- 1Department of Health Behavior, School of Public Health, University of Alabama at Birmingham, Birmingham, AL, United States
- 2Department of Pharmacology and Experimental Therapeutics, LSU Health Sciences Center, New Orleans, LA, United States
Introduction: The majority of contemporary psychedelic research has focused on ayahuasca, lysergic acid diethylamide, and psilocybin, though there are hundreds of novel psychedelic compounds that may have clinical utility. The purpose of the present study was to evaluate the therapeutic potential of classic and novel phenethylamine, tryptamine, and lysergamide psychedelics via a large, nationally representative population-based survey.
Methods: We tested the unique associations of lifetime classic and novel phenethylamine, tryptamine, and lysergamide psychedelics with past month psychological distress and past year suicidality among respondents pooled from years 2008–2017 of the National Survey on Drug Use and Health (weighted N = 260,964,827).
Results: Lifetime classic tryptamine use was associated with a decreased odds of past month psychological distress [aOR = 0.76; (0.69–0.83)] and past year suicidal thinking [aOR = 0.79; (0.72–0.87)]. Lifetime novel phenethylamine use, on the other hand, was associated with an increased odds of past year suicidal thinking [aOR = 1.44; (1.06–1.95)] and past year suicidal planning [aOR = 1.60; (1.06–2.41)]. No other significant associations were found.
Discussion and Conclusions: These findings, which may be driven by differences in pharmacodynamics, suggest that classic tryptamines may hold the greatest therapeutic potential of the psychedelics, whereas novel phenethylamines may pose risk for harm. The present findings thus support continued research on the clinical application of classic tryptamines. Though the current results caution against the clinical utility of novel phenethylamines, further study of these and other novel psychedelic substances is nonetheless warranted to better understand their potential application.
Introduction
Classic psychedelics, which include dimethyltryptamine (DMT), lysergic acid diethylamide (LSD), mescaline, and psilocybin, have been studied clinically, anthropologically, and sociologically (1, 2). Classic psychedelics appear to be both generally safe and potentially therapeutic in the treatment of anxiety disorders, mood disorders, and substance use disorders (3–7). Consistent with findings from clinical trials, population-level analyses demonstrate that lifetime classic psychedelic use is associated with a reduced likelihood of past month psychological distress and past year suicidality (8). Lifetime psilocybin use in particular evinced these protective associations above and beyond other lifetime classic psychedelic use in one analysis, suggesting that psilocybin may have unique therapeutic potential (9), however, this analysis collapsed all non-psilocybin classic psychedelics across the three primary categories of classic psychedelics: phenethylamines (mescaline and the mescaline-containing cacti peyote and San Pedro), tryptamines (DMT and the DMT-containing admixture ayahuasca; psilocybin is also a tryptamine), and lysergamides (LSD). Whether the unique protective associations of psilocybin apply to all tryptamines, and whether tryptamines in general may have unique therapeutic potential relative to phenethylamines and lysergamides is unknown.
Novel psychedelics, which also comprise phenethylamines, tryptamines, and lysergamides, are distinct from classic psychedelics in that they lack both the long history of human use and substantial research data investigating their general safety, though there are notable pharmacologic and chemical data on these substances (10–13). From 2005 to 2017, novel phenethylamines (i.e. 2,5-Dimethoxy-4-"X"-phenethylamine or 2C-X, N-Benzyl Derivatives or NBOME’s) accounted for the majority of novel drug mentions in the National Survey on Drug Use and Health (NSDUH) (14), suggesting naturalistic use of these substances is on the rise. One population-level analysis found that lifetime novel psychedelic use is rare, accounted for primarily by phenethylamines, and associated with an increased likelihood of past month psychological distress and past year suicidality relative to lifetime use of classic psychedelics only (15). This suggests that novel psychedelics may be distinct from and carry reduced therapeutic potential relative to classic psychedelics. However, as with the abovementioned analysis, this analysis collapsed all classic psychedelics across phenethylamines, tryptamines, and lysergamides, and collapsed all novel psychedelics across a variety of subcategories, potentially obscuring any meaningful differences between the three primary categories of novel psychedelics. Whether each of the three categories of novel psychedelics may be distinct from and carry reduced therapeutic potential relative to each of the three categories of classic psychedelics is unknown.
Exploring the therapeutic potential of classic and novel phenethylamine, tryptamine, and lysergamide psychedelics is relevant considering that psychedelic research is experiencing a modest but growing resurgence. Whereas almost all contemporary research is accounted for by ayahuasca, LSD, and psilocybin (16), there are hundreds of novel psychedelic compounds that might have clinical utility (17, 18), with population-based survey respondents reporting the use of over 40 such compounds (15). Winnowing down this extensive list of psychedelic substances to those most likely to carry therapeutic benefit would help direct future study. Though classic and novel phenethylamine, tryptamine, and lysergamide psychedelics share important similarities (e.g. 5-HT2A receptor agonism), they differ in chemical structure, which appears to account for differences in reported subjective effects (19). It is known that psychedelics interact differently with their target 5-HT2A receptor (20). That is to say, they engage with different sets of amino acid residues in the binding pocket of the receptor to produce slightly different active state conformations of the receptor. The differences in conformational states lead to known differential or biased recruitment of second messenger and effector pathways that ultimately alter the physiology of the cell or neuron such that how the classic lysergamide psychedelic LSD alters cellular physiology is slightly different from how the classic phenethylamine psychedelic mescaline does. Indeed, it is has been hypothesized that these functional differences in receptor/ligand interactions and differential effects on cellular physiology are linked to their respective subjective experiences (21).
The purpose of this study was to test for unique associations of lifetime use of classic and novel phenethylamines, tryptamines, and lysergamide psychedelics with mental health outcomes using data from a large, nationally representative population-based survey. Considering the regulatory and other complexities associated with administering psychedelic substances to humans, population-based surveys represent useful springboards for exploring the therapeutic potential of these compounds (8). Thus, the present analysis will provide preliminary evidence with regard to which categories of classic and novel psychedelics might hold the greatest therapeutic potential, thereby informing future clinical research.
Methods
Data
Data were obtained from the publicly available NSDUH, a survey of the general, non-institutionalized United States population aged 12 and older administered by the Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services. The survey uses a multistage probability sampling design where individuals are randomly selected within a roster that accounts for state population size and housing inventory. NSDUH interviewers met with respondents in their homes, who listened to pre-recorded interview guides on headphones and responded via computer prompt. We combined the data from 2008–2017 in order to maximize sample size while maintaining standardized assessment procedures introduced in 2008. The comprehensive NSDUH sampling and questionnaire methodology can be found on their website https://nsduhweb.rti.org/respweb/about_nsduh.html.
Respondents
Using SPSS syntax, individual respondents from the 2008–2017 NSDUH were given a unique identifier and combined into a single database using the Cantor pairing function for a total unweighted sample of 562,072 cases. The analytic sample included all respondents with valid responses to the primary and secondary variables, yielding a total unweighted sample size of 354,535 (see Supplementary Table 1 for psychosocial characteristics of the sample). The Analysis section includes sample sizes for each regression model as the sample sizes varied based upon the dependent variable used. Respondents reporting mescaline (MESC2 = 1 and code 603 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1), peyote or San Pedro (cacti that contains mescaline; PEYOTE2 = 1 and code 602 and 6077 respectively from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1), were coded as positive for lifetime classic phenethylamine use. Respondents reporting they had ever, even once used DMT (code 616 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1), ayahuasca (an admixture that contains DMT; code 6103 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1), or psilocybin (PSILCY2 = 1 and code 604 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1) were coded as positive for lifetime classic tryptamine use. Respondents who reported using LSD (LSDFLAG = 1, and code 601 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1) were coded positive for lifetime classic lysergamide use, whereas those reporting they had never used any of the aforementioned substances were coded as negative for each respective drug category (8, 9, 15). Respondents were given the option to write-in other “hallucinogens” they had used, and novel psychedelics were gathered from write-in responses as per Sexton et al. (15). Table 1 lists both classic and novel psychedelic compounds and their classification for the purposes of this analysis. Respondents who indicated they had ever taken a substance that was classified as a novel phenethylamine (code in Table 1 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1) were coded as positive for lifetime novel phenethylamine use. Respondents who indicated they had ever taken a substance that was classified as a novel tryptamine (code in Table 1 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1) were coded as positive for lifetime novel tryptamine use. Respondents who indicated they had ever taken a substance that was classified as a novel lysergamide (code in Table 1 from variables HALNEWA, HALNEWB, HALNEWC, HALNEWD, HALNEWE = 1) were coded as positive for lifetime novel lysergamide use, whereas those reporting they had never used novel phenethylamines, tryptamines, or lysergamides were coded as negative for lifetime use of those respective compounds. Respondents who responded to the write-in query with “no” and those who did not provide a write-in a response were coded as negative for each of the novel psychedelic use variables. Supplementary Table 2 presents correlations among lifetime classic and novel phenethylamine, tryptamine, and lysergamide use. It is noted that these correlations ranged from very modest (e.g., lifetime classic phenethylamine use with lifetime novel lysergamide use) to moderate (lifetime classic phenethylamine use with lifetime classic tryptamine use and lifetime classic lysergamide use) to strong (lifetime classic tryptamine use with lifetime classic lysergamide use).
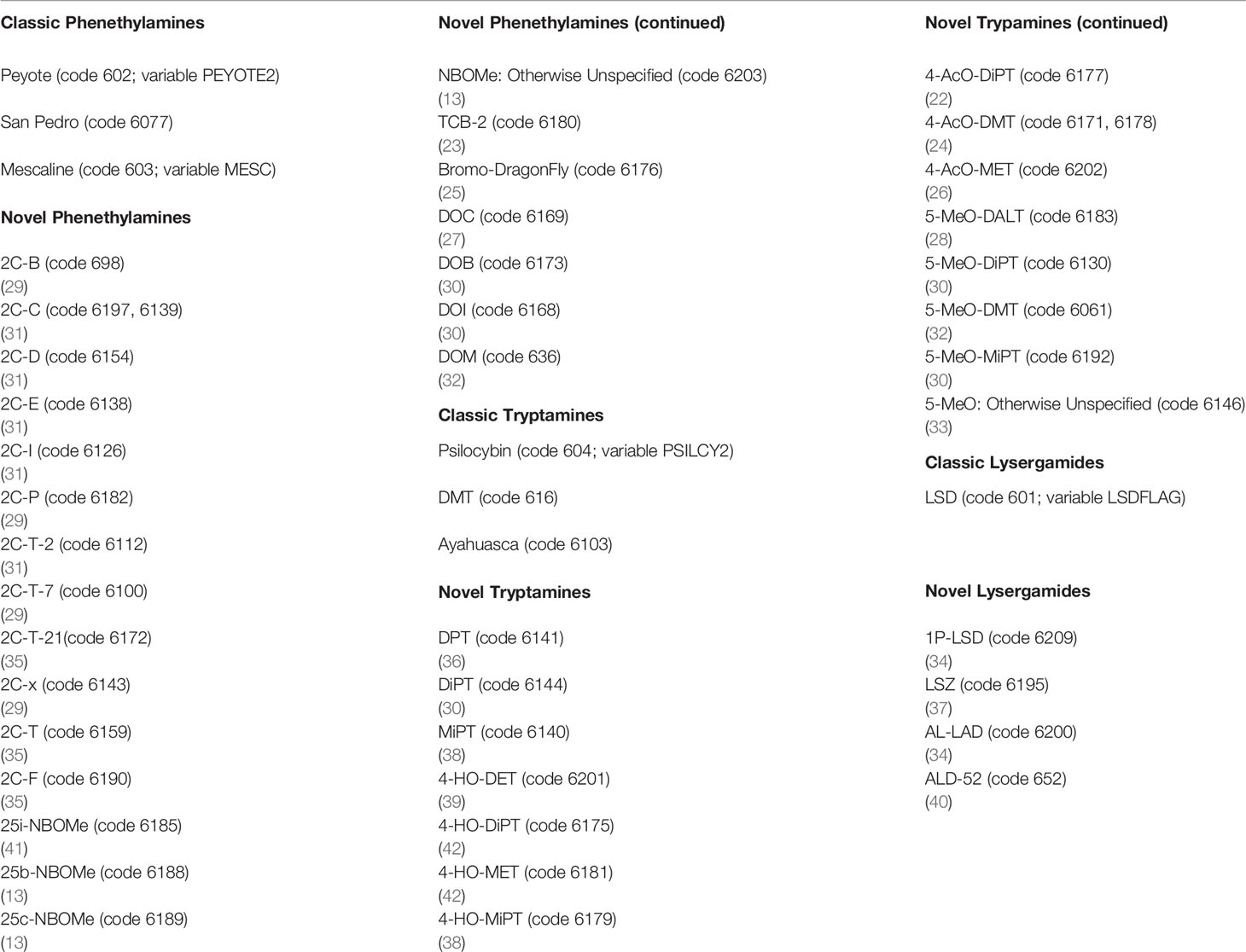
Table 1 Psychedelic compounds reported by respondents from the 2008–2017 National Survey on Drug Use and Health (NSDUH), respective NSDUH codes, and citations to supporting literature.
Analysis
Four multivariate logistic regression models were created to test the associations of 1) past month psychological distress (unweighted n = 356,046; variable SPDMON; yes = 1 or no = 0) as measured by the widely-used and well-validated six-item Kessler Psychological Distress Scale (K6; consistent with K6 scoring guidelines and its application in research, the NSDUH uses a dichotomous cutoff score ≥13; 43, 44), 2) past year suicidal thinking (unweighted n = 354,580; “At any time in the past 12 months … did you seriously think about trying to kill yourself? ”; variable MHSUITHK; yes = 1 or no = 0), 3) past year suicidal planning (unweighted n = 354,555; “During the past 12 months, did you make any plans to kill yourself? ”; variable MHSUITRY; yes = 1 or no = 0), and 4) past year suicide attempt (unweighted n = 354,552; “During the last 12 months, did you try to kill yourself? ”; variable MHSUITRY; yes = 1 or no = 0) with the following independent variables: lifetime use of classic phenethylamines (yes = 1 or no = 0), lifetime use of classic tryptamines (yes = 1 or no = 0), lifetime use of classic lysergamides (yes = 1 or no = 0), lifetime use of novel phenethylamines (yes = 1 or no = 0), lifetime use of novel tryptamines (yes = 1 or no = 0), and lifetime use of novel lysergamides (yes = 1 or no = 0; all independent variables were entered simultaneously). Consistent with prior analyses making use of NSDUH data (8, 15), the following covariates were included in the regression models to control for potential sources of confounding: age in years (12–17, 18–25, 26–34, 35–49, 50–64, or 65 or older); sex (male or female); ethnoracial identity (non-Hispanic White, non-Hispanic African American, non-Hispanic Native American/Alaska Native, non-Hispanic Native Hawaiian/Pacific Islander, non-Hispanic Asian, non-Hispanic more than one race, or Hispanic); educational attainment (5th grade or less, 6th grade, 7th grade, 8th grade, 9th grade, 10th grade, 11th grade, 12th grade, freshman college year, sophomore or junior college year, or senior college year or more); annual household income (less than $20,000, $20,000–$49,999, $50,000–$74,999, or $75,000 or more); marital status (married, divorced/separated, widowed, or never married); self-reported engagement in risky behavior (“How often do you like to test yourself by doing something a little risky? ”; never, seldom, sometimes, or always); and lifetime use of cocaine, other stimulants, sedatives, tranquilizers, heroin, pain relievers, marijuana, phencyclidine, 3,4-methylenedioxymethamphetamine (MDMA/ecstasy), and inhalants (each aforementioned drug category coded as separate covariates). Logistic regression models were created in R version 3.5.1 using the package “survey” and the svydesign and svyglm functions to account for the complex survey design used by the NSDUH (45, 46), and the package “jtools” to generate 95% confidence intervals and adjusted odds rations for each model (47). Lifetime novel lysergamide use, though quite rare (N = 9 unweighted respondents) was included in the regression models despite the fact that all novel lysergamide users also reported classic lysergamide use. Despite this overlap, multi-collinearity was not present within the model. However, associations of lifetime novel lysergamide use are not reported here given difficulty in interpretation. Indeed, adjusted ORs (all non-significant) revealed values well outside the range of all other variables included in regression models. All of the SPSS syntax, R source code, and datasets used to conduct these analyses are hosted on the Open Science Framework at the following link https://osf.io/xgqmd/.
Results
The weighted frequency of lifetime use of each psychedelic category and lifetime use of specific substances within each of these categories can be found in Table 2. As shown in this table, lifetime use of classic psychedelics was much more common than lifetime use of novel psychedelics. Lysergamides were the most commonly used category of classic psychedelic with approximately 10% of the United States population reporting lifetime use, whereas phenethylamines were the most commonly used category of novel psychedelic with one-tenth of one percent of the United States population reporting lifetime use. Psilocybin accounted for the vast majority of those reporting lifetime classic tryptamine use.
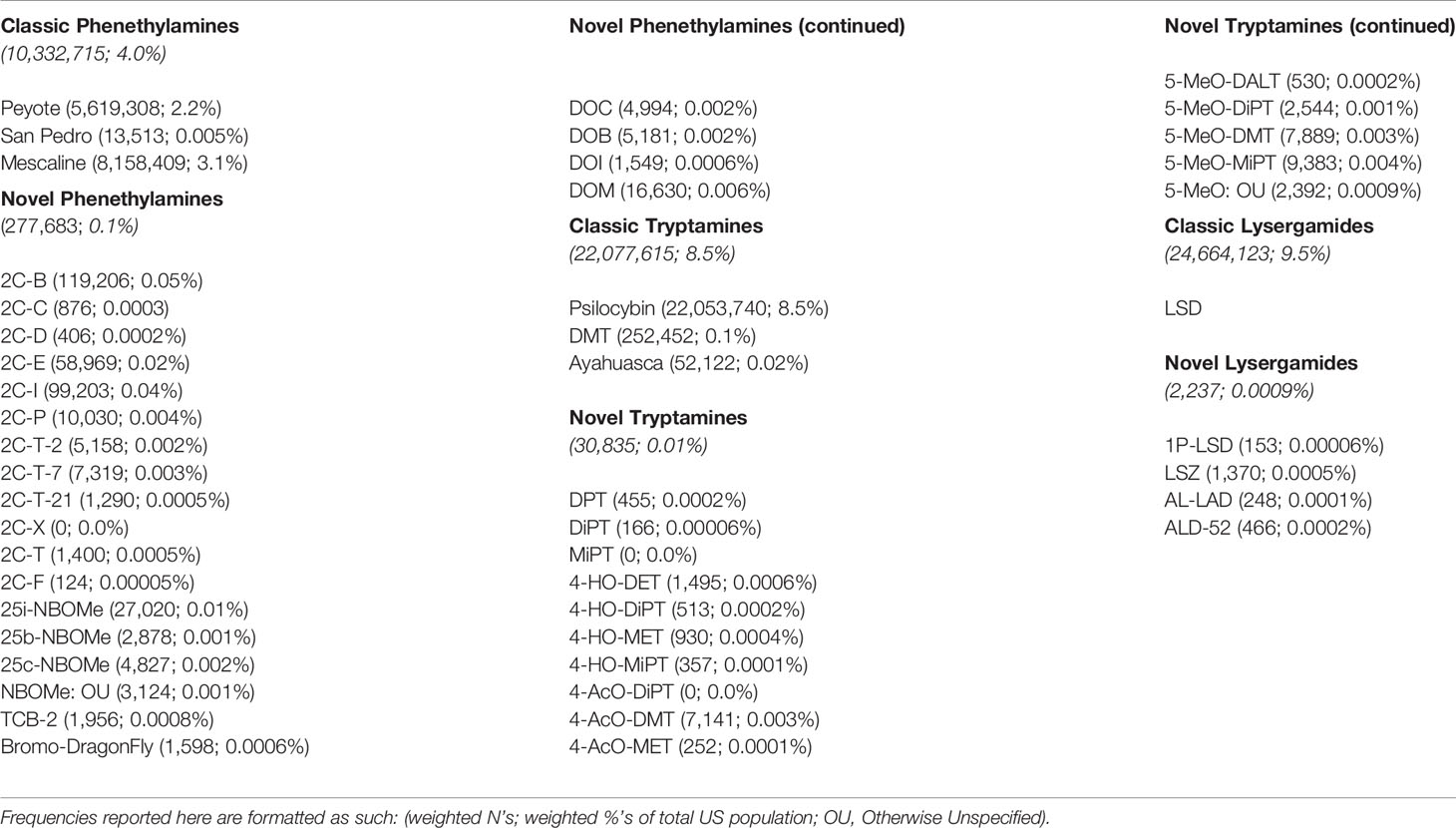
Table 2 Weighted frequencies of lifetime use of each psychedelic category and lifetime use of specific substances within each of these categories from the 2008–2017 NSDUH.
Findings generated from the four multivariate logistic regression models can be seen in Figure 1. These models show that lifetime classic tryptamine use was associated with a decreased odds of past month psychological distress [adjusted odds ratio or aOR = 0.76; (0.69–0.83)] and past year suicidal thinking [aOR = 0.79; (0.72–0.87)]. Novel phenethylamine use, however, was associated with an increased odds of past year suicidal thinking [aOR = 1.44; (1.06–1.95)] and past year suicidal planning [aOR = 1.60; (1.06–2.41)]. No other significant associations were found.
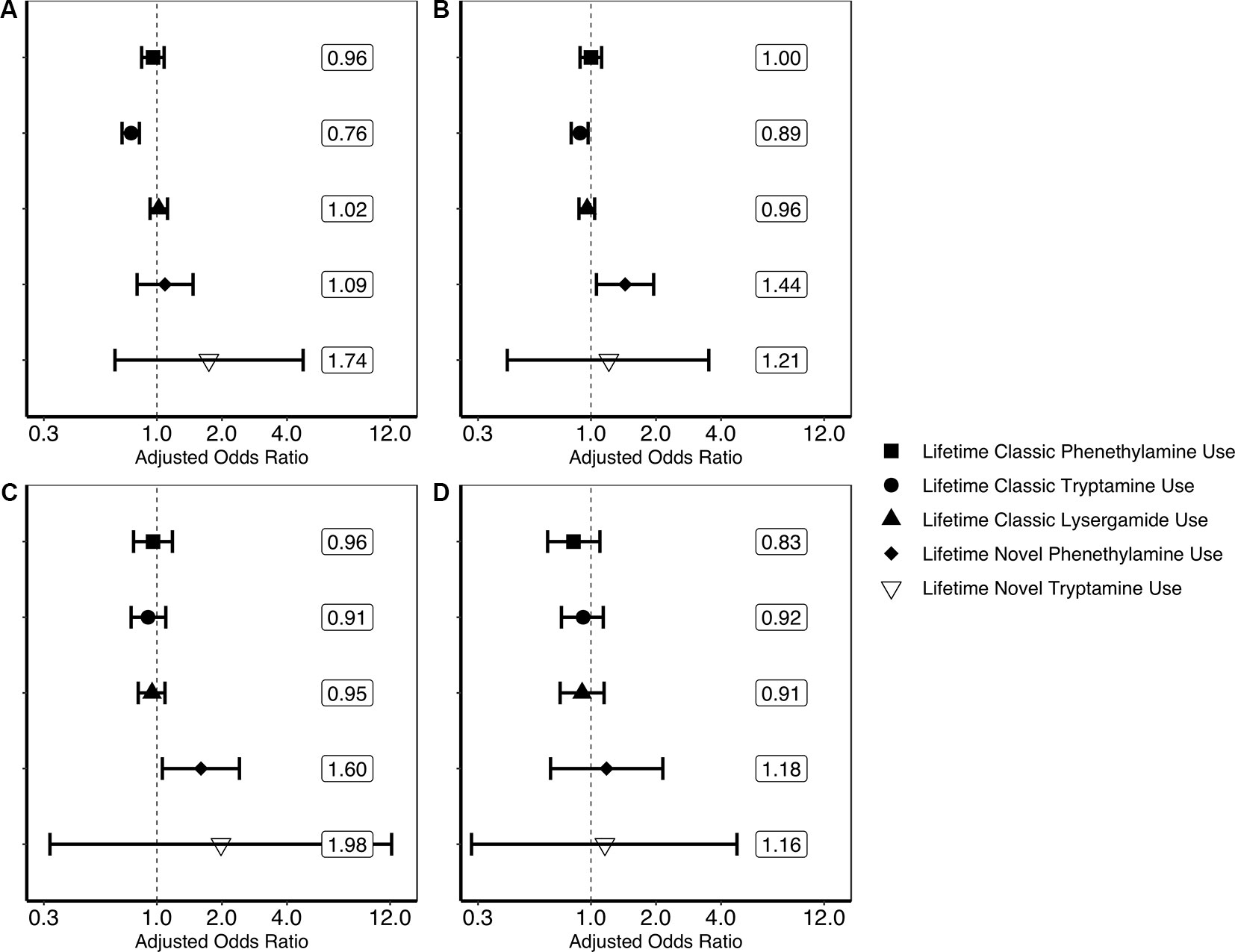
Figure 1 Results of multivariate logistic regression models predicting past month psychological distress and past year suicidality. (A) Result of multivariate logistic regression model predicting past month psychological distress (unweighted n = 356,046). (B) Result of multivariate logistic regression model predicting past year suicidal thinking (unweighted n = 354,580). (C) Result of multivariate logistic regression model predicting past year suicidal planning (unweighted n = 354,555). (D) Result of multivariate logistic regression model predicting past year suicide attempt (unweighted n = 354,552). Each plotted shape relates to the drug category and represent weighted adjusted odds ratio point estimates and error bars are 95% confidence intervals. Associations are adjusted for the following covariates: age in years (12–17, 18–25, 26–34, 35–49, 50–64, or 65 or older); sex (male or female); ethnoracial identity (non-Hispanic White, non-Hispanic African American, non-Hispanic Native American/Alaska Native, non-Hispanic Native Hawaiian/Pacific Islander, non-Hispanic Asian, non-Hispanic more than one race, or Hispanic); educational attainment (5th grade or less, 6th grade, 7th grade, 8th grade, 9th grade, 10th grade, 11th grade, 12th grade, freshman college year, sophomore or junior college year, or senior college year or more); annual household income (less than $20,000, $20,000–$49,999, $50,000–$74,999, or $75,000 or more); marital status (married, divorced/separated, widowed, or never married); self-reported engagement in risky behavior (“How often do you like to test yourself by doing something a little risky?”; never, seldom, sometimes, or always); and lifetime use of cocaine, other stimulants, sedatives, tranquilizers, heroin, pain relievers, marijuana, phencyclidine (PCP), 3,4-methylenedioxymethamphetamine (MDMA/ecstasy), and inhalants (each aforementioned drug category coded as separate covariates). Associations of covariates with psychological distress and suicidality are not reported here. The associations of lifetime novel lysergamide use are not evaluated here as noted in the Discussion.
Discussion
The objective of the present analysis was to test unique population-level associations of classic and novel phenethylamine, tryptamine, and lysergamide use with psychological distress and suicidality, thereby providing one line of evidence regarding which categories of psychedelics might hold the greatest therapeutic potential. We found that lifetime classic tryptamine use, the vast majority of which was accounted for by psilocybin, was associated with a reduced likelihood of past month psychological distress and past year suicidal thinking above and beyond a range of covariates including lifetime use of other classic psychedelics and lifetime use of novel psychedelics. These findings are consistent with a prior analysis indicating that lifetime psilocybin use may be especially protective against psychological distress and suicidality as compared to other classic psychedelics (9). Results were also consistent with a number of recent clinical trials suggesting that psilocybin is a promising therapeutic agent for end-of-life anxiety, treatment-resistant depression, alcohol dependence, and tobacco dependence (3, 4, 48–50). It is noted that though very few respondents reported lifetime use of ayahuasca, recent clinical trials suggest a substantial and rapid antidepressant effect of this DMT-containing admixture (51, 52). It may be, therefore, that classic tryptamines are among the most promising therapeutic agents of the psychedelics.
Sexton et al. found that lifetime use of novel psychedelics increased the likelihood of past year suicidal thinking and planning compared to lifetime classic psychedelic use only (15). In the present study, we found that novel phenethylamine use was associated with an increased likelihood of past year suicidal thinking and planning above and beyond several covariates including lifetime use of classic psychedelics and lifetime use of other novel psychedelics. Lifetime use of novel tryptamines was not associated with psychological distress or suicidality. The same was true of novel lysergamides, though interpretation of this finding is complicated by very few respondents reporting the use of novel lysergamides and the fact that all novel lysergamide users also reported the use of classic lysergamides. Nevertheless, this suggests that novel phenethylamine use accounts for the prior associations of Sexton et al., and that novel phenethylamines may be, to some degree, potentially harmful to mental health (15). Indeed, there have been a number of adverse event reports from novel phenethylamine use including psychosis, neurovascular hemorrhages, and seizures (53–56). These findings support the conclusion that novel phenethylamine psychedelics may be distinct from other psychedelic categories in that they may confer harm.
Tryptamine-based compounds in general have affinity for and agonist activity at primarily several different serotonin receptors. For example, psilocin, the active metabolite of the prodrug classic tryptamine psychedelic psilocybin, has varying but appreciable affinity for all serotonin receptors, with the exception of the 5-HT3 receptor, where it acts as an agonist, and the 5-HT7 receptor, where it is an antagonist. Significantly, all known tryptamines that have been tested have affinity for and agonist activity at 5-HT1A receptors. Activation of this receptor has been associated with antidepressant activity, and proposed as an important mechanism of the antidepressant effects of selective serotonin reuptake inhibitor medications (57, 58). Indeed, new antidepressant medications on the market were specifically designed to have at least partial agonist activity at 5-HT1A receptors (59). It is possible that activation of 5-HT1A receptors within the brain by classic tryptamine psychedelics confers positive effects to affective states and the observed reduction of psychological distress and suicidality in users. This may also apply to novel tryptamine psychedelics, though lifetime use of novel tryptamine psychedelics was not associated with psychological distress or suicidality in the current study, perhaps due to a lack of statistical power.
The phenethylamine compounds listed in Table 2, especially the novel phenethylamine 2C class, more often have affinity for and activity at the alpha-adrenergic receptor as well as moderate affinity for blockade of norepinephrine and dopamine transporters, whereas most tryptamines do not (60–63). Further, there is little to no activation of 5-HT1A receptors by these drugs. Together, activation of alpha adrenergic receptors with increases in synaptic norepinephrine and dopamine would be predicted to induce behavioral outcomes similar to amphetamines, including negative effects on cognitive behavioral control (64). These pharmacological outcomes, predicted to occur more frequently with phenethylamine (and especially the novel 2C phenethylamine) drugs than tryptamines, could underlie the observed associations of these novel phenethylamines with negative psychological health. In support of this view, 2C-B, the most commonly reported novel phenethylamine, is often substituted for MDMA among electronic music party goers secondary to its purported psychostimulant properties (15, 20, 65). Indeed, novel phenethylamines are often described in terms of psychostimulant effects (20, 29), whereas challenging, emotional breakthrough, and mystical-type experiences appear to underlie the therapeutic outcomes of the classic tryptamine psychedelic psilocybin (16, 66, 67). Thus, with regard to acute subjective effects, it may be that novel phenethylamines are characterized more so by problematic psychostimulant outcomes and less so by salubrious challenging, emotional breakthrough, and mystical-type experiences. It is important to interpret these associations with caution, however, as the NSDUH only provides data on naturalistic psychedelic use and it is quite possible that certain novel phenethylamines hold therapeutic potential when administered in a controlled environment.
A strength of the current study includes the assessment of a large, nationally representative sample of respondents from real-world settings. Additionally, the code used to conduct these analyses and the data sets that were analyzed are freely available online on the Open Science Framework. As in prior analyses, this analysis used a range of covariates to control for a number of sources of confounding (8, 9, 15). Furthermore, when estimating the associations of one independent variable (e.g., lifetime classic tryptamine use), our models controlled for the other five independent variables (e.g., lifetime classic phenethylamine use, lifetime classic lysergamide use, lifetime novel phenethylamine use, lifetime novel tryptamine use, and lifetime novel lysergamide use). Despite this approach, a number of limitations should be noted. First, an obvious limitation is reliance on self-report, which may have obfuscated true relationships between classic and novel psychedelic use and mental health outcomes. Second, as with any population-based survey, we could not control for every possible source of confounding. Any number of unassessed covariates may account for the associations reported here. For instance, perhaps classic tryptamine users are especially open to new experience and spiritual, and therefore the reported associations reflect the influence of these traits, rather than an effect of classic tryptamine use. Moreover, novel phenethylamine users may be especially prone to neuroticism, and therefore associations with suicidal thinking and planning may capture the impact of this characteristic on these outcomes (see 8). As noted above, the novel phenethylamine 2C-B may have a reputation as a “party drug, ” and thus the associations reported here may reflect the influence of recreational use motives. One such motive may be sensation seeking (see 68–71) which can be defined as a trait characterized by “the seeking of varied, novel, complex, and intense sensations and experiences, and the willingness to take physical, social, legal, and financial risks for the sake of such experience” (72, page 27). Though the inclusion of self-reported engagement in risky behavior as a covariate in analyses likely accounted for some of the variance in this trait, sensation seeking itself, in addition to a number of other relevant psychological constructs (e.g., openness and neuroticism), was not assessed by the NSDUH. In any event, as with any cross-sectional survey, the present results may not necessarily indicate causation. Third, as analyses were restricted to the available data (i.e., whether or not a respondent had used a classic or novel phenethylamine, tryptamine, or lysergamide psychedelic in his or her lifetime), dose-response relationships as well as associations with frequency of use, age of first use, recency of use, and any number of other variables pertaining to use patterns could not be tested. Future surveys including the NSDUH that seek to better understand the relationships of psychedelic use with mental health would benefit from the assessment of more complex use patterns rather than simple lifetime use. Additionally, there was overlap among lifetime classic and novel phenethylamine, tryptamine, and lysergamide psychedelic use, which might have limited the ability to detect the unique associations of these predictor variables with the outcomes (e.g., lifetime classic lysergamide use might be associated with a reduced likelihood of psychological distress and suicidality, but not above and beyond lifetime classic tryptamine use, with which it was strongly correlated). Fourth, population-level associations may obscure effects at the individual level. Thus, despite the reported trends, it is possible that some individuals were harmed by classic tryptamine use, whereas others benefited from novel phenethylamine use. Finally, as noted in Sexton et al., the write-in nature of lifetime novel psychedelic use likely lead to underreporting of these substances, which potentially affected the current estimates, including limiting power to detect associations (15). This is especially true in the case of lifetime novel lysergamide use (N = 9 unweighted respondents), where all lifetime novel lysergamide users reported lifetime classic lysergamide use. It is quite possible that data from surveys with predetermined items assessing novel psychedelic use would yield different findings.
Conclusions
The present research suggests that classic tryptamine psychedelics (i.e., ayahuasca, DMT, and psilocybin) may hold the greatest therapeutic potential of the psychedelics in that lifetime use of these substances was uniquely associated with a decreased likelihood of psychological distress and suicidal thinking. Novel phenethylamines, by contrast, might be distinct from other psychedelics in that lifetime use of these substances was independently associated with an increased likelihood of suicidal thinking and planning. Of course, the present data are by no means definitive, and it is possible that the range of psychedelic substances have clinical utility. Nevertheless, as clinical research with psychedelics remains in its infancy, the current study points to classic tryptamines as the best candidates for further study, with novel phenethylamines posing the potential for harm. Future research should aim to combine population-level methodology with chemical and pharmacological data to further investigate the therapeutic potential of classic and novel phenethylamine, tryptamine, and lysergamide psychedelics.
Data Availability Statement
All datasets generated for this study are available on the Open Science Framework at the following link: https://osf.io/xgqmd/.
Author Contributions
JS was the primary author who cleaned data, conducted analyses and drafted the manuscript summarizing the findings. CN contributed meaningful pharmacological expertise to inform methodology and aid in the interpretation of results. PH, corresponding author, was responsible for ensuring analyses were conducted and interpreted correctly.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00896/full#supplementary-material
References
1. Guerra Doce E. Psychoactive substances in prehistoric times: examining the archaeological evidence. Time Mind (2015) 8:91–112. doi: 10.1080/1751696X.2014.993244
2. Schultes RE. Hallucinogens of Plant Origin. Science (1969)(80-), 163:245. LP – 254. doi: 10.1126/science.163.3864.245
3. Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry (2016) 3:619–7. doi: 10.1016/S2215-0366(16)30065-7
4. Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol (2014) 28(11):983–92. doi: 10.1177/0269881114548296
5. Gasser P, Holstein D, Michel Y, Doblin R, Yazar-Klosinski B, Passie T, et al. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J Nerv Ment Dis (2014) 202:513–0. doi: 10.1097/NMD.0000000000000113
6. Krebs TS, Johansen PØr. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol (2012) 26:994–1002. doi: 10.1177/0269881112439253
7. Halpern JH. Hallucinogens in the treatment of alcoholism and other addictions. Psychedelic Med New Evid Hallucinog Subst Treat (2007) 2:1–14. doi: 10.1017/CBO9781107415324.004
8. Hendricks PS, Thorne CB, Clark CB, Coombs DW, Johnson MW. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J Psychopharmacol (2015) 29(3):280–8. doi: 10.1177/0269881114565653
9. Hendricks PS, Johnson MW, Griffiths RR. Psilocybin, psychological distress, and suicidality. J Psychopharmacol (2015) 29:1041–3. doi: 10.1177/0269881115598338
11. Halberstadt AL, Geyer MA. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology (2014) 77:200–7. doi: 10.1016/j.neuropharm.2013.08.025
12. Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res (2015) 277:99–120. doi: 10.1016/j.bbr.2014.07.016
13. Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, et al. Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci (2014) 5:243–9. doi: 10.1021/cn400216u
14. Palamar JJ, Le A. Use of new and uncommon synthetic psychoactive drugs among a nationally representative sample in the United States, 2005–2017. Hum Psychopharmacol Clin Exp (2019) 34:e2690. doi: 10.1002/hup.2690
15. Sexton JD, Crawford MS, Sweat NW, Varley A, Green EE, Hendricks PS. Prevalence and epidemiological associates of novel psychedelic use in the United States adult population. J Psychopharmacol (2019) 33(9):1058–67. doi: 10.1177/0269881119827796
16. Johnson MW, Hendricks PS, Barrett FS, Griffiths RR. Classic psychedelics: an integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacol Ther (2019) 197:83–102. doi: 10.1016/j.pharmthera.2018.11.010
17. Shulgin AT, Shulgin A. TiHKAL: Tryptamines I have known and loved. Transform Press: Berkeley (1997).
18. Shulgin AT, Shulgin A. PiHKAL: Phenethylamine I have known and loved. Transform Press: Berkeley (1991).
19. Nichols DE. Chemistry and structure-activity relationships of psychedelics. Curr Top Behav Neurosci (2018) 36:1–43. doi: 10.1007/7854_2017_475
20. Papaseit E, Farré M, Pérez-Mañá C, Torrens M, Ventura M, Pujadas M, et al. Acute pharmacological effects of 2C-B in humans: an observational study. Front Pharmacol (2018) 9:1–10. doi: 10.3389/fphar.2018.00206
21. Zamberlan F, Sanz C, Martínez Vivot R, Pallavicini C, Erowid F, Erowid E, et al. The Varieties of the Psychedelic Experience: a preliminary study of the association between the reported subjective effects and the binding affinity profiles of substituted phenethylamines and tryptamines. Front Integr Neurosci (2018) 12:1–22. doi: 10.3389/fnint.2018.00054
22. Meyer MR, Caspar A, Brandt SD, Maurer HH. A qualitative/quantitative approach for the detection of 37 tryptamine-derived designer drugs, 5 β-carbolines, ibogaine, and yohimbine in human urine and plasma using standard urine screening and multi-analyte approaches. Anal Bioanal Chem (2014) 406:225–7. doi: 10.1007/s00216-013-7425-9
23. Fox MA, French HT, Laporte JL, Blackler AR, Murphy DL. The serotonin 5-HT2Areceptor agonist TCB-2: A behavioral and neurophysiological analysis. Psychopharmacol (Berl) (2010) 212:13–3. doi: 10.1007/s00213-009-1694-1
24. Nichols DE. Improvements to the synthesis of psilocybin and a facile method for preparing the O-Acetyl prodrug of psilocin. Synthesis (Stuttg) (1999) 1999:935–8. doi: 10.1055/s-1999-3490
25. Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE. A Novel (Benzodifuranyl)aminoalkane with Extremely Potent Activity at the 5-HT2A Receptor. J Med Chem (1998) 41:5148–9. doi: 10.1021/jm9803525
26. McIntyre IM, Trochta A, Gary RD, Storey A, Corneal J, Schaber B. A Fatality related to two novel hallucinogenic compounds: 4-Methoxyphencyclidine and 4-Hydroxy-N-methyl-N-ethyltryptamine. J Anal Toxicol (2015) 39:751–5. doi: 10.1093/jat/bkv089
27. Coutts RT, Malicky JL. The synthesis of some analogs of the hallucinogen 1-(2,5-Dimethoxy-4-methylphenyl)-2-aminopropane (DOM). Can J Chem (1973) 51:1402–9. doi: 10.1139/v73-210
28. Cozzi NV, Daley PF. Receptor binding profiles and quantitative structure-affinity relationships of some 5-substituted-N,N-diallyltryptamines. Bioorganic Med Chem Lett (2016) 26:959–4. doi: 10.1016/j.bmcl.2015.12.053
29. Villalobos CA, Bull P, Sáez P, Cassels BK, Huidobro-Toro JP. 4-Bromo-2,5-dimethoxyphenethylamine (2C-B) and structurally related phenylethylamines are potent 5-HT 2A receptor antagonists in Xenopus laevis oocytes. Br J Pharmacol (2004) 141:1167–4. doi: 10.1038/sj.bjp.0705722
30. Ray TS. Psychedelics and the human receptorome. PloS One (2010) 5(2):e9019. doi: 10.1371/journal.pone.0009019
31. Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: Mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacol (Berl) (2014) 231:875–8. doi: 10.1007/s00213-013-3303-6
32. Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci (1984) 35:2505–1. doi: 10.1016/0024-3205(84)90436-3
33. Cheetham SC, Yamaguchi Y, Horton RW. [3H]5-hydroxytryptamine binding sites in postmortem human brain. Neuropharmacology (1989) 28:1055–0. doi: 10.1016/0028-3908(89)90117-2
34. Brandt SD, Kavanagh PV, Westphal F, Elliott SP, Wallach J, Colestock T, et al. Return of the lysergamides. Part II: Analytical and behavioural characterization of N6-allyl-6-norlysergic acid diethylamide (AL-LAD) and (2’S,4’S)-lysergic acid 2,4-dimethylazetidide (LSZ). Drug Test Anal (2016) 9:38–0. doi: 10.1002/dta.1985
35. Halpern JH. Hallucinogens: an update. Curr Psychiatry Rep (2003) 5:347–54. doi: 10.1007/s11920-003-0067-4
36. Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC. Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT(1A) and 5-HT(2A) receptors in rodents. Pharmacol Biochem Behav (2008) 88:358–5. doi: 10.1016/j.pbb.2007.09.007
37. Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM. Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD). J Med Chem (2002) 45:4344–9. doi: 10.1021/jm020153s
38. Repke DB, Grotjahn DB, Shulgin AT. Psychotomimetic N-methyl-N-isopropyltryptamines. Effects of variation of aromatic oxygen substituents. J Med Chem (1985) 28:892–6. doi: 10.1021/jm00145a007
39. Sard H, Kumaran G, Morency C, Roth BL, Toth BA, He P, et al. SAR of psilocybin analogs: discovery of a selective 5-HT2C agonist. Bioorg Med Chem Lett (2005) 15:4555–9. doi: 10.1016/j.bmcl.2005.06.104
40. Brandt SD, Kavanagh PV, Westphal F, Stratford A, Elliott SP, Hoang K, et al. Return of the lysergamides. Part I: Analytical and behavioural characterization of 1-propionyl-d-lysergic acid diethylamide (1P-LSD). Drug Test Anal (2016) 8:891–2. doi: 10.1002/dta.1884
41. Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. High Specific Activity Tritium-Labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): a high affinity 5-HT(2A) Receptor-Selective Agonist Radioligand. Bioorg Med Chem (2008) 16:6116–3. doi: 10.1016/j.bmc.2008.04.050
42. Rickli A, Moning OD, Hoener MC, Liechti ME. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol (2016) 26:1327–7. doi: 10.1016/j.euroneuro.2016.05.001
43. Hendricks PS, Crawford MS, Cropsey KL, Copes H, Sweat NW, Walsh Z, et al. The relationships of classic psychedelic use with criminal behavior in the United States adult population. J Psychopharmacol (2017) 32:37–8. doi: 10.1177/0269881117735685
44. Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med (2002) 32:959–6. doi: 10.1017/s0033291702006074
45. SAMHSA. How To Prepare and Analyze Pair Data in the National Survey on Drug Use and Health. (2017). Available at: https://www.samhsa.gov/data/report/how-prepare-and-analyze-pair-data-national-survey-drug-use-and-health.
46. Lumley T. “Survey: analysis of complex survey samples.” R package version 3.35-1. (2019). Available at: https://cran.r-project.org/web/packages/survey/index.html
47. Long JA. Jtools: analysis and presentation of social scientific data. (2019). Available at: https://cloud.r-project.org/web/packages/jtools/index.html.
49. Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology (2018) 147:143–66. doi: 10.1016/j.neuropharm.2018.05.012
50. Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstad AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry (2011) 68:71–8. doi: 10.1001/archgenpsychiatry.2010.116
51. Palhano-Fontes F, Barreto D, Onias H, Andrade KC, Novaes MM, Pessoa JA, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med (2018) 49(4):655–63. doi: 10.1017/S0033291718001356
52. dos Santos RG, Osório FL, Crippa JAS, Riba J, Zuardi AW, Hallak JEC. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol (2016) 6:193–3. doi: 10.1177/2045125316638008
53. Huang HH, Bai YM. Persistent psychosis after ingestion of a single tablet of "2C-B." Prog Neuropsychopharmacol Biol Psychiatry (2011) 35(1):293–4. doi: 10.1016/j.pnpbp.2010.10.018
54. Suzuki J, Poklis JL, Poklis A. My friend said it was good LSDA suicide attempt following analytically confirmed 25I-NBOMe ingestion. J Psychoactive Drugs (2014) 46:379–2. doi: 10.1080/02791072.2014.960111
55. Caudevilla F, Ventura M, Fornís I, Barratt MJ, Vidal C, lladanosa CG, et al. Results of an international drug testing service for cryptomarket users. Int J Drug Policy (2016) 35:38–1. doi: 10.1016/j.drugpo.2016.04.017
56. Caudevilla-Gálligo F, Riba J, Ventura M, González D, Farré M, Barbanoj MJ, et al. 4-Bromo-2,5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects. J Psychopharmacol (2012) 26:1026–5. doi: 10.1177/0269881111431752
57. Kulikov AV, Gainetdinov RR, Ponimaskin E, Kalueff AV, Naumenko VS, Popova NK. Interplay between the key proteins of serotonin system in SSRI antidepressants efficacy. Expert Opin Ther Targets (2018) 22:319–0. doi: 10.1080/14728222.2018.1452912
58. Köhler S, Cierpinsky K, Kronenberg G, Adli M. The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. J Psychopharmacol (2016) 30:13–2. doi: 10.1177/0269881115609072
59. Alvarez E, Perez V, Artigas F. Pharmacology and clinical potential of vortioxetine in the treatment of major depressive disorder. Neuropsychiatr Dis Treat (2014) 10:1297–7. doi: 10.2147/NDT.S41387
60. Luethi D, Liechti ME. In vitro pharmacological profiles as predictors of effects and clinical potency of monoaminergic new psychoactive substances. 39th International Congress of the European Association of Poison Centres and Clinical Toxicologists (2019) 479.
61. Luethi D, Liechti ME. Monoamine transporter and receptor interaction profiles in vitro predict reported human doses of novel psychoactive stimulants and psychedelics. Int J Neuropsychopharmacol (2018) 21:926–1. doi: 10.1093/ijnp/pyy047
62. Lobos M, Borges Y, Gonzalez E, Cassels BK. The action of the psychoactive drug 2C-B on isolated rat thoracic aorta. Gen Pharmacol Vasc Syst (1992) 23:1139–2. doi: 10.1016/0306-3623(92)90301-Y
63. C Cheng H, P Long J, Nichols D, F Barfknecht C, Rusterholz D. Effects of rigid amphetamine analogs on vascular strips: studies of 2-aminotetrahydronaphthalene and 2-aminoindan derivatives. Arch Int Pharmacodyn Thérapie (1974) 208:264–3.
64. Faraone SV. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev (2018) 87:255–0. doi: 10.1016/j.neubiorev.2018.02.001
65. González D, Ventura M, Caudevilla F, Torrens M, Farre M. Consumption of new psychoactive substances in a Spanish sample of research chemical users. Hum Psychopharmacol (2013) 28:332–0. doi: 10.1002/hup.2323
66. Barrett FS, Bradstreet MP, Leoutsakos JMS, Johnson MW, Griffiths RR. The challenging experience questionnaire: characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol (2016) 30:1279–5. doi: 10.1177/0269881116678781
67. Roseman L, Haijen E, Idialu-Ikato K, Kaelen M, Watts R, Carhart-Harris R. Emotional breakthrough and psychedelics: validation of the emotional breakthrough inventory. J Psychopharmacol (2019) 33(9):1076–87. doi: 10.1177/0269881119855974
68. Bardo M, Williams Y, Dwoskin L, Moynahan S, Perry I, Martin C. The sensation seeking trait and substance use: research findings and clinical implications. Curr Psychiatry Rev (2007) 3:3–13. doi: 10.2174/157340007779815682
69. Khodarahimi S. Sensation-seeking and risk-taking behaviors: a study on young Iranian adults. Appl Res Qual Life (2015) 10:721–4. doi: 10.1007/s11482-014-9350-2
70. Zhang L, Zhang C, Shang L. Sensation-seeking and domain-specific risk-taking behavior among adolescents: risk perceptions and expected benefits as mediators. Pers Individ Dif (2016) 101:299–5. doi: 10.1016/j.paid.2016.06.002
71. Tomko RL, Bountress KE, Gray KM. Personalizing substance use treatment based on pre-treatment impulsivity and sensation seeking: a review. Drug Alcohol Depend (2016) 167:1–7. doi: 10.1016/j.drugalcdep.2016.07.022
Keywords: phenethylamines, tryptamines, lysergamides, psychedelic-assisted therapy, mental health outcomes
Citation: Sexton JD, Nichols CD and Hendricks PS (2020) Population Survey Data Informing the Therapeutic Potential of Classic and Novel Phenethylamine, Tryptamine, and Lysergamide Psychedelics. Front. Psychiatry 10:896. doi: 10.3389/fpsyt.2019.00896
Received: 26 July 2019; Accepted: 13 November 2019;
Published: 11 February 2020.
Edited by:
Felix Müller, University Psychiatric Clinic Basel, SwitzerlandReviewed by:
Anna Brancato, University of Palermo, ItalyMax Wolff, Dresden University of Technology, Germany
Copyright © 2020 Sexton, Nichols and Hendricks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter S. Hendricks, cGhlbmRyaWNrc0B1YWIuZWR1
 James D. Sexton
James D. Sexton Charles D. Nichols
Charles D. Nichols Peter S. Hendricks
Peter S. Hendricks