- 1Department of Food Science, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, United Arab Emirates
- 2Food Research Section, Applied Research and Capacity Building Division, Abu Dhabi Agriculture and Food Safety Authority (ADAFSA), Abu Dhabi, United Arab Emirates
- 3Food Technology Department, Arid Lands Cultivation Research Institute (ALCRI), City of Scientific Research and Technological Applications (SRTACITY), New Borg El-Arab, Egypt
- 4Agriculture Research Section, Applied Research and Capacity Building Division, Abu Dhabi Agriculture and Food Safety Authority, Al Ain, United Arab Emirates
- 5Department of Plant Protection and Molecular Diagnosis, Arid Lands Cultivation Research Institute, City of Scientific Research and Technological Applications (SRTACity), Alexandria, Egypt
- 6Department of Poultry Science, College of Agriculture, Auburn University, Auburn, AL, United States
Based on their geographical origins, the functional compound profile of beehive propolis varies significantly. The present study evaluates the phenolic and flavonoid, antioxidant, and antimicrobial activities of propolis extracts (PE) sourced from four different geographical areas in the UAE. The Kuwaitat and Al-Wathba propolis extracts were further used as an additive in minced beef burger to demonstrate their natural preservative effects to enhance shelf life and keeping quality over 15 days of refrigerated storage. Kuwaitat and Al-Wathba propolis ethanol extracts using DPPH assays with IC50 0.30 ± 0.052 and 0.28 ± 0.002 mg/mL, respectively, showed highest antioxidant activities. The HPLC analysis of phenolic profile in Kuwaitat and Al-Wathba propolis extracts confirmed the presence of polyphenolic compounds including vanillic acid, p-coumaric acid, resveratrol, and quercetin. Furthermore, Al-Wathba and Kuwaitat sourced propolis exhibited good antimicrobial activity against various pathogenic strains. The total aerobic counts in meat burger products incorporated with Kuwaitat and Al-Wathba propolis extracts were 25 and 25.6% lower than the untreated meat products, respectively, with the Al-Wathba extract fortified burger exhibiting a shelf life of 9 days in chilled storage which was 67% higher than the untreated samples. These fortified meat burger formulations also showed significant inhibition rates against Escherichia coli and Salmonella senftenberg after 15 days of refrigerated storage, respectively. These findings suggest that beehive propolis from four different UAE regions have good antioxidant and antimicrobial properties and can be safely used to improve shelf-life safety in minced beef products.
Introduction
Minced beef is a key ingredient in many food preparations across the world, including beef burgers and patties which exhibits high perishability (Del Nobile et al., 2009; Chuang et al., 2023). Due to a high protein, fat and water content along with a large surface area created due to mincing, such meat products are susceptible to microbial and oxidative spoilage (Djordjević et al., 2018; Wang et al., 2023). The addition of natural additives in minced meat formulations having antimicrobial and antioxidant properties are gaining significant importance to improve the storage stability (Estévez, 2021; Efenberger-Szmechtyk et al., 2021). Natural plant-based sources of bioactive compounds such as, blackberry, blueberry, black chokeberry, red currant, rosemary, garlic, red onion, pepper etc. are continuously being explored for functional components having potential applications in extending the shelf life of meat and meat products (Olivas-Méndez et al., 2022; Babaoğlu et al., 2022; Sarvinehbaghi et al., 2021; Dai et al., 2022). Extracts from natural sources prevent oxidative degradation and ensure microbial safety in meat formulations due to their rich polyphenolic profile (Babaoğlu et al., 2022). Besides, improving the storage stability, such additives also impart particular health benefits including antidiabetic, anticancer cardio-protective and antiallergen properties in the fortified meat products (Abd-El-Aziz et al., 2021; Lorenzo et al., 2018).
Propolis, a resinous substance is usually an adhesive material prepared by honeybees (Apis mellifera L.) by mixing resins collected from cracks in barks of diverse trees and leaf buds with salivary enzymes and partially digested materials along with beeswax (Yosri et al., 2021; Burdock, 1998). These resourceful insects utilize propolis as a bioderived adhesive within the beehive’s structure (Wagh, 2013). Propolis constitutes of a combination of wax, resin, balsam, pollen, essential oils, organic compounds, and honeybee saliva (Zulhendri et al., 2021). Bees utilize this material to seal hexagonal cells and protect the hive from external threats and environmental factors (Kieliszek et al., 2023). Propolis primarily consists of polyphenolic compounds (58%), including flavonoids (28%)—a significant subclass within polyphenols—as well as terpenoids, steroids, sugars, and amino acids (Shehata et al., 2020a,b; Zullkiflee et al., 2022). Polyphenols, a diverse group of chemical compounds with multiple phenol units, include various sub-classes, with flavonoids being notable for their potent antioxidant and antimicrobial properties (Cianciosi et al., 2020; Hassanpour and Doroudi, 2023). The diversity of polyphenolic compounds and chemical composition in propolis are influenced by its geographical and botanical origins (Arruda et al., 2019; Shehata et al., 2020a). Propolis extract (PE) have shown antioxidant, anticancer and antimicrobial properties, therefore attracting significant research interest (Ding et al., 2021, 2024; Touzani et al., 2019). Numerous studies have investigated the potential of PE in improving storage stability of meat products, including beef patties (Vargas-Sánchez et al., 2014), beef meatballs (Gedikoğlu, 2022), sausages (Casquete et al., 2016), and fish fillets (Ucak et al., 2020). Additionally, due to its strong antimicrobial properties, South Korean authorities approved new oral formulations based on propolis in response to the coronavirus outbreak (Berretta et al., 2020). The potential antioxidant and antimicrobial applications of propolis in different food processing applications are further reviewed by Irigoiti et al. (2021) and Segueni et al. (2023).
This study investigates the chemical composition of beehive propolis collected from four different regions in the UAE. Propolis extracts using different solvents were screened for their potential antioxidant and antimicrobial activities. Furthermore, fortified meat burgers were used as a food application of Emirati propolis extracts to evaluate their natural preservative potential for extending shelf life and ensuring food safety. The impact of these additions on microbial safety was analyzed during refrigerated storage at 4°C over a period of 15 days.
Materials and methods
Sample collection
Samples for beehive propolis were collected from four district locations, Kuwaitat, Al-Wathba, Mazyed, and Fujairah. The samples from Kuwaitat and Al-Wathba were obtained from honeybee hives located at Kuwaitat Research Station and Al-Wathba Forest, respectively. While the propolis samples from Mazyed and Fujairah were collected from local beekeepers.
Propolis Extract preparation
For maximum extraction, various solvents are required due to the wide variety of bioactive compounds and concentrations of these compounds across natural sources. In this study, a comparison was made between beehive propolis from four different geographical regions of the UAE using several types of extracts: water, ethanol, acetone and mix equal volume of ethanol, and acetone. The extraction procedure was conducted following the protocol of Wang et al. (2021) with some modifications. Briefly, 20 g of raw propolis were macerated and subjected to extraction with 200 mL of four different solvents (1:10 w/v) including, water, ethanol, acetone, and a 1:1 ethanol-acetone (E/A) mixture at room temperature for 6 h. The solvents were later separated from the extracts inside a vacuum assisted rotary evaporator at 40°C (KV400, Karl Kolb, West Germany), followed by freeze drying at −50°C and 0.05 mbar (LyoAlfa 15, Telstar, Barcelona, Spain) and the component yields were assessed. The solubilized freeze-dried extracts were later used as a functional ingredient in the minced beef formulations.
Analysis of total phenolic and flavonoid contents
The total phenolic and flavonoid contents were analyzed following methods as available in Mostafa et al. (2022) and Ashraf et al. (2024). Gallic acid and catechin were used as standards for phenolics and flavonoids. The overall phenolic content was expressed as milligrams of gallic acid equivalent per gram of sample (mg GAE/g) and flavonoid content was quantified as milligrams of catechin equivalents per gram of sample.
Color analysis
Color parameters of propolis from different geographical regions, prior to extraction, were assessed using a Hunter Lab Color Flex (Reston, VA), calibrated with white, black, and green tiles. The color analyses were conducted in triplicate on samples processed under identical conditions, with the results presented as mean values along with their standard deviations.
Determination of antioxidant activity
Antioxidant activity of the propolis extracts, in terms of the radical scavenging potential of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was analysed per Shehata et al. (2020b) and Gu et al. (2020) with some modifications. Three mL of 600 μM DPPH solution (absorbance of 0.7 ± 0.02 at 517 nm) was added to 30 μL of propolis extracts and incubated in dark for 30 min. The scavenging of characteristic dye color was measured in terms of absorbance using a 96 well microplate reader (Epoch 2, BioTek, Agilent Technologies, US), and the extract activity was evaluated using:
The IC50 value (mg extract/mL) represents the concentration of the test sample needed to scavenge 50% of DPPH radicals, determined through interpolation from linear regression analysis. Antioxidant activity is reported as the IC50, indicating the extract concentration or reference compound needed to prevent 50% of DPPH radical generation.
Bacterial strains and determination of antimicrobial activity
The antimicrobial efficacy of propolis extracts and minimum inhibition concentration (MIC) was assessed using the agar well diffusion technique (Airouyuwa et al., 2024; Shehata et al., 2017). Nine pathogenic bacteria (Table 1) were tested using the agar well assay. Each microorganism was mixed with a specific medium and spread in Petri dishes. After placing 100 μL of propolis extracts into wells on the agar plates, they were incubated for 48 h at 37°C. The inhibition zones were then measured in millimeters and MIC was determined as the minimum extract concentration where there was no visible microbial growth after the incubation period.
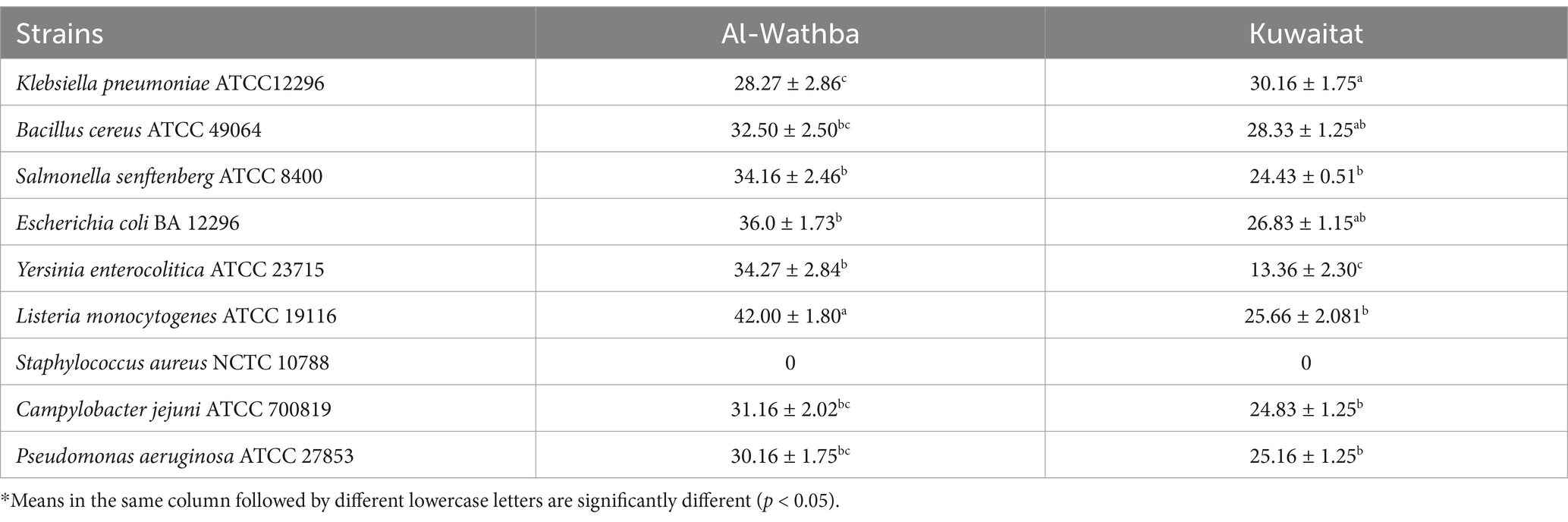
Table 1. Antimicrobial activity of Kuwaitat and Al-Wathba propolis extracts expressed as mm of inhibition due to well diffusion.
Determination of polyphenolic profile by RP-HPLC
The polyphenolic profiles of the propolis extracts were further analyzed through high pressure liquid chromatography (HPLC) (Agilent 1,260, Santa Clara, CA, United States) following the method of Fadil et al. (2022) with some modifications. The separation was carried out on a Zorbax Eclipse Plus C18 column (100 mm × 4.6 mm, Agilent technologies, United States) with detector set at 284 nm. The mobile phase comprised of 0.2% H3PO4 (Solvent A), 100% methanol (Solvent B) and acetonitrile (Solvent C). The main phenolic compounds were expressed as mg/kg of sample.
Scanning electron microscopy (SEM)
Samples of treated foodborne pathogen cells were fixed in 3% glutaraldehyde. The samples were then prepared for scanning electron microscopy (SEM) to examine cell morphology. SEM imaging was performed at the SEM Lab. at Khalifa University (15 kV; JEOL JSM6360LA SEM, Japan). The sample preparation and imaging procedures followed the method detailed by Munir et al. (2016), ensuring imaging for clear observation of morphological changes.
Microbiological profile analysis of meat burger
The microbial growth on the propolis-extract fortified meat burgers was analyzed from a representative 10 g sample at regular intervals during 15 days of storage at 4°C. Each sample (10 g) was homogenized with 90 mL of sterile saline solution (0.9% w/v) using the method outlined by Gull et al. (2021). Total mesophilic bacteria, Salmonella and E. coli counts were quantified via plate count agar (PCA), SS agar and MacConkey Agar (Himedia, India). The plates were incubated at 30°C for 48 h for total mesophilic bacteria whereas Salmonella and E. coli were incubated at 37°C for 48 h. Yeasts and molds were quantified on acidified potato dextrose agar (PDA) from Himedia, India, incubated at 30°C for 48 to 72 h. All microbiological assessments were carried out using the pour plate technique as outlined by Elshobary et al. (2020). The microbial counts were reported as log10 colony-forming units per gram (log10 CFU/g) of the meat burgers.
Antimicrobial assessment against foodborne pathogens
Approximately 100 g of meat burger samples were portioned into individual sterile plastic packages. To investigate the effects on each pathogen, the meat burger samples were allocated into three distinct experimental treatments (control, Escherichia coli and Salmonella senftenberg). The pathogenic strains and propolis extract were introduced at a rate of 1 mL per gram of meat burger, yielding a system with a starting load of 7 log10 CFU/g for both Escherichia coli BA 12296 and Salmonella senftenberg ATCC 8400, with each pathogenic strain being inoculated separately.
After inoculation, the samples were stored in dark at 4°C for 15 days, resulting in 36 samples (comprising 2 pathogenic strains, each subjected to 3 treatments, and analyzed at 6 different time intervals during storage). Viable cell counts were conducted on each sample at the 0th, 3rd, 6th, 9th, 12th, and 15th days of storage. For the enumeration of viable Escherichia coli and Salmonella senftenberg strains, Violet Red Bile Agar (VRBA) and SS agar was used, and the plates were incubated for 24 h at 37°C (Balogun et al., 2023). The results were then presented as means of log10 CFU/ g of sample.
Statistical analysis
The results presented in this work are mean ± standard deviation of a minimum of three replications. The statistical analysis was performed using SPSS (IBM, SPSS Inc., v20). One way analysis of variance was performed and means were compared through Duncan’s post-hoc test at a significance level of p < 0.05.
Results and discussion
Solvent specificity and its effect on propolis extract yield
The effect of solvent polarization on the effectiveness of extracting phytochemical components from different types of propolis was studied. The extraction of polyphenolic compounds is strongly related to the solubility of individual components in the solvent (Sridhar et al., 2021). Solvents with sequential polarization, ranging from the high polar solvent (water = 1) via the medium polar solvent (ethanol = 0.654) to the low polar solvent (acetone = 0.355), were evaluated. Data in Figure 1a show a significant difference in yield % between solvents. Among the propolis varieties from different regions, Fujairah propolis yielded the maximum extract yield. On the other hand, among the solvents, maximum extractability was exhibited by ethanol for Kuwaitat (17.75%), Al-Wathba (34.33%) and Mazyad (41.67%) propolis varieties, whereas, ethanol/acetone (1:1) mixture yielded highest extract from Fujairah (55.54%) propolis (p < 0.05). Using water as a solvent resulted in the lowest extraction from different propolis varieties. For acetone solvent, the highest yields were obtained for the Mazyad propolis (39.76%) and Fujairah propolis (34.43%), followed by the Al-Wathba propolis (28.41%) and Kuwaitat propolis (9.10%). These findings support the ability of ethanol to effectively penetrate propolis matrices and extract biologically active compounds. Similar results have been reported in the literature by Ibrahim and Alqurashi (2022), wherein the authors observed a significantly higher extract yield of phenolic content from propolis using ethanolic solution in comparison to water extracts. This may be due to specific solubility of extracted components with non-polar solvents like ethanol and acetone (Chisté et al., 2011).
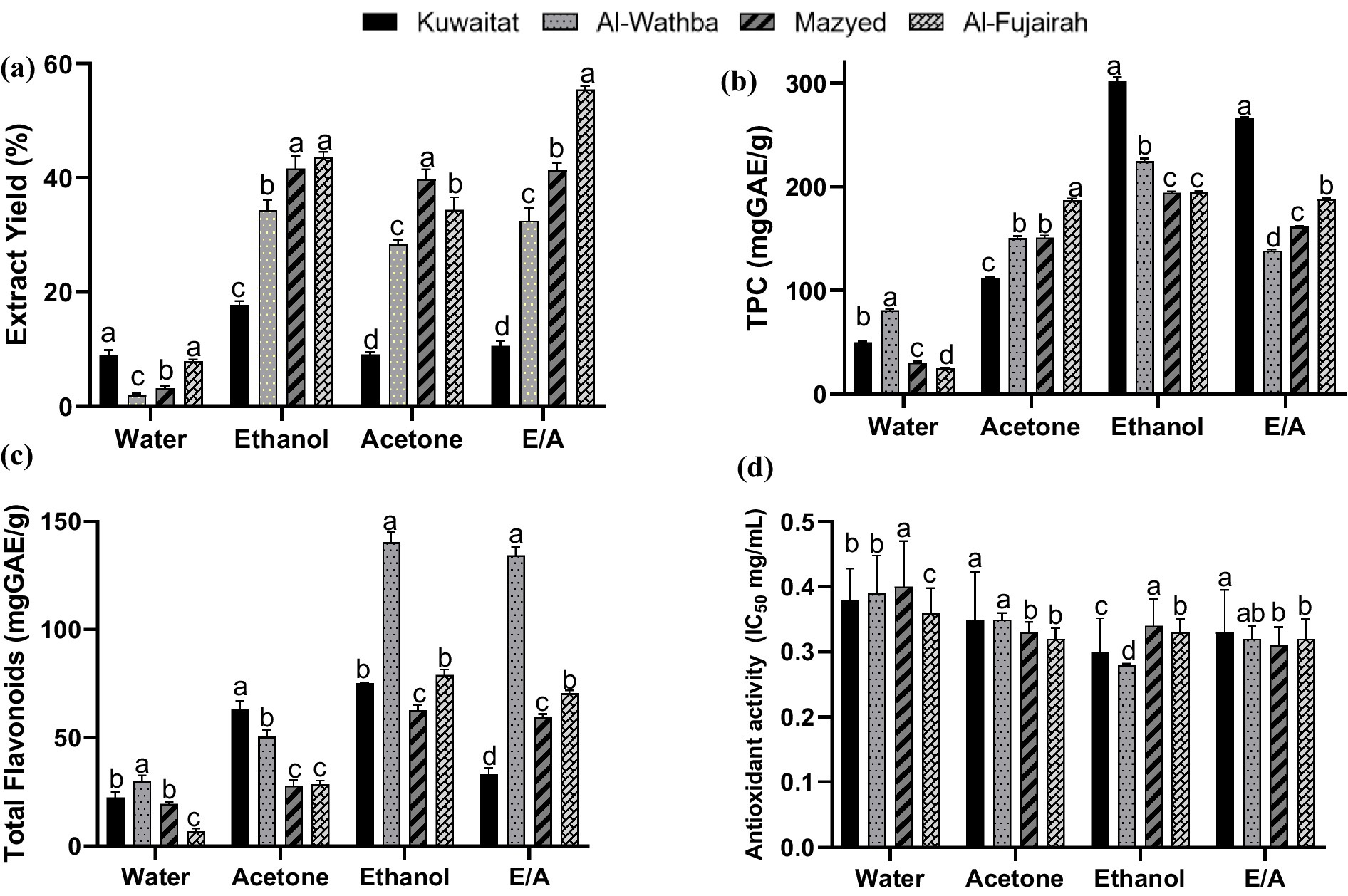
Figure 1. Characteristics of propolis extracts from different geographic regions in UAE (a) Extract yield (%); (b) Total Phenolic Content (mg GAE/G of dry extract); (c) Total Flavonoids Content (mg GAE/G of dry extract); and (d) Antioxidant activity (IC50 mg/mL) of propolis from different regions in Emirates. Means in the same group associated with different lowercase letters are significantly different (p < 0.05).
Physicochemical characteristics of propolis
Color attributes
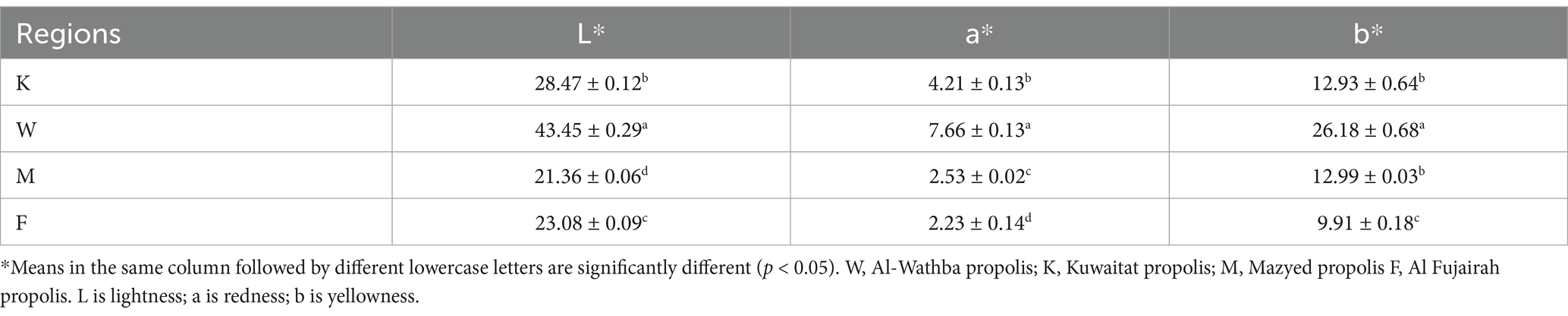
The results shown in Table 2 indicate significant differences in the L*, a*, and b* values of propolis samples collected from different areas. This suggests that the color characteristics of propolis vary depending on their geographical origin. Additionally, the color intensity in terms of darkness (lower L* values) of Al Fujairah propolis was higher than Al-Wathba propolis probably due to the diversity of botanical source. For the different types of propolis, lightness (L*) value of Al-Wathba propolis was higher than other propolis extracts. This was reflected in terms of visual perception through the light color of Al-Wathba propolis and may have a positive effect when extracts are added in moderate concentration to food (such as juices) without affecting their natural color. On the other hand, Al-Wathba propolis had highest a* and b* values, followed closely by Kuwaitat, Mazyed and Fujairah propolis, respectively, (Table 2). Previously, Pant et al. (2021) reported L* values of Indian propolis to be in the range of 38.15–41.99, while as a* (1.09–2.64) and b* (5.43–10.2) were also observed. These findings highlight the significance of considering the color characteristics of propolis extracts when utilizing them in the food industry. Careful selection of propolis extract type and concentration should be exercised to prevent any negative impact on the natural color of food products, ensuring that the desired aesthetic qualities are maintained. Further investigations are needed to identify the compounds that are responsible for imparting dark color in propolis extracts and to explore methods to minimize their potential negative effects on food coloration.
Assessment of total phenolic and flavonoid content in propolis extracts
The total phenolic content in different propolis extracts is presented in Figure 1b. The total phenolic content of the different types of propolis ranged from 24.96 ± 0.75 to 302.02 ± 3.28 mg GAE/g of dry sample. It can be seen that the highest phenolic content was detected in ethanol extract of Kuwaitat propolis (302.02 ± 3.28 mg GAE equivalent/g). On the other hand, the flavonoid content ranged from 6.84 ± 1.31 to 140.42 ± 4.64 mg QE equivalent/g of dry sample. The ethanol extract of Al-Wathba propolis had the highest flavonoid content (140.42 ± 4.64 mg QE equivalent/g), followed by the Mix (Ethanol: Acetone 1:1) extract of Al-Wathba propolis (134.44 ± 3.76 mg QE equivalent/g) (Figure 1c). It is noteworthy that the matrices investigated in this study contained approximately twice the total phenolic content in comparison to previously published research (Banwo et al., 2021; Kurek et al., 2022). Importantly, this study is the first to examine the concentrations of total phenolics and flavonoids in various types of Emirati propolis, as these specific details have not been previously documented. Natural sources of bioactive compounds including phenolics and antioxidants are continuously being sought by the food industry for the development of functional products having health benefits. Consequently, propolis has garnered significant interest among those focused on health-enhancing foods, owing to its rich content of bioactive compounds. These compounds are known for their health benefits, particularly due to their capacity to neutralize harmful free radicals (Shehata et al., 2020a,b; Šuran et al., 2021).
Phenolic compound profile (RP-HPLC)
As previously reported, it is well documented that ethanolic and methanolic propolis extracts predominantly comprise of polyphenols, especially flavonoids (Hossain et al., 2022). Table 3 summarizes polyphenols and flavonoid compounds quantified using HPLC on ethanol extracts of Kuwaitat and Al-Wathba propolis. These two propolis types were analysed due to their higher phenolic activities in comparison to Fujairah and Mazyed propolis types (Figure 1). Most identified polyphenolic compounds (=16) were detected in Kuwaitat propolis extract, followed by 10 components in Al-Wathba propolis extracts. While the quantity of compounds identified in the Al-Wathba propolis extract is lower than that in the Kuwaitat propolis extract, it was observed that certain phenolic compounds in Al-Wathba exhibit a concentration that was 10 times higher than those found in Kuwaitat propolis extract. Kuwaitat propolis had the highest concentration of Vanillic acid among all polyphenolic compounds detected. In contrast, in Al-Wathba propolis, the highest concentrations were observed for Vanillic acid, p-coumaric acid, Resveratrol, and Quercetin compounds.

Table 3. High-performance liquid chromatography (HPLC) analysis of phenolic compounds expressed as amount (mg/kg), present in Kuwaitat and Al-Wathba propolis extract.
The chemical profiles of Emirate propolis collected from Kuwaitat and Al-Wathba were similar to various propolis types reported in literature. Polyphenolic compounds such as quercetin, rutin, gallic acid, ferulic acid, caffeic acid, chlorogenic acid, cinnamic acid, p-coumaric acid etc. have been previously reported in propolis from different origins (Hossain et al., 2022; Touzani et al., 2019). HPLC analysis results corroborate these findings. Notably, phenolic acid and flavonoid compounds have been identified to exhibit antibacterial and antioxidant properties (Huang et al., 2014; Halagarda et al., 2020). Additionally, Supplementary Figure S1 demonstrates that numerous compounds have not been identified due to the substantial and diverse amounts of compounds present in the Kuwaitat propolis extract samples.
Antioxidant activity
Antioxidant activity of the beehive propolis extracts from four different places in the Emirates are presented in Figure 1d in terms of IC50 values. The IC50 values of standard ascorbic acid and the ethanol extracts of propolis from Kuwaitat, Al-Wathba, Mazyed and Al Fujairah, UAE were 0.14 ± 0.032, 0.28 ± 0.002, 0.30 ± 0.052, 0.34 ± 0.041 and 0.33 ± 0.020, mg/mL, respectively. The results indicate that the Kuwaitat propolis extracts had the highest free radical scavenging activity. This can be attributed to the types and concentrations of polyphenolic compounds present in this propolis, which are derived from the buds of trees native to the region where the bees foraged. The results demonstrated that the propolis extracts are highly effective antioxidants. Antioxidants are widely recognized for their critical role in preventing oxidative stress (Kocot et al., 2018). Touzani et al. (2021) previously reported that the antioxidant activity of the Fez, Morocco; Sefrou, Morocco; Boulemane, Morocco; Jenin, Ramallah, Palestine samples had IC50 values of 0.08 ± 0.02, 0.02 ± 0.02, 0.07 ± 0.01, 0.04 ± 0.001, and 0.14 ± 0.01 mg/mL, respectively. Results observed in this study for ethanol extracts of propolis from Kuwaitat, Al-Wathba, Mazyed and Al Fujairah are comparatively higher than those observed by Touzani et al. (2021), suggesting that propolis from different regions of UAE have higher antioxidant activity. In addition, Ibrahim and Alqurashi (2022) observed that ethanolic extracts of propolis had higher antioxidant activity, in terms of DPPH free radical scavenging activity (94.45%) in comparison to water extracts (90.01%). High antioxidant activity of the propolis extracts from Portugese urban apiaries was also confirmed by Pobiega et al. (2023), who reported ABTS, DPPH and FRAP antioxidant activities of extracts to be in the range of 16.80–51.53, 7.54–22.13 and 10.93–29.55 mg Trolox equivalents (TE)/mL, respectively. The potential health benefits of such antioxidant rich polyphenolic compounds are well researched (Lobo et al., 2010).
Antimicrobial activity
Previous research has demonstrated that propolis extracts show significant antimicrobial activity against several food-borne pathogens (Bouchelaghem, 2022). The Kuwaitat and Al-Wathba propolis extracts were assessed against a range of both Gram positive and negative bacterial strains. The inhibition zones (in millimeters) for these extracts against selected microorganisms are detailed in Table 1. The results observed suggest that Listeria monocytogenes ATCC 19116 showed greater sensitivity to the Al-Wathba propolis extracts compared to the other tested pathogens. Conversely, Klebsiella pneumoniae ATCC12296 displayed a higher sensitivity to the Kuwaitat propolis extracts.
Kuwaitat and Al-Wathba propolis extracts (100 mg/mL) exhibited the strongest antimicrobial activity against all tested microorganisms, with the exception of Staphylococcus aureus NCTC 10788, which was resistant to both propolis extracts even at this higher concentration. The resistance of Staphylococcus aureus to these propolis extracts may be due to its robust cell wall, formation of biofilms, and efflux pumps, which together limit antimicrobial agent penetration and efficacy (Wang et al., 2021). Among the two extracts, Al-Wathba propolis demonstrated superior antimicrobial efficacy against the same pathogens. However, it is worth noting that the minimum inhibitory concentration (MIC) values were between 25 to 50 mg/mL, indicating that the propolis extracts can hinder the growth of both Gram +ve and Gram -ve bacteria (Table 4).
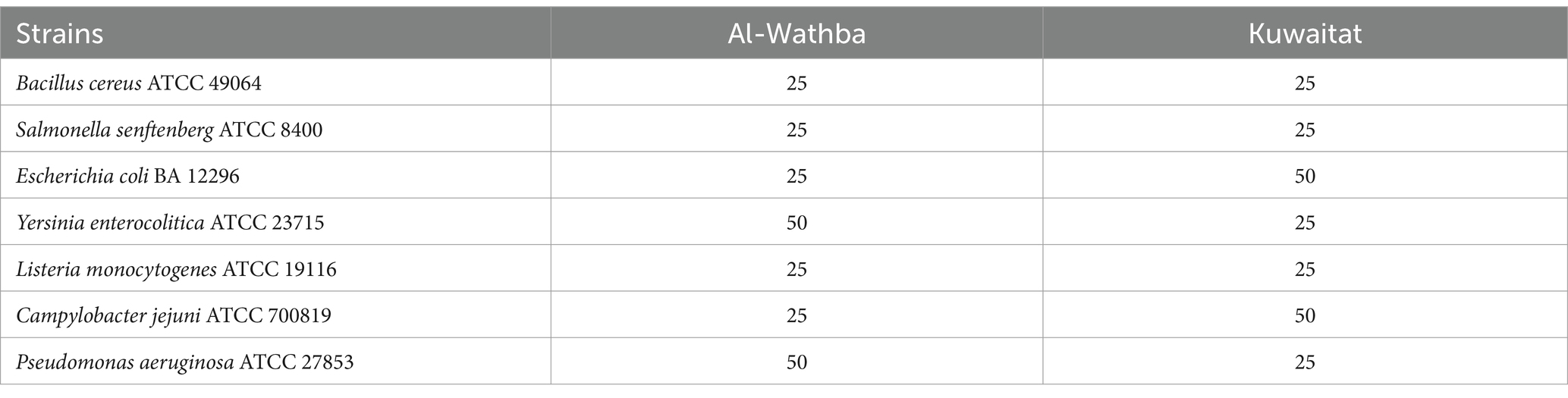
Table 4. Minimum inhibitory concentration (MIC) value (mg/mL) of Kuwaitat and Al-Wathba propolis extracts.
Propolis extracts have shown significant effects against foodborne pathogens, largely due to a rich polyphenolic profile (Pobiega et al., 2019). Research indicates that these phenolic compounds, including rutin, quercetin, and naringenin, are essential for the antimicrobial activity observed in propolis. These compounds have been found to interact with bacterial cell membranes, increasing their permeability and causing disruptions in membrane integrity. Additionally, phenolic compounds can inhibit ATP production and bind to metabolic enzymes, leading to the damage to the bacterial cells (Bordes et al., 2019). Furthermore, propolis extracts are also rich in flavonoids, which have previously been reported to effectively hamper bacterial metabolism and DNA and RNA synthesis in bacteria, further contributing to their antimicrobial effects (Mirzoeva et al., 1997).
Bacterial cell morphology through scanning electron microscopy (SEM)
The morphology of Salmonella senftenberg ATCC 8400 cells treated with Al-Wathba and Kuwaitat propolis extracts (25 mg/mL) was studied using SEM (Figure 2). After exposure to propolis extracts at 37°C for 24 h, Salmonella senftenberg ATCC 8400 cells witnessed damages to the structural integrity of cells which was visualized in terms of cell lysis caused due to the separation of cell wall and cytoplasmic membrane followed by leaching of cell components. The effect of Al-Wathba propolis extract was particularly interesting as different holes were created in the external bacterial cell walls. In contrast, untreated and intact Salmonella senftenberg ATCC 8400 cells can be visualized as short rod-shaped cells (Figure 2). Extract of Al-Wathba propolis had a very strong effect on Salmonella senftenberg ATCC 8400 cells that showed maximum sensitivity to propolis extracts. On the other hand, Al-Wathba propolis extract showed mild effect on the cells of Salmonella senftenberg ATCC 8400.
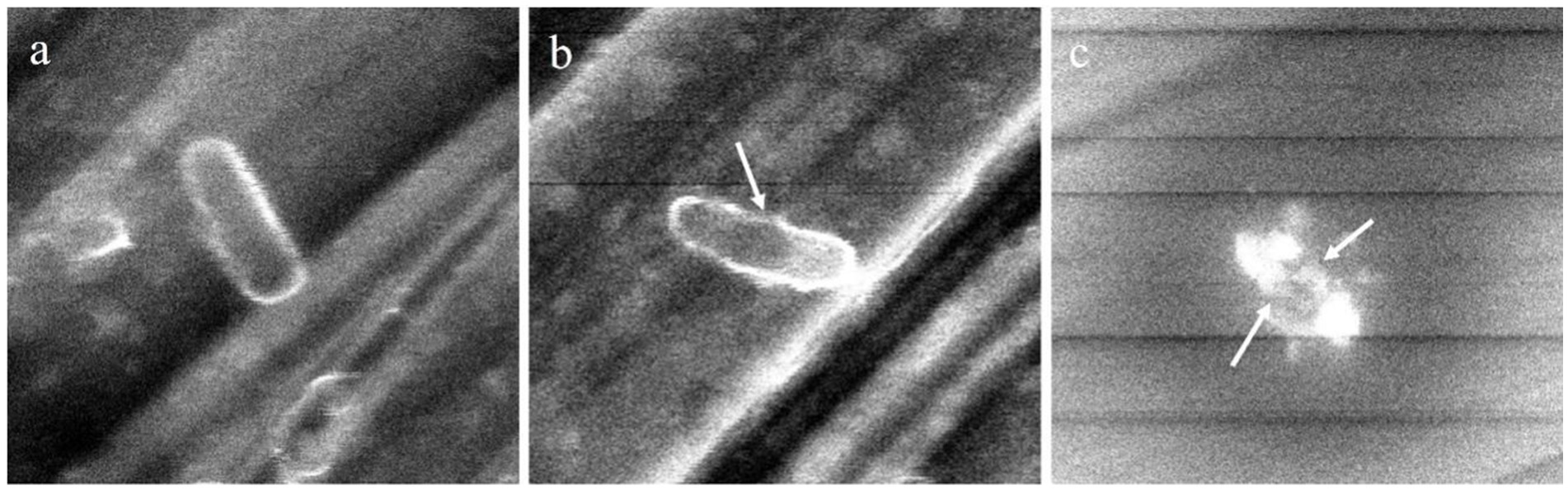
Figure 2. Morphology of Salmonella senftenberg ATCC 8400 cells treated with propolis extracts. (a) control cell that was not treated with extract, (b) cells treated with kuwaitat propolis extracts, (c) cells treated with Al-wathba propolis extracts. The white arrow shows pores in the cell membrane.
Previously, changes in the morphology of pathogenic microorganisms have been documented to have a crucial impact on intracellular processes (Kalchayanand et al., 2004). Consequently, damage to these microorganisms can lead to cell inactivation and/or death when treated with propolis extract (Corrêa et al., 2020; Wang et al., 2021, and Bouchelaghem, 2022). The observed morphological changes in the Salmonella senftenberg could possibly be due to a high oxidative stress imparted by the propolis extracts, thereby increasing permeability and decreasing its structural integrity which results in the cell lysis (Bajpai et al., 2009; Tassou et al., 2000).
Microbiological analysis of meat burger fortified with propolis extracts
The antimicrobial properties of Kuwaitat and Al-Wathba propolis extracts were further studied by incorporating them into minced meat for burger formulations. The microbiological changes of the meat burger during chilled storage at 4°C for 15 days are shown in Table 5. The findings reveal a gradual increase in microbial count over time for all samples during the storage period.
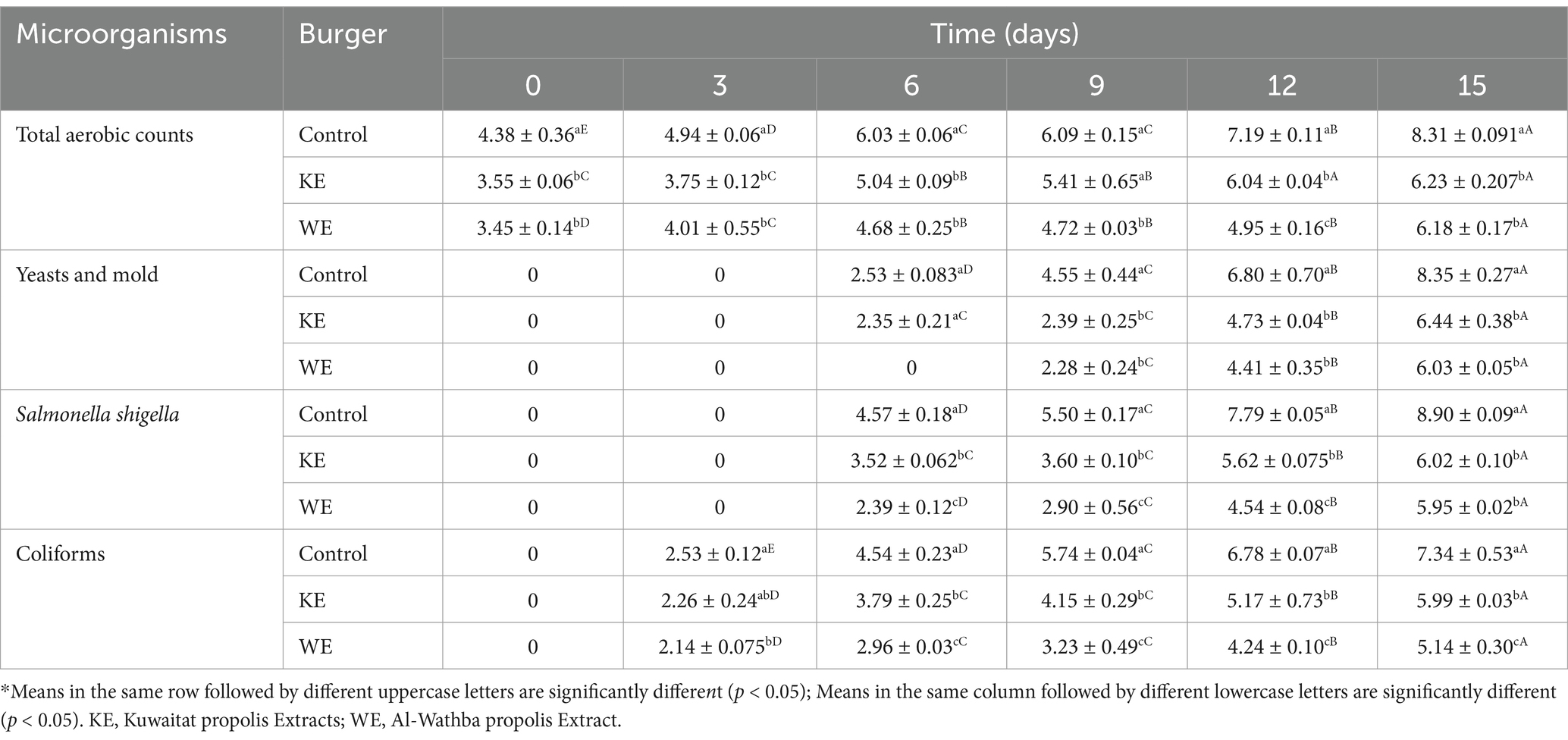
Table 5. Microbial populations (log CFU/g) of functional meat burger products fortified with Kuwaitat and Al-Wathba propolis extract during 15 days of storage at 4 ͦ C.
The control sample witnessed a significant increase of total microbial load with storage from 4.38 ± 0.36 to 8.31 ± 0.091 log CFU/g for total aerobic counts, from 0 to 8.35 ± 0.27 log CFU/g for Yeasts and mold, from 0 to 8.90 ± 0.09 log CFU/g for salmonella shigella and from 0 to 7.34 ± 0.53 log CFU/g for coliforms on the 15th day of chilled storage.
At the end of the storage time, bacterial populations in the samples treated with Al-Wathba propolis extract and Kuwaitat propolis extract were significantly lower than in the control sample which demonstrated a strong antimicrobial effect of studied propolis extracts. The total aerobic counts in meat burger products incorporated with Kuwaitat and Al-Wathba propolis extracts were 25 and 25.6% lower than the untreated meat products, respectively. The highest percentage difference was observed in the case of coliforms wherein the samples treated with Al-Wathba propolis extracts showed 30% lower count than control (Table 5). The bacterial counts were observed to be within the maximum permissible limit of 6 log CFU/g as specified in the sanitary specifications (Vargas-Sánchez et al., 2014) up to 12 days of storage period for Al-Wathba propolis extract. Accordingly, propolis extract could retard the microbial growth in beef burgers during chilled storage for 12 days, and shelf-life of the product was consequently extended in comparison to control (by 9 days). Similar results have been reported in literature. For example, the impact of propolis extract on enhancing the oxidative stability of protein and lipid within freshly prepared beef and pork patties during a 9-day refrigerated storage period was attributed to the notable antioxidant properties of the propolis extract (Vargas-Sánchez et al., 2019). Mahdavi-Roshan et al. (2022) investigated the effect of propolis aqueous extract on microbial, physical, chemical, and sensory attributes of marinated chicken breasts. The results showed that utilizing propolis extract at concentrations of 8 and 12% v/w for marinating chicken breasts resulted in a reduction of Staphylococcus aureus and Escherichia coli microbial counts, as well as decreases in pH and total Volatile nitrogen (TVN) levels during 12 days of storage at 5°C.
Inhibitory effect of propolis extract against pathogenic bacteria
The inhibition rates caused by propolis extract against E. coli and S. senftenberg are shown in (Figure 3). The meat burger fortification with Kuwaitat and Al-Wathba propolis extract showed significant differences (p < 0.05) in inhibition rates against E. coli from 8.11 ± 0.65 to 5.03 ± 0.55; 8.16 ± 0.49 to 5.26 ± 0.37 log10 CFU/g after 15th day of storage. The meat burgers fortified with Kuwaitat and Al-Wathba propolis extract also showed significant differences (p < 0.05) in inhibition rates against S. senftenberg from 8.13 ± 0.45 to 5.33 ± 0.35; 8.09 ± 0.19 to 5.07 ± 0.50 log10 CFU/g after 15th day of storage. The presence of Kuwaitat and Al-Wathba propolis extracts in meat burger treatments succeeded in decreasing the E. coli and S. senftenberg counts during 12th storage day (p >0.05), suggesting that the Kuwaitat and Al-Wathba propolis extracts had a significant antimicrobial activity against these foodborne pathogens. After 12 days of storage, E. coli and S. senftenberg counts reduced from 8.11 ± 0.65 to 3.03 ± 0.36 and 8.09 ± 0.19 to 3.64 ± 0.57 log10 CFU/g in treatments with Al-Wathba propolis extract, with the same trend in Kuwaitat propolis extract (Figure 3).
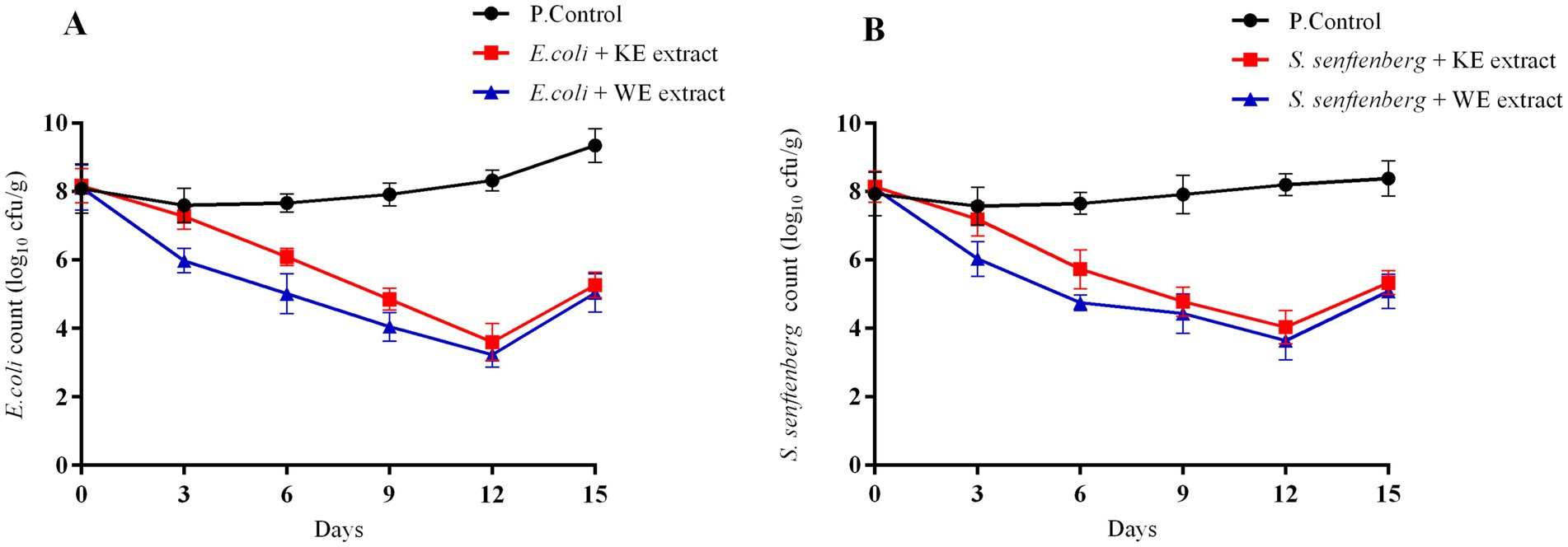
Figure 3. Inhibition rate of E. coli and S. senftenberg in meat burger products added with 1 mL/g propolis extracts throughout 15 days of storage at 4 ͦ C. Inhibition rate expressed as mean values ± standard deviation. (A), E. coli where; Positive Control: E. coli (P. Control), Treatments: Meat Burger with KE extract + E. coli; Meat Burger with WE extract + E. coli. (B), S. senftenberg where; Positive Control: S. senftenberg (P. Control), Treatments: Meat Burger with KE extract + S. senftenberg; Meat Burger with WE extract + S. senftenberg.
The reduction in foodborne pathogens observed in formulations fortified with Kuwaitat and Al-Wathba propolis extract compared to the negative control after 12 days of storage may be attributed to the presence of various antimicrobial compounds, including rutin, quercetin, and naringenin, which are capable of inhibiting pathogenic bacteria and fungi (Šuran et al., 2021). As a result, propolis extracts with antimicrobial properties against spoilage or pathogenic bacteria in their applied matrices are of significant interest for industrial use, as they help extend product shelf life (Touzani et al., 2021; Osaili et al., 2022).
Conclusion
In conclusion, this study sheds light on the distinct functional attributes of beehive propolis in UAE and the effect of geographical origin on its polyphenolic profile. The phenolic and flavonoid content along with the antioxidant and antimicrobial activities varied among propolis extracts from different locations. Importantly, our study reports the phytochemical and antimicrobial properties of Emirate propolis extracts for the first time. A novel application of propolis extracts to enhance the shelf life and safety of meat products was also presented. Incorporating Kuwaitat and Al-Wathba propolis extracts into meat burgers exhibited significant inhibitory effects on E. coli and S. senftenberg during storage, implying their utility as natural preservatives. The shelf life of Al-Wathba propolis extract fortified meat burgers was extended by almost 9 days in comparison to control samples under chilled storage conditions. These findings open up avenues for leveraging propolis extracts as valuable additions to the food industry, contributing to safer food products. As consumer demand for natural and sustainable preservatives grows, the potential of propolis as a preservative agent gains importance, promising a positive impact on both food quality and safety. Further exploration of propolis extract incorporation in various food products and its effects on sensory attributes would be of great interest for future research and application.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HM: Data curation, Investigation, Formal analysis, Methodology, Software, Writing – original draft. MS: Investigation, Writing – review & editing. HA: Conceptualization, Investigation, Writing – review & editing. SaaM: Writing – review & editing. RA: Software, Writing – original draft, Writing – review & editing. SS: Supervision, Writing – review & editing. SajM: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Authors would like to acknowledge United Arab Emirates University and Abu Dhabi Agriculture and Food Safety Authority (ADAFSA) for providing facilities to conduct this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1574880/full#supplementary-material
References
Abd-El-Aziz, N. A., El Sesy, T. A., and Hashem, S. M. (2021). Evaluation of nutritional value and acceptability of chicken nuggets produced by chicken wings and dehydrated shellfish. Food Nutr. Sci. 12, 805–817. doi: 10.4236/fns.2021.128060
Airouyuwa, J. O., Khan, H., Mostafa, H., Mudgil, P., and Maqsood, S. (2024). A comparative study on sequential green hybrid techniques (ultrasonication, microwave and high shear homogenization) for the extraction of date seed bioactive compounds and its application as an additive for shelf-life extension of Oreochromis niloticus. Ultrason. Sonochem. 111:107094. doi: 10.1016/j.ultsonch.2024.107094
Arruda, S., Almeida, C., Oliveira, S., Basílio-Júnior, I. D., Porto, M., Sabino, A. R., et al. (2019). Comprehensive multivariate correlations between climatic effect, metabolite-profile, antioxidant capacity and antibacterial activity of Brazilian red propolis metabolites during seasonal study. Sci. Rep. 9:18293. doi: 10.1038/s41598-019-54591-3
Ashraf, W., Rehman, A., Hussain, A., Karim, A., Sharif, H. R., Siddiquy, M., et al. (2024). Optimization of extraction process and estimation of flavonoids from fenugreek using green extracting deep eutectic solvents coupled with ultrasonication. Food Bioprocess Technol. 17, 887–903. doi: 10.1007/s11947-023-03170-6
Babaoğlu, A. S., Unal, K., Dilek, N. M., Poçan, H. B., and Karakaya, M. (2022). Antioxidant and antimicrobial effects of blackberry, black chokeberry, blueberry, and red currant pomace extracts on beef patties subject to refrigerated storage. Meat Sci. 187:108765. doi: 10.1016/j.meatsci.2022.108765
Bajpai, V. K., Al-Reza, S. M., Choi, U. K., Lee, J. H., and Kang, S. C. (2009). Chemical composition, antibacterial and antioxidant activities of leaf essential oil and extracts of Metasequioa glyptostroboides Miki ex Hu. Food Chem. Toxicol. 47, 1876–1883. doi: 10.1016/j.fct.2009.04.043
Balogun, M. A., Sobande, O. S., and Oyeyinka, S. A. (2023). Antimicrobial properties of onion and garlic extracts in beef and chicken. Food Chem. Adv. 3:100519. doi: 10.1016/j.focha.2023.100519
Banwo, K., Olojede, A. O., Adesulu-Dahunsi, A. T., Verma, D. K., Thakur, M., Tripathy, S., et al. (2021). Functional importance of bioactive compounds of foods with potential health benefits: a review on recent trends. Food Biosci. 43:101320. doi: 10.1016/j.fbio.2021.101320
Berretta, A. A., Silveira, M. A. D., Capcha, J. M. C., and De Jong, D. (2020). Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 131:110622. doi: 10.1016/j.biopha.2020.110622
Bordes, P., Sablé, S., Michel, G., and Louis, M. (2019). Antimicrobial peptides and self-assembling cyclic lipopeptides: a promising approach to tackle gram-negative bacteria. Probiotics Antimicrob. Proteins 11, 1010–1024.
Bouchelaghem, S. (2022). Propolis characterization and antimicrobial activities against Staphylococcus aureus and Candida albicans: a review. Saudi J. Biol. Sci. 29, 1936–1946. doi: 10.1016/j.sjbs.2021.11.063
Burdock, G. A. (1998). Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 36, 347–363. doi: 10.1016/S0278-6915(97)00145-2
Casquete, R., Castro, S. M., Jácome, S., and Teixeira, P. (2016). Antimicrobial activity of ethanolic extract of propolis in “Alheira”, a fermented meat sausage. Cogent Food Agric. 2:1125773. doi: 10.1080/23311932.2015.1125774
Chisté, R. C., Benassi, M. T., and Mercadante, A. Z. (2011). Effect of solvent type on the extractability of bioactive compounds, antioxidant capacity and colour properties of natural annatto extracts. Int. J. Food Sci. Technol. 46, 1863–1870. doi: 10.1111/j.1365-2621.2011.02693.x
Chuang, L., Jiyong, S., Chenguang, Z., Xiaowei, H., Xiaodong, Z., Zhikun, Y., et al. (2023). Effects of sodium chloride substitutes on physicochemical properties of salted beef. Food Chem. X 20:100885. doi: 10.1016/j.fochx.2023.100885
Cianciosi, D., Forbes-Hernandez, T. Y., Ansary, J., Gil, E., Amici, A., Bompadre, S., et al. (2020). Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: the effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 325:126881. doi: 10.1016/j.foodchem.2020.126881
Corrêa, J. L., Veiga, F. F., Jarros, I. C., Costa, M. I., Castilho, P. F., de Oliveira, K. M. P., et al. (2020). Propolis extract has bioactivity on the wall and cell membrane of Candida albicans. J. Ethnopharmacol. 256:112791. doi: 10.1016/j.jep.2020.112791
Dai, J., Hu, W., Yang, H., Li, C., Cui, H., Li, X., et al. (2022). Controlled release and antibacterial properties of PEO/casein nanofibers loaded with thymol/β-cyclodextrin inclusion complexes in beef preservation. Food Chem. 382:132369. doi: 10.1016/j.foodchem.2022.132369
Del Nobile, M. A., Conte, A., Cannarsi, M., and Sinigaglia, M. (2009). Strategies for prolonging the shelf life of minced beef patties. J. Food Saf. 29, 14–25. doi: 10.1111/j.1745-4565.2008.00145.x
Ding, Q., Sheikh, A. R., Gu, X., Li, J., Xia, K., Sun, N., et al. (2021). Chinese Propolis: ultrasound-assisted enhanced ethanolic extraction, volatile components analysis, antioxidant and antibacterial activity comparison. Food Sci. Nutr. 9, 313–330. doi: 10.1002/fsn3.1997
Ding, Q., Zheng, Y., Zhu, Y., Yang, H., Luo, L., Ma, H., et al. (2024). Propolis extract and Hermetia illucens larval proteins synergistically inhibit the growth of Aspergillus niger. Food Biosci. 61:104661. doi: 10.1016/j.fbio.2024.104661
Djordjević, J., Bošković, M., Starčević, M., Ivanović, J., Karabasil, N., Dimitrijević, M., et al. (2018). Survival of Salmonella spp. in minced meat packaged under vacuum and modified atmosphere. Braz. J. Microbiol. 49, 607–613. doi: 10.1016/j.bjm.2017.09.009
Efenberger-Szmechtyk, M., Nowak, A., and Czyzowska, A. (2021). Plant extracts rich in polyphenols: antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 61, 149–178. doi: 10.1080/10408398.2020.1722060
Elshobary, M. E., El-Shenody, R. A., Ashour, M., Zabed, H. M., and Qi, X. (2020). Antimicrobial and antioxidant characterization of bioactive components from Chlorococcum minutum. Food Biosci. 35:100567. doi: 10.1016/j.fbio.2020.100567
Estévez, M. (2021). Critical overview of the use of plant antioxidants in the meat industry: opportunities, innovative applications and future perspectives. Meat Sci. 181:108610. doi: 10.1016/j.meatsci.2021.108610
Fadil, M., Lebrazi, S., Aboulghazi, A., Guaouguaou, F. E., Rais, C., Slimani, C., et al. (2022). Multi-response optimization of extraction yield, total phenols-flavonoids contents, and antioxidant activity of extracts from moroccan Lavandula stoechas leaves: predictive modeling using simplex-centroid design. Biocatal. Agric. Biotechnol. 43:102430. doi: 10.1016/j.bcab.2022.102430
Gedikoğlu, A. (2022). Antimicrobial and antioxidant activities of commercialized Turkish Propolis extract, and application to beef meatballs. Turk. J. Agric. Food Sci. Technol. 10, 2021–2029. doi: 10.24925/turjaf.v10i10.2021-2029.5340
Gu, J., Zhang, H., Yao, H., Zhou, J., Duan, Y., and Ma, H. (2020). Comparison of characterization, antioxidant and immunological activities of three polysaccharides from Sagittaria sagittifolia L. Carbohydr. Polym. 235:115939. doi: 10.1016/j.carbpol.2020.115939
Gull, A., Bhat, N., Wani, S. M., Masoodi, F. A., Amin, T., and Ganai, S. A. (2021). Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 349:129149. doi: 10.1016/j.foodchem.2021.129149
Halagarda, M., Groth, S., Popek, S., Rohn, S., and Pedan, V. (2020). Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants 9:44. doi: 10.3390/antiox9010044
Hassanpour, S. H., and Doroudi, A. (2023). Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 13, 354–376. doi: 10.22038/AJP.2023.21774
Hossain, R., Quispe, C., Khan, R. A., Saikat, A. S. M., Ray, P., Ongalbek, D., et al. (2022). Propolis: an update on its chemistry and pharmacological applications. Chin. Med. 17:100. doi: 10.1186/s13020-022-00651-2
Huang, S., Zhang, C. P., Wang, K., Li, G. Q., and Hu, F. L. (2014). Recent advances in the chemical composition of Propolis. Molecules 19, 19610–19632. doi: 10.3390/molecules191219610
Ibrahim, M. E. E. D., and Alqurashi, R. M. (2022). Anti-fungal and antioxidant properties of propolis (bee glue) extracts. Int. J. Food Microbiol. 361:109463. doi: 10.1016/j.ijfoodmicro.2021.109463
Irigoiti, Y., Navarro, A., Yamul, D., Libonatti, C., Tabera, A., and Basualdo, M. (2021). The use of propolis as a functional food ingredient: a review. Trends Food Sci. Technol. 115, 297–306. doi: 10.1016/j.tifs.2021.06.041
Kalchayanand, N., Dunne, P., Sikes, A., and Ray, B. (2004). Viability loss and morphology change of foodborne pathogens following exposure to hydrostatic pressures in the presence and absence of bacteriocins. Int. J. Food Microbiol. 91, 91–98. doi: 10.1016/S0168-1605(03)00324-6
Kieliszek, M., Piwowarek, K., Kot, A. M., Wojtczuk, M., Roszko, M., Bryła, M., et al. (2023). Recent advances and opportunities related to the use of bee products in food processing. Food Sci. Nutr. 11, 4372–4397. doi: 10.1002/fsn3.3411
Kocot, J., Kiełczykowska, M., Luchowska-Kocot, D., Kurzepa, J., and Musik, I. (2018). Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxid. Med. Cell. Longev. 2018:7074209. doi: 10.1155/2018/7074209
Kurek, M., Benaida-Debbache, N., Garofulić, I. E., Galić, K., Avallone, S., Voilley, A., et al. (2022). Antioxidants and bioactive compounds in food: critical review of issues and prospects. Antioxidants 11:742. doi: 10.3390/antiox11040742
Lobo, V., Patil, A., Phatak, A., and Chandra, N. (2010). Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 4, 118–126. doi: 10.4103/0973-7847.70902
Lorenzo, J. M., Pateiro, M., Domínguez, R., Barba, F. J., Putnik, P., Kovačević, D. B., et al. (2018). Berries extracts as natural antioxidants in meat products: a review. Food Res. Int. 106, 1095–1104. doi: 10.1016/j.foodres.2017.12.005
Mahdavi-Roshan, M., Gheibi, S., and Pourfarzad, A. (2022). Effect of propolis extract as a natural preservative on quality and shelf life of marinated chicken breast (chicken kebab). LWT 155:112942. doi: 10.1016/j.lwt.2021.112942
Mirzoeva, O. K., Grishanin, R. N., and Calder, P. C. (1997). Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 152, 239–246. doi: 10.1016/S0944-5013(97)80034-1
Mostafa, H., Airouyuwa, J. O., and Maqsood, S. (2022). A novel strategy for producing nano-particles from date seeds and enhancing their phenolic content and antioxidant properties using ultrasound-assisted extraction: a multivariate based optimization study. Ultrason. Sonochem. 87:106017. doi: 10.1016/j.ultsonch.2022.106017
Munir, H., Shahid, M., Anjum, F., and Mudgil, D. (2016). Structural, thermal and rheological characterization of modified Dalbergia sissoo gum—a medicinal gum. Int. J. Biol. Macromol. 84, 236–245. doi: 10.1016/j.ijbiomac.2015.12.001
Olivas-Méndez, P., Chávez-Martínez, A., Santellano-Estrada, E., Guerrero Asorey, L., Sánchez-Vega, R., Rentería-Monterrubio, A. L., et al. (2022). Antioxidant and antimicrobial activity of rosemary (Rosmarinus officinalis) and garlic (Allium sativum) essential oils and chipotle pepper oleoresin (Capsicum annum) on beef hamburgers. Food Secur. 11:2018. doi: 10.3390/foods11142018
Osaili, T. M., Giatrakou, V., Ntzimani, A., Tsiraki, M., and Savvaidis, I. N. (2022). Application of quantitative microbiology and challenge tests to reach a suggested food safety objective in a middle eastern-style ready-to-cook chicken product. Food Secur. 11:1900. doi: 10.3390/foods11131900
Pant, K., Thakur, M., Chopra, H. K., Nanda, V., Ansari, M. J., Pietramellara, G., et al. (2021). Characterization and discrimination of Indian propolis based on physico-chemical, techno-functional, thermal and textural properties: a multivariate approach. J. King Saud Univ. Sci. 33:101405. doi: 10.1016/j.jksus.2021.101405
Pobiega, K., Kot, A. M., Przybył, J. L., Synowiec, A., and Gniewosz, M. (2023). Comparison of the chemical composition and antioxidant properties of propolis from urban apiaries. Molecules 28:6744. doi: 10.3390/molecules28186744
Pobiega, K., Kraśniewska, K., and Gniewosz, M. (2019). Application of propolis in antimicrobial and antioxidative protection of food quality–a review. Trends Food Sci. Technol. 83, 53–62. doi: 10.1016/j.tifs.2018.11.007
Sarvinehbaghi, M. B., Ahmadi, M., Shiran, M., and Azizkhani, M. (2021). Antioxidant and antimicrobial activity of red onion (Allium cepa, L.) extract nanoencapsulated in native seed gums coating and its effect on shelf-life extension of beef fillet. J. Food Meas. Charact. 15, 4771–4780. doi: 10.1007/s11694-021-00985-9
Segueni, N., Boutaghane, N., Asma, S. T., Tas, N., Acaroz, U., Arslan-Acaroz, D., et al. (2023). Review on propolis applications in food preservation and active packaging. Plants 12:1654. doi: 10.3390/plants12081654
Shehata, M. G., Ahmad, F. T., Badr, A. N., Masry, S. H., and El-Sohaimy, S. A. (2020a). Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 65, 209–217. doi: 10.1016/j.aoas.2020.12.001
Shehata, M. G., Badr, A. N., Abdel-Razek, A. G., Hassanein, M. M., and Amra, H. A. (2017). Oilbioactive films as an antifungal application to save post-harvest food crops. Annu. Res. Rev. Biol. 16, 1–16. doi: 10.9734/ARRB/2017/36149
Shehata, M. G., Darwish, A. M., and El-Sohaimy, S. A. (2020b). Physicochemical, structural and functional properties of water-soluble polysaccharides extracted from Egyptian agricultural by-products. Ann. Agric. Sci. 65, 21–27. doi: 10.1016/j.aoas.2020.05.004
Sridhar, A., Ponnuchamy, M., Kumar, P. S., Kapoor, A., Vo, D. V. N., and Prabhakar, S. (2021). Techniques and modeling of polyphenol extraction from food: a review. Environ. Chem. Lett. 19, 3409–3443. doi: 10.1007/s10311-021-01217-8
Šuran, J., Cepanec, I., Mašek, T., Radić, B., Radić, S., Tlak Gajger, I., et al. (2021). Propolis extract and its bioactive compounds-from traditional to modern extraction technologies. Molecules 26:2930. doi: 10.3390/molecules26102930
Tassou, C., Koutsoumanis, K., and Nychas, G. J. E. (2000). Inhibition of Salmonella enteritidis and Staphylococcus aureus in nutrient broth by mint essential oil. Food Res. Int. 33, 273–280. doi: 10.1016/S0963-9969(00)00047-8
Touzani, S., Embaslat, W., Imtara, H., Kmail, A., Kadan, S., Zaid, H., et al. (2019). In vitro evaluation of the potential use of propolis as a multitarget therapeutic product: physicochemical properties, chemical composition, and immunomodulatory, antibacterial, and anticancer properties. Biomed. Res. Int. 2019, 1–11. doi: 10.1155/2019/4836378
Touzani, S., Imtara, H., Katekhaye, S., Mechchate, H., Ouassou, H., Alqahtani, A. S., et al. (2021). Determination of phenolic compounds in various propolis samples collected from an African and an Asian region and their impact on antioxidant and antibacterial activities. Molecules 2021:4589. doi: 10.3390/molecules26154589
Ucak, I., Khalily, R., Carrillo, C., Tomasevic, I., and Barba, F. J. (2020). Potential of propolis extract as a natural antioxidant and antimicrobial in gelatin films applied to rainbow trout (Oncorhynchus mykiss) fillets. Food Secur. 9:1584. doi: 10.3390/foods9111584
Vargas-Sánchez, R. D., Torrescano-Urrutia, G. R., Acedo-Félix, E., Carvajal-Millán, E., González-Córdova, A. F., Vallejo-Galland, B., et al. (2014). Antioxidant and antimicrobial activity of commercial propolis extract in beef patties. J. Food Sci. 79, C1499–C1504. doi: 10.1111/1750-3841.12533
Vargas-Sánchez, R. D., Torrescano-Urrutia, G. R., Torres-Martínez, M., Pateiro, M., Lorenzo, J. M., and Sánchez-Escalante, A. (2019). Propolis extract as antioxidant to improve oxidative stability of fresh patties during refrigerated storage. Food Secur. 8:614. doi: 10.3390/foods8120614
Wagh, V. D. (2013). Propolis: a wonder bees product and its pharmacological potentials. Adv. Pharmacol. Pharm. Sci. 2013:308249. doi: 10.1155/2013/308249
Wang, F., Liu, H., Li, J., Zhang, W., Jiang, B., and Xuan, H. (2021). Australian propolis ethanol extract exerts antibacterial activity against methicillin-resistant Staphylococcus aureus by mechanisms of disrupting cell structure, reversing resistance, and resisting biofilm. Braz. J. Microbiol. 52, 1651–1664. doi: 10.1007/s42770-021-00547-7
Wang, H., Wang, J., Wang, Y., Gao, S., Xu, S., Zou, X., et al. (2023). Characterization and correlation of dominant microbiota and flavor development in different post-mortem processes of beef. Food Secur. 12:3266. doi: 10.3390/foods12173266
Yosri, N., Abd El-Wahed, A. A., Ghonaim, R., Khattab, O. M., Sabry, A., Ibrahim, M. A., et al. (2021). Anti-viral and immunomodulatory properties of propolis: chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Food Secur. 10:1776. doi: 10.3390/foods10081776
Zulhendri, F., Chandrasekaran, K., Kowacz, M., Ravalia, M., Kripal, K., Fearnley, J., et al. (2021). Antiviral, antibacterial, antifungal, and Antiparasitic properties of Propolis: a review. Food Secur. 10:1360. doi: 10.3390/foods10061360
Keywords: propolis extract, shelf-life extension, beef burger, antimicrobial potential, natural preservatives, functional food
Citation: Al Marzooqi HM, Shehata MG, Afifi HS, Masry SHD, Aslam R, Srikumar S and Maqsood S (2025) Antioxidant and antimicrobial properties of propolis from different geographic regions in UAE and its applications in shelf-life extension of beef burger. Front. Sustain. Food Syst. 9:1574880. doi: 10.3389/fsufs.2025.1574880
Edited by:
Wee Sim Choo, Monash University Malaysia, MalaysiaReviewed by:
Gulden Goksen, Tarsus University, TürkiyeAvik Mukherjee, Central Institute of Technology, Kokrajhar, India
Copyright © 2025 Al Marzooqi, Shehata, Afifi, Masry, Aslam, Srikumar and Maqsood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sajid Maqsood, c2FqaWQubUB1YWV1LmFjLmFl
 Hassan Mohamed Al Marzooqi
Hassan Mohamed Al Marzooqi Mohamed Gamal Shehata2,3
Mohamed Gamal Shehata2,3 Saad H. D. Masry
Saad H. D. Masry Raouf Aslam
Raouf Aslam Shabarinath Srikumar
Shabarinath Srikumar Sajid Maqsood
Sajid Maqsood