- 1Japan International Research Center for Agricultural Sciences (JIRCAS), Tsukuba, Ibaraki, Japan
- 2Rice and Cash Crops Research Center (RCCRC), National Agriculture and Forestry Research Institute (NAFRI), Vientiane, Laos
Black rice is an essential agricultural commodity in the upland ecosystems of northern Laos. Considering the low yield potential of black rice and the low-fertility soils in the region, breeding improved black rice with high yield potential and phytic acid (PA) content in grains as a phosphorus source for vigorous seedling growth is necessary. This study included genetic analyses conducted in Laos and Japan using an F2 population derived from a cross between Kampeng, a black rice variety with a high PA content, and Non, a white rice variety with more panicles, even in low-fertility soils. A major quantitative trait locus (QTL) for grain color was detected in Kala4, which is involved in the expression of the black color in the rice variety. Two QTLs were detected for PA content. One of these, a novel QTL named qPA1, was detected at the end of the short arm of chromosome 1 in both Japan and Laos. The effect of the Kampeng allele on increasing the PA content was demonstrated in the F3 lines. An additional QTL identified exclusively in Japan was suggested to be due to an environmental response via Hd6. PA accumulation in late-heading F2 individuals by Hd6 may have been suppressed due to low temperatures. In addition, no QTLs were detected for the Non allele that increased the panicle number, although Non exhibited more panicles than Kampeng. These results indicate that incorporating the Kampeng allele of Kala4 and qPA1 into the Non genetic background may serve as an effective breeding strategy. This approach could enhance the development of black rice with high yield potential and PA content, thereby contributing to increased black rice production in the upland ecosystems of northern Laos.
Background
Rice is a staple food in Laos, a country covering an area of 236,800 km2 of mountainous terrain. The rice-planted area in the country is approximately 873,000 ha, which is only 3.7% of the national land area owing to the mountainous terrain (Lao Statistics Bureau, 2019). Although 77% of the rice is grown in lowland ecosystems located in the Mekong River Valley in the central and southern parts of the country, 9% is grown in upland ecosystems in the mountainous region of northern Laos (Basnayake et al., 2006; Lao Statistics Bureau, 2019). Laos achieved 100% rice self-sufficiency in 1999 through the development of agricultural infrastructure, such as irrigation systems, reservoirs, and water pump stations (Schiller et al., 2006); however, the current yield of upland rice remains at 2.0 t ha−1, which is only half that of lowland rice (Lao Statistics Bureau, 2019). The low productivity of upland rice in mountainous regions has been attributed to its low yield potential and poor soil fertility (Saito et al., 2006; Asai et al., 2009). Due to the challenges in implementing modern agricultural practices to enhance rice production in mountainous areas, the government established a comprehensive Agricultural Development Strategy (ADS) to produce comparative and competitive potential agricultural commodities, such as black rice, in the region (Ministry of Agriculture and Forestry, 2015) since black rice is traded at a higher price than white rice (Hasada and Phonhnachit, 2023).
Black rice, characterized by a black pericarp, is traditionally cultivated in the upland ecosystems of northern Laos. Although black rice has low yield potential, ranging from 1.4–3 t ha−1, it provides several nutritional advantages and health benefits compared to white rice, such as higher levels of protein, vitamins, minerals, and antioxidants (Appa Rao et al., 2006; Sompong et al., 2011). The characteristics of black rice can contribute to its market value. Myo-inositol hexaphosphate, also known as phytic acid (PA), is a natural antioxidant that constitutes 1–5% by weight in the majority of cereals, nuts, legumes, and oil seeds (Graf and Eaton, 1990). Importantly, PA is the principal form of phosphorus (P) in cereals, accounting for 60–90% of the total P in mature seeds (Lolas et al., 1976). Although in vitro and in vivo studies have demonstrated its beneficial effects in the prevention of diseases, including cancer (Silva and Bracarense, 2016), PA also functions as an antinutritional component by acting as a strong chelator of metal cations and reducing the bioavailability of micronutrients (Perera et al., 2018). Therefore, breeding rice varieties with low-PA content effectively enhances micronutrient bioavailability (Liu et al., 2007; Tp et al., 2022). However, since PA in grains is a substantial source of P necessary for the initial growth of seedlings, rice varieties with low PA frequently have low germination rates and reduced seedling vigor, resulting in low yields, particularly in P-deficient soil (Zhao et al., 2008; Oo et al., 2023a). A reduced germination rate has been demonstrated in the low PA mutant lines (Qamar et al., 2019; Qamar et al., 2024). This is a critical issue for rice cultivation in the upland ecosystem of northern Laos because the low-P concentration soils do not provide sufficient P to rice plants (McDowell et al., 2024). Black rice, because of its low yield potential, will further reduce the actual yield for lower PA content in seeds. Therefore, developing improved black rice varieties with high PA and yield potential is necessary in the upland ecosystem of northern Laos.
A recent study identified a black rice germplasm, Kampeng, from Houaphanh province in northern Laos (Asai et al., 2020). Kampeng has been shown to accumulate high levels of rutin (quercetin-3-O-rutinoside), a health-promoting flavonoid, highlighting its potential as a functional food and expecting more accumulation of other compounds such as PA (Asai et al., 2020). Another study observed a stable and high-yield performance for a white rice germplasm, Non, under low-fertility soil conditions in the upland ecosystem of northern Laos (Asai and Soisouvanh, 2017). Non achieves high performance by producing many panicles, although shoot branching in rice is often restricted under low-fertility soil conditions (Takai et al., 2021). These results indicate that combining the superior traits of the two germplasms could lead to the development of an improved black rice variety suitable for northern Laos. However, this breeding method has not yet been implemented in northern Laos.
This study first compared PA content in mature grains and panicle number between Kampeng and Non. Then, we conducted quantitative trait locus (QTL) analysis for the two traits and grain color using genetic materials derived from a cross between the two germplasms. These experiments were conducted in northern Laos and Japan to analyze the interactions between QTL and the environment. Subsequently, we attempted to validate the effects of the detected QTLs on PA content using advanced genetic materials. The effect of air temperature on PA content during grain filling was also investigated. Finally, based on the results, we discuss future breeding strategies for developing an improved black rice variety with high PA content and enhanced yield potential for use in upland ecosystems in northern Laos.
Materials and methods
Plant materials
Two upland rice germplasm lines from northern Laos, Kampeng and Non, were used in this study. Kampeng is a tropical japonica black rice domestically utilized as traditional medicine in rural villages of Houaphanh province. This landrace has recently been identified as a high rutin-accumulating variety with excellent grain appearance, particularly its strong black pigmentation, which is an important trait for black rice quality (Figures 1A,B) (Asai et al., 2020). In line with the national strategy for promoting value-added rice production, Kampeng has attracted increasing attention as a health-promoting rice; however, its yield performance is generally low (Asai et al., 2020). Non is a traditional indica white rice with strong shoot-branching characteristics (Figures 1A,B) (Asai and Soisouvanh, 2017). Due to its high yield potential in low-input upland systems, Non has been officially selected for seed distribution in northern Laos. Kampeng was crossed with Non, and the F1 seeds were harvested. After growing the F1 plants, self-pollinated F2 seeds were harvested for QTL analysis. Based on the results of this analysis, four genotypes were selected from the F2 individuals: (i) homozygous for Kampeng for detected QTLs qPA1 and Hd6; (ii) homozygous for Kampeng for qPA1 and Non for Hd6; (iii) homozygous for Non for qPA1 and Kampeng for Hd6; and (iv) homozygous for Non for qPA1 and Hd6. F3 lines derived from the four genotypes were used to validate the effects of the QTLs.
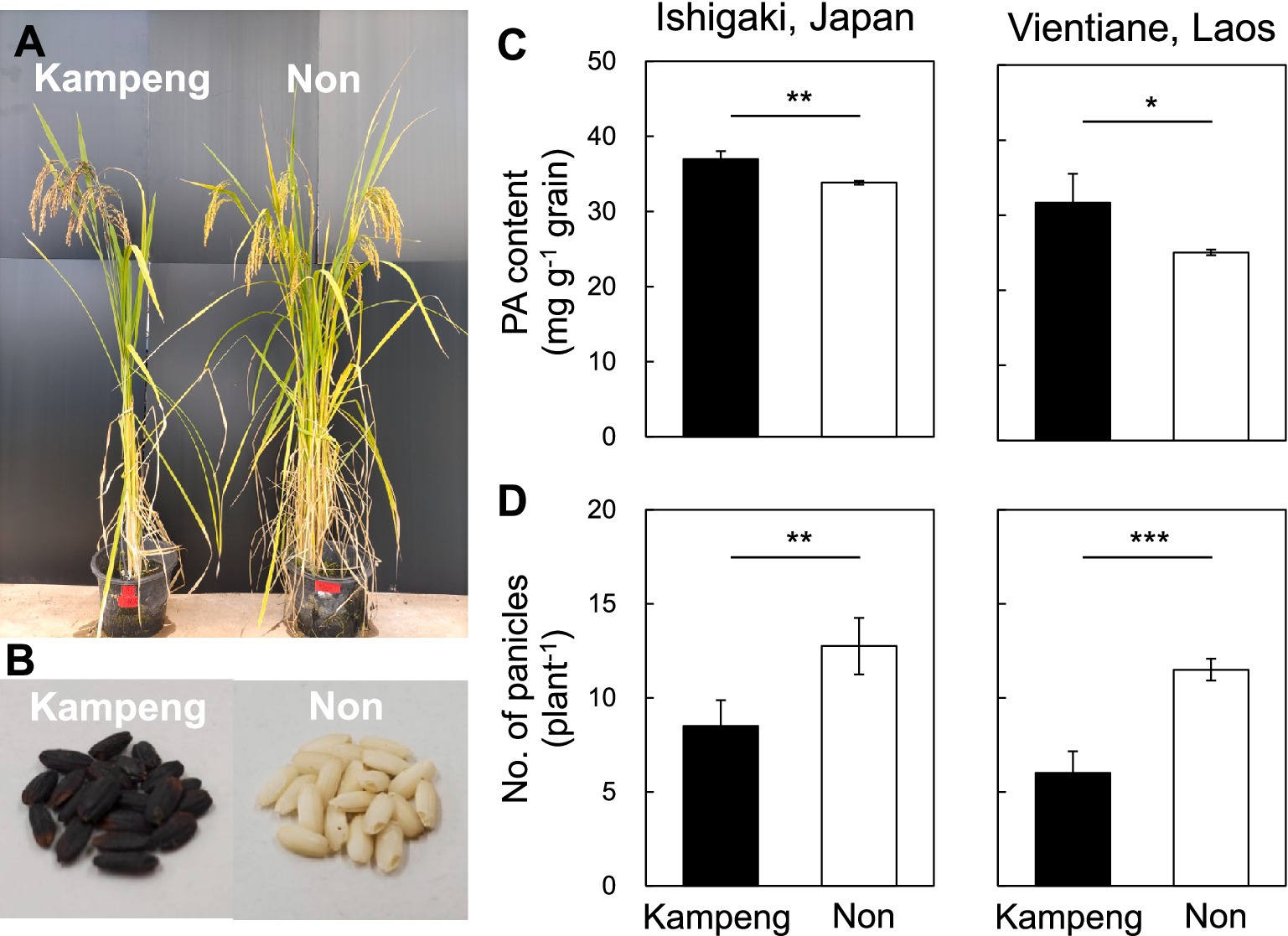
Figure 1. Phenotypic differences between Kampeng and Non. (A,B) Kampeng and Non plant types and dehulled grains (brown rice). (C,D) Comparisons of phytic acid (PA) content and panicle number between Kampeng and Non. *, **, and *** represent significant differences at p < 0.05, 0.01, and 0.001, respectively, with Student’s t-test.
Phenotyping
Phenotyping was conducted at two locations with different climate conditions: Ishigaki, Japan, and Vientiane, Lao PDR. In 2021, more than 200 F2 individuals and their parents were cultivated in a paddy field at the Japan International Research Center for Agricultural Sciences (JIRCAS), Ishigaki, Japan (24° 22′45.92” N, 124° 11′49.12″E, 30 m altitude). The trial was conducted under irrigated lowland conditions to ensure the growth potential in the environmental conditions of Japan. On 13 July 2021, the seeds were sown in seedling nursery boxes, and on 11 August 2021, the seedlings were transplanted into the experimental paddy field, one seedling per hill. The planting density was 18.5 hills m−2, with 18 cm between each hill and 30 cm between the rows. Each row had 10 hills. At transplanting, nitrogen (N), phosphorus (P), and potassium (K) were applied as basal fertilizers at rates of 64 kg N ha−1, 17.5 kg P ha−1, and 33.2 kg K ha−1, respectively. Thirty days after transplanting, 20 kg N ha−1 was applied as a top dressing. A total of 158 F2 individuals, excluding border plants, were used for phenotyping.
In 2022, 124 F2 individuals and their parents were grown in pots at an experimental pool in the Rice and Cash Crop Research Center (RCCRC), Vientiane, Laos (18° 08′51.53” N, 102° 44′10.18″E, 170 m altitude). Pots were used to ensure precise data collection for genetic analysis under homogeneously simulated upland conditions. Soil (4 kg) was mixed with 1 kg of farmyard manure, and each pot (25 cm in diameter) was filled with the mixed soil. The pots had small holes at the bottom to allow water uptake, and all pots were placed in the experimental pool. On 15 June 2022, seeds were sown in pots (one per pot). The water level in the pool was maintained at 2–3 cm to simulate the upland conditions in the pots.
In 2023, the four F3 genotypes selected from the QTL analysis of the F2 populations were grown in a paddy field at JIRCAS, Ishigaki, Japan. On 11 July 2023, the seeds were sown in seedling nursery boxes, and on 8 August 2023, the seedlings were transplanted into an experimental paddy field, one seedling per hill. The planting density and fertilizer application were similar to those used in 2021.
In all experiments, the heading date was recorded when the first panicle emerged on each plant. The number of days to heading was calculated as the number of days from the sowing date to the heading date. The number of panicles was counted at maturity, and the panicles were harvested and threshed. The grains were then dehulled, and the dehulled grains (brown rice) were used for grain color and phytic acid analysis. Grain color was assessed using the Grain Scanner instrument (Satake Engineering Co., Ltd., Tokyo, Japan). Brown rice was scanned, and the lightness parameter L* was calculated, with 100 representing white and 0 representing black, according to the Lab color system of Commission Internationale d’Eclairage (1976). PA analysis was conducted following the method reported by Matsuo et al. (2005), with some modifications. Brown rice (3 g) was placed in the grinding container and chilled in a freezer at −80°C before being ground using the automated TK-AM5 (Tokken Inc., Chiba, Japan). The ground powder samples were stored at −20°C until analysis. The powdered sample (0.3 g) was mixed with 1.2 mL of 0.8 N HCl in a 5-mL microcentrifuge tube and vortexed at 2,400 rpm for 1 h at 4°C using a Multi-Tube Vortexer BSR-MTV100 (Bio Medical Science, Tokyo, Japan), followed by centrifugation at 18,000 × g for 15 min at 4°C. The clear supernatant (15 μL) was mixed with 285 μL of sterilized reverse osmosis water in a 1.5 mL microcentrifuge tube and heated at 100°C for 20 min, followed by centrifugation at 18,000 × g for 15 min at 4°C. The clear supernatant was filtered through a 0.22 μm membrane filter and subjected to high-performance liquid chromatography (HPLC) using a binary HPLC system equipped with an anion-exchange column, TSKgel DEAE-5PW (7.5 mm φ × 75 mm, Tosoh Corporation, Tokyo, Japan). Phytic acid was eluted from an injected sample (100 μL) using a linear gradient of 0.01 M HNO3 to 0.4 M NaNO3 and 0.01 M HNO3 over 40 min at a flow rate of 1.0 mL min−1. Eluted phytic acid reacted with Wade reagent (0.06% FeCl3∙6H2O, 0.3% sulfosalicylic acid) in a post-column tube at a flow rate of 0.5 mLmin−1, and the decrease in absorbance at 500 nm due to phytic acid was measured. The phytic acid concentration in an injected sample was calculated using standard solutions at concentrations of 0.1, 0.2, 0.3, and 0.5 mg mL−1.
Daily air temperatures were recorded at JIRCAS, Ishigaki, Japan, and RCCRC, Vientiane, Laos.
Genotyping
The total DNA of each F2 individual was extracted from the leaves using a conventional method with phenol-chloroform-isoamyl alcohol (Nippon Gene, Tokyo, Japan) (Gautam, 2022) and used for single-nucleotide polymorphism (SNP) analysis. 1K-Rice Custom Amplicon panel (1k-RiCA) (Arbelaez et al., 2019) was used to genotype F2 individuals. The majority of the 1,024 SNP markers showed homozygosity with Kampeng and Non. Among the 1,024 SNP markers, 365 in the whole genome showed polymorphisms between Kampeng and Non and were used for QTL analysis.
Statistical analysis
Construction of the linkage map for the F2 population and subsequent QTL analysis were conducted using R/qtl software (Broman et al., 2003). The chromosomal positions and effects of the putative QTLs were determined using composite interval mapping. The threshold for QTL detection was based on 1,000 permutation tests at a 5% significance level. The additive and dominant effects and the phenotypic variance (R2) explained by each QTL were estimated using the peak LOD score.
A Student’s t-test was conducted using SPSS software (version 23.0; IBM, Chicago, IL, United States) to compare the PA content and panicle number between Kampeng and Non. To compare PA content and days to heading among the four F3 genotypes, a two-way analysis of variance (ANOVA) was performed to assess the effect of the two QTLs, qPA1 and Hd6, and their interaction. qPA, Hd6, and their interaction were considered as fixed effects, while replication was considered a random effect. The significance of the fixed effects was assessed using Tukey’s honest significant difference test at the 5% level. Normality and homogeneity of the variance were confirmed using the Shapiro–Wilk test and Levene’s test, respectively, before conducting the Student t-test and two-way ANOVA. Correlations between PA content, days to heading, panicle number, average daily mean temperature during grain filling, and PA content in F2 individuals were also assessed using SPSS 23.0.
Results
Comparison between Kampeng and Non varieties
In both experiments in Japan and Laos, Kampeng (37.0 and 31.7 mg g−1 grain, respectively) showed a significantly higher PA content than Non (33.8 and 25.0 mg g−1 grain, respectively) (Figure 1C), whereas Non (12.8 and 11.5 plant−1, respectively) produced significantly more panicles than Kampeng (8.5 and 6.0 plant−1, respectively) (Figure 1D). The absolute values of the PA content and panicle number were lower in Laos than those in Japan.
Phenotypic variation, correlation, and QTL analysis
Continuous variations were observed in days to heading, grain color, PA content, and panicle number among F2 individuals in both experiments (Figure 2). The days to heading were transgressively segregated, ranging from 67 to 100 days (85.5 days in Kampeng and 75.3 days in Non) in Japan and from 61 to 117 days (84.8 days in Kampeng and 88 days in Non) in Laos (Figure 2A). The values for grain color, represented as L*, ranged from 23.0–77.0 (25.9 in Kampeng and 74.3 in Non) in Japan and from 34.7 to 77.6 (41.8 in Kampeng and 74.0 in Non) in Laos (Figure 2B). PA content also showed transgressive segregation, ranging from 25.3–54.5 mg g−1 grain in Japan and from 23.9–54.4 mg g−1 grain in Laos (Figure 2C). The panicle numbers ranged from 4 to 27 plant−1 in Japan and from 7 to 19 plant−1 in Laos (Figure 2D).
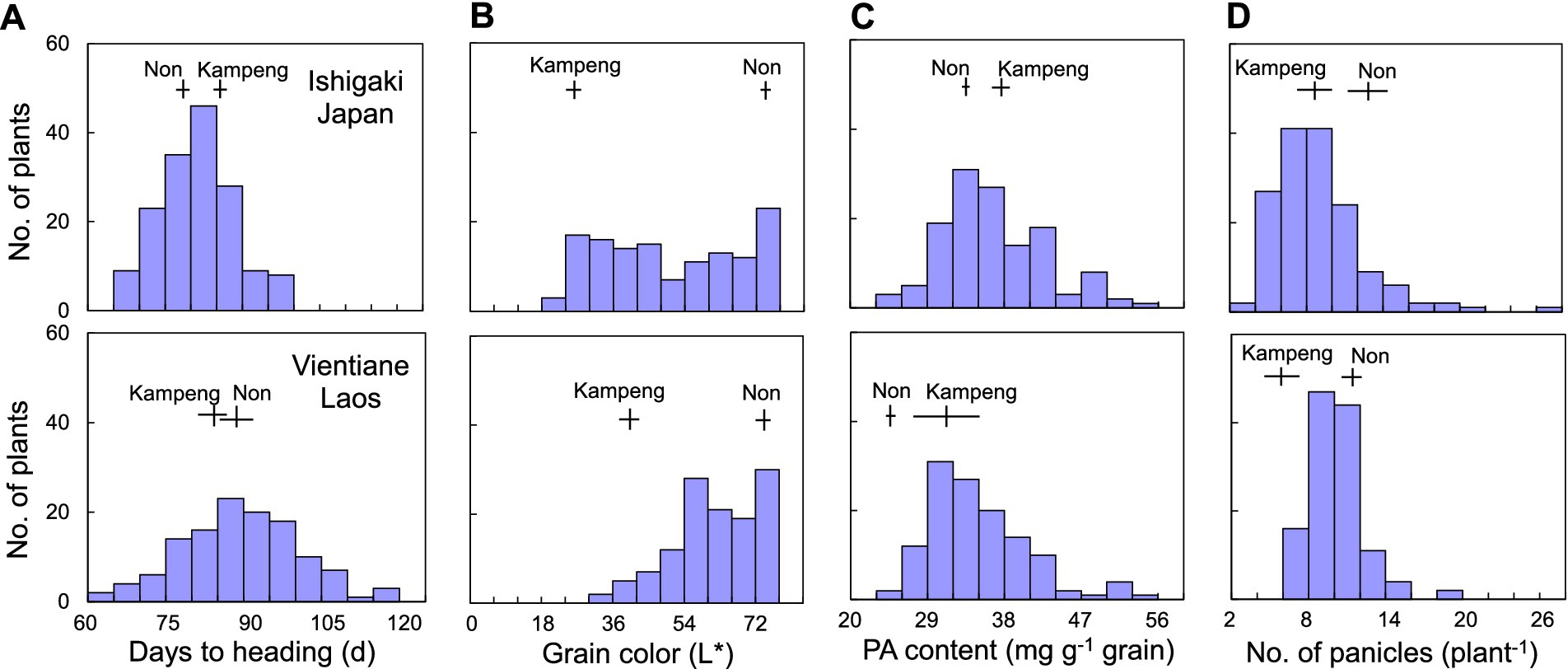
Figure 2. Frequency distribution of (A) days to heading, (B) grain color, (C) PA content, and (D) panicle number in 158 and 124 F2 individuals derived from a cross between Kampeng and Non grown in Ishigaki, Japan and Vientiane, Laos, respectively. Vertical lines denote mean parental values, and horizontal lines denote standard deviation.
Since days to heading can affect agronomic traits, the relationship between days to heading and grain color, PA content, and panicle number was investigated. Days to heading were negatively correlated with grain color only in Laos (Figure 3A). Days to heading were also negatively correlated with the PA content in both Japan and Laos (Figure 3B). The absolute value of the correlation coefficient was higher in Japan (0.47) compared to Laos (0.22). No correlation was observed between days to heading and the panicle number (Figure 3C).
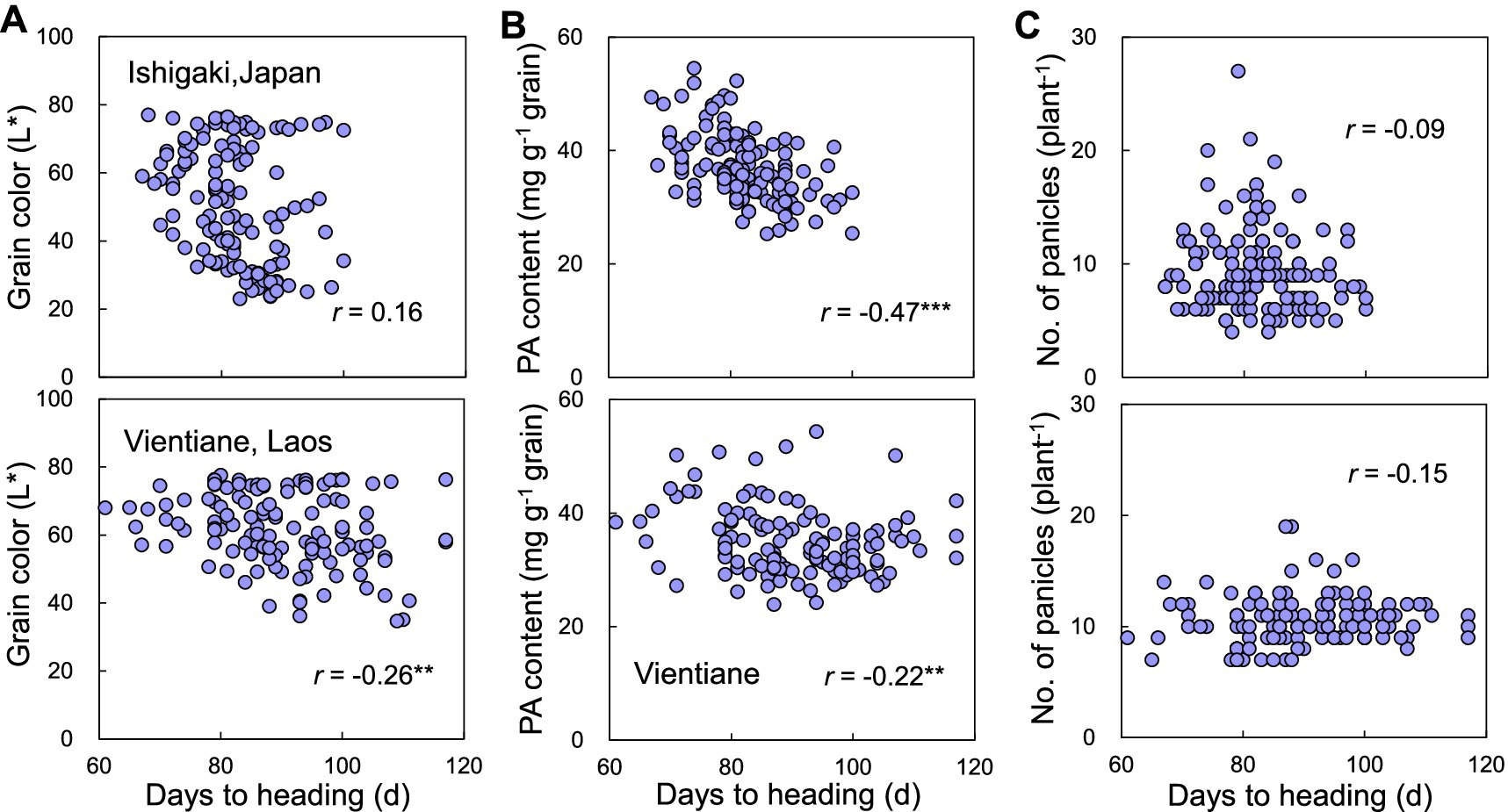
Figure 3. Relationships between days to heading and (A) grain color, (B) PA content, and (C) panicle number in F2 individuals grown in Ishigaki, Japan and Vientiane, Laos. r represents the correlation coefficient. ** and *** represent significant differences at p < 0.01 and 0.001, respectively.
The QTL analysis of F2 individuals detected three QTLs for days to heading in both Japan and Laos, among which two were commonly detected on the short and long arms of chromosome 3 (Figure 4A). The QTL on the long arm of chromosome 3 had a considerable effect, explaining 48.2–48.8% of the total variance in this trait (Table 1). The additive effect of the Kampeng allele was 7.3–12.0 days, indicating that the Kampeng allele increased the days to heading. Based on its location, the QTL was suggested to be Hd6 (Takahashi et al., 2001). One and three QTLs were detected for grain color in Japan and Laos, respectively. In both regions, one QTL was detected on the long arm of chromosome 4 (Figure 4B). The QTL showed a strong effect, explaining 48.3–36.4% of the total variance in this trait (Table 1). Based on its location and decreased additive effect with the Kampeng allele (blackening brown rice), the QTL was suggested to be Kala4, a gene from black rice that regulates anthocyanin pigmentation (Oikawa et al., 2015). Two and one QTLs were detected for PA content in Japan and Laos, respectively, in which one QTL was commonly detected at the end of the short arm of chromosome 1 (Figure 4C). The QTL explained 11.1–15.6% of the total variance in the trait. The additive effect of the Kampeng allele was 2.6 to 3.0 mg g−1 grain, showing that the Kampeng allele increased the PA content (Table 1). Another QTL detected only in Japan was located near Hd6, and the Kampeng allele decreased the PA content. Two and one QTLs were detected for panicle numbers in Japan and Laos, respectively (Figure 4D). Each of these QTLs was detected in different chromosomal regions. Although Non produced more panicles than Kampeng in both experiments, the Kampeng allele of the three QTLs increased the panicle number (Table 1).
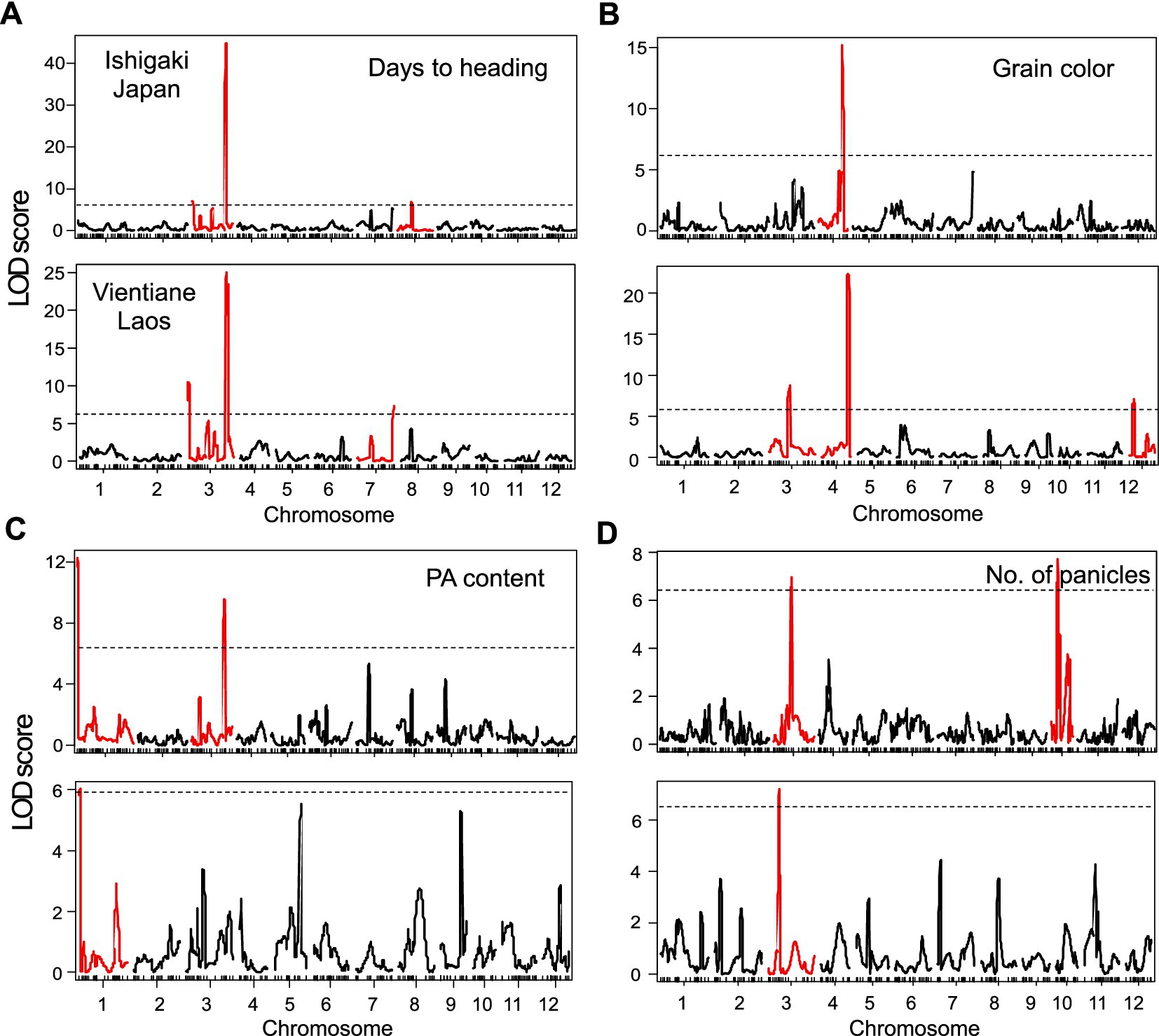
Figure 4. LOD scores associated with (A) days to heading, (B) grain color, (C) PA content, and (D) panicle number computed by composite interval mapping in the F2 population grown in Ishigaki, Japan and Vientiane, Laos. The dotted lines represent thresholds of QTL detection. LOD scores in red represent QTLs above the thresholds.
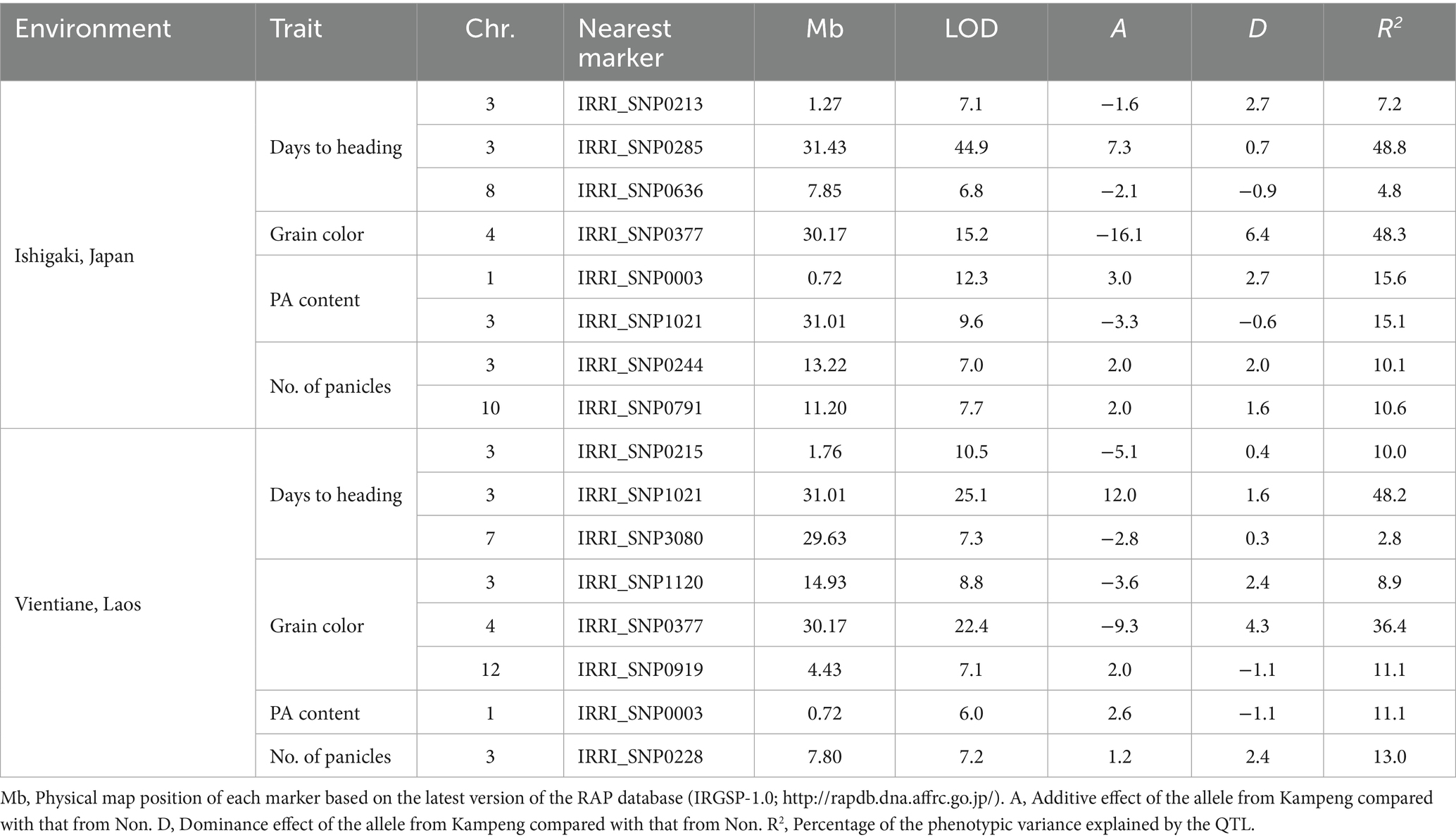
Table 1. Putative QTLs for days to heading, grain color, phytic acid (PA) content in grains, and panicle number detected in the F2 population between Kampeng and Non grown in Ishigaki, Japan and Vientiane, Laos.
Validation of QTL effects
We named the QTL for PA content located at the end of the short arm of chromosome 1 qPA1 and attempted to validate the effects of qPA1 and Hd6 on days to heading and PA content using four different F3 genotypes in Japan. Days to heading were significantly influenced by Hd6, whereas the effects of qPA1 and its interaction were not significant (Figure 5A). The mean number of days to heading in Kampeng homozygous for Hd6 was 15.2 days later than that of Non homozygous. The PA content was affected by both qPA1 and Hd6, but the effect of the interaction was not significant (Figure 5B), suggesting that qPA1 contributed to the enhancement of PA content, irrespective of Hd6. The mean PA content in genotypes of Kampeng homozygous for qPA1 was 7.1 mg g−1 grain higher than that of Non homozygous. In contrast, the mean PA content in genotypes of Kampeng homozygous for Hd6 was 3.4 mg g−1 grain lower than that of Non homozygous.
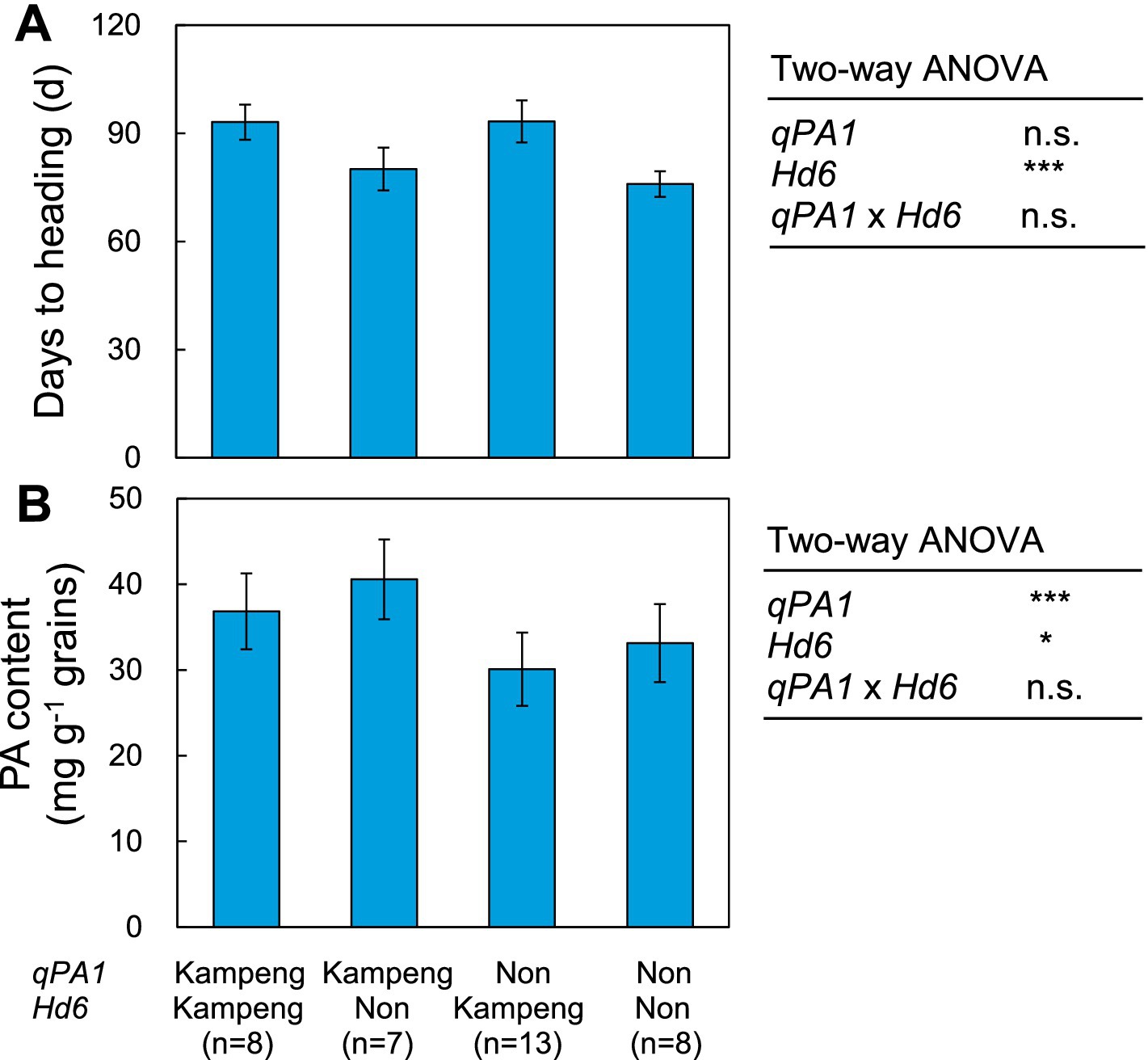
Figure 5. Comparisons of (A) days to heading and (B) PA contents among the four different F3 genotypes for qPA1 and Hd6. Vertical bars show standard variation. n.s. represents no significance. *, **, and *** represent significance at p < 0.05, 0.01, and 0.001.
Effect of air temperature during grain filling on PA content
Finally, the effect of air temperature on the PA content during grain filling was investigated because the PA content in grains is typically determined 30 days after heading (Perera et al., 2019). The daily mean temperature during grain filling among F2 individuals decreased by 10.5°C from 28.6 to 18.1°C in Japan and 7.3°C from 30.2 to 22.9°C in Laos (Figure 6A). Therefore, we calculated the average daily mean temperature for 30 days after heading for each F2 individual. This ranged between 23.1 and 27.3°C in Japan and 24.8 and 27.8°C in Laos and was positively correlated with PA content in both Japan and Laos (Figure 6B). The absolute value of the correlation coefficient was higher in Japan (0.44) than that in Laos (0.22).
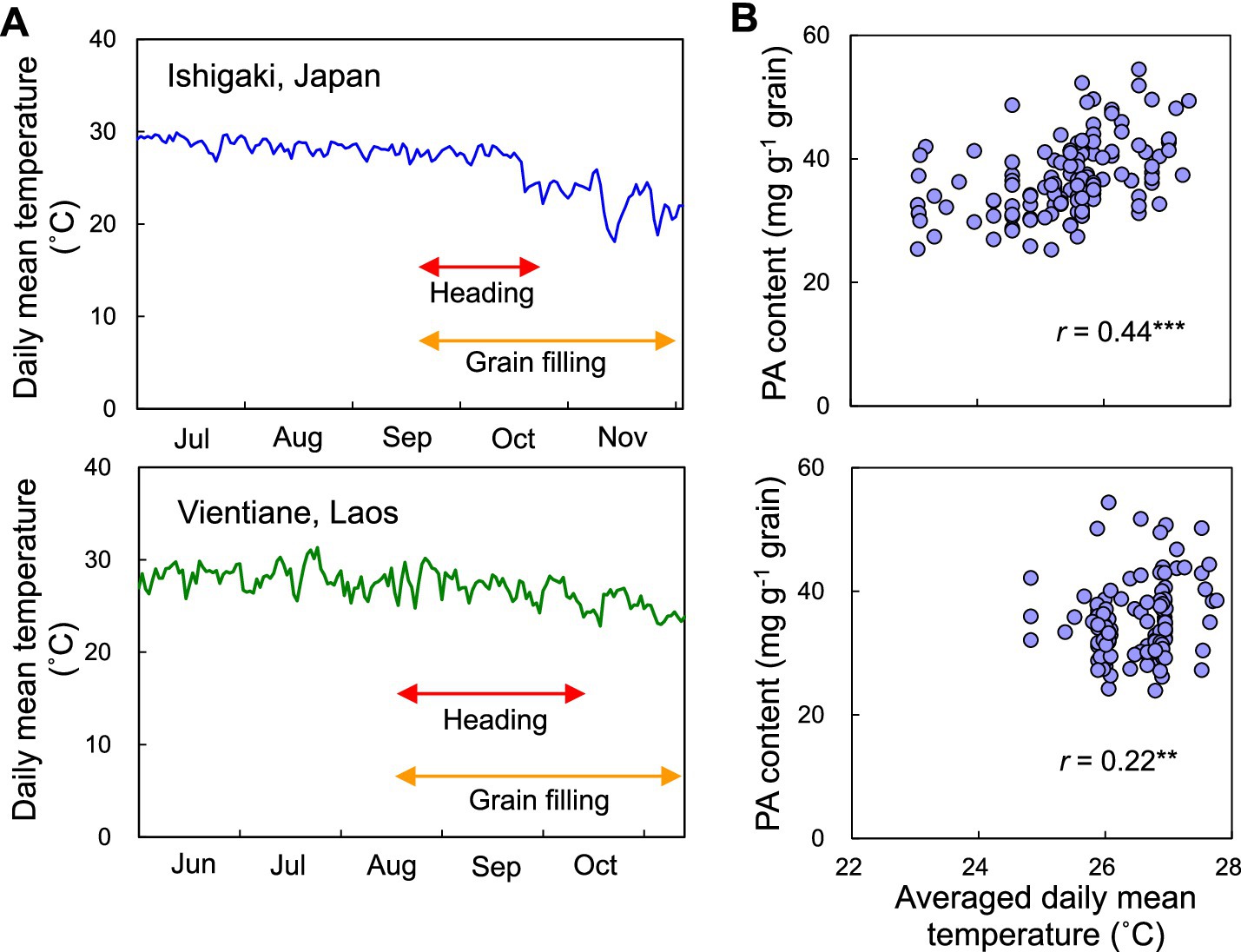
Figure 6. Influence of air temperature during grain filling on the accumulation of PA in grains. (A) Change in daily mean temperature during the rice growing period in Ishigaki, Japan, and Vientiane, Laos. Arrows represent the duration of heading and grain filling among F2 individuals. (B) Relationship between averaged daily mean temperature over 30 days after heading and PA content in F2 individuals grown in Ishigaki, Japan and Vientiane, Laos. r represents the correlation coefficient. ** and *** represent significant difference at p < 0.01 and 0.001, respectively.
Discussion
In this study, we detected qPA1 at the end of the short arm of chromosome 1 in Japan and Laos and demonstrated the effect of the Kampeng allele on increasing PA content in Japan. Our validation using the F3 lines also confirmed the stable contribution of qPA1 to PA enhancement, irrespective of Hd6. Previous studies have detected QTLs for PA content on chromosomes 2, 3, 5, 7, 8, and 12 (Stangoulis et al., 2007; Perera et al., 2019; Gyani et al., 2020). To the best of our knowledge, no QTLs have been detected on chromosome 1. However, the Rice Annotation Project Database (RAP-DB) (Sakai et al., 2013) annotated a gene of Os01g0102000 at the region of qPA1, which may be involved in the synthesis of myo-inositol according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (Kanehisa and Goto, 2000).1 Therefore, qPA1 is a novel QTL underlying PA content in rice grains and could be useful for increasing PA content in the variety, Non, under different environmental conditions.
Notably, a QTL for PA content was detected at Hd6 in Japan but not in Laos, whereas a QTL for days to heading was detected at Hd6 in both Japan and Laos. Although variations in heading date often have pleiotropic effects on other traits (Keurentjes et al., 2007; Takai et al., 2009), the inconsistency in our study may be explained by the assumption that PA accumulation in grains is temperature-dependent (Thavarajah et al., 2010). Japan exhibited a more substantial decrease in daily air temperature during grain filling (10.5°C) than Laos (7.3°C). Thus, in Japan, some F2 individuals matured at average daily mean temperature below 25°C, whereas in Laos, the majority of F2 individuals matured at temperatures above 25°C. Consequently, the average daily mean temperature in Japan showed a stronger correlation with PA content compared to that in Laos (r = 0.44 vs. 0.22). Our findings suggest that PA accumulation in rice grains is temperature-sensitive, and suppressed accumulation was observed below 25°C. Similar results were reported by Thavarajah et al. (2010), who found that lentils grown under cooler seed-filling temperatures had a lower PA content. The detection of the QTL for PA content at Hd6 in Japan may be because the Kampeng allele of Hd6 had a delayed heading date in some F2 individuals, exposing them to low temperatures during grain filling and resulting in suppressed PA accumulation in the grains. Conversely, the QTL for PA content was not detected at Hd6 in Laos because the majority of F2 individuals, including late-heading ones by Hd6, matured above 25°C, at which PA accumulation was not suppressed. Although the influence of soil conditions such as P availability on PA accumulation in rice grains has been well-studied (Rose et al., 2016; Oo et al., 2023b), information on the effects of temperature on PA accumulation in rice grains is limited (Perera et al., 2018). The key enzyme activity in the PA biosynthesis pathway and/or the transport of P into the developing grains may be reduced by low temperatures. Further studies using growth chambers for controlled environments at different temperatures are needed to prove the temperature dependence of PA accumulation in rice grains and to more precisely decouple heading date from exposed temperature.
We observed higher panicle numbers in Non than in Kampeng. However, no QTLs were detected for the Non allele associated with increased panicle number. Non possibly had QTLs below the detection threshold that increased the panicle number. In addition, QTLs for the panicle number were detected in different chromosomal regions in Japan and Laos. These results indicate that the environment strongly influences the panicle number in F2 individuals and that minor genetic factors may be involved in Non’s vigorous panicle production, unlike several QTLs for the panicle number that have been previously cloned as single genes (Takai, 2024). Based on the results of this study, we developed a targeted breeding strategy integrating marker-assisted selection to improve black rice with high PA and yield potential suitable for upland ecosystems in northern Laos. First, Non is to be used as the genetic background in the breeding program because we could not detect QTLs for the Non allele to increase the panicle number. Second, the Kampeng allele of qPA1 is to be introduced into the Non genetic background because it is not the result of an environmental response regulated by heading genes and would genuinely increase PA content in Non grains. Third, the Kampeng allele of Kala4 is to be introduced into the Non genetic background to convert white rice into black rice (Oikawa et al., 2015) because no QTLs for days to heading, PA content, and panicle number were detected at the location of Kala4 on chromosome 4 in this study. Introducing Kala4 also increases the accumulation of anthocyanins and other antioxidants in grains because Kala4 regulates several enzymes involved in the anthocyanin biosynthesis pathway (Oikawa et al., 2015). We are breeding new black rice varieties by backcrossing Non with Kampeng and using marker-assisted selection. The agronomic performance and nutritional quality of the new black rice will be evaluated in the upland ecosystems of northern Laos in the near future to meet the government’s ADS in Laos.
Conclusion
We detected two QTLs for PA content in the F2 population derived from a cross between black rice Kampeng and white rice Non. One QTL resulted from an environmental response via Hd6, whereas another QTL, qPA1, was not affected by the heading QTLs and contributed to the increased PA content with the Kampeng allele. Therefore, qPA1 can be useful for breeding improved black rice with high PA content and yield potential in the Non genetic background. Fine-mapping and cloning qPA1 can also reveal the genetic and physiological mechanisms controlling PA content in rice grains. As described in the Introduction, PA has dual roles as a P source for seedling vigor and as an antinutritional component, particularly for reducing the bioavailability of micronutrients such as iron and zinc. Furthermore, the two aspects must be balanced. The antinutritional properties of PA could be mitigated by various processing approaches, such as the production of germinated brown rice and koji-amazake, a fermented rice drink that dephosphorylates PA and converts it into inositol, also known as a vitamin-like compound in breast milk and infant formula (an infant growth factor) with no antinutritional properties (Koletzko et al., 2005; Patil and Khan, 2011; Marui et al., 2025). The utilization of black rice in these processes can also increase its value.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
TT: Writing – original draft, Investigation, Writing – review & editing, Formal analysis, Methodology, Data curation, Conceptualization. HS: Investigation, Resources, Writing – review & editing. JM: Investigation, Data curation, Writing – review & editing. AO: Investigation, Writing – review & editing. KV: Writing – review & editing, Investigation. SP: Investigation, Writing – review & editing. HA: Formal analysis, Conceptualization, Methodology, Writing – review & editing, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the JIRCAS research program “Indigenous crops and food design.”
Acknowledgments
We express our gratitude to Y. Iijima, E. Kojima, H. Sato, and M. Monma for their technical support with phenotyping and genotyping, as well as the staff of the technical support section for their assistance in the paddy fields at JIRCAS. We also thank A. Dethvongsa, B. Tansoukhang, and K. Keoonlah for their technical assistance with the pot experiments at RCCRC. We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Appa Rao, S., Schiller, J. M., Bounphanousay, C., Inthapanya, P., and Jackson, M. T. (2006). “The colored pericarp (black rice) of Laos” in Rice in Laos. eds. J. M. Schiller, M. B. Chanphengxay, B. Linquist, and S. Appa Rao (Los Baños: IRRI), 175–186.
Arbelaez, J. D., Dwiyanti, M. S., Tandayu, E., Llantada, K., Jarana, A., Ignacio, J. C., et al. (2019). 1k-RiCA (1K-Rice custom amplicon) a novel genotyping amplicon-based SNP assay for genetics and breeding applications in rice. Rice 12:55. doi: 10.1186/s12284-019-0311-0
Asai, H., Maruyama, K., and Boualapanh, C. (2020). Potential for high-functional rice production identified in black rice genetic resource of shifting cultivation in Lao. Jpn. J. Crop Sci. 89:99.
Asai, H., Saito, K., Samson, B., Songyikhangsuthor, K., Homma, K., Shiraiwa, T., et al. (2009). Yield response of indica and tropical japonica genotypes to soil fertility conditions under rainfed uplands in northern Laos. Field Crop Res. 112, 141–148. doi: 10.1016/j.fcr.2009.02.010
Asai, H., and Soisouvanh, P. (2017). Yield performance of upland rice, maize and job's tears in a rainfed upland ecosystem in mountainous Laos. Jpn. Agric. Res. Q. 51, 309–318. doi: 10.6090/jarq.51.309
Basnayake, J., Inthavong, T., Kam, S. P., Fukai, S., Schiller, J. M., and Chanphengxay, M. (2006). “Climatic diversity within the rice environments in Laos” in Rice in Laos. eds. J. M. Schiller, M. B. Chanphengxay, B. Linquist, and S. Appa Rao (Los Baños: IRRI), 47–64.
Broman, K. W., Wu, H., Sen, S., and Churchill, G. A. (2003). R/QTL: QTL mapping in experimental crosses. Bioinformatics 19, 889–890. doi: 10.1093/bioinformatics/btg112
Commission Internationale d’Eclairage. (1976). Colorimetry—part 4: CIE 1976 L*a*b* colour space. Available online at: https://cie.co.at/publications/colorimetry-part-4-cie-1976-lab-colour-space-0 (Accessed 25 April, 2025).
Gautam, A. (2022). “Phenol-chloroform DNA isolation method” in DNA and RNA isolation techniques for non-experts. ed. A. Gautam (Cham: Springer), 33–39.
Graf, E., and Eaton, J. W. (1990). Antioxidant functions of phytic acid. Free Radic. Biol. Med. 8, 61–69. doi: 10.1016/0891-5849(90)90146-a
Gyani, P. C., Bollinedi, H., Gopala Krishnan, S., Vinod, K. K., Sachdeva, A., Bhowmick, P. K., et al. (2020). Genetic analysis and molecular mapping of the quantitative trait loci governing low phytic acid content in a novel LPA rice mutant, PLM11. Plan. Theory 9:1728. doi: 10.3390/plants9121728
Hasada, K., and Phonhnachit, P. (2023). Estimation of black rice distribution and comparison of prices among regions in Lao PDR. JSIDRE 23, 469–470.
Kanehisa, M., and Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. doi: 10.1093/nar/28.1.27
Keurentjes, J. J., Bentsink, L., Alonso-Blanco, C., Hanhart, C. J., Blankestijn-De Vries, H., Effgen, S., et al. (2007). Development of a near-isogenic line population of Arabidopsis thaliana and comparison of mapping power with a recombinant inbred line population. Genetics 175, 891–905. doi: 10.1534/genetics.106.066423
Koletzko, B., Baker, S., Cleghorn, G., Neto, U. S., Gopalan, S., Hernell, O., et al. (2005). Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 41, 584–599. doi: 10.1097/01.mpg.0000187817.38836.42
Liu, Q. L., Xu, X. H., Ren, X. L., Fu, H. W., Wu, D. X., and Shu, Q. Y. (2007). Generation and characterization of low phytic acid germplasm in rice (Oryza sativa L.). Theor. Appl. Genet. 114, 803–814. doi: 10.1007/s00122-006-0478-9
Lolas, G. M., Palamidis, N., and Markakis, P. (1976). The phytic acid-total phosphorus relation in barley, oats, soybeans and wheat. Cereal Chem. 53, 867–871.
Marui, J., Shiraishi, Y., Takeura, M., Shompoosang, S., Varichanan, P., and Boulom, S. (2025). Combined use of rice-koji cultured with aspergillus oryzae and aspergillus luchuensis for enhanced dephosphorylation of phytate to myo-inositol in brown rice-koji-amazake, a sweet brown rice beverage. Food Sci. Technol. Res. 31, 147–153. doi: 10.3136/fstr.FSTR-D-24-00170
Matsuo, A., Sato, K., Nakamura, Y., and Ohtsuki, K. (2005). Hydrolysis of phytic acid in brown-rice bread by Aspergillus niger phytase. Nippon Eiyo Shokuryo Gakkaishi. 58, 267–272. doi: 10.4327/jsnfs.58.267
McDowell, R. W., Pletnyakov, P., and Haygrth, P. M. (2024). Phosphorus applications adjusted to optimal crop yields can help sustain global phosphorus reserves. Nat. Food 5, 332–339. doi: 10.1038/s43016-024-00952-9
Ministry of Agriculture and Forestry (2015). Agriculture development strategy to 2025 and vision to the year 2030. Vientiane Capital, Lao PDR:Ministry of Agriculture and Forestry.
Oikawa, T., Maeda, H., Oguchi, T., Yamaguchi, T., Tanabe, N., Ebana, K., et al. (2015). The birth of a black rice gene and its local spread by introgression. Plant Cell 27, 2401–2414. doi: 10.1105/tpc.15.00310
Oo, A. Z., Asai, H., Kawamura, K., Marui, J., Nakahara, K., Takai, T., et al. (2023b). Optimizing phosphorus management to increase grain yield and nutritional quality while reducing phytic acid concentration in black rice (Oryza sativa L.). Front. Sustain. Food Syst. 7:1200453. doi: 10.3389/fsufs.2023.1200453
Oo, A. Z., Asai, H., Win, K. T., Marui, J., and Saito, H. (2023a). Seed phytic acid concentration affects rice seedling vigor irrespective of soil phosphorus bioavailability. Physiol. Plant. 175:e13913. doi: 10.1111/ppl.13913
Patil, S. B., and Khan, M. K. (2011). Germinated brown rice as a value added rice product: a review. J. Food Sci. Technol. 48, 661–667. doi: 10.1007/s13197-011-0232-4
Perera, I., Fukushima, A., Akabane, T., Horiguchi, G., Seneweera, S., and Hirotsu, N. (2019). Expression regulation of myo-inositol 3-phosphate synthase 1 (INO1) in determination of phytic acid accumulation in rice grain. Sci. Rep. 9:14866. doi: 10.1038/s41598-019-51485-2
Perera, I., Seneweera, S., and Hirotsu, N. (2018). Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability. Rice 11:4. doi: 10.1186/s12284-018-0200-y
Qamar, Z., Hameed, A., Ashraf, M., Rizwan, M., and Akhtar, M. (2019). Development and molecular characterization of low phytate basmati rice through induced mutagenesis, hybridization, backcross, and marker assisted breeding. Front. Plant Sci. 10:1525. doi: 10.3389/fpls.2019.01525
Qamar, Z., Uzair, M., Hameed, A., Zafar, S. A., and Li, X. (2024). Identification of a novel mutation in the OsMRP5 gene in low phytate basmati rice mutant and development of CAPS marker for marker-assisted breeding. Front. Plant Sci. 15:1455219. doi: 10.3389/fpls.2024.1455219
Rose, T., Kretzschmar, T., Liu, L., Lancaster, G., and Wissuwa, M. (2016). Phosphorus deficiency alters nutrient accumulation patterns and grain nutritional quality in rice. Agronomy 6:52. doi: 10.3390/agronomy6040052
Saito, K., Linquist, B., Keobualapha, B., Phanthaboon, K., Shiraiwa, T., and Horie, T. (2006). Cropping intensity and rainfall effects on upland rice yields in northern Laos. Plant Soil 284, 175–185. doi: 10.1007/s11104-006-0049-5
Sakai, H., Lee, S. S., Tanaka, T., Numa, H., Kim, J., Kawahara, Y., et al. (2013). Rice annotation project database (RAP-DB): an integrative and interactive database for rice genomics. Plant Cell Physiol. 54:e6. doi: 10.1093/pcp/pcs183
Schiller, J. M., Hatsadong, S., and Doungsila, K. (2006). “A history of rice in Laos” in Rice in Laos. eds. J. M. Schiller, M. B. Chanphengxay, B. Linquist, and S. Appa Rao (Los Baños: IRRI), 9–28.
Silva, E. O., and Bracarense, A. P. F. R. L. (2016). Phytic acid: from antinutritional to multiple protection factor of organic systems. J. Food Sci. 81, R1357–R1362. doi: 10.1111/1750-3841.13320
Sompong, R., Siebenhandl-Ehn, S., Linsberger-Martin, G., and Berghofer, E. (2011). Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 124, 132–140. doi: 10.1016/j.foodchem.2010.05.115
Stangoulis, J. C. R., Huynh, B. L., Welch, R. M., Choi, E. Y., and Graham, R. D. (2007). Quantitative trait loci for phytate in rice grain and their relationship with grain micronutrient content. Euphytica 154, 289–294. doi: 10.1007/s10681-006-9211-7
Takahashi, Y., Shomura, A., Sasaki, T., and Yano, M. (2001). Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2. Proc. Natl. Acad. Sci. USA 98, 7922–7927. doi: 10.1073/pnas.111136798
Takai, T. (2024). Potential of rice tillering for sustainable food production. J. Exp. Bot. 75, 708–720. doi: 10.1093/jxb/erad422
Takai, T., Ohsumi, A., San-oh, Y., Laza, M. R., Kondo, M., Yamamoto, T., et al. (2009). Detection of a quantitative trait locus controlling carbon isotope discrimination and its contribution to stomatal conductance in japonica rice. Theor. Appl. Genet. 118, 1401–1410. doi: 10.1007/s00122-009-0990-9
Takai, T., Sakata, M., Rakotoarisoa, N. M., Razafinarivo, N. T., Nishigaki, T., Asai, H., et al. (2021). Effects of quantitative trait locus MP3 on number of panicles and rice productivity in nutrient-poor soils of Madagascar. Crop Sci. 61, 519–528. doi: 10.1002/csc2.20344
Thavarajah, D., Thavarajah, P., See, C. T., and Vandenberg, A. (2010). Phytic acid and Fe and Zn concentration in lentil (Lens culinaris L.) seeds is influenced by temperature during seed filling period. Food Chem. 122, 254–259. doi: 10.1016/j.foodchem.2010.02.073
Tp, M. A., Kumar, A., Anilkumar, C., Sah, R. P., Behera, S., and Marndi, B. C. (2022). Understanding natural genetic variation for grain phytic acid content and functional marker development for phytic acid-related genes in rice. BMC Plant Biol. 22:446. doi: 10.1186/s12870-022-03831-2
Keywords: black rice, breeding, Laos, phytic acid, quantitative trait locus
Citation: Takai T, Saito H, Marui J, Oo AZ, Vilayheuang K, Phongchanmixay S and Asai H (2025) Detection and characterization of quantitative trait loci for phytic acid in grains toward improved black rice in northern Laos. Front. Sustain. Food Syst. 9:1620644. doi: 10.3389/fsufs.2025.1620644
Edited by:
Manosh Kumar Biswas, University of Leicester, United KingdomReviewed by:
Francesco Sestili, University of Tuscia, ItalyZia- ul- Qamar, Nuclear Institute for Agriculture and Biology, Pakistan
Copyright © 2025 Takai, Saito, Marui, Oo, Vilayheuang, Phongchanmixay and Asai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshiyuki Takai, dGFrYWl0MDI3MEBqaXJjYXMuZ28uanA=
 Toshiyuki Takai
Toshiyuki Takai Hiroki Saito
Hiroki Saito Junichiro Marui1
Junichiro Marui1