- 1School of Hospitality Management and Culinary Arts, Tourism College of Zhejiang, Hangzhou, Zhejiang, China
- 2Department of Biotechnology, College of Life and Environmental Sciences, Hangzhou Normal University, Hangzhou, Zhejiang, China
The genus Polygonatum is recognized for both its medicinal and food applications. It demonstrates a range of beneficial activities, making it a strong candidate for the development of health-promoted products. The activities, including hypoglycaemic, antioxidant, anti-fatigue, immune regulatory, anticancer, antibacterial and anti-atherosclerotic properties, are highly associated with various substances, including polysaccharides, saponins, alkaloids, flavonoids, and many others. Flavonoids in the genus Polygonatum are regarded as being one of the primary functional constituents, exhibiting a broad spectrum of molecular structures and bioactivities. Among them, flavonoids such as homoisoflavonoids, chalcones, isoflavones, and flavones, have been identified in the genus Polygonatum. Many studies have indicated its capacity to manifest various kinds of potently biological functions, for instance, anti-tumor, anti-viral, and glycemic control properties. Various processing methods, notably nine steam-nine bask, have been investigated. It has been observed that various chemical constituents including flavonoids, and pharmacological activities undergo significant alterations following processing. This present study aims to offer a review of the current research state on the extraction and processing of Polygonatum flavonoids, providing a theoretical foundation for their scientific advancement and reasonable utilization of Polygonati Rhizoma in food industry.
Introduction
The genus Polygonatum, which is classified within the Asparagaceae family, is typically distributed across temperate areas of the northern hemisphere, including China, India, Japan, Russia, South Korea, Europe, and North America (Zhao et al., 2018; Zhang et al., 2024). To date, approximately 81 species in the genus Polygonatum have been identified, 39 of which are found in China (Luo et al., 2022). Dried rhizomes from the genus Polygonatum, such as Polygonatum sibiricum, Polygonatum kingianum, and Polygonatum cyrtonema have been incorporated into the “Chinese Pharmacopeia” (2020 edition) (Nie et al., 2023, 2024; Ye et al., 2025). As a homologous medicine and food, it has been extensively utilized as an intervention drug and nutritional supplement (Zhao et al., 2018; Zong et al., 2024). It is extensively employed in clinical settings for treating a variety of ailments, such as hypertension, cough, chronic hepatitis, diabetes, and cardio-cerebrovascular diseases (Liu and Si, 2018). In addition, the genus Polygonatum is also employed in food industry to produce a variety of functional health products, including Polygonati Rhizoma wine, functional beverages, yogurt thickeners, confectioneries, noodle formulations, and fermented foods (Zheng et al., 2023). It is noteworthy that the combination of rice wine and Polygonatum, known for its immune-modulating properties, has been reported. Modern pharmacological studies demonstrate that the genus Polygonatum possesses significant benefits, including hypoglycaemic, antioxidant, anti-fatigue, immune regulatory, anticancer, antibacterial and anti-atherosclerotic properties (Bu et al., 2024; Li H. M. et al., 2022; Li J. et al., 2022; Shen et al., 2021; Pan et al., 2024; Wang Y. F. et al., 2020; Wang Y. J. et al., 2020). The abundant biological activities exhibited by the genus Polygonatum are ascribe to the existence of multiple constituents, for example, polysaccharides, flavonoids, saponins, phytosterols, and amino acids (Cui et al., 2018; Pan et al., 2024; Zhou L. et al., 2024; Zhou T. et al., 2024). Of these, polysaccharides, saponins and flavonoids are considered to be the key active ingredients in the genus Polygonatum (Zhou T. et al., 2024).
Flavonoids represent a significant chemical compound class within the genus Polygonatum, with recent studies focusing on P. kingianum, P. cyrtonema, and P. sibiricum (Ye et al., 2025; Zhang et al., 2024). The flavonoids show a variety of bioactivities, including the ability to delay aging, reduce blood glucose and lipids, and prevent atherosclerosis, in addition to their antimicrobial properties (Yang G. et al., 2024; Yang L. et al., 2024; Ye et al., 2025). The process of extraction and purification is integral to the efficient recovery of bioactive substances from their natural and unprocessed sources (Shen et al., 2024). The primary extraction methods for flavonoids from the genus Polygonatum have been investigated, such as solvent extraction, ultrasonic-assisted extraction, microwave-assisted extraction, and enzymatic-assisted extraction (Pan et al., 2024). The stability of various flavonoids, for example flavonols, flavanones, and flavones, is affected by both their structural characteristics and the processing. In the work of Liang Z. et al. (2022), 12 flavonoids from P. cyrtonema rhizomes processed by steaming-dying technology, were identified. It is also found that vitexin 2′′-O-xyloside, one of flavonoids, was completely destroyed after seven processing cycles. Prolonged processing has been shown to induce the change in the abundance of various flavonoids, including the generation of new flavonoids and the decrease of original flavonoids. Throughout history, a variety of methodologies have been employed in the processing of the genus Polygonatum, with the objective of mitigating the irritation caused by fresh rhizomes. The processing of the genus Polygonatum can be categorized into various methods, including single steaming, re-steaming, wine processing, honey processing, black bean steaming, and nine steam-nine bask processing (Sun et al., 2023; Wang et al., 2024b). Presently, the traditional nine steam-nine bask processing method is the most extensively adopted (Mei et al., 2025; Yao et al., 2022). This method involves subjecting Polygonati Rhizoma to steaming for several hours, followed by drying, and repeating this cycle nine times (Mei et al., 2025; Wang et al., 2024b). As demonstrated by several studies, the genus Polygonatum rhizomes have been observed to undergo a transformation in color, becoming black, soft, and notably sweet in taste following the process (Li et al., 2021). Furthermore, an alteration in the chemical composition was observed, with changes in the levels of polysaccharides, fructose, amino acids, steroidal saponins and flavonoids being reported (Nie et al., 2023; Zhu et al., 2022). The steaming and drying process of the genus Polygonatum results in significant alterations to its chemical composition, leading to the destruction of potentially irritating components. This process effectively mitigates the side effects of irritation in the throat, thereby conferring a beneficial effect on patients. These alterations have the potential to significantly impact the biological activities of the genus Polygonatum (Su et al., 2024).
In consideration of the substantial and varied bioactivities of flavonoids in the genus Polygonatum, their potential utilization in food relative industries are encouraging. Many reports have reviewed the chemical constituents, biological activities, and food utilization of the genus Polygonatum (Bi et al., 2023; Cui et al., 2018; Khan and Rauf, 2015; Singh et al., 2013; Wujisguleng et al., 2012; Zhao and Li, 2015; Zhao et al., 2018). However, as far as we know, the studies regarding the extraction of flavonoids from the genus Polygonatum, as well as effect of the processing on flavonoids content and its activities, have not been reported. This paper, therefore, aims to provide a review on recent development in the extraction and processing of flavonoids in the genus Polygonatum. The work would facilitate the further utilization and development of the genus Polygonatum.
General overview of flavonoids in the genus Polygonatum
In recent years, the quality evaluation for the genus Polygonatum has been mainly based on the analysis of total polysaccharides, total saponins, and some other active components, including total flavonoids (Jiang et al., 2017). As one of the most prevalent secondary metabolites in various plants, flavonoids have attracted considerable attention because of their validated bioactivities, including anti-tumor, anti-aging, anti-bacterial, anti-inflammatory, anti-atherosclerosis, cardiovascular protection, among others (Jing et al., 2021). It can be reasonably deduced that flavonoids in the genus Polygonatum may prove to be one of effective constituents for treating many kinds of diseases (Tao et al., 2018; Xiang et al., 2024). Flavonoids possess a structural skeleton comprising 15 carbon atoms, which are arranged in two benzene rings (A and B) and a heterocycle (C). This carbon skeleton structure, which is abbreviated as C6-C3-C6, is generally referred to as the parent nucleus (Luo et al., 2022). The classification of these compounds is based on their structure traits, specifically the configuration of the C-ring, which allows for the categorization into a variety of structural classifications. The difference between many structural classifications depends on the oxidation level and substitution position of the C-ring. Flavonoids in the genus Polygonatum can be classified according to their parent nucleus structure, which leads to the following divisions: homoisoflavones, isoflavones, flavones, chalcones and dihydroflavonoids (Tao et al., 2018). Homoisoflavones are the characteristic components of the genus Polygonatum (Jiang et al., 2017).
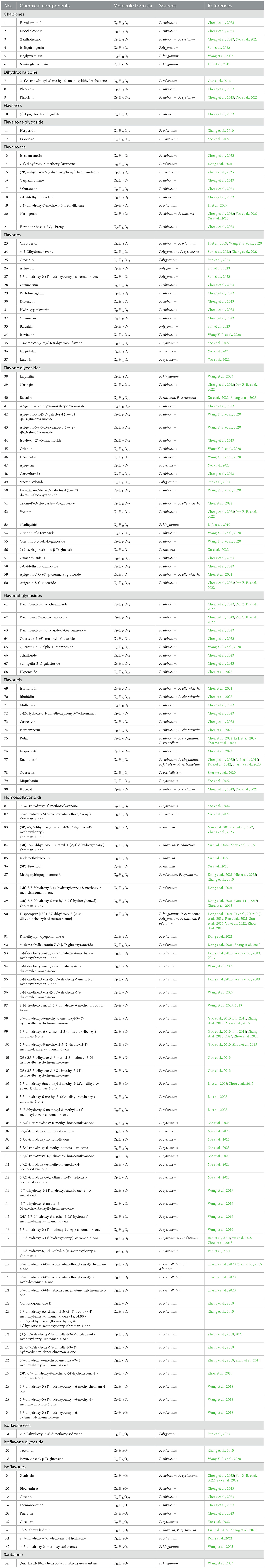
The employment of chromatography and HPLC techniques has facilitated the identification of 90 flavonoids in the genus Polygonatum, on the basis of their physicochemical and spectral characteristics (Wang M. et al., 2024; Yang G. et al., 2024; Yang L. et al., 2024). Qi et al. (2022) identified 873 kinds of metabolites from four Polygonatum species (P. sibiricum, P. kingianum, P. cyrtonema, and P. macropodium), which included 185 flavonoids, 54 alkaloids, 88 amino acids and derivatives, 105 phenolic acids, 74 organic acids, 31 terpenoids, 36 steroids, and 24 lignans and coumarins. In Jiang et al. (2017)'s study, flavonoids from P. sibiricum and P. kingianum, including four homoisoflavones, two dihydroflavones, two chalcones, and four isoflavones, were found. Wang et al. (2019) successfully obtained 15 flavonoids from P. cyrtonema, including homoisoflavones and dihydroflavonoids. In recent years, Han et al. (2023) isolated 53 flavonoids in the genus Polygonatum through UPLC-ESI-MS/MS analysis techniques. A total of 22 flavonoids in P. cyrtonema rhizomes, for example chalcones, dihydroflavonols, flavonoid carbonosides, dihydroflavones, flavonoids, flavones, and flavonols, were found. To date, many kinds of flavonoids belonging to the genus Polygonatum have been obtained. Various flavonoids types in the genus Polygonatum are showed in Table 1. Additionally, the genus Polygonatum is a good reservoir of other flavonoids, for example, rutin, quercetin, isorhamnetin, kaempferol, and baicalein (Lin et al., 2024; Mu et al., 2021; Wang et al., 2022).
Flavonoids can be employed as a crucial quality marker for the genus Polygonatum (Jiang et al., 2017). Furthermore, it has been illustrated that different flavonoid compounds may exhibit varied pharmacological effects, a prime example of which is homoisoflavonoids from the gensus Polygonatum. For instance, 3-(2′,4′-dihydroxybenzyl)-5,7-dihydroxy-6-methyl-chroman-4-one has been shown to possess the capability to inhibit the proliferation of tumor cells (Li et al., 2012). Odoratumone A has been shown to possess stronger DPPH radical scavenging abilities (Zhou et al., 2015). In addition, the homoisoflavonoids from P. Verticillatum, 5,7-dihydroxy-3-(2-hydroxy-4-methoxybenzyl)-8-methyl-chroman-4-one, have been shown to possess significant antibacterial properties (Sharma et al., 2018).
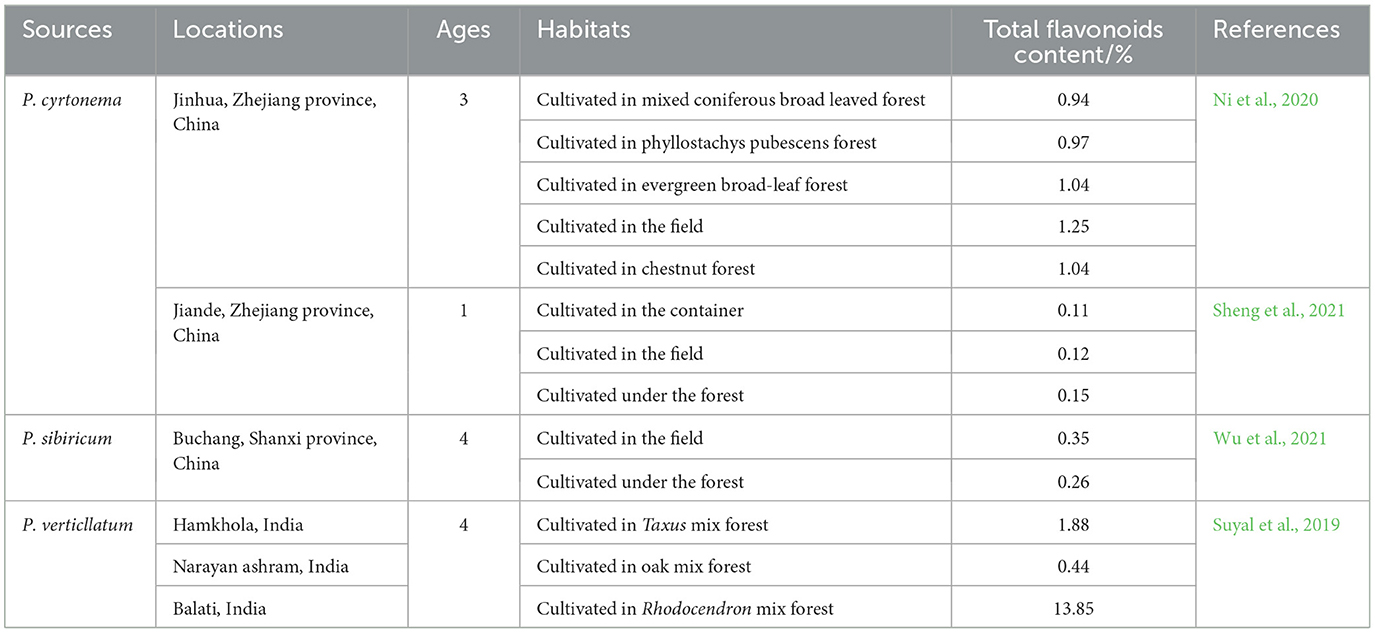
The flavonoids composition and content are not only dependent on the Polygonatum species, but also on various environmental factors, including climate, geographical location, habitat, light, latitude, water quality, and fertilizer (Tables 2, 3) (Ni et al., 2020; Sheng et al., 2021; Suyal et al., 2019; Wu et al., 2021). Moreover, it has been demonstrated to be age-related (Table 3) (Suyal et al., 2019; Wu et al., 2021; Zhang et al., 2023). Chen et al. (2020) examined the flavonoids content in different age groups of P. cyrtodonema in an artificial cultivation in the Liuzhi area of Guizhou Province, China. The findings indicated that, with increasing age, the flavonoids level in the roots of P. cyrtodonema initially increased and then declined. The highest flavonoids content expressed on the dry weight basis of the plant aged 2 years was observed, reaching 1.91%. The flavonoids content was found to be the lowest in specimens of the 5-year-old age group, at only 0.66%. Zhang K. et al. (2022) found that the flavonoids content of P. kingianum, P. cyrtonema and P. sibiricum was determined to be relatively low, with the values ranging from 0.11 to 0.14%. Among the three species, P. sibiricum exhibited the highest flavonoids content (0.14%), while P. kingianum demonstrated the lowest (0.11%).
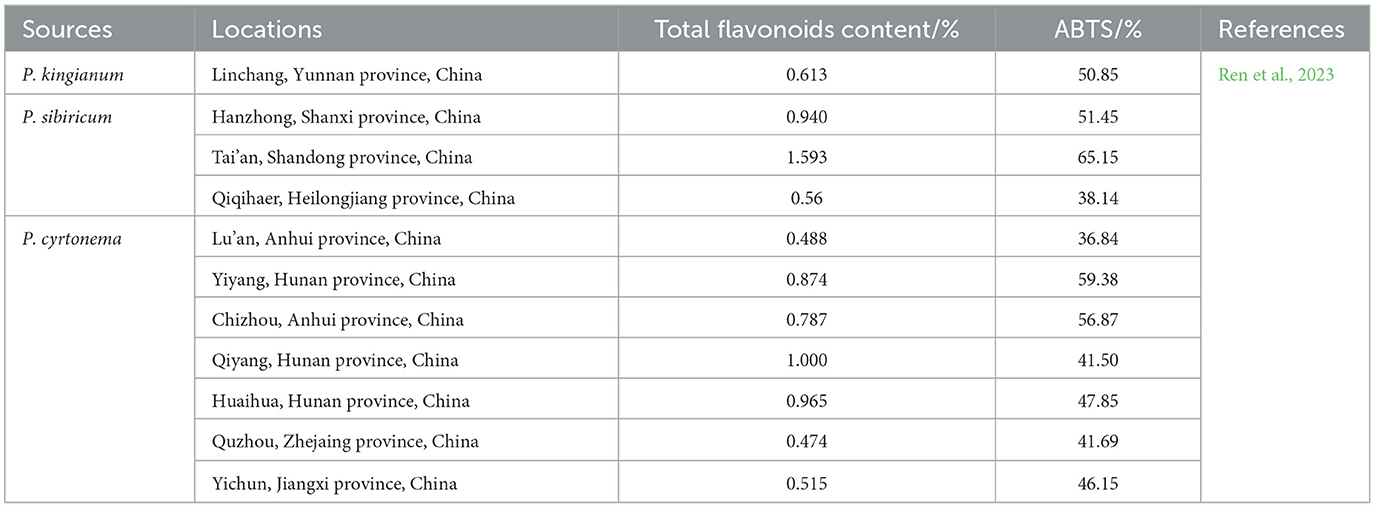
Table 2. Effect of the locations on total flavonoids content and radical scavenging (ABTS protocol) of the genus Polygonatum.
Extraction and purification of flavonoids from the genus Polygonatum
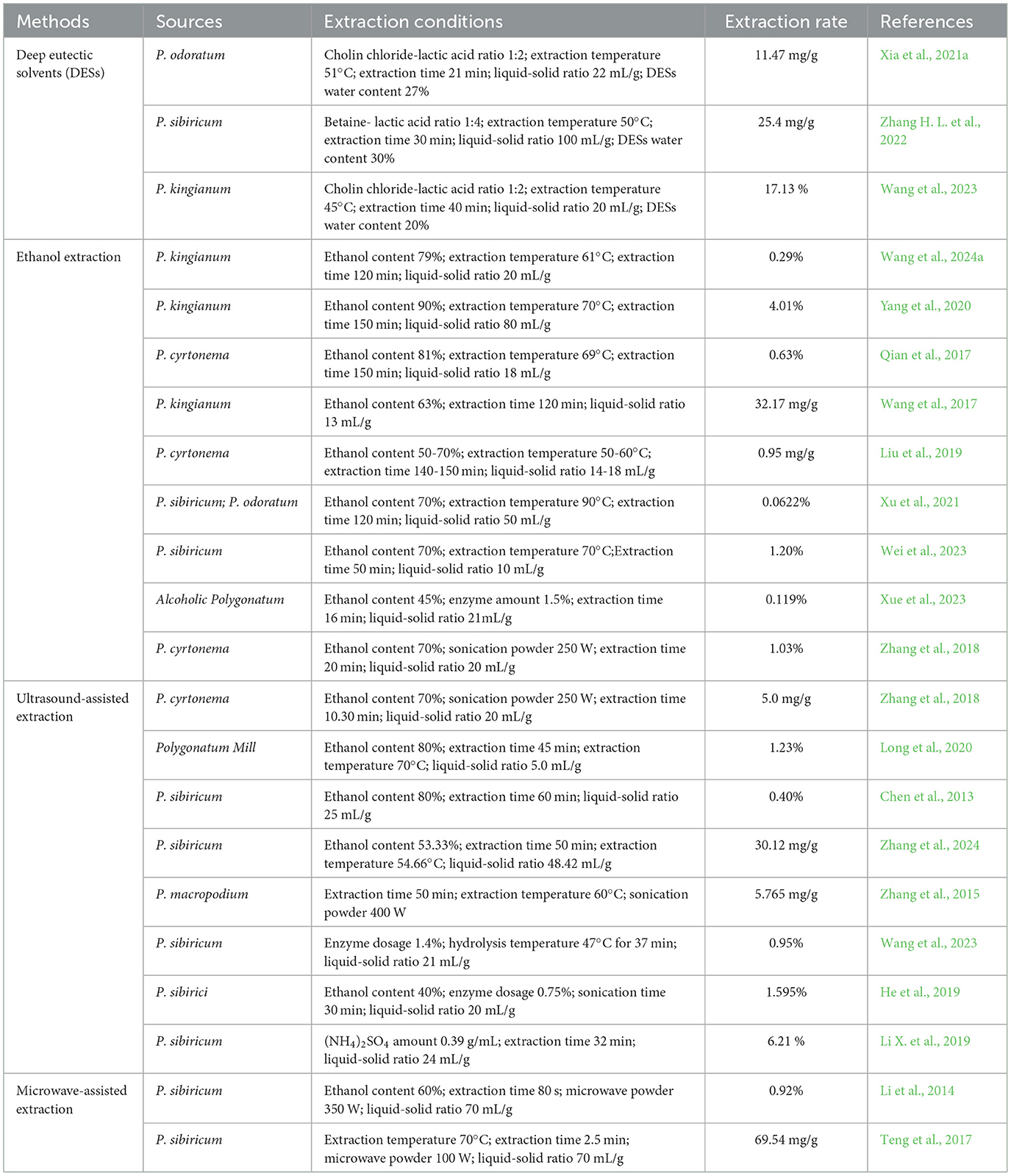
Extraction is a significant and widely utilized process for obtaining flavonoids from the genus Polygonatum. Many methods for extracting flavonoids, for instance, ultrasound-assisted extraction, ethanol extraction, enzymatic method, microwave-assisted extraction, have been reported (Table 4). The extraction rate ranges from 0.04 to 1.25%. The significant difference can be attributed to a number of factors, including extraction method, growth environment, cultivation pattern and growing periods for the genus Polygonatum (Xiang et al., 2024).
Solvent extraction
The remarkable diversity of flavonoids in terms of structure and type, and the structural differences of the functional groups, gives rise to a range of physicochemical properties, including solubility properties, that vary considerably. Accordingly, the choice of an appropriate extract solvent mainly relys on the polarity and water solubility of flavonoids. Own to the poor solubility of flavonoids in water, their primary extraction is undertaken using a range of organic solvents, for instance, ethanol, acetone, methanol, and ethyl acetate (Jing et al., 2021). The solvent extraction method is a conventional and well-established technique, mainly utilizing ethanol for extracting flavonoids from the genus Polygonatum. The principal advantages of this approach are a high extraction rate, a simple operational procedure and a good scalability (Zeng et al., 2020). However, the purity of obtained flavonoids is relatively low. Further purification of the extracts is required. In addition, it should be noted that this process may result in the destruction of flavonoid structures. The rate of flavonoids extraction expressed on the dry weight basis of the plant, is significantly influenced by many factors, including solvent type, extraction time, solid/solvent ratio, extraction temperature, extraction numbers (Chen et al., 2013; Wang et al., 2024a; Xu et al., 2021). As illustrated in Table 4, ethanol concentrations of 60−90%, extraction time of 50−150 min, extraction temperatures of 50−70°C, extraction numbers of 2−3, are usually adopted. Chen et al. (2013) optimized P. kingianum flavonoids extraction conditions. The optimized conditions: ethanol content of 80%, liquid-to-solid ratio of 25:1, extraction time of 60 min, extraction numbers of 3. Under the optimized extraction conditions, P. kingianum flavonoids maximum extraction rate was 0.40%. In addition, Xu et al. (2021) optimized flavonoids extraction rate by back propagation artificial neural network. The results demonstrated that under the specified parameters, namely a solid-liquid ratio of 1:50, an extraction time of 2 h, an extraction temperature of 90°C, and an ethanol content of 70%, the highest extraction efficiency (0.0622%) of flavonoids was achieved. Recently, Wang et al. (2024a) also extracted flavonoids from P. kingianum. The optimized extraction parameters they found were: ethanol content of 79%, liquid-to-solid ratio of 20:1, extraction time of 2 h, extraction temperature 61°C. The extraction rate under the optimized conditions reached 0.29%.
DESs are currently emerging as eco-friendly alternatives to traditionally organic solvents for extracting active constituents from natural products (Wang et al., 2023; Zhang H. L. et al., 2022). DESs have several admirable attributes, including affordability, straightforward preparation and storage, low toxicity, favorable biocompatibility, biodegradability, and good sustainability. While the use of DESs is environmentally friendly, their low volatility also means challenges in purifying the bioactive compounds or reusing the solvent. Xia et al. (2021a) used DESs for extracting total flavonoids derived from P. odoratum rhizomes. Under optimized parameters (choline chloride and lactic acid at a molar ratio of 1:2, DESs water concentration of 27%, extraction temperature of 51°C, extraction time of 21 min, and liquid-solid ratio of 22 mL/g), a total flavonoid extraction rate of (11.47 ± 0.35) mg/g was obtained. In a related study, Zhang H. L. et al. (2022) also extracted flavonoids from P. sibiricum by using betaine–acetic acid DESs. The optimal extraction parameters were determined to be a betaine to acetic acid ratio of 1:4, water concentration of 30%, solid–liquid ratio of 1: 100 g/mL, extraction temperature of 50°C, and extraction time of 30 min. The extraction rate could reach up to 25.4 mg/g, which was approximately eight times the amount obtained through the conventional ethanol extraction method. Therefore, in comparison with the traditional ethanol extraction method, the use of DESs extraction represented a more efficient, environmentally friendly and reusable approach.
Cellulase is a highly efficient cellulolytic enzyme, and its combination with protease or pectinase can disrupt the cell wall and lead to cellular destruction, thus promoting the release and diffusion of active cellular substances (Nguyen et al., 2021, 2024). Li H. M. et al. (2022); Li J. et al. (2022) investigated the enzymatic process conditions of P. odoratum, and the findings indicated that the total flavonoids content was improved with the increase of enzyme dosage, and the enzymatic digestion promoted the conversion of flavonoids from the bound state to the free state. However, the total flavonoids concentration exhibited a decline when the optimized enzyme dosage increased further, which might be attributed to the adsorption between flavonoids and enzyme proteins, resulting in the formation of new binds between free flavonoids. The authors indicated that the flavonoids content could be significantly enhanced through the utilization of enzyme digestion.
Ultrasound-assisted extraction
Ultrasound has been extensively applied in extracting biological components. It can generate a “cavitation effect” within a relatively short period, which leads to the formation of sufficient pores in the cell membrane, thus facilitating the release of flavonoids from the cells. Additionally, ultrasound facilitates the dispersion and homogenisation of flavonoids within the extraction solvent. Furthermore, it exhibits a heat transfer effect, which, to a certain extent, increases extraction yield of flavonoids and decreases extraction time (Long et al., 2020; Wei et al., 2023). However, ultrasonic-assisted extraction has certain drawbacks, including the potential destruction of flavonoids during the process and the high cost of necessary equipment (Wei et al., 2024; Zhou et al., 2022). Zhang et al. (2018) studied the extraction of total flavonoids from P. cyrtonema through ultrasonic-assisted extraction. The optimal conditions (ultrasonic power of 250 W, ultrasonic extraction time of 20 min, ethanol content of 70%, and solid-liquid ratio of 1:20) were found. The highest content of total flavonoids of P. cyrtonema was observed to reach 10.30 mg/g under these conditions. Recently, Long et al. (2020) optimized the ultrasonic-assisted extraction process for total flavonoids from P. mill. The optimized extraction parameters were as follows: extraction temperature of 70°C, ethanol content of 80%, extraction time of 45 min, solid-to-liquid ratio of 1:5. The content of total flavonoids extracted under the optimized parameters was 2.46 mg/g.
To improve extraction rate of bioactive ingredients, the potential of combining ultrasound with enzymatic methods has been investigated. He et al. (2019) optimized the extraction process for the total flavonoids from P. sibiricum using an enzymatic-ultrasonic technique. The optimal parameters were: cellulase dosage of 0.75%, ethanol concentration of 40%, liquid-to-solid ratio of 20 mL/g, and ultrasonic time of 30 min, and the total flavonoids were extracted with a high extraction rate of 1.595% under the optimized conditions. Recently, Guo K. L. et al. (2022) used enzyme-assisted ultrasonication to extract P. sibiricum flavonoids, optimizing the extraction conditions. The findings indicated that the extraction yield of total flavonoids was most significantly influenced by the interaction between the ultrasonication time, solid-to-liquid ratio, ethanol content, and ultrasonication time. The enzymatic-ultrasonic method yielded a significantly higher extraction rate of total flavonoids from the genus Polygonatum than the ethanol extraction and ultrasonic extraction methods.
The biphasic extraction method is according to the different partition coefficients of the separated substances in the alcohol-salt biphasic system. This approach offers several advantages, including favorable conditions for alcohol recovery, enhanced separation performance, and reduced cost. Furthermore, it has been extensively employed in the extraction and separation of biological constituents. Li X. et al. (2019) obtained flavonoids from P. cyrtonema using biphasic-ultrasonic extraction. The optimal extraction parameters were ultrasonication time of 32 min, (NH4)2SO4 amount of 0.39 g/mL, solid-liquid ratio of 24:1. The yield of flavonoids was 6.21%. The samples showed excellent anti-oxidant activity, and could inhibit the lipid autoxidation ability in mouse liver tissue.
Microwave-assisted extraction
The microwave-assisted method represents a relatively innovative approach that employs the instantaneous penetration of electromagnetic waves generated with microwave energy for the purpose of extraction. During this process, the application of microwave energy can result in an increase in temperature within the cells, which subsequently leads to expansion of cell wall. When the pressure exceeds the tolerance threshold of cell wall, cell wall ruptures, resulting in the release of flavonoids from the cells (Guo X. D. et al., 2022). In comparison to the solvent extraction method, microwave-assisted method exhibits the advantages of a shorter extraction time and higher efficiency. Nevertheless, as with the ultrasonic-assisted method, the microwave-assisted method also results in the destruction of flavonoids. Furthermore, the cost of necessary microwave equipment is high (Wei et al., 2024). Li et al. (2014) extracted flavonoids from P. sibiricum using microwave-assisted method. The findings demonstrated that the optimal extraction yield (0.92%) for total flavonoids was obtained under the conditions of microwave powder of 350 W, solid-liquid ratio 70 g/mL, ethanol concentration of 60%, extraction time of 80 s. Teng et al. (2017) also employed microwave-assisted extraction technique to extract flavonoids and polysaccharides derived from P. sibiricum, optimizing the process conditions. The findings indicated that the optimal conditions for extracting Polygonatum polysaccharide and Polygonatum flavonoids were: microwave time 2.5 min, solid-liquid ratio 1:70 g/mL, extraction time 90 min, extraction temperature 70°C. Under the optimized parameters, polysaccharide and flavonoids extraction rate was 69.54 mg/g, corresponding to a yield of 186.35 mg/g.
Purification of Polygonatum flavonoids
In order to obtain a higher purity of flavonoids, the purification process is of great importance. At present, many techniques have been employed to purify flavonoids from nature products, such as membrane separation, column chromatography (macroporous resin, silica gel column, preparative high performance liquid chromatography, polyamide column, sephadex column, high speed counter-current chromatography, etc.) (Gvazava and Kikoladze, 2011; Li et al., 2024; Zhou et al., 2015). As far as we know, the main purification method for flavonoids in the genus Polygonatum is macroporous resin technique. Macroporous resin exhibits a strong adsorption and desorption capacity. Once the sample solution is uploaded onto the macroporous column, the water-soluble impurities are eluted with water initially. Subsequently, the targets are eluted with a series of ethanol solutions of varying concentrations. Finally, different polar components can be obtained. Xia et al. (2021a,b) carried out the adsorption and desorption studies of P. odoratum flavonoids using three kinds of macroporous resins (HPD826, D101, and AB8). The findings revealed that D101 exhibited the highest adsorption/desorption abilities, with AB8 and HPD826 demonstrating progressively lower capacities. The recycling process for DESs yielded an extraction rate of 10.78 mg/g, indicating a reuse rate of 93.98%. The authors indicated that the enrichment using the combination of DESs with macroporous resin represented a potential approach for the effective purification of flavonoids derived from the genus Polygonatum. Recently, Wang et al. (2024a) also studied the adsorption and desorption capacity of flavonoids from P. kingianum using seven kinds of macroporous resins (AB-8, NKA-II, NKA-9, D101, HPD400, HP-20, and X-5). AB-8 macroporous resin showed the most effective purification of flavonoids. The optimal conditions were as follows: a 40 g/L crude extract was applied to the column, and 70% ethanol was eluted at 2.0 g/L. Under these conditions, the purity of flavonoids from P. kingianum was increased to 5.31%, which was 11.8 times of the content of crude extract. The study indicated that the purification of flavonoids derived from P. kingianum using AB-8 macroporous resin was a suitable method. The conclusion also was supported by many other studies (Du et al., 2021; Shu et al., 2009). In other investigation, Zhou et al. (2015) successfully isolated three novel homoisoflavonoids from P. odoratum through the technique of high-speed countercurrent chromatography combined with Sephadex LH-20 CC. All of the obtained homoisoflavonoids exhibited excellent antioxidant properties.
Effect of processing methods on flavonoids from the genus Polygonatum
Steam-bask processing
In order to mitigate the irritation to the throat and enhance its therapeutic effect, it is necessary to process the genus Polygonatum rhizome prior to use or consumption. A variety of processing techniques for the genus Polygonatum, for example steaming, co-processing with auxiliary materials (wine, black bean, and honey), and nine steam-nine bask processing, have been investigated (Table 5) (Luo et al., 2022; Xu P. et al., 2023; Xu Y. L. et al., 2023; Yao et al., 2022). These processes would result in the alterations to the appearance, taste, chemical composition and pharmacological activity of the genus Polygonatum (Li X. et al., 2023; Li Y. et al., 2023; Liang H. H. et al., 2022; Liang Z. et al., 2022; Zhou L. et al., 2024). Among these processing methods, nine steam-nine bask processing has been extensively employed at present. The total flavonoid content and activities of the samples are found to vary significantly with the processing cycle numbers. Generally, the content of total flavonoids increased throughout nine steam-nine bask processing procedure (Pan et al., 2021; Wu et al., 2023; Zhang et al., 2021; Zhou L. et al., 2024). Pan et al. (2021) observed that the flavonoids content was enhanced with the prolongation of steaming-dying cycles, reaching the highest in the ninth steaming-drying cycle. Additionally, Wang et al. (2024b) reported that the flavonoids content was ranged from 0.10 to 0.82% during the process. At the eighth steaming cycle, the flavonoids level reached the maximum. At the same time, DPPH and FRAP reached a maximum of 81.95% and 1.97 mmol/L in the eighth cycle, representing a 50.92% and 1.72 mmol/L increase, respectively, compared to the first cycle. However, Wu et al. (2023) found that the flavonoids content reached the highest in the fourth steaming cycle. The different results might be due to the different pre-treatment for the genus Polygonatum. Recently, Sun et al. (2023) reported that various processing times (two steam-two bask, nine steam-nine bask) had notable impacts on the ingredient and metabolites of the genus Polygonatum. The flavonoids content, including baicalein, disporopsin, and isoliquiritigenin, exhibited a decline with the prolongation of treatment period. Conversely, the 3′-methoxydaidzein content demonstrated an initial increase, followed by a subsequent decline. The data based on network pharmacological analysis revealed that the compounds of 4,5-dihydroxyflavone and liquiritigenin activated PLA2 enzyme, thereby promoting the synthesis of lysophosphatidylcholine. The nine steam-nine bask method was found to be beneficial with regard to the role of both two constituents.
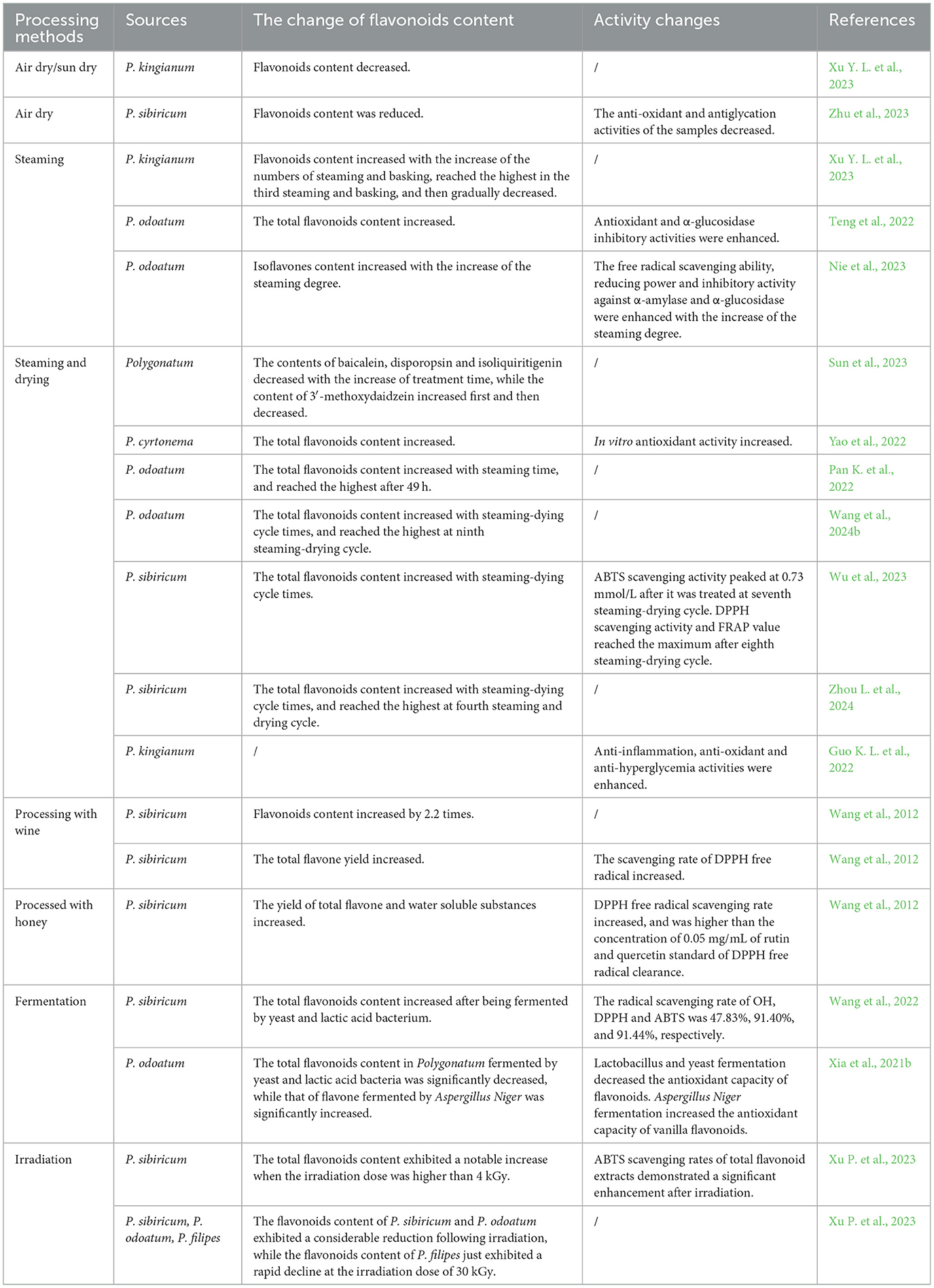
Table 5. Effects of processing methods on flavonoids content of Polygonatum rhizome and its activities.
The traditional nine steam-nine bask method is a labor-intensive process. Studies have been conducted to optimize this process and enhance the efficacy. Yao et al. (2022) carried out the impact of nine steam-nine bask cycles on the functional components of Polygonatum. The obtained data revealed that an increase in the number of nine steam-nine bask cycles resulted in a notable enhancement in the total flavonoids concentration and antioxidant capacity of Polygonatum in vitro. The authors also suggested that four cycles of steaming and basking (four steaming and four basking) might be sufficient to maintain the active component of Polygonatum, thereby negating the necessity for nine cycles of steaming and basking. The findings of this study indicated that the traditional nine steam-nine bask method could be optimized to reduce processing cycle numbers, while maintaining or enhancing its medicinal composition and biological activity. In order to improve processing efficacy, Liang H. H. et al. (2022) investigated effects of nine steam-nine bask processing under high pressure steam boiler on chemical constituents of P. cyrtonema. 3-6 steaming times of P. cyrtonema under high pressure were recommended.
Besides nine steaming-nine basking processing, both steaming and processing with wine are also investigated (Ren et al., 2021; Zhu et al., 2021). Nie et al. (2023) investigated the impacts of steaming (100°C, 110°C, 120°C and 130°C for 60 min) on P. cyrtonema rhizome. The findings indicated that the isoflavones concentration increased with the elevation of the steaming temperature. The content of isoflavones in P. cyrtonema rhizome extracts treated at 120°C and 130°C was significantly higher compared to that of raw P. cyrtonema rhizome extracts. At the same time, the free radical scavenging ability, reducing power and inhibitory activity against α-amylase and α-glucosidase of P. cyrtonema rhizome extracts demonstrated an increase with the elevation of steaming temperatures. In the work of Pan K. et al. (2022), it had been found the concentration of total flavonoids from P. cyrtonema increased with the prolongation of steaming time. The content of the total flavonoids reached the highest when P. cyrtonema was steamed after 49 h. In addition, Guo X. D. et al. (2022) analyzed various chemical components of P. sibiricum after processing with wine, and found that total flavonoids content increased by 2.2 times.
Fermentation processing
Fermentation processing, one of traditional Chinese medicine processing technologies, has been demonstrated to enhance the efficacy of the medical materials and reduce the occurrence of adverse effects (Li J. et al., 2019). The chemical ingredients of the genus Polygonatum, both before and after fermentation, are significantly altered by fermentation process. Li J. et al. (2019) identified the chemical components of P. sibiricum before and after fermentation. The authors observed that 62 chemical components were identified before fermentation, while 18 chemical components were identified after fermentation. The flavonoids content of the genus Polygonatum depends on the employed microorganisms after fermentation. Generally, after being treated by fermentation, the flavonoids content is improved, thereby increasing its antioxidant activity (Table 5) (Liu et al., 2023; Wang et al., 2022). Xia et al. (2021b) investigated the impact of bacterial, fungal and yeast fermentation on P. odoratum flavonoids. The findings revealed, in comparison to untreated P. odoratum, the total flavonoids content of P. odoratum fermented with yeast and lactic acid bacteria significantly decreased, whereas the total flavonoids content of P. odoratum fermented with Aspergillus Niger exhibited a significant increase. Concurrently, the anti-lipid peroxidation free radical scavenging abilities of flavonoids extracts were found to be reduced after yeast and lactic acid bacteria fermentation, while DPPH radical scavenging ability, anti-lipid peroxidation ability, and hydroxyl (OH) radical scavenging ability of flavonoids from Aspergillus Niger fermentation broth increased. Recently, Wang et al. (2022) employed a mixed culture of yeast and lactic acid bacteria to ferment P. sibiricum. In comparison to un-fermented P. sibiricum, the contents of polysaccharides, polyphenols and flavonoids of P. sibiricum in the fermentation broth were found to be significantly improved. Additionally, the antioxidant activity of P. sibiricum fermentation broth was higher than that of the un-fermented samples. The radical scavenging rates of OH, DPPH, ABTS for P. sibiricum fermentation broth were 47.83%, 91.40% and 91.44, respectively.
Drying process
After fresh Polygonatum being gathered, many post-harvest treatments, such as drying, are usually employed, which can affect the flavonoids content significantly. Peng et al. (2022) found that the flavonoids content and activity of P. odoratum were significantly affected by the drying method. Of the three drying methods, freeze drying was observed to result in the greatest retention of flavonoids content in P. odoratum to the greatest extent, followed by vacuum drying. The application of hot air drying, particularly at elevated temperatures (100°C), has been observed to result in a notable decline in the flavonoids content. Recently, Zhu et al. (2023) investigated the impact of air-dying and alkali treatment on the quality and antioxidant activity of P. sibiricum. The findings indicated that there was a reduction in flavonoids and polyphenol concentrations in the alcohol extract after post-harvest air-drying. After 9 d of post-harvest air-drying, flavonoids content was reduced by 64.6%. However, alkali treatment exhibited no significant impact on flavonoids concentration and functional activity of P. sibiricum. As a sterilization technique, electron beam radiation has also been employed to extend the storage of the genus Polygonatum. Xu P. et al. (2023) found that when the irradiation dose exceeded 4 kGy, total flavonoids content exhibited a notable increase. Additionally, ABTS scavenging rates of total flavonoid extracts demonstrated a significant enhancement after irradiation. Xu et al. (2021) studied the impact of irradiation on flavonoids in P. sibiricum, P. odoatum and P. filipes. The flavonoids content demonstrated a decline with the increase of irradiation dose due to the flavonoid monomer in different Polygonatum samples. The flavonoids content of P. sibiricum and P. odoatum exhibited a considerable reduction following irradiation, while the flavonoids content of P. filipes just exhibited a rapid decline at the irradiation dose of 30 kGy.
Conclusion
The genus Polygonatum, a time-honored traditional Chinese medicine, is considered as a medicine and food homologous plant in China. The purpose of present study is to provide an overview of the extraction and processing of flavonoids in the genus Polygonatum. A range of the methods for extracting flavonoids have been developed, for instance, solvent extraction, microwave-assisted extraction, ultrasonic-assisted extraction, and enzymatic-assisted extraction. Furthermore, a range of processing methods, including steaming processing, wine processing, honey processing, black bean steaming and nine steam-nine bask processing, have been investigated. These processing techniques have been shown to have a significant impact on the content and bioactivity of flavonoids. Notwithstanding the substantial progress achieved in Polygonatum flavonoids-related research, numerous challenges must be addressed to fully realize it's potential.
The first challenge is that the methodologies for extracting and purifying flavonoids are currently only at the laboratory stage. Moreover, existing flavonoids extraction techniques have certain shortcomings, including relatively low yields. Despite the development of numerous extraction and purification techniques, no definitive conclusions have been reached regarding the optimal parameters for their application. It is recommended that future investigations should focus on the optimization of these methods, and developing green and highly efficient flavonoids extraction technology that can be applied on an industrial production scale.
Secondly, it is widely acknowledged that the genus Polygonatum can be used as a food or medicinal product after a processing stage, for example steaming, wine-steaming, black bean processing, or nine steam-nine bask. Flavonoids content and activities would be altered as a result of different processing techniques. Up to now, the investigations have been carried out to establish a correlation between the flavonoids content and processing techniques. Accordingly, it is necessary to conduct further in-depth investigations into the impact of processing on the structure-activity relationship of flavonoids.
In conclusion, previous studies have provided a comprehensive and solid foundation for the further research of flavonoids. However, in the future, it is imperative to enhance the isolation of Polygonatum flavonoids and identify their structures, carry out in-depth pharmacological investigations, and utilize extensive data analysis to elucidate the critical targets for the treatment of related diseases.
Author contributions
Z-XT: Funding acquisition, Writing – original draft, Writing – review & editing. X-PY: Investigation, Methodology, Writing – original draft. Z-BJ: Investigation, Methodology, Writing – review & editing. Y-XC: Methodology, Validation, Writing – original draft. H-YS: Formal analysis, Validation, Writing – review & editing. Y-YH: Formal analysis, Investigation, Writing – original draft. L-ES: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the High Level Major Research Achievements Training Program of Tourism College of Zhejiang (No. 2024GCC03) and Natural Science Foundation of Zhejiang Province (No. LY22C020001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bi, J. Y., Fang, H. Y., Zhang, J. Y., Lu, L. T., Gu, X., and Zheng, Y. G. (2023). A review on the application, phytochemistry and pharmacology of Polygonatum odoratum, an edible medicinal plant. J. Future Foods 3, 240–251. doi: 10.1016/j.jfutfo.2023.02.006
Bu, Y., Yin, B., Qiu, Z., Li, L., Zhang, B., Zheng, Z., et al. (2024). Structural characterization and antioxidant activities of polysaccharides extracted from Polygonati rhizoma pomace. Food Chem. X 24:101778. doi: 10.1016/j.fochx.2024.101778
Chen, S. C., Yang, C. S., and Chen, J. J. (2022). Main bioactive components and their biological activities from natural and processed rhizomes of Polygonum sibiricum. Antioxidants 11:1383. doi: 10.3390/antiox11071383
Chen, Y., Yao, Y. S., Chen, S. S., Liu, H. C., and Zhao, Z. (2020). Medicinal quality relating to age of Polygonatum cyrtonema hua plant sections. Fujian J. Agric. Sci. 35, 38–43.
Chen, Y. J., Shi, X., Qu, R., and Peng, W. S. (2013). Study on extraction process of flavonoids from Polygonatum kingianum Coll.et Hemsl and antioxidative activities of flavonoids. Sci. Technol. Food Ind. 34, 222–225.
Cheng, W. Q., Pan, Z. B., Zheng, H. J., Luo, G. L., Liu, Z. B., Xu, S. L., et al. (2023). Characterization of phytochemical profile of rhizome of artificial cultured Polygonatum sibiricum with multiple rhizome buds. Appl. Biol. Chem. 66:35. doi: 10.1186/s13765-023-00792-4
Cui, X., Wang, S., Cao, H., Guo, H., Li, Y., Xu, F., et al. (2018). A review: the bioactivities and pharmacological applications of Polygonatum sibiricum polysaccharides. Molecules 23:1170. doi: 10.3390/molecules23051170
Dong, S. Q., Wang, X. F., Li, W. H., Wang, L., Du, H. X., and Yu, Y. (2021). Phytochemical constituents and chemotaxonomic study of Polygonatum prattii Baker. Biochem. Syst. Ecol. 97:104278. doi: 10.1016/j.bse.2021.104278
Dong, W., Shi, H. B., Ma, H., Miao, Y. B., Liu, T. J., and Wang, W. (2010). Homoisoflavanones from Polygonatum odoratum rhizomes inhibit advanced glycation end product formation. Arch. Pharm. Res. 33, 669–674. doi: 10.1007/s12272-010-0504-y
Du, X. Q., Liang, Z. J., Xia, Y., Zhang, X. Q., Zhang, W. C., Han, J. L., et al. (2021). Comparative study on anti-exercise fatigue effects of different extracts from raw materials of Polygonatum cyrtonema Hua and its processed products. J. Anhui Agric. Uinv. 48, 26–30.
Gan, L. S., Chen, J. J., Shi, M. F., and Zhou, C. X. (2013). A new homoisoflavanone from the rhizomes of Polygonatum cyrtonema. Nat. Prod. Commun. 8:1934578X1300800513. doi: 10.1177/1934578X1300800513
Guo, H. J., Zhao, H. X., Kanno, Y., Li, W., Mu, Y. L., Kuang, X. Z., et al. (2013). A dihydrochalcone and several homoisoflavonoids from Polygonatum odoratum are activators of adenosine monophosphate-activated protein kinase. Bioorg. Med. Chem. Lett. 23, 3137–3139. doi: 10.1016/j.bmcl.2013.04.027
Guo, K. L., Liu, J. P., Zhao, C. B., Shi, Y. H., Wang, B., Quan, L. N., et al. (2022). Enzymatic-ultrasonic assisted extraction of total flavonoids from Shaanxi Polygonatum sibiricum and in vitro evaluation of their anti-oxidant and anti-A549 proliferation activities. Nat. Prod. Res. Dev. 34, 630–638.
Guo, X. D., Yung, X. F., Yang, C. G., and Xiao, C. H. (2022). Study on the changes of the active ingredients in Polygonatum sibiricum after the alcoholic preparation. Lishizhen Med. Mater. Med. Res. 33, 1366–1368.
Gvazava, L. N., and Kikoladze, V. S. (2011). Flavonoids from the plants Polygonatum polyanthemum and P. glaberrimum. Chem. Nat. Compd. 47, 818–819. doi: 10.1007/s10600-011-0072-5
Han, Z. G., Gong, Q. Q., Huang, S. Y., Meng, X. Y., Xu, Y., Li, L. G., et al. (2023). Machine learning uncovers accumulation mechanism of flavonoid compounds in Polygonatum cyrtonema Hua. Plant Physiolo. Biochem. 201:107839. doi: 10.1016/j.plaphy.2023.107839
He, L. X., Ding, K., Xie, M. H., Ji, L. Q., and Ge, M. (2019). Study on enzymatic-ultrasonic assisted extraction of total flavonoids from Polygonatum Sibirici and its antioxidant activities. Chin. J. Mod. Appl. Pharm. 36, 1075−1080.
Jiang, C. X., Zhang, T. J., Chen, C. Q., Li, X. K., and Liu, C. X. (2017). Research progress in Polygonati Rhizoma and predictive analysis on Q-marker. Chin. Tradit. Herb. Drugs 48, 1–16.
Jing, L. P., Tang, X. Y., Gu, L. L., Shang, G. L., Li, R. D., Li, Z. L., et al. (2021). Research progress on extraction methods of flavonoids from plants. J. Cellulose Sci. Technol. 29, 60–70.
Khan, H., and Rauf, A. (2015). Phytochemistry of genus polygonatum: a review. Am. J. Biomed. Life Sci. 3, 5–20. doi: 10.11648/j.ajbls.s.2015030201.12
Li, H. M., Zhang, F. M., Xu, P., Cao, Y. X., Zheng, T., Zhao, L. P., et al. (2022). Enzymatic modification and functional quality evaluation of Polygonatum odoratum whole powder. Nat. Prod. Res. Dev. 34, 2098–2109.
Li, J., Wang, Y. Q., Mei, X. D., Liu, Z. H., Song, S. A., Ma, T., et al. (2019). Characterization of chemical constituents in aqueous extracts and fermentation broth from Polygonati Rhizoma by UHPLC-LTQ-Orbitrap MS combined with solid phase extraction. Chin. Tradit. Herb. Drugs 50, 3029–3036.
Li, J., Wang, Z., Fan, M. X., Hu, G. W., and Guo, M. Q. (2022). Potential antioxidative and anti-hyperuricemic components targeting superoxide dismutase and xanthine oxidase explored from Polygonatum sibiricum red. Antioxidants 11:1651. doi: 10.3390/antiox11091651
Li, L., Li, L., Qiu, X., and Ming, G. (2014). Study on extraction of total flavonoids from microwave-assisted route. J. Baoshan Univ. 33, 39–42.
Li, L. H., Ren, F. Z., Chen, S. H., and Gao, Y. Q. (2009). New homoisoflavanones from Polygonatum odoratum (Mill.) druce. Acta Pharm. Sin. 44, 764–767.
Li, L. H., Ren, F. Z., Chen, S. H., Zheng, Z. H., Gao, Y. Q., Zhang, H., et al. (2008). “Study on the new homoisoflavonoids and their bioactivity from Polygonatum odoratum (Mill.) druce (H. P. P. s. G. Chinese Pharmaceutical Association, Trans.),” in 2008 Chinese Pharmaceutical Association Academic Annual Meeting and the 8th Chinese Pharmacist Week (Heibei: People's Health Publication Press), p. 5.
Li, L. H., Ren, F. Z., Zheng, Z. H., Chen, S. H., Gao, Y. Q., and Zhu, X. L. (2012). Studies on biological activity of homoisoflavanones from Polygonatum odoratum (Mill.) Druce. J. Hebei Norm. Univ. 36, 509–511.
Li, Q., Zeng, J., Gong, P., Wu, Y., and Li, H. (2021). Effect of steaming process on the structural characteristics and antioxidant activities of polysaccharides from Polygonatum sibiricum rhizomes. Glycoconjugate J. 38, 561–572. doi: 10.1007/s10719-021-10013-z
Li, W., Luo, H., Zhang, H., Zhang, P., and Liu, S. (2024). The chemical compounds of homoisoflavonoids in Polygonatum hunanese. J. Anhui Tradit. Chin. Med. Coll. 43, 102–108.
Li, X., Mei, M. J., Pu, X. M., Chen, X. J., Li, X. F., Meng, F. Y., et al. (2023). Protective effect and mechanism of Polygonatum kingianum against hypoxia-induced injury. Heliyon 9:e14353. doi: 10.1016/j.heliyon.2023.e14353
Li, X., Yang, X., Li, A., and Wu, D. (2019). Study on ultrasonic-assisted aqueous two-phase extraction of flavonoids from Polygonatum kingianum and its antioxidant activity. Jiangsu Agric. Sci. 47, 234–238.
Li, Y., Su, Y., Yuan, W. Q., Yang, S. T., Huang, J. Y., Li, P., et al. (2023). Advances in characterizing the main active components, functions, and mechanisms of Polygonatum species. Mod. Food Sci. Technol. 39, 354–363.
Liang, H. H., Jia, Q. Q., Zhu, L. Z., Huang, L. L., and Zhu, P. L. (2022). Changes of chemical constitutes in the steaming process of Polygonatum cyrtnema from different provenances. South China Forest. Sci. 50, 1–5.
Liang, Z., Pan, Y., Qiu, L., Wu, X., Xu, X., Shu, Y., et al. (2022). Analysis on chemical components changes of Polygonati Rhizoma in processing of nine times steaming and nine times sunning by UPLC-Q-TOF-MS/MS. Chin. Tradit. Herb. Drugs 53, 4948–4957.
Lin, H. R. (2015). Two homoisoflavonoids act as peroxisome proliferator-activated receptor agonists. Med. Chem. Res. 24, 2898–2905. doi: 10.1007/s00044-015-1343-7
Lin, H. Y., Wang, W. H., Peng, M. Q., Kong, Y. F., Zhang, X. W., Wei, X. H., et al. (2024). Pharmacological properties of Polygonatum and its active ingredients for the prevention and treatment of cardiovascular diseases. Chin. Med. 19:1. doi: 10.1186/s13020-023-00871-0
Liu, H., Wan, P., Sun, J., Liu, X., Yang, Z., Ma, Y., et al. (2023). Processing technology optimization and functional analysis of Polygonatum sibiricum extract fermented soymilk. Food Res. Dev. 44, 106–115.
Liu, J. J., and Si, J. P. (2018). Herbal textual research on Chinese medicine “Huangjing” (Polygonati Rhizoma) and some enlightenments. China J. Chin. Mater. Med. 43, 631–636.
Liu, Q. H., Gao, H. Y., Chen, G. F., Hua, B. C., and Wang, Y. H. (2019). Optimization of flavonoids extraction process from Polygonatum cyrtonema Hua and visualization analysis. Chin. J. Inform. Tradit. Chin. Med. 26, 89–93.
Long, J., Hu, X., Zhou, B., Wei, G., and Wang, X. (2020). Ultrasonic assisted extraction of total flavonoids from genuine Polygonatum Mill. from Qiandongnan. Guizhou Life Sci. 38, 28–33.
Luo, L., Qiu, Y. X., Gong, L. M., Wang, W., and Wen, R. D. (2022). A review of Polygonatum Mill. genus: its taxonomy, chemical constituents, and pharmacological effect due to processing changes. Molecules 27:4821. doi: 10.3390/molecules27154821
Mei, X., Xia, J., Li, W., Wu, Y., Cheng, H., Chen, S., et al. (2025). Glycan degradation in Polygonati Rhizoma: effect of traditional “nine steaming and nine basking” on low molecular weight fructans and polysaccharides. Food Chem. X 25:102131. doi: 10.1016/j.fochx.2024.102131
Mu, C., Sheng, Y., Wang, Q., Amin, A., Li, X., and Xie, Y. (2021). Potential compound from herbal food of Rhizoma Polygonati for treatment of COVID-19 analyzed by network pharmacology: viral and cancer signaling mechanisms. J. Funct. Foods 77:104149. doi: 10.1016/j.jff.2020.104149
Nguyen, H. C., Ngo, K. N., Tran, H. K., and Barrow, C. J. (2024). Enzyme-assisted coextraction of phenolics and polysaccharides from padina gymnospora. Mar. Drugs 22:24. doi: 10.3390/md22010042
Nguyen, H. C., Nguyen, H. N. T., Huang, M. Y., Lin, K. H., Pham, D. C., Tran, Y. B., et al. (2021). Optimization of aqueous enzyme-assisted extraction of rosmarinic acid from rosemary (Rosmarinusofficinalis L.) leaves and the antioxidant activity of the extract. J. Food Process Pres. 45, 1–10. doi: 10.1111/jfpp.15221
Ni, T. Y., Luo, X. M., Zhang, C. C., Yu, B., Zhang, S. L., and Fan, H. Y. (2020). Comparative analysis on chemical constituents of Polygonatum cyrtonema from different growing areas. Chin. Tradit. Pat. Med. 42, 2948–2953.
Nie, R., Wu, C., Zhang, X., and Deng, P. (2024). Identification markers responsible for differentially processed Polygonatum cyrtonema Hua by ultra-performance liquid chromatography with quadruple time-of-flight mass spectrometry. Molecules 29:1559. doi: 10.3390/molecules29071559
Nie, X. H., Wang, L. Y., Wang, S. Y., Yu, N. X., Lu, Y. C., Lyu, W., et al. (2023). In vitro hypoglycemic and antioxidant activities of steamed Polygonatum cyrtonema Hua with various steaming degrees: relationship with homoisoflavonoids. Food Biosci. 53:102518. doi: 10.1016/j.fbio.2023.102518
Pan, K., Li, D., Wang, H., Chen, S., Luo, C., and Li, J. (2021). Effect of nine steaming and nine processing systems on the quality of Polygonatum cyrtonema Hua in different ages. Spec. Wild Econ. Animal Plant Res. 43, 23–27.
Pan, K., Wang, H., Li, D., Chen, S., Wang, T., and Li, J. (2022). Effect of continuous steaming on the quality of Polygonatum cyrtonema Hua at different ages. Storage Proc. 22, 42–48.
Pan, M., Wu, Y., Sun, C., Ma, H., Ye, X., and Li, X. (2024). Polygonati Rhizoma: a review on the extraction, purification, structural characterization, biosynthesis of the main secondary metabolites and anti-aging effects. J. Ethonopharmacol. 327:118002. doi: 10.1016/j.jep.2024.118002
Pan, Z. B., Cheng, W. Q., Liu, Z. B., Wu, W. B., Yang, B., and Lin, J. H. (2022). Comparative study of the phytochemical profiles of the rhizomes of cultivated and wild-grown Polygonatum sibiricum. Separations 9:398. doi: 10.3390/separations9120398
Park, U. H., Jeong, J. C., Jang, J. S., Sung, M. R., Youn, H., Lee, S. J., et al. (2012). Negative regulation of adipogenesis by kaempferol, a component of Rhizoma Polygonati falcatum in 3T3-L1 cells. Biol. Pharm. Bull. 35, 1525–1533. doi: 10.1248/bpb.b12-00254
Peng, X. W., Peng, Y. L., He, X. H., Kan, H., Deng, J., Zhao, P., et al. (2022). Effects of different drying methods on the drying dynamic characteristics and quality of Polygonatum odoratum. J. Cent. South Uinv. Forest. Technol. 42, 164–172.
Qi, J. J., Wei, J. H., Liao, D. Q., Ding, Z. M., Yao, X., Sun, P., et al. (2022). Untargeted metabolomics analysis revealed the major metabolites in the Seeds of four Polygonatum species. Molecules 27:1445. doi: 10.3390/molecules27041445
Qian, S., Jin, H.: Wei, M., and Cai, R. (2017). Extraction of Polygonati rhizoma flavonoids by response surface methodology and antioxidant activities. J. Anhui Polytech. Uinv. 32, 8–14.
Ren, H. M., Zhang, J. L., Deng, Y. L., Ye, X. W., Xia, L. T., Liu, M. M., et al. (2021). Analysis of chemical constitutions of Polygonatum cyrtonema dried rhizomes before and after processing with wine based on UPLC-Q-TOF-MS. Chin. J. Exp. Tradit. Med. Form. 27, 110–121.
Ren, J. L., Luan, M. B., Deng, X., and Zhen, W. (2023). Active ingredients and antioxidant activity of Polygonatum spp. rhizome with 11 geographical indications. J. Zhejiang For. Sci. Technol. 43, 39–44.
Sharma, S., Joshi, R., and Kumar, D. (2020). Quantitative analysis of flavonols, flavonol glycoside and homoisoflavonoids in Polygonatum verticillatum using UHPLC-DAD-QTOF-IMS and evaluation of their antioxidant potential. Phytochem. Anal. 31, 333–339. doi: 10.1002/pca.2899
Sharma, S., Patial, V., Singh, D., Sharma, U., and Kumar, D. (2018). Antimicrobial homoisoflavonoids from the Rhizomes of Polygonatum verticillatum. Chem. Biodivers. 15:e1800430. doi: 10.1002/cbdv.201800430
Shen, J., Pu, W., Song, Q., Ye, B., Shi, X., Chen, Y., et al. (2024). Traditional processing can enhance the medicinal effects of Polygonatum cyrtonema by inducing significant chemical changes in the functional components in its rhizomes. Pharmaceuticals 17:1074. doi: 10.3390/ph17081074
Shen, W. D., Li, X. Y., Deng, Y. Y., Zha, X. Q., Pan, L. H., Li, Q. M., et al. (2021). Polygonatum cyrtonema Hua polysaccharide exhibits anti-fatigue activity via regulating osteocalcin signaling. Int. J. Biol. Macromol. 175, 235–241. doi: 10.1016/j.ijbiomac.2021.01.200
Sheng, W., Xu, Z., and Wang, X. (2021). Effects of different cultivation patterns on Polygonatum cyrtonema under the camellia oleifera forest. Hubei Forest. Sci. Technol. 50, 22–24.
Shu, X. S., Lv, J. H., Tao, J., Li, G. M., Li, H. D., and Ma, N. (2009). Antihyperglycemic effects of total flavonoids from Polygonatum odoratum in STZ and alloxan-induced diabetic rats. J. Ethnopharmacol. 124, 539–543. doi: 10.1016/j.jep.2009.05.006
Singh, S. K., Singh, S., Verma, S. K., Jain, P., Dixit, V. K., and Solanki, S. (2013). A review on plants of genus Polygonatum. Int. J. Res. Dev. Pharm. Life Sci. 2, 387–397.
Su, H., He, L., Yu, X., Wang, Y., Yang, L., Wang, X., et al. (2024). Structural characterization and mechanisms of macrophage immunomodulatory activity of a novel polysaccharide with a galactose backbone from the processed Polygonati Rhizoma. J. Pharm. Anal. 14:100974. doi: 10.1016/j.jpha.2024.100974
Sun, Y., Zhou, L., Shan, X., Zhao, T. T., Cui, M. R., Hao, W. Q., et al. (2023). Untargeted components and in vivo metabolites analyses of Polygonatum under different processing times. LWT Food Sci. Technol. 173:114334. doi: 10.1016/j.lwt.2022.114334
Suyal, R., Rawat, S., Rawal, R. S., and Bhatt, I. D. (2019). Variability in morphology, phytochemicals, and antioxidants in Polygonatum verticillatum (L.) All. populations under different altitudes and habitat conditions in western himalaya, India. Environ. Monit. Assess. 191:783. doi: 10.1007/s10661-019-7687-6
Tao, A. E., Zhang, X. C., Du, Z. F., Zhao, F. Y., Xia, C. L., and Duan, B. Z. (2018). Research progress on flavonoids in plants of Polygonatum Mill. and their pharmacological activities. Chin. Tradit. Herb. Drugs 49, 2163–2171.
Teng, H. H., Wang, R. Z., Wu, D. L., Jin, C. S., Liu, C. F., Tang, X., et al. (2022). The study on the antioxidant and hypoglycemic activities of different polar extracts from crude and steam-processed Polygonatum cyrtonema Hua. Food Fermentation Ind. 48, 70–75.
Teng, S. R., Liao, L. J., Wu, Y., Jiang, N., and Zheng, X. J. (2017). Optimization of extraction technology of polysaccharides and flavonoids from Polygonatum sibiricum and effects of selenium fertilizer on its content. Hubei Agric. Sci. 56, 4572–4576.
Wang, D., Li, D., Zhu, W., and Peng, P. (2009). A new C-methylated homoisoflavanone and triterpenoid from the rhizomes of Polygonatum odoratum. Nat. Prod. Res. 23, 580–589. doi: 10.1080/14786410802560633
Wang, D. M., Lu, Z. J., Wang, Y. H., and Zhang, H. H. (2012). Effect of different processing methods on the yield of extracts and antioxidant activities of Polygonatum odoratum. Bull. Bot. Res. 32, 621–626.
Wang, D. M., Zeng, L., Li, D. W., and Pu, W. J. (2013). Antioxidant activities of different extracts and homoisoflavanones isolated from the Polygonatum odoratum. Nat. Prod. Res. 27, 1111–1114. doi: 10.1080/14786419.2012.701212
Wang, H. J., Fowler, M. I., Messenger, D. J., Terry, L. A., Gu, X. L., Zhou, L. X., et al. (2018). Homoisoflavonoids are potent Glucose Transporter 2 (GLUT2) inhibitors: a potential mechanism for the glucose-lowering properties of Polygonatum odoratum. J. Agric. Food Chem. 66, 3137–3145. doi: 10.1021/acs.jafc.8b00107
Wang, J., Li, M., Fan, B., Cui, W., Wang, Q., Lu, C., et al. (2024a). Optimization of purification process of flavonoids from P. kingianum Coll. by macroporous resin and comparison of antioxidant activity before and after purification. Feed Res. 7, 74–81.
Wang, J., Lu, Q., Xue, X., Zhang, C., and Yang, D. (2024b). Effects of nine steaming-nine drying on the physicochemical properties and antioxidant activity of Polygonatum sibiricum red. Mod. Food Sci. Technol. 40, 231–245.
Wang, M., Hu, J., Hai, X., Cao, T., Zhou, A., Han, R., et al. (2024). Quality evaluation of Polygonatum cyrtonema Hua based on UPLC-Q-Exactive Orbitrap MS and electronic sensory techniques with different numbers of steaming cycles. Foods 13:1586. doi: 10.3390/foods13101586
Wang, R., Qian, C., Zheng, P., Li, F., Wang, Z., Wang, H., et al. (2022). Optimization of preparation technology and its antioxidant activity analysis of Polygonatum sibiricum fermentation broth. China Brewing 41, 153–158.
Wang, W. X., Dabu, X. L. T., He, J., Yang, H. X., Yang, S. C., Chen, J. W., et al. (2019). Polygonatone H, a new homoisoflavanone with cytotoxicity from Polygonatum Cyrtonema Hua. Nat. Prod. Res. 33, 1727–1733. doi: 10.1080/14786419.2018.1434645
Wang, Y., Li, F., Huang, M., Chen, Y., Lu, J., and Qian, J. (2023). Optimization of enzymatic hydrolysis conditions and antioxidation of Polygonatum sibiricum instant tea. Food Res. Dev. 44, 139–145.
Wang, Y., Xie, W., Hu, L., and Zhang, J. (2017). Optimization of extraction technology for Polygonatum sibiricum Red. by central composite design-response surface method. Shaanxi J. Tradit. Chin. Med. 38, 396–397.
Wang, Y. F., Mu, T. H., Chen, J. J., and Luo, S. D. (2003). Studies on chemical constituents from the root of Polygonatum kingianum. China J. Chin. Mater. Med. 28, 524–527.
Wang, Y. F., Zhang, Z. X., He, R. J., Yang, B. Y., Wang, L., and Huang, Y. L. (2020). Study on the chemical constituents of the aerial parts of Polygonatum sibiricum and its pancreatic lipase inhibitory activity. Nat. Prod. Res. Dev. 32, 1811–1817.
Wang, Y. J., Liu, N., Xue, X., Li, Q., Sun, D. P., and Zhao, Z. X. (2020). Purification, structural characterization and in vivo immunoregulatory activity of a novel polysaccharide from Polygonatum sibiricum. Int. J. Biol. Macromol. 160, 688–694. doi: 10.1016/j.ijbiomac.2020.05.245
Wei, D., Sun, Y., Wang, Z., and Kuang, H. (2024). Research process on extraction and isolation of flavonoids from Astragalus membranaceus. Chem. Eng. 12, 58–62.
Wei, G., Wu, C., Hu, X., Long, F., and Zhang, Q. (2023). Optimization of extraction process and antioxidant study on total flavonoids from Polygonatum sibiricum. Guangzhou Chem. Ind. 51, 127–130.
Wu, K., Wang, F., Chang, H., Liang, Z., Yang, Q., Ma, C., et al. (2021). Effects of different cultivation methods on the accumulation of main chemical constituents of four-year-old Polygonatum sibiricum. Chin. Tradit. Pat. Med. 43, 2433–2437.
Wu, L., Huang, N., Xiong, M., Huang, J., Zhao, H., Xie, W., et al. (2023). Comparison study on the effect of nine-steaming and nine-system process on the active components in genus Polygonatum. Technol. Innov. Appl. 20, 49–53.
Wujisguleng, W., Liu, Y., and Long, C. (2012). Ethnobotanical review of food uses of Polygonatum (convallariaceae) in China. Acta Soc. Bot. Pol. 81, 239–244. doi: 10.5586/asbp.2012.045
Xia, G. H., Li, X. H., and Jiang, Y. H. (2021a). Deep eutectic solvents as green media for flavonoids extraction from the rhizomes of Polygonatum odoratum. Alex. Eng. J. 60, 1991–2000. doi: 10.1016/j.aej.2020.12.008
Xia, G. H., Li, X. H., Zhang, Z., and Jiang, Y. H. (2021b). Effects of fermentation treatments on Polygonatum odoratum flavones' antioxidant activities. Saudi J. Biol. Sci. 28, 5011–5016. doi: 10.1016/j.sjbs.2021.01.026
Xiang, Z., Chen, P., and Liu, Y. (2024). Research progress on active ingredients of Polygonatum cyrtonema Hua. J. Chongqing Techno. Bus. Univ. 41, 1–11.
Xu, H., Dai, L., Deng, P. F., and Xu, X. N. (2022). Study on the anti-inflammatory active components and mechanism of Polygonati-rhizoma based on network pharmacology. J. Anhui Agr. Univ. 49, 144–149.
Xu, P., Jia, X., Gao, P., Meng, A., Wu, G., Huang, M., et al. (2023). Analysis of the effect of electron beam irradiation on antioxidant activity of Polygonati rhizoma based on orthogonal partial least squares-discriminant. J. Food Saf. Qual. 14, 274–281.
Xu, S., Qu, Z., Bi, J., Li, T., Gan, X., Wei, G., et al. (2021). Optimization of extraction by BP-ANN and determination of flavonoids in three varieties of Polygonati rhizoma before and after irradiation by HPLC chromatographic fingerprint. Sci. Technol. Food Ind. 42, 257–264.
Xu, Y. L., Yang, M. Q., Yang, T. M., Yang, W. Z., Wang, Y. Z., and Zhang, J. Y. (2023). Untargeted GC-MS and FT-NIR study of the effect of 14 processing methods on the volatile components of Polygonatum kingianum. Front. Plant Sci. 14:1140691. doi: 10.3389/fpls.2023.1140691
Xue, M., Guo, K., Yuan, P., Liu, J., Shi, J., Guo, Y., et al. (2023). Extraction optimization and antioxidant activities of total flavonoid from alcoholic polygonatum. Cent. South Pharm. 21, 351–355.
Yang, G., Jiang, D., Huang, L. J., Cui, C., Yang, R., Pi, X., et al. (2024). Distinct toxic effects, gene expression profiles, and phytohormone responses of Polygonatum cyrtonema exposed to two different antibiotics. J. Hazard. Mater. 466:133639. doi: 10.1016/j.jhazmat.2024.133639
Yang, L., Yang, Q., Zhang, L., Ren, F., Zhang, Z., and Jia, Q. (2024). Integrated metabolomics and transcriptomics analysis of flavonoid biosynthesis pathway in Polygonatum cyrtonema Hua. Molecules 29:2248. doi: 10.3390/molecules29102248
Yang, Z., Tan, H., Chen, Q., Hu, X., Wu, Y., and Chen, Z. (2020). Optimization of extraction technology of effective components from upper stem and leaf of Polygonatum sibiricum by orthogonal method. Hubei Agric. Sci. 59, 139–144.
Yao, X. J., Deng, Z. Y., Li, H. Y., and Zhang, B. (2022). Effect of processing cycles on the composition of Polygonatum cyrtonema Hua during nine-steam-nine-bask processing. Food Biosci. 50:102081. doi: 10.1016/j.fbio.2022.102081
Ye, X., Hu, Y., Chen, Y., Tang, Z., Jiang, Z., Fu, Y., et al. (2025). Flavonoids from the genus Polygonatum: biological activities and biosynthesis mechanism. Front. Nutr. 12:1574182. doi: 10.3389/fnut.2025.1574182
Yu, J. Q., Chen, W. X., Zhao, L., Yue, T., Yang, W. C., and Wang, X. (2022). Efficient separation of anti-inflammatory isolates from Polygonti rhizome by three different modes of high-speed counter-current chromatography. J. Sep. Sci. 45, 4012–4022. doi: 10.1002/jssc.202200545
Zeng, R. X., Chen, L., Lan, Y. K., and Lei, K. J. (2020). Comparison of microwave extraction versus reflux extraction for melastoma dodecandrum. China Pharm. 29, 39–41.
Zhang, C. H., Lin, Z. L., Li, B. Y., Hua, W. P., Chen, W. H., and Yang, B. (2018). Content analysis and bioactivity evaluation of the total flavonoids extracted from Polygonatum cyrtonema cultivated under forest in north fujian. Nat. Prod. Res. Dev. 30, 225–231.
Zhang, H., Yang, F., Qi, J., Song, X. C., Hu, Z. F., Zhu, D. N., et al. (2010). Homoisoflavonoids from the fibrous roots of Polygonatum odoratum with glucose uptake-stimulatory activity in 3T3-L1 adipocytes. J. Nat. Prod. 73, 548–552. doi: 10.1021/np900588q
Zhang, H., Zheng, H., Zeng, Y., Luo, X., Zhu, J., Tan, J., et al. (2021). Analysis of main components in the processing of Polygonatum cyrtonema Hua. Guangdong Chem. Ind. 48, 5–7.
Zhang, H. L., Hao, F. L., Yao, Z. F., Zhu, J. L., Jing, X., and Wang, X. W. (2022). Efficient extraction of flavonoids from Polygonatum sibiricum using a deep eutectic solvent as a green extraction solvent. Microchem. J. 175:107168. doi: 10.1016/j.microc.2021.107168
Zhang, J., Liang, S., Xiao, Q., and Cui, L. (2024). Extraction and antioxidant activity of total flavonoids of Polygonatum sibiricum. Hubei Forest. Sci. Technol. 53, 37–44.
Zhang, J., Zhang, R., and Ning, W. (2015). Study on polysaccharide content and extraction of total flavonoids in Polygonatm macropodium Turcz. Lishizhen Med. Mater. Med. Res. 26, 2870–2871.
Zhang, K., Zhang, Z., Fan, Y. F., and Zhang, B. G. (2022). Nutritional and bioactive compounds of Polygonati rhizoma and Polygonati rhizoma with medicinal and edible property. Mod. Chin. Med. 24, 1463–1472.
Zhang, T. L., Zhou, D., Chen, M. F., Zou, H., Tang, Q., Lu, Y., et al. (2023). Effects of the fibrous root of Polygonatum cyrtonema Hua on growth performance, meat quality, immunity, antioxidant capacity, and intestinal morphology of white-feathered broilers. Antibiotics 12:1627. doi: 10.3390/antibiotics12111627
Zhao, P., Zhao, C., Li, X., Gao, Q., Huang, L., Xiao, P., et al. (2018). The genus Polygonatum: a review of ethnopharmacology, phytochemistry and pharmacology. J. Ethonopharmacol. 214, 274–291. doi: 10.1016/j.jep.2017.12.006
Zhao, X. Y., and Li, J. (2015). Chemical Constituents of the genus Polygonatum and their role in medicinal treatment. Nat. Prod. Commun. 10, 683–688. doi: 10.1177/1934578X1501000439
Zheng, M., Su, H., Xiao, R., Chen, J., Chen, H., Tan, K. B., et al. (2023). Effects of Polygonatum cyrtonema extracts on the antioxidant ability, physical and structure properties of carboxymethyl cellulose-xanthan gum-flaxseed gum active packaging films. Food Chem. 403:134320. doi: 10.1016/j.foodchem.2022.134320
Zhou, L., Liu, T., Yan, T., Yang, M., Wang, P., and Shi, L. (2024). Nine steaming nine sun-drying processing enhanced properties of Polygonatum kingianum against inflammation, oxidative stress and hyperglycemia. J. Sci. Food Agric. 104, 3123–3138. doi: 10.1002/jsfa.13203
Zhou, P., Song, C., Chi, S., and Feng, J. (2022). Extraction technology of traditional Chinese medicine. J. Trad. Chin. Vet. Med. 41, 37–43.
Zhou, T., Wang, G., Zhen, F., Zhang, Q., Zhang, Z., Qu, B., et al. (2024). Study on efficient extraction of saponins from Polygonatum sibiricum by enzyme assisted cold isostatic processing technology. Ind. Crop Prod. 220:119163. doi: 10.1016/j.indcrop.2024.119163
Zhou, X. L., Zhang, Y. P., Zhao, H. D., Liang, J. S., Zhang, Y., and Shi, S. Y. (2015). Antioxidant homoisoflavonoids from Polygonatum odoratum. Food Chem. 186, 63–68. doi: 10.1016/j.foodchem.2015.02.058
Zhu, S., Liu, P., Wu, W., Li, D., Shang, E., Guo, S., et al. (2022). Multi-constituents variation in medicinal crops processing: investigation of nine cycles of steam-sun drying as the processing method for the rhizome of Polygonatum cyrtonema. J. Pharmaceut. Biomed. Anal. 209:114497. doi: 10.1016/j.jpba.2021.114497
Zhu, X., Wang, F., Su, X., Li, Q., Guo, H., Sun, Z., et al. (2023). Effects of post-harvest air-drying and alkali treatment on the quality and antioxidant activity of Polygonatum sibiricum. Food Res. Dev. 44, 38–45.
Zhu, Z., Yu, L., Chen, M., Yu, X., Wang, Z., and Jiang, J. (2021). Effect of the different wine preparation methods on the final product quality of Polygonatum cyrtonema. Shaanxi Forest Sci. Technol. 49, 9–13.
Keywords: Polygonatum, flavonoids, extraction, nine steam-nine bask processing, fermentation
Citation: Tang Z-X, Ye X-P, Jiang Z-B, Chen Y-X, Shen H-Y, Hu Y-Y and Shi L-E (2025) Recent advances in flavonoids from the genus Polygonatum: extraction and processing methods. Front. Sustain. Food Syst. 9:1569034. doi: 10.3389/fsufs.2025.1569034
Received: 31 January 2025; Accepted: 09 June 2025;
Published: 25 June 2025.
Edited by:
Cristina Rocha, University of Minho, PortugalReviewed by:
Serena Carpentieri, University of Salerno, ItalyCatarina Teixeira, University of Minho, Portugal
Copyright © 2025 Tang, Ye, Jiang, Chen, Shen, Hu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Xing Tang, dGFuZ3poZW54aW5nQDEyNi5jb20=; Lu-E Shi, c2hpbHVlQGh6bnUuZWR1LmNu
 Zhen-Xing Tang
Zhen-Xing Tang Xin-Pei Ye2
Xin-Pei Ye2 Lu-E Shi
Lu-E Shi