There was a mistake in the order of the Figures 2–5 as published. The figures and their corresponding figure captions for these results were all misaligned. The corrected figures and their captions appear below.
FIGURE 2
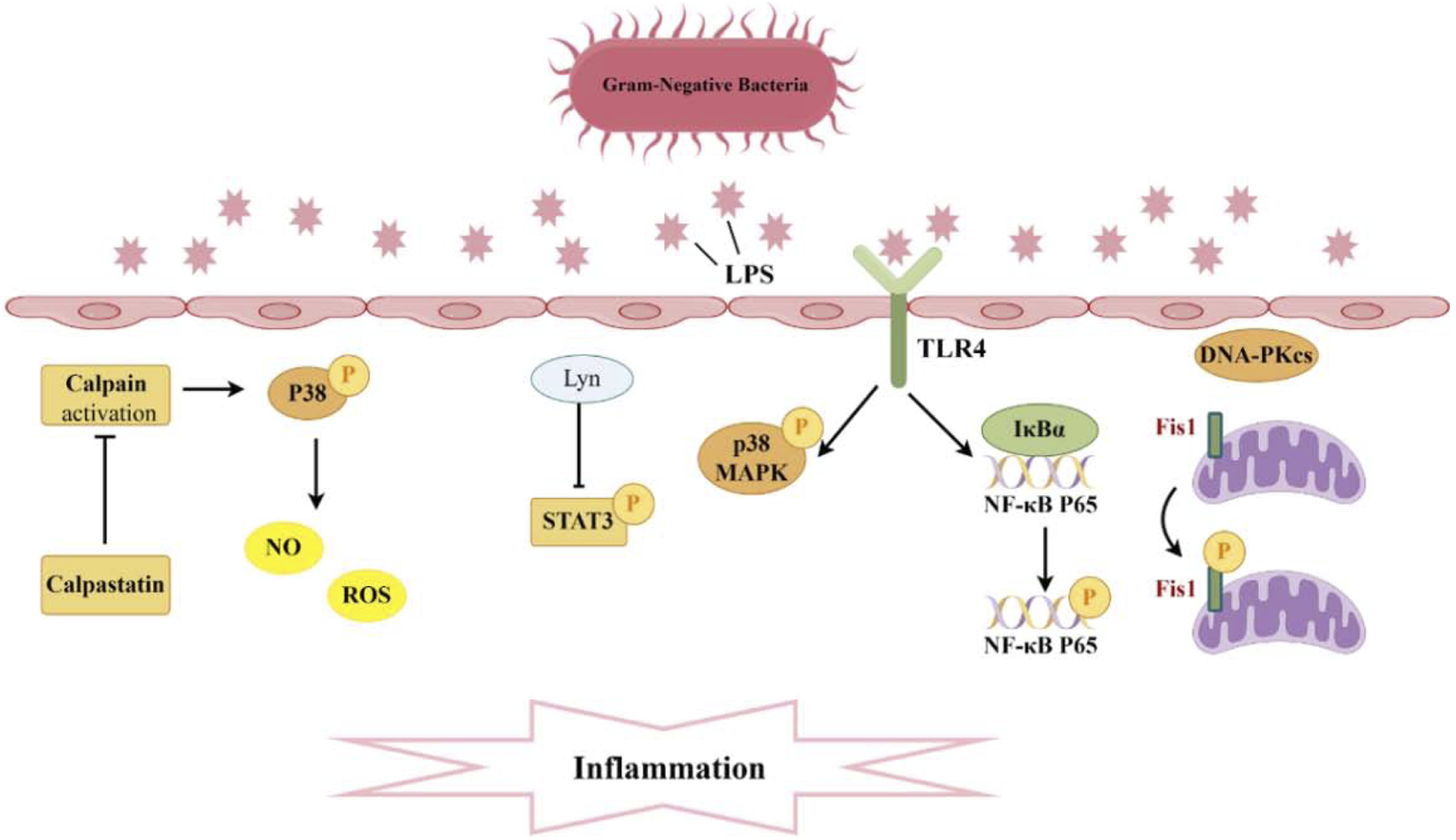
Pathogenesis associated with Phosphorylation and sepsis-induced acute kidney injury. A significant production of inflammatory cytokines occurs within the glomeruli and renal tubular interstitium. The interaction between TLR4 and LPS activates the phosphorylation of P38 MAPK and NF-κB, while Lyn inhibits the phosphorylation of STAT3, thereby diminishing levels of inflammatory mediators. Moreover, the suppression of calpain activation can curtail P38 phosphorylation, reduce ROS, and consequently mitigate endothelial cell apoptosis. Additionally, DNA-PKcs can induce the phosphorylation of Fis1, resulting in mitochondrial dysfunction and subsequent cell apoptosis.
FIGURE 3
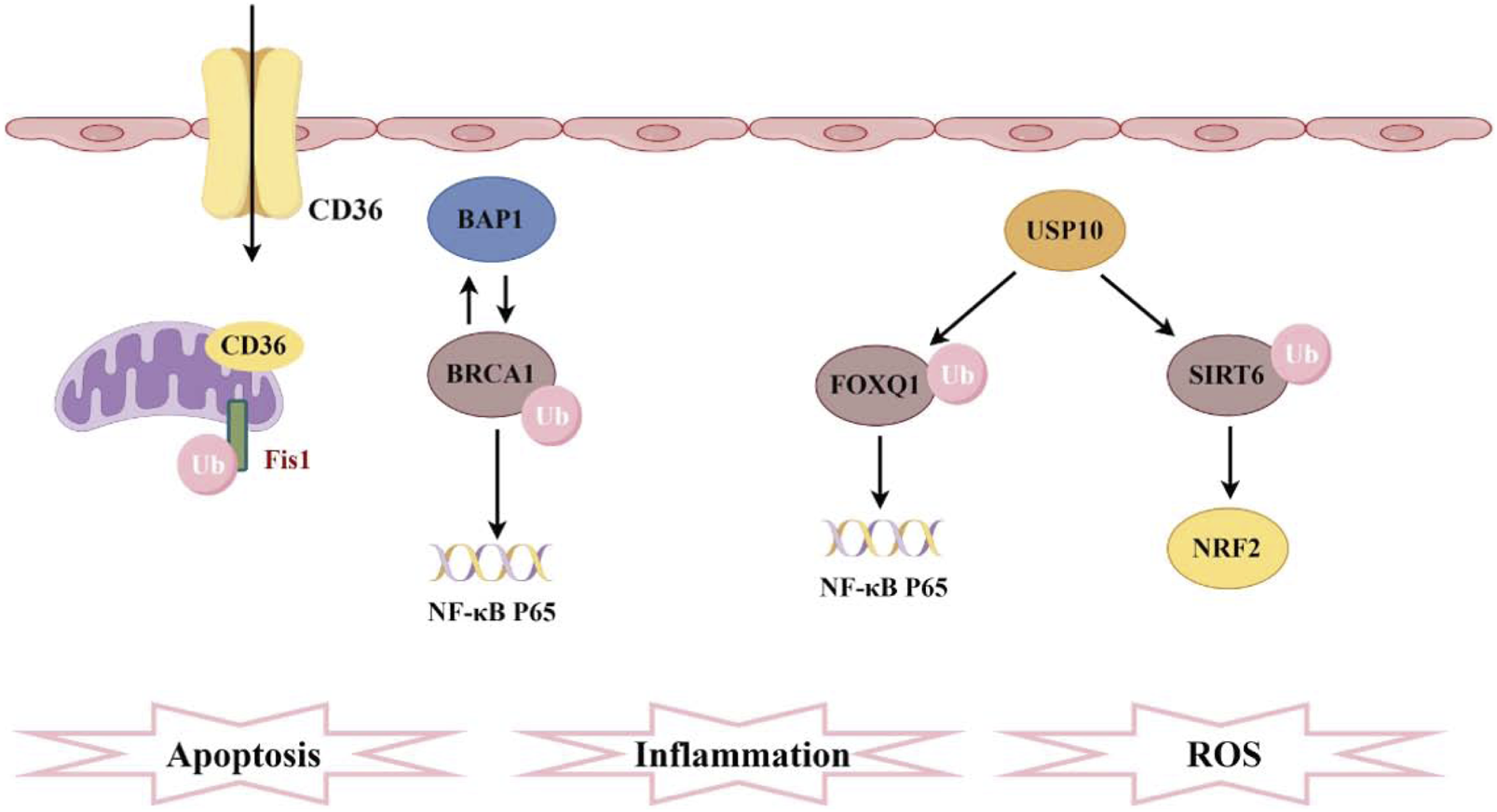
Pathogenesis associated with ubiquitination and sepsis-induced acute kidney injury. CD36 promotes ferroptosis in proximal tubular cells by regulating the ubiquitination of FSP1. The interaction between BAP1 and BRCA1 enhances the stability of BRCA1 protein through deubiquitination, thereby inhibiting NF-κB. Furthermore, FOXQ1, deubiquitinated by USP10, ameliorates cellular inflammation and apoptosis. Additionally, USP10 interacts with SIRT6 to suppress its ubiquitination, alleviating oxidative stress.
FIGURE 4
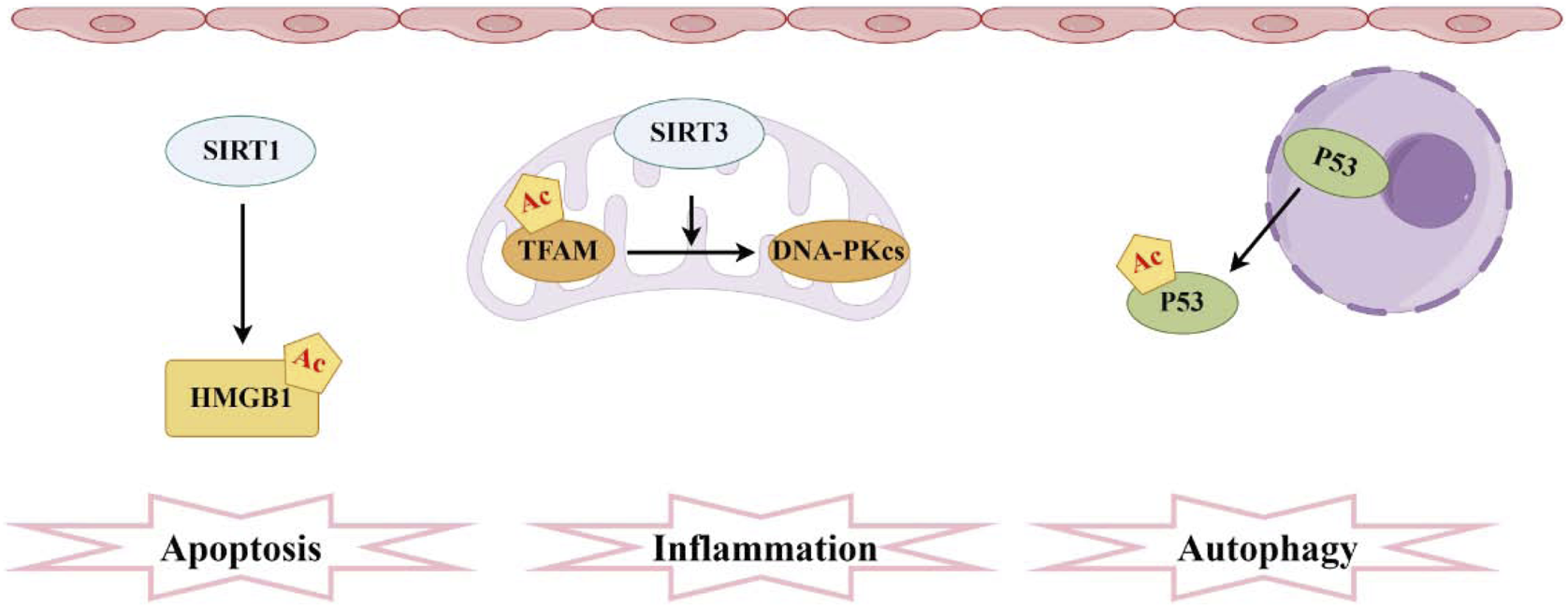
Pathogenesis associated with acetylation and sepsis-induced acute kidney injury. The sirtuin family comprises the most prevalent deacetylases, with SIRT1 mediating the acetylation of HMGB1 and SIRT3 facilitating the acetylation of TFAM. Moreover, elevated levels of acetylated P53 in RTECs hinder autophagy.
FIGURE 5
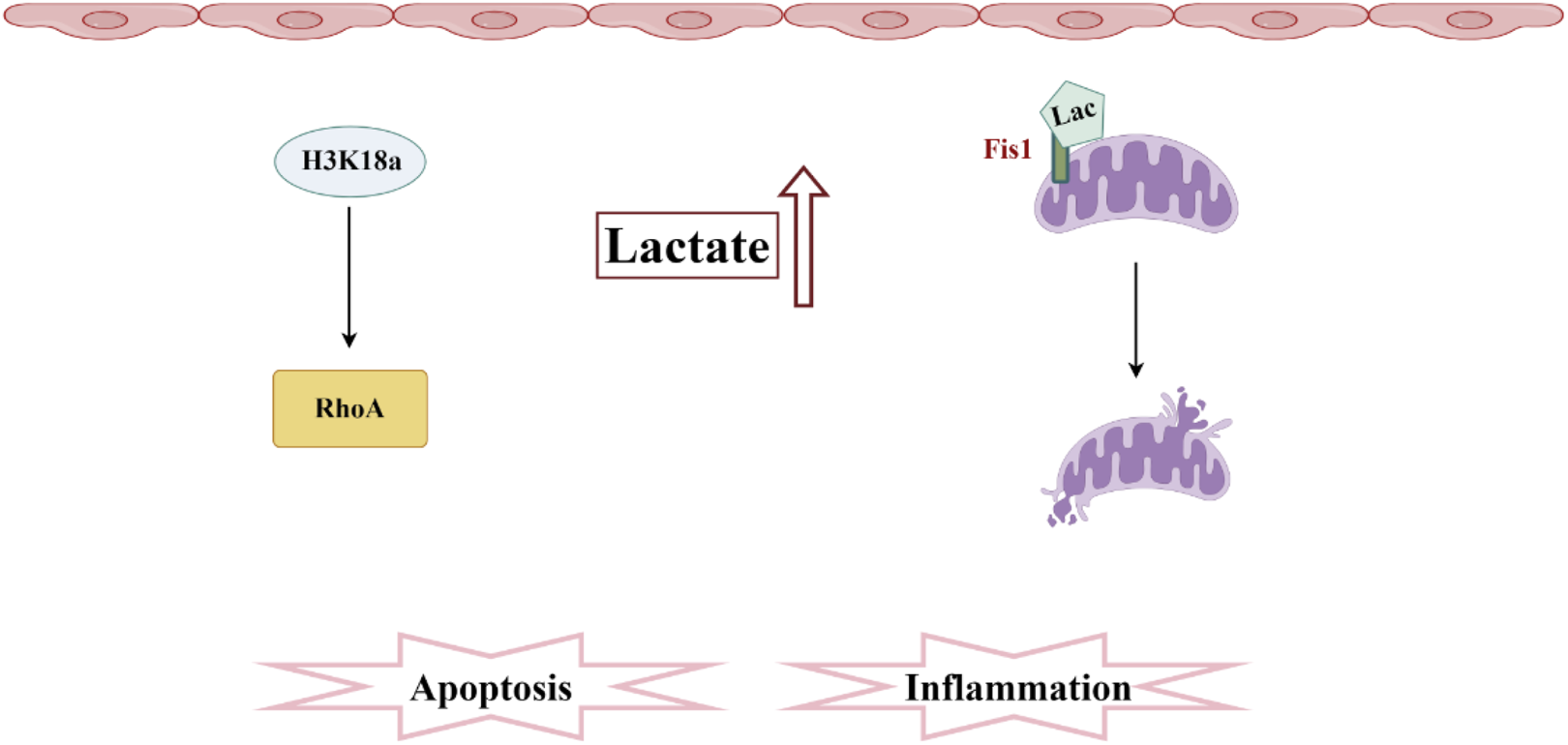
Pathogenesis associated with lactylation and sepsis-induced acute kidney injury. In SA-AKI, elevated levels of lactate and histone lactylation, particularly the increased lactylation of H3K18, activate RhoA protein, thereby triggering inflammation and apoptosis. Additionally, lactate mediates the lactylation of Fis1, promoting mitochondrial fission and exacerbating cellular apoptosis.
There was a mistake in the caption of Figure 5 as published. Instead of “Pathogenesis associated with Ubiquitination and sepsis-induced acute kidney injury” it should be “Pathogenesis associated with lactylation and sepsis-induced acute kidney injury”. The corrected caption of Figure 5 appears below.
The original article has been updated.
Statements
Generative AI statement
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Summary
Keywords
acute kidney injury, sepsis, post-translational modifications, sepsis-induced acute, kidney injury, inflammation
Citation
Song L, Jiang W, Liu K, Wang J, Gong W, Yu J and Zheng R (2025) Correction: Post-translational modifications in sepsis-induced acute kidney injury: mechanisms and perspectives. Front. Pharmacol. 16:1710281. doi: 10.3389/fphar.2025.1710281
Received
22 September 2025
Accepted
25 September 2025
Published
10 October 2025
Volume
16 - 2025
Edited and reviewed by
Edgar Jaimes, Memorial Sloan Kettering Cancer Center, United States
Updates
Copyright
© 2025 Song, Jiang, Liu, Wang, Gong, Yu and Zheng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangquan Yu, yujiangquan2021@163.com; Ruiqiang Zheng, zhengruiqiang2021@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.