- 1Centre for Agri-Environmental Research, School of Agriculture, Policy and Development, University of Reading, Reading, United Kingdom
- 2Centro Regional de Investigación e Innovación para la Sostenibilidad de la Agricultura y los Territorios Rurales (Ceres), Quillota, Chile
- 3Escuela de Agronomía, Pontificia Universidad Católica de Valparaíso, Casilla 4-D, Quillota, Chile
- 4Institute of Ecology and Biodiversity, Concepcion, Chile
- 5Instituto de Investigaciones Agropecuarias, INIA-La Cruz, La Cruz, Chile
Insect pollination is known to increase avocado yields, with wild pollinators likely playing an important role. In central Chile, the rapid expansion of avocado orchards has resulted in highly diverse natural habitats being replaced by plantations, potentially negatively impacting wild pollinators and thus avocado production. This study aimed to understand the role of natural habitats and wild pollinators in avocado production by (1) exploring the relationship between flower visitor abundance and diversity, and proximity to natural habitat, (2) quantifying the pollination effectiveness of different insect taxa, and (3) measuring the contribution to avocado production of insect pollinators and exploring how this varies with proximity to natural habitats. We conducted flower visitor observations and controlled pollination trials at different distances to natural habitat in three orchards in central Chile, across three years. The results showed that flower visitor abundance, visitation, richness, and diversity were significantly higher closer to natural habitats. However, this relationship varied across distances, with wild insect abundance and visitation rates approximately 2.55 times higher, richness around 1.6 times higher, and diversity 1.5 times higher at the natural habitat edge compared to further inside the orchard. Insect pollinators contributed significantly to avocado production, with almost no fruit set when pollinators were excluded. Hoverflies and other flies were identified as potentially important avocado pollinators. This study demonstrates the importance of natural habitats and wild insect pollination services in crop production. We recommend that growers implement land management practices that protect and restore natural areas in and around their orchards to support wild pollinators.
1 Introduction
Pollinators play a crucial role in increasing the quantity and quality of many globally important (Klein et al., 2007) as well as nutritionally valuable crops (Chaplin-Kramer et al., 2014; Eilers et al., 2011). To support production, farmers often introduce managed honeybees (Apis mellifera) into their fields and orchards. However, relying exclusively on a single managed species for pollination carries risks, especially considering the combined threats facing honeybees such as disease and pesticides (Kremen et al., 2002; Potts et al., 2010; Stokstad, 2007; VanEngelsdorp et al., 2008). In addition, the escalating global demand for insect-pollinated crops is expected to surpass the supply of managed honeybees (Mashilingi et al., 2022), stressing the need to develop alternatives to secure pollination and food production. Wild insects, such as wild bees (Klein et al., 2007), flies, beetles, ants, wasps, moths, and butterflies (Rader et al., 2020), also pollinate many important agricultural crops. Moreover, wild pollinators often provide additional benefits. Multiple studies show that an increase in the abundance and diversity of wild pollinators can provide a more efficient and stable pollination service compared to managed honeybees (Blüthgen and Klein, 2011; Garibaldi et al., 2013; Hoehn et al., 2008; Klein et al., 2009; Senapathi et al., 2021). Consequently, there has been a growing recognition in recent years of the importance of wild pollinators and protecting or increasing their role in facilitating the transition toward sustainable agriculture (Garibaldi et al., 2014).
To survive and reproduce, many wild pollinators need resources such as nesting sites (e.g., areas of bare ground or suitable vegetation), and diverse food sources (e.g., a variety of floral resources). These resources are often lacking in managed landscapes (Winfree et al., 2009) but are present in natural or semi natural areas such as native herbaceous habitats (Bartual et al., 2019). Several studies have shown that the proximity of agricultural land to natural areas correlates with increased pollinator diversity and abundance, attributed to spillover effects (Gonzalez-Chaves et al., 2020; Hipólito et al., 2018; Hipólito et al., 2019; Klein et al., 2003; Ricketts, 2004; Sritongchuay et al., 2019), which in turn is often linked to increased yields (Dainese et al., 2019; Martin et al., 2019). However, across the world, natural areas are being diminished and fragmented, primarily due to agricultural expansion. For example, in South America, the cover of various terrestrial natural habitat biomes, including grasslands, forests, and the Mediterranean-climate biomes, have decreased by more than 50% (IPBES, 2018). This reduction poses a potential threat to pollination services and, thus, food production (Campbell et al., 2017; IPBES, 2016; Vanbergen et al., 2020). To protect wild pollinators, practices such as conserving or restoring natural and semi natural areas and implementing hedgerows and flowers strips in agricultural landscapes are known to be effective (Garibaldi et al., 2014). However, to encourage the widescale implementation of such measures, more evidence of the contribution from wild pollinators and natural habitats to agricultural production is needed from understudied crops and regions.
Avocado, Persea americana L. (1753), is a globally important crop grown in many countries. In recent years, avocados have gained recognition for their high nutritional value (Weschenfelder et al., 2015) leading to an increase in economic value and global demand (e.g., global production was around 4.2 million tonnes in 2011 and approximately 8.8 million tonnes in 2023) (FAO, 2023). Insect pollination is important for optimal avocado production, as the flowering process limits self-pollination and strongly promotes cross-pollination (Sedgley, 1977). Avocado exhibits a flowering pattern known as protogynous dichogamy in which the hermaphrodite flowers will first function as female, then later transition to male, with different cultivars opening at different times throughout the day (Nirody, 1922; Stout, 1932). For example, A-Type cultivars are female in the morning of the first day and male in the afternoon of the second day, while B-Type cultivars are female in the afternoon of the first day and male in the morning of the second day (Nirody, 1922). Several studies have demonstrated avocado’s reliance on insect vectors and have shown that when pollinators are excluded from avocado flowers, fruit set or yield is close to zero (Dymond et al., 2021).
Due to the importance of insect pollinators, many growers employ managed honeybees in their avocado orchards. However, it is also known that wild pollinators contribute to avocado pollination, with several studies showing a diverse array of wild insects visiting avocado flowers (Bushuru, 2015; Carabalí-Banguero et al., 2018; Castañeda-Vildózola et al., 1999; Celis-Diez et al., 2023; De la Cuadra-Infante, 2007; Estévez and Martínez, 2020; McNeil and Pidduck, 2003; Monzón et al., 2020; Read et al., 2017; Willcox et al., 2019). Certain species are also known to be effective avocado pollinators. For instance, various wild bees have been shown to visit a similar number of flowers and deposit a comparable amount of pollen compared to honeybees (Bushuru, 2015; Can-Alonzo et al., 2005; Perez-Balam et al., 2012; Vithanage, 1990; Willcox et al., 2019).
Chile is a globally significant producer of avocados and currently has the third-largest production area (FAO, 2023). Avocado orchards are primarily located in the Mediterranean region of central Chile. Within this region, native sclerophyllous forests stand as a biodiversity hotspot due to the high level of endemism among the fauna and flora (Myers et al., 2000). However, over the past two decades, the expansion of avocado production has replaced much of this native sclerophyllous forest and other natural habitats (Armesto et al., 2010; Magrach and Sanz, 2020). Numerous studies have evidenced that natural habitats host an increased abundance and diversity of wild pollinators, resulting in a comprehensive and effective pollination service, thereby enhancing crop production in areas adjacent to natural habitats (Dainese et al., 2019; Garibaldi et al., 2011; Martin et al., 2019; Ricketts et al., 2008). As such, it is likely that the expansion of avocado orchards and the increasing isolation from natural habitats has negatively impacted avocado yield in this region, however, direct evidence is needed.
Using data from field experiments in avocado orchards; this study aims to understand the contribution of natural habitats and wild insect visitors to avocado pollination and production in Chile. Specifically, the objectives of this study are to (1) identify the flower visitors present in avocado orchards and explore the relationship between flower visitor abundance, visits, richness, and diversity, and proximity to natural habitat, (2) understand the pollination effectiveness of different insect taxa by quantifying their flower visitation rate, and (3) measure the contribution of insect pollinators to avocado production and investigate whether this contribution varies with proximity to natural habitats. The findings of this study provide valuable insights for avocado growers regarding land management strategies for enhancing pollination management and achieving sustainable production.
2 Materials and methods
2.1 Study design
This study was conducted in three avocado orchards located in the Mediterranean region of central Chile (Figure 1). Data collection took place from October to December in the years 2020, 2021, and 2022. The orchards primarily cultivated the A-type Hass variety, with intermittent planting of B-type cultivars such as ‘Edranol’ to serve as a pollenizer. On two farms, other crops such as almonds and oranges were cultivated but, in all cases, avocado was the prominent crop. To identify the farms, an initial list of commercial avocado orchards was provided by industry partners and other collaborators. Farm selection involved choosing orchards with a native habitat more than 1 km long surrounding the orchard, ensuring the farms were more than 30 km apart, and verifying a similar topography, such as hillside plantations. At all sites, managed honeybees (Apis mellifera) were located throughout the orchard during the flowering period (around seven to ten hives per hectare).

Figure 1. Details of the avocado study sites in central Chile. The red circles represent the location of the study orchards. The red lines show the location of natural habitat, and the control transects for one of the study orchards. For this farm, the natural habitat border runs alongside the right-hand side of the avocado orchard and the control border starts next to the reservoir which is located between the avocado orchard and an almond orchard.
Three transects at least 1 km apart were established on each farm. Two transects were run from the native vegetation, serving as natural habitat borders, and one transect was run from a non-natural habitat border, such as another agricultural crop (e.g., almond crop) or farm infrastructure (e.g., reservoir), which served as a control (Figure 1). Each transect was 300 m long extending from the border (natural habitat or control) into the centre of the orchard. This distance was chosen as typically, the flight range of wild bees is approximately 100-200 m from their nesting site, usually located in natural habitats (Zurbuchen et al., 2010).
2.2 Flower visitor surveys
To collect data on flower visitor abundance and diversity, observational surveys were conducted along the transects at distances of 0 m, 50 m, 100 m, 200 m, and 300 m from the orchard edge into the centre. Two trees were randomly selected at each distance, and each tree was observed for 5 min. Observations were conducted on four different days each year, however, in 2020, two sites were only visited on three days due to logistical challenges. Data collection spanned 34 days over three years; with each site visited 11 or 12 times, observing two trees at each transect point 11 or 12 times (e.g., each transect point had a total observation time 110 or 120 min). Surveys were carried out during the flowering season which occurred from October to November, depending on the year. The observations took place between 10 a.m. and 3 p.m. to coincide with the warmest part of the day when the avocado flowers were open. Observations only took place on warm days with little wind when more than 10% of the flowers on the trees were open. Before the observation started, a branch at eye level and roughly 1 m square was selected as the observation area, as this was perceived to be a feasible area to observed accurately. Data were recorded on species observed visiting avocado flowers and the number of times that they visited an open flower. If an insect could not be identified to the species level in the field, then the insect was captured in a net and deposited in a collection jar, where it was later taken to taxonomist for identification. In cases where it was not possible to capture the insect or its capture would have distracted from the observations, the insect was either, photographed, or a written description was taken and identified at a later stage. In cases where identification was still not possible, a broad taxonomic group (e.g., honeybee, wild bee, fly, hoverfly, wasp, beetle) was assigned instead (Supplementary Appendix A1). Data were also recorded on the number of open flowers in the observed area.
Species abundance was the number of wild visitors recorded, species richness was the number of different wild species observed, and visitation rate was the total number of times wild visitors visited an open flower. Species diversity was calculated using the Shannon index. For each year and each observation point, we combined the data for all observation days. Although sampling effort was uneven between natural habitat and control transects, for species diversity analysis, we retained data from both transects because rarefaction analysis indicated that species diversity estimates were stable, and sampling completeness curves showed that sampling effort reach 100% in each transect type (Supplementary Appendix A2). This suggests that additional sampling would likely yield few new species, thereby validating the comparability of the datasets.
2.3 Flower visitation rate
We assessed flower visitation rate for different taxa as this is a proxy for pollen deposition and thus an important component of pollinator performance (Ne’eman et al., 2010). In all years, GoPro video (model hero 8) cameras were set up in the avocado orchards to record flower visitation rates. The cameras were located in an area close to the natural habitat, as it was hypothesized that these locations would have a greater diversity of wild insects. Two or three video cameras were used every day that flower visitor surveys were taking place (e.g. 34 days). Video cameras were focused on a flowering branch and recorded data from around 11 a.m. to 2 p.m. Each camera recorded for around one to two hours, depending on the battery quality and daily temperature (on days with extreme temperature the batteries did not last as long). In 2020 and 2021, most recorded observations were of honeybees with limited replication for other taxa. Therefore, to supplement the data set, in 2022, visitation rate data was also collected through Dictaphone voice recordings as this method allowed for more targeted recordings of less frequently observed taxa such as flies, wasps, and wild bees. To achieve this, the observer actively searched for an insect taxon of interest, and once identified, the recording began. The observer recorded when the insect arrived on a flower, when it left the flower, and when it landed on a new flower. The observation was continued for five minutes or until the insect went out of sight.
After the end of each season, the video and Dictaphone recordings were reviewed. The video recordings were watched using a VLC media player as at times it was necessary to use the interactive zoom function available on this software to focus closer on the target branch. After selecting the best view, the videos were watched, and if a flower visiting insect was observed, the taxon, and if possible, the species were identified. However, species identification was not always possible due to the quality of the image. The BORIS software was used to extract observations from the video and Dictaphone recordings (Friard and Gamba, 2016). Data were recorded on when the insect landed on a flower, when it left the flower, when it was moving between flowers, when it landed on a new flower, and when it left the observation area. Using these data, we calculated the average time spent on a flower and the average time moving between flowers for each insect observed in the video and Dictaphone recordings. We then applied the following formula to calculate individual flower visitation rate: 60/ [average time on flower (seconds) + average time moving between flowers (seconds)].
2.4 Controlled pollination trials
To understand pollinator contribution to fruit set, controlled pollination trials were conducted along the same transects used for the flower visitor surveys. In 2022, treatments were established at distances of 0 m, 100 m, and 300 m, and at each distance, five trees were selected (45 trees at each distance across all transects and orchards). On each tree, two panicles on separate branches were selected and labeled as either ‘open’ or ‘exclusion’ treatment (Figure 2). Panicles were chosen as a suitable scale for measuring pollination contribution, due to execution challenges associated with whole tree or branch measurements (Webber et al., 2020). It was ensured that the panicles on the same tree had a similar number of primary branches and pre-flowering buds, were at a comparable height, and had similar access to light. Panicles that received the ‘exclusion’ treatment had a mesh bag placed securely over the panicle to exclude all pollinators. The bags were placed on the panicles in September before the flowers had opened and remained on the panicles until the end of the experiment in late December. Open pollination treatments served as the control; allowing insect pollinators to access the flowers naturally. To calculate the percentage of fruit set, an estimation of the number of flowers per treatment panicle was conducted in the middle of the flowering season. An exact count of the flowers was not possible as new flowers open daily and it was not feasible to be present at every site on every day during the flowering season. The estimation involved counting the number of flowers on 100 primary branches from 100 panicles (from different trees and sites). This data was used to calculate the average number of flowers per primary branch. The number of primary branches per treatment panicle was also counted. To save time in the field, a photo of the treatment panicle was taken, and then counting was done later, on a computer screen. The average number of flowers per primary branch (17) was then multiplied by the number of primary branches on each panicle to provide an estimated number of flowers on that treatment panicle. Six weeks after the end of the flowering season, the number of initial fruit set per treatment panicle was recorded.

Figure 2. Example photograph of one tree in the controlled pollination trials in avocado orchards in Chile. The panicle with the white bag is the “exclusion” treatment and the panicle with the red tape is the “open” pollinated treatment.
2.5 Statistical analysis
2.5.1 Flower visitor surveys
Given the multiple observations per transects and distances, a mixed-effect model was necessary. We applied a generalized linear mixed model to the dependent variables of wild insect visitor abundance, honeybee abundance, wild insect visitation rate, species richness, and species diversity. All models were run using the independent variables of; distance from the edge (categorical variable, 0, 50, 100, 200, 300), habitat type (categorical variable, natural habitat, control), year (categorical variable, 2020, 2021, 2022), the number of open flowers (log-transformed) and all two-way interactions. We chose to include only two-way interactions to focus on the main effects, while simplifying the model and minimizing the risk of overfitting. For most of the models, the random effects were transect nested within site and observation day. However, for species diversity, since the data for observation day were combined, only transect nested in site was used as a random effect. Model fit assessment showed that random effects explained a notable proportion of the variance in all models (wild visitor abundance: Conditional R2 = 0.365, Marginal R2 = 0.169, wild visitor visits: Conditional R2 = 0.416, Marginal R2 = 0.189, species richness: Conditional R2 = 0.801, Marginal R2 = 0.025, species diversity Conditional R2 = 0.41, Marginal R2 = 0.374, and honeybee abundance: Conditional R2 = 0.460, Marginal R2 = 0.234), justifying their inclusion (Supplementary Appendix A3). We used a negative binomial family for wild visitor abundance, honeybee abundance, and visitation rate as the data was highly over-dispersed relative to the expectation of the Poisson distribution. For species richness, we used a generalized Poisson distribution with a log link because the data exhibited under-dispersion, meaning the variance was smaller than the mean, which is inconsistent with the assumption of the standard Poisson distribution. For species diversity, we used a Gamma distribution with a log link. All models were checked for overdispersion, and their assumptions were verified by plotting residuals against fitted values and the covariate ‘number of open flowers’. The models were selected for ‘best fit’ using backwards stepwise deletion based on AIC (Akaike Information Criterion) comparisons and, where necessary, independent variables were dropped from the model (Supplementary Appendix A4). Variables were removed if the difference in AIC between the full model and the reduced model was less than 2, to prevent overfitting. To assess significant differences between all distances at each habitat type, we ran another GLMM model for each dependent variable. The model syntax was the same as before, however, a new independent variable was added to combine all possible distances and habitats (e.g., Natural Habitat0, Natural Habitat 50, etc.). An ANOVA and post hoc Tukey’s test were conducted on this model to identify significant differences for each distance and habitat combination.
2.5.2 Flower visitation rate
Since it was not possible in the video recording to identify many insect species, the flower visitation rate data was analyzed at the taxa level (e.g., honeybees, flies, hoverflies, wild bees, beetles, and wasps). As the data was not normally distributed, we performed a Kruskal-Wallis test and post hoc Dunn test, to compare differences between flower visitation rate. The data for all years and all observation methods (video and Dictaphone) were combined, as the results from one-way ANOVAs conducted for individual taxon and year, and individual taxa and observation method were non-significant, indicating no effect of year or observation method.
2.5.3 Controlled pollination trials
To assess the contribution of proximity to natural habitats to fruit set, we applied a generalized linear mixed model with a binomial distribution and logit link to the dependent variable of ‘proportion of fruit set’. The independent variables included pollination treatment (open and exclusion), habitat type (natural habitat and control), distance from the edge (0 m, 100 m, and 300 m), and all two-way interactions. The random effects were tree nested in transect and transect nested in site, however, transect nested in site was later removed as these effects had no explanatory power on the model and consequently, the model would not converge. This model showed a strong over all fit (conditional R2 = 0.993), with fixed effects accounting for 52.5% of the explained variance (marginal R2 = 0.525).
Additionally, we explored the relationship between the abundance of individual insect taxa and fruit set in the open pollination treatments. For each site, transect, and distance we calculated the average abundance of each insect taxon using the flower visitor survey data, as well as the average fruit set at each distance. For this analysis, only data from 2022 was used given that fruit set data was only collected in this year. We then applied a generalized linear mixed model (binomial family, logit link), using the proportion of fruit set as the dependent variable, the abundance of each insect taxon as the independent variable, and transect nested within site as the random effect. Models followed the same process of model checking and fitting as before.
Data for all the above analyses were carried out in R version 4.2.3 using the R Core Team (2023). The package glmmTMB (Brooks et al., 2017) was used to carry out the GLMM analysis, and base R and the multcomp package (Hothorn et al., 2008) was used to implement the ANOVA, Kruskal-Wallis, and post hoc tests.
3 Results
3.1 Flower visitor surveys
Across the three years of surveys and in the three study orchards, a total of 5,340 flower-visiting insects were observed, representing 75 different species across five orders (Coleoptera, Diptera, Lepidoptera, Hymenoptera, and Hemiptera) (Supplementary Appendix A1). Honeybees accounted for 54% of the observations (n = 2,883), and it is assumed that all these observations were from managed hives, as local knowledge indicates that wild honeybees are not present in the area. Wild insects accounted for 46% of the observations with 29.3% (n = 1,569) beetles, 7.4% (n = 396) hoverflies, 5.8% (n = 311) flies, 1.7% (n = 89) wild bees, 1.6% (n = 85) wasps, 0.2% (n = 11) butterflies, and 0.09% true bugs (n = 5).
For the metrics of wild visitor abundance, visits, richness, and diversity, all independent variables were retained in the model, except for the interaction between distance and year and, in the case of wild insect visitor abundance and wild insect visits, the interaction between habitat and year was also dropped (Supplementary Appendix A4). For all wild insect models, the interaction between distance and habitat type was significant and the results showed no relationship between wild insect variables (abundance, visits, richness, and diversity) and distance to edge in control transects, while a negative relationship was observed in natural habitat transects (Table 1, Figure 3, and Supplementary Appendix A5). Furthermore, the results obtained from the ANOVA and Tukey’s test demonstrated significantly higher wild insect visitor abundance, richness, diversity, and visitation rates at 0 m in natural habitat transects in comparison to nearly all other distances (Supplementary Appendices A6, A7). For instance, wild insect visitor abundance and visitation rates were approximately 2.55 times higher, species richness around 1.6 times higher and species diversity around 1.5 times higher at the natural habitat edge compared to nearly all other distances. For honeybee abundance, the number of open flowers and distance from the edge, were retained in the model, however, only the number of open flowers was significant. (Table 2 and Supplementary Appendix A5).

Table 1. Results of the GLMM models of wild visitor abundance, wild visitor visits, species richness, and species diversity in avocado orchards in Chile. Z-values and p-values are shown for all independent variables and two-way interactions retained in the model. Intercept represents the control transect at 0 m in the year 2020.

Figure 3. Effects of distance from natural habitat edge and control (non-natural habitat) edge in avocado orchards, across years on (a) wild visitor abundance, (b) total wild visitor visits, (c) species richness and (d) species diversity. Point denotes the predicted mean for each distance and the bars represent the standard error.
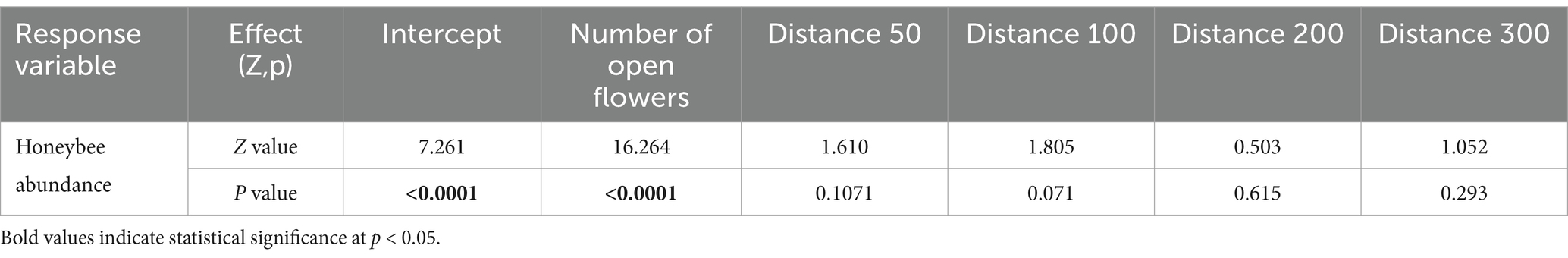
Table 2. Results of the GLMM model of honeybee abundance in avocado orchards in Chile. Z values and p- values are show for all independent variables and two-way interactions retained in the model. Intercept represents the control transect at 0 m in the year 2020.
3.2 Flower visitation rate
The analysis of the flower visitation rate revealed that honeybees and flies visited the highest number of flowers per minute, averaging 8.5 and 7.9, respectively. This was significantly higher than hoverflies, which visited an average of 4.2 flowers per minute (comparison between honeybee: hoverfly p-value <0.0001 and z-value 6.8, comparison between fly: hoverfly p-value 0.001 and z-value 3.9) (Supplementary Appendix A8). Beetles had the lowest visitation rate, with an average of 1.3 visits per minute (Figure 4), visiting significantly fewer flowers per minute compared to all other taxa (p-value <0.05 for all taxa) (Supplementary Appendix A8).
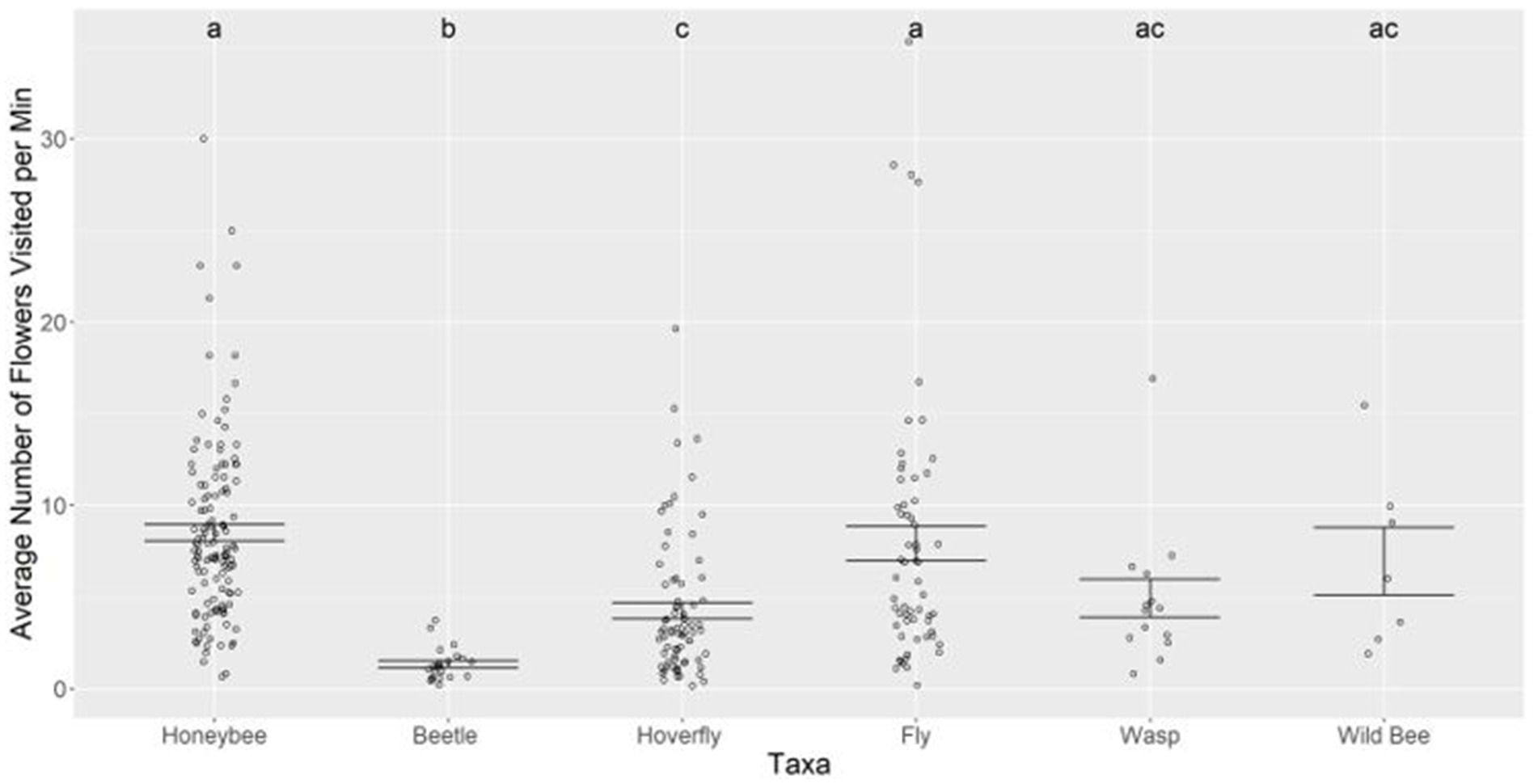
Figure 4. The number of avocado flowers visited per minute for: honeybee (n = 128 observations), beetles (n = 23 observations), hoverflies (n = 77 observations), flies (n = 60 observations), wasps (n = 14 observations), and wild bees (n = 7 observations). Dots show the visitation rate per individual, the bars represent the standard errors and the letters denote significant difference. Beetles had a significantly lower visitation rate compared to all other taxa (p-value <0.05) and honeybees and flies had a higher visitation rate than hoverflies (hoverflies: flies p-value 0.001 and honeybee: flies p-value 0.0001).
3.3 Controlled pollination trials
In the fruit set model, the interaction between distance and habitat, the interaction between pollination treatment and habitat, and habitat were dropped (Supplementary Appendix A4), and the results showed a significant interaction between pollination treatment and distance. Specifically, distance from the edge for open pollinated treatments showed a negative linear trend, while no relationship was observed for exclusion treatments (Table 3, Figure 5, and Supplementary Appendix A9). All open pollinated treatments had significantly higher fruit set than exclusion treatments, except for 300 m open and 100 m exclusion (Supplementary Appendix A10) and, on average, fruit set per panicle was approximately 3.2 fruits in open pollinated treatments compared to 0.6 fruits in exclusion treatments.

Table 3. Results of the GLMM model of proportion fruit set in avocado orchards in Chile. Z-values and p-value are shown for all independent variables and two-way interactions retained in the model. Intercept represents the control transect at 0 m and the open pollination treatment.
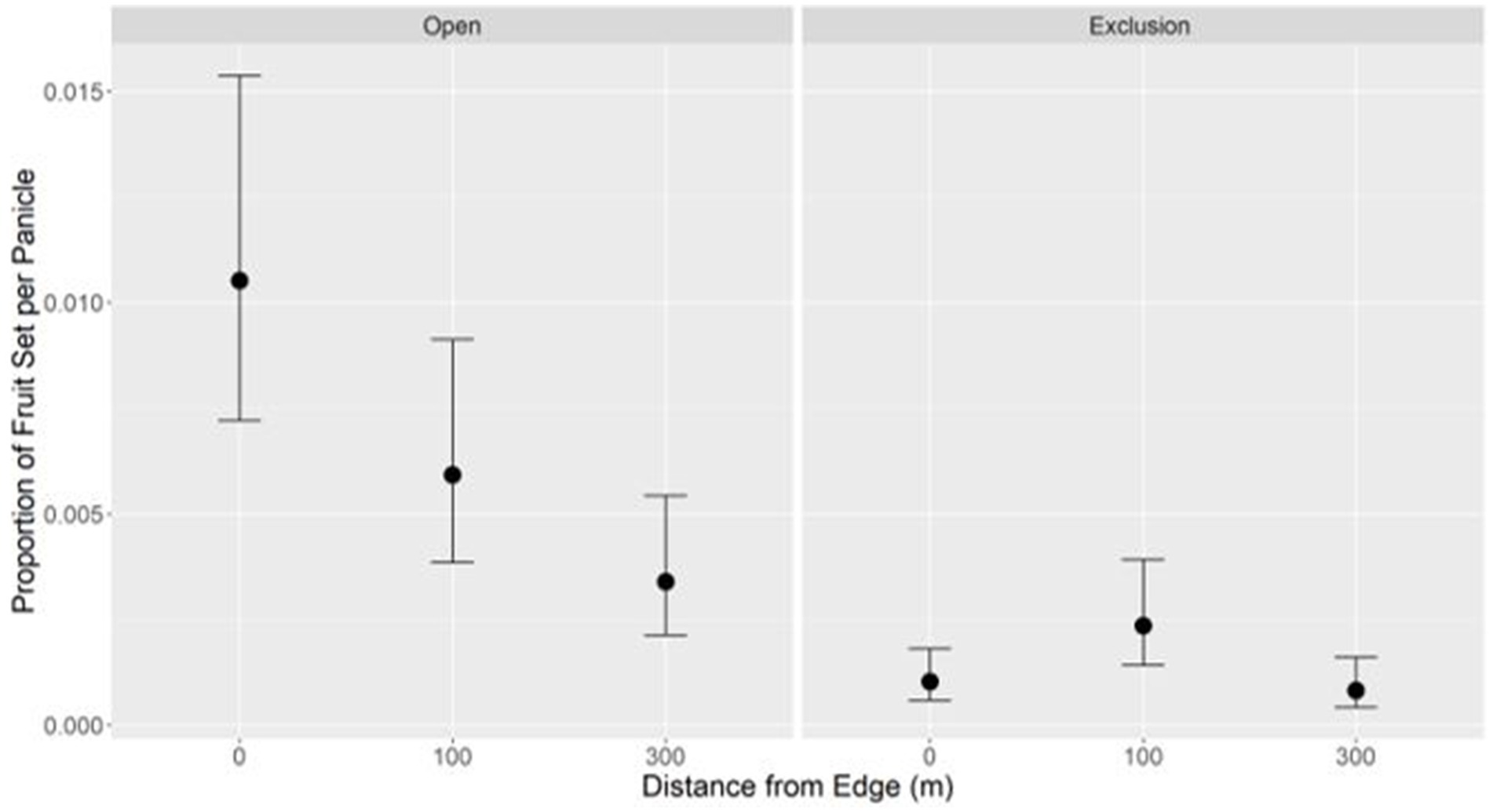
Figure 5. The proportion of fruit set per avocado panicle at increasing distances from the edge for open treatments (pollinators could freely access the panicles) and exclusion treatments (mesh bags were placed on panicles to exclude pollinators). Dots denote the mean and bars represent the standard errors.
The analysis of the relationship between insect taxa and fruit set indicated a positive correlation between hoverflies and fruit set (β = 1.37, SE = 0.46, p-value 0.003). Although the remaining taxa did not yield statistically significant results, the data suggests a potential positive relationship between fruit set and abundance of flies and wasps, while no such relationship was observed for honeybees and beetles (Figure 6).
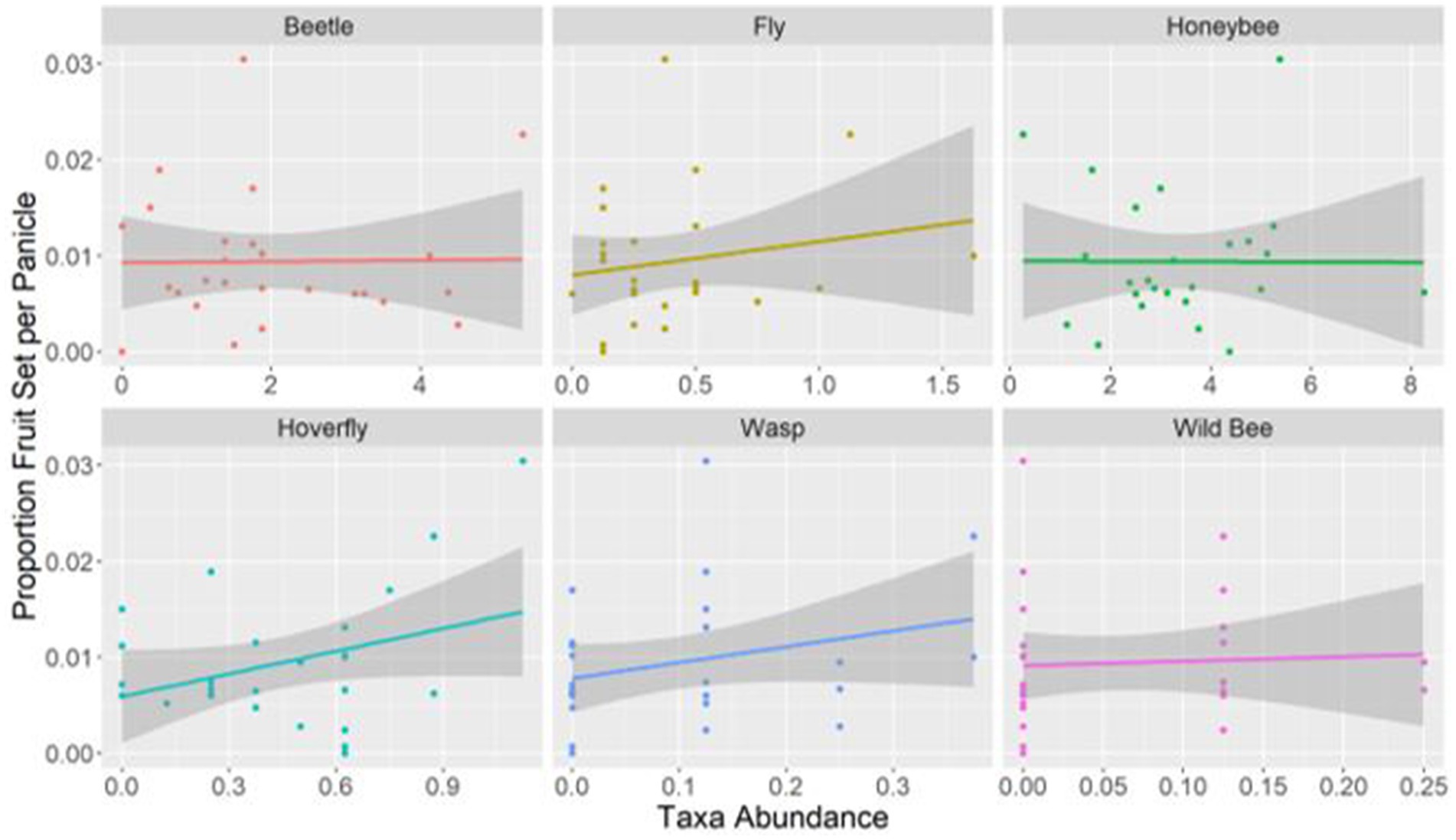
Figure 6. The relationship between proportion of fruit set per panicle and the abundance of beetles, flies, honeybees, hoverflies, wasps, and wild bees. Hoverfly showed a significant relationship (p-value 0.003). Points denote the average abundance of each taxon per experimental point and the grey shaded areas indicate confidence intervals.
4 Discussion
Our study revealed that the abundance, diversity, visitation rate, and richness of avocado flower visitors were all higher closest to natural habitats. Additionally, the significant contribution of insect pollinators to avocado production was evident as pollinator exclusion trials yielded almost no fruit set. The findings also suggested the importance of wild insects (especially hoverflies and other flies) as potential avocado pollinators, with flies displaying a higher flower visitation rate and, avocado fruit set being positively correlated with the abundance of hoverflies. However, further research to confirm the individual contribution of different taxa is needed. Overall, this study contributes to our understanding of the effects of pollination and natural habitats on agricultural practices in Mediterranean central Chile, where robust data, collected over multiple seasons, is limited (Medel et al., 2018).
4.1 Flower visitor surveys
The abundance, diversity, richness, and visitation rate of flower visitors decreased with increasing distances from natural habitats, aligning with findings from other studies and reviews (Bartual et al., 2019; Garibaldi et al., 2011; Klein et al., 2012; Ricketts et al., 2008). This relationship can be attributed to the provision of various resources for wild insects, such as nesting sites and additional food sources, within natural habitats. Consequently, these habitats tend to support higher species abundance and diversity, which spills over into bordering agricultural areas (Potts et al., 2005; Öckinger and Smith, 2007; Evans et al., 2018). In the Mediterranean region of Chile, such resources have been shown to be present in natural habitats, such as remnants of sclerophyllous forest, and host higher diversity and abundance of native bees compared to managed areas (Rodríguez et al., 2021).
Our study highlighted that areas immediately adjacent to natural habitats had higher flower visitor abundance, diversity, and richness. However, beyond a distance of 50 meters into the orchard, there were no further declines. While a sharp decline in abundance was expected for certain taxa, such as solitary wild bees that typically nest in natural habitats and have a limited foraging distance (Ricketts et al., 2008; Woodcock et al., 2016), our observations indicated that hoverflies and flies were much more prevalent compared to wild bees. These taxa often do not exhibit a strong negative relationship with distance from natural habitat edge, as they are generally not central place foragers and can therefore travel further from natural habitat areas (Rader et al., 2020). One possible explanation for this observation is that when feeding resources are abundant in close proximity to the natural habitat, these taxa are less likely to travel long distances in order to conserve energy (Chacoff and Aizen, 2006).
The results showed no significant effect of distance from natural habitat on honeybee abundance. This finding is consistent with previous studies, as the abundance of managed honeybees is often influenced by the positioning of the hives in the agricultural landscape rather than natural habitats (Steffan-Dewenter and Kuhn, 2003).
4.2 Flower visitation rate
Data on flower visitation rates were collected to explore the potential effectiveness of different insect taxa on avocado pollination. Although several factors determine pollinator effectiveness, pollination visitation rate is a key indicator (Ne’eman et al., 2010), and, for crops like avocado, which rely on pollen transfer from polliniser trees during the male flowering stage, a high visitation rate is important as it increases the probability that a male flower has been visited and, consequently, that the insect may deposit pollen. Our analyses showed that honeybees and flies had a higher flower visitation rate per minute compared to other insect taxa, supporting existing research suggesting that flies are important avocado pollinators (Cook et al., 2020; Dymond et al., 2021; Perez-Balam et al., 2012; Vithanage, 1985). However, to gain a more comprehensive understanding of pollinator effectiveness for avocados, future studies should focus on collecting additional metrics, such as single-visit pollen deposition and flower handling behavior by different species (Ne’eman et al., 2010).
4.3 Controlled pollination trials
Our results showed that insect pollinators play a vital role in avocado pollination, as we observed close to zero fruit set following pollinator exclusion. To our knowledge, there are only two studies that have shown significant avocado pollination in pollinator exclusion trials (Davenport, 2019; Davenport et al., 1994) and this anomaly is generally attributed to thrip pollination within the exclusion bags or humid climatic conditions causing overlapping transitions from the male to female stage on the same flower. Thus, our findings contribute to the existing literature highlighting the significance of pollinators in avocado production (Dymond et al., 2021) and additionally underscore the importance of pollinators for avocado production in Chile, for which there is currently limited data.
Our results showed higher fruit set close to the orchard edges, but no effect of habitat type despite the greater abundance and diversity of wild insects at orchard margins near natural habitat. One possible explanation for this is that abiotic factors present exclusively at the orchard edge significantly contribute to fruit set. For example, in our study sites, there were approximately four to five metres of space between the orchard and the edge of the natural habitat or other non-natural habitat areas, thereby increasing the availability of resources. For example, light is a key factor in flowering intensity and duration (Coutanceau, 1964), and flowering and fruiting are significantly reduced in shaded conditions (Meyer Myers, 1960), therefore a greater light exposure near the border may enhance fruit set in these areas. However, another explanation could be that certain pollinator taxa, which are less reliant on natural habitats, contribute more to avocado pollination. Previous studies have shown that hoverflies and other flies are effective avocado pollinators (Can-Alonzo et al., 2005; Castañeda-Vildózola et al., 1999; Ish-Am et al., 1999; Perez-Balam et al., 2012; Sagwe et al., 2022; Vithanage, 1990), and our results support this hypothesis as hoverfly abundance was positively correlated with fruit set and flies had a comparatively high flower visitation rate in comparison to other wild taxa. To investigate this finding further, we attempted to analyze the effect of distance and habitat type on individual flower visiting taxa but the sample size for these less observed taxa was too small to provide robust results. Nonetheless, previous research suggests that hoverflies and other flies, often considered generalists species, may not be as strongly associated with natural habitats compared to other pollinators (Jauker et al., 2009; Jauker and Wolters, 2008; Rader et al., 2020; Schirmel et al., 2018; Speight, 2014). Consequently, the abundances of these taxa in natural and non-natural edges may be more evenly distributed. This could, in part, help explain why we observed similar levels of fruit set in areas close to both habitat types. Additionally, since our study measured only initial fruit set and was implemented for one-year, further research is needed. Future studies should measure final fruit set or yield, as these metrics more accurately reflect production (Webber et al., 2020) and conducting studies over multiple years is necessary to account for annual fluctuations in production.
In line with other avocado pollination studies in Chile (Celis-Diez et al., 2023), our results suggest that an increase in honeybee abundance does not have an impact on fruit set. This could be because even at low honeybee abundances, there are sufficient honeybee numbers to ensure adequate pollination. However, the average fruit set in this study was around 1% whereas other studies have shown that under optimal pollination (manual pollination), fruit set can reach up to 5% (Alcaraz and Hormaza, 2009; Evans et al., 2010; Garner and Lovatt, 2008). Therefore, this could indicate a pollination deficit in our orchards, suggesting that fruit set rates could be increased with improved pollination services. An alternative hypothesis is that honeybees are not efficient avocado pollinators, however, this is unlikely as several other studies have shown a positive correlation between honeybee abundance and avocado pollination, as well as their effective pollen deposition in avocado flowers (Bushuru, 2015; Castañeda-Vildózola et al., 1999; Perez-Balam et al., 2012; Peña and Carabalí, 2018; Sagwe et al., 2022; Vithanage, 1990; Willcox et al., 2019). Therefore, the results likely indicate that pollinator diversity and richness are beneficial for avocado pollination even when honeybee abundance is high. This has been demonstrated in several other crops (Garibaldi et al., 2013) and is likely due to the complementary pollination services provided by a variety of pollinators (Blüthgen and Klein, 2011; Hoehn et al., 2008) as well as functional facilitation, which occurs when honeybee displace wild pollinators, promoting outcrossing and improved pollination (Greenleaf and Kremen, 2006).
4.4 Management implications
Our study highlights the crucial role of insects in avocado pollination and underscores the importance of crop proximity to natural habitats in ensuring wild visitor abundance, diversity, and richness. As such, it is recommended that avocado growers protect and enhance natural habitats throughout the agricultural landscape and ensure that crops are located close (ideally <100 m) to natural habitat edges. Additionally, our results, along with previous research, indicate the likely importance of hoverflies and other flies as key avocado pollinators. These taxa often have a broader foraging range and are not solely reliant on specific plant species that might only be found in natural habitats. Therefore, they can benefit from alternative habitat interventions, such as managed floral plantings within the crop. Several studies have shown that floral strips can improve crop pollination services, and they are often considered a cost-effective and relatively easy pollination management strategy (Albrecht et al., 2020; Blaauw and Isaacs, 2014; Krimmer et al., 2019; Lowe et al., 2021; Muñoz et al., 2021; Rundlöf et al., 2018). Additionally, such habitat interventions can reduce pests due to enhanced pest regulations services, leading to further yield improvements (Albrecht et al., 2020; Martin et al., 2019). However, recent reviews have highlighted that the implementation of floral strips can be ineffective without sufficient natural habitat in the landscape (Albrecht et al., 2020; Dainese et al., 2019) and therefore, we recommend that a combination of both management strategies are employed by growers. The implementation of these practices should enhance local biodiversity, providing a robust approach to sustainable avocado production.
Data availability statement
The datasets generated and analyzed during the current study are available in the Figshare repository at https://doi.org/10.6084/m9.figshare.29145257.v1. Researchers can access the data for verification and further analysis. Any additional information required can be obtained from the corresponding author upon reasonable request.
Ethics statement
Ethical approval was not required for this study as the relevant institutions and local legislations did not require ethical approval for observing and capturing invertebrates.
Author contributions
KD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JC-D: Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. PD-S: Investigation, Writing – review & editing. VR-B: Investigation, Writing – review & editing. JM-H: Investigation, Project administration, Resources, Writing – review & editing. SP: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. MG: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was funded by the University of Reading graduate school and BBSRC and Waitrose Agronomy Group as part of the Waitrose Collaborative Training Partnership (BB/V509747/1). JM-H, VR-B, and JC-D were funded by the FIA PYT-2020-0250, and FONDECYT 1241258.
Acknowledgments
The authors wish to thank the avocado growers Daniela Riegel and Roberto Mayol who allowed us access to their farms to carry out this research and to Carl Lymma-Denis and Juan Enrique-Ortuzar from Westfalia Fruit. We are also extremely gratefully to the entire team at INIA and PUCV who supported with the field data collection, specifically, Camila B. García, Fernanda Montero, Camila Martínez, Francisca Sepúlveda, Marlene Ponce, Natalia Olmos-Moya, and Ana Morales. We would also like to thank the ANID/ R23F0003 project for providing additional support resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1560802/full#supplementary-material
References
Albrecht, M., Kleijn, D., Williams, N. M., Tschumi, M., Blaauw, B. R., Bommarco, R., et al. (2020). The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: a quantitative synthesis. Ecol. Lett. 23, 1488–1498. doi: 10.1111/ele.13576
Alcaraz, L. M., and Hormaza, J. I. (2009). Avocado pollination and fruit set- a perspective from Spain. California Avocado Society Yearbook 92, 113–135.
Armesto, J. J., Manuschevich, D., Mora, A., Smith-Ramirez, C., Rozzi, R., Abarzúa, A. M., et al. (2010). From the Holocene to the Anthropocene: a historical framework for land cover change in southwestern South America in the past 15,000 years. Land Use Policy 27, 148–160. doi: 10.1016/j.landusepol.2009.07.006
Bartual, A. M., Sutter, L., Bocci, G., Moonen, A.-C., Cresswell, J., Entling, M., et al. (2019). The potential of different semi-natural habitats to sustain pollinators and natural enemies in European agricultural landscapes. Agric. Ecosyst. Environ. 279, 43–52. doi: 10.1016/j.agee.2019.04.009
Blaauw, B. R., and Isaacs, R. (2014). Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 51, 890–898. doi: 10.1111/1365-2664.12257
Blüthgen, N., and Klein, A.-M. (2011). Functional complementarity and specialisation: the role of biodiversity in plant–pollinator interactions. Basic and Applied Ecology 12, 282–291. doi: 10.1016/j.baae.2010.11.001
Brooks, M. E., Kristensen, K., Benthem Van, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling in. The R Journal. doi: 10.32614/RJ-2017-066
Bushuru, E. (2015). Diversity and pollination activity of flower visiting insect associated with avocado along the slopes of Taita Hills in Kenya. Kenya: Masinde Muliro University of Science and Technology.
Campbell, B. M., Beare, D. J., Bennett, E. M., Hall-Spencer, J. M., Ingram, J. S. I., Jaramillo, F., et al. (2017). Agriculture production as a major driver of the earth system exceeding planetary boundaries. Ecol. Soc. 22. doi: 10.5751/ES-09595-220408
Can-Alonzo, C., Quezada-Euan, J. J., Xiu-Ancona, P., Moo-Valle, H., Valdovinos-Nunez, G., and Medina-Peralta, S. (2005). Pollination of ‘criollo’ avocados (Persea americana) and the behaviour of associated bees in subtropical Mexico. J. Apic. Res. 44, 3–8. doi: 10.1080/00218839.2005.11101138
Carabalí-Banguero, D., Montoya-Lerma, J., and Carabalí-Muñoz, A. (2018). Dípteros asociados a la floración del aguacate Persea americana Mill cv. Hass en Cauca, Colombia. Biota Colombiana 19, 92–111. doi: 10.21068/c2018v19n01a06
Castañeda-Vildózola, A., Equihua-Martínez, A., Valdés-Carrasco, J., Barrientos-Priego, A. F., Ish-Am, G., and Gazit, S. (1999). Insectos polinizadores del aguacatero en los estados de México y Michoacán. Revista Chapingo Serie Horticultura 5, 129–136.
Celis-Diez, J. L., García, C. B., Armesto, J. J., Abades, S., Garratt, M. P. D., and Fontúrbel, F. E. (2023). Wild floral visitors are more important than honeybees as pollinators of avocado crops. Agronomy 13:1722. doi: 10.3390/agronomy13071722
Chacoff, N. P., and Aizen, M. A. (2006). Edge effects on flower-visiting insects in grapefruit plantations bordering premontane subtropical forest. J. Appl. Ecol. 43, 18–27. doi: 10.1111/j.1365-2664.2005.01116.x
Chaplin-Kramer, R., Dombeck, E., Gerber, J., Knuth, K. A., Mueller, N. D., Mueller, M., et al. (2014). Global malnutrition overlaps with pollinator-dependent micronutrient production. Proc. R. Soc. B Biol. Sci. 281:20141799. doi: 10.1098/rspb.2014.1799
Cook, D. F., Voss, S. C., Finch, J. T. D., Rader, R. C., Cook, J. M., and Spurr, C. J. (2020). The role of flies as pollinators of horticultural crops: An Australian case study with worldwide relevance. Insects 11:341. doi: 10.3390/insects11060341
Dainese, M., Martin, E. A., Aizen, M. A., Albrecht, M., Bartomeus, I., Bommarco, R., et al. (2019). A global synthesis reveals biodiversity-mediated benefits for crop production. Science. Advances 5:eaax0121. doi: 10.1126/sciadv.aax0121
Davenport, T. L. (2019). Cross- vs. self-pollination in ‘Hass’ avocados growing in coastal and inland orchards of Southern California. Sci. Hortic. 246, 307–316. doi: 10.1016/j.scienta.2018.10.051
Davenport, T. L., Parnitzki, P., Fricke, S., and Hughes, M. S. (1994). Evidence and significance of self-pollination of avocados in Florida. J. Am. Soc. Hortic. Sci. 119, 1200–1207. doi: 10.21273/jashs.119.6.1200
De la Cuadra-Infante, S. (2007). Determination of the pollination activity of honeybees (Apis Mellifera) in the avocado tree pollination in the central zone in Chile. Chile: World Avocado Congress.
Dymond, K., Celis-Diez, J. L., Potts, S. G., Howlett, B. G., Willcox, B. K., and Garratt, M. P. D. (2021). The role of insect pollinators in avocado production: a global review. J. Appl. Entomol. 145, 369–383. doi: 10.1111/jen.12869
Eilers, E. J., Kremen, C., Greenleaf, S. S., Garber, A. K., and Klein, A.-M. (2011). Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS One 6:e21363. doi: 10.1371/journal.pone.0021363
Estévez, A. A., and Martínez, A. G. (2020). Visitantes florales del aguacate (Persea americana Mill.) en un terreno urbano en La Habana. Cuba. Acta Botánica Cubana 219.
Evans, L. J., Goodwin, R. M., and McBrydie, H. M. (2010). Factors affecting Hass avocado (Persea americana) fruit set in New Zealand. New Zealand Plant Protection 63, 214–218. doi: 10.30843/nzpp.2010.63.6548
Evans, E. C., Smart, M., Cariveau, D. P., and Spivak, M. (2018). Wild, native bees and managed honey bees benefit from similar agricultural land uses. Agric. Ecosyst. Environ. 268, 162–170. doi: 10.1016/j.agee.2018.09.014
FAO (2023). FAOSTAT statistical database. Rome, Italy. Available at: https://www.fao.org/statistics/en/
Friard, O., and Gamba, M. (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 7, 1325–1330. doi: 10.1111/2041-210X.12584
Garibaldi, L. A., Carvalheiro, L. G., Leonhardt, S. D., Aizen, M. A., Blaauw, B. R., Isaacs, R., et al. (2014). From research to action: enhancing crop yield through wild pollinators. Front. Ecol. Environ. 12, 439–447. doi: 10.1890/130330
Garibaldi, L. A., Steffan-Dewenter, I., Kremen, C., Morales, J. M., Bommarco, R., Cunningham, S. A., et al. (2011). Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 14, 1062–1072. doi: 10.1111/j.1461-0248.2011.01669.x
Garibaldi, L. A., Steffan-Dewenter, I., Winfree, R., Aizen, M. A., Bommarco, R., Cunningham, S. A., et al. (2013). Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339, 1608–1611. doi: 10.1126/science.1230200
Garner, L. C., and Lovatt, C. J. (2008). The relationship between flower and fruit abscission and alternate bearing of ‘Hass’ avocado. J. Am. Soc. Hortic. Sci. 133, 3–10. doi: 10.21273/JASHS.133.1.3
Gonzalez-Chaves, A., Jaffe, R., Metzger, J. P., and de Mp Kleinert, A. (2020). Forest proximity rather than local forest cover affects bee diversity and coffee pollination services. Landsc. Ecol. 35, 1841–1855. doi: 10.1007/s10980-020-01061-1
Greenleaf, S. S., and Kremen, C. (2006). Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. 103, 13890–13895. doi: 10.1073/pnas.0600929103
Hipólito, J., Boscolo, D., and Viana, B. F. (2018). Landscape and crop management strategies to conserve pollination services and increase yields in tropical coffee farms. Agric. Ecosyst. Environ. 256, 218–225. doi: 10.1016/j.agee.2017.09.038
Hipólito, J., Sousa, B., Borges, R. C., de Brito, R. M., Jaffé, R., Dias, S., et al. (2019). Valuing nature’s contribution to people: the pollination services provided by two protected areas in Brazil. Global Ecology Conservation 20:e00782. doi: 10.1016/j.gecco.2019.e00782
Hoehn, P., Tscharntke, T., Tylianakis, J. M., and Steffan-Dewenter, I. (2008). Functional group diversity of bee pollinators increases crop yield. Proc. R. Soc. B Biol. Sci. 275, 2283–2291. doi: 10.1098/rspb.2008.0405
Hothorn, T., Bretz, F., and Westfall, P. (2008). Simultaneous inference in general Parametic models. Biometircal J. 50, 346–363. doi: 10.1002/bimj.200810425
IPBES (2016). “The assessment report of the intergovernmental science-policy platform on biodiversity and Ecosystme service on pollinators, pollination and food production” in Secretariat of the intergovernmental science-policy platform on Biodiverity and ecosystem services. eds. S. G. Potts, V. L. Imperatriz-Fonseca, and H. T. Ngo, vol. 552 (Bonn, Germany: IPBES).
IPBES (2018). “The IPBES regional assessment report on biodiversity and ecosystem services for the Americas” in Secretariat of the intergovernmental science-policy platform on Biodiverity and ecosystem services. eds. J. Rice, C. S. Seixas, M. E. Zaccagnini, M. Bedoya-Gaitan, and N. Valderrama, (Bonn, Germany: IPBES) 656.
Ish-Am, G., Barrientos-Priego, A., Castañeda-Vildózola, A., and Gazit, S. (1999). Avocado (Persea Americana mill.) pollinators in its region of origin. Revista Chapingo Serie Horticultura 5, 137–143. doi: 10.5154/r.rchsh.1999.05.137
Jauker, F., Diekoetter, T., Schwarzbach, F., and Wolters, V. (2009). Pollinator dispersal in an agricultural matrix: opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landsc. Ecol. 24, 547–555. doi: 10.1007/s10980-009-9331-2
Jauker, F., and Wolters, V. (2008). Hover flies are efficient pollinators of oilseed rape. Oecologia 156, 819–823. doi: 10.1007/s00442-008-1034-x
Klein, A. M., Brittain, C., Hendrix, S. D., Thorp, R., Williams, N., and Kremen, C. (2012). Wild pollination services to California almond rely on semi-natural habitat. J. Appl. Ecol. 49, 723–732. doi: 10.1111/j.1365-2664.2012.02144.x
Klein, A. M., Dewenter, S. I, and Tscharntke, T. (2003). Fruit set of highland coffee increases with the diversity of pollinating bees. Proceedings of the Royal Society of London. Series B 270, 955–961. doi: 10.1098/rspb.2002.2306
Klein, M. A., Muller, C., Hoehn, P., and Kremen, C. (2009). “Understanding the role of species richness for crop pollination services” in Biodiversity, ecosystem functioning, and human wellbeing. eds. S. Naeem, D. E. Bunker, A. Hector, M. Loreau, and C. Perring (Oxford, United Kingdom: Oxford University Press).
Klein, A.-M., Vaissiere, B. E., Cane, J. H., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., et al. (2007). Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 274, 303–313. doi: 10.1098/rspb.2006.3721
Kremen, C., Williams, N. M., and Thorp, R. W. (2002). Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. 99, 16812–16816. doi: 10.1073/pnas.26241359
Krimmer, E., Martin, E. A., Krauss, J., Holzschuh, A., and Steffan-Dewenter, I. (2019). Size, age and surrounding semi-natural habitats modulate the effectiveness of flower-rich Agri-environment schemes to promote pollinator visitation in crop fields. Agric. Ecosyst. Environ. 284:106590. doi: 10.1016/j.agee.2019.106590
Lowe, E. B., Groves, R., and Gratton, C. (2021). Impacts of field-edge flower plantings on pollinator conservation and ecosystem service delivery–a meta-analysis. Agric. Ecosyst. Environ. 310:107290. doi: 10.1016/j.agee.2020.107290
Magrach, A., and Sanz, M. J. (2020). Environmental and social consequences of the increase in the demand for ‘superfoods’ world-wide. People and Nature 2, 267–278. doi: 10.1002/pan3.10085
Martin, E. A., Dainese, M., Clough, Y., Báldi, A., Bommarco, R., Gagic, V., et al. (2019). The interplay of landscape composition and configuration: new pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 22, 1083–1094. doi: 10.1111/ele.13265
Mashilingi, S. K., Zhang, H., Garibaldi, L. A., and An, J. (2022). Honeybees are far too insufficient to supply optimum pollination services in agricultural systems worldwide. Agric. Ecosyst. Environ. 335:108003. doi: 10.1016/j.agee.2022.108003
McNeil, R., and Pidduck, W. (2003). The effectiveness of the western bumblebee in pollinating Hass avocado trees. In Proceedings V World Avocado Congress (Junta de Andalucía, Consejería de Agricultura y Pesca: Actas V Congreso Mundial del Aguacate) (pp. 253–256).
Medel, R., González-Browne, C., and Fontúrbel, F. E. (2018). Pollination in the Chilean Mediterranean-type ecosystem: a review of current advances and pending tasks. Plant Biol. 20, 89–99. doi: 10.1111/plb.12644
Monzón, V. H., Avendaño-Soto, P., Araujo, R. O., Garrido, R., and Mesquita-Neto, J. N. (2020). Avocado crops as a floral resource for native bees of Chile. Rev. Chil. Hist. Nat. 93, 1–7. doi: 10.1186/s40693-020-00092-x
Muñoz, A. E., Plantegenest, M., Amouroux, P., and Zaviezo, T. (2021). Native flower strips increase visitation by non-bee insects to avocado flowers and promote yield. Basic Applied Ecol 56, 369–378. doi: 10.1016/j.baae.2021.08.015
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Ne’eman, G., Jürgens, A., Newstrom-Lloyd, L., Potts, S. G., and Dafni, A. (2010). A framework for comparing pollinator performance: effectiveness and efficiency. Biol. Rev. 85, 435–451. doi: 10.1111/j.1469-185X.2009.00108.x
Nirody, B. (1922). Investigations in avocado breeding. Masters thesis 1911. Massachusetts Agricultural College.
Öckinger, E., and Smith, H. G. (2007). Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J. Appl. Ecol. 44, 50–59. doi: 10.1111/j.1365-2664.2006.01250.x
Peña, J. F., and Carabalí, A. (2018). Effect of honey bee (Apis mellifera L.) density on pollination and fruit set of avocado (Persea americana mill.) cv. Hass. J. Apicultural Sci. 62, 5–14. doi: 10.2478/jas-2018-0001
Perez-Balam, J., Quezada-Euan, J. J., Alfaro-Bates, R., Medina, S., McKendrick, L., Soro, A., et al. (2012). The contribution of honey bees, flies and wasps to avocado (Persea americana) pollination in southern Mexico. J Pollination Ecol. 8, 42–47. doi: 10.26786/1920-7603(2012)6
Potts, S. G., Roberts, S. P., Dean, R., Marris, G., Brown, M. A., Jones, R., et al. (2010). Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 49, 15–22. doi: 10.3896/IBRA.1.49.1.02
Potts, S. G., Vulliamy, B., Roberts, S., O’Toole, C., Dafni, A., Ne’eman, G., et al. (2005). Role of nesting resources in organising diverse bee communities in a Mediterranean landscape. Ecological Entomology 30, 78–85. doi: 10.1111/j.0307-6946.2005.00662.x
R Core Team (2023). “R: a language and environment for statistical computing” in R foundation for statistical computing.
Rader, R., Cunningham, S. A., Howlett, B. G., and Inouye, D. W. (2020). Non-bee insects as visitors and pollinators of crops: biology, ecology, and management. Annu. Rev. Entomol. 65, 391–407. doi: 10.1146/annurev-ento-011019-025055
Read, S., Howlett, B., Jesson, L. K., and Pattemore, D. (2017). Insect visitors to avocado flowers in the Bay of Plenty, New Zealand. New Zealand Plant Protection 70, 38–44. doi: 10.30843/nzpp.2017.70.25
Ricketts, T. H. (2004). Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 18, 1262–1271. doi: 10.1111/j.1523-1739.2004.00227.x
Ricketts, T. H., Regetz, J., Steffan-Dewenter, I., Cunningham, S. A., Kremen, C., Bogdanski, A., et al. (2008). Landscape effects on crop pollination services: are there general patterns? Ecol. Lett. 11, 499–515. doi: 10.1111/j.1461-0248.2008.01157.x
Rodríguez, S., Pérez-Giraldo, L. C., Vergara, P. M., Carvajal, M. A., and Alaniz, A. J. (2021). Native bees in Mediterranean semi-arid agroecosystems: unravelling the effects of biophysical habitat, floral resource, and honeybees. Agric. Ecosyst. Environ. 307:107188. doi: 10.1016/j.agee.2020.107188
Rundlöf, M., Lundin, O., and Bommarco, R. (2018). Annual flower strips support pollinators and potentially enhance red clover seed yield. Ecol. Evol. 8, 7974–7985. doi: 10.1002/ece3.4330
Sagwe, R. N., Peters, M. K., Dubois, T., Steffan-Dewenter, I., and Lattorff, H. M. G. (2022). Pollinator efficiency of avocado (Persea americana) flower insect visitors. Ecological Solutions Evidence 3:e12178. doi: 10.1002/2688-8319.12178
Schirmel, J., Albrecht, M., Bauer, P. M., Sutter, L., Pfister, S. C., and Entling, M. H. (2018). Landscape complexity promotes hoverflies across different types of semi-natural habitats in farmland. J. Appl. Ecol. 55, 1747–1758. doi: 10.1111/1365-2664.13095
Sedgley, M. (1977). Reduced pollen tube growth and the presence of callose in the pistil of the male floral stage of the avocado. Sci. Hortic. 7, 27–36. doi: 10.1016/0304-4238(77)90040-1
Senapathi, D., Fründ, J., Albrecht, M., Garratt, M. P. D., Kleijn, D., Pickles, B. J., et al. (2021). Wild insect diversity increases inter-annual stability in global crop pollinator communities. Proc. R. Soc. B Biol. Sci. 288:20210212. doi: 10.1098/rspb.2021.0212
Speight, M. C. D. (2014). Species accounts of European Syrphidae (Diptera). Syrph the net, the database of European Syrphidae. Glasgow 65, 82–90.
Sritongchuay, T., Hughes, A. C., Memmott, J., and Bumrungsri, S. (2019). Forest proximity and lowland mosaic increase robustness of tropical pollination networks in mixed fruit orchards. Landsc. Urban Plan. 192:103646. doi: 10.1016/j.landurbplan.2019.103646
Steffan-Dewenter, I., and Kuhn, A. (2003). Honeybee foraging in differentially structured landscapes. Proc. R. Soc. B Biol. Sci. 270, 569–575. doi: 10.1098/rspb.2002.2292
Stokstad, E. (2007). The case of the empty hives. Science 316, 970–972. doi: 10.1126/science.316.5827.9
Vanbergen, A. J., Aizen, M. A., Cordeau, S., Garibaldi, L. A., Garratt, M. P. D., Kovács-Hostyánszki, A., et al. (2020). “Transformation of agricultural landscapes in the Anthropocene: Nature’s contributions to people, agriculture and food security” in Advances in ecological research, vol. 63 (Amsterdam, Netherlands: Academic Press), 193–253.
VanEngelsdorp, D., Hayes, J. Jr., Underwood, R. M., and Pettis, J. (2008). A survey of honey bee colony losses in the US, fall 2007 to spring 2008. PLoS One 3:e4071. doi: 10.1371/journal.pone.0004071
Vithanage, H. (1985). Insect pollination of avocado and macadamia. 175, 199–204. doi: 10.17660/ActaHortic.1986.175.14
Vithanage, V. (1990). The role of the European honeybee (Apis mellifera L.) in avocado pollination. J. Horticultural Sci. 65, 81–86. doi: 10.1080/00221589.1990.11516033
Webber, S. M., Garratt, M. P. D., Lukac, M., Bailey, A. P., Huxley, T., and Potts, S. G. (2020). Quantifying crop pollinator-dependence and pollination deficits: the effects of experimental scale on yield and quality assessments. Agric. Ecosyst. Environ. 304:107106. doi: 10.1016/j.agee.2020.107106
Weschenfelder, C., dos Santos, J. L., de Souza, P. A. L., de Campos, V. P., and Marcadenti, A. (2015). Avocado and cardiovascular health. Open J Endocrine Metabolic Diseases 5, 77–83. doi: 10.4236/ojemd.2015.57010
Willcox, B., Howlett, B., Robson, A., Cutting, B., Evans, L., Jesson, L., et al. (2019). Evaluating the taxa that provide shared pollination services across multiple crops and regions. Sci. Rep. 9, 13538–13510. doi: 10.1038/s41598-019-49535-w
Winfree, R., Aguilar, R., Vázquez, D. P., LeBuhn, G., and Aizen, M. A. (2009). A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90, 2068–2076. doi: 10.1890/08-1245.1
Woodcock, B. A., Bullock, J. M., McCracken, M., Chapman, R. E., Ball, S. L., Edwards, M. E., et al. (2016). Spill-over of pest control and pollination services into arable crops. Agric. Ecosyst. Environ. 231, 15–23. doi: 10.1016/j.agee.2016.06.023
Keywords: wild pollinators, crop production, ecosystem services, land management, Chile, Mediterranean region
Citation: Dymond K, Celis-Diez JL, Díaz-Siefer P, Rojas-Bravo V, Martínez-Harms J, Potts SG and Garratt MPD (2025) Proximity to natural habitat enhances flower visitor diversity and pollination services in avocado orchards. Front. Sustain. Food Syst. 9:1560802. doi: 10.3389/fsufs.2025.1560802
Edited by:
Daniel Paredes, University of Extremadura, SpainReviewed by:
Mário Boieiro, University of the Azores, PortugalVinicio J. Sosa, Instituto de Ecología (INECOL), Mexico
Copyright © 2025 Dymond, Celis-Diez, Díaz-Siefer, Rojas-Bravo, Martínez-Harms, Potts and Garratt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keira Dymond, ZHltb25ka2VpcmFAZ21haWwuY29t; Jaime Martínez-Harms, amFpbWUubWFydGluZXpAaW5pYS5jbA==
 Keira Dymond
Keira Dymond Juan L. Celis-Diez
Juan L. Celis-Diez Pablo Díaz-Siefer
Pablo Díaz-Siefer Valeska Rojas-Bravo
Valeska Rojas-Bravo Jaime Martínez-Harms
Jaime Martínez-Harms Simon G. Potts1
Simon G. Potts1 Michael P. D. Garratt
Michael P. D. Garratt