Abstract
Objective:
The potential drug-drug interactions (pDDIs) seriously affecting the prognosis of colorectal cancer (CRC) patients. This study aimed to identify the risk factors of pDDIs in hospitalized CRC patients and construct a risk prediction model to provide a reference for clinical rational drug use.
Research design and methods:
A retrospective cohort study was conducted, enrolling 2,868 patients from a tertiary hospital. Medscape was used to assess pDDIs, and a risk prediction model was constructed based on independent risk factors.
Results:
A total of 1,790 (62.41%) patients experienced at least one pDDIs, with 1,458 (50.84%) cases of severe pDDIs. The number of drug varieties, hypoalbuminemia, and treatment were independent risk factors. The area under the receiver operating characteristic curve (AUC) of the model in the training and validation sets were 0.826 and 0.824, respectively. The calibration curve showed a good agreement between the predicted probability and the actual occurrence probability. Decision curve analysis (DCA) demonstrated that the model had a positive net clinical benefit within a wide range of 10%–90%.
Conclusion:
The constructed model has good predictive performance and can be used to identify high-risk patients with pDDIs in clinical practice, thereby improving the safety of drug use.
1 Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide, with a global incidence and mortality rate ranking among the top three of all cancers, imposing a heavy burden on public health and social economy (Bray et al., 2024; Filho et al., 2025). With the advancement of medical technology, the treatment of CRC has evolved into a comprehensive model combining surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy (Wang et al., 2025a; Vilz et al., 2024; Yoshino et al., 2023). However, most CRC inpatients are elderly and often accompanied by multiple chronic comorbidities such as hypertension, diabetes, and cardiovascular diseases, leading to complex medication regimens (Alqenae et al., 2023; Chu et al., 2025; Endalifer et al., 2025; Guo et al., 2025).
Potential drug-drug interactions (pDDIs) refer to the potential changes in the efficacy or toxicity of one drug caused by the simultaneous or sequential use of another drug, which may result in adverse drug reactions (ADRs), treatment failure, or even life-threatening events (Alkathiri et al., 2025; Demirkapu and Cavdar, 2024; Khanna et al., 2024; Meslamani and Abou Hajal, 2025). For CRC inpatients, the risk of pDDIs is significantly increased due to the long treatment cycle, diverse therapeutic drugs, and combined use of drugs for comorbidities. Previous studies have shown that the incidence of pDDIs in cancer patients ranges from 55.7% to 95.0%, and severe pDDIs can increase the length of hospital stay and the mortality rate (Pinto et al., 2023; Choudary et al., 2022; Wondm et al., 2023; Milovanovic and Pejcic, 2025; Ismail et al., 2020). However, there are few studies specifically focusing on the risk factors of pDDIs in CRC inpatients, and the existing studies have small sample sizes and lack reliable risk prediction models, making it difficult to provide effective guidance for clinical prevention and intervention of pDDIs.
Therefore, this study conducted a retrospective study to systematically analyze the risk factors of pDDIs in hospitalized CRC patients, construct a risk prediction model using logistic regression, and evaluate the performance of the model. The study is expected to provide a scientific basis for clinical pharmacists and physicians to identify high-risk patients with pDDIs, formulate individualized medication plans, and reduce the occurrence of ADRs, thereby improving the prognosis and quality of life of CRC inpatients.
2 Materials and methods
2.1 Study design and participants
A retrospective study was conducted, and the study population was hospitalized CRC patients admitted to the Oncology Department of the First Affiliated Hospital of Bengbu Medical University (A large-scale comprehensive teaching hospital in Anhui province, China), from January 1 to 31 October 2023. The inclusion criteria were: (1) Patients diagnosed with CRC; (2) The types of drugs used ≥2; (4) complete medical records, including detailed medication information and clinical data. The exclusion criteria were: (1) The types of drugs used <2; (2) patients with incomplete medical records or missing key data (such as medication records, laboratory test results).
According to the above criteria, a total of 2,868 patients were included in this study. The patients were randomly divided into a training set (n = 2007, 70%) and a validation set (n = 861, 30%) in a 7:3 ratio. The flowchart was shown in Figure 1.
FIGURE 1
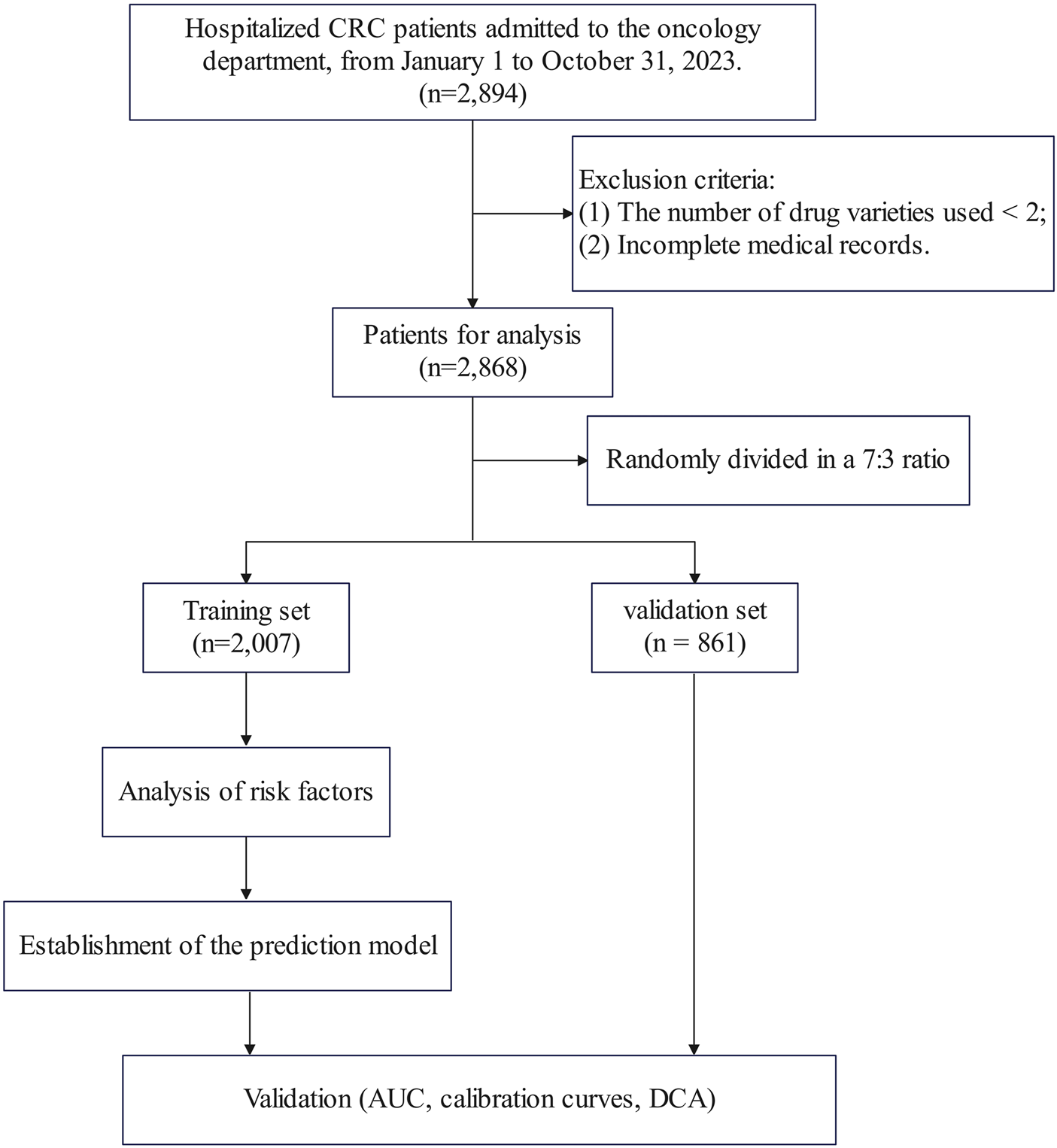
Flowchart of the study.
2.2 Ethical approval
The study protocol was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Bengbu Medical University (2024024), and the requirement for informed consent was waived due to there was no contact with the patient and the patient’s information was processed anonymously.
2.3 Data collection
A standardized data collection form was designed to extract data from the electronic medical records system, including the following aspects: age, gender, length of hospital stay, number of medication types, tumor metastasis status, and types of comorbidities (hypertension, coronary heart disease, diabetes, anemia, gastritis, renal insufficiency, hepatic insufficiency, thrombosis, cerebral infarction, intestinal obstruction, hypoproteinemia, etc.).
2.4 Assessment of pDDIs
Medscape, a widely used and authoritative drug interaction database, was used to assess pDDIs. According to the severity classification, pDDIs were divided into four types: “Contraindicated”, “Serious Use Alternative”, “Monitor Close”, “Minor”. In this study, patients with “Contraindicated” or “Serious Use Alternative” pDDIs were defined as the “Serious pDDIs group”.
The assessment of pDDIs was independently completed by two pharmacists. In case of disagreement, a third pharmacist was invited for consultation to reach a consensus.
2.5 Statistical analysis
All statistical analysis were performed using R software (Version 4.3.1) and SPSS software (Version 26.0). The statistical significance level was set at α = 0.05 (two-tailed).
Descriptive statistics: For measurement data, as they did not conform to a normal distribution, they were expressed as median (interquartile range) [M (Q1, Q3)], and the Mann-Whitney U test was used for comparison between groups. For count data, they were expressed as frequency (percentage) [n (%)], and the chi-square test or Fisher’s exact test was used for comparison between groups.
Screening of risk factors: First, univariate analysis was performed to compare the differences in variables between the serious pDDIs group and the non-serious pDDIs group. Then, variables with P < 0.1 in the univariate analysis were included in the multivariate logistic regression analysis to identify independent risk factors of serious pDDIs.
Construction and evaluation of the risk prediction model: Based on the independent risk factors identified by multivariate logistic regression and Akaike information criterion (AIC), a risk prediction model was constructed, and a nomogram was drawn using R software to visualize the model (R packages including pROC, ggplot2, rms, ResourceSelection, rmda). The receiver operating characteristic (ROC) curve, calibration curve, and decision curve analysis (DCA) are used to evaluate the discriminative ability, calibration ability, and clinical utility of the model, respectively (Xie et al., 2025).
3 Results
3.1 Baseline characteristics
A total of 2,868 hospitalized CRC patients were included in this study, including 1,701 males (59.31%) and 1,167 females (40.69%). The patients were randomly divided into a training set (n = 2,007) and a validation set (n = 861) at a ratio of 7:3. Comparison of clinical characteristics between the training and validation sets showed no statistically significant differences (P > 0.05), as detailed in Table 1.
TABLE 1
| Characteristics | Training set (n = 2,007) | Validation set (n = 861) | P value |
|---|---|---|---|
| Age, years | 60 (54, 69) | 60 (54, 69) | 0.544 |
| Gender, n (%) | | | 0.143 |
| Female | 799 (27.9%) | 368 (12.8%) | |
| Male | 1,208 (42.1%) | 493 (17.2%) | |
| Length of hospital stay, days | 4 (3, 5) | 4 (3, 5) | 0.675 |
| Number of drug varieties | 12 (9, 14) | 12 (9, 14) | 0.909 |
| Hypertension, n (%) | | | 0.157 |
| No | 1,557 (54.3%) | 647 (22.6%) | |
| Yes | 450 (15.7%) | 214 (7.5%) | |
| Coronary heart disease, n (%) | | | 0.924 |
| No | 1971 (68.7%) | 846 (29.5%) | |
| Yes | 36 (1.3%) | 15 (0.5%) | |
| Diabetes, n (%) | | | 0.902 |
| No | 1,773 (61.8%) | 762 (26.6%) | |
| Yes | 234 (8.2%) | 99 (3.5%) | |
| Anemia, n (%) | | | 0.562 |
| No | 1,879 (65.5%) | 811 (28.3%) | |
| Yes | 128 (4.5%) | 50 (1.7%) | |
| Gastritis, n (%) | | | 0.646 |
| No | 1,968 (68.6%) | 842 (29.4%) | |
| Yes | 39 (1.4%) | 19 (0.7%) | |
| Renal insufficiency, n (%) | | | 0.778 |
| No | 1,991 (69.4%) | 855 (29.8%) | |
| Yes | 16 (0.6%) | 6 (0.2%) | |
| Hepatic insufficiency, n (%) | | | 0.121 |
| No | 1,938 (67.6%) | 821 (28.6%) | |
| Yes | 69 (2.4%) | 40 (1.4%) | |
| Thrombosis, n (%) | | | 0.927 |
| No | 1,990 (69.4%) | 854 (29.8%) | |
| Yes | 17 (0.6%) | 7 (0.2%) | |
| Cerebral infarction, n (%) | | | 0.281 |
| No | 1,929 (67.3%) | 820 (28.6%) | |
| Yes | 78 (2.7%) | 41 (1.4%) | |
| Intestinal obstruction, n (%) | | | 0.505 |
| No | 1987 (69.3%) | 850 (29.6%) | |
| Yes | 20 (0.7%) | 11 (0.4%) | |
| Hypoalbuminemia, n (%) | | | 0.736 |
| No | 1,933 (67.4%) | 827 (28.8%) | |
| Yes | 74 (2.6%) | 34 (1.2%) | |
| Metastasis, n (%) | | | 0.456 |
| No | 916 (31.9%) | 406 (14.2%) | |
| Yes | 1,091 (38%) | 455 (15.9%) | |
| Treatment, n (%) | | | 0.391 |
| FOLFOX regimen | 151 (5.3%) | 77 (2.7%) | |
| FOLFIRI regimen | 155 (5.4%) | 66 (2.3%) | |
| CapeOx regimen | 670 (23.4%) | 270 (9.4%) | |
| Targeted therapy | 715 (24.9%) | 295 (10.3%) | |
| Others | 316 (11%) | 153 (5.3%) | |
| Serious pDDIs, n (%) | | | 0.383 |
| No | 976 (34%) | 434 (15.1%) | |
| Yes | 1,031 (35.9%) | 427 (14.9%) | |
Baseline characteristics of patients in the training and validation sets.
Among them, a total of 3,647 pDDIs were identified, and 1,790 patients (62.41%) were found to have experienced at least one pDDIs. Based on severity classification, the numbers of pDDIs categorized as “Contraindicated”, “Serious - Use Alternative”, “Monitor Closely”, and “Minor” were 5, 1,749, 1,876, and 17, respectively. A total of 1,458 patients were divided into the severe pDDIs group, accounting for 50.84%.
3.2 Univariate and multivariate analysis of risk factors for pDDIs
The occurrence of severe pDDIs was set as the outcome variable. Univariate logistic regression analysis was first performed for each influencing factor, the variables with p < 0.1 were then included in a multivariate logistic regression analysis. The results are presented in Table 2. The analysis revealed that the number of drug varieties, hypoalbuminemia, and treatment were risk factors for the occurrence of severe pDDIs.
TABLE 2
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | 2,007 | 0.992 (0.985–1.000) | 0.057 | 1.003 (0.992–1.013) | 0.613 |
| Gender | |||||
| Female | 799 | Reference | | | |
| Male | 1,208 | 0.955 (0.798–1.142) | 0.612 | | |
| Length of hospital stay, days | 2,007 | 1.002 (0.967–1.037) | 0.921 | | |
| Number of drug varieties | 2,007 | 1.108 (1.083–1.133) | <0.001 | 1.097 (1.068–1.128) | <0.001 |
| Hypertension | |||||
| No | 1,557 | Reference | | | |
| Yes | 450 | 0.880 (0.713–1.085) | 0.232 | | |
| Coronary heart disease | |||||
| No | 1,971 | Reference | | | |
| Yes | 36 | 1.498 (0.762–2.945) | 0.241 | | |
| Diabetes mellitus | |||||
| No | 1773 | Reference | | | |
| Yes | 234 | 1.097 (0.835–1.442) | 0.505 | | |
| Anemia | |||||
| No | 1,879 | Reference | | Reference | |
| Yes | 128 | 0.608 (0.422–0.875) | 0.007 | 0.715 (0.433–1.180) | 0.189 |
| Gastritis | |||||
| No | 1,968 | Reference | | Reference | |
| Yes | 39 | 0.414 (0.208–0.822) | 0.012 | 0.535 (0.235–1.220) | 0.137 |
| Renal insufficiency | |||||
| No | 1,991 | Reference | | Reference | |
| Yes | 16 | 0.216 (0.061–0.761) | 0.017 | 0.223 (0.047–1.063) | 0.060 |
| Hepatic insufficiency | |||||
| No | 1,938 | Reference | | | |
| Yes | 69 | 0.863 (0.534–1.396) | 0.549 | | |
| Thrombosis | |||||
| No | 1,990 | Reference | | | |
| Yes | 17 | 1.743 (0.642–4.731) | 0.275 | | |
| Cerebral infarction | |||||
| No | 1,929 | Reference | | Reference | |
| Yes | 78 | 0.648 (0.409–1.026) | 0.064 | 1.189 (0.663–2.131) | 0.561 |
| Intestinal obstruction | |||||
| No | 1,987 | Reference | | Reference | |
| Yes | 20 | 0.234 (0.078–0.701) | 0.010 | 0.392 (0.119–1.289) | 0.123 |
| Hypoalbuminemia | |||||
| No | 1,933 | Reference | | Reference | |
| Yes | 74 | 0.174 (0.093–0.324) | <0.001 | 0.240 (0.113–0.510) | <0.001 |
| Metastasis | |||||
| No | 916 | Reference | | Reference | |
| Yes | 1,091 | 0.579 (0.485–0.692) | <0.001 | 0.989 (0.767–1.276) | 0.933 |
| Treatment | |||||
| FOLFOX regimen | 151 | Reference | | Reference | |
| FOLFIRI regimen | 155 | 3.204 (1.581–6.496) | 0.001 | 3.473 (1.691–7.132) | <0.001 |
| CapeOx regimen | 670 | 1.037 (0.672–1.598) | 0.871 | 1.247 (0.799–1.947) | 0.332 |
| Targeted therapy | 715 | 0.095 (0.062–0.146) | <0.001 | 0.125 (0.080–0.197) | <0.001 |
| Others | 316 | 0.051 (0.031–0.083) | <0.001 | 0.069 (0.042–0.116) | <0.001 |
Univariate and multivariate logistic regression analysis of factors for severe pDDIs.
3.3 Establishment of the risk prediction model
Based on the independent risk factors identified through multivariate logistic regression analysis and AIC, a nomogram model was constructed to predict the occurrence of severe pDDIs in hospitalized CRC patients (Figure 2).
FIGURE 2
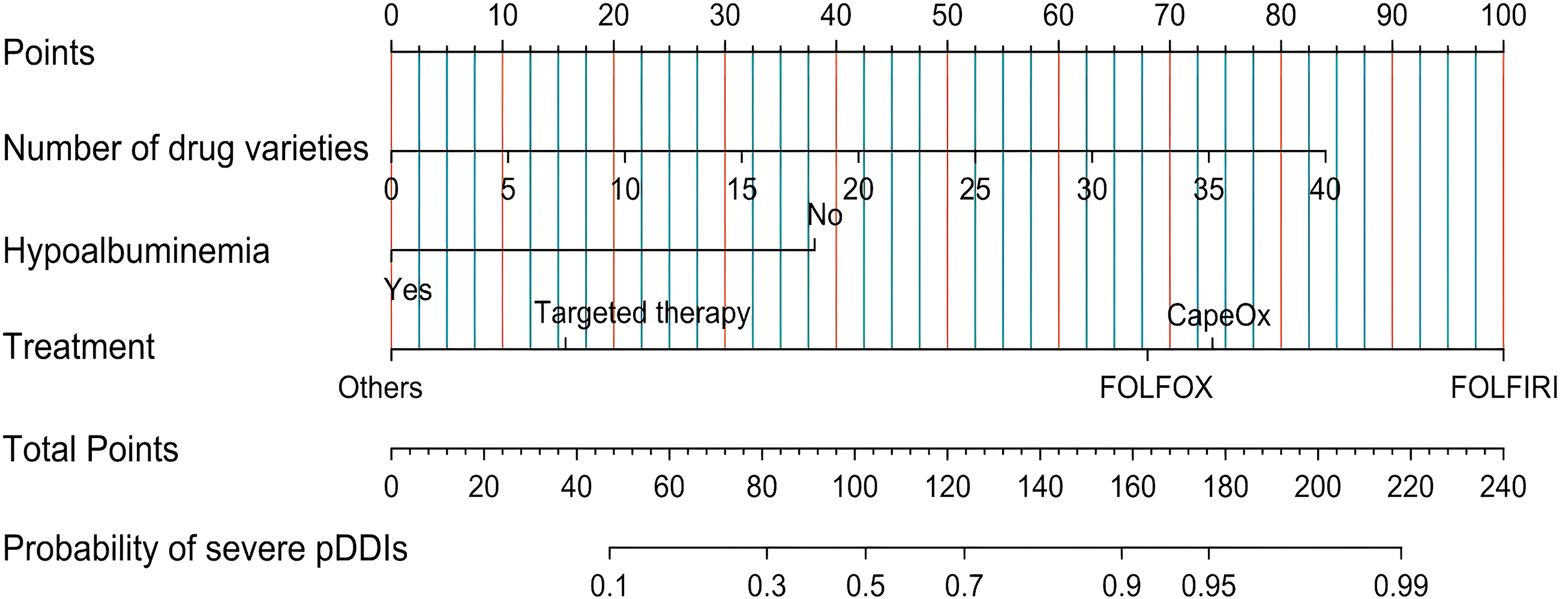
Nomogram for predicting the risk of severe pDDIs in hospitalized CRC patients. Note: For each patient, find the corresponding position of each risk factor on the upper axis, draw a vertical line downward to the “Points” axis to obtain the score of the factor, sum the scores of all factors to get the total score, and then draw a vertical line upward from the “Total Points” axis to the “Probability of severe pDDIs” axis to obtain the predicted probability of severe pDDIs.
3.4 Validation of the predictive nomogram
The performance of the nomogram model was evaluated in the training set and the validation set, respectively.
The AUC of the established nomogram model is larger than when the three influencing factors was applied separately, and the AUC of the nomogram model in the training and validation sets were 0.826 (95% CI: 0.807–0.844) and 0.824 (95% CI: 0.795–0.853), respectively, indicating favorable discriminative ability (Figure 3).
FIGURE 3
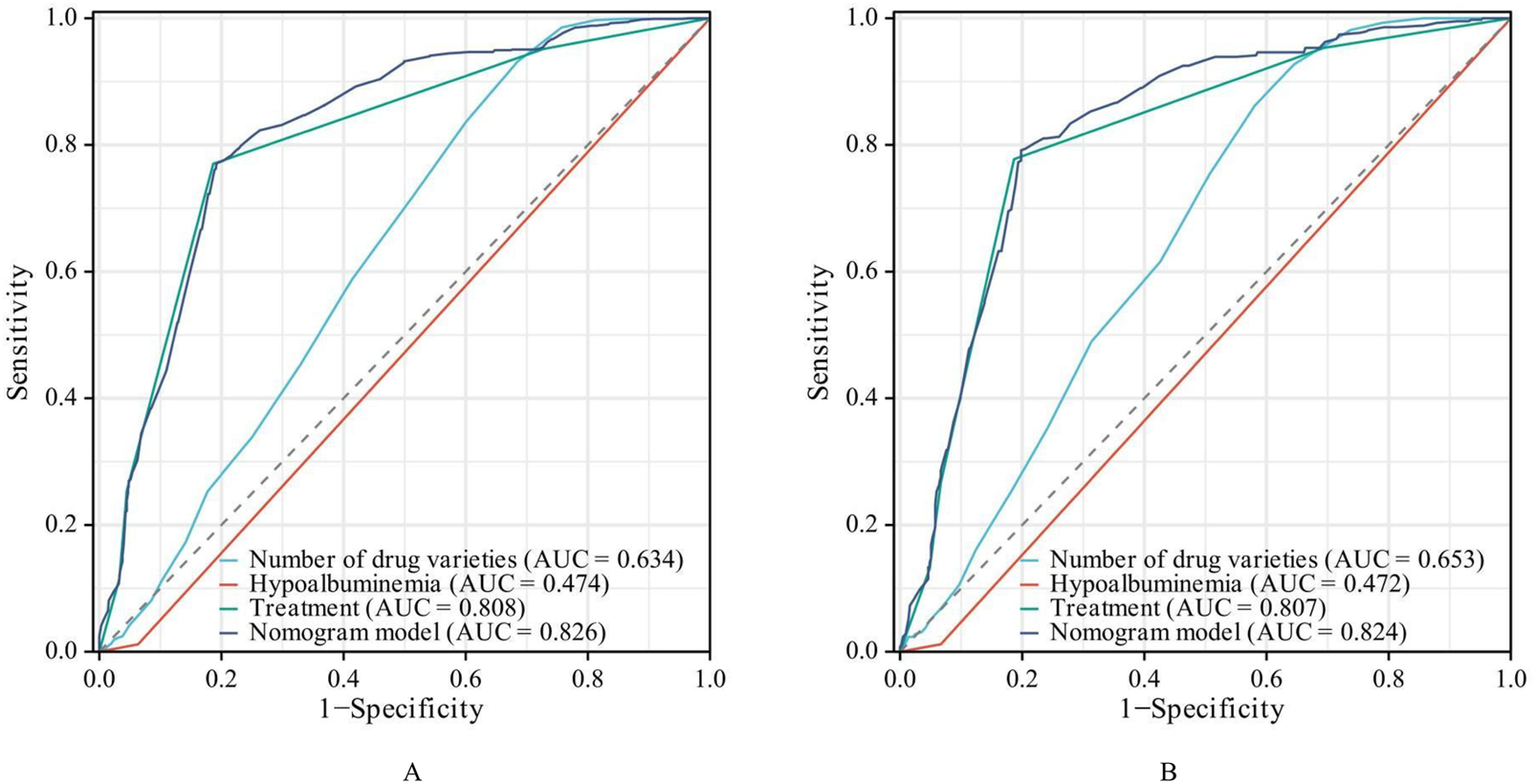
ROC Curves of the risk prediction model. ((A) training set. (B) validation set).
The calibration curve (Figure 4) showed that the predicted probability of pDDIs was in good agreement with the actual occurrence probability in both the training set and the validation set.
FIGURE 4
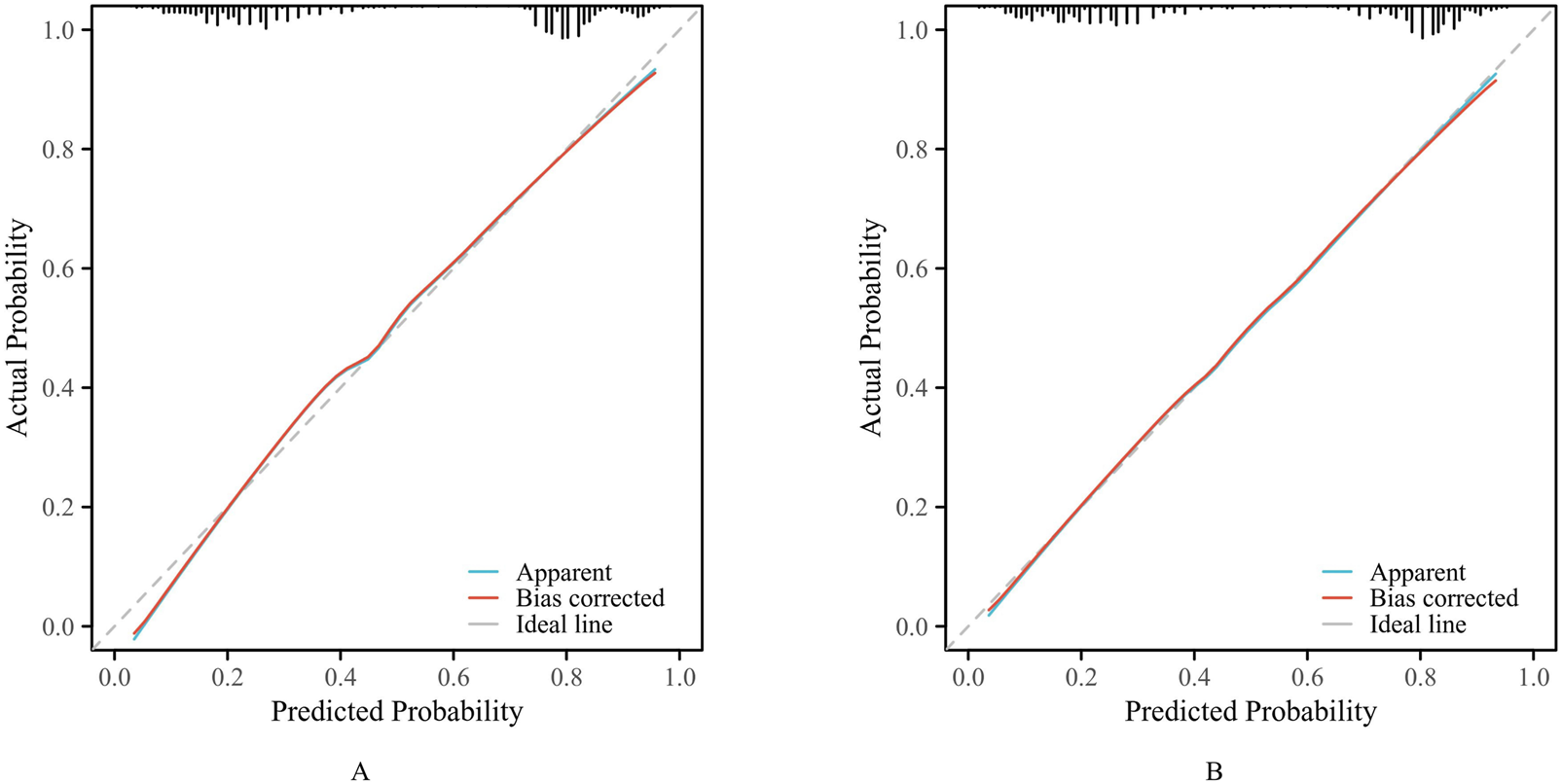
The calibration curves. ((A) training set. (B) validation set).
DCA (Figure 5) showed that when the threshold probability of pDDIs was between 10% and 90%, the net benefit of the model was higher than that of the “treat all” or “treat none” strategies in both the training set and the validation set, indicating that the model had good clinical utility.
FIGURE 5
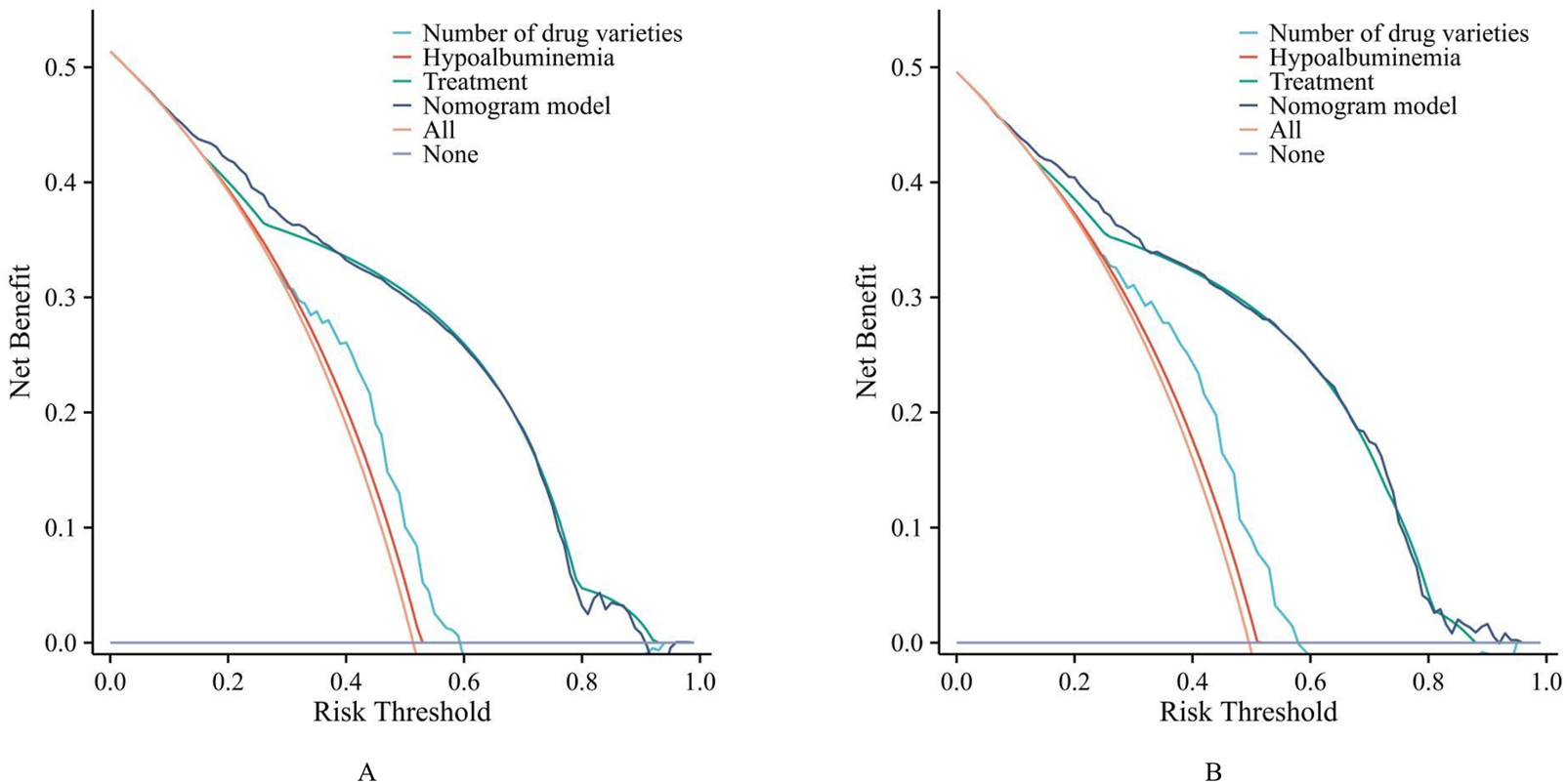
The decision curve analysis (DCA). ((A) training set. (B) validation set).
4 Discussion
This retrospective cohort study systematically analyzed the risk factors of pDDIs in 2,868 hospitalized CRC patients and constructed a risk prediction model based on the identified independent risk factors. The results showed that the incidence of pDDIs in hospitalized CRC patients was 62.41%, which was consistent with the incidence of pDDIs in cancer inpatients reported in previous studies (55.7%–95.0%) (Choudary et al., 2022; Wondm et al., 2023; Milovanovic and Pejcic, 2025; Ismail et al., 2020). In our study, severe pDDIs accounted for 50.84%, indicating that pDDIs are a common and serious problem in hospitalized CRC patients, which requires high attention from clinical medical staff. However, to the best of our knowledge, no previous studies have investigated the prediction of pDDIs risks in hospitalized CRC patients. Based on real-world evidence, this study developed a risk prediction model for PDDIs in this population, which is expected to enhance the safety of treatment.
Based on univariate and multivariate logistic regression analyses, the independent risk factors ultimately identified in this study included the number of medication types, hypoalbuminemia, and treatment plans. Unlike previous studies (Saeed et al., 2025; Ramasubbu et al., 2025), age was not considered a risk factor for pDDIs in this study, possibly due to differences in study design, disease populations and regions.
Multiple studies have shown that the number of drug varieties is closely related to pDDIs, as the probability of pharmacokinetic and pharmacodynamic interactions increases combinatorially with the number of drugs administered. In this study, the OR for the number of drug varieties was 1.097 (95% CI: 1.068–1.128), suggesting that for each additional drug variety, the risk of pDDI increased by 9.7%. Furthermore, the specific treatment regimen employed also emerged as a critical determinant, which may be attributed to differences in metabolic pathways and adverse reactions, ultimately leading to variations in pDDIs (Wu et al., 2025; Peshin et al., 2025). For instance, in this study, the risk of pDDI was 3.47 times higher with the FOLFIRI regimen (Fluorouracil, Calcium Folinate, Irinotecan) compared to the FOLFOX regimen (Fluorouracil, Calcium Folinate, Oxaliplatin). This difference can be attributed to the complex metabolic pathways of irinotecan (involving carboxylesterases, CYP3A4, UGT1A1, etc.), which make it more susceptible to the effects of concomitant medications than oxaliplatin, which is primarily metabolized through non-enzymatic pathways (Zhu et al., 2023; Lentz et al., 2005; Liang et al., 2025).
Notably, hypoalbuminemia appears to be a protective factor against pDDIs, with patients in the hypoalbuminemia group having only 0.24 times the risk of pDDIs compared to those with normal albumin levels. In hypoalbuminemia, reduced plasma protein binding of drugs leads to increased free drug concentrations, significantly raising the risk of toxicity (Alvarez-Elias et al., 2017; Yang J. et al., 2025). Previous clinical studies have primarily focused on the relationship between hypoalbuminemia and clinical efficacy or adverse reactions (Lin et al., 2023; Wang et al., 2023; Liang et al., 2023; Tang et al., 2025). However, unlike earlier research, the clinical outcome in this study was the risk of pDDIs. The lower risk observed in the hypoalbuminemia group may be attributed to more cautious treatment plans (Prudent selection of drug types, reduction in the number of medications, and lower dosages) adopted by clinicians for this patient population (Wang et al., 2025b; Tan et al., 2024). Therefore, hypoalbuminemia should not be regarded as a protective factor against pDDIs, it should be understood as a clinical characteristic associated with more prudent medication management in this condition. What’s more, although the probability of pDDI risk is low, the severity of adverse reactions in real-world settings may be more severe (Ngcobo, 2025; Idasiak-Piechocka et al., 2025; Ling et al., 2024).
The nomogram prediction model, features a simple and intuitive interface along with high accuracy, thereby aiding clinicians in making better clinical decisions, its application in scenarios such as disease diagnosis and prognosis analysis is becoming increasingly widespread (Xu et al., 2025; Xie et al., 2025; Yang Y. et al., 2025; Huang et al., 2025). Based on the identified independent risk factors (aa, bb), this study developed and validated a nomogram for predicting the risk of serious pDDIs in patients with colorectal cancer.
As shown in Figure 2, the ROC curve demonstrated that the AUC of the nomogram constructed by combining these three indicators was higher than that of any single indicator. The AUC values of the nomogram were 0.826 in the training set and 0.824 in the validation set, indicating that the nomogram has a strong ability to identify patients at potential risk of severe DDIs. Calibration curves for both the training and validation sets indicated good agreement between actual and predicted diagnoses. Furthermore, DCA showed that within the threshold probability range of 10%–90%, using the nomogram for assessing patients’ DDI risk yielded satisfactory net benefits, indicating favorable clinical utility of the model. The established nomogram model can assist clinicians in more conveniently and rapidly identifying patients’ DDI risk, enabling targeted early intervention and improving medication safety.
The risk prediction model constructed in this study can help clinical medical staff identify high-risk patients with pDDIs in a timely manner. For patients with a high predicted risk (such as a probability ≥0.5 in the nomogram), clinical medical staff can take the following intervention measures: (1) Conduct a comprehensive medication review, including reviewing the patient’s medication history, comorbidities, and laboratory test results, and discontinuing unnecessary drugs; (2) Select drugs with fewer interactions, and adjust the dosage or administration time of drugs if necessary; (3) Strengthen the monitoring of patients’ clinical symptoms and laboratory indicators (such as liver and kidney function, coagulation function, drug concentration), and detect and handle ADRs in a timely manner; (4) Strengthen the communication between clinical pharmacists and physicians, and provide professional medication guidance for patients. These measures are expected to reduce the incidence and severity of adverse reactions, thereby improving treatment safety. However, the effectiveness of this model requires further validation in clinical practice.
This study also has some limitations: (1) This is a retrospective study, and there may be selection bias and information bias, which may affect the accuracy of pDDI assessment; (2) The study only included CRC patients from one hospitals in Anhui province, and the study population may have regional limitations; (3) The study did not consider the dosage and duration of drug use, which may also affect the occurrence of pDDIs; (4) The study did not conduct a prospective validation of the model, and the long-term predictive value of the model needs to be further verified in future studies.
5 Conclusion
The number of medication types, hypoalbuminemia, and treatment plans were risk factors for severe pDDIs in hospitalized CRC patients. The risk prediction model constructed based on these factors has good discriminative ability, calibration ability, and clinical utility, can be used as a simple and effective tool to assess the risk of pDDIs in CRC patients. This study provides a scientific basis for the prevention and intervention of pDDIs in hospitalized CRC patients, and is of great significance for improving the safety of drug use.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was performed in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Bengbu Medical University (2024024), and the requirement for informed consent was waived due to there was no contact with the patient and the patient's information was processed anonymously.
Author contributions
XP: Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft. XY: Data curation, Formal Analysis, Methodology, Supervision, Validation, Writing – original draft. LK: Conceptualization, Funding acquisition, Project administration, Visualization, Writing – review and editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the talent training plan of Bengbu Medical University (No. by51201316).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Alkathiri M. A. Bamogaddam R. F. Alhabshi H. A. AlAjmi M. N. Alashgaai A. A. Assiri G. A. et al (2025). Potential drug-drug interactions among geriatric oncology patients: a retrospective study in Saudi Arabia. BMC Geriatr.25, 300. 10.1186/s12877-025-05965-y
2
Alqenae F. A. Steinke D. Carson-StevensR A. Keers N. (2023). Analysis of the nature and contributory factors of medication safety incidents following hospital discharge using national reporting and learning system (NRLS) data from England and Wales: a multi-method study. Ther. Adv. Drug Saf.14, 20420986231154365. 10.1177/20420986231154365
3
Alvarez-Elias A. C. Yoo E. C. Todorova E. K. Filler R. N. S. G. (2017). A retrospective study on mycophenolic acid drug interactions: effect of prednisone, sirolimus, and tacrolimus with MPA. Ther. Drug Monit.39, 220–228. 10.1097/FTD.0000000000000403
4
Bray F. Laversanne M. Sung H. Ferlay J. Siegel R. L. Soerjomataram I. et al (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.74, 229–263. 10.3322/caac.21834
5
Choudary N. A. Khan A. Wahid A. Abubakar M. Ahmad M. A. N. (2022). Evaluation of potential drug-drug interactions in cancer patients at a tertiary care hospital in Pakistan. J. Oncol. Pharm. Pract.28, 618–626. 10.1177/10781552221074629
6
Chu F. Yao Y. Gao B. Kong M. H. L. (2025). Incidence and risk factors for potential drug-drug interactions in outpatients receiving opioid analgesics. Expert Opin. Drug Saf.24, 167–175. 10.1080/14740338.2024.2346101
7
Demirkapu J. Cavdar M. E. (2024). Potential drug-drug interactions in outpatient lung cancer patients in a university hospital. Pharmacology109, 231–236. 10.1159/000538742
8
Endalifer B. L. Kassa M. T. EjiguA Y. W. Ambaye S. (2025). Polypharmacy, drug-drug interactions, and potentially inappropriate medications among older adults: a cross-sectional study in northeast Ethiopia. Front. Public Health13, 1525079. 10.3389/fpubh.2025.1525079
9
Filho A. M. Laversanne M. Ferlay J. Colombet M. Pineros M. Znaor A. et al (2025). The GLOBOCAN 2022 cancer estimates: data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer156, 1336–1346. 10.1002/ijc.35278
10
Guo H. Zhou Y. Li X. Zheng Y. Li Z. Liu Y. et al (2025). Real-world analysis of potential drug-drug interactions with nirmatrelvir/Ritonavir based on the hospital prescription analysis (HPA) database in 9 cities of China. Infect. Drug Resist18, 4539–4548. 10.2147/IDR.S536758
11
Huang L. Li H. Ren S. W. Y. (2025). Prediction of early mucosal healing of Crohn's disease after treatment with biologics-a novel nomogram based on radiomics and clinical risk factors. Front. Pharmacol.16, 1586300. 10.3389/fphar.2025.1586300
12
Idasiak-Piechocka I. Lewandowski D. Swigut W. Kalinowski J. Mikosza K. Suchowiejski P. et al (2025). Effect of hypoalbuminemia on drug pharmacokinetics. Front. Pharmacol.16, 1546465. 10.3389/fphar.2025.1546465
13
Ismail M. Khan S. Khan F. Noor S. Sajid H. Yar S. et al (2020). Prevalence and significance of potential drug-drug interactions among cancer patients receiving chemotherapy. BMC Cancer20, 335. 10.1186/s12885-020-06855-9
14
Khanna J. Kumar S. Mehta S. Jain J. C. A. (2024). Clinical pertinence and determinants of potential drug-drug interactions in chronic kidney disease patients: a cross-sectional study. J. Pharm. Technol.40, 142–151. 10.1177/87551225241241977
15
Lentz F. Tran A. Rey E. Pons G. Tréluyer J. M. (2005). Pharmacogenomics of fluorouracil, irinotecan, and oxaliplatin in hepatic metastases of colorectal cancer: clinical implications. Am. Journal Pharmacogenomics Genomics-Related Research Drug Development Clinical Practice5 (1), 21–33. 10.2165/00129785-200505010-00002
16
Liang S. Liang Y. J. Li Z. Wang Y. Guo X. R. Zhang C. Y. et al (2023). Evaluating efficacy and safety of tacrolimus treatment in membranous nephropathy: results of a retrospective study of 182 patients. Ther. Clin. Risk Manag.19, 351–360. 10.2147/TCRM.S399218
17
Liang Y. Lei M. Zhang B. (2025). Comparative effects of oxaliplatin-based versus irinotecan-based regimens combined with capecitabine and bevacizumab in patients with colorectal cancer and liver metastases. Am. Journal Cancer Research15 (10), 4264–4275. 10.62347/WFPE2805
18
Lin D. Lai P. Zhang W. Lin J. Wang H. Hu X. et al (2023). Development and validation of a nomogram to evaluate the therapeutic effects of second-line axitinib in patients with metastatic renal cell carcinoma. Front. Oncol.13, 1071816. 10.3389/fonc.2023.1071816
19
Ling J. Yang X. Dong L. Jiang Y. Hu S. Z. N. (2024). Influence of C-reactive protein on the pharmacokinetics of voriconazole in relation to the CYP2C19 genotype: a population pharmacokinetics analysis. Front. Pharmacol.15, 1455721. 10.3389/fphar.2024.1455721
20
Meslamani Al Abou Hajal A. Z. A. (2025). Language models for drug-drug interactions: current applications, pitfalls, and future directions. Expert Opin. Drug Metab. Toxicol.21, 1083–1102. 10.1080/17425255.2025.2551724
21
Milovanovic I. R. Pejcic A. V. (2025). Drug-drug interactions in hospitalized urological patients: a retrospective cohort study. Pharmacology110, 15–25. 10.1159/000540427
22
Ngcobo N. N. (2025). Malnutrition and its effect on drug pharmacokinetics: a clinical perspective. Clin. Pharmacokinet.64, 1283–1293. 10.1007/s40262-025-01558-5
23
Peshin S. Dharia A. Takrori E. Kaur J. Iyer K. T. R. (2025). Understanding chemotherapy-induced thrombocytopenia: implications for gastrointestinal cancer treatment. Curr. Oncol.32, 455. 10.3390/curroncol32080455
24
Pinto R. A. Rao M. Roy A. Thomas L. Guddattu K. S. U. V. (2023). Potential drug-drug interactions between anti-cancer drugs and other medications in lung cancer patients: a retrospective study. Curr. Drug Saf.18, 175–189. 10.2174/1574886317666220324100356
25
Ramasubbu S. K. Mishra A. Mandal S. P. B C. K. M. B M P. (2025). Therapeutic drug-drug interactions (DDIs) causing QT prolongation in patients with cancer: a systematic review and meta-analysis. Cureus17, e82770. 10.7759/cureus.82770
26
Saeed Z. M. Sridhar S. B. Rashed Alsereidi J. S. A. M. (2025). From prescription patterns to drug safety: a closer look at non-steroidal anti-inflammatory drugs and analgesics in outpatient pharmacy. Front. Pharmacol.16, 1558830. 10.3389/fphar.2025.1558830
27
Tan Y. Xiang W. Chen Y. Sun J. H. D. (2024). Effect of hypoproteinemia on mortality of elderly male patients with chronic heart failure. Med. Baltim.103, e37078. 10.1097/MD.0000000000037078
28
Tang Y. Liu Y. Qin S. Huai C. Zhang J. Ding W. et al (2025). Correlation analysis of pharmacokinetic parameters of docetaxel AUC and adverse reactions in breast cancer patients. Front. Pharmacol.16, 1563506. 10.3389/fphar.2025.1563506
29
Vilz T. O. Post S. Langer T. Follmann M. Nothacker M. Willis M. A. et al (2024). Clinical practice guideline: recommendations for the perioperative management of pancreatic and colorectal cancer patients. Dtsch. Arztebl Int.121, 681–687. 10.3238/arztebl.m2024.0172
30
Wang C. Zhou Y. Ye Y. Z. C. (2023). Ertapenem-induced neurotoxicity: a literature review of clinical characteristics and treatment outcomes. Infect. Drug Resist16, 3649–3658. 10.2147/IDR.S406852
31
Wang F. Chen G. Zhang Z. Yuan Y. Wang Y. Gao Y. H. et al (2025a). The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of colorectal cancer, 2024 update. Cancer Commun. (Lond)45, 332–379. 10.1002/cac2.12639
32
Wang F. Su Z. Z. Guo X. Q. Li M. WangY R. Xu J. (2025b). Development and validation of a risk prediction model for hypoproteinemia after adult cardiac valve surgery: implications for clinical care. PeerJ13, e19676. 10.7717/peerj.19676
33
Wondm S. A. Tamene F. B. Gubae K. Dagnew S. B. WorkuE A. A. Belachew A. (2023). Potential drug-drug interaction and its determinants among patients with cancer receiving chemotherapy in oncology centres of northwest Ethiopia: an institutional-based cross-sectional study. BMJ Open13, e077863. 10.1136/bmjopen-2023-077863
34
Wu Y. Zhao W. Zhang L. Wang Y. Liu Y. W. L. (2025). Machine learning models for predicting chemotherapy-induced adverse drug reactions in colorectal cancer patients. Dig. Liver Dis.57, 1845–1852. 10.1016/j.dld.2025.06.007
35
Xie M. Jiang M. Xu J. Kong Y. Z. L. (2025). Development and validation of a clinical risk score nomogram for predicting voriconazole trough concentration above 5 mg/L: a retrospective cohort study. J. Chemother.37, 229–237. 10.1080/1120009X.2024.2376453
36
Xu J. Peng T. Fan K. Dou Y. Sang L. K. R. (2025). Development of a lung immune prognostic index-based nomogram model for predicting overall survival and immune-related adverse events in non-small cell lung cancer patients treated with sintilimab. Front. Immunol.16, 1569689. 10.3389/fimmu.2025.1569689
37
Yang J. Wang H. Liu D. Cao W. Wang H. X. P. (2025). A population pharmacokinetics study of venetoclax concomitant with voriconazole in patients with hematologic malignancies. Drug Des. Devel Ther.19, 3681–3690. 10.2147/DDDT.S514173
38
Yang Y. Li L. Hu H. Zhou C. Huang Q. Zhao J. et al (2025). A nomogram integrating the clinical and CT imaging characteristics for assessing spread through air spaces in clinical stage IA lung adenocarcinoma. Front. Immunol.16, 1519766. 10.3389/fimmu.2025.1519766
39
Yoshino T. Cervantes A. Bando H. Martinelli E. Oki E. Xu R. H. et al (2023). Pan-asian adapted ESMO clinical practice guidelines for the diagnosis, treatment and follow-up of patients with metastatic colorectal cancer. ESMO Open8, 101558. 10.1016/j.esmoop.2023.101558
40
Zhu J. Zhang Y. Zhao Y. Zhang J. Hao K. He H. (2023). Translational pharmacokinetic/pharmacodynamic modeling and simulation of oxaliplatin and irinotecan in colorectal cancer. Pharmaceutics15 (9), 2274. 10.3390/pharmaceutics15092274
Summary
Keywords
colorectal cancer, inpatients, potential drug-drug interactions, risk factors, riskprediction model
Citation
Pei X, Yang X and Kong L (2026) Risk prediction model for severe potential drug-drug interactions in colorectal cancer patients: a real-world data study. Front. Pharmacol. 16:1714838. doi: 10.3389/fphar.2025.1714838
Received
28 September 2025
Revised
16 December 2025
Accepted
22 December 2025
Published
12 January 2026
Volume
16 - 2025
Edited by
Adina Turcu-Stiolica, University of Medicine and Pharmacy of Craiova, Romania
Reviewed by
Andreas Antzoulas, General University Hospital of Patras, Greece
Soukaina Amniouel, National Center for Advancing Translational Sciences (NIH), United States
Updates
Copyright
© 2026 Pei, Yang and Kong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingti Kong, konglingti@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.