- 1College of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, Jilin, China
- 2Department of Proctology, Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, Jilin, China
- 3Department of Liver, Spleen and Gastroenterology, Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, Jilin, China
Common kidney diseases include acute kidney injury, diabetic kidney disease, kidney cancer, and other related conditions. Ginsenosides, the principal bioactive constituents of ginseng, have been widely reported as therapeutic agents against these disorders. However, recent advances regarding their efficacy in kidney diseases have not been comprehensively synthesized. This review addresses this gap by summarizing current findings on the mechanisms and therapeutic targets of ginsenosides. Literature from PubMed, Web of Science, and other databases was systematically retrieved using keywords such as ginsenosides, acute kidney injury, diabetic nephropathy, renal cell carcinoma, lupus nephritis, and aging-related kidney injury. Evidence from cell-based and animal studies demonstrates that ginsenoside compound K, Rg1, Rg3, Rh2, Rb1, Rb3, Rg2, and Rg5 are the most frequently reported for kidney protection. Mechanistically, ginsenosides modulate multiple signalling networks, including NF-κB, PI3K/AKT, MAPK, TGF-β/Smads, PPAR, SIRT1, NLRP3, and Nrf2, to mitigate inflammation, oxidative stress, apoptosis, epithelial-mesenchymal transition, pyroptosis, autophagy, and endoplasmic reticulum stress. Taken together, these findings provide valuable insights into the therapeutic potential of ginsenosides and underscore their promise as candidates for the prevention and treatment of kidney diseases.
1 Introduction
Kidneys play a central role in maintaining homeostasis by filtering blood to remove metabolic waste and by regulating fluid and electrolyte balance (Robson, 2014). Major kidney diseases include acute kidney injury, diabetic kidney disease, and kidney cancer. Acute kidney injury can be triggered by infection, drug toxicity, heart failure, or shock, is characterized by reduced urine output, electrolyte disturbances, and azotemia (Kellum et al., 2021), which leads to death in 25% of the patients, according to a cohort study (Sohaney et al., 2022). The burden of diabetic kidney disease is primarily driven by type 2 diabetes mellitus and hypertension, with prevalence continuing to rise in aging populations (Li Z. et al., 2025). In 2023, chronic kidney disease occurs in 788 million people aged 20 and older with a global age-standardized prevalence of 14.2%, which is the ninth leading cause of death globally and the 12th leading cause of disability-adjusted life years (Collaborators, 2025). Separately, kidney cancer accounts for an estimated 400,000 new cases and 175,000 deaths annually worldwide (Cirillo et al., 2024). Current therapeutic strategies for these diseases include the elimination of causative factors (e.g., drug withdrawal, antihypertensive or antidiabetic therapy), anti-infective treatment, the correction of electrolyte imbalances, and renoprotective agents such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and chemotherapy or immunotherapy, including immune checkpoint inhibitors (Chen et al., 2019; Chowdhury and Drake, 2020; Tamargo et al., 2024). Given the high prevalence and poor prognosis with these conditions, the development of effective interventions for kidney diseases are both urgent and essential (Padala et al., 2020; Kung and Chou, 2023; Jadoul et al., 2024). Consequently, drug discovery and therapeutic development remain a key research frontier worldwide.
Panax ginseng C.A. Mey. (ginseng) is a widely recognized medicinal herb used in the prevention and treatment of multiple conditions, including cardiovascular (Wan et al., 2023; Chang et al., 2025; Yuan et al., 2025), neurodegenerative (Wang et al., 2025; Zhou et al., 2025), and metabolic diseases (Ding et al., 2023; Liu Q. et al., 2024), as well as kidney diseases (Zhang H. et al., 2025; Zhang Y. X. et al., 2025). Ginsenosides, the principal bioactive components of ginseng, which are triterpene saponins structurally, classified as three types of saponins: dammarane-, oleanane-, or octillol-types based on the structure of their aglycone (Li et al., 2022; Ding et al., 2024). The dammarane-type group, the most abundant group, is characterized by a 4-ring, steroid-like structure, which is further divided into protopanaxadiol (PPD; e.g., Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2), protopanaxatriol (PPT; e.g., Re, Rf, Rg1, Rg2, Rh1) (Li et al., 2022; Ding et al., 2024). The oleanane-type has a different pentacyclic structure and ginsenoside Ro is the representative example (Li et al., 2022; Ding et al., 2024). The octillol-type is characterized by a tetrahydrofuran ring and less abundant in medicinal herbs (Cao et al., 2020). Different ginsenosides regulate similar pathological steps, such as autophagy, gut microbiota, mitochondrial function to display a wide range of therapeutic effects (Huang et al., 2021a; Chen Z. et al., 2022; Ren et al., 2023), which might be not related with ginsenoside structures. Certainly, ginsenosides have various therapeutic efficacies in diverse conditions, such as myocardial ischemia-reperfusion injury, aging, and diabetes and its related complications through different potential targets, such as SIRT1, splicing factor 2 subunit 2 acetylation, nicotinamide adenine dinucleotide metabolism, G protein-coupled receptor 30, and mitosis A-related kinase 7 (Huang et al., 2021b; Wang et al., 2021; Huang et al., 2024; Guo et al., 2025; Tang et al., 2025; Zhu et al., 2025). The unique function and potential target of ginsenosides might be related the glucopyranosyl group at C-3 or C-6 position, which need to be further investigated (Huang et al., 2021b). For kidney protection, it was first reported in 1990 that ginsenosides stimulate p-aminohippurate secretion to reverse its imbalance in renal cortex against acute renal failure (Liu and Gemba, 1990). Gradually, the protective effects of ginsenosides on kidney ischemic damages, cisplatin-induced acute renal failure and hydrogen peroxide or epidermal growth factor-induced cellular injury were studied (Yokozawa et al., 1998; Yokozawa and Liu, 2000; Han et al., 2002). In recent years, increasing evidence specifically highlights the renoprotective effects of ginsenosides in acute kidney injury (Guo et al., 2022), diabetic kidney disease (Chen Q. et al., 2022), kidney cancer (Zhao et al., 2024), and other renal injuries, such as IgA nephropathy (Wu et al., 2020), lupus nephritis (Li Y. et al., 2025), and hypoxia- or aging-related damage (Ji P. et al., 2024; Nguepi Tsopmejio et al., 2025). While several systematic reviews have summarized their benefits of ginsenosides for diabetic nephropathy (Chen et al., 2023) and nephrotoxicity (Luo et al., 2023), the current understanding of their protective mechanisms against kidney diseases remain fragmented and inconclusive. This review aims to consolidate recent advances by comprehensively summarizing the protective effects, pharmacological mechanisms, and therapeutic targets of ginsenosides in kidney diseases, as illustrated in the accompanying figures and tables. By integrating this evidence, the review provides new insights into the therapeutic potential of ginsenosides against renal disorders.
2 Pharmacological effects and molecular mechanisms of ginsenosides against common kidney diseases
Following oral or intravenous administration, ginsenoside monomers such as Rb1, Rc, Rd, Rh3, Rg1, Rg2, and compound K (CK) are detectable in the plasma humans, rats or mice (Wang et al., 2011; Ma et al., 2015; Jeon et al., 2020). Tissue distribution studies show that ginsenoside Rg1 accumulates most abundantly in the kidney, particularly in the renal pelvis, compared to the liver, lung, or heart (Wei W. et al., 2021). Functionally, ginsenosides have demonstrated efficacy in preventing acute kidney injury induced by cisplatin- or lipopolysaccharide (LPS) (Wu et al., 2021; Guo et al., 2024; Ma et al., 2024; Wang L. et al., 2024), ameliorating diabetic nephropathy and fibrosis (He et al., 2022; Han et al., 2023; Ji et al., 2023; Li K. et al., 2025; Zhang H. et al., 2025), and inhibiting renal carcinoma progression (Hwang et al., 2022; Ma et al., 2023; Zhao et al., 2024) in cellular and animal models. These protective and anti-tumor effects are mediated through key biological pathways, including modulation of inflammatory responses, oxidative stress, apoptosis, and autophagy.
2.1 Cisplatin-induced nephrotoxicity
Acute kidney injury is a syndrome of rapid-onset renal dysfunction within hours, characterized by elevated proteinuria, serum creatinine, and blood urea nitrogen levels (Hoste et al., 2018). Its global incidence is rising, contributing to substantial healthcare costs (Gonsalez et al., 2019) and is commonly associated with reduced renal perfusion, major surgery, or drug exposure. Cisplatin, a platinum-based chemotherapeutic widely used for solid tumors, induces nephrotoxicity in nearly one-third of patients and poses an additional risk of long-term renal impairment (Motwani et al., 2022). Once taken up by renal cells, cisplatin forms intra- and interstrand crosslinks with DNA, RNA, and proteins, thereby disrupting replication and transcription (McSweeney et al., 2021). Furthermore, this damage-induced acute tubular necrosis can decrease tubular drainage, elevate intratubular pressure and lower the glomerular filtration rate, thereby exacerbating renal injury (McSweeney et al., 2021). The pathological mechanisms of cisplatin-induced nephrotoxicity are multifactorial, including oxidative stress, inflammation, endoplasmic reticulum (ER) stress, necrosis, and apoptosis with key therapeutic targets, such as CXCL1-CXCR2 axis, NOD-like receptor protein 3 (NLRP3), and TLR4 (Li S. et al., 2019; Wang H. et al., 2020; Badr et al., 2023; Park et al., 2024).
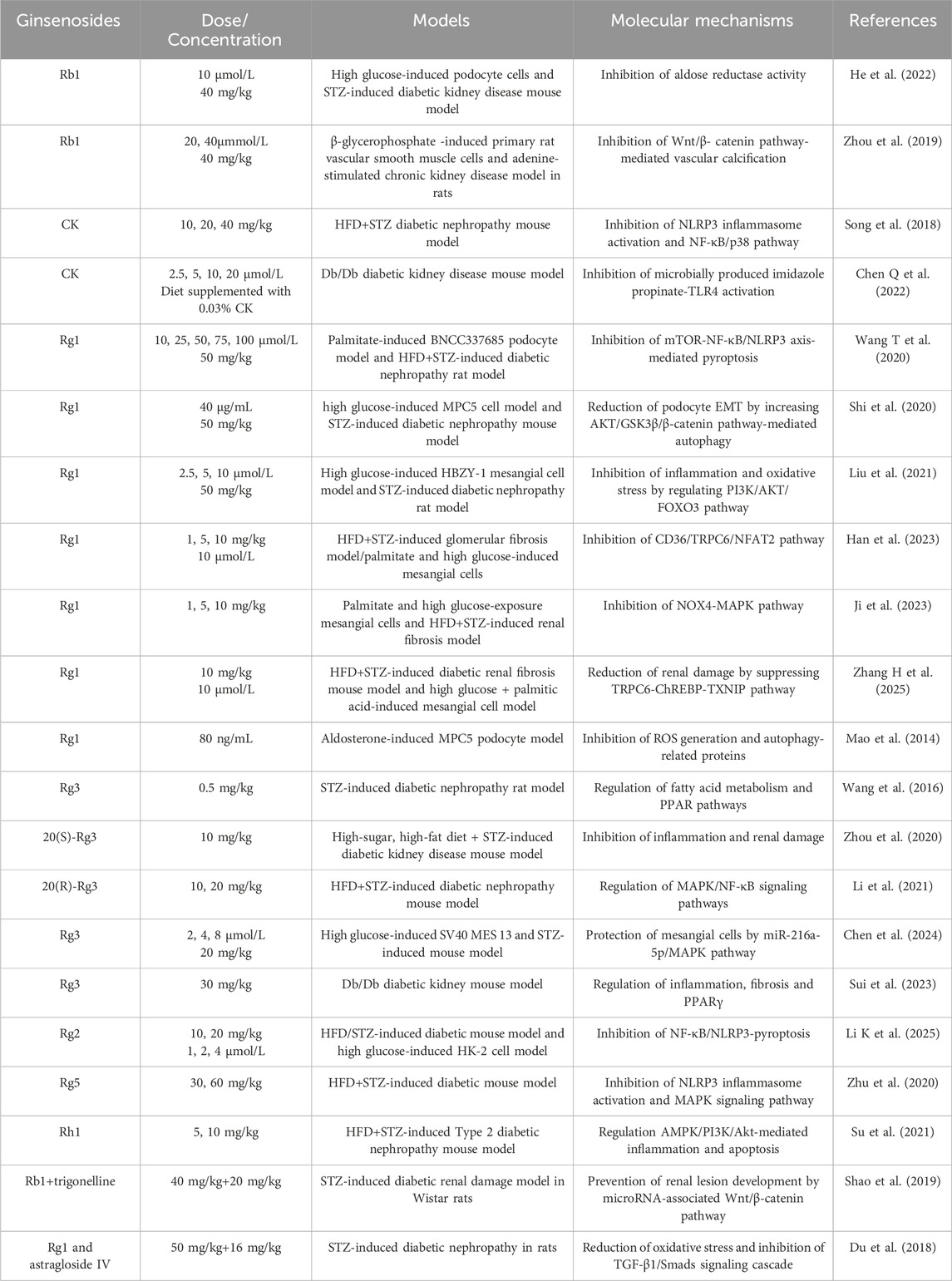
Extensive cell and animal studies demonstrate that various ginsenosides, including Rb3, Rh2, Rg3, and others, confer significant nephroprotection against cisplatin-induced injury. Cisplatin administered at 4–25 mg/kg in animal models typically induces nephrotoxicity and acute renal failure (Katagiri et al., 2016; Ozkok et al., 2016; Chatterjee et al., 2017). Multiple ginsenosides target diverse pathways against cisplatin-induced nephrotoxicity. In ICR mouse and HEK293 models, ginsenoside Rb3 significantly reduces serum creatinine, blood urea nitrogen, histopathological damage, and autophagy-related proteins (p62, Atg3, Atg5, Atg7) by modulating AMPK/mTOR signaling (Xing et al., 2019). After 28 days of administration, ginsenoside Rb3 markedly reduces necrotic lesions and TGF-β expression in the kidneys of oral carcinoma xenograft nude mice. In cisplatin-treated GP-293 cells, Rb3 further inhibits mitochondrial apoptosis by suppressing Smad2/3 phosphorylation and blocking the cleavage of PARP and caspase-3/8/9 (Wu et al., 2021). Ginsenoside Re also exhibits nephroprotective effects by improving renal function, lowering malondialdehyde levels (Wang et al., 2018), and attenuating inflammation and apoptosis. Consistently, two independent studies demonstrates that ginsenoside Rg3 mitigates cisplatin-induced nephrotoxicity (Zhai et al., 2021; Zhang et al., 2021). Mechanistically, Rg3 reduced autophagy and NLRP3 inflammasome-associated proteins, including p62, ASC, caspase-1, and IL-1β, thereby protecting against cisplatin-induced cellular injury in HK-2 cells and a murine kidney injury model (Zhai et al., 2021). Another study reveals that Rg3 modulates the PI3K/Akt and NF-κB signalling pathways to alleviate renal apoptosis and inflammation in the kidney tissues of cisplatin-induced mouse model (Zhang et al., 2021). Similarly, ginsenoside Rg5 exerts nephroprotective effects by suppressing inflammation, oxidative stress, and apoptosis (Li et al., 2016). In a cisplatin-induced HK-2 cell model, ginsenoside Rh2 protects renal tubular epithelial cells by inhibiting ER stress and mitochondria-mediated apoptosis (Qi et al., 2019; Wang L. et al., 2024). Ginsenoside Rh3 also demonstrates protective activity, reducing cisplatin-induced apoptosis in renal proximal LLC-PK1 cells through inhibition of the JNK/ERK signaling cascade (Lee and Kang, 2017). Rk1 activates the Nrf2/HO-1 pathway, enhancing glutathione and reducing reactive oxygen species (ROS) production and apoptosis in HEK293 cells (Hu et al., 2020). Combined Rk3 and Rh4 attenuate oxidative damage in cisplatin-induced rat and LLC-PK1 models (Baek et al., 2017). Beyond cisplatin, ginsenosides also protect against other nephrotoxic insults. Rg1 and Rb1 alleviate cadmium- and lithium-induced renal injury by reducing oxidative stress and inflammation (El-Sheikh and Kamel, 2016; Ren et al., 2021). Rb1 prevents bavachin-induced nephrotoxicity by blocking ER stress, epithelial–mesenchymal transition, and fibrosis via Bip/eIF2α/CHOP signaling in HK-2 and zebrafish models (Ni et al., 2022). Similarly, Rb1 mitigates 3-monochloropropane-1,2-diol-induced pyroptosis in mice and rat renal tubules, with chloroquine confirming the role of autophagy (Zhang et al., 2024). In tacrolimus-induced nephrotoxicity, Rb1 inhibits MAPK and caspase activation to reduce apoptosis in LLC-PK1 cells (Lee et al., 2018). Collectively, diverse ginsenosides, including Rb3, Rg3, and others, attenuate drug-induced nephrotoxicity by targeting central mechanisms such as oxidative stress, dysregulated autophagy, ER stress, and mitochondrial apoptosis, thereby offering promising nephroprotective strategies in preclinical models (Table 1; Figure 1A).
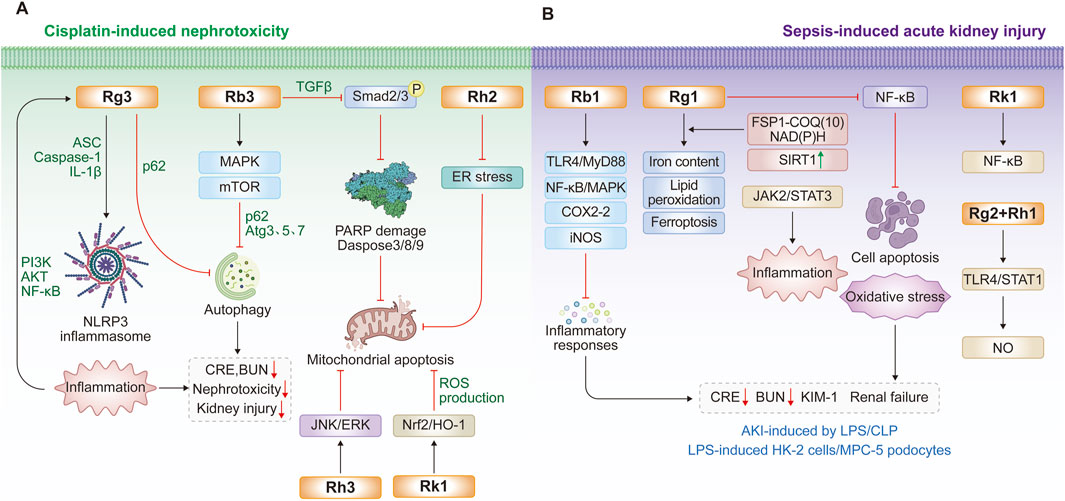
Figure 1. Protective effects and molecular mechanisms of ginsenosides for inhibiting nephrotoxicity and acute kidney injury. (A) Recent findings of ginsenosides for cisplatin-induced nephrotoxicity; (B) Recent findings of ginsenosides for the treatment of acute kidney injury by lipopolysaccharide (LPS) or cecal ligation and puncture (CLP).
2.2 Sepsis-induced acute kidney injury
The bacterial endotoxin, LPS and cecal ligation and puncture (CLP) procedure are widely used experimental models for inducing systemic inflammatory responses and sepsis, conditions that can progress to multiple organ failure or acute injury of the kidney, lung, and liver (Plotnikov et al., 2018; Gonzalez-Nicolas and Lazaro, 2025). Sepsis-associated acute kidney injury is primarily driven by inflammation, microvascular dysfunction, and glomerular/tubular damage. A key mechanism that LPS activates toll-like receptor 4 (TLR4), initiating downstream immune signaling via the NF-κB pathway, which promotes the release of chemokines and cytokines, such as IL-1β, IL-6, IL-8, CXCL2, CXCL10, and CCL20 (Lu et al., 2008). During sepsis, these interleukins, particularly IL-6 and IL-8 further amplify NF-κB signaling, leading to inducible nitric oxide synthase upregulation and nitric oxide (NO) production. Excess NO inhibits cytochrome oxidase and disrupts mitochondrial electron transport, thereby increasing ROS generation (Plotnikov et al., 2018). Similarly, CLP procedure triggers widespread cytokine and chemokine release in the peritoneal cavity, circulation, and peripheral organs (Gonzalez-Nicolas and Lazaro, 2025). Ultimately, multiple signaling axes, including TLR4, IL-18, NADPH oxidase isoform 4 (NOX4), and the Janus kinase/signal transducer and activator of transcription-1/3 (JAK/STAT1/3) pathway, contribute to sepsis-induced acute kidney injury (Nozaki et al., 2017; Yoo et al., 2020; Lee et al., 2024).
Ginsenosides have emerged as promising interventions against LPS- or CLP-induced acute kidney injury, primarily by suppressing renal inflammation, podocyte apoptosis, and ferroptosis (Gao et al., 2020; Guo et al., 2022; Hu Y. et al., 2024). Ginsenoside Rb1 reduces septic mortality in mice challenged with LPS or cantharidin by inhibiting proinflammatory cytokine release, COX-2 and inducible nitric oxide synthase levels in LPS-stimulated RAW264.7 cells and bone marrow-derived macrophages, through modulation of TLR4/MyD88 and NF-κB/MAPK pathways (Gao et al., 2020). Likewise, ginsenoside Rg1 protects against sepsis-induced acute kidney injury in both cellular and animal models (Guo et al., 2022; Guo et al., 2024; Hu Y. et al., 2024). In CLP rats and LPS-stimulated HK-2 cells, Rg1 decreases serum creatinine, blood urea nitrogen, kidney injury molecule-1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL) levels, while reducing iron accumulation, lipid peroxidation, and ferroptosis (Guo et al., 2022). Mechanistically, Rg1 suppresses renal tubular epithelial ferroptosis via the ferroptosis suppressor protein 1–CoQ10–NAD(P)H pathway (Guo et al., 2024). Additionally, the protective role of Rg1 involves in enhancing SIRT1 activity to inhibit NF-κB signaling, thereby reducing apoptosis, oxidative stress, and inflammation in septic kidneys (Hu Y. et al., 2024). Beyond acute injury, Rg1 also protects against low-dose LPS-induced chronic renal injury and fibrosis by inhibiting NOX4 and the NLRP3 inflammasome (Zhang et al., 2022) or through Nrf2-mediated suppression of AIM2 inflammasome activation (Ji P. M. et al., 2024). Ginsenoside Rk1 provides further renoprotection by blocking NF-κB and JAK2/STAT3 signaling in LPS-induced murine and podocyte injury models (Ma et al., 2024). Moreover, a combination of minor ginsenosides Rg2 and Rh1 exerts synergistic anti-inflammatory effects by targeting macrophages and suppressing TLR4/STAT1-dependent cytokine and NO production (Huynh et al., 2020). Collectively, ginsenosides Rg1, Rb1, and Rk1 confer multi-faceted renoprotection against sepsis-induced acute kidney injury through multiple mechanisms, including TLR4, NF-κB, NLRP3, STAT1/3, and ferroptosis-associated pathways (Table 2; Figure 1B).
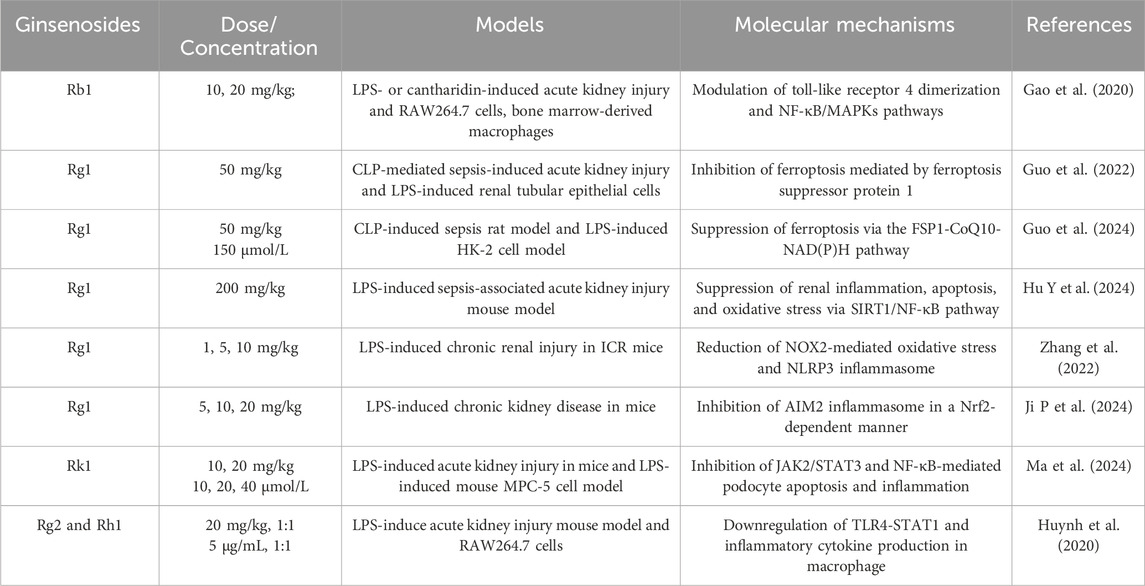
Table 2. Protective effects and molecular mechanisms of ginsenosides for reducing acute kidney injury induced by LPS or CLP.
2.3 Diabetic nephropathy
Diabetic nephropathy, a leading cause of end-stage renal disease, is characterized by persistent albuminuria and a progressive decline in renal function (Selby and Taal, 2020). Its hallmark pathological changes include glomerular basement membrane (GBM) thickening, mesangial expansion, glomerulosclerosis, podocyte injury, extracellular matrix accumulation, epithelial-to-mesenchymal transition (EMT) of tubular epithelial cells, and progressive fibrosis (Zhao et al., 2025). Chronic hyperglycemia induces metabolic dysregulation, abnormal hemodynamics, activation of the renin–angiotensin–aldosterone system, fatty acid accumulation, oxidative stress, and inflammation to drive renal damage. These alterations collectively promote the upregulation of growth factors (transforming growth factor-β1, TGF-β1 and vascular endothelial growth factor, VEGF), cytokines (TNF-α, IL-6), and chemokines (CCL2, CXCL10), reinforcing a vicious cycle of renal injury (Chang and Chen, 2020; Tuttle et al., 2022).
The progression of diabetic nephropathy is driven by multiple molecular mechanisms (Tuttle et al., 2022; Hou et al., 2025), including TGF-β1/Smads, NF-κB, MAPK, PI3K/AKT, NLRP3 inflammasome, peroxisome proliferator-activated receptors (PPARs), JAK/STAT, and sodium-glucose cotransporter-2 (SGLT2) pathways. During the process, high glucose enhances TGF-β signaling and Smad-mediated transcription, leading to inflammatory injury, GBM thickening, and extracellular matrix overproduction, thereby driving diabetic renal fibrosis (Wang Y. et al., 2020; Xu et al., 2020). Similarly, PI3K/AKT signaling contributes to the pathology of diabetic nephropathy by promoting inflammation, apoptosis, and EMT (Wang H. et al., 2024). Concurrently, hyperglycemia-induced MAPK and TLR4 activation also enhance NF-κB-dependent TNF-α expression, aggravating renal damage (Abhirami et al., 2025). Recent studies highlight NLRP3 inflammasome, PPAR subtypes, and JAK/STAT signaling as key therapeutic targets, given their critical roles in regulating inflammation, autophagy, EMT, and fibrosis in diabetic nephropathy (Yang et al., 2021; Gao and Gu, 2022; Liu et al., 2023). Importantly, SGLT2 inhibition offers broad renoprotective benefits, mitigating multiple pathological features of diabetic nephropathy (DeFronzo et al., 2021; Papaetis, 2024).
Ginsenosides have been evaluated in diverse animal models of diabetic kidney disease, including high-fat diet (HFD) and streptozotocin (STZ)-induced rodent models and Db/Db mice with leptin receptor mutations, to assess their protective effects against diabetic kidney disease (Chen et al., 2023; Fan et al., 2023). Ginsenoside Rb1 suppresses high glucose-induced cell death, mitochondrial injury, and diabetes-associated glomerular hypertrophy and mesangial expansion by binding to and inhibiting aldose reductase (He et al., 2022). In chronic kidney disease, vascular calcification characterized by calcium deposition and vascular smooth muscle cell osteogenic transdifferentiation, is attenuated by Rb1 via regulation of the PPARγ/Wnt/β-catenin axis in both chronic kidney disease-induced vascular calcification rats and β-glycerophosphate-stimulated vascular smooth muscle cells (Zhou et al., 2019). Another major metabolite, CK, prevents HFD/STZ-induced diabetic nephropathy and high glucose-mediated mesangial cell damage by inhibiting NLRP3 inflammasome activation and the NF-κB/p38 pathway (Song et al., 2018). Furthermore, 16-week CK supplementation remodels the gut microbiota, reducing the level of its metabolite imidazole propionate, thereby downregulating TLR4 signaling and improving renal morphology and microalbuminuria (Chen Q. et al., 2022).
Additional ginsenosides, including Rg1, Rg2, Rg3, Rg5, and Rh1, protect against diabetic kidney disease by preserving podocyte and mesangial cell integrity and inhibiting renal fibrosis. Podocyte and diabetic rat studies show that ginsenoside Rg1 downregulates the mTOR/NF-κB pathway, suppressing hyperlipidemia-induced NLRP3 inflammasome activation and mitigating podocyte injury (Wang T. et al., 2020). Rg1 also alleviates epithelial-mesenchymal transition in high-glucose podocytes and improves renal function in STZ-injected rats through autophagy induction mediated by the AKT/GSK3β/β-catenin pathway (Shi et al., 2020). In mesangial cells, Rg1 markedly counters high-glucose-induced inflammation, oxidative stress, and apoptosis by increasing phosphorylated PI3K/AKT levels and promoting nuclear export of FOXO3 (Liu et al., 2021). In renal fibrosis, multiple studies demonstrate that Rg1 inhibits renal injury and fibrosis through diverse mechanisms. For instance, in a T2DM mouse model induced by HFD/STZ, 8-week Rg1 treatment reduces urinary protein, serum creatinine, and blood urea nitrogen levels by inhibiting CD36/TRPC6/NFAT2 signaling (Han et al., 2023). Other reports show that Rg1 suppresses NOX4-MAPK and TRPC6-ChREBP-TXNIP pathways to limit lipid deposition, ROS accumulation, and glycoprotein deposition in T2DM-associated fibrosis (Ji et al., 2023; Zhang H. et al., 2025). Importantly, Rg1 targets TRPC6 channels to buffer lipid, inflammatory, and oxidative stress signals in diabetic kidneys, as confirmed in Trpc6 knockout mice and through combination with the TRPC6 antagonist BI749327 (Zhang H. et al., 2025). Additionally, Rg1 alleviates aldosterone-induced podocyte injury in diabetic nephropathy and chronic kidney disease by inhibiting oxidative stress and enhancing autophagy (Mao et al., 2014). Collectively, these findings establish Rg1 as a multi-target protective agent against diabetic nephropathy.
Among the ginsenosides, Rg3 is particularly well-studied for diabetic nephropathy. Transcriptomic analysis of renal cortex tissue from diabetic rats revealed that Rg3 modulates genes involved in fatty acid metabolism and PPAR signaling (Wang et al., 2016). Consistently, Rg3 reduces proteinuria, creatinine, and triglyceride levels, while suppressing inflammation via downregulation of TGF-β1, NF-κB p65, and TNF-α (Zhou et al., 2020). Similar effects are observed in HFD/STZ-induced mice, where Rg3 regulates the MAPK/NF-κB pathway to improve glucose and lipid homeostasis, enhance antioxidant capacity, and alleviate renal inflammation (Li et al., 2021). At the cellular level, Rg3 enhances proliferation and prevents apoptosis in high glucose-stimulated SV40 mesangial cells, while ameliorating renal pathological changes in diabetic mice, in part, through the miR-216a-5p/MAPK pathway (Chen et al., 2024). In Db/Db mice, Rg3 improves renal function by regulating inflammation, fibrosis, and PPARγ expression, with effects comparable to ginsenoside Re (Sui et al., 2023). In summary, Rg3 confers comprehensive renoprotection by concurrently targeting inflammation, fibrosis, and fatty acid metabolism through TGF-β1, NF-κB, and PPAR signaling pathways.
Additionally, other ginsenosides such as Rg2, Rg5, and Rh1 have been shown to regulate novel mechanisms relevant to diabetic kidney disease, particularly inflammasome activation and pyroptosis (Zhu et al., 2020; Su et al., 2021; Li K. et al., 2025). Ginsenoside Rg2 reduces NF-κB p65 phosphorylation, suppresses NLRP3 inflammasome activation, and decreases IL-18 and IL-1β release, thereby improving hyperglycemia, dyslipidemia, and renal dysfunction in HFD/STZ-induced diabetic mice (Li K. et al., 2025). Similarly, ginsenoside Rg5 protects against HFD/STZ-induced renal pathology by inhibiting NLRP3 activation, oxidative stress, and inflammatory responses (Zhu et al., 2020). For ginsenoside Rh1, activation of the AMPK/PI3K/AKT pathway mediates the inhibition of advanced glycation end product accumulation and inflammatory factor release, explaining its renoprotective effects in diabetic nephropathy (Su et al., 2021). Combination therapy with ginsenosides and other bioactive compounds is emerging as a promising strategy for the prevention and treatment of diabetic nephropathy. For example, ginsenoside Rb combined with trigonelline ameliorates renal dysfunction and pathological changes in diabetic rats by regulating miR-3350 expression and modulating the Wnt/β-catenin pathway (Shao et al., 2019). Likewise, co-administration of ginsenoside Rg1 and astragaloside IV inhibits diabetic nephropathy-related renal fibrosis through suppression of oxidative stress and the TGF-β1/Smads signaling pathway (Du et al., 2018). Collectively, these evidences from both single-agent and combination studies confirms that multiple ginsenosides, including Rg1 and Rg3, confer protection against inflammation, oxidative stress, apoptosis, and fibrosis in diabetic nephropathy progression through diverse pathways such as PI3K/AKT, TGF-β1, NF-κB, MAPK, and PPAR signaling (Table 3; Figure 2).
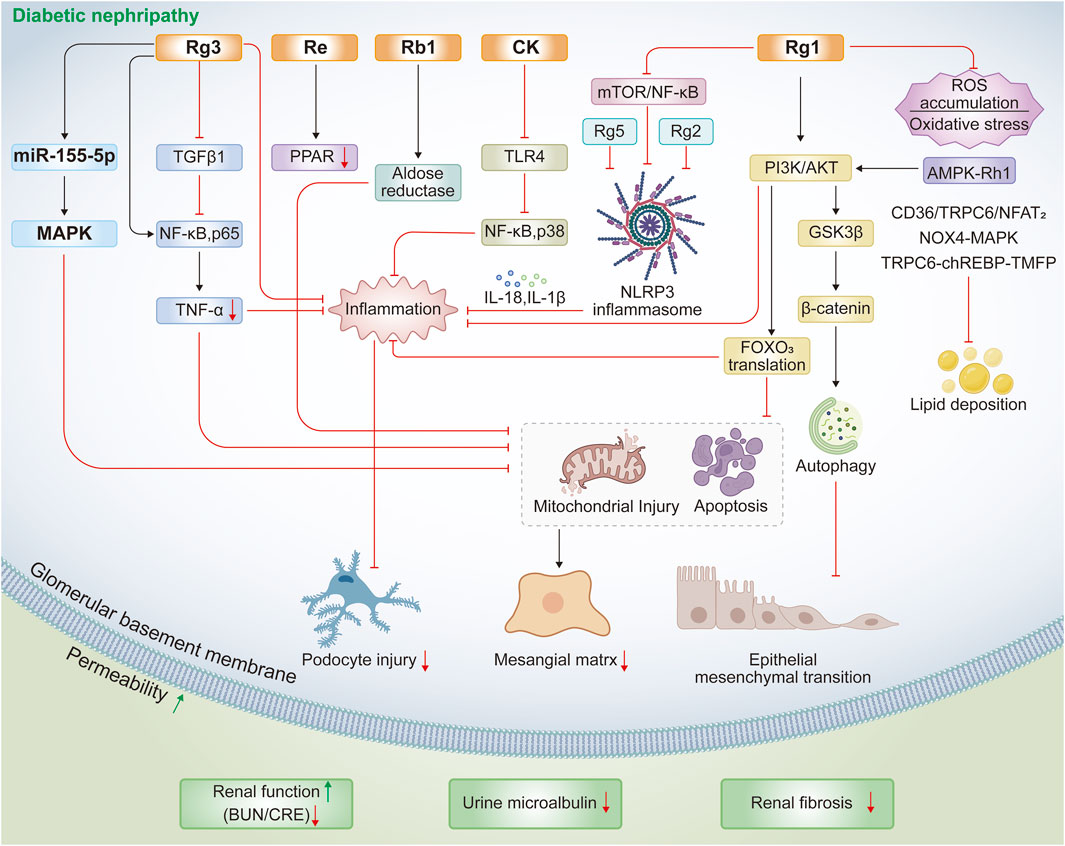
Figure 2. Protective mechanisms of ginsenosides against HFD/STZ- or STZ-induced diabetic nephropathy.
2.4 Renal cell carcinoma
Renal cell carcinoma is the predominant form of kidney cancer, accounting for approximately 90% of cancers (Rose and Kim, 2024). Key signaling pathways implicated in renal cell carcinoma progression include von Hippel–Lindau–HIF-1α/2α, VEGF, BRCA1-associated protein-1/host cell factor-1, mTOR, and PD-1 signaling (Nabi et al., 2018). Commonly used research platforms for drug evaluation include renal cell carcinoma cell lines (786-O, ACHN, Caki-1, A-498), xenograft models, and genetically engineered mouse models (Shapiro et al., 2022). Several ginsenosides, particularly Rg3 and Rh4, demonstrate anti-tumor effects or enhance drug sensitivity in renal cell carcinoma (Ma et al., 2023; Zhao et al., 2024). In both renal cell carcinoma cell and xenograft models, CK inhibits proliferation, invasion, and migration, while inducing cell cycle arrest and caspase-dependent apoptosis through ROS modulation and regulation of lncRNA THOR (Chen et al., 2021). Ginsenoside Rg3 exerts comparable anti-cancer activity, primarily though the promotion of DNA demethylation and histone acetylation (Ma et al., 2023). Importantly, ginsenosides can potentiate the efficacy of tyrosine kinase inhibitors. For instance, ginsenoside Rh2 enhances sunitinib’s inhibitory effect on renal cell carcinoma by promoting oxidative DNA damage and cell cycle arrest (Hwang et al., 2022). Similarly, ginsenoside Rh4 suppresses Nrf2 signaling, thereby reducing the activities of SOD1, GPX4, and catalase to increase ferroptotic sensitivity, which highlights a promising therapeutic strategy for renal cell carcinoma (Zhao et al., 2024). Collectively, ginsenosides exhibit direct anti-cancer activity and improve drug sensitivity for renal cell carcinoma via regulation of lncRNA THOR, epigenetic remodeling, and Nrf2-mediated ferroptosis (Table 4).
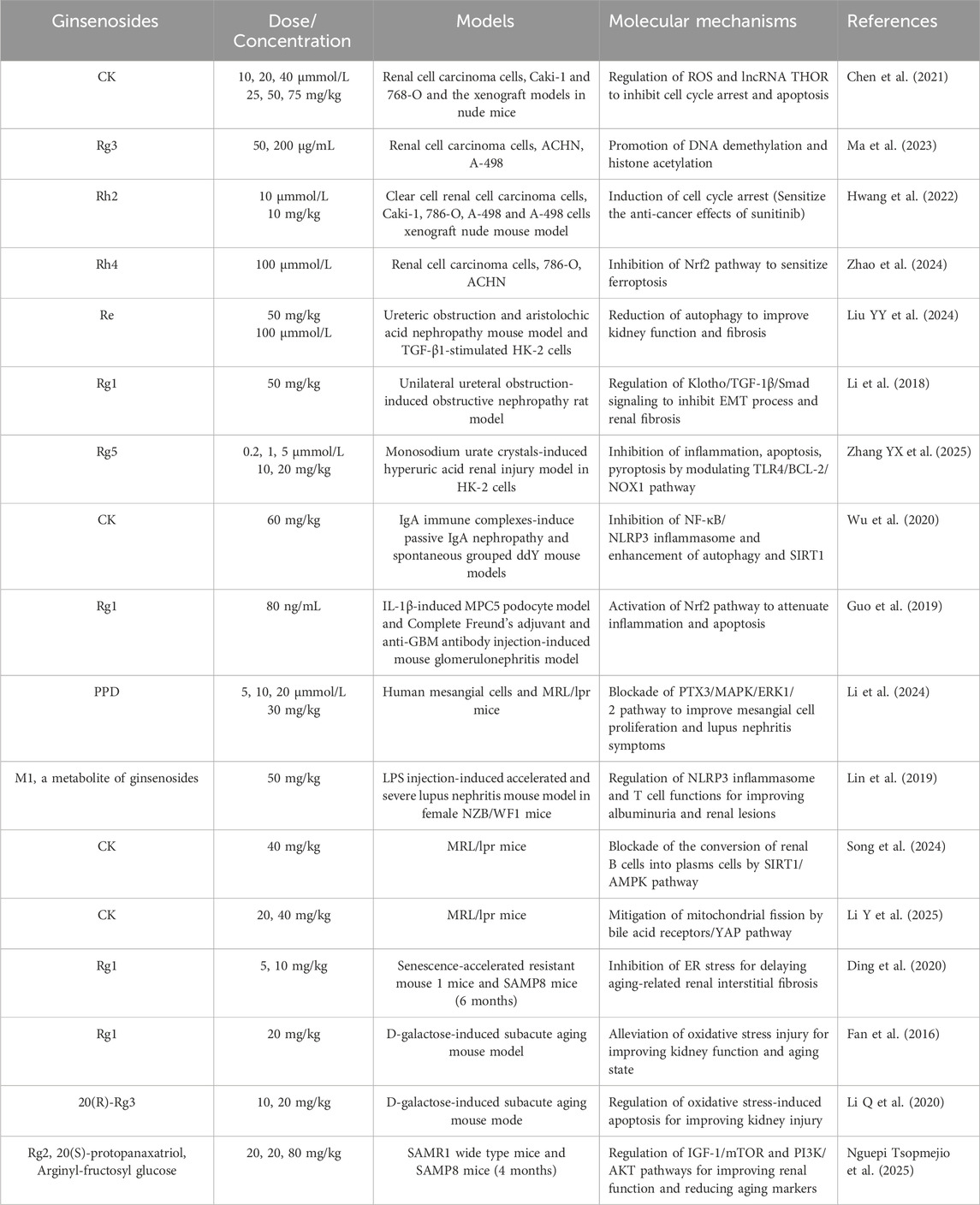
Table 4. Protective effects and molecular mechanisms of ginsenosides against renal cell carcinoma and other kidney diseases.
2.5 Other kidney-related diseases
Multiple studies have demonstrated the renoprotective effects of ginsenosides across a broad spectrum of other kidney diseases, including obstructive nephropathy, IgA nephropathy, glomerulonephritis, and aging-related kidney injury.
In mouse models of ureteral obstruction and aristolochic acid nephropathy, as well as in HK-2 cells, ginsenoside Re treatment suppresses autophagy and reduces TGF-β-stimulated profibrotic markers, thereby mitigating fibrotic injury and preserving kidney function (Liu Y. Y. et al., 2024). Similarly, in rats with unilateral ureteral obstruction, ginsenoside Rg1 inhibits TGF-β1-induced Smad3 phosphorylation while restoring Klotho and Smad7 expression, which collectively attenuate EMT and renal fibrosis (Li et al., 2018). In the monosodium urate-stimulated HK-2 cell model, ginsenoside Rg5 reduces uric acid content, the levels of urate transporter proteins and oxidative stress to reverse the cell damage. In the yeast extract and adenine-induced hyperuricemic mice, it significantly reduces urine uric acid, blood urea nitrogen and serum creatinine levels, reverses pathomorphological changes and uric acid transport. These protective effect of Rg5 above is mediated by the inhibition of oxidative stress, inflammation, and pyroptosis through the modulation of NOX-1-dependent TLR4 and BCL-2 pathways (Zhang Y. X. et al., 2025). In complementary IgA nephropathy models, CK exerts therapeutic effects by suppressing NLRP3 inflammasome activation in renal tissues and macrophages and promoting SIRT1-mediated autophagy (Wu et al., 2020). In an anti-GBM glomerulonephritis mouse model and IL-1β–induced MPC5 podocytes, ginsenoside Rg1 reduces inflammation and apoptosis via Nrf2 activation, an effect confirmed by the Nrf2 inhibitor ML385 (Guo et al., 2019). In lupus nephritis, PPD inhibits PTX3 overexpression, suppresses mesangial cell proliferation, and improves renal pathology in MRL/lpr mice through blockade of the PTX3/MAPK/ERK1/2 pathway (Li et al., 2024). For more aggressive lupus nephritis, M1, a ginsenoside metabolite, markedly reduces albuminuria and renal injury by inhibiting NLRP3 inflammasome, modulating T-helper cell activation, and promoting regulatory T-cell differentiation (Lin et al., 2019). Furthermore, recent multi-omics analyses further reveal that CK alleviates podocyte injury in MRL/lpr mice by regulating bile acid receptor/YAP and SIRT1/AMPK pathways, thereby attenuating mitochondrial fission and suppressing B-cell to plasma cell transition (Song et al., 2024; Li Y. et al., 2025).
For aging-associated kidney injury, ginsenoside monomers consistently demonstrate anti-fibrotic and renoprotective effects across multiple models, including naturally aged mice, SAMP8 mice (a spontaneous amyloid precursor protein-overexpressing strain), and D-galactose-induced subacute aging models. In SAMP8 mice, ginsenoside Rg1 reduces renal tubular injury, fibrosis, glycoprotein deposition, and tubular cell apoptosis by inhibiting ER stress pathways involving GRP78, PERK, and CHOP (Ding et al., 2020). Comparable benefits are observed in D-galactose-treated mice, where Rg1 ameliorates glomerular injury and enhances anti-oxidant capacity (Fan et al., 2016). Similarly, 20(R)-Rg3 prevents oxidative stress-induced renal injury by activating PI3K/AKT signaling (Li W. et al., 2020). A comparative study further indicates that Rg2, PPT, and arginyl-fructosyl glucose attenuate renal dysfunction and aging markers via regulation of insulin/IGF-1, mTOR, and PI3K/AKT pathways (Nguepi Tsopmejio et al., 2025). Collectively, these findings indicate that ginsenoside monomers like CK, Rg1, and Rg5 confer broad protection against nephropathies, including obstructive, hyperuricemic, glomerular, lupus, and aging-related forms through key mechanisms involving inflammasome inhibition, ER stress modulation, pyroptosis suppression, and autophagy regulation (Table 4).
3 Discussion
At present, drug-induced nephrotoxicity, sepsis-associated acute kidney injury, diabetic nephropathy, and renal cell carcinoma remain leading contributors to chronic kidney disease and end-stage renal disease, severely impairing patient survival and quality of life (Turin et al., 2012). This urgent need for novel nephroprotective agents has directed attention to ginseng, a traditional medicinal herb from Jilin Province, China, long recognized as both food and medicine (Song et al., 2025). Among its constituents, ginsenosides, the principal active components of ginseng, have been extensively investigated in both preclinical and clinical studies for their renoprotective effects (Xu et al., 2017; Jin et al., 2021; Fan et al., 2023). While, other constituents, including polysaccharides (Liu et al., 2012; Wei X. M. et al., 2021), arginyl-fructosyl-glucose (Li R. Y. et al., 2019; Tsopmejio et al., 2025), and pectin lyase-modified ginseng extract (Kim et al., 2017; Jung et al., 2021), also show protective effects against nephrotoxicity and diabetic nephropathy, their mechanisms are less well characterized than those of ginsenosides. Reported mechanisms overlap with those of ginsenosides, such as regulation of ER stress, ROS production, and apoptosis, but require further validation in animal and cellular models (Jung et al., 2021; Wei X. M. et al., 2021; Tsopmejio et al., 2025). Comparative studies with other medicinal plants highlight both shared and distinct mechanisms. For instance, Panax notoginseng saponins exhibit renoprotection against cisplatin-induced acute kidney injury and lupus nephritis comparable to those of ginsenosides, but act primarily through HIF-1α/mitochondrial pathways (Li Q. et al., 2020) and macrophage-derived exosome–mediated autophagy (Pan et al., 2025). Differently, astragaloside IV protects podocytes from diabetic nephropathy by modulating the NLRP3 inflammasome (Hu Z. et al., 2024), a mechanism that parallels the action of ginsenosides Rg1, Rg2, and Rg5 (Wang T. et al., 2020; Zhu et al., 2020; Li K. et al., 2025). Together, these findings suggest that saponins from the Araliaceae family provide a broad nephroprotective spectrum, shielding the kidney from diverse drug-induced, inflammatory, and metabolic insults.
This review synthesizes recent advances on the pharmacological efficacy and molecular mechanisms of ginsenosides in protecting against common kidney diseases, including cisplatin-induced nephrotoxicity, sepsis-induced acute kidney injury, diabetic nephropathy, renal cell carcinoma, lupus nephritis, and aging-related kidney injury, as illustrated in the accompanying figures and tables. After reviewing, we find that ginsenosides possess several significant advantages as potential therapies for kidney diseases. Firstly, ginsenosides exhibit a broad-spectrum efficacy from acute injury and chronic diseases to autoimmune diseases and cancer, which enable them to be more holistic therapeutic strategy. Furthermore, they are multi-target agents, which can simultaneously inhibit multiple pathological processes, such as inflammation, oxidative stress, cell death to demonstrate more pronounced effectiveness. This protection is that ginsenosides protect podocytes, mesangial cells, and tubular epithelial cells from various damages to preserve renal structure and function. More importantly, as a bioactive compound mixture, ginsenosides might synergistically enhance their overall efficacy against kidney diseases. Based on these advantages, ginsenosides represent promising candidates for the development of novel therapeutic drugs or potential alternative agents for kidney diseases.
Despite promising preclinical data, several critical issues and future directions warrant attention and could be addressed. First, clinical evaluation remains limited, with only one trial involving 177 patients reporting that ginsenoside Rb1 improves renal function and delays chronic kidney disease progression at early stages by reducing oxidative stress and inflammation to (Xu et al., 2017). Given the lack of robust clinical evidence, more high-quality, large-scale clinical trials are urgently needed to substantiate the therapeutic potential of ginsenosides. Secondly, most studies emphasize the effects of individual ginsenoside on kidney injury and diabetic nephropathy, while their roles in glomerulonephritis, renal pelvis nephritis, and renal failure are rarely investigated. Preclinical studies using diverse animal models of nephritis and chronic kidney disease are crucial to expand our understanding of their pharmacological application. Third, although potential target networks for ginsenoside-mediated renal protection have been proposed, definitive evidence for direct molecular targets is elusive. Gene-edited cell and animal models should be employed to validate these targets, and identify the binding sites using protein interaction technologies or site-directed mutagenesis. Fourth, nanomaterial-based delivery systems, including those incorporating Rg1, Rg3, or combinations of ginsenosides with other drugs, hold significant promise for enhancing bioavailability and therapeutic efficacy (Kim et al., 2018), offering an innovative direction for kidney disease treatment. Collectively, this review consolidates the most recent understanding of ginsenosides against various kidney diseases and discusses ginsenosides’ advantages, and current existing challenges, which provides a clear outlining potential directions for future research.
4 Conclusion
In conclusion, we present the pharmacological efficacy and molecular mechanisms of ginsenosides in cisplatin-induced nephrotoxicity, acute kidney injury, diabetic nephropathy, renal cell carcinoma, lupus nephritis, and aging-related kidney injury, based on evidence from cellular and animal models. Among the commonly studied ginsenosides, CK, Rg1, Rg3, Rh2, Rb1, Rb3, Rg2, and Rg5, are the most extensively investigated. These compounds concurrently modulate diverse signalling pathways, including NF-κB, PI3K/AKT, MAPK, TGF-β/Smad, PPAR, SIRT1, NLRP3, and Nrf2, thereby regulating key pathological processes such as inflammation, oxidative stress, apoptosis, EMT, pyroptosis, aberrant autophagy, and ER stress (Figure 3). This review provides new insights into recent advances for nephroprotective properties and potential mechanisms of ginsenosides. Despite abundant preclinical studies, the clinical investigation of ginsenosides, their renoprotective potential, and direct molecular mechanism still remains slow progression. Future research must prioritize clinical validation, emphasize synergistic potential, and pinpoint precise mechanisms of ginsenosides, which could pave promising avenues for ginsenosides as novel therapeutic agents in kidney diseases.
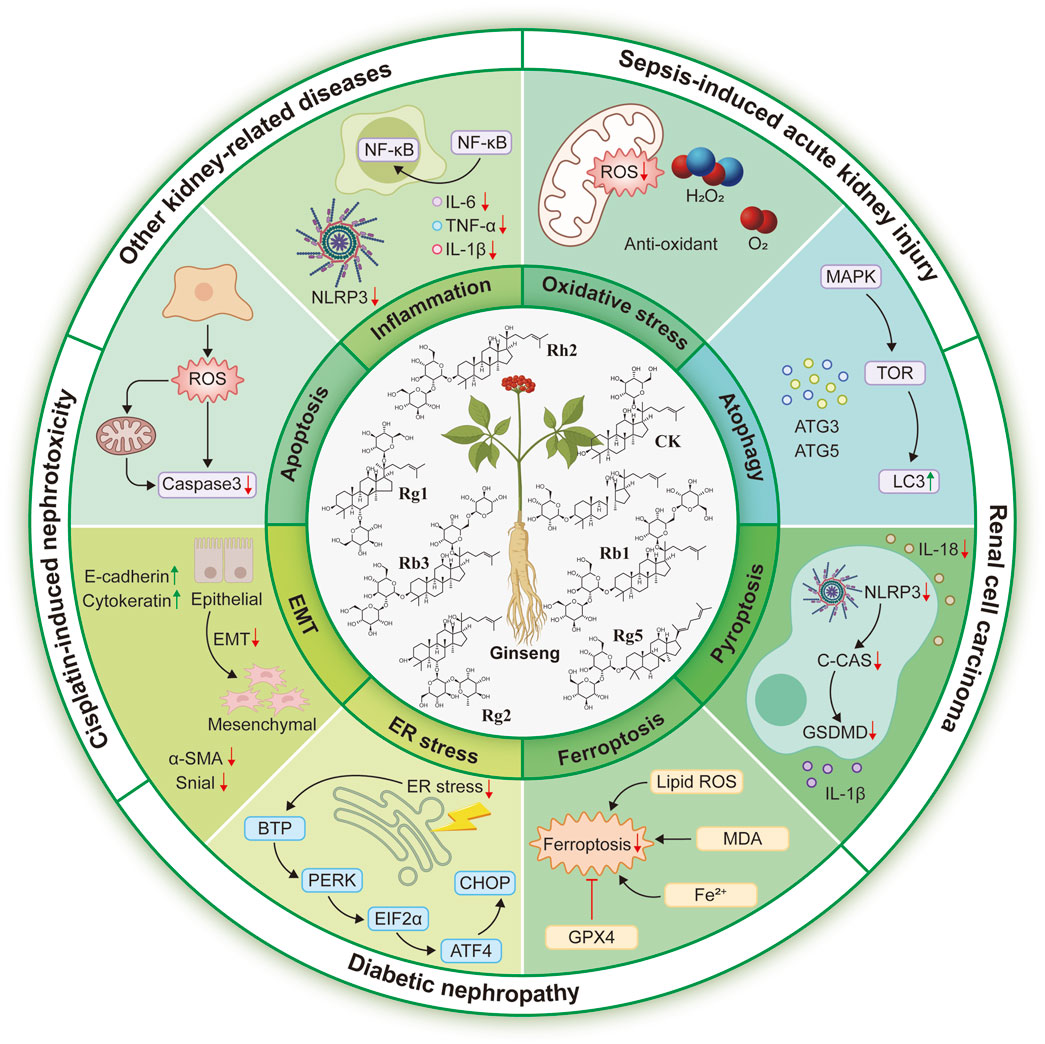
Figure 3. Protective roles and potential mechanisms of ginsenosides against common kidney diseases, including nephrotoxicity, acute kidney injury, diabetic nephropathy, renal cell carcinoma, and other kidney-related injuries.
Author contributions
TJ: Data curation, Methodology, Writing – original draft, Writing – review and editing. YD: Writing – review and editing, Data curation. CL: Writing – review and editing. JZ: Writing – review and editing, Supervision. TL: Conceptualization, Supervision, Writing – review and editing, Visualization.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CLP, Cecal ligation and puncture; EMT, Epithelial-to-mesenchymal transition; ER, Endoplasmic reticulum; GBM, Glomerular basement membrane; HFD, High-fat diet; LPS, Lipopolysaccharide; NLRP3, NOD-like receptor protein 3; PPAR, Peroxisome proliferator-activated receptors; ROS, Reactive oxygen species; STZ, Streptozotocin; TLR4, Toll-like receptor 4.
References
Abhirami, B. L., Krishna, A. A., Kumaran, A., and Chiu, C. H. (2025). Targeting NF-kappaB in diabetic nephropathy: exploring the therapeutic potential of phytoconstituents. Arch. Pharm. Res. 48, 577–637. doi:10.1007/s12272-025-01555-z
Badr, A. M., Al-Kharashi, L. A., Attia, H., Alshehri, S., Alajami, H. N., Ali, R. A., et al. (2023). TLR4/Inflammasomes cross-talk and pyroptosis contribute to N-Acetyl cysteine and chlorogenic acid protection against cisplatin-induced nephrotoxicity. Pharm. (Basel) 16 (3), 337. doi:10.3390/ph16030337
Baek, S. H., Shin, B. K., Kim, N. J., Chang, S. Y., and Park, J. H. (2017). Protective effect of ginsenosides Rk3 and Rh4 on cisplatin-induced acute kidney injury in vitro and in vivo. J. Ginseng Res. 41 (3), 233–239. doi:10.1016/j.jgr.2016.03.008
Cao, Y., Wang, K., Xu, S., Kong, L., Bi, Y., and Li, X. (2020). Recent advances in the semisynthesis, modifications and biological activities of ocotillol-type triterpenoids. Molecules 25 (23), 5562. doi:10.3390/molecules25235562
Chang, T. T., and Chen, J. W. (2020). The role of chemokines and chemokine receptors in diabetic nephropathy. Int. J. Mol. Sci. 21 (9), 3172. doi:10.3390/ijms21093172
Chang, J. J., Xu, L. X., Yi, W. J., Zhang, H. H., Zhang, J. W., Zheng, B., et al. (2025). Ginsenoside Rb1 ameliorates post-doxorubicin treatment myocardial hypertrophy via CaN/NFATc4/GATA4. J. Ginseng Res. 49 (5), 585–593. doi:10.1016/j.jgr.2025.06.003
Chatterjee, P. K., Yeboah, M. M., Solanki, M. H., Kumar, G., Xue, X., Pavlov, V. A., et al. (2017). Activation of the cholinergic anti-inflammatory pathway by GTS-21 attenuates cisplatin-induced acute kidney injury in mice. PLoS One 12 (11), e0188797. doi:10.1371/journal.pone.0188797
Chen, T. K., Knicely, D. H., and Grams, M. E. (2019). Chronic kidney disease diagnosis and management: a review. JAMA 322 (13), 1294–1304. doi:10.1001/jama.2019.14745
Chen, S., Ye, H., Gong, F., Mao, S., Li, C., Xu, B., et al. (2021). Ginsenoside compound K exerts antitumour effects in renal cell carcinoma via regulation of ROS and lncRNA THOR. Oncol. Rep. 45 (4), 38. doi:10.3892/or.2021.7989
Chen, Q., Ren, D., Liu, L., Xu, J., Wu, Y., Yu, H., et al. (2022). Ginsenoside compound K ameliorates development of diabetic kidney disease through inhibiting TLR4 activation induced by microbially produced imidazole propionate. Int. J. Mol. Sci. 23 (21), 12863. doi:10.3390/ijms232112863
Chen, Z., Zhang, Z., Liu, J., Qi, H., Li, J., Chen, J., et al. (2022). Gut microbiota: therapeutic targets of ginseng against multiple disorders and ginsenoside transformation. Front. Cell Infect. Microbiol. 12, 853981. doi:10.3389/fcimb.2022.853981
Chen, X. M., Lin, G. X., Wang, X., Ma, H. Y., Wang, R. S., Wang, S. M., et al. (2023). Beneficial effects of ginsenosides on diabetic nephropathy: a systematical review and meta-analysis of preclinical evidence. J. Ethnopharmacol. 302 (Pt A), 115860. doi:10.1016/j.jep.2022.115860
Chen, Y., Peng, Y., Li, P., Jiang, Y., and Song, D. (2024). Ginsenoside Rg3 induces mesangial cells proliferation and attenuates apoptosis by miR-216a-5p/MAPK pathway in diabetic kidney disease. Aging (Albany NY) 16 (11), 9933–9943. doi:10.18632/aging.205907
Chowdhury, N., and Drake, C. G. (2020). Kidney cancer: an overview of current therapeutic approaches. Urol. Clin. North Am. 47 (4), 419–431. doi:10.1016/j.ucl.2020.07.009
Cirillo, L., Innocenti, S., and Becherucci, F. (2024). Global epidemiology of kidney cancer. Nephrol. Dial. Transpl. 39 (6), 920–928. doi:10.1093/ndt/gfae036
Collaborators, G. B. D. C. K. D. (2025). Global, regional, and national burden of chronic kidney disease in adults, 1990-2023, and its attributable risk factors: a systematic analysis for the global burden of disease study 2023. Lancet 406, 2461–2482. doi:10.1016/S0140-6736(25)01853-7
DeFronzo, R. A., Reeves, W. B., and Awad, A. S. (2021). Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 17 (5), 319–334. doi:10.1038/s41581-021-00393-8
Ding, S. X., Zhang, H., Sun, Z. H., Han, Y. L., Li, Y., Dong, X. N., et al. (2020). Ginsenoside Rg1 protects against aging-induced renal interstitial fibrosis due to inhibition of tubular epithelial cells endoplasmic reticulum stress in SAMP8 mice. J. Funct. Foods 72, 104049. doi:10.1016/j.jff.2020.104049
Ding, H., Dong, J., Wang, Y., Huang, Q., Xu, J., Qiu, Z., et al. (2023). Ginsenoside Rb1 interfered with macrophage activation by activating PPARgamma to inhibit insulin resistance in obesity. Molecules 28 (7), 3083. doi:10.3390/molecules28073083
Ding, M., Cheng, H., Li, X., Li, X., Zhang, M., Cui, D., et al. (2024). Phytochemistry, quality control and biosynthesis in ginseng research from 2021 to 2023: a state-of-the-art review concerning advances and challenges. Chin. Herb. Med. 16 (4), 505–520. doi:10.1016/j.chmed.2024.08.002
Du, N., Xu, Z., Gao, M., Liu, P., Sun, B., and Cao, X. (2018). Combination of ginsenoside Rg1 and astragaloside IV reduces oxidative stress and inhibits TGF-beta1/Smads signaling Cascade on renal fibrosis in rats with diabetic nephropathy. Drug Des. Devel Ther. 12, 3517–3524. doi:10.2147/DDDT.S171286
El-Sheikh, A. A. K., and Kamel, M. Y. (2016). Ginsenoside-Rb1 ameliorates lithium-induced nephrotoxicity and neurotoxicity: differential regulation of COX-2/PGE(2) pathway. Biomed. Pharmacother. 84, 1873–1884. doi:10.1016/j.biopha.2016.10.106
Fan, Y., Xia, J., Jia, D., Zhang, M., Zhang, Y., Huang, G., et al. (2016). Mechanism of ginsenoside Rg1 renal protection in a mouse model of d-galactose-induced subacute damage. Pharm. Biol. 54 (9), 1815–1821. doi:10.3109/13880209.2015.1129543
Fan, M., Lan, X., Wang, Q., Shan, M., Fang, X., Zhang, Y., et al. (2023). Renal function protection and the mechanism of ginsenosides: current progress and future perspectives. Front. Pharmacol. 14, 1070738. doi:10.3389/fphar.2023.1070738
Gao, J., and Gu, Z. (2022). The role of peroxisome proliferator-activated receptors in kidney diseases. Front. Pharmacol. 13, 832732. doi:10.3389/fphar.2022.832732
Gao, H., Kang, N., Hu, C., Zhang, Z., Xu, Q., Liu, Y., et al. (2020). Ginsenoside Rb1 exerts anti-inflammatory effects in vitro and in vivo by modulating toll-like receptor 4 dimerization and NF-kB/MAPKs signaling pathways. Phytomedicine 69, 153197. doi:10.1016/j.phymed.2020.153197
Gonsalez, S. R., Cortes, A. L., Silva, R. C. D., Lowe, J., Prieto, M. C., and Silva Lara, L. D. (2019). Acute kidney injury overview: from basic findings to new prevention and therapy strategies. Pharmacol. Ther. 200, 1–12. doi:10.1016/j.pharmthera.2019.04.001
Gonzalez-Nicolas, M. A., and Lazaro, A. (2025). Induction of sepsis in a rat model by the cecal ligation and puncture technique. Application for the study of experimental acute renal failure. Methods Cell Biol. 192, 69–82. doi:10.1016/bs.mcb.2024.05.004
Guo, X., Zhang, J., Liu, M., and Zhao, G. C. (2019). Protective effect of ginsenoside Rg1 on attenuating anti-GBM glomerular nephritis by activating NRF2 signalling. Artif. Cells Nanomed Biotechnol. 47 (1), 2972–2979. doi:10.1080/21691401.2019.1640712
Guo, J., Wang, R., and Min, F. (2022). Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells. J. Leukoc. Biol. 112 (5), 1065–1077. doi:10.1002/JLB.1A0422-211R
Guo, J., Chen, L., and Ma, M. (2024). Ginsenoside Rg1 suppresses ferroptosis of renal tubular epithelial cells in sepsis-induced acute kidney injury via the FSP1-CoQ(10)- NAD(P)H pathway. Curr. Med. Chem. 31 (15), 2119–2132. doi:10.2174/0929867330666230607125054
Guo, C., Wu, M. X., Zhang, Z. P., Li, J., Chen, J. J., Su, H., et al. (2025). Total ginsenosides and ginsenoside Rb2 delay hepatocyte senescence by regulating NAD(+) metabolism and promoting IDO2/QPRT expression. J. Ginseng Res. 49 (5), 541–552. doi:10.1016/j.jgr.2025.05.001
Han, H. J., Yoon, B. C., Lee, S. H., Park, S. H., Park, J. Y., Oh, Y. J., et al. (2002). Ginsenosides inhibit EGF-induced proliferation of renal proximal tubule cells via decrease of c-fos and c-jun gene expression in vitro. Planta Med. 68 (11), 971–974. doi:10.1055/s-2002-35659
Han, Y., Su, Y., Han, M., Liu, Y., Shi, Q., Li, X., et al. (2023). Ginsenoside Rg1 attenuates glomerular fibrosis by inhibiting CD36/TRPC6/NFAT2 signaling in type 2 diabetes mellitus mice. J. Ethnopharmacol. 302 (Pt A), 115923. doi:10.1016/j.jep.2022.115923
He, J. Y., Hong, Q., Chen, B. X., Cui, S. Y., Liu, R., Cai, G. Y., et al. (2022). Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacol. Sin. 43 (2), 342–353. doi:10.1038/s41401-021-00788-0
Hoste, E. A. J., Kellum, J. A., Selby, N. M., Zarbock, A., Palevsky, P. M., Bagshaw, S. M., et al. (2018). Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 14 (10), 607–625. doi:10.1038/s41581-018-0052-0
Hou, G., Dong, Y., Jiang, Y., Zhao, W., Zhou, L., Cao, S., et al. (2025). Immune inflammation and metabolic interactions in the pathogenesis of diabetic nephropathy. Front. Endocrinol. (Lausanne) 16, 1602594. doi:10.3389/fendo.2025.1602594
Hu, J. N., Xu, X. Y., Jiang, S., Liu, Y., Liu, Z., Wang, Y. P., et al. (2020). Protective effect of ginsenoside Rk1, a major rare saponin from black ginseng, on cisplatin-induced nephrotoxicity in HEK-293 cells. Kaohsiung J. Med. Sci. 36 (9), 732–740. doi:10.1002/kjm2.12220
Hu, Y., Xiang, C., Zhang, D., Zhou, F., and Zhang, D. (2024). Nephroprotective effect of Ginsenoside Rg1 in lipopolysaccharide-induced sepsis in mice through the SIRT1/NF-kappaB signaling. Folia Histochem Cytobiol. 62 (1), 13–24. doi:10.5603/fhc.97140
Hu, Z., Zhou, Y., Gao, C., Liu, J., Pan, C., and Guo, J. (2024). Astragaloside IV attenuates podocyte apoptosis via regulating TXNIP/NLRP3/GSDMD signaling pathway in diabetic nephropathy. Diabetol. Metab. Syndr. 16 (1), 296. doi:10.1186/s13098-024-01546-y
Huang, Q., Gao, S., Zhao, D., and Li, X. (2021a). Review of ginsenosides targeting mitochondrial function to treat multiple disorders: current status and perspectives. J. Ginseng Res. 45 (3), 371–379. doi:10.1016/j.jgr.2020.12.004
Huang, Q., Su, H., Qi, B., Wang, Y., Yan, K., Wang, X., et al. (2021b). A SIRT1 activator, Ginsenoside Rc, promotes energy metabolism in cardiomyocytes and neurons. J. Am. Chem. Soc. 143 (3), 1416–1427. doi:10.1021/jacs.0c10836
Huang, Q., Yao, Y., Wang, Y., Li, J., Chen, J., Wu, M., et al. (2024). Ginsenoside Rb2 inhibits p300-mediated SF3A2 acetylation at lysine 10 to promote Fscn1 alternative splicing against myocardial ischemic/reperfusion injury. J. Adv. Res. 65, 365–379. doi:10.1016/j.jare.2023.12.012
Huynh, D. T. N., Baek, N., Sim, S., Myung, C. S., and Heo, K. S. (2020). Minor ginsenoside Rg2 and Rh1 attenuates LPS-induced acute liver and kidney damages via downregulating activation of TLR4-STAT1 and inflammatory cytokine production in macrophages. Int. J. Mol. Sci. 21 (18), 6656. doi:10.3390/ijms21186656
Hwang, H. J., Hong, S. H., Moon, H. S., Yoon, Y. E., and Park, S. Y. (2022). Ginsenoside Rh2 sensitizes the anti-cancer effects of sunitinib by inducing cell cycle arrest in renal cell carcinoma. Sci. Rep. 12 (1), 19752. doi:10.1038/s41598-022-20075-0
Jadoul, M., Aoun, M., and Masimango Imani, M. (2024). The major global burden of chronic kidney disease. Lancet Glob. Health 12 (3), e342–e343. doi:10.1016/S2214-109X(24)00050-0
Jeon, J. H., Lee, J., Choi, M. K., and Song, I. S. (2020). Pharmacokinetics of ginsenosides following repeated oral administration of red ginseng extract significantly differ between species of experimental animals. Arch. Pharm. Res. 43 (12), 1335–1346. doi:10.1007/s12272-020-01289-0
Ji, P. M., Shi, Q. F., Liu, Y., Han, M., Su, Y., Sun, R., et al. (2023). Ginsenoside Rg1 treatment alleviates renal fibrosis by inhibiting the NOX4-MAPK pathway in T2DM mice. Ren. Fail. 45 (1), 2197075. doi:10.1080/0886022x.2023.2197075
Ji, P., Zhang, Z., Mingyao, E., Liu, Q., Qi, H., Hou, T., et al. (2024). Ginsenosides ameliorates high altitude-induced hypoxia injury in lung and kidney tissues by regulating PHD2/HIF-1alpha/EPO signaling pathway. Front. Pharmacol. 15, 1396231. doi:10.3389/fphar.2024.1396231
Ji, P. M., Shi, Q. F., Kong, L. L., Liu, Y., Su, Y., Sun, R., et al. (2024). Ginsenoside Rg1 attenuates chronic inflammation-induced renal fibrosis in mice by inhibiting AIM2 inflammasome in an Nrf2-dependent manner. J. Funct. Foods 116, 106204. doi:10.1016/j.jff.2024.106204
Jin, D., Zhang, Y., Zhang, Y., Duan, L., Zhou, R., Duan, Y., et al. (2021). Panax Ginseng C.A.Mey. as medicine: the potential use of Panax Ginseng C.A.Mey. as a remedy for kidney protection from a pharmacological perspective. Front. Pharmacol. 12, 734151. doi:10.3389/fphar.2021.734151
Jung, E., Pyo, M. K., and Kim, J. (2021). Pectin-lyase-modified ginseng extract and ginsenoside Rd inhibits high glucose-induced ROS production in mesangial cells and prevents renal dysfunction in db/db mice. Molecules 26 (2), 367. doi:10.3390/molecules26020367
Katagiri, D., Hamasaki, Y., Doi, K., Negishi, K., Sugaya, T., Nangaku, M., et al. (2016). Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int. 89 (2), 374–385. doi:10.1038/ki.2015.327
Kellum, J. A., Romagnani, P., Ashuntantang, G., Ronco, C., Zarbock, A., and Anders, H. J. (2021). Acute kidney injury. Nat. Rev. Dis. Prim. 7 (1), 52. doi:10.1038/s41572-021-00284-z
Kim, C. S., Jo, K., Kim, J. S., Pyo, M. K., and Kim, J. (2017). GS-E3D, a new pectin lyase-modified red ginseng extract, inhibited diabetes-related renal dysfunction in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 17 (1), 430. doi:10.1186/s12906-017-1925-7
Kim, H., Lee, J. H., Kim, J. E., Kim, Y. S., Ryu, C. H., Lee, H. J., et al. (2018). Micro-/nano-sized delivery systems of ginsenosides for improved systemic bioavailability. J. Ginseng Res. 42 (3), 361–369. doi:10.1016/j.jgr.2017.12.003
Kung, C. W., and Chou, Y. H. (2023). Acute kidney disease: an overview of the epidemiology, pathophysiology, and management. Kidney Res. Clin. Pract. 42 (6), 686–699. doi:10.23876/j.krcp.23.001
Lee, H. L., and Kang, K. S. (2017). Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J. Ginseng Res. 41 (2), 227–231. doi:10.1016/j.jgr.2017.01.011
Lee, D., Lee, D. S., Jung, K., Hwang, G. S., Lee, H. L., Yamabe, N., et al. (2018). Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J. Ginseng Res. 42 (1), 75–80. doi:10.1016/j.jgr.2016.12.013
Lee, S. H., Kim, K. H., Lee, S. M., Park, S. J., Lee, S., Cha, R. H., et al. (2024). STAT3 blockade ameliorates LPS-induced kidney injury through macrophage-driven inflammation. Cell Commun. Signal 22 (1), 476. doi:10.1186/s12964-024-01841-1
Li, W., Yan, M. H., Liu, Y., Liu, Z., Wang, Z., Chen, C., et al. (2016). Ginsenoside Rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients 8 (9), 566. doi:10.3390/nu8090566
Li, S. S., He, A. L., Deng, Z. Y., and Liu, Q. F. (2018). Ginsenoside-Rg1 protects against renal fibrosis by regulating the Klotho/TGF-beta1/Smad signaling pathway in rats with obstructive nephropathy. Biol. Pharm. Bull. 41 (4), 585–591. doi:10.1248/bpb.b17-00934
Li, R. Y., Zhang, W. Z., Yan, X. T., Hou, J. G., Wang, Z., Ding, C. B., et al. (2019). Arginyl-fructosyl-glucose, a major maillard reaction product of red ginseng, attenuates cisplatin-induced acute kidney injury by regulating nuclear factor kappaB and phosphatidylinositol 3-Kinase/Protein kinase B signaling pathways. J. Agric. Food Chem. 67 (20), 5754–5763. doi:10.1021/acs.jafc.9b00540
Li, S., Lin, Q., Shao, X., Mou, S., Gu, L., Wang, L., et al. (2019). NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp. Cell Res. 383 (1), 111488. doi:10.1016/j.yexcr.2019.07.001
Li, Q., Liang, X., Yang, Y., Zeng, X., Zhong, X., and Huang, C. (2020). Panax notoginseng saponins ameliorate cisplatin-induced mitochondrial injury via the HIF-1alpha/mitochondria/ROS pathway. FEBS Open Bio 10 (1), 118–126. doi:10.1002/2211-5463.12760
Li, W., Wang, J. Q., Zhou, Y. D., Hou, J. G., Liu, Y., Wang, Y. P., et al. (2020). Rare ginsenoside 20(R)-Rg3 inhibits D-Galactose-Induced liver and kidney injury by regulating oxidative stress-induced apoptosis. Am. J. Chin. Med. 48 (5), 1141–1157. doi:10.1142/S0192415x20500561
Li, Y., Hou, J. G., Liu, Z., Gong, X. J., Hu, J. N., Wang, Y. P., et al. (2021). Alleviative effects of 20(R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-kappaB signaling pathways in C57BL/6 mice. J. Ethnopharmacol. 267, 113500. doi:10.1016/j.jep.2020.113500
Li, X., Liu, J., Zuo, T. T., Hu, Y., Li, Z., Wang, H. D., et al. (2022). Advances and challenges in ginseng research from 2011 to 2020: the phytochemistry, quality control, metabolism, and biosynthesis. Nat. Prod. Rep. 39 (4), 875–909. doi:10.1039/d1np00071c
Li, Z. Y., Gan, H. L., Ji, K., Yang, M. Y., Pan, T., Meng, X. T., et al. (2024). Protopanaxadiol improves lupus nephritis by regulating the PTX3/MAPK/ERK1/2 pathway. J. Nat. Med. 78 (3), 474–487. doi:10.1007/s11418-023-01777-9
Li, K., Wang, Y. J., Wei, K., Li, W. L., Liu, Y. B., Hu, J. N., et al. (2025). Ginsenoside Rg2 alleviates HFD/STZ-Induced diabetic nephropathy by inhibiting pyroptosis via NF-κB/NLRP3 signaling pathways. Am. J. Chin. Med. 53 (3), 909–930. doi:10.1142/S0192415X2550034X
Li, Y., Song, Z., Xu, S., Xu, K., Xu, W., Xu, L., et al. (2025). Ginsenoside compound K mitigates mitochondrial fission through bile acid Receptors/YAP signaling to counteract podocyte injury in lupus nephritis. Phytother. Res., ptr.8492. doi:10.1002/ptr.8492
Li, Z., He, R., Wang, Y., Qu, Z., Liu, J., Yu, R., et al. (2025). Global trends of chronic kidney disease from 1990 to 2021: a systematic analysis for the global burden of disease study 2021. BMC Nephrol. 26 (1), 385. doi:10.1186/s12882-025-04309-7
Lin, T. J., Wu, C. Y., Tsai, P. Y., Hsu, W. H., Hua, K. F., Chu, C. L., et al. (2019). Accelerated and severe lupus nephritis benefits from M1, an active metabolite of ginsenoside, by regulating NLRP3 inflammasome and T cell functions in mice. Front. Immunol. 10, 1951. doi:10.3389/fimmu.2019.01951
Liu, L. Y., and Gemba, M. (1990). Stimulation of p-aminohippurate transport in renal cortical slices prepared from rats treated with ginsenosides. J. Pharmacobiodyn 13 (8), 507–511. doi:10.1248/bpb1978.13.507
Liu, Z., Li, C., Zhang, Q., and Tao, M. (2012). Effect of renshen polysaccharides on oxidative injury in kidney IR rabbits. Carbohydr. Polym. 90 (2), 773–777. doi:10.1016/j.carbpol.2012.05.040
Liu, H., Chen, W., Lu, P., Ma, Y., Liang, X., and Liu, Y. (2021). Ginsenoside Rg1 attenuates the inflammation and oxidative stress induced by diabetic nephropathy through regulating the PI3K/AKT/FOXO3 pathway. Ann. Transl. Med. 9 (24), 1789. doi:10.21037/atm-21-6234
Liu, Y., Wang, W., Zhang, J., Gao, S., Xu, T., and Yin, Y. (2023). JAK/STAT signaling in diabetic kidney disease. Front. Cell Dev. Biol. 11, 1233259. doi:10.3389/fcell.2023.1233259
Liu, Q., Zhang, Z., Ji, P., Liu, J., Chen, B., E, M., et al. (2024). Ginseng polysaccharide components attenuate obesity and liver lipid accumulation by regulating fecal microbiota and hepatic lysine degradation. Int. J. Biol. Macromol. 269 (Pt 1), 131872. doi:10.1016/j.ijbiomac.2024.131872
Liu, Y. Y., Mou, L. Y., Yi, Z. Z., Lin, Q. S., Banu, K., Wei, C. G., et al. (2024). Integrative informatics analysis identifies that ginsenoside re improves renal fibrosis through regulation of autophagy. J. Nat. Med. 78 (3), 722–731. doi:10.1007/s11418-024-01800-7
Lu, Y. C., Yeh, W. C., and Ohashi, P. S. (2008). LPS/TLR4 signal transduction pathway. Cytokine 42 (2), 145–151. doi:10.1016/j.cyto.2008.01.006
Luo, X., Xie, D., Chen, Z., and Ji, Q. (2023). Protective effects of ginsenosides in cisplatin-induced kidney injury: a systematic review, meta-analysis. Indian J. Pharmacol. 55 (4), 243–250. doi:10.4103/ijp.ijp_251_23
Ma, L. Y., Zhang, Y. B., Zhou, Q. L., Yang, Y. F., and Yang, X. W. (2015). Simultaneous determination of eight ginsenosides in rat plasma by liquid chromatography-electrospray ionization tandem mass spectrometry: application to their pharmacokinetics. Molecules 20 (12), 21597–21608. doi:10.3390/molecules201219790
Ma, Z., Zuo, Y., and Wang, W. (2023). Ginsenoside Rg3 inhibits renal cell carcinoma cell migration, invasion, colony formation, and tube formation and enhances apoptosis through promoting the DNA demethylation and histone acetylation. J. Pharm. Pharmacol. 75 (1), 76–86. doi:10.1093/jpp/rgac072
Ma, X., Pang, L., Shi, F., and Guan, B. (2024). Ginsenoside Rk1 exerts protective effects of LPS-induced podocyte apoptosis and inflammation by inactivating JAK2/STAT3 and NF-kappaB pathways. Drug Chem. Toxicol., 1–10. doi:10.1080/01480545.2024.2434900
Mao, N., Cheng, Y., Shi, X. L., Wang, L., Wen, J., Zhang, Q., et al. (2014). Ginsenoside Rg1 protects mouse podocytes from aldosterone-induced injury in vitro. Acta Pharmacol. Sin. 35 (4), 513–522. doi:10.1038/aps.2013.187
McSweeney, K. R., Gadanec, L. K., Qaradakhi, T., Ali, B. A., Zulli, A., and Apostolopoulos, V. (2021). Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers (Basel) 13 (7), 1572. doi:10.3390/cancers13071572
Motwani, S. S., Kaur, S. S., and Kitchlu, A. (2022). Cisplatin nephrotoxicity: novel insights into mechanisms and preventative strategies. Semin. Nephrol. 42 (6), 151341. doi:10.1016/j.semnephrol.2023.151341
Nabi, S., Kessler, E. R., Bernard, B., Flaig, T. W., and Lam, E. T. (2018). Renal cell carcinoma: a review of biology and pathophysiology. F1000Res 7, 307. doi:10.12688/f1000research.13179.1
Nguepi Tsopmejio, I. S., Zhang, J. T., Wang, Z., Tian, Z. F., Zhu, H. Y., Zhang, J., et al. (2025). Comparative study of ginsenoside Rg2, 20(S)-protopanaxatriol, and AFG from ginseng on aging-related kidney injury in SAMP8 mice. J. Ethnopharmacol. 348, 119807. doi:10.1016/j.jep.2025.119807
Ni, Y. H., Deng, H. F., Zhou, L., Huang, C. S., Wang, N. N., Yue, L. X., et al. (2022). Ginsenoside Rb1 ameliorated bavachin-induced renal fibrosis via suppressing Bip/eIF2alpha/CHOP signaling-mediated EMT. Front. Pharmacol. 13, 872474. doi:10.3389/fphar.2022.872474
Nozaki, Y., Hino, S., Ri, J., Sakai, K., Nagare, Y., Kawanishi, M., et al. (2017). Lipopolysaccharide-induced acute kidney injury is dependent on an IL-18 receptor signaling pathway. Int. J. Mol. Sci. 18 (12), 2777. doi:10.3390/ijms18122777
Ozkok, A., Ravichandran, K., Wang, Q., Ljubanovic, D., and Edelstein, C. L. (2016). NF-kappaB transcriptional inhibition ameliorates cisplatin-induced acute kidney injury (AKI). Toxicol. Lett. 240 (1), 105–113. doi:10.1016/j.toxlet.2015.10.028
Padala, S. A., Barsouk, A., Thandra, K. C., Saginala, K., Mohammed, A., Vakiti, A., et al. (2020). Epidemiology of renal cell carcinoma. World J. Oncol. 11 (3), 79–87. doi:10.14740/wjon1279
Pan, F., Lu, Y., and Yang, H. (2025). Panax notoginseng saponins treat steroid-resistant lupus nephritis by inhibiting macrophage-derived exosome-induced injury in glomerular endothelial cells via the mitochondrial Autophagy-NLRP3 pathway. J. Ethnopharmacol. 343, 119475. doi:10.1016/j.jep.2025.119475
Papaetis, G. (2024). SGLT2 inhibitors and diabetic kidney disease: targeting multiple and interrelated signaling pathways for renal protection. Curr. Mol. Pharmacol. 17, e18761429261105. doi:10.2174/0118761429261105231011101200
Park, J., Sim, J., Yi, H. J., Rhee, S. G., and Woo, H. A. (2024). Cisplatin induces kidney damage through the down-regulation of Prx I by autophagic degradation. Free Radic. Biol. Med. 225, 236–246. doi:10.1016/j.freeradbiomed.2024.09.049
Plotnikov, E. Y., Brezgunova, A. A., Pevzner, I. B., Zorova, L. D., Manskikh, V. N., Popkov, V. A., et al. (2018). Mechanisms of LPS-induced acute kidney injury in neonatal and adult rats. Antioxidants (Basel) 7 (8), 105. doi:10.3390/antiox7080105
Qi, Z., Li, W., Tan, J., Wang, C., Lin, H., Zhou, B., et al. (2019). Effect of ginsenoside Rh(2) on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine 61, 152862. doi:10.1016/j.phymed.2019.152862
Ren, T. T., Yang, J. Y., Wang, J., Fan, S. R., Lan, R., and Qin, X. Y. (2021). Gisenoside Rg1 attenuates cadmium-induced neurotoxicity in vitro and in vivo by attenuating oxidative stress and inflammation. Inflamm. Res. 70 (10-12), 1151–1164. doi:10.1007/s00011-021-01513-7
Ren, H., Dai, R., Chen, Y., Xi, Z., and Xu, H. (2023). How ginseng regulates autophagy: insights from multistep process. Biomed. Pharmacother. 158, 114139. doi:10.1016/j.biopha.2022.114139
Robson, L. (2014). The kidney--an organ of critical importance in physiology. J. Physiol. 592 (18), 3953–3954. doi:10.1113/jphysiol.2014.279216
Rose, T. L., and Kim, W. Y. (2024). Renal cell carcinoma: a review. JAMA 332 (12), 1001–1010. doi:10.1001/jama.2024.12848
Selby, N. M., and Taal, M. W. (2020). An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 22 (Suppl. 1), 3–15. doi:10.1111/dom.14007
Shao, X., Chen, C., Miao, C., Yu, X., Li, X., Geng, J., et al. (2019). Expression analysis of microRNAs and their target genes during experimental diabetic renal lesions in rats administered with ginsenoside Rb1 and trigonelline. Pharmazie 74 (8), 492–498. doi:10.1691/ph.2019.8903
Shapiro, D. D., Virumbrales-Munoz, M., Beebe, D. J., and Abel, E. J. (2022). Models of renal cell carcinoma used to investigate molecular mechanisms and develop new therapeutics. Front. Oncol. 12, 871252. doi:10.3389/fonc.2022.871252
Shi, Y., Gao, Y., Wang, T., Wang, X., He, J., Xu, J., et al. (2020). Ginsenoside Rg1 alleviates podocyte EMT passage by regulating AKT/GSK3 β/β-Catenin pathway by restoring autophagic activity. Evid. Based Complement. Altern. Med. 2020, 1903627. doi:10.1155/2020/1903627
Sohaney, R., Yin, H., Shahinian, V., Saran, R., Burrows, N. R., Pavkov, M. E., et al. (2022). In-Hospital and 1-Year mortality trends in a national cohort of US veterans with acute kidney injury. Clin. J. Am. Soc. Nephrol. 17 (2), 184–193. doi:10.2215/CJN.01730221
Song, W., Wei, L., Du, Y., Wang, Y., and Jiang, S. (2018). Protective effect of ginsenoside metabolite compound K against diabetic nephropathy by inhibiting NLRP3 inflammasome activation and NF-kappaB/p38 signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Int. Immunopharmacol. 63, 227–238. doi:10.1016/j.intimp.2018.07.027
Song, Z., Jin, M., Wang, S., Wu, Y., Huang, Q., Xu, W., et al. (2024). Reciprocal regulation of SIRT1 and AMPK by Ginsenoside compound K impedes the conversion from plasma cells to mitigate for podocyte injury in MRL/lpr mice in a B cell-specific manner. J. Ginseng Res. 48 (2), 190–201. doi:10.1016/j.jgr.2023.11.006
Song, X. X., Liu, E. L., and Du, X. W. (2025). Ginseng: a traditional medicine and food homologous plant. J. Asian Nat. Prod. Res., 1–13. doi:10.1080/10286020.2025.2514689
Su, W. Y., Li, Y., Chen, X., Li, X., Wei, H., Liu, Z., et al. (2021). Ginsenoside Rh1 improves type 2 diabetic nephropathy through AMPK/PI3K/Akt-Mediated inflammation and apoptosis signaling pathway. Am. J. Chin. Med. 49 (5), 1215–1233. doi:10.1142/S0192415X21500580
Sui, Z., Sui, D., Li, M., Yu, Q., Li, H., and Jiang, Y. (2023). Ginsenoside Rg3 has effects comparable to those of ginsenoside re on diabetic kidney disease prevention in db/db mice by regulating inflammation, fibrosis and PPARgamma. Mol. Med. Rep. 27 (4), 84. doi:10.3892/mmr.2023.12971
Tamargo, C., Hanouneh, M., and Cervantes, C. E. (2024). Treatment of acute kidney injury: a review of current approaches and emerging innovations. J. Clin. Med. 13 (9), 2455. doi:10.3390/jcm13092455
Tang, K., Huang, C., Huang, Z., Wang, Z., and Tan, N. (2025). GPR30-driven fatty acid oxidation targeted by ginsenoside Rd maintains mitochondrial redox homeostasis to restore vascular barrier in diabetic retinopathy. Cardiovasc Diabetol. 24 (1), 121. doi:10.1186/s12933-025-02638-3
Tsopmejio, I. S. N., Tang, S., Gao, X. F., Liu, Z., Li, X. D., Qi, M. H., et al. (2025). Arginyl-fructosyl-glucose (AFG), a major Maillard reaction product of red ginseng, improved kidney and pancreatic injury in vitro and in vivo in type 2 diabetes mellitus. Int. Immunopharmacol. 164, 115378. doi:10.1016/j.intimp.2025.115378
Turin, T. C., Tonelli, M., Manns, B. J., Ravani, P., Ahmed, S. B., and Hemmelgarn, B. R. (2012). Chronic kidney disease and life expectancy. Nephrol. Dial. Transpl. 27 (8), 3182–3186. doi:10.1093/ndt/gfs052
Tuttle, K. R., Agarwal, R., Alpers, C. E., Bakris, G. L., Brosius, F. C., Kolkhof, P., et al. (2022). Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 102 (2), 248–260. doi:10.1016/j.kint.2022.05.012
Wan, S., Cui, Z., Wu, L., Zhang, F., Liu, T., Hu, J., et al. (2023). Ginsenoside Rd promotes omentin secretion in adipose through TBK1-AMPK to improve mitochondrial biogenesis via WNT5A/Ca(2+) pathways in heart failure. Redox Biol. 60, 102610. doi:10.1016/j.redox.2023.102610
Wang, C. Z., Kim, K. E., Du, G. J., Qi, L. W., Wen, X. D., Li, P., et al. (2011). Ultra-performance liquid chromatography and time-of-flight mass spectrometry analysis of ginsenoside metabolites in human plasma. Am. J. Chin. Med. 39 (6), 1161–1171. doi:10.1142/S0192415X11009470
Wang, J., Cui, C., Fu, L., Xiao, Z., Xie, N., Liu, Y., et al. (2016). Genomic expression profiling and bioinformatics analysis on diabetic nephrology with ginsenoside Rg3. Mol. Med. Rep. 14 (2), 1162–1172. doi:10.3892/mmr.2016.5349
Wang, Z., Li, Y. F., Han, X. Y., Sun, Y. S., Zhang, L. X., Liu, W., et al. (2018). Kidney protection effect of ginsenoside Re and its underlying mechanisms on cisplatin-induced kidney injury. Cell Physiol. Biochem. 48 (5), 2219–2229. doi:10.1159/000492562
Wang, H., Xia, W., Long, G., Pei, Z., Li, Y., Wu, M., et al. (2020). Isoquercitrin ameliorates cisplatin-induced nephrotoxicity Via the inhibition of apoptosis, inflammation, and oxidative stress. Front. Pharmacol. 11, 599416. doi:10.3389/fphar.2020.599416
Wang, T., Gao, Y., Yue, R., Wang, X., Shi, Y., Xu, J., et al. (2020). Ginsenoside Rg1 alleviates podocyte injury induced by hyperlipidemia via targeting the mTOR/NF-kappaB/NLRP3 axis. Evid. Based Complement. Altern. Med. 2020, 2735714. doi:10.1155/2020/2735714
Wang, Y., Zhang, X., Mao, Y., Liang, L., Liu, L., Peng, W., et al. (2020). Smad2 and Smad3 play antagonistic roles in high glucose-induced renal tubular fibrosis via the regulation of SnoN. Exp. Mol. Pathol. 113, 104375. doi:10.1016/j.yexmp.2020.104375
Wang, H., Zhang, S., Zhai, L., Sun, L., Zhao, D., Wang, Z., et al. (2021). Ginsenoside extract from ginseng extends lifespan and health span in Caenorhabditis elegans. Food Funct. 12 (15), 6793–6808. doi:10.1039/d1fo00576f
Wang, H., Gao, L., Zhao, C., Fang, F., Liu, J., Wang, Z., et al. (2024). The role of PI3K/Akt signaling pathway in chronic kidney disease. Int. Urol. Nephrol. 56 (8), 2623–2633. doi:10.1007/s11255-024-03989-8
Wang, L., Hao, X., Li, X., Li, Q., and Fang, X. (2024). Effects of ginsenoside Rh2 on cisplatin-induced nephrotoxicity in renal tubular epithelial cells by inhibiting endoplasmic reticulum stress. J. Biochem. Mol. Toxicol. 38 (8), e23768. doi:10.1002/jbt.23768
Wang, Y., Sun, X., He, B., and Yu, S. (2025). Ginsenoside Rg1 downregulates miR-9-5p expression to modulate SIRT1-Mediated mitochondrial dysfunction and ameliorate Alzheimer's disease. Mol. Neurobiol. 62 (10), 13044–13059. doi:10.1007/s12035-025-05073-3
Wei, W., Li, Z., Li, H., An, Y., Qu, H., Yao, C., et al. (2021). Exploration of tissue distribution of ginsenoside Rg1 by LC-MS/MS and nanospray desorption electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 198, 113999. doi:10.1016/j.jpba.2021.113999
Wei, X. M., Jiang, S., Li, S. S., Sun, Y. S., Wang, S. H., Liu, W. C., et al. (2021). Endoplasmic reticulum stress-activated PERK-eIF2alpha-ATF4 signaling pathway is involved in the ameliorative effects of Ginseng polysaccharides against cisplatin-induced nephrotoxicity in mice. ACS Omega 6 (13), 8958–8966. doi:10.1021/acsomega.0c06339
Wu, C. Y., Hua, K. F., Hsu, W. H., Suzuki, Y., Chu, L. J., Lee, Y. C., et al. (2020). IgA nephropathy benefits from compound K treatment by inhibiting NF-kappaB/NLRP3 inflammasome and enhancing autophagy and SIRT1. J. Immunol. 205 (1), 202–212. doi:10.4049/jimmunol.1900284
Wu, W. J., Tang, Y. F., Dong, S., and Zhang, J. (2021). Ginsenoside Rb3 alleviates the toxic effect of cisplatin on the kidney during its treatment to oral cancer via TGF-beta-Mediated mitochondrial apoptosis. Evid. Based Complement. Altern. Med. 2021, 6640714. doi:10.1155/2021/6640714
Xing, J. J., Hou, J. G., Ma, Z. N., Wang, Z., Ren, S., Wang, Y. P., et al. (2019). Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 52 (4), e12627. doi:10.1111/cpr.12627
Xu, X. F., Lu, Q. D., Wu, J. Y., Li, Y. X., and Sun, J. Z. (2017). Impact of extended ginsenoside Rb1 on early chronic kidney disease: a randomized, placebo-controlled study. Inflammopharmacology 25 (1), 33–40. doi:10.1007/s10787-016-0296-x
Xu, B. H., Sheng, J., You, Y. K., Huang, X. R., Ma, R. C. W., Wang, Q., et al. (2020). Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism 103, 154013. doi:10.1016/j.metabol.2019.154013
Yang, M., Wang, X., Han, Y., Li, C., Wei, L., Yang, J., et al. (2021). Targeting the NLRP3 inflammasome in diabetic nephropathy. Curr. Med. Chem. 28 (42), 8810–8824. doi:10.2174/0929867328666210705153109
Yokozawa, T., and Liu, Z. W. (2000). The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren. Fail 22 (2), 115–127. doi:10.1081/jdi-100100858
Yokozawa, T., Liu, Z. W., and Dong, E. (1998). A study of ginsenoside-Rd in a renal ischemia-reperfusion model. Nephron 78 (2), 201–206. doi:10.1159/000044911
Yoo, J. Y., Cha, D. R., Kim, B., An, E. J., Lee, S. R., Cha, J. J., et al. (2020). LPS-induced acute kidney injury is mediated by Nox4-SH3YL1. Cell Rep. 33 (3), 108245. doi:10.1016/j.celrep.2020.108245
Yuan, H. H., Yi, Z. Y., Xie, L. L., Bu, L. L., Guo, M. H., and Zheng, X. L. (2025). Ginsenoside Rb1 alleviates atherosclerosis by modulating vascular smooth muscle cell proliferation, foam cell formation, and autophagy. Phytomedicine 149, 157503. doi:10.1016/j.phymed.2025.157503
Zhai, J., Gao, H., Wang, S., Zhang, S., Qu, X., Zhang, Y., et al. (2021). Ginsenoside Rg3 attenuates cisplatin-induced kidney injury through inhibition of apoptosis and autophagy-inhibited NLRP3. J. Biochem. Mol. Toxicol. 35 (11), e22896. doi:10.1002/jbt.22896
Zhang, J. J., Zhou, Y. D., Liu, Y. B., Wang, J. Q., Li, K. K., Gong, X. J., et al. (2021). Protective Effect of 20(R)-Ginsenoside Rg3 Against Cisplatin-Induced Renal Toxicity via PI3K/AKT and NF-[Formula: see text]B Signaling Pathways Based on the Premise of Ensuring Anticancer Effect. Am. J. Chin. Med. 49 (7), 1739–1756. doi:10.1142/S0192415X21500828
Zhang, D., Ji, P., Sun, R., Zhou, H., Huang, L., Kong, L., et al. (2022). Ginsenoside Rg1 attenuates LPS-induced chronic renal injury by inhibiting NOX4-NLRP3 signaling in mice. Biomed. Pharmacother. 150, 112936. doi:10.1016/j.biopha.2022.112936
Zhang, R., Guan, S., Meng, Z., Zhang, D., and Lu, J. (2024). Ginsenoside Rb1 alleviates 3-MCPD-induced renal cell pyroptosis by activating mitophagy. Food Chem. Toxicol. 186, 114522. doi:10.1016/j.fct.2024.114522
Zhang, H., Liang, H., Fan, L., Zhu, X., Ji, P., Su, Y., et al. (2025). Ginsenoside Rg1 attenuates T2DM-induced renal damage and fibrosis by inhibiting TRPC6-ChREBP-TXNIP signaling. J. Ethnopharmacol. 348, 119863. doi:10.1016/j.jep.2025.119863
Zhang, Y. X., Wan, H., Shan, G. Y., Cheng, J. Y., Gao, Z. C., Liu, Y. Y., et al. (2025). Ginsenoside Rg5 modulates the TLR4 and BCL-2 pathways by inhibiting NOX1, thereby alleviating inflammation, apoptosis and pyroptosis in hyperuricemia nephropathy. J. Ginseng Res. 49 (4), 426–437. doi:10.1016/j.jgr.2025.03.009
Zhao, H., Ding, R., and Han, J. (2024). Ginsenoside Rh4 facilitates the sensitivity of renal cell carcinoma to ferroptosis via the NRF2 pathway. Arch. Esp. Urol. 77 (2), 119–128. doi:10.56434/j.arch.esp.urol.20247702.16
Zhao, M., Cao, Y., and Ma, L. (2025). New insights in the treatment of DKD: recent advances and future prospects. BMC Nephrol. 26 (1), 72. doi:10.1186/s12882-025-03953-3
Zhou, P., Zhang, X., Guo, M., Guo, R., Wang, L., Zhang, Z., et al. (2019). Ginsenoside Rb1 ameliorates CKD-associated vascular calcification by inhibiting the Wnt/beta-catenin pathway. J. Cell Mol. Med. 23 (10), 7088–7098. doi:10.1111/jcmm.14611
Zhou, T., Sun, L., Yang, S., Lv, Y., Cao, Y., Gang, X., et al. (2020). 20(S)-Ginsenoside Rg3 protects kidney from diabetic kidney disease via renal inflammation depression in diabetic rats. J. Diabetes Res. 2020, 7152176. doi:10.1155/2020/7152176
Zhou, S., Liu, Y., Liu, Y., Duan, Z., Zhu, C., Wang, P., et al. (2025). Ginsenoside Rk3 alleviates neuroinflammation and gastrointestinal dysfunction in Parkinson's disease via modulation of gut microbiota-mediated butyric acid metabolism. J. Agric. Food Chem. 73 (38), 24139–24154. doi:10.1021/acs.jafc.5c06844
Zhu, Y., Zhu, C., Yang, H., Deng, J., and Fan, D. (2020). Protective effect of ginsenoside Rg5 against kidney injury via inhibition of NLRP3 inflammasome activation and the MAPK signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Pharmacol. Res. 155, 104746. doi:10.1016/j.phrs.2020.104746
Keywords: ginsenosides, common kidney disease, diabetic nephropathy, acute kidney injury, ginseng
Citation: Jin T, Du Y, Liu C, Zhao J and Liu T (2025) Advances in ginsenoside treatment for common kidney diseases: pharmacological evaluation and potential mechanisms. Front. Pharmacol. 16:1702234. doi: 10.3389/fphar.2025.1702234
Received: 09 September 2025; Accepted: 24 November 2025;
Published: 03 December 2025.
Edited by:
Abeda Jamadar, University of Kansas Medical Center, United StatesReviewed by:
Aabid Hussain, Cleveland Clinic, United StatesSwetha Thiyagarajan, Independent Researcher, Sunnyvale, CA, United States
Ayantika Sen Gupta, Stowers Institute for Medical Research, United States
Copyright © 2025 Jin, Du, Liu, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingming Zhao, emptNjcwMThAaG90bWFpbC5jb20=; Tiejun Liu, bGl1dGllanVuNjk5QDE2My5jb20=
 Tonghui Jin
Tonghui Jin Yu Du2
Yu Du2 Tiejun Liu
Tiejun Liu