- 1School of Pharmacy, School of Ethnic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Sichuan Institute for Drug Control, Chengdu, China
Pulmonary hypertension (PH) is a progressive cardiovascular disease characterized by increased pulmonary vascular resistance and structural remodeling of pulmonary vessels, leading to poor clinical outcomes and high mortality. pulmonary artery smooth muscle cells (PASMCs) migration, apoptosis and abnormal proliferation are the main pathological features leading to the occurrence of PH. Increasing evidence suggests that inhibition of PASMCs pathological changes contributes to the improvement of PH. However, the current clinical treatment of PH is limited, and medicinal plants or secondary metabolites are gradually recognized as potential treatment options for PH. Therefore, this article focuses on inhibiting the abnormal pathological changes of PASMCs, and analyzes and summarizes the mechanism and process of medicinal plants or secondary metabolites in the treatment of PH by inhibiting the abnormal proliferation of PASMCs, so as to provide a direction for the development of medicinal plants or secondary metabolites for the treatment of PH.
1 Introduction
Pulmonary hypertension (PH) is a severe syndrome characterized by pulmonary vascular obstruction, persistently elevated pulmonary vascular resistance, right ventricular hypertrophy, and progressive functional decline (Zhang et al., 2022). Its core pathological mechanism is pulmonary artery remodeling, which is mainly characterized by dysfunction of pulmonary artery endothelial cells, excessive proliferation of pulmonary artery smooth muscle cells (PASMCs), activation of fibroblasts, and inflammatory cell infiltration. Among them, the imbalance between abnormal proliferation and apoptosis of PASMCs is particularly critical (Cuthbertson et al., 2023; Jiao et al., 2025; Lu et al., 2021).
At present, the global prevalence of PH is about 1% (Yang et al., 2025), and it shows an aging trend. Inhaled vasodilators and nitric oxide are commonly used in clinical practice to improve cardiac function and hemodynamics, but long-term use is prone to drug resistance, side effects and economic burden. Medicinal plants and their secondary metabolites have the characteristics of abundant sources, few adverse reactions, and low cost, which have shown potential in the treatment of PH (Ivy et al., 2024). In particular, some active ingredients can play a role by inhibiting PASMCs (Li M. X. et al., 2016; Miao et al., 2025; Qian et al., 2024). However, there is a lack of systematic analysis of the anti-pulmonary hypertension effects of medicinal plants or secondary metabolites by regulating PASMCs. Therefore, this review focuses on the latest research progress of the role and mechanism of PASMCs in PH, in order to provide new ideas for the development of future treatment strategies.
2 Normal functions of PASMCs
PASMCs are highly differentiated cells in blood vessels with contractile and diastolic functions. They regulate blood vessels by contraction and relaxation, thereby promoting blood circulation and regulating blood pressure and blood flow (Cowan et al., 2003). PASMCs are capable of transitioning between a differentiated, contractile phenotype and a dedifferentiated, synthetic phenotype in response to varying extracellular stimuli. Under normal physiological conditions, PASMCs maintain a relatively quiescent state following their differentiation and maturation into the contractile phenotype (Jin et al., 2018). PASMCs express smooth muscle α-actin (α-SMA), smooth muscle 22α (SM22α) and myocardin, all of which are essential for maintaining the contractile function of blood vessels. The expression of contractile protein in synthetic PASMCs gradually decreased or lost, while the expression of osteopontin (OPN) and bone morphogenetic protein 2 (BMP-2) increased (Allahverdian et al., 2018). In addition, differentiated contractile PASMCs are generally spindle-shaped or fusiform, with strong cell contraction ability, and proliferation and apoptosis are in a dynamic balance, which is mainly responsible for maintaining vascular wall tension and vascular elasticity (Angelini et al., 2013; Gao et al., 2018; Yu et al., 2019; Yue et al., 2019). The synthetic PASMCs were polygonal, with large cell volume and increased proliferation, migration, and apoptosis (Figure 1).
3 The effects and mechanisms of medicinal plants or secondary metabolites on PASMCs in PH
PVR is a key determinant in the progression of pulmonary hypertension. In the early stages of this remodeling process, blood vessels activate a compensatory mechanism aimed at maintaining normal physiological function (Guignabert and Dorfmuller, 2013). However, if vascular homeostasis is broken due to improper repair, excessive proliferation of PASMCs in the injured area will destroy the normal physiological morphology and function of the vessel wall. In general, PVR is a structural change caused by adaptive changes and repair of damaged vessels (Lacolley et al., 2018; Zhu et al., 2019). It has been reported that individuals residing at high altitudes frequently demonstrate right ventricular hypertrophy and increased pulmonary vascular pressure, primarily attributed to irreversible PVR resulting from prolonged exposure to hypoxia (Lee et al., 2019). Moreover, studies have found obvious vascular occlusion and vascular remodeling in PH mouse models. The pathological changes in PASMCs, including excessive proliferation, phenotypic transformation, migration, and apoptosis, are key contributors to PVR in PH.
Medicinal plants or secondary metabolites from natural sources, which are potential drugs for treating diseases. In recent years, substantial evidence has demonstrated that ameliorating PVR through the normalization of PASMCs represents an effective therapeutic strategy for PH. Furthermore, targeting the pathological alterations in PASMCs may offer a promising avenue for the development of novel PH treatments. In this section, we summarize the effects and possible mechanisms of medicinal plants or secondary metabolites on the proliferation, migration, and apoptosis of PASMCs in PH, all relevant medicinal plants or secondary metabolites are summarized in Table 1.
3.1 Migration of PASMCs
Cell migration refers to the directed movement of cells, which is driven by changes in cell morphology in response to external signaling stimuli (Tajsic and Morrell, 2011; Yao et al., 2024). The migration of PASMCs plays a critical role in the development of neointimal formation, neovascularization, and filamentous lesions. The available evidence suggests that PASMCs migration is also regulated by PDGF and can stimulate PASMCs migration from the media to the neointima, and that neointimal thickening is significantly reduced when migration is inhibited by antiplatelet or anti-PDGF antibodies, whereas treatment with PDGF after vascular injury results in marked neointimal thickening (Sun et al., 2025). In addition, matrix metalloproteinase (MMP), and renin-angiotensin system (RAS) were identified to influence PASMCs migration (Jiao et al., 2023). Increased expression of epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), and extracellular matrix secondary metabolites collagen, fibronectin, laminin, and tenascin was found (Zhang Y. X. et al., 2018). It also has the effect of promoting cell migration, thereby participating in PVR in PH (Zeng et al., 2019). (Figure 2).
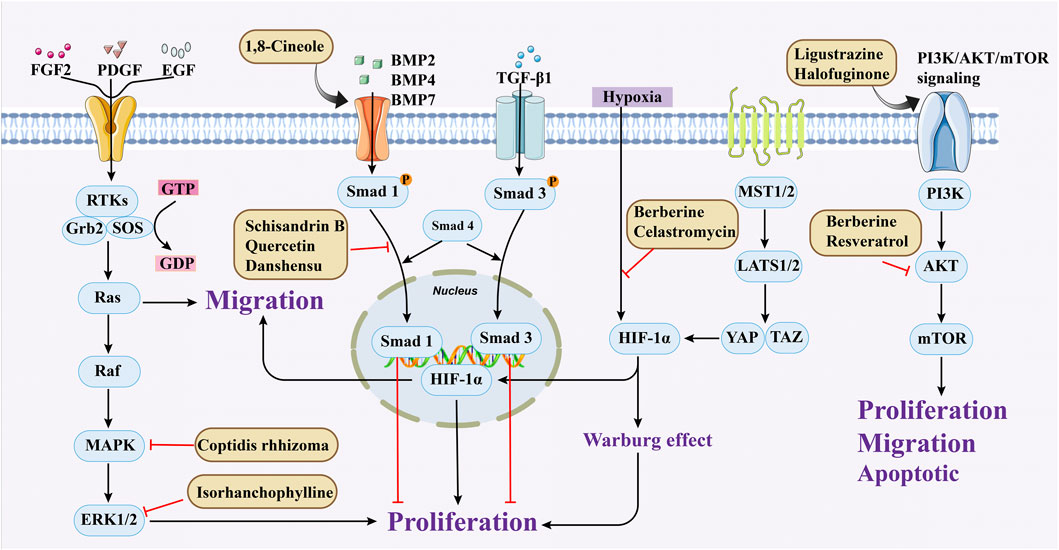
Figure 2. Medicinal plants or secondary metabolites regulate PASMCs cell proliferation and migration through different pathways.
Coptis chinensis Franch., a plant in the Ranunculaceae, contains multiple bioactive compounds. Through the establishment of a rat pulmonary arterial hypertension treatment model, the empirical research results of Luo et al. showed that the effective ingredients of C. chinensis Franch can inhibit the expression of MAPK1, effectively inhibit the migration and proliferation of PASMCs, thereby alleviating pulmonary artery remodeling (Luo et al., 2021). In addition to medicinal plants or secondary metabolites, the importance of traditional Chinese medicine formulas cannot be ignored, and compound preparations are relatively commonly used in clinical treatment. In a study conducted in 2021, it was recorded that Xinmai’an extract (containing Panax ginseng C.A.Mey., Astragalus membranaceus Fisch. ex Bunge., Salvia miltiorrhiza Bunge, Paeonia lactiflora Pall., Ophiopogon japonicus (Thunb.) Ker Gawl., and Dryobalanops aromatica C.F.Gaertn.) can inhibit the MAPK signaling pathway and increase MMP2 and MMP9, effectively alleviating the migration, proliferation, and anti-apoptosis of PASMCs (Zhu Y. et al., 2021).
In 2015, He et al. found through in vitro experiments that quercetin (Que, 10, 30, and 60 μmol/L) may dose dependently reduce the migration of PASMCs by inhibiting the TrkA/AKT signaling pathway (He et al., 2015). In addition, a recent study has shown that Que has more than one anti migration pathway. Through in vitro (60 μmol/mL) and in vivo (5 mg/kg/d) studies, it has been demonstrated that Que suppress PVR by inhibiting the TGF-β1/Smad2/Smad3, reducing migration, proliferation, and phenotype transformation of PASMCs (Gao et al., 2024).
Resveratrol is a phenolic compound primarily obtained from the Polygonaceae plant Reynoutria japonica Houtt. In 2017, Guan et al. established a hypoxia model using rat PASMCs primary cells and found that resveratrol (10 μmol/L) could inhibit phosphorylation of AKT, thereby reducing the migration and proliferation of PASMCs (Guan et al., 2017). Schisandrin B (Sch B) is one of the major bioactive constituents found in Schisandra chinensis (Turcz.) Baill. Research has shown that at any doses (20, 50, 100, 150 μM), Sch B has a strong therapeutic effect by reducing TGF-β1 levels and activating downstream signaling pathways, thereby alleviating PASMCs migration and apoptosis resistance caused by hypoxia (Wu J. et al., 2017). Berberine (BBR) occurs naturally in various plant species and has been extensively utilized in the treatment of conditions such as gastroenteritis and bacterial dysentery. In 2018, Luo et al. found that BBR (100 mg/kg) may suppress migration and proliferation of PASMCs in PH models induced by norepinephrine by activating the PP2A (Luo et al., 2018). Subsequent research in 2019 found that BBR, as a Src inhibitor, can inhibit Src activation and HIF-1α expression, thereby suppressing Akt/mTOR and slowing down the migration and proliferation of PASMCs (Liu et al., 2019).
Andrographolide (ANDR) derived from the medicinal plant Andrographis paniculata (Burm.f.) Wall. ex Nees. It exhibits a broad spectrum of anti-cancer activities and demonstrates significant therapeutic potential in the context of cardiovascular diseases (Gou et al., 2023; Hu et al., 2024). Due to its strong anti-proliferative activity in the treatment of cancer, ANDR has attracted researchers to explore its potential therapeutic role in the process of PVR towards PH. Nie et al. systematically studied its related mechanisms through in vitro and in vivo experiments. ANDR (1 mg/kg/day) restored the signal transduction of BMPR2, inhibited the activation of TLR4/NF-κB and NOX/Nrf2, and jointly caused a reduction in the proliferation and migration of PASMCs, and promoted their apoptosis, demonstrating a multi-target effect (Nie et al., 2021). Taken together, these findings highlight the therapeutic potential of medicinal plants or secondary metabolites for PH by inhibiting multiple molecular mechanisms such as TGF-β1/Smad2/Smad3 axis, TLR4/NF-κB and NOX/Nrf2 pathways to inhibit the migration of PASMCs and improve the pathological symptoms of PASMCs.
3.2 Apoptosis of PASMCs
Apoptosis, a known programmed cell death pathway, is an important way for multicellular organisms to maintain homeostasis in the internal environment (Liu et al., 2025). Apoptosis normally removes cells that migrate into the vascular lumen and eliminates accumulated mast cells within the pulmonary vasculature. A reduction in apoptosis, coupled with increased PASMCs proliferation, contributes to vascular wall thickening, and elevated pulmonary vascular resistance, ultimately leading to the development of PH (Perini et al., 2018). It has been reported that inhibitors of Bcl-2 and Bcl-xl in the Bcl family of anti-apoptotic proteins can promote apoptosis and thus delay the development of PH (Rybka et al., 2018). Therefore, promoting apoptosis can reverse PVR and is a potential therapeutic option against PH (Figure 3).

Figure 3. Medicinal plants or secondary metabolites regulate PASMCs apoptosis through different pathways. (Extrinsic pathway for caspase 8 and 10 and intrinsic pathway for caspase 9. Activation of different starting caspases eventually leads to activation of the same effector caspases 3, 6, and 7).
Puerarin (Pue) is an isoflavone derivative isolated from Pueraria lobata (Willd.) Ohwi. It is often used in diseases such as heart disease and high blood pressure. As early as 2008, some scholars found that Pue could treat PVR in PH rats, but the specific therapeutic mechanism was not mentioned (Li et al., 2008). Subsequently, Chen et al. conducted further studies by culturing HPASMCs in vitro and found that Pue treatment with 50 μM and above could upregulate caspase-9 and Bax, downregulate Bcl-2, and promote cell apoptosis, thereby determining the possible related mechanism (Chen et al., 2012). Salidroside (SAL), a diterpenoid compound from the rhizome of Rhodiola rosea L. (Kosanovic et al., 2013), treated chronic hypoxia mice with different concentrations of SAL (0, 16, 32 and 64 mg/kg). It was found that SAL could activate AMPKα1-P53-P27/P21 to reduce PASMCs proliferation, thereby attenuating chronic hypoxia-induced PH (Huang et al., 2015). Moreover, it promotes cell apoptosis through Bax/Bcl-2-caspase 9-caspase 3 to alleviate pulmonary artery remodeling, thereby alleviating PH (Chen et al., 2016). Interestingly, Aloperine (Alo) is a quinoline alkaloid mainly extracted from the seeds and leaves of Sophora alopecuroides L., and it possesses various biological activities, including anti-inflammatory, anticancer. Researchers found that Alo (10 mg/kg) could downregulate Bcl-2/Bax ratio, promote cell apoptosis, and improve PH-related symptoms in rats (Wei et al., 2025). Subsequently, Chang et al. further explored the mechanism of Alo treatment by using HPASMCs to supplement Alo for PH, and found that Alo (0.125, 0.25, 0.5, 1 mM) could enhance cell apoptosis by inhibiting NK-κB and increasing p27. Thus, the excessive proliferation of PASMCs was reduced (Chang et al., 2019).
Plumbagin (PLU) is a naphthoquinone substance mainly found in the herbaceous plant Plumbago zeylanica L., which has anti-tumor and anti-proliferative pharmacological effects. It is often used as a STAT3 inhibitor in cancer cells to promote cell apoptosis (Hsu et al., 2006). Its pro apoptotic properties are also highly valued in pulmonary arterial hypertension. Researchers have identified a strong correlation between the proliferation and anti-apoptotic effects on PASMCs and the activation of STAT and NFAT signaling pathways, and PLU has been shown to specifically target STAT. A thorough study by Courboulin et al. showed that in vivo, Oral PLU (4 mg/kg) can reduce the distal pulmonary artery remodelling, mean pulmonary artery pressure and right ventricular hypertrophy without affecting systemic circulation in both monocrotaline-and suden/chronic hypoxia-induced PH in rats can inhibit the activation of STAT3/NFAT axis and alleviate the apoptosis resistance and excessive proliferation of PASMCs (Courboulin et al., 2012).
Although traditional Chinese medicine secondary metabolites have significant pharmacological effects, their effects may have many targets, such as affecting different pathways to achieve the same results. Quercetin is a typical example. In addition to balancing PASMCs by inhibiting the TrkA/AKT and the TGF-β1/Smad2/Smad3, other researches have shown that it can also work by upregulating 5-HT2A receptors, restoring Kv current, and reducing phosphorylation of AKT and S6. These effects guide the apoptosis of PASMCs and inhibit its proliferation, thereby reducing the mortality rate of PVR rats (Morales-Cano et al., 2014). Carvacrol (CAR) is a monoterpene phenol and one of the main secondary metabolites of beef tallow (Burt, 2004). In tumor cells, it also exhibits pro apoptotic properties. Scholars hypothesize that it can also be applied to pulmonary arterial hypertension and have found that CAR can induce apoptosis of PASMCs by inhibiting the ERK1/2 and PI3K/Akt, reducing Bcl-2 expression, and promoting caspase-3 activation, providing new insights for the treatment of hypoxic PH (Zhang et al., 2016). Baicalin, a flavonoid and main bioactive compound, is found in Scutellaria baicalensis Georgi, a commonly used herb in traditional Chinese medicine. It can be used to treat cancer, liver and intestinal diseases, and acute lung injury (He et al., 2021; Hu et al., 2021; Li-Weber, 2009). In 2010, a survey in Cell Research found that baicalin inhibited the proliferation of PASMCs by inhibiting PDGFR β-ERK1/2 and the accumulation of p27, thereby reducing the development of atherosclerosis (Dong et al., 2010). The related phenotypes caused by it are highly similar to PASMCs in PH. Therefore, in the in-depth study by Zhang et al., it was found that baicalin can also improve PH induced by MCT in rats, by inhibiting NF-κB and activating bone morphogenetic protein (BMP) mechanisms to promote apoptosis and anti-proliferation of PASMCs (Zhang Z. et al., 2017).
As previously mentioned, resveratrol has been shown to inhibit the migration of PASMCs by downregulating the phosphorylation of AKT. In addition, the role of Res in cell apoptosis cannot be ignored. After establishing a rat model of PH, Yu et al. found that Res administration could improve PVR and right ventricular hypertrophy. In vitro cellular mechanism experiments have shown that Res can enhance the activation of SIRT1, induce mitochondrial permeability transition (mPT) dysfunction, enhance cell apoptosis, and thus resist the proliferation of PASMCs (Yu et al., 2017).
3.3 Proliferation of PASMCs
Cell proliferation and differentiation play a key role in tissue development. Excessive PASMCs proliferation can cause thickening of the pulmonary arteriole walls and narrowing of the lumen, leading to PVR (Solinc et al., 2022; Zucker et al., 2019). PDGF-BB, a member of the PDGF family, is a major regulator of PVR. It accelerates smooth muscle cell proliferation by up-regulating low-density lipoprotein receptor-related protein 1 (LRP1), leading to thickening of the pulmonary vascular media and promoting PH (Qin et al., 2022). This phenomenon may be attributed to the excessive production of ROS during PDGF-BB-induced PASMCs proliferation, which leads to the activation of ataxia-telangiectasia mutated protein (ATM) (Deng et al., 2022; Wujak et al., 2021), thereby inhibiting PASMCs proliferation.
In addition, hypoxia-inducible factor (HIF)-1α plays a key role in the development of pulmonary hypertension by regulating downstream genes that promote PASMCs proliferation. Other studies have shown that the energy metabolism of mitochondria in PH patients does not favor aerobic respiration, but is more inclined to supply energy through anaerobic respiration, which is called the “Warburg effect,” which leads to the excessive proliferation of PASMCs, promotes the thickening of the media, and then leads to the occurrence of PH (Arai et al., 2021). When PASMCs are stimulated by the above factors, BDNF-TrkB-ERK1/2 (65), BMP/TGF-β (Wei et al., 2023), PDGF/Ca2+ (Lan et al., 2024), PI3K-AKT-Mtor (Meng et al., 2019) signaling pathways are activated, which further affect cell proliferation and promote PVR in PH. Therefore, the inhibition of excessive proliferation of PASMCs can be considered a potential therapeutic strategy for patients with PH. (Figure 2).
1,8-Cineole is mainly derived from plants such as Eucalyptus L'Hér. and Mentha canadensis L., and is a monoterpene compound used as a natural aromatic oil, and spice (Ćavar Zeljković et al., 2021). In addition to the above purposes, 1,8-Cineole can also be used in the medical industry. The latest research shows that 1,8-Cineole (25 and 100 mg/kg) mitigated PAH-associated derailment of both BMPR2/Smad1/5 and BMPR2/PPAR-γ pathways and concomitantly reduced interstitial fibrosis and the arterial medial layer thickness in pulmonary arteries. (Alves-Silva et al., 2025). Ginsenoside compound K (GCK) is one of P. ginseng C.A.Mey. secondary metabolites, which has shown anti proliferative effects in cancer, and the proliferation of PASMCs has similar characteristics to cancer cells (Attele et al., 1999). So under the research of Liu et al., after intervention with 5 μmol/L GCK in a cell model, the drug downregulated β-catenin and cyclin, inhibited cell cycle circulation, thus reduced abnormal proliferation of PASMCs (Liu et al., 2023). In the same year, it was also found that cannabidiol has an inhibitory effect on the proliferation of PASMCs at 10 μM, with almost no cytotoxicity (Sadowska et al., 2020). Its possible mechanism is that it upregulates Nrf2 and downstream proteins, improving oxidative stress and mitochondrial function. Similarly, studies have shown that oxymatrine inhibits abnormal PASMCs proliferation and oxidative stress by enhancing Nrf2 and antioxidant protein expression, thereby improving PH (Liu et al., 2014; Zhang et al., 2014).
Crocin is a water-soluble carotenoid compound that can be isolated from Crocus sativus L. or Gardenia jasminoides J.Ellis (Deng et al., 2024). Another study found that crocin not only inhibits collagen fiber proliferation, but also reduces the proliferation of PASMCs by suppressing the CCL2/CCR2 inflammatory pathway, improving PVR induced by MCT in rats with PH (Sheng et al., 2022). The activity of the typical lipophilic component Tanshinone IIA is already very broad (Pang et al., 2016). In PH disease, it can alleviate pulmonary artery remodeling in diseased SD rats, mainly by downregulating Akt/Skp2/P27 pathway proteins and inhibiting PASMCs proliferation (Luo et al., 2013). In addition, another water-soluble component of S. miltiorrhiza Bunge, Danshensu, can inhibit the conduction of TGF-β-Smad3, thereby exerting anti proliferative effects (Zhang N. et al., 2018). From various research results, S. miltiorrhiza Bunge is a key traditional Chinese medicine for treating PH, which can add a new therapeutic drug for this type of disease.
Epigallocatechin-3-gallate present in green tea can inhibit proliferation by upregulating KLF-4 and MFN-2 and downregulating p-Erk (Zhu et al., 2017). Isorhanchophylline, a compound derived from Uncaria rhynchophylla (Miq.)Miq. ex Havil., can also downregulate ERK1/2 and cyclin D1, p-STAT3, Akt/GSK3β, and increase the accumulation of p27Kip1, jointly exerting an anti PASMCs proliferation effect (Guo et al., 2014). It is also worth noting that the PI3K/AKT plays a role in controlling the proliferation of PASMCs. Two studies have demonstrated that Ligustrazine and Halofuginone can inhibit this pathway, prevent cell cycle progression, and improve PH (Huang et al., 2021; Jain et al., 2021).
The diversity of natural products corresponds to a variety of anti-proliferative mechanisms, but they can achieve the same effect. In addition to several common pathways, researchers have also found that paeoniflorin may inhibit the proliferation of PASMCs by activating A2BAR, thereby blocking the cell cycle progression (Qian et al., 2013). Magnolol can inhibit the phosphorylation level of JAK2/STAT3, exert anti proliferative effects, and prevent pulmonary artery remodeling (Xiao et al., 2022). Hydroxysafflor yellow A can also inhibit proliferation and reverse vascular remodeling by reducing PCNA and Ki67 levels, and possibly synergistically activating Kv channels (Li L. et al., 2016). Tetrandrine derived from the traditional Chinese medicine Stephania tetrandra S.Moore can upregulate the expression of PKG-1 while inhibiting iNOS, balancing the NO signaling pathway, and exerting anti proliferative and antioxidant effects, alleviating PH symptoms in rats (Wang et al., 2016).
Most of the research on the treatment of PH with natural products focuses on exploring the effects of herbs and their secondary metabolites, while there is relatively less research on other drugs, such as animals and microorganisms. Interestingly, Kurosawa et al.'s study found that a benzoylpyrrole type compound found in bacteria, Celastromycin (Cel), can improve PH related symptoms. The specific mechanism involved is that Cel downregulates HIF-1α and NF-κB, restores mitochondrial function, and inhibits abnormal proliferation of PASMCs (Kurosawa et al., 2019). This study further enriches the variety of natural products for treating PH and expands new ideas for future drug development. In summary, existing studies fully reveal the potential of medicinal plants or secondary metabolites to treat pulmonary hypertension through multi-target intervention of PASMCs migration, apoptosis and proliferation. However, there is a deeper logic behind this: as a multi-pathway disorder, the pathological network of pulmonary hypertension has a significant “robustness,” that is, the inhibition of a single target is often offset by a compensatory pathway. The multi-target nature of natural products can systematically perturb this pathological network and break its stable state (He et al., 2025). For example, simultaneous inhibition of TGF-β/Smad and MAPK pathways may result in synergistic effects rather than simply additive. Future research should go beyond the linear model of “one component, one pathway” and apply multi-omics and systems biology approaches to quantify the overall remodeling effect of medicinal plants or secondary metabolites on PASMC signaling network. It also focuses on its ability to drive cell “state transitions” such as the transition from a proliferative state to an apoptotic state. This shift in thinking from “target inhibition” to “network remodeling” may provide a new paradigm for the development of more effective multi-target synergistic therapies.
4 Conclusion
The pathogenesis of PH is complex and involves the joint action of many factors, which leads to abnormal migration, apoptosis and excessive proliferation of PASMCs, and then leads to PVR, increased vascular resistance and increased vascular pressure. At present, there is still a lack of completely effective treatment in clinical practice. Medicinal plants or secondary metabolites have attracted more and more attention due to their high safety and abundant resource sources (Bharate and Lindsley, 2024; Peng et al., 2024). This review systematically summarizes the latest research progress of medicinal plants and their secondary metabolites to improve pulmonary arterial hypertension (PH) by regulating the pathological changes of PASMCs, such as inhibiting abnormal proliferation and migration and promoting apoptosis. Medicinal plants and their secondary metabolites alleviate PH mainly by directly interfering with PASMCs function or indirectly exerting anti-inflammatory and anti-oxidation effects, which provides an important basis for the development of new therapeutic candidates.
However, many challenges remain in translating relevant findings into clinical application. At present, most studies are limited to cell and animal models, and the disease process is different from that of humans, and the clinical research is limited, which may lead to bias in the evaluation of efficacy. In addition, systematic evaluation of the safety, toxicity, and pharmacokinetic properties (e.g., solubility, oral bioavailability) of candidate ingredients is still inadequate.Therefore, more well-designed clinical trials are needed in the future to scientifically verify its efficacy and safety. At the same time, more efforts should be focused on optimizing the drug administration strategy and dosage form design to improve patient compliance, and ultimately promote more potential natural products from experimental research to clinical translation.
Author contributions
QL: Writing – original draft. HG: Writing – review and editing. YZ: Writing – review and editing. LH: Writing – review and editing. LC: Writing – review and editing. CZ: Conceptualization, Writing – review and editing. LA: Conceptualization, Writing – review and editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Sichuan Science and Technology Program (2025ZNSFSC0582 and 2024NSFJQ0059)2022 “Tianfu Qingcheng Plan” Tianfu Science and Technology Leading Talents Project (Chuan Qingcheng No. 1090).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allahverdian, S., Chaabane, C., Boukais, K., Francis, G. A., and Bochaton-Piallat, M. L. (2018). Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc Res. 114, 540–550. doi:10.1093/cvr/cvy022
Alves-Silva, J. M., Zuzarte, M., Marques, C., Rodrigues, T., Barbeitos, J., Caetano, R., et al. (2025). 1,8-Cineole reduces pulmonary vascular remodelling in pulmonary arterial hypertension by restoring intercellular communication and inhibiting angiogenesis. Phytomedicine 137, 156334. doi:10.1016/j.phymed.2024.156334
Angelini, D. J., Su, Q., Yamaji-Kegan, K., Fan, C., Skinner, J. T., Poloczek, A., et al. (2013). Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) in chronic hypoxia- and antigen-mediated pulmonary vascular remodeling. Respir. Res. 14, 1. doi:10.1186/1465-9921-14-1
Arai, M. A., Sakuraba, K., Makita, Y., Hara, Y., and Ishibashi, M. (2021). Evaluation of naturally occurring HIF-1 inhibitors for pulmonary arterial hypertension. Chembiochem 22, 2799–2804. doi:10.1002/cbic.202100223
Attele, A. S., Wu, J. A., and Yuan, C. S. (1999). Ginseng pharmacology: multiple constituents and multiple actions. Biochem. Pharmacol. 58, 1685–1693. doi:10.1016/s0006-2952(99)00212-9
Bharate, S. B., and Lindsley, C. W. (2024). Natural products driven medicinal chemistry. J. Med. Chem. 67, 20723–20730. doi:10.1021/acs.jmedchem.4c02736
Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods--a review. Int. J. Food Microbiol. 94, 223–253. doi:10.1016/j.ijfoodmicro.2004.03.022
Ćavar Zeljković, S., Šišková, J., Komzáková, K., De Diego, N., Kaffková, K., and Tarkowski, P. (2021). Phenolic compounds and biological activity of selected mentha species. Plants (Basel) 10, 10. doi:10.3390/plants10030550
Çetinkaya, M., and Baran, Y. (2023). Therapeutic potential of luteolin on cancer. Vaccines (Basel) 11, 11. doi:10.3390/vaccines11030554
Chang, Z., Zhang, P., Zhang, M., Jun, F., Hu, Z., Yang, J., et al. (2019). Aloperine suppresses human pulmonary vascular smooth muscle cell proliferation via inhibiting inflammatory response. Chin. J. Physiol. 62, 157–165. doi:10.4103/cjp.Cjp_27_19
Chen, C., Chen, C., Wang, Z., Wang, L., Yang, L., Ding, M., et al. (2012). Puerarin induces mitochondria-dependent apoptosis in hypoxic human pulmonary arterial smooth muscle cells. PLoS One 7, e34181. doi:10.1371/journal.pone.0034181
Chen, M., Cai, H., Yu, C., Wu, P., Fu, Y., Xu, X., et al. (2016). Salidroside exerts protective effects against chronic hypoxia-induced pulmonary arterial hypertension via AMPKα1-dependent pathways. Am. J. Transl. Res. 8, 12–27.
Courboulin, A., Barrier, M., Perreault, T., Bonnet, P., Tremblay, V. L., Paulin, R., et al. (2012). Plumbagin reverses proliferation and resistance to apoptosis in experimental PAH. Eur. Respir. J. 40, 618–629. doi:10.1183/09031936.00084211
Cowan, D. B., Jones, M., Garcia, L. M., Noria, S., del Nido, P. J., and McGowan, F. X., Jr. (2003). Hypoxia and stretch regulate intercellular communication in vascular smooth muscle cells through reactive oxygen species formation. Arterioscler. Thromb. Vasc. Biol. 23, 1754–1760. doi:10.1161/01.Atv.0000093546.10162.B2
Cuthbertson, I., Morrell, N. W., and Caruso, P. (2023). BMPR2 mutation and metabolic reprogramming in pulmonary arterial hypertension. Circ. Res. 132, 109–126. doi:10.1161/circresaha.122.321554
Deng, H., Tian, X., Sun, H., Liu, H., Lu, M., and Wang, H. (2022). Calpain-1 mediates vascular remodelling and fibrosis via HIF-1α in hypoxia-induced pulmonary hypertension. J. Cell. Mol. Med. 26, 2819–2830. doi:10.1111/jcmm.17295
Deng, J., Wei, R. Q., Zhang, W. M., Shi, C. Y., Yang, R., Jin, M., et al. (2024). Crocin's role in modulating MMP2/TIMP1 and mitigating hypoxia-induced pulmonary hypertension in mice. Sci. Rep. 14, 12716. doi:10.1038/s41598-024-62900-8
Dong, L. H., Wen, J. K., Miao, S. B., Jia, Z., Hu, H. J., Sun, R. H., et al. (2010). Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRβ-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell. Res. 20, 1252–1262. doi:10.1038/cr.2010.111
Gao, S., Xu, L., Zhang, Y., Yu, Q., Li, J., Guan, H., et al. (2018). Salusin-α inhibits proliferation and migration of vascular smooth muscle cell via Akt/mTOR signaling. Cell. Physiol. Biochem. 50, 1740–1753. doi:10.1159/000494792
Gao, R. J., Aikeremu, N., Cao, N., Chen, C., Ma, K. T., Li, L., et al. (2024). Quercetin regulates pulmonary vascular remodeling in pulmonary hypertension by downregulating TGF-β1-Smad2/3 pathway. BMC Cardiovasc Disord. 24, 535. doi:10.1186/s12872-024-04192-4
Gou, T., Hu, M., Xu, M., Chen, Y., Chen, R., Zhou, T., et al. (2023). Novel wine in an old bottle: preventive and therapeutic potentials of andrographolide in atherosclerotic cardiovascular diseases. J. Pharm. Anal. 13, 563–589. doi:10.1016/j.jpha.2023.05.010
Guan, Z., Shen, L., Liang, H., Yu, H., Hei, B., Meng, X., et al. (2017). Resveratrol inhibits hypoxia-induced proliferation and migration of pulmonary artery vascular smooth muscle cells by inhibiting the phosphoinositide 3-kinase/protein kinase B signaling pathway. Mol. Med. Rep. 16, 1653–1660. doi:10.3892/mmr.2017.6814
Guignabert, C., and Dorfmuller, P. (2013). Pathology and pathobiology of pulmonary hypertension. Semin. Respir. Crit. Care Med. 34, 551–559. doi:10.1055/s-0033-1356496
Guo, H., Zhang, X., Cui, Y., Deng, W., Xu, D., Han, H., et al. (2014). Isorhynchophylline protects against pulmonary arterial hypertension and suppresses PASMCs proliferation. Biochem. Biophys. Res. Commun. 450, 729–734. doi:10.1016/j.bbrc.2014.06.044
He, C., Wang, P., Zuo, Y., Guo, J., Zhang, Q., Peng, S., et al. (2025). Constituents-composed Chinese medicine of dajianzhong decoction ameliorates Non-alcoholic fatty liver disease via AMPK/PPARα/CPT1 signaling. Phytomedicine149, 157515. doi:10.1016/j.phymed.2025.157515
He, Y., Cao, X., Liu, X., Li, X., Xu, Y., Liu, J., et al. (2015). Quercetin reverses experimental pulmonary arterial hypertension by modulating the TrkA pathway. Exp. Cell. Res. 339, 122–134. doi:10.1016/j.yexcr.2015.10.013
He, Y. Q., Zhou, C. C., Yu, L. Y., Wang, L., Deng, J. L., Tao, Y. L., et al. (2021). Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 163, 105224. doi:10.1016/j.phrs.2020.105224
He, Y., Zhong, J. H., Wei, X. D., Huang, C. Y., Peng, P. L., Yao, J., et al. (2022). Pachymic acid ameliorates pulmonary hypertension by regulating Nrf2-Keap1-ARE pathway. Curr. Med. Sci. 42, 56–67. doi:10.1007/s11596-021-2414-2
Hsu, Y. L., Cho, C. Y., Kuo, P. L., Huang, Y. T., and Lin, C. C. (2006). Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. J. Pharmacol. Exp. Ther. 318, 484–494. doi:10.1124/jpet.105.098863
Hu, Q., Zhang, W., Wu, Z., Tian, X., Xiang, J., Li, L., et al. (2021). Baicalin and the liver-gut system: pharmacological bases explaining its therapeutic effects. Pharmacol. Res. 165, 105444. doi:10.1016/j.phrs.2021.105444
Hu, J., Li, Y., Xie, X., Song, Y., Yan, W., Luo, Y., et al. (2024). The therapeutic potential of andrographolide in cancer treatment. Biomed. Pharmacother. 180, 117438. doi:10.1016/j.biopha.2024.117438
Huang, X., Zou, L., Yu, X., Chen, M., Guo, R., Cai, H., et al. (2015). Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J. Mol. Cell. Cardiol. 82, 153–166. doi:10.1016/j.yjmcc.2015.03.005
Huang, H., Kong, L., Luan, S., Qi, C., and Wu, F. (2021). Ligustrazine suppresses platelet-derived growth Factor-BB-Induced pulmonary artery smooth muscle cell proliferation and inflammation by regulating the PI3K/AKT signaling pathway. Am. J. Chin. Med. 49, 437–459. doi:10.1142/s0192415x21500208
Ivy, D., Rosenzweig, E. B., Abman, S. H., Beghetti, M., Bonnet, D., Douwes, J. M., et al. (2024). Embracing the challenges of neonatal and paediatric pulmonary hypertension. Eur. Respir. J. 64, 64. doi:10.1183/13993003.01345-2024
Jain, P. P., Zhao, T., Xiong, M., Song, S., Lai, N., Zheng, Q., et al. (2021). Halofuginone, a promising drug for treatment of pulmonary hypertension. Br. J. Pharmacol. 178, 3373–3394. doi:10.1111/bph.15442
Ji, L., Su, S., Xin, M., Zhang, Z., Nan, X., Li, Z., et al. (2022). Luteolin ameliorates hypoxia-induced pulmonary hypertension via regulating HIF-2α-Arg-NO axis and PI3K-AKT-eNOS-NO signaling pathway. Phytomedicine 104, 154329. doi:10.1016/j.phymed.2022.154329
Jiao, X., Yu, H., Du, Z., Li, L., Hu, C., Du, Y., et al. (2023). Vascular smooth muscle cells specific deletion of angiopoietin-like protein 8 prevents angiotensin II-promoted hypertension and cardiovascular hypertrophy. Cardiovasc Res. 119, 1856–1868. doi:10.1093/cvr/cvad089
Jiao, Q., Xu, X., Xu, L., Wang, Y., Pang, S., Hao, J., et al. (2025). Knockdown of eIF3a alleviates pulmonary arterial hypertension by inhibiting endothelial-to-mesenchymal transition via TGFβ1/SMAD pathway. J. Transl. Med. 23, 524. doi:10.1186/s12967-025-06505-3
Jin, L., Lin, X., Yang, L., Fan, X., Wang, W., Li, S., et al. (2018). AK098656, a novel vascular smooth muscle cell-dominant long noncoding RNA, promotes hypertension. Hypertension 71, 262–272. doi:10.1161/hypertensionaha.117.09651
Jin, H., Jiao, Y., Guo, L., Ma, Y., Zhao, R., Li, X., et al. (2021). Astragaloside IV blocks monocrotaline-induced pulmonary arterial hypertension by improving inflammation and pulmonary artery remodeling. Int. J. Mol. Med. 47, 595–606. doi:10.3892/ijmm.2020.4813
Kosanovic, D., Tian, X., Pak, O., Lai, Y. J., Hsieh, Y. L., Seimetz, M., et al. (2013). Rhodiola: an ordinary plant or a promising future therapy for pulmonary hypertension? A brief review. Pulm. Circ. 3, 499–506. doi:10.1086/674303
Kurosawa, R., Satoh, K., Kikuchi, N., Kikuchi, H., Saigusa, D., Al-Mamun, M. E., et al. (2019). Identification of celastramycin as a novel therapeutic agent for pulmonary arterial hypertension. Circ. Res. 125, 309–327. doi:10.1161/circresaha.119.315229
Lacolley, P., Regnault, V., and Avolio, A. P. (2018). Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res. 114, 513–528. doi:10.1093/cvr/cvy009
Lan, C., Fang, G., Qiu, C., Li, X., Yang, F., and Yang, Y. (2024). Inhibition of DYRK1A attenuates vascular remodeling in pulmonary arterial hypertension via suppressing STAT3/Pim-1/NFAT pathway. Clin. Exp. Hypertens. 46, 2297642. doi:10.1080/10641963.2023.2297642
Lee, G. L., Yeh, C. C., Wu, J. Y., Lin, H. C., Wang, Y. F., Kuo, Y. Y., et al. (2019). TLR2 promotes vascular smooth muscle cell chondrogenic differentiation and consequent calcification via the concerted actions of osteoprotegerin suppression and IL-6-Mediated RANKL induction. Arterioscler. Thromb. Vasc. Biol. 39, 432–445. doi:10.1161/atvbaha.118.311874
Li, J. W., Chen, P., Guan, X. Q., Gong, Y. S., and Yang, P. L. (2008). Inhibition of puerarin on pulmonary hypertension in rats with hypoxia and hypercapnia. Zhongguo Zhong Yao Za Zhi 33, 544–549.
Li, M. X., Jiang, D. Q., Wang, Y., Chen, Q. Z., Ma, Y. J., Yu, S. S., et al. (2016a). Signal mechanisms of vascular remodeling in the development of pulmonary arterial hypertension. J. Cardiovasc Pharmacol. 67, 182–190. doi:10.1097/fjc.0000000000000328
Li, L., Dong, P., Hou, C., Cao, F., Sun, S., He, F., et al. (2016b). Hydroxysafflor yellow A (HSYA) attenuates hypoxic pulmonary arterial remodelling and reverses right ventricular hypertrophy in rats. J. Ethnopharmacol. 186, 224–233. doi:10.1016/j.jep.2016.04.004
Li, Y., Wang, Y., Li, Y., Qian, Z., Zhu, L., and Yang, D. (2017). Osthole attenuates pulmonary arterial hypertension in monocrotaline-treated rats. Mol. Med. Rep. 16, 2823–2829. doi:10.3892/mmr.2017.6876
Li-Weber, M. (2009). New therapeutic aspects of flavones: the anticancer properties of scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat. Rev. 35, 57–68. doi:10.1016/j.ctrv.2008.09.005
Liu, Y., Xu, Y., Ji, W., Li, X., Sun, B., Gao, Q., et al. (2014). Anti-tumor activities of matrine and oxymatrine: literature review. Tumour Biol. 35, 5111–5119. doi:10.1007/s13277-014-1680-z
Liu, P., Gu, Y., Luo, J., Ye, P., Zheng, Y., Yu, W., et al. (2019). Inhibition of src activation reverses pulmonary vascular remodeling in experimental pulmonary arterial hypertension via Akt/mTOR/HIF-1<alpha> signaling pathway. Exp. Cell. Res. 380, 36–46. doi:10.1016/j.yexcr.2019.02.022
Liu, T., Xie, L., Liu, H. M., and Liu, B. (2021). The effects of ginsenoside compound K on PDGF-BB-Induced PASMCs proliferation and phenotypic conversion of pulmonary arterial smooth muscle cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 52, 643–648. doi:10.12182/20210760101
Liu, P., Gao, S., Li, Z., Pan, S., Luo, G., and Ji, Z. (2023). Endothelial progenitor cell-derived exosomes inhibit pulmonary artery smooth muscle cell in vitro proliferation and resistance to apoptosis by modulating the Mitofusin-2 and Ras-Raf-ERK1/2 signaling pathway. Eur. J. Pharmacol. 949, 175725. doi:10.1016/j.ejphar.2023.175725
Liu, Z. P., Tang, W. S., Wang, G. Z., Xu, J. W., Zhu, L. J., Lyu, Q. Y., et al. (2025). Ferulic acid inhibiting Colon cancer cells at different Duke’s stages. Food and Med. Homol. 2 (3), 9420063. doi:10.26599/FMH.2025.9420063
Lu, X., Zhang, J., Liu, H., Ma, W., Yu, L., Tan, X., et al. (2021). Cannabidiol attenuates pulmonary arterial hypertension by improving vascular smooth muscle cells mitochondrial function. Theranostics 11, 5267–5278. doi:10.7150/thno.55571
Luitel, H., Novoyatleva, T., Sydykov, A., Petrovic, A., Mamazhakypov, A., Devkota, B., et al. (2020). Yarsagumba is a promising therapeutic option for treatment of pulmonary hypertension due to the potent anti-proliferative and vasorelaxant properties. Med. Kaunas. 56, 56. doi:10.3390/medicina56030131
Luo, Y., Xu, D. Q., Dong, H. Y., Zhang, B., Liu, Y., Niu, W., et al. (2013). Tanshinone IIA inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation via Akt/Skp2/p27-associated pathway. PLoS One 8, e56774. doi:10.1371/journal.pone.0056774
Luo, J., Gu, Y., Liu, P., Jiang, X., Yu, W., Ye, P., et al. (2018). Berberine attenuates pulmonary arterial hypertension via protein phosphatase 2A signaling pathway both in vivo and in vitro. J. Cell. Physiol. 233, 9750–9762. doi:10.1002/jcp.26940
Luo, S., Kan, J., Zhang, J., Ye, P., Wang, D., Jiang, X., et al. (2021). Bioactive compounds from coptidis rhizoma alleviate pulmonary arterial hypertension by inhibiting pulmonary artery smooth muscle cells' proliferation and migration. J. Cardiovasc Pharmacol. 78, 253–262. doi:10.1097/fjc.0000000000001068
Meng, L., Liu, X., Teng, X., Gu, H., Yuan, W., Meng, J., et al. (2019). Osteopontin plays important roles in pulmonary arterial hypertension induced by systemic-to-pulmonary shunt. FASEB J. 33, 7236–7251. doi:10.1096/fj.201802121RR
Miao, Y. H., Wang, X., Zhao, X. M., Hu, Y. W., Liu, X., and Deng, D. W. (2025). Co-assembly strategies of natural plant compounds for improving their bioavailability. Food and Med. Homol. 2 (2), 9420022. doi:10.26599/fmh.2025.9420022
Morales-Cano, D., Menendez, C., Moreno, E., Moral-Sanz, J., Barreira, B., Galindo, P., et al. (2014). The flavonoid quercetin reverses pulmonary hypertension in rats. PLoS One 9, e114492. doi:10.1371/journal.pone.0114492
Nie, X., Shen, C., Tan, J., Yang, X., Wang, W., Dai, Y., et al. (2021). Andrographolide attenuates established pulmonary hypertension via rescue of vascular remodeling. Biomolecules 11, 11. doi:10.3390/biom11121801
Niu, Z., Fu, M., Li, Y., Ren, H., Zhang, X., and Yao, L. (2022). Osthole alleviates pulmonary vascular remodeling by modulating microRNA-22-3p mediated lipid metabolic reprogramming. Phytomedicine 96, 153840. doi:10.1016/j.phymed.2021.153840
Pang, H., Wu, L., Tang, Y., Zhou, G., Qu, C., and Duan, J. A. (2016). Chemical Analysis of the Herbal Medicine Salviae miltiorrhizae Radix et Rhizoma (Danshen). Molecules 21, 51. doi:10.3390/molecules21010051
Peng, W., He, C. X., Li, R. L., Qian, D., Wang, L. Y., Chen, W. W., et al. (2024). Zanthoxylum bungeanum amides ameliorates nonalcoholic fatty liver via regulating gut microbiota and activating AMPK/Nrf2 signaling. J. Ethnopharmacol. 318, 116848. doi:10.1016/j.jep.2023.116848
Perini, G. F., Ribeiro, G. N., Pinto Neto, J. V., Campos, L. T., and Hamerschlak, N. (2018). BCL-2 as therapeutic target for hematological malignancies. J. Hematol. Oncol. 11, 65. doi:10.1186/s13045-018-0608-2
Qian, G., Cao, J., Chen, C., Wang, L., Huang, X., Ding, C., et al. (2013). Paeoniflorin inhibits pulmonary artery smooth muscle cells proliferation via upregulating A2B adenosine receptor in rat. PLoS One 8, e69141. doi:10.1371/journal.pone.0069141
Qian, D., Zhang, Q., He, C. X., Guo, J., Huang, X. T., Zhao, J., et al. (2024). Hai-honghua medicinal liquor is a reliable remedy for fracture by promotion of osteogenic differentiation via activation of PI3K/Akt pathway. J. Ethnopharmacol. 330, 118234. doi:10.1016/j.jep.2024.118234
Qin, C., Zan, Y., Xie, L., and Liu, H. (2022). Ataxia telangiectasia mutated: the potential negative regulator in platelet-derived growth factor-BB promoted proliferation of pulmonary arterial smooth muscle cells. Front. Cardiovasc Med. 9, 942251. doi:10.3389/fcvm.2022.942251
Rai, N., Sydykov, A., Kojonazarov, B., Wilhelm, J., Manaud, G., Veeroju, S., et al. (2022). Targeting peptidyl-prolyl isomerase 1 in experimental pulmonary arterial hypertension. Eur. Respir. J. 60, 60. doi:10.1183/13993003.01698-2021
Rybka, V., Suzuki, Y. J., and Shults, N. V. (2018). Effects of Bcl-2/Bcl-x(L) inhibitors on pulmonary artery smooth muscle cells. Antioxidants (Basel) 7, 7. doi:10.3390/antiox7110150
Sadowska, O., Baranowska-Kuczko, M., Gromotowicz-Popławska, A., Biernacki, M., Kicman, A., Malinowska, B., et al. (2020). Cannabidiol ameliorates monocrotaline-induced pulmonary hypertension in rats. Int. J. Mol. Sci., 21. doi:10.3390/ijms21197077
Sheng, Y., Gong, X., Zhao, J., Liu, Y., and Yuan, Y. (2022). Effects of crocin on CCL2/CCR2 inflammatory pathway in monocrotaline-induced pulmonary arterial hypertension rats. Am. J. Chin. Med. 50, 241–259. doi:10.1142/s0192415x22500082
Siddique, M. A. H., Satoh, K., Kurosawa, R., Kikuchi, N., Elias-Al-Mamun, M., Omura, J., et al. (2019). Identification of emetine as a therapeutic agent for pulmonary arterial hypertension: novel effects of an old drug. Arterioscler. Thromb. Vasc. Biol. 39, 2367–2385. doi:10.1161/atvbaha.119.313309
Solinc, J., Ribot, J., Soubrier, F., Pavoine, C., Dierick, F., and Nadaud, S. (2022). The platelet-derived growth factor pathway in pulmonary arterial hypertension: still an interesting target? Life (Basel) 12, 12. doi:10.3390/life12050658
Sun, N., Wang, Y. B., Huang, J., Deng, R. W., He, H. Y., Gao, L., et al. (2025). Irisin attenuates pulmonary vascular remodeling in pulmonary arterial hypertension via ubiquitin-mediated regulation of ENO1. Adv. Sci. (Weinh) 12, e00096. doi:10.1002/advs.202500096
Tajsic, T., and Morrell, N. W. (2011). Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr. Physiol. 1, 295–317. doi:10.1002/cphy.c100026
Wande, Y., Jie, L., Aikai, Z., Yaguo, Z., Linlin, Z., Yue, G., et al. (2020). Berberine alleviates pulmonary hypertension through Trx1 and β-catenin signaling pathways in pulmonary artery smooth muscle cells. Exp. Cell. Res. 390, 111910. doi:10.1016/j.yexcr.2020.111910
Wang, X., Yang, Y., Yang, D., Tong, G., Lv, S., Lin, X., et al. (2016). Tetrandrine prevents monocrotaline-induced pulmonary arterial hypertension in rats through regulation of the protein expression of inducible nitric oxide synthase and cyclic guanosine monophosphate-dependent protein kinase type 1. J. Vasc. Surg. 64, 1468–1477. doi:10.1016/j.jvs.2015.09.016
Wei, S., Lin, L., Jiang, W., Chen, J., Gong, G., and Sui, D. (2023). Naked cuticle homolog 1 prevents mouse pulmonary arterial hypertension via inhibition of Wnt/β-catenin and oxidative stress. Aging 15, 11114–11130. doi:10.18632/aging.205105
Wei, S., Ju, F., Xiao, J., Li, J., Liu, T., and Hu, Z. (2025). Aloperine alleviates myocardial injury induced by myocardial ischemia and reperfusion by activating the ERK1/2/β-catenin signaling pathway. Cardiovasc Drugs Ther. 39, 533–551. doi:10.1007/s10557-024-07566-0
Wu, J., Jia, J., Liu, L., Yang, F., Fan, Y., Zhang, S., et al. (2017a). Schisandrin B displays a protective role against primary pulmonary hypertension by targeting transforming growth factor β1. J. Am. Soc. Hypertens. 11, 148–157.e141. doi:10.1016/j.jash.2016.12.007
Wu, F., Yao, W., Yang, J., Zhang, M., Xu, Y., Hao, Y., et al. (2017b). Protective effects of aloperin on monocroline-induced pulmonary hypertension via regulation of rho A/Rho kinsase pathway in rats. Biomed. Pharmacother. 95, 1161–1168. doi:10.1016/j.biopha.2017.08.126
Wujak, M., Veith, C., Wu, C. Y., Wilke, T., Kanbagli, Z. I., Novoyatleva, T., et al. (2021). Adenylate kinase 4-A key regulator of proliferation and metabolic shift in human pulmonary arterial smooth muscle cells via akt and HIF-1α signaling pathways. Int. J. Mol. Sci. 22, 22. doi:10.3390/ijms221910371
Xiao, X. H., Luo, F. M., Wang, E. L., Fu, M. Y., Li, T., Jiang, Y. P., et al. (2022). Magnolol alleviates hypoxia-induced pulmonary vascular remodeling through inhibition of phenotypic transformation in pulmonary arterial smooth muscle cells. Biomed. Pharmacother. 150, 113060. doi:10.1016/j.biopha.2022.113060
Xie, S., Zhao, J., Zhang, F., Li, X., Yu, X., Shu, Z., et al. (2025). Dehydrodiisoeugenol inhibits PDGF-BB-induced proliferation and migration of human pulmonary artery smooth muscle cells via the mTOR/HIF1-α/HK2 signaling pathway. Toxicol. Appl. Pharmacol. 495, 117212. doi:10.1016/j.taap.2024.117212
Yang, J., Liu, J., Gu, H., Song, W., Zhang, H., Wang, J., et al. (2025). Gut microbiota, metabolites, and pulmonary hypertension: mutual regulation and potential therapies. Microbiol. Res. 299, 128245. doi:10.1016/j.micres.2025.128245
Yao, Y., Song, L., Zuo, Z., Chen, Z., Wang, Y., Cai, H., et al. (2024). Parthenolide attenuates hypoxia-induced pulmonary hypertension through inhibiting STAT3 signaling. Phytomedicine 134, 155976. doi:10.1016/j.phymed.2024.155976
Yu, L., Tu, Y., Jia, X., Fang, K., Liu, L., Wan, L., et al. (2017). Resveratrol protects against pulmonary arterial hypertension in rats via activation of silent information regulator 1. Cell. Physiol. Biochem. 42, 55–67. doi:10.1159/000477115
Yu, W., Chen, H., Yang, H., Ding, J., Xia, P., Mei, X., et al. (2019). Dissecting molecular mechanisms underlying pulmonary vascular smooth muscle cell dedifferentiation in pulmonary hypertension: role of mutated Caveolin-1 (Cav1(F92A))-Bone marrow mesenchymal stem cells. Heart Lung Circ. 28, 1587–1597. doi:10.1016/j.hlc.2018.08.002
Yue, H., Febbraio, M., Klenotic, P. A., Kennedy, D. J., Wu, Y., Chen, S., et al. (2019). CD36 enhances vascular smooth muscle cell proliferation and development of neointimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 39, 263–275. doi:10.1161/atvbaha.118.312186
Yue, Y., Li, Y. Q., Fu, S., Wu, Y. T., Zhu, L., Hua, L., et al. (2020). Osthole inhibits cell proliferation by regulating the TGF-β1/Smad/p38 signaling pathways in pulmonary arterial smooth muscle cells. Biomed. Pharmacother. 121, 109640. doi:10.1016/j.biopha.2019.109640
Zeng, H. H., Dong, W., Ding, C., Xie, Y. P., Liu, L. J., and Wang, L. X. (2009). Effect of safflower injection on pulmonary hypertension in rat during chronic hypoxia and hypercapnia. Zhongguo Ying Yong Sheng Li Xue Za Zhi 25, 36–40.
Zeng, Z. H., Wu, W. H., Peng, Q., Sun, Y. H., and Liu, J. X. (2019). MicroRNA-132 mediates proliferation and migration of pulmonary smooth muscle cells via targeting PTEN. Mol. Med. Rep. 19, 3823–3830. doi:10.3892/mmr.2019.10053
Zhang, B., Niu, W., Xu, D., Li, Y., Liu, M., Wang, Y., et al. (2014). Oxymatrine prevents hypoxia- and monocrotaline-induced pulmonary hypertension in rats. Free Radic. Biol. Med. 69, 198–207. doi:10.1016/j.freeradbiomed.2014.01.013
Zhang, Q., Fan, K., Wang, P., Yu, J., Liu, R., Qi, H., et al. (2016). Carvacrol induces the apoptosis of pulmonary artery smooth muscle cells under hypoxia. Eur. J. Pharmacol. 770, 134–146. doi:10.1016/j.ejphar.2015.11.037
Zhang, Z., Zhang, L., Sun, C., Kong, F., Wang, J., Xin, Q., et al. (2017a). Baicalin attenuates monocrotaline-induced pulmonary hypertension through bone morphogenetic protein signaling pathway. Oncotarget 8, 63430–63441. doi:10.18632/oncotarget.18825
Zhang, Y., Cui, Y., Deng, W., Wang, H., Qin, W., Huang, C., et al. (2017b). Isoquercitrin protects against pulmonary hypertension via inhibiting PASMCs proliferation. Clin. Exp. Pharmacol. Physiol. 44, 362–370. doi:10.1111/1440-1681.12705
Zhang, Y. X., Li, J. F., Yang, Y. H., Zhai, Z. G., Gu, S., Liu, Y., et al. (2018a). Renin-angiotensin system regulates pulmonary arterial smooth muscle cell migration in chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 314, L276–l286. doi:10.1152/ajplung.00515.2016
Zhang, N., Dong, M., Luo, Y., Zhao, F., and Li, Y. (2018b). Danshensu prevents hypoxic pulmonary hypertension in rats by inhibiting the proliferation of pulmonary artery smooth muscle cells via TGF-β-smad3-associated pathway. Eur. J. Pharmacol. 820, 1–7. doi:10.1016/j.ejphar.2017.12.010
Zhang, X., Liu, Q., Zhang, C., Sheng, J., Li, S., Li, W., et al. (2019). Puerarin prevents progression of experimental hypoxia-induced pulmonary hypertension via inhibition of autophagy. J. Pharmacol. Sci. 141, 97–105. doi:10.1016/j.jphs.2019.09.010
Zhang, Z. Q., Zhu, S. K., Wang, M., Wang, X. A., Tong, X. H., Wan, J. Q., et al. (2022). New progress in diagnosis and treatment of pulmonary arterial hypertension. J. Cardiothorac. Surg. 17, 216. doi:10.1186/s13019-022-01947-y
Zhang, X., Yang, Z., Su, S., Nan, X., Xie, X., Li, Z., et al. (2023). Kaempferol ameliorates pulmonary vascular remodeling in chronic hypoxia-induced pulmonary hypertension rats via regulating Akt-GSK3β-cyclin axis. Toxicol. Appl. Pharmacol. 466, 116478. doi:10.1016/j.taap.2023.116478
Zhang, Z., Chen, J., Su, S., Xie, X., Ji, L., Li, Z., et al. (2024). Luteolin ameliorates hypoxic pulmonary vascular remodeling in rat via upregulating K(V)1.5 of pulmonary artery smooth muscle cells. Phytomedicine 132, 155840. doi:10.1016/j.phymed.2024.155840
Zhu, T. T., Zhang, W. F., Luo, P., He, F., Ge, X. Y., Zhang, Z., et al. (2017). Epigallocatechin-3-gallate ameliorates hypoxia-induced pulmonary vascular remodeling by promoting mitofusin-2-mediated mitochondrial fusion. Eur. J. Pharmacol. 809, 42–51. doi:10.1016/j.ejphar.2017.05.003
Zhu, N., Xiang, Y., Zhao, X., Cai, C., Chen, H., Jiang, W., et al. (2019). Thymoquinone suppresses platelet-derived growth factor-BB-induced vascular smooth muscle cell proliferation, migration and neointimal formation. J. Cell. Mol. Med. 23, 8482–8492. doi:10.1111/jcmm.14738
Zhu, Y., Sun, Y., Zhang, S., Li, C., Zhao, Y., Zhao, B., et al. (2021a). Xinmai 'an extract enhances the efficacy of sildenafil in the treatment of pulmonary arterial hypertension via inhibiting MAPK signalling pathway. Pharm. Biol. 59, 594–605. doi:10.1080/13880209.2021.1917629
Zhu, L., Li, Y. L., Qian, Z. Q., Hua, L., Yue, Y., and Yang, D. L. (2021b). Osthole improves pulmonary artery hypertension by inducing apoptosis in pulmonary artery smooth muscle cells. J. Pharm. Pharmacol. 73, 1109–1117. doi:10.1093/jpp/rgab068
Keywords: mechanism, medicinal plants or secondary metabolites, PASMCs, pathological changes, pH
Citation: Liang Q, Gou H, Zhu Y, He L, Chen L, Zhang C and Ai L (2026) Inhibiting the pathological changes of PASMCs is an effective approach for medicinal plants or secondary metabolites in treating pulmonary hypertension. Front. Pharmacol. 17:1725809. doi: 10.3389/fphar.2026.1725809
Received: 22 October 2025; Accepted: 19 January 2026;
Published: 12 February 2026.
Edited by:
Narasaiah Kolliputi, University of South Florida, United StatesReviewed by:
Ni Zhu, Changhai Hospital, ChinaSila Özlem Şener, University of Health Sciences, Türkiye
Copyright © 2026 Liang, Gou, Zhu, He, Chen, Zhang and Ai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Ai, YWlsaWNkdXRjbUAxNjMuY29t, YWlsaUBjZHV0Y20uZWR1LmNu; Chuantao Zhang, emhhbmdjaHVhbnRhb0BjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work
 Qi Liang
Qi Liang Hanghang Gou1†
Hanghang Gou1† Yi Zhu
Yi Zhu Lin He
Lin He Chuantao Zhang
Chuantao Zhang Li Ai
Li Ai
