Abstract
Aim:
Suicide is the most severe consequence of Major Depressive Disorder (MDD). Current risk assessments rely heavily on subjective self-reports, which lack reliability. Emerging technologies, such as facial and behavioral recognition devices, are being explored to improve suicide risk evaluation. This study aimed to examine the potential of 3D facial features in identifying suicide risk and uncovering sex-specific characteristics in patients with MDD.
Methods:
We conducted a cross-sectional study involving 222 MDD patients. Suicide-related information was collected from caregivers, while independent raters assessed depressive symptoms and recorded sociodemographic data. Three-dimensional facial scans were acquired using the 3dMDface System, followed by preprocessing to extract key facial landmarks. Sex-stratified subgroup analyses were performed to identify suicide risk-associated facial features. Logistic regression analysis was used to evaluate predictors, including demographic data, clinical characteristics, and the identified facial markers.
Results:
Data from 203 patients were analyzed, including 110 in the suicide-risk group and 93 in the non-suicidal group. The suicidal group exhibited significantly shorter philtrum length (t = 2.137, p < 0.05). Analyses revealed sex-specific facial patterns, with males demonstrating suicide risk association with philtrum depth (t=2.389, p < 0.05) and females showing nose-eye distance variations (U = 1121, p < 0.05). Logistic regression identified female (OR = 2.055, 95% CI: 1.107-3.873, p < 0.05) and shallow philtrum (OR = 0.644, 95% CI: 0.419-0.952, p < 0.05) as potential factors, with a significant interaction effect (OR = 1.963, 95% CI: 0.419-0.952, p < 0.05).
Conclusion:
This study identified sex-specific facial features associated with suicide risk in MDD, with reduced philtrum depth in females emerging as a correlate. These objective measures could complement current clinical risk assessments, though further longitudinal validation is required.
Clinical trial registration:
https://www.chictr.org.cn, identifier ChiCTR2400090458.
1 Introduction
Major Depressive Disorder (MDD) poses a profound and growing public health challenge, with its global incidence having risen by approximately 50% over the past three decades (1, 2). By 2021, depressive disorders ranked as the 12th leading cause of years lived with disability worldwide (3), underscoring the urgent need for effective intervention strategies. Among the most severe consequences of MDD is suicide. Although suicidal ideation and behaviors are transdiagnostic phenomena (4), MDD remains their strongest predictor (5). Supporting this, Lucht et al. (6) demonstrated that negative mood states significantly predict both the escalation and persistence of suicidal thoughts. The scale of the issue is further highlighted by a meta-analysis in China, which found that 53.1% of MDD patients experienced suicidal ideation, 17.5% made plans, and 23.7% attempted suicide at some point in their lives (7). Developmental evidence confirms adolescence as a high-risk period for suicidal behavior. In a UK cohort, 12% of 16-year-olds with suicidal ideation attempted suicide within five years (8), consistent with epidemiological data showing peak onset of suicidality occurs between ages 11-17 (9).
This high prevalence necessitates improved methods for the early identification of suicidality. Current clinical practice relies heavily on self-report instruments, such as the Beck Scale for Suicide Ideation (10) and the Columbia-Suicide Severity Rating Scale (11). While invaluable, these tools share a critical limitation due to their dependence on patients’ subjective recall and willingness to disclose, potentially resulting in unintentional inaccuracy or deliberate concealment. Given the established link between suicide risk and depression severity (5), the search for objective biomarkers has gained momentum. Emerging technologies that analyze facial expressions, which are a known indicator of depressive states, offer a promising avenue to supplement traditional assessments (12). These integrated systems use video-based behavioral analysis for multidimensional evaluation (13–15). For instance, researchers have observed that individuals at high suicide risk often exhibit gaze aversion when questioned about suicide, a behavior that is strongly correlated with suicidal ideation (16, 17). Other subtle cues, such as reduced blinking and decreased eyelid movement, have also been noted (18). The evidence regarding specific facial expressions, however, is mixed. While reduced smiling has been associated with suicidal thoughts (16, 19), one recent study intriguingly found that expressions of disgust might be even more accurate indicators of risk than smiles (20). These inconsistent findings across the literature may stem from methodological constraints, as most existing systems rely on standard two-dimensional (2D) imaging, which captures only surface-level details and lacks the depth information necessary to detect nuanced facial dynamics.
This limitation highlights the potential of three-dimensional (3D) facial imaging, a technology that captures the face’s detailed geometry through x, y, and z coordinates. Unlike 2D photographs, 3D scans generate comprehensive facial models in seconds, enabling precise measurement of subtle morphological traits. In practice, the reliability and efficiency of 3D imaging contribute to its emerging role as a valuable tool in medicine (21–24). Crucially, by quantifying depth and angular relationships, 3D technology permits rigorous symmetry analysis and other detailed assessments that are simply not possible with 2D images (25). The precision of 3D imaging, now applied in psychiatric research, enables the identification of distinct facial phenotypes. In a key study, Haque et al. (26) developed a combined 3D facial-vocal model that detected depression with 83.3% sensitivity and 82.6% specificity, demonstrating the clinical feasibility of this approach for objective mental health assessment.
Despite these technological advances, it is still an open question whether 3D facial features can distinguish suicide risk in MDD. In this initial exploration, we analyzed a cohort of patients stratified by suicide risk level, looking specifically for potential sex-specific markers. By building models that combine multiple data types, we hope to identify any associations. Finding such features would mark a first, crucial advance in developing objective assessment methods.
2 Methods
This analysis is derived from a cross-sectional study investigating facial features and oral microbiota in patients with MDD. The parent study was registered with the Chinese Clinical Trial Registry (ChiCTR-2400090458). We recruited participants diagnosed with MDD from the inpatient unit at Beijing Anding Hospital’s Depression Treatment Center. Trained examiners collected 3D facial imaging data using standardized protocols. All participants completed comprehensive assessments of affective symptoms.
2.1 Participants
The participants were recruited from Beijing Anding Hospital and assessed by inpatient physicians. Using a consecutive enrollment approach, we included patients from April 2022 through December 2023. Eligibility was contingent upon the following criteria: (1) Patients diagnosed with MDD according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (F32-F33) (27); (2) Han Chinese descent, aged between 16 to 50 years. The exclusion criteria encompassed (1) Young Mania Rating Scale (YMRS) score > 6 points; (2) a history of craniofacial trauma or surgery. All participants gave written informed consent. Standard care comprised antidepressants, with antipsychotics or mood stabilizers added based on clinical assessment.
The study received approval from the Ethics Committee of Beijing Anding Hospital, Capital Medical University (Approval number: 2021-Science Research Program-104). Participants were not paid for their involvement, and all provided written informed consent before participating.
2.2 Assessment
2.2.1 Suicide-related assessment
To reduce the risk of patients concealing information, we collected suicide-related data from medical records containing both patient and caregiver reports. Participants with prior suicide attempts, self-harm, or current suicidal ideation were classified as suicide risk (28). This classification aligns with clinical psychiatric practice for suicide risk assessment. Data were extracted directly from medical records to minimize underreporting bias (29).
2.2.2 Affective symptom assessment
-
17-item Hamilton Depression Rating Scale (HDRS-17), a clinician-administered scale with items scored 0-4 (total score range: 0-68) (30). We used the validated Chinese version (31).
-
9-item Patient Health Questionnaire (PHQ-9), a self-report measure with items scored 0-3 (total score range: 0-27) (32). The Chinese version has demonstrated validity (33).
-
Hamilton Anxiety Scale (HAMA): Clinician-administered 14-item scale (0–4 per item; total score range: 0-56), widely used in depression research (34, 35).
-
Generalized Anxiety Disorder-7 (GAD-7): Validated 7-item self-report measure (0–3 per item; total score range: 0-21) for core anxiety symptoms (36).
-
Young Mania Rating Scale (YMRS): Clinician-administered scale assessing manic symptoms across 11 items. Each item is scored from 0 to 4 (items 5, 6, and 8 scored 0-8), yielding a total score range of 0-60 (37). We used the YMRS to exclude participants with manic or hypomanic features.
2.2.3 Momentary mood assessment
Immediate Mood Scale (IMS), a 22-item self-report measure, assessed transient affective states using a validated Chinese version with a 7-point Likert scale (-3 [hopeless] to +3 [hopeful]) (38). Total scores were calculated by summing all items. Participants completed the IMS immediately before undergoing 3D facial imaging to control for the acute effects of mood on facial morphology.
2.3 3D facial features acquisition and processing
We acquired 3D facial images using the 3dMDface System (www.3dmd.com) under standardized lighting conditions (natural or artificial). Participants removed facial accessories to ensure optimal capture quality. The system’s cameras captured images from fixed angles, with proprietary algorithms generating raw 3D data (0.2mm accuracy), including point clouds and texture files. The preprocessing pipeline involved identifying and aligning key facial landmarks, with pose normalization performed through global rotation of the complete facial model centered at the nasal tip (designated as point O’). This rotation step preserved the original point cloud distribution without altering individual morphological characteristics. Subsequent alignment was conducted using multiple landmarks. To reduce noise and facilitate group-level comparisons, surface smoothing was applied using the validated MeshMonk (39), which generates landmark-derived facial features that closely correspond to anatomical measurements. Collectively, these steps standardized the dataset for robust three-dimensional morphometric analysis. Figure 1A shows the 3D facial reconstruction with XYZ axis orientation for spatial analysis.2.5 Face landmarks labeling. Each sample contained 72 standardized facial landmarks (P0-P71; Figure 1B) encompassing key morphological features: facial contours, oral commissures, nasal alae, and ocular canthi. All directional references adopt the observer’s perspective unless specified.
Figure 1
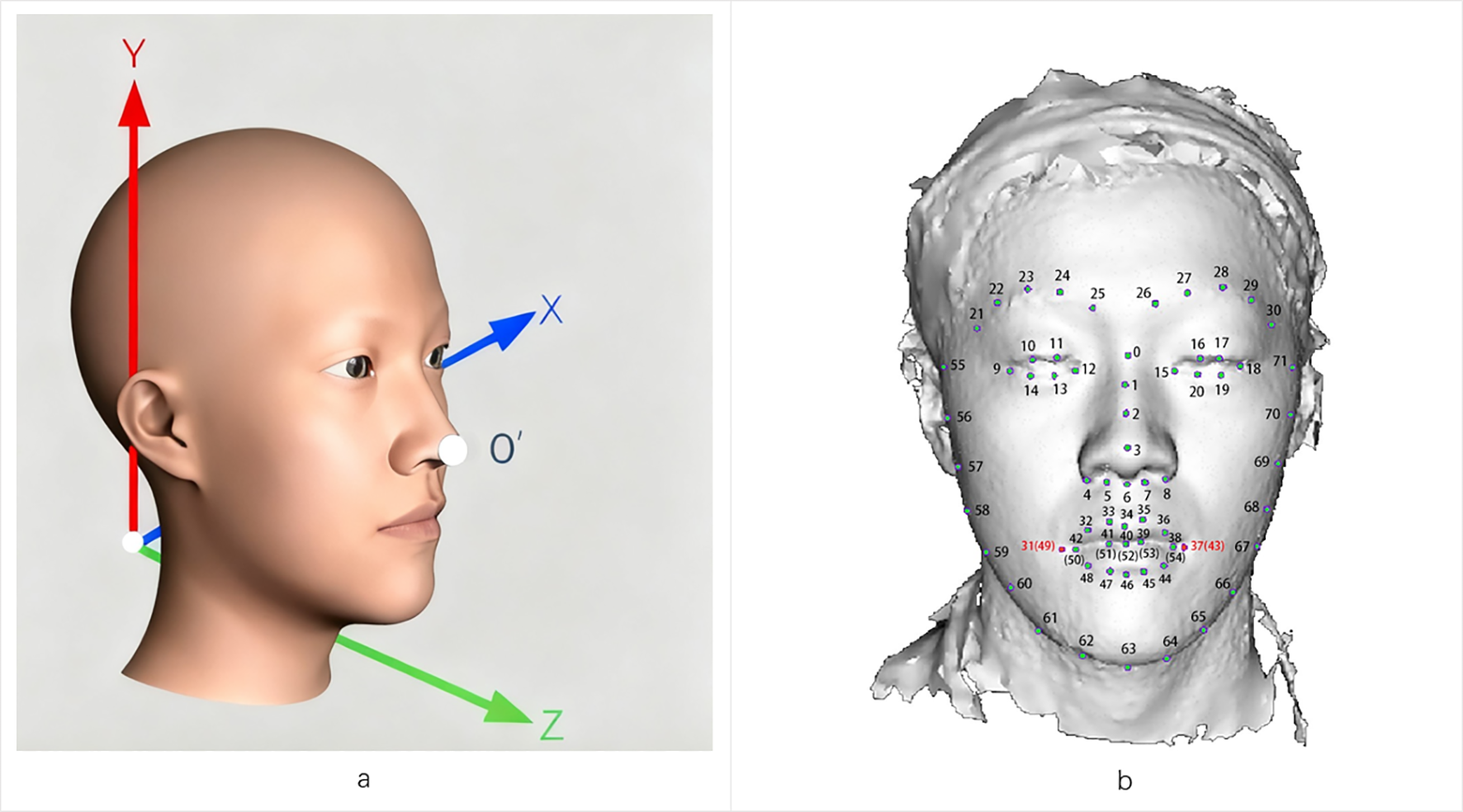
3D facial landmarks are labeled as the basis for morphological quantitative analysis. (a) Representation of a 3D facial image based on meshes within a three-dimensional coordinate space. The face exhibits overall symmetry concerning the X-Y-Z plane. (b) Example of 72 landmarks on a 3D facial surface.
From pre-annotated landmarks, we selected 47 features based on their relevance to psychiatric research. Analyses comprised linear, angular, and surface measurements, enhanced by ratio and midpoint techniques. The photographer-recorded laterality was converted to anatomical orientation. Additional details are provided in the Supplementary Materials.
2.4 Statistical analysis
All statistical analyses were conducted using R software, version 4.3.1. Categorical variables were expressed in terms of frequency and percentage, with intergroup comparisons made using the chi-square (χ2) test. For continuous variables, the mean ± standard deviation (SD) was reported following a Shapiro-Wilk test for normality. Normally distributed variables were analyzed using the independent samples t-test, while the non-parametric Mann-Whitney U test was applied for those not meeting normality criteria. Logistic regression was used to identify clinical and facial variables correlated with suicide risk. To address the issue of multiple comparisons arising from testing numerous facial features against suicide risk, we controlled the false discovery rate using the Benjamini-Hochberg procedure. Statistical significance was set at p < 0.05, indicating a meaningful difference.
3 Results
We screened 222 participants and excluded 19 (10 withdrew consent, 9 had poor-quality 3D images), leaving 203 for analysis. These patients were divided into two groups based on suicide risk: the suicide risk group (SR, n = 110) and the no-suicide risk group (NSR, n = 93).
3.1 Intergroup comparison of demographic, clinical characteristics, and 3D facial features
Demographic and clinical characteristics were largely comparable between the SR and NSR groups. No significant differences were observed in age, body mass index, diagnostic subtype, presence of psychotic symptoms, or scores on depression and anxiety scales. The SR included a significantly higher proportion of female patients (62%) compared to the NSR group (46%; χ² = 4.329, p < 0.05). Detailed demographic and clinical data are provided in Table 1.
Table 1
| Variables | Overall (n=203) | NSR (n=93) | SR (n=110) | U/χ2 | p |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age | 26.95 (9.96) | 27.87 (10.47) | 26.17 (9.49) | 5333.5 | 0.600 |
| Gender, n (%) | 4.329 | 0.038* | |||
| Male | 92 (45%) | 50 (54%) | 42 (38%) | ||
| Female | 111 (55%) | 43 (46%) | 68 (62%) | ||
| BMI | 23.25 (4.66) | 23.74 (4.89) | 22.83 (4.45) | 5580.0 | 0.265 |
| Diagnosis, n (%) | 0.090 | 0.765 | |||
| Single-episode depressive disorder | 106 (52%) | 47 (51%) | 59 (54%) | ||
| Recurrent depressive disorder | 97 (48%) | 46 (49%) | 51 (46%) | ||
| Psychotic symptoms, n (%) | 0.025 | 0.873 | |||
| Without Psychotic symptoms | 183 (90%) | 83 (89%) | 100 (91%) | ||
| With Psychotic symptoms | 20 (10%) | 10 (11%) | 10 (9%) | ||
| IMS | -1.22 (27.44) | 3.23 (27.66) | -4.99 (26.81) | 5934.0 | 0.050 |
| PHQ-9 | 15.30 (6.48) | 14.48 (6.32) | 15.98 (6.56) | 4416.0 | 0.094 |
| GAD-7 | 10.27 (5.74) | 10.62 (5.72) | 9.96 (5.77) | 5484.5 | 0.376 |
| HDRS-17 | 20.51 (7.52) | 20.00 (7.45) | 20.95 (7.58) | 4732.0 | 0.359 |
| HAMA | 18.76 (9.14) | 19.76 (9.39) | 17.92 (8.87) | 5717.0 | 0.149 |
Characteristics of patients with MDD in the NSR and SR.
NSR, No-suicide risk group; SR, Suicide risk group; BMI, Body mass index; IMS, Immediate Mood Scale; PHQ-9, 9-item Patient Health Questionnaire; GAD-7, Generalized Anxiety Disorder; HDRS-17, 17-item Hamilton Depression Rating Scale; HAMA, Hamilton Anxiety Scale; *p < 0.05.
Among the 47 facial features analyzed, philtrum length was the only measure that differed significantly between groups. Patients in the SR group had a shorter philtrum length than those in the NSR group (t = 2.137, p < 0.05). No other 3D facial features showed statistically significant differences in the overall sample (Table 2).
Table 2
| Variables | NSR (n=93) | SR (n=110) | t/U | p |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Distance features (mm) | ||||
| Forehead width | 122.44 (6.64) | 122.30 (5.80) | 0.164 | 0.870 |
| Face width | 155.00 (9.09) | 153.18 (8.17) | 1.486 | 0.139 |
| Face length | 132.68 (8.17) | 131.86 (6.83) | 0.772 | 0.441 |
| Jaw width | 139.56 (10.77) | 135.95 (9.30) | 1.832 | 0.069 |
| Nose width | 28.76 (2.23) | 28.38 (2.30) | 1.214 | 0.226 |
| Nose height | 14.49 (1.80) | 14.47 (1.74) | 5200 | 0.839 |
| Nose depth | 17.53 (2.74) | 17.39 (2.59) | 0.360 | 0.719 |
| Nose length | 41.36 (3.25) | 41.72 (2.79) | -0.833 | 0.406 |
| Part nose x | 28.69 (2.25) | 28.29 (2.27) | 1.258 | 0.210 |
| Part nose y | 50.91 (3.17) | 51.05 (2.73) | -0.331 | 0.741 |
| Philtrum depth | 3.83 (1.75) | 3.57 (1.58) | 1.101 | 0.273 |
| Philtrum length | 16.14 (2.45) | 15.45 (2.13) | 2.137 | 0.034* |
| Philtrum length flat | 15.56 (2.44) | 14.91 (2.28) | 1.964 | 0.051 |
| Mouth width | 49.38 (4.27) | 48.38 (3.89) | 5854 | 0.077 |
| Part mouth x | 49.33 (4.25) | 48.34 (3.88) | 1.712 | 0.089 |
| Dist nose lip | 22.69 (2.40) | 22.34 (2.21) | 1.099 | 0.273 |
| Upper lip thick | 9.52 (1.64) | 9.63 (1.62) | -0.479 | 0.633 |
| Lower lip thick | 10.90 (1.71) | 11.20 (1.67) | -1.257 | 0.210 |
| Chin depth | 24.32 (17.58) | 25.30 (19.88) | 5208 | 0.824 |
| Chin length | 41.53 (13.73) | 41.72 (15.79) | 5323 | 0.619 |
| center eye width | 25.93 (1.55) | 25.93 (1.52) | 0.002 | 0.998 |
| Right eye width | 25.77 (1.64) | 25.94 (1.48) | -0.774 | 0.440 |
| Dist inner eyes | 39.88 (3.01) | 40.08 (2.79) | -0.493 | 0.623 |
| Dist outer eyes | 90.30 (5.11) | 90.66 (4.24) | -0.536 | 0.592 |
| Dist Leye nose | 19.61 (2.04) | 19.91 (1.62) | 4567 | 0.189 |
| Dist Reye nose | 20.20 (1.60) | 20.14 (1.65) | 0.286 | 0.775 |
| Part Leye x | 25.29 (1.47) | 25.31 (1.37) | -0.119 | 0.905 |
| Part Leye y | 7.64 (1.85) | 7.39 (1.84) | -0.604 | 0.547 |
| Part Reye x | 25.17 (1.52) | 25.27 (1.39) | -0.473 | 0.637 |
| Part Reye y | 7.62 (1.77) | 7.88 (1.79) | -1.067 | 0.287 |
| Part Leyepit y | 28.77 (3.05) | 28.58 (3.07) | 0.430 | 0.667 |
| Part Reyepit y | 28.73 (2.79) | 28.82 (3.17) | 4954 | 0.700 |
| Angle features (°) | ||||
| Philtrum slope x | 1.52 (0.04) | 1.53 (0.03) | 4440 | 0.106 |
| Philtrum slope y | 0.25 (0.10) | 0.25 (0.11) | 0.284 | 0.777 |
| Philtrum slope z | 1.33 (0.11) | 1.33 (0.11) | -0.068 | 0.946 |
| Chin slope x | 1.53 (0.04) | 1.53 (0.04) | 5351 | 0.572 |
| Chin slope y | 0.60 (0.25) | 0.61 (0.26) | 5201 | 0.838 |
| Chin slope z | 0.98 (0.25) | 0.96 (0.26) | 4567 | 0.189 |
| center eye slope x | 0.21 (0.08) | 0.21 (0.06) | 4832 | 0.498 |
| center eye slope y | 1.48 (0.05) | 1.47 (0.05) | 1.017 | 0.310 |
| center eye slope z | 1.40 (0.09) | 1.40 (0.07) | 5335 | 0.599 |
| Right eye slope x | 0.21 (0.07) | 0.22 (0.06) | -1.752 | 0.081 |
| Right eye slope y | 1.47 (0.05) | 1.46 (0.05) | 5462 | 0.406 |
| Right eye slope z | 1.41 (0.07) | 1.39 (0.07) | 1.578 | 0.116 |
| Area features (mm2) | ||||
| center eye area | 126.40 (30.97) | 129.19 (30.98) | -0.639 | 0.524 |
| Right eye area | 130.40 (31.82) | 132.07 (33.01) | -0.366 | 0.715 |
| Midpoint projection (mm) | ||||
| Forehead height | 120.04 (4.38) | 120.40 (4.40) | -0.575 | 0.566 |
Differences in 3D facial features between NSR and SR.
NSR, No-suicide risk group; SR, Suicide risk group; *p < 0.05.
3.2 Sex-specific 3D facial features for suicide risk
When analyses were stratified by sex, distinct facial patterns emerged. Among male patients, a deeper philtrum was significantly associated with suicide risk (t = 2.389, p < 0.05). In female patients, a shorter distance between the nose and the left eye (Dist Leye nose) showed a stronger association with suicide risk (U = 1121, p < 0.05). The complete set of sex-stratified analyses is presented in Table 3.
Table 3
| Variables | Male NSR (n=50) vs. SR (n =42) | Female NSR (n=43) vs. SR (n =68) | ||
|---|---|---|---|---|
| t/U | p | t/U | p | |
| Distance features (mm) | ||||
| Forehead width | -0.080 | 0.937 | -0.977 | 0.331 |
| Face width | 0.828 | 0.410 | 0.131 | 0.896 |
| Face length | 1.540 | 0.127 | -1.362 | 0.176 |
| Jaw width | 1.396 | 0.166 | 0.001 | 0.999 |
| Nose width | 1.157 | 0.251 | -0.904 | 0.368 |
| Nose height | 970 | 0.533 | 1430 | 0.849 |
| Nose depth | -0.360 | 0.720 | 0.146 | 0.884 |
| Nose length | -1.308 | 0.194 | -1.046 | 0.299 |
| Part nose x | 1.232 | 0.221 | -0.886 | 0.378 |
| Part nose y | -0.609 | 0.544 | -0.938 | 0.351 |
| Philtrum depth | 2.389 | 0.019 * | -1.112 | 0.269 |
| Philtrum length | 1.799 | 0.075 | 0.418 | 0.677 |
| Philtrum length flat | 1.400 | 0.165 | 0.600 | 0.550 |
| Mouth width | 1217 | 0.192 | 1493 | 0.854 |
| Part mouth x | 1.449 | 0.151 | 0.133 | 0.894 |
| Dist nose lip | 0.730 | 0.467 | 0.067 | 0.947 |
| Upper lip thick | -0.507 | 0.614 | -0.193 | 0.848 |
| Lower lip thick | -0.439 | 0.662 | -1.614 | 0.110 |
| Chin depth | 1130 | 0.533 | 1393 | 0.678 |
| Chin length | 1174 | 0.333 | 1380 | 0.622 |
| center eye width | 0.067 | 0.946 | -0.227 | 0.821 |
| Right eye width | -0.228 | 0.820 | -1.219 | 0.226 |
| Dist inner eyes | -0.603 | 0.548 | -1.196 | 0.234 |
| Dist outer eyes | -0.432 | 0.667 | -1.231 | 0.222 |
| Dist Leye nose | 971 | 0.538 | 1121 | 0.039 * |
| Dist Reye nose | 0.022 | 0.983 | -0.319 | 0.750 |
| Part Leye x | -0.125 | 0.901 | -0.240 | 0.811 |
| Part Leye y | 0.115 | 0.908 | 0.001 | 0.999 |
| Part Reye x | 0.043 | 0.966 | -1.208 | 0.230 |
| Part Reye y | 0.206 | 0.838 | -0.868 | 0.388 |
| Part Leyepit y | 1.385 | 0.170 | -0.892 | 0.375 |
| Part Reyepit y | 1166 | 0.365 | 1234 | 0.168 |
| Angle features (°) | ||||
| Philtrum slope x | 891 | 0.214 | 1297 | 0.319 |
| Philtrum slope y | 1.678 | 0.097 | -1.166 | 0.247 |
| Philtrum slope z | 1.163 | 0.248 | 0.787 | 0.434 |
| Chin slope x | 1022 | 0.829 | 1540 | 0.639 |
| Chin slope y | 1041 | 0.947 | 1436 | 0.877 |
| Chin slope z | 1063 | 0.922 | 1492 | 0.858 |
| center eye slope x | 941 | 0.395 | 1508 | 0.783 |
| center eye slope y | 0.484 | 0.630 | 0.344 | 0.732 |
| center eye slope z | 1143 | 0.468 | 1430 | 0.849 |
| Right eye slope x | -1.389 | 0.168 | -1.389 | 0.168 |
| Right eye slope y | 1118 | 0.597 | 1428 | 0.839 |
| Right eye slope z | 1.163 | 0.248 | 0.787 | 0.434 |
| Area features (mm2) | ||||
| center eye area | 0.634 | 0.528 | -0.907 | 0.367 |
| Right eye area | 0.416 | 0.678 | -0.012 | 0.991 |
| Midpoint projection (mm) | ||||
| Forehead height | 0.212 | 0.833 | -1.092 | 0.278 |
Differences in 3D facial features between NSR and SR by gender.
NSR, No-suicide risk group; SR, Suicide risk group; *p < 0.05.
3.3 Exploratory factors associated with suicide risk in MDD
An exploratory logistic regression was performed to evaluate the joint contribution of sex and facial morphology to suicide risk. The results indicated that female sex (OR = 1.944, 95% CI: 1.107–3.873) and shallower philtrum depth (OR = 0.650, 95% CI: 0.419–0.952) were each independently associated with suicide risk (both p < 0.05). A significant interaction was observed between female sex and philtrum depth (OR = 2.349, 95% CI: 1.079–3.655, p < 0.05), suggesting a combined effect on suicide risk. After adjusting for multiple comparisons using the Benjamini-Hochberg procedure, the association for philtrum depth and the interaction term remained marginally significant (p_adj = 0.068). Full regression results are shown in Table 4.
Table 4
| Variables | Estimate | OR | 95%CI | p | p_adj1 |
|---|---|---|---|---|---|
| Gender (Female/Male) |
0.720 | 2.055 | 1.107-3.873 | 0.024* | 0.068 |
| Philtrum length | -0.260 | 0.771 | 0.560-1.050 | 0.103 | 0.154 |
| Philtrum depth | -0.441 | 0.644 | 0.419-0.952 | 0.034* | 0.068 |
| Dis Leye nose | 0.247 | 1.280 | 0.868-1.949 | 0.217 | 0.260 |
| Female* Philtrum depth | 0.674 | 1.963 | 1.079-3.655 | 0.030* | 0.068 |
| Female* Dis Leye nose | 0.230 | 1.258 | 0.671-2.364 | 0.470 | 0.470 |
Logistic regression of suicide risk by sex and facial features.
1P-values were corrected using the Benjamini-Hochberg method; *p < 0.05.
4 Discussion
Our study identified distinct facial morphology patterns associated with suicide risk in depression, with variations between sexes. Reduced philtrum depth showed a particular association with suicide risk in women, supported by a significant interaction term between female sex and philtrum morphology. While these findings should be interpreted as exploratory, they contribute to the growing interest in developing objective markers for suicide risk assessment.
Women consistently show higher rates of suicidal ideation and attempts (40, 41), which aligns with the sex-specific associations observed in our study. Neurochemical profiles reveal that women typically show higher densities of 5-Hydroxytryptamine receptor 1A (5-HT1A) and lower serotonin transporter (5-HTT) availability in key emotional processing regions like the hippocampus (42). This pattern may contribute to their increased vulnerability to stress and depression (43, 44). Consistent evidence from Arango et al. (45) and Aleksandra et al. (46) demonstrated reduced 5-HT1A receptor and 5-HTT function across multiple brain regions, including the dorsal raphe nucleus, prefrontal cortex, and hypothalamus, in depressed individuals who died by suicide, indicating that serotonergic dysfunction is a key neurobiological basis of suicide. Furthermore, estrogen fluctuations during pregnancy and menopause in females can modulate serotonin activity and serotonin transporter availability (47, 48). From a developmental perspective, the link between philtrum morphology and suicide risk in women may share embryological origins. During weeks 4–7 of gestation, neural crest cells give rise to both the midfacial prominences forming the philtrum and the limbic structures regulating emotional processing (49, 50). Lower testosterone levels in female embryos may be associated with shallower philtrum development (51) and concurrently influence the maturation of emotion-regulation pathways such as the prefrontal-amygdala circuit (52). It remains unclear whether a shallower philtrum arises primarily from female sex characteristics or reflects atypical development of emotional neurocircuitry. Future studies should focus on high-risk female populations to examine how philtrum depth correlates with emotional regulation function and suicide vulnerability.
Facial features capture both biological traits and emotional expression. Muscle movements around the mouth directly alter philtrum morphology. For instance, contraction of the orbicularis oris shortens the upper lip and flattens the philtrum, an expression linked to anxiety (53). Similarly, activation of the depressor anguli oris elongates and shallowens the philtrum in sad or fearful expressions (54). These expressive patterns are consistent across cultures (55). Our findings reveal that a shallower philtrum depth remains detectable even at rest and is significantly associated with suicide risk, suggesting that emotion-related facial movements may leave stable morphological traces or reflect early neurodevelopmental shaping of facial structures. Supporting evidence comes from studies of Chinese youth linking resting angry or anxious expressions to suicide risk (20). Most notably, the combination of reduced philtrum depth and female sex shows an association with suicide risk (OR = 1.963, p < 0.05), further suggesting potential neurodevelopmental interactions. The persistence of these morphological markers across emotional states underscores their value as indicators of vulnerability, complementing traditional behavioral assessments in clinical risk evaluation.
Conventional methods relying on 2D images or videos remain constrained by sensitivity to lighting, pose, and other environmental variables. Our approach leverages 3D facial geometry to overcome these limitations, aligning with the growing interest in more robust and physiologically meaningful features. This direction is supported by a number of recent methodological innovations, which also highlight the potential advantages of integrating 3D data. Wang et al. (56) developed a sophisticated multimodal spatiotemporal feature set to capture depression-related manifestations; their framework’s reliance on 2D video leaves it susceptible to lighting and pose variations. The incorporation of 3D features could directly mitigate these challenges, simplifying data normalization and enhancing model generalization. The behavioral primitives proposed by Song et al. (57) for spectral analysis could be more accurately defined using 3D facial data, as the geometry provides a direct correlate of muscle activity, free from appearance-based artifacts. The value of 3D integration is further evident in architectures like the Depression Multi-view Graph Neural Network by Wu et al. (58), where anatomically grounded spatial coordinates from 3D scans would provide a more physically accurate and interpretable basis for modeling interactions between facial regions. Beyond enhancing existing models, 3D features are particularly promising for identifying cross-modal depression characteristics in real-world contexts. The multimodal corpus contributed by Zou et al. (59), derived from semi-structured interviews, underscores the importance of nuanced behavioral cues. 3D facial quantification is ideally suited to this task, enabling precise measurement of subtle dynamics often lost in 2D representations. This capability supports a richer quantitative analysis of non-verbal behavior. These efforts reflect a growing interest in multimodal datasets (60) that incorporate 3D facial data for finer-grained behavioral measurement.
From a methodological perspective, we acknowledge several considerations regarding feature stability. Our preprocessing pipeline, while necessary for standardization, may affect subtle morphological variations. The global rotation around the nasal tip preserves overall point cloud distribution but could obscure orientation-dependent shape details. Similarly, surface smoothing with MeshMonk (39), though effective for noise reduction, might attenuate fine-grained morphological information. These technical factors, combined with the moderate sample size and marginal significance after multiple testing correction, highlight the preliminary nature of our findings. Future studies focusing on highly localized facial features may benefit from alternative registration strategies or multi-scale analytical approaches that better balance noise reduction with feature preservation.
In terms of clinical interpretation, it is important to clarify the temporal relationship between risk assessment and data collection. Suicide risk was determined based on historical medical records, including prior suicide attempts or self-harm episodes, while 3D facial imaging was conducted at a subsequent time point. Therefore, the observed associations reflect retrospective correlations rather than predictive validity. The results suggest that certain facial morphological patterns may persist as stable markers of vulnerability even during non-acute phases among individuals with a history of suicidality. However, the study design does not support the use of these features to predict future suicidal events. Rather, these findings highlight the potential of facial morphology as an enduring indicator of underlying vulnerability, which may complement dynamic risk assessments in future longitudinal studies.
This study has several limitations that should be considered. First, our feature selection was based mainly on univariate differential testing. While this approach detects features with significant group mean differences, it cannot adjust for multicollinearity or capture complex interactive effects between multiple features and the outcome. Future studies should consider applying diverse feature selection methods for validation. Second, the sample size of 203 participants from a single source offers limited statistical power to reliably identify associations with small to moderate effect sizes, even after controlling for potential confounders. This increases the risk of unstable subgroup estimates or overlooking meaningful predictors. Finally, the cross-sectional design restricts insight into how facial morphology changes with symptom progression over time. Accordingly, these findings should be viewed as exploratory. They provide preliminary clues and generate hypotheses for future mechanistic and modeling research, but their robustness and clinical relevance need to be confirmed through larger, prospective, and multi-center studies.
5 Conclusion
This study provides preliminary evidence that 3D facial morphology, particularly philtrum depth, may serve as a sex-specific marker associated with suicide risk in women with major depressive disorder. While these results highlight the potential of objective facial metrics to complement current risk assessment tools, the moderate sample size and cross-sectional design underscore the need for further validation. Future longitudinal and multi-center studies are essential to confirm the stability and predictive value of these facial features, and to explore their integration into clinically actionable frameworks for suicide prevention.
Statements
Data availability statement
The datasets presented in this article are not readily available because the datasets generated during this study are not publicly available due to participant privacy concerns and institutional regulations. Requests to access the datasets should be directed to gangwangdoc@ccmu.edu.cn.
Ethics statement
The studies involving humans were approved by Beijing Anding Hospital, Capital Medical University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JY: Project administration, Writing – original draft, Writing – review & editing, Investigation, Formal Analysis. LC: Writing – original draft, Visualization. LZ: Supervision, Conceptualization, Writing – review & editing. J-DH: Conceptualization, Funding acquisition, Writing – review & editing. GW: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by 1) National Natural Science Foundation of China (No.92049302), 2) Beijing Hospital Authority (No. PX2025065), 3) Beijing Anding Hospital Science Foundation.
Acknowledgments
Special thanks to Linghui Meng, Jia Zhou and Yanjie Zhao (National Center for Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China) for the suggestions on statistics for this study. We thank all the research staff and participants for their contributions to this study.
Conflict of interest
The authors declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1650104/full#supplementary-material
References
1
Monroe SM Harkness KL . Major depression and its recurrences: life course matters. Annu Rev Clin Psychol. (2022) 18:329–57. doi: 10.1146/annurev-clinpsy-072220-021440
2
Liu Q He H Yang J Feng X Zhao F Lyu J . Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J Psychiatr Res. (2020) 126:134–40. doi: 10.1016/j.jpsychires.2019.08.002
3
GBD 2021 Dis Injuries Collaborators . Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403(10440):2133–61. doi: 10.1016/S0140-6736(24)00757-8
4
Oquendo MA Baca-García E Mann JJ Giner J . Issues for DSM-V: suicidal behavior as a separate diagnosis on a separate axis. Am J Psychiatry. (2008) 165:1383–4. doi: 10.1176/appi.ajp.2008.08020281
5
Ribeiro JD Huang X Fox KR Franklin JC . Depression and hopelessness as risk factors for suicide ideation, attempts and death: meta-analysis of longitudinal studies. Br J psychiatry: J Ment science. (2018) 212:279–86. doi: 10.1192/bjp.2018.27
6
Lucht L Spangenberg L Forkmann T Hallensleben N Rath D Strauss M et al . Association of real-time assessed mood, affect and suicidal ideation in psychiatric inpatients with unipolar depression. Clin Psychol Psychother. (2022) 29:1580–6. doi: 10.1002/cpp.2741
7
Lin J-Y Huang Y Su Y-A Yu X Lyu X-Z Liu Q et al . Association between perceived stressfulness of stressful life events and the suicidal risk in chinese patients with major depressive disorder. Chin Med J. (2018) 131:912–9. doi: 10.4103/0366-6999.229898
8
Mars B Heron J Klonsky ED Moran P O’Connor RC Tilling K et al . Predictors of future suicide attempt among adolescents with suicidal thoughts or non-suicidal self-harm: a population-based birth cohort study. Lancet Psychiatry. (2019) 6:327–37. doi: 10.1016/s2215-0366(19)30030-6
9
Hutchinson E Scott L Choukas-Bradley S Silk J . Interpersonal risk factors for suicide in daily life among young people: A review of intensive longitudinal studies. Dev Psychopathol. (2025) 37:2196–216. doi: 10.1017/s0954579424001810
10
Beck AT Kovacs M Weissman A . Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. (1979) 47:343–52. doi: 10.1037//0022-006x.47.2.343
11
Posner K Brown GK Stanley B Brent DA Yershova KV Oquendo MA et al . The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. (2011) 168:1266–77. doi: 10.1176/appi.ajp.2011.10111704
12
Hu B Tao Y Yang M . Detecting depression based on facial cues elicited by emotional stimuli in video. Comput Biol Med. (2023) 165:107457. doi: 10.1016/j.compbiomed.2023.107457
13
Liu D Liu B Lin T Liu G Yang G Qi D et al . Measuring depression severity based on facial expression and body movement using deep convolutional neural network. Front Psychiatry. (2022) 13:1017064. doi: 10.3389/fpsyt.2022.1017064
14
Hunter L Roland L Ferozpuri A . Emotional expression processing and depressive symptomatology: eye-tracking reveals differential importance of lower and middle facial areas of interest. Depression Res Treat. (2020) 2020:1049851. doi: 10.1155/2020/1049851
15
Galatzer-Levy I Abbas A Ries A Homan S Sels L Koesmahargyo V et al . Validation of visual and auditory digital markers of suicidality in acutely suicidal psychiatric inpatients: proof-of-concept study. J Med Internet Res. (2021) 23:e25199. doi: 10.2196/25199
16
Eigbe N Baltrušaitis T Morency L-p Pestian JP . “ Toward Visual Behavior Markers of Suicidal Ideation,” In: M2018 13th IEEE International Conference on Automatic Face & Gesture Recognition (FG 2018), Xi'an, China, (2018) 530–4. doi: 10.1109/FG.2018.00085
17
Shah A Sharma V Vaibhav V Alismail M Morency L . . “Multimodal Behavioral Markers Exploring Suicidal Intent in Social Media Videos.” In: 2019 International Conference on Multimodal Interaction. (2019).
18
Liu S Lu C Alghowinem S Gotoh L Breazeal C Park HW . “ Explainable AI for Suicide Risk Assessment Using Eye Activities and Head Gestures”. In: Degen H, Ntoa S, editors. Artificial Intelligence in HCI. HCII 2022. Lecture Notes in Computer Science, Cham: Springer. (2022) 13336. doi: 10.1007/978-3-031-05643-7_11
19
Laksana E Baltrusaitis T Morency LP Pestian JP . (2017). Investigating facial behavior indicators of suicidal ideation, in: IEEE International Conference on Automatic Face & Gesture Recognition, .
20
Hu CS Zhang H Short LA Hu S . Individuals with higher suicide risk showed more anger and disgust during rest. Death Stud. (2024) 48:9–15. doi: 10.1080/07481187.2023.2186537
21
Dolci C Sansone VA Gibelli D Cappella A Sforza C . Distinctive facial features in Andersen-Tawil syndrome: A three-dimensional stereophotogrammetric analysis. Am J Med Genet A. (2021) 185:781–9. doi: 10.1002/ajmg.a.62040
22
Meng T Guo X Lian W Deng K Gao L Wang Z et al . Identifying facial features and predicting patients of acromegaly using three-dimensional imaging techniques and machine learning. Front Endocrinol (Lausanne). (2020) 11:492. doi: 10.3389/fendo.2020.00492
23
Park H Min J Koh KS . Three-dimensional anthropometry for evaluating philtrum contour in patients with unilateral cleft lip: comparison between photographic assessment and 3-dimensional anthropometry. J craniofacial surgery. (2023) 34:2061–5. doi: 10.1097/scs.0000000000009667
24
Postema FAM Matthews H Hopman SMJ Merks JHM Suttie M Hoskens H et al . 3D analysis of facial morphology in Dutch children with cancer. Comput Methods Programs Biomed. (2021) 205:106093. doi: 10.1016/j.cmpb.2021.106093
25
Berssenbrügge P Berlin NF Kebeck G Runte C Jung S Kleinheinz J et al . 2D and 3D analysis methods of facial asymmetry in comparison. J cranio-maxillo-facial surgery: Off Publ Eur Assoc Cranio-Maxillo-Facial Surgery. (2014) 42:e327–34. doi: 10.1016/j.jcms.2014.01.028
26
Haque A Guo M Miner AS Fei-Fei Li . Measuring Depression Symptom Severity from Spoken Language and 3D Facial Expressions. ArXiv, abs/1811.08592. (2018).
27
World Health Organization . The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization. (1993).
28
Raue PJ Ghesquiere AR Bruce ML . Suicide risk in primary care: identification and management in older adults. Curr Psychiatry Rep. (2014) 16:466. doi: 10.1007/s11920-014-0466-8
29
Yarborough BJH Stumbo SP Schneider JL Richards JE Hooker SA Rossom RC . Patient expectations of and experiences with a suicide risk identification algorithm in clinical practice. BMC Psychiatry. (2022) 22:494. doi: 10.1186/s12888-022-04129-1
30
Hamilton M . Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. (1967) 6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x
31
Zheng YP Zhao JP Phillips M Liu JB Cai MF Sun SQ et al . Validity and reliability of the chinese hamilton depression rating scale. Br J psychiatry: J Ment science. (1988) 152:660–4. doi: 10.1192/bjp.152.5.660
32
Kroenke K Spitzer RL Williams JB . The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
33
Sun XY Li YX Yu CQ Li LM . Reliability and validity of depression scales of Chinese version: a systematic review. Chin J Epidemiol. (2017) 38:110–6. doi: 10.3760/cma.j.issn.0254-6450.2017.01.021
34
Hamilton M . The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
35
Maier W Buller R Philipp M Heuser I . The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. (1988) 14:61–8. doi: 10.1016/0165-0327(88)90072-9
36
Löwe B Decker O Müller S Brähler E Schellberg D Herzog W et al . Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. (2008) 46:266–74. doi: 10.1097/MLR.0b013e318160d093
37
Young RC Biggs JT Ziegler VE Meyer DA . A rating scale for mania: reliability, validity and sensitivity. Br J psychiatry: J Ment science. (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
38
Nahum M Vleet TMV Sohal VS Mirzabekov JJ Rao VR Wallace DL et al . Immediate mood scaler: tracking symptoms of depression and anxiety using a novel mobile mood scale. JMIR mHealth uHealth. (2017) 5:e44. doi: 10.2196/mhealth.6544
39
White JD Ortega-Castrillón A Matthews H Zaidi AA Ekrami O Snyders J et al . MeshMonk: Open-source large-scale intensive 3D phenotyping. Sci Rep. (2019) 9:6085. doi: 10.1038/s41598-019-42533-y
40
Freeman A Mergl R Kohls E Székely A Gusmao R Arensman E et al . A cross-national study on gender differences in suicide intent. BMC Psychiatry. (2017) 17:234. doi: 10.1186/s12888-017-1398-8
41
Nock MK Green JG Hwang I McLaughlin KA Sampson NA Zaslavsky AM et al . Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. (2013) 70:300–10. doi: 10.1001/2013.jamapsychiatry.55
42
Jovanovic H Lundberg J Karlsson P Cerin A Saijo T Varrone A et al . Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage. (2008) 39:1408–19. doi: 10.1016/j.neuroimage.2007.10.016
43
Zhang Z-J Wang D Man SC Ng R McAlonan GM Wong HK et al . Platelet 5-HT(1A) receptor correlates with major depressive disorder in drug-free patients. Prog Neuropsychopharmacol Biol Psychiatry. (2014) 53:74–9. doi: 10.1016/j.pnpbp.2014.03.004
44
Altieri SC Garcia-Garcia AL Leonardo ED Andrews AM . Rethinking 5-HT1A receptors: emerging modes of inhibitory feedback of relevance to emotion-related behavior. ACS Chem Neurosci. (2013) 4:72–83. doi: 10.1021/cn3002174
45
Arango V Underwood MD Boldrini M Tamir H Kassir SA Hsiung S et al . Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology: Off Publ Am Coll Neuropsychopharmacol. (2001) 25:892–903. doi: 10.1016/s0893-133x(01)00310-4
46
Wisłowska-Stanek A Kołosowska K Maciejak P . Neurobiological basis of increased risk for suicidal behaviour. Cells. (2021) 10(10):2519. doi: 10.3390/cells10102519
47
Steiner M . Serotonin, depression, and cardiovascular disease: sex-specific issues. Acta Physiol (Oxf). (2011) 203:253–8. doi: 10.1111/j.1748-1716.2010.02236.x
48
Stacy M Kremer M Schulkin J . Suicide among women and the role of women’s health care providers. Obstet Gynecol Surv. (2022) 77:293–301. doi: 10.1097/ogx.0000000000001025
49
Roth DM Bayona F Baddam P Graf D . Craniofacial development: neural crest in molecular embryology. Head Neck Pathol. (2021) 15:1–15. doi: 10.1007/s12105-021-01301-z
50
Cordero DR Brugmann S Chu Y Bajpai R Jame M Helms JA . Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet A. (2011) 155A:270–9. doi: 10.1002/ajmg.a.33702
51
Whitehouse AJ Gilani SZ Shafait F Mian A Tan DW Maybery MT et al . Prenatal testosterone exposure is related to sexually dimorphic facial morphology in adulthood. Proc Biol Sci. (2015) 282:20151351. doi: 10.1098/rspb.2015.1351
52
Lombardo MV Ashwin E Auyeung B Chakrabarti B Taylor K Hackett G et al . Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci. (2012) 32:674–80. doi: 10.1523/jneurosci.4389-11.2012
53
Peck SR . Atlas of Human Anatomy for the Artist (Galaxy Books). Oxford Univ Pr (2015).
54
Ekman P Sorenson ER Friesen WV . Pan-cultural elements in facial displays of emotion. Sci (New York NY). (1969) 164:86–8. doi: 10.1126/science.164.3875.86
55
Harmonjones E . Handbook of cognition and emotion. Br J Psychiatry. (1999) 176:500. doi: 10.1002/0470013494
56
Wang Y Lin Z Yang C Zhou Y Yang Y . Automatic depression recognition with an ensemble of multimodal spatio-temporal routing features. IEEE Trans Affect Computing. (2025) 16:1855–72. doi: 10.1109/TAFFC.2025.3543226
57
Song S Jaiswal S Shen L Valstar M . Spectral representation of behaviour primitives for depression analysis. IEEE Trans Affect Computing. (2022) 13:829–44. doi: 10.1109/TAFFC.2020.2970712
58
Wu Z Zhou L Li S Fu C Lu J Han J et al . DepMGNN: matrixial graph neural network for video-based automatic depression assessment. Proc AAAI Conf Artif Intell. (2025) 39:1610–9. doi: 10.1609/aaai.v39i2.32153
59
Zou B Han J Wang Y Liu R Zhao S Feng L et al . Semi-structural interview-based chinese multimodal depression corpus towards automatic preliminary screening of depressive disorders. IEEE Trans Affect Computing. (2023) 14:2823–38. doi: 10.1109/TAFFC.2022.3181210
60
Girard JM Chu WS Jeni LA Cohn JF . “ Sayette Group Formation Task (GFT) Spontaneous Facial Expression Database,” In: 2017 12th IEEE International Conference on Automatic Face & Gesture Recognition (FG 2017), Washington, DC, USA. (2017) 581–8. doi: 10.1109/FG.2017.144
Summary
Keywords
major depressive disorder, suicide risk, 3D facial features, 3D imaging, female, sex-specific
Citation
Yang J, Chen L, Zhang L, Han J-DJ and Wang G (2026) Three-dimensional facial features of suicide risk in females with depression. Front. Psychiatry 16:1650104. doi: 10.3389/fpsyt.2025.1650104
Received
19 June 2025
Revised
09 October 2025
Accepted
12 December 2025
Published
22 January 2026
Volume
16 - 2025
Edited by
Jun Ji, Beijing Wanling Pangu Science and Technology Ltd, China
Reviewed by
Linlin Shen, Shenzhen University, China
Yantao Ma, Peking University Sixth Hospital, China
Updates
Copyright
© 2026 Yang, Chen, Zhang, Han and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-Dong J. Han, jackie.han@pku.edu.cn; Gang Wang, gangwangdoc@ccmu.edu.cn
†These authors have contributed equally to this work
ORCID: Jing-Dong J. Han, orcid.org/0000-0002-9270-7139
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.