Abstract
Introduction:
Immune dysregulation and chronic inflammation have been hypothesized as potential pathways in metabolic syndrome and schizophrenia. Anti-inflammatory diets have the potential not only to treat metabolic syndrome but also to reduce the symptom burden in schizophrenia. The aim of this systematic review was to investigate the role of anti-inflammatory diets and vitamin supplementation in the management of metabolic syndrome and in symptom remission in people with schizophrenia.
Methods:
This systematic review included research articles from PubMed, EMBASE, Scopus, PsycINFO, and the Cochrane Central Register for Controlled Trials. The primary outcomes were markers of metabolic syndrome and symptoms of psychosis.
Results:
Our search identified 2,124 potential studies, of which 1,559 were screened based on the title and abstract, resulting in 81 full-text articles assessed for eligibility. A total of 17 studies were included, which demonstrated mixed findings on the impacts of anti-inflammatory diet interventions on metabolic markers and symptom remission in schizophrenia. Prebiotic, probiotic, and fish oil supplementation showed improvements in metabolic markers. Fish oil and vitamin D supplementation demonstrated symptom remission in some trials.
Conclusion:
It is important to consider that people with schizophrenia may experience common external barriers that hinder adherence to dietary interventions. These findings underscore the need for larger trials with standardized dietary protocols and consistent metabolic and symptom outcome measures in order to better understand the potential role of anti-inflammatory interventions in this population.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, identifier CRD42024511596.
1 Introduction
Lifetime history of schizophrenia spectrum disorders, including schizophrenia and schizoaffective and schizophreniform disorders, within the US is estimated at 1.8% (1). People with schizophrenia have an increased risk of premature mortality, with an estimated average potential life loss of 15–20 years (2). The earlier onset of serious medical diseases such as cardiovascular diseases, including coronary heart disease, is estimated to account for up to 80% of deaths within this population (2, 3). Metabolic syndrome has been identified as a patient factor contributing to the increased risk of cardiovascular disease and, ultimately, to premature mortality (2). Recent estimates of people with schizophrenia who met the criteria for metabolic syndrome have been as high as 60%, compared to 30% within the general population (4). The contributing factors to the development of metabolic syndrome include the side effects of antipsychotics and their effects on metabolism, comorbid substance use, lifestyle factors, adverse diets and poor nutrition, and negative symptoms (2).
A systematic review and meta-analysis examining the global prevalence of metabolic syndrome in patients with schizophrenia found a pooled prevalence of 41.3%, with significant variability across regions, ranging from 79.1% in France to 18.03% in China (5). This high prevalence, affecting over one-third of patients with schizophrenia, underscores the critical need for targeted interventions to reduce the associated chronic disease burden and mortality, reinforcing the importance of this study’s focus on the management of metabolic syndrome within this vulnerable population (5). Individuals with schizophrenia face disproportionately high rates of medical comorbidities, with metabolic syndrome present in approximately 32.5% of cases, which is largely influenced by factors such as illness duration and specific antipsychotic medications, particularly clozapine (6). People with severe mental illness (SMI), such as schizophrenia and bipolar disorder, face a 20-year reduction in life expectancy, primarily due to preventable cardiometabolic diseases—a disparity that appears to be worsening (7). While multidisciplinary teams provide varied treatments, lifestyle interventions to improve physical health have historically been lacking (8).
Metabolic syndrome and antipsychotic-induced weight gain negatively impact quality of life and are common reasons for medication non-adherence and premature discontinuation, creating barriers to symptom remission among people with schizophrenia (9–11). Metabolic syndrome is defined as a group of conditions including obesity, high blood pressure, high blood sugar, high triglycerides, and low high-density lipoprotein (HDL) cholesterol (12).
People with schizophrenia and comorbid metabolic syndrome have poorer cognitive performance, possibly resulting in worse symptom remission (13, 14). Medications such as metformin are used in weight reduction due to antipsychotic therapy (11, 15). Lifestyle counseling and exercise interventions have been shown to be more effective for weight reduction compared with low-metabolic-risk antipsychotics and metformin (15). Although lifestyle interventions and dietary modifications are effective methods to reduce metabolic syndrome, no clear consensus exists on the most effective dietary approaches for people with schizophrenia.
Immune dysregulation and chronic inflammation have been hypothesized as potential risk factors for both metabolic syndrome and the development and worsening of symptoms in individuals with schizophrenia. The complement system, particularly C1q and C3, plays a crucial role in both the immune response and the regulation of synaptic pruning. Synaptic pruning, which occurs during a critical period between adolescence and early adulthood, coincides with the typical onset of psychosis symptoms in schizophrenia (16). PET scans of the prefrontal cortex have revealed a reduction in dendritic spine density during this time (16, 17).
Astrocytes upregulate C1q and C3, facilitating opsonization and the targeted destruction of synapses by the microglia (17). Individuals with schizophrenia exhibit elevated pro-inflammatory cytokines (e.g., IL-1β, IL-6, IL-8, and TNF-α) released by the microglia (18, 19). These cytokines affect the downstream neurotransmission pathways, notably increasing the level of kynurenic acid in the kynurenine pathway, which disrupts the neurotransmitter balance and further amplifies inflammation through increased cytokine production (20).
In addition, an imbalance between the production of reactive oxygen species and antioxidants leads to increased oxidative damage (21). Patients with schizophrenia have been shown to have lower levels of the antioxidant glutathione in the medial prefrontal cortex and the striatum, areas that are both implicated in the pathophysiology of psychotic symptoms (21). Oxidative stress also negatively affects neurodevelopment in patients with schizophrenia via dysfunction of the N-methyl d-aspartate (NMDA) receptors in the process of synaptic plasticity (21). Previous studies have demonstrated the potential of antioxidant supplements such as omega-3 polyunsaturated fatty acids and vitamin D as adjunctive treatments in reducing the symptoms of schizophrenia (21). However, these studies focusing on dietary supplements have not directly looked at improvements in the metabolic markers.
Individuals with schizophrenia frequently have adverse, nutrient-poor diets, which include a higher intake of animal fats compared with vegetable fats, a lower intake of fruits, and a higher consumption of instant meals (22–24). Excessive saturated fat intake and low fiber and fruit intake result in higher levels of the inflammatory markers, including tumor necrosis factor (TNF), C-reactive protein (CRP), and interleukin 6 (IL-6), which have been implicated as a possible pathophysiology in schizophrenia development and symptom exacerbation (25). There is evidence that specific anti-inflammatory dietary regimens such as the Mediterranean diet can lead to a significant reduction of these inflammatory markers while also improving metabolic outcomes such body weight and lipids (26). The potential anti-inflammatory nutritional avenues identified are omega-3 fatty acids, vitamin C, vitamin D, resveratrol, Mediterranean diet, ketogenic diet, and dietary approaches to stop hypertension (DASH) (25).
Ultimately, these processes lead to increased neuroinflammation. This contributes to dysregulation in neurotransmission systems, including alterations in the dopamine pathway, a key factor in schizophrenia symptoms and neurodevelopment (20). There is a potential for specialized diets and vitamin supplements to not only improve the metabolic outcomes but also help reduce inflammation, thereby bringing about an improvement in the symptoms of psychosis. The aim of this review was to investigate the possible role of anti-inflammatory diets and vitamin supplementation in addressing both symptom remission and metabolic syndrome in people with schizophrenia, schizoaffective disorder, and first-episode psychosis.
2 Methods and analysis
2.1 Objectives
The aims of this systematic review were as follows:
-
To evaluate the effectiveness of anti-inflammatory diets in the treatment of metabolic syndrome in patients with psychosis;
-
To assess the methodological quality and the strength of evidence regarding anti-inflammatory diets in patients with psychosis, specifically for the treatment of metabolic syndrome and for symptom remission; and
-
To determine the appropriate dietary interventions that enhance symptom control for patients with psychosis.
2.2 Protocol and registration
This systematic review was conducted in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) (27). The protocol was registered in the International Prospective Register for Systematic Reviews, PROSPERO (protocol no. CRD42024511596).
Figure 1
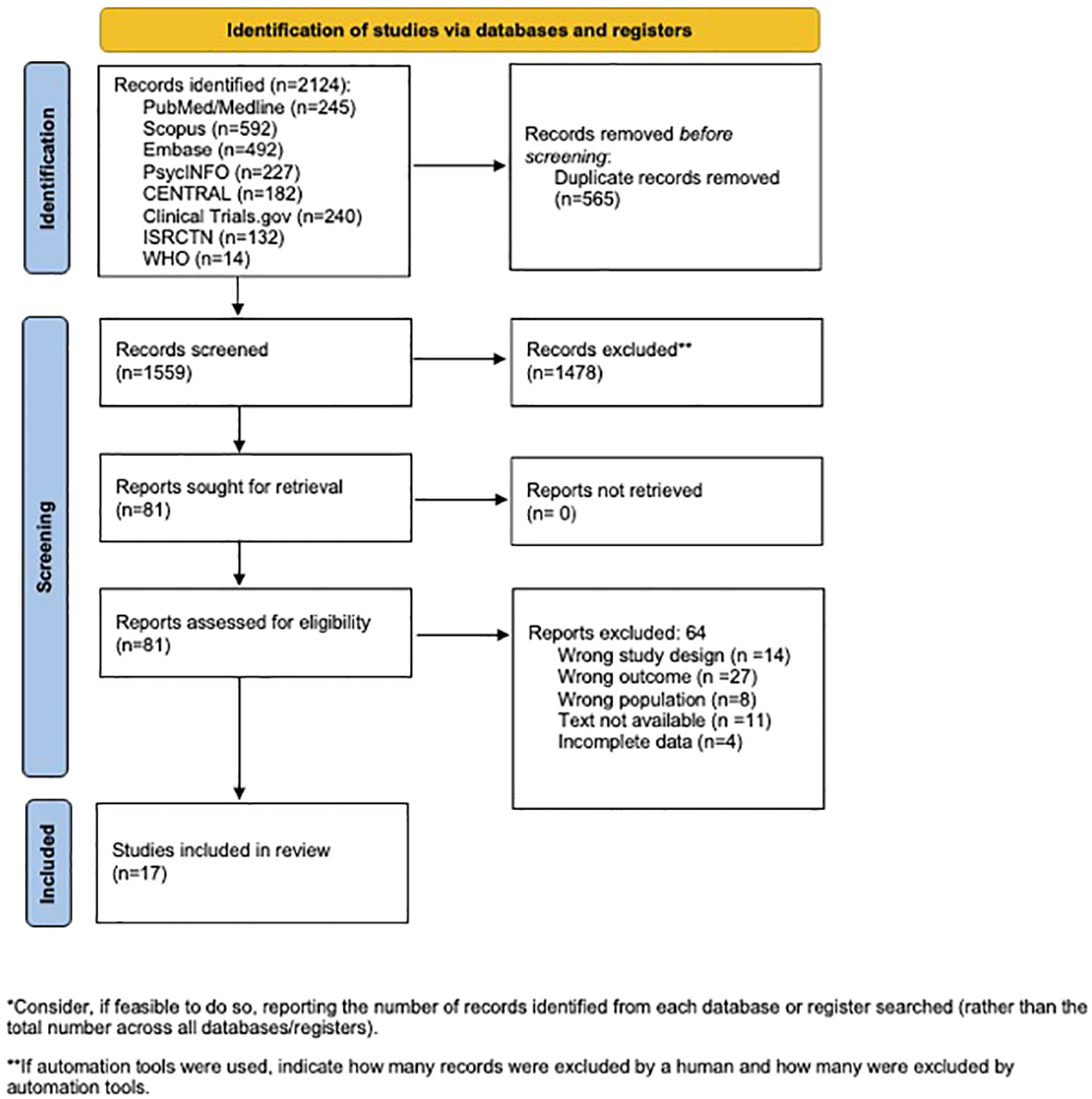
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
2.3 Outcome measures
The primary outcomes included the following measures:
-
Changes in the metabolic profile as measured by body mass index (BMI), blood glucose, lipids, blood pressure, weight, and waist circumference, among others.
-
Changes in the symptoms of psychosis measured using a standardized instrument, such as the Positive and Negative Symptom Scale for Schizophrenia (PANSS), the Scale for the Assessment of Negative Symptoms (SANS), and the Scale for the Assessment of Positive Symptoms (SAPS).
2.4 Eligibility criteria
The eligibility criteria for the participants, intervention, comparison, outcome, and study design (PICOS) domains are presented in Table 1.
Table 1
| Domain | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Patients with schizophrenia, schizoaffective disorder, and first episode psychosis without age or gender predilection | Participants without psychotic disorder; medication-induced psychosis |
| Intervention | Anti-inflammatory diets or vitamins | Non-anti-inflammatory diets or vitamin |
| Comparator | Placebo or treatment as usual | |
| Outcome |
Primary outcomes: Changes in the metabolic profile Changes in the symptoms of psychosis |
|
| Study design | Randomized controlled trials, non-randomized controlled trials, retrospective studies, and cohort studies | Animal studies, case reports, case series, reviews, letters, commentary/editorial articles, and clinical trials with no results |
Description of the population, intervention, comparison, outcome, and study design domains.
2.5 Information sources and search strategy
The search strategies were developed by an experienced health sciences librarian and other members of the research team. The strategy was translated for the other included databases. The librarian searched the following databases and trial registries from the date of inception through to October 24, 2024: PubMed (including pre-MEDLINE and non-MEDLINE; from 1945 to October 2024), EMBASE (Elsevier; from 1974 to October 2024), Scopus (Elsevier; from 1966 to October 2024), PsycINFO (EBSCO, from 1887 to October 2024), and Cochrane Central Register for Controlled Trials (Wiley, through to October 2024). Unpublished literature was also considered in this review through a search of the International Clinical Trials Registry Platform, the International Standard Registered Clinical/Social Study Number, and ClinicalTrials.gov. Unpublished studies with no results were not included in our analysis. A number of items found in CENTRAL fell into this category as well (see Supplementary Table 1 for the search strategy). The Boolean operator NOT, along with the database limiters, was applied when appropriate to omit items deemed undesirable to the project (see above for the exclusion criteria). The citation management tool EndNote (Clarivate, Philadelphia, PA) was used to de-duplicate and to manage all citations.
2.6 Data extraction and coding
Four reviewers independently extracted data (Table 2). Specific data including the means and p-values were recorded as feasible. In the event of missing or ambiguous data in the included studies, the researchers made efforts to contact the corresponding authors. If missing data could not be obtained despite these attempts, the study was excluded from the analysis. Discrepancies were resolved via discussion until a consensus was reached or by a third reviewer. The following data were recorded.
-
Basic information of the study: first author, year, and country of publication.
-
Study characteristics: study inclusion criteria, participant details (i.e., age, gender, diagnosis, baseline symptom severity, and baseline metabolic markers).
-
Details of the study intervention(s) and control intervention(s).
-
Results.
-
Risk of bias assessment.
Table 2
| Study | Study design | Population | Intervention | Limitations | BAS | ||
|---|---|---|---|---|---|---|---|
| Duration | Experimental | Control | |||||
| Deutsch et al. (28) | RCT | 18- to 70-year-olds with schizophrenia or schizoaffective disorder, on a stable second-generation antipsychotic for at least 4 weeks, and at least moderately severe symptoms on PANSSa | 3.5 months |
n = 37 2,000 mg/day of galantamine/CP-coline |
n = 37 Placebo supplementation |
1) Small sample size 2) Inclusion of confounding variables such as smoking |
2/3 |
| Dickerson et al. (29) | RCT | 18- to 65-year-olds with schizophrenia, at least moderately severe psychotic symptoms on PANSS, and compliance with maintenance antipsychotic medicationa | 3.5 months |
n = 33 1 probiotic tablet daily |
n = 32 Not described |
1) Small sample size 2) No description on the placebo group 3) No description of the blinding |
2/3 |
| Fenton et al. (30) | RCT | 18- to 65-year-olds with schizophrenia with no medication changes in the prior 30 days, on medication, and with presence of significant residual symptoms on PANSSa | 4 months |
n = 43 3 g of omega-3 fatty acid daily |
n = 44 Mineral oil placebo pill |
1) Small sample size | 2/3 |
| Jamilian et al. (31) | RCT | 15- to 55-year-olds with schizophreniaa | 2 months |
n = 30 1,000 mg omega-3 |
n = 30 Placebo supplementation |
1) Small sample size 2) Short duration of study |
2/3 |
| Kelly et al. (32) | RFS | Diagnosis of schizophrenia or schizoaffective disorder, on a stable medication dose for at least 4 weeks, and positive AGA IgG screeninga | 1.25 months |
n = 9 10 g of rice flour (gluten-free diet) in a protein shake and standardized gluten-free diet |
n = 9 10 g of gluten four in a protein shake and standardized gluten-free diet |
1) Small sample size 2) Short duration |
2/3 |
| Kelly et al. (33) | PCTE | Inpatients aged 16–64 years with schizophrenia and with no antipsychotic changes in the past 14 daysa | 2 weeks |
n = 5 4 g of oligofructose-enriched inulin three times daily |
None | 1) Small sample size 2) Open-label design |
0/3 |
| Roffman et al. (34) | RCT | 18- to 68-year-olds with schizophrenia with psychiatric stability and persistent symptoms despite at least 6 months of antipsychotic treatment, including 6 weeks at a stable dose, and a PANSS score of 60 or morea | 4 months |
n = 94 2 mg folic acid and 400 µg vitamin B12 |
n = 46 Placebo supplementation |
1) Focused on European ancestry 2) Small sample size |
3/3 |
| Fadai et al. (35) | RCTB | Male patients 18–65 years old with schizophrenia and on a stable olanzapine regimen with no evidence of metabolic syndromeb | 3 months | Arm 1: n = 20 15 mg of saffron twice daily Arm 2: n = 20 15 mg of crocin twice daily |
n = 21 Not described |
1) Small sample size 2) Only male patients included 3) No inclusion criteria for baseline symptom severity |
3/3 |
| Neriman et al. (36) | Quasi-experimental | 18 – 65 years old with schizophrenia and had stable medication regimens and low 25-OHD levelsb | 4 months |
n = 40 Oral vitamin D supplementation until sufficient levels are reached |
None | 1) Small sample size 2) Only included participants with low vitamin D levels 3) No control group |
*** |
| Pawelckyz et al. (37) | RCT | 16- to 35-year-olds with first episode of schizophrenia and currently psychotica, c | 2–3 months |
n = 36 2.2 g fish oil |
n = 35 Olive oil |
1) Small sample size 2) Only included first episode of schizophrenia 3) Limited age range |
2/3 |
| Qiao et al. (38) | RCT | Inpatients 18–60 years old with schizophrenia, a PANSS >50, violent behavior (MOAS score >4), and elevated aggression scores while on antipsychoticsa, c | 3 months |
n = 28 900 mg fish oil |
n = 22 Vitamin E |
1) Small sample size 2) Short intervention period 3) Variable intervention periods between participants depending on discharge from the inpatient unit |
2/3 |
| Qiao et al. (39) | RCT | Acutely hospitalized with schizophrenia and demonstrating violent behavior | 2 months |
n = 32 900 mg fish oil |
n = 35 10 mg vitamin E |
1) Small sample size 2) Gender imbalance 3) Placebo was vitamin E, which may be an “active” compound |
2/3 |
| Rensburg et al. (40) | RCT | 18- to 65-year-olds with schizophrenia and persistent symptoms (PANSS score >50) while on fixed doses of antipsychotics at least 6 months prior to the trial | 3 months |
n = 16 3 g ethyl-EPA daily |
n = 16 Placebo capsule |
1) Variable dietary intake 2) Small sample 3) Short intervention |
1/3 |
| Sevillano-Jimenez et al. (41) | RCT | 18- to 65-year-olds with schizophrenia and clinical stability 6 months prior to the initiation of the trail | 6 months |
n = 23 Dietary counseling to increase prebiotic and probiotic intake |
n = 21 Conventional dietary advice |
1) Assessment of mainly parametrics of dietary modification, not outcomes 2) During COVID-19 pandemic, leading to variability 3) High dropout rate in the intervention group |
3/3 |
| Soric et al. (42) | RCT | Hospitalized patients 18–67 years old with schizophrenia and clinical stability | 3 months |
n = 33 DASH diet with caloric restriction of 400 kcal/day |
n = 34 Normal hospital diet |
1) Short time 2) Lack of standardized diet as participants could purchase outside food |
2/3 |
| Vaughan and McConaghy (43) | RCT | Outpatient patients with schizophrenia | 5 months |
n = 11 Megavitamin |
n = 9 25 mg vitamin C |
1) Small sample size 2) Placebo was a vitamin supplementation |
3/3 |
| Zhang et al. (44) | RCT | 19- to 65-year-olds with schizophrenia, serum total cholesterol (TC) ≥6.22 mmol/L or serum triglycerides (TG) ≥2.26 mmol/L | 1 month |
n = 39 Konjac flour 1,800 kcal diet for women 2,200 kcal diet for men |
n = 37 Maltodextrin supplementation 1,800 kcal diet for women 2,200 kcal diet for men |
1) Short intervention 2) Small sample size 3) Inadequate sample size for subgroup analysis |
3/3 |
Description of the study characteristics, limitations, and the bias assessment score (BAS).
BAS, bias assessment score; RCT, randomized controlled trial; PCT, pilot clinical trial; RFS, randomized feasibility trial; DASH, dietary approaches to stop hypertension.
In these studies, the participants were diagnosed using the DSM-V criteria.
In these studies marked, the participants were diagnosed using the DSM-IV criteria.
In these studies marked, the participants were diagnosed using ICD-10 diagnostic criteria.
2.7 Bias assessment
All reviewers independently evaluated the risk of bias of the included studies. Each study was rated using a validated three-point questionnaire that included randomization, double blinding, and the withdrawal and dropout descriptions (Table 3) (45). A score of 1 out of 3 was defined as high risk of bias. A score of greater than 2 out of 3 was defined as low risk of bias.
Table 3
| Risk of bias assessment validated questionnaire |
|---|
| Studies were assigned 1 point for yes or 0 point for no in the following categories. The maximum score is 3 points. 3 points = low risk of bias 2 points = moderate risk of bias 1 point = high risk of bias |
| Randomization: 1 point was given for description of appropriate randomization (table of random numbers, computer generated, etc.). A point of 0 was given if there was no description of randomization or the randomization was not appropriate (date of birth, date of admission, hospital number, or alternation). |
| Double blinding: 1 point was given if the word “double blind” was used or the method of blinding was appropriate, including neither the study personnel nor the participants would identify the intervention or statements such as active placebos, identical placebos, or dummies were mentioned. |
| Withdrawals and dropouts: 1 point was given for a description of the dropouts and withdrawals, defined as participants who did not complete the observation period or who were not included. |
Description of the risk bias assessment and scoring via a validated questionnaire.
3 Results
Our search identified 2,124 potential studies, of which 1,559 were screened based on the title and abstract, resulting in 81 full-text articles assessed for eligibility. Ultimately, 17 studies met the inclusion criteria and were included in the analysis (Table 2). Most of the studies were randomized controlled trials (RCTs) conducted in adults with schizophrenia aged 18–65 years in an outpatient setting, with sample sizes ranging from 5 to 80. The duration of intervention ranged from 2 weeks to 6 months, with most interventions lasting 3 months or more. Only three studies included dietary interventions, such as a gluten-free diet or the DASH diet. Most interventions were of vitamin or mineral supplementation of various doses. The most common vitamin supplementation across the studies was fish oil or omega-3s.
Most of the studies did not assess metabolic markers. One study that looked at prebiotic and probiotic intake found a significant reduction in BMI (p < 0.001) and waist circumference (p < 0.001) between the control and experimental groups (Table 4). In one study, fish oil supplementation resulted in a significant difference in the levels of triglycerides (p = 0.094), fasting glucose (p = 0.045), and low-density lipoprotein (LDL) cholesterol (p = 0.094) between the control and experimental groups (Table 4). Another study compared crocin and saffron supplementation to a control group and noted a significant difference in the fasting glucose level (p = 0.004), but no other metabolic markers (Table 4).
Table 4
| Study | Metabolic markers | |||||
|---|---|---|---|---|---|---|
| BMI | WC | TG | FBG | LDL | SBP | |
| Fadai et al. (35) Saffron, crocin |
– | At 3 months: -Saffron: 92.1 -Crocin: 91.9 -Control: 98.4 p = 0.19 |
At 3 months: -Saffron: 85 -Crocin: 102.8 -Control: 102.8 p = 0.723 |
At 3months: -Saffron: 100.5 -Crocin: 97.9 -Control: 108.6 p = 0.004* |
At 3 months: -Saffron: 168.5 -Crocin: 178.5 -Control: 181.7 p = 0.598 |
At 3 months: -Saffron: 116.5 -Crocin: 109.7 -Control: 115.9 p = 0.065 |
| Kelly et al. (32) Gluten-free diet |
– | – | No change | Cohen’s d = −0.36 | – | – |
| Pawelckyz et al. (37) Fish oil |
– | At 2 months, mean change: -Control: 4.32 -Experimental: 3.99 Mean difference = −0.33 p = 0.819 |
At 2 months, mean change: -Control: 8.48 -Experimental: −16.02 Mean difference = −24.5 p = 0.094 |
At 2 months, mean change: -Control: 10.55 -Experimental: 3.55 Mean difference = −7.0 p = 0.045* |
At 2 months, mean change: -Control: 16.37 -Experimental: 2.52 Mean difference = −13.85 p = 0.094 |
At 2 months, mean change: -Control: 4.07 -Experimental: 0.68 Mean difference = −3.39 p = 0.1576 |
| Sevillano-Jimenez et al. (41) Pre-/probiotic |
Control: -Pre: 27.5 -Post: 27.2 p = 0.323 Experimental: -Pre: 29.5 -Post: 27.9 p < 0.001* |
Control -Pre: 97.6 -Post: 101.2 p = 0.322 Experimental -Pre: 105.7 -Post: 102.1 p < 0.001* |
– | – | – | – |
| Soric et al. (42) DASH and calorie restriction |
Control: -Pre: 27.47 -Post: 26.96 Experimental: -Pre: 28.95 -Post: 28.16 p = 0.281 |
Control: -Pre: 106.92 -Post: 104.47 Experimental: -Pre: 109.09 -Post: 105.55 p = 0.690 |
Control: -Pre: 1.81 -Post: 1.83 Experimental: -Pre: 2.15 -Post: 2.32 p = 0.056 |
– | Control: -Pre: 3.07 mmol/L -Post: 3.27 mmol/L Experimental: -Pre: 3.19 mmol/L -Post: 3.04 mmol/L p = 0.255 |
Control: -Pre: 129.41 -Post: 126.25 Experimental: -Pre: 130.38 -Post: 126.52 |
| Zhang et al. (44) Konjac flour and calorie restriction |
Non-significant increase in BMI in both the control and experimental groups | – | Non-significant decrease in the experimental group | Overall: Non-significant change Women: Statistically significant decrease in FBG |
Total cholesterol: decreased significantly. −3.90 ± 14.38 p = 0.005 (overall) HDL and LDL change not significant |
Non-significant increase in SBP in both the control and experimental groups |
Description of the changes in metabolic markers.
BMI, body mass index; WC, waist circumference; TG, triglycerides; FBG, fasting blood glucose; LDL, low-density lipoprotein; SBP, systolic blood pressure; HDL, high-density lipoprotein.
*Indicates that the p-value was statistically significant.
All but four studies assessed the symptoms of schizophrenia, including the total symptom scores, the positive symptom scores, and the negative symptom scores, using various validated symptom scales (Table 5). The symptom scales used included the Positive and Negative Syndrome Scale (PANSS), the Brief Psychiatric Rating Scale (BPRS), the Scale for the Assessment of Negative Symptoms (SANS), the Scale for the Assessment of Positive Symptoms (SAPS), Beck’s Depression Inventory (BDI), and Brief Symptom Inventory (BSI). Supplementation with fish oil and vitamin D led to significant improvements in the total PANSS scores, positive symptom scores, and negative symptom scores in a few included studies, but insignificant improvements in others (Table 5).
Table 5
| Study | Schizophrenia symptoms | ||
|---|---|---|---|
| Total symptom score | Positive symptoms | Negative symptoms | |
| Deutsch (28) Galantamine/CP-choline |
No significant difference between groups | No significant difference between groups | No significant difference between groups |
| Dickerson et al. (29) Probiotic |
No significant difference between groups | No significant difference between groups | No significant difference between groups |
| Fenton et al. (30) Omega-3 fatty acids |
Total Positive and Negative Syndrome Scale: -Control: 18 -Experimental: 16 Mean difference = −2.0 p = 0.84 |
– | – |
| Jamilian et al. (46) Omega-3 fatty acid |
Total score on PANNS: -Control: 52.43 -Experimental: 49.13 Mean difference = −3.3 p < 0.05* |
PANSS: -Control: 14.66 -Experimental: 14.00 Mean difference = −2.66 p > 0.05 |
PANSS: -Control: 11.26 -Experimental: 12.13 Mean difference = 0.87 p > 0.050 |
| Kelly et al. (32) Gluten-free diet |
– | BPRS: -No change |
SANS: -Cohen’s d = −0.53 |
| Kelly et al. (33) OEI |
– | BPRS: -Pre-intervention: 14.4 -Post-intervention: 12.2 Mean difference = −2.2 p = not statistically significant |
SANS: -No change between pre- and post-intervention |
| Roffman et al. (34) Folic acid and vitamin B12 |
Total score on PANNS: -Control: −0.22 -Experimental: −0.21 Mean difference = 0.01 p = 0.89 |
PANSS: -Control: -0.04 -Experimental: -0.06 Mean difference = −0.02 p = 0.61 |
SANS: -Control: 0.02 -Experimental: -0.19 Mean difference = −0.21 p = 0.15 |
| Neriman et al. (36) Vitamin D |
– | SAPS: -Pre-intervention: 18.58 -Post-intervention: 15.51 p < 0.001* |
SANS: -Pre-intervention: 51.51 -Post-intervention: 23.6 p < 0.001* |
| Pawelckyz et al. (37) Fish oil |
– | Correlation between triglyceride levels and total PANSS p = 0.031* |
Correlation between triglyceride levels and the general psychopathology subscale of PANSS p = 0.0008* |
| Qiao et al. (38) Fish oil |
Total score on PANNS: -Control: 59.91 -Experimental: 67.38 Mean difference = +7.47 p = 0.19 |
PANSS: -Control: 16.00 -Experimental: 17.17 Mean difference = 1.17 p = 0.27 |
PANSS: -Control: 14.65 -Experimental: 16.63 Mean difference = 1.98 p = 0.05* |
| Qiao et al. (39) Fish oil |
Total score on PANNS: -Control: 63.00 -Experimental: 61.65 Mean difference = −1.35 p = 0.86 |
PANSS: No significant difference between groups; both groups decreased from baseline. |
PANSS: No significant difference between groups; both groups decreased from baseline. |
| Rensburg et al. (40) Ethyl-EPA |
Correlation between symptom improvement and PUFA concentrations in the experimental group with >20% improvement on the total PANSS score | Correlation between symptom improvement and PUFA concentrations in the experimental group with >20% improvement on the positive symptom PANSS score | Correlation between symptom improvement and PUFA concentrations in the experimental group with >20% improvement on the negative symptom PANSS score |
| Vaughan and McConaghy (43) Megavitamin |
BDI and BSI: No significant difference |
– | – |
Description of the changes in the psychiatric symptom scale scores.
PANSS, Positive and Negative Syndrome Scale; BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; BDI, Beck’s Depression Inventory; BSI, Brief Symptom Inventory; PUFA, polyunsaturated fatty acid.
* indicates that the p-value was statistically significant.
4 Discussion
Current management of schizophrenia includes administration of antipsychotics, which are well known for increasing the risk of metabolic syndrome. Dysregulation of the inflammatory pathways seen in patients with schizophrenia might play a potential role in its pathogenesis and the subsequent development of metabolic syndrome often seen in these patients. Mental illnesses such as depression, anxiety, and bipolar disorder contribute heavily to global disability; however, the current treatments address less than half of this burden, highlighting the need for complementary strategies. Nutritional psychiatry suggests that the diet quality could be a modifiable risk factor, with evidence linking diet to mental health via pathways such as inflammation, oxidative stress, the gut microbiome, and neuroplasticity (8). An analysis of systematic reviews found that dietary interventions combined with lifestyle therapies can help reduce body weight, BMI, and waist circumference in people with schizophrenia on antipsychotic medications; however, the effects on glycemic control, blood pressure, and triglycerides have been mixed (31). This evidence was rated as suggestive to weak, highlighting the need for higher-quality research and standardized reporting to better understand effective intervention elements and support future dietetic practice and policy (31). Recent research has suggested that lifestyle modifications may be equally, if not more, impactful as a pharmacological treatment for reducing the risk of the metabolic side effects of antipsychotics (15). Although the exact pathogenesis is not well defined, increased inflammation may play an important role in schizophrenia. Specialized diets might not only improve the metabolic outcomes but also reduce inflammation and improve symptoms in people with schizophrenia.
A systematic review in 2017 of RCTs found that adjunctive vitamin B supplementation, particularly B6, B8, and B12, significantly reduced psychiatric symptoms in people with schizophrenia compared with placebo, although effects on the specific symptom domains (positive and negative) were not observed (47). Notably, a shorter illness duration correlated with greater effectiveness of vitamin B, while other supplements, such as antioxidant vitamins and minerals, showed no significant impact (47). These findings suggest that targeted nutritional supplementation could offer symptom relief in schizophrenia, although further research is needed to refine the nutrient formulations and explore the underlying mechanisms (47). Similarly, a review of studies up to 2015 found stronger support for fatty acid supplementation in improving psychotic symptoms and reducing extrapyramidal side effects; however, limitations such as a small sample size and a short study duration highlighted the need for larger, longer-term studies focused on varied patient profiles and cognitive outcomes (48). Thus, our review analyzing the effects of anti-inflammatory diets and supplementation makes strides in addressing the knowledge gap by incorporating more recent studies and analyzing both metabolic and psychiatric symptoms.
Our systematic review revealed mixed findings regarding whether anti-inflammatory diets and supplementation of omega-3 fatty acids, fish oil, and vitamin D are effective in the treatment of metabolic markers or schizophrenia symptoms. The results across multiple studies with similar interventions had various degrees of statistical significance. People with schizophrenia have decreased nutritional intake, including a higher intake of animal fats compared with vegetable fats and instant meals and a lower intake of fruits (22–24). This underlying poor dietary pattern might generate higher baseline inflammation levels that could counteract the potential benefits from targeted interventions.
4.1 Strengths and limitations
This review has several important methodological strengths. Firstly, a comprehensive search was conducted across multiple databases, including unpublished literature, to minimize publication bias. Secondly, both dietary and supplementation interventions were evaluated, allowing for broader insights into anti-inflammatory approaches. Thirdly, both metabolic and psychiatric outcomes were assessed, where available, providing a more complete picture of the intervention effects.
However, several limitations affected the interpretation of our findings. Firstly, most of the included studies had small sample sizes and relatively short intervention periods, potentially insufficient to detect meaningful clinical changes. Secondly, there was poor standardization of the interventions, with varying supplement doses and dietary protocols, making direct comparisons challenging. Thirdly, most of the studies failed to measure intervention adherence, particularly in outpatient settings. Fourthly, only a few studies controlled for baseline dietary intake or measured both metabolic and psychiatric outcomes. Finally, there was minimal assessment of cost-effectiveness, which limits our understanding of real-world feasibility.
4.2 Implementation challenges and future directions
The implementation of dietary interventions faces significant practical challenges. People with schizophrenia often experience external barriers, including limited financial resources, inadequate social support, and impaired decision-making capacity (49). Internal barriers such as emotional eating and entrenched dietary habits further complicate the adoption of lifestyle changes (49). A 2013 systematic review identified key drivers of medication non-adherence in patients with schizophrenia, such as lack of insight, medication beliefs, and substance abuse, which contribute to negative outcomes such as increased hospitalization and relapse rates. While medication non-adherence and lifestyle intervention and supplementation non-adherence cannot be compared, the barriers to medication adherence might inform the challenges in the implementation of other treatments (50). Another study identified several modifiable barriers to healthy eating for individuals with serious mental illnesses, including both internal factors (e.g., taste preferences, comfort eating, and prioritization of mental health) and external factors (e.g., limited access to healthy foods, social pressures, and medication side effects) (7). To support dietary improvements and to reduce cardiovascular risks, the recommended interventions included individualized nutrition education, opportunities for hands-on healthy food preparation, and involving family and friends. The authors of the aforementioned study additionally recommended that community mental health centers and group homes should offer only healthy foods and that practitioners should discuss emotional eating and the dietary effects of psychiatric medications with patients (7). While there is limited evidence of nutrition interventions significantly improving metabolic syndrome risk factors in people with serious mental illness, one study found that interventions showed more effectiveness when delivered individually or by dietitians. Integrating dietitian-led, personalized dietary programs within mental health services—using behavior change techniques, goal setting, and self-monitoring alongside peer support—could enhance adherence, reduce attrition, and potentially yield cost savings for healthcare systems (31). A recent scoping review found that only 25% of dietary intervention studies in mental health populations demonstrated clear cost-effectiveness (51).
These findings underscore the need for more comprehensive studies that ensure that effective, affordable dietary interventions can be widely implemented, particularly in low-resource settings where the burden of mental illness is highest. Future studies into the impact of anti-inflammatory diets should target larger sample sizes, longer duration of interventions, and more standardized dietary interventions. Supplementation alone may not be adequate to combat the inflammatory disruption associated with either metabolic syndrome or schizophrenia. Future dietary interventions may need to combine an anti-inflammatory diet with other lifestyle changes and/or dietary supplementation to sufficiently impact metabolic markers and improve schizophrenia symptoms.
5 Conclusion
The present systematic review reveals important insights into the potential role of anti-inflammatory supplements and diets in schizophrenia while highlighting specific gaps in current research. The mixed findings across studies reflect both the complexity of the implementation of dietary interventions in this population and the challenges of the current research methodology. While some interventions, particularly supplementation with fish oil and vitamin D, showed promise in symptom reduction—and prebiotics/probiotics demonstrated metabolic benefits—the overall evidence remains inconclusive due to methodological limitations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
ES: Conceptualization, Data curation, Methodology, Project administration, Writing – original draft, Writing – review & editing. TA: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. CH: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. AN: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. JS: Conceptualization, Data curation, Methodology, Writing – review & editing. MS: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1506353/full#supplementary-material.
References
1
Ringeisen H Edlund MJ Guyer H Geiger P Stambaugh LF Dever JA et al . Mental and substance use disorders prevalence study: findings report. North Carolina: RTI International (2023).
2
Peritogiannis V Ninou A Samakouri M . Mortality in schizophrenia-spectrum disorders: recent advances in understanding and management. Healthcare. (2022) 10:2366. doi: 10.3390/healthcare10122366
3
Westman J Eriksson SV Gissler M Hällgren J Prieto ML Bobo WV et al . Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiol Psychiatr Sci. (2018) 27:519–27. doi: 10.1017/S2045796017000166
4
Papanastasiou E . The prevalence and mechanisms of metabolic syndrome in schizophrenia: a review. Ther Adv Psychopharmacol. (2013) 3:33–51. doi: 10.1177/2045125312464385
5
Salari N Maghami N Ammari T Mosafer H Abdullahi R Rasoulpoor S et al . Global prevalence of metabolic syndrome in schizophrenia patients: A systematic review and meta-analysis. J Prev. (2024) 45:973–86. doi: 10.1007/s10935-024-00798-8
6
Mitchell AJ Vancampfort D Sweers K Van Winkel R Yu W De Hert M . Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders: A systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. doi: 10.1093/schbul/sbr148
7
Teasdale SB Samaras K Wade T Jarman R Ward PB . A review of the nutritional challenges experienced by people living with severe mental illness: a role for dietitians in addressing physical health gaps. J Hum Nutr Diet. (2017) 30:545–53. doi: 10.1111/jhn.2017.30.issue-5
8
Marx W Moseley G Berk M Jacka F . Nutritional psychiatry: the present state of the evidence. Proc Nutr Soc. (2017) 76:427–36. doi: 10.1017/S0029665117002026
9
Allison DB Mackell JA McDonnell DD . The impact of weight gain on quality of life among persons with schizophrenia. PS. (2003) 54:565–7. doi: 10.1176/appi.ps.54.4.565
10
Kane JM Kishimoto T Correll CU . Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. (2013) 12:216–26. doi: 10.1002/wps.20060
11
Fitzgerald I O’Connell J Keating D Hynes C McWilliams S Crowley EK . Metformin in the management of antipsychotic-induced weight gain in adults with psychosis: development of the first evidence-based guideline using GRADE methodology. Evid Based Ment Health. (2022) 25:15–22. doi: 10.1136/ebmental-2021-300291
12
Rochlani Y Pothineni NV Kovelamudi S Mehta JL . Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. (2017) 11:215–25. doi: 10.1177/1753944717711379
13
Boyer L Testart J Michel P Richieri R Faget-Agius C Vanoye V et al . Neurophysiological correlates of metabolic syndrome and cognitive impairment in schizophrenia: A structural equation modeling approach. Psychoneuroendocrinology. (2014) 50:95–105. doi: 10.1016/j.psyneuen.2014.07.019
14
Adamowicz K Kucharska-Mazur J . Dietary behaviors and metabolic syndrome in schizophrenia patients. JCM. (2020) 9:537. doi: 10.3390/jcm9020537
15
Vancampfort D Firth J Correll CU Solmi M Siskind D De Hert M et al . The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: A meta-review of meta-analyses of randomized controlled trials. FOC. (2021) 19:116–28. doi: 10.1176/appi.focus.19103
16
Keshavan MS Anderson S Pettergrew JW . Is Schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. (1994) 28:239–65. doi: 10.1016/0022-3956(94)90009-4
17
Hogenaar JTT Van Bokhoven H . Schizophrenia: complement cleaning or killing. Genes. (2021) 12:259. doi: 10.3390/genes12020259
18
Dennison U McKernan D Cryan J Dinan T . Schizophrenia patients with a history of childhood trauma have a pro-inflammatory phenotype. Psychol Med. (2012) 42:1865–71. doi: 10.1017/S0033291712000074
19
Soulet D Rivest S . Microglia. Curr Biol. (2008) 18:R506–8. doi: 10.1016/j.cub.2008.04.047
20
Kindler J Lim CK Weickert CS Boerrigter D Galletly C Liu D et al . Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. (2020) 25:2860–72. doi: 10.1038/s41380-019-0401-9
21
Perkins DO Jeffries CD Do KQ . Potential roles of redox dysregulation in the development of schizophrenia. Biol Psychiatry. (2020) 88:326–36. doi: 10.1016/j.biopsych.2020.03.016
22
Strassnig M Singh Brar J Ganguli R . Dietary fatty acid and antioxidant intake in community-dwelling patients suffering from schizophrenia. Schizophr Res. (2005) 76:343–51. doi: 10.1016/j.schres.2005.03.002
23
Simonelli-Muñoz AJ Fortea MI Salorio P Gallego-Gomez JI Sánchez-Bautista S Balanza S . Dietary habits of patients with schizophrenia: A self-reported questionnaire survey. Int J Ment Health Nurs. (2012) 21:220–8. doi: 10.1111/j.1447-0349.2012.00821.x
24
Amani R . Is dietary pattern of schizophrenia patients different from healthy subjects? BMC Psychiatry. (2007) 7:15. doi: 10.1186/1471-244X-7-15
25
Cha HY Yang SJ . Anti-inflammatory diets and schizophrenia. Clin Nutr Res. (2020) 9:241. doi: 10.7762/cnr.2020.9.4.241
26
Koelman L Egea Rodrigues C Aleksandrova K . Effects of dietary patterns on biomarkers of inflammation and immune responses: A systematic review and meta-analysis of randomized controlled trials. Adv Nutr. (2022) 13:101–15. doi: 10.1093/advances/nmab086
27
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 10(89):n71. doi: 10.1186/s13643-021-01626-4
28
Deutsch SI Schwartz BL Schooler NR Brown CH Rosse RB Rosse SM . Targeting alpha-7 nicotinic neurotransmission in schizophrenia: A novel agonist strategy. Schizophr Res. (2013) 148:138–44. doi: 10.1016/j.schres.2013.05.023
29
Dickerson FB Stallings C Origoni A Katsafanas E Savage CLG Schweinfurth LAB et al . Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim care companion CNS disord (2014). Available online at: https://www.psychiatrist.com/pcc/effect-probiotic-supplementation-schizophrenia-symptoms (Accessed 2024 Nov 18).
30
Fenton WS Dickerson F Boronow J Hibbeln JR Knable M . A placebo-controlled trial of omega-3 fatty acid (Ethyl eicosapentaenoic acid) supplementation for residual symptoms and cognitive impairment in schizophrenia. AJP. (2001) 158:2071–4. doi: 10.1176/appi.ajp.158.12.2071
31
Kelly B Dawson S Jacka F Dunbar JA O’Neil A . Effectiveness of nutrition and dietary interventions for people with serious mental illness: systematic review and meta-analysis. Med J Aust. (2022) 217:21. doi: 10.5694/mja2.51680
32
Kelly DL Demyanovich HK Rodriguez KM Čiháková D Talor MV McMahon RP et al . Randomized controlled trial of a gluten-free diet in patients with schizophrenia positive for antigliadin antibodies (AGA IgG): a pilot feasibility study. jpn. (2019) 44:269–76. doi: 10.1503/jpn.180174
33
Kelly DL Kane MA Fraser CM Sayer MA Grant-Beurmann S Liu T et al . Prebiotic treatment increases serum butyrate in people with schizophrenia: results of an open-label inpatient pilot clinical trial. J Clin Psychopharmacol. (2021) 41:200–2. doi: 10.1097/JCP.0000000000001364
34
Roffman JL Lamberti JS Achtyes E Macklin EA Galendez GC Raeke LH et al . Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry. (2013) 70:481. doi: 10.1001/jamapsychiatry.2013.900
35
Fadai F Mousavi B Ashtari Z Ali Beigi N Farhang S Hashempour S et al . Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: A randomized triple blind placebo controlled study. Pharmacopsychiatry. (2014) 47:156–61. doi: 10.1055/s-0034-1382001
36
Neriman A Hakan Y Ozge U . The psychotropic effect of vitamin D supplementation on schizophrenia symptoms. BMC Psychiatry. (2021) 21:309. doi: 10.1186/s12888-021-03308-w
37
Pawełczyk T Grancow-Grabka M Żurner N Pawełczyk A . Omega-3 fatty acids reduce cardiometabolic risk in first-episode schizophrenia patients treated with antipsychotics: Findings from the OFFER randomized controlled study. Schizophr Res. (2021) 230:61–8. doi: 10.1016/j.schres.2021.02.012
38
Qiao Y Mei Y Han H Liu F Yang XM Shao Y et al . Effects of Omega-3 in the treatment of violent schizophrenia patients. Schizophr Res. (2018) 195:283–5. doi: 10.1016/j.schres.2017.08.026
39
Qiao Y Liu CP Han HQ Liu FJ Shao Y Xie B . No impact of omega-3 fatty acid supplementation on symptoms or hostility among patients with schizophrenia. Front Psychiatry. (2020) 11:312. doi: 10.3389/fpsyt.2020.00312
40
Van Rensburg SJ Smuts CM Hon D Kidd M van der Merwe S Myburgh C et al . Changes in erythrocyte membrane fatty acids during a clinical trial of eicosapentaenoic acid (EPA) supplementation in schizophrenia. Metab Brain Dis. (2009) 24:659–72. doi: 10.1007/s11011-009-9160-7
41
Sevillano-Jiménez A Romero-Saldaña M García-Rodríguez M Molina-Luque R Molina-Recio G . Nutritional impact and eating pattern changes in schizophrenic spectrum disorders after health education program on symbiotic dietary modulation offered by specialised psychiatric nursing–two-arm randomised clinical trial. Nutrients. (2022) 14:5388. doi: 10.3390/nu14245388
42
Sorić T Mavar M Rumbak I . The effects of the dietary approaches to stop hypertension (DASH) diet on metabolic syndrome in hospitalized schizophrenic patients: A randomized controlled trial. Nutrients. (2019) 11:2950. doi: 10.3390/nu11122950
43
Vaughan K McConaghy N . Megavitamin and dietary treatment in schizophrenia: A randomised, controlled trial. Aust N Z J Psychiatry. (1999) 33:84–8. doi: 10.1046/j.1440-1614.1999.00527.x
44
Zhang L Han Y Zhao Z Liu X Xu Y Cui G et al . Beneficial effects of konjac powder on lipid profile in schizophrenia with dyslipidemia: A randomized controlled trial. Asia Pac J Clin Nutr. (2020) 29:505–512. doi: 10.6133/apjcn.202009_29(3).0009
45
Jadad AR Moore RA Carroll D Jenkinson C Reynolds DJM Gavaghan DJ et al . Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
46
Jamilian H Solhi H Jamilian M . Randomized, placebo-controlled clinical trial of omega-3 as supplemental treatment in schizophrenia. Glob J Health Sci. (2014) 6:103–8. doi: 10.5539/gjhs.v6n7p103
47
Firth J Stubbs B Sarris J Rosenbaum S Teasdale S Berk M et al . The effects of vitamin and mineral supplementation on symptoms of schizophrenia: a systematic review and meta-analysis. Psychol Med. (2017) 47:1515–27. doi: 10.1017/S0033291717000022
48
Chia SC Henry J Mok YM Honer WG Sim K . Fatty acid and vitamin interventions in adults with schizophrenia: a systematic review of the current evidence. J Neural Transm. (2015) 122:1721–32. doi: 10.1007/s00702-015-1451-z
49
Barre LK Ferron JC Davis KE Whitley R . Healthy eating in persons with serious mental illnesses: understanding and barriers. Psychiatr Rehabil J. (2011) 34:304–10. doi: 10.2975/34.4.2011.304.310
50
Higashi K Medic G Littlewood KJ Diez T Granström O De Hert M . Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. (2013) 3:200–18. doi: 10.1177/2045125312474019
51
Burrows T Teasdale S Rocks T Whatnall M Schindlmayr J Plain J et al . Cost effectiveness of dietary interventions for individuals with mental disorders: A scoping review of experimental studies. Nutr Diet. (2022) 79:291–302. doi: 10.1111/1747-0080.12703
Summary
Keywords
schizophrenia, anti-inflammatory diets, inflammation, vitamins, supplements, metabolic syndrome
Citation
Suschana E, Anderson T, Hong C, Narikatte A, Silverberg J and Sharma MS (2025) The role of anti-inflammatory diets and supplementation in metabolic syndrome and symptom remission in adults with schizophrenia: a systematic review. Front. Psychiatry 15:1506353. doi: 10.3389/fpsyt.2024.1506353
Received
05 October 2024
Accepted
02 December 2024
Published
07 January 2025
Volume
15 - 2024
Edited by
Massimo Tusconi, University of Cagliari, Italy
Reviewed by
Francesco Monaco, Azienda Sanitaria Locale Salerno, Italy
Pasquale Pezzella, University of Campania Luigi Vanvitelli, Italy
Octavian Vasiliu, Dr. Carol Davila University Emergency Military Central Hospital, Romania
Updates
Copyright
© 2025 Suschana, Anderson, Hong, Narikatte, Silverberg and Sharma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth Suschana, suschana@uchc.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.