Abstract
Background:
Farsetia aegyptia and Zilla spinosa belonging to the Brassicaceae family, well-known for their biological and therapeutic activities.
Objective:
To our knowledge, this is the first comprehensive study to explore the composition, antibacterial, anticandidal, antibiofilm activities using in vitro, and molecular docking analysis assays of F. aegyptia and Z. spinosa herb extracts collected from the Hail region of Saudi Arabia.
Methods:
The antibacterial and anticandida effects of chloroform, ethanolic, and aqueous extracts from the aerial parts of F. aegyptia and Z. spinosa were determined by conventional assays. The compositions of extracts were determined by Gas Chromatography–Mass Spectrometry (GC–MS) and High-Resolution Liquid Chromatography–Mass Spectrometry (HR–LC–MS). Molecular docking simulations were carried out with the major identified compounds against penicillin-binding protein 4.
Results:
The chloroform and ethanolic extracts of these plants exhibited substantial activities against Enterococcus faecalis ATCC 29212, and Listeria monocytogenes ATCC 19115, with minimum inhibitory concentration (MIC), minimal bactericidal concentration (MBC) levels varying between 625 and 2,500 μg/mL, and MBC/MIC was equal to 1. The chloroformic extract of F. aegyptia demonstrated the most significant anticandidal activity against Candida albicans ATCC 90028 and Candida krusei ATCC 6258, with MIC of 625 μg/mL. Interestingly, the chloroform extract of F. aegyptia demonstrated the most important antibiofilm activity against Staphylococcus aureus, with Minimum Biofilm Inhibition Concentration (MBIC50) of 700 μg/mL, while the chloroform extract of Z. spinosa showed the most potent antibiofilm activity against Pseudomonas aeruginosa ATCC 27853 with MBIC50 = 630 μg/mL. Candida albicans showed the highest sensitivity to the ethanolic extract of Z. spinosa, with MBIC50 = 660 μg/mL. The GC–MS analysis identified β-sitosterol (40.39%), stigmasterol (22.24%), and coumarin (9.25%), as the main components of Z. spinosa; and linolenic acid (12.68%), linolenic acid ethyl ester (7.31%), arachidonic acid (6.96%), and (Z)-13-docosenamide (6.47%) as predominant compounds in F. aegyptia. The docking analysis revealed that the key compound stigmasterol from Z. spinosa served as superior ligands, penicillin-binding protein four (PBP4) (−7.7 kcal/mol).
Conclusion:
These findings highlight the powerful antimicrobial and antibiofilm activities of F. aegyptia and Z. spinosa, and supporting their prospective role in developing novel anti-infective agents.
1 Introduction
One of the world’s greatest challenges is the rise, the rapid and widespread spread of antimicrobial resistance in pathogenic Gram-negative and Gram-positive bacterial strains, which were associated with multi-drug resistant (MDR) bacterial infections responsible for millions of deaths each year for several important reasons: current medications are often ineffective against them, the scarcity of new antibiotics, and synthetic antibiotics can have harmful side effects (WHO, 2024; Khameneh et al., 2019).
Pathogenic microorganisms can form biofilms, which are structured communities encased in an extracellular matrix of polymeric materials (Coppola et al., 2025; Kungwani et al., 2024; Alibi et al., 2021). These biofilms can be highly detrimental and are a significant cause of nosocomial infections (Kungwani et al., 2024; Alibi et al., 2021). The National Institutes of Health reported that 80% of chronic infections and 65% of microbial infections are associated with biofilm formation.
To address these challenges, there is a critical need for new, effective drugs to treat pathogenic and MDR bacterial infections. In this setting, the World Health Organization (WHO) has announced an urgent need to encourage the research and development of new drugs or strategies (WHO, 2024). Thus, numerous studies have been conducted to discover novel antimicrobials for clinical use for the eradication of MDR bacteria. However, as no new antimicrobial has been found to combat MDR Gram-negative and Gram-positive bacteria, other strategies are proposed and evaluated in vitro. One of these approaches is based on investigating natural products due to their great diversity and relative abundance. Hence, natural products, such as essential oils, which comprise a wide range of chemical compounds, are becoming an attractive and popular mainstream platform for researchers in drug discovery. Therefore, our studies and others have previously reported interesting effects of different plant essential oils extracted from medicinal plants such Thymus capitatus (Ben Selma et al., 2024a), Thymus algeriensis (Jilani et al., 2025), Thymus vulgaris (Ben Selma et al., 2024a; Alibi et al., 2020), Grantia aucheri (Sharifi-Rad et al., 2022a), Cinnamomum zeylanicum (Ben Selma et al., 2024b; Alibi et al., 2022), Teucrium polium (L.) (Sharifi-Rad et al., 2022b), Astragalus species (Zengin et al., 2022) against MDR and extensively resistant Gram-negative and Gram-positive pathogenic bacteria.
Accordingly, secondary metabolites derived from medicinal plants have shown promising effects in combating these infections and are generally considered safe due to their minimal side effects (Cowan, 1999; Gyawali and Ibrahim, 2014; Narayana et al., 2017). This growing interest in plant-based compounds has led to increased engagement from the pharmaceutical industry (Anand et al., 2020). With over 350,000 plant species on Earth (Borris, 1996), many plant-derived products are already being used as commercial antimicrobial drugs (Khameneh et al., 2019).
The Brassicaceae family, a group of dicotyledonous angiosperms (Chen et al., 2011), contains 3,709 species, across 338 genera (Al-Shehbaz et al., 2006). This family is of significant nutritional and economic value and has been a focus of research for many years. The species Farsetia aegyptia and Zilla spinosa, selected in this study, both belong to the Brassicaceae family.
Zilla Spinosa is also an important source of phytoconstituents, such as flavonoids, sterols, glycosides, tannins, triterpenoids, carbohydrates, and glucosinolates (Karawya 1974). These components confer it powerful antiviral, hepatoprotective, antifungal and antioxidant (El-Sharkawy et al., 2013; El-Toumy et al., 2011), hepatoprotective, important anti-inflammatory, antipyretic, and analgesic prospective effects and anti-cancer potential (Ullah et al., 2020; El-Sharabasy and Mohamed, 2013Suleiman and Ateeg, 2020; Alghanem et al., 2018; Salma et al., 2018; Ullah et al., 2018) of the different fractions of this plant. Additionnally, it has been frequently employed in folk medicine to treat a variety of ailments, including diabetes, gastrointestinal issues (Sekkoum et al., 2015), urinary tract pain (Sekkoum et al., 2011), kidney stones, gallbladder problems, pancreatic and hepatic pain, diarrhea, and respiratory infections (Berreghioua et al., 2014).
Farsetia aegyptia organic extracts are rich in bioactive phytocompounds including phenolic acids, glycosinolates, and flavonoids such as kaempferol-7, 8-dilucoside and apienin; flavanols and thier glycosides such as botulin, friedelin, β-amyrin, scopoletin and coumarin (El-Sharkawy et al., 2013). These compounds exhibited different promising biological activities including antimicrobial, antioxidative (Suleiman, and Ateeg, 2020; Atta et al., 2013), anti-inflammatory (Mecheri et al., 2023) hepatoprotective, anticancer and cytotoxic effects against certain selected cancerous cells (Madouh, and Davidson, 2024; Marzouk 2009). Moreover, this plant has been used in traditional medicine for its antispasmodic and anti-diabetic properties and is also utilized to treat rheumatic pains (Youssef, 2013). Moreover, a decoction of this plant can be used for mouth disinfection (Abdelshafeek et al., 2016; Bhandari et al., 2015).
A literature survey indicated that no data are available on the chemical composition and antimicrobial effects of the two plants F. aegyptia and Z. spinosa growing in Hail region of Saudi Arabia. Thus, the current study aimed to determinate the phytochemical composition of most active herb extracts of these two plants by GC-MS and HR–LC–MS, as well as, to explore their extracts’ eventual antibacterial, anticandidal, antibiofilm activities, and to conduct a docking analysis to predict molecular interactions between major compounds of active extracts and penicillin-binding protein 4 (PBP4) of Enterococcus faecalis (PDB: 6mki) implicated in the pathway of biosynthesis of peptidoglycans.
2 Methods
2.1 Reagents and chemicals
All solvents used in this study—ethanol (99.99%, C2H6O), chloroform (90%, CHCl3), and dimethyl sulfoxide (DMSO)—as well as the Mueller–Hinton broth, resazurin, filter paper, Amphotericin B, and ciprofloxacin employed in the experiments—were purchased from Merck (Darmstadt, Germany).
2.2 Preparation of plants extract
Farsetia aegyptia and Z. spinosa (Figure 1) were collected from farms in the Simira governorate, located south of Hail, Saudi Arabia, in 2022, after receiving verbal permission from the owners. These plants were taxonomically identified by Dr. Assia Hamdi at the Faculty of Pharmacy, Monastir University, Tunisia. Voucher specimens were prepared for each species and deposited at the herbarium of the Laboratory of Biology (HLB) at the College of Sciences, University of Hail, Saudi Arabia, as follow: Plant 1: F. aegyptia Turra Voucher No. F.a 1, and Plant 2: Z. spinosa L. Voucher No. Z.s 1.
FIGURE 1

(a) Zilla spinosa (L): Prantl (Z.s) (b) Farsetia aegyptia Turra (F.a).
These voucher specimens confirm the botanical identities and serve as references for future use.
Following the extraction protocol established by our research group (Besbes et al., 2025), the preparation of extracts was performed as follows: after drying and grinding approximately 700 g of plant material, the maceration method was used to extract compounds in chloroform, ethanol, and water. The extraction yields were 1.4% and 2.1% (chloroform), 5.17% and 6.21% (ethanol), and 7.2% and 8.6% (water) for F. aegyptia and Z. spinosa, respectively. All samples were stored in airtight glass vials sealed with aluminum foil at −4 °C until further use.
2.3 Microorganisms strains
Three Gram-positive bacterial strains including: Staphylococcus aureus ATCC 25923, Listeria monocytogenes ATCC 19115, and E. faecalis ATCC 29212; three Gram-negative bacteria strains comprising: Escherichia coli ATCC 35218, Salmonella Typhimurium ATCC 1408, and Pseudomonas aeruginosa ATCC 27853; and three strains of candida: Candida neoformans ATCC 14116, Candida albicans ATCC 90028, and Candida krusei ATCC 6258.
2.4 Antibacterial and anticandida activities
2.4.1 Well diffusion assay
The antibacterial and anticandida activities of F. aegyptia and Z. spinosa herb extracts were tested as previously recommended against S. aureus ATCC 25923, L. monocytogenes ATCC 19115, E. faecalis ATCC 29212, E. coli ATCC 35218, Salmonella Typhimurium ATCC 1408, P. aeruginosa ATCC 27853, Candida neoformans ATCC 14116, Candida albicans ATCC 90028, and Candida krusei ATCC 6258 (EUCAST 2024; CLSI, 2018). Briefly, the strains were grown in Mueller–Hinton (MH) broth (Oxoid, USA) at 37 °C for 24 h, and suspensions were adjusted to 0.5 McFarland standard turbidity. Then, 100 µL of each precultured suspension was spread onto plates containing MH agar. Wells were cut with a sterile borer (four to eight mm in diameter), and impregnated with 100 µL of the different plant extracts used. Ciprofloxacin and Amphotericin B (Oxoid, USA) served as positive controls. The treated plates were placed at 4 °C for 1 h and then incubated at 37 °C overnight (Memmert, Germany). After incubation, the inhibition zone surrounding the wells was measured. Each sample was tested in triplicate.
To determine the antibacterial and anticandida effects of F. aegyptia and Z. spinosa herb extracts, the diameter of the transparent zone around the well (zone of inhibition) was measured, and the results were interpreted as follows: not sensitive = diameter ˂8 mm; sensitive = diameter varying between 8 and 14 mm; very sensitive = diameter varying between 15 and 19 mm; extremely sensitive = diameter ≥20 mm.
2.4.2 Minimal inhibitory concentration assay
The minimal inhibitory concentration (MIC) is defined as the lowest concentration showing no visible growth after 24 h incubation. For the MIC assay, the tested extracts of F. aegyptia and Z. spinosa were diluted to 10,000 μg/mL in a 5% DMSO solution and placed in 96-well microtiter plates (SARSTEDT AG and Co. KG, Numbrecht, Allemagne). It is important to note that the concentration of DMSO used in the tested extracts of the two plants solutions experiments did not exceed 0.05%, which is non-toxic to bacteria (Cirino et al., 2023).
The MIC was determined using the broth microdilution assay as a standard method (EUCAST 2024; CLSI, 2018). Thus, these extracts were then further serially diluted in Mueller-Hinton broth (Biolife, Italy) to achieve concentrations ranging from 10,000 μg/mL down to 312 μg/mL. Next, 10 μL of each bacterial and candida selected isolates, adjusted to a 0.5 McFarland turbidity standard, were added to each well, which contained 100 μL of the serially diluted extracts. Finally, 10 µL of resazurin (100 μg/mL) was added to each well as an indicator dye. The microtiter plates were incubated for 24 h at 37 °C (Besbes Hlila et al., 2016).
2.5 Minimal bactericidal/fungicidal concentration (MBC/MFC) assay
To estimate the MBC and the Minimum Fungicidal Concentration (MFC) of each herb extracts of F. aegyptia and Z. spinosa, 10 μL of medium from each well that showed no growth were transferred to Mueller-Hinton agar plates (Biolife, Italy) as previously recommended (EUCAST 2024; CLSI, 2018). These plates were incubated for 24 h at 37 °C, and we examined bacterial and candidal growth after incubation. The concentration at which no microbial growth occurred was identified as the MBC or MFC. To characterize the effectiveness of the analyzed samples, we calculated the MFC/MIC and MBC/MIC ratios. A ratio of less than or equal to four indicated a bactericidal effect, while a ratio greater than four indicated a bacteriostatic action (Hlila et al., 2017).
2.6 Antibiofilm assay
2.6.1 Biofilm production test
For the biofilm formation assay among clinical isolates of S. aureus and P. aeruginosa, we used the microtiter method using 96-well polystyrene microtiter sterile plates as previously recommended (CLSI, 2018). Briefly, clinical isolates were cultured on nutrient agar plates at 37 °C overnight, and 0.5 McFarland standard was prepared from each bacterial sample in phosphate-buffered saline (PBS). Then, 20 µL of bacterial suspension was mixed with 180 µL trypticase soy broth (TSB, Condalab Co., Madrid, Spain) supplemented with 1% glucose and added to sterile 96-well polystyrene microtiter plates. After incubation overnight at 37 °C, the microplates were carefully washed three times with sterile PBS, and then microplates were inverted to dry for 20 min at room temperature. For biofilm quantification, 200 µL of 2% safranin dye solution was added to the well for 40 min at room temperature and washed three times with sterile PBS. Safranin bound to the biofilm in each well was extracted with 200 µL of pure ethanol, and the absorbance of the extracted safranin was measured at 490 nm in an ELISA reader (BioTek, Agilent Technologies, Inc., Santa Clara, CA 95051, USA). Each assay was performed in triplicate. TSB +1% glucose medium was used as a negative control to determine background optical density (OD). The cut-off ODs for biofilm formation were determined as the average OD of the negative control +3 × standard deviation (SD) of the negative control. The OD value was calculated for each microtiter plate separately. OD > 4 × ODc was considered a strong biofilm formation; 2 × ODc < OD ≤ 4 × ODc was considered a moderate biofilm formation; ODc < OD ≤ 2 × ODc was considered a weak biofilm formation; and OD ≤ ODc was considered a nonbiofilm formation.
2.6.2 Biofilm inhibition assay
Briefly, the antibiofilm activity was assessed using the crystal violet test in 96-well polystyrene sterile plates with a flat-bottom microplate format according to according to the conventional guidelines (CLSI, 2018). Five dilutions of the samples (625–10,000 μg/mL) were prepared and combined with a suspension of pathogenic bacteria cultured in brain heart infusion (BHI) for 24 h at 37 °C, at 105 CFU/mL. We used 96 U-bottomed plates containing BHI with 2% glucose (w/v). The positive control consisted of BHI with 2% glucose inoculated with pathogenic bacteria, while the negative control contained only BHI with 2% glucose. After incubation at 37 °C for 24 h, the plates were washed three times with phosphate-buffered saline. The biofilm cells were fixed with methanol for 15 min, air-dried, and stained with 1% crystal violet. Biofilm formation was quantified using a microplate reader, by measuring absorbance (A) at 595 nm. All experiments were performed in triplicate. The percentage of inhibition (%) was calculated using the following formula: % Inhibition = [(A of control − A of extract)/A of control] × 100.
2.7 Chemical composition of active extracts
2.7.1 GC-MS analysis
The phytocompounds present in the most active samples were determined by the GC-MS as previously described (Besbes et al., 2025). Briefly, this analysis used a Perkin Elmer Clarus 600 T coupled to a single quadrupole mass spectrometer. An elite 5-M column with helium gas as a carrier, flowing at 1 mL per minute, was employed. The temperature started at 40 °C for 1 minute, then increased to 150 °C at 10 °C per minute, and finally to over 300 °C at the same rate for 3 minutes. The inlet line and ion source temperatures were set to 220 °C and 200 °C, respectively. Ionization occurred at 70 eV, with a mass scanning range of 40–618 m/z. Peaks were identified by comparing them to Wiley sixth. edition mass spectral library, standards, and published data. Percentages of detected compounds were calculated using GC peak areas. Kovats index of each compound was determined using retention times of C6-C26 n-alkanes and compared to literature values (Adams, 2017).
2.7.2 LC-HRMS analysis
The phytochemistry of the extracts was analyzed using a UHPLC-PDA-Detector Mass Spectrophotometer (HR-L = CMS 1290 Infinity UHPLC System, Agilent Technologies®, Santa Clara, CA, USA). The liquid chromatographic system consists of a binary gradient solvent pump, a HiP sampler, a quadrupole time-of-flight mass spectrometer (MS Q-TOF) equipped with a dual Agilent Jet Stream Electrospray (AJS ES) ion source, and a column compartment. A 10 µL sample was injected into the system, followed by separation in an SB-C18 column (2.1 mm × 50 mm, 1.8 µm particle size). The mobile phase consisted of solvent A (1% formic acid in deionized water) and solvent B (acetonitrile). A flow rate of 0.350 mL/min was used for MS detection in the MS Q-TOF. Phytocompounds were identified based on their mass spectra and unique mass fragmentation patterns. The primary tools for identifying the phytochemical products included Compound Discoverer 2.1, ChemSpider, and PubChem (Reddy et al., 2020).
2.8 Molecular docking study
In-silico interaction analysis was performed using the AutoDock 4.2 program package (Trott and Olson, 2010). The crystal structure of the target receptor (PDB: 6mki) (Moon et al., 2018) was retrieved from the RCSB Protein Data Bank (https://www.rcsb.org/). The target protein was prepared by removing the co-crystallized ligand and water molecules, and by adding any missing hydrogen atoms and Gasteiger charges. AutoDock Tools were used to save the files for the tested ligands and the target protein (PDBQT format). The optimization of all compound geometries was done using ACD (3D viewer) software (http://www.filefacts.com/acd3d-viewer-freeware-info). At the same time, the visualization and analysis of interactions were conducted using Discovery Studio 2017R2 (https://www.3dsbiovia.com/products/collaborative-science/biovia-discovery-studio/) and PyMOL (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC).
2.9 Statistical analysis
The antimicrobial and antibiofilm analyses were performed using two tests for each sample. All data were first assessed for normality using a Kolmogorov–Smirnov test. The results were found to be normally distributed (p > 0.05 in the K-S test) and were analyzed using a one-way ANOVA test and expressed as mean values ± standard (mean ± SEM) (SPSS 22). The Duncan test (p < 0.05) was applied to compare the averages.
3 Results and discussion
3.1 Antibacterial activities of Farsetia aegyptia and Zilla spinosa herb extracts
Historically, MDR, extensively drug-resistant (XDR), and pan-drug-resistant (PDR) Gram-negative and Gram-positive bacterial strains, such as E. coli, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, S. aureus, L. monocytogenes, E. faecalis, Salmonella Typhimurium, and Mycobacterium tuberculosis, have been detected worldwide to have numerous resistance mechanisms (WHO 2024; Srinivas and Rivard, 2017; Tarchouna et al., 2013; Magiorakos et al., 2012; Ben Kahla et al., 2011). Therefore, the world is now facing a significant and growing threat from the rapid emergence of bacteria resistant to almost all available antibiotics (Alibi et al., 2021; Sherry and Howden, 2018; Magiorakos et al., 2012). As highlighted by the Infectious Diseases Society of America in the “Bad Bugs, No Drugs” paper, “as antibiotic discovery stagnates, a public health crisis brews” (Talbot et al., 2006).
According to the literature, due to their multiple bioactive compounds, medicinal plants have been extensively used as an essential source for synthesizing diverse drugs for many decades. In this setting, our studies and others previously reported interesting effects of essential oils extracted from medicinal plants on a broad spectrum of pathogenic and multi-drug-resistant bacterial strains, such as K. pneumoniae, Salmonella enteritidis, Corynebacterium striatum, and E. coli (Ben Selma et al., 2025; Alibi et al., 2022; Alibi et al., 2020).
The antibacterial effects of F. aegyptia and Z. spinosa chloroformic, ethanolic, and water were evaluated by using two methods: the well diffusion method and the microdilution test against three Gram-positive bacterial strains: S. aureus ATCC 25923, L. monocytogenes ATCC 19115, E. faecalis ATCC 29212, and three Gram-negative bacteria: E. coli ATCC 35218, Salmonella Typhimurium ATCC 14080, and P. aeruginosa ATCC 27853.
Table 1 presents the results of the scusseptibility method. This assay revealed that the chloroform extract of Z. spinosa exhibited the strongest antibacterial activity, producing inhibition zones of 13.0 ± 0.5 mm against S. aureus, 15.0 ± 0.5 mm against L. monocytogenes, 15.8 ± 1.33 mm against E. faecalis, 12.0 ± 0.5 mm against E. coli, and 12.4 ± 0.5 mm against Salmonella Typhimurium. The ethanol extract of F. aegyptia showed selective activity, with the highest effect against L. monocytogenes (14.5 ± 1.33 mm) and moderate inhibition of E. faecalis (11.8 ± 0.5 mm). Meanwhile, the ethanol extract of Z. spinosa was particularly active against S. aureus (16.0 ± 0.5 mm) and moderately effective against E. faecalis (13.1 ± 0.33 mm). Both aqueous extracts demonstrated weak activity, with inhibition zones not exceeding 9.5 mm.
TABLE 1
| Strains | Fa CH | Zs CH | FaET | Zs ET | Fa W | Zs W | Ciprofloxacin | Amphotericin B |
|---|---|---|---|---|---|---|---|---|
| DIZ | DIZ | DIZ | DIZ | DIZ | DIZ | DIZ | ||
| Staphylococcus aureus ATCC 25923 | 12.5 ± 1.33hi | 13 ± 0.5h | 6.5 ± 0.5L | 16 ± 0.5e | 6 ± 0.8L | 0.00m | 25 ± 0.2a | - |
| Listeria monocytogenes ATCC 19115 | 10 ± 0.5j | 15 ± 0.5f | 14.5 ± 1.33g | 12 ± 0.5hi | 6.7 ± 0.5L | 0.00m | 25.3 ± 1.33a | - |
| Enterococcus faecalis ATCC 29212 | 14.2 ± 0.5g | 15.8 ± 1.33f | 11.8 ± 0.5i | 13.1 ± 0.33h | 9.5 ± 0.5j | 8.3 ± 1.33k | 18 ± 0.1day | - |
| Escherichia coli ATCC 35218 | 11.8 ± 0.33i | 12 ± 0.5hi | 6.4 ± 0.5L | 11 ± 0.1i | 4.8 ± 0.33lm | 0.00m | 21.8 ± 0.1b | - |
| Salmonella typhimurium ATCC 14080 | 0.00m | 12.4 ± 0.5hi | 7.6 ± 1.33kL | 0.00m | 8.1 ± 1.33k | 7.2 ± 0.33kL | 19.3 ± 0.33c | - |
| Pseudomonas aeruginosa ATCC 27853 | 7 ± 0.5kL | 5.3 ± 1.33lm | 6.8 ± 0.5L | 8.8 ± 0.33k | 5 ± 0.5lm | 0.00m | 19 ± 0.1c | - |
| Candida albicans ATCC 90028 | 8 ± 0.2k | 10.5 ± 0.2j | 9 ± 0.2k | 10.9 ± 1.33i | 8.4 ± 0.2k | 11 ± 1.33i | - | 13.3 ± 0.2h |
| Candida krusei ATCC 6258 | 13-±0.5h | 11.4 ± 0.5i | 8.5 ± 0.2k | 8.02 ± 1.5k | 0.00m | 8 ± 0.5k | - | 12.8 ± 1.33hi |
| Candida neoformans ATCC 14116 | 7 ± 1.33kL | 7 ± 0.5kL | 7.2 ± 1.33kL | 11.5 ± 0.2i | 4.3 ± 0.2lm | 7.2 ± 0.5kL | - | 13 ± 0.5h |
Antimicrobial activities of Farsetia aegyptia and Zilla spinosa extracts by disk method.
DIZ: Diameter Inhibition zone expressed as mm. Fa CH: farsetia aegyptia chloroformic sample; Zs CH: zilla spinosa chloroformic sample; Fa ET: farsetia aegyptia ethanolic sample; Zs ET: zilla spinosa ethanolic sample; Fa W: farsetia aegyptia water sample; Zs W: Zilla spinosa water sample. The letters (a–m) show an important difference between the different doses of extracts in accordance with the Duncan assay (p < 0.05).
Our findings indicate that the water and organic extracts of the two plants, F. aegyptia and Z. spinosa, exhibit varying degrees of antibacterial activity, with MIC values ranging from 625 μg/mL to over 1,000 μg/mL. Among the extracts tested, the chloroform extracts of these plants showed stronger antibacterial activity. Thus, the chloroform extracts of both F. aegyptia and Z. spinosa showed the lowest MIC values against all Gram-positive and Gram-negative bacterial strains selected, ranging from 625 μg/mL to 5,000 μg/mL, except for Salmonella Typhimurium ATCC 14080, which had a MIC value greater than 1,000 μg/mL (Table 1). Furthermore, it exhibited substantial inhibition against E. faecalis ATCC 29212, with both the MBC and MIC at 625 μg/mL. Additionally, they showed significant inhibition against L. monocytogenes ATCC 19115 and S. aureus ATCC 25923, with MIC values ranging from 625 μg/mL to 2,500 μg/mL. Interestingly, these two extracts exhibit bactericidal effects against E. faecalis ATCC 29212 and L. monocytogenes ATCC 19115 (MBC/MIC = 1) and bacteriostatic activities against S. aureus ATCC 25923 (MBC/MIC >4).
The ethanolic extracts of Z. spinosa and F. aegyptia also displayed notable antibacterial activity against S. aureus ATCC 25923 and L. monocytogenes ATCC 19115, with MIC values of 625 μg/mL and 1,250 μg/mL, respectively.
According to the literature, a previous study reported antibacterial activity of aqueous-ethanol and aqueous-methanol extracts derived from the roots and aerial parts of Z. spinosa collected from Asir, Saudi Arabia (Suleiman and Ateeg, 2020). Thus, by using the disk diffusion and microdilution assays, they showed that the aqueous methanolic extract of aerial parts of Z. spinosa exerted antibacterial effect, especially against S. aureus strain, with a zone of inhibition of 26.5 mm and a MIC of 128 μg/mL (Suleiman and Ateeg, 2020). These results were in the same line as our results, which argue for the importance of organic technique extraction of aerial parts of Z. spinosa. In another earlier study, Atta and collaborators demonstrated that the antimicrobial activity of methanolic extract of Farsetia aegyptia from Sinai, Egypt, was more active against S. aureus than against Salmonella Typhimurium and E. coli by using a sensitivity test, the disk method (Atta et al., 2013). To explain the differences between the results of this study and those of our study, some factors could be implicated, such as the solvents, extraction methods, biotic and abiotic characteristics of the plant species used.
Additionally, other studies have evaluated the antimicrobial properties of different Farsetia species (Hayat and Uzair, 2019). Results indicated that the polar methanolic extract was less active than the medium-polar dichloromethane sample, which aligns with our findings, except for Salmonella Typhimurium. In this case, the F. aegyptia water extract had a lower MIC than the chloroformic sample. An acetone extract of Farsetia hamiltonii demonstrated notable effects against S. aureus and P. aeruginosa (Ejaz et al., 2023). Another Farsetia species tested for its antibacterial properties was Farsetia heliophila, which was collected from Pakistan. This extract exhibited significant antibacterial activity against the same Gram-negative bacteria examined in our study, confirming that Salmonella Typhimurium is the most sensitive among the Gram-negative strains. Our study found that the F. aegyptia water extract notably affected Salmonella Typhimurium, with a MIC of 2,500 μg/mL (Burki et al., 2022). Furthermore, in the study by Salma et al. (2018), the 80% alcoholic and water extracts of Farsetia stylosa and Farsetia longiliqua showed no activity against P. aeruginosa, E. coli, Salmonella Typhimurium, and S. aureus.
Furthermore, in this study, the aqueous extracts of both plants exhibited a moderate inhibitory effect on Gram-positive and Gram-negative growth, with MIC values ranging from 5,000 μg/mL to more than 10,000 μg/mL. These aqueous samples showed bactericidal effects against E. faecalis ATCC 29212 and L. monocytogenes ATCC 19115 (MBC/MIC = 1).
Moreover, the disk diffusion assay showed that the aqueous extract of Z. spinosa exhibited no detectable antibacterial activity, whereas the ethanolic extract produced a moderate inhibition zone of 16 mm. This variation may be explained by the differences in the solubility of bioactive constituents, suggesting that the synergistic interaction of ethanol and water extracted compounds could yield a more pronounced antibacterial effect than their individual activities.
It is important to indicate that in this study, practically all tested chloroformic, ethanolic and aqueous extracts of the plants Z. spinosa and Farsetia aegyptia used significantly affected Gram-positive bacteria more than Gram-negative strains. This difference of action of these extracts could be explained by one or more of these factors: i) the difference in phytochemical composition of different extracts used of these plants, and ii) the high sensitivity of Gram-positive bacteria as explained and reported by previous studies (Cosentino et al., 1999; Soković et al., 2007). Thus, this sensitivity is primarily due to the composition of their membranes. In contrast, Gram-negative strains possess double membranes that act as a protective barrier against antibacterial agents.
3.2 Anticandida effects of Farsetia aegyptia and Zilla spinosa herb extracts
The F. aegyptia and Z. spinosa chlorophormic, ethanolic, and water extracts were evaluated against three strains of Candida: Candida albicans ATCC 90028, Candida krusei ATCC 6258, and Candida neoformans ATCC 14116. Table 1 presents the results of the disk diffusion method. The chloroform extract of F. aegyptia showed the highest activity, recording 13.0 ± 0.5 mm against Candida krusei, comparable to that of amphotericin B (12.8 ± 1.33 mm).
The ethanol extract of Z. spinosa also demonstrated consistent antifungal potential, with inhibition zones of 10.9 ± 1.33 mm against Candida albicans and 11.5 ± 0.2 mm against Candida neoformans. In comparison, the water extract of Z. spinosa showed moderate activity against Candida albicans (11.0 ± 1.33 mm). Overall, Z. spinosa chloroform extract was the most potent antibacterial agent, F. aegyptia chloroform extract was the strongest antifungal, and Z. spinosa ethanol extract exhibited the broadest antimicrobial spectrum. Thus, we suggest that the heterogeneity of anticandida effects of these plants’ different extracts could be related to differences between their phytochemical compositions.
Table 2 presents the MIC and MFC values obtained for chloroformic, ethanolic, and water extracts of F. aegyptia and Z. spinosa against the three Candida strains. Our results demonstrated that MIC values ranged from 625 μg/mL to over 10,000 μg/mL, while MFC values ranged from 1,250 μg/mL to over 10,000 μg/mL. The chloroformic extract of F. aegyptia demonstrated the most significant anticandidal activity against Candida albicans ATCC 90028 and Candida krusei ATCC 6258, exhibiting the lowest MIC values of 625 μg/mL. This value was followed by the Z. spinosa extract, which had MIC values of 1,250 μg/mL for these two Candida species.
TABLE 2
| Bacteria | Fa CH | Zs CH | Fa ET | Zs ET | Fa W | Zs W | Ciprofloxacin | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC* | MBC** | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | MIC | MBC | MBC/MIC ratio | |
| S. a | 625 ± 1.25e | 10,000 ± 4.23i | 16 | 1,250 ± 2.1f | >10,000 | - | 10,000 ± 5.2i | >10,000 | - | 625 ± 0.2e | 10,000 ± 2.6i | 16 | 10,000 ± 3.2i | >10,000 | - | >10,000 | >10,000 | - | 31 ± 0.2a | 125 ± 0.1c | 4 |
| L. m | 2,500 ± 4.2g | 2,500 ± 2.1g | 1 | 1,250 ± 2.08f | 1,250 ± 1.3f | 1 | 1,250 ± 0.36f | 1,250 ± 0.36f | 1 | 2,500 ± 2.3g | 2,500 ± 1.3g | 1 | 10,000 ± 3.4i | 10,000 ± 2.3i | 1 | >10,000 | >10,000 | - | 31 ± 0.3a | 125 ± 0.2c | 4 |
| E. f | 625 ± 1.2e | 625 ± 0.39e | 1 | 625 ± 0.68e | 625 ± 1.7e | 1 | 2,500 ± 2.9g | 2,500 ± 0.98g | 1 | 2,500 ± 1.2g | 500 ± 2.5g | 1 | 5,000 ± 2.1h | 5,000 ± 1.3h | 1 | 5,000 ± 2h | 5,000 ± 3h | 1 | 125 ± 0.1c | >125 | - |
| E. c | 5,000 ± 3.5h | >10,000 | - | 5,000 ± 2.69h | >10,000 | - | 10,000 ± 5.9i | 10,000 ± 3.65i | 1 | 5,000 ± 2.6h | >10,000 | - | >10,000 | >10,000 | - | >10,000 | >10,000 | - | 62 ± 0.2b | 62 ± 0.3b | 1 |
| S. T | >10,000 | >10,000 | - | 5,000 ± 3.06h | >10,000 | - | 10,000 ± 4.9i | >10,000 | - | >10,000 | >10,000 | - | 2,500 ± 0.8g | >10,000 | - | 5,000±2h | >10,000 | - | 125 ± 0.1c | 125 ± 0.2c | 1 |
| P. a | 5,000 ± 3.4h | >10,000 | - | 10,000 ± 2.35i | >10,000 | - | 10,000 ± 4.98i | >10,000 | - | 5,000 ± 2.9h | >10,000 | - | 10,000 ± 3.1i | >10,000 | - | >10,000 | >10,000 | - | 125 ± 0.3c | >125 | - |
| Candida | MIC | MFC*** | MBC/MFC ratio | MIC | MFC | MFC/MIC ratio | MIC | MFC | MFC/MIC ratio | MIC | MFC | MFC/MIC ratio | MIC | MFC | MFC/MIC ratio | MIC | MFC | MFC/MIC ratio | Amphotericin B | ||
| MIC | |||||||||||||||||||||
| C. a | 625 ± 2.61e | 5,000 ± 2.63h | 8 | 1,250 ± 0.69f | 5,000 ± 3.5h | 4 | 1,250 ± 0.97i | >10,000 | - | 1,250 ± 0.28i | >10,000 | - | 5,000 ± 2.3h | 5,000 ± 2.4h | 1 | 1,250 ± 0.36i | 1,250 ± 0.5i | 1 | 500 ± 0.5days | ||
| C. k | 625 ± 0.9e | >10,000 | - | 1,250 ± 0.98f | 10,000 ± 2.5i | 8 | 2,500 ± 1.65f | >10,000 | - | 5,000 ± 2.9h | >10,000 | - | >10,000 | >10,000 | - | 5,000 ± 2.1h | >10,000 | - | 500 ± 0.6days | ||
| C. n | 2,500 ± 2.61g | >10,000 | - | 5,000 ± 2.36h | >10,000 | - | 2,500 ± 1.69f | >10,000 | - | 1,250 ± 0.2i | 10,000 ± 3.4i | 8 | 10,000 ± 4.3i | >10,000 | - | 5,000 ± 2.3h | 10,000 ± 2.5i | 2 | 500 ± 0.41day | ||
Antimicrobial effects of Farsetia aegyptia and Zilla spinosa extracts determined by microdilution method.
MIC* : minimal inhibitory concentration is mentioned in μg/mL; MBC** : minimal bactericidal concentration is mentioned in μg/mL, MFC*** : minimal fungicidal concentration is mentioned in μg/mL. Fa CH: farsetia aegyptia chloroformic sample; Zs CH: zilla spinosa chloroformic sample; Fa ET: farsetia aegyptia ethanolic sample; Zs ET: zilla spinosa ethanolic sample; Fa W: farsetia aegyptia water sample; Zs W: Zilla spinosa water sample. S.a: Staphylococcus aureus ATCC, 25923, L.m: Listeria monocytogenes ATCC, 19115, E.f: Enterococcus faecalis ATCC, 29212, E.c: Escherichia coli ATCC, 35218, S.T: Salmonella Typhimurium ATCC, 1408, P.a: Pseudomonas aeruginosa ATCC, 27853, C.a: Candida albicans ATCC, 90028, C.k: Candida krusei ATCC, 6258, C.n: Candida neoformans ATCC, 14116. The letters (a–i) show an important difference between the different doses of extracts in accordance with the Duncan assay (p < 0.05).
The F. aegyptia chloroformic extract showed a fungistatic effect against Candida albicans ATCC 90028, with an MFC/MIC ratio of 8. In contrast, the F. aegyptia aqueous sample, Z. spinosa chloroformic sample, and Z. spinosa aqueous extract exhibited a fungicidal effect against this yeast, with MFC/MIC ratios of four or lower. Notably, the Z. spinosa extract demonstrated fungicidal activity against Candida neoformans ATCC 14116, with an MFC/MIC ratio of 2, whereas the Z. spinosa ethanolic sample displayed a fungistatic effect against this strain, with an MFC/MIC ratio of 8.
In a previous study, organic extracts of Z. spinosa obtained using solvents such as methyl alcohol, acetone, ethyl acetate, methylene chloride, and petroleum ether were evaluated for their antifungal activity against Candida albicans (Alghanem et al., 2018). The authors reported that this Candida demonstrated moderate sensitivity to the ethyl acetate, acetone, and methyl alcohol extracts. This finding aligns with our results, as the chloroform, ethanolic, and water samples of this plant had an important effect on Candida albicans. However, these results were in disagreement with those of two other studies, which reported no antifungal effects of the methanol extract of Z. spinosa collected from Riyadh, Saudi Arabia (Shahat et al., 2017), and the chloroform extract of Z. spinosa collected from the Suez-Cairo desert road in Egypt (El-Sharkawy et al., 2013). The heterogeneity of results reported in our study and other studies could be related to one or more of the following factors: solvents used, extraction methods, and environmental effects on the plant specimens collected.
To explain the antibacterial, antifungal, and antibiofilm activities of the organic extracts of the two plants used in the current study, several previous studies have evaluated the precise mechanisms of action of the major compounds in these extracts. In this setting, El-Toumy and his collaborators have demonstrated that both stigmasterol and β-sitosterol, which are the primary compounds in the chloroform extract of the aerial parts of Z. spinosa collected in Egypt exhibit antimicrobial properties against S. aureus (El-Toumy et al., 2011). Moreover, other studies have shown that stigmasterol, in particular, has important antimicrobial effects against Candida albicans, P. aeruginosa, S. aureus, and E. coli (Odiba et al., 2014; Yinusa et al., 2014; Mailafiya et al., 2018).
Moshi and coworkers indicated that a dichloromethane extract of Uvaria scheffleri contains a mixture of stigmasterol and β-sitosterol, which is active against Candida albicans (Moshi et al., 2004). The antimicrobial activity of these steroids is attributed to their ability to inhibit the enzyme known as 'sortase,' which plays a role in the secretion and anchoring of cell wall proteins (Kanokmedhakul et al., 2005). Additionally, it was reported that disruption of the cell membrane may also be a potential mechanism for the action of steroids on bacteria and fungi (Tamokou et al., 2011).
Palmitic acid was identified as the dominant compound in the chloroform extract of Z. spinosa, and exhibited bactericidal activity against E. faecalis, with a minimal inhibitory concentration (MIC) of 2 μg/mL (Cheung Lam et al., 2016). Several studies have highlighted the effective antibacterial properties of linolenic acid, a prominent compound in the chloroform extract of F. aegyptia (12.68%), particularly against S. aureus (Kusumah et al., 2020; Butt et al., 2016). Additionally, it was reported that long-chain fatty acids, such as arachidonic acid, linoleic acid, and oleic acid found in both F. aegyptia and Z. spinosa extracts, showed interesting antibacterial activities against S. aureus (Zheng et al., 2005). Additionally, 13-docosenamide, a fatty amide, and phytol, an acyclic monounsaturated diterpene alcohol also possess antimicrobial properties (Islam et al., 2018; Kumaradevan et al., 2015; Ghaneian et al., 2015).
3.3 Anti-biofilm activities of Farsetia aegyptia and Zilla spinosa
Microbial biofilms consist of colonies of microorganisms embedded in a polysaccharide matrix, adhering to surfaces. They pose substantial biological threats across various settings, including food, drinking water, industrial environments, and clinical settings, as the physical barriers formed by these biofilms hinder the access of antimicrobials to their target sites (Yasbolaghi Sharahi et al., 2025).
Recently, there has been increasing interest in exploring the processes involved in inhibiting bacterial biofilm growth and formation. The initial step in most biofilm formation is attachment. Consequently, the anti-adhesive properties of natural compounds have become a focal point of research aimed at preventing microcolony formation (Kungwani et al., 2024; Alibi et al., 2021). In this setting, the current study investigated the inhibitory effects of chloroform, ethanol, and water extracts from F. aegyptia and Z. spinosa, both members of the Brassicaceae family, on the adhesion of three Gram-positive bacteria, three Gram-negative bacteria, and three Candida strains. Thus, Extracts that demonstrated more than 50% inhibition of biofilm formation are classified as having a good anti-biofilm (ABF) effect. In contrast, those that exhibited less than 50% inhibition are considered to have a weak ABF effect (Sandasi et al., 2008).
Importantly, in the current study, all Gram-positive bacterial strains used, including S. aureus ATCC 25923, L. monocytogenes ATCC 19115, and E. faecalis ATCC 29212; Gram-negative bacterial strains comprising E. coli ATCC 35218, Salmonella Typhimurium ATCC 1408, and P. aeruginosa ATCC 27853, and strains of Candida: Candida neoformans ATCC 14116, Candida albicans ATCC 90028, and Candida krusei ATCC 6258, were considered biofilm producers according to the microtiter method results.
The term MBIC50 refers to the minimum biofilm inhibition concentration required to achieve 50% inhibition. This term indicates the lowest antimicrobial dose that produce a ≥50% reduction in biofilm formation (Genovese et al., 2021).
In the current study, the MBIC50 values are presented in Table 3. The results indicate that the samples effectively inhibit biofilm formation by the selected pathogens when compared to the negative control used in the experiment (Figures 2–4). Notably, the antibiofilm activity of the two plants samples against the tested microorganisms is dose-dependent, with the percentage inhibition increasing as sample concentration increases (p < 0.05). To our knowledge, the current study is the first to show these findings.
TABLE 3
| Extracts | Fa CH | Zs CH | Fa ET | Zs ET | Fa W | Zs W |
|---|---|---|---|---|---|---|
| Gram-positive bacteria | MBIC50 (μg/mL) | |||||
| Staphylococcus aureus ATCC 25923 | 700 ± 0.01abc | 940 ± 0.89abcde | 710 ± 0.79abc | 840 ± 0.21abc | 1,200 ± 0.31def | 840 ± 0.05abc |
| Enterococcus faecalis ATCC 29212 | >10,000 | 2,660 ± 0.21jk | 750 ± 0.78abc | 1820 ± 0.23i | 1,530 ± 0.22ghi | 5,050 ± 0.04n |
| Listeria monocytogenes ATCC 19115 | 1,650 ± 0.16hi | 5,010 ± 0.32n | 1,020 ± 0.32bcde | 3,840 ± 0.54m | 5,020 ± 0.25n | >10,000 |
| Gram-negative bacteria | MBIC50 (μg/mL) | |||||
| Pseudomonas aeruginosa ATCC 27853 | 3,100 ± 0.03L | 630 ± 0.25a | 700 ± 0.24ab | 1,250 ± 0.33efg | >10,000 | >10,000 |
| Escherichia coli ATCC 35218 | >10,000 | 1820 ± 0.33i | 870 ± 0.35abcd | 970 ± 0.34abcde | >10,000 | >10,000 |
| Salmonella typhimurium ATCC 14080 | >10,000 | >10,000 | 2,800 ± 0.24jkl | >10,000 | >10,000 | 2,850 ± 0.95kL |
| Candida strains | MBIC50 (μg/mL) | |||||
| Candida albicans ATCC 90028 | 3,900 ± 0.5m | 1,350 ± 0.05fgh | 660 ± 0.65a | 970 ± 0.24abcde | >10,000 | >10,000 |
| Candida krusei ATCC 6258 | 750 ± 1.23abc | 5,100 ± 0.3n | 770 ± 0.42abc | >10,000 | >10,000 | 1,650 ± 0.32hi |
| Candida neoformans ATCC 14116 | 920 ± 1.33abcde | 1850 ± 0.09i | 970 ± 0.3abcde | 960 ± 0.32abcde | 2,500 ± 0.54j | 1,000 ± 0.24bcde |
Minimal biofilm inhibitory concentration (MBIC) of Farsetia aegyptia and Zilla spinosa chloroformic, ethanolic, and water samples against Gram-positive, Gram-negative, and Candida strains.
Fa CH: farsetia aegyptia chloroformic sample; Zs CH: zilla spinosa chloroformic sample; Fa ET: farsetia aegyptia ethanolic sample; Zs ET: zilla spinosa ethanolic sample; Fa W: farsetia aegyptia water sample; Zs W: Zilla spinosa water sample. The letters (a–n) demonstrate a significant difference between the different samples by the Duncan test (p < 0.05).
FIGURE 2
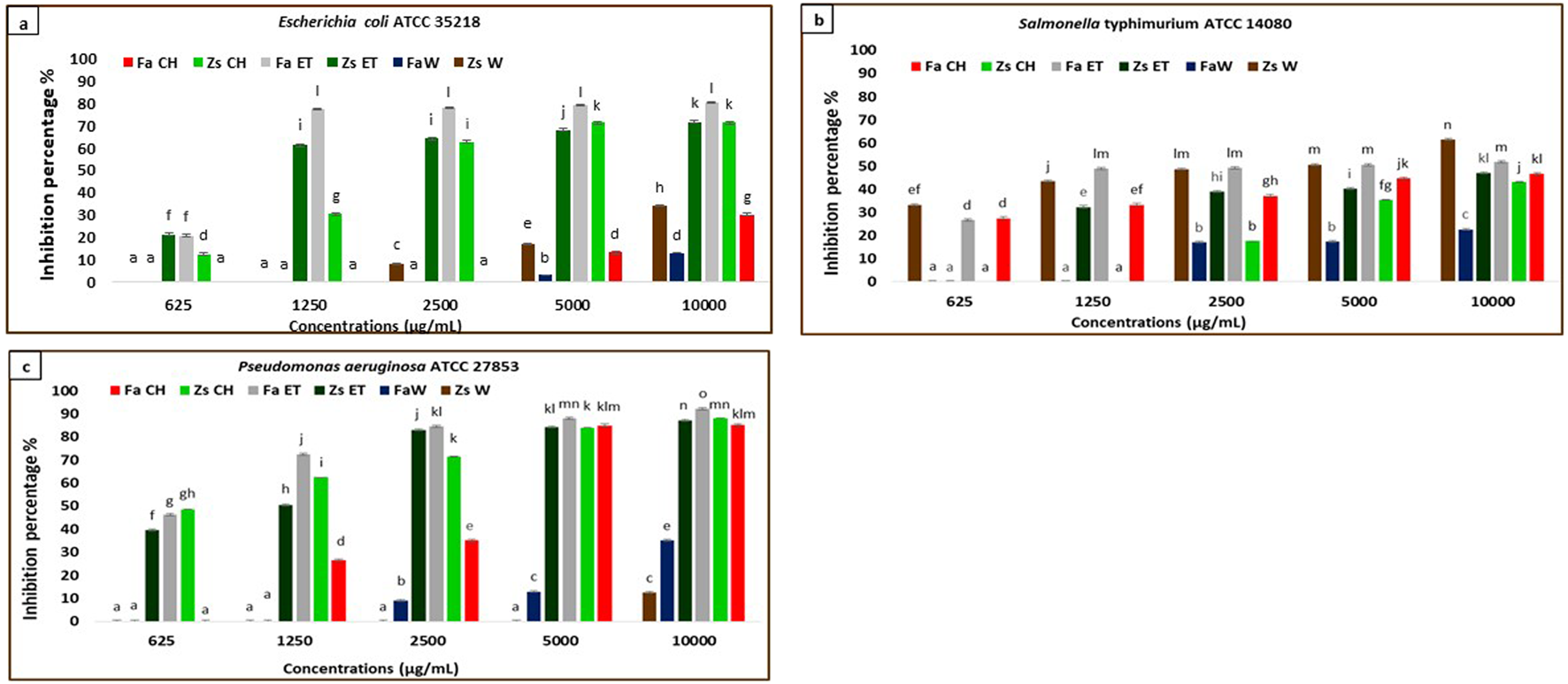
Anti-biofilm activity of F. aegyptia chloroformic sample (Fa CH), Z. spinosa chloroformic sample (Zs CH), F. aegyptia ethanolic sample (Fa ET), Z. spinosa ethanolic sample (Zs ET), F. aegyptia water sample (Fa W), and Z. spinosa water sample (Zs W), against (a) Escherichia coli ATCC 35218, (b)Salmonella Typhimurium ATCC 14080, and (c)Pseudomonas aeruginosa ATCC 27853. The letters (a -p) on the bars present important differences (p < 0.05).
3.3.1 Gram-negative bacteria anti-biofilm assay
The effects of different extracts of the two plants used in this study on inhibiting biofilm formation against the Gram-negative strains: E. coli ATCC 35218, Salmonella Typhimurium ATCC 14080, and P. aeruginosa ATCC 27853 were indicated in Figures 2a–c. The chloroformic and ethanolic extracts of Z. spinosa, and Z. spinosa, and the chloroformic extract of Z. spinosa showed significant biofilm inhibition against E. coli ATCC 35218 (p < 0.05). The most effective sample was the ethanolic extract of F. aegyptia, which exhibited biofilm inhibition percentages of 77.38% at 1,250 μg/mL, 78.14% at 2,500 μg/mL, 79.08% at 5,000 μg/mL, and 80.44% at 10,000 μg/mL (Figure 2a) with an MBIC50 of 870 ± 350 μg/mL.
Regarding the second g-negative bacterium, Salmonella Typhimurium ATCC 14080, the tested samples showed moderate biofilm inhibition, ranging from 22.65% (for the water extract of F. aegyptia) to 61.44% (for the aqueous sample of Z. spinosa) at a concentration of 10,000 μg/mL (Figure 2b) (p < 0.05). At both 10,000 μg/mL and 5,000 μg/mL, Z. spinosa and F. aegyptia, including their chloroformic and ethanolic extracts, demonstrated considerable biofilm inhibition against P. aeruginosa ATCC 27853 (greater than 83%) (Figure 2c), with MBIC50 values ranging from 630 ± 250 μg/mL to 3,100 ± 30 μg/mL (Table 3) (p < 0.05).
3.3.2 Gram-positive bacteria anti-biofilm assay
The treatment of S. aureus ATCC 25923 with F. aegyptia and Z. spinosa samples for 24 h resulted in a decrease in biofilm formation across all concentrations tested (1,000 μg/mL, 5,000 μg/mL, 2,500 μg/mL, 1,250 μg/mL, and 625 μg/mL). For the highest concentration of 10,000 μg/mL, all tested samples of these plants extracts showed significant inhibition of biofilm formation, with more than 80% inhibition. However, the F. aegyptia water extract showed a lower inhibition rate of 67.49% (Figure 3A). The best MBIC50 values were observed at 700 ± 10 μg/mL for the F. aegyptia chloroform extract and at 710 ± 790 μg/mL for the F. aegyptia ethanolic extract (Table 3).
FIGURE 3
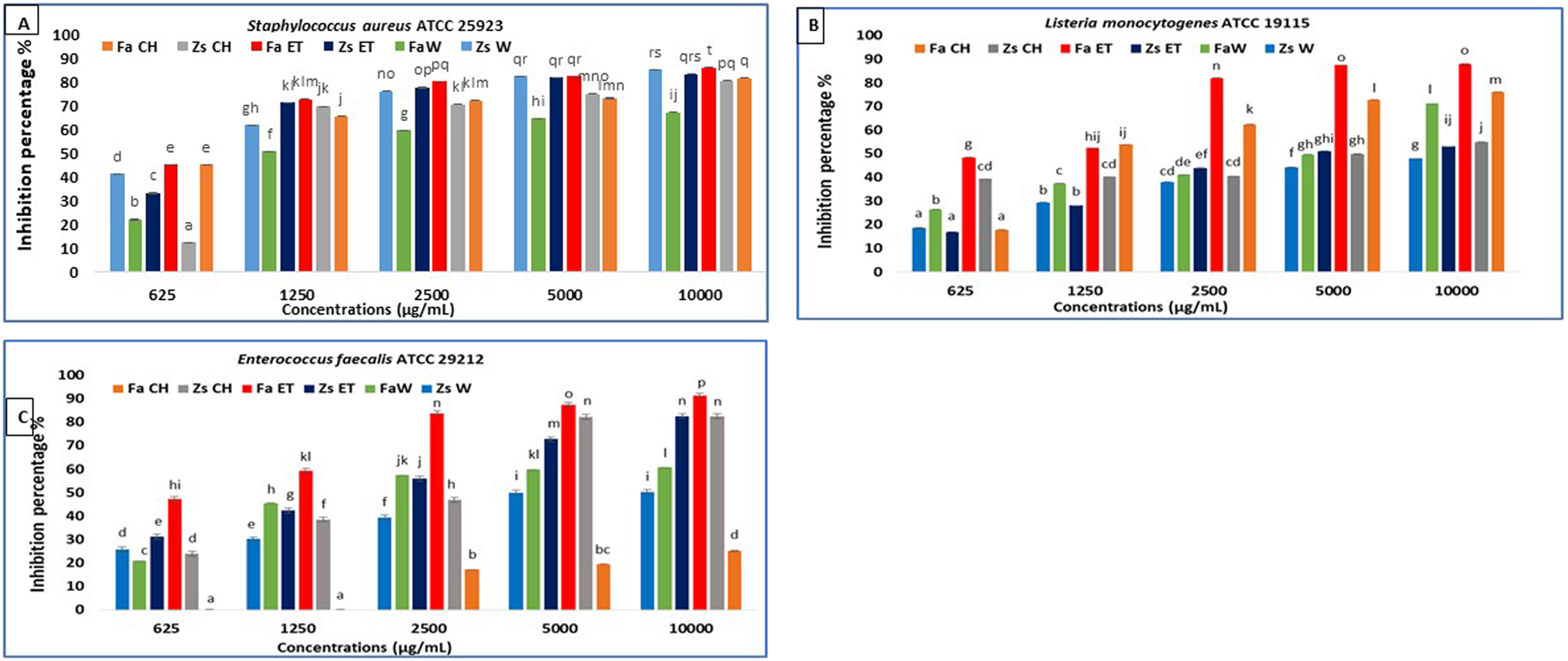
Anti-biofilm activity of F. aegyptia chloroformic sample (Fa CH), Z. spinosa chloroformic sample (Zs CH), F. aegyptia ethanolic sample (Fa ET), Z. spinosa ethanolic sample (Zs ET), F. aegyptia water sample (Fa W), and Z. spinosa water sample (Zs W), against (A) Staphylococcus aureus ATCC 25923, (B)Listeria monocytogenes ATCC 19115, and (C) Enterococcus faecalis ATCC 29212. The letters (a -p) on the bars present important differences (p < 0.05).
For the second g-positive bacterium, L. monocytogenes ATCC 19115, extracts from F. aegyptia were more effective than those from Z. spinosa. Notably, the ethanolic extract exhibited the best antibiofilm effect, achieving 87.94% inhibition at 10,000 μg/mL, 87.53% at 5,000 μg/mL, and 82.02% at 2,500 μg/mL (Figure 3B). The MBIC50 for this extract was 1,020 ± 320 μg/mL, followed by the chloroform extract, which showed a 76.02% inhibition at 10,000 μg/mL with an MBIC50 of 1,650 ± 160 μg/mL (Table 3). For E. faecalis ATCC 29212, the F. aegyptia ethanolic extract demonstrated more than 91% inhibition at 10,000 μg/mL. The chloroform and ethanol extracts of Z. spinosa also significantly inhibited biofilm formation, with inhibition rates of 82.4% and 82.61%, respectively (Figure 3C). In contrast, the F. aegyptia chloroform extract exhibited weak inhibition against E. faecalis, with only 25.06% inhibition at 10,000 μg/mL.
The formation of biofilm plays an important role in pathogenesis, and biofilm development is mediated by a signal-based quorum-sensing system (Yasbolaghi Sharahi et al., 2025; Kungwani et al., 2024; Alibi et al., 2020). Hens, biofilm infections have become a threat in injured and post-surgical patients, leading to prolonged wound healing as well as high patient morbidity and mortality.
In the early stages of the wound, the host immune system destroys microbes. However, when these microbes adhere to the wound surface and cells, they multiply rapidly through quorum sensing, thereby forming biofilms (Yasbolaghi Sharahi et al., 2025; Kungwani et al., 2024). The well-formed biofilm develops resistance (1,000 times more) to destruction by the host immune system and antibiotics (Kungwani et al., 2024). In this setting, the current study showed reduced biofilm biomass production in Gram-positive, Gram-negative, and Candida strains when treated with chloroformic or ethanolic extracts of Z. spinosa and F. aegyptia. MBIC50 was strain-dependent, showing different resistance levels against these extracts. Moreover, we suggest that compounds from these antimicrobial extracts could have disrupted the mechanism of attachment and the formation of the extracellular polymeric substance (EPS), or interfered with the maturation stage of biofilm formation and the quorum sensing system, thereby preventing the development of bacterial biofilms.
It is well established that biofilm resistance to antibiotics is due to intrinsic structural properties, such as limited antibiotic permeability through the EPS, the production of efflux pumps, and the release of inactivating enzymes. They may acquire resistance through gene expression and mutational changes, obstructing antibiotic permeation, inducing slow growth and adaptive stress responses, and leading to persistent cell proliferation (Gedefie et al., 2023; Garousi et al., 2022; Zhao et al., 2020; Hosseini et al., 2020). Biofilms exhibit varying physiological and functional properties; thus, a combination of therapeutics could be better than a single antibiotic intervention for managing and treating biofilm infections (Yasbolaghi Sharahi et al., 2025; Kungwani et al., 2024).
Hence, recent research has focussed on exploring plants as a potential therapeutic solution to biofilm infections. Plants contain bioactive phytochemicals that work together to prevent antibiotic resistance with minimal side effects (Yasbolaghi Sharahi et al., 2025; Kungwani et al., 2024; Silva et al., 2023).
In this context, even the chloroformic or ethanolic extracts of the two plants used in this study showed significant antibiofilm activity against Gram-positive, Gram-negative, and Candida strains, but the MBIC50 values were considerably high. These MBIC50 values were approximately similar to those of previous MBIC50 levels for standard antibiotics (Ferreira et al., 2024; Silva et al., 2023). Thus, to reduce the MBIC50 values of the organic extracts in this study, additional studies will be needed to evaluate the combined use of these extracts or their major compounds with conventional antibiotics to inhibit biofilm formation.
3.3.3 Candida antibiofilm assay
The findings regarding the in vitro antibiofilm effects of different Z. spinosa and F. aegyptia extracts against three Candida species are illustrated in Figures 4d–f. For a concentration of 10,000 μg/mL, the ethanolic extract of F. aegyptia inhibits 83.52% of biofilm formation of Candida albicans ATCC 90028. Additionally, the MBIC50 was determined to be 660 ± 650 μg/mL, which resulted in a 50% reduction in biofilm formation (Table 3). The chloroform extracts from the two tested plants, along with the ethanolic extract of Z. spinosa, also demonstrated significant antibiofilm activity, exceeding 71% against this yeast (p < 0.05). Conversely, the water extracts of Z. spinosa and F. aegyptia exhibited weak biofilm inhibition rates of 35.64% and 41.74%, respectively (Figure 4d). The impact on biofilm formation varies among the different Candida strains tested. The ethanolic extract of Z. spinosa showed similar effects on biofilm formation of both Candida albicans ATCC 90028 and Candida neoformans ATCC 14116, with inhibition percentages of 71.73% and 71.77%, respectively (Figures 4d,f). However, this extract had a limited effect on biofilm formation by Candida Krusei ATCC 6258, with an inhibition percentage of only 30.41% (Figure 4e). In contrast, the ethanolic extract of F. aegyptia significantly inhibited biofilm formation by all three Candida strains, achieving over 80% inhibition. Overall, our results indicate that organic extracts significantly inhibit biofilm formation of Candida strains compared to aqueous extracts, except for the Z. spinosa water extract against Candida neoformans ATCC 14116, which demonstrated an inhibition rate of 75.25%. The MBIC50 values ranged from 660 ± 650 μg/mL to more than 10,000 μg/mL (Table 3).
FIGURE 4
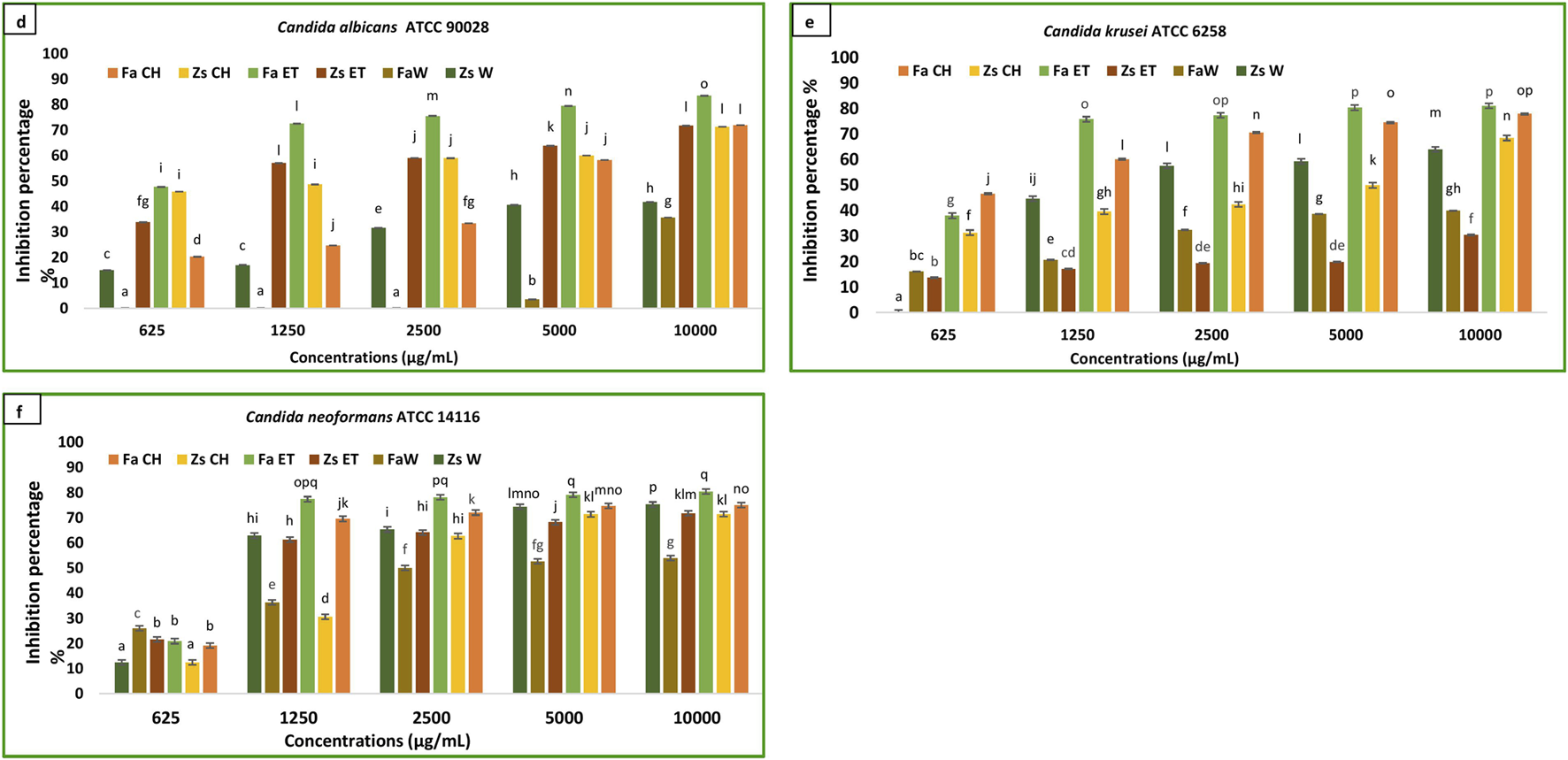
Anti-biofilm activity of F. aegyptia chloroformic sample (Fa CH), Z. spinosa chloroformic sample (Zs CH), F. aegyptia ethanolic sample (Fa ET), Z. spinosa ethanolic sample (Zs ET), F. aegyptia water sample (Fa W), and Z. spinosa water sample (Zs W) against (d)Candida albicans ATCC 90028, (e)Candida Krusei ATCC 6258, and (f)Candida neoformans ATCC 14116. The letters (a–p) on the bars present important differences (p < 0.05).
3.4 Chemical composition of Farsetia aegyptia and Zilla spinosa chloroformic extract by GC-MS and LC-HRMS
In this study, we selected chloroform extracts from F. aegyptia and Z. spinosa for qualitative analysis using GC-MS due to their notable antimicrobial properties. The goal of this experiment is to identify the pharmacologically active constituents present in these extracts. This technique involves comparing retention times, molecular formulas, and other relevant factors. Our findings indicate that both non-polar and polar compounds are present, with a total of 28 phytocomponents identified. Figures 5a,b show the chromatograms of the two chloroform extracts, and the detected compounds are summarized in Table 4. Each compound is described with its molecular formula, retention time, structural information, and area percentages. The chloroform extract of Z. spinosa includes 10 compounds, such as: Phytosterols: β-sitosterol (40.39%) and stigmasterol (22.24%) - Benzopyrone: coumarin (9.25%) - Fatty acids: n-decanoic acid (6.40%), arachidonic acid (3.73%), oleic acid (3.73%), and hexanoic acid (3.38%) - Terpene: neophytadiene (1.24%) - Benzofuran: loliolide (0.88%) - Terpenoid: cyclolaudenol (3.73%). This comprehensive analysis highlights the rich phytochemical profile of these extracts.
FIGURE 5
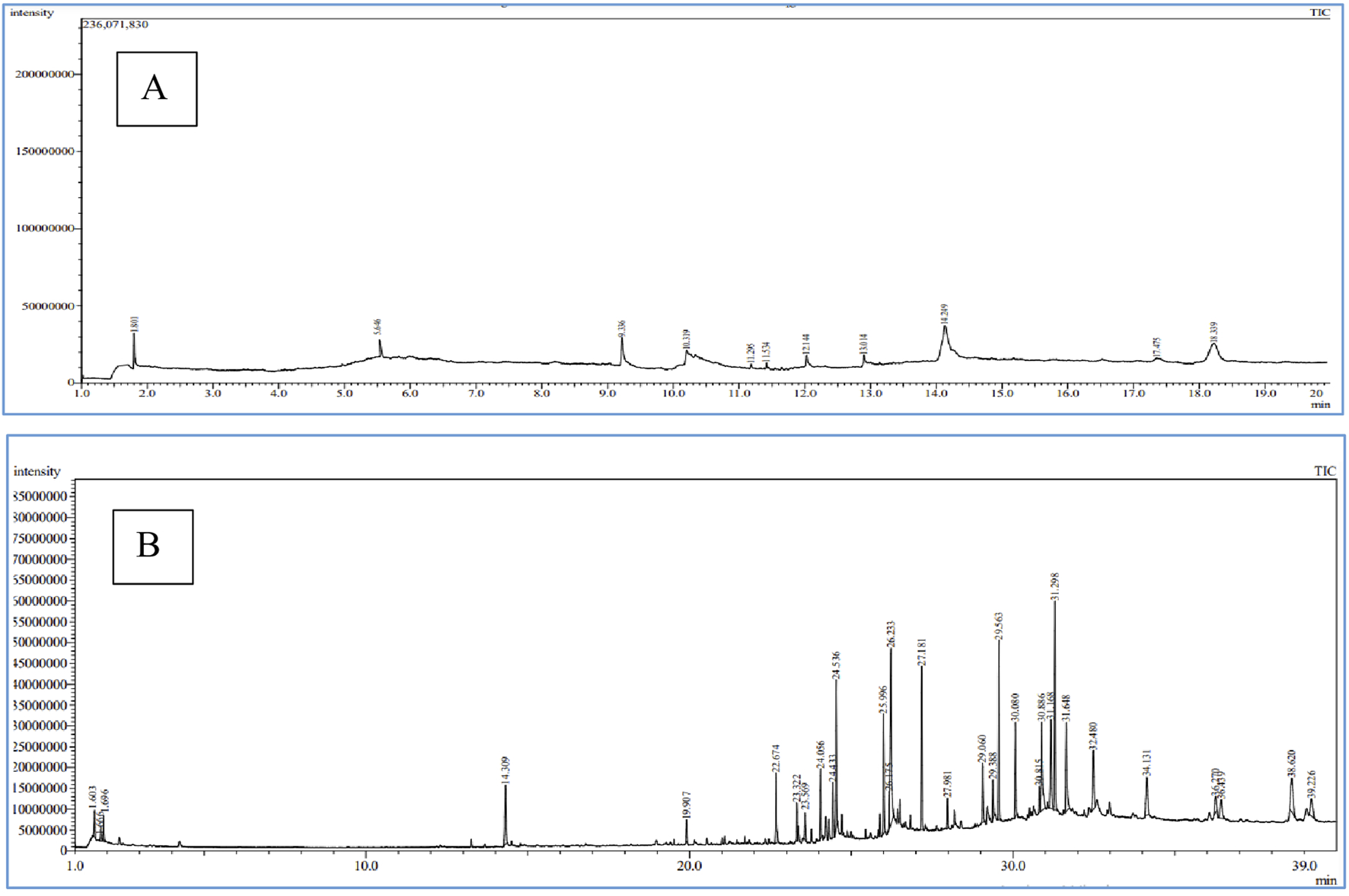
Chromatograms of Z. spinosa (A) and F. aegyptia (B) chloroform extracts by GC-MS method.
TABLE 4
| Compound number | Compounds | Class | Molecular formula | Retention time | Area percentage % | |
|---|---|---|---|---|---|---|
| FaCH | ZsCH | |||||
| 1 | Pentanoic acid, 3-methyl-4-oxo- | Fatty acid esters | C6H10O3 | 1.696 | 3.85 | |
| 2 | Hexanoic acid | Fatty acid | C6H12O2 | 5.646 | 3.38 | |
| 3 | Coumarin | Chromenones | C9H6O2 | 9.336 | 9.25 | |
| 4 | n-Decanoic acid | Fatty acid | C10H20O2 | 10.319 | 6.40 | |
| 5 | Loliolide | Benzofurans | C11H16O3 | 11.295 | 3.70 | 0.88 |
| 6 | Neophytadiene | Terpene | C20H38 | 11.534 | 1.65 | 1.24 |
| 7 | Arachidonic acid | Fatty acid | C20H40O2 | 12.144 | 6.96 | 3.73 |
| 8 | Oleic acid | Fatty acid | C18H34O2 | 13.014 | 3.73 | |
| 9 | Beta-sitosterol | Phytosterol | C29H50O | 14.249 | 40.39 | |
| 10 | Naphtalene | Naphthalenes | C10H8 | 14.309 | 3.76 | |
| 11 | Cyclolaudenol | Steroid | C31H52O | 17.475 | 3.73 | |
| 12 | Stigmasterol | Phytosterol | C29H48O | 18.339 | 1.84 | 22.24 |
| 13 | 2 (4H)-benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)- | Benzofurans | C11H16O2 | 19.907 | 0.82 | |
| 14 | 1,2-Benzenedicarboxylic acid, bis (2-methylpropyl) ester | Benzoic acid esters | C16H22O4 | 24.056 | 3.41 | |
| 15 | Phytol | Alcohol | C20H40O | 25.996 | 5.6 | |
| 16 | Linoleic acid | Fatty acid | C18H32O2 | 26.175 | 1.6 | |
| 17 | Linolenic acid | Fatty acid | C18H30O2 | 26.233 | 12.68 | |
| 18 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | Fattyacidethylesterofglycerol | C19H38O4 | 29.388 | 1.88 | |
| 19 | Linoleic acid ethyl ester | Fatty acid esters | C20H36O2 | 30.815 | 1.15 | |
| 20 | Linolenic acid, ethyl ester | Fatty acid esters | C20H34O2 | 30.886 | 7.31 | |
| 21 | 13-Docosenamide, (Z)- | Fatty amides | C22H43NO | 31.648 | 6.47 | |
| 22 | Cholesterol | Steroid | C27H46O | 36.439 | 4.64 | |
| 23 | Campesterol | Phytosterol | C28H48O | 38.620 | 2.17 | |
Components detected in the chloroform samples of Farsetia aegyptia and Zilla spinosa by GC-MS.
In the chloroform extract of F. aegyptia, as illustrated in Figure 5b and Table 4, a rich diversity of compounds is observed. This extract contains 17 different compounds with varying area percentages. The major constituents include linolenic acid (12.68%), linolenic acid ethyl ester (7.31%), arachidonic acid (6.96%), (Z)-13-docosenamide (6.47%), and phytol (5.6%). Additionally, the extract features several minor compounds, ranging from cholesterol at 4.64% to 2(4H)-benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-, (R)- at 0.82%. Common constituents found in the chloroform extracts of both tested plants include the fatty acid arachidonic acid (6.96% in F. aegyptia and 3.73% in Z. spinosa), the phytosterol stigmasterol (1.84% in F. aegyptia and 22.24% in Z. spinosa), the benzofuran loliolide (3.70% in F. aegyptia and 0.88% in Z. spinosa), and the terpene neophytadiene (1.65% in F. aegyptia and 1.24% in Z. spinosa). Notably, many of these compounds are reported for the first time in these plants. Another qualitative method, LC-HRMS (as shown in Figures 6a,b), was used to detect phytocompounds in these active extracts. We identified 19 compounds in the chloroform extract of F. aegyptia and 38 compounds in the chloroform extract of Z. spinosa (Table 5). These constituents belong to various chemical classes, including polyols, alkaloids, carboxylic acids, phenolic compounds, lactones, glycosides, terpene glycosides, indoles, N-acylethanolamines, amines, fatty acyls, p-benzoquinones, phenylpropanes, terpenoids, fatty amides, quassinoids, fatty acids, brassinolides, macrolides, cyclic peptides, coumaric acid, brassinosteroids, steroids, triterpenoid saponins, and acetogenins. In the F. aegyptia extract, the major compounds include (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid (15.87%), 3-tert-butyl-5-methylcatechol (15.31%), 3-methylbutyl 2-methylpropanoate (6.94%), retronecine (6.91%), and palmitic amide (6.91%). For the Z. spinosa extract, the predominant compound was palmitic acid (13.31%), followed by (3β,22R,23R,24S)-3,22,23-trihydroxystigmastan-6-one (12.76%), α-licanic acid (9.11%), cyclopassifloside IV (5.42%), and embelin (4.79%). Only one compound was common to both extracts: quercuslactone, which was present at 4.34% in the F. aegyptia chloroform extract and 0.59% in the Z. spinosa chloroform extract.
FIGURE 6
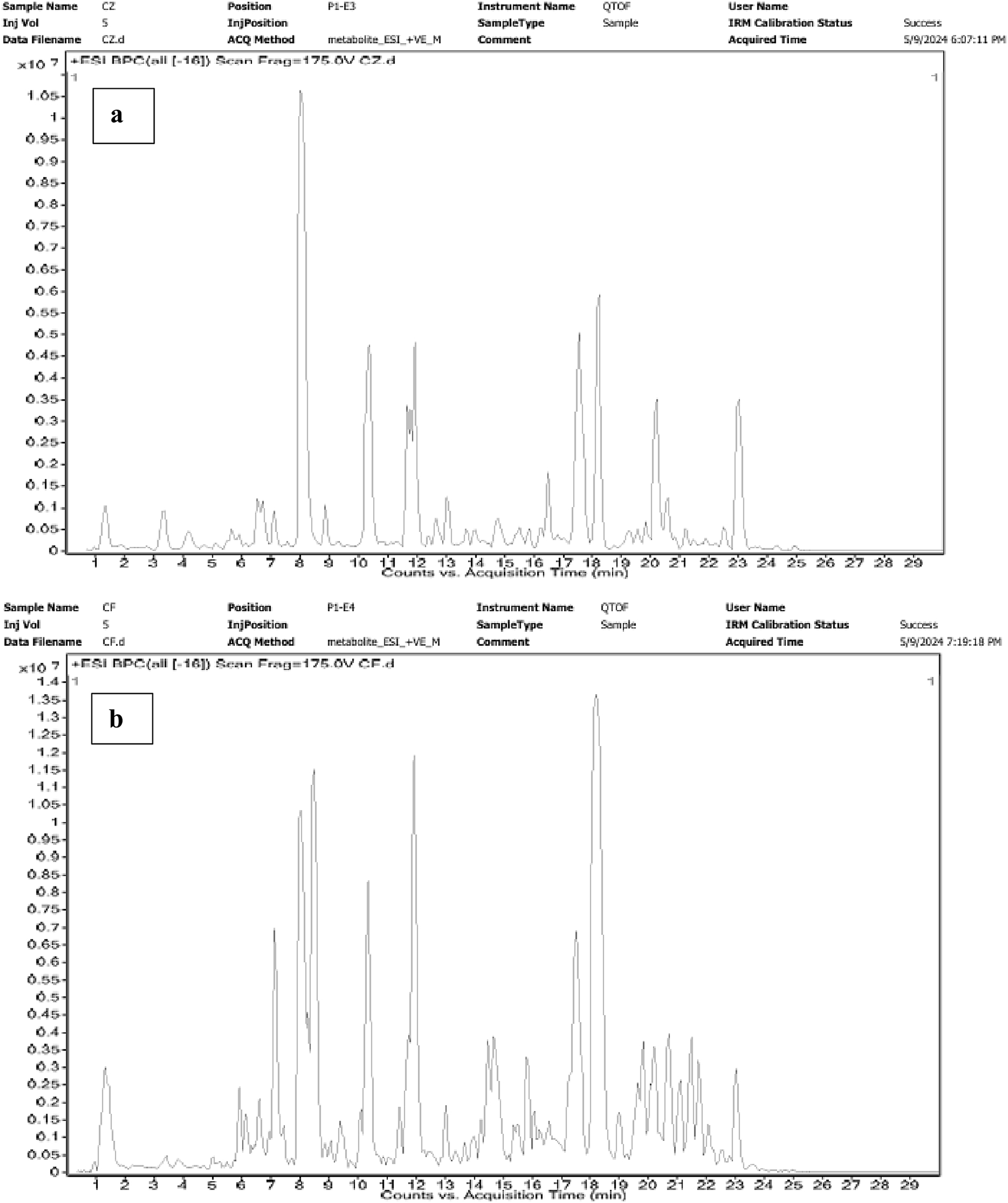
Chromatograms of Z. spinosa (a) and F. aegyptia (b) chloroform extracts by LC-HRMS method.
TABLE 5
| Compounds | Class | Retention time | Formula | [M-H]+ (m/z) | [M-H]-(m/z) | Percentage area % | |
|---|---|---|---|---|---|---|---|
| Fa CH | Zs CH | ||||||
| Quinic acid | Polyol | 1.183 | C7 H12 O6 | 191.0555 | 0.59 | ||
| Retronecine | Alkaloid | 1.251 | C8 H13 N O2 | 156.1007 | 6.91 | ||
| L-Malic acid | Carboxylic acids | 1.268 | C4 H6 O5 | 133.0145 | 2.14 | ||
| 2,6-Dihydroxybenzoic acid | Phenolic compound | 3.042 | C7 H6 O4 | 153.0199 | 0.12 | ||
| Resorcinol | Phenolic compound | 3.51 | C6 H6 O2 | 169.0512 | 0.08 | ||
| (Cyclohexylmethyl) pyrazine | Alkaloid | 3.707 | C11 H16 N2 | 177.1371 | 2.03 | ||
| Chlorogenic acid | Phenolic compound | 4.691 | C16 H18 O9 | 353.0869 | 0.10 | ||
| Genistic acid | Phenolic compound | 4.838 | C7 H6 O2 | 121.0297 | 2.75 | ||
| Caffeic acid | Phenolic compound | 4.872 | C9 H8 O4 | 179.036 | 0.11 | ||
| O-Cresol | Phenolic compound | 5.803 | C7 H8 O | 167.0704 | 0.23 | ||
| Vanillin acetate | Phenolic compound | 6.517 | C10 H10 O4 | 193.0523 | 0.46 | ||
| Quercuslactone a | Lactone | 6.557 | C9 H16 O2 | 179.1051 | 4.34 | 0.59 | |
| Carnegine | Alkaloid | 6.653 | C13 H19 N O2 | 222.1471 | 3.43 | ||
| Solanocapsine | Alkaloid | 6.726 | C27 H46 N2 O2 | 453.341 | 1.08 | ||
| Isorhamnetin 3-O-[b-D-glucopyranosyl-(1->2)-a-L-rhamnopyranoside] | Glycoside | 7.198 | C28 H32 O16 | 623.1652 | 1.30 | ||
| (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid | Phenolic compound | 7.36 | C17 H30 O7 | 347.2059 | 15.87 | ||
| 3-tert-Butyl-5-methylcatechol | Phenylpropanes | 10.236 | C11 H16 O2 | 181.1213 | 15.31 | ||
| 3-Methylbutyl 2-methylpropanoate | Carboxylic acids esters | 10.549 | C9 H18 O2 | 181.1209 | 6.94 | ||
| Cyclobrassinone | Indole | 10.268 | C11 H8 N2 O2 S | 231.0243 | 1.28 | ||
| Linoleoyl ethanolamide | n-acylethanolamines | 11.29 | C20 H37 N O2 | 346.2715 | 0.96 | ||
| Cinnamoside | Terpene glycosides | 11.411 | C24 H38 O12 | 541.2223 | 1.15 | ||
| Phytosphingosine | Amines | 11.841 | C18 H39 N O3 | 318.2978 | 1.63 | ||
| (1R,2R,4S)-p-Menthane-1,2,8-triol 8-glucoside | Glycoside | 11.97 | C16 H30 O8 | 373.1836 | 1.48 | ||
| Lauroyl diethanolamide | Fatty acyls | 12.214 | C16 H33 N O3 | 288.2512 | 2.55 | ||
| Embelin | p-benzoquinones | 12,45 | C17 H26 O4 | 293.1776 | 4.79 | ||
| 2,6-Di-tert-butyl-4-ethylphenol | Phenylpropanes | 12.907 | C16 H26 O | 257.1878 | 1.19 | ||
| 3-Methyl-alpha-ionyl acetate | Terpenoids | 12.954 | C16 H26 O2 | 273.1819 | 1.04 | ||
| Palmitic amide | Fatty amides | 13.415 | C16 H33 N O | 300.2554 | 0.07 | ||
| Nigakilactone B | Quassinoids | 13.644 | C22 H32 O6 | 415.2095 | 1.57 | ||
| (S)-Nerolidol 3-O-[a-L-Rhamnopyranosyl-(1->4)-a-L-rhamnopyranosyl-(1->2)-b-D-glucopyranoside] | Glycoside | 14.768 | C33 H56 O14 | 675.3639 | 1.28 | ||
| Gnididilatin | Terpenoid | 14.831 | C37 H48 O10 | 711.3424 | 1.30 | ||
| Terminaline | Alkaloid | 15.003 | C23 H41 N O2 | 364.3184 | 2.06 | ||
| Alpha-licanic acid | Fatty acid | 15.515 | C18 H28 O3 | 291.1985 | 9.11 | ||
| Homodolicholide | Brassinolides | 17.137 | C29 H48 O6 | 491.339 | 1.67 | ||
| Ethylene brassylate | Macrolides | 17.698 | C15 H26 O4 | 293.1721 | 1.93 | ||
| Pandamine | Cyclic peptide | 18.046 | C31 H44 N4 O5 | 549.3326 | 1.54 | ||
| 16-Feruloyloxypalmitate | Coumaric acids | 18.98 | C26 H40 O6 | 447.2776 | 1.52 | ||
| Kanokoside D | Terpene glycosides | 19.86 | C27 H44 O16 | 623.2565 | 1.30 | ||
| Pteroside A | Glycoside | 20.11 | C21 H30 O8 | 455.1934 | 1.29 | ||
| Gallic acid | Phenolic compound | 20.158 | C7 H6 O5 | 169.0143 | 0.05 | ||
| 19-Hydroxycinnzeylanol 19-glucoside | Terpene glycosides | 20.188 | C26 H42 O13 | 607.2593 | 1.28 | ||
| Oleamide | Fatty amides | 20.443 | C18 H35 N O | 282.2771 | 1.04 | ||
| N-(14-Methylhexadecanoyl) pyrrolidine | Alkaloid | 21.214 | C21 H41 N O | 368.3186 | 1.57 | ||
| Palmitic acid | Fatty acids | 21.43 | C16 H32 O2 | 255.2347 | 13.31 | ||
| 4-Methoxycinnamoyloleanolic acid methyl ester | Terpenoid | 21.473 | C41 H58 O5 | 629.4246 | 1.26 | ||
| 6-Deoxotyphasterol | Brassinosteroid | 21.87 | C28 H50 O3 | 479.374 | 2.00 | ||
| Teasterone | Steroid | 21.885 | C28 H48 O4 | 447.3514 | 4.31 | ||
| N-Hexadecanoylpyrrolidine | Alkaloid | 22.507 | C20 H39 N O | 310.3085 | 2.1 | ||
| Cyclopassifloside IV | Triterpenoid saponin | 22.965 | C37 H62 O12 | 697.4115 | 5.42 | ||
| (3beta,22R,23R,24S)-3,22,23-trihydroxystigmastan-6-one | Steroid | 22.97 | C29 H50 O4 | 461.3658 | 12.76 | ||
| Annoglaxin | Acetogenins | 22.988 | C35 H62 O8 | 669.4559 | 1.49 | ||
| 3-cis-Hydroxy-b,e-Caroten-3′-one | Terpenoid | 23.139 | C40 H54 O | 605.4016 | 1.49 | ||
| Cholesteryl beta-D-glucoside | Glycoside | 23.287 | C33 H56 O6 | 607.4235 | 1.73 | ||
| Cyclopassifloside II | Triterpene | 23.303 | C37 H62 O11 | 681.4183 | 1.41 | ||
| Soyasapogenol B 24-O-b-D-glucoside | Triterpenoid saponin | 23.349 | C36 H60 O8 | 679.4378 | 1.35 | ||
| 3,7-Dihydroxy-25-methoxycucurbita-5,23-dien-19-aL | Steroid | 23.518 | C31 H50 O4 | 531.371 | 1.24 | ||
Chemical composition in chloroform extracts by LC-HRMS.
3.5 Molecular docking study
Molecular docking is a computational method commonly used to obtain insights into the molecular mechanisms of pharmacologically active substances. Several previous studies have highlighted the significant interactions of molecules related to their antibacterial potential through docking simulations (Lahmadi et al., 2023; Horchani et al., 2022; Horchani et al., 2020).
This approach allows us to identify a ligand (the tested compound) in chloroformic extracts of F. aegyptia and Z. spinosa that inhibits a specific target protein through its interactions. We selected several targets to predict the effectiveness of the tested ligands in inhibiting the relevant enzymes. According to the results of GC/MS and LC-HRMS, the major compounds found in the chloroformic extract of Farsetia. aegyptia include linolenic acid, (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid, and 3-tert-butyl-5-methylcatechol. Concerning Z. spinosa, the major components of the chloroformic extract are beta-sitosterol, stigmasterol, alpha-linolenic acid, palmitic acid, and (3beta,22R,23R,24S)-3,22,23-trihydroxystigmastan-6-one. These compounds were selected as ligands for docking simulations with the receptor, penicillin-binding protein 4 (PBP4) of E. faecalis, in the ceftaroline-bound form (PDB: 6mki), which is implicated in the biosynthesis of peptidoglycan. Both extracts exhibited similar antibacterial potential, with a MIC and MBC of 625 μg/mL against the E. faecalis. The in silico study aims to identify the compounds responsible for enhancing biological activity. Validation of the docking results for the main abundant compounds from the extracts of the two plants can be observed in Figures 7, 8, which demonstrate that the docked ligands fit well within the target receptor’s active site.
FIGURE 7
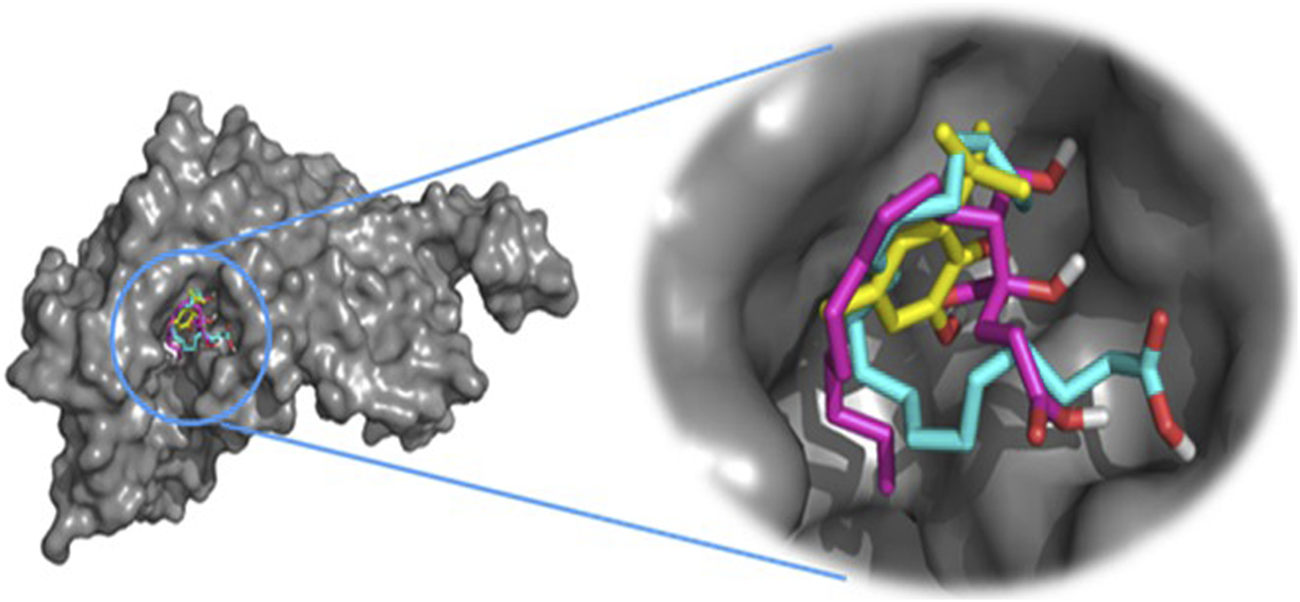
The three-dimensional binding modes of the docked ligands. Linolenic acid (cyan color), (38,48)-3-hydroxytetradecane-1,3,4-tricarboxylic acid (purple color), and 3-tert-Butyl-5-methylcatechol (yellow color)) of the Farsetia aegyptia chloroformic extract in the binding cavity of penicillin-binding protein four (PBP4) from Enterococcus faecalis in the ceftaroline-bound form (pdb: 6mki).
FIGURE 8
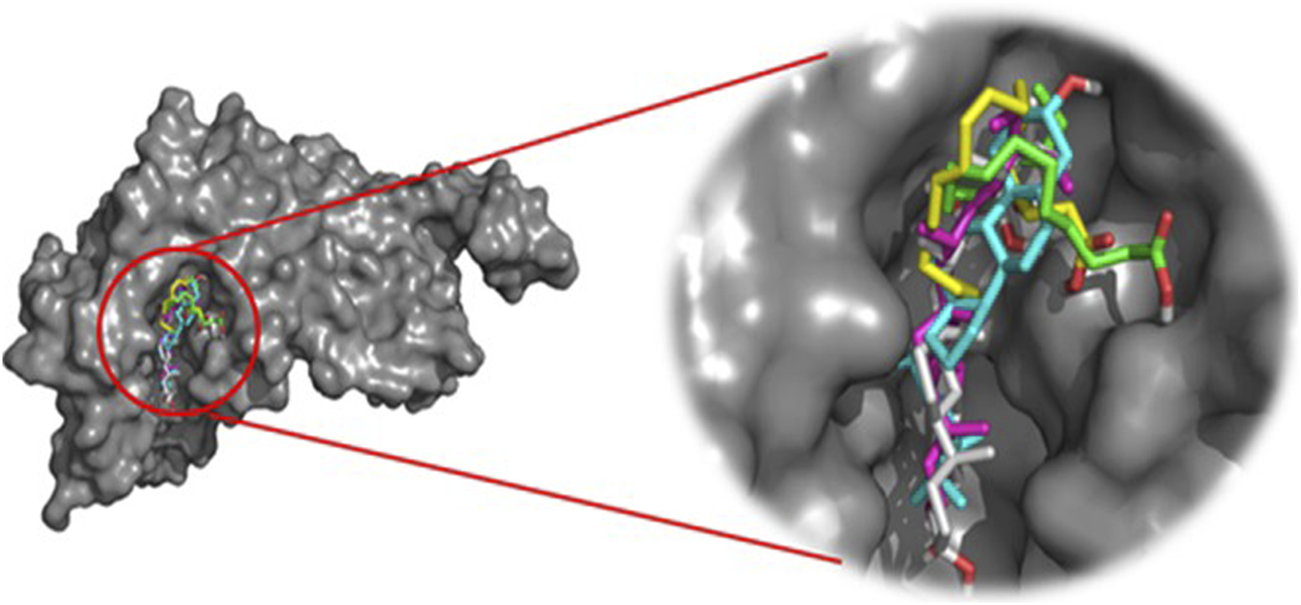
The 3D binding modes of the docked ligands: beta-Sitosterol (cyan color), Stigmasterol (purple color), Alpha-licanic acid (yellow color), Palmitic Acid (green color) and (3beta,22R,23R,248)-3,22,23- Trihydroxystigmastan-6-one (white color)) of the Zilla spinosa chloroformic extract in the binding cavity of penicillin-binding protein four (PBP4) from Enterococcus faecalis in the ceftaroline-bound form (pdb: 6 mki).
The results presented in Table 6, shown as free-binding energy values, highlight the effects of the selected molecules on the target receptor when compared to the standard, Cefalotin, which has a binding energy of −5.5 kcal/mol. Some of the docked phytocompounds demonstrate higher activity than this standard.
TABLE 6
| Docked molecule | Interacting residues | Binding energy (kcal/mol) |
|---|---|---|
| Linolenic acid (f) | Van der Waals: Ser424, Lys427, Thr465, Arg466, Ser482, Asn484, Tyr605, Thr620, Gly621, Thr622, Ser663, Thr665; H bond: Asp666; Alkyl/Pi–Alkyl: Val467 | −4.4 |
| (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid (f) | Van der Waals: Val467, Ser482, Tyr605, Gly621, Ser637, Phe638, Ser658, Ala664, Asp666; H bond: Thr620, Thr622, Glu635, Ser663, Thr665; carbon hydrogen bond: Thr620 | −5.0 |
| 3-tert-Butyl-5-methylcatechol (f) | Van der Waals: Gly621, Ser637, Phe638, Ser663, Thr665; H bond: Thr622; Pi-donor hydrogen bond: Thr620; Pi-sigma: Tyr605; Pi-Pi-Stacked: Tyr605; Alkyl/Pi–Alkyl: Val467, Tyr605 | −5.1 |
| Beta-sitosterol (z) | Van der Waals: Ser424, Ser482, Asn484, Gln542, Thr620, Gly621, Thr622, Glu624, Ser663, Thr665; H bond: Asp666; Alkyl/Pi–Alkyl: Val467, Tyr540, Tyr605 | −7.4 |
| Stigmasterol (z) | Van der Waals: Ser482, Thr620, Gly621, Thr622, Glu624, Ser663, Thr665; H bond: Asp666; Alkyl/Pi–Alkyl: Val467, Tyr605; carbon hydrogen bond: Tyr605 | −7.7 |
| Alpha-licanic acid (z) | Van der Waals: Ser424, Val467, Ser482, Tyr605, Thr620, Gly621, Glu635, Ser663; H bond: Thr622 | −4.2 |
| Palmitic acid (z) | Van der Waals: Val467, Tyr605, Thr620, Gly621, Thr622, Ser637, Ser658, Gly659, Gly662, Thr665; H bond: Glu635, Ser663 | −3.7 |
| (3beta,22R,23R,24S)-3,22,23-trihydroxystigmastan-6-one (z) | Van der Waals: Ser424, Ser482, Asn484, Thr465, Tyr540, Gln542, Gly621, Glu624, Ser637, Ser663, Thr665; H bond: Thr620, Thr622 Pi-sigma: Tyr605; alkyl: Val467 | −7.5 |
| Cefalotin (standard) | Van der Waals: Thr465, Arg466, Val467, Ser482, Tyr540, Gln542, Tyr605, Thr620, Gly621; H bond: Thr622, Ser637; carbon hydrogen bond: Ser663; Pi-donor hydrogen bond: Asn484 | −5.5 |
Docking results of the docked ligands with the lowest binding energy score and interacting residues within the binding cavity of penicillin-binding protein four (PBP4) from Enterococcus faecalis in the ceftaroline-bound form (pdb:6mki).
(z): Zilla spinosa; (f): Farsetia aegyptia.
As is known, the docking is used to predict which compounds within an extract exhibit the most favorable binding scores and are therefore responsible for the biological activity. This biological potential is expressed by inhibiting of the selected target receptor, which is evaluated during the docking process. Such inhibition occurs when the tested molecule forms intermolecular interactions with the amino acids located in the receptor’s active site. Among these interactions, hydrogen bonds are generally the most significant, followed by hydrophobic contacts, including π-related interactions. Thus, molecular interactions such as hydrogen bonds (H-bonds) and π-interactions often correlate strongly with a compound’s the in vitro biological activity because they determine how well a ligand binds to its biological target. For example, in our case, the target is “penicillin-binding protein four (PBP4) of E. faecalis in the ceftaroline-bound form (PDB: 6mki,” which designates the most sensitive strain according to the in vitro test: E. faecalis ATCC 29212. In this light, as shown in Figure 9, regarding the F. aegyptia extract, we note that 3-tert-Butyl-5-methylcatechol (−5.1 kcal/mol) is the most effective ligand. It forms a hydrogen bond with Thr622 via its hydroxyl group, as well as a Pi-donor hydrogen bond with Thr620, a Pi-sigma bond with Tyr605, and both Pi-Pi stacking and alkyl/Pi-alkyl interactions with Val467 and Tyr605 (Figure 9c). The compound (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid (−5 kcal/mol) exhibits similar interactions as follows: hydrogen bonds with Thr620, Thr622, Glu635, Ser663, and Thr665, along with a carbon-hydrogen bond with Thr620. This compound is the second most active among those tested (Figure 9b). In contrast, linolenic acid is the least active ligand when compared to the other phytocompounds from F. aegyptia, showing only a hydrogen bond with Asp666 and alkyl/Pi-alkyl contact with Val467 (Figure 9a). On the other hand, concerning the phytoligand from Z. spinosa, stigmasterol (−7.7 kcal/mol) has the best docking score. It exhibits a hydrogen bond with Asp666 through its hydroxyl group, as well as alkyl/Pi-alkyl interactions with Val467 and Tyr605. The residue Tyr605 also forms a carbon-hydrogen bond with the carbon skeleton of the molecule (Figure 10e). Additionally, (3beta,22R,23R,24S)-3,22,23-trihydroxystigmastan-6-one, the second-most effective ligand, interacts through two hydrogen bonds with Thr620 and Thr622, a Pi-sigma bond with Tyr605, and an alkyl contact with Val467 (Figure 10h). Beta-sitosterol (−7.4 kcal/mol), like stigmasterol, forms a hydrogen bond with Asp666 via its hydroxyl group, in addition to alkyl/Pi-alkyl interactions with Val467, Tyr540, and Tyr605 (Figure 10d). Meanwhile, the two acids, alpha-licanic acid and palmitic acid, were found to be the weakest docked ligands, displaying some interactions detailed in Table 6 (Figures 10f,g). The above findings demonstrate that the predicted antibacterial effect of the Z. spinosa extract against PBP4 from E. faecalis in the ceftaroline-bound form primarily results from the three docked compounds: stigmasterol, (3beta,22R,23R,24S)-3,22,23-trihydroxystigmastan-6-one, and beta-sitosterol. Additionally, the individual biological potential of the three major compounds from the F. aegyptia extract is lower than that of the compounds from the Z. spinosa extract, and even lower than that of the standard. We conclude that the significant antibacterial activity arises from the combination of these three molecules.
FIGURE 9
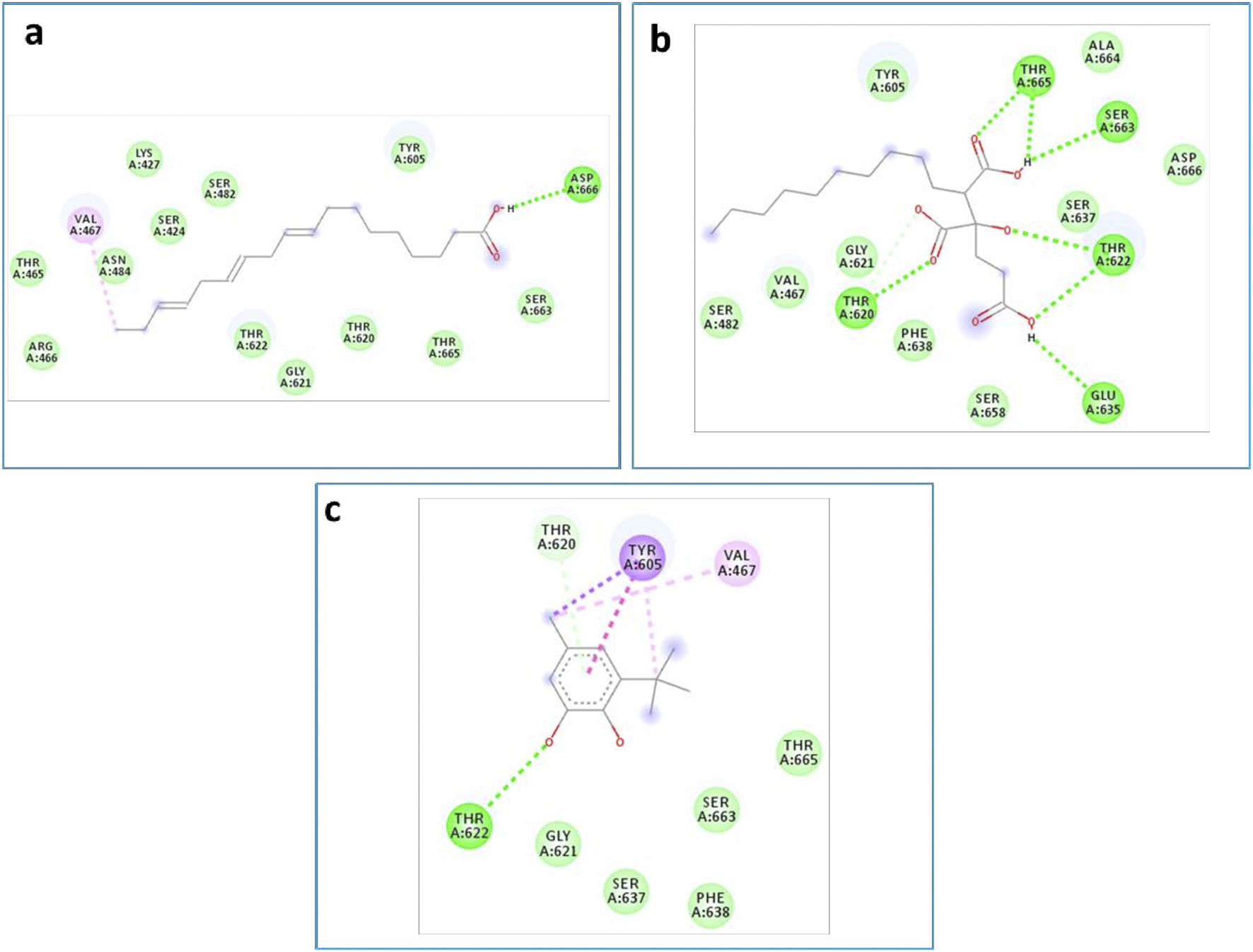
2D docking models of different interactions formed by the docked molecules: (a) Linolenic acid, (b) (38,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid and (c): 3-tert-Butyl-5-methylcatechol of the F. aegyptia extract.
FIGURE 10
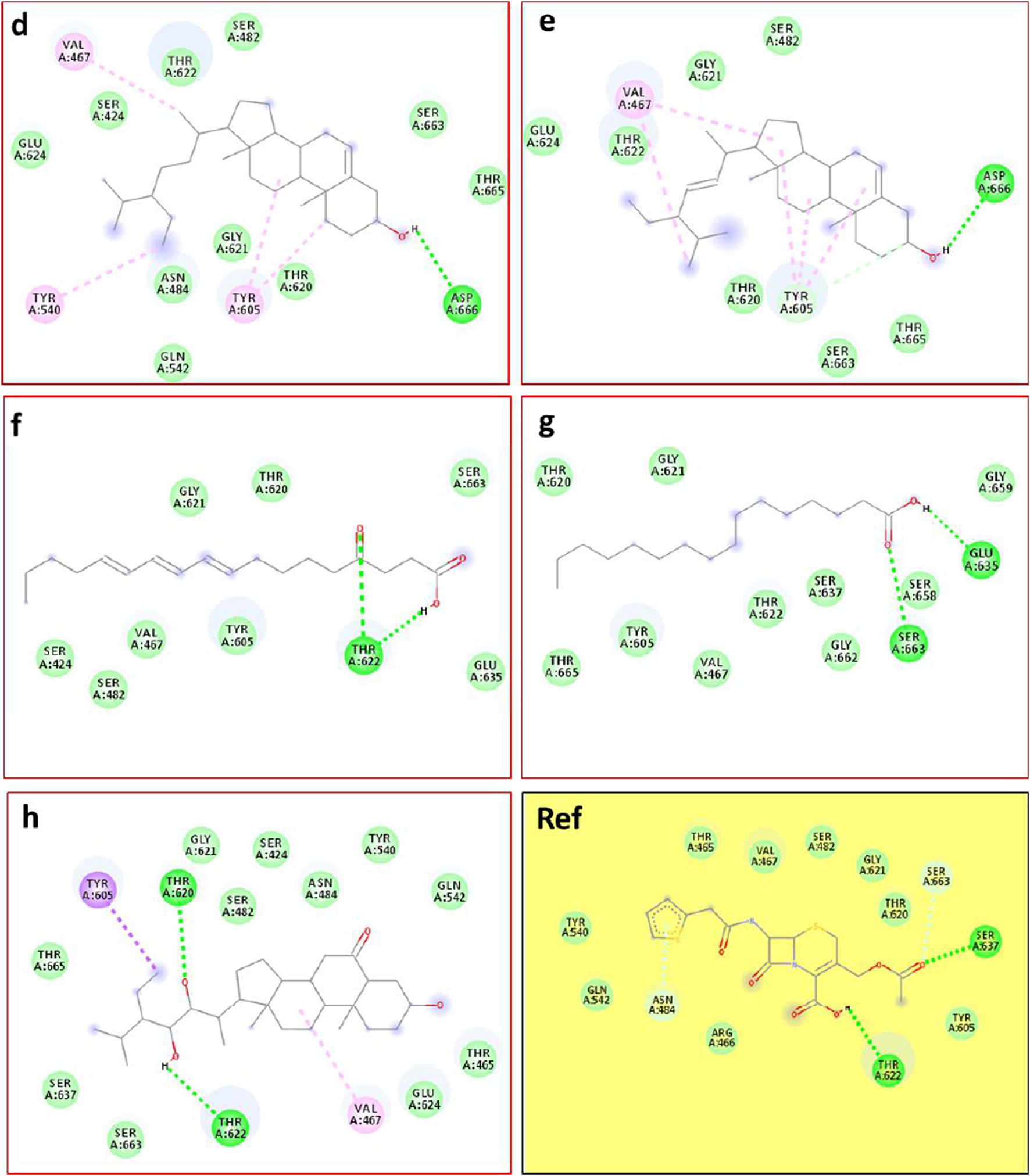
2D docking models of different interactions formed by the docked molecules: (d) Beta-sitosterol, (e) Stigmasterol, (f) Alpha-licanic acid, (g) Palmitic acid, (h): (3beta,22R,23R,24S)-3,22,23- trihydroxystigmastan-6-one of the Z. spinosa extract and Ref: Cefalotin in the binding cavity of penicillin-binding protein four (PBP4) from Enterococcus faecalis in the ceftaroline-bound form (pdb: 6mki).
The in silico molecular docking analysis provided valuable insights into the antibacterial potential of compounds derived from F. aegyptia and Z. spinosa.
In particular, the phytoconstituents from Z. spinosa showed stronger binding affinities and more extensive interactions with the active residues of PBP4 in E. faecalis than those from F. aegyptia. This result is encouraging for the development of new antibacterial agents. Particularly, stigmasterol (−7.7 kcal/mol), (3β,22R,23R,24S)-3,22,23-trihydroxystigmastan-6-one, and β-sitosterol formed multiple hydrogen bonds, π–σ interactions, and alkyl/π–alkyl contacts with key residues, including Thr620, Thr622, Tyr605, Asp666, and Val467. This accentuates their potent stabilizing potential at the active site. On the other hand, the most active phytoconstituents from F. aegyptia, namely, 3-tert-butyl-5-methylcatechol and (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid, displayed weaker binding energies (−5.0 to −5.1 kcal/mol) and fewer stabilizing interactions. In addition, palmitic acid and linolenic acid, which are found in both species, were estimated to be the least efficient ligands. These results are consistent with earlier publications that emphasize the antimicrobial activity of phytosterols, mainly those found in Z. spinosa (Kanokmedhakul et al., 2005), aligning well with our docking findings, which identified sterols as the most effective ligands. Altogether, the docking study findings suggest that the important antibacterial activity of Z. spinosa derives from a synergistic effect of its three active sterols, although the inferior activity of F. aegyptia may be linked to its lower levels of interactive fatty acids and phenolic compounds.
4 Conclusion
This is the first report of advanced bactericidal activities of the organic extracts of F. aegyptia and Z. spinosa from Ha’il, Saudi Arabia. Furthermore, it demonstrated antibiofilm activity against Gram-positive, Gram-negative bacterial strains, and anti-candida strains. The molecular docking showed that the major components of Zilla spinose have the highest binding free energy with penicillin-binding protein 4 (PBP4) of E. faecalis in the ceftaroline-bound form (PDB: 6mki), which is implicated in the biosynthesis and survival of this bacterium. Further in vivo studies will be needed to elucidate the specific mechanisms of action and the safety of these important phytochemical components before translating their therapeutic potential.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MB: Formal Analysis, Project administration, Data curation, Methodology, Funding acquisition, Visualization, Conceptualization, Writing – original draft, Writing – review and editing. AH: Writing – original draft, Data curation, Resources, Formal Analysis, Writing – review and editing, Methodology. MH: Software, Writing – original draft, Formal Analysis, Writing – review and editing. KM: Formal Analysis, Writing – original draft, Data curation, Methodology, Funding acquisition, Writing – review and editing. AD: Formal Analysis, Writing – original draft, Methodology, Writing – review and editing, Validation. SJ: Writing – review and editing, Funding acquisition, Writing – original draft, Formal Analysis. AA: Writing – original draft, Funding acquisition, Validation, Writing – review and editing. SL: Funding acquisition, Writing – review and editing, Formal Analysis, Writing – original draft, Methodology, Validation. KA: Data curation, Validation, Formal Analysis, Writing – review and editing, Writing – original draft. RL: Validation, Resources, Writing – review and editing, Investigation, Writing – original draft. HBJ: Writing – original draft, Visualization, Writing – review and editing, Supervision. JK: Funding acquisition, Writing – original draft, Supervision, Investigation, Writing – review and editing. WBS: Conceptualization, Formal Analysis, Writing – original draft, Writing – review and editing, Visualization.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This research was supported by the University of Ha’il, grant number RG- 23-132.
Acknowledgments
This research has been funded by Scientific Research Deanship at University of Ha’il—Saudi Arabia through project number RG 23-132.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdelshafeek K. Elmissiry M. M. Hussiny H. A. Elnasr M. M. (2016). The flavonoids and anticomplement activity of two cruciferous plants growing in Egypt. Int. J. Pharmacogn. Phytochem. Res.8 (2), 223–227.
2
Adams R. P. (2017). Identification of essential oil components by gas chromatography/mass spectrometry. 5th online ed. Nashville, TN, USA: Texensis Publishing.
3
Al-Shehbaz I. A. Beilstein M. A. Kellogg E. A. (2006). Systematics and phylogeny of the brassicaceae (cruciferae): an overview. Plant Syst. Evol.259, 89–120. 10.1007/s00606-006-0415-z
4
Alghanem S. M. Al-Hadithy O. N. Milad M. (2018). Regular article antimicrobial activity and phytochemical characterization from Zilla spinosa. J. Med Bot2, 24–27. 10.25081/jmb.2018.v2.987
5
Alibi S. Ben Selma W. Ramos-Vivas J. Smach M. A. Touati R. Boukadida J. et al (2020). Anti-oxidant, antibacterial, anti-biofilm, and anti-quorum sensing activities of four essential oils against multidrug-resistant bacterial clinical isolates. Curr. Research Translational Medicine68 (2), 59–66. 10.1016/j.retram.2020.01.001
6
Alibi S. Ramos-Vivas J. Ben Selma W. Ben Mansour H. Boukadida J. Navas J. (2021). Virulence of clinically relevant multidrug resistant Corynebacterium striatum strains and their ability to adhere to human epithelial cells and inert surfaces. Microb. Pathogenesis155, 104887. 10.1016/j.micpath.2021.104887
7
Alibi S. Selma W. B. Mansour H. B. Navas J. (2022). Activity of essential oils against multidrug-resistant salmonella Enteritidis. Curr. Microbiology79 (9), 273. 10.1007/s00284-022-02938-x
8
Anand U. Nandy S. Mundhra A. Das N. Pandey D. K. Dey A. (2020). A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resistance Updates Reviews Commentaries Antimicrobial Anticancer Chemotherapy51, 100695. 10.1016/j.drup.2020.100695
9
Atta E. M. Hashem A. I. Eman R. E. (2013). A novel flavonoid compound from Farsetia aegyptia and its antimicrobial activity. Chem. Nat. Compd.49, 432–436. 10.1007/s10600-013-0631-z
10
Ben Kahla I. Ben Selma W. Marzouk M. Ferjeni A. Ghezal S. Boukadida J. (2011). Evaluation of a simplified IS6110 PCR for the rapid diagnosis of Mycobacterium tuberculosis in an area with high tuberculosis incidence. Pathologie-biologie59 (3), 161–165. 10.1016/j.patbio.2009.04.001
11
Ben Selma W. Alibi S. Ferjeni M. Ghezal S. Gallala N. Belghouthi A. et al (2024a). Synergistic activity of Thymus capitatus essential oil and cefotaxime against ESBL-producing Klebsiella pneumoniae. Int. Journal Environmental Health Research34 (8), 2936–2946. 10.1080/09603123.2023.2280149
12
Ben Selma W. Farouk A. Ban Z. Ferjeni M. Alsulami T. Ali H. et al (2024b). Thymus algeriensis essential oil: phytochemical investigation, bactericidal activity, synergistic effect with colistin, molecular docking, and dynamics analysis against Gram-negative bacteria resistant to colistin. Heliyon10 (19), e38281. 10.1016/j.heliyon.2024.e38281
13
Ben Selma W. Ferjeni S. Farouk A. Marzouk M. Boukadida J. (2025). Antimicrobial activity of Cinnamomum zeylanicum essential oil against colistin-resistant gram-negative bacteria. Int. Journal Environmental Health Research35 (1), 169–181. 10.1080/09603123.2024.2348094
14
Besbes M. Hamdi A. Horchani M. Majouli K. Ghorbel M. Lotfi S. et al (2025). Phytochemical screening, phytotoxic effects and in silico studies of Zilla Spinosa L. and Farsetia Aegyptia turra extracts growing in hail Region. J. Soil Sci. Plant Nutr.25 (1), 2052–2069. 10.1007/s42729-025-02256-8
15
Besbes Hlila M. Mosbah H. Majouli K. Ben Nejma A. Ben Jannet H. Mastouri M. et al (2016). Antimicrobial activity of Scabiosa arenaria Forssk. Extracts and pure compounds using bioguided fractionation. Chem. and Biodiversity13 (10), 1262–1272. 10.1002/cbdv.201600028
16
Bhandari S. R. Jo J. S. Lee J. G. (2015). Comparison of glucosinolate profiles in different tissues of nine brassica crops. Mol. Basel, Switz.20 (9), 15827–15841. 10.3390/molecules200915827
17
Borris R. P. (1996). Natural products research: perspectives from a major pharmaceutical company. J. Ethnopharmacology51 (1-3), 29–38. 10.1016/0378-8741(95)01347-4
18
Berreghioua A. Cheriti A. Belboukhari N. (2014). Antibacterial activity of Zilla macroptera extracts from Algerian Sahara. Phyto.Chem. Bio. Sub. J.8 (2), 92–96.
19
Burki S. Burki Z. G. Khan M. Ahmed I. Ali S. (2022). GC/MS profiling, antibacterial and atomic force microscopic study of bacterial cell membrane affected by Farsetia heliophila bark extract along with wound healing activity. JAPS J. Animal and Plant Sci.32 (3), 877–885. 10.36899/JAPS.2022.3.0489
20
Butt U. ElShaer A. Snyder L. A. Chaidemenou A. Alany R. G. (2016). Fatty acid microemulsion for the treatment of neonatal conjunctivitis: quantification, characterisation and evaluation of antimicrobial activity. Drug Delivery Translational Research6 (6), 722–734. 10.1007/s13346-016-0338-3
21
Chen S. Nelson M. N. Chèvre A. M. Jenczewski E. Li Z. Mason A. S. et al (2011). Trigenomic bridges for Brassica improvement. Crit. Rev. Plant Sci.30 (6), 524–547. 10.1080/07352689.2011.615700
22
Cheung Lam A. H. Sandoval N. Wadhwa R. Gilkes J. Do T. Q. Ernst W. et al (2016). Assessment of free fatty acids and cholesteryl esters delivered in liposomes as novel class of antibiotic. BMC Research Notes9, 337. 10.1186/s13104-016-2138-8
23
Cirino I. C. D. S. de Santana C. F. Bezerra M. J. R. Rocha I. V. Luz A. C. O. Coutinho H. D. M. et al (2023). Comparative transcriptomics analysis of multidrug-resistant Acinetobacter baumannii in response to treatment with the terpenic compounds thymol and carvacrol. Biomed. Pharmacother.165, 115189. 10.1016/j.biopha.2023.115189
24
CLSI (2018). “Performance standards for antimicrobial susceptibility testing,” in CLSI supplement M100. 28th Edn. Wayne, PA: Clinical and Laboratory Standards Institute.
25
Coppola F. Abdalrazeq M. Fratianni F. Ombra M. N. Testa B. Zengin G. et al (2025). Rosaceae honey: antimicrobial activity and prebiotic properties. Antibiot. Basel, Switz.14 (3), 298. 10.3390/antibiotics14030298
26
Cosentino S. Tuberoso C. I. Pisano B. Satta M. Mascia V. Arzedi E. et al (1999). In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Applied Microbiology29 (2), 130–135. 10.1046/j.1472-765x.1999.00605.x
27
Cowan M. M. (1999). Plant products as antimicrobial agents. Clin. Microbiology Reviews12 (4), 564–582. 10.1128/CMR.12.4.564
28
EUCAST (2024). Clinical Breakpoint (v 14.0). Available online at: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf.
29
Ejaz S. Nasim F. U. Abdullah I. Rashid S. Ashraf M. (2023). Analysis of antibacterial and cytotoxic potential of medicinal plants from Cholistan desert, Pakistan. Saudi Journal Biological Sciences30 (9), 103750. 10.1016/j.sjbs.2023.103750
30
El-Sharabasy F. Mohamed N. Z. (2013). Chemical constituents and biological activity from chloroform extract of Zilla Spinosa. Int. J. Pharm. Pharm. Sci.5 (1), 422–427.
31
El-Sharkawy E. Azza A. M. Emad M. A. (2013). Cytotoxity of new flavonoid compound isolated from Farsetia aegyptia. Int. J. Pharm. Sci. Inven.2, 23–27.
32
El-Toumy S. A. El-Sharabasy F. S. Ghanem H. Z. El-Kady M. U. Kassem A. F. (2011). Phytochemical and pharmacological studies on Zilla spinosa. Aust. J. Basic Appl. Sci.5 (8), 1362–1370.
33
Favela-González K. M. Hernández-Almanza A. Y. De la Fuente-Salcido N. M. (2020). The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: a review. J. Food Biochemistry44 (10), e13414. 10.1111/jfbc.13414
34
Ferreira M. Pinto M. Aires-da-Silva F. Bettencourt A. Gaspar M. M. Aguiar S. I. (2024). Rifabutin: a repurposed antibiotic with high potential against planktonic and biofilm staphylococcal clinical isolates. Front. Microbiology15, 1475124. 10.3389/fmicb.2024.1475124
35
Garousi M. Monazami Tabar S. Mirazi H. Asgari P. Sabeghi P. Salehi A. et al (2022). A global systematic review and meta-analysis on correlation between biofilm producers and non-biofilm producers with antibiotic resistance in Uropathogenic Escherichia coli. Microb. Pathogenesis164, 105412. 10.1016/j.micpath.2022.105412
36
Gedefie A. Alemayehu E. Mohammed O. Bambo G. M. Kebede S. S. Kebede B. (2023). Prevalence of biofilm producing Acinetobacter baumannii clinical isolates: a systematic review and meta-analysis. PloS One18 (11), e0287211. 10.1371/journal.pone.0287211
37
Genovese C. D'Angeli F. Bellia F. Distefano A. Spampinato M. Attanasio F. et al (2021). In vitro antibacterial, anti-adhesive and anti-biofilm activities of Krameria lappacea (Dombey) Burdet and B.B. simpson Root extract against Methicillin-resistant Staphylococcus aureus strains. Antibiot. Basel, Switz.10 (4), 428. 10.3390/antibiotics10040428
38
Ghaneian M. T. Ehrampoush M. H. Jebali A. Hekmatimoghaddam S. Mahmoudi M. (2015). Antimicrobial activity, toxicity and stability of phytol as a novel surface disinfectant. Environ. Health Eng. Manag. J.2 (1), 13–16.
39
Gyawali R. Ibrahim S. A. (2014). Natural products as antimicrobial agents. Food Control.46, 412–429. 10.1016/j.foodcont.2014.05.047
40
Hayat M. M. Uzair M. (2019). Biological potential and GC-MS analysis of phytochemicals of Farsetia hamiltonii (Royle). Biomed. Res.30, 609–616. 10.35841/biomedicalresearch.30-19-241
41
Hlila M. B. Hichri A. O. Mahjoub M. A. Mighri Z. Mastouri M. (2017). Antioxidant and antimicrobial activities of Padina pavonica and Enteromorpha sp. from the Tunisian Mediterranean coast. J. Coast. Life Med.5, 336–342. 10.12980/jclm.5.2017J7-107
42
Horchani M. Hajlaoui A. Harrath A. H. Mansour L. Jannet H. B. Romdhane A. (2020). New pyrazolo-triazolo-pyrimidine derivatives as antibacterial agents: design and synthesis, molecular docking and DFT studies. J. Mol. Struct.1199, 127007. 10.1016/j.molstruc.2019.127007
43
Horchani M. Edziri H. Harrath A. H. Jannet H. B. Romdhane A. (2022). Access to new schiff bases tethered with pyrazolopyrimidinone as antibacterial agents: design and synthesis, molecular docking and DFT analysis. J. Mol. Struct.1248, 131523. 10.1016/j.molstruc.2021.131523
44
Hosseini M. Shapouri Moghaddam A. Derakhshan S. Hashemipour S. M. A. Hadadi-Fishani M. Pirouzi A. et al (2020). Correlation between biofilm Formation and antibiotic resistance in MRSA and MSSA isolated from clinical samples in Iran: a systematic review and meta-analysis. Microb. Drug Resistance Larchmt. N.Y.26 (9), 1071–1080. 10.1089/mdr.2020.0001
45
Islam M. T. Ali E. S. Uddin S. J. Shaw S. Islam M. A. Ahmed M. I. et al (2018). Phytol: a review of biomedical activities. Food Chemical Toxicology121, 82–94. 10.1016/j.fct.2018.08.032
46
Jilani S. Ferjeni M. Al-Shammery K. Rashid Mohammed AlTamimi H. Besbes M. Ahmed Lotfi S. et al (2025). The synergistic effect of Thymus vulgaris essential oil and carvacrol with imipenem against carbapenem-resistant Acinetobacter baumannii: in vitro,, molecular docking, and molecular dynamics studies. Front. Pharmacology16, 1582102. 10.3389/fphar.2025.1582102
47
Kanokmedhakul K. Kanokmedhakul S. Phatchana R. (2005). Biological activity of Anthraquinones and Triterpenoids from Prismatomeris fragrans. J. Ethnopharmacology100 (3), 284–288. 10.1016/j.jep.2005.03.018
48
Karawya M. S. Wassel G. M. el-Menshawi B. S. (1974). Phytochemical study of Zilla spinosa (Turra) Prantl. General analysis. Carbohydrates and lipids. Pharmazie29 (1), 60–61.
49
Khameneh B. Iranshahy M. Soheili V. Fazly Bazzaz B. S. (2019). Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob. Resistance Infection Control8, 118. 10.1186/s13756-019-0559-6
50
Kumaradevan G. Damodaran R. Mani P. Dineshkumar G. Jayaseelan T. (2015). Phytochemical Screening and GC-MS Analysis of Bioactive Components of Ethanol Leaves Extract of Clerodendrum Phlomidis (L.). American J. Biol. Pharm. Res2 (3), 142–148.
51
Kungwani N. A. Panda J. Mishra A. K. Chavda N. Shukla S. Vikhe K. et al (2024). Combating bacterial biofilms and related drug resistance: role of phyto-derived adjuvant and nanomaterials. Microb. Pathogenesis195, 106874. 10.1016/j.micpath.2024.106874
52
Kusumah D. Wakui M. Murakami M. Xie X. Yukihito K. Maeda I. (2020). Linoleic acid, α-linolenic acid, and monolinolenins as antibacterial substances in the heat-processed soybean fermented with Rhizopus oligosporus. Biosci. Biotechnology, Biochemistry84 (6), 1285–1290. 10.1080/09168451.2020.1731299
53
Lahmadi G. Horchani M. Dbeibia A. Mahdhi A. Romdhane A. Lawson A. M. et al (2023). Novel oleanolic acid-phtalimidines tethered 1,2,3 triazole hybrids as promising antibacterial agents: design, synthesis, in vitro experiments and in silico docking studies. Mol. Basel, Switz.28 (12), 4655. 10.3390/molecules28124655
54
Madouh A. T. Davidson M. K. (2024). Phytochemical screening and potential effects of Farsetia aegyptia turra seeds: a native desert herb, from Kuwait. J. Trop. Agric.62 (1), 81–96.
55
Magiorakos A. P. Srinivasan A. Carey R. B. Carmeli Y. Falagas M. E. Giske C. G. et al (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiology Infection The Official Publication Eur. Soc. Clin. Microbiol. Infect. Dis.18 (3), 268–281. 10.1111/j.1469-0691.2011.03570.x
56
Mailafiya M. M. Yusuf A. J. Abdullahi M. I. Aleku G. A. Ibrahim I. A. Yahaya M. et al (2018). Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J. Med. Plants Econ. Dev.2 (1), 1–5. 10.4102/jomped.v2i1.38
57
Marzouk M. M. Kawashty S. A. Saleh N. A. M. "https://link.springer.com/article/10.1007/s10600-009-9402-2#auth-Abdel_Salam_M_-Al_Nowaihi-Aff2"Al-Nowaihi A. S. M. (2009). A new kaempferol trioside from Farsetia aegyptia. Chem. Nat. Compd.45, 483–486. 10.1007/s10600-009-9402-2
58
Mecheri R. Mahfouf N. Smati D. Boutefnouchet A. (2023). Antioxidant and anti-inflammatory activities of different extracts from aerial parts of Zilla spinosa (L.) Prantl. Curr. Bot.14, 62–66. 10.25081/cb.2023.v14.8323
59
Moon T. M. D’Andréa É. D. Lee C. W. Soares A. Jakoncic J. Desbonnet C. et al (2018). The structures of penicillin-binding protein 4 (PBP4) and PBP5 from Enterococci provide structural insights into β-lactam resistance. J. Biol. Chem.293 (48), 18574–18584. 10.1074/jbc.RA118.006052
60
Moshi M. Joseph C. Innocent E. Nkunya M. (2004). In vitro antibacterial and antifungal activities of extracts and compounds from Uvaria scheffleri. Pharm. Biology42 (4-5), 269–273. 10.1080/13880200490511035
61
Narayana D. V. Gopichandramukhi T. Kumar K. A. Nikhil P. Mahesh R. Akbar J. A. (2017). A critical review on natural antimicrobial agents. World J. Pharm. Pharm. Sci., 329–341. 10.20959/wjpps20175-9064
62
Odiba J. Musa A. Hassan H. Yahay S. Okolo E. (2014). Antimicrobial activity of isolated stigmast-5-en-3-β-ol (β-sitosterol) from honeybee propolis from North-Western, Nigeria. Int. J. Pharm. Sci. Res.5 (12), 908–918.
63
Reddy M. N. Adnan M. Alreshidi M. M. Saeed M. Patel M. (2020). Evaluation of anticancer, antibacterial and antioxidant properties of a medicinally treasured fern Tectaria coadunata with its phytoconstituents analysis by HR-LCMS. Anti-cancer Agents Medicinal Chemistry20 (15), 1845–1856. 10.2174/1871520620666200318101938
64
Salma A. Motawe H. M. Ahmad S. S. Ibrahim M. E. (2018). Survey and assessment of chemical composition and biological activity of some wild plants growing in the Egyptian eastern desert. J. Mater. Environ. Sci.9 (5), 1495–1502.
65
Sandasi M. Leonard C. M. Viljoen A. M. (2008). The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control.19 (11), 1070–1075. 10.1016/j.foodcont.2007.11.006
66
Sekkoum K. Cheriti A. Taleb S. Bourmita Y. Belboukhari N. (2011). Traditional phytotherapy for urinary diseases in Bechar district (south west of Algeria). Electron J. Environ. Agric. Food Chem.10 (8), 2616–2622.
67
Sekkoum K. Belboukhari N. Cheriti A. Y Aboul-Enein H. (2015). Chemical composition of the essential oil of Zilla macroptera coss. From Algerian sahara. Curr. Bioact. Compd.11 (2), 100–103. 10.2174/1573407211666150130191436
68
Shahat A. A. Mahmoud E. A. Al-Mishari A. A. Alsaid M. S. (2017). Antimicrobial activities of some saudi arabian herbal plants. Afr. Journal Traditional, Complementary, Alternative Medicines AJTCAM14 (2), 161–165. 10.21010/ajtcam.v14i2.17
69
Sharifi-Rad M. Mohanta Y. K. Pohl P. Jaradat N. Aboul-Soud M. A. M. Zengin G. (2022a). Variation of phytochemical constituents, antioxidant, antibacterial, antifungal, and anti-inflammatory properties of Grantia aucheri (Boiss.) at different growth stages. Microb. Pathogenesis172, 105805. 10.1016/j.micpath.2022.105805
70
Sharifi-Rad M. Pohl P. Epifano F. Zengin G. Jaradat N. Messaoudi M. (2022b). Teucrium polium (L.): phytochemical screening and biological activities at different phenological stages. Mol. Basel, Switz.27 (5), 1561. 10.3390/molecules27051561
71
Sherry N. Howden B. (2018). Emerging Gram negative resistance to last-line antimicrobial agents fosfomycin, colistin and ceftazidime-avibactam - epidemiology, laboratory detection and treatment implications. Expert Review Anti-infective Therapy16 (4), 289–306. 10.1080/14787210.2018.1453807
72
Silva E. Teixeira J. A. Pereira M. O. Rocha C. M. R. Sousa A. M. (2023). Evolving biofilm inhibition and eradication in clinical settings through plant-based antibiofilm agents. Phytomedicine International Journal Phytotherapy Phytopharmacology119, 154973. 10.1016/j.phymed.2023.154973
73
Soković M. Marin P. D. Brkić D. Van Griensven L. J. L. D. (2007). Chemical composition and antibacterial activity of essential oils of ten aromatic plants against human pathogenic bacteria. Food1 (2), 220–226.
74
Srinivas P. Rivard K. (2017). Polymyxin resistance in gram-negative pathogens. Curr. Infectious Disease Reports19 (11), 38. 10.1007/s11908-017-0596-3
75
Suleiman M. H. A. Ateeg A. A. (2020). Antimicrobial and antioxidant activities of different extracts from different parts of Zilla spinosa (L.) Prantl. Evidence-based Complementary Alternative Medicine eCAM2020, 6690433. 10.1155/2020/6690433
76
Talbot G. H. Bradley J. Edwards J. E. Jr Gilbert D. Scheld M. Bartlett J. G. et al (2006). Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infectious Diseases An Official Publication Infect. Dis. Soc. Am.42 (5), 657–668. 10.1086/499819
77
Tamokou J. deD. Kuiate J. R. Tene M. Kenla Nwemeguela T. J. Tane P. (2011). The antimicrobial activities of extract and compounds isolated from Brillantaisia lamium. Iran. Journal Medical Sciences36 (1), 24–31.
78
Tarchouna M. Ferjani A. Ben-Selma W. Boukadida J. (2013). Distribution of uropathogenic virulence genes in Escherichia coli isolated from patients with urinary tract infection. Int. Journal Infectious Diseases IJID Official Publication Int. Soc. Infect. Dis.17 (6), e450–e453. 10.1016/j.ijid.2013.01.025
79
Trott O. Olson A. J. (2010). AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Computational Chemistry31 (2), 455–461. 10.1002/jcc.21334
80
Ullah R. Alsaid M. S. Shahat A. A. Naser A. A. Al-Mishari A. A. Adnan M. et al (2018). Antioxidant and hepatoprotective effects of methanolic extracts of Zilla spinosa and Hammada elegans against carbon tetrachloride induced hepatotoxicity in rats. Open Chem.16 (1), 133–140. 10.1515/chem-2018-0021
81
Ullah R. Shahat A. A. Alqahtani A. S. Almarfadi O. M. Alharbi M. S. M. Ahamad S. R. et al (2020). Anti-Inflammatory, antipyretic and analgesic potential of Zilla spinosa in animal model. Indian J. Animal Res.54 (7), 889–894.
82
WHO (2024). WHO Bacterial Priority Pathogens List, 2024: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Available online at: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (Accessed October 1, 2025).
83
Yasbolaghi Sharahi J. Fayyazi A. Kodori M. Hosseinpour M. Hashemi A. Mahmoudi M. et al (2025). Dispersal mechanisms in biofilm control: characteristics, induction, impacts, and therapeutic potential. Curr. Microbiology83 (1), 3. 10.1007/s00284-025-04584-5
84
Yinusa I. George N. I. Shuaibu U. O. Ayo R. G. (2014). Bioactivity of Stigmasterol Isolated from the Aerial Part of Spillanthes Acmella (Murr) on Selected Microorganism. Int. J. Curr. Microbiol. Appl. Sci.3 (2), 475–479.
85
Youssef R. S. (2013). Medicinal and non-medicinal uses of some plants found in the middle region of Saudi Arabia. J. Med. Plants Res.7 (34), 2501–2513.
86
Zengin G. Uba A. I. Ocal M. Sharifi-Rad M. Caprioli G. Angeloni S. et al (2022). Integration of in vitro and in silico approaches to assess three Astragalus species from Turkey flora: a novel spotlight from lab bench to functional applications. Food Biosci.49, 101858. 10.1016/j.fbio.2022.101858
87
Zhao F. Yang H. Bi D. Khaledi A. Qiao M. (2020). A systematic review and meta-analysis of antibiotic resistance patterns, and the correlation between biofilm formation with virulence factors in uropathogenic E. coli isolated from urinary tract infections. Microb. Pathogenesis144, 104196. 10.1016/j.micpath.2020.104196
88
Zheng C. J. Yoo J. S. Lee T. G. Cho H. Y. Kim Y. H. Kim W. G. (2005). Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Letters579 (23), 5157–5162. 10.1016/j.febslet.2005.08.028
Summary
Keywords
antibiofilm effects, antimicrobial activities, Farsetia aegyptia , molecular docking, phytochemical analysis, Zilla spinosa
Citation
Besbes M, Hamdi A, Horchani M, Majouli K, Dbeibia A, Jilani S, Alshammari AA, Lotfi SA, Alabbosh KF, Lajimi RH, Ben Jannet H, Kraiem J and Ben Selma W (2026) Phytochemical profiling, antimicrobial, antibiofilm, and molecular docking of Farsetia aegyptia and Zilla spinosa from Saudi Arabia. Front. Pharmacol. 16:1723207. doi: 10.3389/fphar.2025.1723207
Received
11 October 2025
Revised
27 November 2025
Accepted
28 November 2025
Published
03 February 2026
Volume
16 - 2025
Edited by
Hanen Najjaa, Arid Regions Institute, Tunisia
Reviewed by
Majid Sharifi-Rad, Zabol University, Iran
Rukiye Aslan, Cumhuriyet University, Türkiye
Updates
Copyright
© 2026 Besbes, Hamdi, Horchani, Majouli, Dbeibia, Jilani, Alshammari, Lotfi, Alabbosh, Lajimi, Ben Jannet, Kraiem and Ben Selma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walid Ben Selma, walid.bensalma@issatmh.u-monastir.tn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.