- 1Faculty of Science and Technology, Department of Agricultural and Fisheries Science, Prince of Songkla University, Pattani, Thailand
- 2Urban Agriculture Technology Research Group, Faculty of Science and Technology, Prince of Songkla University, Pattani, Thailand
- 3Department of Mathematics and Computer Science, Faculty of Science and Technology, Prince of Songkla University, Pattani, Thailand
- 4Kasetsart University International College, Kasetsart University, Bangkok, Thailand
Recycling food waste and agricultural byproducts into the food production cycle is essential for minimizing waste, consequently promoting sustainable global agricultural and food systems. This study aimed to support the goals of sustainable nutrition for both animals and humans by evaluating the nutritional composition, energy potential, and antioxidant properties of different anatomical components of the Parkia timoriana pod —namely, the exocarp, mesocarp, and endocarp. The analysis revealed that the mesocarp exhibited the highest levels of total amino acids, moisture, crude protein, ether extract, crude ash, nitrogen-free extract, non-structural carbohydrates, digestible energy, metabolizable energy, net energy for maintenance, gain, and lactation, as well as relative feeding values. It also contained the highest contents of phosphorus, potassium, iron, manganese, magnesium, copper, and zinc. In contrast, the exocarp had the highest levels of acid detergent fiber, acid detergent lignin, gross energy, calcium, total phenolic content, and DPPH radical scavenging activity, indicating strong antioxidant potential. The endocarp demonstrated the highest levels of dry matter, organic matter, total carbonhydrate, crude fiber, neutral detergent fiber, hemicellulose, cellulose, and the highest calcium-to-phosphorus ratio. Based on the nutritional classification for animals, all pod wall components were categorized as roughage and energy-rich feedstuffs. Forage quality grading classified the mesocarp as “Prime,” the exocarp as “Poor,” and the endocarp as “Reject,” reflecting their respective suitability in feed formulations. This is the first study to systematically characterize the internal anatomical components of P. timoriana pods, offering new insights into their heterogeneous nutritional and functional potential and supporting their targeted use as sustainable feed and functional ingredient resources.
1 Introduction
The recycling of food waste and its byproducts into food production is one of the key methods of minimizing waste, conserving vital resources, and addressing environmental and social problems as part of global sustainable agriculture and food systems (Swetha et al., 2024). Organic waste, once regarded as useless and hazardous, has now become a valuable raw material for producing a wide range of products (Jayeola et al., 2018). Technological advancements have significantly increased its economic and nutritional potential, as countries shift their focus from exporting waste for minimal foreign exchange to developing technologies that enable more efficient use (Jayeola et al., 2018). In the agri-food sector, the processing of fruits and vegetables generates waste that is abundant in bioactive compounds, which poses both great potential and hurdles for zero-waste strategies as well as the creation of functional foods (Naik et al., 2023). Furthermore, when enhanced with modern technologies, agro residues possess enormous potential in resolving global problems such as the deficiency of animal feed and protein (Ajila et al., 2012). Nowadays, apple pomace, carrot pomace, and mango peels are considered rich in bioactive compounds and have been recently used as functional food ingredients in bakery products (Naik et al., 2023) and are also being developed into value-added bioproducts for the agri-food and pharmaceutical industries using micro- and nano-formulation technologies (Bala et al., 2023).
P. timoriana, a member of the Fabaceae family, is a fast-growing leguminous species that grows throughout Southeast Asia and the Pacific region (Singha et al., 2021). This plant, growing mostly in the Southern Thailand and India, is known as a food and traditional medicinal plant in which its various parts, such as the leaf, pod (fruit), and seed, have been reported to exhibit antioxidant and numerous biological activities (Angami et al., 2018; Apirattananusorn, 2017; Singha et al., 2021). This large perennial tree typically flowers from November to December and produces pods (fruits) from December to April (Apirattananusorn, 2017). The ripe dry pods or black pods, generally falling down from its tree, are collected to obtain seeds for producing sprout vegetables (Apirattananusorn, 2017). The black pod mainly consists of four different parts, including husk (56.30%), seed (17.07%), pod powder (12.33%), and endocarp (12.11%; Wanapat et al., 2024). Among these, only the seeds are used, whereas the other parts are normally discarded as waste. Nevertheless, in some local communities in Southern Thailand, these pod wastes have been repurposed for culinary use, such as in the preparation of jelly-like desserts (Apirattananusorn, 2017). The earlier studies on P. timoriana have been primarily concerned with seeds and young pods as sources of food and feed, while the pod wall byproduct remains mostly ignored. There are studies which have focused on the young pods and their nutritional uses (Angami et al., 2018), and more recent studies that focused on the extraction of pectin from pod husk and pod powder (Buathongjan et al., 2020), and the use of pod by products as alternative feeds, especially for ruminants (Ruangrak et al., 2025). However, the nutritional composition, energy value, and antioxidant capacity of various anatomical parts of the P. timoriana pod have different anatomical components of the P. timoriana pod and still remain as gaps in the literature. This gap is addressed in the current study by investigating the exocarp, mesocarp, and endocarp, and their potential for enhancing the sustainable nutrition of animals and humans. There is still a limited amount of scientific literature on the nutritional composition on the various pod components and their antioxidant activities, and it is in this respect that this research attempts to find a solution. The focus of this study is to analyze nutrition and antioxidant activity in exocarp, mesocarp and endocarp of P. timoriana pod for sustainable animal and human nutrition.
2 Materials and methods
2.1 Sample preparation
In this study, the P. timoriana pod samples were taken from a commercial seed production company which is based in Surat Thani Province, Thailand. The samples were taken at the threshing phase of the seed production cycle. During this stage of threshing, the pods wall (pericarp) was sorted into three anatomical layers by hand: exocarp (the outer shell), the mesocarp (the fleshy middle layer), and the endocarp (the inner layer which lines the seed) as shown in Figures 1A–C. Any dirt or debris adhering to the samples were cleaned and then placed at 70 °C until they reached a constant weight. About 500–700 g of dried samples were then well ground using a laboratory grinder and then kept at room temperature in sealed containers until they were analyzed.
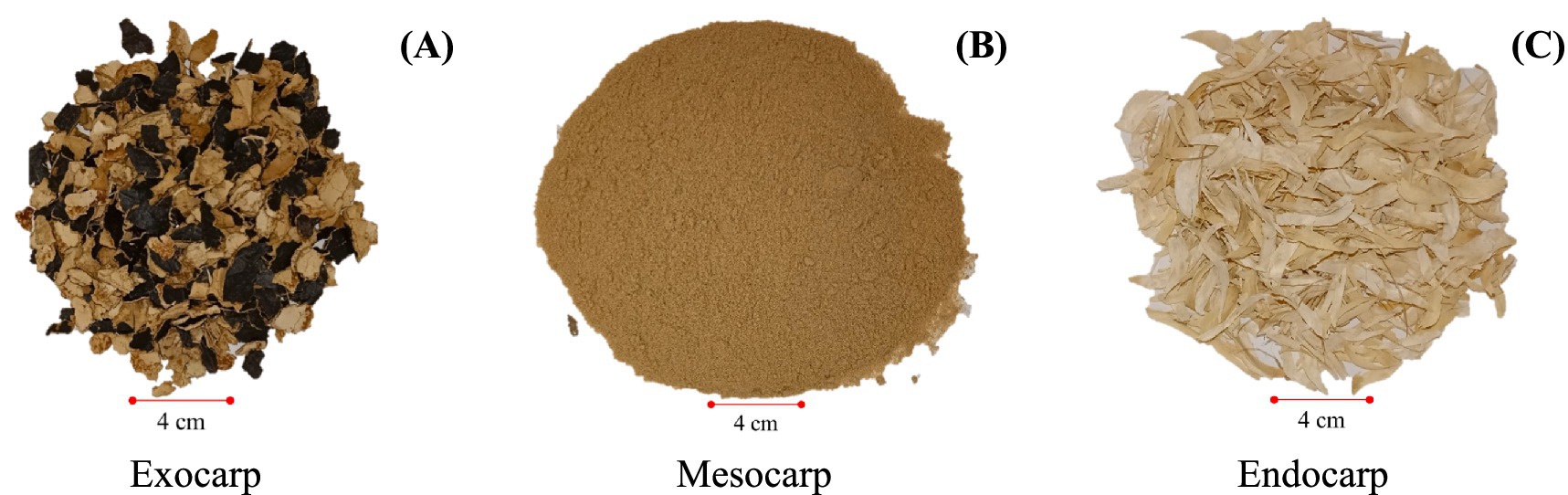
Figure 1. Sample preparation of P. timoriana pods. The pods were mechanically dissected and then hand sorted into three anatomical components: (A) exocarp (outer shell), (B) mesocarp (middle fleshy layer), and (C) endocarp (inner lining surrounding the seeds).
2.2 Amino acid composition analysis
The amino acid composition of the samples was determined by the method (Liu et al., 1995) using HPLC.
2.3 Chemical composition analysis
To determine the nutritional quality of the exocarp, mesocarp and endocarp, the proximate composition analysis including crude protein (CP), ether extract (EE), crude fiber (CF), and crude ash (CA) was performed by following the standard methods described by the Association of Official Analytical Chemists (AOAC, 1990). Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were analyzed using the Van-Soest et al. (1991). Dry matter (DM) content was determined using the procedure outlined by Chen et al. (2024). Moisture content was measured by using the AOAC method (AoOA and Horwitz, 2000). Organic matter (OM) was analyzed using the method of Liu et al. (2021). The value of nitrogen-free extract (NFE) was determined as per Sasu et al. (2023). The hemicellulose (HEM) and cellulose (CEL) contents were derived using the equations established by Van-Soest et al. (1991). Non-structural carbohydrates (NSC) were estimated in accordance with the guidelines of the National Research Council (Council, 1989), and the total carbohydrate (TC) content was computed following the method of AOAC (1995). The formulas used for calculating the respective components were as follows:
2.4 Analysis of relative feeding value
Dry matter intake (DMI), digestible dry matter (DDM), total digestible nutrients (TDN), relative feeding value (RFV), and relative forage quality (RFQ) were assessed from exocarp, mesocarp and endocarp to determine consumption and nutritional digestibility of the forage. The equations used in the calculations were mentioned from Chen et al. (2024) and stated as follows:
2.5 Effective energy analysis
We determined the sample’s gross energy (GE) content through the AOAC (1990) method. Also, to assess the energy availability and utilization, Chen et al. (2024) equations on digestible energy (DE), metabolizable energy (ME), net energy for maintenance (NEm), net energy for gain (NEg), and net energy for milk production (NEL) were used. These formulas are presented below:
2.6 Mineral content analysis
The samples were evaluated for mineral value determining their contribution to nutrition, with special focus on dietary minerals. Calcium (Ca), potassium (K), sodium (Na), magnesium (Mg), iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn) were analyzed. Using Atomic Absorption Spectroscopy (AAS), the contents of these minerals were measured in accordance with the standard procedures set by the Association of Official Analytical Chemists (AOAC, 1990). Phosphate (P) was also analyzed using spectrophotometric method following the Association of Official Analytical Chemists (AOAC, 1990).
2.7 Phenolic compounds and antioxidant activity (DPPH) analysis
Total phenolic compounds were analyzed by the Folin–Ciocalteu phenol colorimetric assay (Kupina et al., 2018). Antioxidant activity was determined by the DPPH radical scavenging assay (Kumar and Chaiyasut, 2017). Ascorbic acid was used as a standard. DPPH radical scavenging activity of each extract was reported in terms of milligrams of ascorbic acid equivalent per mL of extract (mg VCE/mL extract).
2.8 Statistical analysis
The data was analyzed using a one-way analysis of variance (ANOVA) in Microsoft Excel. The analysis was followed with a least significant difference (LSD) test at the level of p < 0.05.
3 Results and discussion
This research focused on harvesting the values of nutrition as well as the antioxidant action from the exocarp, mesocarp, and endocarp of the walls of the P. timoriana pods for the sustainable nutrition of animal and human. This research analyzed the amino acid and chemical compositions, effective energy, relative feeding value, mineral content, total phenolic compounds, and antioxidant activity.
3.1 Amino acid composition
Amino acids are essential nutrients for animal and human health, functioning as fundamental constituents of protein-rich diets and dietary supplements (Karau and Grayson, 2014). An analysis of the amino acid composition of the exocarp, mesocarp, and endocarp of P. timoriana pod was conducted to evaluate their nutritional potential for sustainable animal and human nutrition, as shown in Table 1. The findings show that the mesocarp holds the highest total amino acid (TAA) content and concentration of 4.29 mg/100 mg. The exocarp and endocarp contained lower amounts of 2.91 mg/100 mg and 2.22 mg/100 mg, respectively. The mesocarp’s values are greater than exocarp and endocarp by 1.5-fold and 2.0-fold respectively, proving further that the mesocarp is the most protein-rich tissue of the pod wall. The findings of this study emphasize that the mesocarp can serve as an important additional protein granule in diets of humans and livestock in resource-scarce areas. Despite the relatively high amino acid content in P. timoriana, the values were consistently lower compared with those reported for tender, immature, and mature pods as well as mature kernels of Parkia roxburghii (8.65, 8.84, 8.69, and 14.26 mg/100 mg, respectively; Longvah and Deosthale, 1998; Table 1). These differences can be caused by interspecific variability, genetic differences, stage of maturity, or external factors related to the environment in regard to the nutrients. However, mesocarp of P. timoriana is rather limited in comparison with closely related species. The amino acid profile of P. timoriana pod tissues is lower when compared to the agricultural byproducts T. cacao husk (6.09 mg/100 mg) and T. cacao bean shell (10.74 mg/100 mg; Soares and Oliveira, 2022). Besides, employing the pod tissues, particularly the mesocarp, as livestock feed would enhance dietary nutrition and simultaneously diversify protein sources.
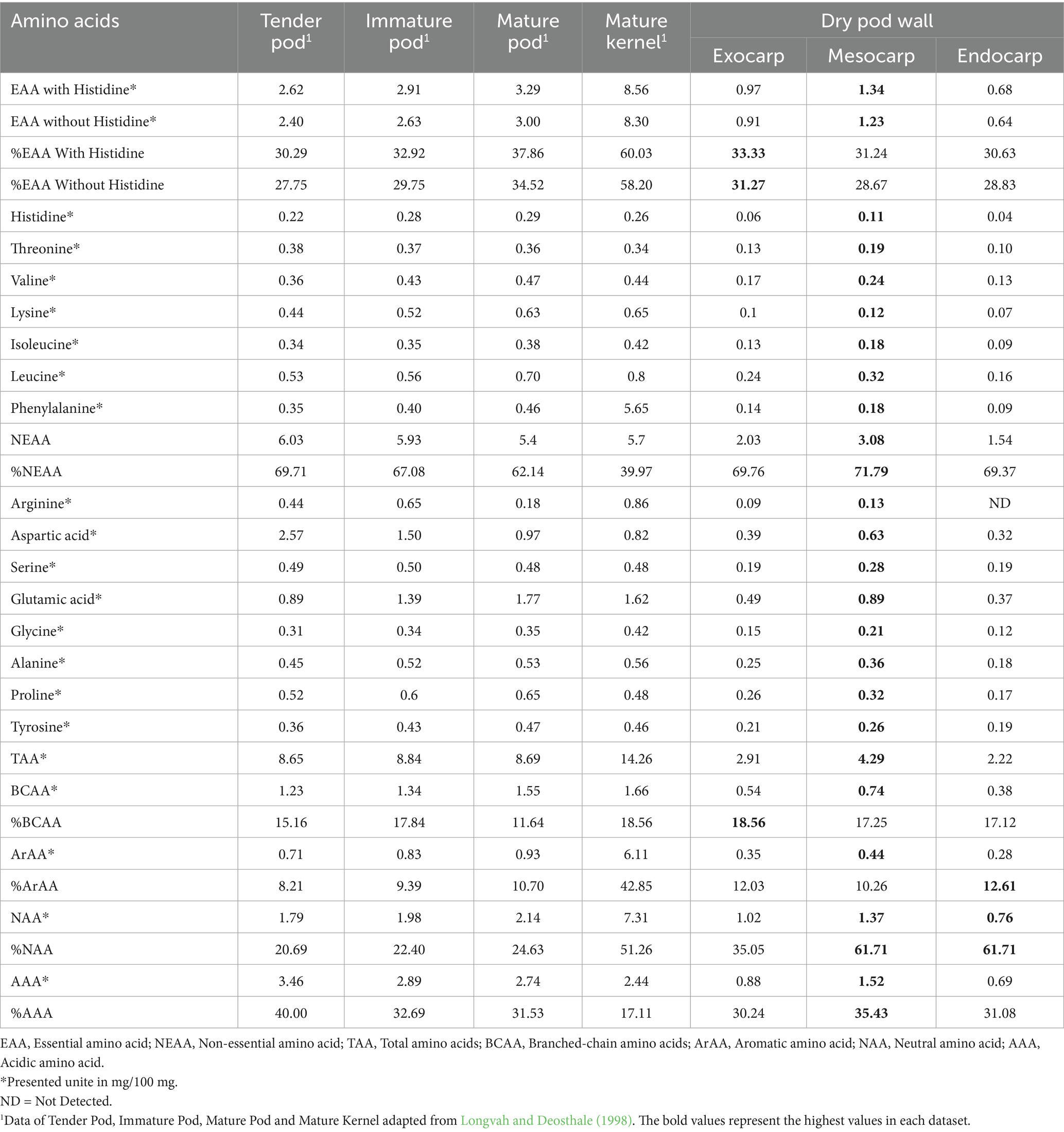
Table 1. The amino acid composition of the exocarp, mesocarp, and endocarp derived from P. timoriana pods.
Essential amino acids (EAAs) are amino acids that are not able to be produced by the cells of humans and other animals or their metabolic processes are unable to produce sufficiently to meet their requirements (Wu, 2021). These amino acids include histidine, threonine, valine, lysine, isoleucine, leucine, phenylalanine, methionine, tryptophan, tyrosine, and cysteine (Hou and Wu, 2018). As shown in Table 1, the mesocarp had the highest EAA value with 1.34 mg/100 mg and 1.23 mg/100 mg when histidine is added or excluded, respectively. The exocarp contained 0.97 mg/100 mg with histidine and 0.91 mg/100 mg without histidine, while the endocarp had the lowest EAA content at 0.68 mg/100 mg (with histidine) and 0.64 mg/100 mg (without histidine). For results of the EAAs with histidine, the mesocarp exhibits approximately 1.4- and 2.0-fold higher values than the exocarp and endocarp, respectively. Besides the EAAs without histidine, the mesocarp exhibits approximately 1.4- and 1.9-fold higher values than the exocarp and endocarp. The value of the EAAs as a percentage of the TAA in the three pod wall components was relatively similar, in the range of 30.63% (endocarp) to 33.33% (exocarp) with histidine and 28.67% (mesocarp) to 31.27% (exocarp) without histidine. Leucine was the individually most abundant EAA in all pod wall components, and the content was the highest in mesocarp (0.32 mg/100 mg), followed by exocarp (0.24 mg/100 mg) and endocarp (0.16 mg/100 mg). Besides these, other EAAs that were present in considerable amounts also included valine and phenylalanine. The possible influence of leucine-rich P. timoriana pod walls suggests that these may help to enhance muscle and metabolic health in animals and humans (Davis et al., 2010; Rehman et al., 2023). In comparison to related species like P. roxburghii, the EAA content in P. timoriana pod walls was consistently lower. P. roxburghii pods had 2.62 to 3.29 mg/100 mg EAAs (with histidine) and 2.40 to 3.00 mg/100 mg (without histidine). Mature kernels had 8.56 mg/100 mg and 8.30 mg/100 mg, respectively (Longvah and Deosthale, 1998). In P. roxburghii, the percentage contributions were higher as well, with mature kernels reaching 60.03% (with histidine) and 58.20% (without) compared to ~30–33% in P. timoriana. This suggests that in P. timoriana pod walls, the functional amino acids provide some nutritional benefit, but the kernels are significantly richer nutritionally in comparison. Likewise, T. cacao byproducts such as husk and bean shell have also shown higher EAA contents in comparison to P. timoriana pod walls. T. cacao husks contained 2.66 mg/100 mg (with histidine) and 2.45 mg/100 mg (without histidine) while T. cacao bean shells showed 4.15 mg/100 mg and 3.88 mg/100 mg, respectively, (Soares and Oliveira, 2022). The EAAs’ relative proportion from P. timoriana pod fractions is similar and emphasize the prospect of these fractions as supplementary protein sources, particularly in areas where P. timoriana pods are abundantly available and T. cacao byproducts are less available. From a sustainability standpoint, the P. timoriana pod walls offer several benefits as animal or human feeds. Their EAA concentrations, while lower than those from kernels or T. cacao byproducts, the steady amino acid profile, particularly the high leucine content, provide functional value. Moreover, the incorporation of exocarp and endocarp in feed could enhance protein and metabolic efficiency while curtailing agricultural waste. For human nutrition, processed pod wall fractions could serve as diversifying protein sources in diet for amino acid profile diversification.
Branched-chain amino acids (BCAAs), which consist of valine, leucine, and isoleucine, participate in numerous physiological and metabolic processes, such as the synthesis of insulin, lipid metabolism, maintenance of glucose homeostasis, the barrier function of the intestine, nutrient absorption, the quality of milk, the health of the mammary gland, the development of the embryo, and immune modulation (Doi et al., 2003; Lei et al., 2012; Li et al., 2009; Negro et al., 2008; Nishimura et al., 2010; Teodoro et al., 2012). Given their wide-ranging functions, the evaluation of BCAAs in P. timoriana pod tissues provides insight into their nutritional and functional potential for sustainable animal and human diets. In this study, BCAAs constituted 17–18% of the TAA across the exocarp, mesocarp, and endocarp of P. timoriana pods, indicating a stable proportional contribution among pod fractions (Table 1). This ratio is similar to what was found in the tender, immature, and mature pods and also the mature kernel of P. roxburghii which was 11–18% (Longvah and Deosthale, 1998; Table 1). The uniformity of the data suggests that P. timoriana pod tissues, though containing lower absolute amino acid concentrations relative to P. roxburghii, still had a relative BCAA contribution that was consistent with other related species. Among the pod tissues of P. timoriana, the mesocarp displayed the highest BCAA content (0.74 mg/100 mg), followed by the exocarp (0.54 mg/100 mg) and endocarp (0.38 mg/100 mg). The mesocarp had about 1.4- and 1.9-fold more than the exocarp and endocarp, respectively. This suggests that the mesocarp is the most promising pod fraction for protein enrichment and dietary supplementation. From a nutritional standpoint, the consistent proportion of BCAAs across fractions indicates that even the non-edible parts, such as the exocarp and endocarp, may be processed into value-added feed ingredients, supporting circular utilization of agricultural byproducts. In comparison, the P. timoriana shows lower values than the P. roxburghii. For example, BCAA concentrations in the tender, immature, and mature pods, as well as in the mature kernel of P. roxburghii, were from 1.23 to 1.66 mg/100 mg (Longvah and Deosthale, 1998; Table 1), considerably greater than the pod wall tissues of P. timoriana. Likewise, some T. cacao byproducts like T. cacao husk (1.11 mg/100 mg; 18.23% of TAA) and T. cacao bean shell (1.18 mg/100 mg; 10.99% of TAA; Soares and Oliveira, 2022) displayed greater BCAA content than the P. timoriana pod fractions. This comparison shows that although P. timoriana pod walls serve as a secondary source of BCAAs, the functional potential of these secretions is preserved because of the well-balanced amino acid profile. Even though the pod walls have lower BCAA concentrations than P. roxburghii kernels or T. cacao byproducts, the pod walls BCAA concentration’s relative scarcity and the two raw materials’ utilization potential highlight prospects for sustainable feed development.
Non-essential amino acids (NEAAs) are considered to be amino acids that are produced endogenously in sufficient quantities to sustain processes such as maintenance, growth, development, and health, thereby reducing the need for such amino acids in the diet (Hou et al., 2015). The important NEAAs are alanine, arginine, asparagine, aspartate, cysteine, glutamate, glutamine, glycine, proline, serine, taurine, and tyrosine (Trumbo et al., 2002; Wu, 2021). In this case, the amino acid composition of P. timoriana pod tissues showed that the NEAAs were of major significance, making up 69–71% of the TAA (Table 1). Of the pod fractions, NEAA content was highest in the mesocarp (3.08 mg/100 mg) followed by the exocarp (2.03 mg/100 mg) and the endocarp (1.54 mg/100 mg). NEAA content was highest in mesocarp followed by exocarp then endocarp. The mesocarp had about 1.5- and 2.0-fold greater concentrations than exocarp and endocarp, respectively, indicating high nutritional value. The results indicate the mesocarp had the highest metabolic activity in the plant tissue in the regard of nitrogen metabolism and amino acid metabolism, NEAA accumulation (Liao et al., 2023; Teh et al., 2013). Glutamic acid was the most abundant NEAA in all the fractions, especially in the mesocarp (0.89 mg/100 mg) followed by aspartic acid and alanine. The predominance of glutamic acid is noted in the pods of P. roxburghii (Longvah and Deosthale, 1998; Table 1), which corroborates its important functions in nitrogen catabolism as well as in the enhancement of flavor as a precursor of umami taste (Bellisle, 1999). In addition, glutamic acid is a precursor of γ-aminobutyric acid (GABA), a neuroactive compound of nutraceutical significance (Kulkarni et al., 2005; Montevecchi et al., 2012). When compared, P. timoriana pod tissues contained lower NEAA levels than P. roxburghii. In the case of P. roxburghii, the tender and immature pods, mature pod, and the mature kernel contained NEAA values of 5.4–6.03 mg/100 mg at about 39.97–69.71% of TAA (Longvah and Deosthale, 1998; Table 1). Also, T. cacao byproducts, such as T. cacao husk (3.43 mg/100 mg; 56.32% of TAA) and T. cacao bean shell (6.59 mg/100 mg; 61.36% of TAA; Soares and Oliveira, 2022), had NEAA concentrations that were higher than P. timoriana pod walls. However, the range of the proportional contribution of NEAAs in P. timoriana aligns with these reference materials and thus strengthens the argument for its nutritional value. From a health perspective, NEAAs, even though they are not categorized as “essential,” are important for overall well-being. In animal nutrition, glycine and serine are critical for collagen synthesis and tissue repair, while alanine and glycine improve feed acceptance (Suehs et al., 2024). As for P. timoriana, the pod fractions appear to contain considerable amounts of NEAAs, particularly glutamate and aspartate, which are associated with valuable feed and food applications.
Aromatic amino acids (ArAAs) are crucial for functioning physiologically in animals and humans, as they serve as biologically and neurologically active precursors for important compounds in the body. They are important in the structure of proteins and as precursors for phenotypically active and neurologically important compounds (Han et al., 2019). In the current analysis, ArAAs were found in all fractions of P. timoriana pods, with the lowest and highest concentrations in the endocarp and mesocarp, respectively (0.28 mg/100 mg and 0.44 mg/100 mg; Table 1). The mesocarp, as a metabolically active tissue, serves to support seed development (Setia et al., 1987) and contained the highest ArAA concentration, which was 1.3 to 1.6-fold higher than the exocarp and endocarp. The proportional contribution of ArAAs to TAA was 12.03, 10.26, and 12.61% in exocarp, mesocarp, and endocarp, respectively. These values indicate that while the mesocarp had the highest absolute concentration of ArAAs, the relative distribution of ArAAs across fractions was balanced. In comparison to P. roxburghii, P. timoriana pod walls did contain ArAAs, but the absolute quantities were significantly lower. For instance, P. roxburghii pods and kernels contained 0.71–6.11 mg/100 mg ArAAs, with the kernel showing particularly high levels (Longvah and Deosthale, 1998; Table 1). The proportional contribution of ArAAs to the TAA (8.21–42.85%) was roughly the same which shows the protein quantity in P. timoriana is lower than in P. roxburghii, the quality of the amino acids is similar. This indicates that even with lower overall protein levels, the pod tissues of P. timoriana have some robust functional amino acid profiles. For other agricultural byproducts, P. timoriana pod tissues ArAA concentration is lower than T. cacao husk (0.83 mg/100 mg; 13.63% of TAA) and T. cacao bean shell (1.26 mg/100 mg; 11.73% of TAA; Soares and Oliveira, 2022). However, the absolute values may be lower, the relative values are still comparable which reinforces the argument for their incorporation as animal feed supplements and possibly as ingredients in functional foods. From a nutritional perspective, the presence of ArAAs in P. timoriana pod fractions aids in protein metabolism, and enhances taste, which may increase the acceptance of livestock feeds. In regard to human nutrition, ArAAs serve as important for protein synthesis and precursors for vital compounds of neurotransmitters and hormones (Pencharz et al., 2007). P. timoriana pod wall tissues may not contain ArAAs as abundantly as P. roxburghii kernels or byproducts of T. cacao, but their functional value is improved by the preservation of relative proportions as well as the mesocarp’s greater concentration. This emphasizes the possibility of augmenting pod wall fractions, especially mesocarp and exocarp, as supplemental sources of bioactive amino acids for sustainable nutrition in animals and humans.
Acidic amino acids (AAAs), primarily aspartic acid and glutamic acid, were abundant in all pod fractions of P. timoriana. The highest amounts were recorded in the mesocarp, which had aspartic acid and glutamic acid levels of 0.63 and 0.89 mg/100 mg, respectively. The exocarp and endocarp showed lower levels of these acids, as indicated by their 0.39 and 0.49 mg/100 mg and 0.32 and 0.37 mg/100 mg, respectively. In general, the mesocarp had the highest overall abundance of AAAs, which was around 1.7- and 2.2-fold greater than exocarp and endocarp, respectively. In the context of TAA, AAAs were observed to constitute 30.24, 35.43, and 31.08% in exocarp, mesocarp, and endocarp, respectively (Table 1). In comparison to P. roxburghii pods and kernels (Longvah and Deosthale, 1998), P. timoriana pod walls had lower absolute AAA concentrations. From a nutritional point of view, aspartic acid and glutamic acid are invaluable. Aspartic acid is involved in amino group transfer, and in the metabolism of nucleotides and energy, whereas glutamic acid is a key player in nitrogen metabolism and is a precursor for several important molecules, including the nutraceutical GABA, an inhibitory neurotransmitter (Chen et al., 2021; Li et al., 2018; Robinson, 1998). Moreover, glutamic acid is important because it helps to boost the umami flavor which increases flavors, and is helpful in improving voluntary feed intake in livestock. However, when comparing the levels to P. roxburghii, the levels may be lower, but the functional importance of aspartic and glutamic acids emphasizes the possibility of considering P. timoriana pod walls as supplementary feed and functional food.
Neutral amino acids (NAAs) are essential for cognitive performance and general health (Douglas et al., 2019). NAA supplementation has been proven effective in enhancing cognitive performance in patients with phenylketonuria and poor metabolic control (Scala et al., 2020). As shown in Table 1, the highest NAA content was recorded in the mesocarp histidine, leucine, isoleucine, valine, phenylalanine, threonine, and tyrosine, which amounted to 1.37 mg/100 mg. The exocarp came second with 1.02 mg/100 mg, followed closely by the endocarp at 0.76 mg/100 mg. The contribution of NAAs to the TAA pool was greatest in the exocarp (35.05%) compared to the mesocarp (31.93%) and endocarp (34.23%). This suggests that the endocarp is less protein dense than the exocarp and mesocarp, but is relatively richer in NAAs in comparison to other pod fractions. These results are in agreement with the earlier findings on pods and kernels of P. roxburghii where, tender, immature pods and mature pods showed NAAs at 1.79, 1.98 and 2.14 mg/100 mg, while matured kernels contained 7.31 mg/100 mg (Longvah and Deosthale, 1998). Consistently, the percentage contribution of NAAs to TAA in P. roxburghii was documented as 20.69, 22.40, 24.63, and 51.26% for tender pods, immature pods, mature pods, and kernels. This relative trend reinforces the functioning of metabolically active tissues like the mesocarp, which serve to accumulate greater quantities of NAA, while storage or protective tissues, like the endocarp, are proportionally richer. Comparing to other agro-industrial byproducts, like T. cacao husk and T. cacao bean shell which contained 3.51 and 5.18 mg/100 mg NAAs with 57.64 and 48.23% proportional contributions (Soares and Oliveira, 2022), the fractions of P. timoriana pods show even greater or at least comparable proportional enrichment. Even though the quantities of NAAs in P. timoriana are lower than in the byproducts of T. cacao, the overall distribution NAAs in P. timoriana indicates that it can be used as a protein supplement. The most important changes from a nutritional perspective in this study are from the view of animal nutrition and human food. From the animal nutrition perspective, especially ruminant nutrition, pod wall byproducts containing NAA can enhance protein in the animal diet, thus improving protein metabolism and productivity. From a human diet perspective, the functional amino acids in the non-kernel tissues suggest that pod walls could be used as functional food ingredients aimed at enhancing the nutritional value of foods. Such applications of agro-wastes are the goals of sustainable nutrition that transform these agricultural byproducts into prized nutritional food resources.
3.2 Chemical composition
This study examined the chemical makeup of the exocarp, mesocarp, and endocarp of the P. timoriana pods with a particular focus on dry matter (DM; Table 2). The findings showed significant differences among pod fractions. The mesocarp contained 82.75% of DM, and the endocarp contained a higher percentage of 90.63%. These results support the physiological functions of pod tissues. The mesocarp needs to retain more water to support seed development, and the endocarp, which is more structural and denser, retains less moisture (Setia et al., 1987). DM is one of the most essential criteria in the evaluation of feeds, as it serves as a basis for comparing the nutritional value of some feeds, estimating nutrient value precisely, and in strictly measuring animal feeds (Cebra et al., 2013). The DM results for the pod fractions of P. timoriana align with some other leguminous species of plants that have traditionally been used in livestock feeding. For example, García-Winder et al. (2009) noted complete pods of A. farnesiana were composed of 90.7% DM, which is in agreement with the endocarp of P. timoriana. Pedro et al. (2023) reported remarkable DM values in the green pods of various Acacia species such as A. melanoxylon (91.41%), A. dealbata (96.79%), A. cyclops (96.22%), A. retinodes (91.03%), A. mearnsii (96.20%), A. pycnantha (93.21%), and A. longifolia (93.80%). In addition, Abraham et al. (2023) noted A. tortilis pods had 91.26% DM, which further confirmed the trend of elevated DM values in leguminous pods. The endocarp of P. timoriana is 90.63% DM, which is within the range of some of these leguminous references, reinforcing its claim to being a nutrient dense fraction compared to well-known feed resources. The mesocarp, on the other hand, is lower in DM (82.75%), having higher moisture. This lower DM makes the mesocarp less favorable for long-term storage, as it is more prone to microbial spoilage (Alp and Bulantekin, 2021). Nonetheless, these fractions still contain substantial nutrient density and could serve as supplementary feed materials. An ecological point of view highlights sustainability where high DM of P. timoriana pods as well as potential alternative feed and food supplies in areas with limited access are of great value and where feed is overpriced. In animal nutrition, the mesocarp and endocarp could enhance the stability and nutrient provision of the ration. For human uses, the mesocarp’s composition indicates it may be usable as a raw material for food product formulation with appropriate processes in place to guarantee safety and stability for storage.
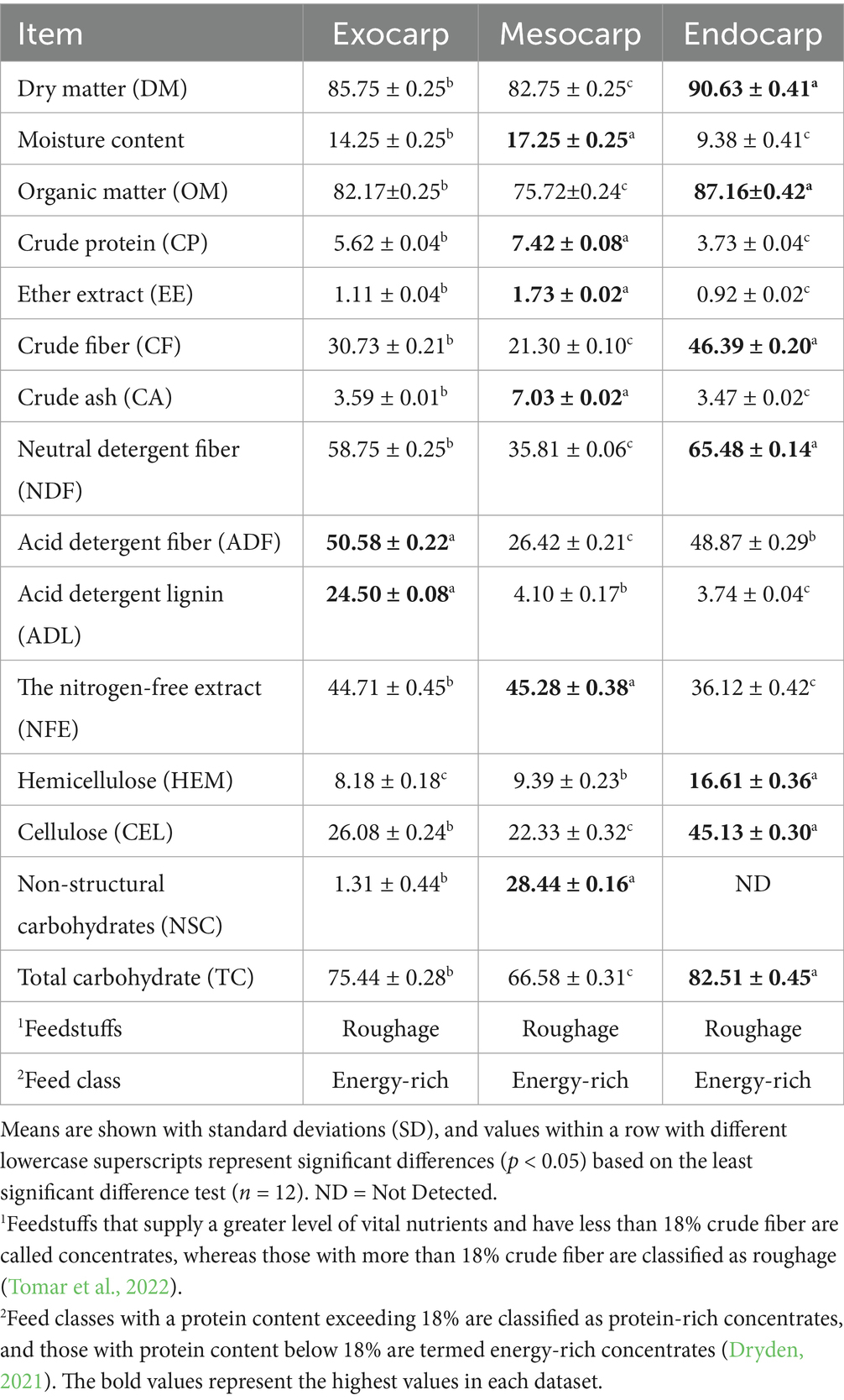
Table 2. Chemical composition of exocarp, mesocarp, and endocarp derived from P. timoriana pods (DM basis, %).
Moisture content in P. timoriana pods was diverse in comparison to their anatomical parts, with the mesocarp showing the greatest value (17.25 ± 0.25%) followed by the exocarp (14.25 ± 0.25%) and the endocarp (9.38 ± 0.41%; Table 2). This trend corresponds to the different biological roles of pod tissues: the mesocarp is the metabolically active and water-rich layer, rich in nutrients for seed development, while the endocarp is more protective and structural, moisture content is lower (Setia et al., 1987). Compared to related and non-related leguminous species, the P. timoriana pod fractions show relatively higher moisture content. In P. roxburghii, moisture contents reported for tender, immature and mature pods, as well as mature kernels, were 8.4, 7.1, 6.7, and 10.0%, respectively, (Longvah and Deosthale, 1998). In the same way, pods of Ceratonia siliqua were shown to contain 8.79% moisture (Ishag et al., 2024). These values are especially lower than those of P. timoriana, especially within the mesocarp and exocarp, which indicates that there are differences at the species level regarding the morphology of pods and the composition of the tissues and their capacity for water binding. From a nutritional and feed perspective, higher moisture present in the mesocarp has its advantages and disadvantages. Increased moisture content enhances ease of digestion and may enhance the fresh feed’s appeal to the livestock, allowing animals to nutritionally hydrate and receive more nutrients (Pasha et al., 1994). On the other hand, excess water can compromise long-term storage by increasing microbial spoilage in these feed fractions if not adequately processed or preserved (Gilpin et al., 2002). On the contrary, the relatively lower moisture content of the endocarp enhances its potential for long-term storage.
Organic matter (OM) is vital for both animal and human food, as it constitutes their building elements, including carbon, hydrogen, oxygen, nitrogen, and organic compounds like carbohydrates, proteins, lipids, and vitamins that support growth, metabolism, and health (Council et al., 2001; Khan, 2015; Vincent et al., 2021). In animal feed, OM is the main energy and digestible nutrient source. Its composition influences the efficiency with which feed is used, its overall digestibility, and the level of microbial activity in the rumen (Council et al., 2001; Khan, 2015). The results of this study showed high OM values, with the mesocarp containing 75.72% and the endocarp showing 87.16% (Table 2). Based on Ruangrak et al. (2025), all three sizes of black pod byproducts from a P. timoriana demonstrated exceptional OM content of 83.96, 88.45, and 88.33% for small, medium, and large sizes, respectively. This suggests that the byproducts pose significant possibilities as energy feed sources for ruminants given the high digestible organic nutrients and OM levels. OM is the primary source of energy and nutrients in ruminant feeds. Its composition influences the efficiency, digestibility, and microbial activity of the rumen (Council et al., 2001; Khan, 2015). High OM levels in nutrition feeds improve their value, but high fiber fractions like lignin can hinder digestibility and energy access (Adesogan et al., 2019).
Crude protein (CP) plays an important role in the growth, metabolism, and health of an individual in both human and animal nutrition (Ajomiwe et al., 2024; Wu, 2022). In the pod parts of P. timoriana, the CP showed some variability in its distribution. The greatest values were in the mesocarp with 7.42 ± 0.08%, followed by exocarp 5.62 ± 0.04%, and endocarp 3.73 ± 0.04% (Table 2). This distribution supports the biological function of the mesocarp that acts as a seed tissue and the endocarp which, while protein contributes lower protein due to being more fibrous and structural. The protein content of the fractions of P. timoriana was low compared to other leguminous pods. P. roxburghii for example had considerably higher protein content in tender (12.1%), immature (15.6%) and mature pods (18.8%) and in the mature kernel (28.8%; Longvah and Deosthale, 1998). In the same manner, other leguminous pods exhibit higher CP values, including C. siliqua (13.27%; Ishag et al., 2024), A. tortilis (11.86%; Abraham et al., 2023), and A. farnesiana (12.1%; García-Winder et al., 2009). Pedro et al. (2023) mentioned that green pods of Acacia have a greatly higher protein content than other parts of the plant, with A. cyclops containing 11.82% and A. retinodes 21.66%. Moreover, A. farnesiana’s mature pods were recorded to have 22.1% CP, while Prosopis laevigata and Leucaena leucocephala’s mature pods had 11.9 and 17.2% CP (Zapata-Campos et al., 2020). Even though the CP content in P. timoriana pods is lower in comparison to other plants, the CP content in the mesocarp still remains of nutritional value. The CP content of P. timoriana pod fractions, particularly the mesocarp, though lower when compared to other plants, is sufficient to support the nutritional needs of small P. timorianas, whose small type byproducts were reported to contain 6.95% CP, while the medium, and large types were 5.54 and 5.16%, respectively (Ruangrak et al., 2025). This consistency suggests that P. timoriana pods could potentially function as supplementary protein sources and especially as an added protein in mixed diets that contain other high protein feed ingredients. In livestock nutrition, protein dense feedstuffs such as soybean meal, aid in more efficient animal growth. They also contain amino acids essential to animal development, reproduction, and optimized productivity (Johnson et al., 2012). Specifically, the mesocarp fraction of P. timoriana pods would increase the protein value of P. timoriana pods when fed in conjunction with other forages and byproducts from agro-industries. With regards to human nutrition, the increasing popularity of diets high in protein for aiding in weight loss or managing type 2 diabetes, excessive amounts of protein can lead to health concerns, particularly an increased risk of chronic kidney disease or kidney dysfunction arising from glomerular injury and intraglomerular hypertension (Ko et al., 2020). Looking from a human nutrition view, a moderate amount of protein supports dietary diversity especially in rural populations where legumes and tree foods are part of traditional diets.
Ether extract (EE) holds considerable importance in the context of nutrition in animals and humans since it serves as an economical source of energy and as a component in many biological structures such as essential fatty acids, vitamins, and various other compounds which perform physiological functions (Jayaprakash et al., 2024; Kerr et al., 2024; Kujoana et al., 2024; Palmquist and Jenkins, 2003). In the area of animal nutrition, EE serves in enhancing the nutrition of the animal, the nutrition of the animal, the animal’s appetite, and even reproductive activities in high-yielding poultry and dairy cows (Kujoana et al., 2024). For humans, omega 3 fatty acids, which are lipid sourced from plants and animals, are vital in nutrition as they have remarkable, antiapoptotic, safeguarding of cells from apoptosis, antioxidant and anti-inflammatory functions, and are considered useful in the treatment of most organs (liver, kidney, heart and brain; Singh et al., 2023). In this study, EE content of P. timoriana pod fractions was markedly different. Mesocarp had the highest EE concentration of 1.73 ± 0.02%, exocarp 1.11 ± 0.04%, and endocarp 0.92 ± 0.02% (Table 2). The observed distribution aligns with the mesocarp’s role in seed development in as much as the mesocarp serves as a nutrient dense tissue, while the exocarp and the endocarp are primarily protective and structural (Setia et al., 1987). Comparisons with other species shows that the pod walls of P. timoriana have relatively lower values of EE. For instance, P. roxburghii demonstrated a significantly greater concentration of EE in its tender, immature, and mature pods, which were 1.0, 7.8, and 15.5%, respectively, and the mature kernel had as high as 33.5% (Longvah and Deosthale, 1998). Furthermore, pods of C. siliqua were noted to have 6.32% EE (Ishag et al., 2024), and A. farnesiana pods had around 2.0% EE (García-Winder et al., 2009). For other leguminous pods, the EE concentration was also low, but slightly higher than P. timoriana. The EE content in L. leucocephala, P. laevigata, and A. farnesiana is modest and comparable to that of P. timoriana, with mature pods containing 2.1, 1.7, and 1.9% EE, respectively. Studies conducted by Pedro et al. (2023) suggests that A. melanoxylon (1.48%), A. retinodes (1.37%), and A. longifolia (1.82%) are within the same range as P. timoriana while A. pycnantha had diminished levels (0.87%), which was lower than all the other species. Concerning P. timoriana pod byproducts, Ruangrak et al. (2025) reported EE values of 1.30, 0.93, and 0.86% for small, medium, and large-type pod byproducts, which aligns with the current observations for pod fractions. Even though the total lipid content of the pod walls of P. timoriana is low in comparison to the kernels of P. roxburghii and leguminous plants with high EE content, the mesocarp does offer some dietary lipids which could be used to improve the energy density and palatability of animal feeds. From an animal feed formulation perspective, the low EE values of the exocarp and endocarp indicate these parts will be more useful in fiber and carbohydrates as these will be of lesser value as lipid sources. For community nutrition, the relatively low lipid value of pod walls in composite foods and supplements may still offer benefits in communities with limited global access to oils and fats.
Crude fiber (CF) is obtained from plant remains after several extraction processes using dilute acid and alkali, while dietary fiber (DF) is obtained from plant residues and includes a broader range which undergo no hydrolysis by human enzymes (Trowell, 1976). Analysis of CF content in different anatomical parts of P. timoriana pods showed some significant differences (Table 2). The CF content in the different anatomical sections was as follows: 21.30% in the mesocarp, 30.73% in the exocarp, and a maximum of 46.39% in the endocarp. These data emphasize the distinct layered structural and compositional architectural roles of each pod layer, where there is a marked increase in fiber concentration from the mesocarp toward the endocarp. This material is domianted by indigestible fractions such as cellulose, hemicellulose, lignin, and pectin. Its nutritional value is context-specific: in non-ruminants, it has a low caloric contribution but a valuable role in improving gut health and antimicrobial balance as well as hindgut fermentation (Jha et al., 2019). The rumen of ruminants converts CF into volatile fatty acids and microbial protein, which can later be used in their metabolism, and thus, they make effective use of CF (Cherian, 2020). In comparison to other leguminous pods, the CF levels of P. timoriana are considerably higher. For example, pods of A. farnesiana (García-Winder et al., 2009) and C. siliqua pods having 13.2% CF and 8.62% CF, respectively (Ishag et al., 2024). The CF values of several species of Acacia green pods are also lower, such as A. melanoxylon (19.51%), A. dealbata (22.11%), and A. cyclops (21.16%), which have 17–23% (Pedro et al., 2023) which is considerably lower than the exocarp and endocarp of P. timoriana. In addition, three pod byproducts of P. timoriana seed processing (small-type, medium-type, and large-type byproducts) displayed CF values of 20.19, 30.29, and 31.32% (Ruangrak et al., 2025). These results are negligible in comparison to the pod wall tissues reported in this study. The CF content of the endocarp stands out due to is exceptionally high value of 46.39% which fortifies the claim that the endocarp is lignified and serves as mechanical protection to the developing seeds (Ghimire and Chen, 2022). The concentration of them also distinguishes P. timoriana pods from other legumes. It also gives an indication of which animal groups could be better suited for feeding them. In livestock feeding, these pods would be beneficial for ruminants and could be incorporated into diets, lessening dependence on traditional feed, particularly for cattle, goats, and sheep. For human nutrition, DF has gained attention as a functional ingredient, surpassing CF as it includes all non-digestible plant remains (Cherian, 2020). Relating to human nutrition, the functional health benefits of DF is a growing focal point. Epidemiological studies have demonstrated that increased DF consumption is associated with a lower risk of obesity, type 2 diabetes, colorectal cancer, and cardiovascular disease (He et al., 2022). Therefore, the high fiber content associated with P. timoriana pod tissues has the potential as a functional food ingredient, particularly in foods that are processed to enhance taste and ease of digestion like flour or dietary supplements.
Crude Ash (CA) also known as total mineral content is defined as the residue of the inorganic components after the complete combustion of organic matter in food and feed (Liu, 2019; Quirino et al., 2023). In this study, the CA content was different in all the sections of the P. timoriana pod wall, the CA was 3.47% in the endocarp and 7.03% in the mesocarp (Table 2). These results imply that the mineral content of the P. timoriana pods is specific to the tissues, the mesocarp is mineral enriched and serves as a reservoir of macro and micro minerals. The CA observed in the present study for P. timoriana are in the range of some leguminous pods reported in the literature. For instance, CA values of L. leucocephala, P. laevigata, and A. farnesiana mature pods were 7.4, 3.5, and 3.5% (Zapata-Campos et al., 2020), which strongly align with the mesocarp and endocarp fractions of P. timoriana. Also, the C. siliqua pods ash value of 3.55% (Ishag et al., 2024) is very close to P. timoriana endocarp value. Other Acacia pod species like A. melanoxylon (3.24 ± 0.08%), A. dealbata (2.25%), and A. retinodes (5.89%) also exhibited CA values within the range observed for P. timoriana pod fractions (Pedro et al., 2023). Abraham et al. (2023) observed that A. tortilis pods contained 3.72% CA. This was attributed to the pods’ unique physiology and ecological adaptations, the way the samples were handled, or the species’ deep adaptations. This study illustrates, more strikingly, the diversity of the mineral profiles of leguminous species. In relation to other species, the ash content of P. timoriana pod fractions also aligned with that of P. roxburghii, whose tender, immature, mature pods, and mature kernels had ash content of 7.4, 6.9, 6.1, and 5.7%, respectively. These similarities strengthen the evidence for the nutritional similarity amongst the Parkia species. Moreover, the values are consistent with those for the ash content of pod byproducts from P. timoriana seed production, in which the small-, medium-, and large-type residues showed 5.94, 3.85, and 3.97% CA content (Ruangrak et al., 2025). The CA values, from a nutritional perspective, bolster the consideration of P. timoriana pods as potentially mineral-dense feed and food resources. Minerals such as calcium, magnesium, potassium, iron, and zinc which are usually present in legumes are important as co-factors for enzymatic processes and catalyze numerous reactions as structural tissues and metabolic processes in both animals and humans (Grela et al., 2017; Princewill, 2015; White and Broadley, 2009). Thus, this indicates that the revolving CA content in the mesocarp of P. timorianna’s may add significantly in sustainable livestock diets. While the exocarp and endocarp fractions, which are of lower mineral density, may still have some value as dietary components or in processed foods. Moreover, the CA content variation among the fractions of the pod may help in investigating P. timoriana byproducts in the context of a circular bioeconomy. Such fraction-specific nutrient profiling supports the hypothesis that it sustains P. timoriana pods and helps increase food security, support sustainable livestock production, and reduce waste.
Neutral detergent fiber (NDF) is an essential component of carbohydrate animal feeds and foods because it encompasses cellulose, hemicellulose, and lignin fibers (Van-Soest et al., 1991). As shown in Table 2, this study found notable differences on the different portions of NDF value for P. timoriana pod wall, mesocarp 35.81% and endocarp 65.48%. This clear disparity reflects the structural and compositional differences of the softer, fleshy mesocarp and the denser, fibrous endocarp layers of the pod wall. From an animal nutrition standpoint, the endocarp NDF value suggests low digestibility but usefulness for some maintenance of rumen health and stimulation of chewing in ruminants. On the other hand, the higher NDF value in mesocarp implies lower energy and higher digestibility, which makes mesocarp more beneficial in livestock diets. For NDF, the value is the same for human nutrition, concerning improving digestion, gut microbiota regulation, bowel movement, and reducing chronic cardiovascular and diabetes diseases (Brauer et al., 1981; Slavin, 2005; Yatoo et al., 2013). Therefore, the NDF value of different pod wall fractions of P. timoriana illustrates its importance in sustainable animal and human nutrition. The NDF values recorded for the mesocarp of P. timoriana 35.81% are comparable to the small-type seed byproduct fraction (33.77%) reported by Ruangrak et al. (2025), but are considerably lower than the large-type (50.56%) and medium-type (53.59%) fractions. Moreover, the endocarp’s NDF concentration (65.48%) is well above the reported values for several Acacia pod species, which include A. melanoxylon (44.41%), A. dealbata (51.17%), A. cyclops (50.15%), and A. tortilis (42.10%; Abraham et al., 2023; Pedro et al., 2023). This is also higher than the NDF contents of mature pods of L. leucocephala (52.0%), P. laevigata (39.4%), and A. farnesiana (24.6%; Zapata-Campos et al., 2020). From these comparisons, it can be inferred that while the mesocarp of P. timoriana provides moderate levels of fiber that are comparable to other commonly used leguminous feed resources, the endocarp is unique for its high content of structural fiber, thus suggesting its potential as a raw material for high-fiber dietary formulations within livestock production systems. Regarding human nutrition, controlled use of mesocarp-derived fractions may offer balanced fiber intake while preserving digestibility. On the other hand, the endocarp parts are better suited for the applications of functional foods focused on dietary fiber enrichment.
Acid detergent fiber (ADF) is a crucial factor when analyzing dietetic fiber. It is made up of a tough feed plant animal and human cellulose and lignin diet material portion (Caballero et al., 2003). In this study, ADF content measurement showed highest in exocarp and lowest in mesocarp (50.58 and 26.42% respectively). The ADF content of exocarp suggests its structural complexity and low digestibility, which limits its nutritional value especially for monogastric animals (Garrison et al., 1978). For ruminants, such fibrous fractions, however, can serve as a structural carbohydrate source that support rumen function and aid in satiety (Niwińska, 2012). In contrast, the mesocarp, which has lower ADF, is more digestible and nutritionally favorable for dietary applications. The intermediate level in the endocarp signifies its transitional structural role within the pod. Moreover, comparisons with related species emphasize the nutritional importance of these results. The ADF content of the mesocarp (26.42%) is similar to those recorded for A. dealbata (25.74%), A. retinodes (26.92%), and A. tortilis pods (26.03%; Abraham et al., 2023; Pedro et al., 2023). This similarity indicates that the mesocarp of P. timoriana would serve as a competitive alternative fiber source in animal feed formulations. On the other hand, the ADF value of the exocarp is 50.58%, which is greatly higher than the ADF values reported for leguminous pods L. leucocephala (35.7%), P. laevigata (30.3%), and A. farnesiana (17.1%; Zapata-Campos et al., 2020). This suggests the strength and possible reductions in the potential for digestion of the exocarp’s processes which would require some kind of treatment or processing. Moreover, when comparing ADF values of the organic byproducts from P. timoriana seed production reported by Ruangrak et al. (2025), the mesocarp (26.42%) is almost the same as the small-type byproduct (24.72%). However, the exocarp’s ADF (50.58%) exceeds the values reported for the medium-type (45.84%) and large-type (41.50%) byproducts. These differences suggest that some portions of the pod may be designed for use according to the nutritional objective high fiber for ruminants or low fiber for better digestibility in monogastrics. While excessively high ADF levels may compromise digestibility, moderate levels, as noted in the mesocarp, may be advantageous in developing functional foods, especially fiber-enriched formulations. ADF serves an essential purpose in the analysis of the digestibility of forage and fibrous foods. Therefore, it is vital in the case of animal diet formulation and also in human nutrition assessment, especially in evaluating the dietary fibers intake (Lattimer and Haub, 2010).
Acid detergent lignin (ADL) is a subclass of DF and a complex component of plant tissue. ADL consists mainly of lignin which is one of the most complex and indigestible plant components (Council et al., 2001). Phenolic polymers which lignin consists of help strengthen the cell walls of plants (Council et al., 2001). This study found a significant difference in ADL content in different parts of the P. timoriana pod wall. ADL content ranged from 3.74% in the endocarp to 24.50% in the exocarp (Table 2). These changes reveal the diversity of fiber fractions of pod wall tissues as they show different structural and physiological roles. The ADL content suggests that the exocarp functions as an outer protective barrier which microbial degradation and nutrient access is restricted in the context of animal feeding. The lower ADL content of the endocarp, in contrast, may promote easier digestibility and energy release making this fraction easier to incorporate in livestock diets. Previous study shows that higher lignin content tends to decrease voluntary intake, feed digestibility, and energy expenditure, because lignin slows down access of microbes to cellulose and hemicellulose in the rumen (Stypinski et al., 2024). Thus, the differing distributions of ADL within the pod wall of P. timoriana strengthen the conclusions of feed optimization for ruminants and potential functional foods for humans. The ADL values of P. timoriana are both comparable to and different from other leguminous and multipurpose tree pods. For instance, Acacia green pods had a wide range of ADL contents, with A. mearnsii as low as 7.25% and A. melanoxylon reaching 18.82% (Pedro et al., 2023). A. tortilis pod ADL content was reported at 10.06% (Abraham et al., 2023) as well. In the meantime, mature pods of L. leucocephala, P. laevigata, and A. farnesiana had lignin concentrations of 8.4, 4.9, and 3.7%, respectively (Zapata-Campos et al., 2020) which are equal to or lower than the values found in the mesocarp and endocarp of P. timoriana pods. These comparisons suggest that although the endocarp of P. timoriana is pod compositional similar to other well-explored pods with moderate lignin content, the P. timoriana exocarp is distinct in having extremely high ADL which greatly limits its use as animal feed without extensive processing. Furthermore, the ADL values for the byproducts of P. timoriana seeds are known with a lot of variability with 2.34% for small, 21.05% for medium, and 15.45% for large-type byproducts (Ruangrak et al., 2025). This reinforces the variability of lignin content not only between pod wall fractions but also byproducts of the same species. From an ecological standpoint, the significant concentration of ADL in the exocarp may hinder its digestion as a feed for ruminants. However, this could be of greater value through other means, for example, the removal of bioactive compounds or added as functional ingredients in food due to the phenolic structure antioxidants linked to lignin. On the other hand, the relatively lower ADL concentration of the endocarp suggests a greater potential as a feed resource with improved digestibility and nutrition.
Nitrogen-free extract (NFE) refers to the carbohydrate component of feeds and foods that are not part of crude fiber. It consists largely of soluble sugars, starches, and non-fibrous carbohydrates aiding in energy provision (He et al., 2022; Maynard, 1940). The investigation revealed differences in NFE value in the P. timoriana pod wall and endocarp and mesocarp portions with 36.12 and 45.28% respectively, which are notable percentages. The results suggest the mesocarp may be a moderately energy dense part of the pod, which corroborates its proposed use in balanced ruminant feed formulations and as a carbohydrate resource in human nutrition. Higher NFE values are often linked to better palatability and digestibility, especially in livestock feeds (Council et al., 2001). In comparison with other studies, the NFE value of P. timoriana pod tissues was lower than those reported for the three organic byproducts from P. timoriana seed production which were categorized as small-type, medium-type, and large-type and contained 55.72, 52.13, and 51.19% NFE, respectively, (Ruangrak et al., 2025). Similar analyses of leguminous pods show that NFE content varies depending on pod age, pod type, and the specific cultivar. For instance, the pods of Acacia species tend to have high NFE because of the abundant soluble carbohydrates they possess, which aids ruminant feeding (Abraham et al., 2023; Pedro et al., 2023).
Hemicellulose (HEM), a crucial element of dietary fiber, is located inside the plant cell wall. It consists of several polysaccharides including, xylans, mannans, and glucuronoxylans which aid in the strength of plant tissues (Li, 2021). In this investigation, exocarp contained 8.18 ± 0.18% HEM, and the endocarp was remarkably higher with 16.61 ± 0.36% HEM. The mesocarp, with 9.39 ± 0.23% HEM, was intermediate but leaned closer to exocarp value. This particular pattern of HEM distribution within the pod wall also illustrates the interplay of structure and function of the different tissues, where the endocarp is more sclerified, lignified, and fibrous to provide protective support for the seeds (Dardick and Callahan, 2014). In addition, HEM has some nutritional value as it acts as a source of energy for some animals, either ruminant or non-ruminant. In ruminants, specific gut microbes are capable of breaking down HEM into volatile fatty acids, which are then absorbed and used as a primary energy source (Weimer, 2022). For non-ruminants, which include pigs and poultry, that inclusion of HEM in their diets has proven to be useful for gut health, nutrient assimilation, and feeding efficacy (Jha and Berrocoso, 2016). In regards to humans, HEM is well-known as a functional fiber which supports gastrointestinal health, helps to maintain regular bowel movements, and promotes the growth of beneficial microbes in the gut (Holloway et al., 1980). The HEM content in the pod walls of P. timoriana is comparable to that of other leguminous tree pods. Mature pods of L. leucocephala, P. laevigata, and A. farnesiana have HEM contents of 16.2, 16.0, and 7.5%, respectively (Zapata-Campos et al., 2020). It is noteworthy that P. timoriana endocarp HEM concentration of 16.61% is comparable to L. leucocephala and P. laevigata, which are already known as valuable candidates in sustainable animal feeding systems (Hernández-Ruiz et al., 2024; Rusdy, 2020). On the other hand, the exocarp value of 8.18% is more in line with A. farnesiana’s HEM content. Concerning the P. timoriana seed by-products, Ruangrak et al. (2025) mentioned the HEM content for small-, medium-, and large-type residues as 9.05, 7.75, and 9.07%, respectively. These findings are in rough alignment with the exocarp value determined in the current study, bolstering the sustainable use of P. timoriana by-products as animal feed.
Cellulose (CEL), one of the main plant cell wall polysaccharides, is important for the nutrition of animals and humans as fiber and for influencing the gastrointestinal tract (Fujimori, 2021; Hao et al., 2021). Based on the results of the present study, there is notable variation in CEL content within different fractions of the pod walls of P. timoriana (Table 2). The mesocarp had CEL content of 22.33%, whereas the endocarp had a significantly higher CEL concentration of 45.13%, which demonstrates CEL’s protective, structural role to the seed. In the nutrition of ruminants, cellulose constitutes a major fraction of dietary fiber, which is fermentable by a highly complex microbial ecosystem present in the rumen. There are several microorganisms, such as Ruminococcus and Fibrobacter species, which degrade cellulose to SCFAs like acetate, propionate, and butyrate, all of which are utilized as primary energy metabolites for the host (Weimer, 2022). In general, ruminants are able to digest constituents of plant cell walls, like hemicellulose, to a greater extent than cellulose from grasses. However, legumes tend to offer a more intricate composite structure which hampers CEL fermentation (Wedig et al., 1987). Therefore, the endocarp’s high CEL content is advantageous in ruminant nutrition as a slow release energy and fiber source. In human nutrition, cellulose is an important dietary fiber that is not digestible and functions as a gut motility aid, enhancing fecal bulk and regular bowel movements (Hao et al., 2021). Additionally, it decreases the likelihood of constipation, diverticular diseases, and several other gastrointestinal disorders (Rana et al., 2012; Suresh et al., 2024). The endocarp’s higher cellulose content than that of the mesocarp suggests that targeted harvesting of pod fractions could enhance the fiber content of functional foods. In comparison, the cellulose content in the pods of P. timoriana still aligns with values recorded for other legumes. Mature pods of L. leucocephala, P. laevigata, and A. farnesiana contained 24.4, 20.8, and 13.4% CEL, respectively (Zapata-Campos et al., 2020). In the same manner, the cellulose content of byproducts of P. timoriana seed processing was classified into three types: small-type, medium-type, and large-type, which had 22.38, 25.05, and 26.05% CEL, respectively (Ruangrak et al., 2025). This is consistent with the mesocarp values from this study.
Non-structural carbohydrates (NSC) are carbohydrates in plants that are simple sugars as well as storage polysaccharides which can provide energy in the form of fuel (Hartmann and Trumbore, 2016). In the case of this study, NSC content differentiated considerably among the different parts of P. timoriana pod walls (Table 2). The mesocarp showed the highest NSC content of 28.44% while the exocarp contained 1.31% and the endocarp had no NSC at all. This distribution illustrates the mesocarp’s role as an active tissue during metabolism which economically stores carbohydrates to aid in seed development while the endocarp’s role is mainly structural, as a lignified barrier (Setia et al., 1987). In the case of ruminants, diets containing NSCs are fermented swiftly in the rumen, creating volatile fatty acids which serve as a significant energy source and improve feed efficiency and productivity of the animal (Panneerselvam et al., 2024). That NSC is present in the mesocarp implies that it can serve as a natural and energy-dense feed ingredient and can be strategically used in the ruminants rations to boost growth, and increase milk production. The exocarp’s lower NSC and the endocarp’s absent NSC suggest that these parts are better used as structural components to energy feed. From the perspective of human nutrition, NSCs derived from plants such as non-starchy vegetables, legumes, fruits, as well as whole grains, support metabolic energy, regulate blood glucose levels, and promote health (Ludwig et al., 2018). The mesocarp part of P. timoriana pods, which is high in NSC, may be used in formulating functional foods or as a natural energy ingredient in plant-based diets. In comparison, the NSC content of P. timoriana byproducts which had been reported for small-type, medium-type, and large-type byproducts were 52.05, 36.10, and 39.45%, respectively (Ruangrak et al., 2025). Although these values exceed those of the individual pod fractions analyzed in this study, the mesocarp accumulation pattern is consistent with the function of metabolically active pod tissues in carbohydrate storage.
Total carbohydrates (TC) are both a significant part of animal feed and a human dietary component as they provide the necessary energy and impact metabolism as well as the digestive system and health (Holesh et al., 2020; Polakof et al., 2012). In the case of P. timoriana, TC content varied within the pod fractions (Table 2). Endocarp pods had the highest TC content of 82.51% and mesocarp pods 66.58%. This difference illustrates the metabolic role of the endocarp, which aids in accumulating both structural and non-structural carbohydrates required for the development of the seed (Setia et al., 1987). In animal nutrition, TC is non-structural carbohydrates (sugars and starches) and structural carbohydrates (Fiber) which are vital to energy metabolism, microbial fermentation, and proper function of the rumen. Growth, feed conversion efficiency, and the quality of the carcass in livestock are positively correlated with TC intake (Nutrient Requirements of Dairy Cattle, 2021). The endocarp is rich in TC and can therefore serve as an energy-dense feed ingredient, whereas the exocarp, with moderate TC levels, serves primarily as a source of fiber to aid in gut motility and rumen health. In the case of humans, TC serves as a metabolic energy source, carrying considerable TC dietary implications. Whole legumes and fibrous plant materials have high-quality carbohydrates which help with weight control, heart health, and lowered risk of diabetes (Polak et al., 2015). In contrast, refined carbohydrates and added sugars can lead to obesity, insulin resistance, and chronic diseases (Ludwig et al., 2018; Nutrient Requirements of Dairy Cattle, 2021). The TC distribution in P. timoriana pod fractions indicates that products derived from endocarp may be utilized in functional food development or in nutritionally balanced diets. In comparison, the TC values of the three organic byproducts from P. timoriana seed production: small-, medium- and large-types were 98.58, 98.97, and 99.00%, respectively (Ruangrak et al., 2025). While these values are considerably greater than the pod fractions, likely because of processing concentration during byproduct processing, the tendency of greater carbohydrate accumulation in metabolically active fractions support the present study.
Feedstuffs are divided into two types: concentrates and roughages, based on their nutritional value and CF content (Tomar et al., 2022). Concentrates are feedstuff with oilseeds, animal proteins, and cereal grains with CF less than 18% (Tomar et al., 2022). These are also high in energy and protein. Concentrates are a vital source of nutrients that support livestock growth, reproduction, and production efficiency (Kırkpınar et al., 2018). Roughages are bulky and high in fiber with more than 18% CF (Tomar et al., 2022). Current research showed that exocarp, mesocarp, and endocarp of P. timoriana pods are all roughages based on their CF and CP composition (Table 2). The three fractions had CF values above the 18% threshold. The fractions had moderate levels of CP, and did not meet the requirements for concentrates, which are higher in nutrients and lower in fiber. The pod fractions did not qualify for concentrates because of their moderate CP values and met the criteria of high fiber and low nutrients. This aligns with previous research on P. timoriana by-products where small-type, medium-type, and large-type residues were categorized as roughages because of their high CF content (Ruangrak et al., 2025). The consistency of pod wall fractions with other studied residues reinforces the reliability of placing P. timoriana pod components into roughage feeds. Most importantly, this consistency affirms the P. timoriana pod wall’s potential value in livestock diets for sustainable feeding systems that require structural fiber.
In animal nutrition, feed classes are differentiated based on protein content, as energy and protein sources are used to formulate balanced diets. Materials with lower than 18% CP are considered energy-rich concentrates, and those with more than 18% CP are classified as protein-rich concentrates (Dryden, 2021). This study sought to classify nourishments for P. timoriana pods by evaluating the exocarp, mesocarp, and endocarp’s of the pods for their protein and fiber content. The data showed that all three pod fractions fit the roughage class because of the high fiber content. However, their protein content classified them as concentrates of high energy, rather deficient protein energy (Table 2). The mesocarp, which has relatively higher protein, soluble carbohydrate, and moisture content, supports Setia et al. (1987) who noted that it serves biologically as nutrient-rich tissue assisting in the seed’s nutrient-rich tissue sustenance. On the other hand, the endocarp’s fibrous, lignin, cellulose, and hemicellulose-rich composition served its protective and structural role for the seed (Dardick and Callahan, 2014). The observations made earlier regarding P. timoriana are also valid as the small-type, medium-type and large-type residues are classified as energy-rich and compliant due to their protein and fiber contents (Ruangrak et al., 2025). The differentiation of the pod fractions by their nutrient composition and feed type as well as their classification by nutrient content and feed type underscores the possibility of using them as sustainable feed resources, with mesocarp offering digestible nutrients and endocarp serving as structural fiber critical for ruminant digestion and efficient feed use.
3.3 Effective energy analysis
The energy values associated with P. timoriana pod fractions are vital in assessing their value for animal and human nutrition. In particular, the energy value of each feed component and its calories are prominent in its nutritional value. In this case, the gross energy (GE), which is the chemical energy released during complete combustion of a given material, had a significant difference in the pod wall fractions (Table 3). The mesocarp had a GE of 16.20 MJ/kg, while the exocarp had a higher value of 18.09 MJ/kg DM. This shows that separated pod fractions are different in energy value. In animal nutrition, GE is critical in providing a rough estimate for the potential energy that can be supplied by a given feed (Velayudhan et al., 2015). Some energy is lost in the process of digestion, therefore, in the case of formulating a diet that is aimed to maximize growth, reproduction, and production, more precise measures like digestible energy (DE) and metabolizable energy (ME) are preferred (Cooke, 2020). The relatively high GE of the exocarp indicates that its use in animal rations would be as an energy-contributing component yielding good results. On the other hand, the mesocarp although providing energy also provides better protein and soluble carbohydrates which assist in better metabolic and productive efficiency. In human nutrition, the use of GE pertains to caloric intake, although the actual energy available varies with the digestibility and the metabolism of the calories (Fao, 2005). The functional foods or dietary supplements that are based on P. timoriana are likely to bolster health and energy needs by providing a balanced combination of calories and nutrients when the mesocarp pod fractions are incorporated in them. These results also align with previous studies on byproducts of P. timoriana, where small-type, medium-type, and large-type residues were reported to have GE values of 15.39, 17.45, and 16.88 MJ/kg DM, respectively. This shows that various processing fractions incorporate comparable energy contributions (Ruangrak et al., 2025). Thus, the finding of this study demonstrates that the different pod fractions should be aimed to be utilized in the formulation of animal feeds or as functional ingredients in human nutrition.
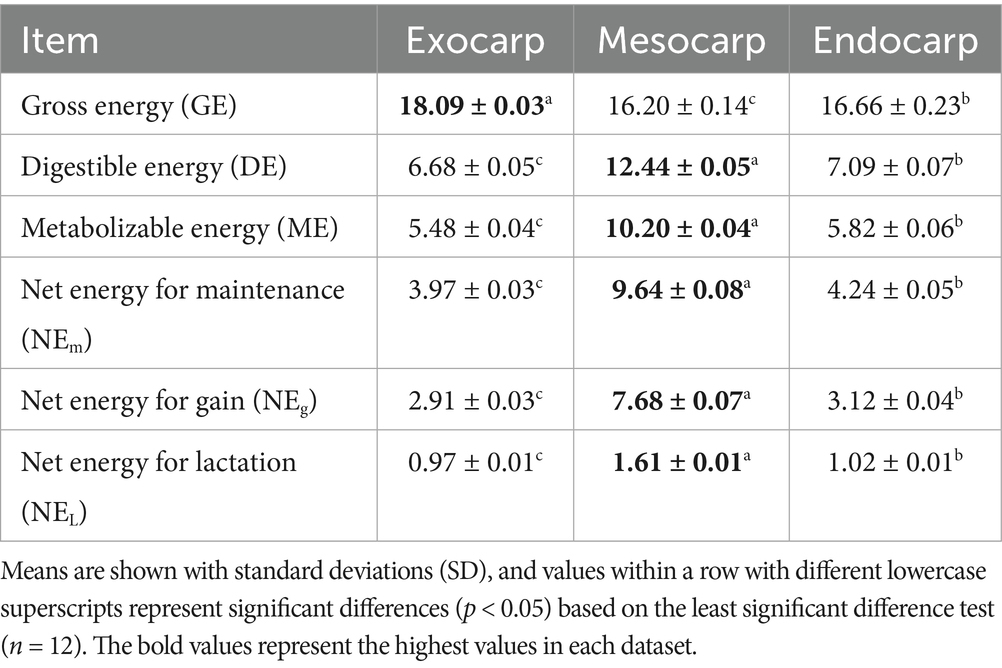
Table 3. Effective energy analysis of exocarp, mesocarp, and endocarp derived from P. timoriana pods (MJ/kg DM).
Digestible energy (DE) indicates the portion of energy unused due to energy loss from feces; this makes DE more precise relative to fodder quality for animals (Cooke, 2020; Maclean et al., 2003; Weiss and Tebbe, 2018). DE was very different among the fractions of the P. timoriana pods (Table 3). Mesocarp’s DE value was highest among the fractions at 12.44 MJ/kg DM. The exocarp and endocarp DE values were lower at 6.68 MJ/kg DM and 7.09 MJ/kg DM, respectively. The higher DE of the mesocarp is consistent with its some nutrient tissues which have higher crude protein, soluble carbohydrates, and moisture (Setia et al., 1987). The exocarp and endocarp do have some nutrients, but because of higher fiber and lignin, they have lower DE due to the reduced accessible carbohydrates for microbial fermentation in the rumen. Measuring DE together with other nutrient values helps nutritionist tailor feeds to the digestive abilities of specific species (Cooke, 2020). As for the DE values of P. timoriana byproducts from seed production, the small-type, medium-type, and large-type byproducts had 12.81, 7.78, and 8.81 MJ/kg DM, respectively (Ruangrak et al., 2025), exhibiting the same small-nutrient dense fraction yields higher digestible energy. This study supports the notion that smaller, more nutrient dense particles yield higher digestible energy. This study highlights the mesocarp fractions’ energy-dense feed component potential, while exocarp and endocarp fractions are better suited as feed with structural fiber.
Metabolizable energy (ME) refers to the part of DE which can be utilized for growth and reproduction after the expenses in urine and gaseous form (Cherian, 2020; Cooke, 2020). In this study, ME values, particularly in the mesocarp, were significantly higher with 10.20 MJ/kg DM, showing better energy potential when compared to the endocarp and exocarp which had 5.82 MJ/kg DM and 5.48 MJ/kg DM respectively, as shown in Table 3. The higher ME of mesocarp is in agreement with its greater crude protein, soluble carbohydrates, and digestible nutrients, supporting its biological role as the primary nutritionally dense tissue facilitating seed development (Setia et al., 1987). On the other hand, exocarp and endocarp, which are dense and fibrous and high in lignin, offer very limited energy potential due to poor digestibility and microbial access. In animal nutrition, ME is critical for formulating balanced rations to optimize growth, feed and reproductive efficiency, and production, especially in poultry and other monogastric animals that have low gaseous energy losses (Cherian, 2020). Using the ME benchmark, nutritionists can formulate energy-appropriate diets without the risk of over- or under-feeding. When it comes to human nutrition, ME is used as a base measurement for caloric intake, estimated at 4 kcal/g for proteins and carbohydrates and 9 kcal/g for fats, although it underestimates the actual energy yield from protein and carbohydrate metabolism (Council et al., 1989; Sturkie, 2000). In comparison to other legumes, the ME for P. timoriana pod fractions is in line with the previous P. tortilis pod finding of 7.95 MJ/kg DM (Abraham et al., 2023). In the same manner, small-type byproducts from P. timoriana seed production had ME values of 10.50 MJ/kg DM while the medium-type and large-type byproducts gave lower values at 6.38 and 7.23 MJ/kg DM, respectively (Ruangrak et al., 2025). The comparison illustrates the mesocarp’s value as an energy dense feed for boosting animal productivity while the exocarp and endocarp fractions are better utilized as fibrous or structural feed ingredients.
Net energy (NE) is the leftover ME after an animal’s feeding and heat increment has been subtracted and reflects energy that can be used in maintenance, growth, and production (Saha et al., 2010). In this case, the mesocarp of P. timoriana pod walls showed the highest NE across all categories with NEm being 9.64 MJ/kg DM, NEg (7.68 MJ/kg DM) and NEL (1.61 MJ/kg DM) as shown in Table 3. However, the exocarp and endocarp fractions showed lower NE values, which is consistent with their lower digestible nutrient NE and higher structural fiber constituents. The higher NE in the mesocarp portion is due to the greater amounts of digestible protein, soluble carbohydrates, and lower fiber fractions compared to exocarp and endocarp. This is consistent with its biological function as “storage” tissue, which serves to nourish the embryo and seed (Setia et al., 1987). On the other hand, the endocarp and exocarp tissues are very rich in cellulosic and semi-cellulosic compounds, which are the supportive scaffolding for the plant, and while offering limited energy, these tissues are useful in providing fiber. In animal nutrition, NE is necessary for accurately balancing rations for maintenance (NEm), excess body weight gain (NEg), and energy for lactation or production (NEL). Accurate NE metrics enable optimum feed efficiency and improvement in growth, reproductive performance, and reduction in energy wastage in ruminants and monogastric livestock (Saha et al., 2010). In human nutrition, NE is seldom used, but it yields a more relevant estimate in physiology than gross energy or metabolizable energy (Livesey, 2001). In comparison with other P. timoriana byproducts, the small-type, medium-type, and large-type byproducts from seed production had NEm values of 10.21, 4.73, and 5.54 MJ/kg DM, respectively, NEg of 8.21, 3.50, 4.13 MJ/kg DM, respectively and NEL values of 1.65, 1.10, and 1.21 MJ/kg DM, respectively (Ruangrak et al., 2025). These comparisons reinforce the superior energy density and nutritional potential of the mesocarp, justifying its use as a high-value feed component for sustainable animal nutrition, while the exocarp and endocarp would better serve as fiber-enriching or structural ingredients.
3.4 The relative feeding value
Dry matter intake (DMI) is an important indicator of the amount of feed consumed in relation to the animal’s body weight. It indicates the acceptability, palatability, and the nutritive value of the feed (Allen, 2000; Madilindi et al., 2022). In this research, the estimated DMI of P. timoriana pod fractions showed significant differences, with the mesocarp having the highest DMI at 3.35% of body mass (Table 4). The DMI of mesocarp is relatively high because the digestible nutrients are greater, including protein and soluble carbohydrates, along with lower levels of structural fiber and lignin. Lower structural fiber and lignin levels enhance mesocarp palatability and easier digestion, boosting voluntary feed consumption. The higher proportion of lignin, hemicellulose, and cellulose in the exocarp and endocarp, which is more rigid, and lower digestibility is likely to have contributed to lower DMI. These tissues are serving to give mechanical protection and structural support to the developing seed. Based on the findings of P. timoriana byproducts the small-, medium-, and large-type byproducts have DMI values of 3.6, 2.2, and 2.4% of body mass, respectively (Ruangrak et al., 2025). As previously noted, the mesocarp is by far the best feed component, not only providing biomass, but also offering better nutrients. While the exocarp and the endocarp exhibit low DMI, they can still serve as valuable low demand, fiber-rich feed ingredients for ruminants, where fiber is essential for microbial health and rumen function. In general, the multiphasic diets for sustainable animal nutrition need careful and strategic pod fraction selection for mesocarp to maximize feed and nutrient efficiency.
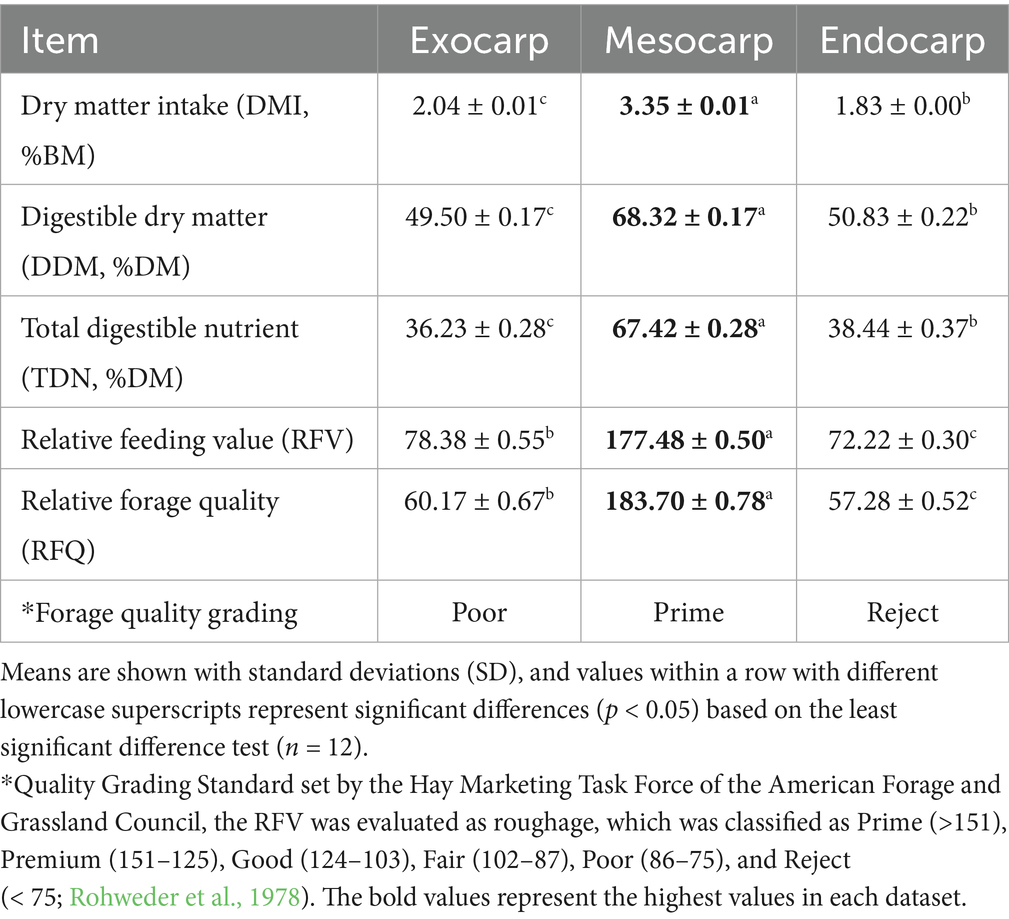
Table 4. The relative feeding value analysis of exocarp, mesocarp, and endocarp derived from P. timoriana pods.
Dry matter digestibility (DMD) or digestible dry matter (DDM) refers to the quantifiable portion of feed that is digestible for the animal; in this case, it relates to the animal’s nutrient and energy requirements (Amiri, 2012). In this study, DDM values for different sections of P. timoriana pod wall showed significant variation (Table 4). The mesocarp had the highest DDM value of 68.32%, followed by endocarp (50.83%) and exocarp (49.50%) in that order. The DDM mesocarp’s superiority stems from its protein, soluble carbohydrates, and moderate fiber level which support microbial fermentation and enzymatic breakdown throughout the gastrointestinal tract. On the other hand, exocarp and endocarp DDM values were lower due to the higher content of the more insoluble and less degradable cellulose, hemicellulose, and lignin, which limits nutrient and energy release. These results support the reasoning that the exocarp and endocarp are primarily protective and supportive for the seed; hence their structurally protective, nutrient-poor, and low-digestibility tissues. Evaluating previously studied byproducts of P. timoriana shows a consistent small-scale trend where small-type, medium-type, and large-type byproducts exhibited DDM values of 69.6, 53.2, and 56.6% DM, respectively (Ruangrak et al., 2025). This accentuates the remarkable multiplicative value of the mesocarp and its high nutritive value when compared to other byproducts, especially for ruminants and also monogastric animals that need energy-dense feeds. The overall DDM results also justify the importance of mesocarp selection as the principal fraction which greatly improves feed efficiency and energy optimization while enhancing the animal performance. Moreover, the exocarp and endocarp byproducts can serve as fiber-rich materials to support the functionality of the rumen and gut health.
In the same context, total digestible nutrients (TDN) serve as an estimation of the total energy value of the feed (Jayanegara et al., 2019). The TDN value for mesocarp also ranked the highest (67.42%), followed by endocarp (38.44%) and exocarp (36.23%) as shown in Table 4. These results show that the pod components exhibit clear nutritional differences, as mesocarp has a marked energetic advantage over the exocarp and endocarp, which are comparatively more fibrous. The high TDN in the mesocarp may be due to lower proportions of lignin and structural carbohydrates that enhance energy and fermentative efficiency in the rumen. The exocarp and endocarp are richer in indigestible fiber fractions like ADF and lignocellulosic material which are likely to diminish nutrient bioavailability and, thus, the effective energy value. These results are in agreement with the TDN values reported earlier for P. timoriana seed production byproducts, in which small-type byproducts had the highest TDN (69.4% DM) followed by medium- (42.2% DM) and large-type (47.8% DM) byproducts (Ruangrak et al., 2025). From a nutritional viewpoint, the high TDN value of mesocarp suggests that it can serve as a promising feed resource, particularly to increase energy intake and efficiency in ruminants. Diets with higher TDN are associated with improvements in growth performance, productivity, milk yield, and feed conversion efficiency (Davis et al., 2014). On the other hand, exocarp and endocarp fractions, which possess low TDN, may still be valuable as roughage due to their high fiber content that promotes ruminant digestion. This demonstrates the promise of pod fractionation for enhancing the nutritional value of P. timoriana pods by focusing on mesocarp for energy supplementation and using exocarp and endocarp as fiber-rich bulk feed. Additionally, the relatively close TDN values of mesocarp and the small-type byproduct reported by Ruangrak et al. (2025), 67.42 and 69.4% DM respectively, is evidence of uniformity across the developmental and processing stages of pods. This uniformity bolsters the argument for mesocarp being regarded as the best adapted feed for sustainable animal nutrition in areas where traditional feed resources are limited or expensive. Integrating these underfed byproducts into livestock feeding frameworks is a way to diversify feed but also support approaches to circular bioeconomy by lower agricultural waste.
The relative feed value (RFV) is an important measure used for evaluating the quality of forage, with full-bloom alfalfa hay serving as the benchmark forage with an RFV of 100 (H. Chen et al., 2024). Although this index is dimensionless, it allows for comparisons between different lots of forage, which is useful for evaluation, pricing, and marketing purposes. As illustrated in Table 5, the RFV values of P. timoriana pod fractions RFV values measured differed significantly, indicating varying nutritional potential. The mesocarp had the highest RFV of 177.48, indicating high forage value, better digestibility, and greater energy compared to the other fractions. The exocarp and endocarp, however, had low RFV values of 78.38 and 72.22, respectively, grading them as low-quality roughage. These three fractions differ in their ADF and NDF fiber content, which in the case of the exocarp and endocarp, higher values of these components make them less energy efficient. The current data supports earlier findings on the organic byproducts of P. timoriana seed production, where the small, medium, and large-type byproducts exhibited RFV values of 191.85, 92.35, and 104.08, respectively (Ruangrak et al., 2025). Both the mesocarp fraction and small-type byproduct not only met but exceeded the RFV cut-off of 150, which is associated with a high-quality forage good for ruminant livestock. This suggests that certain fractions of the pods, especially the mesocarp, can be considered as energy-dense feeds, almost on par with high-valued forages. From the standpoint of nutritional sustainability, the high RFV of the mesocarp suggests its potential importance in food systems for both livestock and humans. From the perspective of nutritional sustainability, the high RFV of mesocarp supports its value in animal and human nutrition systems. From the standpoint of human nutrition, the lower fiber density and higher digestibility of the mesocarp fraction functions as a potential food ingredient or flour functional food where enhanced nutrient bioavailability is favorable. On the other hand, while the exocarp and endocarp are low-quality roughages, they could still serve as fibrous to ruminant livestock that need structural fiber for proper rumen function.
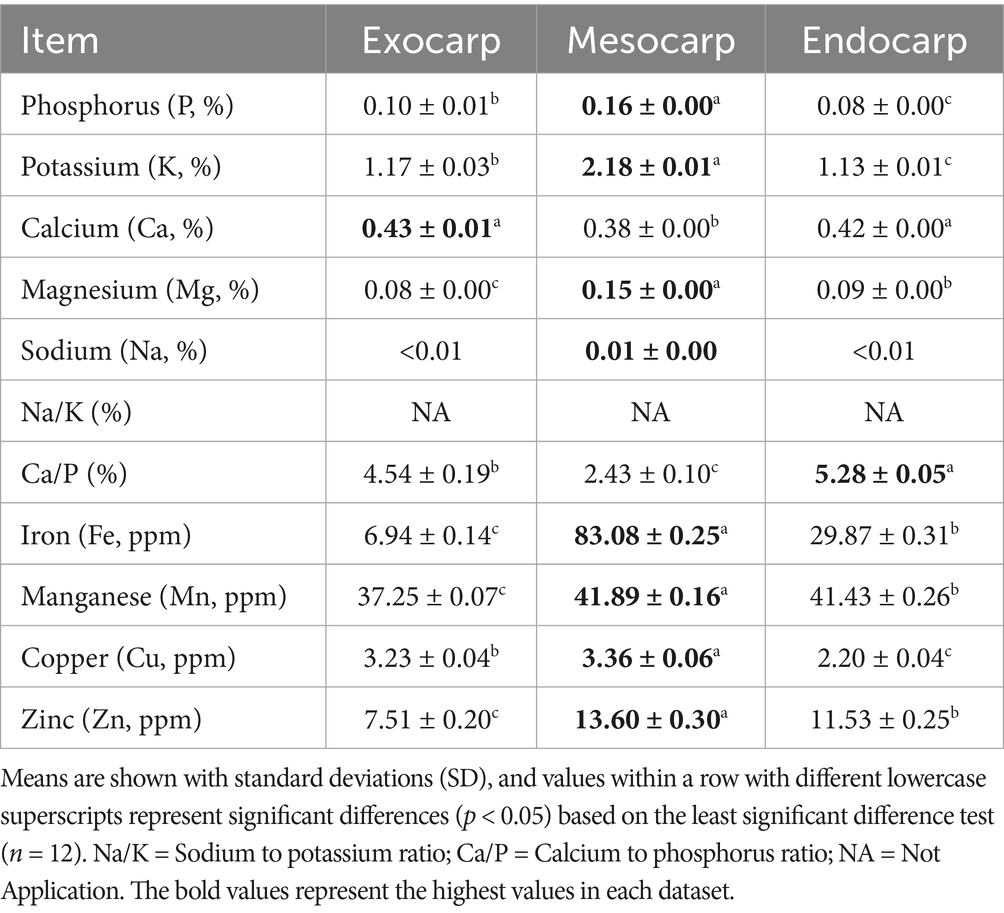
Table 5. Mineral content analysis of exocarp, mesocarp, and endocarp derived from P. timoriana pods (DM basis, %).
Relative forage quality (RFQ) serves as an improvement over RFV since it considers fiber digestibility, providing a better assessment of an animal’s performance with a given feed (Moore and Undersander, 2002). The RFQ values for the P. timoriana pod fractions of exocarp, mesocarp and endocarp are given in Table 4. The mesocarp RFQ score was the highest at 183.70 while exocarp and endocarp values were significantly lower at 60.17 and 57.28, respectively. The substantially greater RFQ of the mesocarp signifies better forage quality with greater DDM and intake potential. This means that the mesocarp forage is more accessible during digestion, making it ideal for high-yielding dairy rations for lactating dairy cows or for growing animals that demand energy-dense forages. The exocarp and endocarp, on the other hand, are best classified as low quality roughages, useful only as bulk feed for animals that do not need much nutrition. The trends seen in this study support those published by Ruangrak et al. (2025) where small-, medium-, and large-type byproducts of P. timoriana seed production were assigned RFQ values of 200.62, 76.79, and 92.18, respectively. The findings emphasize that in P. timoriana pod derivatives, size and tissue composition markedly dictate forage quality. The mesocarp, or mesocarp-dominant portions in both instances, delivered the highest RFQ, thereby reinforcing the notion of P. timoriana as a sustainable and nutritionally efficient feed resource. Nutritionally, the RFQ of mesocarp is high due to low fiber and high soluble carbohydrate levels that enhance digestibility and voluntary intake. Moreover, the exocarp and endocarp’s nutritive value is low due to high lignocellulosic levels, thus reducing these layers to mere fiber supply instead of energy. The above findings have significant implications from the standpoint of sustainable nutrition. The advantage of using the mesocarp as a feed resource is that it improves animal productivity, and at the same time, it helps in waste valorization because pod fractions are minimally used byproducts of P. timoriana production. Also, while the exocarp and endocarp lack high nutritive value, their fiber could still support rumen health when incorporated into well-balanced diets, or could be used for other purposes such as bioenergy, or as a functional dietary fiber in human nutrition.
Grading forage quality assesses feed adequacy using a criterion called RFV, which measures a combination of fiber and digestibility of the feed to estimate the performance of the animal (Rohweder et al., 1978). Following the standards set by the Hay Marketing Task Force (Rohweder et al., 1978), the mesocarp of P. timoriana pods was marked as “Prime” (>151) and received a score of above “151,” which suggests that the feed was of very high quality meant for highly productive animals. On the other hand, the exocarp and endocarp were branded as “Poor” (86–75) and “Reject” (<75), respectively (Table 4). These findings show that although the mesocarp could contribute as a valuable feed component, the exocarp and endocarp do not satisfy the requirements for standalone ruminant feed. Their use in pre-formulated diets is still favorable, as they would boost the fiber content in the diets while promoting gut health. The same trend was noted in the forage quality grading of byproducts of P. timoriana, where small-, medium-, and large-types were graded as Prime, Fair, and Good, respectively (Ruangrak et al., 2025). This further highlights the consistent under over the considerable range of natural value within and across the pod and byproduct fractions. With these characteristics, it is possible to achieve sustainable animal nutrition by using a feedstuff that increases animal performance and decreases the need for conventional feeds. On the other hand, the lower ranking exocarp and endocarp, are still useful and may serve as filler in formulated animal feeds as balancing bulk fiber and nutrient density. This multifunctional use supports circular bioeconomy principles by reducing the waste generated from P. timoriana seed processing while broadening its use in livestock feeding.
3.5 Mineral composition
In both animals and humans, minerals are important in metabolism, enzymes, bone formation, and immune function (Razzaque and Wimalawansa, 2025; Sampath et al., 2023; Weyh et al., 2022). The mineral study of P. timoriana pod wall parts shows important differences across tissues, which gives various nutritional advantages.
Both humans and animals require phosphorus (P) in large amounts, mainly as P plays a major role in bone and teeth formation, muscle, and tissue energy metabolism, and in the synthesis of nucleic acids (Wagner, 2023). In the current research, the phosphorus content in P. timoriana pods exocarp, mesocarp, and endocarp showed in Table 5. The concentration of mesocarp P (0.16%) was also the highest, and was greater than exocarp (0.10%) and endocarp (0.08%). Thus, the mesocarp is the most valuable part of the pods, and can be used as mineral-rich constituents of animal diet and as supplementary foods for humans, especially in areas where P is deficient in food. Moreover, the comparison with other studies suggests the other fractions of P. timoriana pods also have high nutritional value. The 0.16% mesocarp P tilts the balance of P. timoriana seed production waste in small-type byproducts (0.14%), and is greater than the medium-type (0.09%) and large-type (0.08%) byproducts (Ruangrak et al., 2025). Among comparative studies with other legumes, P. timoriana is more advantageous. The P content in the pods of C. siliqua has been cited as being 0.07% (Ayaz et al., 2007), while members of the Acacia genus such as A. melanoxylon, A. dealbata, A. cyclops, A. retinodes, A. mearnsii, A. pycnantha and A. longifolia have been shown to contain very low concentrations of this element, (< 0.01%; Pedro et al., 2023). The P in P. timoriana mesocarp, and thus L. leucocephala (0.19%), P. laevigata (0.25%), and A. farnesiana (0.12%; Zapata-Campos et al., 2020) has shown to either equal or exceed the concentration of P which further allows this species to fall within the range of competitively nutritious legume feed and food resources.
Potassium (K) is a primary micronutrient considered crucial for fluid retention, neural signaling, and muscle activity, hence vital for human and animal wellness (Egert et al., 2023). In the current study, the mesocarp from pods of P. timoriana had K concentrations of 2.18% which is almost double to that of the K concentration of the exocarp (1.17%) and endocarp (1.13%; Table 5). This suggests that particularly the mesocarp is a source of dietary K, which is the more reason for the nourishing value it has for animal feeds and functional foods. This is in conjunction to comparative studies that emphasize the K content P. timoriana mesocarp has. For instance, Ruangrak et al. (2025) studies K concentrations in seed production by products and found that small type residues (2.08%) were relatively high, unlike the medium (1.28%) and large (1.42%) type byproducts which were low. Pedro et al. (2023) presented research indicating that in relation to Acacia green pod species, the highest value of P. timoriana mesocarp was 2.18%, A. melanoxylon was 1.88%, A. dealbata was 1.10%, A. cyclops was 1.32%, A. mearnsii was 1.76%, A. pycnantha was 1.62%, and A. longifolia was 1.47%, while A. retinodes was 2.32% (Pedro et al., 2023) which is slightly higher. Compared to other leguminous pods, the mesocarp was also advantageous: C. siliqua was 0.97% (Ayaz et al., 2007), while L. leucocephala (0.73%), P. laevigata (0.48%), and A. farnesiana (0.50%) were considerably lower (Zapata-Campos et al., 2020). The benefits of the P. timoriana mesocarp which is a substantial source of K is critically important in animal nutrition. Moreover, the valorization of P. timoriana pod fractions, particularly the mesocarp, as potential alternative resources of minerals, still retains the principles of sustainability concerning nutrition and waste disposal. The agricultural by-products in the P. timoriana pods, when fully utilized, not only improve nutrition security but also support circular bioeconomy models in animal and human nutrition systems.
Calcium (Ca) is described as one of the macro-minerals critical to the forming the bone structure, and is also crucial to the contraction of muscles, transmission of the neural impulses and the clotting of blood (Del-Valle et al., 2011). In the current study, the Ca content showed some differences in the three fractions of the P. timoriana pods, and the exocarp had the greatest concentration (0.43%), followed in descending order by the endocarp (0.42%) and the mesocarp (0.38%; Table 5). In particular, the exocarp was richest in Ca content. Although the differences were relatively small, these findings suggest that all pod fractions are of Ca nutrition coherent for animal and human diets. In relation to the pod fractions, the greatest values of Ca: P ratios were in the endocarp (5.28) and exocarp (4.54) for the mesocarp exhibited a low ratio Ca: P due to a relatively high P content. A ratio of 1.3 or higher is considered acceptable for the maintenance of bone and soft tissue development (Medicine, I. of, Board, F. and N., and Intakes, S. C. on the S. E. of D. R 1999), and a ratio of 2 is considered supportive in infants (Medicine, I. of, Board, F. and N., and Intakes, S. C. on the S. E. of D. R 1999). For adults, wider ranges of Ca: P ratios (0.08–2.40) have been reported without any significant effect on calcium absorption or retention (He et al., 2021; Spencer et al., 1965, 1978). In the case of livestock, the ratio has little effect on mineral retention until it reaches extreme values greater than 7 or less than 1 (Nutrient Requirements of Dairy Cattle, 2021). Therefore, the ratios of the P. timoriana pod fractions remain above the optimal range for human consumption, but the values are below the upper limit for animals, and especially for ruminants, which have evolved specialized mechanisms for adaptive use of retainable minerals. Analysis of P. timoriana in the context of other plant species has shown that P. timoriana has untapped nutritional value. Ruangrak et al. (2025) has Ca levels in seed by products of P. timoriana which small-type 0.42% and medium-type 0.39% Ca by weight are comparable to the values presented here while considerably lower 0.33% in large-type fraction was previously reported. Ca content in the green pods of the Acacia ranged from 0.28% in A. melanoxylon to 1.01% in A. retinodes (Pedro et al., 2023), demonstrating that P. timoriana is in the mid to higher range, which is common across species but shows variability. Ayaz et al. (2007) reported that C. siliqua pods contained Ca at 0.30%, which remains lower than that of P. timoriana. Among L. leucocephala (0.80%) has also higher Ca concentrations while P. laevigata (0.43%) and A. farnesiana (0.53%) came close to the P. timoriana exocarp and endocarp. The fractioned pods of P. timoriana offer a moderate concentration of Ca which is especially important for Ca deficient animal diet. The addition of Ca expecially the exocarp and endocarp tissues could prove useful for ruminants where bone growth is important in the diet. In fortified foods for P. timoriana pod fractions which are constantly paired with Ca deficient foods could help people to improve mineral intake. In conclusion, the exocarp and endocarp of P. timoriana pods are rich in Ca and P with a good balance of both elements that can be used to formulate animal and human foods.
Magnesium (Mg) is important because it has over 300 functions including muscle action, blood pressure moderation, insulin metabolism, and DNA, RNA, and protein synthesis (Gröber et al., 2015; Kirkland et al., 2018). It is also essential for the proper advancement and maintenance of Mg consumption because it is needed both for humans and animals (Pinotti et al., 2021). Considering the results of the current study, the mesocarp of the P. timoriana pods had the highest Mg concentration of all the sampled parts, at 0.15%, followed by the endocarp (0.09%) and the exocarp (0.08%; Table 5). These findings support the idea of using the mesocarp for Mg enrichment, as it could be a functional food candidate to help with the dietary magnesium deficiency numerous people face (Al-Alawi et al., 2021). For animals, magnesium also has positive effects on feed digestibility, reproductive performance of cows and sows, growth of broilers, egg and shell quality of layers, and hatchability (Gaál et al., 2005). These findings, along with the relatively high Mg concentration of the mesocarp, strongly support the idea that it can be added to animal feed to increase overall production performance. This also makes P. timoriana particularly valuable, as the financial gains from it can be expected to be substantial. Among the Acacia green pods, Mg concentrations ranged from 0.10% in A. dealbata to 0.25% in A. retinodes, with several species like A. melanoxylon, A. pycnantha, and A. longifolia matching the mesocarp value of 0.15% (Pedro et al., 2023). This indicates that mesocarp of P. timoriana is competitive amongst other leguminous pods. On the other hand, C. siliqua has considerably less Mg content of 0.06% (Ayaz et al., 2007). L. leucocephala (0.17%) was at the uppermost range, while A. farnesiana (0.11%) and P. laevigata (0.12%) contained even less (Zapata-Campos et al., 2020). This suggests that P. timoriana pods have high Mg content, and thus valuable nutritional content.
Sodium (Na) is an essential mineral that is macro-mineral and is important for balancing extracellular fluids, regulating osmotic pressure, and facilitating normal nerve and muscle activity in humans and other animals. Physiologically, Na deficiency may greatly decrease blood volume and blood pressure, which is corroborated by the fact that Na deficiency can often be self-diagnosed by the salty taste often described (McCaughey, 2007). In this study, the concentrations of Na in the pod wall fractions of P. timoriana was extremely low. In the exocarp and endocarp, the proportions were less than 0.01%. In the mesocarp, it was 0.01% (Table 5). Thus, the calculation of the Na/K ratio was not applicable (NA). The very low Na concentrations in the pods suggest they might be useful for other minerals, but as a source of Na, they are not adequate and salt deficiency would be essential to satisfy the dietary needs of both humans and animals. Compared to other reports, P. timoriana does not contain a very high concentration of Na. Ruangrak et al. (2025) reported the same 0.01% Na concentration in small-type byproducts and less than 0.01% in medium and large type as well as in other byproducts derived from the residues of P. timoriana seed production. In comparison, Acacia green pods display a considerably higher Na percentage, from 0.06% in A. dealbata to 0.70% in A. longifolia (Pedro et al., 2023). Likewise, Na concentrations in L. leucocephala (0.18%), P. laevigata (0.23%), and A. farnesiana (0.12%; Zapata-Campos et al., 2020) were all greater than in P. timoriana. Moreover, the Na concentration in C. siliqua pods was 30 ppm (Ishag et al., 2024), and illustrates the very low contributions of Na of most leguminous pod species.
Trace minerals, including iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn), are critical for the metabolism, immune system, oxidative defense, and enzyme functions in both man and animal (Silva et al., 2019; Zhang et al., 2024). As shown in Table 5, the mesocarp of P. timoriana pod walls exhibited the highest content of all four trace elements, indicating its utility for nutrition-focused methods.
Fe is a component in oxygen transportation, in muscular respiration, in respiration at the cellular level, and in the synthesis of DNA (Crichton, 2016). In the pod fractions, the mesocarp had the highest value of iron 83.08 ppm, followed by the endocarp 29.87 ppm and the exocarp 6.94 ppm, as indicated in Table 5. This clearly identifies the mesocarp as the richest in Fe concentration. In humans, Fe is important for the synthesis of hemoglobin and also for several other processes, and it is important to note that the intake of Fe also has negative effects. The exess Fe in the body is harmful, as redox-active Fe is responsible for oxidative stress through the generation of reactive oxygen species (ROS; Kawabata, 2022). Therefore, the exess of Fe in the body is highly undesirable, and one has to be careful regarding the dosage in the Fe-rich mesocarp in human nutrition to avoid Fe overload. In animal nutrition, Fe supplementation is important for growth, immune function and productivity. For example, 25 mg Fe supplements added to lactating sheep milk resulted in improved health and productivity of suckling lambs (Asadi et al., 2022). Thus, the Fe concentration in mesocarp supports its use as a mineral source in livestock feeding, particularly in young and growing animals with higher Fe needs. Comparing P. timoriana to more well-characterized resources provide more context on its nutrition. Ayaz et al. (2007) states that Fe content in C. siliqua mesocarp is 18.80 ppm, considerably less than that in P. timoriana. However, Fe content in C. siliqua pods may be higher, as per Ishag et al. (2024), who documents 663.8 ppm, exposing stark differences in Fe content that may be due to geographic, phenotypic, or methodological variety. Leguminous pods such as L. leucocephala, P. laevigata and A. farnesiana had considerably higher Fe content at 47,300, 40,300 and 11,600 ppm, respectively, (Zapata-Campos et al., 2020), all far greater than the P. timoriana values. In the same way, Acacia green pods such as A. melanoxylon (45.54 ppm), A. dealbata (30.61 ppm), A. cyclops (30.35 ppm), A. retinodes (44.13 ppm), A. mearnsii (25.70 ppm), A. pycnantha (46.50 ppm) and A. longifolia (46.85 ppm) posse Fe contents comparable to or slightly higher than the P. timoriana endocarp but still, the mesocarp is greater (Pedro et al., 2023). All these hint that the P. timoriana pods mesocarp may still be a fair source of Fe in comparison to many other leguminous pod species, though not as much as some forage legumes like L. leucocephala or P. laevigata. Regarding animal feed, the mesocarp might enhance feed efficiency and support hematological status due to the readily bioavailable Fe it contains. For human nutrition, its use would necessitate specific inclusion into Fe fortified food, with Fe sluggish regulation to oxidative stress that the excess Fe may trigger.
Mn is vital for bone tissue mineralization, metabolism, cellular defense, and the biosynthesis of glycosaminoglycans, thus demonstrating its value as a critical nutrient for humans and other animals (Klimis-Zacas, 1993). In our study, the highest content of Mn within the pod was in the mesocarp (41.89 ppm) and endocarp (41.43 ppm) and the exocarp had slightly lower amounts (37.25 ppm) as shown in Table 5. These values of Mn concentration within pods, were notably high and consistent, indicating that the pod constituents of P. timoriana might be good candidates for the natural source of Mn for use in dietary supplements for animals and humans. P. timoriana pods contain more manganese compared to C. siliqua (Ayaz et al., 2007) which has a concentration of 12.90 ppm. On the other hand, the concentration is lower compared to what has been reported recently for the same species, 86.70 ppm (Ishag et al., 2024). In addition, the Mn concentrations in P. timoriana are markedly greater than for several species of Acacia green pods—A. dealbata (15.04 ppm), A. cyclops (13.15 ppm), and A. mearnsii (14.73 ppm)—but are similar to A. melanoxylon (48.78 ppm) and A. pycnantha (50.20 ppm; Pedro et al., 2023). This indicates that the Mn profile of P. timoriana is comparable to some of the Acacia species that are known to be nutritionally valuable and considered as mineral-rich fodder. However, in comparison to certain multipurpose tree legumes, such as L. leucocephala, P. laevigata, and A. farnesiana, which exhibit Mn levels of 14,700 ppm, 13,000 ppm, and 3,900 ppm, respectively (Zapata-Campos et al., 2020), the Mn content in P. timoriana pods is considerably lower. The extremely high concentrations of Mn in the pods of L. leucocephala and P. laevigata may pose some threats of toxicity, as elevated Mn levels in the diet are known to Mn neurological function and is considered to reduce the palatability of feed to livestock. In contrast, the moderate Mn levels observed in P. timoriana place it within a safer and more nutritionally balanced range for dietary applications. It is notable that the constant levels of Mn, measured per the exocarp, mesocarp and endocarp fractions, points to the geometric pod constituent’s nutrition value for inclusion in the sustainable animal feeds and the nutritionally supplemented diets for humans. In ruminants the Mn deficiency is prevalent and for livestock Mn addition is vital for bone growth, animal fertility, and enzymatic stimulation. In human nutrition, the P. timoriana pods could be beneficial in alleviating dietary Mn deficiency, which is common in regions that rely on cereal-based meals and often underestimate the intake of essential trace minerals.
Cu is equally essential to human and animals. Though animals deficient in Cu is one of the major nutritional problems. The human body has an estimated 100 mg of Cu, predominantly stored in the skeleton and muscles, and the remaining distributed in the liver, brain, blood, heart, and kidneys (Burkhead and Collins, 2021). As indicated in Table 5, the mesocarp again exhibited the highest Cu level (3.36 ppm), followed by the exocarp (3.23 ppm) and endocarp (2.20 ppm). This pattern suggests that the Cu composition of exocarp and mesocarp is greater than that of endocarp, and thus exocarp and mesocarp may serve as potential dietary Cu sources. The copper concentration in P. timoriana in comparison to other leguminous and non-leguminous pods is lower than that in C. siliqua (8.50 ppm; Ayaz et al., 2007) and Acacia species, which was between 10.75 and 14.62 ppm (Pedro et al., 2023). Likewise, the pods of L. leucocephala (8,200 ppm), P. laevigata (8,900 ppm), and A. farnesiana (1,000 ppm) contained considerably higher Cu concentrations (Zapata-Campos et al., 2020). The results on the relatively lower Cu concentrations in P. timoriana would indicate that while it may not be the primary source of Cu in the diet, its addition to the diet formulations may have favorable contribution towards the mineral balance. In terms of nutrition, animal feeds made with pod fractions (especially the mesocarp) from P. timoriana could mitigate Cu deficiency in farmlands with low available copper and subsequently low Cu forage. In the same way, the dietary incorporation of Cu has not been well addressed, however P. timoriana pod products could be used as an adjunct to other source of micronutrients as a natural supply of Cu.
Zn is essential for the metabolism of all living organisms, from plants to animals and human beings, and plays an important role in more than 300 enzyme and protein molecules in catalysis, structure, and regulation of body systems, as well as in central nervous system activities, DNA transcription, etc. (Bolan et al., 2025). The mesocarp was found to have the highest Zn concentration of 13.60 ppm, while the endocarp and exocarp had 11.53 ppm and 7.51 ppm, respectively (Table 5). The high concentration of Zn in mesocarp suggests the possible use of mesocarp as a natural Zn supplement, particularly for the correction of Zn deficient conditions which hinder enzymatic and metabolic activities in humans and animals alike. In comparison with the other leguminous and non-leguminous pods, the Zn content of P. timoriana fractions is considered to be moderate. For example, it was stated that the concentration for C. siliqua pods contains Zn at 7.50 ppm (Ayaz et al., 2007) and 43.00 ppm (Ishag et al., 2024). It is worth mentioning that the pods from L. leucocephala, P. laevigata and A. farnesiana report considerably higher Zn concentrations, which are 57,000, 73,700 and 10,700 ppm, respectively (Zapata-Campos et al., 2020). In the same way, the Acacia green pods of certain species showed Zn values at 15.84 and 23.13 at certain ranges (A. melanoxylon, A. dealbata, A. cyclops, A. retinodes, A. mearnsii, A. pycnantha and A. longifolia; Pedro et al., 2023). P. timoriana pods, even though these values are lower than that of L. leucocephala and P. laevigata, are comparable to or higher than those of some Acacia species and range within C. siliqua. These pods have the ability to serve as a dietary source of zinc, especially from the mesocarp which contains a considerably higher concentration of Zn. The obtained prompt is very important for animals in which it is incorporated into diets for supporting growth, fertility, reproductive function, and providing efficient enzymatic activities. For humans, zinc is especially vital for immune function, growth and reproduction.
3.6 Total phenolic compound and antioxidant activity
Analyzing the antioxidant activities and phenolic contents in the exocarp, mesocarp, and endocarp of the walls of the P. timoriana pods deepens our understanding of the potential nutritional and functional values. Figure 2 shows that the total phenolic content (TPC; Figure 2A) and DPPH radical scavenging activity (Figure 2B) both exhibited significant differences between the fractions of the pod wall, revealing variability in the composition of the bioactive compounds. The exocarp had the highest phenolic content, measuring antioxidant potential of 39.13 mg GAE/g and DPPH radical scavenging activity of 22.60 mg VCE/g, indicating a high concentration of phenolic compounds that have been shown to eliminate free radicals and reduce oxidative stress (Ezez, 2022; Shahidi and Ambigaipalan, 2015). In animal nutrition, phenolics are related to better growth performance, immune response, oxidative stress balance, and product value (Waqas et al., 2023). Therefore, the exocarp of P. timoriana, which is rich in antioxidants, can be considered as a potential functional ingredient, as well as a natural feed supplement to improve livestock health and productivity. The antioxidant potential of mesocarp is moderate, given the TPC value of 9.52 ± 0.10 mg GAE/g and the DPPH activity of 7.43 ± 0.14 mg VCE/g. As lesser quantity of phytochemicals than the exocarp, they do contribute to modest antioxidant activity. The constituent value of phenolic biomolecules facilitates the potential of mesocarp to serve in structural carbohydrates and bioactive compounds, thus enhancing the dietary fiber of both humans and animals. The value of endocarp is still positive as structural fiber, particularly in ruminants, and it is considered to exhibit the least antioxidant potential with a TPC of 2.09 mg GAE/g and DPPH activity of 1.26 mg VCE/g. Though endocarp contributes the least phenolic molecules to the system, alongside other legume and non-legume pods, it is likely to serve as non-nutritive and bulk energy when paired with energy dense feeds. The pod walls of P. timoriana exhibited a certain degree of variability in antioxidant activity when compared with its leguminous and non-leguminous counterparts. Even within the same genus, interspecific differences marked by the TPC of the Parkia speciosa pods, ranging from 71.39 to 84.24 mg GAE/g DW (Wonghirundecha et al., 2014), show the inter generic disparity. Like other tissues examined, the pods and pulps of the C. siliqua showed TPC values from 0.14–12.70 mg/g DW depending on the region and the type of tissue sampled (El-Chami et al., 2025; Goulas and Georgiou, 2019; Ouzounidou et al., 2012). This concentration range places P. timoriana exocarp on the higher side of the phenolic scale, as some C. siliqua varieties and the P. speciosa remain lower. In P. timoriana exocarp, the DPPH radical scavenging activity values (22.60 mg VCE/g) are similar to those reported in C. siliqua from Spain (25.10 mg TE/g DW) and Morocco (16.70 mg TE/g DW; El-Chami et al., 2025) and higher than the Tunisian (11.47 mg TE/100 g DW; Othmen et al., 2019) and Algerian (1.26 mg TE/100 g DW; Benchikh et al., 2014) samples. These differences underscore the specific diversity of phytochemicals in the P. timoriana pod walls, especially the exocarp. In general, the data shows that the exocarp of the P. timoriana pods is highly concentrated with phenolic antioxidants, the mesocarp has moderate levels, and the endocarp primarily structural fibers with weak antioxidant activity. Based on the data these findings suggest the use of pod wall residues as sustainable human and animal nutrition ingredients used for the active intake of health-promoting bio-actives and also for the valorization of agro-industrial by-products.
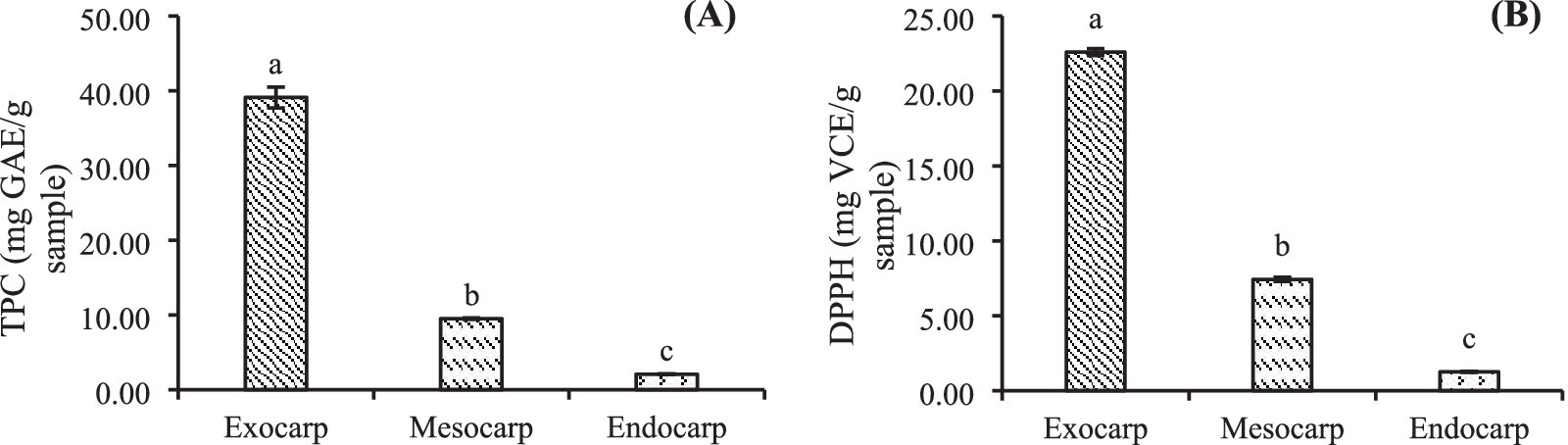
Figure 2. Total phenolic content (TPC) (A) and DPPH radical scavenging activity (B) of the exocarp, mesocarp, and endocarp of Parkia timoriana pods. Values are expressed as mean ± standard deviation (n = 12). TPC is expressed in mg gallic acid equivalents (GAE)/g sample; DPPH activity is expressed in mg vitamin C equivalents (VCE)/g sample.
4 Conclusion
This study reported the nutritional value, energetics, and antioxidant potential of the exocarp, mesocarp, and endocarp of the P. timoriana pods to promote sustainable nutrition for humans and animals. With respect to the total amino acids, moisture, crude protein, ether extract, crude ash, nitrogen-free extract, non-structural carbohydrates, digestible energy, metabolizable energy, net energy for maintenance, net energy for gain, net energy for lactation, relative feed value, and certain minerals such as phosphorus, potassium, iron, manganese, magnesium, copper, and zinc, the constituents were highest in the mesocarp. On the other hand, the exocarp was dominant in acid detergent fiber, acid detergent lignin, gross energy, calcium and total phenolic content as well as DPPH radical scavenging activity. The endocarp had the highest amount of dry matter, organic matter, total carbonhydrate, crude fiber, neutral detergent fiber, hemicellulose, cellulose, and even the ratio of calcium to phosphorus. According to feed classification, all components of the pod were classified as roughage and energy-rich materials. Forage quality grading divided the mesocarp as “Prime,” and the exocarp and the endocarp as “Poor” and “Reject” respectively, emphasizing the value of the mesocarp as a feed resource in sustainable animal nutrition. This research is the first to systematically describe and reveal new insights into the internal anatomical structures of P. timoriana pods, their diverse nutritional and functional capacities, and how they can be reinforced as sustainable feed and functional ingredient resources. This study does have some limitations. These include the possible variation of pod composition due environmental and genetic influences, the absence of in vivo confirmation on the nutritive and antioxidant activity, and doubts of scale of industrial or commercial use. These gaps should be addressed in future research by: (i) studying a broader and more representative sample in varying cultivation conditions to capture the geographic or climatic range, (ii) designing and executing animal feeding trials or human clinical studies to substantiate the proved nutritional and bioactive benefits, and (iii) studying the processing and techno-economic evaluation for large-scale use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ER: Validation, Conceptualization, Methodology, Data curation, Funding acquisition, Writing – original draft, Formal analysis, Visualization. SS: Conceptualization, Methodology, Writing – review & editing, Funding acquisition. SC-A: Writing – review & editing, Visualization, Validation. AJ: Writing – review & editing, Validation. AB: Methodology, Validation, Data curation, Writing – review & editing, Formal analysis. NH: Writing – review & editing, Validation, Data curation, Conceptualization, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project was supported by funds of the Innovation and Technology Assistance Program (ITAP) and Lan Lan Sub Turakit kasat Co. Ltd.
Acknowledgments
The authors extend their heartfelt appreciation to the Urban Agriculture Technology Research Group and the Department of Agricultural and Fisheries Science, Faculty of Science and Technology, Prince of Songkla University, Pattani Campus, for their willing help towards the provided laboratory services.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abraham, G., Kechero, Y., and Andualem, D. (2023). Nutritional quality of indigenous legume browse in southern Ethiopia: farmers’ preference and correlation of local valuation of feed value with scientific indicators. Front. Vet. Sci. 10:1198242. doi: 10.3389/fvets.2023.1198212
Adesogan, A. T., Arriola, K. G., Jiang, Y., Oyebade, A., Paula, E. M., Pech-Cervantes, A. A., et al. (2019). Symposium review: technologies for improving fiber utilization. J. Dairy Sci. 102, 5726–5755. doi: 10.3168/jds.2018-15334
Ajila, C. M., Brar, S. K., Verma, M., Tyagi, R. D., Godbout, S., and Valéro, J. R. (2012). Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 32, 382–400. doi: 10.3109/07388551.2012.659172
Ajomiwe, N., Boland, M., Phongthai, S., Bagiyal, M., Singh, J., and Kaur, L. (2024). Protein nutrition: understanding structure, digestibility, and bioavailability for optimal health. Foods 13:11. doi: 10.3390/foods13111771
Al-Alawi, A. M., Al Badi, A., Al Huraizi, A., and Falhammar, H. (2021). “Chapter six - magnesium: the recent research and developments” in Advances in food and nutrition research. ed. N. A. M. Eskin, vol. 96 (Cambridge, USA: Academic Press), 193–218.
Allen, M. S. (2000). Effects of diet on short-term regulation of feed intake by lactating dairy cattle. J. Dairy Sci. 83, 1598–1624. doi: 10.3168/jds.S0022-0302(00)75030-2
Alp, D., and Bulantekin, Ö. (2021). The microbiological quality of various foods dried by applying different drying methods: a review. Eur. Food Res. Technol. 247, 1333–1343. doi: 10.1007/s00217-021-03731-z
Amiri, F. (2012). Comparison of nutritive values of grasses and legume species using forage quality index. Songklanakarin J. Sci. Technol. 34, 577–586. Available online at: https://doaj.org/article/9545889411914146a88a35e7eab084a5
Angami, T., Bhagawati, R., Touthang, L., Makdoh, B., Khatri, N., Singson, L., et al. (2018). Traditional uses, phytochemistry and biological activities of Parkia timoriana (DC.) Merr., an underutilized multipurpose tree bean: a review. Genet. Resour. Crop. Evol. 65, 679–692. doi: 10.1007/s10722-017-0595-0
AOAC, C. (1990). Association of Official Analytical Chemists, official methods of analysis 15th Ed Washington DC. USA.
AOAC, C. (1995). Animal feeds. In Official methods of analysis 6th Ed. AOAC International Gaithersburg, MD. USA.
AoOA, C., and Horwitz, W. (2000). Official methods of analysis: Association of Official Analytical Chemists Washington. DC.
Apirattananusorn, S. (2017). Some chemical and functional properties of dry pulp from Riang (Parkia timoriana (DC.) Merr.). Int. J. Agr. Technol. 13, 869–881. Available online at: https://search.tci-thailand.org/article.html?b3BlbkFydGljbGUmaWQ9MjE4ODg5
Asadi, M., Toghdory, A., Hatami, M., and Ghassemi Nejad, J. (2022). Milk supplemented with organic Iron improves performance, blood Hematology, Iron metabolism parameters, biochemical and immunological parameters in suckling Dalagh lambs. Animals 12:4. doi: 10.3390/ani12040510
Ayaz, F. A., Torun, H., Ayaz, S., Correia, P. J., Alaiz, M., Sanz, C., et al. (2007). Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): sugars, amino and organic acids, minerals and phenolic compounds. Journal of food quality. J. Food Qual. 30, 1040–1055. doi: 10.1111/j.1745-4557.2007.00176.x
Bala, S., Garg, D., Sridhar, K., Inbaraj, B. S., Singh, R., Kamma, S., et al. (2023). Transformation of Agro-waste into value-added bioproducts and bioactive compounds: Micro/Nano formulations and application in the Agri-food-pharma sector. Bioengineering 10:152. doi: 10.3390/bioengineering10020152
Bellisle, F. (1999). Glutamate and the UMAMI taste: sensory, metabolic, nutritional and behavioural considerations. A review of the literature published in the last 10 years. Neurosci. Biobehav. Rev. 23, 423–438. doi: 10.1016/S0149-7634(98)00043-8
Benchikh, Y., Louaileche, H., George, B., and Merlin, A. (2014). Changes in bioactive phytochemical content and in vitro antioxidant activity of carob (Ceratonia siliqua L.) as influenced by fruit ripening. Ind. Crop. Prod. 60, 298–303. doi: 10.1016/j.indcrop.2014.05.048
Bolan, N., Srinivasarao, C., Rocco, C., Bolan, S., Mansoor, S., Wani, O. A., et al. (2025). “Chapter one—zinc in soil-crop-animal-human health continuum” in Advances in agronomy. ed. D. L. Sparks, vol. 189 (Cambridge, USA: Academic Press), 1–61.
Brauer, P. M., Slavin, J. L., and Marlett, J. A. (1981). Apparent digestibility of neutral detergent fiber in elderly and young adults. Am. J. Clin. Nutr. 34, 1061–1070. doi: 10.1093/ajcn/34.6.1061
Buathongjan, C., Israkarn, K., Sangwan, W., Outrequin, T., Gamonpilas, C., and Methacanon, P. (2020). Studies on chemical composition, rheological and antioxidant properties of pectin isolated from Riang (Parkia timoriana (DC.) Merr.) pod. Int. J. Biol. Macromol. 164, 4575–4582. doi: 10.1016/j.ijbiomac.2020.09.079
Burkhead, J. L., and Collins, J. F. (2021). Nutrition information brief—copper. Adv. Nutr. 13, 681–683. doi: 10.1093/advances/nmab157
Caballero, B., Trugo, L., and Finglas, P. (2003). Encyclopedia of food sciences and nutrition: volumes 1-10. Available online at: https://www.cabidigitallibrary.org/doi/full/10.5555/20033096645 (Accessed September 01, 2025).
Cebra, C., Anderson, D., Tibary, A., Van Saun, R., and Johnson Larue, W. (2013). Llama and alpaca care: Medicine, surgery, reproduction. St. Louis, Missouri, USA: Nutrition, and Herd Health.
Chen, H., Sun, Q., Tian, C., Tang, X., Ren, Y., and Chen, W. (2024). Assessment of the nutrient value and in vitro rumen fermentation characteristics of garlic peel, sweet potato vine, and cotton straw. Fermentation 10:9. doi: 10.3390/fermentation10090464
Chen, S., Wu, X., Duan, J., Huang, P., Li, T., Yin, Y., et al. (2021). Low-protein diets supplemented with glutamic acid or aspartic acid ameliorate intestinal damage in weaned piglets challenged with hydrogen peroxide. Anim. Nutr. 7, 356–364. doi: 10.1016/j.aninu.2020.12.005
Cherian, G. (2020). A guide to the principles of animal nutrition. Oregon State University Corvallis, OR, USA. Available online at: http://trva-ind-media.s3.amazonaws.com/media/knowledgehub/A_Guide_to_the_Principles_of_Animal_Nutrition_Gita_Cherian.pdf (Accessed September 01, 2025).
Cooke, R. (2020). The importance of energy nutrition for cattle. Ag - Beef/Cattle. Available online at: https://extension.oregonstate.edu/animals-livestock/beef/importance-energy-nutrition-cattle (Accessed March 12, 2025).
Council, N. R. (1989). Nutrient requirements of horses, vol. 6. Washington, D.C., USA: National Academies Press.
Council, N. R., Resources, B. A. N., Nutrition, C. A., and Nutrition, S. D. C. (2001). Nutrient requirements of dairy cattle: Seventh revised edition, 2001. Washington, D.C., USA: National Academies Press.
Council, N. R., Sciences, C. L., and Allowances, S. T. E. R. D. (1989). Recommended dietary allowances.
Crichton, R. (2016). Iron metabolism: From molecular mechanisms to clinical consequences. Chichester, West Sussex, UK: John Wiley and Sons.
Dardick, C., and Callahan, A. M. (2014). Evolution of the fruit endocarp: molecular mechanisms underlying adaptations in seed protection and dispersal strategies. Front. Plant Sci. 5:284. doi: 10.3389/fpls.2014.00284
Davis, M. P., Freetly, H. C., Kuehn, L. A., and Wells, J. E. (2014). Influence of dry matter intake, dry matter digestibility, and feeding behavior on body weight gain of beef steers. J. Anim. Sci. 92, 3018–3025. doi: 10.2527/jas.2013-6518
Davis, T., Suryawan, A., Orellana, R. A., and Fiorotto, M. L. (2010). Leucine acts as a nutrient signal to stimulate protein synthesis. J. Anim. Sci. 88:2232. doi: 10.2527/jas.2009-2232
Del-Valle, H. B., Yaktine, A. L., Taylor, C. L., and Ross, A. C. (2011). Dietary reference intakes for calcium and vitamin D.
Doi, M., Yamaoka, I., Fukunaga, T., and Nakayama, M. (2003). Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes. Biochem. Biophys. Res. Commun. 312, 1111–1117. doi: 10.1016/j.bbrc.2003.11.039
Douglas, T. D., Nucci, A. M., Berry, A. M., Henes, S. T., and Singh, R. H. (2019). Large neutral amino acid status in association with P:T ratio and diet in adult and pediatric patients with phenylketonuria. JIMD Reports 50, 50–59. doi: 10.1002/jmd2.12076
Egert, A., Lee, K., and Gill, M. (2023). Nursing fundamentals. Fundamentals of Nursing Pharmacology-Mohawk College Edition.
El-Chami, M. A., Palacios-Rodriguez, G., Ordonez-Diaz, J. L., Rodriguez-Solana, R., Navarro-Cerrillo, R. M., and Moreno-Rojas, J. M. (2025). Proximate analysis, total phenolic content, and antioxidant activity of wild carob pulp from three Mediterranean countries. Appl. Sci. 15:1340. doi: 10.3390/app15031340
Ezez, D. (2022). Antioxidant properties of phenolic compounds to manage oxidative stress / a review. J. Am. Chem. Soc. 2:202. doi: 10.17303/jacs.2023.2.103
Fao, J. (2005). Human energy requirements: report of a joint FAO/WHO/UNU expert consultation. Food Nutr. Bull. 26:166. doi: 10.4060/cd1600es
Fujimori, S. (2021). Humans have intestinal bacteria that degrade the plant cell walls in herbivores. World J. Gastroenterol. 27, 7784–7791. doi: 10.3748/wjg.v27.i45.7784
Gaál, K., Sáfár, O., Gulyás, L., and Stadler, P. (2005). Magnesium in animal nutrition. J. Am. Coll. Nutr. 23, 754S–757S. doi: 10.1080/07315724.2004.10719423
García-Winder, L., Goñi-Cedeño, S., Olguín-Lara, P., Salgado Diaz, G., and Arriaga-Jordán, C. (2009). Huizache (Acacia farnesiana) whole pods (flesh and seeds) as an alternative feed for sheep in Mexico. Trop. Anim. Health Prod. 41, 1615–1621. doi: 10.1007/s11250-009-9355-2
Garrison, M. V., Reid, R. L., Fawley, P., and Breidenstein, C. P. (1978). Comparative digestibility of acid detergent Fiber by laboratory albino and wild Polynesian rats. J. Nutr. 108, 191–195. doi: 10.1093/jn/108.2.191
Ghimire, A., and Chen, P.-Y. (2022). Seed protection strategies of the brainy Elaeocarpus ganitrus endocarp: gradient motif yields fracture tolerance. Acta Biomater. 138, 430–442. doi: 10.1016/j.actbio.2021.10.050
Gilpin, A., Herrman, T., Behnke, K., and Fairchild, F. (2002). Feed moisture, retention time, and steam as quality and energy utilization determinants in the pelleting process. Appl. Eng. Agric. 18:8585. doi: 10.13031/2013.8585
Goulas, V., and Georgiou, E. (2019). Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: extraction optimization and application in food models. Foods 9:20. doi: 10.3390/foods9010020
Grela, E. R., Samolińska, W., Kiczorowska, B., Klebaniuk, R., and Kiczorowski, P. (2017). Content of minerals and fatty acids and their correlation with phytochemical compounds and antioxidant activity of leguminous seeds. Biol. Trace Elem. Res. 180, 338–348. doi: 10.1007/s12011-017-1005-3
Gröber, U., Schmidt, J., and Kisters, K. (2015). Magnesium in prevention and therapy. Nutrients 7, 8199–8226. doi: 10.3390/nu7095388
Han, Q., Phillips, R. S., and Li, J. (2019). Aromatic amino acid metabolism. Front. Mol. Biosci. 6:22. doi: 10.3389/fmolb.2019.00022
Hao, Z., Wang, X., Yang, H., Tu, T., Zhang, J., Luo, H., et al. (2021). PUL-mediated plant Cell Wall polysaccharide utilization in the gut Bacteroidetes. Int. J. Mol. Sci. 22:3077. doi: 10.3390/ijms22063077
Hartmann, H., and Trumbore, S. (2016). Understanding the roles of nonstructural carbohydrates in forest trees – from what we can measure to what we want to know. New Phytol. 211, 386–403. doi: 10.1111/nph.13955
He, Y., Wang, B., Wen, L., Wang, F., Yu, H., Chen, D., et al. (2022). Effects of dietary fiber on human health. Food Sci. Human Wellness 11, 1–10. doi: 10.1016/j.fshw.2021.07.001
He, R., Zhang, Y., Song, S., Su, W., Hao, Y., and Liu, H. (2021). UV-A and FR irradiation improves growth and nutritional properties of lettuce grown in an artificial light plant factory. Food Chem. 345:128727. doi: 10.1016/j.foodchem.2020.128727
Hernández-Ruiz, J., Aguilar-Marcelino, L., Marín, J. A. H., Avalos, A. M. C., Huerta, I. A., and Arriaga, A. I. M. (2024). Use of Prosopis on feed nutrition: challenges and opportunities. Complementary and Alternative Medicine: Feed Additives, pp. 163–170.
Holesh, J. E., Aslam, S., and Martin, A. (2020). Physiology, Carbohydrates. StatPearls, Treasure Island, FL.
Holloway, W. D., Tasman-Jones, C., and Bell, E. (1980). The hemicellulose component of dietary fiber. Am. J. Clin. Nutr. 33, 260–263. doi: 10.1093/ajcn/33.2.260
Hou, Y., and Wu, G. (2018). Nutritionally essential amino acids. Adv. Nutr. 9, 849–851. doi: 10.1093/advances/nmy054
Hou, Y., Yin, Y., and Wu, G. (2015). Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. 240, 997–1007. doi: 10.1177/1535370215587913
Ishag, O., Hamid, M., Hamad, M., Nour, A., Erwa, I., and Ssempala, M. (2024). Evaluation of proximate composition, minerals content and antioxidant properties of carob (Ceratonia Siliqua L.) pod. Int. J. Multidiscip. Res. 6, 1–7. doi: 10.36948/ijfmr.2024.v06i03.21796
Jayanegara, A., Ridla, M., Nahrowi, N., and Laconi, E. (2019). Estimation and validation of total digestible nutrient values of forage and concentrate feedstuffs. IOP Conf. Ser. Mater. Sci. Eng. 546:042016. doi: 10.1088/1757-899X/546/4/042016
Jayaprakash, J., Nath, L. R., Gowda, S. G., Gowda, D., and Hui, S.-P. (2024). Analysis and functions of bioactive lipids in food. Discov. Food 4:107. doi: 10.1007/s44187-024-00186-5
Jayeola, C., Adebowale, B., Yahaya, L., Ogunwolu, S., and Olubamiwa, O. (2018). “Production of bioactive compounds from waste” in Therapeutic, probiotic, and unconventional foods, (Cambridge, USA: Academic Press), 317–340.
Jha, R., and Berrocoso, J. F. D. (2016). Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. Technol. 212, 18–26. doi: 10.1016/j.anifeedsci.2015.12.002
Jha, R., Fouhse, J. M., Tiwari, U. P., Li, L., and Willing, B. P. (2019). Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 6:48. doi: 10.3389/fvets.2019.00048
Johnson, A., Cantrill, R., and Clapper, G. (2012). “9—implementing a uniform process for measurement of soybean quality traits” in Designing soybeans for 21st century markets. ed. R. F. Wilson (Urbana, Illinois, USA: AOCS Press), 161–173.
Karau, A., and Grayson, I. (2014). “Amino acids in human and animal nutrition” in Biotechnology of food and feed additives. eds. H. Zorn and P. Czermak, vol. 143 (Heidelberg, Germany: Springer Berlin Heidelberg), 189–228.
Kawabata, T. (2022). Iron-induced oxidative stress in human diseases. Cells 11:2152. doi: 10.3390/cells11142152
Kerr, B. J., Dozier, W. A., and Lee, D. T. (2024). Energy and ether extract digestibility of commercially available lipids fed to broilers. J. Appl. Poult. Res. 33:100406. doi: 10.1016/j.japr.2024.100406
Khan, M. (2015). Dynamics of microbial protein synthesis in the rumen –a review. Dhaka, Bangladesh: Annals of Veterinary and Animal Science.
Kirkland, A. E., Sarlo, G. L., and Holton, K. F. (2018). The role of magnesium in neurological disorders. Nutrients 10:730. doi: 10.3390/nu10060730
Kırkpınar, F., Açıkgöz, Z., Kırkpınar, F., and Açıkgöz, Z. (2018). “Feeding” in Animal husbandry and nutrition. Eds. B. Yücel and T. Taşkin. (London, United Kingdom: IntechOpen).
Klimis-Zacas, D. (1993). Manganese in health and disease, vol. 2. Boca Raton, Florida, USA: CRC Press.
Ko, G.-J., Rhee, C. M., Kalantar-Zadeh, K., and Joshi, S. (2020). The effects of high-protein diets on kidney health and longevity. J. Am. Soc. Nephrol. 31, 1667–1679. doi: 10.1681/ASN.2020010028
Kujoana, T. C., Mabelebele, M., and Sebola, N. A. (2024). Role of dietary fats in reproductive, health, and nutritional benefits in farm animals: a review. Open Agricult. 9:244. doi: 10.1515/opag-2022-0244
Kulkarni, C., Kulkarni, K. S., and Hamsa, B. R. (2005). L-glutamic acid and glutamine: exciting molecules of clinical interest. Indian J. Pharm. 37, 148–154. doi: 10.4103/0253-7613.16210
Kumar, N., and Chaiyasut, C. (2017). Health promotion potential of vegetables cultivated in northern Thailand: a preliminary screening of tannin and flavonoid contents, 5α-reductase inhibition, astringent activity, and antioxidant activities. J. Evid. Based Comp. Alternat. Med. 22, 573–579. doi: 10.1177/2156587216686689
Kupina, S., Fields, C., Roman, M. C., and Brunelle, S. L. (2018). Determination of Total phenolic content using the Folin-C assay: single-laboratory validation, first action 2017.13. J. AOAC Int. 101, 1466–1472. doi: 10.5740/jaoacint.18-0031
Lattimer, J. M., and Haub, M. D. (2010). Effects of dietary fiber and its components on metabolic health. Nutrients 2, 1266–1289. doi: 10.3390/nu2121266
Lei, J., Feng, D., Zhang, Y., Dahanayaka, S., Li, X., Yao, K., et al. (2012). Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino Acids 43, 2179–2189. doi: 10.1007/s00726-012-1302-2
Li, X. (2021). Plant Cell Wall chemistry: implications for ruminant utilisation. J. Appl. Anim. Nutr. 9, 1–26. doi: 10.3920/JAAN2020.0017
Li, Y., Han, H., Yin, J., Li, T., and Yin, Y. (2018). Role of D-aspartate on biosynthesis, racemization, and potential functions: a mini-review. Anim. Nutr. 4, 311–315. doi: 10.1016/j.aninu.2018.04.003
Li, P., Knabe, D. A., Kim, S. W., Lynch, C. J., Hutson, S. M., and Wu, G. (2009). Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J. Nutr. 139, 1502–1509. doi: 10.3945/jn.109.105957
Liao, Z., Fan, J., Lai, Z., Bai, Z., Wang, H., Cheng, M., et al. (2023). “Chapter three—response network and regulatory measures of plant-soil-rhizosphere environment to drought stress” in Advances in agronomy. ed. D. L. Sparks, vol. 180 (Cambridge, Massachusetts, USA: Academic Press), 93–196.
Liu, K. (2019). Effects of sample size, dry ashing temperature and duration on determination of ash content in algae and other biomass. Algal Res. 40:101486. doi: 10.1016/j.algal.2019.101486
Liu, H. J., Chang, B. Y., Yan, H. W., Yu, F. H., and Liu, X. X. (1995). Determination of amino acids in food and feed by derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate and reversed-phase liquid chromatographic separation. J. AOAC Int. 78, 736–743. doi: 10.1093/jaoac/78.3.736
Liu, Z., Zhong, R., Li, K., Chen, L., Zhang, B., Liu, L., et al. (2021). Evaluation of energy values of high-fiber dietary ingredients with different solubility fed to growing pigs using the difference and regression methods. Anim. Nutr. 7, 569–575. doi: 10.1016/j.aninu.2020.07.010
Livesey, G. (2001). A perspective on food energy standards for nutrition labelling. Br. J. Nutr. 85, 271–287. doi: 10.1079/BJN2000253
Longvah, T., and Deosthale, Y. G. (1998). Nutrient composition and food potential of Parkia roxburghii, a less known tree legume from Northeast India. Food Chem. 62, 477–481. doi: 10.1016/S0308-8146(97)00179-9
Ludwig, D. S., Hu, F. B., Tappy, L., and Brand-Miller, J. (2018). Dietary carbohydrates: role of quality and quantity in chronic disease. BMJ 361:k2340. doi: 10.1136/bmj.k2340
Maclean, W., Harnly, J., Chen, J., Chevassus-Agnes, S., Gilani, G., Livesey, G., et al. (2003). Food energy–methods of analysis and conversion factors. Food and Agriculture Organization of the United Nations Technical Workshop Report, 77, 8–9.
Madilindi, M. A., Zishiri, O. T., Dube, B., and Banga, C. B. (2022). Technological advances in genetic improvement of feed efficiency in dairy cattle: a review. Livest. Sci. 258:104871. doi: 10.1016/j.livsci.2022.104871
Maynard, L. A. (1940). Nitrogen-free extract in animal nutrition. J. Assoc. Off. Agric. Chem. 23, 156–161. doi: 10.1093/jaoac/23.1.156
McCaughey, S. (2007). “4-Dietary salt and flavor: mechanisms of taste perception and physiological controls” in Reducing salt in foods. eds. D. Kilcast and F. Angus (Sawston, Cambridge, UK: Woodhead Publishing), 77–98.
Medicine, I. of, Board, F. and N., and Intakes, S. C. on the S. E. of D. R (1999). Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, D.C., USA: National Academies Press.
Montevecchi, G., Masino, F., and Antonelli, A. (2012). “Glutamic acid in food and its thermal degradation in acidic medium” in Glutamic acid: Chemistry, food sources & health benefits. Eds. D. M. J. Balcazar and E. A. Reinoso Perez. (Hauppauge, New York, USA: Nova Science Publishers Inc.), 79–96.
Moore, J., and Undersander, D. (2002). “Relative forage quality: an alternative to relative feed value and quality index.” in Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium.
Naik, B., Kumar, V., and Gupta, A. K. (2023). Valorization of tender coconut mesocarp for the formulation of ready-to-eat dairy-based dessert (kheer): utilization of industrial by-product. J. Agric. Food Res. 12:100572. doi: 10.1016/j.jafr.2023.100572
Negro, M., Giardina, S., Marzani, B., and Marzatico, F. (2008). Branched-chain amino acid supplementation does not enhance athletic performance but affects muscle recovery and the immune system. J. Sports Med. Phys. Fitness 48:347. Available online at: https://pubmed.ncbi.nlm.nih.gov/18974721/
Nishimura, J., Masaki, T., Arakawa, M., Seike, M., and Yoshimatsu, H. (2010). Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARα and uncoupling protein in diet-induced obese mice. J. Nutr. 140, 496–500. doi: 10.3945/jn.109.108977
Niwińska, B. (2012). “Digestion in Ruminants” in Carbohydrates—Comprehensive studies on glycobiology and Glycotechnology Ed. C.-F. Chang. (London, UK: IntechOpen).
Nutrient Requirements of Dairy Cattle. (2021). Nutrient Requirements of Dairy Cattle: Eighth Revised Edition (with Committee on Nutrient Requirements of Dairy Cattle, Board on Agriculture and Natural Resources, Division on Earth and Life Studies, & National Academies of Sciences, Engineering, and Medicine) National Academies Press.
Othmen, K., Elfalleh, W., Lachiheb, B., and Haddad, M. (2019). Evolution of phytochemical and antioxidant activity of Tunisian carob (Ceratonia siliqua L.) pods during maturation. Eurobiotech J. 3, 135–142. doi: 10.2478/ebtj-2019-0016
Ouzounidou, G., Vekiari, S., Asfi, M., Gork, M. G., Sakcali, M. S., and Ozturk, M. (2012). Photosynthetic characteristics of carob tree (Ceratonia siliqua L.) and chemical composition of its fruit on diurnal and seasonal basis.
Palmquist, D. L., and Jenkins, T. C. (2003). Challenges with fats and fatty acid methods. J. Anim. Sci. 81, 3250–3254. doi: 10.2527/2003.81123250x
Panneerselvam, S., Palanisamy, V., Balasubramaniam, M., Palanisamy, S., Jaganathan, M., and Kannan, T. A. (2024). Effect of nonstructural carbohydrates on production performance, rumen metabolism and rumen health in lambs fed with isocaloric and isonitrogenous complete diets. Trop. Anim. Health Prod. 56:181. doi: 10.1007/s11250-024-04029-4
Pasha, T. N., Prigge, E. C., Russell, R. W., and Bryan, W. B. (1994). Influence of moisture content of forage diets on intake and digestion by sheep. J. Anim. Sci. 72, 2455–2463. doi: 10.2527/1994.7292455x
Pedro, S. I., Antunes, C. A. L., Horta, C., Pitacas, I., Gonçalves, J., Gominho, J., et al. (2023). Characterization of mineral composition and nutritional value of Acacia green pods. Plants 12:1853. doi: 10.3390/plants12091853
Pencharz, P. B., Hsu, J. W., and Ball, R. O. (2007). Aromatic amino acid requirements in healthy human subjects. J. Nutr. 137, 1576S–1578S. doi: 10.1093/jn/137.6.1576S
Pinotti, L., Manoni, M., Ferrari, L., Tretola, M., Cazzola, R., and Givens, I. (2021). The contribution of dietary magnesium in farm animals and human nutrition. Nutrients 13:509. doi: 10.3390/nu13020509
Polak, R., Phillips, E. M., and Campbell, A. (2015). Legumes: health benefits and culinary approaches to increase intake. Clin. Diabetes 33, 198–205. doi: 10.2337/diaclin.33.4.198
Polakof, S., Panserat, S., Soengas, J. L., and Moon, T. W. (2012). Glucose metabolism in fish: a review. J. Comp. Physiol. B. 182, 1015–1045. doi: 10.1007/s00360-012-0658-7
Princewill, O. (2015). Interactions between dietary minerals and reproduction in farm animal. Glob. J. Anim. Sci. Res. 3, 524–535. Available online at: https://www.researchgate.net/publication/290883761_Interactions_between_Dietary_Minerals_and_Reproduction_in_farm_Animal
Quirino, D. F., Palma, M. N., Franco, M. O., and Detmann, E. (2023). Variations in methods for quantification of crude ash in animal feeds. J. AOAC Int. 106, 6–13. doi: 10.1093/jaoacint/qsac100
Rana, V., Bachheti, R., Chand, T., and Barman, A. (2012). Dietary fibre and human health. Int. J. Food Saf. Nutr. Public Health 4, 101–118. doi: 10.1504/IJFSNPH.2011.044528
Razzaque, M. S., and Wimalawansa, S. J. (2025). Minerals and human health: from deficiency to toxicity. Nutrients 17:3. doi: 10.3390/nu17030454
Rehman, S. U., Ali, R., Zhang, H., Zafar, M. H., and Wang, M. (2023). Research progress in the role and mechanism of leucine in regulating animal growth and development. Front. Physiol. 14:1252089. doi: 10.3389/fphys.2023.1252089
Robinson, M. B. (1998). Review article the family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem. Int. 33, 479–491. doi: 10.1016/S0197-0186(98)00055-2
Rohweder, D. A., Barnes, R. F., and Jorgensen, N. (1978). Proposed hay grading standards based on laboratory analyses for evaluating quality. J. Anim. Sci. 47, 747–759. doi: 10.2527/jas1978.473747x
Ruangrak, E., Jeanmas, A., Kraiprom, T., Longnapa, K., Sornnok, S., and Htwe, N. M. P. S. (2025). Nutritional composition and ruminant feed potential of black pod by-products from tree bean (Parkia timoriana (DC.) Merr.) in southern Thailand. Trees For. People 20:100890. doi: 10.1016/j.tfp.2025.100890
Rusdy, M. (2020). Silvopastoral system using Leucaena leucocephala for sustainable animal production in the tropics. LRRD2020. Available online at: https://repository.unhas.ac.id/id/eprint/5242/ (Accessed September 02, 2025).
Saha, U. K., Sonon, L. S., Hancock, D. W., Hill, N. S., Stewart, L., Heusner, G. L., et al. (2010). Common terms used in animal feeding and nutrition.
Sampath, V., Sureshkumar, S., Seok, W. J., and Kim, I. H. (2023). Role and functions of micro and macro-minerals in swine nutrition: a short review. J. Anim. Sci. Technol. 65, 479–489. doi: 10.5187/jast.2023.e9
Sasu, P., Attoh-Kotoku, V., Akorli, D. E., Adjei-Mensah, B., Tankouano, R. A., and Kwaku, M. (2023). Nutritional evaluation of the leaves of Oxytenanthera abyssinica, Bambusa balcooa, Moringa oleifera, Terminalia catappa, Blighia sapida, and Mangifera indica as non-conventional green roughages for ruminants. J. Agric. Food Res. 11:100466. doi: 10.1016/j.jafr.2022.100466
Scala, I., Riccio, M. P., Marino, M., Bravaccio, C., Parenti, G., and Strisciuglio, P. (2020). Large neutral amino acids (LNAAs) supplementation improves neuropsychological performances in adult patients with phenylketonuria. Nutrients 12:1092. doi: 10.3390/nu12041092
Setia, R. C., Setia, N., and Malik, C. P. (1987). The podwall structure and function in relation to seed development in some legumes. Phyton 27, 205–220.
Shahidi, F., and Ambigaipalan, P. (2015). Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects – a review. J. Funct. Foods 18, 820–897. doi: 10.1016/j.jff.2015.06.018
Silva, C. S., Moutinho, C., Ferreira da Vinha, A., and Matos, C. (2019). Trace minerals in human health: Iron, zinc, copper, manganese and fluorine. Int. J. Sci. Res. Methodol. 13, 57–80. Available online at: https://ijsrm.humanjournals.com/wp-content/uploads/2019/10/5.Carla-Sousa-Carla-Moutinho-Ana-F.-Vinha-Carla-Matos.pdf
Singh, P. K., Gupta, M. K., and Nath, R. (2023). “Chapter 16—Omega-3 fatty acid as a protectant in lead-induced neurotoxicity” in Treatments, nutraceuticals, supplements, and herbal medicine in neurological disorders. eds. C. R. Martin, V. B. Patel, and V. R. Preedy (Cambridge, Massachusetts, USA: Academic Press), 285–301.
Singha, W., Kurmi, B., Sahoo, U., Sileshi, G., Nath, A., and Das, A. (2021). Parkia roxburghii, an underutilized tree bean for food, nutritional and regional climate security. Trees For. People. 4:65. doi: 10.1016/j.tfp.2021.100065
Slavin, J. L. (2005). Dietary fiber and body weight. Nutrition 21, 411–418. doi: 10.1016/j.nut.2004.08.018
Soares, T. F., and Oliveira, M. B. P. P. (2022). Cocoa by-products: characterization of bioactive compounds and beneficial health effects. Molecules 27:1625. doi: 10.3390/molecules27051625
Spencer, H., Kramer, L., Osis, D., and Norris, C. (1978). Effect of phosphorus on the absorption of calcium and on the calcium balance in man. J. Nutr. 108, 447–457. doi: 10.1093/jn/108.3.447
Spencer, H., Menczel, J., Lewin, I., and Samachson, J. (1965). Effect of high phosphorus intake on calcium and phosphorus metabolism in man. J. Nutr. 86, 125–132. doi: 10.1093/jn/86.2.125
Sturkie, P. D. (2000) in Sturkie’s avian physiology. ed. G. C. Whittow (San Diego, California, USA: Academic Press).
Stypinski, J. D., Weiss, W. P., Carroll, A. L., and Kononoff, P. J. (2024). Effect of acid detergent lignin concentration for diets formulated to be similar in neutral detergent fiber content on energy utilization in lactating Jersey cows. J. Dairy Sci. 107, 5699–5708. doi: 10.3168/jds.2023-24318
Suehs, B. A., Gatlin, D. M. III, and Wu, G. (2024). Glycine nutrition and biochemistry from an aquaculture perspective. Anim. Front. 14, 17–23. doi: 10.1093/af/vfae014
Suresh, A., Shobna,, Salaria, M., Morya, S., Khalid, W., Afzal, F. A., et al. (2024). Dietary fiber: an unmatched food component for sustainable health. Food Agric. Immunol. 35:2384420. doi: 10.1080/09540105.2024.2384420
Swetha, B. S., Prabhakar, I., and Ainapur, S. D. (2024). Food waste reduction and sustainable food systems: strategies, challenges, and future directions. J. Sci. Res. Rep. 30, 328–336. doi: 10.9734/jsrr/2024/v30i51948
Teh, H. F., Neoh, B. K., Hong, M. P. L., Low, J. Y. S., Ng, T. L. M., Ithnin, N., et al. (2013). Differential metabolite profiles during fruit development in high-yielding oil palm mesocarp. PLoS One 8:e61344. doi: 10.1371/journal.pone.0061344
Teodoro, G. F. R., Vianna, D., Torres-Leal, F. L., Pantaleao, L. C., Matos-Neto, E. M., Donato, J. Jr., et al. (2012). Leucine is essential for attenuating fetal growth restriction caused by a protein-restricted diet in rats. J. Nutr. 142, 924–930. doi: 10.3945/jn.111.146266
Tomar, K., Tiwari, S., Singh, D., and Kumar, D. (2022). Feeding Management of Ruminant Livestock. Smart Agricultural Technologies, September, pp. 71–84.
Trowell, H. (1976). Definition of dietary fiber and hypotheses that it is a protective factor in certain diseases. Am. J. Clin. Nutr. 29, 417–427. doi: 10.1093/ajcn/29.4.417
Trumbo, P., Schlicker, S., Yates, A. A., and Poos, M. (2002). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. (Commentary. J. Am. Diet. Assoc. 102, 1621–1631. doi: 10.1016/s0002-8223(02)90346-9
Van-Soest, P. v., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Velayudhan, D. E., Kim, I. H., and Nyachoti, C. M. (2015). Characterization of dietary energy in swine feed and feed ingredients: a review of recent research results. Asian Australas. J. Anim. Sci. 28, 1–13. doi: 10.5713/ajas.14.0001R
Vincent, S. G. T., Jennerjahn, T., and Ramasamy, K. (2021). “Chapter 1—source and composition of organic matter and its role in designing sediment microbial communities” in Microbial communities in coastal sediments. eds. S. G. T. Vincent, T. Jennerjahn, and K. Ramasamy (Amsterdam, Netherlands: Elsevier), 1–45.
Wagner, C. A. (2023). The basics of phosphate metabolism. Nephrol. Dial. Transplant. 39, 190–201. doi: 10.1093/ndt/gfad188
Wanapat, M., Suriyapha, C., Dagaew, G., Prachumchai, R., Phupaboon, S., Sommai, S., et al. (2024). The recycling of tropical fruit peel waste-products applied in feed additive for ruminants: food manufacturing industries, phytonutrient properties, mechanisms, and future applications. J. Agric. Food Res. 17:101234. doi: 10.1016/j.jafr.2024.101234
Waqas, M., Salman, M., and Sharif, M. S. (2023). Application of polyphenolic compounds in animal nutrition and their promising effects. J. Anim. Feed Sci. 32, 233–256. doi: 10.22358/jafs/159718/2023
Wedig, C. L., Jaster, E. H., and Moore, K. J. (1987). Hemicellulose monosaccharide composition and in vitro disappearance of orchard grass and alfalfa hay. J. Agric. Food Chem. 35, 214–218. doi: 10.1021/jf00074a012
Weimer, P. J. (2022). Degradation of cellulose and hemicellulose by ruminal microorganisms. Microorganisms 10:2345. doi: 10.3390/microorganisms10122345
Weiss, W., and Tebbe, A. (2018). Estimating digestible energy values of feeds and diets and integrating those values into net energy systems. Transl. Anim. Sci. 3, 953–961. doi: 10.1093/tas/txy119
Weyh, C., Krüger, K., Peeling, P., and Castell, L. (2022). The role of minerals in the optimal functioning of the immune system. Nutrients 14:644. doi: 10.3390/nu14030644
White, P. J., and Broadley, M. R. (2009). Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 182, 49–84. doi: 10.1111/j.1469-8137.2008.02738.x
Wonghirundecha, S., Benjakul, S., and Sumpavapol, P. (2014). Total phenolic content, antioxidant and antimicrobial activities of stink bean (Parkia speciosa Hassk.) pod extracts. Songklanakarin J. Sci. Technol. 36, 301–308. Available online at: https://www.thaiscience.info/journals/Article/SONG/10968311.pdf
Wu, G. (2022). Nutrition and metabolism: foundations for animal growth, development, reproduction, and health. Adv. Exp. Med. Biol. 1354, 1–24. doi: 10.1007/978-3-030-85686-1_1
Yatoo, D. M., Saxena, A., Pm, D., Habeeb, B., Devi, S., Jatav, R., et al. (2013). Role of trace elements in animals: a review. Vet. World 6, 963–967. doi: 10.14202/vetworld.2013.963-967
Zapata-Campos, C., García, J., Salinas-Chavira, J., Ascacio-Valdés, J., Medina-Morales, M. A., and Mellado, M. (2020). Chemical composition and nutritional value of leaves and pods of Leucaena leucocephala, Prosopis laevigata and Acacia farnesiana in a xerophilous shrubland. Emirates J. Food Agric. 32, 723–730. doi: 10.9755/ejfa.2020.v32.i10.2148
Keywords: recycling food waste, antioxidant activity, amino acid profile, animal feed, alternative food, bioactive compounds
Citation: Ruangrak E, Sornnok S, Chuai-Aree S, Jeanmas A, Bourchookarn A and Htwe NMPS (2025) Nutritional evaluation and antioxidant activity of the exocarp, mesocarp, and endocarp from Parkia timoriana (DC.) Merr. pods for sustainable animal and human nutrition. Front. Sustain. Food Syst. 9:1628357. doi: 10.3389/fsufs.2025.1628357
Edited by:
Rakesh Bhardwaj, Indian Council of Agricultural Research (ICAR), IndiaReviewed by:
Juliana Morales-Castro, Durango Institute of Technology, MexicoSapna Langyan, Indian Council of Agricultural Research (ICAR), India
Copyright © 2025 Ruangrak, Sornnok, Chuai-Aree, Jeanmas, Bourchookarn and Htwe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nang Myint Phyu Sin Htwe, bmFuZ215aW50cGh5dXNpbi5oQGt1LnRo; Apichai Bourchookarn, YXBpY2hhaS5iQHBzdS5hYy50aA==
 Eaknarin Ruangrak
Eaknarin Ruangrak Somnuek Sornnok1
Somnuek Sornnok1 Somporn Chuai-Aree
Somporn Chuai-Aree Apichai Bourchookarn
Apichai Bourchookarn Nang Myint Phyu Sin Htwe
Nang Myint Phyu Sin Htwe