruotti et al., 2017). Additionally, osteocytes display impaired perilacunar/canalicular remodeling and diminished mechanosensing capacity, further disrupting local bone homeostasis (Zhu et al., 2020; Donell, 2019). In the late stage of OA, subchondral bone shows advanced structural damage. Prominent features include subchondral bone cysts, also known as intraosseous lesions, that form in regions subjected to repetitive mechanical overload. These cysts are characterized by osteoclastic resorption at their periphery and poorly mineralized new bone formation internally, reflecting highly dysregulated turnover and structural compromise (Feng and McDonald, 2011; Ott, 2008). Other features include necrotic marrow, fibrosis, and persistent abnormal vascularization, all of which correlate with disease severity and increased joint pain. Cellular dysfunction continues in the late stage, with a persistent imbalance between osteoclast and osteoblast activity. Osteoblasts continue producing excessive osteoid with poor mineral quality, contributing to bone fragility and structural weakness (Maruotti et al., 2017). Osteocyte dysfunction remains a key contributor to abnormal bone remodeling in advanced disease (Zhu et al., 2020; Donell, 2019).
Recent studies have also highlighted the molecular mechanisms underlying these pathological changes. Dysregulated signaling pathways such as Wnt/β-catenin, TGF-β, and PI3K/Akt have been shown to modulate osteoblast and osteoclast activity in OA subchondral bone. Furthermore, subchondral angiogenesis, often driven by VEGF and mechanical stress, disrupts the osteochondral interface and promotes cartilage deterioration (Grässel et al., 2021; Noh et al., 2020).
2.2 Synovium and infrapatellar fat pad
In OA, the synovial membrane exhibits marked pathological remodeling characterized by lining layer hyperplasia, sublining fibrosis, increased vascularization, and infiltration of immune cells, particularly macrophages, lymphocytes, and mast cells. Fibroblast-like synoviocytes (FLS) in the intimal layer adopt a pro-inflammatory phenotype under chronic stimulation, secreting cytokines such as IL-1β, TNF-α, IL-6, and chemokines that attract monocytes and perpetuate synovitis. This altered microenvironment enhances production of matrix-degrading enzymes (e.g., MMPs, ADAMTS), which degrade cartilage matrix and disrupt joint homeostasis (Sanchez-Lopez et al., 2022; Scanzello and Goldring, 2012). Moreover, tissue-resident type A synoviocytes (macrophage-like) are key mediators of innate immune responses and interact with FLSs to potentiate inflammatory cascades, further contributing to cartilage destruction and joint pain. Synovial inflammation is closely associated with peripheral sensitization via nerve growth factor (NGF) signaling, and imaging studies reveal strong correlations between synovial thickening, effusion, and OA symptom severity (Panichi et al., 2024; Bai et al., 2022). A comprehensive bioinformatics analysis review revealed 143 differentially expressed genes in OA synovial tissue, primarily involved in immune response, inflammation, and chemokine signaling pathways. Using protein-protein interaction networks, key hub genes, including IL6, CXCL8, and IL1B, were identified as central to OA pathogenesis (Zou et al., 2023). Lin et al. identified a distinct subset of OA-associated macrophages (OA-macrophages) that exhibit heightened pro-inflammatory and pro-fibrotic activity in synovial and infrapatellar fat pad, contributing significantly to leukocyte recruitment, chondrocyte apoptosis, and angiogenesis. They further developed a diagnostic model, OAMGS, based on four marker genes (VEGFA, CDKN1A, PIM3, MAFF) using machine learning, validated in both human and rat models (Lin et al., 2025). In addition, Huang et al. explored synovial cell heterogeneity and demonstrated the feasibility of computationally deconvolving bulk RNA-seq data into single-cell resolution using customized gene signature matrices, especially via CIBERSORTx. They identified seven major synovial cell types and emphasized the underestimated role of T cells and macrophage subtypes in OA pathogenesis (Huang Z. Y. et al., 2022).
The infrapatellar fat pad (IFP), or Hoffa’s fat pad, is an intra-articular but extrasynovial structure rich in adipocytes, immune cells, blood vessels, and nerve fibers (Braun et al., 2022). In OA, the IFP undergoes significant morphological and molecular alterations, including adipocyte hypertrophy, fibrosis of interlobular septa, neovascularization, and infiltration by pro-inflammatory immune cells, particularly M1-polarized macrophages (Figure 3). This tissue becomes a potent source of adipokines (e.g., leptin, resistin, visfatin), cytokines, and prostaglandins that act locally and systemically to exacerbate joint inflammation, cartilage catabolism, and pain perception (Braun et al., 2022; Wang M. G. et al., 2024). Recent evidence has demonstrated that aging and obesity drive maladaptive remodeling of the IFP, characterized by chronic low-grade inflammation, accumulation of senescent cells, and expression of senescence-associated secretory phenotype (SASP) factors, such as MMP-13, IL-1β, and RANTES. These mediators promote extracellular matrix remodeling, vascular activation, and further immune recruitment, creating a self-sustaining inflammatory niche that facilitates OA progression (Wang B. et al., 2024; Musale et al., 2023). Emerging molecular and histological evidence suggests that the IFP and synovium form a single anatomo-functional entity. Single-cell RNA-sequencing has revealed that both tissues originate from shared mesenchymal progenitor cells, particularly Dpp4+ stromal populations, which give rise to synovial fibroblasts, adipocytes, and myofibroblasts under OA conditions. These progenitors exhibit pro-fibrotic and pro-inflammatory differentiation trajectories in response to joint injury. Coordinated pathological changes include fibrosis, stromal stiffening, and myofibroblast expansion in both compartments, which compromise joint biomechanics and serve as sources of persistent nociceptive signaling (Tang et al., 2024; Li et al., 2024). Furthermore, the IFP and synovium serve as reservoirs of mesenchymal stromal cells (MSCs) with potential immunomodulatory functions. However, under OA conditions, these MSCs are reprogrammed toward pro-inflammatory phenotypes, losing their reparative capacity and instead contributing to disease propagation. Fibrosis alters IFP biomechanics, reducing its cushioning capacity and increasing mechanical stress on adjacent joint structures, thereby accelerating cartilage degeneration (Jeyaraman et al., 2022; Garcia et al., 2016; Zupan et al., 2020).

Figure 3. M1 macrophage activation, characterized by specific surface marker expression and secretion of pro-inflammatory cytokines.
2.3 Cartilage
Cartilage in healthy joints maintains a precise balance between synthesis and degradation of ECM components, primarily type II collagen and aggrecan, orchestrated by chondrocytes. In OA, this balance is disrupted by catabolic signaling, inflammation, and mechanical stress, culminating in progressive cartilage erosion and exposure of subchondral bone (Peng Z. et al., 2021). The onset of OA pathology in cartilage is marked by an early shift in chondrocyte phenotype from a homeostatic to a catabolic and hypertrophic state. Pro-inflammatory cytokines, particularly IL-1β, TNF-α, and IL-6, are key mediators that stimulate chondrocytes to upregulate degradative enzymes such as matrix metalloproteinases (MMP-1, MMP-3, MMP-13) and aggrecanases (ADAMTS-4 and ADAMTS-5). These enzymes target and cleave aggrecan and collagen fibrils, leading to loss of tensile strength, increased water content, and impaired load-bearing capacity of the matrix (Yunus et al., 2020; Charlier et al., 2019). At the same time, anabolic signaling pathways are suppressed, with downregulation of COL2A1 and ACAN expression (Table 1). These events are compounded by oxidative stress and mitochondrial dysfunction, which contribute to DNA damage, chondrocyte senescence, and impaired autophagy. Reactive oxygen species (ROS) exacerbate matrix degradation and promote further inflammation, establishing a self-sustaining loop of cartilage damage (Lepetsos and Papavassiliou, 2016; Hong et al., 2024). A pivotal early event in OA cartilage pathology is the degradation of the pericellular matrix (PCM), which surrounds chondrocytes and facilitates mechanotransduction. As shown by high-resolution imaging and proteomic analyses, PCM damage impairs cellular responses to mechanical stimuli and nutrient diffusion, leading to chondrocyte death and clustering. These clusters are typically observed in the middle and deep zones of the cartilage and are indicative of attempted, but dysregulated, repair responses. The loss of PCM integrity also allows deeper penetration of cytokines and enzymes, accelerating matrix breakdown (Goldring, 2012; Umlauf et al., 2010). The phenotypic transformation of chondrocytes toward a hypertrophic state is evidenced by upregulation of type X collagen (COL10A1), alkaline phosphatase (ALP), and VEGF. This hypertrophic shift recapitulates features of endochondral ossification and contributes to cartilage calcification, tidemark duplication, and eventual osteophyte formation at joint margins (Rim et al., 2020). Moreover, chondrocyte apoptosis, triggered by ER stress, nitric oxide, and oxidative insults, plays a critical role in cartilage degradation. Apoptotic bodies and released damage-associated molecular patterns (DAMPs) further stimulate inflammatory signaling in surrounding tissues, promoting joint-wide degeneration (Guan et al., 2024; Takada et al., 2011). Furthermore, chondrocyte ferroptosis is a type of regulated cell death driven by the accumulation of lipid peroxides and disruption of redox homeostasis, which severely affects cartilage integrity (Yao et al., 2021). In chondrocytes, ferroptosis occurs primarily due to oxidative stress, iron overload, and impaired antioxidant defense mechanisms such as reduced activity of glutathione peroxidase 4 (GPX4) and the cystine/glutamate antiporter system Xc. These disruptions lead to excessive lipid ROS accumulation, resulting in cellular damage and matrix degradation. Inflammatory stimuli further aggravate ferroptosis in chondrocytes, contributing to pathological changes in the cartilage environment (Yao et al., 2021; Fan et al., 2024). The JNK-JUN-NCOA4 pathway has been identified as a critical regulator, promoting iron accumulation via ferritinophagy (Sun et al., 2023). Protective factors such as Nrf2 and SLC7A11/GPX4 can counteract this process by reducing oxidative damage (Wan et al., 2023). Li et al. combined single-cell RNA sequencing and population-level genetic analyses to reveal two key chondrocyte subpopulations, inflammatory and fibrocartilage chondrocytes, that are expanded in hand OA and enriched for ferroptosis-related and inflammatory pathways, with elevated expression of FTH1, implicating iron overload in OA pathogenesis (Li H. et al., 2023). Supporting this, Sun et al. identified CD8+ T cells in the synovium as drivers of cartilage degeneration and subchondral bone damage through interactions with chondrocytes, offering mechanistic insight into immune-mediated OA progression (Sun et al., 2024). Nevertheless, chondrocyte senescence refers to the irreversible growth arrest of cartilage cells, characterized by altered gene expression, impaired matrix production, and secretion of pro-inflammatory and degradative factors collectively known as the senescence-associated secretory phenotype (SASP) (Loeser, 2009). This process is primarily triggered by cumulative oxidative stress, DNA damage, and telomere attrition, which activate key pathways such as p53/p21 and p16^INK4a/Rb, leading to permanent cell cycle arrest (Loeser, 2009). Mechanical stress and mitochondrial dysfunction also contribute significantly to the induction of senescence in chondrocytes, disrupting their homeostasis and regeneration potential (Jiang et al., 2023). Furthermore, the overexpression of caveolin-1 has been associated with promoting senescent phenotypes, indicating its involvement in age-related cartilage degeneration (Aigner et al., 2007). These mechanisms collectively lead to the progressive deterioration of cartilage, establishing senescence as a central player in joint aging and osteoarthritis pathogenesis. OA disrupts the natural zonal architecture of cartilage. The superficial zone, typically rich in lubricin (PRG4) and oriented collagen fibers, is eroded early, compromising lubrication and tensile resistance. The middle zone, responsible for absorbing compressive forces, becomes disorganized as proteoglycan loss advances. In the deep zone, vertical collagen bundles are fragmented, and the calcified cartilage zone expands abnormally, with advanced mineral deposition leading to stiffening and altered load transmission across the osteochondral unit (Sophia Fox et al., 2009; Eschweiler et al., 2021).
On the other hand, the bidirectional crosstalk between synovium and articular cartilage amplifies inflammation and matrix degradation across the whole joint. In OA, fibroblast-like synoviocytes and synovial macrophages acquire a pro-inflammatory phenotype, secreting high levels of cytokines and chemokines and prostaglandins, together with matrix-degrading enzymes and nitric oxide. These mediators diffuse through the synovial fluid to the cartilage surface, where they drive chondrocytes toward catabolic, hypertrophic, or senescent phenotypes, upregulate MMPs and aggrecanases, suppress COL2A1 and ACAN expression, and promote oxidative stress and ferroptosis, thereby accelerating ECM breakdown (Chou et al., 2020; Wang et al., 2022). Conversely, stressed or dying chondrocytes release DAMPs, apoptotic bodies, cartilage fragments, and pro-inflammatory mediators that further activate synovial macrophages and fibroblasts, maintaining a chronic synovitic state and perpetuating pain and swelling (Fan et al., 2024; Li and Sun, 2024). In addition to soluble cytokines, synovium-cartilage communication is increasingly recognized to be mediated by extracellular vesicles and exosomes carrying microRNAs, long non-coding RNAs, and bioactive proteins (Wessler and Meisner-Kober, 2025; Asghar et al., 2020).
2.4 Ligaments, meniscus, and peri-articular muscles
In OA, ligaments, especially the cruciate (ACL/PCL) and collateral ligaments, undergo both structural and biochemical deterioration. Histological studies have demonstrated disorganization of collagen fiber alignment, increased vascularity, mucoid degeneration, and loss of fibroblast density (Cho et al., 2012). Cruciate ligaments often show dystrophic mineralization and partial ruptures in advanced disease stages, particularly in knees with varus or valgus malalignment. Molecularly, ligament fibroblasts in OA upregulate MMP and inflammatory mediators, contributing to ECM degradation and mechanical instability (Table 1) (Nyland et al., 2020). Aging and OA synergistically affect the mechanical properties of ligaments, reducing tensile strength and altering viscoelasticity, thereby impairing joint proprioception and load distribution. These ligamentous alterations also predispose to further structural deterioration in the meniscus and cartilage (Peters et al., 2018; Loeser, 2017). The meniscus, a fibrocartilaginous structure essential for load transmission, shock absorption, and joint congruency, exhibits significant degenerative changes in OA. Macroscopically, OA-associated meniscal pathology includes partial maceration, radial and horizontal tears, and extrusion from the joint line. MRI studies frequently reveal meniscal degeneration in early OA, often preceding radiographic evidence of cartilage loss (Makris et al., 2011; Melrose, 2019). Histopathologically, OA menisci exhibit surface fibrillation, collagen disorganization, hypocellularity, calcification, and formation of fibrocartilaginous metaplasia. These changes compromise the biomechanical function of the meniscus and reduce its capacity to distribute load, thereby increasing stress on adjacent cartilage and subchondral bone. Meniscal extrusion has been associated with accelerated medial joint space narrowing and correlates strongly with knee pain and OA progression (Langhans et al., 2023; Ozeki et al., 2022). Emerging evidence also suggests that meniscal cells actively contribute to the inflammatory microenvironment. Degenerated menisci produce catabolic mediators such as prostaglandin E2 (PGE2), nitric oxide, and cytokines that can diffuse into synovial fluid and exacerbate articular cartilage degeneration (Krupkova et al., 2018). In addition, meniscectomy, whether partial or total, significantly contributes to the development and progression of OA through structural and biomechanical alterations in the knee joint. Studies have shown that total meniscectomy leads to a substantially higher risk of OA progression compared to partial meniscectomy, with increased incidence of joint space narrowing and osteophyte formation (Ma X. et al., 2025; Cabarcas et al., 2025; Migliorini et al., 2023). Moreover, partial meniscectomy, though considered less invasive, still results in long-term joint degeneration, especially when combined with factors such as limb malalignment or ACL deficiency (Zhang H. et al., 2025; Wechsler and Rotem, 2025). Histopathological evaluations demonstrate increased cartilage fibrillation, matrix loss, and inflammatory cytokine expression post-meniscectomy (Kwok et al., 2016; Haemer et al., 2011). Thus, meniscal preservation remains critical in delaying OA onset, underscoring the importance of repair technique over excision whenever possible. Furtheoremore, Varus and valgus alignments alter load-bearing dynamics across the tibiofemoral joint, leading to compartment-specific degeneration. Varus alignment, often associated with medial compartment OA, increases compressive forces on the medial meniscus and cartilage, accelerating cartilage breakdown and osteophyte formation (Valente et al., 2025; Terauchi et al., 2025). Conversely, valgus morphotype predisposes the lateral compartment to degenerative changes, especially in conjunction with lateral meniscal damage (Slynarski et al., 2025). Recent gait and imaging analyses further support that morphological features such as tibial plateau slope, femoral condyle shape, and joint congruency significantly affect joint biomechanics, contributing to early onset OA in at-risk individuals (Zhang K. et al., 2025). Therefore, understanding and assessing individual knee morphology is vital in predicting OA risk and optimizing preventive or surgical strategies.
Furthermore, peri-articular muscle weakness and atrophy are common but under-recognized features of OA. Quadriceps and hamstring muscle mass and strength are often significantly reduced in patients with symptomatic knee OA. This sarcopenia-like phenotype arises from a combination of disuse, altered neuromuscular recruitment patterns, systemic inflammation, and age-related muscle loss (Kim et al., 2023; Shorter et al., 2019). Peri-articular muscle biopsies in OA demonstrate fiber-type atrophy (especially type II fast-twitch fibers), increased infiltration by adipocytes and macrophages, fibrosis, and mitochondrial abnormalities. Inflammatory cytokines such as IL-6 and TNF-α have been implicated in muscle catabolism and impaired regeneration. These changes impair dynamic joint stabilization and contribute to further joint instability and pain, forming a deleterious feedback loop with intra-articular degeneration (Table 1) (Cruz Ayala et al., 2022; Terracciano et al., 2013). Quantitative MRI and ultrasound have confirmed reductions in muscle cross-sectional area and quality (e.g., increased fat infiltration), even in early-stage OA. Notably, reduced muscle mass is strongly predictive of radiographic progression and poorer functional outcomes (Huber et al., 2020; Diaz et al., 2025; Guzmán-David et al., 2023).
3 Management and treatment of osteoarthritis
3.1 Non-pharmacological management
Non-pharmacological interventions form the foundation of contemporary OA management and are universally recommended as first-line therapy across all disease stages and phenotypes. They serve as symptomatic treatments and as disease-modifying strategies aimed at optimizing joint function, minimizing structural deterioration, and reducing long-term pharmacologic and surgical burden (Bierma-Zeinstra et al., 2020). The efficacy of these interventions is maximized when delivered as part of an integrated, patient-centered model of care. A central tenet of non-pharmacological management is patient education, which empowers individuals with knowledge about OA pathophysiology, prognosis, and the value of self-management. Educational programs, particularly those that include shared decision-making and goal-setting components, have been shown to significantly improve treatment adherence and functional outcomes. Patients who understand the biomechanical and inflammatory underpinnings of OA are more likely to engage consistently with lifestyle modifications (Moseng et al., 2024). Rannou et al. highlighted patient education as a cornerstone of OA management. Endorsed by EULAR with level A evidence, education helps patients understand the purpose and benefits of interventions like exercise and weight management. In addition, they emphasized that education should be delivered by both physicians and therapists, and reinforced through follow-ups to sustain engagement and behavioral change (Rannou and Poiraudeau, 2010). Exercise therapy remains the most extensively validated non-pharmacological intervention for OA, supported by numerous randomized controlled trials and systematic reviews. Both aerobic and resistance-based regimens improve pain, enhance mobility, and delay functional decline. Structured programs that combine strength training with proprioceptive and neuromuscular exercises are particularly effective in targeting periarticular muscle weakness, joint instability, and gait abnormalities, hallmarks of lower limb OA (Ambr et al., 2015; Golightly et al., 2012). Supervised physiotherapy may be necessary during the initial stages to ensure safe technique and progressive loading, particularly in frail or deconditioned individuals (Marriott and Birmingham, 2023; Skou and Roos, 2019; Bennell et al., 2014). Conley et al. reviewed multiple high-quality clinical practice guidelines and concluded that exercise, particularly strength training, aerobic activity, and mind-body practices such as tai chi, should be considered a core intervention in OA management, with strong consensus supporting its effectiveness in improving pain and function. They emphasized the importance of individualized, progressive exercise programs tailored to patient needs and capabilities (Conley et al., 2023). Furthermore, Verhagen et al. conducted a meta-epidemiological analysis of 42 randomized controlled trials and demonstrated that exercise yields a clinically meaningful reduction in pain in patients with knee OA, with individually supervised interventions showing greater effectiveness. They concluded that exercise is a well-established, evidence-based treatment for knee OA, making further trials against minimal treatment unnecessary (Verhagen et al., 2019). Closely linked to exercise is weight management, a critical intervention in patients with knee or hip OA who are overweight or obese. Excess body weight increases mechanical load on weight-bearing joints and contributes to systemic inflammation through adipokine dysregulation (Huffman et al., 2024). Clinical evidence demonstrates that a sustained weight loss of ≥10% significantly reduces pain and improves joint function, particularly when combined with physical activity (Wood et al., 2021; Ryan and Yockey, 2017). Behavioral interventions that integrate nutritional counseling with physical rehabilitation have been shown to yield superior outcomes compared to either approach in isolation. Furthermore, physical therapy modalities, including manual therapy, stretching, and functional task training, are frequently incorporated to optimize joint biomechanics and movement quality. For example, interventions targeting hip flexor tightness, quadriceps inhibition, or valgus knee alignment can reduce joint stress and enhance locomotor efficiency. Gait retraining using real-time feedback and corrective bracing may further improve joint load distribution and proprioceptive acuity (Page, 2012; Ni et al., 2024). In patients with biomechanical malalignment or instability, assistive and supportive devices play a complementary role. Off-loading knee braces, lateral wedge insoles, canes, and shock-absorbing footwear can all provide symptomatic relief by modifying joint loading patterns. The prescription of such aids should be tailored by a physiotherapist or orthotist based on a comprehensive biomechanical assessment (Guilak, 2011; Zhang et al., 2020). Finally, the integration of psychosocial interventions, such as cognitive behavioral therapy (CBT) and mindfulness-based stress reduction, addresses the affective dimension of chronic OA pain. Central sensitization, fear-avoidant behavior, and depression are common comorbidities that attenuate treatment response. By enhancing pain coping skills and emotional resilience, these interventions contribute to better functional recovery and health-related quality of life (Zhang et al., 2018; Ordoñez-Mora et al., 2022). Although first-line management strategies for OA are underpinned by robust evidence, including systematic reviews and international guideline recommendations, real-world adherence remains suboptimal. Despite global consensus on the recommended interventions and increasing recognition of the disease’s growing public health burden, many individuals with OA do not receive care aligned with these standards. Furthermore, both healthcare providers and patients demonstrate low levels of engagement with and implementation of these evidence-based protocols (Cunningham et al., 2021; Jönsson et al., 2019; Dziedzic and Allen, 2018). Mazzei et al. reported that although 74% of patients engaged in some form of exercise and 64% received educational support, only 19% adhered to all first-line guideline-consistent treatments, highlighting implementation gaps in clinical settings (Mazzei et al., 2022). Hofstede et al. similarly observed that while many patients received individual interventions (e.g., 80% education, 73% physical therapy), a mere 6% received the full spectrum of recommended therapies, with dietary guidance markedly underutilized, particularly among overweight patients (Hofstede et al., 2015). Cronström et al. further emphasized that only 40% of patients received guideline-based OA management, such as the better management of patients with osteoarthritis program, before being placed on a surgical waitlist, with even fewer receiving such care during the wait, underscoring systemic deficiencies in guideline implementation (Cronström et al., 2020).
3.2 Pharmacological management of osteoarthritis
3.2.1 Non-opioid analgesics, anti-inflammatory agents, and senescence/ferroptosis-targeted drugs
Non-opioid analgesics, particularly non-steroidal anti-inflammatory drugs (NSAIDs), are the pharmacological mainstay for symptom control in OA, particularly in cases presenting with moderate to severe pain (Table 2). NSAIDs exert their effect by inhibiting cyclooxygenase (COX) enzymes, thereby reducing the synthesis of prostaglandins involved in pain and inflammation (Williams Benzon et al., 2018; Sohail et al., 2023). Both oral and topical formulations are widely used, with treatment tailored to the patient’s comorbidity profile and risk factors. Oral NSAIDs, such as ibuprofen, naproxen, and celecoxib, have demonstrated consistent efficacy in reducing pain intensity and improving joint function across numerous randomized controlled trials and meta-analyses (Pelletier et al., 2016; Ong et al., 2007). However, their long-term use is constrained by well-documented risks of gastrointestinal, cardiovascular, and renal adverse events. To mitigate these risks, the concurrent use of gastroprotective agents, such as proton pump inhibitors (PPIs), is advised in at-risk populations (Pelletier et al., 2016; Osteoarthritis Group of the Rheumatology and Immunology Physicians Branch of the Chinese Medical Doctor Association, 2024). Moreover, topical NSAIDs, especially topical diclofenac, are strongly recommended as first-line pharmacological therapy for patients with localized OA, particularly of the knee and hand, and in individuals for whom systemic NSAIDs pose unacceptable risk. Their efficacy in providing analgesia is comparable to oral NSAIDs in mild-to-moderate OA, with a substantially lower incidence of systemic side effects (Table 2) (Osteoarthritis Group of the Rheumatology and Immunology Physicians Branch of the Chinese Medical Doctor Association, 2024; Bariguian Revel et al., 2020; Roth and Fuller, 2011). Acetaminophen (paracetamol), once widely used for OA-related pain, is now considered a second-line agent due to its modest analgesic efficacy and potential for hepatotoxicity at higher doses. Current guidelines restrict its role to short-term use in individuals with contraindications to NSAIDs or those who experience intolerable adverse effects from alternative therapies (Conaghan et al., 2019; Klotz, 2012). Meta-analytical evidence has demonstrated limited benefit over placebo in reducing OA pain, particularly in knee and hip OA, and its use is increasingly de-emphasized in clinical guidelines (Bannuru et al., 2019; Fr et al., 2024).
Recent preclinical studies have identified two promising pharmacological strategies for OA management. Senolytics, which are agents that selectively eliminate senescent cells, and ferroptosis inhibitors, which target iron-dependent cell death pathways implicated in cartilage degeneration, are emerging as potential disease-modifying therapies. Senescent chondrocytes accumulate in osteoarthritic joints, contributing to inflammation and tissue breakdown via the senescence-associated secretory phenotype (SASP). Preclinical models have shown that senolytic compounds like navitoclax or flavonoid-based combinations (e.g., fisetin) can reduce cartilage degradation and synovial inflammation by clearing senescent cells (Fernandez-Moreno et al., 2025; de Magalhães, 2025).Additionally, the combination of senolytics with autophagy enhancers or anti-inflammatory agents has shown synergistic effects on OA symptom relief and structural repair (Garcia-Dominguez et al., 2025).Parallel advances have emerged in ferroptosis research, revealing that this iron-dependent oxidative cell death plays a key role in chondrocyte dysfunction in OA. Several natural compounds and small molecules, such as tetrandrine, proanthocyanidins, sodium tanshinone IIA sulfonate, and dehydrotanshinone II A,have been demonstrated to inhibit ferroptosis, reduce ROS levels, and preserve cartilage matrix in OA animal models (Xing et al., 2025; Xu M. et al., 2025; Guan et al., 2025). Notably, CDO1, a key regulator of cellular redox balance, has been identified as a ferroptosis-related therapeutic target in OA via integrated multi-omics analyses (Zhao et al., 2025). Moreover, multi-targeted nanoparticle systems combining ferroptosis inhibition with anti-inflammatory or antioxidant therapy show promise in enhancing drug delivery and therapeutic efficacy (Li et al., 2026).
3.2.2 Intra-articular therapies
Intra-articular (IA) therapies represent a key component of localized pharmacologic management in OA, particularly for patients who exhibit inadequate response to oral medications or are contraindicated for systemic therapy. These interventions aim to deliver targeted symptom relief directly within the joint space, with the principal agents including corticosteroids, hyaluronic acid (HA), and emerging biologics such as platelet-rich plasma (PRP) and cell-based products (Wehling et al., 2017; Testa et al., 2021). IA corticosteroids (e.g., methylprednisolone, triamcinolone) are among the most frequently used IA agent in OA treatment. Their anti-inflammatory action is mediated through inhibition of pro-inflammatory cytokines and leukocyte trafficking within the synovium. Clinical evidence supports their efficacy in providing short-term pain relief (3–6 weeks) and functional improvement, especially in patients with inflammatory phenotypes of OA or joint effusions (Iannitti et al., 2011; Paik et al., 2019; Najm et al., 2021). However, repeated or frequent injections may pose risks, including chondrotoxicity and acceleration of cartilage degeneration. As such, their use is generally limited to a few administrations per year per joint (Bensa et al., 2024; Guermazi et al., 2023). HA is a major component of synovial fluid, serving a structural and lubricating function within the joint. In OA, both the molecular weight and concentration of endogenous HA are reduced, contributing to impaired viscoelasticity and increased mechanical stress on cartilage. IA administration of exogenous HA aims to restore synovial fluid rheology, improve joint mechanics, and potentially exert chondroprotective effects (Tamer, 2013; Gupta et al., 2019). Clinical outcomes from HA therapy remain variable. Some randomized controlled trials report modest improvements in pain and function, particularly in patients with mild-to-moderate knee OA, while others show minimal benefit compared to placebo (Chavda et al., 2022; Lana et al., 2016). A 2023 OARSI consensus report concluded that HA may offer limited but clinically relevant benefit in select patients, though guideline recommendations remain inconsistent due to heterogeneity in formulations and trial quality (Macri et al., 2023). Furthermore, PRP has garnered increasing attention due to its autologous origin and potential regenerative properties. Rich in growth factors such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and insulin-like growth factor-1 (IGF-1), PRP may promote tissue repair and modulate synovial inflammation (Gato-Calvo et al., 2019). Bensa et al. demonstrated that PRP injections provide statistically and clinically significant improvements in both pain and function in patients with knee OA. Their meta-analysis of 18 randomized controlled trials involving 1995 patients showed that PRP significantly outperformed placebo at 1, 3, 6, and 12 months follow-up, with the greatest clinical relevance observed at 3 and 6 months. Notably, PRP preparations with higher platelet concentrations were associated with superior outcomes, suggesting that platelet dose is a key determinant of therapeutic efficacy in intra-articular PRP administration for knee OA (Bensa et al., 2024). In addition, Adrien et al. found that PRP injections, when combined with a structured rehabilitation program, led to clinically significant and sustained improvements in pain and joint function in patients with large joint OA. Their retrospective analysis of 252 patients demonstrated a 49% reduction in pain (VAS) and a 44% improvement in function (SANE) at 6 months, with benefits maintained at 12 months. Moreover, multiple PRP sessions and higher levels of sports activity, particularly at the competitive level, were associated with more favorable outcomes, suggesting a dose-response relationship and the influence of biomechanical conditioning on PRP efficacy (Schwitzguébel et al., 2025). However, variation in PRP preparation methods, injection protocols, and lack of standardization have limited widespread adoption and regulatory endorsement. Other IA, including mesenchymal stem cells (MSCs), autologous protein solutions, and liposomal corticosteroids, are under investigation for their potential to modify disease progression. While early-phase studies have demonstrated safety and some efficacy signals, larger randomized trials are necessary to validate their long-term effects, cost-effectiveness, and comparative utility (Rodríguez-Merchán, 2022; Cao M. et al., 2025; Hunter et al., 2022).Hernigou et al. found that subchondral injection of bone marrow-derived MSCs is significantly more effective than intra-articular delivery in delaying total knee arthroplasty (TKA), with a 15-year randomized trial showing a 70% TKA rate in knees treated intra-articularly versus only 20% in those treated subchondrally (Hernigou et al., 2021a). In a separate study comparing subchondral MSC injection to contralateral TKA in elderly patients, MSC therapy provided comparable long-term pain relief and functional outcomes, with only 18% of MSC-treated knees requiring later arthroplasty. Notably, persistent subchondral bone marrow lesions were predictive of eventual TKA, underscoring their role as imaging biomarkers of therapeutic response (Hernigou et al., 2021b).
In contrast, a randomized controlled trial by Nouri et al. compared the efficacy of IA injections of PRP, HA, and a combination of both in hip OA. While all groups showed some improvement in pain and function at 2 and 6 months post-injection, the outcomes were modest and not significantly different in terms of long-term structural benefit. The combination therapy showed slightly better scores in WOMAC and Lequesne indices than HA alone, but not enough to support strong clinical superiority (Nouri et al., 2022). Similarly, another randomized study by Ghorbani et al. involving patients with knee OA reported that PRP injections were more effective than HA in reducing pain and stiffness scores. However, the clinical benefit remained confined to the short term. At the 5-month follow-up, although both groups improved, the magnitude of improvement was moderate and did not lead to complete functional recovery. Moreover, despite statistical significance, the improvements did not reach minimal clinically important difference (MCID) thresholds for all patients (Ghorbani et al., 2024). These findings align with broader systematic reviews and meta-analyses which suggest that while IA PRP and HA may offer symptomatic relief in early-stage OA, their impact on disease progression, cartilage regeneration, or long-term pain control remains questionable. The variability in product preparation, lack of standardization, and placebo effects further cloud their clinical utility (Tschopp et al., 2023), (Paget et al., 2023).
3.3 Surgical intervention
Surgical management of OA is typically reserved for individuals with advanced disease who have not responded to conservative measures, including pharmacologic and non-pharmacologic therapies. The primary goal of surgical intervention is to alleviate pain, restore joint function, and improve quality of life. The choice of procedure is guided by the joint involved, severity of structural damage, patient age, activity level, and comorbidities (Table 3) (Katz et al., 2010; Brumat et al., 2022).
3.3.1 Autologous chondrocyte implantation (ACI)
ACI is a cell-based surgical technique designed to repair focal articular cartilage defects, particularly in the knee, to restore hyaline-like cartilage and delay OA progression. The procedure involves harvesting a small sample of healthy cartilage from a non-weight-bearing area, isolating and expanding chondrocytes ex vivo, and implanting them into the cartilage defect, often under a periosteal flap or collagen membrane (Welch et al., 2016; Karuppal, 2017). ACI is primarily indicated for younger, active patients with isolated, symptomatic full-thickness defects larger than 2–2.5 cm2, and is generally not recommended in advanced OA with diffuse degeneration (Minas et al., 2010; Jones and Peterson, 2006).
Clinical success with ACI is highly dependent on defect etiology, morphology, and location. Traumatic chondral lesions, such as those from sports injuries,are among the most responsive to ACI due to their well-circumscribed borders and otherwise healthy surrounding cartilage matrix, which provides a favorable environment for graft integration (Rodríguez-Merchán et al., 2012; Mithoefer et al., 2011). Similarly, early-stage focal osteoarthritic lesions, where degenerative changes are limited and biomechanical alignment is preserved, can also benefit from ACI,though outcomes are generally less predictable compared to purely traumatic defects (Brittberg et al., 2016; Kurz et al., 2023). Importantly, diffuse osteoarthritis, characterized by widespread cartilage thinning, subchondral bone sclerosis, and inflammatory milieu, is a contraindication for ACI due to poor regenerative potential and high failure rates (Muthu et al., 2023; Liu et al., 2022).
Anatomical defect location plays a critical role in outcome predictability. The medial femoral condyle is the most commonly treated and tends to yield better outcomes compared to the patellofemoral compartment, which presents with complex biomechanics and greater shear forces during flexion (Ebert et al., 2024; Trattnig et al., 2011). A meta-analysis by Mithoefer et al. demonstrated that medial condylar defects had significantly higher return-to-activity rates and more durable repair compared to patellar lesions (Mithoefer et al., 2011). Additionally, defect size and containment are key determinants,well-contained lesions >2 cm2 show better matrix fill and integration, while uncontained or “kissing lesions” have shown less favorable histological and clinical results (Brittberg et al., 2016; Choe et al., 2022). Recent advances, such as atrix-assisted autologous chondrocyte implantation (MACI), have improved surgical handling and defect conformity, especially in irregular or hard-to-access locations, by providing a pre-seeded scaffold that promotes even chondrocyte distribution and better ECM deposition (Zhou et al., 2024).
Furthermore, radiological and histological evaluations underscore the importance of defect characterization. MRI-based biomarkers, such as T2 mapping and dGEMRIC, can identify early OA and assess repair quality over time, aiding both in patient selection and long-term monitoring (Trattnig et al., 2011; Zhang et al., 2023). In the context of early osteoarthritis, recent trials suggest that MACI may slow degenerative progression when applied to isolated focal areas, though longitudinal studies remain limited and are complicated by variable definitions of early OA across trials (Muthu et al., 2023). Ultimately, clear distinction between focal cartilage injury and diffuse degenerative joint disease is essential to determine ACI eligibility, with emerging imaging, biomaterials, and scaffold innovations contributing to expanding its therapeutic window.
3.3.2 Arthroscopy
Arthroscopic debridement and lavage were historically used in the management of knee OA, especially in the presence of mechanical symptoms such as locking or catching (Medical Advisory Secretariat, 2005). However, several large randomized controlled trials, including the pivotal study by Moseley et al., and subsequent meta-analyses have demonstrated no long-term benefit over placebo or conservative therapy in terms of pain, function, or disease progression (Moseley et al., 2002). As a result, contemporary guidelines, including those from the American Academy of Orthopaedic Surgeons (AAOS) and OARSI, strongly advise against routine arthroscopic surgery for degenerative OA in the absence of clear mechanical indications such as unstable meniscal tears or loose bodies (Moseley et al., 2002; Brophy and Lowry, 2023).
3.3.3 Osteotomy (joint preservation strategy)
Osteotomy, particularly high tibial osteotomy (HTO) for varus-aligned medial compartment knee OA, is a joint-preserving surgical option primarily indicated in younger, active patients (<60 years) with unicompartmental disease and intact lateral joint surfaces (Murray et al., 2021). The procedure involves realigning the mechanical axis of the limb to offload the diseased compartment, thereby redistributing joint stress and delaying the need for total knee arthroplasty (Peng H. et al., 2021; Ferrera and Menetrey, 2022). Clinical studies report significant improvements in pain and function, with survivorship rates of up to 75%–85% at 10 years postoperatively (Mustamsir et al., 2025; Ollivier et al., 2021). Lateral distal femoral osteotomy (LDFO) is similarly employed for valgus-aligned lateral compartment disease, though with more limited long-term data. Key predictors of success include preoperative alignment correction, joint congruency, and preservation of joint space width (Cameron et al., 2015; O'Malley et al., 2016). In parallel, joint distraction has emerged as another joint-preserving approach, particularly for patients with end-stage OA who are otherwise candidates for arthroplasty. Unlike osteotomy, joint distraction does not rely on realignment but instead involves temporary mechanical separation of the joint surfaces using an external fixation device. This unloading period promotes cartilage regeneration and modifies the subchondral bone environment (Shtroblia et al., 2025). A study has shown that distraction can lead to symptomatic relief and radiographic evidence of cartilage repair, with some studies demonstrating benefits lasting up to 10 years (Jansen et al., 2022). Mechanistically, the benefits are thought to arise from partial unloading, synovial fluid pressure oscillation, enhanced activity of mesenchymal stem cells, and modulation of inflammatory and reparative pathways within the joint (Jansen et al., 2022). As with osteotomy, patient selection is critical, and this modality is especially suited for relatively young, active individuals in whom delaying prosthetic joint replacement is a priority (McGonagle et al., 2017).
3.3.4 Total joint arthroplasty (TJA)
TJA, particularly TKA and total hip arthroplasty (THA), represents the most effective and durable surgical treatment for end-stage OA. These procedures consistently demonstrate high levels of pain relief, restoration of function, and improved health-related quality of life (HRQoL), with implant survival exceeding 90% at 15–20 years in well-selected patients (Feng et al., 2018; Pollock et al., 2016; Madry, 2022). THA is considered among the most successful surgical procedures in all of medicine, with patient satisfaction rates surpassing 95% (Serfaty, 2020; Shon et al., 2019). Advances in implant technology (e.g., highly cross-linked polyethylene, cementless fixation), computer-assisted navigation, and perioperative care protocols (e.g., enhanced recovery after surgery [ERAS]) have improved short- and long-term outcomes while reducing complications and hospitalization times (Min et al., 2020; Altman et al., 2019). However, the risk of surgical site infection, thromboembolism, aseptic loosening, and periprosthetic fracture must be carefully weighed against the anticipated benefit, particularly in elderly or frail patients (Antonelli and Chen, 2019; Li et al., 2022). Moreover, pain relief after TKA is not assured. Wylde et al. emphasize that chronic pain after TKA affects up to 34% of patients and significantly impacts quality of life, often persisting for months or years postoperatively despite technically successful surgeries (Wylde et al., 2018). Beswick et al. systematically reviewed prospective studies and found that 9%–20% of patients continue to experience moderate to severe pain after THA or AKA, underscoring a critical need to identify predictors and improve pain management strategies. However, a 2025 review by Trojian and Naik emphasized that TKA and THA remain the gold standard interventions for patients with severe hip or knee OA who do not respond to non-operative therapy, with evidence supporting significant pain reduction and sustained functional improvement across large cohort studies and registry data (Trojian and Naik, 2025).
3.3.5 Unicompartmental knee arthroplasty (UKA)
UKA arthroplasty is indicated in patients with isolated medial or lateral compartment OA and intact cruciate ligaments. Compared to TKA, UKA offers the advantage of a smaller incision, faster recovery, and preservation of native joint kinematics (Mittal et al., 2020; Rodríguez-Merchán and Gómez-Cardero, 2018). However, registry data show a higher revision rate for UKA than TKA, particularly in younger and more active patients. Careful patient selection and surgical precision are essential for optimal outcomes. Long-term studies have shown survivorship rates of approximately 80%–90% at 10 years (Rodríguez-Merchán and Gómez-Cardero, 2018; Deschamps et al., 2012).
4 Prevention
4.1 Weight management and obesity prevention
Obesity is a major modifiable risk factor for OA, particularly affecting weight-bearing joints such as the knees. Excess adipose tissue contributes to increased mechanical loading and to systemic inflammation via adipokine dysregulation. Miao et al. found that high body mass index (BMI) significantly correlates with both global and regional OA burden, reinforcing weight management as a central preventive strategy (Miao et al., 2025). Emerging evidence also highlights the role of lean body mass (LBM) in joint health. A large-scale NHANES-based study (n = 31,172) identified a nonlinear inverse association between LBM and OA risk, with a protective threshold at 52.26 kg (Lu H. et al., 2025). Lower LBM, often seen in obesity-related sarcopenia, was associated with greater OA prevalence, underscoring the importance of preserving muscle mass to maintain joint stability and reduce mechanical load (Lu H. et al., 2025). In addition, myokines, particularly irisin, a hormone released during muscle contraction, have been shown to exert anti-inflammatory and chondroprotective effects. Irisin has been found to enhance collagen synthesis, reduce oxidative stress, and inhibit MMP activity, thereby supporting cartilage integrity (Ning et al., 2022; Wang F. S. et al., 2020). However, weight loss programs should prioritize preservation of LBM, using resistance training and adequate protein intake, to optimize joint support and stimulate beneficial myokine responses (Olson and Eccleston, 2024; Messier et al., 2018).
4.2 Joint protection and injury prevention
Trauma to intra-articular structures such as the ACL, meniscus, and articular cartilage, is a well-established risk factor for the development of post-traumatic osteoarthritis (PTOA) (Wang L. J. et al., 2020). It is estimated that approximately 50% of individuals who sustain major joint injuries, such as ACL ruptures, will develop radiographic OA within 10–15 years, even following surgical reconstruction. These injuries disrupt joint biomechanics, induce inflammatory cascades, and initiate cartilage degradation, all of which contribute to the early onset of OA, often in younger, otherwise healthy individuals (Carbone and Rodeo, 2017; Kraus and Hsueh, 2024). Primary prevention of joint injuries is a critical public health strategy, especially in youth and amateur sports settings. Neuromuscular and plyometric training programs (e.g., FIFA 11+, PEP) have demonstrated efficacy in reducing ACL injury risk by enhancing dynamic alignment, balance, and movement control (Viswanathan et al., 2025; Emery and Pasanen, 2019). Systematic implementation of such protocols across athletic populations has shown a 40%–60% reduction in non-contact lower limb injuries, suggesting a potential long-term effect on OA prevention at the population level (Ding et al., 2022; Stephenson et al., 2021). In occupational settings, repetitive joint loading, heavy lifting, kneeling, and squatting have all been linked to increased OA risk, particularly in the knees and hips (Fransen et al., 2011). Ergonomic interventions, job redesign, and protective equipment use (e.g., knee pads) can reduce joint strain and cumulative trauma. Public health policies that enforce workplace standards and promote injury surveillance may contribute to reducing OA burden across physically demanding professions (Hoe et al., 2018; Wang X. et al., 2020). Post-injury secondary prevention is equally critical. Early identification and comprehensive rehabilitation following joint trauma are essential to restore function and minimize risk of PTOA. Strategies such as load management, progressive strengthening, proprioceptive retraining, and regular follow-up imaging can delay or prevent OA progression in high-risk individuals (Figure 4) (Holm-Jensen et al., 2025; Whittaker et al., 2022).
Recent research has also implicated the gut microbiome in OA pathogenesis. Dysbiosis, alterations in gut microbial composition, has been linked to systemic low-grade inflammation, elevated lipopolysaccharides (LPS), and increased cartilage catabolism via immune modulation (Boer et al., 2019). In animal models, transplantation of microbiota from obese donors exacerbated cartilage damage, while prebiotic and probiotic interventions were shown to modulate systemic inflammation and improve joint integrity (Shock et al., 2021; Cao B. et al., 2025; Collins et al., 2021). This suggests that obesity-associated OA may in part be mediated by microbiota-derived inflammatory mediators, highlighting gut health as a novel preventive target in metabolic OA. Moreover, dietary omega-3 polyunsaturated fatty acids (n-3 PUFAs), such as EPA and DHA, have shown chondroprotective and anti-inflammatory effects. Omega-3s suppress the NF-κB pathway, reduce the production of pro-inflammatory cytokines (IL-1β, TNF-α), and inhibit MMP-13 expression in cartilage cells (Chen F. et al., 2024; Kar et al., 2023). In both clinical and preclinical studies, higher omega-3 intake has been associated with slower OA progression, reduced synovitis, and improved cartilage volume. Importantly, dietary omega-3s may also modulate the gut microbiome, creating synergistic effects for inflammation reduction (Shawl et al., 2024; Huang et al., 2024).
4.3 Physical activity and muscle strengthening
Regular, moderate-intensity physical activity is protective against OA by enhancing joint lubrication, improving muscle support, and reducing inflammation. Exercise programs focused on quadriceps strengthening, proprioception, and balance training can delay or prevent the onset of symptomatic OA, particularly in individuals with early cartilage changes or biomechanical abnormalities. Telerehabilitation approaches are also emerging as scalable tools to deliver preventive care remotely (Plavoukou et al., 2025).
5 Future direction
The landscape of OA research is rapidly evolving from symptom-based management to a deeper understanding of the molecular, biomechanical, and systemic drivers of disease progression. This paradigm shift is propelling innovation across diagnostics, therapeutics, and patient care delivery, with an emphasis on precision medicine, regenerative approaches, and digital health integration (Grässel et al., 2021; Mobasheri et al., 2019). A promising avenue in OA research is the development of precision medicine frameworks, leveraging molecular profiling to stratify patients based on inflammatory, metabolic, or mechanical phenotypes. The integration of genomics, proteomics, and metabolomics, alongside advanced imaging biomarkers, can enable earlier diagnosis, individualized treatment selection, and more accurate monitoring of disease progression (Zhai, 2021; Veillette et al., 2015). For instance, Lu et al. emphasize the need to develop and validate molecular signatures that predict structural worsening and therapeutic response in OA, which could guide tailored interventions at earlier stages of the disease (Lu J. S. et al., 2025). Recent imaging advances also enhance the biomarker-driven approach. Quantitative MRI techniques, including T2 mapping and dGEMRIC, can noninvasively assess early cartilage degeneration. Gao et al. developed a “digital twin” of the knee using quantitative MRI data and AI-driven modeling to forecast disease progression and support precision interventions (Hoyer et al., 2025). On the genomic front, several single-nucleotide polymorphisms (SNPs) have been associated with OA susceptibility and progression, including variants in the GDF5, SMAD3, and MMP13 genes. These markers hold potential for predictive modeling and risk stratification (Waheed and Rai, 2024; Wang et al., 2016; Hong et al., 2018). A recent study by Pan also underscores the role of Mendelian randomization in confirming causal relationships between biomarker profiles and OA outcomes, reinforcing the biological relevance of circulating factors (Pan, 2025). Furthermore, synovial tissue proteomics and RNA sequencing are being applied to detect local biomarkers reflective of active joint inflammation, enabling more accurate subtyping of inflammatory OA phenotypes (Carr et al., 2020). Patient phenotyping is increasingly recognized as a pivotal factor in optimizing future OA clinical trials. OA encompasses diverse phenotypes, including inflammatory, metabolic, mechanical, and post-traumatic subtypes, each driven by distinct pathophysiologic mechanisms (Saxer et al., 2024). Applying a “one-size-fits-all” approach in past DMOAD trials likely diluted therapeutic effects and contributed to negative outcomes. To address this, modern clinical trial designs are incorporating biomarker-guided stratification and multi-omics profiling to enrich study populations with patients most likely to respond to specific interventions (Schäfer and Grässel, 2022; Kim et al., 2022). For example, patients with synovitis may benefit more from anti-inflammatory biologics, while those with biomechanical OA may respond better to structural modulators or orthobiologics (Knights et al., 2023; Kl and oppenburg, 2024). Regulatory authorities are increasingly supportive of these strategies, recognizing that precision trial enrollment can enhance efficacy signals, reduce variability, and align therapeutic outcomes with specific disease subtypes. In this evolving paradigm, phenotype-driven research is no longer optional, it is essential for regulatory approval, clinical impact, and the successful translation of next-generation OA therapies (Chu et al., 2025; Edwards et al., 2016). Furthermore, Chen et al. identified dipeptidyl peptidase-4 (DPP-4) as a candidate biomarker linked to chondrocyte catabolism and synovial activation, suggesting a novel target for endotype-specific intervention (Chen et al., 2025). Ge et al. conducted a comprehensive multi-omics analysis of synovial tissue and fluid in knee OA and identified distinct metabolic and proteomic profiles associated with disease progression, highlighting key roles for fibronectin 1 (FN1), TGFBI, and the tricarboxylic acid cycle (TCA) in synovial osteogenesis. Their findings suggest that alterations in collagen metabolism, ECM remodeling, and energy metabolism serve as mechanistic drivers and offer a set of candidate biomarkers for diagnostic and therapeutic targeting (Ge et al., 2025). In a pivotal study, Huo et al. identified a panel of seven m7G-related hub genes, including SNUPN, RNMT, NUDT1, LSM1, LARP1, CYFIP2, and CYFIP1, that were differentially expressed in OA synovial tissue compared to healthy controls. Using integrated computational modeling and validation via RT-qPCR, these genes were incorporated into a diagnostic risk model with high predictive accuracy (AUC >0.9). Their functional enrichment analyses linked these genes to mRNA metabolic processes, immune cell regulation, and RNA transport pathways, confirming their potential role in OA pathogenesis and immune-mediated joint degradation (Huo et al., 2025). Recent targeted therapies are focusing on molecular pathways implicated in OA progression, including TGF-β/ALK5 signaling, Wnt/β-catenin modulation, NF-κB suppression, and ferroptosis inhibition (Tian et al., 2025; Hadzic and Beier, 2023). Agents that block ADAMTS-5, neutralize nerve growth factor, or reduce cellular senescence have shown disease-modifying potential (Jiang et al., 2021). Moreover, significant progress has been made in biological therapies, particularly engineered cytokine modulators, macrophage-reprogramming strategies, and extracellular vesicle-based treatments derived from MSCs, all of which have demonstrated anti-inflammatory, chondroprotective, and immunomodulatory effects (Cotter et al., 2020; Xu G. et al., 2025) (Figure 5). Exosome-based therapeutics, in particular, have shown the ability to deliver microRNAs and regulatory proteins to modify chondrocyte metabolism, reduce synovial fibrosis, and attenuate subchondral angiogenesis (Ni et al., 2020; Yang et al., 2025). Furthermore, advanced drug-delivery systems, including nanoparticle formulations, ROS-responsive hydrogels, thermo-sensitive polymers, and sustained-release microspheres, are also rapidly evolving. These platforms improve intra-articular retention, enhance drug bioavailability, and allow localized release of antioxidants, growth factors, or small-molecule inhibitors precisely within inflamed or mechanically stressed regions of the joint (Yin et al., 2024; Huang H. et al., 2022). In the field of tissue engineering, multifunctional scaffolds integrating bioactive molecules, immunomodulatory cues, and stem cells are emerging as next-generation regenerative platforms (Im, 2018). Recent investigations have shown that composite scaffolds incorporating growth factors (e.g., TGF-β3, BMP-7), gene-delivery vectors, or mechanical-responsive components can simultaneously promote cartilage regeneration, modulate synovial inflammation, and restore osteochondral integrity (Shanto et al., 2025; Liang et al., 2023). Advances in 3D bioprinting enable anatomically accurate osteochondral constructs with zonal organization and gradient stiffness, improving integration and long-term repair quality (Chartrain et al., 2022; Lai et al., 2025), (Turunen et al., 2023). Nevertheless, the pursuit of disease-modifying osteoarthritis drugs (DMOADs) represents another transformative direction (Li S. et al., 2023). While current treatments largely focus on symptom relief, agents such as Wnt pathway modulators (e.g., lorecivivint), ADAMTS-5 inhibitors, and nerve growth factor (NGF) antagonists like tanezumab are under investigation for their potential to alter disease progression (Jiang et al., 2021; Cho et al., 2021; Kou et al., 2023). Although clinical trial outcomes have been mixed, ongoing research aims to optimize patient selection, dosing regimens, and combinatory strategies. Furthermore, novel formulations of intra-articular therapeutics, such as glucosinolates and enhanced hyaluronic acid, are being explored for their potential to provide both analgesic and structural benefits (Gambari et al., 2025).
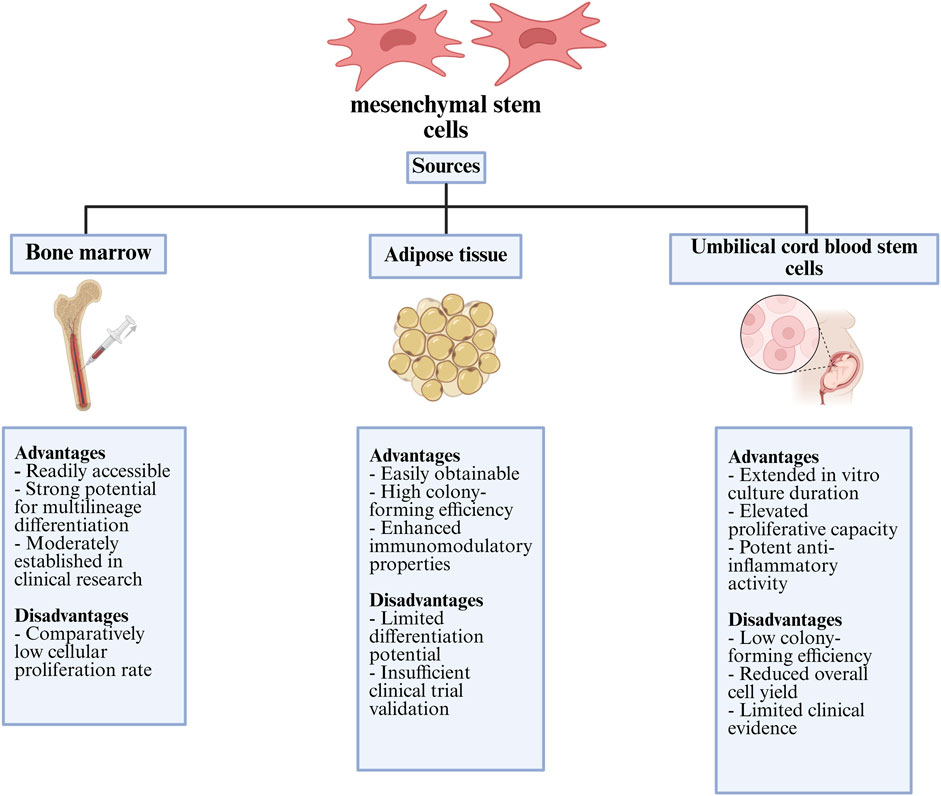
Figure 5. Comparative summary of the main advantages and limitations of mesenchymal stem cells from different tissue origins with clinical and regenerative use.
Artificial intelligence (AI) and digital health technologies are increasingly being integrated into OA care. AI-powered image analysis systems can detect subtle cartilage changes and joint space narrowing before they become clinically apparent, offering the possibility of earlier intervention. In the omics domain, AI facilitates the processing and interpretation of complex high-dimensional datasets, which is critical for identifying cell-type-specific gene expression patterns, post-transcriptional regulators, and predictive biomarkers. For instance, Ou et al. describe how transcriptomic and proteomic analyses, enhanced by machine learning classifiers like random forests and support vector machines, have identified OA-relevant genes such as RIPK3, PDK1, and CDH2. These genes are associated with chondrocyte necroptosis, metabolic dysregulation, and immune cell infiltration, offering a more nuanced understanding of disease mechanisms compared to traditional markers like CTX-II or IL-6 (Ou et al., 2025). AI also enables multimodal integration of data sources, including clinical records, electronic health records (EHRs), and laboratory tests, to construct predictive models for disease onset, progression, and surgical outcomes (Figure 6). Notably, predictive models incorporating AI have demonstrated high accuracy in forecasting the need for joint replacement surgery and in stratifying patients based on their expected response to specific interventions (Mohsen et al., 2022; Lee et al., 2022). Wearable devices and mobile health platforms are also emerging as tools for remote monitoring of physical activity, gait abnormalities, and treatment adherence. According to Takase et al., such technologies could play a central role in the real-time assessment and personalization of therapy for OA patients (Takase et al., 2025). Another important emerging concept is the role of mitochondrial dysfunction and oxidative stress in chondrocyte senescence and cartilage degradation. Novel therapies targeting mitochondrial quality control pathways, such as mitophagy enhancers and antioxidant regulators, may represent a new class of interventions capable of modulating OA pathophysiology at the cellular level (Picca et al., 2020; Guo et al., 2025). Wu et al. describe how these mechanisms are increasingly linked to the metabolic-inflammatory axis of OA, offering future therapeutic targets beyond conventional anti-inflammatories (Wu et al., 2025).
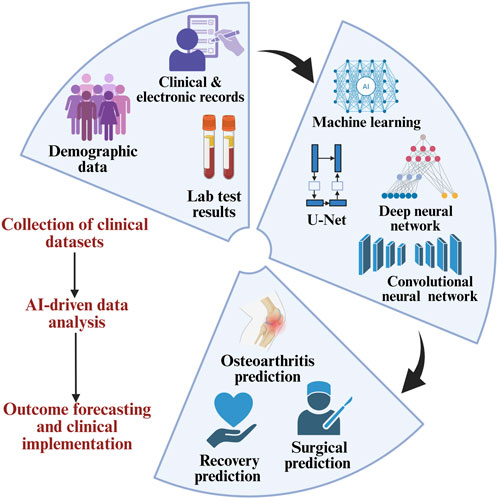
Figure 6. AI-based analysis of clinical data enables the prediction of osteoarthritis outcomes and supports clinical decision-making.
The future of OA care is poised to be transformed by innovations in systems biology, biomaterials, digital health, and pharmacology. These advancements underscore the transition from a reactive, symptom-based approach to a proactive, mechanism-driven model of care. Success will depend on collaborative efforts across basic science, clinical research, and health technology sectors to translate these promising developments into safe, effective, and accessible therapies for the global OA population.
6 Conclusion
Advances in molecular profiling, imaging, and regenerative strategies are redefining our understanding of osteoarthritis beyond structural degeneration. Bridging basic science with precision therapeutics offers a path toward earlier diagnosis, targeted intervention, and long-term disease modification. Continued interdisciplinary research will be essential to translate these innovations into accessible, patient-centered care.
Author contributions
LQ: Writing – original draft, Conceptualization, Data curation. AA: Supervision, Data curation, Writing – original draft. SM: Supervision, Writing – review and editing, Conceptualization.
Funding
The author(s) declared that financial support was received for this work and/or its publication. Scientific research project of Zhejiang Education Department NO.Y202352378.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aho, O. M., Finnilä, M., Thevenot, J., Saarakkala, S., and Lehenkari, P. (2017). Subchondral bone histology and grading in osteoarthritis. PLoS One 12 (3), e0173726. doi:10.1371/journal.pone.0173726
Aigner, T., Söder, S., Gebhard, P. M., McAlinden, A., and Haag, J. (2007). Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis—structure, chaos and senescence. Nat. Clin. Pract. Rheumatol. 3 (7), 391–399. doi:10.1038/ncprheum0534
Altman, A. D., Helpman, L., McGee, J., Samouëlian, V., Auclair, M. H., Brar, H., et al. (2019). Enhanced recovery after surgery: implementing a new standard of surgical care. CMAJ 191 (17), E469–e475. doi:10.1503/cmaj.180635
Ambrose, K. R., and Golightly, Y. M. (2015). Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best. Pract. Res. Clin. Rheumatol. 29 (1), 120–130. doi:10.1016/j.berh.2015.04.022
Anandacoomarasamy, A., and March, L. (2010). Current evidence for osteoarthritis treatments. Ther. Adv. Musculoskelet. Dis. 2 (1), 17–28. doi:10.1177/1759720X09359889
Antonelli, B., and Chen, A. F. (2019). Reducing the risk of infection after total joint arthroplasty: preoperative optimization. Arthroplasty 1 (1), 4. doi:10.1186/s42836-019-0003-7
Arden, N., and Nevitt, M. C. (2006). Osteoarthritis: epidemiology. Best Pract. Res. Clin. Rheumatol. 20 (1), 3–25. doi:10.1016/j.berh.2005.09.007
Asghar, S., Litherland, G. J., Lockhart, J. C., Goodyear, C. S., and Crilly, A. (2020). Exosomes in intercellular communication and implications for osteoarthritis. Rheumatol. Oxf. 59 (1), 57–68. doi:10.1093/rheumatology/kez462
Bai, L. K., Su, Y. Z., Wang, X. X., Bai, B., Zhang, C. Q., Zhang, L. Y., et al. (2022). Synovial macrophages: past life, current situation, and application in inflammatory arthritis. Front. Immunol. 13, 905356. doi:10.3389/fimmu.2022.905356
Bannuru, R. R., Schmid, C. H., Kent, D. M., Vaysbrot, E. E., Wong, J. B., and McAlindon, T. E. (2015). Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann. Intern Med. 162 (1), 46–54. doi:10.7326/M14-1231
Bannuru, R. R., Osani, M. C., Vaysbrot, E. E., Arden, N. K., Bennell, K., Bierma-Zeinstra, S. M. A., et al. (2019). OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 27 (11), 1578–1589. doi:10.1016/j.joca.2019.06.011
Bariguian Revel, F., Fayet, M., and Hagen, M. (2020). Topical diclofenac, an efficacious treatment for osteoarthritis: a narrative review. Rheumatol. Ther. 7 (2), 217–236. doi:10.1007/s40744-020-00196-6
Bennell, K. L., Dobson, F., and Hinman, R. S. (2014). Exercise in osteoarthritis: moving from prescription to adherence. Best Pract. Res. Clin. Rheumatol. 28 (1), 93–117. doi:10.1016/j.berh.2014.01.009
Bensa, A., Salerno, M., Moraca, G., Boffa, A., McIlwraith, C. W., and Filardo, G. (2024). Intra-articular corticosteroids for the treatment of osteoarthritis: a systematic review and meta-analysis on the comparison of different molecules and doses. J. Exp. Orthop. 11 (3), e12060. doi:10.1002/jeo2.12060
Bierma-Zeinstra, S., van Middelkoop, M., Runhaar, J., and Schiphof, D. (2020). Nonpharmacological and nonsurgical approaches in OA. Best Pract. Res. Clin. Rheumatol. 34 (2), 101564. doi:10.1016/j.berh.2020.101564
Boer, C. G., Radjabzadeh, D., Medina-Gomez, C., Garmaeva, S., Schiphof, D., Arp, P., et al. (2019). Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 10 (1), 4881. doi:10.1038/s41467-019-12873-4
Braun, S., Zaucke, F., Brenneis, M., Rapp, A. E., Pollinger, P., Sohn, R., et al. (2022). The Corpus adiposum infrapatellare (Hoffa’s fat Pad)-The role of the infrapatellar fat pad in osteoarthritis pathogenesis. Biomedicines 10 (5), 1071. doi:10.3390/biomedicines10051071
Brittberg, M., Gomoll, A. H., Canseco, J. A., Far, J., Lind, M., and Hui, J. (2016). Cartilage repair in the degenerative ageing knee. Acta Orthop. 87 (Suppl. 363), 26–38. doi:10.1080/17453674.2016.1265877
Brophy, R. H., and Lowry, K. J. (2023). American Academy of Orthopaedic Surgeons Clinical Practice Guideline summary: management of anterior cruciate ligament injuries. JAAOS - J. Am. Acad. Orthop. Surg. 31 (11), 531–537. doi:10.5435/JAAOS-D-22-01020
Brumat, P., Kunšič, O., Novak, S., Slokar, U., Pšenica, J., Topolovec, M., et al. (2022). The surgical treatment of osteoarthritis. Life (Basel) 12 (7), 982. doi:10.3390/life12070982
Cabarcas, B., Peairs, E., Iyer, S., Ina, J., Hevesi, M., Tagliero, A. J., et al. (2025). Long-term results for meniscus repair. Curr. Rev. Musculoskelet. Med. 18 (7), 229–245. doi:10.1007/s12178-025-09966-7
Cameron, J. I., McCauley, J. C., Kermanshahi, A. Y., and Bugbee, W. D. (2015). Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin. Orthop. Relat. Res. 473 (6), 2009–2015. doi:10.1007/s11999-014-4106-8
Cao, M., Ou, Z., Sheng, R., Wang, Q., Chen, X., Zhang, C., et al. (2025a). Efficacy and safety of mesenchymal stem cells in knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 16 (1), 122. doi:10.1186/s13287-025-04252-2
Cao, B., Sun, Y., Lam, C., Chen, Y., Dri, C. E., and McIntyre, R. S. (2025b). Interaction between dietary omega-3 polyunsaturated fatty acids, obesity and gut microbiota in preclinical models: a systematic review of randomized controlled trials. Diabetes Obes. Metab. 27 (9), 4643–4661. doi:10.1111/dom.16535
Carbone, A., and Rodeo, S. (2017). Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J. Orthop. Res. 35 (3), 397–405. doi:10.1002/jor.23341
Carr, H. L., Turner, J. D., Major, T., Scheel-Toellner, D., and Filer, A. (2020). New developments in transcriptomic analysis of synovial tissue. Front. Med. (Lausanne) 7, 21. doi:10.3389/fmed.2020.00021
Chappell, A. S., Desaiah, D., Liu-Seifert, H., Zhang, S., Skljarevski, V., Belenkov, Y., et al. (2011). A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 11 (1), 33–41. doi:10.1111/j.1533-2500.2010.00401.x
Charlier, E., Deroyer, C., Ciregia, F., Malaise, O., Neuville, S., Plener, Z., et al. (2019). Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 165, 49–65. doi:10.1016/j.bcp.2019.02.036
Chartrain, N. A., Gilchrist, K. H., Ho, V. B., and Klarmann, G. J. (2022). 3D bioprinting for the repair of articular cartilage and osteochondral tissue. Bioprinting 28, e00239. doi:10.1016/j.bprint.2022.e00239
Chavda, S., Rabbani, S. A., and Wadhwa, T. (2022). Role and effectiveness of intra-articular injection of hyaluronic acid in the treatment of knee osteoarthritis: a systematic review. Cureus 14 (4), e24503. doi:10.7759/cureus.24503
Chen, W., Wang, Q., Tao, H., Lu, L., Zhou, J., Wang, Q., et al. (2024a). Subchondral osteoclasts and osteoarthritis: new insights and potential therapeutic avenues. Acta Biochim. Biophys. Sin. (Shanghai) 56 (4), 499–512. doi:10.3724/abbs.2024017
Chen, F., Zhang, Z., Wang, W., Liu, C., Huang, Z., Yu, C., et al. (2024b). Omega-3 fatty acids protect cartilage from acute injurie by reducing the mechanical sensitivity of chondrocytes. J. Orthop. Surg. Res. 19 (1), 591. doi:10.1186/s13018-024-05081-4
Chen, Y.-H., Wang, C.-C., and Peng, Y.-J. (2025). Identification of dipeptidyl Peptidase-4 (DPP-4) related pathways in osteoarthritis chondrocytes using rna-seq analysis. Osteoarthr. Cartil. 33, S385–S386. doi:10.1016/j.joca.2025.02.560
Cho, S. D., Youm, Y. S., Lee, C. C., Seo, D. K., and Kim, T. W. (2012). Mucoid degeneration of both ACL and PCL. Clin. Orthop. Surg. 4 (2), 167–170. doi:10.4055/cios.2012.4.2.167
Cho, Y., Jeong, S., Kim, H., Kang, D., Lee, J., Kang, S. B., et al. (2021). Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp. Mol. Med. 53 (11), 1689–1696. doi:10.1038/s12276-021-00710-y
Choe, R., Devoy, E., Jabari, E., Packer, J. D., and Fisher, J. P. (2022). Biomechanical aspects of osteochondral regeneration: implications and strategies for three-dimensional bioprinting. Tissue Eng. Part B Rev. 28 (4), 766–788. doi:10.1089/ten.TEB.2021.0101
Chou, C.-H., Jain, V., Gibson, J., Attarian, D. E., Haraden, C. A., Yohn, C. B., et al. (2020). Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 10 (1), 10868. doi:10.1038/s41598-020-67730-y
Chu, C. R., Hochberg, M., White, D., Rodeo, S., Huard, J., Shapiro, S., et al. (2025). Transformative approaches for effective clinical trials to reduce the disease burden of osteoarthritis. Semin. Arthritis Rheum. 71, 152652. doi:10.1016/j.semarthrit.2025.152652
Coaccioli, S., Sarzi-Puttini, P., Zis, P., Rinonapoli, G., and Varrassi, G. (2022). Osteoarthritis: new insight on its pathophysiology. J. Clin. Med. 11 (20), 6013. doi:10.3390/jcm11206013
Collins, K. H., Schwartz, D. J., Lenz, K. L., Harris, C. A., and Guilak, F. (2021). Taxonomic changes in the gut microbiota are associated with cartilage damage independent of adiposity, high fat diet, and joint injury. Sci. Rep. 11 (1), 14560. doi:10.1038/s41598-021-94125-4
Conaghan, P. G., Arden, N., Avouac, B., Migliore, A., and Rizzoli, R. (2019). Safety of paracetamol in osteoarthritis: what does the literature say? Drugs Aging 36 (Suppl. 1), 7–14. doi:10.1007/s40266-019-00658-9
Conley, B., Bunzli, S., Bullen, J., O'Brien, P., Persaud, J., Gunatillake, T., et al. (2023). Core recommendations for osteoarthritis care: a systematic review of Clinical Practice Guidelines. Arthritis Care Res. Hob. 75 (9), 1897–1907. doi:10.1002/acr.25101
Cotter, E. J., Frank, R. M., and Mandelbaum, B. (2020). Management of osteoarthritis - biological approaches: current concepts. J. ISAKOS 5 (1), 27–31. doi:10.1136/jisakos-2019-000377
Cronström, A., Nero, H., Lohmander, L. S., and Dahlberg, L. E. (2020). On the waiting list for joint replacement for knee osteoarthritis: are first-line treatment recommendations implemented? Osteoarthr. Cartil. Open 2 (2), 100056. doi:10.1016/j.ocarto.2020.100056
Cruz Ayala, J. A., Crawford, M., Gatterer, M. C., Tovar, M., Rivera, J. C., Dasa, V., et al. (2022). Using the articularis genu to test peri-articular muscle health during knee osteoarthritis. Sci. Rep. 12 (1), 12896. doi:10.1038/s41598-022-17046-w
Cunningham, J., M Briggs, A., Cottrell, E., Doyle, F., Dziedzic, K., Finney, A., et al. (2021). Barriers and facilitators to the implementation of osteoarthritis management programmes in primary or community care settings: a systematic review and qualitative framework synthesis protocol. HRB Open Res. 4, 102. doi:10.12688/hrbopenres.13377.1
de Magalhães, J. P. (2025). Senolytics under scrutiny in the quest to slow aging. Nat. Biotechnol. 43 (9), 1413–1415. doi:10.1038/s41587-025-02740-7
Derry, S., Conaghan, P., Da Silva, J. A., Wiffen, P. J., and Moore, R. A. (2016). Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 4 (4), Cd007400. doi:10.1002/14651858.CD007400.pub3
Deschamps, G., Dodd, P., Hernigou, A., Franz, Z., Parratte, J. M., et al. (2012). “Unicompartmental knee arthroplasty in medial osteoarthritis: the basics,” in ESSKA instructional course lecture book: geneva 2012. Editor J. Menetrey, S. Zaffagnini,, D. Fritschy, and N. van Dijk (Berlin, Heidelberg: Springer Berlin Heidelberg), 31–40. doi:10.1007/978-3-642-29446-4_4
Di Nicola, V. (2020). Degenerative osteoarthritis a reversible chronic disease. Regen. Ther. 15, 149–160. doi:10.1016/j.reth.2020.07.007
Diaz, F., Thornton, J. S., Wastling, S. S., Asaab, A., Morrow, J. M., Zafeiropoulos, N., et al. (2025). Longitudinal quantitative MRI provides responsive outcome measures for early and late muscle changes in ALS. Muscle Nerve 71 (2), 171–182. doi:10.1002/mus.28306
Ding, L., Luo, J., Smith, D. M., Mackey, M., Fu, H., Davis, M., et al. (2022). Effectiveness of Warm-Up intervention programs to prevent sports injuries among children and adolescents: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health 19 (10), 6336. doi:10.3390/ijerph19106336
Donell, S. (2019). Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 4 (6), 221–229. doi:10.1302/2058-5241.4.180102
Dziedzic, K. S., and Allen, K. D. (2018). Challenges and controversies of complex interventions in osteoarthritis management: recognizing inappropriate and discordant care. Rheumatol. Oxf. 57 (Suppl. l_4), iv88–iv98. doi:10.1093/rheumatology/key062
Ebert, J. R., Zheng, M., Fallon, M., Wood, D. J., and Janes, G. C. (2024). 10-Year prospective clinical and radiological evaluation after matrix-induced autologous chondrocyte implantation and comparison of tibiofemoral and patellofemoral graft outcomes. Am. J. Sports Med. 52 (4), 977–986. doi:10.1177/03635465241227969
Edwards, R. R., Dworkin, R. H., Turk, D. C., Angst, M. S., Dionne, R., Freeman, R., et al. (2016). Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 157 (9), 1851–1871. doi:10.1097/j.pain.0000000000000602
Emery, C. A., and Pasanen, K. (2019). Current trends in sport injury prevention. Best Pract. Res. Clin. Rheumatol. 33 (1), 3–15. doi:10.1016/j.berh.2019.02.009
Eschweiler, J., Horn, N., Rath, B., Betsch, M., Baroncini, A., Tingart, M., et al. (2021). The biomechanics of Cartilage-an overview. Life (Basel) 11 (4), 302. doi:10.3390/life11040302
Fan, F., Yang, C., Piao, E., Shi, J., and Zhang, J. (2024). Mechanisms of chondrocyte regulated cell death in osteoarthritis: focus on ROS-triggered ferroptosis, parthanatos, and oxeiptosis. Biochem. Biophysical Res. Commun. 705, 149733. doi:10.1016/j.bbrc.2024.149733
Farinelli, L., Riccio, M., Gigante, A., and De Francesco, F. (2024). Pain management strategies in osteoarthritis. Biomedicines 12 (4), 805. doi:10.3390/biomedicines12040805
Felson, D. T. (2004). Risk factors for osteoarthritis: understanding joint vulnerability. Clin. Orthop. Relat. Research® 427, S16–S21. doi:10.1097/01.blo.0000144971.12731.a2
Feng, X., and McDonald, J. M. (2011). Disorders of bone remodeling. Annu. Rev. Pathol. 6, 121–145. doi:10.1146/annurev-pathol-011110-130203
Feng, J. E., Novikov, D., Anoushiravani, A. A., and Schwarzkopf, R. (2018). Total knee arthroplasty: improving outcomes with a multidisciplinary approach. J. Multidiscip. Healthc. 11, 63–73. doi:10.2147/JMDH.S140550
Fernandez-Moreno, M., Hermida-Gomez, T., Vaamonde-Garcia, C., Paniagua-Barro, S., Larkins, N., Reynolds, A., et al. (2025). Appa showed senolytic capacity in senescent chondrocytes. Osteoarthr. Cartil. 33, S330–S331. doi:10.1016/j.joca.2025.02.464
Ferrera, A., and Menetrey, J. (2022). Optimizing indications and technique in osteotomies around the knee. EFORT Open Rev. 7 (6), 396–403. doi:10.1530/EOR-22-0057
French, H. P., Cunningham, J., Bennett, K., Cadogan, C. A., Clyne, B., Doyle, F., et al. (2024). Patterns of pain medication usage and self-reported pain in older Irish adults with osteoarthritis: a latent class analysis of data from the Irish Longitudinal Study on Ageing. BMC Musculoskelet. Disord. 25 (1), 773. doi:10.1186/s12891-024-07854-8
Fransen, M., Agaliotis, M., Bridgett, L., and Mackey, M. G. (2011). Hip and knee pain: role of occupational factors. Best Pract. Res. Clin. Rheumatology 25 (1), 81–101. doi:10.1016/j.berh.2011.01.012
Gambari, L., Cellamare, A., D'Alessandro, M., Grigolo, B., Gualandi, C., Velino, C., et al. (2025). Towards a nutraceutical-based injectable treatment for early osteoarthritis: preclinical evidence from a glucosinolate-releasing hyaluronic acid hydrogel. Osteoarthr. Cartil. 33, S206–S207. doi:10.1016/j.joca.2025.02.292
Garcia, J., Wright, K., Roberts, S., Kuiper, J. H., Mangham, C., Richardson, J., et al. (2016). Characterisation of synovial fluid and infrapatellar fat pad derived mesenchymal stromal cells: the influence of tissue source and inflammatory stimulus. Sci. Rep. 6 (1), 24295. doi:10.1038/srep24295
Garcia-Dominguez, C., Nogueira-Recalde, U., Copena Soutelo, A., Diaz-Rodriguez, P., Blanco, F., Dominguez, E., et al. (2025). ABS0006 integrating peroxisome proliferator-activated receptor A and circadian rhythm insights as novel underlying mechanisms of osteoarthritis. Ann. Rheumatic Dis. 84, 1732. doi:10.1016/j.ard.2025.06.1190
Gato-Calvo, L., Magalhaes, J., Ruiz-Romero, C., Blanco, F. J., and Burguera, E. F. (2019). Platelet-rich plasma in osteoarthritis treatment: review of current evidence. Ther. Adv. Chronic Dis. 10, 2040622319825567. doi:10.1177/2040622319825567
Ge, M., Sun, W., Xu, T., Yang, R., Zhang, K., Li, J., et al. (2025). Multi-omics analysis of synovial tissue and fluid reveals differentially expressed proteins and metabolites in osteoarthritis. J. Transl. Med. 23 (1), 285. doi:10.1186/s12967-025-06310-y
Ghorbani, O., Mahdibarzi, D., and Yousefi-Tooddeshki, P. (2024). Comparison of the short-term effect of intra-articular hyaluronic acid and platelet-rich plasma injections in knee osteoarthritis: a randomized clinical trial. J. Prev. Med. Hyg. 65 (2), E214–e220. doi:10.15167/2421-4248/jpmh2024.65.2.3270
Goldring, M. B. (2012). Articular cartilage degradation in osteoarthritis. Hss J. 8 (1), 7–9. doi:10.1007/s11420-011-9250-z
Golightly, Y. M., Allen, K. D., and Caine, D. J. (2012). A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Phys. Sportsmed. 40 (4), 52–65. doi:10.3810/psm.2012.11.1988
Grässel, S., and Muschter, D. (2017). Peripheral nerve fibers and their neurotransmitters in Osteoarthritis pathology. Int. J. Mol. Sci. 18 (5), 931. doi:10.3390/ijms18050931
Grässel, S., Zaucke, F., and Madry, H. (2021). Osteoarthritis: novel molecular mechanisms increase our understanding of the disease pathology. J. Clin. Med. 10 (9), 1938. doi:10.3390/jcm10091938
Guan, M., Yu, Q., Zhou, G., Wang, Y., Yu, J., Yang, W., et al. (2024). Mechanisms of chondrocyte cell death in osteoarthritis: implications for disease progression and treatment. J. Orthop. Surg. Res. 19 (1), 550. doi:10.1186/s13018-024-05055-6
Guan, W., Yuan, F., and Wang, X. (2025). Dehydrotanshinone II A alleviates osteoarthritis via activating PPARγ to inhibit ferroptosis in chondrocytes. Sci. Rep. 15 (1), 29602. doi:10.1038/s41598-025-14896-y
Guermazi, A., Hunter, D. J., and Kloppenburg, M. (2023). Debate: intra-articular steroid injections for osteoarthritis - harmful or helpful?(☆,☆☆). Osteoarthr. Imaging 3 (3), 100163. doi:10.1016/j.ostima.2023.100163
Guilak, F. (2011). Biomechanical factors in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 25 (6), 815–823. doi:10.1016/j.berh.2011.11.013
Guo, P., Alhaskawi, A., Adel Abdo Moqbel, S., and Pan, Z. (2025). Recent development of mitochondrial metabolism and dysfunction in osteoarthritis. Front. Pharmacol. 16, 1538662. doi:10.3389/fphar.2025.1538662
Gupta, R. C., Lall, R., Srivastava, A., and Sinha, A. (2019). Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 6, 192. doi:10.3389/fvets.2019.00192
Guzmán-David, C. A., Ruiz-Ávila, H. A., Camargo-Rojas, D. A., Gómez-Alegría, C. J., and Hernández-Álvarez, E. D. (2023). Ultrasound assessment of muscle mass and correlation with clinical outcomes in critically ill patients: a prospective observational study. J. Ultrasound 26 (4), 879–889. doi:10.1007/s40477-023-00823-2
Hadzic, E., and Beier, F. (2023). Emerging therapeutic targets for osteoarthritis. Expert Opin. Ther. Targets 27 (2), 111–120. doi:10.1080/14728222.2023.2185133
Haemer, J., Song, Y., Carter, D. R., and Giori, N. J. (2011). Changes in articular cartilage mechanics with meniscectomy: a novel image-based modeling approach and comparison to patterns of OA. J. Biomechanics 44, 2307–2312. doi:10.1016/j.jbiomech.2011.04.014
Henrotin, Y., Pesesse, L., and Sanchez, C. (2012). Subchondral bone and osteoarthritis: biological and cellular aspects. Osteoporos. Int. 23 (8), 847–851. doi:10.1007/s00198-012-2162-z
Hernigou, P., Bouthors, C., Bastard, C., Flouzat Lachaniette, C. H., Rouard, H., and Dubory, A. (2021a). Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: what better postpone knee arthroplasty at fifteen years? A randomized study. Int. Orthop. 45 (2), 391–399. doi:10.1007/s00264-020-04687-7
Hernigou, P., Delambre, J., Quiennec, S., and Poignard, A. (2021b). Human bone marrow mesenchymal stem cell injection in subchondral lesions of knee osteoarthritis: a prospective randomized study versus contralateral arthroplasty at a mean fifteen year follow-up. Int. Orthop. 45 (2), 365–373. doi:10.1007/s00264-020-04571-4
Hoe, V. C., Urquhart, D. M., Kelsall, H. L., Zamri, E. N., and Sim, M. R. (2018). Ergonomic interventions for preventing work-related musculoskeletal disorders of the upper limb and neck among office workers. Cochrane Database Syst. Rev. 10 (10), Cd008570. doi:10.1002/14651858.CD008570.pub3
Hofstede, S. N., Vliet Vlieland, T. P. M., van den Ende, C. H. M., Nelissen, R. G. H. H., Marang-van de Mheen, P. J., and van Bodegom-Vos, L. (2015). Variation in use of non-surgical treatments among osteoarthritis patients in orthopaedic practice in the Netherlands. BMJ Open 5 (9), e009117. doi:10.1136/bmjopen-2015-009117
Holm-Jensen, A., Vlachos, E., Storm, L. K., and Myburgh, C. (2025). The consistency of primary, secondary and tertiary prevention definitions in the context of musculoskeletal sports injuries: a rapid review and critical exploration of common terms of usage. Sports Med. Open 11 (1), 28. doi:10.1186/s40798-025-00823-y
Hong, J. Q., Wang, Y. X., Li, S. H., Jiang, G. Y., Hu, B., Yang, Y. T., et al. (2018). Association between SMAD3 gene polymorphisms and osteoarthritis risk: a systematic review and meta-analysis. J. Orthop. Surg. Res. 13 (1), 232. doi:10.1186/s13018-018-0939-2
Hong, Y., Boiti, A., Vallone, D., and Foulkes, N. S. (2024). Reactive oxygen species signaling and oxidative stress: transcriptional regulation and evolution. Antioxidants (Basel) 13 (3), 312. doi:10.3390/antiox13030312
Hoyer, G., Gao, K. T., Gassert, F. G., Luitjens, J., Jiang, F., Majumdar, S., et al. (2025). Foundations of a knee joint digital twin from qMRI biomarkers for osteoarthritis and knee replacement. Npj Digit. Med. 8 (1), 118. doi:10.1038/s41746-025-01507-3
Huang, Z. Y., Luo, Z. Y., Cai, Y. R., Chou, C. H., Yao, M. L., Pei, F. X., et al. (2022a). Single cell transcriptomics in human osteoarthritis synovium and in silico deconvoluted bulk RNA sequencing. Osteoarthr. Cartil. 30 (3), 475–480. doi:10.1016/j.joca.2021.12.007
Huang, H., Lou, Z., Zheng, S., Wu, J., Yao, Q., Chen, R., et al. (2022b). Intra-articular drug delivery systems for osteoarthritis therapy: shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 29 (1), 767–791. doi:10.1080/10717544.2022.2048130
Huang, S., Jiang, J., and Gong, H. (2024). Association between dietary omega-3 fatty acid intake and all-cause mortality in patients with osteoarthritis: a population-based prospective cohort study. Sci. Rep. 14 (1), 26516. doi:10.1038/s41598-024-78486-0
Huber, F. A., Del Grande, F., Rizzo, S., Guglielmi, G., and Guggenberger, R. (2020). MRI in the assessment of adipose tissues and muscle composition: how to use it. Quant. Imaging Med. Surg. 10 (8), 1636–1649. doi:10.21037/qims.2020.02.06
Huffman, K. F., Ambrose, K. R., Nelson, A. E., Allen, K. D., Golightly, Y. M., and Callahan, L. F. (2024). The critical role of physical activity and weight management in knee and hip osteoarthritis: a narrative review. J. Rheumatol. 51 (3), 224–233. doi:10.3899/jrheum.2023-0819
Hunter, D. J., Chang, C. C., Wei, J. C. C., Lin, H. Y., Brown, C., Tai, T. T., et al. (2022). TLC599 in patients with osteoarthritis of the knee: a phase IIa, randomized, placebo-controlled, dose-finding study. Arthritis Res. Ther. 24 (1), 52. doi:10.1186/s13075-022-02739-4
Huo, Z., Fan, C., Li, K., Xu, C., Niu, Y., and Wang, F. (2025). Identification and validation of hub m7G-related genes and infiltrating immune cells in osteoarthritis based on integrated computational and bioinformatics analysis. BMC Musculoskelet. Disord. 26 (1), 333. doi:10.1186/s12891-025-08539-6
Iannitti, T., Lodi, D., and Palmieri, B. (2011). Intra-articular injections for the treatment of osteoarthritis: focus on the clinical use of hyaluronic acid. Drugs R. D. 11 (1), 13–27. doi:10.2165/11539760-000000000-00000
Im, G. I. (2018). Tissue engineering in osteoarthritis: current status and prospect of mesenchymal stem cell therapy. BioDrugs 32 (3), 183–192. doi:10.1007/s40259-018-0276-3
Jansen, M. P., and Mastbergen, S. C. (2022). Joint distraction for osteoarthritis: clinical evidence and molecular mechanisms. Nat. Rev. Rheumatol. 18 (1), 35–46. doi:10.1038/s41584-021-00695-y
Jeyaraman, M., Muthu, S., Jeyaraman, N., Ranjan, R., Jha, S. K., and Mishra, P. (2022). Synovium derived mesenchymal stromal cells (Sy-MSCs): a promising therapeutic paradigm in the management of knee osteoarthritis. Indian J. Orthop. 56 (1), 1–15. doi:10.1007/s43465-021-00439-w
Jiang, L., Lin, J., Zhao, S., Wu, J., Jin, Y., Yu, L., et al. (2021). ADAMTS5 in osteoarthritis: biological functions, regulatory network, and potential targeting therapies. Front. Mol. Biosci. 8, 703110. doi:10.3389/fmolb.2021.703110
Jiang, W., Chen, H., Lin, Y., Cheng, K., Zhou, D., Chen, R., et al. (2023). Mechanical stress abnormalities promote chondrocyte senescence - the pathogenesis of knee osteoarthritis. Biomed. Pharmacother. 167, 115552. doi:10.1016/j.biopha.2023.115552
Jones, D. G., and Peterson, L. (2006). Autologous chondrocyte implantation. JBJS 88 (11), 2501–2520. doi:10.2106/00004623-200611000-00025
Jönsson, T., Eek, F., Dell'Isola, A., Dahlberg, L. E., and Ekvall Hansson, E. (2019). The better management of patients with Osteoarthritis program: outcomes after evidence-based education and exercise delivered nationwide in Sweden. PLoS One 14 (9), e0222657. doi:10.1371/journal.pone.0222657
Kar, A., Ghosh, P., Patra, P., Chini, D. S., Nath, A. K., Saha, J. K., et al. (2023). Omega-3 fatty acids mediated cellular signaling and its regulation in human health. Clin. Nutr. Open Sci. 52, 72–86. doi:10.1016/j.nutos.2023.10.004
Karuppal, R. (2017). Current concepts in the articular cartilage repair and regeneration. J. Orthop. 14 (2), A1–a3. doi:10.1016/j.jor.2017.05.001
Katz, J. N., Earp, B. E., and Gomoll, A. H. (2010). Surgical management of osteoarthritis. Arthritis Care Res. Hob. 62 (9), 1220–1228. doi:10.1002/acr.20231
Kim, H., Seo, J., Lee, Y., Park, K., Perry, T. A., Arden, N. K., et al. (2022). The current state of the osteoarthritis drug development pipeline: a comprehensive narrative review of the present challenges and future opportunities. Ther. Adv. Musculoskelet. Dis. 14, 1759720x221085952. doi:10.1177/1759720X221085952
Kim, J. R., Pham, T. H. N., Kim, W. U., and Kim, H. A. (2023). A causative role for periarticular skeletal muscle weakness in the progression of joint damage and pain in OA. Sci. Rep. 13 (1), 21349. doi:10.1038/s41598-023-46599-7
Kloppenburg, M. (2024). Synovial inflammation in osteoarthritis. A treatable target? Seminars Arthritis Rheumatism 64, 152326. doi:10.1016/j.semarthrit.2023.152326
Klotz, U. (2012). Paracetamol (Acetaminophen) - a popular and widely used nonopioid analgesic. Arzneimittelforschung 62 (8), 355–359. doi:10.1055/s-0032-1321785
Knights, A. J., Redding, S. J., and Maerz, T. (2023). Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr. Opin. Rheumatol. 35 (2), 128–134. doi:10.1097/BOR.0000000000000923
Kou, H., Qing, Z., Zhao, G., Sun, X., Zhi, L., Wang, J., et al. (2023). Effect of lorecivivint on osteoarthritis: a systematic review and meta-analysis. Heliyon 9 (8), e18682. doi:10.1016/j.heliyon.2023.e18682
Kraus, V. B., and Hsueh, M.-F. (2024). Molecular biomarker approaches to prevention of post-traumatic osteoarthritis. Nat. Rev. Rheumatol. 20 (5), 272–289. doi:10.1038/s41584-024-01102-y
Krupkova, O., Smolders, L., Wuertz-Kozak, K., Cook, J., and Pozzi, A. (2018). The pathobiology of the meniscus: a comparison between the human and dog. Front. Vet. Sci. 5, 73. doi:10.3389/fvets.2018.00073
Kurz, B., Lange, T., Voelker, M., Hart, M. L., and Rolauffs, B. (2023). Articular Cartilage—from basic science structural imaging to non-invasive clinical quantitative molecular functional information for AI classification and prediction. Int. J. Mol. Sci. 24 (19), 14974. doi:10.3390/ijms241914974
Kwok, J., Onuma, H., Olmer, M., Lotz, M. K., Grogan, S. P., and D'Lima, D. D. (2016). Histopathological analyses of murine menisci: implications for joint aging and osteoarthritis. Osteoarthr. Cartil. 24 (4), 709–718. doi:10.1016/j.joca.2015.11.006
Lai, Y., Fan, J., Li, P., You, X., Pan, H., Zhou, Z., et al. (2025). Recent advances in 3D bioprinting for cartilage and osteochondral regeneration. Int. J. Bioprinting 11 (3), 154–184. doi:10.36922/IJB025120098
Lana, J. F., Weglein, A., Sampson, S. E., Vicente, E. F., Huber, S. C., Souza, C. V., et al. (2016). Randomized controlled trial comparing hyaluronic acid, platelet-rich plasma and the combination of both in the treatment of mild and moderate osteoarthritis of the knee. J. Stem Cells Regen. Med. 12 (2), 69–78. doi:10.46582/jsrm.1202011
Langhans, M. T., Lamba, A., Saris, D. B. F., Smith, P., and Krych, A. J. (2023). Meniscal extrusion: diagnosis, etiology, and treatment options. Curr. Rev. Musculoskelet. Med. 16 (7), 316–327. doi:10.1007/s12178-023-09840-4
Lee, L. S., Chan, P. K., Wen, C., Fung, W. C., Cheung, A., Chan, V. W. K., et al. (2022). Artificial intelligence in diagnosis of knee osteoarthritis and prediction of arthroplasty outcomes: a review. Arthroplasty 4 (1), 16. doi:10.1186/s42836-022-00118-7
Leifer, V. P., Katz, J. N., and Losina, E. (2022). The burden of OA-health services and economics. Osteoarthr. Cartil. 30 (1), 10–16. doi:10.1016/j.joca.2021.05.007
Lepetsos, P., and Papavassiliou, A. G. (2016). ROS/oxidative stress signaling in osteoarthritis. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1862 (4), 576–591. doi:10.1016/j.bbadis.2016.01.003
Li, R., and Sun, K. (2024). Regulation of chondrocyte apoptosis in osteoarthritis by endoplasmic reticulum stress. Cell Stress Chaperones 29 (6), 750–763. doi:10.1016/j.cstres.2024.11.001
Li, G., Yin, J., Gao, J., Cheng, T. S., Pavlos, N. J., Zhang, C., et al. (2013). Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res. Ther. 15 (6), 223. doi:10.1186/ar4405
Li, T., Zhang, H., Chan, P. K., Fung, W. C., Fu, H., and Chiu, K. Y. (2022). Risk factors associated with surgical site infections following joint replacement surgery: a narrative review. Arthroplasty 4 (1), 11. doi:10.1186/s42836-022-00113-y
Li, H., Jiang, X., Xiao, Y., Zhang, Y., Zhang, W., Doherty, M., et al. (2023a). Combining single-cell RNA sequencing and population-based studies reveals hand osteoarthritis-associated chondrocyte subpopulations and pathways. Bone Res. 11 (1), 58. doi:10.1038/s41413-023-00292-7
Li, S., Cao, P., Chen, T., and Ding, C. (2023b). Latest insights in disease-modifying osteoarthritis drugs development. Ther. Adv. Musculoskelet. Dis. 15, 1759720x231169839. doi:10.1177/1759720X231169839
Li, J., Gui, T., Yao, L., Guo, H., Lin, Y. L., Lu, J., et al. (2024). Synovium and infrapatellar fat pad share common mesenchymal progenitors and undergo coordinated changes in osteoarthritis. J. Bone Min. Res. 39 (2), 161–176. doi:10.1093/jbmr/zjad009
Li, Y., Liu, H., Liu, Y., Wang, S., Yin, C., and Huo, F. (2026). Electronic cloud-regulation strategy enabling rapid and sensitive near-infrared detection of ONOO- for dynamic monitoring of ferroptosis-mediated drug-induced liver injury. Nano Today 66, 102900. doi:10.1016/j.nantod.2025.102900
Liang, J., Liu, P., Yang, X., Liu, L., Zhang, Y., Wang, Q., et al. (2023). Biomaterial-based scaffolds in promotion of cartilage regeneration: recent advances and emerging applications. J. Orthop. Transl. 41, 54–62. doi:10.1016/j.jot.2023.08.006
Lin, C., Wan, Y., Xu, Y., Zou, Q., and Li, X. (2025). Molecular features and diagnostic modeling of synovium- and IPFP-derived OA macrophages in the inflammatory microenvironment via scRNA-seq and machine learning. J. Orthop. Surg. Res. 20 (1), 382. doi:10.1186/s13018-025-05793-1
Liu, T. P., Ha, P., Xiao, C. Y., Kim, S. Y., Jensen, A. R., Easley, J., Yao, Q., et al. (2022). Updates on mesenchymal stem cell therapies for articular cartilage regeneration in large animal models. Front. Cell Dev. Biol. 10, 2022. doi:10.3389/fcell.2022.982199
Loeser, R. F. (2009). Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 17 (8), 971–979. doi:10.1016/j.joca.2009.03.002
Loeser, R. F. (2017). The role of aging in the development of osteoarthritis. Trans. Am. Clin. Climatol. Assoc. 128, 44–54.
Lu, H., Zhuang, Z., Wang, G., Zhang, M., Yang, C., and Wang, D. (2025a). Association between lean body mass and osteoarthritis: a cross-sectional study from the NHANES 2007–2018. Sci. Rep. 15 (1), 14726. doi:10.1038/s41598-025-98795-2
Lu, J. S., Song, C. Y., Cheng, W. J., and Wang, K. Y. (2025b). Mechanisms and challenges of mesenchymal stem cells in the treatment of knee osteoarthritis. World J. Stem Cells 17 (4), 102923. doi:10.4252/wjsc.v17.i4.102923
Ma, W., Chen, H., Yuan, Q., Chen, X., and Li, H. (2025a). Global, regional, and national epidemiology of osteoarthritis in working-age individuals: insights from the global burden of disease study 1990–2021. Sci. Rep. 15 (1), 7907. doi:10.1038/s41598-025-91783-6
Ma, X., Feng, L., Lyu, Y., Hua, X., Sugita, N., and Shu, L. (2025b). Computational dynamic analysis of medial meniscus with redial tear, repair, and meniscectomy. Ann. Biomed. Eng. 53 (10), 2524–2535. doi:10.1007/s10439-025-03803-1
Machado, G. C., Maher, C. G., Ferreira, P. H., Pinheiro, M. B., Lin, C. W. C., Day, R. O., et al. (2015). Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ 350, h1225. doi:10.1136/bmj.h1225
Macri, E. M., Selles, R. W., Stefanik, J. J., and Reijman, M. (2023). OARSI year in review 2023: rehabilitation and outcomes. Osteoarthr. Cartil. 31 (12), 1534–1547. doi:10.1016/j.joca.2023.08.011
Madry, H. (2022). Surgical therapy in osteoarthritis. Osteoarthr. Cartil. 30 (8), 1019–1034. doi:10.1016/j.joca.2022.01.012
Makris, E. A., Hadidi, P., and Athanasiou, K. A. (2011). The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32 (30), 7411–7431. doi:10.1016/j.biomaterials.2011.06.037
Marriott, K. A., and Birmingham, T. B. (2023). Fundamentals of osteoarthritis. Rehabilitation: exercise, diet, biomechanics, and physical therapist-delivered interventions. Osteoarthr. Cartil. 31 (10), 1312–1326. doi:10.1016/j.joca.2023.06.011
Maruotti, N., Corrado, A., and Cantatore, F. P. (2017). Osteoblast role in osteoarthritis pathogenesis. J. Cell Physiol. 232 (11), 2957–2963. doi:10.1002/jcp.25969
Mazzei, D. R., Whittaker, J. L., Kania-Richmond, A., Faris, P., Wasylak, T., Robert, J., et al. (2022). Do people with knee osteoarthritis use guideline-consistent treatments after an orthopaedic surgeon recommends nonsurgical care? A cross-sectional survey with long-term follow-up. Osteoarthr. Cartil. Open 4 (2), 100256. doi:10.1016/j.ocarto.2022.100256
McAlindon, T. E., LaValley, M. P., Harvey, W. F., Price, L. L., Driban, J. B., Zhang, M., et al. (2017). Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. Jama 317 (19), 1967–1975. doi:10.1001/jama.2017.5283
McGonagle, D., Baboolal, T. G., and Jones, E. (2017). Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Rev. Rheumatol. 13 (12), 719–730. doi:10.1038/nrrheum.2017.182
Medical Advisory Secretariat (2005). Arthroscopic lavage and debridement for osteoarthritis of the knee: an evidence-based analysis. Ont. Health Technol. Assess. Ser. 5 (12), 1–37.
Melrose, J. (2019). The importance of the knee joint meniscal fibrocartilages as stabilizing weight bearing structures providing global protection to human knee-joint tissues. Cells 8 (4), 324. doi:10.3390/cells8040324
Messier, S. P., Resnik, A. E., Beavers, D. P., Mihalko, S. L., Miller, G. D., Nicklas, B. J., et al. (2018). Intentional weight loss in overweight and Obese patients with knee osteoarthritis: is more better? Arthritis Care Res. Hob. 70 (11), 1569–1575. doi:10.1002/acr.23608
Miao, Z., Li, S., Luo, Y., Li, S., Chu, Z., Zheng, W., et al. (2025). Trends, inequalities and time-series based prediction of knee osteoarthritis attributed to high body-mass-index: findings from global burden of disease 2021. J. Orthop. Transl. 52, 209–219. doi:10.1016/j.jot.2025.03.022
Migliorini, F., Schäfer, L., Bell, A., Weber, C. D., Vecchio, G., and Maffulli, N. (2023). Meniscectomy is associated with a higher rate of osteoarthritis compared to meniscal repair following acute tears: a meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 31 (12), 5485–5495. doi:10.1007/s00167-023-07600-y
Min, B. W., Bae, K. C., Cho, C. H., Son, E. S., Lee, K. J., and Lee, S. W. (2020). Highly cross-linked polyethylene in cementless total hip arthroplasty in patients with previous acetabular fractures: a minimum 5-Year Follow-Up study. Indian J. Orthop. 54 (Suppl. 2), 239–245. doi:10.1007/s43465-020-00137-z
Minas, T., Gomoll, A. H., Solhpour, S., Rosenberger, R., Probst, C., and Bryant, T. (2010). Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin. Orthop. Relat. Research® 468 (1), 147–157. doi:10.1007/s11999-009-0998-0
Mithoefer, K., Saris, D. B. F., Farr, J., Kon, E., Zaslav, K., Cole, B. J., et al. (2011). Guidelines for the design and conduct of clinical studies in knee articular cartilage repair:international cartilage repair society recommendations based on current scientific evidence and standards of clinical care. Cartilage 2 (2), 100–121. doi:10.1177/1947603510392913
Mittal, A., Meshram, P., Kim, W. H., and Kim, T. K. (2020). Unicompartmental knee arthroplasty, an enigma, and the ten enigmas of medial UKA. J. Orthop. Traumatol. 21 (1), 15. doi:10.1186/s10195-020-00551-x
Mobasheri, A., Saarakkala, S., Finnilä, M., Karsdal, M. A., Bay-Jensen, A. C., and van Spil, W. E. (2019). Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 8, F1000 Faculty Rev-2091. doi:10.12688/f1000research.20575.1
Mohsen, F., Ali, H., El Hajj, N., and Shah, Z. (2022). Artificial intelligence-based methods for fusion of electronic health records and imaging data. Sci. Rep. 12 (1), 17981. doi:10.1038/s41598-022-22514-4
Morgan, M., Nazemian, V., Harrington, K., and Ivanusic, J. J. (2022). Mini review: the role of sensory innervation to subchondral bone in osteoarthritis pain. Front. Endocrinol. (Lausanne) 13, 1047943. doi:10.3389/fendo.2022.1047943
Moseley, J. B., O'Malley, K., Petersen, N. J., Menke, T. J., Brody, B. A., Kuykendall, D. H., et al. (2002). A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 347 (2), 81–88. doi:10.1056/NEJMoa013259
Moseng, T., Vliet Vlieland, T. P. M., Battista, S., Beckwée, D., Boyadzhieva, V., Conaghan, P. G., et al. (2024). EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 83 (6), 730–740. doi:10.1136/ard-2023-225041
Murray, R., Winkler, P. W., Shaikh, H. S., and Musahl, V. (2021). High tibial osteotomy for varus deformity of the knee. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 5 (7), e21.00141. doi:10.5435/JAAOSGlobal-D-21-00141
Musale, V., Wasserman, D. H., and Kang, L. (2023). Extracellular matrix remodelling in obesity and metabolic disorders. Life Metab. 2 (4), load021. doi:10.1093/lifemeta/load021
Mustamsir, E., Aji, A. P., Adiwangsa, A. A., and Akmalizzan, A. A. (2025). Clinical outcomes and long-term efficacy of high tibial osteotomy in treating knee instability: an updated systematic review. Sicot J. 11, 6. doi:10.1051/sicotj/2024061
Muthu, S., Korpershoek, J. V., Novais, E. J., Tawy, G. F., Hollander, A. P., and Martin, I. (2023). Failure of cartilage regeneration: emerging hypotheses and related therapeutic strategies. Nat. Rev. Rheumatol. 19 (7), 403–416. doi:10.1038/s41584-023-00979-5
Najm, A., Alunno, A., Gwinnutt, J. M., Weill, C., and Berenbaum, F. (2021). Efficacy of intra-articular corticosteroid injections in knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Jt. Bone Spine 88 (4), 105198. doi:10.1016/j.jbspin.2021.105198
Nedunchezhiyan, U., Varughese, I., Sun, A. R., Wu, X., Crawford, R., and Prasadam, I. (2022). Obesity, inflammation, and immune system in osteoarthritis. Front. Immunol. 13, 907750. doi:10.3389/fimmu.2022.907750
Ni, Z., Zhou, S., Li, S., Kuang, L., Chen, H., Luo, X., et al. (2020). Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 8, 25. doi:10.1038/s41413-020-0100-9
Ni, X., Hu, L., Zhang, X., Wang, Z., Yan, C., Peyrodie, L., et al. (2024). Physical therapy options for knee osteoarthritis: a review. Med. Baltim. 103 (30), e38415. doi:10.1097/MD.0000000000038415
Ning, K., Wang, Z., and Zhang, X. A. (2022). Exercise-induced modulation of myokine irisin in bone and cartilage tissue-positive effects on osteoarthritis: a narrative review. Front. Aging Neurosci. 14, 934406. doi:10.3389/fnagi.2022.934406
Noh, J. Y., Yang, Y., and Jung, H. (2020). Molecular mechanisms and emerging therapeutics for osteoporosis. Int. J. Mol. Sci. 21 (20), 7623. doi:10.3390/ijms21207623
Nouri, F., Babaee, M., Peydayesh, P., Esmaily, H., and Raeissadat, S. A. (2022). Comparison between the effects of ultrasound guided intra-articular injections of platelet-rich plasma (PRP), high molecular weight hyaluronic acid, and their combination in hip osteoarthritis: a randomized clinical trial. BMC Musculoskelet. Disord. 23 (1), 856. doi:10.1186/s12891-022-05787-8
Nyland, J., Huffstutler, A., Faridi, J., Sachdeva, S., Nyland, M., and Caborn, D. (2020). Cruciate ligament healing and injury prevention in the age of regenerative medicine and technostress: homeostasis revisited. Knee Surg. Sports Traumatol. Arthrosc. 28 (3), 777–789. doi:10.1007/s00167-019-05458-7
O’Malley, M. P., Pareek, A., Reardon, P. J., Stuart, M. J., and Krych, A. J. (2016). Distal femoral osteotomy: lateral opening wedge technique. Arthrosc. Tech. 5 (4), e725–e730. doi:10.1016/j.eats.2016.02.037
Ollivier, B., Berger, P., Depuydt, C., and Vandenneucker, H. (2021). Good long-term survival and patient-reported outcomes after high tibial osteotomy for medial compartment osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 29 (11), 3569–3584. doi:10.1007/s00167-020-06262-4
Olson, K. L., and Eccleston, C. (2024). Reconsidering the role of weight loss in treatment for chronic pain: Knee osteoarthritis as an exemplar. J. Pain 25 (12), 104647. doi:10.1016/j.jpain.2024.104647
Ong, C. K., Lirk, P., Tan, C. H., and Seymour, R. A. (2007). An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin. Med. Res. 5 (1), 19–34. doi:10.3121/cmr.2007.698
Ordoñez-Mora, L. T., Morales-Osorio, M. A., and Rosero, I. D. (2022). Effectiveness of interventions based on pain neuroscience education on pain and psychosocial variables for osteoarthritis: a systematic review. Int. J. Environ. Res. Public Health 19 (5), 2559. doi:10.3390/ijerph19052559
Osteoarthritis Group of the Rheumatology and Immunology Physicians Branch of the Chinese Medical Doctor Association (2024). Consensus on clinical drug therapy for knee osteoarthritis in China (2023). Zhonghua Nei Ke Za Zhi 63 (6), 560–578. doi:10.3760/cma.j.cn112138-20240402-00215
Ott, S. M. (2008). Histomorphometric measurements of bone turnover, mineralization, and volume. Clin. J. Am. Soc. Nephrol. 3 (Suppl. 3), S151–S156. doi:10.2215/CJN.04301206
Ou, J., Zhang, J., Alswadeh, M., Zhu, Z., Tang, J., Sang, H., et al. (2025). Advancing osteoarthritis research: the role of AI in clinical, imaging and omics fields. Bone Res. 13 (1), 48. doi:10.1038/s41413-025-00423-2
Ozeki, N., Koga, H., and Sekiya, I. (2022). Degenerative meniscus in knee osteoarthritis: from pathology to treatment. Life (Basel) 12 (4), 603. doi:10.3390/life12040603
Page, P. (2012). Current concepts in muscle stretching for exercise and rehabilitation. Int. J. Sports Phys. Ther. 7 (1), 109–119.
Paget, L. D. A., Reurink, G., de Vos, R. J., Weir, A., Moen, M. H., Bierma-Zeinstra, S. M. A., et al. (2023). Platelet-Rich plasma injections for the treatment of ankle osteoarthritis. Am. J. Sports Med. 51 (10), 2625–2634. doi:10.1177/03635465231182438
Paik, J., Duggan, S. T., and Keam, S. J. (2019). Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs 79 (4), 455–462. doi:10.1007/s40265-019-01083-3
Pan, X. (2025). Editorial: mendelian randomization: an approach for precision medicine and public health. Front. Genet. 16, 1602592. doi:10.3389/fgene.2025.1602592
Panichi, V., Costantini, S., Grasso, M., Arciola, C. R., and Dolzani, P. (2024). Innate immunity and synovitis: key players in osteoarthritis progression. Int. J. Mol. Sci. 25 (22), 12082. doi:10.3390/ijms252212082
Pelletier, J.-P., Martel-Pelletier, J., Rannou, F., and Cooper, C. (2016). Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: evidence from real-life setting trials and surveys. Seminars Arthritis Rheumatism 45 (4, Suppl.), S22–S27. doi:10.1016/j.semarthrit.2015.11.009
Peng, Z., Sun, H., Bunpetch, V., Koh, Y., Wen, Y., Wu, D., et al. (2021a). The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 268, 120555. doi:10.1016/j.biomaterials.2020.120555
Peng, H., Ou, A., Huang, X., Wang, C., Wang, L., Yu, T., et al. (2021b). Osteotomy around the knee: the surgical treatment of osteoarthritis. Orthop. Surg. 13 (5), 1465–1473. doi:10.1111/os.13021
Peters, A. E., Akhtar, R., Comerford, E. J., and Bates, K. T. (2018). The effect of ageing and osteoarthritis on the mechanical properties of cartilage and bone in the human knee joint. Sci. Rep. 8 (1), 5931. doi:10.1038/s41598-018-24258-6
Picca, A., Calvani, R., Coelho-Junior, H. J., Landi, F., Bernabei, R., and Marzetti, E. (2020). Mitochondrial dysfunction, oxidative stress, and neuroinflammation: intertwined roads to neurodegeneration. Antioxidants (Basel) 9 (8), 647. doi:10.3390/antiox9080647
Plavoukou, T., Iosifidis, M., Papagiannis, G., Stasinopoulos, D., and Georgoudis, G. (2025). The effectiveness of telerehabilitation in managing pain, strength, and balance in adult patients with knee osteoarthritis: systematic review. JMIR Rehabil. Assist. Technol. 12, e72466. doi:10.2196/72466
Pollock, M., Somerville, L., Firth, A., and Lanting, B. (2016). Outpatient total hip arthroplasty, total knee arthroplasty, and unicompartmental knee arthroplasty: a systematic review of the literature. JBJS Rev. 4 (12), e4. doi:10.2106/JBJS.RVW.16.00002
Rannou, F., and Poiraudeau, S. (2010). Non-pharmacological approaches for the treatment of osteoarthritis. Best. Pract. Res. Clin. Rheumatol. 24 (1), 93–106. doi:10.1016/j.berh.2009.08.013
Rim, Y. A., Nam, Y., and Ju, J. H. (2020). The role of chondrocyte hypertrophy and senescence in osteoarthritis initiation and progression. Int. J. Mol. Sci. 21 (7), 2358. doi:10.3390/ijms21072358
Rodríguez-Merchán, E. C. (2022). Intraarticular injections of mesenchymal stem cells in knee osteoarthritis: a review of their current molecular mechanisms of action and their efficacy. Int. J. Mol. Sci. 23 (23), 14953. doi:10.3390/ijms232314953
Rodríguez-Merchán, E. C., and Gómez-Cardero, P. (2018). Unicompartmental knee arthroplasty: current indications, technical issues and results. EFORT Open Rev. 3 (6), 363–373. doi:10.1302/2058-5241.3.170048
Rodríguez-Merchán, E. C., and De la Corte-García, H. (2012). “Alignment osteotomies,” in Articular cartilage defects of the knee: diagnosis and treatment. Editor E. C. Rodrìguez-Merchán (Milan: Milano: Springer), 79–85.
Roelofs, A. J., Kania, K., Rafipay, A. J., Sambale, M., Kuwahara, S. T., Collins, F. L., et al. (2020). Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis. Ann. Rheum. Dis. 79 (12), 1625–1634. doi:10.1136/annrheumdis-2020-218350
Roth, S. H., and Fuller, P. (2011). Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis. J. Pain Res. 4, 159–167. doi:10.2147/JPR.S20965
Ryan, D. H., and Yockey, S. R. (2017). Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr. Obes. Rep. 6 (2), 187–194. doi:10.1007/s13679-017-0262-y
Sanchez-Lopez, E., Coras, R., Torres, A., Lane, N. E., and Guma, M. (2022). Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 18 (5), 258–275. doi:10.1038/s41584-022-00749-9
Saxer, F., Hollinger, A., Bjurström, M. F., Conaghan, P. G., Neogi, T., Schieker, M., et al. (2024). Pain-phenotyping in osteoarthritis: current concepts, evidence, and considerations towards a comprehensive framework for assessment and treatment. Osteoarthr. Cartil. Open 6 (1), 100433. doi:10.1016/j.ocarto.2023.100433
Scanzello, C. R., and Goldring, S. R. (2012). The role of synovitis in osteoarthritis pathogenesis. Bone 51 (2), 249–257. doi:10.1016/j.bone.2012.02.012
Schäfer, N., and Grässel, S. (2022). Targeted therapy for osteoarthritis: progress and pitfalls. Nat. Med. 28 (12), 2473–2475. doi:10.1038/s41591-022-02057-x
Schnitzer, T. J., Easton, R., Pang, S., Levinson, D. J., Pixton, G., Viktrup, L., et al. (2019). Effect of tanezumab on joint pain, physical function, and patient global assessment of osteoarthritis among patients with osteoarthritis of the hip or knee: a randomized clinical trial. Jama 322 (1), 37–48. doi:10.1001/jama.2019.8044
Schwitzguébel, A., Corzo, A. H., Theodoridou, E., Bogoev, M., Grange, M., Boudabbous, S., et al. (2025). Platelet-rich plasma treatment for large joint osteoarthritis: retrospective study highlighting a possible treatment protocol with long-lasting stimulation of the joint with an adequate dose of platelets. BMC Musculoskelet. Disord. 26 (1), 412. doi:10.1186/s12891-025-08663-3
Seref-Ferlengez, Z., Kennedy, O. D., and Schaffler, M. B. (2015). Bone microdamage, remodeling and bone fragility: how much damage is too much damage? Bonekey Rep. 4, 644. doi:10.1038/bonekey.2015.11
Serfaty, A. (2020). Hip arthroplasty: current concepts and potential complications. Radiol. Bras. 53 (1). Vii. doi:10.1590/0100-3984.2020.53.1e2
Shanto, P. C., Park, S., Fahad, M. A. A., Park, M., and Lee, B. T. (2025). 3D bio-printed proteinaceous bioactive scaffold loaded with dual growth factor enhanced chondrogenesis and in situ cartilage regeneration. Bioact. Mater. 46, 365–385. doi:10.1016/j.bioactmat.2024.12.021
Shawl, M., Geetha, T., Burnett, D., and Babu, J. R. (2024). Omega-3 supplementation and its effects on osteoarthritis. Nutrients 16 (11), 1650. doi:10.3390/nu16111650
Shock, T., Badang, L., Ferguson, B., and Martinez-Guryn, K. (2021). The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 95, 108631. doi:10.1016/j.jnutbio.2021.108631
Shon, W. Y., Park, B. Y., R, R. N., Park, P. S., Im, J. T., and Yun, H. H. (2019). Total hip arthroplasty: past, present, and future. What has been achieved? Hip Pelvis 31 (4), 179–189. doi:10.5371/hp.2019.31.4.179
Shorter, E., Sannicandro, A. J., Poulet, B., and Goljanek-Whysall, K. (2019). Skeletal muscle wasting and its relationship with osteoarthritis: a mini-review of mechanisms and current interventions. Curr. Rheumatol. Rep. 21 (8), 40. doi:10.1007/s11926-019-0839-4
Shtroblia, V., Petakh, P., Kamyshna, I., Halabitska, I., and Kamyshnyi, O. (2025). Recent advances in the management of knee osteoarthritis: a narrative review. Front. Med. (Lausanne) 12, 1523027. doi:10.3389/fmed.2025.1523027
Skou, S. T., and Roos, E. M. (2019). Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice. Clin. Exp. Rheumatol. 37 (Suppl. 120), 112–117.
Slynarski, K., and Lane, J. G. (2025). “Orthobiologics in meniscus pathology,” in Regenerative medicine in sports and orthopaedics: a new approach. Editor A. Gobbi, N. Nakamura, J. G. Lane, and I. Dallo (Cham: Springer Nature Switzerland), 243–250. doi:10.1007/978-3-031-84693-9_18
Sohail, R., Mathew, M., Patel, K. K., Reddy, S. A., Haider, Z., Naria, M., et al. (2023). Effects of non-steroidal anti-inflammatory drugs (NSAIDs) and gastroprotective NSAIDs on the gastrointestinal tract: a narrative review. Cureus 15 (4), e37080. doi:10.7759/cureus.37080
Sophia Fox, A. J., Bedi, A., and Rodeo, S. A. (2009). The basic science of articular cartilage: structure, composition, and function. Sports Health 1 (6), 461–468. doi:10.1177/1941738109350438
Stephenson, S. D., Kocan, J. W., Vinod, A. V., Kluczynski, M. A., and Bisson, L. J. (2021). A comprehensive summary of systematic reviews on sports injury prevention strategies. Orthop. J. Sports Med. 9 (10), 23259671211035776. doi:10.1177/23259671211035776
Sun, K., Hou, L., Guo, Z., Wang, G., Guo, J., Xu, J., et al. (2023). JNK-JUN-NCOA4 axis contributes to chondrocyte ferroptosis and aggravates osteoarthritis via ferritinophagy. Free Radic. Biol. Med. 200, 87–101. doi:10.1016/j.freeradbiomed.2023.03.008
Sun, Z., Yan, M., Wang, J., Zhang, H., Ji, X., Xiao, Y., et al. (2024). Single-cell RNA sequencing reveals different chondrocyte states in femoral cartilage between osteoarthritis and healthy individuals. Front. Immunol. 2024, 15. doi:10.3389/fimmu.2024.1407679
Takada, K., Hirose, J., Senba, K., Yamabe, S., Oike, Y., Gotoh, T., et al. (2011). Enhanced apoptotic and reduced protective response in chondrocytes following endoplasmic reticulum stress in osteoarthritic cartilage. Int. J. Exp. Pathol. 92 (4), 232–242. doi:10.1111/j.1365-2613.2010.00758.x
Takase, K., McCulloch, P. C., Yik, J. H. N., and Haudenschild, D. R. (2025). Clinical and molecular landscape of post-traumatic osteoarthritis. Connect. Tissue Res. 66, 1–7. doi:10.1080/03008207.2025.2490797
Tamer, T. M. (2013). Hyaluronan and synovial joint: function, distribution and healing. Interdiscip. Toxicol. 6 (3), 111–125. doi:10.2478/intox-2013-0019
Tang, S. A., Yao, L., Ruan, J., Kang, J., Cao, Y., Nie, X., et al. (2024). Single-cell atlas of human infrapatellar fat pad and synovium implicates APOE signaling in osteoarthritis pathology. Sci. Transl. Med. 16 (731), eadf4590. doi:10.1126/scitranslmed.adf4590
Terauchi, M., Hatayama, K., and Saito, K. (2025). Step-off between the lateral femoral condyle and the lateral tibial Plateau: Association with degenerative lateral meniscal tears and lateral osteoarthritis of the knee. J. Knee Surg. doi:10.1055/a-2693-0944
Terracciano, C., Celi, M., Lecce, D., Baldi, J., Rastelli, E., Lena, E., et al. (2013). Differential features of muscle fiber atrophy in osteoporosis and osteoarthritis. Osteoporos. Int. 24 (3), 1095–1100. doi:10.1007/s00198-012-1990-1
Testa, G., Giardina, S. M. C., Culmone, A., Vescio, A., Turchetta, M., Cannavò, S., et al. (2021). Intra-articular injections in knee osteoarthritis: a review of literature. J. Funct. Morphol. Kinesiol 6 (1), 15. doi:10.3390/jfmk6010015
Tian, Y., Li, J., Cai, X., Huang, Y., Wang, X., Liu, Q., et al. (2025). Molecular drivers of epithelial-mesenchymal transition (EMT) in glioblastoma and impact on therapy resistance. Pathology - Res. Pract. 272, 156111. doi:10.1016/j.prp.2025.156111
Trattnig, S., Winalski, C. S., Marlovits, S., Jurvelin, J. S., Welsch, G. H., and Potter, H. G. (2011). Magnetic resonance imaging of cartilage repair:a review. CARTILAGE 2 (1), 5–26. doi:10.1177/1947603509360209
Tschopp, M., Pfirrmann, C. W. A., Fucentese, S. F., Brunner, F., Catanzaro, S., Kühne, N., et al. (2023). A randomized trial of intra-articular injection therapy for knee osteoarthritis. Invest Radiol. 58 (5), 355–362. doi:10.1097/RLI.0000000000000942
Turunen, S., Kalpio, T., Lindahl, C., Shanthinathan, C. J. M., Akhter, T., Concaro, S., et al. (2023). “Chapter 12 - future solutions for osteoarthritis using 3D bioprinting of articular cartilage,” in Handbook of surgical planning and 3D printing. Editor P. Gargiulo (Academic Press), 335–369.
Umlauf, D., Frank, S., Pap, T., and Bertrand, J. (2010). Cartilage biology, pathology, and repair. Cell Mol. Life Sci. 67 (24), 4197–4211. doi:10.1007/s00018-010-0498-0
Valente, G., Grenno, G., Benedetti, M. G., Dal Fabbro, G., Grassi, A., Leardini, A., et al. (2025). Altered motor function during daily activities in patients eligible for high tibial osteotomy is primarily driven by knee varus deformity. Bone Jt. Open 6 (4), 454–462. doi:10.1302/2633-1462.64.BJO-2024-0189.R1
Veillette, C. J. H., and Jurisica, I. (2015). “Precision medicine for osteoarthritis, in osteoarthritis: pathogenesis, diagnosis,” in Available treatments, drug safety, regenerative and precision medicine. Editors M. Kapoor, and N. N. Mahomed (Cham: Springer International Publishing), 257–270.
Verhagen, A. P., Ferreira, M., Reijneveld-van de Vendel, E. A. E., Teirlinck, C. H., Runhaar, J., van Middelkoop, M., et al. (2019). Do we need another trial on exercise in patients with knee osteoarthritis? no new trials on exercise in knee OA. Osteoarthr. Cartil. 27 (9), 1266–1269. doi:10.1016/j.joca.2019.04.020
Viswanathan, V. K., Vaishya, R., Iyengar, K. P., Jain, V. K., and Vaish, A. (2025). Strategies for preventing anterior cruciate ligament injuries in athletes: insights from a scoping review. J. Orthop. 67, 101–110. doi:10.1016/j.jor.2025.01.001
Waheed, A., and Rai, M. F. (2024). Osteoarthritis year in review 2023: genetics, genomics, and epigenetics. Osteoarthr. Cartil. 32 (2), 128–137. doi:10.1016/j.joca.2023.11.006
Wan, Y., Shen, K., Yu, H., and Fan, W. (2023). Baicalein limits osteoarthritis development by inhibiting chondrocyte ferroptosis. Free Radic. Biol. Med. 196, 108–120. doi:10.1016/j.freeradbiomed.2023.01.006
Wandel, S., Jüni, P., Tendal, B., Nüesch, E., Villiger, P. M., Welton, N. J., et al. (2010). Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 341, c4675. doi:10.1136/bmj.c4675
Wang, T., Liang, Y., Li, H., Li, H., He, Q., Xue, Y., et al. (2016). Single nucleotide polymorphisms and osteoarthritis: an overview and a meta-analysis. Med. Baltim. 95 (7), e2811. doi:10.1097/MD.0000000000002811
Wang, F. S., Kuo, C. W., Ko, J. Y., Chen, Y. S., Wang, S. Y., Ke, H. J., et al. (2020a). Irisin mitigates oxidative stress, chondrocyte dysfunction and osteoarthritis development through regulating mitochondrial integrity and autophagy. Antioxidants (Basel) 9 (9), 810. doi:10.3390/antiox9090810
Wang, L. J., Zeng, N., Yan, Z. P., Li, J. T., and Ni, G. X. (2020b). Post-traumatic osteoarthritis following ACL injury. Arthritis Res. Ther. 22 (1), 57. doi:10.1186/s13075-020-02156-5
Wang, X., Perry, T. A., Arden, N., Chen, L., Parsons, C. M., Cooper, C., et al. (2020c). Occupational risk in knee osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res. Hob. 72 (9), 1213–1223. doi:10.1002/acr.24333
Wang, M., Tan, G., Jiang, H., Liu, A., Wu, R., Li, J., et al. (2022). Molecular crosstalk between articular cartilage, meniscus, synovium, and subchondral bone in osteoarthritis. Bone Jt. Res. 11 (12), 862–872. doi:10.1302/2046-3758.1112.BJR-2022-0215.R1
Wang, M. G., Seale, P., and Furman, D. (2024a). The infrapatellar fat pad in inflammaging, knee joint health, and osteoarthritis. Npj Aging 10 (1), 34. doi:10.1038/s41514-024-00159-z
Wang, B., Han, J., Elisseeff, J. H., and Demaria, M. (2024b). The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 25 (12), 958–978. doi:10.1038/s41580-024-00727-x
Wechsler, R., and Rotem, R. (2025). ABS0267 coating of meniscus and cartilage of osteoarthritic rats and rabbits following intraarticular injection of mm-ii, a dispersion of empty large multilamellar liposomes. Ann. Rheumatic Dis. 84, 1740–1741. doi:10.1016/j.ard.2025.06.1204
Wehling, P., Evans, C., Wehling, J., and Maixner, W. (2017). Effectiveness of intra-articular therapies in osteoarthritis: a literature review. Ther. Adv. Musculoskelet. Dis. 9 (8), 183–196. doi:10.1177/1759720X17712695
Welch, T., Mandelbaum, B., and Tom, M. (2016). Autologous chondrocyte implantation: past, present, and future. Sports Med. Arthrosc. Rev. 24 (2), 85–91. doi:10.1097/JSA.0000000000000115
Wessler, S., and Meisner-Kober, N. (2025). On the road: extracellular vesicles in intercellular communication. Cell Commun. Signal 23 (1), 95. doi:10.1186/s12964-024-01999-8
Whittaker, J. L., Losciale, J. M., Juhl, C. B., Thorlund, J. B., Lundberg, M., Truong, L. K., et al. (2022). Risk factors for knee osteoarthritis after traumatic knee injury: a systematic review and meta-analysis of randomised controlled trials and cohort studies for the OPTIKNEE consensus. Br. J. Sports Med. 56 (24), 1406–1421. doi:10.1136/bjsports-2022-105496
Williams, B. S. (2018). “Chapter 51 - nonopioid analgesics: nonsteroidal antiinflammatory drugs, Cyclooxygenase-2 inhibitors, and acetaminophen,” in Essentials of pain medicine. Editor H. T. Benzon Fourth Edition (Elsevier), 457–468.e2.
Wong, S. H., Chiu, K. Y., and Yan, C. H. (2016). Review article: osteophytes. J. Orthop. Surg. (Hong. Kong) 24 (3), 403–410. doi:10.1177/1602400327
Wood, G. C., Bailey-Davis, L., Benotti, P., Cook, A., Dove, J., Mowery, J., et al. (2021). Effects of sustained weight loss on outcomes associated with obesity comorbidities and healthcare resource utilization. PLoS One 16 (11), e0258545. doi:10.1371/journal.pone.0258545
Wu, J., Xu, J., Zhang, M., Gao,, W., and Wu, M. (2025). Chondrocyte mitochondrial quality control: a novel insight into osteoarthritis and cartilage regeneration. Adv Wound Care (New Rochelle). doi:10.1089/wound.2024.0270
Wylde, V., Beswick, A., Bruce, J., Blom, A., Howells, N., and Gooberman-Hill, R. (2018). Chronic pain after total knee arthroplasty. EFORT Open Rev. 3 (8), 461–470. doi:10.1302/2058-5241.3.180004
Xing, C., Wu, Y., Huang, Y., Li, W., Wang, X., and Chen, H. (2025). Self-assembled carrier free oligomeric proanthocyanidin/tetrandrine nanoparticles ameliorate osteoarthritis via anti-inflammatory and anti-ferroptotic pathways. Int. J. Nanomedicine 20, 13165–13181. doi:10.2147/IJN.S531873
Xu, M., Sun, X., Ma, X., Qin, Z., Gao, X., Jin, X., et al. (2025a). Sodium tanshinone IIA sulfonate alleviates osteoarthritis through targeting SIRT1. Chin. Med. 20 (1), 142. doi:10.1186/s13020-025-01166-2
Xu, G., Jin, J., Fu, Z., Wang, G., Lei, X., Xu, J., et al. (2025b). Extracellular vesicle-based drug overview: research landscape, quality control and nonclinical evaluation strategies. Signal Transduct. Target. Ther. 10 (1), 255. doi:10.1038/s41392-025-02312-w
Yang, X.-H., Chen, S. Y., Zhou, Q. F., and Cai, Y. Z. (2025). Exosomes in osteoarthritis: breakthrough innovations and advanced tissue engineering for cartilage regeneration since 2020. Biomedicines 13 (10), 2486. doi:10.3390/biomedicines13102486
Yao, X., Sun, K., Yu, S., Luo, J., Guo, J., Lin, J., et al. (2021). Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Transl. 27, 33–43. doi:10.1016/j.jot.2020.09.006
Yin, B., Xu, J., Lu, J., Ou, C., Zhang, K., Gao, F., et al. (2024). Responsive hydrogel-based drug delivery platform for osteoarthritis treatment. Gels 10 (11), 696. doi:10.3390/gels10110696
Yunus, M. H. M., Nordin, A., and Kamal, H. (2020). Pathophysiological perspective of osteoarthritis. Med. Kaunas. 56 (11), 614. doi:10.3390/medicina56110614
Zhai, G. (2021). The role of metabolomics in precision medicine of osteoarthritis: how far are we? Osteoarthr. Cartil. Open 3 (4), 100170. doi:10.1016/j.ocarto.2021.100170
Zhang, L., Fu, T., Zhang, Q., Yin, R., Zhu, L., He, Y., et al. (2018). Effects of psychological interventions for patients with osteoarthritis: a systematic review and meta-analysis. Psychol. Health Med. 23 (1), 1–17. doi:10.1080/13548506.2017.1282160
Zhang, L., Liu, G., Han, B., Wang, Z., Yan, Y., Ma, J., et al. (2020). Knee joint biomechanics in physiological conditions and how pathologies can affect it: a systematic review. Appl. Bionics Biomech. 2020, 7451683. doi:10.1155/2020/7451683
Zhang, W., Zha, K., Hu, W., Xiong, Y., Knoedler, S., Obed, D., et al. (2023). Multifunctional hydrogels: advanced therapeutic tools for osteochondral regeneration. Biomaterials Res. 27 (1), 76. doi:10.1186/s40824-023-00411-9
Zhang, H., Zhao, Z., Zhou, F., and Liu, X. (2025a). Evaluation of radiographic knee OA progression after arthroscopic meniscectomy compared with IACI for degenerative meniscus tear. Sci. Rep. 15 (1), 11538. doi:10.1038/s41598-025-95649-9
Zhang, K., Song, L., Liu, J., Tan, J., Mei, Z., Xu, Q., et al. (2025b). Systemic toxicity and transient locked-in-like syndrome following intra-articular ropivacaine injection. Br. J. Anaesth. 135 (5), 1341–1343. doi:10.1016/j.bja.2025.07.071
Zhao, Z., Wu, X., Wang, D., Luo, W., Ma, J. X., and Ma, X. L. (2025). Integrated multiomics analysis identifies CDO1 as a novel therapeutic target for osteoarthritis. Endocr. Metab. Immune Disord. Drug Targets 25. doi:10.2174/0118715303387442250918111006
Zhou, H., Zhang, Z., Mu, Y., Yao, H., Zhang, Y., and Wang, D. A. (2024). Harnessing nanomedicine for cartilage repair: design considerations and recent advances in biomaterials. ACS Nano 18 (16), 10667–10687. doi:10.1021/acsnano.4c00780
Zhu, S., Zhu, J., Zhen, G., Hu, Y., An, S., Li, Y., et al. (2019). Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest 129 (3), 1076–1093. doi:10.1172/JCI121561
Zhu, X., Chan, Y. T., Yung, P. S. H., Tuan, R. S., and Jiang, Y. (2020). Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front. Cell Dev. Biol. 8, 607764. doi:10.3389/fcell.2020.607764
Zou, Z., Li, H., Yu, K., Ma, K., Wang, Q., Tang, J., et al. (2023). The potential role of synovial cells in the progression and treatment of osteoarthritis. Explor. (Beijing) 3 (5), 20220132. doi:10.1002/EXP.20220132
Keywords: osteoarthritis, chondrocyte ferroptosis, synovial inflammation, regenerative therapy, meniscus degeneration
Citation: Qiu L, Alhaskawi A and Moqbel SAA (2026) Osteoarthritis: multitissue pathology, molecular mechanisms, clinical management, and emerging precision and regenerative therapies. Front. Pharmacol. 16:1697192. doi: 10.3389/fphar.2025.1697192
Received: 01 September 2025; Accepted: 30 November 2025;
Published: 05 January 2026.
Edited by:
Silvia Barbon, University of Padua, ItalyReviewed by:
Girish Pattappa, University of Wurzburg, GermanyMalay K. Das, Dibrugarh University, India
Tudor Sorin Pop, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, Romania
Copyright © 2026 Qiu, Alhaskawi and Moqbel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Safwat Adel Abdo Moqbel, MjMyMjIwN0B6anUuZWR1LmNu
†These authors have contributed equally to this work
 Lina Qiu1†
Lina Qiu1† Ahmad Alhaskawi
Ahmad Alhaskawi




