- 1Department of Biomedical and NeuroMotor Sciences, University of Bologna, Bologna, Italy
- 2IRCCS Istituto delle Scienze Neurologiche di Bologna, Epilepsy Center (full member of the European Reference Network EpiCARE), Bologna, Italy
- 3Department of Medicine and Surgery, University of Milano Bicocca, Milan, Italy
Epileptologists and psychiatrists have long observed a correlation between epilepsy and personality disorders (PDs) in their clinical practice. We conducted a comprehensive PubMed search looking for evidence on PDs in people with epilepsy (PwE). Out of over 600 results obtained without applying any time restriction, we selected only relevant studies (both analytical and descriptive) limited to English, Italian, French and Spanish languages, with a specific focus on PDs, rather than traits or symptoms, thus narrowing our search down to 23 eligible studies. PDs have been investigated in focal epilepsy (predominantly temporal lobe epilepsy - TLE), juvenile myoclonic epilepsy (JME) and psychogenic non-epileptic seizures (PNES), with heterogeneous methodology. Prevalence rates of PDs in focal epilepsy ranged from 18 to 42% in surgical candidates or post-surgical individuals, with Cluster C personality disorders or related traits and symptoms being most common. In JME, prevalence rates ranged from 8 to 23%, with no strong correlation with any specific PDs subtype. In PNES, prevalence rates ranged from 30 to 60%, with a notable association with Cluster B personality disorders, particularly borderline personality disorder. The presence of a PD in PwE, irrespective of subtype, complicates treatment management. However, substantial gaps of knowledge exist concerning the neurobiological substrate, effects of antiseizure medications and epilepsy surgery on concomitant PDs, all of which are indeed potential paths for future research.
Introduction
Epilepsy is a chronic disease of the brain defined as “an enduring predisposition to generate epileptic seizures, and by the neurobiologic, cognitive, psychological, and social consequences of this condition” (1). It is a common neurological disease, with a calculated incidence rate of 61.4 per 100,000 person-years and a prevalence of 7.60 per 1,000 population (2), it affects approximately 46 million people, therefore representing a significant fraction of the worldwide disease burden (3). Remarkably, nearly half of people with epilepsy (PwE) experience comorbidities, with certain conditions exhibiting a higher prevalence among PwE compared to the general population, often impacting on epilepsy prognosis itself (4). Psychiatric disorders have garnered particular attention, due to their association with poor seizure outcome, drug-resistance, heightened suicide risk (5–7). While extensive research has been devoted to psychotic, anxiety and mood disorders, limited attention has been directed to the relationship between epilepsy and personality disorders (PDs). This neglect is somewhat surprising given historical observations dating back to the last century, originating from studies involving institutionalized PwE. Several clinical conditions have been described, such as Geschwind syndrome (8), gliscroid personality or Blumer syndrome (9). These data were not universally supported or agreed upon (10) and were related, in particular, to the presence of temporal lobe epilepsies (TLE) with drug-resistant seizures, social isolation, and the use of certain medications, such as phenobarbital or bromine. Subsequent studies showed that other types of epilepsy, such as the Idiopathic Generalized Epilepsies (IGE), showed very different personological characteristics from those described for TLE (11). Features such as superficiality, tendency to elation, poor compliance and critical traits were noted, often attributed to frontal lobe involvement (11). However the debate about the existence of personological alterations during epilepsy has waned in subsequent years, partly in connection with the use of medications with less impact on cognitive function and with the improved social integration of PwE, so much so that the Commission on Epilepsy, Risks and Insurance of the International Bureau for Epilepsy regarded the risk for psychological disorders in epilepsy to be negligible, at least when considered as such (12). Furthermore, the emergence of a revised classification system both for epilepsies and for personality disorders (DSM-5) has provided more rigorous and precise definitions, warranting a reevaluation of the association between epilepsy and PDs. In light of these developments, it is imperative to revisit the nexus between epilepsy and PDs, incorporating contemporary scientific evidence and examining diverse populations.
Our review focuses specifically on PDs in PwE, with the aim to collect and synthesize existing evidence, identify gaps of knowledge and propose potential avenues for future research.
Material and methods
In November 2023 we performed a search on PubMed database using the following terms: “Epilepsy”[Mesh] AND [“personality disorder”(All Fields) OR “paranoid personality disorder” (MeSH) OR “schizoid personality disorder” (Mesh) OR “schizotypal personality disorder” (MeSH) OR “antisocial personality disorder” [MeSH] OR “borderline personality disorder “(MeSH) OR “histrionic personality disorder” (MeSH) OR “narcissistic personality disorder” (All Fields) OR “avoidant personality disorder” (All Fields) OR “dependent personality disorder” (Mesh) OR “obsessive compulsive disorder” (MeSH)].
We selected studies (both descriptive and analytical) written in English, Italian, French and Spanish investigating the prevalence of personality disorders in PwE. Exclusion criteria were reviews, case reports, case series with small populations (i.e. less than 10 patients) and editorial comments. Additionally, studies emphasizing personality traits or symptoms without a formal diagnosis of personality disorder were excluded. Studies focusing on personality traits or symptoms without a diagnosis of personality disorder were excluded. No time restrictions were applied, and the entire selection process was carried out manually without the use of any automated tool.
The identified references were screened and provisionally selected for inclusion on the basis of title and abstract, when available. The full texts of articles meeting the inclusion criteria were then assessed.
Results
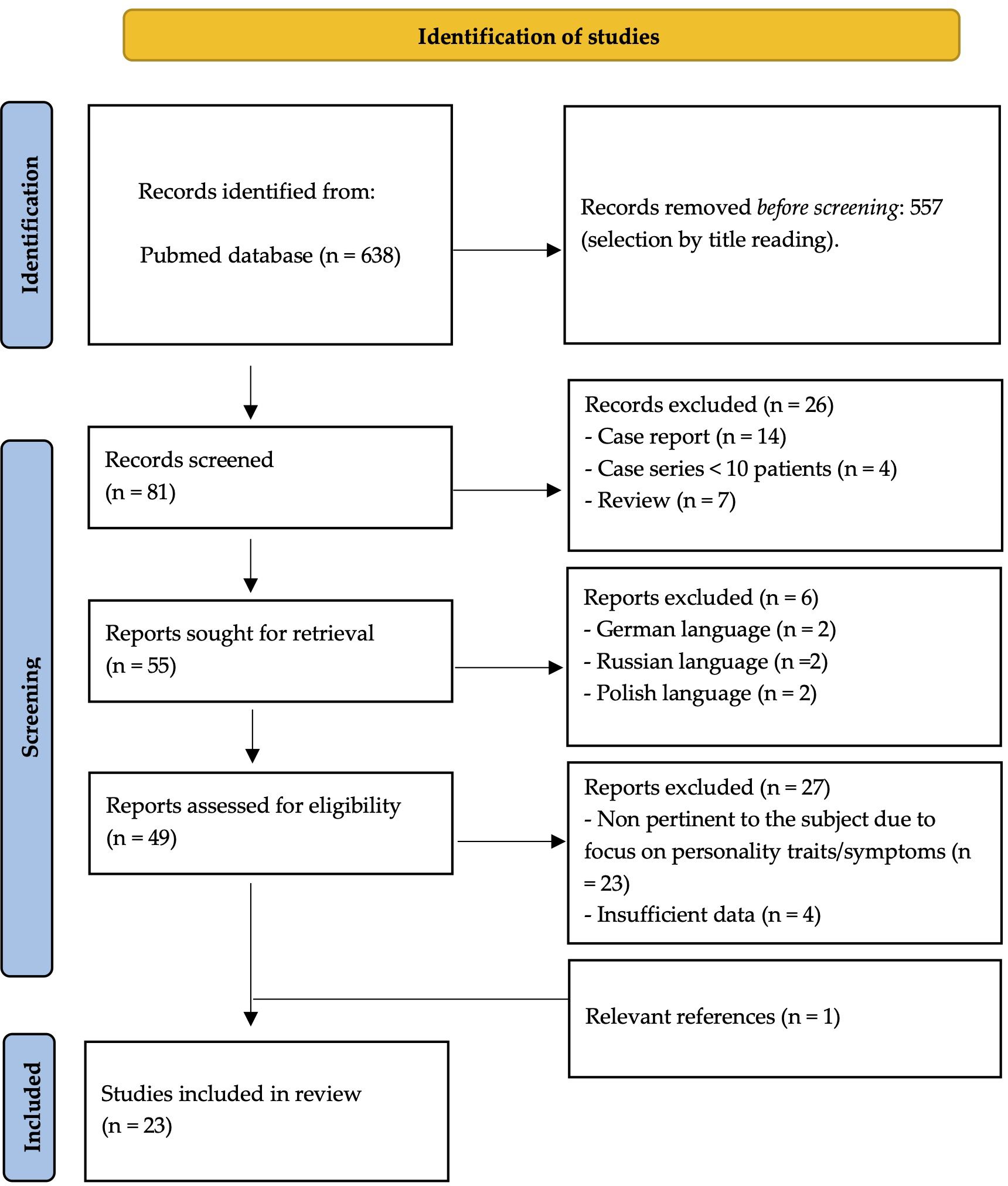
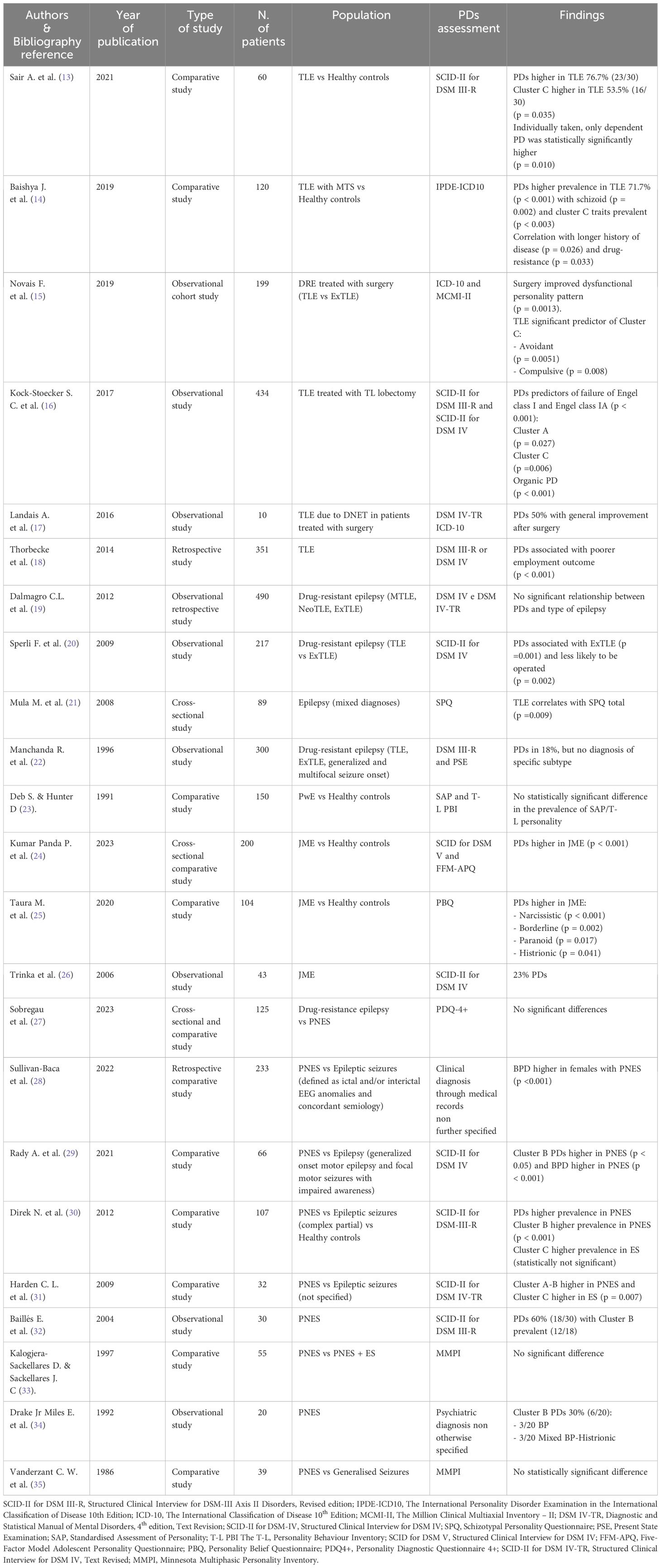
The initial PubMed search yielded a total of 638 articles. Following a multi-step selection process, 23 studies met the inclusion criteria and were considered eligible for analysis (see Figure 1 for further details). The findings of these studies are presented in distinct subchapters, according to the main themes investigated (see Table 1).
Personality disorders and focal epilepsy
Among the included studies, eleven investigated the relationship between PDs and focal epilepsy. These studies showed a significant prevalence of PDs within the studied population, with the strongest association observed between TLE and Cluster C PDs (13–15) and, to a lesser extent, between TLE and schizotypal personality (21).
Several studies investigated PDs in people with focal epilepsy who underwent epilepsy surgery, investigating its impact on both medical conditions. Notably, one study reported a statistically significant improvement in pre-surgical PD symptoms, although details on seizure outcomes were not provided (15). In another work, surgical resection of the epileptogenic zone led to remarkable improvements in all five patients diagnosed with concomitant PD, with two even being able to discontinue antipsychotic medications. Post-surgical seizure outcomes, assessed using the Engel Surgical Outcome Scale, were also highly favorable, with patients either becoming completely seizure-free or experiencing only rare seizures (12). Interestingly, a large population- based study in people with TLE revealed that PDs were predictive of surgery failure, defined as significantly lower rates of Engel class I and IA (16). The authors speculated that complex brain microstructural abnormalities in people with TLE and psychiatric comorbidities might underlie this association, emphasizing the need for further research.
In a separate comparative study, PDs were more commonly associated with extratemporal lobe epilepsy (ExTLE) rather than TLE, leading to lower rates of surgical intervention for individuals with this comorbidity. Nevertheless, those who did undergo surgery exhibited similarly successful outcomes (20). Moreover, people with epilepsy and PDs seem to suffer from an even bleaker stigma, as shown by lower employment rates two years after surgery (18).
Conversely, three studies did not detect significant differences in PD prevalence among PwE. One uncontrolled study focused only on people with intellectual disability and epilepsy, thus this finding could be attributed to a population selection bias (23). In the other two studies, discrepancies in results might be attributed to the specific assessment scales utilized – as questioned by the Authors themselves (17) - or the comparison of PD prevalence across different subtypes of focal epilepsy (19) that did not yield statistically significant differences.
Personality disorders and juvenile myoclonic epilepsy
Three studies on PDs and juvenile myoclonic epilepsy (JME) were identified. Two recent comparative studies showed that PDs were significantly more prevalent in JME compared to healthy controls. While one study did not identify specific correlation between PD subtypes and JME (24), the other reported strong associations between narcissistic, borderline, paranoid and histrionic PDs and JME (25). These discrepancies may stem from variations in assessment scales used, as the populations examined exhibited otherwise similar characteristics. A third older and purely descriptive study focused on assessing the prevalence of PDs in a medium-size sample of patients with JME, without providing statistical analyses (23).
Personality disorders and psychogenic non-epileptic seizures
Among the 9 studies investing the association between PDs and psychogenic non-epileptic seizures (PNES), seven were comparative studies comparing people with PNES to various control groups, including those with epilepsy/epileptic seizures alone (27–31, 35), PwE and PNES (33), or healthy controls (30). The remaining two studies were observational uncontrolled studies that gathered data on people with PNES through medical history and hospital charts (32, 34). While the evidence from these latter two studies was undoubtedly of lower quality due to the lack of proper statistical analysis and, in one case, insufficient details on the methods used to diagnose PD (34), we deemed it important to include them as seminal works that highlighted a higher prevalence of Cluster B PDs in people with PNES, a finding largely confirmed by subsequent literature and deemed statistically significant in the majority of our results (28–31). However, three studies presented discordant findings in this regard, potentially influenced by factors such as the assessments tools used. For instance, one study (27) reported statistically significant high scores in the “extraversion” section of the NEO-Personality Inventory-Revised among patients with PNES. The other two studies assessed PDs by the Minnesota Multiphasic Personality Inventory (MMPI), despite criticisms regarding its limitations and inconsistency of results obtained using this tool (36, 37).
Regarding gender differences, two studies show a statistically significant higher prevalence of PDs in women with PNES (28, 29).
Discussion
Despite the known higher prevalence of psychiatric comorbidities in PwE, the relationship between epilepsy and PDs has been inadequately investigated in the most recent scientific literature (38).
Our review demonstrates that the literature available on this topic is extremely heterogeneous in terms of methodology, population size, type of epilepsy investigated and assessment tools used for evaluating the disorder, with many works even failing to differentiate between psychiatric disorders in general and PDs (38–43). This last issue seems particularly relevant as the vast majority of the examined studies tend to combine and investigate various psychiatric conditions together (44). Conversely, when studies do focus on personality comorbidities, they often center on traits or symptoms rather than formally diagnosed PDs (39–41). Such methodological inconsistencies present a significant barrier to the comprehensive inclusion of pertinent evidence in our analysis, consequently constraining our capacity to derive meaningful insights regarding the relationship between PDs and epilepsy.
Another issue encountered in reviewing the existing literature on this subject is the lack of strong evidence due to the limited scope of studies carried out thus far, as, in spite of the higher prevalence of psychiatric comorbidities in PwE and the impact they seem to have on epilepsy prognosis itself, the number of well-designed case-control studies investigating their correlation remains insufficient (24). Ideally, to ensure statistical validity and clinical relevance, systematic comparisons should be made among different types of epilepsy and between PWE and healthy controls. Moreover, it seems very interesting to observe that there is relatively little standardized work on the issue of diagnostic tools used to identify PDs in epilepsy. Many studies relied on the MMPI, despite concerns raised by some researchers (36, 37). The consequence of it, however, is the vast array of diagnostic tools used subsequently, such as the Bear-Fedio Inventory (BFI), the DSM-IV axis I and axis II, the Structured Clinical Interview for DSM-III-R PDs (SCID-II), the Clinical Interview Schedule (CIS) and many others, making the different results obtained arduous to analyse in a comprehensive fashion (38). Moreover, as reiterated by Trimble (36), the diagnosis made by scales cannot be considered valid tout court for a clinical diagnosis.
On the other hand, the change in the classification of the same disorders in relation to different editions of the DSM, the same different classification of epilepsies, the change in the treatment of them in recent years and, in particular, the increased social integration of individuals with epilepsy do not allow the historically acquired data to be considered valid.
Furthermore, the literature revision is complicated by its heterogeneity, as specific different types of epilepsy were investigated in individual studies, while, on the contrary, some neglected to differentiate between epilepsy and isolated seizures. There is a long-standing and established interest in exploring the relationship between PDs and PNES (35) and, more recently, a rapidly growing body of evidence on PDs in people with focal epilepsy has emerged (19–21), even applying the recently proposed dimensional approach to personality profile (45). However, there are only scattered studies on other subtypes of epilepsy, such as JME (24), and, to our best knowledge, very few works on the correlation between PDs and epilepsy as a whole, most of which also happen to be quite dated (36, 46–48).
Given these limitations, our study aimed to aggregate and summarize available findings to offer a current perspective on the state of research in this area. By highlighting these constraints, we hope to identify potential research directions for future exploration. The relationship between PDs and focal epilepsy, particularly TLE, is well-established, with prevalence rates ranging from 18 to 42% in surgical candidates or post-surgical individuals (49). While there is no clear agreement as to what type of PD is the most prevalent, cluster C personality disorders or related traits and symptoms seem to be the most frequent (13–15, 31, 40, 50). However, the correlation between TLE, PDs and surgical treatment remains inconclusive, highlighting the need for future works to delve in this complex relationship, due to its profound impact on patients’ outcome.
In the context of JME, the prevalence and specific types of PDs remain contentious among the limited available studies (51). Nevertheless, the significance of exploring this relationship is undeniable, given the prevalence of JME. Analysing the correlation between JME and PDs within the broader framework of the idiopathic generalized epilepsies, ideally comparing JME with the other subtypes, could yield robust evidence on their psychiatric and personality profiles.
Our research underscores a strong connection PDs and PNES, particularly in females, with a strong association with cluster B personality disorders, notably borderline personality disorder (52). Delayed diagnosis of PNES, averaging 7-9 years from the initial clinical episode, leads to unnecessary hospitalizations and inappropriate treatment with antiseizure medications (ASMs) (53). Regarding the association with PNES, it must, in any case, be considered, that since PNES is a psychiatric disorder, many authors wonder whether we are really facing an association of different pathologies or a single one (11).
Regarding ASMs in general, our review highlights that the presence of a PD in PwE, regardless of the subtype, complicates treatment management. Clinicians face challenges in maintaining a delicate balance due to the potential for ASMs to worsen or exacerbate underlying PDs, as observed with drugs like perampanel and levetiracetam (7, 54–57).
This review has methodological limitations. We conducted our research through only one database, potentially excluding relevant evidence available from other online sources. Moreover, few studies were not included for language reasons (58–61). Lastly, we did not offer an analysis of the evidence strength provided by each work as the grand variety of statistical methodology used, population size, type of epilepsy and parameters considered made us opt for a purely descriptive presentation of our findings. However, we do not think that these limits significantly reduce the strength of our results, which allowed us to identify research paths worthy of further investigation.
Conclusions and directions for future research
While the relationship between psychiatric comorbidities and epilepsy has been discussed since time immemorial (47), our study reveals a notable lack of definitive data on various aspects concerning PDs in PwE. The literature in this domain is limited and methodologically diverse, yet our findings suggest that PDs are a prevalent comorbidity in PwE.
Future research should prioritize the identification of significant correlations between PDs and specific types of epilepsy, as well as elucidate how their co-occurrence influence patients’ prognosis. Additionally, it is crucial to investigate the effects of current ASMs on concomitant PDs.
Given the increasing number of patients being considered for epilepsy surgery, it is of the utmost importance to deepen our understanding of the neurological basis and pathological mechanisms that intertwine PDs and epilepsy, given the current scarcity of evidence that may inadvertently hinder optimal treatment decisions for some patients.
Based on our findings and with the aim of enhancing patient care and wellbeing, we advocate for a collaborative, multidisciplinary approach involving neurologists and psychiatrists, which should begin from the early stages of diagnosis and extend to the selection of personalized therapeutic strategies for each patient.
Author contributions
VV: Writing – original draft, Conceptualization, Investigation, Methodology. FB: Writing – review & editing. CC: Writing – review & editing. LF: Writing – review & editing. LL: Writing – review & editing. LM: Writing – review & editing. BM: Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The publication of this article was supported by the “Ricerca Corrente” funding from the Italian Ministry of Health.
Acknowledgments
The authors would like to thank Loretta Giuliano for her help in creating our search string for PubMed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. (2014) 55:475–82. doi: 10.1111/epi.12550
2. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. (2020) 54:185–91. doi: 10.1159/000503831
3. Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, et al. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–80. doi: 10.1016/S1474-4422(18)30499-X
4. Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. (2016) 15:106–15. doi: 10.1016/S1474-4422(15)00225-2
5. Taylor RS, Sander JW, Taylor RJ, Baker GA. Predictors of health-related quality of life and costs in adults with epilepsy: a systematic review. Epilepsia. (2011) 52:2168–80. doi: 10.1111/j.1528-1167.2011.03213.x
6. Ioannou P, Foster DL, Sander JW, Dupont S, Gil-Nagel A, Drogon O’Flaherty E, et al. The burden of epilepsy and unmet need in people with focal seizures. Brain Behav. (2022) 12:e2589. doi: 10.1002/brb3.2589
7. Memon AM, Katwala J, Douille C, Kelley C, Monga V. Exploring the complex relationship between antiepileptic drugs and suicidality: A systematic literature review. Innov Clin Neurosci. (2023) 20:47–51.
9. Blumer D. Evidence supporting the temporal lobe epilepsy personality syndrome. Neurology. (1999) 53:S9–12.
10. Devinsky O, Najjar S. Evidence against the existence of a temporal lobe epilepsy personality syndrome. Neurology. (1999) 53:S13–25.
11. Beghi M, Beghi E, Cornaggia CM, Gobbi G. Idiopathic generalized epilepsies of adolescence. Epilepsia. (2006) 47 Suppl 2:107–10. doi: 10.1111/j.1528-1167.2006.00706.x
13. Sair A, Şair YB, Saracoğlu İ, Sevincok L, Akyol A. The relation of major depression, OCD, personality disorders and affective temperaments with Temporal lobe epilepsy. Epilepsy Res. (2021) 171:106565. doi: 10.1016/j.eplepsyres.2021.106565
14. Baishya J, Ravish Rajiv K, Chandran A, Unnithan G, Menon RN, Thomas SV, et al. Personality disorders in temporal lobe epilepsy: What do they signify? Acta Neurol Scand. (2020) 142:210–5. doi: 10.1111/ane.13259
15. Novais F, Franco A, Loureiro S, Andrea M, Figueira ML, Pimentel J, et al. Personality patterns of people with medically refractory epilepsy - Does the epileptogenic zone matter? Epilepsy Behav EB. (2019) 97:130–4. doi: 10.1016/j.yebeh.2019.05.049
16. Koch-Stoecker SC, Bien CG, Schulz R, May TW. Psychiatric lifetime diagnoses are associated with a reduced chance of seizure freedom after temporal lobe surgery. Epilepsia. (2017) 58:983–93. doi: 10.1111/epi.13736
17. Landais A, Crespel A, Moulis JL, Coubes P, Gelisse P. Psychiatric comorbidity in temporal DNET and improvement after surgery. Neurochirurgie. (2016) 62:165–70. doi: 10.1016/j.neuchi.2016.02.002
18. Thorbecke R, May TW, Koch-Stoecker S, Ebner A, Bien CG, Specht U. Effects of an inpatient rehabilitation program after temporal lobe epilepsy surgery and other factors on employment 2 years after epilepsy surgery. Epilepsia. (2014) 55:725–33. doi: 10.1111/epi.12573
19. Dalmagro CL, Velasco TR, Bianchin MM, Martins APP, Guarnieri R, Cescato MP, et al. Psychiatric comorbidity in refractory focal epilepsy: a study of 490 patients. Epilepsy Behav EB. (2012) 25:593–7. doi: 10.1016/j.yebeh.2012.09.026
20. Sperli F, Rentsch D, Despland PA, Foletti G, Jallon P, Picard F, et al. Psychiatric comorbidity in patients evaluated for chronic epilepsy: a differential role of the right hemisphere? Eur Neurol. (2009) 61:350–7. doi: 10.1159/000210547
21. Mula M, Cavanna A, Collimedaglia L, Viana M, Barbagli D, Tota G, et al. Clinical correlates of schizotypy in patients with epilepsy. J Neuropsychiatry Clin Neurosci. (2008) 20:441–6. doi: 10.1176/appi.neuropsych.20.4.441
22. Manchanda R, Schaefer B, McLachlan RS, Blume WT, Wiebe S, Girvin JP, et al. Psychiatric disorders in candidates for surgery for epilepsy. J Neurol Neurosurg Psychiatry. (1996) 61:82–9. doi: 10.1136/jnnp.61.1.82
23. Deb S, Hunter D. Psychopathology of people with mental handicap and epilepsy. III: Personality disorder. Br J Psychiatry J Ment Sci. (1991) 159:830–4. doi: 10.1192/bjp.159.6.830
24. Panda PK, Ramachandran A, Tomar A, Elwadhi A, Kumar V, Sharawat IK. Prevalence, nature, and severity of the psychiatric comorbidities and their impact on quality of life in adolescents with Juvenile myoclonic epilepsy. Epilepsy Behav EB. (2023) 142:109216. doi: 10.1016/j.yebeh.2023.109216
25. Taura M, Gama AP, Sousa AVM, Noffs MHS, Alonso NB, Yacubian EM, et al. Dysfunctional personality beliefs and executive performance in patients with juvenile myoclonic epilepsy. Epilepsy Behav EB. (2020) 105:106958. doi: 10.1016/j.yebeh.2020.106958
26. Trinka E, Kienpointner G, Unterberger I, Luef G, Bauer G, Doering LB, et al. Psychiatric comorbidity in juvenile myoclonic epilepsy. Epilepsia. (2006) 47:2086–91. doi: 10.1111/j.1528-1167.2006.00828.x
27. Sobregrau P, Baillès E, Carreño M, Donaire A, Boget T, Setoain X, et al. Psychiatric and psychological assessment of Spanish patients with drug-resistant epilepsy and psychogenic nonepileptic seizures (PNES) with no response to previous treatments. Epilepsy Behav EB. (2023) 145:109329. doi: 10.1016/j.yebeh.2023.109329
28. Sullivan-Baca E, Weitzner DS, Choudhury TK, Fadipe M, Miller BI, Haneef Z. Characterizing differences in psychiatric profiles between male and female veterans with epilepsy and psychogenic non-epileptic seizures. Epilepsy Res. (2022) 186:106995. doi: 10.1016/j.eplepsyres.2022.106995
29. Rady A, Elfatatry A, Molokhia T, Radwan A. Psychiatric comorbidities in patients with psychogenic nonepileptic seizures. Epilepsy Behav EB. (2021) 118:107918. doi: 10.1016/j.yebeh.2021.107918
30. Direk N, Kulaksizoglu IB, Alpay K, Gurses C. Using personality disorders to distinguish between patients with psychogenic nonepileptic seizures and those with epileptic seizures. Epilepsy Behav EB. (2012) 23:138–41. doi: 10.1016/j.yebeh.2011.11.013
31. Harden CL, Jovine L, Burgut FT, Carey BT, Nikolov BG, Ferrando SJ. A comparison of personality disorder characteristics of patients with nonepileptic psychogenic pseudoseizures with those of patients with epilepsy. Epilepsy Behav EB. (2009) 14:481–3. doi: 10.1016/j.yebeh.2008.12.012
32. Baillés E, Pintor L, Fernandez-Egea E, Torres X, Matrai S, De Pablo J, et al. Psychiatric disorders, trauma, and MMPI profile in a Spanish sample of nonepileptic seizure patients. Gen Hosp Psychiatry. (2004) 26:310–5. doi: 10.1016/j.genhosppsych.2004.04.003
33. Kalogjera-Sackellares D, Sackellares JC. Personality profiles of patients with pseudoseizures. Seizure. (1997) 6:1–7. doi: 10.1016/S1059-1311(97)80045-3
34. Drake MEJ, Pakalnis A, Phillips BB. Neuropsychological and psychiatric correlates of intractable pseudoseizures. Seizure. (1992) 1:11–3. doi: 10.1016/1059-1311(92)90048-6
35. Vanderzant CW, Giordani B, Berent S, Dreifuss FE, Sackellares JC. Personality of patients with pseudoseizures. Neurology. (1986) 36:664–8. doi: 10.1212/WNL.36.5.664
36. Trimble MR. Personality disturbances in epilepsy. Neurology. (1983) 33:1332–4. doi: 10.1212/WNL.33.10.1332-a
37. Perini GI, Tosin C, Carraro C, Bernasconi G, Canevini MP, Canger R, et al. Interictal mood and personality disorders in temporal lobe epilepsy and juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry. (1996) 61:601–5. doi: 10.1136/jnnp.61.6.601
38. Swinkels WAM, Duijsens IJ, Spinhoven P. Personality disorder traits in patients with epilepsy. Seizure. (2003) 12:587–94. doi: 10.1016/S1059-1311(03)00098-0
39. Guo X, Lin W, Zhong R, Han Y, Yu J, Yan K, et al. Factors related to the severity of obsessive-compulsive symptoms and their impact on suicide risk in epileptic patients. Epilepsy Behav EB. (2023), 146:109362. doi: 10.1016/j.yebeh.2023.109362
40. Kilicaslan EE, Türe HS, Kasal Mİ, Çavuş NN, Akyüz DA, Akhan G, et al. Differences in obsessive-compulsive symptom dimensions between patients with epilepsy with obsessive-compulsive symptoms and patients with OCD. Epilepsy Behav EB. (2020) 102:106640. doi: 10.1016/j.yebeh.2019.106640
41. Kim SJ, Lee SA, Ryu HU, Han SH, Lee GH, Jo KD, et al. Factors associated with obsessive-compulsive symptoms in people with epilepsy. Epilepsy Behav EB. (2020) 102:106723. doi: 10.1016/j.yebeh.2019.106723
42. Ertekin BA, Kulaksizoğlu IB, Ertekin E, Gürses C, Bebek N, Gökyiğit A, et al. A comparative study of obsessive-compulsive disorder and other psychiatric comorbidities in patients with temporal lobe epilepsy and idiopathic generalized epilepsy. Epilepsy Behav EB. (2009) 14:634–9. doi: 10.1016/j.yebeh.2009.01.016
43. Isaacs KL, Philbeck JW, Barr WB, Devinsky O, Alper K. Obsessive-compulsive symptoms in patients with temporal lobe epilepsy. Epilepsy Behav EB. (2004) 5:569–74. doi: 10.1016/j.yebeh.2004.04.009
44. de Oliveira GNM, Kummer A, Salgado JV, Portela EJ, Sousa-Pereira SR, David AS, et al. Psychiatric disorders in temporal lobe epilepsy: an overview from a tertiary service in Brazil. Seizure. (2010) 19:479–84. doi: 10.1016/j.seizure.2010.07.004
45. Kustov G, Zhuravlev D, Zinchuk M, Popova S, Tikhonova O, Yakovlev A, et al. Maladaptive personality traits in patients with epilepsy and psychogenic non-epileptic seizures. Seizure. (2024) 117:77–82. doi: 10.1016/j.seizure.2024.02.005
46. Jeżowska-Jurczyk K, Kotas R, Jurczyk P, Nowakowska-Kotas M, Budrewicz S, Pokryszko-Dragan A. Mental disorders in patients with epilepsy. Psychiatr Pol. (2020) 54:51–68. doi: 10.12740/PP/93886
47. Trimble MR. Personality disorders and epilepsy. Acta Neurochir Suppl (Wien). (1988) 44:98–101. doi: 10.1007/978-3-7091-9005-0_20
49. Gaitatzis A, Trimble MR, Sander JW. The psychiatric comorbidity of epilepsy. Acta Neurol Scand. (2004) 110:207–20. doi: 10.1111/j.1600-0404.2004.00324.x
50. Hamed SA, Elserogy YM, Abd-Elhafeez HA. Psychopathological and peripheral levels of neurobiological correlates of obsessive-compulsive symptoms in patients with epilepsy: a hospital-based study. Epilepsy Behav EB. (2013) 27:409–15. doi: 10.1016/j.yebeh.2013.01.022
51. Gélisse P, Thomas P, Samuelian JC, Gentin P. Psychiatric disorders in juvenile myoclonic epilepsy. Epilepsia. (2007) 48:1032–3. doi: 10.1111/j.1528-1167.2007.01009_4.x
52. Lacey C, Cook M, Salzberg M. The neurologist, psychogenic nonepileptic seizures, and borderline personality disorder. Epilepsy Behav EB. (2007) 11:492–8. doi: 10.1016/j.yebeh.2007.09.010
53. Oto MM. The misdiagnosis of epilepsy: Appraising risks and managing uncertainty. Seizure. (2017) 44:143–6. doi: 10.1016/j.seizure.2016.11.029
54. Villanueva V, Garcés M, López-González FJ, Rodriguez-Osorio X, Toledo M, Salas-Puig J, et al. Safety, efficacy and outcome-related factors of perampanel over 12 months in a real-world setting: The FYDATA study. Epilepsy Res. (2016) 126:201–10. doi: 10.1016/j.eplepsyres.2016.08.001
55. Yumnam S, Bhagwat C, Saini L, Sharma A, Shah R. Levetiracetam-associated obsessive-compulsive symptoms in a preschooler. J Clin Psychopharmacol. (2021) 41:495–6. doi: 10.1097/JCP.0000000000001402
56. Fujikawa M, Kishimoto Y, Kakisaka Y, Jin K, Kato K, Iwasaki M, et al. Obsessive-compulsive behavior induced by levetiracetam. J Child Neurol. (2015) 30:942–4. doi: 10.1177/0883073814541471
57. Mbizvo GK, Dixon P, Hutton JL, Marson AG. The adverse effects profile of levetiracetam in epilepsy: a more detailed look. Int J Neurosci. (2014) 124:627–34. doi: 10.3109/00207454.2013.866951
58. Usykina MV, Kornilova SV, Lavrushchik MV. [Cognitive impairment and social functioning in organic personality disorder due to epilepsy]. Zh Nevrol Psikhiatr Im S Korsakova. (2021) 121:21–6. doi: 10.17116/jnevro202112106121
59. Dvirskiĭ AE, Shevtsov AG. [Clinical manifestations of epilepsy in hereditary schizophrenia]. Zhurnal Nevropatol Psikhiatrii Im SS Korsakova Mosc Russ 1952. (1991) 91:28–30.
60. Ekiert H, Jarzebowska E, Nurowska K, Welbel L. [Personality of epileptic patients and their relatives with special regard to traits and symptoms not included in the epileptic character]. Psychiatr Pol. (1967) 1:15–21.
Keywords: epilepsy, personality disorders (PDs), PNES, juvenile myoclonic epilepsy (JME), temporal lobe epilepsy, epilepsy surgery
Citation: Viola V, Bisulli F, Cornaggia CM, Ferri L, Licchetta L, Muccioli L and Mostacci B (2024) Personality disorders in people with epilepsy: a review. Front. Psychiatry 15:1404856. doi: 10.3389/fpsyt.2024.1404856
Received: 21 March 2024; Accepted: 24 April 2024;
Published: 10 May 2024.
Edited by:
Massimiliano Beghi, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyReviewed by:
Rosa Patrizia Sant’Angelo, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyAntonino Romeo, Fatebenefratelli Hospital, Italy
Copyright © 2024 Viola, Bisulli, Cornaggia, Ferri, Licchetta, Muccioli and Mostacci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barbara Mostacci, Yi5tb3N0YWNjaUBpc25iLml0
 Veronica Viola1
Veronica Viola1 Francesca Bisulli
Francesca Bisulli Laura Licchetta
Laura Licchetta Lorenzo Muccioli
Lorenzo Muccioli Barbara Mostacci
Barbara Mostacci