- 1Department of Chemistry, College of Science, Taif University, Taif, Saudi Arabia
- 2Research Center of Basic Sciences, Engineering and High Altitude, Taif University, Taif, Saudi Arabia
- 3Department of Food Science and Nutrition, College of Sciences, Taif University, Taif, Saudi Arabia
- 4Department of Agricultural Engineering, Delta State University of Science and Technology, Ozoro, Nigeria
- 5Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, University of Tabuk, Tabuk, Saudi Arabia
- 6Department of Clinical Nutrition, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia
- 7Program of Food Sciences and Nutrition, Turabah University College, Taif University, Taif, Saudi Arabia
- 8Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia
- 9Department of Food and Nutrition, Faculty of Human Sciences and Design, King Abdulaziz University, Jeddah, Saudi Arabia
- 10Department of Public Health, College of Health Sciences, Saudi Electronic University, Riyadh, Saudi Arabia
- 11Department of Home Economic, Prince Sattam Bin Abdulaziz University, Al-kharj, Saudi Arabia
- 12Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 13Embryonic Stem Cell Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
This research was focused on developing a suitable and optimizing model, for dried meat preservation (storability), through the application of natural essential oils and extracts. The dried meat (beef) samples were treated with onion peels extract (OPE), onion peels oil (OPO), plantain peels extract (PPE), and plantain peels oil (PPO), in accordance with concentrations and hybridization levels, provided by Central Composite Design (CCD) model. Two basic independent variables – treatment type and storage time – were evaluated, to establish their impact on six responses: total bacterial viable content (TBC), Staphylococcus aureus, Salmonella spp., total viable fugal content (TFC), aflatoxin B1 and aflatoxin B2. Based on the CCD optimization guidance, the meat samples were treated with 10 different natural additives, and coded T0 to T9, respectively. Nutritional and microbial evaluations of the samples were done by utilizing standard procedures. Specifically, the High-Performance Liquid Chromatography (HPLC) approach was used to explore the B vitamins and aflatoxins concentrations. The results specified that the plantain and onion waste products safeguarded the meat nutritional integrity and public health safety. Additionally, the optimization result depicted that the hybridized PPO and OPO treatment (T9), demonstrated the optimal efficacy in inhibiting microbial growth, and formation of aflatoxins. The maximum TBC and TFC populations of 3,130 and 2,180 cfu/g, were recorded in the control (T1) meat samples, respectively. The study found that the essential oils and extracts, effectively reduced the aflatoxins B1/B2 levels, in preserved beef to safe limits, established by the World Health Organization for meat products suitable for human consumption. This study’s results have highlighted the effectiveness of using oils and extracts derived from discarded onion and plantain peels, in improving meat quality, integrity and inhibiting pathogens survival.
Highlights
• Essential oils and plants extract influences on meat nutritional composition
• Essential oils and extracts play essential roles in inhibiting pathogens
• Plants materials are eco-friendly alternatives for meat preservation
• Employing the HPLC approach ensures accurate aflatoxins quantification
Introduction
Food is a vital requirement of the human body, as inadequate nutrition can lead to malnutrition-related issues, which are among the leading causes of death worldwide (Pawlak and Kołodziejczak, 2020). Protein and vitamins play crucial roles in maintaining health and supporting various bodily functions. Meat, as an animal-based protein unlike plant-based protein, is classified as complete protein since it naturally comprises of the nine essential amino acids in a proportions required for human health. However, by combining various plant foods, it is possible to achieve a complementary amino acid profile, which can result in the formation of a complete protein (Qin et al., 2022; Stadnik, 2024). Animal-based food items are prefect source of essential nutrients, some of which are challenging to obtain from plant-based sources alone. Meat and its products have been crucial in human nutrition, contributing to a balanced diet and supporting growth and performance of the body systems (Ruxton and Gordon, 2024; Sheffield et al., 2024). Despite its high cholesterol content, meat is one the most staple protein source in many human diets worldwide, resulting from its high concentration of essential amino acids, vital metals and vitamins (Stadnik, 2024).
Effective food storage is one of the major objectives of achieving food security, helping to reduce food wastage, maintains economic stability, and ensures food availability (Alirezalu et al., 2019; Pawlak and Kołodziejczak, 2020; Uguru et al., 2023; Ashraf et al., 2025). Food storage can be categorized into wet and dry methods, and the choice of method adopted is dependent on the type of the agricultural product, desired shelf life, and available resources (Sayed and Elsharkawy, 2021). The dry storage category is the widespread storage method used globally, due to its low level of complexity, availability of the materials, energy efficiency, and lower technical know-how (Uguru et al., 2023; Bradford et al., 2020; Lisboa et al., 2024). Fungal and bacteria growth is a major concern in dried meat storage, largely because this storage environment conditions (humidity, temperature and moisture content fluctuation conditions) often favors their survival (Ivane et al., 2024; Kunová et al., 2021; Kahraman and Tutun, 2021).
The key consequences in dried meat during storage are, decline in sensory quality, nutritional value, and microbial invasion (Sayed and Elsharkawy, 2021). Salmonella spp., Pseudomonas, Staphylococcus spp., E. coli, Aspergillus, Penicillium, and Fusarium have adverse effect on animal products quality, and posing significant public health issues (Kunová et al., 2021; Paswan and Park, 2020). Aflatoxins produced by some fungi (Aspergillus flavus and Aspergillus parasiticus) are potent toxic compounds known for their carcinogenic and mutagenic effects; which are directly linked to severe health issues - organ (liver and kidney) failure, endocrine disruption and respiratory issues (Gruber-Dorninger et al., 2019; Ekwomadu et al., 2022; Uguru et al., 2023; Kahraman and Tutun, 2021).
Animal products are often treated with various natural additives (agro-based materials) during processing and storage operations, to improve their dietary integrity, shelf life, and consumer prevalence (Umaru et al., 2019; Beya et al., 2021; Djenane et al., 2024). Plant extracts and oils are rich in bioactive compounds, with antioxidants and antimicrobial properties (Gál et al., 2023; Enciso-Martínez et al., 2024), leasing to improve food functional/nutritional properties (Kunová et al., 2021; Kačániová et al., 2024). Although natural extracts and oils offer many benefits in food preservation, their excessive applications can result in the emergence of resistant strains of microorganisms, thereby complicating food quality and safety (Zhou et al., 2023; Ashraf et al., 2025). Most meat processing industry uses synthetic preservatives; however, these inorganic chemicals toxicity (health challenges) includes - allergies, carcinogenic issues, and organs damage (Gál et al., 2023; Kačániová et al., 2024). This has spurred interest in the development of natural and environmental friendly alternatives, which include plant essential oils (EOs) and extracts having minimal health issues (Unar, 2022; Ivane et al., 2024). Additionally, the hybridization of EOs and extracts leverages antimicrobial and antioxidant properties of the treatments, which can combat spoilage-causing bacteria and delay oxidation (Al-Hijazeen, 2022).
Though numerous researches have been done on the use of organic preservatives in food preservation, most of them utilized plants edible parts as bio-preservatives (Turgut et al., 2017; Pateiro et al., 2021; Kačániová et al., 2024; Ashraf et al., 2025), inadvertently, contributing to food insecurity. Additionally, prior research primarily focused on the impact of green additives (bio-preservatives) on pathogenic infestation of preserved foods, and very little attention paid to the dietetic quality and human safety (Prakash et al., 2015; Yuan and Yuk, 2018; Kunová et al., 2021; Fu et al., 2022). This study tends to bridge these gaps by using agricultural waste materials (plantain finger peels and onion bulb wastes) as organic preservatives - essential oils (EOs) and extracts – for meat quality and integrity preservation, through mathematical modeling and optimization. Apart from microbial in inhibition and nutrients stabilization, this research appraised the aflatoxins toxicity status of the preserved meat. Specifically, this research uses a novel combination of High-Performance Liquid Chromatography (HPLC) for the aflatoxins evaluation, and predictive modeling techniques (Centre Composite Design “CCD” and Prediction Interval “PI” analysis), to create mathematical models capable of predicting meat quality alterations during storage; as well as, the optimal levels (concentrations) of these bio-preservatives, needed for maximize microbial defense and food safety. Eventually, this study’s outcomes will bridge essential knowledge gaps, connecting meat quality, food safety and computational optimization. This contributes to defensible and innovative methods in food preservation, which is in conformity with global sustainability trends like UN SDGs, European Union and Green Deal.
Materials and methods
Meat sampling
Ten kilogram of high-quality beef was collected from a local abattoir in Delta State, Nigeria on August 18th, 2023. The samples were placed in sterilized, ice-cooled plastic containers, and promptly transported to the microbiology laboratory for analysis at Delta State University of Science and Technology, Ozoro, Nigeria, and Docchy analytical laboratories and environmental services limited, Nigeria.
Quality control
The HPLC system, Plate Count Agar (PCA), Salmonella-Shigella (SS) agar, Baird-Parker Agar (BPA), Potato Dextrose Agar (PAD), methanol, helium (99% purity) and other solvents used for the analyses were manufactured by Thermo Fisher Scientific Inc. USA. All the materials and equipment used were sterilized to prevent cross-contamination. All measurements were conducted in triplicate, and the relative standard deviation was less than 4% across the measurements.
Extract and essential oil (EO) samples preparation
The onion bulb peels and plantain peels were sorted to eliminate contaminants, sun-dried and then ground into powder form using the laboratory mill (Ultra Centrifugal Mill ZM 300, produced by Retsch GmbH Ltd. Germany). Thereafter, the onion bulbs peels extract (OPE) and plantain peels extract (PPE) were prepared in accordance with standard procedures, through cold maceration approach using ethanol as the carrier solvent. 100 g of the pulverized onions and plantain peels wastes were soaked in 1 L of ethanol for four days, and filtered using a 0.22 μm membrane. Thereafter, the filtrate was evaporated (concentrated) utilizing a rotary evaporator (model Rotavapor R-300, manufactured by Thermo Fisher Scientific Inc. America) at 45°C, to evaporate the excess methanol leaving the onion bulbs peels extract (OPE) and plantain peels extract (PPE) behind.
Additionally, the onion bulbs peels oil (OPO) and plantain peels oil (PPO), were extracted through the solvent extraction technique, and using n-hexane as the solvent. 200 g of the sieved plants materials were poured into a Soxhlet extractor, containing 600 mL of n-hexane. The mixture was subjected to temperature of 67°C for 6 h, ensuring constant n-hexane cycling, and the high quality oil obtained was collected into a cylinder (Ashraf et al., 2025). Thereafter, the excess solvent in the EO was evaporated at 40°C, and dried with anhydrous sodium sulfate, and kept in cool dark environment.
Experiment design
The following treatment plans as presented in Table 1, was used to achieve the goals of this research. The experimental design was generated by Design Expert Software (Version 23.1), through CCD and considering these factors: storage duration, treatment concentration, treatment hybridization, and their interactions. Additionally, the PI analysis was employed to confirm (validate) the experiment results.
The optimization will involve all the treatments of T0–T9, and storage durations of 0–10 weeks. The extract and oil concentrations used as treatments in this research, were selected based on the range of concentrations previously applied by other authors (Kunová et al., 2021; Kačániová et al., 2024). This approach controls existing research to optimize their effectiveness and public safety in the stored beef quality control.
Meat samples preparation
Approximately 30 g of each meat sample was diced into 0.05 m x 0.05 m pieces. The cook-dried method was adopted for Treatments 1, 3, 5, 6, 7, and 8. For treatments involving plant extract application (Treatments 1 and 3), the meat was diced into 0.05 m × 0.05 m pieces, steamed with the appropriate extract concentration for 20 min, and then immediately cooled to room temperature. Also, for the treatments involving hybridized EO and extract application (Treatments 5, 6, 7, and 8), the meat pieces, already prepared and inoculated with the extract, were then sprayed with the appropriate volume of oil. The data indicated that, for treatments involving only oil application (Treatments 2, 4, and 9), the oil-dried method was utilized: the meat cubes were blanched for 20 min, cooled and coated with the essential oils. Afterward, all the prepared meat samples were dried in a laboratory oven at 105°C to a moisture content of approximately 7%.
Storage condition
The dried meat samples were stored in a dry ambient environment (30 ± 5°C, 80 ± 11% RH), covered with netting to protect them from direct insect and pest exposure. This is similar to traditional storage practices commonly used in markets and households across many developing and underdeveloped nations. The nutritional quality of the meat and microbial analyses were conducted every 2 weeks over a 10-week period.
Proximate analyses
Essential oils and extracts phytochemicals composition
The composition of phytochemicals in the onion peels and plantain peels extracts and oils were analyzed using gas chromatography with flame ionization detection (GC-FID), manufactured by Chromatography Direct Ltd., United Kingdom. Firstly, the samples (extracts and oils) were prepared by mixing the oil/extract with n-hexane at a ratio of 1:10, trembled vigorously for 5 min and sieved through a 0.45 μm syringe filter. One μL of the prepared sample was injected into the system, which uses HP5 capillary column (30 m × 0.25 mm × 0.25 μm), and helium as the carrier medium with a flow rate of 1.0 mL/min. The injector temperature and detector temperature were 250°C and 280°C, respectively. The initial system’s oven temperature was pre-set at 60°C for 2 min, before it was ramp at 10°C/min, and then a final temperature of 280°C. Additionally, the operational run time was 25 min, and standards were employed quantification and documentation of the phytochemicals.
Moisture content
The moisture content (MC) of the meat samples was determined using the gravimetric method, following the Association of Official Analytical Chemists (AOAC) guidelines.
Vitamins determination
The vitamin B profile and vitamin E level of the dried meat samples was measure through the HPLC method. To measure the vitamin E level in the samples, 2 g of the ground specimen was mixed with 202 mL of methanol, and strained using a 0.45 μm strainer. Basically, these factors/parameters were used to determine the vitamin E level of each specimen – injector volume of 20 μL, methanol mobile phase, C18 reversed-phase column, 25°C column temperature, flow speed of 0.06 L/h, UV detection of 295 nm, run duration of 15 min, retention time of 10 min, and recovery rate of 105%.
Likewise, to measure the B vitamins content of each sample, these were the preliminary preparations: 3 g of the meat the ground meat was normalized with acidified distill water, centrifuge at the speed of 10,000 rpm, sieved with a 0.45 μm filter. These were the major HPLC factors/parameters considered for the B vitamins evaluation: 20 μL injector volume, methanol carrier, ≤30 min run time (depending on the B vitamin to be measured), of 0.06 L/h flow rate, C18 column, 30°C column temperature. Specifically, the UV detection wavelengths for Vitamins B1, B2, B6, B9 and B12 were 246, 270, 290, 290 and 361 nm, respectively; while the retention time for Vitamins B1, B2, B6, B9 and B12 were 7, 10, 12, 15, 17, and 24, respectively, (Albawarshi et al., 2022).
Protein determination
The Kjeldahl method was employed to assess the protein content in the meat samples. A 10 g portion of the meat was digested with sulfuric acid (H₂SO₄) at 90°C, utilizing copper as a catalyst. Following the digestion process, the resulting acidified solution was neutralized with sodium hydroxide (NaOH) to release ammonia gas, which was captured as ammonium. This ammonium solution was subsequently titrated with hydrochloric acid to measure the nitrogen content in the meat.
Microbial analyses
Preparation of sample
Twenty grams of each sample was blended in a laboratory mill, with the addition of 0.180 L of sterile saline solution, to produce a suspension having a 1:10 dilution factor. This dilution helps to homogenize the sample concentration, thereby enhancing the accuracy of microbial counts evaluation.
Bacterial count
One milliliter (1 mL) of the dilution was evenly spread onto sterile PCA, utilizing a disinfected glass spreader, incubated at 37°C for 48 h, and the bacterial clusters formed were counted. The total viable bacterial load (TBC) produced expressed as colony forming units per gram (cfu/g) (Ashraf et al., 2025).
Staphylococcus aureus determination
One (1) mL of the meat dilution, was spread across a BPA plate, and placed on incubation operation for 48 h at 37°C. Staphylococcus aureus colonies were identified through their black or dark gray coloration.
Salmonella spp. determination
The homogenized meat dilution (1 mL) was incubated (37°C for 24 h), before it was transferred to the Rappaport-Vassiliadis (RV) broth, and further incubated at 40°C for 24 h. This enriched sample was plated onto a SS agar plate, incubated at 37°C for 24 h, and the Salmonella spp. produced were identified and counted (Ashraf et al., 2025).
Fungal count
One mL of the diluted solution was uniformly spread across a sterile Potato Dextrose Agar (PDA) plate, and incubated at 25°C for 3 days. After incubation, the colony produced were counted and expressed as cfu/g.
Aflatoxins determination
The HPLC technique was used to determine aflatoxins (afB1 and afB2) count of the dried meat. Thirty gram of the meat sample was ground and mixed with 303 mL of methanol - Methanol: water (80:20), and filtered with a Whatman No. 1 filter paper. 20 μL of the liquid (extract) specimen was introduced into the HPLC system, with a mobile phase that consist a mixture of water, methanol and acetonitrile, mixed at a ratio of 60:20:20. The system was operating at a flow speed of 1 mL/min, column temperature of 30°C, UV- diode array detector (DAD) detector, and typical emission wavelength of 460 nm. The retention period of AFB₁ and AFB₂ were 12 and 9 min, respectively. The retention times and peak areas were associated with individual aflatoxins, and the concentration of each aflatoxin was assessed by comparing the results to established aflatoxins standards (Algammal et al., 2021). The assay was conducted in triplicate, with recovery rates for aflatoxins B1 and B2 at 93 and 94%, respectively.
Statistical analysis
The laboratory data evaluated using Analysis of Variance (ANOVA), by utilizing the SPSS software (version 20.0) to establish the impact of the treatments on the meat quality and stability. Also the differences between the means were investigated using the Duncan’s Multiple Range Test (DMRT), at 5% significance level (p ≤ 0.5). Furthermore, descriptive statistic was conducted on the data and the findings presented in tables, to provide a clear and organized summary of the results. The experiments were conducted in triplicate to ensure consistency.
Results and discussion
Phytochemical properties of the bio-agents
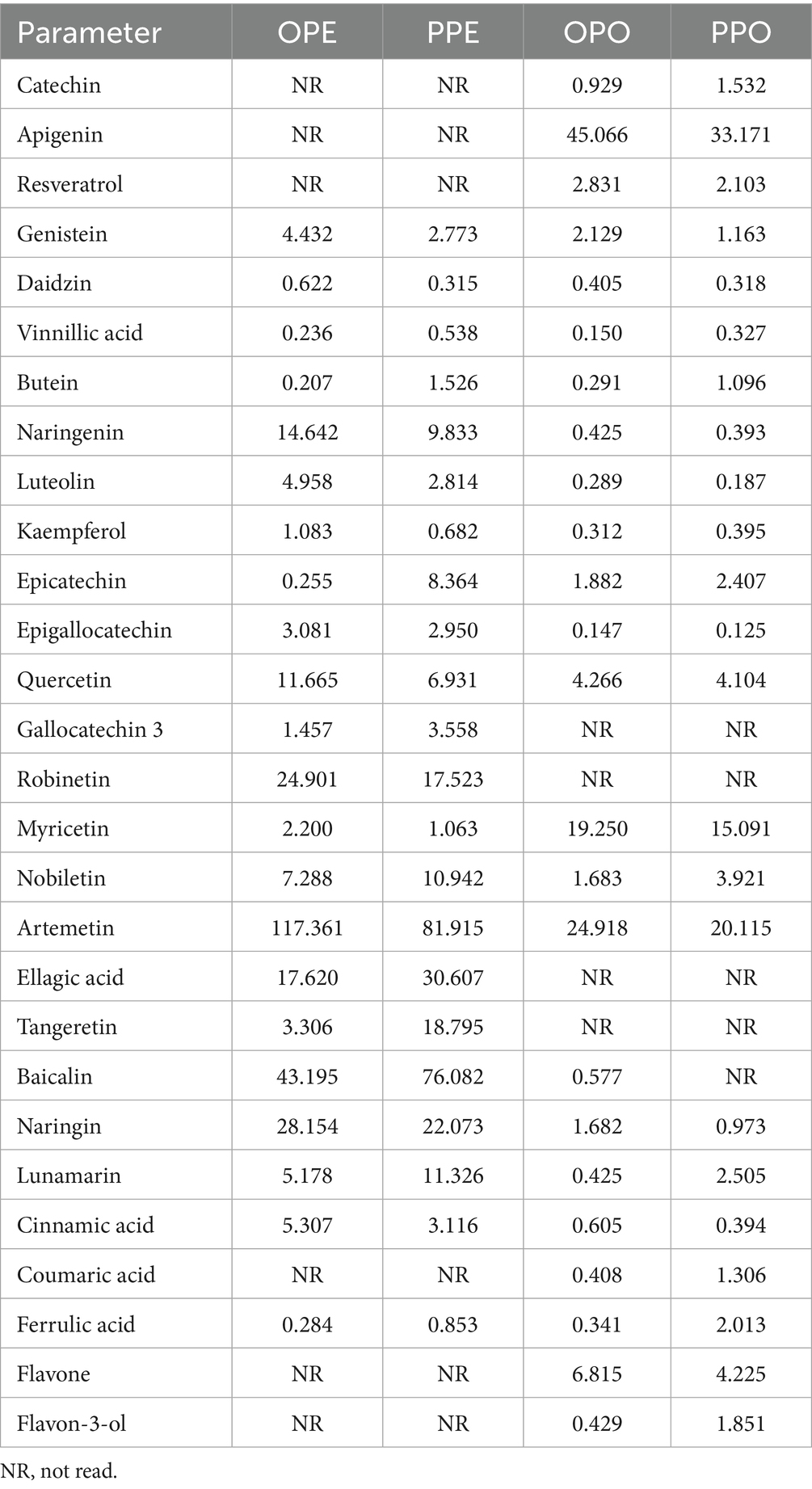
The results of the oils and extracts phytochemical properties were presented in Table 2. The results indicated that in the OPE and OPO samples, the most prevalent phytochemicals were Naringenin, Quercetin, Artemetin, Baicalin, and Naringin. In contrast, the PPE and PPO samples contained Epicatechin, Naringenin, Robinetin, Ellagic acid, Baicalin, Lunamarin, and Tangeretin as their primary phytochemicals. The results illustrated that out of the parameters measured, the plants extracts generally had higher concentration of phytochemicals than the plants oils, which is in conformity with earlier reports on bioactive compounds availability in plants derivatives (Umaru et al., 2019). The high phytochemical concentrations in the extracts and oils contribute to their effectiveness in inhibiting microbial growth. This makes the OPE, PPE, OPO and PPO viable options for extending the shelf life of dried meat products. Apigenin, Ellagic acid, Naringenin, Cinnamic acid, and Resveratrol demonstrate antimicrobial properties against a wide range of bacteria and fungi, including Staphylococcus aureus, Escherichia coli, Candida albicans, Salmonella spp., and several yeast species (Kačániová et al., 2024; Mandal and Domb, 2024).
Nutritional quality of the raw meat
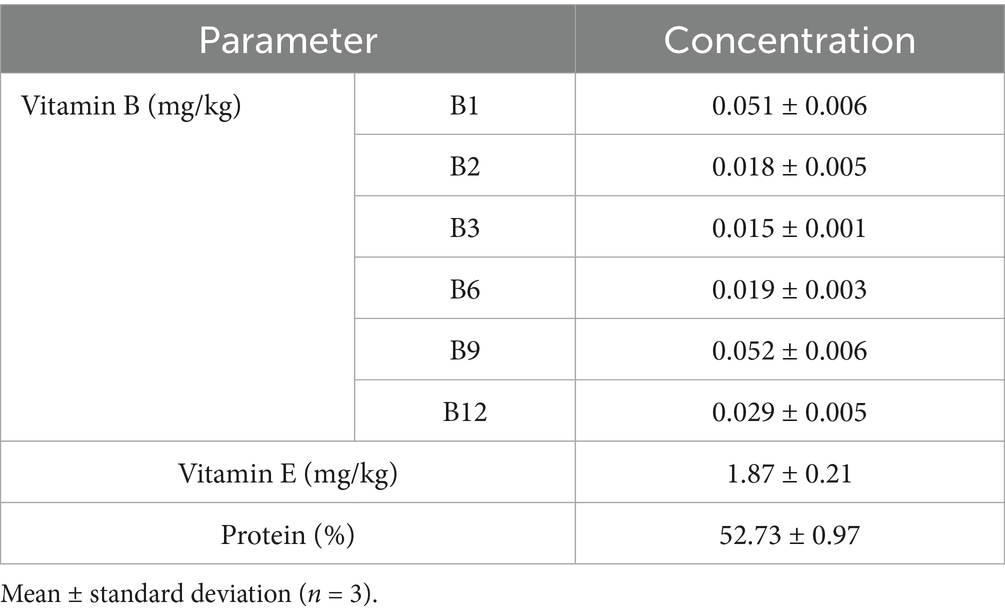
The results of the vitamins and protein levels of the beef before its preservation are presented in Table 3. It was noted that the Vitamin B1, B2, B3, B5 B9, and B12 of the raw beef was 0.051, 0.018, 0.015, 0.019, 0.052 and 0.029 mg/kg, respectively, while the total vitamin E and protein contents were 1.87 mg/kg and 52.73%, respectively. This is an indication that the raw meat flesh was rich in vitamins B and E; making beef a beneficial component of balanced diet. Vitamins and protein play crucial roles in energy production, nervous system functionality, and fetus development (Kačániová et al., 2024).
Meat quality after storage
Nutritional value
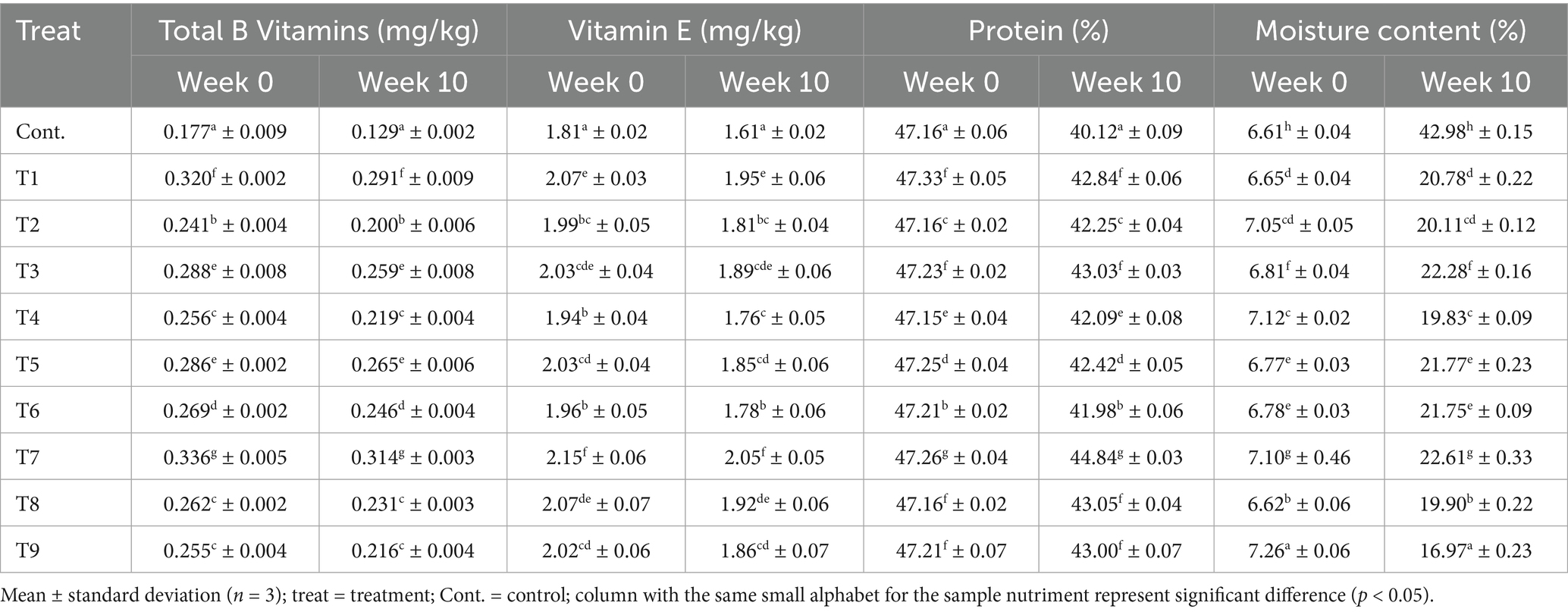
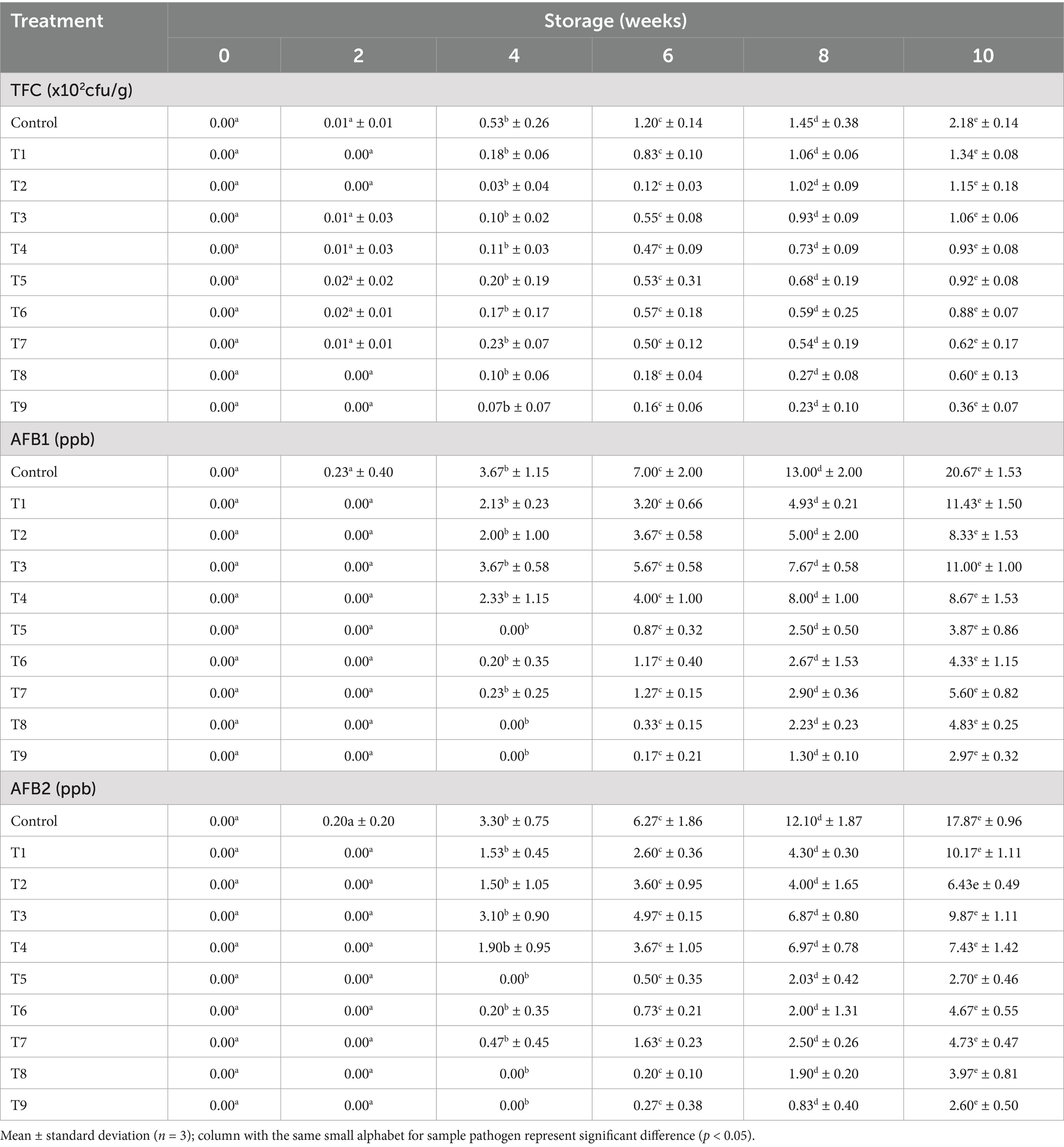
The results of the meat nutritional quality before and after the storage period are presented in Table 4. Following the processing of the meat samples, the levels of vitamin B, vitamin E, protein, and moisture content were observed to range from 0.177 to 0.336 mg/kg, 1.81 to 2.15 mg/kg, 47.16 to 47.26%, and 6.61 to 7.26%, respectively. It was observed that both the extract and oil treatments had an insignificant impact on the protein content of the meat after treatment. In contrast, the plant extracts and oils enhanced the vitamin B complex and Vitamin E levels in the beef. This could be attributed to the rich antioxidant properties and vitamins levels of onions peels and plantain peels extracts and oils. Antioxidants and other essential compounds present in essential oils and plant extracts, tend to enhance the total vitamins concentration in the meat product during processing, and inhibit nutrients degradation during storage (Pateiro et al., 2018). These results are comparable to the findings reported by Baker (2023), for various meat samples treated with different natural additives.
Furthermore, at the end of the 10-week experimental period, each treatment had a distinct and significant impact on the proximate composition of the meat. The levels of vitamins Band E, protein, and moisture content of the preserved meat varied from 0.129 to 0.314 mg/kg, 1.61 to 2.05 mg/kg, 40.12 to 47.26%, 16.97 to 42.98%, respectively. The research data revealed that the control and T1–T9 meat samples showed total vitamin B reductions of 27.07, 8.86, 17.01, 10.19, 14.34, 7.23, 8.66, 6.64, 11.83, and 15.29%, respectively. Furthermore, the control and T1–T9 meat samples recorded vitamin E depreciation level 11.05, 5.80, 9.05, 6.90, 9.28, 8.87, 9.18, 4.65, 7.25 and 7.92%, respectively. Meanwhile, the protein content of the meat showed depreciation rates of 14.93, 9.49, 10.41, 8.89, 10.73, 10.22, 11.08, 5.12, 8.72, and 8.92% in the control and T1–T9 samples, respectively. Additionally, it was observed that the moisture level in the control sample was the highest at 84.62%. In contrast, the oil-based treatment samples T2, T4, and T9 exhibited the lowest moisture contents, measuring 63.94, 64.09, and 57.21%, respectively. Meanwhile, the extract-based treatment samples T1, T3, and T7 had average moisture contents of 87.99, 69.43, and 68.59%, respectively.
Notably, the control sample showed the greatest decline in vitamins and protein levels during storage; whereas, the sample treated with hybridized extracts (T7) experienced the least depreciation in B vitamins (6.64% depreciation rate) and protein content (5.11%). Similar to Turgut et al. (2017) observation, where pomegranate (Punica granatum) peels extract hinders beef protein oxidation storage. These findings present perfect insight into plant extracts and EOs antioxidants attributes, in effectively retarding vitamins and proteins degradation during storage (Fu et al., 2022; Petcu et al., 2023). The higher B vitamins degradation rate, compared to vitamin E in the preserved meat samples, can be associated with the distinct chemical properties of these two vitamins. B vitamins been water-soluble are more vulnerable to degradation when exposed to heat and light (Hrubša et al., 2022); whereas, Vitamin E which is a fat-soluble vitamin had elevated stability under high temperatures and UV light (Napolitano et al., 2019).
Additionally, it was observed that the moisture level in the control sample was the highest at 84.62%. In contrast, the oil-based treatment samples - T2, T4, and T9 exhibited the lowest moisture contents, measuring 63.94, 64.09, and 57.21%, respectively. Meanwhile, the extract-based treatment samples - T1, T3, and T7 had average moisture contents of 87.99, 69.43, and 68.59%, respectively. These findings highlight the effectiveness of the oil, in reducing moisture absorption by the meat compared to the extracts. Oils act as impermeable barrier, which slow down water migration, microbial growth and food decay (Umaru et al., 2019). The reduced moisture content, especially in T9 (57.21%), points to improved storage stability, as lower moisture typically correlates with a lower risk of microbial contamination and oxidation (Uguru et al., 2023). By retarding nutrient breakdown, these preservatives have helped to extend the shelf life of the treated meat samples.
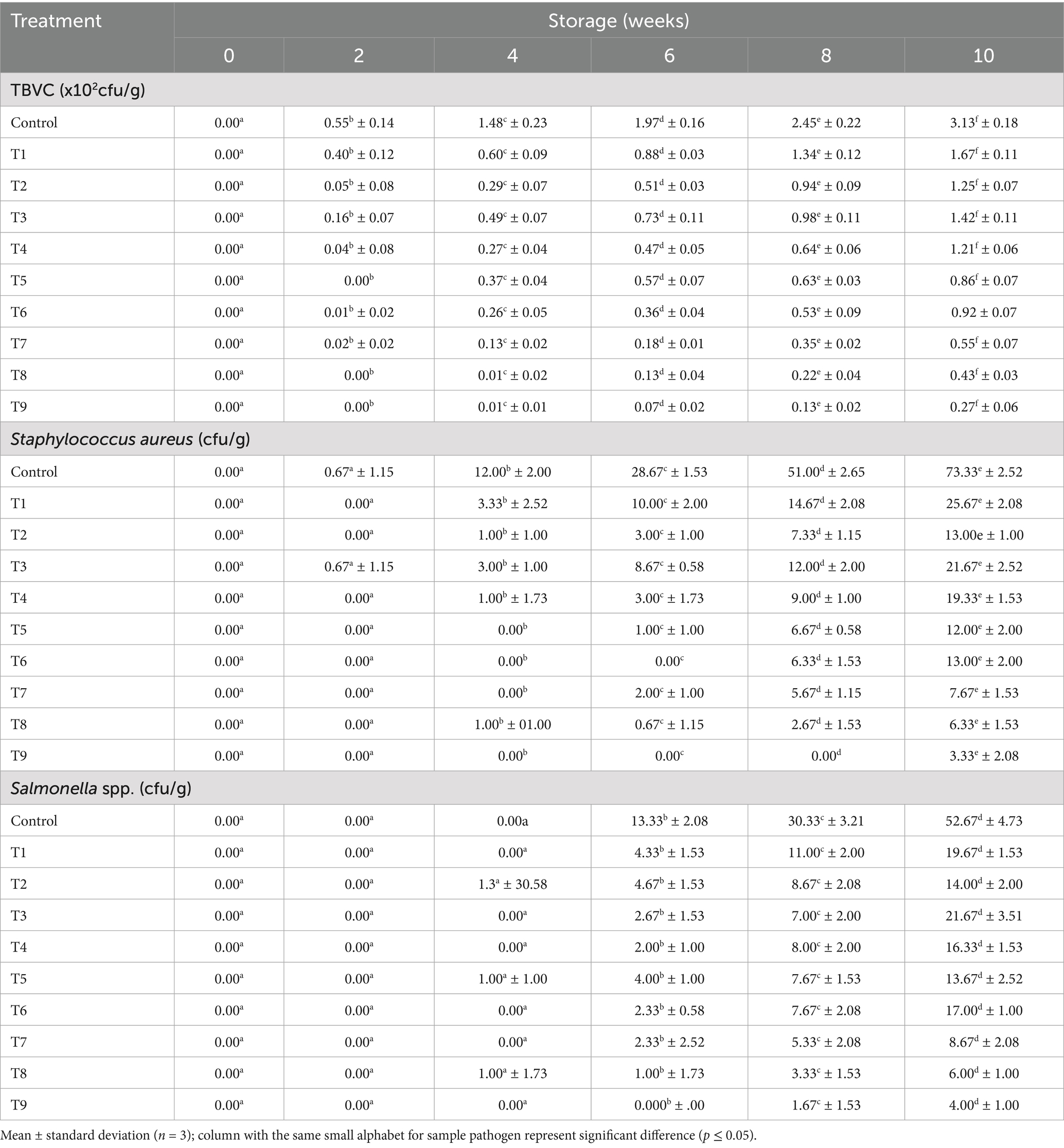
Microbial population evaluation
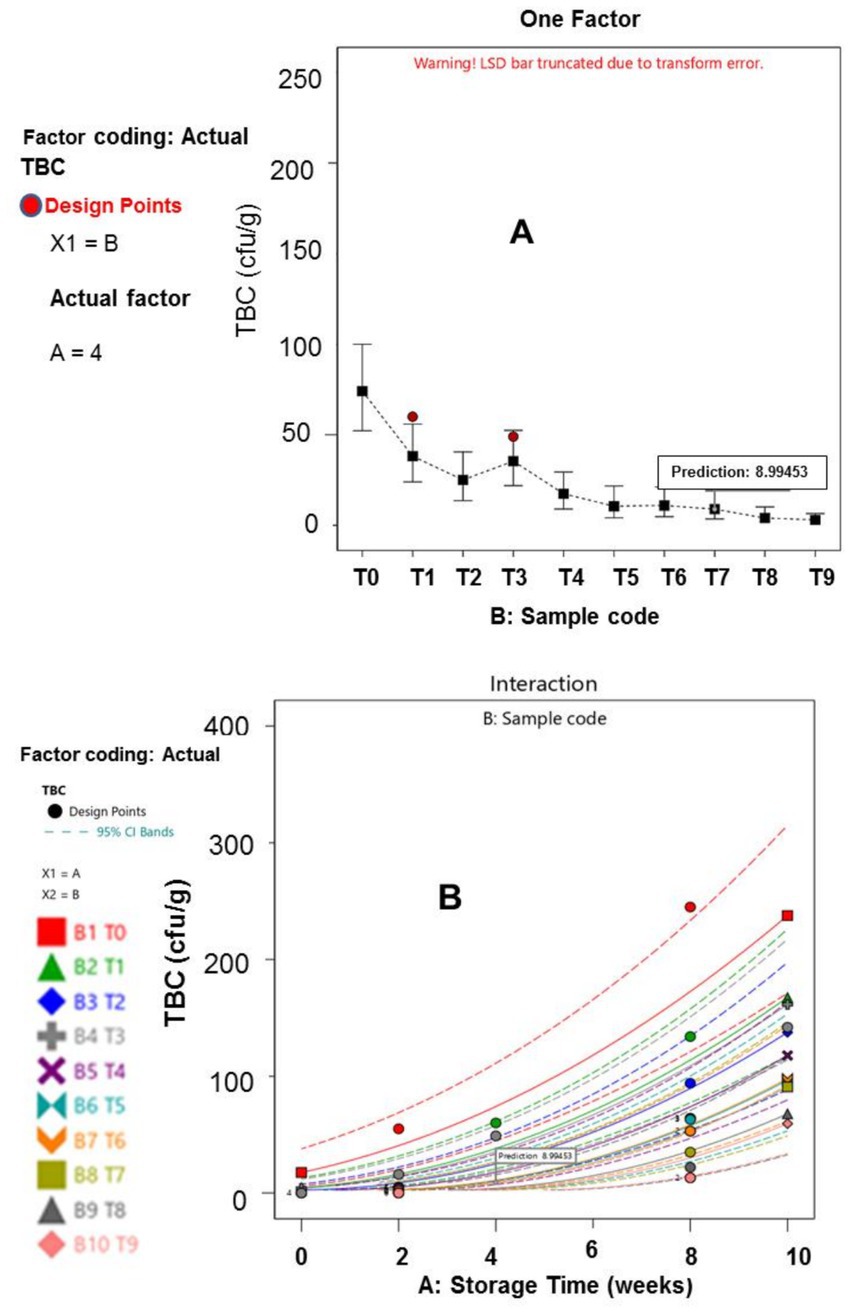
The microbiology results for the meat samples are presented in Tables 5, 6. The results highlight that both the storage duration and treatment plan, have significant effect on dried meat samples microbial growth (p ≤ 0.05). These findings confirmed earlier reports of these scholars (Gál et al., 2023; Ivane et al., 2024; Djenane et al., 2024), stating that EOs and extracts treatments, substantially retard microorganisms’ growth and survival. Notably, at the end of the experimental duration (tenth week), the total viable bacterial counts (TVBC) for the T1 to T9 samples were 167, 125, 142, 121, 86, 92, 55, 43, and 27 cfu/g, respectively; the Staphylococcus aureus populations in the T1 to T9 treated meat specimens were 25.67, 13.00, 21.67, 19.33, 12.00, 13.00, 7.67, 6.33 and 3.33 cfu/g, respectively; the Salmonella spp. counts in the T1 to T9 meat samples were 19.67, 14.00, 21.67, 16.33, 13.67, 17.00, 8.67, 6.00 and 4.00 cfu/g, respectively. In terms of the fungal contamination, the TFC recorded in the T1 – T9 samples were 134, 115, 106, 93, 92, 88, 52, 60 and 56 cfu/g, respectively; the afB1values among the treated samples ranged from 2.97 to 11.43 ppb, while the afB2 load varied from 2.60 to 10.17 ppb.
Generally, the mean samples treated with essential oils demonstrated better preservative attributes, than those treated with the extracts, similar to the findings of Ashraf et al. (2025). This study’s outcomes depicted that meat samples preserved using hybridized treatments (T3–T9), exhibited a longer shelf life and stability, compared to those samples preserved with single treatments (T1 and T2). The hybridization of onion waste and plantain peel EOs at a concentration of 0.5% each (T9), demonstrated effective control over bacterial and fungal performance, particularly against afB1 and afB2. This could be attributed to the broad-spectrum antimicrobial properties of the oils (Alirezalu et al., 2019; Kačániová et al., 2024). The lower Staphylococcus aureus, Salmonella spp., and aflatoxins populations, observed in T9 meat sample can be attributed to the consistently low moisture content, this specimen maintained throughout the storage period. Lower moisture levels create an inhospitable environment, for microbial growth and toxin production (Uguru et al., 2023; Kačániová et al., 2024). The statistical analysis revealed no significant difference in the populations of fungi, afB1, and afB2 between Weeks 0 and 2 (p ≤ 0.05), an indication that the fungal growth and aflatoxins has very poor functionality during the initial storage period, which can be linked to the immediate effect of the organic additives (Beya et al., 2021).
The zero microbial count recorded at the first storage day, affirmed that the meat primarily picked up pathogenic microorganisms during storage, through contaminated environment and atmospheric depositions (Pleadin et al., 2021; Uguru et al., 2024’ Djenane et al., 2024). Similarly, the rapid microbial growth observed after sixth week storage period, can be associated with the substantial decline in the meat’s antimicrobial and antioxidant properties over time; hence, creating favorable conditions for pathogen proliferation (Punia et al., 2021). Similar findings were documented by Mojaddar et al. (2018), when sumac (Rhus coriaria) extract was able to inhibit bacteria and fungi survivability. According to Lupia et al. (2024), due to the volatile nature of essential oils, they have greater ability of penetrating and disrupting microbial cellular structures, and metabolic functions, leading to enhance antimicrobial actions.
This experimental outcome aligned with the previous reports of these authors (Barbosa et al., 2009; Yuan and Yuk, 2018; Petcu et al., 2023; Kačániová et al., 2024; Ashraf et al., 2025), which stated that EOs and extracts derived from edible portions of peppermint (Mentha piperita L), lemongrass (Cymbopogon citratus), ginger (Zingiber officinale Roscoe), clove (Syzygium aromaticum) and Syzygium antisepticum, successfully retarded Salmonella spp., Staphylococcus aureus and Coliform reproduction in chicken meat stored inside the refrigeration for 12 days. This affirms that EOs and extracts produced from agricultural waste materials, rather than the comestible plant parts, can be effectively utilized for meat preservation, thereby combating food insecurity problem, as well as waste valorization.
The fungal analysis depicted that Aflatoxins B1 (afB1) and Aflatoxins (afB2) presence were relatively small in T5, T8, and T9 samples, which can be linked to anti aflatoxins properties (bioactive compounds) of the extract and oil (Algammal et al., 2021; Baker, 2023). Specifically, the study revealed that the optimization and treatment combination provided by the CCD model revealed that, plants waste materials can be processed into EO and extract, with the capability of retarding aflatoxins formation within permissible limits for human safety, established by the European Council (EU) and World Health Organization (WHO). The WHO and European council approved maximum aflatoxins acceptable level of 10 ppb and 4 ppb, respectively, for food items (Bradford et al., 2020; Uguru et al., 2023). These outcomes underscore the importance of further research into pharmaceutical (antimicrobial) impact of agricultural waste materials, thereby ratifying the efficacy of the natural antioxidants as bio-preservation approach.
Modeling and optimization of the meat quality during storage
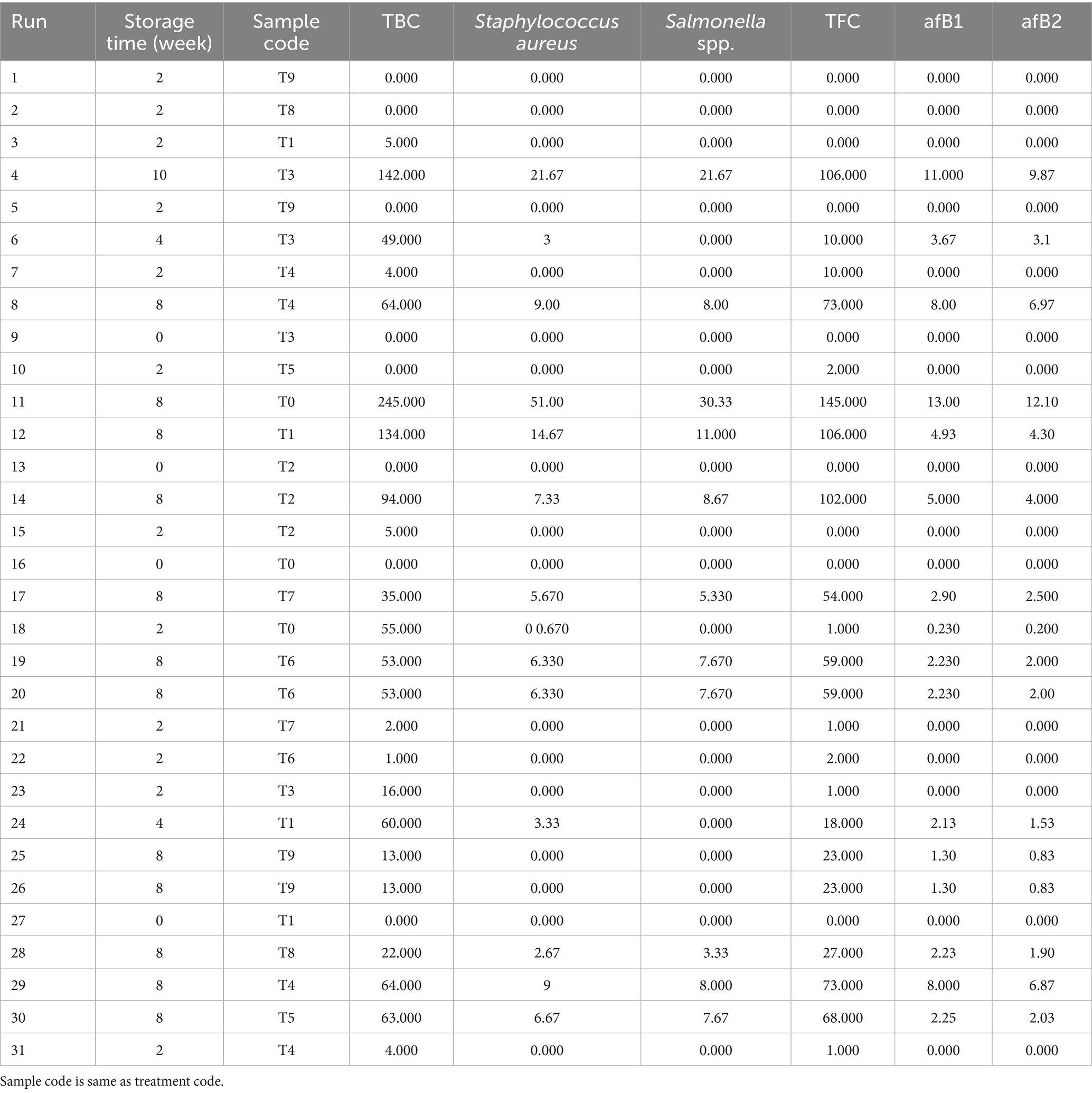
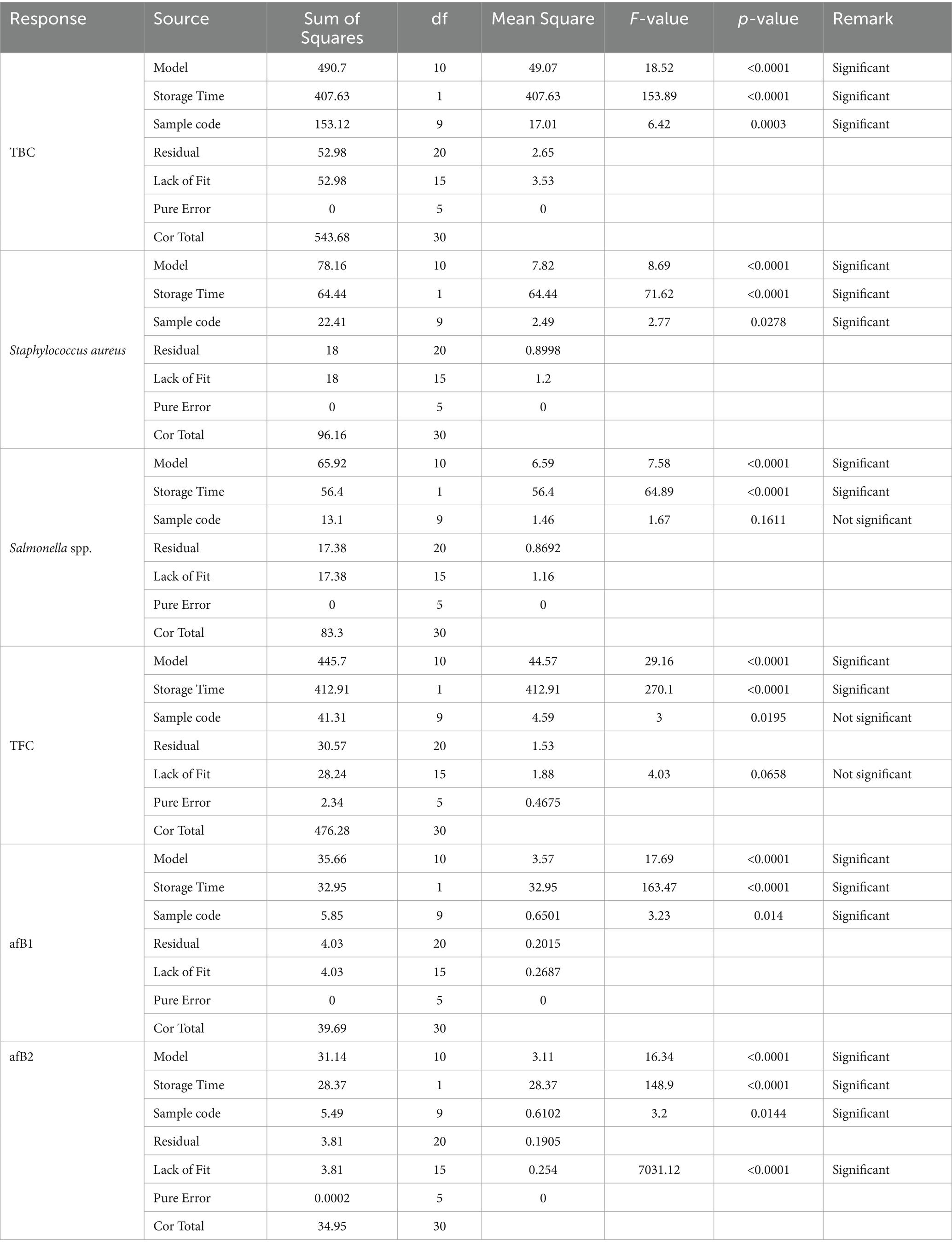
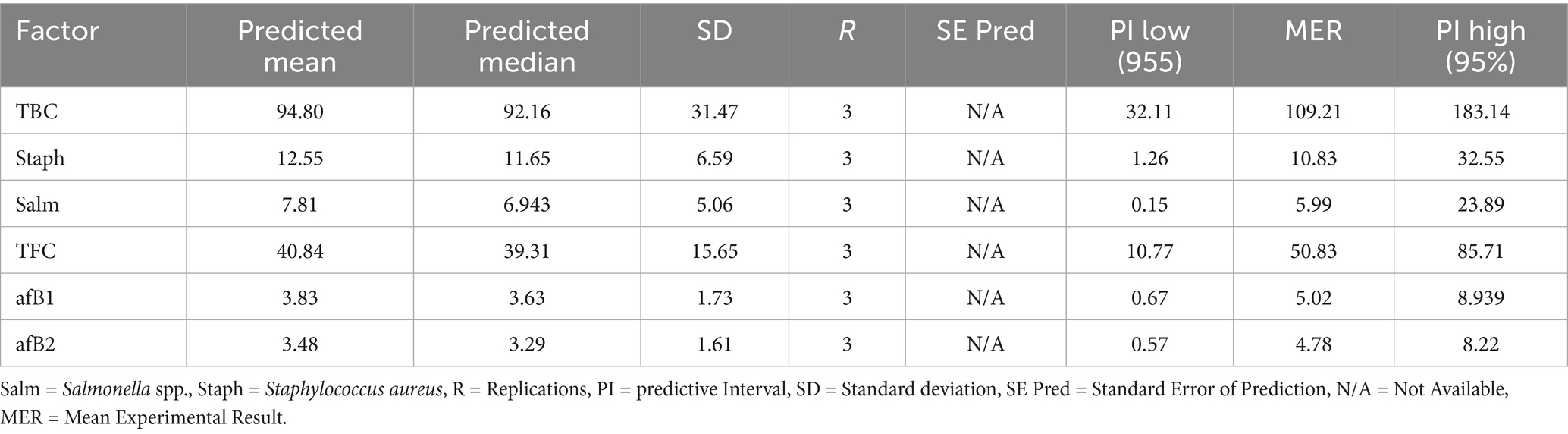
The results of the modeling and optimization of the meat storability, with respect to the treatment plans, were presented in Tables 7–9 and Supplementary Tables S1–S4. Table 7 displays the microbial results of the meat samples during storage for the 31 runs (based on Table 1), through the combination of these factors: treatment and storage duration. Table 8 shows the ANOVA values for response model and parameters. Staphylococcus spp., Salmonella spp. Aflatoxins B1 and B2 classified in the treated meat samples (Table 7) were active pathogenic microbes, which have been linked to serious foodborne diseases (Bradford et al., 2020; Petcu et al., 2023; Kačániová et al., 2024). The data shown that in experimental runs 1, 2, 5, 9, 10, 13, 16, and 27, regardless of the response investigated, the contamination level was 0.000 (0%), which is an indication that the particular treatment successfully inhibited the pathogen persistence, at that exact storage period. Furthermore, the optimal response surface result (Table 7) depicts that, the untreated experimental batch (T0), had the worst microbial prevalence and toxins formation; while T8 and T9 units were very persuasive in microbial suppression, further buttressing the worth of the treatment agents in meat preservation (Alirezalu et al., 2019; Ashraf et al., 2025). Based on the optimization outcomes, T9 had the optimal results, yielding almost 0% microbial growth and toxin formation after several storage weeks; though T6–T8 recorded appreciable anti- pathogenic actions, and T1–T3 demonstrated moderate antimicrobial management behaviors. Fascinatingly, the reliability evaluation outcomes performed with runs 19, 10, 25, and 26, affirmed the optimization model constancy.
Specifically, Supplementary Tables S1–S4 displayed the following - optimization model selection for responses, response model equations used for optimization, response models statistic and optimization solutions, respectively. The information provided by Supplementary Table S4 (optimization solutions) revealed that, the minimal values solutions can be achieved in serial numbers 5, 14, 19, and 23. This sustained the fact that the distill water used for this research, was devoid of microbial pollution. Additionally, Supplementary Table S4 pointed out that, some of the natural agents used for the meat treatment have traces of microbial contamination, which can be attributed to their mode of preparation and cross-contamination effects. Table 9 shows the constrain use for this study’s optimization action. The values in Table 9 provide safe and suitable boundaries, for the parameters investigated in this work, primarily by identifying the combinations–treatments and storage period, which will yield minimal pathogenic contamination. Also, the importance level (3) signifies moderate primacy meant for each restraint. These conditions facilitate the attainment of reliable storage and treatment results.
Furthermore, the results of the interactions between the responses, storage time and treatment plan are presented in Figures 1–6. The models and optimization Figures revealed that Treatments 5, 8, and 9 had the best inhibiting power against TBC survival; while T7 and T8 exhibited the optimal Staphylococcus and Salmonella spp. survival inhibition. Similarly, the results indicated that T8 and T9 effectively retard fungal performance within the specimens; while T6, T8 and T9, exhibited high efficiency against afB1 and afB2 survival. Regarding the stability and storability of the meat products, the Figures 5, 6 revealed the reproduction tracks of the microbial within the preserved meat samples, as the storage period progresses from week 0 to week 10. Generally, there was sharp increment in pathogenic microorganism’s performance, in the T0, T1, T2, T3, and T4 specimens, as storage progresses; which indicates that these treatments has poor preservation qualities. Also, the gentle slope curves, as reflected by T7, T8, and T9, are a sign of good microbial steadiness, irrespective of the microorganism examined in this research. Based on the treatments optimization outcomes, samples treated with T9 show the optimal microbial inhibition potential; though samples treated with Treatments 5 to 8, display good effectiveness in hindering microbial growth. Specifically, samples T5 to T9 can be considered to be more shelf-stable, due to the higher resistivity to microbial invasion, which can lead to food spoilage. The prediction and optimization outcomes established that, the treatments have different antimicrobial potencies. Therefore, some treatments were able to retard microbial resilience significantly, when compared to others, signifying superior microbial control. Conversely, the rapid surge in microbial growth in T0, recorded across microorganisms evaluated, suggested that the untreated meat product is more vulnerable to microbial contamination during storage.
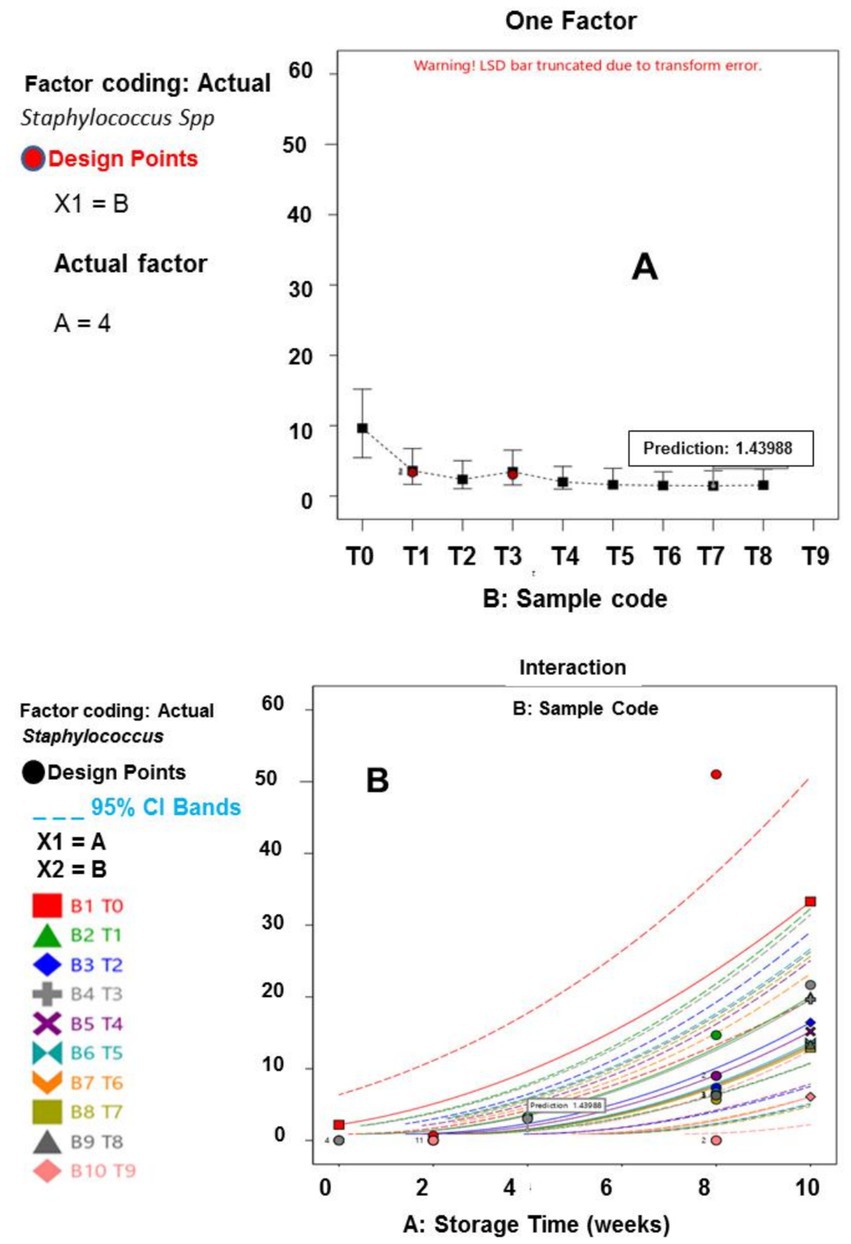
Figure 2. (A) Influence of Staphylococcus aureus across samples. (B) Influence of Staphylococcus aureus on storage duration across sample.
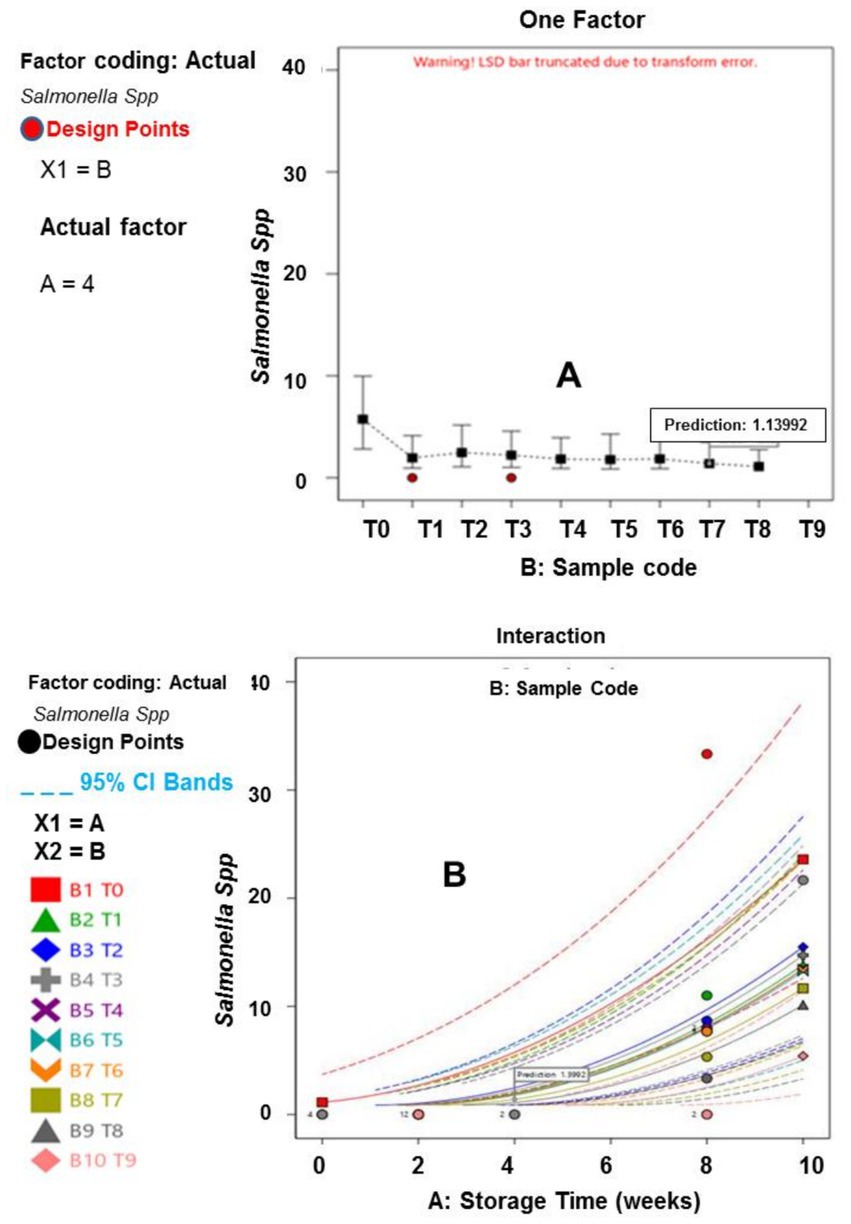
Figure 3. (A) Influence of Salmonella spp. across samples. (B) Influence of Salmonella spp. on Storage time across sample.
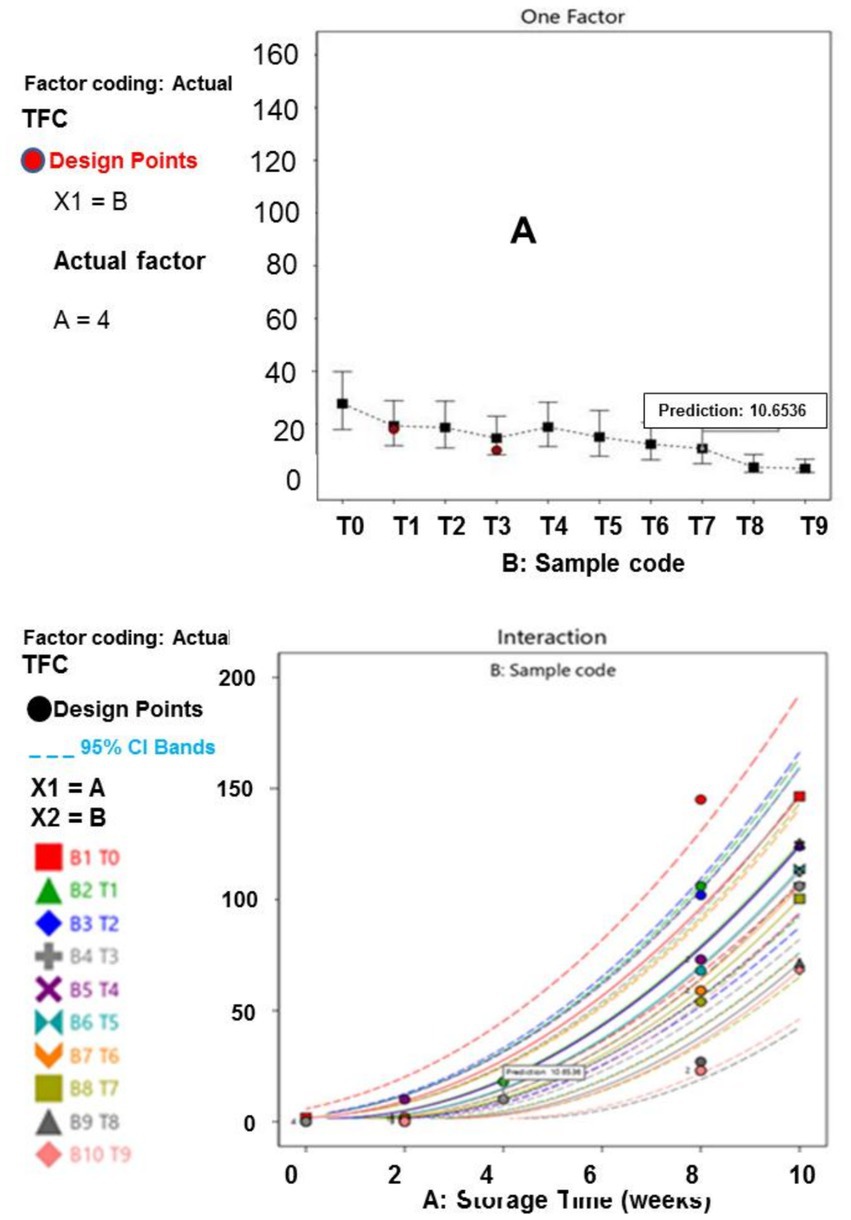
Figure 4. (A) Inspiration of TFC across samples. (B) Effect of TFC on storage duration across sample.
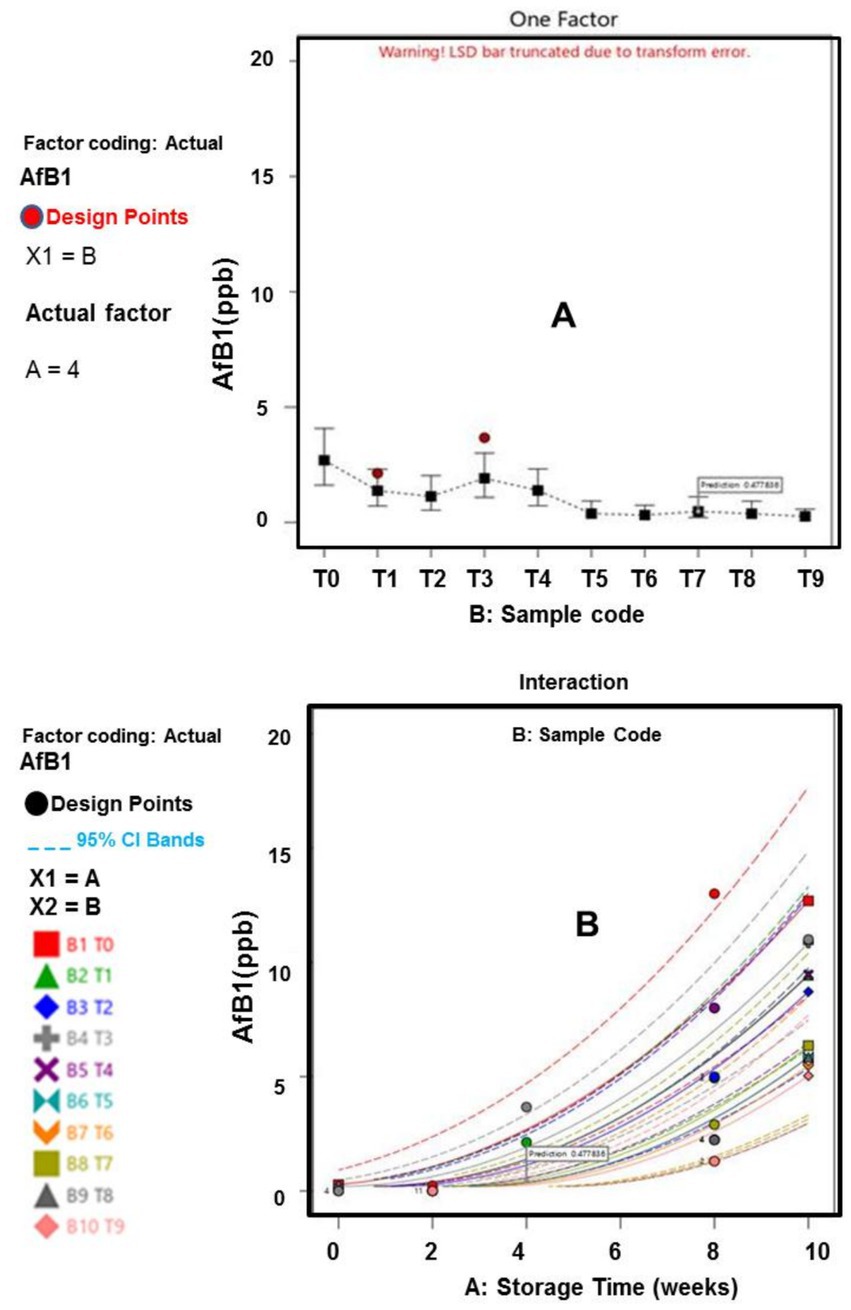
Figure 5. (A) Influence of afB1 across samples; (B) Influence of afB1 on Storage time across sample.
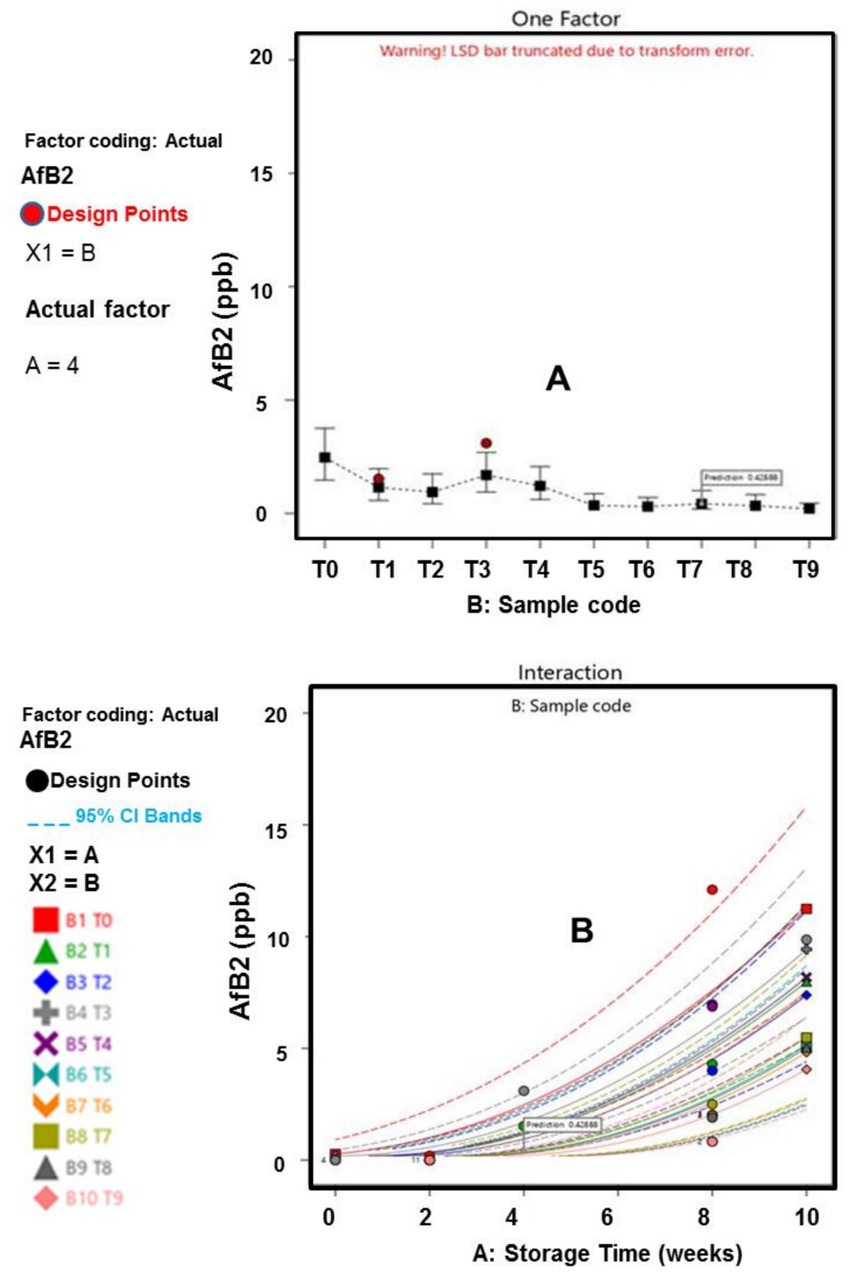
Figure 6. (A) Influence of afB2 across samples. (B) Influence of afB2 on Storage time across sample.
Confirmation of the model created and the optimization process
The result of the confirmation test preformed on the model is presented in Table 10. Validation test is necessary in optimization process, as it helps to authenticate the consistency and reproducibility of the outcomes documented from the experimental procedures (Teja et al., 2023); hence, boosting the assurance level of the optimized final results. Table 10 establishes that all the authentication experimental outcomes, were within the 95% prediction ranges, portraying substantial microbial growth inhibition, and high functionality of the model used. Categorically, it can be seen that the system has high predictive precision, and does not require further improvement, since the microbial burden documented by the validation exercise, did not significantly surpassed the model expectations.
Conclusion
Food insecurity resulting from nutrient degradation and microbial contamination during storage is indeed a significant challenge for the food industry. This study was conducted to optimize the utilization of natural additives, such as onion bulb peel oil (OPO), plantain peel oil (PPO), onion peel extract (OPE), and plantain peel extract (PPE), in enhancing meat quality and inhibit microbial growth in beef during storage. The hybridized effect of these process variables–treatment concentration and storage time, on the microbial reproductively and toxins production was comprehensively explored. The experimental outcomes revealed that the plant extracts (OPE and PPE), effectively slowed down nutrients degradation during storage; while the essential oils (OPO and PPO) demonstrated strong efficacy in inhibiting pathogens survival. The findings confirmed that moisture content significantly promotes microbial survival. The survival of aflatoxins, Staphylococcus aureus, and Salmonella spp. was negatively affected by OPO and PPO, when applied at a concentration of 1% (w/w). Precisely, the optimization program successfully pinpoints the treatments options, which are the most suitable for meat preservation, with minimum spoilage possibilities. It was noted that T9 had the best microbial inhibition potential; while the T1 to T3 samples were vulnerable to microbial infestation, and aflatoxins production. Furthermore, the predictive modeling analysis established the optimal of EOs and extracts concentrations for maximal stabilizing efficiency. Notably, this study outcome emphasizes that EOs and extracts derived from agricultural by-products, instead of the edible parts can be effectively used to preserve meat integrity during storage, thereby conserving the public health safety and enhancing human performance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SA: Methodology, Software, Writing – original draft. RS: Conceptualization, Methodology, Writing – review & editing. HU: Data curation, Methodology, Writing – review & editing. RB: Methodology, Supervision, Writing – review & editing. AMA: Investigation, Methodology, Writing – original draft. SuAA: Formal Analysis, Methodology, Writing – review & editing. RK: Methodology, Software, Writing – original draft. BA: Investigation, Methodology, Writing – original draft. EA: Methodology, Resources, Writing – review & editing. AAA: Data curation, Methodology, Writing – review & editing. SSA: Methodology, Supervision, Writing – original draft. SaAA: Investigation, Methodology, Writing – original draft. DA: Methodology, Project administration, Writing – review & editing. FA: Methodology, Software, Writing – original draft. AB: Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Taif University, Saudi Arabia, Project No. (TU-DSPP-2024-10).
Acknowledgments
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2025.1626489/full#supplementary-material
References
Albawarshi, Y., Amr, A., Al-Ismail, K., Shahein, M., Majdalawi, M., Saleh, M., et al. (2022). Simultaneous determination of B1, B2, B3, B6, B9, and B12 vitamins in premix and fortified flour using HPLC/DAD: effect of detection wavelength. J. Food Qual. 2022, 1–11. doi: 10.1155/2022/9065154
Algammal, A. M., Elsayed, M. E., Hashem, H. R., Ramadan, H., Sheraba, N. S., El-Diasty, E. M., et al. (2021). Molecular and HPLC-based approaches for detection of aflatoxin B1 and ochratoxin a released from toxigenic Aspergillus species in processed meat. BMC Microbiol. 21, 1–13. doi: 10.1186/s12866-021-02144-y
Al-Hijazeen, M. (2022). The combination effect of adding rosemary extract and oregano essential oil on ground chicken meat quality. Food Sci. Technol. 42, 1–14. doi: 10.1590/fst.57120
Alirezalu, K., Hesari, J., Nemati, Z., Munekata, P. E. S., Barba, F. J., and Lorenzo, J. M. (2019). Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 120, 839–850. doi: 10.1016/j.foodres.2018.11.048
Ashraf, N., Arshad, Z., Sami, R., Almehmadi, A. M., Alsanei, W. A., Bedaiwi, R. I., et al. (2025). Effect of peppermint essential oil and ultrasonication on microbiology evaluation and quality parameters of stored chicken meat. Front. Sustain. Food Syst. 9, 1552570–1552585. doi: 10.3389/fsufs.2025.1552570
Baker, I. A. (2023). Study on the effectiveness of adding some plant extracts with BHT on meat quality of lamb patties during chilling storage. Iraqi J. Agric. Sci. 54, 147–155. doi: 10.36103/ijas.v54i1.1686
Barbosa, L. N., Rall, V. L. M., Fernandes, A. A. H., Ushimaru, P. I., Da Silva Probst, I., and Fernandes, A. (2009). Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 6, 725–728. doi: 10.1089/fpd.2009.0282
Beya, M. M., Netzel, M. E., Sultanbawa, Y., Smyth, H., and Hoffman, L. C. (2021). Plant-based phenolic molecules as natural preservatives in comminuted meats: a review. Antioxidants (Basel, Switzerland) 10, 263–278. doi: 10.3390/antiox10020263
Bradford, K. J., Dahal, P., Van Asbrouck, J., Kunusoth, K., Bello, P., Thompson, J., et al. (2020). The dry chain: reducing postharvest losses and improving food safety in humid climates. Food Industry Wastes 71, 375–389. doi: 10.1016/B978-0-12-817121-9.00017-6
Djenane, D., Khaled, B. M., Ben Miri, Y., Metahri, M. S., Montañés, L., Aider, M., et al. (2024). Improved functionality, quality, and shelf life of merguez-type camel sausage fortified with spirulina as a natural ingredient. Food Secur. 14, 59–77. doi: 10.3390/foods14010059
Ekwomadu, T., Mwanza, M., and Musekiwa, A. (2022). Mycotoxin-linked mutations and cancer risk: a global health issue. IJERPH 19, 7754–7781. doi: 10.3390/ijerph19137754
Enciso-Martínez, Y., Zuñiga-Martínez, B. S., Ayala-Zavala, J. F., Domínguez-Avila, J. A., González-Aguilar, G. A., and Viuda-Martos, M. (2024). Agro-industrial by-products of plant origin: therapeutic uses as well as antimicrobial and antioxidant activity. Biomol. Ther. 14, 762–779. doi: 10.3390/biom14070762
Fu, Q., Song, S., Xia, T., and Wang, R. (2022). Effects of cherry (Prunus cerasus L.) powder addition on the physicochemical properties and oxidation stability of Jiangsu-type sausage during refrigerated storage. Food Secur. 11, 3590–3612. doi: 10.3390/foods11223590
Gál, R., Čmiková, N., Kačániová, M., and Mokrejš, P. (2023). Sage essential oil as an antimicrobial agent against Salmonella Enterica during beef sous vide storage. Food Secur. 12, 4172–4193. doi: 10.3390/foods12224172
Gruber-Dorninger, C., Jenkins, T., and Schatzmayr, G. (2019). Global mycotoxin occurrence in feed: a ten-year survey. Toxins 11, 375–391. doi: 10.3390/toxins11070375
Hrubša, M., Siatka, T., Nejmanová, I., Vopršalová, M., Kujovská Krčmová, L., Matoušová, K., et al. (2022). Biological properties of vitamins of the B-complex, part 1: vitamins B1, B2, B3, and B5. Nutrients 14, 484–503. doi: 10.3390/nu14030484
Ivane, N. M. A., Wang, W., Ma, Q., Wang, J., Liu, Y., and Sun, J. (2024). Antioxidative effects of incorporated ginger peel extracts on beef patties subjected to refrigerated storage. Food Human. 2, 100251–100277. doi: 10.1016/j.foohum.2024.100251
Kačániová, M., Čmiková, N., Kluz, M. I., Akacha, B. B., Saad, R. B., and Mnif, W. (2024). Anti-Salmonella activity of Thymus serpyllum essential oil in sous vide cook–chill rabbit meat. Food Secur. 13, 200–221. doi: 10.3390/foods13020200
Kahraman, H. A., and Tutun, H. (2021). Monitoring of aflatoxin B1 in dry aged meats using a modified HPLC-FLD method. Rom. Biotechnol. Lett. 26, 2885–2891. doi: 10.25083/rbl/26.4/2885-2891
Kunová, S., Sendra, E., Hašˇcík, P., Vukovic, N. L., Vukic, M., and Kǎcániová, M. (2021). Influence of essential oils on the microbiological quality of fish meat during storage. Animals 11, 3145–3162. doi: 10.3390/ani11113145
Lisboa, H. M., Pasquali, M. B., dos Anjos, A. I., Sarinho, A. M., de Melo, E. D., Andrade, R., et al. (2024). Innovative and sustainable food preservation techniques: enhancing food quality, safety, and environmental sustainability. Sustain. For. 16, 8223–8248. doi: 10.3390/su16188223
Lupia, C., Castagna, F., Bava, R., Naturale, M. D., Zicarelli, L., Marrelli, M., et al. (2024). Use of essential oils to counteract the phenomena of antimicrobial resistance in livestock species. Antibiotics (Basel, Switzerland) 13, 163–187. doi: 10.3390/antibiotics13020163
Mandal, M. K., and Domb, A. J. (2024). Antimicrobial activities of natural bioactive polyphenols. Pharmaceutics 16, 718–733. doi: 10.3390/pharmaceutics16060718
Mojaddar, L. A., Tajik, H., Mehdizadeh, T., Moradi, M., Moghaddas Kia, E., and Mahmoudian, A. (2018). Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss oil on the shelf-life of meat in modified atmosphere packaging. LWT 98, 372–380. doi: 10.1016/j.lwt.2018.08.063
Napolitano, G., Fasciolo, G., Di Meo, S., and Venditti, P. (2019). Vitamin E supplementation and mitochondria in experimental and functional hyperthyroidism: a Mini-review. Nutrients 11, 2900–2921. doi: 10.3390/nu11122900
Paswan, R., and Park, Y. W. (2020). Survivability of Salmonella and Escherichia coli O157: H7 pathogens and food safety concerns on commercial powder milk products. Dairy 1, 189–201.
Pateiro, M., Barba, F. J., Domínguez, R., Sant’Ana, A. S., Mousavi Khaneghah, A., Gavahian, M., et al. (2018). Essential oils as natural additives to prevent oxidation reactions in meat and meat products: a review. Food Res. Int. 113, 156–166. doi: 10.1016/j.foodres.2018.07.014
Pateiro, M., Munekata, P. E. S., Sant’Ana, A. S., Domínguez, R., Rodríguez-Lázaro, D., and Lorenzo, J. M. (2021). Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 337:108966. doi: 10.1016/j.ijfoodmicro.2020.108966
Pawlak, K., and Kołodziejczak, M. (2020). The role of agriculture in ensuring food security in developing countries: considerations in the context of the problem of sustainable food production. Sustain. For. 12, 5488–5495. doi: 10.3390/su12135488
Petcu, C. D., Tăpăloagă, D., Mihai, O. D., Gheorghe-Irimia, R. A., Negoiță, C., Georgescu, I. M., et al. (2023). Harnessing natural antioxidants for enhancing food shelf life: exploring sources and applications in the food industry. Foods (Basel, Switzerland) 12:3176. doi: 10.3390/foods12173176
Pleadin, J., Lešić, T., Milićević, D., Markov, K., Šarkanj, B., Vahčić, N., et al. (2021). Pathways of mycotoxin occurrence in meat products: a review. PRO 9, 2122–2143. doi: 10.3390/pr9122122
Prakash, B., Kedia, A., Mishra, P. K., and Dubey, N. K. (2015). Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of Agri-food commodities – potentials and challenges. Food Control 47, 381–391. doi: 10.1016/j.foodcont.2014.07.023
Punia, B. S., Chaudhary, V., Thakur, N., Kajla, P., Kumar, M., and Trif, M. (2021). Natural antimicrobials as additives for edible food packaging applications: a review. Foods (Basel, Switzerland) 10, 2282–2306. doi: 10.3390/foods10102282
Qin, P., Wang, T., and Luo, Y. (2022). A review on plant-based proteins from soybean: health benefits and soy product development. J. Agric. Food Res. 7, 100265–100282. doi: 10.1016/j.jafr.2021.100265
Ruxton, C. H. S., and Gordon, S. (2024). Animal board invited review: the contribution of red meat to adult nutrition and health beyond protein. Animal 18, 101103–101125. doi: 10.1016/j.animal.2024.101103
Sayed, A., and Elsharkawy, E. (2021). Incidence of aflatoxins in local and imported canned beef consumed in Sohag City, upper Egypt. Alex. J. Vet. Sci. 70, 80–97. doi: 10.5455/ajvs.94911
Sheffield, S., Fiorotto, M. L., and Davis, T. A. (2024). Nutritional importance of animal-sourced foods in a healthy diet. Front. Nutr. 11, 1015–1032. doi: 10.3389/fnut.2024.1424912
Stadnik, J. (2024). Nutritional value of meat and meat products and their role in human health. Nutrients 16, 1446–1461. doi: 10.3390/nu16101446
Teja, G. S., Archana, D., Srinu, B., Ali, S. K. A., Reddy, S. S. N., Parvez, S. K., et al. (2023). A comprehensive guide for analytical method validation. Int. J. Pharm. Sci. Rev. Res. 82, 5–17. doi: 10.47583/ijpsrr.2023.v82i02.002
Turgut, S. S., Işıkçı, F., and Soyer, A. (2017). Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage. Meat Sci. 129, 111–119. doi: 10.1016/j.meatsci.2017.02.019
Uguru, H., Akpokodje, O. I., Essaghah, A. E., Aljaadi, A. M., Sami, R., Aljahani, A. H., et al. (2023). Toxic metals and aflatoxins occurrence in smoked-dried fish and their health risks assessment. Mater. Express 13, 316–326. doi: 10.1166/mex.2023.2343
Uguru, H., Efeoghene, E. A., Akpokodje, O. I., Sami, R., Baakdah, F., and Pareek, S. (2024). Exposure to airborne pollutants in urban and rural areas: levels of metals and microorganisms in PM10 and gaseous pollutants in ambient air. Air Quality Atmosphere Health 2024, 1–16. doi: 10.1007/s11869-024-01644-w
Umaru, I. J., Badruddin, F. A., and Umaru, H. A. (2019). Phytochemical screening of essential oils and antibacterial activity and antioxidant properties of Barringtonia asiatica (L) leaf extract. Biochem. Res. Int. 2019, 7143989–7143997. doi: 10.1155/2019/7143989
Unar, N. D. (2022). Efficacy of some plant extracts in extending keeping quality of beef patties during storage. Pure Appl. Biol. 11, 271–295. doi: 10.19045/bspab.2022.110035
Yuan, W., and Yuk, H. G. (2018). Antimicrobial efficacy of Syzygium antisepticum plant extract against Staphylococcus aureus and methicillin-resistant S. Aureus and its application potential with cooked chicken. Food Microbiol. 72, 176–184. doi: 10.1016/j.fm.2017.12.002
Keywords: aflatoxins, food security, meat products, microbial invasion, public health
Citation: Alharthi S, Sami R, Uguru H, Bedaiwi RI, Almehmadi AM, Abushal SA, Kadi RH, Aljehany BM, Abduljawad EA, Aljehani AA, Aggad SS, Alasmari SA, Alkhudhayri DA, Alsulaimani F and Basri AM (2025) Modeling and optimization of essential oils and extracts for meat quality evaluation during storage, with high-performance liquid chromatography analysis of aflatoxin levels. Front. Sustain. Food Syst. 9:1626489. doi: 10.3389/fsufs.2025.1626489
Edited by:
Laurent Dufossé, Université de la Réunion, FranceReviewed by:
Nada Benajiba, Ibn Tofail University, MoroccoAhmet Hulusi Dinçoğlu, Mehmet Akif Ersoy University, Türkiye
Wassila Benabderrahmane, University of Skikda, Algeria
Copyright © 2025 Alharthi, Sami, Uguru, Bedaiwi, Almehmadi, Abushal, Kadi, Aljehany, Abduljawad, Aljehani, Aggad, Alasmari, Alkhudhayri, Alsulaimani and Basri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rokayya Sami, cm9rYXl5YS5kQHR1LmVkdS5zYQ==; Sarah Alharthi, c2FyYWguYWxoYXJ0aGlAdHUuZWR1LnNh
 Sarah Alharthi1,2*
Sarah Alharthi1,2* Rokayya Sami
Rokayya Sami Hilary Uguru
Hilary Uguru Ruqaiah I. Bedaiwi
Ruqaiah I. Bedaiwi Buthaina M. Aljehany
Buthaina M. Aljehany Eman A. Abduljawad
Eman A. Abduljawad Abeer A. Aljehani
Abeer A. Aljehani Dalal A. Alkhudhayri
Dalal A. Alkhudhayri