- 1Department of Pharmacy, Zhoushan Hospital, Zhoushan, China
- 2Department of Pharmacy, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 3Department of Pharmacy, The Affiliated Taian City Central Hospital of Qingdao University, Taian, China
Introduction
Voriconazole is a widely used anti-fungal drug with narrow therapeutic window (McCreary et al., 2023). It has shown large inter-individual variation in pharmacokinetics. Given the special pathophysiological condition of intensive care patients, the variation in voriconazole exposure would be even larger (Hinze et al., 2024). Thus, injection formulation could avoid absorption variation and is preferred in ICU. Continuous renal replacement therapy (CRRT) is a common organ support treatment for ICU patients, and it is challenge for dosing voriconazole for these patients. Recently, Wang et al. developed a population pharmacokinetic model of voriconazole injections for patients with pulmonary aspergillosis, and CRRT was a covariate of the final model (Wang et al., 2025). Another earlier study also included CRRT as covariate for voriconazole PPK model (Wang et al., 2024). These evidences would guide voriconazole dosing in patients receiving CRRT. However, we would like to share our opinion about voriconazole injection use in these patients.
Formulation and pharmacokinetic considerations of voriconazole injection application in CRRT patients
Owing to the low water solubility of voriconazole, sulphobutylether beta-cyclodextrin (SBECD) was added as a solubilization agent in its injection formulation. Each bottle of voriconazole injection contained approximately 3200 mg of SBECD. As SBECD could accumulate in patients with renal impairment, patients with creatinine clearance <50 mL/min were advised to receive an oral formulation. According to its insert, the clearance rates of voriconazole and SBECD during hemodialysis were 121 mL/min and 55 mL/min, respectively. These findings indicate that 4-h hemodialysis can only remove small parts of voriconazole and SBECD. Real-world evidence concerning SBECD clearance by CRRT is limited. A previous study in three intensive patients receiving intravenous voriconazole and intermittent dialysis therapy revealed that the SBECD plasma concentrations were greater than 400 mg/mL (von Mach et al., 2006). A subsequent case series also detected SBECD accumulation in four critically ill patients with acute kidney injury under extended daily dialysis (Burkhardt et al., 2010). The trough concentrations of SBECD on day 5 were several times higher than those on day 1. Hafner et al. performed a three-period randomized crossover PK study of 15 patients with end-stage renal failure during treatment with two hemodialysis systems and hemodiafiltration, and found that SBECD recoveries in dialysate samples were 67% of the administered doses (Hafner et al., 2010). Although the half-life during renal replacement therapy nearly normalized and SBECD could be effectively eliminated by 6 h of renal replacement therapy via all methods, the prediction results indicated that SBECD still exceeded the exposure of patients with normal renal function by a factor of 6.2 in the steady state. Kiser et al. also performed an in vivo study in 10 patients receiving CVVH, and reported that CVVH accounted for 86% of the total body clearance of SBECD, with the majority of the dose being recovered in the effluent (Kiser et al., 2015). They reported that standard dosages of intravenous voriconazole can be utilized in patients undergoing CVVH without a significant risk of SBECD accumulation. However, some experts have suggested that the results should be interpreted with caution in different scenarios (Honore et al., 2015). Thus, caution should always be taken when intravenous voriconazole is dosed to CRRT patients to avoid SBECD accumulation.
Clinical evidence of the safety of voriconazole injection in patients receiving CRRT
Considering the difficulty in assessing renal function fluctuations in CRRT patients, there are few data concerning the safety of intravenous voriconazole. However, there is some evidence in patients with renal impairment. Lashof et al. performed a retrospective study to evaluate the safety and tolerability of intravenous voriconazole in 41 patients with baseline renal insufficiency (Oude Lashof et al., 2012). The median duration of intravenous voriconazole treatment was 7 days. Worsening of renal function or newly emerged renal adverse events were reported in 39% of voriconazole-treated patients but were lower than those associated with the alternative drug amphotericin B. In contrast, Lilly et al. evaluated the safety of IV voriconazole compared with two other IV antifungals not containing SBECD in patients with compromised renal function (baseline Clcr <50 mL/min), and formulation with SBECD was not a predictor of AKI (Lilly et al., 2013). They concluded that the decision on which antifungal to use should not be determined by the incorporation of SBECD in the IV formulation. However, the sample size was also limited (19 patients received voriconazole). Kim also performed a prospective observation study in 25 patients (7 patients with baseline Clcr <50 mL/min) and did not find a high ADR incidence of SBECD-containing formulations (Kim et al., 2016).
The argument about intravenous voriconazole safety in patients with renal impairment, as well as in patients receiving CRRT, continues until convincing evidence emerges (Tragiannidis and Groll, 2022; Wang and Zhang, 2022).
Comparison of voriconazole PPK parameters in CRRT patients
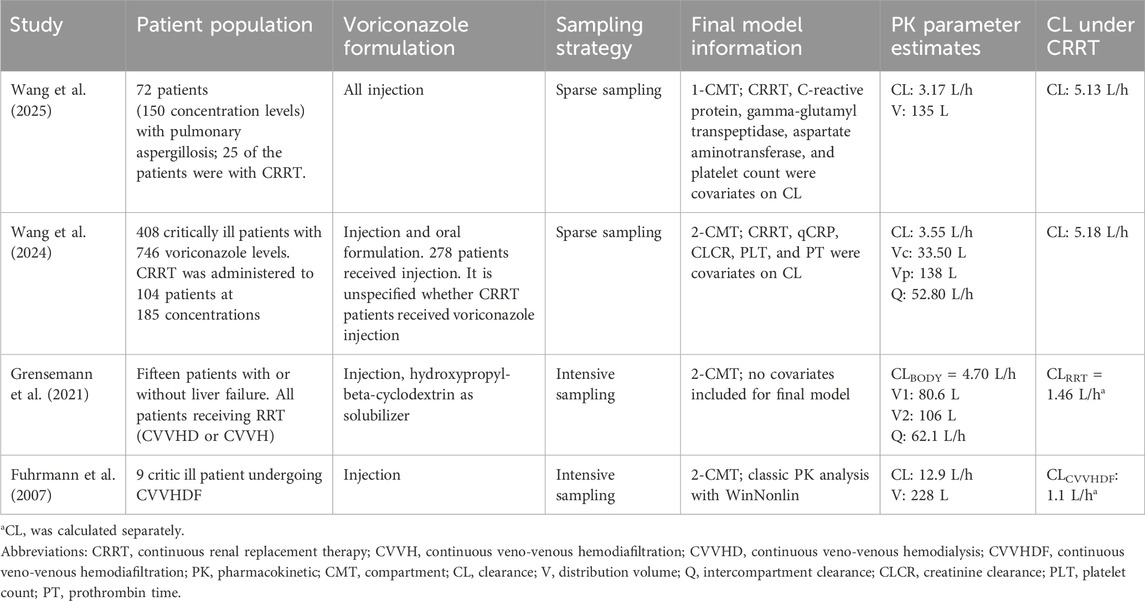
We searched the literature concerning the PK parameters of voriconazole under CRRT, excluding case reports. The data are shown in Table 1. The design of these studies has several limitations, such as a limited sample size or limited sampling points. The PK parameter estimates varied greatly among studies. Multiple factors, such as CRRT modality and patient characteristics, could influence voriconazole clearance and contribute to the variance in voriconazole concentrations. Unfortunately, most studies have not clarified the modality and parameter of CRRT. From the limited data we could see that three studies reported comparable clearance of voriconazole (Grensemann et al., 2021; Wang et al., 2024; Wang et al., 2025), but a study focused on continuous veno-venous hemodiafiltration (CVVHDF) modality reported a much high clearance and may have additional drug removal (Fuhrmann et al., 2007). Current models may be insufficient to estimate voriconazole exposure precisely in CRRT patients, and therapeutic monitoring may be the only way to ensure efficacy and avoid toxicity.
Practical considerations for clinicians
Voriconazole injection could be used in patients receiving CRRT after risk-benefit evaluation. Due to the large inter-individual variability of voriconazole and additional removal by CRRT (especially CVVHDF), therapeutic drug monitoring (TDM) should be performed to ensure optimal exposure of voriconazole. Moreover, there is a risk of SBECD accumulation for these patients and TDM for SBECD is not feasible for most institutes. Thus, symptoms and biomarkers related to kidney injury should be closely monitored, and the duration of voriconazole injection treatment should not be prolonged. Otherwise, alternate antifungals should be considered.
In conclusion, evidence concerning voriconazole injection in patients receiving CRRT is insufficient. Well-designed trials addressing this issue are needed. Caution should be taken when dosing voriconazole injections in these patients.
Author contributions
LZ: Formal Analysis, Data curation, Methodology, Investigation, Writing – original draft. ZY: Writing – original draft, Methodology, Resources, Validation, Data curation, Investigation. XW: Conceptualization, Writing – review and editing, Supervision. HZ: Writing – review and editing, Project administration, Conceptualization, Supervision.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Burkhardt, O., Thon, S., Burhenne, J., Welte, T., and Kielstein, J. T. (2010). Sulphobutylether-beta-cyclodextrin accumulation in critically ill patients with acute kidney injury treated with intravenous voriconazole under extended daily dialysis. Int. J. Antimicrob. Agents 36, 93–94. doi:10.1016/j.ijantimicag.2010.02.017
Fuhrmann, V., Schenk, P., Jaeger, W., Miksits, M., Kneidinger, N., Warszawska, J., et al. (2007). Pharmacokinetics of voriconazole during continuous venovenous haemodiafiltration. J. Antimicrob. Chemother. 60, 1085–1090. doi:10.1093/jac/dkm349
Grensemann, J., Pfaffendorf, C., Wicha, S. G., König, C., Roedl, K., Jarczak, D., et al. (2021). Voriconazole pharmacokinetics are not altered in critically ill patients with acute-on-chronic liver failure and continuous renal replacement therapy: an observational study. Microorganisms 9, 1–13. doi:10.3390/microorganisms9102087
Hafner, V., Czock, D., Burhenne, J., Riedel, K. D., Bommer, J., Mikus, G., et al. (2010). Pharmacokinetics of sulfobutylether-beta-cyclodextrin and voriconazole in patients with end-stage renal failure during treatment with two hemodialysis systems and hemodiafiltration. Antimicrob. Agents Chemother. 54, 2596–2602. doi:10.1128/AAC.01540-09
Hinze, C. A., Fuge, J., Grote-Koska, D., Brand, K., Slevogt, H., Cornberg, M., et al. (2024). Factors influencing voriconazole plasma level in intensive care patients. JAC-Antimicrobial Resist 6, dlae045. doi:10.1093/jacamr/dlae045
Honore, P. M., Jacobs, R., Hendrickx, I., De Waele, E., Van Gorp, V., and Spapen, H. D. (2015). Continuous renal replacement therapy for safe and adequate voriconazole intravenous treatment: enough reason to be confident? Crit. Care 19, 13054. doi:10.1186/s13054-015-0946-1
Kim, S.-H., Kwon, J.-C., Park, C., Han, S., Yim, D.-S., Choi, J.-K., et al. (2016). Therapeutic drug monitoring and safety of intravenous voriconazole formulated with sulfobutylether β-cyclodextrin in haematological patients with renal impairment. Mycoses 59, 644–651. doi:10.1111/myc.12517
Kiser, T. H., Fish, D. N., Aquilante, C. L., Rower, J. E., Wempe, M. F., MacLaren, R., et al. (2015). Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit. Care 19, 1–9. doi:10.1186/s13054-015-0753-8
Lilly, C. M., Welch, V. L., Mayer, T., Ranauro, P., Meisner, J., and Luke, D. R. (2013). Evaluation of intravenous voriconazole in patients with compromised renal function. BMC Infect. Dis. 13, 14. doi:10.1186/1471-2334-13-14
McCreary, E. K., Davis, M. R., Narayanan, N., Andes, D. R., Cattaneo, D., Christian, R., et al. (2023). Utility of triazole antifungal therapeutic drug monitoring: insights from the society of infectious diseases pharmacists: endorsed by the Mycoses Study group education and research consortium. Pharmacotherapy 43, 1043–1050. doi:10.1002/phar.2850
Oude Lashof, A. M. L., Sobel, J. D., Ruhnke, M., Pappas, P. G., Viscoli, C., Schlamm, H. T., et al. (2012). Safety and tolerability of voriconazole in patients with baseline renal insufficiency and candidemia. Antimicrob. Agents Chemother. 56, 3133–3137. doi:10.1128/AAC.05841-11
Tragiannidis, A., and Groll, A. (2022). Reply to letter to the editor to “Comment about the safety of intravenous voriconazole formulated with sulfobutylether beta-cyclodextrin”. Expert Opin. Drug Saf. 21, 135–136. doi:10.1080/14740338.2021.1979183
von Mach, M. A., Burhenne, J., and Weilemann, L. S. (2006). Accumulation of the solvent vehicle sulphobutylether beta Cyclodextrin sodium in critically ill patients treated with intravenous voriconazole under renal replacement therapy. BMC Clin. Pharmacol. 6, 6. doi:10.1186/1472-6904-6-6
Wang, L., and Zhang, Z. (2022). Comment about the safety of intravenous voriconazole formulated with sulfobutylether beta-cyclodextrin. Expert Opin. Drug Saf. 21, 133–134. doi:10.1080/14740338.2021.1978976
Wang, Y., Ye, Q., Li, P., Huang, L., Qi, Z., Chen, W., et al. (2024). Renal replacement therapy as a new indicator of voriconazole clearance in a population pharmacokinetic analysis of critically ill patients. Pharmaceuticals 17, 665. doi:10.3390/ph17060665
Keywords: CRRT, injection, pharmacokinetic, sulphobutylether beta-cyclodextrin, therapeutic drug monitoring, voriconazole
Citation: Zhou L, Yu Z, Wang X and Zheng H (2025) Voriconazole injection use in patients receiving continuous renal replacement therapy. Front. Pharmacol. 16:1736767. doi: 10.3389/fphar.2025.1736767
Received: 31 October 2025; Accepted: 09 December 2025;
Published: 18 December 2025.
Edited by:
Cyprian Onyeji, University of Nigeria, NigeriaReviewed by:
Bilgen Basgut, Baskent University, TürkiyeCopyright © 2025 Zhou, Yu, Wang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Wang, d2FuZ3hpYTFAcWR1LmVkdS5jbg==; Huaiyu Zheng, MTU5MjQwMDI3NzZAMTYzLmNvbQ==
 Ling Zhou
Ling Zhou Zhenwei Yu
Zhenwei Yu Xia Wang3*
Xia Wang3*