1 Introduction
Brain-Computer Interface (BCI) is an advanced system that enables direct communication between the human brain and external devices, bypassing the need for muscle activity. BCI can translate brain signals into commands to control assistive technologies, offering support for individuals with severe motor impairments, such as those caused by stroke or spinal cord injury. BCI hold applications in healthcare, especially for restoring movement and interaction in patients with limited voluntary motor control. By leveraging neural activity, BCIs open pathways for motor rehabilitation, assistive device control, and even cognitive assessment, enhancing quality of life and promoting greater independence.
A widely used control strategy in BCI research is motor imagery (MI), which involves mentally simulating specific physical movements without any actual muscle activation. MI is considered a cognitively driven process that mimics the planning stages of real movements and activates neural networks involved in motor execution. This includes regions such as the primary motor cortex, supplementary motor area (SMA), and premotor cortex, supporting the “functional equivalence” hypothesis (Naseer and Hong, 2015). Because MI can be performed without physical movement, it is especially suitable for patients who are paralyzed, amputated, or recovering from neurological trauma, making it highly relevant for neurorehabilitation and assistive technology development.
Over the past decades, a variety of neuroimaging modalities have been employed to capture the neural correlates of MI for BCI applications. These include invasive techniques like intracranial EEG (iEEG) and non-invasive modalities such as electroencephalography (EEG), magnetoencephalography (MEG), functional magnetic resonance imaging (fMRI), and functional near-infrared spectroscopy (fNIRS) (Cutini and Brigadoi, 2014). EEG is widely favoured for its high temporal resolution and affordability; however, it is highly susceptible to electrical interference and motion artefacts. On the other hand, fMRI offers excellent spatial resolution but is often limited by its high cost, immobility, and operational complexity. These limitations restrict their practical use, especially in real-world or bedside BCI applications.
Among non-invasive techniques, fNIRS has emerged as a promising neuroimaging modality for BCI systems. fNIRS uses near-infrared light to measure cerebral hemodynamic responses, specifically tracking changes in oxygenated (ΔHbO) and deoxygenated hemoglobin (ΔHbR) concentrations in the cortex (Khan et al., 2020). When a brain region becomes active, local blood flow increases, leading to a rise in ΔHbO and a decrease in ΔHbR. fNIRS offers a balance between spatial and temporal resolution, is portable, non-invasive, relatively affordable, and less sensitive to movement artifacts compared to EEG or fMRI (Nazeer et al., 2020a). Its quiet, mobile nature also makes it more comfortable for users and suitable for long-duration BCI sessions. These advantages make fNIRS a particularly suitable tool for developing MI-based BCI systems aimed at real-world rehabilitation scenarios.
While MI has been extensively studied for upper limb movements, there is increasing interest in lower limb motor imagery tasks due to their clinical importance. Lower limb movements such as ankle dorsiflexion/plantarflexion and knee flexion/extension are fundamental to essential motor functions like walking, balance, and postural control (Brockett and Chapman, 2016). Understanding how these movements are represented in the brain during MI can help in developing effective rehabilitation protocols and control strategies for assistive devices. MI of lower limb tasks engages similar motor areas to actual movement, supporting its use in BCIs that aim to stimulate recovery or control robotic assistance.
Datasets focusing on lower limb MI are essential for advancing research in rehabilitation and assistive robotics. They provide the foundation for developing and training machine learning models that can accurately decode movement intent from brain signals. Several studies have shown that MI of knee or ankle movements can generate meaningful patterns suitable for decoding and can be used to control prosthetic limbs or lower limb exoskeletons (Colucci et al., 2022). For instance, joint EEG-fNIRS studies have demonstrated the effectiveness of decoding ankle dorsiflexion and its relevance to balance recovery in stroke patients (Liang et al., 2022). Similarly, several studies has been conducted to improve classification accuracy of fNIRS (Li et al., 2024; Li and Li, 2025; Li and Sun, 2025) and hybrid EEG-fNIRS signals (Xu et al., 2023; Wang et al., 2025) for motor imagery/execution tasks. Such insights are crucial for improving the responsiveness and reliability of BCI-driven assistive devices.
To support this line of research, we introduce a novel fNIRS dataset focused on lower limb MI tasks, specifically ankle dorsiflexion/plantarflexion and knee flexion/extension. The dataset is designed to facilitate the development of BCI systems intended for controlling lower limb exoskeletons in rehabilitation applications (Minhas et al., 2024a). By capturing the hemodynamic responses associated with these specific MI tasks, the dataset enables researchers to explore neural patterns linked to lower limb motor control and develop machine learning models for real-time BCI applications. The unique focus on lower limb motor tasks, combined with the use of fNIRS, distinguishes this dataset and highlights its potential for impactful contributions to neurorehabilitation, exoskeleton design, and assistive technology research.
2 Methodology
This section details the study’s methodology, including participant selection, data acquisition procedures, and the experimental paradigm for MI tasks.
2.1 Subjects
The study included 21 healthy participants (12 males and 9 females) with a mean age of 24 ± 2.1 years, along with one male participant (subject 22) who had a right above-knee ultra-short stump amputation. Ethical approval for the study was granted by the Ethical Committee at Air University (Approval No. AU/EA/2021/03/003). A pre-screening questionnaire was used to confirm that none of the participants had cardiovascular or neurological conditions, as such conditions could affect blood flow regulation or neural activation patterns (Pelicioni et al., 2019). Participants were instructed to avoid smoking and caffeine for at least 3 hours prior to the experiment (Sargent et al., 2020). The study adhered to the ethical principles of the Declaration of Helsinki (World Medical Association, 2013). All participants provided written informed consent after receiving a full explanation of the procedure and demographic details are shown in Table 1.
TABLE 1
| Participants demographic data | ||||||
|---|---|---|---|---|---|---|
| S.No | Gender | Age | Education | Dominance | Neurological disorder | Psychiatric disorder |
| 1 | M | 22 | Undergraduate | Right | No | No |
| 2 | M | 21 | Undergraduate | Right | No | No |
| 3 | M | 27 | Graduate | Right | No | No |
| 4 | F | 23 | Graduate | Right | No | No |
| 5 | M | 23 | Graduate | Right | No | No |
| 6 | M | 28 | DAE | Right | No | No |
| 7 | M | 34 | Undergraduate | Right | No | No |
| 8 | M | 26 | Graduate | Right | No | No |
| 9 | F | 21 | Undergraduate | Right | No | No |
| 10 | F | 21 | Undergraduate | Right | No | No |
| 11 | F | 18 | Undergraduate | Right | No | No |
| 12 | M | 21 | Undergraduate | Right | No | No |
| 13 | F | 20 | Undergraduate | Right | No | No |
| 14 | M | 18 | Undergraduate | Right | No | No |
| 15 | F | 27 | Undergraduate | Right | No | No |
| 16 | F | 21 | Undergraduate | Right | No | No |
| 17 | F | 19 | Undergraduate | Right | No | No |
| 18 | F | 18 | Undergraduate | Right | No | No |
| 19 | M | 23 | Graduate | Right | No | No |
| 20 | M | 24 | Graduate | Right | No | No |
| 21 | M | 21 | Undergraduate | Right | No | No |
| 22 | M | 28 | Graduate | Right | No | No |
Demographic details of all participants.
2.2 Data acquisition
Eight sources and eight detectors were used to record fNIRS signals from the motor cortex, yielding twenty physiological channels. Optode placement followed the internationally recognized EEG 10–20 system (Homan et al., 1987), which ensures standardized and accurate localization of brain regions in neurophysiological research. A fixed source-detector distance of 3 cm was maintained using optode holders, as this spacing is optimal for capturing neural activity from the motor cortex, (Gagnon et al., 2014). Brain signals were recorded using the NIRSport2 system (NIRx Medical Technologies, Germany) using 760 nm and 850 nm wavelengths at a sampling rate of 10.1725 Hz. Data collection was managed using Aurora fNIRS software (NIRx Medical Technologies, Germany), while PsychoPy was used to design the experiment, send triggers, and run the task.
To ensure signal accuracy, secure attachment of optodes to the scalp was prioritized, particularly in participants with thick hair, which can obstruct optical signals (Kwasa et al., 2023). This was achieved using appropriately sized elastic caps equipped with spring-loaded grommets and holders for consistent scalp contact. Real-time signal monitoring via Aurora facilitated the identification and correction of poor contact points. Participants were also advised to wash their hair prior to the session to minimize interference from oil and dandruff.
2.3 Experimental paradigm
In this study, MI tasks included dorsiflexion and plantarflexion of the ankle joint, as well as flexion and extension of the knee joint. These tasks were performed sequentially using the right leg, left leg, and both legs. Participants were positioned in Fowler’s position (a 45-degree recline), which was selected to enhance cerebral blood flow (Kubota et al., 2017). The experiment took place in a quiet environment with reduced ambient light to minimize noise and optical interference (Solovey et al., 2009), thereby improving data accuracy by reducing artifacts. To differentiate between MI and ME, inertial measurement unit (IMU) sensors were secured to the participants’ ankle joints using adjustable straps. These sensors were placed for monitoring purpose and helped detect any unintended physical movements during the tasks (Mizuguchi et al., 2012). Participants were seated in front of a computer screen placed approximately 2 m away. Prior to the experiment, they were thoroughly briefed on the procedure and instructed to avoid any unnecessary movements (Shin et al., 2017). Trials were discarded if any physical movement was detected during the task. Visual cues were presented on the screen to guide participants throughout the experiment. Figure 1 shows the overall experimental protocol.
FIGURE 1

Overview of the experimental protocol. Participants received instructions and gave consent (1), followed by MI tasks of the ankle (2) and knee (3).
The task began with a 45-s rest period to allow the hemodynamic response to stabilize. During this time, participants were instructed to remain still and avoid any voluntary movements. Following the initial rest, participants performed an MI task involving ankle plantarflexion, guided by on-screen instructions for a duration of 5 s. This was followed by a 10-s rest period to allow the hemodynamic signals to return to baseline (Nazeer et al., 2020b). Subsequently, participants engaged in an MI task involving ankle dorsiflexion for 5 s. This cycle of MI tasks and rest was repeated for three trials, followed by a final 45-s rest period to return to baseline. The same protocol was repeated for knee joint movements, specifically flexion and extension, following the same sequence and timing as used for ankle tasks. Figures 2A,B illustrate the experimental paradigms for ankle and knee movements, respectively.
FIGURE 2
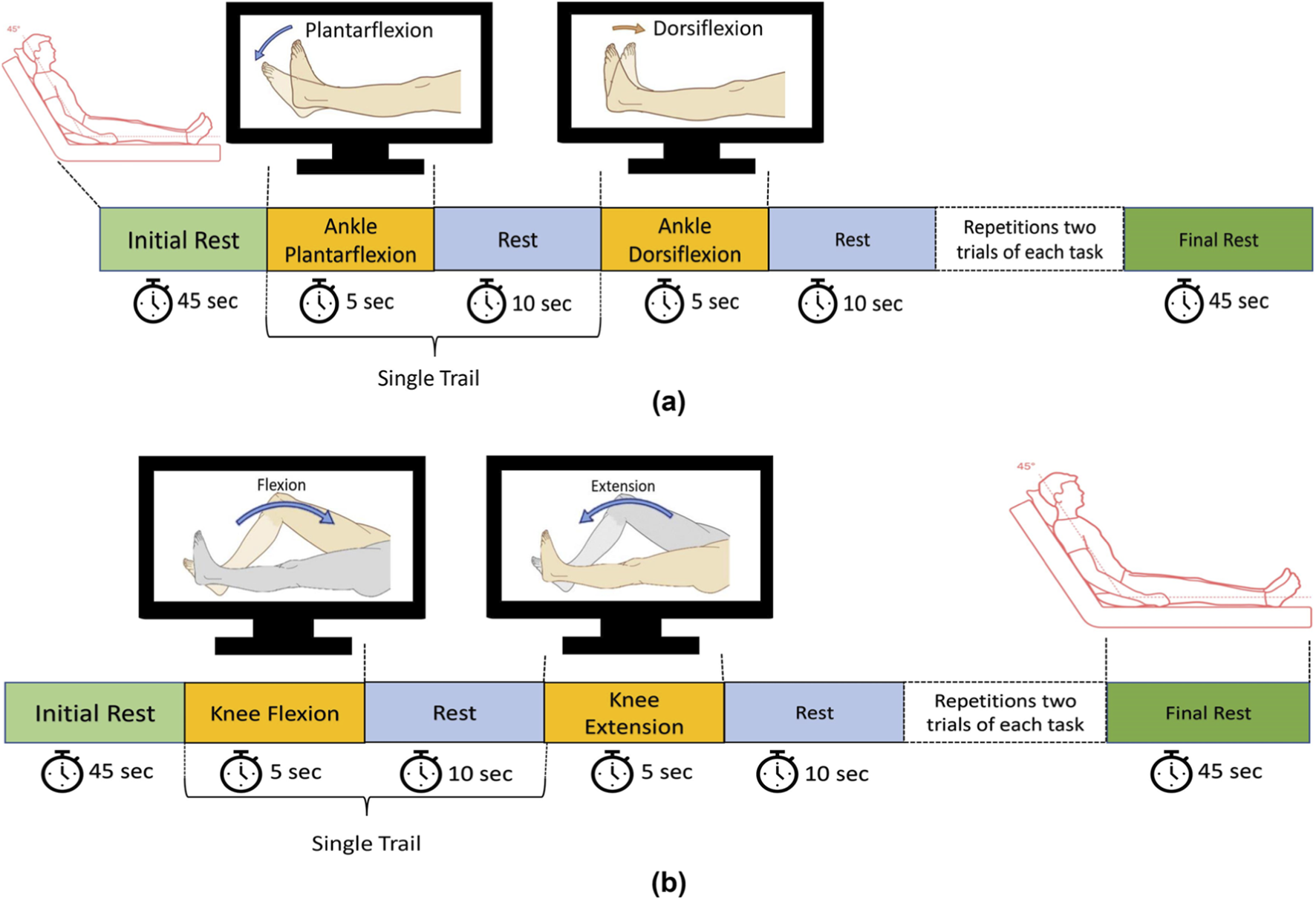
(a) Schematic of the experimental paradigm for ankle dorsiflexion and plantarflexion tasks; (b) Schematic of the experimental paradigm for knee flexion and extension tasks. Reprinted with permission from Minhas et al. (2024a), Copyright © 2024, IEEE.
2.4 Data preprocessing
Data preprocessing was carried out using Satori 2.0 (NIRx Medical Technologies, Germany). fNIRS signals are often affected by physiological artifacts such as respiration, cardiac pulsation, Mayer waves, and motion-related noise (Hakimi et al., 2023). To reduce artifacts, a fourth-order Butterworth bandpass filter was applied with a frequency range of 0.01–0.3 Hz (Minhas et al., 2024b). Z-transform normalization was also applied to standardize the data, making it easier to compare across subjects or conditions. Additionally, baseline-zero adjustment was performed to shift the starting point of the signal to zero, helping to clearly observe task-related changes over time. After filtering, the modified Beer-Lambert law was used to convert the optical signals into concentration changes of oxyhemoglobin (ΔHbO) and deoxyhemoglobin (ΔHbR) (Kocsis et al., 2006).
2.5 Data structure
The dataset is organized and distributed in the shared near infrared file format (.snirf), a standardized format designed to facilitate the sharing and analysis of fNIRS data. It comprises recordings from 22 participants, each performing six distinct motor imagery tasks. Correspondingly, the dataset is divided into six separate folders, each representing a specific activity (e.g., Left Ankle), with each folder containing data from all 22 participants for that particular task. Within these activity-specific folders, individual subject data are stored in separate subfolders named by participant ID. Each subfolder contains a pre-processed CSV file (e.g., sub1.csv) with filtered fNIRS signals, which can be used for classification and analysis tasks.
3 Summary
In summary, we present a structured and preprocessed fNIRS dataset focused on lower limb motor imagery tasks involving the ankle and knee joints. Collected from 22 participants, this dataset supports the development of real-time BCI applications for rehabilitation and assistive technologies. Its standardized format, clear organization, and emphasis on lower limb MI tasks make it a valuable resource for researchers aiming to advance decoding algorithms, improve motor function restoration strategies, and enhance the design of BCI-driven exoskeleton systems.
The study recruited limited number of participants, i.e., 22, though it is sufficient for initial testing, however the sample size may not fully capture the variability present in larger or more diverse populations.
Statements
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://figshare.com/s/c63ff90363263a4dd965; https://figshare.com/s/7ed1702717ae27dd3a03; https://figshare.com/s/307c1c7535a39b1cccfb; https://figshare.com/s/1577643d373cfde64399; https://figshare.com/s/fa8db647f18fe9696032; https://figshare.com/s/f99b3c84fbb148e87f7f.
Ethics statement
Ethical approval for the study was granted by the Ethical Committee at Air University (Approval No. AU/EA/2023/02/008). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HK: Conceptualization, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. HN: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review and editing. HM: Data curation, Methodology, Software, Visualization, Writing – original draft. NN: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review and editing. PM: Conceptualization, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The work is a collaboration between the Neuroimaging Research Group (NRG) at the Department of Mechatronics Engineering, Air University, and Advanced Health Intelligence and Brain-Inspired Technologies (ADEPT) at the Department of Mechanical, Electronics, and Chemical Engineering, Oslo Metropolitan University, Norway. We are thankful to all the participants for taking part in the experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Brockett C. L. Chapman G. J. (2016). Biomechanics of the ankle. Orthop. Trauma30 (3), 232–238. 10.1016/j.mporth.2016.04.015
2
Colucci A. Vermehren M. Cavallo A. Angerhöfer C. Peekhaus N. Zollo L. et al (2022). Brain–computer interface-controlled exoskeletons in clinical neurorehabilitation: ready or not?Neurorehabilitation Neural Repair36 (12), 747–756. 10.1177/15459683221138751
3
Cutini S. Brigadoi S. (2014). Unleashing the future potential of functional near-infrared spectroscopy in brain sciences. J. Neuroscience Methods232, 152–156. 10.1016/j.jneumeth.2014.05.024
4
Gagnon L. Yücel M. A. Boas D. A. Cooper R. J. (2014). Further improvement in reducing superficial contamination in NIRS using double short separation measurements. NeuroImage85 (Pt 1), 127–135. 10.1016/j.neuroimage.2013.01.073
5
Hakimi N. Shahbakhti M. Horschig J. M. Alderliesten T. Van Bel F. Colier W. N. J. M. et al (2023). Respiratory rate extraction from neonatal near-infrared spectroscopy signals. Sensors23 (9), 4487. 10.3390/s23094487
6
Homan R. W. Herman J. Purdy P. (1987). Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiology66 (4), 376–382. 10.1016/0013-4694(87)90206-9
7
Khan R. A. Naseer N. Saleem S. Qureshi N. K. Noori F. M. Khan M. J. (2020). Cortical tasks-based optimal filter selection: an fNIRS Study. J. Healthc. Eng.2020, 1–15. 10.1155/2020/9152369
8
Kocsis L. Herman P. Eke A. (2006). The modified Beer–Lambert law revisited. Phys. Med. Biol.51 (5), N91–N98. 10.1088/0031-9155/51/5/N02
9
Kubota S. Endo Y. Kubota M. Shigemasa T. (2017). Assessment of effects of differences in trunk posture during Fowler’s position on hemodynamics and cardiovascular regulation in older and younger subjects. Clin. Interventions Aging12, 603–610. 10.2147/CIA.S132399
10
Kwasa J. Peterson H. M. Karrobi K. Jones L. Parker T. Nickerson N. et al (2023). Demographic reporting and phenotypic exclusion in fNIRS. Front. Neurosci.17, 1086208. 10.3389/fnins.2023.1086208
11
Li Y. Xu T. Li J. Wan F. Wang H. (2024). Improved dilation CapsuleNet for motor imagery and mental arithmetic classification based on fNIRS. Brain-Apparatus Commun. A J. Bacomics3 (1), 2335886. 10.1080/27706710.2024.2335886
12
Li Y. Li S. Yuan Z. Zhao S. Wan F. Xu T. et al (2025). IVCAN: an improved visual curve attention network for fNIRS-Based motor imagery/execution classification. Biomed. Signal Process. Control104, 107679. 10.1016/j.bspc.2025.107679
13
Li Y. Sun Y. Wan F. Yuan Z. Jung T. P. Wang H. (2025). MetaNIRS: a general decoding framework for fNIRS based motor execution/imagery. Neural Netw.192, 107873. 10.1016/j.neunet.2025.107873
14
Liang J. Song Y. Belkacem A. N. Li F. Liu S. Chen X. et al (2022). Prediction of balance function for stroke based on EEG and fNIRS features during ankle dorsiflexion. Front. Neurosci.16, 968928. 10.3389/fnins.2022.968928
15
Minhas H. S. Nazeer H. Naseer N. Kainat I. Khan H. Mirtaheri P. (2024a). Analysis of motor imagery-based fNIRS-BCI for knee flexion and extension. Int. Conf. Robotics Automation Industry (ICRAI), 1–6. 10.1109/ICRAI62391.2024.10894687
16
Minhas H. S. Nazeer H. Naseer N. Khan U. S. Ansari A. R. Nawaz R. (2024b). Enhancing classification accuracy of fNIRS-BCI for gait rehabilitation. IEEE Access, 1. 10.1109/ACCESS.2024.3443066
17
Mizuguchi N. Nakata H. Uchida Y. Kanosue K. (2012). Motor imagery and sport performance. J. Phys. Fit. Sports Med.1 (1), 103–111. 10.7600/jpfsm.1.103
18
Naseer N. Hong K.-S. S. (2015). fNIRS-based brain-computer interfaces: a review. Front. Hum. Neurosci.9 (January), 3–15. 10.3389/fnhum.2015.00003
19
Nazeer H. Naseer N. Khan R. A. Noori F. M. Qureshi N. K. Khan U. S. et al (2020a). Enhancing classification accuracy of fNIRS-BCI using features acquired from vector-based phase analysis. J. Neural Eng.17 (5), 056025. 10.1088/1741-2552/abb417
20
Nazeer H. Naseer N. Mehboob A. Khan M. J. Khan R. A. Khan U. S. et al (2020b). Enhancing classification performance of fNIRS-BCI by identifying cortically active channels using the z-Score method. Sensors20, 6995. 10.3390/S20236995
21
Pelicioni P. H. S. Tijsma M. Lord S. R. Menant J. (2019). Prefrontal cortical activation measured by fNIRS during walking: effects of age, disease and secondary task. PeerJ7, e6833. 10.7717/peerj.6833
22
Sargent A. Watson J. Topoglu Y. Ye H. Suri R. Ayaz H. (2020). Impact of tea and coffee consumption on cognitive performance: an fNIRS and EDA Study. Appl. Sci.10 (7), 2390. 10.3390/app10072390
23
Shin J. von Luhmann A. Blankertz B. Kim D. W. Jeong J. Hwang H. J. et al (2017). Open access dataset for EEG+NIRS single-trial classification. IEEE Trans. Neural Syst. Rehabilitation Eng.25 (10), 1735–1745. 10.1109/TNSRE.2016.2628057
24
Solovey E. T. Girouard A. Chauncey K. Hirshfield L. M. Sassaroli A. Zheng F. et al (2009). “Using fNIRS brain sensing in realistic HCI settings,” in Proceedings of the 22nd annual ACM symposium on user interface software and technology (New York, NY, USA: ACM), 157–166. 10.1145/1622176.1622207
25
Wang H. Yuan Z. Zhang H. Wan F. Li Y. Xu T. (2025). Hybrid EEG-fNIRS decoding with dynamic graph convolutional-capsule networks for motor imagery/execution. Biomed. Signal Process. Control104, 107570. 10.1016/j.bspc.2025.107570
26
World Medical Association (2013). World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA310 (20), 2191–2194. 10.1001/jama.2013.281053
27
Xu T. Zhou Z. Yang Y. Li Y. Li J. Bezerianos A. et al (2023). Motor imagery decoding enhancement based on hybrid EEG-fNIRS signals. IEEE Access11, 65277–65288. 10.1109/ACCESS.2023.3289709
Summary
Keywords
brain-computer interface, functional near-infrared spectroscopy, open access, motor imagery, dataset
Citation
Khan H, Nazeer H, Minhas HS, Naseer N and Mirtaheri P (2025) Open-access fNIRS dataset for motor imagery of lower-limb knee and ankle joint tasks. Front. Robot. AI 12:1695169. doi: 10.3389/frobt.2025.1695169
Received
29 August 2025
Revised
11 November 2025
Accepted
19 November 2025
Published
05 December 2025
Volume
12 - 2025
Edited by
Zhehao Jin, Nanyang Technological University, Singapore
Reviewed by
Hongtao Wang, Wuyi University, China
Weiyong Si, University of Essex, United Kingdom
Updates
Copyright
© 2025 Khan, Nazeer, Minhas, Naseer and Mirtaheri.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haroon Khan, haroonkh@oslomet.no; Hammad Nazeer, hammad@au.edu.pk
‡These authors have contributed equally to this work and share first authorship
† Present address:Hamza Shabbir Minhas , Human-Centred Technology Research Centre, Faculty of Science and Technology, University of Canberra, Canberra, ACT, Australia
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.