- 1Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 2Embryonic Stem Cell Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 3Department of Food and Nutrition Sciences, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 4Department of Agricultural Engineering, Delta State University of Science and Technology, Ozoro, Nigeria
- 5Department of Mechanical Engineering, Delta State University of Science and Technology, Ozoro, Nigeria
- 6Department of Food Science and Nutrition, College of Sciences, Taif University, Taif, Saudi Arabia
- 7Department of Mechanical Engineering, Faculty of Engineering, Taif University, Taif, Saudi Arabia
This study investigated the effects of turmeric extract (TE), banana peel extract (PE), and onion extract (OE) on the physiological health status and meat quality of broilers. Two-week-old “Cobb 500” chicks were divided into seven treatment groups: the control (distilled water), T1 (100 mL of TE), T2 (100 mL of PE), T3 (100 mL of OE), T4 (100 mL of TE + 100 mL of PE), T5 (100 mL of TE + 100 mL of OE), and T6 (100 mL of PE + 100 mL of OE). Each treatment solution (100 mL or 200 mL, as applicable) was diluted in 4 L of water and administered to the birds in their drinking water three times a week throughout the experiment. After the 8-week experimental period, the birds’ body weight gain, protein content, water-holding capacity, total lipid content, microbial loads, and the mechanical properties of boiler offal were assessed using standard techniques. The results showed that all supplements had significant effects on all the parameters investigated (p ≤ 0.05). The bodyweight gains for the control and treatments T1 through T6 were 1.87, 2.20, 2.03, 2.12, 2.34, 2.42, and 2.26 kg, respectively. Additionally, the extracts substantially reduced the lipid content in broiler meat while enhancing water-holding capacity (WHC), protein content, packed cell volume (PCV), red blood cell (RBC) count, and white blood cell (WBC) count, antimicrobial activity, and shear force. Moreover, the data revealed that the hybridized additive treatment yielded better results compared to single-extract treatments. These findings hold promising implications for the livestock industry, particularly in promoting sustainable growth, enhancing animal performance, and improving meat quality.
Introduction
The animal production industry is a vital component of the economy and national development, as it provides essential compounds required for human development. Meat is a crucial component of a balanced diet, playing a significant role in global food security (Ponnampalam et al., 2022). It contains substantial amounts of high-quality essential amino acids, iron, zinc, vitamin B12, and selenium—nutrients critical for energy production, immune function, enzymatic activity, and neurological health (Apata et al., 2023; Gu et al., 2024). Modern livestock management emphasizes sustainable practices aimed at enhancing productivity while reducing production costs. The majority of sustainability methods focus on the application of natural additives, primarily for their nutritional and pharmaceutical benefits. Therefore, incorporating natural additives into livestock production not only supports animal health and performance but also contributes to environmental protection and reduction in production costs (Abigarl et al., 2023).
Natural antioxidants play a vital role in enhancing the functionality of animal tissues and promoting microstructural development. These can be linked to their capacity to boost the concentrations of essential minerals and bioactive compounds in the animal’s flesh, thus reducing the infection rate and oxidative stress in animal tissues. Poor health conditions and oxidative stress can degrade collagen, elastin, and protein levels in the animal body, resulting in weaker tissues and poor structural development (Ponnampalam et al., 2022; Liu et al., 2023). Natural additives rich in antioxidants and similar essential compounds (minerals and phytochemicals) facilitate cell proliferation, enhancing tissue formation and development. Antioxidants help maintain nutrient integrity and lower inflammation rates, hence assisting in building the body systems and enhancing the mechanical strength of tissues (Rodríguez-Negrete et al., 2024). Additionally, certain minerals, such as calcium, zinc, and selenium, which are present in most bio-additives, play crucial roles in bone and muscle formation, as well as in enzymatic reactions. The multifunctional properties of natural additives enhance cellular functionality and vitality, thereby supporting various metabolic processes within the animal body (Weyh et al., 2022; Ding et al., 2023).
Mechanical properties play a significant role in animal production. These properties significantly impact the quality and quantity of animal tissues, animal welfare, processing operations, storage processes, and consumer preferences (Park et al., 2023; Gu et al., 2024). Tissue shear strength is primarily used to evaluate the textural quality, structural integrity, and consumer preference of animal offal. Shear strength is one of the major indicators of meat quality, as meat tenderness influences consumers’ purchasing decisions (Apata et al., 2023). Although tenderness is one of the primary benchmarks used in meat selection, excessive tenderness can lead to undesirable texture and shrinkage, resulting in a dry, unpleasant texture and reduced juiciness (Ježek et al., 2019). The mechanical behaviors of animal products are significantly dependent on the animal’s age, genetics, climatic conditions, health status, nutrition level, and handling practices. Different animal breeds exhibit distinct cellular architectures, fat deposition patterns, behavioral traits, and types of muscular fibers. Notably, an adequate understanding of these mechanical properties is indispensable in livestock mechanization (Park et al., 2023).
Plant extracts have been widely used as natural additives in livestock production, particularly in the poultry sector, to enhance body immunity and productivity (Ding et al., 2023). Turmeric (Curcuma longa), banana (Musa x paradisiaca), and onion (Allium cepa) are some of the tropical plants that are widely used in animal production as feed supplements due to their potent bioactive compounds (Wang et al., 2015; Aditya et al., 2016; Akram et al., 2022; Ashayerizadeh et al., 2023). Khan et al. (2022) reported that administering Aloe vera (Aloe barbadensis Miller) extract to chickens significantly increased weight gain and improved feed efficiency. Vispute et al. (2019) studied the influence of hemp (Cannabis sativa) and dill seed (Anethum graveolens) extracts on chickens’ growth and development and found that these bio-additives positively influenced the broilers’ serum lipid status and intestinal health. Puvača et al. (2020) investigated the utilization of natural supplements in chicken production and reported that garlic supplements contribute to a drop in production costs. Though intensive research has been carried out on the use of natural additives in animal production, most of the aforementioned investigations into the use of plant extracts in livestock management are principally based on the utilization of lone turmeric, banana peels, and onion extracts (Hidayat et al., 2017; Oyeyinka and Afolayan, 2019; Chounna et al., 2020; Zakiyatulyaqin et al., 2020; Malematja et al., 2023; Sureshbabu et al., 2023; Saeed et al., 2025). The related literature review revealed no clear evidence evaluating the effects of combined multiple extracts on chicken productivity within a single experimental design. Therefore, this current study aimed to examine the impact of hybridized plant extracts on the growth performance, nutritional quality, and microbial condition of broilers. Additionally, this experimental framework investigates the impacts of treatments with multiple extracts on the mechanical properties of chicken muscles, which have been previously neglected in research. The outcomes of this research will help to understand the synergistic effects resulting from hybridizing (combining) multiple plant extracts in animal production.
Materials and methods
Preparation of natural additives (plant extracts)
The turmeric, banana peels, and onion bulbs were cut into small pieces and sun-dried (31 ± 6°C) for 2 weeks. These dried materials were pulverized into a powder using a laboratory blender (produced by Thermo Fisher Scientific Inc., America). 2 kg of the powder was soaked in 10 L of ethanol (96% v/v purity, Merck KGaA, Germany) for 4 days at ambient room temperature (28 ± 3°C), stirred for 30 min four times daily, and sieved at the end of the 4 days using a Whatman No. 2 strainer. Thereafter, the product (extract) was evaporated using a rotary evaporator (model R-300, manufactured by Thermo Fisher Scientific Inc.) at a temperature of 50°C (with the aid of a water bath) and under reduced pressure to remove the remaining ethanol from the extract. The turmeric extract (TE), banana peel extract (PE), and onion extract (OE) were carefully poured into glass bottles and stored in a dark cupboard at room temperature (28 ± 3°C).
Animal of choice and experimental design
The broilers (Cobb 500 breed) were purchased at 2 weeks of age from a certified poultry farm in Delta State, Nigeria. The birds had already been vaccinated with Marek’s disease vaccine, along with the 1st and 2nd doses of the Newcastle disease vaccine and the first and second doses of the Gumboro vaccine.
The birds were divided into seven experimental groups with the following treatment plans: control (200 mL of distilled water), T1 (100 mL ~ TE), T2 (100 mL ~ PE), T3 (100 mL ~ OE), T4 (100 mL TE + 100 mL PE), T5 (100 mL TE + 100 mL OE), and T6 (100 mL PE + 100 mL OE). Each treatment involves adding 25 mL of solution per liter of water, applied three times weekly. The dosage of these treatments was chosen because previous literature had revealed that high dosages (≥300 mL or 300 mg/kg) of natural extracts tend to have negative impacts on animal productivity (Wang et al., 2015; Hidayat et al., 2017; Hafez et al., 2022; Yusuf et al., 2024). The treatments were administered orally through drinking water, at a rate of three doses per week, to enhance the bioavailability of the extract’s bioactive compounds. Additionally, the treatments (extract supplements) were not specific to each bird; rather, they were applied to each experimental unit. In this study, each experimental unit (plan) consisted of six birds, and each unit was replicated three times, resulting in a total of 126 birds used in the experiment. This comparatively high number of birds was intentionally selected to preserve the integrity and statistical validity of the results (sample size) in the event of mortality.
The research was conducted under the supervision of a certified veterinary doctor affiliated with the Delta State Ministry of Agriculture and Natural Resources in Nigeria. All operations were approved by the Department of Agricultural Engineering at Delta State University of Science and Technology and the Ozoro Ethics Committee. All the birds were raised under uniform environmental conditions (31 ± 8°C and 83 ± 9% RH). Furthermore, the broilers were raised at a density of 10 broilers per square meter. The total experimental period lasted 8 weeks, and the broilers were 10 weeks old (Week 2 to Week 10) when they were randomly sampled for laboratory analyses. Only one feed type (produced by Hybrid Feeds Ltd., Kaduna, Nigeria) was used to feed the birds throughout the experimental period, thereby ensuring consistency of results and minimizing the influence of confounding variables.
Laboratory analyses
Sample preparation
The birds were euthanized after 8 experimental weeks and were 10 weeks old. Each bird was gently restrained, its jugular vein cut with a surgical knife, leading to the swift draining of its blood into a container until the bird died. Thereafter, the breast and thigh specimens were obtained through the carcass dissection technique. The cutting board, stainless steel knife, hand gloves, and other tools used for the operation were sterilized. The defeathered bird was placed on a clean platform, and the breast and thigh sections were carefully cut away from the chicken. All the materials used for the operation were washed and sanitized. Then, the samples were labeled accordingly, kept inside an ice-cooled container (9 ± 4°C), and transported immediately, without the addition of extract preservative, to the bio-processing laboratory for laboratory analyses.
Bodyweight gain and feed conversion ratio
The broilers were weighed individually using a digital scale (Fisherbrand, Model: 88861022, manufactured by Richmond Scientific, United Kingdom). Their weight was recorded at the commencement and end of the experiment to calculate individual body weight gain. Specifically, the amount of feed provided to each experimental unit was adjusted according to the mortality rate (i.e., the number of birds remaining per unit) to ensure an accurate calculation of the feed conversion rate. Thereafter, their feed conversion ratio (FCR) was calculated using the total feed intake and the weight increment, as expressed in Equation 1 (Hafez et al., 2022).
Mechanical properties (shear force)
The specimen’s shear force was determined using the Warner-Bratzler shear force apparatus, aided by a universal testing machine (Testometric brand, SKU: XFS Categories, manufactured in England) equipped with a 500 N load cell. Each meat sample was cut in dimensions of length (0.1 m), breadth (0.05 m), and breadth (0.05 m), placed into the apparatus, and sheared using a 1 mm stainless steel blade at a speed of 300 mm/min. The test was performed in 10 replicates for both breast and thigh muscles.
Microbiological assessment
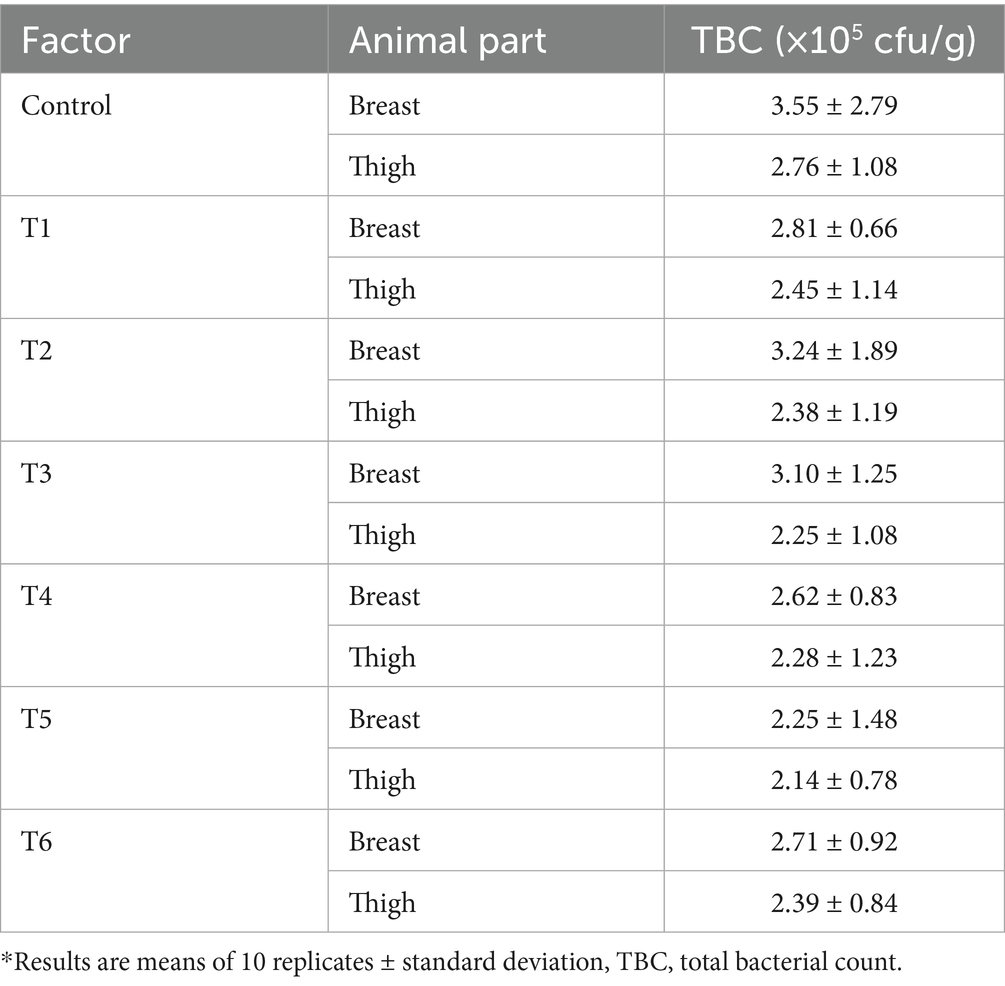
Twenty grams of the meat sample was crushed using a laboratory blender. A dilution was prepared by adding 180 mL of sterile saline to the ground meat sample. Thereafter, 1 mL of the dilution was spread across a sterile Plate Count Agar (Merck KGaA, Germany). The plate was incubated at 36°C for 40 h, and the bacterial colonies produced on the plate surface were counted. The total viable bacterial count (TBC) was computed as colony-forming units per gram (cfu/g). The test was conducted with 10 replicates for both breast and thigh muscles.
Protein and lipid level determination
The meat sample was cut into 20 mm × 20 mm pieces, dried in a laboratory oven (Model PR305225M, manufactured in the United States by Thermo Fisher Scientific Inc.) at 105°C for 5 h, and then ground using a laboratory blender (Thermo Fisher Scientific Inc., United States). Following this, the Kjeldahl approach was employed to measure the protein level of the dried chicken muscles in accordance with the Association of Official Analytical Chemists (AOAC) 981.10 guidelines (Mæhre et al., 2018; Malematja et al., 2023). Additionally, the total lipid level of the meat samples was determined using the Soxhlet extraction method with n-hexane as the solvent. The protein and lipid contents were computed through Equations 2 and 3.
Water holding capacity
The centrifugation approach was used to evaluate the water-holding capacity (WHC) of the muscle specimens using a centrifuge machine (Thermo Scientific, Brand, and Model: ST-8). Five grams of the fresh sample were ground and then poured into a centrifuge tube. The centrifuge machine was operated at a speed of 5,000 × g for 20 min at a temperature of 6 ± 1°C (Katemala et al., 2021). The free water expelled from the meat was decanted, its weight taken, and the sample’s WHC value was computed through Equation 4.
Bioactive compound determination
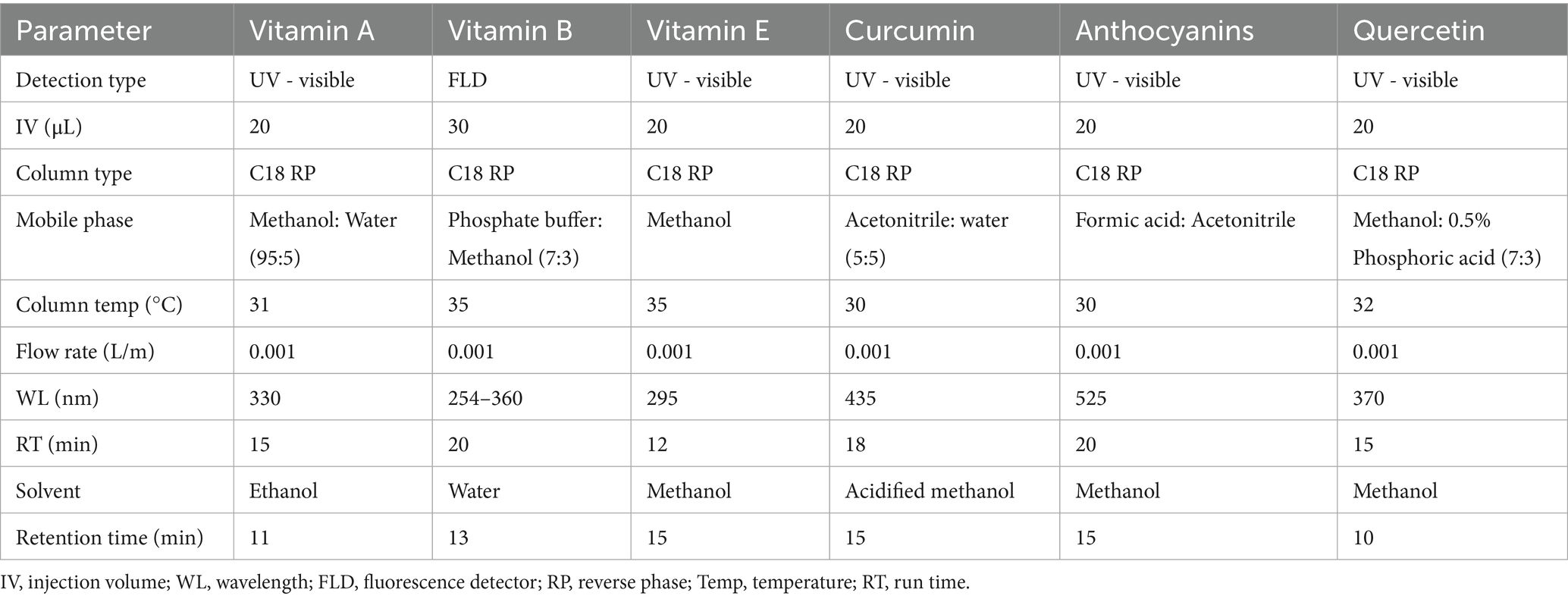
The concentrations of vitamin A, vitamin B, vitamin E, curcumin, anthocyanins, and quercetin were determined using the HPLC approach, while vitamin C levels were quantified using UV–Vis spectrophotometry. Similarly, total phenolic content was measured using the Folin–Ciocalteu assay, and total flavonoid content was measured using the AlCl3 colorimetric assay. The HPLC system used for the analysis was equipped with a reverse-phase C18 column unit.
HPLC analysis
For the nonpolar bioactive compounds investigated—vitamins A and E, as well as curcumin —20 mL of the extract was diluted with 80 mL of n-hexane and centrifuged at a speed of 5,000 × g for 10 min. The upper layer was separated and then evaporated using a rotary evaporator at a temperature of 40°C, followed by filtration through a 0.22 μm membrane mesh. Similarly, for the polar bioactive compounds (B vitamins, “vitamin B-complex,” anthocyanins, and quercetin), 20 mL of the extract was added to 80 mL of a methanol–water mixture (ratio 8:2), then mixed, centrifuged at 6,000 × g for 7 min, and sieved through a 0.22 μm membrane. The filtrate was used for the HPLC analysis, and the HPLC injections were run separately for each parameter to be tested (Pan et al., 2020). Specifically, the operating parameters used to determine the concentrations of vitamins A, B, and E, as well as curcumin, anthocyanins, and quercetin in the extract, are presented in Table 1.
Total phenolics content (TPC) evaluation: 1 mL of the extract was added to 5 mL of 10% Folin–Ciocalteu reagent and 4 mL of 7.5% sodium carbonate solution. This mixture is incubated at 30oC for 1 h. The total phenolic content (TPC) was measured by ultraviolet and visible (UV–Vis) spectrophotometry (manufactured by Thermo Fisher Scientific Inc., America) at a wavelength of 765 nm (Lawag et al., 2023). The TPC was calculated via Equation 5.
Vitamin C determination
The vitamin C level in the extract was quantified using UV–Vis spectrophotometry. A total of 10 mL of the extract was added to 80 mL of distilled water and sieved with Whatman No. 1 filter paper. The filtrate volume was adjusted to 100 mL using 0.1 M tetraoxosulphate (VI) acid. Then, the vitamin C concentration in the prepared sample was measured with a UV–visible spectrophotometer using a wavelength of 265 nm.
Hematological parameters determination
The packed cell volume (PCV), red blood cells (RBC), and white blood cells (WBC) count of the blood specimens were performed through an automated approach using an automated hematology analyzer (model ~ Countess 3 FL, manufactured by Thermo Fisher Scientific Inc., United States) in accordance with standard procedures as explained by Hafez et al. (2022). The blood specimen was collected from the jugular vein using a disinfected needle and syringe. Each blood sample (30 μL) was collected into an ethylenediaminetetraacetic acid (EDTA)-coated tube (as a blood preservative to prevent clotting), inserted into the machine, and hematocrit (HCT), WBC and RBC were analyzed by the machine. The results were displayed digitally on the screen, and the PCV is equivalent to the HCT. The test was conducted at ambient laboratory temperature (28 ± 4°C), and each analysis period lasted approximately 3 min per sample. The blood sample analysis was conducted within 3 h after sampling in order to ensure the accuracy and reliability of the results.
Statistical analysis
SPSS (version 22.0) was employed to analyze the laboratory data. Then, the ANOVA test was conducted to assess the significant impact of the treatments on the quality of broiler meat. The differentiation in means among the groups was carried out using Duncan’s Multiple Range Test (DMRT) at a 5% significance level (p ≤ 0.05). The tests were primarily conducted in five replicates.
Results and discussion
Bioactive compound concentrations of the extracts
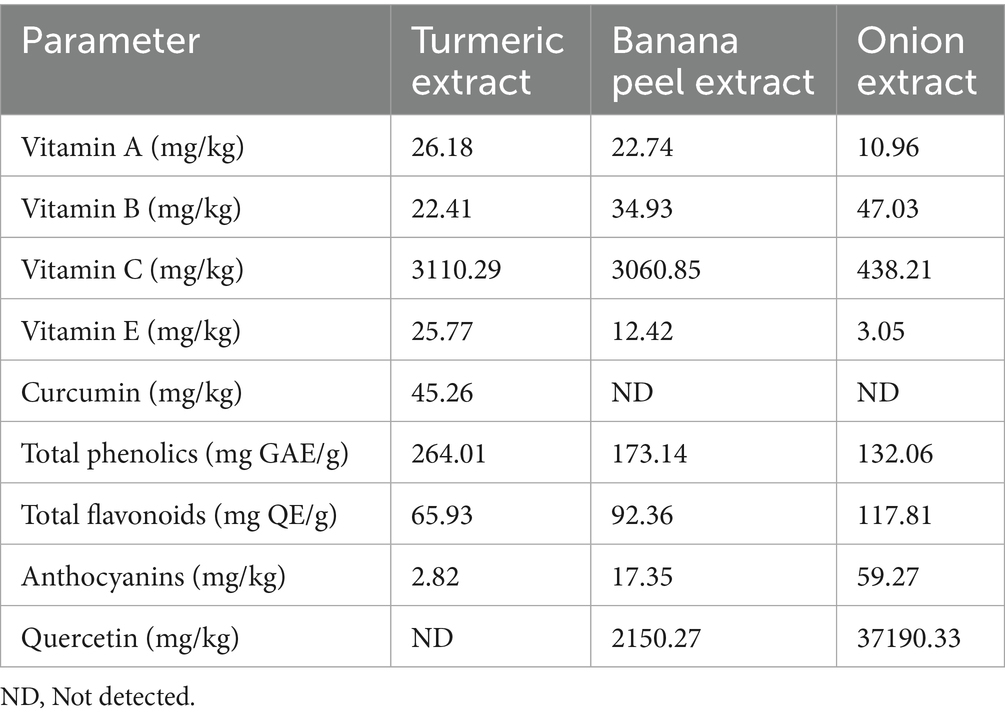
Table 2 presents the concentrations of the various essential bioactive compounds detected in the three extracts used for the experimental investigation. Curcumin was exclusive to the turmeric extract, while the quercetin compound was also found in banana peels and onion extracts. Additionally, vitamin A is extremely concentrated in the turmeric extract (26.18 mg/kg) and banana peel extract (22.74 mg/kg). The OE contains a high vitamin B level (47.03 mg/kg) but a very low vitamin C concentration (438.21 mg/kg). Furthermore, the TE and PE exhibited high concentrations of vitamin E and total phenolics, whereas the OE showed a high presence of total flavonoids, anthocyanins, and quercetin. These results indicate that the extracts (OE, TE, and PE) contain substantial amounts of essential phytochemical compounds, which will likely enhance their antioxidant activity (Bhavani et al., 2023). Concentrations of the various bioactive compounds obtained in this study are similar to those reported by Zhao et al. (2021), Jesumirhewe et al. (2022), and Wu et al. (2024) for turmeric, banana peels, and onion bulb extracts. Phenolic compounds are potent antioxidants that enhance animals’ immune responses and feed efficiency, resulting in a substantial reduction in oxidative stress and high resistance to pathogenic microbial invasion (Bešlo et al., 2023). Flavonoids play vital roles in animal productivity and are considered natural antibiotics with anti-inflammatory properties, which regulate immune responses, enhance cellular function, and promote gut microbiota activity (Jomova et al., 2025).
Productive performance and nutritional content
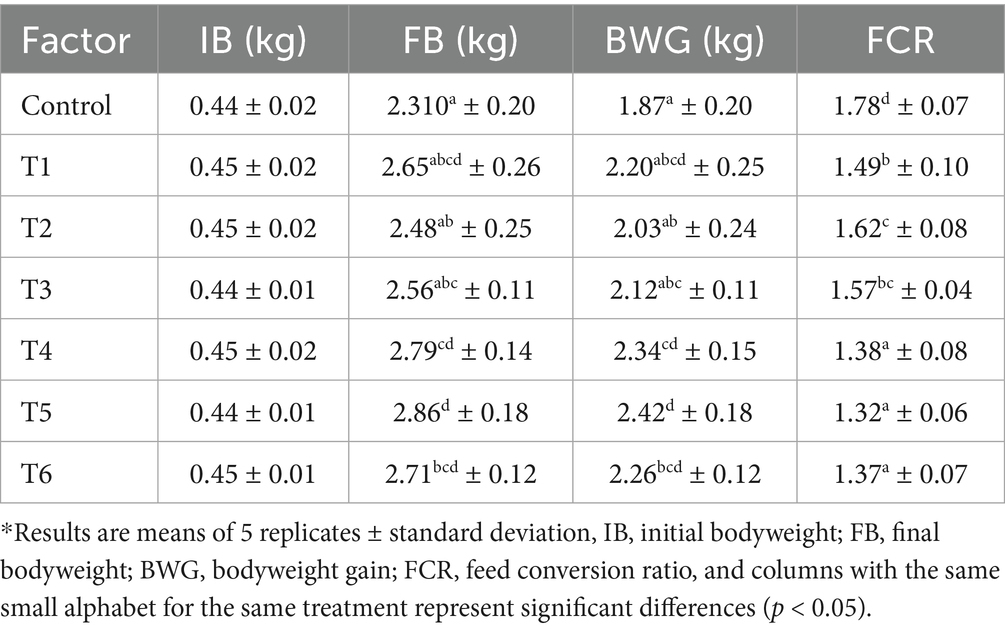
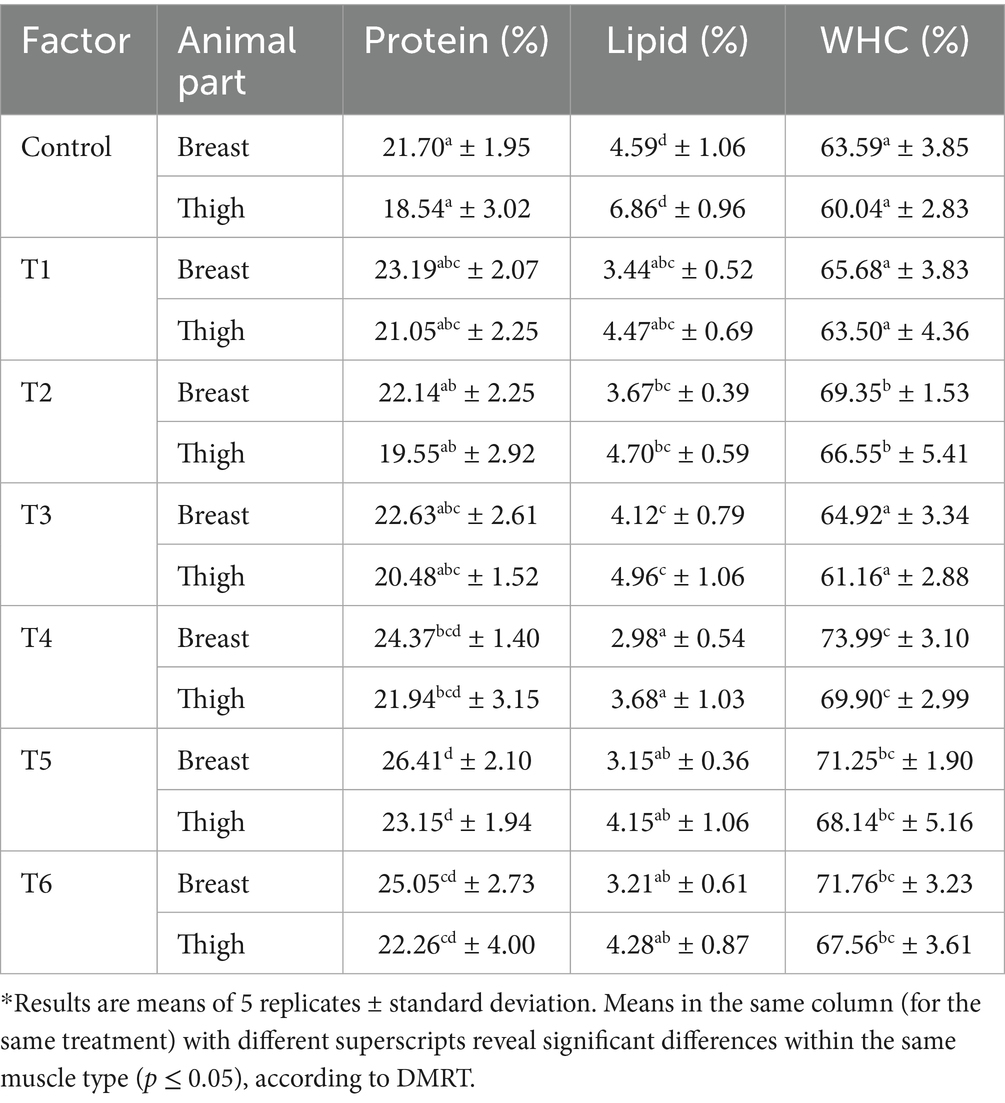
The results of the growth rate and nutrient level of the chickens from the various experimental units are presented in Tables 3, 4. It was noted that treatments had a significant effect on the birds’ body weight and the nutrient content of their muscles (p ≤ 0.05). The weight gained by the control group and Treatments 1 to 6 of the broilers were 1.87, 2.20, 2.03, 2.12, 2.34, 2.42, and 2.26 kg, respectively, while their FCR values were 1.78, 1.49, 1.62, 1.57, 1.38, 1.32, and 1.37, respectively. Notably, the protein levels in the breast meat of the control unit and the experimental units 1 to 6 were found to be 21.70, 23.19, 22.14, 22.63, 24.37, 26.41, and 25.05%, respectively. Similarly, the thigh muscle protein levels were 18.54, 21.05, 19.55, 20.48, 21.94, 23.15, and 22.26%, respectively.
Additionally, the total lipid levels of the breast and thigh muscles under the control unit, T1 to T6, were found to be 4.59, 3.44, 3.67, 4.12, 2.98, 3.15, and 3.21%, respectively, for the breast meat, and 6.86, 4.475, 4.70, 4.96, 3.68, 4.15, and 4.28%, respectively, for the thigh meat. Furthermore, the WHC levels for the tissues found in the breast and thigh muscles were 63.59, 65.68, 69.35, 64.92, 73.99, 71.25, and 71.76% for the breast meat and 60.04, 63.50, 66.55, 61.16, 69.90, 68.14, and 67.56% for the thigh tissues, respectively. The study’s outcomes revealed that the breast tissue protein and WHC levels were higher than those recorded for the thigh tissue. In contrast, the breast has a lower lipid level compared to the thigh. These observations were similar to the findings documented by Soriano-Santos (2010) and Katemala et al. (2021) in their studies on chicken meat quality. Malematja et al. (2023) assessed the effect of onion extract supplements in the Ross 308 broiler breed and reported that those birds that received the extracts had heavier breast meat compared with the untreated broilers. This suggests that the essential bioactive compounds in the supplements (the extracts) enhance feed absorption and improve metabolism, leading to higher meat yields.
Notably, throughout the experimental phase, the weight gains and feed conversion ratio (FCR) of the chicks placed under the hybridized supplement groups (T4, T5, and T6) were statistically greater than those of the other treatment groups (single treatment units). This observation aligned with the findings reported by Ashayerizadeh et al. (2023) during their appraisal of extract potency optimization. A combination of supplements helps increase the concentration of antioxidants and other essential nutrients, thereby enhancing the animal’s productivity compared to a single supplement. This can be associated with the diverse and varying concentrations of bioactive compounds present in the plant extracts (Alem, 2024).
The control unit had the lowest body weight compared to the treated birds, which is an indication that plant extracts considerably influenced the birds’ weight gain. Bodyweight gain is one of the indices used to evaluate the efficacy of a natural supplement because it reveals the animal’s growth performance, health status, and feed conversion ability (Aditya et al., 2016; Yusuf et al., 2024). The bodyweight gain and FCR patterns observed in this study are comparable to those published by Utami et al. (2020) and Hafez et al. (2022). These authors reported that organic supplements led to rapid weight gains and improved feed conversion ratio (FCR) in poultry birds.
Notably, the lower FCR values and higher FBW values recorded in the combined treatments indicate that these treatments had better feed utilization efficiency, resulting in reduced production costs and optimized nutrient availability (Yusuf et al., 2024). Extracts from onion, turmeric, and other plants enhance the workability of the digestive system, positively influencing feed intake, digestibility, and nutrient absorption (Aditya et al., 2016; Hidayat et al., 2017; Yusuf et al., 2024; Sanwo et al., 2020; Abidinsyah et al., 2024). Furthermore, the weight gain can be attributed to the high concentration of B-complex vitamins in the extracts used as the supplements. The B vitamins have the potential to boost energy metabolism and stimulate appetite, leading to an increase in the formation of red blood cells and tissue development. Additionally, curcumin, phenolics, and flavonoids possess strong antioxidant and anti-inflammatory properties, which enhance the birds’ immune system and indirectly support tissue growth and development, thereby contributing to overall body weight gain (Tardy et al., 2020; Aditya et al., 2016; Malematja et al., 2023).
Interestingly, the lowest lipid content (2.98%) was recorded in the breast muscles of broilers administered hybridized TE and PE supplements, while the thigh meat of the birds given OE supplements had the maximum lipid value of 4.96% (p ≤ 0.05). The high concentrations of curcumin, anthocyanins, and quercetin present in the TE and PE supplements could be linked to their effectiveness in reducing lipid synthesis and limiting lipid deposition in broiler meat. These compounds have stronger lipogenesis retardation ability and improve lipid metabolism, hence lowering cholesterol and LDL production rates in animals (Wang et al., 2020; Hafez et al., 2022; Li et al., 2023). The lipid inhibition rate observed in the broilers administered the supplements was comparable to the findings of previous experimental frameworks (Utami et al., 2020; Sanwo et al., 2020). According to these authors (Hidayat et al., 2017; Utami et al., 2020; Akram et al., 2022), the reduction of fat deposits and the increase in fat oxidation lead to greater availability of carbohydrates and proteins, as well as higher antioxidant levels. Furthermore, curcumin and quercetin help facilitate fat oxidation and mobilization, thereby decreasing the rate of lipid deposition in body tissues (Sigolo et al., 2021; Aderemi and Alabi, 2023).
In addition to these research results, these studies (Wang et al., 2015; Sanwo et al., 2020) reported that turmeric supplementation has the potential for retarding fat production through regulation of the lipid metabolism and consequently increasing chicken offal’s protein and WHC levels. Controversially, this research’s findings contradict those of Negari et al. (2015), which indicated that turmeric extract decreased the water-holding capacity (WHC) of chicken flesh. Notably, this study’s outcome differed from the findings of Yusuf et al. (2024), which showed that supplementation with Tahongai (Kleinhovia Hospital L.) leaf extract increased chicken abdominal fat levels. The variations noted in this research’s findings, in comparison to those of the authors (Negari et al., 2015; Yusuf et al., 2024), could be attributed to differences in extract processing techniques, bird variety, farming systems adopted, storage conditions, and human and analytical errors. According to Bharti et al. (2024), analytical techniques and other anthropogenic actions can substantially affect the concentrations of bioactive compounds in food items.
Significantly, the highest protein value (26.41%) was found in the breast tissues of broilers given hybridized TE and OE supplements (p ≤ 0.05). Aditya et al. (2016) documented a similar increment of protein levels in birds administered with OE supplements. The higher protein levels documented in birds given supplements, primarily those that contain OE, can be linked to higher vitamin B levels. The B vitamins aid amino acid metabolism and improve nitrogen balance in animals, consequently leading to protein formation (Alagawany et al., 2020). Similarly, the higher protein concentration found in the breast, compared to the thigh, could be linked to the distinct muscle and fat compositions of these two meat products (Wang et al., 2015). Breast tissue primarily consists of white muscle fibers with a lower fat content, whereas thigh tissues, which predominantly contain red muscle fibers, have a higher fat content and are involved in various metabolic activities that can lead to reduced protein levels in the muscles (Katemala et al., 2021).
Based on the unit effectiveness of the treatment plans, it was noted that the TE-based experimental units (T1, T4, and T7) have a superior impact on chicken body weight increment, protein synthesis, and the synthesis of other essential nutrients. This could be attributed to the presence of curcumin in the TE-based treatments, which helps enhance protein synthesis and other essential body-building compounds in the animal’s body. Curcumin is a powerful antioxidant that stimulates anabolic processes and promotes nitrogen retention, both of which are essential for protein synthesis and decrease oxidative stress (Utami et al., 2020; Hafez et al., 2022; Ashayerizadeh et al., 2023). According to these authors (Utami et al., 2020; Awad et al., 2022), the efficacy of natural supplements in improving animal productivity depends on several factors, including dosage and duration of administration, plant maturation, processing technique, and storage conditions.
Microbiology
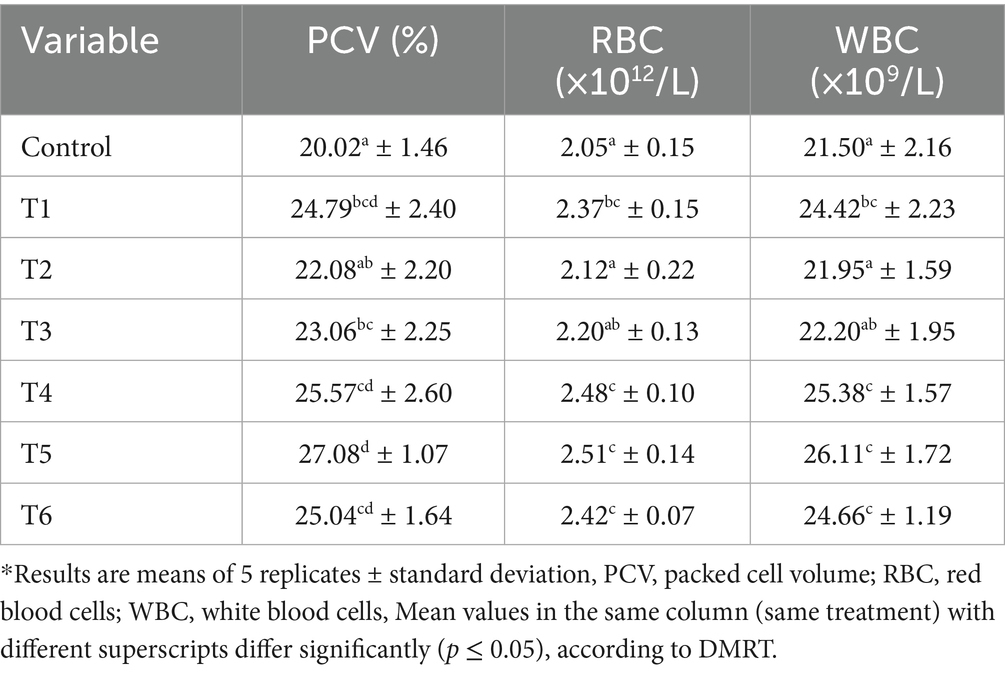
Tables 5, 6 present the bacteria loads and hematological parameters of the chicken specimens. Notably, the meant TBC for the control, T1–T6 breast tissues were 3.55 ×105, 2.81 ×105, 3.24 ×105, 3.10 ×105, 2.62 ×105, 2.25 ×105 and 2.71 ×105 cfu/g, respectively; while in the thigh muscles, an average TBC amounts were 2.76 ×105, 2.45 ×105, 2.38 ×105, 2.25 ×105, 2.28 ×105, 2.14 ×105 and 2.39 ×105 cfu/g, respectively. Regarding the hematological parameters, the control group and treatment units 1 to 6 had the following mean PCV, RBC, and WBC counts: PCV—20.02, 22.08, 23.06, 25.57, 27.08, and 25.04%, respectively; RBC—2.05, 2.12, 2.20, 2.48, 2.51, and 2.42 × 1012/L, respectively, and WBC (white blood cell count per liter): 21.50, 21.95 × 10, 22.20, 25.38, 26.11, and 24.66 × 109/L, respectively. The antimicrobial effects of these treatments, as documented in this study, are similar to those reported by Ashayerizadeh et al. (2023) and Yusuf et al. (2024). These researchers noted that tahongai leaf, black pepper, and turmeric extract successfully retard bacteria performance in broilers’ muscles and improve gut health.
The strong antimicrobial effects observed in the extracts can be attributed to their high curcumin, quercetin, and total phenolic contents. Curcumin is able to disrupt the bacterial membrane and inhibit biofilm formation, resulting in reduced microbial growth. Similarly, quercetin has a strong potential to retard microorganisms’ DNA gyrase and membrane reliability, while phenolic compounds disrupt the cell structure and protein production in microorganisms. These conditions lead to poor microbial performance and survival (Hussain et al., 2022; Sionov and Steinberg, 2022).
Interestingly, the present study outcomes revealed that the breast part of the bird harbored more microbial populations than the thigh part. Similarly, research conducted by Dourou et al. (2021) and Mussa et al. (2022) has shown that the concentrations of microbes in chicken breasts are higher than those reported for chicken thighs. This could be attributed to the lower fat and myoglobin levels in the breast muscles compared to those in the thigh muscles, conditions that may encourage microbial survival (Dourou et al., 2021). The results revealed a significant improvement in blood PCV, RBC, and WBC levels following the administration of the treatments. This suggests that the essential compounds present in the three extracts significantly contribute to blood formation and improve the animal’s physiological performance. This is further supported by the higher protein levels recorded in the treated chicken muscles (Table 4) and the increased vitamin levels in the extracts (Table 3). Vitamins A and B have been shown to stimulate the production of packed cell volume, red blood cells, and white blood cells in animals. These vitamins facilitate DNA synthesis and enhance immune cell and hemoglobin levels, resulting in improved blood cell production and epithelial health (Ponnampalam et al., 2022).
The appreciable amounts of phenolic acids, flavonoids, and other antioxidants present in the treatments tend to act as antibacterial, immunomodulatory, and anti-inflammatory compounds, hence improving the broilers’ health status and impeding free radical activity and oxidative stress (Diniyah and Lee, 2020; Aditya et al., 2016; Yusuf et al., 2024). These factors result in enhanced physiological conditions, improved nutrient utilization, and elevated growth performance. Additionally, Ashayerizadeh et al. (2023) reported that the phenolic and flavonoid contents found in plant extracts enhance the performance and meat quality of poultry birds.
This study’s outcomes further highlight that natural additives have a substantial influence on microbial survival within the chicken’s body, which could be attributed to their potent antioxidant compounds (Manso et al., 2021; Sigolo et al., 2021; Hafez et al., 2022). These compounds possess strong antioxidant and antimicrobial properties, which significantly enhance the bird’s health condition and immune system, thereby inhibiting the reproduction of most pathogenic bacteria within the bird’s muscle (Mudalal et al., 2021). Most herbal supplements are potent antibiotics, having high efficacy in suppressing bacteria growth, thereby making them vital components of livestock management. Ingesting additives rich in antioxidants promotes the performance of beneficial microbes while constraining the reproduction of pathogenic microbes, particularly in the alimentary canal (Attia et al., 2018; Mudalal et al., 2021). Additionally, these bioactive compound supplements enhance protein synthesis and the production of other essential body-building compounds in the body, resulting in effective microbial suppression through the formation of potent antibiotics (Yusuf et al., 2024). Differences in bacterial infestation noted in the birds’ muscles, as reported by various authors, could be attributed to the different anti-microbial activities of the plants (extract) used by these researchers.
Generally, the hybridized experimental units recorded higher antimicrobial impacts than the mono agent’s experimental units (T1, T2, and T3). Specifically, it was found that the turmeric-based supplements (T1, T4, and T5) had more antimicrobial potency compared to the other treatment units. This could be attributed to the high curcumin level in the turmeric extract, which has a strong capacity for retarding pathogenic growth (Aderemi and Alabi, 2023; Ashayerizadeh et al., 2023). It has been proven that onion extract has effective antibacterial and hypolipidemic properties, which helps promote the growth and development of broilers (Malematja et al., 2023); this could be linked to the lower bacterial counts found in T3, T5, and T6 muscle samples. Additionally, the lower bacterial loads identified in the treated samples can be associated with the higher amounts of WBCs and proteins, as well as the lower lipid levels present in these samples. These vital parameters help enhance the broiler’s immune system, thereby improving gut health and resistance mechanisms against bacterial invasion.
Textural profile
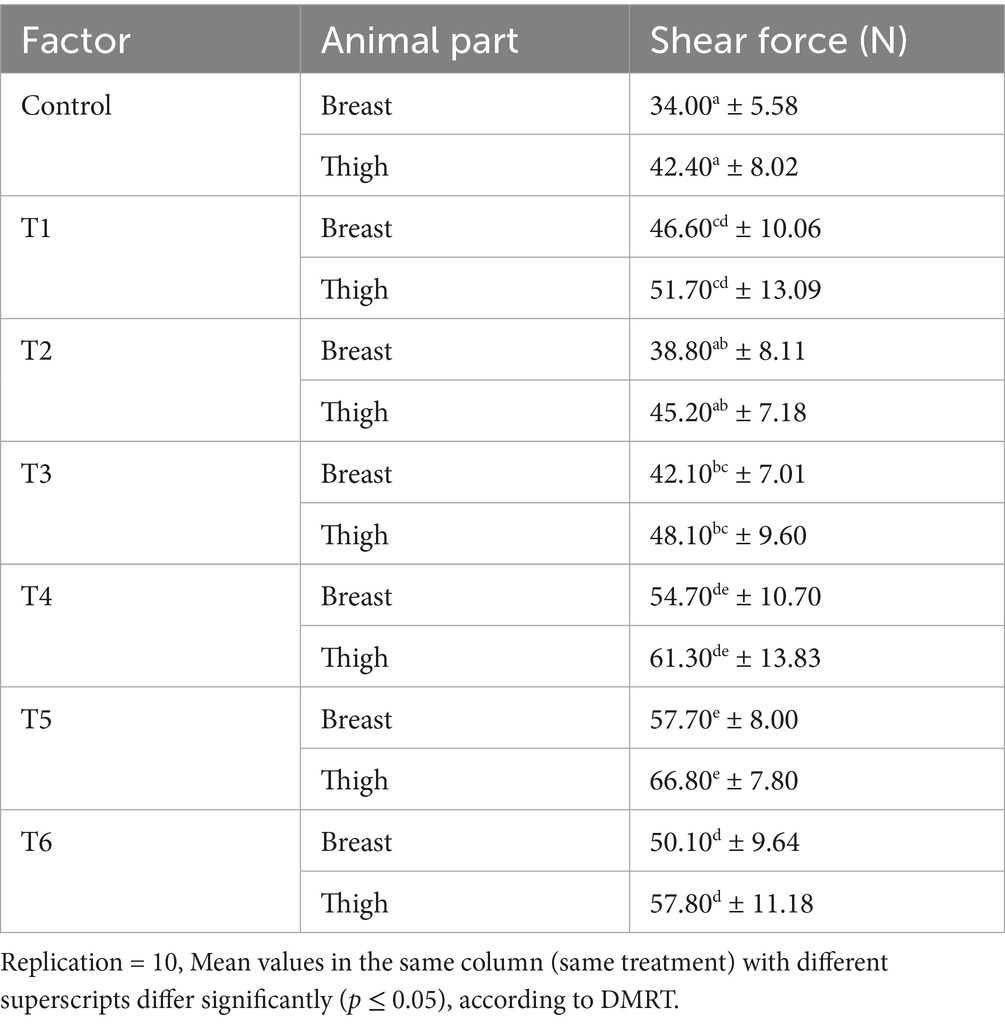
The shear force test results are given in Table 7. It was noted that the breast muscle average shear force for the control, T1-T6 samples were 34.00, 44.60, 38.80, 42.10, 54.70, 57.70, and 50.10 N, respectively, while the thigh muscle had a mean shear force of 42.40, 51.70, 45.20, 48.10, 61.30, 66.80 and 57.80 N, respectively. Additionally, the results show that the thigh tissues absorbed a greater amount of shear force when compared to the breast tissues. This outcome is similar to the conclusions of Park et al. (2023) but contrasts with the observations made by Mbaga et al. (2014) regarding the mechanical properties of chicken parts. The control group shear force values were lower than the values (44.30 to 51.2 N) obtained for the Ufipa chicken breed (Mussa et al., 2022) but were within the range of results (28.00 to 35.80 N) found in Ross chicken and commercial broiler breeds (Katemala et al., 2021; Mussa et al., 2022). Research by Chen et al. (2016) and Katemala et al. (2021) revealed that chicken breast tissues are generally firmer than thigh tissues, and this mechanical property (firmness) is highly influenced by the bird’s maturity stage, nutrition, and health conditions. Animal products with lower shear force values tend to have better palatability but are prone to mechanical damage during packaging, handling, and storage operations (Apata et al., 2023).
Furthermore, the study’s findings highlighted that the treatment had a significant effect on the shear force of the chicken tissues (p ≤ 0.05). This aligns with the reports of Apata et al. (2023), which indicate that various treatment options had a substantial effect on the meat’s mechanical attributes. Malematja et al. (2023) documented that onion extract at a dosage of 5 g/kg significantly enhanced the shear force of chicken muscles. It can be seen from the results that the turmeric extract-based treatments (T1, T4, and T5) demonstrated superior results. The elevated shear force observed in treatments T1, T4, and T5 can be linked to the curcumin and other phytochemical content of the TE. These compounds help to improve the animal protein structure, vigor, and performance of the animal systems, thereby resulting in an increased shear force. Phytochemical compounds enhance animal metabolisms and collagen formation, thereby leading to increased body tissue strength and productivity (Lee et al., 2016). Contrary to our findings, Ali and Zahran (2010) reported a substantial decline in the shear force of chicken tissues administered 2 g/kg onion and garlic extract additives, which can be attributed to anthropogenic factors, such as the concentration of the supplement’s active ingredient and the duration of supplement administration.
Additionally, the hybridized bio-agent treatments (T4, T5, and T6) yielded results with greater shear force compared to the single bio-agent treatments (T1, T2, and T3). This can be attributed to the synergistic collaboration between the distinct essential compounds of the various natural additives, which enhance the mechanical and textural properties of the animal’s tissues. Vitamins B and E play a vital role in muscle development in animals, as they enhance protein synthesis and promote connective tissue formation. These compounds aid in the enhancement of muscle density and cohesion, leading to improved (increased) structural attributes of the animal flesh (Sathyabhama et al., 2022; Malematja et al., 2023). Though natural additives enhance tissue mechanical properties, their effectiveness depends on factors such as bioavailability, dosage, and the specific type of tissue (Sigolo et al., 2021). Apart from the increase in feed intake, higher muscle protein levels can contribute to the increased body weight and mechanical properties of the broiler muscles through the production of myofibrils within the bird’s tissues.
Conclusion
This research was conducted to investigate the sustainability of using natural additives as bio-supplements in animal production. Broilers Cobb 500 were administered turmeric extract, banana peel extract, and onion extract for 8 weeks. The research’s findings indicated that adding these organic extracts as supplements in bird production led to increased body weight, improved nutritional profiles, enhanced mechanical properties, and better meat quality. Notably, the treatments containing turmeric extract produced better results among the seven experimental units. Additionally, the hybridized treatments performed better, as the birds exhibited enhanced nutrient absorption and improved metabolism. Specifically, these treatments (supplements) have led to the production of animal flesh with superlative nutritive values, shear force, and antibacterial behaviors. Though this study’s outcome highlighted the sustainability of hybridized natural supplements, it did not investigate the impact of the extract on individual bacterial or fungal isolates. It is recommended that future research examine the gut microbes and evaluate their effect on pathogenic loads, such as Salmonella spp., Penicillium spp., and Aspergillus spp. This will enhance the understanding of bio-supplement strategies in animal production.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
All the operations were approved by the Department of Agricultural Engineering, Delta State University of Science and Technology, Ozoro Ethics Committee.
Author contributions
AA: Writing – review & editing, Writing – original draft. FA: Writing – review & editing. AB: Writing – original draft. NA: Writing – original draft. HU: Writing – review & editing, Writing – original draft. OE: Writing – original draft. DE: Writing – original draft. RS: Writing – original draft. MH: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Taif University, Saudi Arabia (Project no. TU-DSPP-2024-79).
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-79).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abidinsyah, D. A., Suyub, I. B., Jusoh, S., and Yaakub, H. (2024). The digestibility, ruminal fermentation and methane product of Cajanus cajan forage as a concentrate substitute in goats. J. Indonesian Trop. Anim. Agricult. 49, 307–315. doi: 10.14710/jitaa.49.4.307-315
Abigarl, N., Jessica, P., Tapiwanashe, M., Sakhile, N., Mongameli, M., Samantha, S., et al. (2023). Incorporation of functional feed ingredients to substitute antimicrobials in animal nutrition: opportunities for livestock production in developing countries. Int. J. Livest. Prod. 14, 44–57. doi: 10.5897/ijlp2023.0820
Aderemi, F. A., and Alabi, O. M. (2023). Turmeric (Curcuma longa): an alternative to antibiotics in poultry nutrition. Transl. Anim. Sci. 7:200. doi: 10.1093/tas/txad133
Aditya, S., Ahammed, M., Jang, S. H., and Ohh, S. J. (2016). Effects of dietary onion (Allium cepa) extract supplementation on performance, apparent total tract retention of nutrients, blood profile and meat quality of broiler chicks. Asian Australas. J. Anim. Sci. 30, 229–235. doi: 10.5713/ajas.16.0553
Akram, T., Mustafa, S., Ilyas, K., Tariq, M. R., Ali, S. W., Ali, S., et al. (2022). Supplementation of banana peel powder for the development of functional broiler nuggets. PeerJ. 10:e14364. doi: 10.7717/peerj.14364
Alagawany, M., Elnesr, S. S., Farag, M. R., Tiwari, R., Yatoo, M. I., Karthik, K., et al. (2020). Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health - a comprehensive review. Vet. Q. 41, 1–29. doi: 10.1080/01652176.2020.1857887
Alem, W. T. (2024). Effect of herbal extracts in animal nutrition as feed additives. Heliyon 10:e24973. doi: 10.1016/j.heliyon.2024.e24973
Ali, F. H., and Zahran, D. A. (2010). Effect of growth enhancers on quality of chicken meat during cold storage. Adv. J. Food Sci. Technol. 2, 219–226.
Apata, E., Ahmad, B., Apata, O., Olaleye, O., and Hashem, M. (2023). Quality evaluation of chicken meat preserved with local packaging materials. Meat Res. 3, 1–6. doi: 10.55002/mr.3.6.75
Ashayerizadeh, O., Dastar, B., Shams Shargh, M., Soumeh, E. A., and Jazi, V. (2023). Effects of black pepper and turmeric powder on growth performance, gut health, meat quality, and fatty acid profile of Japanese quail. Front. Physiol. 14:1218850. doi: 10.3389/fphys.2023.1218850
Attia, Y. A., Bakhashwain, A. A., and Bertu, N. K. (2018). Utilisation of thyme powder (thyme vulgaris L.) as a growth promoter alternative to antibiotics for broiler chickens raised in a hot climate. Eur. Poult. Sci. 82, 238–255. doi: 10.1399/eps.2018.238
Awad, N. A., Mohamed, E., El-Bassel, E. H., Ismail, A. S. M., Abd El-Aziz, Y. S. G., Gawish, M. S., et al. (2022). Evaluation of the effect of elite jojoba strains on the chemical properties of its seed oil. Molecules 27, 3904–3913. doi: 10.3390/molecules27123904
Bešlo, D., Golubić, N., Rastija, V., Agić, D., Karnaš, M., Šubarić, D., et al. (2023). Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants (Basel, Switzerland) 12:1141. doi: 10.3390/antiox12061141
Bharti, A., Jain, U., and Chauhan, N. (2024). Progressive analytical techniques utilized for the detection of contaminants attributed to food safety and security. Talanta Open 10:100368. doi: 10.1016/j.talo.2024.100368
Bhavani, M., Morya, S., Saxena, D., and Awuchi, C. G. (2023). Bioactive, antioxidant, industrial, and nutraceutical applications of banana peel. Int. J. Food Prop. 26, 1277–1289. doi: 10.1080/10942912.2023.2209701
Chen, Y., Qiao, Y., Xiao, Y., Chen, H., Zhao, L., Huang, M., et al. (2016). Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian Australas. J. Anim. Sci. 29, 855–864. doi: 10.5713/ajas.15.0840
Chounna, A., Tendonkeng, F., Fualefac, H. D., Lemoufouet, J., Miegoue, E., Watsop, H. M., et al. (2020). Effect of plantain (Musa paradisiaca) leaves ash extract and the source of rumen fluid on in vitro digestibility of rice straw complemented with Calliandra calothyrsus leaves. J. Anim. Health Prod. 8, 122–129. doi: 10.17582/journal.jahp/2020/8.3.122.129
Ding, X., Giannenas, I., Skoufos, I., Wang, J., and Zhu, W. (2023). The effects of plant extracts on lipid metabolism of chickens - A review. Anim. Biosci. 36, 679–691. doi: 10.5713/ab.22.0272
Diniyah, N., and Lee, S. H. (2020). Phenolic com-position and antioxidant potential of legumes - a review. Jurnal Agroteknologi 14, 91–102.
Dourou, D., Spyrelli, E. D., Doulgeraki, A. I., Argyri, A. A., Grounta, A., Nychas, G. J. E., et al. (2021). Microbiota of chicken breast and thigh fillets stored under different refrigeration temperatures assessed by next-generation sequencing. Food Secur. 10:765. doi: 10.3390/foods10040765
Gu, S., Gao, J., Li, Z., Zhang, S., Wen, C., Sun, C., et al. (2024). Comparative analysis of Myofiber characteristics, shear force, and amino acid contents in slow- and fast-growing broilers. Food Secur. 13:3997. doi: 10.3390/foods13243997
Hafez, M. H., El-Kazaz, S. E., Alharthi, B., Ghamry, H. I., Alshehri, M. A., Sayed, S., et al. (2022). The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals 12:958. doi: 10.3390/ani12080958
Hidayat, M., Zuprizal, Z., Sundari, S., Kurniawati, A., Wati, A. K., and Kusmayadi, A. (2017). The effect of liquid tumeric extract supplementation on carcass production and chemical quality of broiler meat. J. Indonesian Trop. Anim. Agricult. 42, 6–13. doi: 10.14710/jitaa.42.1.6-13
Hussain, Y., Alam, W., Ullah, H., Dacrema, M., Daglia, M., Khan, H., et al. (2022). Antimicrobial potential of curcumin: therapeutic potential and challenges to clinical applications. Antibiotics (Basel, Switzerland) 11:322. doi: 10.3390/antibiotics11030322
Jesumirhewe, C., Tobi, N. U., Owolabi, T. A., Oluwatobi, O. O., and Oluwasegun, A. (2022). The proximate analysis, mineral composition, phytochemical screening and antimicrobial activity of ripe and unripe peel extract of Musa paradisiaca. IJPMBS 2, 280–286. doi: 10.47191/ijpbms/v2-i8-04
Ježek, F., Kameník, J., Macharáčková, B., Bogdanovičová, K., and Bednář, J. (2019). Cooking of meat: effect on texture, cooking loss and microbiological quality – a review. Acta Vet. Brno 88, 487–496. doi: 10.2754/avb201988040487
Jomova, K., Alomar, S. Y., Valko, R., Liska, J., Nepovimova, E., Kuca, K., et al. (2025). Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 413:111489. doi: 10.1016/j.cbi.2025.111489
Katemala, S., Molee, A., Thumanu, K., and Yongsawatdigul, J. (2021). Meat quality and Raman spectroscopic characterization of Korat hybrid chicken obtained from various rearing periods. Poult. Sci. 100, 1248–1261. doi: 10.1016/j.psj.2020.10.027
Khan, R. U., Naz, S., De Marzo, D., Dimuccio, M. M., Bozzo, G., Tufarelli, V., et al. (2022). Aloe vera: a sustainable green alternative to exclude antibiotics in modern poultry production. Antibiotics 12:44. doi: 10.3390/antibiotics12010044
Lawag, I. L., Nolden, E. S., Schaper, A. A. M., Lim, L. Y., and Locher, C. (2023). A modified Folin-Ciocalteu assay for the determination of total phenolics content in honey. Appl. Sci. 13:2135. doi: 10.3390/app13042135
Lee, M. T., Lin, W. C., Yu, B., and Lee, T. T. (2016). Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals — a review. Asian Australas. J. Anim. Sci. 30, 299–308. doi: 10.5713/ajas.16.0438
Li, C., Gao, J., Guo, S., He, B., and Ma, W. (2023). Effects of curcumin on the egg quality and hepatic lipid metabolism of laying hens. Animals 14:138. doi: 10.3390/ani14010138
Liu, H. M., Cheng, M. Y., Xun, M. H., Zhao, Z. W., Zhang, Y., Tang, W., et al. (2023). Possible mechanisms of oxidative stress-induced skin cellular senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. Int. J. Mol. Sci. 24:3755. doi: 10.3390/ijms24043755
Mæhre, H. K., Dalheim, L., Edvinsen, G. K., Elvevoll, E. O., and Jensen, I. J. (2018). Protein determination-method matters. Foods (Basel, Switzerland) 7:5. doi: 10.3390/foods7010005
Malematja, E., Manyelo, T. G., Ng'ambi, J. W., Nemauluma, M. F. D., and Kolobe, S. D. (2023). Effects of onion extracts (Allium cepa) inclusion in diets on growth performance, carcass characteristics, and bone morphometric of broiler chickens. Anim. Biosci. 36, 1075–1082. doi: 10.5713/ab.22.0399
Manso, T., Lores, M., and de Miguel, T. (2021). Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics (Basel). 11, 46–75. doi: 10.3390/antibiotics11010046
Mbaga, S. H., Sanka, Y. D., Katule, A. M., and Mushi, D. (2014). Effects of storage time on the quality of local chicken meat. TAJAS 13, 48–54.
Mudalal, S., Zaazaa, A., and Omar, J. A. (2021). Effects of medicinal plants extract with antibiotic free diets on broilers growth performance and incidence of muscles abnormalities. Braz. J. Poult. Sci. 23:325. doi: 10.1590/1806-9061-2020-1342
Mussa, N. J., Kibonde, S. F., Boonkum, W., and Chankitisakul, V. (2022). The comparison between Tanzanian indigenous (Ufipa breed) and commercial broiler (Ross chicken) meat on the physicochemical characteristics, collagen and nucleic acid contents. Food Sci. Anim. Resour. 42, 833–848. doi: 10.5851/kosfa.2022.e35
Negari, I. P., Isroli, I., and Nurwantoro, N. (2015). The effect of turmeric (Curcuma domestica) extract on water holding capacity, cooking loss, pH values and tenderness of broiler chicken meat. Anim. Prod. 16:188. doi: 10.20884/1.jap.2014.16.3.467
Oyeyinka, B. O., and Afolayan, A. J. (2019). Comparative evaluation of the nutritive, mineral, and antinutritive composition of Musa sinensis L. (banana) and Musa paradisiaca L. (plantain) fruit compartments. Plan. Theory 8:598. doi: 10.3390/plants8120598
Pan, Q., Shen, M., Yu, T., Yang, X., Li, Q., Zhao, B., et al. (2020). Liquid chromatography as candidate reference method for the determination of vitamins A and E in human serum. J. Clin. Lab. Anal. 34:342. doi: 10.1002/jcla.23528
Park, S. Y., Byeon, D. S., Kim, G. W., and Kim, H. Y. (2023). Carcass and retail meat cuts quality properties of broiler chicken meat based on the slaughter age. J. Anim. Sci. Technol. 63, 180–190. doi: 10.5187/jast.2021.e2
Ponnampalam, E. N., Kiani, A., Santhiravel, S., Holman, B. W. B., Lauridsen, C., and Dunshea, F. R. (2022). The importance of dietary antioxidants on oxidative stress, meat and Milk production, and their preservative aspects in farm animals: antioxidant action, animal health, and product quality—invited review. Animals 12:3279. doi: 10.3390/ani12233279
Puvača, N., Brkić, I., Jahić, M., Roljević Nikolić, S., Radović, G., Ivanišević, D., et al. (2020). The effect of using natural or biotic dietary supplements in poultry nutrition on the effectiveness of meat production. Sustain. For. 12:4373. doi: 10.3390/su12114373
Rodríguez-Negrete, E. V., Morales-González, Á., Madrigal-Santillán, E. O., Sánchez-Reyes, K., Álvarez-González, I., Madrigal-Bujaidar, E., et al. (2024). Phytochemicals and their usefulness in the maintenance of health. Plan. Theory 13:523. doi: 10.3390/plants13040523
Saeed, M., Hassan, F., Al-Khalaifah, H., Islam, R., Kamboh, A. A., and Liu, G. (2025). Fermented banana feed and nanoparticles: a new eco-friendly, cost-effective potential green approach for poultry industry. Poult. Sci. 104:105171. doi: 10.1016/j.psj.2025.105171
Sanwo, K. A., Adegoke, A. V., Akinola, O. S., Njoku, C. P., Okolo, S. O., Oladipo, N. A., et al. (2020). Meat quality characteristics of improved indigenous chickens (FUNAAB-alpha) fed turmeric (Curcuma longa) or clove (Syzygium aromaticum) as feed additives. J. Agricult. Sci. Environ. 19, 102–112. doi: 10.51406/jagse.v19i1.2018
Sathyabhama, M., Priya Dharshini, L. C., Karthikeyan, A., Kalaiselvi, S., and Min, T. (2022). The credible role of curcumin in oxidative stress-mediated mitochondrial dysfunction in mammals. Biomol. Ther. 12:1405. doi: 10.3390/biom12101405
Sigolo, S., Milis, C., Dousti, M., Jahandideh, E., Jalali, A., Mirzaei, N., et al. (2021). Effects of different plant extracts at various dietary levels on growth performance, carcass traits, blood serum parameters, immune response and ileal microflora of Ross broiler chickens. Ital. J. Anim. Sci. 20, 359–371. doi: 10.1080/1828051x.2021.1883485
Sionov, R. V., and Steinberg, D. (2022). Targeting the holy triangle of quorum sensing, biofilm formation, and antibiotic resistance in pathogenic Bacteria. Microorganisms 10:1239. doi: 10.3390/microorganisms10061239
Soriano-Santos, J. (2010). Chemical composition and nutritional content of raw poultry meat. Handb. Poul. Sci. Technol. 14, 467–489. doi: 10.1002/9780470504451.ch25
Sultana, S., Lawag, I. L., Lim, L. Y., Foster, K. J., and Locher, C. (2024). A critical exploration of the Total flavonoid content assay for honey. Methods Prot. 7:95. doi: 10.3390/mps7060095
Sureshbabu, A., Smirnova, E., Karthikeyan, A., Moniruzzaman, M., Kalaiselvi, S., Nam, K., et al. (2023). The impact of curcumin on livestock and poultry animal's performance and management of insect pests. Front. Vet. Sci. 10:1048067. doi: 10.3389/fvets.2023.1048067
Tardy, A. L., Pouteau, E., Marquez, D., Yilmaz, C., and Scholey, A. (2020). Vitamins and minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence. Nutrients 12:228. doi: 10.3390/nu12010228
Utami, M. M. D., Dwiani, H. P., and Agus, A. (2020). Addition turmeric extract on ration to reduce fat deposit of broiler. J. Phys. Conf. Ser. 1569:042090. doi: 10.1088/1742-6596/1569/4/042090
Vispute, M. M., Sharma, D., Mandal, A. B., Rokade, J. J., Tyagi, P. K., and Yadav, A. S. (2019). Effect of dietary supplementation of hemp (Cannabis sativa) and dill seed (Anethum graveolens) on performance, serum biochemicals and gut health of broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl.) 103, 525–533. doi: 10.1111/jpn.13052
Wang, D., Huang, H., Zhou, L., Li, W., Zhou, H., Hou, G., et al. (2015). Effects of dietary supplementation with turmeric rhizome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of Wenchang broiler chickens. Ital. J. Anim. Sci. 14:3870. doi: 10.4081/ijas.2015.3870
Wang, M., Mao, Y., Wang, B., Wang, S., Lu, H., Ying, L., et al. (2020). Quercetin improving lipid metabolism by regulating lipid metabolism pathway of ileum mucosa in broilers. Oxidative Med. Cell. Longev. 2020:8686248. doi: 10.1155/2020/8686248
Weyh, C., Krüger, K., Peeling, P., and Castell, L. (2022). The role of minerals in the optimal functioning of the immune system. Nutrients 14:644. doi: 10.3390/nu14030644
Wu, H., Liu, Z., Zhang, Y., Gao, B., Li, Y., He, X., et al. (2024). Chemical composition of turmeric (Curcuma longa L.) ethanol extract and its antimicrobial activities and free radical scavenging capacities. Food Secur. 13:1550. doi: 10.3390/foods13101550
Yusuf, R., Ismadi, V. D. Y. B., Kismiati, S., and Sugiharto, S. (2024). Effect of encapsulated Tahongai (Kleinhovia hospita l.) leaf extract on growth performance, intestinal condition and antioxidative status of broilers raised in high stocking density pens. J. Indonesian Trop. Anim. Agricult. 49, 286–296. doi: 10.14710/jitaa.49.4.286-296
Zakiyatulyaqin, Z., Setiawan, D., and Purnomosidi, M. (2020). The performance of broiler chickens with the addition of Dayak onion extract in drinking water. J. Ternak 11, 91–96. doi: 10.30736/jt.v11i2.77
Keywords: antioxidant, bacteriology, digestibility, hematological parameters, shear strength
Citation: Abdulfattah AM, Alsulaimani F, Basri AM, Alqahtani NK, Uguru H, Eboibi O, Edafiadhe DE, Sami R and Helal M (2025) Synergistic effects of turmeric, banana peels, and onion extracts on broiler performance, mechanical properties, microbial quality, and chicken meat quality assessment. Front. Sustain. Food Syst. 9:1602014. doi: 10.3389/fsufs.2025.1602014
Edited by:
Miguel Cerqueira, International Iberian Nanotechnology Laboratory (INL), PortugalCopyright © 2025 Abdulfattah, Alsulaimani, Basri, Alqahtani, Uguru, Eboibi, Edafiadhe, Sami and Helal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rokayya Sami, cm9rYXl5YS5kQHR1LmVkdS5zYQ==
†ORCID: Ahmed M. Abdulfattah, orcid.org/0009-0002-7822-9537
Fayez Alsulaimani, orcid.org/0009-0001-1897-6955
Ahmed M. Basri, orcid.org/0009-0004-8343-7810
Nashi K. Alqahtani, orcid.org/0000-0002-7190-4256
Hilary Uguru, orcid.org/0000-0002-6132-5082
Okeoghene Eboibi, orcid.org/0000-0002-8169-5423
D. E. Edafiadhe, orcid.org/0000-0001-9115-3229
Rokayya Sami, orcid.org/0000-0003-3162-9453
Mahmoud Helal, orcid.org/0000-0002-3772-012X
 Ahmed M. Abdulfattah
Ahmed M. Abdulfattah Fayez Alsulaimani1,2†
Fayez Alsulaimani1,2† Nashi K. Alqahtani
Nashi K. Alqahtani Hilary Uguru
Hilary Uguru Okeoghene Eboibi
Okeoghene Eboibi Rokayya Sami
Rokayya Sami