- 1Department of Biology, College of Science, University of Ha'il, Ha'il, Saudi Arabia
- 2Department of Biology, Faculty of Sciences, University of Bisha, Bisha, Saudi Arabia
- 3Department of Information and Computer Science, College of Computer Science and Engineering, University of Ha'il, Ha'il, Saudi Arabia
- 4Biology Department, College of Science, University of Jazan, Jazan, Saudi Arabia
Background and objectives: One of main challenges in tomato farming are root-knot nematodes (Meloidogyne incognita). By means of improved plant nutrition, induced systemic resistance, and competitive exclusion, arbuscular mycorrhizal fungus (AMF) helps to control nematodes. An arbuscular mycorrhizal fungus (AMF), Scutellospora heterogama's biocontrol potential is assessed in this work as a non-chemical substitute for synthetic nematicides.
Materials and methods: After 1,000 eggs of M. incognita were injected into tomato seedlings (Solanum lycopersicum), three doses of S. heterogama spores (1,000, 1,250, and 1,500 spores per plant) were treated upon them. Three replicates per treatment and a randomized complete block design were used in greenhouses studies. Evaluated were root colonization, gall index, nematode egg count, and plant biomass. The grid-line intersect approach was used to assess AMF colonization; galling index and egg count helped to measure nematode suppression.
Results: All AMF treatments greatly decreased nematode infestation (from 9.33% in control to 3.78%−4.00%) and increased plant biomass. Optimal 1,250 spore dose would have increased shoot dry weight from 2.14 g to 3.40 g. In treated plants, root colonization came at 89% while in controls it came at 0%. Three sequential experimental replicas carried out under the same controlled greenhouse environment produce the results shown below.
Conclusion: In conclusions Scutellospora heterogama reduces M. incognita stress in tomato quite dramatically. Its application might improve environmentally friendly nematode control. Recommendations for field testing help to confirm its broad relevance.
Introduction
Nematodes are microscopic organisms that parasitize plant roots, affecting trees and crops. They are found in many different locations. Across ecosystems, including deep oceans, arid regions, the Arctic, and geothermal springs. Studies show that nematodes, soil-dwelling microorganisms, have widespread negative consequences (Jones et al., 2013; Palomares-Rius et al., 2021). According to the Commission on Genetic Resources for Food, about 80% of food crops are harmed by parasites, especially nematodes (Allender, 2011).
Nematodes' damage is typically overlooked; thus, it is minimized. Root-knot sedentary endoparasitic nematodes induce the formation of large feeding cells in plant roots (Baldacci-Cresp et al., 2015). Singh et al. (2015) and Poveda et al. (2020) estimate a 12.3% global yield loss caused by plant-parasitic nematodes, totaling ~$157 billion. Bradshaw et al. (2016) estimate that invasive insects produce US$70 billion in harm, but the loss is much worse for nematodes.
Parasitic nematodes modify plant form and metabolism, cause root for king, nutritional deficiencies, and abnormal plant growth (Moens et al., 2009; Tileubayeva et al., 2021). Annual crop losses due to plant-parasitic nematodes are estimated at 14.6% in tropical and subtropical regions (Nicol et al., 2011). Elling projected global agricultural losses caused by these nematodes to reach $157 billion, including $13 billion in the United States alone (Elling, 2013). These figures may be underestimated, as nematode-induced damage often goes undetected or is misattributed to other causes. High-value and root-based crops are particularly vulnerable.
Global use of nematicides, a cornerstone of conventional chemical management, has steadily declined in recent decades. Regulatory bodies have tightened controls in response to health and safety concerns, limiting or banning usage. The environmental impacts of synthetic pesticides have driven interest in natural alternatives (Kim et al., 2005). Nematicides are costly, potentially carcinogenic, environmentally hazardous, and ineffective under poor environmental conditions (Desaeger et al., 2020; Pulavarty et al., 2021).
Numerous pests and diseases, including plant-parasitic nematodes, affect tomato (Solanum lycopersicum L.) quality and productivity. These pests present a significant challenge to food security, particularly in vulnerable agricultural systems. Meloidogyne species are recognized as major root-knot nematodes impacting tomato crops (Mandal et al., 2021; Mukhtar and Kayani, 2020). Historically, root-knot disease in tomatoes has been documented since the late 19th century. Through several channels, arbuscular mycorrhizal fungus (AMF) has become rather successful biological agents for controlling root-knot nematodes. These include improvement of host plant nutrition and vigor, especially by higher phosphorus absorption, which so indirectly increases the plant's resistance against nematode invasion (Wang, 2017). Root shape and architecture are changed by AMF colonization, hence forming a physical barrier preventing nematode access (Schouteden et al., 2015). Furthermore, in plants, AMF can cause systemic resistance (ISR), thereby activating defense-related genes and increasing the synthesis of phytoalexins and defense enzymes (Vos et al., 2013). Additionally seen is competitive exclusion, whereby AMF occupy cortical root cells that might otherwise be targeted by nematodes, therefore limiting the locations for nematode infection (Azcón-Aguilar and Barea, 1997). These synergistic effects place AMF as interesting biocontrol agents in environmentally friendly nematode control plans.
This study investigates the potential of arbuscular mycorrhizal fungi (AMF) as a biological control agent to suppress populations of root-knot nematodes and promote the growth of tomato plants under nematode pressure.
Materials and methods
Study area
The research was carried out in Ha'il City (Figure 1), in the northwestern area of the Kingdom of Saudi Arabia (KSA) (27° 31′ 0″ N, 41° 41′ 0″ E). Ha'il is an agrarian and pastoral region distinguished by abundant water supplies, arable land, and temperate temperatures. Due to its strategic location, Ha'il has historically played a vital role in the Arabian Peninsula. The region benefits from water availability, fertile soil, and a favorable climate, supporting the cultivation of fruits, vegetables, cereals, barley (Hordeum vulgare), and livestock farming.
Nematode sample collection
Root samples showing root-knot nematode symptoms were collected from infected tomato plants. From every 10 symptomatic plants, five root samples were taken, totaling fifty samples. These were transported in labeled polythene bags to the laboratory and stored at 4°C to preserve nematode egg viability for subsequent analysis.
Nematode identification and egg extraction
Eggs were extracted from heavily galled tomato roots using the method (Hussey and Boerma, 1981). Roots were rinsed, chopped into 1–2 cm segments, and blended for 15–20 s. The resulting suspension was mixed with 1.25% sodium hypochlorite (NaOCl) and gently swirled for 3 min to release eggs. The mixture was passed through a 200 to 25 μm sieve sequence, and the retained eggs on the 25 μm sieve were rinsed and collected in distilled water.
Preparation of tomato seedlings
Seeds of tomato cultivar Beto-86 were surface-sterilized using 1.25% NaOCl for 20 min, rinsed thoroughly, and germinated in peat-based media. At the 4–5 leaf stage, seedlings were transplanted into pots containing sterilized 2:1 clay-to-sand mix. Soil sterilization was conducted at 121°C and 15 psi for 30 to 60 min.
Nematode inoculation
Nematode suspensions were prepared by adjusting egg concentrations to ~200 eggs/mL. Using 5 mL of suspension (~1,000 eggs), each plant was inoculated by creating a small hole near the stem and covering it with soil. Controls received 5 mL of sterile distilled water.
Greenhouse conditions
Plants were maintained under controlled conditions (25°C−30°C, 60%−70% RH) with a 12 h light/dark cycle. Soil moisture was monitored using sensors, and watering was done with deionized water as needed. Artificial lighting supplemented natural light to ensure uniform growth.
Arbuscular mycorrhizal fungi (AMF) inoculation
Spores of Scutellospora heterogama were isolated from the rhizosphere of Bermuda grass using wet sieving (250, 180, 63, and 45 μm sieves) and decanting. Viability was confirmed via trypan blue exclusion. AMF suspensions were standardized to 250 spores/mL. Each seedling received 4, 5, or 6 mL of the suspension (1,000, 1,250, or 1,500 spores) applied 5 cm below the roots at transplanting. Controls received equal volumes of sterile distilled water.
Experimental design and treatments
A randomized complete block design (RCBD) with five plants per treatment was used. The experiment included:
• Control (no AMF, no nematode)
• Nematode only
• AMF only
• AMF + Nematode (1,000, 1,250, and 1,500 spores per plant)
Each treatment had three replicated experiments conducted under identical greenhouse conditions. Plants were randomized and repositioned weekly.
Evaluation of root colonization and plant growth
Shoot and root lengths, fresh and dry biomass were recorded at harvest. Root colonization by AMF was quantified using the grid-line intersect method (Giovannetti and Mosse, 1980). Roots were stained with 0.05% ink-acetic acid solution and evaluated microscopically.
Gall and egg mass indexing
Galls and egg masses were stained with 0.15 g/L Phloxine B and evaluated under a stereomicroscope using a 0–5 scale. The same scale was used to assess nematode infection severity.
Measurement of parameters
Root galling was graded on a 0–5 scale; egg masses were counted under a stereomicroscope; the grid-line intersect method was used to estimate mycorrhizal colonization. Harvest saw fresh/dry shoot and root weights recorded (Giovannetti and Mosse, 1980).
Evaluations of root colonization and plant growth
Measuring shoot and root lengths, as well as fresh and dry biomass, allowed one to assess plant growth at the end of the experiment. To check how much arbuscular mycorrhizal fungi had grown on the roots, we used the grid-line intersect method described by Giovannetti and Mosse (1980). Root samples were cleaned, stained with 0.05% ink in acetic acid, and dried for 48 h at 62°C. Colonization was quantified via microscopic inspection (Giovannetti and Mosse, 1980).
The percentage of root colonization was calculated using the following formula:
Root Colonization = Number of intersections with mycorrhizal structures × 100.
Total Number of intersections observed.
Statistical analysis
One-way ANOVA was used to compare treatment effects, and Duncan's Multiple Range Test (DMRT) was applied for mean separation at p < 0.05. Assumptions of normality and variance homogeneity were validated using Shapiro-Wilk and Levene's tests, respectively (Shapiro and Wilk, 1965; Chialva et al., 2023; Duncan, 1955). Effect sizes were also calculated to assess treatment impact.
Results
Effect of mycorrhizal fungi on plant growth and nematode suppression
Dose-response trend
Increasing AMF spore concentrations from 1,000 to 1,250 enhanced plant growth metrics; 1,500 demonstrated a plateau, implying an ideal threshold.
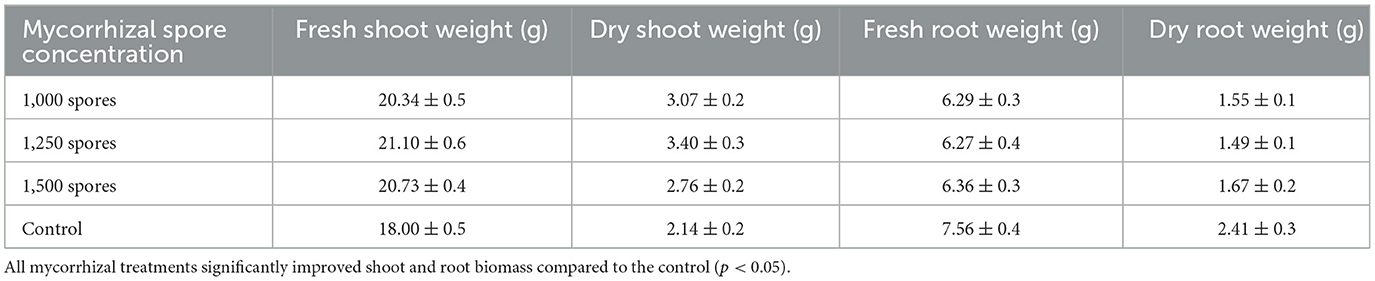
In tomato (Solanum lycopersicum L.), plants infected with Meloidogyne incognita and Scutellospora heterogamy spores improved growth indices and worm control. Mycorrhizal treatments at 1,000, 1,250, and 1,500 spores per plant considerably improved fresh and dry biomass relative to the control group (Table 1).
However, raising the spore concentration above 1,250 did not increase shoot or root biomass, implying a saturation point in mycorrhizal advantage. Furthermore, a notable decrease in nematode-related indicators, including gall formation and egg mass count, was observed in the treated plants (Table 2).
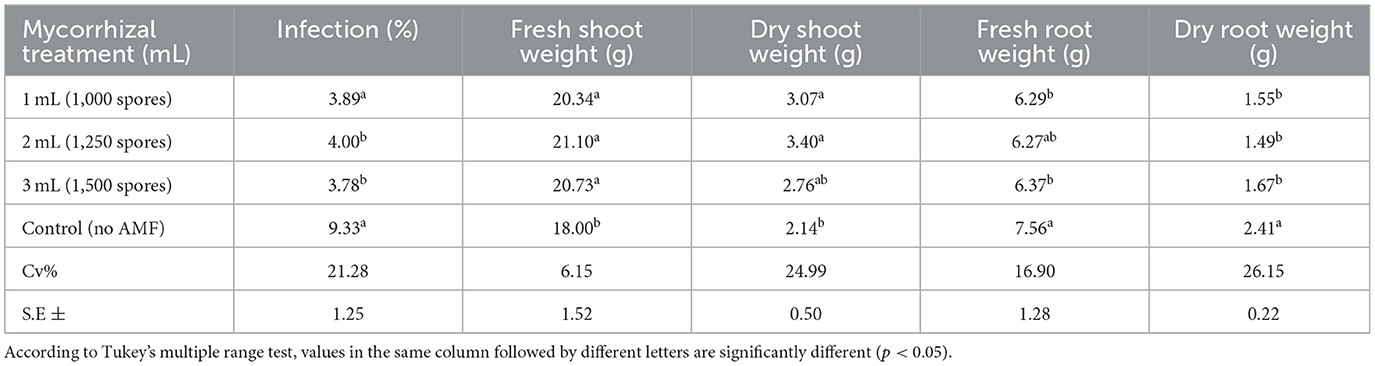
Table 2. Infection rate and growth parameters of tomato plants inoculated with Meloidogyne incognita and treated with Scutellospora heterogamy.
The nematode infection percentage fell from 9.33% in the control to 3.78%−4.00% in treated plants. Likewise, in treated groups, the count of galls and eggs per plant dropped significantly. In injected plants, mycorrhizal colonization rose to 89%, while it was absent in controls.
Dry shoot weight rose from 2.14 g to 3.40 g; fresh shoot weight rose from 18.00 g (control) to 21.10 g (at 1,250 spores). Nematode control reflected root dry weight dropped from 2.41 g in controls to 1.49–1.67 g in treated plants (Tables 2–4; Figure 2).
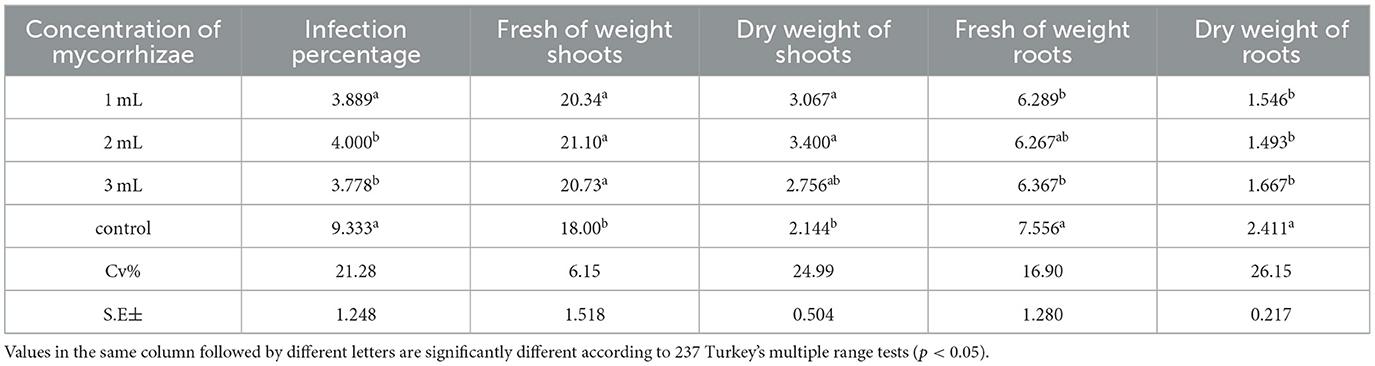
Table 3. Infection % of shoots and root fresh and dry weights of tomato (Solanum lycopersicum L.) plants infected with root-knot nematodes, Melodogyne spp. infected with three concentrations of mycorrhizae.
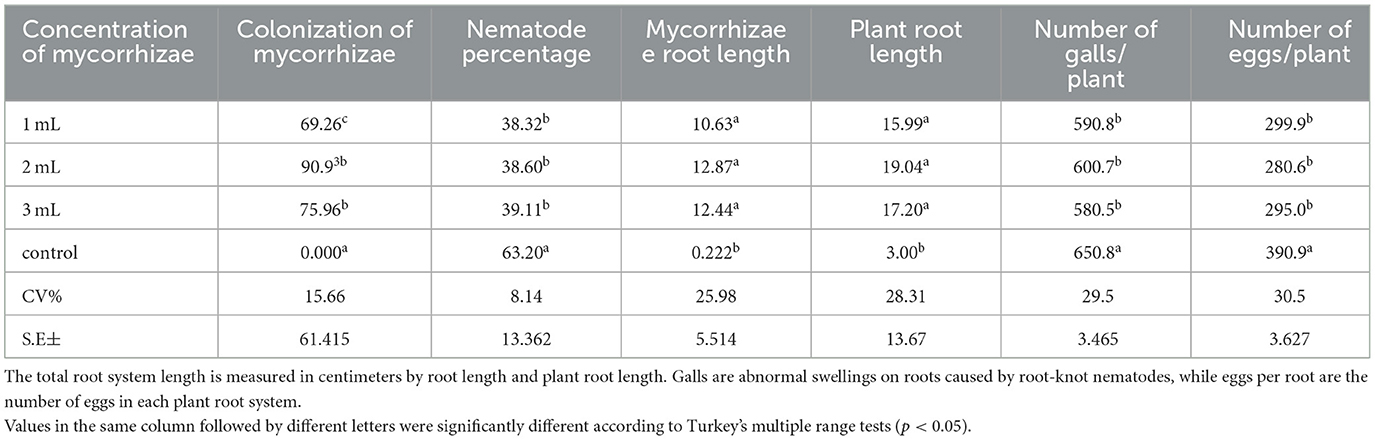
Table 4. The legend shows data from the second growing season, including several mycorrhizal colonies, which indicate the presence and abundance of mycorrhizal fungal structures in plant roots, and the percentage of root-knot nematodes, which is the proportion of Meloidogyne spp. in the soil or plant roots to the total nematode population.

Figure 2. Segments of galled tomato (Solanum lycopersicum L.) in a plastic Petri dish with a five by 5 mm grid.
The several concentrations of nematodes investigated produced diverse degrees of plant reaction. Application of mycorrhizal fungus significantly reduced gall development and inhibited nematode population density in inoculated plants. At 4, 5, and 6 mL (equivalent to 1,000, 1,250, and 1,500 spores, respectively), mycorrhizal treatments reduced nematode infection levels. Mycorrhiza-treated plants demonstrated statistically significant improvements in all evaluated parameters—including root galling, egg mass count, nematode population, and plant growth measures—as shown in Figure 2 against untreated controls.
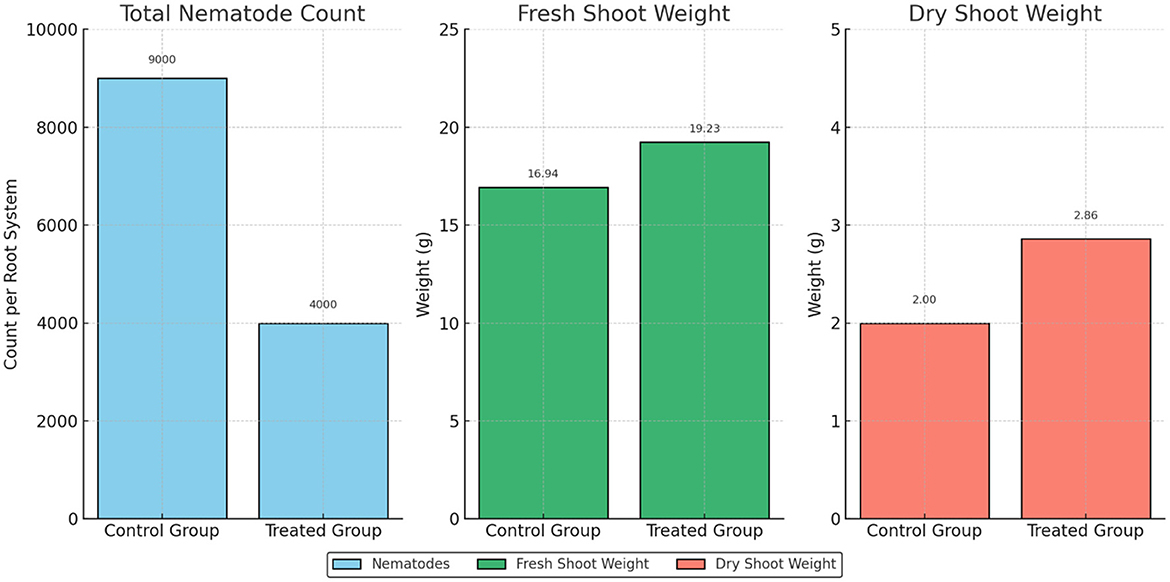
For instance, the total nematode count per root system in the control group was almost 9,000; in the treated group, it dropped to almost 4,000. In plants injected with mycorrhizal fungus, the galls, and egg masses count was likewise much reduced.
Regarding plant development, fresh shoot weight increased from 16.94 g in the control group to 19.23 g in the mycorrhizal treatment group. This suggests that mycorrhizal inoculation increased biomass production. Additional evidence supports this effect, including the increase in shoot dry weight, which rose from 2.00 g in the control plants to 2.86 g in the treated plants (Figure 3).
Unlike 6.6–2 g, the treatment caused fresh and dry roots to weigh less from 5.7 g to 1.1 g (Table 1). Concerning the control zero, the mycorrhizae colonization of 89% in treated plants increased. With a nematode percentage of 62%, mycorrhizae-treated plants were much less than the proportion of 34% of the control plants. Of the treated plants, 34 to 43 percent had roots with mycorrhizae spanning 10 cm when compared to the control group. Whereas, the control plant measured eight centimeters, the treatment plant measured sixteen. Whereas, the number of eggs generated by the control plant rose to 734.7a, the number of eggs generated by the treatment plant dropped to 590 per plant. Furthermore, the number of galls generated by the treated plant dropped to 275 per plant, whereas the number of galls generated by the control plant rose to 328.5 per plant (Table 2).
Seasonal consistency
Reliability of the results was strengthened by similar trends in nematode suppression and growth augmentation reported throughout repetitions.
The treatment resulted in fresh and dry roots weighing less from 5.7 g to 1.1 g, unlike 6.6–2 g (Table 1). Regarding the control zero, the mycorrhizae colonization of 89% in treated plants got higher. Plants treated with a nematode percentage of 62% were far less than the proportion of 34% of the control plants. Comparatively, 34 to 43 percent of the treated plants showed roots with mycorrhizae extending 10 cm compared to the control group. The treatment plant measured 16 m, while the control plant measured eight. The number of eggs produced by the treatment plant was reduced to 590 per plant, while the number of eggs produced by the control plant grew to 734.7a. Moreover, whereas the number of galls produced by the control plant climbed to 328.5 per plant, the number of galls produced by the treated plant reduced to 275 per plant (Table 2).
Discussion
Because of its culinary adaptability, nutritional worth, and economic relevance, tomato (Solanum lycopersicum L.) is among the most grown vegetable crops worldwide. Root-knot nematodes (Meloidogyne spp.) compromise root structure, limit nutrient absorption, and function as persistent soilborne diseases (Phani et al., 2021; Ma et al., 2023), greatly restricting its productivity. Conventional management methods may raise environmental and health-related issues, from chemical nematicides to resistant cultivars. Therefore, biological solutions like arbuscular mycorrhizal fungus (AMF) become increasingly important.
Treatment comparison
Among treatments, 1,250 spores per plant showed the best combination of reduced nematode infection and increased biomass. In this work, tomato plants pre-inoculated with Scutellospora heterogama showed notably increased resistance to Meloidogyne incognita. AMF-treated plants displayed lower gall development, decreased nematode infection rates, and better plant growth than untreated controls. These results fit earlier studies by Calvet et al. (2001) and Talavera et al. (2001), which found that early AMF inoculation promoted symbiosis growth and provided defense against nematodes.
Akbar et al. (2023) also noted that AMF-treated plants showed higher shoot and root biomass, most likely because of improved phosphorus absorption and general nutritional efficiency. These results show AMF's ability to stimulate plant vigor and reduce stress caused by nematodes. Furthermore, supporting AMF's function in limiting nematode growth is the decrease in gall numbers and egg mass generation per root system. The present results confirm findings from earlier research involving various crops and AMF species (Liu et al., 2020; da Silva Campos et al., 2017; Cofcewicz et al., 2001).
AMF's suppressive mechanisms likely include physical barrier creation via improved root architecture, competition for root colonization sites, and induction of systemic resistance (ISR) pathways. Newsham et al. (1995) and Wang (2017) proposed that AMF may prime the plant immune system, enabling faster and stronger defenses against nematode attack.
Field limitations
Results derived from sterilized soil under greenhouse conditions must be validated in field trials to confirm effectiveness under real-world scenarios.
Although the outcomes in a greenhouse environment show promise, using AMF in field conditions presents challenges such as soil variability, microbial competition, and cost of AMF production. Sterilized soil may have artificially boosted AMF colonization, and real soil conditions must be tested through field studies.
The variation in outcomes associated with different concentrations of mycorrhizal inocula is a key factor that warrants further exploration. This study found that inoculation levels (1,000, 1,250, and 1,500 spores per plant) reduced the impact of root-knot nematodes in a dose-dependent manner. Environmental factors such as soil type, moisture, and temperature also influence AMF effectiveness.
According to Detrey et al. (2022), tomato plants had fewer galls when inoculated with AMF. Similarly, Schouteden et al. (2015) showed increased resistance to both M. javanica and M. incognita. This confirms that AMF plays a dual role in growth promotion and biocontrol.
The timing of AMF inoculation is critical. Nematodes invade roots quickly, but AMF colonization takes time. Pre-inoculation offers superior protection, as supported by Rausch et al. (2009) and Sikora et al. (1990).
Using only one AMF species in this work limits the findings. Indigenous or mixed AMF species could provide broader resistance and growth benefits under varied environments. Ultimately, although AMF lowered worm infection and stimulated development, the study did not assess long-term resistance under repeated nematode infestation or secondary pathogen attack. Future studies should explore this interaction in more depth. The findings support integrating AMF into sustainable pest management strategies for tomato crops.
Strengths and weaknesses
This work has great merit in its controlled design, unambiguous dose-response pattern, and exhaustive quantification of plant growth and nematode reduction. Limitations include dependency on greenhouse settings with sterilized soil and absence of long-term studies, nevertheless. Next field research should fill in these voids.
Our findings reaffirm AMF's potential in integrated pest management (IPM) frameworks and warrant further evaluation in diverse agroecological zones.
Conclusions
In conclusion, our work supports the possible biocontrol capacity of Scutellospora heterogamy against Meloidogyne incognita in tomatoes. Important next steps for converting these discoveries into useful agricultural uses, meanwhile, are improving inoculation time, investigating several AMF species, and confirming efficacy in field settings.
Mycorrhizal fungus considerably lowers root-knot nematode infestation, increasing tomato plant development. Improving plant defenses and nitrogen absorption is a natural and sustainable method that reduces the stress in agriculture by nematodes. Mycorrhiza can be combined with organic additions, less tillage, and crop rotation techniques for pest control. Still, good implementation calls for farmer education and economic viability analysis. Future research should consider natural field trials, dose-response models, temporal investigations, and knowledge of species interactions for best colonization.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AS: Conceptualization, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. GE: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. NAA: Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. MA: Funding acquisition, Software, Supervision, Writing – original draft, Writing – review & editing. AA: Methodology, Resources, Writing – original draft, Writing – review & editing. NSA: Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We express our gratitude to the Ha'il University for their financing support for this project (R.G. 23/194).
Acknowledgments
The University of Hail Project (RG-23/194) financed this study. We are grateful to the University of Hail KSA for the financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akbar, M., Chohan, S. A., Yasin, N. A., Ahmad, A., Akram, W., and Nazir, A. (2023). Mycorrhizal inoculation enhanced tillering in field grown wheat, nutritional enrichment and soil properties. PeerJ. 11:e15686. doi: 10.7717/peerj.15686
Allender, C. (2011). The Second Report on the State of the World's Plant Genetic Resources for Food and Agriculture. Rome: Food and Agriculture Organization of the United Nations.
Azcón-Aguilar, C., and Barea, J. M. (1997). Arbuscular mycorrhizas and biological control of soil-borne plant pathogens–an overview of the mechanisms involved. Mycorrhiza 6, 457–464. doi: 10.1007/s005720050147
Baldacci-Cresp, F., Maucourt, M., Deborde, C., Pierre, O., Moing, A., Brouquisse, R., et al. (2015). Maturation of nematode-induced galls in Medicago truncatula is related to water status and primary metabolism modifications. Plant Sci. 232, 77–85. doi: 10.1016/j.plantsci.2014.12.019
Bradshaw, C. J., Leroy, B., Bellard, C., Roiz, D., Albert, C., Fournier, A., et al. (2016). Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 7, 1–8. doi: 10.1038/ncomms12986
Calvet, C., Pinochet, J., Hernández-Dorrego, A., Estaún, V., and Camprubí, A. (2001). Field microplot performance of the peach-almond hybrid GF-677 after inoculation with arbuscular mycorrhizal fungi in a replant soil infested with root-knot nematodes. Mycorrhiza 10, 295–300. doi: 10.1007/PL00009998
Chialva, M., Patono, D. L., de Souza, L. P., Novero, M., Vercellino, S., Maghrebi, M., et al. (2023). The mycorrhizal root-shoot axis elicits Coffea arabica growth under low phosphate conditions. New Phytol. 239, 271–285. doi: 10.1111/nph.18946
Cofcewicz, E. T., Medeiros, C. A., Carneiro, R. M., and Pierobom, C. R. (2001). Interação dos fungos micorrízicos arbusculares Glomus etunicatum e Gigaspora margarita e o nematóide das galhas Meloidogyne javanica em tomateiro. Fitopatol. Bras. 26, 65–70. doi: 10.1590/S0100-41582001000100011
da Silva Campos, M. A., Da Silva, F. S. B., Yano-Melo, A. M., De Melo, N. F., and Maia, L. C. (2017). Application of arbuscular mycorrhizal fungi during the acclimatization of Alpinia purpurata to induce tolerance to Meloidogyne arenaria. Plant Pathol. J. 33, 329–336. doi: 10.5423/PPJ.OA.04.2016.0094
Desaeger, J., Wram, C., and Zasada, I. (2020). New reduced-risk agricultural nematicides-rationale and review. J. Nematol. 52, 1–16. doi: 10.21307/jofnem-2020-091
Detrey, J., Cognard, V., Djian-Caporalino, C., Marteu, N., Doidy, J., Pourtau, N., et al. (2022). Growth and root-knot nematode infection of tomato are influenced by mycorrhizal fungi and earthworms in an intercropping cultivation system with leeks. Appl. Soil Ecol. 169:104181. doi: 10.1016/j.apsoil.2021.104181
Duncan, D. B. (1955). Multiple range and multiple F tests. biometrics 11, 1–42. doi: 10.2307/3001478
Elling, A. A. (2013). Major emerging problems with minor Meloidogyne species. Phytopathol. 103, 1092–1102. doi: 10.1094/PHYTO-01-13-0019-RVW
Giovannetti, M., and Mosse, B. (1980). An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 489–500. doi: 10.1111/j.1469-8137.1980.tb04556.x
Hussey, R. S., and Boerma, H. R. (1981). A greenhouse screening procedure for root-knot nematode resistance in soybeans1. Crop Sci. 21, 794–796. doi: 10.2135/cropsci1981.0011183X002100050041x
Jones, J. T., Haegeman, A., Danchin, E. G., Gaur, H. S., Helder, J., Jones, M. G., et al. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Molecul. Plant Pathol. 14, 946–961. doi: 10.1111/mpp.12057
Kim, D. I., Park, J. D., Kim, S. G., Kuk, H., Jang, M. S., and Kim, S. S. (2005). Screening of some crude plant extracts for their acaricidal and insecticidal efficacies. J. Asia Pac. Entomol. 8, 93–100. doi: 10.1016/S1226-8615(08)60076-X
Liu, H., Wu, M., Liu, J., Qu, Y., Gao, Y., and Ren, A. (2020). Tripartite interactions between endophytic fungi, arbuscular mycorrhizal fungi, and Leymus chinensis. Microbial. Ecol. 79, 98–109. doi: 10.1007/s00248-019-01394-8
Ma, M., Taylor, P. W., Chen, D., Vaghefi, N., and He, J. Z. (2023). Major soilborne pathogens of field processing tomatoes and management strategies. Microorganisms 11:263. doi: 10.3390/microorganisms11020263
Mandal, H. R., Katel, S., Subedi, S., and Shrestha, J. (2021). Plant parasitic nematodes and their management in crop production: a review. J. Agric. Nat. Res. 4, 327–338. doi: 10.3126/janr.v4i2.33950
Moens, M., Perry, R. N., and Starr, J. L. (2009). “Meloidogyne species-a diverse group of novel and important plant parasites,” in Root-knot Nematodes (Wallingford: CABI), 1–17.
Mukhtar, T., and Kayani, M. Z. (2020). Comparison of the damaging effects of Meloidogyne incognita on a resistant and susceptible cultivar of cucumber. Bragantia 79, 83–93. doi: 10.1590/1678-4499.20190359
Newsham, K. K., Fitter, A. H., and Watkinson, A. R. (1995). Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 10, 407–411. doi: 10.1016/S0169-5347(00)89157-0
Nicol, J. M., Turner, S. J., Coyne, D. L., Nijs, L.D, Hockland, S., and Maafi, Z. T. (2011). “Current nematode threats to world agriculture,” in Genomics and Molecular Genetics of Plant-Nematode Interactions, eds. J. Jones, G. Gheysen, and C. Fenoll (Dordrecht: Springer), 21–43. doi: 10.1007/978-94-007-0434-3_2
Palomares-Rius, J. E., Hasegawa, K., Siddique, S., and Vicente, C. S. (2021). Protecting our crops-approaches for plant parasitic nematode control. Front. Plant Sci. 12:726057. doi: 10.3389/fpls.2021.726057
Phani, V., Khan, M. R., and Dutta, T. K. (2021). Plant-parasitic nematodes as a potential threat to protected agriculture: current status and management options. Crop Prot. 144:105573. doi: 10.1016/j.cropro.2021.105573
Poveda, J., Abril-Urias, P., and Escobar, C. (2020). Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 11:992. doi: 10.3389/fmicb.2020.00992
Pulavarty, A., Egan, A., Karpinska, A., Horgan, K., and Kakouli-Duarte, T. (2021). Plant parasitic nematodes: a review on their behaviour, host interaction, management approaches and their occurrence in two sites in the republic of Ireland. Plants 10:2352. doi: 10.3390/plants10112352
Rausch, S., Huehn, J., Loddenkemper, C., Hepworth, M. R., Klotz, C., Sparwasser, T., et al. (2009). Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur. J. Immunol. 39, 3066–3077. doi: 10.1002/eji.200939644
Schouteden, N., De Waele, D., Panis, B., and Vos, C. M. (2015). Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front. Microbiol. 6:1280. doi: 10.3389/fmicb.2015.01280
Shapiro, S. S., and Wilk, M. B. (1965). An analysis of variance test for normality (complete samples). Biometrika 52, 591–611. doi: 10.1093/biomet/52.3-4.591
Sikora, R. A., Greco, N., and Silva, J. F. V. (1990). “Nematode parasites of food legumes,” in Plant Parasitic Nematodes in Subtropical and Tropical Agriculture (Wallingford: CAB International), 259–318.
Singh, S., Singh, B., and Singh, A. P. (2015). Nematodes: a threat to the sustainability of agriculture. Procedia Environ. Sci. 29, 215–216. doi: 10.1016/j.proenv.2015.07.270
Talavera, M., Itou, K., and Mizukubo, T. (2001). Reduction of nematode damage by root colonization with arbuscular mycorrhiza (Glomus spp.) in tomato-Meloidogyne incognita (Tylenchida: Meloidogynidae) and carrot-Pratylenchus penetrans (Tylenchida: Pratylenchidae) pathosystems. Appl. Entomol. Zool. 36, 387–392. doi: 10.1303/aez.2001.387
Tileubayeva, Z., Avdeenko, A., Avdeenko, S., Stroiteleva, N., and Kondrashev, S. (2021). Plant-parasitic nematodes affecting vegetable crops in greenhouses. Saudi J. Biol. Sci. 28, 5428–5433. doi: 10.1016/j.sjbs.2021.05.075
Vos, C., Schouteden, N., van Tuinen, D., Chatagnier, O., Elsen, A., De Waele, D., et al. (2013). Mycorrhiza-induced resistance against the root–knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 60, 45–54. doi: 10.1016/j.soilbio.2013.01.013
Keywords: nematicide, arbuscular mycorrhizal fungi, tomato, synthetic pesticides, pathogen
Citation: Sulieman AME, Elbadri GM, Alanazi NA, Alazmi M, Alrashidi A and Alothman NS (2025) Mitigation of root-knot nematode infestation in tomato plants by mycorrhizal fungi. Front. Sustain. Food Syst. 9:1609286. doi: 10.3389/fsufs.2025.1609286
Received: 10 April 2025; Accepted: 02 June 2025;
Published: 23 June 2025.
Edited by:
Matteo Balderacchi, Independent Researcher, Piacenza, ItalyReviewed by:
Stanlous Juma Waswa, Kibabii University, KenyaFatma Gül Göze Özdemir, Isparta University of Applied Sciences, Türkiye
Copyright © 2025 Sulieman, Elbadri, Alanazi, Alazmi, Alrashidi and Alothman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdel Moneim Elhadi Sulieman, YW0uc3VsaWVtYW5AdW9oLmVkdQ==
 Abdel Moneim Elhadi Sulieman
Abdel Moneim Elhadi Sulieman Gamal Mohammed Elbadri2
Gamal Mohammed Elbadri2 Meshari Alazmi
Meshari Alazmi