- 1Department of Psychiatry, Yale University School of Medicine, New Haven, CT, United States
- 2University of California, San Diego, San Diego, CA, United States
- 3Department of Psychology, Yale University, New Haven, CT, United States
- 4Yale Child Study Center, Yale University School of Medicine, New Haven, CT, United States
- 5Department of Neuroscience, Yale University School of Medicine, New Haven, CT, United States
- 6Center for Brain and Mind Health, Yale University School of Medicine, New Haven, CT, United States
- 7Wu-Tsai Institute, Yale University, New Haven, CT, United States
Obsessive-compulsive disorder (OCD) is a debilitating psychiatric condition characterized by intrusive thoughts and repetitive behaviors, with significant barriers to timely diagnosis and effective treatment. Deep learning, a subset of machine learning, offers promising tools to address these challenges by leveraging large, complex datasets to identify OCD, classify symptoms, and predict treatment outcomes. This narrative review synthesizes findings from 10 studies that applied deep learning to OCD research. Results demonstrate high accuracy in diagnostic classification (80–98%) using neuroimaging, EEG, and clinical data, as well as promising applications in symptom classification and treatment response prediction. However, current models are limited by small sample sizes, lack of comparative treatment predictions, and minimal focus on early response detection or scalable monitoring solutions. Emerging opportunities include leveraging passively collected data, such as wearable sensors or electronic medical records, to enhance early detection and continuous symptom tracking. Future research should prioritize multimodal datasets, prospective study designs, and clinically implementable models to translate deep learning advancements into precision psychiatry for OCD.
Introduction
Two to three percent of individuals in the United States are diagnosed with obsessive-compulsive disorder (OCD), a debilitating psychiatric illness characterized by the presence of unwanted or intrusive thoughts (obsessions) that provoke distress and by behaviors (compulsions) performed to reduce that distress (1–3). OCD is frequently misdiagnosed, increasing patient concerns about receiving high-quality care (4, 5). For individuals seeking treatment, the two primary recommendations are exposure and response prevention (ERP), a specialized form of cognitive-behavioral therapy, and pharmacotherapy with selective serotonin reuptake inhibitors (SSRIs). ERP is an evidence-based therapy that involves gradually exposing patients to feared stimuli while providing them the therapeutic skills needed to resist compulsive behaviors (6). ERP is highly efficacious in randomized clinical trials and in routine clinical care, with large to very large effect sizes (g = 0.74 to 2.30; 7, 8). SSRIs are also standard-of-care for individuals with OCD, contributing to meaningful clinical improvement (e.g., an average decrease of 4 points on the Yale-Brown Obsessive-Compulsive Scale [YBOCS]; 9, 10). Nevertheless, up to 50% of patients do not respond to ERP (7, 11–13), and the high SSRI doses used for optimal OCD treatment increases risk of side-effects and drop-out (9).
Within this context, it is of paramount importance for researchers and clinicians to understand how to develop accurate models for identifying OCD and its symptoms, and to predict who will benefit from treatment. One challenge to achieving this has been that the volume and types of data in modern research pose novel analytic challenges. As an example, in a study on the NOCD mobile app, 25,369 individuals across 108 countries contributed lexical descriptions of obsessions, triggers, exposures, and compulsions (14). Relatedly, the ENIGMA OCD Consortium collection of neuroimaging data continues to grow, with 47 datasets from 34 institutes in 15 countries on 5 continents, with a total sample of 4,648 participants. Of these participants, 2,323 have OCD and 2,325 are healthy controls, with the majority being adults, followed by adolescents and children. Data types span various MRI machines with different field strengths and scan sequences, as well as information on clinical phenotypes. Although such datasets can answer novel and exciting questions, new analytic methods are required to manage their size and heterogeneity, generate hypotheses, and account for the nonlinear relationships between variables.
Machine learning has been used as one approach to this problem (15). Machine learning encompasses algorithms that learn patterns from data and make predictions or decisions based on those patterns (16). Machine learning accounts for multivariate, nonlinear relationships that more traditional, linear analyses overlook (17). Additionally, it increases confidence that models will generalize to unseen data, allowing researchers to predict what will happen for individuals outside of their research samples (18). Researchers can also use it to combine multiple types of input data, like clinical scales, therapy session recordings, images, and psychological assessment scores, into the same model.
Deep learning, a subset of machine learning, has been rapidly gaining popularity (19, 20), boasting several comparative advantages. First, machine learning typically relies on domain experts pre-identifying the most relevant data features for the model. For example, to predict OCD treatment outcomes, researchers might pre-select theoretically related variables, including scores on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS), frequency of intrusive thoughts, or levels of impairment caused by compulsive behaviors. By contrast, deep learning automatically identifies patterns in data without predefined feature selection, saving researchers time and reducing bias for complex data tasks like image recognition, natural language processing, and speech analysis. Of note, deep learning can be computationally expensive, require large amounts of data, and can be challenging to interpret. Despite these drawbacks, improved computing power and more advanced techniques have driven its use in schizophrenia (21), attention-deficit/hyperactivity disorder (22), autism (23), depression (24), and OCD (25).
While reviews surveying the application of deep learning across psychiatry provide valuable broad context (24, 26–28), OCD presents a unique constellation of diagnostic, neurobiological, and treatment-related challenges (see 29) that warrants a dedicated examination of how deep learning is being applied. OCD is characterized by a profound heterogeneity spanning distinct symptom dimensions (e.g., contamination/washing, symmetry/ordering, hoarding, taboo thoughts) and highly ritualized compulsive behaviors (30). This complexity makes traditional statistical and even basic machine learning approaches potentially insufficient for capturing the nuanced patterns needed for accurate diagnosis, subtyping, and outcome prediction.
Furthermore, OCD research is heavily driven by specific neural circuits, particularly the cortico-striato-thalamo-cortical (CSTC) loops encompassing regions like the frontal cortex, striatum, and thalamus. Within this circuit, a proposed mechanism is an imbalance between the excitatory direct and inhibitory indirect pathways, leading to excess excitation and thought to underlie OCD symptoms. Accumulating evidence also points to OCD being mediated by multiple parallel CSTC circuits involved in sensorimotor, cognitive, and affective processes. Furthermore, significant evidence indicates a heritable component to OCD, with studies searching for specific risk genes and variants (31). These insights offer a unique opportunity for deep learning models applied to neuroimaging data to test and refine these biologically-grounded models in ways less defined in other disorders. Moreover, the prominent behavioral component of OCD—the compulsions—presents both a challenge for traditional, often subjective, assessment and a unique opportunity for deep learning-powered objective monitoring using sensor data (32, 33), a focus potentially less applicable or developed for conditions primarily defined by internal states.
Therefore, this review fills a crucial knowledge gap by synthesizing how deep learning is being tailored to address the unique symptomatic heterogeneity, neurobiological targets, objective behavioral monitoring needs, and treatment prediction challenges inherent to OCD potentially missed by broader psychiatric reviews. In this manuscript, we focus on studies examining classification of symptoms and diagnostic status, as well as prediction of treatment outcomes. We discuss the results and conceptual takeaways of these models and their clinical applications. We end on a note of optimism for deep learning’s future as a tool for diagnostics and treatment prediction in OCD research.
Methods
Although this was a narrative review, and so not all PRISMA guidelines applied (e.g., extracting effect measures or synthesizing data), we followed the PRISMA checklist (34) as closely as possible to increase transparency and to ensure that we located all studies meeting inclusion criteria. To cast a comprehensive search net, we utilized PubMed, Google Scholar, as well as searched references by hand. PubMed and Google Scholar were chosen to provide broad coverage across biomedical and general scientific literature, including conference proceedings often captured by Google Scholar, balancing comprehensiveness with feasibility. To be included, studies were required to use at least one deep learning technique (e.g., convolutional neural networks, recurrent neural networks, autoencoders, generative adversarial neural networks) for symptom classification, diagnostic classification, or treatment prediction. The article must have been from a peer-reviewed journal in English. Studies using only machine learning, not deep learning, were excluded. Studies synthesizing empirical data, such as reviews/meta-analyses, and data simulations, were also excluded. There was no exclusion criteria for year of publication.
Search terms included “obsessive-compulsive disorder (OCD)” along with the “AND” operator and the following terms: “Deep Learning,” “Neural Network,” “Neural Networks,” “CNN,” “Convolutional Neural network,” “Recurrent Neural network,” “LSTM,” “GRU,” “Auto Encoders,” “Deep Belief Networks,” “Generative Adversarial Network,” “Ensemble Neural Network,” “Artificial Neural Network,” “Deep Neural Network.” To minimize bias, two independent reviewers (KA and LB) assessed each study to ensure that it met inclusion/exclusion criteria. A third reviewer (BZ) resolved conflicts between the two reviewers.
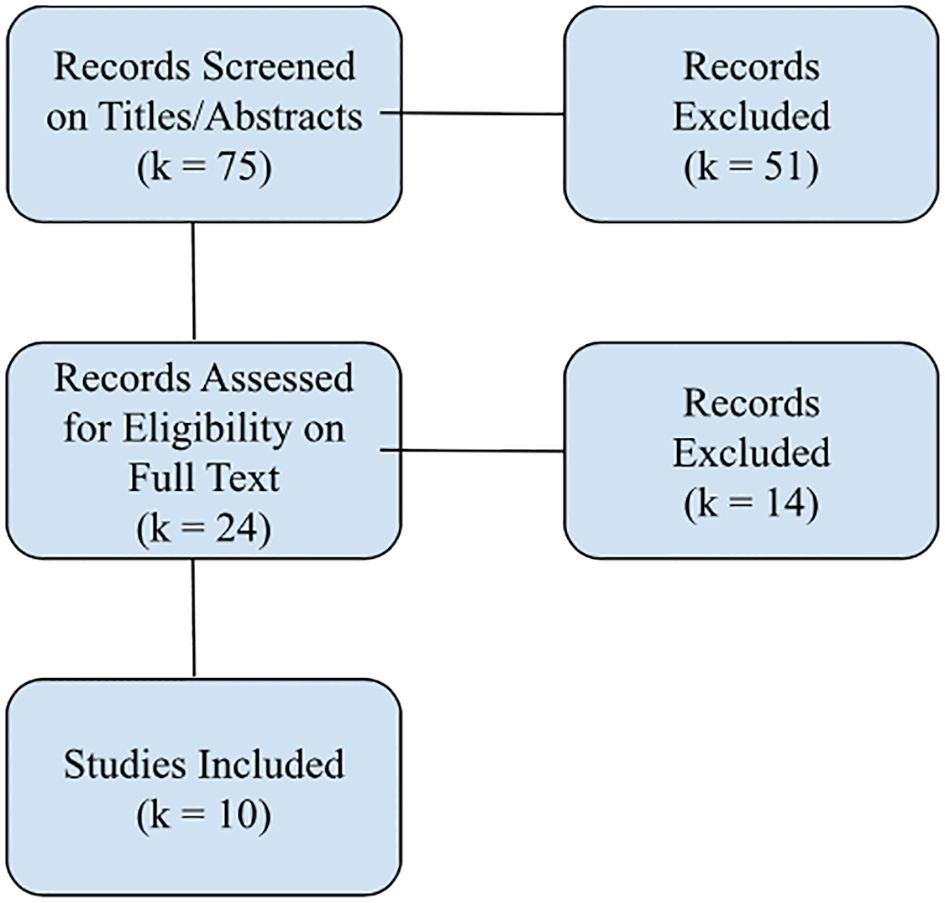
Our process is illustrated in the flow diagram in Figure 1.
To determine the effectiveness of the models in each study, accuracy, F1 score, and area under the curve (AUC; 35, 36) were extracted when possible. Model accuracy represents the total number of correct predictions divided by the total number of predictions, expressed as a percentage. While easily interpretable, accuracy alone can be misleading, especially with imbalanced datasets. To mitigate this shortcoming, we also used the F1 score, which provides a more balanced evaluation by combining precision (accuracy of positive predictions) and recall (ability to find all positive cases) into a single metric (36). For papers that did not include an F1 score, we calculated it from the confusion matrix provided by the study with the following formula (36):
Lastly, we considered AUC, a comprehensive performance metric that measures a model’s ability to discriminate between classes. This is the probability that a model will rank a randomly chosen positive instance higher than a randomly chosen negative instance (35).
Study characteristics were further organized into descriptive categories including the country of the corresponding author, research design, and prediction outcome. We collected details on the model architectures the researchers used, input data for the models, training sample/strategy, and primary model results. Lastly, we included conceptual take-aways for each paper to summarize findings.
Results
All results are displayed in Tables 1–3.
In total, k = 10 studies met inclusion criteria. Studies were published from 2009 to 2024, though we did find two early exceptions not meeting inclusion criteria. One older study using neural networks in OCD (45) was excluded as it did not classify OCD diagnoses or predict OCD treatment outcomes. Another study was excluded for using only simulated data in a neural network to assess pathophysiological theories of OCD (46). We eliminated one study that met inclusion criteria because we could not ascertain key characteristics of the sample (e.g., sample size; 47).
Of the included studies, corresponding authors were from Switzerland (k = 1), Turkey (k = 3), the Netherlands (k = 1), Canada (k = 1), Denmark (k = 1), Italy (k = 1), Pakistan (k = 1), and China (k = 1). The most common research design was a retrospective analysis (k = 5). Quasi-experimental designs were the second most frequent (k = 3). The remaining studies were distributed across other designs, one a mixed (survey, RCT) approach and another feasibility study. This distribution suggests a predominance of observational and retrospective approaches, with fewer studies employing experimental or prospective designs.
Symptom classification studies
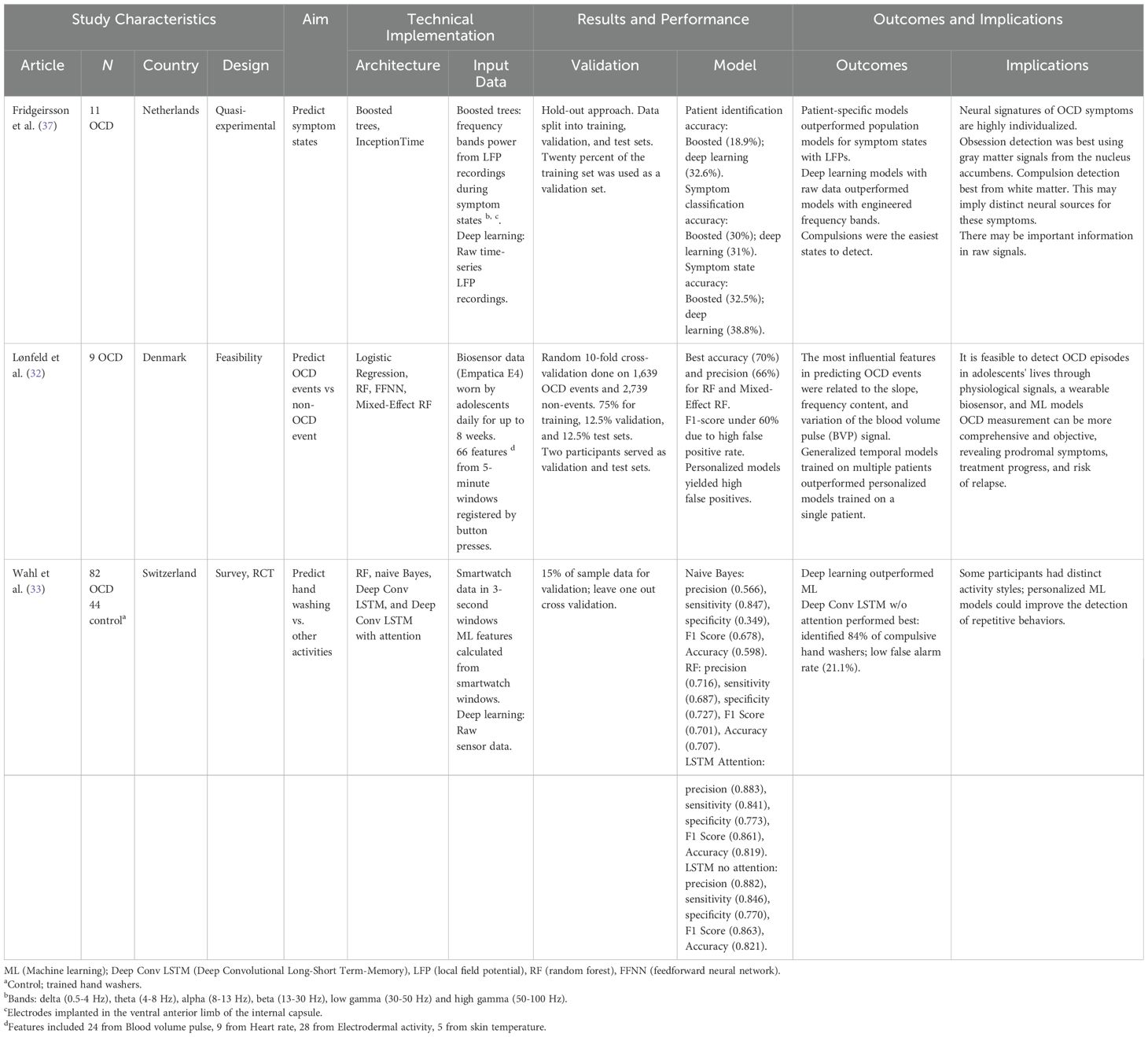
Three studies examined the ability of deep learning to classify symptom types in OCD; all used physiological data. Wahl et al. (33) used machine learning and deep learning models, including random forests and deep convolutional long short-term memory networks (LSTMs), to distinguish compulsive hand washing from other repetitive activities using smartwatch sensor data. Participants (n = 82 with OCD, n = 44 without OCD) wore a smartwatch while hand washing, brushing teeth, cleaning a cup, and peeling a carrot. Sensor data were collected in 3-second sliding windows. The models correctly identified 84% of compulsive hand washing episodes. This showcased the capability of analyzing and distinguishing OCD-related vs non-OCD related behaviors in daily life. Moreover, the analysis revealed distinct activity styles among participants, suggesting the need for personalized models.
Fridgeirsson et al. (37) applied machine and deep learning to classify OCD symptom states from local field potential recordings in patients who underwent deep brain stimulation. They collected data from 11 OCD patients with electrodes implanted in the ventral anterior limb of the internal capsule. Recordings were made during different symptom states (baseline, obsessions, compulsions, and relief) induced by a symptom provocation task. The researchers found that patient-specific models outperformed population-level models, suggesting neural signatures of OCD symptoms are highly individualized. Deep learning models using raw time series data performed better than using frequency band features. The models distinguished obsessive and compulsive states with moderate accuracy in some patients, with compulsions being easier to detect overall. Obsession detection was best using signals from gray matter areas like the nucleus accumbens, while compulsion detection was most successful with signals from nearby white matter.
Lastly, machine and deep learning models were used to classify OCD events using physiological data collected from wearable biosensors in 9 adolescents for up to 8 weeks (32). Participants manually tagged OCD events by pressing a button when bothered by symptoms. The researchers then extracted 66 features from 5-minute windows of physiological data, including pulse, heart rate, electrodermal activity, and skin temperature. They used 1,639 OCD events and 2,739 non-events for model training. Tree-based models like random forests performed best, reaching 70% accuracy in detecting OCD events. Features related to pulse were most predictive. In contrast to Wahl et al. (33), generalized temporal models trained on multiple patients outperformed personalized models trained on a single patient. These differences may be due to the data types used, model architectures, or sample sizes.
Diagnostic classification studies
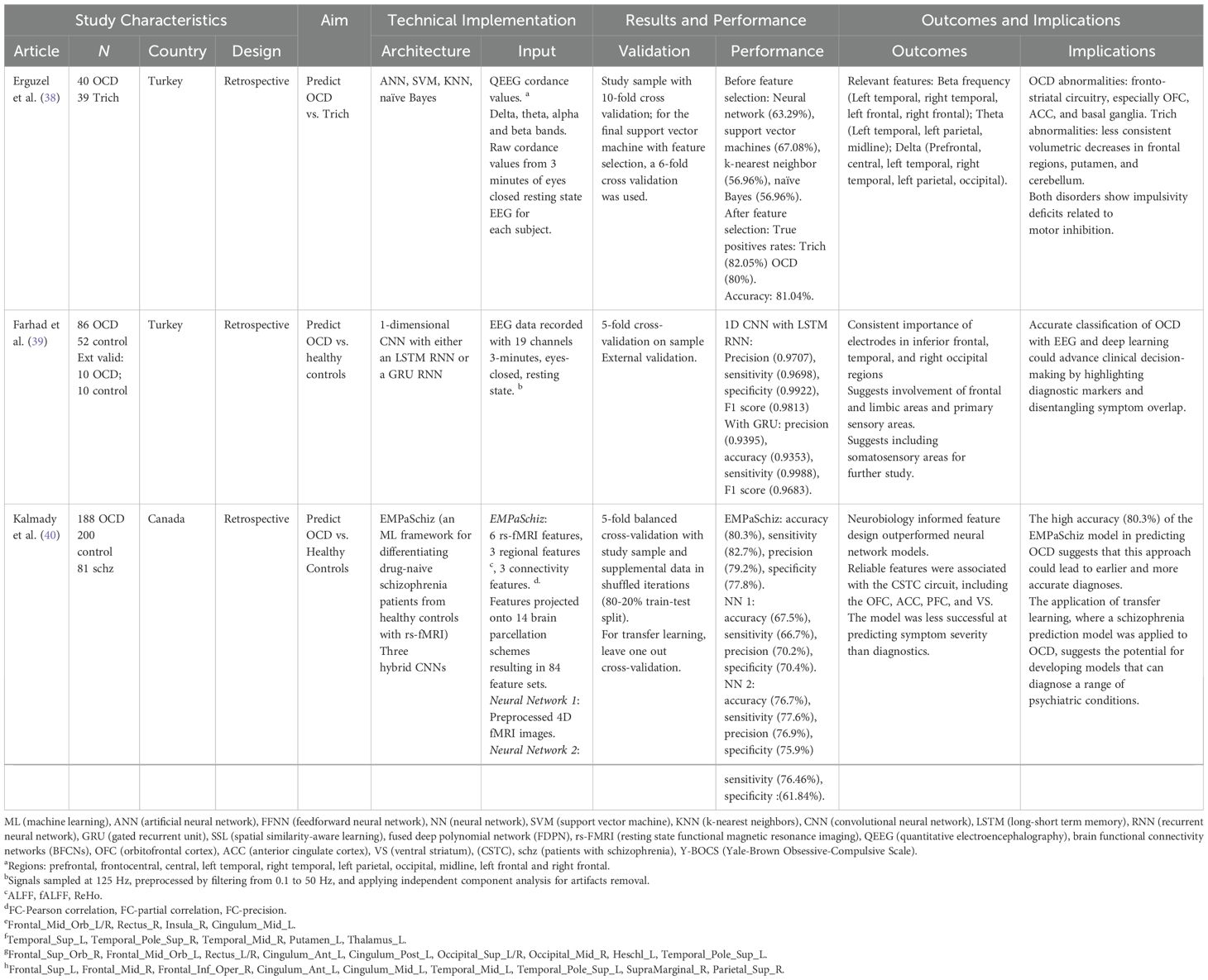
Five studies applied deep learning techniques to distinguish OCD patients from healthy controls, two using functional magnetic resonance imaging (fMRI) data, two using electroencephalography (EEG), and one using clinical data (Table 2). In one investigation, convolutional and recurrent neural networks differentiated between groups based on resting-state EEG data from 86 OCD patients and 52 healthy controls (39). Using input data from a 19-channel EEG system and a 3-minute eyes-closed resting state condition, models achieved over 93% accuracy, with 97% precision, 97% accuracy, and 99% specificity. Electrodes in inferior frontal, temporal, and right occipital regions were most important for classification, suggesting involvement of frontal-limbic and sensory areas in OCD pathophysiology.
Kalmady et al. (40) examined resting-state functional magnetic resonance imaging (rs-fMRI) in 188 OCD patients and 200 controls. The authors used three regional features and three connectivity-based features (FC-Pearson correlation, FC-partial correlation, FC-precision). They were projected onto 14 different brain parcellation schemes, resulting in 84 feature sets used as input supplied to a machine learning algorithm (EMPaSchiz; 40). The ensemble algorithm achieved 80.3% accuracy, 82.7% sensitivity, 79.2% precision, and 77.8% specificity. Features selected for schizophrenia prediction in previous work transferred well to OCD classification, demonstrating usefulness for cross-diagnostic transfer learning in psychiatry. The successful transfer of features from schizophrenia prediction to OCD classification suggests some overlap in the underlying pathophysiological mechanisms and may facilitate the development of cross-diagnostic tools. Such transfer learning is crucial for psychiatry research, as it reduces the need for large, diagnosis-specific datasets, which are often difficult to obtain, accelerates model training, and improves generalization (21).
In a study using a novel application of deep learning, Yang et al. (42) combined spatial similarity-aware learning and fused deep polynomial networks to construct and analyze brain functional connectivity networks from rs-fMRI in 62 OCD patients, 65 controls, and 53 unaffected first-degree relatives. The brain was parcellated into 90 regions of interest. Next, a brain functional connectivity network (BFCN) was constructed with a smooth sparse network (SSN) model to reduce the density of the network and incorporate similarity constraints between subjects. A fused sparse auto-encoder (FSAE) learned deep features of the BFCN to reduce its dimensionality, and then a support vector machine classifier output the OCD diagnosis based on the high-level features extracted by the auto-encoder. This two-stage process–BFCN construction using the SSN followed by deep learning and OCD classification–achieved 88.7% accuracy in distinguishing OCD from controls. Using this method, the model also identified discriminative brain regions aligned with known OCD neurocircuitry, including frontal, insular, cingulate, temporal, and subcortical areas.
Two studies used comparatively simpler deep learning models for diagnostic classification. One used support vector machines, k-nearest neighbor, naïve Bayes, and deep neural networks to separate individuals with OCD (n = 40) and trichotillomania (n = 39; 38). Input consisted of quantitative EEG cordance values from 19 electrodes in 10 brain regions across four frequency bands. After feature selection, the model achieved 81.04% accuracy, with true positive rates of 82.05% for trichotillomania and 80% for OCD. With only clinical and demographic data, Shahzad et al. (41) used a feedforward neural network to classify OCD vs. healthy controls in 200 OCD patients and 400 healthy controls. Model input consisted of 6 factors calculated from Y-BOCS interviews, including a newly added factor, “worth of an individual in family.” Their model achieved 98% accuracy in differentiating OCD patients from healthy controls, with contamination and cleaning being the most important factors for prediction. The strikingly high accuracy may reflect the presence of relevant clinical data embedded in the Y-BOCS input. The new “family” factor was the third most important in the models, supporting longstanding work identifying family dynamics as a pivotal variable in understanding OCD (48, 49).
Treatment prediction studies
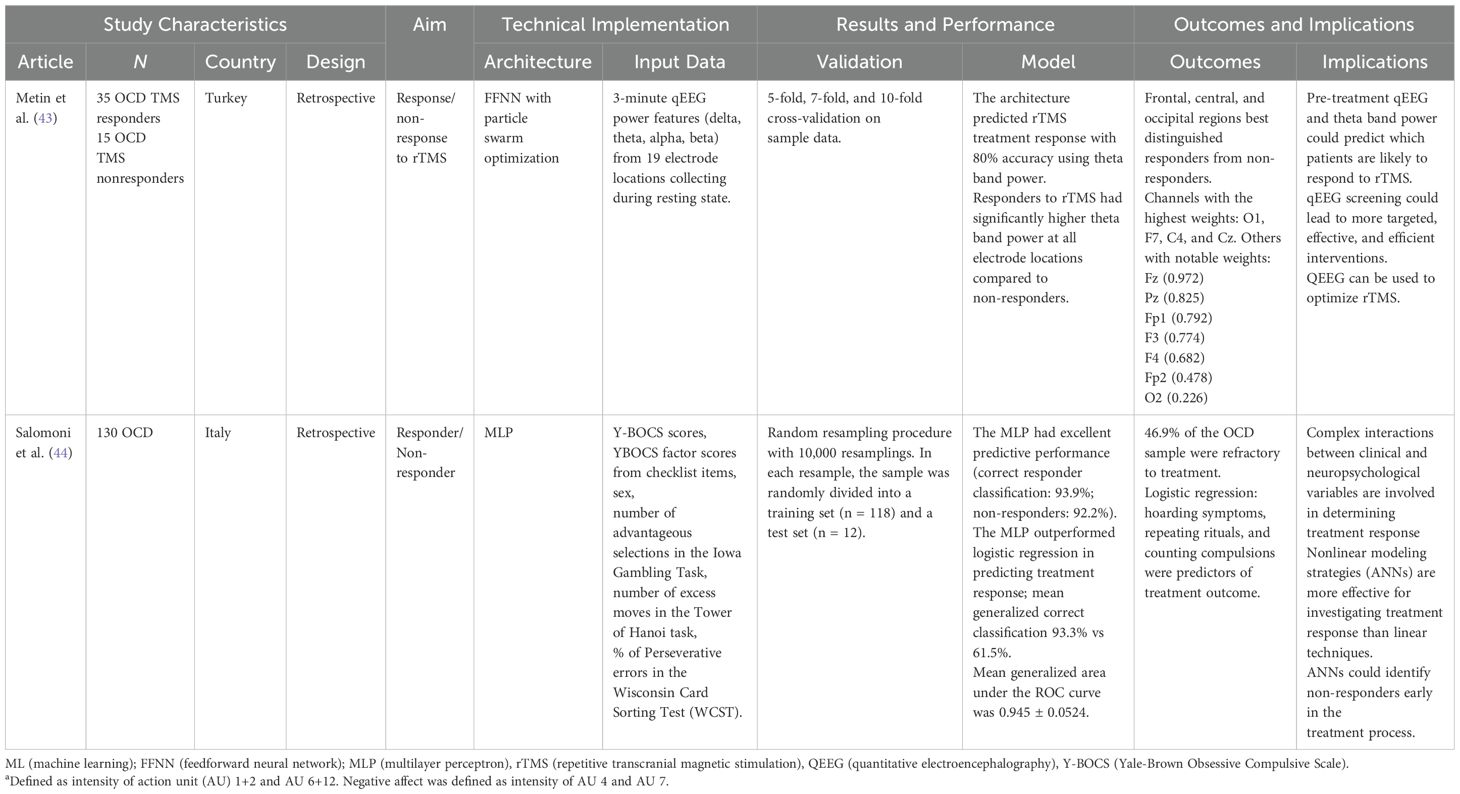
Only two treatment studies met inclusion criteria (Table 3). Metin et al. (43) used artificial neural networks with particle swarm optimization to predict response to repetitive transcranial magnetic stimulation (TMS) in 35 TMS responders and 15 TMS non-responders based on quantitative EEG features collected prior to treatment. The input consisted of quantitative electroencephalography (qEEG) delta, theta, alpha, and beta band power features from 19 electrodes locations placed according to the 10–20 system, with linked ear electrodes (A1-A2) as reference. Features were extracted from 3 minutes of resting-state EEG recordings with eyes closed. Treatment responders showed higher theta power across all electrodes compared to non-responders, with 80% model accuracy. Frontal and central electrodes were the most predictive.
Multilayer neural networks were used to predict response to pharmacological and psychotherapeutic treatment in a study on 130 OCD patients based on clinical, epidemiological, and neuropsychological variables (44). Y-BOCS scores (for obsessions, compulsions, and insight), Y-BOCS symptom checklist items (7 obsession and 6 compulsion subtypes), sex, and neuropsychological test scores (Iowa Gambling Task performance, Tower of Hanoi excess moves, Wisconsin Card Sorting Test perseverative errors) were included. Factor analysis of the 13 symptom subtypes resulted in a 4-factor solution; these factors were used as predictors instead of the 13 original items. Their model significantly outperformed logistic regression, achieving 93.3% accuracy in classifying treatment responders vs. non-responders. This study highlights the potential of neural networks to capture nuanced understanding of OCD treatment response beyond traditional, linear statistical modeling methods.
Discussion
Identifying OCD: bridging technical innovation and clinical scalability
Current deep learning models demonstrate impressive performance in distinguishing OCD from healthy controls, with accuracies ranging from 80% to 98% across studies using neuroimaging, EEG, and structured clinical assessments (Table 2). We are optimistic for deep learning in the near-term as it becomes a more accurate diagnostic tool. However, in the mid-term, as long as these approaches still require resource-intensive data collection (e.g., fMRI protocols, 19-channel EEG arrays, or a clinician-administered Y-BOCS), they will need to go beyond the high accuracy already afforded by specialists to demonstrate their incremental utility. Although these models show clear promise validating the neurobiological and behavioral correlates of OCD, their long-term translational value is constrained without sufficiently scaling, which could address systemic barriers to early detection or frequent misdiagnosis (4, 50, 51). Future work should prioritize scalable identification frameworks, some of which can leverage passively collected or electronic medical record (EMR) data—advances absent in the reviewed literature but rich with opportunity. For example, natural language processing of primary care visit notes could detect undiagnosed OCD through lexical markers of doubt, contamination fears, or ritualistic behavior, akin to methods successfully deployed for psychosis risk (52–54). Similarly, smartphone sensors analyzing movement patterns (e.g., repetitive hand motions) or app engagement metrics (e.g., frequent calendar rechecking) could provide digital biomarkers, building on Wahl and colleagues’ (33) proof-of-concept for wearables.
Although deep learning has proven itself in diagnostic classification, by shifting focus from rivaling expert diagnosis to augmenting frontline detection, deep learning could transform OCD identification from a specialist-dependent process to a population-level public health tool in the years to come. Large scale OCD detection models, like those based on EMR data, represent a notable path forward. EMRs can contain longitudinal data on medication trials, comorbidities, and somatic complaints (e.g., dermatitis from overwashing)—all potential predictors of OCD. Deep learning’s capacity to model nonlinear interactions between these sparse, heterogeneous variables makes it uniquely suited for this task. For instance, a multimodal network combining structured diagnostic codes, unstructured clinician notes, and pharmacy records could identify high-risk patients for targeted screening, mirroring suicide risk models (e.g., 55). However, significant challenges remain: EMR data suffers from selection bias (overrepresentation of severe cases), fragmented care documentation, and privacy constraints that complicate model training. Collaborative frameworks like the ENIGMA OCD Consortium could mitigate these issues by curating multisite EMR repositories with standardized phenotyping.
Commonalities with broader deep learning trends
Our findings indicate that OCD deep learning research aligns with broader trends across psychiatry. The promising results in diagnostic classification of OCD using neuroimaging, EEG, and clinical data mirror successes reported in automated detection and diagnosis for depression (24, 27), schizophrenia (28), and autism (26). Moreover, they often utilize similar data modalities—fMRI and EEG—along with common architectures like CNNs and LSTMs. Furthermore, the challenges identified in the OCD deep learning literature, like small sample sizes, the need for improved scalability, the lack of external validation, and the necessity for enhanced model interpretability, persist across the field (24, 27, 28). The growing interest in treatment response prediction for OCD also parallels efforts in depression, where deep learning is being explored to predict outcomes for repetitive transcranial magnetic stimulation (rTMS) and pharmacotherapy (24).
While sharing common ground, deep learning applications in OCD have also presented unique opportunities. The nascent but promising use of deep learning with wearable sensor data and intracranial recordings for granular symptom classification (e.g., compulsive hand washing, specific symptom states) appears uniquely focused within OCD research compared to the broader diagnostic detection goals often emphasized for depression or schizophrenia (24, 28). While there seems to be some interest in using similar technology to track suicidal behavior with recurrent neural networks (56, 57), these EMA analyses still seem dominated by traditional machine learning algorithms (58).
By comparison to the broader literature, our review highlights a comparative lack of focus in OCD deep learning research on leveraging large-scale, passively collected data streams like electronic medical records or extensive social media analysis, areas where significant progress or potential has been demonstrated for depression and suicide risk assessment (27). To bridge the gap towards clinical translation, OCD research needs to integrate methodologies becoming crucial across precision psychiatry, such as prospective study designs embedding deep learning algorithms, robust external validation on diverse datasets, and developing models capable of comparative treatment prediction (e.g., predicting differential response to ERP vs. SSRIs) (59). Addressing these points, alongside the shared challenges of interpretability and scalability, will be essential for translating the technical advancements of deep learning into tangible improvements in precision care for individuals with OCD, moving beyond diagnostic biomarkers towards personalized and adaptive treatment strategies.
Integrating prediction into clinical decision-making
Current deep learning studies show clinically relevant accuracy in predicting OCD treatment response, including rTMS (80% accuracy) and pharmacotherapy/psychotherapy (93.3% accuracy). This work answers the call for psychological investigations emphasizing prediction (60). However, for deep learning to be successful in the long-term, it must move from simple binary predictions to providing insight into matching patients to more complex, evidence-based treatment options (e.g., outpatient ERP vs. intensive ERP vs. SSRIs). This current limitation reflects broader precision psychiatry challenges, where models prioritize preselected interventions rather than guiding initial modality choice (61). A reasonable solution might be to integrate multimodal biomarkers—for example, wearable-derived movement patterns (33) with clinical profiles to predict which patients respond better to varying levels of ERP or SSRIs (44). Implementing this approach demands hybrid study designs that embed machine/deep learning analyses within randomized controlled trials—a methodology absent from current OCD research but critical for clinical translation (62).
Three implementation challenges must be addressed to move from prediction to clinical action. First, prediction confidence should align with intervention risk (e.g., higher confidence is required for neurosurgical referrals than ERP). Second, temporal resolution must match clinical needs: EEG biomarkers enable rapid 3-minute triage for time-sensitive cases (43), while longitudinal wearable data better informs chronic care adjustments (32). Third, model explanations should be stakeholder-adapted—providing clinicians neurobiological plausibility (e.g., frontostriatal engagement) while giving patients behaviorally actionable insights (e.g., “Hoarding reduces your ERP response likelihood by X%”). Hybrid expert-AI systems could bridge this gap, embedding predictions within clinical workflows. Such systems require human-in-the-loop frameworks, which keep clinicians accountable and involved with AI decision making (63).
Early identification of response
This review shows deep learning’s capacity to predict treatment outcomes using baseline biomarkers—such as pre-treatment EEG patterns or clinical symptom profiles. A key area for future research is to identify early response signals during therapeutic interventions. Current practice often recommends long sessions or multiple weeks of ERP that sufficiently violate a patient’s expectation of an aversive event occurring (64, 65). Yet prolonging patient exposure to potentially ineffective interventions or delaying alternative care pathways can be inefficient and costly. Detecting movement patterns (33) and longitudinal physiological data (32) provide foundational evidence that symptom-linked digital phenotypes evolve detectably within days to weeks. Adapting these modalities to track early response could help identify divergence between responders and non-responders at critical junctures—for instance, detecting stalled habituation curves in ERP via reduced skin conductance variability, or flagging SSRI non-response through persistent ritual frequency in smartphone sensor data. Such approaches would require shifting from static, single-time-point models to recurrent architectures that process temporal sequences with patient-specific models (e.g., 37).
Monitoring symptoms
We found an emerging capability for passive sensor data to transform OCD symptom monitoring by capturing granular, objective behavioral signatures that circumvent recall bias and clinician-dependent rating scales. Wahl et al. (33) demonstrated that smartwatch data could distinguish compulsive hand washing from similar non-OCD behaviors with 84% accuracy, identifying unique kinematic patterns (e.g., repetitive and inertial motion) that traditional clinical assessments cannot detect or quantify. When extended longitudinally, such real-time digital phenotyping could map symptom trajectories at hourly resolution, detecting subtle response signals like reduced ritual duration or altered movement variability weeks before Y-BOCS scores reflect improvement. Lønfeldt et al. (32) further validated this approach, showing that wearable-derived physiological markers (heart rate, electrodermal activity, and skin temperature) predicted OCD event onset with 70% accuracy in adolescents. Integrating motor kinematics and autonomic arousal patterns could yield composite digital biomarkers that differentiate perseverative compulsions from adaptive behaviors while controlling for contextual confounds like exercise or stress (66, 67).
Data security and bias
For clinical-research translation, the collection, use, and protection of sensitive personal data are paramount. Safeguarding patient confidentiality requires not only adherence to stringent data protection protocols but also the implementation of advanced security measures to prevent unauthorized access or misuse (68, 69). Beyond data security, bias can influence algorithms in several ways, amplifying negative health outcomes for marginalized and underserved patients. For example, AI could miscalibrate risk, leading to under-prioritization and undertreatment of minority groups; racial stereotypes can influence data in electronic health records (70). Addressing these challenges demands a proactive approach that emphasizes cultural competence, inclusivity, and fairness in AI system design. This includes curating datasets that are diverse and representative of different demographic groups, employing robust bias-detection and mitigation strategies, and continuously evaluating AI performance to ensure equitable application across varied populations (71).
Conclusion
This review highlights the promise of deep learning to revolutionize OCD diagnosis and treatment by addressing critical gaps in current clinical practice. At the same time, this work is in its infancy. While current architectures achieve high accuracy in diagnostic classification and treatment response prediction, limited scalability and lack of integration into real-world settings are essential for them to have clinical impact. Key areas for future development include early identification of treatment response through dynamic temporal modeling, comparative predictions across therapeutic modalities, and continuous symptom monitoring using passive data streams like wearable sensors or electronic medical records. Collaborative efforts to standardize multimodal datasets and incorporate diverse patient populations will be essential for building robust, generalizable models. By shifting the focus from technical optimization to clinical implementation, deep learning can increase the precision, personalization, and accessibility of OCD care.
Author contributions
BZ: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Supervision. LB: Writing – original draft, Writing – review & editing, Conceptualization, Investigation. KA: Writing – review & editing, Writing – original draft, Investigation, Visualization. CP: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Brain and Behavior Research Foundation (NARSAD: Grant no. 31149) funded a portion of BZ’s effort and training for this work.
Conflict of interest
For the past three years, BZ has consulted with Biohaven Pharmaceuticals and received royalties from Oxford University Press. In the past three years, CP has consulted for Biohaven Pharmaceuticals, Ceruvia Neurosciences, UCB BioPharma, Freedom Biosciences, Transcend Therapeutics, Alco Therapeutics, Lucid.Care, Nobilis Therapeutics, Mind Therapeutics, F-Prime Capital, and Madison Avenue Partners. He holds equity in Alco Therapeutics, Mind Therapeutics, and Lucid/Care. He receives or has received research support from Biohaven Pharmaceuticals, Freedom Biosciences, and Transcend Therapeutics. He receives royalties from Oxford University Press and UpToDate. He has filed patents on pharmacological treatments for OCD and related disorders, psychedelic therapeutics, and autoantibodies in OCD. None of these relationships are of relevance to the work described here.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Karpiak CP and Zaboski BA. Lifetime prevalence of mental disorders in the general population. In: Koocher GP, Norcross JC, and Greene BA, editors. Psychologists’ Desk Reference, 3rd ed. Oxford: Oxford University Press (2013). p. 3–16).
2. Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, and Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. (2012) 21:169–84. doi: 10.1002/mpr.1359
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
3. Ruscio AM, Stein DJ, Chiu WT, and Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. (2010) 15:53–63. doi: 10.1038/mp.2008.94
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
4. Glazier K, Wetterneck C, Singh S, and Williams M. Stigma and shame as barriers to treatment in obsessive-compulsive and related disorders. J Depression Anxiety. (2015) 4:1000191. doi: 10.4191/2167-1044.1000191
5. Senter MS, Patel SR, Dixon LB, Myers RW, and Simpson HB. Defining and addressing gaps in care for obsessive-compulsive disorder in the United States. Psychiatr Serv. (2021) 72:784–93. doi: 10.1176/appi.ps.202000296
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
6. Craske MG, Treanor M, Conway CC, Zbozinek T, and Vervliet B. Maximizing exposure therapy: An inhibitory learning approach. Behav Res Ther. (2014) 58:10–23. doi: 10.1016/j.brat.2014.04.006
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
7. Öst L-G, Enebrink P, Finnes A, Ghaderi A, Havnen A, Kvale G, et al. Cognitive behavior therapy for obsessive-compulsive disorder in routine clinical care: A systematic review and meta-analysis. Behav Res Ther. (2022) 159:104170. doi: 10.1016/j.brat.2022.104170
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
8. Reid AM, Guzick AG, Fernandez AG, Deacon B, McNamara JP, Geffken GR, et al. Exposure therapy for youth with anxiety: Utilization rates and predictors of implementation in a sample of practicing clinicians from across the United States. J Anxiety Disord. (2018) 58:8–17. doi: 10.1016/j.janxdis.2018.06.002
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
9. Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, and Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. (2010) 15:850–5. doi: 10.1038/mp.2009.50
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
10. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry. (1989) 46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
11. Fisher PL and Wells A. How effective are cognitive and behavioral treatments for obsessive–compulsive disorder? A clinical significance analysis. Behav Res Ther. (2005) 43:1543–58. doi: 10.1016/j.brat.2004.11.007
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
12. Law C and Boisseau CL. Exposure and response prevention in the treatment of obsessive-compulsive disorder: Current perspectives. Psychol Res Behav Manage. (2019) 12:1167–74. doi: 10.2147/PRBM.S211117
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
13. Springer KS, Levy HC, and Tolin DF. Remission in CBT for adult anxiety disorders: A meta-analysis. Clin Psychol Rev. (2018) 61:1–8. doi: 10.1016/j.cpr.2018.03.002
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
14. Feusner JD, Mohideen R, Smith S, Patanam I, Vaitla A, Lam C, et al. Semantic linkages of obsessions from an international obsessive-compulsive disorder mobile app data set: Big data analytics study. J Med Internet Res. (2021) 23:e25482. doi: 10.2196/25482
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
15. Li X, Kang Q, and Gu H. A comprehensive review for machine learning on neuroimaging in obsessive-compulsive disorder. Front Hum Neurosci. (2023) 17:1280512. doi: 10.3389/fnhum.2023.1280512
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
16. James G, Witten D, Hastie T, and Tibshirani R. An introduction to statistical learning. New York, NY: Springer (2013). doi: 10.1007/978-1-4614-7138-7
18. Hindman M. Building better models: Prediction, replication, and machine learning in the social sciences. Ann Am Acad Political Soc Sci. (2015) 659:48–62. doi: 10.1177/0002716215570279
19. Lakhani P, Gray DL, Pett CR, Nagy P, and Shih G. Hello world deep learning in medical imaging. J Digital Imaging. (2018) 31:283–9. doi: 10.1007/s10278-018-0079-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
20. Zhang L, Tan J, Han D, and Zhu H. From machine learning to deep learning: Progress in machine intelligence for rational drug discovery. Drug Discov Today. (2017) 22:1680–5. doi: 10.1016/j.drudis.2017.08.010
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
21. Cortes-Briones JA, Tapia-Rivas NI, D’Souza DC, and Estevez PA. Going deep into schizophrenia with artificial intelligence. Schizophr Res. (2021) 245:122–40. doi: 10.1016/j.schres.2021.05.018
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
22. Hernández-Capistran J, Sánchez-Morales LN, Alor-Hernández G, Bustos-López M, and Sánchez-Cervantes JL. Machine and deep learning algorithms for ADHD detection: A review. In: Rivera G, Rosete A, Dorronsoro B, and Rangel-Valdez N, editors. Innovations in Machine and Deep Learning: Case Studies and Applications. Springer Nature, Switzerland (2023). p. 163–91. doi: 10.1007/978-3-031-40688-1_8
23. Uddin M, Shahriar M, Mahamood M, Alnajjar F, Pramanik M, and Ahad MAR. Deep learning with image-based autism spectrum disorder analysis: A systematic review. Eng Appl Artif Intell. (2024) 127:107185. doi: 10.1016/j.engappai.2023.107185
24. Squires M, Tao X, Elangovan S, Gururajan R, Zhou X, Acharya UR, et al. Deep learning and machine learning in psychiatry: A survey of current progress in depression detection, diagnosis and treatment. Brain Inf. (2023) 10:10. doi: 10.1186/s40708-023-00188-6
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
25. Zaboski BA, Wilens A, McNamara JP, and Muller GN. Predicting OCD severity from religiosity and personality: A machine learning and neural network approach. J Mood Anxiety Disord. (2024) 8:100089. doi: 10.1016/j.xjmad.2024.100089
26. Ding Y, Zhang H, and Qiu T. Deep learning approach to predict autism spectrum disorder: A systematic review and meta-analysis. BMC Psychiatry. (2024) 24:739. doi: 10.1186/s12888-024-06116-0
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
27. Malhotra A and Jindal R. Deep learning techniques for suicide and depression detection from online social media: A scoping review. Appl Soft Computing. (2022) 130:109713. doi: 10.1016/j.asoc.2022.109713
28. Sharma M, Patel RK, Garg A, Tan RS, and Acharya UR. Automated detection of schizophrenia using deep learning: A review for the last decade. Physiol Measurement. (2023) 44:03TR01. doi: 10.1088/1361-6579/acb24d
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
29. Pittenger C. Obsessive-compulsive disorder: Phenomenology, pathophysiology, and treatment. Oxford:Oxford University Press (2017).
30. Cervin M, Miguel EC, Güler AS, Ferrão YA, Erdoğdu AB, Lazaro L, et al. Towards a definitive symptom structure of obsessive- compulsive disorder: A factor and network analysis of 87 distinct symptoms in 1366 individuals. psychol Med. (2021) 52:3267–79. doi: 10.1017/S0033291720005437
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
31. Zhang Y-D, Shi D-D, and Wang Z. Neurobiology of obsessive–compulsive disorder from genes to circuits: Insights from animal models. Neurosci Bull. (2024) 40:1975–94. doi: 10.1007/s12264-024-01252-9
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
32. Lønfeldt NN, Olesen KV, Das S, Mora-Jensen A-RC, Pagsberg AK, and Clemmensen LKH. Predicting obsessive-compulsive disorder episodes in adolescents using a wearable biosensor–A wrist angel feasibility study. Front Psychiatry. (2023) 14:1231024. doi: 10.3389/fpsyt.2023.1231024
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
33. Wahl K, Scholl PM, Miché M, Wirth S, Burchard R, and Lieb R. Real-time detection of obsessive-compulsive hand washing with wearables: Research procedure, usefulness and discriminative performance. J Obsessive-Compulsive Related Disord. (2023) 39:100845. doi: 10.1016/j.jocrd.2023.100845
34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. doi: 10.1136/bmj.n71
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
35. Çorbacıoğlu Ş.K and Aksel G. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turkish J Emergency Med. (2023) 23:195. doi: 10.4103/tjem.tjem_182_23
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
36. Hicks SA, Strümke I, Thambawita V, Hammou M, Riegler MA, Halvorsen P, et al. On evaluation metrics for medical applications of artificial intelligence. Sci Rep. (2022) 12:5979. doi: 10.1038/s41598-022-09954-8
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
37. Fridgeirsson EA, Bais MN, Eijsker N, Thomas RM, Smit DJA, Bergfeld IO, et al. Patient specific intracranial neural signatures of obsessions and compulsions in the ventral striatum. J Neural Eng. (2023) 20:026008. doi: 10.1088/1741-2552/acbee1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
38. Erguzel TT, Ozekes S, Sayar GH, Tan O, and Tarhan N. A hybrid artificial intelligence method to classify trichotillomania and obsessive compulsive disorder. Neurocomputing. (2015) 161:220–8. doi: 10.1016/j.neucom.2015.02.039
39. Farhad S, Metin SZ, Uyulan Ç., Makouei STZ, Metin B, Ergüzel TT, et al. Application of hybrid deep learning architectures for identification of individuals with obsessive compulsive disorder based on EEG data. Clin EEG Neurosci. (2024) 55:543–52. doi: 10.1177/15500594231222980
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
40. Kalmady SV, Paul AK, Narayanaswamy JC, Agrawal R, Shivakumar V, Greenshaw AJ, et al. Prediction of obsessive-compulsive disorder: Importance of neurobiology-aided feature design and cross-diagnosis transfer learning. Biol Psychiatry: Cogn Neurosci Neuroimaging. (2022) 7:735–46. doi: 10.1016/j.bpsc.2021.12.003
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
41. Shahzad MN, Suleman M, Ahmed MA, Riaz A, and Fatima K. Identifying the symptom severity in obsessive-compulsive disorder for classification and prediction: An artificial neural network approach. Behav Neurol. (2020) 2020:1–7. doi: 10.1155/2020/2678718
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
42. Yang P, Wei Z, Yang Q, Xiao X, Wang T, Lei B, et al. OCD diagnosis via smooth sparse network and fused sparse auto-encoder learning. Expert Syst Appl. (2023) 216:119389. doi: 10.1016/j.eswa.2022.119389
43. Metin SZ, Balli Altuglu T, Metin B, Erguzel TT, Yigit S, Arıkan MK, et al. Use of EEG for predicting treatment response to transcranial magnetic stimulation in obsessive compulsive disorder. Clin EEG Neurosci. (2020) 51:139–45. doi: 10.1177/1550059419879569
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
44. Salomoni G, Grassi M, Mosini P, Riva P, Cavedini P, and Bellodi L. Artificial neural network model for the prediction of obsessive-compulsive disorder treatment response. J Clin Psychopharmacol. (2009) 29:343. doi: 10.1097/JCP.0b013e3181aba68f
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
45. Stein DJ and Ludik J. A neural network of obsessive–compulsive disorder: Modelling cognitive disinhibition and neurotransmitter dysfunction. Med Hypotheses. (2000) 55:168–76. doi: 10.1054/mehy.1999.1028
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
46. Ownby RL. Computational model of obsessive-compulsive disorder: Examination of etiologic hypothesis and treatment strategies. Depression Anxiety. (1998) 8:91–103. doi: 10.1002/(SICI)1520-6394(1998)8:3<91::AID-DA1>3.0.CO;2-Q
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
47. Patel K, Tripathy AK, Padhy LN, Kar SK, Padhy SK, and Mohanty SP. Accu-Help: A machine-learning-based smart healthcare framework for accurate detection of obsessive compulsive disorder. SN Comput Sci. (2023) 5:36. doi: 10.1007/s42979-023-02380-1
48. Boeding SE, Paprocki CM, Baucom DH, Abramowitz JS, Wheaton MG, Fabricant LE, et al. Let me check that for you: Symptom accommodation in romantic partners of adults with obsessive–compulsive disorder. Behav Res Ther. (2013) 51:316–22. doi: 10.1016/j.brat.2013.03.002
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
49. Turner C, O’Gorman B, Nair A, and O’Kearney R. Moderators and predictors of response to cognitive behaviour therapy for pediatric obsessive-compulsive disorder: A systematic review. Psychiatry Res. (2018) 261:50–60. doi: 10.1016/j.psychres.2017.12.034
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
50. Olatunji BO, Deacon BJ, and Abramowitz JS. The cruelest cure? Ethical issues in the implementation of exposure-based treatments. Cogn Behav Pract. (2009) 16:172–80. doi: 10.1016/j.cbpra.2008.07.003
51. Wang PS, Berglund P, Olfson M, Pincus HA, Wells KB, and Kessler RC. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. (2005) 62:603–13. doi: 10.1001/archpsyc.62.6.603
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
52. Corcoran CM, Mittal VA, Bearden CE, Gur RE, Hitczenko K, Bilgrami Z, et al. Language as a biomarker for psychosis: A natural language processing approach. Schizophr Res. (2020) 226:158–66. doi: 10.1016/j.schres.2020.04.032
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
53. He R, Palominos C, Zhang H, Alonso-Sánchez MF, Palaniyappan L, and Hinzen W. Navigating the semantic space: Unraveling the structure of meaning in psychosis using different computational language models. Psychiatry Res. (2024) 333:115752. doi: 10.1016/j.psychres.2024.115752
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
54. Hua Y, Blackley S, Shinn A, Skinner J, Moran L, and Zhou L. Identifying psychosis episodes in psychiatric admission notes via rule-based methods, machine learning, and pre-trained language models. Research Square (2024). doi: 10.21203/rs.3.rs-4126574/v1
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
55. Zheng L, Wang O, Hao S, Ye C, Liu M, Xia M, et al. Development of an early-warning system for high-risk patients for suicide attempt using deep learning and electronic health records. Trans Psychiatry. (2020) 10:72. doi: 10.1038/s41398-020-0684-2
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
56. Choo T-H, Galfalvy H, and Stanley B. Effects of life events on suicidal ideation in EMA data using recurrent neural network prediction. Biol Psychiatry. (2022) 91:S371. doi: 10.1016/j.biopsych.2022.02.931
57. Peis I, Olmos PM, Vera-Varela C, Barrigón ML, Courtet P, Baca-Garcia E, et al. Deep sequential models for suicidal ideation from multiple source data. IEEE J Biomed Health Inf. (2019) 23:2286–93. doi: 10.1109/JBHI.6221020
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
58. Melia R, Schafer KM, Rogers ML, Wilson-Lemoine E, and Joiner TE. The application of AI to ecological momentary assessment data in suicide research: Systematic review. J Med Internet Res. (2025) 27:e63192. doi: 10.2196/63192
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
59. Zaboski BA and Bednarek L. Precision psychiatry for obsessive-compulsive disorder: Clinical applications of deep learning architectures. J Clin Med. (2025) 14:2442. doi: 10.3390/jcm14072442
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
60. Yarkoni T and Westfall J. Choosing prediction over explanation in psychology: Lessons from machine learning. Perspect psychol Sci. (2017) 12:1100–22. doi: 10.1177/1745691617693393
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
61. Salazar de Pablo G, Studerus E, Vaquerizo-Serrano J, Irving J, Catalan A, Oliver D, et al. Implementing precision psychiatry: A systematic review of individualized prediction models for clinical practice. Schizophr Bull. (2021) 47:284–97. doi: 10.1093/schbul/sbaa120
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
62. Plana D, Shung DL, Grimshaw AA, Saraf A, Sung JJ, and Kann BH. Randomized clinical trials of machine learning interventions in health care: A systematic review. JAMA Network Open. (2022) 5:e2233946–e2233946. doi: 10.1001/jamanetworkopen.2022.33946
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
63. Chandler C., Foltz P. W., and Elvevåg B.. (2022). Improving the applicability of AI for psychiatric applications through human-in-the-loop methodologies. Schizophrenia Bulletin, 48(5), 949-957. https://doi.org/10.1093/schbul/sbac038
64. Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, and Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. (2008) 46:5–27. doi: 10.1016/j.brat.2007.10.003
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
65. Song Y, Li D, Zhang S, Jin Z, Zhen Y, Su Y, et al. The effect of exposure and response prevention therapy on obsessive-compulsive disorder: A systematic review and meta-analysis. Psychiatry Res. (2022) 317:114861. doi: 10.1016/j.psychres.2022.114861
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
66. Bottoms L, Prat Pons M, Fineberg NA, Pellegrini L, Fox O, Wellsted D, et al. Effects of exercise on obsessive-compulsive disorder symptoms: A systematic review and meta-analysis. Int J Psychiatry Clin Pract. (2023) 27:232–42. doi: 10.1080/13651501.2022.2151474
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
67. Sousa-Lima J, Moreira PS, Raposo-Lima C, Sousa N, and Morgado P. Relationship between obsessive compulsive disorder and cortisol: Systematic review and meta-analysis. Eur Neuropsychopharmacol. (2019) 29:1185–98. doi: 10.1016/j.euroneuro.2019.09.001
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
68. Balthazar P, Harri P, Prater A, and Safdar NM. Protecting your patients’ interests in the era of big data, artificial intelligence, and predictive analytics. J Am Coll Radiol. (2018) 15:580–6. doi: 10.1016/j.jacr.2017.11.035
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
69. Malik AS, Acharya S, and Humane S. Exploring the impact of security technologies on mental health: A comprehensive review. Cureus. (2024) 16:e53664. doi: 10.7759/cureus.53664
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
70. Hussain SA, Bresnahan M, and Zhuang J. The bias algorithm: How AI in healthcare exacerbates ethnic and racial disparities–A scoping review. Ethnicity Health. (2025) 30:197–214. doi: 10.1080/13557858.2024.2422848
PubMed Abstract | PubMed Abstract | Crossref Full Text | Google Scholar
Keywords: obsessive-compulsive disorder, precision psychiatry, machine learning, deep learning, neuroimaging, treatment prediction, diagnostic prediction
Citation: Zaboski BA, Bednarek L, Ayoub K and Pittenger C (2025) Deep learning in obsessive-compulsive disorder: a narrative review. Front. Psychiatry 16:1581297. doi: 10.3389/fpsyt.2025.1581297
Received: 21 February 2025; Accepted: 15 May 2025;
Published: 13 June 2025.
Edited by:
Pedro Morgado, University of Minho, PortugalReviewed by:
Na Liu, Nanjing Brain Hospital Affiliated to Nanjing Medical University, ChinaHilal Uygur, University of Health Sciences, Türkiye
Copyright © 2025 Zaboski, Bednarek, Ayoub and Pittenger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian A. Zaboski, YnJpYW4uemFib3NraUB5YWxlLmVkdQ==
†ORCID: Brian A. Zaboski, orcid.org/0000-0003-4567-8694
Lora Bednarek, orcid.org/0000-0003-2214-7858
Karen Ayoub, orcid.org/0009-0001-3059-8475
Christopher Pittenger, orcid.org/0000-0003-2117-9321
 Brian A. Zaboski
Brian A. Zaboski Lora Bednarek
Lora Bednarek Karen Ayoub
Karen Ayoub Christopher Pittenger
Christopher Pittenger