- Federal Scientific Agroengineering Center VIM, Moscow, Russia
This research presents quantitative assessment of the biological risk level associated with spread of infectious and parasitic potato plant (Solanum tuberosum) diseases, based on the parameters of transmission and contagion mechanisms and pathways. The authors conducted a point-rating analysis of threats of potato infection or infestation with pathogens of different etiologies: bacterial, viral, helminthic and mycotic. The developed system of analysis is flexible and implies regular monitoring and data update in case of the globalization and world trade bring new strains of infections and varieties of parasites, which will be able to adapt to the climatic conditions of a particular region and the means of chemical plant protection. As a result, the combination of modern approaches to the field of potato plant disease detection and the proposed methodology will allow the development of a comprehensive system for monitoring and forecasting the spread of infections, which will make it possible to take timely and effective measures to protect the crop. The use of optical methods in combination with the analysis of data on pathogens will increase the accuracy of diagnostics and the effectiveness of prevention, contributing to the sustainable development of agriculture.
Introduction
Modern research methods that determine morphophysiology of plant objects require an integrated approach. As noted by Yudina et al. (2022) and Konchekov et al. (2023), the integrated approach should combine different methods of obtaining data on plant physiology and morphology in real time. Arshaghi et al. (2023), Afzaal et al. (2021), Bangari et al. (2022), Ivanyuk et al. (2020), Mohanty et al. (2016), and Dorokhov et al. (2019) believe that compliance with certain methodological approaches and technical requirements in obtaining information on the state of the plant organism allows to identify the presence of pathogens that negatively affect the growth and development of plants. On one hand, real-time monitoring of plant diseases at early stages and in real-time is an urgent and important problem of food security, since pathogens can destroy crops and synthesize a wide range of toxins dangerous for animals and humans (Rahman et al., 2022; Das et al., 2021), on the other hand, a wide range of different optical methods are already used in agriculture (Ferentinos, 2018; Kumar et al., 2021; Kumar et al., 2023; Onozuka et al., 2021). We would like to point out that unlike canonical common laboratory methods (such as polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA) and other methods used in biochemistry), optical methods of analysis are non-invasive, fast, simple, have relatively low cost, and allow rapid analysis and results.
Among optical methods, the most common are reflectance spectroscopy in visible spectra and IR region, Raman spectroscopy, hyperspectral imaging, temporally resolved THz spectroscopy, and fluorescence. IR spectroscopy and Raman spectroscopy are most common optical methods for diagnosis of mycoses (Zubler and Yoon, 2020; Zedler et al., 2023; Patel et al., 2023).
However, to develop fundamentally new methods of analysis, diagnosis and prevention of diseases it is necessary to construct a system of a fundamentally new methodology for assessing the risks of plant infection and spread of infectious and parasitic diseases. For potato crops as the most traditional crop for cultivation on private farms, soil and organic matter used as fertilizer can be a base for development and spread of the studied infectious and parasitic diseases.
The issue of pathogen adaptation to climate change (Yanagisawa et al., 2021; Parums, 2024; Yi et al., 2019), disinfectants (Lyashchuk et al., 2022; Naciri et al., 2011; Lyashchuk et al., 2023) and even specially developed ‘resistant’ varieties is extremely acute. In medicine, this issue was raised several decades ago. With the increase in the number of antimicrobial compounds, mankind received a surge in the growth of resistant strains (Zhanel et al., 2019; Zhang et al., 2024; Bhat et al., 2023; Zhou et al., 2025). In this ‘race’, pathogens have an advantage: with a lucky coincidence of circumstances, new generations of resistant bacteria can infect plant material within a few days, and a new antimicrobial drug or resistant strain will be developed and tested in several years.
Moreover, the use of synthetic biology technologies in working with genetic material has expanded the list of pathogenic biological objects, which, in addition to biological risk factors that are integral organisms, prions (protein-like infectious particles) (Casey and Sleator, 2024; Supattapone, 2020), insertion sequences (Is elements) (Shintani et al., 2023; Cheng et al., 2020), DNA transposons (bacterial and eukaryotic), retrotransposons (viral and non-viral), plasmids and other mobile genetic elements (Ahmadi et al., 2020; Kerkvliet et al., 2024). Some researchers point to a correlation between the increase in the number of resistant strains of plant disease pathogens and the rise in electromagnetic pollution levels, including background ultra-high frequency radiation. They hypothesize that electromagnetic exposure may hypothetically influence the physiological processes of microorganisms in a particular way, altering their resistance to adverse environmental factors and phytosanitary measures (Kerkvliet et al., 2024; Leuthner and Meyer, 2021; Xu et al., 2020; Dashti et al., 2022).
Also quite widespread is the theory of chemical stimulated mutations, which finds confirmation in studies of the appearance of resistant strains of microorganisms, as well as the formation of L-forms of bacteria (Xu et al., 2020; Dashti et al., 2022) and viral complexes (Lico et al., 2009; Ksenofontov et al., 2018; Dutta et al., 2024), which lead to long-term carriage and chronic forms of latent infection (most often these are viral complexes XA and XS). As a result, the plant organism is slowly and subtly destroyed under the influence of infectious agents, which may eventually lead to secondary infections and lethal intoxication in case of a sharp weakening of immunity or severe stress caused by drought or nutrient deficiency. Thus, not only the development of resistant varieties, but also regular monitoring, threat analysis and the application of phytoclearance measures against infected plants are necessary. Only an integrated approach to addressing the problem of yield losses and seed infection due to high potato disease incidence can minimize the annual damage to agriculture caused by the emergence and spread of infectious diseases of potato and vegetable crops.
Materials and methods
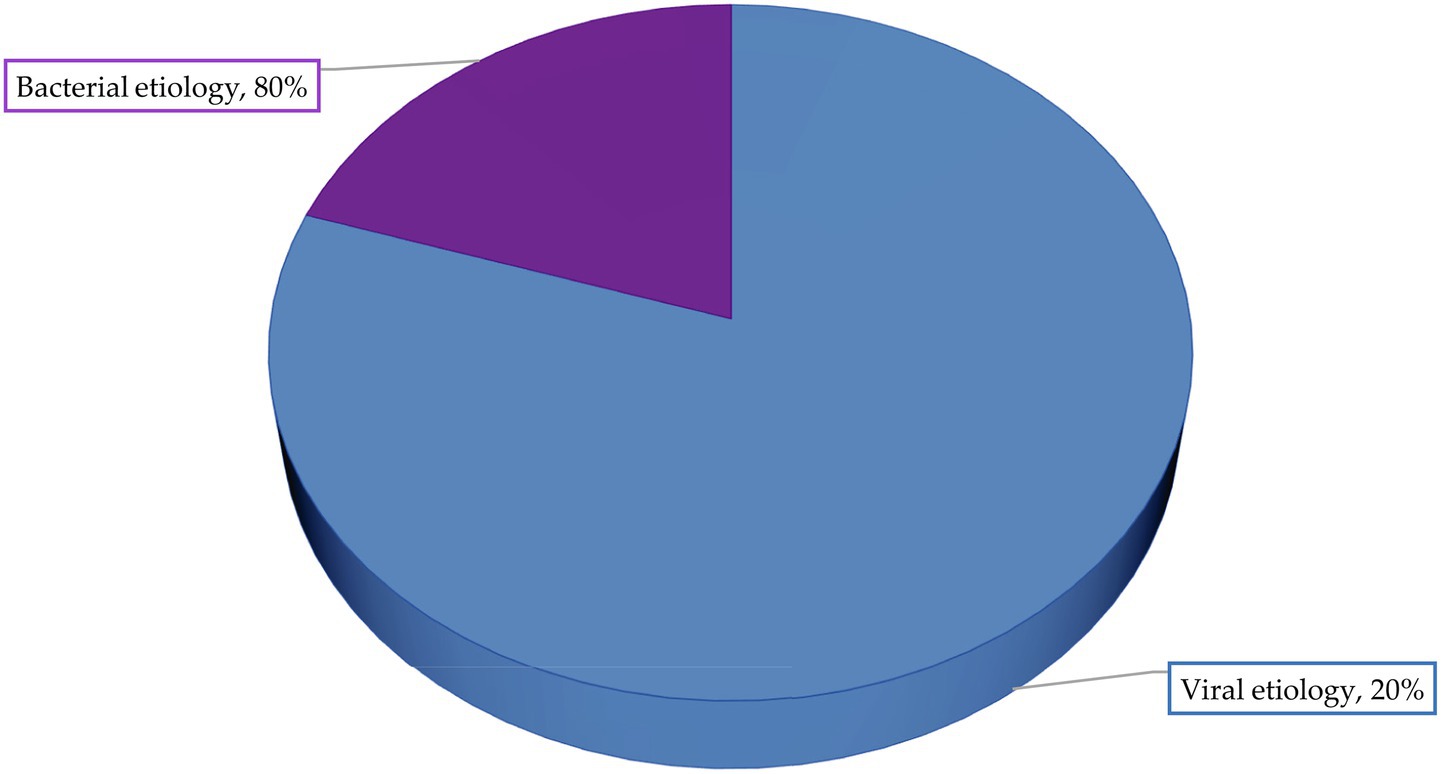
There are many different mechanisms and routes of infectious and parasitic diseases transmission. The prevalence of a particular contagion route will depend on the etiological, morphological and physiological characteristics of the pathogen. Analyses have shown that three mechanisms and five contagion routes are specific for infectious and parasitic diseases of potato (Figure 1).
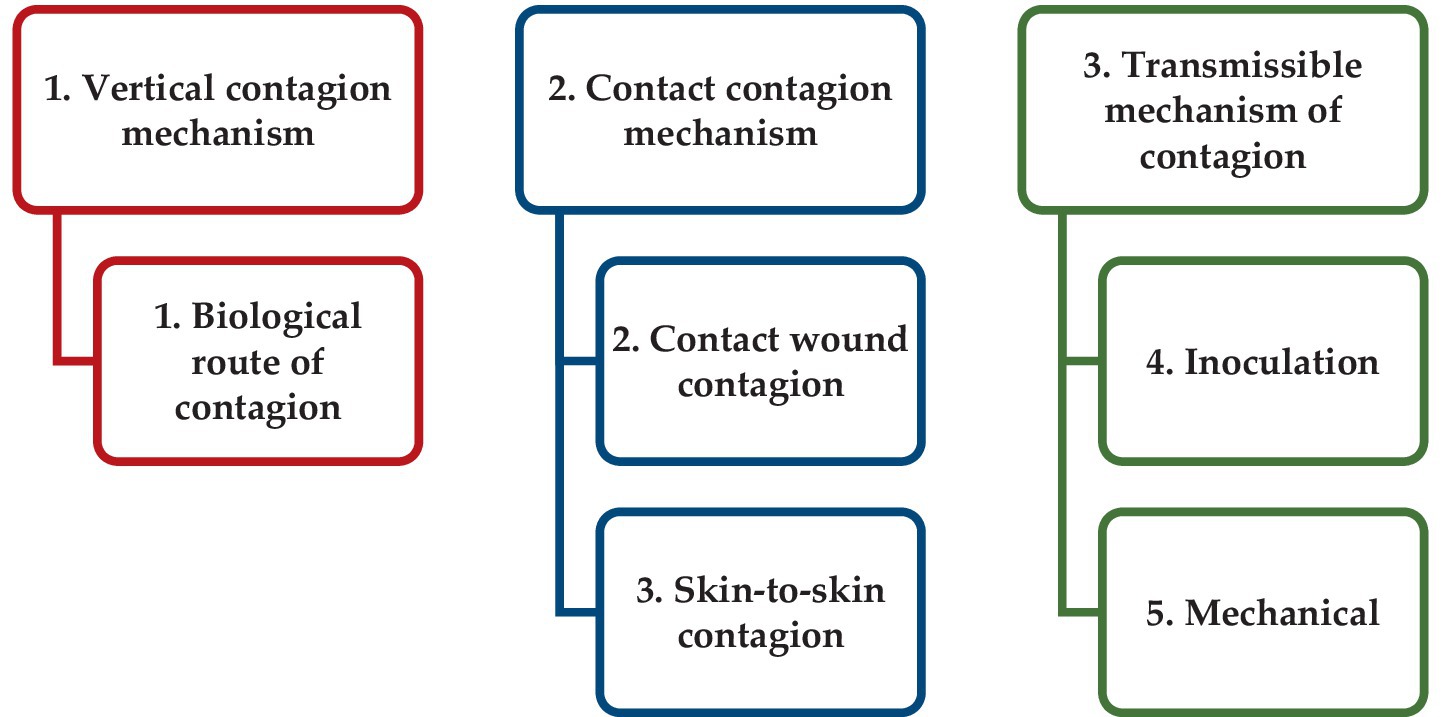
Figure 1. Mechanisms and pathways of biological risk factors of infectious and parasitic diseases of potato and vegetable crops spread.
Brief description of biological risk factors by parameters of mechanisms and pathways of contagion and spread of infectious and parasitic diseases of potato is presented in Supplementary Table S1. To process the results obtained during the study, databases were created in Microsoft Excel.
Results
The methodology for assessing the level of biological risk of the emergence and spread of infectious and parasitic diseases of potatoes based on the parameters of the mechanisms and routes of pathogen transmission includes the following stages:
1. Analysis of the relationship between the reservoir of infection and the prevalence of certain routes of transmission of pathogens of potato diseases.
2. Ranking the weight coefficients of the prevalence of a particular route of transmission in the process of pathogen spread.
3. Assessment and ranking of the level of biological risk based on the parameters of the mechanisms and routes of pathogen transmission.
Let us consider each of them in more detail.
Analysis of the relationship between the reservoir of infection and the prevalence of certain routes of transmission of pathogens of potato diseases
Analyses of the information presented in Supplementary Table S1 revealed some regularities in the relationship between the reservoir of infection and the prevalence of certain routes of contagion of potato pathogens (Figure 2).
Figure 2. Structure of share distribution of the main infection reservoirs characteristic for potato infectious and parasitic pathogens.
The diagram shows that for the considered pathogens of infectious and parasitic diseases of potato the main reservoirs of infection are planting material (40.4%) and soil (46.1%). It should be noted that soil as a typical habitat is specific for reservoir of infection for 11.5% of pathogens, soil contaminated with fungal spores—for 5.8% of pathogens, and soil with contaminated plant residues—for 28.8% of pathogens. Live vectors, as a reservoir of infection, are characteristic for 7.7% of the pathogens considered, wild and weedy plants—for 5.8% of IPPC pathogens.
Ranking of the weight coefficients of the prevalence of one or another transmission route in the process of spreading pathogens
The analysis revealed that infectious and parasitic diseases of potato can be described by five routes of contagion: biological, contact-wound, contact-coat, inoculative and mechanical.
The weighting coefficients of predominance of one or another route of contagion in the spread of pathogens, corresponding to the frequency of cases of infection by this route, are presented in Table 1.
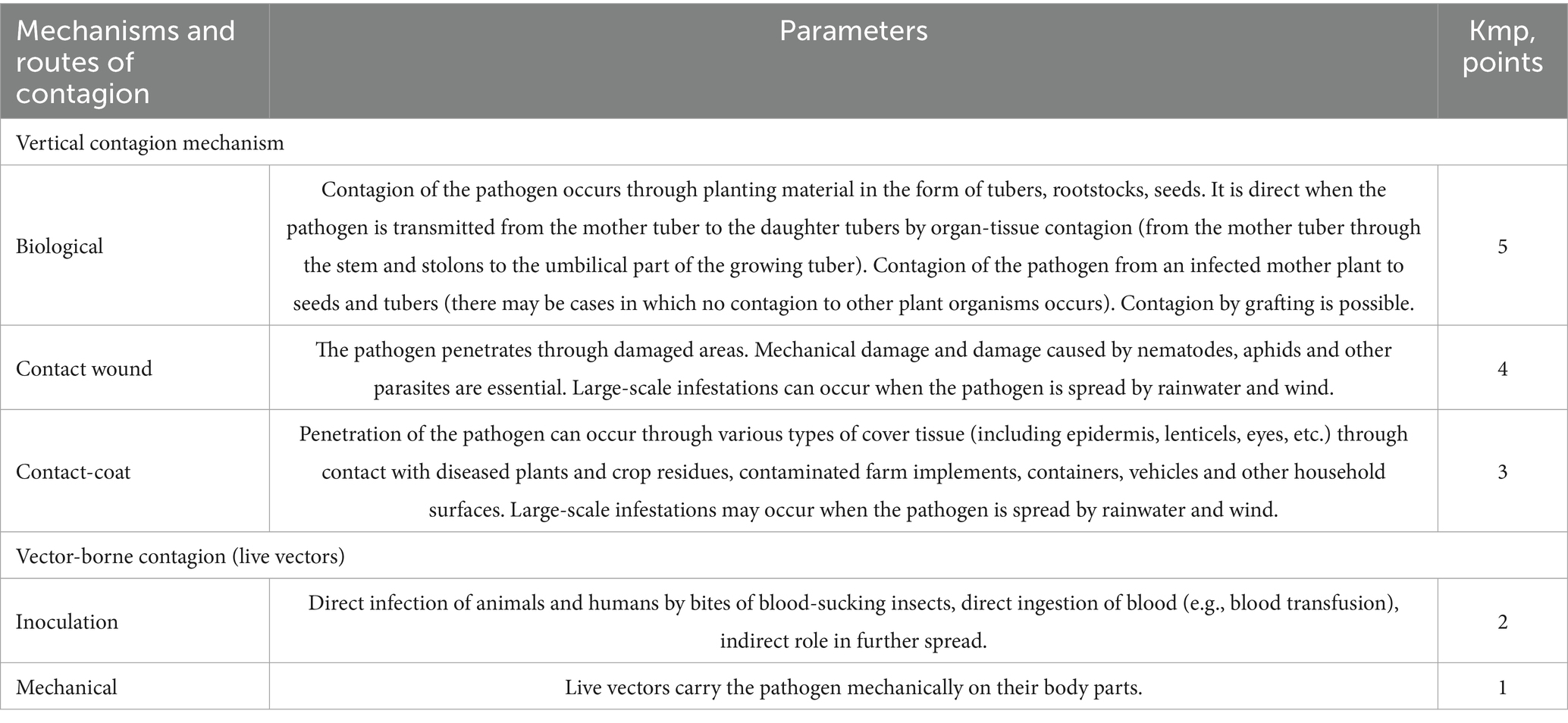
Table 1. Quantitative parameters for assessing mechanisms and pathways of contagion of biological risk factors.
Assessment and ranking of the level of biological risk according to the parameters of the mechanisms and routes of transmission of pathogens
The assessment of the level of biological risk based on the parameters of the mechanisms and routes of transmission of pathogens was carried out using the Equation 1:
Yrp – the level of risk of contagion of the pathogen;
KV – weighting factor;
Kmp – an indicator of the importance of contagion mechanisms and pathways on the rating scale (Table 2).
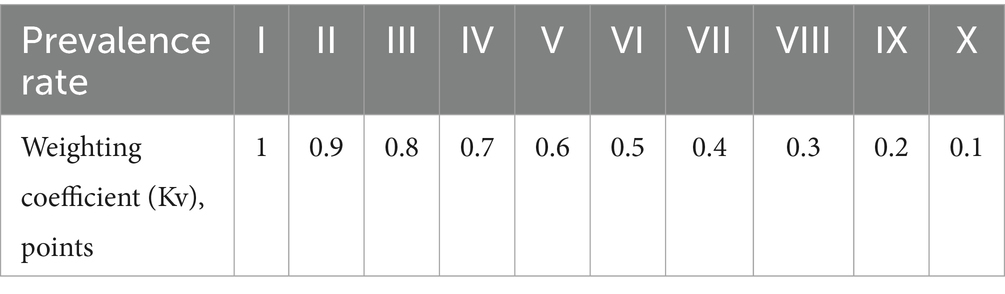
Table 2. Distribution of weighting coefficients in assessing the significance of mechanisms and routes of contagion of biological risk factors.
Results of the calculations were summarized in Tables 3, 4. Based on the values obtained, each pathogen was assigned to one of five groups corresponding to a certain level of biological risk according to the parameters of contagion mechanisms and routes.
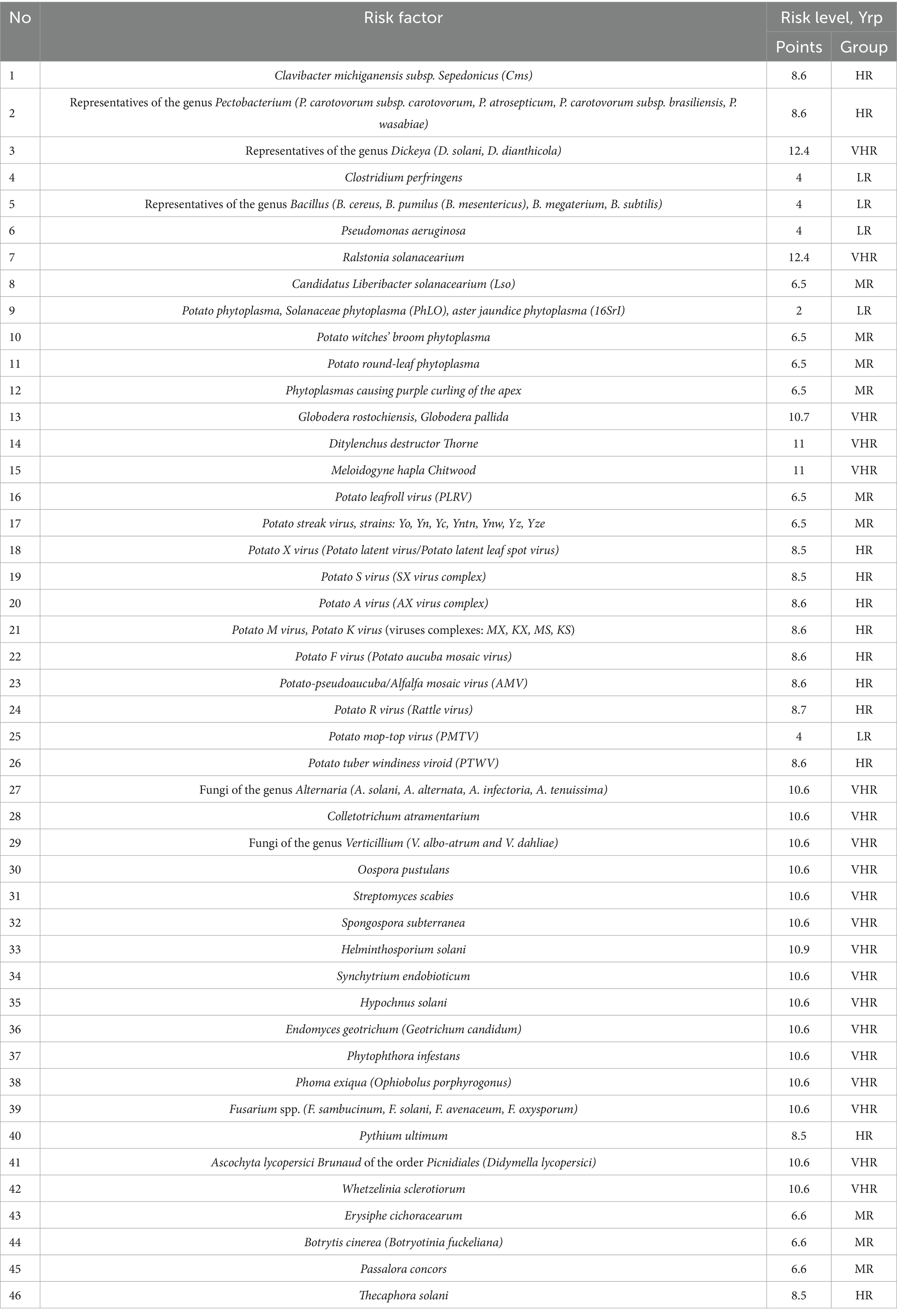
Table 3. Quantification of the biological risk level by mechanism and route of contagion (aetiological groups).
These were characterized according to the following scale:
1. Up to 2 points—very low risk (VLR);
2. Between 2 and 4 points—low risk (LR);
3. Between 5 and 7 points—medium risk (MR);
4. Between 8 and 10 points—high risk (HR);
5. Above 10 points—very high risk (VHR).
A quantitative assessment of the level of biological risk according to the parameters of contagion mechanisms and routes is presented below.
On the basis of the obtained data, graphical dependencies of biological risk level distribution by parameters of contagion mechanisms and pathways for pathogens of different etiologies were plotted in Figures 3–7.
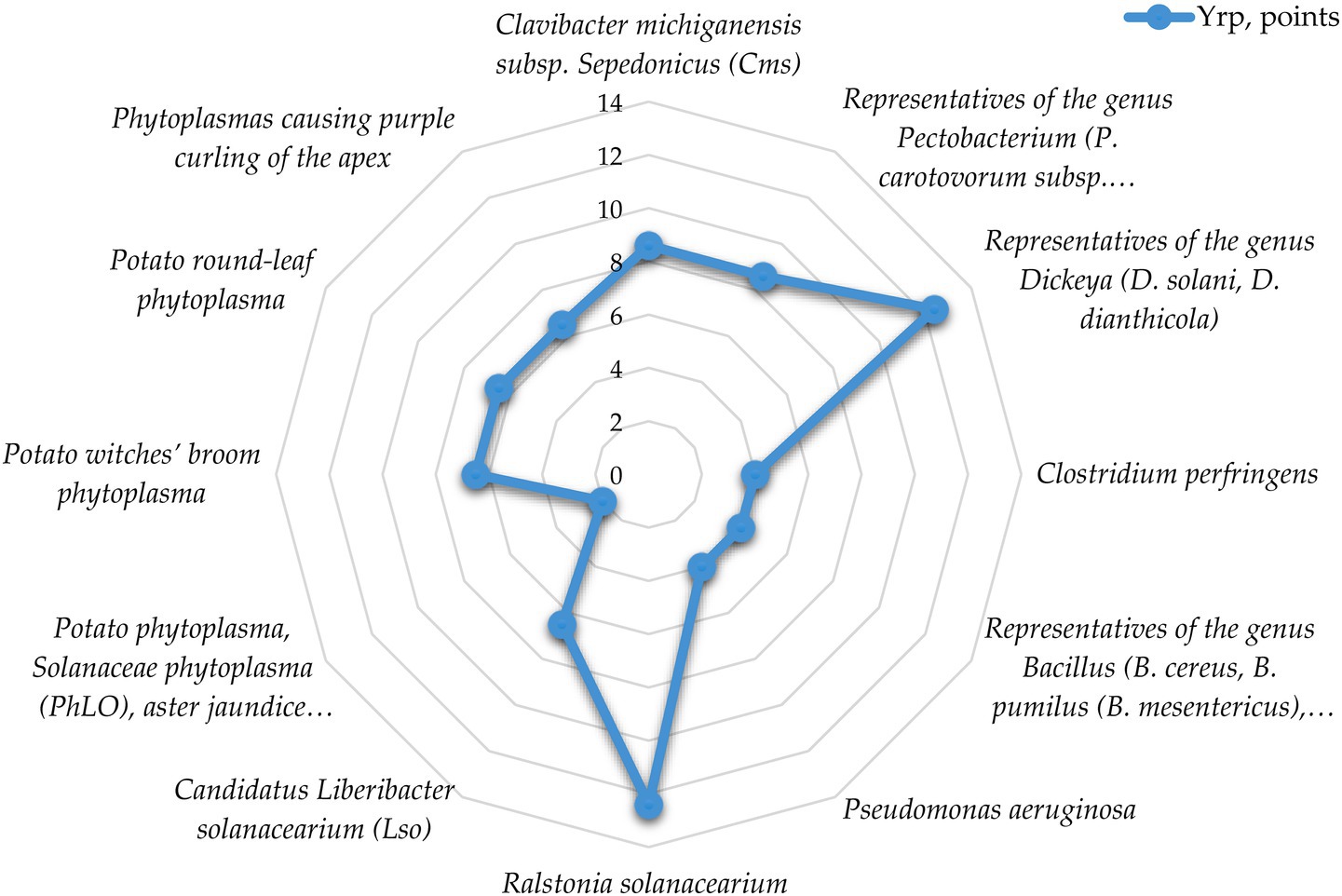
Figure 3. Distribution of biological risk level by mechanism and pathway of contagion for bacterial pathogens.
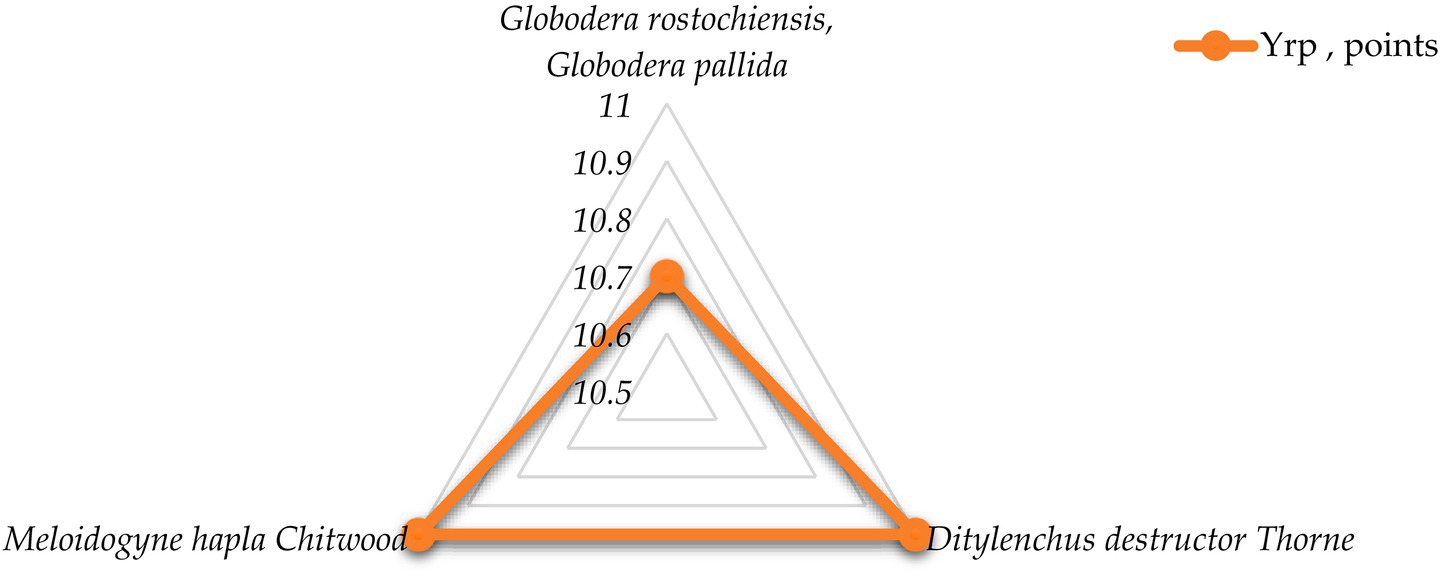
Figure 4. Distribution of biological risk level by contagion mechanisms and pathways for helminthic pathogens.
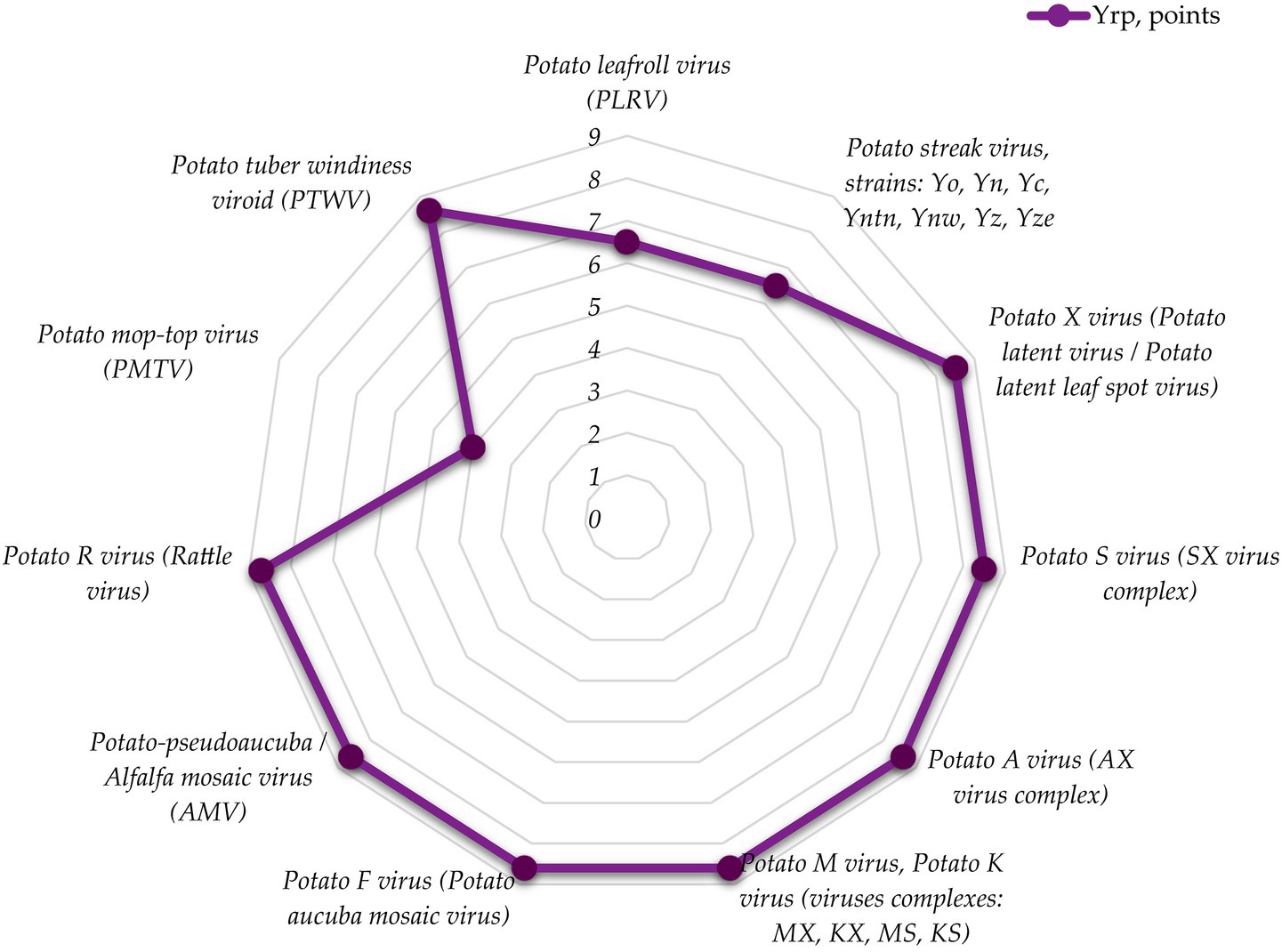
Figure 5. Distribution of biological risk level by mechanism and pathway of contagion for viral pathogens.
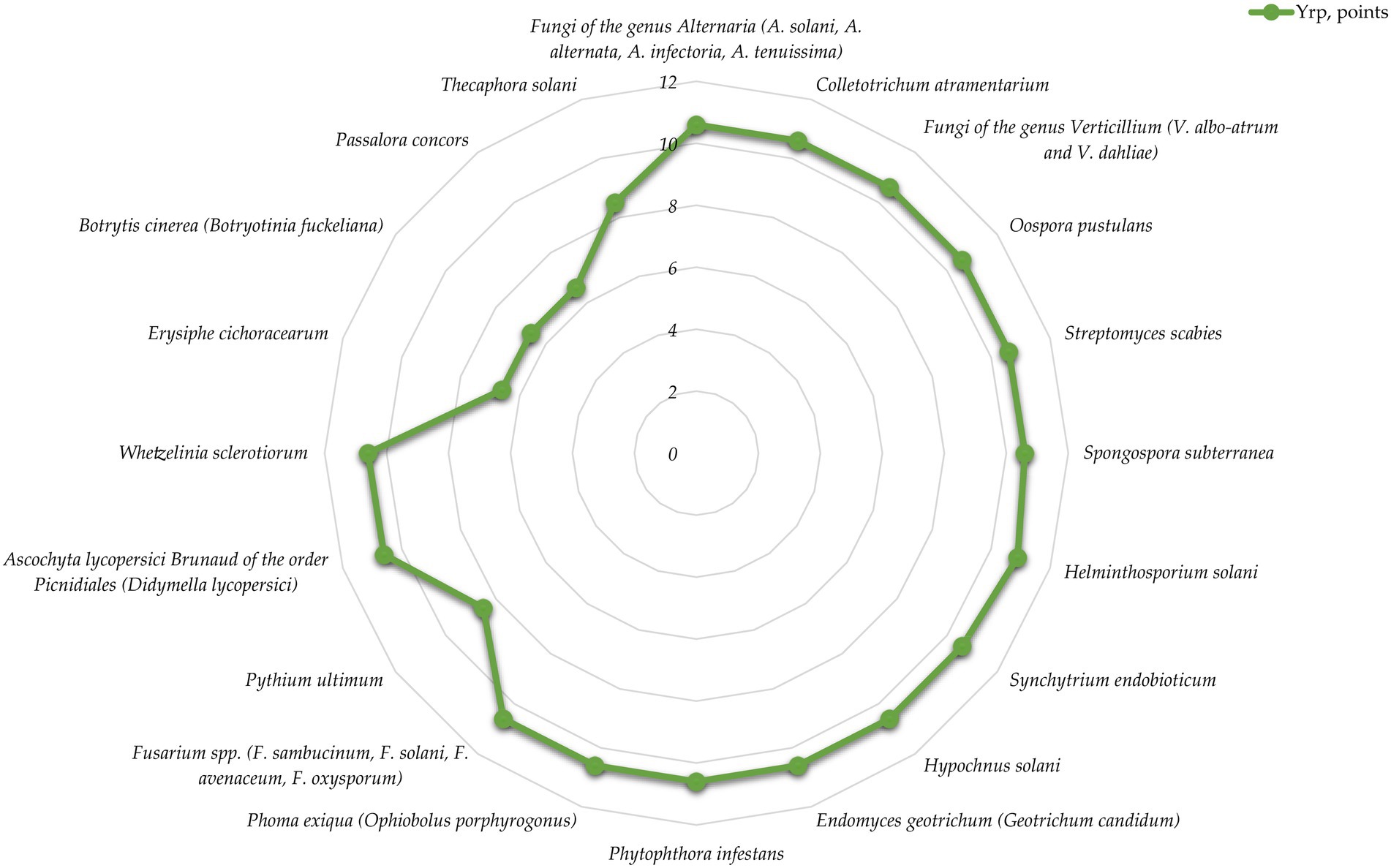
Figure 6. Distribution of biological risk level by mechanism and pathway of contagion for mycotic pathogens.
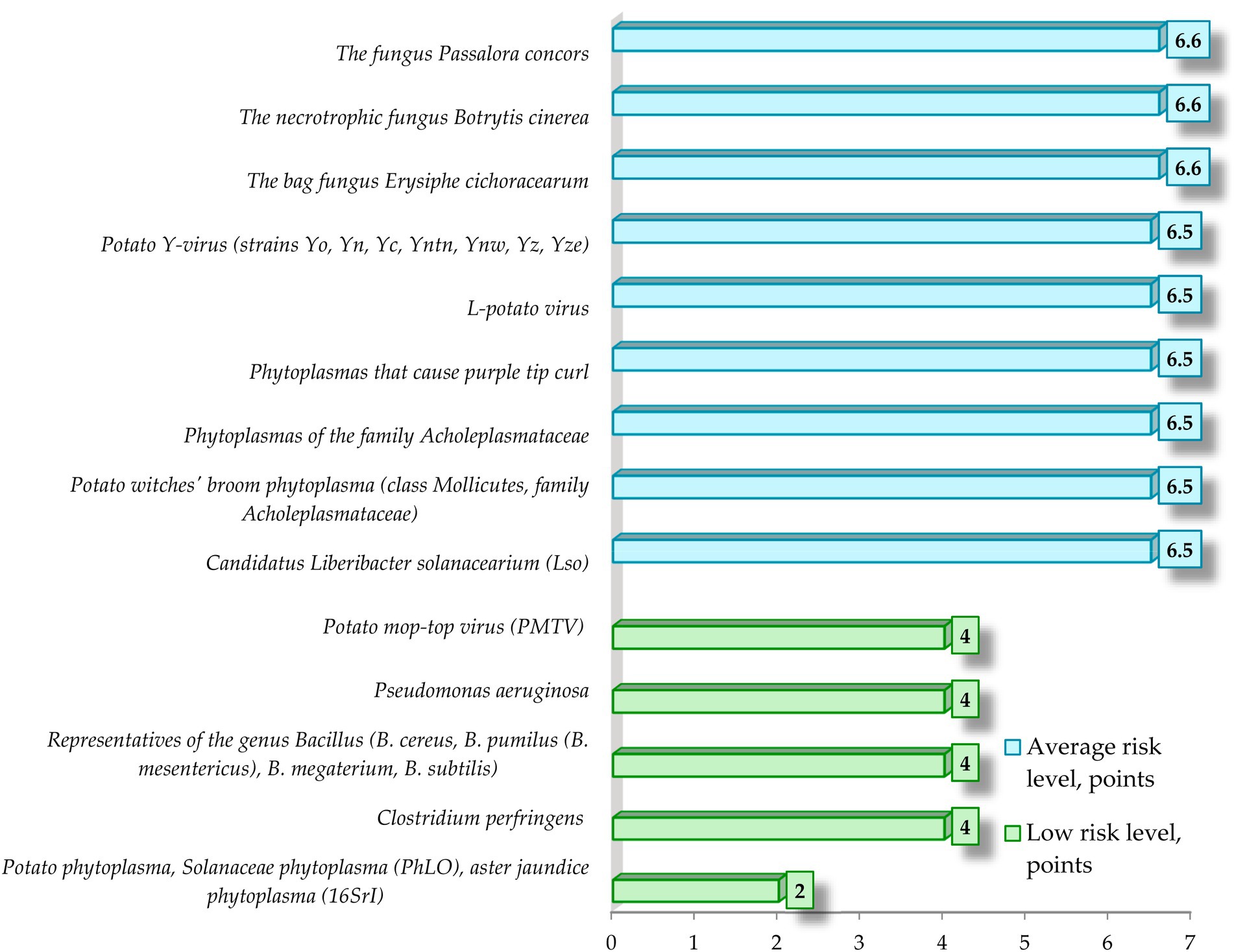
Figure 7. Low and medium level of biological risk of spread of infectious and parasitic diseases of potato by parameters of contagion mechanisms and pathways.
Analysis of the data shows that low (33.3%) and medium (33.3%) risk levels are characteristic of IPPC pathogens of bacterial etiology in terms of contagion mechanisms and routes, while high (16.7%) and very high (16.7%) risk levels are much less common.
At the same time, the average risk level is mainly found in phytoplasmas, high and very high—in species-specific phytopathogens causing various types of rots, including potato brown rot pathogen (Ralstonia solanacearium, low-temperature strain R2b3 is widespread in our country), which is a quarantine object. The average score for the group is 6.8, which corresponds to an average risk level.
The most specific feature of all the helminthic pathogens is very high risk level (group mean score 10.9). Members of the solanaceae family are parasitized by a variety of helminths, most commonly nematodes. Due to violations of storage and planting standards development of phytohelminthic diseases may happen, such as: globoderosis (causative agents: Globodera rostochiensis and Globodera pallida, TRL = 10.7), ditylenchosis, which is quarantined in some countries (causative agent: Ditylenchus destructor Thorne, TRL = 11) and meilodoginosis (causative agent: Meloidogyne hapla Chitwood, TRL = 11).
Analysis of the data shows that the high-risk level (72.7%) is characteristic of viral pathogens in terms of contagion mechanisms and routes. For 18.2% of viral EPIC pathogens, medium risk level is specific, and only 9.1% are characterized by a low biological risk level. The average score for the group is 7.8, which corresponds to a high risk level.
It should be noted that viral complexes have the greatest plasticity in terms of pathogen contagion, which in most cases is due to the latency of manifestation of clinical signs of the disease. For most of the considered pathogens of viral etiology, the reservoir of infection is planting material (63.6%), as latent viral forms are practically not detected in tubers neither at the harvesting nor at the storage stage. Viral agents become active after potato planting and germination, reducing productivity and adversely affecting yields.
Live vectors (27.3%) are also an important reservoir for contagion of IPPC pathogens of viral etiology: peach aphids (Myzus persicae for L-virus and potato Y-virus) and nematodes Paratrichodorus minor, P. pachydernus, Tr. pachydernus, Trichodorus similis, within which R-virus virions (Rattle virus/Tobacco rattle virus/Tobacco variegation virus/Tuber necrosis virus) remain virophoric for up to 5 years.
It is noteworthy that for Potato mop-top virus (PMTV), the reservoir of infection is soil infected with zoospores of powdery mildew (Spongospora subterranea) carrying PMTV virions. The spores can persist in the soil for up to 18 years, waiting for favorable germination conditions. Thus, potato is infected with a complex infection (virus + fungus).
Data analysis shows that according to the parameters of contagion mechanisms and pathways, 75% of mycotic pathogens are characterized by a very high risk level, 10% by a high risk level and 15% by a medium biorisk level. The average score for the group is 9.8, which corresponds to a high risk level, almost threshold with very high.
It should be noted that fungi are the most widespread group of potato pathogens.
The most significant among them are:
• Alternaria spp. (Yrp = 10.6) causing early blight (may cause allergies if ingested);
• Oospora pustulans (Yrp = 10.6) causing potato cancer (quarantine facility);
• Endomyces geotrichum (Yrp = 10.6) causing “rubber rot” of potatoes;
• Phytophthora infestans (Yrp = 10.6), which causes phytophthorosis (the disease has a high rate of development);
• Phoma exiqua (Yrp = 10.6) causing phomosis (the disease can become epiphytotic with serious consequences and high yield losses);
• Fusarium spp. (Yrp = 10.6) causing fusariosis (the disease has a high rate of development and can take a latent form when the tubers are affected, which negatively affects the quality of planting material and is a threat to future yield loss).
Data analysis shows that low level of risk of occurrence and spread of infectious and parasitic diseases of potato according to the parameters of contagion mechanisms and pathways is specific for 10.9% of considered pathogens of infectious and parasitic diseases of potato, medium level of risk is specific for 19.6%, high level is specific for 26.1%, very high level of risk is specific for 43.5% of considered pathogens of IPPC.
Based on the obtained data, graphs that describe levels of biological risk of emergence and spread of infectious and parasitic diseases of potato by parameters of contagion mechanisms and pathways were made (Figures 7, 8).
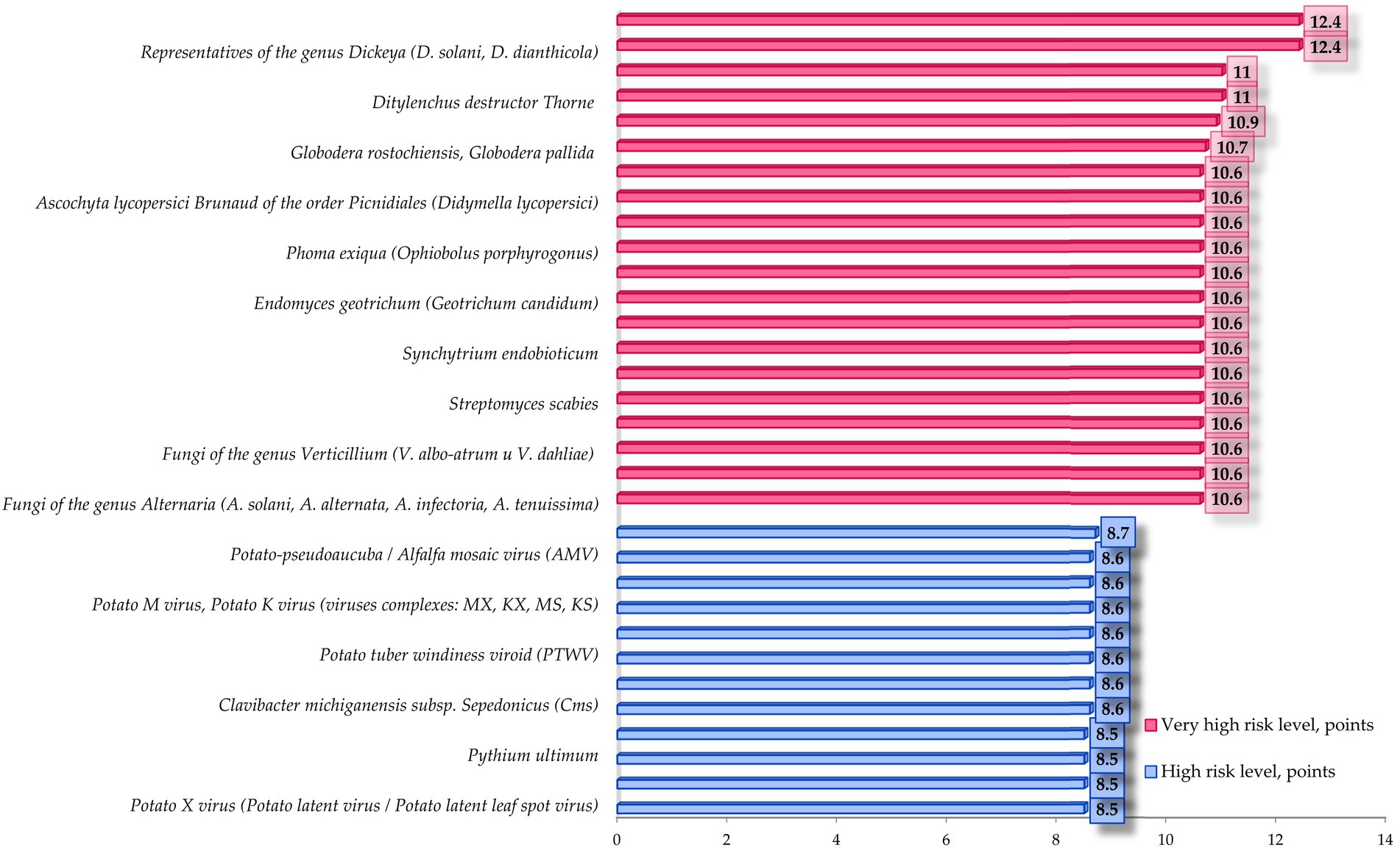
Figure 8. High and very high level of biological risk of emergence and spread of infectious and parasitic diseases of potato according to the parameters of contagion mechanisms and pathways.
The analysis of the graphs shows that there are some regularities between the risk of IPPC emergence and spread and the taxonomic affiliation of the pathogens. Therefore, structural diagrams characterizing the proportion of pathogens of each etiology were constructed for groups with low, medium, high and very high risk levels (Figures 9–12).
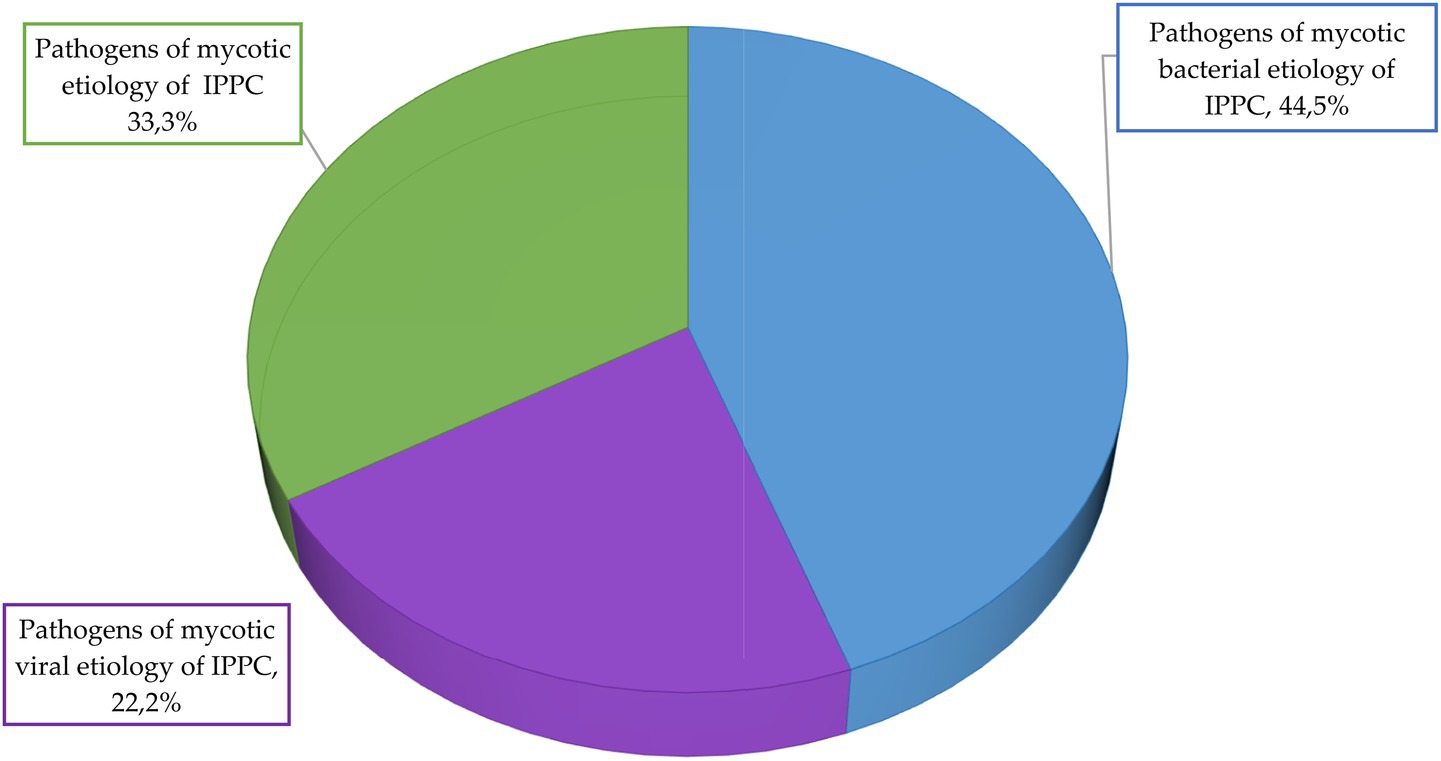
Figure 10. Etiological structure of potato infectious and parasitic pathogens for the group with medium risk level.
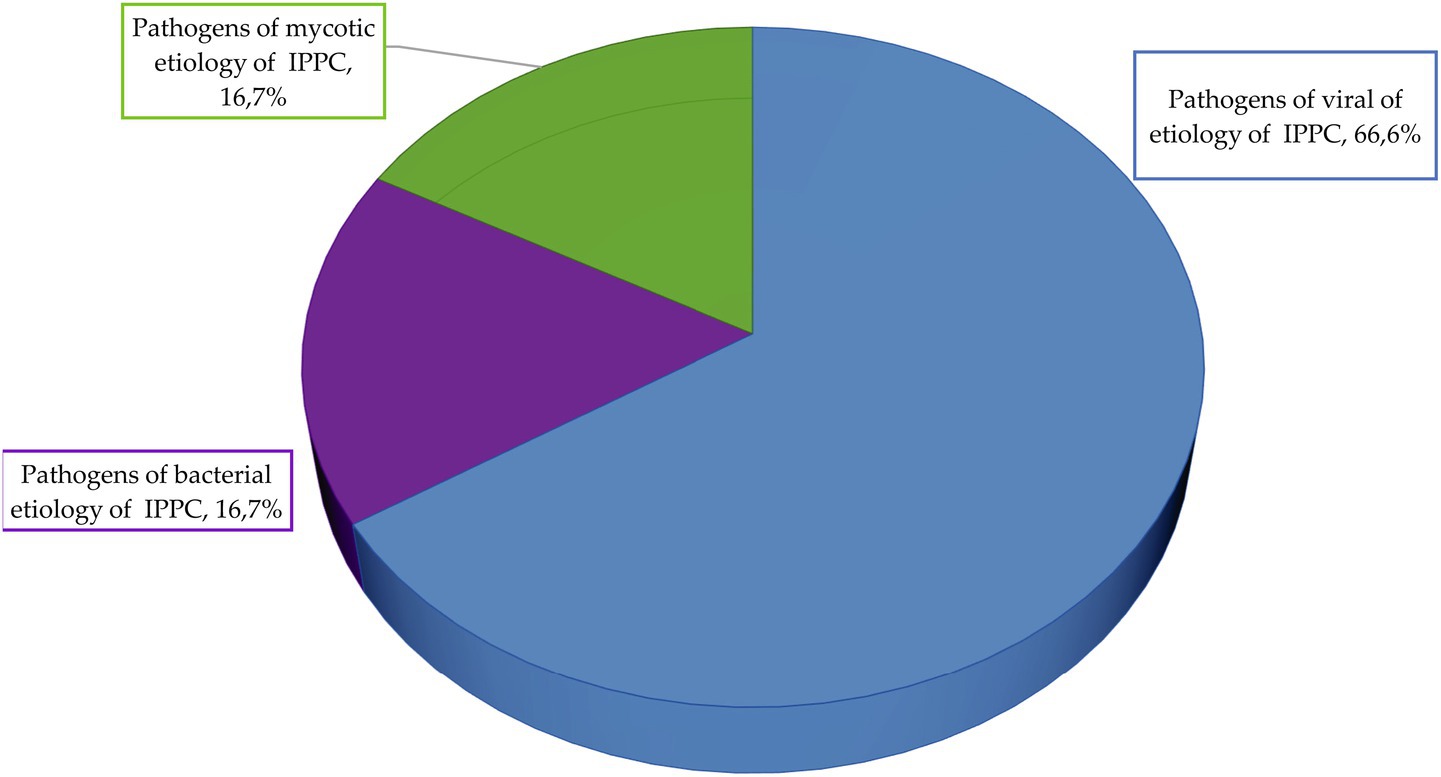
Figure 11. Etiological structure of potato infectious and parasitic pathogens for the high-risk group.
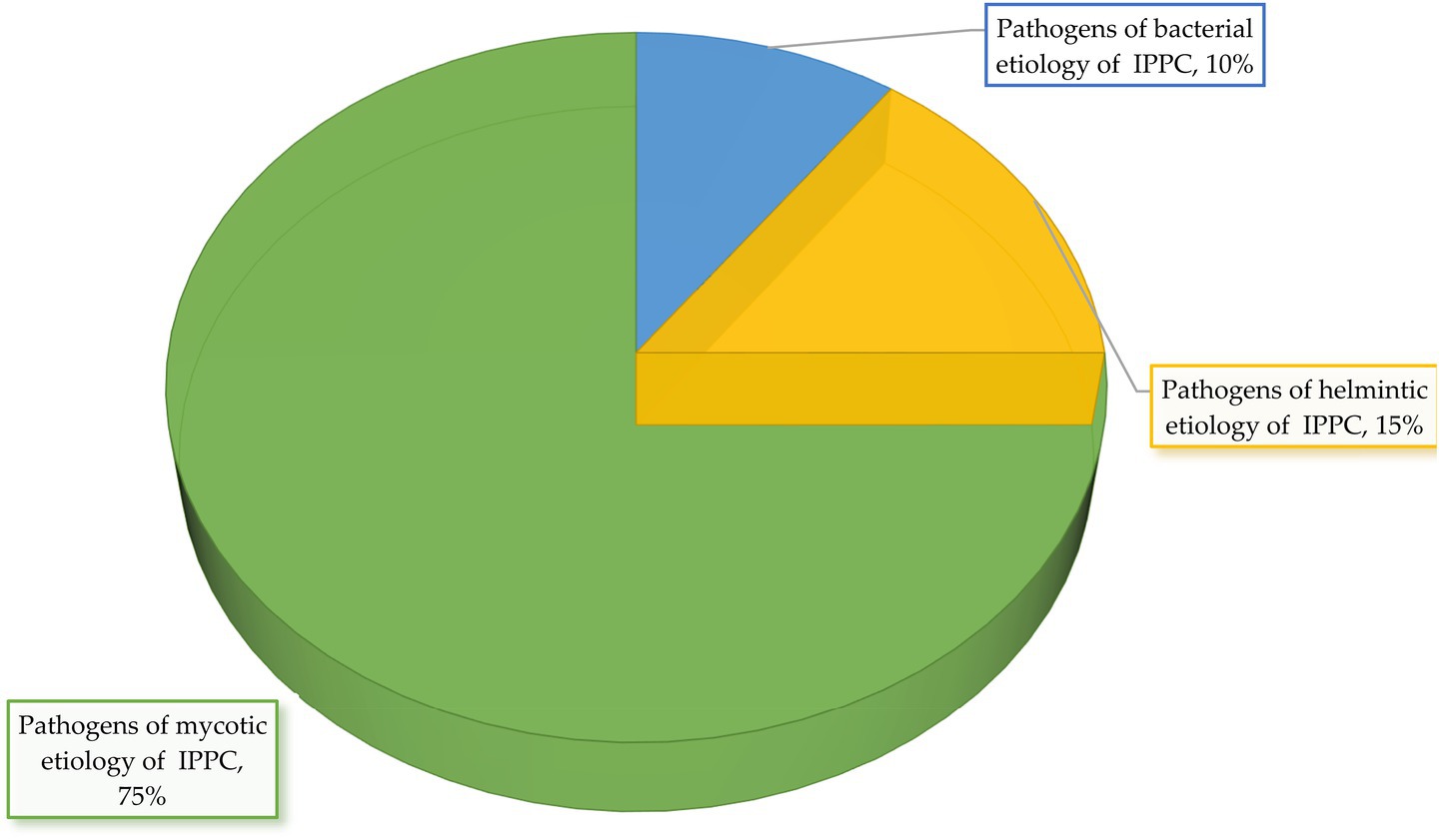
Figure 12. Etiological structure of potato infectious and parasitic pathogens for the very high risk group.
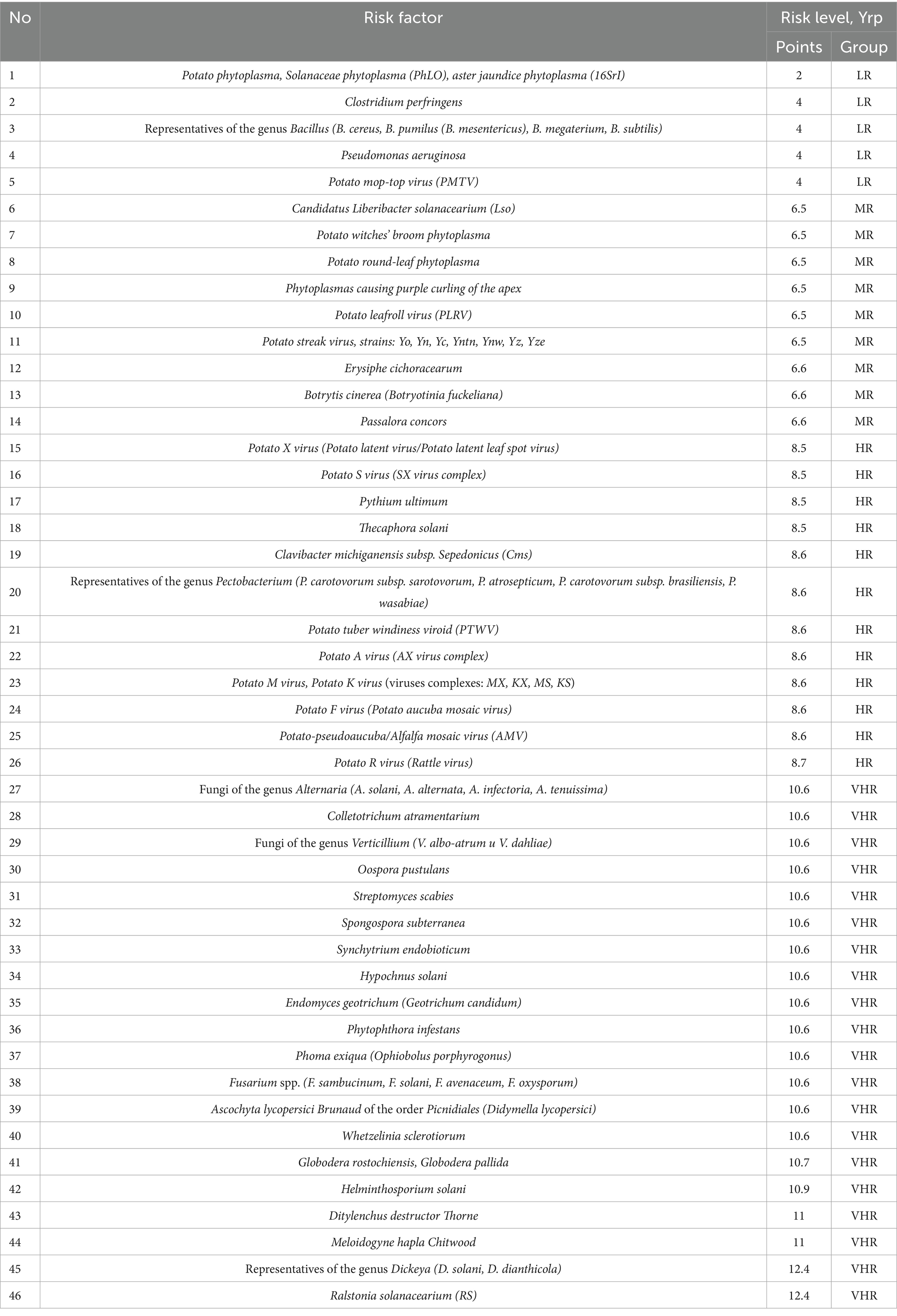
Data analysis shows that in the low-risk group 80% of potato infectious and parasitic diseases have bacterial etiology and only 20% have viral etiology. There were no pathogens of infectious and parasitic potato diseases of helminthic and mycotic etiology in this group. The average score of the low-risk group is 3.6.
Data analysis shows that in the group with medium risk level, 44.5% of potato infectious and parasitic diseases have bacterial etiology, 33.3%—mycotic etiology, 22.2%—viral etiology. There are no pathogens of infectious and parasitic potato diseases of helminthic etiology in this group. The average score for the group with medium risk level is 6.5.
The data analysis shows that in the group with high risk level the pathogens of 66.6% of infectious and parasitic potato diseases have viral etiology, 16.7%—mycotic etiology, 16.7%—bacterial etiology.
There are no pathogens of infectious and parasitic potato diseases of helminthic etiology in this group. The average score of the high-risk group is 8.6.
Data analysis shows that in the group with a very high risk level, 75% of potato infectious and parasitic diseases have mycotic etiology, 15%—helminthic, 10%—bacterial. There are no pathogens of infectious and parasitic potato diseases of viral etiology in this group. The average score of the very high risk group is 10.8.
To assess the relationship between the spread of potato diseases and the biological risk of pathogen dissemination based on the parameters of transmission mechanisms and pathways, statistical data from the Rosselkhozcenter on the areas affected by potato diseases characteristic of Russia for the period from 2020 to 2024 were analyzed.
The dynamics of potato crop infection by the main causative agents of infectious and parasitic diseases are presented in Table 5.
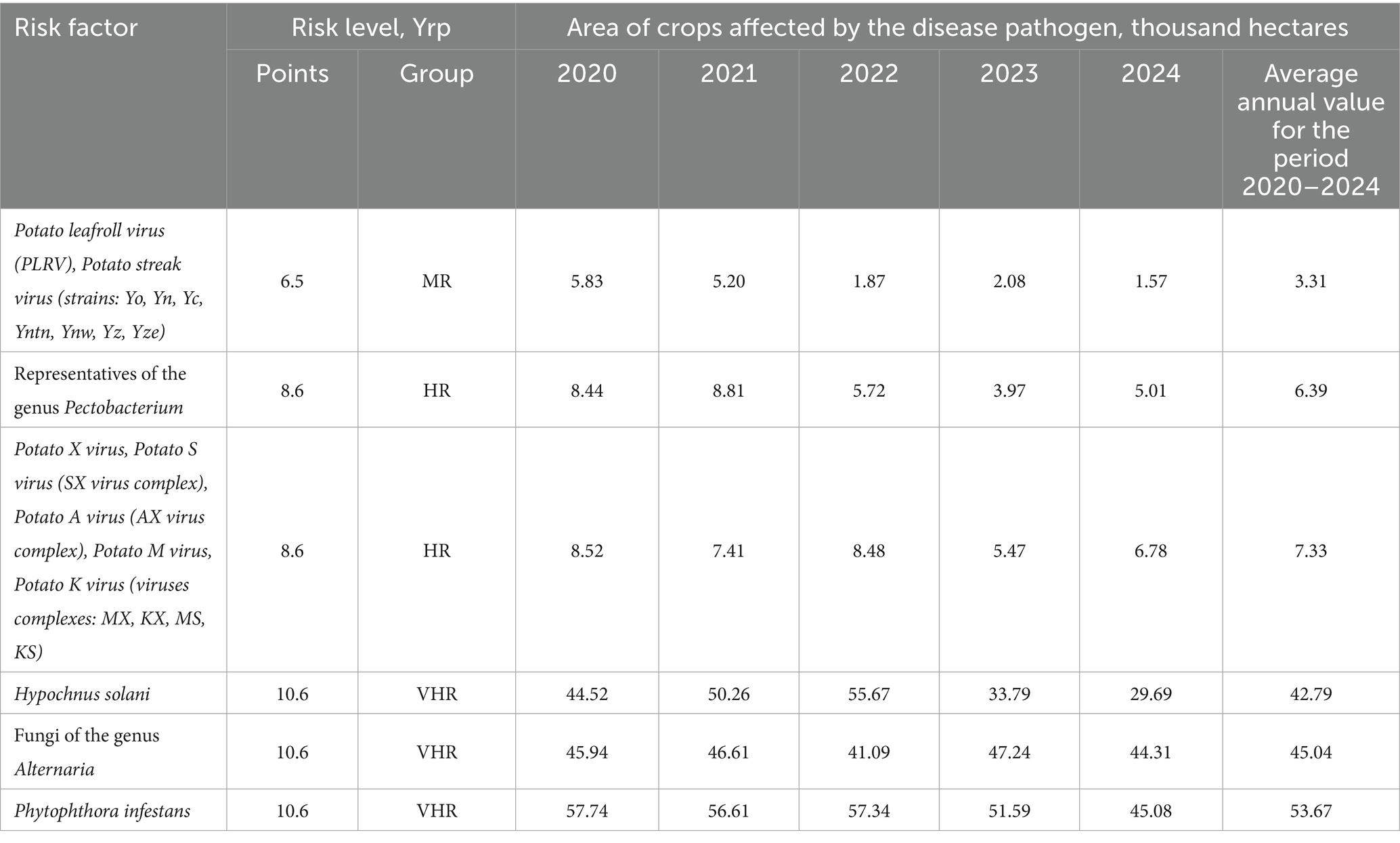
Table 5. Dynamics of potato crop infection by the main causative agents of infectious and parasitic diseases.
Using the available statistical data and the results of the point-rating assessment characterizing the level of biological risk according to the parameters of the mechanisms and routes of transmission, a diagram of the relationship between the level of biological risk according to the parameters of the mechanisms and routes of transmission of pathogens and the average annual area of damage to plantings by the main pathogens of infectious and parasitic diseases of potatoes was constructed, presented in Figure 13.
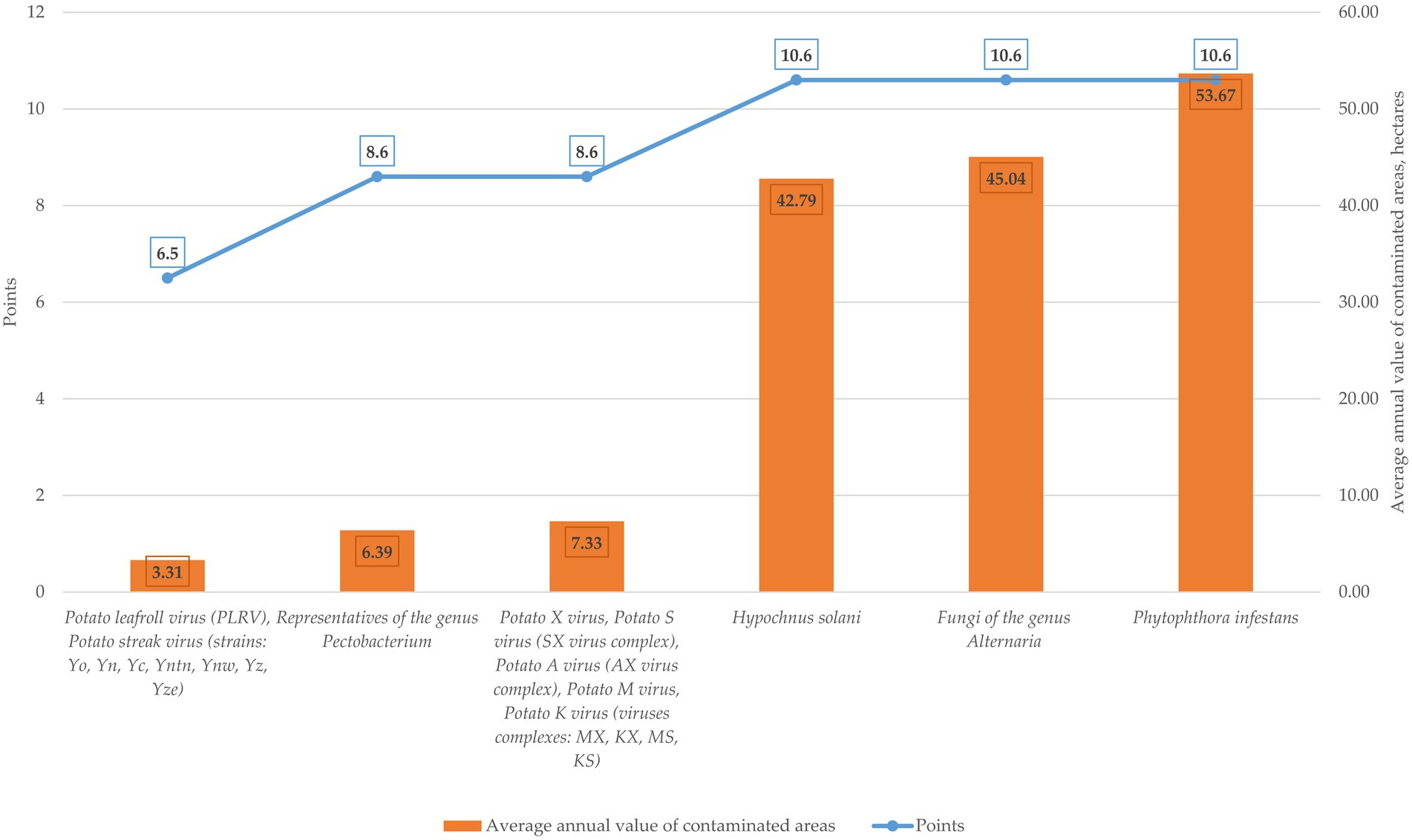
Figure 13. Analysis of the correlation between the level of biological risk according to the parameters of the mechanisms and routes of transmission of pathogens and the average annual area of damage to crops by the main pathogens of infectious and parasitic diseases of potatoes.
The analysis of Table 5 and Figure 13 shows that among the pathogens of infectious and parasitic diseases of potatoes registered in the Russian Federation over the past five years, the most widespread was the pathogen of late blight—Phytophthora infestans (over a five-year period, the average annual value of the affected area of plantings is 53.67 hectares), in second place are the pathogens of alternariosis—fungi of the genus Alternaria (over a five-year period, the average annual value of the affected area of plantings is 45.04 hectares), and third place is occupied by the pathogens of rhizoctonia—Hypochnus solani (over a five-year period, the average annual value of the affected area of plantings is 42.79 hectares).
All the above-described pathogens have mycotic etiology and belong to a group with a very high risk level (VHR—10.6) according to the parameters of the mechanisms and routes of transmission of infectious and parasitic diseases of potatoes.
The group with a high risk level (HR—8.6) is represented by viruses and viral complexes that cause the appearance of “mosaic” and leaf curling: Potato X virus, Potato S virus (SX virus complex), Potato A virus (AX virus complex), Potato M virus, Potato K virus (viruses complexes: MX, KX, MS, KS), as well as pathogens that cause the appearance of necrosis of vascular tissues of the plant—“black leg” (representatives of the genus Pectobacterium). The average annual values of the planting areas affected by pathogens of this group are comparable, amounting to 7.33 hectares for pathogens of viral etiology and 6.39 hectares for pathogens of bacterial etiology (P. carotovorum subsp. сarotovorum, P. atrosepticum, P. carotovorum subsp. brasiliensis, P. wasabiae).
The group with an average risk level (MR—6.5) closes the rating, represented by pathogens of viral etiology causing leaf curling—Potato leafroll virus (PLRV) and leaf mottling—Potato streak virus (strains: Yo, Yn, Yc, Yntn, Ynw, Yz, Yze). Over a five-year period, the average annual value of the affected area of plantings affected by these pathogens is 3.31 hectares.
The analysis of the correlation dependence between the level of biological risk for the parameters of the mechanisms and routes of transmission of pathogens and the average annual area of damage to crops by the main pathogens of infectious and parasitic diseases of potatoes shows that the point values of risk levels reflect the dynamics of the average annual damage to crops by pathogens of infectious and parasitic diseases of potatoes from the point of view of their ranking by risk groups.
Discussion
Various optical methods are widely used in agriculture. Unlike canonical conventional laboratory methods such as PCR, ELISA, etc., optical methods of analysis are non-invasive, fast, simple, relatively low cost, and allow rapid analysis and results. Among optical methods, the most common are reflectance spectroscopy in the visible and IR region, Raman spectroscopy, hyperspectral imaging, temporally resolved THz spectroscopy and fluorescence. IR spectroscopy and Raman spectroscopy (Onozuka et al., 2021; Zubler and Yoon, 2020; Zedler et al., 2023; Patel et al., 2023; Yanagisawa et al., 2021) are examples of the application of optical techniques to diagnose fungal infestation.
In Ferentinos (2018), Kumar et al. (2021), and Kumar et al. (2023), differences in the optical characteristics of pathogenic and nonpathogenic fungal species and strains were investigated using fluorescence and Raman spectroscopy. Differences in the fluorescence spectra of some pathogenic and non-pathogenic fungal species were determined using excitation-emission matrices and principal component method. It was found that there are two zones in the excitation-emission matrix showing maximum differences in fluorescence of pathogenic and non-pathogenic fungi.
However, the above presented studies do not allow to determine and establish the nature of the occurrence of infestations, as well as to perform an assessment of the spread of diseases among plants, which together with the developed methodology of infection spread will allow to provide forecasting of the spread of infection and to develop preventive measures to eliminate its spread.
The occurrence and spread of potato diseases caused by viruses, viroids and virus complexes have been investigated in the works of such Russian and foreign scientists as: Ksenofontov et al. (2018), Dutta et al. (2024), Visser et al. (2024), and Karasev and Gray (2013), etc.
Peculiarities of pathogenic effect on potato of pathogens of bacterial etiology (Clavibacter michiganensis subsp. Sepedonicus (Cms), Ralstonia solanacearium (RS), Candidatus Liberibacter solanacearium (Lso), representatives of the genera: Pectobacterium, Dickeya, Bacillus, Pseudomonas and phytoplasmas of Mollicutes class) were considered in the works of such scientists as: Chen et al. (2022), Rivera-Zuluaga et al. (2023), Wang et al. (2022), Citti et al. (2018), and Waleron et al. (2019), etc.
The dynamics of potato diseases caused by golden, pale and stem nematodes have been studied and described in Wainer and Dinh (2021), Wainer and Dinh (2021), and Yu et al. (2012), etc.
The peculiarities of pathogenic effect on potato of pathogens of mycotic etiology (Colletotrichum atramentarium, Oospora pustulans, Streptomyces scabies, Spongospora subterranea, Helminthosporium solani, Synchytrium endobioticum, Phoma exiqua, Fusarium spp., Pythium ultimum, Ascochyta lycopersici Brunaud, fungi of genera Alternaria, Verticillium, etc.) were considered in the works of such scientists as: Goodell et al. (1982), Przetakiewicz (2015), Deb et al. (2020), Woudenberg et al. (2013), Woudenberg et al. (2015), Schmey et al. (2024), and Johnson and Dung (2010).
Available data from research results show the diversity of approaches and depth of study of potato disease problems. The proposed methodology covers the features of 46 potato pathogens of viral (Ksenofontov et al., 2018; Dutta et al., 2024; Visser et al., 2024; Karasev and Gray, 2013; Dissanayaka Mudiyanselage et al., 2018), bacterial (Chen et al., 2022; Rivera-Zuluaga et al., 2023; Wang et al., 2022; Citti et al., 2018; Waleron et al., 2019), helminthic (Wainer and Dinh, 2021; Yu et al., 2012; Pan et al., 2022) and mycotic etiologies (Goodell et al., 1982; Przetakiewicz, 2015; Deb et al., 2020; Woudenberg et al., 2013; Woudenberg et al., 2015).
Modeling of plant disease detection systems using artificial intelligence has been studied by researchers such as Abbas et al. (2024), Rathor et al. (2024), and Núñez-Muñoz et al. (2025).
As a result, the combination of modern approaches in the field of potato plant disease detection and the proposed methodology will make it possible to develop a comprehensive system for monitoring and forecasting the spread of infections, which will make it possible to take timely and effective measures to protect the crop. The use of optical methods in combination with the analysis of data on pathogens will increase the accuracy of diagnosis and efficiency of prevention, contributing to sustainable agricultural development.
Conclusion
According to the results of analysis and assessment of the level of biological risk of occurrence and spread of infectious and parasitic diseases of potato according to the parameters of contagion mechanisms and pathways, the following conclusions can be drawn:
1. High (26.1%) and very high (43.5%) level of risk of occurrence and spread of infectious and parasitic diseases of potato according to the parameters of contagion mechanisms and pathways are specific for the majority of the considered pathogens.
2. According to the parameters of contagion mechanisms and pathways, the considered pathogens of infectious and parasitic potato diseases of bacterial etiology are characterized by low (33.3%) and medium (33.3%) risk level, viral etiology—high risk level (72.7%), mycosis etiology—very high risk level (75%), helminth etiology—very high risk level (100%).
3. The etiological groups of pathogens according to the parameters of contagion mechanisms and pathways revealed regularities with respect to grouping by risk levels.
In the group with low risk level the majority of pathogens of infectious and parasitic diseases of potato have bacterial etiology (80%), for average risk level the most typical pathogens are bacterial (44.5%) and mycosis etiology (33.3%), in the group with high risk level the majority of pathogens have viral etiology (66.6%), in the group with very high risk level the majority of pathogens have mycosis etiology (75%).
1. High and very high risk levels are most specific of species-specific bacterial phytopathogens causing various types of rots, phytohelminthic diseases, viral complexes and mycoses. It should be noted that virus complexes and fungi have the greatest plasticity in terms of pathogen contagion, fungi being the most common group of potato pathogens.
Plasticity of contagion of viral complexes in most cases is due to the latency of manifestation of clinical signs of the disease. The reservoir of infection is planting material, as latent viral forms are practically not detected in tubers neither at the stage of harvesting nor at the storage stage. Viral agents become active after potato planting and germination, reducing productivity and adversely affecting yields.
The plasticity of fungal contagion is due not only to the high probability of latent disease, but also to the high rate of disease spread due to the possibility of spore formation. Spores are spread over long distances by wind and rainwater, which causes unpredictability of spread and infestation of plants in the field and leads to poor detectability of pathogens during storage of planting material.
Latent forms of tuber contamination by viruses and fungal spores require close attention, as infected planting material has lower quality and is a threat to future yield losses.
Thus, the methodology proposed by our research team will enhance the possibilities for monitoring, threat analysis and application of measures for treatment of potato plants and separation of healed plants from infected ones.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AD: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing, Supervision, Data curation, Conceptualization. AS: Writing – original draft, Funding acquisition, Software, Resources, Visualization, Formal analysis, Conceptualization, Supervision, Project administration, Validation, Data curation, Writing – review & editing, Methodology, Investigation. YL: Writing – original draft, Formal analysis, Methodology, Writing – review & editing. VT: Software, Formal analysis, Writing – original draft, Writing – review & editing, Validation. NP: Validation, Investigation, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was carried out with the support of the Ministry of Science and Higher Education of the Russian Federation in the form of subsidies in accordance with paragraph 4 of Article 78.1 of the Budget Code of the Russian Federation No. 075-15-2024-540 dated April 24, 2024 for a major scientific project “Development of nature-like technologies for managing properties and quality indicators of plants using biophotonics methods and digital systems.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1614949/full#supplementary-material
References
Abbas, J., Nabila, B., Rizwan, A. N., Abolghasem, S. N., and Daesik, J. (2024). Revolutionizing agriculture with artificial intelligence: plant disease detection methods, applications, and their limitations. Front. Plant Sci. 15:1356260. doi: 10.3389/fpls.2024.1356260
Afzaal, H., Farooque, A. A., Schumann, A. W., Hussain, N., McKenzie-Gopsill, A., Esau, T., et al. (2021). Detection of a potato disease (early blight) using artificial intelligence. Remote Sens 13:411. doi: 10.3390/rs13030411
Ahmadi, A., De Toma, I., Vilor-Tejedor, N., Eftekhariyan Ghamsari, M. R., and Sadeghi, I. (2020). Transposable elements in brain health and disease. Ageing Res. Rev. 64:101153. doi: 10.1016/j.arr.2020.101153
Arshaghi, A., Ashourian, M., and Ghabeli, L. (2023). Potato diseases detection and classification using deep learning methods. Multimed. Tools Appl. 82, 5725–5742. doi: 10.1007/s11042-022-13390-1 [Google Scholar]
Bangari, S., Rachana, P., Gupta, N., Sudi, P. S., and Baniya, K. K. (2022). A Survey on Disease Detection of a potato Leaf Using CNN. Second International Conference on Artificial Intelligence and Smart Energy (ICAIS). Coimbatore, India: IEEE, 144–149.
Bhat, M. P., Chakraborty, B., Nagaraja, S. K., Gunagambhire, P. V., Kumar, R. S., Nayaka, S., et al. (2023). Aspergillus niger CJ6 extract with antimicrobial potential promotes in-vitro cytotoxicity and induced apoptosis against MIA PaCa-2 cell line. Environ. Res. 229:116008. doi: 10.1016/j.envres.2023.116008
Casey, C., and Sleator, R. D. (2024). Prions: structure, function, evolution, and disease. Arch. Microbiol. 207:1. doi: 10.1007/s00203-024-04200-3
Chen, X., Tan, Q., Lyu, Q., Yu, C., Jiang, N., Li, J., et al. (2022). Unmarked gene editing in Clavibacter michiganensis using CRISPR/Cas9 and 5-fluorocytosine counterselection. Mol. Plant-Microbe Interact. 35, 4–14. doi: 10.1094/MPMI-07-21-0179-TA
Cheng, L., Min, D., Liu, D. F., Zhu, T. T., Wang, K. L., and Yu, H. Q. (2020). Deteriorated biofilm-forming capacity and electroactivity of Shewanella oneidnsis MR-1 induced by insertion sequence (IS) elements. Biosens. Bioelectron. 156:112136. doi: 10.1016/j.bios.2020.112136
Citti, C., Dordet-Frisoni, E., Nouvel, L. X., Kuo, C. H., and Baranowski, E. (2018). Horizontal gene transfers in mycoplasmas (Mollicutes). Curr. Issues Mol. Biol. 29, 3–22. doi: 10.21775/cimb.029.003
Das, S., Mitra, B., Saha, A., Mandal, S., Paul, P. K., El-Sharnouby, M., et al. (2021). Evaluation of quality parameters of seven processing type potato (Solanum tuberosum L.) cultivars in the eastern sub-Himalayan Plains. Foods. 10:1138. doi: 10.3390/foods10051138
Dashti, Y., Tajabadi, F. M., Wu, L. J., Sumang, F. A., Escasinas, A., Ellis Allenby, N. E., et al. (2022). Discovery of Demurilactone a: a specific growth inhibitor of L-form Bacillus subtilis. ACS Infect. Dis. 8, 2253–2258. doi: 10.1021/acsinfecdis.2c00220
Deb, D., Khan, A., and Dey, N. (2020). Phoma diseases: epidemiology and control. Plant Pathol. 7, 1203–1217. doi: 10.1111/ppa.13221
Dissanayaka Mudiyanselage, S. D., Qu, J., Tian, N., Jiang, J., and Wang, Y. (2018). Potato spindle tuber viroid RNA-templated transcription: factors and regulation. Viruses 10:503. doi: 10.3390/v10090503
Dorokhov, A. S., Sibirev, A. V., and Aksenov, A. G. (2019). Dynamic systems modeling using artificial neural networks for agricultural machines. INMATEH Agric Eng. 58, 63–74. doi: 10.35633/INMATEH-58-07
Dutta, P., Lõhmus, A., Ahola, T., and Mäkinen, K. (2024). The replicase protein of potato virus X is able to recognize and trans-replicate its RNA component. Viruses 16:1611. doi: 10.3390/v16101611
Ferentinos, K. P. (2018). Deep learning models for plant disease detection and diagnosis. Comput. Electron. Agric. 145, 311–318 [Google Scholar] [CrossRef]. doi: 10.1016/j.compag.2018.01.009
Goodell, J. J., Powelson, M. L., and Allen, T. C. (1982). Interrelations between potato virus X, Verticillium dahliae, and Colletotrichum atramentarium in potato. Phytopathology 72, 631–634.
Ivanyuk, V. V., Shkirin, A. V., Belosludtsev, K. N., Gudkov, S. V., Dubinin, M. V., Kozlov, V. A., et al. (2020). Influence of fluoropolymer film modified with nanoscale photoluminophor on growth and development of plants. Front. Phys. 8, 1–6. doi: 10.3389/fphy.2020.616040
Johnson, D. A., and Dung, J. K. S. (2010). Verticillium wilt of potato–the pathogen, disease and management. Can. J. Plant Pathol. 1, 58–67. doi: 10.1080/07060661003621134
Karasev, A. V., and Gray, S. M. (2013). Continuous and emerging challenges of potato virus Y in potato. Annu. Rev. Phytopathol. 51, 571–586. doi: 10.1146/annurev-phyto-082712-102332
Kerkvliet, J. J., Bossers, A., Kers, J. G., Meneses, R., Willems, R., and Schürch, A. C. (2024). Metagenomic assembly is the main bottleneck in the identification of mobile genetic elements. PeerJ 12:e16695. doi: 10.7717/peerj.16695
Konchekov, E. M., Gusein-Zade, N., Burmistrov, D. E., Kolik, L. V., Dorokhov, A. S., Izmailov, A. Y., et al. (2023). Advancements in plasma agriculture: a review of recent studies. Int. J. Mol. Sci. 24:15093. doi: 10.3390/ijms242015093
Ksenofontov, A. L., Dobrov, E. N., Fedorova, N. V., Arutyunyan, A. M., Golanikov, A. E., Järvekülg, L., et al. (2018). Structure of potato virus a coat protein particles and their dissociation. Mol. Biol. (Mosk) 52, 1055–1065. doi: 10.1134/S0026898418060101
Kumar, S., Chaudhary, V., and Chandra, S. K. (2021). Plant disease detection using CNN. Turk. J. Comput. Math. Educ. 12, 2106–2112. doi: 10.13140/RG.2.2.36485.99048
Kumar, R., Kaundal, P., Tiwari, R. K., Lal, M. K., Kumari, H., Kumar, R., et al. (2023). Development of reverse transcription recombinase polymerase amplification (RT-RPA): a methodology for quick diagnosis of potato leafroll viral disease in potato. Int. J. Mol. Sci. 24:2511. doi: 10.3390/ijms24032511
Leuthner, T. C., and Meyer, J. N. (2021). Mitochondrial DNA mutagenesis: feature of and biomarker for environmental exposures and aging. Curr. Environ. Health Rep. 8, 294–308. doi: 10.1007/s40572-021-00329-1
Lico, C., Mancini, C., Italiani, P., Betti, C., Boraschi, D., Benvenuto, E., et al. (2009). Plant-produced potato virus X chimeric particles displaying an influenza virus-derived peptide activate specific CD8+ T cells in mice. Vaccine 27, 5069–5076. doi: 10.1016/j.vaccine.2009.06.045
Lyashchuk, Y. O., Ovchinnikov, A. Y., Ivanishchev, K. A., and Shchur, A. V. (2022). Assessment of the resistance of alimentary-related risk factors to the effects of chemical disinfectants. Agrar. Vestn. Urala. 12, 54–61. doi: 10.32417/1997-4868-2022-227-12-54-61
Lyashchuk, Y. O., Teterin, V. S., Panferov, N. S., Pekhnov, S. A., and Ovchinnikov, A. Y. (2023). Rating analysis of the sensitivity of biorisk factors to the effects of chemical disinfectants in the context of food safety. Agrar. Sci. J. 7, 69–76. doi: 10.28983/asj.y2023i7pp69-76
Mohanty, S. P., Hughes, D. P., and Salathe, M. (2016). Using deep learning for image-based plant disease detection. Front. Plant Sci. 7:1419. doi: 10.3389/fpls.2016.01419
Naciri, M., Mancassola, R., Fort, G., Danneels, B., and Verhaeghe, J. (2011). Efficacy of amine-based disinfectant KENO™COX on the infectivity of Cryptosporidium parvum oocysts. Vet. Parasitol. 179, 43–49. doi: 10.1016/j.vetpar.2011.01.066
Núñez-Muñoz, L. A., Calderón-Pérez, B., Ruiz-Medrano, R., Xoconostle-Cázares, B., and de la Torre-Almaraz, R. (2025). Viral tropism in plants, reproductive tissues, and seeds. Arch. Microbiol. 207:152. doi: 10.1007/s00203-025-04353-9
Onozuka, N., Ohki, T., Oka, N., and Maoka, T. (2021). One-step real-time multiplex reverse transcription-polymerase chain reaction assay with melt curve analysis for detection of potato leafroll virus, potato virus S, potato virus X, and potato virus Y. Virol. J. 18:131. doi: 10.1186/s12985-021-01591-3
Pan, S., Wang, L., Zhang, R., Wei, P., Yang, Y., Peng, D., et al. (2022). First report of Meloidogyne hapla infecting Salvia miltiorrhiza in Shaanxi, China. Plant Dis. 107:585. doi: 10.1094/PDIS-01-22-0088-PDN
Parums, D. V. (2024). Editorial: climate change and the spread of vector-borne diseases, including dengue, malaria, Lyme disease, and West Nile virus infection. Med. Sci. Monit. 29:e943546. doi: 10.12659/MSM.943546
Patel, R., Mitra, B., Vinchurkar, M., Adami, A., Patkar, R., Giacomozzi, F., et al. (2023). Plant pathogenicity and associated/related detection systems. a review. Talanta 251:123808. doi: 10.1016/j.talanta.2022.123808
Przetakiewicz, J. (2015). The viability of winter sporangia of Synchytrium endobioticum (Schilb.) Perc. From Poland. Am. J. Potato Res. 92, 704–708. doi: 10.1007/s12230-015-9480-6
Rahman, M., Hasan, M. S., Islam, R., Rana, R., Sayem, A., Sad, M. A. A., et al. (2022). Plasma-activated water for food safety and quality: a review of recent developments. Int. J. Environ. Res. Public Health 19:6630. doi: 10.3390/ijerph19116630
Rathor, A. S., Choudhury, S., Sharma, A., Nautiyal, P., and Shah, G. (2024). Empowering vertical farming through IoT and AI-driven technologies: a comprehensive review. Heliyon 10:e34998. doi: 10.1016/j.heliyon.2024.e34998
Rivera-Zuluaga, K., Hiles, R., Barua, P., Caldwell, D., and Iyer-Pascuzzi, A. S. (2023). Getting to the root of Ralstonia invasion. Semin. Cell Dev. Biol. 148, 3–12. doi: 10.1016/j.semcdb.2022.12.002
Schmey, T., Tominello-Ramirez, C. S., Brune, C., and Stam, R. (2024). Alternaria diseases on potato and tomato. Mol. Plant Pathol. 25:e13435. doi: 10.1111/mpp.13435
Shintani, M., Vestergaard, G., Milaković, M., Kublik, S., Smalla, K., Schloter, M., et al. (2023). Integrons, transposons and IS elements promote diversification of multidrug resistance plasmids and adaptation of their hosts to antibiotic pollutants from pharmaceutical companies. Environ. Microbiol. 25, 3035–3051. doi: 10.1111/1462-2920.16481
Supattapone, S. (2020). Cofactor molecules: essential partners for infectious prions. Prog. Mol. Biol. Transl. Sci. 175, 53–75. doi: 10.1016/bs.pmbts.2020.07.009
Visser, N., Herreman, L. C. M., Vandooren, J., Pereira, R. V. S., Opdenakker, G., Spelbrink, R. E. J., et al. (2024). Novel high-yield potato protease inhibitor panels block a wide array of proteases involved in viral infection and crucial tissue damage. J. Mol. Med. 102, 521–536. doi: 10.1007/s00109-024-02423-x
Wainer, J., and Dinh, Q. (2021). Taxonomy, morphological and molecular identification of the potato cyst nematodes, Globodera pallida and G. Rostochiensis. Plants 10:184. doi: 10.3390/plants10010184
Waleron, M., Misztak, A., Waleron, M., Jonca, J., Furmaniak, M., and Waleron, K. (2019). Pectobacterium polonicum sp. nov. isolated from vegetable fields. Int. J. Syst. Evol. Microbiol. 69, 1751–1759. doi: 10.1099/ijsem.0.003387
Wang, X., Mandadi, K., Peña, L., Cao, M., and Zhou, C. (2022). Editorial: evolutionary genomics of Candidatus Liberibacter spp. and their interactions with plant and insect-vector hosts. Front. Microbiol. 13:1025795. doi: 10.3389/fmicb.2022.1025795
Woudenberg, J. H. C., Woudenberg, J. H., Groenewald, J. Z., Binder, M., and Crous, P. W. (2013). Alternaria redefined. Stud. Mycol. 75, 171–212. doi: 10.3114/sim0015
Woudenberg, J. H. C., Seidl, M. F., Groenewald, J. Z., de Vries, M., Stielow, J. B., Thomma, B. P., et al. (2015). Alternaria section Alternaria: species, formae speciales or pathotypes? Stud. Mycol. 82, 1–21. doi: 10.1016/j.simyco.2015.07.001
Xu, Y., Zhang, B., Wang, L., Jing, T., Chen, J., Xu, X., et al. (2020). Unusual features and molecular pathways of Staphylococcus aureus L-form bacteria. Microb. Pathog. 140:103970. doi: 10.1016/j.micpath.2020.103970
Yanagisawa, H., Matsushita, Y., Khiutti, A., Mironenko, N., Ohto, Y., and Afanasenko, O. (2021). Occurrence and distribution of viruses infecting potato in Russia. Lett. Appl. Microbiol. 73, 64–72. doi: 10.1111/lam.13476
Yi, L., Xu, X., Ge, W., Xue, H., Li, J., Li, D., et al. (2019). The impact of climate variability on infectious disease contagion in China: current knowledge and further directions. Environ. Res. 173, 255–261. doi: 10.1016/j.envres.2019.03.043
Yu, Q., Zaida, M. A., Hughes, B., and Celetti, M. (2012). Discovery of potato rot nematode, Ditylenchus destructor, infesting garlic in Ontario, Canada. Plant Dis. 96:297. doi: 10.1094/PDIS-08-11-0697
Yudina, L., Sukhova, E., Mudrilov, M., Nerush, V., Pecherina, A., Vodeneev, V., et al. (2022). Ratio of intensities of blue and red light at cultivation influences photosynthetic light reactions, respiration, growth, and reflectance indices in lettuce. Biology 11:60. doi: 10.3390/biology11010060
Zedler, M., Tse, S. W., Ruiz-Gonzalez, A., and Haseloff, J. (2023). Paper-based multiplex sensors for the optical detection of plant stress. Micromachines (Basel) 14:314. doi: 10.3390/mi14020314
Zhanel, G. G., Golden, A. R., Zelenitsky, S., Wiebe, K., Lawrence, C. K., Adam, H. J., et al. (2019). Cefiderocol: a Siderophore cephalosporin with activity against Carbapenem-resistant and multidrug-resistant gram-negative Bacilli. Drugs 79, 271–289. doi: 10.1007/s40265-019-1055-2
Zhang, L., Tian, X., Sun, L., Mi, K., Wang, R., Gong, F., et al. (2024). Bacterial efflux pump inhibitors reduce antibiotic resistance. Pharmaceutics 16:170. doi: 10.3390/pharmaceutics16020170
Zhou, J., Jia, Q., Liu, L., Liang, L., Zhang, H., He, C., et al. (2025). Epidemiology and clinical outcomes in skin and soft tissue nontuberculous mycobacteria infections: a retrospective study. J. Infect. Public Health 18:102655. doi: 10.1016/j.jiph.2025.102655
Keywords: potato, infectious and parasitic diseases, methodology, assessment, risk factors, spread, prevention
Citation: Dorokhov A, Sibirev A, Lyashchuk Y, Teterin V and Panferov N (2025) Methodology for assessing the level of biological risk of emergence and spread of infectious and parasitic diseases of potato by parameters of mechanisms and pathways of pathogen transmission. Front. Sustain. Food Syst. 9:1614949. doi: 10.3389/fsufs.2025.1614949
Edited by:
Mohamed Trigui, Institut Préparatoire aux Etudes d'Ingénieur de Sfax (IPEIS), TunisiaReviewed by:
Yasmine Abdallah, Minia University, EgyptLaura Meno Fariñas, University of Vigo, Spain
Copyright © 2025 Dorokhov, Sibirev, Lyashchuk, Teterin and Panferov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexey Sibirev, c2liaXJldjIwMTFAeWFuZGV4LnJ1
 Alexey Dorokhov
Alexey Dorokhov Alexey Sibirev
Alexey Sibirev Yulia Lyashchuk
Yulia Lyashchuk Vladimir Teterin
Vladimir Teterin Nikolay Panferov
Nikolay Panferov