- 1Chengdu Southwest Children's Rehabilitation Hospital, Chengdu, China
- 2The Second Affiliated Hospital of Chengdu Medical College, China National Nuclear Corporation 416 Hospital, Chengdu, China
Background: Major depressive disorder (MDD) is a prevalent mental health condition characterized by persistent low mood, diminished interest in pleasurable activities, and anhedonia. Some patients with depression experience high levels of anxiety, complicating clinical treatment. However, the underlying pathological mechanisms remain unclear.
Methods: The sample comprised 178 participants, including 73 MDD with high anxiety symptom subjects, 55 MDD with low anxiety symptom, and 50 healthy controls registered from multiple sites based on the REST-meta-MDD Project in China. Resting-state functional magnetic resonance imaging (rs-fMRI) data were recorded. Large-scale static and dynamic functional connectivity analyses were conducted to identify specific brain connectivity distinguishing MDD with low and high anxiety symptoms.
Results: While MDD patients with high and low anxiety symptoms exhibit overlapping alterations in dynamic functional connectivity between the auditory cortex and nodes of the salience network, their distinct clinical profiles may be associated with differential functional connectivity patterns between the components of the default mode network (DMN) and the visual network (VN), as well as between the components of the basal ganglia network (BGN) and VN.
Conclusion: The VN–DMN–BGN functional circuit may help elucidate the underlying pathological mechanisms associated with varying levels of anxiety in depressive disorders. Understanding this neural correlation could contribute to the development of targeted therapeutic strategies for MDD.
1 Introduction
Major depressive disorder (MDD) is one of the most common mental disorders and is a highly heterogeneous condition characterized by depressed mood, loss of interest or pleasure, and anhedonia (1). Understanding the neuropathological mechanisms of MDD could enhance our ability to diagnose and treat the disorder more effectively. Notably, some patients with MDD exhibit significant anxiety symptoms, while others do not experience pronounced anxiety (2). This variability highlights the complexity of the disorder and suggests that different underlying mechanisms may be involved, potentially influencing treatment strategies. However, the biological mechanisms remain unclear. Elucidating these mechanisms is essential for identifying novel targets for clinical intervention in MDD.
Researchers have indicated that MDD may be associated with dysfunction in emotional regulation, involving the core brain regions such as the hippocampus, prefrontal lobe, amygdala, and hypothalamus (3, 4). Abnormalities in the connectivity and activity of these regions may contribute to the impaired emotional and social functioning in individuals with MDD. Additionally, alterations in sensory thresholds may lead to either heightened sensitivity or diminished responsiveness to sensory input, further affecting daily functioning and social interactions (5). Recent evidence also suggests that the visual network is implicated in the neuropathological mechanism of both MDD and anxiety disorder (5–7). Understanding how sensory perception networks contribute to depression may offer valuable insights for developing targeted therapeutic interventions aimed at restoring normal sensory processing and improving emotional regulation (8).
Moreover, MDD is related to mediated neural activity in non-motor regions, such as the nodes within the default mode network (DMN) (9–13). Most interestingly, a recent study indicated that the symptoms of MDD subjects are associated with reduced visual network (VN) and DMN regions during stimulation with especially rapid visual stimuli (13). Different treatment approaches have been shown to modulate GABA levels in the VN in individuals with MDD (14). The visual cortex has also been reported to exhibit globally reduced activity, which is associated with impaired visual psychophysical performance (15, 16). These findings suggest that the DMN and VN may be involved in the neuropathological mechanisms of MDD. However, the distinct neural pathways differentiating MDD with high anxiety symptoms from MDD with low anxiety symptoms remain unclear.
The resting-state functional magnetic resonance imaging (rs-fMRI) has emerged as a powerful tool in clinical neuroscience (17–20). Clinical neuroscience has adopted transdiagnostic methodologies to explore the neurobiological abnormalities of mental disorders (21, 22). A highly synchronized network at the resting state is thought to represent distinct primary sensorimotor, emotional, or cognitive processes. Specifically, static and dynamic functional connectivity are crucial methodologies for investigating brain function and elucidating the pathological mechanisms underlying depression. Static functional connectivity examines the consistent patterns of brain activity over time, providing insights into the stable relationships between different brain regions (23). This approach has revealed altered connectivity in networks such as the DMN in individuals with depression, highlighting disruptions in emotional regulation and self-referential thought processes. In contrast, dynamic functional connectivity captures the temporal fluctuations in brain connectivity, reflecting the brain’s adaptability and responsiveness to changing cognitive and emotional demands (24, 25). Dynamic connectivity analyses can reveal periods of heightened connectivity that correlate with depressive episodes, as well as moments of disconnection that may indicate resilience or recovery. Together, these approaches provide a comprehensive framework for understanding the complex interplay of brain networks in depression (26). By integrating both static and dynamic perspectives, researchers can better identify biomarkers for depression, inform treatment strategies, and ultimately enhance our understanding of MDD.
Based on previous research, we hypothesized that MDD patients with high anxiety symptoms would exhibit distinct functional connectivity (FC) within primary systems, as well as in associated modulatory networks, compared to those with low anxiety symptoms. To test this hypothesis, we conducted large-scale static and dynamic FC analyses using multi-site datasets of patients with MDD. We propose that these differential functional changes may offer insights into the underlying biological mechanisms of MDD.
2 Materials and methods
2.1 Participants
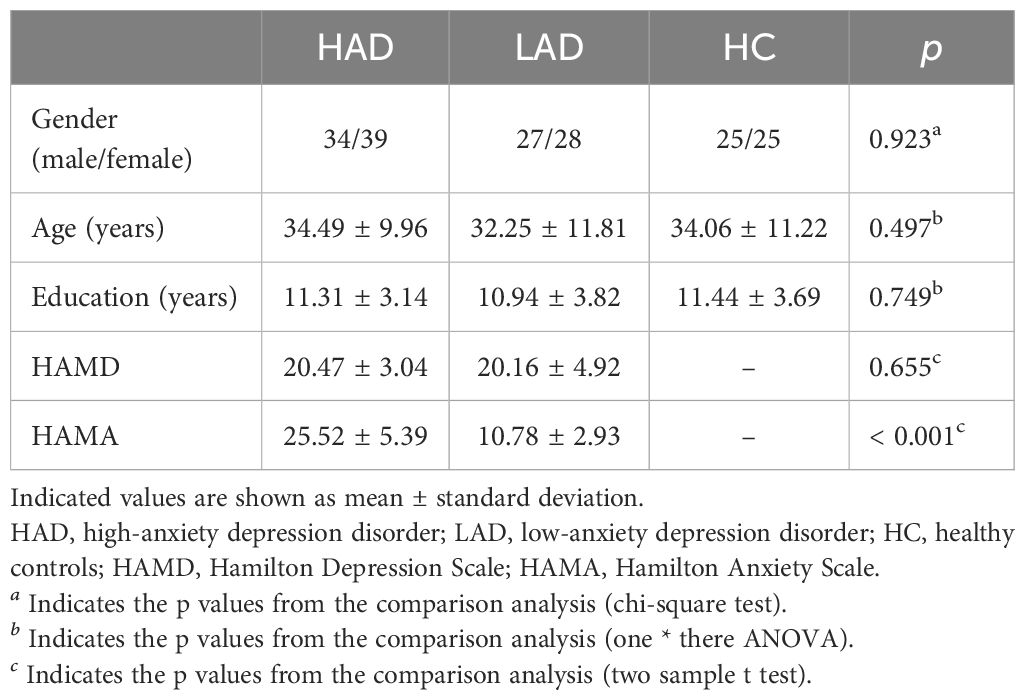
This study was based on the REST-meta-MDD Project of resting-state fMRI initiated in China (27–29). The fMRI data were downloaded from the website of a public database (rfmri.org/REST-meta-MDD). In the REST-meta-MDD Project, the Hamilton Depression Scale (HAMD) was used to evaluate the depression symptom severity of patients. The severity of anxiety was examined using the Hamilton Anxiety Scale (HAMA) in MDD patients. Depression patients with a HAMA score greater than 14 were defined as the high-anxiety depression (HAD) group, while those with a score less than 14 were defined as the low-anxiety depression (LAD) group. Matched healthy controls (HCs) were also recruited. The sample comprised 178 participants, including 73 MDD with high anxiety symptom subjects, 55 MDD with low anxiety symptom subjects, and 50 healthy controls. Subject demographics are displayed in Table 1. All data were identified and anonymized. All subjects provided informed consent in accordance with the requirements of the ethics committee of the local institutional review boards.
2.2 Data acquisition and preprocessing
All resting-state fMRI data were preprocessed at each site according to a standardized preprocessing protocol on Data Processing Assistant for Resting-State fMRI (DPARSF) (27). The detailed preprocessing steps were as follows: discarding the first 10 volumes, slice-timing correction, realignment, coregistration, normalization, and nuisance regression. Nuisance signals include the Friston-24 head motion parameters, white matter, and cerebrospinal fluid. Participants with mean framewise displacement (FD) larger than 0.2 were excluded. Finally, a linear trend was included as a regressor to account for drifts in the Blood Oxygen Level-Dependent (BOLD) signal, and temporal band-pass filtering (0.01–0.1 Hz) was applied to all time series.
2.3 Atlas-based static and dynamic functional connectivity analyses
Static and dynamic FC analyses were performed. An overview of the analytic steps is shown in Figure 1. For each subject, key nodes within the whole brain were defined based on Power’s 264 functional regions of interest (ROIs). To assess the static and dynamic FC among these regions, a series of steps were performed. Pearson’s correlation coefficients were calculated among 264 ROIs. The resulting values were transformed to approximate a Gaussian distribution using Fisher’s r-to-z transformation. The FC score based on the whole time course was considered as the static (rstatic) FC. Dynamic FC was also measured using a sliding window. The time courses were segmented into windows to efficiently capture cognitive status. Previous research has indicated that the minimum window length should be no less than 1/fmin. Thus, the time courses were segmented into 100-s windows, sliding by 2 s of data. Within each window, Pearson’s correlations were computed among 264 ROIs. Across n windows, the dynamic FC was defined as the coefficient of variation score in each connectivity.
2.4 Statistical analysis
Based on prior studies, ComBat has been recognized as one of the most effective harmonization techniques, as it successfully eliminates site-related unwanted variation while retaining inter-subject biological differences (30, 31). Thus, for the static and dynamic FC, the ComBat method was used to remove site effects. In the ComBat model, the age, sex, education years, and FD were included as covariates. ComBat harmonization analyses were performed using a publicly available Matlab package hosted at https://github.com/Jfortin1/ComBatHarmonization. Then, the ANOVA was performed to assess the difference between static and dynamic FC among the HAD group, the LAD group, and the HC group. The significance threshold of group differences for the ANOVA was set to false discovery rate (FDR)-corrected p < 0.05. The different matrix was obtained. Then, the altered FC was extracted for the post-hoc analysis through a two-sample t-test between the two groups (uncorrected p < 0.05).
2.5 Correlations with HAMD and HAMA scores
The relationship was assessed between changed FC and coupling symptoms of depression and anxiety. First, the HAMD value was divided by the HAMA value. This score was defined as the coupling of symptoms of each patient. Then, the partial correlation analysis was calculated between the changed FCs and coupling symptoms in the LAD and HAD groups, with age, sex, education years, and FD as covariates (uncorrected p < 0.05).
3 Results
3.1 Altered static functional connectivity
The altered static FC was observed between the key node of the dorsal visual network and the subcortical region through ANOVA. Post-hoc analysis revealed that the reduced static FC between the left paracentral lobule and putamen within the basal ganglia network (BGN) was observed in the HAD group compared to the HC and LAD groups, but did not show a difference between the LAD and HC groups (Figure 2A). Moreover, through ANOVA, altered static FCs were observed between the region of the VN and the region of the DMN. Post-hoc analysis found that reduced static FC was observed between the posterior cingulate cortex (PCC) and the lingual gyrus in the LAD group compared to the HAD and HC groups, but did not show a difference between the HC and HAD groups (Figure 2B).
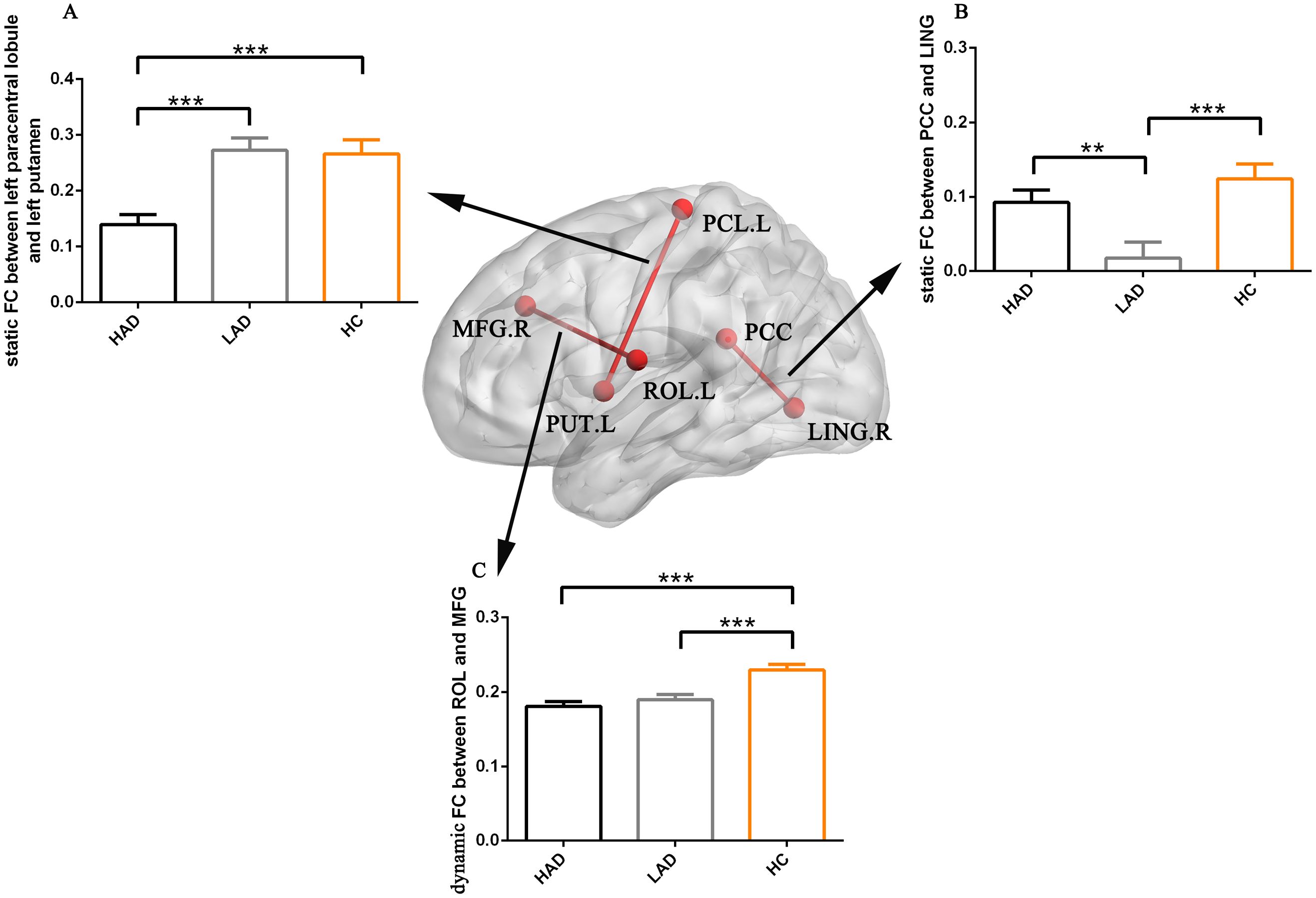
Figure 2. The bar maps represent the post-hoc results. The data are expressed as the mean value + standard error. *** p < 0.001 and **p < 0.01. (A) The difference in static FC between left paracentral lobule and left putamen. (B) The difference in static FC between PCC and LING. (C) The differences in dynamic FC between ROL and MFG. PCC, posterior cingulate cortex; LING, lingual gyrus; MFG, middle frontal gyrus; ROL, Rolandic operculum; FC, functional connectivity; HAD, high-anxiety depression group; LAD, low-anxiety depression group; HC, healthy control.
3.2 Altered dynamic functional connectivity
The abnormal dynamic FC was found between the region of the auditory network (AN) and the region of the salience network (SN). Post-hoc analysis found high static FC between the left Rolandic operculum and right middle frontal gyrus in the HC group compared to the HAD and LAD groups, but did not show a difference between the LAD and HAD groups (Figure 2C).
3.3 Relationship among altered functional connectivities
Using a post-hoc analysis to determine the relationship among altered FCs (Figure 3), in the HC group, we observed a positive relationship (r = 0.434, p = 0.0016) between static FC (left paracentral lobule and left putamen) and static FC (PCC and lingual gyrus), whereas this correlation was not apparent in the LAD (r = 0.037, p = 0.7835) and HAD (r = 0.201, p = 0.0879) groups. This is the common deficient functional coupling within the VN–DMN–BGN circuit in the LAD and HAD groups.
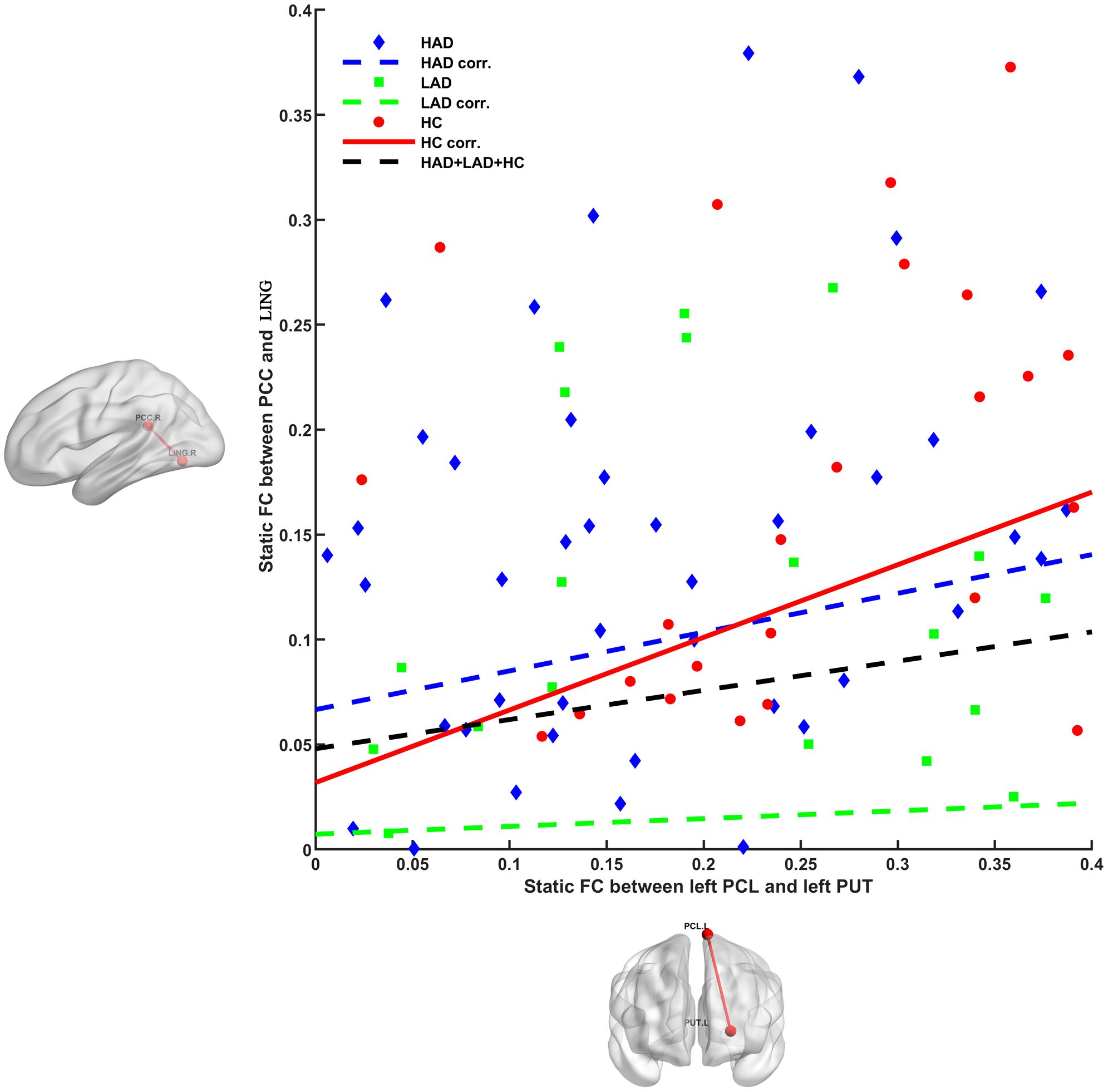
Figure 3. Different relationships between PCL–PUT static FC and PCC–LING static FC in HAD, LAD, and HC groups. Positive relationship was observed in HC group (r = 0.434, p = 0.0016), whereas this correlation was not apparent in the LAD (r = 0.037, p = 0.7835) and HAD (r = 0.201, p = 0.0879) groups. PCL, paracentral lobule; PUT, putamen; PCC, posterior cingulate cortex; LING, lingual gyrus; HAD, high-anxiety depression group; LAD, low-anxiety depression group; HC, healthy control.
3.4 Relationship between altered FC and coupling score of HAMD and HAMA
A negative relationship (r = −0.328, p = 0.018) was observed between HAMD/HAMA score and dynamic Rolandic operculum–middle frontal gyrus (ROL–MFG) FC in the LAD group (Figure 4).
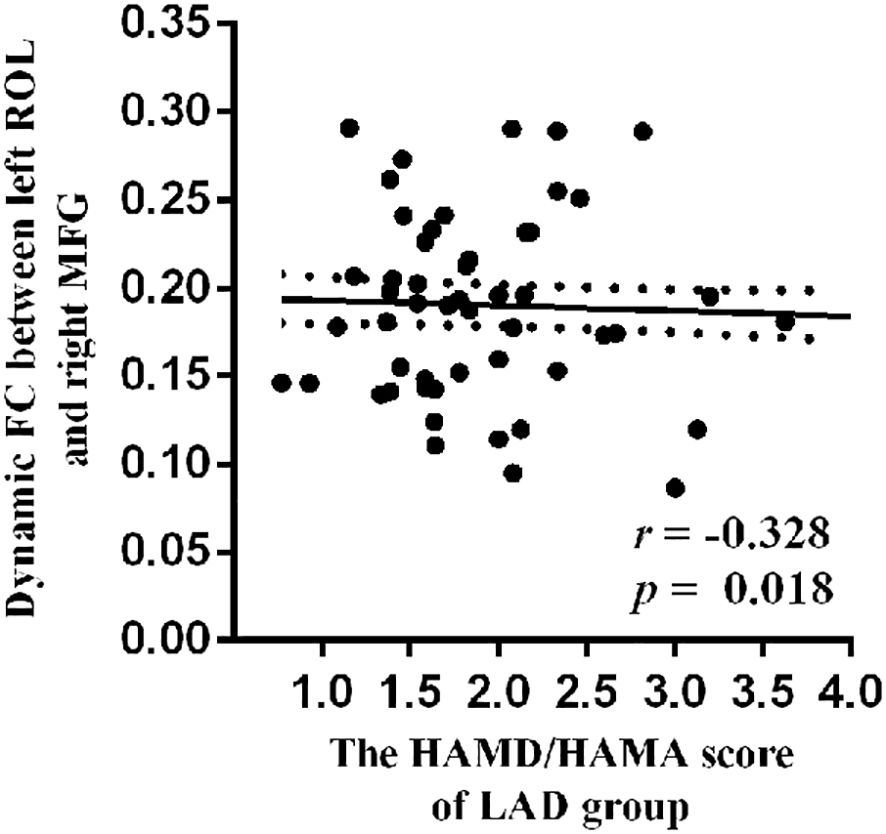
Figure 4. The negative relationship between HAMD/HAMA score and dynamic ROL–MFG FC in LAD group. ROL, Rolandic operculum; MFG, middle frontal gyrus; FC, functional connectivity; LAD, low-anxiety depression group.
4 Discussion
In this study, we applied static and dynamic large-scale functional connectivity analyses to multi-site MDD datasets. While both MDD patients with high anxiety and those with low anxiety symptoms exhibited overlapping alterations in dynamic FC between the auditory region and nodes of the salience network, their distinct characteristics may be attributed to differences in FC between regions of the DMN and the VN, as well as between the putamen and the paracentral lobule. Elucidating the relationships between specific patterns of aberrant FC and MDD may enhance our understanding of the neuropathophysiological mechanisms underlying the symptom heterogeneity of this complex disorder.
The first main finding is the reduced FC between the putamen and the paracentral lobule, a key region within the dorsal visual pathway, in MDD patients with high anxiety symptoms. The dorsal visual pathway is involved in spatial awareness and motion processing (32). MDD has been associated with reduced FC in visual processing regions, which has been linked to difficulties in visual attention and the interpretation of emotional cues (33). These abnormalities may contribute to the cognitive and emotional symptoms of depression, influencing how individuals perceive and interact with their environment. Conversely, research has shown that individuals with anxiety disorders often exhibit heightened sensitivity to visual stimuli, which may be reflected in altered FC patterns between the visual network and the basal ganglia network (34, 35). In this study, altered static FC was observed in MDD patients with high anxiety symptoms between regions of the dorsal visual network and nodes within the BGN. This difference was not observed in MDD patients with low anxiety symptoms. Together, our findings highlight that abnormal FC between the VN and BGN may be a key feature associated with the neuropathological mechanisms of MDD in individuals with high anxiety symptoms.
Our next key finding consists of the decreased FC between PCC and the lingual gyrus in MDD subjects with low anxiety symptoms. Previous research has indicated that the symptoms of MDD may be related to a psychomotor source with neural changes outside motor regions, for example, regions within the DMN (36) and regions within the visual network (37–39). The DMN is commonly active during rest, and self-referential thought (40) shows increased activity patterns in depressed patients (41, 42). Studies using fMRI have demonstrated that the lingual gyrus, which is associated with processing social and emotional information, exhibits increased connectivity with the occipital lobe (43, 44). This enhanced connectivity may reflect a maladaptive neural response in depression with low anxiety symptoms, where the integration of emotional and visual information becomes dysregulated. In contrast, individuals with anxiety disorders often show reduced DMN–frontoparietal network (FPN) connectivity, which may reflect a reduced state of vigilance and a tendency to focus on external threats rather than internal thoughts (45). Finally, our findings are well in line with the previous finding of decreased local and global synchronization of the visual cortex in MDD (39). In this study, reduced static FC was observed between the PCC and lingual gyrus in the LAD group compared to the HAD and HC groups, but did not show a difference between the HC and HAD groups. Therefore, reduced PCC–lingual gyrus connectivity may represent a specific feature of MDD patients with low anxiety symptoms.
Despite delineating several critical observations, there are several limitations that should be considered. First, the causality between altered functional networks and patient symptoms was not examined in this study. Second, participants did not complete any emotion-related task-based fMRI assessments. Finally, we acknowledge that validation using independent datasets, particularly from our own clinical center, is crucial to confirm the robustness of our results and will be a key focus of our future work.
5 Conclusion
Using a data-driven approach with multi-site datasets, the present study provides robust evidence for different DMN–VN and VN–BGN FCs between MDD with low anxiety symptoms and MDD with high anxiety symptoms, supporting a sub-categorization of depression. These networks create an interplay of VN–DMN–BGN functional circuit, which may contribute to our understanding of the neuropathophysiological mechanisms underlying the heterogeneity of symptoms in this complex disorder.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was based on the REST-meta-MDD Project of resting-state fMRI initiated in China. We downloaded the fMRI data from the website of public database (rfmri.org/REST-meta-MDD). All subjects provided informed consent in accordance with requirement of the ethics committee of the local institutional review boards. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LL: Formal analysis, Methodology, Writing – original draft. ZZ: Investigation, Writing – original draft. YZ: Project administration, Validation, Writing – original draft. JFL: Data curation, Investigation, Writing – review & editing. JYL: Methodology, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Thanks for the support of the REST-meta-MDD Project. The R-fMRI indices of the patients with major depressive disorder and normal controls are available through the R-fMRI Maps Project (rfmri.org/REST-meta-MDD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1539702/full#supplementary-material
References
1. Goldberg D. The heterogeneity of “major depression. World Psychiatry. (2011) 10:226–8. doi: 10.1002/j.2051-5545.2011.tb00061.x
2. Nutt D. Management of patients with depression associated with anxiety symptoms. J Clin Psychiatry. (1997) 58 Suppl 8:11–6.
3. Gold PW. The PPARg system in major depression: pathophysiologic and therapeutic implications. Int J Mol Sci. (2021) 22:9248. doi: 10.3390/ijms22179248
4. Sokolowska E, Viitanen R, Misiewicz Z, Mennesson M, Saarnio S, Kulesskaya N, et al. The circadian gene Cryptochrome 2 influences stress-induced brain activity and depressive-like behavior in mice. Genes Brain Behav. (2021) 20:e12708. doi: 10.1111/gbb.12708
5. Serafini G, Gonda X, Canepa G, Pompili M, Rihmer Z, Amore M, et al. Extreme sensory processing patterns show a complex association with depression, and impulsivity, alexithymia, and hopelessness. J Affect Disord. (2017) 210:249–57. doi: 10.1016/j.jad.2016.12.019
6. Mallorquí-Bagué N, Bulbena A, Pailhez G, Garfinkel SN, and Critchley HD. Mind-body interactions in anxiety and somatic symptoms. Harv Rev Psychiatry. (2016) 24:53–60. doi: 10.1097/HRP.0000000000000085
7. Wu F, Lu Q, Kong Y, and Zhang Z. A comprehensive overview of the role of visual cortex malfunction in depressive disorders: opportunities and challenges. Neurosci Bull. (2023) 39:1426–38. doi: 10.1007/s12264-023-01052-7
8. Chung S, Li X, and Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. (2002) 34:437–46. doi: 10.1016/S0896-6273(02)00659-1
9. Videbech P, Ravnkilde B, and Pedersen TH. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. (2002) 106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x
10. Narita H, Odawara T, Iseki E, Kosaka K, and Hirayasu Y. Psychomotor retardation correlates with frontal hypoperfusion and the Modified Stroop Test in patients under 60-years-old with major depression. Psychiatry Clin Neurosci. (2004) 58:389–95. doi: 10.1111/j.1440-1819.2004.01273.x
11. Walther S, Hügli S, Höfle O, Federspiel A, Horn H, Bracht T, et al. Frontal white matter integrity is related to psychomotor retardation in major depression. Neurobiol Dis. (2012) 47:13–9. doi: 10.1016/j.nbd.2012.03.019
12. Han S, Wang X, and He Z. Decreased static and increased dynamic global signal topography in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 94:109665. doi: 10.1016/j.pnpbp.2019.109665
13. Lu X, Zhang JF, Gu F, Zhang HX, Zhang M, Zhang HS, et al. Altered task modulation of global signal topography in the default-mode network of unmedicated major depressive disorder. J Affect Disord. (2022) 297:53–61. doi: 10.1016/j.jad.2021.09.093
14. Runia N, Yücel DE, Lok A, de Jong K, Denys DA, van Wingen G, et al. The neurobiology of treatment-resistant depression: A systematic review of neuroimaging studies. Neurosci Biobehav Rev. (2022) 132:433–48. doi: 10.1016/j.neubiorev.2021.12.008
15. Song XM, Hu XW, Li Z, Gao Y, Ju X, Liu DY, et al. Reduction of higher-order occipital GABA and impaired visual perception in acute major depressive disorder. Mol Psychiatry. (2021) 26:6747–55. doi: 10.1038/s41380-021-01090-5
16. Liu DY, Ju X, Gao Y, Han JF, Li Z, Hu XW, et al. From molecular to behavior: higher order occipital cortex in major depressive disorder. Cereb Cortex. (2022) 32:2129–39. doi: 10.1093/cercor/bhab343
17. Jiang Y, Duan M, Li X, Huang H, Zhao GC, Li X, et al. Function-structure coupling: White matter functional magnetic resonance imaging hyper-activation associates with structural integrity reductions in schizophrenia. Hum Brain Mapp. (2021) 42:4022–34. doi: 10.1002/hbm.v42.12
18. Zhang X, Yang X, Wu B, Pan NF, He M, Wang S, et al. Large-scale brain functional network abnormalities in social anxiety disorder. Psychol Med. (2023) 53:6194–204. doi: 10.1017/S0033291722003439
19. Gao K, He H, Lu B, Xie QS, Lu J, Yao DZ, et al. Discrepant changes in structure-function coupling in dancers and musicians. Cereb Cortex. (2024) 34:bhae068. doi: 10.1093/cercor/bhae068
20. Zhang Y, Duan M, and He H. Deficient salience and default mode functional integration in high worry-proneness subject: a connectome-wide association study. Brain Imaging Behav. (2024) 18:1560–8. doi: 10.1007/s11682-024-00951-1
21. He H, Yang M, Duan M, Chen X, Lai YX, Xia Y, et al. Music intervention leads to increased insular connectivity and improved clinical symptoms in schizophrenia. Front Neurosci. (2017) 11:744. doi: 10.3389/fnins.2017.00744
22. He H, Luo C, Luo Y, Duan MJ, Yi QZ, Biswal BB, et al. Reduction in gray matter of cerebellum in schizophrenia and its influence on static and dynamic connectivity. Hum Brain Mapp. (2019) 40:517–28. doi: 10.1002/hbm.24391
23. Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. (2010) 107:4734–9. doi: 10.1073/pnas.0911855107
24. Chang C and Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. (2010) 50:81–98. doi: 10.1016/j.neuroimage.2009.12.011
25. Hansen EC, Battaglia D, Spiegler A, Deco G, and Jirsa VK. Functional connectivity dynamics: modeling the switching behavior of the resting state. Neuroimage. (2015) 105:525–35. doi: 10.1016/j.neuroimage.2014.11.001
26. Chavanne AV and Robinson OJ. The overlapping neurobiology of induced and pathological anxiety: A meta-analysis of functional neural activation. Am J Psychiatry. (2021) 178:156–64. doi: 10.1176/appi.ajp.2020.19111153
27. Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci U S A. (2019) 116:9078–83. doi: 10.1073/pnas.1900390116
28. Liu PH, Li Y, Zhang AX, Sun N, Li GZ, Chen X, et al. Brain structural alterations in MDD patients with gastrointestinal symptoms: Evidence from the REST-meta-MDD project. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 111:110386. doi: 10.1016/j.pnpbp.2021.110386
29. Yang H, Chen X, Chen ZB, Li L, Li XY, Castellanos FX, et al. Disrupted intrinsic functional brain topology in patients with major depressive disorder. Mol Psychiatry. (2021) 26:7363–71. doi: 10.1038/s41380-021-01247-2
30. Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. (2017) 161:149–70. doi: 10.1016/j.neuroimage.2017.08.047
31. Yu M, Linn KA, Cook PA, Phillips ML, McInnis M, Fava M, et al. Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Hum Brain Mapp. (2018) 39:4213–27. doi: 10.1002/hbm.v39.11
32. Young MP, Scannell JW, Burns GA, and Blakemore C. Analysis of connectivity: neural systems in the cerebral cortex. Rev Neurosci. (1994) 5:227–50. doi: 10.1515/REVNEURO.1994.5.3.227
33. Jaworska N, Yang XR, Knott V, and MacQueen G. A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry. (2015) 16:448–71. doi: 10.3109/15622975.2014.885659
34. Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. (2016) 3:472–80. doi: 10.1016/S2215-0366(15)00579-9
35. Zhang Y, Cai X, Duan M, and He H. The influence of high worry on static and dynamic insular functional connectivity. Front Neurosci. (2023) 17:1062947. doi: 10.3389/fnins.2023.1062947
36. Zeng LL, Shen H, Liu L, Wang LB, Li BJ, Fang P, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. (2012) 135:1498–507. doi: 10.1093/brain/aws059
37. Kaiser RH, Andrews-Hanna JR, Wager TD, and Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. (2015) 72:603–11. doi: 10.1001/jamapsychiatry.2015.0071
38. Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. (2016) 41:1822–30. doi: 10.1038/npp.2015.352
39. Song XM, Liu DY, Hirjak D, Hu XW, Han JF, Roe AW, et al. Motor versus psychomotor? Deciphering the neural source of psychomotor retardation in depression. Adv Sci (Weinh). (2024) 11:e2403063. doi: 10.1002/advs.202403063
40. Killingsworth MA and Gilbert DT. A wandering mind is an unhappy mind. Science. (2010) 330:932. doi: 10.1126/science.1192439
41. Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
42. Steinfurth EC, Alius MG, Wendt J, and Hamm AO. Physiological and neural correlates of worry and rumination: Support for the contrast avoidance model of worry. Psychophysiology. (2017) 54:161–71. doi: 10.1111/psyp.2017.54.issue-2
43. Norris CJ, Chen EE, Zhu DC, Small SL, and Cacioppo JT. The interaction of social and emotional processes in the brain. J Cognit Neurosci. (2004) 16:1818–29. doi: 10.1162/0898929042947847
44. Wang J, Yang Z, Klugah-Brown B, Zhang T, Yang JM, Yuan JJ, et al. The critical mediating roles of the middle temporal gyrus and ventrolateral prefrontal cortex in the dynamic processing of interpersonal emotion regulation. Neuroimage. (2024) 300:120789. doi: 10.1016/j.neuroimage.2024.120789
Keywords: major depressive disorder, anxiety, default model network, functional connectivity, fMRI
Citation: Li L, Zeng Z, Zhou Y, Lin J and Li J (2025) Altered static and dynamic functional connectivity in major depressive disorder accompanied by high anxiety: evidence from the REST-meta-MDD consortium. Front. Psychiatry 16:1539702. doi: 10.3389/fpsyt.2025.1539702
Received: 06 December 2024; Accepted: 12 May 2025;
Published: 09 June 2025.
Edited by:
Takashi Nakano, Fujita Health University, JapanReviewed by:
Masahiro Takamura, Shimane University, JapanWenjian Tan, Central South University, China
Copyright © 2025 Li, Zeng, Zhou, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lujun Li, enpqMjFsbGpAMTYzLmNvbQ==
 Lujun Li
Lujun Li Zhijun Zeng2
Zhijun Zeng2