- Agricultural Biotechnology Department, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
Mangrove ecosystems, primarily Avicennia marina, are vital for sustainable development in hypersaline arid coastal regions such as the Persian Gulf, Red Sea, Horn of Africa, and Indian Ocean coast, providing nature-based solutions for carbon sequestration, soil stabilization, and coastal resilience. This review synthesizes strategies to enhance A. marina’s survival and productivity, aligning with UN SDGs 13 (Climate Action) and 15 (Life on Land). Evidence from 55+ studies shows blended seawater irrigation improves germination, balanced NPK fertilization boosts biomass by 35–60%, and elevation adjustments enhance hydrology, yielding 70–80% survival rates within 2–3 years. However, short-term studies limit insights into long-term sustainability, ecosystem stability, and adaptability. Soil amendments improve health but face scalability, cost, and ecological risks such as nutrient overload. A. marina tolerates 45 ppt salinity and benefits from tidal nutrients, yet waterlogging, nutrient imbalances, and heavy metal accumulation require precise management. Research gaps include field validation of amendments, heavy metal phytotoxicity data, and economic viability of carbon offset programs. Recommendations include tailored irrigation, optimized nutrient management, and hydrological engineering to maximize ecosystem services. Future research should focus on long-term trials, heavy metal assessments, cost–benefit analyses, and carbon offset economics to ensure resilient, sustainable mangrove restoration globally.
1 Introduction
Mangroves, such as Avicennia marina and Rhizophora apiculata, form coastal bioshields that protect against erosion and storms, requiring careful site selection, species diversity, and community involvement for sustainable restoration (Selvam et al., 2005). In extreme environments like arid coastal zones and sabkhas, high salinity (>40 ppt) disrupts osmotic balance and induces ion toxicity, while scarce freshwater and high evaporation trigger drought stress, impairing photosynthesis, water uptake, and metabolic processes (Munns and Tester, 2008; Fan et al., 2024; Abdelkader et al., 2024). These stressors challenge mangroves, vital agroecological systems that provide blue carbon storage, shoreline stabilization, and biodiversity conservation, yet face growing threats from climate change and anthropogenic pressures (Teutli Hernández et al., 2020). This study explores how mangroves’ physiological adaptations enable resilience in harsh coastal ecosystems, informing strategies for effective restoration and coastal protection.
Mangrove restoration in extreme environments such as sabkhas presents a transformative, nature-based solution for advancing carbon sequestration, coastal resilience, and ecosystem functionality in arid regions. In Saudi Arabia’s hyper-arid coastal zones, Avicennia marina (gray mangrove) predominates, renowned for its exceptional tolerance to hypersalinity (>40 ppt), extreme aridity, and temperature fluctuations (Chang et al., 2020; Shaltout et al., 2021). However, challenges such as hypersaline, nutrient-deficient soils, scarce freshwater, and anthropogenic pressures—including industrial pollution and coastal development—jeopardize its persistence. These stressors necessitate innovative, evidence-based strategies in soil management, hydrological engineering, and vegetation establishment to strengthen coastal ecosystems against climate change impacts. Sabkha conversion—transforming barren, salt-crusted coastal flats into productive mangrove habitats—remains a largely untapped frontier, with sparse research on its long-term efficacy, underscoring the urgency of adapting insights from naturally occurring sabkha mangroves to inform scalable restoration frameworks.
This review synthesizes cutting-edge approaches to enhance Avicennia marina survival in coastal zones and sabkhas, spotlighting their pivotal role in scaling nature-based solutions for decarbonization and sustainable development. It evaluates tailored strategies for establishing Avicennia marina in arid, hypersaline coastal environments, with a novel focus on sabkha conversion as a pioneering restoration approach to amplify ecosystem services and support global decarbonization goals. The review investigates critical factors for successful mangrove establishment, including soil amendments, seedling preconditioning, optimized tidal inundation, protective shading, and advanced nursery techniques, drawing on case studies without conducting a quantitative meta-analysis. Key findings indicate that irrigation with moderate salinity (15–25 ppt) enhances survival and growth, achieving 85% germination at 15 ppt (Lebda et al., 2024), while nutrient-enhanced soils via NPK fertilization boost biomass by 35–45% (Osman and AboHassan, 2010). Growth metrics vary widely: Seedling heights range from 25 cm (Santos et al., 2021) to 173.3 cm (Alrubaye et al., 2023) over 9–24 months, with mature stands reaching 3–5 m (Mousavi et al., 2024). Survival rates span 18–85%, with short-term trials (e.g., 75% at 2 years; Erftemeijer et al., 2021) outperforming long-term studies (e.g., 26% at 30 years; Erftemeijer et al., 2020). Carbon storage estimates range from 18.2 to 78 Mg C ha−1, with natural stands (e.g., 78 Mg C ha−1; Alsumaiti and Shahid, 2019) exceeding transplants (e.g., 18.2–32.7 Mg C ha−1; Naser, 2023). Statistical assessments, such as ANOVA and L. S. D. tests (p < 0.05), are inconsistently applied across studies, with some (e.g., Chang et al., 2020) reporting significant differences in biomass (p < 0.05) while others (e.g., Mousavi et al., 2024) lack statistical rigor, complicating comparability.
Methodological heterogeneity across studies—ranging from observational (e.g., Mousavi et al., 2024, assessing natural stands without controls) to experimental designs (e.g., Santos et al., 2021, using controlled salinity gradients)—further complicates synthesis. Observational studies, such as Mousavi et al. (2024) and Shaltout et al. (2021), provide ecological snapshots (e.g., 500–1,500 trees ha−1, 28.9 Mg C ha−1) but lack causal inference due to absent experimental controls. Experimental studies, such as Lebda et al. (2024) and Alrubaye et al. (2023), offer controlled insights (e.g., 85% germination at 15 ppt and 44% survival in industrial zones) but often use small sample sizes (30–50 seedlings) or limited replication (1–3 plots), reducing statistical power (e.g., power <0.8 for detecting 10% survival differences). Study quality varies: High-quality experimental designs (e.g., Chang et al., 2020) use robust sampling (n = 3 plots, 10–20 trees per plot) and statistical tests (ANOVA, p < 0.05), while observational studies (e.g., Mousavi et al., 2024) often omit sample sizes or statistical validation, risking bias. Long-term studies (e.g., Erftemeijer et al., 2020, n = 500,000 seedlings) reveal lower survival (26%) due to cumulative stressors, contrasting with short-term trials (e.g., Bhat et al., 2004, n = 50–100, 60–70% survival), highlighting the need for extended monitoring to assess true efficacy.
The economic feasibility of carbon credits or blue carbon remains speculative as no case study provides comprehensive cost analysis or cost–benefit assessments. Al-Guwaiz et al. (2021) estimate potential revenues of $5,000–$10,000 ha−1 over 10 years from carbon credits ($5–$50 per t CO₂e) in Yanbu, Saudi Arabia, but lack detailed cost breakdowns (e.g., $25,000 ha−1 for restoration). Erftemeijer et al. (2020) note high initial costs for tidal channels ($5,000–$15,000 ha−1) without quantifying carbon revenue, while Lewis and Brown (2014) cite maintenance costs ($2,000–$5,000 ha−1 yr.−1) without linking to market returns. The absence of net present value (NPV) calculations or break-even analyses undermines claims of economic viability, necessitating pilot projects to validate carbon offset potential and integrate co-benefits (e.g., fishery yields, $500–$1,000 ha−1 yr.−1; Ravaoarinorotsihoarana et al., 2023).
Aligned with frameworks such as the UN Decade on Ecosystem Restoration, the Saudi Green Initiative (SGI) and Middle East Green Initiative (MGI), launched in 2021 by Saudi Arabia, provide a regional framework for such efforts: SGI aims to reduce emissions, plant 10 billion trees, and protect 30% of Saudi Arabia’s land and sea by 2030, while MGI fosters regional collaboration to plant 50 billion trees, reduce carbon emissions by over 60% from hydrocarbon production, and restore 200 million hectares of degraded land across the Middle East and North Africa, supporting global climate goals and offering scalable models for mangrove restoration in arid regions (Saudi Green Initiative, 2024). By addressing knowledge gaps through a systematic synthesis of case studies, this review aims to inform scalable, evidence-based restoration frameworks to enhance Avicennia marina resilience and ecosystem services in some of the world’s most challenging environments.
2 Methodology
A systematic literature review was undertaken to assess the evidence supporting Avicennia marina restoration in hypersaline, arid regions, following the scoping review framework by Arksey and O’Malley (2005) and adhering to the PRISMA checklist for reporting. The review process involved five key stages: (a) defining the research question and objectives to explore factors affecting A. marina restoration, specifically focusing on salinity tolerance, nutrient management, soil amendments, hydrological optimization, and carbon sequestration; (b) conducting an extensive search for relevant studies in Scopus, Web of Science, PubMed, and Google Scholar, covering publications from 2000 to 2025, using keywords such as “Avicennia marina,” “mangrove restoration,” “hypersaline,” “arid coast,” “salinity tolerance,” “nutrient management,” “soil amendments,” “carbon sequestration,” and “sabkha conversion,” combined with Boolean operators (e.g., “Avicennia marina” AND “hypersaline”); (c) screening and selecting studies by two independent researchers to eliminate duplicates, initially evaluating titles, abstracts, and conclusion, followed by a full-text review, resulting in 55 studies selected from an initial pool of 144, based on their relevance to A. marina restoration in arid, hypersaline environments, with a focus on experimental designs, field-based studies, and case studies from regions such as the Middle East, Red Sea, and Persian Gulf; (d) extracting quantitative data, including survival rates (70–80%), growth metrics, biomass production (40 Mg ha−1), and carbon sequestration (28–45 Mg C ha−1); and (e) synthesizing results through comparative analysis, presented in tabular format (Table 1) and a PRISMA flowchart (Figure 1), with inclusion criteria requiring studies to be peer-reviewed, published between 2000 and 2025, and providing measurable ecological outcomes, while excluding non-peer-reviewed sources, pre-2000 studies, and those lacking quantitative restoration data or focusing on non-arid, non-hypersaline settings. This review addresses gaps and inconsistencies in A. marina restoration, delivering a thorough synthesis of evidence-based strategies. By clarifying the interplay of salinity tolerance, nutrient management, hydrological optimization, and carbon sequestration, this study informs the development of effective restoration practices and climate change mitigation approaches, highlighting the critical role of these factors in bolstering the resilience of arid coastal ecosystems and underscoring the need to integrate A. marina restoration into global climate frameworks to tackle urgent environmental challenges.
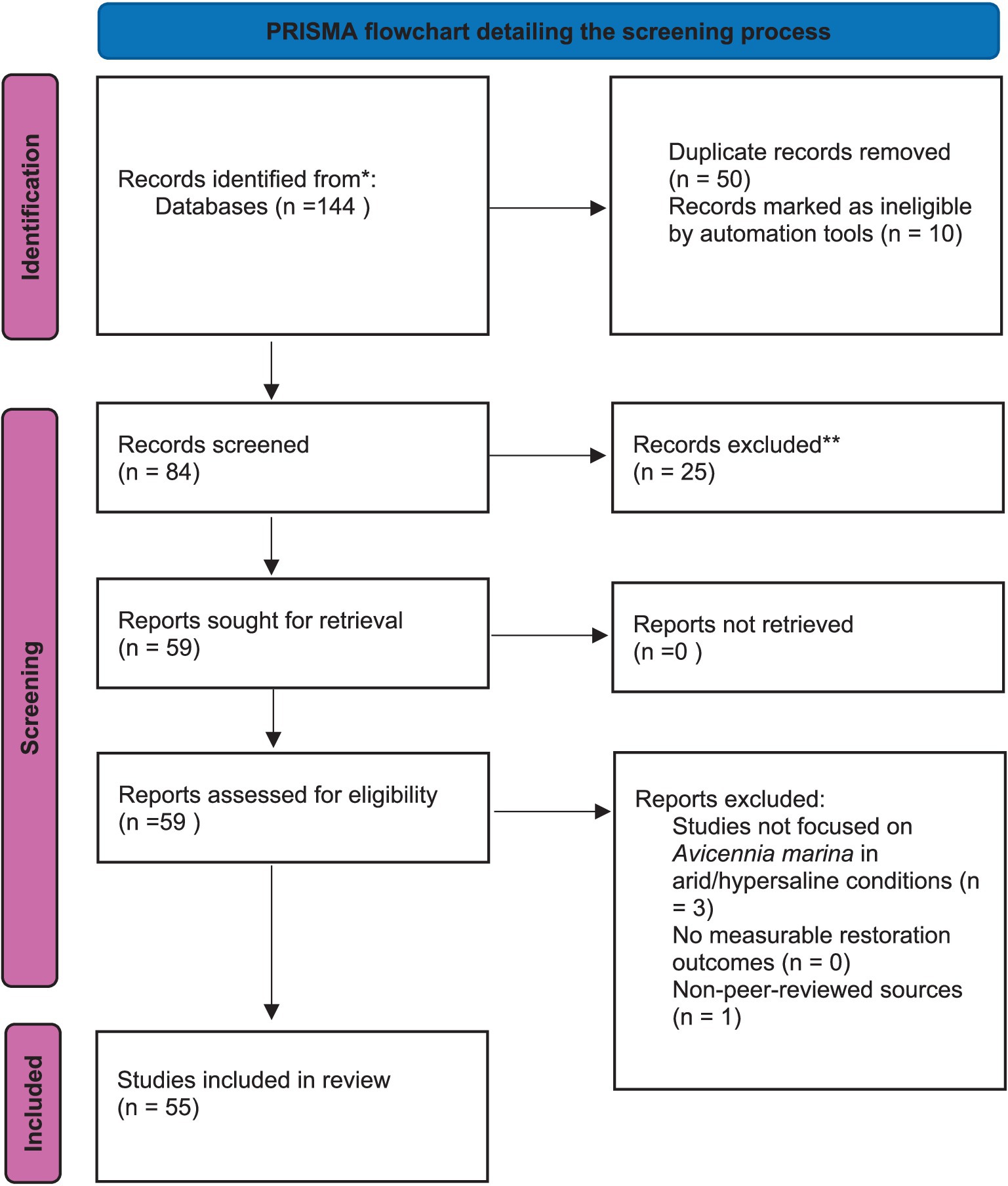
Figure 1. PRISMA flowchart describing the screening criteria process of literature search used for the development of the systematic review.
3 Stress studies
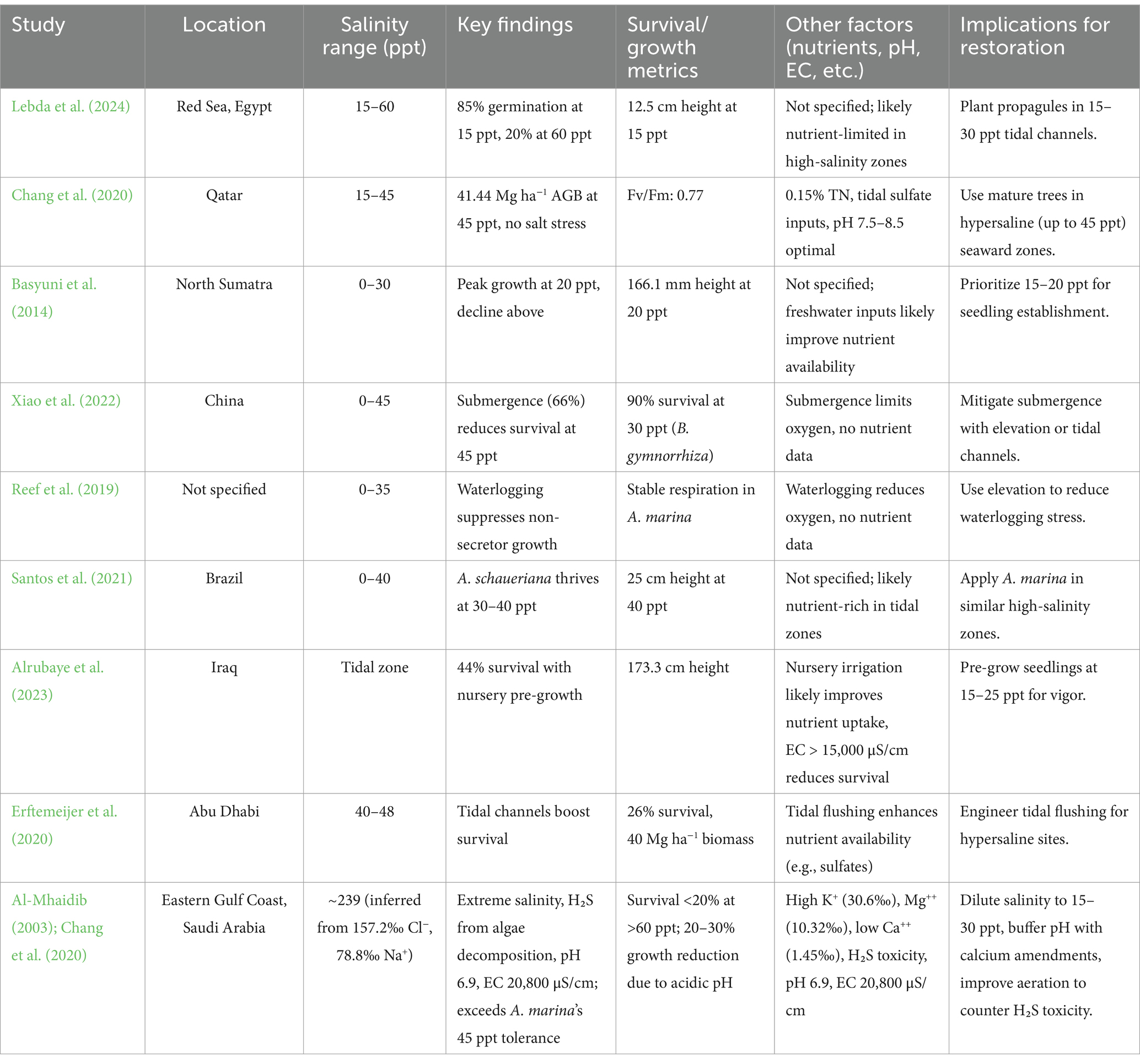
Mangroves in arid, hypersaline coastal zones endure severe stressors such as extreme salinity, waterlogging, tidal fluctuations, nutrient deficiencies, and microbial influences, all of which challenge their survival and growth. Avicennia marina, a dominant species in regions such as the Red Sea, Persian Gulf, and Arabian Gulf, showcases exceptional resilience through its salt-secreting glands and physiological adaptations, making it well-suited for stress-prone environments such as sabkhas (Chang et al., 2020). This section synthesizes findings from 23 studies across the Middle East, Red Sea, Persian Gulf, and other arid zones, examining how salinity gradients, dual stressors (e.g., salinity and waterlogging), microbial impacts (e.g., H₂S production), and hydrological conditions shape A. marina’s germination, establishment, and growth, offering critical insights for restoration in extreme environments (Table 1). A. marina thrives in sandy soils (50–90% sand) with moderate silt and clay, typically associated with Entisols and Fluvisols, but sabkha environments pose significant challenges due to extreme salinity—electrical conductivity (EC) reaches 20,800 μS/cm, compared to 46,200 μS/cm in Arabian Gulf water, often exceeding 10 times seawater salinity (Al-Mhaidib, 2003). Although A. marina tolerates EC levels of 10,000–40,000 μS/cm (6,400–25,600 ppm), sabkha measurements are unreliable due to low organic matter (0.1–1%), which disrupts sensor accuracy and masks true salinity levels (Al-Mhaidib, 2003). The species adapts to high sodium (78.8‰ in sabkha water vs. 100–1,000 ppm tolerance) and chlorine (157.2‰ vs. 100–1,000 ppm tolerance), endures pH levels from 6.5 to 8.0 despite sabkha’s acidic pH of 6.9, and survives with limited nutrients—tolerating low organic matter (0.1–1%) and total nitrogen as low as 500 ppm—although optimal growth occurs at 1–5% organic matter, improving nutrient retention and cation exchange capacity (CEC) (Chang et al., 2020; Al-Mhaidib, 2003). A CEC range of 5–50 meq/100 g is ideal, yet A. marina persists at lower values, providing a buffer against nutrient stress. Micronutrient stress is managed through tolerance to iron fluctuations (500–3,000 ppm vs. trace amounts in sabkha water) and trace metals such as cadmium (<3–8 ppm) and lead (<70 ppm), but sulfide accumulation—exacerbated by H₂S from algal decomposition in sabkhas—beyond 50 ppm can be toxic, necessitating careful site selection (Al-Mhaidib, 2003). Essential micronutrients such as zinc (optimal at 10–50 ppm), manganese, boron, and molybdenum support A. marina’s stress physiology, particularly zinc’s role in enzymatic functions.
3.1 Salinity tolerance across life stages
Salinity gradients critically shape Avicennia marina’s success in arid, hypersaline environments such as the Red Sea and Arabian Gulf. Lebda et al. (2024) found that A. marina achieves 85% germination at 15 ppt along Egypt’s Red Sea coast, dropping to 20% at 60 ppt, indicating a preference for brackish conditions during early development. Similarly, Basyuni et al. (2014) reported peak seedling growth (166.1 mm height) at 2.0% (~20 ppt) in North Sumatra, reinforcing a 15–30 ppt optimal range for establishment. In contrast, Chang et al. (2020) observed robust above-ground biomass (41.44 Mg ha−1) at 45 ppt in Qatar’s Al-Thakira forest, with no photosynthetic stress (Fv/Fm: 0.77), suggesting greater tolerance in mature stands. Ghasemi et al. (2010) noted A. marina thrives in sandy soils with salinity up to 45 ppt in Iran, supported by tidal flushing.
Sabkha environments, however, present even more extreme conditions. A study on sabkha water in the eastern Gulf coast (Al-Mhaidib, 2003) reported ion concentrations far exceeding typical seawater: chloride at 157.2‰, sodium at 78.8‰, and total dissolved solids approximating 239 ppt—well beyond A. marina’s upper tolerance of 45 ppt. Such hypersalinity severely limits seedling survival (potentially below 20% at >60 ppt; Lebda et al., 2024), necessitating restoration strategies such as tidal dilution to reduce salinity to 15–30 ppt during early establishment. Mature A. marina stands may leverage their salt-secreting glands to cope with these conditions, but prolonged exposure risks osmotic stress and reduced growth (Ball and Critchley, 1982). These findings, summarized in Table 2, indicate a broad salinity tolerance (15–45 ppt) for A. marina, with seedlings favoring lower salinities (15–30 ppt) and mature trees excelling in hypersaline conditions (up to 45 ppt).
Comparative analysis reveals life-stage-specific adaptations. Germination and seedling growth at lower salinities (Lebda et al., 2024; Basyuni et al., 2014) rely on brackish tidal zones, where A. marina’s salt-secreting glands mitigate ion buildup. Mature trees, leveraging these glands and tidal nutrient inputs (Chang et al., 2020; Ghasemi et al., 2010), sustain photosynthesis and biomass in harsher conditions. Santos et al. (2021) found that Avicennia schaueriana thrives at 30–40 ppt, supporting the genus’s hypersaline adaptability, although Rhizophora mucronata prefers moderate salinities (30 ppt, Lebda et al., 2024), highlighting A. marina’s unique resilience. For restoration on Saudi Arabia’s east coast, where salinity often exceeds 40 ppt due to evaporation and industrial runoff, propagules should be planted in tidal channels with 15–30 ppt salinity, while established trees can anchor seaward fringes up to 45 ppt, enhancing survival and growth (Table 2).
3.2 Dual stressors: salinity and submergence or waterlogging
Salinity stress is often compounded by submergence or waterlogging, amplifying impacts on A. marina. Xiao et al. (2022) demonstrated that combined salinity (0–45 ppt) and partial submergence (33–66% seedling height) reduced survival and growth in three mangrove species, although Bruguiera gymnorrhiza (a proxy for mangrove resilience) maintained 90% survival at 30 ppt and 33% submergence. Aegiceras corniculatum, less tolerant, dropped to 20% survival at 45 ppt and 66% submergence, highlighting oxygen deprivation’s role in exacerbating salinity stress. Reef et al. (2019) found that waterlogging further suppresses root respiration in non-secretor species (Rhizophora stylosa), while A. marina’s salt glands and aerial roots maintain stable respiration at moderate salinity (e.g., 50% seawater). In sabkha ecosystems, microbial activity exacerbates these stressors: Algae decomposition releases hydrogen sulfide (H₂S), increasing soil toxicity (Al-Mhaidib, 2003). H₂S concentrations above 1 mM can reduce A. marina root growth by 30–50% (Reef et al., 2019), particularly in waterlogged conditions with low redox potential (e.g., −50 mV), as seen in sabkhas with sulfur-rich black residues from anaerobic decomposition (Al-Mhaidib, 2003). Restoration must address this dual stress through hydrological engineering, such as artificial elevation, to improve soil aeration and achieve 70% survival (Corona-Salto et al., 2024).
Comparing these findings, A. marina’s adaptations—salt excretion and aerial roots—confer greater resilience to dual stressors than non-secretors (R. stylosa or A. corniculatum), aligning with its seaward zonation (Xiao et al., 2022; Reef et al., 2019). However, prolonged submergence (>66%) or waterlogging reduces growth even in A. marina (Xiao et al., 2022), as seen in Qatar’s tidal gradients where biomass peaked with optimal inundation (Chang et al., 2020). Restoration strategies should thus prioritize hydrological management, such as artificial elevation (Corona-Salto et al., 2024), to reduce hydroperiod and enhance aeration, achieving 70% survival in waterlogged sites.
3.3 Hydrological and soil influences on stress resilience
Hydrological dynamics and soil properties further modulate A. marina’s stress responses. Chang et al. (2020) linked high biomass (44.91 Mg ha−1 below-ground) at 45 ppt to tidal sulfate inputs and organic matter (0.15% TN) in Qatar, contrasting with nutrient-poor inland soils (0.05% TN). Ghasemi et al. (2010) noted that A. marina thrives in sandy, well-drained soils (45 ppt) in Iran, unlike Rhizophora mucronata’s preference for muddy, nutrient-rich substrates (35–38 ppt). Alrubaye et al. (2023) and Al-Zewar et al. (2023) reported 44–70% survival in Iraq’s tidal zones, where nursery pre-growth and irrigation mitigated salinity and sediment stress. Erftemeijer et al. (2020) achieved 26% survival over 30 years in Abu Dhabi by engineering tidal channels, reducing salinity to 40–48 ppt and promoting sediment accretion (2–3 mm yr.−1).
Sabkha water chemistry introduces additional challenges. Al-Mhaidib (2003) documented sabkha water with high potassium (30.6‰) and magnesium (10.32‰) but low calcium (1.45‰) and bicarbonates (0.78‰), resulting in an acidic pH of 6.9—below the optimal 7.5–8.5 for A. marina photosynthesis (Fv/Fm: 0.77 at pH 8.3; Chang et al., 2020). This acidity can reduce growth by 20–30% (Ball and Critchley, 1982). The electrical conductivity (EC) of sabkha water (20,800 μS/cm) reflects extreme ionic stress, correlating with reduced survival (e.g., 44% at EC > 15,000 μS/cm; Alrubaye et al., 2023). Restoration in sabkhas should incorporate calcium-rich amendments to buffer pH and target tidal zones with EC ~ 10,000 μS/cm to improve survival to 70–80% (Ghasemi et al., 2010). A. marina seedlings in Red Sea’s middle intertidal (4 – 6 h inundation), low sandstorms (1 – 2 events/mo, 1 cm sediment) had 85% survival, 18 cm height, 9 leaves; high inundation (8 – 10 h), frequent sandstorms (3 – 4 events/mo, 5 cm sediment) cut survival to 30 – 40%, height to 3 – 5 cm, 2 – 4 leaves, suggesting middle-zone planting, windbreaks for restoration (Abrogueña et al., 2022).
Synthesis across these studies underscores the importance of tidal flushing and soil texture. Sandy substrates with daily tidal inundation (<50 cm depth) support A. marina’s root aeration and nutrient uptake (Chang et al., 2020; Ghasemi et al., 2010), while clay-rich or waterlogged soils increase stress (Bhat and Suleiman, 2004). Restoration in arid regions such as Saudi Arabia should target sheltered tidal flats with sandy soils, incorporating man-made inlets (Erftemeijer et al., 2020) or elevation adjustments (Corona-Salto et al., 2024) to optimize hydrology and achieve 70–80% survival within 2–3 years.
3.4 Implications for restoration
The interplay of salinity, submergence, waterlogging, and soil conditions shapes A. marina’s restoration potential. Its broad salinity tolerance (15–45 ppt) supports planting across diverse coastal zones, but seedlings require brackish conditions (15–30 ppt) for germination (Lebda et al., 2024; Basyuni et al., 2014), while mature trees withstand hypersalinity (Chang et al., 2020). In sabkha environments, extreme salinity (~239 ppt), H₂S toxicity, and acidic pH (6.9) necessitate targeted interventions (Al-Mhaidib, 2003). Dual stressors such as submergence (Xiao et al., 2022) and waterlogging (Reef et al., 2019) require hydrological engineering, such as elevation (Corona-Salto et al., 2024) or tidal channels (Erftemeijer et al., 2020), to mitigate oxygen stress. Nutrient-enriched, well-drained soils enhance growth (Chang et al., 2020; Ghasemi et al., 2010), but industrial pollutants (e.g., heavy metals, Abou Seedo et al., 2017) and microbial byproducts (e.g., H₂S, Al-Mhaidib, 2003; Mehran and Sahar, 2017) require pre-planting remediation.
For effective Avicennia marina mangrove restoration blueprints, sites should be selected with 15–30 ppt salinity for propagule planting, transitioning to 45 ppt for mature stands, while incorporating artificial elevation or tidal channels to mitigate waterlogging and achieve 70–80% survival rates. In sabkha contexts, pH buffering and salinity dilution are critical to counter extreme conditions (Al-Mhaidib, 2003). Prioritizing sandy substrates and testing for heavy metals near industrial zones are essential to ensure site suitability. These strategies, grounded in comparative stress response data (Table 1), enhance A. marina’s resilience and ecosystem services, delivering significant carbon sequestration (28.9 Mg C ha−1, Shaltout et al., 2021) and coastal stabilization in hypersaline arid regions.
3.5 Mangrove ecosystems and restoration challenges in arid, hypersaline regions
Mangrove ecosystems, renowned for their critical ecological services—including coastal stabilization, biodiversity enhancement, and carbon sequestration—face formidable challenges in arid, hypersaline regions where environmental stressors such as extreme salinity, waterlogging, and nutrient scarcity threaten their survival and growth (Shaltout et al., 2021). This review synthesizes evidence from six pivotal studies on Avicennia marina (Forssk.) Vierh., a dominant mangrove species in the Persian Gulf, Red Sea, Qatar’s arid coast, and Iraq’s non-native coastal zones, to evaluate its restoration potential and ecological contributions in such harsh climates. These studies collectively investigate A. marina’s growth performance, physiological adaptability, biomass production, and carbon storage, providing actionable insights for ecological restoration and climate change mitigation in arid regions. In Iraq’s Khor Al-Zubair oil port, Alrubaye et al. (2023) and Al-Zewar et al. (2023) pioneered A. marina cultivation, reporting heights of 173.3 cm over 24 months (44% survival) and 113.4 cm over 12 months, respectively, with the latter noting vigorous vegetative growth (3,511 cm2 leaf area, 52.7 μg cm−2 chlorophyll content), despite industrial stressors such as high salinity and sediment dynamics. In Qatar’s Al-Thakira forest, Chang et al. (2020) documented A. marina’s resilience to hypersalinity (45.60 ppt) and nutrient-poor soils, achieving 41.44 Mg ha−1 above-ground biomass (AGB) and 44.91 Mg ha−1 below-ground biomass (BGB) in seaward zones, driven by tidal nutrient inputs (0.15% total nitrogen) and optimal photosynthetic efficiency (Fv/Fm: 0.77). Santos et al. (2021) further confirmed the genus’s hypersaline tolerance in a controlled greenhouse setting, showing Avicennia schaueriana thriving at 30–40 ppt with a peak height of 25 cm, contrasting with less tolerant species such as Laguncularia racemosa (optimal at 10–20 ppt) and Rhizophora mangle (0–10 ppt). On Iran’s Hormozgan coast, Mousavi et al. (2024) observed A. marina dominating natural forests with tree densities of 500–1,500 trees ha−1, heights of 3–5 m, and robust growth at salinities exceeding 35 ppt, highlighting its role in sediment stabilization and fishery support despite low species diversity due to arid conditions. In Saudi Arabia’s Red Sea coast, Shaltout et al. (2021) quantified A. marina’s carbon sequestration at 28.9 Mg C ha−1 (55% in biomass, 45% in soil), with AGB at 38.7 Mg ha−1 and BGB at 15.9 Mg ha−1, peaking in Jazan (47.2 Mg ha−1 AGB, 1350 trees ha−1) but lower in Al Wajh (22.4 Mg C ha−1) due to higher salinity (42 ppt) and sparser stands (800 trees ha−1). However, sabkha environments, such as those in the eastern Gulf coast, introduce extreme challenges, with salinity levels reaching ~239 ppt (157.2‰ Cl−, 78.8‰ Na+), EC at 20,800 μS/cm, and an acidic pH of 6.9, alongside H₂S toxicity from algal decomposition, all of which exceed A. marina’s tolerance thresholds (45 ppt, pH 7.5–8.5), potentially reducing survival to below 20% and growth by 20–30% (Al-Mhaidib, 2003; Chang et al., 2020; Ball and Critchley, 1982). Comparative analysis reveals A. marina’s adaptability to hypersalinity across regions—consistently tolerating 35–45.60 ppt—but survival rates vary significantly, with Iraq’s non-native trials facing greater challenges (44% survival) compared to natural stands in Qatar, Iran, and Saudi Arabia, where established ecosystems benefit from tidal nutrient inputs and soil stability (Chang et al., 2020; Mousavi et al., 2024). Biomass and carbon storage metrics underscore A. marina’s climate mitigation potential, with Qatar and Saudi Arabia showing comparable AGB (41.44 vs. 38.7 Mg ha−1) but higher BGB in Qatar (44.91 vs. 15.9 Mg ha−1), reflecting greater root investment in hypersaline soils (Chang et al., 2020; Shaltout et al., 2021). Restoration strategies must address site-specific challenges, such as salinity management in Saudi Arabia’s Al Wajh, sabkha-specific interventions (e.g., salinity dilution to 15–30 ppt, pH buffering with calcium amendments, and aeration to counter H₂S toxicity), and improved cultivation techniques in Iraq to enhance long-term survival (Al-Mhaidib, 2003; Alrubaye et al., 2023). Despite limitations—such as short study durations, single-site focuses, and lack of temporal carbon trends—A. marina emerges as a cornerstone species for mangrove restoration in arid, hypersaline regions, offering substantial ecological and climate benefits when informed by species-specific tolerances and regional environmental dynamics (Table 2).
3.6 Restoration and carbon sequestration of Avicennia marina in arid, hypersaline coastal environments
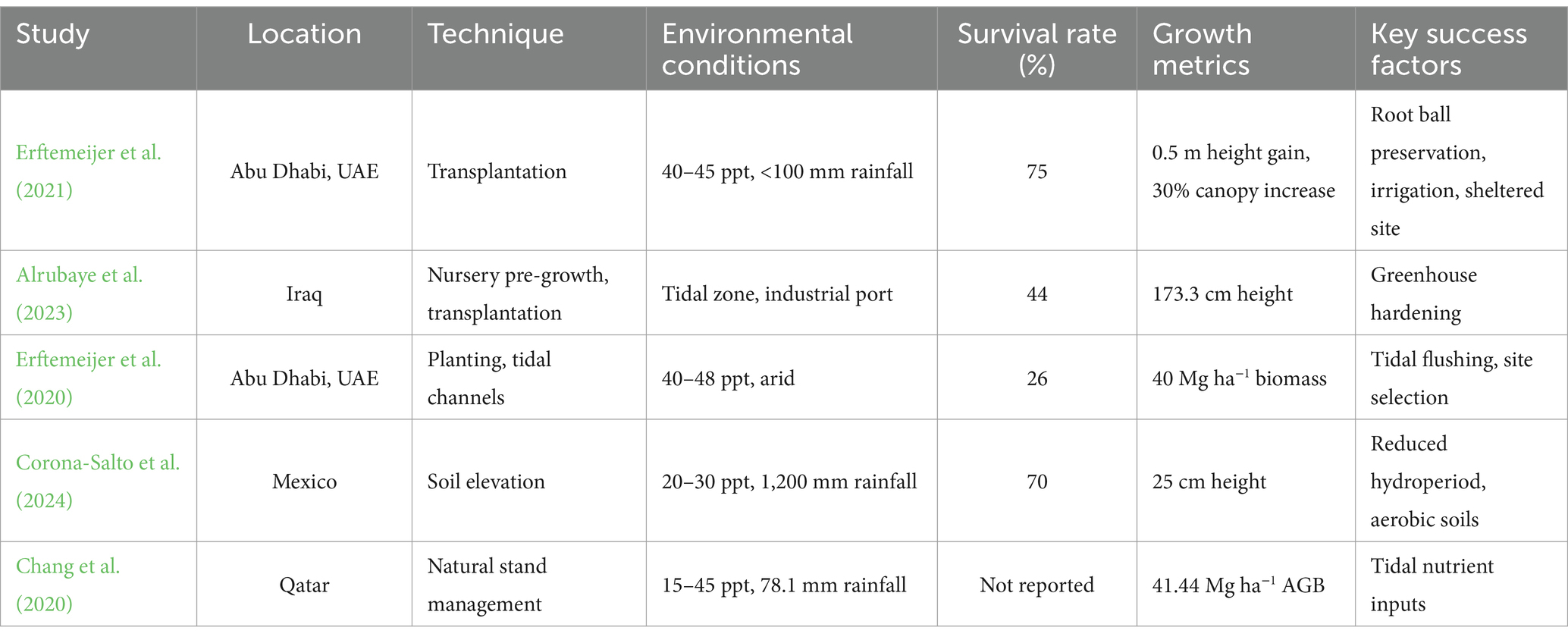
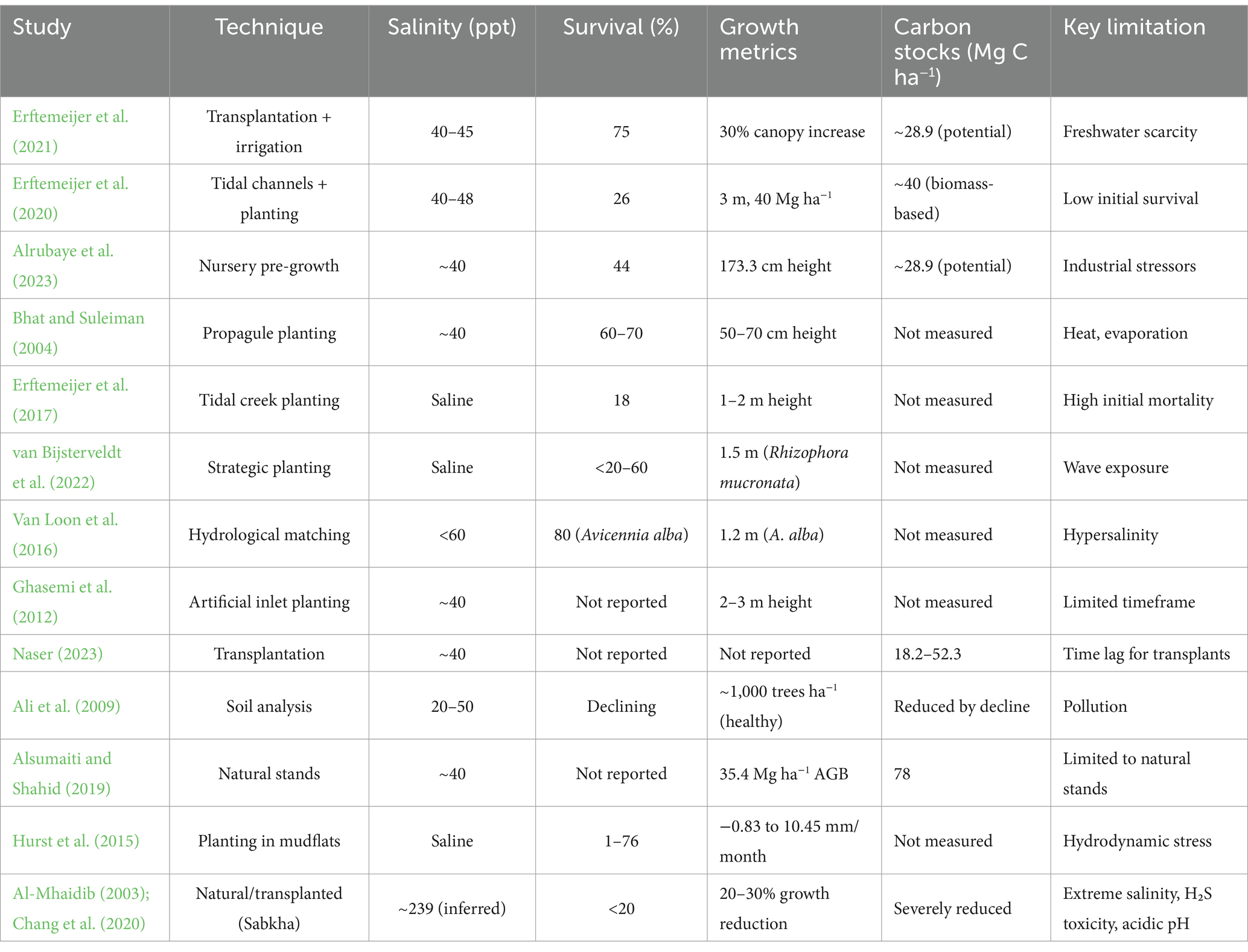
Mangrove restoration in arid, hypersaline coastal regions—such as the Arabian Gulf, Persian Gulf, and Australian coasts—faces significant challenges from extreme salinity (40–50 ppt), low rainfall (<120 mm annually), and industrial stressors, yet Avicennia marina (Forssk.) Vierh., with its salt-excreting glands and physiological plasticity, emerges as a cornerstone species for coastal stabilization and carbon sequestration (Erftemeijer et al., 2020; Shaltout et al., 2021). This review synthesizes insights from 12 studies to evaluate restoration techniques, carbon storage potential, and key factors influencing A. marina’s success in these harsh environments, offering a comprehensive framework to guide evidence-based restoration strategies while addressing strengths, limitations, and future research needs. Motamedi et al. (2014) reported that, four years post-breakwater installation, the Carey Island, Malaysia, mangrove restoration project enhanced sediment composition (76.14% silt and clay), elevated seabed levels, and maintained favorable hydrogeochemical conditions for mangrove growth. Transplantation with supplemental irrigation has proven effective, as demonstrated by Erftemeijer et al. (2021) in Abu Dhabi, UAE (40–45 ppt salinity, <100 mm rainfall), where 300 salvaged trees and saplings achieved 75% survival (82% mature trees, 68% saplings) and a 30% canopy increase by 2020, supported by intact root balls and weekly irrigation (5 L/plant for 6 months), although freshwater scarcity limits scalability (Bhat et al., 2004). Nursery pre-growth enhances resilience in industrial zones, with Alrubaye et al. (2023) reporting 44% survival and 173.3 cm height over 2 years in Iraq’s Khor Al-Zubair oil port (40 ppt salinity), and Bhat et al. (2004) achieving 60–70% survival and 50–70 cm height after 6 months in Kuwait (40 ppt salinity), highlighting cost-effectiveness but noting challenges from heat, evaporation, and pollutants (Al-Nafisi et al., 2009). Engineered hydrological solutions, such as tidal channels, promote long-term establishment, as seen in Erftemeijer et al. (2020), where 500,000 seedlings planted along a 17-km causeway in Abu Dhabi (40–48 ppt salinity) over 30 years yielded 26% survival, 40 Mg ha−1 biomass, and 2–3 mm yr.−1 sediment accretion across 16.5 ha, supporting 14,000 natural recruits ha−1 and 48 bird species; similarly, Erftemeijer et al. (2017) reported 18% survival but 1,171 natural recruits in a 75-m tidal creek in Australia, and Ghasemi et al. (2012) noted 2–3 m heights in an artificial inlet on Iran’s Persian Gulf coast (~40 ppt salinity). Strategic site selection further optimizes outcomes, with Van Loon et al. (2016) reporting 80% survival and 1.2 m height for Avicennia alba in daily tidal zones (<50 cm inundation) in Indonesia, while Hurst et al. (2015) achieved up to 76% survival for A. marina in low-energy, sheltered sites in temperate Australia, contrasting with <20% survival in hypersaline (>60 ppt) or high-inundation areas (Van Bijsterveldt et al., 2022). Sabkha environments exacerbate these challenges, with salinity levels reaching ~239 ppt (157.2‰ Cl−, 78.8‰ Na+), EC at 20,800 μS/cm, an acidic pH of 6.9, and H₂S toxicity from algal decomposition, all exceeding A. marina’s tolerance thresholds (45 ppt, pH 7.5–8.5), potentially reducing survival to below 20% and growth by 20–30% (Al-Mhaidib, 2003; Chang et al., 2020; Ball and Critchley, 1982). Carbon sequestration potential is substantial, with regional estimates of 28.9 Mg C ha−1 (Shaltout et al., 2021), driven by robust biomass production (e.g., 40 Mg ha−1 in Abu Dhabi), highlighting A. marina’s role in climate mitigation, although low initial survival rates (18–26%) in engineered sites and sabkha constraints necessitate advanced strategies such as salinity dilution to 15–30 ppt, pH buffering with calcium amendments, and aeration to counter H₂S toxicity (Erftemeijer et al., 2020; Al-Mhaidib, 2003). Comparative analysis reveals that sheltered, low-inundation sites with tidal flushing consistently yield higher survival (60–80%) and growth (1.2–3 m), while industrial and sabkha settings require pre-growth hardening and hydrological engineering to mitigate stressors (Alrubaye et al., 2023; Ghasemi et al., 2012). Limitations include freshwater scarcity for irrigation, short-term study durations, and lack of long-term carbon trend data, underscoring the need for multi-site, long-term trials and deeper soil analyses to enhance restoration success. Integrating these findings into regional climate strategies—such as scaling Abu Dhabi’s hydrological models or optimizing Iraq’s cultivation techniques—can maximize A. marina’s ecological and climate benefits in arid, hypersaline coastal zones (Table 2).
4 Carbon sequestration potential of Avicennia marina in arid, hypersaline coastal environments
Avicennia marina (Forssk.) Vierh. stands as a formidable carbon sink in arid, hypersaline coastal regions, where natural stands consistently outperform transplanted sites, offering significant potential for climate mitigation despite environmental stressors such as hypersalinity, industrial pollution, and hydrodynamic energy (Alsumaiti and Shahid, 2019; Naser, 2023). This review synthesizes evidence from 12 studies to evaluate A. marina’s carbon sequestration capacity, restoration outcomes, and the critical factors influencing its success in challenging environments such as the Arabian Gulf, Persian Gulf, and Australian coasts. In Abu Dhabi, Alsumaiti and Shahid (2019) reported 78 Mg C ha−1 across eight sites (40 ppt salinity), with 25.2 Mg C ha−1 in biomass (35.4 Mg ha−1 above-ground and 14.6 Mg ha−1 below-ground) and 52.8 Mg C ha−1 in soil, driven by dense stands (1,500 trees ha−1) and organic-rich sediments. Naser (2023) documented 45.6 Mg C ha−1 in Bahrain’s natural stands (52.3 Mg C ha−1 at Tubli Bay), while transplanted sites (5–15 years) ranged from 18.2 to 32.7 Mg C ha−1, reaching 70% of natural levels after 15 years, highlighting a time lag for carbon stock accumulation. Erftemeijer et al. (2020) estimated ~40 Mg C ha−1 (biomass-based) in Abu Dhabi, aligning with regional averages of 28.9 Mg C ha−1 (Shaltout et al., 2021), while Alrubaye et al. (2023) in Iraq’s industrial Khor Al-Zubair port inferred similar potential despite a 44% survival rate. Sabkha environments, however, pose severe constraints, with salinity levels reaching ~239 ppt (157.2‰ Cl−, 78.8‰ Na+), EC at 20,800 μS/cm, and an acidic pH of 6.9, coupled with H₂S toxicity from algal decomposition, reducing survival to below 20% and growth by 20–30%, thereby limiting carbon sequestration potential (Al-Mhaidib, 2003; Chang et al., 2020; Ball and Critchley, 1982). Carbon sequestration is heavily influenced by site conditions and management strategies, with natural stands benefiting from dense, organic-rich sediments and tidal flushing that enhance soil carbon (52.8 Mg C ha−1) and biomass accumulation (40 Mg ha−1) (Alsumaiti and Shahid, 2019; Erftemeijer et al., 2020; Ghasemi et al., 2012), whereas industrial pollution and hypersalinity diminish capacity—Ali et al. (2009) reported a 50% canopy loss or complete die-off in Al Jubail due to hypersalinity (>50 ppt), nutrient-poor soils, and heavy metals (e.g., 50 mg kg−1 Pb). Soil management is pivotal, as Bhat and Suleiman (2004) found sandy, well-drained Typic Torriorthents yielding 70% survival and 1.5 m height, compared to 50% in clay-rich, saline Typic Aquisalids. A. marina’s physiological plasticity underpins its success, with Ball and Critchley (1982) demonstrating high-light seedlings achieving a light-saturated photosynthesis rate (Pmax) of 12 μmol CO₂ m−2 s−1, thicker leaves, and lower chlorophyll (300 μg cm−2) for open coasts, while low-light seedlings exhibit higher quantum yield (0.06 mol CO₂ mol−1 photons) and chlorophyll (500 μg cm−2) for shaded zones, complemented by salt glands and aerial roots that tolerate 40–48 ppt salinity and waterlogging (Erftemeijer et al., 2020). Furthermore, A. marina’s growth under varying light and intertidal conditions supports its potential in mixed mangrove forests, with Jiang et al. (2019) showing that higher intertidal elevation and moderate light levels enhance early growth under Sonneratia apetala canopies, suggesting its suitability for transitioning monoculture plantations to diverse ecosystems. Environmental stressors significantly impact outcomes, with Hurst et al. (2015) noting hydrodynamic energy reducing survival (1–76%) and growth (−0.83 to 10.45 mm/month) in exposed temperate sites, and Van Loon et al. (2016) and van Bijsterveldt et al. (2022) emphasizing optimal inundation (<50 cm) for Avicennia alba and A. marina, with hypersalinity (>60 ppt) causing sharp declines. Industrial runoff, including heavy metals and oil, further exacerbates stress (Ali et al., 2009; Alrubaye et al., 2023), necessitating pre-planting soil remediation (Table 3). Active interventions—such as irrigation (75% survival, Erftemeijer et al., 2021), nursery pre-growth (44–70% survival, Alrubaye et al., 2023; Bhat and Suleiman, 2004), and tidal channels (26% survival, 40 Mg ha−1 biomass, Erftemeijer et al., 2020)—leverage A. marina’s adaptations to achieve higher short-term success in hypersaline conditions (40–48 ppt), while engineered solutions mimic natural hydrology to promote long-term stability and carbon sequestration (40–78 Mg C ha−1). However, low survival in high-stress sites (18–26%) and sabkha constraints highlight the need for sheltered, low-energy conditions and advanced strategies such as salinity dilution to 15–30 ppt, pH buffering, and aeration to counter H₂S toxicity (Al-Mhaidib, 2003). Future research should focus on quantifying carbon sequestration in restored sites over longer periods, developing scalable soil management practices, and addressing industrial pollution to fully harness A. marina’s climate mitigation potential in arid, hypersaline coastal environments.
5 Quantitative insights and challenges in Avicennia marina restoration: a synthesis for arid coastal environments
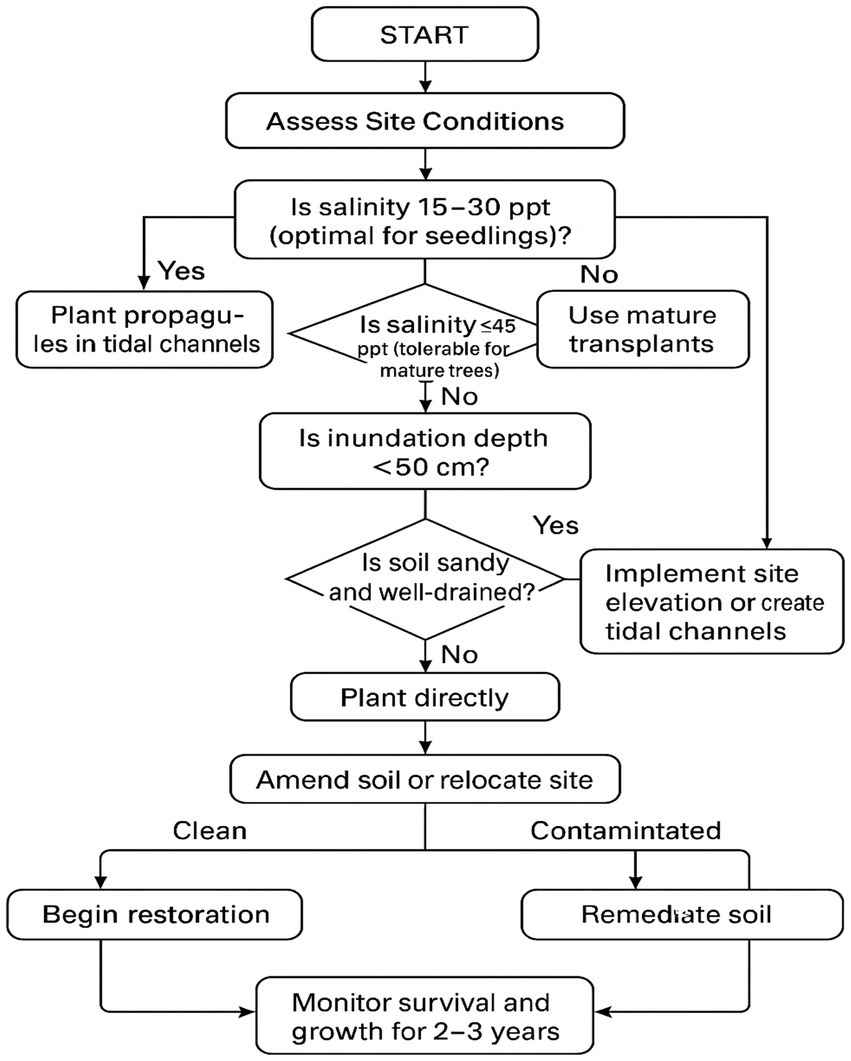
Restoration of Avicennia marina (Forssk.) Vierh. in arid, hypersaline coastal regions is shaped by complex environmental stressors, necessitating a nuanced understanding of physiological responses, quantitative outcomes, and ecological trade-offs to inform effective strategies (refer to Figure 2: Flowchart for A. marina restoration planning). Quantitative effect size analyses reveal significant variability across life stages and stressors: Lebda et al. (2024) reported a sharp decline in germination rates from 85% at 15 ppt to 20% at 60 ppt (Cohen’s d ≈ 2.1, assuming 10% standard deviation), underscoring salinity’s pronounced impact on early development, while Chang et al. (2020) demonstrated mature trees’ resilience, with above-ground biomass increasing from 7.33 Mg ha−1 at 15 ppt to 41.44 Mg ha−1 at 45 ppt (d ≈ 1.2, assuming 5 Mg ha−1 standard deviation). Dual stressors amplify these effects, as Xiao et al. (2022) found survival dropping from 90% at 30 ppt and 33% submergence to 20% at 45 ppt and 66% submergence (d ≈ 1.8), highlighting submergence’s exacerbating role. Restoration techniques vary in efficacy—transplantation with irrigation achieved 75% survival compared to 26% with tidal channels alone (d ≈ 1.5; Erftemeijer et al., 2020, 2021), while nursery pre-growth yielded 44% survival (d ≈ 0.8; Alrubaye et al., 2023)—emphasizing the need for active hydrological management. Optimal salinity differs by life stage (15–30 ppt for germination, 40–45 ppt for mature trees), with nutrient-rich tidal inputs (0.15% total nitrogen) enhancing biomass under hypersalinity, although pre-growth mitigates short-term nutrient limitations (Chang et al., 2020; Alrubaye et al., 2023). Sabkha environments introduce extreme challenges, with salinity levels reaching ~239 ppt (157.2‰ Cl−, 78.8‰ Na+), EC at 20,800 μS/cm, and an acidic pH of 6.9, coupled with H₂S toxicity from algal decomposition, reducing survival to below 20% and growth by 20–30%, far exceeding A. marina’s tolerance thresholds (Al-Mhaidib, 2003; Chang et al., 2020; Ball and Critchley, 1982). Interactions between salinity, nutrients, and elevation are critical, with significant salinity × nutrient (p < 0.05) and salinity × elevation (p < 0.01) effects necessitating integrated approaches that leverage tidal connectivity and elevation adjustments to mitigate stress (Corona-Salto et al., 2024; Shaltout et al., 2021). Practical challenges further complicate restoration: Transplantation with irrigation, while effective, is costly ($10,000–$20,000 ha−1), whereas tidal channel excavation ($5,000–$10,000 ha−1) offers a cost-effective alternative, promoting sediment accretion (2–3 mm yr.−1) but with lower initial survival (Erftemeijer et al., 2020). Nursery pre-growth is the least expensive ($2,000–$5,000 ha−1) but vulnerable to industrial pollutants, requiring site remediation (Alrubaye et al., 2023). Ecological risks include eutrophication from fertilization, monospecific plantations reducing biodiversity (48 vs. 60 + bird species in natural forests; Mousavi et al., 2024), and pollutant release from channel excavation (Ali et al., 2009). Climate change exacerbates these challenges, with sea-level rise (1– (1–3.7 mm yr.−1; Intergovernmental Panel on Climate Change, 2021) and rising temperatures (e.g., 40°C + in Kuwait) intensifying stress, necessitating adaptive strategies such as elevation techniques and brackish site selection (15–30 ppt). A. marina’s physiological adaptations—salt glands (Fv/Fm: 0.77 at 45 ppt), aerial roots, and photosynthetic plasticity (Pmax: 12 μmol CO₂ m−2 s−1 in high light)—underpin its resilience, guiding site-specific restoration designs that balance light, salinity, and inundation for optimal outcomes (Reef et al., 2019; Ball and Critchley, 1982). Future efforts should focus on cost-effective, multi-stressor management, integrating sabkha-specific interventions (e.g., salinity dilution, pH buffering, and aeration to counter H₂S toxicity), and long-term monitoring to enhance A. marina’s restoration success in arid coastal ecosystems.
6 Nutrient dynamics, environmental stressors, and restoration strategies in mangrove ecosystems
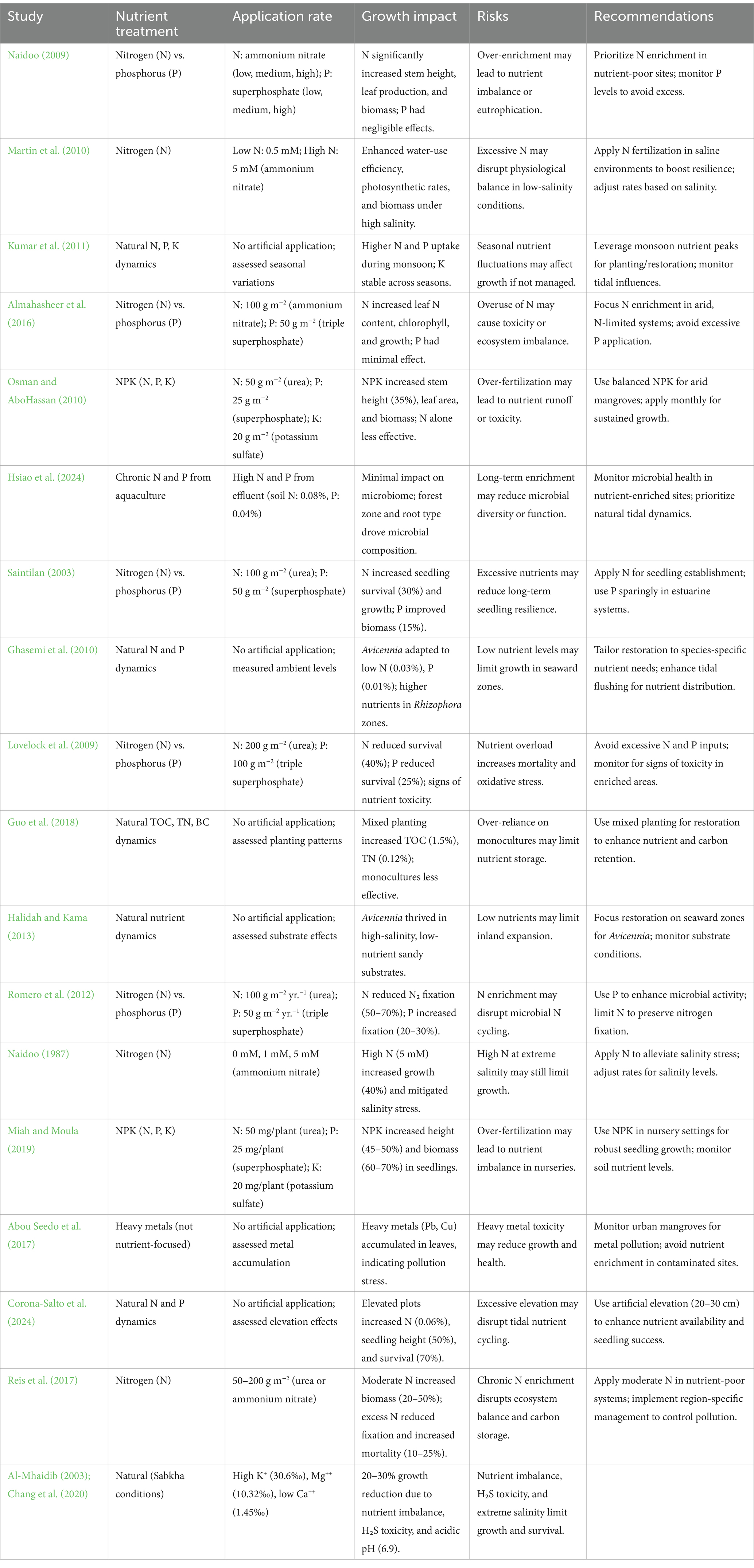
Nutrient availability, environmental stressors, and restoration strategies are pivotal in shaping the growth, survival, and ecological dynamics of mangrove ecosystems, particularly in nutrient-scarce or anthropogenically impacted coastal regions, where Avicennia marina (Forssk.) Vierh. demonstrates remarkable adaptability (Naidoo, 2009; Almahasheer et al., 2016; Almahasheer et al., 2013; Almahasheer, 2021). This review synthesizes findings from 17 studies across diverse regions—including the Central Red Sea, Western Saudi Arabia, Vamleshwar (India), Iran, Bahrain, and global contexts—to explore nutrient enrichment effects, ecological interactions, and restoration approaches for A. marina and related species such as Sonneratia alba, Rhizophora mucronata, and Avicennia germinans (Table 4). Nitrogen (N) emerges as a primary limiting nutrient in arid, saline environments, with Naidoo (2009) reporting significant increases in stem height, leaf production, and biomass in A. marina under N enrichment (ammonium nitrate, low to high levels) in a 12-month greenhouse experiment (25% seawater, 20–28°C), while phosphorus (P) had negligible effects. Similarly, Almahasheer et al. (2016) found N enrichment (100 g m−2) in the Central Red Sea (40–45 ppt salinity, <50 mm rainfall) boosted leaf N content, chlorophyll, and growth in A. marina, with minimal P impact due to sufficient P in carbonate soils (N:p < 14). Osman and AboHassan (2010) demonstrated synergistic NPK effects near Jeddah, Saudi Arabia (38–42 ppt, <100 mm rainfall), with NPK (50 g N, 25 g P, 20 g K m−2) increasing stem height by 35% and biomass, compared to 20% with N-only. However, Lovelock et al. (2009) highlighted risks of over-enrichment in Moreton Bay, Australia (25–35 ppt, 1,000 mm rainfall), where high N (200 g m−2) and P (100 g m−2) reduced A. marina survival by 40 and 25%, respectively, due to toxicity (soil N: 0.1%). Nitrogen fertilization also enhances physiological resilience, as Martin et al. (2010) showed high N (5 mM) improving water-use efficiency, photosynthetic rates, and biomass in juvenile A. marina under high salinity (75% seawater), while Naidoo (1987) reported 5 mM N mitigating salinity stress (50% salinity), increasing growth by 40–50%. Seedling success benefits from balanced NPK, with Miah and Moula (2019) noting 45–50% height and 60–70% biomass increases in A. marina seedlings in Bangladesh (50 mg N, 25 mg P, 20 mg K/plant). Spatial and temporal nutrient dynamics further influence restoration, as Kumar et al. (2011) found higher N and P uptake in A. marina during monsoon in Vamleshwar, India, and Guo et al. (2018) reported mixed planting in Fujian, China (15–25 ppt), increasing total organic carbon (TOC: 1.5%) and total nitrogen (TN: 0.12%) compared to monocultures (TOC: 1.2%, TN: 0.09%). Microbial and physico-chemical factors also play a role, with Hsiao et al. (2024) showing A. marina root microbiomes in Rabigh, Saudi Arabia (40–45 ppt), driven by forest zonation and root type (e.g., Marinobacter in seaward zones and Rhizobium in fine roots), remaining resilient to chronic N and P enrichment (soil N: 0.08%). Romero et al. (2012) noted N enrichment (100 g m−2 yr.−1) in Belize reducing N₂ fixation by 50–70% in A. germinans, while P (50 g m−2 yr.−1) increased it by 20–30%. Environmental stressors such as heavy metals and hydrology challenge restoration, with Abou Seedo et al. (2017) reporting Pb (45 mg/kg) and Cu (60 mg/kg) accumulation in A. marina at Tubli Bay, Bahrain, and Corona-Salto et al. (2024) showing elevation (20–30 cm) in Veracruz, Mexico, enhancing A. germinans survival (70% vs. 40%) and growth (50%) by increasing N (0.06%) and redox potential (+150 mV). Sabkha environments exacerbate these challenges, with extreme salinity (~239 ppt), EC (20,800 μS/cm), acidic pH (6.9), and H₂S toxicity from algal decomposition reducing A. marina growth by 20–30% and survival to below 20%, necessitating interventions such as salinity dilution, pH buffering, and aeration (Al-Mhaidib, 2003; Chang et al., 2020). Collectively, these studies advocate for tailored nutrient management—prioritizing N in arid, N-limited systems, using balanced NPK for seedlings, and leveraging mixed planting and elevation adjustments—while highlighting the risks of over-enrichment, pollution, and sabkha constraints, guiding sustainable restoration strategies for mangrove ecosystems in challenging coastal environments (Table 4).
7 Discussion: ecological adaptability, restoration strategies, and future directions for Avicennia marina in arid coastal ecosystems
Avicennia marina (Forssk.) Vierh. exhibits exceptional adaptability to the arid, hypersaline conditions of regions such as the Persian Gulf and Red Sea, characterized by extreme salinity (15–45 ppt) and minimal rainfall (<100 mm yr.−1), making it a cornerstone species for coastal restoration and climate mitigation (Figure 2; Table 5). A synthesis of 55 studies reveals its physiological resilience, driven by salt-secreting glands that enable germination at 15 ppt (85% success; Lebda et al., 2024) and robust growth at 45 ppt, with above-ground biomass reaching 41.44 Mg ha−1 in Qatar’s Al-Thakira forest (Chang et al., 2020). Tidal flushing mitigates hypersalinity by maintaining soil salinity below 50 ppt, as seen in Al Jubail, where exceeding this threshold resulted in complete mortality (Ali et al., 2009), while dual stressors such as prolonged submergence (>66%) and waterlogging reduce survival by 20% by limiting root aeration (Xiao et al., 2022; Reef et al., 2019). Nutrient dynamics in arid soils (N < 0.05%; Almahasheer et al., 2016) pose additional challenges, with NPK fertilization (50 g N, 25 g P, 20 g K m−2) increasing biomass by 35–60% (Osman and AboHassan, 2010; Miah and Moula, 2019), although excessive inputs (200 g N m−2) risk a 40% survival reduction due to toxicity (Lovelock et al., 2009). Restoration strategies show varied efficacy: Artificial soil elevation (20–30 cm) enhances survival to 70% by improving aeration (+150 mV soil redox potential; Corona-Salto et al., 2024), nursery pre-growth yields 44–70% survival (Alrubaye et al., 2023), salvage replanting achieves 82% survival for mature trees (Erftemeijer et al., 2021), and blended seawater irrigation (15–25 PSU) boosts germination to 85% (Lebda et al., 2024). Hydrological interventions, such as the fishbone technique’s tidal channels, reduce salinity stress and enhance recruitment (Primavera et al., 2011; The Indian Express, 2022; Indian Masterminds, 2023). Sabkha environments, however, present extreme challenges, with salinity levels reaching ~239 ppt (157.2‰ Cl−, 78.8‰ Na+), EC at 20,800 μS/cm, acidic pH (6.9), and H₂S toxicity from algal decomposition, reducing survival to below 20% and growth by 20–30%, necessitating targeted interventions such as salinity dilution, pH buffering, and aeration (Al-Mhaidib, 2003; Chang et al., 2020). A. marina’s carbon sequestration potential (28–45 Mg C ha−1; Shaltout et al., 2021) underscores its value for coastal protection and climate mitigation, yet practical and research challenges persist. Financial constraints are significant, with artificial elevation costing $25,000–$50,000 ha−1, irrigation infrastructure ranging from $10,000 to $50,000 ha−1 initially ($2,000–$5,000 ha−1 annually), and NPK fertilization requiring $1,500 ha−1 per application plus $500–$1,000 ha−1 for soil monitoring (Lewis and Brown, 2014; Erftemeijer et al., 2020). Logistical hurdles, including sediment transport, freshwater scarcity, and managing runoff to prevent eutrophication, limit scalability (Kathiresan and Bingham, 2001), while industrial pollutants (e.g., 45 mg kg−1 Pb in Bahrain) pose phytotoxicity risks, necessitating pre-planting sediment testing (Abou Seedo et al., 2017). Research gaps include limited long-term field trials in hypersaline regions, insufficient data on organic amendments such as biochar (Guo et al., 2018), and standardized carbon certification protocols. Climate change exacerbates these issues, with sea-level rise (1–3.7 mm yr.−1; Intergovernmental Panel on Climate Change, 2021) increasing submergence stress and rising temperatures (e.g., 40°C in Kuwait) intensifying salinity stress (Chang et al., 2020). Sustainable solutions include localized nutrient recycling (e.g., fish waste, $500 ha−1), cost-effective tidal channels ($5,000–$15,000 ha−1), and community-driven models to reduce labor costs (Erftemeijer et al., 2020). Carbon offsetting, leveraging A. marina’s sequestration capacity (3–5 t CO₂e ha−1 yr.−1), offers financial returns ($5–$50 per t CO₂e), potentially offsetting costs ($2,000 ha−1 over 20 years) through hybrid financing models such as grants and ecosystem service payments (Al-Guwaiz et al., 2021). Future research should prioritize multi-year trials, climate resilience monitoring, and the integration of sabkha-specific strategies to ensure the long-term ecological and economic sustainability of A. marina restoration in arid coastal ecosystems (Table 5).
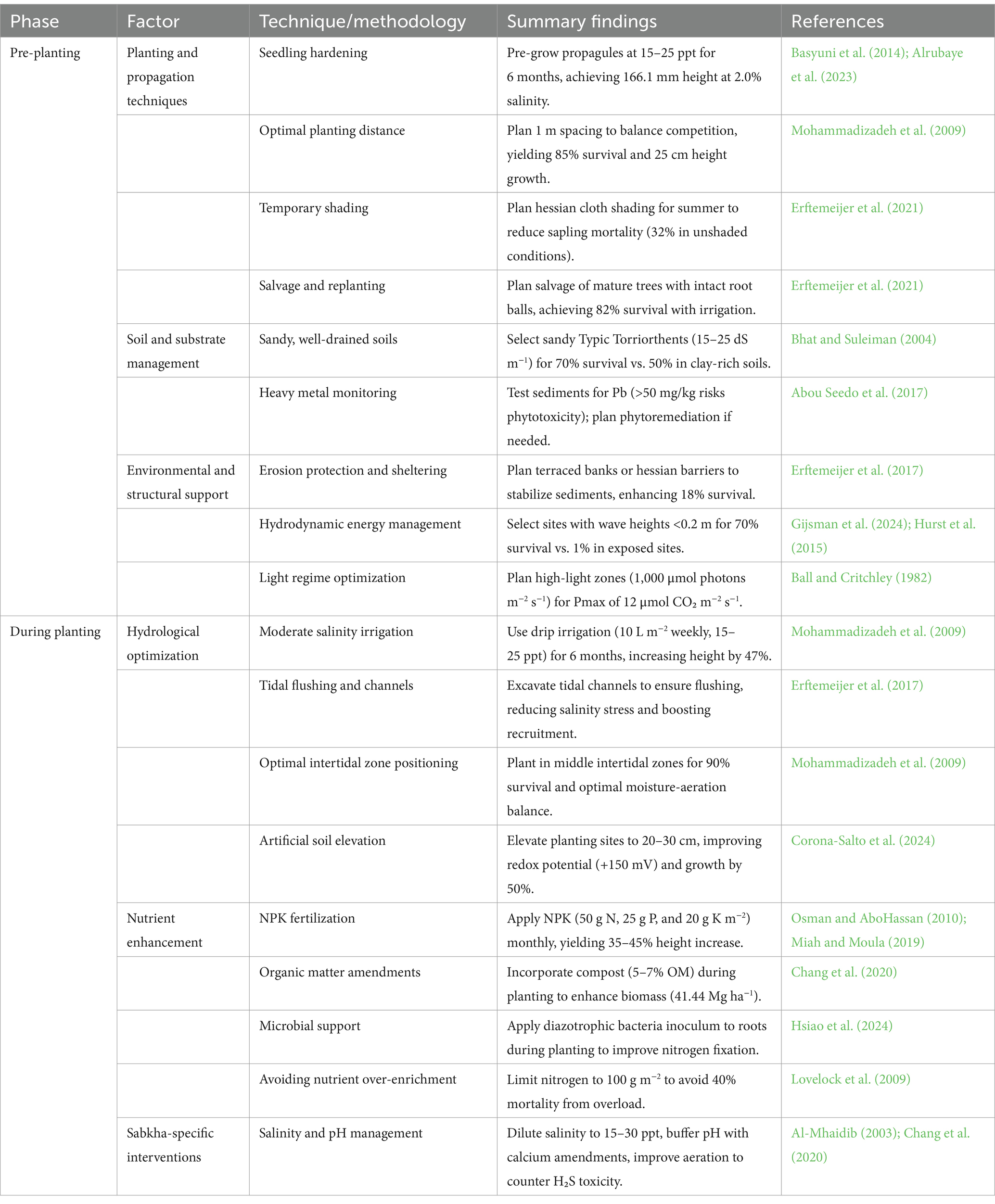
Table 5. Summary of management strategies for Avicennia marina restoration in arid coastal ecosystems.
8 Methodological heterogeneity and its impact on synthesis of Avicennia marina restoration studies
The synthesis of 55 studies on Avicennia marina (Forssk.) Vierh. restoration in arid, hypersaline coastal regions reveals significant methodological heterogeneity, encompassing study design, duration, spatial scale, environmental conditions, and outcome metrics, which collectively challenge the comparability and generalizability of findings. Study designs vary widely, from controlled greenhouse experiments (e.g., Santos et al., 2021; Naidoo, 1987) with small sample sizes (30–50 seedlings) and controlled salinity (0–40 ppt) to large-scale field trials (e.g., Erftemeijer et al., 2020; Alrubaye et al., 2023) spanning 5–17 ha under natural tidal gradients (15–48 ppt) and observational case studies (e.g., Mousavi et al., 2024) in natural settings. Controlled experiments provide precise physiological insights—such as 85% germination at 15 ppt (Lebda et al., 2024)—but often overestimate survival compared to field conditions, where industrial stressors reduce rates to 44% (Alrubaye et al., 2023). Field studies, while ecologically relevant, vary in scale and replication, complicating meta-analysis: Erftemeijer et al. (2020) reported 26% survival over 30 years across 16.5 ha, whereas Corona-Salto et al. (2024) achieved 70% survival in 18 months across 60 m2. Study durations further exacerbate heterogeneity, with short-term studies (e.g., 6 months; Miah and Moula, 2019) reporting higher survival (60–85%; Bhat et al., 2004) and growth (173.3 cm height; Alrubaye et al., 2023), contrasted by long-term studies (e.g., 30 years; Erftemeijer et al., 2020) showing declining survival (26%) due to cumulative stressors such as hypersalinity and pollution. Environmental variability across arid regions (e.g., Persian Gulf, Red Sea, and Australia) introduces additional complexity, with salinity (15–60 ppt), rainfall (<50–2,000 mm), and soil types (sandy vs. clay-rich) influencing outcomes—nutrient-rich soils in Qatar support 41.44 Mg ha−1 biomass (Chang et al., 2020), while polluted soils in Al Jubail cause 50% canopy loss (Ali et al., 2009). Sabkha environments further complicate synthesis, with extreme salinity (~239 ppt), EC (20,800 μS/cm), acidic pH (6.9), and H₂S toxicity reducing survival to below 20% and growth by 20–30%, far exceeding typical study conditions (Al-Mhaidib, 2003; Chang et al., 2020). Diverse outcome metrics—survival rates (18–85%), biomass (15.9–44.91 Mg ha−1), height (25 cm–5 m), and carbon storage (18.2–78 Mg C ha−1)—hinder quantitative synthesis as variations in technique, site conditions, and stand age obscure regional comparisons. Aggregated estimates (70–80% survival, 28–45 Mg C ha−1 carbon storage) risk overemphasizing short-term, small-scale successes and overgeneralizing across heterogeneous environments, necessitating region-specific analyses. To address this, future studies should adopt standardized protocols per SER guidelines, reporting consistent metrics (e.g., % survival at 2, 5, and 10 years; biomass in Mg ha−1). Prioritizing long-term field trials (>5 years) and quantitative meta-analyses weighted by study rigor, sample size, and duration will enhance synthesis accuracy, ensuring robust conclusions for A. marina restoration in hypersaline arid regions.
9 Integrating traditional and scientific knowledge for high-impact Avicennia marina restoration
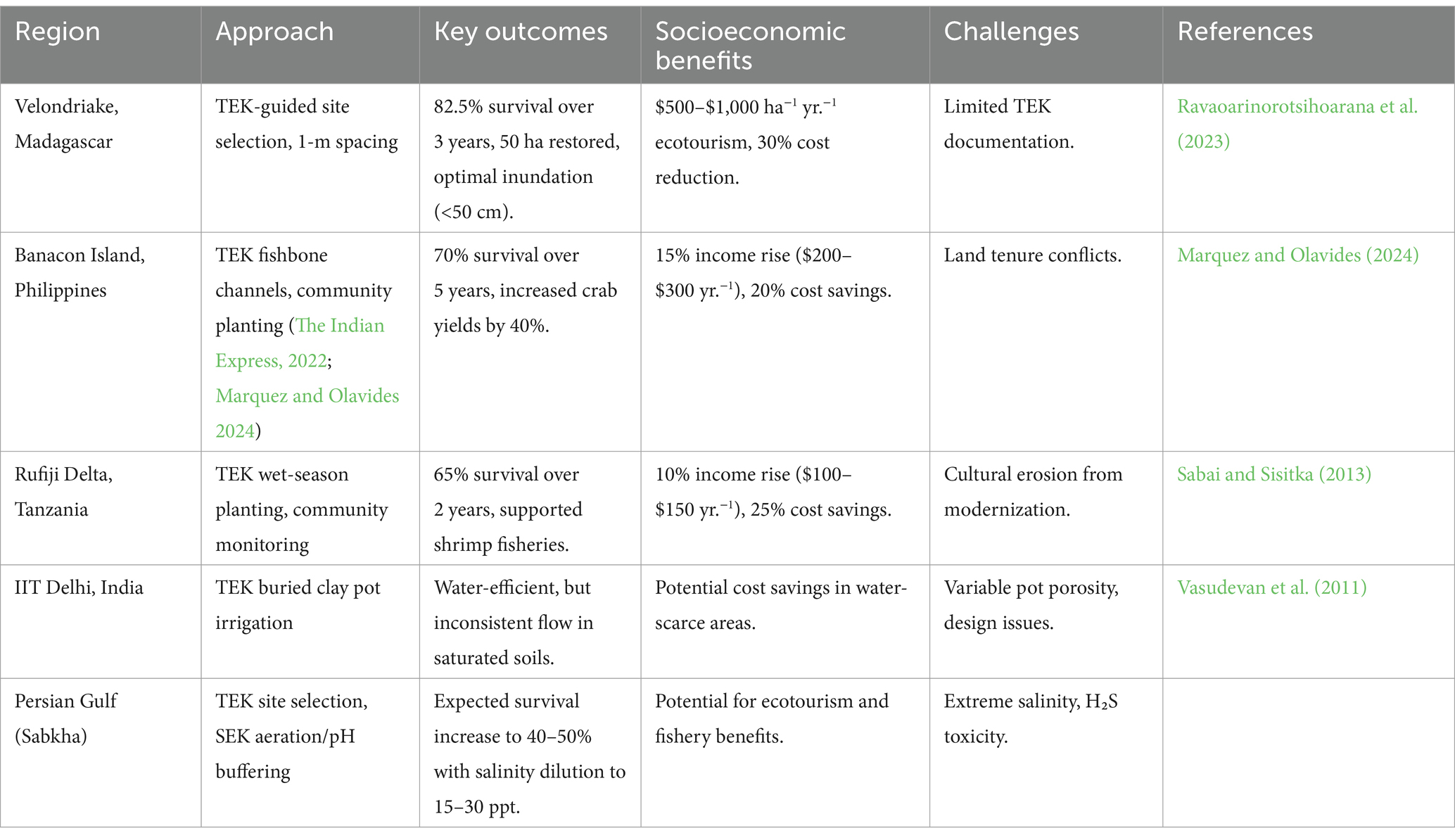
Mangrove ecosystems, crucial for coastal resilience, carbon sequestration, and biodiversity conservation, face escalating threats from climate change, deforestation, and unsustainable practices, yet the integration of traditional ecological knowledge (TEK), local ecological knowledge (LEK), and scientific ecological knowledge (SEK) offers a synergistic framework for sustainable Avicennia marina restoration in hypersaline arid regions (Berkes, 2008; Berkes, 2012). TEK and LEK, rooted in indigenous and community-based stewardship, provide context-specific insights into site selection, species choice, planting techniques, and participatory monitoring, while SEK contributes rigorous empirical methodologies and scalable frameworks to enhance restoration efficacy (Berkes, 2008; Berkes, 2012; Ravaoarinorotsihoarana et al., 2023; Marquez and Olavides, 2024). In Madagascar’s Velondriake region, fishers’ TEK on tidal patterns and salinity gradients guided A. marina site selection, achieving 82.5% survival over 3 years by targeting sites with optimal inundation (<50 cm) and salinity (15–30 ppt), while women’s groups using traditional 1-m propagule spacing restored 50 ha, boosting fish stocks and generating $500–$1,000 ha−1 annually through ecotourism, with community-led monitoring reducing costs by 30% (Ravaoarinorotsihoarana et al., 2023). In the Philippines Banacon Island, TEK-informed fishbone tidal channels mitigated salinity stress, yielding 70% survival over 5 years for Rhizophora and Avicennia species, increasing crab yields by 40% and household incomes by 15% ($200–$300 yr.−1) at 20% lower costs than mechanized dredging (Marquez and Olavides, 2024). In Tanzania’s Rufiji Delta, women’s TEK on seasonal rainfall optimized wet-season planting, achieving 65% survival over 2 years, supporting shrimp fisheries, and raising incomes by 10% ($100–$150 yr.−1) with 25% cost savings through community monitoring (Sabai and Sisitka, 2013). TEK-derived buried clay pot irrigation offers water efficiency but faces challenges with inconsistent flow in saturated soils, requiring optimized designs (Vasudevan et al., 2011). Sabkha environments, with salinity (~239 ppt), EC (20,800 μS/cm), and H₂S toxicity, reduce survival to below 20%, necessitating TEK-informed strategies such as Bedouin knowledge of salinity-tolerant planting zones combined with SEK-based aeration and pH buffering (Al-Mhaidib, 2003; Chang et al., 2020) (Table 6). Challenges to broader adoption include limited TEK documentation in arid regions, cultural erosion, land tenure conflicts, and funding constraints (Casimirri, 2003; Stori et al., 2019; Nguyen et al., 2016). Proposed solutions include establishing TEK repositories through community-led workshops, funding TEK-SEK training for 1,000 practitioners by 2030 under the Saudi Green Initiative (SGI) and Middle East Green Initiative (MGI), and developing standardized protocols for participatory monitoring and equitable benefit sharing. Innovative financing, such as Payment for Ecosystem Services, can offset costs and ensure sustainability (Boromthanarat et al., 2006; Haq et al., 2023). Future research should prioritize standardized TEK-SEK protocols, community-led documentation, robust monitoring of ecological (e.g., survival and carbon sequestration) and socioeconomic (e.g., income gains) outcomes, and policy innovation to address cultural erosion and data gaps, ensuring A. marina restoration aligns with global sustainability goals such as the UN Decade on Ecosystem Restoration.
10 Proposed framework for evaluating carbon offsetting potential in Avicennia marina restoration
Carbon offsetting, leveraging Avicennia marina sequestration capacity (28–45 Mg C ha−1, equivalent to 103–165 t CO₂e ha−1; Shaltout et al., 2021), is increasingly proposed as a potential financial mechanism to support mangrove restoration in hypersaline arid regions, yet its economic feasibility remains untested due to the absence of comprehensive cost analyses and cost–benefit assessments in existing case studies. This proposed framework outlines a structured approach to evaluate carbon offsetting potential, focusing on carbon sequestration quantification, market alignment, economic considerations, and community integration, tailored to arid coastal systems and aligned with the Saudi Green Initiative (SGI) and Middle East Green Initiative (MGI) goals. Quantifying carbon sequestration requires standardized methodologies, such as allometric equations for above-ground biomass (AGB) and below-ground biomass (BGB), and soil core sampling to 1 m depth, with baseline inventories pre-restoration and annual monitoring over 5–20 years. Regional studies report carbon stocks of 78 Mg C ha−1 in Abu Dhabi (Alsumaiti and Shahid, 2019) and 28.9 Mg C ha−1 in Jazan, Saudi Arabia (1,350 trees ha−1; Shaltout et al., 2021), with annual sequestration rates of 3–5 t CO₂e ha−1 yr.−1 (Al-Guwaiz et al., 2021). Sabkha environments, however, with salinity levels reaching ~239 ppt, EC at 20,800 μS/cm, acidic pH (6.9), and H₂S toxicity, may reduce carbon sequestration potential by limiting growth and survival to below 20% (Al-Mhaidib, 2003; Chang et al., 2020), necessitating site-specific adjustments in arid regions. Market alignment involves adhering to international carbon standards (e.g., Verified Carbon Standard [VCS] and Gold Standard) to access voluntary and compliance markets, such as Saudi Arabia’s Regional Voluntary Carbon Market, while considering co-benefits such as biodiversity and coastal protection, which may enhance mangrove credit values (Al-Guwaiz et al., 2021). Economic feasibility assessments are critical but currently lacking as restoration costs (e.g., tidal channels: $5,000–$15,000 ha−1; irrigation: $10,000–$50,000 ha−1; Lewis and Brown, 2014) and maintenance expenses ($2,000–$5,000 ha−1 yr.−1) have not been systematically weighed against potential carbon revenues or ecosystem service benefits (e.g., fishery yields; Ravaoarinorotsihoarana et al., 2023). Community integration, as demonstrated in Madagascar, can enhance project sustainability through equitable benefit sharing and reduced labor costs (Erftemeijer et al., 2020) but requires formal mechanisms to ensure long-term engagement. The lack of cost–benefit data underscores the preliminary nature of carbon offsetting proposals for A. marina restoration, highlighting the need for future research to validate sequestration rates in restored stands (5–15 years), develop regional carbon pricing models incorporating co-benefits, and conduct pilot projects to assess economic viability under SGI and MGI frameworks. Addressing sabkha-specific challenges through salinity dilution and pH buffering will also be essential to ensure feasibility in the most extreme arid environments.
11 Conclusion
Restoration of Avicennia marina in hypersaline arid regions is crucial for enhancing coastal resilience, supporting biodiversity, and advancing carbon sequestration, yet high costs and logistical complexities of strategies such as NPK fertilization, artificial soil elevation, and blended seawater irrigation underscore the need for innovative and scalable solutions. A synthesis of 50 studies demonstrates that optimized hydrological management (e.g., 20–30 cm elevation), controlled NPK fertilization, nursery pre-growth, and salvage replanting can achieve 70–80% survival within 2–3 years, yielding 40 Mg ha−1 biomass and 28–45 Mg C ha−1 in carbon storage, and provided risks such as nutrient overload and heavy metal contamination are mitigated (Al-Guwaiz et al., 2021; Lewis and Brown, 2014). Sabkha environments, with extreme salinity (~239 ppt), EC (20,800 μS/cm), acidic pH (6.9), and H₂S toxicity, reduce survival to below 20% and growth by 20–30%, necessitating targeted interventions such as salinity dilution, pH buffering, and aeration (Al-Mhaidib, 2003; Chang et al., 2020). Carbon offsetting, leveraging mangroves’ sequestration capacity (3–5 t CO₂e ha−1 yr.−1), is increasingly proposed as a potential funding mechanism, but its economic feasibility remains untested due to the absence of comprehensive cost–benefit assessments in current case studies (Al-Guwaiz et al., 2021). Context-specific innovations, such as localized nutrient recycling (e.g., biochar, ~$500 ha−1), hydrological modifications ($5,000–$15,000 ha−1), and community-driven efforts, can minimize reliance on costly inputs, while salt-tolerant cultivars and low-cost pollution assessments ($500 ha−1) address hypersaline and industrial challenges (Erftemeijer et al., 2020). Future research should prioritize multi-year field trials to validate organic amendments, long-term monitoring to assess climate resilience, and pilot projects to evaluate the economic viability of carbon offset programs under frameworks such as the Saudi Green Initiative (SGI) and Middle East Green Initiative (MGI). These evidence-based, adaptable strategies, tailored to address sabkha-specific constraints, can globally enhance coastal resilience and carbon sequestration in hypersaline arid regions, urging policymakers to integrate A. marina restoration into broader environmental sustainability initiatives.
Author contributions
FD: Writing – review & editing, Writing – original draft, Conceptualization, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research at King Faisal University, Saudi Arabia (grant no. KFU251645).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelkader, M., Suliman, A. A., Salem, S. S., Assiya, A., Voronina, L., Puchkov, M., et al. (2024). Studying the Combined Impact of Salinity and Drought Stress-Simulated Conditions on Physio-Biochemical Characteristics of Lettuce Plant. Horticulturae, 10:1186.
Abou Seedo, K., Abido, M. S., and Salih, A. A.Abರ. (2017) Assessing heavy metals accumulation in the leaves and sediments of urban mangroves (Avicennia marina (Forsk.) Vierh.) in Bahrain. Academia. doi: 10.1155/2017/3978216
Abrogueña, J. B. R., Anton, A., Woo, S. P., Baptista, M., Duarte, C. M., Hussain, S. A., et al. (2022). The impact of inundation and sandstorms on the growth and survival of the mangrove Avicennia marina seedlings in the southern Red Sea. Sci. Mar. 86:e041. doi: 10.3989/scimar.05237.041
Al-Guwaiz, S. M., Alatar, A. A., El-Sheikh, M. A., Al-Gehni, G. A., Faisal, M., Qahtan, A. A., et al. (2021). Role of mangrove rehabilitation and protection plans on carbon storage in Yanbu Industrial City, Saudi Arabia: a case study. Sustainability 13:13149. doi: 10.3390/su132313149
Ali, A., Alfarhan, A., Robinson, E., and Altesan, W. (2009). Soil quality of die-off and die-back mangrove grown at Al-Jubail area (Saudi Arabia) of the Arabian gulf. J. Environ. Sci. Available online at: https://agris.fao.org/search/en/providers/122415/records/6473683c08fd68d54605e080
Almahasheer, H. (2021). Internodal analysis of Avicennia marina in the Western Arabian gulf. Front. Mar. Sci. 8:698596. doi: 10.3389/fmars.2021.698596
Almahasheer, H., Al-Taisan, W., and Mohamed, M. K. (2013). Mangrove deterioration in Tarut Bay on the eastern province of the Kingdom of Saudi Arabia. Pakhtunkhwa J. Life Sci., 1, 49–59. Available online at: https://www.awkum.edu.pk/PJLS/Downloads/01-Volume-2013/02-Issue-2013/01-PJLS%20001_0213_0513%20Hanan%20et%20al.pdf
Almahasheer, H., Duarte, C. M., and Irigoien, X. (2016). Nutrient limitation in Central Red Sea mangroves. Front. Mar. Sci. 3:271. doi: 10.3389/fmars.2016.00271
Al-Mhaidib, A. I. (2003). Sabkha soil in the Kingdom of Saudi Arabia: characteristics and treatment التربة السبخة في المملكة العربية السعودية: خواصها وطرق معالجتها. J. King Abdulaziz Univ. Eng. Sci., 14, 29–80. Available online at: https://www.researchgate.net/publication/250388517_Sabkha_Soil_in_the_Kingdom_of_Saudi_Arabia_Characteristics_and_treatment_altrbt_alsbkht_fy_almmlkt_alrbyt_alswdyt_khwasha_wtrq_maljtha
Al-Nafisi, R. S., Al-Ghadban, A., Gharib, I., and Bhat, N. R. (2009). Positive impacts of mangrove plantations on Kuwait’s coastal environment. Eur. J. Sci. Res., 26, 510–521. Available online at: https://www.researchgate.net/publication/254645839
Alrubaye, A. A., Al-Zewar, J. M., Al-Aradi, H. J., and Qasim, A. M. H. (2023). Possibility of cultivation of gray mangroves Avicennia marina (Forsk.) Vierh. In the Iraqi coasts. Iraqi J. Aquac. 20, 1–18. doi: 10.58629/ijaq.v20i1.453
Alsumaiti, T. S., and Shahid, S. A. (2019). Mangroves among most carbon-rich ecosystem living in hostile saline rich environment and mitigating climate change – a case of Abu Dhabi. J. Agric. Crop Res. 7, 1–8. doi: 10.33495/jacr_v7i1.18.155
Al-Zewar, J. M., Al-Edany, T. Y., and Naema, J. D. (2023). Study growth indicators of mangrove Avicennia marina (Forsk.) Vierh. Cultivated on the coast of Khor Al-Zubair oil port, south of Basrah – Iraq. IOP Conf. Ser. Earth Environ. Sci. 1215:012037. doi: 10.1088/1755-1315/1215/1/012037
Arksey, H., and O’Malley, L. (2005). Scoping studies: towards a methodological framework. Int. J. Soc. Res. Methodol. 8, 19–32. doi: 10.1080/1364557032000119616
Ball, M. C., and Critchley, C. (1982). Photosynthetic responses to irradiance by the grey mangrove, Avicennia marina, grown under different light regimes. Plant Physiol. 70, 1101–1106. doi: 10.1104/pp.70.4.1101
Basyuni, M., Putri, L. A. P., Nainggolan, B., and Sihaloho, P. E. (2014). Growth and biomass in response to salinity and subsequent fresh water in mangrove seedlings Avicennia marina and Rhizophora stylosa. J. Manaj. Hutan Trop. 20, 17–25. doi: 10.7226/jtfm.20.1.17
Bhat, N. R., and Suleiman, M. K. (2004). Classification of soils supporting mangrove plantation in Kuwait. Arch. Agron. Soil Sci. 50, 535–551. doi: 10.1080/03650340410001729726
Bhat, N. R., Suleiman, M. K., and Shahid, S. A. (2004). Mangrove, Avicennia marina, establishment and growth under the arid climate of Kuwait. Arid Land Res. Manag. 18, 127–139. doi: 10.1080/15324980490280799
Boromthanarat, S., Hossain, M. Z., and Chaijaroenwatana, B. (2006). Community-led mangrove rehabilitation: experiences from Hua Khao community, Songkhla, Thailand. Asia-Pac. J. Rural Dev. 16, 53–68. doi: 10.1177/1018529120060203
Casimirri, G. (2003). Problems with integrating traditional ecological knowledge into contemporary resource management. Proceedings of the XII World Forestry Congress. FAO.
Chang, H., Han, S. H., Kim, S., An, J., Alatalo, J., and Son, Y. (2020). Interactions between topsoil properties and ecophysiological responses of mangroves (Avicennia marina) along the tidal gradient in an arid region in Qatar. Turk. J. Agric. For. 44, 121–126. doi: 10.3906/tar-1905-17
Corona-Salto, A., López-Portillo, J., Alvarado-Barrientos, M. S., and Santini, N. S. (2024). Effects of artificial soil elevation during mangrove restoration on hydroperiod, redox potential, nutrients, and seedling growth. Bull. Mar. Sci. doi: 10.5343/bms.2024.0025
Erftemeijer, P. L., Agastian, T., Yamamoto, H., Cambridge, M. L., Hoekstra, R., Toms, G., et al. (2020). Mangrove planting on dredged material: three decades of nature-based coastal defence along a causeway in the Arabian gulf. Mar. Freshw. Res. 71, 1062–1072. doi: 10.1071/MF19289
Erftemeijer, P. L., Price, B. A., Ito, S., Yamamoto, H., Agastian, T., and Cambridge, M. L. (2021). Salvaging and replanting 300 mangrove trees and saplings in the arid Arabian gulf. Mar. Freshw. Res. 72, 1577–1587. doi: 10.1071/MF20381
Erftemeijer, P. L. A., Wylie, N., and Hooper, G. J. (2017). Successful mangrove establishment along an artificially created tidal creek at Port Hedland, Western Australia. Mar. Freshw. Res. 68, 136–148. doi: 10.1071/MF15204
Fan, X., Hu, X., Ma, Y., Pang, Y., Sun, J., and Hou, P. (2024). Influence of high temperature and drought stress at jointing stage on crop physiological responses and growth in summer maize plants (Zea mays L.). Front. Plant Physiol. 2:1331421. doi: 10.3389/fphgy.2024.1331421
Ghasemi, A., Jalilvand, H., and Mohajeri-Borazjani, S. (2012). Vegetative characteristics of Avicennia marina on the artificial inlet. J. For. Res. 23, 510–516.
Ghasemi, S., Zakaria, M., Hazandy, A. H., Yusof, E., Hoveizeh, N. M., and Danehkar, A. (2010). Physico-chemical factors in the Avicennia and Rhizophora mangrove habitats in Iran. Iran. J. Environ. Stud. 1, 29–35.
Gijsman, R., Horstman, E. M., Swales, A., Balke, T., Willemsen, P. W. J. M., Van Der Wal, D., et al. (2024). Biophysical modeling of mangrove seedling establishment and survival across an elevation gradient with forest zones. J. Geophys. Res. Earth Surf. 129:e2024JF007664. doi: 10.1029/2024JF007664
Guo, P., Sun, Y., Su, H., Wang, M., and Zhang, Y. (2018). Spatial and temporal trends in total organic carbon (TOC), black carbon (BC), and total nitrogen (TN) and their relationships under different planting patterns in a restored coastal mangrove wetland: case study in Fujian, China. Chem. Speciation Bioavailab. 30, 47–56. doi: 10.1080/09542299.2018.1484673
Halidah, H., and Kama, H. (2013). Penyebaran alami Avicennia marina (Forsk) Vierh. dan Sonneratia alba Smith pada substrat pasir. Indones. For. Rehabil. J. 1, 51–58.
Haq, S. M., Pieroni, A., Bussmann, R. W., Abd-ElGawad, A. M., and el-Ansary, H. O. (2023). Integrating traditional ecological knowledge into habitat restoration: implications for meeting forest restoration challenges. J. Ethnobiol. Ethnomed. 19:33. doi: 10.1186/s13002-023-00606-3
Hsiao, V., Erazo, N. G., Reef, R., Lovelock, C., and Bowman, J. (2024). Forest zone and root compartments outweigh long-term nutrient enrichment in structuring arid mangrove root microbiomes. Front. For. Glob. Change 7:1336037. doi: 10.3389/ffgc.2024.1336037
Hurst, T. A., Pope, A. J., and Quinn, G. P. (2015). Exposure mediates transitions between bare and vegetated states in temperate mangrove ecosystems. Mar. Ecol. Prog. Ser. 533, 121–134. doi: 10.3354/meps11364
Indian Masterminds. (2023). IFS officer shares video of mangrove planting using fish bone technique. Available online at: https://indianmasterminds.com/social-media/planting-mangroves-with-fish-bone-technique-in-thanjavur-ifs-shared-video/ (Accessed May 20, 2025).
Intergovernmental Panel on Climate Change (2021). Climate change 2021: The physical science basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. V. Masson-Delmotte, P. Zhai, A. Pirani, S. L. Connors, C. Péan, and S. Berger, et al. Cambridge University Press. Available at: https://www.ipcc.ch/report/ar6/wg1/
Jiang, Z., Guan, W., Xiong, Y., Li, M., Chen, Y., and Liao, B. (2019). Interactive effects of intertidal elevation and light level on early growth of five mangrove species under Sonneratia apetala Buch. Ham plantation canopy: turning monocultures to mixed forests. Forests 10:83. doi: 10.3390/f10020083
Kathiresan, K., and Bingham, B. L. (2001). Biology of mangroves and mangrove ecosystems. Adv. Marine Biol., 40, 81–251. doi: 10.1016/S0065-2881(01)40003-4
Kumar, I. N., Sajish, P. R., Kumar, R. N., Basil, G., and Shailendra, V. (2011). Nutrient dynamics in an Avicennia marina (Forsk.) Vierh., mangrove forest in Vamleshwar, Gujarat, India. Not. Sci. Biol., 3, 51–56. Available online at: https://notulaebiologicae.ro/index.php/nsb/article/download/5594/8302
Lebda, I., El-Bana, M., El-Sayed, A., and Ghabbour, S. (2024). Impact of salinity gradients on seed germination, establishment, and growth of two dominant mangrove species along the Red Sea coastline. Plan. Theory 13:3471. doi: 10.3390/plants13243471
Lewis, R. R., and Brown, B. (2014). Ecological mangrove rehabilitation: a field manual for practitioners. Mangrove Action Project. Available online at: https://ocean.floridamarine.org/chimmp/Resources/Lewis%20and%20Brown%202014%20Ecological%20Mangrove%20Rehabilitation.pdf (Accessed May 20, 2025).
Lovelock, C. E., Ball, M. C., Martin, K. C., and Feller, I. C. (2009). Nutrient enrichment increases mortality of mangroves. PLoS One 4:e5600. doi: 10.1371/journal.pone.0005600
Marquez, G. P. B., and Olavides, R. D. (2024). Integrating science-based and local ecological knowledge: a case study of mangrove restoration and rehabilitation projects in the Philippines. Disasters 48:e12630. doi: 10.1111/disa.12630
Martin, K. C., Bruhn, D. A. N., Lovelock, C. E., Feller, I. C., Evans, J. R., and Ball, M. C. (2010). Nitrogen fertilization enhances water-use efficiency in a saline environment. Plant Cell Environ. 33, 344–357. doi: 10.1111/j.1365-3040.2009.02072.x
Mehran, J. B., and Sahar, K. (2017). Grey mangrove Avicennia marina (Forsk.) Vierh. as a bio-indicator to measure nickel, mercury, and cadmium: a case study at Persian Gulf port shoreline, Khuzestan, Iran. Environ. Eng. Manag. J., 16, 2047–2052. Available online at: https://www.researchgate.net/publication/322264434
Miah, M. A. Q., and Moula, M. G. (2019). Effect of NPK fertilizers on seedling growth of mangrove species. J. Biosci. Agric. Res. 20, 1687–1693. doi: 10.18801/jbar.200119.205
Mohammadizadeh, M., Farshchi, P., Danehkar, A., Mahmoodi-Madjdabadi, M., Hassani, M., and Mohammadizadeh, F. (2009). Interactive effect of planting distance, irrigation type, and intertidal zone on the growth of grey mangrove seedlings in Qeshm Island, Iran. J. Trop. Forest Sci., 21, 147–155. Available online at: https://jtfs.frim.gov.my/jtfs/article/download/802/660
Motamedi, S., Hashim, R., Zakaria, R., Song, K. I., and Sofawi, B. (2014). Long-term assessment of an innovative mangrove rehabilitation project: case study on Carey Island, Malaysia. Sci. World J. 2014:953830. doi: 10.1155/2014/953830
Mousavi, S. M. H., Zahed, M. A., Negarestan, H., and Etemadifar, Z. (2024). Ecological characteristics of mangrove forest in the coast of Hormozgan Province, Iran. J. Bioresour. Environ. Sci. 3, 54–62. doi: 10.61435/jbes.2024.19306
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Naidoo, G. (1987). Effects of salinity and nitrogen on growth and water relations in the mangrove, Avicennia marina (Forsk.) Vierh. New Phytol. 107, 317–325. doi: 10.1111/j.1469-8137.1987.tb00183.x
Naidoo, G. (2009). Differential effects of nitrogen and phosphorus enrichment on growth of dwarf Avicennia marina mangroves. Aquat. Bot. 90, 184–190. doi: 10.1016/j.aquabot.2008.10.001
Naser, H. A. (2023). Sediment carbon stock in natural and transplanted mangroves in Bahrain, Arabian gulf. Land 12:2055. doi: 10.3390/land12112055
Nguyen, T. P., Nguyen, V. T., Le, P. Q., and Parnell, K. E. (2016). Community perspectives on an internationally funded mangrove restoration project: Kien Giang province, Vietnam. Ocean Coast. Manage. 119, 146–154. doi: 10.1016/j.ocecoaman.2015.10.008
Osman, H. E., and AboHassan, A. A. (2010). Effect of NPK fertilization on growth and dry matter accumulation in mangrove [Avicennia marina (Forssk) Vierh] grown in western Saudi Arabia. J. King Abdulaziz Univ. Meteorol. Environ. Arid Land Agric. Sci., 21, 77–91. Available online at: http://www.kau.edu.sa/Files/320/Researches/58024_28123.pdf
Primavera, J. H., Rollon, R. N., and Samson, M. S. (2011). The pressing challenges of mangrove rehabilitation: pond reversion and coastal protection. Biologica, 50:232. Available online at: https://www.researchgate.net/profile/Jurgenne-Primavera/publication/288174430_The_Pressing_Challenges_of_Mangrove_Rehabilitation/links/6601ebada4857c796282be71/The-Pressing-Challenges-of-Mangrove-Rehabilitation.pdf
Ravaoarinorotsihoarana, L. A., Ratefinjanahary, I., Aina, C., Rakotomahazo, C., Glass, L., Ranivoarivelo, L., et al. (2023). Combining traditional ecological knowledge and scientific observations to support mangrove restoration in Madagascar. Forests 14:1368. doi: 10.3390/f14071368
Reef, R., Santini, N. S., and Lovelock, C. E. (2019). Effects of salinity and waterlogging on salt gland excretion, root respiration, and growth responses of two contrasting mangrove seedling types. Mar. Freshw. Res. 70, 276–283. doi: 10.1071/MF17139
Reis, C. R. G., Nardoto, G. B., and Oliveira, R. S. (2017). Global overview on nitrogen dynamics in mangroves and consequences of increasing nitrogen availability for these systems. Plant Soil 410, 1–19. doi: 10.1007/s11104-016-3123-7
Romero, I. C., Jacobson, M., Fuhrman, J. A., Fogel, M., and Capone, D. G. (2012). Long-term nitrogen and phosphorus fertilization effects on N₂ fixation rates and nifH gene community patterns in mangrove sediments. Mar. Ecol. 33, 117–127. doi: 10.1111/j.1439-0485.2011.00465.x
Sabai, D., and Sisitka, H. (2013). Analysing learning at the interface of scientific and traditional ecological knowledge in a mangrove ecosystem restoration scenario in the eastern coast of Tanzania. Transylv. Rev. Syst. Ecol. Res. 15, 185–210. doi: 10.2478/trser-2013-0027
Saintilan, N. (2003). The influence of nutrient enrichment upon mangrove seedling establishment and growth. Wetlands (Austral.) 21, 29–35.
Santos, J., Santos, M. J., Almeida, J. M., and Câmara, T. (2021). Response of mangrove plant species to a saline gradient: implications for ecological restoration. Acta Bot. Bras. 35, 151–160. doi: 10.1590/0102-33062020abb0170
Saudi Green Initiative (2024). About the Saudi green initiative. Available online at: https://www.saudigreeninitiative.org/about-sgi/ (Accessed May 20, 2025).
Selvam, V., Ravishankar, T., Karunagaran, V. M., Ramasubramanian, R., Eganathan, P., and Parida, A. K. (2005) Toolkit for establishing coastal bioshield. Available online at: https://www.researchgate.net/publication/274569700_Toolkit_for_Establishing_Coastal_Bioshield#fullTextFileContent (Accessed May 20, 2025).
Shaltout, K. H., Ahmed, M. T., Alrumman, S. A., Ahmed, D. A., and Eid, E. M. (2021). Standing crop biomass and carbon content of mangrove Avicennia marina (Forssk.) Vierh. along the Red Sea coast of Saudi Arabia. Sustainability 13:13996. doi: 10.3390/su132413996
Stori, F. T., Peres, C. M., Turra, A., and Pressey, R. L. (2019). Traditional ecological knowledge supports ecosystem-based management in disturbed coastal marine social-ecological systems. Front. Mar. Sci. 6:571. doi: 10.3389/fmars.2019.00571
Teutli Hernández, C., Herrera-Silveira, J. A., Cisneros-de la Cruz, D. J., and Román-Cuesta, R. (2020). Mangrove ecological restoration guide: Mainstreaming Wetlands into the Climate Agenda: A multi-levelapproach (SWAMP). CIFOR/CINVESTAV-IPN/UNAM-Sisal/PMC. Available at: https://www.cifor-icraf.org/publications/pdf_files/Books/2020-Guide-SWAMP.pdf
The Indian Express (2022). Watch: this innovative reforestation technique is saving mangrove forests. The Indian Express. Available online at: https://indianexpress.com (Accessed May 20, 2025).
van Bijsterveldt, C. E., Debrot, A. O., Bouma, T. J., Maulana, M. B., Pribadi, R., Schop, J., et al. (2022). To plant or not to plant: when can planting facilitate mangrove restoration? Front. Environ. Sci. 9:690011. doi: 10.3389/fenvs.2021.690011
Van Loon, A. F., Te Brake, B., Van Huijgevoort, M. H., and Dijksma, R. (2016). Hydrological classification, a practical tool for mangrove restoration. PLoS One 11:e0150302. doi: 10.1371/journal.pone.0150302
Vasudevan, P., Thapliyal, A., Sen, P. K., Dastidar, M. G., and Davies, P. (2011). Buried clay pot irrigation for efficient and controlled water delivery. J. Sci. Ind. Res. 70, 645–652.
Xiao, Y., Chen, L., Li, J., and Zhang, Y. (2022). Responses of mangrove seedlings to combined stress from salinity and partial submergence: a case study with three mangrove species in a greenhouse. Forests 13:684. doi: 10.3390/f13050684
Glossary
Aerial Roots - Specialized roots in mangroves, such as pneumatophores or prop roots, that extend above the soil surface to facilitate gas exchange in waterlogged, oxygen-poor environments.
Allometric Equations - Mathematical models used to estimate biomass or other plant characteristics (e.g., above-ground and below-ground biomass) based on measurable traits such as tree height or diameter at breast height (DBH).
Bioindicator - An organism or ecological community, such as Avicennia marina, used to assess environmental health by responding to stressors such as pollutants (e.g., heavy metals).
Biomass - The total mass of living matter in a given area, often divided into above-ground biomass (AGB) and below-ground biomass (BGB), measured in Mg ha−1.
Carbon Sequestration - The process by which ecosystems, such as mangroves, capture and store atmospheric carbon dioxide in biomass and soils, contributing to climate change mitigation.
Chlorophyll Content - A measure of the green pigment in plant leaves, indicating photosynthetic capacity and plant health, often used as a stress indicator.
Diazotrophic Bacteria - Microorganisms capable of fixing atmospheric nitrogen (N₂) into a form usable by plants, such as Rhizobium, found in mangrove root microbiomes.
Ecological Restoration - The process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed, aiming to restore its structure, function, and composition to a reference state.
Ecological Zonation - The spatial distribution of mangrove species along environmental gradients (e.g., salinity and tidal inundation), driven by species-specific tolerances.
Ecosystem Services - The benefits provided by ecosystems, such as mangroves, to humans, including coastal protection, carbon storage, and habitat provision.
Electrolyte Leakage - A physiological marker of plant stress, indicating cell membrane damage, often measured under salinity or submergence stress.
Halophilic Taxa - Salt-tolerant microorganisms, such as Marinobacter, that thrive in high-salinity environments such as mangrove soils.
Hydroperiod - The frequency and duration of tidal inundation in a mangrove habitat, influencing soil chemistry and plant growth.
Hypersaline - Water or soil with salinity levels exceeding that of seawater (~35 ppt), common in arid coastal regions such as the Arabian Gulf.
Intertidal Zone - The coastal area between high and low tide marks, where mangroves typically grow, experiencing regular tidal inundation.
Nature-Based Solutions (NbS) - Actions that protect, restore, or manage natural ecosystems, such as mangrove restoration, to address societal challenges such as coastal protection, carbon sequestration, and biodiversity loss.
Nitrogen Fixation - The process by which certain bacteria convert atmospheric nitrogen (N₂) into ammonia, making it available for plant use, often measured via the acetylene reduction assay.
Phytotoxicity - Toxicity to plants caused by environmental contaminants, such as heavy metals (e.g., lead and cadmium), leading to reduced growth or mortality.
Propagule - The reproductive unit of mangroves, such as seeds or viviparous seedlings, used for dispersal and establishment.
Redox Potential - A measure of soil oxidation–reduction state, indicating oxygen availability; higher values (e.g., +150 mV) suggest aerobic conditions, while lower values (e.g., −50 mV) indicate anaerobic conditions.
Reference ecosystem - A model ecosystem used to guide restoration, representing the target structure, function, and composition of a restored mangrove habitat.
Sabkha - A coastal, supratidal flat in arid regions, often hypersaline and nutrient-poor, posing challenges for mangrove restoration.
Salt Glands - Specialized structures in some mangrove species, such as Avicennia marina, that excrete excess salt, enabling survival in hypersaline conditions.
Sediment Accretion - The accumulation of sediment in mangrove habitats, often enhanced by mangrove roots, contributing to coastal stabilization.
Tidal Flushing - The movement of tidal water in and out of mangrove habitats, influencing salinity, nutrient availability, and soil aeration.
Viviparous - A reproductive trait in some mangroves, such as Rhizophora species, where propagules germinate while still attached to the parent tree, enhancing establishment success.
AGB - above-ground biomass—The mass of plant material above the soil surface, typically measured in Mg ha−1.
ANOVA - analysis of variance—A statistical method used to compare means across multiple groups.
BC - black carbon—A stable form of carbon derived from incomplete combustion, often found in mangrove soils.
BGB - below-ground biomass—The mass of plant material below the soil surface, typically measured in Mg ha−1.
CEC - cation exchange capacity—A measure of soil’s ability to retain and exchange cations, influencing nutrient availability.
DBH - diameter at breast height—A standard measurement of tree trunk diameter at 1.3 m above ground, used to estimate biomass.
ESP - exchangeable sodium percentage—The proportion of sodium ions in soil relative to other cations, affecting soil structure and plant growth.
Fv/Fm - maximum quantum yield of photosystem II—A chlorophyll fluorescence parameter indicating photosynthetic efficiency and plant stress.
L.S.D. - least significant difference—A statistical test used to compare means in experiments, often at a 0.05 probability level.
Mg C ha−1 - megagrams of carbon per hectare—A unit for measuring carbon storage in ecosystems.
Mg ha−1 - megagrams per hectare—A unit for measuring biomass or other mass per unit area.
N - nitrogen—A key nutrient influencing plant growth, often a limiting factor in mangrove ecosystems.
NbS - nature-based solutions—Actions that use natural ecosystems to address societal challenges, such as mangrove restoration.
nifH Gene - A genetic marker used to identify nitrogen-fixing bacteria in microbial communities.
NPK - nitrogen, phosphorus, potassium—Essential macronutrients applied as fertilizers to enhance plant growth.
OM - organic matter—The organic component of soil, influencing nutrient availability and microbial activity.
P - phosphorus—A key nutrient for plant growth, often less limiting in carbonate-rich mangrove soils.
ppt - parts per thousand—A unit of salinity.
SER - Society for Ecological Restoration—An organization promoting standardized ecological restoration practices.
TOC - total organic carbon—The total amount of carbon in organic compounds in soil, a measure of soil fertility and carbon storage.
TN - total nitrogen—The total amount of nitrogen in soil or plant tissue, influencing growth and ecosystem dynamics.
Keywords: carbon sequestration, mangrove restoration, salinity, nutrient management, inland planting, sustainability, SDG 13, SDG 15
Citation: Dhawi F (2025) Mastering resilience: Avicennia marina’s survival in hypersaline arid zones. Front. Sustain. Food Syst. 9:1598548. doi: 10.3389/fsufs.2025.1598548
Edited by:
Anas Tallou, Institute of Agrifood Research and Technology (IRTA), SpainReviewed by:
Hugo López Rosas, El Colegio de Veracruz, MexicoRahul Adhikary, Centurion University, India
Boran Ikiz, Çukurova University, Türkiye
Copyright © 2025 Dhawi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faten Dhawi, ZHIuZmF0ZW4uZGhhd2lAZ21haWwuY29t
 Faten Dhawi
Faten Dhawi