- 1International Centre of Insect Physiology and Ecology (icipe), Nairobi, Kenya
- 2Tamil Nadu Dr. J. Jayalalithaa Fisheries University, Nagapattinam, India
- 3Kenya Marine and Fisheries Research Institute (KMFRI), Sangoro Research Centre, Pap-Onditi, Kenya
- 4Kenya Marine and Fisheries Research Institute (KMFRI), National Aquaculture Research Development and Training Centre, Sagana, Kenya
- 5WorldFish, Bayan Lepas, Malaysia
The global significance of mycotoxins in aquaculture is evident. However, regional vulnerabilities, effects, and inconsistent regulations on mycotoxin contamination remain underexplored. This study integrates a scientometric analysis of research on mycotoxins in aquafeed, published from 1992 to 2023 in Web of Science, with a conventional review of their occurrence in aquafeed and feed ingredients. Bibliometric tools, VOSviewer, and biblioshiny, were used to analyze global research trends, collaborations, and themes. We found a total of 181 publications, authored by 938 researchers from 49 countries, with Brazil leading (25 publications). The Toxins journal accounted for the most publications (23). Aflatoxins, particularly aflatoxin B1, were the most reported mycotoxins, alongside fumonisins, deoxynivalenol, and zearalenone. Mycotoxin occurrence was highest in tropical regions, particularly in East African countries (aflatoxins, fumonisins, deoxynivalenol, acetyldeoxynivalenol, ochratoxin A, roquefortine C, alternariol, T-2 toxin, zearalenone, and zivalenol), and the Southeast Asian countries (aflatoxins, fumonisins, deoxynivalenol, zearalenone, and ochratoxin A), where climatic conditions exacerbate fungal growth and mycotoxin production. The findings highlight the global regulations on mycotoxins, the risks associated with the different mycotoxins, and their effects on the health of fish and humans. Our findings emphasize the need for stringent monitoring and regulation of mycotoxins in aquafeeds. Future research should focus on developing effective mitigation strategies and understanding the regional variations in mycotoxin prevalence to safeguard aquaculture productivity and consumer health.
1 Introduction
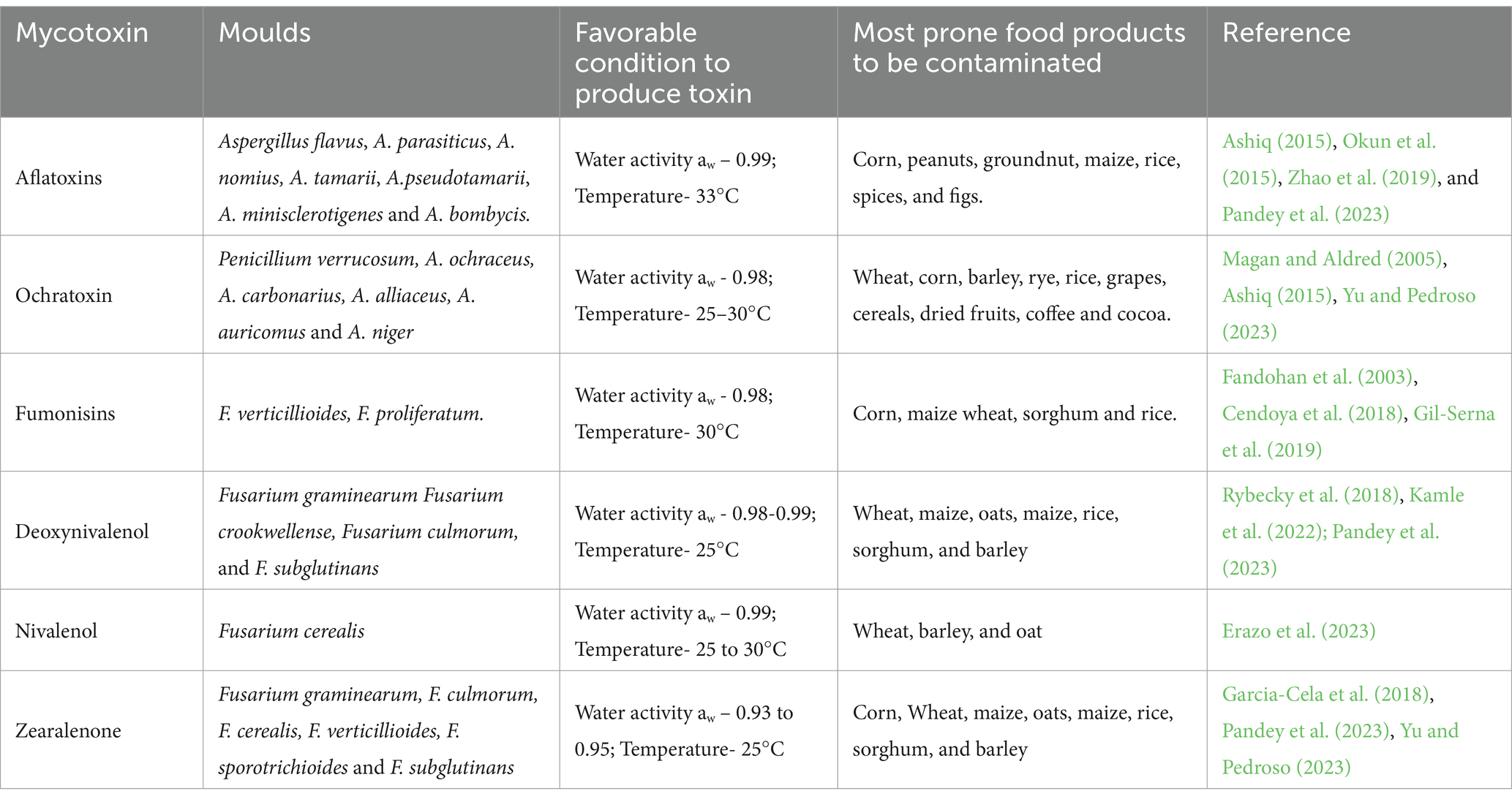
The term “mycotoxin” is derived from the Greek word “mykes” meaning fungus (mould), and the Latin word “toxicum” meaning poison; as such, mycotoxins refer to poisons produced by fungi (Iqbal et al., 2016a, 2016b; Mwihia et al., 2020). The intoxications that occur in animals and people due to ingesting one or more mycotoxins into the body is referred to as mycotoxicosis, and can cause sickness or death (Gallo et al., 2015; Patil and Kakde, 2017). The primary health impact of mycotoxin exposure is due to chronic poisoning (Krogh, 1969). Toxigenic fungi can be categorized into two groups: field fungi (e.g., Alternaria and Fusarium spp.), which access the crop during the development of the plant, or storage fungi (e.g., Aspergillus spp., Penicillium spp.), which mostly contaminate the crop post-harvest (Magan and Lacey, 1984; Kebede et al., 2020). Moreover, poor hygienic practices during transportation and storage, such as high temperature, heavy rain, and high moisture content, are the main predisposing factors which play an important role on mycotoxins production (Kebede et al., 2020). Detecting these fungi in feed or its raw materials does not necessarily mean that mycotoxins will contaminate them (Oliveira and Vasconcelos, 2020). These fungi invade stored feed crops and other farm products used in animal feed production, mostly maize, groundnuts and cotton (Thompson and Henke, 2000; Mwihia et al., 2018).
Mycotoxins have diverse structures with varying biological effects in their chemical form, which can cause cancer, mutation, neural damage, or suppression of the immune system in humans (Ahmed et al., 2017; Shahba et al., 2021). Aspergillus, Penicillium, and Fusarium are the most common genera of mycotoxigenic fungus, but Trichoderma, Trichothecium, and Alternaria are also present as food contaminants or plant pathogens, causing huge economic and health impacts (Richard, 2007; Viegas et al., 2015; Albero et al., 2022). At present, about 300 to 400 mycotoxin types have been identified (Berthiller et al., 2007), with aflatoxins (AFs) (AFB1, B2, G1, and G2) and fumonisins (FBs) (FB1, FB2, and FB3) being the most significant (Pitt, 2000; Cimbalo et al., 2020). They show a great structural diversity, resulting in different chemical and physicochemical properties. Generally, mycotoxins are chemically and thermally stable compounds, capable of surviving storage and most cereal production processes (Köppen et al., 2010). Aside from AFs and FBs, ochratoxin A (OTA), zearalenone, and trichothecenes (THs) are also significant (Bryden, 2012; Marroquín-Cardona et al., 2014; Cimbalo et al., 2020).
Contamination of food items with dangerous and unfavorable compounds restricts their trade in international markets (Frenich et al., 2014). Contamination by mycotoxins can cause degradation and decrease the dietary value of the feed materials (Chukwudi et al., 2021). Animal feed contamination with mycotoxins due to mold growth on living and stored plants presents a global challenge to farmers (Nakavuma et al., 2020). Essentially, raw materials for compound food and feeds are good substrates for mold growth; the Food and Agricultural Organization (FAO) estimated up to 25% of the world’s food crops and a significant proportion of the world’s animal feedstuff are contaminated by mycotoxins (Nakavuma et al., 2020).
A variety of environmental factors influence the presence of mycotoxins in food (Koletsi et al., 2021). Climate is the key driver of mycotoxin production by altering environmental conditions that affect fungal growth and toxin biosynthesis (Marroquín-Cardona et al., 2014). Rising global temperatures create favorable conditions for toxigenic fungi such as Aspergillus and Fusarium, leading to increased production of mycotoxins (Medina et al., 2017). Changes in precipitation patterns, such as prolonged droughts, followed by sudden rainfall, create stress conditions that weaken crops, making them more susceptible to fungal invasion, enhancing mycotoxin contamination in agricultural produce (Battilani et al., 2016). Additionally, climate change can lead to shifts in fungal populations, where non-toxigenic species are outcompeted by toxigenic strains, further exacerbating the risk of mycotoxin contamination in new geographical areas (Moretti et al., 2019). Post-harvest conditions are also affected, as increased temperatures and humidity levels in storage facilities create ideal environments for fungal growth and secondary contamination. Inadequate drying and poor storage conditions under warmer climates contribute to the persistence and proliferation of mycotoxins in stored food and feed products (Paterson and Lima, 2010). Preventing this contamination is critical because various products might be contaminated along the animal production chain, and it is difficult to identify the infected product (Magnoli et al., 2019).
The occurrence of mycotoxin in livestock was emphasized after the epidemics of aflatoxicosis in the 1960s in farm-reared turkeys (Meleagris gallopavo) in the UK and rainbow trout (Oncorhynchus mykiss) in the USA (Monson et al., 2015; Gonçalves et al., 2018). The source of contamination was found to be raw materials like peanut meal for turkeys and cottonseed meal for trout used in feed formulation (Wolf and Jackson, 1963; Kumar et al., 2013). Mycotoxin in fish feeds (henceforth referred to as aquafeed in this paper) is a significant concern, particularly in tropical climates and developing nations where farmers frequently produce aquafeeds under unsuitable conditions, including incorrect milling and storage (Marijani et al., 2019). To regulate mycotoxins in food, nodal agencies like the Food and Drug Administration of the USA (FDAF, 2021) and the European Commission [European Commission (EC), 2006] have set regulations for storage and maximum residual limits (MRLs) (Chong, 2022). A report from the Food and Agriculture Organization of the United Nations (Food and Agriculture Organization, 2003) states that the establishment of regulations on mycotoxins can be influenced by scientific factors such as data on the occurrence of mycotoxin in different commodities, including the toxicity of mycotoxin and their effects, the detection and analytical methods for mycotoxins, and the legislative elements regarding the control of mycotoxins. As research on mycotoxins in aquafeeds develops in response to the expanding health concerns, scientists face information constraints that may stymie creative investigation and scholarly collaboration. Conventional literature review studies are often performed to understand the extent of existing knowledge in a given domain (e.g., mycotoxins in aquafeeds). For instance, Matejova et al. (2017) reviewed the effects of mycotoxins on cultured fish. Marijani et al. (2019) reviewed the fungal and mycotoxin contamination of aquafeed, feed ingredients, and their effects on fish health. Oliveira and Vasconcelos (2020) reviewed the most important mycotoxins found in crops and in finished aquafeed, their effects on the health of fish and humans, and their regulations in the European Union.
Although these studies are relevant and provide valuable insights, they do not encompass the full scope of knowledge and research trends on mycotoxins in aquafeeds. This limitation is expected given the extensive and growing body of research in this area. The conventional study review process may be too subjective to fully capture the body of knowledge on mycotoxins in aquafeeds since it draws from a proportion of existing published research and may not capture the whole of the knowledge on the topic. This is because conventional review studies fail to accurately and thoroughly connect various elements within the literature (Amin et al., 2022). Consequently, it is crucial to develop and use an approach that allows scientists to gather essential information from multiple sources simultaneously. This limitation could be addressed by a scientometric review approach using a software tool. In order to produce statistical indexes that show research dynamics and emerging trends, scientometric reviews statistically analyze the relationships between various scientific publications (Andriamamonjy et al., 2019). This study intends to conduct a combination of a conventional review of mycotoxins in aquafeeds and a scientometric analysis of bibliographic records published on the topic up to 2023. Specifically, the goals of this study are to (I) identify the global publication trend on mycotoxin in aquafeed; (II) identify the most influential countries, authors, journals, articles, and keywords in the area of mycotoxin in aquafeed; (III) provide an overview of mycotoxin occurrence in aquafeeds; and (IV) identify the gaps and future research directions concerning this topic.
2 Methodology
This research was based on a scientometric analysis of studies on mycotoxins in aquafeed published between 1982 and 2023. To achieve the research objectives, academic publications within the field were identified in the Web of Science (WoS) database, the most reliable and prominent data source comprising the leading journals worldwide (Chadegani et al., 2013; Iftikhar et al., 2023). Both the Web of Science Core Collection (WoSCC) and Scopus are popular search tools for bibliometric analysis. Considering the long history and wide recognition of the WoSCC, as well as its rich citation indexing capabilities, we selected the WoSCC as the search tool (Li et al., 2024). WoSCC is widely recognized as a reliable source for bibliometric research, encompassing the Science Citation Index Expanded (SCI-EXPANDED), Social Sciences Citation Index (SSCI), Arts and Humanities Citation Index (A&HCI), Conference Proceedings Citation Index-Science (CPCI-S), Conference Proceedings Citation Index-Social Science and Humanities (CPCI-SSH), and Emerging Sources Citation Index (ESCI) (Yan et al., 2024). Therefore, the WoS database was used as the source of information in this study. It contains an extensive metadata collection, including author lists, abstracts, references, citation counts, organizations, journal impact factors, and nations (Gaviria-Marin et al., 2019; Wilson et al., 2021).
The search string was constructed using Boolean operators (OR and AND), an asterisk (*) symbol, and quotation marks (“”) in the Topics search (TS) of the WoSCC database. The asterisk symbol was used to account for variations in keywords, and the quotation marks were used to ensure that the keywords were interpreted with exact meanings. TS = (“Mycotoxin*” OR “Mycotoxicosis” OR “Aflatoxin*” OR “AFB1” OR “Aflatoxicosis” OR “Zearalenone*” OR “Ochratoxin*” OR “Fumonisin*” OR “T-2 toxin” OR “Patulin” OR “Trichothecene*” OR “Deoxynivalenol” OR “Vomitoxin”) AND TS = (“Aquafeed*” OR “Shrimp feed*” OR “Aquaculture feed*” OR “Aquafeed Ingredient*” OR “Aquaculture” OR “Aqua farming” OR “Aquafeed”). After removing the duplicated and irrelevant literature, 181 papers were selected and used for further bibliometric and visualized analysis.
The types of documents that were considered for inclusion in the study were journal articles and conference papers. The source types “journal” and “conference proceedings” were selected. The “year of publication” limitation was set from “1982 to 2023” and there was no language restriction. No grey literature was included in this study. To uphold data integrity throughout our bibliometric analysis, we implemented a meticulous three-step process encompassing data cleaning, duplicate removal, and validation. Initially, we standardized author names, institutional affiliations, and keywords to address inconsistencies and variations, ensuring uniformity across the dataset. Subsequently, we identified and eliminated duplicate records to prevent redundancy and potential skewing of results. Finally, we conducted a thorough cross-verification of the dataset to confirm the accuracy of publication counts and citation metrics. These steps align with established best practices in bibliometric research, as highlighted in recent literature. After applying these conditions, a total of 181 records were maintained.
The records were saved in the text files (.txt) files for further evaluation using appropriate computer software. The extracted raw data was mapped using VOSviewer (Van Eck and Waltman, 2010) and Biblioshiny (Aria and Cuccurullo, 2017). VOSviewer is a mapping tool that is easily available, based on open source software, utilized in various disciplines, and recommended by scientists (van Eck and Waltman, 2010; Wong, 2018). Biblioshiny is an open source tool programmed in R and designed to perform extensive scientific analysis. It enables creating thematic maps based on information found in the dataset, such as institutions, countries and keywords (Aria and Cuccurullo, 2017). The VOS viewer bibliometric analysis software was preferred for this study over other similar software mainly because of its efficiency in analyzing research outputs in clusters. Additionally, VOS viewer constructs and offers a visual representation of research networks in bibliometric analysis (Obileke et al., 2020; George et al., 2021). As a result, the objectives of this study are effectively achieved through the application of VOSviewer and Biblioshiny. The.txt files that were generated were imported into VOSviewer, and additional analysis was carried out while ensuring that the data’s integrity and consistency were not compromised. The publications types and sources, keywords, authors with the most citations, and the countries and institutions were all assessed in the bibliographic analysis. Statistical results were reported in tables and figures, while their relationships and term co-occurrence were shown using maps. The flowchart of the scientometric review process is shown in Figure 1. However, for a more detailed and better grasp of the topic, a traditional review of existing body of literature related to mycotoxins in aquafeed was performed in this study. Emphasis was placed on the occurrence of mycotoxins in aquafeeds, specifically on documented mycotoxin occurrence in aquafeeds, raw materials used in aquafeed, and the risk of contamination by various mycotoxins. Additionally, the detection methods for mycotoxins in aquafeeds and the maximum permissible limits of mycotoxins in aquafeed and animal feed were identified. As a result, this paper combines scientometric analysis with a traditional literature review to better describe the main research interest and focus of mycotoxins in aquafeed.
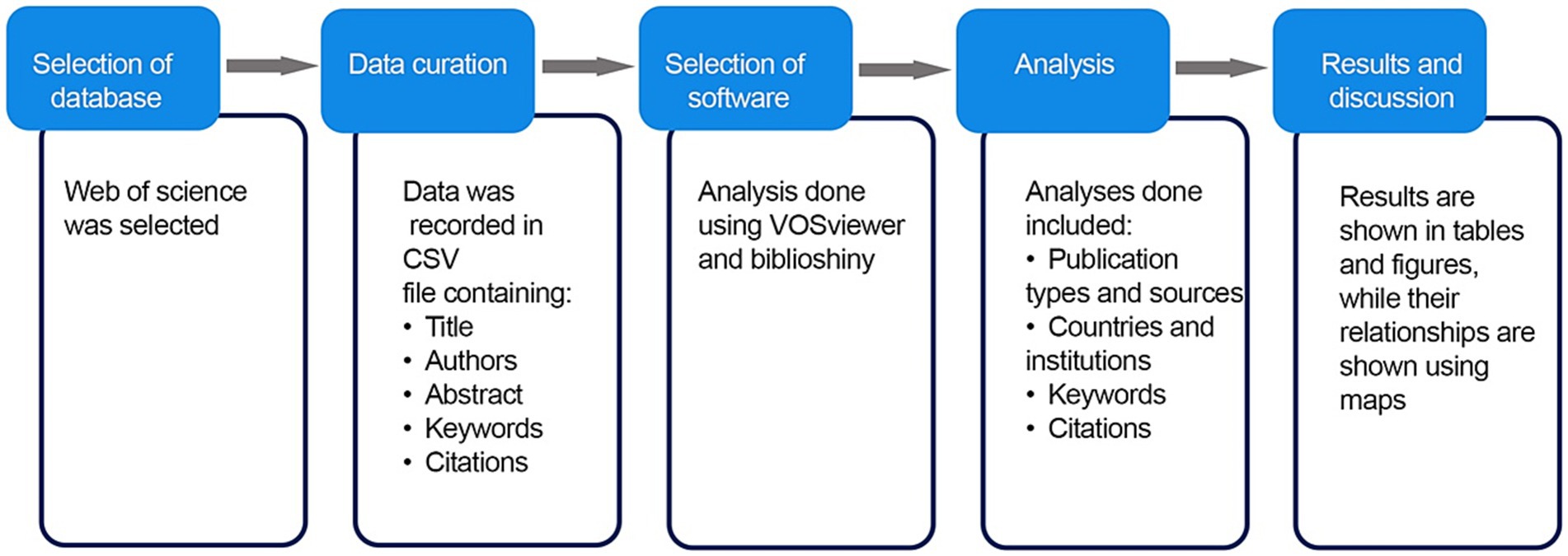
Figure 1. Flowchart of the scientometric review process from the database selection to the analysis and discussion of the results.
3 Results
3.1 Main information extracted
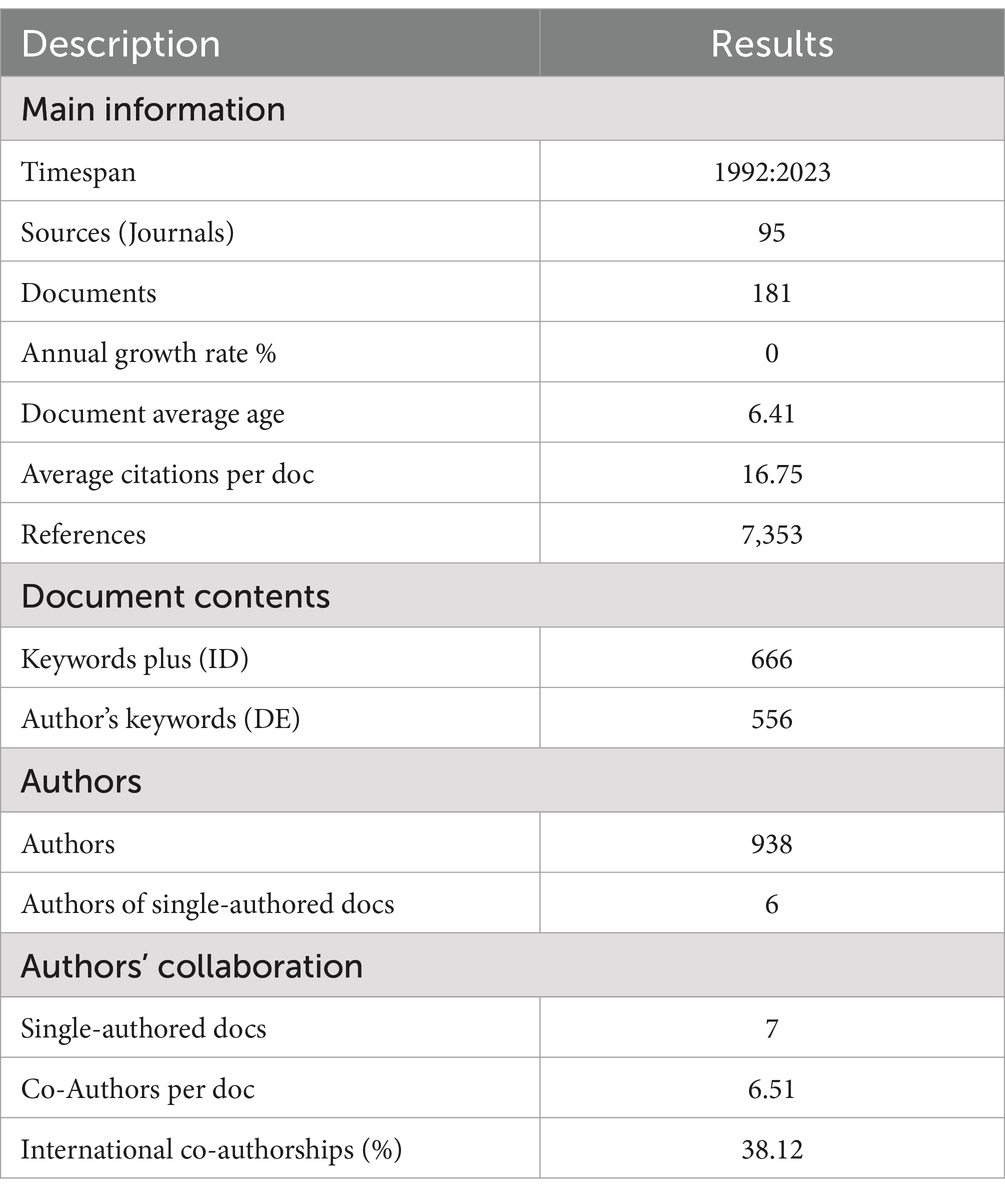
The main information extracted from the analysis is summarized in Table 1. There were 181 publications in the field of “mycotoxins” and “aquafeed” on the Web of Science from 1992 to 2023, written by 938 authors. The data collection had 2,517 citations in total as of May 2023, with a mean of 16.75 citations per document. Out of the 181 publications, 178 were in English, and only three were in other languages: French (1), Turkish (1) and Polish (1).
3.2 Yearly publications
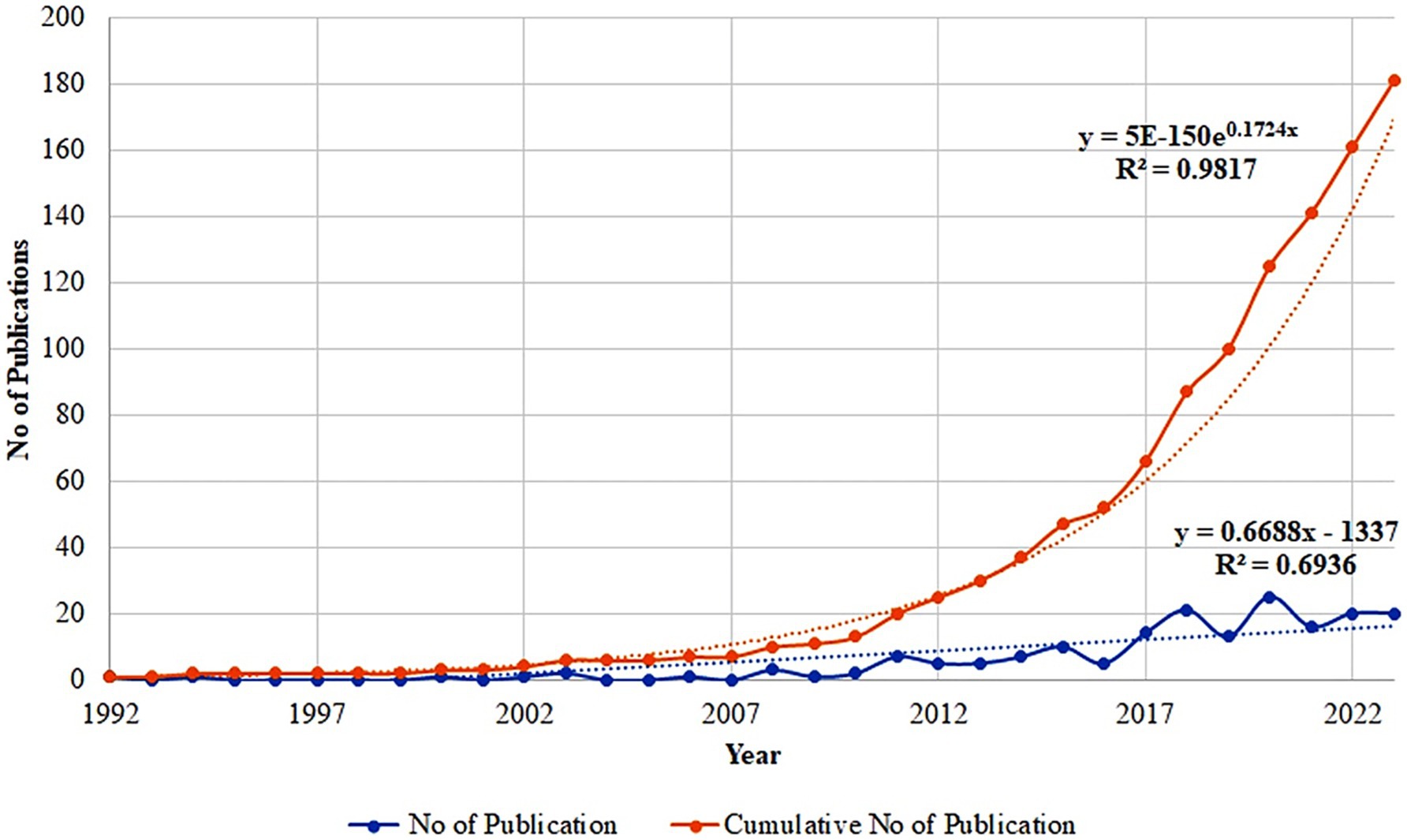
The yearly trend in publications in the present research area from 1992 to 2023 is depicted in Figure 2. A slow increase in the number of publications was observed, with the yearly publications increasing from 1 in 1992 to 20 in 2023. The highest number of publications was recorded in 2020, followed by 2022 and 2023 (25, 20 and 20 articles, respectively).
3.3 Publication types and sources
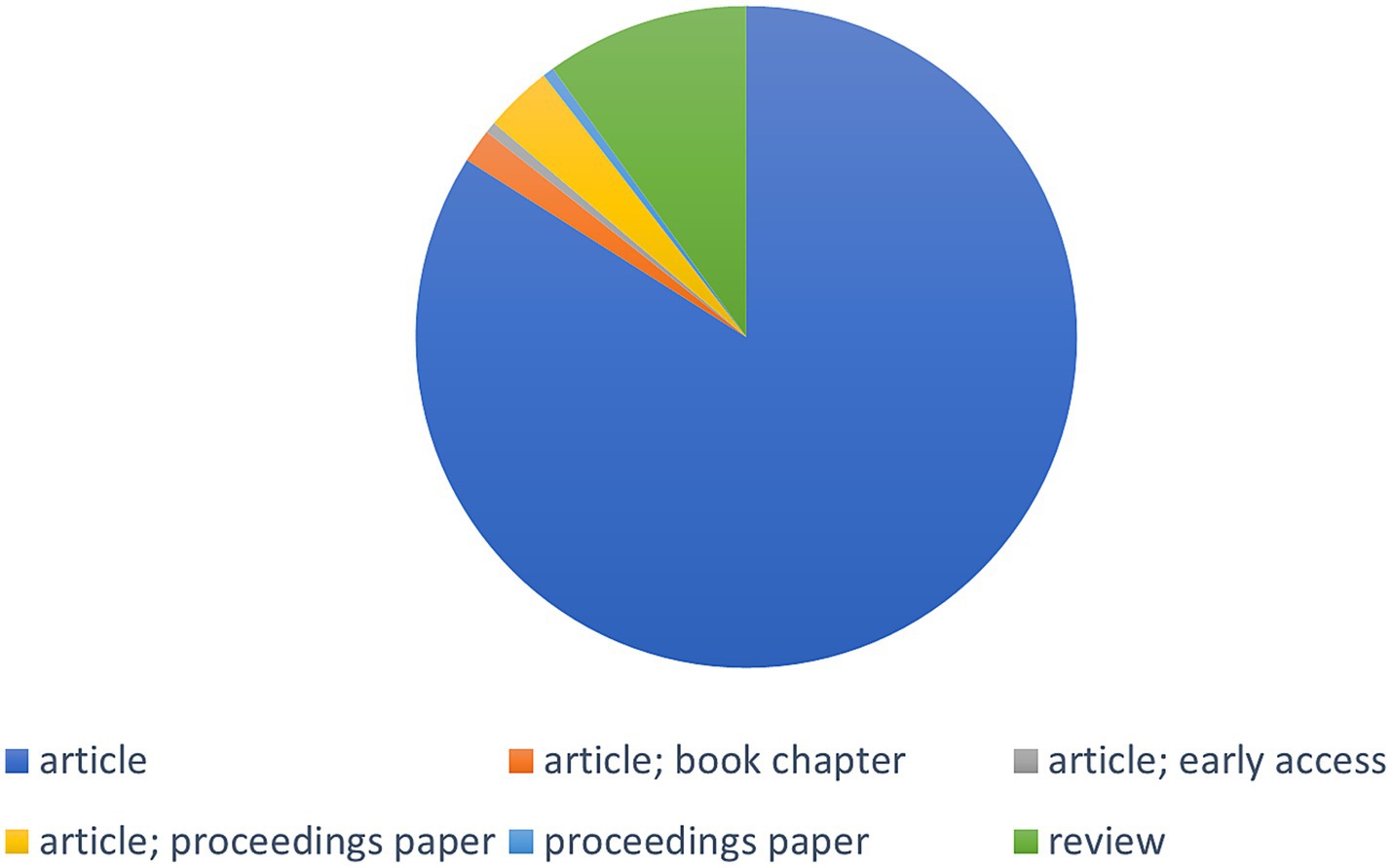
Out of the 181 results, there were 152 original research articles, 18 review articles, six proceeding papers, three book chapters, and one Early Access publication (Figure 3). Systematic reviews become crucial in this situation to comprehensively understand the issues and hazards of mycotoxin.
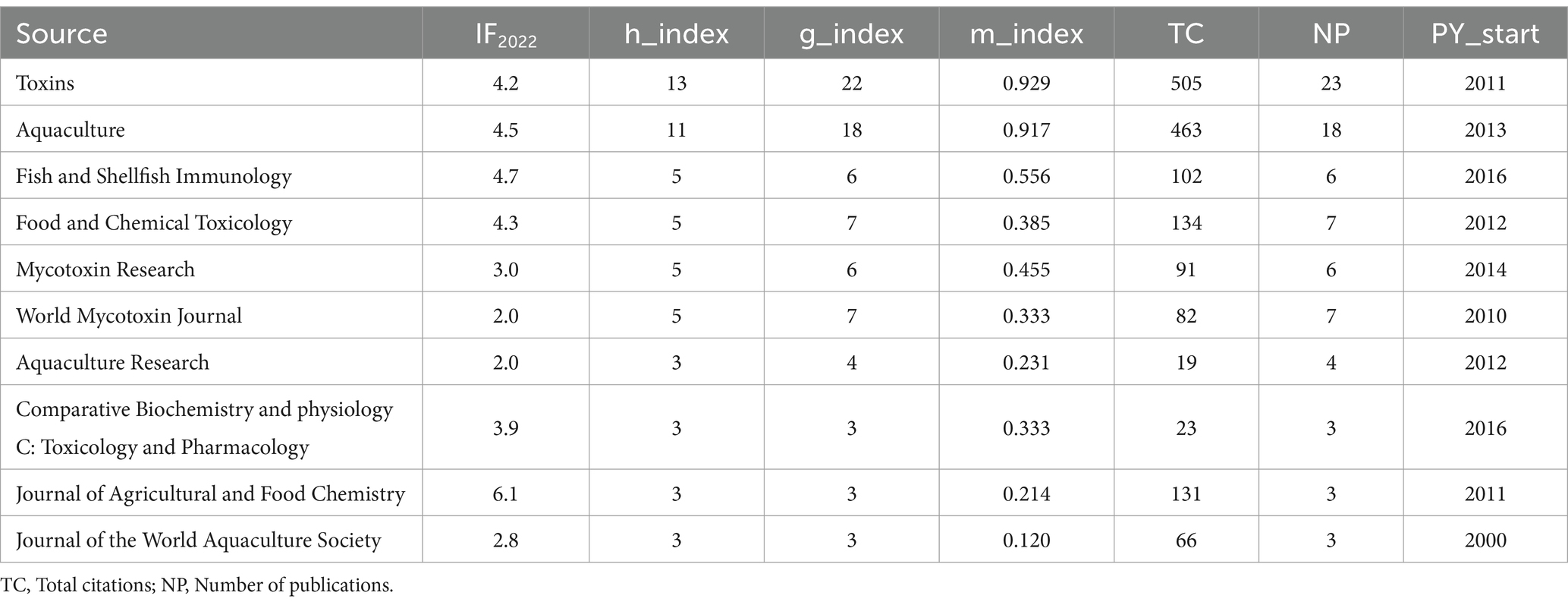
Studies on mycotoxins in aqua feed have been published in 95 journals. The top 10 journals were chosen based on the number of papers submitted. Table 2 lists the number of citations, number of publications, and impact factor (IF) in 2022. Toxins was the journal that had the most publications, with 23 total number of publications (NP), or 12.70% of the total, and 505 citations.
Two prominent measures for analyzing the quantity and quality of articles are the impact factor (IF) and the h-index. The impact factor (IF) was established by the Institute of Scientific Information (ISI) to quantify how frequently a journal’s articles are referenced over time, indicating the value of a journal or a series of scientific investigations. The h-index, which considers both number of papers and citations, is another statistic used to assess the publication impact of journals, nations, organizations, or people. The number of papers with at least h citations is specified as the h-index value, while other publications (Np-h) have citation counts less than h. The impact of journals and countries on scientific research is assessed in this study using the IF 2022 and the h-index 2022.
3.4 Countries and institutions
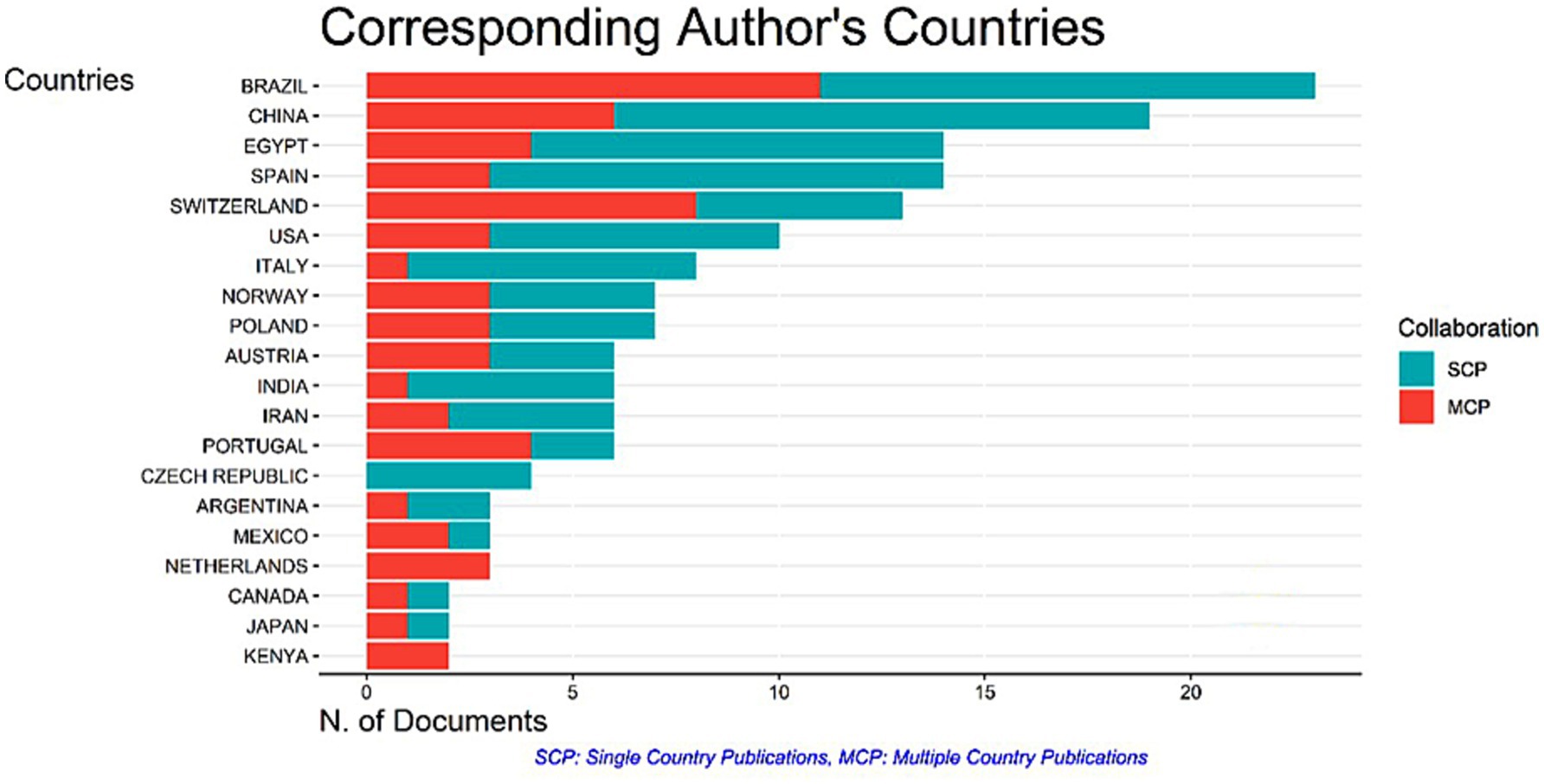
Throughout the publishing process, including final proofreading, the corresponding author is the primary point of contact for the manuscript and any associated correspondence. The standards for identifying the relevant author(s) differ from one publisher to the next. For example, although some publishers are liberal when there are multiple authors on a single document, others are strict when there is only one. We analyzed the data based on the authors’ countries of affiliation to identify the most relevant corresponding authors. According to our results (Figure 4), Brazil maintained its dominance with a total of 11 Multiple Country Publications (MCP) and 12 Single Country Publications (SCP), followed by China with 6 MCP and 13 SCP.
Figure 5 depicts the involvement of several universities in the research. We discovered that UNIV FED SANTA MARIA (Federal University of Santa Maria1) ranks first with 21 articles and KAFRELSHEIKH UNIV (Kafrelsheikh University2) ranks second with 20 articles.
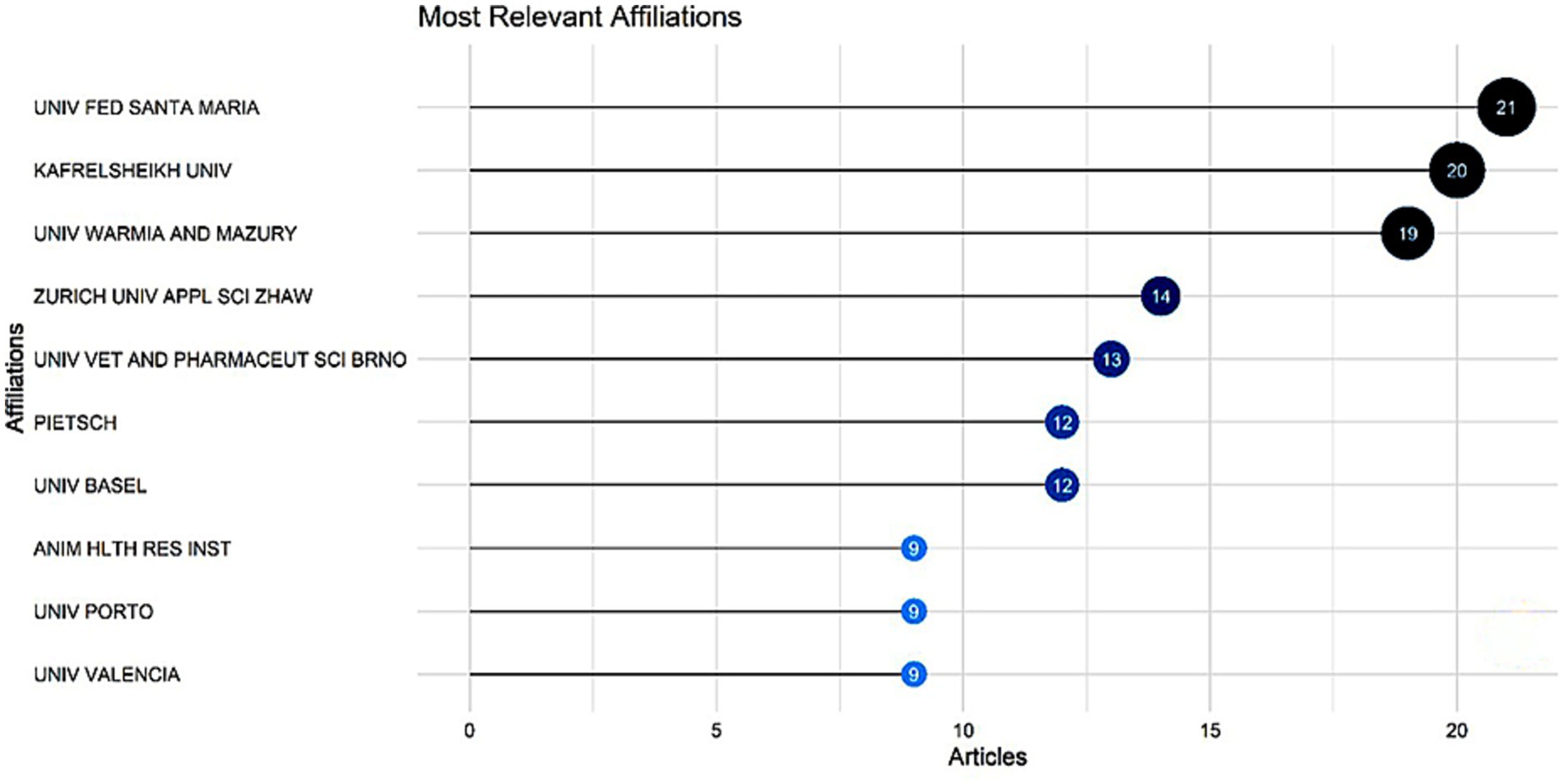
Figure 5. Top 10 most relevant institutional affiliations and the number of articles found per institution.
3.5 Citations
Citations to documents illustrate their usefulness to the general public and researchers. As illustrated in Figure 6, we evaluated the data to find the most commonly referenced countries. Switzerland got the first position with 366 citations, followed by Brazil with 303 citations.
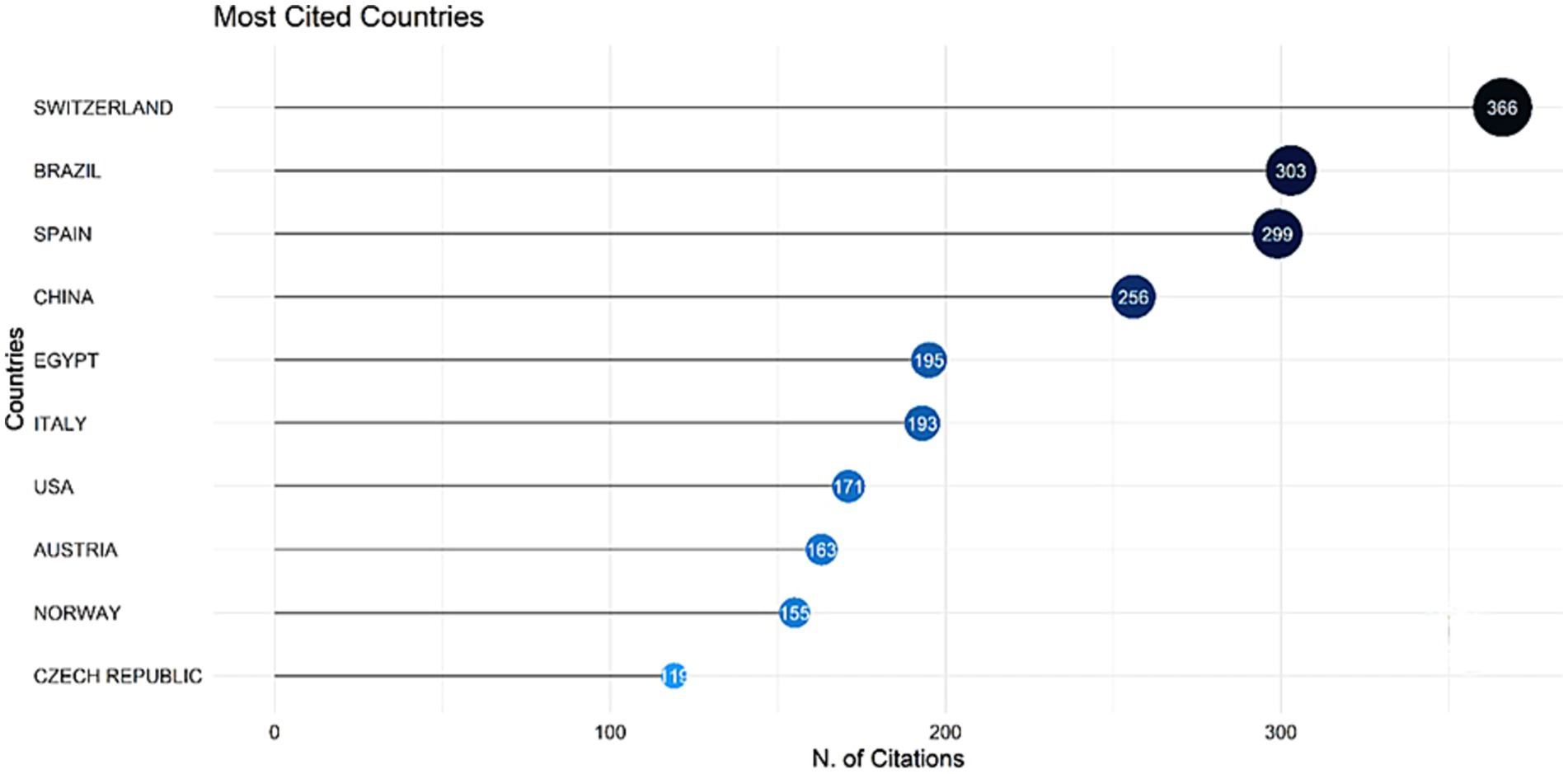
Figure 6. Top 10 most cited countries on the research on mycotoxin in aquafeed, and the number of citations per country.
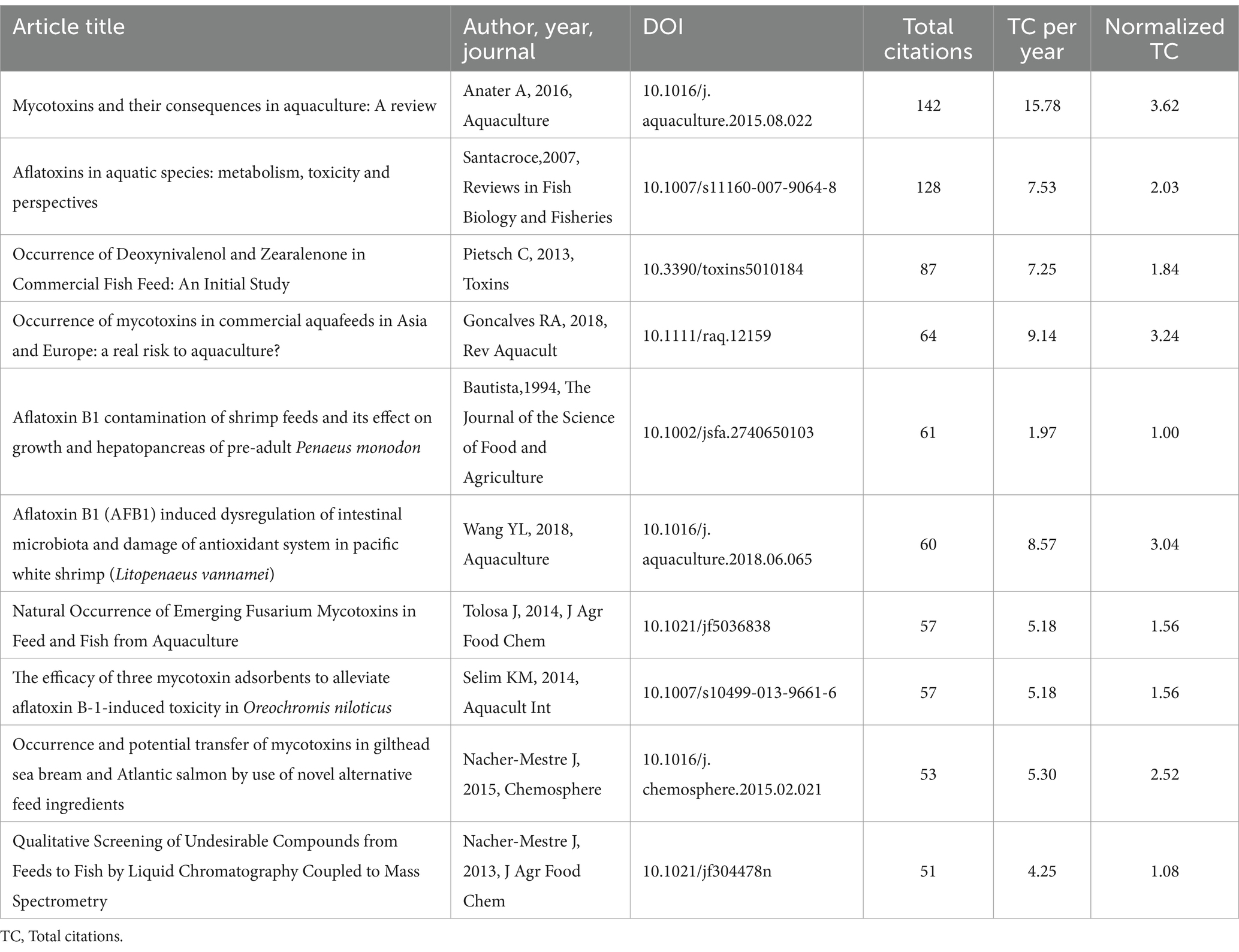
Researchers typically cite a document based on its value to the general public and the scholarly community. A document with more citations has a greater influence. We analyzed the data to extract the documents with the broadest scope. Table 3 lists the top 10 most often cited documents. The most cited publication was a review article titled “Mycotoxins and their consequences in aquaculture: A review,” which was published in the Aquaculture journal and has a total of 142 citations.
3.6 Keywords
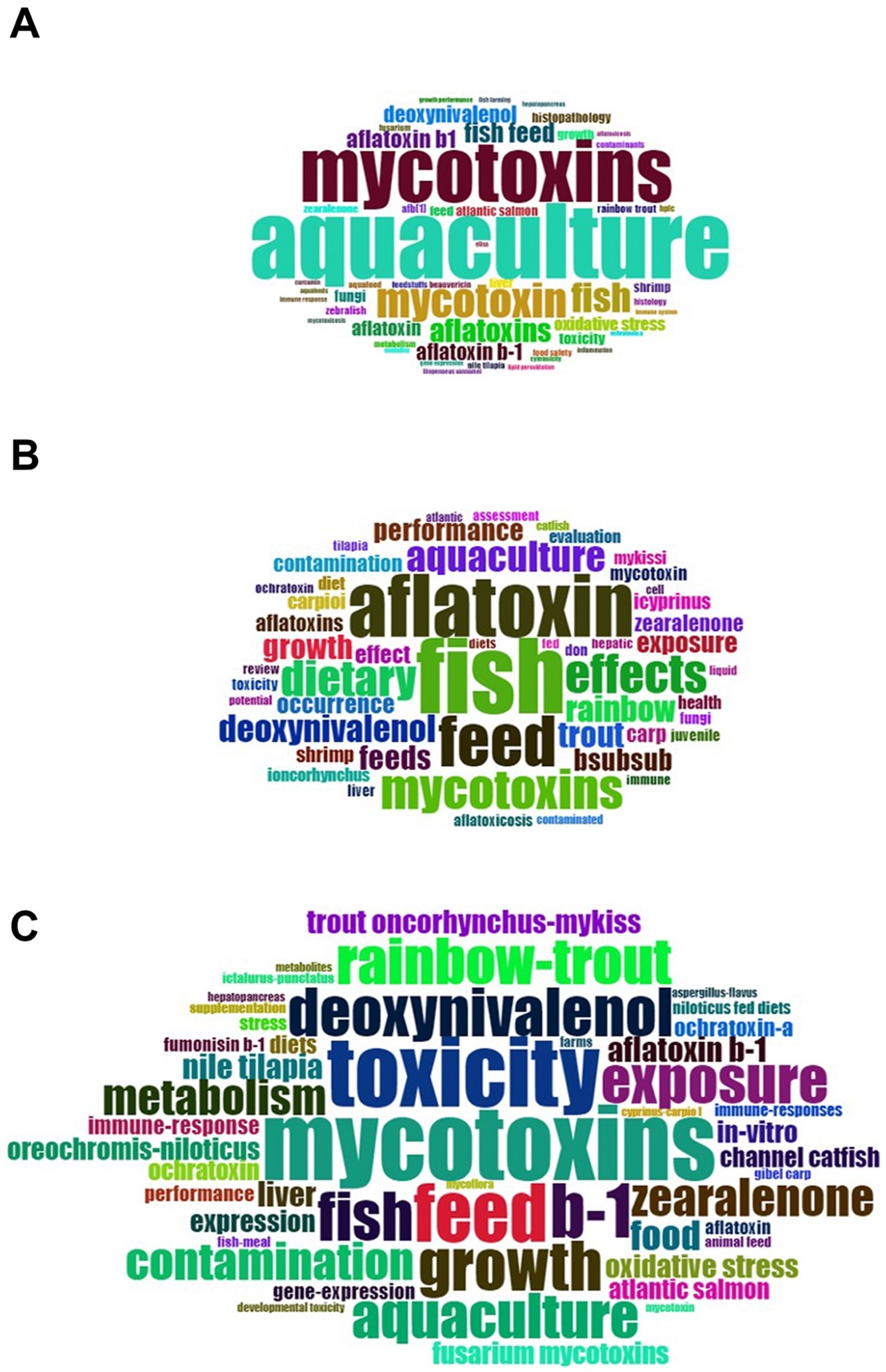
We analyzed the data to create WordCloud and extracted the words for WordCloud using the author’s keywords and titles. We discovered that “Aquaculture” and “Mycotoxins” were the leading terms recovered from the author’s keywords, as shown in Figure 7A, but “Fish” and “feed,” among other things, were the leading words retrieved from the titles (Figure 7B). Figure 7C shows similar findings for terms extracted from keywords plus. We discovered that “Mycotoxins” and “toxicity” were the leading terms recovered from the keywords plus.
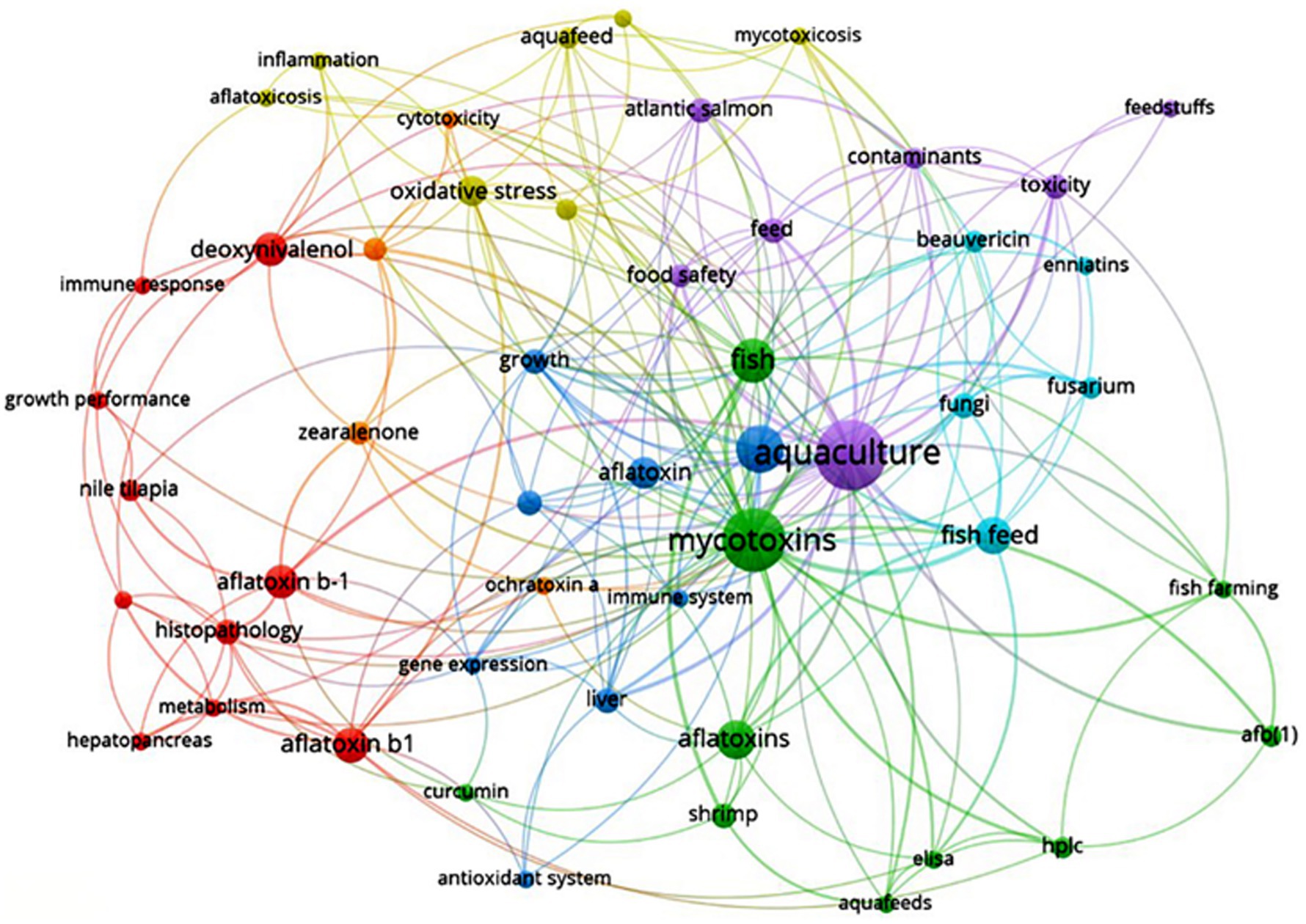
From the term co-occurrence map (terms taken from “author’s keywords”), 51 terms out of a possible 523 fulfilled the requirement for the term’s minimum number of occurrences of three. As shown in Figure 8, “aquaculture,” “mycotoxins” and “aflatoxin” appeared the most times, respectively. The term “aquaculture” appeared 44 times, “mycotoxins” appeared 37 times and “aflatoxin,” which was the third most often used term, appeared 21 times.
The ability to extract the most relevant terms from the data is crucial. Figure 9 depicts the extraction of the most relevant terms from four distinct groups. The most common phrase in the author’s keywords was “aquaculture,” which occurred 45 times, followed by “mycotoxins,” which appeared 37 times (Figure 9A). “Mycotoxins” were the most relevant phrase in the keywords plus, appearing 42 times, followed by “toxicity” (39 times) and “feed” (32 times) (Figure 9B). As shown in Figure 9C, “fish” were the most often common term in the titles, appearing 58 times, followed by “aflatoxin,” which appeared 48 times. The most commonly used phrase in abstracts was “fish,” which occurred 615 times, followed by “feed,” which appeared 406 times, as shown in Figure 9D.
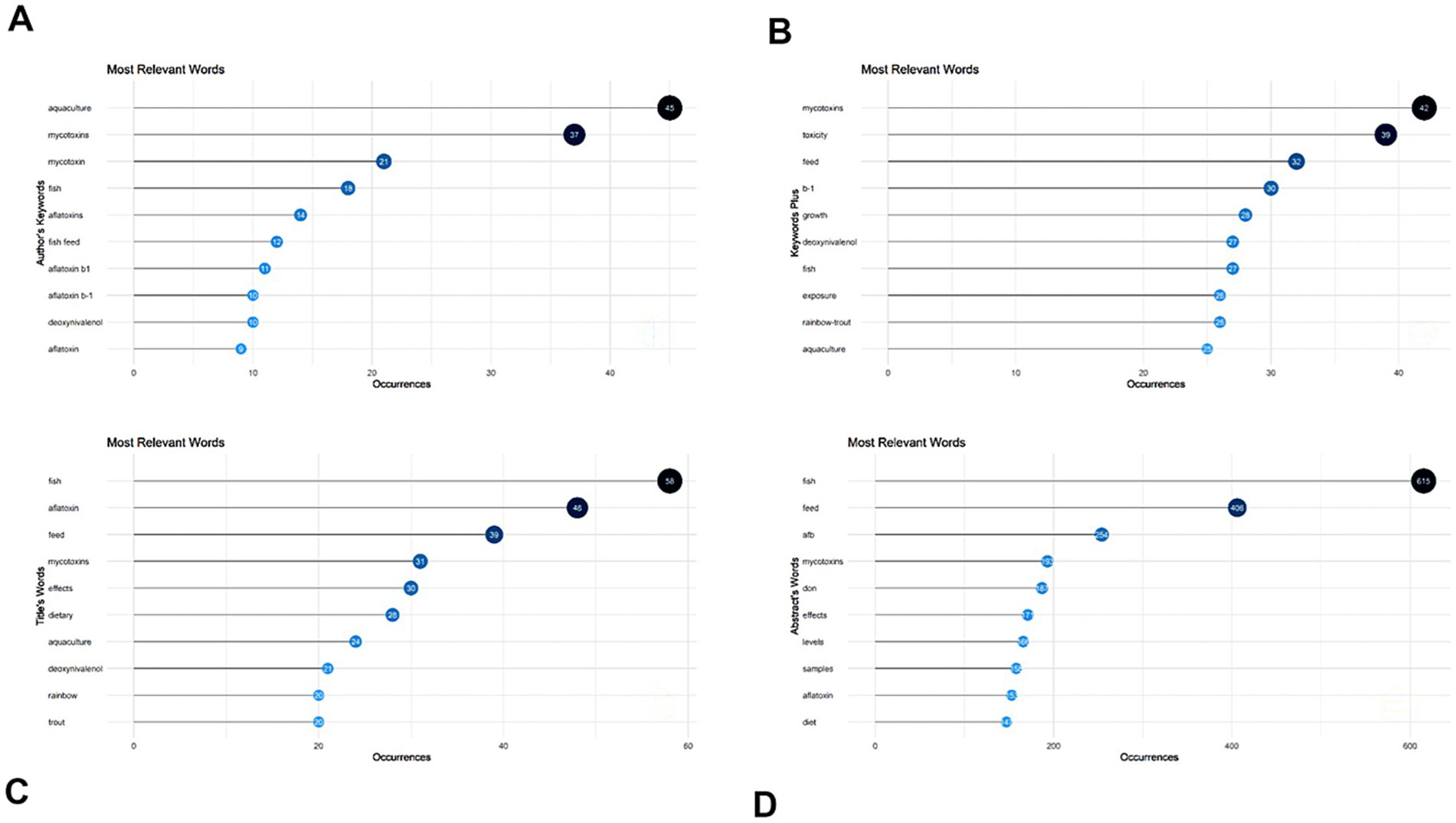
Figure 9. The author’s keywords (A), Keywords plus (B), titles (C), and abstracts (D) were used to extract the most relevant term.
3.7 Country and institution linkage
In total, 49 countries had publications on mycotoxin in aquaculture, of which 19 had at least five (5) papers. The linkages across these countries are as shown in Figure 10.
A total of 322 institutions were included in the analysis of the institutional linkage based on citations. We selected a minimum number of documents for each institution as three (3), and 37 institutions met this threshold. University of Basel and the Kafrelsheikh University came out on top with a total of eight (8) documents and a total of 276 and 68 citations, respectively, followed by Biomin Holding GmbH, which had 7 papers and 182 citations (Figure 11).

Figure 11. Density visualization map of institutional linkage based on collaborations on publications on mycotoxin in aquaculture.
3.8 Country research collaborations
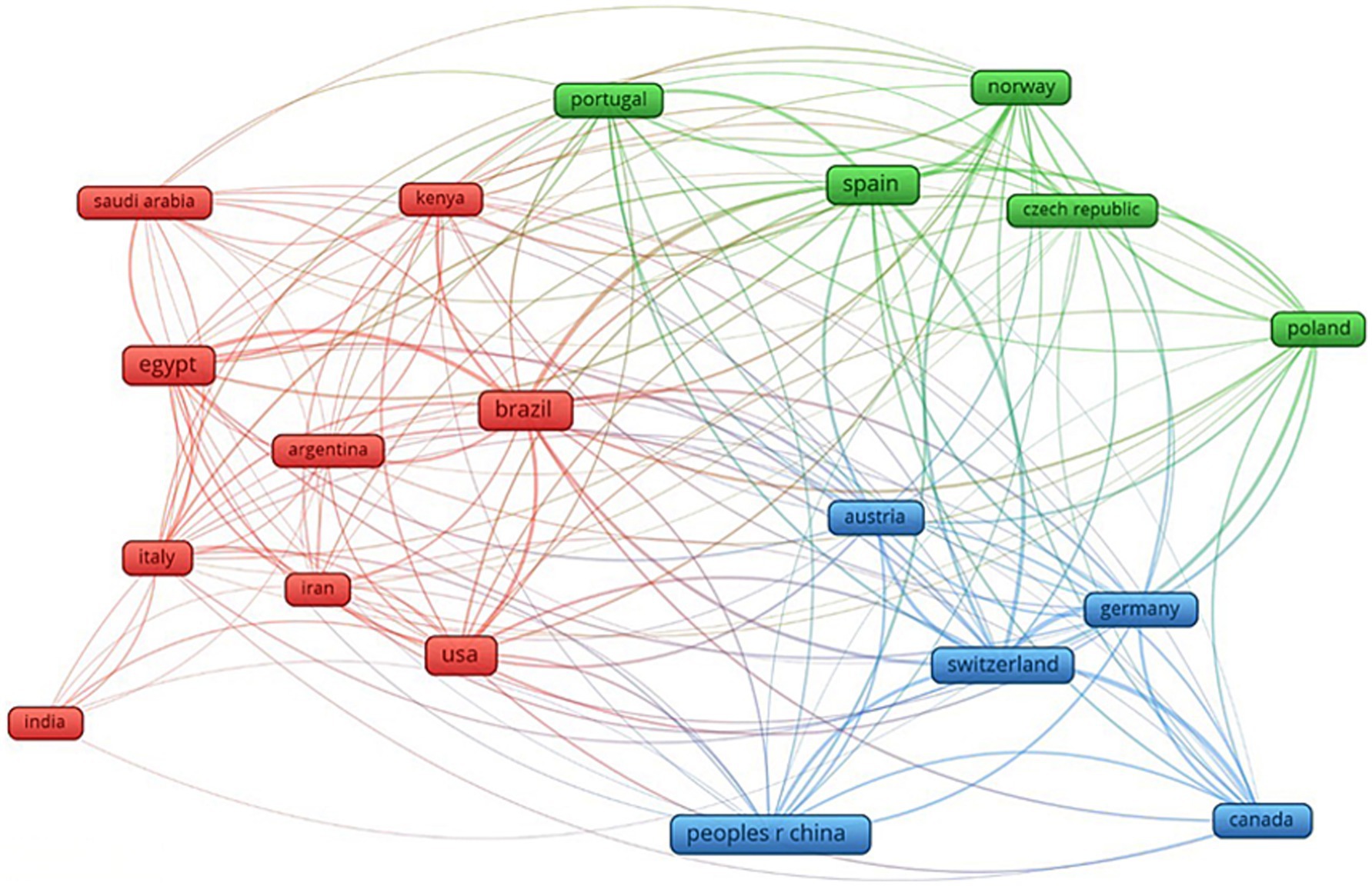
Science mapping facilitates the exploration of connections among different scholars, organizations, and nations within a particular field of study. The process of establishing science mapping involves analyzing co-citation, citation, co-word, co-authorship, and theme groupings. Research collaborations on mycotoxins in aquaculture indicate that African nations conducted very less research and participated in research collaborations less frequently than other regions of the world. Among South American countries, Brazil had strong collaborations with Argentina, Spain, the USA, Iran, and Colombia. There were also strong research collaborations between China, the USA, Switzerland, Egypt, Saudi Arabia, the United Kingdom, and Portugal. Among Asian countries, China had strong collaborations with the rest of the world.
3.9 Mycotoxin occurrence in aquafeeds
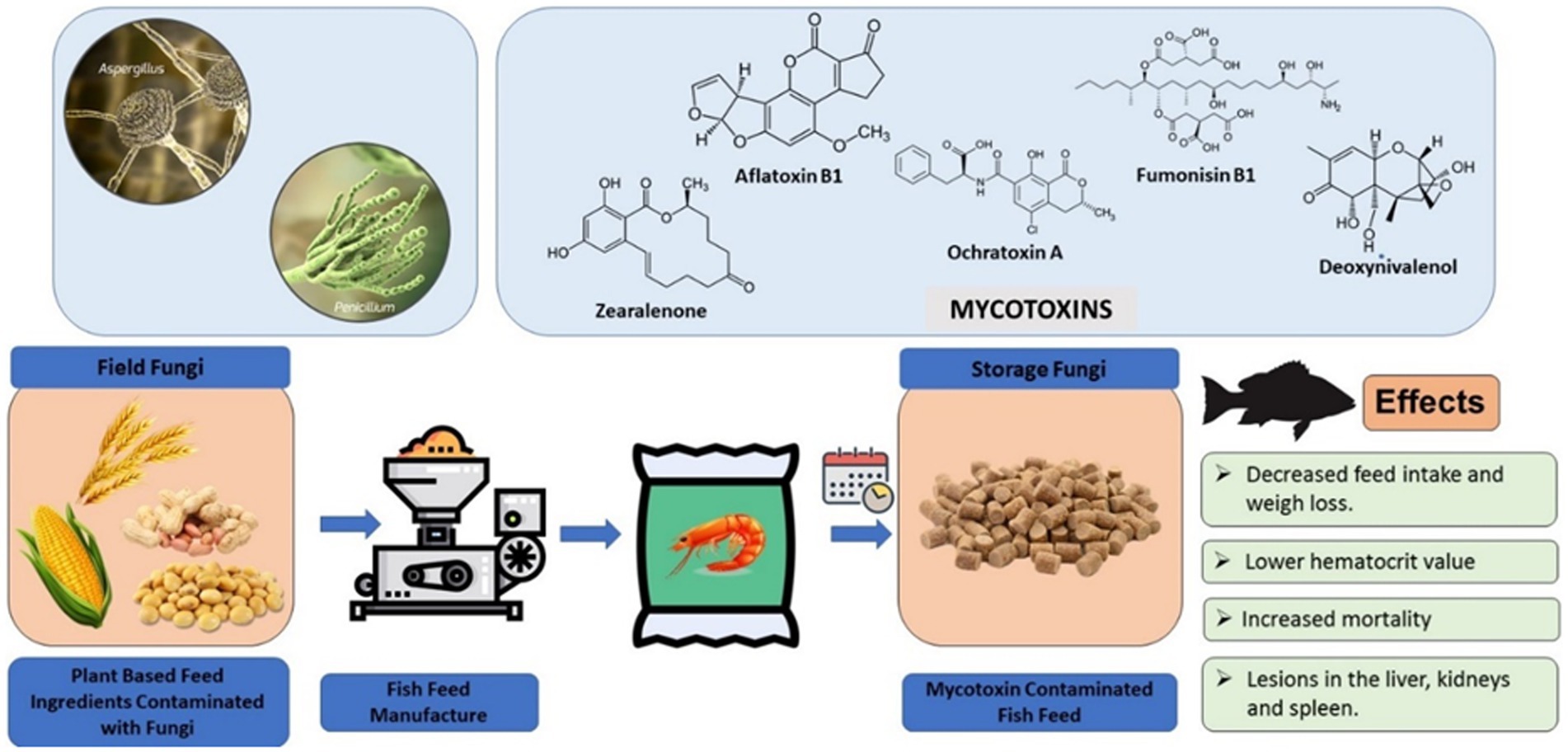
The inclusion rate of traditionally used finite and expensive protein and fat sources from wild-caught fish (i.e., fishmeal and fish oil) in the diets of farmed fish species continues to decline. However, the consequential increase in the use of more economical plant protein and energy sources in commercial aquafeeds has significantly increased the potential of exposing intensively cultured fishes to low, but non-negligible, concentrations of mycotoxins (Hooft et al., 2011). Figure 12 illustrates the risk and effects associated with mycotoxin occurrence in aquafeeds.
3.9.1 Mycotoxin contamination in aquafeed and feed ingredients
Plant-based feed ingredients currently used in aquafeeds as substitutes for fishmeal include soybean meal, cotton seed meal, corn gluten meal, peanut meal, sunflower meal, rapeseed/canola meal, maize/corn, wheat bran and wheat (Kaiser et al., 2022) and they are prone to mycotoxin contamination (Table 4). Of all the plant protein sources, soybean meal currently represents a major protein source in aquafeeds due to its relatively higher protein content and balanced amino acid profile, except for sulphur-containing amino acids (Jannathulla et al., 2019).
Mycotoxins are included as potential contaminants in plant-based fish feed as natural contaminants in cereals and oilseeds (Albero et al., 2022). They are primarily found in subtropical and tropical regions, where they contaminate feeds made from corn, soybean, cottonseed, and wheat (Guerre, 2016; Gonçalves et al., 2018). In aquafeed ingredients such as wheat, corn and soybean meal, the risk of mycotoxin production especially AFs and OTA, is heightened during prolonged storage in hot and humid environments, which facilitate active fungal colonization, predominantly by Aspergillus and Penicillium spp. (Bashorun et al., 2023). Fungal growth requirements for minimal and optimal water activity (aW) differ among genera. Fusarium and Alternaria are plant pathogens and hygrophilic (1.00 aW), meaning they proliferate in substrates with high water availability and, therefore, predominate in the fields at pre-harvest. Aspergillus and Penicillium are xerophilic (<0.95 aW), meaning they can proliferate at low water availability and are the main mycotoxigenic fungi post-harvest, during storage (Koletsi et al., 2021). The higher inclusion of less-expensive plant sources may introduce a series of anti-nutritional factors (e.g., protease inhibitors, phytates, saponins, glucosinolates, tannins, non-starch polysaccharides) and/or increase the occurrence of animal feed contaminants; factors that might affect the quality and safety of aquafeeds (Koletsi et al., 2021).
Most farmers use on-farm or locally-made commercial fish feed produced using locally available ingredients, rarely using imported feed (Marijani et al., 2017). It is important to note that mycotoxins are more commonly found in higher concentrations in farm-made feed than in commercial feed (Foluke et al., 2016; Marijani et al., 2017; Oliveira and Vasconcelos, 2020). Since farm-made feed is more commonly formulated in developing countries, this might explain why contamination by mycotoxins is more frequent in developing countries in these regions (Barbosa et al., 2013; Fallah et al., 2014).
Various mycotoxins in aquafeed have been reported in different parts of the world, and Figure 13 shows the global map of the various mycotoxins and the number of publications on mycotoxins in aquafeed in various countries by 2024. The number of publications in the map is the total of SCP and MCP. The countries leading in the number of publications in different continents were Brazil (25) in South America, Spain (23) in Europe, China (20) in Asia, Egypt (18) in Africa, and the USA (19) in North America. From the map, it is evident that most mycotoxins in aquafeed have been reported in the East African countries (e.g., aflatoxin B1, B2, G1, and G2, fumonisin B1 and B3, deoxynivalenol, acetyldeoxynivalenol, ochratoxin A, roquefortine C, alternariol, T-2 toxin, zearalenone, and zivalenol), especially in Kenya, Uganda and Tanzania, the Southeast Asian countries (e.g., aflatoxin B1, B2, G1, and G2, fumonisin B1 and B3, deoxynivalenol, zearalenone, and ochratoxin A), including Thailand, Singapore and Myanmar, and the European countries (e.g., aflatoxin B1, B2, G1, and G2, fumonisin B1 and B3, deoxynivalenol, zearalenone, and ochratoxin A), including Austria, Croatia and Portugal, and the United Kingdom (e.g., deoxynivalenol, fumonisin B1 and B3).
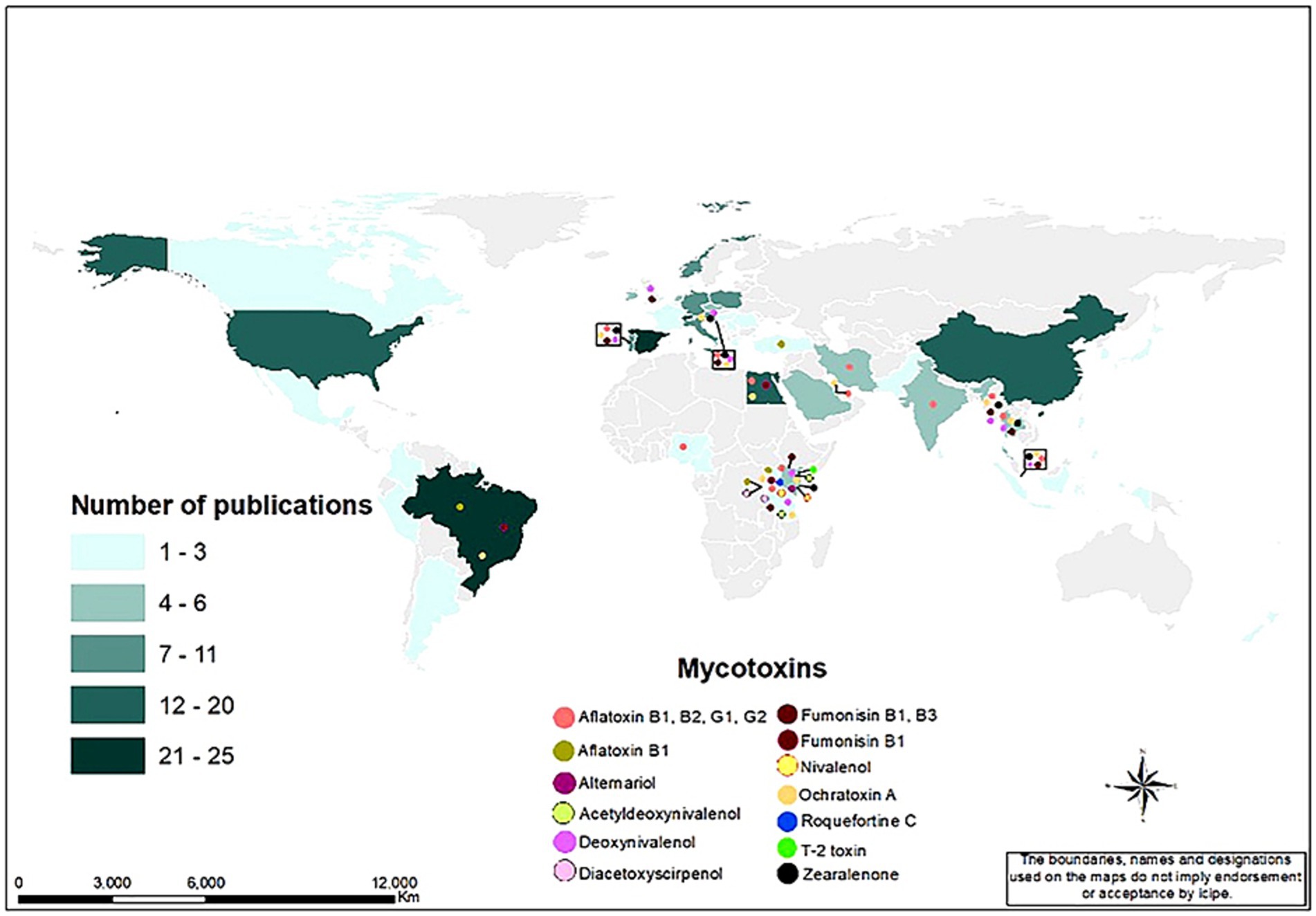
Figure 13. Global map of the mycotoxin occurrence in aquafeed and the number of publications in different countries.
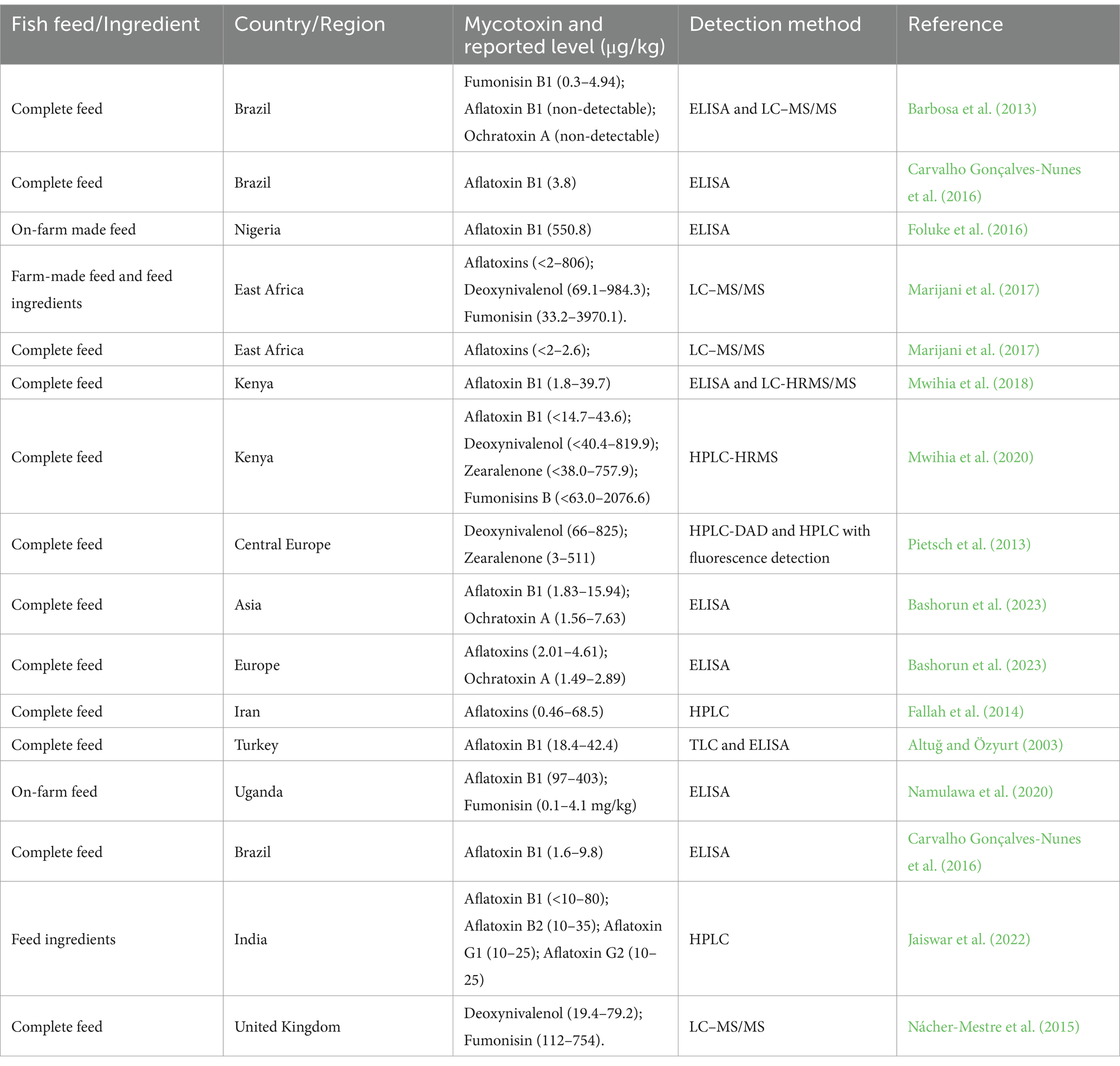
Some of the studies on mycotoxin occurrence in aquafeeds and their detection methods in various regions are also summarized in Table 5. For instance, In Brazil, aflatoxin B1 was detected in low doses (1.1 μg/kg to 7.4 μg/kg), in samples of soybean bran, corn bran and other cereals from fish farms (Carvalho Gonçalves-Nunes et al., 2016). In Turkey, Altuğ and Özyurt (2003) verified the presence of aflatoxins in 49.5% of aquafeed samples, with 23.5% exceeding levels of 20 μg/kg. In East Africa, Marijani et al. (2017) found 14 mycotoxins (AFs B1, B2, G1 and G2, fumonisin B1 and B3, deoxynivalenol (DON) and acetyldeoxynivalenol (sum of 3-ADON and 15-ADON), OTA, roquefortine C, alternariol, T-2 toxin, diacetoxyscirpenol (DAS) and nivalenol) in locally manufactured feeds in Kenya, Tanzania, Rwanda and Uganda. They found that DON (92.9%), aflatoxins (64.3%) and fumonisins (57.1%) were the most prevalent mycotoxins in the feeds. In Kenya, another study by Mwihia et al. (2018) reported aflatoxin levels in aquafeeds ranging from 1.76 to 39.7 μg/kg. In Uganda, Namulawa et al. (2020) detected AFB1 levels of 90 to 211 μg/kg in factory samples and 70 to 374 μg/kg in farm samples of aquafeeds collected from nine fish farms and seven aquafeed factories. Additionally, they reported fumonisin contamination levels of between 0.1 to 0.8 μg/kg in factory samples and between 0.1 to 1.86 μg/kg in farm samples.
3.10 Detection and analytical methods for mycotoxins in aquafeeds
Due to the varied structures of these compounds it is not possible to use one standard technique to detect all mycotoxins, as each requires a different method (Turner et al., 2009). Since the discovery of mycotoxins, many different methods have been used to analyze the mycotoxins (Table 5) including traditional quantitative methods viz. chromatography, immunological, and the advanced methods viz. ultrahigh-performance liquid chromatography, fluorescence polarization immunoassay, nanoparticle-based methods, microfluidics, and phage display methods (Singh and Mehta, 2020). Mycotoxin toxicity occurs at very low concentrations, necessitating sensitive and reliable methods for their detection. In most cases, the extracted samples are analyzed by the LC–MS chromatographic method. In addition, the development of the LC–MS/MS technique for the simultaneous identification of multiple mycotoxins has achieved much attention (Turner et al., 2009). Chromatographic methods such as Thin Layer Chromatography (TLC) and High-Performance Liquid Chromatography (HPLC) are noted to be regular and the global gold techniques in mycotoxin analysis in laboratories. Thin Layer Chromatography (TLC) was the most commonly used chromatographic technique applied to mycotoxins in the early 1980s. However, it has certain drawbacks, such as low sensitivity and poor accuracy (Singh and Mehta, 2020). High-Performance Liquid Chromatography (HPLC) has become the main method for mycotoxin analysis. Coupled with a variety of detectors, practically all mycotoxins have been separated and detected by HPLC. Fumonisins, AFs, ZEA, and OTA are routinely analyzed by HPLC (Rahmani et al., 2009). Thin Layer Chromatography (TLC) enables the screening of large numbers of samples with low operating cost as well as the identification of target compounds, using UV–vis spectral analysis. Enzyme linked immuno-sorbent assays (ELISA) is a quick and a relatively cheap analytical method, which has been previously used in the rapid screening of mycotoxin contamination in different commodities. Enzyme linked immuno-sorbent assays (ELISA) is widely used for routine mycotoxin measurement due to the availability of test kits for practically all relevant mycotoxins (Köppen et al., 2010). Nowadays, ELISAs have also become widespread in mycotoxin determination (Rodríguez-Cervantes et al., 2013; Namulawa et al., 2020).
The four important AFs found are Aflatoxin B1 (AFB1), Aflatoxin B (AFB2), Aflatoxin G1 (AFG1), and Aflatoxin G2 (AFG2), distinguishable by their fluorescence under UV light (green or blue) and comparative chromatographic movement during thin-layer chromatography (Barbosa et al., 2013; Singh and Mehta, 2020). As fumonisins lack a useful chromophore or fluorophore, their detection by HPLC involves appropriate derivatization and sensitive, specific fluorescence detection. Fumonisins can be detected using HPLC-UV or HPLC fluorescence detectors after derivatization (Ndube et al., 2009). The LC–MS/MS technique allows for the determination of co-occurrence and concentration of several mycotoxins within a feed sample in a single run (Marijani et al., 2017).
3.11 The toxicity of mycotoxins and their effects on fish and humans
3.11.1 Effects on fish
Mycotoxins can be responsible for the induction of many disorders in fish, such as inducing cell and organ alterations, producing functional and morphological effects, and in more severe cases mortality, resulting in economic loss on fish production (Anater et al., 2016). Various mycotoxins have varying effects on fish. Aflatoxins increase the chances of cancer development in rainbow trout (Oncorhynchus mykiss), causing liver tumors (Russo and Yanong, 2010). Additionally, it causes low immunity in O. mykiss (Ottinger and Kaattari, 1998), sea bass (Dicentrarchus labrax) (El-Sayed and Khalil, 2009), and red drum (Sciaenops ocellatus) (Zychowski et al., 2013).
Fumonisin causes reduction in haematocrit and increased free sphinganine/free sphingosine ratio in channel catfish (Ictalurus punctus) and Nile tilapia (Oreochromis niloticus) liver (Deng et al., 2010; Tuan et al., 2003), and increases mortality due to Cytophaga columnaris infection in channel catfish (Ictalurus punctus) (Lumlertdacha et al., 1995).
Effects of ochratoxin A include hemorrhagic patches on the dorsal surface, erosion of the fins and rusty spot formation in the belly region and dorsal musculature, kidney and gill congestion, and spots of congestion on the periphery of the liver in D. labrax (El-Sayed and Khalil, 2009), lesions in the liver and posterior kidney, that is, increased incidence and severity of melanomacrophage centers in the hepatopancreatic tissue and posterior kidney and reduced number or absence of exocrine pancreatic cells surrounding the portal veins in I. punctus (Manning et al., 2003), and lesions in the liver, kidneys and spleen, enlargement and congestion of kidney and liver, dilation of blood vessels and necrosis of the kidney, degeneration and necrosis of hepatocytes, increased levels of alanine aminotransferase, aspartate transaminase, creatine and urea in O. niloticus (Diab et al., 2018).
Trichothecenes, specifically deoxynivalenol (DON), can cause gastrointestinal and liver haemorrhaging, anaemia, decrease in metabolism, lower whole-body crude protein concentration, and lesions in the liver in O. mykiss (Pietsch et al., 2011; Matejova et al., 2014). It can also cause deduced packed cell volume, decrease in concentration of alkaline phosphatase, cholesterol, triglycerides, total proteins and albumin, and reduced vaccination response against Aeromonas salmonicidae [EFSA Panel on Contaminants in the Food Chain (CONTAM), 2011].
Zearalenone can cause increased weight gain and body length on female fish, feminization, induction of plasma vitellogenin, increased condition factor of the next generation, and decreased reproductive performance in zebrafish (Danio rerio) (Schwartz et al., 2013). It can also cause increased feeding efficiency and growth rate, modulation of the adaptative and innate immune system, inflammation likely caused by Tetracapsuloides bryosalmonae infection, and changes in kidney morphology leading to atypical kidney structure and fibrosis in rainbow O. mykiss (Woźny et al., 2019). Overall, mycotoxins cause organ damage, impair the immune system, reduce weight gain, and cause metabolic alterations which can result in cancer and increased mortality in fish (Oliveira and Vasconcelos, 2020).
3.11.2 Effects on humans
The main source of human exposure to mycotoxins is the ingestion of contaminated food, with the burden of dietary exposure being particularly high in developing countries (Barbosa et al., 2013). Humans can be exposed to mycotoxins indirectly by consuming fish with aflatoxin residue accumulation. Among the different types of mycotoxins produced, AFB1 is recognized as a group I carcinogen by International Agency for Research on Cancer (IARC), mainly affecting the liver when consumed (Claeys et al., 2020). It has potent genotoxic and carcinogenic effects on humans. It has been classified as a group-1 carcinogen by the International Agency for Research on Cancer (IARC) of the World Health Organization,3 being particularly toxic to individuals who are infected by the hepatitis B virus (World Health Organization, 2002).
Chronic exposure of humans to fumonisins can be dangerous (Wangia-Dixon and Nishimwe, 2020). Fumonisins B1 are a group-2B carcinogen, according to IARC and, as such, are cancer-promoting toxins. They have been associated with a higher incidence of esophageal and hepatic cancer in China (Sun et al., 2007) and in Africa (Wangia-Dixon and Nishimwe, 2020), in regions where contamination by fumonisins is highly frequent. Additionally, exposure to fumonisins during pregnancy appears to be related to a higher neural tube deformity risk in offspring (Gelineau-van Waes et al., 2009; Kyei et al., 2020).
Ochratoxin A (OTA) has been considered a group-2B carcinogen by IARC (Iqbal et al., 2016a). As such, exposure to this toxin may be involved in the development of hepatic cancer, urinary tract tumors and testicular cancer, among other diseases [Malir et al., 2016; EFSA Panel on Contaminants in the Food Chain (CONTAM) et al., 2020]. It also induces human kidney tubular epithelial cell apoptosis of human kidney tubular epithelial cell (HK-2) (Song et al., 2021).
Deoxynivalenol (DON) does not pose a health threat to humans compared to other mycotoxins as its effects are generally gastrointestinal (Kamle et al., 2022). However, it has been reported to have potential health concern in infants, toddlers and other children, and at high exposure also in adolescents and adults [EFSA Panel on Contaminants in the Food Chain (CONTAM) etal., 2017]. In fact, trichothecenes in general, but particularly DON, have been associated with an outbreak of acute mycotoxicosis which occurred in India after consumption of bread made using mold-damaged wheat and led to severe gastrointestinal problems (Bhat et al., 1989).
There are no studies that have clearly elucidated the effects of zearalenone on human beings. Nevertheless, it is classified as a xenoestrogen, an exogenous compound which resembles the structure of naturally occurring estrogens with its chemical structure (Fink-Gremmels and Malekinejad, 2007; Balló et al., 2023). This property of zearalenone determines its ability to bind to estrogen receptors of cell and its bioaccumulation, which leads to disorders of the hormonal balance of the body, which in consequence may lead to numerous diseases of reproductive system such as prostate, ovarian, cervical or breast cancers (Rogowska et al., 2019; Balló et al., 2023).
3.12 Legislative and regulatory elements regarding the control of mycotoxins
The epidemiological risk of mycotoxin contamination has attracted the attention of international bodies such as the Food and Agriculture Organization of the United Nations (FAO), the United Nations (UN) through the United Nations Environment Program (UNEP), and the World Health Organization (WHO) (Poroșnicu et al., 2023). These organizations are actively involved in providing information on various aspects of mycotoxin control globally (Khitska and Gerard, 2019). This has led to the push for national and international legislations to control the occurrence and effects of mycotoxins. Food legislation serves to protect the economic interests of food producers and traders, and over 100 countries have specific regulations for mycotoxins (Jiménez Medina et al., 2021). However, regulations regarding mycotoxins have been harmonized, especially among countries that have trade agreements such as the European Union and MERCUSOR (trade agreement between Argentina, Brazil, Uruguay, Paraguay and Venezuela) and Australia and New Zealand (van Egmond et al., 2007; Poroșnicu et al., 2023).
3.12.1 Maximum permissible limit of mycotoxins in aquafeed and animal feed
Regulations regarding the maximum levels of mycotoxins which can be present in foodstuff and feed have been established in different countries or regions. These limits differ due to varying dietary patterns and consequent crop intake (Poroșnicu et al., 2023), including those set by the European Union (EU), and other regional organizations. These regulations stipulate the maximum levels of mycotoxin in foods and feeds in accordance with the provisional maximum acceptable daily intake (Anukul et al., 2013). The Food and Agriculture Organization (FAO) and the World Health Organization (WHO) established the CODEX Alimentarius Commission in 1963 to develop CODEX standards, guidelines, and other food-related documents, such as the “Code of Practice,” to safeguard consumer health and promote ethical food trade standards. With over 180 countries, the CODEX Alimentarius Commission represents 99% of the global population. To prevent and mitigate mycotoxin contamination in various foods and feeds, the Codex Committee on Food Additives and Contaminants has issued different codes of practice (Anukul et al., 2013).
Many countries follow FDA guidelines, ESFA, CODEX Alimentarius and EC for acceptable levels of mycotoxins. However, the regulations set by the European Commission appear to be the most followed (Oliveira and Vasconcelos, 2020). Table 6 shows the maximum allowable limits of some mycotoxins in animal feed in various countries. The maximum permissible limits for aflatoxin are 20 μg/kg for FDA and 5 μg/kg for WHO (Namulawa et al., 2020). The maximum permissible limits for fumonisin are 10 μg/kg as per EC regulations, 20 μg/kg for FDA and 5,000 μg/kg for WHO (Marijani et al., 2017; Namulawa et al., 2020). The maximum permissible limits are 5 μg/kg for OTA and 5,000 μg/kg for DON and zearalenone according to EC (Bashorun et al., 2023; Marijani et al., 2019).
4 Discussion
The term mycotoxin was coined in 1962 in response to an enormous veterinary crisis near London, England, during which approximately 100,000 turkey poults perished. Scientists determined that this peculiar turkey X illness was caused by a peanut (groundnut) meal contaminated with secondary metabolites from Aspergillus flavus (aflatoxins), alerting them to the potential lethality of other hidden mold substances (Bennett and Klich, 2003). While all mycotoxins originate from fungi, not all fungal metabolites are toxic substances. The concentration of both the metabolite and its target is crucial. Mycotoxins produced by fungi are detrimental to vertebrates and other animal species even at low doses. They are distinct from ethanol or other low-molecular-weight fungal metabolites, which are lethal only at high doses (Bennett, 1987). To the best of our knowledge, this is the first comprehensive study that has attempted to summarize the mycotoxin research trends in aquafeed using a combination of scientometric and conventional review approaches.
Based on statistics and visualization tools, bibliometric analysis has emerged as a way to display a specific topic’s knowledge structures and evolutionary patterns (Devos and Menard, 2019; Chaudhuri et al., 2020). It can measure author and institution productivity, map international collaboration networks, and investigate geographic dispersion using published data to detect research trends and hot subjects (Lu et al., 2021; Ou et al., 2022). Various tools are available for bibliometric analysis, each with its own advantages and disadvantages (Choudhri et al., 2015; Karanatsiou et al., 2017; Chen, 2020; Markscheffel and Schröter, 2021).
Mycotoxins pose a significant concern to both human and animal health and welfare. This field requires more study due to the diminishing publication growth rate despite growing mycotoxin concerns in the global feed market. Zyoud (2019) conducted a bibliometric study from Scopus in 2019 and found 9,845 articles on aflatoxin. In contrast, the current study searched WoS for papers addressing mycotoxins in farmed aquafeed and found 181 articles. The low number of documents underscores the need for additional research on mycotoxins in aquafeed. Nevertheless, the documents and the top journals publishing the work on mycotoxin in aquafeed play an important role in providing the scientific evidence needed by researchers, industry players, and policymakers in tackling mycotoxin contamination in feeds and feed ingredients.
The bibliometric analysis reveals a significant underrepresentation of African countries in mycotoxin research within the aquaculture sector, reflecting broader systemic challenges that hinder scientific output on the continent. This can be attributed to challenges such as the chronic underfunding of research and development, which limits access to essential infrastructure, equipment, and long-term project support (Akuru, 2019). Additionally, a shortage of specialized human capital, exacerbated by brain drain, further constrains the capacity of institutions to engage in high-quality research (Sheikheldin and Mohamed, 2021). Fragmented linkages among universities, research institutes, and industry also contribute to low research productivity by hindering interdisciplinary collaboration and knowledge transfer (Okoroigwe et al., 2022). Yet collaboration plays a critical role in enhancing scientific knowledge through the exchange of expertise, joint experimentation, and shared access to resources (Bégin-Caouette et al., 2023). The low level of collaboration in Africa, therefore, not only limits co-authored publications but also stifles opportunities for innovation and broader scientific impact in addressing pressing issues like mycotoxin contamination.
Based on our conventional literature review, we see evidence for the occurrence of mycotoxins in aquafeed and feed ingredients in various countries. The contamination of aquafeed with mycotoxins appears to be common and a worldwide issue, but the type and prevalence of mycotoxin contamination in feed appears to highly depend on the geographical region. This might be due to the difference in climatic conditions of various regions, the type of samples analyzed, or the methodology used to identify mycotoxins, among other factors. According to Moretti et al. (2019), climate change has the potential to have a dramatic impact on the growth, dispersal, and production of mycotoxin in fungi. Different regions have different climatic conditions (Milani, 2013), and mycotoxin occurrence is more prevalent in regions dominated by warm climatic conditions. Increased temperatures favor the proliferation of heat-tolerant mycotoxigenic fungi, particularly aflatoxin-producing Aspergillus species. While Penicillium species, producing OTA and patulin, are typically associated with mycotoxin production in temperate climates, Aspergillus species, producing AFs and OTA, are more prevalent in tropical and subtropical regions due to their ability to thrive at elevated temperatures and lower water activity levels (Zingales et al., 2022).
Moreover, the findings indicate that mycotoxin contamination is more prevalent in developing countries (Barbosa et al., 2013; Fallah et al., 2014), which might be due to the lack of stringent regulations and monitoring and surveillance due to weak institutional efforts (Chilaka et al., 2022). Moreover, farmers in developing countries often produce aquafeeds under unsuitable conditions, including incorrect milling and storage (Marijani et al., 2019), which could increase the chances of mycotoxin contamination.
The detrimental effects of mycotoxins to fish and to humans have necessitated the establishment of stringent international regulations on mycotoxins, and various permissible limits have been set. Although mycotoxin legislation around the world varies, the lack of a clear approach has resulted in variations in standards, particularly regarding the maximum permissible levels imposed among countries and international organizations. Furthermore, regulations may not exist or may exist but not always be followed in many developing countries, especially where there are problems with food availability (Poroșnicu et al., 2023).
5 Research gaps and future research
Despite the growing body of research on mycotoxins in aquafeed, several gaps remain that limit the full understanding of this issue. Research reveals the global prevalence of mycotoxin contamination, especially in developing countries, but lacks a more detailed examination of specific regions and their unique vulnerabilities. Additionally, while the detrimental effects of mycotoxins on aquaculture species are acknowledged, there is limited focus on species-specific research, particularly in terms of tolerance and sensitivity to different mycotoxins. Another significant gap lies in the inconsistent regulations across countries, where a deeper analysis of the impact of these regulatory variations on aquafeed trade and local aquaculture industries, especially in nations with weaker institutional frameworks, is needed. Moreover, the existing research does not sufficiently address the role of climate change, which may exacerbate mycotoxin contamination, particularly in tropical regions where favorable conditions for toxin production exist. Furthermore, while the research highlights the negative effects of mycotoxins to fish and to human, practical strategies for mitigation, such as detoxification methods or the use of alternative feed ingredients, are not discussed in enough detail.
Future research should focus on species-specific mycotoxin tolerance to provide tailored solutions for different aquaculture species. Regional studies that dive deeper into the specific conditions of developing countries can offer more nuanced insights into the factors contributing to mycotoxin occurrence. The harmonization of global regulations on permissible mycotoxin levels is another key area for future research. Priority should be given to aligning regulations across key aquaculture-producing regions, such as the African Union (AU), Association of Southeast Asian Nations (ASEAN), and Latin America’s MERCOSUR bloc. Leveraging existing global frameworks like the Codex Alimentarius, particularly its Code of Practice for the Prevention and Reduction of Mycotoxin Contamination in Cereals, could help standardize permissible limits, analytical protocols, and surveillance strategies.
As climate change is expected to alter environmental conditions, its influence on the prevalence and dynamics of mycotoxin contamination in aquafeed warrants further exploration. Developing and evaluating practical, cost-effective mitigation strategies, such as detoxification techniques or the introduction of alternative feed ingredients, is also crucial. Future studies should explore and validate interventions such as the use of mycotoxin binders (e.g., bentonite, activated carbon), biological detoxification through fermentation, and biotransformation using microbial enzymes capable of degrading aflatoxins and other common mycotoxins. These approaches are particularly relevant for small-scale and resource-limited producers in developing countries, where access to advanced technology is limited.
Additionally, interdisciplinary research networks that bring together experts in toxicology, aquaculture, feed technology, and policy could foster more comprehensive solutions to manage the mycotoxin challenge. These efforts are necessary to safeguard both the aquaculture industry and the populations reliant on fish as a key food source, particularly in the most vulnerable regions.
6 Limitations of the study
Despite its comprehensive scope, the current study was not without its limitations. The first limitation was the inability of the bibliometric software to distinguish between articles focusing on human and animal models. Secondly, a few authors in the data may have duplicate names, and some may have held honorary or part-time positions at different universities. Additionally, the terms “mycotoxin” and “mycotoxins” have been treated as two distinct terms because the program cannot distinguish the difference in writing. Moreover, VOSviewer’s visualizations are mostly limited to network graphs, and it does not support other types of visual representations like geospatial maps, tree maps, spectrograms, or data different from the bibliography dataset (Bukar et al., 2023). In recent years, software writers have gradually improved these deficiencies, and appropriate improvements can be used to reduce the impact of these limitations. In future research, we will further revise these deficiencies, strengthen the analysis, and obtain more detailed and accurate research conclusions.
The bibliometric analysis relied solely on a single scholarly database (WoS). As WoS consistently updates its database, data downloaded for a specific time frame may not include other publications added after the time frame. Furthermore, while WoS is extensive, it may not capture all relevant research outputs, particularly from non-English sources or studies indexed in other major databases such as Scopus, Pubmed and Google Scholar. Future research could address these limitations by incorporating data from additional databases, such as Scopus, Pubmed, and Google Scholar, to provide a more comprehensive and global perspective on research in mycotoxins on aquafeed.
7 Conclusion
This study conducted a combined bibliometric analysis and conventional review of global mycotoxin research in aquafeed. The study’s findings showed that publications on the topic have gradually expanded over the last 10 years, with substantial international participation. It reveals several publications in this field, from various countries, and published in various journals. The publications on mycotoxin research in aquafeed have garnered several citations, depicting the scientific impact of these studies. The scientometric analysis mapped research trends using statistical indices, revealing knowledge gaps and emerging priorities. From the conventional review, there is evidence of the occurrence of mycotoxins in aquafeed and feed ingredients in various countries and regions, indicating that contamination of aquafeed with mycotoxins is a common and global problem. Mycotoxin occurrence is more prevalent in regions dominated by warm climatic conditions, especially in developing countries, which lack stringent regulations, monitoring, and surveillance due to weak institutional efforts. The detrimental effects of mycotoxins have necessitated the establishment of international regulations and permissible limits. However, standards, particularly regarding the maximum permissible levels imposed, vary significantly across countries and international organizations.
This study connects various elements within the literature on mycotoxin in aquafeeds by gathering essential information from sources that are most reliable. The most important studies in this discipline are highlighted in this paper, which will likely become the benchmark for mycotoxin research and regulations in the future. Although significant research has been conducted on mycotoxins in aquafeed, important gaps remain. These include a lack of regional specificity, limited species-specific studies on mycotoxin tolerance, and inconsistent regulatory frameworks across countries. Research also overlooks the role of climate change in exacerbating mycotoxin contamination and lacks practical mitigation strategies. Future research should focus on species-specific tolerance, regional analyses, harmonization of regulations, and the impact of climate change. Moreover, developing cost-effective mitigation techniques and fostering interdisciplinary research networks will be crucial for managing mycotoxins and safeguarding aquaculture industries, especially in vulnerable regions.
Author contributions
MM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. SuS: Methodology, Writing – original draft. JBM: Methodology, Writing – original draft, Writing – review & editing. FS: Conceptualization, Methodology, Resources, Validation, Writing – review & editing. KO: Validation, Writing – review & editing. JM: Validation, Writing – review & editing. SE: Funding acquisition, Resources, Validation, Writing – review & editing. SeS: Funding acquisition, Resources, Validation, Writing – review & editing. SC: Validation, Visualization, Writing – review & editing. DB: Validation, Visualization, Writing – review & editing. RY: Funding acquisition, Validation, Writing – review & editing. CT: Conceptualization, Funding acquisition, Methodology, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the following organizations and agencies: the Norwegian Agency for Development Cooperation (NORAD) (Grant No: SAFA-21/0004); Australian Centre for International Agricultural Research (ACIAR) (Protein Africa -Grant No: LS/2020/154), Netherlands Organization for Scientific Research, WOTRO Science for Global Development (NWO-WOTRO) (ILIPA-W 08.250.202), IKEA Foundation (G-2204-02144), Global Affairs Canada (BRAINS project: P011585), European Commission (HORIZON 101060762 NESTLER and HORIZON 101136739 INNOECOFOOD), Postkode Lottery, Sweden [Waste for Cash Eco Project (WACEP-PJ1651)], The French Ministry of Europe and Foreign Affairs (BIO Kenya project-FEF N°2024-53), Novo Nordisk Foundation (RefIPro: NNF22SA0078466), the Rockefeller Foundation (WAVE-IN-Grant No: 2021 FOD 030); Bill and Melinda Gates Foundation (INV-032416); the Curt Bergfors Foundation Food Planet Prize Award; Norwegian Agency for Development Cooperation, the Section for research, innovation, and higher education grant number RAF-3058 KEN-18/0005 (CAP-Africa); the Swedish International Development Cooperation Agency (SIDA); the Swedish International Development Cooperation Agency (Sida); the Swiss Agency for Development and Cooperation (SDC); the Australian Centre for International Agricultural Research (ACIAR); the Government of Norway; the German Federal Ministry for Economic Cooperation and Development (BMZ); and the Government of the Republic of Kenya.
Acknowledgments
We are grateful to the Data Management, Modelling, and Geo-Information (DMMG) Unit of the International Centre of Insect Physiology and Ecology (icipe), especially Mr. Raphael Mongare, and colleagues at Tamil Nadu Dr. J. Jayalalithaa Fisheries University for their support in developing this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views expressed herein do not necessarily reflect the official opinion of the donors.
Footnotes
2. ^https://kfs.edu.eg/engkfs/
3. ^International Agency for Research on Cancer List of Classifications. Available online: https://monographs.iarc.fr/list-of-classifications.
References
Ahmed, M., Tabana, Y., Musa, K., and Sandai, D. (2017). Effects of different mycotoxins on humans, cell genome and their involvement in cancer. Oncol. Rep. 37, 1321–1336. doi: 10.3892/or.2017.5424
Akuru, U. B. (2019). Research funding issues in African universities: penalties and pathways. J. Sustain. Dev. Stud. 12, 305–327.
Albero, B., Fernández-Cruz, M. L., and Pérez, R. A. (2022). Simultaneous determination of 15 mycotoxins in aquaculture feed by liquid chromatography–tandem mass spectrometry. Toxins 14:316. doi: 10.3390/toxins14050316
Altuğ, G., and Özyurt, G. (2003). Level of aflatoxin in some fish feeds from fish farming processes, feed factories and imported feeds. Turkish J. Vet. Anim. Sci. 27, 1247–1252.
Amin, M., Ahmad, W., Khan, K., and Sayed, M. (2022). Mapping research knowledge on rice husk ash application in concrete: a scientometric review. Materials 15:3431. doi: 10.3390/ma15103431
Anater, A., Manyes, L., Meca, G., Ferrer, E., Luciano, F. B., Pimpão, C. T., et al. (2016). Mycotoxins and their consequences in aquaculture: a review. Aquaculture 451, 1–10. doi: 10.1016/j.aquaculture.2015.08.022
Andriamamonjy, A., Saelens, D., and Klein, R. (2019). A combined scientometric and conventional literature review to grasp the entire BIM knowledge and its integration with energy simulation. J. Build. Eng. 22, 513–527. doi: 10.1016/j.jobe.2018.12.021
Anukul, N., Vangnai, K., and Mahakarnchanakul, W. (2013). Significance of regulation limits in mycotoxin contamination in Asia and risk management programs at the national level. J. Food Drug Anal. 21, 227–241. doi: 10.1016/j.jfda.2013.07.009
Aria, M., and Cuccurullo, C. (2017). Bibliometrix: an R-tool for comprehensive science mapping analysis. J. Informet. 11, 959–975. doi: 10.1016/j.joi.2017.08.007
Ashiq, S. (2015). Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Comp. Rev. Food Sci. Food Safe 14, 159–175. doi: 10.1111/1541-4337.12122
Balló, A., Busznyákné Székvári, K., Czétány, P., Márk, L., Török, A., Szántó, Á., et al. (2023). Estrogenic and non-estrogenic disruptor effect of zearalenone on male reproduction: a review. Int. J. Mol. Sci. 24:1578. doi: 10.3390/ijms24021578
Barbosa, T. S., Pereyra, C. M., Soleiro, C. A., Dias, E. O., Oliveira, A. A., Keller, K. M., et al. (2013). Mycobiota and mycotoxins present in finished fish feeds from farms in the Rio de Janeiro state, Brazil. Int. Aquat. Res. 5:3. doi: 10.1186/2008-6970-5-3
Bashorun, A., Hassan, Z. U., Al-Yafei, M. A.-A., and Jaoua, S. (2023). Fungal contamination and mycotoxins in aquafeed and tissues of aquaculture fishes and their biological control. Aquaculture 576:739892. doi: 10.1016/j.aquaculture.2023.739892
Battilani, P., Toscano, P., Van der Fels-Klerx, H. J., Moretti, A., Camardo Leggieri, M., Brera, C., et al. (2016). Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific reports, 6:24328. doi: 10.1038/srep24328
Bégin-Caouette, O., Aarrevaara, T., Rose, A. L., and Arimoto, A. (2023). “International research collaboration practices and outcomes: a comparative analysis of academics’ international research activities” in Internationalization and the academic profession: Comparative perspectives. eds. A. Çalıkoğlu, G. A. Jones, and Y. Kim (Cham: Springer International Publishing), 191–215.
Bennett, J. W. (1987). Mycotoxins, mycotoxicoses, mycotoxicology and mycopathologia. Mycopathologia 100, 3–5. doi: 10.1007/BF00769561
Bennett, J. W., and Klich, M. (2003). Mycotoxins. Clin. Microbiol. Rev. 16, 497–516. doi: 10.1128/CMR.16.3.497-516.2003
Berthiller, F., Sulyok, M., Krska, R., and Schuhmacher, R. (2007). Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int. J. Food Microbiol. 119, 33–37. doi: 10.1016/j.ijfoodmicro.2007.07.022
Bhat, R., Ramakrishna, Y., Beedu, S., and Munshi, K. L. (1989). Outbreak of trichothecene mycotoxicosis associated with consumption of mould-damaged wheat products in Kashmir Valley, India. Lancet 333, 35–37. doi: 10.1016/S0140-6736(89)91684-X
Bryden, W. L. (2012). Mycotoxin contamination of the feed supply chain: implications for animal productivity and feed security. Anim. Feed Sci. Technol. 173, 134–158. doi: 10.1016/j.anifeedsci.2011.12.014
Bukar, U. A., Sayeed, M. S., Razak, S. F. A., Yogarayan, S., Amodu, O. A., and Mahmood, R. A. R. (2023). A method for analyzing text using VOSviewer. MethodsX 11:102339. doi: 10.1016/j.mex.2023.102339
Carvalho Gonçalves-Nunes, E. M., Gomes-Pereira, M. M., Raposo-Costa, A. P., da Rocha-Rosa, C. A., Pereyra, C. M., Calvet, R. M., et al. (2016). Screening of aflatoxin B1 and mycobiota related to raw materials and finished feed destined for fish. Lat. Am. J. Aquat. Res. 43, 595–600. doi: 10.3856/vol43-issue3-fulltext-22
Cendoya, E., Chiotta, M. L., Zachetti, V., Chulze, S. N., and Ramirez, M. L. (2018). Fumonisins and fumonisin-producing fusarium occurrence in wheat and wheat by products: a review. J. Cereal Sci. 80, 158–166. doi: 10.1016/j.jcs.2018.02.010
Chadegani, A., Salehi, H., Yunus, M., Farhadi, H., Fooladi, M., Farhadi, M., et al. (2013). A comparison between two main academic literature collections: Web of science and Scopus databases. arXiv [Preprint]. doi: 10.48550/arXiv.1305.0377
Chaudhuri, R., Chavan, G., Vadalkar, S., Vrontis, D., and Pereira, V. (2020). Two-decade bibliometric overview of publications in the journal of knowledge management. J. Knowl. Manag. doi: 10.1108/JKM-07-2020-0571
Chen, C. (2020). A glimpse of the first eight months of the COVID-19 literature on Microsoft academic graph: themes, citation contexts, and uncertainties. Front. Res. Metr. Anal. 5:607286. doi: 10.3389/frma.2020.607286
Chilaka, C. A., Obidiegwu, J. E., Chilaka, A. C., Atanda, O. O., and Mally, A. (2022). Mycotoxin regulatory status in Africa: a decade of weak institutional efforts. Toxins 14:442. doi: 10.3390/toxins14070442
Chong, R. S.-M. (2022). “Fungal toxins in feedstuffs.” Aquaculture Pathophysiology. Academic Press, 673–676. doi: 10.1016/B978-0-12-812211-2.00057-3
Choudhri, A. F., Siddiqui, A., Khan, N. R., and Cohen, H. L. (2015). Understanding bibliometric parameters and analysis. Radiographics 35, 736–746. doi: 10.1148/rg.2015140036
Chukwudi, U. P., Kutu, F. R., and Mavengahama, S. (2021). Mycotoxins in maize and implications on food security: a review. AG. doi: 10.18805/ag.R-140
Cimbalo, A., Alonso-Garrido, M., Font, G., and Manyes, L. (2020). Toxicity of mycotoxins in vivo on vertebrate organisms: a review. Food Chem. Toxicol. 137:111161. doi: 10.1016/j.fct.2020.111161
Claeys, L., Romano, C., De Ruyck, K., et al. (2020). Mycotoxin exposure and human cancer risk: a systematic review of epidemiological studies. Compr. Rev. Food Sci. Food Saf. 19, 1449–1464. doi: 10.1111/1541-4337.12567
Deng, S. X., Tian, L. X., Liu, F. J., Jin, S. J., Liang, G. Y., Yang, H. J., et al. (2010). Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus × O. aureus) during long-term dietary exposure. Aquaculture 307, 233–240. doi: 10.1016/j.aquaculture.2010.07.029
Devos, P., and Menard, J. (2019). Bibliometric analysis of research relating to hypertension reported over the period 1997–2016. J. Hypertens. 37, 2116–2122. doi: 10.1097/HJH.0000000000002143
Diab, A. M., Salem, R. M., Abeer, E. K., Ali, G. I., and El-Habashi, N. (2018). Experimental ochratoxicosis a in Nile tilapia and its amelioration by some feed additives. Int. J. Vet. Sci. Med. 6, 149–158. doi: 10.1016/j.ijvsm.2018.09.004
EFSA Panel on Contaminants in the Food Chain (CONTAM) (2011). Scientific opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 9:2481. doi: 10.2903/j.efsa.2011.2481
EFSA Panel on Contaminants in the Food Chain (CONTAM) Knutsen, H. K., Alexander, J., Barregård, L., Bignami, M., Brüschweiler, B., et al. (2017). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 15:e04718. doi: 10.2903/j.efsa.2017.4718
EFSA Panel on Contaminants in the Food Chain (CONTAM) Schrenk, D., Bodin, L., Chipman, J. K., del Mazo, J., Grasl-Kraupp, B., et al. (2020). Risk assessment of ochratoxin a in food. EFSA J. 18:e06113. doi: 10.2903/j.efsa.2020.6113
El-Sayed, Y. S., and Khalil, R. H. (2009). Toxicity, biochemical effects and residue of aflatoxin B1 in marine water-reared sea bass (Dicentrarchus labrax L.). Food Chem. Toxicol. 47, 1606–1609. doi: 10.1016/j.fct.2009.04.008
Erazo, J. G., Palacios, S. A., Veliz, N. A., del Canto, A., Plem, S., Ramirez, M. L., et al. (2023). Effect of temperature, water activity and incubation time on Trichothecene production by fusarium cerealis isolated from durum wheat grains. Pathogens 12:736. doi: 10.3390/pathogens12050736
European Commission (EC) (2006) Commission regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs (text with EEA relevance). Official Journal of the European Union. L 364, 5–24.
Fallah, A. A., Pirali-Kheirabadi, E., Rahnama, M., Saei-Dehkordi, S. S., and Pirali-Kheirabadi, K. (2014). Mycoflora, aflatoxigenic strains of aspergillus section Flavi and aflatoxins in fish feed. Qual. Assuran. Safety Crops Foods 6, 419–424. doi: 10.3920/QAS2012.0186
Fandohan, P., Hell, K., Marasas, W. F. O., and Wingfield, M. J. (2003) Review- infection of maize by fusarium species and contamination with fumonisin in Africa. African Journal of biotechnology, 2, 570–579. doi: 10.5897/AJB2003.000-1110
FDAF (2021). Compliance policy guide sec. 555.400 aflatoxins in human food. Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/compliance-policy-guide-sec-555400-aflatoxins-human-food. (Accessed July 23, 2023).
Fink-Gremmels, J., and Malekinejad, H. J. (2007). Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 137, 326–341. doi: 10.1016/j.anifeedsci.2007.06.008
Foluke, O. M., Adegboyega, C. O., and Chibundu, N. E., (2016) Detection of aspergillus section Flavi and aflatoxins in locally formulated fish feeds from South-Western Nigeria. In: International conference on Mycology & Mushrooms. San Antonio, TX
Food and Agriculture Organization (2003). Worldwide regulations for mycotoxins in food and feed in 2023. Available online at: https://www.fao.org/3/y5499e/y5499e00.htm#Contents (Accessed July 23, 2023)
Frenich, A. G., Romero-González, R., and del Mar, A. L. M. (2014). Comprehensive analysis of toxics (pesticides, veterinary drugs and mycotoxins) in food by UHPLC-MS. TrAC Trends Anal. Chem. 63, 158–169. doi: 10.1016/j.trac.2014.06.020
Gallo, A., Giuberti, G., Frisvad, J., Bertuzzi, T., and Nielsen, K. (2015). Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 7, 3057–3111. doi: 10.3390/toxins7083057
Garcia-Cela, E., Kiaitsi, E., Sulyok, M., Medina, A., and Magan, N. (2018). Fusarium graminearum in stored wheat: use of CO2 production to quantify dry matter losses and relate this to relative risks of Zearalenone contamination under interacting environmental conditions. Toxins 10:86. doi: 10.3390/toxins10020086
Gaviria-Marin, M., Merigó, J. M., and Baier-Fuentes, H. (2019). Knowledge management: a global examination based on bibliometric analysis. Technol. Forecast. Soc. Chang. 140, 194–220. doi: 10.1016/j.techfore.2018.07.006
Gelineau-van Waes, J., Voss, K. A., Stevens, V. L., Speer, M. C., and Riley, R. T. (2009). Maternal fumonisin exposure as a risk factor for neural tube defects. Adv. Food Nutr. Res. 56, 145–181. doi: 10.1016/S1043-4526(08)00605-0
George, T. T., Obilana, A. O., Oyenihi, A. B., and Rautenbach, F. G. (2021). Moringa oleifera through the years: a bibliometric analysis of scientific research (2000-2020). S. Afr. J. Bot. 141, 12–24. doi: 10.1016/j.sajb.2021.04.025
Gil-Serna, J., Vázquez, C., and Patiño, B. (2019). “Mycotoxins | toxicology” in Reference module in food science (Elsevier).
Gonçalves, R. A., Naehrer, K., and Santos, G. A. (2018). Occurrence of mycotoxins in commercial aquafeeds in Asia and Europe: a real risk to aquaculture? Rev. Aquac. 10, 263–280. doi: 10.1111/raq.12159
Guerre, P. (2016). Worldwide mycotoxins exposure in pig and poultry feed formulations. Toxins 8:350. doi: 10.3390/toxins8120350
Hooft, J. M., Elmor, A. E. H. I., Encarnação, P., and Bureau, D. P. (2011). Rainbow trout (Oncorhynchus mykiss) is extremely sensitive to the feed-borne fusarium mycotoxin deoxynivalenol (DON). Aquaculture 311, 224–232. doi: 10.1016/j.aquaculture.2010.11.049
Iftikhar, B., Alih, S., Vafaei, M., Alrowais, R., Bashir, M., Khalil, A., et al. (2023). A scientometric analysis approach on the plastic sand. Heliyon 9:e14457. doi: 10.1016/j.heliyon.2023.e14457
Iqbal, S. Z., Asi, M. R., Hanif, U., Zuber, M., and Jinap, S. (2016a). The presence of aflatoxins and ochratoxin a in rice and rice products; and evaluation of dietary intake. Food Chem. 210, 135–140. doi: 10.1016/j.foodchem.2016.04.104
Iqbal, S. Z., Selamat, J., and Ariño, A. (2016b). “Mycotoxins in food and food products: current status” in Food safety. eds. J. Selamat and S. Z. Iqbal (Cham: Springer International Publishing), 113–123. doi: 10.1007/978-3-319-39253-0_6
Jaiswar, R., Sarathchandra, G., Shanmugam, S. A., Felix, N., and Narayanan, A. L. (2022). Assessment of total aflatoxin (AFB1, AFB2, AFG1 and AFG2) in fish feed and feedstuffs by using high performance thin layer chromatography. Pharma Innov. J. 11, 1371–1377. doi: 10.22271/tpi.2022.v11.i9Sq.15535
Jannathulla, R., Rajaram, V., Kalanjiam, R., Ambasankar, K., Muralidhar, M., and Dayal, J. S. (2019). Fishmeal availability in the scenarios of climate change: inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 50, 3493–3506. doi: 10.1111/are.14324
Jiménez Medina, M. L., Lafarga, T., Garrido Frenich, A., and Romero-González, R. (2021). Natural occurrence, legislation, and determination of aflatoxins using chromatographic methods in food: a review (from 2010 to 2019). Food Rev. Intl. 37, 244–275. doi: 10.1080/87559129.2019.1701009
Kaiser, F., Harbach, H., and Schulz, C. (2022). Rapeseed proteins as fishmeal alternatives: a review. Rev. Aquac. 14, 1887–1911. doi: 10.1111/raq.12678
Kamle, M., Mahato, D. K., Gupta, A., Pandhi, S., Sharma, B., Dhawan, K., et al. (2022). Deoxynivalenol: an overview on occurrence, Chemistry, biosynthesis, health effects and its detection, management, and control strategies in food and feed. Microbiol. Res. 13, 292–314. doi: 10.3390/microbiolres13020023
Karanatsiou, D., Misirlis, N., and Vlachopoulou, M. (2017). Bibliometrics and altmetrics literature review: performance indicators and comparison analysis. PMM 18, 16–27. doi: 10.1108/PMM-08-2016-0036
Kebede, H., Liu, X., Jin, J., and Xing, F. (2020). Current status of major mycotoxins contamination in food and feed in Africa. Food Control 110:106975. doi: 10.1016/j.foodcont.2019.106975
Khitska, O., and Gerard, R. (2019). International and national legislation to control mictoxins in food. Науковий вісник ветеринарної медицини 1, 30–40. doi: 10.33245/2310-4902-2019-149-1-30-40
Koletsi, P., Schrama, J. W., Graat, E. A. M., Wiegertjes, G. F., Lyons, P., and Pietsch, C. (2021). The occurrence of mycotoxins in raw materials and fish feeds in Europe and the potential effects of Deoxynivalenol (DON) on the health and growth of farmed fish species—a review. Toxins 13:403. doi: 10.3390/toxins13060403
Köppen, R., Koch, M., Siegel, D., Merkel, S., Maul, R., and Nehls, I. (2010). Determination of mycotoxins in foods: current state of analytical methods and limitations. Appl. Microbiol. Biotechnol. 86, 1595–1612. doi: 10.1007/s00253-010-2535-1
Krogh, P. (1969). The pathology of mycotoxicoses. J. Stored Prod. Res. 5, 259–264. doi: 10.1016/0022-474X(69)90041-1
Kumar, V., Roy, S., Barman, D., Kumar, A., Paul, L., and Meetei, W. A. (2013). Importance of mycotoxins in aquaculture feeds. Aquaculture Asia, 18, 25–29.
Kyei, N. N., Boakye, D., and Gabrysch, S. (2020). Maternal mycotoxin exposure and adverse pregnancy outcomes: a systematic review. Mycotoxin Res. 36, 243–255. doi: 10.1007/s12550-019-00384-6
Li, J., Fei, X., Wang, S., Xu, Z., Xu, F., Wang, J., et al. (2024). A bibliometric analysis of the WoSCC literature on the use of selective serotonin reuptake inhibitors as antidepressants. Drug Des. Devel. Ther. 18, 4961–4974. doi: 10.2147/DDDT.S476680
Lu, X., Lu, C., Yang, Y., Shi, X., Wang, H., Yang, N., et al. (2021). Current status and trends in peptide receptor radionuclide therapy in the past 20 years (2000–2019): a bibliometric study. Front. Pharmacol. 12:624534. doi: 10.3389/fphar.2021.624534
Lumlertdacha, S., Lovell, R. T., Shelby, R. A., Lenz, S. D., and Kemppainen, B. W. (1995). Growth, hematology, and histopathology of channel catfish, Ictalurus punctatus, fed toxins from fusarium moniliforme. Aquaculture 130, 201–218. doi: 10.1016/0044-8486(94)00219-E
Magan, N., and Aldred, D. (2005). Conditions of formation of ochratoxin a in drying, transport and in different commodities. Food Addi. Contamin. 22, 10–16. doi: 10.1080/02652030500412154
Magan, N., and Lacey, J. (1984). Effect of temperature and pH on water relations of field and storage fungi. Trans. Br. Mycol. Soc. 82, 71–81. doi: 10.1016/S0007-1536(84)80213-2
Magnoli, A. P., Poloni, V. L., and Cavaglieri, L. (2019). Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 29, 99–108. doi: 10.1016/j.cofs.2019.08.009
Malir, F., Ostry, V., Pfohl-Leszkowicz, A., Malir, J., and Toman, J. (2016). Ochratoxin a: 50 years of research. Toxins 8:191. doi: 10.3390/toxins8070191
Manning, B. B., Ulloa, R. M., Li, M. H., Robinson, E. H., and Rottinghaus, G. E. (2003). Ochratoxin a fed to channel catfish (Ictalurus punctatus) causes reduced growth and lesions of hepatopancreatic tissue. Aquaculture 219, 739–750. doi: 10.1016/S0044-8486(03)00033-4
Marijani, E., Kigadye, E., and Okoth, S. (2019). Occurrence of Fungi and mycotoxins in fish feeds and their impact on fish health. Int. J. Microbiol. 2019, 1–17. doi: 10.1155/2019/6743065
Marijani, E., Wainaina, J. M., Charo-Karisa, H., Nzayisenga, L., Munguti, J., Joselin Benoit Gnonlonfin, G., et al. (2017). Mycoflora and mycotoxins in finished fish feed and feed ingredients from smallholder farms in East Africa. Egypt. J. Aquat. Res. 43, 169–176. doi: 10.1016/j.ejar.2017.07.001
Markscheffel, B., and Schröter, F. (2021). Comparison of two science mapping tools based on software technical evaluation and bibliometric case studies. Collnet J. Scientometrics Inf. Manag. 15, 365–396. doi: 10.1080/09737766.2021.1960220
Marroquín-Cardona, A. G., Johnson, N. M., Phillips, T. D., and Hayes, A. W. (2014). Mycotoxins in a changing global environment – a review. Food Chem. Toxicol. 69, 220–230. doi: 10.1016/j.fct.2014.04.025
Matejova, I., Modra, H., Blahova, J., Franc, A., Fictum, P., Sevcikova, M., et al. (2014). The effect of mycotoxin deoxynivalenol on haematological and biochemical indicators and histopathological changes in rainbow trout (Oncorhynchus mykiss). Biomed. Res. Int. 2014, 1–5. doi: 10.1155/2014/310680
Matejova, I., Svobodova, Z., Vakula, J., Mares, J., and Modra, H. (2017). Impact of mycotoxins on aquaculture fish species: a review. J. World Aquacult. Soc. 48, 186–200. doi: 10.1111/jwas.12371
Medina, A., Mohale, S., Samsudin, N. I. P., Rodriguez-Sixtos, A., Rodriguez, A., and Magan, N. (2017). Biocontrol of mycotoxins: Dynamics and mechanisms of action. Current Opinion in Food Science, 17, 41–48. doi: 10.1016/j.cofs.2017.09.008
Milani, J. M. (2013). Ecological conditions affecting mycotoxin production in cereals: a review. Vet. Med. 58, 405–411. doi: 10.17221/6979-VETMED
Monson, M. S., Coulombe, R. A., and Reed, K. M. (2015). Aflatoxicosis: lessons from toxicity and responses to aflatoxin B1 in poultry. Agriculture 5, 742–777. doi: 10.3390/agriculture5030742
Moretti, A., Pascale, M., and Logrieco, A. F. (2019). Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 84, 38–40. doi: 10.1016/j.tifs.2018.03.008
Mwihia, E. W., Lyche, J. L., Mbuthia, P. G., Ivanova, L., Uhlig, S., Gathumbi, J. K., et al. (2020). Co-occurrence and levels of mycotoxins in fish feeds in Kenya. Toxins 12:627. doi: 10.3390/toxins12100627
Mwihia, E., Mbuthia, P., Eriksen, G., et al. (2018). Occurrence and levels of aflatoxins in fish feeds and their potential effects on fish in Nyeri, Kenya. Toxins 10:543. doi: 10.3390/toxins10120543
Nácher-Mestre, J., Serrano, R., Beltrán, E., Pérez-Sánchez, J., Silva, J., Karalazos, V., et al. (2015). Occurrence and potential transfer of mycotoxins in gilthead sea bream and Atlantic salmon by use of novel alternative feed ingredients. Chemosphere 128, 314–320. doi: 10.1016/j.chemosphere.2015.02.021
Nakavuma, J. L., Kirabo, A., Bogere, P., Nabulime, M. M., Kaaya, A. N., and Gnonlonfin, B. (2020). Awareness of mycotoxins and occurrence of aflatoxins in poultry feeds and feed ingredients in selected regions of Uganda. Food Contamin. 7:1. doi: 10.1186/s40550-020-00079-2
Namulawa, V. T., Mutiga, S., Musimbi, F., Akello, S., Ngángá, F., Kago, L., et al. (2020). Assessment of fungal contamination in fish feed from the Lake Victoria Basin, Uganda. Toxins 12:233. doi: 10.3390/toxins12040233
Ndube, N., van der Westhuizen, L., and Shephard, G. S. (2009). Determination of fumonisins in maize by HPLC with ultraviolet detection of o-phthaldialdehyde derivatives. Mycotoxin Res. 25, 225–228. doi: 10.1007/s12550-009-0031-1
Obileke, K., Onyeaka, H., Omoregbe, O., Makaka, G., Nwokolo, N., and Mukumba, P. (2020). Bioenergy from bio-waste: a bibliometric analysis of the trend in scientific research from 1998–2018. Biomass Convers. Biorefinery 12, 1077–1092. doi: 10.1007/s13399-020-00832-9
Okoroigwe, C. E., Agbasi, S. I., Okoroigwe, C. F., and Okoroigwe, C. (2022) University-industry linkages: the gateway for accelerating National Development in Africa. In Proceedings of the international conference on industrial engineering and operations management Nsukka (pp. 5–7).
Okun, D. O., Khamis, F. M., Muluvi, G. M., Ngeranwa, J. J., Ombura, F. O., Yongo, M. O., et al. (2015). Distribution of indigenous strains of atoxigenic and toxigenic aspergillus flavus and aspergillus parasiticus in maize and peanuts agro-ecological zones of Kenya. Agric. Food Secur. 4:14. doi: 10.1186/s40066-015-0033-5
Oliveira, M., and Vasconcelos, V. (2020). Occurrence of mycotoxins in fish feed and its effects: a review. Toxins 12:160. doi: 10.3390/toxins12030160
Ottinger, C. A., and Kaattari, S. L. (1998). Sensitivity of rainbow trout leucocytes to aflatoxin B1. Fish Shellfish Immunol. 8, 515–530. doi: 10.1006/fsim.1998.0154
Ou, Z., Qiu, L., Rong, H., Li, B., Ren, S., Kuang, S., et al. (2022). Bibliometric analysis of chimeric antigen receptor-based immunotherapy in cancers from 2001 to 2021. Front. Immunol. 13:822004. doi: 10.3389/fimmu.2022.822004
Pandey, A. K., Samota, M. K., Kumar, A., Silva, A. S., and Dubey, N. K. (2023). Fungal mycotoxins in food commodities: present status and future concerns. Front. Sustain. Food Syst. 7:1162595. doi: 10.3389/fsufs.2023.1162595
Paterson, R. R. M., and Lima, N. (2010). How will climate change affect mycotoxins in food?. Food research international, 43, 1902–1914. doi: 10.1016/j.foodres.2009.07.010
Patil, N. S., and Kakde, U. B. (2017). Assessment of fungal bioaerosol emission in the vicinity of a landfill site in Mumbai, India. IJEWM 20:75. doi: 10.1504/IJEWM.2017.086031
Pietsch, C., Bucheli, T. D., Wettstein, F. E., and Burkhardt-Holm, P. (2011). Frequent biphasic cellular responses of permanent fish cell cultures to deoxynivalenol (DON). Toxicol. Appl. Pharmacol. 256, 24–34. doi: 10.1016/j.taap.2011.07.004
Pietsch, C., Kersten, S., Burkhardt-Holm, P., Valenta, H., and Dänicke, S. (2013). Occurrence of Deoxynivalenol and Zearalenone in commercial fish feed: an initial study. Toxins 5, 184–192. doi: 10.3390/toxins5010184
Pitt, J. I. (2000). Toxigenic fungi and mycotoxins. Br. Med. Bull. 56, 184–192. doi: 10.1258/0007142001902888
Poroșnicu, I., Ailincăi, L. I., Neculai-Văleanu, A. S., Ariton, M. A., and Mareș, M. (2023). Legislative aspects regarding the control of mycotoxins. Sci. Papers Anim. Sci. Biotechnol. 56:153.
Rahmani, A., Jinap, S., and Soleimany, F. (2009). Qualitative and quantitative analysis of mycotoxins. Comp. Rev. Food Sci. Food Safe 8, 202–251. doi: 10.1111/j.1541-4337.2009.00079.x
Richard, J. L. (2007). Some major mycotoxins and their mycotoxicoses—an overview. Int. J. Food Microbiol. 119, 3–10. doi: 10.1016/j.ijfoodmicro.2007.07.019
Rodríguez-Cervantes, C. H., Ramos, A. J., Robledo-Marenco, M. L., Sanchis, V., Marín, S., and Girón-Pérez, M. I. (2013). Determination of aflatoxin and fumonisin levels through ELISA and HPLC, on tilapia feed in Nayarit, Mexico. Food Agric. Immunol. 24, 269–278. doi: 10.1080/09540105.2012.684202
Rogowska, A., Pomastowski, P., Sagandykova, G., and Buszewski, B. (2019). Zearalenone and its metabolites: effect on human health, metabolism and neutralisation methods. Toxicon 162, 46–56. doi: 10.1016/j.toxicon.2019.03.004
Russo, J. R., and Yanong, R. P. (2010). Molds in fish feeds and aflatoxicosis. Gainesville, Florida, USA: Institute of Food and Agriculture Sciences, University of Florida.
Rybecky, A. I., Chulze, S. N., and Chiotta, M. L. (2018). Effect of water activity and temperature on growth and trichothecene production by Fusarium meridionale. Int. J. Food Microbiol. 285, 69–73. doi: 10.1016/j.ijfoodmicro.2018.07.028
Schwartz, P., Bucheli, T. D., Wettstein, F. E., and Burkhardt-Holm, P. (2013). Life-cycle exposure to the estrogenic mycotoxin zearalenone affects zebrafish (Danio rerio) development and reproduction. Environ. Toxicol. 28, 276–289. doi: 10.1002/tox.20718
Shahba, S., Mehrzad, J., and Malvandi, A. (2021). Neuroimmune disruptions from naturally occurring levels of mycotoxins. Environ. Sci. Pollut. Res. 28, 32156–32176. doi: 10.1007/s11356-021-14146-4
Sheikheldin, G. H., and Mohamed, A. A. (2021). Skills for science systems in Africa: The case of ‘brain drain’. Building science Systems in Africa: Conceptual foundations and empirical considerations. Nairobi: Mkuki na Nyota publishers ltd.
Singh, J., and Mehta, A. (2020). Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: a review. Food Sci. Nutr. 8, 2183–2204. doi: 10.1002/fsn3.1474
Song, Y., Liu, W., Zhao, Y., Zang, J., and Gao, H. (2021). Ochratoxin a induces human kidney tubular epithelial cell apoptosis through regulating lipid raft/PTEN/AKT signaling pathway. Environ. Toxicol. 36, 1880–1885. doi: 10.1002/tox.23308
Sun, G., Wang, S., Hu, X., Su, J., Huang, T., Yu, J., et al. (2007). Fumonisin B1 contamination of home-grown corn in high-risk areas for esophageal and liver cancer in China. Food Addit. Contam. 24, 181–185. doi: 10.1080/02652030601013471
Thompson, C., and Henke, S. (2000). Effect of climate and type of storage container on aflatoxin production in corn and its associated risks to wildlife species. J. Wildl. Dis. 36, 172–179. doi: 10.7589/0090-3558-36.1.172
Tuan, N. A., Manning, B. B., Lovell, R. T., and Rottinghaus, G. E. (2003). Responses of Nile tilapia (Oreochromis niloticus) fed diets containing different concentrations of moniliformin or fumonisin B1. Aquaculture 217, 515–528. doi: 10.1016/S0044-8486(02)00268-5
Turner, N. W., Subrahmanyam, S., and Piletsky, S. A. (2009). Analytical methods for determination of mycotoxins: a review. Anal. Chim. Acta 632, 168–180. doi: 10.1016/j.aca.2008.11.010
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
van Egmond, H. P., Schothorst, R. C., and Jonker, M. A. (2007). Regulations relating to mycotoxins in food. Anal. Bioanal. Chem. 389, 147–157. doi: 10.1007/s00216-007-1317-9
Viegas, C., Gomes, A. Q., Faria, T., and Sabino, R. (2015). Prevalence of Aspergillus fumigatus complex in waste sorting and incineration plants: an occupational threat. IJEWM 16:353. doi: 10.1504/IJEWM.2015.074939
Wangia-Dixon, R. N., and Nishimwe, K. (2020). Molecular toxicology and carcinogenesis of fumonisins: a review. J. Environ. Sci. Health C 39, 44–67. doi: 10.1080/26896583.2020.1867449
Wilson, M., Sampson, M., Barrowman, N., and Doja, A. (2021). Bibliometric analysis of neurology articles published in general medicine journals. JAMA Netw. Open 4:e215840. doi: 10.1001/jamanetworkopen.2021.5840
Wolf, H., and Jackson, E. W. (1963). Hepatomas in rainbow trout: descriptive and experimental epidemiology. Science 142, 676–678. doi: 10.1126/science.142.3593.676.b
World Health Organization (2002). Evaluation of certain mycotoxins in food: Fifty-sixth report of the joint FAO/WHO expert committee on food additives. Geneva: World Health Organization.
Woźny, M., Obremski, K., Hliwa, P., Gomułka, P., Różyński, R., Wojtacha, P., et al. (2019). Feed contamination with zearalenone promotes growth but affects the immune system of rainbow trout. Fish Shellfish Immunol. 84, 680–694. doi: 10.1016/j.fsi.2018.10.032
Yan, W., Liao, J., and Zhai, H. (2024). Place-making research: a bibliometric, visualization, and thematic analysis. Buildings 14:2855. doi: 10.3390/buildings14092855
Yu, J., and Pedroso, I. R. (2023). Mycotoxins in cereal-based products and their impacts on the health of humans, livestock animals and pets. Toxins 15:480. doi: 10.3390/toxins15080480
Zhao, Y., Wang, Q., Huang, J., Chen, Z., Liu, S., Wang, X., et al. (2019). Mycotoxin contamination and presence of mycobiota in rice sold for human consumption in China. Food Control 98, 19–23. doi: 10.1016/j.foodcont.2018.11.014
Zingales, V., Taroncher, M., Martino, P. A., Ruiz, M. J., and Caloni, F. (2022). Climate change and effects on molds and mycotoxins. Toxins 14:445. doi: 10.3390/toxins14070445
Zychowski, K. E., Hoffmann, A. R., Ly, H. J., Pohlenz, C., Buentello, A., Romoser, A., et al. (2013). The effect of aflatoxin-B1 on red drum (Sciaenops ocellatus) and assessment of dietary supplementation of NovaSil for the prevention of aflatoxicosis. Toxins 5, 1555–1573. doi: 10.3390/toxins5091555
Keywords: aflatoxin, aquaculture, aquafeed, bibliometric analysis, feed ingredients, mycotoxin
Citation: Meenakshisundaram M, Shanmugam S, Mboya JB, Sugantham F, Obiero K, Munguti J, Ekesi S, Subramanian S, Chia SY, Beesigamukama D, Yossa R and Tanga CM (2025) Mapping global research on mycotoxins in aquafeed from scientometric and critical perspectives. Front. Sustain. Food Syst. 9:1609489. doi: 10.3389/fsufs.2025.1609489
Edited by:
Christian Larbi Ayisi, University of Environment and Sustainable Development, GhanaReviewed by:
Gertrude Dzifa Mensah, University of Environment and Sustainable Development, GhanaSamuel Ayeh Osei, Shanghai Ocean University, China
Copyright © 2025 Meenakshisundaram, Shanmugam, Mboya, Sugantham, Obiero, Munguti, Ekesi, Subramanian, Chia, Beesigamukama, Yossa and Tanga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jimmy B. Mboya, amJyaWFuQGljaXBlLm9yZw==
 Menaga Meenakshisundaram
Menaga Meenakshisundaram Sudarshan Shanmugam
Sudarshan Shanmugam Jimmy B. Mboya
Jimmy B. Mboya Felix Sugantham2
Felix Sugantham2 Kevin Obiero
Kevin Obiero Jonathan Munguti
Jonathan Munguti Sevgan Subramanian
Sevgan Subramanian Dennis Beesigamukama
Dennis Beesigamukama Chrysantus M. Tanga
Chrysantus M. Tanga