- 1Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, Cairo, Egypt
- 2Department of Laboratory Medicine, Karolinska Institute, Stockholm, Sweden
- 3Department of Cellular Therapy and Allogeneic Stem Cell Transplantation (CAST), Karolinska University Hospital Huddinge and Karolinska Comprehensive Cancer Center, Stockholm, Sweden
- 4Department of Microbiology and Immunology, Faculty of Pharmacy, Cairo University, Cairo, Egypt
- 5School of Life and Medical Sciences, University of Hertfordshire Hosted By Global Academic Foundation, Cairo, Egypt
Introduction: Vaginal candidiasis remains a recurrent fungal infection affecting millions of women worldwide, necessitating innovative local delivery systems to overcome poor drug solubility and mucosal barriers. This study introduces Dual-Functional Hybrid Cationic PEGylated Proniosomes (DHCPP) as a smart nano-platform designed to boost the vaginal delivery of Fenticonazole Nitrate (FTN).
Methods: DHCPP systems were fabricated via the coacervation phase separation method and optimized using a full 23 factorial design, achieving a high desirability value of 0.931. The optimized DHCPP exhibited an encapsulation efficiency of 91.61%, particle size of 238.85 nm, and zeta potential of +58.85 mV, ensuring colloidal stability and efficient mucosal interaction.
Results: In-vitro characterization using TEM and FTIR verified the formation of uniform spherical vesicles and successful FTN encapsulation without chemical incompatibility. Mucoadhesion testing revealed superior adhesive strength, indicating prolonged vaginal residence, while drug release followed a diffusion-controlled pattern. The optimized DHCPP was incorporated into a carbopol-based gel exhibiting pseudo-plastic rheology and pH compatibility with the vaginal environment. Multi-scale ex vivo and in vivo evaluations revealed a 2.36-fold permeation enhancement compared to FTN gel, consistent with deeper mucosal penetration observed via confocal microscopy. The microbiological assessment indicated a pronounced reduction in MIC and MFC values and a remarkable improvement in biofilm inhibitory effect, highlighting enhanced antifungal efficacy. Histopathological examination verified the mucosal safety of the optimized gel.
Conclusion: The developed DHCPP represents an innovative, multifunctional, and biocompatible delivery system offering enhanced vaginal permeation, prolonged retention, and potent antifungal activity for the effective management of vaginal candidiasis.
Highlights
• Innovative Dual-Functional Hybrid Cationic PEGylated Proniosomes (DHCPP) were developed to enhance vaginal delivery of Fenticonazole Nitrate (FTN).
• Formulation optimization using a full 23 factorial design achieved a high desirability value (0.931), producing stable nano-sized vesicles (238.85 nm) with high EE% (91.61%) and strong positive ZP (+58.85 mV).
• FTIR and TEM analyses confirmed molecular compatibility, successful PEGylation, and the formation of uniform spherical vesicles ensuring effective drug entrapment.
• DHCPP revealed enhanced mucoadhesion, diffusion-controlled release, prolonged vaginal retention, supported by pseudo-plastic rheology and physiological pH compatibility.
• Comprehensive in-vitro, ex-vivo, microbiological, and in-vivo evaluations demonstrated a 2.36-fold permeation enhancement, pronounced biofilm inhibition, and significant reduction in MIC and MFC values.
• Histopathological findings verified mucosal safety, positioning DHCPP as an innovative, biocompatible, and clinically promising nanocarrier for effective management of vaginal candidiasis.
1 Introduction
Fungal infections remain a persistent and expanding global health challenge, particularly among immunocompromised individuals and women of reproductive age. Among the diverse pathogenic fungi, Candida species are the predominant culprits, responsible for approximately 90% of fungal infections worldwide (Campelo et al., 2021). Vaginal candidiasis, primarily induced by Candida albicans, represents one of the most prevalent gynecological disorders, affecting around three out of every four women will experience an episode during their lifetime, and nearly half of them may suffer from repeated recurrences over time (Bhosale et al., 2025). Such alarming recurrence rates underscore the inadequacy of conventional antifungal therapies in providing durable clinical resolution. The etiology of vaginal candidiasis is multifactorial, involving hormonal fluctuations linked to oral contraceptive use, uncontrolled diabetes, and, most prominently, the widespread administration of broad-spectrum antibiotics that disrupt the protective vaginal microbiota (Chassot et al., 2008). The ensuing dysbiosis compromises mucosal immunity, enabling fungal overgrowth and recurrent infections. Clinically, patients present with pruritus, burning, irritation, and profuse malodorous discharge, all of which collectively diminish quality of life and psychological wellbeing (Ahmed et al., 2025a). Currently, azole antifungals, particularly fluconazole, remain the cornerstone of therapy due to their inhibition of the fungal cytochrome P450-dependent ergosterol synthesis pathway (Ahmed et al., 2025b). However, increasing reports of azole resistance, coupled with systemic adverse effects such as gastrointestinal distress, hepatotoxicity, and cardiac arrhythmias, have intensified the demand to achieve enhanced site-specific efficacy with improved safety and minimal systemic exposure (Picheta et al., 2024). In this context, the vaginal route offers an attractive and targeted alternative for antifungal delivery. It allows site-specific administration, enhanced drug concentration at the infection locus, and reduced systemic exposure. Nevertheless, challenges such as poor drug solubility, rapid mucosal clearance, and limited penetration across the vaginal epithelium necessitate the development of advanced nanocarrier systems (Albash et al., 2020).
The accelerating advancements in nanotechnology have revolutionized the pharmaceutical landscape, providing transformative approaches designed to address and mitigate the drawbacks associated with conventional drug delivery systems (Farag et al., 2025). Emerging nanocarrier-based systems enable precise modulation of drug pharmacokinetics and overall bio-distribution, leading to improved treatment effectiveness with reduced risk of systemic side effects (Elmahboub et al., 2025). These next-generation delivery platforms are meticulously engineered to achieve controlled and site-specific drug release, ensuring optimal spatiotemporal availability of active pharmaceutical ingredients and improved patient compliance (Ahmadi et al., 2023). The present study introduces Dual-Functional Hybrid Cationic PEGylated Proniosomes (DHCPP) as an innovative nano-platform engineered to enhance the vaginal delivery of Fenticonazole Nitrate (FTN). This hybrid system strategically combines cationic charge-induced mucoadhesion with PEGylation-driven stability and biocompatibility, yielding a synergistic architecture that improves mucosal residence, permeability, and antifungal efficacy. The system is primarily composed of di-dodecyl-dimethyl-ammonium bromide (DDAB), a cationic quaternary ammonium surfactant that constitutes the structural backbone of the vesicular membrane (Albash et al., 2021). The positive surface charge imparted by DDAB facilitates ionic attraction with the anionic mucosal surface, resulting in stronger adherence and extended residence time within the vaginal environment. Phosphatidylcholine (PC), a biocompatible phospholipid, was incorporated as a bilayer-forming agent to mimic natural biological membranes, facilitating drug encapsulation and controlled release while maintaining structural integrity (Ahmed et al., 2024a). To further reinforce membrane rigidity and minimize premature drug leakage, cholesterol was included to intercalate within the lipid bilayer, conferring both mechanical strength and thermodynamic stability to the vesicles (Younes et al., 2024). Additionally, Pluronic® L121, a non-ionic triblock copolymer, acts as a surface-active stabilizer that enhances flexibility and prevents vesicle aggregation, contributing to improved dispersibility and sustained release behavior (Ahmed et al., 2025c). Complementing this design, PEGylation, the covalent attachment of polyethylene glycol (PEG) chains, was introduced to the vesicular surface to impart steric stabilization and improve the nano-system’s resistance to enzymatic degradation. PEGylation also mitigates immunogenic responses and enhances residence time within the mucosal milieu, ensuring prolonged therapeutic exposure and consistent antifungal action (Fahmy et al., 2025; Megahed et al., 2022).
Fenticonazole Nitrate (FTN) is a synthetically derived imidazole-based antifungal agent with broad-spectrum activity known for its concentration-dependent activity (Ahmed et al., 2023). At sub-inhibitory concentrations, FTN demonstrates a potent biofilm-suppressive effect, disrupting early fungal adhesion and thereby minimizing the likelihood of infection recurrence and chronic colonization (Sanguinetti et al., 2019). Conversely, at higher concentrations, FTN exerts a fungicidal mechanism by enhancing membrane permeability and causing disruption of the fungal cell membrane architecture, which ultimately results in irreversible cell rupture and death (Ahmed et al., 2022). FTN exhibits good thermal stability in its solid crystalline state, with a reported melting point in the range of approximately 135 °C–137 °C, indicating a stable crystal lattice capable of withstanding moderate thermal conditions. This relatively high melting point suggests that FTN can tolerate the temperatures commonly employed during formulation and processing without undergoing thermal degradation (Ahmed et al., 2025a; Nemr et al., 2025). Clinical investigations have confirmed the therapeutic effectiveness of FTN through short-course vaginal ovule regimens, which notably improved patient adherence and tolerability compared to conventional azole therapies (Lawrence et al., 1990). However, despite its advantageous pharmacodynamic properties, FTN’s poor aqueous solubility (<0.1 mg/mL) and limited systemic bioavailability significantly restrict its therapeutic potential and consistent local retention (Nemr et al., 2024). These challenges emphasize the necessity for a next-generation localized delivery system that can improve drug solubility, facilitate deeper mucosal diffusion, and maintain prolonged antifungal effectiveness.
The objective of this research was to design and optimize FTN-loaded DHCPP formulations with the goal of attaining maximal encapsulation efficiency and surface charge stability, alongside reduced particle size and a narrow poly-dispersity index, ensuring a homogeneous nanoscale distribution. A full 23 factorial experimental design was employed through Design Expert® software (v13, Stat-Ease Inc., Minneapolis, USA) to systematically explore formulation variables and determine the most optimized composition. Before incorporating the optimized formula into a gel, comprehensive in-vitro evaluations were performed. These included drug release studies, surface morphology using TEM, and interaction analysis by FTIR. In addition, mucoadhesion testing was carried out to confirm the enhanced adhesive properties of the cationic PEGylated system, and stability testing was performed under refrigerated conditions for 3 months to ensure formulation integrity. The optimized DHCPP were then incorporated into a carbopol-based gel designed for vaginal application. The prepared gel was examined for rheological behavior and pH compatibility to confirm suitability for mucosal use. Ultimately, ex-vivo permeation experiments combined with in-vivo confocal laser scanning microscopy (CLSM) imaging demonstrated enhanced vaginal tissue penetration, and histological examination further validated the biocompatibility and safety of the developed formulation on the mucosal surface.
2 Materials and methodology
2.1 Materials
FTN, possessing a molecular weight of 518.41 g/mol and a purity exceeding 99%, was kindly supplied by Andalous Pharmaceutical Company. PC; (egg yolk, ∼60%), DDAB (98% purity, melting point 157-162
2.2 Design of experiments
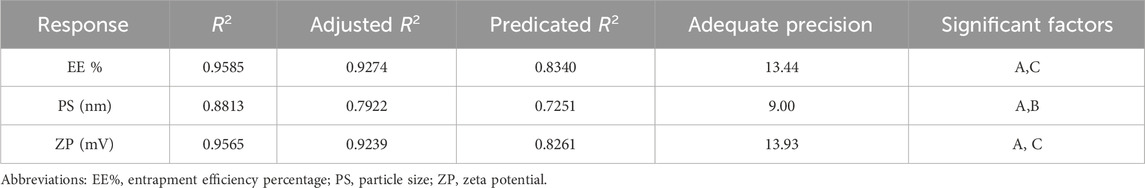
To methodically evaluate how different formulation parameters affect the physicochemical behavior of the prepared FTN–loaded DHCPPs, a full 23 factorial experimental design was employed utilizing Design-Expert® software (version 13; Stat-Ease Inc., Minneapolis, MN, USA).This statistical tool was employed for its efficiency in simultaneously evaluating multiple factors and their possible interactions while minimizing experimental runs (Ahmed et al., 2025d). Based on preliminary trials, three key formulation parameters were chosen as independent variables: the ratio of DDAB to drug (Factor A), the PEG-to-DDAB ratio (Factor B), and the PC-to-PEG ratio (Factor C). Each parameter was examined at two distinct levels to provide a comprehensive understanding of its impact on formulation performance. The evaluated dependent variables comprised encapsulation efficiency (EE%; Y1), particle size (PS; Y2), poly-dispersity index (PDI; Y3), and zeta potential (ZP; Y4), which collectively serve as key determinants (pre-optimization measurements) of the formulation’s drug encapsulation capability, nanoscale uniformity, and overall colloidal stability. This factorial approach enabled the identification of significant main effects, facilitating the optimization of formulation composition toward achieving high EE%, nanoscale particle size, and stable charge characteristics (El Hassab et al., 2025; Ahmed et al., 2025e). A detailed overview of the experimental layout, outlining the factor levels alongside the corresponding measured responses, is provided in Table 1, whereas Table 2 summarizes the obtained results for all formulated batches.
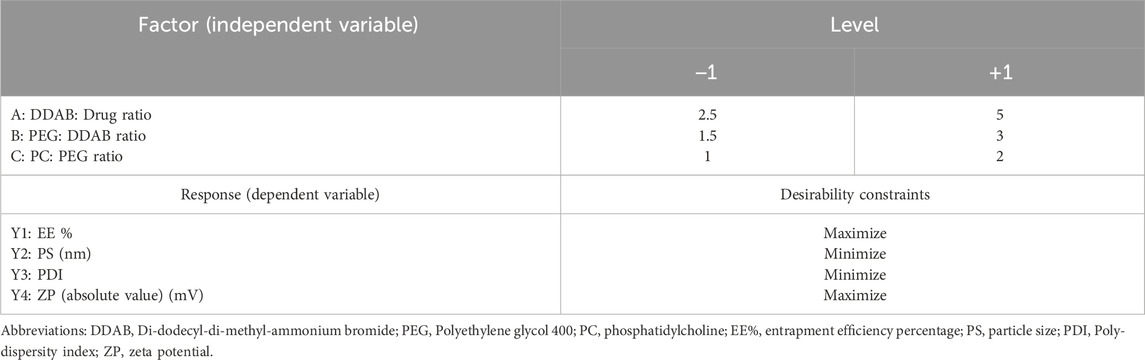
Table 1. 23 full factorial experimental design highlighting different levels of independent variables and targeted optimization criteria for the responses.
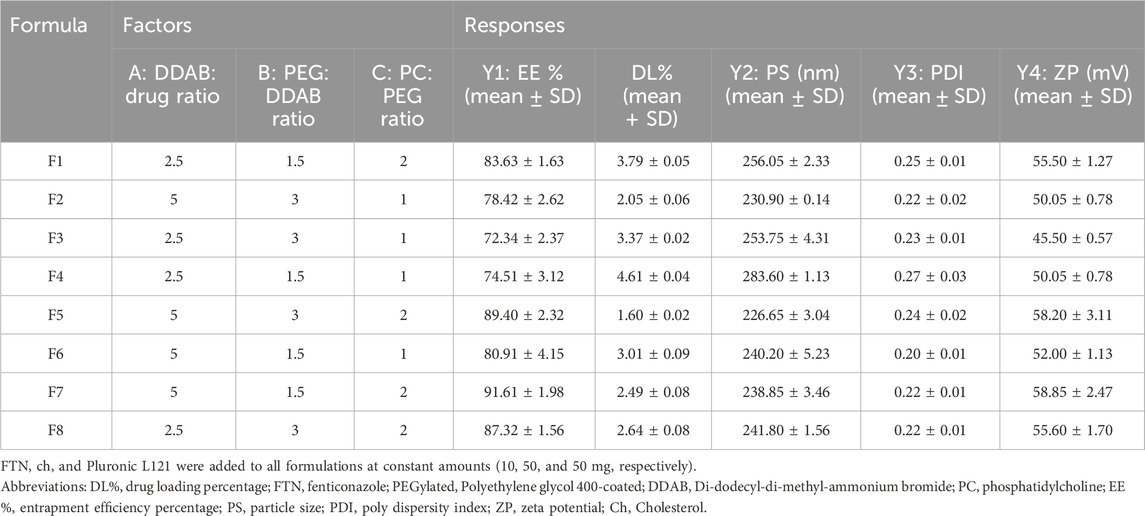
Table 2. Composition and Characterization of the prepared FTN-loaded Hybrid Cationic PEGylated Proniosomes (mean ± SD).
2.3 Development of FTN-loaded DHCPP
The FTN-loaded DHCPPs were fabricated using a modified coacervation phase separation method, as adapted from previously reported with slight modifications (Ahmed et al., 2025f; Khatoon et al., 2017). In brief, accurately weighed quantities of FTN (10 mg), cholesterol (50 mg), and Pluronic L121 (50 mg) were incorporated in all formulations as fixed components, whereas the proportions of DDAB, PEG, and PC were varied according to the factorial design parameters. All components were solubilized in a tightly closed glass vial containing 2 mL of a methanol and acetone mixture prepared in a 1:1 volume ratio. The mixture was maintained at 60 °C in a water bath and sonicated (Model SH 150–41, MTI Corporation, Richmond, CA) for 30 min to obtain a homogeneous clear dispersion. Afterward, three drops of phosphate-buffered saline (PBS, pH 7.4), pre-warmed to the required temperature, were gradually introduced while maintaining continuous sonication for a further 15 min. The resulting solution was left to slowly reach ambient temperature and then stored overnight. Before further characterization, the stored formulations were preheated to 60 °C for 1 minute, then hydrated with 10 mL of double-distilled water under vortex agitation for 5 min to yield uniform, nano-sized niosomal dispersions (Younes et al., 2024).
2.4 Analysis of the developed FTN-loaded DHCPP
2.4.1 Evaluation of encapsulation efficiency (EE%) and drug loading (DL%)
The encapsulation efficiency (EE%) of the FTN-loaded DHCPP formulations was assessed indirectly by ultracentrifugation, measuring the amount of drug remaining in the aqueous phase that was not encapsulated (Nemr et al., 2025). Accurately measured aliquots of the freshly prepared formulations (1 mL, equivalent to 1000 µg FTN) were transferred into centrifuge tubes. The samples were then centrifuged at 21,000 rpm for 1 h at 4 °C using a refrigerated centrifuge (Model 8,880, Centurion Scientific Ltd., West Sussex, UK). This step ensured complete sedimentation of the vesicular fraction, leaving the supernatant containing the free, non-encapsulated FTN. The transparent supernatant was gently collected, suitably diluted to a final volume of 10 mL using methanol, corresponding to a 1:10 (v/v) dilution ratio, and its absorbance was measured at 252 nm using a UV–visible spectrophotometer (UV-1601 PC, Shimadzu, Kyoto, Japan). Quantification was performed against a validated calibration curve (R2 = 0.9997, n = 3) to ensure analytical accuracy and reproducibility. For drug loading (DL%) measurement, a methanolic solution of the sample volume (0.5 mL; equivalent to 500 µg FTN) was prepared and measured for FTN content. The encapsulation efficiency (EE%) and drug loading (DL%) was subsequently determined using the Equations 1, 2 (Ahmed et al., 2025a; Teama et al., 2025; Herdiana et al., 2024):
2.4.2 Evaluation of particle size, poly-dispersity index and surface charge
The particle size (PS), poly-dispersity index (PDI), and zeta potential (ZP) of the developed FTN-loaded DHCPP formulations were analyzed to evaluate their colloidal characteristics and stability. Dynamic Light Scattering (DLS) analysis was performed using a Zetasizer Nano ZS instrument (Malvern Instruments Ltd., Worcestershire, UK) at 25 °C ± 1 °C with a backscattering angle of 173° (Ahmed et al., 2025g). Before measurement, 50 μL of the freshly prepared sample was diluted with double-distilled water to a final volume of 10 mL and mixed gently using a vortex mixer to ensure a uniform, translucent dispersion suitable for accurate light scattering. The particle size provided insight into the nanoscale dimensions of the vesicles, The polydispersity index (PDI) indicated the degree of uniformity in particle size distribution, with smaller PDI values corresponding to a more uniform vesicular population (Albash et al., 2025a). The zeta potential, determined through electrophoretic mobility within the same instrument, indicated the surface charge of the vesicles and was used to predict colloidal stability and the potential for electrostatic interaction with the vaginal mucosa (El-Naggar et al., 2026). All measurements were carried out in triplicate, and the data are presented as mean ± standard deviation to guarantee reliability and reproducibility of the analysis (Bazaz et al., 2021).
2.5 Choice of best-performing DHCPP
FTN-loaded DHCPP formulations were systematically optimized using Design-Expert® software (version 13, Stat-Ease Inc., Minneapolis, USA) to determine the formulation with the most favorable physicochemical characteristics. The optimization parameters were established to achieve maximum encapsulation efficiency (EE%; Y1) and high absolute zeta potential (ZP; Y4), while minimizing particle size (PS; Y2) and keeping the polydispersity index (PDI; Y3) within a suitable range to ensure uniform nanoscale distribution and stable colloidal behavior (Ahmed et al., 2024b). Analysis of variance (ANOVA) was employed to statistically examine the significance of the model terms and the interactions between formulation variables. A numerical multi-response optimization approach was then applied, where each response was transformed into an individual desirability function ranging from 0 (least desirable) to 1 (most desirable) (Ahmed et al., 2025e). These individual functions were subsequently integrated into a composite desirability index, allowing simultaneous consideration of all targeted parameters. The formulation displaying the highest overall desirability value was identified as the optimized system and selected for confirmatory evaluation (Albash et al., 2025b). The close agreement between predicted and experimental values, expressed as a low percentage deviation, verified the accuracy, stability, and predictive capability of the optimization model was determined using Equation 3 (Albash et al., 2025c).
2.6 Evaluation of the selected DHCPP
2.6.1 TEM visualization
TEM (JEOL JEM-2100, Frankfurt, Germany) was used to scan the surface morphology and structure of the optimized DHCPP formulation, confirming vesicle formation and uniform nanoscale characteristics (Elgendy et al., 2024). Before imaging, the chosen formulation was diluted with deionized water to ensure adequate dispersion and reduce vesicle aggregation. First, the sample was properly diluted, and a drop was taken and gently applied onto a carbon-coated copper grid. The grid was left to dry in an open atmosphere at room temperature. To improve contrast and clearly visualize the vesicle boundaries, the sample was negatively stained with a 0.1% (w/v) phosphotungstic acid solution and allowed to dry for additional 15 min. The prepared specimens were subsequently examined at an accelerating voltage of 80 kV, enabling detailed visualization of the vesicle morphology, surface smoothness, and structural integrity (Tawfik et al., 2025).
2.6.2 FT-IR analysis
FTIR analysis was carried out to investigate potential physicochemical interactions among the components of the optimized FTN-loaded DHCPP and to confirm successful drug encapsulation within the nanocarrier matrix. The newly prepared optimum formulation was first frozen at −20 °C and subsequently lyophilized at −45 °C using a Novalyphe-NL 500 freeze-dryer (Savant Instruments, NY, USA) to obtain a completely dry powder (Ahmed et al., 2022). The effect of lyophilization on the nanoscale characteristics of the optimized formulation was evaluated by measuring particle size and poly-dispersity index (PDI) before and after freeze-drying. After that, an accurately weighed portion of the lyophilized sample (2 mg) was thoroughly mixed with anhydrous potassium bromide (KBr) and pressed into a clear pellet using hydraulic pressure (Ahmed et al., 2025h). The FTIR spectra were acquired at room temperature using a Shimadzu FTIR spectrophotometer (Model 8400 S, Kyoto, Japan) across a wavenumber range of 4,000–400 cm-1 (Elgendy et al., 2023). For comparative analysis, spectra of pure FTN, DDAB, PC and cholesterol were also obtained under identical conditions.
2.6.3 In-vitro release study
In vitro drug release was evaluated using the dialysis bag diffusion method to compare the release behavior of the optimized FTN-loaded DHCPP formulation with that of the plain drug suspension. For the preparation of the FTN suspension, the drug was uniformly dispersed in distilled water containing methylcellulose as a suspending agent. Methylcellulose was chosen owing to its biological inertness, excellent suspending properties, and minimal interference with drug stability or analytical assessment. The suspension was prepared under continuous stirring to ensure complete homogeneity and was freshly prepared prior to each use to maintain consistency and reliability.
Dialysis membranes with a molecular weight cut-off of 12–14 kDa were pretreated by soaking overnight in phosphate-buffered saline (PBS, pH 4.5) and subsequently filled with a precisely measured amount of the optimized formulation (1 mL, equivalent to 1000 µg FTN) or an equivalent drug suspension. Each dialysis membrane was tightly sealed and placed into 50 mL of release medium, composed of phosphate-buffered saline (PBS, pH 4.5) and absolute ethanol in a 3:1 (v/v) ratio, to ensure sink conditions (Ahmed et al., 2025b). The assemblies were placed in a thermostatically controlled shaking water bath (Unimax, IKA, Germany) maintained at 37 °C ± 0.2 °C and agitated at 100 rpm to simulate physiological conditions (Weng et al., 2020). At specific time intervals (0.5, 1, 2, 4, 6, and 8 h), 3 mL samples were collected and promptly replaced with an equal volume of fresh pre-warmed medium. The collected samples were examined using spectrophotometry to quantify the cumulative release of FTN. The results were plotted as cumulative percentage of drug released versus time, and Q2h and Q8h values were calculated and compared with those from the plain drug suspension. The release profiles were analyzed using zero-order, first-order, Korsmeyer–Peppas, and Higuchi kinetic models, with the correlation coefficient (R2) employed to determine the model that most accurately represented FTN release from the optimized DHCPP formulation (Ahmed et al., 2025c; Abdelhakeem et al., 2025).
2.6.4 Mucoadhesion assessment
The mucoadhesive properties of the optimized FTN-loaded DHCPP formulation were investigated by examining its electrostatic interactions with mucin. A 1% (w/v) mucin suspension was combined with an equal volume of the optimized DHCPP formulation in a 1:1 (v/v) ratio (Nemr et al., 2024). The mixture was carefully agitated using a magnetic stirrer for 5 min to ensure homogeneity, followed by overnight equilibration at room temperature to allow sufficient interaction between mucin glycoproteins and the positively charged vesicular surface (Tawfik et al., 2025). The zeta potential (ZP) of the mucin dispersion and the optimized DHCPP formulation was determined individually and compared to that of the mucin–DHCPP mixture employing a Zetasizer (Model ZEN3600, Malvern Instruments Ltd., Worcestershire, UK). A significant change in the ZP of the mixture compared to the individual components was interpreted as an indication of strong electrostatic interaction and effective mucin binding, confirming the enhanced mucoadhesive behavior of the optimized formulation which is an essential attribute for prolonged retention and improved drug residence at the vaginal mucosa (Albash et al., 2021; Ahmed et al., 2025d).
2.6.5 Short term stability assessment
The influence of storage on the physicochemical stability, as well as the release behavior, of the selected FTN-loaded DHCPP formulation was studied (Fahmy et al., 2025). Newly prepared samples were evaluated for encapsulation efficiency (EE%, Y1), particle size (PS, Y2), zeta potential (ZP, Y4), and in-vitro drug release profiles. After characterization, the samples were placed in tightly sealed amber glass vials and stored at 4 °C–8 °C for 3 months to protect them from light- and temperature-induced degradation (Sayed et al., 2021). This duration is widely used in preliminary assessments of nanoscale systems and has been shown in previous studies to reliably predict the possible stability trends of similar formulations (Younes et al., 2024; Ahmed et al., 2025f). After the 3-month storage period, the same parameters were re-evaluated under identical experimental conditions to detect any potential alterations in vesicular integrity or drug retention. One-way ANOVA was applied to statistically compare EE%, PS, and ZP between freshly prepared and stored samples, assessing the significance of any observed differences (Al-Mahallawi et al., 2017). Additionally, the in-vitro release profiles of both formulations were compared by calculating the similarity factor (ƒ2), using Equation 4 (Emad Eldeeb et al., 2019):
where n is the number of sampling points, Rt represents the cumulative percentage of FTN released from the fresh sample at time t, and Tt denotes the corresponding release from the stored sample. An ƒ2 value between 50 and 100 indicated no significant difference in release behavior, confirming that the optimized formulation retained its colloidal stability, encapsulation capacity, and release performance throughout refrigerated storage (Abdelbary et al., 2016; Ahmed et al., 2025).
2.7 Microbiological assay
2.7.1 Determination of minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) of the optimized DHCPP formulation and the pure drug suspension was determined using the broth microdilution assay, performed in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines (Humphries et al., 2018). A series of two-fold serial dilutions of each tested sample was prepared within a concentration range of 1000–0.976 μg/mL, using double-strength Sabouraud Dextrose Broth (SDB) as the nutrient medium. Precisely 150 µL of each dilution was transferred into sterile 96-well microtiter plates with U-shaped wells, followed by inoculation with 30 µL of a standardized C. albicans (ATCC 60193) suspension containing approximately 105–106 CFU/mL. Appropriate controls were included: a positive control containing inoculum without treatment to confirm fungal viability, and a negative control containing un-inoculated broth to ensure sterility of the medium. Immediately, the plates were placed in an incubator at 28 °C ± 2 °C for 48 h. Following incubation, fungal growth inhibition was evaluated both visually and spectrophotometrically by measuring optical density at 600 nm using a microplate reader (Biotek Synergy 2, SLFA model, USA). The MIC value was defined as the lowest concentration of the tested formulation exhibiting no visible growth or turbidity compared to the control wells. All experiments were carried out in biological and technical triplicates to ensure data reproducibility.
2.7.2 Determination of minimum fungicidal concentration (MFC)
Serial two-fold dilutions of both the pure drug suspension and the optimized DHCPP were prepared and inoculated with 30 μL of a C. albicans culture standardized to an inoculum density of 105–106 CFU/mL. The mixtures were placed in 96-well microplates and incubated at 28 °C ± 2 °C for 48 h. Following incubation, 10 μL samples from each well were aseptically transferred and spotted onto Sabouraud Dextrose Agar (SDA) plates. These plates were further incubated under the same conditions (28 °C ± 2 °C for an additional 48 h). The minimum fungicidal concentration (MFC) was identified as the lowest concentration that exhibited complete inhibition of visible fungal growth. All assays were conducted in triplicate, covering both biological and technical replicates to ensure reproducibility (Ahmed et al., 2025b).
2.7.3 Determination of biofilm inhibitory effect
The anti-biofilm potential of both the plain drug suspension and the optimized formulation was assessed against C. albicans standard strain using the microtiter plate method as previously described (O'Toole, 2011; Van Dijck et al., 2018). Briefly, 75 μL of the optimized DHCPP was combined with an equal volume (75 μL) of C. albicans inoculum (approximately 108 CFU/mL in Sabouraud Dextrose Broth, SDB) in sterile, non-pyrogenic, flat-bottom 96-well polystyrene plates. The resulting final concentrations within the wells corresponded to 1/16, 1/8, ¼, and ½ X, where X denotes the previously determined minimum inhibitory concentration. The plates were incubated statically at 28 °C ± 2 °C for 48 h, allowing biofilm development. Following incubation, the optical density at 600 nm (OD600) was measured via a spectrophotometric plate reader (Synergy 2, USA) to quantify planktonic growth. The same procedure was repeated for drug suspension and the results were compared to that of the optimized formulation.
After measuring planktonic growth, the wells were gently decanted, rinsed twice with sterile phosphate-buffered saline (PBS) to remove the supernatant, and air-dried thoroughly. The remaining adherent biofilms were stained with 175 μL of 0.5% crystal violet for 30 min at room temperature. Excess dye was carefully removed by 3 washing cycles of the plates with sterile distilled water, followed by complete drying. The bound dye, representing the residual biofilm biomass, was then solubilized with 200 μL of 95% ethanol and incubated for 15 min under gentle agitation (110 rpm). The absorbance of the resulting solution was determined at 570 nm (OD570) and normalized to the corresponding OD600 values of planktonic cultures. All experiments were conducted in triplicate, including both biological and technical replicates, with positive and negative controls incorporated to validate the assay. The statistics were performed in version 4.1.2 of R and visualized in Rstudio (Racine, 2012). The percentage of biofilm inhibition was subsequently calculated according to the following Equation 5 (Ahmed et al., 2025e):
2.8 Development and characterization of optimum DHCPP gel
2.8.1 Development of DHCPP gel
Following the selection of the optimized DHCPP formulation, a carbopol-based gel was developed to facilitate vaginal application and ensure prolonged drug retention at the target site. To prepare the gel, an accurately weighed amount of Carbopol 940 (1% w/w) was gradually dispersed into the freshly prepared optimized DHCPP under continuous magnetic stirring (300 rpm) at 40 °C, ensuring uniform polymer hydration and avoiding lump formation (Shamma et al., 2019). Once a clear dispersion was obtained, triethanolamine (TEA) was cautiously introduced dropwise to neutralize the mixture and trigger gelation through pH adjustment. The prepared gel was subsequently allowed to stand overnight at room temperature to ensure complete polymer swelling and stabilization of the network structure. The resulting transparent, homogenous DHCPP gel exhibited smooth texture and was stored under refrigerated conditions (4 °C–8 °C) until further evaluation of its physicochemical and performance attributes (Ahmed et al., 2025b; Younes and Habib, 2022).
2.8.2 Rheological study
The rheological characteristics of the optimized DHCPP gel were systematically evaluated to determine its flow profile, and suitability for vaginal application. Measurements were carried out using a rotational cone-and-plate rheometer (Brookfield DV3THB, spindle CPE-41) maintained at a controlled temperature of 25 °C ± 2 °C. An accurately weighed 0.5 g sample of the gel was placed on the plate, and the shear rate was gradually increased from 0.5 to 100 rpm, with a 10-s equilibration period at each speed increment to ensure steady-state readings (Ahmed et al., 2025e). Only torque values within the 10%–100% range were included in the analysis to maintain precision and minimize measurement error. The resulting data were plotted to construct a comprehensive flow curve correlating shear stress, shear rate, and apparent viscosity. Rheological data were fitted using the Ostwald–de Waele (Power Law) model due to its proven accuracy in describing the shear-dependent behavior of non-Newtonian vesicular and semisolid systems. The model is widely reported in the literature for proniosomal and vaginal formulations and showed an excellent fit to the experimental data in the present study (Ahmed et al., 2025a; Ahmed et al., 2025b). The experimental data were fitted to the following Equation 6 (Ahmed et al., 2025):
where Ʈ represents shear stress, γ denotes shear rate, K is the consistency index, and n is the flow behavior index. The value of n was used to classify the type of flow: n = 1 corresponds to Newtonian behavior, n < 1 indicates pseudoplastic (shear-thinning) flow, and n > 1 reflects dilatant (shear-thickening) properties (Fahmy et al., 2018). To gain deeper insight into the viscoelastic and non-Newtonian characteristics of the system, additional rheological models (Bingham, Casson, and Carreau) were also applied. The coefficient of determination (R2) was used to evaluate model fitting accuracy and identify the best representation of the gel’s flow dynamics (Younes et al., 2024).
2.8.3 pH measurement
The pH of the optimized formulation was assessed to verify its physiological compatibility and ensure safe application at the intended administration site without inducing irritation or discomfort. Measurements were conducted using a calibrated benchtop pH meter (Jenway®, Model 3510, Cole-Parmer®, Illinois, USA). A defined quantity of the gel was dispersed in distilled water (1:10 w/v) to facilitate consistent readings, and measurements were performed in triplicate at room temperature. The results were recorded as mean ± standard deviation (SD) (Ahmed et al., 2024b; Fouda et al., 2018).
2.9 Animal studies
2.9.1 Ethical approval
Animal studies were conducted following strict ethical and scientific standards to ensure animal welfare and data reliability. The study protocol received prior approval from the Research Ethics Committee of the Faculty of Pharmacy, Cairo University, Egypt (Approval No. PI 3771). All procedures adhered to the ethical principles outlined in the Guide for the Care and Use of Laboratory Animals (U.S. National Institutes of Health, NIH Publication No. 85–23, revised 2011) and complied with the ARRIVE guidelines for transparent reporting of in vivo experiments.
Adult female albino rabbits (weighing 2 ± 0.5 kg) were selected as the biological model (Ahmed et al., 2025a). Animals were housed individually in well-aerated cages under strict laboratory conditions including a temperature of 25 °C ± 2 °C, a 12 h-light/dark cycle, and properly regulated humidity. Throughout the experimental period, rabbits were provided ad libitum access to a standard laboratory diet and clean drinking water. Prior to study initiation, each rabbit underwent a veterinary health screening to confirm the absence of infection or inflammatory disorders that could interfere with the results.
2.9.2 Ex-vivo permeation study
Six Adult female albino rabbits (2 ± 0.5 kg) were randomly divided into two groups (n = 3). They were anesthetized using I.M. injection of ketamine (35 mg/kg) and xylazine (5 mg/kg) to facilitate tissue excision (Sayed et al., 2020). Following anesthesia, the vaginal canal was carefully dissected, isolated from adjacent tissues, and thoroughly defatted with isopropyl alcohol. The membrane was then gently washed several times with saline and deionized water to eliminate residual contaminants, after which it was stored at −20 °C until further use in permeation experiments (Ahmed et al., 2025a; Satapathy et al., 2023). The ex vivo permeation behavior of the optimized DHCPP gel was examined using a modified Franz diffusion cell specifically designed to mimic physiological vaginal conditions. The apparatus consisted of two compartments, a donor and a receptor cell, separated by the excised rabbit vaginal membrane, oriented with the epithelial surface towards the donor compartment to simulate in vivo orientation. An accurately weighed amount of the optimized DHCPP gel (equivalent to 1000 µg FTN) was applied to the donor chamber. The receptor compartment contained 25 mL of phosphate-buffered saline (PBS, pH 4.5) mixed with absolute ethanol (3:1 v/v) to maintain sink conditions (Ahmed et al., 2025b). The system was maintained at a controlled temperature of 37 °C ± 0.2 °C and continuously agitated at 100 rpm. At predetermined intervals (1, 2, 4, 6, 8, and 10 h), 1 mL samples were withdrawn from the receptor medium and replenished with equal volumes of fresh pre-warmed medium to maintain equilibrium. Collected samples were first filtered through a 0.22 µm nylon filter to remove any biological particulates and then analyzed spectrophotometrically (Ahmed et al., 2025c; Ahmed et al., 2025f). UV spectrophotometry has been widely reported in the literature as a validated and reliable method for determination, and several studies have successfully employed this technique for similar nanoscale formulations (Ahmed et al., 2024a; Mohy et al., 2026).
To provide a comparative analysis, a similar quantity of plain FTN gel (negative control) was similarly tested under identical experimental conditions. The cumulative drug permeation per unit area (Q, μg/cm2) was plotted against time to derive the permeation profile, from which key kinetic parameters, namely, the maximum flux (Jmax) and enhancement ratio (ER) were calculated using Equations 7, 8 (Ahmed et al., 2024a; ElMeshad and Mohsen, 2016):
2.9.3 Confocal laser scanning microscopy (CLSM)
The in vivo vaginal permeation profile of the optimized DHCPP gel was evaluated using Confocal Laser Scanning Microscopy (CLSM) to visualize and quantify the extent of mucosal penetration. To enable fluorescence-based tracking, Rhodamine B (RhB), a well-established fluorescent probe, was incorporated into the optimized DHCPP gel at a concentration of 0.1% w/w, replacing FTN. This dye served as a surrogate marker due to its inherent ability to emit distinct fluorescence upon excitation (Nemr et al., 2024). At this concentration, no separation step was required, as the dye provided sufficient fluorescence without affecting vesicle integrity (Ahmed et al., 2024a; Ahmed et al., 2025i). Female albino rabbits were randomly assigned into two groups (n = 3 per group). The treatment group received 2 g of RhB-loaded DHCPP gel, whereas the negative control group was administered 2 g of plain RhB gel. Formulations were applied intra-vaginally using a soft polyethylene applicator to ensure uniform coverage and minimize mucosal trauma. After 6 h of exposure, animals were anesthetized with ketamine (35 mg/kg) and xylazine (5 mg/kg), then euthanized following the same previous procedures. The vaginal mucosa was immediately excised, thoroughly rinsed with normal saline and deionized water, and then mounted between a glass slide and coverslip for microscopic analysis (Ahmed et al., 2025a). Fluorescence imaging was conducted using a CLSM (LSM 710, Carl Zeiss, Jena, Germany) equipped with argon and helium–neon lasers at excitation wavelengths of 485 nm and 595 nm, respectively (Ahmed et al., 2022). The resulting confocal images were processed and analyzed using LSM software version 4.2 (Carl Zeiss Microimaging, Jena, Germany) to assess the fluorescence distribution and penetration depth across the vaginal epithelium (Sayed et al., 2021). This study provided direct visual evidence of enhanced mucosal permeation conferred by the DHCPP system, validating the findings from the ex vivo diffusion studies and confirming the formulation’s potential for improved intravaginal drug delivery performance.
2.9.4 Histopathological assessment
To assess the local safety and mucosal tolerance of DHCPP gel following vaginal application, a histopathological study was performed using female albino rabbits. The animals were randomly divided into two groups (n = 3 each): a negative control group that received no treatment and a test group treated with the optimized DHCPP gel. The DHCPP gel (equivalent to 1000 µg FTN) was administered intra-vaginally twice daily for 1 week using a soft polyethylene applicator. After treatment termination, the animals were anesthetized and subsequently euthanized. Vaginal tissue specimens were excised and immediately fixed in 10% neutral buffered formalin for 24 h to preserve structural integrity. The samples were then sequentially dehydrated using graded alcohol concentrations (methanol, ethanol, and absolute ethanol), cleared with xylene, and embedded in paraffin wax at 56 °C to obtain tissue blocks. Thin sections (approximately 4 µm) were cut using a rotary microtome, mounted on glass slides, deparaffinized, and stained with hematoxylin and eosin (H&E) following standard histological protocols (Nemr et al., 2025; Abdelbary and AbouGhaly, 2015). The stained sections were examined under a light microscope (CX21, Olympus, Tokyo, Japan) to detect any signs of epithelial damage, edema, inflammatory infiltration, or other histological abnormalities. Comparison with control tissues confirmed the biocompatibility and non-irritant nature of the developed formulation toward the vaginal mucosa (Helal et al., 2025).
2.10 Statistical analysis
Statistical evaluation of the experimental design was conducted using one-way analysis of variance (ANOVA) implemented in Design Expert® software (Version 13, Stat-Ease Inc., Minneapolis, MN, USA). All experimental measurements were performed in triplicate (n = 3), and the results are expressed as mean ± standard deviation (SD). When significant differences among groups were observed, post-hoc comparisons were carried out using the Least Significant Difference (LSD) test to determine pairwise variations. A p-value of less than 0.05 was considered statistically significant for all analyses.
3 Results and discussion
3.1 Factorial model interpretation and response analysis
To achieve a precise optimization of DHCPPs, a 23 factorial design was systematically employed. This design enabled an in-depth investigation of the critical formulation factors influencing the nanocarrier’s physicochemical properties and performance. The selected independent variables were: the DDAB: drug ratio (Factor A), the PEG: DDAB ratio (Factor B), and the PC: PEG ratio (Factor C). Using the factorial matrix, eight experimental runs were generated to evaluate the effects of the studied variables while minimizing experimental workload (Zhang et al., 2010). The responses selected as critical quality attributes included EE%, PDI, ZP and PS. These parameters collectively reflect encapsulation capability, nanoscale uniformity, and electrostatic stability, which are essential for ensuring efficient vaginal delivery. The experimental data were analyzed using Design-Expert® software. Regression modeling and response surface methodology (RSM) were applied to derive polynomial equations, predict optimal conditions, and visualize the influence of each factor on the measured responses (Habib et al., 2018). Statistical validation confirmed the reliability and accuracy of the developed models, as indicated by high coefficients of determination (R2) and minimal differences (<0.2) between adjusted and predicted R2 values. Furthermore, adequate precision values for all responses exceeded the recommended threshold of 4, signifying strong model predictability and a favorable signal-to-noise ratio (Younes et al., 2024; Younes et al., 2025) (Table 3).
3.1.1 Statistical evaluation of EE%
Efficient drug encapsulation is a critical determinant of nanocarrier performance, directly influencing therapeutic retention, stability, and localized bioavailability (Vanic et al., 2021). The EE% of the prepared Dual-Functional Hybrid Cationic PEGylated Proniosomes (DHCPP) exhibited high values ranging from 72.34% ± 2.37% to 91.61% ± 1.98%, reflecting the system’s strong ability to retain the lipophilic antifungal agent within the vesicular bilayer matrix. The relationship between the formulation variables and EE% was mathematically described by the regression equation:
where A, B, and C correspond to the DDAB: drug ratio, PEG: DDAB ratio, and PC: PEG ratio, respectively. Statistical analysis confirmed that factors A and C exerted significant positive effects (p < 0.05) on the response, as illustrated in Figure 1A, signifying their pivotal role in optimizing the encapsulation of FTN. While factor B was non-significant (p > 0.05).
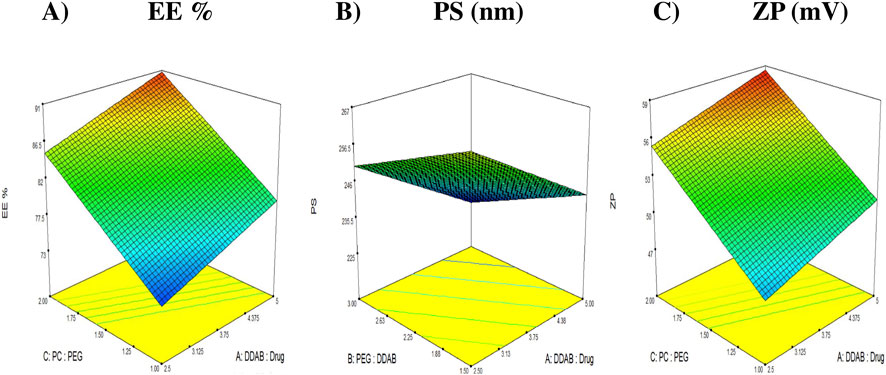
Figure 1. 3D response plots illustrating the effect of DDAB: drug ratio (factor X1), PEG: DDAB ratio (factor X2) and PC: PEG ratio (factor X3) on entrapment efficiency (%EE), particle size (PS), and zeta potential (ZP). (A) %EE. (B) PS (nm). (C) ZP (mV).
The DDAB: drug ratio (Factor A) markedly influenced EE%. Increasing the DDAB proportion improved drug retention owing to its pronounced lipophilicity (log P ≈ 11.8) (Carbone et al., 2012), which reinforced the hydrophobic core of the proniosomal bilayer. The long alkyl chains of DDAB enhance bilayer compactness and reduce drug diffusion into the aqueous medium, resulting in superior vesicular integrity. Moreover, the quaternary ammonium head group of DDAB imparted a strong cationic charge that not only stabilized the vesicular surface electrostatically but also improved the organization of the amphiphilic molecules within the lipid phase (Nemr et al., 2025). These combined hydrophobic and electrostatic contributions established a rigid yet cohesive bilayer structure, favoring efficient encapsulation of the lipophilic FTN molecules (Chou et al., 2014; Cooper and Harirforoosh, 2014).
Similarly, the PC: PEG ratio (Factor C) exhibited a positive and statistically significant impact on EE%. Formulations containing higher proportions of phosphatidylcholine (PC) exhibited greater %EE, possibly due to the naturally high phase transition temperature (Tc) of lecithin (Ahmed et al., 2025f). This characteristic reduces bilayer fluidity and permeability, thereby minimizing drug leakage and effectively preserving the encapsulated cargo (Emad Eldeeb et al., 2019; Sayed et al., 2020). In addition, the amphiphilic nature of PC, with hydrophobic tails forming the core and hydrophilic phosphate head groups aligning toward the aqueous interface, provides a stable, cohesive bilayer that accommodates lipophilic drugs efficiently (Abdelbary et al., 2017; Abdelbary and Aburahma, 2015). The strong hydrophobic interactions between PC and DDAB tails reinforce bilayer compactness, while hydrogen bonding between PC and PEG chains contributes to interfacial flexibility and steric stabilization. In summary, both DDAB and PC play synergistic roles in establishing a stable and hydrophobic bilayer architecture, while PEG enhances colloidal stability and prolongs vesicular lifespan.
3.1.2 Statistical evaluation of PS
Particle size (PS) plays a decisive role in determining the biological behavior, diffusion dynamics, and therapeutic efficiency of nanosystems intended for vaginal delivery. Maintaining an optimal nanoscale dimension ensures intimate contact with the mucosal surface, deeper tissue penetration, and improved drug retention within the target site (Ahmed et al., 2025a). In the present study, the particle size of the prepared DHCPPs ranged from 226.7 ± 3.04 to 256.1 ± 2.33 nm, confirming the successful fabrication of homogeneously dispersed vesicles with suitable dimensions for mucosal application. Statistical analysis revealed that factors A and B exerted significant negative effects (p < 0.05), indicating their prominent roles in governing vesicular size (Figure 1B). While factor C was non-significant (p > 0.05). The relationship between the independent variables and the observed PS values was expressed by the following regression equation:
The DDAB: drug ratio (Factor A) demonstrated a pronounced inverse relationship with PS, where increasing the DDAB proportion led to a reduction in vesicle diameter. This downsizing can be attributed to the strong stabilizing influence of DDAB, which enhances bilayer cohesion through its cationic quaternary ammonium head group and long hydrophobic alkyl chain. The resulting electrostatic repulsion between positively charged vesicles minimizes aggregation, promoting the formation of discrete, well-separated nanoparticles (Carbone et al., 2014). Moreover, DDAB’s inherent lipophilicity (log P ≈ 11.8) contributes to a more compact lipid arrangement, decreasing inter-vesicular fusion and yielding smaller, uniformly distributed particles. Similar findings were reported by Cooper et al., who observed that DDAB incorporation into celecoxib-loaded PLGA nanoparticles produced smaller, more stable systems with higher encapsulation efficiency (Cooper and Harirforoosh, 2014). Beyond size reduction, this phenomenon also supports improved drug loading, as smaller vesicles often possess greater surface area and tighter packing density, favoring retention of hydrophobic drugs within the bilayer.
The PEG: DDAB ratio (Factor B) also exhibited a significant negative effect on particle size, further reinforcing the crucial stabilizing role of PEGylation in vesicle formation. PEG reduces interfacial tension between the aqueous and lipid phases, facilitating vesicle curvature and spontaneous nanoscale assembly (Fahmy et al., 2025). Increasing PEG content leads to the development of a steric stabilization layer around the vesicles, effectively preventing coalescence and aggregation. This surface coating provides hydration repulsion forces that preserve vesicular individuality and sustain smaller particle dimensions (Zeb et al., 2016). At higher PEG ratios, this effect becomes more prominent, whereas at lower concentrations, incomplete surface coverage may lead to partial aggregation and consequently larger vesicles (Yousry et al., 2016). Comparable outcomes were reported by and Kim et al. (2009), and Wolfram et al. (2014), who observed that PEGylation markedly decreased the vesicular size of the formulated systems. Collectively, the synergistic contribution of DDAB and PEG results in a balance between electrostatic and steric stabilization forces that finely control vesicle size.
3.1.3 Statistical evaluation of PDI
The poly-dispersity index (PDI) serves as a critical indicator of nanoparticle uniformity, reflecting the degree of homogeneity in size distribution and, consequently, the reproducibility and stability of the formulation. In general, PDI values approaching zero signify a highly monodisperse system with uniform vesicle populations, whereas values nearing one indicate heterogeneous dispersions with wide size variability (Danaei et al., 2018). For pharmaceutical nanocarriers, PDI values below 0.5 are typically deemed acceptable, denoting well-controlled size distribution that supports consistent drug release, and stable behavior during storage and administration (Nemr et al., 2021).
In the present study, the prepared formulations demonstrated PDI values ranging between 0.20 ± 0.01 and 0.27 ± 0.03, confirming their narrow particle size distribution. Statistical analysis revealed that none of the investigated formulation factors (A, B, or C) exerted a statistically significant effect on PDI values (p > 0.05). Although the PDI model was thus excluded from the optimization criteria due to its lack of sensitivity to variable changes, the consistently favorable results underscore the robustness and reproducibility of the fabrication process.
3.1.4 Statistical evaluation of ZP
Zeta potential (ZP) is a fundamental indicator of the electrostatic stability of nanocarrier systems, as it reflects the degree of repulsion between dispersed particles and, consequently, their tendency to aggregate. Nanovesicles with surface charges exceeding ±20 mV are typically considered electrostatically stable, since strong repulsive forces prevent particle flocculation and ensure a well-dispersed colloidal system (El et al., 2023). In the present study, the formulated DHCPPs exhibited ZP values ranging from 45.50 ± 0.57 mV to 58.85 ± 2.47 mV (Table 2), confirming excellent electrostatic stability throughout the design space. As illustrated in Figure 1C, factor A (DDAB: drug ratio) and factor C (PC: PEG ratio), showed statistically significant positive effects (p < 0.05) on the zeta potential, indicating that increasing either factor enhanced the surface charge of the nanovesicles. While factor B was non-significant (p > 0.05). The statistical model describing the relationship between formulation factors and ZP was expressed by the following coded equation:
The positive impact of Factor A can be attributed to the intrinsic cationic nature of DDAB. The quaternary ammonium head groups of DDAB contribute to the generation of a stronger positive surface potential as their proportion increases. This effect arises from the higher density of positively charged sites on the vesicular surface, which enhances electrostatic repulsion between particles, minimizing aggregation and improving overall dispersion stability (Albash et al., 2021; Nemr et al., 2025). Moreover, the presence of DDAB promotes ordered molecular packing within the bilayer structure, strengthening interfacial organization and contributing to enhanced vesicle rigidity and physical stability (Gossmann et al., 2015).
The significant positive effect of Factor C (PC: PEG ratio) can be explained by the amphiphilic and structural characteristics of PC. As PC concentration increases relative to PEG, the lipid bilayer becomes more compact and densely packed, leading to enhanced exposure of positively oriented head groups on the vesicle surface (Younes et al., 2024). This contributes to an increase in the overall surface potential, further stabilizing the formulation. A higher PC content also strengthens the bilayer’s mechanical integrity, reducing the risk of vesicle fusion and drug leakage (Ahmed et al., 2025f). Although PEGylation may provide steric stabilization by creating a hydration shell around vesicles, it does not directly affect electrostatic interactions in this system.
3.2 Selection of optimum DHCPP
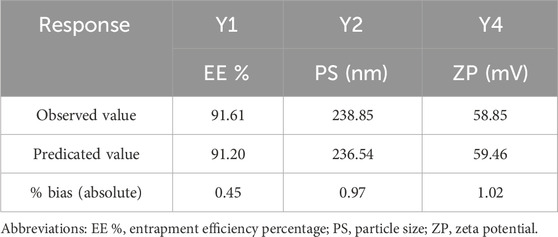
Optimization of the developed DHCPPs was carried out using Design-Expert® software (version 13, Stat-Ease Inc., Minneapolis, USA). The software employed a multi-response desirability approach, integrating all critical formulation responses into a single composite index to identify the most favorable formulation conditions (Helal et al., 2024). Among the evaluated responses, encapsulation efficiency (EE%), particle size (PS), and zeta potential (ZP) were chosen as the key quality attributes, as they collectively determine the vesicular integrity, stability, and drug delivery efficiency. The poly-dispersity index (PDI) was excluded from the optimization model due to its consistently low values, confirming homogeneity of vesicle distribution across all experimental runs. Based on the software-generated model, the optimal formulation was identified at the factor levels of DDAB: drug ratio (A) = 5:1, PEG: DDAB ratio (B) = 1.5:1, and PC: PEG ratio (C) = 2:1, achieving an overall desirability score of 0.931.
The optimized formulation exhibited experimental values in close agreement with the predicted ones, confirming the validity and reliability of the model (Table 4) (Ahmed et al., 2025e). The final formulation displayed an encapsulation efficiency of 91.61% ± 1.98%, a mean particle size of 238.85 ± 3.46 nm, a PDI of 0.22 ± 0.01 and a zeta potential of +58.85 ± 2.47 mV, indicating the formation of stable, nano-sized vesicles with strong electrostatic repulsion and efficient drug retention. The high positive surface potential further suggested excellent colloidal stability, minimizing aggregation and ensuring prolonged mucosal residence. Therefore, the aforementioned formulation was further evaluated to assess its efficacy for the underlying treatment condition.
3.3 Characterization of the optimum DHCPP
3.3.1 TEM visualization
TEM was employed to visualize the morphological characteristics of the optimized DHCPP. The obtained micrograph (Figure 2) revealed well-defined, spherical vesicles with smooth surfaces and uniform size distribution, confirming the successful formation of nanoscale DHCPP. The vesicles appeared distinctly separated and non-agglomerated, reflecting effective stabilization by the incorporated PEG, DDAB and phosphatidylcholine components, which provided both steric and electrostatic repulsion (Fahmy et al., 2025; Tawfik et al., 2025).
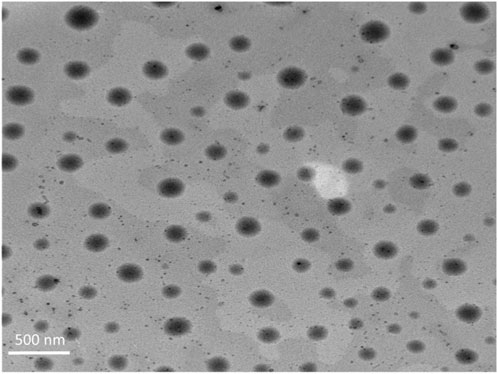
Figure 2. TEM images of the optimum formula showing the spherical vesicles, lacking any sort of aggregation.
3.3.2 FT-IR analysis
The effect of lyophilization on the optimized formulation was evaluated by comparing particle size and poly-dispersity index (PDI) before and after freeze-drying. Statistical analysis demonstrated no significant difference in particle size or PDI (p > 0.05), indicating that the lyophilization process did not adversely affect the nanoscale characteristics or size uniformity of the formulation.
The FTIR spectra of the individual components and the optimized DHCPP are presented in Figure 3.
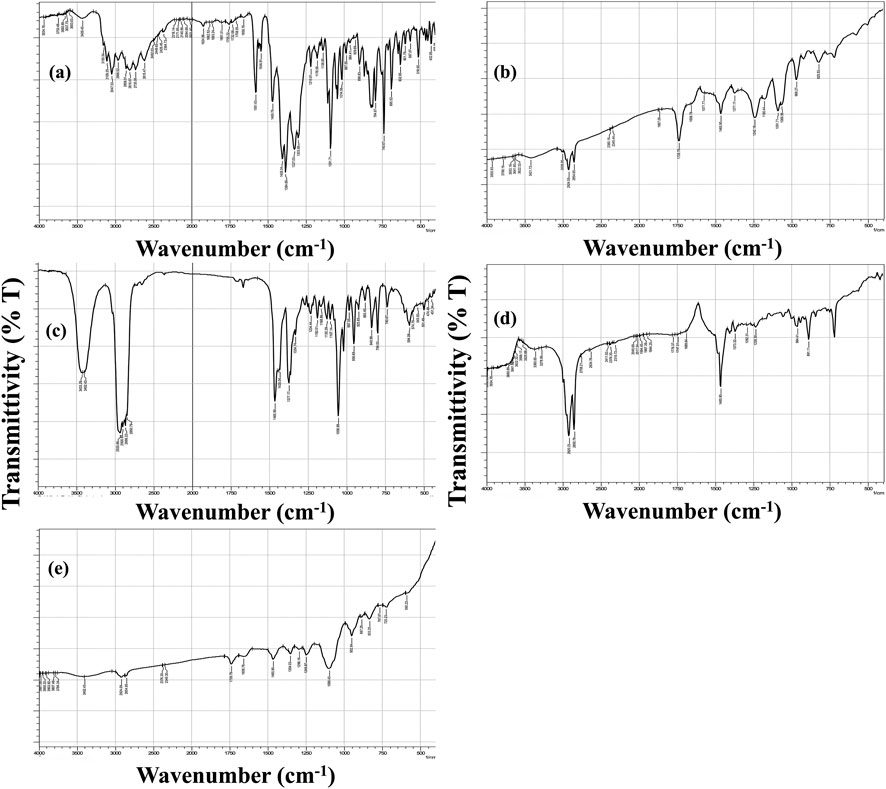
Figure 3. FT-IR spectra of (a) fenticonazole, (b) phosphatidylcholine, (c) cholesterol, (d) didodecyldimethylammonium bromide (DDAB), and (e) the optimum DHCPP.
The spectrum of pure FTN (Figure 3a) exhibited distinct and sharp absorption bands characteristic of its molecular structure. The prominent peak observed at 3047 cm-1 corresponds to N–H stretching vibrations, while the band at 1581 cm-1 is attributed to the stretching of the imine (C=N) group. Additional peaks appearing at 1469 cm-1 and 1091 cm-1 were assigned to C=C stretching and aromatic ring vibrations, respectively, consistent with previously reported findings (Ahmed et al., 2022; Castro et al., 2016).
The FTIR spectrum of phosphatidylcholine (PC) (Figure 3b) closely matched the literature profile (Ahmed et al., 2025f). A broad absorption band at 3421 cm-1 represented the O–H stretching of the carboxylic group, whereas a strong signal at 1739 cm-1 confirmed the presence of ester carbonyl (C=O) stretching. Furthermore, the peaks at 2,924 cm-1 and 2,854 cm-1 corresponded to the asymmetric and symmetric stretching vibrations of aliphatic C–H bonds, respectively, while the band at 1465 cm-1 was linked to aromatic ring vibrations (Younes et al., 2024). Similarly, the cholesterol (CH) spectrum (Figure 3c) displayed a broad peak near 3433 cm-1, indicative of the phenolic O–H stretching, accompanied by two overlapping bands at 2,935 cm-1 and 2,900 cm-1 corresponding to C–H stretching vibrations (Romano et al., 2024). The FTIR spectrum of DDAB (Figure 3d) displayed characteristic vibrational bands corresponding to its aliphatic structure. Prominent methylene-associated peaks appeared at 721, 886, and 1467 cm-1, while the symmetric and asymmetric stretching vibrations of C–H bonds were clearly observed at approximately 2,852 and 2,919 cm-1, respectively (Mishra et al., 2017). In contrast, the FTIR spectrum of the optimized DHCPP (Figure 3e) demonstrated significant attenuation of FTN’s distinctive peaks. The marked reduction in the intensity of characteristic FTN absorption bands suggests that the drug was successfully encapsulated within the proniosomal bilayer rather than existing in its crystalline form (Sharma et al., 2023).
3.3.3 In-vitro release study
The in-vitro release profiles of the optimized DHCPP and the FTN suspension are illustrated in Figure 4A. The optimized formulation exhibited a distinctive biphasic release pattern, typical of proniosomal systems (Alshora et al., 2023; Darson et al., 2023). An initial burst phase was observed within the first 2 hours, attributed to the rapid diffusion of surface-associated FTN molecules, followed by a sustained release phase extending throughout the remainder of the study. This sustained profile reflects the gradual diffusion of the drug from the inner lipidic core of the vesicles, demonstrating the ability of the proniosomal matrix to control and prolong drug release (Younes et al., 2024).
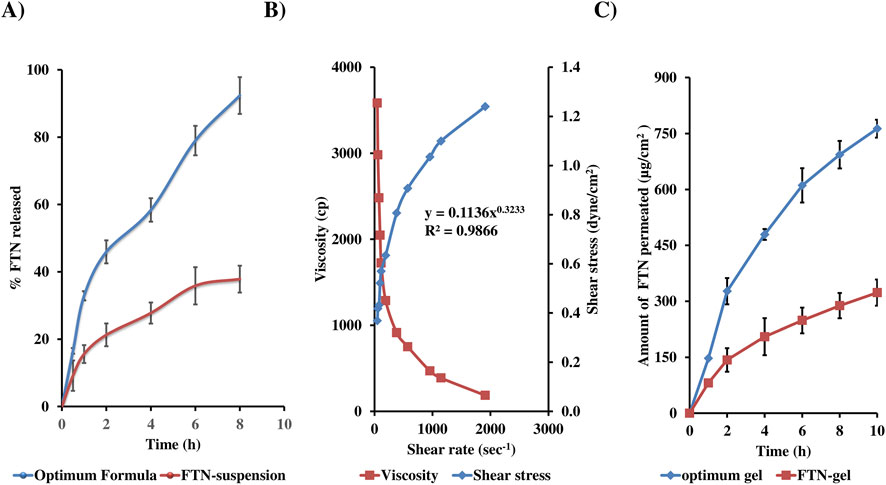
Figure 4. In vitro release (A), rheological characteristics (B) and ex-vivo permeation (C) profiles from optimum formula, demonstrating biphasic drug release, pseudo-plastic (shear-thinning) rheology, and enhanced vaginal permeation.
Quantitatively, the optimized DHCPP achieved a markedly higher release compared to the FTN suspension, with Q2h = 45.93 ± 3.41% versus 21.27% ± 3.37%, and Q8h = 92.36 ± 5.46% versus 37.82% ± 3.99%, respectively. This pronounced enhancement can be attributed to the improved solubilization capacity and nanoscale encapsulation within the proniosomal system, which increases the apparent solubility and diffusion rate of the otherwise poorly water-soluble FTN (solubility <0.1 mg/mL) (Khudair et al., 2020). Kinetic modeling of the release data revealed that the profile best fitted the Higuchi diffusion model (R2 = 0.987), confirming that FTN release from DHCPP was predominantly diffusion-controlled. These findings substantiate the formulation’s capacity to provide an initial therapeutic burst followed by a sustained, controlled release that is required for enhancing and maintaining prolonged antifungal efficacy at the vaginal site.
3.3.4 Mucoadhesion study
The mucoadhesive potential of the optimized DHCPP was thoroughly investigated to evaluate its ability to interact with and remain attached to the vaginal mucosal surface to ensure localized, prolonged antifungal action. Since vaginal mucosa is rich in negatively charged mucin glycoproteins, its interaction with the cationic DHCPP vesicles was examined using mucin dispersion as a biological mimic of the mucosal environment (Ahmed et al., 2025d). Upon mixing the DHCPP formulation with mucin, a marked reduction in zeta potential was observed, signifying the formation of electrostatic complexes between the positively charged vesicles and the negatively charged mucin chains. This interaction confirms the establishment of strong electrostatic bonding forces, leading to the adsorption of mucin onto the vesicular surface (Tawfik et al., 2025). The high positive charge imparted by DDAB, combined with the stabilizing PEG coating, not only strengthened these electrostatic attractions but also created a hydrated interfacial layer that enhances mechanical adhesion (Ahmed et al., 2025b).
3.3.5 Short term stability study
The short-term stability of the optimized DHCPP was systematically assessed to ensure the preservation of its physicochemical and functional integrity during storage. The formulation was stored under refrigerated conditions (4 °C ± 2 °C) for a period of 3 months. Post-storage analysis revealed EE% of 89.21% ± 0.67%, PS of 237.75 ± 3.32 nm, and ZP of 51.35 ± 4.45 mV, which were statistically comparable (p > 0.05) to those of the freshly prepared sample (EE% = 91.83 ± 1.11%, PS = 234.55 ± 3.61 nm, ZP = 56.9 ± 2.4 mV). The negligible variations indicate excellent formulation stability, with minimal drug leakage, aggregation, or vesicle coalescence during storage (Hegazy et al., 2025). Such stability can be primarily attributed to the protective effect of PEGylation, which provides a steric barrier on the vesicular surface, reducing particle–particle interaction and preventing aggregation (El-Naggar et al., 2026). Additionally, the cationic nature of DDAB reinforces electrostatic repulsion, contributing to the formulation’s resistance against structural destabilization and fusion over time (Nemr et al., 2025).
Furthermore, a comparative evaluation of the in-vitro release profiles between the fresh and stored samples yielded an f2 similarity factor of 73.73, confirming the in-vitro equivalence. The absence of significant differences in Q8h values (87.40% ± 4.32% vs. 92.36% ± 5.46%) further corroborates that storage did not adversely affect the drug release kinetics or diffusion behavior (Diaz et al., 2016).
3.4 Microbiological assay
3.4.1 Determination of MIC
The MIC assay was performed using the broth microdilution technique to quantitatively assess the antifungal potency of the optimized DHCPP and its plain suspension against C. albicans. This evaluation aimed to confirm whether the DHCPP enhanced the antifungal efficacy of FTN compared to its plain suspension. Remarkably, the optimized DHCPP demonstrated a MIC value of 0.244 μg/mL, which was approximately 64-fold lower than that of the FTN suspension (15.6 μg/mL). This pronounced improvement underscores the significant potentiation of antifungal activity achieved through nanoscale encapsulation. The enhanced performance can be attributed to multiple synergistic mechanisms: first, the nano-sized vesicular structure increases the surface area available for fungal cell interaction; second, the positive surface charge promotes electrostatic attraction with the negatively charged fungal cell wall, thereby facilitating enhanced adhesion and membrane permeation; and third, the PEGylated bilayer composition ensures greater stability and prolonged drug availability at the infection site, enabling sustained antifungal action.
Furthermore, the improved drug solubilization and dispersion within the vesicular matrix likely increased FTN’s effective concentration at the fungal cell interface, leading to better inhibition of ergosterol biosynthesis and disruption of membrane integrity. These findings collectively confirm that the optimized DHCPP not only enhances drug delivery efficiency but also amplifies antifungal efficacy, positioning it as a promising therapeutic strategy for the management of vaginal candidiasis, especially in resistant or recurrent cases.
3.4.2 Determination of MFC
Similarly, the MFC values further reinforced these findings. Both the optimized DHCPP and the FTN suspension exhibited fungicidal activity after 48 h of incubation at 28 °C ± 2 °C; however, the optimized DHCPP displayed a significantly lower MFC (1.953 μg/mL) compared to the drug suspension (62.5 μg/mL). This notable difference reflects the improved fungicidal capability of the nanosystem, which can be linked to its enhanced interaction with fungal membranes and more efficient drug delivery to intracellular targets responsible for cell death.
3.4.3 Determination of biofilm inhibitory effect
The crystal violet staining method was employed to assess and compare the biofilm inhibition potential of the optimized DHCPP and the FTN suspension. This assay was conducted using C. albicans as a representative fungal strain, focusing on sub-inhibitory concentrations corresponding to 1/16, 1/8, ¼, and ½ X, where X denotes the previously determined MIC value. As illustrated in Figure 5, the optimized DHCPP exhibited a remarkably stronger anti-biofilm effect compared to the free drug suspension, particularly at 1/8, ¼, and ½ MIC concentrations (p < 0.05). These results clearly demonstrate the enhanced ability of the optimized DHCPP to prevent biofilm formation at concentrations below the minimal inhibitory threshold. The pronounced improvement in biofilm inhibition may be attributed to the superior mucoadhesive characteristics and nanoscale architecture of the proniosomal system, which facilitate intimate interaction with the fungal surface and prolonged drug retention within the biofilm microenvironment. Moreover, the cationic nature of DDAB promotes electrostatic binding to the negatively charged fungal cell membrane, leading to disruption of the biofilm matrix and hindering fungal adhesion and colonization. At the lowest tested concentration (1/16 MIC), no statistically significant difference (p > 0.05) was observed, indicating that at extremely low drug levels, the available concentration of active molecules might be insufficient to achieve effective biofilm disruption.
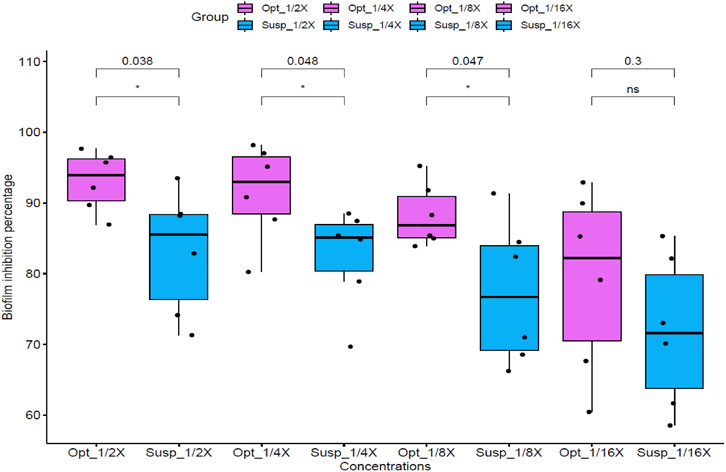
Figure 5. Anti-biofilm activity of optimized formula (Opt) and FTN suspension (Susp) against Candida albicans standard strain ATCC 60193 at different concentrations (1/16, 1/8, ¼, and ½ X, where X is the calculated MIC). Optimized formula concentrations are as follows: 1/16 X = 0.015 μg/mL, 1/8 X = 0.0305 μg/mL, 1/4 X = 0.061 μg/mL, 1/2 X = 0.122 μg/mL. FTN suspension concentrations are as follows: 1/16 X = 0.975 μg/mL, 1/8 X = 1.95 μg/mL, 1/4 X = 3.9 μg/mL, 1/2 X = 7.8 μg/mL. Statistical significance was assessed, within each concentration between optimized formula and FTN suspension, by Student’s t-test, and all p values are shown.
3.5 Development and characterization of optimum DHCPP gel
3.5.1 Rheological study
Looking at the rheogram in Figure 4B, it is evident that as the shear rate increased, a fall in viscosity was noticed, suggestive of a shear-thinning behavior. This shear-thinning characteristic is indicative of non-newtonian flow behavior, confirmed by the power law index (n) of 0.3233 (Ansari et al., 2020). Such flow property is not only suitable for easy application of the gel within the vaginal cavity, but also with maintaining the gel in-situ to ensure prolonged drug release. Moreover, based on the R2 of the investigated models, the optimum DHCPP gel was found to follow Carreau’s model.
3.5.2 pH measurement
The measured pH of the prepared gel was 4.21 ± 0.24, placing it comfortably within the physiological vaginal range of 4.0–4.5. Maintaining this acidic environment is crucial, as it aligns with the natural vaginal pH, thereby supporting mucosal compatibility while minimizing the risk of irritation, inflammation, or microbial imbalance. Such a pH ensures the formulation’s suitability for safe and well-tolerated vaginal application (El-Naggar et al., 2026).
3.6 Animal studies
3.6.1 Ex-vivo permeation study
During the ex vivo permeation study, a lag time of approximately 1 h was observed before the onset of drug permeation. Several findings can be interpreted from Figure 4C, illustrating the cumulative permeation profiles of the DHCPP gel and FTN suspension. Evidently, an increase in the amount of FTN permeated can be seen from the DHCPP gel as compared to the control FTN gel (763.1
3.6.2 Confocal laser scanning microscopy (CLSM)
To further consolidate the findings of ex-vivo permeation, CLSM was carried. The microscopy images in Figure 6 indicate the higher penetrability of RhB from the prepared DHCPP gel (240
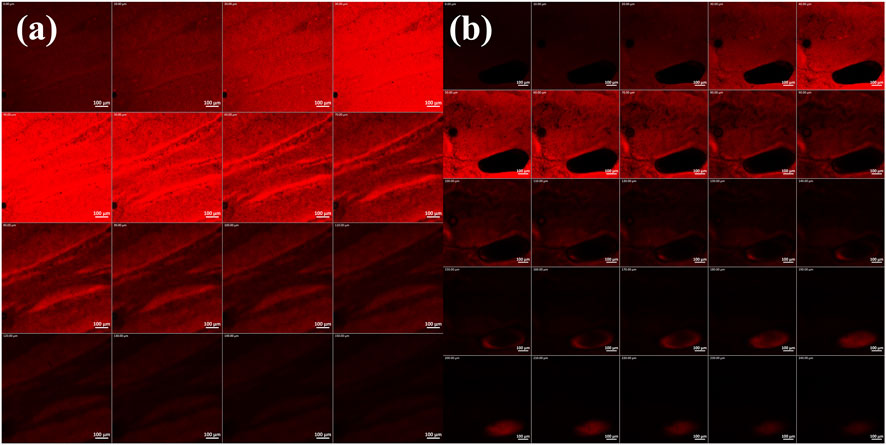
Figure 6. Confocal laser scanning microscopic images of (a) rhodamine B aqueous solution and (b) Rhodamine B-loaded DHCPP gel. The images suggest the deeper penetration of the DHCPP gel.
3.6.3 Histopathological assessment
Consistent with the previously observed pH findings, histological evaluation using hematoxylin and eosin staining (Figure 7) revealed comparable results between the treatment (DHCPP gel) and control groups. The control group (Figure 7A) displayed normal vaginal mucosal architecture, characterized by healthy epithelial cells, well-differentiated epithelium, and intact connective tissue beneath. Similarly, the treatment group (Figure 7B) exhibited a histological appearance closely resembling that of the control, with no noticeable tissue alterations. These findings, in harmony with the pH observed, further support the biocompatibility and safety of the applied DHCPP gel.
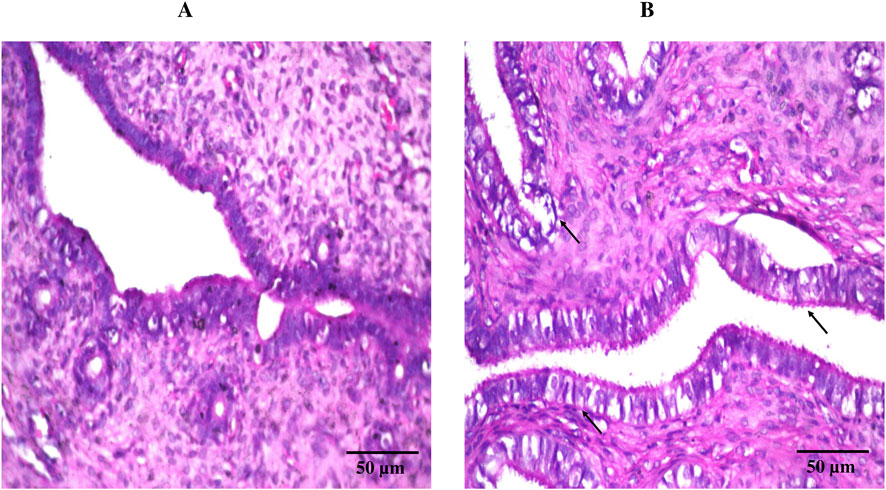
Figure 7. Histopathological images of (A) control group (left untreated) and (B) treatment group (DHCPP gel), showing lack of alteration in normal tissue structure in both groups following staining with hematoxylin and eosin.
4 Limitation of the study
Although the current animal study provided valuable insights, inclusion of a larger number of animals could have further increased the statistical power of the findings. However, the study intentionally employed a minimal number of animals to adhere to the 3R principles of animal research (reduce, reuse, and recycle), ensuring ethical compliance while maintaining reliable and reproducible results.
5 Conclusion
The present investigation successfully introduced and optimized Dual-Functional Hybrid Cationic PEGylated Proniosomes (DHCPP) as an innovative nano-platform for the localized management of vaginal candidiasis. The strategic integration of PEGylation and cationic components within a hybrid proniosomal matrix conferred synergistic advantages, resulting in enhanced physicochemical stability, efficient mucosal interaction, and improved biological performance. The optimized formulation, selected using a factorial design with a high desirability value (0.931), exhibited superior encapsulation efficiency, nanoscale particle size, and a highly positive zeta potential, collectively ensuring colloidal stability and effective interaction with the negatively charged vaginal mucosa.
Comprehensive in vitro, ex vivo, microbiological, and in vivo evaluations confirmed the multifunctional nature of the DHCPP system. Enhanced mucoadhesion and pseudo-plastic gel behavior promoted prolonged vaginal residence and improved local drug retention, while diffusion-controlled drug release enabled sustained antifungal activity. FTIR analysis verified molecular compatibility and successful encapsulation of FTN. Notably, the significant enhancement in vaginal permeation, pronounced biofilm inhibition, and marked reductions in MIC and MFC values highlight the system’s ability to enhance antifungal efficacy and potentially reduce infection recurrence. Histopathological examination further confirmed the safety, tolerability, and biocompatibility of the optimized gel following repeated administration.
Importantly, the clinical relevance of the developed DHCPP system is underscored when compared with conventional topical FTN formulations. Beyond improved vaginal permeation, the DHCPP system offers clinically meaningful advantages, including enhanced mucoadhesion resulting in prolonged vaginal residence time, controlled and sustained drug release, and superior antifungal performance. These attributes may translate clinically into reduced dosing frequency, improved therapeutic outcomes, and enhanced patient compliance. Moreover, incorporation of the optimized DHCPP into a pH-compatible, non-irritant carbopol gel supports local safety and comfort, as confirmed by histopathological findings.
Collectively, these features highlight the feasibility, clinical applicability, and therapeutic utility of the developed DHCPP system, positioning it as a promising alternative to conventional FTN topical products for the effective management of vaginal candidiasis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Faculty of pharmacy, Ethical committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SA: Writing – review and editing, Investigation, Conceptualization, Resources, Formal Analysis. OS: Resources, Writing – review and editing, Conceptualization. HA: Writing – original draft, Investigation, Resources, Formal Analysis, Conceptualization. AF: Resources, Writing – review and editing, Conceptualization. IA: Conceptualization, Writing – original draft.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1746918/full#supplementary-material
References
Abdelbary, A. A., and AbouGhaly, M. H. (2015). Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: application of box-behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. Journal Pharmaceutics 485 (1-2), 235–243. doi:10.1016/j.ijpharm.2015.03.020
Abdelbary, G. A., and Aburahma, M. H. (2015). Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J. Liposome Res. 25 (2), 107–121. doi:10.3109/08982104.2014.941861
Abdelbary, A. A., Abd-Elsalam, W. H., and Al-Mahallawi, A. M. (2016). Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: in vitro characterization, ex vivo permeation and in vivo safety assessment. Int. Journal Pharmaceutics 513 (1-2), 688–696. doi:10.1016/j.ijpharm.2016.10.006
Abdelbary, G. A., Amin, M. M., and Zakaria, M. Y. (2017). Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 24 (1), 309–319. doi:10.1080/10717544.2016.1247928
Abdelhakeem, E., Nemr, A. A., Rashed, H. M., Selim, A. A., Essa, B. M., and Hegazy, D. (2025). Revitalizing itraconazole: unleashing its anticancer potential through oral nanosystems for liver targeting and biodistribution profiling in an animal model using radiolabeling technique. J. Drug Deliv. Sci. Technol. 104, 106463. doi:10.1016/j.jddst.2024.106463
Ahmadi, F., Saeedi, M., Akbari, J., Seyedabadi, M., Ebrahimnejad, P., Morteza-Semnani, K., et al. (2023). Nanohybrid based on (mn, zn) ferrite nanoparticles functionalized with chitosan and sodium alginate for loading of curcumin against human breast cancer cells. AAPS PharmSciTech 24 (8), 222. doi:10.1208/s12249-023-02683-9
Ahmed, S., Amin, M. M., El-Korany, S. M., and Sayed, S. (2022). Pronounced capping effect of olaminosomes as nanostructured platforms in ocular candidiasis management. Drug Deliv. 29 (1), 2945–2958. doi:10.1080/10717544.2022.2120926
Ahmed, S., Amin, M. M., and Sayed, S. (2023). A comprehensive review on recent nanosystems for enhancing antifungal activity of fenticonazole nitrate from different routes of administration. Drug Deliv. 30 (1), 2179129. doi:10.1080/10717544.2023.2179129
Ahmed, S., Aziz, D. E., Sadek, M. A., and Tawfik, M. A. (2024a). Capped flexosomes for prominent anti-inflammatory activity: development, optimization, and ex vivo and in vivo assessments. Drug Deliv. Transl. Res. 14, 2474–2487. doi:10.1007/s13346-024-01522-z
Ahmed, S., Attia, H., Saher, O., and Fahmy, A. M. (2024b). Augmented glycerosomes as a promising approach against fungal ear infection: optimization and microbiological, ex vivo and in vivo assessments. Int. J. Pharm. 8, 100295. doi:10.1016/j.ijpx.2024.100295
Ahmed, S., Mehana, D., Attia, H., and El-Ashmoony, M. M. (2025). Terpene-enhanced olaminogel for superior vaginal permeation: robust assessment through in vitro, microbiological, ex vivo, and in vivo evaluations. Naunyn-Schmiedeberg's Archives Pharmacol. doi:10.1007/s00210-025-04838-w
Ahmed, S., Saher, O., Attia, H., Fahmy, A. M., and Adel, I. M. (2025a). Development and characterization of fenticonazole nitrate-loaded cubogel for the management of vaginal candidiasis. Int. J. Pharm. X 10, 100355. doi:10.1016/j.ijpx.2025.100355
Ahmed, S., Mehana, D., Attia, H., and El-Ashmoony, M. M. (2025b). Engineered terconazole-loaded flexogel for targeted vaginal delivery: In-Depth analysis using in-vitro,, microbiological, Ex-Vivo and in-vivo methodologies. J. Drug Deliv. Sci. Technol. 111, 107192. doi:10.1016/j.jddst.2025.107192
Ahmed, S., Farag, M. M., Attia, H., Balkhi, B., Adel, I. M., and Nemr, A. A. (2025c). Terconazole loaded edge-activated hybrid elastosome for revamped corneal permeation in ocular mycosis: in-vitro characterization, statistical optimization, microbiological assessment, and in-vivo evaluation. Int. J. Pharm. 9, 100333. doi:10.1016/j.ijpx.2025.100333
Ahmed, S., Attia, H., and Saher, O. (2025d). Novel hybrid tri-polymer hyalurosomes: unlocking next-generation trans-tympanic therapeutics through multi-scale evaluations. Int. J. Pharm. X, 100425. doi:10.1016/j.ijpx.2025.100425
Ahmed, S., Ibrahim, M. M., Balkhi, B., Attia, H., and Aziz, D. E. (2025e). From bench to biology: unraveling the efficiency of novel brijosomes for trans-tympanic drug delivery. J. Drug Deliv. Sci. Technol. 114, 107493. doi:10.1016/j.jddst.2025.107493
Ahmed, S., Farag, M. M., Attia, H., Balkhi, B., Adel, I. M., and Nemr, A. A. (2025f). Exploring the potential of antifungal-loaded proniosomes to consolidate corneal permeation in fungal keratitis: a comprehensive investigation from laboratory characterization to microbiological evaluation. Int. J. Pharm. X 9, 100322. doi:10.1016/j.ijpx.2025.100322
Ahmed, S., Fayez, A., Attia, H., and El-Setouhy, D. A. (2025g). Terpene-augmented novasomal carriers for trans-tympanic drug delivery: a comprehensive optimization and in vivo evaluation. Naunyn-Schmiedeberg's Archives of Pharmacology. doi:10.1007/s00210-025-04659-x
Ahmed, S., Farag, M. M., Sadek, M. A., and Aziz, D. E. (2025h). Transdermal application of diacerin loaded-terpene enriched invasomes: an approach to augment anti-edema and nociception inhibition activity. J. Liposome Res. 35 (1), 1–14. doi:10.1080/08982104.2024.2382974
Ahmed, S., Saher, O., ElBishbishy, R. M., and Ibrahim, M. M. (2025i). Revolutionary hyaluronic acid-modified edge-activated spanlastics as a novel approach to boost Hepatoprotective activity of Curcumin: Optimization, biochemical analysis and in-vivo assessment. Int. J. Pharm. 10, 100430. doi:10.1016/j.ijpx.2025.100430
Al-Mahallawi, A. M., Khowessah, O. M., and Shoukri, R. A. (2017). Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. Journal Pharmaceutics 522 (1-2), 157–164. doi:10.1016/j.ijpharm.2017.03.005
Albash, R., Elmahboub, Y., Baraka, K., Abdellatif, M. M., and Alaa-Eldin, A. A. (2020). Ultra-deformable liposomes containing terpenes (terpesomes) loaded fenticonazole nitrate for treatment of vaginal candidiasis: Box-Behnken design optimization, comparative ex vivo and in vivo studies. Drug Deliv. 27 (1), 1514–1523. doi:10.1080/10717544.2020.1837295
Albash, R., M, M. A., Hassan, M., and N, M. B. (2021). Tailoring terpesomes and leciplex for the effective ocular conveyance of Moxifloxacin hydrochloride (comparative assessment): in-vitro,, Ex-vivo, and in-vivo evaluation. Int. J. Nanomedicine 16, 5247–5263. doi:10.2147/IJN.S316326
Albash, R., Hassan, M., Agiba, A. M., Mohamed, H. W., Hassan, M. S., Ali, R. M., et al. (2025a). Advanced vaginal nanodelivery of losartan potassium via PEGylated zein nanoparticles for Methicillin-Resistant Staphylococcus aureus. Pharmaceutics 17 (10), 1344. doi:10.3390/pharmaceutics17101344
Albash, R., Fahmy, A. M., Shamsel-Din, H. A., Ibrahim, A. B., Bogari, H. A., Malatani, R. T., et al. (2025b). Intranasal propranolol hydrochloride-loaded PLGA-lipid hybrid nanoparticles for brain targeting: optimization and biodistribution study by radiobiological evaluation. Eur. J. Pharm. Sci. 208, 107061. doi:10.1016/j.ejps.2025.107061
Albash, R., Yousry, C., El Hassab, M. A., Eldehna, W. M., and Alaa-Eldin, A. A. (2025c). Investigation of Moxifloxacin-loaded terpenes enriched cationic cerosomes (TECs) as an adjunct pulmonary therapy for COVID-19: in-silico study; D-optimal optimization; aerodynamic simulation assessment and cytotoxic evaluation. J. Drug Deliv. Sci. Technol. 106, 106683. doi:10.1016/j.jddst.2025.106683
Alshora, D., Ibrahim, M., Alanazi, N., Alowyid, M., Ali Alnakhli, Z., Mohammed Alshiban, N., et al. (2023). Formulation of Glibenclamide proniosomes for oral administration: pharmaceutical and pharmacodynamics evaluation. Saudi Pharm. J. 31 (12), 101830. doi:10.1016/j.jsps.2023.101830
Ansari, S., Rashid, M. A. I., Waghmare, P. R., and Nobes, D. S. (2020). Measurement of the flow behavior index of Newtonian and shear-thinning fluids via analysis of the flow velocity characteristics in a mini-channel. SN Appl. Sci. 2 (11), 1787. doi:10.1007/s42452-020-03561-w
Bazaz, S., Lehto, T, Tops, R., Gissberg, O., Gupta, D., and Bestas, B. (2021). Novel orthogonally hydrocarbon-modified cell-penetrating peptide nanoparticles mediate efficient delivery of splice-switching antisense oligonucleotides in vitro and in vivo. Biomedicines. 9 (8). doi:10.3390/biomedicines9081046
Bhosale, V. B., Koparde, A. A., and Thorat, V. M. (2025). Vulvovaginal candidiasis-an overview of current trends and the latest treatment strategies. Microb. Pathog. 200, 107359. doi:10.1016/j.micpath.2025.107359
Campelo, M. S., Melo, E. O., Arrais, S. P., Nascimento, FBSAd, Gramosa, N. V., Soares, S. A., et al. (2021). Clove essential oil encapsulated on nanocarrier based on polysaccharide: a strategy for the treatment of vaginal candidiasis. Colloids Surfaces A Physicochem. Eng. Aspects 610, 125732. doi:10.1016/j.colsurfa.2020.125732
Carbone, C., Tomasello, B., Ruozi, B., Renis, M., and Puglisi, G. (2012). Preparation and optimization of PIT solid lipid nanoparticles via statistical factorial design. Eur. J. Med. Chem. 49, 110–117. doi:10.1016/j.ejmech.2012.01.001
Carbone, C., Campisi, A., Manno, D., Serra, A., Spatuzza, M., Musumeci, T., et al. (2014). The critical role of didodecyldimethylammonium bromide on physico-chemical, technological and biological properties of NLC. Colloids Surf. B Biointerfaces 121, 1–10. doi:10.1016/j.colsurfb.2014.05.024
Castro, G., Roca, M., Rodriguez, I., Ramil, M., and Cela, R. (2016). Identification and determination of chlorinated azoles in sludge using liquid chromatography quadrupole time-of-flight and triple quadrupole mass spectrometry platforms. J. Chromatogr. A 1476, 69–76. doi:10.1016/j.chroma.2016.11.020
Chassot, F., Negri, M. F., Svidzinski, A. E., Donatti, L., Peralta, R. M., Svidzinski, T. I., et al. (2008). Can intrauterine contraceptive devices be a Candida albicans reservoir? Contraception 77 (5), 355–359. doi:10.1016/j.contraception.2008.01.007
Chou, T. H., Liang, C. H., Lee, Y. C., and Yeh, L. H. (2014). Effects of lipid composition on physicochemical characteristics and cytotoxicity of vesicles composed of cationic and anionic dialkyl lipids. Phys. Chem. Chem. Phys. 16 (4), 1545–1553. doi:10.1039/c3cp54176b
Cooper, D. L., and Harirforoosh, S. (2014). Effect of formulation variables on preparation of celecoxib loaded polylactide-co-glycolide nanoparticles. PLoS One 9 (12), e113558. doi:10.1371/journal.pone.0113558
Danaei, M., Dehghankhold, M., Ataei, S., Hasanzadeh Davarani, F., Javanmard, R., Dokhani, A., et al. (2018). Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10 (2), 57. doi:10.3390/pharmaceutics10020057
Darson, J., Thirunellai Seshadri, R., Katariya, K., Mohan, M., Srinivas Kamath, M., Etyala, M. A., et al. (2023). Design development and optimisation of multifunctional Doxorubicin-loaded Indocynanine Green proniosomal gel derived niosomes for tumour management. Sci. Rep. 13 (1), 1697. doi:10.1038/s41598-023-28891-8
Diaz, D. A., Colgan, S. T., Langer, C. S., Bandi, N. T., Likar, M. D., and Van Alstine, L. (2016). Dissolution similarity requirements: how similar or dissimilar are the global regulatory expectations? AAPS J. 18 (1), 15–22. doi:10.1208/s12248-015-9830-9
El, T. M. M., Tawfik, M. A., Soliman, K., Khattab, M. S., and Farag, M. M. (2023). Tailoring of topically applied curcumin loaded pro-novasomes for skin cancer treatment: in-vitro characterization, statistical optimization and histopathological assessment of subcutaneous Ehrlich carcinoma mice model. J. Drug Deliv. Sci. Technol. 88, 104957. doi:10.1016/j.jddst.2023.104957
El Hassab, M. A., Ibrahim, M. H., Abdel, M. S. S., Mahmoud, A. M. A., Othman Ahmed, Z. S., Mosallam, S., et al. (2025). Formulation of zein nanoparticles for augmenting the anti-inflammatory activity of dexketoprofen. Front. Pharmacol. 16, 1560585. doi:10.3389/fphar.2025.1560585
El-Naggar, M. M., El-Nabarawi, M. A., Hassan, M., Hamed, M. I. A., Elrashedy, A. A., Teaima, M. H., et al. (2026). PEG-decorated emulsomal gel loaded levocetirizine dihydrochloride as repurposed cure for effective management of methicillin-resistant Staphylococcus aureus (MRSA) vaginal infection: D-optimal design optimization, ex-vivo, in-silico,, and in-vivo studies. J. Drug Deliv. Sci. Technol. 115, 107680. doi:10.1016/j.jddst.2025.107680
Elgendy, H. A., Makky, A. M. A., Elakkad, Y. E., Ismail, R. M., and Younes, N. F. (2023). Syringeable atorvastatin loaded eugenol enriched PEGylated cubosomes in-situ gel for the intra-pocket treatment of periodontitis: statistical optimization and clinical assessment. Drug Deliv. 30 (1), 2162159. doi:10.1080/10717544.2022.2162159
Elgendy, H. A., Makky, A. M. A., Elakkad, Y. E., Awad, H. H., Hassab, M. A. E., and Younes, N. F. (2024). Atorvastatin loaded lecithin-coated zein nanoparticles based thermogel for the intra-articular management of osteoarthritis: in-silico,, in-vitro,, and in-vivo studies. J. Pharm. Investigation 54, 497–518. doi:10.1007/s40005-024-00666-x
Elmahboub, Y., Albash, R., Ahmed, S., and Salah, S. (2025). The road to precision nanomedicine: an Insight on drug repurposing and advances in nanoformulations for treatment of cancer. AAPS PharmSciTech 26 (8), 237. doi:10.1208/s12249-025-03233-1
ElMeshad, A. N., and Mohsen, A. M. (2016). Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 23 (7), 2115–2123. doi:10.3109/10717544.2014.942811
Emad Eldeeb, A., Salah, S., and Ghorab, M. (2019). Formulation and evaluation of cubosomes drug delivery system for treatment of glaucoma: Ex-vivo permeation and in-vivo pharmacodynamic study. J. Drug Deliv. Sci. Technol. 52, 236–247. doi:10.1016/j.jddst.2019.04.036
Fahmy, A. M., El-Setouhy, D. A., Ibrahim, A. B., Habib, B. A., Tayel, S. A., and Bayoumi, N. A. (2018). Penetration enhancer-containing spanlastics (PECSs) for transdermal delivery of haloperidol: in vitro characterization, ex vivo permeation and in vivo biodistribution studies. Drug Deliv. 25 (1), 12–22. doi:10.1080/10717544.2017.1410262
Fahmy, A. M., Balkhi, B., Sadek, M. A., ElBishbishy, R. M., and Ahmed, S. (2025). PEGylated terpesomes of curcumin for prominent hepatoprotective activity: fabrication, optimization, biochemical analysis and in vivo evaluation. J. Drug Deliv. Sci. Technol. 108, 106876. doi:10.1016/j.jddst.2025.106876
Farag, M. M., Abdelmalak, N. S., El Menshawe, S. F., Omara, A. S., and Hamad, D. S. (2025). Repurposing linagliptin-loaded novasomes as a neuroprotectant for Alzheimer's disease: in-vitro characterisation, statistical optimisation and ex-vivo permeation study. J. Microencapsul. 42 (5), 531–545. doi:10.1080/02652048.2025.2500542
Fouda, N. H., Abdelrehim, R. T., Hegazy, D. A., and Habib, B. A. (2018). Sustained ocular delivery of Dorzolamide-HCl via proniosomal gel formulation: in-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 25 (1), 1340–1349. doi:10.1080/10717544.2018.1477861
Gossmann, R., Langer, K., and Mulac, D. (2015). New perspective in the formulation and characterization of didodecyldimethylammonium bromide (DMAB) stabilized Poly(Lactic-co-Glycolic acid) (PLGA) nanoparticles. PLoS One 10 (7), e0127532. doi:10.1371/journal.pone.0127532
Habib, B. A., Sayed, S., and Elsayed, G. M. (2018). Enhanced transdermal delivery of ondansetron using nanovesicular systems: fabrication, characterization, optimization and ex-vivo permeation study-box-cox transformation practical example. Eur. J. Pharm. Sci. 115, 352–361. doi:10.1016/j.ejps.2018.01.044
Harasym, J., and Banas, K. (2024). Lecithin's roles in oleogelation. Gels 10 (3), 169. doi:10.3390/gels10030169
Hegazy, D., Fayed, N. M., Nour El-Din, H. T., Habib, B. A., and Abdelrehim, R. T. (2025). Optimized tazarotene transfersomes versus previously optimized tazarotene cubosomes: exploring their in vivo anti-acne activity in a Cutibacterium acnes inflammatory Murine model. BioNanoScience 15 (3), 410. doi:10.1007/s12668-025-02009-y
Helal, A. M., Yossef, M. M., Seif, I. K., Abd El-Salam, M., El Demellawy, M. A., Abdulmalek, S. A., et al. (2024). Nanostructured biloalbuminosomes loaded with berberine and berberrubine for alleviating heavy Metal-Induced male infertility in rats. Int. Journal Pharmaceutics 667 (Pt A), 124892. doi:10.1016/j.ijpharm.2024.124892
Helal, D. A., Osama, A., El-Nabarawi, M. A., Teaima, M. H., and Ibrahim Al-Samadi, I. E. (2025). Dual-action of clotrimazole loaded - nanosponges vaginal gel for spermicidal action and treatment of vaginal candidiasis: optimization, in-vitro,, ex-vivo, and in-vivo experiments. Int. Journal Pharmaceutics 670, 125193. doi:10.1016/j.ijpharm.2025.125193
Herdiana, Y., Febrina, E., Nurhasanah, S., Gozali, D., Elamin, K. M., and Wathoni, N. (2024). Drug loading in chitosan-based nanoparticles. Pharmaceutics 16 (8), 1043. doi:10.3390/pharmaceutics16081043
Humphries, R. M., Ambler, J., Mitchell, S. L., Castanheira, M., Dingle, T., Hindler, J. A., et al. (2018). CLSI Methods Development and Standardization Working Group best practices for evaluation of antimicrobial susceptibility tests. J. Clin. Microbiol. 56 (4), 1934–1937. doi:10.1128/JCM.01934-17
Khatoon, M., Shah, K. U., Din, F. U., Shah, S. U., Rehman, A. U., Dilawar, N., et al. (2017). Proniosomes derived niosomes: recent advancements in drug delivery and targeting. Drug Deliv. 24 (Suppl. 1), 56–69. doi:10.1080/10717544.2017.1384520
Khudair, N., Agouni, A., Elrayess, M. A., Najlah, M., Younes, H. M., and Elhissi, A. (2020). Letrozole-loaded nonionic surfactant vesicles prepared via a slurry-based proniosome technology: formulation development and characterization. J. Drug Deliv. Sci. Technol. 58, 101721. doi:10.1016/j.jddst.2020.101721
Kim, J. Y., Kim, J. K., Park, J. S., Byun, Y., and Kim, C. K. (2009). The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials 30 (29), 5751–5756. doi:10.1016/j.biomaterials.2009.07.021
Lawrence, A. G., Houang, E. T., Hiscock, E., Wells, M. B., Colli, E., and Scatigna, M. (1990). Single dose therapy of vaginal candidiasis: a comparative trial of fenticonazole vaginal ovules versus clotrimazole vaginal tablets. Curr. Med. Res. Opin. 12 (2), 114–120. doi:10.1185/03007999009110479
Megahed, M. A., El-Sawy, H. S., Reda, A. M., Abd-Allah, F. I., Abu Elyazid, S. K., Lila, A. E., et al. (2022). Effect of nanovesicular surface-functionalization via chitosan and/or PEGylation on cytotoxicity of tamoxifen in induced-breast cancer model. Life Sci. 307, 120908. doi:10.1016/j.lfs.2022.120908
Mishra, R., Mishra, S., Upadhyay, C., and Prakash, R. (2017). DDAB-Triggered, Size-Sorted, instant phase-switching of silver nanoparticles. ChemistrySelect 2 (10), 3028–3034. doi:10.1002/slct.201602012
Mohy, K., Elhabak, M., Elkasabgy, N. A., Fahmy, R. H., and Younes, N. F. (2026). Bilosomes-mediated trans-ocular delivery of azelastine hydrochloride for the treatment of allergic conjunctivitis: in vitro,, ex vivo and in vivo investigations. J. Drug Deliv. Sci. Technol. 115, 107673. doi:10.1016/j.jddst.2025.107673
Nemr, A. A., El-Mahrouk, G. M., and Badie, H. A. (2021). Development and evaluation of proniosomes to enhance the transdermal delivery of cilostazole and to ensure the safety of its application. Drug Dev. Ind. Pharm. 47 (3), 403–415. doi:10.1080/03639045.2021.1890111
Nemr, A. A., Ahmed, S., and Adel, I. M. (2024). Limonene-enriched ultra-structural cubosomes to augment ocular delivery of a poorly water soluble anti-fungal drug: fabrication, characterization, statistical optimization, in vivo corneal uptake and histopathological evaluation in rabbits. J. Drug Deliv. Sci. Technol. 98, 105886. doi:10.1016/j.jddst.2024.105886
Nemr, A. A., Farag, M. M., Hegazy, D., Attia, H., and Abdelhakeem, E. (2025). Visionary NanoBoost: revolutionizing ocular treatment with positively charged Leciplex for enhanced Fenticonazole nitrate ocular delivery. J. Drug Deliv. Sci. Technol. 114, 107477. doi:10.1016/j.jddst.2025.107477
Picheta, N., Piekarz, J., Burdan, O., Satora, M., Tarkowski, R., and Kulak, K. (2024). Phytotherapy of vulvovaginal candidiasis: a narrative review. Int. J. Mol. Sci. 25 (7), 3796. doi:10.3390/ijms25073796
Racine, J. S. (2012). RStudio: a platform-independent IDE for R and sweave. J. Appl. Econ. Chichester. Engl. 27 (1), 167–172. doi:10.1002/jae.1278
Romano, E., Cataldo, P. G., Iramain, M. A., Castillo, M. V., Manzur, M. E., and Antonia Brandán, S. (2024). Identification of cholesterol in different media by using the FT-IR, FT-Raman and UV–visible spectra combined with DFT calculations. J. Mol. Liq. 403, 124879. doi:10.1016/j.molliq.2024.124879
Salahuddin, Z., Farrukh, S., and Hussain, A. (2018). Optimization study of polyethylene glycol and solvent system for gas permeation membranes. Int. J. Polym. Analysis Charact. 23 (5), 483–492. doi:10.1080/1023666X.2018.1485613
Sanguinetti, M., Canton, E., Torelli, R., Tumietto, F., Espinel-Ingroff, A., and Posteraro, B. (2019). In vitro activity of Fenticonazole against candida and bacterial vaginitis isolates determined by Mono- or dual-species testing assays. Antimicrob. Agents Chemother. 63 (7), e02693-18. doi:10.1128/AAC.02693-18
Satapathy, B. S., Biswal, B., Pattnaik, S., Parida, R., and Sahoo, R. N. (2023). A mucoadhesive nanolipo gel containing Aegle marmelos gum to enhance transdermal effectiveness of linezolid for vaginal infection: in vitro evaluation, in vitro-ex vivo correlation, pharmacokinetic studies. Int. Journal Pharmaceutics 648, 123542. doi:10.1016/j.ijpharm.2023.123542
Sayed, S., Abdelmoteleb, M., Amin, M. M., and Khowessah, O. M. (2020). Effect of formulation variables and gamma sterilization on transcorneal permeation and stability of proniosomal gels as ocular platforms for antiglaucomal drug. AAPS PharmSciTech 21 (3), 87. doi:10.1208/s12249-020-1626-2
Sayed, S., Abdel-Moteleb, M., Amin, M. M., and Khowessah, O. M. (2021). Cubogel as potential platform for glaucoma management. Drug Deliv. 28 (1), 293–305. doi:10.1080/10717544.2021.1872740
Shamma, R. N., Sayed, S., Sabry, N. A., and El-Samanoudy, S. I. (2019). Enhanced skin targeting of retinoic acid spanlastics: in vitro characterization and clinical evaluation in acne patients. J. Liposome Res. 29 (3), 283–290. doi:10.1080/08982104.2018.1552706
Sharma, D., Rani, A., Singh, V. D., Shah, P., Sharma, S., and Kumar, S. (2023). Glycerosomes: novel nano-vesicles for efficient delivery of therapeutics. Recent Adv. Drug Deliv. Formul. 17 (3), 173–182. doi:10.2174/0126673878245185230919101148
Tawfik, M. A., Ahmed, S., El-Dahmy, R. M., and Aziz, D. E. (2025). Oleic acid enriched leciplexes as novel mucoadhesive cationic nanocarriers of agomelatine for glaucoma treatment. AAPS PharmSciTech 27 (1), 4. doi:10.1208/s12249-025-03250-0
Teama, M. T., Abdelmalak, N. S., Naguib, M. J., and Ahmed, S. (2025). Polymeric micelles for pulmonary drug delivery: a comprehensive review. BioNanoScience 15 (3), 504. doi:10.1007/s12668-025-02105-z
Van Dijck, P., Sjollema, J., Cammue, B. P., Lagrou, K., Berman, J., d'Enfert, C., et al. (2018). Methodologies for in vitro and in vivo evaluation of efficacy of antifungal and antibiofilm agents and surface coatings against fungal biofilms. Microb. Cell 5 (7), 300–326. doi:10.15698/mic2018.07.638
Vanic, Z., Joraholmen, M. W., and Skalko-Basnet, N. (2021). Nanomedicines for the topical treatment of vulvovaginal infections: addressing the challenges of antimicrobial resistance. Adv. Drug Deliv. Rev. 178, 113855. doi:10.1016/j.addr.2021.113855
Weng, J., Tong, H. H. Y., and Chow, S. F. (2020). In vitro release Study of the polymeric drug nanoparticles: development and validation of a novel method. Pharmaceutics 12 (8), 732. doi:10.3390/pharmaceutics12080732
Wolfram, J., Suri, K., Yang, Y., Shen, J., Celia, C., Fresta, M., et al. (2014). Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf. B Biointerfaces 114, 294–300. doi:10.1016/j.colsurfb.2013.10.009
Younes, N. F., and Habib, B. A. (2022). Augmented local skin accumulation efficiency of sertaconazole nitrate via glycerosomal hydrogel: formulation, statistical optimization, ex vivo performance and in vivo penetration. J. Drug Deliv. Sci. Technol. 72, 103364. doi:10.1016/j.jddst.2022.103364
Younes, N. F., Sayed, S., Hassan, M., and Ahmed, S. (2024). Engineered lecithin-based proniosomes for enhanced trans-tympanic permeation: in vitro,, microbiological, ex vivo and in vivo evaluation. J. Drug Deliv. Sci. Technol. 96, 105728. doi:10.1016/j.jddst.2024.105728
Younes, N. F., Latif, R., Badawi, A., and Hegazy, K. (2025). Optimized buccoadhesive repaglinide-loaded cubogel: in-vitro characterization and in-vivo hypoglycemic activity in a streptozotocin-induced diabetic rat model. Int. J. Pharm. X 10, 100357. doi:10.1016/j.ijpx.2025.100357
Yousry, C., Fahmy, R. H., Essam, T., El-Laithy, H. M., and Elkheshen, S. A. (2016). Nanoparticles as tool for enhanced ophthalmic delivery of vancomycin: a multidistrict-based microbiological study, solid lipid nanoparticles formulation and evaluation. Drug Dev. Ind. Pharm. 42 (11), 1752–1762. doi:10.3109/03639045.2016.1171335
Zeb, A., Qureshi, O. S., Kim, H. S., Cha, J. H., Kim, H. S., and Kim, J. K. (2016). Improved skin permeation of methotrexate via nanosized ultradeformable liposomes. Int. J. Nanomedicine 11, 3813–3824. doi:10.2147/IJN.S109565
Zhang, Y., Wang, X., Lin, X., Liu, X., Tian, B., and Tang, X. (2010). High azithromycin loading powders for inhalation and their in vivo evaluation in rats. Int. Journal Pharmaceutics 395 (1-2), 205–214. doi:10.1016/j.ijpharm.2010.05.043
Zimmermann, E. S., Ferreira, L. M., Denardi, L. B., Sari, M. H. M., Cervi, V. F., Nogueira, C. W., et al. (2021). Mucoadhesive gellan gum hydrogel containing diphenyl diselenide-loaded nanocapsules presents improved anti-candida action in a mouse model of vulvovaginal candidiasis. Eur. J. Pharm. Sci. 167, 106011. doi:10.1016/j.ejps.2021.106011
Keywords: biofilm inhibition, ex-vivo permeation, fenticonazole nitrate, hybrid cationic PEGylated proniosomes, Mucoadhesion Enhancement, vaginal drug delivery
Citation: Ahmed S, Saher O, Attia H, Fahmy AM and Adel IM (2026) Innovative dual-functional hybrid cationic PEGylated proniosomes as a smart nano-platform for Boosted vaginal delivery: multi-level in-vitro, ex-vivo, microbiological, and in-vivo studies. Front. Pharmacol. 16:1746918. doi: 10.3389/fphar.2025.1746918
Received: 15 November 2025; Accepted: 25 December 2025;
Published: 28 January 2026.
Edited by:
Donato Cosco, University of Catanzaro “Magna Graecia”, ItalyReviewed by:
Velichka Andonova, Medical University of Varna, BulgariaMohhammad Ramzan, Lovely Professional University, India
Copyright © 2026 Ahmed, Saher, Attia, Fahmy and Adel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Osama Saher, b3NhbWEuc2FoZXJAa2kuc2U=; Sadek Ahmed, c2FkZWsuc2FkZWtAcGhhcm1hLmN1LmVkdS5lZw==
†ORCID: Sadek Ahmed, orcid.org/0000-0002-0190-9502; Osama Saher, orcid.org/0000-0003-3286-5706; Heba Attia, orcid.org/0000-0001-9880-1751; Abdurrahman M. Fahmy, orcid.org/0000-0003-3003-6541; Islam M. Adel, orcid.org/0000-0002-0347-0513
 Sadek Ahmed
Sadek Ahmed Osama Saher
Osama Saher Heba Attia
Heba Attia Abdurrahman M. Fahmy
Abdurrahman M. Fahmy Islam M. Adel1†
Islam M. Adel1†