Abstract
Background:
Neurodevelopmental disorders (NDDs) such as Intellectual Disability, Autism Spectrum Disorder (ASD), and Attention-Deficit/Hyperactivity Disorder (ADHD) impact cognitive, behavioral, and social functions. The Zinc finger MYM-type protein 3, located on the X-chromosome, has been implicated in neurodevelopment, but its effects in females remain poorly understood due to limited research.
Case presentation:
We report a 19-year-old female with a de novo heterozygous variant in ZMYM3 (NM_201599.3:c.1927C>G, p.(His643Asp)), presenting with ADHD symptoms, poor motor coordination, and mild cognitive impairments. Although her language development was normal, she exhibited motor delays, learning and social difficulties, leading to anxiety and academic struggles. Neuropsychological assessment revealed an IQ of 85, with significant deficits in working memory and visuospatial reasoning but relative strengths in verbal comprehension. Brain MRI showed an incomplete left-sided hippocampal inversion. Genetic analysis confirmed the presence of the ZMYM3.
Discussion and conclusion:
This case contributes to the limited literature on ZMYM3-related NDDs in females, highlighting potential variability in phenotypic expression due to X-inactivation and penetrance effects. The patient’s symptoms emphasize how ADHD and other neurodevelopmental traits may manifest differently in females, often with more subtle and internalized features. Our findings underscore the importance of sex-specific research on ZMYM3-associated disorders and the need for comprehensive genetic and neuropsychological assessments to guide diagnosis and intervention in affected individuals.
Introduction
Neurodevelopmental disorders (NDDs), such as Intellectual Disability, Autism Spectrum Disorder (ASD) and Attention Deficit/Hyperactivity Disorder (ADHD), profoundly affect multiple aspects of life beyond cognitive functioning, beginning from early childhood and often persisting through adulthood. Individuals with NDDs often face challenges in academic attainment, employment, social relationships, and emotional regulation (1). These disorders are also associated with lower self-esteem and increased risks for psychiatric comorbidities, including anxiety and depression, contributing to lifelong impairments in quality of life (1–3).
Often, the impact extends beyond the individual, influencing family dinamics, access to healthcare and inclusion in society, underscoring the importance of early diagnosis, tailored interventions and supportive environments (3).
Zinc finger MYM-type protein 3, located on the X-chromosome, plays a role in neurodevelopment and raises unique issues regarding inheritance and expression (4). Most studies focus on males, creating a gap in understanding its impact in females, where symptoms may be subtler. Hiatt reviewed ZMYM3-variants in 27 patients, mostly males, with only three females, highlighting the need for further research (5, 6). This case report discusses a 19-year-old female with a de novo ZMYM3-variant, emphasizing her clinical, neuropsychological, and behavioral features.
Case presentation
A 19-year-old female with a family history of neurodevelopmental disorders, including delayed language acquisition in her younger sister and a maternal cousin diagnosed with ASD. A timeline of key clinical events is shown in Figure 1.
Figure 1
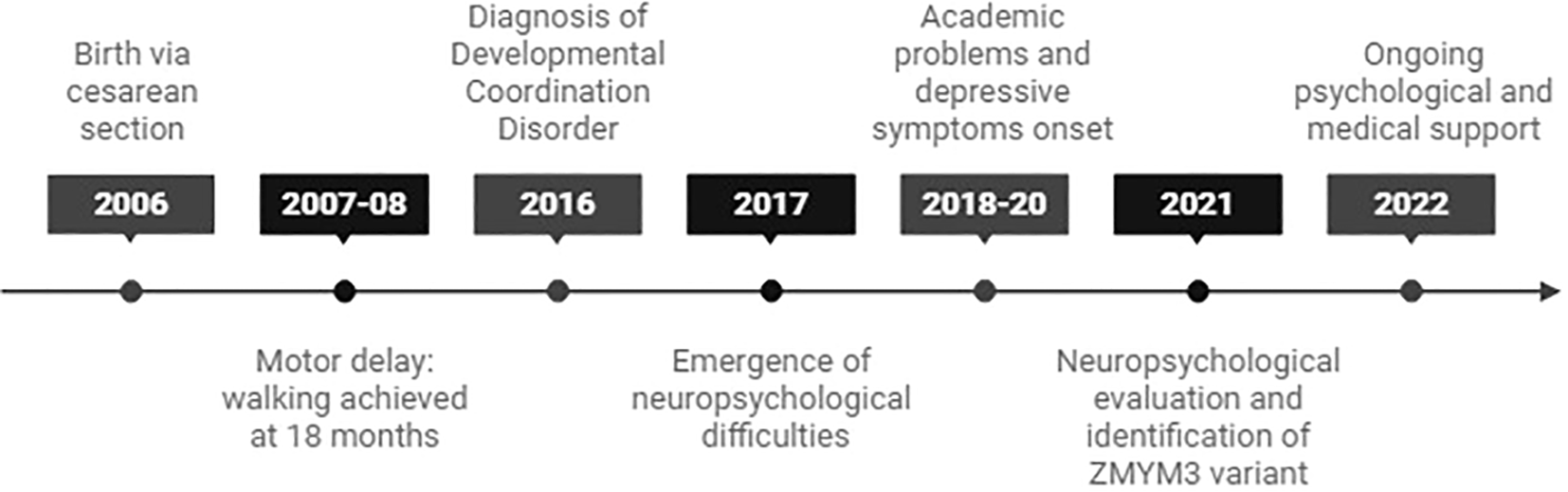
Timeline of key clinical events in the patient’s development.
She was born via emergency cesarean section due to fetal distress and showed mild motor delays, walking at 18 months with hypotonia. Despite motor challenges, her language development was normal. She has bilateral myopia, motor incoordination, and Scheuermann’s disease.
Clinically, she is 176 cm tall with a long face, prominent auricles, narrow palpebral fissures, and a bulbous nasal tip. She has asymmetric pectus carinatum, dorsal kyphosis, and elbow joint hypermobility (Beighton score 2/9). Throughout early schooling in Italy, she struggled with coordination, motor skills, and learning, particularly in math. Diagnosed with developmental coordination disorder at age 10, she later moved to Switzerland, where additional difficulties with attention, reading comprehension, and anxiety emerged. She exhibits symptoms of ADHD, including difficulties focusing and impulsivity, leading to social withdrawal and behavioral outbursts.
Psychologically, she faced bullying and social isolation, which contributed to the onset of depressive episodes. She was described by her parents as socially withdrawn, with increasing signs of anxiety and frustration in academic settings. Academic and social challenges led her to change educational paths, but she eventually discontinued formal education.
Cognitive functions (intelligence, executive functions, attention, visuospatial functions, emotional regulation and behavioral) were assessed. Wechsler Adult Intelligence Scale (WAIS-IV) indicated a heterogeneous cognitive profile. The Verbal Comprehension Index (VCI) was in the average range with a composite score of 96 (39th percentile; 95% CI: 89–103). The Perceptual Reasoning Index (PRI) was in the low average range with a score of 85 (17th percentile; 95% CI: 79–93). The Working Memory Index (WMI) fell in the borderline range with a score of 77 (7th percentile; 95% CI: 72–86). The Processing Speed Index (PSI) was in the average range with a score of 92 (28th percentile; 95% CI: 84–101). The Full Scale IQ (FSIQ) was 85 (15th percentile; 95% CI: 80–91), placing it in the low average range. The General Ability Index (GAI) was 90 (26th percentile; 95% CI: 85–96), and the Cognitive Proficiency Index (CPI) was 82 (12th percentile; 95% CI: 76–90). Both indices were considered interpretable. Due to a significant discrepancy among index scores (range = 19 points), the FSIQ should be interpreted with caution, as it may not reflect a unitary cognitive ability.
Additional neuropsychological assessments showed impairments in executive functions (Five point test and planning in Rey figure copy under 5° percentile, working memory in Digit span backward under 16° percentile), sustained attention (D2-R under 5° percentile), visuospatial processing (Lines orientation under 16° percentile). Emotional regulation and behavioral symptoms were assessed using self-report instruments and informant questionnaires completed by caregivers and teachers (see Supplementary Data Sheet 1 for details).
Brain-MRI showed a left-sided incomplete hippocampal inversion; no other brain parenchymal findings were noted (Figure 2).
Figure 2
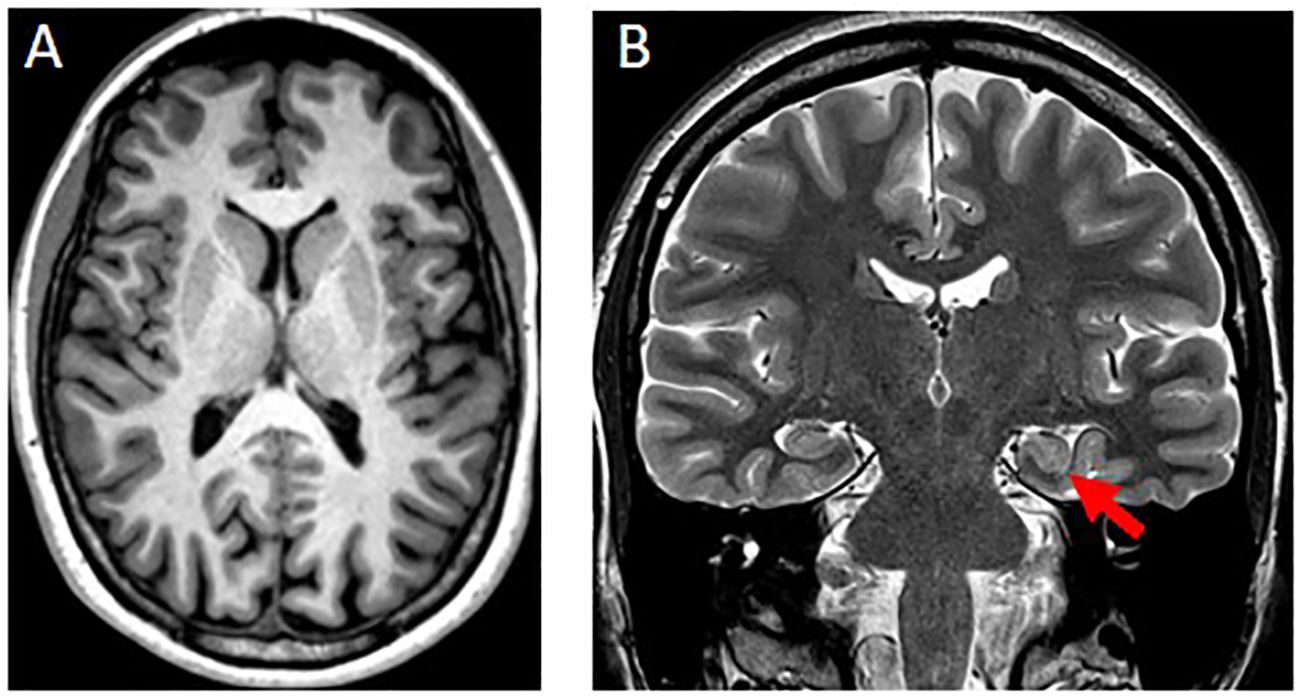
(A) Axial T1w-MPRAGE image at the level of the basal ganglia was unremarkable. (B) Coronal T2w image showed a round-shaped of the hippocampal head on the left side (red arrow) with normal signal intensity, size and visualization of the internal structure. The left collateral sulcus is more vertical than the controlateral side. These findings are consistent with an incomplete hippocampal inversion.
Initial genetic testing for Fragile X and search for CNVs with microarray technology returned negative results, leading to Next Generation Sequencing of over 100 genes in 2021, which revealed a de novo heterozygous variant in the ZMYM3-gene (NM_201599.3:c.1927C>G, p.(His643Asp)), classified as likely pathogenic according to ACMG criteria: PS2, PM1, PM2.
The ADHD diagnosis was formulated using DSM-5 criteria, applied by experienced clinicians based on all available assessment data (7).
The patient provided informed consent for the publication of this case report.
The patient underwent occupational therapy between ages of 17 and 19, as well as targeted educational support in Switzerland. Behavioral interventions were provided with partial improvement in attentional control and emotional regulation. No pharmacological treatment has been initiated to date.
Actually, the patient is currently not engaged in formal education, but participates in community-based social and occupational support programs. Anxiety symptoms have stabilized, though attentional difficulties and reduced autonomy remain a concern. There have been no hospitalizations or major behavioral problems in the past 12 months. Continued psychological monitoring is ongoing.
In the context of ultra rare disorders, such as those caused by ZMYM3 variants, we strongly recommend follow-up consultations with a clinical geneticist, as planned for our patient.
The main aim is to reassess the patient’s phenotypic evolution over time, reinterpret genetic findings, provide family counseling and reproductive planning, encourage participation in research and registries, and most importantly, keep the patient and her family informed about scientific and clinical developments relating to the genetic diagnosis.
From the patient’s point of view, the most difficult challenges have been managing attention and emotional regulation, particularly in academic and social environments. Despite this, she preferred not to undertake psychostimulant therapy. She expressed that understanding her condition provided some relief and helped reframe past difficulties. However, uncertainty about future support and outcomes remains a source of anxiety.
“For years, I felt misunderstood in my struggles. When I received the diagnosis and learned about the genetic condition, I felt relieved—it all made sense. It doesn’t fix everything, but now I know it’s not all my fault.”
– Patient, age 19
Discussion and conclusion
This case provides rare insight into ZMYM3-associated neurodevelopmental disorders in females, a group less understood compared to males.
ZMYM3 encodes a zinc finger MYM-type protein involved in transcriptional regulation and chromatin remodeling. Pathogenic variants are believed to impair its ability to regulate gene expression in the developing brain. This dysregulation may affect genes critical for neuronal migration, synaptogenesis, and cortical connectivity—all essential components of neurodevelopment (5, 13). While ZMYM3-variants have been linked to developmental delays and intellectual disabilities in males, their phenotypic expression in females remains underexplored.
In this paper, we seek to highlight the potential role of skewed X-inactivation and dosage effects in the clinical presentation of ZMYM3-variants in females, showing variability in symptoms (8, 9).
Our patient presented with ADHD symptoms, cognitive deficit in working memory and visuospatial processing, and emotional challenges such as anxiety and depression. These features align with previously described ZMYM3-related phenotypes but also point to potential sex-specific manifestations (10–12). As shown in Supplementary Data Sheet 2, we compared the phenotype of our patient with the spectrum observed in previously reported case (5).
Similarities in behavioral and cognitive phenotypes across genetic syndromes, such as Klinefelter syndrome (14, 15), highlight shared pathways underlying NDDs. ADHD-like symptoms, executive dysfunction, and social communication difficulties are common across distinct syndromes, underscoring the complexity and overlap within neurodevelopmental presentations and neurobiological mechanisms (impairment in synaptic development, social cognition or executive function)This case suggests an expansion of the phenotypic spectrum associated with ZMYM3 variants. It highlights variability that is likely influenced by gene dosage, X-inactivation patterns and the individual’s genetic background. Although functional studies on ZMYM3 are limited, these observations provide valuable clinical insights. However, definitive causality cannot yet be established without additional functional validation and corroborative evidence. While this case contributes to the understanding of ZMYM3-related phenotypes in females, it also raises important questions regarding the underlying molecular mechanisms. In particular, the potential contribution of X-inactivation patterns and dosage sensitivity requires further study. In addition, this case includes a comprehensive neuropsychological profile, which has not previously been reported in female carriers of ZMYM3 variants.
The main limitation of this report is its single-case design and the absence of functional validation of the identified variant. Although the de novo status of the variant, along with the patient’s clinical profile and brain MRI findings, supports a likely pathogenic role, the involvement of additional genetic or environmental modifiers cannot be excluded. Furthermore, no X-inactivation studies were performed in this patient, which prevents us from drawing conclusions about mechanisms such as skewing or allelic dosage imbalance. This is particularly relevant, as skewed X-inactivation is a relatively frequent phenomenon: studies have shown that approximately 25–35% of females in the general population exhibit moderate skewing (>70:30), and 8–10% exhibit extreme skewing (>80:20) in blood-derived DNA (16). Such skewing can influence the expression and penetrance of X-linked variants, as demonstrated in female carriers of Fabry disease or Lesch–Nyhan syndrome, where phenotypic variability has been associated—though not always conclusively—with X-inactivation profiles (17, 18).
In our case, hypotheses involving X-inactivation or gene dosage are raised based on existing literature and the known biology of the gene, but in the absence of direct data they must be interpreted cautiously. Future studies will benefit from incorporating molecular assays such as HUMARA-based methylation testing or RNA-based allelic expression analysis to clarify the relationship between X-inactivation and phenotype expression in female carriers of ZMYM3 variants.
Therefore, integrated research strategies that combine genomic, epigenetic, and transcriptomic data are necessary to elucidate the mechanisms underlying ZMYM3-related disorders in females and to better understand the factors driving phenotypic variability across the sex spectrum.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AC: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. SM: Data curation, Investigation, Writing – review & editing. DD: Data curation, Investigation, Writing – review & editing. ML: Investigation, Writing – review & editing. LM: Investigation, Writing – review & editing. LS: Project administration, Supervision, Validation, Writing – review & editing, Data curation, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
In the writing of this manuscript, the authors used ChatGPT-4 to improve readability and language. The authors have reviewed the content and take full responsibility for the content of the publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1604523/full#supplementary-material
References
1
Lord C Elsabbagh M Baird G . Autism spectrum disorder. Lancet. (2018) 392(10146):508–20. doi: 10.1016/S0140-6736(18)31129-2
2
Klin A Jones W Schultz RT . The neurodevelopmental basis of autism spectrum disorder. Annu Rev Neurosci. (2002) 43:261–85. doi: 10.1146/annurev-neuro-080317-062507
3
Zaneva M Coll-Martin T Hejja-Brichard Y . Point of View: An annotated introductory reading list for neurodiversity. eLife. (2024) 13:e102467. doi: 10.7554/eLife.10246
4
National Center for Biotechnology Information (NCBI) . ZMYM3 zinc finger MYM-type containing 3 [Gene ID: 9203]. In NCBI Gene. National Library of Medicine (US) (2025). Available online at: https://www.ncbi.nlm.nih.gov/gene/9203 (Accessed August 12, 2025).
5
Hiatt SM Trajkova S Rossi Sebastiano M Partridge EC Abidi FE Anderson A et al . Deleterious, protein-altering variants in the transcriptional coregulator ZMYM3 in 27 individuals with a neurodevelopmental delay phenotype. Am J Hum Genet. (2023) 110:215–27. doi: 10.1016/j.ajhg.2022.12.007
6
National Center for Biotechnology Information . ClinVar: Public archive of relationships among sequence variation and human phenotype [Database]. National Library of Medicine (US) (2024). Available online at: https://www.ncbi.nlm.nih.gov/clinvar/ (Accessed August 12, 2025).
7
American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Publishing. (2013). doi: 10.1176/appi.books.9780890425596.
8
Lyon MF . Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. (1961) 191(4797):945–8. doi: 10.1038/1911210a0
9
Quinn P Madhoo M . Review of attention-deficit/hyperactivity disorder in women and girls: uncovering this hidden diagnosis. Prim Care Companion CNS Disord. (2014) 16(3):PCC.13r01596. doi: 10.4088/PCC.13r01596
10
Gershon J . A meta-analytic review of gender differences in ADHD. J Attention Disord. (2002) 6:1–8. doi: 10.1177/108705470200500302
11
Lukin J Smith CM De Rubeis S . Emerging X-linked genes associated with neurodevelopmental disorders in females. Curr Opin Neurobiol. (2024) 87:102902. doi: 10.1016/j.conb.2024.102902
12
Moeschler JB Shevell M . Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics. (2014) 134:e903–18. doi: 10.1542/peds.2014-1839
13
Afshar H Khamse S Alizadeh F Delbari A Najafipour R Bozorgmehr A et al . Evolving evidence on a link between the ZMYM3 exceptionally long GA-STR and human cognition. Sci Rep. (2020) 10:13586. doi: 10.1038/s41598-020-76461-z
14
Zamboni L Fusina F Casari R Lugoboni F Zandonai T Cappelletti S et al . Klinefelter Syndrome and ADHD: a short systematic review. Front Psychiatry. (2025) 16:1585259. doi: 10.3389/fpsyt.2025.1585259
15
Tragantzopoulou P Giannouli V . Understanding the neuropsychological implications of klinefelter syndrome in pediatric populations: current perspectives. Pediatr Rep. (2024) 16:420–31. doi: 10.3390/pediatric16020036
16
Amos-Landgraf JM Cottle A Plenge RM Friez M Schwartz CE Longshore J et al . X chromosome-inactivation patterns of 1,005 phenotypically unaffected females. Am J Hum Genet. (2006) 79:493–9. doi: 10.1086/507565
17
Carrel L Willard HF . X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. (2005) 434:400–4. doi: 10.1038/nature03479
18
Plenge RM Stevenson RA Lubs HA Schwartz CE Willard HF Hendrich BD et al . Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. Am J Hum Genet. (2002) 71:168–73. doi: 10.1086/341123
Summary
Keywords
neurodevelopmental disorders, ZMYM3 gene, ADHD, gender differences, genetic testing, neuropsychological assessment, case report
Citation
Cordella A, Maitz SB, Distefano D, Lissi M, Morellini L and Sacco L (2025) The gender-sensitive spectrum of neurodevelopmental disorders: a case report on a ZMYM3 variant in a 19-year-old female. Front. Psychiatry 16:1604523. doi: 10.3389/fpsyt.2025.1604523
Received
02 April 2025
Accepted
31 July 2025
Published
15 August 2025
Volume
16 - 2025
Edited by
Fengmei Lu, Chengdu No.4 People’s Hospital, China
Reviewed by
Vaitsa Giannouli, Aristotle University of Thessaloniki, Greece
Ruohao Wu, Sun Yat-sen Memorial Hospital, China
Bayram Toraman, Karadeniz Technical University, Türkiye
Updates
Copyright
© 2025 Cordella, Maitz, Distefano, Lissi, Morellini and Sacco.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Cordella, alberto.cordella@eoc.ch
†ORCID: Alberto Cordella, orcid.org/0000-0001-8002-7462; Silvia B. Maitz, orcid.org/0000-0003-0637-5033; Distefano Daniela, orcid.org/0000-0002-7591-1314; Lucia Morellini, orcid.org/0000-0002-0733-8122; Leonardo Sacco, orcid.org/0000-0002-9494-7742
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.