- 1Regional Centre of Agricultural Research of Sidi Bouzid, Sidi Bouzid, Tunisia
- 2Laboratory of Agriculture Production Systems and Sustainable Development (LR03AGR02), Department of Agricultural Production, Higher School of Agriculture of Mograne, University of Carthage, Zaghouan, Tunisia
- 3Department of Environmental Management, Institute of Environmental Engineering, RUDN University, Moscow, Russia
This study provides the first comprehensive evaluation of the efficacy of three Trichoderma species (Trichoderma longibrachiatum, Trichoderma harzianum, and Trichoderma asperellum) in controlling Curvularia spicifera on tomato plants under both in vitro and in vivo conditions. Laboratory-based experiments assays, including direct and indirect confrontation, application of culture filtrates, and inhibition of spore germination, demonstrated significant antagonistic activity by the Trichoderma species. These treatments markedly reduced the mycelial growth (<2.63 cm), mycelial growth rate (<1.28 mm/h), and spore germination (<0.40) of C. spicifera, with T. longibrachiatum exhibiting the strongest antagonistic effect. The efficacy of three Trichoderma spp. and salicylic acid was evaluated under greenhouse conditions. Greenhouse trials further confirmed that T. longibrachiatum (2.83) significantly reduced disease severity compared to the control inoculated with C. spicifera (5.50) at 90 days post-inoculation (dpi). Biochemical analysis revealed an increase in enzyme activity and total protein content in the leaves and roots of Trichoderma-treated plants, with values of 10.09 and 10.44 mg g−1, respectively. These changes reflect an induced defense response. Specifically, T. longibrachiatum consistently induced higher activities of catalase (74.58 and 73.1 μmol H2O2 mg protein−1, respectively), peroxidase (5.35 and 54.91 μmol mg−1 min−1, respectively), ascorbate peroxidase (54.91 and 60.29 μmol mg−1 min−1, respectively), and polyphenol oxidase (14.07 and 9.37 units mg−1 min−1, respectively) in tomato leaves and roots at 90 dpi. Furthermore, T. longibrachiatum significantly enhanced chlorophyll content and other agronomic traits, including root and shoot biomass, fruit yield, and overall plant growth. These findings suggest that T. longibrachiatum is a promising biocontrol agent against C. spicifera in tomato plants, promoting both plant growth and the activation of defense mechanisms.
1 Introduction
Food security remains one of the most pressing challenges facing humanity today. According to the Food and Agriculture Organization (FAO), food security encompasses not only access to food but also its nutritional quality and safety. To address this challenge, ecological farming systems have gained increasing attention in recent years (Muhie, 2022). These systems integrate a range of environmentally sustainable agricultural practices, including the cultivation of high-yielding and stress-resistant crop varieties, integrated pest and disease management, the application of biological fertilizers, and other agroecological approaches that collectively support both productivity and food safety (Temirbekova et al., 2021; Rebouh et al., 2019; Kherif et al., 2021).
Among these practices, integrated pest and disease management (IPDM) is of particular importance, as it seeks to replace or reduce chemical inputs with effective biological alternatives (Deguine et al., 2021; Rebouh et al., 2020). However, the current efficacy of IPDM still requires substantial improvement to match the performance of conventional chemical-based methods (Williams et al., 2005). Therefore, the development and implementation of novel biological agents for the control of pests and diseases is both timely and of high scientific and practical relevance.
Tomato (Solanum lycopersicum L.) is among the most valuable crops worldwide, representing a plant of important nutritional and economic interest for agricultural systems (Simoglou et al., 2024). However, tomato crops are often threatened by a wide variety of fungal pathogens, among which one of the most aggressive agents is the fungus C. spicifera, causing leaf spot disease (Cui et al., 2020; Baral et al., 2022). This pathogen is highly virulent, causing foliar symptoms such as leaf spot and blight that result in significant yield losses, lower quality fruits, and higher production costs for farmers (Manzar et al., 2022; Rabaaoui et al., 2022). The impact of C. spicifera on plants is further enhanced by it’s the preference for warm, and usually humid conditions, which are typically characteristic of many tomato-producing regions (Connally et al., 2022).
The chemical management of C. spicifera and similar phytopathogens has traditionally depended on the widespread use of synthetic fungicides. While these chemical agents can offer rapid and effective suppression of disease symptoms, their long-term application presents several critical challenges. Continuous and excessive use of fungicides contributes to environmental contamination, including the accumulation of toxic residues in soil and water bodies (Manjarres-Lopez et al., 2021), which disrupts ecological balance and negatively impacts soil microbiota (Wang et al., 2025). Furthermore, the selective pressure exerted by repeated fungicide applications accelerates the evolution of fungicide-resistant strains of pathogens, rendering these chemicals progressively less effective (Ishii, 2006). In addition to ecological concerns, there are increasing apprehensions regarding human health and the safety of non-target organisms exposed to fungicide residues through food chains or environmental contact (Tao et al., 2020; Pathak et al., 2022).
As a result of these growing concerns, biological control has emerged as a promising and sustainable alternative for plant disease management. Among various strategies, the use of antagonistic microorganisms such as Trichoderma spp., Bacillus spp., and Pseudomonas fluorescens has received particular attention due to their capacity to inhibit plant pathogens through mechanisms like mycoparasitism, competition, production of antimicrobial compounds, and induction of host plant resistance (Ojha and Chatterjee, 2011; Güçlü and Özer, 2022; Riera et al., 2023). These biocontrol agents offer a safer, more ecologically harmonious approach, aligning with the principles of integrated pest management and sustainable agriculture (Al-Shuaibi et al., 2024; Köhl et al., 2019).
Among these microorganisms, Trichoderma species have emerged as effective biocontrol agents against various tomato diseases, particularly those caused by soil-borne pathogens like Fusarium oxysporum f. sp. lycopersici (Jamil, 2021), F. solani (Shams et al., 2023), and Phytophthora nicotianae (Dini et al., 2021). It has been reported that species belonging to Trichoderma spp. employ various mechanisms for the biocontrol of plant pathogens, including competition for nutrients and space, production of antifungal metabolites, direct mycoparasitism, and the induction of systemic resistance in host plants (Behiry et al., 2023; Chávez-Avilés et al., 2024; Hernández et al., 2024; Huang et al., 2024). These attributes make Trichoderma spp. highly effective in controlling fungal pathogens, while promoting plant growth and enhancing overall crop health (Mahmoud et al., 2021). However, despite their potential for managing many plant diseases, the efficacy of Trichoderma spp. against C. spicifera has been scarcely explored in previous research. Furthermore, salicylic acid’s effectiveness extends to direct antifungal actions, contributing to plant resistance against a spectrum of fungal pathogens. There is limited research investigating the efficacy of Trichoderma spp. against C. spicifera under in vitro conditions, and to date, no studies have evaluated their effectiveness under in vivo conditions (Rao et al., 2020). This knowledge gap need for focused research efforts to evaluate the potential of Trichoderma spp. as a biocontrol agent against this pathogen.
Given the demonstrated efficacy of Trichoderma spp. in controlling C. spicifera and other phytopathogens under in vitro conditions, we hypothesize that these species may also suppress C. spicifera under field conditions, while concurrently improving tomato yield and quality. Thus, the present study investigated the ability of Trichoderma spp. to control C. spicifera in tomato plants both in vitro and in vivo conditions, with the aim of developing an effective, sustainable, and ecologically safe approach for disease management. Additionally, the study determined the mode of action through which Trichoderma spp. antagonize C. spicifera, suppress disease, and promote plant growth, with a view to establishing a complete understanding of its biocontrol potentials. The successful application of Trichoderma spp. in controlling C. spicifera could contribute to improved tomato yields, improved fruit quality, and enhanced economic stability for farmers. The study finally stands in line with the rise of sustainable agriculture worldwide, offering durable practice to one of the big challenges in tomato cultivation while supporting more general objectives of food security and environmental conservation (Bouanaka et al., 2021; Dourou and La Porta, 2023; Ferreira et al., 2024). Therefore, the aim of this study was to evaluate the antagonistic and antifungal potential of three Trichoderma species against Curvularia spicifera, a pathogen associated with gray mold in tomato. In addition, the study assessed the effectiveness of Trichoderma spp. and salicylic acid under greenhouse conditions to manage disease severity and promote plant health.
2 Materials and methods
2.1 Fungal strains
Three Trichoderma species isolates, T. longibrachiatum (Tr1), T. harzianum (Tr2), and T. asperellum (Tr3), obtained from the Plant Protection and Biological Sciences laboratory at the Regional Center of Agricultural Research of Sidi Bouzid, Tunisia. Trichoderma species were isolated previously from the rhizosphere of tomato plants. The three isolates (Tr1, Tr2, and Tr3) were submitted to GenBank and assigned under the accession numbers: OP799680, OP799678, OP799679, respectively (Hajji-Hedfi et al., 2023a).
The phytopathogen fungus, C. spicifiera was isolated from tomato fruits exhibiting symptoms of gray mold disease and maintained on Potato Dextrose Agar (PDA) medium at 25 ± 2°C for subsequent tests. Macroscopic and microscopic observation were performed to identify the fungi using a colony appearance and morphological keys as described by Ellis (1971) and Sivanesan (1987) for the Curvularia genus. Pure fungal cultures were preserved in 20% glycerol and then stored at −20°C.
2.2 Molecular identification
To confirm pathogen species identity, molecular identification was performed. DNA extraction was performed according to the method described by White et al. (1990), and using the universal primers ITS1 (5´-TCCGTAGGTGAACCT TGCGG-3′) and ITS4 (5´-TCC TCCGCTTATTGATATGC-3′). PCR cycling conditions were as follows: initial denaturation at 94°C for 1 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, 72°C for 1 min, and then a final extension at 72°C for 10 min, according to Glass and Donaldson (1995). All PCR products were separated by electrophoresis on a 1.5% agarose gel, stained with SYBR Safe DNA Gel Stain (Invitrogen, Carlsbad, CA, United States), and visualized under UV illumination. Three positive amplified PCR products were excised from the gel, and subsequently purified and sequenced by Applied Biosystems (Bedford, MA, United States).
2.3 Sequence alignment and phylogenetic analysis
The obtained sequences of the pathogen were edited and quality checked by analyzing the chromatogram peaks using BioEdit 7.2.5 software (Hall, 1999). The identity and similarity of sequences were checked by Blast in the NCBI database (Madden et al., 1996), and then were aligned and compared with reference sequences, using the MEGA V.7 software (Kumar et al., 2016). Phylogenetic trees were constructed using a Maximum Likelihood (ML) method. Consensus sequence of the pathogen was deposited in GenBank under accession numbers: PQ892128.
2.4 In vitro evaluation of Trichoderma species against Curvularia spicifera
2.4.1 Antagonistic interaction
A dual culture assay on potato dextrose agar (PDA) plates was used to examine the antagonistic interaction between Trichoderma spp. and the C. spicifera pathogen. Two agar plugs, each with a diameter of 0.5 cm, were prepared: one containing Trichoderma spp. and the other containing the C. spicifera pathogen. Both plugs were taken from 4-day-old cultures. These plugs were placed on opposite sides of a single 9-cm diameter PDA plate, maintaining 2 cm from the plate edge toward the center for the antagonist plug and a distance of 5 cm between the two plugs. A control plate was included, containing only a PDA plug on one side and the C. spicifera plug on the opposite side (Hajji-Hedfi et al., 2023a).
Antagonistic interactions between Trichoderma spp. and C. spicifera were also investigated also using indirect confrontation. Disks (5 mm diameter) of both fungi were placed on separate Petri dishes containing PDA medium. The dishes were then superimposed, with Trichoderma spp. on the bottom and C. spicifera on the top. Parafilm was used to seal the junction between the dishes and prevent the loss of volatile compounds. A control plate was prepared with a blank PDA plug on one side and a C. spicifera plug on the other (Bouanaka et al., 2021). Three replicates (five plates/replicate) were conducted for each individual treatment, and the plates were incubated at 25 ± 2°C for 7 days.
2.4.2 Antifungal activity
The antifungal activity of Tr1, Tr2, and Tr3 filtrates against the mycelial growth of C. spicifera was assessed in vitro using a culture filtrate method. Mycelia plugs of Trichoderma spp. were cultured in potato dextrose broth (PDB) for 4 days, then filtered to obtain culture filtrates. These filtrates were incorporated into molten PDA medium at three concentrations (C1: 60%; C2: 80%; C3: 100%) and inoculated with C. spicifera (Hajji-Hedfi et al., 2023b).
Three replicates of each treatment were conducted, with each replicate containing five plates. All plates were maintained at 28 ± 2°C for a week. After incubation, the percentage of inhibition (PI) of C. spicifera radial growth was determined using the formula presented by Abdelmoteleb et al. (2023); as follows:
Where C0 is the radial growth diameter of the pathogen, Cn is the pathogen colony’s radial growth in the presence of the antagonist fungus.
2.4.3 Mycelial growth
Mycelial growth was also measured daily in cm, for 7 days post-incubation following Hajji-Hedfi et al. (2024). The mycelial growth rate (MGR) of C. spicifera was calculated using the formula reported by Hajji-Hedfi et al. (2023b) as follows:
The formula considers the radial growth diameter of the fungus over a period of 7 days (D) and the corresponding incubation time (Te). The MGR is determined by summing the incremental changes in diameter divided by the respective incubation time intervals.
A series of microtubes were prepared containing 200 μL of Trichoderma strains (106 spores/ml) and 200 μL of C. spicifera (106 spores/ml), suspended in 1 mL of sterile distilled water containing 5% glucose. The spore counts were standardized using a hemocytometer. These microtubes were incubated at 25°C for 24 h. After incubation, the inhibition of spore germination was assessed microscopically using a Malassez cell. The number of germinated and non-germinated spores was recorded. The percentage of germinated spores (%SG) was calculated using the formula:
Where SG is the number of germinated spores and SNG is the number of non-germinated spores (Benslim et al., 2016).
2.5 In vivo, greenhouse evaluation of Trichoderma spp. against Curvularia spicifera
2.5.1 Disease severity assessment
The experiment was conducted to investigate the potential of Trichoderma spp. to manage gray mold in tomato plants. Tomato seeds (cv. Firenze) were provided from a certified nursery. Thirty-day-old seedlings were treated by root-dipping in Trichoderma spp. (106 spores/ml) conidial suspensions for 30 min. Subsequently, the seedlings were inoculated with C. spicifera conidial suspension (106 spores/ml). The experiment included three replicates of 10 plants each. A randomized complete block design was used to evaluate the effects of three experimental treatments (Tr1, Tr2, and Tr3) and salicylic acid (by root-dipping) on plant response to C. spicifera infection. Each experimental block contained two control groups: a positive control inoculated only with C. spicifera, and a negative control treated with sterile distilled water. Seedlings were subjected to six treatments: T1 (Tr1 + C. spicifera), T2 (Tr2 + C. spicifera), T3 (Tr3 + C. spicifera), T4 (salicylic acid (SA 1%) + C. spicifera), T5 (C. spicifera only), and T6 (untreated). After treatment, plants were incubated in a greenhouse at 25°C for a duration of 90 days. To enhance data reliability, the entire experiment was replicated twice.
Disease assessment was conducted at 5, 10, 20, 30, 60, and 90 days after inoculation (dpi), utilizing a disease severity scale. A 0–6 scale was employed to evaluate fruit rot symptoms, as outlined by Hajji-Hedfi et al. (2023b). Scores on this scale corresponded to the extent of leaf surface covered by lesions: 0 (no lesions), 1 (1–5% leaf surface), 2 (6–10% leaf surface), 3 (11–20% leaf surface), 4 (21–35% leaf surface), 5 (36–50% leaf surface), and 6 (51–100% leaf surface) (AbdElfatah et al., 2021).
2.5.2 Enzymatic activities and defense marker
To investigate the biochemical effects of pre-treating tomato plants with Trichoderma spp. and salicylic acid, enzyme activities in root and leaf samples were measured. Five samples were collected per treatment and block at 7, 30, 60, and 90 days post-inoculation (dpi). Enzyme analyses included catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), polyphenol oxidase (PPO), and total protein content (TPC). Root and leaf samples were immediately flash-frozen in liquid nitrogen to prevent enzyme degradation. Subsequently, the samples were homogenized in a chilled phosphate buffer containing EDTA. The homogenate was centrifuged, and the supernatant was used for enzyme activity assays.
CAT activity was measured by monitoring the decrease in absorbance at 240 nm, following the method of Hajji-Hedfi et al. (2023a). POX activity was assayed using the protocol described by Reddy et al. (1995). APX activity was determined according to the method of Hajji-Hedfi et al. (2024). PPO activity was assessed based on the procedure outlined by Mayer et al. (1965), by measuring the increase in absorbance at 408 nm. TPC was quantified using the Bradford (1976) method.
Chlorophyll content was measured using a portable fluorometer (OS1p; NH 03051-United States). A Minolta SPAD-502 meter was used for non-destructive assessment of leaf chlorophyll content in tomato plants. This instrument measures the transmittance of the tomato leaf to determine the relative amount of chlorophyll present. The resulting dimensionless SPAD units are directly proportional to the chlorophyll content. Readings were recorded at 7, 30, 60, and 90 dpi, as detailed by Almansoori et al. (2021). Agronomic measurements, including fresh and dry weights of roots and aerial parts, as well as the number of fruits, leaves, flowers, and branches, were recorded, along with plant and root lengths, at 30, 60, and 90 dpi.
2.6 Statistical analyses
ANOVA one way was conducted in SPSS version 20.0 statistical software (SPSS, SAS Institute, United States) to assess differences among treatment groups. Normality and homogeneity assumptions were verified before proceeding. Duncan’s Multiple Range Test was used to identify significant differences (p ≤ 0.05) among treatment means. Post-hoc test allowed for detailed comparisons of treatment effects and identified variations in measured parameters across Trichoderma spp. and control groups.
3 Results
3.1 In vitro, antifungal activities of Trichoderma spp. against Curvularia spicifera
Over the seven assessment days, the growth of C. spicifera under direct confrontation with Trichoderma spp. remained lower compared to the control. The mycelial growth for the control was 5.34 cm, while that of Tr1/CS was 2.58 cm, Tr2/CS was 2.74 cm, and Tr3/CS was 2.94 cm at 7 days of incubation. Although all Trichoderma species inhibited C. spicifera, their strengths were not identical. T. longibrachiatum was the strongest inhibitor, resulting in the lowest C. spicifera growth across most incubation time (Figure 1a). Figure 1b presented the temporal variation of mycelial growth of C. spicifera during indirect confrontation with the three same species of Trichoderma. As observed in direct confrontation methods, the indirect one also reveals an inhibitory effect of Trichoderma spp. on C. spicifera growth, though their magnitude of inhibition from this approach seems a little reduced compared to the results shown in the direct method. Furthermore, C. spicifera growth remained generally lower in the presence of T. longibrachiatum (0.77–2.63 cm at J1 and J7, respectively) compared to the positive control (1.70–5.23 cm at J1 and J7, respectively) (Figure 1b).
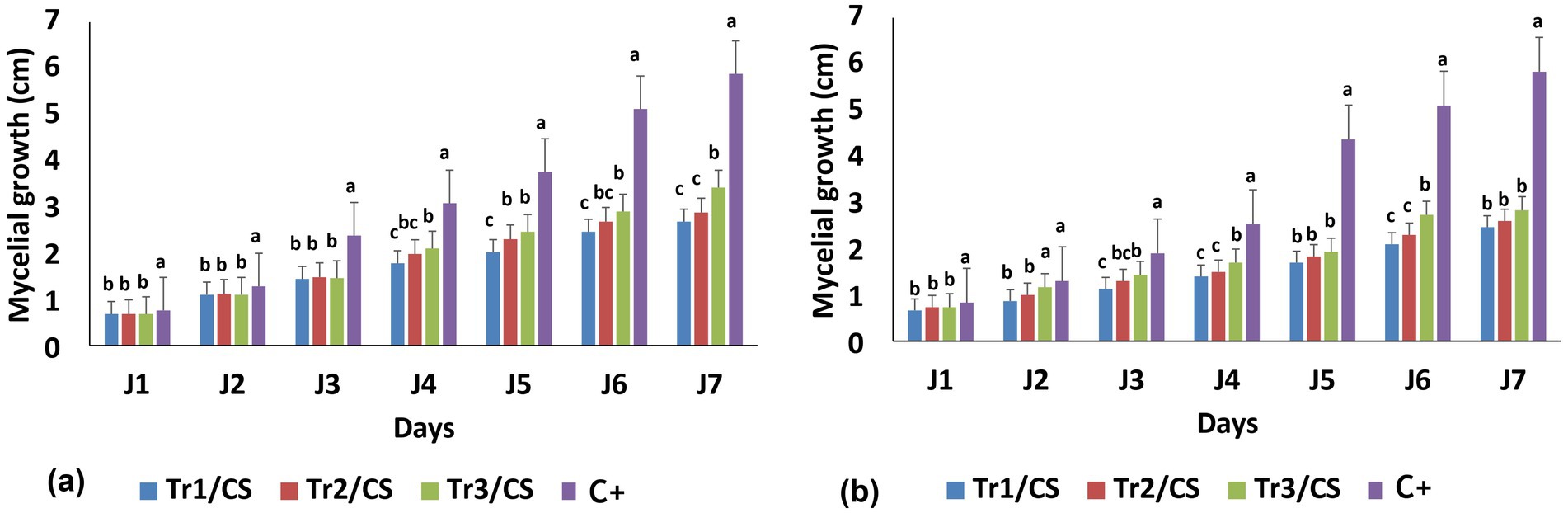
Figure 1. Temporal variation of Curvularia spicifera (CS) mycelial growth in response to Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) using direct (a) and indirect (b) confrontation methods. Small letters are used to compare different treatments. Different letters above bars indicate statistically significant differences within the experiments (p ≤ 0.5) according to Duncan’s multiple range tests. Bars without letters are not significantly different.
Though all three species of Trichoderma are inhibiting growth of C. spicifera in comparison to positive control (2.58 mm/h), T. longibrachiatum exhibited the most effect with 1.28 mm/h, followed by T. harzianum (1.37 mm/h) and T. asperellum (1.43 mm/h) (Figure 2a). The results obtained revealed the effect of volatile compound produced by Trichoderma species on C. spicifera. Where the volatile compounds of Trichoderma treatments were exhibiting an inhibitory effect, a reduction in growth rate for C. spicifera was observed. Among all treatments, T. longibrachiatum expressed the strongest inhibition with a growth rate of 1.33 mm/h (Figure 2b).
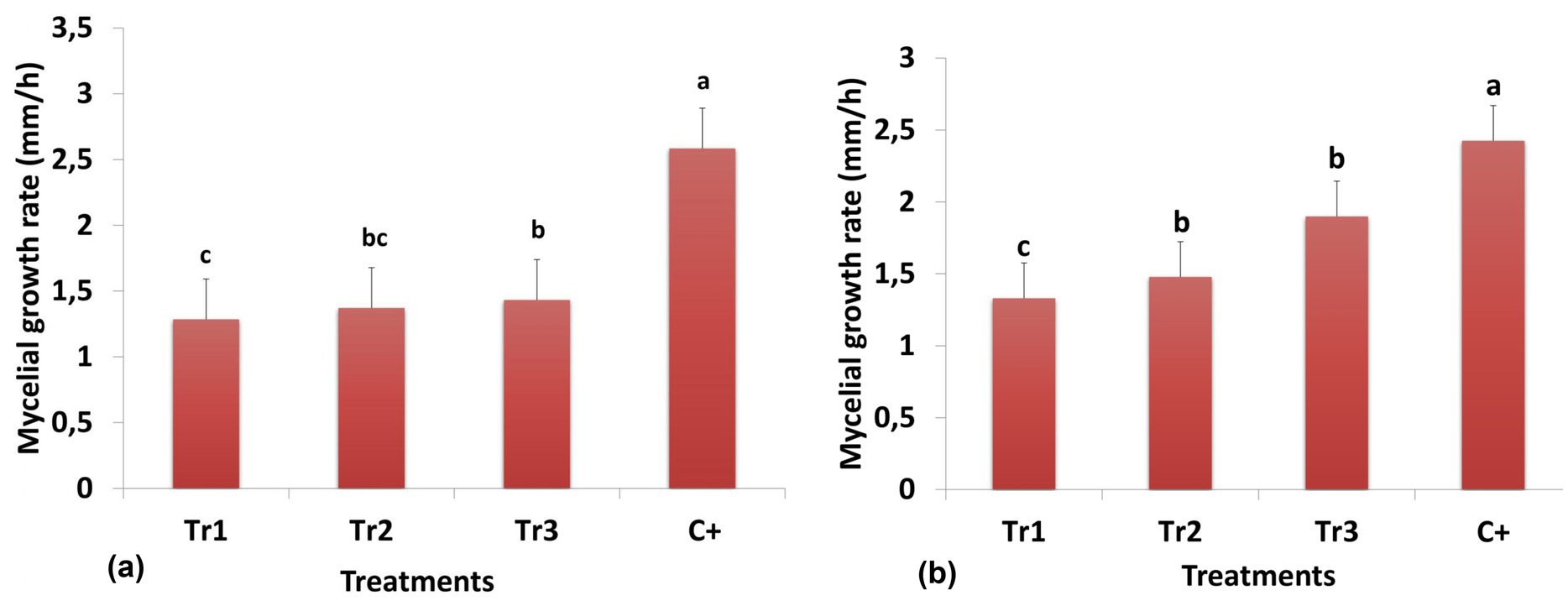
Figure 2. Mycelial growth rate of Curvularia spicifera in response to Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) after 7 days of incubation according to the direct (a) and indirect (b) confrontation methods. Small letters are used to compare different treatments. Different letters above the bars indicate statistically significant differences within the experiments (p ≤ 0.5) according to Duncan’s multiple range tests. Bars without letters are not significantly different.
3.2 Impact of Trichoderma spp. filtrates on mycelial growth
Table 1 points out the temporal variation in mycelial growth of C. spicifera under different concentrations of filtrates from three species of Trichoderma during the 7-day incubation period. In this study, all Trichoderma treatments significantly inhibited the mycelial growth of C. spicifera compared to the control (p < 0.01). This indicates that across all assessed time points and concentrations, the mycelial growth values were considerably lower in the Trichoderma treated groups. Mycelial growth in the positive control reached 5.97 cm on 7th day, while the growth in Trichoderma treatments ranged from 1.20 cm (Tr3/C3) to 1.47 cm (Tr2/C1), depending on the species and concentrations (Table 1). In the same context, Figure 3 illustrated the mycelial growth rate of C. spicifera at different filtrate concentrations of three species of Trichoderma, showing significant variation among them. The lower concentration of Trichoderma filtrates as 60% are associated with higher mycelial growth rates which compared to the higher concentrations, 80 and 100%, indicating that lower concentrations are less inhibitory. Among the Trichoderma species, T. longibrachiatum confirmed the most inhibitory effect at higher concentrations, as indicated by the lower growth rates at 80 and 100% (0.80 and 0.79 mm/h, respectively; Figure 3).
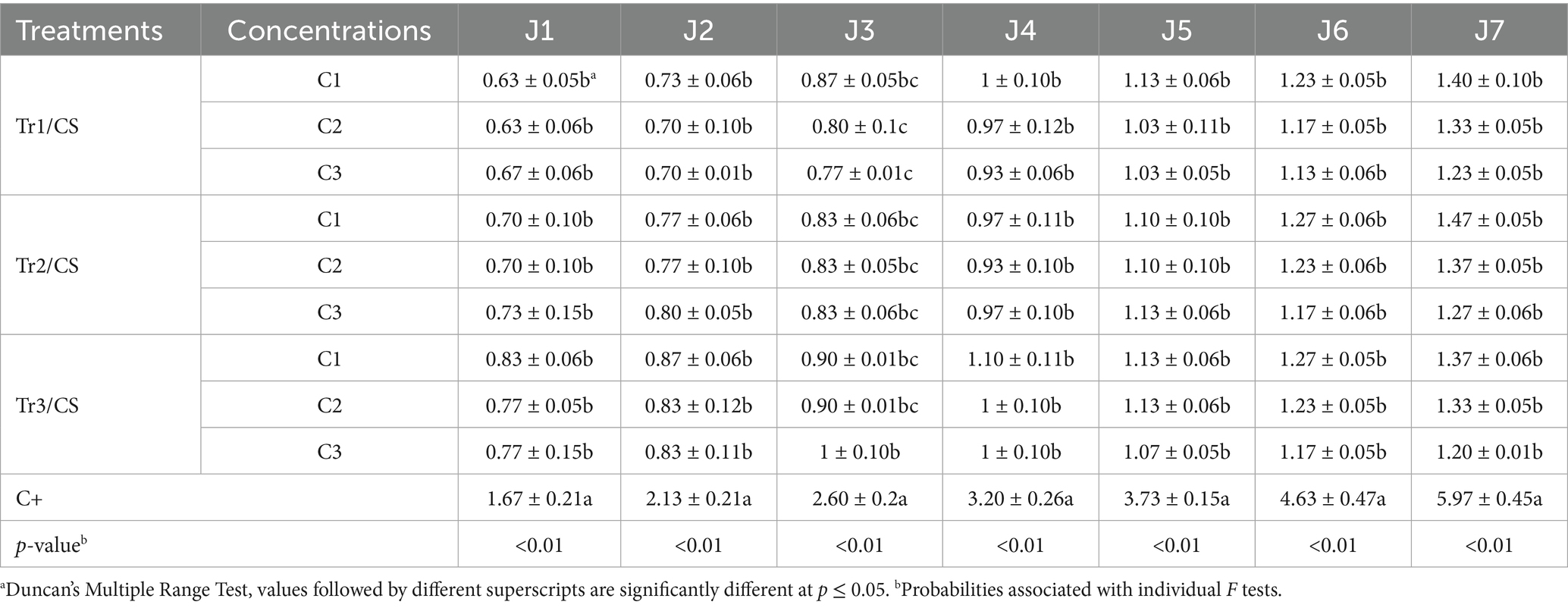
Table 1. Temporal variation of Curvularia spicifera (CS) mycelial growth (cm) in response to different concentrations (C1: 60%, C2: 80%, and C3: 100%) of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) filtrates during 7 days of incubation (J1, J2, J3, J4, J5, J6, and J7).
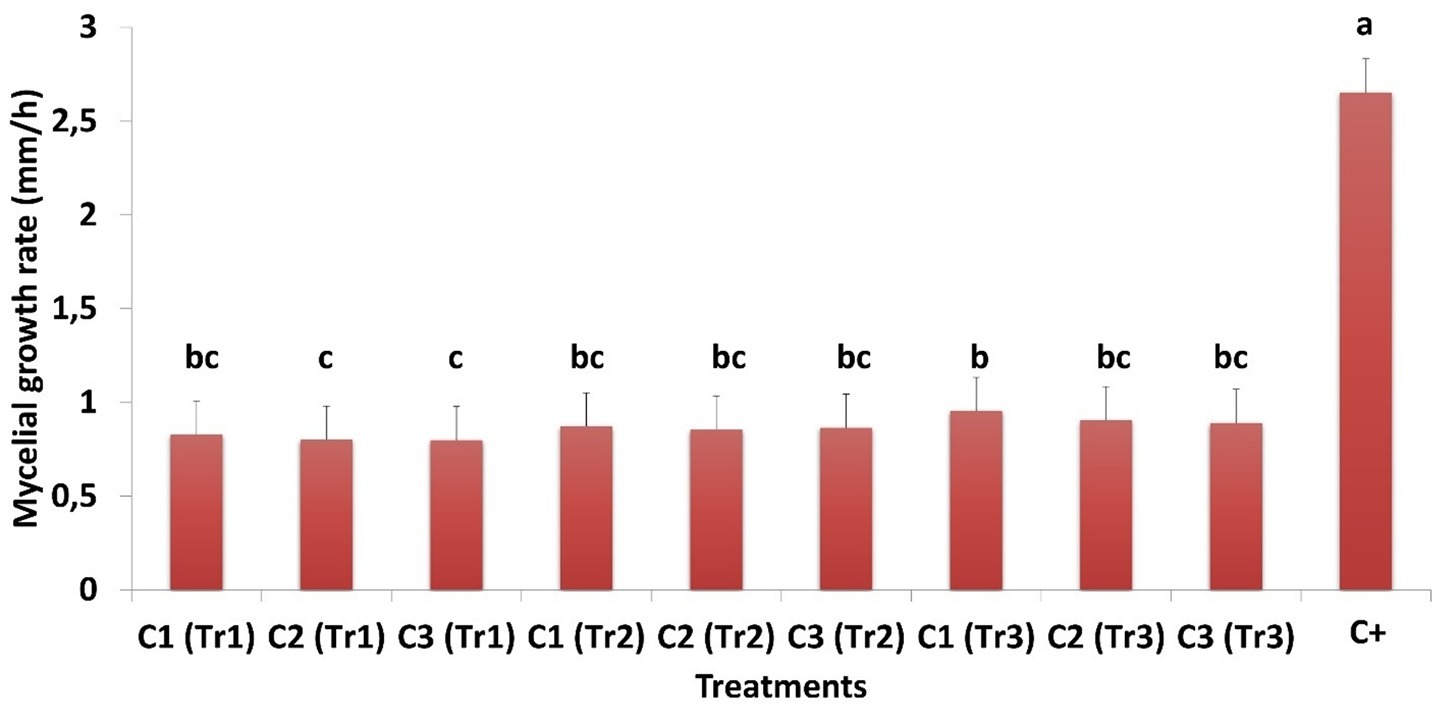
Figure 3. Mycelial growth rate of Curvularia spicifera in response to different concentrations (C1: 60%, C2: 80%, and C3: 100%) of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) filtrates. Small letters are used to compare different treatments. Different letters above bars indicate statistically significant differences within the experiments (p ≤ 0.5) according to Duncan’s multiple range tests. Bars without letters are not significantly different.
The results indicate that spore germination of C. spicifera is highly influenced by the presence of Trichoderma spp. Generally, the number of germinated spores was significantly lower in the treatments with Trichoderma filtrates compared to the positive control (17.60), confirming an inhibitory effect. Among the Trichoderma species, T. longibrachiatum revealed the highest inhibitory activity, by reducing the spore germination at (0.40), which is the lowest number of germinated spores. T. harzianum (2.20) and T. asperellum (4.40) also exhibited inhibitory effects (Figure 4).
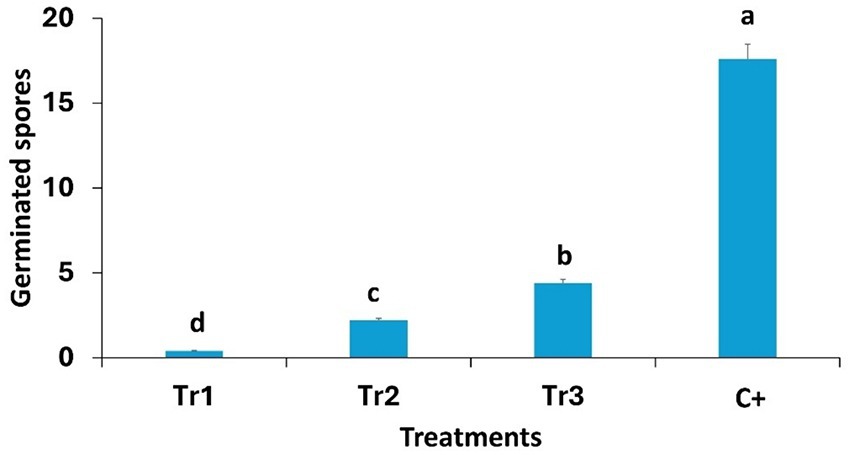
Figure 4. Effect of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) on the number of germinated spores of Curvularia spicifera after 24 h of incubation. Small letters are used to compare different treatments. Different letters above bars indicate statistically significant differences within the experiments (p ≤ 0.5) according to Duncan’s multiple range tests. Bars without letters are not significantly different.
3.3 Impact of Trichoderma species under greenhouse conditions
3.3.1 Disease severity assessment
The results of the preventative treatments using three Trichoderma species and salicylic acid against C. spicifera on tomato plants under greenhouse conditions are presented in Figure 5. At 5 dpi, none of the disease severity treatments exceeded 0 compared to the highly significant positive control (0.67). This result continued at 10 dpi, where all the treatments and the negative control maintained a disease severity of between 0 and 0.5, which is significantly lower than the positive control (1.67). At 90 dpi, during the final assessment, the disease severity reached its maximum in all treatments and the value ranged between 2.83 (T. longibrachiatum and salicylic acid) and 3.67 (T. harzianum) (positive control = 5.50; negative control = 0; Figure 5).
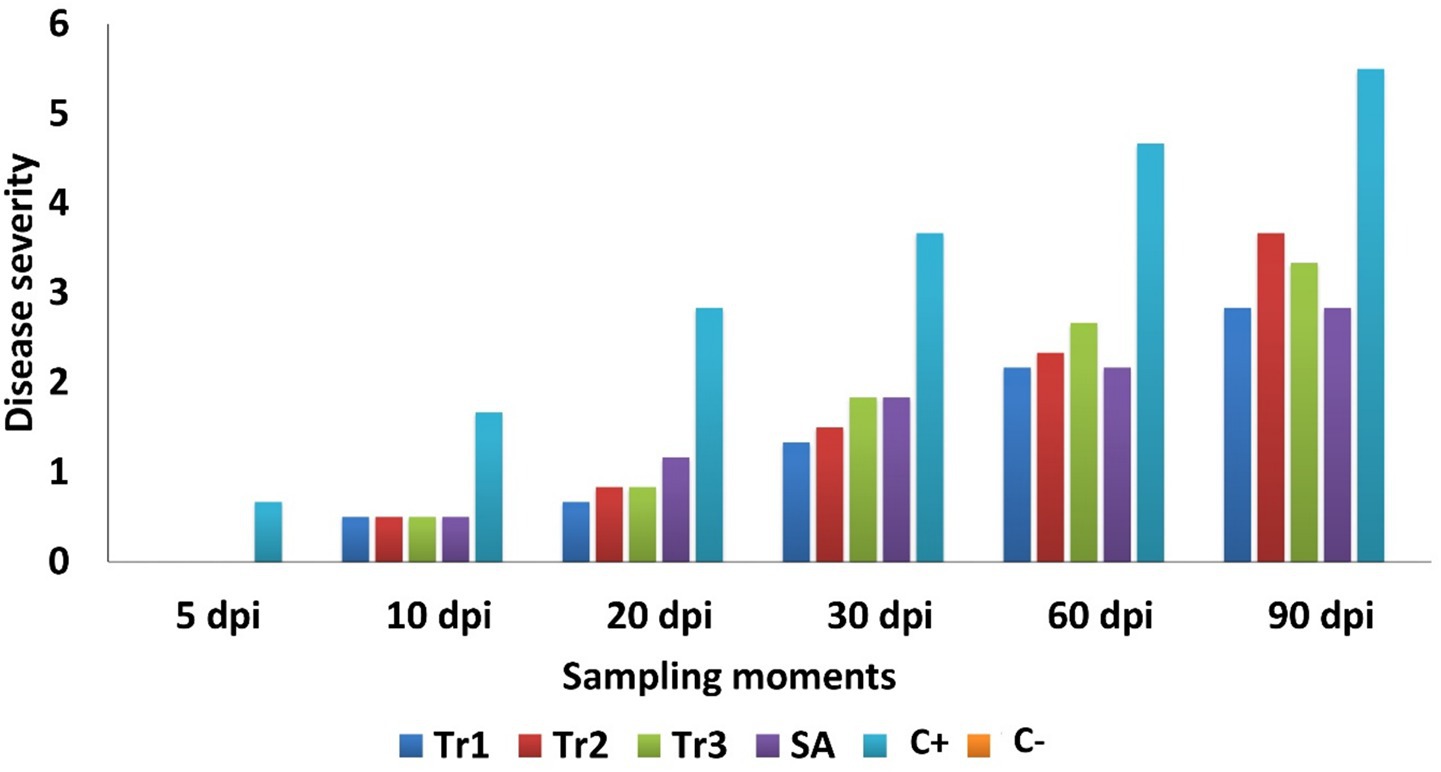
Figure 5. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the disease severity in tomato plants inoculated with Curvularia spicifera at six sampling moments [days after pathogen inoculation (dpi)] under experimental greenhouse conditions.
3.3.2 Enzymatic activities and stress markers
3.3.2.1 Catalase activity
Table 2 presents the catalase activity in tomato leaves and roots, subjected to the mentioned treatments above. Catalase activity,is ann important indicator of oxidative stress and marker for evaluating the effectiveness of preventive treatments against C. spicifera, varied significantly among treatments and sampling times. At 7 dpi, salicylic acid had the highest catalase activity of 52.04 μmol H2O2 mg protein−1, followed by T. asperellum with 49.72 μmol H2O2 mg protein−1, while the lowest was T. harzianum with 22.98 μmol H2O2 mg protein−1. At 30 dpi, the highest activity was recorded in the positive control (51.64 μmol H2O2 mg protein-1), followed by salicylic acid (48.4 μmol H2O2 mg protein−1), and T. harzianum (44.96 μmol H2O2 mg protein−1). At 60 dpi, T. longibrachiatum demonstrated the highest activity (57.42 μmol H2O2 mg protein−1), significantly surpassing the other treatments, including positive control (51.76 μmol H2O2 mg protein−1) and T. harzianum (50.8 μmol H2O2 mg protein-1). By 90 dpi, T. longibrachiatum maintained the highest activity (74.58 μmol H2O2 mg protein−1), followed by T. harzianum (70.18 μmol H2O2 mg protein−1) and positive control (59.70 μmol H2O2 mg protein−1). Notably, T. asperellum and salicylic acid showed a decline in activity over time, with the lowest values at 90 dpi (28.18 and 23.52 μmol H2O2 mg protein−1, respectively) (Table 2). In tomato roots, at 7 dpi, the highest catalase activity was recorded in salicylic acid (5.24 μmol H2O2 mg protein−1) and the lowest in T. asperellum (1.02 μmol H2O2 mg protein−1). At 30 dpi, salicylic acid again showed the highest activity of 66.1 μmol H2O2 mg protein−1, followed by T. longibrachiatum, which accounted 44.7 μmol H2O2 mg protein−1. At 60 dpi, T. longibrachiatum maintained the highest activity of 67.6 μmol H2O2 mg protein−1, significantly higher than positive control (58.02 μmol H2O2 mg protein−1) and other treatments. At 90 dpi, T. longibrachiatum continued to show the highest activity of 73.1 μmol H2O2 mg protein−1, followed by T. harzianum with 60.64 μmol H2O2 mg protein−1. In contrast, at later stages of T. asperellum and salicylic acid exhibited low catalase activity, with T. asperellum recording the lowest values at 90 dpi (1.36 μmol H2O2 mg protein−1) (Table 2).
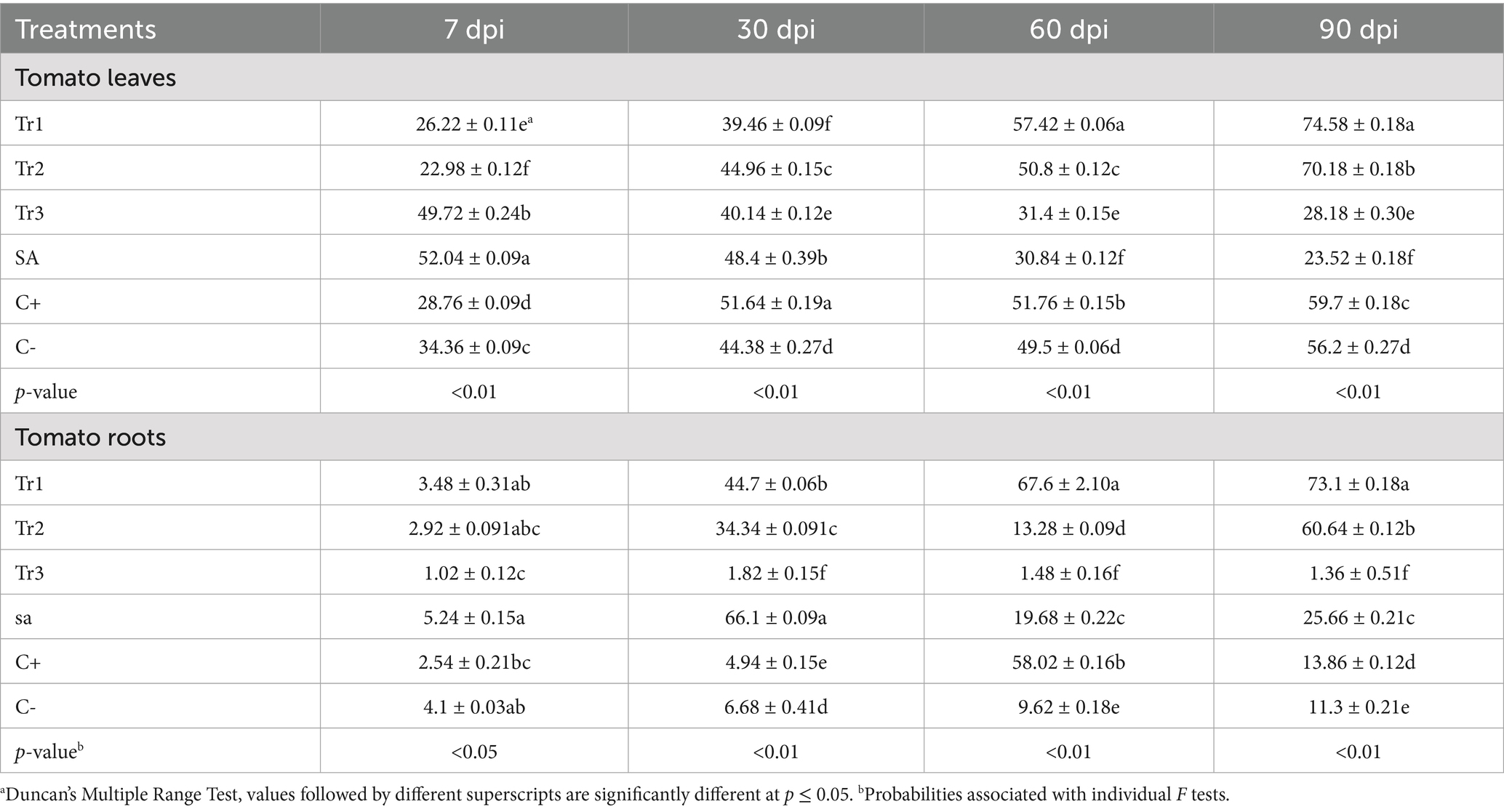
Table 2. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the catalase activity (μmol H2O2 mg protein−1) in tomato leaves and roots inoculated with Curvularia spicifera at four sampling moments [7, 30, 60, and 90 days after pathogen inoculation (dpi)] under experimental greenhouse conditions.
3.3.2.2 Peroxidase activity
Results mentioned in Table 3 revealed that peroxidase activity varied significantly in treatments, sampling times, and different plant tissues. In tomato leaves, the highest peroxidase activity was recorded in T. longibrachiatum at 7 dpi, accounted 4.34 units mg−1 min−1 and followed by 90 dpi (5.35 units mg−1 min−1), which reflects a strong and sustained induction of plant defense-related mechanisms. On the other hand, T. asperellum showed moderate activity at all-time points, and the value levels ranged from 3.36 to 1.52 units mg−1 min−1 (at 7 and 90 dpi, respectively). Salicylic acid was moderate at the beginning (4.17 units mg−1 min−1 at 7 dpi) but declined significantly by 90 dpi to a value as low as 2.07 units mg−1 min−1, indicating a short-term effect. T. harzianum showed intermediate activity, which reached a maximum at 60 dpi (4.37 units mg−1 min−1), but by 90 dpi, the activity declined to 4.77 units mg−1 min−1 (Table 3). As well roots, T. longibrachiatum showed the highest peroxidase activity (4.67 to 5.24 units mg−1 min−1 from 7 to 90 dpi). The enzymatic activity was also relatively high for T. harzianum, though it was lower in comparison with T. longibrachiatum activity, constituting 4.78 units mg-1 min-1 at 90 dpi. The lowest activity found to T. asperellum with values varied from 3.47 and 2.23-units mg−1 min−1 (at 7 and 90 dpi, respectively), evidencing its minimal efficiency in the root tissues. Salicylic acid showed a moderate activity that decreased along time, from 4.18 units mg−1 min−1 at 7 dpi to 2.57 units mg−1 min−1 at 90 dpi, as previously observed in leaves (Table 3).
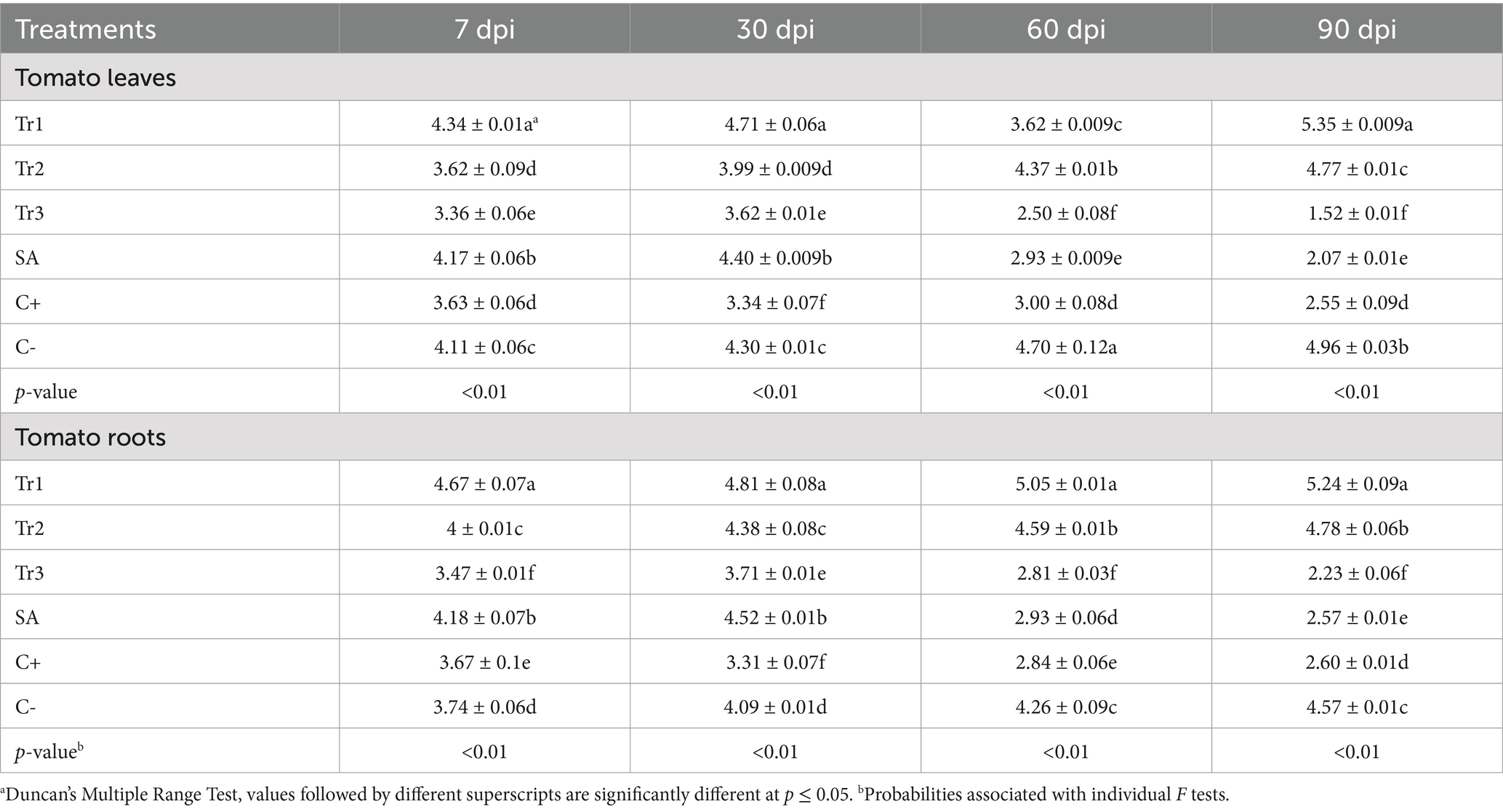
Table 3. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the peroxydase activity (units mg−1 min−1) in tomato leaves and roots inoculated with Curvularia spicifera at four sampling moments [7, 30, 60, and 90 days after pathogen inoculation (dpi)] under experimental greenhouse conditions.
3.3.2.3 Ascorbate peroxidase activity
Ascorbate peroxidase activity in tomato leaves was the highest under T. longibrachiatum treatment ranging from 45.21 to 54.91 μmol mg−1 min−1 at 7 and 90 dpi, respectively. In term of potential activity, T. longibrachiatum was followed by T. harzianum, which showed relatively high ascorbate peroxidase activity, with values rising from 42.82 to 50.96 μmol mg−1 min−1 at 7 and 90 dpi. Howevere, a less ascorbate peroxidase activity was recorded in T. asperellum and salicylic acid. Their activity declined with increase in days, starting from 21.38 to 18.03 μmol mg−1 min−1 at 7 and 90 dpi for T. asperellum and 38.58 to 18.16 μmol mg−1 min−1 at 7 and 90 dpi for salicylic acid (Table 4). In the roots, a similar patterns were revealed, where the maximum ascorbate peroxidase activity was recorded in T. longibrachiatum, increasing from 27.01 to 60.29 μmol mg−1 min−1 at 7 and 90 dpi. Compared to negative control, an increase from 26.82 μmol mg−1 min−1 at 7 dpi to 47.89 μmol mg−1 min−1 at 90 dpi was revealed, whereas positive control, a significant drop from 19.22 μmol mg−1 min−1 at 7 dpi to 9.20 μmol mg−1 min−1 at 90 dpi (Table 4).
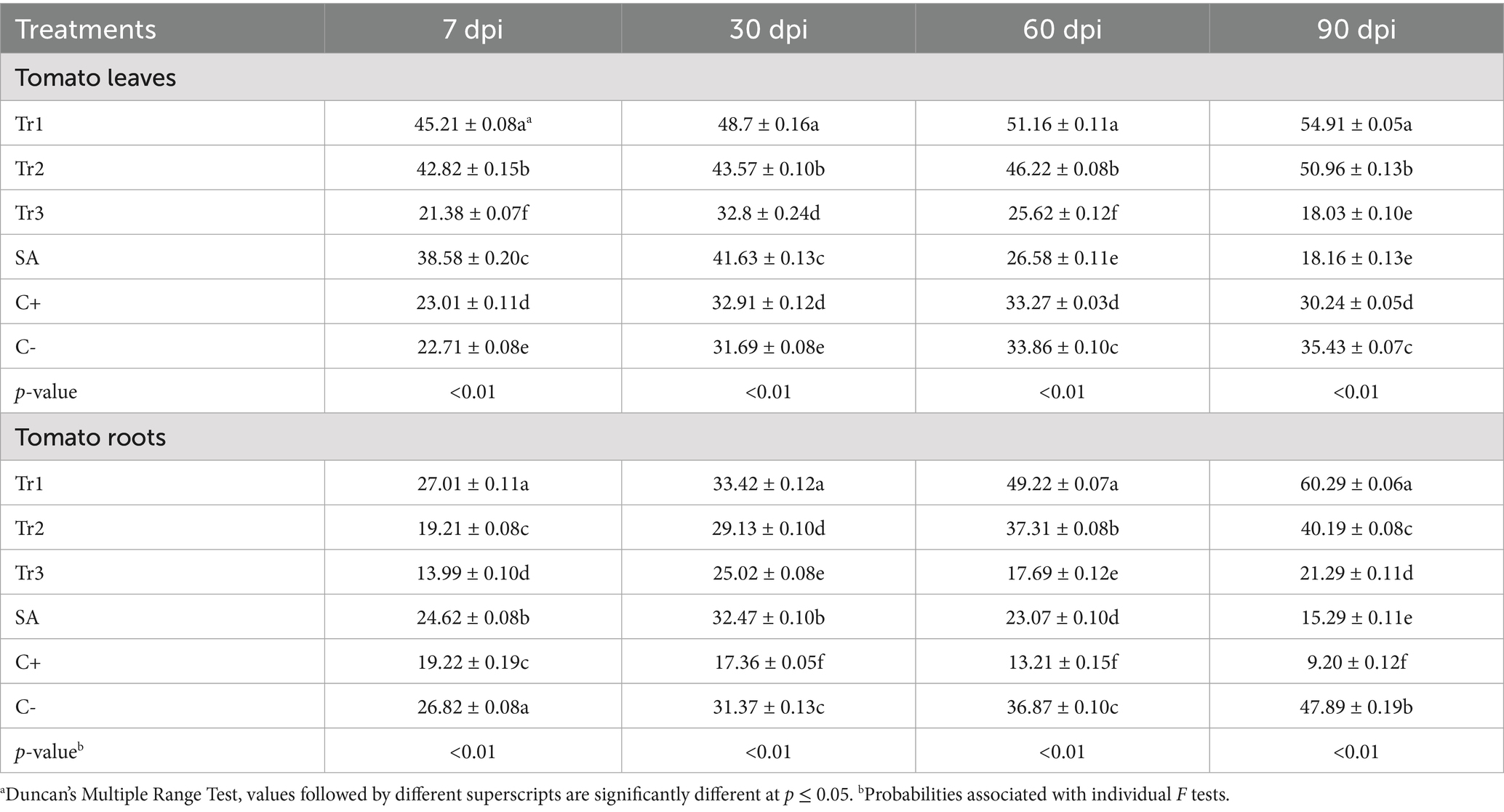
Table 4. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the ascorbate peroxidase activity (μmol mg−1 min−1) in tomato leaves and roots inoculated with Curvularia spicifera at four sampling moments [7, 30, 60, and 90 days after pathogen inoculation (dpi)] under experimental greenhouse conditions.
3.3.2.4 Polyphenol activity
Trichoderma longibrachiatum consistently exhibited the highest polyphenol oxidase activity in tomato leaves at all the sampling periods, which varied between 9.89 and 14.07 units mg−1 min−1 at 7 and 90 dpi, respectively. These activities were significantly important than those the other applied treatments. T. harzianum and T. asperellum increased the activity of polyphenol oxidase over the controls, but activities were less than those of T. longibrachiatum. Salicylic acid showed a moderate polyphenol oxidase activity at 7 and 30 dpi (5.48 and 10.10 units mg−1 min−1, respectively), but decreased considerably at 90 dpi (2.22 units mg−1 min−1) (Table 5). In the same context, in tomato roots, T. longibrachiatum yielded the maximum polyphenol oxidase activity with increasing values over time (2.83 to 9.37 units mg−1 min−1 at 7 and 90 dpi, respectively). Tomato plants treated with T. asperellum showed the lowest enzyme activity, where the polyphenol oxidase activity reduced significantly at 60 dpi (1.82 units mg−1 min−1) and persisted to be low at 90 dpi (3.24 units mg−1 min−1) (Table 5).
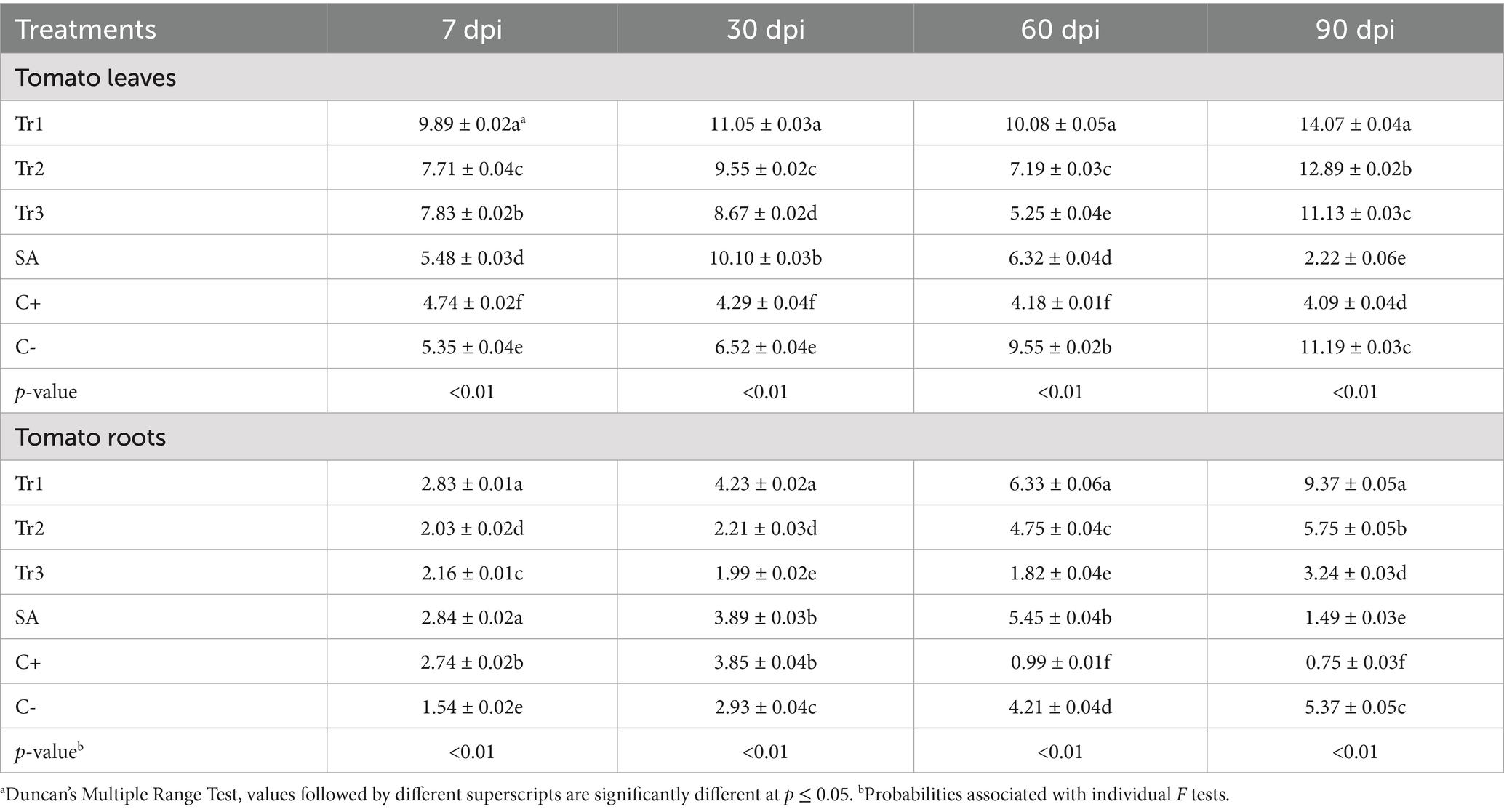
Table 5. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the polyphenol oxidase activity (units mg−1 min−1) in tomato leaves and roots inoculated with Curvularia spicifera at four sampling moments [7, 30, 60, and 90 days after pathogen inoculation (dpi)] under experimental greenhouse conditions.
3.3.2.5 Total protein
Table 6 documented the influence of preventive treatments with three species of Trichoderma spp. and salicylic acid on the total protein content in tomato leaves and roots inoculated with C. spicifera. The total protein content in the leaves varied significantly among treatments and sampling periods. At 30 dpi, T. longibrachiatum showed the highest protein content (11.23 mg g−1). At 60 dpi, negative control revealed the highest protein content (11.28 mg g−1) followed by T. longibrachiatum (11.12 mg g−1). At 90 dpi, salicylic acid (10.35 mg g−1), T. longibrachiatum (10.09 mg g−1) once again contained the highest protein content in tomato leaves (Table 6). Protein content in the roots of tomato also differed greatly during treatment and sampling time. At 7 dpi, positive control (11.46 mg g−1) exhibited maximum protein content followed by salicylic acid (10.67 mg g−1) and T. asperellum (10.12 mg g−1). At 90 dpi, positive control exhibited a remarkable rise in protein content (14.53 mg g−1) that was far higher than all treatments, indicating its ability to maintain protein synthesis over time. T. longibrachiatum (10.44 mg g−1) and negative control (10.39 mg g−1) followed, while T. asperellum (9.42 mg g−1) showed the lowest protein content (Table 6).
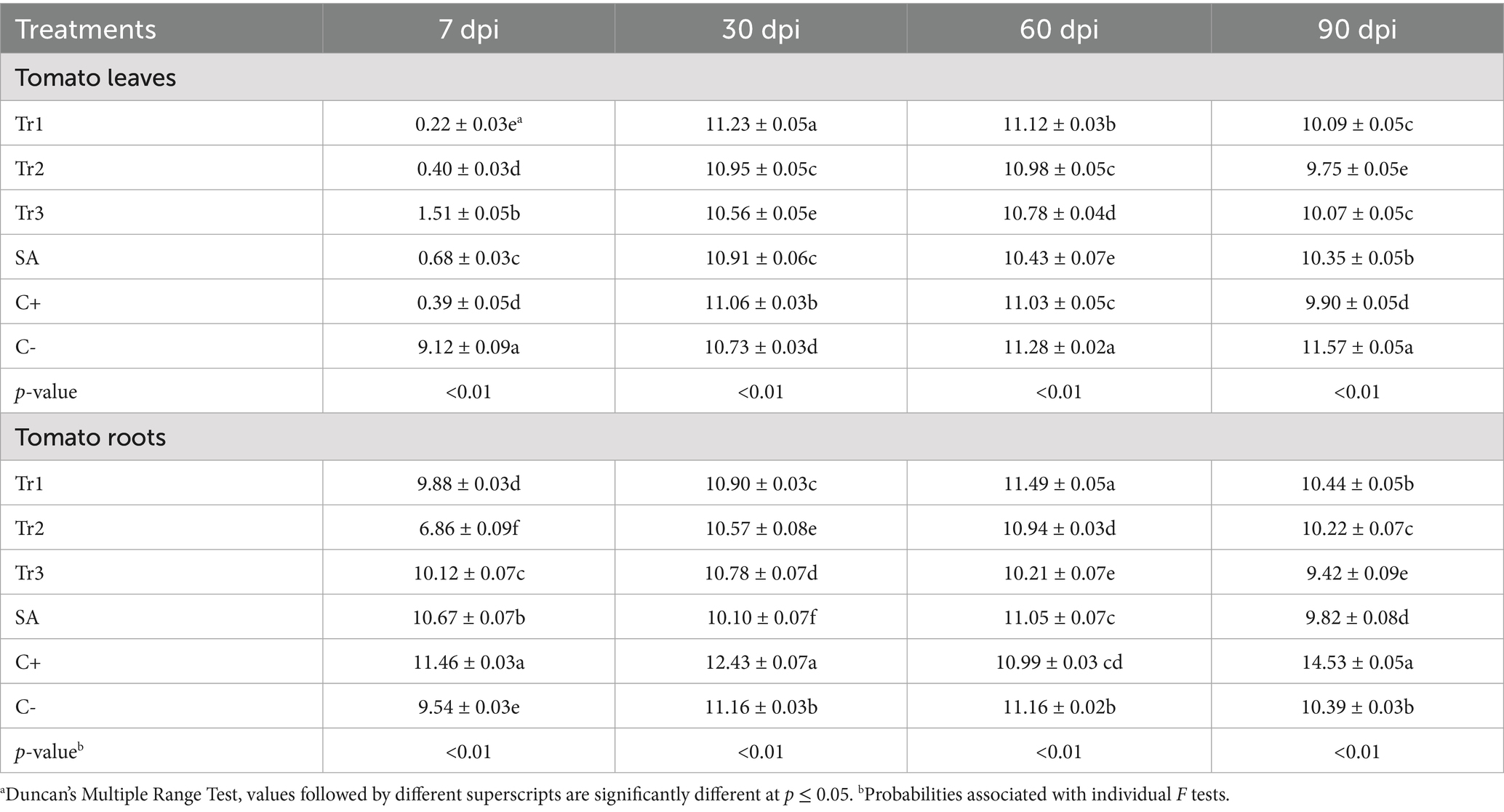
Table 6. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the total protein content (mg g−1) in tomato leaves and roots inoculated with Curvularia spicifera at four sampling moments [7, 30, 60, and 90 days after pathogen inoculation (dpi)] under experimental greenhouse conditions.
3.3.3 Impact of Trichoderma spp. on plant growth promoting parameters
Statistical analysis showed a highly significant difference (p < 0.01) between treatments and sampling moments. The highest chlorophyll content was observed in the tomato plants treated with salicylic acid (38.77) and T. longibrachiatum (38.69), which were both significantly higher than the other applied treatments at 7 dpi, compared to control. At 30 dpi, chlorophyll content was boosted in most of the treatments, with salicylic acid (46.44) and T. longibrachiatum (46.31) showing the highest values, followed by T. harzianum (45.88) and T. asperellum (44.81), which were all significantly higher than the positive control (36.19), indicating long term protective effect of the treatments. At 60 dpi, salicylic acid (50.77) still maintained the highest chlorophyll content, outperforming significantly all the other treatments and controls. T. longibrachiatum (42.92) and T. harzianum (43.43) had comparatively more chlorophyll, while T. asperellum (35.68) showed a notable reduction, and it was nearly approaching the value observed in positive control (36.20). Salicylic acid (56.74) maintained the maximum content of chlorophyll at 90 dpi and exceeded that of all other controls and treatments. T. longibrachiatum (42.50) and T. harzianum (39.68) continued to decline but were still much higher in comparison to the positive control (30.60) (Table 7). The growth responses of the tomato plants varied significantly between treatments (p < 0.01) and was most enhanced in Trichoderma-treated plants at 90 dpi. The preventative application of T. longibrachiatum against C. spicifera significantly increased growth over all the other treatments. Specifically, T. longibrachiatum recorded 29 cm root length, 46 cm plant length, 13 leaves per plant, 2 fruits per plant, 8 flowers per plant, and 12 ramifications per plant at 90 dpi. This treatment also recorded the highest fresh weight of aerial (25.87 g) and root (15.24 g) parts, and dry weight of aerial (3.25 g) and root (3.17 g) parts. Plants inoculated with the pathogen only, however, recorded a notable decrease in all the growth parameters measured (Figure 6).
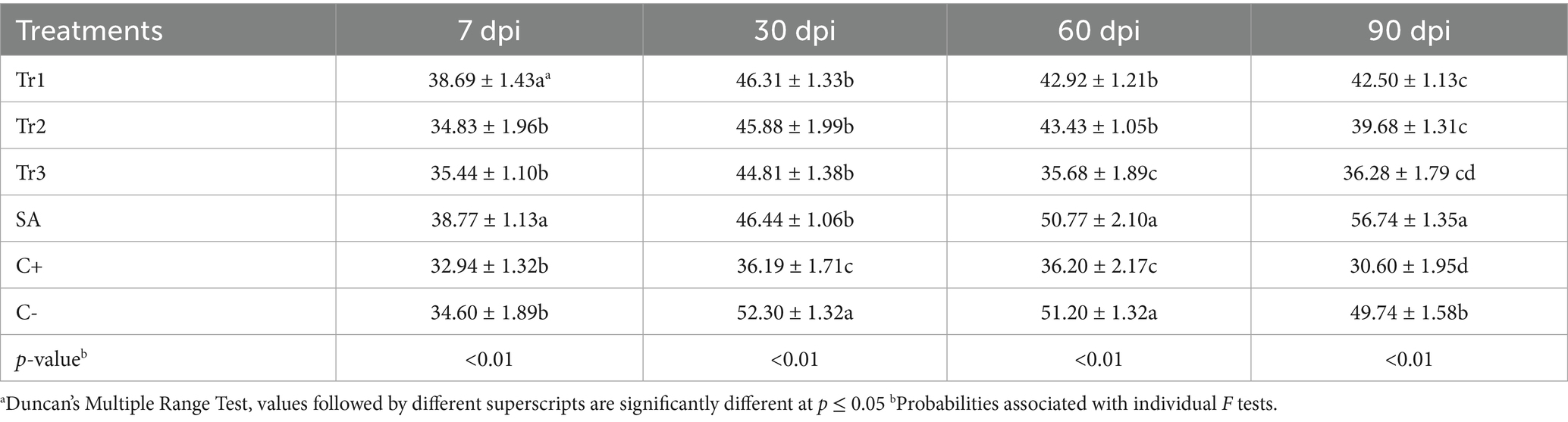
Table 7. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the chlorophyll content in tomato leaves inoculated with Curvularia spicifera at four sampling moments [7, 30, 60, and 90 days after pathogen inoculation (dpi)] under experimental greenhouse conditions.
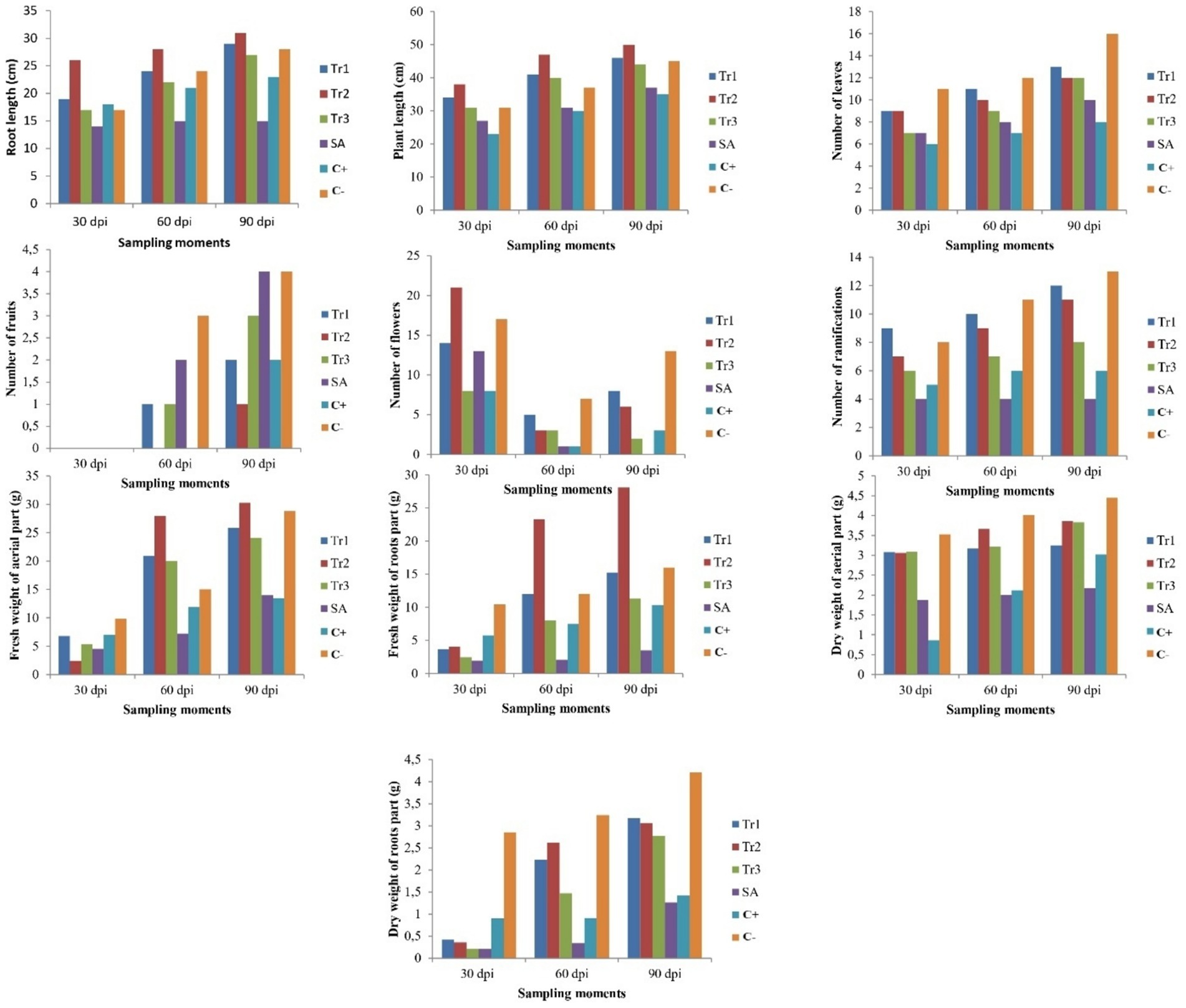
Figure 6. Effect of preventive treatments of Trichoderma spp. (Tr1: T. longibrachiatum, Tr2: T. harzianum, and Tr3: T. asperellum) and salicylic acid (SA) on the agronomic parameters of tomato plants inoculated with Curvularia spicifera at three sampling moments (30, 60, and 90 dpi) under experimental greenhouse conditions.
4 Discussion
Plant disease management is a critical component of sustainable crop production, and the use of biological control agents (BCAs), such as Trichoderma spp., represents a promising strategy (Becker et al., 2025). Trichoderma species are known to employ multiple modes of action against plant pathogens, including mycoparasitism (direct infection and lysis of the pathogen), antibiosis (production of inhibitory secondary metabolites), competition for nutrients and space, enzymatic degradation of pathogen cell walls (López-López et al., 2022), and the induction of host plant defense mechanisms (Gouit et al., 2024), potentially mediated through the emission of volatile organic compounds (Rubio et al., 2023).
In addition, Trichoderma spp. are capable of upregulating plant defense-related genes, thereby enhancing the plant’s resistance to subsequent infections. These fungi have demonstrated strong antagonistic activity against a wide spectrum of phytopathogenic fungi—including C. spicifera—under diverse environmental conditions, confirming their broad-spectrum potential as effective BCAs (Baral et al., 2022; Manzar et al., 2022; Becker et al., 2025).
In vitro assays demonstrated the strong inhibitory effects of T.longibrachiatum on the mycelial growth and spore germination of C. spicifera, indicating a high level of antagonistic activity. These findings are consistent with previous studies by Hajji-Hedfi et al. (2023a, 2023b), which reported the production of both volatile and non-volatile bioactive metabolites by Trichoderma spp. as key mechanisms of pathogen suppression. Trichoderma spp. are known to synthesize a wide array of volatile secondary metabolites, including ethylene, hydrogen cyanide, various aldehydes, and ketones. These compounds have been shown to play a pivotal role in the biological control of numerous plant pathogens (Khan et al., 2020). Extensive research has confirmed that such volatile organic compounds VOCs, particularly those produced by Trichoderma spp., possess strong antifungal properties. They can effectively inhibit the growth of several economically important pathogenic fungi, including Aspergillus spp. and Fusarium spp., and in many cases, their inhibitory potential exceeds that of classical mycoparasitism (Awad-Allah et al., 2022; Modrzewska et al., 2022; Ren et al., 2022; Rebouh et al., 2022; Napolitano et al., 2024; Becker et al., 2025). Moreover, VOCs emitted by Trichoderma spp. have also been shown to suppress the growth of other phytopathogens, such as Rhizoctonia spp. and Pythium spp., further highlighting their broad-spectrum biocontrol potential (Behiry et al., 2023; Al-Shuaibi et al., 2024). These findings collectively underscore the role of T. longibrachiatum as an effective biological control agent, capable of reducing fungal pathogen viability through multiple biochemical mechanisms, with volatile metabolite production being a major contributing factor.
Trichoderma spp. have a very advanced mechanism of action against phytopathogenic fungi, including cell wall-degrading enzymes and antibiotic biosynthesis (Becker et al., 2025). In addition to the reported chitinolytic activity for the degradation of pathogen cell walls, Trichoderma spp. biosynthesize antibiotics that directly inhibit growth of the pathogenic mycelial growth (Rubio et al., 2023). Main extracellular enzymes such as endochitinases, β-1,3-glucanases, and proteases play an important role in Trichoderma mycoparasitism and bring about extensive disruption of structural integrity of pathogenic fungal cell walls (Suriani Ribeiro et al., 2019; Tomah et al., 2023). Our findings are consistent with these studies, as disease severity was significantly reduced in all treatments involving Trichoderma strains.
Recent studies have demonstrated that Trichoderma spp. can alleviate the negative impacts of both biotic and abiotic stress in plants by modulating reactive oxygen species (ROS) and enhancing the activity of antioxidant enzymes (Fu et al., 2017; Chen et al., 2019; Cornejo-Ríos et al., 2021). In our study, all tested Trichoderma species significantly increased enzymatic activity in plants, with T. longibrachiatum exhibiting the highest levels among them. These findings highlight species-specific variation in the capacity of Trichoderma to induce plant defense responses. Indeed, outcomes often vary depending on the Trichoderma species or isolate used and the target phytopathogen. For example, Almaghasla et al. (2023) reported that T. asperellum strain KSATR11 exhibited the weakest antagonistic activity against Rhizoctonia solani in cucumber when compared to six other Trichoderma species. Similarly, Yao et al. (2023) reported that T. asperellum and T. harzianum exhibited varying degrees of inhibitory activity against 29 plant pathogenic fungi across 18 genera. This finding highlights the broad-spectrum biocontrol potential of Trichoderma spp. and reinforces the concept of strain-specific effectiveness. The observed variability in antagonistic activity suggests that the efficacy of Trichoderma is not consistent across species or even strains, but rather depends on the specific interaction between the isolate and the target pathogen. These results underscore the importance of screening and selecting the most effective Trichoderma strains for particular pathogens and crops. Furthermore, they support the use of diverse Trichoderma isolates in biocontrol programs to enhance effectiveness across a wide range of plant diseases (Yao et al., 2023). This research was mainly directed toward the beneficial effects of Trichoderma spp. as a biocontrol agent, but it is also crucial to use salicylic acid in co-application and investigate its possible effect on the results achieved. Salicylic acid, a significant plant hormone, has a special role in triggering systemic acquired resistance against a range of phytopathogens by usually inducing defense-related genes and enabling the synthesis of antimicrobial compounds (Decsi et al., 2025).
The effectiveness of T. longibrachiatum compared to T. harzianum and T. asperellum in biocontrol applications is typically attributed to several unique biological traits and metabolic processes (Cao et al., 2025). T. longibrachiatum can grow in a variety of environmental conditions (Bint-e-Zahira et al., 2024). Its effectiveness often results from an effective complement of extracellular enzymes, especially cell wall-degrading enzymes. These enzymes are directly involved in mycoparasitism by degrading the cell walls of phytopathogenic fungi, thereby inhibiting or lysing them (Zhu et al., 2022; Díaz-García et al., 2024). Although other Trichoderma spp. also produce these enzymes, some strains of T. longibrachiatum can produce them to a larger degree for a longer duration, or in a more synergistic combination (Boamah et al., 2025). Besides, T. longibrachiatum is a rich source of numerous secondary metabolites such as peptaibols, polyketides, pyrones, terpenes, and diketopiperazine-type compounds, some of which have been shown to display potent antimicrobial or antifungal activity (Yu et al., 2023; Li et al., 2025). These molecules can act by directly inhibiting the growth of the pathogen, disrupting its development, or triggering systemic resistance in the plant, thus creating several layers of defense (Caracciolo et al., 2023).
The finding that Trichoderma treatments significantly enhanced tomato plant growth aligns with a substantial body of research demonstrating the growth-promoting effects of Trichoderma spp. on tomato plants. For instance, a study by Mwangi et al. (2011) reported that inoculation with T. harzianum improved various growth parameters of tomato seedlings, including shoot length, root length, and dry biomass, compared to untreated controls. Similarly, Fontenelle et al. (2011) observed that 12 out of 28 Trichoderma isolates promoted an increase in dry matter mass of tomato seedlings by over 100%, indicating a significant enhancement in plant growth.
Moreover, a study by Palacios-Torres et al. (2019) found that application of Trichoderma spp. resulted in increased tomato yields and fruit quality compared to untreated controls, further supporting the role of Trichoderma in promoting tomato plant growth. These studies collectively underscore the potential of Trichoderma spp. as effective biostimulants for enhancing tomato plant growth.
The variability in growth responses among different Trichoderma species, suggests that the efficacy of Trichoderma treatments can be influenced by several factors, including the specific strain used and the genetic background of the host plant. Therefore, selecting appropriate Trichoderma strains tailored to specific tomato cultivars and environmental conditions is crucial for maximizing growth promotion and disease suppression.
5 Conclusion
This study demonstrates the potential of Trichoderma species, particularly T. longibrachiatum, as effective biocontrol agents against C. spicifera in tomato plants. Both in vitro and greenhouse experiments confirmed the strong antagonistic effects of T. longibrachiatum, which significantly reduced pathogen growth and disease severity. Additionally, treated plants exhibited enhanced biochemical defenses, including increased antioxidant enzyme activities, elevated protein content, improved chlorophyll levels, and better agronomic performance. These results highlight T. longibrachiatum as a promising, eco-friendly alternative to chemical control methods for managing C. spicifera, one of the most harmful pathogens of tomato, offering the dual benefits of disease suppression and plant growth promotion. Further field studies are recommended to validate these findings under diverse environmental conditions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
LH-H: Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. AR: Investigation, Software, Writing – original draft. TW: Formal analysis, Resources, Writing – original draft. AU: Investigation, Software, Writing – original draft. NR: Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This paper was supported by the RUDN University Strategic Academic Leadership Program. The financial support has been provided by SIRAM project within the framework of PRIMA, a program supported by H2020, the European Program for Research and Innovation and the Tunisian Ministry of Higher Education and Scientific Research (MERS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AbdElfatah, H. A. S., Sallam, N. M. A., Mohamed, M. S., and Bagy, H. M. (2021). Curvularia lunata as new causal pathogen of tomato early blight disease in Egypt. Mol. Biol. Rep. 48, 3001–3006. doi: 10.1007/s11033-021-06254-8
Abdelmoteleb, A. L., Moreno-Ramírez, B., Valdez-Salas, M. F., Seleiman, S., El-Hendawy, K. J., Aldhuwaib, M., et al. (2023). New Bacillus subtilis strains isolated from Prosopis glandulosa rhizosphere for suppressing Fusarium Spp. and enhancing growth of Gossypium hirsutum L. Biology 12:73. doi: 10.3390/biology12010073
Almaghasla, M. I., El-Ganainy, S. M., and Ismail, A. M. (2023). Biological activity of four Trichoderma species confers protection against Rhizoctonia solani, the causal agent of cucumber damping-off and root rot diseases. Sustain. For. 15:7250. doi: 10.3390/su15097250
Almansoori, T., Salman, M., and Aljazeri, M. (2021). Rapid and nondestructive estimations of chlorophyll concentration in date palm (Phoenix dactylifera L.) leaflets using SPAD-502+ and CCM-200 portable chlorophyll meters. Emir. J. Food Agric. 33, 544–554. doi: 10.9755/ejfa.2021.v33.i7.2723
Al-Shuaibi, B. K., Kazerooni, E. A., Al-Maqbali, D., Al-Kharousi, M., Al-Yahya’ei, M. N., Hussain, S., et al. (2024). Biocontrol potential of Trichoderma Ghanense and Trichoderma Citrinoviride toward Pythium aphanidermatum. J. Fungi 10:284. doi: 10.3390/jof10040284
Awad-Allah, E. F. A., Shams, A. H. M., Helaly, A. A., and Ragheb, E. I. M. (2022). Effective applications of Trichoderma spp. as biofertilizers and biocontrol agents mitigate tomato Fusarium wilt disease. Agriculture 12:1950. doi: 10.3390/agriculture12111950
Baral, D., Thapa, S., and Saha, J. (2022). First report of Curvularia alcornii as a plant pathogen causing post-harvest rot of tomatoes. New Dis. Rep. 45:e12078. doi: 10.1002/ndr2.12078
Becker, E., Rajakulendran, N., and Shamoun, S. F. (2025). Biocontrol potential of Trichoderma spp. against Phytophthora ramorum. Pathogens 14:136. doi: 10.3390/pathogens14020136
Behiry, S., Soliman, S. A., Massoud, M. A., Abdelbary, M., Kordy, A. M., Abdelkhalek, A., et al. (2023). Trichoderma pubescens elicit induced systemic resistance in tomato challenged by Rhizoctonia solani. J. Fungi 9:167. doi: 10.3390/jof9020167
Benslim, A., Mezaache-Aichour, S., Haichour, N., Chebel, S., and Mihoub Zerroug, M. (2016). Evaluation of inhibition of fungal spore germination by rhizospheric bacterial extracts. ARRB 11, 1–7. doi: 10.9734/arrb/2016/31228
Bint-e-Zahira, S., Khalid, A. N., Yousaf, N., Iqbal, M., Anwar, T., Qureshi, H., et al. (2024). Exploring Trichoderma species in industrial wastewater: morphological and molecular insights from isolates. Life 14:750. doi: 10.3390/life14060750
Boamah, S., Zhang, S., Xu, B., Zhu, N., and Li, E. (2025). Trichoderma longibrachiatum TG1 colonization and signal pathway in alleviating salinity and Fusarium pseudograminearum stress in wheat. Int. J. Mol. Sci. 26:4018. doi: 10.3390/ijms26094018
Bouanaka, H., Bellil, I., Harrat, W., Boussaha, S., Benbelkacem, A., and Khelifi, D. (2021). On the biocontrol by Trichoderma afroharzianum against Fusarium culmorum responsible of fusarium head blight and crown rot of wheat in Algeria. Egypt. J. Biol. Pest Control 31:68. doi: 10.1186/s41938-021-00416-3
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Cao, X., Liang, J., Wu, Z., Zhang, M., Li, H., Liu, T., et al. (2025). Biocontrol mechanisms of Trichoderma longibrachiatum SMF2 against Lanzhou lily wilt disease caused by Fusarium oxysporum and Fusarium solani. Horticulturae 11:660. doi: 10.3390/horticulturae11060660
Caracciolo, R., Sella, L., De Zotti, M., Bolzonello, A., Armellin, M., Trainotti, L., et al. (2023). Efficacy of Trichoderma longibrachiatum Trichogin GA IV Peptaibol analogs against the black rot pathogen Xanthomonas campestris pv. Campestris and other Phytopathogenic Bacteria. Microorganisms 11:480. doi: 10.3390/microorganisms11020480
Chávez-Avilés, M. N., García-Álvarez, M., Ávila-Oviedo, J. L., Hernández-Hernández, I., Bautista-Ortega, P. I., and Macías-Rodríguez, L. I. (2024). Volatile organic compounds produced by Trichoderma asperellum with antifungal properties against Colletotrichum acutatum. Microorganisms 12:2007. doi: 10.3390/microorganisms12102007
Chen, S. C., Ren, J. J., Zhao, H. J., Wang, X. L., Wang, T. H., Jin, S. D., et al. (2019). Trichoderma harzianum improves defense against Fusarium oxysporum by regulating ROS and RNS metabolism, redox balance, and energy flow in cucumber roots. Phytopathology 109, 972–982. doi: 10.1094/PHYTO-09-18-0342-R
Connally, A., Smith, D., Marek, S., Wu, Y., and Walker, N. (2022). Phylogenetic evaluation of Bipolaris and Curvularia species collected from turfgrasses. Int. Turfgrass Soc. Res. J. 14, 916–930. doi: 10.1002/its2.16
Cornejo-Ríos, K., Osorno-Suárez, M. D. P., Hernández-León, S., Reyes-Santamaría, M. I., Juárez-Díaz, J. A., Pérez-España, V. H., et al. (2021). Impact of Trichoderma asperellum on chilling and drought stress in tomato (Solanum lycopersicum). Horticulturae 7:385. doi: 10.3390/horticulturae7100385
Cui, W. L., Lu, X. Q., Bian, J. Y., Qi, X. L., Li, D. W., and Huang, L. (2020). Curvularia spicifera and Curvularia muehlenbeckiae causing leaf blight on Cunninghamia lanceolata. Plant Pathol. 69, 1139–1147. doi: 10.1111/ppa.13198
Decsi, K., Ahmed, M., Abdul-Hamid, D., and Tóth, Z. (2025). The role of salicylic acid in activating plant stress responses results of the past decade and future perspectives. Int. J. Mol. Sci. 26:4447. doi: 10.3390/ijms26094447
Deguine, J. P., Aubertot, J. N., Flor, R. J., Lescourret, F., Wyckhuys, K. A. G., and Ratnadass, A. (2021). Integrated pest management: good intentions, hard realities. A review. Agron. Sustain. Dev. 41:38. doi: 10.1007/s13593-021-00689-w
Díaz-García, E., Valenzuela-Quintanar, A. I., Sánchez-Estrada, A., González-Mendoza, D., Tiznado-Hernández, M. E., Islas-Rubio, A. R., et al. (2024). Phenolic compounds synthesized by Trichoderma longibrachiatum native to semi-arid areas show antifungal activity against phytopathogenic fungi of horticultural interest. Microbiol. Res. 15, 1425–1440. doi: 10.3390/microbiolres15030096
Dini, I., Pascale, M., Staropoli, A., Marra, R., and Vinale, F. (2021). Effect of selected Trichoderma strains and metabolites on olive drupes. Appl. Sci. 11:8710. doi: 10.3390/app11188710
Dourou, M., and La Porta, C. A. M. (2023). A pipeline to investigate fungal–fungal interactions: Trichoderma isolates against plant-associated Fungi. J. Fungi 9:461. doi: 10.3390/jof9040461
Ferreira, N. C. D., Ramos, M. L. G., and Gatto, A. (2024). Use of Trichoderma in the production of forest seedlings. Microorganisms 12:237. doi: 10.3390/microorganisms12020237
Fontenelle, A. D. B., Guzzo, S. D., Lucon, C. M. M., and Harakava, R. (2011). Growth promotion and induction of resistance in tomato plant against Xanthomonas euvesicatoria and Alternaria solani by Trichoderma spp. Crop Prot. 30, 1492–1500. doi: 10.1016/j.cropro.2011.07.019
Fu, J., Liu, Z., Li, Z., Wang, Y., and Yang, K. (2017). Alleviation of the effects of saline-alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS One 12:e0179617. doi: 10.1371/journal.pone.0179617
Glass, N. L., and Donaldson, G. C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 61, 1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995
Gouit, S., Chair, I., Belabess, Z., Legrifi, I., Goura, K., Tahiri, A., et al. (2024). Harnessing Trichoderma spp.: a promising approach to control apple scab disease. Pathogens 13:752. doi: 10.3390/pathogens13090752
Güçlü, T., and Özer, N. (2022). Trichoderma harzianum antagonistic activity and competition for seed colonization against seedborne pathogenic fungi of sunflower. Lett. Appl. Microbiol. 74:698. doi: 10.1111/lam.13698
Hajji-Hedfi, L., Hlaoua, W., Al-Judaibi, A. A., Rhouma, A., Horrigue-Raouani, N., and Abdel-Azeem, A. M. (2023a). Comparative effectiveness of filamentous fungi in biocontrol of Meloidogyne javanica and activated defense mechanisms on tomato. J. Fungi 9:37. doi: 10.3390/jof9010037
Hajji-Hedfi, L., Rhouma, A., Al-Judaibi, A. A., Hajlaoui, H., Hajlaoui, F., and Abdel Azeem, A. M. (2024). Valorization of Capsicum annuum seed extract as an antifungal against Botrytis cincera. Waste Biomass Valor. 15, 2559–2573. doi: 10.1007/s12649-023-02322-1
Hajji-Hedfi, L., Rhouma, A., Hajlaoui, H., Hajlaoui, F., and Rebouh, N. Y. (2023b). Understanding the influence of applying two culture filtrates to control gray mold disease (Botrytis cinerea) in tomato. Agronomy 13:1774. doi: 10.3390/agronomy13071774
Hall, T. A. (1999). Bioedit: a user-friendly biological sequence alignment editor and analysis program for window 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Hernández, G., Ponce de la Cal, A., Louis, Y., Baró Robaina, Y., Coll, Y., Spengler, I., et al. (2024). Identification of secondary metabolites by UHPLC-ESI-HRMS/MS in antifungal strain Trichoderma harzianum (LBAT-53). J. Fungi 10:547. doi: 10.3390/jof10080547
Huang, L., Bian, Q., Liu, M., Hu, Y., Chen, L., Gu, Y., et al. (2024). Structure and fungicidal activity of secondary metabolites isolated from Trichoderma hamatum b-3. J. Fungi 10:755. doi: 10.3390/jof10110755
Ishii, H. (2006). Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. Jpn. Agric. Res. Q. 40, 205–211. doi: 10.6090/jarq.40.205
Jamil, A. (2021). Antifungal and plant growth promoting activity of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici colonizing tomato. J. Plant Prot. Res. 61, 243–253. doi: 10.24425/jppr.2021.137950
Khan, R. A. A., Najeeb, S., Hussain, S., Xie, B., and Li, Y. (2020). Bioactive secondary metabolites from Trichoderma spp. against Phytopathogenic Fungi. Microorganisms 8:817. doi: 10.3390/microorganisms8060817
Kherif, O., Keskes, M. I., Pansu, M., Ouaret, W., Rebouh, Y. N., Dokukin, P., et al. (2021). Agroecological modeling of nitrogen and carbon transfers between decomposer micro-organisms, plant symbionts, soil and atmosphere in an intercropping system. Ecol. Model. 440:109390. doi: 10.1016/j.ecolmodel.2020.109390
Köhl, J., Kolnaar, R., and Ravensberg, W. J. (2019). Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front. Plant Sci. 10:845. doi: 10.3389/fpls.2019.00845
Kumar, S., Stecher, G., and Tamura, K. (2016). Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Li, E., Zhu, N., Zhang, S., Xu, B., Liu, L., and Zhang, A. (2025). Efficacy of Trichoderma longibrachiatum SC5 fermentation filtrate in inhibiting the Sclerotinia sclerotiorum growth and development in sunflower. Int. J. Mol. Sci. 26:201. doi: 10.3390/ijms26010201
López-López, M. E., Del-Toro-Sánchez, C. L., Gutiérrez-Lomelí, M., Ochoa-Ascencio, S., Aguilar-López, J. A., Robles-García, M. A., et al. (2022). Isolation and characterization of Trichoderma spp. for antagonistic activity against avocado (Persea americana mill) fruit pathogens. Horticulturae 8:714. doi: 10.3390/horticulturae8080714
Mahmoud, G. A. E., Abdel-Sater, M. A., Al-Amery, E., and Hussein, N. A. (2021). Controlling Alternaria cerealis MT808477 tomato phytopathogen by Trichoderma harzianum and tracking the plant physiological changes. Plan. Theory 10:1846. doi: 10.3390/plants10091846
Madden, T. L., Tatusov, R. L., and Zhang, J. (1996). Applications of network BLAST server. Methods Enzymol 266:131–141. doi: 10.1016/S0076-6879(96)66011-X
Manjarres-Lopez, D. P., Andrades, M. S., Sanchez-Gonzalez, S., Rodriguez-Cruz, M. S., Sanchez-Martin, M. J., and Herrero-Hernandez, E. (2021). Assessment of pesticide residues in waters and soils of a vineyard region and its temporal evolution. Environ. Pollut. 284:117463. doi: 10.1016/j.envpol.2021.117463
Manzar, N., Kashyap, A. S., Maurya, A., Rajawat, M. V. S., Sharma, P. K., Srivastava, A. K., et al. (2022). Multi-gene phylogenetic approach for identification and diversity analysis of Bipolaris maydis and Curvularia lunata isolates causing foliar blight of Zea mays. J. Fungi 8:802. doi: 10.3390/jof8080802
Mayer, A. M., Harel, E., and Shaul, R. B. (1965). Assay of catechol oxidase: a critical comparison of methods. Phytochemistry 5, 783–789. doi: 10.1016/S0031-9422(00)83660-2
Modrzewska, M., Błaszczyk, L., Stępień, Ł., Urbaniak, M., Waśkiewicz, A., Yoshinari, T., et al. (2022). Trichoderma versus Fusarium inhibition of pathogen growth and mycotoxin biosynthesis. Molecules 27:8146. doi: 10.3390/molecules27238146
Muhie, S. H. (2022). Novel approaches and practices to sustainable agriculture. J. Agric. Food Res. 10:100446. doi: 10.1016/j.jafr.2022.100446
Mwangi, M. W., Monda, E. O., Okoth, S. A., and Jefwa, J. M. (2011). Inoculation of tomato seedlings with Trichoderma Harzianum and Arbuscular Mycorrhizal Fungi and their effect on growth and control of wilt in tomato seedlings. Braz. J. Microbiol. 42, 508–513. doi: 10.1590/S1517-838220110002000015
Napolitano, A., Senatore, M., Coluccia, S., Palomba, F., Castaldo, M., Spasiano, T., et al. (2024). Development and evaluation of a Trichoderma-based bioformulation for enhancing sustainable potato cultivation. Horticulturae 10:664. doi: 10.3390/horticulturae10070664
Ojha, S., and Chatterjee, N. C. (2011). Mycoparasitism of Trichoderma spp. in biocontrol of fusarial wilt of tomato. Arch. Phytopathol. Plant Protect. 44, 771–782. doi: 10.1080/03235400903187444
Palacios-Torres, R. E., Bustamante-Ortiz, A. G., Prieto-Baeza, L. A., Hernández-Hernández, H., Ramírez-Seañez, A. R., Yam-Tzec, J. A., et al. (2019). Effect of foliar application of Trichoderma on the quality of tomato fruits grown in different hydroponic substrates. Folia Hortic. 31, 355–364. doi: 10.2478/fhort-2019-0028
Pathak, V. M., Verma, V. K., Rawat, B. S., Kaur, B., Babu, N., Sharma, A., et al. (2022). Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: a comprehensive review. Front. Microbiol. 13:962619. doi: 10.3389/fmicb.2022.962619
Rabaaoui, A., Masiello, M., Somma, S., Crudo, F., Dall’Asta, C., Righetti, L., et al. (2022). Phylogeny and mycotoxin profiles of pathogenic Alternaria and Curvularia species isolated from date palm in southern Tunisia. Front. Microbiol. 13:1034658. doi: 10.3389/fmicb.2022.1034658
Rao, Y. H., Yadhuvamsha, H., Devi, P. H. S., Vemavarapu, V. V., and Chowdary, K. R. (2020). In vitro evaluation of antagonistic potential of native Trichoderma spp.,botanicals and fungicides against Curvularia spicifera causing Curvularia leaf spot of tomato in Manipur. Int. J. Curr. Microbiol. Appl. Sci. 9, 1815–1823. doi: 10.20546/ijcmas.2020.910.221
Rebouh, N. Y., Aliat, T., Polityko, P. M., Kherchouche, D., Boulelouah, N., Temirbekova, S. K., et al. (2022). Environmentally friendly wheat farming: biological and economic efficiency of three treatments to control fungal diseases in winter wheat (Triticum aestivum L.) under field conditions. Plan. Theory 11:1566. doi: 10.3390/plants11121566
Rebouh, N. Y., Latati, M., Polityko, P., Kucher, D., Hezla, L., Norezzine, A., et al. (2020). Influence of three cultivation technologies to control Fusarium spp. in winter wheat (Triticum aestivum L.) production under Moscow conditions. Res. Crops. 21, 17–25. doi: 10.31830/2348-7542.2020.003
Rebouh, N. Y., Polityko, P. M., Pakina, E., Plushikov, V. G., Norezzine, A., Gadzhikurbanov, A., et al. (2019). Impact of three integrated crop protection treatments on the varieties of winter wheat (Triticum aestivum L.) in Moscow area, Russia. Res. Crops 20, 161–168. doi: 10.31830/2348-7542.2019.022
Reddy, K. P., Subhani, S. M., Khan, P. A., and Kumar, K. B. (1995). Effect of light and benzyladenine on dark treated graving rice (Oryza sativa) leaves - changes in peroxidase activity. Plant Cell Physiol. 26, 987–994.
Ren, X., Branà, M. T., Haidukowski, M., Gallo, A., Zhang, Q., Logrieco, A. F., et al. (2022). Potential of Trichoderma spp. for biocontrol of aflatoxin-producing aspergillus flavus. Toxins 14:86. doi: 10.3390/toxins14020086
Riera, N., Davyt, D., Durán, R., Iraola, G., Lemanceau, P., and Bajsa, N. (2023). An antibiotic produced by Pseudomonas fluorescens CFBP2392 with antifungal activity against Rhizoctonia solani. Front. Microbiol. 14:1286926. doi: 10.3389/fmicb.2023.1286926
Rubio, M. B., Monti, M. M., Gualtieri, L., Ruocco, M., Hermosa, R., and Monte, E. (2023). Trichoderma harzianum volatile organic compounds regulated by the THCTF1 transcription factor are involved in antifungal activity and beneficial plant responses. J. Fungi 9:654. doi: 10.3390/jof9060654
Shams, A. H. M., Helaly, A. A., Algeblawi, A. M., and Awad-Allah, E. F. A. (2023). Efficacy of seed-biopriming with Trichoderma spp. and foliar spraying of zno-nanoparticles induce cherry tomato growth and resistance to Fusarium wilt disease. Plan. Theory 12:3117. doi: 10.3390/plants12173117
Simoglou, K. Β., Stavrakaki, M., Alipranti, K., Mylona, K., and Roditakis, E. (2024). Understanding greenhouse tomato (Solanum lycopersicum L.) growers’ perceptions for optimal Phthorimaea absoluta (Meyrick) management a survey in Greece. Agriculture 14:2291. doi: 10.3390/agriculture14122291
Sivanesan, A. (1987). Graminicolous species of Bipolaris, Curvularia, Drechslera, Exsorohilum and their teleomorphs. Mycological Papers, No. 158, pp. 1–259.
Suriani Ribeiro, M., Graciano de Paula, R., Raquel Voltan, A., de Castro, R. G., Carraro, C. B., José de Assis, L., et al. (2019). EEndo-β-1,3-glucanase (GH16 family) from Trichoderma harzianum participates in Cell Wall biogenesis but is not essential for antagonism against plant pathogens. Biomol. Ther. 9:781. doi: 10.3390/biom9120781
Tao, H., Bao, Z., Jin, C., Miao, W., Fu, Z., and Jin, Y. (2020). Toxic effects and mechanisms of three commonly used fungicides on the human colon adenocarcinoma cell line Caco-2. Environ. Pollut. 263:114660. doi: 10.1016/j.envpol.2020.114660
Temirbekova, S. K., Kulikov, I. M., Afanasyeva, Y. V., Beloshapkina, O. O., Kalashnikova, E. A., Kirakosyan, R. N., et al. (2021). The evaluation of winter wheat adaptation to climate change in the central non-black region of Russia: study of the gene pool resistance of wheat from the N.I. Vavilov Institute of Plant Industry (VIR) world collection to abiotic stress factors. Plan. Theory 10:2337. doi: 10.3390/plants10112337
Tomah, A. A., Alamer, I. S. A., Khattak, A. A., Ahmed, T., Hatamleh, A. A., Al-Dosary, M. A., et al. (2023). Potential of Trichoderma virens HZA14 in controlling Verticillium wilt disease of eggplant and analysis of its genes responsible for Microsclerotial degradation. Plan. Theory 12:3761. doi: 10.3390/plants12213761
Wang, Z., Yun, S., An, Y., Shu, L., Li, S., Sun, K., et al. (2025). Effect of fungicides on soil respiration, microbial community, and enzyme activity: a global meta-analysis (1975-2024). Ecotoxicol. Environ. Saf. 289:117433. doi: 10.1016/j.ecoenv.2024.117433
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics” in PCR protocols: A guide to methods and applications. eds. M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White, vol. 315 (San Diego, CA: Academic Press), 322.
Williams, G. M., Linker, H. M., Waldvogel, M. G., Leidy, R. B., and Schal, C. (2005). Comparison of conventional and integrated pest management programs in public schools. J. Econ. Entomol. 98, 1275–1283. doi: 10.1603/0022-0493-98.4.1275
Yao, X., Guo, H., Zhang, K., Zhao, M., Ruan, J., and Chen, J. (2023). Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 14:1160551. doi: 10.3389/fmicb.2023.1160551
Yu, C., Jiang, X., Xu, H., and Ding, G. (2023). Trichoderma longibrachiatum inoculation improves drought resistance and growth of Pinus massoniana seedlings through regulating physiological responses and soil microbial community. J. Fungi 9:694. doi: 10.3390/jof9070694
Keywords: antifungal activity, biotic stress, biocontrol, Solanum lycopersicum, biostimulant
Citation: Hajji-Hedfi L, Rhouma A, Wannassi T, Utkina AO and Rebouh NY (2025) Biocontrol assessment of Trichoderma species on tomato crops infested by Curvularia Spicifera: toward sustainable farming systems. Front. Sustain. Food Syst. 9:1627903. doi: 10.3389/fsufs.2025.1627903
Edited by:
Vuk M. Maksimović, University of Belgrade, SerbiaReviewed by:
Elsherbiny A. Elsherbiny, Mansoura University, EgyptEman F. A. Awad-Allah, Alexandria University, Egypt
Nicolás Pastor, National University of Río Cuarto, Argentina
Copyright © 2025 Hajji-Hedfi, Rhouma, Wannassi, Utkina and Rebouh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nazih Y. Rebouh, bi55YWNlcjE2QG91dGxvb2suZnI=; Lobna Hajji-Hedfi, bG9ibmEuaGFqamlAaXJlc2EuYWdyaW5ldC50bg==
 Lobna Hajji-Hedfi
Lobna Hajji-Hedfi Abdelhak Rhouma
Abdelhak Rhouma Takwa Wannassi
Takwa Wannassi Aleksandra O. Utkina
Aleksandra O. Utkina Nazih Y. Rebouh
Nazih Y. Rebouh