- 1Department of Seed Science and Technology, College of Agriculture, Odisha University of Agriculture and Technology, Bhubaneswar, Odisha, India
- 2AICRP on Seed (Crops), Odisha University of Agriculture and Technology, Bhubaneswar, Odisha, India
- 3College of Agriculture, Odisha University of Agriculture and Technology, Chiplima, Odisha, India
- 4Department of Forest, Environment and Climate Change, Government of Odisha, Bhubaneswar, Odisha, India
- 5Director PME, Odisha University of Agriculture and Technology, Bhubaneswar, Odisha, India
- 6AICRP on Honeybee and Pollinators, Odisha University of Agriculture and Technology, Bhubaneswar, Odisha, India
- 7College of Horticulture, Odisha University of Agriculture and Technology, Chiplima, Odisha, India
Seed aging adversely affects germination, vigor, and biochemical efficiency, resulting in reduced seed performance and crop growth. The present study addressed the effects of SA priming at varying concentrations (0.10, 0.25, 0.50, 0.75, and 1.00 mM) on the physiological and biochemical attributes of aged (10-, 11-, 12-, 13-, and 14-month-old) tomato seeds. Results revealed that 0.50 mM SA priming significantly enhanced the seed vigor index–I (SVI-I) from 19.6% to 41.3% over the control in 10- and 14-month-old seeds, respectively. Similarly, protein levels were increased by 27%, dehydrogenase (29%) and α-amylase (19%) compared to the control. At 14-month-old seed, electrical conductivity was reduced to 28% over the control. In contrast, higher SA concentrations (0.75 and 1.00 mM) negatively impacted seedling growth, highlighting a threshold beyond which SA becomes inhibitory. The lowest root length was recorded in 1.00 mM SA-primed seeds. The controlled treatment showed higher root length than 1.00 mM SA-treated seeds. The seed germination (%) of 10-month-old seeds varied between 78.66% and 89%, which were significantly different (P = 0.05). This study demonstrates that the optimal SA concentration is crucial for effective seed priming, providing new insights into the physiological and biochemical pathways activated in aged tomato seeds. These findings contribute to the unexplored field of seed aging and establish 0.50 mM SA as an effective concentration for improving seed quality.
1 Introduction
Tomato (Solanum lycopersicum L.) is one of the most important vegetable crops globally, which ranks second in terms of production and consumption among horticultural commodities (FAOSTAT, 2019). With a global production surpassing 189 million metric tons in 2022, tomatoes are extensively cultivated across temperate, tropical, and increasingly arid and semi-arid regions (FAOSTAT, 2023; Collins et al., 2022). The crop is highly rich in essential vitamins (A, C, and E), minerals, dietary fiber, and bioactive compounds such as lycopene and flavonoids, which contribute to human health and disease prevention (Shafe et al., 2024; Mallick, 2021). A major concern in tomato production is the deterioration of seed quality due to aging, which significantly inhibits germination, seedling vigor, and ultimately crop productivity (Ray and Bordolui, 2022). Seed aging is characterized by the accumulation of reactive oxygen species (ROS), loss of membrane integrity, decreased enzymatic activity, and reduced metabolic function (Nickas et al., 2025). The loss of seed viability and vigor during storage is due to significant changes in the physiological characteristics of the seed (Pirredda et al., 2024). These physiological and biochemical changes result in non-uniform sprouting, poor seedling establishment, and reduced tolerance to environmental stress (Diya et al., 2024). The leakage of cellular components and electrolytes leads to a decline in seed viability (El-Maarouf-Bouteau, 2022). Seed priming, a pre-sowing hydration-dehydration technique, has emerged as an effective strategy to improve the germination performance of aged seeds (Saxena et al., 2021). Among various priming agents, salicylic acid (SA), a naturally occurring phenolic compound and plant hormone, plays a crucial role in modulating plant growth and development. The SA regulates multiple processes relevant to seed performance, including membrane protection, antioxidant defense, carbon metabolism, protein synthesis, and the activation of stress-responsive genes and enzymes (Karimi et al., 2025; Mallick, 2021).
Studies have shown that SA enhances the physio-biochemical attributes of aged seeds, SA improves photosynthetic efficiency, induces the accumulation of proline and protein, and boosts activities of antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD; Barwal et al., 2024; Copeland and Li, 2019). Priming with SA leads to a steady accumulation of oxidative species as the aging period increases, including ROS that are detrimental to seed storage by inducing oxidative damage (Alam et al., 2022). These oxidative damages generally occur in mitochondria, chloroplasts, DNA, soluble sugars, proteins, and lipids of storage seed (Nickas et al., 2025). This oxidative stress induces programmed cell death (PCD) in seeds, consequently reducing seed viability, which is reflected in biochemical changes such as alterations in seed physiological quality (Kumar et al., 2021; Nickas et al., 2025). Apart from oxidative damage, environmental factors such as temperature and moisture affect the fluidity and stability of the membrane, which leads to a reduction in seed viability. The endosperm of tomato seed contains a higher amount of galacto (gluco) mannans, which are generally degraded by endo-β-mannanase, β-mannosidase, and α-galactosidase enzymes through hydrolysis (Moles et al., 2019). These enzymes help in producing oligo- and monosaccharides in seeds and act as a source of energy for embryo growth (Szablinska-Piernik and Lahuta, 2023) and endo-β-mannanase activity has been generally recognized as a marker for tomato germination (Ashoknarayanan et al., 2025). The enzyme activity is required for seed germination, but a higher concentration of SA shows inhibitory effects (Galviz et al., 2020).
Recent work by Habibi et al. (2025) demonstrated the role of SA in maintaining seed turgidity and enhancing recovery during rehydration in aged tomato seeds. The SA has a significant role in plant development, flowering, root growth, and seed germination under stress conditions (Kurniawan et al., 2025). Similarly, Alam et al. (2022) reported that SA priming significantly improves the germination rate, seedling vigor, and antioxidant enzyme activity in aged tomato seeds, helping counteract oxidative deterioration. Several researchers have reported the influence of SA on seed characteristics and yield, such as in crops like Cantaloupe (Alam et al., 2022), Barley (Ellouzi et al., 2023; Youssef et al., 2025), and Phaseolus vulgaris (Karimi et al., 2025). Despite these findings, there remains a significant knowledge gap regarding the optimal use of SA in aged tomato seeds, particularly in identifying effective concentrations and elucidating the precise biochemical and molecular mechanisms of its action. The present study seeks to investigate the efficacy of SA priming in restoring the physio-biochemical quality of aged tomato seeds. Specifically, it aims to (1) evaluate the impact of SA priming on germination performance and seedling vigor in aged seeds, (2) analyze key biochemical indicators such as antioxidant enzymatic activity and protein content, and (3) identify an effective SA priming concentration for improving the performance of aged tomato seeds after storing for about 10 months.
2 Material and methods
2.1 Seed material and experimental site
A 10-month-old seed (stored at a room temperature of 28 °C and RH is about 60% inside a cloth bag tied with a rubber band) of the tomato cv. Utkal Kumari (BT 10) was collected from All India Coordinated Research Project (AICRP) on Vegetable Crops, OUAT, Odisha, to conduct the experiment. Laboratory work has been carried out in Dr. G.V. Chalam Odisha state-notified Seed Testing Laboratory under the Department of Seed Science and Technology, College of Agriculture, OUAT, Bhubaneswar, Odisha, India. Seeds of different ages were taken, viz: 10 (M0), 11-(M1), 12-(M2), 13-(M3), and 14-(M4)-month-old and the six treatments, viz: 0.10, 0.25, 0.50, 0.75, and 1.00 mM SA, were imposed on each seed, and the experiment was replicated three times.
2.2 Seed priming with SA
Different concentrations (0.10, 0.25, 0.50, 0.75, and 1.00 mM) of SA (Baker and Baker) were prepared. Priming of seeds was carried out by soaking in SA solutions for 24 h (Karami et al., 2020). After priming, the solutions were decanted off, and the settled seeds were taken for shade drying to reduce the moisture content.
2.3 Estimation of physiological assay
Initially, seed moisture content (%) was estimated by the hot air oven method, and seed germination (%) was estimated through the top of paper (TP) method [International Seed Testing Association (ISTA), 1985]. Ten standard seedlings were randomly selected from each replication to assess root length, shoot length and seedling length. The seedling dry weight (mg) was estimated by the oven dry method [International Seed Testing Association (ISTA), 2020]. Speed of germination (SOG) (Equation 1), SVI-I (Equation 2) and SVI-II (Eqn-3) were calculated by using the formulae given by Abdul-Baki and Anderson (1973). The distance from the collar area to the apex of the primary root was measured for root length, and the average value was recorded in centimeters. SOG was calculated by taking daily counts of germinated seeds throughout the duration of the standard germination test (Maguire, 1962).
where n = No. of seeds germinated (radicle has emerged)
d = Days from sowing
2.4 Biochemical assay
2.4.1 Electrical conductivity
Eight grams of seeds were placed in a 100-ml beaker containing 40 ml of distilled water and maintained at 27 °C for 12 h. The leachate was then collected into another beaker, and its conductivity was measured using an electrical conductivity (EC) meter, expressed in dS m−1 (Matthews and Powell, 2006).
2.4.2 Dehydrogenase activity
A seed sample of 0.125 g was ground with 2 ml of 0.1-ml sodium phosphate buffer (Solution A: 1.38 g of NaH2 PO4 in 100 ml of water, Solution B: 1.78 g of Na2HPO4 in 100 ml of distilled water; 39 ml of Solution A + 61 ml of Solution B, made the volume to 200 ml) in a test tube. Then, 3 g of 2,3,5-trichlorotriphenyl tetrazolium chloride was added to the solution and incubated at 25 °C and 95% RH for 24 h. Then, the sample was centrifuged for 6 min at 1200 rpm, and the reaction mixture contained 2 ml of buffer, 0.1 ml of supernatant, 0.9 ml of distilled water and absorbance was recorded at 470 nm in a spectrophotometer and expressed as μmol TPF min−1 (Kittock and Law, 1968).
2.4.3 α-amylase activity
Germinated seed of 1 g was added to a CaCl2 10 mM solution (1.11 g of anhydrous CaCl2 in 100 ml of water). Centrifuged the sample at 7,000 rpm for 16 min, then 1 ml of starch solution and 1 ml of enzyme were added to it and incubated for 15 min at room temperature. Then, 2 ml of di-nitro salicylic acid (DNSA) was added and kept in a water bath for 5 min. After 5 min, 1 ml of sodium potassium tartrate was added to it, then cooled by running water. After cooling, the final volume was made up to 10 ml by adding distilled water. The absorbance was recorded at 560 nm in a spectrophotometer and finally expressed as μmol min−1 g−1 (Sadasivam and Manickam, 1992).
2.4.4 Superoxide dismutase activity
The ground seed sample of 0.15 g was mixed with 2 ml of potassium phosphate buffer (Solution A: KH2PO4 3.4 g in 100 ml of distilled water, Solution B: K2HPO4 of 4.35 g in 100 ml of distilled water; 4 ml of Solution A + 33 ml of Solution B and made up the volume to 100 ml). Then, the sample was centrifuged for 15-20 min at 15,000 rpm and kept at room temperature for some time. The reaction mixture of 3 ml was added in each test tube contained distilled H2O 1.54, 600 μl of phosphate buffer, 60 μl of EDTA (0.019 g 10 ml−1 of water), 390 μl of methionine (0.149 g in 10 ml of distilled H2O), 60 μl of riboflavin (0.038 g in 10 ml of water), 50 μl of supernatant and finally 0.06 g of nitro blue tetrazolium (NBT) in 10 ml. Then, prepared two blank solutions (blank 1: without sample and NBT; blank 2: without sample only). Then, the test tubes were exposed to a 400 W bulb for 15 min inside the incubator, and color change was observed to dark green; then, the absorbance was taken at 560 nm using a spectrophotometer and expressed as (μmol min−1 g−1) (Zahra et al., 2010).
2.4.5 Peroxidase activity
The POD was estimated by following the method given by Summer and Gjessing (1943). In this method, 0.1 g of seed was ground with 2 ml of sodium phosphate buffer (Solution A: 1.38 g of NaH2 PO4 in 100 ml of water, Solution B: 1.78 g of Na2HPO4 in 100 ml of distilled water; 39 ml of Solution A + 61 ml of Solution B, made the volume to 200 ml). Then, the sample was centrifuged at 16,000 g for 20 min at 4 °C. Then, reading mixture was contained 1 ml of buffer, 0.5 ml of guaicol (0.223 ml in 100 ml of distilled water), 0.1 ml of supernatant, 0.9 ml of distilled water and 0.5 ml of H2O2 (0.14 ml in 100 ml of water) was added at last and recorded the observations at 0, 1, 2, and 3 min at 470 nm against the blank. Value was expressed in μmol min−1 g−1.
2.4.6 Catalase activity
The CAT activity of the seed was estimated by following the method given by Barber (1980). In this method, 0.1 g of ground seed sample was grinded with pre-chilled mortar and pestle. Then, 0.1 M of potassium phosphate buffer (PH 7.0) (Solution A: KH2PO4 3.4 g in 100 ml of distilled water, Solution B: KH2PO4 4.35 g in 100 ml of distilled water; 4 ml of Solution A + 33 ml of Solution B and made up the volume to 100 ml). Then, the sample was centrifuged at 15,000 g for 30 min at 4 °C, and then 3 ml of phosphate buffer, 2 ml of H2O2 (0.005 M H2O2), and 1 ml of enzyme extract were pipetted out and incubated at 20 °C for 1 min. After 1 min, 10 ml of 0.7 N diluted H2SO4 was titrated into the reaction mixture against 0.01N KMNO4 and the residual H2O2 was found by observing purple color for at least 15 s. Then, a blank was prepared by adding the enzyme extract to an acidified solution of the reaction mixture at zero time, and it was expressed in (μmol min−1 g−1).
2.4.7 Protein content
A 0.2 g of ground seed sample was taken in a test tube, then added 10 ml of buffer solution (Solution C = 50 ml of Solution A + 1 ml of solution B; Solution A: 2% sodium carbonate (anhyd.) in 0.1 N NaOH + Solution B: 0.5% Copper sulfate (CuSO4.5H2O) in 1% sodium potassium tartrate). Centrifuged the sample for 8 min at 7000 rpm. Then, pipetted out 0.2 ml of supernatant, poured it into a test tube, and added 0.8 ml of distilled water, the volume was made up to 1 ml, then 5 ml of reagent C was kept at room temperature for 10 min, and finally, 0.5 ml of freshly prepared Folin-Ciocalteau reagent (FCR) was added immediately and incubated in dark conditions for 30 min. Then, an observation was taken at 660 nm in a spectrophotometer against the blank, and the reading was expressed in mg g−1 (Lowry et al., 1951).
2.5 Statistical analysis
The replicated data with respect to different physio-biochemical assays of the seed were analyzed by using a completely randomized design by following Gomez and Gomez (1984). For each parameter with respect to each time period, the sample size will be 18. The correlogram and DMRT were performed by using GRAPES-KAU software (Gopinath et al., 2021). The principal component analysis (PCA) was performed by using the R-Studio software (R 3.6.0+).
3 Results
3.1 Morphological parameters
3.1.1 Root length and seedling length
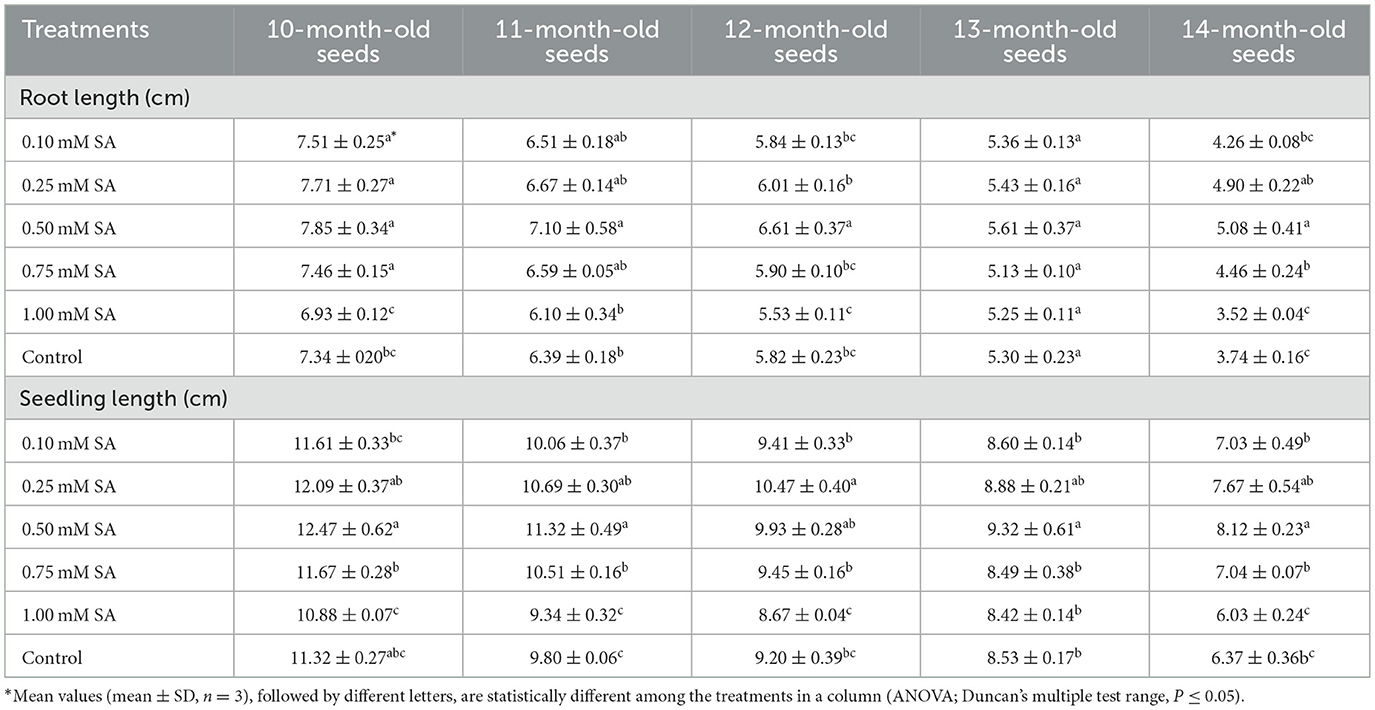
The data related to root length and seedling length have been depicted in Table 1. The root length of the 10-month-old seed was varied between 6.93 and 7.85 cm. In the 11-month-old seed, the root length varied between 6.10 and 7.10 cm. Similarly, in 12, 13, and 14-month-old seeds, 5.53-6.61, 5.13-5.61, and 3.52-5.08, respectively. The highest root length was recorded in 0.50 mM SA-treated seeds of each age. The lowest was recorded in 1.00 mM SA-primed seeds. The controlled treatment showed higher root length than the 1.00 mM SA-treated seeds. Similarly, in the 10-month-old seed, the seedling length varies from 10.88 to 12.47. In the 11-month-old seed, the seedling length varies from 9.34 to 11.32. In 12, 13, and 14-month-old seeds, the values were showing 8.67-9.93, 8.42-9.32, and 6.03-8.12, respectively. The highest seedling length was observed in 0.50 mM SA-treated seed, and the lowest in 1.00 mM SA-treated seed. The controlled treatment is showing higher seedling length than the 1.00 mM of SA treatment.
3.1.2 Seedling dry weight (mg)
The data related to seedling dry weight, germination, and SOG have been presented in Table 2. The seedling dry weight of the 10-month-old seed was varied between 0.015 and 0.018 mg. In the 11-month-old seed, the seedling dry weight varied between 0.015 and 0.018 mg. Similarly, in 12, 13, and 14-month-old seeds, 0.013-0.016 mg, 0.012-0.015 mg, and 0.011-0.014 mg, respectively. The highest seedling dry weight was recorded in 0.50 mM SA-treated seeds of each age. The lowest was recorded in 1.00 mM SA-primed seeds. The controlled treatment showed higher root length than the 1.00 mM SA-treated seeds. The seed germination (%) of the 10-month-old seed was varied between 78.66 and 89%. In the 11-month-old seed, the germination (%) varied between 61.33 and 75.33. Similarly, in 12, 13, and 14-month-old seeds, 52.66-70.66, 52.33-57, and 47.33-54.33, respectively. The controlled treatment showed higher germination (%) than 1.00 mM SA-treated seeds. The data on the SOG in the table showed that the 10-month-old seed varied between 6.48 and 7.20. In the 11-month-old seed, the SOG varied between 6.34 and 7.17. Similarly, in 12, 13, and 14-month-old seeds, 5.30-6.20, 4.70-5.17, and 3.83-4.31, respectively. The highest SOG was recorded in 0.50 mM SA-treated seeds of each age. The lowest was recorded in 1.00 mM SA-primed seeds. The controlled treatment showed higher SOG than the 1.00 mM SA-treated seeds.

Table 2. Effect of treatments on seedling dry weight (mg), germination (%) and speed of germination (nos day−1) over time.
3.2. Physiological parameters
3.2.1 Seed vigor index-I and seed vigor index-II
The data pertaining to SVI-I are presented in Table 3. In 10-month-old seeds, the index ranged from 856.09 to 1110.25, while in 11-month-old seeds, it varied from 601.89 to 852.56. Similarly, values ranged between 456.54 and 706.33, 440.66, and 530.38, and 285.62 and 441.97 in 12, 13, and 14-month-old seeds, respectively. The highest SVI-I was consistently observed in seeds primed with 0.50 mM SA across all seed ages. Conversely, the lowest values were recorded in seeds treated with 1.00 mM SA. Notably, the control treatment exhibited higher vigor index values than those treated with 1.00 mM SA. The data related to SVI-II. The SVI-II of the 10-month-old seed was varied between 1.23 and 1.63. In the 11-month-old seed, the SVI-II varied between 0.94 and 1.40. Similarly, in 12, 13, and 14-month-old seeds, 0.71-1.12, 0.62-0.87, and 0.52-0.77, respectively. The highest SVI-II was recorded in 0.50 mM SA-treated seeds of each age. The lowest was recorded in 1.00 mM SA-primed seeds. The controlled treatment showed a higher SVI-II than 1.00 mM SA-treated seeds.
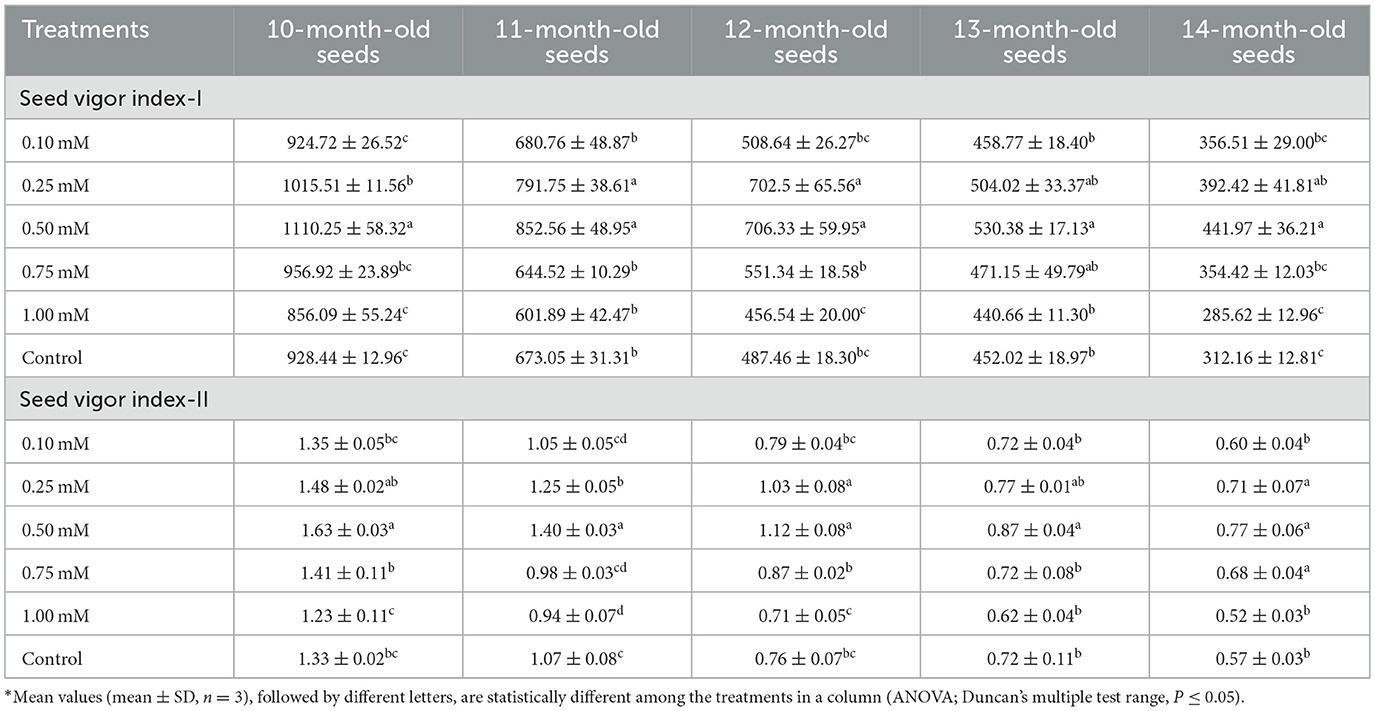
Table 3. Effect of different treatments on seed vigor index-I (SVI-I) and seed vigor index-II (SVI-II) over time.
3.3 Biochemical assay
3.3.1 Electrical conductivity and protein
It is an indicator of membrane integrity and solute leakage from internal issues of the seed. Figure 1 shows that the EC value is consistently higher at M0 than M4, which shows high solute leakage with reduced membrane stability over time. In M0, the EC value is showing the lowest (0.30 dS m−1) at 0.50 mM and the highest (0.75 dS m−1) in 1.00 mM of SA treatment. However, in M4, the EC value is showing the lowest (0.41 dS m−1) and highest (0.91 dS m−1) in 1.00 mM of SA treatment. Low EC value signifies high vigor of the seed. Similarly, Figure 2 depicts the protein content in both M0 and M4 tomato seeds across different concentrations of SA. The highest (4.10 mg g−1) protein content was observed at 0.50 mM SA in M4 and (5.53 mg g−1) in M0. Whereas, the lowest (2.48 mg g−1) protein content was observed at 1.00 mM SA in M4, and (2.46 mg g−1) in M0. It shows less leakage of solute and shows high vigor.
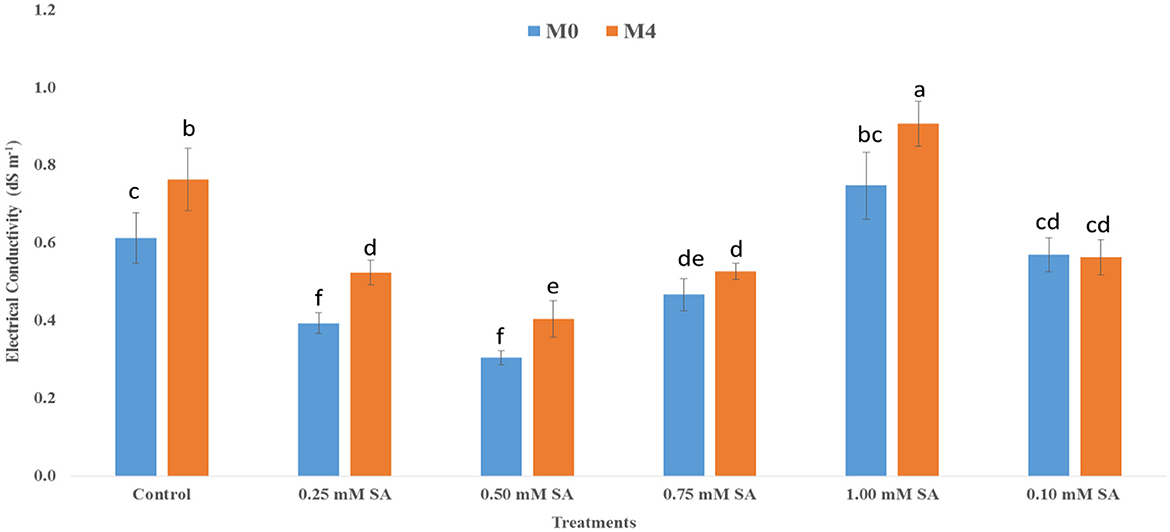
Figure 1. Electrical conductivity of 10-month-old seed (M0) and 14-month-old seed (M4) as influenced by different SA concentrations. The error bar represents standard error. Differences among treatments were analyzed by Duncan's multiple test range, P ≤ 0.05. Different letters on top of each bar indicates significantly different and vice-versa is statistically at par.
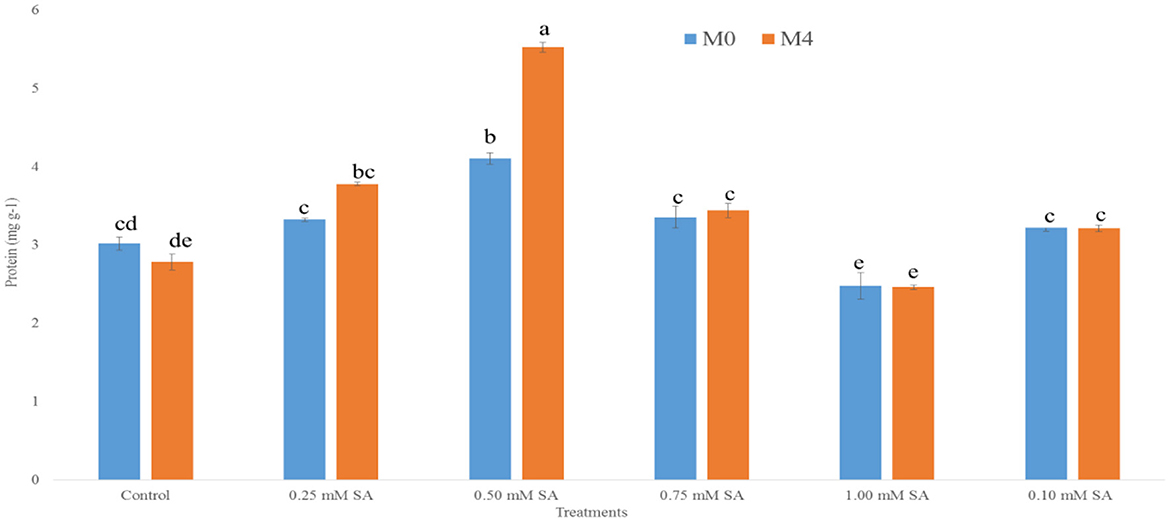
Figure 2. Protein content of 10-month-old seed (M0) and 14-month-old seed (M4) as influenced by different SA concentrations. The error bar represents standard error. Differences among treatments were analyzed by Duncan's multiple test range, P ≤ 0.05. Different letters on top of each bar indicates significantly different and vice-versa is statistically at par.
3.3.2 Enzyme activities
The activity of the dehydrogenase enzyme among six treatments at different phases, M0 and M4, has been depicted in Figure 3. The presence of this enzyme indicates the viability and liveliness of the embryo inside the seed. Across all treatments, dehydrogenase activity was higher at M0 than M4, indicating a general decline in enzymatic activity over time. The 0.50 mM of SA treatment is exhibiting the highest (1,887 μmol TPF min−1) dehydrogenase activity at both time points, and it reduced to 1,620 μmol TPF min−1. In contrast, 1.00 mM SA and control treatments showed the lowest dehydrogenase activity, at both time points, where values dropped below 1,250 μmol TPF min−1. Figure 4 illustrates that in M0, the 0.50 SA treatment recorded the highest α-amylase activity (3581 μmol min−1 g−1), followed by 0.75 SA with a value of 3,417 μmol min−1 g−1. These treatments suggest enhanced enzymatic activation, potentially leading to improved vigor. Similarly, in M4, the highest α-amylase activity (3,443 μmol min−1 g−1) is shown at 0.50 SA treatment, and the lowest (2,105 μmol min−1 g−1) is at 1.00 mM. In both time frames, 0.50 SA is showing the highest and 1.00 mM SA is the lowest. The activity of SOD varied notably across different concentrations of SA in both M0 and M4 tomato seeds (Figure 5). The highest SOD (0.62 μmol min−1 g−1) activity was observed at 0.50 mM SA in M0 and 0.60 μmol min−1 g−1 in M4, indicating that this concentration is optimal for enhancing enzymatic antioxidant defense. In M0, SOD activity ranged from 0.45 μmol min−1 g−1 (1.00 mM SA) to 0.62 μmol min−1 g−1(0.50 mM SA). Similarly, in M4, the activity ranged from 0.39 μmol min−1 g−1 (1.00 mM SA) to 0.60 μmol min−1 g−1 (0.50 mM SA). POD activity exhibited significant variation in response to SA treatments in both M0 and M4 seeds (Figure 6). Among the treatments, 0.50 mM SA resulted in the highest (0.15 μmol min−1 g−1) in M0 and 0.04 μmol min−1 g−1 in M4, indicating that this concentration most effectively enhanced POD-mediated antioxidant defense. In M0, POD activity ranged from 0.04 U μmol min−1 g−1 (1.00 mM SA) to 0.15 μmol min−1 g−1 (0.50 mM SA), while in M4, the activity varied between 0.05 μmol min−1 g−1 (1.00 mM SA) and 0.13 μmol min−1 g−1 (0.50 mM SA). A steady increase in activity was observed from 0.10 to 0.50 mM SA, followed by a decline at higher concentrations (0.75 and 1.00 mM SA), indicating a concentration-dependent response. CAT activity showed significant variation across SA treatments in both M0 (10-month-aged) and M4 (14-month-aged) seeds (Figure 7). Among the treatments, 0.50 mM SA resulted in the highest CAT (229 μmol min−1 g−1) activity in M0 and (211 μmol min−1 g−1) at M4 conditions, indicating enhanced antioxidant defense at this concentration. In M0, CAT activity ranged from 156.67 μmol min−1 g−1 (1.00 mM SA) to 229 μmol min−1 g−1 (0.50 mM SA), while in M4, it ranged from 106.67 μmol min−1 g−1 (1.00 mM SA) to 211.67 μmol min−1 g−1 (0.50 mM SA). A gradual decline in CAT activity was observed at higher SA concentrations (0.75 and 1.00 mM), in both M0 and M4 conditions.

Figure 3. Dehydrogenase activity of 10-month-old seed (M0) and 14-month-old seed (M4) as influenced by different SA concentrations. Differences among treatments were analyzed by Duncan's multiple test range, P ≤ 0.05; Different letters on top of each bar indicates significantly different and vice-versa is statistically at par.
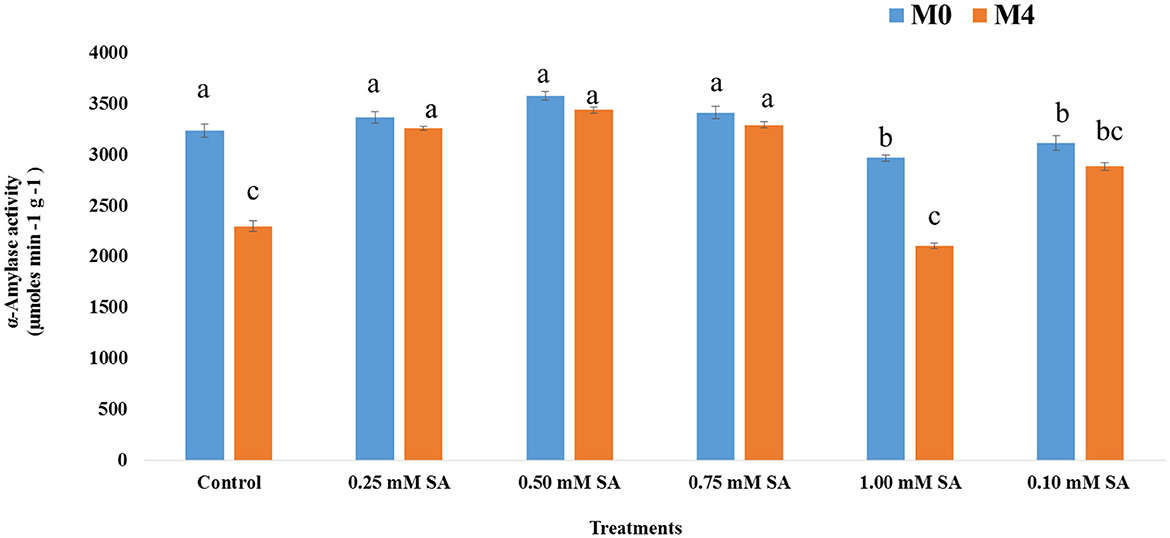
Figure 4. α-amylase activity of 10-month-old seed (M0) and 14-month-old seed (M4) as influenced by different SA concentrations. Differences among treatments were analyzed by Duncan's multiple test range, P ≤ 0.05; Different letters on top of each bar indicates significantly different and vice-versa is statistically at par.
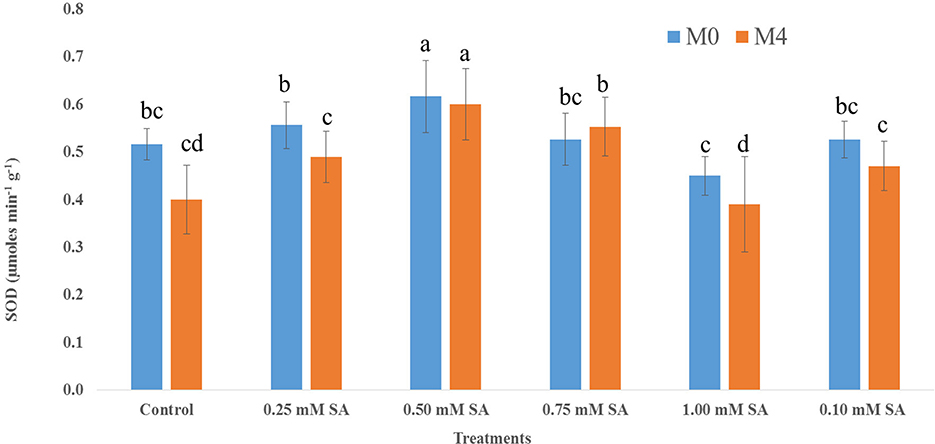
Figure 5. SOD activity of 10-month-old seed (M0) and 14-month-old seed (M4) as influenced by different SA concentrations. Differences among treatments were analyzed by Duncan's multiple test range, P ≤ 0.05; Different letters on top of each bar indicates significantly different and vice-versa is statistically at par.
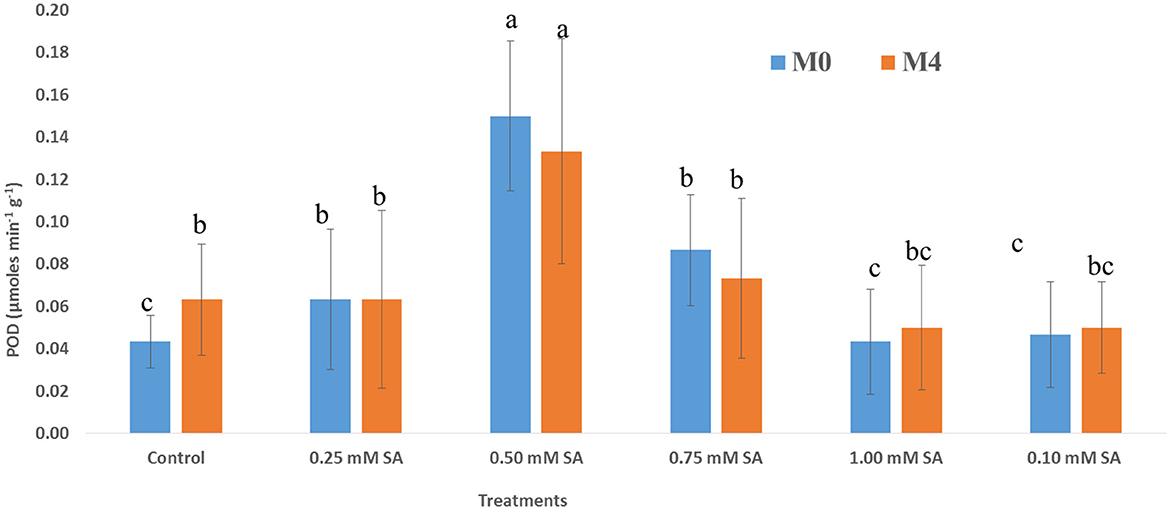
Figure 6. POD activity of 10-month-old seed (M0) and 14-month-old seed (M4) as influenced by different SA concentrations. Differences among treatments were analyzed by Duncan's multiple test range, P ≤ 0.05; Different letters on top of each bar indicates significantly different and vice-versa is statistically at par.
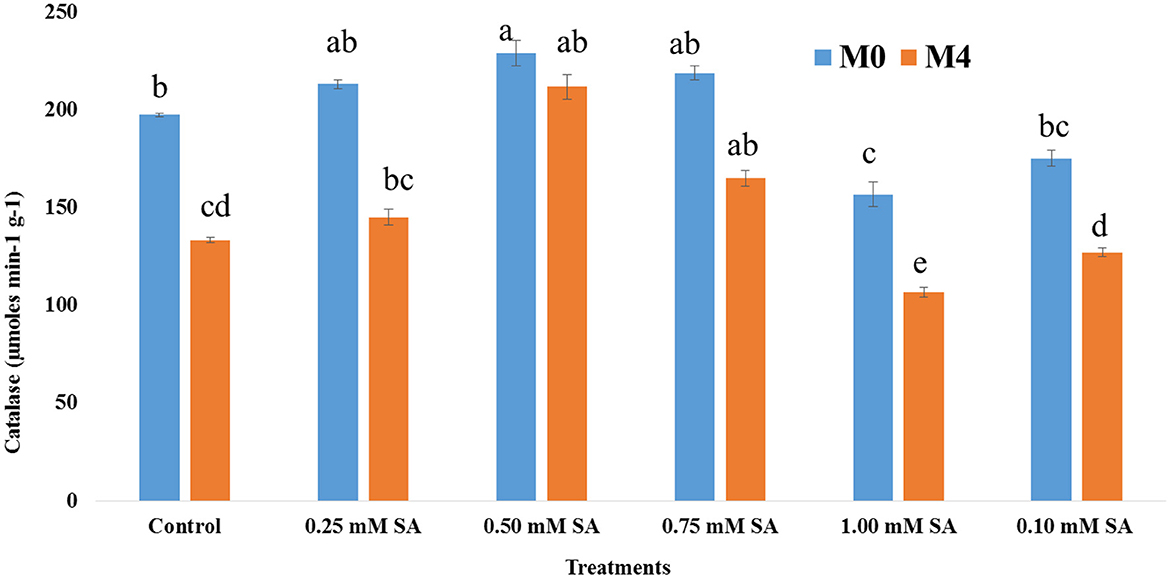
Figure 7. Catalase activity of 10-month-old seed (M0) and 14-month-old seed (M4) as influenced by different SA concentrations. The error bar represents standard error. Differences among treatments were analyzed by Duncan's multiple test range, P ≤ 0.05. Different letters on top of each bar indicates significantly different and vice-versa is statistically at par. Correlation of physiological and biochemical assays as influenced by SA priming.
To elucidate the interrelationships among physiological and biochemical responses under SA treatments, a Pearson's correlation matrix was constructed (Figures 8A, B). Notably, strong positive correlations (r > 0.90, p < 0.001) were observed among enzyme activities, including M0-AM and M0-CAT (r = 0.95), M0-AM and M4-POD (r = 0.94), and M0-SOD with both M0-AM (r = 0.99) and M0-CAT (r = 0.97), suggesting a coordinated upregulation of the antioxidant defense system. The EC, an indicator of membrane damage and electrolyte leakage, showed strong negative correlations with enzymatic activities, including M0-SOD (r = −0.89), M0-AM (r = −0.89), and M0-CAT (r = −0.85), all statistically significant (p < 0.05 to p < 0.001). These findings reinforce the role of protection of SA-induced antioxidant enzymes in maintaining membrane integrity under stress conditions. Together, the data underscore a tightly coordinated network between anti-oxidative responses and physiological performance, highlighting the efficacy of salicylic acid in modulating stress resilience during tomato seed germination and early seedling development. Furthermore, variables associated with seedling growth and metabolic activity, such as amino acid metabolism (AM), protein content, and dehydrogenase activity (DHA), were positively correlated with enzymatic antioxidant activity and negatively correlated with EC, suggesting that enhanced redox balance via salicylic acid application supports early seedling vigor and stress tolerance. Overall, the correlation structure delineates a robust and interconnected response mechanism, whereby salicylic acid induces enzymatic antioxidant systems that, in turn, safeguard physiological integrity and enhance seedling development under abiotic stress.

Figure 8. Correlogram of physio-biochemical assay of 10-month-old seed (A) and 14-month-old seed (B).
3.4 Principal component analysis
Principal component analysis was carried out to investigate the multivariate relationships among physiological and biochemical assays under control (M0) and salicylic acid-treated (M4) conditions. The first two principal components (PC1 and PC2) accounted for 90.58% of the total variance, with PC1 explaining a dominant 82.69%, indicating strong data structuring along this axis. The biplot (Figure 9) illustrates a clear separation between variables associated with salicylic acid treatment (M4) and the control (M0), particularly along PC1. Traits such as M4-CAT, M4-AM, and M4-PC, which lie positively along PC1 and PC2, are closely grouped, indicating a synergistic enhancement of enzymatic activity and metabolic performance in response to salicylic acid. Conversely, EC under both M0 and M4 conditions is oriented negatively along PC1, reinforcing its inverse association with the beneficial physiological traits and its role as a stress indicator. The strong vector magnitudes of enzymes (SOD, CAT, and POD) under M4, in addition to close alignment of M4-DHA and M4-AM, signify a coordinated upregulation of both protective enzymatic defenses and growth-associated assays under SA application. Notably, the proximity of M4-DHA and M4-PC to M4-AM suggests that membrane stability and osmolyte accumulation contribute significantly to metabolic improvement during early seedling development. These findings highlight that salicylic acid induces a tightly integrated physiological response, predominantly captured by PC1, promoting antioxidant defense and osmotic balance, while downregulating damage-related indicators, such as EC. The PCA thus affirms the pivotal role of SA in modulating multiple interdependent pathways to enhance seedling resilience.
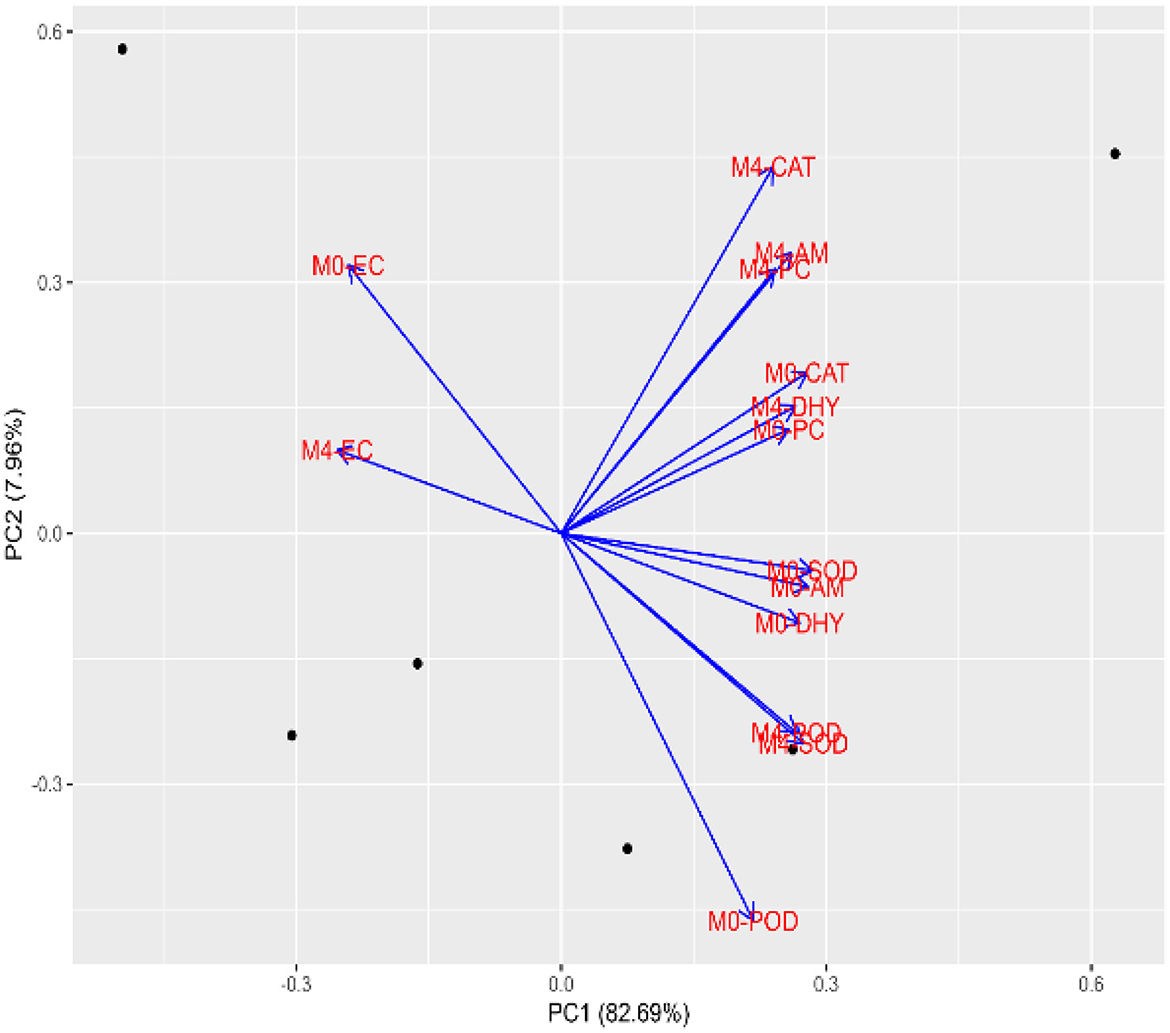
Figure 9. Grouping patterns of aged tomato seed samples treated with varying concentrations of salicylic acid.
4 Discussion
Salicylic acid exhibits a well-documented concentration-dependent influence on seed germination and seedling development, acting as a biostimulant at lower concentrations and a stressor at higher levels. In the present study, aged tomato seeds treated with 0.25–0.50 mM SA demonstrated significantly enhanced germination percentage, SVI, and improved root and shoot development. In contrast, higher concentrations (1.00 mM) led to a decline in germination assay and overall seedling performance, indicating possible phytotoxic effects. The increase in seed germination rate might be due to a decrease in the accumulation of abscisic acid (ABA), as well as the prevention of water deficit-induced decline in cytokinin and indole-3-acetic acid (IAA) content in the seed (Alam et al., 2022). Youssef et al. (2025) reported that 0.5 mM SA priming significantly improved germination and seedling traits in tomato, with similar promotive effects observed in other crops, such as faba bean and soybean (Collins et al., 2022; Choudhury and Bordolui, 2023). Conversely, high SA concentrations inhibited germination and early seedling growth, likely due to oxidative stress, hormonal imbalance, and disruption of enzymatic functions (Karimi et al., 2025; Habibi and Abdoli, 2013). Similarly, Youssef et al. (2025) and Fujikura et al. (2020) reported that seed priming with SA at 0.5 mM enhanced salinity stress tolerance of barley seeds and higher root length was measured. This might be due to an increase in mitotic cell division of root cells. The seed priming of SA also increased the germination of tomato (Adhikari et al., 2020), Alfalfa (Boukari et al., 2019) and basil (Damalas, 2019). In our study, the 1.00 mM SA treatment led to visible reductions in root and shoot lengths, suggesting a toxic accumulation of ROS and inhibition of cellular metabolism. At an optimum concentration, SA enhances the growth and germination, but at higher concentrations, it inhibits plant growth (Sofy et al., 2020; Tang et al., 2023).
Moderate SA doses significantly enhanced the activities of enzymatic antioxidants, including SOD, CAT, and POD. These responses were associated with improved seedling vigor (Habibi et al., 2025; Khan et al., 2021). Similar antioxidant enhancements were reported in cabbage, maize, and mung bean (Hassanuzzaman et al., 2020; Minaric et al., 2021; Desci et al., 2025). Seed priming with SA reduced H2O2 and MDA accumulation more than gibberellic acid (GA3) and NaCl priming, which might be due in part to the restoration of seed membranes and organelles after priming (Ellouzi et al., 2021). The SA treatment was found to modulate the hormonal balance by reducing ABA and increasing GA3, IAA, and cytokinins, all of which are essential for promoting germination and early seedling growth (Praveen et al., 2021; Collins et al., 2022). The SA also interferes with ethylene synthesis by inhibiting ACC synthase activity, which may contribute to improved root elongation and delayed senescence.
Priming with SA stimulated key enzymes such as malate synthase and isocitrate lyase, which play crucial roles in the glyoxylate cycle, facilitating the mobilization of stored lipids into carbohydrates during early seedling growth. Enhanced respiration, ATP production and protein synthesis under SA treatment further supported improved seedling establishment.
At concentrations of 1.00 mM, SA negatively affected germination and growth. Reports suggest that excess SA may inhibit CAT and POD activity, leading to H2O2 accumulation and oxidative stress (Ignatenko et al., 2021). The SA is an effective redox state regulator, which is regulated by ascorbate-glutatheion cycle, resulting in the reduction of H2O2 detoxification (Wiciarz et al., 2018). Additionally, inhibition of sucrose transport and increased ABA content has been linked to poor germination under high SA levels. Peroxynitrite formation from the reaction of nitric oxide (NO) with superoxide may also trigger programmed cell death in developing seedlings.
Similar inhibitory outcomes were documented in maize embryos treated with 3.00 mM SA, Vicia faba (Soliman et al., 2016), and tomato (Szepesi et al., 2005). In our study, 1.00 mM SA significantly delayed emergence, reduced seedling biomass, and increased oxidative markers, consistent with the aforementioned reports.
5 Conclusion
Seed priming with salicylic acid at a concentration of 0.50 mM is showing excellent results in all attributes of seed physio-biochemical qualities by reducing H2O2, MDA, and ROS accumulation and enhancing internal antioxidants and metabolic functions, which is a key tool in repairing and restoration mechanisms to seed membranes as well as organelles after priming. Tomato seed was classified under the microbiotic (< 3 years lifespan) category; the deterioration starts from 3 months, and subsequently, the physio-biochemical seed qualities, including germination, will reduce significantly with respect to time. The 0.5 mM SA treatment can be followed for restoring and improving the seed physio-biochemical quality as a reclamation measure to seed deterioration in tomato. However, high doses or more than 0.50 mM of SA treatment also show a negative impact on seed quality, so we should also be cautious about it. The treatment of 0.50 mM is the cheapest, easiest, low-cost, and sustainable practice. Therefore, we can conclude that the treatment with moderate, i.e., 0.50 mM SA-priming treatment, can be a useful eco-friendly recoupment method in aged tomato seed for farmers in crop production as well as productivity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AD: Data curation, Formal analysis, Conceptualization, Writing – original draft, Writing – review & editing, Investigation, Methodology. SB: Visualization, Formal analysis, Project administration, Writing – original draft, Investigation, Data curation, Supervision, Writing – review & editing, Methodology. SM: Writing – original draft, Writing – review & editing, Visualization, Methodology, Validation, Resources. CP: Validation, Data curation, Writing – review & editing, Methodology, Writing – original draft. SD: Data curation, Writing – original draft, Formal analysis, Writing – review & editing, Investigation. UB: Writing – original draft, Formal analysis, Data curation, Writing – review & editing, Conceptualization, Supervision. MR: Investigation, Writing – original draft, Conceptualization, Formal analysis, Writing – review & editing, Data curation, Methodology. CS: Data curation, Project administration, Supervision, Investigation, Writing – review & editing, Formal analysis, Writing – original draft. SS: Writing – original draft, Data curation, Investigation, Writing – review & editing, Conceptualization, Software, Supervision. PB: Formal analysis, Writing – review & editing, Writing – original draft, Methodology, Software, Investigation, Data curation. DS: Writing – original draft, Validation, Investigation, Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul-Baki, A. A., and Anderson, J. D. (1973). Vigour determination in soybean seed by multiple criteria. Crop Sci. 13, 630–633. doi: 10.2135/cropsci1973.0011183X001300060013x
Adhikari, B., Adhikari, M., Ghimire, B., Adhikari, B. C., Park, G., and Choi, E. H. (2020). Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radic. Biol. Med. 156, 57–69. doi: 10.1016/j.freeradbiomed.2020.06.003
Alam, A., Ullah, H., Thuenprom, N., Tisarum, R., and Dutta, A. (2022). Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters parameters of cantaloupe under water-deficit stress. S. Afr. J. Bot. 150, 1–12. doi: 10.1016/j.sajb.2022.06.056
Ashoknarayanan, S., Murugeshwari, T., Umarani, R., Vanitha, C., Eevera, T., and Tilak, M. (2025). Dysfunctional carbohydrate metabolism and seed vigour in sweet corn: a review of structural and physiological implications. Trop. Plant Biol. 18:25. doi: 10.1007/s12042-025-09393-5
Barber, J. M. (1980). Catalase and peroxidase in primary leaves during development and senescence. Zentrum für Molekularbiologie der Pflanzen. 97, 135–144. doi: 10.1016/S0044-328X(80)80027-4
Barwal, S. K., Shah, S. H., Parwar, A., Siddiqui, M. H., Agnihotri, R. K., Vimala, Y., et al. (2024). Mechanistic insights of salicylic acid-mediated salt stress tolerance in Zea mays L. seedlings. Heliyon. 10:e34486. doi: 10.1016/j.heliyon.2024.e34486
Boukari, N., Jelali, N., Renaud, J. B., Youssef, R. B., Abdelly, C., and Hannoufa, A. (2019). Salicylic acid seed priming improves tolerance to salinity, iron deficiency and their combined effect in two ecotypes of Alfalfa. Environ. Exp. Bot. 167:103820. doi: 10.1016/j.envexpbot.2019.103820
Choudhury, A., and Bordolui, S. (2023). Concept of seed deterioration: reason, factors, changes during deterioration and preventive measures to overcome seed degradation. Am. Int. J. Agric. Stud. 7, 41–56. doi: 10.46545/aijas.v7i1.291
Collins, J. E., Bowyer, C., Tsouza, A., and Chopra, M. (2022). Tomatoes: an extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 11:239. doi: 10.3390/biology11020239
Copeland, C., and Li, X. (2019). Regulation of plant immunity by the proteasome pathway. Int. Rev. Cell. Mol. Biol. 343, 37–63. doi: 10.1016/bs.ircmb.2018.06.004
Damalas, C. A. (2019). Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Scientia Hort. 246, 360–365. doi: 10.1016/j.scienta.2018.11.005
Desci, K., Ahmad, M., and Toth, Z. (2025). The role of salicylic acid in activating plant stress responses—results of the past decade and future perspectives. Int. J. Mol. Sci. 26:4447. doi: 10.3390/ijms26094447
Diya, A., Beena, R., and Jayalekshmy, V .G. (2024). Physiological, biochemical and molecular mechanisms of seed priming: a review. Legume Res. 47, 159–166.
Ellouzi, H., Oueslati, O., Hessini, K., Rabhi, M., and Abdelly, C. (2021). Seed-priming with H2O2 alleviates subsequent salt stress by preventing ROS production and amplifying antioxidant defense in cauliflower seeds and seedlings. Scientia Hort. 288:110360. doi: 10.1016/j.scienta.2021.110360
Ellouzi, H., Zorrig, W., Amraoui, S., Oueslati, S., Abdelly, C., Rabhi, M., et al. (2023). Seed priming with salicylic acid alleviates salt stress toxicity in barley by suppressing ROS accumulation and improving antioxidant defense systems, compared to halo- and gibberellin priming. Antioxidants 12:1779. doi: 10.3390/antiox12091779
El-Maarouf-Bouteau, H. (2022). The seed and the metabolism regulation. Biology 11:168. doi: 10.3390/biology11020168
FAOSTAT (2019). FAO Statistics, Crops and Livestock Products. Rome: Food and Agriculture Organization of the United Nations.
FAOSTAT (2023). FAO Statistics: Global Tomato Production in 2022. Rome: Food and Agriculture Organization of the United Nations.
Fujikura, U., Kazune, E., Horiguchi, G., Seo, M., Yuri, K., and Yuji, K. (2020). Suppression of class l compensated cell enlargement by xs2 mutation is mediated by salicylic acid signaling. PLoS Gen. 16:e1008873. doi: 10.1371/journal.pgen.1008873
Galviz, Y., Bortolin, G. S., Deuner, S., and Moraes, D .M. D. (2020). Seed priming with salicylic acid potentiates water restriction-induced effects in tomato seed germination and early seedling growth. J. Seed Sci. 42:202042031. doi: 10.1590/2317-1545v42234256
Gomez, K. A., and Gomez, A. A. (1984). Statistical Procedures for Agricultural Research, 2nd Edn. New York, NY: John Wiley and Sons, 680.
Gopinath, P. P., Adarsh, V. S., and Parsad, R. (2021). Grapes agri1: collection of shiny apps for data analysis in agriculture. J. Open Source Softw. 6:3437. doi: 10.21105/joss.03437
Habibi, A., and Abdoli, M. (2013). Influence of salicylic acid pre-treatment on germination, vigour and growth parameters of garden cress (Lepidium sativum) seedlings underwater potential loss at salinity stress. Int. Res. J. Appl. Basic. Sci. 4, 1393–1399.
Habibi, N., Aryan, S., Sediqui, N., Terada, N., Sanada, A., Kamata, A., et al. (2025). Enhancing salt tolerance in tomato plants through PEG 6000 seed priming: inducing antioxidant activity and mitigating oxidative stress. Plants 14:1296. doi: 10.3390/plants14091296
Hassanuzzaman, M., Zulfiqr, F., and Fuzita, M. (2020). Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. Int. J. Mol. Sci. 21:8695. doi: 10.3390/ijms21228695
Ignatenko, A., Repkina, N. S., and Titov, A. F. (2021). Effect of salicylic acid on antioxidant enzymes and cold tolerance of cucumber plants. Russ. J. Plant Physiol. 68, 491–498. doi: 10.1134/S1021443721020059
International Seed Testing Association (ISTA) (1985). International Rules for Seed Testing. Zurich: ISTA.
International Seed Testing Association (ISTA) (2020). Bassersdorf, Switzerland, International Rules for Seed Testing. Bassersdorf: ISTA.
Karami, L., Hedayat, M., and Farrahbaksh, S. (2020). Effect of salicylic acid priming on seed germination and morphophysiological and biochemical characteristics of tomato seedling (Lycopersicom esculentun). Iranian J. Seed Res. 7, 165–180. doi: 10.29252/yujs.7.1.165
Karimi, R. K., Manijesh, S., Beheshti, H. K., Abbasi, A. R., and Bihamta, M. R. (2025). Seed priming with salicylic acid enhances salt stress tolerance by boosting antioxidant defense in Phaseolus vulgaris genotypes. BMC Plant Biol. 25:489. doi: 10.1186/s12870-025-06376-2
Khan, I., Seleiman, M. F., Chattha, M. U., Jalal, R. S., Mahmood, F., Hassan, F. A. S., et al. (2021). Enhancing antioxidant defense system of mungbean with a salicylic acid exogenous application to mitigate cadmium toxicity. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 49:12303. doi: 10.15835/nbha49212303
Kittock, D. L., and Law, A. G. (1968). Relationship of seedling vigour to respiration and tetrazolium reduction in germinating wheat seeds. Agron. J. 60, 268–288. doi: 10.2134/agronj1968.00021962006000030012x
Kumar, S. P. J., Chintagunta, A. D., Reddy, Y. M., Rajjou, L., Garlapati, V. K., Agarwal, D. K., et al. (2021). Implications of reactive oxygen and nitrogen species in seed physiology for sustainable crop productivity under changing climate conditions. Curr. Plant Biol. 26:100197. doi: 10.1016/j.cpb.2021.100197
Kurniawan, A., Noviandi, W. D., Mauilidah, N. I., Mullatif, I. A., Rahmandhias, T., Karim, M. F., et al. (2025). Mitigating drought stress effects in tomato through seed priming and foliar application of salicylic acid: impacts on germination and plant growth. J. Ecol. Eng. 26, 369–383. doi: 10.12911/22998993/200337
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275. doi: 10.1016/S0021-9258(19)52451-6
Maguire, J. D. (1962). Speed of germination—aids in selection and evaluation for seedling emergence and vigor. Crop Sci. 2, 176–177. doi: 10.2135/cropsci1962.0011183X000200020033x
Mallick, P. K. (2021). Medicinal values of tomato (Lycopersicon esculentum). Int. J. Appl. Sci. 9, 166–168. doi: 10.3126/ijasbt.v9i3.39789
Matthews, S., and Powell, A. (2006). Electrical Conductivity Vigour Test: Physiological Basis and Use. Seed science. Zurich: ISTA.
Minaric, S., Smolko, A., and Sondi, B. (2021). Ferulic acid and salicylic acid foliar treatments reduce short-term salt stress in chinese cabbage by increasing phenolic compounds accumulation and photosynthetic performance. Plants 10:2346. doi: 10.3390/plants10112346
Moles, T. M., Guglielminetti, L., and Reyes, T. H. (2019). Differential effects of sodium chloride on germination and post-germination stages of two tomato genotypes. Sci. Hort. 257:108730. doi: 10.1016/j.scienta.2019.108730
Nickas, J., Danikou, S .N, Shango, A. J., and Kilasi, N. (2025). Physio-biochemical response of vegetable seeds to ageing: a systematic review. Plant Physiol. Biochem. 228:110223. doi: 10.1016/j.plaphy.2025.110223
Pirredda, M., FañanásPueyo, I., Oñate-Sánchez, L., and Mira, S. (2024). Seed longevity and ageing: a review on physiological and genetic factors with an emphasis on hormonal regulation. Plants 13:41. doi: 10.3390/plants13010041
Praveen, A., Hussain, I., and Abdallah, F. E. (2021). Promotion of growth and physiological characteristics in water-stressed wheat (Triticum aestivum) in relation to foliar-application of salicylic acid. Water 13:1316. doi: 10.3390/w13091316
Ray, J., and Bordolui, S. K. (2022). Seed quality deterioration of tomato during storage: effect of storing containers and condition. Biol. For.14, 327–334.
Sadasivam, S., and Manickam, A. (1992). Biochemical Methods for Agricultural Sciences. New Delhi: Wiley Eastern Ltd.
Saxena, R., Kumar, M., and Tomar, R. S. (2021). Seed priming: an effective approach to improve seed germination and abiotic stress tolerance. Indian J. Nat. Sci. 12, 32346–32357.
Shafe, O. M., Gumede, M. N., Nyakudiya, T. T., and Chivandi, E. (2024). Lycopene: a potent antioxidant with multiple health benefits. J. Nutr. Metab. 62, 24–26. doi: 10.1155/2024/6252426
Sofy, M. R., Seleiman, M. F., Alhammad, B. A., Alharbi, B. M., and Mohamed, H. I. (2020). Minimizing adverse effects of Pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 10:699. doi: 10.3390/agronomy10050699
Soliman, M. H., Rawan, S., Al-Juhani, R. S., Hashash, M., and Al-Juhani, F. M. (2016). Effect of seed priming with salicylic acid on seed germination and seedling growth of broad bean (Vicia faba L.). J. Agric. Technol. 12, 1125–1138.
Summer, J. B., and Gjessing, E. C. (1943). Determination of peroxidase activity. Arch. Biochem. Biophys. 2:291.
Szablinska-Piernik, J., and Lahuta, L. B. (2023). Changes in polar metabolites during seed germination and early seedling development of pea, cucumber, and wheat. Agriculture 13:2278. doi: 10.3390/agriculture13122278
Szepesi, A., Csiszar, J., Bajkan, S., Gemes, K., Horvath, E., Erdei, L., et al. (2005). Role of salicylic acid pre-treatment on the acclimation of tomato plants to salt stress. Acta Biol. Szeged 49, 123–125.
Tang, S., Xie, L., Li, W., Guo, C., Han, J., and Yu, L. (2023). Nutrient resorption responses of female and male Populus cathayana to drought and shade stress. Physiol. Plant 175:13980. doi: 10.1111/ppl.13980
Wiciarz, M., Niewiadomska, E., and Kruk, J. (2018). Effects of salt stress on low molecular antioxidants and redox state of plastoquinone and P700 in Arabidopsis thaliana (glycophyte) and Eutrema salsugineum (halophyte). Photosynthetica 56, 811–819. doi: 10.1007/s11099-017-0733-0
Youssef, R. B., Jelali, N., Abdelli, C., and Albacette, A. (2025). Salicylic acid seed priming: a key frontier in conferring salt stress tolerance in barley seed germination and seedling growth. Agronomy 15:154. doi: 10.3390/agronomy15010154
Keywords: electrical conductivity, germination, protein, seed vigor, seedling dry weight
Citation: Dalapati A, Behera S, Mohanty S, Patra C, Dash S, Behera UK, Rout MK, Sahoo C, Swain SK, Behera P and Sahoo D (2025) Effects of salicylic acid on the physio-biochemical quality of aged tomato seeds. Front. Sustain. Food Syst. 9:1647031. doi: 10.3389/fsufs.2025.1647031
Received: 14 June 2025; Accepted: 25 August 2025;
Published: 16 September 2025.
Edited by:
Matteo Balderacchi, Independent Researcher, Piacenza, ItalyReviewed by:
Sushil K. Sharma, National Institute of Biotic Stress Management, IndiaAndi Kurniawan, Universitas Brawijaya Fakultas Pertanian, Indonesia
Copyright © 2025 Dalapati, Behera, Mohanty, Patra, Dash, Behera, Rout, Sahoo, Swain, Behera and Sahoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soubhagya Behera, c2JlaGVyYS5zc3RAb3VhdC5hYy5pbg==; Subhashree Dash, c3ViaGFzaHJlZWRhc2gyMkBnbWFpbC5jb20=
 Anupam Dalapati1
Anupam Dalapati1 Soubhagya Behera
Soubhagya Behera Uttam Kumar Behera
Uttam Kumar Behera