- 1Program of Food Sciences and Nutrition, Turabah University College, Taif University, Taif, Saudi Arabia
- 2Department of Food and Nutrition Sciences, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, Saudi Arabia
- 3Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 4Embryonic Stem Cell Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 5Special Infectious Agents Unit—BSL3, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 6Department of Food Science and Nutrition, College of Sciences, Taif University, Taif, Saudi Arabia
- 7Department of Biology, College of Science, Taif University, Taif, Saudi Arabia
- 8Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
- 9Department of Biology, College of Sciences, Umm Al-Qura University, Makkah, Saudi Arabia
- 10Department of Biology, Aljumum University College, Umm Al-Qura University, Makkah, Saudi Arabia
- 11Department of Biology, College of Science and Humanities in Al-Kharj, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
- 12Department of Zoology, Faculty of Science, Suez Canal University, Ismailia, Egypt
Background: The growing prevalence of cardiac diseases has heightened the necessity to adjust modern lifestyles to achieve a better balance and diversification of the nutrients and oil consumption in the daily diet.
Methods: This study explored the impact of various edible oils (0.4 mL/100 g B. W./day) on the biochemical cardiovascular risks in hyperlipidemic rats (olive, coconut, palm, soybean, sunflower, and flaxseed) which coded as OLO, COO, PAO, SOO, SUO, and FLO groups, respectively. The study designed for 60 days, utilized male Sprague–Dawley rats weighing 330–350 g. The rats were divided into seven sections each containing eight rats. Rats were fed a high-fat diet for 21 days to induce hyperlipidemia. Several parameters were assessed, including body weight, glucose, insulin, lipid profile, and key metabolic indicators such as oxidative stress and inflammatory markers, as well as parameters related to the heart and kidneys.
Results: The FLO and OLO groups presented a reduction in body weight of 21.70 g and 20.27 g, respectively. All cardiovascular risk markers and lipid profile values were improved by FLO consumption, except triglycerides (TG) (97.11 mg/dL) and low-density lipoprotein cholesterol (LDL-c) (55.12 mg/dL), which were improved by OLO consumption. FLO and OLO groups also have positive effects on glucose and insulin resistance levels (IRL) and inflammatory cytokines. Rats in the PAO group reported the highest value of IRL, at 23.28 μU/mL, compared to the COL group, at 8.87 μU/mL. SUO group detected a lower value of TNF-α to the COL group at 37.42 pg./mL and 37.55 pg./mL, respectively. The heart cardiac hypertrophy (CH) index of 0.40 mg/dL was found to be the same for the COO, SOO, SUO, and FLO groups.
Conclusion: According to the results, extra experimental work is needed on PAO to find out the relationship between cardiovascular disease risk in the long run compared with the other edible oils. Administration of OLO, FLO, and SUO demonstrated protective activity against these factors.
Introduction
Recent studies on humans and animals have demonstrated that malnutrition may have significant impacts on subsequent anatomical, biochemical, physiological, and behavioral processes (Kharazmi-Khorassani et al., 2021). Low protein and high-fat malnutrition have major impacts on fat metabolism and contributing factors to obesity. Excess body fat is the property of obesity, which is different from insulin resistance (dos Santos et al., 2024). It might not be considered an imminent threat as long as the fat is transported by the lipoproteins and stored in healthy fat cells that react to insulin. Insulin resistance occurs when cells do not react well to circulating insulin (Chen et al., 2023). Reduced insulin sensitivity, poor glucose absorption, hyperglycemia, and type 2 diabetes are all largely caused by changes in lipid homeostasis in living and insulin-sensitive tissues. Dietary changes may be able to lessen or even reverse these changes (Campos et al., 2023). Numerous facets of fatty acid metabolism can be significantly impacted by energy intake and dietary combinations, including dietary fats, carbohydrates, and fibers (Shetty and Kumari, 2021).
A broad variety of packaged foods and pastries, frequently rich in calories, are now widely available at reasonable prices because of the food industry’s progressive globalization (Lopes et al., 2024). Saturated fatty acids (SFA) and hydrogenated or trans fatty acids (TFA) are abundant in the majority of these foods. They are regarded as significant contributors to non-communicable diseases, which the WHO states primarily come through improper eating habits and behaviors, particularly when consistently taken as part of an insufficient diet (Bangulzai et al., 2022). It is recommended to consume more monounsaturated FA (MUFAs), polyunsaturated FA (PUFAs), and particularly omega-3 (Ω-3) PUFAs, while reducing consumption of SFA, and restricted to moderate consumption of animal proteins and simple carbs (Ündağ and Dönmez, 2023; Al-Hoshani et al., 2023; Grover et al., 2021).
Dietary improvements are only achievable through the use of vegetable oils with balanced and beneficial FA profiles (Al-Hoshani et al., 2023). The immune cell populations in adipose tissue undergo dynamic changes as obesity worsens (Çalışkan and Emin, 2023). Low-grade inflammation progresses as a result of this shift from an anti-inflammatory state to pro-inflammatory settings (Salman and Imran, 2022). It is important since all nutrients in the diet have a natural capacity to promote inflammation as they can cause molecular interactions that intensify inflammation when they are converted to energy or metabolized into other biological components (Kaseke et al., 2021). Globally, some of the main causes of morbidity and mortality are cardiovascular illnesses, hepatic disorders, and kidney failure. Furthermore, the growing number of illnesses has brought attention to the topic of nutrition and prompted food specialists to develop natural preventative solutions (Rabail et al., 2021).
The fact that edible oils play an essential part in everyday meals and have a substantial impact on general well-being, it is imperative to inquire into their effects on health. Different oils have different amounts of saturated, unsaturated, and trans fats, which can have an impact on heart health, cholesterol, and the risk of developing chronic illnesses. Establishing rational dietary decisions, maintaining heart health, controlling weight, and avoiding lifestyle-related diseases are all significantly easier with an understanding of their nutritional profiles (Pandey et al., 2023). Rats with hyperlipidemia are frequently employed as experimental models to assess how dietary elements, including edible oils, affect cardiovascular health and lipid metabolism and how various dietary interventions affect oxidative stress indicators, liver function, and plasma lipid profile. Researching the effect of edible oils in hyperlipidemic rats offers important information about their possible benefits or disadvantages, which can guide dietary guidelines and the creation of functional foods.
The current study aimed to investigate how biochemical cardiovascular risks measured by body weight, glucose, insulin, lipid profile, and important metabolic indicators such as oxidative stress, inflammatory markers, heart and kidney-related parameters are affected by various consumption of edible oils in hyperlipidemic rats.
Materials and methods
Animals and edible oils
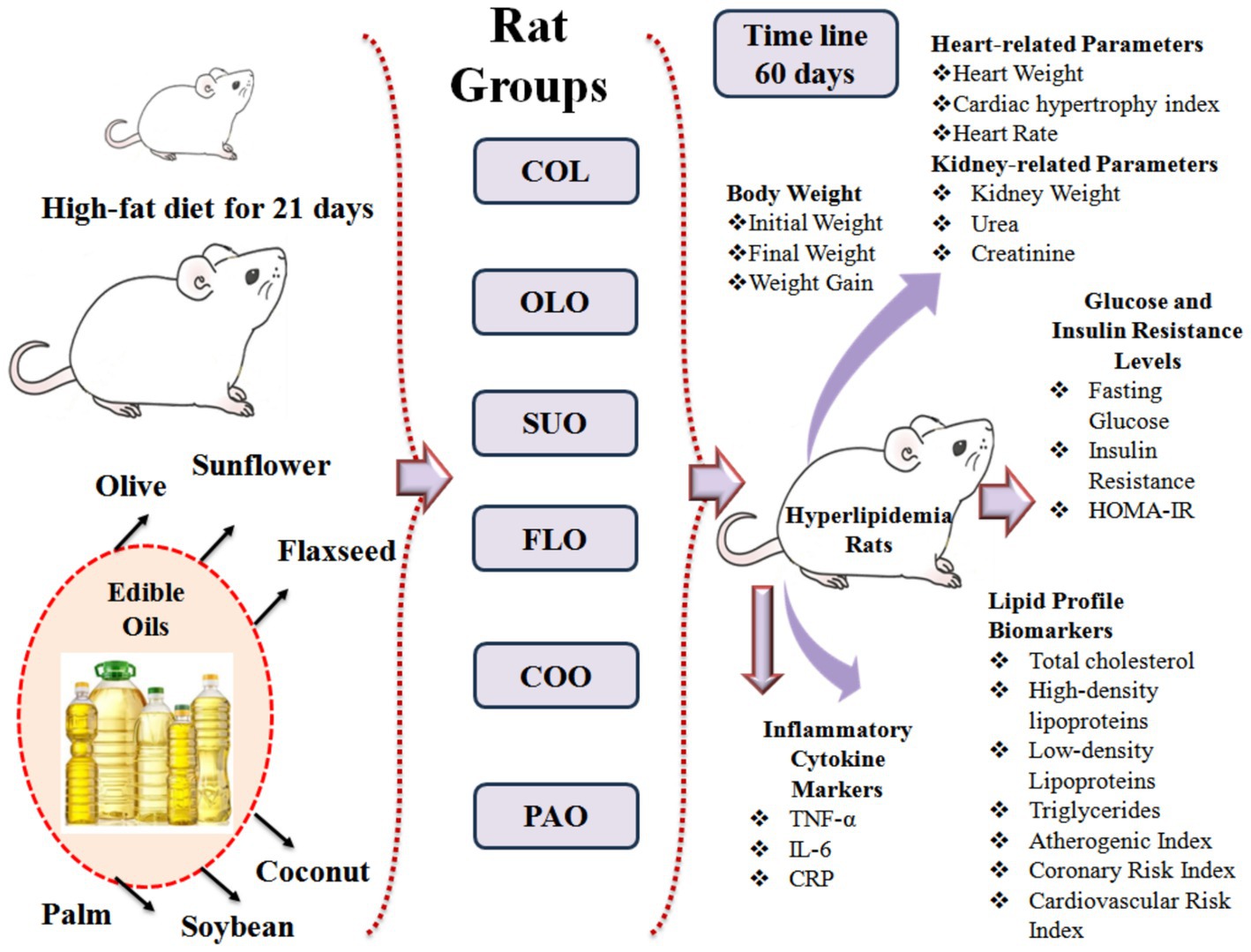
In order to investigate the possible advantages of edible vegetable oils for the biochemical cardiovascular risks in the diet, 60-day efficacy research known as the “treatment trial” was conducted on rats. For this experiment, 56 mature male Sprague–Dawley rats weighing between 330 g and 350 g were selected from the Animal Holding Unit of Biological Science in Jeddah, Saudi Arabia. Rats were housed under regular settings, one-square-foot metallic wire cages with adequate ventilation, a temperature range of 27°C to 30°C, and 12 h of darkness and night cycle were employed. Throughout the trial, the animals were given full access to tap water and the basic chow diet that included all of the food components. Eight rats in one group were appropriately housed in a single cage with a label. Food detritus, feces, and urine were cleared every day to avoid contaminating the food and water. Rats were acclimated for 2 weeks before the trial began by feeding them the typical basic chow diet. They were fed a high-fat diet for 21 days to induce hyperlipidemia. Rats fed a diet high in fats frequently showed several physical symptoms that signal the start of hyperlipidemia. Increased fat deposition usually results in weight gain. Furthermore, fatty liver alterations or hepatomegaly may be indicated by the liver appearing bigger, pale, and greasy. In terms of behavior, afflicted rats may exhibit decreased activity or lethargy, which is indicative of metabolic stress. Changes in fur quality, such as greasiness, dullness, or an untidy appearance, can also occasionally be observed (Rabail et al., 2024). Olive, coconut, palm, soybean, sunflower, and flaxseed oils were purchased from a local market, in Taif City, KSA.
Experimental design
Seven distinct experimental groups were randomly selected among the rats: The first control group (COL) departed without receiving any special care. Approximately (0.4 mL/100 g B. W./day) olive, coconut, palm, soybean, sunflower, and flaxseed oils were administered to rat groups and coded as OLO, COO, PAO, SOO, SUO, and FLO groups, respectively (Lashin et al., 2020). For 60 days, the therapies were administered orally via gastric gavage to compare the improvements in hyperlipidemic biomarkers achieved by different oil groups. On the 61st day, the rats were applied in an anesthesia-producing chamber and given chloral hydrate (250 mg/kg B. W. pentobarbital) to render them unconscious following a complete fast for 12 h (Tai et al., 2010). As soon as the rats seemed to be unconscious, their hearts were punctured to draw blood from the abdominal aorta and centrifuged right away at 4,000 rpm for 10 min at 4°C in tubes containing heparin to produce plasma. They were stored at −80°C until routine chemical examination analysis after being aliquoted in Eppendorf tubes. For the biochemical studies, the resultant plasma (supernatant) was stored at −20°C after careful decantation. After the blood was extracted, the tubes containing the blood samples were allowed to coagulate at room temperature. The rats were slaughtered and their important organs such as kidneys and hearts were removed. The weight of the organs was determined in a Shimadzu AX 200 analytical scale to be expressed as (g/100 g). Organs were carefully rinsed twice with ice-cold phosphate-buffered saline (PBS pH 7.4) before being drained with filter paper. Tissue samples were homogenized in ice-cold PBS using a mechanical homogenizer to maintain enzyme activity and prevent protein denaturation. After centrifuging the homogenates for 15 min at 4°C at 10,000 rpm, the supernatants were gathered for additional biochemical analyses, such as assessments of enzyme and lipid activity. The homogenates were kept at −20°C. Figure 1 presents the experimental design to maintain their integrity until they were used again.
Body weight
Using a laboratory scale in grams (WT2003CH, China), the rats’ body weight as one of the primary sensitive indicators of hyperlipidemia, was consistently measured gravimetrically at the light cycle for each rat during the experimental period (60 days) at the initial and final periods throughout the trial. Body weights during the experiment were calculated based on the measurements (Sokoła-Wysoczańska et al., 2024).
Plasma lipid profile
Standard techniques were applied based on the manufacturer’s specifications of the commercial enzymatic kits for the lipid profile which were purchased from (Zhongsheng B. B. Co., China) to assess the levels calorimetrically of plasma using a blood chemistry analyzer (910, Munich, Germany). Approximately 0.5 mL of each sample were centrifuged for 15 min at 4°C at 3,500 rpm to detect the total cholesterol (CHOL), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and triglycerides (TG). The lipid profile results were used to calculate the cardiovascular risk indicators such as atherogenic index (AI), coronary risk index (CoRI), and cardiovascular risk index (CaRI) according to the formulas and expressed as mg/dL; (LDL-c/HDL-c), (CHOL/HDL-c), and (TG/HDL-c), respectively (Sa’adah et al., 2017). Each analysis was carried out in triplicate to ensure the precision and correctness of the results.
Glucose and insulin resistance levels
Rats were fasted for 6 h from 8:00 am to 2:00 pm. Blood was then drawn from the saphenous vein. Basal conditions were used to obtain fasting blood samples (0.25 mL) for each group, which were centrifuged for 15 min at 4°C at 3,500 rpm. Fasting plasma glucose level (FGL) was measured by a glucometer (Bionime®, Switzerland). In order to determine the insulin resistance level (IRL), an enzyme-linked immunosorbent assay kit (ELISA) (Sigma, MO, United States) was used. The homeostasis model assessment of insulin resistance index (HOMA-IR), which was computed as the product of the fasting blood glucose (mg/dL) and the insulin levels (μU/mL) divided by 2,430, was used to assess HOMA-IR level (Keita et al., 2013).
Inflammatory markers
The commercially available rat tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), and C-reactive protein (CRP) were detected by the manufacturer’s instructions of the enzyme-linked immunosorbent assay (ELISA) kits (Thermo F. S., MA, United States). Every step and circumstance complied with the guidelines provided by these products. The amount of TNF-α contained in the sample divided by the sample’s protein concentration was used to compute the TNF-α concentration; results are given in pg./mg (Baakdah et al., 2023). The cytokine-specific capture antibody was applied to the bottom of the 96-well plate. The specific conjugated detection antibodies were used to wash, dilute, and detect the plates. As directed by the manufacturer, cytokines were measured at 450 nm (Al-Eisa et al., 2023).
Heart-related parameters
The heart cardiac hypertrophy (CH) index was evaluated after being cleaned with saline and weighed (Shahidi et al., 2024). Coronary health index (CHI) presented the ratio of heart weight to body weight (g/g) × 100 (Equation 1).
Heart Rate (BPM), which is equal to (the number of beats measured in seconds) times 60, (beats/min) was calculated manually (Wojciechowski et al., 2010).
Kidney-related parameters
Based on the conversion of the hydrolysis of the urease enzyme into ammonia and carbon dioxide, the urea content was evaluated colorimetrically at a wavelength of 546 nm by a commercial kit from Diamond, Egypt. Based on the interaction of creatinine and picric acid with an alkaline solution, the concentration of creatinine was measured colorimetrically at 492 nm by a commercial kit from Biomed Diagnostics, Egypt (Baazeem et al., 2024). Results were represented as (mg/dL) (Bazzano et al., 2015).
Statistical analysis
The mean and its standard errors were used to express all the results. For every continuous variable that was measured (such as organ weights, inflammatory markers, and biochemical markers), the means of all experimental groups were compared using the one-way ANOVA. For these characteristics, each treatment group was specifically compared to the control group using Dunnett’s t-test after a significant ANOVA result, statistically significant effects were then examined further. The statistical significance was set at p < 0.05. Version 3.0 of the GraphPad software was used for the statistical analyses.
Results
Effects of edible oils on body weight
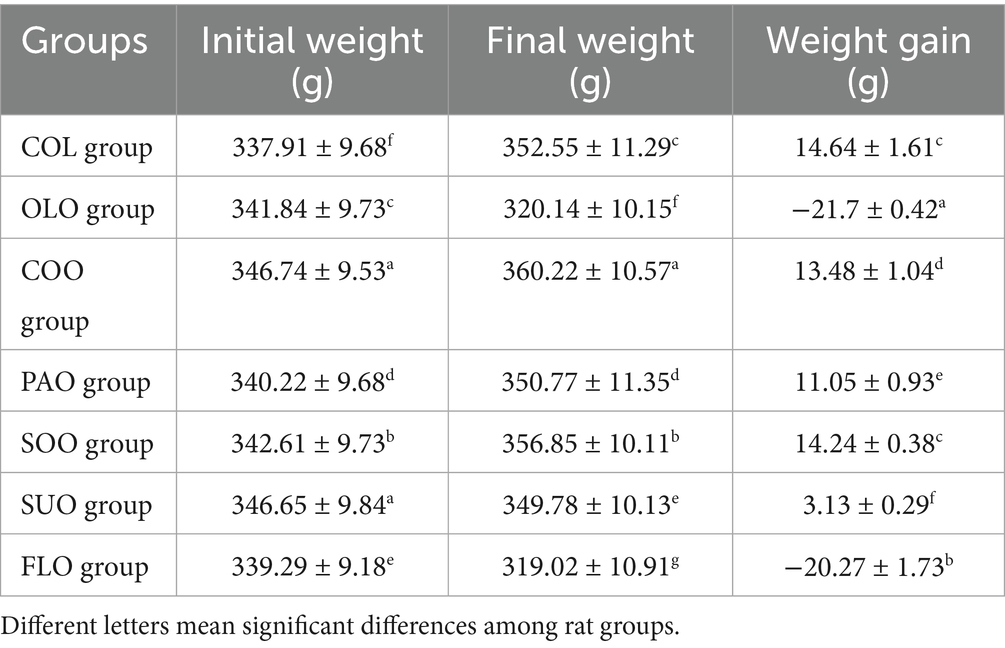
Rat models can be used in experiments to create obesity as they gain weight rapidly after feeding with high-fat meals. Neither of the experimental groups experienced any negative effects or deaths during the investigation. All treatment groups continued to consume the same amount of food. Within each group, the effect of feeding various edible oils on changes in body weight grew progressively each week. Table 1 presents the changes in body weights throughout the treatment trial among rat groups. The initial weight ranged from 337.91 g to 346.65 g. The COO group reported a maximum weight of 360.22 g followed by SOO, PAO, and SUO groups 356.85 g, 350.77 g, and 349.78 g, respectively. On the other hand, the FLO and OLO groups presented a reduction in body weight of 21.70 g and 20.27 g, respectively.
Effects of edible oils on lipid profile and cardiovascular risk indicators
The biochemical assay results are shown in Figures 2, 3. Data indicated that the lipid profile has changed in comparison to the COL group values. Total CHOL, LDL-c, HDL-c, TG, and cardiovascular risk indicators in plasma after 60 days after the consumption of various edible oils fluctuated throughout the experimental groups. The study’s overall lipid profile results fall within the normal ranges (Rabail et al., 2024). The consumption of flaxseed, sunflower, and olive oils in FLO, SUO, and OLO groups reduced the total CHOL values to reach 125.56 mg/dL, 128.47 mg/dL, and 144.87 mg/dL. On the other hand rats in the SOO group reported the highest value of total CHOL 209.12 mg/dL compared to the COL group 177.24 mg/dL.
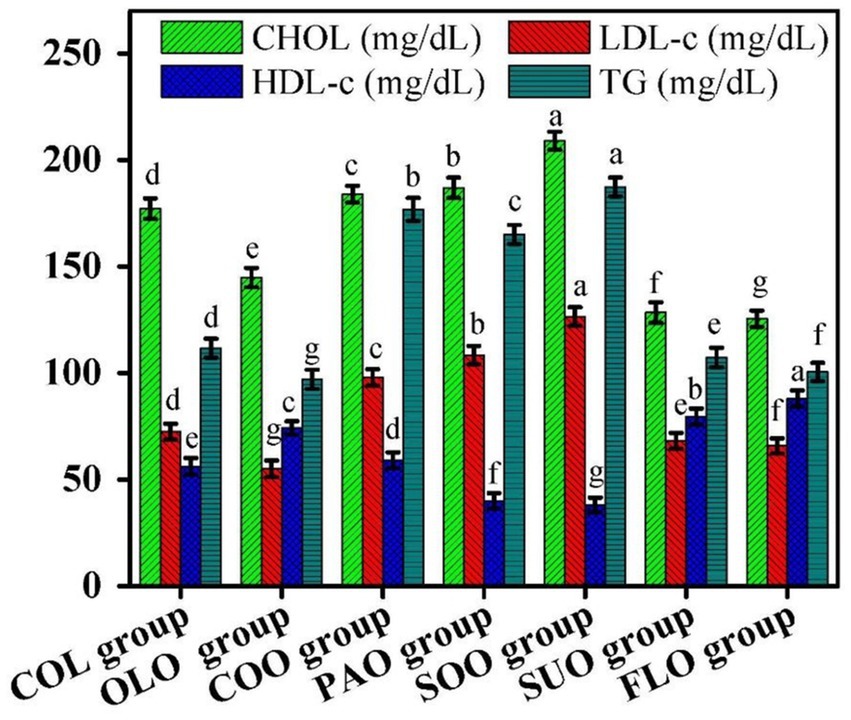
Figure 2. Effects of edible oils on lipid profile; total cholesterol (CHOL), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and triglycerides (TG). Different letters mean significant differences among rat groups.
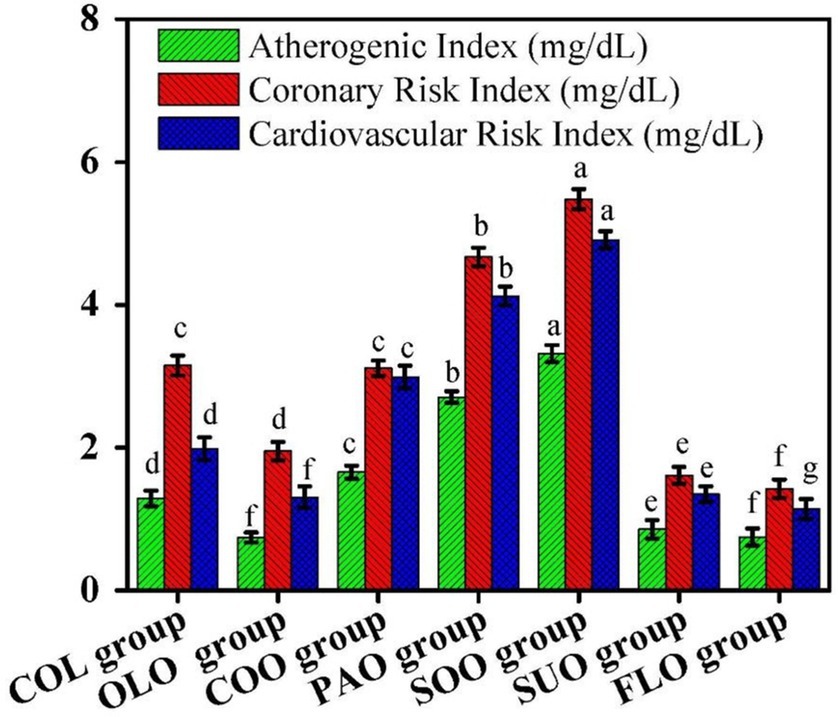
Figure 3. Effects of edible oils on cardiovascular risk indicators; atherogenic index (AI), coronary risk index (CoRI), and cardiovascular risk index (CaRI). Different letters mean significant differences among rat groups.
On the other hand, rats in the SOO group reported the highest value of total LDL-c, at 126.58 mg/dL, compared to the COL group, at 72.58 mg/dL. The consumption of soybean and palm oils in SOO and PAO groups reduced the total HDL-c values to 38.15 mg/dL and 40.02 mg/dL. Rats in the FLO group reported the highest value of total HDL-c, at 88.06 mg/dL, compared to the COL group, at 56.25 mg/dL. Rats in the SOO group reported the highest value of total TG, at 187.41 mg/dL, compared to the COL group, at 111.72 mg/dL.
The atherogenic index reported the highest value for the SOO group at 3.32 mg/dL, while the OLO group was 0.74 mg/dL compared to the COL group at 1.29 mg/dL. The coronary risk index reported the highest value for the SOO group at 5.48 mg/dL, while the FLO group at 1.43 mg/dL compared to the COL group at 3.15 mg/dL. The cardiovascular risk index reported the highest value for the SOO group at 4.91 mg/dL, while the FLO group at 1.14 mg/dL compared to the COL group at 1.99 mg/dL.
Effects of edible oils on glucose and insulin resistance levels
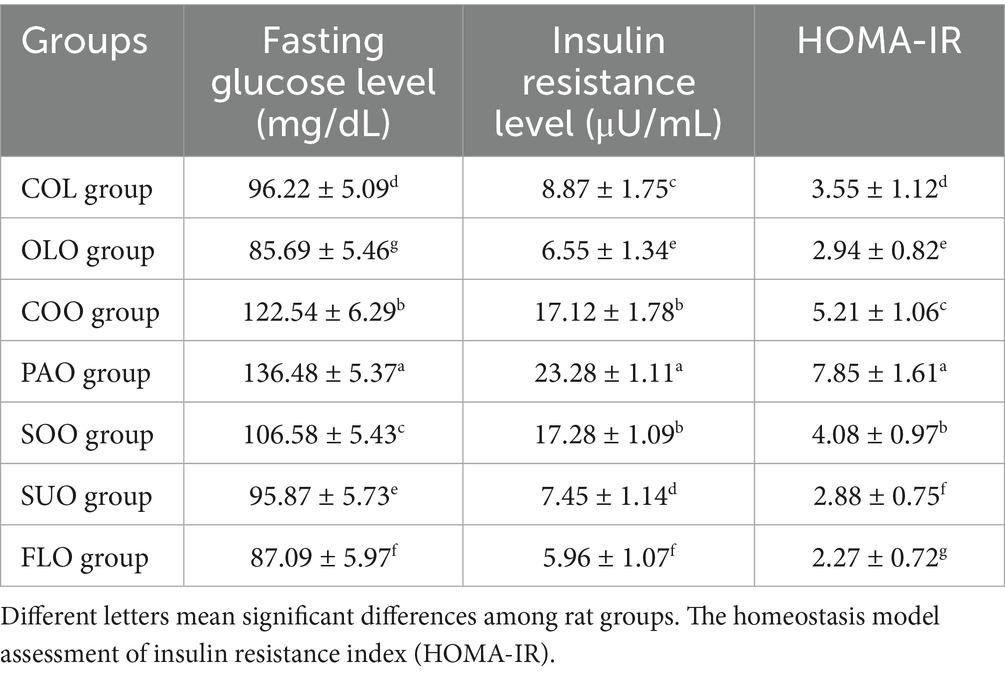
The results of the changes in glucose and insulin resistance are shown in Table 2. Data indicated that the consumption of edible oils has changed in comparison to the COL group values throughout the experimental groups. The consumption of olive and flaxseed oils in OLO and FLO groups reduced the FGL to reach 85.69 mg/dL and 87.09 mg/dL. SUO group detected lower value to the COL group at 95.87 mg/dL and 96.22 mg/dL, respectively. On the other hand, rats in the PAO group reported the highest value of FGL 136.48 mg/dL.
The consumption of flaxseed and olive oils in FLO and OLO groups reduced the IRL to reach 5.96 μU/mL and 6.55 μU/mL. The COO group detected lower value to the SOO group at 17.12 μU/mL and 17.28 μU/mL, respectively. On the other hand, rats in the PAO group reported the highest value of IRL 23.28 μU/mL compared to the COL group 8.87 μU/mL. The consumption of flaxseed oil in the FLO group reduced the HOMA-IR to reach 2.27. SUO group detected lower value to the OLO group at 2.88 and 2.94, respectively. On the other hand, rats in the PAO group reported the highest value of HOMA-IR 7.85 compared to the COL group 3.55.
Effects of edible oils on inflammatory cytokines
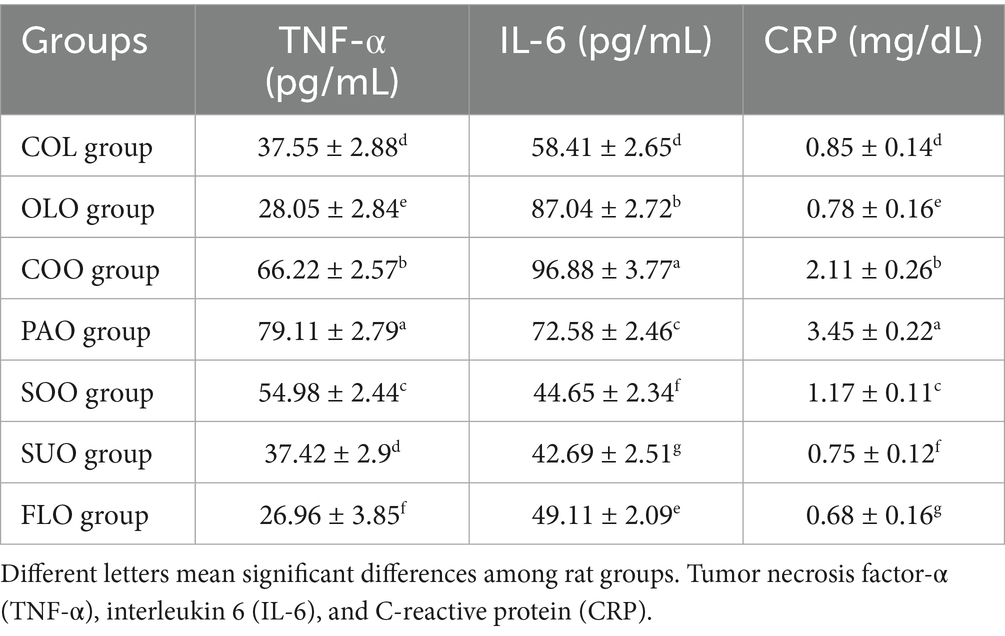
The ELISA results of inflammatory cytokines TNF-α, IL-6, and CRP are shown in Table 3. The consumption of flaxseed and olive oils in FLO and OLO groups reduced the TNF-α immune response to reach 26.96 pg./mL and 28.05 pg./mL. SUO group detected lower value to the COL group at 37.42 pg./mL and 37.55 pg./mL, respectively. On the other hand, rats in the PAO group reported the highest value of TNF-α immune response 79.11 pg./mL.
On the other hand, rats in the COO group reported the highest production of the pro-inflammatory (IL-6) cytokine value 96.88 pg./mL compared to the COL group 58.41 pg./mL. The consumption of flaxseed oil in the FLO group reduced the production of the pro-inflammatory CRP cytokine to reach 0.68 mg/dL. SUO group detected lower value to the OLO group at 0.75 mg/dL and 0.78 mg/dL, respectively. On the other hand, rats in the PAO group reported the highest production of the pro-inflammatory CRP cytokine value at 3.45 mg/dL compared to the COL group at 0.85 mg/dL.
Effects of edible oils on heart-related parameters
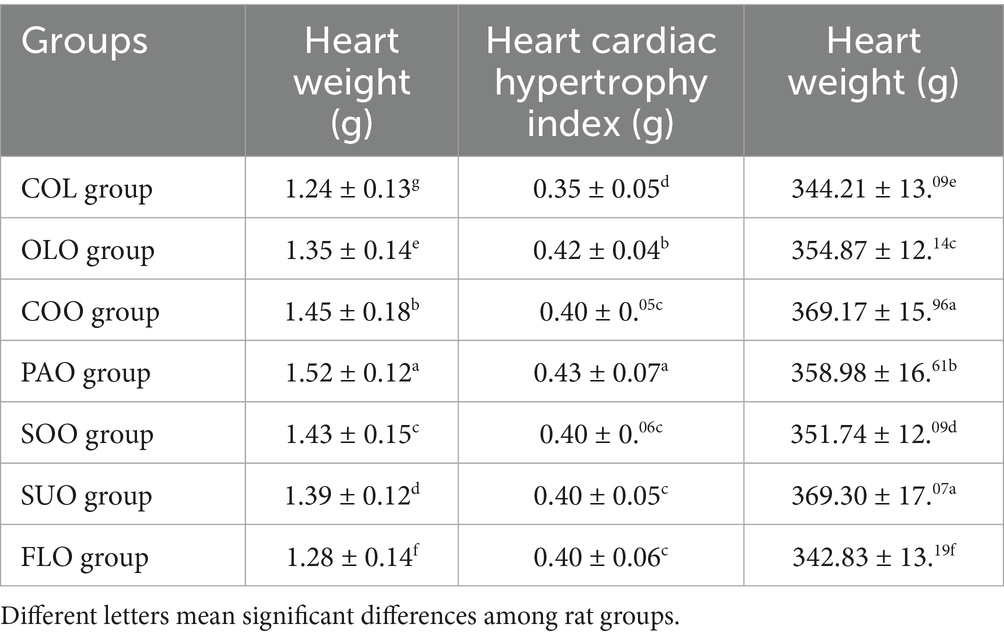
The consumption of flaxseed oil in the FLO group detected higher value of heart weight to the COL group at 1.28 g and 1.24 g, respectively. On the other hand, rats in the PAO group reported the highest heart weight 1.52 g compared to the other edible oils (Table 4). The consumption of coconut, soybean, sunflower, and flaxseed oils in COO, SOO, SUO, and FLO groups detected the same values of CH index 0.40 g. On the other hand, rats in the PAO group reported the highest value of CH index 0.43 g compared to the COL group 0.43 g. The consumption of flaxseed oil in the FLO group detected lower value of heart rate to the COL group at 342.83 BPM and 344.21 BPM, respectively. The consumption of coconut and sunflower oils in COO and SUO groups detected the highest and same values of heart rate 369 BPM.
Effects of edible oils on kidney-related parameters
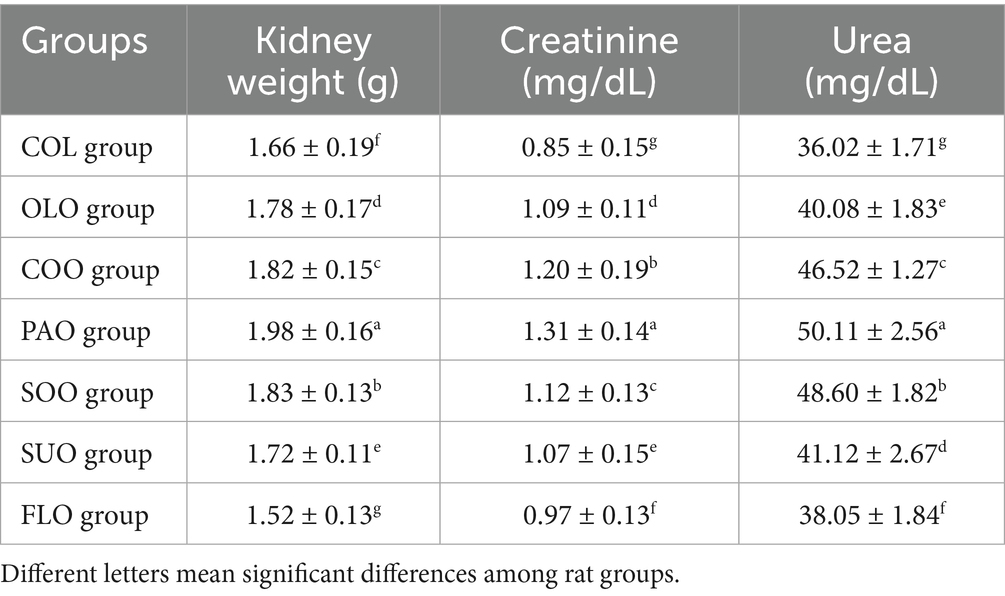
Following the 60-day observation period, the urea and creatinine levels were assessed in experimental obese rats that had been administered various edible oils. Both biomarkers have risen compared to the COL group (Table 5). The consumption of flaxseed oil in the FLO group detected the lowest value of kidney weight compared to the COL group at 1.52 g and 1.66 g, respectively. On the other hand, rats in the PAO group reported the highest kidney weight 1.98 g compared to the other edible oils. Kidney weights in the COO and SOO groups were a little high 1.82 g and 1.83 g, followed by SUO and OLO groups 1.72 g and 1.78 g, respectively. The consumption of edible oils increased the creatinine levels from 0.85 mg/dL in the COL group to 1.31 mg/dL in the PAO group. The consumption of flaxseed oil in the FLO group detected a little high value of creatinine 0.97 mg/dL. Creatinine levels in SUO and OLO groups were at 1.07 mg/dL and 1.09 mg/dL, respectively. The consumption of edible oils increased the urea levels from 36.02 mg/dL in the COL group to 50.11 mg/dL in the PAO group. The consumption of flaxseed oil in the FLO group detected a little high value of urea 38.05 mg/dL. Urea levels in OLO and SUO groups were 40.08 mg/dL and 41.12 mg/dL, respectively.
Discussion
It is increasingly recognized that chronic over nutrition, such as consuming a high-fatty diet (HFD), contributes to pro-inflammatory diseases like type 2 diabetes and some types of cancer. However not all lipids cause metabolic inflammation; for instance, SFAs are linked to this condition. On the other hand, MUFAs and PUFAs, which are UFAs have been shown to reduce metabolic inflammation and preserve energy balance (Keita et al., 2013).
The reduction in body weight for olive and flaxseed oils groups can be due to the presence of higher lipoprotein lipase (LPL) activities compared to the other edible oils (Li et al., 2014). Coconut and corn oils include short-chain FA and MUFAs, which have a detrimental effect on lipid absorption and the activation of oxidative energy production (Abudigin et al., 2024). In contrast to our findings, some researchers suggest that olive oil has an anti-obesogenic impact (Keita et al., 2013), another study reported that longer-term studies show that prolonged olive oil consumption causes weight gain (Buettner et al., 2006). The dietary treatment (4–12 weeks) or the amount of olive oil given may related to some of this disparity (Alsaif and Duwaihy, 2004). The amount, and duration of dietary therapy of fat intake all influence obesity. Following the Mediterranean diet has been linked to a decreased risk of cardiovascular disease. High intakes of fruits, vegetables, grains, fish, and olive oil define this diet. The low energy density and high fiber content, which encourage satiety, may account for the decreased body weight, belly fat deposition, and fasting blood glucose levels associated with this diet (Di Daniele et al., 2013). According to Patel et al. (2022), diets high in SFA typically encourage more weight gain than diets high in unsaturated fatty acids (UFA). Our results, however, were consistent with those of Shiraev et al. (2009), who showed that if caloric intake is maintained, differences in dietary fat sources may not have a significant effect on body weight. The theory is that olive and flaxseed oils can aid in preventing obesity in hyperlipidemic rats by providing them with balanced fatty acid profiles and cardioprotective dietary parameters according to the results of obesity indicators. Similar findings have been reported that palm oil may marginally raise CHOL levels (Edem, 2002; Ullah et al., 2021).
Coconut, palm, and soybean oils, which have plenty amounts of SFA, raised the total TG and CHOL levels, while the consumption of olive and flaxseed oils correlated with the lower level of TG and CHOL in the blood (Abudigin et al., 2024). The expected effects of oral consumption of those edible oils in rats may be due to the high levels of linoleic acid with low levels of oleic acid, and alpha-linolenic acid. Elevated consumption of linoleic acid raises the amount of linoleic acid in HDL, increasing their vulnerability to oxidation, and raising the risk of CVD (Lashin et al., 2020).
Olive and flaxseed oils showed the greatest reductions for AI, CoRI, and CaRI among cardiovascular risk markers. Its balanced quantities of SFAs, MUFAs, PUFAs, and Ω-3 FA are considered as the reason for these greatest reductions, which are true indicators of the heart-protective capability, where lower values denote better heart health. Those values were in agreement with Abdelfattah et al. (2024) who studied the cardiovascular risk indicators on Sprague–Dawley rats given a fatty diet. The cardioprotective properties of olive, flaxseed, and sunflower oils are attributed to their high content of phenolic compounds as tocopherols, and UFAs, particularly oleic acid, while other edible oils contain lower values of MUFAs and PUFAs with higher values of UFA. The idea that oleic acid lowers the risk of CVD is supported by the Food and Drug Administration (Keita et al., 2013). It may be a product that is good for vascular health because oils have up to 60% oleic acid. High quantities of oleic acid was thus considered to lower the risk of CVD (Lashin et al., 2020). The consumption of lauric, myristic, and palmitic acids can increase LDL-c, while stearic acids have no effect on LDL-c, and substituting PUFAs for SFAs may reduce the concentration of LDL-c as well as the ratio of total CHOL to HDL-c (Morsi et al., 2014). These results highlighted the significance of edible oil choices in preventing cardiovascular disease.
Obesity is thought to be primarily caused by a high intake of energy-dense vegetable fat and a lack of physical activity. High-fat diets have been linked in numerous studies to the emergence of diabetes in genetically predisposed people and groups as well as to the higher incidence of insulin resistance. The primary characteristic of diabetes is hyperglycemia, or persistently elevated blood glucose levels (Abdelazez et al., 2018). Changes in lipid homeostasis in living and insulin-sensitive tissues are a major cause of reduced insulin sensitivity, poor glucose absorption, hyperglycemia, and type 2 diabetes (Abudigin et al., 2024).
Excessive consumption of dietary fatty acids stimulates the lipogenesis signaling proteins by reducing glucose homeostasis, which is involved in TG production and IRL. Nuclear receptors are transcription factors that are activated by ligands, that control glucose metabolism and lipid homeostasis, particularly peroxisome proliferator-activated receptors (Abudigin et al., 2024).
The results were corroborated by the decreased weight gain and increased insulin levels in flaxseed and olive oils. Diets rich in saturated fats, such as those in coconut, palm, soybean, and sunflower, block the insulin response to LPL activity in adipose tissue. This disruption leaded to the increased obesity, which in turn induces insulin resistance and obesity due to the faster rate of TG construction (Memetimin et al., 2022). Furthermore, the consumption of flaxseed and olive oils successfully reduced the inflammatory cytokines that cause atherosclerosis and IRL linked to obesity caused by HFD. In this regard, the current dietary guidelines for preventing type 2 diabetes, addressing oxidative stress, and metabolic inflammation raise awareness about the need to consume more vegetable fats and less animal fat overall (Schlesinger et al., 2019). Significant variations were also observed in HOMA-IR analysis, which detects insulin resistance during fasting. Although it has been shown that HF diets cause glucose intolerance and the consequent development of insulin hypersecretion after a glucose load, the body attempts to maintain glucose at normal physiological levels (Keita et al., 2013). Consuming edible oils was found to have a beneficial impact on TNF-α immunological response as an anti-inflammatory agent by promoting the production of keratinocytes and cytokines (Baakdah et al., 2023).
Edible oils contain flavonoid components that serve as inhibitory agents and have immune-suppressive effects on cytokine production. The presence of glycosylated proteins in various types of oil may be the cause of elevated cytokine production in rats, as these proteins can activate cells and cause synergistic activities (Al-Eisa et al., 2023).
Proinflammatory cytokines are linked to CVD and are used as inflammatory markers to predict atherosclerotic risk (Xu et al., 2012). Consuming sunflower, soybean, flaxseed, and olive oils significantly decreased the plasma levels of both IL-6 and CRP in the current investigation, suggesting that these oils may help to ameliorate inflammation status.
The heart is renowned for its capacity to convert fatty acids into energy because it is better able to carry out β-oxidation due to the high levels and activity of associated enzymes (Oliveira et al., 2017). Heart weight results have likewise revealed typical growth trends (Rabail et al., 2024). CH index can be influenced by several indexes such as obesity, aging-induced cardiac hypertrophy, high blood pressure, and a decrease of mitochondrial biogenesis via the heart’s Sirtuin 1 (Shahidi et al., 2024). The heart can only guarantee a few seconds of beating because of its limited capacity to store the adenosine triphosphate. When there is a greater supply of those nutrients, cardiac muscle may swiftly adjust to the energy demand and boost its energy production from fatty acids by up to 100% (Oliveira et al., 2017). Edible oils’ anti-oxidative and anti-inflammatory properties may help to control the cardiac hypertrophy and dysfunction linked to myocardial infarction (Al-Shudiefat et al., 2022). In a rat model, Csepanyi et al. (2015) showed that consuming carotenoid-rich oils enhanced cardiac function at reduced reperfusion periods. These effects were attributed to the chemicals’ antioxidant qualities. Finding possible novel treatment targets for heart hypertrophy is aided by new regulatory mechanisms (Shahidi et al., 2024). It has been demonstrated that high oleic oils have positive effects on heart structure and function in vivo, even though they may also have positive effects on cardiovascular risk factors, such as lowering LDL-c in hypercholesterolemic rats (Thandapilly et al., 2017).
Levels of creatinine and urea are considered crucial indicators of kidney functions. Elevated urea and creatinine levels serve as significant indicators of kidney failure, reflecting a lack of glomerular filtration rate activity (Mohammed et al., 2023). The enhanced weight gain of the kidney after consumption of edible oils may be related to the improved immunity observed in these rats (Rabail et al., 2024). According to another earlier study, urea and creatinine levels decreased in animal models (Mahfouz, 2020). Through kidney neutralization and active transport in the kidney tubules, the kidneys release some removed chemicals, including penicillin, urea, creatinine, and the ions H+ and K+, into the urine, maintaining the chemical compositions of body fluids steady (Nuru et al., 2024).
Conclusion
The growing prevalence of heart disease has rendered it more important than ever to change modern lives in order to achieve a better balance and diversification of nutrient and oil consumption in the daily diet. It is crucial to change the consumption of fats to improve current dietary lifestyles. This research assessed the therapeutic effects of several common edible oils through a 60-day bio-efficacy trial on rats that had been given a high-fat diet for 21 days to induce hyperlipidemia. Strong cardiovascular preventive advantages were demonstrated by rats given flaxseed and olive oils, which also resulted in decreased body weight and better lipid profile, glucose, insulin resistance levels, and inflammatory markers. Flaxseed oil consumption was more successful in improving lipid profile values and lowering cardiovascular risk indicators, even though both flaxseed and olive oils enhanced cardiovascular health. The rats in the palm oil group had the highest levels of insulin resistance, suggesting that consumption of palm oil may increase the risk of cardiovascular disease. Sunflower oil showed protective effects similar to olive oil in terms of inflammatory markers and heart hypertrophy index, suggesting it a is moderate but beneficial dietary oil option. The administration of olive, flaxseed, and sunflower oils exhibited protective effects against cardiovascular risk factors. Although flaxseed oil demonstrates superiority compared to olive oil.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Gulf Countries Association of Sciences in Experimentation on Animals (8,901/3177). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SAb: Supervision, Writing – original draft. NA: Software, Writing – original draft. AA: Methodology, Writing – original draft. MA: Validation, Writing – original draft. AI: Formal analysis, Writing – original draft. RS: Investigation, Writing – review & editing. RZ: Data curation, Writing – review & editing. HH: Software, Writing – original draft. NA-S: Investigation, Writing – original draft. SAl: Project administration, Writing – review & editing. TA: Visualization, Writing – review & editing. SQ: Methodology, Writing – original draft. EH: Data curation, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Taif University, Saudi Arabia (Project no. TU-DSPP-2024-10).
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelazez, A., Abdelmotaal, H., Evivie, S. E., Melak, S., Jia, F.-F., Khoso, M. H., et al. (2018). Screening potential probiotic characteristics of Lactobacillus brevis strains in vitro and intervention effect on type I diabetes in vivo. Biomed. Res. Int. 2018:7356173. doi: 10.1155/2018/7356173
Abdelfattah, D. S. E., Fouad, M. A., Elmeshad, A. N., El-Nabarawi, M. A., and Elhabal, S. F. (2024). Anti-obesity effect of combining white kidney bean extract, Propolis Ethanolic extract and CrPi3 on Sprague-Dawley rats fed a high-fat diet. Nutrients 16:310. doi: 10.3390/nu16020310
Abudigin, W. I., Bajaber, A., and Subash-Babu, P. (2024). Impact of various dietary lipids on amelioration of biomarkers linked to metabolic syndrome in both healthy and diabetic Wistar rats. BMC Nutr. 10:75. doi: 10.1186/s40795-024-00881-7
Al-Eisa, R. A., Helal, M., Aljahani, A. H., Sami, R., Aljaadi, A. M., Alshahrani, M. Y., et al. (2023). The immunological effects against invasive aspergillosis disease on inbred mice after the dietary intake of honey varieties with the determination of diastase and invertase enzyme activities. Mater. Express 13, 1088–1094. doi: 10.1166/mex.2023.2439
Al-Hoshani, N., Al Syaad, K. M., Saeed, Z., Kanchev, K., Khan, J. A., Raza, M. A., et al. (2023). Anticoccidial activity of star anise (Illicium verum) essential oil in broiler chicks. Pak. Vet. J. 43, 553–558.
Al-Hoshani, N., Zaman, M. A., Al Syaad, K. M., Salman, M., Rehman, T. U., and Olmeda, A. S. Assessment of repellency and acaricidal potential of Nigella sativa essential oil using Rhipicephalus microplus ticks. Pak Vet J. (2023) 43, 606–610.
Alsaif, M. A., and Duwaihy, M. M. S. (2004). Influence of dietary fat quantity and composition on glucose tolerance and insulin sensitivity in rats. Nutr. Res. 24, 417–425. doi: 10.1016/j.nutres.2003.11.011
Al-Shudiefat, A. A.-R., Ludke, A., Malik, A., Jassal, D. S., Bagchi, A. K., and Singal, P. K. (2022). Olive oil protects against progression of heart failure by inhibiting remodeling of heart subsequent to myocardial infarction in rats. Physiol. Rep. 10:e15379. doi: 10.14814/phy2.15379
Baakdah, F., Ashi, A., Almaghrabi, S., Ismail, K. A., Sami, R., Alshehry, G., et al. (2023). Healing processes of burn wounds with honey and vaseline as ointment forms: an in-vivo study in Wistar rats. Mater. Express 13, 2042–2048. doi: 10.1166/mex.2023.2569
Baazeem, A., Helal, M., Sami, R., Alshehry, G., Algarni, E., Hilary, U., et al. (2024). Positive effects of dietary honey and aflatoxin B1 on serum enzymes, superoxide dismutase activity, β-glucuronidase enzyme activity, and colonic probiotic bacteria on rats. Mater. Express 14, 60–65. doi: 10.1166/mex.2024.2584
Bangulzai, N., Ahmed, S. F., Kashif, M., Fatima, M., Ahmed, M., and Mushtaq, N. (2022). Antifungal activity of essential oils extracted from different plants against Penicillium digitatum causing green mold of citrus. Int. J. Agric. Biosci. 11, 75–83. doi: 10.47278/journal.ijab/2022.011
Bazzano, T., Restel, T. I., Porfirio, L. C., Souza, A. S., and Silva, I. S. (2015). Renal biomarkers of male and female Wistar rats (Rattus norvegicus) undergoing renal ischemia and reperfusion. Acta Cir. Bras. 30, 277–288. doi: 10.1590/S0102-865020150040000007
Buettner, R., Parhofer, K. G., Woenckhaus, M., Wrede, C. E., Kunz-Schughart, L. A., Scholmerich, J., et al. (2006). Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 36, 485–501. doi: 10.1677/jme.1.01909
Çalışkan, G. Ü., and Emin, N. (2023). Protective efficacy of fresh and aged macerated garlic oils in safflower oil against intra-abdominal adhesions in rats. Pak. Vet. J. 43, 290–296. doi: 10.29261/pakvetj/2023.030
Campos, S. B., Oliveira Filho, J. G., Salgaço, M. K., Jesus, M. H., and Egea, M. B. (2023). Effects of peanuts and pistachios on gut microbiota and metabolic syndrome: a review. Foods 12:4440. doi: 10.3390/foods12244440
Chen, X., Gu, J., and Huang, Y. (2023). High dietary intake of unsaturated fatty acids is associated with improved insulin resistance – a cross-sectional study based on the NHANES database. Lipids Health Dis. 22:216. doi: 10.1186/s12944-023-01982-1
Csepanyi, E., Czompa, A., Haines, D., Lekli, I., Bakondi, E., Balla, G., et al. (2015). Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol. Res. 100, 148–156. doi: 10.1016/j.phrs.2015.07.021
Di Daniele, N., Petramala, L., Di Renzo, L., Sarlo, F., Della Rocca, D. G., Rizzo, M., et al. (2013). Body composition changes and cardiometabolic benefits of a balanced Italian Mediterranean diet in obese patients with metabolic syndrome. Acta Diabetol. 50, 409–416. doi: 10.1007/s00592-012-0445-7
dos Santos, R. M., Miyamoto, J. É., Siqueira, B. P., Araujo, T. R., Vettorazzi, J. F., Menta, P. L. R., et al. (2024). Interesterified palm oil promotes insulin resistance and altered insulin secretion and signaling in Swiss mice. Food Res. Int. 177:113850. doi: 10.1016/j.foodres.2023.113850
Edem, D. O. (2002). Palm oil: biochemical, physiological, nutritional, hematological and toxicological aspects: a review. Plant Foods Hum. Nutr. 57, 319–341. doi: 10.1023/A:1021828132707
Grover, S., Kumari, P., Kumar, A., Soni, A., Sehgal, S., and Sharma, V. (2021). Preparation and quality evaluation of different oil blends. Lett Appl Nano Bio Science 10, 2126–2137. doi: 10.33263/LIANBS102.21262137
Kaseke, T., Opara, U. L., and Fawole, O. A. (2021). Blending of sunflower oil with pomegranate seed oil from blanched seeds: impact on functionality, oxidative stability, and antioxidant properties. PRO 9:635. doi: 10.3390/pr9040635
Keita, H., Ramírez-San Juan, E., Paniagua-Castro, N., Garduño-Siciliano, L., and Quevedo, L. (2013). The long-term ingestion of a diet high in extra virgin olive oil produces obesity and insulin resistance but protects endothelial function in rats: a preliminary study. Diabetol. Metab. Syndr. 5, 1–10. doi: 10.1186/1758-5996-5-53
Kharazmi-Khorassani, J., Ghafarian Zirak, R., Ghazizadeh, H., Zare-Feyzabadi, R., Kharazmi-Khorassani, S., Naji-Reihani-Garmroudi, S., et al. (2021). The role of serum monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) in cardiovascular disease risk. Acta Biomed. Atenei Parmensis 92:e2021049. doi: 10.23750/abm.v92i2.9235
Lashin, F. M., Rizk, H. A., Ahmed-Farid, O. A., and Shehata, A. M. (2020). A screening study of corn oil versus fish oil and coconut oil on biochemical cardiovascular risk factors in rats. Cur Sci Inter 9, 418–430. doi: 10.36632/csi/2020.9.3.36
Li, Y., Ma, W. J., Qi, B. K., Rokayya, S., Li, D., Wang, J., et al. (2014). Blending of soybean oil with selected vegetable oils: impact on oxidative stability and radical scavenging activity. Asian Pac. J. Cancer Prev. 15, 2583–2589. doi: 10.7314/APJCP.2014.15.6.2583
Lopes, M. C. O. S., Kaippert, V. C., Crovesy, L., de Carvalho, D. P., and Rosado, E. L. (2024). Monounsaturated fat-rich diet reduces body adiposity in women with obesity, but does not influence energy expenditure and substrate oxidation: a parallel randomized controlled clinical trial. Eur. J. Clin. Nutr. 78, 335–343. doi: 10.1038/s41430-024-01401-3
Memetimin, H., Zhu, B., Lee, S., Katz, W. S., Kern, P. A., and Finlin, B. S. (2022). Improved β-cell function leads to improved glucose tolerance in a transgenic mouse expressing lipoprotein lipase in adipocytes. Sci. Rep. 12:22291. doi: 10.1038/s41598-022-26995-1
Mahfouz, M. Z. (2020). Using pan bread enriched with chia seeds to reduce some side effects of fatty liver induced with fructose in male rats. J. Home Econ. 36, 167–204.
Mohammed, A., Abdulrasak, M. A., Musa, A. L., Liman, J. H., Israel, E., Harry, U., et al. (2023). Anti-hyperglycaemic and selected organ protective effects of Ziziphus spina-christi hydroethanol leaf extract in alloxan induced diabetic rats. Gadau J. Pure Appl. Sci. 2, 146–155. doi: 10.54117/gjpas.v2i2.102
Morsi, M. K. S., Galal, S. M., Abd El-Rahman, K., and Katry, A. (2014). Palm kernel oil increases the risk of coronary heart disease in rats compared with ghee. IOSR J. Pharm. Biol. Sci. 9, 34–40. doi: 10.9790/3008-09163440
Nuru, K. A., Garkuwa, U. A., Yusuf, H., Tijjani, H., and Kura, A. U. (2024). Toxicity evaluation of Ziziphus spina-christi fruit on Wistar rats. Public Health Toxicol 4, 1–9. doi: 10.18332/pht/189288
Oliveira, L. G., Moreno, L. G., Melo, D. S., Costa-Pereira, L. V., Carvalho, M. M. F., Silva, P. H. E., et al. (2017). Caryocar brasiliense oil improves cardiac function by increasing Serca 2a/PLB ratio despite no significant changes in cardiovascular risk factors in rats. Lipids Health Dis. 16, 1–8. doi: 10.1186/s12944-017-0422-9
Pandey, V. K., Dar, A. H., Rohilla, S., Mahanta, C. L., Shams, R., Khan, S. A., et al. (2023). Recent insights on the role of various food processing operations towards the development of sustainable food systems. Circ. Econ. Sustain. 3, 1491–1514. doi: 10.1007/s43615-022-00248-9
Patel, S., Patel, A., Singh, L., Katiyar, A., and Singh, R. (2022). Effect of saturated and unsaturated fats on popping quality of popcorn variety-amber. Indian J. Agric. Biochem. 35, 105–107. doi: 10.5958/0974-4479.2022.00016.8
Rabail, R., Altemimi, A. B., Maerescu, C. M., Socol, C. T., Criste, F. L., Khalid, A. R., et al. (2024). Consumption of edible oil blended with flax, coconut, sunflower, and olive oil can significantly improve the negative health consequences of high-fat/high-cholesterol diet in Sprague Dawley rats. Front. Nutr. 11:1469601. doi: 10.3389/fnut.2024.1469601
Rabail, R., Shabbir, M. A., Sahar, A., Miecznikowski, A., Kieliszek, M., and Aadil, R. M. (2021). An intricate review on nutritional and analytical profiling of coconut, flaxseed, olive, and sunflower oil blends. Molecules 26:7187. doi: 10.3390/molecules26237187
Sa’adah, N. N., Purwani, K. I., Nurhayati, A. P. D., and Ashuri, N. M. Analysis of lipid profile and atherogenic index in hyperlipidemic rat (Rattus norvegicus Berkenhout, 1769) that given the methanolic extract of Parijoto (Medinilla speciosa). AIP Conf Proc (2017); 1854:020031.
Salman, M., and Imran, A. (2022). In vitro anticoccidial evaluation of Citrus sinensis essential oil against Eimeria oocysts. Agrobiol. Rec. 10, 15–18. doi: 10.47278/journal.abr/2022.020
Schlesinger, S., Schwingshackl, L., and Neuenschwander, M. (2019). Dietary fat and risk of type 2 diabetes. Curr. Opin. Lipidol. 30, 37–43. doi: 10.1097/MOL.0000000000000567
Shahidi, S., Ramezani-Aliakbari, K., Sarihi, A., Heshmati, A., Shiri, E., Nosrati, S., et al. (2024). Olive oil protects against cardiac hypertrophy in D-galactose induced aging rats. BMC Cardiovasc. Disord. 24:626. doi: 10.1186/s12872-024-04278-z
Shetty, S. S., and Kumari, S. (2021). Fatty acids and their role in type-2 diabetes (review). Exp. Ther. Med. 22:706. doi: 10.3892/etm.2021.10138
Shiraev, T., Chen, H., and Morris, M. J. (2009). Differential effects of restricted versus unlimited high-fat feeding in rats on fat mass, plasma hormones and brain appetite regulators. J. Neuroendocrinol. 21, 602–609. doi: 10.1111/j.1365-2826.2009.01877.x
Sokoła-Wysoczańska, E., Czyż, K., and Wyrostek, A. (2024). Different sources of omega-3 fatty acid supplementation vs. blood lipid profiles—a study on a rat model. Foods 13:385. doi: 10.3390/foods13030385
Tai, C.-J., Chen, C.-H., Chen, H.-H., and Liang, H.-J. (2010). Differential effect of high dietary fat intakes on haemorheological parameters in rats. Br. J. Nutr. 103, 977–983. doi: 10.1017/S0007114509992704
Thandapilly, S. J., Raj, P., Louis, X. L., Perera, D., Yamanagedara, P., Zahradka, P., et al. (2017). Canola oil rich in oleic acid improves diastolic heart function in diet-induced obese rats. J. Physiol. Sci. 67, 425–430. doi: 10.1007/s12576-016-0504-x
Ullah, R., Rauf, N., Nabi, G., Yi, S., Yu-Dong, Z., and Fu, J. (2021). Mechanistic insight into high-fat diet-induced metabolic inflammation in the arcuate nucleus of the hypothalamus. Biomed. Pharmacother. 142:112012. doi: 10.1016/j.biopha.2021.112012
Ündağ, İ., and Dönmez, H. H. (2023). Protective effect of Nigella sativa oil on hippocampus in acrylamide-induced toxicity in rats. Pak. Vet. J. 43, 616–622. doi: 10.29261/pakvetj/2023.046
Wojciechowski, P., Juric, D., Louis, X. L., Thandapilly, S. J., Yu, L., Taylor, C., et al. (2010). Resveratrol arrests and regresses the development of pressure overload- but not volume overload-induced cardiac hypertrophy in rats. J. Nutr. 140, 962–968. doi: 10.3945/jn.109.115006
Keywords: edible oils, lipid profile, inflammation, cardiovascular risk, hyperlipidemia
Citation: Abushal SA, Alqahtani NK, Abdulfattah AM, Abuzinadah MF, Izmirly AM, Sami R, Zarah RK, Hamdi H, Al-Sulami N, Almasoudi SH, Albishi TS, Qari SH and Hassan EA (2025) Consumption effects of edible oils on the biochemical cardiovascular risks in hyperlipidemic rats. Front. Sustain. Food Syst. 9:1613633. doi: 10.3389/fsufs.2025.1613633
Edited by:
Amélia Delgado, University of Algarve, PortugalReviewed by:
Waseem Khalid, Government College University, Faisalabad, PakistanMira Dewi, IPB University, Indonesia
Anna Wyrostek, Wroclaw University of Environmental and Life Sciences, Poland
Copyright © 2025 Abushal, Alqahtani, Abdulfattah, Abuzinadah, Izmirly, Sami, Zarah, Hamdi, Al-Sulami, Almasoudi, Albishi, Qari and Hassan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rokayya Sami, cm9rYXl5YS5kQHR1LmVkdS5zYQ==
 Suzan A. Abushal1
Suzan A. Abushal1 Nashi K. Alqahtani
Nashi K. Alqahtani Ahmed M. Abdulfattah
Ahmed M. Abdulfattah Rokayya Sami
Rokayya Sami Hamida Hamdi
Hamida Hamdi Nadiah Al-Sulami
Nadiah Al-Sulami Sameer H. Qari
Sameer H. Qari