- The Water Environment and Animal Safety Laboratory, Henan University of Science and Technology, Luoyang, China
In recent years, with the accelerated development of science, technology and industry, aquatic ecosystems have been severely damaged, which not only profoundly affects the survival and metabolic stability of aquatic animals, but also has a certain impact on the flavor of aquatic products. The key flavor substances affecting the flavor of aquatic products include free amino acids (FAA), nucleotides and organic acids, which form the basis of the key taste of aquatic products. This review focuses on the research on main flavor substances in aquatic animals, as well as the physiological and metabolic changes of flavor substances under the action of typical marine pollutants (including persistent organic pollutants (POPs) and heavy metal stress). However, there are relatively few studies on the molecular mechanisms of taste substance metabolism, and most of them are conducted in the field setting. This review aims to provide a reference for in-depth exploration of the metabolic mechanism of aquatic organisms’ taste substances in response to marine pollution.
1 Introduction
Aquatic products are highly favored due to their excellent nutritional value and rich flavor, and they play a crucial role in the global food system (Golden et al., 2021). According to the latest data published by the Food and Agriculture Organization of the United Nations (FAO), the total global output from fisheries and aquaculture reached 223.2 million tons in 2022. Notably, around 62% of this production was derived from marine sources, significantly contributing to the dietary protein requirements of approximately 3.2 billion people worldwide (FAO1). As a result, the ocean is recognized as a crucial source of high-quality animal protein for global food security. Simultaneously, aquatic products are instrumental in enhancing nutritional well-being and public health at a global scale (Cheng et al., 2023; Li C. et al., 2022). The market demand for high-quality aquatic foods has shown a steady increase. Flavor, particularly its distinctive taste, is a major determinant of product quality and consumer preference (Wu et al., 2023).
Aquatic organisms exhibit five fundamental taste modalities: sourness, sweetness, bitterness, saltiness, and umami. These taste-active substances are not only central to defining the sensory attributes of aquatic species but also significantly influence consumer purchasing behavior (Jones et al., 2022). However, accelerated technological and industrial advancements have expanded human influence over natural systems, leading to serious ecological degradation and extensive pollution of marine environments (Thiagarajan and Devarajan, 2025). Common forms of marine pollution include petroleum contamination, heavy metals, radioactive substances, harmful algal blooms, and marine debris. The expansion of chemical and petrochemical industries has intensified problems such as oil spills and elevated concentrations of heavy metals in seawater (Sharma et al., 2024). Given that aquatic organisms are entirely dependent on water for their survival, fluctuations in water quality can disrupt physiological homeostasis (Bartley et al., 2006). Environmental pollutants, including persistent organic pollutants (POPs) and heavy metals, pose a serious threat to the survival and physiological stability of these organisms and have severe toxic effects on certain species (Ribeiro et al., 2005; Corsolini et al., 2014; Yan et al., 2020). How marine pollution affects the taste and quality of aquatic products and how it interferes with the metabolism of taste substances in aquatic animals are important scientific issues of concern to people.
This article thoroughly introduces the main flavor substances and flavor characteristics of aquatic organisms, as well as their sensitivity to POPs and heavy metals. The purpose is to clarify the impact of marine pollution on flavor metabolism, provide scientific basis for marine ecological toxicology protection, and improve the nutrition, safety, and economic value of aquatic products.
2 Flavor substances of aquatic animals
Food flavor arises from the integrated sensory experience of smell, taste, and trigeminal nerve stimulation, with the balance of various substances shaping the final flavor (Khan et al., 2015). Food flavor encompasses volatile and non-volatile components, in which volatile substances, such as unsaturated aldehydes and ketones from fat oxidation, evoke aroma through olfactory stimulation (Wang et al., 2020). And non-volatile substances, responsible for taste, include the five basic tastes: acid, sweet, bitter, salty, and umami. In addition, taste substances are categorized as nitrogen-containing (e.g., free amino acids, nucleotides, organic acids) and non-nitrogen-containing (e.g., organic acids, sugars, inorganic compounds) (Zhang et al., 2003).
Amino acids, nucleotides, and organic acids constitute the basic taste characteristics of aquatic products. Fish, shrimp, and shellfish contain various auxiliary flavor enhancing compounds that interact to produce different tastes (Liang et al., 2008; Lim et al., 2017; Michihata et al., 2000). Although flavor enhancing amino acids and nucleotides are the main sources of taste in aquatic animals, their metabolic pathways still need to be explored. Research mainly focuse on the genes that affect the flavor of fish meat, but the metabolic processes of flavor substances in shellfish and shrimp have not been studied to a large extent.
2.1 Flavor amino acids
Studies have shown that free amino acids (FAA) have a significant impact on the muscle flavor of aquatic animals. It is worth noting that glutamic acid (Glu) and aspartic acid (Asp) are the main sources of umami, while glycine (Gly) and alanine (Ala) have a sweet and refreshing taste. Arginine can enhance freshness, while sodium chloride, monosodium glutamate or adenosine can reduce the inherent bitterness of arginine. Histidine (His) enhances the flavor. In addition, methionine (Met) and valine (Val) are related to bitterness (Cheng et al., 2024). The research has found that the main umami and sweet amino acids in tilapia filets were Asp and Gly (Li R. et al., 2022).
Studies have revealed that Glu, Ser, proline (Pro), arginine (Arg), and lysine (Lys) are the main taste-active substances in Takifugu obscurus (Zhang et al., 2019a). For tilapia fillets, the primary umami and sweet-tasting amino acids are Asp and Gly (Li R. et al., 2022). In metabolomic analyses of Mercenaria mercenaria, researchers identified Glu, Gly, Arg, Ala, and Asp as the five free amino acids with the greatest contribution (Zhang et al., 2023). Additionally, the main umami sources in Chinese soft-shelled turtles (Pelodiscus sinensis) are Glu, Asp., Gly and Ala; the umami taste of their skirt tissues is superior to that of muscle, but the balance of amino acid composition is inferior to that of muscle (Xie et al., 2021). Thus, the amino acids contributing to umami substances vary across different species.
Shrimp cultured in low-salinity brackish water exhibit better amino acid nutritional status, with a total essential amino acid (TEAA) content of 238.41 ± 46.24 mg/mL, which is significantly higher than that in the standard seawater group (Qin et al., 2024). Supplementing the feed with an appropriate amount of Lys can increase the levels of alanine and glutamic acid in the muscle tissue of Litopenaeus vannamei, ultimately improving the palatability of this species in freshwater aquaculture (Wu et al., 2022). Koyama et al. (2018) found that high salinity enhances the expression of the Glu ligase gene in Marsupenaeus japonicus. Conversely, under low-salinity conditions, alanine may be converted to pyruvate via the Ala-glyoxylate aminotransferase gene, leading to a decrease in Ala concentration. Similarly, other scholars evaluated changes in key amino acid metabolism genes in Crassostrea gigas. The results showed that under salinity stress, oysters regulate Gly levels primarily through the Gly dehydrogenase and aminomethyltransferase pathways (Meng et al., 2013).
2.2 Flavor nucleotides
At present, more than 30 kinds of taste-active nucleotides and their derivatives have been identified, among which inosine monophosphate (IMP), guanosine monophosphate (GMP) and adenosine monophosphate (AMP) are representative (Zhang et al., 2016). IMP is abundant in the muscles of animals such as chickens, cattle and pigs, while aquatic species like shrimp, clams and abalones mainly contain AMP. The GMP levels of seafood are usually low (Chen et al., 2021).
Studies have found that IMP is the main nucleotide in fresh rainbow trout Oncorhynchus mykiss (Duan et al., 2020). Exogenous IMP improves the flesh quality, composition, and flavor of Carassius auratus gibelio by increasing the AMP/ATP ratio and activating the AMPK signaling pathway (Cai et al., 2022). In Antarctic krill Euphausia superba and Penaeus vannamei Boone, both IMP and AMP can significantly enhance umami flavor and show a synergistic effect (Chang and Fang, 2024). The IMP is generated through two metabolic pathways of ATP degradation, one of which produces AMP, which is then degraded into IMP or adenosine (AdR) (Seki et al., 2017). However, the existence of IMP in shellfish has long been controversial. Soldatov et al. (2022) identified two pathways for IMP degradation in the Anadara kagoshimensis, including the adenosine monophosphate dehydrogenase and adenosine monophosphate deaminase pathways. Recent studies have found the IMP in refrigerated M. meretrix, C. gigas, and Chlamys farreri (He et al., 2025; Liu et al., 2024; Zhang et al., 2023).
2.3 Flavor organic acids
In fish, shrimp and shellfish, organic acids can affect pH regulation and flavor. The organic acids that play a key role in flavor include succinic acid, lactic acid and pyruvic acid, whose composition and concentration are closely related to the biochemical reactions in the metabolic process (Cheng et al., 2023). Studies show that crustaceans contain organic acids such as succinic acid, lactic acid, acetic acid and oxalic acid, while lactic acid and succinic acid are dominant in the muscle tissues of fish and shellfish (Bampidis et al., 2023; Ding et al., 2022). Among shellfish, succinic acid, sodium succinate and disodium succinate are essential for maintaining freshness and acidity (Ma et al., 2020).
It was found that the levels of lactic acid and succinic acid increasing with the size of rainbow trout O. mykiss in muscles (Duan et al., 2020). In addition, researches have determined that the major organic acid in raw and high hydrostatic pressure-treated oysters Crassostrea hongkongensis is succinic acid (Liu et al., 2021). Furthermore, succinic acid, citric acid and betaine are the major organic acids in oysters Crassostrea ariakensis (Liu et al., 2022).
2.4 Other flavor substances
Umami in aquatic products primarily arises from organic bases like betaine and trimethylamine oxide (TMAO) (Niizeki and Tanimoto, 2024). Betaine contributes to enhancing the sweetness of shellfish and shrimps. Additionally, betaine aldehyde dehydrogenase (BADH) may play a role in the osmoregulatory capacity of Litopenaeus vannamei (Chen et al., 2021; Delgado-Gaytán et al., 2017; Hefni et al., 2021). Existing studies have shown that by-products of betaine induce a positive feeding response in abalones and can affect their unique umami and sweet tastes (Hernández et al., 2019). TMAO is widely present in the muscles of marine teleosts, which can counteract the harmful effects of urea on proteins and serves as an important flavor substance in fish and shrimps (Zerbst-Boroffka et al., 2005). It has been found that adding TMAO to the diet of Chinese mitten crab (Eriocheir sinensis) can improve the freshness of its muscle, and dietary TMAO may activate the mTOR pathway by influencing amino acid metabolism (Hua et al., 2025). However, there are currently no reports on the impact of marine pollution on TMAO in aquatic animals. Furthermore, inorganic ions also play a key role in the flavor of seafood. Among them, ions such as Na+, K+, Cl−, and PO₄3− are considered to have significant impacts on the flavor of shellfish, with the effects of Na+ and Cl− being particularly prominent (Fu et al., 2025; Liu et al., 2023).
2.5 Interactions among flavor substances
The synergistic effect between nucleotides and certain amino acids is usually quantified by equivalent umami concentration (EUC). The higher the EUC value, the stronger the synergistic effect (Zhang et al., 2019b). Using EUC calculations to predict that most mushrooms can enhance the flavor of Japanese fish (Mau, 2005). They suggest mixing fish and mushrooms in a 1:1 ratio to maximize the umami of the stock. The umami flavor in clams of Meretrix petechialis, Mactra veneriformis and Ruditapes philippinarum is mainly driven by the proliferative effects of Glu, Asp and nucleotides (Wang et al., 2019). A small amount of IMP can significantly improve the sweetness of Gly and Ala (Kawai et al., 2002). In addition, the combination of nucleotide umami enhancers can significantly lower the threshold and enhance the umami effect (Vasilaki et al., 2021). Unlike fish, the taste components of shellfish are significantly different. Similar organisms such as squid, octopuses, shellfish, and similar organisms lack 5’-IMP. Therefore, their umami taste comes from the combination of amino acids, polypeptides, succinic acid and inorganic ions. These ions are important flavor enhancers in seafood and are closely related to inorganic ions such as Na+, K+, PO43−, and Cl− (Sikorski, 2020).
3 Effects of marine pollutant stress on the metabolism of flavor substances in aquatic animals
3.1 POPs
It is well known that POPs have carcinogenic, teratogenic and mutagenic effects, posing significant risks to the reproductive, genetic, immune, nervous and endocrine systems of humans and animals (Ashraf, 2015; Nguyen et al., 2020). Once persistent organic pollutants enter the environment, they will persist in the food chain, bioaccumulate and bioamplify, reaching toxic concentrations in the atmosphere, water and soil, causing serious adverse effects (Akhtar et al., 2021).
Dichlorodiphenyltrichloroethane (DDT) and benzo[a]pyrene (B[a]P) are typical persistent organic pollutants, posing severe threats to aquatic organisms and environmental health. The results showed that DDT stress significantly altered metabolites in the gonads, including increased levels of alanine, glutamic acid, and glycine, while decreased arginine levels. Both DDT and B[a]P could induce signal transduction and oxidative stress, and the mixed stress group showed a similar trend to the DDT group (Song et al., 2016). In a metabolomic study on the adductor muscles of Mytilus galloprovincialis from the western regions of Italy, which were contaminated with excessive levels of polycyclic aromatic hydrocarbons (PAHs) and mercury, the results revealed disturbances in energy metabolism, alterations in amino acid metabolism, and disorders in osmoregulatory processes. Among flavor substances, Ala, Gly, and inosine significantly increased, while the levels of aspartic acid, arginine, taurine, and betaine significantly decreased (Cappello et al., 2017). The results of the toxic effects of B[a]P on the gills of Pinctada martensii showed that under exposure to 1 μg/L and 10 μg/L B[a]P, arginine levels were significantly higher than those in the control group, while the levels of flavor amino acids Thr and Glu were significantly lower compared with the control group, inducing signal transduction, transcriptional regulation, cell growth, stress response, and energy metabolism (Chen et al., 2018).
Studies have indicated that B[a]P has significant effects on the composition and taste activity values (TAV) of free amino acids, nucleotides, organic acids, flavor peptides, organic bases, carbohydrates, and inorganic ions in Ruditapes philippinaru, as well as the gene expressions during their synthesis and decomposition processes. This suggests that B[a]P affects the levels of these taste substances by interfering with their metabolic processes, thereby altering the taste characteristics of R. philippinarum (reducing umami and sweetness, and enhancing bitterness) (Bi et al., 2024). Researchers pointed out that 38 μg/L B[a]P significantly reduced the content of flavor amino acids in C. farreri, leading to a significant decrease in TAV and EUC. Transcriptome analysis identified key pathways related to the metabolism of taste substances, and the expression levels of genes involved in the synthesis of Glu, Gly, Ala, and Arg were generally inhibited (He et al., 2025). However, current research on the molecular mechanisms of POPs on typical flavor substances in aquatic animals is relatively scarce.
3.2 Heavy metals
In addition to organic pollutants, heavy metals represent another class of marine pollutants that cannot be ignored. Heavy metals, known for their toxicity, wide sources, persistence, and non-degradability, originate from various human activities such as mining, smelting, agriculture, petrochemical industry, printing, aquaculture, electronic industry, and municipal waste (Rainbow and Luoma, 2011). These metals are released into the marine environment, where they can accumulate in marine organisms and magnify through the food chain, leading to higher concentrations in predatory species, and the elevated levels of heavy metals in marine ecosystems raise ecological concerns and public health risks about seafood safety (Wang et al., 2005). Currently, the heavy metal elements polluting the ocean mainly include mercury, cadmium, lead, zinc, chromium, copper, etc., assessing the current pollution levels of heavy metals in coastal ecosystems is crucial for the seafood industry, public health, and the sustainable development of marine ecosystems (Wang et al., 2013).
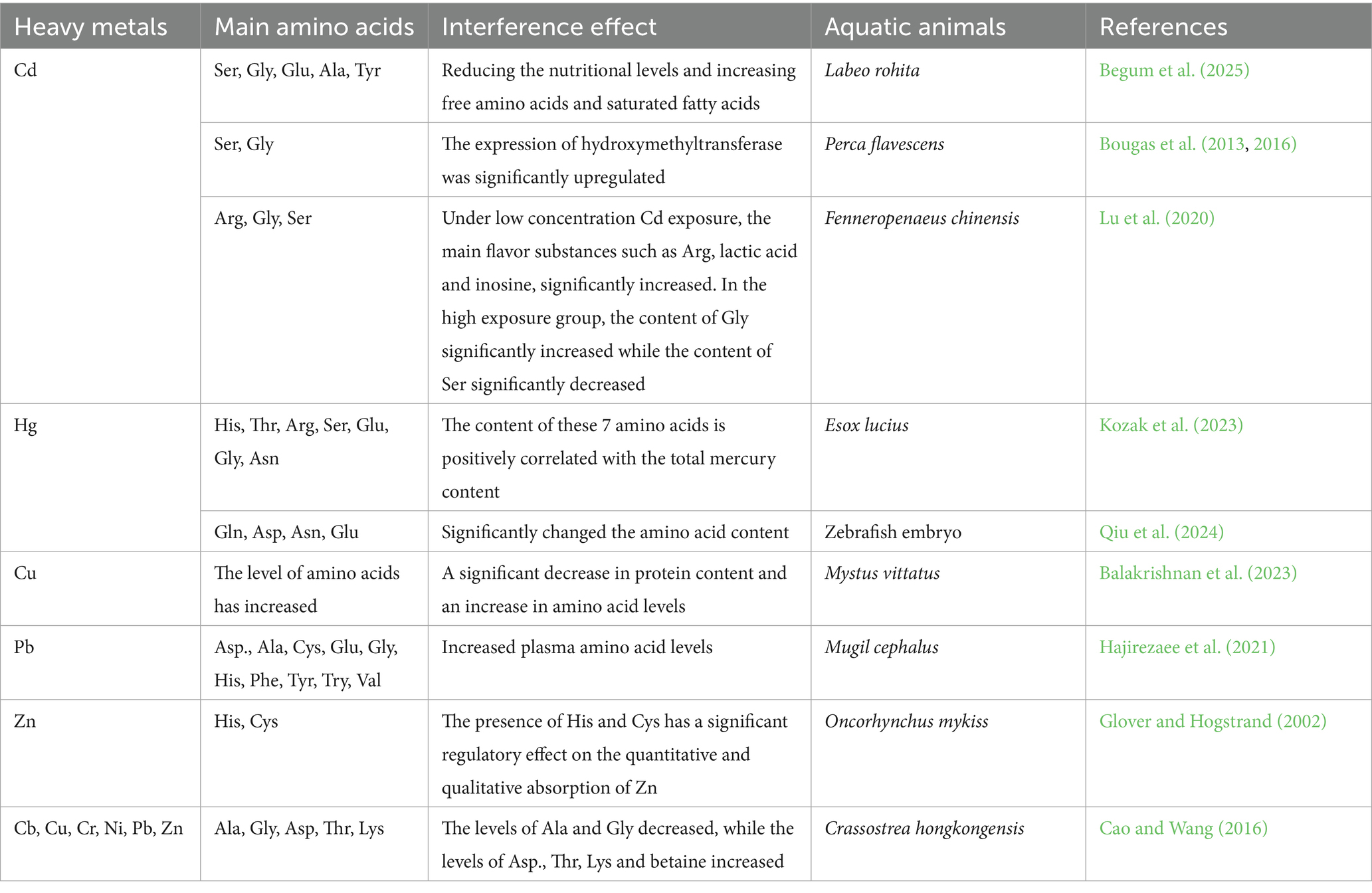
Currently, the main heavy metal elements polluting the ocean include Cd, Hg, Cu, Pb, and Zn. Assessing the current pollution levels of heavy metals in coastal ecosystems is crucial for the seafood industry, public health, and the sustainable development of marine ecosystems (Wang et al., 2013). When the marine environment is contaminated by heavy metals, these metals accumulate in aquatic organisms and enter the human body through the food chain. The examination of scalp hair samples from whale meat-eaters and large fish-eaters revealed that all the essential amino acids such as Ala, Val, Leu, etc., increased as the mercury concentration increased (Endo et al., 2017). Long-term exposure can lead to chronic poisoning, lung damage, and carcinogenic risks (Cao et al., 2021; Petrovic et al., 2022; Tek and Ng, 2024). As exemplified in Table 1 (line 521), different aquatic animals exhibit varying degrees of changes in their metabolic levels after being exposed to heavy metal pollution, with differences in the primary amino acids affected. This suggests that the toxic mechanisms of different heavy metals on aquatic animals may vary. Studies on the impact of heavy metal pollution on aquatic animals mainly focus on amino acids, while there are relatively few studies on organic acids and nucleotides and others. Moreover, experimental sampling is mainly conducted in the field, which could not rule out other influencing factors in the environment.
4 Conclusion and perspectives
The research on the impact of marine pollution on aquatic animals mainly covers two areas: the accumulation and elimination of pollutants in economically important aquatic organisms, and the influence of pollution-induced stress on physiological processes such as osmoregulation, energy metabolism, reproduction, and immunity. However, research on the molecular mechanism of flavor compound metabolism in aquatic products is still relatively limited, with most focusing on flavor amino acids. Additionally, studies on the effects of marine pollutant stress are mostly conducted through field sampling experiments.
In the future, metabolomics and transcriptomics technologies can be used to explore the interference mechanisms of pollutants on key flavor compounds in aquatic animals, and laboratory single-pollutant exposure experiments can be conducted to exclude the impact of other environmental factors on the research results.
Author contributions
RX: Data curation, Funding acquisition, Writing – original draft, Writing – review & editing, Investigation. WG: Writing – original draft, Data curation. PZ: Writing – original draft, Data curation. CZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation of China (2023) (Grant No. 42207331) and the Doctoral Initial Funding of Henan University of Science and Technology (Grant No. 13480097).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Akhtar, A. B. T., Naseem, S., Yasar, A., and Naseem, Z. (2021). “Persistent organic pollutants (POPs): sources, types, impacts, and their remediation” in Environmental pollution and remediation. ed. R. prasad (Singapore: Springer), 213–246.
Ashraf, M. A. (2015). Persistent organic pollutants (POPs): a global issue, a global challenge. Environ. Sci. Pollut. Res. 24, 4223–4227. doi: 10.1007/s11356-015-5225-9
Balakrishnan, K., Veerakumar, D., Mariappan, M., and Leon, J. P. S. (2023). Sublethal toxicity of copper Sulphate (CuSO4) on protein, amino acids changes in liver, kidney and muscle of the fish Mystus vittatus. EEC 29, 432–437. doi: 10.53550/EEC.2023.v29i01s.067
Bampidis, V., Azimonti, G., de Bastos, M. L., Christensen, H., Durjava, M., Dusemund, B., et al. (2023). Assessment of the safety of the feed additives acetic acid, calcium acetate and sodium diacetate for fish (FEFANA asbl). EFSA J. 21:e08176. doi: 10.2903/j.efsa.2023.8176
Bartley, D. M., Bondad-Reantaso, M. G., and Subasinghe, R. P. (2006). A risk analysis framework for aquatic animal health management in marine stock enhancement programmes. Fish. Res. 80, 28–36. doi: 10.1016/j.fishres.2006.03.011
Begum, A., Rabbane, M. G., Moniruzzaman, M., Hasan, M. R., and Chang, X. (2025). Cadmium pollution deteriorates the muscle quality of Labeo rohita by altering its nutrients and intestinal microbiota diversity. Biol. Trace Elem. Res. 203, 4835–4852. doi: 10.1007/s12011-025-04524-1
Bi, Y., Song, A., Pan, L., Miao, J., Zhou, Y., and Li, Z. (2024). Interference mechanism of benzo[a]pyrene exposure on the taste substance metabolisms in Ruditapes philippinarum. Environ. Sci. Pollut. Res. 31, 12019–12035. doi: 10.1007/s11356-024-31906-0
Bougas, B., Normandeau, E., Grasset, J., Defo, M. A., Campbell, P. G. C., Couture, P., et al. (2016). Transcriptional response of yellow perch to changes in ambient metal concentrations—a reciprocal field transplantation experiment. Aquat. Toxicol. 173, 132–142. doi: 10.1016/j.aquatox.2015.12.014
Bougas, B., Normandeau, E., Pierron, F., Campbell, P. G. C., Bernatchez, L., and Couture, P. (2013). How does exposure to nickel and cadmium affect the transcriptome of yellow perch (Perca flavescens) – results from a 1000 candidate-gene microarray. Aquat. Toxicol. 142-143, 355–364. doi: 10.1016/j.aquatox.2013.09.009
Cai, W., Liu, H., Fu, L., Han, D., Zhu, X., Jin, J., et al. (2022). Dietary inosine monophosphate improved liver health and flesh quality of gibel carp (Carassius auratus gibelio) via activating AMPK signalling pathway and enhancing the contents of muscle fat and flavour substance. Front. Mar. Sci. 9:940732. doi: 10.3389/fmars.2022.940732
Cao, X., Fu, M., Bi, R., Zheng, X., Fu, B., Tian, S., et al. (2021). Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 263:128346. doi: 10.1016/j.chemosphere.2020.128346
Cao, C., and Wang, W.-X. (2016). Bioaccumulation and metabolomics responses in oysters Crassostrea hongkongensis impacted by different levels of metal pollution. Environ. Pollut. 216, 156–165. doi: 10.1016/j.envpol.2016.05.047
Cappello, T., Maisano, M., Mauceri, A., and Fasulo, S. (2017). 1H NMR-based metabolomics investigation on the effects of petrochemical contamination in posterior adductor muscles of caged mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 142, 417–422. doi: 10.1016/j.ecoenv.2017.04.040
Chang, L.-T., and Fang, M. (2024). Establishment of the flavor quality standard for white shrimp (Litopenaeus vannamei) by instrumental and sensory approaches. ACS Food Sci. Technol. 4, 3016–3024. doi: 10.1021/acsfoodscitech.4c00567
Chen, H., Diao, X., Wang, H., and Zhou, H. (2018). An integrated metabolomic and proteomic study of toxic effects of benzo[a]pyrene on gills of the pearl oyster Pinctada martensii. Ecotoxicol. Environ. Saf. 156, 330–336. doi: 10.1016/j.ecoenv.2018.03.040
Chen, L., Zeng, W., Rong, Y., and Lou, B. (2021). Characterisation of taste-active compositions, umami attributes and aroma compounds in Chinese shrimp. Int. J. Food Sci. Technol. 56, 6311–6321. doi: 10.1111/ijfs.15304
Cheng, H., Mei, J., and Xie, J. (2024). Analysis of changes in volatile compounds and evolution in free fatty acids, free amino acids, nucleotides, and microbial diversity in tilapia (Oreochromis mossambicus) fillets during cold storage. J. Sci. Food Agric. 104, 2959–2970. doi: 10.1002/jsfa.13188
Cheng, H., Wang, J., and Xie, J. (2023). Progress on odor deterioration of aquatic products: characteristic volatile compounds, analysis methods, and formation mechanisms. Food Biosci. 53:102666. doi: 10.1016/j.fbio.2023.102666
Corsolini, S., Ancora, S., Bianchi, N., Mariotti, G., Leonzio, C., and Christiansen, J. S. (2014). Organotropism of persistent organic pollutants and heavy metals in the Greenland shark Somniosus microcephalus in NE Greenland. Mar. Pollut. Bull. 87, 381–387. doi: 10.1016/j.marpolbul.2014.07.021
Delgado-Gaytán, M. F., Rosas-Rodríguez, J. A., Yepiz-Plascencia, G., Figueroa-Soto, C. G., and Valenzuela-Soto, E. M. (2017). Cloning and molecular characterization of the betaine aldehyde dehydrogenase involved in the biosynthesis of glycine betaine in white shrimp (Litopenaeus vannamei). Chem. Biol. Interact. 276, 65–74. doi: 10.1016/j.cbi.2017.02.006
Ding, Q., Lu, C., Hao, Q., Zhang, Q., Yang, Y., Olsen, R. E., et al. (2022). Dietary succinate impacts the nutritional metabolism, protein succinylation and gut microbiota of zebrafish. Front. Nutr. 9:894278. doi: 10.3389/fnut.2022.894278
Duan, Z., Zhou, Y., Liu, W., Shi, C. C., Li, L., Dong, Y., et al. (2020). Variations in flavor according to fish size in rainbow trout (Oncorhynchus mykiss). Aquaculture 526:735398. doi: 10.1016/j.aquaculture.2020.735398
Endo, T., Ogasawara, H., Hayasaka, M., Hotta, Y., Kimura, O., and Petzke, K. J. (2017). Correlations between mercury concentration, and stable isotope ratios of carbon and nitrogen of amino acids in scalp hair from whale meat eaters and heavy fish eaters. Rapid Commun. Mass Spectrom. 31, 745–752. doi: 10.1002/rcm.7841
Fu, B., Li, M., Chang, Z., Yi, J., Cheng, S., and Du, M. (2025). Identification of novel umami peptides from oyster hydrolysate and the mechanisms underlying their taste characteristics using machine learning. Food Chem. 473:142970. doi: 10.1016/j.foodchem.2025.142970
Glover, C. N., and Hogstrand, C. (2002). Amino acid modulation of in vivo intestinal zinc absorption in freshwater rainbow trout. J. Exp. Biol. 205, 151–158. doi: 10.1242/jeb.205.1.151
Golden, C. D., Zachary Koehn, J., Shepon, A., Passarelli, S., Free, C. M., Viana, D. F., et al. (2021). Aquatic foods to nourish nations. Nature 598, 315–320. doi: 10.1038/s41586-021-03917-1
Hajirezaee, S., Ajdari, A., and Azhang, B. (2021). Metabolite profiling, histological and oxidative stress responses in the grey mullet, Mugil cephalus exposed to the environmentally relevant concentrations of the heavy metal, Pb (NO3)2. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 244:109004. doi: 10.1016/j.cbpc.2021.109004
He, Z., Pan, L., Xu, R., Zhou, Y., Gao, Z., Miao, J., et al. (2025). The impact of PAHs on the taste substances of Chlamys farreri during the reproductive period: changes in flavor characteristics and metabolic mechanisms. J. Food Compos. Anal. 138:107011. doi: 10.1016/j.jfca.2024.107011
Hefni, M. E., Bergström, M., Lennqvist, T., Fagerström, C., and Witthöft, C. M. (2021). Simultaneous quantification of trimethylamine N-oxide, trimethylamine, choline, betaine, creatinine, and propionyl-, acetyl-, and l-carnitine in clinical and food samples using HILIC-LC-MS. Anal. Bioanal. Chem. 413, 5349–5360. doi: 10.1007/s00216-021-03509-y
Hernández, J., Viana, M. T., Lastra, M., Matus De La Parra, A., and Toledo-Agüero, P. (2019). The possible use of beet-vinasse as carbohydrate replacer in formulated diets for the juvenile abalone, Haliotis tuberculata. J. Appl. Aquac. 31, 271–288. doi: 10.1080/10454438.2018.1547672
Hua, H., Guo, H., Liu, W., Liu, Z., He, C., Huang, Y., et al. (2025). Trimethylamine N-oxide promotes the growth of Chinese mitten crab (Eriocheir sinensis), deposition of amines, and activates the mTOR pathway by affecting metabolism of amino acids. Anim. Adv. 2:e008. doi: 10.48130/animadv-0025-0005
Jones, B. C., Rocker, M. M., Keast, R. S. J., Callahan, D. L., Redmond, H. J., Smullen, R. P., et al. (2022). Systematic review of the odorous volatile compounds that contribute to flavour profiles of aquatic animals. Rev. Aquac. 14, 1418–1477. doi: 10.1111/raq.12657
Kawai, M., Okiyama, A., and Ueda, Y. (2002). Taste enhancements between various amino acids and IMP. Chem. Senses 27, 739–745. doi: 10.1093/chemse/27.8.739
Khan, M. I., Jo, C., and Tariq, M. R. (2015). Meat flavor precursors and factors influencing flavor precursors--a systematic review. Meat Sci. 110, 278–284. doi: 10.1016/j.meatsci.2015.08.002
Koyama, H., Mizusawa, N., Hoashi, M., Tan, E., Yasumoto, K., Jimbo, M., et al. (2018). Changes in free amino acid concentrations and associated gene expression profiles in the abdominal muscle of kuruma shrimp (Marsupenaeus japonicus) acclimated at different salinities. J Exp Biol. 221. doi: 10.1242/jeb.168997
Kozak, N., Kahilainen, K. K., Pakkanen, H. K., Hayden, B., Østbye, K., and Taipale, S. J. (2023). Mercury and amino acid content relations in northern pike (Esox lucius) in subarctic lakes along a climate-productivity gradient. Environ. Res. 233:116511. doi: 10.1016/j.envres.2023.116511
Li, C., Li, W., Li, L., Chen, S., Wu, Y., and Qi, B. (2022). Microbial community changes induced by a newly isolated salt-tolerant Tetragenococcus muriaticus improve the volatile flavor formation in low-salt fish sauce. Food Res. Int. 156:111153. doi: 10.1016/j.foodres.2022.111153
Li, R., Sun, Z., Zhao, Y., Li, L., Yang, X., Chen, S., et al. (2022). Effect of different thermal processing methods on water-soluble taste substances of tilapia fillets. J. Food Compos. Anal. 106:104298. doi: 10.1016/j.jfca.2021.104298
Liang, M., WANG, S., WANG, J., CHANG, Q., and MAI, K. (2008). Comparison of flavor components in shrimp Litopenaeus vannamei cultured in sea water and low salinity water. Fish. Sci. 74, 1173–1179. doi: 10.1111/j.1444-2906.2008.01637.x
Lim, L.-S., Lai, S.-K. J., Yong, A. S.-K., Shapawi, R., and Kawamura, G. (2017). Feeding response of marble goby (Oxyeleotris marmorata) to organic acids, amino acids, sugars and some classical taste substances. Appl. Anim. Behav. Sci. 196, 113–118. doi: 10.1016/j.applanim.2017.06.014
Liu, C., Gu, Z., Lin, X., Wang, Y., Wang, A., Sun, Y., et al. (2022). Effects of high hydrostatic pressure (HHP) and storage temperature on bacterial counts, color change, fatty acids and non-volatile taste active compounds of oysters (Crassostrea ariakensis). Food Chem. 372:131247. doi: 10.1016/j.foodchem.2021.131247
Liu, C., Ji, W., Jiang, H., Shi, Y., He, L., Gu, Z., et al. (2021). Comparison of biochemical composition and non-volatile taste active compounds in raw, high hydrostatic pressure-treated and steamed oysters Crassostrea hongkongensis. Food Chem. 344:128632. doi: 10.1016/j.foodchem.2020.128632
Liu, C., Sun, Y., Hong, X., Yu, F., Yang, Y., Wang, A., et al. (2023). Effects of Ammonia and salinity stress on non-volatile and volatile compounds of ivory Shell (Babylonia areolata). Foods 12:3200. doi: 10.3390/foods12173200
Liu, Y., Teng, X., Chen, L., Wu, S., Xue, C., and Li, Z. (2024). Changes in flavor-related biomarkers in Pacific oysters (Crassostrea gigas) following microplastic exposure. Foods 13:765. doi: 10.3390/foods13050765
Lu, Z., Wang, S., Ji, C., Shan, X., and Wu, H. (2020). Evaluation of metal pollution-induced biological effects in Chinese shrimp Fenneropenaeus chinensis by NMR-based metabolomics. Mar. Pollut. Bull. 150:110688. doi: 10.1016/j.marpolbul.2019.110688
Ma, J., Chen, Y., Zhu, Y., Ayed, C., Fan, Y., Chen, G., et al. (2020). Quantitative analyses of the umami characteristics of disodium succinate in aqueous solution. Food Chem. 316:126336. doi: 10.1016/j.foodchem.2020.126336
Mau, J.-L. (2005). The umami taste of edible and medicinal mushrooms. Int. J. Med. Mushrooms 7, 119–126. doi: 10.1615/intjmedmushr.v7.i12.120
Meng, J., Zhu, Q., Zhang, L., Li, C., Li, L., She, Z., et al. (2013). Genome and transcriptome analyses provide insight into the euryhaline adaptation mechanism of Crassostrea gigas. PLoS One 8:e58563. doi: 10.1371/journal.pone.0058563
Michihata, T., SADO, Y., YANO, T., and ENOMOTO, T. (2000). Free amino acids, oligopeptides, organic acids and nucleotides of ISHIRU (fish sauce). Nippon Shokuhin Kagaku Kogaku Kaishi 47, 241–248. doi: 10.3136/nskkk.47.241
Nguyen, V.-H., Smith, S. M., Wantala, K., and Kajitvichyanukul, P. (2020). Photocatalytic remediation of persistent organic pollutants (POPs): a review. Arab. J. Chem. 13, 8309–8337. doi: 10.1016/j.arabjc.2020.04.028
Niizeki, N., and Tanimoto, S. (2024). Trimethylamine N-oxide (TMAO) in Seafoods: shared mechanisms between fish and humans for forming gut-microbial TMAO: overview of animal TMAO-yield potential. Food Rev. Intl. 40, 3471–3486. doi: 10.1080/87559129.2024.2358949
Petrovic, J., Jovetic, M., Štulić, M., Vujadinović, D., Lorenzo, J. M., Iammarino, M., et al. (2022). Exposure assessment in the Serbian population and occurrence of histamine and heavy metals in fish and seafood. Int. J. Food Sci. Technol. 57, 7517–7527. doi: 10.1111/ijfs.15342
Qin, K., Feng, W., Ji, Z., Jiang, X., Hu, Y., Li, Y., et al. (2024). Shrimp cultured in low-salt saline–alkali water has a better amino acid nutrition and umami─comparison of flavors between saline–alkali water- and seawater-cultured Litopenaeus vannamei. J. Agric. Food Chem. 72, 6585–6592. doi: 10.1021/acs.jafc.3c08435
Qiu, X., Zhang, Y., Gao, J., Cui, Y., Dong, K., Chen, K., et al. (2024). Exposure to thimerosal induces behavioral abnormality in the early life stages of zebrafish via altering amino acid homeostasis. J. Hazard. Mater. 478:135548. doi: 10.1016/j.jhazmat.2024.135548
Rainbow, P. S., and Luoma, S. N. (2011). Metal toxicity, uptake and bioaccumulation in aquatic invertebrates—modelling zinc in crustaceans. Aquat. Toxicol. 105, 455–465. doi: 10.1016/j.aquatox.2011.08.001
Ribeiro, C. A. O., Vollaire, Y., Sanchez-Chardi, A., and Roche, H. (2005). Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue nature reserve, France. Aquat. Toxicol. 74, 53–69. doi: 10.1016/j.aquatox.2005.04.008
Seki, H., Nakazato, K., and Hamada-Sato, N. (2017). Adenosine monophosphate degradation and inosinic acid accumulation in the shrimp Penaeus japonicus. Int. Aquat. Res. 9, 37–52. doi: 10.1007/s40071-017-0154-5
Sharma, K., Shah, G., Singhal, K., and Soni, V. (2024). Comprehensive insights into the impact of oil pollution on the environment. Reg. Stud. Mar. Sci. 74:103516. doi: 10.1016/j.rsma.2024.103516
Sikorski, ZE. (2020). The nutritive composition of the major groups of marine food organisms. Seafoods: Chemistry, Processing Technology and Quality, 29–54. Available online at: https://cir.nii.ac.jp/crid/1571135650034783872.
Soldatov, A. A., Golovina, I. V., Kolesnikova, E. E., Sysoeva, I. V., and Sysoev, A. A. (2022). Effect of hydrogen sulfide loading on the activity of energy metabolism enzymes and the adenylate system in tissues of the Anadara kagoshimensis clam. Inland Water Biol. 15, 632–640. doi: 10.1134/S1995082922050194
Song, Q., Zheng, P., Qiu, L., Jiang, X., Zhao, H., Zhou, H., et al. (2016). Toxic effects of male Perna viridis gonad exposed to BaP, DDT and their mixture: a metabolomic and proteomic study of the underlying mechanism. Toxicol. Lett. 240, 185–195. doi: 10.1016/j.toxlet.2015.10.031
Tek, P. P. Y., and Ng, C. C. (2024). Accumulation of potentially toxic elements in fourfinger threadfin (Eleutheronema tetradactylum) and black pomfret (Parastromateus niger) from Selangor, Malaysia. Environ. Monit. Assess. 196:382. doi: 10.1007/s10661-024-12508-2
Thiagarajan, C., and Devarajan, Y. (2025). The urgent challenge of ocean pollution: impacts on marine biodiversity and human health. Reg. Stud. Mar. Sci. 81:103995. doi: 10.1016/j.rsma.2024.103995
Vasilaki, A., Panagiotopoulou, E., Koupantsis, T., Katsanidis, E., and Mourtzinos, I. (2021). Recent insights in flavor-enhancers: definition, mechanism of action, taste-enhancing ingredients, analytical techniques and the potential of utilization. Crit. Rev. Food Sci. Nutr. 62, 9036–9052. doi: 10.1080/10408398.2021.1939264
Wang, S., He, Y., Zhang, J., Chen, S., and He, B. (2019). Comparison of taste and odour characteristics of three mass-produced aquaculture clams in China. Aquac. Res. 51, 664–673. doi: 10.1111/are.14415
Wang, X., Sato, T., Xing, B., and Tao, S. (2005). Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 350, 28–37. doi: 10.1016/j.scitotenv.2004.09.044
Wang, S.-L., Xu, X.-R., Sun, Y.-X., Liu, J.-L., and Li, H.-B. (2013). Heavy metal pollution in coastal areas of South China: a review. Mar. Pollut. Bull. 76, 7–15. doi: 10.1016/j.marpolbul.2013.08.025
Wang, W., Zhou, X., and Liu, Y. (2020). Characterization and evaluation of umami taste: a review. Trends Anal. Chem. 127:115876. doi: 10.1016/j.trac.2020.115876
Wu, H., Hou, J., and Wang, X. (2023). A review of microplastic pollution in aquaculture: sources, effects, removal strategies and prospects. Ecotoxicol. Environ. Saf. 252:114567. doi: 10.1016/j.ecoenv.2023.114567
Wu, M., Li, M., Wen, H., Yu, L., Jiang, M., Lu, X., et al. (2022). Dietary lysine facilitates muscle growth and mediates flesh quality of Pacific white shrimp (Litopenaeus vannamei) reared in low-salinity water. Aquac. Int. 31, 603–625. doi: 10.1007/s10499-022-00997-2
Xie, Z., Deng, S., CHENG, X., CHEN, X., HE, Z., LI, J., et al. (2021). Evaluation of amino acid nutrition of Pelodiscus sinensi during hibernation period. J. Hunan Agricult. Univ. (Nat. Sci.) 47, 231–239. doi: 10.13331/j.cnki.jhau.2021.02.017
Yan, W., Hamid, N., Deng, S., Jia, P.-P., and Pei, D.-S. (2020). Individual and combined toxicogenetic effects of microplastics and heavy metals (cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J. Hazard. Mater. 397:122795. doi: 10.1016/j.jhazmat.2020.122795
Zerbst-Boroffka, I., Kamaltynow, R. M., Harjes, S., Kinne-Saffran, E., and Gross, J. (2005). TMAO and other organic osmolytes in the muscles of amphipods (Crustacea) from shallow and deep water of Lake Baikal. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 142, 58–64. doi: 10.1016/j.cbpa.2005.07.008
Zhang, N., Ayed, C., Wang, W., and Liu, Y. (2019a). Sensory-guided analysis of key taste-active compounds in puffer fish (Takifugu obscurus). J. Agric. Food Chem. 67, 13809–13816. doi: 10.1021/acs.jafc.8b06047
Zhang, Z., Chen, S., Chen, A., Xu, Y., Zhang, Y., Yu, W., et al. (2023). Comparison of the flavor qualities between two varieties of Mercenaria mercenaria. Sci. Rep. 13:13047. doi: 10.1038/s41598-023-39757-4
Zhang, Y., Hoon, M. A., Chandrashekar, J., Mueller, K. L., Cook, B., Wu, D., et al. (2003). Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301. doi: 10.1016/S0092-8674(03)00071-0
Zhang, Y., Venkitasamy, C., Pan, Z., Liu, W., and Zhao, L. (2016). Novel umami ingredients: umami peptides and their taste. J. Food Sci. 82, 16–23. doi: 10.1111/1750-3841.13576
Keywords: flavor substances metabolism, aquatic products, marine pollution, flavor amino acids, flavor nucleotides
Citation: Xu R, Guo W, Zhang P and Zhang C (2025) Metabolic disruption of flavor substance in aquatic animals: a review of POPs and heavy metal effects. Front. Sustain. Food Syst. 9:1651556. doi: 10.3389/fsufs.2025.1651556
Edited by:
Roberto Anedda, Porto Conte Ricerche, ItalyReviewed by:
Manjun Yang, Sun Yat-sen University, ChinaCopyright © 2025 Xu, Guo, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruiyi Xu, eHVydWl5aUBoYXVzdC5lZHUuY24=
 Ruiyi Xu
Ruiyi Xu Weili Guo
Weili Guo Chunnuan Zhang
Chunnuan Zhang