Abstract
Abelmoschus manihot (L.) Medicus (AM), commonly known as Huangshukui in China, is a traditional medicinal herb. Its flowers serve as the primary active component of Huangkui Capsule (HKC), which has demonstrated therapeutic potential in various conditions such as chronic kidney disease (CKD), inflammatory bowel disease (IBD), ischemic cardiac/cerebral injuries, hepatic injury, and diabetes mellitus. In order to reveal that AM has extensive clinical applications and significant development value, this paper collates the pharmacological effects of AM and the clinical data of traditional Chinese medicine (TCM) formulations containing AM. This review aims to systematically summarize the pharmacological effects and clinical applications of AM, with a focus on its underlying mechanisms—including immunomodulation, antifibrotic activity, metabolic regulation, intestinal flora modulation, organ protection, antioxidant effects, and analgesia. Although most clinical data currently center on HKC, this article also examines other TCM formulations containing AM, such as Jiahua Tablets, Chuangling Liquid, Huangkui Lianchang Decoction, Huangkui Siwu Formula, Yu Kui Qing, Qikui Granules, Huangshukui paste, and Er Huang Ointment. By consolidating current evidence on the pharmacology and clinical use of AM, this review highlights its broad therapeutic potential and promote further research and development of AM-based treatments.
1 Introduction
Abelmoschus manihot (L.) Medicus (AM), known as Huangshukui in Chinese, is a versatile herbaceous plant of the Malvaceae family (Abelmoschus genus) with significant medicinal and edible value (Hong et al., 2021). Its therapeutic importance is well-established both in history and in modern medicine. Ancient Chinese medical books, such as Jiayou Materia Medica and the Compendium of Materia Medica, document AM’s use for treating conditions like carbuncles, toxic swellings, and scalds (Liu et al., 2023; Xue et al., 2023). The 2020 edition of the Pharmacopoeia of the People’s Republic of China (Chinese Pharmacopoeia) formally recognizes the AM flower for clearance of dampness and heat from the body, diminishing swelling, and eliminating toxins (Luan et al., 2020). Beyond China, AM (also known as Aibika or Sunset Muskmallow) is widely used in countries including India, Nepal, Papua New Guinea, Vanuatu, Fiji, and New Caledonia for various medicinal purposes. In these regions, its applications range from using crushed seeds to relieve pain and foot spasms, as well as to manage lactation or menorrhagia, to the practice of applying root juice for sprains (Todarwal et al., 2011; Zhong et al., 2024).
Although the dried flower is the primary part used in medicine, other plant components—such as the seeds, roots, stems, and leaves—are also utilized to address conditions like indigestion, poor appetite, and traumatic injuries (Yin et al., 2021). Phytochemical studies have identified diverse constituents in AM, such as flavonoids, polysaccharides, steroids, volatile oils, and amino acids (Liu et al., 2016; Guo et al., 2021; Xue et al., 2023). Modern pharmacological research validates and expands upon traditional uses, revealing that AM possesses various bioactivities, including antimicrobial, antioxidant, analgesic, and anti-inflammatory properties. These mechanisms contribute to the treatment of various ailments, such as the amelioration of inflammatory bowel disease (IBD), restoration of heart and brain damage, improvement of renal and liver function, and regulation of intestinal flora and glucose/lipid metabolism (Ding et al., 2013; Zhang et al., 2017; Zhu et al., 2018; Luan et al., 2020; Gao et al., 2022; Zhou et al., 2022; Wei et al., 2023; Miao et al., 2024b; Song et al., 2024; Xu et al., 2024; Zhang et al., 2024; Wen and Chen, 2017; Zhou and Chen, 2016).
The most widespread application of AM in clinical practice is Huangkui Capsule (HKC), a single-herb traditional Chinese medicine (TCM) preparation derived from AM, approved for treating chronic kidney disease (CKD) and rheumatoid arthritis by the National Medical Products Administration of China (Luan et al., 2020; Wei et al., 2023). HKC is also commonly prescribed for conditions including primary chronic glomerulonephritis, nephrotic syndrome (NS), diabetic nephropathy (DN), hepatitis B-associated glomerulonephritis, and chronic renal failure (Luan et al., 2020; Wei et al., 2023). Similarly, Huangkui Lianchang decoction (HLD), formulated with AM as the principal ingredient, has shown efficacy in alleviating ulcerative colitis (UC) (Xu et al., 2024). Other AM-containing prescriptions—such as Jiahua Tablets, Chuangling Liquid, Huangkui Siwu Formula, Yu Kui Qing, Qikui Granules, Huangshu Kuihua paste, and Er Huang Ointment—have demonstrated therapeutic effects on CKD, skin ulcers, and chronic inflammatory diseases (Guo and Wang, 2017; Yan et al., 2018; Lu T. et al., 2020). This review synthesizes current reports on the ethnobotany, phytochemistry and their bioactive properties, pharmacological activities, and potential mechanisms of AM and its formulations. It also highlights limitations in existing research and discusses prospects for future applications, underscoring AM’s potential to bridge traditional and modern medicine.
2 Literature search methods
This review used the keywords “A. manihot (L.) Medicus” to search PubMed, Science Direct, Web of Science, Sarcandra, Baidu Scholar, Google Scholar, Connected Papers, Springer Search, and CNKI databases from 1981 to 2024, and 675 pieces of information were collected. This article aligns with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021). The botanical information was obtained through the Flora of China (www.iplant.cn). The inclusion criteria for this review were the availability of relevant studies on botany, traditional uses, pharmacology, and TCM formulas of AM. This article included all published studies in Chinese and English, in vitro and in vivo trials, and clinical studies. Duplicate studies, abstracts or partial/incomplete manuscripts, lack of transparent methods and objectives, and papers on agricultural practices, engineering, and technology development were excluded (Figure 1).
FIGURE 1

Flowchart of study selection for this review.
3 Plant ethnobotany and distribution
Abelmoschus manihot (L.) Medicus (AM) is an annual or perennial robust, erect herb with vigorous growth of aboveground parts and a plant height of 1–2 m (Figure 2A). The root of AM is slightly conical, with many lateral roots (Shao, 2012). AM leaves are palmately divided and have irregular, coarsely toothed margins. The flower is divided into five petals; the periphery is yellowish or pale yellow, and the center is purplish. The flower diameter is 10–20 cm, flowering from bottom to top, and the ovary is 5-chambered (Figure 2B). The plant flowers from August to October and is considered highly ornamental value (Chen et al., 2016). The capsule is oblong, pointed, and hirsute, 5.0–7.5 cm long, and contains about 50 seeds per capsule. At maturity, the seeds are gray-black and kidney-shaped, with many stripes on the surface.
FIGURE 2
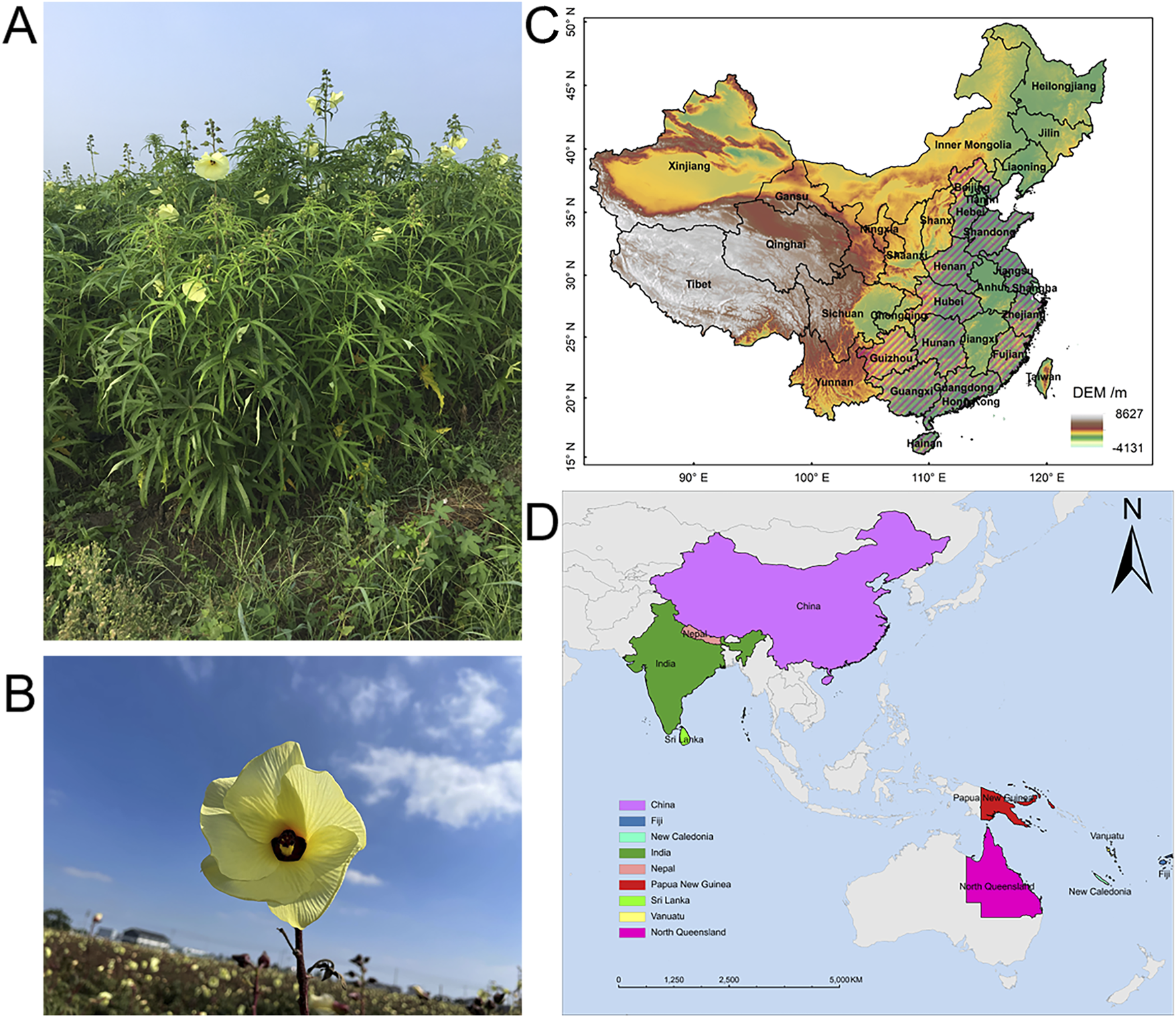
Plant morphology and natural distribution of Abelmoschus manihot (L.) Medicus. (A,B) Photographs of Abelmoschus manihot (L.). Medicus. (C) AM is native to provinces in China including Hebei, Shandong, Henan, Hubei, Hunan, Hainan, Guizhou, Guangxi, Guangdong, Jiangsu, Zhejiang, and Fujian, which are marked with slashes in figure. (D) AM is distributed in China, India, Nepal, Papua New Guinea, Vanuatu, Fiji, New Caledonia, Sri Lanka, and North Queensland.
AM originated in the southern part of China. It prefers to be grown in warm and humid weather with abundant rainfall and adequate sunshine in good drainage and loose, fertile soil. However, it is adaptable and now is widely distributed in tropical and subtropical regions. The natural distribution of AM in China is mainly concentrated in plain areas, such as Hebei, Henan, Shandong, and Fujian (Figure 2C). AM is also distributed in India, Sri Lanka, North Queensland, and other countries (Figure 2D).
4 Phytochemistry and their bioactive properties
Chemical investigations have revealed that different parts of A. manihot (L.) Medicus (AM) contain distinct chemical components. The flowers are primarily rich in flavonoids, while the seeds are abundant in amino acids and unsaturated fatty acids. The roots, stems, and leaves mainly contain polysaccharides (Li Z. et al., 2016). While the chemical composition of AM has been well-studied, research into the pharmacological effects of its main components is still in the early stages.
4.1 Flavonoids
Flavonoids represent the primary chemical constituents and the most significant bioactive components of AM. Forty-eight flavonoids have been isolated and identified from AM flowers. These compounds can be broadly categorized into four groups: 1) Quercetin and its glycoside derivatives, including quercetin, rutin, hyperoside, quercetin-3′-O-β-D-glucoside, quercetin-3-O-β-D-glucoside, quercetin-3-O-β-D-6′-acetylglucopyranoside, quercetin-3-O-β-robinobioside, quercetin-3-O-β-rutinoside, and quercetin-3-O-β-D-xylopyranosyl (1→2)-β-D-galactopyranoside; 2) Gossypetin and its glycoside derivatives, such as gossypetin, hibifolin, and gossypetin-3′-O-β-D-glucoside; 3) Myricetin and its glycoside derivatives, exemplified by myricetin, myricetin-3-O-β-D-glucoside, cannabiscitrin, myricetin-3-O-β-D-galactopyranoside, myricetin-3-O-rutinoside, myricetin-3-O-robinobioside, and myricetin-3-O-β-D-xylopyranosyl-(1→2)-β-D-glucopyranoside; 4) Other flavonoid compounds, such as tiliroside, hibiscetin-3-O-glucoside, floramanoside B, floramanoside C, and 5-hydroxy-4′,7,8-trimethoxyflavone. Based on current literature, Table 1 summarizes the pharmacological effects and associated mechanisms of action for key representative flavonoid constituents of AM, including: hyperoside, quercetin, isoquercitrin, gossypetin-3′-O-β-D-glucoside, quercetin-3′-O-β-D-glucoside, gossypetin-8-O-β-D-glucuronide, myricetin-3-O-β-D-galactopyranoside, and abelmanihotols A-C.
TABLE 1
| Compounds | Compounds type and composition | Content in AM | Pharmacological effect | Mechanism of action | Ref |
|---|---|---|---|---|---|
| Hyperoside | Flavonoids | Hyperoside is ≥0.5% in AM flowers and is required to be ≥1% in HKC | Anti-adipogenic effect Anti-inflammatory effect Anti-fibrotic effect Antioxidant effect Antidepressant effect Neuroprotective effect |
Regulating NMDA receptors in the periaqueductal gray Inhibiting NLRP3 inflammasome Improving the function of vascular endothelium Myocardial SOD↑, oxygen free radicals↓, and improve the lipid metabolism disorder in diabetic mice Regulating the AMPK-ULK1 signaling pathway |
Wang et al. (2022a), Fernando et al. (2024), Gao et al. (2024), Li et al. (2024a), Ogunro and Olasehinde (2024), Wang et al. (2024c), Xia et al. (2024), Gao et al. (2025), Zhao et al. (2025), Zhou et al. (2025) |
| Quercetin | Flavonoids | 72.0 mg in 1000 mg total favone of AM flowers | Anti-inflammatory effect Antioxidant effect Antiplatelet effect Diuresis Glycolipid metabolism improvement |
ROS↓, DDAH II↑ The level of ADMA↓, the content of NO and the ratio of p-eNOS/eNOS↑ Inhibiting the expression of ICAM-1, VCAM-1 and E-selectin to reduce the adhesion between endothelial cells and neutrophils Increase proliferation of EPCs through PI3K/AKT signaling pathway Inhibition of HIF-1a on the NEAT1/HMGB1 signaling pathway Inhibition non-enzymatic glycation and oxidative damage in the kidney of diabetic rats |
Sun et al. (2019), Chen et al. (2020), Riemschneider et al. (2021), Luo et al. (2022), Zhang et al. (2022a), Topcu-Tarladacalisir et al. (2024), Zhao et al. (2024b), Abdou et al. (2025) |
| Isoquercitrin | Flavonoids | 121.2 mg in 1000 mg total favone of AM flowers 0.0116–0.5064 mg in 1000 mg AM stems and leaves |
Antioxidant effect Anti-inflammatory effect Anti-diabetic effect Anti-tumor effect Anti-apoptotic effect Neuroprotective properties |
Inhibition oxidative stress and neuronal apoptosis via Nrf2-mediated NOX4/ROS/NF-κB signaling pathway Regulation the expression of CREB, Bax, Bcl-2, and caspase-3 to alleviate hippocampus neuron apoptosis Activating AMPK pathway and suppressing TGF-β signaling to alleviate hepatic lipid accumulation and oxidative stress suppressing the activation of TLR4, NF-κB and MAPK Mediation of the stimulatory effect on glucose uptake independent of insulin receptor activation through PI3K, MAPK, MEK/ERK pathways and de novo protein synthesis to GLUT-4 translocation Regulating the proliferation and differentiation of preadipocytes Regulation angiogenesis-relevant proteins, such as vasohibin-1 and vasohibin-2 expressions |
Cai et al. (2016), Cao et al. (2017), Wang et al. (2017), Dai et al. (2018), Qin et al. (2018), Jayachandran et al. (2019), Jayachandran et al. (2020), Rey et al. (2020), Silva et al. (2022) |
| Gossypenin -3′-O-β-D-glucoside | Flavonoids | Not detected | Anti-steatotic and anti-fibrotic effects | Glutamic-pyruvic transaminase↓ Glutamic-oxaloacetic transaminase↓ SOD↑ |
Cheng et al. (2010), Chen et al. (2012a) |
| Quercetin-3′-glucoside | Flavonoids | ≈4.5 mg/g in AM flowers and seeds (Yin et al., 2021) | Glycolipid metabolism improvement Anti-depressant activity |
Glucose and lipid metabolism-related factors (PPARγ, C/EBPα, SREBP-1, adiponectin, lactone and resistin) ↑ The utilization of glucose↑ Improve insulin resistance The expression of BDNF↑ |
Cai et al. (2016), Cai et al. (2017b) |
| Gossypolin-8-O-β-glucuronic acid | Flavonoids | 126.8 mg in 1000 mg total favone of AM flowers (Xue et al., 2023) | Glycolipid metabolism improvement Renal tubulointerstitial fibrosis improvement |
Glucose and lipid metabolism-related factors (PPARγ, C/EBPα, SREBP-1, adiponectin, lactone and resistin) ↑ The utilization of glucose↑ Improve insulin resistance Inhibiting NADPH/ROS/ERK signaling pathway |
Cai et al. (2016), Cai et al. (2017a) |
| Gossyptin-8-O-β-D-glucuronide | Flavonoids | Not detected | Anti-depressant activity | The expression of BDNF↑ | Cai et al. (2017b) |
| Myricetin-3-O-β-D-galactopyranoside | Flavonoids | ≈27 μg/g in AM roots, seeds and leaves ≈1.2 mg/g in AM flowers (Yin et al., 2021) |
Antiphotoaging properties Anti-osteoporotic effect |
Repressing MAPK/AP-1 signaling and stimulating the TGFβ/Smad signaling | Oh et al. (2020), Karadeniz et al. (2021) |
| Abelmanihotols A | Flavonoids | Not detected | Anti-inflammatory effect | Inhibiting NLRP3 inflammasome | Su et al. (2022) |
| Abelmanihotols B | Flavonoids | Not detected | Anti-inflammatory effect | Inhibiting NLRP3 inflammasome | Su et al. (2022) |
| Abelmanihotols C | Flavonoids | Not detected | Anti-inflammatory effect | Inhibiting NLRP3 inflammasome | Su et al. (2022) |
| AMPS-a | Polysaccharides (glucose, mannose, galactose, and fucose) | Not detected | Anti-tumor effect | Proliferation of hepatoma cells (SMMC-7721, HepG2) and gastric cancer cells (MGC-803, MKN-45) ↓ | Zheng et al. (2016) |
| KSK-JT | Polysaccharides (arabinose, galactose, glucose, galacturonic acid, rhamnose, and mannose) | Not detected | Immunomodulatory activity | Proliferation of immune cells activates the phagocytosis of macrophages↑ The release of NO↑ |
Liu et al. (2016), Zheng et al. (2016) |
| S-SLAMP-a3 | Polysaccharides (Mannose, rhamnose, glucuronic acid, glucose, galactose, and arabinose) | Not detected | Immunomodulator activity | Lymphocyte proliferation↑ The content of TNF-α and IL-6 in RAW264.7↑ |
Pan et al. (2018) |
| SLAMP-a | Polysaccharides (Mannose, rhamnose, glucuronic acid, glucose, galactose, and arabinose) | Not detected | Immunomodulatory activity | Lymphocyte proliferation↑ The content of TNF-α and IL-6 in RAW264.7↑ |
Pan et al. (2018) |
| SLAMP-c | Polysaccharides (Mannose, rhamnose, glucuronic acid, glucose, galactose, and arabinose) | Not detected | Immunomodulatory activity | Lymphocyte proliferation↑ The content of TNF-α and IL-6 in RAW264.7↑ |
Pan et al. (2018) |
| SLAMP-d | Polysaccharides (Mannose, rhamnose, glucuronic acid, glucose, galactose, and arabinose) | Not detected | Immunomodulatory Activity |
Lymphocyte proliferation↑ The content of TNF-α and IL-6 in RAW264.7↑ |
Pan et al. (2018) |
The pharmacological effects of the active ingredients of AM.
4.2 Polysaccharides
AM flowers, roots, stems, and leaves are rich in soluble polysaccharides. Notably, the total polysaccharide content in the stems can reach as high as 10.86% (Li et al., 2025). Recent studies have confirmed that AM polysaccharides possess skincare, immunomodulatory, and anti-tumor activities (Liu et al., 2016; Pan et al., 2018; Wang J. et al., 2024). Specific polysaccharide fractions—such as AMPS-a, KSK-JT, S-SLAMP-a3, SLAMP-c, and SLAMP-d—contribute significantly to these immunomodulatory and anti-tumor activities (Table 1).
4.3 Other compounds
A total of 22 amino acids and 16 nucleosides have been identified in AM. Among these, the amino acid content is higher in the flowers, reaching 4.737 mg/g, while nucleoside levels in the leaves are relatively lower at 1.474 mg/g (Liu et al., 2016). Leaf composition analysis reveals 1.77% lipids, 2.20% protein, 1.61% ash, and 88.4% moisture. Steroids and triterpenoids have also been detected in AM stems (Wen et al., 2015).
AM seeds are rich in fatty acids, soluble polysaccharides, soluble proteins, amino acids, nucleosides, and mineral elements (Liu et al., 2012; Wen et al., 2015). Total fatty acids constitute 10.22% of seed composition, with unsaturated fatty acids accounting for 78.01%–79.40% of this fraction (Liu et al., 2016). Amino acids are abundant (10.08%–10.15%), and essential amino acids represent 38.42%–39.40% of total free amino acids (Liu et al., 2016). Nucleoside content is relatively low (3.01–3.11 mg/g) (Liu et al., 2016). AM seeds also contain a diverse profile of 24 mineral elements—including K, Ca, Fe, Mn, Cu, Zn, and Mo—with levels of harmful elements (Hg, As, Cd) below food hygiene standards (Wen et al., 2015). Studies report that unsaturated fatty acids from AM seeds reduce serum levels of total cholesterol (TC), triglycerides (TG), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) in hyperlipidemic rats, while increasing high-density lipoprotein (HDL) (Wu, 2011). These findings demonstrate the significant nutritional and medicinal value of AM seeds.
5 Pharmacological activities of AM
Abelmoschus manihot (L.) Medicus (AM), particularly its flowers, exhibits therapeutic potential against various diseases including CKD, IBD, ischemic cardiac/cerebral injuries, hepatic injury, and diabetes mellitus (Figure 3). Advances in research methodologies have enabled systematic investigation into the pharmacological activities of AM. This section summarizes the established pharmacology and underlying mechanisms of AM and HKC (Table 2; Figure 4).
FIGURE 3
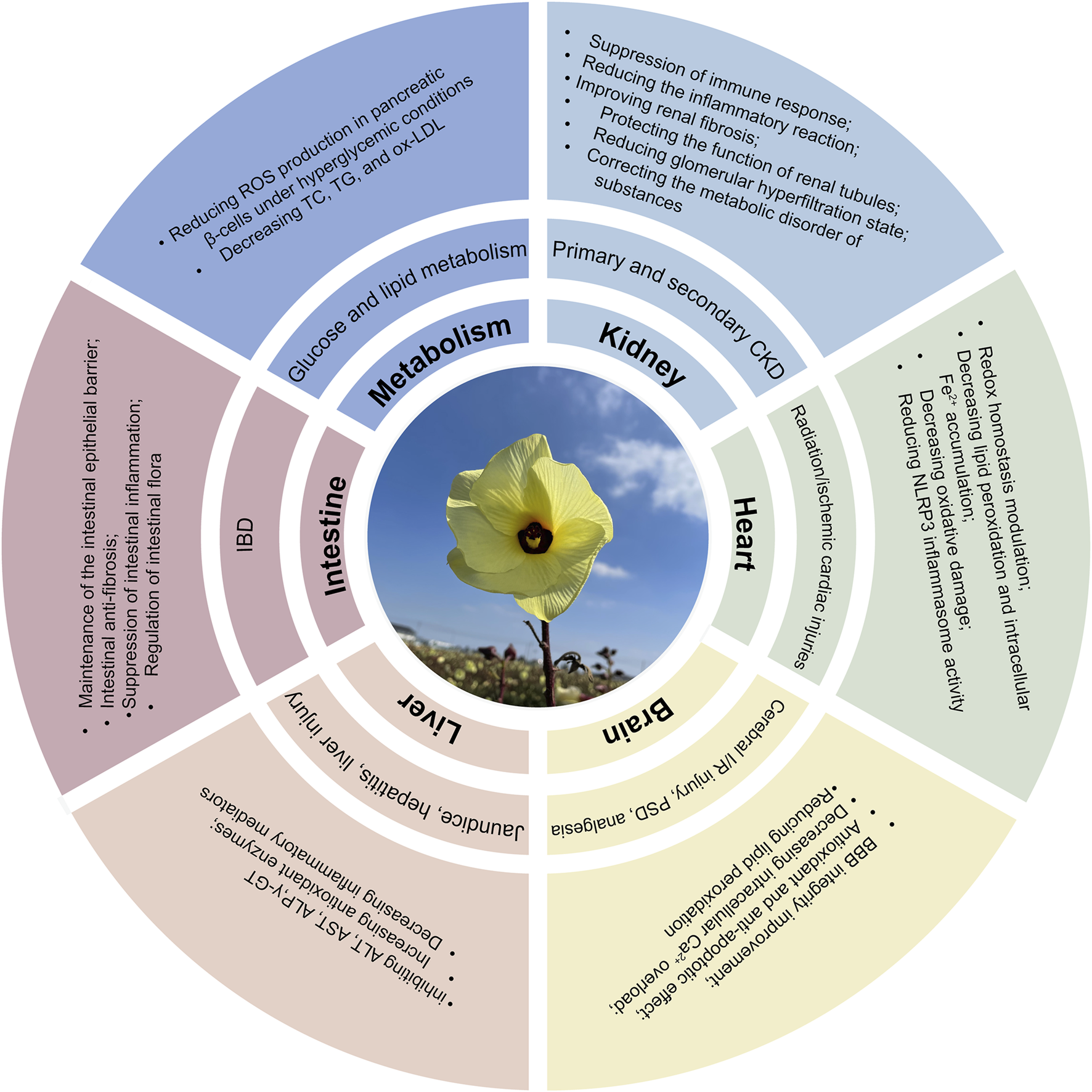
Multifunctional mechanisms of AM in protecting multiple organs and treating diseases.
TABLE 2
| Biological activity | Study type | Extract/Compound | Testing subject | Mechanisms/Effects | Dosage | Ref |
|---|---|---|---|---|---|---|
| Anti-inflammatory effects | In vivo | Flowers; 70% ethanol extract | LPS-induced cystitis in mice | The expression of TLR4, MYD88, IκBα, p-IκBα, NF-κB p65, and p-NF-κB p65↓ | 0.75, 1.5, 3 g/kg, i.g | Zhou et al. (2022) |
| Anti-inflammatory effects | In vivo | AM Seeds | Collagen-induced rat arthritis | The expression of IFN-γ, IL-6, IL-17, IL-1β, and TNF-α↓; the expression of IL-10, IL-4 ↑ the protein Bcl-2/Bax, STAT3, and JAK2 levels↓ the expression of Caspase3, SOCS1, and SOCS3 in the JAK2/STAT3 pathway ↑ |
157.5, 315, 630 mg/kg, i.g | Tao et al. (2024b) |
| Anti-inflammatory effects | In vitro | A mixed neutral polysaccharide (SLAMP-a) and two acidic polysaccharides (SLAMP-c and SLAMP-d) were obtained from stems and leaves of AM. | Spleen lymphocyte proliferation assay in vitro Cytotoxicity assay and nitric oxide assay on RAW264.7 |
S-SLAMP-a3, SLAMP-c and SLAMP-d exhibited significant immunomodulatory activity, while SLAMP-a showed little effects. | 50, 100, and 200 μg/mL in vitro | Pan et al. (2018) |
| Anti-inflammatory effects | In vitro | Abelmanihotols A−C (1–3) from AM seeds | LPS-induced NO release in THP-1 cells | AM blocked the formation of NLRP3 inflammasome formation bysuppressing apoptosis-associated speck-like protein oligomerization, thereby attenuating caspase-1 activation and IL-1β release | 10 μΜin vitro | Su et al. (2022) |
| Anti-inflammatory effects | In vivo, in vitro | Total flavones of AM flowers | Influenza A virus-induced lung inflammation | Inflammatory responses↓ MAPK signaling pathway↓; viral eradiation↑ |
125, 250, 500 mg/kg, i.g 25, 50, 100 μg/mL in vitro |
Gao et al. (2022) |
| Anti-inflammatory and anti-oxidative effects | In vivo, in vitro | HKC | LPS-Induced Acute Lung Injury and Macrophage Activation |
Glutathione peroxidase and catalase activities↑; the expression of miR-451↑ the production of nitric oxide, TNF-α, and IL-6↓ |
150, 300, and 600 mg/kg, i.g 25, 50, 100, 200 μg/mL |
Deng et al. (2021) |
| Anti-inflammatory effects | In vivo | Flowers; 80% ethanol extract | DSS-induced UC | UC signs, symptoms, colon macroscopic lesion scores, and disease activity index (DAI) scores↓; the levels of proinflammatory cytokines (IL-6, IL-1β, IL-18, IL-17, and TNF-α) ↓ The mRNA expression levels of NLRP3, ASC, and caspase 1 in colon tissue ↓; the expression of occludin-1, claudin-1, and ZO-1↑ |
2.05, 4.1, 8.2 g/kg, i.g | Wu et al. (2021) |
| Anti-inflammatory effects | In vivo, in vitro | Total flavones of AM flowers | TNBS-induced Colitis in mice LPS-induced RAW264.7 cells |
The levels of cytokines in the serum↓ MPO activity in the colon tissues↓ NF-κB and MAPK signaling pathways↓ |
125, 250 and 500 mg/kg, i.g 50, 100, 200 μg/mL in vitro |
Zhang et al. (2019a) |
| Anti-inflammatory effects | In vivo, in vitro | Total flavones of AM flowers | the chronic renal failure rat models were induced by uninephrectomy, potassium oxonate, and proinflammatory diet LPS-induced RAW 264.7 cells |
Renal dysfunction and renal tubulointerstitial lesions↓; the content of Bacteroidales and Lactobacillales↓ the content of Erysipelotrichales↑ modulation of macrophage polarization, including markers of M1/M2 macrophages TFA reversed the expression of BECN1 and phosphorylation of p62 protein and LC3 conversion by activating the AMPK-SIRT1 signaling |
136 mg/kg, i.g 20 μg/mL in vitro |
Tu et al. (2020) |
| Anti-inflammatory effects | In vivo, in vitro | Total flavones of AM flowers | DSS-induced colitis TNF-α-induced MAECs |
DAI score, colon shortening, and histological injuries↓; the expression of cytokines (IL-1β and TNF-α) and adhesion molecules (ICAM-1, VCAM-1, and MAdCAM-1) ↓ the phosphorylation and nuclear translocation of NF-κB in MAECs↓ |
30, 60, 120 mg/kg, i.g 10, 50 μg/mL in vitro |
Xue et al. (2023) |
| Anti-inflammatory effects and Regulation of Intestinal Flora | In vivo | Flowers; 75% ethanol extract | DSS-induced colitis in mice | Microbial diversity↑; the abundance of short chain fatty acids (SCFAs)-producing gut microbiota↑ Treg generation↑ and Th17 development↓ |
0.25, 0.5, 1 mg/g, i.g | Zhang et al. (2019b) |
| Regulation of Intestinal Flora | In vivo | HKC | non-obese diabetes mice with DN |
Faecalitalea and Muribaculum ↑ Phyllobacterium, Weissella and Akkermansia ↓ |
0.45 g/kg, i.g | Shi et al. (2023) |
| Regulation of Intestinal Flora | In vivo | Total flavones of AM flowers | DSS-induced colitis | Akkermansia muciniphila (A. muciniphila)↑; colonic inflammatory response and intestinal epithelial barrier dysfunction↓ | 62.5, 125 mg/kg, i.g | Bu et al. (2021) |
| Regulation of Intestinal Flora | In vivo | Total flavones of AM flowers | DSS-induced colitis; chronic stress-induced depression | TFA treatment improved the depression-like phenotype, the disturbed gut microbiota, and the intestinal barrier function in chronic stress mice | 62.5, 125 mg/kg, i.g | Wang et al. (2021) |
| Renal protective effect | In vivo | Total flavones of AM flowers | db/db mice | Urinary albumin-to-creatinine ratio ↓ The expression of slc2a2, slc4a1, slc5a2, slc5a3, slc5a8, slc6a20, slc27a2, slc12a3, slc34a1 and slc38a2↑ |
0.076 g/kg, i.g | Yu et al. (2023a) |
| Renal protective effect | In vivo | HKC | db/db mice | The urinary albumin-to-creatinine ratio↓ The activities of col4a3, slc5a2, slc34a1, slc12a3, and slc4a1↓ |
0.84 g/kg, i.g | Yu et al. (2023b) |
| Renal protective effect | In vivo | HKC | Immunoglobulin A nephropathy rat model | TGF-b1/Smad3 signaling pathway↓ CCL20, CCL22, and CCL27 levels↓ |
2, 5 g/kg, i.g | Pei and Li (2021) |
| Renal protective effect | In vivo | Total flavones of AM flowers | db/db mice | In db/db mice administered with HKC and TFA, 7 flavonoid prototypes and 38 metabolites were identified | HKC (0.84 g/kg) and TFA (0.076 g/kg), i.g | Diao et al. (2024) |
| Renal protective effect | In vitro | Total flavones of AM flowers | Iopromide induced renal tubular cell injury | Iopromide induced renal tubular cell injury and apoptosis ↓ The phosphorylation of AKT↑ |
0.6 mg/mL in vitro | Xu et al. (2022) |
| Renal protective effect | In vivo | Total flavones of AM flowers | Streptozotocin-induced DN | The urinary microalbumin to creatinine ratio and 24-h urinary total protein↓; glomerular cell apoptosis↓ | 200 mg/kg, i.g | Zhou et al. (2012) |
| Renal protective effect | In vivo | Flower or leaf extracts of AM | A DN model by combining unilateral nephrectomy, a high-fat diet, and streptozotocin in C57BL/6 mice | Hepatic injury, proinflammatory cytokines, and lipid accumulation↓; the expression of proteins by regulating autophagy and mitochondrial dynamics↑ | 100 mg/kg, i.g | Kim et al. (2018) |
| Renal protective effect | In vivo, in vitro | Total flavones of AM flowers | a DKD rat model and the NRK-52E cells | TFA improved biochemical parameters, renal tubular injury, and ferroptosis in the DKD rats. TFA inhibited ferroptosis by ameliorating iron deposition, lipid peroxidation capacity, and ferroptosis-related proteins expression in vitro |
136 mg/kg TFA suspension, i.g 50, 100, 150, 200, 250 μg/mL in vitro |
Wang et al. (2023b) |
| Renal protective effect | In vivo, in vitro | Flowers; 75% ethanol extract | Adriamycin-induced NRK-52E cells Adriamycin-induced nephropathy in rats |
TEA ameliorated Adriamycin-induced cellular morphological changes, cell viability, and apoptosis TEA suppressed NLRP3 inflammasomes via inhibition of ERK1/2 signal transduction |
1.5 g/kg, i.g 100 μg/mL in vitro |
Li et al. (2019) |
| Renal protective effect | In vivo, in vitro | Total flavones of AM flowers | DN rats via unilateral nephrectomy and intraperitoneal injection of streptozotocin AGEs-induced HK-2 injury |
IC50 of TFA is 35.6 µM in HK2 and 39.6 µM in HRMC; the activation of iRhom2/TACE signalling↓ the expression of proinflammatory cytokines↓ |
300, 135 and 75 mg/kg, i.g 20 μg/mL in vitro |
Liu et al. (2017) |
| Renal protective effect | In vitro | Total flavones of AM flowers | MPC-5 cells under high glucose (HG) conditions | The protein expression levels of gasdermin D, interleukin-1β, and interleukin-18↓; the protein expression levels of nephrin, ZO-1, WT1 and podocalyxin↑ the protein levels of NIMA-related kinase7, NLRP3, ASC, and caspase-1↓ the protein expression levels of p-PI3K and p-Akt↑ |
5, 10, and 20 μg/mL in vitro | Liu et al. (2021a) |
| Gastroprotective Activity | In vivo | Total flavones of AM flowers | Ethanol-induced gastric ulcer | The activity of SOD and GSH↑; the levels of MDA↓ the levels of Bax, TNF-α, and NF-κB (p65) expressions↓ the Bcl-2 expression level↑ |
300, 600, and 1200 mg/kg, i.g | Zhang et al. (2020) |
| Hepatoprotective effect | In vivo, in vitro | Total flavones of AM flowers | Carbon tetrachloride (CCl4) induced hepatocyte damage in vitro and liver injury in vivo | Levels of ALT, AST and ALP↓; the MDA level ↓ and the content of GSH ↑ in the liver activities of antioxidative enzymes (SOD, GPx, CAT and GST) ↑ the inflammatory mediators (TNF-α, IL-1β and NO) ↓ |
125, 250 and 500 mg/kg, i.g 4.5–72 mg/L in vitro |
Ai et al. (2013) |
| Hepatoprotective effect | In vivo | Total flavones of AM flowers | α-naphthylisothiocyanate-induced cholestatic liver injury in rats | Levels of ALT, AST, LDH, ALP, GGT, TBIL, DBIL and TBA↓; polymorphonuclear neutrophil in-filtration and histological damages↓ | 125, 250 and 500 mg/kg, i.g | Yan et al. (2015) |
| Cardioprotective effect | In vivo | Total flavones of AM flowers | myocardial ischemia/reperfusion in rats | Myocardial infarction area, serum creatinine kinase, LDH levels, serum IL-6, IL-1β and TNF-α production↓; the activities of SOD↑ and the amounts of MDA↓ NLRPR3 inflammasome↓ |
40, 80 mg/kg, i.g | Lv et al. (2017) |
| Neuroprotective actions | In vitro | Total flavones of AM flowers | cultured rat hippocampal neurons | TFA rapidly and reversibly inhibited the INMDA in a concentration-dependent manner TFA non-competitively inhibited the INMDA by enhancement of the NMDA receptor desensitization Intracellular application of TFA did not alter the TFA inhibition of INMDA. |
0.2, 0.8 mg/mL in vitro | Cheng et al. (2006) |
| Neuroprotective Effect | In vitro | Flowers; 95% ethanol extract | H2O2-induced cytotoxicity, oxidative stress and inflammation in PC12 cells | The pro-inflammatory cytokines and mediators (TNF-α, IL-1β, IL-6, COX-2 and iNOS) ↓; the production of nucleotide excision repair (NER)-related proteins↑ | 125, 250, 500 μg/mL in vitro | Wang et al. (2022c) |
| Neuroprotective Effect | In vivo | Total flavones of AM flowers | Cerebral ischemic reperfusion injury in rats | Serum LDH activity and MDA level↓ | 20, 40, 80, 160 mg/kg, i.g | Wen and Chen (2006) |
| Neuroprotective Effect | In vivo | Total flavones of AM flowers | Poststroke Depression Injury in Mice | Escape-directed behavioral impairment induced by PSD MDA levels↓; the activity of SOD, GSH-Px↑ neuronal death/losses ↓ BDNF both at mRNA and protein levels↑ CREB mRNA levels↑ |
40, 80, 160 mg/kg, i.g | Liu et al. (2009) |
| Anticonvulsant, Atidepressant | In vivo | Flowers; 75% ethanol extract | PTZ-induced clonic convulsions and mortality | Immobility time in the FST in mice↓ Fiveparent components including isoquercitrin, hyperoside, hibifolin, quercetin-3-O-glucoside, quercetin and threemetabolites were detected in rat brain |
50, 100, 200 mg/kg, i.g | Guo et al. (2011) |
| Anti-cancer | In vivo | Flowers; 75% ethanol extract | Multiple Myeloma in Mouse Model | Survival rate↑ | 3.75 g/kg, 3 times/week, i.g | Hou et al. (2020) |
| Metabolic Regulation | In vivo | Flowers; 75% ethanol extract | Adriamycin -induced CKD model rats | Deglycosylation and methylation are the major metabolic pathways | 3 g/kg, i.g | Du et al. (2017) |
| Sex hormones Regulation | In vivo | Flowers; 75% ethanol extract | Wild-type adult zebrafish | The expression levels of sex-related genes (lhcgr, ar, cyp19a1a, and cyp19a1b) ↑ The chasing number, fertilized egg production, and hatching rate↑ |
0.2%, 1%, 10% extract diet | Chang et al. (2022) |
| Antioxidant and Anti-Adipogenic Activity | In vitro | Total flavones of AM flowers | DPPH free radical-scavenging assay 3T3-L1 cell line |
IC50 = 0.288 mg/mL The expression of PPARγ and C/EBPα↓ |
25, 50, 100, and 200 μg/mL in vitro | Cai et al. (2016) |
| anti-oxidative effects | In vivo, in vitro | Ethyl acetate fraction of AM flowers | H2O2-induced HepG2 cells and D-galactose-induced aging mice | Viability of H2O2-induced HepG2 cells↑; the ROS level, apoptotic cells, and activities of caspase 3/9↓ the levels of SOD and GSH-Px ↑ MDA generation and LDH release↓ |
25, 50, 100 mg/kg, i.g 15.6–1000 μg/mL in vitro |
Liu et al. (2021b) |
| anti-oxidative effects | In vivo | Total flavones of AM flowers | D-galactose-induced oxidative stress in mouse liver | Antioxidant enzymes (CAT, GPx, SOD, and T-AOC) ↑ MDA production↓; expression of Nrf2 and its target antioxidants (HO-1 and NQO1)↑ |
40, 80, and 160 mg/kg, i.g | Qiu et al. (2017) |
| Anti-cancer effect | In vitro | AMPS-a (from the ethanol-extracted debris of AM flowers) | Hepatic (SMMC-7721, HepG2) and gastric (MGC-803, MKN-45) cancer cells | AMPS-a exhibited potent inhibitory effects on the proliferation of hepatic and gastric cancer cells | 25, 50, 100, 200, 400 μg/mL in vitro | Zheng et al. (2016) |
| Bone loss improvement | In vivo | leaves of AM | Osteopenia induced by ovariectomy in rats | Bone mineral density↑; bone mineral content↑ | 10% (2.2 g/day/rat; 10% leaves) or 15% leaves (3.3 g/day/rat; 15% leaves) of AM | Puel et al. (2005) |
| Anti-fibrosis effect | In vivo | Total flavones of AM flowers | (TNBS)-induced chronic colonic inflammation | Body weight loss, colon length shortening, the morphological damage index score, and inflammatory response↓; the colonic expression of col1a2, col3a2, and hydroxyproline↓ α-SMA, TGF-β, vimentin, TIMP-1 expression↓ |
250 mg/kg, i.g | Qiao et al. (2021) |
| Pro-angiogenic ability | In vivo, in vitro | Total flavones of AM flowers | HUVECs in vitro; chick chorioallantoic membrane (CAM) in vivo | TFA promoted HUVECs proliferation TFA increased HUVECs migratory ability and the number of tubular structure, promoted vessel formation in HUVECs culture and CAM model; the expression of VEGF and KDR↑ |
5, 10, 20 μg/mL in vitro | Tang et al. (2017) |
| Pro-angiogenic ability | In vitro | Total flavones of AM flowers | HUVEC | Cell viability, wounding healing, transwell invasion, tube formation↑ PI3K and Akt phosphorylation↑ VEGF-A and VEGFR2 ex pression↑ |
5, 10, and 20 μg/mL in vitro | Zhu et al. (2018) |
Summary of the pharmacological activities of AM.
FIGURE 4
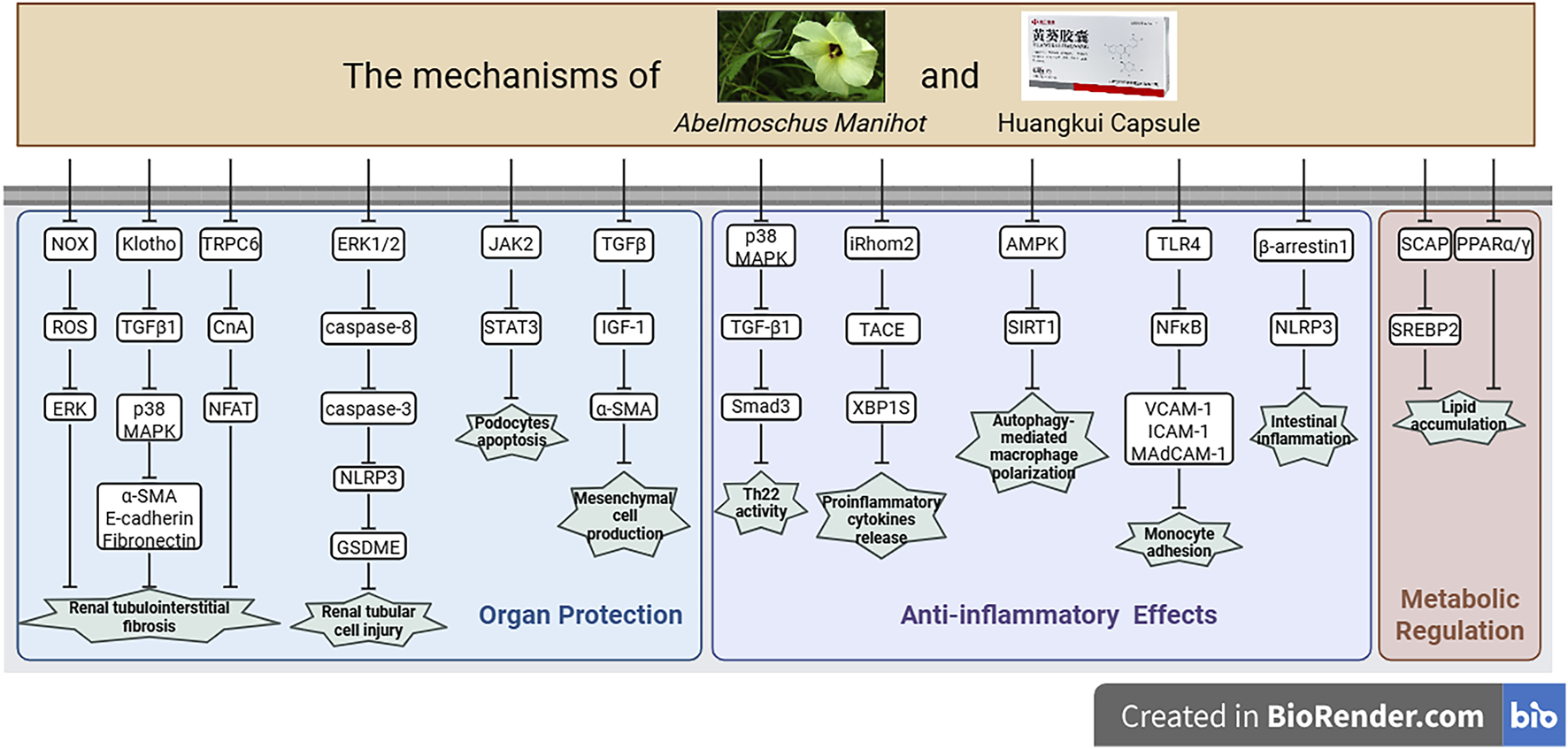
Potential mechanisms of AM or HKC in disease treatment, created with BioRender.com. This figure summarizes the multi-target mechanisms of AM in organ protection, anti-inflammatory effects, and metabolic regulation. The mechanisms include: organ protection via inhibiting renal tubulointerstitial fibrosis by regulating the NOX/ROS/ERK pathway (e.g., α-SMA, E-cadherin), reducing podocyte apoptosis through JAK2/STAT3, suppressing mesangial cell generation via TGFβ/IGF-1, and alleviating renal tubular cell injury through the ERK1/2/caspase pathway and NLRP3/GSDME; anti-inflammatory effects by suppressing inflammatory activity through p38 MAPK/TGFβ/Smad3, decreasing pro-inflammatory cytokine release via iRhom2/TACE/XBP1S, regulating macrophage polarization via AMPK/SIRT1, and inhibiting monocyte adhesion through TLR4/NFκB and β-arrestin1/NLRP3; as well as metabolic regulation through ameliorating lipid accumulation via the SCAP/SREBP2 and PPARα/γ pathways.
5.1 Anti-inflammatory and immunomodulatory effects
Inflammation accelerates organ injury through multiple mechanisms, while targeted anti-inflammatory intervention can significantly delay the progression of the disease associated with the inflammation. Current evidence demonstrates that AM downregulates pivotal inflammatory mediators—including M1 macrophage-derived cytokines (IL-1β, IL-12, IL-6, MMP-12), chemokines (MCP-1), and adhesion molecules (VCAM-1)—in diabetic nephropathy (DN) models (Ge et al., 2016; Gu et al., 2020). This anti-inflammatory action appears central to AM’s therapeutic mechanism, with additional research revealing its ability to modulate ER stress through iRhom2/tumor necrosis factor-α converting enzyme (TACE)/Spliced X-box binding protein-1 (XBP1S) pathways and regulate autophagy via adenosine monophosphate-activated protein kinase (AMPK)- sirtuin 1 (SIRT1) signaling (Liu et al., 2017; Tu et al., 2020). Parallel mechanistic studies reveal that HKC attenuates inflammatory cell activation and infiltration via p38 mitogen-activated protein kinase (MAPK) pathway inhibition, thereby reducing transforming growth factor beta 1 (TGF-β1) expression in nephropathy models (Chen P. et al., 2012). Current evidence establishes AM and HKC as renal protective agents targeting pathogenic T-lymphocyte subsets including Th22 cells, with single-cell RNA sequencing revealing critical renal receptor networks mediating HKC’s suppression of T-cell activation and infiltration (Wu et al., 2024). Simultaneously, these interventions modulate TGF-β1/Smad family member 3 (Smad3) signaling to reduce immune complex deposition in IgA nephropathy (IgAN) and mesangial proliferative glomerulonephritis (MsPGN) (Pei and Li, 2021; Wu et al., 2024), while enhancing erythrocyte-mediated clearance via redistribution of circulating complexes to erythrocyte surfaces—a mechanism shown to limit renal deposition (Hu et al., 2011). These multimodal actions collectively substantiate HKC’s clinical efficacy in IgAN through integrated immunoregulation (Pei and Li, 2021).
In addition, intestinal inflammation drives IBD progression by mediating tissue damage, fibrosis, and carcinogenesis. In 2,4,6-trinitrobenzenesulfonic acid solution (TNBS)/dextran sodium sulfate (DSS)-induced colitis models, AM ethanol extract attenuates pro-inflammatory cytokines (IL-6, IL-1β, IL-18, IL-17, TNF-α) and reduced myeloperoxidase activity in serum and colonic tissues (Cheng et al., 2015; Xue et al., 2023). Mechanistically, AM suppresses Nod-like receptor protein 3 (NLRP3) inflammasome activation via β-arrestin1 inhibition—evidenced by reducing NLRP3/apoptosis-associated speck-like protein containing caspases recruitment domain (ASC)/caspase-1 expression (Wu et al., 2021). Immune modulation studies revealed AM’s capacity to normalize T helper 17 cell (Th17)/regulatory T cell (Treg) balance through peroxisome proliferator-activated receptor gamma (PPAR-γ)-mediated enhancement of Treg cytokines (IL-10, TGF-β) with concurrent Th17 marker suppression (Qiao et al., 2021). In vitro studies confirmed AM’s inhibition of nuclear factor kappa B (NF-κB) and MAPK signaling in lipopolysaccharide (LPS)-stimulated macrophages (Zhang D. et al., 2019). Immune cell infiltration is critical in initiating and propagating the immune response against pathogens during the progression of UC (Neurath, 2014). This process is tightly regulated by adhesion molecules that mediate interactions between vascular endothelial cells and immune cells (Besendorf et al., 2022). Recent work identified the total flavonoids of AM (TFA) suppression of tumor necrosis factor-alpha (TNF-α)-induced NF-κB activation in colonic endothelial cells, thereby reducing vascular adhesion molecule expression (ICAM-1, VCAM-1, MAdCAM-1) and monocyte recruitment (Xue et al., 2023).
5.2 Anti-fibrotic effect
When tissues are damaged (such as due to infection, toxins, ischemia, mechanical injury, or chronic inflammation), the body activates its repair mechanism. The core of fibrosis lies in the activation and proliferation of fibroblasts. By differentiating into myofibroblasts with strong contractile and synthetic capabilities, they produce and secrete extracellular matrix components, thereby isolating the damaged area and preventing the spread of inflammation (Wang et al., 2025). However, as the injury stimulus persists or the repair process becomes imbalanced, the fibrotic response will proceed excessively, persistently and eventually lead to the aggravation of the disease state.
Renal fibrosis, representing the terminal pathological endpoint in CKD, is driven primarily by myofibroblast activation triggered by inflammatory mediators (TNF-α, IL-1β), metabolic disturbances (high glucose/lipid-induced reactive oxygen species (ROS)), mechanical stress, or hypoxia. These insults activate key signaling pathways (such as TGF-β/Smad3) to induce phenotypic transformation. In DN models, HKC suppresses TGF-β1 expression and reduces α-SMA levels (Gu et al., 2021). Furthermore, in unilateral ureteral obstruction models, HKC targets calcium-permeable TRPC channels, specifically modulating TRPC6/NFAT signaling. This mechanism is critical, as the protective effect of HKC is abolished in TRPC6-knockout mice (Gu et al., 2020). The protective role of HKC in renal disease is primarily mediated through the critical TRPC6/NFAT pathway, as complemented by its suppression of the TGF-β1/α-SMA axis. In addition, mechanistic studies reveal HKC’s capacity to inhibit fibrogenesis through coordinated p38 MAPK/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) pathway downregulation (Mao et al., 2015), NLRP3 inflammasome suppression with toll-like receptor 4 (TLR4)/NF-κB blockade to prevent epithelial-mesenchymal transition (Han et al., 2019), NOX/ROS/ERK axis modulation (Li et al., 2019), and angiogenic receptor (VEGFR, PDGFR) inhibition coupled with pericyte myofibroblast transdifferentiation suppression (Wang M. et al., 2022). These actions are further complemented by connective tissue growth factor/osteopontin reduction via Smad7/SnoN upregulation and Klotho/TGF-β1/p38 pathway normalization in renal tubular cells (Gu et al., 2021). These studies collectively demonstrate that HKC orchestrates a coordinated suppression of critical pro-fibrotic pathways—including p38 MAPK/PI3K/Akt, TLR4/NF-κB/NLRP3, and NOX/ROS/ERK—thereby counteracting fibrogenesis at multiple levels.
Intestinal fibrosis—a pivotal complication in IBD progression with over 50% incidence in Crohn’s disease—drives intestinal stenosis, obstruction, and surgical intervention through excessive ECM deposition and pathological myofibroblast activation. AM counteracts this remodeling by reducing TNBS-induced weight loss, disease activity index (DAI) scores, and collagen deposition while improving histopathological features (Zhang et al., 2017). Mechanistically, AM suppresses fibrotic markers (col1a2, col3a2, α-SMA) via dual inhibition of TGF-β and IGF-1 signaling (Qiao et al., 2021; Zhang et al., 2024), remodels ECM through altered MMP/TIMP balance (elevated MMP-9/MMP-2 with suppressed TIMP-1), and attenuates TGF-β1-induced epithelial-mesenchymal transition (EMT) by blocking Smad2/3 phosphorylation and MAPK cascades (Yang et al., 2018). In vitro evidence confirms AMPK/mTOR-regulated autophagy mediates AM’s inhibition of IGF-1-driven collagen synthesis in intestinal fibroblasts (Zhang H. et al., 2022), establishing a multi-target therapeutic profile against fibrogenesis.
5.3 Metabolic and anti-oxidant regulation
The ethanol extract showed superior efficacy and reduced hyperglycemia-induced ROS in pancreatic β-cells (Wang S. W. et al., 2024). AM total flavonoids, TFA, enhanced glucose metabolism through dose-dependent promotion of glucose uptake in 3T3-L1 adipocytes and exerted hypoglycemic effects in diabetic murine models. TFA administration elevated superoxide dismutase (SOD) activity while reducing malondialdehyde (MDA) levels in diabetic rats, suggesting β-cell protection against oxidative damage (Zhou and Chen, 2016).
The ethanol extract of AM inhibited TG accumulation in 3T3-L1 adipocytes and lowered serum TC, TG, low-density lipoprotein cholesterol (LDL-C), and ox-LDL levels in hyperlipidemic rats, achieved through downregulation of PPARγ and CCAAT/enhancer-binding protein alpha (C/EBPα) mRNA to inhibit adipogenesis (An et al., 2011; Li J. et al., 2016). At the molecular level, AM ethanol extract orchestrates lipid homeostasis by normalizing adipogenic regulators (PPARα/γ, C/EBPα, SREBP-1), correcting adipokine imbalances (adiponectin/resistin), and alleviating ER stress-induced lipid dysmetabolism. Synergistically, TFA combined with glipizide reduced plasma lipids, blood urea nitrogen (BUN), creatinine, urinary microalbumin, and blood viscosity in DN rats (Li et al., 2018). Metabolic profiling reveals HKC’s systemic modulation of circulating metabolites including methionine sulfoxide, branched-chain amino acids, and cis-7-hexadecenoic acid (Shi et al., 2023). Complementary renal protection involves improved sodium handling via inhibition of renal medullary Na+-K+-ATPase activity, reducing pathological edema and lipid deposition in diabetic kidneys (Cai et al., 2016; Li et al., 2018; Qian et al., 2023).
AM also has a significant antioxidant effect. AM accelerates scald wound healing by modulating MDA/SOD via nuclear factor erythroid 2-related factor 2 (Nrf2) (Song et al., 2024), protects NIH-3T3 fibroblasts from H2O2 damage through the same pathway, and alleviates gastric mucosal injury by boosting glutathione synthesis (Hsuan et al., 2024). Furthermore, AM flower extracts enhance human fibroblast proliferation through cyclin D1 upregulation (Park et al., 2020) and HKC reduces ROS/NO in LPS-activated macrophages and increases lung catalase (CAT)/glutathione peroxidase (GSH-Px) activity in acute lung injury models (Deng et al., 2021). Several studies have revealed that AM exhibits antioxidant activity through scavenging DPPH/ABTS/OH radicals while enhancing antioxidant enzymes (GSH-Px, CAT, SOD, T-AOC) and reducing MDA levels (Qiu et al., 2017; Liu et al., 2021c; Sun et al., 2024). Its total flavonoid fraction TFA activates Nrf2 signaling to upregulate heme oxygenase-1 (HO-1)/NAD(P)H dehydrogenase quinone 1 (NQO1), mitigating D-galactose-induced hepatocyte cytotoxicity (Qiu et al., 2017).
5.4 Regulation of intestinal flora
The intestinal microbiota’s pivotal role in IBD pathogenesis is increasingly recognized, with microbial dysbiosis driving immune activation through commensal flora infiltration into lamina propria. Clinical observations reveal reduced microbial diversity in IBD patients, where therapeutic strategies like probiotics and fecal microbiota transplantation (FMT) demonstrate clinical efficacy by restoring ecological balance (Weingarden and Vaughn, 2017; Tao P. et al., 2024; Zhao H. et al., 2024). In DSS-induced colitis models, AM intervention reverses Firmicutes depletion and Bacteroidetes expansion while enriching beneficial taxa (Akkermansia muciniphila, Lachnospiraceae, Bifidobacterium) and suppressing pathogens (Escherichia coli, Proteobacteria) (Zhang W. et al., 2019; Bu et al., 2021). Cohousing experiments demonstrate AM-treated mice attenuate colitis severity in cohabited counterparts (Gao et al., 2022), with fecal microbiota transplantation confirming microbiota-dependent protection via increased Bacteroides and Ruminiclostridium [sp. cluster 9] in UC recipients (Wu et al., 2021). Notably, AM selectively enhances butyrate-producing Lachnospiraceae to boost short-chain fatty acid (SCFA) production—critical for mucosal integrity and inflammation suppression (Zhang W. et al., 2019) —and directly stimulates A. muciniphila proliferation in vitro (Bu et al., 2021). In addition, AM polysaccharides enhance mucin production via IL-10-dependent pathways, with bacterial transfer studies confirming A. muciniphila as a key mediator of this protective effect (Wang Y. et al., 2024), establishing microbial-metabolic modulation as a viable barrier rehabilitation strategy.
5.5 Organ protection
Cerebral ischemia, characterized by insufficient cerebral blood flow leading to oxygen/glucose deficiency, underlies pathologies like cerebral infarction and vascular dementia with high disability/recurrence rates (Chen et al., 2025). AM administration mitigates cerebral edema, attenuates infarct volume, and improves blood-brain barrier integrity while reducing serum biomarkers (LDH, MDA, and PGE2) of neuronal damage in cerebral ischemia-reperfusion injury model (Guo and Chen, 2002; Gao et al., 2003; Wen and Chen, 2006). AM further enhances hippocampal neuron survival by inhibiting intracellular Ca2+ overload and lactate dehydrogenase (LDH) release during hypoxia/reoxygenation, while its total flavonoids (TFA) suppress N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity via non-competitive receptor desensitization—validated by patch-clamp electrophysiology (Cheng et al., 2006; Wen and Chen, 2017). In post-stroke depression (PSD) models, TFA enhances locomotor activity and improves hemorheological parameters by reducing blood viscosity while increasing erythrocyte deformability. Concurrently, it elevates SOD/GSH-Px activities and reduces levels of MDA, adrenocorticotropic hormone (ACTH), and cortisol in both brain tissue and the hypothalamus (Hao et al., 2007; Hao and Zhou, 2009). These effects are achieved by TFA’s suppression of lipid peroxidation and upregulation of brain-derived neurotrophic factor (BDNF)/cAMP response element-binding protein (CREB) neurotrophic pathways (Liu et al., 2009). In neuronal oxidative stress models, AM ethanol extract directly protects neuronal PC12 cells against oxidative stress through antioxidant enzyme induction, glutathione (GSH) synthesis promotion, DNA repair enhancement, and ROS reduction (Wang S. W. et al., 2022). Critically, AM modulates the gut-brain axis to alleviate depression-associated intestinal barrier damage and microbial dysbiosis, concurrently ameliorated depressive behaviors and DSS-induced colitis (Wang et al., 2021).
AM, traditionally used in Jiangsu and Anhui provinces for jaundice and hepatitis management, exerts hepatoprotection primarily through its total flavonoid component (Liu et al., 2010). In CCl4-induced liver injury models, TFA significantly reduces serum markers (ALT, AST, ALP, γ-GT) and hepatic MDA while elevating GSH content, concurrently enhancing antioxidant enzyme activities (SOD, GSH-Px, CAT, GST) and suppressing inflammatory mediators (TNF-α, IL-1β, NO) (Hu et al., 2011; Ai et al., 2013). TFA’s dose-dependent cytoprotection in CCl4-exposed hepatocytes, evidenced by improved cell viability and reduced ALT/AST/ALP leakage (Ai et al., 2013). In cholestatic models, TFA upregulates bile transporters (BSEP, MRP2, NTCP) at protein/mRNA levels (Yan et al., 2015). In D-galactose-induced aging models, TFA activates the Nrf2 pathway to boost antioxidant capacity (CAT, T-SOD, GSH-Px, T-AOC) and inhibit peroxidation (Qiu et al., 2017). In vitro analyses confirm AM’s ethyl acetate fraction protects HepG2 cells from H2O2-induced damage through ROS scavenging, membrane stabilization, and apoptosis modulation via Nrf2/HO-1/NQO1 pathway activation and caspase-3/9/Bax regulation (Liu et al., 2021c).
5.6 Antiviral, antibacterial, and ulcer repair
AM and its total flavonoid component exhibit broad-spectrum antimicrobial activity against viral and bacterial pathogens. TFA potently inhibits herpes simplex virus types 1/2 (HSV-1 IC50 = 1.01 mg/L; HSV-2 IC50 = 1.21 mg/L) with high therapeutic indices (>10), indicating selective antiviral action (Jiang et al., 2006). Gao et al. found that AM ethanol extract reduces influenza A viral loads and suppresses pulmonary IL-1β/IL-6/TNF-α via MAPK pathway modulation in murine models (Gao et al., 2022). Against bacterial pathogens, AM demonstrates efficacy against Gram-positive/negative species, particularly Neisseria gonorrhoeae, with TFA exhibiting bactericidal activity against Staphylococcus epidermidis and Staphylococcus aureus (MIC = 3.125 g/L) and fungistatic effects on Candida albicans (MIC = 1.562–3.125 g/L) (Zhang et al., 2006). These properties translate to therapeutic efficacy in mucosal infections: TFA accelerates healing of C. albicans- or S. epidermidis-induced oral ulcers in guinea pigs, and AM flower extract reduces inflammation in S. aureus-infected rabbit oral mucosa (Li et al., 2006; Zhang et al., 2006).
5.7 Antipyretic and analgesic effects
AM and its total flavonoid component exhibit antipyretic and analgesic properties through distinct biological pathways. In rabbit fever models induced by turpentine oil or E. coli, TFA reduced body temperature within 150 minutes—albeit with slower onset than aspirin due to delayed systemic absorption and hepatic metabolism (Fan et al., 2003b). For analgesia, TFA (5–20 mg/kg) significantly inhibited acetic acid-induced writhing responses (42.81%–57.53% reduction) and attenuated potassium chloride-evoked nociception without inducing addictive behaviors even at 280 mg/kg (Fan et al., 2003a). TFA significantly reduced pain responses in rabbits following potassium chloride injections (Fan et al., 2003a). Petroleum ether and methanol extracts of AM also exhibited a dose-dependent inhibition on thermopain (Jain et al., 2011), and AM extracts alleviate burn-induced inflammatory exudation and elevate pain thresholds in scalded models (Song et al., 2024).
6 Traditional Chinese medicine formulas containing AM
Currently, the only drug related to A. manihot (L.) Medicus (AM) approved for production by the National Medical Products Administration is HKC. Given that there have been a large number of systematic reviews and meta-analyses on the efficacy and safety of HKC (Li et al., 2017; An et al., 2021; Li Y. et al., 2024), we have sorted out the clinical application status of other TCM compounds containing AM (such as Jiahua Tablets, Chuangling Liquid, Yu Kui Qing, etc.), as shown in Table 3. In addition, this section summarizes the traditional prescriptions that contain AM, along with their pharmacological effects (Table 4).
TABLE 3
| Prescription name | Composition and proportion | Source of the prescription | Clinical application | Ref |
|---|---|---|---|---|
| Huangkui Capusle | Abelmoschus manihot (Huang Shu Kui) | Jiangsu Provincial Hospital of Chinese Medicine | Primary/secondary CKD | Zhang et al. (2014), An et al. (2021) |
| Jiahua Tablets (甲花片) | Abelmoschus manihot (Huang Shu Kui) | Jiangsu Provincial Hospital of Chinese Medicine | Acute and chronic nephritis | Lin et al. (2020a), Wu et al. (2020), Zha et al. (2020) |
| Chuangling Liquid (疮灵液) | Abelmoschus manihot (Huang Shu Kui), Rheum palmatum (Da Huang), Carthamus tinctorius (Hong Hua), and Terminalia chebula (He Zi), and the composition ratio is 1:4:2:2 | Jiangsu Provincial Hospital of Traditional Chinese Medicine | Various types of infectious ulcers with significant inflammation | Chen et al., 2017; Pei (2018), Wang et al. (2020) |
| Huangkui Lianchang Decoction (黄葵敛肠汤) | Abelmoschus manihot (Huang Shu Kui), Euphorbia humifusa Willd (Di Jin Cao), Pteris multifida Poir (Feng Wei Cao), Lithospermum erythrorhizon Siebold & Zucc (Zi Cao), Rubia cordifolia L. (Qian Cao), and Rhus chinensis Mill (Wu Bei Zi), and the composition ratio is 6:6:6:3:3:1 | Jiangsu Provincial Hospital of Traditional Chinese Medicine | UC | He et al. (2023), Yang et al. (2023) |
| Huangkui Siwu Formula (黄葵四物方) | Abelmoschus manihot (Huang Shu Kui Hua), Astragalus mongholicus (Huang Qi), Polygonum cuspidatum (Hu Zhang), and Curcuma longa L (Jiang Huang), and the composition ratio is 3.5:5:1.5:1 | Nanjing University of Chinese Medicine | CKD | Lu et al. (2020a), Lu et al. (2020b), Lu et al. (2021) |
| Yu Kui Qing (玉葵清) | Abelmoschus manihot (Huang Shu Kui), Astragalus membranaceus (Huang Qi), and processed Polygonum multiflorum Thunb. (Zhi Shou Wu), and the composition ratio is 30:1.5:1 | Not found | Type 2 DN | Chen and Yu (2008), 2009 |
| Qikui Granules (芪葵颗粒) | Astragalus membranaceus (Huang Qi), Abelmoschus manihot (Huang Shu Kui Hua), and processed Polygonum multiflorum (Zhi Shou Wu), and the composition ratio is 3:3:1 | Jiangsu Provincial Hospital of Traditional Chinese Medicine | Early to mid-stage DN | Zhang et al. (2021), Wang et al. (2023a) |
| Huangshu Kuihua paste (黄蜀葵花贴剂) | Abelmoschus manihot (Huang Shu Kui) | the First Affiliated Hospital of Anhui Medical University | Managing pain associated with oral ulcers | Han et al. (1997) |
| Er Huang Ointment (二黄油膏) | Abelmoschus manihot (Huang Shu Kui Hua), Scutellaria baicalensis (Huang qin), Phellodendron amurense (Huang bai), Gardenia jasminoides (Zhi zi), and Ampelopsis japonica (Bai lian), and the composition ratio is 5:5:5:3:3 | Not found | Managing burns and enhancing wound healing | Yang, 2016; Guo and Wang (2017) |
A summary of traditional Chinese medicine compound prescriptions containing AM.
TABLE 4
| Prescription | Application | Intervention | Subject size | Duration | Outcomes | Conclusion | Ref | |
|---|---|---|---|---|---|---|---|---|
| Control group | Treatment group | |||||||
| Jiahua Tablets | Contrast agent-induced nephropathy | Routine treatment: provided, includingSacubitril/Valsartan (100mg, oral, twice daily), Clopidogrel (75mg, oral, once daily), Atorvastatin (20mg, oral, once daily) | Routine treatment + Jiahua Tablets (4 tablets, PO tid) 3 days preoperatively for 1 week | Control group: 30 cases; Observation group: 30 cases | Preoperative 24h, postoperative 48 h | KIM-1, MCP-1, BUN, SCr, eGFR levels | Jiahua tablet has a certain preventive and treating effect on contrast agent-induced renal injury,we can apply Jiahua tablets during the perioperative period of coronary angiography | Niu et al. (2023) |
| Jiahua Tablets | Contrast agent-induced nephropathy | Standard treatment (antihypertensives, statins, aspirin) + coronary angiography/PCI; nephrotoxic drugs discontinued 24–48 h prior | Standard treatment + Jiahua Tablets (4 tablets, PO tid) 72 h preoperatively to 72 h postoperatively | Control group: 40 cases; Observation group: 40 cases | Preoperative 24h, postoperative 72 h | SCr, BUN, eGFR, hs-CRP, Cys C, urine NAG/GAL | Jiahua tablet can prevent and treat contrast-induced nephropathy for patients with the perioperative period of coronary intervention | Lin et al. (2020a) |
| Jiahua Tablets | Postoperative AKI in the patients with PCI | Iodixanol as contrast agent; standard coronary angiography/PCI. | Control treatment + Jiahua Tablets (4 tablets, tid) | Control group: 40 cases; Observation group: 40 cases | Preoperative 72h, postoperative 72 h | Serum SCr, BUN, eGFR, IL-6, IL-8 | Jiahua Tablets can be used for the patients with PCI, which can protect and repair AKI by inhibiting the inflammatory response | Lin et al. (2020b) |
| Jiahua Tablets | Early-stage DN | Diabetic diet + antidiabetic/antihypertensive drugs (ACEIs/ARBs avoided) | Control treatment + Jiahua Tablets (1.8g, tid) | Control group: 30 cases; Observation group: 30 cases | 3 months | Serum CRP, TNF-α, Cys C; urinary albumin-to-creatinine ratio (UACR) | The use of Jiahua Tablets based on conventional therapy can effectively reduce serum inflammatory factors levels and improve renal function in patients with diabetic nephropathy | Zha et al. (2020) |
| Jiahua Tablets | Contrast agent-induced nephropathy | Standard treatment (antihypertensives, statins, aspirin) + coronary angiography/PCI; nephrotoxic drugs discontinued 24–48 h prior | Control treatment + Jiahua Tablets (4 tablets, tid) 72 h preoperatively to 72 h postoperatively for 1 week | Control group: 40 cases; Observation group: 40 cases | Preoperative 72h, postoperative 72 h | SCr, BUN, eGFR, NAG, GAL. | Jiahua tablets can improve the expression of renal injury indexes such as Scr, BUN, NAG and GAL, and exert a better renal protective effect, thereby achieving the purpose of preventing and treating contrast-induced nephropathy | Wu et al. (2020) |
| Chuangling Liquid | Oral lip ulcer caused by endotracheal intubation | No specific intervention. was implemented | Secure endotracheal tube, clear secretions, clean ulcer with saline; Chuangling Liquid-soaked gauze dressing, bid | 20 cases | 3–7 days | Wound area, healing rate, healing time | Chuangling Liquid has a good therapeutic effect on lip pressure sores in patients with oral tracheal intubation | Sheng (2014) |
| Chuangling Liquid | Postoperative wound healing after anal fistula surgery | Debridement + Huangqin Ointment-soaked gauze packing | Debridement + Chuangling Liquid-soaked gauze packing | Huangqin Ointment group: 30 cases; Chuangling Liquid group: 30 cases | 2 weeks | Wound area, pain, healing rate, healing time | The wound healing effect of changing the dressing with Chuangling Liquid after surgery for postoperative wound healing after anal fistula surgery is better than Huangqin ointment | Cao et al. (2014) |
| Chuangling Liquid | Stage III-IV pressure ulcers induced by stroke in the elderly | Routine disinfection/debridement; infected wounds: silver ion/alginate dressings, daily changes | Debridement + heat-sensitive moxibustion + Chuangling Liquid-soaked dressing, once daily | Control group: 15 cases; Observation group: 20 cases | 10 days | Granulation growth, wound healing status | The treatment of stage III-IV pressure ulcers in elderly stroke patients with wet compresses using Chuangling Liquid combined with heat-sensitive moxibustion has a remarkable effect | Miao (2013) |
| Chuangling Liquid | After anal fistula and perianal abscess surgery | Iodophor disinfection + Huangqin Ointment-soaked gauze packing, bid until healing | Iodophor disinfection + Chuangling Liquid-soaked gauze packing, bid until healing | Huangqin Ointment group: 20 cases; Chuangling Liquid group: 20 cases | Not specified | Wound exudate, skin temperature, necrotic sloughing time, healing time | The Chuangling Liquid has a satisfactory effect in reducing exudate from the wound surface, lowering the skin temperature around the wound, accelerating the shedding of necrotic flesh, and promoting healing | Yin et al. (2013) |
| Chuangling Liquid | Ulcer stage bedsores | Routine disinfection + gentamicin spray + sterile dressing, once/twice daily | Routine disinfection + Chuangling Liquid dressing (daily for exudative wounds); switch to Shengji Yuhong Ointment (bid) when exudate decreases | Control group: 28 cases; Observation group: 28 cases | 20 days | Healing rate, granulation growth time, healing timedetect | The clinical effect of the combination Chuangling liquid and Shengji Yuhong ointment used in ulcers of bedsores is significant | Huang and Chen (2012) |
| Chuangling Liquid | Stage IV pressure ulcer | Infection control + nutrition support; disinfection + ConvaTec dressing (changed when discolored) | Control treatment + Chuangling Liquid moist dressing (weekly changes; extended to 2-3 days with granulation) | Control group: 16 cases; Observation group: 16 cases | 4 weeks | Healing rate, granulation time, healing time, dressing change frequency | Five to 7 days after changing the Chuangling Liquid dressing, it can be seen that the necrotic tissue on the wound surface falls off, the black scabs gradually loosen and dissolve, the pus disappears, and the infection is under control | Xu (2010) |
| Chuangling Liquid | Stage IV pressure ulcer | Infection control + nutrition support; iodophor wet compress + debridement + saline irrigation | Control treatment + Chuangling Liquid moist dressing (weekly changes; extended to 2-3 days with granulation) | Control group: 26 cases; Observation group: 29 cases | 30 days | Healing rate, granulation growth time, healing time | The treatment effect of wet compress with Chuangling Liquid combined with iodophor for stage IV pressure ulcers is significantly better than that of wet compress with Chuangling Liquid alone | Xu and Cai (2010) |
| Chuangling Liquid | Ischemic ulcer | Kangfuxin Solutionappli | Chuangling Liquid-soaked gauze dressing, daily/every other day | Control group: 34 cases; Observation group: 38 cases | 3 weeks | Healing rate, pain intensity | The pain-relieving effect of Chuangling Liquid is significantly better than that of other topical liquids, and it is convenient to use with no toxic or side effects at all | Yang (1997) |
| Chuangling Liquid | Stage II pressure ulcer | Mepilex dressing | Chuangling Liquid + Mepilex dressing | Control group: 30 cases; Observation group: 30 cases | 20 days | Wound healing ratedetect | The combination of Chuangling Liquid and Mepilex has a definite therapeutic effect on stage II pressure ulcers, which can shorten the healing time of the ulcer surface and increase the healing rate | Teng and Ji (2016) |
| Chuangling Liquid | Chronic lower limb ulcers | Vaseline-soaked gauze, dressing changes every other day | Two subgroups: Chuangling Liquid- soaked gauze or Chuangling Liquid- loaded collagen, changes every other day | Chuangling Liquid collagen group: 20 cases; Chuangling Liquid group: 20 cases; Vaseline group: 20 cases | 14 days | Healing rate, inflammation/ granulation scores, infection rate | After the Chuangling Liquid is loaded into the collagen sponge, it helps to enhance the therapeutic effect of the Chuangling Liquid in regulating wound inflammation and the collagen sponge in promoting granulation tissue growth, further promoting the healing of chronic wounds | Wang et al. (2016) |
| Chuangling Liquid | Postoperative mixed hemorrhoids | Vaseline-soaked gauze strips for dressing changes | Chuangling Liquid-soaked gauze + photon therapy device irradiation | Control group: 20 cases; Observation group: 20 cases | 7 days | Edema, exudate, pain, healing time | Chuangling solution and photon therapeutic instrument combined with high-quality effective nursing measures for postoperative mixed hemorrhoids has exactly curative effect | Li (2017) |
| Chuangling Liquid | After anal fistula and perianal abscess surgery | Huangqin Ointment-coated gauze strips for dressing changes | Chuangling Liquid-soaked gauze strips for dressing changes | Control group: 20 cases; Observation group: 20 cases | 11 days | Exudate, skin temperature, necrotic sloughing time, healing time | The anal fistula and perianal abscess after surgery using Chuangling lotion for dressing exchange can reduce the wound-surface exudate and inflammation reaction and promote wound-surface healing | Xu (2017) |
| Chuangling Liquid | Diabetic foot | Glucose/blood pressure/lipid control + antibiotics + microcirculation improvement + debridement + saline irrigation | Control treatment + herbal fumigation + Chuangling Liquid moist dressing, bid for 4 weeks (total 16 weeks) | Control group: 30 cases; Observation group: 30 cases | 16 weeks | Wound healing status, hospitalization costs | Chuangling Liquid combined with herbal fumigation and washing can promote the healing of diabetic foot | Pei (2018) |
| Chuangling Liquid | Diabetic foot | Photon therapy device irradiation, bid for 15–20min | Photon therapy + Chuangling Liquid moist dressing, daily changes | Control group: 60 cases; Observation group: 60 cases | 2 weeks | Exudate score, dressing change pain score, healing time, granulation time | In observation group, significant effects were achieved in reducing the wound exudate score, lowering the pain score during dressing change, shortening the healing time and granulation tissue growth time | Liu (2019) |
| Chuangling Liquid | Puncture site infection after PICC placement | Diluted iodophor wet compress on puncture site | Chuangling Liquid wet compress on puncture site | Control group: 18 cases; Observation group: 18 cases | 5 days | Healing effect, healing time, dressing change frequency, cost | Chuangling solution wet-compressing is effective in the treatment of puncture site infection after PICC placement,and associated with shorter healing time and less frequency and cost of dressing change compared with povidone-iodine-diluent wet-compressing | Wang et al. (2020) |
| Chuangling Liquid | Plasma cell mastitis (PCM) | Iodophor disinfection + pus drainage + granulation scraping (if any) + saline irrigation + saline gauze drainage | Control treatment + Chuangling Liquid irrigation + Chuangling Liquid-soaked gauze drainage, dressing changes twice weekly | Control group: 20 cases; Observation group: 20 cases | 4 weeks | Tumor size, patient pain score, and levels of IL-1β, IL-2, IL-6, IFN-γ, and TNF-α were measured | External use of Chuangling Liquid inhibited local inflammation and reduced local mass of PCM patients. | Xue et al. (2020) |
| Chuangling Liquid | Non-lactating mastitis | After abscess incision and drainage, the wound and surrounding area were routinely disinfected with iodophor. The wound and secretions were rinsed with normal saline, and regular gauze strips or Vaseline gauze strips were used for drainage | After abscess incision and drainage, the wound and surrounding area were routinely disinfected with iodophor. The wound and secretions were rinsed with Chuangling Liquid, and Chuangling Liquid-soaked gauze strips were used for drainage | Control group: 33 cases; Observation group: 31 cases | 4 weeks | Wound symptom score and levels of wound tissue inflammatory markers (TNF-α, IFN-γ, IL-1β, IL-2, IL-6) were measured | The external application of Chuangling Liquid can effectively improve the local symptoms and improve the curative effect by reducing the inflammation of the wound tissue in the treatment of non-lactating mastitis patients with local ulceration | Ye et al. (2021) |
| Huangkui Lianchang Decoction | UC | Mesalazine enteric-coated tablets, 1 g PO TID. | Huangkui Lianchang Decoction administered via retention enema | Control group: 60 cases; Observation group: 60 cases | 12 weeks | Baron endoscopy score, Mayo score, and scores of main UC symptoms (diarrhea, abdominal pain, mucus, rectal bleeding, tenesmus) were evaluated | The retention enema of Huangkui Lianchang Decoction has a good therapeutic effect on UC with damp-heat internal accumulation syndrome. It can inhibit the inflammatory response of the intestinal mucosa in patients, reduce mucosal damage, and has good safety | Yang et al. (2023) |
| Yu Kui Qing | DN | Yu Kui Qing placebo (dextrin granules), 1 packet PO BID daily | Yu Kui Qing granules, 1 packet PO BID. | Control group: 30 cases; Observation group: 31 cases | 12 months | Urinary mAlb/Cr levels were measured before and after treatment. Symptom scores were observed, quantifying symptoms such as fatigue, lower limb edema, back pain, frequent urination, increased nocturia, and dry mouth | Yukuiqing has the effect of reducing urinary microalbumin and can improve the symptoms of fatigue, weakness and lower back pain in patients with early type 2 DN. | Chen and Yu (2008) |
| Qikui Granules | DN | Standard hypoglycemic (excluding thiazolidinediones) and antihypertensive (ARB as first-line) treatment, with FBG 4.4–7.0 mmol/L, PBG 4.4–10.0 mmol/L, BP < 130/85 mmHg | Control group treatment + Qikui Granules (1 bag PO TID, 150–200 mL warm water 1 h post-meal) | Control: 32 cases; Treatment: 31 cases | 12 weeks | UACR, UAER, inflammatory factors (IL-6, TNF-α, TGF-β1, MCP-1) | Qikui Granules can significantly reduce urinary protein in patients with type 2 DN. Its mechanism of action may be to exert a protective effect on the kidneys by improving the inflammatory state of the glomerulus | Xie et al. (2017a) |
| Qikui Granules | DN | Standard hypoglycemic (conventional agents/insulin) and antihypertensive (excluding ACEI/ARB) treatment, maintaining normal BP. | Control group treatment + Qikui Granules (1 bag PO TID) | Control: 50 cases; Treatment: 52 cases | 6 months | BP, BG, BUN, CR, 24hUTP, urinary CTGF, serum sICAM-1 | Qikui Granules can significantly reduce urinary protein in patients with type 2 DN. Its mechanism of action may be to exert a protective effect on the kidneys by improving the inflammatory state of the glomerulus | Zhang et al. (2021) |
| Qikui Granules | DN in elderly patients | Dulaglutide (1.5 mg SC QW) | Dulaglutide (1.5 mg SC QW) + Qikui Granules (10 g PO TID) | Control: 40 cases; Treatment: 40 cases | 12 weeks | BG (FPG, HbA1c), BP (SBP, DBP), lipids (TC, TG, LDL-C, HDL-C), renal indicators (SCr, UACR, 24hUTP) | The combination of Qikui Granules and Dulagtide can significantly reduce the levels of Scr and urine protein in elderly patients with DKD in the clinical stage, and has a good protective effect on the kidneys | Wang et al. (2023a) |
| Huangshu Kuihua paste | Oral mucosal ulcer | Compound borax gargle + Xileisan (blown on lesion 5 times/d) | Compound borax gargle + Huangshu Kuihua Paste (covered lesion, held 15min before swallowing, 3 times/d) | Control: 36 cases; Treatment: 82 cases | 5 days | Pain, eating status, ulcer healing | Compared with the Xileisan group, the total effective rate of the Huangshu Kuihua paste was significantly higher | Han et al. (1997) |
| Er Huang Ointment | Superficial second-degree burns | SD-Ag cream (QD) | Er Huang Ointment + rhGM-CSF gel (BID-TID) | Control: 49 cases; Treatment: 49 cases | 2 weeks | Wound healing rate, scarring, healing time, pain, adverse reactions | The combination of Er Huang Ointment and rhGM-CSF gel can effectively promote wound healing in patients with superficial second-degree burns and reduce scarring | Guo and Wang (2017) |
| Er Huang Ointment | Superficial second-degree burns | Vaseline (QD) | Er Huang Ointment (QD) | Control: 45 cases; Treatment: 45 cases | 1 week | Healing time, healing rate, visual analogue scale, skin irritation, appearance satisfaction | The clinical efficacy of Er Huang Ointment in treating superficial second-degree burns is remarkable. It can accelerate the wound healing time, relieve pain, avoid severe irritation to the skin, and has a relatively high safety level | Yang (2016) |
Clinical trial of Chinese herbal formula containing AM.
6.1 Jiahua tablets
Jiahua Tablets are semi-extracted TCM tablets made from the raw powder and extracts of AM flowers, containing 10% starch slurry and an appropriate amount of ethanol. These tablets have been clinically used for over a decade and have been demonstrated to display significant therapeutic efficacy (Lin et al., 2020a; Zha et al., 2020). Both HKC and Jiahua Tablets are derived from the single medicinal ingredient of AM flowers. They share properties that clear heat, promote diuresis, support kidney function, and eliminate toxins, primarily addressing acute and chronic nephritis (Zhang and Zhou, 2012). According to the Chinese Pharmacopoeia, the recommended dosage of AM flowers is 10–30 g/day, with both HKC and Jiahua Tablets containing a daily dosage of 30 g of AM flowers.
In addition to conventional early DN treatments, Jiahua Tablets can reduce serum inflammatory factors such as C-reactive protein (CRP) and TNF-α. It also lowers levels of Cystatin C (Cys-C) and urinary albumin-to-creatinine ratio (UACR), thereby improving kidney function (Cha et al., 2020). In patients undergoing coronary intervention, those treated with Jiahua Tablets exhibited significantly lower levels of serum creatinine (SCr), BUN, Cys-C, IL-6, IL-8, high-sensitivity C-reactive protein (hs-CRP), urinary N-acetyl-β-D-glucosaminidase (NAG), and urinary β-galactosidase (GAL) 72 h post-operation compared to the control group. Additionally, the estimated glomerular filtration rate (eGFR) was significantly higher in the treatment group than in the control group, indicating that Jiahua Tablets can effectively prevent contrast-induced nephropathy. The underlying mechanism likely involves inhibiting inflammatory responses and protecting renal tubules, thereby repairing kidney damage and preserving renal function (Wu et al., 2020).
6.2 Chuangling liquid
Chuangling Liquid was developed by the Jiangsu Provincial Hospital of TCM in the 1980s. Its formulation including A. manihot (Huang Shu Kui), Rheum palmatum (Da Huang), Carthamus tinctorius (Hong Hua), and Terminalia chebula (He Zi) (Yang, 1997; Xu, 2010; Huang and Chen, 2012; Miao, 2013; Yin et al., 2013; Cao et al., 2014; Sheng, 2014; Teng and Ji, 2016; Wang et al., 2016; Li, 2017; Liu, 2019; Wang et al., 2020; Xue et al., 2020; Ye et al., 2021). The primary active components of Chuangling Liquid include gallic acid, hyperoside, isoquercitrin, myricetin, quercetin, rhein, emodin, chrysophanol, and physcion (Shu et al., 2016). This preparation promotes blood circulation and removes blood stasis, effectively treating various types of infectious ulcers with significant inflammation (Chen et al., 2017).
Chuangling Liquid has demonstrated a range of therapeutic properties in various experimental and clinical settings. Zhang et al. showed that it can counteract acute exudative inflammation induced by croton oil in mice and inhibit granulation tissue proliferation in cotton pellet granuloma models (Zhang et al., 1997). In rabbit ulcer models, Chuangling Liquid promotes early wound healing processes such as epithelial tissue proliferation, granulation formation, and accessory regeneration, while inhibiting collagen fiber scar formation, leading to faster healing with less scarring (Zhang et al., 1997). Additionally, it exhibits strong antibacterial activity against S. aureus, methicillin-resistant S. aureus (MRSA), and Proteus species, being particularly effective at eliminating MRSA from infected wounds and promoting recovery (Liu et al., 2018). Moreover, Chuangling Liquid also inhibits thrombosis and platelet aggregation, thereby improving blood circulation and alleviating blood stasis (Chen et al., 2017). Clinically, it has been proven effective in treating postoperative infected wounds, chronic ulcers, diabetic foot ulcers, venous ulcers of the lower limbs, and anal fistulas (Hu, 2016; Li, 2016; Xu, 2017). Furthermore, modifying the formulation process and incorporating collagen into Chuangling Liquid enhances its anti-inflammatory effects, wound healing, angiogenesis, and overall recovery (Zhao and Zhu, 2013). Recent studies have also found that Chuangling Liquid promotes the proliferation of immortalized melanocytes in mice and activates tyrosinase activity, enhancing melanin synthesis. This suggests potential applications in treating pigmentary disorders such as vitiligo (Zhou et al., 2019).
6.3 Huangkui Lianchang decoction
Huangkui Lianchang Decoction (HLD) is a traditional Chinese medicinal formulation used to treat UC. It consists of six key ingredients: the primary herb is A. manihot (Huang Shu Kui), combined with Euphorbia humifusa Willd (Di Jin Cao), Pteris multifida Poir (Feng Wei Cao), Lithospermum erythrorhizon Siebold & Zucc (Zi Cao), Rubia cordifolia L. (Qian Cao), and Rhus chinensis Mill (Wu Bei Zi) (He et al., 2019; Yang et al., 2023). HLD is rich in flavonoids, including rutin, isoquercitrin, gossypetin, and quercetin (Cheng et al., 2022). The proposed functions of HLD are to clear heat and dampness, activate blood circulation, protect the intestines, and detoxify the body. These properties make it particularly beneficial for managing the symptoms associated with UC.
HLD has demonstrated significant therapeutic effects on DSS-induced UC in mouse models. Studies indicate that HLD exerts anti-inflammatory effects by modulating key signaling pathways, including NF-κB, IL-6/signal transducer and activator of transcription 3 (STAT3), and the IL-23/IL-17 inflammatory axis (He et al., 2019; He et al., 2020; Cheng et al., 2022). In vitro experiments with drug serum containing HLD (HLD-DS) showed that it reduces inflammatory cytokine levels while increasing IL-10 levels in NCM460 cells. HLD-DS also decreased the expression of p-NF-κB p65, LC3II/I, and Beclin 1, suggesting that HLD alleviates colitis by inhibiting the NF-κB pathway and autophagy (Cheng et al., 2022). Clinically, HLD is used for treating mild to moderate E1 and E2 type UC via enema alone. In contrast, severe or E3-type UC is treated with HLD enemas and oral medications (He et al., 2019). Clinical studies have shown that modified HLD, when used alongside corticosteroids, can effectively improve gut microbiota and exhibit significant anti-inflammatory effects, aiding in managing IBD (He et al., 2023). Additionally, the retention enema method of HLD has been found to inhibit the intestinal mucosal inflammatory response in UC patients and reduce mucosal damage (Yang et al., 2023).
6.4 Huangkui Siwu Formula
Huangkui Siwu Formula (HKSWF) is a traditional Chinese medicinal formulation comprised of four key herbs: A. manihot (Huang Shu Kui), Astragalus mongholicus (Huang Qi), Polygonum cuspidatum (Hu Zhang), and Curcuma longa L (Jiang Huang) (Lu T. et al., 2020). The formula contains eleven identified components, including polydatin, hyperoside, isoquercitrin, hibifolin, myricetin, resveratrol, quercetin, didemethoxycurcumin, demethoxycurcumin, curcumin, and emodin (Lu T. et al., 2020). The herbal combination in HKSWF clears heat, promotes blood circulation, facilitates urination, and reduces swelling, effectively addressing the symptoms of CKD.
Research on HKSWF has shown promising therapeutic effects in kidney health, particularly in an anti-Thy-1 nephritis model. One study indicates that HKSWF can alleviate glomerular injury by attenuating pyruvate dehydrogenase activity, contributing to its protective effects on renal function (Lu T. et al., 2020). Further investigations by Lu et al. revealed that HKSWF does not alter the expression of organic anion transporters in the kidneys or the transport of p-cresyl sulfate (PCS) from blood to kidneys. Instead, it regulates the synthesis pathway of PCS within host cells, inhibiting its endogenous production. This action helps reduce the accumulation of uremic toxins and slows the progression of CKD (Lu et al., 2021). Moreover, HKSWF modulates uremic toxin metabolism pathways within the gut microbiota, inhibiting the formation of uremic toxin precursors at various stages. This modulation alleviates the symptoms associated with uremic toxin accumulation and further delays CKD progression, highlighting the multifaceted benefits of HKSWF for managing kidney health (Lu J. et al., 2020).
6.5 Yu Kui Qing
Yu Kui Qing (YKQ) is composed of A. manihot (Huang Shu Kui), Astragalus membranaceus (Huang Qi), and Polygonum multiflorum Thunb. (Zhi Shou Wu) (30:1.5:1) (Chen and Yu, 2008; Chen and Yu, 2009). YKQ clears heat, detoxifies, promotes diuresis, tonifies Qi, strengthens the spleen, activates blood circulation, and nourishes Yin and kidneys. Modern pharmacological studies have demonstrated that YKQ can reduce the expression and chemotactic effects of chemokines in human renal mesangial cells (HRMC) induced by advanced glycation end-products bound to bovine serum albumin (AGE-BSA). Additionally, YKQ intervenes in the expression of connective tissue growth factor (CTGF) mRNA and protein in HRMC, which may contribute to reducing renal inflammation associated with DN (Yang et al., 2010; Sun et al., 2014). Clinical studies have shown that YKQ effectively treats microalbuminuria in patients with type 2 DN, reduces urinary microalbumin levels, and improves symptoms (such as fatigue and lower back pain) in early-stage disease. These findings underscore the potential of YKQ as a valuable therapeutic option for managing diabetes-related complications (Chen and Yu, 2009).
6.6 Qikui Granules
Qikui Granules is composed with A. membranaceus (Huang Qi), A. manihot (Huang Shu Kui), and processed P. multiflorum (Zhi Shou Wu) (Wang L. et al., 2023). Specifically, they help to benefit Qi, nourish Yin, and clear heat. Clinically, Qikui Granules have been utilized to treat early to mid-stage DN (Yang et al., 2016).
The study by Lou et al. shows that Qikui Granules provide renal protection in DN by lowering blood glucose, reducing inflammatory markers like IL-6, monocyte chemoattractant protein-1 (MCP-1), and TGF-β1, and inhibiting the p38 MAPK signaling pathway (Yang et al., 2016; Shao et al., 2017). In addition to renal benefits, Qikui Granules were observed to improve bone density, likely linked to improved blood glucose regulation (Lou et al., 2022). In vitro experiments revealed that these granules enhance the proliferation of mesenchymal stem cells (MSCs) and promote their osteogenic differentiation while simultaneously reducing their potential for adipogenic differentiation (Lou et al., 2022). Clinically, Qikui Granules have been shown to reduce microalbuminuria in patients with early DN. It slows disease progression and alleviates symptoms such as fatigue, weakness, lower back and knee soreness, and facial and limb edema (Yan et al., 2018). Observations indicate a marked reduction in urine protein levels, UACR, urinary albumin excretion rate (UAER), and urinary MCP-1/creatinine (uMCP-1/Cr) levels in patients with early DN (Yan et al., 2018). The underlying mechanisms of action for Qikui Granules may involve inhibiting CTGF expression and soluble intercellular adhesion molecule-1 (sICAM-1) in urine and serum (Xie et al., 2017b; Zhang et al., 2021). Overall, the findings suggest that Qikui Granules could be a valuable therapeutic approach for managing complications associated with diabetes, particularly DN, by addressing metabolic and inflammatory pathways.
6.7 Huangshu Kuihua paste
The Huangshu Kuihua paste, which combines 0.3–10 parts of A. manihot (Huang Shu Kui) with 0.1–10 parts of propolis extract and 0.1-5 parts of borneol, is an innovative formulation widely used at the First Affiliated Hospital of Anhui Medical University (Han and Situ, 1997; Chen et al., 2017). Its strong analgesic properties make it particularly effective for managing pain associated with oral ulcers. Notably, the formulation is characterized by minimal tissue irritation and does not cause local numbness or discomfort, which enhances patient comfort during treatment. Moreover, the paste exhibits excellent adhesion to mucous membranes, facilitating prolonged contact and promoting the healing process of oral ulcers without any toxic side effects. In clinical trials involving 82 patients with oral ulcers, the formulation achieved an impressive cure rate of 97.5%, underscoring its efficacy and safety in treating this condition (Han et al., 1997). These findings make Huangshu Kuihua paste a valuable option in managing oral ulcer pain and healing.
6.8 Er Huang ointment
Er Huang Ointment, formulated with A. manihot (Huang Shu Kui), Scutellaria baicalensis (Huang qin), Phellodendron amurense (Huang bai), Gardenia jasminoides (Zhi zi), and Ampelopsis japonica (Bai lian) (5:5:5:3:3), has garnered attention for its therapeutic effects on burns and wounds (Yang, 2016). Clinical studies have affirmed that the overall efficacy of Er Huang Ointment in treating burns and scalds and reducing wound healing time is comparable to that of Moist Burn Ointment (Yang et al., 2016; Guo and Wang, 2017). Furthermore, Er Huang Ointment has notable antibacterial activity against common pathogens such as S. aureus, Pseudomonas aeruginosa, and E. coli, suggesting its potential in preventing infections associated with burns and wounds (Li, 1993). These findings position Er Huang Ointment as a promising clinical practice option for managing burns and enhancing wound healing.
7 Research challenges and future perspectives
The traditional Chinese medicinal herb AM features pale yellow flowers with a delicate fragrance and holds significant ornamental, medicinal, and edible value. Modernizing AM faces two core challenges: degree of development and utilization, and gaps in translation from laboratory to clinic. Jiangsu Suzhong Pharmaceutical Co., Ltd. in Taizhou (Jiangsu, China) has successfully developed and commercially produced HKC—a Category III new TCM—after years of research. These capsules are widely used clinically to treat conditions such as nephritis and rheumatoid arthritis, serving as an adjunctive therapy for glomerulonephritis and demonstrating considerable value in preventing gadolinium-based contrast agent-induced nephrogenic systemic fibrosis. Utilizing AM flowers as the primary raw material, the proven efficacy and substantial market potential of these capsules have driven high demand, consequently stimulating the flourishing cultivation industry across Taizhou, Jiangsu, surrounding regions, and nationwide. However, other AM parts—including leaves, roots, stems, and seeds—are typically discarded, resulting in resource wastage and environmental pollution. Literatures indicate that the entire AM plant possesses medicinal properties, suggesting that non-capsule components also hold therapeutic value. To enable more scientific, comprehensive, and rational utilization of this medicinal resource, intensified research on its other bioactive parts is essential. This will maximize economic and social benefits while accelerating the development of the AM industry.
Transitioning to evidence-based medicine is hampered by significant clinical evidence gaps, including small randomized controlled trials with fewer than 300 cases, short follow-up periods of 6 months or less, reliance on surrogate endpoints like proteinuria instead of hard endpoints such as end-stage renal disease incidence, and insufficient long-term safety data on aspects like reproductive toxicity. Currently, only HKC has gained broad clinical acceptance, primarily for treating nephropathy. This is due to its robust clinical data, characterized by rigorous record-keeping, standardized patient grouping, sufficient sample sizes, and sustained post-treatment follow-up. Other AM-containing TCM formulations suffer from significant clinical evidence gaps: their studies often involve small, non-systematic patient cohorts and lack post-administration observation. This evidence deficit severely limits the broader clinical adoption and justification for using AM. Therefore, building upon the systematically compiled data on AM-containing TCM formulations presented in this study, it is recommended to prioritize 1-2 promising prescriptions for development. Conducting standardized clinical research on these selected formulations is crucial. This focused approach will enhance the understanding of AM’s therapeutic potential and ultimately expand its clinical applications.
8 Conclusion
Based on the current body of evidence derived from both basic research and clinical practice, AM has demonstrated significant therapeutic potential. Its pharmacological activities, including the improvement of liver and kidney functions, regulation of material metabolism, and anti-fibrotic effects, have been scientifically validated, providing a rationale for its application in conditions such as CKD and IBD (Miao et al., 2024a; Wang Y. et al., 2024; Zhao H. et al., 2024). However, the predominant clinical application remains limited to the single-component preparation HKC (Geng et al., 2023), underscoring the need to broaden the development and utilization of AM-derived formulations.
Notwithstanding this promise, current research faces notable limitations that must be addressed in future studies. These include a narrow focus on the flowers and total flavonoids, neglecting other plant parts and individual bioactive compounds; the use of animal models that may not accurately replicate human diseases; insufficient mechanistic insights at cellular and molecular levels; and a lack of integrated pharmacokinetic-pharmacodynamic (PK-PD) studies to represent the whole herb’s in vivo behavior. Consequently, future efforts should prioritize expanding phytochemical investigations, pinpointing specific bioactive compounds and their mechanisms, establishing integrated PK-PD models, and developing improved formulations through rigorous clinical trials. Overcoming these challenges via a multidisciplinary approach is essential to fully realize the clinical potential of AM.
Statements
Author contributions
CX: Conceptualization, Visualization, Writing – original draft. HG: Conceptualization, Writing – review and editing. YL: Formal Analysis, Investigation, Methodology, Writing – review and editing. YZ: Conceptualization, Resources, Writing – review and editing. WH: Conceptualization, Resources, Writing – review and editing. ZL: Formal Analysis, Methodology, Project administration, Writing – review and editing. QY: Investigation, Resources, Writing – review and editing. XC: Investigation, Resources, Writing – review and editing. ZC: Conceptualization, Funding acquisition, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by High-value Patent Cultivation Project from Jiangsu Provincial Intellectual Property Administration (GJ20231209-14), Major Program of Jiangsu Provincial Administration for Market Regulation (KJ2024014), Tibet Autonomous Region Science and Technology Plan Project Key Project (XZ202301ZY0014G), the Natural Science Foundation of Jiangsu Province of China (BK20241599), the Postdoctoral Fellowship Program of CPSF (GZC20242019), the Fundamental Research Funds for the Central Universities (2632024PY10), China Postdoctoral Science Foundation (2024M763667).
Conflict of interest
Author HG was employed by Jiangsu Suzhong Pharmaceutical Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- ACEI
angiotensin-converting enzyme inhibitor
- ACTH
adrenocorticotropic hormone
- AGEs
advanced glycation end products
- AGE-BSA
advanced glycation end-products bound to bovine serum albumin
- AKI
acute kidney injury
- AKT
protein kinase B
- ALI
acute lung injury
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AM
Abelmoschus manihot (L.) Medicus
- AMPK
adenosine monophosphate-activated protein kinase
- ARB
angiotensin II receptor blocker
- ASC
apoptosis-associated speck-like protein containing caspases recruitment domain
- AST
aspartate aminotransferase
- Bax
Bcl-2-associated x protein
- Bcl-2
B-cell lymphoma-2
- BDNF
brain-derived neurotrophic factor
- BECN1
beclin 1
- BG
blood glucose
- bid
bis in die
- BP
blood pressure
- BSEP
bile salt export pump
- BUN
blood urea nitrogen
- [Ca2+]i
calcium concentrations
- CAM
chick chorioallantoic membrane
- CAT
catalase
- CCL
C-C Motif Chemokine Ligand
- C/EBPα
CCAAT/enhancer-binding protein alpha
- CKD
chronic kidney disease
- COX-2
cyclooxygenase-2
- CTGF
connective tissue growth factor
- Cys C
cystatin C
- CREB
cAMP response element-binding protein
- CRP
C-reactive protein
- DAI
disease activity index
- DBP
diastolic blood pressure
- DN
diabetic nephropathy
- DKD
diabetic kidney disease
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- DSS
dextran sodium sulfate
- EPCs
endothelial progenitor cells
- ERK1/2
extracellular signal-regulated kinase 1/2
- EMT
epithelial-mesenchymal transition
- eGFR
estimated glomerular filtration rate
- FBG
fasting blood glucose
- FPG
fasting plasma glucose
- FST
forced swimming test
- GAL
β-galactosidase
- GLUT-4
glucose transporter type 4
- GSH
glutathione
- GSH-Px
glutathione peroxidase
- GST
glutathione S-transferase
- HbA1c
glycated hemoglobin A1c
- HG
high glucose
- HIF-1a
hypoxia-inducible factors 1a
- HKC
Huangkui Capsule
- HLD
Huangkui Lianchang decoction
- HLD-DS
drug serum containing HLD
- HO-1
heme oxygenase-1
- HRMC
human renal mesangial cells
- HKSWF
Huangkui Siwu Formula
- HUVECs
human umbilical vein endothelial cells
- hs-CRP
high-sensitivity C-reactive protein
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon-gamma
- i.g
intragastric administration
- IgA nephropathy
immunoglobulin a nephropathy
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- IκBα
nhibitor of nuclear factor kappa b alpha
- INMDA
N-methyl-D-aspartate receptor-activated current
- JAK2
janus kinase 2
- LC3
microtubule - associated protein light chain 3
- LDL-C
low-density lipoprotein cholesterol
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- mAlb/Cr
microalbumin/creatinine ratio
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MDA
malondialdehyde
- MPO
myeloperoxidase
- MRSA
methicillin-resistant Staphylococcus aureus
- MRP2
multidrug resistance-associated protein 2
- MYD88
myeloid differentiation primary response 88
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAG
N-acetyl-β-D-glucosaminidase
- NF-κB
nuclear factor kappa B
- NLRP3
nod-like receptor protein 3
- NMDA
N-methyl-D-aspartate
- Nrf2
nuclear factor erythroid 2-related factor 2
- NO
nitric oxide
- NOX4
nicotinamide adenine dinucleotide phosphate oxidases 4
- NOS
nitric oxide synthase
- NS
nephrotic syndrome
- NTCP
sodium taurocholate cotransporting polypeptide
- ox-LDL
oxidized low-density lipoprotein
- PBG
postprandial blood glucose
- PCI
percutaneous coronary intervention
- PCS
p-cresyl sulfate
- PGE2
prostaglandin E2
- PICC
peripherally inserted central catheter
- PI3K
phosphoinositide 3-kinase
- po
peros
- PPARγ
peroxisome proliferator-activated receptor gamma
- PSD
post-stroke depression
- PTZ
pentylenetetrazol
- qd
quaque die
- qw
quaque week
- rhGM-CSF
granulocyte-macrophage colony-stimulating factor
- ROS
reactive oxygen species
- SBP
systolic blood pressure
- SC
subcutaneous
- SCFAs
short-chain fatty acids
- SCr
serum creatinine
- sICAM-1
soluble intercellular adhesion molecule-1
- SIRT1
sirtuin 1
- Slc
solute carrier family
- Smad3
smad family member 3
- STAT3
signal transducer and activator of transcription 3
- SOCS1
suppressor of cytokine signaling 1
- SOD
superoxide dismutase
- KDR
kinase insert domain receptor
- KIM-1
kidney injury molecule-1
- TACE
tumor necrosis factor-α converting enzyme
- T-AOC
total antioxidant capacity
- TBA
total bile acid
- TC
total cholesterol
- TCM
traditional Chinese medicine
- TFA
total flavones of Abelmoschus manihot
- TG
triglyceride
- TGF-β
transforming growth factor beta
- Th17
T helper 17 cell
- TNBS
2,4,6-trinitrobenzenesulfonic acid solution
- TNF-α
tumor necrosis factor-alpha
- tid
ter in die
- TLR4
toll-like receptor 4
- Treg
regulatory T cell
- UACR
urinary albumin-to-creatinine ratio
- UAER
urinary albumin excretion rate
- UC
ulcerative colitis
- UTP
urinary total protein
- VEGF-A
vascular endothelial growth factor-A
- VEGFR2
vascular endothelial growth factor receptor-2
- VCAM-1
vascular cell adhesion molecule-1
- YKQ
Yu Kui Qing
- ZO-1
zonula occludens-1
References
1
Abdou H. M. Elmageed G. M. A. Hussein H. K. Yamari I. Chtita S. El-Samad L. M. et al (2025). Antidiabetic effects of quercetin and silk sericin in attenuating dysregulation of hepatic gluconeogenesis in diabetic rats through potential modulation of PI3K/Akt/FOXO1 signaling: in vivo and in silico studies. J. Xenobiot.15, 16–24. 10.3390/jox15010016
2
Ai G. Liu Q. Hua W. Huang Z. Wang D. (2013). Hepatoprotective evaluation of the total flavonoids extracted from flowers of abelmoschus manihot (L.) medic: in vitro and in vivo studies. J. Ethnopharmacol.146, 794–802. 10.1016/j.jep.2013.02.005
3
An Y. Zhang Y. Li C. Qian Q. He W. Wang T. (2011). Inhibitory effects of flavonoids from Abelmoschus manihot flowers on triglyceride accumulation in 3T3-L1 adipocytes. Fitoterapia82, 595–600. 10.1016/j.fitote.2011.01.010
4
An W. Huang Y. Chen S. Teng T. Liu J. Xu Y. (2021). Efficacy and safety of Huangkui capsule for diabetic nephropathy: a protocol for systematic review and meta-analysis. Medicine100. 10.1097/MD.0000000000027569
5
Besendorf L. Mueller T. M. Geppert C.-I. Schneider I. Muehl L. Atreya I. et al (2022). Vedolizumab blocks α4β7 integrin-mediated T cell adhesion to MAdCAM-1 in microscopic colitis. Ther. Adv. Gastroenterol.15, 175–186. 10.1177/17562848221098899
6
Bu F. Ding Y. Chen T. Wang Q. Wang R. Zhou J.-y. et al (2021). Total flavone of Abelmoschus Manihot improves colitis by promoting the growth of Akkermansia in mice. Sci. Rep.11, 20787–20796. 10.1038/s41598-021-00070-7
7
Cai H. Su S. Guo S. Zhu Y. Qian D. Tao W. et al (2016). Effect of flavonoids from Abelmoschus manihot on proliferation, differentiation of 3T3-L1 preadipocyte and insulin resistance. China J. Chin. Mater Med.41, 4635–4641. 10.4268/cjcmm20162424
8
Cai H. Su S. Qian D. Guo S. Tao W. Cong X. et al (2017a). Renal protective effect and action mechanism of Huangkui capsule and its main five flavonoids. J. Ethnopharmacol.206, 152–159. 10.1016/j.jep.2017.02.046
9
Cai H. Tao W. Su S. Guo S. Zhu Y. Guo J. et al (2017b). Antidepressant activity of flavonoid ethanol extract of Abelmoschus manihot corolla with BDNF up-regulation in the hippocampus. Acta Pharm. Sin.52, 222–228. 10.16438/j.0513-4870.2016-0764
10
Cao L. Yin C. Zhou Q. Hu W. Zhou Y. (2014). Observation on curative effect of chuangling fluid and skul lcap ointment used for postoperative dressing in patients with low simple anal fistula patients. Nurs. Res.28, 3127–3129. 10.3969/j.issn.1009-6493.2014.25.022
11
Cao H. Xu H. Zhu G. Liu S. (2017). Isoquercetin ameliorated hypoxia/reoxygenation-induced H9C2 cardiomyocyte apoptosis via a mitochondrial-dependent pathway. Biomed. Pharmacother.95, 938–943. 10.1016/j.biopha.2017.08.128
12
Cha M. Sun H. Zhang S. Ye L. Huang L. Chen S. et al (2020). Effect of Jiahua Tablets as adjuvant treatment on serum inflammatory factors levels and renal function in patients with early diabetic nephropathy. Guangxi Med. J.42, 2059–2061.
13
Chang C. Houng J. Peng W. Yeh T. Wang Y. Chen Y. et al (2022). Effects of abelmoschus manihot flower extract on enhancing sexual arousal and reproductive performance in zebrafish. Molecules27, 2218–2229. 10.3390/molecules27072218
14
Chen X. Yu J. (2008). Clinical Study on the intervention treatment of early type 2 diabetic nephropathy with Yu Kui qing. Jiangsu J.TCM, 48–49. 10.3969/j.issn.1672-397X.2008.07.028
15
Chen X. Yu J. (2009). Clinical Study of yukuiqing on early Type-2 diabetic kidney cell factors. Zhejiang J. Trad. Chin. Med.33, 56–57. 10.16466/j.issn1005-5509.2009.01.038
16
Chen L. Su C. Liu Q. Xu X. Yu S. Li R. et al (2012a). Protective effect of F-6 from abelmoschus manihot on acute liver injury of mice induced by CCl4. Pharm. J. Chin. PLA.28, 214–217. 10.3969/j.issn.1008-9926.2012.03.07
17
Chen P. Wan Y. Wang Z. Zhao Q. Wei Q. Tu Y. et al (2012b). Mechanisms and effects of Abelmoschus manihot preparations in treating chronic kidney disease. China J. Chin. Mater Med.37, 2252–2256. 10.4268/cjcmm20121514
18
Chen Y. Cai G. Sun X. Chen X. (2016). Treatment of chronic kidney disease using a traditional Chinese medicine, flos Abelmoschus manihot (Linnaeus) Medicus (Malvaceae). Clin. Exp. Pharmacol. Physiol.43, 145–148. 10.1111/1440-1681.12528
19
Chen S. Chai C. Ge H. (2017). Research progress of the topical preparations containing abelmoschus coroll. Shanghai Yiyao38, 75–78. 10.3969/j.issn.1006-1533.2017.05.025
20
Chen X. Li H. Wang Z. Zhou Q. Chen S. Yang B. et al (2020). Quercetin protects the vascular endothelium against iron overload damages via ROS/ADMA/DDAHⅡ/eNOS/NO pathway. Eur. J. Pharmacol.868, 172885–172893. 10.1016/j.ejphar.2019.172885
21
Chen J. Lin R. Jiang C. Chen F. Li W. Wang L. et al (2025). Brain endothelial HIF-1α exacerbates diabetes-associated cognitive impairment by accelerating glycolysis-driven lactate production. Acta Pharm. Sin. B15, 5772–5788. 10.1016/j.apsb.2025.09.028
22
Cheng L. Hu K. Chen X. Huang Z. (2010). The flavonoid components of abelmoschus manihot flowers-experimental study on the treatment of non-alcoholic fatty liver in rats using F-6. Pharm. J. Chin.7–12.
23
Cheng X. Qin S. Dong L. Zhou J. (2006). Inhibitory effect of total flavone of abelmoschus manihot L. medic on NMDA receptor-mediated current in cultured rat hippocampal neurons. Neurosci. Res.55, 142–145. 10.1016/j.neures.2006.02.011
24
Cheng Y. Chen Y. Zhou J. Qu D. Zhou Z. Yang B. et al (2015). Effects of abelmoschi corolla extracts on IBD treatment in mice and expression of TNF-α and IFN-γ. J. Nanjing Univ. Tradit. Chin. Med.31, 32–34. 10.14148/j.issn.1672-0482.2015.0032
25
Cheng X. Du J. Zhou Q. Wu B. Wang H. Xu Z. et al (2022). Huangkui lianchang decoction attenuates experimental colitis by inhibiting the NF-κB pathway and autophagy. Front. Pharmacol.13, 951558. 10.3389/fphar.2022.951558
26
Dai Y. Zhang H. Zhang J. Yan M. (2018). Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway. Chem. Biol. Interact.284, 32–40. 10.1016/j.cbi.2018.02.017
27
Deng J. He Z. Li X. Chen W. Yu Z. Qi T. et al (2021). Huangkui capsule attenuates lipopolysaccharide-induced acute lung injury and macrophage activation by suppressing inflammation and oxidative stress in mice. Evid. Based Complement. Altern. Med.2021, 6626483–6626505. 10.1155/2021/6626483
28
Diao Z. Yu H. Wu Y. Sun Y. Tang H. Wang M. et al (2024). Identification of the main flavonoids of Abelmoschus manihot (L.) medik and their metabolites in the treatment of diabetic nephropathy. Front. Pharmacol.14, 1290868. 10.3389/fphar.2023.1290868
29
Ding L. Li S. Jiang Q. Guo Y. Chen Z. Dong L. (2013). Effects of total flavone of Abelmoschus manihot (L.) Medic on hemodynamics and myocardial oxygen consumption in anesthetized dogs. Chin. J. Pharmacol. Clin.29, 79–82. 10.13412/j.cnki.zyyl.2013.03.032
30
Du L.-y. Tao J.-h. Jiang S. Qian D.-w. Guo J.-m. Duan J.-a. (2017). Metabolic profiles of the Flos Abelmoschus manihot extract by intestinal bacteria from the normal and CKD model rats based on UPLC‐Q‐TOF/MS. Biomed. Chromatogr.31, e3795. 10.1002/bmc.3795
31
Fan L. Dong L. Jiang Q. Cen D. Chen Z. Ma C. (2003a). Analgesic effect of Total Flavone of Abelmoschl manihot L medic. Chin. J. Tradit. Med., 12–14. 10.13412/j.cnki.zyyl.2003.01.007
32
Fan L. Dong L. Jiang Q. Cen D. Chen Z. Ma C. (2003b). Anti inflammatory and antipyretic effects of TFA. J Anhui Med Univ.25–27. 10.19405/j.cnki.issn1000-1492.2003.01.009
33
Fernando P. D. S. M. Piao M. J. Herath H. M. U. L. Kang K. A. Hyun C. L. Kim E. T. et al (2024). Hyperoside reduced particulate matter 2.5-induced endoplasmic reticulum stress and senescence in skin cells. Toxicol. Vitro99, 105870. 10.1016/j.tiv.2024.105870
34
Gao S. Fan L. Dong L. Zhao W. Chen Z. (2003). Effect of TFA on cell apoptosis in MCAO rats. Chin. Pharmacol. Bull., 704–707. 10.3321/j.issn:1001-1978.2003.06.029
35
Gao Y. Liang Z. Lv N. Shan J. Zhou H. Zhang J. et al (2022). Exploring the total flavones of Abelmoschus manihot against IAV-induced lung inflammation by network pharmacology. BMC Complement. Med. Ther.22, 36–45. 10.1186/s12906-022-03509-0
36
Gao R.-J. Aikeremu N. Cao N. Chen C. Ma K.-T. Li L. et al (2024). Quercetin regulates pulmonary vascular remodeling in pulmonary hypertension by downregulating TGF-β1-Smad2/3 pathway. BMC Cardiovasc Disord.24, 535. 10.1186/s12872-024-04192-4
37
Gao T. Wang C. Yang X. He Z. Wang Y. Mi W. (2025). Hyperoside ameliorates neuropathic pain by modulating the astroglial reactivity in the vlPAG. Neuropharmacology.266, 110276. 10.1016/j.neuropharm.2024.110276
38
Ge J. Miao J. Sun X. Yu J. (2016). Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, improves diabetic nephropathy via activating peroxisome proliferator–activated receptor (PPAR)-α/γ and attenuating endoplasmic reticulum stress in rats. J. Ethnopharmacol.189, 238–249. 10.1016/j.jep.2016.05.033
39
Geng Y. Dong Z. Wang Y. Zhang P. Tang J. Li P. et al (2023). Efficacy of huangkui capsules in the treatment of diabetic kidney disease: a systematic review and using network pharmacology. Integr. Med. Nephrol. Androl.10, 1–8. 10.1097/IMNA-D-22-00020
40
Gu L. Ge H. Zhao L. Wang Y. Zhang F. Tang H. et al (2020). Huangkui capsule ameliorates renal fibrosis in a unilateral ureteral obstruction mouse model through TRPC6 dependent signaling pathways. Front. Pharmacol.11, 996–1005. 10.3389/fphar.2020.00996
41
Gu L. Yun S. Tang H. Xu Z. (2021). Huangkui capsule in combination with metformin ameliorates diabetic nephropathy via the Klotho/TGF-β1/p38MAPK signaling pathway. J. Ethnopharmacol.281, 113548–113561. 10.1016/j.jep.2020.113548
42
Guo Y. Chen Z. (2002). Protective effect of abelmischl manihot medic against cerebral ischemia-reperfusion injury. Chin. Pharmacol. Bull., 692–695. 10.3321/j.issn:1001-1978.2002.06.026
43
Guo Y. Wang S. (2017). Clinical Study of Erhuang Ointment combined with recombinant human granulocyte-macrophage colony stimulating factor gel in the treatment of superficial second-degree burns. J. Tradit. Chin. Med. Pharm.23, 90–92. 10.13862/j.cnki.cn43-1446/r.2017.14.030
44
Guo J. Xue C. Duan J. Qian D. Tang Y. You Y. (2011). Anticonvulsant, antidepressant-like activity of abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine18, 1250–1254. 10.1016/j.phymed.2011.06.012
45
Guo X. Liu Y. Zhang Q. Shang L. (2021). Comparative study on contents of flavonoids indifferent parts of Abelmoschi Corolla. Food Drug23, 514–520.
46
Han X. Situ M. (1997). 82 cases of oral mucosal ulcer treated with abelmoschus manihot paste. Anhui J. Clin.TCM, 308–309.
47
Han X. Si T. Man L. (1997). The treatment of oral mucosal ulcers with Huang Shukui Paste: a Study of 82 cases. Anhui Clin. J. Tradit. Chin. Med., 308–310. 10.16448/j.cjtcm.1997.06.024
48
Hao J. Zhou L. (2009). Effects of total flavone of Abelmoschus Manihot L. Medic on the expression of corticotropin releasing factor in rats with post stroke depression. Anhui Med. Pharm. J.13, 1025–1027. 10.3969/j.issn.1009-6469.2009.09.008
49
Hao J. Zhou L. Si L. Jiang Q. Ma Y. Yan H. (2007). Effects of total flavone of abelmoschus manihot on pos-stroke depression in rats. China Pharm., 885–887. 10.3969/j.issn.1001-0408.2007.12.002
50
He Z. Zhou Q. Wen K. Wu B. Sun X. Wang X. et al (2019). Huangkui Lianchang Decoction ameliorates DSS-Induced ulcerative colitis in mice by inhibiting the NF-kappaB signaling pathway. Evid. Based Complement. Altern. Med.2019. 10.1155/2019/1040847
51
He Z. Zhou Q. Wu B. Wen K. Wang X. Chen Y. (2020). Huangkui Lianchang decoction improving inflammatory effect of DSS-Induced UC mice by regulating IL-6/STAT3 Pathway and IL-23/IL-17 inflammatory axis. China J. Chin. Med.35, 821–826. 10.16368/j.issn.1674-8999.2020.04.183
52
He X. Wei X. Wang Q. Xi Z. (2023). Curative effect of Huangkui Lianchang Decoction and Western medicine in treatment of inflammatory bowel disease. Liaoning J. Tradit. Chin. Med.50, 90–93. 10.13192/j.issn.1000-1719.2023.05.025
53
Hong G. Quan H. Yu K. Quan X. (2021). The relationship between the size of abelmoschus manihot buds and pollen development stages. Mod. Hortic.44, 4–5. 10.14051/j.cnki.xdyy.2021.19.002
54
Hou J. Qian J. Li Z. Gong A. Zhong S. Qiao L. et al (2020). Bioactive compounds from abelmoschus manihot L. alleviate the progression of multiple myeloma in mouse model and improve bone marrow microenvironment. Onco Targets Ther.13, 959–973. 10.2147/OTT.S235944
55
Hsuan C. Tsai I. T. Fang L. Chang T. Chen Y. Houng H. et al (2024). Aibika flower Flavonoid extract exhibits antiulcer activity in a murine model of ethanol-induced Acute Gastric injury. J. Med. Food27, 615–626. 10.1089/jmf.2024.k.0015
56
Hu Y. (2016). Application of chuangling liquid in skin damage caused by Winter disease treatment in summer with pasting therapy. Chin. J. Emerg. Tradit. Chin. Med.25, 2008–2010. 10.3969/j.issn.1004-745X.2016.10.060
57
Hu C. Dai M. Chen J. Xuan Z. (2011). Therapeutic effects of total flavones of abelmoschus Manihot (L.) medic on chronic nephritis of heat-dampness type and its influence on immune adherence ability of red blood cell. J. Anhui TCM Coll.30, 57–61. 10.3969/j.issn.1000-2219.2011.01.021
58
Huang Z. Chen J. (2012). Efficacy analysis of treating 28 cases of ulcer stage bedsores by the Chuangling liquid plus Shengji Yuhong ointment. J. TCM Clin. Res.4, 108–110. 10.3969/j.issn.1674-7860.2012.18.066
59
Jain P. S. Todarwal A. A. Bari S. B. Surana S. J. (2011). Analgesic Activity of Abelmoschus manihot extracts. Int. J. Pharmacol.7, 716–720. 10.3923/ijp.2011.716.720
60
Jayachandran M. Wu Z. Ganesan K. Khalid S. Chung S. M. Xu B. (2019). Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact.303, 62–69. 10.1016/j.cbi.2019.02.017
61
Jayachandran M. Zhang T. Wu Z. Liu Y. Xu B. (2020). Isoquercetin regulates SREBP-1C via AMPK pathway in skeletal muscle to exert antihyperlipidemic and anti-inflammatory effects in STZ-induced diabetic rats. Mol. Biol. Rep.47, 593–602. 10.1007/s11033-019-05166-y
62
Jiang Q. Dong L. Fang M. Li J. Chen Z. Ma C. (2006). Antiviral action of total flavone of abelmoschus Manihot L medic in vitro. Anhui Med. J., 93–94. 10.3969/j.issn.1009-6469.2006.02.005
63
Karadeniz F. Oh J. H. Jo H. J. Seo Y. Kong C. S. (2021). Myricetin 3-O-β-D-Galactopyranoside exhibits potential anti-osteoporotic properties in human bone marrow-derived mesenchymal stromal cells via stimulation of osteoblastogenesis and suppression of adipogenesis. Cells10, 1–12. 10.3390/cells10102690
64
Kim H. Dusabimana T. Kim S. R. Je J. Jeong K. Kang M. C. et al (2018). Supplementation of abelmoschus manihot ameliorates diabetic nephropathy and hepatic steatosis by activating autophagy in mice. Nutrients10, 1703. 10.3390/nu10111703
65
Li D. (2016). Observation on the effect and nursing of chuangling liquid combined with Zhilou Fumigationand wash lotion on postoperative wound of mixed hemorrhoids. Mod. Distance Educ. Chin. Med.14, 116–117. 10.3969/j.issn.1672-2779.2016.09.050
66
Li D. (2017). Observation on the curative effect and nursing of chuangling solution combined with Photon therapeutic instrument on postoperative mixed hemorrhoids. China J. TCMDE15, 121–123. 10.3969/j.issn.1672-2779.2017.10.053
67
Li J. (1993). Erhuangointment for the treatment of erosive hand-foottinea (Athlete’s foot). Jiangxi Univ. Tradit. Chin. Med.62.
68
Li J. Yu R. Lin X. Wang K. (2006). Pharmacological Study of abelmoschus manihot flower extract on oral mucosal ulcers in rabbits. J. Shandong Univ. Tradit. Chin. Med., 497–498. 10.16294/j.cnki.1007-659x.2006.06.028
69
Li J. Zhang J. Wang M. (2016a). Extraction of flavonoids from the flowers of abelmoschus manihot (L.) medic by modified supercritical CO2 extraction and determination of antioxidant and anti-adipogenic activity. Molecules21, 1–9. 10.3390/molecules21070810
70
Li Z. Wu S. Quan X. Jin M. (2016b). Study on flowering and seed setling biology of abelmoschus manihot. Seed35, 25–28. 10.16590/j.cnki.1001-4705.2016.02.025
71
Li P. Chen Y. Lin H. Ni Z. Zhan Y. Wang R. et al (2017). Abelmoschus manihot – a traditional Chinese medicine versus losartan potassium for treating IgA nephropathy: study protocol for a randomized controlled trial. Trials18, 170–173. 10.1186/s13063-016-1774-6
72
Li M. Song C. Wang S. Xue Y. Ma Y. (2018). Effects of total flavone of Abelmoschl manihot combined with glipizide on model rats of diabetic nephropathy. Chin. J. Pharmacol. Vigil.15, 12–15. 10.3969/j.issn.1672-8629.2018.01.003
73
Li W. He W. Xia P. Sun W. Shi M. Zhou Y. et al (2019). Total extracts of abelmoschus manihot L. Attenuates Adriamycin-Induced renal tubule injury via suppression of ROS-ERK1/2-Mediated NLRP3 inflammasome activation. Front. Pharmacol.10, 567–578. 10.3389/fphar.2019.00567
74
Li D. Chang J. Wang Y. Du X. Xu J. Cui J. et al (2024a). Hyperoside mitigates photoreceptor degeneration in part by targeting cGAS and suppressing DNA-induced microglial activation. Acta Neuropathol. Commun.12, 76–88. 10.1186/s40478-024-01793-0
75
Li Y. Wu C. Song C. Liu S. Nan Z. (2024b). Efficacy and safety of Huangkui capsule for diabetic nephropathy: a systematic review and meta-analysis. Medicine103, e38417–e38417. 10.1097/MD.0000000000038417
76
Li Q. Zhang H. He Y. Zhang H. Han C. (2025). Inhibition of colorectal cancer metastasis by total flavones of abelmoschus manihot via LncRNA AL137782-mediated STAT3/EMT pathway regulation. Curr. Pharm. Des.31, 219–232. 10.2174/0113816128298998240828060306
77
Lin X. Gao K. Miao X. Wu Q. (2020a). Study on prevention and treatment effect of Jiahua Tablets on postoperative AKI in patients with coronary intervention. Chongqing Med.49, 4055–4057. 10.3969/j.issn.1671-8348.2020.24.003
78
Lin X. Wang Y. Miao X. Gao K. Wu Q. (2020b). Clinical study on Jiahua tablet in preventing and treating contrast-medium nephropathy in patients undergoing coronary intervention. Mod. J. Integr. Tradit. West Med.29, 3882–3885. 10.3969/j.issn.1008-8849.2020.35.002
79
Liu Y. (2019). Research on the application value of photon therapeutic apparatus irradiation combined with chuangling liquid wet compression in the nursing of lung cancer patients with diabetic foot. Chin. J. Feet Health28, 49–50. 10.19589/j.cnki.issn1004-6569.2019.13.049
80
Liu M. Jiang Q. Rao J. Zhou L. (2009). Protective effect of total flavones of Abelmoschus manihot L. medic against poststroke depression injury in mice and its action mechanism. Anat. Rec. Hob.292, 412–422. 10.1002/ar.20864
81
Liu S. Jiang W. Wu B. (2010). Research progress on the chemical composition and pharmacological activity of abelmoschus manihot Mod Chin med. 12, 5–9. 10.13313/j.issn.1673-4890.2010.08.006
82
Liu Z. Li Y. Zhou Y. Yong T. Xu J. Lu Y. (2012). The progress of research on the chemical composition and pharmacokinetics of abelmoschus manihot. Cent. South Pharm.10, 839–841. 10.3969/j.issn.1672.2981.2012.11.012
83
Liu J. Guo S. Duan J. Yan H. Qian D. Tang H. et al (2016). Analysis and utilization value discussion of multiple chemical composition in different tissues of Abelmoschus manihot. Zhongguo Zhong Yao Za Zhi41, 3782–3791. 10.4268/cjcmm20162013
84
Liu S. Ye L. Tao J. Ge C. Huang L. Yu J. (2017). Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm. Biol.56, 1–11. 10.1080/13880209.2017.1412467
85
Liu L. Duan P. Yan S. Wang H. Wang Z. Huo X. (2018). Antibacterial effeet of Chuanglingye in vitro and its effeet on rat skin and external diseases model withsensitive bacteria infection. Jiangsu Med.44, 1370–1372. 10.19460/j.cnki.0253-3685.2018.12.004
86
Liu B. Tu Y. Ni G. Yan J. Yue L. Li Z. et al (2021a). Total flavones of abelmoschus manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-Dependent m6A modification-mediated NLRP3-Inflammasome activation and PTEN/PI3K/Akt signaling. Front. Pharmacol.12, 667644–667673. 10.3389/fphar.2021.667644
87
Liu C. Jia Y. Qiu Y. (2021b). Ethyl acetate fraction of Abelmoschus manihot (L.) medic flowers exerts inhibitory effects against oxidative stress in H2O2-Induced HepG2 cells and D-Galactose-Induced aging mice. J. Med. Food24, 997–1009.
88
Liu C. Jia Y. Qiu Y. (2021c). Ethyl acetate fraction of Abelmoschus manihot (L.) medic flowers exerts inhibitory effects against oxidative stress in H2O2-Induced HepG2 cells and D-Galactose-Induced aging mice. J. Med. Food24, 997–1009. 10.1089/jmf.2021.K.0053
89
Liu H.-J. Miao H. Yang J.-Z. Liu F. Cao G. Zhao Y.-Y. (2023). Deciphering the role of lipoproteins and lipid metabolic alterations in ageing and ageing-associated renal fibrosis. Ageing Res. Rev.85, 101861. 10.1016/j.arr.2023.101861
90
Lou Y. Yu X. Sun X. Miao Y. Xu W. Zha M. (2022). Experimental Study on the intervention effects of qikui granules on type 2 diabetes rats with osteoporosis. J. West Chin. Med.35, 25–28. 10.12174/j.issn.2096-9600.2022.06.07
91
Lu J. Wang Y. Zhang S. Li J. Li C. Xu X. et al (2020a). Huang-Kui-Si-Wu Formula decreases uremic toxin production by modulating intestinal microbial metabolic pathways. Acta Pharm. Sin.55, 1229–1236. 10.16438/j.0513-4870.2019-0852
92
Lu T. Bian Y. Zhu Y. Guo M. Yang Y. Guo J. et al (2020b). HUANGKUISIWUFANG inhibits pyruvate dehydrogenase to improve glomerular injury in anti-Thy1 nephritis model. J. Ethnopharmacol.253, 112682. 10.1016/j.jep.2020.112682
93
Lu J. Wang Y. Peng Y. Xu X. Chen C. Duan J. et al (2021). Effect of Huangkui Siwu Formula on metabolism and transportation pathway of urotoxin p-cresyl sulfate in vivo. Zhong Cao Yao52, 176–185. 10.7501/j.issn.0253-2670.2021.01.021
94
Luan F. Wu Q. Yang Y. Lv H. Liu D. Gan Z. et al (2020). Traditional uses, chemical constituents, biological properties, clinical settings, and toxicities of abelmoschus manihot L.: a comprehensive review. Front. Pharmacol.11, 1068. 10.3389/fphar.2020.01068
95
Luo M. Liu Z. Hu Z. He Q. (2022). Quercetin improves contrast-induced acute kidney injury through the HIF-1α/lncRNA NEAT1/HMGB1 pathway. Pharm. Biol.60, 889–898. 10.1080/13880209.2022.2058558
96
Lv D. Cheng X. Tang L. Jiang M. (2017). The cardioprotective effect of total flavonoids on myocardial ischemia/reperfusion in rats. Biomed. and Pharmacother.88, 277–284. 10.1016/j.biopha.2017.01.060
97
Mao Z. Shen S. Wan Y. Sun W. Chen H. Huang M. et al (2015). Huangkui capsule attenuates renal fibrosis in diabetic nephropathy rats through regulating oxidative stress and p38MAPK/Akt pathways, compared to α-lipoic acid. J. Ethnopharmacol.173, 256–265. 10.1016/j.jep.2015.07.036
98
Miao H. (2013). Observation on the efficacy of wet compression with chuangling liquid combined with heat-sensitive moxibustion in the treatment of stage Ⅲ - Ⅳ Pressure Ulcers in Elderpy StrokeuPatients.eJ. Nurs. sci.20p 68–70. 10.16460/j.issn1008-9969.2013.22.005
99
Miao H. Liu F. Wang Y.-N. Yu X.-Y. Zhuang S. Guo Y. et al (2024a). Targeting Lactobacillus johnsonii to reverse chronic kidney disease. Signal Transduct. Target. Ther.9, 195. 10.1038/s41392-024-01913-1
100
Miao H. Wang Y.-N. Yu X.-Y. Zou L. Guo Y. Su W. et al (2024b). Lactobacillus species ameliorate membranous nephropathy through inhibiting the aryl hydrocarbon receptor pathway via tryptophan-produced indole metabolites. Br. J. Pharmacol.181, 162–179. 10.1111/bph.16219
101
Neurath M. F. (2014). New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol.7, 6–19. 10.1038/mi.2013.73
102
Niu R. Lin X. Xu Z. Liu J. Wang Y. Miao X. et al (2023). Efficacy observation of Jiahua tablet on contrast agent-induced kidney injury based on KIM-1 and MCP-1. Shanxi J. TCM39, 18–20. 10.20002/j.issn.1000-7156.2023.04.007
103
Ogunro O. B. Olasehinde O. R. (2024). Neuroinflammatory response and redox-regulation activity of hyperoside in manganese-induced neurotoxicity model of Wistar rats. Curr. Aging Sci.17, 220–236. 10.2174/0118746098277166231204103616
104
Oh J. H. Karadeniz F. Lee J. I. Park S. Y. Seo Y. Kong C. S. (2020). Anticatabolic and anti-inflammatory effects of myricetin 3-O-β-d-Galactopyranoside in UVA-Irradiated dermal cells via repression of MAPK/AP-1 and activation of TGFβ/Smad. Molecules25, 1331–1345. 10.3390/molecules25061331
105
Page M. J. McKenzie J. E. Bossuyt P. M. Boutron I. Hoffmann T. C. Mulrow C. D. et al (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ372, 71–88. 10.1136/bmj.n71
106
Pan X. Tao J. Jiang S. Zhu Y. Qian D. Duan J. (2018). Characterization and immunomodulatory activity of polysaccharides from the stems and leaves of Abelmoschus manihot and a sulfated derivative. Int. J. Biol. Macromol.107, 9–16. 10.1016/j.ijbiomac.2017.08.130
107
Park Y. I. Cha Y. E. Jang M. Park R. Namkoong S. Kwak J. et al (2020). The flower extract of abelmoschus manihot (Linn.) increases cyclin D1 expression and activates cell proliferation. J. Microbiol. Biotechnol.30, 1044–1050. 10.4014/jmb.2002.02024
108
Pei W. (2018). Observation on the efficacy of chuangling liquid combined with traditional Chinese medicine fumigation and washing in the treatment of diabetic foot. Prac. J. Clin. Nurs.3, 73–74. 10.3969/j.issn.2096-2479.2018.39.059
109
Pei S. Li Y. (2021). Huangkui capsule in combination with leflunomide improves immunoglobulin A nephropathy by inhibiting the TGF-β1/Smad3 signaling pathway. Clinics76, 2904–2911. 10.6061/clinics/2021/e2904
110
Puel C. Mathey J. Kati-Coulibaly S. Davicco M. J. Lebecque P. Chanteranne B. et al (2005). Preventive effect of Abelmoschus manihot (L.) medik. On bone loss in the ovariectomised rats. J. Ethnopharmacol.99, 55–60. 10.1016/j.jep.2005.01.047
111
Qian Q. Zhou Y. An X. (2023). Research progress of treating diabetic kidney disease with abelmoschus manihot. Henan J. Tradit. Chin. Med.43, 1257–1264. 10.16367/j.issn.1003-5028.2023.08.0249
112
Qiao L. Fang L. Zhu J. Xiang Y. Xu H. Sun X. et al (2021). Total flavone of abelmoschus manihot ameliorates TNBS-induced colonic fibrosis by regulating Th17/Treg balance and reducing extracellular matrix. Front. Pharmacol.12, 769793. 10.3389/fphar.2021.769793
113
Qin G. Ma J. Huang Q. Yin H. Han J. Li M. et al (2018). Isoquercetin improves hepatic lipid accumulation by activating AMPK pathway and suppressing TGF-β signaling on an HFD-Induced nonalcoholic Fatty Liver Disease rat model. Int. J. Mol. Sci.19, 4126–4154. 10.3390/ijms19124126
114
Qiu Y. Ai P. Song J. Liu C. Li Z. (2017). Total flavonoid extract from Abelmoschus manihot (L.) medic flowers attenuates D-Galactose-Induced oxidative stress in mouse liver through the Nrf2 pathway. J. Med. Food20, 557–567. 10.1089/jmf.2016.3870
115
Rey D. Fernandes T. A. Sulis P. M. Goncalves R. Sepulveda R M. Silva Frederico M. J. et al (2020). Cellular target of isoquercetin from Passiflora ligularis Juss for glucose uptake in rat soleus muscle. Chem. Biol. Interact.330, 109198. 10.1016/j.cbi.2020.109198
116
Riemschneider S. Hoffmann M. Slanina U. Weber K. Hauschildt S. Lehmann J. (2021). Indol-3-Carbinol and Quercetin Ameliorate chronic DSS-Induced colitis in C57BL/6 mice by AhR-Mediated anti-inflammatory mechanisms. Int. J. Environ. Res. Public Health18, 2262. 10.3390/ijerph18052262
117
Shao G. (2012). The characteristics and cultivation techniques of abelmoschus manihot. Mod. Agric. Sci. Technol., 110–119. 10.3969/j.issn.1007-5739.2012.23.069
118
Shao X. Yu J. Ni W. (2017). The Study of the renal protective effects and mechanisms of qikui granules on STZ-Induced diabetic nephropathy in rats. Zhong Yao Cai40, 2437–2440. 10.13863/j.issn1001-4454.2017.10.045
119
Sheng J. (2014). Treatment and nursing experience of chuangling liquid in 20 patients with oral pressure ulcers caused by tracheal intubation. Hunan J. TCM30, 102–103. 10.16808/j.cnki.issn1003-7705.2014.12.057
120
Shi R. Tao Y. Tang H. Wu C. Fei J. Ge H. et al (2023). Abelmoschus Manihot ameliorates the levels of circulating metabolites in diabetic nephropathy by modulating gut microbiota in Non-obese diabetes mice. Microb. Biotechnol.16, 813–826. 10.1111/1751-7915.14200
121
Shu X. Chen H. Zhou H. Jiang Y. Zou J. Liu Z. (2016). HPLC determination of 9 components in chuanglingye. Cent. South Pharm.14, 1354–1358. 10.7539/j.issn.1672-2981.2016.12.017
122
Silva D. d.C. Orfali G. D. C. Santana M. G. Palma J. K. Y. Assuncao I. R. d.O. Marchesi I. M. et al (2022). Antitumor effect of isoquercetin on tissue vasohibin expression and colon cancer vasculature. Oncotarget13, 307–318. 10.18632/oncotarget.28181
123
Song Y. Fu Z. Zhu X. Zhang J. Bai W. Song B. (2024). The flower of Abelmoschus manihot (L.) medik exerts antioxidant effects by regulating the Nrf2 signalling pathway in scald injury. Wound Repair Regen.32, 123–134. 10.1111/wrr.13146
124
Su J. Yang F. Kang X. Liu J. Tao Y. Diao Q. et al (2022). Chalcone derivatives from abelmoschus manihot seeds restrain NLRP3 inflammasome assembly by inhibiting ASC oligomerization. Front. Pharmacol.13, 932198. 10.3389/fphar.2022.932198
125
Sun H. Yuan Y. Zhou L. Yu J. Sun Z. (2014). Effeet of YuKuiOing on the expression of monoeyte chemoattractant protein-1 and fractalkine induced by AGEs. Chin. J. Diabetes22, 942–946. 10.3969/j.issn.1006-6187.2014.10.017
126
Sun J. Wen C. Zhang B. Bao X. (2019). The protective effect of quercetin on vascular endothelial progenitor cells by regulating PI3K/Akt signaling pathway and its mechanisms. Chin. Pharmacol. Bull.35, 85–90. 10.3969/j.issn.1001-1978.2019.01.017
127
Sun Z. Liu W. Zhang S. Tian S. Aikemu A. (2024). Optimization of flavonoid extraction from abelmoschus manihot flowers using ultrasonic techniques: predictive modeling through response surface methodology and deep neural network and biological activity assessment. Molecules29, 2610. 10.3390/molecules29112610
128
Tang L. Pan W. Zhu G. Liu Z. Lv D. Jiang M. (2017). Total flavones of abelmoschus manihot enhances angiogenic ability both in vitro and in vivo. Oncotarget8, 69768–69778. 10.18632/oncotarget.19264
129
Tao P. Huo J. Chen L. (2024a). Bibliometric analysis of the relationship between Gut Microbiota and chronic kidney disease from 2001–2022. Integr. Med. Nephrol. Androl.11. 10.1097/IMNA-D-23-00017
130
Tao Y. Liu J. Li M. Wang H. Fan G. Xie X. et al (2024b). Abelmoschus manihot (L.) medik. seeds alleviate rheumatoid arthritis by modulating JAK2/STAT3 signaling pathway. J. Ethnopharmacol.325, 117641. 10.1016/j.jep.2023.117641
131
Teng D. Ji C. (2016). Clinical observation of 30 cases of stage II pressure ulcers treated with the combination of chuangling liquid and mepilex. Jiangsu J. TCM.48, 33–34.
132
Todarwal A. Jain P. Bari S. (2011). Abelmoschus manihot Linn: ethnobotany, phytochemistry and pharmacology. Asian J. Tradit. Med.6, 114–129.
133
Topcu-Tarladacalisir Y. Sapmaz-Metin M. Mercan Z. Ercetin D. (2024). Quercetin Attenuates endoplasmic Reticulum stress and apoptosis in TNBS-Induced colitis by inhibiting the glucose regulatory protein 78 activation. Balk. Med. J.41, 30–37. 10.4274/balkanmedj.galenos.2023.2023-10-9
134
Tu Y. Fang Q. Sun W. Liu B. Liu Y. Wu W. et al (2020). Total flavones of abelmoschus manihot remodels Gut Microbiota and inhibits microinflammation in chronic renal failure progression by targeting autophagy-mediated macrophage polarization. Front. Pharmacol.11, 566611. 10.3389/fphar.2020.566611
135
Wang C. Feng Z. Xu Y. Yao C. Shi Y. (2016). Clinical Study on the treatment of 20 cases of chronic lower extremity ulcers with chuangling liquid-loaded collagen. Jiangsu J. TCM48, 37–40.
136
Wang C. Shi Y. Tang M. Zhang X. Gu Y. Liang X. et al (2017). Isoquercetin ameliorates cerebral impairment in focal ischemia through Anti-Oxidative, Anti-Inflammatory, and anti-apoptotic effects in primary culture of rat hippocampal neurons and hippocampal CA1 Region of rats. Mol. Neurobiol.54, 2126–2142. 10.1007/s12035-016-9806-5
137
Wang Q. Wei Y. Dong Y. (2020). Clinical effect between chuangling solution wet-compressing and povid one iodine-diluent wet-compressing in thetreatment of puncture site infection afterperipherally inserted central catheter placement. Int J Nurs Integr Med (Chin and Eng).6, 61–64. 10.11997/nitcwm.202001014
138
Wang R. Chen T. Wang Q. Yuan X. Duan Z. Feng Z. et al (2021). Total flavone of abelmoschus manihot ameliorates stress-induced microbial alterations drive intestinal barrier injury in DSS colitis. Drug Des. Devel Ther.15, 2999–3016. 10.2147/dddt.s313150
139
Wang K. Lu C. Wang T. Qiao C. Lu L. Wu D. et al (2022a). Hyperoside suppresses NLRP3 inflammasome in Parkinson's disease via Pituitary Adenylate Cyclase-Activating Polypeptide. Neurochem. Int.152, 105–121. 10.1016/j.neuint.2021.105254
140
Wang S. W. Chang C. C. Hsuan C. F. Chang T. H. Chen Y. L. Wang Y. Y. et al (2022c). Neuroprotective effect of abelmoschus manihot flower extracts against the H2O2-Induced cytotoxicity, oxidative stress and inflammation in PC12 cells. Bioeng. (Basel)9, 596–611. 10.3390/bioengineering9100596
141
Wang L. Feng F. Zhou J. Ruan Y. Zha M. Pu Q. et al (2023a). Short-term effect of Qi-Kui granules combined with dulaglutide in the treatment of clinical stage of diabetic kidney disease in the elderly. Pract. J. Geriatr. Med.37, 348–351. 10.3969/j.issn.1003-9198.2023.04.007
142
Wang M. Cai Y. Fang Q. Liu Y. Wang J. Chen J. et al (2023b). Inhibition of ferroptosis of renal tubular cells with total flavones of Abelmoschus manihot alleviates diabetic tubulopathy. Anat. Rec. Hob.306, 3199–3213. 10.1002/ar.25123
143
Wang J. Liao E. Ren Z. Wang Q. Xu Z. Wu S. et al (2024a). Extraction and in vitro skincare effect assessment of polysaccharides extract from the roots of abelmoschus manihot (L.). Molecules29, 2109. 10.3390/molecules29092109
144
Wang S. W. Lee T. L. Chang T. H. Chen Y. L. Houng H. Y. Chang N. et al (2024b). Antidiabetic potential of abelmoschus manihot flower extract: in vitro and intracellular studies. Medicina60, 1211–1212. 10.3390/medicina60081211
145
Wang T. Hu L. Li R. Ren H. Li S. Sun Q. et al (2024c). Hyperoside inhibits EHV-8 infection via alleviating oxidative stress and IFN production through activating JNK/Keap1/Nrf2/HO-1 signaling pathways. J. Virol.98, 159–174. 10.1128/jvi.00159-24
146
Wang Y. Li C. Li J. Zhang S. Zhang Q. Duan J. et al (2024d). Abelmoschus manihot polysaccharide fortifies intestinal mucus barrier to alleviate intestinal inflammation by modulating Akkermansia muciniphila abundance. Acta Pharm. Sin. B14, 3901–3915. 10.1016/j.apsb.2024.06.002
147
Wang Y.-n. Wu X. Shan Q.-y. Yang Q. Yu X.-y. Yang J.-h. et al (2025). Acteoside-containing caffeic acid is bioactive functional group of antifibrotic effect by suppressing inflammation via inhibiting AHR nuclear translocation in chronic kidney disease. Acta Pharmacol. Sin.46, 2975–2988. 10.1038/s41401-025-01598-4
148
Wei C. Wang C. Li R. Bai Y. Wang X. Fang Q. et al (2023). The pharmacological mechanism of Abelmoschus manihot in the treatment of chronic kidney disease. Heliyon9, 22017. 10.1016/j.heliyon.2023.e22017
149
Weingarden A. R. Vaughn B. P. (2017). Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes8, 238–252. 10.1080/19490976.2017.1290757
150
Wen J. Chen Z. (2006). Protective effect of pharmacological preconditioning of total flavone of abelmoschl Manihot on cerebral ischemicre perfusion injury in rats. J Anhui Med Univ.667–669. 10.19405/j.cnki.issn1000-1492.2006.06.018
151
Wen J. Feng Z. Xu Y. Yao C. Shi Y. (2017). Protective effect of total flavones of abelmoschl manihot on hippocampal neurons subjected to anoxia-reoxygenation injury. Chin. J. Clin. Pharmacol. Ther.22, 367–372.
152
Wen R. Xie G. Li X. Qin M. (2015). Advance research on chemical constituents and pharmacological activities of abelmoschus manihot (L.) medic. Chin. Wild Plant Res.34, 37–44. 10.3969/j.issn.1006-9690.2015.02.010
153
Wu Z. (2011). The effects of unsaturated fatty acids from Jin Hua Kui seeds on blood lipids and liver function in experimental hyperlipidemic rats. Zhong Cheng Yao33, 1245–1247. 10.3969/j.issn.1001-1528.2011.07.045
154
Wu Q. Lin X. Miao X. Liu F. (2020). Effects of Jiahua Tablet on renal injury index of patients with nephropathy after coronary angiography or PCI. Nanjing J. TCM36, 189–192. 10.14148/j.issn.1672-0482.2020.0189
155
Wu B. Zhou Q. He Z. Wang X. Sun X. Chen Y. (2021). Protective effect of the Abelmoschus manihot flower extract on DSS-Induced ulcerative colitis in mice. Evid. Based Complement. Altern. Med.2021, 7422792–7427431. 10.1155/2021/7422792
156
Wu C. Tang H. Cui X. Li N. Fei J. Ge H. et al (2024). A single-cell profile reveals the transcriptional regulation responded for Abelmoschus manihot (L.) treatment in diabetic kidney disease. Phytomedicine130, 155642–155655. 10.1016/j.phymed.2024.155642
157
Xia J. Wan Y. Wu J.-j. Yang Y. Xu J.-F. Zhang L. et al (2024). Therapeutic potential of dietary flavonoid hyperoside against non-communicable diseases: targeting underlying properties of diseases. Crit. Rev. Food Sci. Nutr.64, 1340–1370. 10.1080/10408398.2022.2115457
158
Xie S. Cao W. Hu Y. Chu X. Zhu B. Yu J. et al (2017a). Effects of Qi Kui granules on urine protein and inflammatory markers in patients with type 2 diabetic nephropathy. World Sci-Tech J. TCM Mod.19, 149–153.
159
Xie S. Cao W. Hu Y. Chu X. Zhu B. Yu J. et al (2017b). Effects of Qi Kui granules on urine protein and inflammatory markers in patients with type 2 diabetic nephropathy. World Sci. Technol. - Mod. Tradit. Chin. Med.19, 149–153. 10.11842/wst.2017.01.022
160
Xu Z. (2010). Clinical observation of chuangling liquid in the treatment of 16 cases of stage Ⅳ Pressure Ulcprs. Jiangsu J. TCM42, 47–49. 10.3969/j.issn.1672-397X.2010.11.033
161
Xu F. (2017). Influence of chuangling lotion on wound-surface healing after operation of anal fistula and abscess. China J. Coloproctol.37, 65–66. 10.3969/j.issn.1000-1174.2017.08.029
162
Xu Z. Cai H. (2010). Treatment of stage Ⅳ Pressure Ulcprs with uhe Combination cf Chuangling Lcquid and Ildophor WetiCompresswon.cJ. Nurs. Sci.25, 56–57. 10.3870/hlxzz.2010.21.056
163
Xu Z. Qian L. Niu R. Wang Y. Yang Y. Liu C. et al (2022). Mechanism of abelmoschus manihot L. in the treatment of contrast-induced nephropathy on the basis of network pharmacology analysis. Front. Nephrol.2, 834513–834515. 10.3389/fneph.2022.834513
164
Xu Z. Wang Y. Yang W. Han W. Ma B. Zhao Y. et al (2024). Total extracts from Abelmoschus manihot (L.) alleviate radiation-induced cardiomyocyte ferroptosis via regulating redox imbalances mediated by the NOX4/xCT/GPX4 axis. J. Ethnopharmacol.334, 1185–1198. 10.1016/j.jep.2024.118582
165
Xue J. Cao S. Tang T. Wen Y. Ye B. (2020). External use of chuangling liquid for reducing mass caused by plasma cell mastitis. Chin. J. Integr. Med.40, 418–421. 10.7661/j.cjim.20200119.135
166
Xue C. Zhang X. Ge H. Tang Q. Jeon J. Zhao F. et al (2023). Total flavone of flowers of Abelmoschus manihot (L.) Medic inhibits the expression of adhesion molecules in primary mesenteric arterial endothelial cells and ameliorates dextran sodium sulphate-induced ulcerative colitis in mice. Phytomedicine112, 154713–154722. 10.1016/j.phymed.2023.154713
167
Yan J. Y. Ai G. Zhang X. J. Xu H. J. Huang Z. M. (2015). Investigations of the total flavonoids extracted from flowers of Abelmoschus manihot (L) Medic against α-naphthylisothiocyanate-induced cholestatic liver injury in rats. J. Ethnopharmacol.172, 202–213. 10.1016/j.jep.2015.06.044
168
Yan Q. Sheng M. Yu J. Huang L. Gu L. Zhang S. et al (2018). Effect of Qikui granule on microalbuminuria and progress in patients with early diabetic nephropathy. Chin. J. Integr. Tradit. West Med.38, 430–434. 10.7661/j.cjim.20180326.080
169
Yang N. (1997). Treatment of 38 Cases of Ischemic Ulcers with Chuangling Liquid. Nanjing J TCM Univ.310–311. 10.14148/j.issn.1672-0482.1997.05.035
170
Yang J. (2016). Erhuang ointment for the treatment of superficial second-degree burns. Jilin Univ. Tradit. Chin. Med.36, 905–908. 10.13463/j.cnki.jlzyy.2016.09.012
171
Yang B. Sun Z. Yu J. Gao F. Liu S. (2010). Effect of yukuiqing on expression of connective tissue growth factor in human real mesangial cells incubated with AGEs. China J. Tradit. Chin. Med. Pharm.25, 1102–1105.
172
Yang C. Zhu X. Yu J. Zhang D. (2016). Effect of Qikui Granules on the expression of TGF -β1 and MCP -1 in kidney of rats with diabetic nephropathy. Chin. J. Med. Guid13, 8–11.
173
Yang B. L. Zhu P. Li Y. R. Xu M. M. Wang H. Qiao L. C. et al (2018). Total flavone of Abelmoschus manihot suppresses epithelial-mesenchymal transition via interfering transforming growth factor-β1 signaling in Crohn's disease intestinal fibrosis. World J. Gastroenterol.24, 3414–3425. 10.3748/wjg.v24.i30.3414
174
Yang W. Cai L. He N. Meng H. (2023). Clinical Study of retention enema of Huangkui Lianchang Decoction in the treatment of ulcerative colitis with internal accumulation of damp - heat syndrome. Chin. Pharm.32, 100–103. 10.3969/j.issn.1006-4931.2023.15.022
175
Ye B. Xue J. Feng Z. Tang T. Yao C. (2021). Study on the effect of Chuangling Liquid on wound inflammation of non-actating mastitis. Mod. J. Integr. Med.30, 1825–1829. 10.3969/j.issn.1008-8849.2021.17.001
176
Yin C. Zhou Q. Hu W. Zhou Y. (2013). Application of Chuangling fluid in postoperative wound healing in patients with anal fistula or perianal abscess. Nurs. Res.27, 1491–1492. 10.3969/j.issn.1009-6493.2013.15.041
177
Yin S. Mei Y. Wei L. Zou L. Cai Z. Wu N. et al (2021). Comparison of multiple bioactive constituents in the corolla and other parts of Abelmoschus manihot. Molecules26, 1864. 10.3390/molecules26071864
178
Yu H. Tang H. Wang M. Xu Q. Yu J. Ge H. et al (2023a). Effects of total flavones of Abelmoschus manihot (L.) on the treatment of diabetic nephropathy via the activation of solute carriers in renal tubular epithelial cells. Biomed. Pharmacother.169, 115899. 10.1016/j.biopha.2023.115899
179
Yu H. Wang M. Yu J. Tang H. Xu Q. Cheng N. et al (2023b). Evaluation of the efficacy of Abelmoschus manihot (L.) on diabetic nephropathy by analyzing biomarkers in the glomeruli and proximal and distal convoluted tubules of the kidneys. Front. Pharmacol.14, 1215996–1216012. 10.3389/fphar.2023.1215996
180
Zha M. Sun H. Zhang S. Ye L. Huang L. Chen S. et al (2020). Effect of Jiahua Tablets as adjuvant treatment on serum inflammatory factors levels and renal function in patients with early diabetic nephropathy. Guangxi Med.42, 2059–2061. 10.11675/j.issn.0253-4304.2020.16.01
181
Zhang Y. Zhou Q. (2012). Granulation process and prescription for Jiahua Tablet. Chin. Pharm.23, 2519–2521. 10.6039/j.issn.1001-0408.2012.27.08
182
Zhang A. Lu Y. Xu H. Chen B. Xue P. (1997). Pharmacology Study of chuamgningyie. Jiangsu Pharm. Clin. Res., 14–18. 10.13664/j.cnki.pcr.1997.04.006
183
Zhang H. Dong L. Jiang Q. Fang M. Li J. Chen Z. et al (2006). Effect of anti-infection oral mucosa ulcers of guinea-pig and antibacterial in vitro of total flavone of abelmoschus Manihot L medic. Anhui Med. J., 810–811. 10.3969/j.issn.1009-6469.2006.11.005
184
Zhang L. Li P. Xing C. Zhao J. He Y. Wang J. et al (2014). Efficacy and safety of abelmoschus manihot for primary glomerular disease: a prospective, Multicenter randomized controlled clinical trial. Am. J. Kidney Dis.64, 57–65. 10.1053/j.ajkd.2014.01.431
185
Zhang D. Qian H. Yang B. Chen Y. (2017). The effect of total flavonoids from abelmoschus manihot on intestinal fibrosis in TNBS-Induced crohn’s disease mice. Jiangsu J. Tradit. Chin. Med.49, 76–79. 10.3969/j.issn.1672-397X.2017.03.029
186
Zhang D. Zhu P. Liu Y. Shu Y. Zhou J. Y. Jiang F. et al (2019a). Total flavone of Abelmoschus manihot ameliorates Crohn's disease by regulating the NF-B and MAPK signaling pathways. Int. J. Mol. Med.44, 324–334. 10.3892/ijmm.2019.4180
187
Zhang W. Cheng C. Han Q. Chen Y. Guo J. Wu Q. et al (2019b). Flos Abelmoschus manihot extract attenuates DSS-induced colitis by regulating gut microbiota and Th17/Treg balance. Biomed. Pharmacother.117, 109162. 10.1016/j.biopha.2019.109162
188
Zhang J. Fu Z. L. Chu Z. X. Song B. W. (2020). Gastroprotective activity of the total flavones from Abelmoschus manihot (L.) medic flowers. Evid. Based Complement. Altern. Med.2020, 6584945. 10.1155/2020/6584945
189
Zhang S. Ruan Y. Zhou J. Ye L. Jia J. Yu J. et al (2021). Effect of Qikui particles on diabetic kidney disease and its influence on urinary CTGF and serum sICAM-1. Shaanxi J. Tradit. Chin. Med.42, 308–311. 10.3969/j.issn.1000-7369.2021.03.009
190
Zhang H.-X. Li Y.-Y. Liu Z.-J. Wang J.-F. (2022a). Quercetin effectively improves LPS-induced intestinal inflammation, pyroptosis, and disruption of the barrier function through the TLR4/NF-κB/NLRP3 signaling pathwa in vivo and in vitro. Food Nutr. Res.66, 1–23. 10.29219/fnr.v66.8948
191
Zhang H. Zhang D. Qian H. Wang Y. Zeng L. (2022b). TFA regulating autophagy through activation of AMPK/mTOR pathway to improve intestinal fibrosis of CD. Inf. TCM39, 1–10. 10.19656/j.cnki.1002-2406.20220701
192
Zhang D. Liu J. Lv L. Chen X. Qian Y. Zhao P. et al (2024). Total flavone of Abelmoschus manihot regulates autophagy through the AMPK/mTOR signaling pathway to treat intestinal fibrosis in Crohn's disease. J. Gastroenterol. Hepatol.39, 1586–1596. 10.1111/jgh.16560
193
Zhao H. Zhu Y. (2013). Study on the promotion of wound healing by Collagen modified with chuangling liquid. World J. Integr. Tradit. West Med.8, 417–418. 10.13935/j.cnki.sjzx.2013.04.018
194
Zhao H. Zhao T. Li P. (2024a). Gut Microbiota-derived metabolites: a new perspective of traditional Chinese medicine against diabetic kidney disease. Integr. Med. Nephrol. Androl.11, 1–11. 10.1097/IMNA-D-23-00024
195
Zhao X. Wang S. He X. Wei W. Huang K. (2024b). Quercetin prevents the USP22-Snail1 signaling pathway to ameliorate diabetic tubulointerstitial fibrosis. Food Funct.15, 11990–12006. 10.1039/d4fo03564j
196
Zhao K. Zhou F. Lu Y. Gao T. Wang R. Xie M. et al (2025). Hyperoside alleviates depressive-like behavior in social defeat mice by mediating microglial polarization and neuroinflammation via TRX1/NLRP1/Caspase-1 signal pathway. Int. Immunopharmacol.145, 113731. 10.1016/j.intimp.2024.113731
197
Zheng X. Liu Z. Li S. Wang L. Lv J. Li J. et al (2016). Identification and characterization of a cytotoxic polysaccharide from the flower of Abelmoschus manihot. Int. J. Biol. Macromol.82, 284–290. 10.1016/j.ijbiomac.2015.10.004
198
Zhong X. Jia J. Tan R. Wang L. (2024). Hederagenin improves Adriamycin-induced nephropathy by inhibiting the JAK/STAT signaling pathway. Integr. Med. Nephrol. Androl.11, 1–6. 10.1097/IMNA-D-22-00016
199
Zhou L. An X. F. Teng S. C. Liu J. S. Shang W. B. Zhang A. H. et al (2012). Pretreatment with the total flavone glycosides of Flos Abelmoschus manihot and hyperoside prevents glomerular podocyte apoptosis in Streptozotocin-Induced diabetic nephropathy. J. Med. Food15, 461–468. 10.1089/jmf.2011.1921
200
Zhou L. Peng W. Zhou M. Jiang J. Xu P. Tan C. (2019). Effect of chuangling liquid on melanogenesis of rat immortalized melanocytes. Acta Chin. Med. Pharmacol.47, 19–23. 10.19664/j.cnki.1002-2392.190039
201
Zhou S. Fan K. K. Gu L. F. Yu B. Y. Chai C. Z. (2022). Anti-inflammatory effects of Abelmoschus manihot (L.) Medik. on LPS-induced cystitis in mice: potential candidate for cystitis treatment based on classic use. Chin. J. Nat. Med.20, 321–331. 10.1016/s1875-5364(22)60140-7
202
Zhou Q. Tang H. Wang Y. Hua Y. Ouyang X. Li L. (2025). Hyperoside mitigates PCOS-associated adipogenesis and insulin resistance by regulating NCOA2-mediated PPAR-γ ubiquitination and degradation. Life Sci.364, 123417–123431. 10.1016/j.lfs.2025.123417
203
Zhou X. Chen Z. (2016). Effects of total flavones of abelmoschl manihotonrats with experimental hyperglycemia. Chin. J. Clin. Pharmacol. Ther.21, 617–620.
204
Zhu G. S. Tang L. Y. Lv D. L. Jiang M. (2018). Total flavones of abelmoschus manihot exhibits pro-angiogenic activity by activating the VEGF-A/VEGFR2-PI3K/Akt signaling axis. Am. J. Chin. Med.46, 567–583. 10.1142/s0192415x18500295
Summary
Keywords
Abelmoschus manihot , Huangkui Capsule, total flavonoids of Abelmoschus Manihot, Jiahua Tablets, Chuangling Liquid, Huangkui Lianchang Decoction
Citation
Xue C, Ge H, Liu Y, Zhao Y, Huang W, Lu Z, Ye Q, Chen X and Cao Z (2025) Therapeutic potential of Abelmoschus manihot: mechanisms of action and clinical use in traditional Chinese medicine formulas. Front. Pharmacol. 16:1709530. doi: 10.3389/fphar.2025.1709530
Received
20 September 2025
Revised
24 November 2025
Accepted
26 November 2025
Published
10 December 2025
Volume
16 - 2025
Edited by
James Olukayode Olopade, University of Ibadan, Nigeria
Reviewed by
Ling Chen, Shanghai Municipal Hospital of Traditional Chinese Medicine, China
Carsten Tsun Ka Kwok, Hong Kong Polytechnic University, Hong Kong SAR, China
Cheng Wang, Chengdu Medical College, China
Aditya Singh Ranout, Institute of Himalayan Bioresource Technology (CSIR), India
Updates
Copyright
© 2025 Xue, Ge, Liu, Zhao, Huang, Lu, Ye, Chen and Cao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengyu Cao, zycao@cpu.edu.cn; Xiaoli Chen, 540935437@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.