- 1Faculty of Engineering, Huanghe Science and Technology University, Zhengzhou, China
- 2School of Geography and Tourism, Shaanxi Normal University, Xian, China
Introduction: There is a limited amount of research available on how changes in soil hydrothermal cycles impact soil N2O emissions in greenhouses that use a tomato irrigation system with micro-sprinkler irrigation mulched (MSM).
Methods: This study examined the effects of different irrigation frequency (F, F1 which is every 3 days, F2 which is every 5 days, F3 which is every 7 days) and irrigation amount (I, I1 which is 0.7 Epan, I2 which is 1.0 Epan, I3 which is 1.2 Epan) on soil N2O emissions in tomato cultivation. The research was carried out using a randomized experimental design over two consecutive growing seasons for greenhouse tomatoes in Northwest China.
Results: The findings revealed that F1 and F3 did not support the accumulation of microbial biomass carbon and nitrogen in the tomato soil under MSM. This limitation hindered the enhancement of soil extracellular enzymes BG and LAP, and decreased the diversity of the bacterial community structure. The functional genes related to bacterial nitrogen metabolism were abundant. The application of I2 treatment can result in a high accumulation of microbial biomass carbon and nitrogen in tomato soil, leading to enhanced soil BG and LAP activities and contributing to the stability of the soil bacterial community structure. As the F decreased, the cumulative emission flux of N2O in tomato soil initially decreased, then increased. Increasing the I showed a rising trend in the cumulative emission flux of N2O in tomato soil. The yield of spring and autumn tomatoes in F2 was higher compared to F1 and F3 at approximately 5.27 and 3.24%, and 19.31 and 11.30%, respectively. The yield of spring and autumn tomatoes in I2 was around 24.44 and 26.15% higher than in I1 and 1.64 and 3.06% higher than in I3. The regulation of the irrigation system in MSM resulted in a favorable interaction among tomato soil, microbial biomass carbon and nitrogen, soil extracellular enzymes, and soil bacterial community. When the I increased by 1.00%, the cumulative N2O emission flux and yield of tomato soil increased by at least 30.68 and 39.24%, respectively. For every 1.00% increase in F, the cumulative N2O emission flux and yield of tomato soil decreased by at least 7.41% and 11.23%, respectively. A quadratic relationship was observed between soil N2O emission flux and the abundance and yield of soil bacterial nitrogen metabolism functional genes. The assessment of tomato yield potential in the area could be indirectly done by examining the abundance of soil bacterial nitrogen metabolism functional genes. The study demonstrates the feasibility of regulating soil N2O emissions under the MSM irrigation system. Moreover, the findings indicate that F2I2 can significantly improve tomato yield without causing a considerable rise in soil N2O emission flux.
Discussion: This conclusion can provide a scientific basis for the optimization of irrigation system in facility agriculture, so as to ensure the high yield of crops and reduce the negative impact on the environment. It is also of great significance for the green development of agriculture under the background of global climate change.
1 Introduction
Currently, CO2, CH4 and N2O are widely acknowledged as the primary greenhouse gases (Li et al., 2022). N2O, a significant greenhouse gas in the atmosphere, has a warming potential 298 times greater than that of CO2 and can engage in various photochemical reactions (Li et al., 2025; Chen et al., 2018). Temperature, moisture, oxygen content and many other factors restrict the emission of N2O. Agricultural activities contribute around 13.80% to global greenhouse gas emissions, with 66% of N2O emissions stemming from crop production. It is projected that N2O emissions will double by 2050 (Bracken et al., 2021; Gu et al., 2023). Irrigation plays a crucial role in maintaining stable farmland yields. However, it impacts soil enzyme activity, respiration, nitrification, denitrification and mineralization, thereby affecting N2O emissions in farmlands (Fernández-Ortega et al., 2023; Kong et al., 2024; Ortega-Farias et al., 2022). Hence, determining the optimal balance between irrigation and N2O emissions is vital for the sustainable development of agriculture and the environment.
Facility agriculture is characterized by rapid crop growth and a high demand for soil water and fertilizer. Currently, there is a significant focus on research related to measuring soil water and fertilizer levels. The nutrients crucial for plant development primarily originate from the soil, where soil microorganisms and soil enzyme activities play a crucial role in the breakdown of soil nutrients and their absorption by plant roots (Jafari et al., 2018; Morio et al., 2022). This process results in the greenhouse gas N2O being produced under both aerobic and anaerobic conditions. Studies have indicated that soil nitrogen-fixing bacteria can transform atmospheric nitrogen into usable sources, enhance plant root growth to meet plant requirements, and minimize plant nutritional stress (Gandhamanagenahalli et al., 2024; Wang J. et al., 2022). The feedback loop between plants and soil can be positive when plant roots respond to soil dryness by developing fine roots that create conducive micro-environments for microbial activity and nutrient release (Veresoglou et al., 2022; Xiao et al., 2023). Furthermore, the substances released by root growth provide energy for soil microorganisms, influencing the soil nitrogen cycle and subsequently impacting N2O emissions (Helfrich et al., 2024; Zhang et al., 2025). Thus, investigating the interplay among soil bacterial communities, soil microbial biomass, soil extracellular enzyme activity and N2O emissions in facility agriculture is crucial for controlling greenhouse gas emissions in this type of agricultural system.
The redistribution of water and heat in soil is closely related to field irrigation management measures. These practices directly or indirectly alter soil enzyme activity, soil respiration, nitrification, denitrification, and mineralization. Consequently, they influence the development of soil microbial communities and plant root morphology, which in turn affects the emission of the greenhouse gas N2O (Feng et al., 2023; Lou et al., 2024; Vera et al., 2023). Irrigation frequency and irrigation amount not only affect the nitrogen cycle in soil, but also play a key role in the production and emission of N2O. Frequent irrigation may lead to reduced soil aeration, thereby increasing N2O emissions from denitrification; the unreasonable irrigation amount may affect the soil moisture content, change the living environment of soil microorganisms, and further affect the emission of N2O. In order to ensure the increase of plant yield and limit the emission of greenhouse gas N2O, researchers have proposed methods such as microbial inoculation and growth regulator addition. However, the implementation of the above methods has many problems such as high investment cost and environmental pollution (Boddington and Dodd, 1999; Zhao et al., 2017). At the same time, some researchers have found that yield increase and emission limitation can be achieved by adjusting crop irrigation management methods. For example, in the high-temperature forage planting system, water management synergistically increases or decreases forage yield and soil N2O emissions (Andrews et al., 2022). High and low frequency irrigation helps to increase soil N2O emissions (Mumford et al., 2019). Insufficient irrigation helps to reduce N2O emissions from winter wheat soil (Zhong et al., 2021). Therefore, exploring the changing patterns and correlation between soil greenhouse gas N2O emission and crop yield under changes in irrigation management is of significant guidance for reducing agricultural crop emissions and increasing yield.
Micro-sprinkler mulched (MSM) system, as a new type of water-saving irrigation technology, has been widely used in facility agricultural production in recent years. A large number of studies have shown that the MSM system has significant water-saving and yield-increasing effects. For example, in the greenhouse crop planting in the northwest region, the MSM system evenly penetrates the water droplets into the soil through its fine atomization nozzle, which effectively avoids the problems of soil compaction and water waste caused by traditional irrigation methods. At the same time, combined with plastic film mulching technology, the soil moisture retention ability and fertilizer retention level are further improved, creating a good soil environment for crop growth. At present, the MSM system has achieved good application results in the cultivation of cucumber, celery, watermelon and other greenhouse crops. Previous studies have found that different micro-sprinkler irrigation frequencies have significant effects on the growth characteristics and quality of greenhouse mini-watermelon. The growth and yield of celery under MSM system showed that the irrigation technology could significantly improve the yield and quality of celery (Zeng et al., 2021; Xie, 2019; Yin et al., 2021). However, although there are many studies on the MSM system in terms of water saving and yield increase, there are relatively few studies on how it regulates N2O emissions from greenhouse tomato soil. Previous studies have focused on the effects of traditional irrigation methods on soil N2O emissions. For example, some studies have shown that irrigation methods such as drip irrigation can affect soil microbial activity by changing soil moisture and ventilation conditions, thereby affecting soil N2O production and emissions. However, these studies are different from the unique mechanisms of MSM systems in soil improvement, water and nutrient cycling, and greenhouse gas emission reduction. In addition, the combined effects of different irrigation frequency and irrigation amount on soil microbial community, soil microbial biomass carbon and nitrogen, soil exoenzyme activity and soil bacterial community structure in MSM system, and the quantitative relationship between these factors and soil N2O emission and tomato yield are still unclear. Therefore, under the background of MSM system, this study systematically explored the effects of different irrigation frequency and irrigation amount on greenhouse tomato soil N2O emission and tomato yield, and deeply analyzed its relationship with soil microbial related factors.
The purpose of this study was to comprehensively and systematically study the comprehensive effects of different irrigation frequency and irrigation amount on greenhouse tomato soil N2O emission, soil microbial biomass carbon and nitrogen, soil exonuclease activity, soil bacterial community structure and tomato yield, and to construct a multi-factor MSM combination. The comprehensive analysis system of these factors provides a more comprehensive perspective for understanding the role of irrigation system in the soil ecosystem of facility agriculture. Advanced experimental techniques and analytical methods were used, such as chloroform fumigation-K2SO2 extraction method for the determination of soil microbial biomass carbon and nitrogen content, and Illumina MiSeq sequencing technology for the analysis of soil bacterial community structure. At the same time, combined with a variety of models and analytical methods, such as Cobb–Douglas model, Pearson correlation and regression analysis, the experimental data were deeply excavated and comprehensively analyzed, and the complex relationship between irrigation frequency and irrigation amount and soil N2O emission and tomato yield was quantitatively described (Zhang et al., 2022). The influence mechanism of MSM irrigation system on soil N2O emission and tomato yield was discussed, which provided a new perspective for understanding the relationship between soil ecological process and greenhouse gas emission in facility agriculture, and also provided a scientific basis for realizing the green and sustainable development of facility agriculture.
2 Materials and methods
2.1 Experimental site and management
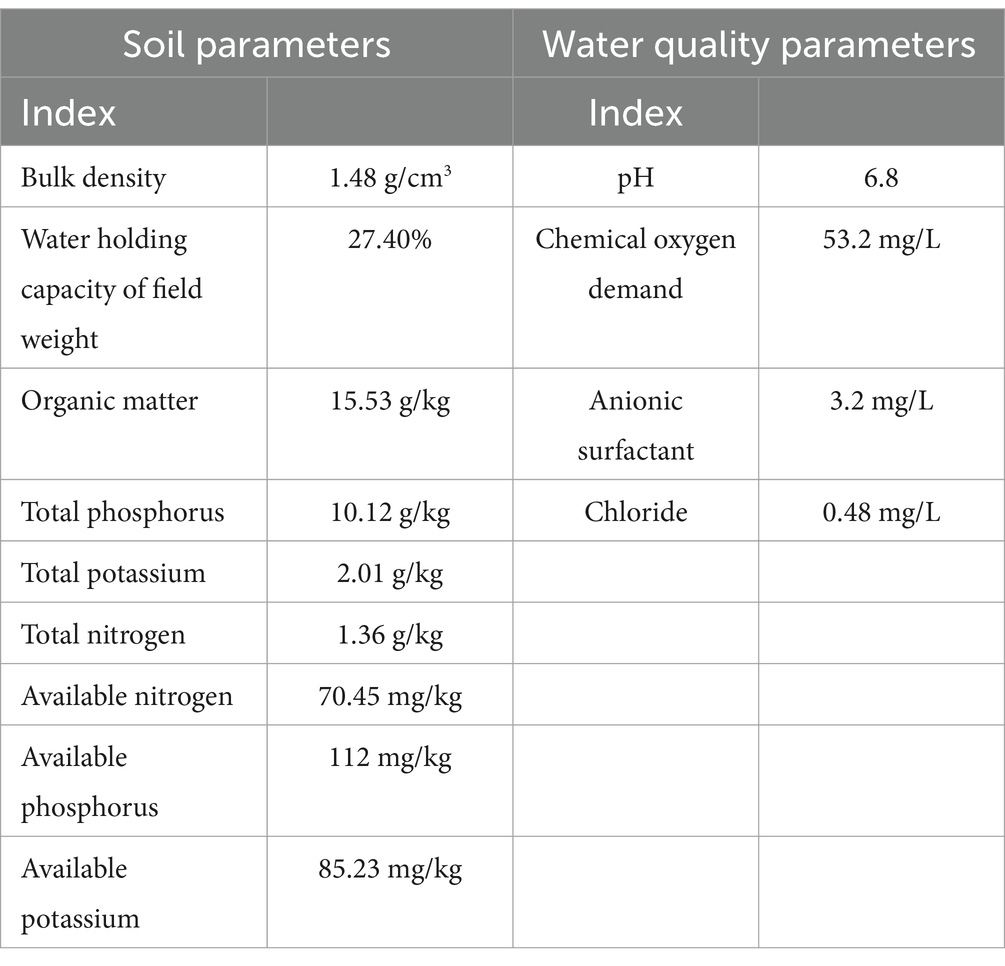
A controlled cultivation experiment was conducted from March 27, 2019, to January 30, 2020, at the Xi’an Modern Agricultural Science and Technology Extension Center (34.03°N, 108.52°E) in Shaanxi Province, China. The experimental setup utilized greenhouse facilities with detailed soil characteristics and irrigation water quality parameters documented in Table 1. The ‘Jingfan 401’ tomato cultivar was systematically planted using a spatial arrangement of 50 cm row spacing and 40 cm plant spacing within rows, with experimental plots measuring 3.4 m in length. Figure 1 illustrates the phenological development stages of the tomato plants. Throughout the growth cycle, all plots received standardized management practices including scheduled fertilization, regulated irrigation, and coordinated pest control measures to maintain experimental consistency.
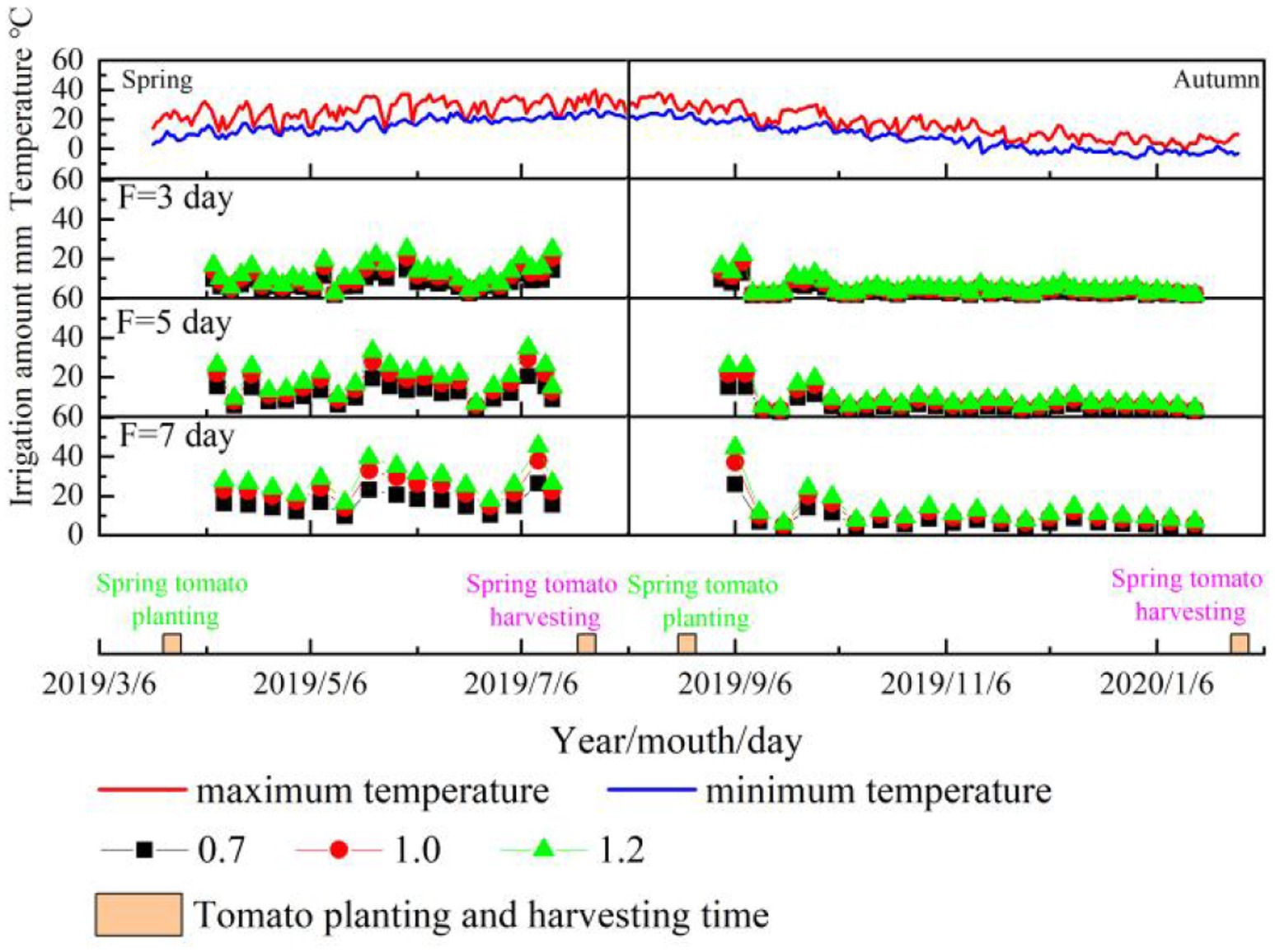
Figure 1. Greenhouse tomato growth period temperature, spring and autumn tomato growth period and irrigation amount under different treatments. Spring represents the spring tomato; Autumn represents the autumn tomato.
2.2 Experimental design
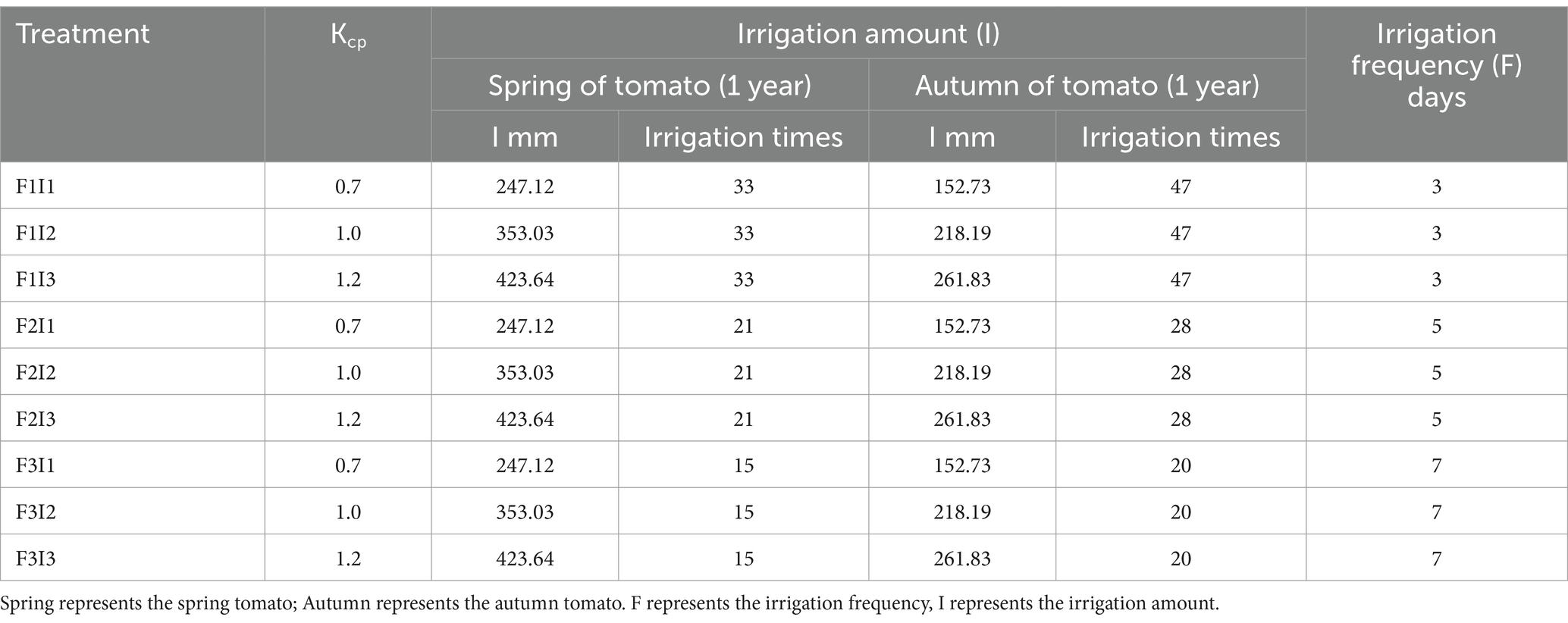
In this study, two factors were identified as irrigation frequency (F) and irrigation amount (I) as depicted in Table 2. Irrigation frequency and irrigation amount are set at 3 levels, in which the interval of irrigation frequency was 3 days, 5 days and 7 days respectively; the irrigation amount was controlled by the basis of the cumulative evaporation (Epan, a 20-cm diameter standard pan), which was realised by a control coefficient (kcp), The kcp (the crop pan coefficient) was 0.7, 1.0, and 1.2. A completely randomized trial design was used, the treatments are shown in Table 3. Each of treatments were repeated 3 times, a total of 27 experimental plots.
Pan evaporation amount was monitored at 8.00 am on the same day of each irrigation frequency. Equation 1 is used to calculate irrigation amount (Table 2) (Zhang et al., 2021).
In the formula, represents the evaporation within the interval of two irrigation, based on the cumulative evaporation from a 20 cm diameter pan (mm); represents the capillary control area (mm) and represents the crop-pan coefficient.
2.3 Measurements and computational methods
2.3.1 Soil greenhouse gas N2O emission flux
Samples of N2O gas in the soil were obtained using the static dark box method, with the sampling device consisting of a box and a base. The box, measuring 25 cm in length, width, and height, was constructed from 6 mm thick polyvinyl chloride (PVC) material. To ensure accuracy in sampling, the outer surface of the box was covered with sponge and tin foil paper, and a small fan was installed at the top for proper air circulation. The base of the static box (25 × 25 cm2) was buried between two tomato seedlings in the center of each ridge on the day of transplanting. It was inserted 5 cm deep into the soil as a sampling point until the tomatoes were harvested. Regular weeding was conducted during the tomato growth phase. A shallow groove at the top of the base was used to hold the static box securely, and a water injection seal was applied to isolate the box from the external environment during sampling. Gas samples were collected at 18, 36, 51, 72, 91, and 110 days after planting the tomatoes. Four gas sampling sessions took place at 10:00, 10:20, 10:40, and 10:60 using a 50 mL syringe with a three-way valve, drawing 30 mL of gas each time for concentration analysis on the same day. Outliers were eliminated to obtain a linear regression coefficient of R2 ≥ 0.90 for the concentration measurements of the four samples over time (Table 4).
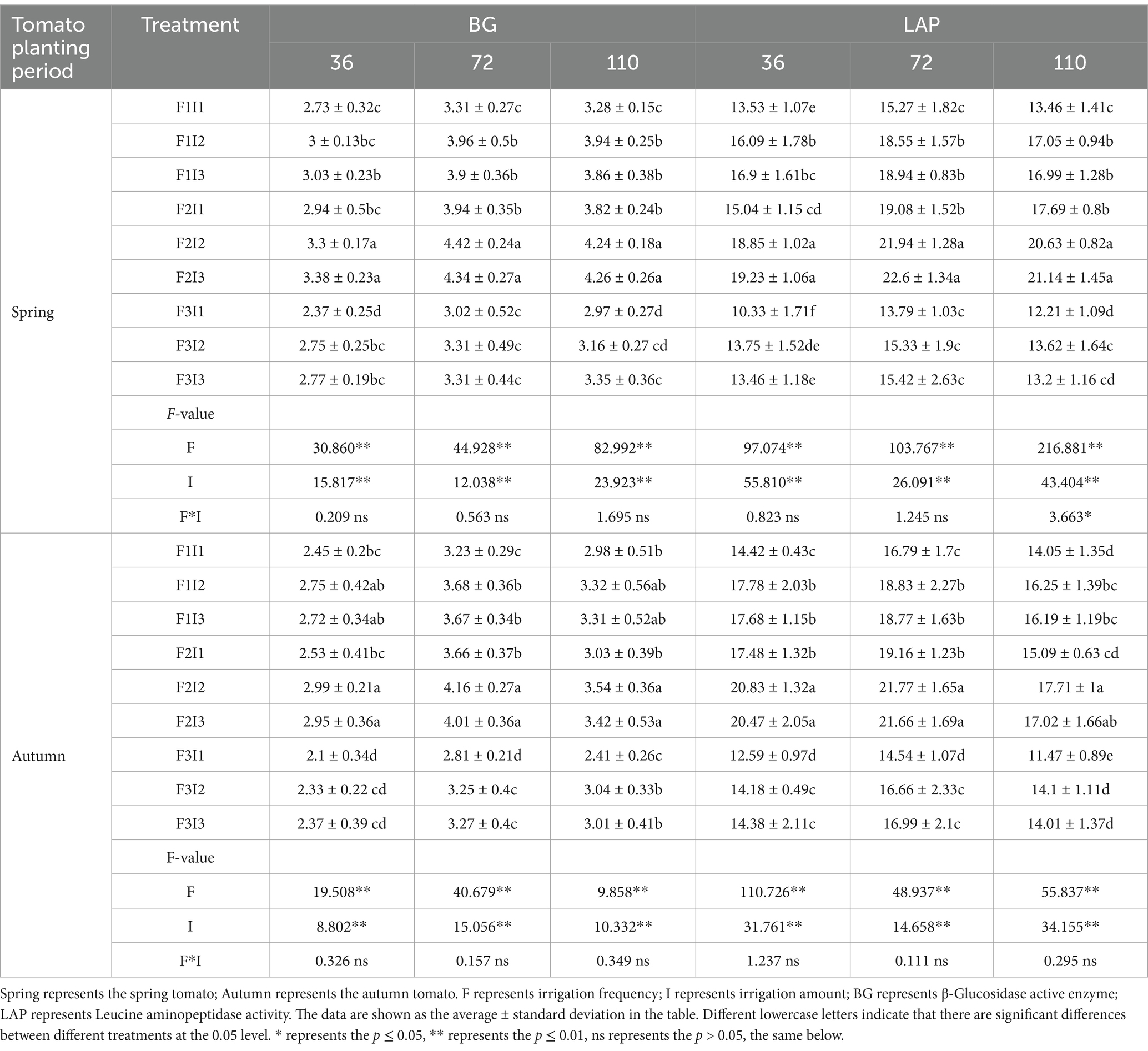
Table 4. Effects of different treatments on soil extracellular enzyme activities of greenhouse tomato.
The concentration of N2O in the soil was determined using an Agilent Technologies 7890 A GC System (USA), and the flux of N2O emission from the soil was calculated as described in Li et al. (2022). While collecting gas samples, the temperature inside the container was monitored using a mercury thermometer placed at the top. The air temperature during sampling was recorded with a mercury thermometer located about 1.5 m above the ground. Furthermore, the soil temperature of the soil layer that was 5 cm deep was measured using a soil thermometer.
2.3.2 Soil microbial biomass, soil extracellular enzyme activity, soil bacterial community
1. Seventy-two days after planting in both spring and autumn, soil samples were taken from the rhizosphere of tomato plants. The soil was collected using the shaking soil method, which involved excavating a 5–25 cm portion of the tomato root system and shaking off loose soil. The soil adhering closely to the greenhouse tomato root system was gently brushed off with a soft brush to obtain the greenhouse tomato rhizosphere soil. Three random soil samples were selected, brought to the laboratory and plant residues were removed. According to the specific requirements of different analysis, the soil samples were divided into two parts. One part was used for the timely determination of soil microbial biomass carbon and nitrogen and soil extracellular enzyme activity, and the other part was stored in a refrigerator at −20°C and immediately contacted Shanghai Meiji Biomedical Technology Co., Ltd. (China) for the determination of soil bacterial community. Analysis of soil-related indicators was completed within 10 days.
2. The microbial biomass carbon and nitrogen content in the tomato rhizosphere soil were determined using the chloroform fumigation-K2SO4 extraction method. The levels of microbial biomass carbon and nitrogen in the extracted filtrate were measured utilizing a Multi N/C 3100 total organic carbon/nitrogen analyzer.
3. The activity of soil extracellular enzymes β-glucosidase (BG) and leucine aminopeptidase (LAP), was determined by extracting them using an enzyme-linked immunosorbent assay and analyzing them with a microplate reader (RT-6100, Shanghai Precision Instrument Co., Ltd., China) (Puissant et al., 2019).
4. The analysis of the soil bacterial community involved three key steps: DNA extraction and PCR amplification, Illumina MiSeq sequencing and processing of sequencing data. Specific analyses were conducted using the Shanghai Meiji Biological Cloud Platform (Shanghai Meiji Biomedical Technology Co., Ltd., China), with references to the Kyoto Encyclopedia of Genes and Genomes and relevant literature (Wang et al., 2020). Functional genes related to nitrogen fixation, nitrification, denitrification, and phosphorus metabolism were identified in the soil bacteria. The nitrogen metabolism of soil bacteria was evaluated based on the collective abundance of functional genes associated with nitrogen fixation, nitrification, and denitrification.
2.3.3 Yield
Yield quantification was conducted through random sampling of four representative tomato plants at harvest maturity. For each sampled plant, four fruits reaching commercial ripeness were selected, and their fresh mass was determined using an analytical balance (±0.01 g). Measurements were standardized to per-hectare productivity using established agricultural conversion metrics, accounting for planting density (row spacing: 50 cm; plant spacing: 40 cm) and plot dimensions (3.4 m length). Total yield was extrapolated to estimate metric tons per hectare (kg/ha) based on the sampled fresh weight data and spatial planting parameters.
2.4 Data analysis
The Cobb-Douglas model was utilized to both qualitatively and quantitatively depict the impact of F and I on tomato ANG, NCL, and yield. The relationship between soil bacterial community, soil microbial biomass, soil extracellular enzyme activity, N2O emission flux and yield was quantitatively explained through Pearson correlation and regression analyses. Significance testing was conducted using the F test in SPSS 22.0 (IBM Crop., Armonk, New York, NY, USA) with a significance level set at p < 0.05. Pearson’s two-tailed test was performed using SPSS 22.0 (IBM Crop., Armonk, New York, NY, USA). Graphs were generated using OriginPro 2019 (Origin Lab Corporation, Northampton, MA, USA), while Excel 2016 (Microsoft Excel, Microsoft, Washington, USA) was employed for regression analysis.
2.5 Abbreviations
The abbreviations of this article are explained in Table 3.
3 Results
3.1 Effects of different treatments on soil microbial biomass carbon and nitrogen in greenhouse tomato
From Figure 2, it is evident that as the tomato growth period progresses, the soil MBC and MBN of both spring and autumn tomatoes treated with MSM show an upward trend. F and I significantly affect the soil MBC and MBN of spring and autumn tomatoes. As F decreases, soil MBC and MBN first increase and then decrease. Specifically, under F2 treatment, the soil MBC of spring and autumn tomatoes is approximately 7.40 and 8.94% higher than under F1 treatment, and about 8.96 and 8.78% higher than under F3 treatment. The soil MBN under F2 treatment is around 5.51 and 6.93% higher than under F1 treatment, and approximately 14.32 and 11.29% higher than under F3 treatment. Similarly, as I increases, soil MBC and MBN first rise and then fall. Under I2 treatment, the soil MBC of spring and autumn tomatoes is 9.34 and 11.18% higher than under I1 treatment, and about 2.13 and 2.83% higher than under I3 treatment. The soil MBN under I2 treatment is 10.62 and 7.90% higher than under I1 treatment, and approximately 1.90 and 1.30% higher than under I3 treatment. This trend can be attributed to the balance between soil aeration and moisture retention under different irrigation regimes. F1 leads to excessive soil moisture, reducing soil aeration and inhibiting microbial activity. In contrast, F3 results in soil drying and re-wetting cycles, which also stress microbial communities. The F2 provides optimal conditions for microbial growth. Similarly, increasing I initially enhances soil MBC and MBN by improving moisture availability, but I3 causes water-logging, reducing microbial biomass due to hypoxic conditions.
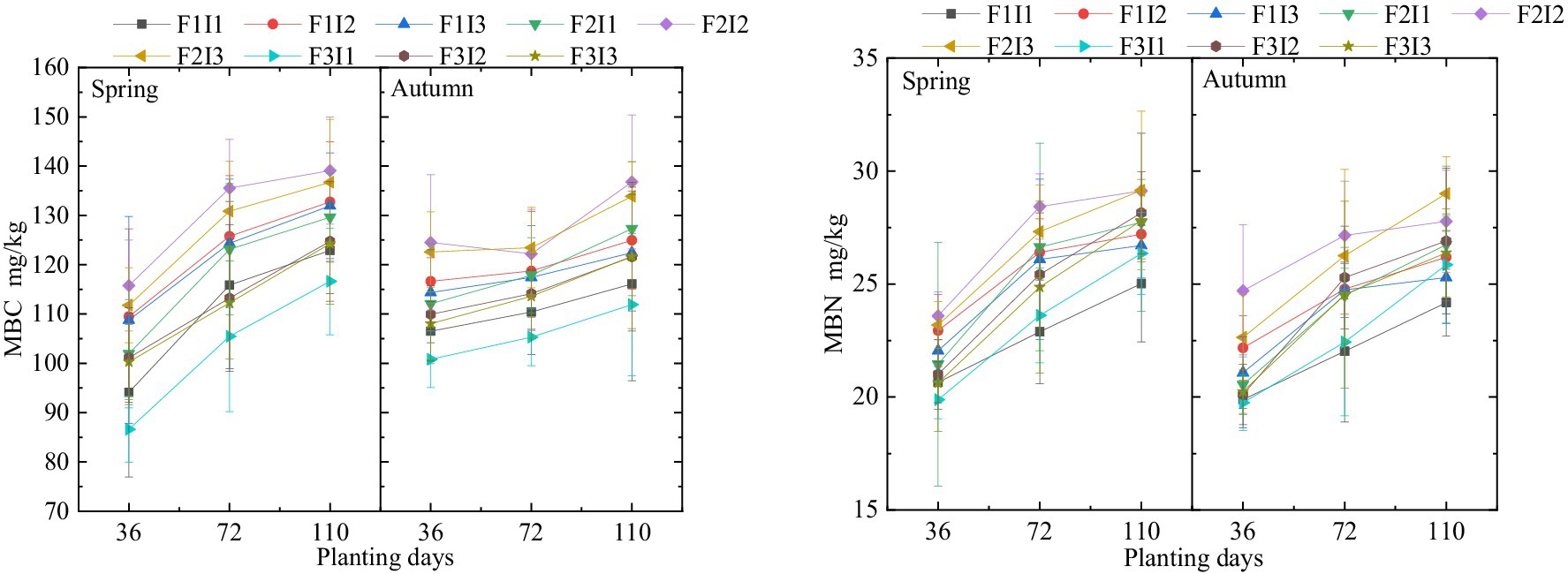
Figure 2. Effect of irrigation scheme regulation on soil microbial biomass carbon and nitrogen content. Spring represents the spring tomato; Autumn represents the autumn tomato. F represents irrigation frequency; I represents irrigation amount. The MBC represents the soil microbial biomass carbon, the MBN represents the soil microbial biomass nitrogen. The data are shown as the average ± standard deviation in the figure.
3.2 Effects of different treatments on soil extracellular enzyme activity of greenhouse tomato
It can be seen from Table 5 that the F and I significantly impacted soil BG and LAD activities at 36, 72, and 110 days after spring and autumn tomato planting. For BG activity, a decrease in F initially increased and then decreased activity. During the growth period, F2 treatment resulted in BG activity that was approximately 11.56 and 7.61% higher than F1, and 27.93 and 23.10% higher than F3. An increase in I showed a similar pattern, with I2 treatment leading to BG activity that was about 14.02 and 1.35% higher than I1, and 14.02 and 1.81% higher than I3. For LAD activity, the same trend was observed: F2 treatment increased LAD activity by approximately 19.99 and 13.36% compared to F1, and by 45.55 and 32.85% compared to F3. I2 treatment increased LAD activity by about 20.38 and 2.33% compared to I1, and by 16.85 and 1.30% compared to I3. This can be explained by the fact that F1 reduced soil oxygen levels, suppressing aerobic microbial activity and enzyme production. F3 caused soil desiccation, limiting enzyme activity by reducing substrate availability. In contrast, the F2 maintained adequate soil moisture and aeration, promoting enzyme synthesis and activity. Similarly, I2 supported a balanced bacterial community structure.
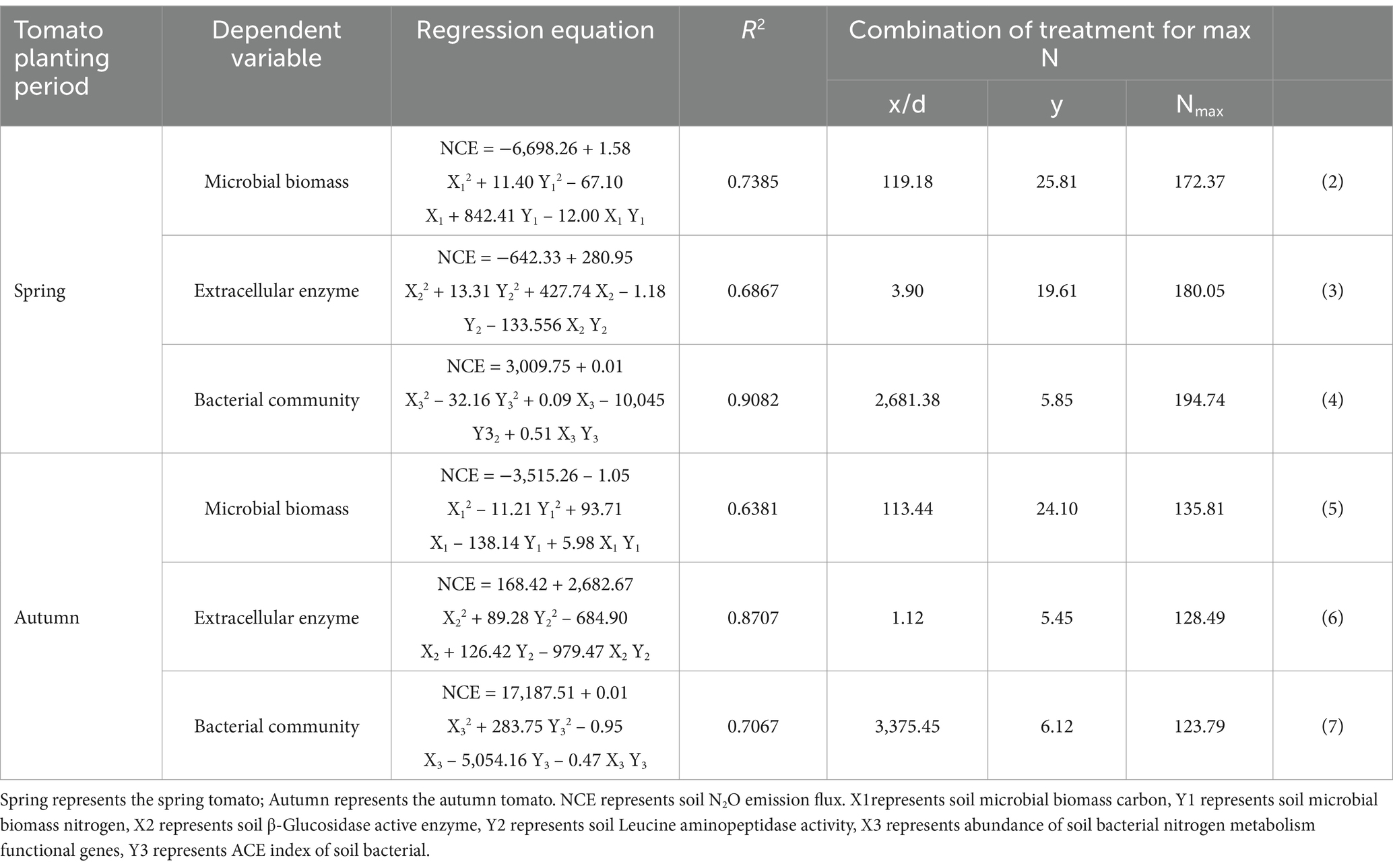
Table 5. Effects of soil bacterial community-soil microbial biomass-soil extracellular enzyme activity on soil N2O emission flux.
3.3 Effects of different treatments on bacterial community structure diversity and nitrogen functional gene abundance in greenhouse tomato soil
3.3.1 Diversity of soil bacterial community structure
It can be seen from Figure 3 that F and I had significant effects on soil bacteria ACE and SHN in spring tomato and autumn tomato (p ≤ 0.05). With the decrease of F, the soil bacteria ACE and SHN increased first and then decreased. Among them, the soil bacteria ACE and SHN in F2 were higher than those in F1 by about 13.43 and 6.09%, 8.47% and −0.64%, respectively (p ≤ 0.05). The ACE and SHN of soil bacteria in I2 was also higher than F3 by about 7.68% and 18.03%, 2.32% and 1.12% (p ≤ 0.05). With the increase of I, the ACE and SHN of soil bacteria showed a trend of increasing first and then decreased (p ≤ 0.05). Among them, the ACE and SHN of soil bacteria in I2 were about 23.56% and 12.79%, 5.97% and 4.12% higher than that of I1 (p ≤ 0.05). The ACE and SHNN of soil bacteria in I2 was also higher than I3 by about 2.78% and 2.76%, 0.63% and 1.72% (p ≤ 0.05).
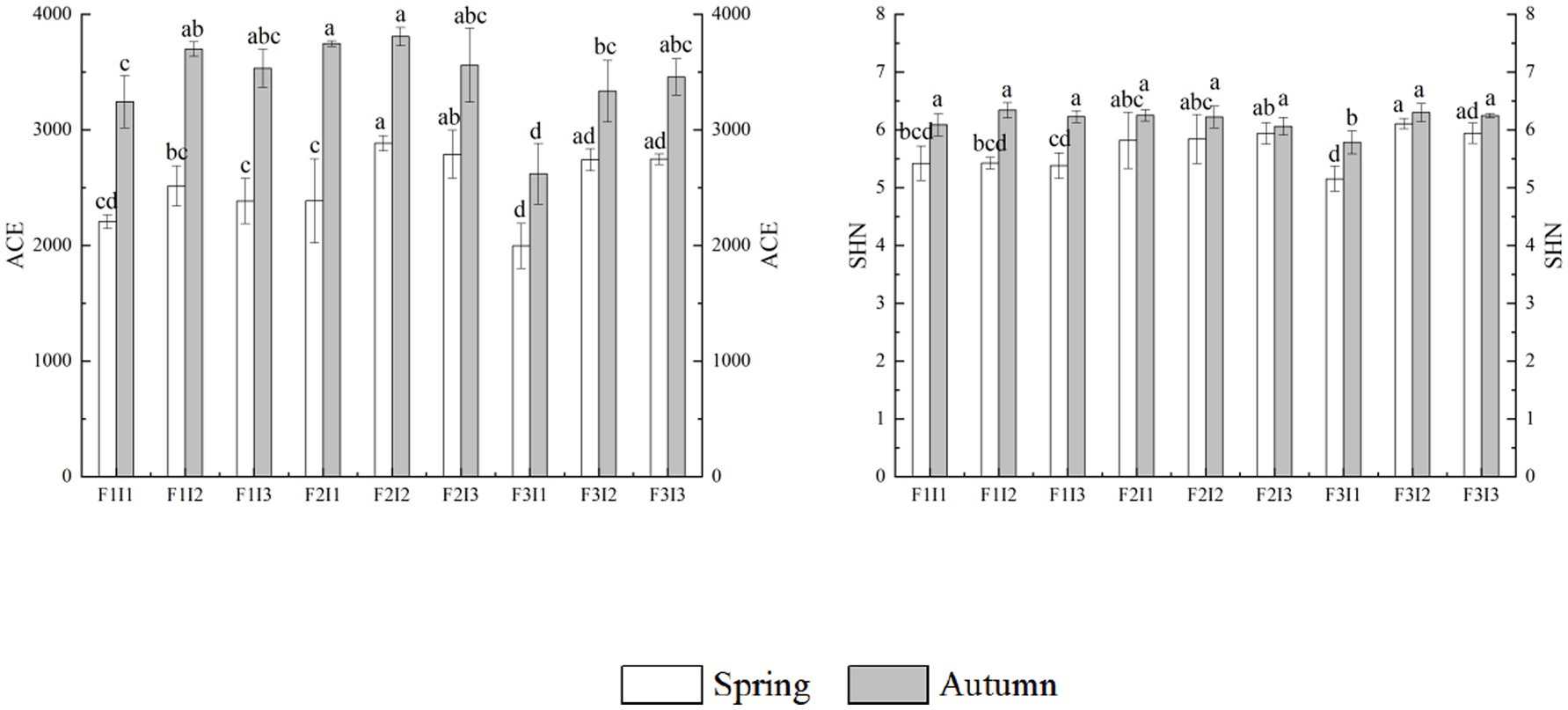
Figure 3. Effects of different treatments on bacterial community structure diversity in greenhouse tomato soil. The white histogram represents spring tomatoes, and the gray histogram represents autumn tomatoes. Spring represents the spring tomato; Autumn represents the autumn tomato. F represents irrigation frequency; I represents irrigation amount. The ACE represents ACE index of soil bacterial, SHN represents SHN index of soil bacterial. The data are all average ± standard deviation in the figure. Different lowercase letters indicate that there are significant differences between different treatments at the 0.05 level, the same as blow.
3.3.2 Abundance of functional genes related to soil bacterial nitrogen metabolism
In Figure 4, The ANFG, ANIG and ANG in spring tomato and autumn tomato soil treated with F2I2 was not significantly lower than that of F2I3, but higher than that of F1I1, F1I2, F1I3, F2I1, F3I1, F3I2 and F3I3, indicating that F2I2 treatment could improve the abundance of nitrogen metabolism functional genes and promote soil nitrogen cycle. With the decrease of F, the ANFG, ANIG and ANG in tomato soil increased first and then decreased. Among them, the ANFG, ANIG and ANG in spring tomato and autumn soil of F2 was higher than that of F1 by about 77.66% and 5.69%, 37.53% and 10.76%, 40.01% and 1.10%, respectively (p ≤ 0.05). The F2 was about 35.69% and 1.85%, 19.37% and 2.62%, 21.61% and 0.99% higher than that of F3 (p ≤ 0.05). With the increase of I, the ANFG, ANIG and ANG in tomato soil increased first and then decreased (p ≤ 0.05). The ANG in spring tomato and autumn tomato with I2 was higher than that of I1 by about 12.36% and 8.33% (p ≤ 0.05). The I2 was also higher than that of I3 treatment by about 15.33% and 7.90% (p ≤ 0.05).
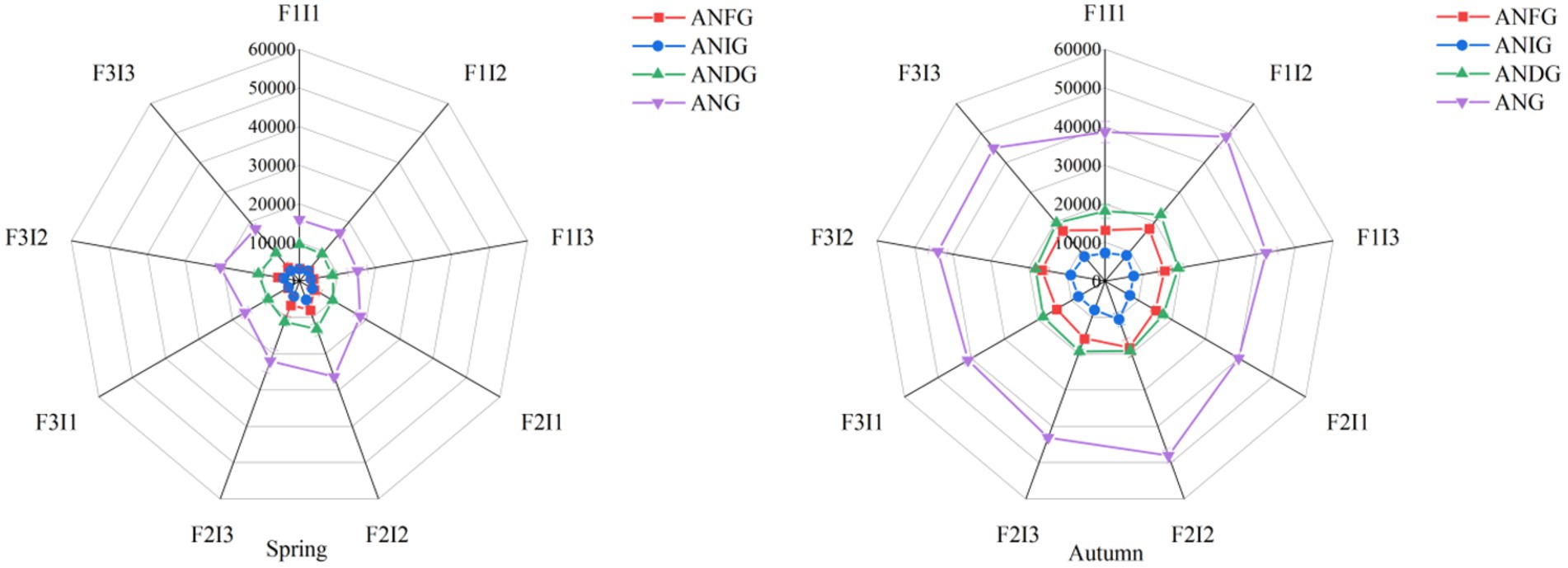
Figure 4. Irrigation schedule regulates the abundance of functional genes related to soil bacterial nitrogen metabolism. Spring represents the spring tomato; Autumn represents the autumn tomato. F represents irrigation frequency; I represents irrigation amount. The data are all average ± standard deviation in the figure. ANFG represents abundance of soil bacterial nitrogen-fixing functional genes, ANIG represents abundance of soil bacterial nitrification functional genes, ANDG represents abundance of soil bacterial denitrification functional genes, ANG represents abundance of soil bacterial nitrogen metabolism functional genes.
3.4 Effects of different treatments on greenhouse tomato soil N2O emissions
3.4.1 N2O emission flux of tomato soil
From Figure 5, it can be seen that with the advance of tomato growth period, the N2O emission flux of spring tomato soil increased first and then decreased, and the N2O emission flux of autumn tomato soil decreased, indicating that the effect of temperature on soil N2O emission flux was significantly higher than the regulation of irrigation system in this study. During the growth period of tomato, F and I had significant effects on N2O emission flux of tomato soil (p ≤ 0.05). With the decrease of F, the N2O emission flux of tomato soil decreased first and then increased. Under F2 (mean of tomato growth period, the same below) treatment, the N2O emission flux of spring tomato and autumn tomato soil was about 28.38% and 15.26% lower than that of F1 treatment and also lower than that of F3 treatment by about 15.59% and 10.15%. With the increase of I, the N2O emission flux of tomato soil showed an increasing trend.
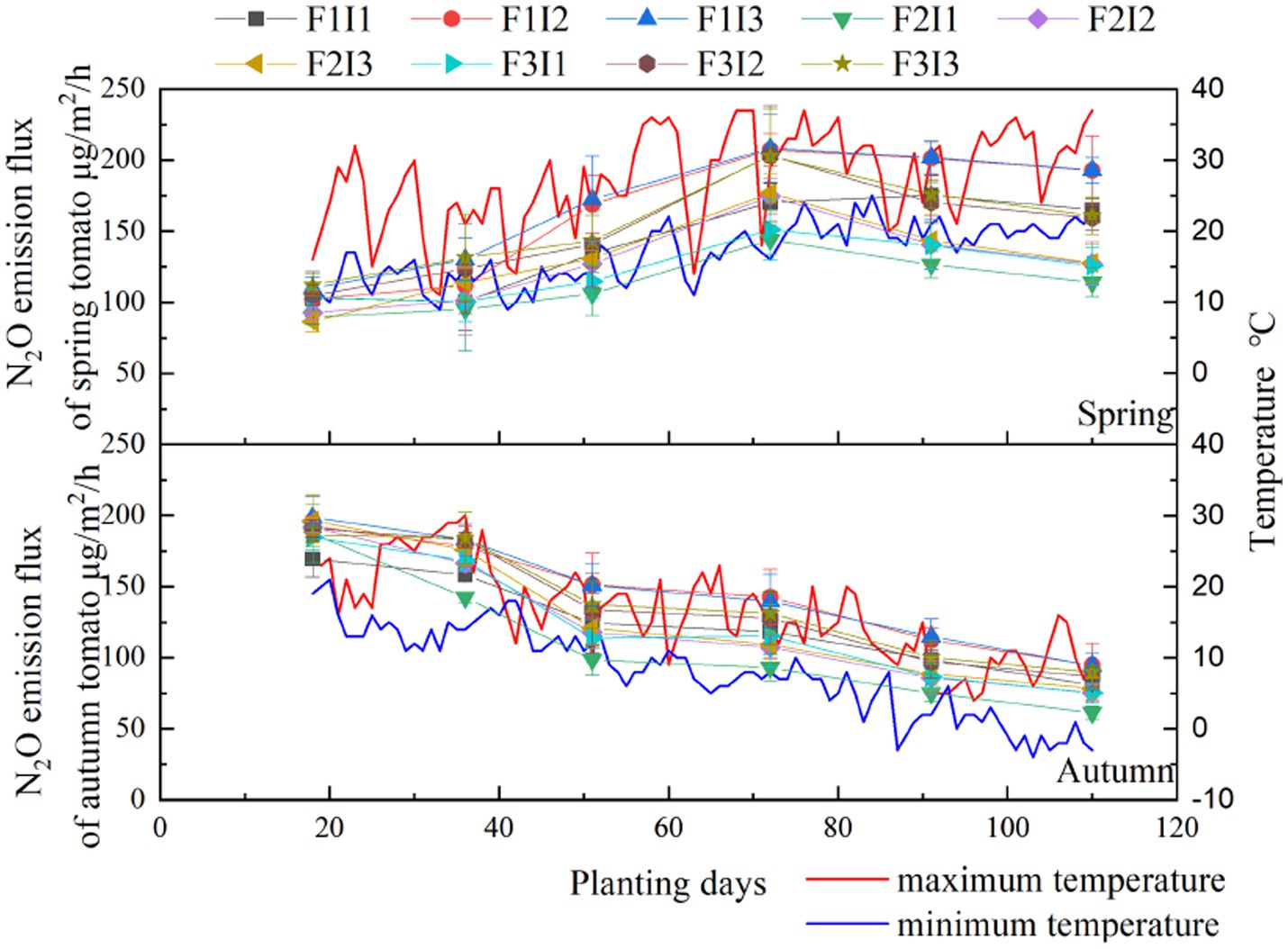
Figure 5. Effects of different treatments on greenhouse tomato soil N2O emission flux. Spring represents the spring tomato; Autumn represents the autumn tomato. F represents irrigation frequency; I represents irrigation amount. The data are shown as the average ± standard deviation in the figure.
3.4.2 Cumulative N2O emission flux of tomato soil
It can be seen from Figure 6 that F and I had a significant effect on the cumulative N2O emission flux of tomato soil (p ≤ 0.05). With the decrease of F, the cumulative N2O emission flux of tomato soil decreased first and then increased. Among them, the cumulative N2O emission flux of spring tomato and autumn tomato soil under F2 treatment was lower than that of F1 by about 28.26% and 15.46%. The F2 was also lower than F3 about 15.72% and 10.37%. When the I increased from I1 to I3, the cumulative N2O emission flux of tomato soil increased by about 20.50% and 15.19%.
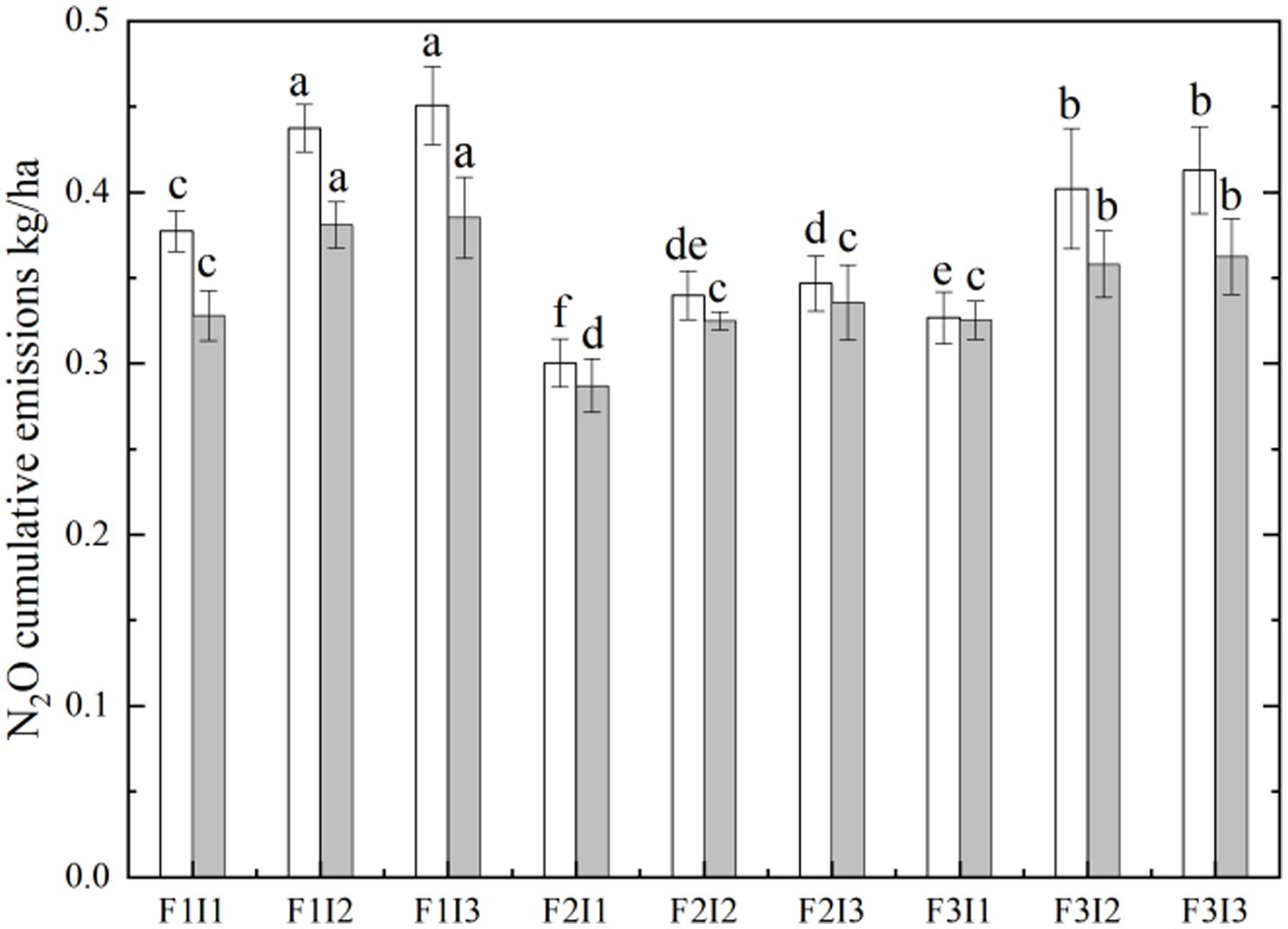
Figure 6. Effects of different treatments on cumulative N2O emission flux of tomato soil. The white histogram represents spring tomatoes, and the gray histogram represents autumn tomatoes. Spring represents the spring tomato; Autumn represents the autumn tomato. F represents irrigation frequency; I represents irrigation amount. The data are all average ± standard deviation in the figure. Different lowercase letters indicate that there are significant differences between different treatments at the 0.05 level, the same below.
3.5 Effect of different treatments on yield of greenhouse tomato
The data presented in Figure 7 that the yield of F2I2 of spring tomato and autumn tomato was significantly higher than that of F1I1, F2I1, F3I1, F3I2 and F3I3 by about 33.44% and 31.00%, 31.88% and 28.03%, 44.08% and 43.38%, 27.49% and 16.73%, 21.04% and 32.03%. The yield of F2I2 spring tomato and autumn tomato was higher than F1, F3 was about 5.27% and 3.24%, 19.31% and 11.30%. The yield of F2I2 spring tomato and autumn tomato in I2 was about 24.44% and 26.15% higher than that in I1, and 1.64% and 3.06% higher than that in I3.
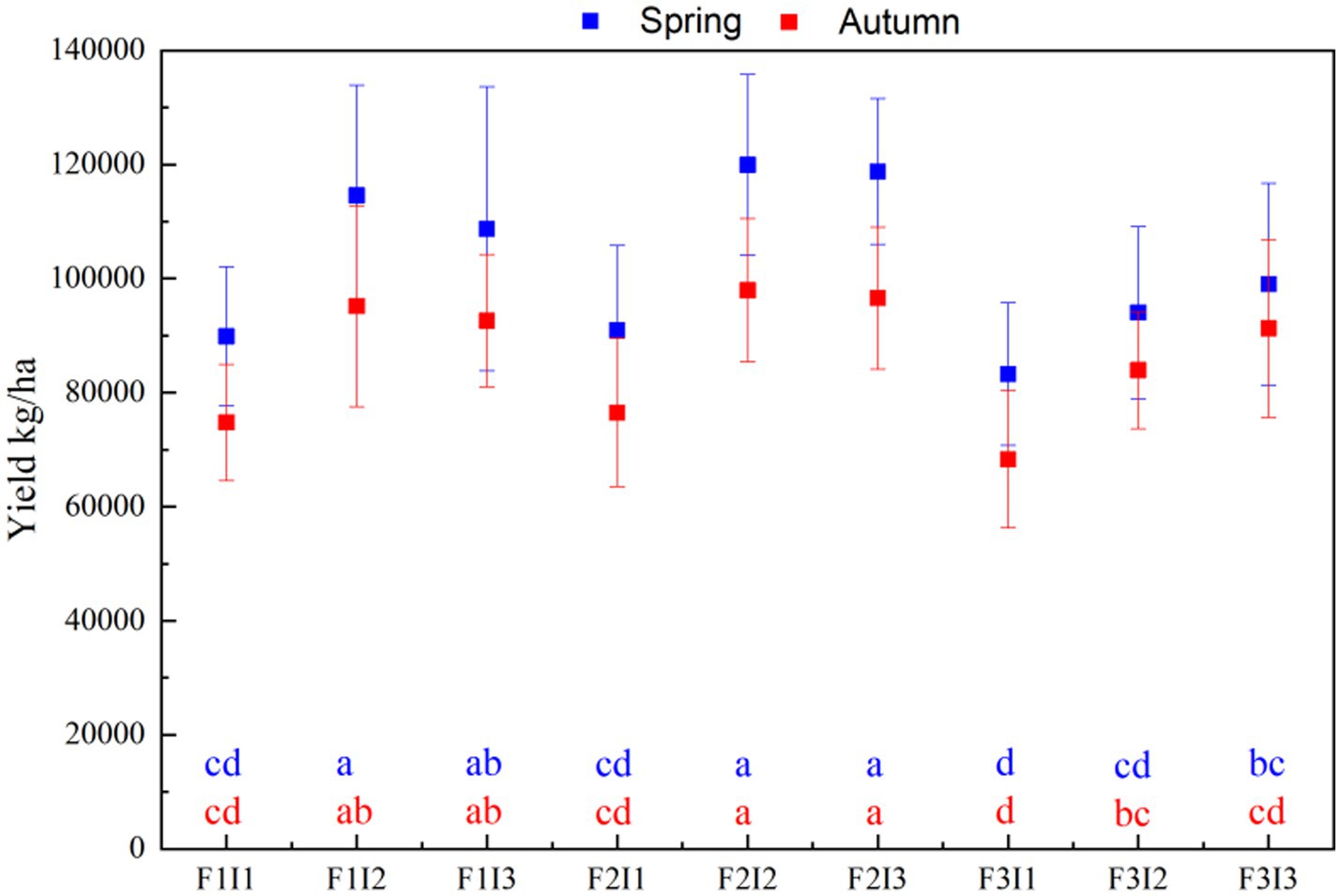
Figure 7. Effect of different treatments on yield of greenhouse tomato. Spring represents the spring tomato; Autumn represents the autumn tomato. F represents irrigation frequency; I represents irrigation amount. The data are all average ± standard deviation in the figure. Different lowercase letters indicate in the same column that there are significant differences between different treatments at the 0.05 level, the same below.
3.6 Effects of soil bacteria-microbial biomass-extracellular enzymes on N2O emission and yield of greenhouse tomato
As shown in Figure 8, through the Pearson double tail test, it was found that there was a pairwise correlation among soil microbial biomass carbon and nitrogen, soil extracellular enzyme, soil bacterial community, soil N2O emission flux and yield. Indicating that there was a positive interaction between soil microbial biomass carbon and nitrogen, soil extracellular enzyme and soil bacterial community. Rich soil microbial biomass carbon and nitrogen, high soil extracellular enzyme activity and stable bacterial community positively promoted the increase of tomato yield. However, the positive promotion of soil N2O emission flux was small. In order to further quantitatively describe how soil microbial biomass carbon and nitrogen, soil extracellular enzymes, and soil bacterial communities affect tomato soil N2O emission flux. Therefore, this study used multiple regression analysis to qualitatively and quantitatively describe the correlation between soil microbial biomass carbon and nitrogen, soil extracellular enzymes, soil bacterial community and soil N2O emission flux (Table 5). The higher abundance of nitrogen metabolism functional genes increased the soil N cycle. Although the risk of soil N2O emission flux was slightly increased, the increase of tomato yield was significant. In order to further express the balance relationship between the three, regression analysis was used to quantitatively describe the relationship between the abundance of nitrogen metabolism functional genes and soil N2O emission flux, soil N2O emission flux and tomato yield, in order to obtain the balance relationship between the micro-sprinkler irrigation system under the membrane to regulate the tomato soil N cycle, N2O emission flux and yield (Figure 9).
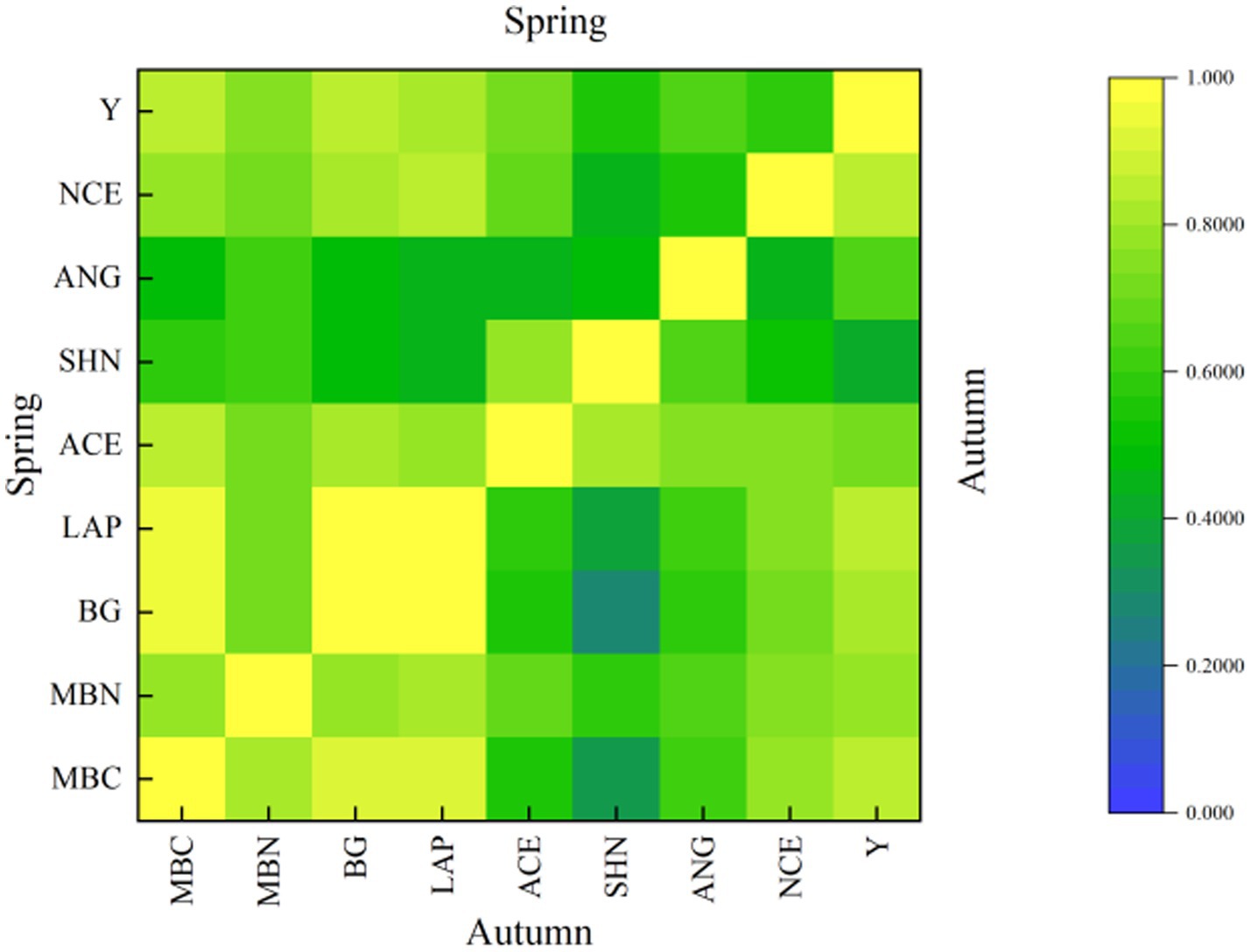
Figure 8. Correlation between soil bacterial community, soil microbial biomass, soil extracellular enzyme activity, N2O emission flux and yield. Spring represents the spring tomato; Autumn represents the autumn tomato. The MBC represents the soil microbial biomass carbon, the MBN represents the soil microbial biomass nitrogen, the BG represents the soil β-glucosidase, the LAP represents the soil leucine aminopeptidase, the ACE represents ACE index of soil bacterial, SHN represents SHN index of soil bacterial, the ANG represents the abundance of soil bacterial nitrogen metabolism functional genes, the NCE represents the soil N2O emission flux, the Y represents the yield.
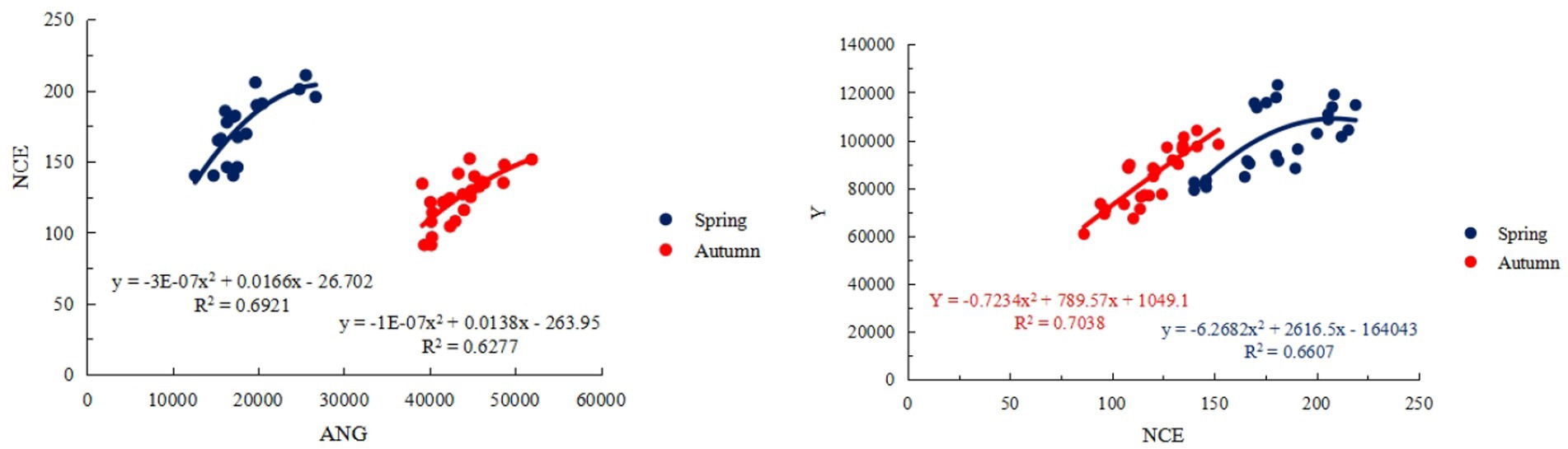
Figure 9. Regression analysis of soil N2O emission and soil bacterial nitrogen metabolism functional gene abundance and yield. Spring represents the spring tomato; Autumn represents the autumn tomato. NCE represents the soil N2O emission flux; ANG represents the abundance of soil bacterial nitrogen metabolism functional genes; Y represents the yield.
It can be seen from Figure 9 that the abundance of soil bacterial nitrogen metabolism functional genes and soil N2O emission flux showed a quadratic parabolic curve relationship, in which the determination coefficient R2 ≥ 0.6277, indicating that the change of soil bacterial nitrogen metabolism functional gene abundance in the regression model can explain 62.77% of the change of N2O emission flux of tomato soil. The relationship between tomato soil N2O emission flux and yield showed a quadratic parabolic curve, and the determination coefficient R2 ≥ 0.6607, The results showed that the N2O emission flux of tomato soil in the regression model could explain 66.07% of the change of tomato yield. The tomato soil N2O emission flux can be used to estimate the yield, and the tomato production potential in the region can also be indirectly evaluated by the abundance of soil bacterial nitrogen metabolism functional genes. In this study, the changes of gene activity were indirectly inferred by analyzing the structure of soil bacterial community and the abundance of functional genes, combined with the changes of soil enzyme activity and microbial biomass. For example, under F2I2 treatment, soil microbial biomass carbon and nitrogen increased significantly, and soil enzyme activities (BCG and LAP) also increased significantly, indicating that the activity of functional genes related to nitrogen metabolism may be enhanced.
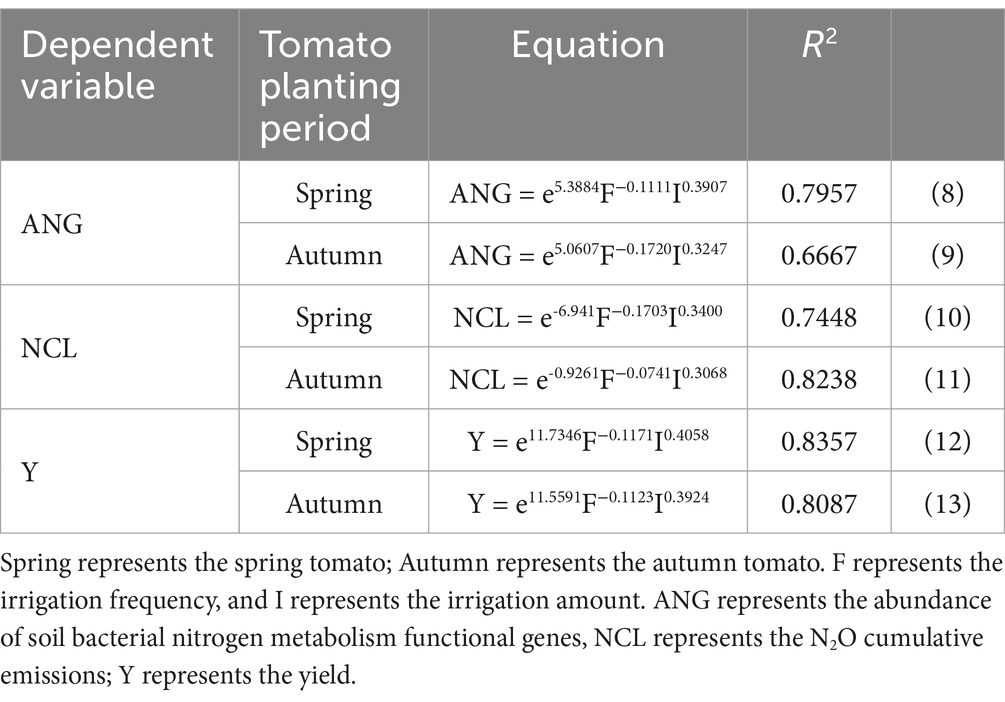
The data from Equations (8)–(13) in Table 6 reveal that controlling I and F of MSM has a greater impact on the abundance of bacterial nitrogen metabolism functional genes in tomato soil, the total emission of soil N2O, and the yield elasticity of tomatoes. Specifically, a 1.00% increase in I led to at least a 32.47% increase in the abundance of bacterial nitrogen metabolism genes, a 30.68% increase in N2O emissions, and a statistically significant 39.24% increase in yield. On the other hand, a 1.00% increase in F resulted in at least a statistically significant 11.11% decrease in the abundance of nitrogen metabolism genes, a statistically significant 7.41% decrease in N2O emissions, and a statistically significant 11.23% decrease in tomato yield. The Cobb–Douglas model fitting indicated that the impacts of F and I on the total N2O emissions of tomato soil aligned with the F test. The regulation of the irrigation system in MSM through optimizing irrigation frequency and amount resulted in a favorable interaction among tomato soil, microbial biomass carbon and nitrogen, soil extracellular enzymes, and soil bacterial community. This interaction notably boosted tomato yield and without a statistically significant increased soil N2O emissions.
4 Discussion
4.1 Effects of different treatments on soil micro-environment of tomato
Previous studies have found that differences in irrigation management measures can significantly affect soil microbial biomass carbon and nitrogen (Han et al., 2010). This study shows that as F decreases, the content of microbial biomass carbon and nitrogen initially increases and then decreases, which may be due to the positive effect of microbial biomass carbon and nitrogen, and soil water distribution. When high-frequency irrigation is executed, the water potential in the environment increases sharply. High osmotic pressure can kill microorganisms that cannot withstand the pressure, thereby reducing soil microbial biomass carbon and nitrogen content (Kumar et al., 2017; Sang et al., 2024). At the same time, when shallow soil moisture is maintained at a high level for a long period, the evaporation of ineffective water increases, and soil denitrification and N2O and CH4 emissions also increase (Tan et al., 2025; Vogeler et al., 2019). There are larger dry and humid areas and longer dry and humid duration in low frequency irrigation soil. Crop roots are vulnerable to soil drought and low oxygen stress, which reduces root morphological development and activity, slows root metabolism, and is not conducive to the accumulation of crop root metabolism (secretion), litter and microbial residues (Jiang et al., 2023; Leng et al., 2022). Previous studies have shown that soil microorganisms and plant residues are the main components of soil microbial biomass carbon and nitrogen (Buckeridge et al., 2020; Zhang et al., 2009). This conclusion is consistent with Xue’s study, which found that an appropriate increase in F helps improve the soil microbial biomass carbon of winter wheat (Xue et al., 2018). The study also found that, under MSM conditions, an irrigation efficiency of 5 days could significantly increase the activity of extracellular enzymes related to the soil N cycle. This may be because the irrigation efficiency of 3 days in sandy loam is too high, reducing soil aeration which the irrigation efficiency of 7 days would led to the soil being over-dried or over-wet for a long time. Soil microorganisms cannot adapt to environmental changes due to soil moisture and hypoxia stress, which reduces the source of soil enzymes. This is inconsistent with Li’s research, which found that the number of soil microorganisms is large and the soil enzyme activity is high after 8 days of irrigation. This conclusion may be due to differences in irrigation methods, regional climate and soil texture. Further research and demonstration are needed (Li et al., 2014). Changing F can alter the frequency of soil dry-wet alternation, thereby affecting the diversity and abundance of soil microbial communities. According to previous studies, dry-wet alternation can lead to about 58.00% of microbial death, but the active organic matter released by soil dry-wet changes is conducive to the growth of surviving microorganisms (Qin et al., 2025; Van Gestel et al., 1993). In this study, we found that the abundance of soil nitrogen metabolism functional genes was higher under F2, which may be due to the small wetting body and long wetting time of the soil with F1. Soil with long-term wetting state has a large amount of evaporation and poor soil aeration. Smaller wetting body and higher frequency irrigation can easily increase surface soil water filling porosity, promote the decrease of soil aeration, lead to competition among aerobic bacteria for survival in soil nitrogen fixation, nitrification and denitrification, and limit the increase of soil bacterial population abundance (Adu and Oades, 1978; Wang, 2017). However, when F was too low (F3), the average soil volumetric water content of spring and autumn tomato in the cultivated layer was lower than that of F2 by about 2.16% and 6.02%, respectively. The soil moisture in the cultivated layer became the main factor limiting the bacteria in the rhizosphere soil of greenhouse tomato under MSM (Xu et al., 2024; Wang, 2017). In addition, the spatial and temporal distribution of soil volumetric water content is uneven when the F is F3, and the soil at the root of the crop will be dry or wet for a long time. The abundance of soil microorganisms decreases8 due to the environmental filtration mechanism (Gregorutti and Caviglia, 2019; Wang et al., 2020). Water and low oxygen stress led to a decrease in the number of roots in the cultivated layer, and the amount of root exudates also decreased, which reduced the source of bacterial survival substrates. Moreover, the growth and reproduction of functional bacteria related to nitrogen metabolism were squeezed by the niche of other functional bacteria in the soil, resulting in a decrease in their abundance (Maheshwari, 2011; Wang et al., 2019). The abundance of soil phosphorus metabolism functional genes was higher than that of F1 or F3 when the F of MSM was F2, which further indicated that the irrigation frequency of F2 was beneficial to improve the soil P cycle metabolism (Balemi and Negisho, 2012; Monokrousos et al., 2020).
This study also showed that as I increased, the total organic carbon in soil initially increased and then decreased, which may be due to the uniform distribution of soil moisture in I2. There was no hypoxia and water stress caused by too high or too low soil volumetric water content in soil wetted body, which promoted the development of crop root morphology. The better root morphology development was beneficial to improve the abundance of root microbial population. The residues of plants and microorganisms provided sufficient carbon for crop rhizosphere soil (Buckeridge et al., 2020; Yao et al., 2019). With the increase of I, the activities of soil enzymes (β-glucosidase and leucine aminopeptidase) under micro-sprinkler irrigation under greenhouse tomato film increased first and then decreased. It may be that soil moisture is one of the main factors limiting soil enzyme activity under small I. When the I was too high, the soil aeration was poor (the soil water filling porosity of 1.20 Epan was higher than that of I2 by about 3.55 and 2.25%). The low oxygen soil micro-environment would inhibit the increase of aerobic microorganisms, resulting in the decrease of soil microbial community structure diversity and population abundance, the decrease of root absorption and utilization of soil nutrients, and the restriction of crop root metabolic cycle. The lower root cycle metabolites, root exudates and soil microbial biomass directly limit the improvement of soil enzyme activity (Wang et al., 2025; Zhang et al., 2019; Wang, 2017). With the increase of I, the soil volumetric water content increased. Weakening of soil permeability. This study also showed that the abundance of bacterial nitrogen metabolism functional genes in the rhizosphere soil of spring tomato with I2 was better. It may be due to the I1, the soil is easy to produce water stress limitation, and the soil volumetric heat capacity decreases with the decrease of soil water content, which is not conducive to the daily variation of soil temperature in a small range, and the fluctuation of soil internal fine structure also increases. The unstable soil structure limits the rapid increase of soil dominant flora abundance (Meisner et al., 2021; Han et al., 2020). At the same time, the lower irrigation amount is not conducive to the development of crop root morphology, resulting in a decrease in the input of organic matter such as root exudates and litter, which limits the increase in the abundance of soil bacterial nitrogen metabolism functional genes (Lin et al., 2024; Xiao C. et al., 2024; Xiao H. et al., 2024; Zhu et al., 2022). I3 makes the water fill the soil pores, and the external air is difficult to enter, which is easy to cause the decrease of soil oxygen content. The hypoxia stress of crop roots is easy to reduce the soil pH, and the reproduction of aerobic bacteria (nitrogen-fixing bacteria and rhizobia) in soil bacteria will be limited (González et al., 2015; Jiang et al., 2006). At the same time, there is a phenomenon of oxygen competition between crop roots and soil aerobic bacteria, which will greatly reduce the diversity and abundance of soil bacterial community structure (Zhou et al., 2024; Jiang et al., 2006; Yuan et al., 2020).
4.2 Effects of different treatments on N2O emission of tomato soil
Soil moisture is a limiting factor for the growth of plant roots and soil microorganisms (Xia et al., 2025). Different irrigation regimes can significantly affect a variety of soil abiotic factors, such as water availability, gas, heat distribution and porosity. At the same time, changes in soil environmental factors will affect the growth of microorganisms and plant roots. The growth of plant roots affects soil nutrient nitrification and denitrification, which in turn changes soil N2O gas emissions (He et al., 2024; Storch et al., 2023). This study found that too high (F1) or too low (F3) irrigation frequency could increase soil N2O emissions. Soil wetting and drying increased the substrate availability generated by soil microbial senescence and degradation of soil aggregates that protect organic matter, accelerated soil C and N mineralization rates, promoted aerobic respiration and reduced soil O2 levels. Subsequently, the anoxic zone formed on the soil promoted the rapid growth and activity of denitrifying bacteria, resulting in a significant increase in N2O emissions. Wu et al. (2022) found that the increase of rainfall frequency will increase the N2O emission flux of grassland soil. The conclusion is inconsistent, which may be due to the difference in the cycle of water entering the soil or the type of crop. The highest irrigation frequency of Wu grassland is 1 week, and the lowest irrigation frequency of vegetables in this study is 1 week. It is inconsistent with the results of Mumford et al. (2019) that high frequency and low frequency irrigation can increase soil N2O emission. It may be because Owens et al. (2016) believed that increasing F will reduce N2O emission by increasing surface soil moisture and reducing soil O2 concentration, which is consistent with the conclusion that N2O emission is lower than F1 under F2 in this study.
Soil water content often leads to changes in soil physical and chemical properties, thereby regulating soil denitrification (Li et al., 2024; Wu et al., 2022). Researchers generally believe that soil denitrification increases with the increasing water content, but some studies have found that soil N2O flux is the largest under critical saturated water content (Thiloka Edirisooriya et al., 2024). This study found that with the increase of I, tomato soil N2O emissions showed an increasing trend, may be due to the increase of soil water content will reduce the gas diffusion rate, and by limiting the main electron acceptor (such as oxygen) and substrate supply to directly affect the activity of microorganisms in the soil and its dominant nitrification and denitrification, improve soil microorganisms and plant root anaerobic respiration, increase tomato soil N2O emissions. It is consistent with the conclusion of Shang et al. (2020) and Gultekin et al. (2023) that drip irrigation can increase the N2O emission of greenhouse tomato soil, which further indicates that the effect of MSM on N2O emission of greenhouse tomato soil is similar to that of drip irrigation.
4.3 The correlation among soil micro-environment, N2O emission and yield of tomato under MSM
Previous research has shown that differences in soil water distribution resulting from irrigation can have a significant impact on the structure of soil bacterial communities, soil enzyme activity, microbial biomass carbon and nitrogen, as well as influencing soil N2O emissions and crop yield (Wang Z. et al., 2022; Wang et al., 2021). The current study revealed a strong positive correlation between soil microbial biomass carbon and nitrogen, extracellular enzymes and the soil bacterial community in the rhizosphere soil of tomatoes. This correlation is likely influenced by the diverse and abundant structure of the soil bacterial community, which facilitates the decomposition of soil organic matter, accelerating the carbon and nitrogen cycling in the soil. Increased levels of soil microbial biomass carbon and nitrogen, extracellular enzymes and soil bacterial communities can enhance plant roots’ ability to access soil moisture and nutrients, leading to higher tomato yields. Interestingly, the study discovered that the impact of I on tomato soil N2O emissions and yield was more pronounced compared to that of F, possibly due to the increased irrigation. The increased irrigation led to a notable rise in soil moisture content compared to the control group, reducing the frequency of soil dry and wet cycles. With higher moisture, oxygen levels in the soil diminish, promoting the production of N2O by microorganisms in low oxygen conditions. Previous research has shown a consistent linear relationship between irrigation amounts and soil N2O emissions. Altering the micro-sprinkler irrigation schedule under plastic film influenced the soil bacterial community composition, enzyme activities, and the interplay between soil microbial carbon and nitrogen, subsequently affecting N2O emissions (Xiao et al., 2024; Xiao et al., 2024). Depletion of soil nitrogen would likely hinder the growth of tomato plants. Remarkably, the F2I2 treatment resulted in increased soil microbial carbon and nitrogen biomass, enhanced enzyme activities, a stable bacterial community, and reduced N2O emissions, facilitating nitrogen uptake by tomato plants and ensuring a consistent tomato yield (Liu et al., 2024; Yang et al., 2023). The success of the F2I2 treatment may stem from increased nitrogen absorption by tomatoes, encouraging root colonization by microorganisms, strengthening root-microorganism interactions, limiting available nitrogen sources for soil microbes and ultimately reducing N2O emissions under this treatment.
4.4 Insights for facility agriculture from MSM irrigation system regulation
The present study provides valuable insights into optimizing the MSM irrigation system for enhanced tomato yield and controlled soil N2O emissions in facility agriculture. By systematically investigating the effects of different irrigation frequencies and amounts, we have demonstrated that a balanced irrigation strategy (F2I2 treatment) can significantly improve crop yield while maintaining N2O emissions at a relatively low level. These findings imply that precise irrigation management is crucial for achieving sustainable agricultural production in greenhouse settings. The interaction between soil microbial biomass, extracellular enzyme activity, and bacterial community diversity under varying irrigation regimes offers a deeper understanding of soil ecological processes. This knowledge can guide farmers and agricultural practitioners in making informed decisions regarding irrigation practices, thereby promoting efficient resource utilization and mitigating environmental impacts. Furthermore, the observed correlation between soil bacterial nitrogen metabolism functional gene abundance and tomato yield provides a potential biological indicator for assessing soil fertility and crop production potential in facility agriculture. Overall, these insights highlight the importance of tailoring irrigation strategies to specific crop requirements and soil conditions to optimize productivity and sustainability.
5 Conclusion
By exploring the response mechanism of tomato soil N2O emission under the regulation of MSM, the results showed that high frequency irrigation (F1) and low frequency irrigation (F3) were not conducive to the accumulation of microbial biomass carbon and nitrogen in tomato soil under MSM, limiting the increase of soil extracellular enzymes BCG and LAP, reducing the diversity of bacterial community structure and the abundance of functional genes related to bacterial nitrogen metabolism. The I2 can ensure the accumulation of microbial biomass carbon and nitrogen in tomato soil, promote the increase of soil BCG and LAP activities, and facilitate the stability of soil bacterial community. With the decrease of F, the cumulative N2O emission flux of tomato soil decreased first and then increased. With the increase of I, the cumulative emission flux of soil N2O also increased first and then decreased. The yield of spring tomato and autumn tomato under F2 was higher than that of F1 and F3 about 5.27% and 3.24%, 19.31% and 11.30%. The yield of spring tomato and autumn tomato under I2 was higher than that of I1 about 24.44% and 26.15%, also higher than that of I3 about 1.64% and 3.06%. Pearson two-tailed test and regression analysis showed that there was a positive interaction among soil microbial biomass carbon and nitrogen, soil extracellular enzymes and soil bacterial community structure, which was beneficial to the increase of tomato yield. There was a quadratic curve relationship among soil N2O emission flux, the abundance of soil bacterial nitrogen metabolism functional genes and yield. The tomato production potential and greenhouse gas emissions in this region could be indirectly evaluated by the abundance of soil bacterial nitrogen metabolism functional genes. In this study, it is feasible to regulate soil N2O emissions under the MSM irrigation system. The specific performance is that F2I2 treatment can increase tomato yield on the basis of limiting N2O emission flux of tomato soil. This study provides data support for yield increase and emission limitation and soil production potential prediction in facility agriculture.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
HY: Data curation, Writing – original draft. MZ: Writing – original draft, Writing – review & editing. WW: Formal analysis, Writing – original draft. NX: Supervision, Writing – original draft. MS: Resources, Writing – original draft. YL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research is funded by the Natural Science Foundation of China (No. 41807041), the science and technology research project of Henan Province (No. 242102111101), key research projects of higher education institutions in Henan Province (No. 25B610011), Zhengzhou basic research and applied basic research project (No. ZZSZX202432) the Mechanical Design, Manufacturing, and Automation key discipline of Henan Province (No. JG [2018] No.119). We are grateful for the helpful comments of the anonymous reviewers.
Acknowledgments
We thank Xuchang Irrigation Experimental Station for providing experimental sites and equipment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adu, J. K., and Oades, J. M. (1978). Physical factors influencing decomposition of organic materials in soil aggregates. Soil Biol. Biochem. 10, 109–115. doi: 10.1016/0038-0717(78)90080-9
Andrews, H. M., Homyak, P. M., Oikawa, P. Y., Wang, J., and Jenerette, G. D. (2022). Water-conscious management strategies reduce per-yield irrigation and soil emissions of CO2, N2O, and NO in high-temperature forage cropping systems. Agric. Ecosyst. Environ. 332:107944. doi: 10.1016/j.agee.2022.107944
Balemi, T., and Negisho, K. (2012). Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J. Soil Sci. Plant Nutr. 12, 547–562. doi: 10.4067/S0718-95162012005000015
Boddington, C. L., and Dodd, J. C. (1999). Evidence that differences in phosphate metabolism in mycorrhizas formed by species of Glomus and Gigaspora might be related to their life-cycle strategies. New Phytol. 142, 531–538. doi: 10.1046/j.1469-8137.1999.00422.x
Bracken, C. J., Lanigan, G. J., Richards, K. G., Müller, C., Tracy, S. R., Grant, J., et al. (2021). Source partitioning using N2O isotopomers and soil WFPS to establish dominant N2O production pathways from different pasture sward compositions. Sci. Total Environ. 781:146515. doi: 10.1016/j.scitotenv.2021.146515
Buckeridge, K. M., Mason, K. E., McNamara, N. P., Ostle, N., Puissant, J., Goodall, T., et al. (2020). Environmental and microbial controls on microbial necromass recycling, an important precursor for soil carbon stabilization. Commun. Earth Environ. 1, 1–9. doi: 10.1038/s43247-020-00031-4
Chen, H., Li, L., Cai, H. J., Zhu, Y., Wang, Y. F., and Xu, J. T. (2018). Response of soil N2O fluxes to soil nitrifying and denitrifying bacteria under aerated irrigation. Trans. Chinese Soc. Agric. Mach. 49:303. doi: 10.6041/j.issn.1000-1298.2018.04.035
Feng, S., Ding, W., Shi, C., Zhu, X., Hu, T., and Ru, Z. (2023). Optimizing the spatial distribution of roots by supplemental irrigation to improve grain yield and water use efficiency of wheat in the North China plain. Agric. Water Manag. 275:107989. doi: 10.1016/j.agwat.2022.107989
Fernández-Ortega, J., Álvaro-Fuentes, J., and Cantero-Martínez, C. (2023). The use of double-cropping in combination with no-tillage and optimized nitrogen fertilization reduces soil N2O emissions under irrigation. Sci. Total Environ. 857:159458. doi: 10.1016/j.scitotenv.2022.159458
Gandhamanagenahalli, A. R., Dass, A., Venkatesh, P., Choudhary, A. K., Upadhyay, P. K., Chandrashekar, A. B., et al. (2024). Soil moisture dynamics, rooting traits, crop and water productivity of wheat under different tillage, irrigation and nutrition conditions. Farm. Syst. 2:100087. doi: 10.1016/j.farsys.2024.100087
González, E. M., Larrainzar, E., Marino, D., Wienkoop, S., Gil-Quintana, E., and Arrese-Igor, C. (2015). Physiological responses of N2-fixing legumes to water limitation. Cham: Springer International Publishing, 5–33.
Gregorutti, V. C., and Caviglia, O. P. (2019). Impact of crop aerial and root biomass inputs on soil nitrifiers and cellulolytic microorganisms. Soil Tillage Res. 191, 85–97. doi: 10.1016/j.still.2019.03.018
Gu, B., Zhang, X., Lam, S. K., Yu, Y., van Grinsven, H. J. M., Zhang, S., et al. (2023). Cost-effective mitigation of nitrogen pollution from global croplands. Nature 613, 77–84. doi: 10.1038/s41586-022-05481-8
Gultekin, R., Avağ, K., Görgİşen, C., Öztürk, Ö., Yeter, T., and Alsan, P. B. (2023). Effect of deficit irrigation practices on greenhouse gas emissions in drip irrigation. Sci. Hortic. 310:111757. doi: 10.1016/j.scienta.2022.111757
Han, Y., Qiao, D. M., Qi, X., Li, P., Guo, W., Cui, B., et al. (2020). Effects of reclaimed water irrigation amounts on soil salinity and composition of soil bacteria community. Trans. CSAE 364, 106–117.
Han, L., Zhang, Y., Jin, S., Wang, J., Wei, Y., Cui, N., et al. (2010). Effect of different irrigation methods on dissolved organic carbon and microbial biomass carbon in the greenhouse soil. Agric. Sci. China 9, 1175–1182. doi: 10.1016/S1671-2927(09)60205-4
He, M., Tian, R., Vancov, T., Ma, F., Fang, Y., and Liang, X. (2024). Response of N2O emission and denitrifying genes to iron (II) supplement in root zone and bulk region during wetting-drying alternation in paddy soil. Appl. Soil Ecol. 194:105193. doi: 10.1016/j.apsoil.2023.105193
Helfrich, M., Dechow, R., Merl, S., Fuß, R., Räbiger, T., Kühling, I., et al. (2024). Winter cover crops decreased soil mineral N contents and increased soil organic C stocks and N2O emission. Agric. Ecosyst. Environ. 367:108985. doi: 10.1016/j.agee.2024.108985
Jafari, M., Yari, M., Ghabooli, M., Sepehri, M., Ghasemi, E., and Jonker, A. (2018). Inoculation and co-inoculation of alfalfa seedlings with root growth promoting microorganisms (Piriformospora indica, Glomus intraradices and Sinorhizobium meliloti) affect molecular structures, nutrient profiles and availability of hay for ruminants. Anim. Nutr. 4, 90–99. doi: 10.1016/j.aninu.2017.08.008
Jiang, O., Li, L., Duan, G., Gustave, W., Zhai, W., Zou, L., et al. (2023). Root exudates increased arsenic mobility and altered microbial community in paddy soils. J. Environ. Sci. 127, 410–420. doi: 10.1016/j.jes.2022.05.036
Jiang, J. Y., Tang, W., Zhu, Q. L., and Ren, J. J. (2006). N2O production from soil nitrification and denitrification bacteria at different water-filled pore space. J. Agro-Environ. Sci. 25, 1535–1540. doi: 10.3321/j.issn:1672-2043.2006.06.028
Kong, D., Zhang, X., Yu, Q., Jin, Y., Jiang, P., Wu, S., et al. (2024). Mitigation of N2O emissions in water-saving paddy fields: evaluating organic fertilizer substitution and microbial mechanisms. J. Integr. Agric. 23, 3159–3173. doi: 10.1016/j.jia.2024.03.047
Kumar, A., Nayak, A. K., Pani, D. R., and Das, B. S. (2017). Physiological and morphological responses of four different rice cultivars to soil water potential based deficit irrigation management strategies. Field Crop Res. 205, 78–94. doi: 10.1016/j.fcr.2017.01.026
Leng, Z., Wu, Y., Li, J., Nie, Z., Jia, H., Yan, C., et al. (2022). Phenolic root exudates enhance Avicennia marina tolerance to cadmium under the mediation of functional bacteria in mangrove sediments. Mar. Pollut. Bull. 185:114227. doi: 10.1016/j.marpolbul.2022.114227
Li, L., Awada, T., Shi, Y., Jin, V. L., and Kaiser, M. (2025). Global greenhouse gas emissions from agriculture: pathways to sustainable reductions. Glob. Chang. Biol. 31:e70015. doi: 10.1111/gcb.70015
Li, H., He, H. J., and Li, T. (2014). Microbial activity and functional diversity in rhizosphere of cucumber under different subsurface drip irrigation scheduling. Chin. J. Appl. Ecol. 25, 2349–2354. doi: 10.13287/j.1001-9332.20140530.021
Li, H., Song, X., Wu, D., Wei, D., and Ju, X. (2024). Digestate induces significantly higher N2O emission compared to urea under different soil properties and moisture. Environ. Res. 241:117617. doi: 10.1016/j.envres.2023.117617
Li, Y., Zhang, M., Lu, Z., Zhang, Y., and Wang, J. (2022). Effects of irrigation strategy and plastic film mulching on soil N2O emissions and fruit yields of greenhouse tomato. Agriculture 12:296. doi: 10.3390/agriculture12020296
Lin, H., Lai, C., Yu, G., Sunahara, G. I., Liu, L., Ullah, H., et al. (2024). Root exudate-driven rhizospheric recruitment of plant growth-promoting rhizobacteria. Pedosphere 35, 216–228. doi: 10.1016/j.pedsph.2024.03.005
Liu, X., Liu, J., Huang, C., Liu, H., Meng, Y., Chen, H., et al. (2024). The impacts of irrigation methods and regimes on the water and nitrogen utilization efficiency in subsoiling wheat fields. Agric. Water Manag. 295:108765. doi: 10.1016/j.agwat.2024.108765
Lou, Y., Li, J., Guo, J., Pan, D., Zhang, Z., Ma, L., et al. (2024). Water-saving irrigation and delayed sowing increased the emission intensity of CH4 and N2O in the rice-wheat rotated field under nighttime warming. Agric. Ecosyst. Environ. 365:108896. doi: 10.1016/j.agee.2024.108896
Maheshwari, D. (2011). Plant growth and health promoting bacteria, vol. 18. Berlin: Springer-Verlag, 45–79.
Meisner, A., Snoek, B. L., Nesme, J., Dent, E., Jacquiod, S., Classen, A. T., et al. (2021). Soil microbial legacies differ following drying-rewetting and freezing-thawing cycles. ISME J. 15, 1207–1221. doi: 10.1038/s41396-020-00844-3
Monokrousos, N., Papatheodorou, E. M., Orfanoudakis, M., Jones, D. G., Scullion, J., and Stamou, G. P. (2020). The effects of plant type, AMF inoculation and water regime on rhizosphere microbial communities. Eur. J. Soil Sci. 71, 265–278. doi: 10.1111/ejss.12882
Morio, K. A., Sternowski, R. H., and Brogden, K. A. (2022). Dataset of endodontic microorganisms killed at 265 nm wavelength by an ultraviolet C light emitting diode in root canals of extracted, instrumented teeth. Data Brief 40:107750. doi: 10.1016/j.dib.2021.107750
Mumford, M. T., Rowlings, D. W., Scheer, C., De Rosa, D., and Grace, P. R. (2019). Effect of irrigation scheduling on nitrous oxide emissions in intensively managed pastures. Agric. Ecosyst. Environ. 272, 126–134. doi: 10.1016/j.agee.2018.11.011
Ortega-Farias, S., Meza, S. E., López-Olivari, R., Araya-Alman, M., and Carrasco-Benavides, M. (2022). Effects of four irrigation regimes on yield, fruit quality, plant water status, and water productivity in a furrow-irrigated red raspberry orchard. Agric. Water Manag. 273:107885. doi: 10.1016/j.agwat.2022.107885
Owens, J., Clough, T. J., Laubach, J., Hunt, J. E., Venterea, R. T., and Phillips, R. L. (2016). Nitrous oxide fluxes, soil oxygen, and denitrification potential of urine- and non-urine-treated soil under different irrigation frequencies. J. Environ. Qual. 45, 1169–1177. doi: 10.2134/jeq2015.10.0516
Puissant, J., Jones, B., Goodall, T., Mang, D., Blaud, A., Gweon, H. S., et al. (2019). The pH optimum of soil exoenzymes adapt to long term changes in soil pH. Soil Biol. Biochem. 138:107601. doi: 10.1016/j.soilbio.2019.107601
Qin, J., Dodd, I. C., Bian, C., Li, G., and Jin, L. (2025). Deficit irrigation differentially modulates rhizosphere microbial community and metabolites of two potato genotypes differing in drought tolerance. J. Environ. Manag. 373:123836. doi: 10.1016/j.jenvman.2024.123836
Sang, J., Zhao, Y., Shen, Y., Shurpali, N. J., and Li, Y. (2024). Optimizing irrigation and nitrogen addition to balance grassland biomass production with greenhouse gas emissions: a mesocosm study. Environ. Res. 249:118387. doi: 10.1016/j.envres.2024.118387
Shang, Z. H., Cai, H. J., Chen, H., Sun, Y. N., Li, L., Zhu, Y., et al. (2020). Effect of water-fertilizer-gas coupling on soil N2O emission and yield in greenhouse tomatoes. Huanjing kexue. 49, 1–18. doi: 10.13227/j.hjkx.201910056
Storch, L. C., Schulz, K., Rißmann, C., Cerull, E., Plakias, A., Schlichting, I., et al. (2023). Nitrogen fertilization and irrigation types do not affect the overall N2O production potential of a sandy soil, but the microbial community structure and the quantity of functional genes related to the N cycle. Appl. Soil Ecol. 192:105083. doi: 10.1016/j.apsoil.2023.105083
Tan, M., Cui, N., Jiang, S., Xing, L., Wen, S., Liu, Q., et al. (2025). Effect of practicing water-saving irrigation on greenhouse gas emissions and crop productivity: a global meta-analysis. Agric. Water Manag. 308:109300. doi: 10.1016/j.agwat.2025.109300
Thiloka Edirisooriya, E. M. N., Wang, H., Banerjee, S., Longley, K., Wright, W., Mizuno, W., et al. (2024). Economic feasibility of developing alternative water supplies for agricultural irrigation. Curr. Opin. Chem. Eng. 43:100987. doi: 10.1016/j.coche.2023.100987
Van Gestel, M., Merckx, R., and Vlassak, K. (1993). Microbial biomass responses to soil drying and rewetting: the fate of fast- and slow-growing microorganisms in soils from different climates. Soil Biol. Biochem. 25, 109–123. doi: 10.1016/0038-0717(93)90249-B
Vera, A., Bastida, F., Patiño-García, M., and Moreno, J. L. (2023). The effects of boron-enriched water irrigation on soil microbial community are dependent on crop species. Appl. Soil Ecol. 181:104677. doi: 10.1016/j.apsoil.2022.104677
Veresoglou, S. D., Li, G. C., Chen, J., and Johnson, D. (2022). Direction of plant–soil feedback determines plant responses to drought. Glob. Chang. Biol. 28, 3995–3997. doi: 10.1111/gcb.16204
Vogeler, I., Thomas, S., and van der Weerden, T. (2019). Effect of irrigation management on pasture yield and nitrogen losses. Agric. Water Manag. 216, 60–69. doi: 10.1016/j.agwat.2019.01.022
Wang, J. (2017). Effect of mulched drip irrigation on crop root-zone soil microenvironment and crop growth in plastic greenhouse : Northwest A&F University. Available at: http://cdmd.cnki.com.cn/Article/CDMD-10712-1017064536.htm
Wang, J., Du, Y., Niu, W., Han, J., Li, Y., and Yang, P. (2022). Drip irrigation mode affects tomato yield by regulating root–soil–microbe interactions. Agric. Water Manag. 260:107188. doi: 10.1016/j.agwat.2021.107188
Wang, Y., Hui, W., Zhang, D., Chen, X., Wang, R., Xu, Y., et al. (2025). Absorption and transport of different ZnO nanoparticles sizes in Agrostis stolonifera: impacts on physiological, biochemical responses, root exudation, and microbial community structure. Plant Physiol. Biochem. 219:109369. doi: 10.1016/j.plaphy.2024.109369
Wang, J., Li, Y., and Niu, W. (2021). Effect of alternating drip irrigation on soil gas emissions, microbial community composition, and root–soil interactions. Agric. Water Manag. 256:107123. doi: 10.1016/j.agwat.2021.107123
Wang, Z., Li, X., Wang, J., Qi, S., Dai, Z., and Du, D. (2022). Effect of nitrogen-fixing bacteria on resource investment of the root system in an invasive clonal plant under low nutritional environment. Flora 297:152166. doi: 10.1016/j.flora.2022.152166
Wang, J., Niu, W., and Li, Y. (2020). Nitrogen and phosphorus absorption and yield of tomato increased by regulating the bacterial community under greenhouse conditions via the alternate drip irrigation method. Agronomy 10:315. doi: 10.3390/agronomy10030315
Wang, Y., Zhang, F., Wang, H., Bi, L., Cheng, M., Yan, F., et al. (2019). Effects of the frequency and amount of drip irrigation on yield, tuber quality and water use efficiency pf potato in sandy soil. Chin. J. Appl. Ecol. 30, 4159–4168. doi: 10.13287/j.1001-9332.201912.021
Wu, J., Wang, H., Li, G., Wu, J., Gong, Y., and Wei, X. (2022). Unimodal response of N2O flux to changing rainfall amount and frequency in a wet meadow in the Tibetan plateau. Ecol. Eng. 174:106461. doi: 10.1016/j.ecoleng.2021.106461
Xia, H., Shen, J., Riaz, M., Ran, F., Cheng, T., Wang, X., et al. (2025). Effects of agricultural plastic films on crop growth and soil health in tobacco fields: a comparative study. Appl. Soil Ecol. 206:105795. doi: 10.1016/j.apsoil.2024.105795
Xiao, H., Rong, Y., Li, P., and Liu, Y. (2024). Soil moisture drives the response of soil microbial nutrient limitation to N and P additions in an inner Mongolian meadow steppe. Eur. J. Soil Biol. 120:103601. doi: 10.1016/j.ejsobi.2024.103601
Xiao, C., Zhang, F., Li, Y., Fan, J., Ji, Q., Jiang, F., et al. (2024). Optimizing drip irrigation and nitrogen fertilization regimes to reduce greenhouse gas emissions, increase net ecosystem carbon budget and reduce carbon footprint in saline cotton fields. Agric. Ecosyst. Environ. 366:108912. doi: 10.1016/j.agee.2024.108912
Xiao, W., Zhang, Q., Zhao, S., Chen, D., Gao, N., Huang, M., et al. (2023). Citric acid secretion from rice roots contributes to reduction and immobilization of Cr(VI) by driving microbial sulfur and iron cycle in paddy soil. Sci. Total Environ. 854:158832. doi: 10.1016/j.scitotenv.2022.158832
Xie, N. (2019). Effects of micro-sprinkling irrigation under film on growth and yield of celery in greenhouse. Water Conserv. Sci. Cold Region Eng. 2, 30–32.
Xu, Z., Wang, Y., Li, H., Dong, Y., Wang, Z., Liu, Z., et al. (2024). Soil moisture and bacterial carbon limitation regulate the soil organic carbon in mountain peatlands. Catena 234:107610. doi: 10.1016/j.catena.2023.107610
Xue, R., Shen, Y., and Marschner, P. (2018). Watering frequency and Total water input influence wheat growth, soil microbial biomass and nutrient availability in a silt loam. Commun. Soil Sci. Plant Anal. 49, 380–388. doi: 10.1080/00103624.2018.1427264
Yang, X., Hou, R., Fu, Q., Li, T., Wang, J., Su, Z., et al. (2023). Effect of freeze-thaw cycles and biochar coupling on the soil water-soil environment, nitrogen adsorption and N2O emissions in seasonally frozen regions. Sci. Total Environ. 893:164845. doi: 10.1016/j.scitotenv.2023.164845
Yao, X., Xiao, B., Kidron, G. J., and Hu, K. (2019). Respiration rate of moss-dominated biocrusts and their relationships with temperature and moisture in a semiarid ecosystem. Catena 183:104195. doi: 10.1016/j.catena.2019.104195
Yin, Y. L., Li, K., Hou, Y., Qi, Q., Wang, L., Ding, W. W., et al. (2021). Design of simplified water fertilizer integrated machine and its application effect test in cucumber planting in solar greenhouse. Water Saving Irrig. 9, 7–11. doi: 10.3969/j.issn.1007-4929.2021.09.002
Yuan, J. Y., Li, G., Yan, L. J., Chen, G. P., Zhang, S. W., and Teng, R. (2020). Soil and plant carbon, nitrogen, and phosphorus content and their stoichiometry in spring wheat under different irrigation treatments in the loess plateau. Pratacult. Sci. 379, 1803–1812. doi: 10.11829/j.issn.1001-0629.2019-0538
Zeng, Y., Li, C., Dai, Y. X., Ha, X. J., Tian, L., Li, C., et al. (2021). Effects of different micro-spray irrigation frequency on growth characteristics and quality of mini watermelon in greenhouse. China Cucurbits Veg. 34, 73–76. doi: 10.3969/j.issn.1673-2871.2021.12.013
Zhang, X., Dippold, M. A., Kuzyakov, Y., and Razavi, B. S. (2019). Spatial pattern of enzyme activities depends on root exudate composition. Soil Biol. Biochem. 133, 83–93. doi: 10.1016/j.soilbio.2019.02.010
Zhang, L., Lin, W., Sardans, J., Xu, E., Hui, D., Liu, X., et al. (2025). Contrasting effects of litter and root removals on soil N2O emissions of an evergreen broadleaved forest in southeastern China. Appl. Soil Ecol. 206:105867. doi: 10.1016/j.apsoil.2025.105867
Zhang, F., Shen, J., and Feng, G. (2009). Rhizosphere ecology - process and regulation. 1st Edn. Beijing: China Agricultural University Press.
Zhang, M., Xiao, N., Li, Y., Li, Y., Zhang, D., Xu, Z., et al. (2022). Growth and fruit yields of greenhouse tomato under the integrated water and fertilizer by Moistube irrigation. Agronomy 12:1630. doi: 10.3390/agronomy12071630
Zhang, M., Yan, X., Lu, Z., Bai, Q., Zhang, Y., Wang, D., et al. (2021). Effects of micropore group spacing and irrigation amount on soil respiration and yield of tomato with microsprinkler irrigation under plastic film in greenhouse. J Sens 2021, 1–16. doi: 10.1155/2021/6658059
Zhao, Q. Y., Xing, Y. Z., Sun, Y., Lin, X. J., and Zhu, F. F. (2017). Effects of different soil conditioner application on coffee seedlings growth and soil enzyme activities in acidic continuous cropping soil. Chinese J. Trop. Crops 38, 1868–1873. doi: 10.3969/j.issn.1000-2561.2017.10.016
Zhong, Y., Li, J., and Xiong, H. (2021). Effect of deficit irrigation on soil CO2 and N2O emissions and winter wheat yield. J. Clean. Prod. 279:123718. doi: 10.1016/j.jclepro.2020.123718
Zhou, Z., Lu, J., Widyastuti, R., Scheu, S., Potapov, A., and Krashevska, V. (2024). Plant roots are more strongly linked to microorganisms in leaf litter rather than in soil across tropical land-use systems. Soil Biol. Biochem. 190:109320. doi: 10.1016/j.soilbio.2024.109320
Keywords: microbial biomass carbon and nitrogen, extracellular enzyme, bacterial community, N2O emission flux, yield
Citation: Yang H, Zhang M, Wu W, Xiao N, Sun M and Li Y (2025) Irrigation frequency and irrigation amount of micro-sprinkler irrigation mulched regulate N2O emission of tomato (Solanum lycopersicum) soil. Front. Sustain. Food Syst. 9:1570994. doi: 10.3389/fsufs.2025.1570994
Edited by:
Rameshwar Kanwar, Iowa State University, United StatesReviewed by:
Uday Bhanu Prakash Vaddevolu, Texas A&M University, United StatesXing Wang, Northwest A&F University, China
Copyright © 2025 Yang, Zhang, Wu, Xiao, Sun and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzhi Zhang, bWluZ3poaXpAaGhzdHUuZWR1LmNu
 Haijian Yang
Haijian Yang Mingzhi Zhang
Mingzhi Zhang Wenqian Wu
Wenqian Wu Na Xiao
Na Xiao Mengmeng Sun
Mengmeng Sun Yuan Li
Yuan Li