- 1China National Engineering Research Center of Juncao Technology, College of Life Science, Fujian Agriculture and Forestry University, Fuzhou, China
- 2Institute of Environmental Microbiology, College of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou, China
- 3College of Juncao Science and Ecology, Fujian Agriculture and Forestry University, Fuzhou, China
Malnutrition in underdeveloped regions is exacerbated by the lack of accessible, nutrient-dense foods and energy-intensive agricultural practices. This study introduces a sustainable non-sterile cultivation method for Pleurotus sapidus (an oyster mushroom species) using fresh Pennisetum giganteum (Giant Juncao grass), a fast-growing C4 grass cultivated on marginal soils. Unlike conventional sterilization-dependent approaches, our method employs lime-saturated water (LW, 4 mL·g−1) to pretreat fresh grass, eliminating the need for drying; a critical advantage in humid regions where biomass degradation occurs rapidly. Coupled with optimized substrate composition (2% CaO, 0.5% corn flour), this strategy achieved robust mycelial growth (0.53 cm·d−1) and high biological efficiency (112.78%). LW treatment altered substrate metabolites and reduced soluble nutrients. L-malic acid and soluble sugars promoted P. sapidus growth, whereas amino acids, available nitrogen (N), phosphorus (P) and potassium (K), and lactic acid inhibited its growth. The Mucor circinelloides was identified as a key contaminant. This scalable strategy transforms underutilized lignocellulosic biomass into nutrient-rich food, aligning with SDG 2 (Zero Hunger) and SDG 12 (Responsible Consumption and Production).
1 Introduction
Globally, over 800 million people face chronic hunger, with underdeveloped regions disproportionately affected by protein-energy malnutrition and micronutrient deficiencies (Jha et al., 2024). Edible mushrooms, notably Pleurotus spp., offer a sustainable protein source rich in essential amino acids, vitamins, and antioxidants (Jayasuriya et al., 2020), yet their cultivation remains inaccessible to low-income populations due to reliance on sterilization and lignocellulosic substrates like sawdust, which compete with livestock feed and fuel production (Albertia et al., 2021). Sterilized cultivation technology, involving mixing raw materials (e.g., sawdust, cottonseed hulls) with additives (e.g., bran, calcium carbonate), bagging, autoclaving, and aseptic inoculation, offers high standardization, stable production, and high conversion rates. However, it requires advanced equipment, high energy consumption, and skilled personnel (Xue et al., 2019). Non-sterile cultivation, which mixes cultivation materials (e.g., cottonseed hulls, bran) with fungicides and bags the mixture, is simple, low-cost, and energy-efficient. Nevertheless, it is prone to microbial contamination and relies on chemicals, conflicting with the goals of green organic agriculture (Wang et al., 2016; Zhang and Jiao, 2022; Hou et al., 2023). Fermented cultivation, achieved by mixing and piling ingredients (e.g., corn cobs, calcium oxide), turning piles, and bagging, features low cost, simple techniques, and high first-flush yield. However, it faces issues such as pest and disease problems, high spawn usage, and labor-intensive pile and bag turnings (Li, 2013). Consequently, traditional approaches encounter substantial obstacles in underdeveloped regions, including scarcities of grass and wood, pesticide residues, elevated technical requirements, and high costs associated with equipment and energy consumption.
To resolve the issues of forest resource depletion due to timber use and pesticide residue risks from traditional agricultural residues (e.g., cottonseed hulls, corn cobs) in mushroom cultivation, green organic fresh grasses like Giant Juncao Grass have become ideal alternatives, inspired by leafcutter ants’ fungal cultivation strategy (Catalani et al., 2019). Giant Juncao Grass (Pennisetum giganteum Zhan X. Lin, synonym Cenchrus fungigraminus), a C4 plant, offers rapid growth (5 m in 6 months), high yield (225–450 t/ha annually), perennial harvest (6–7 years), simple management, and strong stress resistance (suitable for marginal lands) (Zheng et al., 2023). It promotes organic cultivation, reduces pesticide residues, eases the conflict between mushroom cultivation and forestry, and optimizes marginal land use (Liu, 2018). However, dry P. giganteum, commonly used for Pleurotus spp. cultivation, is costly to dry and prone to fermentation and decay in humid environments, reducing cultivation efficiency (Lei et al., 2019). Fresh P. giganteum eliminates drying costs but contains bioactive metabolites that inhibit Pleurotus spp. growth and excessive soluble nutrients that increase contamination risks during non-sterile cultivation (Ma et al., 2024). To address these challenges, we propose a new cultivation method for underdeveloped regions: treating fresh P. giganteum with saturated lime water, which effectively kills microbial contaminants and insect eggs (Liu et al., 2020) and inhibits contaminating fungi (Hu et al., 2004); and adding calcium oxide and corn flour, which are readily available and commonly used in non-sterile Pleurotus spp. cultivation (Yin and He, 2004).
Here, we optimized a non-sterile protocol using lime-saturated water (LW) to mitigate the contaminants, and modulate nutrient availability. We hypothesized that the lime water (LW) treatment could selectively enrich metabolites beneficial to Pleurotus sapidus (a Pleurotus species characterized by high adaptability, rapid growth rate, rich nutritional profile, and palatable flavor) while inhibiting microbial contamination. The specific hypotheses are: (1) creating an alkaline microenvironment (pH > 10) to selectively inhibit high pH - sensitive microbial contamination such as Mucor spp. (Wu, 2005); (2) reducing soluble nutrients to restrict microbial proliferation without affecting P. sapidus mycelial growth; (3) altering metabolites (e.g., reducing P. sapidus - inhibitory substances and increasing P. sapidus - promoting substances) to enhance P. sapidus growth. Through integrated biochemical, metabolomic, and phenotypic analyses, we (1) identified the optimal ratios of LW, CaO, and corn flour, (2) characterized metabolic shifts in the substrate, and discovered two indicators that can be used to determine whether the cultivation raw materials are qualified, (3) isolated and identified key contaminants, and (4) validated key growth-promoting and inhibitory factors. This work provides a basis for further raw material expansion, strain screening, formulation optimization, and method simplification. Previous studies have explored lime treatment in mushroom cultivation and non - sterile substrates; yet, the use of fresh grass for non - sterile cultivation remains unexplored. Herein, we employed P. giganteum—a high - biomass C4 plant with broad adaptability—to provide a continuous supply of raw materials from marginal lands while eliminating drying costs. By optimizing lime water (LW), calcium oxide, and corn flour concentrations and analyzing changes in nutrient components and metabolites, this study enables large - scale, non - sterilized, green organic cultivation of P. sapidus. This work aims to address malnutrition and food shortages in underdeveloped regions and contribute novel strategies for the sustainable development of the edible fungus industry and global agriculture.
2 Materials and methods
2.1 Non-sterile cultivation of P. sapidus with fresh P. giganteum in tubes
The P. sapidus strain P969 and P. giganteum were provided by the China National Engineering Research Center of Juncao Technology. P969, extensively used in Juncao substrate cultivation (Xue et al., 2019), is registered under the NCBI GeneBank accession number PP090953. To mitigate the inhibitory effects of endophytes, microbial contamination, and antimicrobial substances on mycelium, fresh P. giganteum shreds was soaked in saturated LW for disinfection and improved substrate aeration. Corn flour (CF) optimized the substrate’s nutritional profile by increasing available carbon, thereby fostering a conducive environment for the recovery of P. sapidus. Calcium oxide (CaO, Solarbio, China) was used to adjust the pH value, of the substrate capitalizing on the alkaline tolerance of P. sapidus. As investigated earlier; this adjustment provided essential calcium and suppressed the growth of microbial contaminants (Sato et al., 2019; He et al., 2023). Referencing the results of previous studies, in order to systematically evaluate the interactive effects of lime-saturated water (LW, set at 5 levels: 0, 1, 2, 4, 8 mL·g−1), corn flour (CF, set at 5 levels: 0, 0.25, 0.5, 0.75, 1%, c) and calcium oxide (CaO, set at 5 levels: 0, 1, 2, 3, 4%, mass fraction) on the mycelial growth and contamination of P. sapidus in test tube cultures, a Latin square design was adopted. This design controlled two confounding variables—the substrate water content (fixed at 65%) and particle size (≤2 cm), and five biological replicates were set for each treatment combination to isolate the main effects of each factor. The process of non-sterile cultivation using fresh P. giganteum is shown in Figure 1, and the specific operations are as follows:
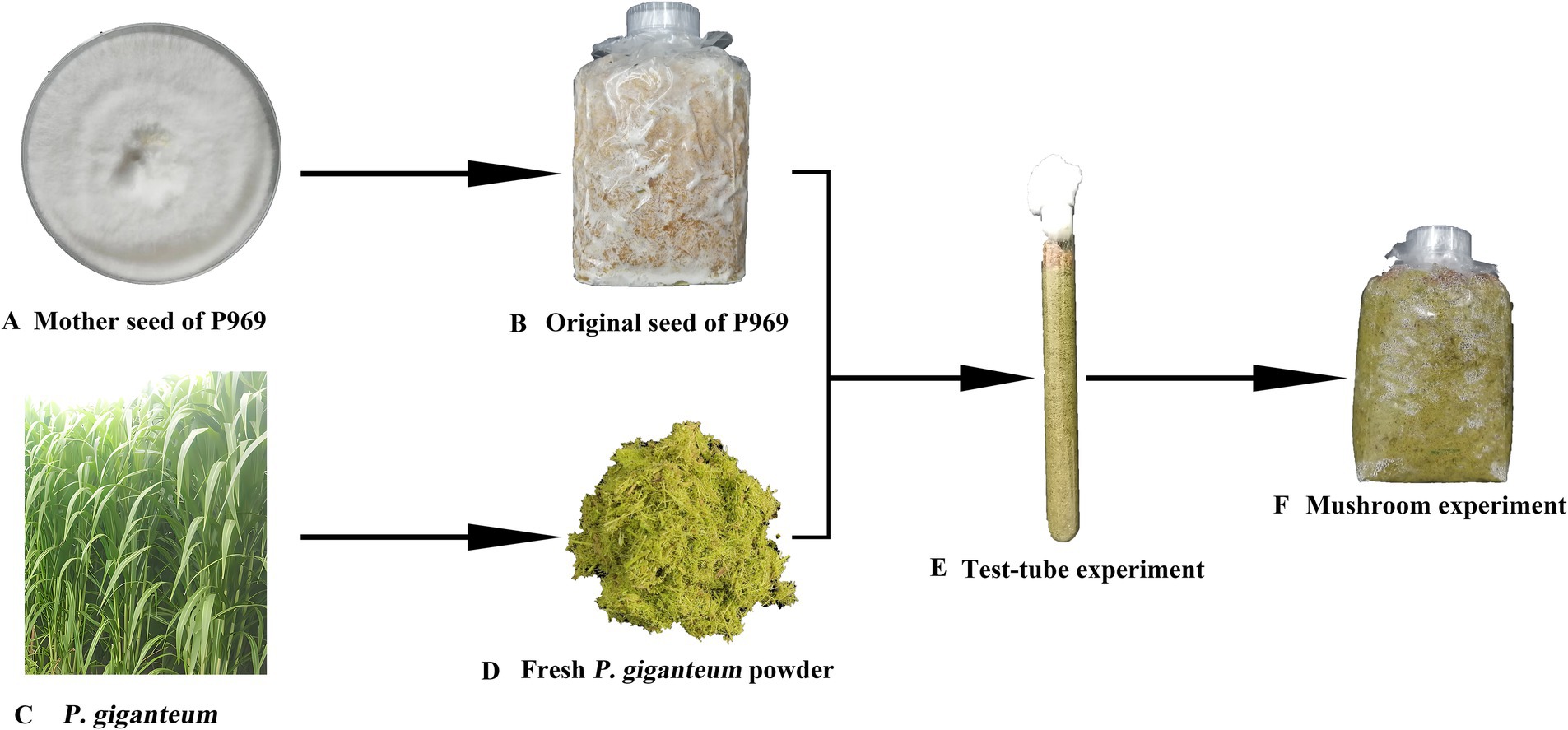
Figure 1. Schematic diagram of non-sterile cultivation of P. sapidus using fresh P. giganteum. (A,B) Inoculation of P969 mother seed into polypropylene bags for original seed preparation. (C,D) Crushing of fresh P. giganteum into shreds. (B,D,E) Treatment of fresh shreds with LW (0, 1, 2, 4, and 8 mL·g−1), and blending with components (CF (0, 0.25, 0.5, 0.75, and 1% w/w) and CaO (0, 1, 2, 3, and 4% w/w)) as per the experimental design, then filling into test tubes and inoculating with original seed for cultivation. (E,F) Subsequent cultivation in polypropylene bags based on test tube screening outcomes.
The original seed was prepared by mixing 78% fresh P. giganteum grass shreds, 20% wheat bran, and 2% gypsum by mass, with a moisture content of 62%. The mixture was packed into polypropylene mushroom bags (12 cm × 24 cm × 0.5 cm), each containing 250 g grass followed by sterilization at 121°C for 2 h. Sterile inoculation with P969 was done, followed by bags incubated at 25°C until mycelial coverage was achieved, forming the reserve original seed for subsequent experiments.
P. giganteum was sourced from healthy plants that had been growing for 8 months and had a high lignin content. The fresh P. giganteum was crushed for 30 s using a high-speed grinder (DFY-300, Linda Machinery, China) to a particle size of ≤2 cm.
Lime-saturated water (LW) was prepared by dissolving 1.74 grams per liter of calcium hydroxide (Ca(OH)₂, purchased from Solarbio, China) in a volumetric flask. The pH value of the lime-saturated water was measured to be 12.5 ± 0.1 using a digital pH meter (PB-10, Sartorius, Germany).
For test tube cultivation, the fresh P. giganteum grass shreds was soaked in lime-saturated water at concentrations of 0, 1, 2, 4 and 8 mL·g−1 for 30 min, and manually stirred every 15 min to ensure full and uniform contact between the substrate and the liquid. Then, excess liquid was filtered off using coarse cotton cloth, and the water content of the substrate was adjusted to 65% ± 2%. According to the Latin square design, the treated grass shreds was subsequently mixed with corn flour (CF, contents were 0, 0.25, 0.5, 0.75 and 1%, mass fraction) and calcium oxide (CaO, contents were 0, 1, 2, 3 and 4%, mass fraction). The substrate was adjusted to 65 moisture, and 30 g was liquated into each test tube (20 mm × 25 mL) with five replicates per treatment. After open inoculation, tubes were incubated at 25°C to assess P. sapidus growth and contamination. Mycelial growth rates were calculated using Equation 1.
2.2 Impact of lime-saturated water on the non-sterile cultivation of P. sapidus with fresh P. giganteum
In tube culture, optimal concentrations of CaO and CF were identified to be 2 and 0.5%, respectively. Lime-saturated water (LW) was selected for further studies for three reasons: (1) The maximum growth rate of P. sapidus mycelium was achieved at 4 mL·g−1 and 8 mL·g−1. (2) Reducing microbial contamination and substrate darkening. (3) Spearman’s correlation analysis revealed positive correlations with mycelial growth and negative correlations with contamination (p < 0.05). Despite the stronger correlation by CaO, LW was more direct and effective in inhibiting contamination and preventing substrate darkening, significantly impacting P. sapidus growth.
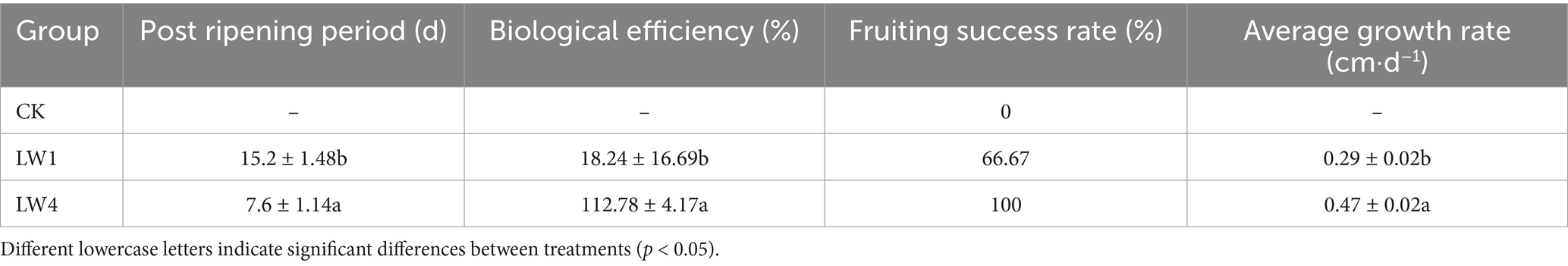
Further experiments utilized polypropylene mushroom bags (12 cm × 24 cm × 0.5 cm). Fresh shredded P. giganteum was treated with LW at three dosage gradients (CK: 0 mL·g−1, LW1: 1 mL·g−1, LW4: 4 mL·g−1) based on fresh weight. Each bag contained 250 g of substrate with five replicates per group, maintaining tube culture conditions. P. sapidus growth and microbial contamination were monitored. The average mycelial growth rate, biological efficiency, and fruiting success rate were evaluated using above mentioned Equation 1, and below mentioned Equations 2, 3.
2.3 Nutrients and physicochemical properties analysis of non-sterile cultivation substrates
Samples from CK, LW1 and LW4 substrate groups were collected with five replicates each, with each being over 50 g. Samples were dried at 65°C for 24 h until reaching a constant weight, ground, and sieved through an 80-mesh screen for subsequent analysis. Substrate composition, including lignin, cellulose, hemicellulose, proteins, nitrogen, phosphorus, potassium, sugars, and amino acids, were analyzed using kits from Shanghai UPLC-MS Ltd. (Shanghai, China). Crude fat was measured by GB/T 6433–2006 (NFQCC, 2006), crude ash by GB/T 6438–2007 (NFQIWC, 2007), total carbon by T/NAIA 070–2021 (NAIA, 2021), total nitrogen by NY/T 53–1987 (Cau, 1987), total phosphorus by HJ 632–2011 (Gansu Provincial Environmental Monitoring Station, Qualified Provincial Environmental Monitoring, 2011), and total potassium by NY/T 87–1988 (SAAS, 1988). The electrical conductivity (EC) value was determined by fully inserting a soil speedometer (JXBS-3001, Jingxun Changtong, China) into the sample and recording the reading once it stabilized. The refractive index (RI) was measured by placing a drop of the substrate exudate on the detection area of a refractometer (0–32% Brix, Shanghai Lichen, China) and recording the reading. The method for determining the pH value is as follows: Homogenize 5 g of the sample with 50 mL of distilled water, shake it at room temperature for 1 h, and then centrifuge it using a centrifuge (5415R, Sigma, Germany) at 4°C and 10,000 × g for 5 min. Subsequently, filter the obtained supernatant through a 0.45 μm cellulose acetate membrane (Millipore, USA) to remove particulates, and then measure the pH value of the filtrate using a digital pH meter (PB-10, Sartorius, Germany).
2.4 Metabolomic analysis of non-sterile cultivation substrates
Samples were collected from the CK, LW1, and LW4 substrate groups, with five replicates taken from each, each weighing more than 50 g. All samples underwent analysis via GC-QTOFMS and UHPLC–MS/MS (Allwegene Technology Co., Ltd., Beijing, China). See the Supplementary material for the detailed method.
2.4.1 GC-QTOFMS analysis
The extracts were analyzed with an Agilent 7,890 gas chromatograph system equipped with a DB-5MS capillary column (30 m × 250 μm × 0.25 μm, Agilent, SC, USA) and a time-of-flight mass spectrometer (GC-TOF-MS) operated in negative chemical ionization mode (NCI). Raw data analysis, including peak extraction, baseline adjustment, deconvolution, alignment, and integration, was finished with Chroma TOF (V 4.3x, LECO) software and LECO-Fiehn Rtx5 database was used for metabolite identification by matching the mass spectrum and retention index. Finally, the peaks detected in less than half of QC samples or RSD > 30% in QC samples were removed.
2.4.2 UHPLC–MS/MS analysis
The extracts were analyzed with an Acquity UHPLC system equipped with a Waters UPLC column (ACQUITY UPLC BEH Amide 1.8 μm, 2.1 × 100 mm, Waters, Milford, MA) and a Q Exactive HFX mass spectrometer (Orbitrap MS, Thermo) operated in positive electrospray ionization mode (ESI +). The raw data were converted to the mzXML format using ProteoWizard and processed with a built-in program, which was developed using R and based on XCMS, for peak detection, extraction, alignment, and integration. Metabolite structure identification was carried out by means of accurate mass matching (<25 ppm) and secondary spectrum matching, using a self-written R package to identify peaks.
2.5 Isolation and identification of microbial contaminants in the substrates
From the CK, LW1 and LW4 groups cultivated for 7 days, five bags were selected from each group. In each bag, three random sites containing microbial contamination were inoculated onto Potato Dextrose Agar (PDA) and incubated at 25°C for 5 days. Mycelial growth was observed, and the morphology of mycelium, sporangia and spores was examined microscopically for preliminary identification.
Fungal DNA was extracted using the HP Fungal DNA Kit D3195 (Omega Bio-Tek, California, USA). The ITS region was amplified with 2 × EasyTaq PCR SuperMix (+ dye) (TransGen Biotech, Beijing, China). PCR products were electrophoresed, and the ITS fragment was recovered using the Gel Extraction Kit D2500 (Omega Bio-Tek, California, USA). The fragment was cloned into the pEASY-T5 Zero vector (TransGen Biotech, Beijing, China) and transformed into E. coli Trans1-T1 competent cells (TransGen Biotech, Beijing, China). Positive clones were identified and sequenced for ITS by Fujian Shangya Biotechnology Co., Ltd. Sequences were compared with the NCBI BLAST database and uploaded to GenBank for accession numbers. A Neighbor-Joining phylogenetic tree was constructed for phylogenetic analysis using NCBI BLAST (NCBI, Bethesda, MD, USA).
2.6 Verification of key substances
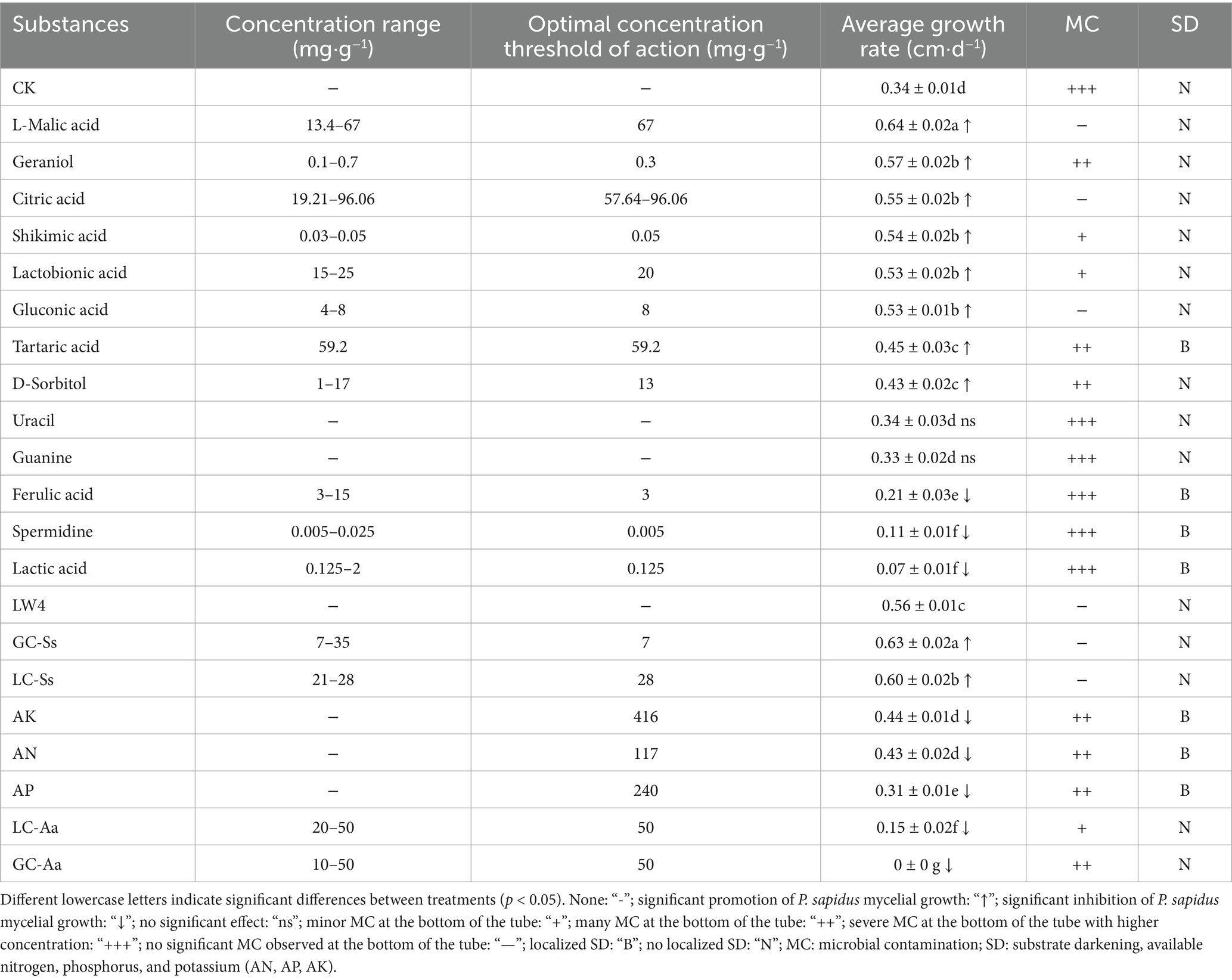
In this study, we simulated the addition of nutritional elements to the LW4 group, including soluble sugars, amino acids, urea (simulating available nitrogen, AN), calcium superphosphate (simulating available phosphorus, AP), and potassium chloride (simulating available potassium, AK). Among the top 20 differential metabolites identified by GC-QTOFMS and UHPLC-QE-MS with Log2FC values, we selected several functionally relevant substances for addition to the CK group. The amounts of AN, AP, and AK were determined based on biochemical analysis results. The quantities of soluble sugars and amino acids were established using data from metabolomic analysis, while the amounts of functional substances were based on relevant literature (Gao and Zheng, 2018; Liu et al., 2018; Ding et al., 2019; Li, 2019; Zhao et al., 2019; Liu et al., 2021; Zhang et al., 2021; Li et al., 2022; Ma et al., 2022; Wang, 2022; Li S et al., 2023; Si et al., 2023; Weng et al., 2023).
Substances were added to the substrate based on dry weight (Supplementary Table S1). The CK group contained 97.5% fresh P. giganteum shreds, 2% CaO, and 0.5% CF. The LW4 group used fresh P. giganteum shreds treated with 4 mL·g−1 LW, with other components unchanged from the CK group. Each concentration had five replicates under the conditions of section 2.1, for monitoring P. sapidus growth and contamination, and for collecting data.
2.7 Data analysis
Metabolomic data were analyzed by GC-QTOFMS and UHPLC-QE-MS. For other data, analysis of variance (ANOVA) was performed with the mean of five replicate values for each treatment data value, and significant differences at 95% (p < 0.05) were determined using Duncan’s multiple range test with SPSS Statistics 25 (IB, Armonk, NY, USA) and Microsoft Office Excel 2010 (Microsoft, Redmond, WA, USA), which were indicated by different lowercase letters. Spearman’s correlation coefficient was used for correlation analysis, with p < 0.05 indicating significance. All figures were generated using Adobe Photoshop 2020 (Adobe, San Jose, CA, USA), and Origin 2018 (OriginLab, Northampton, MA, USA).
3 Results
3.1 Optimization of non-sterile cultivation methods for P. sapidus using fresh P. giganteum
The appropriate concentration of calcium oxide (CaO) significantly promoted the growth of P. sapidus mycelium. The 0 mL·g−1, 1 mL·g−1, and 2 mL·g−1 groups exhibited maximum growth at 3% CaO (Figures 2A–C), while the 4 mL·g−1 and 8 mL·g−1 groups peaked at 2% CaO (Figures 2D,E). Similarly, growth increased with corn flour (CF) concentration, peaking at 0.5% CF across all groups (Figures 2A–E). Notably, the maximum growth rate of P. sapidus mycelium increased with lime-saturated water (LW), with the highest rates in the 4 mL·g−1 and 8 mL·g−1 groups (0.53 ± 0.00 cm·d−1).
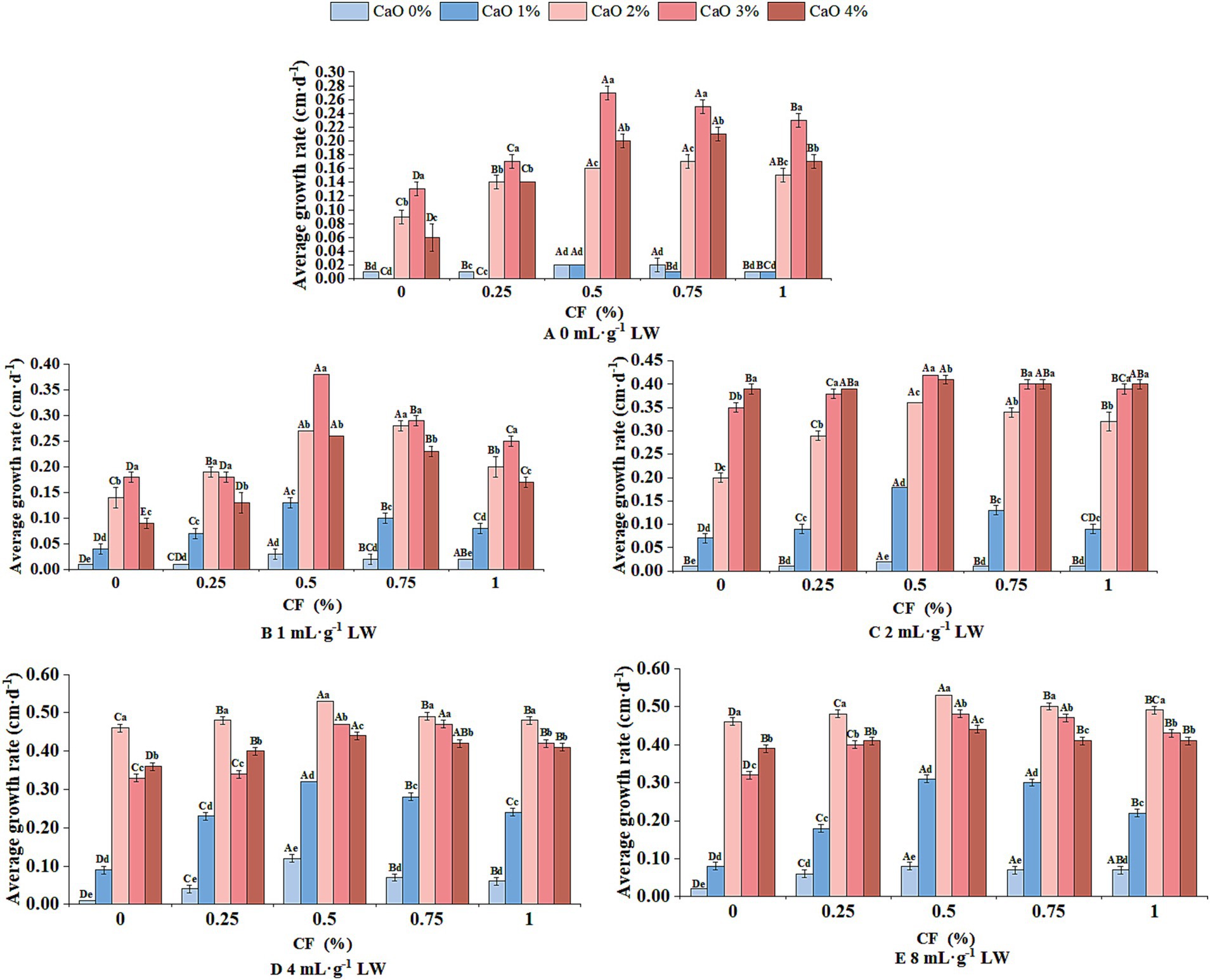
Figure 2. Mycelial growth rate of P. sapidus in non-sterile cultivation of fresh P. giganteum. A-E represent the growth rates of P. sapidus at 0, 1, 2, 4, and 8 mL·g−1 lime-saturated water (LW), respectively. Calcium oxide (CaO), corn flour (CF). Different uppercase letters indicate significant differences between the same CaO concentration at different CF concentrations (p < 0.05), while different lowercase letters indicate significant differences between the same CF concentration at different CaO concentrations (p < 0.05), n = 5.
Elevated LW concentration decreased microbial contamination and substrate darkening (Supplementary Table S2 and Supplementary Figure S1). The 4 mL·g−1 groups with 2 and 4% CaO exhibited no significant contamination or darkening, allowing P. sapidus mycelia to overgrow test tubes successfully. In contrast, despite the highest growth rates at 3% CaO for groups with 0 mL·g−1, 1 mL·g−1, and 2 mL·g−1, 73.33% of test tubes exhibited contamination, and 46.67% showed darkening, adversely affecting P. sapidus mycelial growth.
Spearman correlation analysis (Table 1) showed CF content positively correlated with P. sapidus mycelial growth rate and substrate darkening, negatively with contamination (p < 0.05). CaO content had similar correlations (p < 0.05), and its coefficients were higher than LW’s, indicating a stronger impact on growth and suppressing issues.
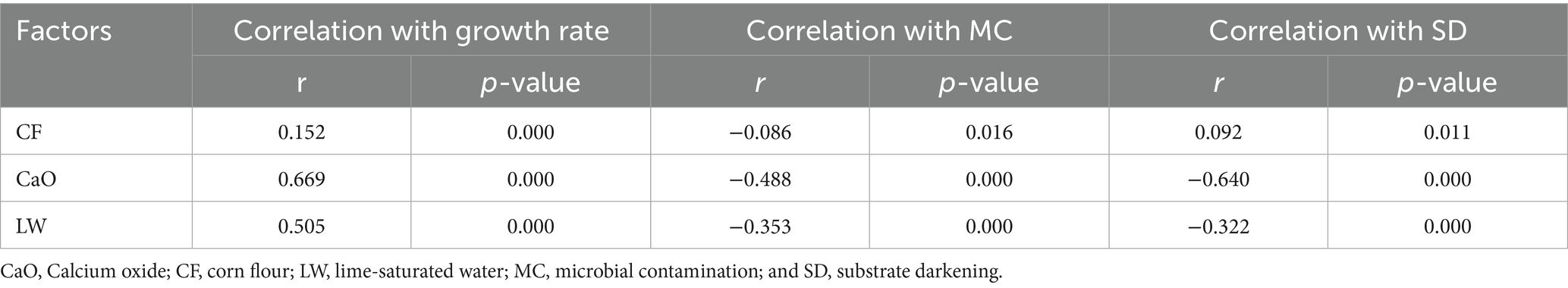
Table 1. Correlation analysis of CF, CaO and LW on P. sapidus mycelium growth and resistance to contamination.
Optimal concentrations for non-sterile cultivation of P. sapidus using fresh P. giganteum were identified as 0.5% CF and 2% CaO, based on growth rate, microbial contamination, and substrate darkening (Figure 2, Table 1 and Supplementary Figure S1).
3.2 Fruiting in non-sterile cultivation of P. sapidus using fresh P. giganteum
Lime-saturated water significantly mitigated contaminant mycelium spread, which was observed as isolated patches or filamentous growth (Figure 3), reducing its inhibition on P. sapidus mycelium. By day 12, contaminant mycelium partly receded yet some inhibition remained. LW4 group’s P. sapidus mycelium was uncontaminated and grew 1.62 times faster than LW1’s, covering cultivation bag by day 21 with growth surge from day 8. Both groups produced fruiting bodies. LW4 (biological conversion rate: 121%, fresh mushrooms/dry culture material; post-ripening period: 7.6 days) had a biological efficiency 6.18 times higher and a post-ripening period half as long as that of LW1 (Table 2).
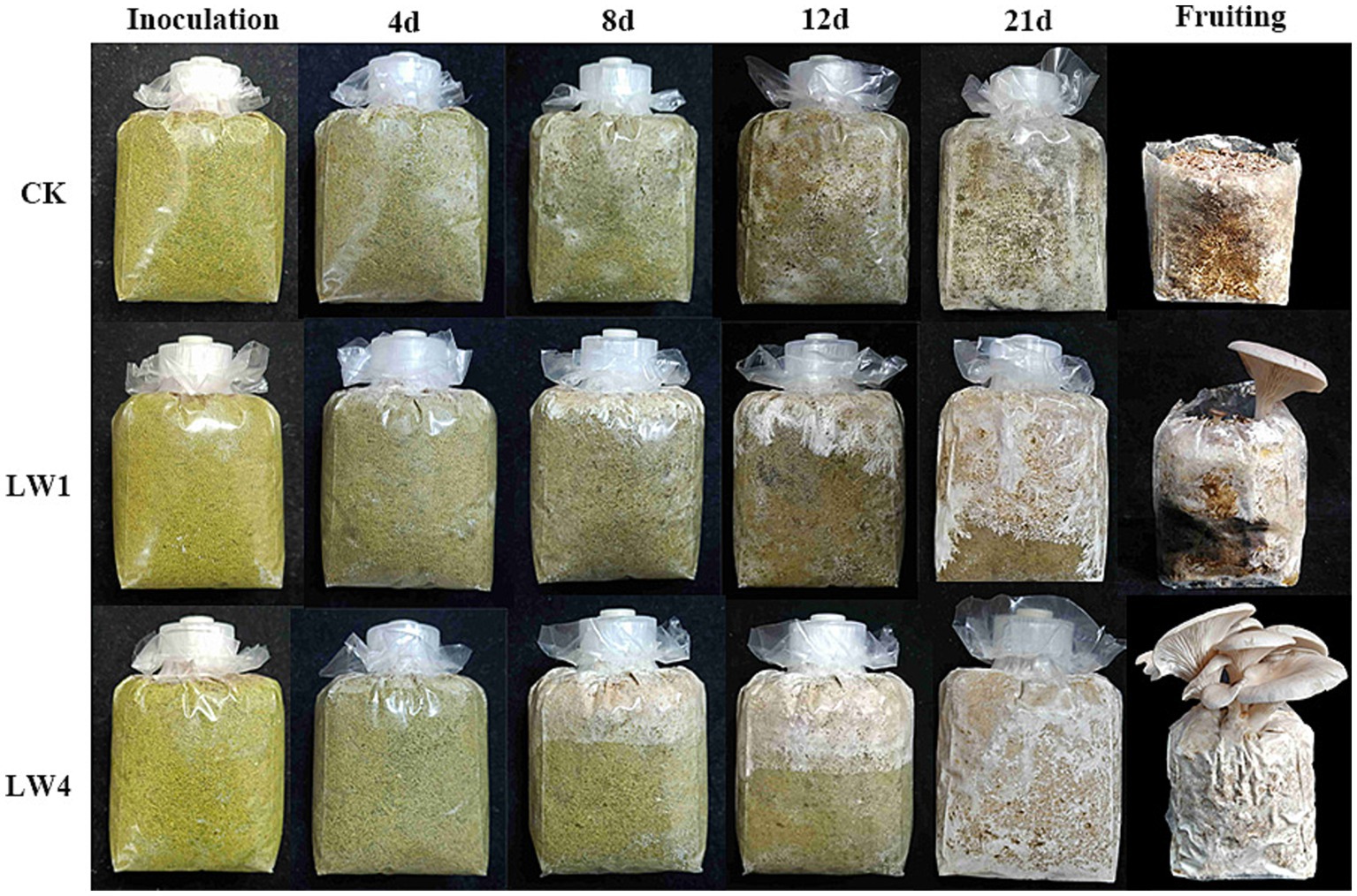
Figure 3. Effect of lime-saturated water (LW) in non-sterile cultivation of P. sapidus. Each row, from left to right, depicts the cultivation process from inoculation to fruiting body formation in the polypropylene bags of the CK, LW1, and LW4 groups.
3.3 Effects of lime-saturated water treatment on the nutrients and physicochemical properties of non-sterile cultivation substrates
Lime-saturated water (LW) had no significant effect on major substrate nutrients (e.g., total sugars, hemicellulose, cellulose, lignin, crude fat, total protein, total carbon, total nitrogen, total phosphorus, and total potassium; Figures 4A–C). However, it markedly reduced soluble nutrients. As LW increased, amino acids, soluble sugars, available nitrogen, phosphorus, and potassium decreased, with the LW4 group having the lowest levels. Compared to the CK group, reductions were: amino acids 63.90%, soluble sugars 68.18%, available nitrogen 45.80%, available phosphorus 65.08%, and available potassium 69.63% (Figures 4B–D).
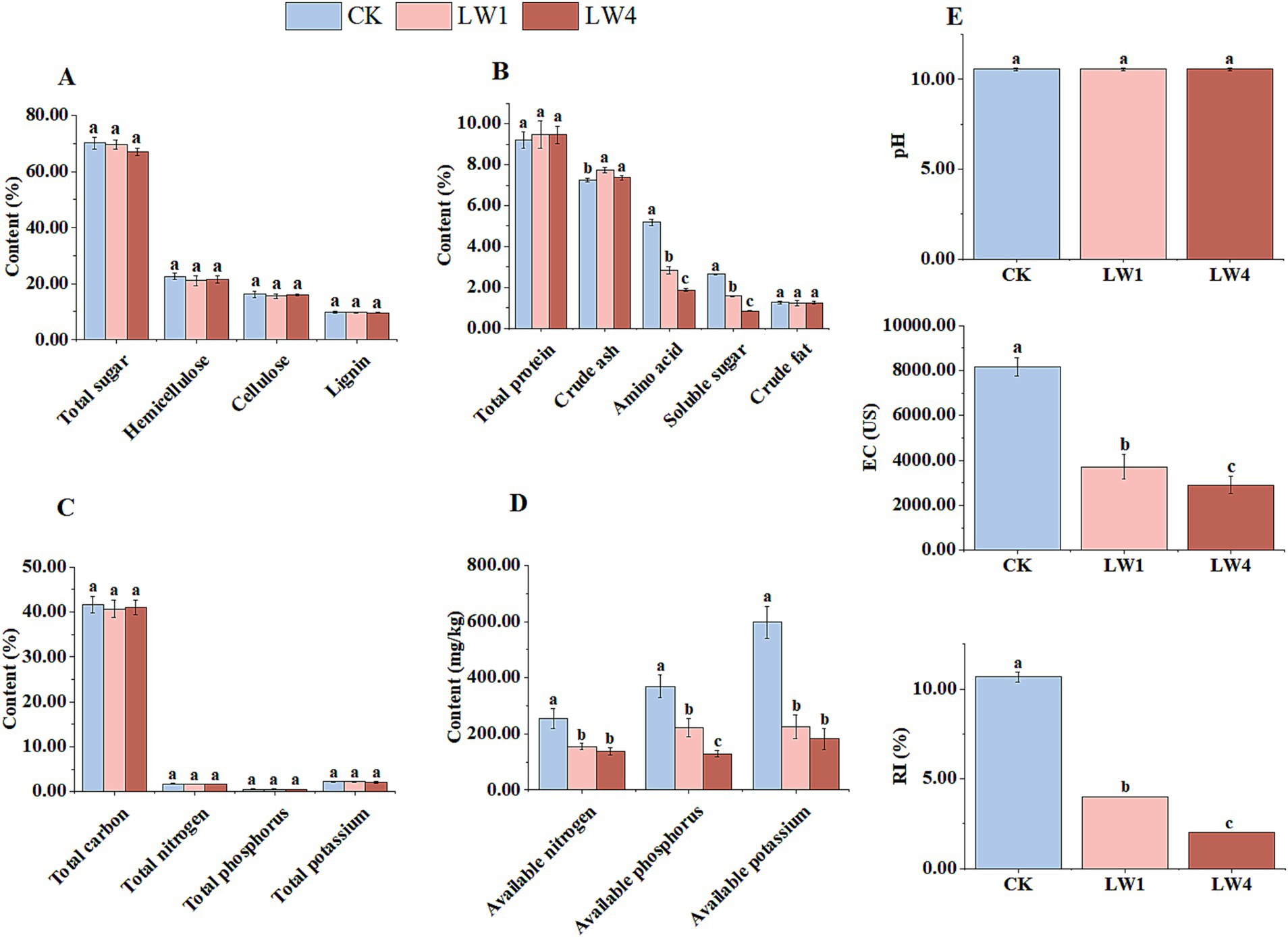
Figure 4. Effects of LW on substrate major nutrients, pH value, EC, and RI. (A) Major nutrients (high content). (B) Major nutrients (low content). (C) Total carbon, total nitrogen, total phosphorus, and total potassium. (D) Available nitrogen, phosphorus, potassium. (E) pH value, electrical conductivity (EC), and refractive index (RI). Lime-saturated water (LW). Different lowercase letters indicate significant differences between treatments (p < 0.05).
With the increase in the addition amount of lime-saturated water (LW), the electrical conductivity (EC) and refractive index (RI) showed a downward trend, among which the decrease in the LW4 treatment group was the most significant. Compared with the control group (CK), the EC value in the LW4 treatment group decreased from 8176.67 US to 2908.67 US, with a decrease rate of 64.43%; the RI value decreased from 10.67 %Brix to 2.00 %Brix, with a decrease rate of 81.31% (Figure 4E).
3.4 Effects of lime-saturated water treatment on the metabolomics of non-sterile cultivation substrates
PCA analysis of non-sterile cultivation substrates treated with different concentrations of lime-saturated water (LW) was shown in Supplementary Figure S2. For GC-QTOFMS, PC1 and PC2 accounted for 61.5 and 31.5% of variance, respectively. UHPLC-QE-MS showed PC1 and PC2 explained 65.4 and 13.3%. Tight clustering across both techniques indicated system stability, methodological reliability and sample quality.
Clustering and heatmap analysis of GC-QTOFMS and UHPLC-QE-MS revealed metabolite expression differences influenced by LW concentration (Figure 5A). GC-QTOFMS indicated significant upregulation of metabolites in LW1 and LW4 treatments versus CK, with increased concentrations enhancing the effect. Conversely, UHPLC-QE-MS suggested metabolite downregulation at higher LW concentrations.
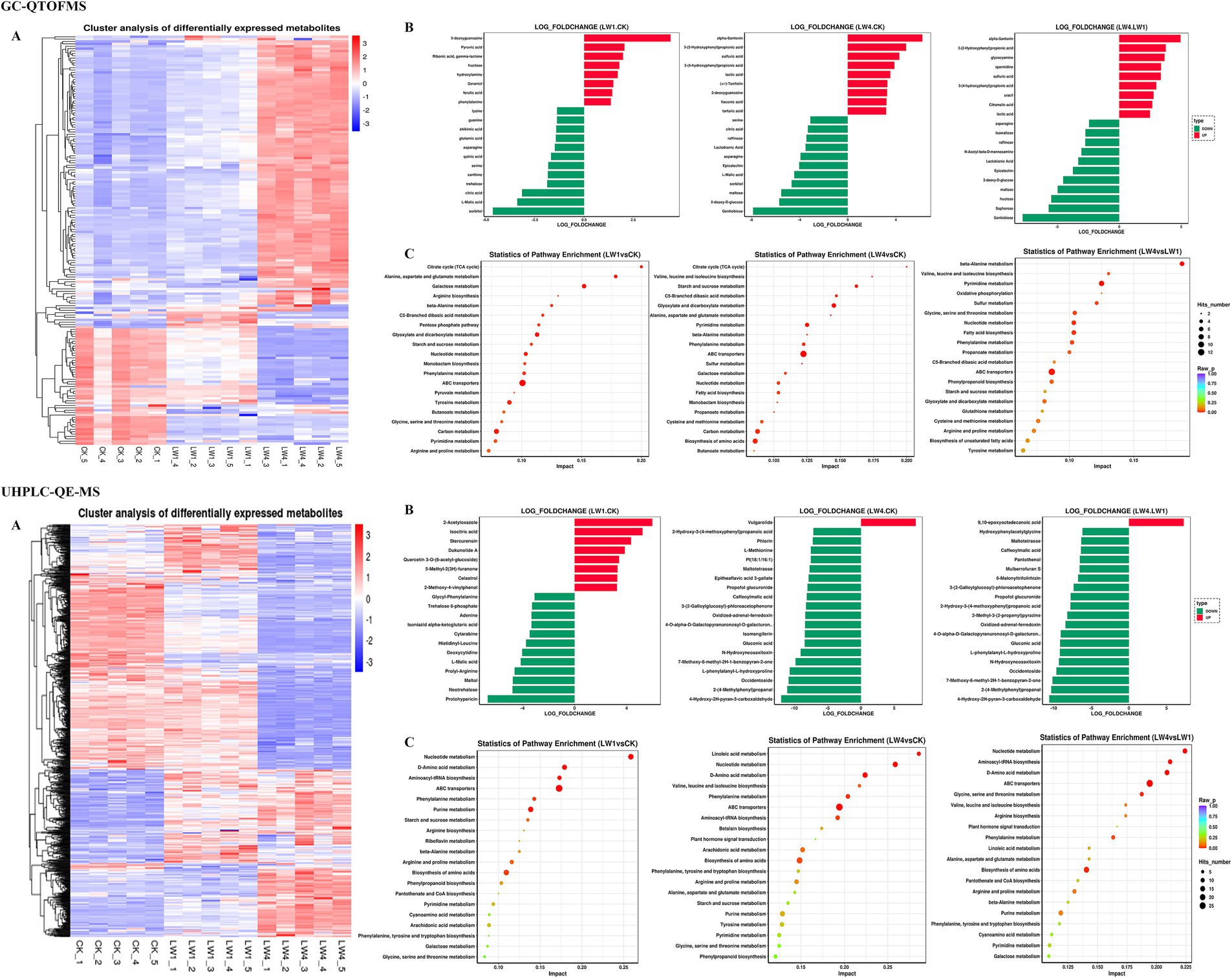
Figure 5. Key differential metabolite screening in non-sterile cultivation substrates with GC-QTOFMS and UHPLC-QE-MS. (A) Metabolite clustering analysis. The different colors indicate expression profiles. Each column in the Figure. represents one sample treatment, and each grid represents one metabolite. (B) Statistical analysis of differential metabolites. The horizontal coordinate represents the log value (Log2FC) of the quantitative multiple difference of metabolites in the two samples. The ordinate is VIP > 1, p < 0.05, Log2FC value Top 20. (C) KEGG enrichment analysis of differential metabolites. The horizontal coordinate and ordinate represent the enrichment factor and metabolic pathway name corresponding to each pathway, respectively.
Venn diagrams were employed to assess differential metabolites among treatment groups (Supplementary Figure S3). GC-QTOFMS detected 108, 135, and 144 unique metabolites in groups A, B, and C, respectively, with 69 common to all. UHPLC-QE-MS identified 534, 777, and 936 unique metabolites in these groups, with 231 common metabolites.
Top 20 most significantly altered metabolites after log2 transformation of metabolite data Figure 5B. For GC-QTOFMS, LW1 had 8 upregulated and 12 downregulated metabolites as compared to CK. LW4 had 9 upregulated and 11 downregulated metabolites compared to CK. When comparing LW4 with LW1, 9 metabolites were upregulated and 11 downregulated. Regarding UHPLC-QE-MS, LW1 had 8 upregulated and 12 downregulated metabolites compared to CK. LW4 had 1 upregulated and 19 downregulated metabolites compared to CK. When comparing LW4 with LW1, 1 metabolite was upregulated and 19 were downregulated.
Differential metabolites were mapped onto KEGG pathways for annotation and enrichment analysis to determine metabolic pathway alterations (Figure 5C). This method identified the top 20 enriched pathways among differential metabolites. GC-QTOFMS analysis primarily revealed carbohydrate, lipid, nucleotide, amino acid metabolism, and secondary metabolic pathways. UHPLC-QE-MS analysis covered similar pathways, excluding energy metabolism, and included additional secondary metabolic pathways.
GC-QTOFMS and UHPLC-QE-MS analyses of the substrate revealed that LW conditions significantly reduced soluble sugars and amino acids, aligning with biochemical assays. GC-QTOFMS resulted decreases in galactose, ribose, sucrose, alanine, β-alanine and valine as compared with CK to LW1 and LW4 groups. UHPLC-QE-MS confirmed this trend for D-tagatose, L-galactose, D-xylose, maltose, β-D-galactose, sucrose, D-alanine, L-phenylalanine, L-valine, D-proline, L-proline, L-methionine, tryptophan, D-glutamine and L-glutamine (Supplementary Tables S3, S4).
3.5 Isolation and identification of M. circinelloides
Microbial contamination was consistent across substrate groups on PDA medium, exhibiting black sporangia in the center, curly aerial mycelium, and white peripheral mycelium (Figure 6A). Microscopy showed the absence of clamp connections and the spores were oval shaped (Figures 6B,C), preliminarily identifying the contaminant as Mucor genus. DNA sequencing of the ITS region (Figure 6D) confirmed 100% sequence identity with M. circinelloides (GenBank accession number MN744376.1) after PCR amplification and TA cloning. A neighbor-joining phylogenetic tree (Supplementary Figure S4) supported the ITS sequence alignment and morphological data, confirming M. circinelloides as the key contaminant in non-sterile cultivation of P. sapidus on fresh P. giganteum substrates.
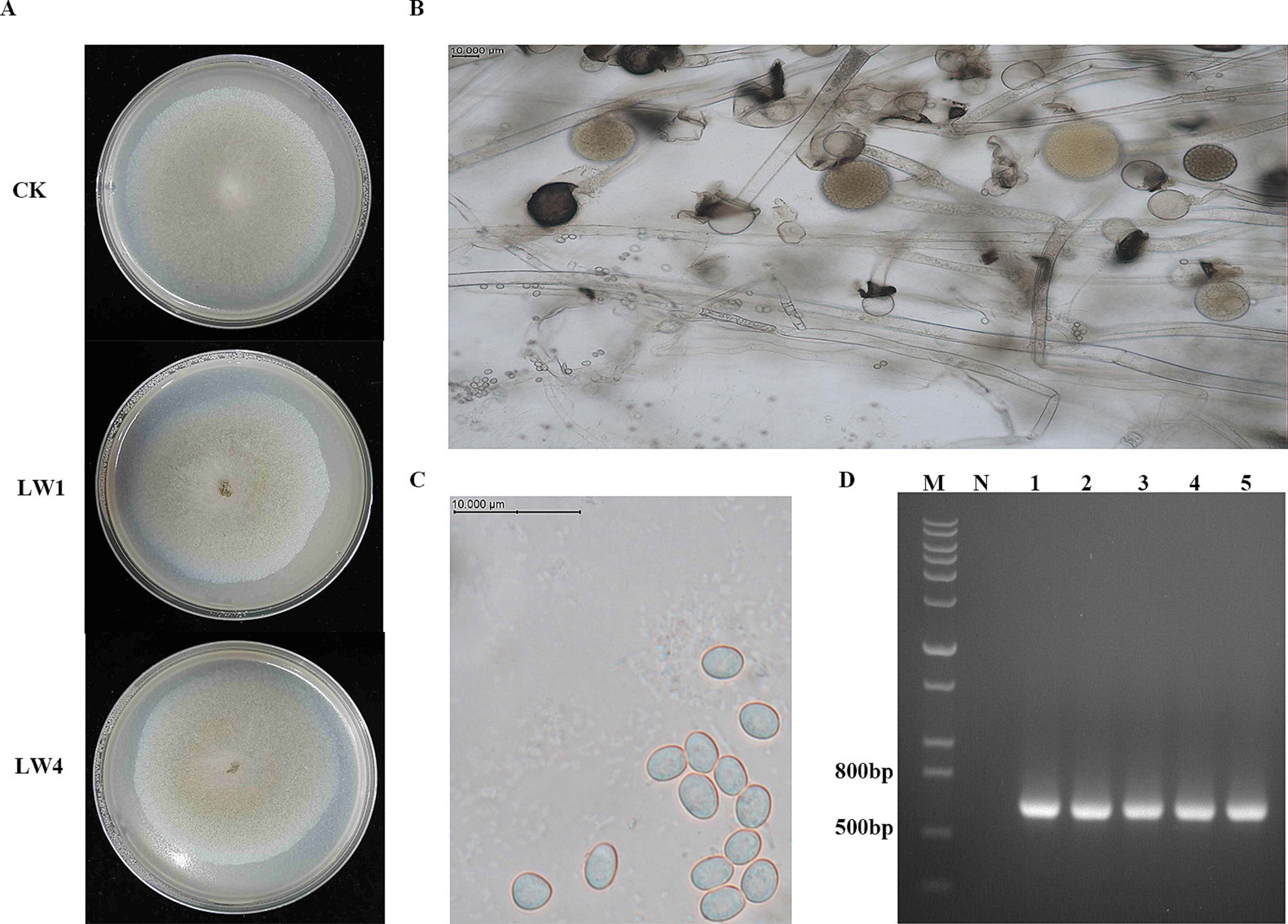
Figure 6. Isolation and identification of M. circinelloides. (A) Morphological characteristics of M. circinelloides after 5 days of cultivation on PDA medium. (B) Mycelium of M. circinelloides. (C) Spores of M. circinelloides. (D) ITS fragments of M. circinelloides. (M represents DNA marker, N represents blank control, and lanes 1–5 represent PCR amplified ITS fragments).
3.6 Influence of key substances on the non-sterile cultivation of P. sapidus on fresh P. giganteum substrates
Key substance validation was summarized in Table 3. P. sapidus growth conditions and average growth rates at various substance concentrations were detailed in Supplementary Figure S5 and Supplementary Table S5. Additions to the CK group substrates, such as spermidine, ferulic acid, and lactic acid, significantly inhibited P. sapidus growth, with lactic acid showing the highest inhibition at 79.41%. Except for guanine and uracil, which did not enhance growth, all other tested substances notably promoted P. sapidus growth. L-malic acid had the most substantial effect, increasing growth by 88.24% over CK, without significant microbial contamination.
In the LW4 group, both GC-Ss and LC-Ss significantly promoted P. sapidus growth, with GC-Ss showing a 12.50% increase over the LW4 group. However, the addition of amino acids and available nitrogen, phosphorus and potassium significantly inhibited P. sapidus mycelium growth, with increased concentrations leading to more pronounced inhibition, particularly GC-Aa, which showed complete (100%) inhibition.
4 Discussion
4.1 Substrate optimization for sustainable non-sterile cultivation
The development of low-cost, non-sterile cultivation methods for P. sapidus addressed critical barriers to scaling edible fungi production in resource-limited regions. By replacing energy-intensive sterilization with substrate formulation engineering—specifically, a combination of lime-saturated water (LW, 4 mL·g−1), calcium oxide (CaO, 2%), and corn flour (CF, 0.5%)—this study demonstrated a viable pathway to reduce production costs compared to traditional methods (Portillo et al., 2018; Rawat et al., 2020). The synergistic effects of these components create a selective environment favoring P. sapidus mycelial dominance: LW suppresses competitor microbes through pH elevation and antimicrobial action (Zanin et al., 2019), while CF provides a controlled carbon flux that sustains fungal growth without triggering saprophytic contamination (Dutta et al., 2024). Notably, the narrow optimal range for CaO (2–3%) highlights the delicate balance required between microbial suppression and substrate toxicity. This finding challenges earlier proposals advocating higher CaO concentrations (Park et al., 2017). This discrepancy underscores the importance of context-specific substrate design, particularly when integrating agricultural byproducts like P. giganteum into circular food systems.
4.2 Nutrient dynamics and microbial competition
The inhibitory effect of lime-saturated water (LW) on microbial contamination, especially M. circinelloides, is mainly achieved through multiple mechanisms: (1) Its high pH value (12.5 ± 0.1) disrupts the integrity of microbial cell membranes and inhibits enzymatic activities (Zanin et al., 2019). This imposes growth stress particularly on alkali-intolerant microbial contamination, while P. sapidus can grow normally in this highly alkaline environment. (2) It reduces the soluble nutrients readily accessible to microbial contamination, including amino acids, soluble sugars, and available nitrogen, phosphorus, and potassium, thereby limiting their energy sources and competitive advantages, without significantly affecting the normal growth of P. sapidus. (3) It increases some antibacterial substances that are unfavorable to the growth of microbial contamination but have no significant impact on P. sapidus, such as geraniol (Scariot et al., 2021) and tartaric acid (Shokri, 2011). The application of lime-saturated water (LW) induced marked alterations in substrate physicochemical properties, particularly through its interaction with soluble nutrient fractions and microbial communities (Li Y. et al., 2023). While primary macronutrients (e.g., lignocellulose and total protein) remained largely unaffected by LW treatment, a significant reduction in soluble nutrients was observed. This reduction, evidenced by decreased electrical conductivity (EC) and refractive index (RI) values (Pedcharat et al., 2023), directly correlated with suppressed P. sapidus mycelial proliferation. These two indicators can potentially be used for the rapid identification of whether the cultivation raw materials meet the standards. Soluble sugars, when maintained at optimal concentrations, enhanced fungal growth; whereas elevated amino acids and available N/P/K levels exhibited inhibitory effects, suggested nutrient-specific modulation of fungal physiology. However, these dynamics were further complicated by microbial competition in non-sterile substrates: excessive available N/P/K stimulated rival microbial populations, which outcompeted P. sapidus for limited resources. The interplay between LW-induced physicochemical shifts (e.g., pH modulation and ionic balance) and microbial activity underscores the necessity for precise substrate optimization to mitigate nutrient over-enrichment and stabilize soluble nutrient fluxes. Future strategies should prioritize balancing microbial suppression via LW with targeted nutrient supplementation to favor P. sapidus dominance in competitive environments.
4.3 Metabolic reprogramming and antimicrobial synergy
Metabolic processes are fundamental to biological activities, significantly affecting organismal growth and development. In edible fungi cultivation, understanding how various factors affect the metabolic dynamics of the substrate is essential for optimizing growth conditions. Previous studies have shown that lime-saturated water treatment modified the metabolic pathways of Lentinula edodes substrates, thereby enhancing nutritional conditions for growth and development (Li Y. et al., 2023). In non-sterile cultivation of P. sapidus, lime-saturated water is a critical factor that may interact complexly with substrate metabolism.
KEGG pathway analysis revealed that metabolite alterations in our substrates influenced multiple pathways, affecting P. sapidus growth and fruiting. Carbohydrate metabolism influenced the growth and fruiting rates; lipid metabolism affected cell membrane structure and function; nucleotide metabolism influenced genetic information transmission and expression; amino acid metabolism altered protein synthesis. Changes in secondary metabolic pathways produced compounds with varying effects on P. sapidus growth (Nelson, 2021). These changes also affected substrate microorganisms, potentially influencing their activity, competition, community structure, antimicrobial properties, and interactions with P. sapidus (Braat et al., 2022). Furthermore, lime-saturated water may degrade harmful substances or decompose beneficial ones, further impacting metabolic pathways and P. sapidus growth.
The identification of key substances such as spermidine (Zhang et al., 2024), ferulic acid (Zhang et al., 2024), geraniol (Scariot et al., 2021), tartaric acid (Shokri, 2011), gluconic acid (Kaur et al., 2006) with antifungal properties; L-malic acid, lactic acid, citric acid (Ji et al., 2023), and shikimic acid (Bai et al., 2022) as an antibiotic agent, and lactobionic acid (Sáez-Orviz et al., 2022) as antimicrobial agents; along with D-sorbitol (Zhang et al., 2008) as a carbon source, that influenced the balance between P. sapidus and microbial contamination, inhibit or promote P. sapidus growth, provides a basis for targeted manipulation of cultivation conditions.
4.4 Biocontrol of M. circinelloides on non-sterile cultivation
In modern mycology, the intricate ecological interactions within cultivation systems have become a focal point of research. Microbial contamination can drastically hinder the growth and productivity of target fungi. Non-sterile cultivation inherently involves the cohabitation of diverse microorganisms, which can markedly affect the target organism’s growth and productivity. Earlier research highlighted how microbial competition could significantly modify fungal growth dynamics and yield (Pii et al., 2015).
The presence of M. circinelloides in non-sterile cultivation environments posed a substantial challenge due to resources competition with P. sapidus. Our study revealed a negative correlation between M. circinelloides activity and the application of saturated LW, indicating that LW might aid in controlling this contamination. This observation is particularly pertinent considering the industrial utility of M. circinelloides and its relative safety compared to other contaminants (Fazili et al., 2022).
5 Conclusion
Our findings show the efficacy of a non-sterile cultivation method for P. sapidus using fresh P. giganteum, which reduces M. circinelloides contamination and improves the mycelial growth environment. Through physiological, biochemical, metabolomic analyses and validation experiments, we have demonstrated that key substances can promote mycelial growth and suppress microbial contamination. This cost-effective and simple method can directly contribute to UN Sustainable Development Goals 2 (Zero Hunger) and 12 (Responsible Consumption and Production). Future research will optimize concentrations, additives, strains, and equipment, and apply the method to high-biomass substrates and agro-forestry waste.
Data availability statement
All datasets generated during this study are publicly available in the Zenodo repository under the permanent DOI: https://doi.org/10.5281/zenodo.15804844.
Author contributions
YZ: Writing – original draft, Writing – review & editing. YL: Writing – original draft, Writing – review & editing. ZhiL: Writing – original draft, Writing – review & editing. MX: Writing – original draft, Writing – review & editing. FW: Writing – original draft, Writing – review & editing. JL: Writing – original draft, Writing – review & editing. CR: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. JC: Writing – original draft, Writing – review & editing. LL: Writing – original draft, Writing – review & editing. FY: Writing – original draft, Writing – review & editing. EO: Writing – original draft, Writing – review & editing. NA: Writing – original draft, Writing – review & editing. ZhaL: Writing – original draft, Writing – review & editing. DL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Special project for the protection and utilization of agricultural resources of the Department of Agriculture and Rural Affairs of Fujian Province “Research and application of key technologies for innovation and industrialized utilization of Juncao and Juncao mushroom” (KKY22001XA).
Acknowledgments
We thank Yingxing Lin, Yunlong Ma, Zehui Wang, and Changjiang Lai from the National Engineering Research Center of Juncao Technology for their help during the pre-treatment and collection of experimental samples.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. During the preparation of this work the authors used Kimi in order to aid in polishing the sentences, enhancing their accuracy, standardization, and overall readability. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1582869/full#supplementary-material
References
Albertia, M. M., Cunha, M. L. O., Mendes, D. W., Vieira Júnior, W. G., and Zied, D. C. (2021). Tecnologic development on Pleurotus cultivation: specific practices used in Brazil. Braz. Arch. Biol. Technol. 64:e21200198. doi: 10.1590/1678-4324-2021200198
Bai, J., Wu, Y., Bu, Q., Zhong, K., and Gao, H. (2022). Comparative study on antibacterial mechanism of shikimic acid and quinic acid against Staphylococcus aureus through transcriptomic and metabolomic approaches. Lwt 153:112441. doi: 10.1016/j.lwt.2021.112441
Braat, N., Koster, M. C., and Wösten, H. A. B. (2022). Beneficial interactions between bacteria and edible mushrooms. Fungal Biol. Rev. 39, 60–72. doi: 10.1016/j.fbr.2021.12.001
Catalani, G. C., Camargo, R. D. S., Sousa, K. A., Caldato, N., Silva, A. A. C., and Forti, L. C. (2019). Fat-soluble substance flow during symbiotic fungus cultivation by leaf-cutter ants. Neotrop. Entomol. 49, 116–123. doi: 10.1007/s13744-019-00718-0
Cau, C. A. U. (1987). Method for the determination of soil total nitrogen (semi-micro Kjeldahl method) : Industry Standard - Agriculture.
Ding, S., Li, Y., and Guo, Y. (2019). Effect of exogenous spermidine treatment on lignification and related enzyme activities in shiitake mushrooms (Lentinula edodes). Food Sci. 40, 226–232. doi: 10.7506/spkx1002-6630-20180615-313
Dutta, A., Dracatos, P. M., and Khan, G. A. (2024). Balancing act: the dynamic relationship between nutrient availability and plant defence. Plant J. 120, 1724–1734. doi: 10.1111/tpj.17098
Fazili, A. B. A., Shah, A., Zan, X., Naz, T., Nosheen, S., Nazir, Y., et al. (2022). Mucor circinelloides: a model organism for oleaginous fungi and its potential applications in bioactive lipid production. Microb. Cell Factories 21:29. doi: 10.1186/s12934-022-01758-9
Gansu Provincial Environmental Monitoring Station, Qualified Provincial Environmental Monitoring (2011). Soil-determination of Total phosphorus by alkali fusion. Mo-Sb anti spectrophotometric method : Industry standard - Environmental protection.
Gao, L., and Zheng, Y. (2018). Preservation effect of coating antistaling agent including lactobionic acid to chilled meat. Sci. Technol. Food Ind. 39, 244–251. doi: 10.13386/j.issn1002-0306.2018.14.046
He, Y., Geng, X., Zhou, H., Luo, K., and Li, Z. (2023). Comparative experiment on lime addition in culture material of Pleurotus citrinopileatus. Edib. Fungi 45, 37–38+53. doi: 10.3969/j.issn.1000-8357.2023.04.011
Hou, X., Luo, C., Chen, S., Zhang, X., Jiang, J., Yang, Z., et al. (2023). Progress in research on diseases of edible fungi and their detection methods: a review. Crop Prot. 174:106420. doi: 10.1016/j.cropro.2023.106420
Hu, J., Gong, M., Peng, Y., and Guo, C. (2004). Effects of Carbendazim and lime solution on the Bateriostasis of 5 Mold strains. Xinjiang Agric. Sci. S1, 40–41. doi: 10.3969/j.issn.1001-4330.2004.z1.014
Jayasuriya, W. J. A. B., Handunnetti, S. M., Wanigatunge, C., Fernando, G. H., Abeytunga, D. T. U., and Suresh, T. S. (2020). Anti-inflammatory activity of Pleurotus ostreatus, a culinary medicinal mushroom, in Wistar rats. Evid. Based Complement. Alternat. Med. 2020:6845383. doi: 10.1155/2020/6845383
Jha, R., Zhang, K., He, Y., Mendler-Drienyovszki, N., Magyar-Tábori, K., Quinet, M., et al. (2024). Global nutritional challenges and opportunities: buckwheat, a potential bridge between nutrient deficiency and food security. Trends Food Sci. Technol. 145:104365. doi: 10.1016/j.tifs.2024.104365
Ji, Q., Wang, W., Yan, H., Qu, H., Liu, Y., Qian, Y., et al. (2023). The effect of different organic acids and their combination on the cell barrier and biofilm of Escherichia coli. Food Secur. 12:3011. doi: 10.3390/foods12163011
Kaur, R., Macleod, J. K., Foley, W. J., and Nayudu, M. (2006). Gluconic acid: an antifungal agent produced by Pseudomonas species in biological control of take-all. Phytochemistry 67, 595–604. doi: 10.1016/j.phytochem.2005.12.011
Lei, Y., Chen, M., Liu, X., Li, J., Liu, B., and Lin, Z. (2019). Effects of fresh Pennisetum giganteum z.x.lin on physical and nutrient components of Pleurotus ostreatus fruiting bodies. Edib. Fungi China 38, 61–68. doi: 10.13629/j.cnki.53-1054.2019.11.013
Li, H. (2013). Studies on fermented material culti-vation technology of Pleurotus ostreatus : Master, Henan Agricultural University.
Li, L. L. (2019). Optimization of culture conditions for antifungal lactic acid bacteria and application in whey antisepsis : Master, Inner Mongolia University.
Li, S., Cao, Y., Liu, C., Zhao, M., Yan, H., and Gao, H. (2023). Leaching of nickel-contaminated soil by tartaric acid and oxalic acid. Environ. Prot. Chem. Indus. 43, 87–93. doi: 10.3969/j.issn.1006-1878.2023.01.013
Li, Y., Wang, H., Zhang, Y., Xiang, Q., Chen, Q., Yu, X., et al. (2023). Hydrated lime promoted the polysaccharide content and affected the transcriptomes of Lentinula edodes during brown film formation. Front. Microbiol. 14:1290180. doi: 10.3389/fmicb.2023.1290180
Li, X., Xie, M., Yan, X., Hu, Z., and Wang, D. (2022). Effects of malic acid on growth performance, slaughter performance, meat quality, muscle biochemical indexes and antioxidant function of cyan-shank partridge chickens. Chin. J. Anim. Nutr. 34, 7747–7757. doi: 10.3969/j.issn.1006-267x.2022.12.026
Liu, X. (2018). Study on the quality and antioxidant activity in vitro of Pleurotus ostreatus cultivated with fresh Juncao : Master, Fujian Agriculture and Forestry University.
Liu, T., Miao, R., Peng, W., Huang, Z., Gan, B., and Tan, H. (2020). Preliminary study on non-sterile cultivation Technology of Pleurotus ostreatus with wheat straw pretreated by saturated lime water. Edib. Fungi 42, 38–77.
Liu, Y., Yang, H., Song, Z., Chen, X., Huang, Y., and Wang, Z. (2018). Guanine and Ile promote the cell growth of Clostridium butyricum. Food Ferment. Ind. 44, 34–40. doi: 10.13995/j.cnki.11-1802/ts.016987
Liu, X., Zhao, Y., and Li, H. (2021). Study on the preservation effect of glutathione, citric acid and sorbitol on chicken. Hans J. Agric. Sci. 11, 840–847. doi: 10.12677/HJAS.2021.119112
Ma, J., Lin, L., Lu, Y., Weng, B., Feng, Y., Du, C., et al. (2024). The influence of silage additives supplementation on chemical composition, aerobic stability, and in vitro digestibility in silage mixed with Pennisetum giganteum and Rice straw. Agriculture 14:1953. doi: 10.3390/agriculture14111953
Ma, R., Wang, J. E. J., Chen, Z., and Zhang, J. (2022). Effect of uracil on the freeze drying survival rate of Lactiplantibacillus plantarum LIP-1 and its action mechanism. Trans. Chin. Soc. Agric. Eng. 38, 308–316. doi: 10.11975/j.issn.1002-6819.2022.07.034
NFQCC (2006). “Determination of crude fat in feeds” in General Administration of Quality Supervision, inspection and quarantine of the People's Republic of China (National Standardization Administration).
NFQIWC (2007). “Animal feeding stuffs—determination of crude ash” in General Administration of Quality Supervision, inspection and quarantine of the People's Republic of China (National Standardization Administration).
Park, S. Y., Jung, S., Kang, I., and Ha, S.-D. (2017). Application of calcium oxide (CaO, heated scallop-shell powder) for the reduction of Listeria monocytogenes biofilms on eggshell surfaces. Poult. Sci. 97, 1681–1688. doi: 10.3382/ps/pex324
Pedcharat, K., Jangchud, K., Jangchud, A., Harnsilawat, T., and Prinyawiwatkul, W. (2023). Selected physicochemical properties of rice flour, modified tapioca starch, and their mixtures after a limewater soaking treatment. Int. J. Food Sci. Technol. 58, 5914–5925. doi: 10.1111/ijfs.16694
Pii, Y., Mimmo, T., Tomasi, N., Terzano, R., Cesco, S., and Crecchio, C. (2015). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 51, 403–415. doi: 10.1007/s00374-015-0996-1
Portillo, E. A., Benigno, L. R., Buenaobra, M. S., and Mediodia, C. J. (2018). Alternatives substrates for the production of Pleurotus ostreatus (oyster mushroom). Agric. Food Sci.
Rawat, N., Negi, R., and Singh, S. (2020). Cost-benefit analysis of different mushroom production for diversification of income in Srinagar Garhwal Valley, Uttarakhand. J. Sci. Technol. Res. 2, 1–5. doi: 10.51514/JSTR.2.4.2020.1-5
SAAS (1988). Method for determination of total potassium in soils : Industry Standard - Agriculture.
Sáez-Orviz, S., Marcet, I., Rendueles, M., and Díaz, M. (2022). The antimicrobial and bioactive properties of lactobionic acid. J. Sci. Food Agric. 102, 3495–3502. doi: 10.1002/jsfa.11823
Sato, Y., Ohata, H., Inoue, A., Ishihara, M., Nakamura, S., Fukuda, K., et al. (2019). Application of colloidal dispersions of bioshell calcium oxide (BiSCaO) for disinfection. Polymers 11:1991. doi: 10.3390/polym11121991
Scariot, F. J., Pansera, M. S., Delamare, A. P. L., and Echeverrigaray, S. (2021). Citral and geraniol induce necrotic and apoptotic cell death on Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 37:42. doi: 10.1007/s11274-021-03011-8
Shokri, H. (2011). Evaluation of inhibitory effects of citric and tartaric acids and their combination on the growth of Trichophyton mentagrophytes, Aspergillus fumigatus, Candida albicans, and Malassezia furfur. Comp. Clin. Pathol. 20, 543–545. doi: 10.1007/s00580-011-1195-6
Si, P., Shao, W., Xu, G., Yu, H., Xie, N., and Du, G. (2023). Effects of different concentrations of citric acid and oxalic acid on yield, quality and nutrient absorption of grape. J. Fruit Sci. 40, 1606–1614. doi: 10.13925/j.cnki.gsxb.20230006
Wang, L. (2022). Effects of exogenous shikimic acid on the growth and development of jujube and wild jujube : Master, Hebei Agricultural University.
Wang, Y., Zhang, Z., Wu, X., and Fan, Z. (2016). Inhibition effect of natamax on mould diseases in Pleurotus ostreatus cultivation. J. Henan Inst. Sci. Technol. 44, 30–32+37. doi: 10.3969/j.issn.1008-7516.2016.06.008
Weng, T., Wang, Y., and Long, C. A. (2023). Inhibitory mechanism of geraniol against Geotrichum citri-aurantii in citrus. Food Sci. 44, 14–21. doi: 10.7506/spkx1002-6630-20220214-087
Wu, M. (2005). How to prevent contamination by contaminant fungi in Pleurotus ostreatus cultivation. New Agric. 48. doi: 10.3969/j.issn.1002-4298.2005.09.044
Xue, Z., Zeng, F., Cao, X., Cai, Y., Tong, J., and Lin, Z. (2019). Medium formulae screening for Pleurotus ostreatus cultivation with fresh JUNCAO. Northern Hortic. 6, 147–150. doi: 10.11937/bfyy.20182757
Yin, D., and He, Z. (2004). Method for non-sterile cultivation of Pleurotus ostreatus with corncob. Agric. Jilin 35. doi: 10.3969/j.issn.1674-0432.2004.02.037
Zanin, M., Lambert, H., and du Plessis, C. A. (2019). Lime use and functionality in sulphide mineral flotation: a review. Miner. Eng. 143:105922. doi: 10.1016/j.mineng.2019.105922
Zhang, H., Ji, H., and Liu, C. (2024). Antifungal metabolites of biocontrol stain LB-1 and their inhibition mechanism against Botrytis cinerea. Front. Microbiol. 15:1444996. doi: 10.3389/fmicb.2024.1444996
Zhang, R., and Jiao, Y. (2022). Key techniques for non-sterile cultivation of Pleurotus ostreatus. Edib. Fungi 44, 67–69. doi: 10.3969/j.issn.1000-8357.2022.04.019
Zhang, W., Ma, Y., Yuan, Y., Sun, X., and Zhang, C. (2021). Effect and mechanism of ferulic acid on major microorganisms in silage. China Feed 1, 28–32. doi: 10.15906/j.cnki.cn11-2975/s.20210708
Zhang, J., Wang, X., Zhang, J., and Wei, D. (2008). Oxygen vectors used for S-adenosylmethionine production in recombinant Pichia pastoris with sorbitol as supplemental carbon source. J. Biosci. Bioeng. 105, 335–340. doi: 10.1263/jbb.105.335
Zhao, S., Li, G., and Zhao, Y. (2019). Effects of dietary gluconic acid on growth performance and content of shortchain fatty acids in chyme of weaned piglets. China Feed 18, 68–71. doi: 10.15906/j.cnki.cn11-2975/s.20191815
Keywords: non-sterile mushroom cultivation, fresh Pennisetum giganteum, food security, marginal-land agriculture, SDGs
Citation: Zhang Y, Lu Y, Li Z, Xie M, Wen F, Li J, Rensing C, Azam SM, Chen J, Luo L, Yan F, Okal EJ, Aimable N, Lin Z and Lin D (2025) Non-sterile substrate cultivation of oyster mushrooms on fresh Giant Juncao Grass: a scalable strategy for sustainable nutrition in underdeveloped regions. Front. Sustain. Food Syst. 9:1582869. doi: 10.3389/fsufs.2025.1582869
Edited by:
Liyou Qiu, Henan Agricultural University, ChinaReviewed by:
Tyler John Barzee, University of Kentucky, United StatesMohd. Rashid Mohd. Rakib, Universiti Malaysia Sabah (Sandakan), Malaysia
Copyright © 2025 Zhang, Lu, Li, Xie, Wen, Li, Rensing, Azam, Chen, Luo, Yan, Okal, Aimable, Lin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhanxi Lin, bHp4anVuY2FvQDE2My5jb20=; Dongmei Lin, bGluZG1fanVuY2FvQDE2My5jb20=
 Yulong Zhang
Yulong Zhang Yiting Lu1
Yiting Lu1 Jing Li
Jing Li Christopher Rensing
Christopher Rensing Syed Muhammad Azam
Syed Muhammad Azam Nsanzinshuti Aimable
Nsanzinshuti Aimable Dongmei Lin
Dongmei Lin