Abstract
Background:
Electronic Patient-Reported Outcome Measures (ePROMs) have emerged as valuable tools in cancer care, facilitating the comprehensive assessment of patients’ physical, psychological, and social well-being. This study synthesizes literature on the utilization of ePROMs in oncology, highlighting the diverse array of measurement instruments and questionnaires employed in cancer patient assessments. By comprehensively analyzing existing research, this study provides insights into the landscape of ePROMs, informs future research directions, and aims to optimize patient-centred oncology care through the strategic integration of ePROMs into clinical practice.
Methods:
A systematic review was conducted by searching peer-reviewed articles published in academic journals without time limitations up to 2024. The search was performed across multiple electronic databases, including PubMed, Scopus, and Web of Science, using predefined search terms related to cancer, measurement instruments, and patient assessment. The selected articles underwent a rigorous quality assessment using the Mixed Methods Appraisal Tool (MMAT).
Results:
The review of 85 studies revealed a diverse range of measurement instruments and questionnaires utilized in cancer patient assessments. Prominent instruments such as the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) and the Patient Reported Outcome-Common Terminology Criteria for Adverse Events (PRO-CTCAE) were frequently referenced across multiple studies. Additionally, other instruments identified included generic health-related quality of life measures and disease-specific assessments tailored to particular cancer types. The findings indicated the importance of utilizing a variety of measurement tools to comprehensively assess the multifaceted needs and experiences of cancer patients.
Conclusion:
Our systematic review provides a comprehensive examination of the varied tools and ePROMs employed in cancer care, accentuating the perpetual requirement for development and validation. Prominent instruments like the EORTC QLQ-C30 and PRO-CTCAE are underscored, emphasizing the necessity for a thorough assessment to meet the multifaceted needs of patients. Looking ahead, scholarly endeavours should prioritize the enhancement of existing tools and the creation of novel measures to adeptly address the evolving demands of cancer patients across heterogeneous settings and populations.
Introduction
Cancer care involves a complex and multifaceted approach, encompassing diagnosis, treatment, and supportive care interventions aimed at enhancing patient outcomes and quality of life. Within the landscape of cancer care, the selection and application of appropriate assessment tools are pivotal in ensuring the accuracy, reliability, and validity of patient symptom evaluations. In oncology, effectively managing symptoms related to both the disease and treatment toxicity is paramount for improving patients’ quality of life (QoL). However, under-detection and under-reporting of symptoms can hinder optimal supportive care delivery. Symptom monitoring through Patient-reported outcomes (PROs) offers an evidence-based solution to bridge the gap between clinician recognition and patient self-reporting (1). PROs offer an evidence-based solution to bridge the gap between clinician recognition and patient self-reporting, allowing for a comprehensive assessment of physical, psychological, and social well-being. PROs play a crucial role in modern oncology care, allowing patients to directly report on their health status without interpretation by clinicians (2, 3).
The emergence of electronic Patient-Reported Outcome Measures (ePROMs) represents a promising advancement, with digital solutions showing significant benefits in improving satisfaction, treatment adherence, symptom control, and overall clinical outcomes (2–4). Interest in integrating ePROMs into regular cancer care has grown, driven by a desire to enhance health-related quality of life (HRQOL) and other patient-centred outcomes (5). Dedicated tools known as Patient-Reported Outcome Measures (PROMs), such as questionnaires or standardized interview schedules, facilitate the collection of PROs and promote communication between patients and clinicians (1). These tools serve as invaluable instruments in capturing the complex interplay of disease burden, treatment efficacy, and patient-reported experiences, thereby informing tailored interventions and optimizing the delivery of patient-centred care (6, 7).
ePROM questionnaires provide standardized instruments for eliciting patient-reported information across diverse domains, such as symptomatology, functional status, and treatment satisfaction, thereby supplying clinicians and researchers with structured data to inform clinical decision-making and research endeavours. They can facilitate data collection, management, and analysis, streamlining the process of patient assessment and enabling real-time monitoring of symptoms and treatment outcomes (8) ePROMs have been utilized in questionnaires to gather information from patients, who consistently report that ePROMs are easy to comprehend, timely to fill out, and enhance communication with their oncology team. Clinicians also find that ePROMs help communication with patients, increase patient engagement in consultations, and alter clinical decision-making (5, 9).
Recent studies highlight the positive impact of ePROM interventions, offering features like remote symptom reporting and real-time clinician feedback (5). Moreover, ePROMs have shown promising benefits in improving communication, symptom control, prolonging survival, and reducing hospital admissions and emergency department visits (10). A systematic review by Warnecke et al. found that ePROMs improve the assessment of underrated physical and psychological symptom burdens among oncological patients, highlighting their potential to enhance patient-centred care. However, further research is needed to fully understand their clinical utility and address the challenges associated with their implementation (11, 12).
ePROMs have the potential to significantly enhance patient-centred cancer care by improving symptom assessment, communication, and patient engagement. The use of electronic platforms and mobile technologies enables remote data collection, enhancing accessibility, convenience, and patient engagement while minimizing logistical barriers associated with traditional paper-based assessments. Addressing these challenges requires a comprehensive approach that encompasses technological Despite the evident advantages of ePROMs, their widespread adoption and implementation present notable challenges, such as technological barriers, health literacy disparities, and concerns regarding data security, privacy, and confidentiality innovation, healthcare policy reform, and patient education initiatives to improve the equitable and ethical integration of assessment tools in cancer care. This systematic review aims to provide a comprehensive analysis of the landscape of assessment tools, questionnaires, and ePROMs utilized in cancer care, informing future research directions, clinical practice guidelines, and policy initiatives aimed at optimizing patient-centred oncology care.
To guide this analysis, the research questions for our systematic review are formulated as follows:
RQ1. What are the existing ePROMs utilized in cancer care?
RQ2. What are the key characteristics and functionalities of ePROMs used in cancer care, and how do they vary across different measurement tools?
These research questions will facilitate a systematic evaluation of the effectiveness of ePROMs in cancer care and help identify optimal strategies for their successful implementation and broader adoption.
Methods
Study design
We conducted a systematic review and addressed all research articles focusing on the utilization of ePROM and their potential for patient-centred solutions in cancer care. The final report follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews. Our study encompasses several key steps including search strategy, inclusion and exclusion criteria, study selection, quality appraisal, and data extraction and synthesis to ensure a comprehensive and rigorous analysis of the available literature (13).
Search strategy
We searched for articles published in electronic databases up to 2024, using three databases: Scopus, Web of Science and PubMed review. The searches used the following keywords and medical subject heading (MeSH) terms in various combinations. We derived two broad themes that were then combined with the Boolean operators “AND” and “OR”. The first theme in Mesh “electronic Patient Reported Outcomes” was created by the Boolean operator “OR” to combine text words (“electronic Patient-Self Reporting”, OR “electronic Patient Reported Measures”). The second theme “Cancer” was the broad aspect created for the search strategy. Additionally, a backward snowball search will be employed to ensure comprehensive coverage of relevant articles (See Appendix Table A1).
Inclusion and exclusion criteria
We included papers based on eligibility criteria with the following characteristics: (1) published in English, (2) papers related to electronic patient-reported outcomes, electronic patient-reported measures, and electronic self-reporting (3) articles with various research types like quantitative, qualitative, and mixed methods, (4) Studies assessing quality of life, symptoms, psychological well-being, treatment satisfaction, or other relevant outcomes in cancer patients.
We excluded studies that were inaccessible in full text, studies exclusively focused on technical infrastructure, and those emphasizing paper-based or manual Patient Reported Outcome (PRO) versions, books, protocols, standards, framework and guidelines, conference proceedings, dissertations, conference abstracts, reviews, short reports, posters, newspapers, editorials and commentary. Furthermore, unrelated subjects were excluded such as feasibility, paper vs. electronic systems, terminology criteria, clinical alerts, health equity, perspective, experiences, and perception, data and machine learning, models, associations, not related and not cancer, editorial, biology, telemedicine, ethical principles, wearables, gamification, system design and technical innovations, oncology informatics, precision oncology, validity and reliability, economic. We also excluded studies lacking indicators or outcomes for cancer, not using the system as the intervention tool.
Study selection
Three investigators independently reviewed papers based on titles and abstracts in alignment with the inclusion and exclusion criteria. Irrelevant studies were removed at this stage. One reviewer (HS) conducted the data extraction, while two other reviewers (MA, SN) rechecked the accuracy of the results. All researchers then read and reviewed the full texts to make final inclusion decisions.
Quality appraisal
The selected articles underwent a rigorous quality assessment using the Mixed Methods Appraisal Tool (MMAT), comprising components for qualitative, quantitative (clinical trials), quantitative (non-clinical trials), descriptive, and mixed methods, incorporating 25 questions. Each affirmative response contributes to a 25% score. Articles surpassing the average in the number of positive responses or fully specified items are categorized as high quality. Those with positive responses ranging from 25% to 50% are classified as medium quality, while those falling below 25% are considered low quality. Following full-text screening and quality appraisal, a total of 85 articles met the inclusion criteria.
Data extraction and synthesis
An initial data extraction form was developed at this stage of the review. Data elements were extracted from each article that was organized into two sections: general items (author, year, country/state, objective, and participants) and specific items (system tools & metrics, cancer type and type of treatment). The selected papers were summarized in the final step of our methodology and important factors were identified. Thus, the statistical results of systematic reviews were described for outcomes reported in the studies. Subsequently, data extracted from these pertinent articles will undergo thorough narrative analysis and be presented in organized tables and diagrams (See Appendix Table A2).
Result
In our systematic review, we identified 672 papers, out of which 85 academic papers were included in our systematic review, providing a comprehensive exploration of electronic patient-reported outcome measures for cancer care. We present the key findings regarding the characteristics of the included studies, measurements, and their use as revealed in our systematic review (See Figure 1).
Figure 1

PRISMA flow diagram for selected studies.
Characteristics of included studies
The distribution of articles about electronic patient-reported outcome measures in cancer by year indicates that the majority of publications were from the years 2022 and 2023, with 17 and 15 contributions, respectively. Additionally, there were 12 publications from 2021, 13 from 2020, 7 from 2019, 7 from 2016, 5 from 2017, 3 from 2015, and 1 publication each from 2024, 2014, 2013, 2012, and 2010 (See Figure 2).
Figure 2
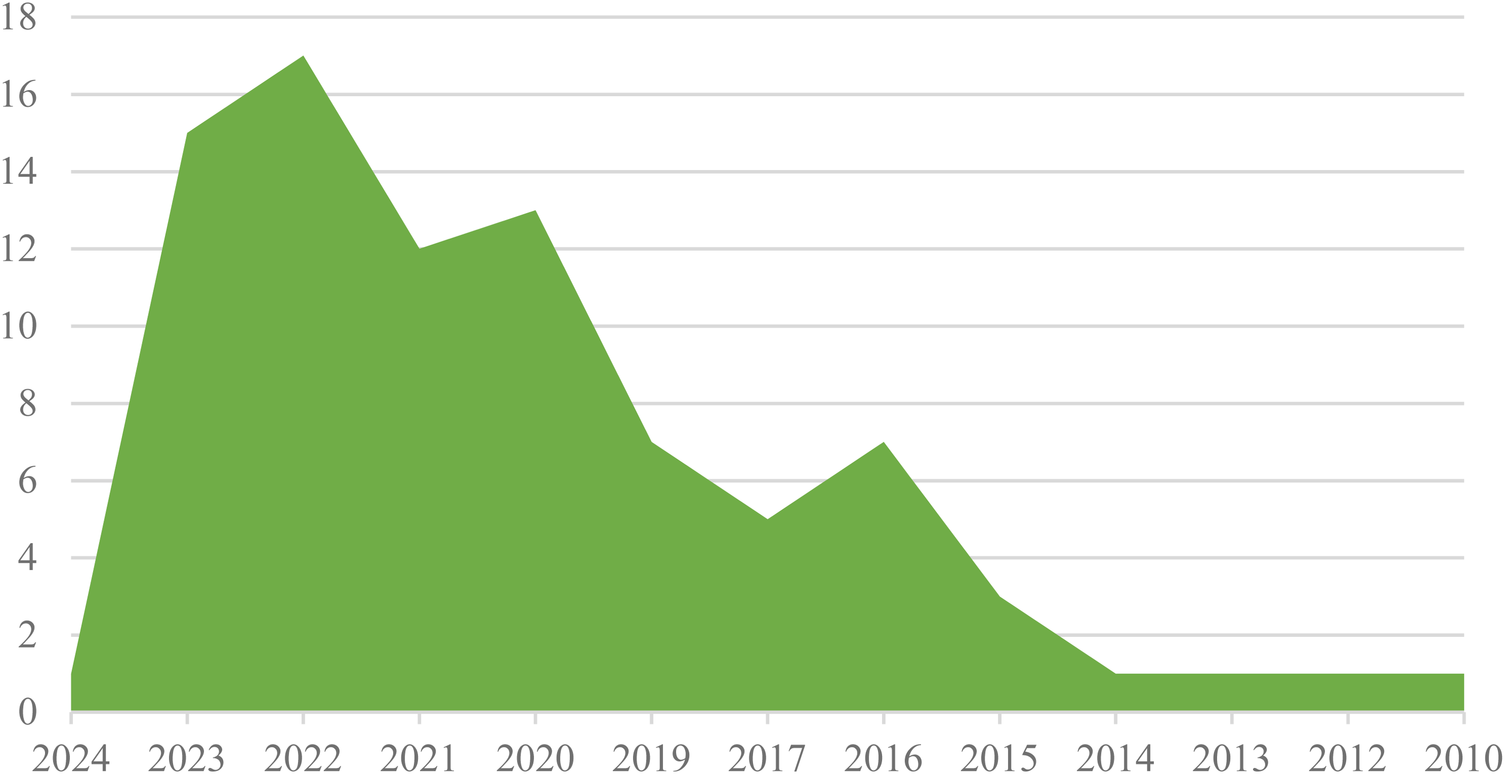
Frequency distribution of articles about ePROM in cancer by year.
According to Figure 3, the United States emerged as the leading source of publications, followed by the United Kingdom in second place and Austria in third. Additionally, Belgium, France, Greece, Iran, Ireland, Japan, and Norway each made a single contribution.
Figure 3

Frequency distribution of articles about ePROM in cancer by country.
Type of cancers
The type and frequency of cancer within the ePROM study are indicated in Figure 4. This figure highlights the prevalence of various malignancies across the studies. A total of 32 different types of cancer were identified, with breast cancer emerging as the most frequently studied.
Figure 4

Frequency distribution of studies articles about ePROM according to cancer types.
Type of cancer treatments
The evaluation of ePROM according to the type of cancer treatment is shown in Figure 5. In terms of the types of treatments, 114 treatments had been found and chemotherapy was the most commonly reported treatment, followed by radiotherapy and surgery.
Figure 5
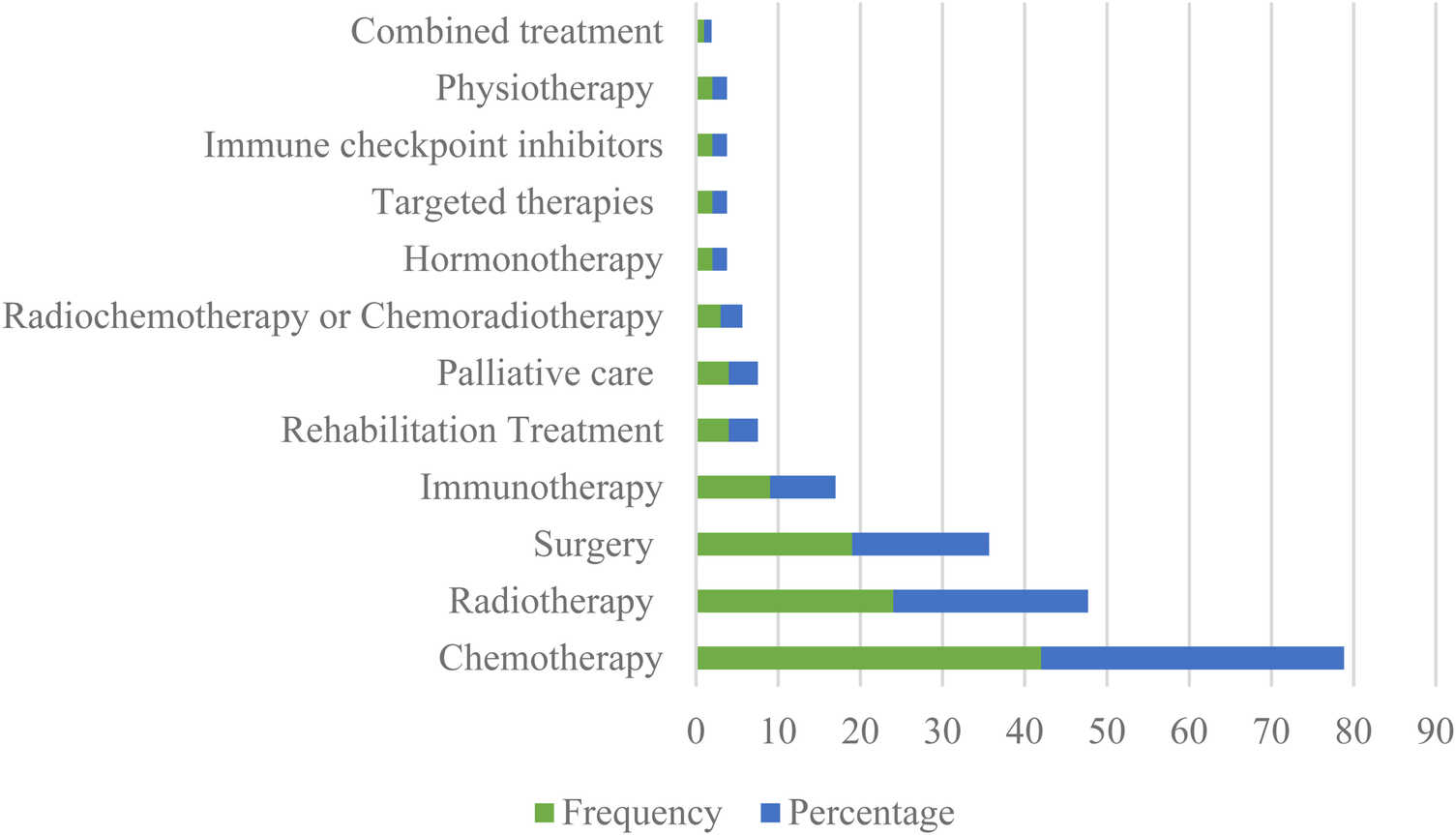
Frequency distribution of studies about ePROM in cancer according to the type of treatments.
ePRO questionnaires and measures
This systematic review identified a diverse range of tools, questionnaires and measurements designed to capture patients’ symptoms and outcomes in ePRO in cancer. According to Figure 6, the most frequently referenced measurements were the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) contributing valuable insights into the physical, psychological, and social functions of cancer patients (14–29) and the Patient Reported Outcome-Common Terminology Criteria for Adverse Events (PRO-CTCAE) (29–42), providing a comprehensive approach to monitoring symptoms associated with various cancer treatments, each cited in 16 and 14 studies across different cancer types and treatment modalities, respectively.
Figure 6
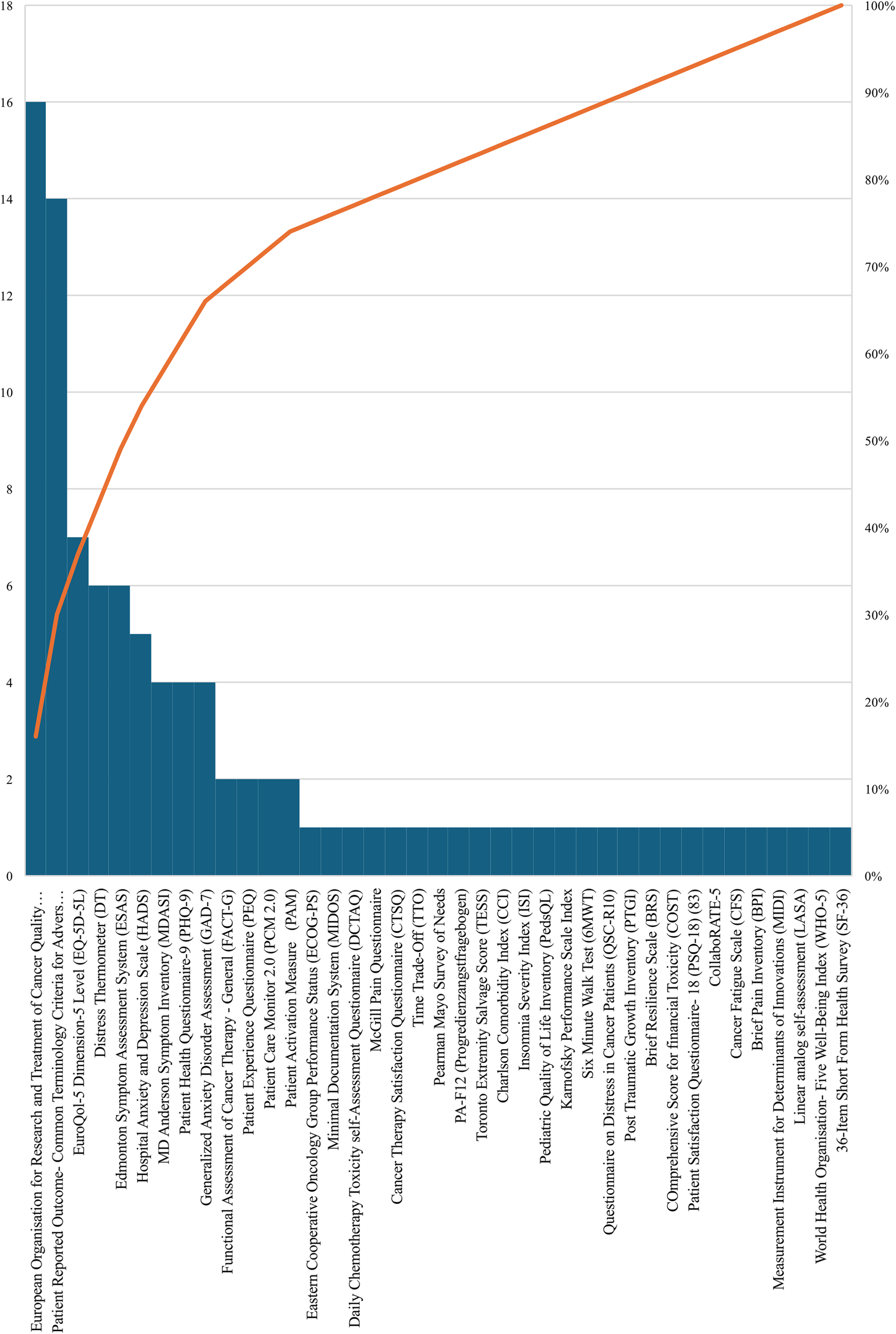
The distribution of ePRO measurements and tools in cancer.
Among other instruments, the EuroQol-5 Dimension-5 Level (EQ-5D-5l) was cited 7 times, providing significant perspectives on patients’ health status across multiple dimensions (43–49), followed by the Distress Thermometer (DT) (45, 48, 50–53) which provides a succinct yet powerful means for patients to express and quantify emotional distress levels on an 11-point Likert-type scale and the Edmonton Symptom Assessment System (ESAS), tailored for advanced cancer patients to express the intensity of symptoms like pain, fatigue, and anxiety, each referenced 6 times (50–55). Furthermore, the Hospital Anxiety and Depression Scale (HADS) is cited 5 times and categorizing scores into varying levels of anxiety and depression (23, 44, 45, 56, 57), while, the MD Anderson Symptom Inventory (MDASI) is referenced 4 times (44, 45, 47, 58), providing a comprehensive patient-reported outcome measure, investigating into the severity of multiple symptoms impacting cancer patients’ daily lives. Additionally, both the Patient Health Questionnaire-9 (PHQ-9) (11, 45, 55, 59) and the Generalized Anxiety Disorder Assessment (GAD-7) (11, 34, 55, 59) are each mentioned 4 times. Finally, the Functional Assessment of Cancer Therapy - General (FACT-G) (25, 58), the Patient Experience Questionnaire (PEQ) (16, 17), Patient Care Monitor 2.0 (PCM 2.0) (58, 60) and the Patient Activation Measure (PAM) (43, 61) are each referenced 2 times in our study. Table 1 shows a summary of ePRO instruments in oncology and their usage.
Table 1
| Number | Instrument/Questionnaire | Description |
|---|---|---|
| 1. | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) (14–29) | The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) is a validated instrument designed to measure the physical, psychological, and social functions of cancer patients. It includes multi-item scales and individual items to holistically evaluate patients’ quality of life. |
| 2. | Patient Reported Outcome- Common Terminology Criteria for Adverse Events (PRO- CTCAE) (29–42) | The National Cancer Institute's PRO-CTCAE Measurement System enables cancer patients to self-report symptomatic toxicities during clinical trials. It complements the CTCAE, the standard for adverse event reporting, by incorporating the patient perspective. The system's item library includes 124 items representing 78 toxicities. The PRO-CTCAE questionnaire consists of 41 items covering 22 symptoms commonly associated with cancer treatments, improving the precision of symptom monitoring. |
| 3. | EuroQol-5 Dimension-5 Level (EQ-5D-5l) (43–49) | The EuroQol-5 Dimension-5 Level (EQ-5D-5l) is a comprehensive instrument introduced to enhance sensitivity and reduce ceiling effects. It consists of two parts: a descriptive system with five dimensions and a visual analogue scale (VAS) for self-rated health. Patients provide a nuanced view of their health status across various dimensions. |
| 4. | Distress Thermometer (DT) (45, 48, 50–53) | The Distress Thermometer (DT) is a succinct yet powerful self-assessment tool utilizing an 11-point Likert-type scale represented graphically as a thermometer. Ranging from 0 (no distress) to 10 (extreme distress), patients use the DT to articulate and quantify their emotional distress levels effectively. |
| 5. | Edmonton Symptom Assessment System (ESAS) (50–55) | The Edmonton Symptom Assessment System (ESAS) is a practical self-reporting tool specifically designed for advanced cancer patients. Covering nine common symptoms, it allows patients to express the intensity of symptoms, including pain, fatigue, nausea, and anxiety. |
| 6. | Hospital Anxiety and Depression Scale (HADS) (23, 44, 45, 56, 57) | The Hospital Anxiety and Depression Scale (HADS) is a well-established tool for assessing anxiety and depression levels in cancer patients over the prior week. Comprising two 7-item subscales (HADS-D for depression and HADS-A for anxiety), it categorizes scores into normal, mild, moderate, and severe levels. |
| 7. | MD Anderson Symptom Inventory (MDASI) (44, 45, 47, 58) | The MD Anderson Symptom Inventory (MDASI) serves as a comprehensive patient-reported outcome measure, designed to assess the severity of multiple symptoms experienced by cancer patients. It encompasses physical and psychological symptoms, providing valuable insights into the impact on daily living. |
| 8. | Patient Health Questionnaire-9 (PHQ-9) (11, 45, 55, 59) | The Patient Health Questionnaire-9 (PHQ-9) is a valuable instrument used for diagnosing and monitoring the severity of depression. With nine questions, it includes a specific item screening for suicide ideation, offering a comprehensive view of the patient's mental health. |
| 9. | Generalized Anxiety Disorder Assessment (GAD-7) (11, 34, 55, 59) | The Generalized Anxiety Disorder Assessment (GAD-7) is a concise seven-item instrument designed to measure the severity of generalized anxiety disorder symptoms over the past two weeks. It provides valuable insights into the patient's anxiety levels. |
| 10. | Functional Assessment of Cancer Therapy—General (FACT-G) (25, 58) | The Functional Assessment of Cancer Therapy—General (FACT-G) is a widely used questionnaire for measuring health-related quality of life (HRQOL) in cancer patients. It covers four domains: physical, social/family, emotional, and functional well-being. Version 4 includes 27 items rated on a 0–4 scale, with a total score range of 0 to 108, where higher scores indicate better quality of life. |
| 11. | Patient Experience Questionnaire (PEQ) (16, 17) | The Patient Experience Questionnaire (PEQ) is a comprehensive survey aimed at gathering patient feedback on various aspects of their interaction with healthcare services. It covers communication, accessibility, coordination of care, and overall satisfaction, providing valuable insights for improvement. |
| 12. | Patient Care Monitor 2.0 (PCM 2.0) (58, 60) | The Patient Care Monitor 2.0 (PCM 2.0) is a robust instrument comprising 86 items for women and 80 items for men, rated on an 11-point scale. It covers six subscales, including general physical symptoms, treatment side effects, distress, despair, impaired performance, and impaired ambulation. PCM 2.0 offers a nuanced assessment of patients’ experiences. |
| 13. | Patient Activation Measure (PAM) (43, 61) | The Patient Activation Measure® (PAM) is a versatile survey assessing individuals’ knowledge, skills, and confidence in managing their own health. Available in multiple versions and languages, it provides insights into patients’ ability to take an active role in their health. |
| 14. | Eastern Cooperative Oncology Group Performance Status (ECOG-PS) (44) | The Eastern Cooperative Oncology Group Performance Status (ECOG-PS) is a reliable 6-point scale evaluating functional impairment in cancer patients. It ranges from fully active to restricted in physically strenuous activity, providing valuable insights into patients’ overall well-being. |
| 15. | Minimal Documentation System (MIDOS) (11) | The Minimal Documentation System (MIDOS) is a validated measure for self-assessment of pain and other symptoms in palliative care patients. It allows patients to articulate their experiences, facilitating effective communication with healthcare providers. |
| 16. | Daily Chemotherapy Toxicity self-Assessment Questionnaire (DCTAQ) (36) | The Daily Chemotherapy Toxicity self-Assessment Questionnaire (DCTAQ) is an 11-item self-reported tool specifically developed to assess 10 core chemotherapy-related symptoms. Patients provide information based on symptoms experienced in the past 24 h, offering real-time insights. |
| 17. | McGill Pain Questionnaire (62) | The McGill Pain Questionnaire is a comprehensive tool primarily consisting of three classes of word descriptors—sensory, affective, and evaluative. Patients use these descriptors to specify their subjective pain experience, providing detailed information for effective pain management. |
| 18. | Cancer Therapy Satisfaction Questionnaire (CTSQ) (27) | The Cancer Therapy Satisfaction Questionnaire (CTSQ) is a 21-item instrument assessing various domains related to patient satisfaction with cancer therapy. It covers expectations, feelings about side effects, adherence, convenience, and overall satisfaction, providing a comprehensive view of the patient experience. |
| 19. | Time Trade-Off (TTO) (27) | The Time Trade-Off (TTO) is a choice-based method for eliciting health state utility. It reflects the length of remaining life expectancy a person is willing to trade-off to avoid remaining in a sub-optimal health state, providing valuable insights into patients’ preferences. |
| 20. | Pearman Mayo Survey of Needs (48) | The Pearlman-Mayo Survey of Needs is a comprehensive survey developed to assess various dimensions of cancer patients’ needs. It categorizes needs into physical effects, social issues, psychological aspects, spiritual aspects, and other issues, providing a holistic view for tailored support. |
| 21. | PA-F12 (Progredienzangstfragebogen) (48) | Fear of Progression using the standardized PA-F12 (Progredienzangstfragebogen) questionnaire is a validated measure assessing the fear of disease progression in cancer patients. It covers various aspects of everyday life, offering valuable insights into patients’ emotional well-being. |
| 22. | Toronto Extremity Salvage Score (TESS) (48) | The Toronto Extremity Salvage Score (TESS) is a specialized physical disability measure developed for patients undergoing surgery for extremity tumours. It demonstrates superior measurement properties compared to other scales, offering detailed insights into patients’ physical well-being. |
| 23. | Charlson Comorbidity Index (CCI) (44) | The Charlson Comorbidity Index (CCI) is a widely used tool for classifying comorbid conditions that may influence mortality risk. It provides a comprehensive assessment of comorbidities, aiding in determining survival rates in patients with multiple health conditions. |
| 24. | Insomnia Severity Index (ISI) (45) | The Insomnia Severity Index (ISI) is a seven-item self-report questionnaire assessing the severity of insomnia disorder, its impact on daily life, and treatment response. It provides valuable insights into patients’ sleep-related experiences. |
| 25. | Pediatric Quality of Life Inventory (PedsQL) (63) | The Pediatric Quality of Life Inventory (PedsQL) is a brief measure assessing health-related quality of life in children and young people. It includes proxy reports from parents as well as self-reports from children, offering a comprehensive view of pediatric patients’ well-being. |
| 26. | Karnofsky Performance Scale Index (63) | The Karnofsky Performance Scale Index is a valuable assessment tool for functional impairment in cancer patients. It aids in comparing the effectiveness of different therapies and provides prognostic information based on patients’ performance status. |
| 27. | Six Minute Walk Test (6MWT) (63) | The Six Minute Walk Test (6MWT) is a sub-maximal exercise test designed to assess aerobic capacity and endurance in cancer patients. It provides insights into patients’ physical capabilities and overall fitness. |
| 28. | Questionnaire on Distress in Cancer Patients (QSC-R10) (26) | The Questionnaire on Distress in Cancer Patients (QSC-R10) is a self-reported measure assessing cancer-specific distress. With 10 items covering relevant aspects of everyday life, it offers a nuanced understanding of the psychosocial impact on cancer patients. |
| 29. | Post Traumatic Growth Inventory (PTGI) (57) | The Post Traumatic Growth Inventory (PTGI) is a 21-item scale assessing positive outcomes reported by individuals who have experienced traumatic events. It includes factors such as new possibilities, relating to others, personal strength, spiritual change, and appreciation of life, providing insights into patients’ psychological resilience. |
| 30. | Brief Resilience Scale (BRS) (57) | The Brief Resilience Scale (BRS) is designed to assess the perceived ability to bounce back or recover from stress. A unitary construct of resilience, it includes both positively and negatively worded items, offering a concise yet comprehensive view of patients’ resilience levels. |
| 31. | COmprehensive Score for financial Toxicity (COST) (57) | Financial toxicity, a burden of cancer care itself, is assessed using the COmprehensive Score for financial Toxicity (COST) tool. It is a patient-reported outcome measurement evaluating the financial impact of cancer care on quality of life. |
| 32. | Patient Satisfaction Questionnaire- 18 (PSQ-18) (64) | The Patient Satisfaction Questionnaire- 18 (PSQ-18) is a short-form instrument with 18 items, tapping into seven dimensions of satisfaction with medical care. It covers general satisfaction, technical quality, interpersonal manner, communication, financial aspects, time spent with the doctor, and accessibility and convenience. |
| 33. | CollaboRATE-5 (16) | CollaboRATE-5 is a 5-point Likert scale survey assessing the degree of shared decision-making between patients and healthcare providers. With responses ranging from no effort to every effort, it offers valuable insights into the collaborative nature of healthcare interactions. |
| 34. | Cancer Fatigue Scale (CFS) (55) | The Cancer Fatigue Scale (CFS) is a comprehensive tool assessing cancer-related fatigue. It comprises physical, affective, and cognitive subscales, providing a detailed understanding of the impact of cancer-related fatigue on patients’ lives. |
| 35. | Brief Pain Inventory (BPI) (55) | The Brief Pain Inventory (BPI) rapidly assesses pain severity and its impact on functioning. With a well-defined scale, it categorizes pain into mild, moderate, and severe, offering insights into patients’ pain experiences |
| 36. | Measurement Instrument for Determinants of Innovations (MIDI) (65) | A tool applicable pre- or post-introduction of an innovation, designed to enhance understanding of critical determinants affecting implementation, aiding in targeted innovation strategy. |
| 37. | Linear analog self-assessment (LASA) (39) | Utilized to gauge general well-being and specific factors (mood, pain, nausea, vomiting, appetite, breathlessness, physical activity) in patients undergoing therapy for malignant melanoma, small cell bronchogenic carcinoma (SCBC), or ovarian cancer. |
| 38. | World Health Organisation- Five Well-Being Index (WHO-5) (23) | A brief self-reported measure assessing current mental well-being with five positively worded items, rated on a 6-point Likert scale (0–5). |
| 39. | 36-Item Short Form Health Survey (SF-36) (23) | A comprehensive set of generic quality-of-life measures, including eight scales: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). |
Summary of ePRO instruments in oncology and their usage.
Table 2 provides a summary of disease-specific ePRO instruments in oncology and their usage. According to Table 2, our systematic review further highlighted the application of disease-specific instruments, Among these, the Quality of Life Questionnaire-Head and Neck 35 (QLQ-H&N35) was referenced in two studies (40, 66), indicating its relevance in assessing health-related quality of life in head-and-neck cancer patients. Similarly, the Quality of Life Questionnaire-Breast 23 (QLQ-BR23) European Organisation for Research appeared in two studies, emphasizing its importance in evaluating various aspects related to breast cancer, such as body image and systemic therapy side effects (16, 17). Another measurement in brain cancer was the EORTC QLQ-BN20 questionnaire (n = 1) for assessing the health-related quality of life (HRQoL) in brain cancer patients extracted from EORTIC QLQ to evaluate the quality of life of cancer patients (43). Additionally, the FACT-Melanoma (FACT-M) (49) and Functional Assessment of Cancer Therapy—Ovary (Fact-O) (57) instruments were each cited in one study, showcasing their utility in assessing melanoma and ovarian cancer-specific factors, respectively. Moreover, the FACT-B (Functional Assessment of Cancer Therapy—Breast) (60), European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Ovarian Cancer 28 (EORTC QLQ-OV28) (61), and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Colorectal 29 (EORTC QLQ-CR29) (61) were each referenced once, highlighting their role in evaluating health-related quality of life in breast, ovarian, and colorectal cancer patients, respectively. In terms of prostate cancer, the Expanded Prostate Cancer Index Composite (EPIC) and 8-item Functional Assessment of Cancer Therapy Advanced Prostate Symptom Index (FAPSI) have been used in monitoring prostate cancer (67).
Table 2
| Instrument/questionnaire | Description |
|---|---|
| Quality of Life Questionnaire-Head and Neck 35 (QLQ-H&N35) (40, 66) | The Quality of Life Questionnaire-Head and Neck 35 (QLQ-H&N35) is a disease-specific module assessing health-related quality of life in head-and-neck cancer patients. With seven multiple-item scales, it covers various aspects such as pain, swallowing ability, and social functioning. |
| Quality of Life Questionnaire-Breast 23 (QLQ-BR23) (16, 17) | The Quality of Life Questionnaire-Breast 23 (QLQ-BR23) is a specialized module incorporating five multi-item scales to assess various aspects related to breast cancer, including body image, sexual functioning, and systemic therapy side effects. |
| FACT-Melanoma (FACT-M) (49) | The FACT-Melanoma (FACT-M) is a validated quality-of-life instrument specifically designed for melanoma patients. Developed in 2005, it incorporates the FACT-G along with melanoma-specific items to assess patients’ well-being. FACT-M consists of 51 items, including 27 from the FACT-G subscale, a 16-item Melanoma Subscale (MS), and an 8-item Melanoma Surgery Scale (MSS). |
| Functional Assessment of Cancer Therapy—Ovary (Fact-O) (57) | The Fact-O (Functional Assessment of Cancer Therapy—Ovary) is a comprehensive instrument combining the FACT-G and an ovarian cancer-specific scale. It provides a detailed assessment of health-related quality of life, considering both general and ovarian cancer-specific factors. |
| Functional Assessment of Cancer Therapy—Breast (FACT-B) (60) | The FACT-B (Functional Assessment of Cancer Therapy—Breast) is a 37-item instrument measuring multiple domains of health-related quality of life in breast cancer patients. With an emphasis on brevity and patient values, it offers a nuanced understanding of the impact on patients’ well-being. |
| European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Ovarian Cancer 28 (EORTC QLQ-OV28) (61) | The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Ovarian Cancer 28 (EORTC QLQ-OV28) are specialized instrument for assessing the quality of life and symptoms in ovarian cancer patients. OV28 covers abdominal/GI symptoms. |
| European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Colorectal 29 (EORTC QLQ-CR29) (61) | The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Colorectal 29 (EORTC QLQ-CR29) demonstrates validity and reliability to supplement the QLQ-C30 in assessing patient-reported outcomes during treatment for colorectal cancer. It covers various aspects specific to colorectal cancer treatment. |
| Expanded Prostate Cancer Index Composite (EPIC) (67) | A comprehensive instrument evaluating patient function and bother post-prostate cancer treatment. Developed with input from a development cohort of localized prostate cancer patients and an expert panel of urological oncologists, radiation oncologists, survey researchers, and prostate cancer nurses. |
| 8-item Functional Assessment of Cancer Therapy Advanced Prostate Symptom Index (FAPSI) (67) | Symptoms/concerns endorsed at a frequency greater than chance probability (17%) were retained for the symptom index and called the FACT Advanced Prostate Symptom Index-8 (FAPSI-8): pain (three items), fatigue, weight loss, urinary difficulties (two items), and concern about the condition becoming worse. |
| European Organisation for Research and Treatment of Cancer Brain Tumor Questionnaire (EORTC QLQ-BN20) (43) | The European Organization for Research and Treatment of Cancer (EORTC) QLQ-BN20 is a QoL assessment specific to brain neoplasms. |
Summary of disease-specific ePRO instruments in oncology and their usage.
This comprehensive analysis not only shows the prevalence of specific instruments but also indicates the diverse dimensions of patients’ experiences and outcomes that researchers aim to capture in cancer-related studies. The significant use of these tools contributes to a complete understanding of the impact of cancer and its treatment on patients’ well-being, informing tailored interventions and improving the quality of care.
Discussion
In our study, we conducted a systematic review aimed at synthesizing literature into the landscape of ePROMs and optimising patient-centred oncology care. Additionally, a distribution analysis by year indicates a majority of publications from 2022 to 2023, with the United States as the leading source. Breast cancer emerges as the most frequent cancer, with chemotherapy being the primary treatment. This study explored the electronic Patient Reported Outcomes (ePRO) measurement tools and metrics in cancer care, aiming to provide a comprehensive analysis for future research and clinical practice guidelines.
Our findings emphasize the importance of using a broad set of tools to comprehensively assess the needs and experiences of cancer patients, indicating the necessity of personalizing assessments to accurately record the multidimensional impact of cancer diagnosis and treatment on patients’ quality of life and well-being. This study identifies differences, similarities, and implications for advancing cancer patient-centered care through a comparative analysis with existing studies.
Additionally, a wide range of other instruments were identified, including generic health-related quality of life measures such as the EuroQol-5 Dimension-5 Level (EQ-5D-5l) and disease-specific modules such as the Quality of Life Questionnaire-Head and Neck 35 (QLQ-H&N35) and Quality of Life Questionnaire-Breast 23 (QLQ-BR23). The European Organization for Research and Treatment Quality of Life Test (EORTC QLQ-C30) emerges as the most widely used measure, alongside other significant tools such as PRO-CTCAE, EQ-5D-5l, DT, ESAS, HADS, and MDASI, in evaluating cancer patients. These instruments encompassed domains such as physical functioning, symptoms, psychological distress, and treatment satisfaction, providing a comprehensive evaluation of cancer patients’ experiences. This study is aligned with Samit et al.'s study, which highlights the use of an electronic patient-reported outcome measurement system to improve distress management in oncology (60). Our study explores important tools like EORTC QLQ-C30 and PRO-CTCAE as reference instruments, emphasizing the multidimensional aspects of patients’ outcomes.
Tang et al.'s comparative effectiveness study on patient-reported outcome assessment methods in cancer care complements our work by focusing on improving patient outcomes (45). While our study identifies types of tools and metrics for measuring ePROs in cancer and describes different tools, it contributes to understanding electronic measurement in cancer care. Consistent with Lee et al.'s emphasis on technological approaches like ePRO in enhancing patient participation and treatment monitoring (68) and Wagner et al.'s focus on bringing Patient Reported Outcome Measures to practice for symptom screening in ambulatory cancer care (69), our study indicates the role of technology and the use of ePROs in advancing patient-centered care and symptom monitoring in oncology.
A diverse array of studies further enriches the discourse by exploring varied contexts and interventions related to ePROM implementation. Studies by Patt et al. (70) and Harper et al. (54) delve into the impact of ePROs on adverse events, cost of care, and symptom severity, providing valuable insights into the economic and clinical implications of ePRO integration across different cancer types and treatment modalities. In addition, Gressel et al. utilized the Patient Reported Outcomes Measurement Information System (PROMIS®) to increase referral to ancillary support services for severely symptomatic patients with gynecologic cancer (71). Moreover, investigations into the comparative effectiveness of ePROs against traditional assessment methods, as demonstrated by Warnecke et al. (11) and Moradian et al. (36), elucidate the potential advantages of leveraging technology in symptom management, treatment monitoring, and survivorship care.
In conclusion, our study contributes to a significant understanding of measurement instruments and ePRO measures in cancer care, drawing parallels with existing research to highlight key insights and implications for advancing patient-centered oncology care. By synthesizing diverse perspectives and methodologies, we aim to inform future research, clinical practice, and policy initiatives aimed at optimizing patient outcomes in oncology. Through interdisciplinary collaboration, innovative technology solutions, and patient-centered approaches, we advocate for evidence-based, holistic oncology practice, highlighting the importance of continued research and innovation in leveraging electronic patient-reported outcome (ePRO) measures to enhance cancer care delivery and patient outcomes.
Limitations
While this study offers valuable insights into electronic patient-reported outcome measures (ePROMs) in cancer care, it is important to acknowledge certain limitations. Firstly, the rapidly evolving nature of healthcare technology raises concerns about the relevance of our findings over time. Additionally, the subjective nature of tool selection across different healthcare settings introduces potential biases in our analysis. The scope of our review may also overlook niche instruments, highlighting the need for further exploration in future studies. Moreover, our survey was limited to published papers from three main databases, suggesting that this study serves as a foundational landscape for prospective research endeavours.
Secondly, while our review included the impact of ePROMs, we did not explore documents related to other technological advancements such as artificial intelligence and wearables. This represents a potential gap in our research that warrants consideration in future investigations. Thirdly, our study excluded other types of papers such as opinion pieces, editorials, and viewpoints, as well as publications in languages other than English. This may have limited the breadth of perspectives included in our analysis. Additionally, we did not solely focus on feasibility studies or patient perspective and experience tests, which could provide valuable insights into the practical implementation and user experience of ePROMs in cancer care. These considerations should be addressed in future research to provide a more comprehensive understanding of the role of technology in improving patient outcomes in oncology.
Implications
Our study presents several significant implications for both research and clinical practice in oncology. Firstly, the study underscores the pivotal role of electronic patient-reported outcome measures (ePROMs) in improving patient assessment accuracy and advancing patient-centered care delivery within oncology practice. By employing a diverse range of measurement tools tailored to different aspects of cancer care, healthcare providers can gain deeper insights into patients’ needs and experiences, enabling personalized care strategies that address individual patient concerns more effectively.
Moreover, the implications of the study extend beyond clinical practice to research endeavours in oncology. By elucidating the various system tools, questionnaires, and ePRO measurements utilized in cancer patient assessment, the review provides valuable insights into methodological approaches. This knowledge empowers researchers to make informed decisions regarding the selection of appropriate tools tailored to assess specific domains of interest, ultimately contributing to the advancement of knowledge in oncology.
Finally, the findings emphasize the importance of ongoing innovation and refinement in tool development to meet the evolving needs of cancer patients. As the landscape of cancer care continues to evolve, there is a need for continuous improvement and adaptation of ePROMs to ensure their relevance and effectiveness. This underscores the importance of investing in research and development efforts aimed at enhancing the usability, accuracy, and relevance of ePROMs in oncology care.
Conclusion
In conclusion, our systematic review enhances our understanding of the complex array of system tools, questionnaires, and electronic patient-reported outcome (ePRO) measurements utilized in cancer care. By synthesizing existing literature, we offer valuable insights into the methodologies and technologies shaping oncology research and practice. Moving forward, sustained efforts in tool development, validation, and implementation are crucial for comprehensively assessing and addressing the multifaceted needs of cancer patients, ultimately improving the standard of care and patient outcomes. Our review identifies frequently referenced tools like the EORTC QLQ-C30 and PRO-CTCAE, alongside less commonly utilized instruments, providing a comprehensive overview of available assessment tools. By utilizing a variety of instruments that capture different dimensions of patients’ experiences, clinicians and researchers can enhance their understanding of cancer patients’ quality of life, symptom burden, psychological well-being, and treatment satisfaction. Future research should focus on validating and refining existing instruments while also developing new tools to meet the evolving needs of cancer patients across diverse settings and populations.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SN: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MAl: Conceptualization, Supervision, Writing – review & editing. MAh: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Iran University of Medical Sciences for supporting this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Meirte J Hellemans N Anthonissen M Denteneer L Maertens K Moortgat P et al Benefits and disadvantages of electronic patient-reported outcome measures: systematic review. JMIR Perioper Med. (2020) 3(1):e15588. 10.2196/15588
2.
Di Maio M Basch E Denis F Fallowfield LJ Ganz PA Howell D et al The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO clinical practice guideline⋆. Ann Oncol. (2022) 33(9):878–92. 10.1016/j.annonc.2022.04.007
3.
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. (2006) 4(1):79. 10.1186/1477-7525-4-79
4.
Basch E Stover AM Schrag D Chung A Jansen J Henson S et al Clinical utility and user perceptions of a digital system for electronic patient-reported symptom monitoring during routine cancer care: findings from the PRO-TECT trial. JCO Clin Cancer Inform. (2020) 4:947–57. 10.1200/CCI.20.00081
5.
Valsecchi AA Battista V Terzolo S Dionisio R Lacidogna G Marino D et al Adoption of electronic patient-reported outcomes in cancer clinical practice: the point of view of Italian patients. ESMO Real World Data Digit Oncol. (2024) 3:100025. 10.1016/j.esmorw.2024.100025
6.
Valsangkar N Fernandez F Khullar O . Patient reported outcomes: integration into clinical practices. J Thorac Dis. (2020) 12(11):6940–6. 10.21037/jtd.2020.03.91
7.
Elkefi S Asan O . The impact of patient-centered care on cancer patients’ QOC, self-efficacy, and trust towards doctors: analysis of a national survey. J Patient Exp. (2023) 10:23743735231151533.
8.
Krzyszczyk P Acevedo A Davidoff EJ Timmins LM Marrero-Berrios I Patel M et al The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). (2018) 6(3-4):79–100.
9.
Payne A Horne A Bayman N Blackhall F Bostock L Chan C et al Patient and clinician-reported experiences of using electronic patient reported outcome measures (ePROMs) as part of routine cancer care. J Patient Rep Outcomes. (2023) 7(1):42. 10.1186/s41687-023-00544-4
10.
Bennett AV Jensen RE Basch E . Electronic patient-reported outcome systems in oncology clinical practice. CA Cancer J Clin. (2012) 62(5):336–47. 10.3322/caac.21150
11.
Warnecke E Salvador Comino MR Kocol D Hosters B Wiesweg M Bauer S et al Electronic patient-reported outcome measures (ePROMs) improve the assessment of underrated physical and psychological symptom burden among oncological inpatients. Cancers (Basel). (2023) 15(11):3029. 10.3390/cancers15113029
12.
Hughes J Flood T . Patients’ experiences of engaging with electronic patient reported outcome measures (PROMs) after the completion of radiation therapy for breast cancer: a pilot service evaluation. J Med Radiat Sci. (2023) 70(4):424–35. 10.1002/jmrs.711
13.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research ed). (2021) 372:n71.
14.
Riedl D Lehmann J Rothmund M Dejaco D Grote V Fischer MJ et al Usability of electronic patient-reported outcome measures for older patients with cancer: secondary analysis of data from an observational single center study. J Med Internet Res. (2023) 25:e49476. 10.2196/49476
15.
Graf J Sickenberger N Brusniak K Matthies LM Deutsch TM Simoes E et al Implementation of an electronic patient-reported outcome app for health-related quality of life in breast cancer patients: evaluation and acceptability analysis in a two-center prospective trial. J Med Internet Res. (2022) 24(2):e16128. 10.2196/16128
16.
Riis CL Stie M Bechmann T Jensen PT Coulter A Möller S et al ePRO-based individual follow-up care for women treated for early breast cancer: impact on service use and workflows. J Cancer Surviv. (2021) 15(4):485–96. 10.1007/s11764-020-00942-3
17.
Riis CL Jensen PT Bechmann T Möller S Coulter A Steffensen KD . Satisfaction with care and adherence to treatment when using patient reported outcomes to individualize follow-up care for women with early breast cancer—a pilot randomized controlled trial. Acta Oncol (Madr). (2020) 59(4):444–52. 10.1080/0284186X.2020.1717604
18.
Kikawa Y Hatachi Y Rumpold G Tokiwa M Takebe S Ogata T et al Evaluation of health-related quality of life via the computer-based health evaluation system (CHES) for Japanese metastatic breast cancer patients: a single-center pilot study. Breast Cancer. (2019) 26(2):255–9. 10.1007/s12282-018-0905-1
19.
Hartkopf AD Graf J Simoes E Keilmann L Sickenberger N Gass P et al Electronic-based patient-reported outcomes: willingness, needs, and barriers in adjuvant and metastatic breast cancer patients. JMIR Cancer. (2017) 3(2):e11. 10.2196/cancer.6996
20.
Graf J Simoes E Wißlicen K Rava L Walter CB Hartkopf A et al Willingness of patients with breast cancer in the adjuvant and metastatic setting to use electronic surveys (ePRO) depends on sociodemographic factors, health-related quality of life, disease status and computer skills. Geburtshilfe Frauenheilkd. (2016) 76(5):535–41. 10.1055/s-0042-105872
21.
Wintner LM Giesinger JM Zabernigg A Rumpold G Sztankay M Oberguggenberger AS et al Evaluation of electronic patient-reported outcome assessment with cancer patients in the hospital and at home. BMC Med Inform Decis Mak. (2015) 15:110. 10.1186/s12911-015-0230-y
22.
Wintner LM Giesinger JM Zabernigg A Sztankay M Meraner V Pall G et al Quality of life during chemotherapy in lung cancer patients: results across different treatment lines. Br J Cancer. (2013) 109(9):2301–8. 10.1038/bjc.2013.585
23.
Schougaard LM Larsen LP Jessen A Sidenius P Dorflinger L de Thurah A et al Ambuflex: tele-patient-reported outcomes (telePRO) as the basis for follow-up in chronic and malignant diseases. Qual Life Res. (2016) 25(3):525–34. 10.1007/s11136-015-1207-0
24.
Mayrbäurl B Giesinger JM Burgstaller S Piringer G Holzner B Thaler J . Quality of life across chemotherapy lines in patients with advanced colorectal cancer: a prospective single-center observational study. Support Care Cancer. (2016) 24(2):667–74. 10.1007/s00520-015-2828-0
25.
Holch P Absolom KL Henry AM Walker K Gibson A Hudson E et al Online symptom monitoring during pelvic radiation therapy: randomized pilot trial of the eRAPID intervention. Int J Radiat Oncol Biol. (2023) 115(3):664–76.
26.
Nordhausen T Lampe K Vordermark D Holzner B Al-Ali HK Meyer G et al An implementation study of electronic assessment of patient-reported outcomes in inpatient radiation oncology. J Patient Rep Outcomes. (2022) 6(1):77. 10.1186/s41687-022-00478-3
27.
Hofer S Hentschel L Richter S Blum V Kramer M Kasper B et al Electronic patient reported outcome (ePRO) measures in patients with soft tissue sarcoma (STS) receiving palliative treatment. Cancers (Basel). (2023) 15(4):1233. 10.3390/cancers15041233
28.
Zabernigg A Giesinger JM Pall G Gamper EM Gattringer K Wintner LM et al Quality of life across chemotherapy lines in patients with cancers of the pancreas and biliary tract. BMC Cancer. (2012) 12:390. 10.1186/1471-2407-12-390
29.
Cowan RA Suidan RS Andikyan V Rezk YA Einstein MH Chang K et al Electronic patient-reported outcomes from home in patients recovering from major gynecologic cancer surgery: a prospective study measuring symptoms and health-related quality of life. Gynecol Oncol. (2016) 143(2):362–6. 10.1016/j.ygyno.2016.08.335
30.
McMullan C Hughes SE Aiyegbusi OL Calvert M . Usability testing of an electronic patient-reported outcome system linked to an electronic chemotherapy prescribing and patient management system for patients with cancer. Heliyon. (2023) 9(6):e16453. 10.1016/j.heliyon.2023.e16453
31.
Macanovic B O'Reilly D Harvey H Hadi D Cloherty M O'Dea P et al A pilot project investigating the use of ONCOpatient®-an electronic patient-reported outcomes app for oncology patients. Digit Health. (2023) 9:20552076231185428. 10.1177/20552076231185428
32.
Rocque GB Dent DAN Ingram SA Caston NE Thigpen HB Lalor FR et al Adaptation of remote symptom monitoring using electronic patient-reported outcomes for implementation in real-world settings. JCO Oncol Pract. (2022) 18(12):e1943–e52. 10.1200/OP.22.00360
33.
Patt D Wilfong L Hudson KE Patel A Books H Pearson B et al Implementation of electronic patient-reported outcomes for symptom monitoring in a large multisite community oncology practice: dancing the Texas two-step through a pandemic. JCO Clin Cancer Inform. (2021) 5:615–21. 10.1200/CCI.21.00063
34.
Lapen K Sabol C Tin AL Lynch K Kassa A Mabli X et al Development and pilot implementation of a remote monitoring system for acute toxicity using electronic patient-reported outcomes for patients undergoing radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. (2021) 111(4):979–91. 10.1016/j.ijrobp.2021.07.1692
35.
Dickson NR Beauchamp KD Perry TS Roush A Goldschmidt D Edwards ML et al Real-world use and clinical impact of an electronic patient-reported outcome tool in patients with solid tumors treated with immuno-oncology therapy. J Patient Rep Outcomes. (2024) 8(1):23. 10.1186/s41687-024-00700-4
36.
Moradian S Ghasemi S Boutorabi B Sharifian Z Dastjerdi F Buick C et al Development of an eHealth tool for capturing and analyzing the immune-related adverse events (irAEs) in cancer treatment. Cancer Inform. (2023) 22:11769351231178587. 10.1177/11769351231178587
37.
Iivanainen S Alanko T Peltola K Konkola T Ekström J Virtanen H et al ePROs in the follow-up of cancer patients treated with immune checkpoint inhibitors: a retrospective study. J Cancer Res Clin Oncol. (2019) 145(3):765–74. 10.1007/s00432-018-02835-6
38.
Helissey C Parnot C Rivière C Duverger C Schernberg A Becherirat S et al Effectiveness of electronic patient reporting outcomes, by a digital telemonitoring platform, for prostate cancer care: the protecty study. Front Digit Health. (2023) 5:1104700. 10.3389/fdgth.2023.1104700
39.
Niska JR Halyard MY Tan AD Atherton PJ Patel SH Sloan JA . Electronic patient-reported outcomes and toxicities during radiotherapy for head-and-neck cancer. Qual Life Res. (2017) 26(7):1721–31. 10.1007/s11136-017-1528-2
40.
Peltola MK Lehikoinen JS Sippola LT Saarilahti K Mäkitie AA . A novel digital patient-reported outcome platform for head and neck oncology patients-A pilot study. Clin Med Insights Ear Nose Throat. (2016) 9:1–6. 10.4137/CMENT.S40219
41.
Tolstrup LK Bastholt L Dieperink KB Möller S Zwisler AD Pappot H . The use of patient-reported outcomes to detect adverse events in metastatic melanoma patients receiving immunotherapy: a randomized controlled pilot trial. J Patient Rep Outcomes. (2020) 4(1):88. 10.1186/s41687-020-00255-0
42.
Stormoen DR Baeksted C Taarnhøj GA Johansen C Pappot H . Patient reported outcomes interfering with daily activities in prostate cancer patients receiving antineoplastic treatment. Acta Oncol (Madr). (2021) 60(4):419–25. 10.1080/0284186X.2021.1881818
43.
Gvozdanovic A Jozsa F Fersht N Grover PJ Kirby G Kitchen N et al Integration of a personalised mobile health (mHealth) application into the care of patients with brain tumours: proof-of-concept study (IDEAL stage 1). BMJ Surg Interv Health Technol. (2022) 4(1):e000130.
44.
Zhang Y Li Z Pang Y He Y He S Su Z et al Changes in patient-reported health Status in advanced cancer patients from a symptom management clinic: a longitudinal study conducted in China. J Oncol. (2022) 2022:7531545.
45.
Tang L He Y Pang Y Su Z Li J Zhang Y et al Implementing symptom management follow-up using an electronic patient-reported outcome platform in outpatients with advanced cancer: longitudinal single-center prospective study. JMIR Form Res. (2022) 6(5):e21458. 10.2196/21458
46.
Convill J Blackhall F Yorke J Faivre-Finn C Gomes F . The role of electronic patient-reported outcome measures in assessing smoking Status and cessation for patients with lung cancer. Oncol Ther. (2022) 10(2):481–91. 10.1007/s40487-022-00210-7
47.
Williams LA Whisenant MS Mendoza TR Peek AE Malveaux D Griffin DK et al Measuring symptom burden in patients with cancer during a pandemic: the MD anderson symptom inventory for COVID-19 (MDASI-COVID). J Patient Rep Outcomes. (2023) 7(1):48. 10.1186/s41687-023-00591-x
48.
Geese F Kaufmann S Sivanathan M Sairanen K Klenke F Krieg AH et al Exploring the potential of electronic patient-reported outcome measures to inform and assess care in sarcoma centers: a longitudinal multicenter pilot study. Cancer Nurs. (2023). 10.1097/NCC.0000000000001248. [Epub ahead of print].
49.
Tolstrup LK Pappot H Bastholt L Möller S Dieperink KB . Impact of patient-reported outcomes on symptom monitoring during treatment with checkpoint inhibitors: health-related quality of life among melanoma patients in a randomized controlled trial. J Patient Rep Outcomes. (2022) 6(1):8. 10.1186/s41687-022-00414-5
50.
Brant JM Hirschman KB Keckler SL Dudley WN Stricker C . Patient and provider use of electronic care plans generated from patient-reported outcomes. Oncol Nurs Forum. (2019) 46(6):715–26. 10.1188/19.ONF.715-726
51.
Girgis A Bamgboje-Ayodele A Rincones O Vinod SK Avery S Descallar J et al Stepping into the real world: a mixed-methods evaluation of the implementation of electronic patient reported outcomes in routine lung cancer care. J Patient Rep Outcomes. (2022) 6(1):70. 10.1186/s41687-022-00475-6
52.
Ayodele A Avery S Pearson J Mak M Smith K Rincones O et al Adapting an integrated care pathway for implementing electronic patient reported outcomes assessment in routine oncology care: lessons learned from a case study. J Eval Clin Pract. (2022) 28(6):1072–83.
53.
Girgis A Durcinoska I Arnold A Descallar J Kaadan N Koh E-S et al Web-based patient-reported outcome measures for personalized treatment and care (PROMPT-care): multicenter pragmatic nonrandomized trial. J Med Internet Res. (2020) 22(10):e19685. 10.2196/19685
54.
Harper A Maseja N Parkinson R Pakseresht M McKillop S Henning JW et al Symptom severity and trajectories among adolescent and young adult patients with cancer. JNCI Cancer Spectr. (2023) 7(6):pkad049. 10.1093/jncics/pkad049
55.
Howell D Rosberger Z Mayer C Faria R Hamel M Snider A et al Personalized symptom management: a quality improvement collaborative for implementation of patient reported outcomes (PROs) in ‘real-world’ oncology multisite practices. J Patient Rep Outcomes. (2020) 4(1):47. 10.1186/s41687-020-00212-x
56.
Licht T Nickels A Rumpold G Holzner B Riedl D . Evaluation by electronic patient-reported outcomes of cancer survivors’ needs and the efficacy of inpatient cancer rehabilitation in different tumor entities. Support Care Cancer. (2021) 29(10):5853–64. 10.1007/s00520-021-06123-x
57.
Hlubocky FJ Daugherty CK Peppercorn J Young K Wroblewski KE Yamada SD et al Utilization of an electronic patient-reported outcome platform to evaluate the psychosocial and quality-of-life experience among a community sample of ovarian cancer survivors. JCO Clin Cancer Inform. (2022) 6:e2200035. 10.1200/CCI.22.00035
58.
Abernethy AP Ahmad A Zafar SY Wheeler JL Reese JB Lyerly HK . Electronic patient-reported data capture as a foundation of rapid learning cancer care. Med Care. (2010) 48(6):S32–8. 10.1097/MLR.0b013e3181db53a4
59.
Lee J Jung JH Kim WW Kang B Woo J Rim H-D et al Short-term serial assessment of electronic patient-reported outcome for depression and anxiety in breast cancer. BMC Cancer. (2021) 21(1):1065. 10.1186/s12885-021-08771-y
60.
Smith SK Rowe K Abernethy AP . Use of an electronic patient-reported outcome measurement system to improve distress management in oncology. Palliat Support Care. (2014) 12(1):69–73. 10.1017/S1478951513000345
61.
Ravn S Thaysen HV Verwaal VJ Seibæk L Iversen LH . Cancer follow-up supported by patient-reported outcomes in patients undergoing intended curative complex surgery for advanced cancer. J Patient Rep Outcomes. (2021) 5(1):120. 10.1186/s41687-021-00391-1
62.
Judge MKM Luedke R Dyal BW Ezenwa MO Wilkie DJ . Clinical efficacy and implementation issues of an electronic pain reporting device among outpatients with cancer. Support Care Cancer. (2021) 29(9):5227–35. 10.1007/s00520-021-06075-2
63.
Riedl D Licht T Nickels A Rothmund M Rumpold G Holzner B et al Large improvements in health-related quality of life and physical fitness during multidisciplinary inpatient rehabilitation for pediatric cancer survivors. Cancers (Basel). (2022) 14(19):4855. 10.3390/cancers14194855
64.
Sprave T Pfaffenlehner M Stoian R Christofi E Rühle A Zöller D et al App-controlled treatment monitoring and support for patients with head and neck cancer undergoing radiotherapy: results from a prospective randomized controlled trial. J Med Internet Res. (2023) 25:e46189. 10.2196/46189
65.
Schepers SA Sint Nicolaas SM Haverman L Wensing M van Meeteren AYN S Veening MA et al Real-world implementation of electronic patient-reported outcomes in outpatient pediatric cancer care. Psychooncology. (2017) 26(7):951–9. 10.1002/pon.4242
66.
Pollom EL Wang E Bui TT Ognibene G von Eyben R Divi V et al A prospective study of electronic quality of life assessment using tablet devices during and after treatment of head and neck cancers. Oral Oncol. (2015) 51(12):1132–7. 10.1016/j.oraloncology.2015.10.003
67.
Tran C Dicker A Leiby B Gressen E Williams N Jim H . Utilizing digital health to collect electronic patient-reported outcomes in prostate cancer: single-arm pilot trial. J Med Internet Res. (2020) 22(3):e12689. 10.2196/12689
68.
Lee M Kang D Kim S Lim J Yoon J Kim Y et al Who is more likely to adopt and comply with the electronic patient-reported outcome measure (ePROM) mobile application? A real-world study with cancer patients undergoing active treatment. Support Care Cancer. (2022) 30(1):659–68. 10.1007/s00520-021-06473-6
69.
Wagner LI Schink J Bass M Patel S Diaz MV Rothrock N et al Bringing PROMIS to practice: brief and precise symptom screening in ambulatory cancer care. Cancer. (2015) 121(6):927–34. 10.1002/cncr.29104
70.
Patt DA Patel AM Bhardwaj A Hudson KE Christman A Amondikar N et al Impact of remote symptom monitoring with electronic patient-reported outcomes on hospitalization, survival, and cost in community oncology practice: the Texas two-step study. JCO Clin Cancer Inform. (2023) 7:e2300182. 10.1200/CCI.23.00182
71.
Gressel GM Dioun SM Richley M Lounsbury DW Rapkin BD Isani S et al Utilizing the patient reported outcomes measurement information system (PROMIS®) to increase referral to ancillary support services for severely symptomatic patients with gynecologic cancer. Gynecol Oncol. (2019) 152(3):509–13. 10.1016/j.ygyno.2018.10.042
72.
Oldenburger E Isebaert S Coolbrandt A Van Audenhove C Haustermans K . The use of electronic patient reported outcomes in follow-up after palliative radiotherapy: a survey study in Belgium. PEC Innovation. (2023) 3:100243. 10.1016/j.pecinn.2023.100243
73.
Mohseni M Ayatollahi H Arefpour AM . Electronic patient-reported outcome (ePRO) application for patients with prostate cancer. PLoS One. (2023) 18(8):e0289974. 10.1371/journal.pone.0289974
74.
Zhang L Zhang X Shen L Zhu D Ma S Cong L . Efficiency of electronic health record assessment of patient-reported outcomes after cancer immunotherapy: a randomized clinical trial. JAMA Netw Open. (2022) 5(3):e224427. 10.1001/jamanetworkopen.2022.4427
75.
Wickline M Wolpin S Cho S Tomashek H Louca T Frisk T et al Usability and acceptability of the electronic self-assessment and care (eSAC) program in advanced ovarian cancer: a mixed methods study. Gynecol Oncol. (2022) 167(2):239–46.
76.
Daly B Nicholas K Flynn J Silva N Panageas K Mao JJ et al Analysis of a remote monitoring program for symptoms among adults with cancer receiving antineoplastic therapy. JAMA Netw Open. (2022) 5(3):e221078. 10.1001/jamanetworkopen.2022.1078
77.
Boeke S Hauth F Fischer SG Lautenbacher H Bizu V Zips D et al Acceptance of physical activity monitoring in cancer patients during radiotherapy, the GIROfit phase 2 pilot trial. Tech Innov Patient Support Radiat Oncol. (2022) 22:16–21. 10.1016/j.tipsro.2022.03.004
78.
Takala L Kuusinen T-E Skyttä T Kellokumpu-Lehtinen P-L Bärlund M . Electronic patient-reported outcomes during breast cancer adjuvant radiotherapy. Clin Breast Cancer. (2021) 21(3):e252–e70. 10.1016/j.clbc.2020.10.004
79.
Strachna O Cohen MA Allison MM Pfister DG Lee NY Wong RJ et al Case study of the integration of electronic patient-reported outcomes as standard of care in a head and neck oncology practice: obstacles and opportunities. Cancer. (2021) 127(3):359–71. 10.1002/cncr.33272
80.
Peltola MK Poikonen-Saksela P Mattson J Parkkari T . A novel digital patient-reported outcome platform (noona) for clinical use in patients with cancer: pilot study assessing suitability. JMIR Form Res. (2021) 5(5):e16156. 10.2196/16156
81.
Generalova O Roy M Hall E Shah SA Cunanan K Fardeen T et al Implementation of a cloud-based electronic patient-reported outcome (ePRO) platform in patients with advanced cancer. J Patient Rep Outcomes. (2021) 5(1):91. 10.1186/s41687-021-00358-2
82.
Doolin JW Berry JL Forbath NS Tocci NX Dechen T Li S et al Implementing electronic patient-reported outcomes for patients with new oral chemotherapy prescriptions at an academic site and a community site. JCO Clin Cancer Inform. (2021) 5:631–40. 10.1200/CCI.20.00191
83.
Absolom K Warrington L Hudson E Hewison J Morris C Holch P et al Phase III randomized controlled trial of eRAPID: eHealth intervention during chemotherapy. J Clin Oncol. (2021) 39(7):734–47. 10.1200/JCO.20.02015
84.
Zylla DM Gilmore GE Steele GL Eklund JP Wood CM Stover AM et al Collection of electronic patient-reported symptoms in patients with advanced cancer using epic MyChart surveys. Support Care Cancer. (2020) 28(7):3153–63. 10.1007/s00520-019-05109-0
85.
Sandhu S King Z Wong M Bissell S Sperling J Gray M et al Implementation of electronic patient-reported outcomes in routine cancer care at an academic center: identifying opportunities and challenges. JCO Oncol Pract. (2020) 16(11):e1255–e63. 10.1200/OP.20.00357
86.
Richards HS Blazeby JM Portal A Harding R Reed T Lander T et al A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery. BMC Cancer. (2020) 20(1):543. 10.1186/s12885-020-07027-5
87.
Mowlem FD Sanderson B Platko JV Byrom B . Optimizing electronic capture of patient-reported outcome measures in oncology clinical trials: lessons learned from a qualitative study. J Comp Eff Res. (2020) 9(17):1195–204. 10.2217/cer-2020-0143
88.
Karamanidou C Maramis C Stamatopoulos K Koutkias V . Development of a ePRO-based palliative care intervention for cancer patients: a participatory design approach. Stud Health Technol Inform. (2020) 270:941–5.
89.
Dronkers EAC Baatenburg de Jong RJ van der Poel EF Sewnaik A Offerman MPJ . Keys to successful implementation of routine symptom monitoring in head and neck oncology with “healthcare monitor” and patients’ perspectives of quality of care. Head Neck. (2020) 42(12):3590–600. 10.1002/hed.26425
90.
Biran N Anthony Kouyaté R Yucel E McGovern GE Schoenthaler AM Durling OG et al Adaptation and evaluation of a symptom-monitoring digital health intervention for patients with relapsed and refractory multiple myeloma: pilot mixed-methods implementation study. JMIR Form Res. (2020) 4(11):e18982. 10.2196/18982
91.
Warrington L Absolom K Holch P Gibson A Clayton B Velikova G . Online tool for monitoring adverse events in patients with cancer during treatment (eRAPID): field testing in a clinical setting. BMJ Open. (2019) 9(1):e025185. 10.1136/bmjopen-2018-025185
92.
Krogstad H Sundt-Hansen SM Hjermstad MJ Hågensen LÅ Kaasa S Loge JH et al Usability testing of EirV3—a computer-based tool for patient-reported outcome measures in cancer. Support Care Cancer. (2019) 27(5):1835–44. 10.1007/s00520-018-4435-3
93.
Avery KNL Richards HS Portal A Reed T Harding R Carter R et al Developing a real-time electronic symptom monitoring system for patients after discharge following cancer-related surgery. BMC Cancer. (2019) 19(1):463. 10.1186/s12885-019-5657-6
94.
Lucas SM Kim T-K Ghani KR Miller DC Linsell S Starr J et al Establishment of a web-based system for collection of patient-reported outcomes after radical prostatectomy in a statewide quality improvement collaborative. Urology. (2017) 107:96–102. 10.1016/j.urology.2017.04.058
95.
Holch P Warrington L Bamforth LCA Keding A Ziegler LE Absolom K et al Development of an integrated electronic platform for patient self-report and management of adverse events during cancer treatment. Ann Oncol. (2017) 28(9):2305–11. 10.1093/annonc/mdx317
96.
Absolom K Holch P Warrington L Samy F Hulme C Hewison J et al Electronic patient self-reporting of adverse-events: patient information and aDvice (eRAPID): a randomised controlled trial in systemic cancer treatment. BMC Cancer. (2017) 17(1):318. 10.1186/s12885-017-3303-8
97.
Duregger K Hayn D Nitzlnader M Kropf M Falgenhauer M Ladenstein R et al Electronic patient reported outcomes in paediatric oncology—applying Mobile and near field communication technology. Stud Health Technol Inform. (2016) 223:281–8.
Appendix
Table A1
| Database | Search Strategy |
|---|---|
| Scopus | TITLE-ABS-KEY (((“electronic patient-reported outcome” OR “electronic patient reported outcome” OR “electronic patient-reported outcome measure” OR “electronic patient reported outcome measure” OR “ePRO” OR “electronic Patient-Self Reporting”) AND (“neoplasms” OR “Oncology” OR “Cancer” OR “tumor”))) AND (LIMIT-TO (DOCTYPE, “re”) OR LIMIT-TO (DOCTYPE, “ar”)) AND [(LIMIT-TO (LANGUAGE, “English”)]) |
| Web of Science | ((“electronic patient-reported outcome” OR “electronic patient reported outcome” OR “electronic patient-reported outcome measure” OR “electronic patient reported outcome measure” OR “ePRO” OR “electronic Patient-Self Reporting”) AND (“neoplasms” OR “Oncology” OR “Cancer” OR “tumor”)) (Topic) and Review Article or Article (Document Types) and English (Languages) |
| PubMed | ((“electronic patient-reported outcome"[Title/Abstract] OR “electronic patient reported outcome” OR “electronic patient-reported outcome measure"[Title/Abstract] OR “electronic patient reported outcome measure"[Title/Abstract] OR “ePRO"[Title/Abstract] OR “electronic Patient-Self Reporting"[Title/Abstract]) AND (“neoplasms"[Title/Abstract] OR “Oncology"[Title/Abstract] OR “Cancer"[Title/Abstract] OR “Tumor"[Title/Abstract])) Filter: English |
Search strategy.
Table A2
| Number | Author | Year | Country/State | Objective | Participants | System tools and metrics | Cancer type | Type of treatment |
|---|---|---|---|---|---|---|---|---|
| 1. | Dickson, et al. (35) | 2024 | USA | To evaluate the utilization and clinical impact of an electronic patient-reported outcome (ePRO) tool in patients with solid tumours undergoing immuno-oncology (IO) therapy | 538 patients in the historical control (HC) cohort, and 1,014 patients in the ePRO cohort, with 319 ePRO users and 695 non-users | Electronic platform for patients to report health status, training for healthcare providers, PRO-Common Terminology Criteria for Adverse Events (PRO-CTCAE) questionnaires, symptom tracking, real-time alerts | Solid tumours [non-small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, bladder cancer, head and neck cancer] | Immuno-oncology therapy |
| 2. | Riedl et al. (14) | 2023 | Austria | To investigate the ability of adult patients of different age ranges to complete routine ePRO assessments and to identify factors associated with completion and the need for assistance. | 5,571 patients (mean age: 60.3 years, range 18 to 93 years) in Inpatient Rehabilitation setting | Electronic assessment of patient-reported outcomes (ePRO), European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), Hospital Anxiety and Depression Scale | Various cancer types (breast, hemoblastoses, prostate, uterine/ovarian, colon, head/neck, lung, stomach, rectum) | Rehabilitation Treatment |
| 3. | Sprave et al. (64) | 2023 | Germany |
|
100 enrolled, 93 evaluable | Electronic patient-reported outcomes were collected via a dedicated mobile app (myoncare, Oncare GmbH) using the European Organisation for Research and Treatment of Cancer Core Quality of Life Questionnaire (QLQ-C30), the head and neck cancer module (H&N35), and the validated Patient Satisfaction Questionnaire Short Form (PSQ-18). | Head and neck cancer (HNC) (Oropharynx, Larynx, Hypopharynx, Nasopharynx, and Parotid glands) | Radiotherapy (Definitive radiotherapy, Adjuvant radiotherapy, Reirradiation, and Palliative radiotherapy) |
| 4. | Patt et al. (70) | 2023 | United States | To evaluate the impact of electronic patient-reported outcomes (ePROs) on adverse events and total cost of care among patients with metastatic cancer enrolled in the Centers for Medicare & Medicaid Services Oncology Care Model (OCM) program | Initially, 1,630 patients with cancer; 831 met the selection criteria, 458 matched patients were identified | Utilized HIPAA-compliant software, Navigating Cancer (NC), Medicare claims data, Adverse Events (AEs), Total Cost of Care Metrics, Outcome Metrics | Metastatic Breast, Chronic leukaemia, Lung, Lymphoma, Multiple myeloma, Prostate, and Small intestine/colorectal cancer | Radiotherapy, and Surgery |
| 5. | Harper, et al. (54) | 2023 | Canada |
|
937 adolescents and young adults; 473 at diagnosis, 322 at 1 year after diagnosis | Edmonton Symptom Assessment System-Revised (ESAS-r) tool measuring:—Pain—Tiredness—Drowsiness—Nausea—Lack of appetite—Shortness of breath—Depression—Anxiety—Overall well-being | Various cancer types, including Breast, Central nervous system, Endocrine, Gastrointestinal, Genitourinary, Gynecologic, Head and neck, Hematologic, Intrathoracic, Melanoma, Sarcoma, etc. | Chemoradiotherapy, Chemotherapy, and Radiotherapy |
| 6. | Oldenburger et al. (72) | 2023 | Belgium | Explore the opinions of healthcare providers (HCP) active in radiation oncology in Belgium on using ePROMs for symptom follow-up after palliative radiotherapy. | 128 respondents, including Radiation Oncologists, Nurses, Radiation Therapy Technologists, Clinical Support Managers, and Quality Managers. | PROMS questions: covering symptoms, side effects of treatment, quality of life, | N/A | Palliative radiotherapy |
| 7. | LA et al. (47) | 2023 | USA | To rapidly develop, launch through an electronic patient portal, and provide initial validation for a PRO measure of COVID-19 symptom burden in patients with cancer. | 600 participants diagnosed with both cancer and COVID-19 | MDASI-COVID questionnaire, advanced psychometric validation methods
|
Various types of cancer in individuals also diagnosed with COVID-19 | N/A |
| 8. | Warnecke et al. (11) | 2023 | Germany | To compare the information provided by ePROMs and nurse-reported assessments to identify overlaps and differences in the assessment of current symptom burden among oncological inpatients. | 230 inpatients | Patient Health Questionnaire 8 (PHQ-8), Generalized Anxiety Disorder Scale 7 (GAD-7), Hornheider Screening Instrument (HSI), Minimal Documentation System 2 (MIDOS2) | Soft-tissue sarcoma, Lung, Uveal melanoma, Gastrointestinal, Hepatobiliary and pancreatic cancer | Palliative care |
| 9. | Moradian et al. (36) | 2023 | Canada | To develop an eHealth platform for cancer patients to manage symptoms and interact with healthcare professionals. | N/A | Common Terminology Criteria for Adverse Events (CTCAE), PRO-CTCAE library, DCTAQ, international guidelines, irAEs observed during clinical trials | Various types of cancer in individuals | Immunotherapy |
| 10. | Mohseni et al. (73) | 2023 | Iran | Develop a smartphone-based app for electronic reporting of outcomes by patients with prostate cancer | Specialists (n = 15), Patients (n = 21) | Post-study system usability questionnaire (PSSUQ) Quality of life questionnaire | Prostate cancer | All types of treatment |
| 11. | McMullan et al. (30) | 2023 | United Kingdom | To assess the usability of the ChemoPRO® app among people with lived experience of cancer. | 10 participants with lived experience of cancer. | Included two Patient-Reported Outcome Measures (PROMs): Euroqol EQ5D5l and PRO-CTCAE™. | Leukaemia, Breast, Multiple, Myeloma, Stomach, Bowel, Rectal, Sarcoma | Chemotherapy |
| 12. | Macanovic et al. (31) | 2023 | Ireland | To investigate the feasibility of implementing a remote patient monitoring system using an electronic patient-reported outcomes (ePROs) platform in a tertiary cancer centre in the Republic of Ireland. | 13 patients and 5 staff | Symptom questionnaires through an ePRO mobile phone application (app), NCI-PRO-CTCAE™ assessment scale, patient-facing interface, clinician-facing interface, medication reminders, symptom tracking, communication with the care team | Breast Melanoma Colorectal Lung | Oral Chemotherapy |
| 13. | Holch et al. (25) | 2023 | United Kingdom | Establish feasibility and acceptability of the eRAPID system | 167 (73.2% consented and randomized) | FACT-G (overall and PWB score), EORTC QLQ-C30 summary score, QLQ-C30 global health/QOL score, and EQ5D-VAS | Prostate, lower gastrointestinal, and gynaecological cancers | Chemotherapy |
| 14. | Silvia Hofer et al. (27) | 2023 | Switzerland | To assess the impact of treatment on health-related quality of life (HRQoL) and patient-reported outcomes in palliative STS treatment. | The study was terminated early due to the COVID-19 pandemic, and only 11 patients were randomized and 10 evaluated. | EORTC QLQ-C30, Cancer Therapy Satisfaction Questionnaire (CTSQ), Time Trade-off (TTO) method. | Soft Tissue Sarcoma (STS) | Chemotherapy |
| 15. | Helissey. et al (38) | 2023 | Helsinki and France | Effectiveness of electronic patient reporting outcomes, by a digital telemonitoring platform, for prostate cancer care: the Protecty study | 61 patients |
|
Prostate cancer | Chemotherapy, Hormonotherapy, Combined treatment |
| 16. | Geese, et al. (48) | 2023 | Switzerland | Explore the potential of ePROMs in clinical practice for assessing quality of life, functionality, needs, fear of progression, distress, and care quality in sarcoma centres | 55 patients from three sarcoma centres | EQ-5D-5l for quality of life, PMSN for unmet needs, NCCN Distress Thermometer (DT) for distress levels, PA-F12 for fear of progression, Toronto extremity salvage score (TESS) for physical functionality | Sarcoma | Surgery, Radiotherapy, Chemotherapy |
| 17. | Gvozdanovic et al. (43) | 2022 | United Kingdom | To assess the feasibility of Vinehealth integration into brain tumour care | Six patients were initially recruited, and four engaged with the Vinehealth application throughout the study period. | Symptom, activity, well-being, medication logs; EORTC QLQ-BN20 (European Organisation for Research and Treatment of Cancer Brain Tumor Questionnaire), EQ-5D-5l (EuroQol 5 dimensions of health questionnaire), and PAM (Patient Activation Measure). | Brain tumours include glioblastoma, metastasis from triple-negative breast carcinoma, and haemangioblastoma. | Surgery, chemoradiotherapy, and radiotherapy |
| 18. | Zhang et al. (44) | 2022 | China | To track patient-reported health status changes over time in Chinese advanced cancer patients and explore the risk factors affecting their health status. | 103 patients completed a baseline survey (T = 0) and two follow-up surveys (T1 = 14 days, T2 = 28 days). | EQ-5D-5l instrument (including EQ-VAS) for assessing health status.—MD Anderson Symptom Inventory (MDASI-C) for assessing symptom severity and life interference.—Hospital Anxiety and Depression Scale (HADS) for measuring anxiety and depression symptoms.—Case report form (CRF) for collecting demographic and medical data.—Charlson Comorbidity Index (CCI) for evaluating complications.—Eastern Cooperative Oncology Group Performance Status (ECOG-PS) scale for measuring functional status. | Advanced stages of cancers, including Stage III without curative treatment chance and Stage IV) Lung, gastric, oesophageal, liver, colorectal, and breast cancer | N/A |
| 19. | Zhang et al (74) | 2022 | China | To compare the efficiency between electronic patient-reported outcomes (ePRO) and traditional follow-up models in cancer immunotherapy. | 278 patients (141 in the intervention group, 137 in the control group) | The ePRO follow-up model included: Assessment of immune-related adverse events (irAEs) Quality of life (QOL) assessment using the European Organization for Research and Treatment of Cancer QLQ-C30 Time spent implementing the ePRO model | Gastric, Esophageal, Lung, Pancreatic, Colorectal, Breast, Brain, Liver, Kidney, and others | Patients receiving cancer immunotherapy. |
| 20. | Wickline et al. (75) | 2022 | United States | Usability and acceptability of the electronic self-assessment and care (eSAC) program in advanced ovarian cancer | Total Sample (N = 134); Device Interview Sample (n = 18); Usability Interview Sample (n = 19) in Ambulatory Setting: | Acceptability E-Scale Score (AES), Usability Interviews, Clinician Surveys, Focus Groups. | Advanced ovarian cancer | Palliative care |
| 21. | Tolstrup et al (49) | 2022 | Denmark |
|
Patients (N = 138) | EuroQol EQ-5D-5l Index, FACT-M questionnaire | Melanoma | Adjuvant 1st line 2nd line 3rd line, Immune checkpoint inhibitors (CPIs) |
| 22. | Tang et al. (45) | 2022 | China | To describe the implementation process and evaluation of an ePRO platform for symptom management in cancer patients, share experiences, and assess feasibility, safety, and efficacy. | A total of 161 patients with advanced cancer were enrolled in the study, although completion rates varied across the seven follow-up assessments. | Various validated instruments were used for symptom assessment, including the MD Anderson Symptom Inventory (MDASI), Insomnia Severity Index (ISI), Hospital Anxiety and Depression Scale (HADS), Patient Health Questionnaire-9 Items (PHQ-9), EuroQol 5 Dimensions Questionnaire-5l Version (EQ-5D-5l), and Distress Thermometer. These tools measured a range of symptoms, from pain and fatigue to insomnia and distress. | Patients with advanced cancer, including lung, liver, gastric, oesophagal, colorectal, and breast cancer. | Follow up |
| 23. | Rocque et al. (32) | 2022 | United States | To adopt a remote symptom monitoring intervention developed in research settings for implementation in real-world clinical settings at two cancer centres. | Phase I: 23 patients; Phase II: 35 patients (Myeloma and Acute Leukemia) | PRO-CTCAE (Common Terminology Criteria for Adverse Events), electronic health data | Lymphoma, Breast, Gastrointestinal, Genitourinary, Myeloma, Acute Leukemia | Chemotherapy, Immunotherapy, or Targeted therapies |
| 24. | Riedl et al. (63) | 2022 | Austria | To assess the impact of multidisciplinary inpatient rehabilitation on the health-related quality of life (HRQOL) and physical fitness of pediatric cancer survivors. | 236 pediatric cancer survivors aged 5-21 years and 478 parents (as proxy respondents). |
|
leukemias, lymphomas, Central Nervous System (CNS) tumours Brain, Bone, Soft tissue, Blood and immune system and others. | Rehabilitation Treatment: Set of multidisciplinary therapies including medical and nursing treatment, nutritional counselling, physiotherapy, and psychological therapies |
| 25. | Nordhausen et al. (26) | 2022 | Germany | To evaluate the implementation of electronic patient-reported outcomes (e-PRO) in inpatient radiation oncology | The study involved a total of 568 patients. | Initial assessment: EORTC QLQ-C30, QSC-R10—Daily symptom monitoring: EORTC single items—Final assessment: EORTC QLQ-C30 | Patients with various cancer | Chemotherapy, Radiotherapy |
| 26. | Lee et al. (68) | 2022 | Korea | To identify factors associated with the adoption and compliance of electronic patient-reported outcome measure (ePROM) among cancer patients in a real-world setting | 580 cancer patients | Symptom Assessment, Summary Report, Information for Self-management | Various cancers (e.g., breast, lung, gastric, colorectal, lymphoma, head and neck, others) | Chemotherapy or radiation therapy |
| 27. | Hlubock et al. (57) | 2022 | United States | To examine the prevalence of psychosocial factors affecting quality of life in ovarian cancer survivors using an electronic patient-reported outcome (ePRO) platform | 174 out of 300 ovarian cancer survivors | Hospital Anxiety/Depression Scale (HADS), Post-traumatic Growth Inventory, Brief Resilience Scale, Comprehensive Score for Financial Toxicity (COST), Functional Assessment of Cancer Therapy-Ovarian [FACT-O/health-related quality of life (HRQOL)], Clinical Characteristics | Ovarian cancer | Surgery, Chemotherapy or Radiation therapy |
| 28. | Graf et al. (15) | 2022 | Germany | To analyze the acceptance and evaluation of a tablet-based ePRO app for breast cancer patients and examine its suitability, effort, and difficulty in the context of HRQoL and sociodemographic factors. | 106 women with adjuvant or advanced breast cancer at 2 major university hospitals in Germany. | EORTC QLQ-C30 and FACT-B HRQoL questionnaires, self-reported technical skills, usability evaluation. | Breast Cancer | Adjuvant therapy (Chemotherapy) |
| 29. | Girgis et al. (51) | 2022 | Australia | To evaluate the processes and success of implementing the PRM system in the routine care of patients diagnosed with lung cancer. | 48 patients diagnosed with lung cancer completed 90 assessments during the 5-month implementation period. |
|
Lung cancer | Chemotherapy, Radiotherapy, Surgery, Immunotherapy |
| 30. | Daly et al. (76) | 2022 | United States | Assess the clinical value of daily electronic patient-reported outcomes (ePROs) for cancer patients undergoing antineoplastic treatment | 217 patients (median age 66, 103 women, and 114 men) | ePRO assessments with red and yellow symptom alerts a system-generated alert system with red and yellow alerts triggered by severe or concerning symptoms
|
Breast, head and neck, gastrointestinal, genitourinary, gynaecology, lymphoma, melanoma, thoracic, and soft cancers | Chemotherapy only (includes cytotoxic, antibodies), Immunotherapy only, Combination chemotherapy and immunotherapy, Radiation within 14 d of antineoplastic |
| 31. | Convill et al. (46) | 2022 | United Kingdom |
|
195 patients were included in the primary analysis. |
|
Lung cancer | Palliative, Curative, Neo-adjuvant |
| 32. | Boeke, et al. (77) | 2022 | Germany | To assess patient acceptance of physical activity (PA) monitoring in an outpatient setting during radiotherapy and to correlate changes in PA with toxicity and changes in quality of life (QoL). | 23 patients | Activity trackers, Functional Assessment of Chronic Illness Therapy questionnaire, daily step counts, quality of life measurements | Breast Head and Neck, Lung Anal, Esophageal, and Pancreatic cancer | Radio chemotherapy: Chemotherapy, Radiotherapy |
| 33 | Bamgboje-Ayodele, et al. (52) | 2022 | Australia | To detail the development and implementation of integrated care pathways (ICPs) for electronic collection of patient-reported outcomes (ePROs) in lung cancer patients in oncology settings | 96 staff members participated in engagement activities across three hospitals. | Cancer Institute New South Wales (CINSW) Patient Reported Outcome Measures (PRMs) system, Edmonton Symptom Assessment System (ESAS), Distress Thermometer (DT), problem checklist | Lung Cancer | N/A |
| 34. | Takala, et al. (78) | 2020 | Finland | Assess the usefulness of electronic patient-reported outcomes (ePROs) during adjuvant radiotherapy (RT) in patients with early breast cancer. | 253 patients with breast cancer receiving RT | Performance status, anxiety, oedema, skin symptoms, pain in the radiated area, tiredness and fatigue, respiratory symptoms, and other symptoms. | Breast cancer | Radiotherapy Chemotherapy, Surgery, and Hormonal therapy |
| 35. | Strachna (79) | 2021 | United States | To develop an electronic PROs (ePROs) program for head and neck cancer patients and evaluate its feasibility and impact. | 4,154 patients | Patient-reported outcomes measures, electronic survey application (MSK Engage), Tableau Software | Head and neck cancer | Radiotherapy, Surgery, and Chemotherapy |
| 36. | Stormoenet et al (42) | 2021 | Denmark | To describe Patient-Reported Outcomes (PROs) from patients with metastatic castration-resistant prostate cancer (mCRPC) receiving oncological treatment and compare them with adverse events from registration studies | 54 patients with mCRPC receiving medical oncological treatment | The ePRO-CTCAE questionnaire contained 41 items corresponding to 22 symptoms/adverse events associated with the treatment regimens commonly used for mCRPC. Data was stratified by antineoplastic agent administered, and the severity of interference was rated on a scale of 0 to 4. | Metastatic castration-resistant prostate cancer (mCRPC) | Antineoplastic treatment for prostate cancer |
| 37. | Riis et al. (16) | 2021 | Denmark | To examine the impact on service use, workflow, and workload after introducing ePRO-based individual follow-up for early breast cancer treatment. | Initially, 129 women were assessed, 64 in SFU, and 60 in PIFU; the final assessment included 47 participants for PREMs. | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30), EORTC Breast Cancer-Specific Module (QLQ-BR23), CollaboRATE measure, Patient Experience Questionnaire (PEQ), DEFACTUM Patient Involvement Indicator Objectives, Additional electronic Patient-Reported Outcome measures, Various Patient Satisfaction Tools, Additional ad hoc Surveys or Questionnaires | Early-stage breast cancer | Surgery, and Chemotherapy in SFU and PIFU, |
| 38. | Ravn, et al. (61) | 2021 | Denmark | To evaluate the effect of a follow-up supported by electronic patient-reported outcomes (ePRO) on Patient Activation (PA) and Patient Involvement (PI) in patients undergoing intended curative complex surgery for advanced cancer. | 187 patients who had undergone intended curative complex surgery for advanced cancer at two different departments at Aarhus University Hospital. | The ePRO included validated questionnaires, including the European Organization for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QlQ) C30, CR29, OV28, and items 6 and 11 from the Hospital and Anxiety Depression scale. | Patients with metastases to the peritoneal surface undergoing intended curative complex surgery for advanced cancer. | Surgery, and systemic chemotherapy |
| 39. | Peltola et al (80) | 2021 | Finland | To assess the suitability of the Noona ePRO application for patients with cancer, nurses, and doctors at Helsinki University Hospital |
|
|
Various solid tumour types | Radiotherapy, Surgery, and Chemotherapy, rehabilitation |
| 40. | Patt et al. (33) | 2021 | United States | To determine the feasibility of real-world implementation of electronic patient-reported outcomes (ePROs) among patients with cancer at a large community oncology practice. | 4,375 patients | The system includes the following tools and metrics:—Patient-Reported Symptoms: Patients were asked to report 14 common cancer-related symptoms each week.
–Data Visualization: The system provided data visualization of patient-reported symptoms, which were immediately available to a care coordination dashboard. - Risk Stratification: The system stratified patient-reported symptoms by the level of risk, enabling triage nurses and healthcare providers to prioritize and provide immediate care to patients at high risk. –Free-Form Text Box: Patients could add additional toxicities in a free-form text box for reporting. |
Breast cancer, Chronic leukaemia, Lung cancer, Lymphoma, Multiple myeloma. And Prostate cancer, Small intestine/colorectal cancer | Radiotherapy, and Surgery |
| 41. | Licht et al. (56) | 2021 | Austria | Investigate cancer survivors’ health-related quality of life (HRQOL), specific deficiencies related to underlying disease or treatment, and benefits of rehabilitation in a variety of cancer entities. | 4,401 cancer survivors |
|
Various cancer entities, including head and neck, esophageal, gastric, colon, rectal, liver, pancreatic, lung, skin, breast, uterine, ovarian, prostate, testicular, renal, bladder, brain, thyroid, malignant lymphomas, multiple myeloma, leukaemias, and other cancer types | Rehabilitation program:Guidance and treatment by a physician Nursing procedures Psychooncology, including individual counselling and biofeedback Group psycho-oncological counselling Psychological counselling, including sexual therapy Psychoeducative lectures Educational presentations focusing on motivation and lifestyle modification Cognitive and perception training Creative therapies Social counseling Speech therapy Nutritional advice Individual and group occupational therapy Physiotherapy, both individual and group sessions Medical training therapy, including aerobic and resistance training Remedial massages Manual lymphatic drainage Hydrogymnastics Electrotherapy Therapeutic ultrasound Thermotherapy Inhalation therapies |
| 42. | Lee et al. (59) | 2021 | South Korea | To evaluate the degree of depression and anxiety in patients with breast cancer during the treatment period and short-term follow-up. | 137 patients with breast cancer | PHQ-9 and GAD-7 for depression and anxiety assessment | Breast cancer | Chemotherapy or radiation and at 6-month follow-up examination after the Surgery |
| 43. | Lapen et al. (34) | 2021 | United States | Develop and study the implementation of a remote system for toxicity assessment and management of acute breast radiation side effects using electronic patient-reported outcomes (ePROs) | 678 patients | - Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE)—Generalized Anxiety Disorder 2-item (GAD-2) screening tool—Likert-type scale questions for frequency, severity, and distress related to symptoms—Questions about anxiety using the GAD-2 | Breast cancer | Radiotherapy, and Chemotherapy |
| 44. | Judge et al. (62) | 2021 | United States | To identify implementation issues and evaluate the efficacy of an electronic patient self-reporting pain device in community-based cancer clinics. | 178 cancer patients (33 in the pilot phase and 145 in the RCT phase) in community-based clinics | PAINReportIt® is an electronic version of the McGill Pain Questionnaire, which measures pain parameters including location, intensity, quality, and pattern. | Various types of Cancer patients | Radiotherapy |
| 45. | Generalova et al. (81) | 2021 | United States | Feasibility, implementation, and healthcare utilization outcomes of an electronic PRO (ePRO) application for cancer patients at an academic medical centre. | 72 patients | Patient feasibility (greater than 70% completion of questionnaires), patient acceptability (neutral or higher satisfaction), symptom responses, ambulatory healthcare utilization | Patients with advanced cancer in the thoracic, gastrointestinal, and genitourinary oncology groups | Medicine treatment, chemotherapy, or immunotherapy |
| 46. | Doolin et al. (82) | 2021 | United States | To assess the feasibility of implementing an electronic patient-reported outcomes (ePRO) system for patients starting oral chemotherapy at a cancer centre improving patient monitoring, and symptom assessment. | 62 patients who started a new oral chemotherapy regimen agreed to receive online ePROs, and 25 of them completed the ePRO (40% completion rate). A historical cohort of 50 patients was also used for comparison. | An ePRO questionnaire was designed in REDCap and included questions related to the treatment start date, administration issues, new or concerning symptoms, and financial toxicity concerns. Responses triggered clinical outreach. The primary outcome was time to first symptom assessment, along with other outcome measures such as time to first clinical action and ED visits/hospitalizations. | N.A | Oral Chemotherapy |
| 47. | Absolom et al. (83) | 2021 | United Kingdom | To evaluate the impact of eRAPID on symptom control, healthcare use, patient self-efficacy, and quality of life in a patient population predominantly treated with curative intent during chemotherapy | 508 consenting patients and 55 health professionals | Online symptom self-reporting, automated algorithm-driven severity-dependent patient advice, and immediate integration of the self-reports in electronic patient records (EPRs). | Colorectal, breast, or gynaecological cancers | Chemotherapy |
| 48. | Zylla et al. (84) | 2020 | United States | To assess the feasibility of using electronic patient-reported outcomes (ePROs) for symptom monitoring in patients with advanced cancer | 80 patients with stage IV non-hematologic malignancies on chemotherapy | The study used the Patient-Reported Symptom Monitoring (PRSM) survey, which is a 23-question ePRO tool for oncology patients. Symptom responses were categorized on a scale from 0 (none) to 4 (very severe). Severe symptoms were highlighted if the pain rating was ≥7 or any other symptom rated ≥3 (severe or very severe). | Stage IV non-hematologic malignancies include lung, colorectal, prostate, pancreas, head and neck, oesophagal and stomach, breast, ovarian, cervical, endometrial, liver/bile duct, and kidney cancers. | Chemotherapy |
| 49. | Tran et al. (67) | 2020 | United States | To explore the feasibility and acceptability of collecting electronic patient-reported outcomes (ePROs) using validated health-related quality of life (HRQoL) questionnaires for prostate cancer. | 29 patients in total; 1 patient excluded from analysis | Apple ResearchKit software, 26-item Expanded Prostate Cancer Index Composite (EPIC), 8-item Functional Assessment of Cancer Therapy Advanced Prostate Symptom Index, qualitative interviews | Prostate cancer | N/A |
| 50. | Tolstrup et al. (41) | 2020 | Denmark | Assess the electronic tool's impact on reducing severe adverse events by 50% in melanoma patients undergoing immunotherapy. | 146 melanoma patients participated in the study. | Patients in the intervention group received a tablet computer with a SIM card to ensure participation in the web-based evaluation. The PRO-CTCAE item library was used for patient reporting. Weekly reporting was chosen since it is the preferred recall period in PRO-CTCAE questionnaires. | Metastatic melanoma patients receiving immunotherapy. | Immunotherapy |
| 51. | Sandhu et al. (85) | 2020 | United States | To gain insights into oncologists’ perspectives regarding the incorporation of electronic patient-reported outcomes (ePROs) into routine cancer care at an academic centre | 16 oncologists with diverse subspecialties and experience in various cancer types |
|
Genitourinary, Breast, GI, Sarcoma, Urologic, Thoracic | Palliative medicine |
| 52. | Riis et al. (17) | 2020 | Denmark | Evaluate patients’ satisfaction with care provided using electronic patient-reported outcomes (ePROs) to individualize follow-up care for women with early breast cancer receiving adjuvant endocrine therapy | 134 women | ePROs, Patient Experience Questionnaire (PEQ), EORTC QLQ-C30, EORTC breast cancer module (QLQ-BR23) | Early breast cancer | Surgery, and Adjuvant endocrine therapy |
| 53. | Richards et al. (86) | 2020 | United Kingdom | To evaluate the feasibility of a real-time electronic symptom monitoring system for patients after discharge following cancer-related upper gastrointestinal surgery | 40 participants in the study |
|
Cancer-related upper gastrointestinal surgery (oesophageal, gastric, hepato-pancreato biliary cancer) | Surgery, Chemotherapy |
| 54. | Mowlem et al. (87) | 2020 | United Kingdom | To understand the impact of anticancer treatment on oncology patients’ ability to use electronic solutions for completing patient-reported outcomes (ePRO). | Seven individuals with a cancer diagnosis and treatment experience. | The researchers assessed the usability of the eCOA software solutions, including electronic diaries, questionnaires, and response scale types, on both tablet and mobile devices. | Breast, Prostate, and Colon/bowel | Chemotherapy |
| 55. | Karamanidoua et al. (88) | 2020 | Greece | To develop a novel ePRO-based palliative care intervention for cancer patients by eliciting end-users needs and judgments of the MyPal system and recommendations for improvement. | Nine patients with Chronic Lymphocytic Leukemia (CLL) | MyPal uses eHealth technologies to support cancer patients and healthcare professionals. It includes electronic Patient Reported Outcome (ePRO) assessments, smartphone applications, smart wristbands, and personalized medical information searches. Metrics and measures include symptom reporting, information access, personalized data, and more. | Chronic Lymphocytic Leukemia (CLL) and Myelodysplastic Syndromes (MDS) | Palliative Care |
| 56. | Howell et al. (55) | 2020 | Canada | To implement electronic Patient Reported Outcomes (e-PROs) in ‘real-world’ oncology practices for personalized management of generic and targeted symptoms of pain, fatigue, and emotional distress (depression, anxiety). | Over 6000 patients completed e-PROs |
|
Lung and sarcoma cancer | chemotherapy, Radiotherapy, Supportive and palliative care |
| 57. | Girgis et al. (53) | 2020 | Australia | Evaluate the effectiveness of the PROMPT-Care web-based system in a diverse population of cancer patients by reducing emergency department presentations and other health service outcomes. | 328 patients received the intervention, and 1312 patients were matched as controls. | Electronic PRO physical symptom and psychosocial well-being assessments, automated electronic clinical alerts, and online patient self-management resources. Distress Thermometer (DT), Edmonton Symptom Assessment Scale (ESAS), and Supportive Care Needs Survey-Screening Tool 9 were used for assessments. Clinical feedback reports provided recommended clinical actions and referrals. Clinical alerts were generated when individual ePRO item scores breached predefined thresholds on two consecutive assessments. Patient self-management resources included domain-specific webpages and information resources. | Patients with solid tumors | Chemotherapy, Radiotherapy, Surgery, and Immunotherapy |
| 58. | Dronkers et al. (89) | 2020 | Netherlands | To evaluate the implementation of an electronic patient-reported outcome measures (ePROs) system, in the routine care of head and neck cancer (HNC) patients. | -Quantitative: HM group (45 patients), Standard care group (46 patients)—Qualitative: Interviews with 15 HM patients | - Internationally validated questionnaires measuring physical problems, psychosocial symptoms, and health-related quality of life (HRQoL) of HNC patients.—Results are graphically displayed in the electronic health record (EHR) and used for monitoring and feedback to patients. | Head and neck cancer | N/A |
| 59. | Biran et al. (90) | 2020 | United States | Evaluate the acceptability and appropriateness of an electronic patient-reported outcome (ePRO) intervention for patients with relapsed and refractory multiple myeloma (RRMM) and explore its impact on clinic workflow | 11 patients with RRMM were recruited, and 9 patients completed the study | The app facilitated the reporting of 17 common RRMM Patient-Reported Outcomes (PROs), including severity, frequency, and interference measures. The data generated alerts for the clinic and provided self-management guidance to patients. | Relapsed and Refractory Multiple Myeloma (RRMM) | N/A |
| 60. | Warringtonet al. (91) | 2019 | United Kingdom | To field test the eRAPID system, an online tool for monitoring and managing adverse events in patients with cancer during treatment. | 12 patients receiving chemotherapy for early breast cancer and 10 health professionals (oncologists and specialist nurses). | The eRAPID system allows patients to complete weekly online symptom reports, provides severity-based self-management advice, and sends notifications to contact the hospital for severe symptoms. Patient data is available in electronic records for staff to review. Metrics include the frequency of online symptom report completion and severe symptom notifications. | Patients with early breast cancer were tested in the field usability study. The eRAPID system is being evaluated in a larger population, including patients with breast, gynaecology, or colorectal cancer. | Chemotherapy |
| 61. | Krogstad et al. (92) | 2019 | Norway | To evaluate the usability of the EirV3 system used for patient-reported outcome measures (PROMs) in cancer care | 37 patients, 17 physicians | EirV3 includes questions assessing 19 common cancer-related symptoms and additional questions on functioning and nutritional status. It presents graphical representations of symptom intensity over time and patient answers to follow-up questions. | Breast, Gastrointestinal, Lymphomas, Prostate, Gynecological, Lung, Malignant melanoma, and Testicular cancers | Palliative, Chemotherapy, and Radiotherapy |
| 62. | Kikawa et al. (18) | 2019 | Japan | Evaluation of health-related quality of life (HRQOL) monitoring from home among metastatic breast cancer (MBC) patients using the Computer-Based Health Evaluation System (CHES) | 16 MBC patients who received outpatient chemotherapy or endocrine therapy, both with and without targeted therapy. | CHES is a platform that electronically collects patient questionnaires developed by the EORTC QOL group. The system uses a Japanese version of the EORTC QLQ C30 questionnaire for HRQOL evaluation. | Metastatic breast cancer (MBC) | Outpatient chemotherapy or endocrine therapy both with and without targeted therapy. |
| 63. | Iivanainen et al. (37) | 2019 | Finland | Investigate whether symptoms collected by the Kaiku Health ePRO tool on cancer patients receiving immune checkpoint inhibitors (ICI) | 37 patients |
|
Various types of cancer | Immune checkpoint inhibitor (ICI) therapy |
| 64. | Gressel et al. (71) | 2019 | United States | To establish feasibility and acceptability of PROMIS ePRO integration in a gynecologic oncology outpatient clinic and assess if it can help identify severely symptomatic patients and increase referral to supportive services. | 336 patients in the Gynecologic Oncology Clinic: |
|
Gynecologic cancer |
|
| 65. | Brant et al. (50) | 2019 | United States | To determine the perception of patients and providers from patient-reported outcomes | 121 women (51 with gynecologic cancer and 70 with breast cancer) |
|
Breast Cancer, Gynecologic Cancer | N/A |
| 66. | Kerry N. L. Avery, et al. (93) | 2019 | United Kingdom | To develop a hospital EHR-integrated ePRO system to improve the detection and management of complications post-discharge following cancer-related surgery | Phase 1: 18 patients, Phase 2: 59 participants who provided 444 complete self-reports | The ePRO system employed 35 symptom-report items from validated European Organisation for Research and Treatment of Cancer (EORTC) questionnaires. Clinical algorithms were created for symptom severity-dependent patient advice and clinician alerts. Self-management advice was informed by clinician-patient consultations, patient interviews, and a review of hospital patient information leaflets and patient support websites. | Cancer-related major abdominal surgery | Surgery |
| 67. | Schepers, et al. (65) | 2016 | Netherlands | Determine the fidelity of the KLIK method as implemented in outpatient pediatric cancer care | 205 children with newly diagnosed cancer |
|
Pediatric cancer: Leukemias/lymphomas, Solid Tumors, and Brain Tumors | N/A |
| 68. | Niska et al. (39) | 2017 | United States | To assess changes in quality of life (QOL) and adverse events (AEs) during radiotherapy (RT) for head-and-neck cancer using electronic patient-reported QOL (PROQOL) data. | 65 patients |
|
Head and neck cancer | Radiotherapy |
| 69. | Lucas et al. (94) | 2017 | United States | To report on the establishment of a unified, electronic PRO infrastructure and | 773 eligible patients, with 688 (89%) enrolled preoperatively | Validated 21-item web-based questionnaire for urinary function, erection function, and sexual interest and satisfaction. Additional questions related to quality of life, relationship status, and use of erectile aids. | Prostate cancer | Surgery |
| 70. | Holch et al. (95) | 2017 | United Kingdom | To develop a system for patients to self-report and manage adverse events (AE) during and after cancer treatment | Patient advocates (N = 9), patients (N = 13), and staff (N = 19) participated in usability testing |
|
Breast, gynaecological, colorectal, pelvic radiotherapy, upper gastrointestinal surgery | Surgery, Radiotherapy |
| 71. | Hartkopf et al. (19) | 2017 | Germany | To investigate the willingness, and assess specific needs, and barriers of adjuvant breast cancer (aBC) and metastatic breast cancer (mBC) patients in nonexposed and exposed settings before implementing digital electronic Patient-Reported Outcome (ePRO) | 202 participants (nonexposed group: 96, exposed group: 106) | Socioeconomic variables, Health-Related Quality of Life (HRQoL) according to EORTC QLQ-C30, preexisting technical skills, the general attitude toward electronic-based surveys, and potential barriers about health status. | Breast cancer | Chemotherapy |
| 72. | Absolom et al. (96) | 2017 | United Kingdom | To improve the safe delivery of cancer treatments, enhance patient care, and standardize adverse event (AE) documentation | Internal pilot phase with 87 participants, full trial target sample of 504 participants |
|
Breast, colorectal and gynaecological cancer | Chemotherapy |
| 73. | Schougaard et al. (23) | 2016 | Denmark | To implement telepatient-reported outcomes (telePRO) as the basis for follow-up in chronic and malignant diseases using the generic PRO system AmbuFlex | AmbuFlex was implemented in nine diagnostic groups in Denmark. A total of 13,135 outpatients from 15 clinics have been individually referred. -Response rates for the initial questionnaire ranged from 81% to 98% in different patient groups | WHO-5, SF-36, HADS, EORTC QLQ-C30. ad hoc items were developed in five projects PRO data collection, PRO-based automated decision algorithm, PRO-based graphical overview for clinical decision support | Prostate, colorectal cancer | Chemotherapy, and Radiotherapy |
| 74. | Peltola et al. (40) | 2016 | Finland | Assess the suitability of Kaiku® (an ePRO application) for collecting patient-reported outcomes (PROs) related to early side effects of radiotherapy and health-related quality of life in head and neck cancer (HNC) patients. | Nine HNC patients were approached, and five consented to participate. |
|
Head and neck cancer (HNC) | Radiotherapy |
| 75. | Mayrbäurl et al. (24) | 2016 | Austria | To assess health-related quality of life (HRQOL) in patients with advanced colorectal cancer across different lines of palliative chemotherapy | 100 consecutive patients with colorectal carcinoma |
|
Colorectal cancer | Palliative chemotherapy |
| 76. | Graf et al. (20) | 2016 | Germany | To determine the extent to which existing computer skills, disease status, health-related quality of life, and sociodemographic factors affect patients’ willingness to use electronic methods of data collection (ePRO) | 96 | EORTC QLQ-C30, EQ VAS, technology skills, willingness to use electronic PRO surveys | Breast cancer | N/A |
| 77. | Duregger et al. (97) | 2016 | Austria | To develop a concept and implement a prototype for introducing electronic Patient Reported Outcomes (ePRO) into the existing neuroblastoma research network by applying Near Field Communication (NFC) and mobile technology. | N/A |
|
Neuroblastoma (the primary focus of the study), but the system's applicability extends to other pediatric cancers and rare diseases. | N/A |
| 78. | Cowan et al. (29) | 2016 | United States | The assessment, and feasibility of acceptability and satisfaction of a Web-based system for capturing patient-reported outcomes (PROs) in the immediate postoperative period in gynecologic cancer surgery patients. | 96 eligible patients | The system included surveys based on the patient adaptation of the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 and the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 version 3.0. Alerts were generated based on patient-reported symptoms. | Gynecologic cancer | Surgery |
| 79. | Wintner et al. (21) | 2015 | Austria | Assessment, and the feasibility of routine clinic-ePRO/home-ePRO with the Computer-based Health Evaluation System (CHES) software. |
|
|
Gastrointestinal, glioma, gynaecological, lung, neuroendocrine, and testicular cancers | Follow-up, and chemotherapy |
| 80. | Wagner et al. (69) | 2015 | United States | To integrate electronic patient-reported outcome (ePRO) assessment into the electronic health record (EHR) and clinical workflow for symptom screening in ambulatory cancer care. | 636 women receiving gynecologic oncology outpatient care | PROMIS CATs for fatigue, pain interference, physical function, depression, and anxiety. Checklists for psychosocial concerns, information needs, and nutritional concerns. PROMIS T-scores with severity thresholds. | Ovarian, Uterine, Cervical, Other female genital malignancy | Gynecologic oncology outpatient care |
| 81. | Erqi L. Pollom, et al. (66) | 2015 | United States | To evaluate the feasibility of eQOL data collection using a touch-screen tablet device in patients undergoing treatment for head and neck cancer | 50 patients |
|
Head and neck cancers | Radiotherapy, and chemotherapy |
| 82. | Smith et al. (60) | 2014 | United States | Demonstrate how an electronic patient-reported outcome (ePRO) system can aid in distress management in oncology. | 17,338 |
|
Breast, lung, and gastrointestinal cancer patients | N/A |
| 83. | Wintner et al. (22) | 2013 | Austria | To assess the quality of life (QOL) of lung cancer patients undergoing chemotherapy (CT) across multiple treatment lines. | 187 | The EORTC QLQ-C30 questionnaire was used, which includes Functioning Scales (Physical, Social, Role, Cognitive, and Emotional Functioning), a Global QOL scale, and Symptom Scales (Fatigue, Pain, Dyspnoea, Appetite Loss, Sleep Disturbance, Constipation, Diarrhoea, Financial Difficulties), and additional items for taste alterations. | Lung cancer | Chemotherapy |
| 84. | Zabernigg et al. (28) | 2012 | Austria | To investigate QOL trajectories from adjuvant treatment to palliative 3rd-line therapy | 80 patients (Pancreatic cancer and cancer of the bile ducts) | EORTC QLQ-C30 questionnaire, Computer-based Health Evaluation System (CHES) | Pancreatic cancer and Bile duct Cancer | Chemotherapy |
| 85. | Abernethy et al. (58) | 2010 | United States | Demonstrate a rapid learning healthcare model in an academic oncology clinic using electronic patient-reported outcomes (ePROs) as foundational data | Metastatic breast cancer (n = 65) and gastrointestinal cancer (n = 113) patients in Duke Cancer Clinics | e/Tablets, Patient Care Monitor (PCM), FACT-G, MD Anderson Symptom Inventory (MDASI), NCCN Distress Scale | Breast and gastrointestinal cancer | N/A |
Summary characteristics of articles included.
Summary
Keywords
electronic patient-reported outcome measures, ePROMs, cancer care, measurement instruments, patient assessment
Citation
Salmani H, Nasiri S, Alemrajabi M and Ahmadi M (2024) Advancing patient-centered cancer care: a systematic review of electronic patient-reported outcome measures. Front. Rehabil. Sci. 5:1427712. doi: 10.3389/fresc.2024.1427712
Received
08 May 2024
Accepted
12 September 2024
Published
25 September 2024
Volume
5 - 2024
Edited by
Saeid Shahraz, Tufts Medical Center, United States
Reviewed by
Annemarie L. Lee, Monash University, Australia
Suleman Atique, Norwegian University of Life Sciences, Norway
Updates
Copyright
© 2024 Salmani, Nasiri, Alemrajabi and Ahmadi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Maryam Ahmadi ahmadi.m@iums.ac.ir
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.