- 1enviroCORE, Department of Science and Health, Institute of Technology Carlow, Carlow, Ireland
- 2Teagasc-Agriculture and Food Development Authority, Environment Research Centre, Wexford, Ireland
Phosphorus (P) is an essential plant macro-nutrient applied to soil in agriculture, mainly sourced from non-renewable mined phosphate-rock, of which readily accessible reserves are currently under pressure, while global food demand continues to grow. Meanwhile, an abundance of P is lost in waste-streams. Hence, bio-based fertilizers are increasingly produced using nutrient-recovery technologies and evaluated as a sustainable fertilizer alternative. However, there is little knowledge of how these products affect soil microorganisms. In this study, four new phosphate bio-based fertilizers (two struvite and two incinerator ashes) were assessed in permanent grassland-plots to understand their impact on soil bacterial, fungal, and nematode community responses. The experiment consisted of 40 plots (each 6 × 2 m2) of 8 treatments (2 struvite, 2 ash, cattle slurry, 100% mineral fertilizer, zero P fertilizer, and a control without fertilization) with 5 replications arranged in a randomized complete block design. Community data were obtained by amplicon sequencing of DNA extracted from soil samples and subsequent analysis of community composition, diversity, structure and influencing environmental variables. Diversity of the soil microorganisms was maintained by all bio-based fertilizer treatments. Results showed that soil bacterial, fungal, and nematode communities of the struvite-treatments were similar to those in 100% mineral treatment. Communities in ash-treatments were more disturbed in their compositions, abundances and structures, possibly due to their high pH and heavy metal content. From canonical correspondence analysis, available P, K, and Mg, as well as plant P uptake and biomass yield, were identified as factors significantly influencing bacterial and nematode communities across different treatment groups. In particular, the abundance of environmental disturbance sensitive nematodes (e.g., Dorylaimida) was significantly reduced by one of the ash products. Overall, results indicate that both struvites are benign to soil bacterial, fungal, and nematode communities and can be safely applied as a source of renewable P to meet crop nutrition requirement. The ash products require further investigations before recommending their regular application as fertilizer. As the application of novel bio-based fertilizers will increase in the foreseeable future, the findings of this study would be valuable to feed into developing environmental risk assessment protocols.
Introduction
Phosphorus (P) is a critically important plant macro-nutrient, playing several key roles in their successful development and productivity. Hence, it is a routinely applied nutrient in agriculture. However, P is chiefly sourced from finite phosphate rock reserves, which are currently under pressure due to an increasing human population, and thus, global food demand. It is estimated that the existing and easily accessible global P resources will be exhausted within the coming 50–100 years (Steen, 1998; Smil, 2000; Ashley et al., 2009). Additionally, phosphate rock is known to contain contaminants such as cadmium (Cd) rendering some reserves unsuitable for use without its prior treatment (Roberts, 2014). At the same time, there is an abundance of phosphorus-rich organic waste being produced municipally, agriculturally, and industrially (Karunanithi et al., 2015). The development of technologies for nutrient-recovery from such waste streams presents an opportunity to develop a circular nutrient economy, sustainable agricultural practices and maintain global food security (Diaz-Ambrona and Maletta, 2014; Harder et al., 2021). However, not all phosphate fertilizers are made equal, particularly those recovered from organic waste, which can contain components such as antibiotic residues and heavy metals that may affect soil ecosystems (Vollú et al., 2018).
Soil microorganisms are critical to the maintenance of soil quality and health status, and are involved in a multitude of key processes, such as the decomposition of organic matter, recycling of nutrients, mineralization, nitrogen fixation, and enhancing soil structure (Verstraete and Mertens, 2004). Furthermore, they play extensive roles in maintaining plant health, such as protection against pathogens, production of growth stimulating hormones, stimulation of the plant immune system and enhanced stress responses (Hayat et al., 2010). By modeling the increase of functions with increasing diversity using the KEGG orthology database of molecular functions, one recent study predicted bacteria to be responsible for a global total of 35.5 million functions, of which just 0.02% are currently known, and fungi to be responsible for 3.2 million functions, of which only 0.14% are known (Starke et al., 2020). A vast range of biotic and abiotic factors can influence the composition and diversity of soil microbial communities (Fierer and Jackson, 2006). Loss of soil microbial diversity and variation in community structure are associated with the loss and disruption of essential ecosystem functions provided by soils (Singh et al., 2014; Zhen et al., 2014). Low microbial diversity in soil indicates stressful conditions, while high soil microbial diversity is indicative of healthy, productive soil (Rao, 2007). Thus, microbial diversity is considered an indicator of soil health (Nielsen and Winding, 2002).
Nematodes are the most numerous and widespread animals in nature (Wilson and Kakouli-Duarte, 2009) and can be used as bioindicators of environmental disturbance because of their diversity, feeding behaviors and quick respond to changes in soil management (Bongers and Bongers, 1998). The diversity and high abundance of nematode communities influence the global carbon cycle and highlight their functional importance in nutrient cycling and agricultural soil food-web functioning (Hoogen et al., 2019). The nematode assemblage in agricultural soil is affected by anthropogenic disturbances such as organic and inorganic fertilization, the application of herbicides and pesticides, tillage, and crop rotation (Treonis et al., 2010; Thiele-Bruhn et al., 2012; Zhang et al., 2017). Nematodes are assigned to a colonizers and persisters (C-P) scale according to their life strategies (Bongers and Ferris, 1999). Environmental disturbance causes a decrease in the number of persister nematode species and shifts toward dominance of colonizing species (Wardle and Yeates, 1993). The reduction in persisters, such as dorylaimids, is a sign of disturbance and stress in the soil. This project is the first to employ the application of nematode communities, using the C-P scale, for the study of the impact of these novel bio-based fertilizers, globally, and also in an Irish context.
The focus of this study is on struvite and ash products, herein termed as recycling derived fertilizers (RDFs), which have been recovered from phosphate-rich sources including municipal waste, industrial potato waste, poultry litter, and sewage sludge. Grass-based farming systems are of significant importance in Irish agriculture. In 2020, ~82.1% of the agricultural land area utilized in Ireland was grassland (CSO, 2020). It is the most abundant and cheapest available feed for ruminant livestock in Ireland and provides a significant competitive advantage in profitable and sustainable milk and meat production. Traditionally, conventional mineral fertilizers like superphosphate, calcium ammonium nitrate, muriate of potash, sulfate of potash, and organic fertilizer like cattle slurry are commonly used in grasslands of Ireland to meet major crop nutrition (i.e., nitrogen, phosphorus, potassium, and sulfur) requirement (Ashekuzzaman et al., 2021a). Herein, cattle slurry and mineral superphosphate based treatments were also included to compare with new struvite and ash based RDF products. Struvite is an ammonium magnesium phosphate mineral (MgNH4PO4.6H20) that can be produced by crystallization and precipitation technologies (Le Corre et al., 2009; Lorick et al., 2020), while incineration technologies can be used to produce ashes that are rich in phosphorus (Franz, 2008). Struvite and ash products have been evaluated as agricultural fertilizers in several studies (Codling et al., 2002; Severin et al., 2014; Herzel et al., 2016; Rech et al., 2019; Hertzberger et al., 2020; Saerens et al., 2021; O'Donnell et al., 2022). However, there have been few attempts to extensively investigate the response of soil microorganism communities to their application. Recently, we published a study on the response of soil microbial and nematode communities to the application of RDFs in a phosphate fertilizer replacement value (P-FRV) trial (Karpinska et al., 2021). The aim of the current study was to assess the affects of four RDF product on the ecological diversity of grassland soil. We analyzed the response of the communities when the RDFs were integrated into a conventional grassland fertilization programme for two consecutive years, to study their behavior under a typical farmer use case, where these new phosphate-based RDFs are combined with mineral fertilizers, to give a balanced nutrient programme for the grassland crop. Due to the differing physiochemical properties of the P sources used and the initial low P index of the soil, we hypothesized some changes to be observed in microbial and nematode community dynamics.
Methods and Materials
Field Description and Experimental Design
The field experiment was established in 2019 and conducted on a P deficient site (P index 1, 1–3 mg P/L by Morgan's extractant) at the Teagasc, Johnstown Castle, Soils, Environment and Land Use Research Center, County Wexford (52°17′46.9″N 6°30′32.1″W). The soil had initial average pH of 5.6 and is of sandy loam texture. Lime was applied at 1.5 t ha−1 at the beginning of the experiment as per agronomic recommendations. Perennial ryegrass [vars. AberGreen (40%), AberChoice (30%), and AberGain (30%), Germinal Ireland Ltd. (Thurles, County Tipperary, Ireland)] was sown in September 2018 and initial fertilizer application occurred 7 months later, in April 2019. The N, K, and S fertilizers were applied in the mineral form at the recommended dosages of 125 kg N/ha, 155 kg K/ha, and 20 kg S/ha. The P fertilizer was applied at a rate of 40 kg P/ha, in the form of conventional fertilizers or RDF under test. Depending on the source, some RDFs can contain other nutrients (Table 1). Where RDF also contained N, K, or S, these nutrients were considered, and only enough chemical fertilizer was supplied to meet the recommended dosage to achieve balanced crop nutrition. Second and third applications of mineral fertilizers N, K, and S took place in May and July 2019, at dosages of 100 kg N/ha, 75 kg K/ha, 20 kg S/ha, and 10 kg P/ha. This fertilization program was repeated in 2020. Supplementary Table 1 details the balanced nutrient amounts applied for each fertilization treatment. The trial was arranged in a randomized block experiment, with individual treatment plots of 2 m × 6 m size. There were five replicates per treatment. Negative controls included a no fertilizer treatment (NF) and a zero phosphate treatment (SP0) with addition of N, K, S only. Also, two commonly practiced conventional fertilizer programmes—(1) superphosphate (SP40) based 100% mineral treatment and (2) cattle slurry with combination of mineral N, P, K, and S to meet crop nutrient requirement were used (application rates in Supplementary Table 1). Both these treatments served as positive controls. Recycling-derived fertilizer treatments included two struvite—one derived from potato processing waste (PWS) and the other from municipal waste (MWS), and two ashes—one derived from poultry litter (PLA) and the other from sewage sludge (SSA).
Soil Sampling and Preparation for DNA Extraction and Sequencing
Soil sampling was conducted in the second growing season, following cutting of grass silage (July 2020), 6 weeks after fertilizer application. Nine sample cores were taken in a “W” shaped pattern to a depth of 10 cm per plot and stored at −20°C until ready for analysis. Forty composite samples were obtained in total (8 treatments × 5 replicate plots). Defrosted samples were sieved through a 2 mm mesh to homogenize. For bacterial and fungal analysis, 0.25 g sub-samples were further processed immediately. For nematode analysis, 25 g sub-samples of soil were shaken in 25 ml of deionized water for 10 min at 95 rpm and centrifuged for 2 min at 3,500 rpm. The supernatant was discarded, and the remaining material was dried overnight at 28°C in petri dishes before thoroughly homogenizing again using a mortar and pestle. Finally, 0.25 g sub-samples were used for further processing. Total DNA, for all taxa analyzed, was extracted from 0.25 g soil sub-samples using the DNeasy® PowerSoil® Pro kit (QIAGEN Ltd., Manchester, UK), as per the manufacturer's instructions. Total DNA quality and quantity was assessed by NanoDrop™ instrument (Fisher Scientific, Cork, Ireland) and agarose gel electrophoresis using 1% agarose gels. Once samples of sufficient quality were obtained, they were outsourced to Novogene Co., Ltd. (Milton Road, Cambridge, UK) for amplicon sequencing. Bacterial 16S V4–V5 region rRNA, fungal ITS1 region rRNA and nematode 18S V4 region rRNA were sequenced using 515F and 907R (Caporaso et al., 2011; Armitage et al., 2012), ITS5-1737F and ITS2-2043R (Schoch et al., 2012), and MN18F and 22R (Bhadury et al., 2006) primer pairs, respectively, on Illumina paired-end platform.
Sequence Data Analysis
Sequenced data was processed and clustered into operational taxonomic units (OTUs) based on a 97% similarity threshold by the sequencing company. Taxonomy was assigned to bacterial and nematode OTUs in QIIME2 (version 2020.11) (Bolyen et al., 2019), using the SILVA (release 138 SSURef_NR99) database (Quast et al., 2012), and to fungal OTUs using UNITE (version 8.2) database (Nilsson et al., 2018). Targeting the ITS1 region of fungi also results in sequencing of this region in other eukaryotic organisms. Therefore, the data was filtered to remove OTUs which did not belong to the fungal kingdom. For subsequent alpha and beta diversity analyses, the number of sequences per sample were normalized to a number which achieved sufficient sequencing depth, while retaining as many samples as possible. Levels of bacterial, fungal, and nematode diversity were assessed by Observed OTU and Shannon alpha diversity indices, and statistically compared by Kruskal-Wallis H test. Beta diversity was measured by Weighted Unifrac distances followed by permutational multivariate analysis of variance (PERMANOVA), based on 999 Monte-Carlo permutations. Differences between the nematode communities were detected via a non-parametric test ANOSIM (Analysis of Similarity) and MetaStat. Statistical analyses of bacterial and fungal abundances and correlation analysis were performed in IBM SPSS Statistics for Windows, version 27 (IBM Corp, 2020). To test for significant changes in the relative abundances of the microorganisms, one-way ANOVA with Tukey's Honestly Significant Difference (HSD) post-hoc test was employed. Where data violated the homogeneity of variance or normality assumptions, the non-parametric Kruskal-Wallis H test with Dunn's post-hoc pairwise comparisons corrected with the Bonferonni adjustment were used. Pearson's correlation analysis was performed to identify relationships between soil and plant variables and the abundances of bacteria, fungi and nematodes at specific taxon levels. Bacterial and fungal correlation analysis was performed at genus level on the core genera, while nematode correlation analysis was performed at order level. Canonical correspondence analysis (CCA) was performed using R software (version 4.0.4) (R Core Team, 2021), based on OTU presence/absence data, to examine whether the communities of the microorganisms and nematodes were significantly influenced by soil physiochemical and plant variables. Soil variables included total carbon (TotC), organic carbon (OC), organic matter (OM), pH, Morgan's reagent extractable available P (MorgP), K (MorgK) and Mg (MorgMg), and total N (N). Plant variables included dry matter yield (DMYield) and phosphorus uptake (PUptake). Visualizations including principal coordinates analysis (PCoA) were also performed with R software. Standard sample preparation and analytical techniques as detailed in Ashekuzzaman et al. (2021b) were used to determine the above soil and plant parameters. For example, fresh soil samples were dried at 40°C for 72 h and subsequently ground to <2 mm using a soil sieving machine in preparation for chemical analysis. Morgan's reagent (prepared as per Morgan, 1941) solution was used to extract soil to determine extractable P, K, Mg, and LR. The analysis was conducted on the Lachat system (Lachat QuickChem 8500 Series 2 continuous flow analyzer, Hach Lange GmbH, Düsseldorf, Germany) colorimetrically for P and Mg, and photometrically for K. Soil pH was determined using a Metler-Toledo pH electrode in the pH autoanalyser system (Gilson 215 Liquid Handler, Gilson Inc., Middleton, WI, USA). Total carbon (TC) and total N were measured by high temperature combustion method using LECO TruSpec CN analyser (LECO Corporation, St. Joseph, MI, USA). Fresh subsamples of grass were weighed and then dried in perforated plastic bags in an oven at 70°C for 72 h. Once dried dry weight was recorded for dry mass analysis and subsequently, dried samples were grounded and sieved to 2 mm size and used for nutrient analysis. Total crop was analyzed using an Agilent 5100 synchronous vertical dual view inductively coupled plasma optical emission spectrometer (Agilent 5100 ICP-OES, Agilent Technologies, Inc., Santa Clara, CA, USA) following the microwave assisted acid digestion of sieved samples (USEPA, 1996).
Results
The responses of soil bacterial, fungal, and nematode communities to bio-based RDFs, including two struvite and two ashes, were analyzed to assess the effects of integrating these products into a conventional grassland fertilization program. The pH and nutrient contents of the RDFs studied varied depending on the source, which are detailed in Supplementary Table 1. The pHs of ash products applied were high, while struvite products were mildly alkaline. Ashes also contained considerably higher quantities of S and Ca, compared to struvites, while struvites were richer in N. Poultry litter ash (PLA) was particularly rich in K, while SSA had high levels of Al and Fe present.
Composition of Microbial and Nematode Communities
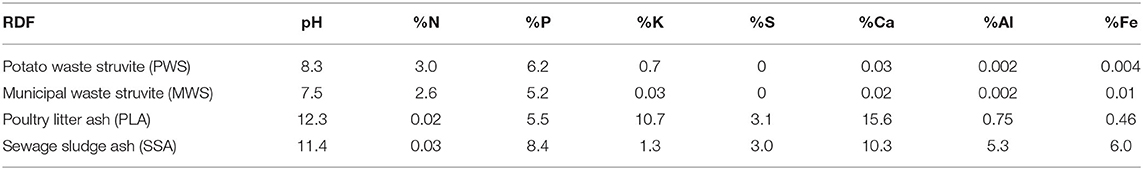
After quality filtering, totals of 3,732,486 (42,366–115,480 sequences per sample), 1,951,491 (23,769–64,421 sequences per sample) and 5,576,906 (123,666–149,634 sequences per sample) 16S rRNA, ITS and 18S rRNA sequences were obtained, respectively. Microbial relative abundance data was used to characterize and compare the dominant phyla and genera of the various treatment groups. The top 10 bacterial phyla covered an average of 96.6% of total sequences across samples (Figure 1A). Actinobacteriota was the dominant phylum of all treatment groups and covered an average of 29.7% bacterial sequences among the samples. The remaining phyla dominating samples included Proteobacteria (19.1%), Firmicutes (18.4%), Acidobacteriota (9.4%), Planctomycetota (6.1%), Chloroflexi (5.3%), Verrucomicrobiota (4.3%), Myxococcota (2.1%), Bacteroidota (1.1%), and Gemmatimonadota (0.9%). In individual treatment groups, this order of dominance was consistent across all treatments, except for MWS and both ash treatments (PLA, SSA). In these treatments, the Firmicutes were more abundant than Proteobacteria. In the case of MWS, this difference was marginal, and the abundances of both phyla were practically equivalent to one another. However, among ash treatments, the increased abundance of Firmicutes was more evident, accounting for 19.2% of bacteria in the PLA treatment and 20.8% in the SSA treatment, while Proteobacteria covered 18.0% and 18.5% in PLA and SSA treatments, respectively. While a number of significant differences were detected between treatments in the abundances of the top 10 bacterial phyla, just one was detected between fully fertilized treatments (Supplementary Table 2). This was between the two positive controls (SP40 and CS), due to the enrichment of Actinobacteriota in the CS treatment. A small number of archaeal sequences (<0.01%) were present among the 16S data. The core bacterial microbiome, which is here defined as those bacterial genera accounting for >1% of total sequences, consisted of 19 genera, which together represented just over half (50.2%) of the total bacterial community (Figure 1B). The top two genera, Bacillus (8.9%) and an uncultured genus belonging to the order Gaiellales (5.5%), were the most abundant bacteria in all samples. Therein, the order of genera dominance was variable among samples and treatment groups. Statistical significance tests (p < 0.05) proved CS and MWS to show the most variability in abundances of the core bacterial genera (Supplementary Table 3). The most considerable of these was the significant enrichment of Nocardioides under the CS treatment, compared with the majority of other fertilization treatments.
Figure 1. The average relative abundances of (A) the top 10 bacterial phyla and (B) the core bacterial genera, observed in each of the treatment groups (n = 5). Treatment group name: NF, No fertilizer; SP0, No phosphate; SP40, mineral super phosphate; CS, cattle slurry; PWS, potato waste struvite; MWS, municipal waste struvite; PLA, poultry litter ash; SSA, sewage sludge ash.
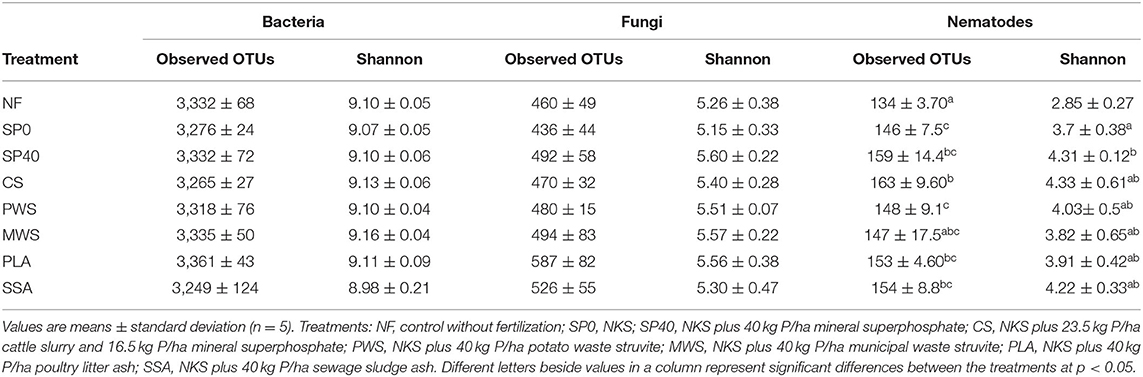
On average, over half of fungal sequences in samples were assigned to the phylum Ascomycota (55.9%), while 12.6% were assigned to Basidiomycota and 4.0% to Mortierellomycota (Figure 2A). The remaining sequences belonged to either unidentified phyla within the fungal kingdom, or very low abundance fungal phyla. The Basidiomycota phylum was found to be significantly enriched in the CS treatment compared to SP40, PWS and PLA treatments (Supplementary Table 4). Fourteen fungal genera constituted the core fungal microbiome, representing an average of 78.6% of total fungal sequences across samples (Figure 2B). However, the dominant genus, which comprised 19.4% of these, could only be identified as belonging to the fungal kingdom. Otherwise, Pseudeurotium (12.4%) was the dominant genus among samples. The order of dominant genera and their abundances were highly variable among treatments. Despite this, few significant differences were observed in fungal abundances (Supplementary Table 5). The most substantial of these was the increased abundance of Apiotrichum in CS treatment, though this was only significantly higher than in the PLA and SSA treatments (p = 0.002, p = 0.043).
Figure 2. The average relative abundances of (A) the top three fungal phyla and (B) the top 10 fungal genera, observed in each of the treatment groups (n = 5). Treatment group name: NF, No fertilizer; SP0, No phosphate; SP40, mineral super phosphate; CS, cattle slurry; PWS, potato waste struvite; MWS, municipal waste struvite; PLA, poultry litter ash; SSA, sewage sludge ash.
The soil nematode community was dominated by the order Dorylaimida (52%), followed by Monhysterida (18.8%), Tylenchida (10%), Triplonchida (9.5%), Rhabditida (5.5%), and Araeolaimida (3.3%), together accounting for an average of 99% of the total nematode sequences in samples (Figure 3). The relative abundance of sensitive to environmental disturbance dorylaimids decreased in SSA treatment when compared with that in NF (p = 0.02) and SP 40 (p = 0.02) control treatments (Supplementary Table 6). There was also a significant increase of environmental stress tolerant monhysterids in the SSA treatment, as well as food opportunistic rhabditids, and plant parasitic tylenchids, when compared with the NF control (p = 0.01, p = 0.001, and p = 0.003, respectively). The CS treatment significantly increased the numbers of enrichment opportunistic rhabditids, when compared with NF (p < 0.001) and SP 40 (p = 0.008) controls, due to the input material rich in organic matter. Poultry litter ash significantly (p = 0.007) increased the relative abundance of plant parasitic tylenchids when compared with NF unfertilized control. On the other hand, the numbers of tylenchids significantly (p = 0.01) decreased in PLA treatment when compared with SP 40, the mineral control. The numbers of bacteria feeding araeolaimids were significantly lower in NF treatment when compared with those in CS (p = 0.03), PWS (p = 0.01), and MWS (p = 0.02) treatment.
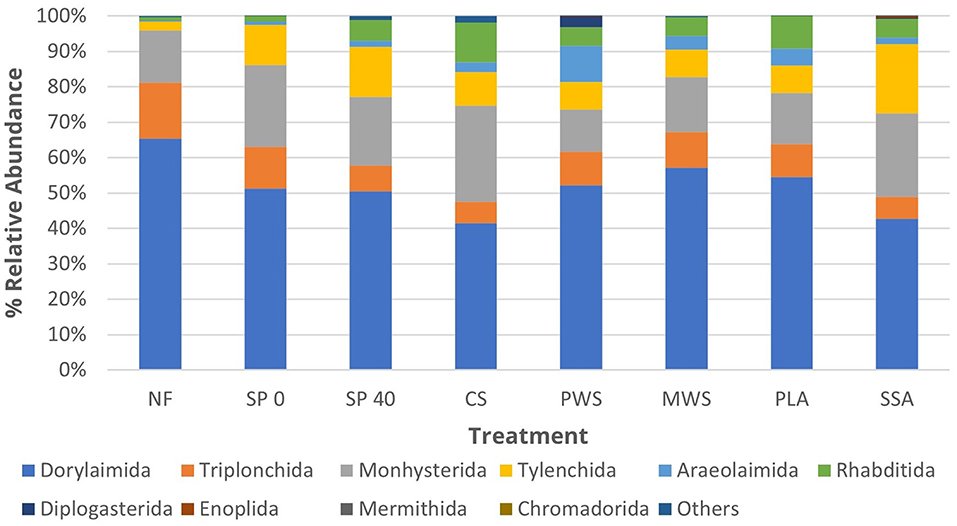
Figure 3. The average relative abundance of the top 10 nematode orders in each treatment group (n = 5). Treatment group name: NF, No fertilizer; SP0, No phosphate; SP40, mineral super phosphate; CS, cattle slurry; PWS, potato waste struvite; MWS, municipal waste struvite; PLA, poultry litter ash; SSA, sewage sludge ash.
Alpha Diversity
Bacterial, fungal, and nematode sequence data were rarefied to 64,910, 28,568 and 33,198 sequences per sample, respectively. This resulted in the loss of two samples in bacterial community analysis (one from PLA and one from SSA) and the loss of one sample in fungal community analysis from the NF treatment, in order to achieve a higher sequencing depth. Alpha diversity metrics, used to measure richness and evenness of bacterial, fungal, and nematode communities within treatment groups, included observed OTUs and Shannon's index. Treatment groups were compared statistically for differences of significance in alpha diversity (p ≤ 0.05). On average, the PLA treatment had the richest (observed OTUs) bacterial community, while the community of MWS was the most even (Shannon's index). The lowest levels of bacterial richness and evenness were observed in SSA treatment. The PLA treatment also exhibited the richest fungal communities, while the SP40 had the most even fungal community. Fungal community richness and evenness were lowest in SP0 treatment. However, statistically, bacterial and fungal communities were found to be equally diverse across all treatment groups, with no significant differences between the treatments to report. Levels of nematode diversity within the different fertilization treatment groups were found to be similar, apart from the NF control treatment, in which the number of observed species, as well as species richness and evenness, were significantly lower when compared with those in the remaining treatments (Table 2).
Beta Diversity
Significant differences in bacterial and fungal communities among the various fertilization treatments (p ≤ 0.05) were determined by PERMANOVA statistical procedure performed on both Weighted and Unweighted versions of Unifrac. Global significance was detected among the bacterial communities of treatments based on both Weighted (p = 0.002, F = 2.16) and Unweighted (p = 0.001, F = 1.07) Unifrac distances. This was also observed among the fungal communities of treatments based on Weighted (p = 0.003, F = 2.04) and Unweighted Unifrac distances (p = 0.001, F = 1.72). A full list of PERMANOVA pairwise comparisons and their significance values are in Supplementary Table 7. Based on Weighted Unifrac data, which accounts for both the phylogenetic relatedness of communities, as well as the abundances of community members, both the bacterial and fungal communities of CS treatment were significantly different to those in all other treatment groups. Two RDF (MWS and PLA) also displayed significantly different bacterial communities to control treatments, while the only other significantly different fungal communities to report were between the unfertilized soil treatment (NF) and the RDF treatments PWS and PLA. Weighted Unifrac distance data was visualized by Principal Coordinates Analysis (PCoA), which captured 55.8% of bacterial and 57.1% of fungal community variability on the first two axes, respectively (Figures 4A, B). However, minimal separation of treatment communities was evident, with many treatment samples more closely related to samples of other treatments than to other samples within their own treatment group. Despite that, the bacterial community of MWS treatment and fungal community of CS treatment appeared to form distinct clusters. Various RDF treatments, as well as CS treatment had significantly different bacterial communities compared to the negative control treatments (NF and SP0) when analyzed using the Unweighted Unifrac metric (Supplementary Table 8). This version considers only the phylogenetic relatedness of communities and is more sensitive to differences in low-abundance features. Only 8.4% of the bacterial community variation could be captured by PCoA (Figure 4C). Meanwhile, the fungal communities of both ash RDF treatments (PLA and SSA) significantly differed to most other treatments. In PCoA, which represented 34.3% of community variation, there was coherent separation of the fungal communities of both these ash treatments from the remaining treatments (Figure 4D).
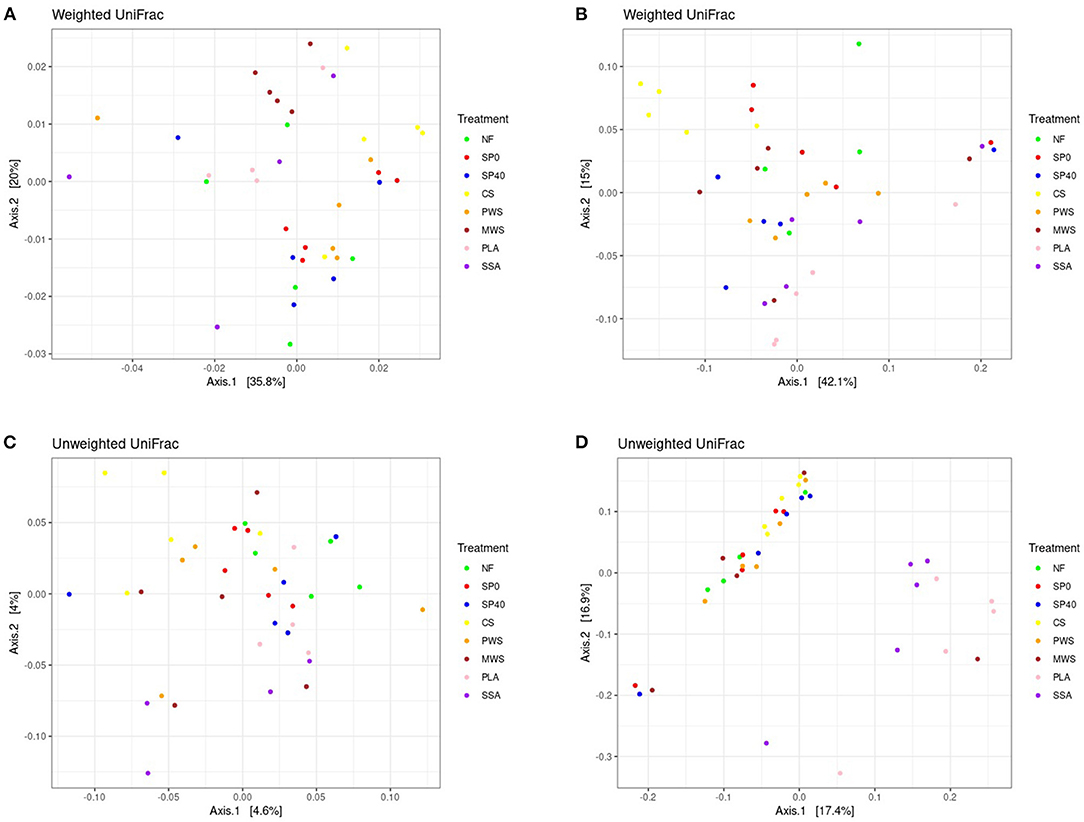
Figure 4. Principal coordinates analysis of (A) bacterial communities and (B) fungal communities based on Weighted Unifrac distances and of (C) bacterial communities and (D) fungal communities based on Unweighted Unifrac distances between samples. Samples are colored by treatment group. NF, No fertilizer; SP0, No phosphate; SP40, mineral super phosphate; CS, cattle slurry; PWS, potato waste struvite; MWS, municipal waste struvite; PLA, poultry litter ash; SSA, sewage sludge ash.
The analysis of similarity (ANOSIM) performed on Weighted Unifrac distances revealed that nematode communities in the NF control treatment differed significantly (p ≤ 0.05) from those in all other treatments, with the exception of the MWS treatment. The nematode communities of soil treated with SSA were found to be significantly different to those in NF and SP0 treatments, while the communities in CS differed significantly from those in SP 40. Based on Unweighted Unifrac distances, the communities of the CS treatment were significantly (p ≤ 0.05) different from those in NF, SP0, PWS, MWS, and PLA treatments. Pairwise comparisons of treatment groups with significantly different nematode communities are listed in Supplementary Tables 9, 10.
Environmental Variables
Several correlations were found between the variables and bacterial (Supplementary Table 11), fungal (Supplementary Table 12), and nematode (Supplementary Table 13) taxa, but only a small proportion of these fell into the class of strong correlations (r > ±0.5). Most notably, the bacterial genus Nocardioides had a strong positive correlation with four of the soil variables, including total C (r = 0.548, p = <0.001), available K (r = 0.642, p = <0.001), available Mg (r = 0.537, p = <0.001), and N content (r = 0.594, p = <0.001). Other strong relationships observed were negative correlations between available K and Bacillus (r = −0.553, p = <0.001) and an unidentified genus belonging to the Planococcaceae family (r = −0.530, p = <0.001), and between dry matter yield and an unidentified genus of the order Vicinamibacterales (r = −0.558, p = <0.001). Four strong negative correlations were identified among the fungal genera and environmental variables. An unidentified genus of the order Coniochaetales was negatively correlated with dry matter yield (r = −0.617, p = <0.001) and P uptake (r = −0.562, p = <0.001). The remaining strong negative relationships were between dry matter yield and Metarhizium (r = −0.523, p = <0.001) and between organic carbon content and an unidentified genus belonging to the order Hypocreales (r = −0.509, p = <0.001). The analysis revealed a strong positive relationship between available P content and the relative abundance of the nematode orders Rhabditida (r = 0.537, p = <0.001) and Araeolaimida (r = 0.552, p = <0.001), while available K was positively correlated with the relative abundance of monhysterids (r = 0.524, p = 0.001). The analysis also revealed a strong positive relationship between uptake P and the numbers of rhabditids (r = 0.509, p = 0.001), and a weak positive relationship with araeolaimids (r = 0.321, p = 0.043). The DM yield was also weakly positively correlated with the relative abundance of rhabditids (r = 0.340, p = 0.032).
Canonical correspondence analysis (CCA) showed several of the soil and plant variables to have significant influences on bacterial and nematode communities, while fungal communities were not significantly affected. The total variations in bacterial and nematode communities explained by the variables were 18.2 and 20.3%, respectively. Table 3 details the average measurements obtained for soil and plant variables. The bacterial communities of the CS treatment, and to a lesser extent, the MWS treatment, were significantly influenced by available K (MorgK), available Mg (MorgMg) and N content (Figure 5A). The CCA plot also showed that dry matter yield (DMYield), P uptake and available P (MorgP) were the major determinants of bacterial community compositions in the RDF phosphate containing treatments, particularly PWS. The uptake P, soil available P and DM yield, appeared to be significant driving factors in shaping the nematode communities in the SSA, and to a lesser extent, MWS treatments, while available K and Mg played a significant role in shaping the community of the CS treatment (Figure 5B). In contrast, NF and SP0 treatments that received no P during the application of the fertilizers showed the least association with DM yield, available and uptake P.
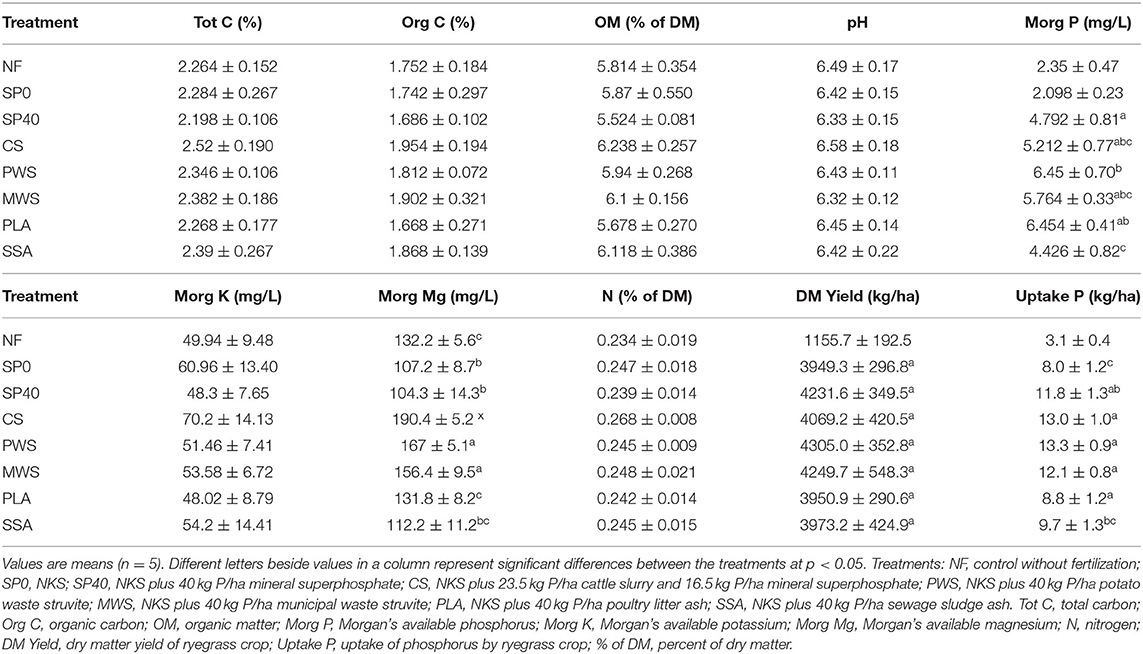
Table 3. Soil physiochemical properties of treatments, crop yield and P uptake after the second ryegrass harvest.
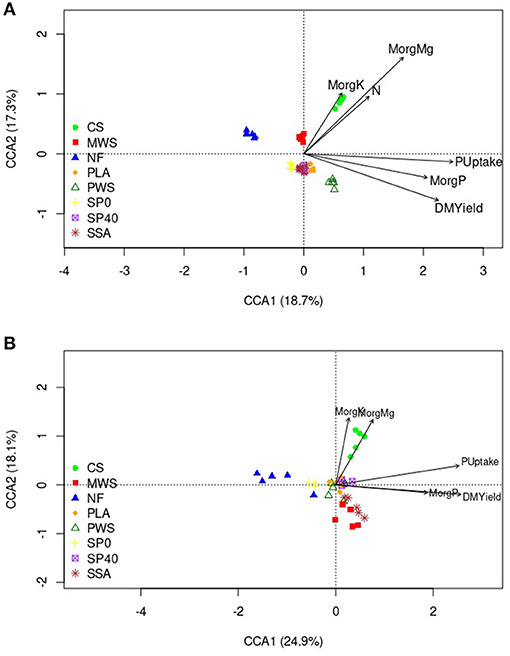
Figure 5. CCA biplot of (A) bacterial OTU data (B) nematode OTU data and the soil variables that were significantly associated. Arrows indicate the direction and magnitude of measurable variables significantly associated with the community structures. Treatment group name: NF, No fertilizer; SP0, No phosphate; SP40, mineral super phosphate; CS, cattle slurry; PWS, potato waste struvite; MWS, municipal waste struvite; PLA, poultry litter ash; SSA, sewage sludge ash.
Discussion
In this study, we aimed to characterize the soil microbial and nematode responses to the application of bio-based phosphate RDFs, when incorporated into conventional grassland management programmes in Ireland. This was achieved by means of amplicon sequencing, and subsequent analysis of sequenced data, including community composition and abundance analysis, alpha and beta diversity analysis and investigations of relationships between communities and environmental variables. Results showed differing effects of the various RDF on bacterial, fungal, and nematode communities. Briefly, communities in struvite treated soil were similar in composition and structure to the 100% mineral P control treatment, while the communities of ash treatments displayed more disturbance, possibly as a result of their properties, such as pH and heavy metal content. Of the soil and plant variables measured, available P, K, and Mg, as well as plant P uptake and dry matter yield were found to have significant influence on soil bacterial and nematode communities, while fungal communities were not affected by any of the measured variables.
Community Composition and Abundance
Bacterial community compositions of the various fertilizer treatment groups remained relatively consistent at phylum level, with very few significantly altered abundances detected. While some significant alterations in bacterial phylum abundance resulted from the addition of nutrients to the soil, more were expected, particularly when the full recommended doses of NPKS were applied. No significant changes were found in the most abundant phylotypes between positive control treatments (mineral SP40 and CS) and RDF treatments. This suggested that the resident bacterial phylotypes of grasslands are resilient to abundance changes when different types of phosphate fertilizer are used.
Actinobacteriota, which are typically dominant in soils, with a diverse range of roles (Zhang et al., 2019), were the dominant bacterial phylotype. Unfertilized soil (NF) had the lowest abundance of Actinobacteriota, which appeared to be stimulated in the other treatments with nutrient additions, though only to a significant level in CS and MWS treatments. Actinobacteriota are generally considered copiotrophic bacteria (Ramirez et al., 2012), which was further supported by the positive correlations observed between Actinobacteriota and all environmental variables (except pH), which typically increased with N and P addition.
Acidobacteriota were of higher abundance in unfertilized soil (NF), though only the SSA treatment had a significantly lower abundance in comparison. This was reflected in correlation analysis, which showed that plant dry matter yield and P uptake had significantly negative relationships with increasing Acidobacteriota. While the life strategy of Acidobacteriota is debated, they are more consistently associated with oligotrophic environments (Ho et al., 2017), which supports these findings. The lower abundance of Bacteroidota in soil fertilized with NKS, but without P, suggested they thrived with P addition, which was supported by the positive correlation with soil P availability and plant P uptake. Soil phosphate content has previously been shown to have a significant positive influence on the abundance of Bacteroidota (Wolińska et al., 2017).
The increase of the Firmicutes phylum in ash treatments (PLA and SSA) which led to an alteration in the order of dominant bacteria, surpassing the Proteobacteria, could not be explained via Pearson's correlation analysis, as the negative relationships detected were skewed by the lower abundance of Firmicutes and higher values of the environmental variables in the CS treatment. Although not reflected in the soil's pH, a likely explanation for this shift in dominance was the high pH of the ash products applied, which were 12.3 and 11.4 in PLA and SSA, respectively. This is supported by previous studies which found wood ash application (Bang-Andreasen et al., 2017, 2020), as well as rapid increases in pH (Anderson et al., 2018), to increase the abundance of Firmicutes.
Just one of the significantly different bacterial genera between treatments were identifiable, with the remainder only identifiable as lineages within higher taxonomic levels. This was among the Nocardioides and was in fact, the most significant abundance change detected throughout the study. The Nocardioides were significantly enriched in the CS treatment in comparison to all other treatments but one, MWS. This genus can utilize a wide range of carbon and nitrogen sources, including unusual organic compounds and toxic environmental pollutants (Evtushenko et al., 2015). A strong positive correlation was observed between Nocardioides and total C and N, while organic C and organic matter displayed moderate positive correlations. These inputs were highest when cattle slurry (CS) organic amendment was added to soil, indicating its application allowed the Nocardioides to thrive.
Abundance changes in fungal phyla and genera were more obvious compared to those in bacteria. However, statistically, few differences were observed due to the high variability of taxa abundances in samples. Ascomycota, which are generally dominant in soils worldwide due to their dispersal ability and their frequency of genomic traits associated with stress-tolerance and competitive abilities (Egidi et al., 2019), remained the dominant fungal phylum in all treatment groups. These were followed by an unidentified fungal phylum in all treatments but NF and CS, in which Basidiomycota were the second most abundant phylum. Increased abundance of Basidiomycota in the CS treatment was largely attributable to the genus Apiotrichum, which are yeasts frequently found in soil. Apiotrichum harbor an abundance of carbohydrate degrading enzymes and are involved in the degradation of various forms of complex substrates (Aliyu et al., 2020), indicating that these were the main fungi involved in the decomposition of the organic component incorporated in the CS treatment.
Myrmecridium, belonging to the Ascomycota, were of significantly higher abundance in SP0 and MWS treated soils, compared to the NF control. Knowledge on this genus is currently lacking, but it potentially consists of saprobial and plant endophytic fungi (Peintner et al., 2016). Another genus of Ascomycota, Metarhizium, was of highest abundance in the unfertilized soil (NF), but only significantly more abundant than in PLA treated soil. This fungal genus decreased in abundance with NKS inputs, and to a greater extent when P was included. Despite being known for their plant growth promoting effects (St. Leger and Wang, 2020), a strong negative correlation was found between Metarhizium and DM yield. Recently, a number of Metarhizium species have been shown to produce high titers of phytase, which can decompose phytic acid, which makes up 60–80% of organic phosphate in soil (Siqueira et al., 2020). Perhaps, these fungi were mining organic phosphorus in NF soil, with their role becoming less important with P inputs. Moreover, Metarhizium are best known as entomopathogenic fungi, acting as biocontrol agents of insects, arachnids, and other arthropod pests, meaning their significant decrease in PLA treatment could potentially make plants vulnerable. However, it is possible that other entomopathogenic fungi became more established in this treatment.
The relative abundance of bacteria feeding rhabditids and araeolaimids was significantly lower in the NF treatment when compared with that in MWS and SSA treatments; nematode communities in both RDF treatments were positively correlated with available P. The NF treatment showed the lowest uptake P and DM yield when compared with the remaining treatments. The CS treatment had significantly higher numbers of bacteria feeding rhabditids, whereas PWS had significantly higher numbers of bacteria feeding araeolaimids in comparison to the NF control. Both these treatments displayed highest average uptake P values. Additionally, Pearson's correlation analysis revealed a positive relationship between the relative abundance of rhabditids and araeolaimids with soil available P and plant uptake P. These findings suggest that during this trial, the nematode orders Rhabditida and Araeolaimida directly positively contributed to either phosphorous uptake by plants or dry matter yield. Nematode species belonging in the orders Rhabditida and Araeolaimida are mostly bacterial feeding organisms (Yeates et al., 1993). Plants growing in soil with bacteria and bacterial feeding nematodes take up more nitrogen and grow faster than plants growing in soil with bacteria only (Ingham et al., 1985). Bacterivorous nematodes play a significant role in N mineralization (Anderson et al., 1983; Hunt et al., 1987; Ferris et al., 1998; Djigal et al., 2004), nutrient cycling (Griffiths, 1990) and enhancing N and P availability (Irshad et al., 2011).
Diversity and Community Structure
Alpha diversity of bacterial and fungal communities remained similar, with no significant effect of fertilization treatment type detected. Our results concur with a study conducted across 25 grassland sites across the globe, which found that nutrient additions did not strongly alter bacterial or fungal diversity (Leff et al., 2015), and suggest that each fertilization regime contributed to equally rich and even microbial communities. This is important due to the threat imposed on ecosystem stability by loss of species diversity, which can ultimately result in compromised ecosystem functioning (Wagg et al., 2021). In nematode diversity analysis, the unfertilized (NF) control treatment was found to be significantly different from the remaining treatments in that it exhibited a lower number of observed species and lower species richness, as well as a different community structure. Although the NF control had a significantly lower number of observed OTUs, the dominance of omnivorous dorylaimids and predacious triplonchids were indicative of structured and undisturbed nematode communities. This highlighted the level of community dissimilarity between the NF control and the remaining treatments enriched with nutrients. These results are similar to those in a study by Steel and Ferris (2016), where the relative abundance and biomass of predaceous and omnivorous nematodes were significantly higher in an undisturbed site when compared to an agricultural site. Over time, nematode abundance and community composition are affected by the application of mineral or organic fertilizer, soil properties, and crop cultivated (Leroy et al., 2009; Briar et al., 2011; Ugarte et al., 2013).
Beta diversity analysis by Weighted and Unweighted Unifrac allowed us to identify significant changes in the overall community structures of bacteria, fungi and nematodes that cannot be determined by analysis of taxonomic composition or diversity analysis. Generally, the bacterial and fungal communities were more responsive to P input than NKS (SP0 treatment), but those of mineral SP40 treatment did not significantly differ to NF or SP0 treatments in any case. This was previously noted in a cut and removed grass management system, which found that soil bacterial abundance, richness and structure was unaffected by inorganic mineral P fertilizer application (Randall et al., 2019). Meanwhile, the effects of CS treatment on soil bacterial and fungal communities were transparent in Weighted Unifrac analysis, demonstrating that the consideration of both abundance and phylogenetic information of samples clearly separated this treatment group from all others. One factor to consider, however, is the external bacterial and fungal community introduced with CS application, which may have influenced the soil community structure (Lentendu et al., 2014). Nonetheless, together these observations are indicative that bio-based RDF, may trigger microbial responses that fall somewhere between the mineral treatment alone (SP40) and the CS combination treatment. This was particularly true for the MWS treatment, which contains only 50% dry matter, as compared to the other RDF, which contain >90%, meaning that the additional moisture of this treatment and CS treatment may have led to more similar communities due to more rapid release of available nutrients. Additionally, in what to our knowledge appears to be the only other literature available on bacterial community response to struvite, it was found that Actinobacterial populations were stimulated by struvite sourced from a wastewater treatment plant (Bastida et al., 2019). This was the same effect as that observed in our study on MWS, which stimulated Actinobacterial populations to an extent similar to CS treatment. Furthermore, the availability of K, Mg, and N content of the soil were found to have the most significant influences on both these treatments in CCA.
Fungal communities were most affected by ash treatments, which was particularly obvious in PCoA of Unweighted Unifrac distances. Because this is a qualitative measure, it presents evidence that application of bio-based ash fertilizers results in a significant change in the phylogenetic composition of the soil fungal community (Lozupone et al., 2007). The average numbers of fungal OTUs were highest in ash treatments. Thus, the additional fungal lineages present were the main reason for the community shifts observed. This could be explained by the high pH of ashes. Fungi typically have a wide pH optimum (Rousk et al., 2010), which may have allowed fungal species with a preference for higher pH to grow. However, there was no effect on the pH of the soil to support this. Another factor shared by the ash treatments is a higher heavy metal content, including Zn, Fe, Cu, Al, Cr, and Mn, compared to the other fertilizers (unpublished data). Literature surrounding soil fungal community responses to heavy metal contamination is limited, with more focus on the bacterial response. However, increased fungal diversity in moderate soil heavy metal contamination has been recorded (Pasqualetti et al., 2012; Lin et al., 2020). It is possible that the observed increase in the number of fungal species could be due to a fungal stress response, in which heavy metals inhibited the growth of otherwise dominant soil fungi, allowing less competitive species to survive (Abdel-Azeem et al., 2007). Additionally, despite the observed shifts in fungal communities across the different fertilization treatments, none of the variables measured were found to be of significant influence in CCA, suggesting some other factor was playing a role in shaping fungal community structure.
Based on Weighted Unifrac distances, the community structure of the MWS treatment was not significantly different from that of the NF treatment, but the number of observed species was significantly higher in the MWS treatment. Nematode communities in the CS treatment differed significantly from those in the SP40 treatment, due to a significant increase in the abundance of food opportunistic rhabditids in CS treatment, where material rich in organic matter was applied. In support of this observation, a study by Ikoyi et al. (2020) also found that the abundance of bacterial feeding nematodes were significantly enriched in soil with a cattle slurry treatment applied, than when an inorganic conventional fertilizer was applied. Ettema and Bongers (1993) showed that the application of animal manure led to changes in nematode fauna, starting with a significant increase of the enrichment opportunists. The succession of nematodes from colonizers to persisters took several weeks. The incorporation of organic amendments into the soil environment stimulates microbial activity and attracts enrichment opportunists. Moreover, in a study by Ferris et al. (1996) 11 taxa of bacterial feeding nematodes, all in the order Rhabditida, became more abundant in organically amended plots than in a conventional system, in more than 30 days after the application.
The application of SSA as the P source resulted in relative abundance changes of nematode taxa which were indicative of environmental disturbance by significantly reducing the abundance of stress intolerant dorylaimids, significantly increasing stress tolerant monhysterids, as well as food opportunistic rhabditids, and plant parasitic tylenchids. Although the SSA treatment did not significantly differ from the SP40 mineral control treatment in terms of beta diversity, it significantly reduced the relative abundance of sensitive to disturbance dorylaimids when compared with SP 40. The relative abundance of dorylaimids in the SSA treatment was reduced by 22.7% when compared with that in the unfertilized control and 7.3% when compared with that in the 100% mineral treatment. The unfavorable shifts on nematode communities in SSA, when compared with those in unfertilized (NF) and SP40 mineral control, could indicate environmental disturbance. The persisters, such as dorylaimids, favor undisturbed habitats (Bongers, 1990), and a decrease in their abundance indicates disturbance or pollution of their habitat. Overall, environmental disturbance causes a decrease in the number of persisters and causes a shift toward the dominance of colonizers (Odum, 1985). A reason that possibly caused the reduction of sensitive to environmental disturbance taxa, was the presence of heavy metals in the ash product (unpublished data). The estimated concentration of copper (Cu) in the ash derived from sewage sludge waste was 609 mg/kg, and the concentration of zinc (Zn) was as high as 1,798 mg/kg. A study examining heavy metal toxicity on nematode communities following addition of Cu and Zn to agricultural soil revealed the nematode community structure, as well as individual nematode taxa, to be significantly affected during exposure by increasing concentrations of Cu and Zn, reaching up to 200 mg/kg (Korthals et al., 2000). In the Karpinska et al. (2021) study, although the ash derived from sewage sludge waste did not adversely affect nematode taxa sensitive to stress and disturbance, it did significantly reduce the number of observed species when compared with the remaining treatments. The presence of persisters is highly important in agricultural soils and nutrient cycling. Placed at the top of the soil nematode food chain, they feed on bacterial and fungal feeding nematodes, releasing more nutrients available for plant uptake, and regulating the colonizer's population (Yeates and Wardle, 1996). According to CCA analysis (Figure 5B), soil available P, appeared to have the strongest influence on the nematode community structure in the SSA treatment. A survey conducted by Herzel et al. (2016) discovered that the bioavailability of phosphorus in the sewage sludge ash is poor and that more than half of the ashes cannot be used as fertilizers due to high heavy metal content. SSA producers are developing new methods of reducing the heavy metal content in the final ash products (Mattenberger et al., 2008, 2010; Adam et al., 2009; Petzet et al., 2011; Stemann et al., 2015) as well as improving the bioavailability of recovered P when in soil (Nanzer et al., 2014; Stemann et al., 2015).
Conclusion
Using a next-generation sequencing approach, this study focused on the effects of four new recycling-derived fertilizer (RDF) products on the major soil microorganisms, when used as a sustainable alternative to non-renewable P fertilizer on Ireland's most cultivated agricultural crop. Changes in community composition and relative abundances of bacteria, fungi and nematodes served as indicators of the soil ecosystem response to RDFs. Statistical comparisons of alpha and beta diversity metrics allowed us to observe changes in the bacterial, fungal, and nematode diversity and community structures of soil with different forms of P applied. Canonical correspondence analysis revealed that available nutrients including P, K, and Mg, as well as plant P uptake and dry matter yield, were the main factors (of those measured) shaping the bacterial and nematode communities of the different grassland fertilization treatments. At the same time, none of the measured variables were found to be influential on fungal communities, suggesting some other factor such as likely the chemical properties of the ashes, were involved in the changes observed in these communities. The results showed promising evidence that the two new struvite RDF products, derived from municipal and potato processing wastes, have the potential to provide an effective and sustainable replacement to mineral non-renewable forms of imported P in temperate Irish grasslands. Dorylaimida is a nematode order sensitive to environmental disturbance. In the present study, their significant reduction, together with the observed alteration in order of dominating bacterial phyla and shifts in fungal community structure, raise questions surrounding the long-term impacts of the sewage ash and poultry litter ash RDF products on soil communities and therefore require further study. In future studies, it would be useful to investigate the heavy metal content of the soil fertilized with RDF to facilitate a deeper understanding of the community changes. Overall, this study provides new insight into soil bacterial, fungal, and nematode community responses to the application of RDF products incorporated into a conventional grassland fertilization programme in Ireland.
Data Availability Statement
The datasets generated for this study can be found in the NCBI Sequence Read Archive database, project accession PRJNA762644. Bacterial sequence accession numbers: SRR16133593–SRR16133851. Fungal sequence accession numbers: SRR161132152–SRR161133455. Nematode sequence accession numbers: SRR16230014–SRR16230053.
Author Contributions
PF and SA designed and managed the field experiment. DR and AK conducted the research, analyzed the data, and wrote the manuscript. TK-D, KG, and DD supervised the research. TK-D, KG, DD, PF, and SA provided critical comments on the manuscript and participated in the revision. All authors read and approved the final manuscript.
Funding
This research was part-funded by ReNu2farm: Nutrient Recycling—from pilot production to farms and fields (Project number: NWE601, Interreg North-West Europe).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2022.832841/full#supplementary-material
References
Abdel-Azeem, A., Abdel-Moneim, T. S., Ibrahim, M., Hassan, M., and Saleh, M. (2007). Effects of long-term heavy metal contamination on diversity of terricolous fungi and nematodes in Egypt - a case study. Water Air Soil Pollution 186, 233–254. doi: 10.1007/s11270-007-9480-3
Adam, C., Peplinski, B., Michaelis, M., Kley, G., and Simon, F. G. (2009). Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manage. 29, 1122–1128. doi: 10.1016/j.wasman.2008.09.011
Aliyu, H., Gorte, O., de Maayer, P., Neumann, A., and Ochsenreither, K. (2020). Genomic insights into the lifestyles, functional capacities and oleagenicity of members of the fungal family Trichosporonaceae. Sci. Rep. 10, 2780. doi: 10.1038/s41598-020-59672-2
Anderson, C. R., Peterson, M. E., Frampton, R. A., Bulman, S. R., Keenan, S., and Curtin, D. (2018). Rapid increases in soil pH solubilise organic matter, dramatically increase denitrification potential and strongly stimulate microorganisms from the Firmicutes phylum. PeerJ 6, e6090. doi: 10.7717/peerj.6090
Anderson, R. V., Gould, W. D., Woods, L. E., Cambardella, C., Ingham, R. E., and Coleman, D. C. (1983). Organic and inorganic nitrogenous losses by microbivorous nematodes in soil. Oikos 40, 75–80.
Armitage, D., Gallagher, K., Youngblut, N., Buckley, D., and Zinder, S. (2012). Millimeter-scale patterns of phylogenetic and trait diversity in a salt marsh microbial mat. Front. Microbiol. 3, 293. doi: 10.3389/fmicb.2012.00293
Ashekuzzaman, S. M., Fenton, O., Meers, E., and Forrestal, P. J. (2021b). Differing phosphorus crop availability of aluminium and calcium precipitated dairy processing sludge potential recycled alternatives to mineral phosphorus fertiliser. Agron 11, 427. doi: 10.3390/agronomy11030427
Ashekuzzaman, S. M., Forrestal, P., Richards, K. G., Daly, K., and Fenton, O. (2021a). Grassland phosphorus and nitrogen fertiliser replacement value of dairy processing dewatered sludge. Sustain. Prod. Consump. 25, 363–373. doi: 10.1016/j.spc.2020.11.017
Ashley, K., Mavinic, D., and Koch, F. (2009). “Preferred future phosphorus scenarios: A framework for meeting long-term phosphorus needs for global food demand, ” in International Conference on Nutrient Recovery From Wastewater Streams (Vancouver, BC: IWA Publishing), 20–40.
Bang-Andreasen, T., Anwar, M. Z., Lanzén, A., Kjøller, R., Rønn, R., Ekelund, F., et al. (2020). Total RNA sequencing reveals multilevel microbial community changes and functional responses to wood ash application in agricultural and forest soil. FEMS Microbiol. Ecol. 96, fiaa016. doi: 10.1093/femsec/fiaa016
Bang-Andreasen, T., Nielsen, J. T., Voriskova, J., Heise, J., Rønn, R., Ekelund, F., et al. (2017). Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 8, 1400. doi: 10.3389/fmicb.2017.01400
Bastida, F., Jehmlich, N., Martínez-Navarro, J., Bayona, V., García, C., and Moreno, J. L. (2019). The effects of struvite and sewage sludge on plant yield and the microbial community of a semiarid Mediterranean soil. Geoderma 337, 1051–1057. doi: 10.1016/j.geoderma.2018.10.046
Bhadury, P., Austen, M., Bilton, D., Lambshead, P., Rogers, A., and Smerdon, G. (2006). Development and evaluation of a DNA-barcoding approach for the rapid identification of nematodes. Mar. Ecol. Prog. Ser. 320, 1–9. doi: 10.3354/MEPS320001
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Bongers, T. (1990). The maturity index: an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83, 14–19. doi: 10.1007/BF00324627
Bongers, T., and Bongers, M. (1998). Functional diversity of nematodes. Appl. Soil Ecol. 10, 239–251. doi: 10.1016/S0929-1393(98)00123-1
Bongers, T., and Ferris, H. (1999). Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 14, 224–228. doi: 10.1016/s0169-5347(98)01583-3
Briar, S. S., Miller, S. A., Stinner, D., Kleinhenz, M., and Grewal, P. S. (2011). Effects of organic transition strategies for peri-urban vegetable production on soil properties, nematode community, and tomato yield. Appl. Soil Ecol. 47, 84–91. doi: 10.1016/j.apsoil.2010.12.001
Caporaso, J. G., Lauber, C. L., Walters, W. A., Berg-Lyons, D., Lozupone, C. A., Turnbaugh, P. J., et al. (2011). Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl. 1), 4516–4522. doi: 10.1073/pnas.1000080107
Codling, E. E., Chaney, R. L., and Sherwell, J. (2002). Poultry litter ash as a potential phosphorus source for agricultural crops. J. Environ. Qual. 31, 954–961. doi: 10.2134/jeq2002.9540
CSO (2020). Central Statistics Office. Available online at: https://www.cso.ie/en/releasesandpublications/ep/p-coa/censusofagriculture2020-preliminaryresults/landutilisation/ (accessed January 12, 2022).
Diaz-Ambrona, C. G. H., and Maletta, E. (2014). Achieving global food security through sustainable development of agriculture and food systems with regard to nutrients, soil, land, and waste management. Curr. Sustain. Renew. Energy Rep. 1, 57–65. doi: 10.1007/s40518-014-0009-2
Djigal, D., Brauman, A., Diop, T. A., Chotte, J. L., and Villenave, C. (2004). Influence of bacterial-feeding nematodes (Cephalobidae) on soil microbial communities during maize growth. Soil Biol. Biochem. 36, 323–331. doi: 10.1016/j.soilbio.2003.10.007
Egidi, E., Delgado-Baquerizo, M., Plett, J. M., Wang, J., Eldridge, D. J., Bardgett, R. D., et al. (2019). A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 10, 2369. doi: 10.1038/s41467-019-10373-z
Ettema, C. H., and Bongers, T. (1993). Characterization of nematode colonization and succession in disturbed soil using the maturity index. Biol. Fertil. Soils 16, 79–85. doi: 10.1007/BF00369407
Evtushenko, L. I., Krausova, V. I., and Yoon, J.-H. (2015). “Nocardioides, ” in Bergey's Manual of Systematics of Archaea and Bacteria, eds M. E. Trujillo, S. Dedysh, P. DeVos, B. Hedlund, P. Kámpfer, F. A. Rainey, and W. B. Whitman (John Wiley & Sons, Inc), 1–81.
Ferris, H., Venette, R., van der Meulen, H., and Lau, S. S. (1998). Nitrogen mineralization by bacterial-feeding nematodes: verification and measurement. Plant Soil 203, 159–171. doi: 10.1023/A:1004318318307
Ferris, H., Venette, R. C., and Lau, S. S. (1996). Dynamics of nematode communities in tomatoes grown in conventional and organic farming systems, and their impact on soil fertility. Appl. Soil Ecol. 3, 161–175. doi: 10.1016/0929-1393(95)00071-2
Fierer, N., and Jackson, R. B. (2006). The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. U.S.A. 103, 626. doi: 10.1073/pnas.0507535103
Franz, M. (2008). Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 28, 1809–1818. doi: 10.1016/j.wasman.2007.08.011
Griffiths, B. S. (1990). A comparison of microbial-feeding nematodes and protozoa in the rhizosphere of different plants. Biol. Fertil. Soils 9, 83–88.
Harder, R., Giampietro, M., and Smukler, S. (2021). Towards a circular nutrient economy. A novel way to analyze the circularity of nutrient flows in food systems. Resources Conserv. Recycl. 172, 105693. doi: 10.1016/j.resconrec.2021.105693
Hayat, R., Ali, S., Amara, U., Khalid, R., and Ahmed, I. (2010). Soil beneficial bacteria and their role in plant growth promotion: a review. Ann. Microbiol. 60, 579–598. doi: 10.1007/s13213-010-0117-1
Hertzberger, A. J., Cusick, R. D., and Margenot, A. J. (2020). A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 84, 653–671. doi: 10.1002/saj2.20065
Herzel, H., Krüger, O., Hermann, L., and Adam, C. (2016). Sewage sludge ash—a promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 542, 1136–1143. doi: 10.1016/j.scitotenv.2015.08.059
Ho, A., Di Lonardo, D. P., and Bodelier, P. L. E. (2017). Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 93, fix006. doi: 10.1093/femsec/fix006
Hoogen, J. V. D., Geisen, S., Routh, D., Ferris, H., Traunspurger, W., Wardle, D., et al. (2019). Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198. doi: 10.1038/s41586-019-1418-6
Hunt, H. W., Coleman, D. C., lngham, E. R., lngham, R. E., Elliott, E. T., Moore, J. C., et al. (1987). The detrital food web in a shortgrass prairie. Biol. Fertil. Soils 3, 57–68. doi: 10.1007/BF00260580
Ikoyi, I., Egeter, B., Chaves, C., Ahmed, M., Fowler, A., and Schmalenberger, A. (2020). Responses of soil microbiota and nematodes to application of organic and inorganic fertilizers in grassland columns. Biol. Fertil. Soils 56, 647–662. doi: 10.1007/s00374-020-01440-5
Ingham, R. E., Trofymow, J. A., lngham, E. R., and Coleman, D.C. (1985). Interactions of bacteria, fungi and their nematode grazers: effects on nutrient cycling and plant growth. Ecol. Monogr. 55, 119–140. doi: 10.2307/1942528
Irshad, U., Villenave, C., Brauman, A., and Plassard, C. (2011). Grazing by nematodes on rhizosphere bacteria enhances nitrate and phosphorus availability to Pinus pinaster seedlings. Soil Biol. Biochem. 43, 2121–2126. doi: 10.1016/j.soilbio.2011.06.015
Karpinska, A., Ryan, D., Germaine, K., Dowling, D., Forrestal, P., and Kakouli-Duarte, T. (2021). Soil microbial and nematode community response to the field application of recycled bio-based fertilisers in Irish grassland. Sustainability 13, 12342. doi: 10.3390/su132212342
Karunanithi, R., Szogi, A. A., Bolan, N., Naidu, R., Loganathan, P., Hunt, P. G., et al. (2015). “Chapter three - phosphorus recovery and reuse from waste streams, ” in Advances in Agronomy, Vol. 131, ed D. L. Sparks (Amsterdam: Academic Press), 173–250.
Korthals, G. W., Bongers, M., Kokkema, A., Dueck, T. A., and Lexmond, T. M. (2000). Joint toxicity of copper and zinc to a terrestrial nematode community in an acid sandy soil. Ecotoxicology 9, 219–228. doi: 10.1023/A:1008950905983
Le Corre, K. S., Valsami-Jones, E., Hobbs, P., and Parsons, S. A. (2009). Phosphorus recovery from wastewater by struvite crystallization: a review. Crit. Rev. Environ. Sci. Technol. 39, 433–477. doi: 10.1080/10643380701640573
Leff, J. W., Jones, S. E., Prober, S. M., Barberán, A., Borer, E. T., Firn, J. L., et al. (2015). Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. U.S.A. 112, 10967–10972. doi: 10.1073/pnas.1508382112
Lentendu, G., Wubet, T., Chatzinotas, A., Wilhelm, C., Buscot, F., and Schlegel, M. (2014). Effects of long-term differential fertilization on eukaryotic microbial communities in an arable soil: a multiple barcoding approach. Mol. Ecol. 23, 3341–3355. doi: 10.1111/mec.12819
Leroy, B. L., Reheul, D., Moens, M., Ferris, H., and De Sutter, N. (2009). Short-term nematode population dynamics as influenced by the quality of exogenous organic matter. Nematology 11, 23–38. doi: 10.1163/156854108X398381
Lin, Y., Xiao, W., Ye, Y., Wu, C., Hu, Y., and Shi, H. (2020). Adaptation of soil fungi to heavy metal contamination in paddy fields—a case study in eastern China. Environ. Sci. Pollution Res. 27, 27819–27830. doi: 10.1007/s11356-020-09049-9
Lorick, D., Macura, B., Ahlström, M., Grimvall, A., and Harder, R. (2020). Effectiveness of struvite precipitation and ammonia stripping for recovery of phosphorus and nitrogen from anaerobic digestate: a systematic review. Environ. Evid. 9, 27. doi: 10.1186/s13750-020-00211-x
Lozupone, C. A., Hamady, M., Kelley, S. T., and Knight, R. (2007). Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 73, 1576–1585. doi: 10.1128/aem.01996-06
Mattenberger, H., Fraissler, G., Jöller, M., Brunner, T., Obernberger, I., Herk, P., et al. (2010). Sewage sludge ash to phosphorus fertiliser (II): Influences of ash and granulate type on heavy metal removal. Waste Manage. 30, 1622–1633. doi: 10.1016/j.wasman.2010.03.037
Mattenberger, H., Fraissler, G., Brunner, T., Herk, P., Hermann, L., and Obernberger, I. (2008). Sewage sludge ash to phosphorus fertiliser: variables influencing heavy metal removal during thermochemical treatment. Waste Manag. 28, 2709–2722. doi: 10.1016/j.wasman.2008.01.005
Morgan, M. F. (1941). “Chemical soil diagnosis by the universal soil testing system, ” in Connecticut Agricultural Experiment Station. Bulletin 450. Available online at: https://portal.ct.gov/-/media/CAES/DOCUMENTS/Publications/Bulletins/B450pdf.pdf?la=en (accessed October 01, 2021).
Nanzer, S., Oberson, A., Berger, L., Berset, E., Hermann, L., and Frossard, E. (2014). The plant availability of phosphorus from thermo-chemically treated sewage sludge ashes as studied by 33P labeling techniques. Plant Soil. 377, 439–456. doi: 10.1007/s11104-013-1968-6
Nielsen, M. N., and Winding, A. (2002). Microorganisms as Indicators of Soil Health. National Environmental Research Institute. Available online at: https://www.dmu.dk/1_Viden/2_Publikationer/3_Fagrapporter/rapporter/FR388.pdf
Nilsson, R. H., Larsson, K.-H., Taylor, A. F. S., Bengtsson-Palme, J., Jeppesen, T. S., Schigel, D., et al. (2018). The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264. doi: 10.1093/nar/gky1022
O'Donnell, C., Barnett, D., Harrington, J., and Power, N. (2022). The extended effect of top-dressed recovered struvite fertiliser on residual irish grassland soil phosphorus levels compared to commercial phosphorus fertiliser. Agronomy 12, 8. doi: 10.3390/agronomy12010008
Odum, E. P. (1985). Trends expected in stressed ecosystems. Bioscience 35, 419–422. doi: 10.2307/1310021
Pasqualetti, M., Mulas, B., Canzonetti, G., Benedetti, A., and Tempesta, S. (2012). Effects of long-term heavy metal contamination on soil fungi in the mediterranean area. Cryptogamie Mycologie 33, 43–57. doi: 10.7872/crym.v33.iss1.2012.043
Peintner, U., Knapp, M., Fleischer, V., Walch, G., and Dresch, P. (2016). Myrmecridium hiemale sp. nov. from snow-covered alpine soil is the first eurypsychrophile in this genus of anamorphic fungi. Int. J. Syst. Evolut. Microbiol. 66, 2592–2598. doi: 10.1099/ijsem.0.001090
Petzet, S., Peplinski, B., Bodkhe, S. Y., and Cornel, P. (2011). Recovery of phosphorus and aluminium from sewage sludge ash by a new wet chemical elution process (SESAL-Phos-recovery process). Water Sci. Technol. 64, 29–35. doi: 10.2166/wst.2011.682
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi: 10.1093/nar/gks1219
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Ramirez, K. S., Craine, J. M., and Fierer, N. (2012). Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 18, 1918–1927. doi: 10.1111/j.1365-2486.2012.02639.x
Randall, K., Brennan, F., Clipson, N., Creamer, R., Griffiths, B., Storey, S., et al. (2019). Soil bacterial community structure and functional responses across a long-term mineral phosphorus (Pi) fertilisation gradient differ in grazed and cut grasslands. Appl. Soil Ecol. 138, 134–143. doi: 10.1016/j.apsoil.2019.02.002
Rao, D. (2007). Microbial diversity, soil health and sustainability. J. Indian. Soc. Soil Sci. 55, 392–403.
Rech, I., Withers, P. J. A., Jones, D. L., and Pavinato, P. S. (2019). Solubility, diffusion and crop uptake of phosphorus in three different struvites. Sustainability 11, 134. doi: 10.3390/su11010134
Roberts, T. L. (2014). Cadmium and phosphorous fertilizers: the issues and the science. Procedia Eng. 83, 52–59. doi: 10.1016/j.proeng.2014.09.012
Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G., et al. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351. doi: 10.1038/ismej.2010.58
Saerens, B., Geerts, S., and Weemaes, M. (2021). Phosphorus recovery as struvite from digested sludge – experience from the full scale. J. Environ. Manage. 280, 111743. doi: 10.1016/j.jenvman.2020.111743
Schoch, C. L., Seifert, K. A., Huhndorf, S., Robert, V., Spouge, J. L., Levesque, C. A., et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. U.S.A. 109, 6241–6246. doi: 10.1073/pnas.1117018109
Severin, M., Breuer, J., Rex, M., Stemann, J., Adam, C., Van den Weghe, H., et al. (2014). Phosphate fertilizer value of heat treated sewage sludge ash. Plant Soil Environ. 60, 555–561. doi: 10.17221/548/2014-PSE
Singh, B. K., Quince, C., Macdonald, C. A., Khachane, A., Thomas, N., Al-Soud, W. A., et al. (2014). Loss of microbial diversity in soils is coincident with reductions in some specialized functions. Environ. Microbiol. 16, 2408–2420. doi: 10.1111/1462-2920.12353
Siqueira, A. C. O., Mascarin, G. M., Gonçalves, C. R. N. C. B., Marcon, J., Quecine, M. C., Figueira, A., et al. (2020). Multi-trait biochemical features of Metarhizium species and their activities that stimulate the growth of tomato plants. Front. Sustain. Food Syst. 4, 137. doi: 10.3389/fsufs.2020.00137
Smil, V. (2000). Phosphorus in the environment: natural flows and human interferences. Annu. Rev. Energy Environ. 25, 53–88. doi: 10.1146/annurev.energy.25.1.53
Starke, R., Capek, P., Morais, D., Callister, S. J., and Jehmlich, N. (2020). The total microbiome functions in bacteria and fungi. J. Proteomics 213, 103623. doi: 10.1016/j.jprot.2019.103623
Steel, H., and Ferris, H. (2016). Soil nematode assemblages indicate the potential for biological regulation of pest species. Acta Oecol. 73, 87–96. doi: 10.1016/j.actao.2016.03.004
Steen, I. (1998). Phosphorus availability in the 21st century: management of a non-renewable resource. Phosphorus Potassium. 217, 25–31.
Stemann, J., Peplinski, B., and Adam, C. (2015). Thermochemical treatment of sewage sludge ash with sodium salt additives for phosphorus fertilizer production - analysis of underlying chemical reactions. Waste Manage. 45, 385–390. doi: 10.1016/j.wasman.2015.07.029
St. Leger, R. J., and Wang, J. B. (2020). Metarhizium: jack of all trades, master of many. Open Biol. 10, 200307. doi: 10.1098/rsob.200307
Thiele-Bruhn, S., Bloem, J., de Vries, F. T., Kalbitz, K., and Wagg, C. (2012). Linking soil biodiversity and agricultural soil management. Curr. Opin. Environ. Sustain. 4, 523–528. doi: 10.1016/j.cosust.2012.06.004
Treonis, A. M., Austin, E. E., Buyer, J. S., Maul, J. E., Spicer, L., and Zasada, I. A. (2010). Effects of organic amendment and tillage on soil microorganisms and microfauna. Appl. Soil Ecol. 46, 103–110. doi: 10.1016/j.apsoil.2010.06.017
Ugarte, C. M., Zaborski, E. R., and Wander, M. M. (2013). Nematode indicators as integrative measures of soil condition in organic cropping systems. Soil Biol. Biochem. 64, 103–113. doi: 10.1016/j.soilbio.2013.03.035
USEPA (1996). SW-846 Test Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices. United States Environmental Protection Agency.
Verstraete, W., and Mertens, B. (2004). “Chapter 5: the key role of soil microbes, ” in Developments in Soil Science, Vol. 29, eds P. Doelman and H. J. P. Eijsackers (Amsterdam: Elsevier), 127–157.
Vollú, R. E., Cotta, S. R., Jurelevicius, D., Leite, D. C. de. A., Parente, C. E. T., Malm, O., et al. (2018). Response of the bacterial communities associated with maize rhizosphere to poultry litter as an organomineral fertilizer. Front. Environ. Sci. 6, 118. doi: 10.3389/fenvs.2018.00118
Wagg, C., Hautier, Y., Pellkofer, S., Banerjee, S., Schmid, B., and van der Heijden, M. G. (2021). Diversity and asynchrony in soil microbial communities stabilizes ecosystem functioning. Elife 10, e62813. doi: 10.7554/eLife.62813
Wardle, D. A., and Yeates, G. W. (1993). The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: evidence from decomposer food-webs. Oecologia 93, 303–306. doi: 10.1007/BF00317685
Wilson, M., and Kakouli-Duarte, T. (2009). Nematodes as Environmental Indicators. Wallingford: CABI. p. 1–17.
Wolińska, A., Kuzniar, A., Zielenkiewicz, U., Izak, D., Szafranek-Nakonieczna, A., Banach, A., et al. (2017). Bacteroidetes as a sensitive biological indicator of agricultural soil usage revealed by a culture-independent approach. Appl. Soil Ecol. 119, 128–137. doi: 10.1016/j.apsoil.2017.06.009
Yeates, G. W., Bongers, T., Goede, R. G. M., de, Freckman, D. W., and Georgieva, S. S. (1993). Feeding habits in soil nematode families and genera - an outline for soil ecologists. J. Nematol. 25, 315–331.
Yeates, G. W., and Wardle, D. A. (1996). Nematodes as predators and prey: relationships to biological control and soil processes. Pedobiologia 40, 43–50.
Zhang, B., Wu, X., Tai, X., Sun, L., Wu, M., Zhang, W., et al. (2019). Variation in Actinobacterial community composition and potential function in different soil ecosystems belonging to the arid heihe river basin of Northwest China. Front. Microbiol. 10, 2209. doi: 10.3389/fmicb.2019.02209
Zhang, X., Ferris, H., Mitchell, J., and Liang, W. (2017). Ecosystem services of the soil food web after long-term application of agricultural management practices. Soil Biol. Biochem. 111, 36–43. doi: 10.1016/j.soilbio.2017.03.017
Keywords: phosphorus, nutrient recycling, bio-based fertilizer, grassland, bacteria, fungi, nematodes, soil communities
Citation: Ryan D, Karpinska A, Forrestal PJ, Ashekuzzaman SM, Kakouli-Duarte T, Dowling DN and Germaine KJ (2022) The Impact of Bio-Based Fertilizer Integration Into Conventional Grassland Fertilization Programmes on Soil Bacterial, Fungal, and Nematode Communities. Front. Sustain. Food Syst. 6:832841. doi: 10.3389/fsufs.2022.832841
Received: 10 December 2021; Accepted: 09 February 2022;
Published: 09 March 2022.
Edited by:
Jianjun Cao, Northwest Normal University, ChinaCopyright © 2022 Ryan, Karpinska, Forrestal, Ashekuzzaman, Kakouli-Duarte, Dowling and Germaine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kieran J. Germaine, a2llcmFuLmdlcm1haW5lQGl0Y2FybG93Lmll
†ORCID: Anna Karpinska orcid.org/0000-0001-8647-3068
Thomais Kakouli orcid.org/0000-0001-9200-2166
 Demi Ryan
Demi Ryan Anna Karpinska
Anna Karpinska Patrick J. Forrestal
Patrick J. Forrestal S. M. Ashekuzzaman2
S. M. Ashekuzzaman2 Thomais Kakouli-Duarte
Thomais Kakouli-Duarte David N. Dowling
David N. Dowling Kieran J. Germaine
Kieran J. Germaine