- 1Department of Emergency, Cancer Hospital of Shantou University Medical College, Shantou, China
- 2Department of Radiation Oncology, Jieyang People’s Hospital, Jieyang, China
- 3Shantou University Medical College, Shantou, China
- 4Department of Radiation Oncology, Cancer Hospital of Shantou University Medical College, Shantou, China
Cancer immunotherapy, including immune checkpoint inhibitors and CAR-T cell therapy, has revolutionized oncology but is associated with a broad spectrum of cardiovascular toxicities. This review comprehensively examines the current landscape of these adverse events, which range from myocarditis, pericardial disease, and arrhythmias to heart failure. We delve into the underlying pathophysiological mechanisms, such as T-cell-mediated cross-reactivity via molecular mimicry and cytokine-mediated injury in cytokine release syndrome. The article critically appraises strategies for risk stratification, vigilant monitoring using biomarkers and advanced imaging, and management protocols that encompass immunosuppression, targeted biological therapies, and supportive care. Furthermore, we explore the complex interplay with vaccinations and infections and highlight promising future directions, including novel therapeutic targets, preventive strategies, and advanced monitoring technologies. Ultimately, this review underscores the necessity of a proactive and multidisciplinary cardio-oncology framework to mitigate cardiovascular risks while preserving the anticancer efficacy of immunotherapies.
1 Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment by harnessing the immune system to combat malignancies. These therapies, including antibodies targeting programmed cell death protein 1 (PD-1), programmed cell death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), have demonstrated remarkable efficacy across various tumor types (Liu L. et al., 2024; Panuccio et al., 2024a). However, the enhanced immune activation can lead to immune-related adverse events (irAEs) affecting multiple organ systems, with cardiovascular toxicities representing particularly serious complications (Pi et al., 2024; Keam et al., 2024). Among these, immunotherapy-induced heart failure emerges as a critical concern due to its potential for rapid progression and high mortality rates (Izumi et al., 2024; Nielsen et al., 2024).
The spectrum of cardiovascular complications associated with cancer immunotherapy extends beyond myocarditis to include various forms of cardiac dysfunction that may culminate in heart failure (Munir et al., 2024; Tocchetti et al., 2024). While initial attention focused on ICI-related myocarditis, recent evidence suggests that more subtle forms of cardiac impairment may occur, potentially progressing to overt heart failure (Braghieri et al., 2025; Delombaerde et al., 2024). The complex interplay between enhanced immune activation and cardiovascular homeostasis creates a challenging clinical scenario where early detection and intervention become paramount for optimal patient outcomes (Chen Z. et al., 2024; Toublanc et al., 2024).
This review comprehensively examines the current understanding of immunotherapy-related cardiovascular toxicity, encompassing epidemiological patterns, underlying mechanisms, diagnostic approaches, management strategies, and future directions. By synthesizing evidence from recent clinical studies and mechanistic investigations, we aim to provide clinicians with a practical framework for recognizing, monitoring, and treating this potentially fatal complication while highlighting critical knowledge gaps that warrant further investigation.
2 The spectrum of cardiovascular toxicities associated with cancer immunotherapy
Cancer immunotherapy has unveiled a diverse array of cardiovascular complications that extend beyond the initially recognized myocarditis. The cardiovascular toxicities associated with immune checkpoint inhibitors encompass a broad clinical spectrum, ranging from subclinical cardiac biomarker elevations to fulminant heart failure and fatal arrhythmias (Nielsen et al., 2024; Milutinovic et al., 2024). Myocarditis remains the most extensively studied cardiotoxicity, with incidence rates varying from 0.3% to 1.14% in clinical trials but potentially higher in real-world settings (Lindsay et al., 2025; Li et al., 2025). Combination ICI therapy demonstrates significantly increased risk compared to monotherapy, with hazard ratios reaching 2.38 for myocarditis development (Lindsay et al., 2025).
Beyond inflammatory cardiac conditions, ICIs are associated with various forms of cardiac dysfunction that may progress to heart failure. Pericardial diseases, including pericarditis and pericardial effusions, occur with notable frequency (Piazza et al., 2025; Li and Li, 2024). Arrhythmias represent another significant concern, with atrial fibrillation being the most commonly reported cardiac disorder in some analyses (Koecke et al., 2024). Vascular events, including vasculitis and thromboembolic phenomena, further expand the cardiovascular toxicity profile (Grabska et al., 2024).
Chimeric antigen receptor (CAR) T-cell therapy introduces additional cardiovascular considerations, primarily driven by cytokine release syndrome (CRS) (Daryanani et al., 2024). The pooled incidence of cardiovascular events following CAR-T cell therapy includes 54% for arrhythmias, 30% for heart failure, and 20% for cardiomyopathy. These events frequently occur in the context of CRS, with patients experiencing CRS grade ≥2 having a 2.36-fold increased risk of cardiovascular complications (Maleki et al., 2024). The hemodynamic stress from CRS, combined with direct inflammatory effects on cardiac tissue, creates a perfect storm for cardiac dysfunction development.
The timing of cardiovascular event onset varies considerably across immunotherapeutic approaches. ICI-related events typically occur within the first few months of treatment initiation, with median onset around 49 days (Lipe et al., 2024; Eldani et al., 2024). In contrast, CAR-T cell associated cardiovascular complications generally manifest within days to weeks following infusion, closely aligned with CRS development (Daryanani et al., 2024). This temporal pattern has important implications for monitoring strategies and intervention timing.
Understanding the full spectrum of immunotherapy-associated cardiovascular toxicities is essential for developing comprehensive screening and management protocols. The spectrum and key characteristics of these toxicities are summarized in Table 1. The heterogeneity in clinical presentation, combined with the potential for rapid deterioration, necessitates heightened awareness among oncologists and cardiologists alike to ensure prompt recognition and treatment of these potentially devastating complications.
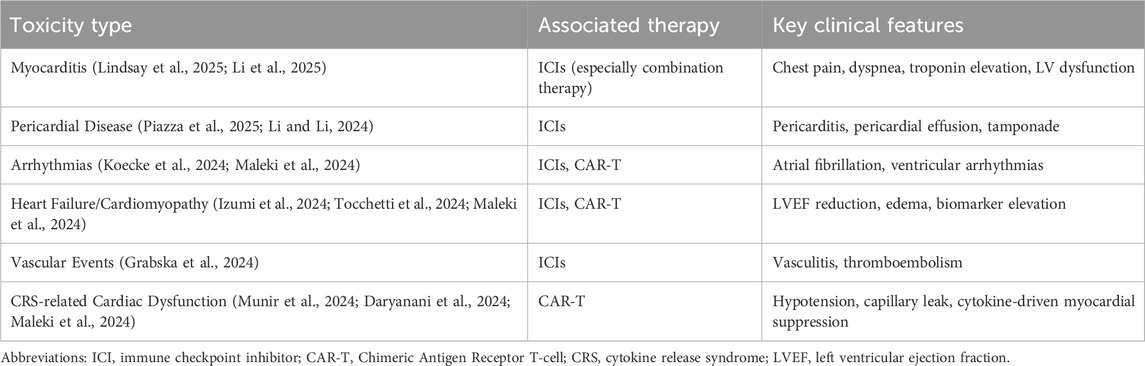
Table 1. Spectrum and characteristics of cardiovascular toxicities associated with cancer immunotherapy.
3 Pathophysiological mechanisms underlying immunotherapy-induced cardiotoxicity
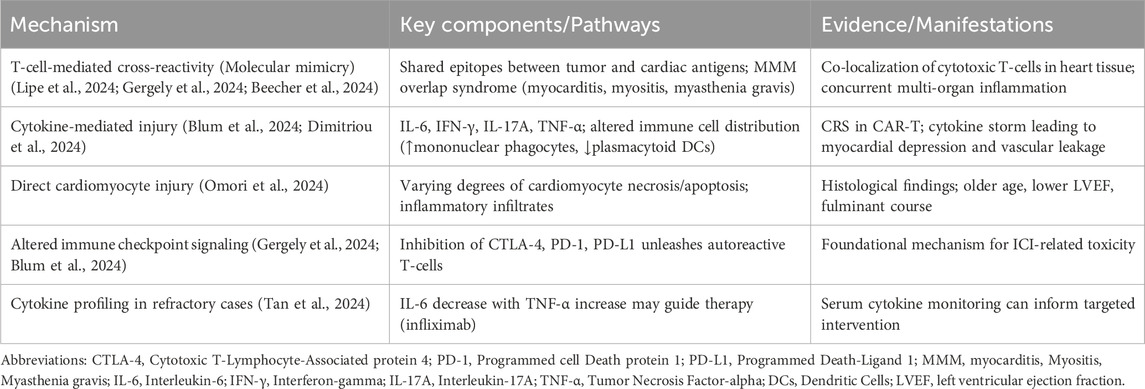
The pathophysiological mechanisms driving immunotherapy-induced cardiotoxicity involve complex interactions between immune activation and cardiovascular homeostasis. The fundamental principle underlying these toxicities revolves around the disruption of immune checkpoint pathways that normally maintain self-tolerance and prevent autoimmunity (Gergely et al., 2024). By inhibiting CTLA-4, PD-1, or PD-L1, ICIs remove inhibitory signals that constrain T-cell activation, potentially unleashing autoreactive T-cells that recognize cardiac antigens (Blum et al., 2024).
Molecular mimicry represents a proposed mechanism whereby T-cells primed against tumor antigens cross-react with similar epitopes expressed on cardiac tissue (Gergely et al., 2024). This phenomenon may explain why myocarditis frequently coincides with other irAEs, particularly myositis and myasthenia gravis, in what is termed the MMM overlap syndrome. The shared epitopes between cardiac muscle, skeletal muscle, and neuromuscular junction components create opportunities for cross-reactivity that manifests as concurrent multi-organ inflammation (Lipe et al., 2024; Beecher et al., 2024).
Cytokine-mediated injury constitutes another significant pathway in immunotherapy-related cardiotoxicity. The analysis of heart tissue from patients with ICI-myocarditis reveals increased frequencies and co-localization of cytotoxic T-cells, conventional dendritic cells, and inflammatory fibroblasts. Simultaneously, peripheral blood shows decreased frequencies of plasmacytoid dendritic cells and B-lineage cells but increased mononuclear phagocytes (Blum et al., 2024). This altered immune cell distribution creates a pro-inflammatory milieu that can compromise cardiac function.
The role of specific cytokine pathways continues to be elucidated. Evidence suggests involvement of type I and type III immune signatures, with particular emphasis on interleukin (IL)-17A expressing CD4 T-cells (Dimitriou et al., 2024). In patients experiencing irAEs, proteomic analyses demonstrate aberrant T-cell activity with differential expression of these immune signatures. The finding that anti-IL-17A administration resolved adverse events in two patients with mild myocarditis, colitis, and skin rash provides preliminary evidence for targeting specific cytokine pathways.
Beyond inflammatory mechanisms, direct cardiomyocyte injury contributes to cardiac dysfunction. Histological examination of myocarditis cases reveals varying degrees of cardiomyocyte injury, ranging from pronounced to absent, along with different types of inflammatory infiltrates (Omori et al., 2024). Those with cardiomyocyte injury present clinically with older age, balanced sex distribution, less frequent chest pain, and lower left ventricular ejection fraction, and more frequently develop fulminant myocarditis requiring mechanical circulatory support.
Emerging evidence also suggests a potential interplay between immune checkpoint inhibition and the progression of coronary atherosclerosis. The systemic inflammatory state induced by ICIs, characterized by elevated cytokines and activated T-cells, may exacerbate endothelial dysfunction, promote plaque instability, and accelerate atherosclerotic progression (Baitsch et al., 2011; Suero-Abreu et al., 2022). This interaction raises the possibility that ICI-related cardiotoxicity may not only manifest as acute myocarditis but also as a worsening of underlying coronary artery disease, potentially leading to acute coronary syndromes (Thuny et al., 2022). In this context, timely coronary revascularization may play a crucial role in managing significant coronary lesions exacerbated by immunotherapy, aiming to restore blood flow, stabilize vulnerable plaques, and prevent ischemic complications. Recent meta-analyses underscore the importance of revascularization in improving cardiovascular outcomes in patients with chronic coronary syndromes, highlighting its relevance even in the setting of cancer therapy-induced vascular injury (Panuccio et al., 2024b).
The cardiovascular effects of cytokine release syndrome in CAR-T cell therapy involve additional mechanisms. CRS generates massive inflammatory responses with elevated levels of IL-6, interferon-γ, and other cytokines that can directly suppress myocardial function and promote vascular leakage (Munir et al., 2024; Camilli et al., 2024). This cytokine storm can induce cardiac dysfunction through both direct myocardial depression and indirect effects on vascular integrity and afterload (Maleki et al., 2024). The key pathophysiological mechanisms underlying immunotherapy-induced cardiotoxicity are outlined in Table 2.
Understanding these multifaceted mechanisms is crucial for developing targeted therapeutic approaches that mitigate cardiotoxicity without compromising antitumor efficacy. The complexity of these pathways underscores the need for continued mechanistic research to identify optimal intervention points for preventing and treating immunotherapy-related heart failure.
4 Risk stratification, monitoring, and diagnostic approaches
Effective management of immunotherapy-related cardiovascular toxicity necessitates robust risk stratification, vigilant monitoring, and accurate diagnostic approaches. Current evidence suggests several potential biomarkers and clinical factors that may help identify patients at elevated risk before and during treatment (Delombaerde et al., 2024). Baseline cardiovascular risk factors, including hypertension and dyslipidemia, appear to increase susceptibility to cardiac events during immunotherapy (Salas et al., 2024; Peng et al., 2024). Older age, male sex, and prior radiation therapy have also been associated with increased major adverse cardiovascular event risk (Li et al., 2025).
Multimodal biomarker assessment shows promise for risk prediction. A scoring strategy incorporating four biomarkers—N-terminal-pro-B-type-natriuretic-peptide (NT-proBNP) (≥125 pg/mL), cardiac troponin T (≥6 ng/L), high-sensitivity C-reactive protein (≥3 mg/L), and coronary artery calcium score (>10 U)—demonstrated ability to stratify patients according to ICIs-related cardiotoxicity risk. Patients with very high scores (4 points) showed 7.29 to 8.83-fold higher risk compared to those with low scores (Chen Z. et al., 2024). An overview of risk stratification tools and monitoring approaches is provided in Table 3. These biomarkers also predicted earlier onset and higher incidence of cardiotoxicity, suggesting potential utility in pre-treatment assessment.
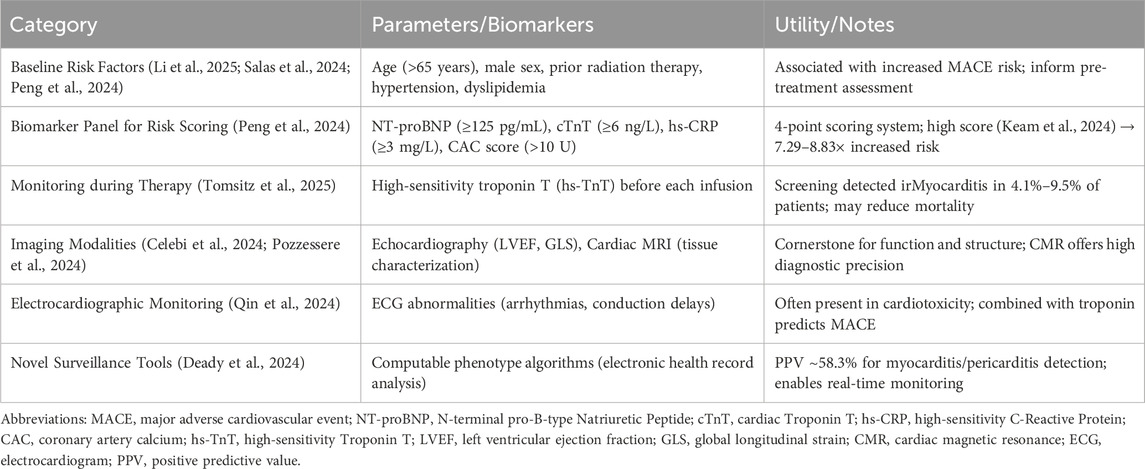
Table 3. Risk stratification and monitoring tools for immunotherapy-related cardiovascular toxicity.
Monitoring during therapy primarily relies on cardiac biomarkers and imaging modalities. Troponin surveillance has emerged as a valuable tool for early detection of myocardial injury. Prospective screening with high-sensitivity cardiac troponin T (hs-TnT) before each ICI infusion identified irMyocarditis in 4.1% of patients with normal baseline hs-TnT and 9.5% of those with elevated baseline. Importantly, no fatalities occurred among the 16 irMyocarditis patients detected through this screening approach, suggesting potential mortality reduction through early intervention (Tomsitz et al., 2025).
Echocardiography remains a cornerstone for monitoring cardiac function during immunotherapy. Left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) represent standard parameters for assessment, though their sensitivity for early detection requires further validation. In one study, reduced left ventricular GLS did not demonstrate significant predictive value for ICI-related myocarditis or cardiac dysfunction, though the small sample size limited definitive conclusions (Celebi et al., 2024). Advanced imaging modalities, particularly cardiac magnetic resonance (CMR) with tissue characterization, provide additional diagnostic precision for detecting inflammatory cardiac conditions (Pozzessere et al., 2024; Efentakis et al., 2024).
Electrocardiographic monitoring offers another valuable tool, with abnormalities frequently present in patients developing cardiotoxicity (Qin et al., 2024). In patients with ICI-related myocarditis, electrocardiogram changes combined with elevated troponin levels helped predict major adverse cardiac events. The combination of these readily available parameters may facilitate early intervention before overt heart failure develops.
For CAR-T cell recipients, monitoring strategies must account for the different timing and mechanism of cardiotoxicity. The strong association between CRS and cardiovascular events suggests that close monitoring should be instituted during and after CRS development (Daryanani et al., 2024). Cardiac biomarkers, including natriuretic peptides and troponins, along with echocardiographic assessment, appear valuable for detecting emerging cardiac dysfunction in this context (Camilli et al., 2024).
The emergence of novel monitoring technologies, including computable phenotype algorithms for automated adverse event detection, may enhance surveillance capabilities (Deady et al., 2024). Such approaches demonstrated positive predictive values around 58.3% for myocarditis/pericarditis detection, though performance varied across healthcare systems. These tools could potentially be integrated into electronic health records to facilitate real-time monitoring of large patient populations.
Despite these advances, significant knowledge gaps remain in optimal monitoring strategies. The frequency of assessments, the most cost-effective approach for different risk groups, and the integration of novel biomarkers require further investigation. Standardized protocols for surveillance and diagnosis will be essential for improving outcomes of patients experiencing immunotherapy-related cardiovascular toxicity.
5 Management strategies for immunotherapy-related cardiovascular adverse events
The management of immunotherapy-related cardiovascular adverse events requires a multidisciplinary approach involving oncologists, cardiologists, and other specialists. The cornerstone of management involves immediate discontinuation of the offending agent and initiation of immunosuppressive therapy, typically starting with high-dose corticosteroids (Tan et al., 2024; Liu Q. et al., 2024). For ICI-associated myocarditis, prompt initiation of methylprednisolone (1–2 mg/kg) is recommended, with escalation to other immunosuppressive agents for severe or refractory cases (Alghabba et al., 2025).
The management approach must be tailored according to severity grading. For grade 2 or higher cardiotoxicity, current guidelines recommend holding ICI therapy and initiating prednisone at 1–2 mg/kg/day. For severe (grade 3–4) events, hospitalization and intravenous methylprednisolone pulse therapy (1,000 mg daily for 3–5 days) is indicated. Refractory cases may require additional immunosuppressive agents, including mycophenolate mofetil, infliximab, or abatacept (Nielsen et al., 2024). A summary of management strategies based on severity grading is presented in Table 4.
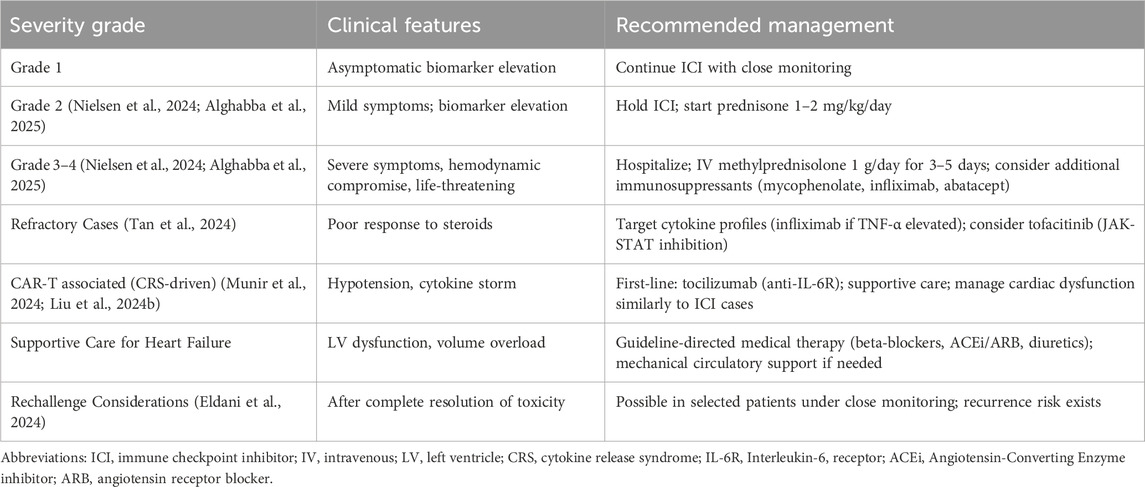
Table 4. Management strategies based on severity of immunotherapy-related cardiovascular adverse events.
Emerging evidence supports targeted approaches based on specific cytokine profiles. In steroid-refractory myocarditis cases where serum interleukin (IL)-6 decreased by >50% while tumor necrosis factor-α (TNF-α) increased, infliximab administration resulted in gradual alleviation of myocarditis. This suggests that cytokine monitoring might guide selection of specific cytokine inhibitors for refractory cases (Tan et al., 2024).
For cardiovascular complications associated with CAR-T cell therapy, management focuses on controlling the underlying CRS. Tocilizumab, an IL-6 receptor antagonist, serves as first-line therapy for moderate to severe CRS (7). The management of subsequent cardiac dysfunction follows similar principles to ICI-related events, though the context of profound cytokine release may necessitate additional supportive measures.
Supportive care represents a critical component of management, particularly for patients developing heart failure. Standard heart failure therapies, including beta-blockers, angiotensin-converting enzyme inhibitors, and diuretics, should be implemented according to current guidelines (Tocchetti et al., 2024). In severe cases with hemodynamic compromise, mechanical circulatory support may be necessary as a bridge to recovery (Izumi et al., 2024).
The question of rechallenge after cardiovascular toxicity remains complex and inadequately studied. Limited evidence suggests that rechallenge may be possible in selected patients after complete resolution of the adverse event and under close monitoring. In one series, among 15 patients retreated with ICIs after severe irAEs (including cardiovascular and neurological events), 73% remained free of subsequent irAEs, while 13% experienced recurrence of the initial irAE and 13% developed new irAEs (Eldani et al., 2024). However, these decisions must be individualized, weighing the oncological benefits against potential risks.
Novel preventive strategies are emerging from mechanistic studies. Statins, particularly atorvastatin, demonstrated protective effects in preclinical models of ICI-related cardiotoxicity (Efentakis et al., 2024; Jiang et al., 2024). The pleiotropic effects of statins, including antioxidative, anti-inflammatory, antifibrotic, endothelial protective, and immune modulatory properties, position them as promising candidates for cardioprotection during immunotherapy. Similarly, glucagon-like peptide-1 agonists showed reduced major adverse cardiovascular events in cancer patients with type 2 diabetes receiving ICIs (Chiang et al., 2025).
The management of immunotherapy-related cardiovascular events continues to evolve as our understanding of mechanisms expands. While corticosteroids remain foundational, targeted approaches based on specific pathological processes hold promise for improving outcomes while preserving anticancer efficacy. Multidisciplinary collaboration is essential for optimizing management strategies for these complex patients.
6 The interplay between vaccinations, infections, and immunotherapy cardiotoxicity
The relationship between vaccinations, infections, and immunotherapy-related cardiotoxicity presents a complex clinical scenario with significant implications for patient management. Emerging evidence suggests that viral infections, particularly SARS-CoV-2, may influence the development and severity of immune-related adverse events (Sumi et al., 2024; Gradone et al., 2024; Watson et al., 2024). Similarly, vaccinations—especially COVID-19 vaccines—have been associated with rare cardiovascular complications that may intersect with immunotherapy toxicities (Omori et al., 2024; Weirauch et al., 2025).
The temporal relationship between COVID-19 vaccination and ICI toxicity warrants careful consideration. Cases of severe myositis with myocarditis have been reported within 1 month of COVID-19 mRNA vaccination followed by PD-1 inhibition (Watson et al., 2024). Analysis of T-cell receptor repertoires from these patients revealed a high degree of clonal expansion and public clones between cases, with several T-cell clones expanded within skeletal muscle putatively recognizing viral epitopes. All patients had recently received COVID-19 mRNA booster vaccination prior to treatment and were positive for SARS-CoV-2 Spike antibody, suggesting potential interaction between vaccine-induced immune activation and ICI effects.
The incidence of vaccine-associated myocarditis/pericarditis must be contextualized within the broader benefit-risk profile of vaccination. The reporting rate for myocarditis/pericarditis following original monovalent COVID-19 mRNA vaccination was approximately 6.91 per million doses, while the rate for bivalent vaccination was significantly lower at 1.24 per million doses (Chen C. et al., 2024). These risks must be balanced against the established cardiovascular benefits of vaccination against infectious diseases.
Influenza vaccination demonstrates particular relevance for cancer patients receiving immunotherapy. Vaccination reduces the risk of major adverse cardiovascular events in patients with ischemic heart disease (Liu et al., 2025). For heart failure patients, influenza vaccination represents a cost-effective intervention that reduces hospitalizations and mortality (Miró et al., 2025; Zhao et al., 2024). In immunocompromised cancer patients, influenza vaccination was associated with reduced overall mortality across all subgroups, with the most pronounced reduction in patients with hematological cancer aged <65 years (adjusted hazard ratio 0.66) (Amdisen et al., 2025).
The potential interaction between infections and ICI toxicity extends beyond viral pathogens. Co-occurring infections in cancer patients treated with checkpoint inhibitors significantly increase the risk of immune-related adverse events. Analysis of over nineteen million reports revealed statistically significant associations between irAEs and concurrent infectious diseases for five out of seven ICIs treatments (Grabska et al., 2024). This finding suggests that infections may lower the threshold for developing irAEs, possibly through enhanced immune activation or molecular mimicry mechanisms.
The management of patients receiving immunotherapy during periods of prevalent infections requires careful consideration. The benefits of vaccination generally outweigh potential risks, but timing relative to immunotherapy cycles may influence both efficacy and safety (Tsang et al., 2024; Le Vu et al., 2024). For COVID-19 vaccination, extending the dosing interval has been employed to reduce myocarditis risk in adolescents, though this approach must balance protection against infection during the extended intra-dose period.
Understanding these complex interactions is essential for optimizing patient care during immunotherapy. Vaccination remains a critical component of supportive care for cancer patients, but awareness of potential intersections with immunotherapy toxicity can guide appropriate monitoring and management strategies.
7 Novel therapeutic targets and future directions in cardio-oncology
The rapidly evolving field of cardio-oncology continues to identify novel therapeutic targets and future directions for managing immunotherapy-related cardiovascular toxicity. Advances in understanding the molecular mechanisms underlying these adverse events have revealed several promising approaches for prevention and treatment (Kong et al., 2024). The recognition that specific immune pathways drive cardiotoxicity provides opportunities for targeted interventions that may preserve antitumor efficacy while mitigating cardiovascular risks.
The IL-17A pathway represents a compelling target based on evidence from patients experiencing irAEs (Dimitriou et al., 2024). The finding that anti-IL-17A administration resolved adverse events in two patients with mild myocarditis, colitis, and skin rash provides proof-of-principle for this approach. Further investigation in clinical trials is warranted to establish the efficacy and safety of IL-17 inhibition for immunotherapy-related cardiotoxicity. Similarly, the JAK-STAT pathway has emerged as another potential target, with tofacitinib showing promising clinical efficacy in patients experiencing irAEs, particularly those who failed to respond to steroids or experienced relapse during steroid tapering (Liu Q. et al., 2024).
Bioorthogonal metabolic engineering-driven extracellular vesicle redirecting (Biomeder) represents an innovative approach for reversing ICI-associated cardiotoxicity (Fan et al., 2024). This strategy involves in situ engineering of tumor-derived extracellular vesicles with myocardial-targeting peptides, which accumulate in the heart and reverse the immune environment by increasing PD-L1 levels in cardiomyocytes and/or directly inhibiting T-cell activity. Remarkably, this approach not only alleviated cardiotoxicity but also enhanced antitumor efficacy by disrupting immunosuppression in tumors. The technique was successfully expanded to prevent ICI-induced type 1 diabetes, suggesting potential applicability across various irAEs.
Statins continue to show promise as preventive agents based on their pleiotropic effects. Preclinical studies demonstrated that atorvastatin mitigated functional deficits in models of ICI-induced cardiotoxicity by inhibiting endothelial dysfunction (Efentakis et al., 2024). The antioxidative stress, anti-inflammatory, antifibrotic, endothelial protective, and immune modulatory properties of statins position them as attractive candidates for cardioprotection during immunotherapy (Jiang et al., 2024). Prospective clinical trials are needed to validate these findings and establish optimal dosing regimens.
The development of novel immunotherapeutic agents with improved safety profiles represents another important direction. PROTAC-based HPK1 degraders have shown high potency and selectivity for cancer immunotherapy with a low risk of cardiotoxicity and wide safety window in preclinical models (Zhang et al., 2024). Similarly, the CD80 variant immunoglobulin domain Fc fusion protein davoceticept (ALPN-202) was designed to mediate PD-L1-dependent CD28 costimulation while inhibiting PD-L1 and CTLA-4 checkpoints, potentially minimizing systemic T-cell activation and untoward toxicities (Cavalcante et al., 2024). However, cases of fatal myocarditis were reported with this agent in combination with pembrolizumab, highlighting the continued challenges in balancing efficacy and safety.
Advanced monitoring technologies offer additional opportunities for improving patient outcomes. Computable phenotype algorithms enable automated detection of adverse events through analysis of electronic health records, facilitating real-time surveillance (Deady et al., 2024). The implementation of such systems required 200–250 h but achieved positive predictive values of 58.3% for myocarditis/pericarditis detection. Further refinement and validation of these tools could significantly enhance early detection and management of cardiovascular toxicities.
The future of cardio-oncology will likely involve increasingly personalized approaches based on individual risk profiles, tumor characteristics, and specific immunotherapeutic regimens. Biomarker-guided therapy, leveraging advances in proteomics, transcriptomics, and immunosequencing, may enable precise targeting of pathological processes while preserving antitumor immunity. Multicenter collaborations and standardized registries will be essential for advancing our understanding of these complex interactions and developing evidence-based management strategies.
8 Discussion
The expanding use of cancer immunotherapies has unmasked a significant spectrum of cardiovascular toxicities that demand vigilant clinical attention and a deepened mechanistic understanding. This review synthesizes current evidence indicating that these adverse events, ranging from fulminant myocarditis to chronic cardiomyopathy, are not merely idiosyncratic reactions but are often rooted in dysregulated immune activation. The pathophysiology involves a complex interplay of T-cell-mediated cross-reactivity, cytokine-driven myocardial injury, and direct cardiomyocyte damage, as outlined in the preceding sections. Importantly, the risk extends beyond the widely recognized immune checkpoint inhibitors to include cellular therapies like CAR-T cells, where cytokine release syndrome serves as a potent driver of cardiac dysfunction.
A key clinical imperative that emerges is the necessity for proactive, multidisciplinary collaboration between oncology and cardiology—the core of the cardio-oncology framework. The management algorithms discussed herein, which hinge on early detection via biomarker surveillance and advanced imaging, followed by graded immunosuppression, underscore a paradigm shift from reactive to pre-emptive care. However, significant knowledge gaps persist. The optimal frequency of monitoring, the definitive role of novel biomarkers, and standardized protocols for re-challenging patients after a cardiotoxic event remain areas of active investigation and debate.
Furthermore, the intersecting influences of concurrent infections and vaccinations on immunotherapy toxicity profiles introduce additional layers of complexity to patient management. While vaccinations remain crucial for patient protection, their timing and potential interaction with immune checkpoint pathways warrant careful consideration.
In conclusion, the cardiovascular toxicities associated with cancer immunotherapy are serious but potentially manageable complications. Their successful mitigation hinges on a dual approach: first, the continued elucidation of underlying biological mechanisms to inform targeted therapies; and second, the rigorous implementation of structured surveillance and management protocols in clinical practice. Future research must focus on validating predictive risk models, refining therapeutic strategies to preserve anticancer efficacy, and fostering robust cardio-oncology partnerships to improve overall patient outcomes.
9 Future perspectives
Building upon the current understanding and management strategies discussed above, the field of cardio-oncology must now address several critical knowledge gaps and translate mechanistic insights into improved patient outcomes. The immediate future should be directed toward validating targeted therapeutic approaches in prospective clinical trials, particularly for steroid-refractory cases. Agents inhibiting specific cytokine pathways, such as IL-17A or JAK-STAT inhibitors, represent promising candidates based on early mechanistic studies and require rigorous evaluation of their efficacy and safety profiles.
Concurrently, advancing preventive strategies is paramount. Large-scale studies are needed to determine whether prophylactic administration of cardioprotective agents, such as statins, can mitigate toxicity without interfering with anticancer immunity. Parallel efforts must focus on refining risk stratification by discovering and validating predictive biomarkers that integrate genomic, proteomic, and clinical data to identify high-risk patients before initiating immunotherapy.
The integration of advanced monitoring technologies into routine clinical practice presents another crucial frontier. The development and standardization of computable phenotype algorithms for automated toxicity detection within electronic health records could enable real-time surveillance across large populations, facilitating earlier intervention. Furthermore, prospective studies must establish the optimal frequency and modality of cardiovascular monitoring tailored to different immunotherapies and patient risk profiles.
Ultimately, the evolution of cardio-oncology hinges on a deeply personalized, patient-centered approach. This will require not only biomarker-guided therapy but also a nuanced understanding of how tumor biology, host immunogenetics, and cardiovascular comorbidities interact to influence toxicity risk. Strengthening multidisciplinary collaboration through structured cardio-oncology programs and international registries will be essential to generate robust real-world evidence, standardize management protocols, and design the next-generation of clinical trials aimed at preserving the revolutionary benefits of cancer immunotherapy while ensuring cardiovascular safety.
Author contributions
LX: Conceptualization, Investigation, Writing – review and editing. WL: Conceptualization, Writing – original draft. YJ: Conceptualization, Writing – original draft, Investigation. QL: Investigation, Writing – review and editing. JP: Investigation, Writing – review and editing. RX: Writing – review and editing, Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alghabban, A., Corke, L., Katzberg, H., Bril, V., Barnett-Tapia, C., Mason, W., et al. (2025). Myasthenia gravis in patients treated with immune checkpoint inhibitors. JTO Clin. Res. Rep. 6, 100772. doi:10.1016/j.jtocrr.2024.100772
Amdisen, L., Pedersen, L., Abildgaard, N., Benn, C. S., Cronin-Fenton, D., and Sørup, S. (2025). Influenza vaccine effectiveness in immunocompromised patients with cancer: a Danish nationwide register-based cohort study. Cancer 131, e35574. doi:10.1002/cncr.35574
Baitsch, L., Baumgaertner, P., Devêvre, E., Raghav, S. K., Legat, A., Barba, L., et al. (2011). Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360. doi:10.1172/JCI46102
Beecher, G., Pinal-Fernandez, I., Mammen, A. L., and Liewluck, T. (2024). Immune checkpoint inhibitor myopathy: the double-edged sword of cancer immunotherapy. Neurology 103, 103. doi:10.1212/WNL.0000000000210031
Blum, S. M., Zlotoff, D. A., Smith, N. P., Kernin, I. J., Ramesh, S., Zubiri, L., et al. (2024). Immune responses in checkpoint myocarditis across heart, blood and tumour. Nature 636, 215–223. doi:10.1038/s41586-024-08105-5
Braghieri, L., Gharaibeh, A., Nkashama, L., Abushouk, A., Abushawer, O., Mehdizadeh-Shrifi, A., et al. (2025). Long-term cardiovascular outcomes of immune checkpoint inhibitor-related myocarditis: a large single-centre analysis. Esc. Heart Fail 12, 1237–1245. doi:10.1002/ehf2.15131
Camilli, M., Viscovo, M., Felici, T., Maggio, L., Ballacci, F., Carella, G., et al. (2024). Inflammation and acute cardiotoxicity in adult hematological patients treated with CAR-T cells: results from a pilot proof-of-concept study. Cardiooncology 10, 18. doi:10.1186/s40959-024-00218-0
Cavalcante, L., Chandana, S., Lakhani, N., Enstrom, A., LeBlanc, H., Schmalz, J., et al. (2024). Case report of fatal immune-mediated myocarditis following treatment with davoceticept (ALPN-202), a PD-L1-dependent CD28 costimulator and dual PD-L1/CTLA-4 checkpoint inhibitor, in combination with pembrolizumab. J. Immunother. Cancer 12, e009475. doi:10.1136/jitc-2024-009475
Celebi, C. E., Coskun, A., Sahin, A. B., Levent, F., Coban, E., Koca, F., et al. (2024). Left ventricular global longitudinal strain in patients treated with immune checkpoint inhibitors. Front. Oncol. 14, 1453721. doi:10.3389/fonc.2024.1453721
Chen, Z., Lan, R., Ran, T., Tao, L., Zhu, Y., Li, Y., et al. (2024a). A multimodality score strategy for assessing the risk of immune checkpoint inhibitors related cardiotoxicity. Sci. Rep. 14, 24821. doi:10.1038/s41598-024-76829-5
Chen, C., Chen, C., Cao, L., Fang, J., and Xiao, J. (2024b). Comparative safety profile of bivalent and original COVID-19 mRNA vaccines regarding myocarditis/pericarditis: a pharmacovigilance study. Int. Immunopharmacol. 133, 112022. doi:10.1016/j.intimp.2024.112022
Chiang, C.-H., Song, J., Chi, K.-Y., Chang, Y.-C., Xanthavanij, N., Chang, Y., et al. (2025). Glucagon-like Peptide-1 agonists reduce cardiovascular events in cancer patients on immune checkpoint inhibitors. Eur. J. Cancer 216, 115170. doi:10.1016/j.ejca.2024.115170
Daryanani, A. E., Abbasi, M. A., Gomez Ardila, M. F., Tellez-Garcia, E., Garzon-Dangond, J. M., Lin, Y., et al. (2024). Baseline echocardiographic variables as predictors of hemodynamically significant cytokine release syndrome in adults treated with CD19 CAR T-cell therapy for hematological malignancies. Cardio-Oncology 10, 91. doi:10.1186/s40959-024-00290-6
Deady, M., Duncan, R., Sonesen, M., Estiandan, R., Stimpert, K., Cho, S., et al. (2024). A computable phenotype algorithm for postvaccination myocarditis/pericarditis detection using real-world data: validation study. J. Med. Internet Res. 26, e54597. doi:10.2196/54597
Delombaerde, D., Vulsteke, C., Van De Veire, N., Vervloet, D., Moerman, V., Van Calster, L., et al. (2024). Close cardiovascular monitoring during the early stages of treatment for patients receiving immune checkpoint inhibitors. Pharmaceuticals 17, 965. doi:10.3390/ph17070965
Dimitriou, F., Cheng, P. F., Saltari, A., Schaper-Gerhardt, K., Staeger, R., Haunerdinger, V., et al. (2024). A targetable type III immune response with increase of IL-17A expressing CD4+ T cells is associated with immunotherapy-induced toxicity in melanoma. Nat. Cancer 5, 1390–1408. doi:10.1038/s43018-024-00810-4
Efentakis, P., Choustoulaki, A., Kwiatkowski, G., Varela, A., Kostopoulos, I. V., Tsekenis, G., et al. (2024). Early microvascular coronary endothelial dysfunction precedes pembrolizumab-induced cardiotoxicity. Preventive role of high dose of atorvastatin. Basic Res. Cardiol. 120, 263–286. doi:10.1007/s00395-024-01046-0
Eldani, C., Kostine, M., Faure, M., Lazaro, E., Rigothier, C., Hiriart, J. B., et al. (2024). Safety of immune checkpoint inhibitor rechallenge after severe immune-related adverse events: a retrospective analysis. Front. Oncol. 14, 1403658. doi:10.3389/fonc.2024.1403658
Fan, M., Zhang, X., Liu, H., Li, L., Wang, F., Luo, L., et al. (2024). Reversing immune checkpoint inhibitor-associated cardiotoxicity via bioorthogonal metabolic engineering-driven extracellular vesicle redirecting. Adv. Mater 36, e2412340. doi:10.1002/adma.202412340
Gergely, T. G., Drobni, Z. D., Sayour, N. V., Ferdinandy, P., and Varga, Z. V. (2024). Molecular fingerprints of cardiovascular toxicities of immune checkpoint inhibitors. Basic Res. Cardiol. 120, 187–205. doi:10.1007/s00395-024-01068-8
Grabska, S., Grabski, H., Makunts, T., and Abagyan, R. (2024). Co-Occurring infections in cancer patients treated with checkpoint inhibitors significantly increase the risk of immune-related adverse events. Cancers 16, 2820. doi:10.3390/cancers16162820
Gradone, A. L., Ma, V. T., Vasbinder, A., Fecher, L. A., Yentz, S., Hayek, S. S., et al. (2024). Increased myositis and possible myocarditis in melanoma patients treated with immune checkpoint inhibitors in the COVID-19 era. Cancer Immunol. Immunother. 73, 259. doi:10.1007/s00262-024-03803-5
Izumi, R., Hashimoto, T., Kisanuki, H., Ikuta, K., Otsuru, W., Asakawa, S., et al. (2024). Clinical and pathological characteristics of immune checkpoint inhibitor-related fulminant myocarditis. Cardio-Oncology 10, 82. doi:10.1186/s40959-024-00288-0
Jiang, R., Lou, L., Shi, W., Chen, Y., Fu, Z., Liu, S., et al. (2024). Statins in mitigating anticancer treatment-related cardiovascular disease. IJMS 25, 10177. doi:10.3390/ijms251810177
Keam, S., Turner, N., Kugeratski, F. G., Rico, R., Colunga-Minutti, J., Poojary, R., et al. (2024). Toxicity in the era of immune checkpoint inhibitor therapy. Front. Immunol. 15, 1447021. doi:10.3389/fimmu.2024.1447021
Koeckerling, D., Reddy, R. K., Barker, J., Eichhorn, C., Divall, P., Howard, J. P., et al. (2024). Cardiovascular events after chimeric antigen receptor T-Cell therapy for advanced hematologic malignant neoplasms: a meta-analysis. JAMA Netw. Open 7, e2437222. doi:10.1001/jamanetworkopen.2024.37222
Kong, Y., Wang, X., and Qie, R. (2024). Immunotherapy-associated cardiovascular toxicities: insights from preclinical and clinical studies. Front. Oncol. 14, 1347140. doi:10.3389/fonc.2024.1347140
Le Vu, S., Bertrand, M., Semenzato, L., Jabagi, M.-J., Botton, J., Drouin, J., et al. (2024). Influence of mRNA Covid-19 vaccine dosing interval on the risk of myocarditis. Nat. Commun. 15, 7745. doi:10.1038/s41467-024-52038-6
Li, X., and Li, D. (2024). Cardiovascular adverse events associated with immune checkpoint inhibitors: a meta-analysis. Front. Immunol. 15, 1394123. doi:10.3389/fimmu.2024.1394123
Li, H., Zheng, Y., Li, B., Zhi, Y., Chen, M., Zeng, J., et al. (2025). Association among major adverse cardiovascular events with immune checkpoint inhibitors: a systematic review and meta-analysis. J. Intern. Med. 297, 36–46. doi:10.1111/joim.20028
Lindsay, A. C., Walker, A. M., and Schneeweiss, S. (2025). Myocarditis in patients starting combination checkpoint inhibitor therapy: analysis of a commercial claims database. JAHA 14, e035689. doi:10.1161/JAHA.124.035689
Lipe, D. N., Qdaisat, A., Krishnamani, P. P., Nguyen, T. D., Chaftari, P., El Messiri, N., et al. (2024). Myocarditis, myositis, and myasthenia gravis overlap syndrome associated with immune checkpoint inhibitors: a systematic review. Diagnostics 14, 1794. doi:10.3390/diagnostics14161794
Liu, L., Yao, W., Wang, M., Wang, B., Kong, F., Fan, Z., et al. (2024a). A systematic review of cardiovascular toxicities induced by cancer immune therapies: underlying mechanisms, clinical manifestations and therapeutic approaches. Seminars Cancer Biol. 106-107, 179–191. doi:10.1016/j.semcancer.2024.10.004
Liu, Q., Liu, M., Zou, Z., Lin, J., Zhang, N., Zhao, L., et al. (2024b). Tofacitinib for the treatment of immune-related adverse events in cancer immunotherapy: a multi-center observational study. J. Transl. Med. 22, 803. doi:10.1186/s12967-024-05617-6
Liu, X., Zhang, J., Liu, F., Wu, Y., Li, L., Fan, R., et al. (2025). Association between influenza vaccination and prognosis in patients with ischemic heart disease: a systematic review and meta-analysis of randomized controlled trials. Travel Med. Infect. Dis. 64, 102793. doi:10.1016/j.tmaid.2024.102793
Maleki, S., Esmaeili, Z., Seighali, N., Shafiee, A., Namin, S. M., Zavareh, M. A. T., et al. (2024). Cardiac adverse events after Chimeric Antigen Receptor (CAR) T cell therapies: an updated systematic review and meta-analysis. Cardio-Oncology 10, 52. doi:10.1186/s40959-024-00252-y
Milutinovic, S., Jancic, P., Jokic, V., Petrovic, M., Dumic, I., Rodriguez, A. M., et al. (2024). Pembrolizumab-Associated cardiotoxicity: a retrospective analysis of the FDA adverse events reporting System. Pharmaceuticals 17, 1372. doi:10.3390/ph17101372
Miró, Ò., Ivars, N., Espinosa, B., Jacob, J., Alquézar-Arbé, A., López-Díez, M. P., et al. (2025). Effect of seasonal influenza and COVID-19 vaccination on severity and long-term outcomes of patients with heart failure decompensations. Eur. J. Heart Fail 27, 152–165. doi:10.1002/ejhf.3469
Munir, M., Sayed, A., Addison, D., and Epperla, N. (2024). Cardiovascular toxicities associated with novel cellular immune therapies. Blood Adv. 8, 6282–6296. doi:10.1182/bloodadvances.2024013849
Nielsen, D. L., Juhl, C. B., Nielsen, O. H., Chen, I. M., and Herrmann, J. (2024). Immune checkpoint inhibitor-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Oncol. 10, 1390–1399. doi:10.1001/jamaoncol.2024.3065
Omori, T., Maruyama, K., Ohta-Ogo, K., Hatakeyama, K., Ishibashi-Ueda, H., Onoue, K., et al. (2024). Clinical and histopathological characteristics of patients with Myocarditis after mRNA COVID-19 vaccination. Circ. J. 89, 120–129. doi:10.1253/circj.CJ-24-0506
Panuccio, G., Correale, P., d’Apolito, M., Mutti, L., Giannicola, R., Pirtoli, L., et al. (2024a). Immuno-related cardio-vascular adverse events associated with immuno-oncological treatments: an under-estimated threat for cancer patients. Basic Res. Cardiol. 120, 153–169. doi:10.1007/s00395-024-01077-7
Panuccio, G., Carabetta, N., Torella, D., and De Rosa, S. (2024b). Percutaneous coronary revascularization versus medical therapy in chronic coronary syndromes: an updated meta-analysis of randomized controlled trials. Eur. J. Clin. Invest. 54, e14303. doi:10.1111/eci.14303
Peng, J.-R., Hsieh, J. C.-H., Chang, C.-H., Chuang, C., Wang, Y.-C., Chen, T.-Y., et al. (2024). Cardiovascular and venous thromboembolism risks in cancer patients treated with immune checkpoint inhibitors compared to non-users- a multi-center retrospective study. Cardiooncology 10, 59. doi:10.1186/s40959-024-00264-8
Pi, J., Chen, X., Zhang, Y., Chen, X., Wang, Y., Xu, J., et al. (2024). Insight of immune checkpoint inhibitor related myocarditis. Int. Immunopharmacol. 143, 113559. doi:10.1016/j.intimp.2024.113559
Piazza, L., Carollo, A., Di Martino, E., Novara, M. E., Cutaia, S., Provenzani, A., et al. (2025). Cardiotoxicity associated with immune checkpoint inhibitors: systematic review and meta-analysis. Crit. Rev. Oncology/Hematology 206, 104587. doi:10.1016/j.critrevonc.2024.104587
Pozzessere, C., Mazini, B., Omoumi, P., Jreige, M., Noirez, L., Digklia, A., et al. (2024). Immune-Related adverse events induced by immune checkpoint inhibitors and CAR-T cell therapy: a comprehensive imaging-based review. Cancers (Basel) 16, 2585. doi:10.3390/cancers16142585
Qin, Y., Zhang, T., Du, Z., Chen, S., Li, Y., Lv, Y., et al. (2024). Prognosis of immune checkpoint inhibitor-related myocarditis: retrospective experience of a single institution. Int. Immunopharmacol. 136, 112385. doi:10.1016/j.intimp.2024.112385
Salas, M. Q., Cascos, E., López-García, A., Pérez-López, E., Baile-González, M., López-Corral, L., et al. (2024). Cardiac events occurring after allogeneic hematopoietic cell transplantation with post-transplant cyclophosphamide. Study conducted on behalf of the GETH-TC. Bone Marrow Transpl. 59, 1694–1703. doi:10.1038/s41409-024-02414-z
Suero-Abreu, G. A., Zanni, M. V., and Neilan, T. G. (2022). Atherosclerosis with immune checkpoint inhibitor therapy: evidence, diagnosis, and management: JACC: cardiooncology state-of-the-art review. JACC CardioOncol 4, 598–615. doi:10.1016/j.jaccao.2022.11.011
Sumi, T., Ikeda, T., Arioka, K., Sakuma, Y., Yamaguchi, M., Ishigooka, T., et al. (2024). Esophagomediastinal fistula during durvalumab plus tremelimumab with chemotherapy in angiotensin-converting enzyme 2-positive non-small cell lung cancer: a case report. Transl. Lung Cancer Res. 13, 2847–2852. doi:10.21037/tlcr-24-444
Tan, S., Qi, C., Zeng, H., Wei, Q., Huang, Q., Pu, X., et al. (2024). Steroid-Refractory Myocarditis induced by immune checkpoint inhibitor responded to infliximab: report of two cases and literature review. Cardiovasc Toxicol. 24, 1174–1191. doi:10.1007/s12012-024-09918-6
Thuny, F., Naidoo, J., and Neilan, T. G. (2022). Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur. Heart J. 43, 4458–4468. doi:10.1093/eurheartj/ehac456
Tocchetti, C. G., Farmakis, D., Koop, Y., Andres, M. S., Couch, L. S., Formisano, L., et al. (2024). Cardiovascular toxicities of immune therapies for cancer – a scientific statement of the Heart Failure Association (HFA) of the ESC and the ESC Council of Cardio-Oncology. Eur. J Heart Fail 26, 2055–2076. doi:10.1002/ejhf.3340
Tomsitz, D., Grabmaier, U., Spiro, J., Nicolai, L., French, L. E., Massberg, S., et al. (2025). Optimized monitoring for immune checkpoint inhibitor induced myocarditis using high-sensitivity troponin-T. Eur. J. Cancer 216, 115186. doi:10.1016/j.ejca.2024.115186
Toublanc, A.-C., Faure, M., Verdy, G., Rabeau, A., Houard, V., Veillon, R., et al. (2024). Prospective cardiovascular events in patients with advanced thoracic cancer treated with immune checkpoint inhibitor. Eur. J. Cancer 207, 114191. doi:10.1016/j.ejca.2024.114191
Tsang, T. K., Sullivan, S. G., Meng, Y., Lai, F. T. T., Fan, M., Huang, X., et al. (2024). Evaluating the impact of extended dosing intervals on mRNA COVID-19 vaccine effectiveness in adolescents. BMC Med. 22, 384. doi:10.1186/s12916-024-03597-4
Watson, R. A., Ye, W., Taylor, C. A., Jungkurth, E., Cooper, R., Tong, O., et al. (2024). Severe acute myositis and myocarditis on initiation of 6-weekly pembrolizumab post-COVID-19 mRNA vaccination. J. Immunother. Cancer 12, e008151. doi:10.1136/jitc-2023-008151
Weirauch, T., Schüttfort, G., and Vehreschild, MJGT (2025). Syncopes, paresis and loss of vision after COVID-19 mRNA-based vaccination and SARS-CoV-2 infection. Infection 53, 741–746. doi:10.1007/s15010-024-02439-y
Zhang, Z., Guo, L., Zhao, M., Pan, H., Dong, Z., Wang, L., et al. (2024). Discovery of novel PROTAC-Based HPK1 degraders with high potency and selectivity for cancer immunotherapy. J. Med. Chem. 67, 18682–18698. doi:10.1021/acs.jmedchem.4c01906
Keywords: cardio-oncology, cardiotoxicity, cytokine release syndrome, heart failure, immune checkpoint inhibitors, immune-related adverse events, myocarditis
Citation: Xiao L, Liu W, Ji Y, Li Q, Pan J and Xie R (2026) Immunotherapy-related cardiovascular toxicity: from mechanisms to management. Front. Pharmacol. 17:1762011. doi: 10.3389/fphar.2026.1762011
Received: 06 December 2025; Accepted: 26 January 2026;
Published: 04 February 2026.
Edited by:
Beshay Zordoky, University of Minnesota Twin Cities, United StatesReviewed by:
Giuseppe Panuccio, Charité Universitätsmedizin Berlin, GermanyCopyright © 2026 Xiao, Liu, Ji, Li, Pan and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renxian Xie, MjFyeHhpZUBzdHUuZWR1LmNu
†These authors have contributed equally to this work
 Lifeng Xiao
Lifeng Xiao Weitong Liu2†
Weitong Liu2† Renxian Xie
Renxian Xie