- 1General Directorate of Animal Health and Production, National Animal Health and Production Research Institute, Phnom Penh, Cambodia
- 2International Livestock Research Institute, Nairobi, Kenya
- 3Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 4Department of Clinical Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 5Department of Medical Biochemistry and Microbiology, Uppsala University, Uppsala, Sweden
- 6Livestock Development for Community Livelihood Organization, Phnom Penh, Cambodia
- 7Natural Resources Institute, University of Greenwich, London, United Kingdom
- 8Emergency Centre for Transboundary Animal Diseases, Food and Agriculture Organization of the United Nations, Phnom Penh, Cambodia
- 9International Centre for Antimicrobial Resistance Solutions, Copenhagen, Denmark
Salmonella is a globally important foodborne bacterial pathogen that poses a high risk to human health. This study aimed to estimate the risk to Cambodian consumers from acquiring salmonellosis after consuming chicken and pork salad, using a quantitative microbial risk assessment (QMRA). Chicken and pork salads are typical Cambodian dishes containing raw vegetables and boiled chicken meat or pork. As previously described, chicken meat and pork samples (n = 204 of each) were collected from traditional markets in 25 Cambodian provinces to generate data on Salmonella contamination. Salad preparation and consumption practices were surveyed in 93 Cambodian households and this information was used to design an experiment to assess Salmonella cross-contamination from raw meat to ready-to-eat salad. In the part of the study reported here, data on consumption, Salmonella in salad, dose-response, and predicted salmonellosis were modeled using Monte Carlo simulations at 10,000 iterations. The prevalence of Salmonella in chicken meat and pork were set to 42.6 and 45.1%, respectively, with average most probable number (MPN) per gram of Salmonella in chicken meat was 10.6 and in pork 11.1 MPN/g, based on an earlier study. Half of the interviewed households cooked meat for the salad directly after purchase. The QMRA model showed that the modeled annual risk of salmonellosis from consuming chicken salad, pork salad and both chicken and pork salad were 11.1% probability of illness per person per year (90% CI 0.0–35.1), 4.0% (90% CI 0.0–21.3), and 14.5% (90% CI 0.0–33.5), respectively. The factors most influencing the estimate were cross-contamination while preparing the salad, followed by the prevalence of Salmonella in chicken meat and pork at the market. The wide confidence interval for the incidence was mainly due to the variability in reducing bacteria concentration by cooking and salad consumption. The predicted risk of salmonellosis due to chicken and pork salad consumption is high, and the study provides evidence supporting control measures of improving the safety of retailed chicken and pork obtained from markets to households and improving food preparation methods in the household.
1. Introduction
The World Health Organization (WHO) has estimated that foodborne diseases (FBD) cause 33 million disability adjusted life years (DALYs) globally, and a loss of more than US$110 billion in productivity and medical expenses each year in low- and middle-income countries (LMIC) (Havelaar et al., 2015; Devleesschauwer et al., 2018). Annually, around 200 different types of foodborne pathogens cause disease in 600 million people, and FBD has been reported to result in around 420,000 deaths every year (World Health Organization Regional Office for South-East Asia., 2016). People living in LMIC are at particular risk for contracting FBD due to challenges related to insufficient hygiene practices, poor knowledge and reduced access to safe food (Grace, 2015; Varijakshapanicker et al., 2019). FBD thus constitute a significant health challenge globally, and non-typhoid Salmonella serovars have been reported as the most common foodborne bacteria causing FBD (Havelaar et al., 2015; World Health Organization Regional Office for South-East Asia., 2016; Boqvist et al., 2018).
Most Salmonella serovars are human pathogens and may cause a wide range of symptoms, of which diarrhea is the most common (Oscar, 2004; Majowicz et al., 2010; Crump and Wain, 2017). Animal-source food (ASF) is often implicated in human salmonellosis. It is estimated that globally Salmonella causes approximately 230,000 deaths annually, mainly in elderly and children under 5 years (Majowicz et al., 2010; Havelaar et al., 2015; World Health Organization Regional Office for South-East Asia., 2016; Devleesschauwer et al., 2018). Salmonella is carried by many animal species and can be transmitted by ASF, contributing to food safety concerns in LMIC (Unger et al., 2020).
In Cambodia, ASF (especially chicken meat and pork) are essential parts of the diet eaten by all age groups (General Directorate of Animal Health Production of Cambodia, 2021) and contribute important micronutrients (Tum, 2008; Sary et al., 2019). Chicken and pork are commonly sold at traditional markets where most people buy fresh food. Meat is commonly stored without chilling at the markets and in most households (People in Need, 2015; Rortana et al., 2022). Several popular dishes are prepared from boiled chicken meat or pork mixed with raw vegetables (Baker, 2009; Saorath, 2019; Cambodia Recipe, 2020).
In LMIC, chicken and pork are easily contaminated with Salmonella, which can occur at slaughterhouse facilities, markets, and storage facilities with insufficient cooling (Cliver, 2006; Carrasco et al., 2012; Aizaabi and Khan, 2017; Possas et al., 2017). Storing of meat in warm temperatures provides good conditions for the growth of Salmonella (Possas et al., 2017; Dang-Xuan et al., 2019). Improper handling and poor practices also contribute to the transmission of bacteria along the food chain, especially from markets to ready-to-eat (RTE) foods (Kristina and Sophal, 2018)). In addition, recent studies have found that poor handling of meat before and during cooking causes bacterial cross-contamination to RTE food, including chicken salad in Cambodia (Rortana et al., 2022) and boiled pork in Vietnam (Dang-Xuan et al., 2018). In Cambodia, a recent study detected 43% prevalence of Salmonella in chicken meat, 41.9% on chicken cutting board, 45% on pork, and 30% on pork cutting board; and the mean MPN of Salmonella per gram was 10.6 MPN/g in chicken and 11.1 MPN/g in pork samples (Rortana et al., 2021).
Quantitative microbial risk assessment (QMRA) can estimate health consequences and help in food safety management and communication. In Cambodia, QMRA has been conducted on Salmonella and different hazards and food type, but there are, to our knowledge, no publications on the risk of salmonellosis related to chicken meat or pork (Tum, 2008; Kristina and Sophal, 2018; Walia et al., 2018; Food and Agriculture Organization of the United Nations, 2021) although QMRA models of salmonellosis have been developed in other countries (Dang-Xuan et al., 2016; Perez-Rodriguez, 2020; Oscar, 2021a,b).
In Cambodia, there is a lack of comprehensive and solid evidence on the impact of FBD that can guide policymakers on health hazards related risks, and support meat production and donors to tackle food safety issues and public health notices (Tum, 2008; Public Health of Canada, 2017; Lam et al., 2019). Moreover, the household knowledge of FBD is low in Cambodia, and most people associate food safety challenges mainly with chemical contamination (Brown et al., 2022; Duong et al., 2022). This study aimed to estimate the risk of consumers acquiring Salmonella infection through consuming contaminated pork and chicken salad at the household level to generated new and actionable information.
2. Materials and methods
2.1. Study location
The study was conducted in Cambodia, located in the Mekong sub-region in Southeast Asia. In 2019, the total population of Cambodia was around 15 million (National Institute of Statistics of Cambodia, 2019). The country is influenced by tropical monsoon winds and has two seasons, the dry season (November–April) and the rainy season (May–October). In the rainy season, the average temperature in 2019 was 29°C, ranging between 27 and 36°C, with a humidity between 45 and 80 % (Department of Meteorology of Cambodia, 2019). Data for Salmonella contamination used in this QMRA was collected from all 25 provinces in Cambodia.
2.2. Study design
The QMRA model was built according to the Codex Alimentarius Commission quantitative microbial risk assessment framework (Codex Alimentatius Commission, 1999), consisting of hazard identification, hazard characterization, exposure assessment, and risk characterization (CAC/GL-30, 1999). This QMRA model was designed using data published earlier. Firstly, a cross-sectional market survey of the Salmonella prevalence in chicken meat and pork had been conducted in traditional markets in all 25 provinces in Cambodia (Rortana et al., 2021). Secondly, a household survey had been carried out in four provinces and cities (Battambang, Preah Sihanouk, Phnom Penh and Siem Reap) to explore handling and consumption patterns of chicken and pork salad in Cambodian (Rortana et al., 2022). Thirdly, experiments to identify cross-contamination scenarios during the preparation of chicken and pork salad at the household level had been done at the National Animal Health and Production Research Institute (Phnom Penh, Cambodia) (Rortana et al., 2022) and in Vietnam (Dang-Xuan et al., 2018). Lastly, data for the hazard characterization, of bacteria growth and dose-response model were obtained from the literature (Teunis et al., 2010; Velugoti et al., 2011). These surveys and experiments were conducted from November 2018 to June 2020 and are described briefly below.
2.3. Salmonellosis risk assessment model
2.3.1. Hazard identification
In a publication from WHO on the global burden of FBD, salmonellosis was identified as the most important bacteria hazard in DALYs (Havelaar et al., 2015). Salmonellosis is also considered one of Cambodia's most critical FBD (Rortana et al., 2021). The hazard identification of this study was made using data from a systematic literature review (Kristina and Sophal, 2018), from key stakeholder meetings in Cambodia (including representatives from a national food safety working group, policymakers, and international partners) (Nguyen-Viet, 2018), from a multi-hazard survey (Rortana et al., 2021), and from the cost of hospitalization for FBD performed by the Ministry of Health in Cambodia (Srey, 2019).
2.3.2. Hazard characterization
Non-typhoid Salmonella serovars are the most common foodborne bacteria causing FBD (Havelaar et al., 2015; World Health Organization Regional Office for South-East Asia., 2016; Boqvist et al., 2018). Most Salmonella serovars are human pathogens and may cause a wide range of symptoms, of which diarrhea is the most common (Oscar, 2004; Majowicz et al., 2010; Crump and Wain, 2017). Moreover, invasive Salmonella infection has been reported in Cambodia (Emary et al., 2012; Vlieghe et al., 2012; Kimsean et al., 2017; Kristina and Sophal, 2018; Kuijpers et al., 2018). Salmonella contamination in ASF is often implicated in human salmonellosis infection (Botteldoorn et al., 2003; Carrasco et al., 2012; Havelaar et al., 2015). In this study, the Beta-Poisson dose-response model developed from Salmonella outbreak data was used (alpha = 0.00853; beta = 3.14) (Teunis et al., 2010). That dose-response model presented an infection ID50 of 7 colony forming unit (CFU) and an illness ID50 of 36 CFU.
2.3.3. Salmonella exposure assessment
2.3.3.1. Meat sampling at market
A previous study investigated the prevalence and concentration of Salmonella in chicken meat and pork sold in traditional Cambodian markets (Rortana et al., 2021). In brief, samples from chicken meat (n = 204) and pork (n = 204) from markets in 25 provinces in Cambodia were included. The prevalence of Salmonella from all the markets in chicken meat was 42.6% and in pork 45.1%. The mean MPN of Salmonella was 10.6 MPN/g in chicken meat and 11.1 MPN/g in pork samples.
2.3.3.2. Cross-contamination study
Cross-contamination of Salmonella has been described in two published papers on chicken salad (Rortana et al., 2022) and boiled pork (Dang-Xuan et al., 2018). According to a recent study, cross-contamination of Salmonella in chicken salad was common in the four scenarios or sets of household practices used for salad preparation in Cambodia (Rortana et al., 2022). Briefly, Salmonella occurrence on cutting boards, knives and hands under four preparation scenarios (Table 1) was assessed. Similarly, Salmonella cross-contamination from raw pork to boiled pork via a hands and kitchen utensils was examined in Vietnam (Dang-Xuan et al., 2018). The similarity of the four scenarios is described in detail in Table 1. The proportion of households using each scenario, as well as the probability of contamination, were part of the modeling.
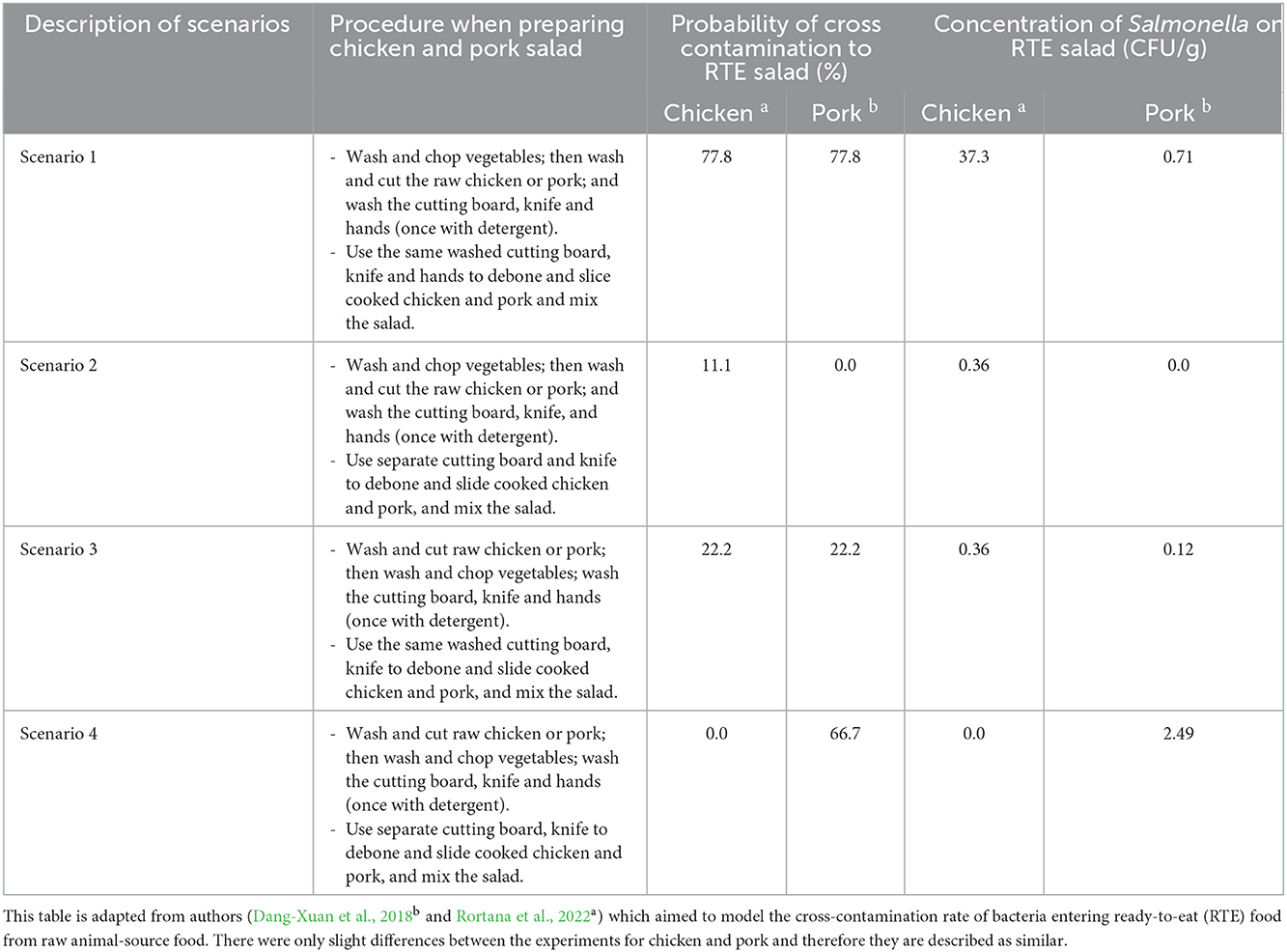
Table 1. Description of the four scenarios where cross-contamination of Salmonella may occur when preparing chicken and pork salad.
2.3.3.3. Chicken and pork salad consumption
Chicken and pork salad consumption were assessed using focus group discussion (FGD) among 93 households in four provinces (Siem Reap, Preah Sihanouk, Battambang, and Phnom Penh) in Cambodia (Rortana et al., 2022). Three FGDs (with participants chosen to represent rural, peri-urban, and urban areas) in each of the four provinces were conducted by randomly selecting households within one commune. A discussion outline was developed in English and translated to Khmer language for FGD and back translated into English for analysis. The FGD was led by trained researchers using flipchart and notes, and lasted about 1.5 h. The information of chicken and pork salad consumption was determined for children below 5 years, youth (6–15 years old), adults (16–60 years old), and the elderly (above 61 years old).
2.3.4. Risk characterization of Salmonella infection
The data presented above was integrated into a stochastic risk model, including different input parameters (Table 2). The risk of salmonellosis (health outcome) was defined as the probability of illness per year per person, simulated by combining different transmission pathways through chicken and pork salad consumption. The parameters, statistics, distribution, and data sources used in the QMRA model are presented in Table 2 and Figure 1. In step 1, the prevalence of Salmonella from samples collected at the markets was used as representative of bacterial contamination in fresh chicken meat and pork (Rortana et al., 2021). In step 2, the rate of Salmonella entering chicken and pork salad was estimated at the household level, the temperature at the study site, duration of storage until cooking, and the laboratory experiment to measure the level of Salmonella in RTE chicken salad (Rortana et al., 2022) and boiled pork (Dang-Xuan et al., 2018). In step 3, the consumption rate was assessed including how often people consume chicken/pork salad and age groups (result from this study).
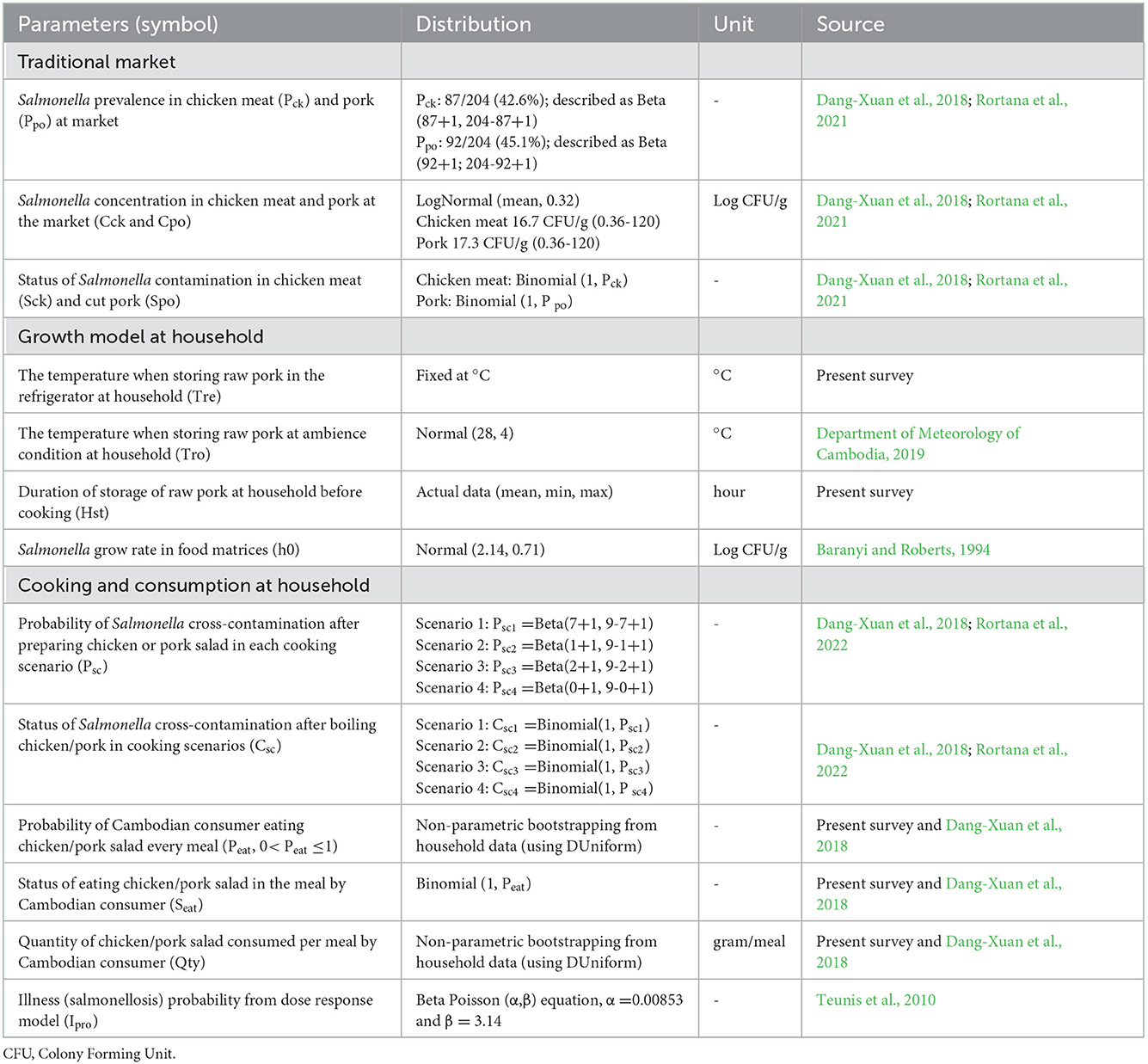
Table 2. Summary of the parameters, statistics, distribution, and data sources used in the QMRA model to estimate the risk of salmonellosis through chicken and pork salad consumption in Cambodia.

Figure 1. Model steps and input parameters in Salmonella QMRA from market to household related to chicken and pork salad preparation and consumption.
2.4. Data management and analysis
Data were managed and processed using MS Excel (Office 365). Descriptive statistical analysis was used to describe Salmonella prevalence using RStudio version 3.2.2 (R Core Team). The risk model was developed, and Monte Carlo simulation was performed using @Risk (Version 8.1, Palisade, Corporation, USA) for 10,000 iterations. The sensitivity analysis was conducted by selecting all the uncertainty parameters and running 1000 iterations at seven quantile values. Consumption data, prevalence and concentration of pathogen were described as mean and median. Final risk estimates were presented as mean and median with 90% confidence interval (CI).
2.5. Ethical considerations
Ethical approval of this study was done under the Safe Food, Fair Food Cambodia project and granted by the National Ethical Committee of Cambodia, coded 300NECHR, dated 26th December 2017. The participating researchers were informed and instructed on the safety procedures and provided their signed informed consent prior to starting the experiment. For the FGDs, participants invited to the discussion were asked for their written consent agreement prior to starting.
3. Results
3.1. Exposure assessment
The consumer survey on consumption of chicken and pork salad was conducted among 93 households in 12 FGDs. Detailed salad eating frequency (times/month) and amount of salad consumed (gram/meal) by age groups are presented in Table 3. In brief, the median frequency of consuming either chicken or pork salad was 1.6 times per month, ranging from 0–24, and the average amount consumed per meal was 130 grams per person (Table 3).
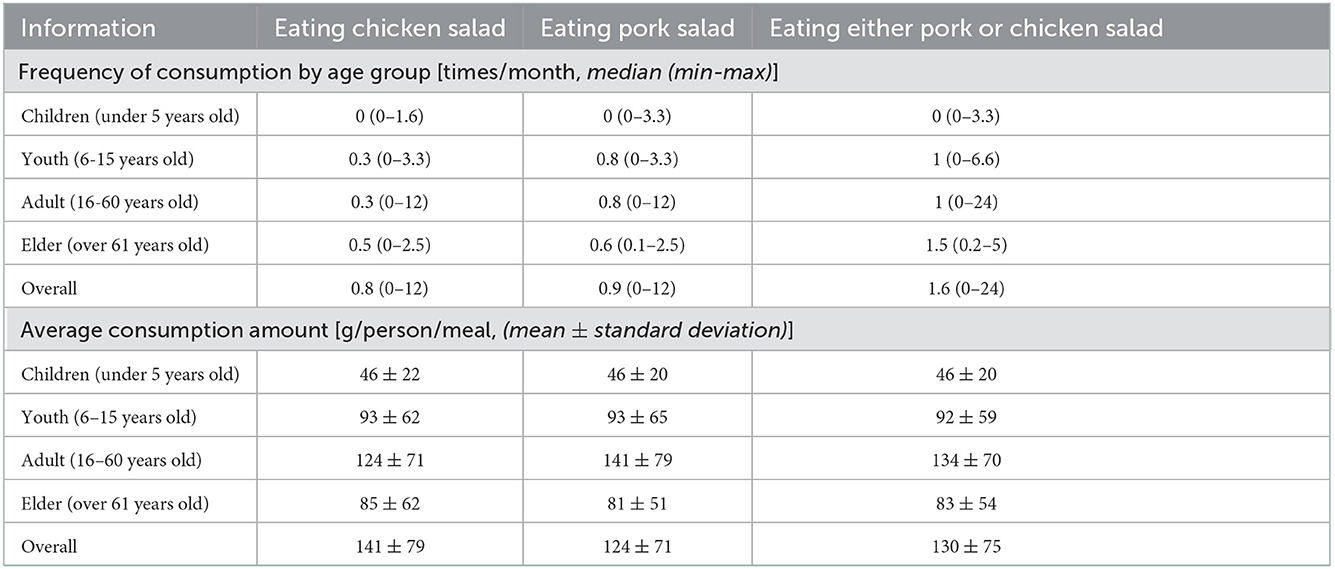
Table 3. Frequency and the average amount of chicken and pork salad consumption by age groups in Cambodian households.
3.2. Risk characterization
The modeled annual incidence rate of salmonellosis was higher for chicken salad (11.1% probability of illness per person per year; 90% CI: 0–35.1) than for pork salad (4.0%, 90% CI: 0–21.3); considering consumption of both chicken and pork salad the annual incidence rate was 14.5% (90% CI: 0–33.5, Table 4; Figure 2). Adults had the highest modeled annual incidence rate (19.1%; 90% CI: 0–48.3): incidence by age categories and types of salad are shown in Table 4.
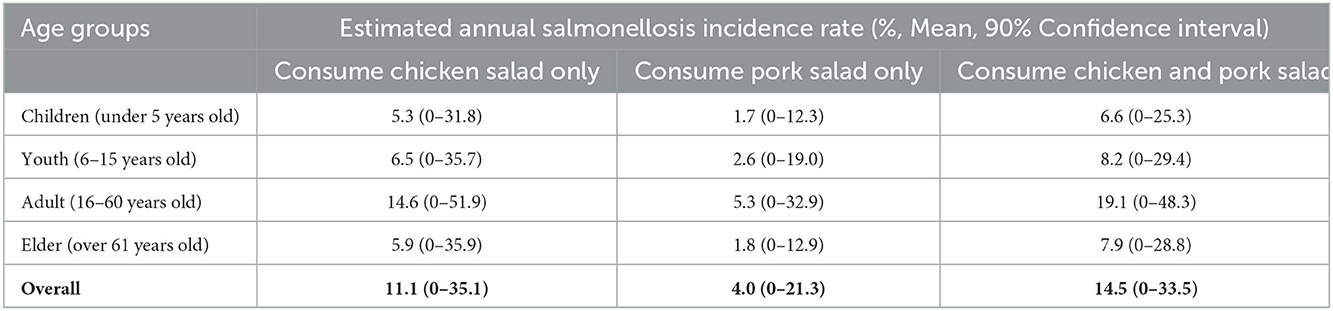
Table 4. The annual incidence rate of human salmonellosis due to chicken and pork salad consumption by age groups in Cambodia.
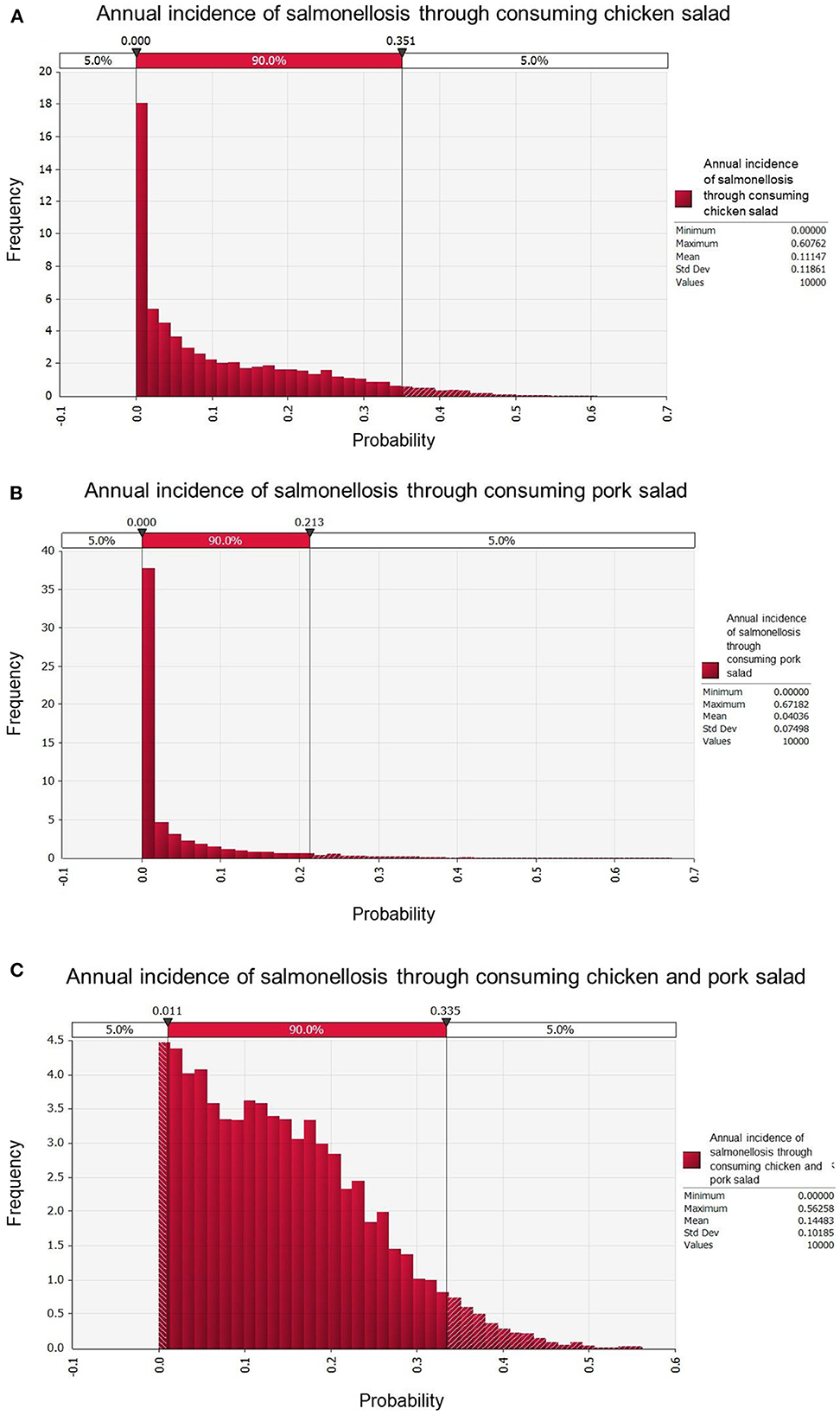
Figure 2. Annual incidence rates of salmonellosis in Cambodian households eating chicken salad (A), pork salad (B), and both chicken and pork salad (C).
3.3. Sensitivity analysis
The sensitivity analysis found the most important influencer of the annual incidence rate of salmonellosis was the probability of cross-contamination in preparing salad in scenario 3 (wash chicken and pork first, use same utensils). This was followed by the prevalence of Salmonella on chicken at the market; probability of cross-contamination in scenario 1 (wash vegetables first, use same utensils for cutting salad and raw chicken and pork); prevalence Salmonella in chicken and pork from the market; and probability of cross-contamination in scenario 4 (wash chicken and pork first, use different utensils, Figure 3; Table 5). The scenarios are described in detail in Table 1.
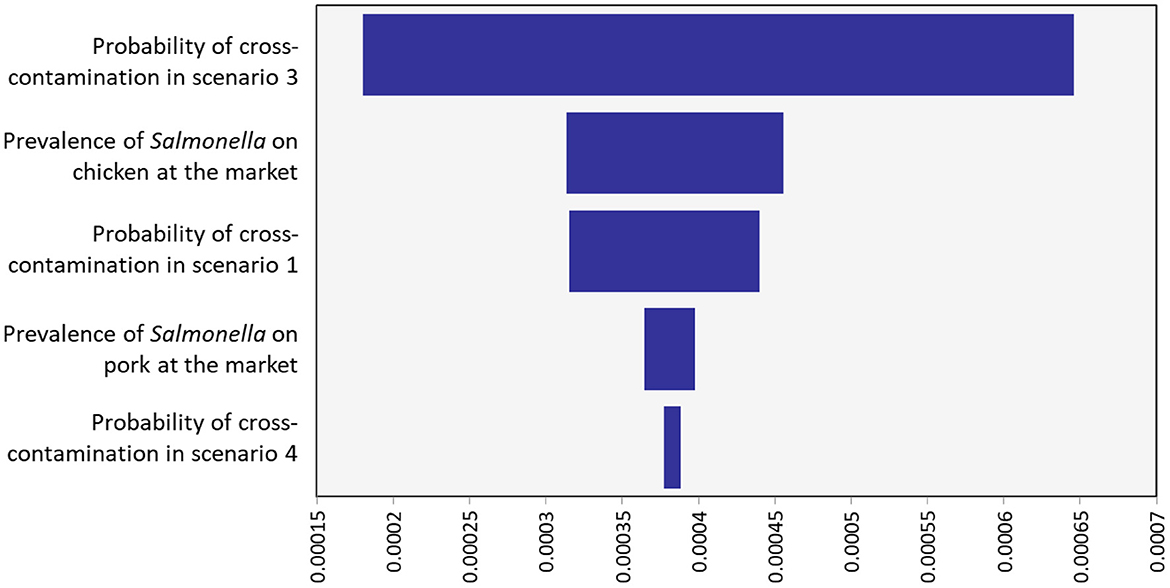
Figure 3. Sensitivity analysis of influence factors on annual salmonellosis incidence due to consuming both pork and chicken salad.
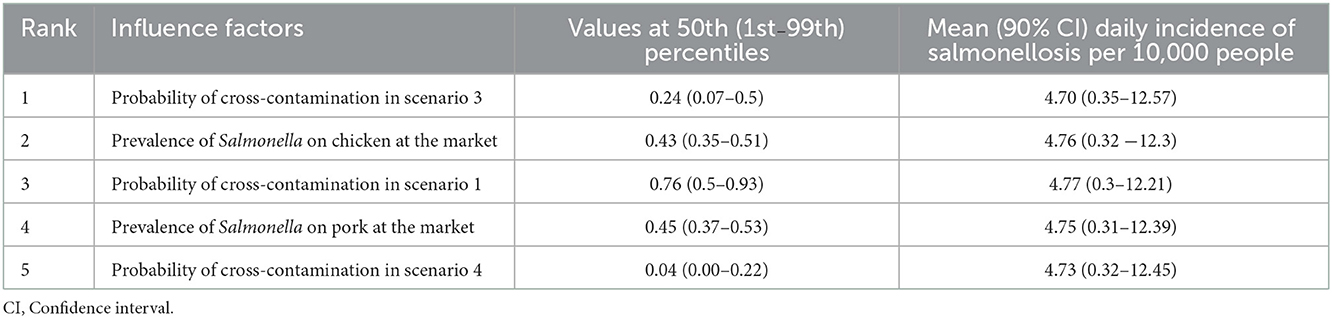
Table 5. Sensitivity analysis of influence factors on daily salmonellosis incidence due to consuming pork and chicken salad.
4. Discussion
This study developed a QMRA model from retail-to-table pathways predicting the likelihood of salmonellosis owing to consumption of chicken and pork salad in the Cambodian setting. The two most crucial factors for bacterial contamination of consumed food were the probability of cross-contamination during preparation in scenario 3 and the Salmonella prevalence in meat from markets. According to Rortana et al. (2022), most (86–96%) households practice preparing salad according to scenario 3, which had less contamination than scenario 1, but the fact that this practice is so common, gave it a larger influence in the model. The high influence of this common practice in the model also shows that there is a great scope of improvements. If the risk of cross-contamination at household level could be reduced, or people could change their habits completely to scenario 2 or 4, the risks could be reduced. All scenarios included rinsing the chicken, since it was the common practice, even if this step should be completely discouraged, as it increases the risks for salmonella contamination.
Most of the meat in Cambodia is sold in traditional markets where temperatures are suitable for bacterial growth (Sary et al., 2019; General Directorate of Animal Health Production of Cambodia, 2021; Rortana et al., 2022). Earlier studies found that meat and vegetables were frequently contaminated with Salmonella at this level (Rortana et al., 2021; Schwan et al., 2021). People in urban and peri-urban areas commonly purchase meat in the morning and cook it the same day (Brown et al., 2022; Rortana et al., 2022), while people in rural areas often keep meat longer before cooking (Duong et al., 2022). The focus of this QMRA was on meat purchased in the traditional value chain, as this is still the most common source of food in Cambodia, and where the prevalence was found the highest (Rortana et al., 2021). The model was built according to how people handle meat in their daily life. Another study in Cambodia used QMRA of Salmonella for risk assessment, specifically on the consumption of cricket powder to treat undernutrition in infants and children (Walia et al., 2018), while our study is the first to build a QMRA on commonly consumed meat. In the future, as supermarkets get more common, it would be interesting to include the origin of the meat in the model, but this was not done here.
This study found that prevalence of Salmonella in the market was the major driver of risk of salmonellosis to salad consumers. As already described, salmonellosis is one of the leading foodborne diseases globally, as well as in Cambodia (Shiowshuh and Cheng-An, 2011; Yates, 2011; Nair and Johny, 2019). This study also found that the prevalence of Salmonella in food sold in markets was an important determinant of the incidence of Salmonella infection, adding insight to discussions on which points in the value chain food safety interventions should target. Recent studies have detected high prevalence of Salmonella in chicken meat and pork in markets in Cambodian (Rortana et al., 2021) and Vietnam (Dang-Xuan et al., 2019; Ngo et al., 2021). Moreover, other studies in Cambodia have found Salmonella in chicken meat (Nadimpalli et al., 2019) and vegetables that are in contact with meat during the selling period at the market (Schwan et al., 2021). In the current study, the prevalence of Salmonella in meat was relatively high compared to studies in nearby countries, including studies from Vietnam and Thailand (Dang-Xuan et al., 2019; Poomchuchit et al., 2021). Pathogenic Salmonella enterica have also been isolated from multiple sources, including humans, animals, and food in Cambodia (van Cuyck et al., 2011; Schwan et al., 2021). Even S. enterica serovar Paratyphi infections have been found earlier in Phnom Penh, Cambodia (Vlieghe et al., 2013). The study found that the average incidence of salmonellosis in adults was higher than in children, youth, and the elderly, which is probably because adults more commonly consume chicken salad. Chicken salad and other similar salads are common foods in Asian countries, including Cambodia (Rortana et al., 2022) and Vietnam (Dang-Xuan et al., 2016). Even though most Salmonella does not cause severe disease in humans, regular exposure to these bacteria could be harmful over long time periods (Bollaerts et al., 2008; Perez-Rodriguez, 2020). Earlier studies also support that the cooking conditions and procedures such as moisture, contact time and pressure could result in higher transfer between the surface of contaminated objects (Cliver, 2006; Pérez-Rodríguez et al., 2008; Van Asselt et al., 2008). Chicken salads and similar salads (e.g. with beef, fish, shrimp, octopus) are very common at ceremonies such as traditional weddings in Cambodia. There is also an earlier report that a group of people got sick from a foodborne pathogen after eating salad in a wedding reception in Kampong Speu province (Vandy et al., 2012).
Two previous studies found that Cambodian people worry more about chemical food safety than microbial contamination (Duong et al., 2021; Brown et al., 2022). Most households also believe that chemicals (additives substances) used to make ASF products look good is the only cause of foodborne illness, leading them to care less of microbially contaminated in ASF (Brown et al., 2022). They tend to pay more attention to purchasing chemical free food than to proper storage, cooking, and good practice to reduce microbial contamination (Kristina and Sophal, 2018; Brown et al., 2022). Yet this study shows a high risk of FBD from a bacterial hazard. Changing perceived risks requires awareness raising data. This in turn, demands a food surveillance system, which is not yet in place in Cambodia (Thompson et al., 2021). Slaughterhouse hygiene improvements are still under development in the country, and the government has only started to monitor microbial contamination in slaughterhouses (Tum, 2008; Thompson et al., 2021) and markets (Rortana et al., 2021) to identify critical control points and prevent cross-contamination.
As consumption varies with age, different age categories were used in this study. However, the dose-response model used did not take differences in susceptibility between age groups into account (Teunis et al., 2010). Therefore, the separate dose-response model of Salmonella according to the categories of age and health condition was uncertain and could not be used as a formal analysis (Bollaerts et al., 2008; Marks and Coleman, 2017; Sanaa, 2021).
Food safety regulations vary between countries, but the usual goal is to combat foodborne diseases (Kunthear, 2022). In Cambodia the government recently adopted the National Plan on Food Safety. Six ministries are currently involved in governing national food safety, and coordinated by a multi-ministries team, the Technical Working Group for Food Security and Nutrition, which included representatives from each ministry. In June 2022, the law on food safety which addresses the entire food chain was adopted and brings Cambodia in line with international food safety standards (Food and Agriculture Organization of the United Nations, 2022; Kunthear, 2022). The law authorizes food inspection and provides a legal basis for action where food safety hazards are identified. This study provides scientific evidence that cross-contamination of Salmonella in the food chain (in this case from market to preparation of RTE salads) is a significant factor for human salmonellosis. This data is of relevance for local and national authorities and could be used to guide future policies, surveillance, and intervention to improve food safety along the food chain.
5. Conclusions
The study presents new results on the risks of contracting salmonellosis after eating chicken and pork salad in Cambodia. It describes household practices that may facilitate Salmonella contamination of RTE food and estimates the probability of salmonellosis caused by consumption of this food. The QMRA suggests that changing meat storage and handling practices from market to household can reduce the likelihood of foodborne disease. The results are evidence for use as a basis for adapting policies in Cambodia. The new knowledge can guide implementation of appropriate and effective intervention strategies to prevent and control the undesirable consequences associated with microbial contamination in animal source food until RTE. Through enhancing food safety practices and responsibility among actors across the value chain, targeting markets, households and RTE food providers and restaurants, the findings can contribute to reduce the burden of FBDs in ASF in Cambodia and elsewhere.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved under the Safe Food, Fair Food Cambodia project and granted by the National Ethical Committee of Cambodia, coded 300NECHR, dated 26th December 2017. The participants provided their written informed consent to participate in this study.
Author contributions
Conception: DG, HN-V, ST, FU, JL, SD-X, SB, and CR. Data curation and writing—original draft: CR and SD-X. Formal analysis: CR, SD-X, and JL. Investigation: CR, SD-X, CT, and ST. Methodology: CR, CT, and SD-X. Supervision: SB, HN-V, ST, SD-X, DG, and JL. Writing—review and editing: SD-X, SB, HN-V, FU, ST, DG, KO, and JL. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the American people through the United States Agency for International Development (USAID) and its Feed the Future Innovation Lab for Livestock Systems managed by the University of Florida. In addition, this project was financially supported by the CGIAR research program on Agriculture for Nutrition and Health A4NH and the CGIAR Initiative on One Health.
Acknowledgments
We would like to acknowledge the support from Livestock Development for Community Livelihood Organization (Mr. Vor Sina, Dr. Huy Sokchea, and Mr. Son Pov), laboratory personnel (Mrs. Sok Koam, Ms. Theng Heng, and Mr. Or Phirum) from the NAHPRI-General Directorate of Animal Health and Production, Ministry of Agriculture Forestry and Fisheries, as well as local veterinary officers and all retailers. Sincerely thank ILRI's team for the statistical consultation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aizaabi, S. E., and Khan, M. A. (2017). A study on foodborne bacterial cross-contamination during fresh chicken preparation. Arab J. Nutr. Exercise (AJNE). 2, 128–138. doi: 10.18502/ajne.v2i2.1251
Baranyi, J., and Roberts, T. A. (1994). A dynamic approach to predicting bacterial growth in food. Int J Food Microbiol. 23, 277–294. doi: 10.1016/0168-1605(94)90157-0
Bollaerts, K., Aerts, M., Faes, C., Grijspeerdt, K., Dewulf, J., and Mintiens, K. (2008). Human salmonellosis: estimation of dose-illness from outbreak data. Risk Anal. 28, 427–440. doi: 10.1111/j.1539-6924.2008.01038.x
Boqvist, S., Söderqvist, K., and Vågsholm, I. (2018). Food safety challenges and One Health within Europe. Acta Vet. Scand. 60, 1–13. doi: 10.1186/s13028-017-0355-3
Botteldoorn, N., Heyndrickx, M., Rijpens, N., Grijspeerdt, K., and Herman, L. (2003). Salmonella on pig carcasses: positive pigs and cross contamination in the slaughterhouse. J. Appl. Microbiol. 95, 891–903. doi: 10.1046/j.1365-2672.2003.02042.x
Brown, S. M., Nguyen-Viet, H., Grace, D., Ty, C., Samkol, P., Sokchea, H., et al. (2022). Understanding how food safety risk perception influences dietary decision making among women in Phenom Phnom Penh, Cambodia: a qualitative study. BMJ Open. 12, e054940. doi: 10.1136/bmjopen-2021-054940
Cambodia Recipe. (2020). Cambodian Chicken Salad Recipe. Available online at: https://www.cambodiarecipe.com (accessed September 15, 2020).
Carrasco, E., Morales-Rueda, A., and García-Gimeno, R. M. (2012). Cross-contamination and recontamination by Salmonella in foods: a review. Food Res. Int. 45, 545–556. doi: 10.1016/j.foodres.2011.11.004
Center for Disease Control and Prevention (US-CDC). (2022). Chicken and Food Poisoning. Available online at: https://www.cdc.gov/foodsafety/chicken.html (accessed November 5, 2022).
Cliver, D. O. (2006). Cutting boards in Salmonella cross-contamination. J. AOAC Int. 89, 538–542. doi: 10.1093/jaoac/89.2.538
Codex Alimentatius Commission. (1999). Principles and Guidelines for the Conduct of Microbiological Risk Assessment. Rome: Codex Alimentatius Commission.
Crump, J. A., and Wain, J. (2017). “Salmonella” in International Encyclopedia of Public Health (Second Edition), Quah, S. R. (Oxford, UK: Academic Press) p. 425–433. doi: 10.1016/B978-0-12-803678-5.00394-5
Dang-Xuan, S., Nguyen-Viet, H., Pham-Duc, P., Grace, D., Unger, F., Nguyen-Hai, N., et al. (2018). Simulating cross-contamination of cooked pork with Salmonella enterica from raw pork through home kitchen preparation in Vietnam. Int. J. Environ. Res. Public Health. 15, 2324. doi: 10.3390/ijerph15102324
Dang-Xuan, S., Nguyen-Viet, H., Pham-Duc, P., Unger, F., Tran-Thi, N., Grace, D., et al. (2019). Risk factors associated with Salmonella spp. prevalence along smallholder pig value chains in Vietnam. Int. J. Food Microbiol. 290, 105–115. doi: 10.1016/j.ijfoodmicro.2018.09.030
Dang-Xuan, S., Nguyen-Viet, H., Unger, F., Pham-Duc, P., Grace, D., Tran-Thi, N., et al. (2016). Quantitative risk assessment of human salmonellosis in the smallholder pig value chains in urban of Vietnam. Int. J. Public Health. 62, 93–102. doi: 10.1007/s00038-016-0921-x
Department of Meteorology of Cambodia. (2019). Announcement: On the Weather Situation on December 01, 2019. Phnom Penh: Department of Meteorology, Ministry of Water Resources and Meteorology.
Devleesschauwer, B., Haagsma, J. A., Mangen, M.-J. J., Lake, R. J., and Havelaar, A. H. (2018). “The global burden of foodborne disease”, in Food Safety Economics. (New York: Springer) p. 107–122. doi: 10.1007/978-3-319-92138-9_7
Duong, M., Brown, S. M., Nguyen-Viet, H., Grace, D., Ty, C., Samkol, P., et al. (2021). Nutrition and Food Safety Perception.
Duong, M.-C., Nguyen-Viet, H., Grace, D., Ty, C., Sokchea, H., Sina, V., et al. (2022). Perceived neighbourhood food access is associated with consumption of animal-flesh food, fruits and vegetables among mothers and young children in peri-urban Cambodia. Public Health Nutr. 25, 717–728. doi: 10.1017/S1368980021004122
Emary, K., Moore, C. E., Chanpheaktra, N., An, K. P., Chheng, K., Sona, S., et al. (2012). Enteric fever in Cambodian children is dominated by multidrug-resistant H58 Salmonella enterica serovar Typhi with intermediate susceptibility to ciprofloxacin. Trans. R. Soc. Trop. Med. Hyg. 106, 718–724. doi: 10.1016/j.trstmh.2012.08.007
Food and Agriculture Organization of the United Nations, the FAOLEX Database. (2022). Cambodia Food Safety Law. Available online at: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC210192/ (accessed September 15, 2022).
Food and Agriculture Organization of the United Nations. (2021). Safe Food Today for a Healthy Tomorrow: Priorities of FAO to Improve Food Safety in Cambodia. Food and Agriculture of the United Nation.
General Directorate of Animal Health and Production of Cambodia. (2021). Annual Report of Animal Health and Production in Cambodia for 2020 and Action Plan for 2021. Phnom Penh, Cambodia: The General Directorate of Animal Health and Production.
Grace, D. (2015). Food safety in low and middle income countries. Int. J. Environ. Res. Public Health 12, 10490–10507. doi: 10.3390/ijerph120910490
Havelaar, A. H., Kirk, M. D., Torgerson, P. R., Gibb, H. J., Hald, T., Lake, R. J., et al. (2015). World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12, 12. doi: 10.1371/journal.pmed.1001923
Kimsean, P., Sreng, K., Has, P., Ly, S., Sim, S., Chhay, S., et al. (2017). An outbreak of gastrointestinal illness associated with khmer noodles: a multipronged investigative approach, Kandal Province, Cambodia, June 2014. OSIR Journal. 9, 1–6. Available online at: http://www.osirjournal.net/index.php/osir/article/view/87
Kristina, R., and Sophal, C. (2018). “Evidence on Foodborne Diseases in Cambodia”, in Safe Food, Fair Food for Cambodia Project Annual Project Meeting 2018. (Phnom Penh, Cambodia).
Kuijpers, L. M. F., Gryseels, C., Uk, S., Chung, P., Bory, S., Sreng, B., et al. (2018). Enteric fever in Cambodia: community perceptions and practices concerning disease transmission and treatment. Am. J. Trop. Med. Hyg. 99, 1369. doi: 10.4269/ajtmh.18-0432
Lam, S., Nguyen-Viet, H., and Unger, F. (2019). Safe Food, Fair Food for Cambodia Project: Report of the Theory of Change Workshop. Nairobi: International Livestock Research Institute.
Majowicz, S. E., Musto, J., Scallan, E., Angulo, F. J., Kirk, M., O'Brien, S. J., et al. (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50, 882–889. doi: 10.1086/650733
Marks, H. M., and Coleman, M. E. (2017). Scientific data and theories for salmonellosis dose-response assessment. Hum. Ecol. Risk Assess. 23, 1857–1876. doi: 10.1080/10807039.2017.1352443
Nadimpalli, M., Fabre, L., Yith, V., Sem, N., Gouali, M., Delarocque-Astagneau, E., et al. (2019). CTX-M-55-type ESBL-producing Salmonella enterica are emerging among retail meats in Phnom Penh, Cambodia. J. Antimicrob. Chemot. 74, 342–348. doi: 10.1093/jac/dky451
Nair, D. V., and Johny, A. K. (2019). “Salmonella in poultry meat production”, in Food Safety in Poultry Meat Production. (New York: Springer) p. 1–24. doi: 10.1007/978-3-030-05011-5_1
National Institute of Statistics of Cambodia. (2019). General Population Census of Cambodia. (Phnom Penh, Cambodia: National Institute of Statistics).
Ngo, H. H. T., Nguyen-Thanh, L., Pham-Duc, P., Dang-Xuan, S., Le-Thi, H., Denis-Robichaud, J., et al. (2021). Microbial contamination and associated risk factors in retailed pork from key value chains in Northern Vietnam. Int. J. Food Microbiol. 346, 109163. doi: 10.1016/j.ijfoodmicro.2021.109163
Nguyen-Viet, H. (2018). Safe Food, Fair Food for Cambodia Project Progress Highlights for the First Year 2018. Nairobi: International Livestock Research Institute.
Oscar, T. (2004). Dose-response model for 13 strains of Salmonella. Risk Analysis. 24, 41–49. doi: 10.1111/j.0272-4332.2004.00410.x
Oscar, T. (2021a). Monte Carlo simulation model for predicting salmonella contamination of chicken liver as a function of serving size for use in quantitative microbial risk assessment Salmonella contamination of chicken liver. J. Food Prot. 84, 1824–1835. doi: 10.4315/JFP-21-018
Oscar, T. (2021b). Salmonella prevalence alone is not a good indicator of poultry food safety. Risk Analysis. 41, 110–130. doi: 10.1111/risa.13563
People in Need. (2015). A Value Chain Analysis of Chicken Production by Cambodian Smallholders: Based on an assessment conducted in Pursat and Kampong Chnnang Provinces, Cambodia. Cambodia: Phnom Penh.
Perez-Rodriguez, F. (2020). Risk Assessment Methods for Biological and Chemical Hazards in Food. Boca Raton, FL: CRC Press. doi: 10.1201/9780429083525
Pérez-Rodríguez, F., Valero, A., Carrasco, E., García, R. M., and Zurera, G. (2008). Understanding and modelling bacterial transfer to foods: a review. Trends Food Sci. Technol. 19, 131–144. doi: 10.1016/j.tifs.2007.08.003
Poomchuchit, S., Kerdsin, A., Chopjitt, P., Boueroy, P., Hatrongjit, R., Akeda, Y., et al. (2021). Fluoroquinolone resistance in non-typhoidal Salmonella enterica isolated from slaughtered pigs in Thailand. J. Med. Microbio. 70, 001386. doi: 10.1099/jmm.0.001386
Possas, A., Carrasco, E., García-Gimeno, R., and Valero, A. (2017). Models of microbial cross-contamination dynamics. Curr. Opin. Food Sci. 14, 43–49. doi: 10.1016/j.cofs.2017.01.006
Public Health of Canada. (2017). Public Health Notice—Outbreak of E. coli Infections Linked to Various Flours and Flour Products. Canada: Public Health Agency of Canada.
Rortana, C., Nguyen-Viet, H., Tum, S., Unger, F., Boqvist, S., Dang-Xuan, S., et al. (2021). Prevalence of Salmonella spp. and Staphylococcus aureus in chicken meat and pork from cambodian markets. Pathogens 10, 556. doi: 10.3390/pathogens10050556
Rortana, C., Sothyra Tum, H. N.-V., Fred, U., Johanna, L., Delia, G., Chhay, T., et al. (2022). Experimental cross-contamination of Salmonella enterica during handling and preparation of chicken salad in Cambodian households. PLoS ONE. 17, e0270425. doi: 10.1371/journal.pone.0270425
Sanaa, M. (2021). “Dose–Response Models for Microbial Risk Assessment,” in Risk Assessment Methods for Biological and Chemical Hazards in Food, Perez-Rodriguez, F. (ed). (Boca Raton, FL: CRC Press) p. 409. doi: 10.1201/9780429083525-16
Sary, S., Shiwei, X., Wen, Y., Darith, S., and Chorn, S. (2019). “Household Food Consumption in Rural, Cambodia Almost Ideal Demand System Analysis”, in Journal of Physics: Conference Series. (Bristol, United Kingdom: IOP Publishing).
Schwan, C. L., Desiree, K., Bello, N. M., Bastos, L., Hok, L., Phebus, R. K., et al. (2021). Prevalence of Salmonella enterica isolated from food contact and nonfood contact surfaces in Cambodian informal markets. J. Food Prot. 84, 73–79. doi: 10.4315/JFP-20-112
Shiowshuh, S., and Cheng-An, H. (2011). Modeling the surface cross-contamination of Salmonella spp. on ready-to-eat meat via slicing operation. Food and Nutrition Sciences. 2, 916–924. doi: 10.4236/fns.2011.29125
Srey, T. (2019). Cost of hospitalization for foodborne diseases. Presented at Safe Food, Fair Food for Cambodia taskforce and stakeholder meeting, Siem Reap, Cambodia, 24-25 October 2019. Phnom Penh, Cambodia: Cambodia Centers for Disease Control and Prevention.
Teunis, P. F., Kasuga, F., Fazil, A., Ogden, I. D., Rotariu, O., and Strachan, N. J. (2010). Dose–response modeling of Salmonella using outbreak data. Int. J. Food Microbiol. 144, 243–249. doi: 10.1016/j.ijfoodmicro.2010.09.026
Thompson, L., Vipham, J., Hok, L., and Ebner, P. (2021). Towards improving food safety in Cambodia: current status and emerging opportunities. Global Food Security. 31, p.100572. doi: 10.1016/j.gfs.2021.100572
Tum, S. (2008). Reducing microbial contamination of meat at slaughterhouses in Cambodia. Policy Brief. Available online at: safetynet2008. com.
Unger, F., Nguyen-Viet, H., Phuc, P. D., Van Hung, P., Thanh, H. L. T., Dang-Xuan, S., et al. (2020). Food safety interventions for traditional pork chains in Vietnam and Cambodia: Success and challenges. Nairobi: International Livestock Research Institute.
Van Asselt, E., De Jong, A., De Jonge, R., and Nauta, M. (2008). Cross-contamination in the kitchen: estimation of transfer rates for cutting boards, hands and knives. J. Appl. Microbiol. 105, 1392–1401. doi: 10.1111/j.1365-2672.2008.03875.x
van Cuyck, H., Farbos-Granger, A., Leroy, P., Yith, V., Guillard, B., Sarthou, J. L., et al. (2011). MLVA polymorphism of Salmonella enterica subspecies isolated from humans, animals, and food in Cambodia. BMC Res. Notes 4, 1–8. doi: 10.1186/1756-0500-4-306
Vandy, S., Leakhann, S., Phalmony, H., Denny, J., and Roces, M. C. (2012). Vibrio parahaemolyticus enteritis outbreak following a wedding banquet in a rural village–Kampong Speu, Cambodia, April 2012. WPSAR. 3, 25. doi: 10.5365/wpsar.2012.3.4.004
Varijakshapanicker, P., McKune, S., Miller, L., Hendrickx, S., Balehegn, M., Dahl, G. E., et al. (2019). Sustainable livestock systems to improve human health, nutrition, and economic status. Anim. Front. 9, 39–50. doi: 10.1093/af/vfz041
Velugoti, P. R., Bohra, L. K., Juneja, V. K., Huang, L., Wesseling, A. L., Subbiah, J., et al. (2011). Dynamic model for predicting growth of Salmonella spp. in ground sterile pork. Food Microbiol. 28, 796–803. doi: 10.1016/j.fm.2010.05.007
Vlieghe, E., Phe, T., De Smet, B., Veng, C., Kham, C., Sar, D., et al. (2013). Increase in salmonella enterica serovar paratyphi a infections in Phnom Penh, Cambodia, january 2011 to august 2013. Eurosurveillance. 18, 20592. doi: 10.2807/1560-7917.ES2013.18.39.20592
Vlieghe, E. R., Phe, T., De Smet, B., Veng, C. H., Kham, C., Bertrand, S., et al. (2012). Azithromycin and ciprofloxacin resistance in Salmonella bloodstream infections in Cambodian adults. PLoS Negl. Trop. Dis. 6, e1933. doi: 10.1371/journal.pntd.0001933
Walia, K., Kapoor, A., and Farber, J. (2018). Qualitative risk assessment of cricket powder to be used to treat undernutrition in infants and children in Cambodia. Food Control. 92, 169–182. doi: 10.1016/j.foodcont.2018.04.047
World Health Organization Regional Office for South-East Asia. (2016). Burden of Foodborne Diseases in the South-East Asia Region. (New Delhi: World Health Organization. Regional Office for South-East Asia.).
Yates, A. (2011). “Salmonella (non-typhoidal)”, Agents of Foodborne Illness. p. 31. Available online at: https://www.foodstandards.gov.au/publications/Documents/salmonella.pdf (accessed November 5, 2022).
Keywords: ASF consumption, Cambodia, QMRA, cross-contamination, Cambodian chicken and pork salad, traditional market
Citation: Rortana C, Dang-Xuan S, Nguyen-Viet H, Unger F, Lindahl JF, Tum S, Ty C, Grace D, Osbjer K and Boqvist S (2022) Quantitative risk assessment of salmonellosis in Cambodian consumers through chicken and pork salad consumption. Front. Sustain. Food Syst. 6:1059235. doi: 10.3389/fsufs.2022.1059235
Received: 01 October 2022; Accepted: 30 November 2022;
Published: 22 December 2022.
Edited by:
M. Leonor Faleiro, University of Algarve, PortugalReviewed by:
Ian Jenson, University of Tasmania, AustraliaPeter S. Evans, Food Safety and Inspection Service (USDA), United States
Copyright © 2022 Rortana, Dang-Xuan, Nguyen-Viet, Unger, Lindahl, Tum, Ty, Grace, Osbjer and Boqvist. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chea Rortana,  cm9ydGFuYS5jaGVhQHNsdS5zZQ==; Sinh Dang-Xuan,
cm9ydGFuYS5jaGVhQHNsdS5zZQ==; Sinh Dang-Xuan,  Uy5EYW5nQGNnaWFyLm9yZw==
Uy5EYW5nQGNnaWFyLm9yZw==
†These authors have contributed equally to this work and share first authorship
 Chea Rortana
Chea Rortana Sinh Dang-Xuan
Sinh Dang-Xuan Hung Nguyen-Viet
Hung Nguyen-Viet Fred Unger
Fred Unger Johanna F. Lindahl
Johanna F. Lindahl Sothyra Tum1
Sothyra Tum1 Chhay Ty
Chhay Ty Delia Grace
Delia Grace Sofia Boqvist
Sofia Boqvist