- 1Department of Fisheries, University of Rajshahi, Rajshahi, Bangladesh
- 2Department of Oceanography and Blue Economy, Habiganj Agricultural University, Habiganj, Bangladesh
- 3Earth Observation Centre, Institute of Climate Change, Universiti Kebangsaan Malaysia, Bangi, Malaysia
- 4Center for Sustainable Tropical Fisheries and Aquaculture, College of Science and Engineering, James Cook University, Townsville, QLD, Australia
- 5Environmental and Life Sciences Programme, Faculty of Science, Universiti Brunei Darussalam, Jalan Tungku Link, Gadong, Brunei Darussalam
- 6Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
- 7School of Engineering and Built Environment, Griffith University, Nathan, QLD, Australia
- 8Department of Fisheries and Marine Science, Noakhali Science and Technology University, Noakhali, Bangladesh
Understanding the reproductive cycle ensures sustainable cultivation practices and facilitates effective management strategies to conserve natural stocks. Lamellidens marginalis, commonly available in South Asian countries, plays an essential role in the aquatic food chain. This study investigated the growth indices and reproductive activity of L. marginalis from a freshwater lake, aiming to understand its stock structure and sustainable exploitation rate. Furthermore, nutritional profiling of tissue was conducted to assess its potential for aquaculture. The results revealed significant variations in the mean biometric parameters across genders and periods. Compared with males, females presented numerically greater dimensions in all body measurements, except for shell height. The overall relationship between body weight and shell length indicated negative allometric growth (‘b’ values ranged between 2.430 and 3.156 for males and between 1.506 and 2.792 for females). The condition index exhibited significant seasonal fluctuations, with a rapid increase during April and June and a sharp decrease in July–August, potentially linked to gonadal maturation and spawning. Histological examination revealed a significantly male-dominated mussel population with an overall sex ratio of 1:0.89 (male: female). However, the histological analysis did not identify sexually undifferentiated and hermaphroditic individuals. This species displayed a continuous reproductive ability year-round, with a peak in July–August at 32.30
1 Introduction
Shellfish farming particularly mussel culture represents a relatively recent and highly sustainable aquaculture practice and renowned for its environmental friendliness. Shellfish farming does not require formulated feed for growth and thus minimizing ecological impact on the environment (Shumway et al., 2012; Nahar, 2020). Bivalves as shellfish species provide high-quality aquatic food and essential nutrients for nourishing the growing human population. Bivalves also contain high levels of n-3 polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which offer numerous health benefits (Anacleto et al., 2014).
Lamellidens marginalis (Lamarck, 1819), a freshwater mussel species, is abundant in freshwater bodies of Bangladesh (Dan et al., 2001). This species is primarily used in lime production and as an aquafeed ingredient. However, it has greater commercial significance owing to production of poultry feed, also rarely as human consumption preferably by the tribal people in Bangladesh (Hossain et al., 2023). Furthermore, this species is also used for pink pearl production at a commercial scale (Saha and Islam, 2000). It can also offer an additional income source to marginal and low-income families. Although this mussel culture in freshwater pond is not so popular, it has enormous potential to grow and thrive in privately owned small ponds by most households in Bangladesh. L. marginalis is currently being collected from natural stock, which is highly unsustainable and poses a threat to the species (Hossain et al., 2023). In the past, the naturally occurring habitats of Bangladesh were bountiful with this mussel species. However, unrestrained exploitation has exerted considerable pressure on the sustainable existence of this potentially commercial species. Therefore, cultural technologies are essential for cultivating L. marginalis in ponds to conserve the natural stock. Sustainable management and conservation of natural stocks of bivalves are involving potential measures enforced by the fisheries authorities. Furthermore, the sustainable management of this species would also require extensive morphological assessment, including the relationships among shell size, body weight, and other morphometric measurements (Grizzle et al., 2017; Rao, 2007). Moreover, a detailed examination of reproduction physiology (Hmida et al., 2010; Calderon-Aguilera et al., 2010) and a thorough insight of the gonadal activities are essential to identify the maturation cycle and spawning behavior. However, collecting bivalves from nature to study their gonadal growth are very difficult and sometimes time-consuming (Camacho-Mondragon et al., 2012).
Analysis of biochemical components such as protein, lipids, carbohydrates, and minerals is essential for the market preference of bivalves (Ramakrishnan and Venkatrao, 1995). The proportions of protein, carbohydrates, and lipids in the bivalve tissue experience temporal oscillations mainly regulated by the food resources, environment (particularly temperature), and reproductive strategy (Idayachandiran et al., 2014). Such factors are also recognized to contribute to the meat yield and body condition index. Therefore, these variables indicate eco-physiological state and gametogenesis activities in highly exploited bivalve stocks worldwide (Yildiz et al., 2006). Therefore, assessing the condition and meat yield indices over time can indicate their culture prospects and optimal harvesting time for higher nutritional and economical quality assurance. Generating data on eco-hydrological parameters of the habitat types, morphometric parameters, gonadal maturation cycles, and biochemical composition of L. marginalis is crucial as they are valuable for assessing the sustainable exploitation rate and estimating its potential for a profitable aquaculture species (Nahar, 2020).
The studies on L. marginalis in Bangladesh are limited to the reproductive cycle (Siddique et al., 2019; Niogee et al., 2019; Hossain et al., 2023) and pearl culture (Saha and Islam, 2000) assessments. However, comprehensive data regarding the global-scale seasonality of biometric indices, growth patterns, gonadal development, sex ratios, spawning activity, reproductive effort, and biochemical composition of L. marginalis is currently lacking. Therefore, this present study investigated the seasonal fluctuations in environmental variables and their subsequent effect on biometric indices, reproductive activities, and biochemical composition of L. marginalis in a freshwater lake in Northwestern Bangladesh. Another aim was to uncover essential missing data and connections crucial for understanding the reproductive biology of L. marginalis and for fostering sustainable production practices in sustainable aquaculture and wild stock management. The findings align with UNSDG Goal 14 (Life Below Water) by advancing strategies for the sustainable management and conservation of wild freshwater mussel populations, contributing to the preservation of aquatic ecosystems and biodiversity. Furthermore, this study supports UNSDG Goal 2 (Zero Hunger) by providing insights into sustainable aquaculture practices that enhance food security through the production of nutrient-dense, eco-friendly protein sources.
2 Materials and methods
2.1 Study area
This was accomplished in a freshwater lake (24° 22′21.56″ N and 88° 38′11.16″E) situated at the University of Rajshahi premises (Figure 1). The lake area is approximately 2.5 ha, with an average depth of nearly 3.0 m (Nahar, 2020). The water depth was maintained in the lake with a continuous water supply. The lake is in good condition as the campus administration regularly monitors it. The regular stocking and harvesting of carp fish species are also practiced in this lake. Therefore, the freshwater mussel L. marginalis is naturally abundant in this water body, rendering it one of the most suitable study sites.
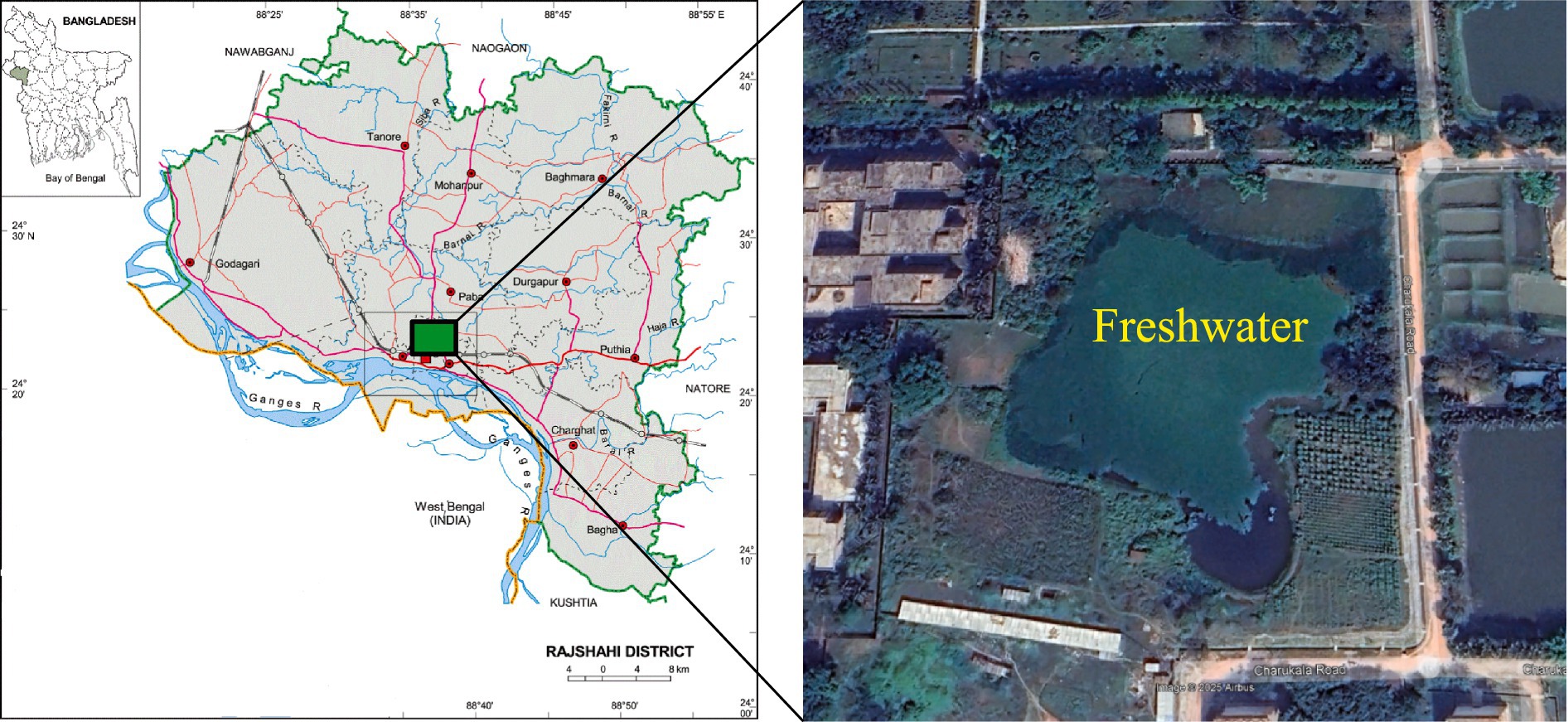
Figure 1. Study area map showing the location of the freshwater lake at the University of Rajshahi, Bangladesh.
2.2 Environmental variables
Environmental variables such as water temperature, transparency, dissolved oxygen (DO), pH, and alkalinity were measured. The temperature was monitored using a Celsius thermometer, whereas the transparency was recorded using a standard Sechi disk. Dissolved oxygen (DO) and alkalinity were analyzed using a convenient aquaculture kit (Model FF2, HACH, USA). The measurements of the leading physicochemical water quality parameters for determining the environmental variables were recorded from the water surface twice on each sampling day.
2.3 L. marginalis sample collection and processing
L. marginalis were sampled monthly from January to December 2018. Live individuals were hand-picked fortnightly by experienced personnel, euthanized with > 250 mg/L buffered (pH ~ 7) tricaine-S (MS222) solution and transferred to the laboratory of the Department of Fisheries, University of Rajshahi, in a cool icebox for further examinations. The samples were washed and scraped to remove all epibiotic materials in the laboratory. Thirty individuals were collected monthly to obtain morphometric measurements. The shell length (maximum anteroposterior distance), height (maximum distance from hinge to the ventral margin), and width or depth or thicknesses (maximum distance between outer edges of two valves) of individual organisms were quantified accurately with the help of a digital caliper to the nearest 0.1 mm below. The total body weight of each L. marginalis sample was determined using a 0.001 g electronic weighing balance. The bivalve shells were opened by scalpels, and soft tissues were removed. The samples were then blotted and weighed individually and preserved in 10% formalin for 12 h before histological analysis. Biochemical analysis was done by dissecting the whole body of 30 individuals each month. Collected samples were chilled in ice (4°C) to transport to the laboratory. In the laboratory, shells were cleaned and opened, and the edible meat was collected following blotting and weighting of the meat. Afterward, the homogenized body tissue was stored at −20°C for further analysis.
2.4 Growth pattern
The growth pattern of L. marginalis was explored by the length-weight relationship following the equation W = aLb (Le Cren, 1951), where ‘a’ and ‘b’ indicate the constants (Pauly, 1983). The linear illustration of the exponential relationship can be expressed by the logarithmic equation (Equation 1) given below.
where, W denotes body weight (total weight, wet tissue weight, and wet shell weight), L signifies length (shell length, shell height, and shell width), while ‘a’ represents the intercept and ‘b’ the slope of the equation. Based on the method of Pauly (1983), the length-weight relationship (LWRs) was determined to understand the growth pattern of the bivalve. The collected samples were grouped according to season and gender to establish gender-specific and seasonally varied LWRs (Zargar et al., 2012). When b = 3, the growth pattern was isometric, while b value greater (b > 3) or lesser (b < 3) than 3 indicates either positive or negative allometric growth (Ricker, 1975).
2.5 Condition index and meat yield
Every month, thirty samples were used to analyze the two indices for growth estimation: condition index (CI) and meat yield index (MYI). The CI (Equation 2) and MYI (Equation 3) were calculated following Lucas and Beninger (1985), and the relationship was described using the following equation:
2.6 Gonadal histology
We performed histological examination to investigate the spawning season and gonadal maturity. Thirty specimens were dissected each month for histological analysis from January to December 2018. After opening, the shell’s soft tissues were removed, and a cross-section (nearly 5 mm thick) of the soft body was cut from the central part of the body containing the gonads, intestine, muscular foot and mantle. The cross-sections of the tissues were attached in the Davidson’s fixative for 24 h. Following the dehydration in graded alcohol series and washing with the help of xylene, the samples were fixed in paraffin. After trimming, the paraffin blocks were split at a 6 μm thickness. The next step involved staining with Harris’ haematoxylin, followed by eosin Y, and then fitted on the slide for a detailed microscopic (Leica microsystems CMS GmbH, Wetzlar, Germany) assessment. The sex selection and gonadal staging were performed via histological examination. The gonadal development of L. marginalis was then categorized into six scales based on Juhel et al. (2003) i.e. (1) early developing; (2) late-developing; (3) ripe; (4) partially spawning; (5) spent; and (6) undifferentiated.
2.7 Gonado-somatic index
The reproductive effort was determined by the gonadosomatic index (GSI), which was calculated from thirty monthly procured samples (Equation 4). The gonado-somatic index (GSI) was calculated as described by Devlaming et al. (1982) following the equation.
2.8 Biochemical analysis
Every month, the tissues procured from the 30 sampled individuals were dried in a refrigerator and pulverized to analyze their biochemical composition. The samples were analyzed in triplicate. The moisture content was calculated by subtracting the wet weight of the tissue from the dry weight at 105 ± 2°C for 24 h, and the results were expressed as percentages (AOAC, 2005). The percent moisture was calculated using the Equation 5:
The Phenol sulfuric acid technique was employed to verify the total carbohydrate content using dextrose as a standard (Taylor, 1995). The protein content (%) was determined followed by the Kjeldahl process (Pearson, 1977) according to the Equations 6, 7:
The lipid content (%) was estimated after extraction with chloroform-methanol (Silva, 2002; Bligh and Dyer, 1959).
2.9 Statistical analyses
The obtained data were analyzed using Statistical Package for Social Science (SPSS ver. 20) for windows, and the findings were stated as means ± standard deviations (SD). The percentage data were arcsine transformed before statistical treatment. The sex ratio was analyzed using the chi-square (χ2) test. After verifying the data normality and homogeneity of variables by the Shapiro-Wilcoxon test, significant variances in variables (environmental variables, CI, MYI, and GSI, biochemical composition) were tested for monthly variations by the one-way analysis of variance (ANOVA) followed by Tukey’s HSD test for mean comparison at the significance level of p < 0.05. ANOVA, at a significance level of p < 0.05 was used to evaluate the statistical significance of the regression model (Gökçe et al., 2010). The coefficient of determination (R2) measures the quality of a linear regression’s prediction (a value close to 1 means a better model). Student’s t -test was accomplished to verify the significant difference in the b value of each measurement from the predictions assigned for isometric growth (b = 3). Growth was considered either positive or negative allometric when the difference of b from 3 was significant (p < 0.05), otherwise, growth was deemed to be isometric (Yilmaz et al., 2012). Furthermore, the statistical differences in the b value of gender and seasons were also assessed by one-way ANOVA (p < 0.05). The Pearson’s correlation was employed to illustrate the predominant relationships between the environmental variables, CI, MYI, gametogenic cycle, GSI, and biochemical composition.
3 Results
3.1 Biometric features
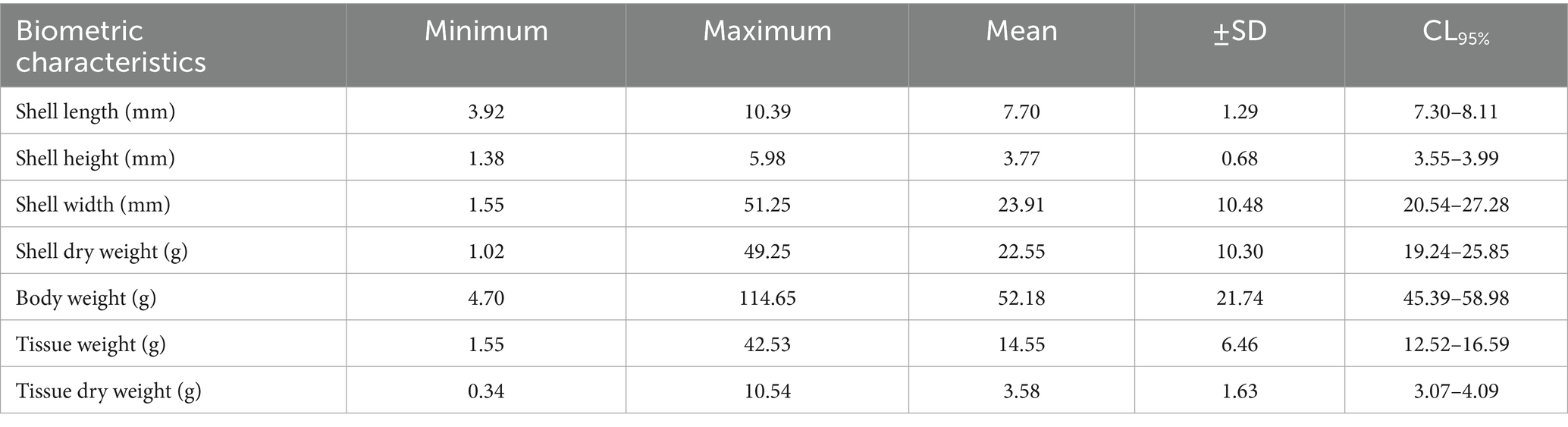
The shell length, shell height, shell width, body weight, tissue weight, dry tissue weight, and dry shell weight varied significantly (p < 0.05) among the seasons, with the highest values recorded in September (Table 1). Furthermore, significantly higher (p < 0.05) tissue weights and dry tissue weights were observed in July. However, significantly (p < 0.05) lower values of these two body dimensions were measured in November and August (Supplementary Table S1). The overall mean of shell length, shell height, and shell width was recorded as 7.70 cm, 3.77 cm, and 23.91 cm, respectively, whereas the total body weight, tissue weight, dry tissue weight, and dry shell weight were 52.18 g, 14.55 g, 3.58 g, and 22.55 g, respectively.
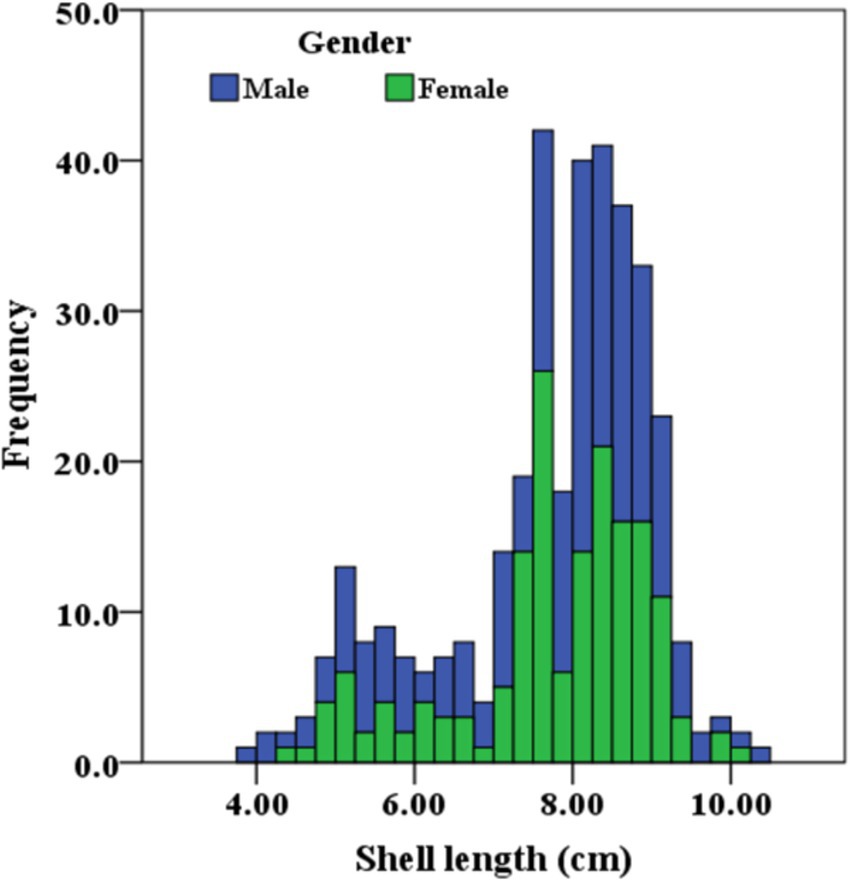
The mean biometric characteristics of L. marginalis differentiated on the basis of sex are shown in Table 2. The t-test revealed insignificant (p > 0.05) diffrences in all the biometric measurements between the two sexes. However, females displayed numerically higher dimensions than males in all the body measurements except for the shell height. In particular, the shell length of the largest male was 10.39 cm, while that of the largest female was 10.22 cm. The size-frequency distributions of L. marginalis males and females are shown with a the size-frequency histogram (Figure 2). The highest abundance contributed to the sizes range (SL) of 7.10–9.80 cm and the uppermost length classes constituted exclusively of females.
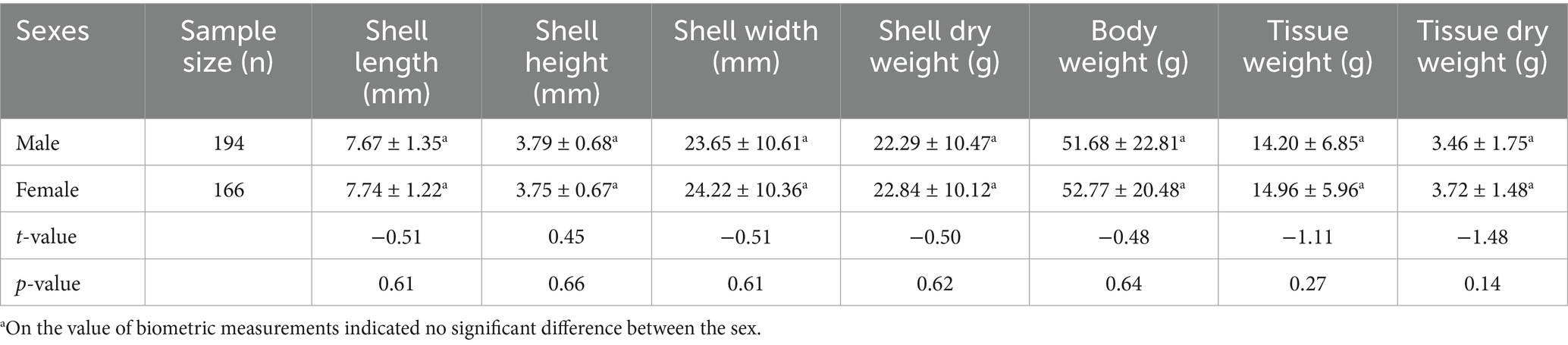
Table 2. Comparison of biometric characteristics of male and female freshwater mussel L. marginalis based on t-test.
3.2 Growth pattern
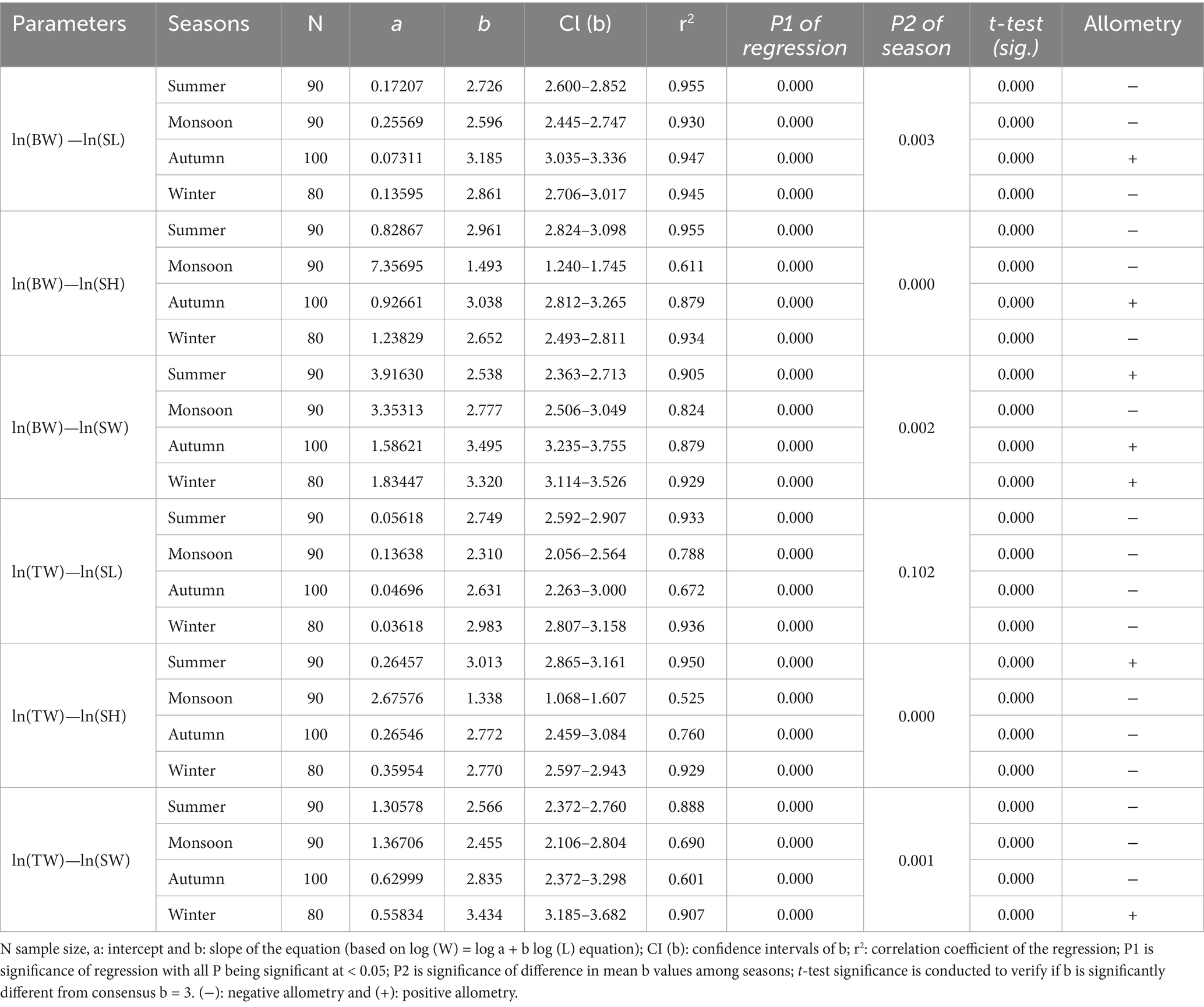
The LWRs of all the body measurements (shell length, height, and shell width against the total body weight and tissue weight) based on sex and season are shown in Tables 3, 4, respectively. For all the body measurements, there was a significant variation in the b values between genders except for BW vs. SL and TW vs. SL. In the case of allometry, all the body measurements showed negative allometry except for the BW vs. SW of the male individuals (Table 3). However, the ‘b’ values observed in all the regression relations ranged between 2.430 and 3.156 for males, and 1.506 and 2.792 for females. The b value was significantly different for all the regression relationships of body measurements among seasons except for the regression relationships of TW vs. SL. Positive allometric relations are observed for BW vs. SL, BW vs. SH and BW vs. SW during Autumn; BW vs. SW and TW vs. SH during Summer; and BW vs. SW and TW vs. SW during winter. While other regression relations of the body measurements are negatively allometric (Table 4).
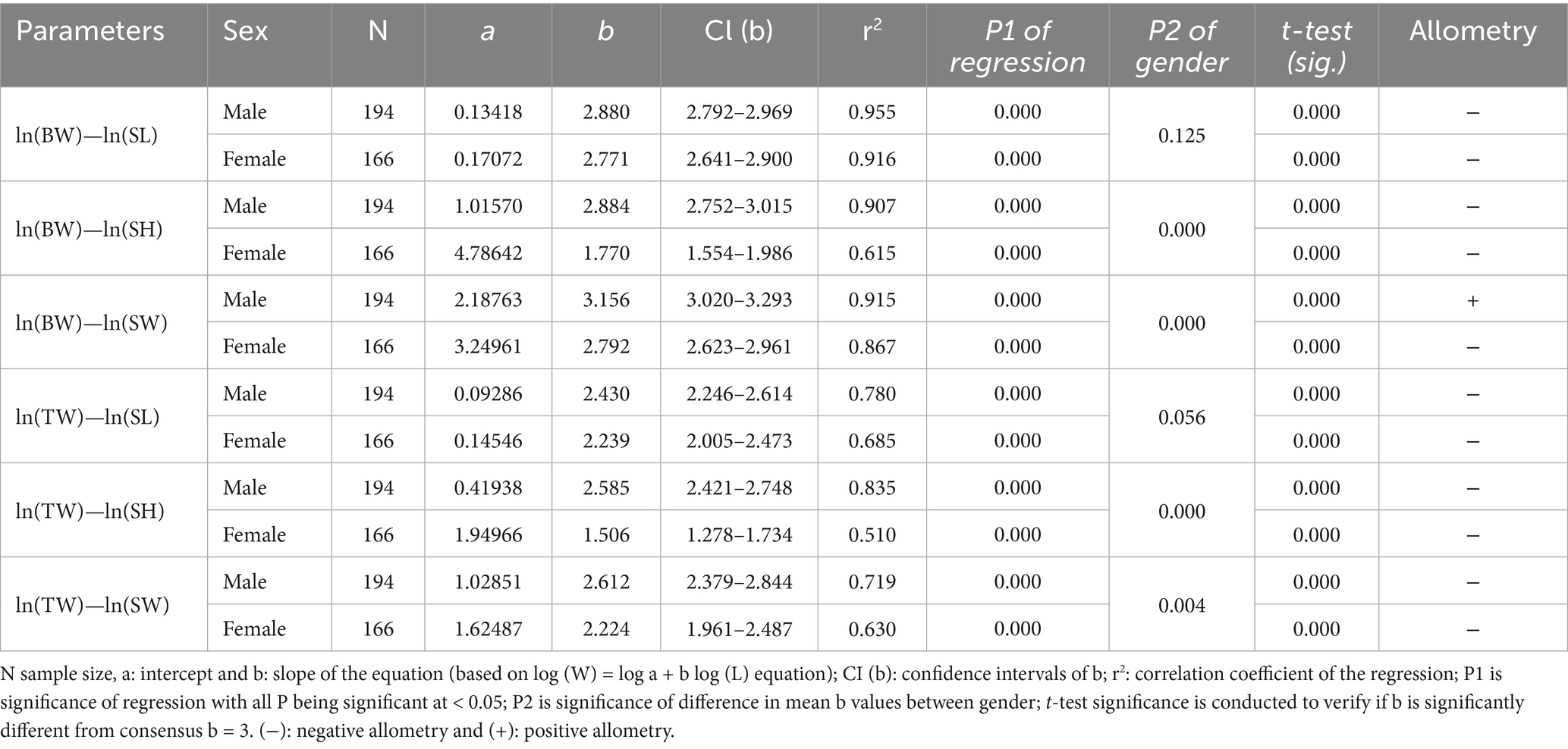
Table 3. Gender-specific growth pattern of L. marginalis collected from a freshwater lake in Bangladesh.
3.3 Condition and meat yield indices
The CI and MYI showed significant monthly fluctuations (ANOVA, p < 0.05) (Figure 3). Starting from 22.06 in January, the CI gradually decreased until April, followed by a sudden peak in June. This trend, however, declined until the annual lowest peak of 10.44 in November followed by a sudden second highest peak in December (21.03). On the other hand, two major peaks of MYI in L. marginalis were observed during January and June, followed by sudden changes in other months. Therefore, the building up of MYI occurred from December onward, which indicated its commercial exploitation period from December to June.
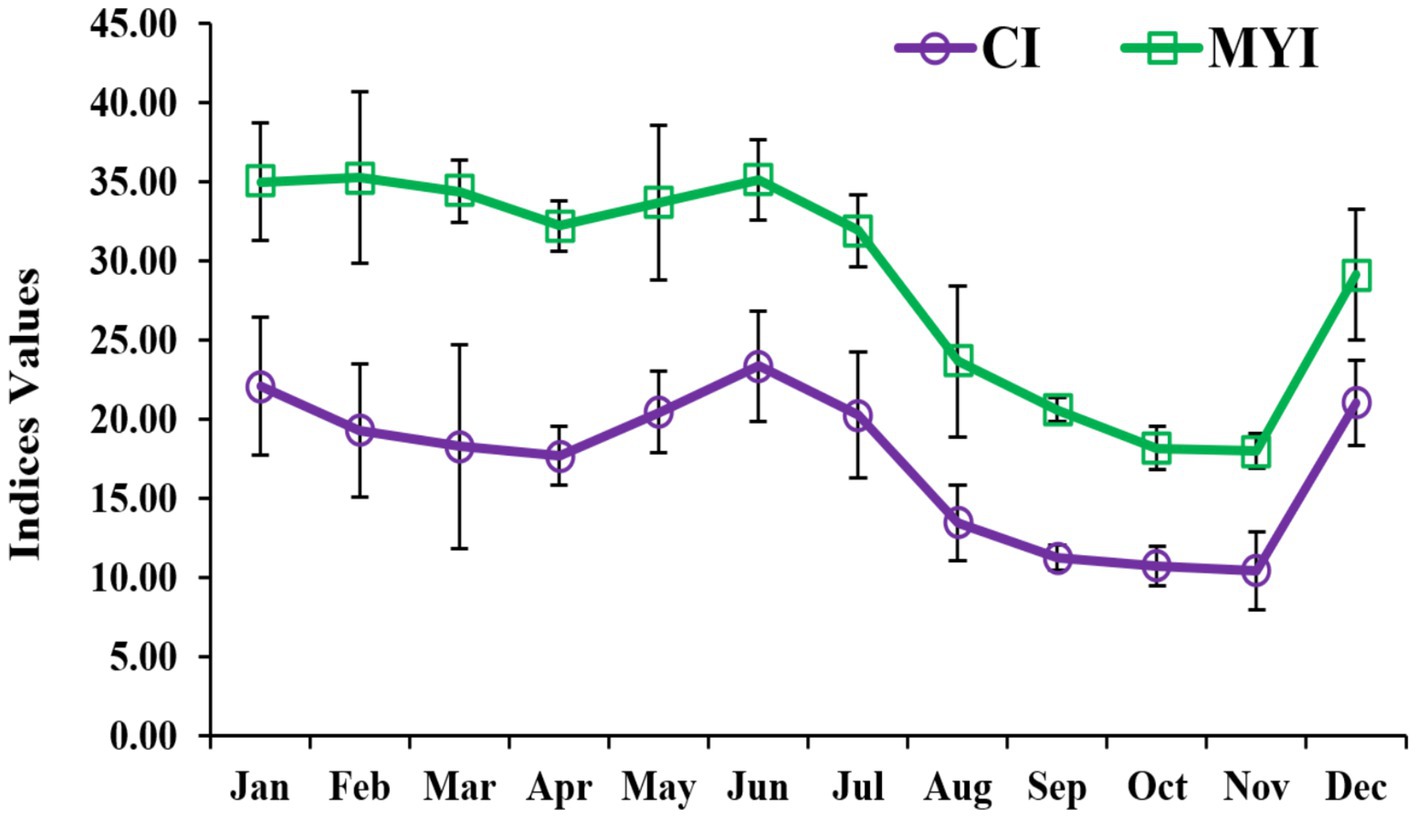
Figure 3. Monthly variations of condition index (CI) and meat yield index (MYI) in freshwater mussel L. marginalis.
3.4 Sex ratio
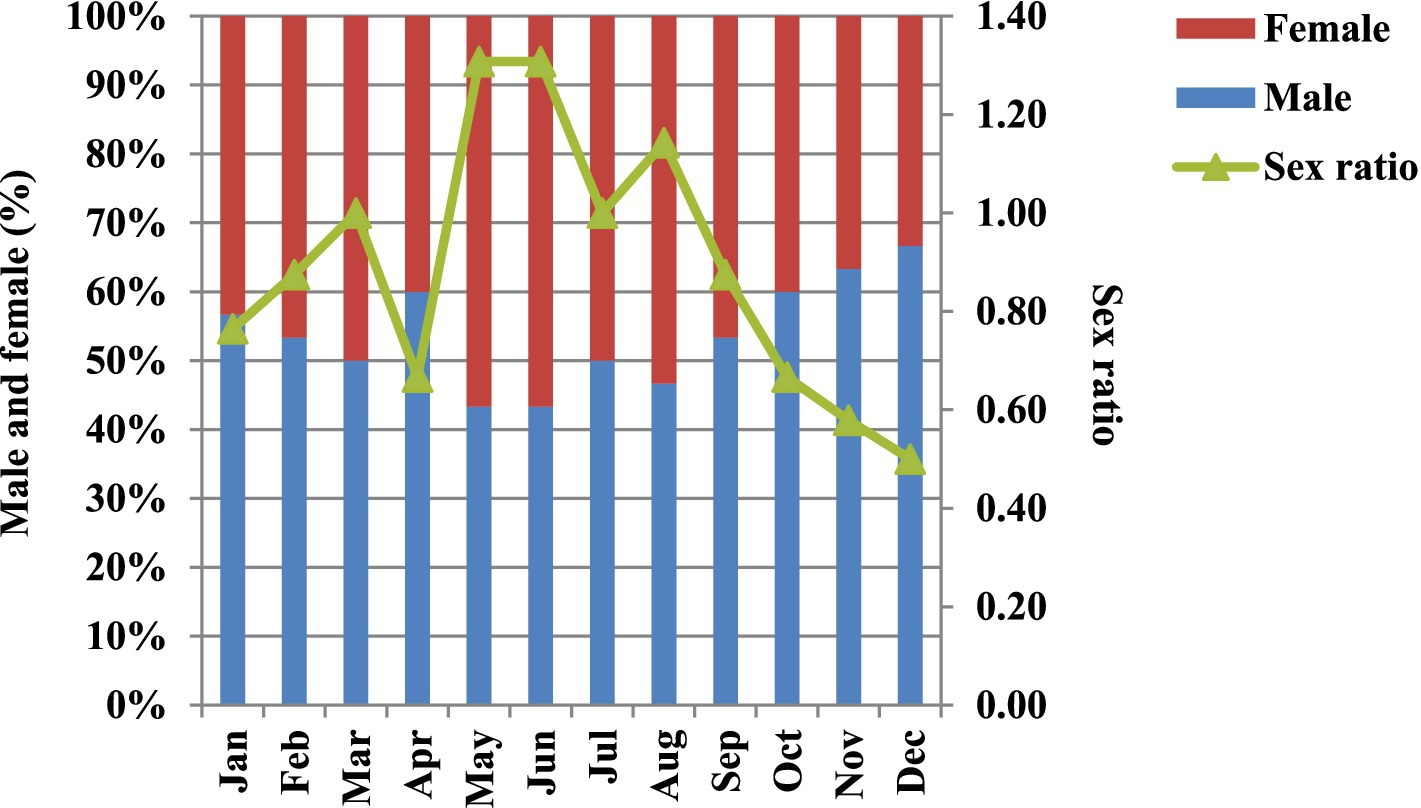
Histological examination revealed that out of the 360 specimens analyzed, 194 (53.89%) were male, and 166 (46.11%) were female. From January, February, April, and September to December, the proportion of male individuals was higher than that of females. The female individuals were observed each month; however, their dominancy was observed during May, June, and August. During March and July, the male and female sexes occurred in equal proportions with the sex ratio of 1:1 (Figure 4). The overall male-to-female sex ratio was 1:0.89 (male: female), which is statistically different from the expected sex ratio of 1:1, indicating the predominance of males over females in the study area during the study period (χ2 = 5.90, p < 0.05).
3.5 Gametogenic cycle
Histological examination revealed five major gonadal development stages: early developing, late-developing, ripe, spawning, and spent, as observed in male and female gonads (Figures 5, 6). The absence of gonadal tissues indicated that there were any sexually undifferentiated hermaphrodite individuals in the investigated samples. During spermatogenesis, the early developing stage was observed during January–April and October–December, whereas oogenesis was exhibited during January–March and November–December. The late-developing stage was observed in all the months except for August and September in males, while August and October were in females. The highest numbers of ripe males and females were observed during June and May, respectively.
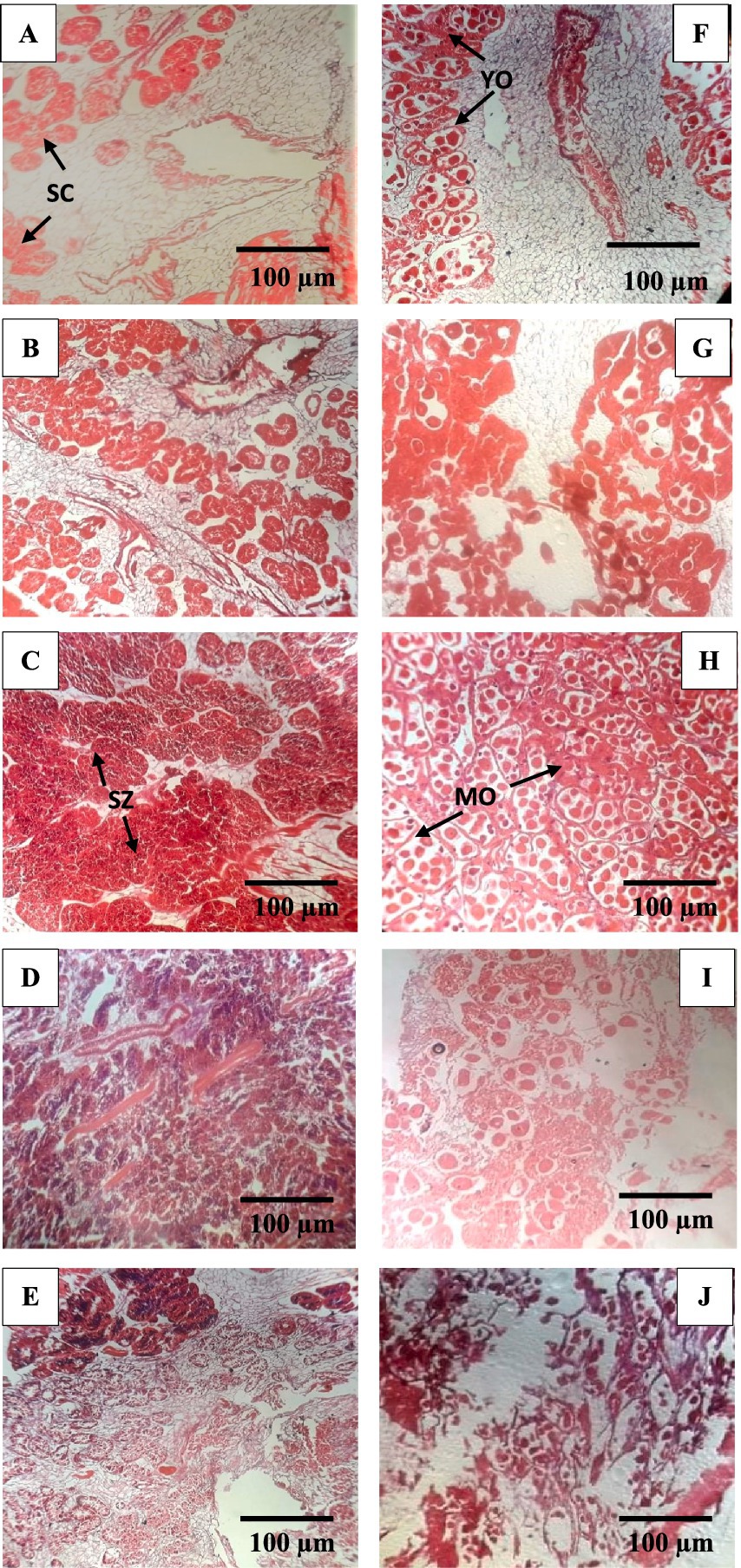
Figure 5. Histological evidence of gonad developmental stages in male (A–E) and female (F–J) freshwater mussel L. marginalis. (A) F, Early developing stage; (B) G, Late developing stage; (C) H, Ripe; (D) I, Spawning and E, J, Spent. SC, Spermatocyte, SZ, Spermatozoa, YO, Young oocytes, MO, Mature oocytes.
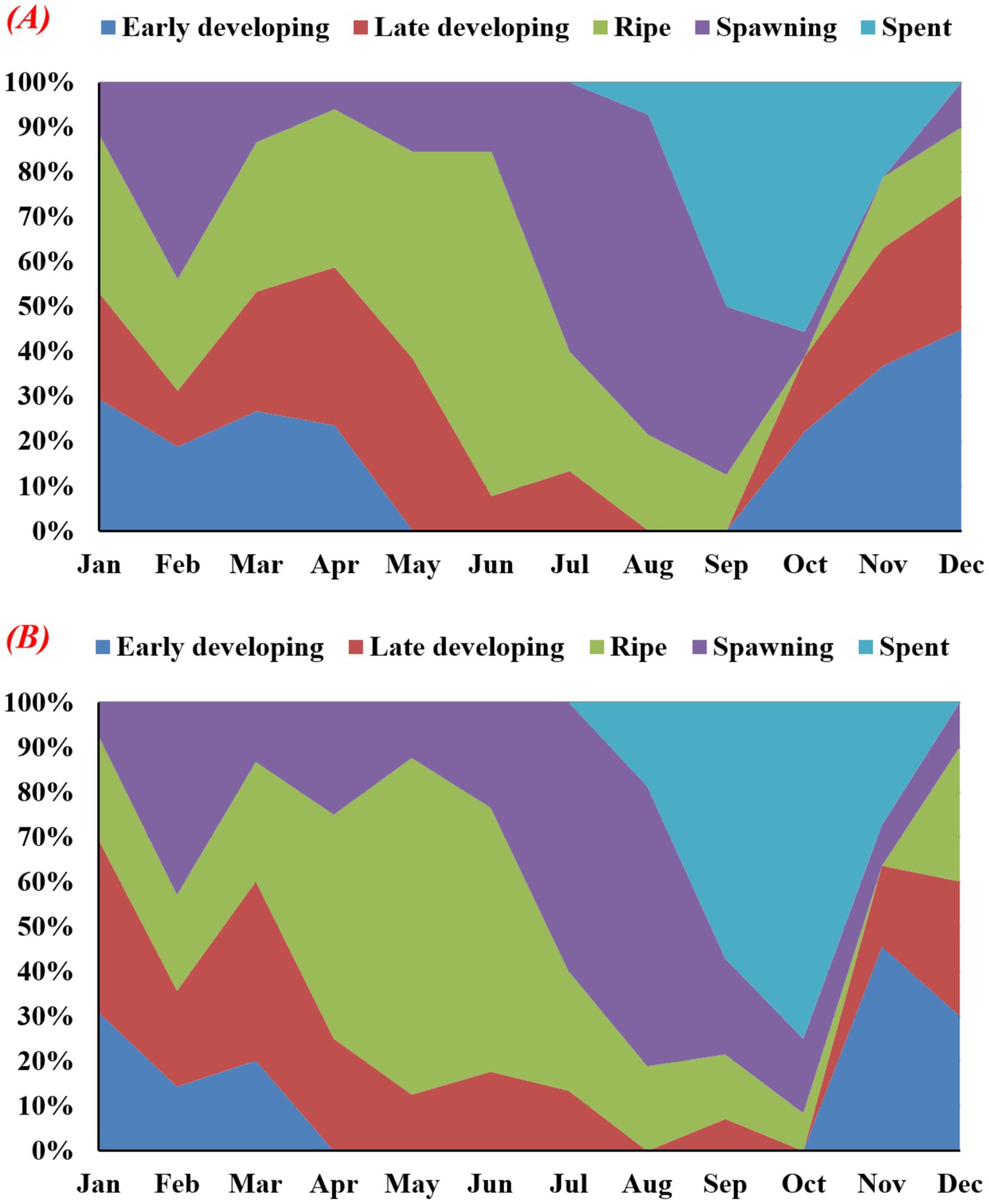
Figure 6. Monthly variations in gonadal maturation stages in freshwater mussel L. marginalis (A = male, B = female).
However, ripe females and males were detected throughout the study except in October for males and November for females. The males and females of L. marginalis demonstrated analogous spawning patterns, whereas spawning occurred throughout the year. However, male and female individuals experienced peak spawning during July and August. Moreover, spent male and female L. marginalis were observed from August to November, whereas the highest numbers of spent individuals were recorded in October (Figure 6).
3.6 Gonado-somatic index
GSI variation was analyzed to precisely quantify the reproductive activity. The GSI in males and females demonstrated significant (p < 0.05) seasonal variations, with the maximum value noted during May in males and during June in females (Figure 7).
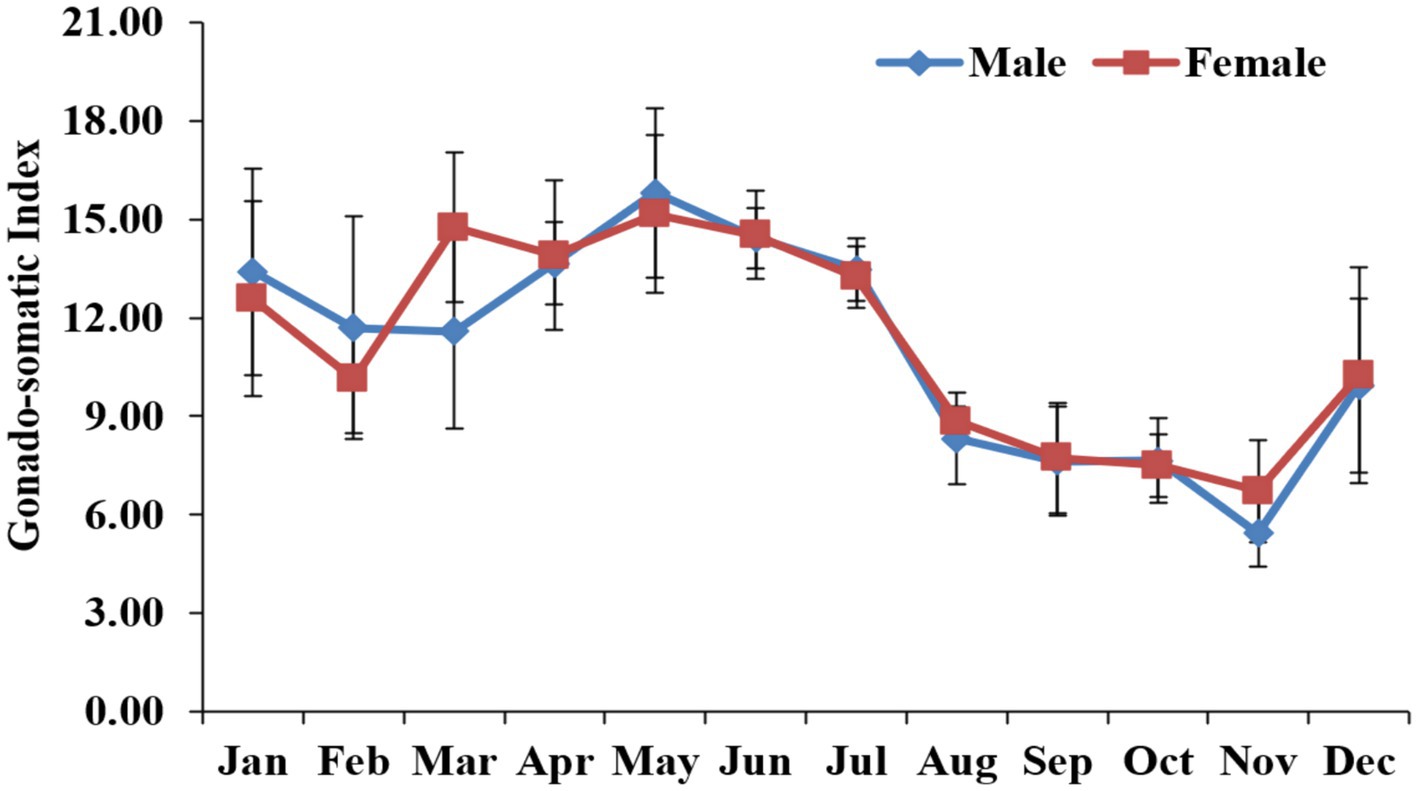
Figure 7. Monthly variations in the gonad index (GI) gonado-somatic index (GSI) in freshwater mussel L. marginalis.
3.7 Influence of environmental parameters on biology of L. marginalis
The environmental variables and the biochemical composition varied significantly (p < 0.05) throughout the study duration, indicating a temporal pattern typical in a tropical region (Supplementary Tables S1, S2). Correlation analysis among the environmental and biological parameters is shown in Table 5. Both CI and MYI positively correlated with alkalinity, while showed negative association with DO. CI also influenced negatively by the moisture and carbohydrate content, while positively with protein and lipid content of the body. However, MYI showed weak negative correlation with carbohydrates, weak positive links with protein and strong positive relationship with lipid content. Moisture content revealed negative correlation with alkalinity, while positive association with the transparency.
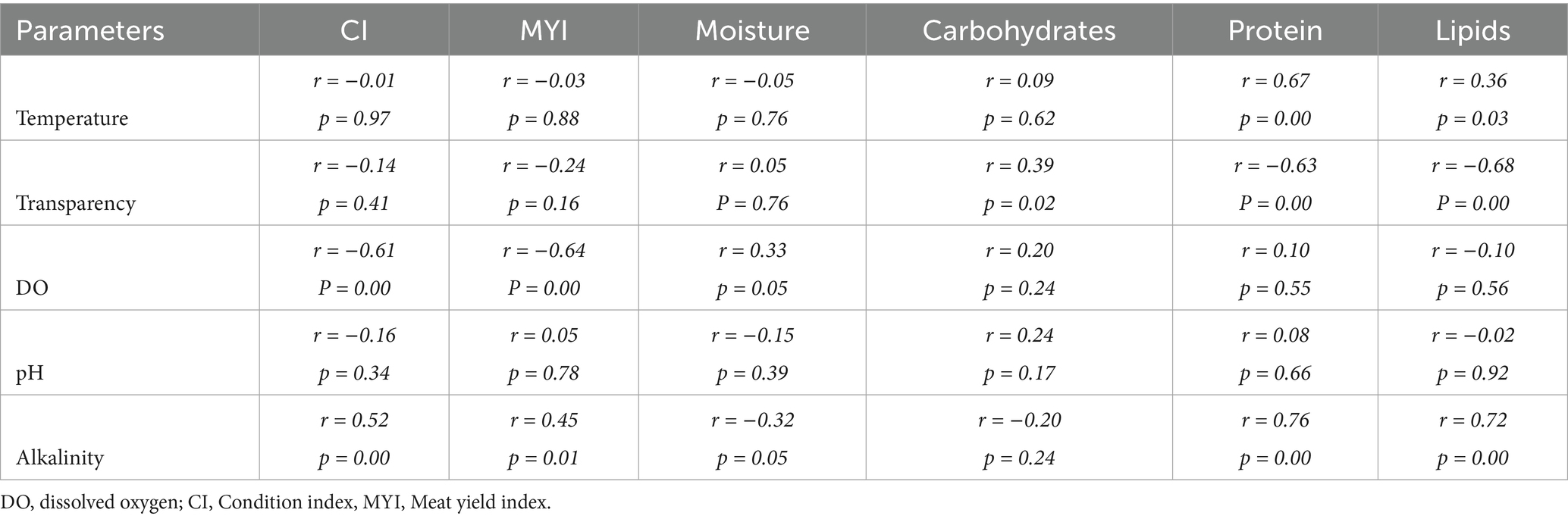
Table 5. Pearson’s correlation coefficients (r) and respective p-values for pairwise correlation analysis (n = 12) on mean monthly environmental parameters, CI, MYI, and biochemical composition of freshwater mussel L. marginalis.
Out of the five gonad developmental stages early developing, late-developing, and ripe stage were influenced by different environmental and biochemical body composition levels (Table 6). For instance, the water temperature was very strongly and negatively correlated with early developing and late developing stages as shown in Table 6. Transparency has shown strong positive association with the early developing stage and strong negative relationship with the ripe stage. On the contrary, alkalinity has robust, strong, and negative correlation with early developing stage and strong positive correlation with ripe stage. Ripe stage also illustrated a strong positive links with protein and lipid content, as well as with CI and MYI in L. marginalis. However, a strong negative association was observed between CI and MYI with the spent stage. The environmental parameters and biochemical composition displayed an influence on the GI and GSI. Transparency, moisture and carbohydrate had negative relation with GI. Only alkalinity showed a strong positive correlation with GSI, while transparency and DO indicate a negative relationship among the ecological parameters. Protein and lipid content of body mass also displayed a strong positive effect on GSI. However, correlations of GSI with moisture and carbohydrate content were strongly negative. On the contrary, the MYI also illustrated a significant positive correlation with GSI.
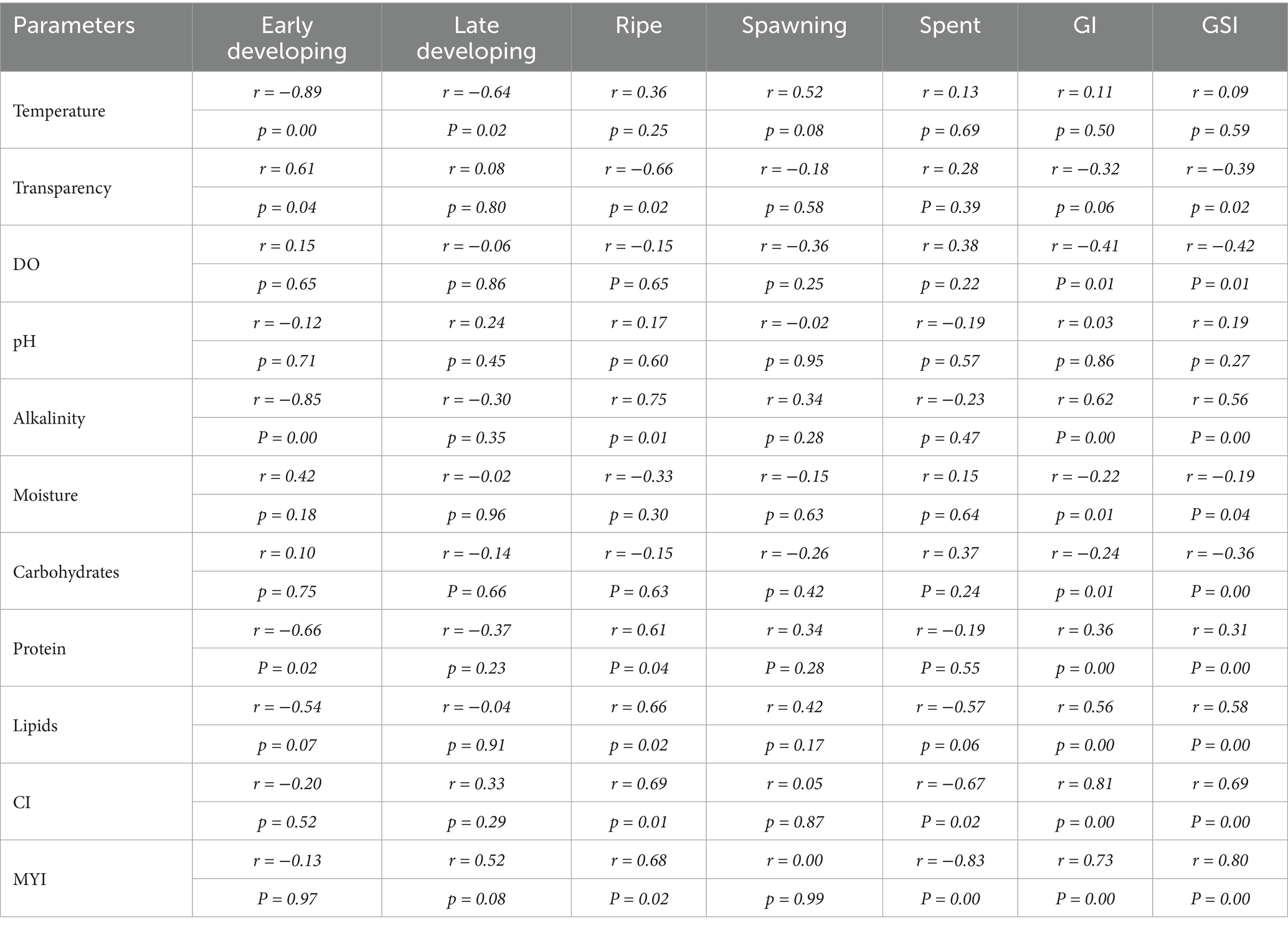
Table 6. Pearson’s correlation coefficients (r) matrix and respective p-values obtained after the pair-wise correlation analysis (n = 12) on monthly means of environmental parameters, gonadal maturation stages, GI, GSI and biochemical composition of freshwater mussel L. marginalis.
4 Discussion
4.1 Growth dynamics and morphometric trends of L. marginalis
This study provided the most comprehensive seasonal and sex-based growth profile of Lamellidens marginalis reported to date from Bangladesh. The length-weight relationships (LWRs) indicated predominantly negative allometric growth across both sexes and multiple body parameters, a finding broadly consistent with earlier work on freshwater mussels such as Parreysia corrugata and P. favidens (Malathi and Thippeswamy, 2011; Ramesha and Sophia, 2015). However, the current study expands on those findings by detecting conditionally positive allometry under certain seasonal-body parameter interactions—such as shell width against body weight during autumn and winter. These results suggest that growth plasticity in L. marginalis is modulated by environmental cues, highlighting a complex interaction between somatic development and ecological seasonality.
Another novel aspect is the temporal variation in biometric traits, with higher body weight, tissue mass, and shell measurements observed during post-monsoon months, especially in September. Such trends may be driven by increased food availability, favorable physicochemical conditions, or physiological compensation following reproductive depletion. Although biometric differences between sexes were not statistically significant, females generally exhibited slightly higher mean values in most dimensions (except shell height), which may reflect the reproductive burden of oocyte development. The lack of significant sexual dimorphism aligns with previous unionid studies and indicates a moderate sexual allocation strategy in this species, advantageous for polyculture systems aiming at uniform growth performance.
In terms of condition and meat yield indices, this study identified two major peaks in CI (June and December) and corresponding high MYI values. The June peak coincided with pre-spawning gonadal maturity, while the December peak likely reflected physiological recovery post-spawning. These patterns were statistically supported and ecologically meaningful, underscoring that L. marginalis follows a cyclical energy allocation model where nutrient reserves are restored in preparation for subsequent reproductive cycles. The identification of December–January as an optimal harvest window is a significant outcome for aquaculture operations, offering practical scheduling guidance to maximize meat yield while avoiding reproductive disruption. Unlike prior studies that briefly reported condition metrics (e.g., Nahar, 2020), this work integrates biometric, seasonal, and reproductive data, offering a much-needed multidimensional framework for evaluating physiological fitness in freshwater mussels.
4.2 Reproductive biology, sex ratio patterns, and environmental influence
The reproductive biology of L. marginalis remains under-documented despite its commercial and ecological importance in South Asia. The present study offers a significant advance by providing histological and index-based evidence of reproductive behavior across a full annual cycle. Unlike prior reports that inferred reproductive timing from limited observations (Behera et al., 2014; Niogee et al., 2019), this investigation utilized monthly gonadal staging to confirm that L. marginalis maintains reproductive activity throughout the year, with asynchronous gametogenic phases often co-occurring in the same individual. This confirms its classification as a partial and successive spawner, capable of continuous recruitment in suitable environments.
The most intense reproductive activity, marked by elevated GSI values and ripe gonadal stages, occurred during May–June, culminating in a spawning peak in July–August, when water temperatures reached 32.3–34.5°C. This seasonal synchrony with rising temperatures and increasing alkalinity reflects an evolutionary adaptation to tropical freshwater systems, enabling reproductive events to align with optimal environmental productivity. Environmental correlates, such as a strong negative relationship between early gametogenic stages and both temperature and alkalinity, suggest that thermal thresholds may regulate the transition from follicle development to spawning readiness. These findings complement global studies on bivalve reproductive ecology (Lefort and Clavier, 1994; Grant and Creese, 1995) but represent the first detailed documentation of such patterns in L. marginalis within a Bangladeshi context.
A further noteworthy result is the male-biased sex ratio (1:0.89), statistically significant and consistent with previous observations in unionid mussels (Mondol et al., 2016). While the underlying cause remains unclear, this pattern may arise from environmental stressors, food availability, or endocrine-disrupting agents, as suggested in other bivalve taxa (Breton et al., 2017). The current study not only quantifies this imbalance but situates it within a broader environmental framework, proposing that such skewed ratios could be indicative of localized ecological pressures or developmental plasticity. These insights are particularly relevant for hatchery programs, where understanding the timing and conditions of peak reproductive output can enhance seed production. Moreover, the clear documentation of year-round breeding challenges traditional assumptions of seasonal spawning in freshwater mussels and suggests greater reproductive flexibility, which may prove beneficial under climate change scenarios.
4.3 Nutritional implications for human and aquaculture
One of the most innovative aspects of this study is the concurrent analysis of biochemical composition alongside reproductive and environmental data. While previous research has investigated protein or lipid dynamics in bivalves (e.g., Camacho et al., 2003; Dridi et al., 2007), few have examined how these metrics interact with environmental variables and reproductive effort in a tropical freshwater mussel across an annual cycle. In L. marginalis, tissue protein and lipid levels increased significantly during the gonadal maturation phase (May–June), reflecting their role as primary constituents in gametogenesis. Proteins are essential for oocyte development, and their peak levels during pre-spawning coincide with the highest GSI values, affirming their energetic significance. This pattern is consistent with studies on Crassostrea gigas and other bivalves (Berthelin et al., 2000; Kang et al., 2003). The subsequent decline in protein and lipid levels during July–August supports the interpretation that these reserves are depleted during spawning, a conclusion reinforced by simultaneous drops in CI and MYI.
Carbohydrate content followed an inverse trend, with the highest values recorded during early gametogenic phases (January–March) and the lowest during peak reproductive activity. This suggests that carbohydrate reserves are mobilized early in the reproductive cycle, likely supporting oocyte formation and early follicular development. Following spawning, carbohydrate levels gradually increased, indicating recovery of glycogen stores. These trends mirror those reported by Gabbott (1975) and Deslous-Paoli and Héral (1988) for marine bivalves but are newly described here for L. marginalis.
Importantly, this biochemical insight is not discussed in isolation. The study connects macronutrient profiles with environmental fluctuations and reproductive indicators, showing that lipid and protein contents are strongly correlated with GSI and ripe gonadal stages, while carbohydrates show negative correlations. This integrative approach offers a valuable diagnostic tool for monitoring the physiological condition of cultured or wild stocks and selecting appropriate harvesting periods for optimal nutritional yield. Unlike earlier studies that mentioned nutritional quality without linking it to reproductive or ecological functions, this study situates biochemical composition as a dynamic trait shaped by environmental and reproductive cycles. These findings can directly inform aquaculture strategies—such as the design of feeding schedules or selective breeding for meat quality—as well as conservation actions aimed at minimizing extraction during peak reproductive periods.
5 Conclusion
This study investigated the growth patterns, reproductive activity, and biochemical composition of a bivalve, Lamellidens marginalis, sourced from a freshwater lake in Northwestern Bangladesh. A significant negative allometric growth pattern was observed in the overall body weight-shell length relationship of L. marginalis. Seasonal fluctuations were evident in the condition index, which reached its highest value between April and June before declining sharply in July and August. The reproductive cycle significantly influenced the seasonal variability of its biochemical composition. Histological analysis revealed a male-dominated sex ratio of 1:0.89, with no sexually undifferentiated or hermaphrodite individuals detected. L. marginalis was observed to reproduce year-round, with a spawning peak during July and August coinciding with higher water temperatures (32.30–34.50°C), accompanied by a sharp decline in the GSI. This continuous and distinct reproductive pattern aligns with typical tropical bivalves, differing from the reproductive cycles observed in temperate species. The study suggests that the most productive harvesting period for supporting commercial-level exploitation of high-quality mussels in the Bangladesh freshwater ecosystem is December and January. During this time, mussels exhibit optimal health conditions with higher potential for meat yield and nutritional content following the peak spawning period and therefore the most productive harvesting time to support the commercial level exploitation of better-quality mussels. This information can be used to optimize stocking schedules in shellfish farming, enhancing production efficiency. The insights from this research will aid in monitoring wild populations, facilitating effective management strategies to conserve natural stocks of L. marginalis. The findings are also aligned with UNSDG Goals 14 and 2 by supporting sustainable management of freshwater mussel stocks, conserving biodiversity, and enhancing food security through eco-friendly aquaculture. However, there are some limitations of this study that may be taken into consideration in future studies. It relied mainly on morphological and histological techniques without considering genetic or molecular strategies that would have shed more meaningful light on population dynamics and sexual differentiation. Despite having emphasized aquaculture potential of L. marginalis, comprehensive socioeconomic evaluation—addressing aspects such as market acceptability, cost-effectiveness, and small-scale farmer suitability—was not undertaken. Finally, biochemical analysis did not account for possible contaminants (e.g., microplastics or heavy metals), which are important in establishing food safety and possible health consequences to consumers in future research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MM: Investigation, Writing – original draft. DN: Project administration, Writing – original draft. MAH: Investigation, Writing – original draft. ST: Formal analysis, Writing – original draft. MRA: Investigation, Writing – review & editing. SJ: Methodology, Writing – review & editing. NM: Software, Writing – review & editing. SD: Formal analysis, Writing – review & editing. TA: Resources, Writing – review & editing. BP: Funding acquisition, Writing – review & editing. MH: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was in part supported by the grant from the Capacity Utilization Program under Special Allocation for Science and Technology, Ministry of Science and Technology, Government of the People’s Republic of Bangladesh, for financial assistance to conduct this study. This is supported by Ongoing Research Funding Program, (ORF-2025-144), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
This article is based on the author’s PhD thesis. The authors are grateful to the members of the Shellfish Research Laboratory at the Department of Fisheries, University of Rajshahi, Bangladesh, for their support in data acquisition. The authors would like to extend their sincere appreciation to the Ongoing Research Funding Program, (ORF-2025-144), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1562686/full#supplementary-material
References
Anacleto, P., Maulvault, A. L., Bandarra, N. M., Repolho, T., Nunes, M. L., Rosa, R., et al. (2014). Effect of warming on protein, glycogen and fatty acid content of native and invasive clams. Food Res. Int. 64, 439–445. doi: 10.1016/j.foodres.2014.07.023
AOAC (2005). International official methods of analysis of AOAC international. 18th Edn. Gaithersburg, Maryland, USA. MD: AOAC International.
Behera, B. K., Meena, D. K., Das, P., and Ram, K. J. (2014). Simulated breeding of Indian pearl mussel, Lamellidens marginalis (L.) in laboratory condition. Int. J. Res. Fish. Aquacult. 4, 145–149.
Berthelin, C., Kellner, K., and Mathieu, M. (2000). Storage metabolism in the Pacific oyster, (Crassostrea gigas) in relation to summer mortalities and reproductive cycle (west coast of France). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 125, 359–369. doi: 10.1016/S0305-0491(99)00187-X
Bligh, E. G., and Dyer, W. J. (1959). A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917.
Breton, S., Capta, C., Guerraa, D., and Stewartb, D. (2017). Sex determining mechanisms in bivalves. Preprints. https://doi.org/10.20944/preprints201706.0127.v1
Calderon-Aguilera, L. E., Arago’n-Noriega, E. A., Hand, C. M., and Moreno-Rivera, V. M. (2010). Morphometric relationships, age, growth, and mortality of the geoduck clam, Panopea generosa, along the Pacific coast of Baja California, Mexico. J. Shellfish Res. 29, 319–326. doi: 10.2983/035.029.0206
Camacho, A. P., Delgado, M., Farnandez-Reiriz, M. J., and Labarta, U. (2003). Energy balance, gonad development and biochemical composition in the clam Ruditapes decussatus. Mar. Ecol. Prog. Ser. 258, 133–145. doi: 10.3354/meps258133
Camacho-Mondragon, M. A., Arellano-Martinez, M., and Ceballos-Vazquez, B. P. (2012). Particular features of gonad maturation and size at first maturity in Atrina maura (Bivalvia: Pinnidae). Sci. Mar. 76, 539–548. doi: 10.3989/scimar.03522.05A
Dan, H., Mazid, M. A., and Hussain, M. G. (2001). Freshwater pearl culture: Principles and techniques. Mymensingh: Bangladesh Fisheries Research Institute.
Deslous-Paoli, J. M., and Héral, M. (1988). Biochemical composition and energy value of Crassostrea gigas (Thunberg) cultured in the bay of Marennes-Oléron. Aqua. Living Resour. 1, 239–149.
DeVlaming, V., Grossman, G., and Chapman, F. (1982). On the use of the gonosomatic index. Comp. Biochem. Physiol. A Physiol. 73, 31–39. doi: 10.1016/0300-9629(82)90088-3
Dridi, S., Romdhane, M. S., and Elcafsi, M. (2007). Seasonal variation in weight and biochemical composition of Pacific oyster, Crassostrea gigas, in relation to gametogenic cycle and environmental condition of the Bizerte lagoon, Tunisia. Aquaculture 263, 238–248. doi: 10.1016/j.aquaculture.2006.10.028
Gabbott, P. A. (1975). “Storage cycles in marine bivalve mollusks: an hypothesis concerning the relation between glycogen and gametogenesis” in Procedings of the ninth Eur. Mar. biol. Symp., Aberdeen Univ. ed. H. Baynes (Aberdeen, Scotland: Press), 191–211.
Gökçe, G., Çekiç, M., and Filiz, H. (2010). Length-weight relationships of marine fishes off Yumurtalık coast (Iskenderun Bay), Turkey. Turk. J. Zool. 34, 101–104. doi: 10.3906/zoo-0905-33
Grant, C. M., and Creese, R. G. (1995). The reproductive cycle of the tuatua Paphiessu btriangulata (wood, 1828), in New Zealand. J. Shellfish Res. 14, 287–292.
Grizzle, R. E., Ward, K. M., Peter, C. R., Cantwell, M., Katz, D., and Sullivan, J. (2017). Growth, morphometrics and nutrient content of farmed eastern oysters, Crassostrea virginica (Gmelin), in New Hampshire, USA. Aquac. Res. 48, 1525–1537. doi: 10.1111/are.12988
Hmida, L., Ayache, N., Haouas, Z., and Romdhane, M. S. (2010). Oocytecohort analysis: criteria for an evaluation of the reproductive cycle in Solen marginatus (Pennnt, 1777), (Bivalvia: Solenacea) in southern Tunisia. J. Shellfish Res. 29, 129–134. doi: 10.2983/035.029.0105
Hossain, M. A., Hussain, M., Sarker, T. R., Saha, S., and Iqbal, M. M. (2023). Reproductive and morphometric traits of freshwater mussel Lamellidens marginalis and associated hydrology in the Ratargul freshwater swamp Forest, Bangladesh. Egypt. J. Aquat. Res. 49, 161–170. doi: 10.1016/j.ejar.2022.11.004
Idayachandiran, G., Muthukumar, A., Kumaresan, S., and Balasubramanian, T. (2014). Nutritional value of marine bivalve, Donax cuneatus (Linnaeus, 1758) from Cuddalore coastal waters, southeast coast of India. Inventi. Impact Life Style 1, 15–19.
Juhel, G., Culloty, R. M., O’Riordan, J., O’Connor, L., and De Faoite, R. (2003). A histological study of the gametogenic cycle of the freshwater mussel Dreissena polymorpha (Pallas, 1771) in lough Derg, Ireland. J. Molluscan Stud. 69, 365–373. doi: 10.1093/mollus/69.4.365
Kang, S. G., Choi, K. S., Bulgacov, A. A., Kim, Y., and Kim, S. Y. (2003). Enzyme–linked immunosorbant assay (ELISA) used in quantification of reproductive output in the Pacific oyster, Crassostrea gigas, in Korea. J. Exp. Mar. Biol. Ecol. 282, 1–21. doi: 10.1016/S0022-0981(02)00444-6
Lamarck, J. B. (1819). Histoire naturelle des animaux sans vertèbres. Tome VI, Paris: Deterville/Verdière.
Le Cren, E. D. (1951). The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 20, 201–219. doi: 10.2307/1540
Lefort, Y., and Clavier, J. (1994). Reproduction of Annachlamys flabellata, Comptopallium radula and Mimachlamys gloriosa (Molluska: Pectinidae) in the south-west lagoon of New Caledonia. Aquat. Living Resour. 7, 39–46. doi: 10.1051/alr:1994005
Lucas, A., and Beninger, P. E. (1985). The use of physiological condition indices in marine bivalve. Aquaculture 44, 187–200. doi: 10.1016/0044-8486(85)90243-1
Malathi, S., and Thippeswamy, S. (2011). Morphometry, length-weight, and condition in Perreysia corrugata (Muller, 1774) (Bivalvia: Unionidae) from river Malthi in the Western Ghats, India. Int. J. Biol. Sci. 2, 43–52.
Mondol, M. R., Nasrin, F., and Nahar, D. A. (2016). Length-weight relationship, condition index and sex ratio of freshwater mussel Lamellidens corrianus (Lea, 1834) from a freshwater lake, Northwest Bangladesh. Croat. J. Fish. 74, 141–152. doi: 10.1515/cjf-2016-0025
Nahar, D. A. (2020) Growth indices and reproductive pattern of freshwater mussel Lamellidens marginalis (Lamarck, 1819) in Rajshahi University, Northwest Bangladesh. Available online at: http://rulrepository.ru.ac.bd/handle/123456789/1091 (Accessed January 28, 2024).
Niogee, S. R., Tonni, K. F., Barman, A. C., Tonu, M. B., Sonia, S., and Uddin, M. J. (2019). Ovarian cycle of freshwater pearl mussel, Lamellidens marginalis (Lamarck, 1819) collected from a culture pond in Bangladesh. Asian Fish. Sci. 32, 117–123. doi: 10.33997/j.afs.2019.32.3.004
Pauly, D. (1983). Some simple methods for the assessment of tropical fish stocks. FAO Fish. Tech. Pap. 235:52.
Ramesha, M. M., and Sophia, S. (2015). Morphometry, length-weight relationships and condition index of Parreysia favidens (Benson, 1862) (Bivalvia: Unionidae) from river Seeta in the Western Ghats, India. Indian J. Fish. 62, 18–24. Available at: https://epubs.icar.org.in/index.php/IJF/article/view/28376
Rao, G. S. (2007). Growth and biometric relationship of the Indian pearl oyster Pinctada fucata (Gould) under long term onshore rearing system. J. Marine Biol. Assoc. India 49, 51–57.
Ricker, W. E. (1975). Computation and interpretation of biological statistics of fish populations. Fish. Res. Board Can. Bull. 191, 1–382.
Saha, J. K., and Islam, M. A. (2000). Culture of pearl in freshwater mussels (Lamellidens marginalis, Lamarck). Bangladesh J. Fish. Res. 4, 57–61.
Shumway, S. E., Davis, C., Downey, R., Karney, R., Kraeuter, J., Parsons, J., et al. (2012). Shellfish aquaculture—in praise of sustainable economies and environments. World Aquacult. 34, 15–17.
Siddique, M. A., Khatun, A. M., Rahman, M., Ahmed, G. U., Moniruzzaman, M., and Uddin, M. J. (2019). Annual gametogenic cycle of the freshwater pearl mussel, Lamellidens marginalis (Lamarck, 1819) collected from a perennial lentic habitat of Bangladesh. Molluscan Res. doi: 10.1080/13235818.2019.1682954
Silva, Q. (2002). Análise de alimentos:Métodos químicos e biológicos. Viçosa, Minas Gerais, Brazil: Editora Universidade Federal de Viçosa.
Taylor, K. A. C. C. (1995). A modification of the phenol/sulfuric acid assay for total carbohydrates giving more comparable absorbance. Appl. Biochem. Biotechnol. 53, 207–214. doi: 10.1007/BF02783496
Yildiz, H., Palaz, M., and Bulut, M. (2006). Condition indices of Mediterranean mussels (Mytilus galloprovincialis L. 1819) growing on suspended ropes in Dardanelles. J. Food Tech. 4, 221–224.
Yilmaz, S., Yazıcıog˘lu, O., Erbas_aran, M., Esen, S., Zengin, M., and Polat, N. (2012). Lengthweight relationship and relative condition factor of white bream, Blicca bjoerkna (L., 1758), from Lake Ladik, Turkey. J. Black Sea Medit. Environ. 18, 380–387.
Keywords: biochemical composition, conservation, growth pattern, Lamellidens marginalis, reproductive cycle, shellfish farming
Citation: Mondol MMR, Nahar DA, Ayenuddin Haque M, Tisha SA, Ali MR, Jasmine S, Md Noor N, Das SK, Arai T, Paray BA and Hossain MB (2025) Growth dynamics, reproductive biology, and nutritional profiling of the freshwater mussel Lamellidens marginalis (Lamarck, 1819): implications for wild stock conservation and sustainable aquaculture development. Front. Sustain. Food Syst. 9:1562686. doi: 10.3389/fsufs.2025.1562686
Edited by:
Roberto Anedda, Parco Scientifico e Tecnologico della Sardegna, ItalyReviewed by:
Federica Montesanto, Aarhus University, DenmarkShailesh Saurabh, Central Institute of Freshwater Aquaculture (ICAR), India
Mohammad Hossain, Sylhet Agricultural University, Bangladesh
Copyright © 2025 Mondol, Nahar, Ayenuddin Haque, Tisha, Ali, Jasmine, Md Noor, Das, Arai, Paray and Hossain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Belal Hossain, bWJobnN0dUBnbWFpbC5jb20=
 Md. Mostafizur Rahman Mondol1
Md. Mostafizur Rahman Mondol1 Md. Ayenuddin Haque
Md. Ayenuddin Haque Noorashikin Md Noor
Noorashikin Md Noor Simon Kumar Das
Simon Kumar Das Takaomi Arai
Takaomi Arai Bilal Ahamad Paray
Bilal Ahamad Paray Mohammad Belal Hossain
Mohammad Belal Hossain