- 1Faculty of Economics and Social Development, Latvia University of Life Sciences and Technologies, Jelgava, Latvia
- 2Faculty of Agriculture and Food Technology, Latvia University of Life Sciences and Technologies, Jelgava, Latvia
- 3Liepaja Academy, Riga Technical University, Liepaja, Latvia
Europe’s food system currently faces significant challenges, including demographic changes, high dependence on synthetic fertilizers, and growing climate change impacts. Aligned with the goals of the European Green Deal and the Farm to Fork policy, this study aims to explore the potential of an algal digestate-based biostimulant to enhance crop productivity and reduce reliance on synthetic fertilizers in sustainable agricultural systems. A vegetation tray trial was conducted using three cover crops representative of palustrine species in the Baltic Sea region: lettuce, radish, and spinach. The effects of full and reduced mineral fertilization with and without algal biostimulant supplementation were assessed. The results showed that using the biostimulant, especially at a 6% application rate, could partially compensate for reduced nutrient input. This approach exceeded the yields of conventional full-strength fertilization while improving soil health and minimizing fertilizer input. Statistical analyses (ANOVA and Tukey HSD) confirmed significant yield increases for treatments with the biostimulant, particularly under nutrient-limited conditions. These results suggest that algal biostimulants have the potential to advance sustainable and resilient food production systems in Europe.
1 Introduction
The European food chain is a complex and vital system that ensures food security, supports rural development, and promotes sustainability across the continent. In this context, agricultural innovation is of particular importance. To that end, the sector must improve productivity, reduce environmental impact, and adapt to climate change while maintaining economic viability. A promising solution is the use of biostimulants, which are natural substances that stimulate plant growth and improve crop productivity by activating physiological processes in plants. Algal biostimulants have been shown to offer benefits such as improved stress tolerance and stimulated root development. This phenomenon, while not overtly evident, exerts an indirect influence on soil microbiological activity, thereby enhancing nutrient use efficiency. These products have the potential to reduce the reliance on fertilizers, mitigate greenhouse gas emissions, and contribute to the realization of EU sustainability objectives (Eurostat, 2023a,b; European Commission, 2022).
In recent years, the European Commission’s Scientific Council (JRC) and the European Innovation Partnerships (EIP-AGRI) have conducted research on using biostimulants, biological soil improvers, and precision agriculture technologies to reduce synthetic fertilizer and pesticide use, improve soil health, and increase plant resilience to climate stress (EIP-AGRI, 2023; Joint Research Centre (JRC), 2022). Legislative actions such as the proposed Sustainable Use of Pesticides Regulation (SUR) further encourage the adoption of alternative crop inputs, including biostimulants, to reduce chemical pesticide dependence in European agriculture (European Commission, 2023a,b). Algal biostimulants represent a particularly promising solution in the context of European agriculture. Recent research has demonstrated that microalgae-based biostimulants significantly enhance nutrient use efficiency and crop productivity while reducing environmental impact (Bajpai et al., 2024; Li et al., 2024). These products are particularly effective in improving plant growth parameters, yield attributes, and soil health indicators (Ruzzi et al., 2024; Neumann et al., 2024).” Additional publications and field trials (e.g., Horizon Europe and LIFE projects) have demonstrated that algae extracts can enhance plant growth, boost yields, minimize fertilizer consumption, and promote soil microbial activity (Chabili et al., 2024; Ruzzi et al., 2024). These products are consistent with the objectives of the European Green Deal and the Farm to Fork Strategy, which prioritize the promotion of sustainable agricultural practices and the minimization of environmental impact.
The objective of this study is to empirically assess the efficacy of an algal biostimulant in agricultural contexts. The primary outcomes of interest include evaluations of its impact on crop productivity, soil health, and synthetic fertilizer consumption. These assessments contribute to the pursuit of sustainable agricultural development, aligning with the objectives outlined by the European Green Deal, the Farm to Fork strategic vision, and recent policy frameworks and scientific initiatives implemented by the European Union. To that end, the following tasks were delineated.
The primary objective is to undertake a thorough analysis of the challenges confronting Europe’s agricultural sector and food supply chain. This analysis will be grounded in the most recent official data and policy documents, complemented by the latest scientific research findings and initiatives.
Secondly, the algal biostimulant starting material must be prepared using appropriate production and extraction methods. This will ensure the material’s quality and improve the reliability of the experiment.
The third objective is to design and implement a covered area experiment with algal biostimulant and evaluate its impact on plant growth, yield, and agronomic efficiency. Additionally, the experiment will assess the impact on soil health (i.e., microbial activity) and fertilizer consumption.
Fourthly, it is imperative to extrapolate the findings from the analysis and the experimental results to formulate conclusions regarding the potential of the algal biostimulant to contribute to a sustainable and resilient food system.
Considering the prevailing challenges confronting the European food chain, the emergence of algal biostimulants as a promising solution to enhance productivity, mitigate environmental impacts, and optimize resource efficiency in agriculture is particularly salient. The prevailing policy frameworks and recent scientific initiatives within the European Union substantiate this assertion. This approach is consistent with the objectives of the European Green Deal, the Farm to Fork strategy, and the Common Agricultural Policy, all of which aim to promote sustainable and resilient agricultural development. The utilization of algae biostimulants in covered area trials enables the assessment of these products’ efficacy through the evaluation of their impact on plant growth, yield, and soil health, in addition to their effect on fertilizer consumption. This experimental approach yielded data that not only advanced scientific discourse but also informed the formulation of recommendations for farmers and policymakers regarding the integration of biostimulants in European agricultural practices. Consequently, algal biostimulants have the potential to serve as a pivotal element in the transformation of European agriculture toward a greener and more sustainable production system, thereby aligning with the EU’s objectives of sustainability and green agriculture. The subsequent sections of the paper meticulously analyze and interpret the experimental results, thereby providing an objective and evidence-based perspective on the viability of this method for implementation.
2 Context
In 2020, the European Union (EU) contained 9.1 million farms, which cultivated 38.4% of the EU’s total land area. These farms employed 8.7 million individuals, constituting 4.2% of the overall labor force (Eurostat, 2023a,b) However, the sector is confronted with numerous challenges, including but not limited to: (1) demographic shifts, with 33.2% of farm managers being over 65 years of age; (2) a decline in the number of farms, with a 24.8% decrease in the number of farms between 2010 and 2020; (3) reduced productivity on smaller farms; and (4) a high dependence on chemical fertilizers and energy inputs (Eurostat, 2023a,b). Additionally, the agricultural sector is responsible for 10.7% of EU greenhouse gas emissions, while organic farming systems account for only 9.9% of total agricultural land (Eurostat, 2023a,b).
To address these challenges, the European Union has developed the Farm to Fork Strategy, a key component of the European Green Deal. This strategy aims to promote sustainable food production, reduce environmental impacts, and ensure a fair economic return for farmers (European Commission, 2022; Eurostat, 2023a,b). The 2023–2027 Common Agricultural Policy (CAP), with a budget of €387 billion, prioritizes the support of small farms, young farmers, and environmentally sustainable agricultural practices (Eurostat, 2023a,b). The agricultural sector plays a pivotal role in the economy. In 2022, the agricultural sector generated €537.5 billion in gross production value, with value added from agriculture accounting for 1.4% of EU GDP (Eurostat, 2023a,b).
However, the sector is facing pressure from multiple fronts. An examination of economic data reveals a 31.4% increase in the input costs, particularly those associated with fertilizer and energy, during the year 2022. Fertilizer prices exhibited a marked increase, reaching nearly 90% higher than the previous year’s figures. This surge was attributed to global market disruptions resulting from Russia’s invasion of Ukraine (Eurostat, 2023a,b). Despite a notable increase in agricultural output prices, these gains were counterbalanced by escalating production costs and variable yields, particularly in the context of extreme weather and climate variability (Eurostat, 2023a,b).
The intricacies of the food chain and the interconnectedness of the global economy serve to compound these challenges. The European Union (EU) maintains a substantial reliance on the importation of specific agricultural commodities and products, including grains, fruits, nuts, and coffee beans. This reliance renders the EU susceptible to external shocks originating in the global market and political arena (Eurostat, 2023a,b). In 2022, the European Union imported €78.1 billion worth of crop products from third countries, while exporting €116 billion worth of processed food and beverages (Eurostat, 2023a,b). This interdependence underscores the necessity of developing resilience and reducing external dependencies within the food system.
3 Materials and methods
The current research was implemented using a mixed research methodology. In the initial phase of the study, a qualitative synthesis of documents and scientific publications was employed. This synthesis was based on contemporary EU policy documents, official reports, and peer-reviewed scientific studies. The objective of this phase was to analyze the challenges facing the European agri-food value chain. Current research emphasizes the critical role of biostimulants in addressing these challenges through enhanced nutrient use efficiency and sustainable agricultural practices (Ludemann et al., 2024). The primary objective of the analysis was to identify and systematize the main challenges affecting the sustainability of the agricultural sector and the food value chain. Additionally, the analysis sought to assess the potential for solutions in the context of different strategic and innovative approaches. The analysis was grounded in official documents from the European Commission and the European Parliament, encompassing the European Union’s Farm to Fork sustainability strategy. This strategy outlines a transition toward a sustainable food system, with the overarching objective being to reduce environmental pollution and enhance food security. Furthermore, an analysis was conducted of the Common Agricultural Policy (CAP) 2023–2027 Strategic Plans and national documents regulating agricultural support and sustainability objectives across Europe. Eurostat’s agricultural and food chain statistics databases were utilized to obtain contemporary and reliable information on socio-economic and environmental indicators, thereby providing a detailed picture of production volumes, climate change impacts, and economic performance. Recent work demonstrates that combining biostimulant technologies with both traditional and modern approaches to nutrient management can substantially improve crop physiology and efficiency, particularly in fruits and vegetables (Ali et al., 2024). In order to achieve a more profound analysis, it is necessary to consult peer-reviewed scientific studies that analyze the use of biostimulants in agriculture, the strengthening of the sustainability of the European food chain, and the impact of innovative solutions on agricultural practices.
3.1 Extraction of algal biostimulant
In addition to biogas, the anaerobic digestion process also yields a residual by-product—digestate—which consists of solid and liquid fractions. The liquid fraction, separated after fermentation, is of particular interest due to its potential application as a plant biostimulant. This fraction contains a variety of bioavailable nutrients, including nitrogen, potassium, and trace elements, as well as organic acids and microbial metabolites that may promote plant growth and soil microbial activity. When Furcellaria lumbricalis is used as a primary feedstock—especially in co-digestion with lignocellulosic biomass—the resulting digestate is enriched with compounds naturally present in red algae, such as polysaccharides and mineral salts. These components may contribute to enhanced plant stress resistance, improved nutrient uptake, and stimulated root development.
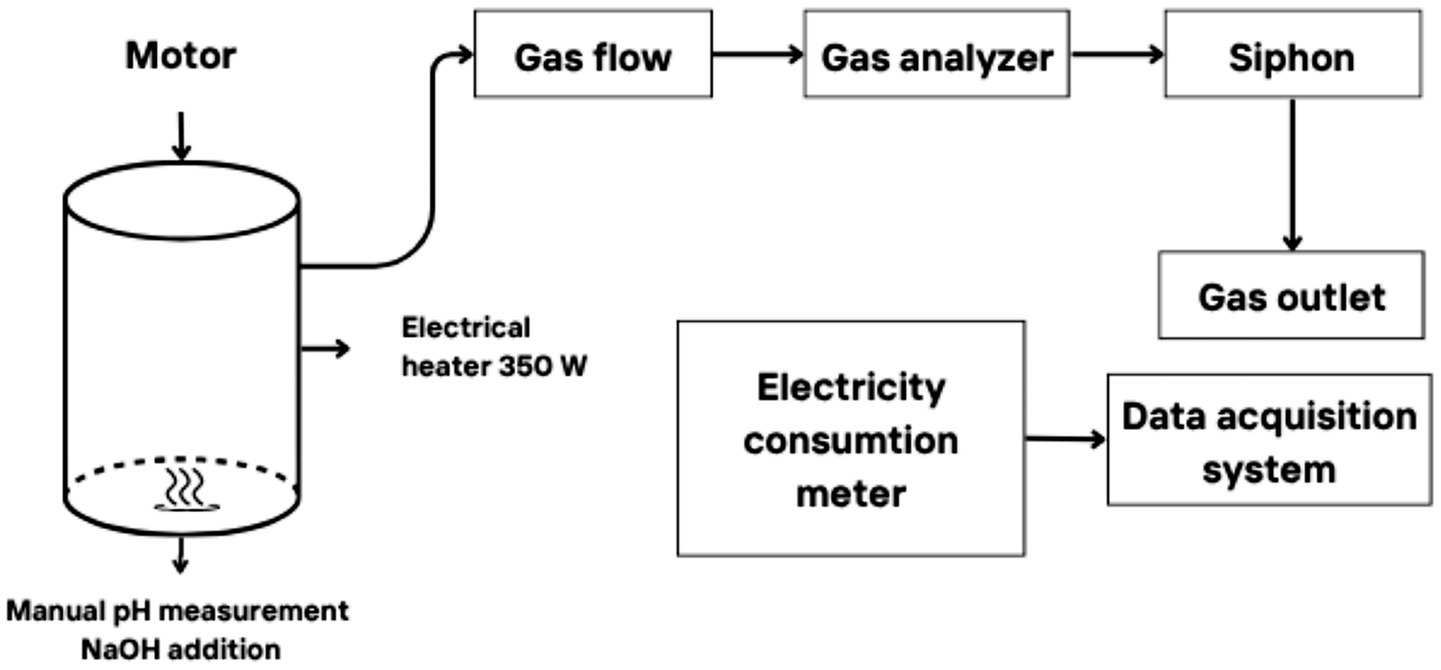
In the second phase of the study, an empirical study was conducted. Technological tests for the extraction of the biostimulant were carried out in a self-constructed plant (Figure 1) that provides the main conditions for anaerobic fermentation of organic substances. This plant is hermetically sealed, and the tank material does not engage in chemical reactions with the materials inserted or extracted during fermentation. It allows for the maintenance of a constant temperature and is equipped with a mixer and equipment for data acquisition and storage. The body of the plant consists of a cylindrical, vertical tank with a capacity of 60 L, constructed from a chemically passive polymer. Reactors of this magnitude are frequently utilized in biochemical research, wherein they are employed for the purpose of evaluating novel substrates and optimizing processes prior to their implementation on a larger scale.
The housing is hermetically sealed to prevent oxygen ingress and maintain anaerobic conditions, as well as to prevent leakage of gases from the process. The lower portion of the biomass is equipped with a stainless steel mixer, which is driven periodically by an external electric motor. The rotor axis is fitted with a seal. At the uppermost point of the reactor is a gas collector that gathers the biogas that have been released. This gas is subsequently sent to a gas analysis plant, where the volume and composition of the gases are measured. Subsequently, the gas exits the facility via a siphon, the function of which is to generate a modest degree of back pressure and to impede the ingress of atmospheric air into the system. In order to ensure that the fermentation temperature remains at the optimal level for the specific microorganisms being cultivated (e.g., 37°C for mesophilic microorganisms), a heating system integrated with an electric thermoregulatory is employed within the reactor. The system incorporates an electric floor heating unit with a power of 350 W, serving as the primary heat source. The controller provides ±0.5°C temperature maintenance accuracy, a level that is sufficient for ensuring the smooth progression of biological processes. The reactor is thermally insulated on all sides. Manual pH measurements are performed at regular intervals to ensure the desired pH level of 6.8–7.5 is maintained. If necessary, the addition of diluted sodium hydroxide (NaOH) in water is used to adjust the pH level.
The acquisition of data is meticulously orchestrated on a Raspberry Pi 4B microcomputer. The process was controlled using temperature, methane (CH4), composition, and gas volume sensors. The temperature readings are obtained from two digital temperature sensors: the Dallas Ds18b20, which has a temperature range of −55°C to +125°C and an accuracy of ±0.5°C. One of these sensors is employed to regulate the overheating of the heater, while the other is positioned centrally within the vessel to directly control the temperature of the biomass. An analogue sensor, designated as MQ4, was utilized to monitor the composition of the combustion gases, which were primarily methane (CH4), with a reading range of 300–10,000 ppm. The sensor exhibited a sensitivity of Rs(in_air)/Rs(in_5000ppmCH4) ≥ 5, indicating its capacity to detect variations in methane concentration with a resolution of at least 5 ppm. The volume of the generated gases was monitored using a Qianwei Kromschroder G-1.6 brand ultrasonic gas flowmeter, with a range of 0–10 L/min and an accuracy of ±1%. The entire plant, inclusive of the data acquisition system, is connected to a GETI PM001 electricity-metering meter, with an accuracy of ±1%.
Along the Latvian coast of the Baltic Sea, the red macroalga Furcellaria lumbricalis is manually collected in the fall, after the storm season, when large amounts of the alga are naturally washed ashore. This selective harvesting method leaves live underwater populations undisturbed and allows for the collection of large quantities of algal detritus without harming the ecosystem. The Baltic Sea Action Plan also encourages harvesting of beach-cast macroalgae as a sustainable practice to support marine environmental protection (HELCOM, 2023).
The collected biomass was carefully rinsed with freshwater to remove sand and debris. Then, it was air-dried to reduce moisture content and ensure stable storage prior to subsequent processing.
After these pre-treatment steps, the biomass was used in the anaerobic fermentation process. After fermentation, the resulting digestate was mechanically separated using a 2 × 2 mm stainless steel mesh. This filtration step effectively removed larger solid particles, yielding a clarified liquid fraction rich in dissolved nutrients and organic compounds that promote plant growth. The filtered liquid digestate was stored in closed containers at room temperature until further use. The principles of the EU Circular Economy Action Plan provide additional policy support for utilizing biogas digestate as a resource in agricultural circularity, closing nutrient loops and valorizing bio-based by-products (European Commission, 2023a,b). Hydrolysates derived from agricultural process residues, such as cauliflower and artichoke, have also shown biostimulant properties, presenting additional avenues for valorizing agri-waste streams (Salvo et al., 2024).
This clarified liquid digestate was employed in agronomic trials as both a direct soil amendment and a foliar spray in diluted form. Preliminary assessments suggest that this product could be a sustainable, eco-friendly alternative to synthetic fertilizers. Thus, products derived from the anaerobic fermentation of Furcellaria lumbricalis support renewable energy generation via biogas while advancing circular bioeconomy practices by providing valuable biostimulants for further agricultural testing.
3.2 Establishment of an algae biostimulant trial to evaluate crop yield and agronomic performance
In the third stage of the study, the Latvia University of Life Sciences and Technologies (LBTU) Institute of Soil and Plant Sciences conducted a covered-area trial of vegetation containers to evaluate the effectiveness of biostimulants in plant production and to obtain up-to-date data on their impact on plant productivity and soil microbial activity.
The greenhouse trial was established at the training and experimental greenhouse of the LBTU Institute of Soil and Plant Sciences from October 18 to December 19, 2024, encompassing a total of 63 days for plant cultivation. The objective of the trial was to evaluate the efficacy of a biostimulant by growing three common, fast-growing crops—lettuce (Lactuca sativa), garden radish (Raphanus raphanistrum subsp. sativus), and spinach (Spinacia oleracea)—on a neutralized peat substrate at pH 5.0. Radishes were cultivated in 5-L containers, with five plants per container. Lettuce and spinach were grown in 1-liter containers, with three replicates for each variant.
Five days prior to sowing, the substrate was transferred into containers and fertilized with urea, simple superphosphate, and potassium sulfate. In the variants at 100% fertilizer rate, 120 mg of nitrogen (N), 120 mg of phosphorus (P2O5), and 300 mg of potassium (K2O) were incorporated into the pure soil. The selection of these rates was informed by scientific recommendations that delineate optimal nutrient supply for the cultivation of leafy vegetables (Nollendorfs et al., 2023). The experimental treatments were meticulously designed for each crop, with three replicates allocated for each treatment. Three variants were selected as controls, not including biostimulant application, and incorporating varying rates of fertilizer application. In contrast, three variants incorporated biostimulant application at 3, 6, or 12% levels, as illustrated in Table 1. Working solutions of the biostimulant (3, 6, and 12%) were obtained from the concentrated biostimulant and deionized water. These solutions were prepared at concentrations of 3, 6, and 12%. The biostimulant solutions were applied to the test variants according to a specific schedule (Table 1) and volume (10 mL of solution per plant). The chemical composition of the biostimulant was subsequently analyzed in the laboratory of Environmental Audits Ltd.
The plant was treated with biostimulant five times during the growing period, with water occurring every 10 days. The biostimulant solution was used at an appropriate concentration for each plant, with a volume of 10 mL. During the growing season, lettuce and spinach were subjected to a 12-h light period, while radishes were exposed to an 8-h light period. This photoperiodic manipulation was implemented to enhance leaf and root biomass, respectively. The plants were watered regularly to ensure optimal substrate moisture.
The effectiveness of the biostimulant was assessed by evaluating the health of the plants. To do so, the relative chlorophyll content values in the leaves were determined using an atLEAF hand-held chlorophyll meter. This was done shortly before the plants were harvested, and three measurements were recorded per plant. In the lettuce and spinach variants, no measurements were recorded in the absence of fertilization (K0) or with a 25% reduced fertilization rate (K75), as the plants exhibited signs of nutrient deficiency and perished.
The microbial activity of the radish trial was ascertained by means of the cellulose degradation intensity method. The experiment utilized linen cloths, which were pre-cut and weighed to the nearest 0.001 g. These clothes were buried in the peat substrate to a depth of 10 cm at the commencement of each variant and replicate. The clothes were affixed to labeled slides with a stapler.
At the conclusion of the experiment, the linen clothes were meticulously removed, thoroughly cleaned, and dried at 60°C to a constant weight. The microbiological activity was calculated from the rate of degradation of the cloths by calculating the mass loss as a percentage of their original weight. The calculation Equation 1 is have to be:
Where:
• m0—initial mass of the linen cloth (g)
• m1—mass of the linen cloth at the end of the experiment (g)
The dry matter yield is calculated by harvesting and drying all parts of the plant at a temperature of 40°C until a constant weight is achieved.
The substrate medium reaction was determined in a suspension of distilled water and 1 M KCl, as per ISO 10390:2005, in duplicate (International Organization for Standardization (ISO), 2005). The potassium and phosphorus content of the substrate was determined in ashy, nitric acid-treated samples. The total potassium content of the extracts was determined by flame photometry, and the total phosphorus content was determined spectrophotometrically. To do so, the samples were treated with a reagent containing ammonium vanadate and ammonium molybdate, and readings were taken at 430 nm. Phosphorus and potassium analyses were carried out in triplicate.
To statistically assess the significance of the results, a one-factor analysis of variance (ANOVA) was employed to analyze the data and determine whether there were significant differences between the mean dry matter yields of the different treatments. To further compare the groups, a Tukey HSD (honestly significant difference) post-hoc analysis was performed, with a confidence level of 0.05. The objective of this study is to accurately identify which variant groups are statistically significantly different from each other. This will allow for the objective interpretation of the results of the experiment.
The methodology described herein enabled a structured and systematic evaluation of the effect of the biostimulant on growth and dry matter yield data of three different crops. Standardized growing conditions (i.e., a neutralized peat substrate, a controlled light regime, and controlled humidity) ensured comparable results between the variants. The utilization of varying concentrations of the biostimulant (3, 6, and 12%) in conjunction with the frequency of its application (five times at 10-day intervals) facilitated a precise analysis of the optimal dose for each plant under the prevailing growing conditions. The data obtained on chlorophyll content, dry matter yield, and macronutrient content of the substrate allow for a quantitative evaluation of the effectiveness of the biostimulant compared to the control. In the subsequent results section, the effects of the experimental variants on each crop will be described in detail.
4 Results
4.1 Effects of fertilizers and algal biostimulants
A cover crop trial was conducted to evaluate the effect of fertilizers and algae biostimulants on dry matter yields of different crops. The trial included radish, lettuce, and spinach. The experiment compared several treatments: control treatments without fertilizer (K0) and with reduced fertilizer rate (K75), optimal fertilizer treatment (K100), and treatments with 75% of the optimal fertilizer rate in combination with different algal biostimulant concentrations (3, 6, and 12%—M75_B3, M75_B6, M75_B12, respectively).
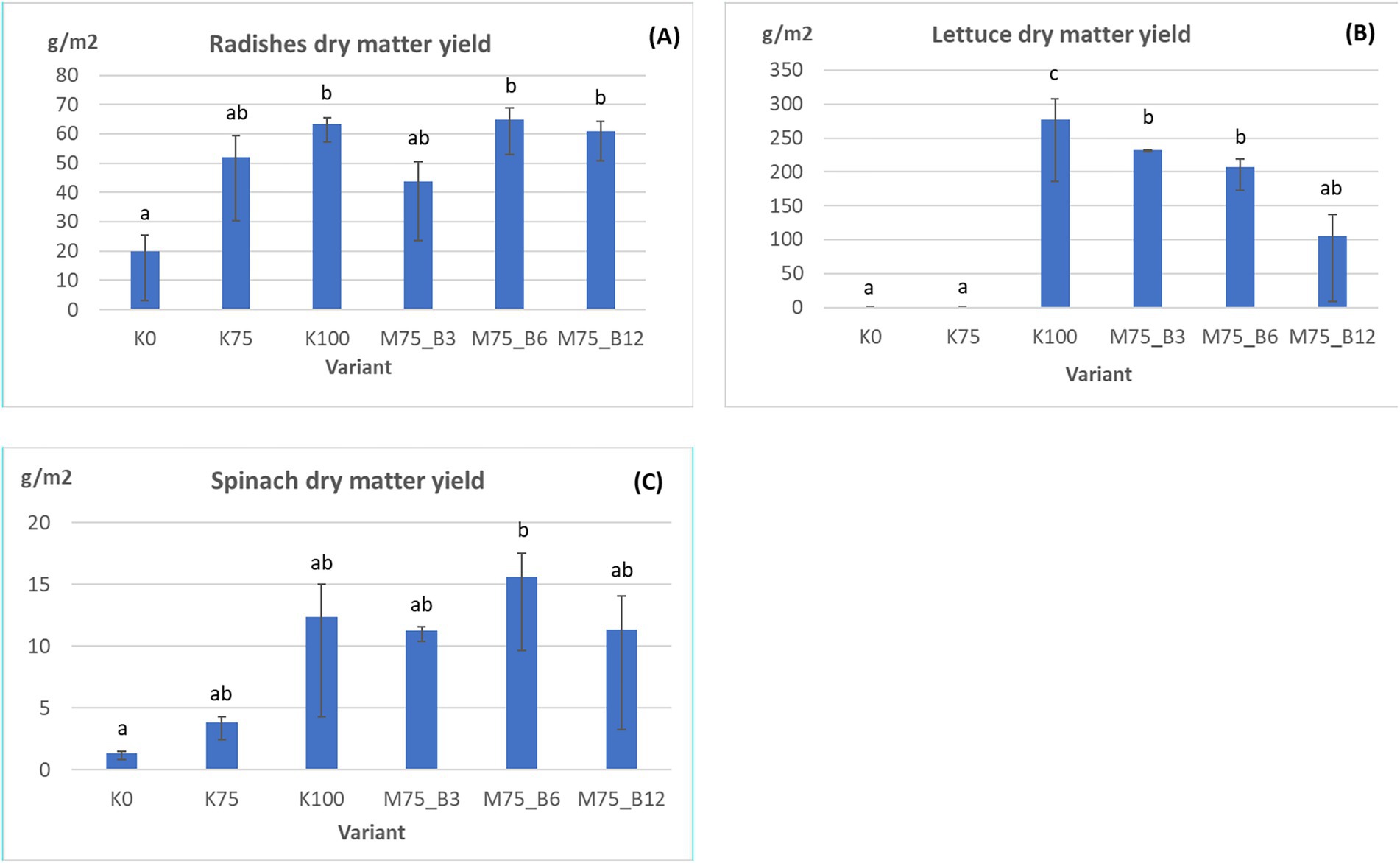
The figure below presents the dry matter yield results (g/m2) for each crop, contingent on the applied fertilizer and biostimulant variant (Figure 2).
One-way ANOVA: radishes F(5,12) = 3.69, p = 0.029; lettuce F(5,12) = 13.75, p = 0.00013; spinach F(5,12) = 3.2, p = 0.046. As demonstrated in Figure 2, across all crops, the control variants lacking fertilization (K0) and those with a reduced fertilization rate (K75) exhibited significantly lower dry matter yields in comparison to the variants receiving full fertilization (K100).
In the case of radish (A), the dry matter yield was approximately 20 g/m2 in the K0 variant and approximately 45 g/m2 in the K75 variant, while the optimum fertilization variant (K100) yielded approximately 65 g/m2. The M75_B6 variant (75% fertilizer + 6% algae biostimulant) exhibited yields comparable to or marginally higher than those of K100, suggesting that the biostimulant enhances plant productivity under reduced fertilizer rates.
In the lettuce trial (B), an inadequate supply of plant nutrients (variants K0 and K75) resulted in conditions that prevented lettuce development and yielded no yield. In K75, where there was a 25% nutrient deficiency, the lettuce ceased to develop after the first true leaves, as can be seen in Figure 3. In the vegetation, no full plants developed in containers with inadequate fertilizer. Only the first true leaves or complete plant death were visible.

Figure 3. Lettuce trial on day 63 after sowing (left to right: K0-K75-K100-M75_B3%-M75_B6%-M75_B 12%) diameter of vegetation containers 11.4 cm.
The highest dry matter yield, which was approximately 280 g/m2, was achieved at the optimum fertilization rate (K100). In contrast, the variants with 75% fertilizer rate and algae biostimulant (M75_B3 and M75_B6) yielded 84 and 74% of the yield of the K100 variant, respectively. As illustrated in Figure 2, the lettuce plants cultivated within these vegetation containers exhibited robust development, demonstrating increased leaf production and enhanced biomass accumulation in comparison to the K0 and K75 variants.
However, increasing the biostimulant concentration to 12% (M75_B12) led to a substantial decline in yields, reaching approximately 100 g/m2. This reduction was not statistically different from the control group, which did not receive fertilizer or had reduced fertilization rates. These results demonstrate the capacity of algal biostimulants to counteract partial nutrient deficiencies and to stimulate plant development under conditions of nutrient deprivation.
A one-factor analysis of variance (ANOVA) in the lettuce trial demonstrated statistically significant differences between groups (Fkrit = 3.10, p = 0.002 < 0.05). A Tukey HSD analysis confirmed that the M75_B3 and M75_B6 variants exhibited high dry matter yields and were statistically significantly different from the control variants, K0 and K75. These results underscore the notion that the incorporation of algal biostimulants can serve as a partial compensatory mechanism for nutrient deficiencies, thereby promoting lettuce development. In contrast, a complete or partial nutrient deficiency in the absence of biostimulant application results in yield loss or the development of plants that are significantly underdeveloped (see Figure 3).
In the spinach trial (C), the K0 and K75 variants yielded approximately 1 g/m2 and 4 g/m2 DM, respectively, which is significantly lower than the K100 variant (approximately 13 g/m2). The M75_B6 variant (75% fertilizer + 6% algae biostimulant) demonstrated the highest yield of approximately 16 g/m2, surpassing the yield of the full fertilizer variant (K100). The M75_B3 and M75_B12 variants also exhibited higher yields than the control variants; however, the 12% biostimulant rate (M75_B12) did not yield statistically significantly higher yields than K75 and K0.
In other trials (spinach, radish) that incorporated algal biostimulant, its efficacy in addressing plant nutrient deficiencies was substantiated. In certain instances, the effectiveness of algal biostimulant surpassed that of the variant containing 75% of the plant nutrients (K75), with an increase of 25% in the radish trial and 26% in the spinach trial. A one-way analysis of variance (ANOVA) was conducted, revealing statistically significant differences between groups in both the spinach and radish variants, with p-values at the confidence level of 0.05. The p-values for the spinach and radish variants were 0.029 and 0.045, respectively. However, the Tukey test revealed that the M75_B6 variant was significantly different from the control (K0) in the spinach trial. While other biostimulants variants containing the biostimulant, as well as the variant with complete fertilizer (K100), tended to produce higher yields in the spinach trial against the K75 variant, were not statistically significant compering to the control variants (K and K75). Statistically significant yield increases in the radish trial relative to the control were found for the fully fertilized variant (K100) and the variant with 6% algal biostimulant application (M75_B6) (Figures 2A,C).
The findings indicate that the utilization of algal biostimulants, particularly at a concentration of 6%, can effectively mitigate partial nutrient deficiencies and enhance dry matter yields of crops. Conversely, an excessively high dose of biostimulant (12%) does not yield additional benefits and may even result in a reduction in yields.
In the lettuce trial, however, the highest dry matter yields, which were statistically significant, were obtained with the full plant nutrient supply (K100) at 277 g/m2 and with the variant with 75% plant nutrient supply and 3% algal biostimulant application at 232 g/m2, respectively. Analogous observations were made in the radish trial, with the salient difference being the concentration of the biostimulant. The highest dry matter yields were achieved with 6% algal biostimulant at a rate of 65 g/m2. The analysis revealed that the incorporation of 6% algal biostimulant (M75_B6) at 16 g/m2 yielded the highest dry matter yields, while all other treatments exhibited significantly lower yields (Figure 2).
These results are consistent with the findings of other studies that have demonstrated the positive effects of algal biostimulants on the development of various crops.
4.2 Agronomic efficiency of fertilizers
In the context of agricultural sustainability, the efficient use of fertilizers is imperative to ensure high yields while mitigating environmental pressures. Agronomic efficiency (AE) analysis facilitates the evaluation of how effectively diverse fertilization strategies contribute to yield growth, considering the amount of fertilizer applied. In this subsection, the data presented in Figure 4 is used to provide a detailed analysis of AE for three crops: lettuce (A), spinach (B), and radish (C). The results for each crop are highlighted, and the overall trend is also discussed. The agronomic efficiency (AE) of fertilizers [IV2] was calculated from the average values using Equation (2).
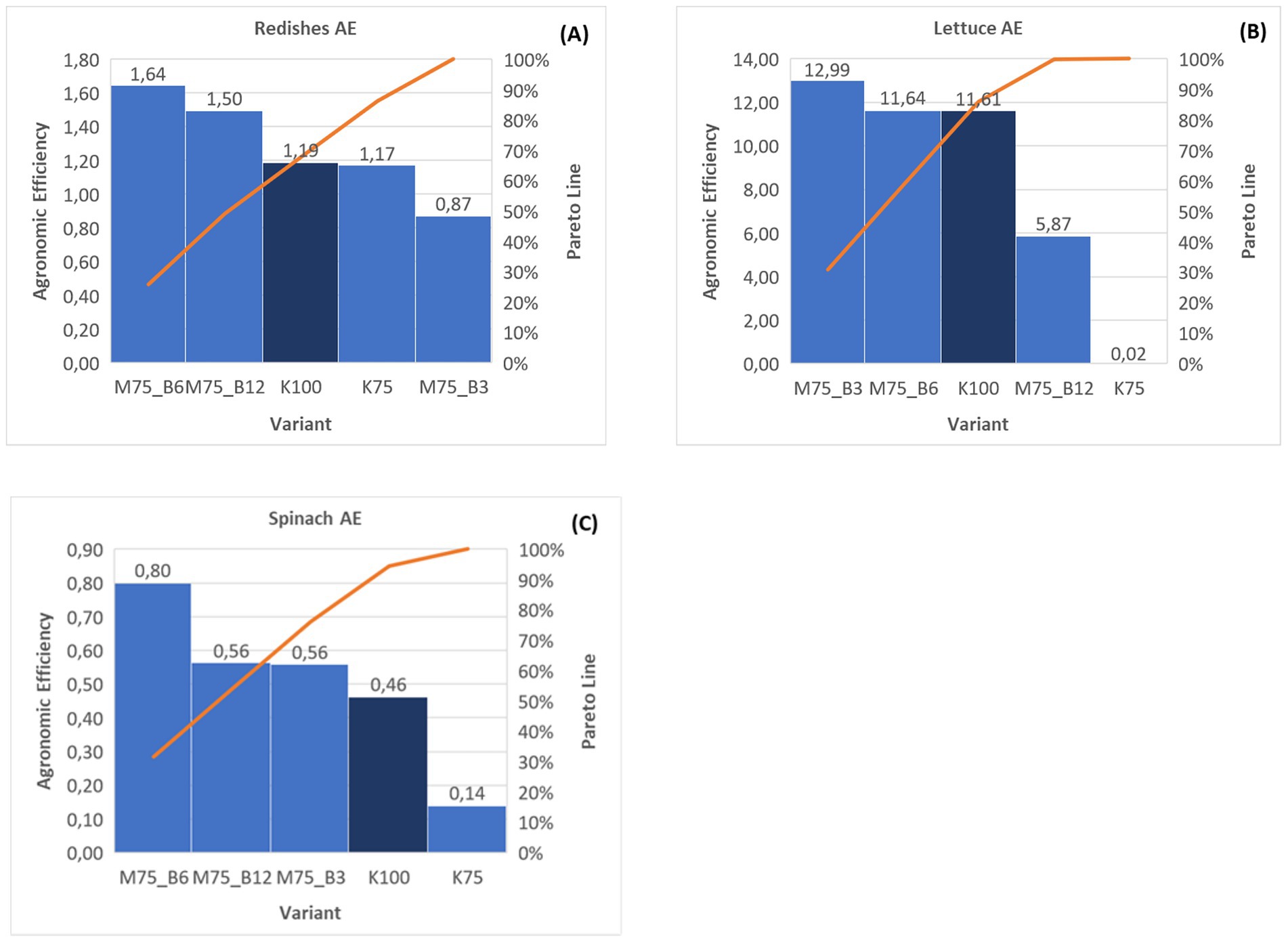
Figure 4. Agronomic efficiency of the experimental variants: radishes (A), lettuce (B), and spinach (C).
Where:
• YF—Yield with fertilization, g/m2
• YC—Control yield without fertilization, g/m2
• AF—Applied fertilizers in total, g/m2
In the lettuce trial, the algae biostimulant at a 6% concentration was able to compensate for a 25% fertilizer deficiency (M75_B6), as the 75% fertilizer rate achieved an AE equivalent to the fully fertilized version (K100) (Figure 4B). In contrast, the variant where 25% of the mineral fertilizer was replaced by 3% biostimulant (M75_B3) exhibited an AE that was almost 12% higher than the variant that was fully fertilized (Figure 4B). Furthermore, in the spinach trial, the utilization of 3% algal biostimulant in the reduced fertilizer variant (M75_B3) exhibited even higher AE, surpassing the full fertilizer variant by 20.74%. A comparable enhancement in yield was observed with the application of a fertilizer with a 12% biostimulant concentration (M75_B12), where AE exhibited superior performance to the full fertilizer option (K100) by 21.73% (Figure 4C). However, the highest agronomic efficiency was achieved for the application of 6% biostimulant solution (M75_B6), as AE was found to be 73% higher in this variant than in the fully fertilized variant (K100) (Figure 4C). Conversely, in the radish trial, the application of 3% biostimulant resulted in negative agronomic efficiency, as the AE was 26.57% lower in this concentration compared to the fully fertilized variant. Conversely, an augmented yield was observed in response to the application of algal biostimulant, with a 6% or 12% replacement of mineral fertilizer, as compared to the control group (Figure 4A). This enhancement in agronomic efficiency was found to be 38.31 and 25.86%, respectively, signifying a substantial improvement in agricultural productivity.
A comprehensive evaluation of the available data suggests that specific concentrations of biostimulants may offer enhanced efficiency in nutrient uptake and increased crop yield, surpassing the performance of 75% fertilization treatments. In accordance with the Pareto principle, 20–40% of the examined samples yielded 80–90% of the total AE. Therefore, it is feasible to select the most effective fertilizer combinations, wherein the concentration of algal biostimulant can range from 3 to 12% according to the specific crop (Figure 4).
This approach has been demonstrated to result in a 25% reduction in fertilizer consumption without compromising yield gains. In certain instances, AE has been observed to outperform the full-rate option. These results underscore the practical importance of biostimulants for the development of sustainable and environmentally friendly agriculture.
Given that the agronomic efficiency (AE) exceeds that of the fully fertilized variant in some instances, it is also imperative to assess the economic benefits of fertilizer application. In order to determine this objectively, it is imperative to calculate the economic efficiency of fertilizers using a gross margin (GM) calculation.
The calculations are based on the gross salad cover data for 2024 of the Latvian Rural Advisory and Training Centre (LLKC). The experimental variant (M75_B6) is distinguished by its 25% reduction in fertilizer costs, with the additional cost of the biostimulant factored into this calculation. The cost items about labor, packaging, peat, and so forth, remain unaltered due to the fact that the application of the biostimulant does not affect these parameters.
As indicated in Table 2, the implementation of biostimulant in lettuce production (variant M75_B6) has been demonstrated to enhance gross margins from €357.61 to €517.00 per 1,000 m2 in comparison to conventional technologies (Latvian Rural Advisory and Training Centre, 2024). Consequently, the gross margin is expected to rise by approximately 45%. This outcome indicates that the utilization of a biostimulant in lettuce cultivation enables a substantial enhancement in economic efficiency, even in the absence of changes in yield and associated costs (labor, machinery, packaging, etc.). The observed increase can be attributed primarily to a 25% reduction in fertilizer costs, which serves to offset the added expense associated with the biostimulant. Consequently, the utilization of scientifically validated biostimulants in crop production technology has the potential to generate substantial economic benefits, constituting a compelling rationale for both practical and scientific agricultural advancement.

Table 2. Economic efficiency of lettuce production with and without biostimulant application (per 1,000 m2).
The results of this study demonstrate that the physiological characteristics of each species—namely, metabolic rate, nutrient uptake mechanisms, stress tolerance, and growth pattern—exert a significant influence on the optimal biostimulant concentration. This conclusion is supported by the crop-specific response to biostimulant concentrations. For instance, lettuce exhibits a high degree of sensitivity to nutrient deficiencies and demonstrates the capacity to respond to lower concentrations of biostimulant, which may be attributable to the crop’s unique physiological processes and its rapid growth rate. Therefore, it can be posited that the effectiveness of the biostimulant concentration is determined by the physiological characteristics of the crop. This hypothesis warrants further investigation.
The results of this particular trial reveal the effects of the fertilizer and algae biostimulant on plant and soil health, as measured by the relative chlorophyll content of plant leaves and the microbiological activity of the peat substrate.
In the radish variant, the relative chlorophyll measurements were statistically significantly different only in the control variant without fertilizer (K0), where it reached 29.8 atLEAF units. In contrast, in the other control variants and the variants with algae biostimulant, the measurements ranged from 45.5 to 48.1, showing no statistically significant differences. However, as previously mentioned, atLEAF measurements could not be made in the K0 and K75 treatments of the leafy vegetable trials. The plants in these treatments died due to nutrient deficiencies. However, statistically significant differences according to the Tukey test for spinach were identified in the M75_B12 group, where the relative chlorophyll averages were lower than for spinach. The values reached 38.5 and were lower than in the other groups, where they ranged from 49.8 to 51.7. In the experimental lettuce trial, the group that received the 12% algae biostimulant exhibited an atLEAF value of 31.3, which was statistically significant and lower than the full fertilization (K100) and the 3 and 6% concentration biostimulant applications (M75_B3 and M75_B6), which ranged from 34.1 to 39.5.
The lowest microbial activity was also observed in control without fertilizer and algae biostimulant addition (K0), indicating an absence of nutrients and metabolism-stimulating compounds in the peat substrate. In the control variant (K0), microbial activity exhibited an average of 8.18%, while in the fertilized variants without biostimulant addition, microbial activity demonstrated an average of 8.18%. The values of microbiological activity ranged from 43.25% (K75) to 31.22% (K100). In the samples with a 25% reduced fertilization rate and an added algal biostimulant, respectively, M75_B3 22.67%; M75_B6 44.17%; M75_B12 36.88%. The findings indicate that the application of mineral fertilizer and algal biostimulant significantly enhances microbial activity in peat substrate. Conversely, the absence of these substances substantially curtails microbial activity. In addition, the Tukey HSD test revealed that only the variant with 6% algal biostimulant application (M75_B6) was statistically significantly different from the control (K0), indicating a specific stimulatory effect of this dose. The results of average microbiological activity in each experimental variant, as well as the corresponding statistical groupings based on the Tukey HSD test, are presented in Table 3.

Table 3. Average values of microbiological activity (%) in the radishes trial and grouping by Tukey HSD test.
5 Discussion
The findings of the experiment demonstrate that the implementation of an algal digestate biostimulant (particularly at a concentration of 6%) under conditions of reduced fertilization significantly enhances the dry matter yield of radishes, lettuce, and spinach, in some cases surpassing the yield of full fertilization options. While microbial-based biostimulants show substantial promise, potential risks and their interaction with soil microbiota must be balanced against agronomic benefits (Kumari et al., 2023). A subsequent statistical analysis (ANOVA, Tukey HSD) confirmed significant differences between the variants, especially under reduced fertilization conditions. These results are consistent with the findings of other studies that have demonstrated the efficacy of biostimulants in various crops.
5.1 Synergy and agronomic efficiency of biostimulant mechanisms
The efficacy of algal biostimulants in the context of fertilizer reduction can be attributed to their distinctive array of bioactive compounds. Recent studies have shown that these compounds, including glycomolecules and polysaccharides, play crucial roles in plant biostimulation and stress tolerance (Boulogne et al., 2024; Mughunth et al., 2024). Furthermore, microbial-based biostimulants have demonstrated significant potential in improving soil health and crop resilience (Samantaray et al., 2024). This intricate regulatory network encompasses multiple levels, from molecular signals within the plant to the stimulation of the soil microbiome and the mobilization of nutrients. Collectively, these elements contribute to plant growth and productivity, even in conditions of reduced fertilization inputs. According to Skapste et al. (2025), phytohormones such as auxins and cytokines, as well as organic acids such as alginic and fulvic acids, promote root morphogenesis, thereby increasing nutrient uptake efficiency by 18–25% even at low biostimulant concentrations (3–6%). This stimulation is of particular significance because the development of the root system is pivotal in determining the capacity of plants to absorb water and nutrients, especially under conditions of limited fertilization. This effect is further enhanced by the capacity of polysaccharides to form complexes with soil trace elements, thereby enhancing their availability to plants.
Research on Furcellaria lumbricalis has demonstrated that a 6% biostimulant dosage enhances phosphorus availability in the substrate by 34%, which is a crucial factor in promoting plant growth (Skapste et al., 2025). This aligns with recent findings showing that microalgae-based biostimulants can improve nutrient uptake efficiency by 15–25% while simultaneously enhancing plant stress tolerance (Nur et al., 2024; Francioso et al., 2024). Phosphorus is one of the primary macronutrients that impede plant development, and its increased availability has been demonstrated to enhance crop yields. Furthermore, algal extracts have been shown to stimulate epigenetic adaptation in plants by activating DNA methylation processes in gene promoter regions associated with abiotic stress tolerance, such as under drought and salinity conditions (El-Beltagi et al., 2022). This epigenetic regulation enables plants to adapt to adverse conditions over time, a process that is particularly important in the context of a changing climate.
The effects of algal biostimulants extend beyond direct stimulation of plant growth, encompassing intricate interactions with soil microorganisms.
This synergy has been demonstrated to promote nutrient mobilization and improve soil health, which has been shown to lead to an overall increase in plant productivity. A recent study by Alharbi et al. (2022) suggests that halotolerant PGPR bacteria, such as Pseudomonas putida and Bacillus subtilis, when utilized in conjunction with algal polysaccharides, can enhance phosphorus availability by up to 40% and increase nitrogen uptake efficiency by 22%. This synergy is of paramount importance because it enables the reduction of fertilization inputs without compromising yields, which can even be enhanced. Oligosaccharides containing algal extracts have been demonstrated to act as prebiotics by stimulating the colonization of such bacteria in the rhizosphere and enhancing microbiome activity (Di Sario et al., 2025; Samantaray et al., 2024). Furthermore, in trials conducted on Pisum sativum, the amalgamation of algal biostimulants with PGPR has been demonstrated to augment root surface area by 35%, thereby facilitating enhanced water and mineral uptake, thus augmenting plant stress tolerance and yield (Khan et al., 2024). The interaction between biostimulants and the microbiome is imperative for the development of sustainable agriculture, as it reduces dependence on synthetic fertilizers and promotes soil viability.
It should be noted that the cellulose degradation method used in this study is incomplete and only provides a general overview of microbial activity. Further research should supplement this method with 16S rRNA and other appropriate methods to provide a more complete picture of the microbiological processes taking place in the soil.
5.2 Epigenetic adaptation and abiotic stress tolerance
The impact of algal biostimulants on plant genetic expression constitutes a primary mechanism contributing to their efficacy. Body research on Triticum durum has demonstrated that algal extracts trigger H3K4me3 modifications in the gene promoter regions implicated in chlorophyll biosynthesis and antioxidant production. These regions include, but are not limited to, superoxide dismutase (SOD) and catalase (CAT) (Kumar et al., 2023). These epigenetic changes persist for a period of at least 60 days after treatment, thereby enabling plants to adapt to drought and salinity stress over an extended timeframe. Furthermore, algal extracts have been demonstrated to modulate reactive oxygen species (ROS) signaling, reducing lipid peroxidation and increasing antioxidant levels, thereby protecting cells from oxidative stress (Hasanuzzaman et al., 2021). For perennial crops such as grapevine and brown algae biostimulants have demonstrated improvements in physiological vitality and yield under various field conditions (Sirbu et al., 2023).
5.3 Concentration optimization and crop-specific response
The experimental data demonstrate that the effectiveness of algal biostimulants is concentration-dependent, forming a U-shaped dose–response curve. Mughunth et al. (2024) developed a dose–response curve, which indicated that optimum efficacy is achieved in the 5–10% concentration range. However, lower or higher doses did not significantly improve the yield. For lettuce, the optimal biostimulant concentration was determined to be 3%, which resulted in an 84% increase in yield compared to full fertilization. In contrast, for spinach and radish, the highest yield was achieved with a 6% biostimulant dose. These disparities may be associated with the physiological characteristics of the crops and the morphology of the root systems. For instance, superficial rhizosphere stimulation is sufficient for aboveground vegetables, while root crops require deeper nutrient mobilization (Vangenechten et al., 2025). The application of biostimulants containing seaweed extracts and amino acids has also significantly improved the biochemical parameters and economic returns in legume cultivation systems, as demonstrated in recent studies (Kocira et al., 2020). Furthermore, Ronga et al. (2019) emphasizes the selective impact of microalgae biostimulants on diverse crops, a factor that must be taken into account when formulating biostimulant application strategies.
5.4 Synergy with soil organic matter and structure
The positive effect of algal biostimulants on soil structure and organic matter dynamics is essential for their agronomic efficiency. Calvo et al. (2014) posits that alginic and fulvic acids form complexes with soil clay particles, thereby increasing the cation exchange capacity (CEC) by 15–20%. This phenomenon is especially evident in sandy soils, where the presence of low levels of organic matter and a comparatively fragile soil structure can amplify its impact. Furthermore, algal polysaccharides have been demonstrated to contribute to the stabilization of soil aggregates, thereby reducing erosion risk by 30% and enhancing water retention by 25%. These effects are critical for achieving water efficiency and soil sustainability (Boulogne et al., 2024; Chabili et al., 2024). This enhancement in soil health has been demonstrated to contribute to long-term yield stability and to reduce the necessity for synthetic fertilizers.
5.5 Economic and environmental efficiency
The incorporation of algae biostimulants into agricultural practices has been demonstrated to yield several benefits. Recent research confirms that these products can reduce fertilizer use by 20–25% while maintaining or improving crop yields (He et al., 2024; Pascoalino et al., 2025). Additionally, the implementation of integrated nutrient management systems incorporating biostimulants shows promising results for sustainable agriculture (Shahrajabian et al., 2021). According to the EIP-AGRI (2023) report, the application of a 6% biostimulant in conjunction with a 75% fertilization application results in yields equivalent to those achieved through full fertilization. However, this approach entails substantially reduced expenditure, with potential savings of up to 40%. Furthermore, the carbon sequestration potential experiences an augmentation of 2.3 t/ha/year, a development that contributes to the fulfillment of the EU Green Deal objectives concerning climate change mitigation (Eurostat, 2023a,b). The economic and environmental benefits of algal biostimulants render them a compelling option for farmers and policymakers seeking to establish a sustainable food system. In summary, algal biostimulants function through a multi-step process that combines molecular, microbiological, and soil physio-chemical mechanisms. Achieving maximum effectiveness necessitates the adaptation of concentration to the particular characteristics of the crop and soil type, in conjunction with an integrated approach that integrates the use of biostimulants with precision agriculture technologies and soil health monitoring. A comprehensive strategy is imperative to ensure a sustainable production system characterized by enhanced productivity and a substantial reduction in environmental impact.
The incorporation of algal biostimulants into agricultural practices has been demonstrated to enhance both crop yield and soil health, thereby contributing to the establishment of a sustainable food value chain. As previously stated in the introduction, the European food chain is confronted with a series of challenges, including its reliance on external resources for fertilization and energy, the impact of climate variability, and the disruption of global markets (Eurostat, 2023a,b, pp. 13–20, 40). The utilization of biostimulants has been demonstrated to curtail these dependencies, enhance productivity, and augment resilience to external shocks. These outcomes are imperative for the long-term stability of food security. This approach aligns with the objectives of the European Green Deal and the Farm to Fork strategy, promoting local, short food chains and value addition in agricultural production (European Commission, 2022; Skapste et al., 2025).
Using algal biostimulants in agriculture has been shown to provide several significant benefits. According to recent Baltic joint initiatives, systemic innovation and knowledge transfer are crucial for ensuring long-term sustainability in the food sector (FofT, 2023). Chief among these benefits is the ability to increase crop yields with minimal reliance on imported fertilizers. Additionally, this approach has been shown to contribute to expanding the value chain by increasing domestic production capacity. This is particularly relevant in the Baltic Sea region, where an increasing reliance on imported raw materials and energy has made the food system more fragile (Eurostat, 2023a,b, pp. 59, 65). Using biostimulants in agriculture promotes local production and contributes to developing short food chains. The broad-scale adoption of these practices is promoted through international programs, such as Interreg Baltic Sea Region’s “For Sustainable Food,” which aims to promote cross-sector cooperation and innovation diffusion (Interreg Baltic Sea Region, 2024). This approach aligns with one of the European Union’s primary sustainability objectives: promoting rural development (European Commission, 2022). Using biostimulants to promote short food chains has been shown to reduce intermediaries, enhance product quality, and increase local consumer confidence in agricultural products. This approach aligns with the objectives of the European Green Deal and the Farm to Fork Strategy, which emphasize the importance of local production and establishing sustainable food systems (European Commission, 2022; Skapste et al., 2025).
Alongside these qualitative improvements, a growing body of evidence highlights the quantifiable environmental benefits of using biostimulants. In line with this study’s finding of a 25% reduction in fertilizer use, quantifying the environmental impact of biostimulant application allows us to evaluate its true potential for mitigating greenhouse gases. Using internationally recognized emissions factors, this technology demonstrates significant environmental benefits. The global carbon intensity of synthetic nitrogen fertilizers can reach 10.5 kg CO2e per kg N, including production, transportation, and field-based N2O emissions (Menegat et al., 2022). In the European context, this value is somewhat lower at 9.2 kg CO2e per kg N, reflecting regional production conditions. When these coefficients are applied to the experimental setup, the treatment involving 75% of the standard fertilizer rate combined with 6% algal biostimulant is estimated to reduce emissions by approximately 389 kg CO2e ha−1 yr−1. Compared to the typical greenhouse vegetable production footprint in the EU (~7 t CO2e ha−1 yr−1), this strategy achieves a 5–6% system-wide emission reduction through a single agronomic adjustment (Liu et al., 2020). Other studies have reported reductions in greenhouse gas emissions ranging from 12 to 66% when mineral nitrogen fertilizers are partially replaced with biostimulants in various cropping systems (Sharma et al., 2024). From an economic perspective, using the average EU ETS carbon price of ~€90/t, this emission reduction has an environmental value of around €35/ha, which, together with fertilizer savings from a 25% reduction, provides a total economic benefit of €203/ha (Yang et al., 2020).
Furthermore, the environmental benefits of using algal biostimulants extend beyond reducing CO2 emissions and encompass a wider set of environmental indicators. In terms of eutrophication potential, lower nitrogen fertilizer input results in savings of 0.1–2.5 kg PO4-eq ha−1, significantly improving water quality and reducing the risk of acidification by 0.5–3.2 kg SO2-eq ha−1 (Renganathan et al., 2024). Improvements in microbial activity of up to 44% compared to the control group reflect strengthened soil health and greater biological nutrient availability. Biostimulant use improves resource efficiency by reducing water consumption by 200–800 L per kg of product and enhancing soil water holding capacity (He et al., 2024). Monte Carlo analysis suggests a range of emission reductions of 320–480 kg CO2e per hectare, allowing for a conservative estimation of the technology’s environmental benefits. Additional improvements in soil structure can reduce erosion risk by 30% and increase water retention by 25%. This is particularly significant in sandy soils with low organic matter content (Boulogne et al., 2024). These broader environmental indicators demonstrate that algal biostimulant application provides a range of multifunctional environmental benefits, supporting the goals of the EU Green Deal and “Farm to Fork” strategy for sustainable agricultural development (Chabili et al., 2024).
The utilization of biostimulants has the potential to prolong the value-added chain, thereby reducing the export of raw or low-value raw materials and enhancing the competitiveness of local firms. This approach fosters economic viability and ensures the long-term sustainability of the food system (Skapste et al., 2025; Eurostat, 2023a,b). The utilization of algae biostimulants constitutes a cross-sectoral innovation that integrates agriculture, science, and technology to foster a sustainable food value chain. These innovations are imperative tools for overcoming the challenges to agricultural productivity and resilience posed by climate change and global food market shocks (European Commission, 2022; Khan et al., 2024).
The utilization of algal biostimulants in agriculture has been demonstrated to be an effective strategy for reducing production costs, enhancing yields and quality, and contributing to the development of a sustainable food system (EIP-AGRI, 2023; European Commission, 2022). This approach aligns with European and global sustainability objectives by reducing environmental pressures and increasing resilience to external shocks (Eurostat, 2023a,b; Khan et al., 2024). Consequently, algal biostimulants emerge as a economically and environmentally viable option that contributes to long-term sustainability and competitiveness along the food chain.
The findings indicate that applying a 12% algal biostimulant with a 25% reduced fertilization rate to leafy vegetables resulted in decreased relative chlorophyll content. A high concentration of biostimulant can overwhelm a plant’s metabolic processes, which inhibits photosynthesis and reduces chlorophyll content (Rouphael et al., 2018). Excessive biostimulant application could theoretically diminish the availability of essential nutrients required for chlorophyll synthesis and plant growth. Therefore, selecting the optimal biostimulant concentration is crucial for adapting it to specific crops and fertilization regimens to ensure maximum efficacy and improved yield quality.
In this study, reducing the biostimulant concentration to 12% was observed to reduce plant growth and development, indicating a nonlinear concentration-response relationship. This underscores the importance of optimizing biostimulant doses to prevent phytotoxicity. This is particularly relevant under the European Union Fertilizing Products Regulation (EU) 2019/1009, which requires the experimental validation of the effectiveness and usage of biostimulants under defined conditions for specific plant species (El Boukhari et al., 2020). As Ricci et al. (2019) noted, this involves precise definitions of dosage, application methods, and assessment protocols.
Several studies have also addressed the impact of seaweed extract concentrations on seed germination and plant growth. For instance, Silva et al. (2019) found that higher biostimulant concentrations inhibited seed germination, and Sathya et al. (2010) showed that increased biostimulant concentrations reduced plant growth parameters. Overall, these findings underscore the importance of carefully selecting biostimulant concentrations tailored to specific crops and growing conditions to optimize results. Scientific studies have demonstrated that mineral fertilizers provide essential nutrients for microorganisms, while algal biostimulants contain phytohormones, amino acids, and organic substances that stimulate microbial activity in soils (Adedayo et al., 2023). This interaction elucidates the reason why the variants with fertilizer and biostimulant exhibit significantly higher microbial activity than the control variant, with the variant containing a 6% algal biostimulant dose (M75_B6) being particularly statistically significant when compared to the control group.
6 Conclusion
The objective of the study has been fully achieved. The effectiveness of algal digestate biostimulant in agriculture has been empirically evaluated through a comprehensive assessment of its impact on crop productivity, soil health, and chemical fertilizer consumption. The findings indicate that the utilization of the biostimulant contributes to the sustainable development of agriculture, aligning with the objectives of the European Green Deal, the Farm to Fork strategy, and recent EU policy and scientific initiatives.
European food systems are confronted with significant challenges, including a 24.8% decrease in the number of farms between 2010 and 2020, 33.2% of farm managers being over the age of 65, a high reliance on synthetic fertilizers and energy sources, and the agricultural sector contributing 10.7% of EU greenhouse gas emissions. Agriculture constitutes a segment of the food system; nevertheless, its sustainability is imperative for the system in its entirety.
The algal biostimulant was prepared according to a rigorous protocol, ensuring a high quality and standardized formulation that was utilized in the experimental trials. The biostimulant was administered at concentrations of 3, 6, and 12% in a five-time application during the growing season, with each application involving the use of 10 mL of solution per plant.
In the radish trial, the dry matter yield of the control version without fertilizer (K0) was approximately 20 g/m2 that of the reduced fertilizer version (K75) was approximately 45 g/m2, and that of the full fertilizer version (K100) was approximately 65 g/m2. The variant with 75% fertilizer and 6% algal biostimulant (M75_B6) achieved yields equivalent to or even slightly higher than the fully fertilized variant (K100), indicating the positive effect of the biostimulant on yield.
In the lettuce trial, the full-rate version (K100) yielded the highest dry matter yield of approximately 280 g/m2. The variants with a 75% fertilizer rate and algae biostimulant (M75_B3 and M75_B6) achieved 84 and 74% of the yield of the K100 variant (approximately 235 g/m2 and 207 g/m2, respectively). An increase in the biostimulant concentration to 12% (M75_B12) led to a substantial reduction in the yield, reaching approximately 100 g/m2.
In the spinach trial, the control variants without fertilizer (K0) and with reduced fertilizer (K75) yielded approximately 1 g/m2 and 4 g/m2, respectively, while the full fertilizer variant (K100) yielded approximately 13 g/m2. The variant with 75% fertilizer and 6% algae biostimulant (M75_B6) demonstrated the highest yield of approximately 16 g/m2, surpassing the yield of the K100 variant.
Preliminary findings, as indicated by statistical analysis (one-factor ANOVA), have demonstrated statistically significant discrepancies between treatments in the lettuce, spinach, and radish trials (p < 0.05). The Tukey HSD test demonstrated that the variants treated with the biostimulant exhibited statistical significance in comparison to the control variants, particularly under conditions of reduced fertilization.
The agronomic efficiency calculation indicates that the use of algal biostimulants at the concentrations studied allows for a 25% reduction in fertilizer application rate and more efficient nutrient uptake, as well as an increase in dry matter yield.
An experimental investigation was conducted to ascertain the economic implications of employing the biostimulant M75_B6 in lettuce production. The findings revealed a substantial economic impact, evidenced by a 45% augmentation in gross margin per 1,000 m2 and a 25% diminution in fertilizer expenditures, while maintaining consistent yield levels. This outcome demonstrates the feasibility of integrating the technology into conventional production schemes and represents a new economic paradigm in the context of resource efficiency.
The development of a sustainable food system necessitates an integrated approach that enhances productivity, mitigates environmental impacts, and guarantees economic viability along the food chain, from farm to consumer. The incorporation of algal biostimulants emerges as a pivotal strategy to attain this objective.
Based on the obtained data, field trials involving a wider range of crop species are recommended. These trials should assess the effect of the biostimulant on plant biomass formation and evaluate the effect on the formation of above-ground, below-ground, and economically important biomass (yield) separately, in terms of quantity and quality. Examples of these species include wheat (Triticum aestivum), potatoes (Solanum tuberosum), and cabbage (Brassica oleracea var. capitata). The data show that the effectiveness of the biostimulant and the most appropriate concentration depend on the plant species. Therefore, the trial variants should continue with all the biostimulant concentrations studied. Future trials should also vary the methods of biostimulant treatment, incorporating them not only into the soil but also spraying them onto plant leaves and treating seeds or planting material.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
IS: Formal analysis, Methodology, Project administration, Investigation, Validation, Writing – review & editing, Software, Data curation, Visualization, Resources, Conceptualization, Writing – original draft. IV: Writing – review & editing, Project administration, Resources, Validation, Formal analysis, Software, Writing – original draft, Data curation, Investigation, Methodology, Visualization, Conceptualization. KS: Data curation, Investigation, Software, Resources, Writing – review & editing, Visualization, Formal analysis. UŽ: Methodology, Writing – review & editing. GG-Z: Writing – review & editing, Funding acquisition, Supervision. AZ: Methodology, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Strengthening the Institutional Capacity of LBTU for Excellence in Studies and Research, funded by The Recovery and Resilience Facility (ANM1) Project No. 5.2.1.1.i.0/2/24/I/CFLA/002.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adedayo, A. A., and Babalola, O. O. (2023). The potential of biostimulants on soil microbial community. Front. Ind. Microbiol. 2:1308641. doi: 10.3389/finmi.2023.1308641
Alharbi, K., Amin, M. A., Ismail, M. A., Ibrahim, M. T. S., Hassan, S. E.-D., Fouda, A., et al. (2022). Alleviate the Drought Stress on Triticum aestivum L. Using the Algal Extracts of Sargassum latifolium and Corallina elongate Versus the Commercial Algal Products. Life, 12, 1757. doi: 10.3390/life12111757
Ali, A., Niu, G., Masabni, J., Ferrante, A., and Cocetta, G. (2024). Integrated nutrient management of fruits, vegetables, and crops through the use of biostimulants, soilless cultivation, and traditional and modern approaches—a mini review. Agriculture 14:1330. doi: 10.3390/agriculture14081330
Bajpai, S., Sharma, P., Kumar, A., and Singh, R. (2024). Editorial: development of next generation bio stimulants for sustainable agriculture. Front. Plant Sci. 15:1383749. doi: 10.3389/fpls.2024.1383749
Boulogne, I., Mirande-Ney, C., Bernard, S., Bardor, M., Mollet, J. C., Lerouge, P., et al. (2024). Glycomolecules: from "sweet immunity" to "sweet biostimulation"? Physiol. Plant. 176:e14640. doi: 10.1111/ppl.14640
Calvo, P., Nelson, L., and Kloepper, J. W. (2014). Agricultural uses of plant biostimulants. Plant Soil 383, 3–41. doi: 10.1007/s11104-014-2131-8
Chabili, A., Minaoui, F., Hakkoum, Z., Douma, M., Meddich, A., and Loudiki, M. (2024). A comprehensive review of microalgae and cyanobacteria-based biostimulants for agriculture uses. Plants 13:159. doi: 10.3390/plants13020159
Di Sario, L., Boeri, P., Matus, J. T., and Pizzio, G. A. (2025). Plant biostimulants to enhance abiotic stress resilience in crops. Int. J. Mol. Sci. 26:1129. doi: 10.3390/ijms26031129
EIP-AGRI. (2023). Focus group on biostimulants in agriculture. Available online at: https://ec.europa.eu/eip/agriculture/en/focus-groups/biostimulants-agriculture (Accessed June 1, 2025).
El Boukhari, M. E. M., Barakate, M., Bouhia, Y., and Lyamlouli, K. (2020). Trends in seaweed extract-based biostimulants: manufacturing process and beneficial effect on soil–plant systems. Plants 9:359. doi: 10.3390/plants9030359
El-Beltagi, H. S., Mohamed, A. A., Mohamed, H. I., Ramadan, K. M. A., Barqawi, A. A., and Mansour, A. T. (2022). Phytochemical and potential properties of seaweeds and their recent applications: a review. Mar. Drugs 20:342. doi: 10.3390/md20060342
European Commission (2022). Farm to fork strategy. Available online at: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (Accessed June 1, 2025).
European Commission (2023a). Circular economy action plan. Available online at: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (Accessed June 1, 2025).
European Commission (2023b). Sustainable use of pesticides regulation (SUR). Available online at: https://food.ec.europa.eu/plants/pesticides/sustainable-use-pesticides_en (Accessed June 1, 2025).
Eurostat. (2023a). Agriculture, forestry and fishery statistics – 2023 edition. Available online at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agriculture,_forestry_and_fishery_statistics (Accessed June 1, 2025).
Eurostat (2023b). Key figures on the European food chain – 2023 edition. Luxembourg: Publications Office of the European Union.
FofT. (2023). Food for thought – Baltic ideas for sustainable food policy. Available online at: https://si.se/en/projects-granted-funding/foft-food-for-thought-baltic-ideas-for-sustainable-food-policy/ (Accessed June 1, 2025).
Francioso, O., Schiavon, M., Nardi, S., Castellani, D., Ferrari, E., Estrada, M. T. R., et al. (2024). Mitigation of salt stress in Lactuca sativa L. var. gentile rossa using microalgae as priming agents. Plants 13:3311. doi: 10.3390/plants13233311
Hasanuzzaman, M., Parvin, K., Bardhan, K., Nahar, K., Anee, T. I., Masud, A. A. C., et al. (2021). Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells, 10, 2537. doi: 10.3390/cells10102537
He, L., Geng, K., Li, B., Li, S., Gustave, W., Wang, J., et al. (2024). Enhancement of nutrient use efficiency with biochar and wood vinegar: a promising strategy for improving soil productivity. J. Sci. Food Agric. 104:e13844. doi: 10.1002/jsfa.13844
HELCOM (2023). Baltic sea action plan. Available online at: https://helcom.fi/baltic-sea-action-plan/ (Accessed June 1, 2025).
International Organization for Standardization (ISO) (2005). Soil quality – Determination of pH (ISO 10390:2005). Geneva: ISO.
Interreg Baltic Sea Region (2024). For sustainable food. Available online at: https://interreg-baltic.eu/solutions/for-sustainable-food/ (Accessed June 1, 2025).
Joint Research Centre (JRC) (2022). Science for policy report: the use of biostimulants in EU agriculture : European Commission JRC129516. Luxembourg: Publications Office of the European Union.
Khan, N., Sudhakar, K., and Mamat, R. (2024). Macroalgae farming for sustainable future: navigating opportunities and driving innovation. Heliyon 10:e28208. doi: 10.1016/j.heliyon.2024.e28208
Kocira, S., Szparaga, A., Hara, P., Treder, K., Findura, P., Bartoš, P., et al. (2020). Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 10:17759. doi: 10.1038/s41598-020-74959-0
Kumar, S., Seem, K., and Mohapatra, T. (2023). Biochemical and Epigenetic Modulations under Drought: Remembering the Stress Tolerance Mechanism in Rice. Life, 13, 1156. doi: 10.3390/life13051156
Kumari, M., Swarupa, P., Kesari, K. K., and Kumar, A. (2023). Microbial inoculants as plant biostimulants: a review on risk status. Life 13:12. doi: 10.3390/life13010012
Latvian Rural Advisory and Training Centre. (2024). Gross margins for 2024. LLKC. Available online at: https://llkc.lv/noderigi/bruto-segumi-par-2024-gadu/ (Accessed April 15, 2025).
Li, J., Lardon, R., Mangelinckx, S., and Geelen, D. (2024). A practical guide to the discovery of biomolecules with biostimulant activity. J. Exp. Bot. 75, 3797–3817. doi: 10.1093/jxb/erae156
Liu, S., Wang, X., Yin, X., Savoy, H. J., McClure, A., and Essington, M. E. (2020). Substitution of mineral fertilizer with organic fertilizer in maize systems: a meta-analysis of reduced nitrogen and carbon emissions. Agronomy 10:1149. doi: 10.3390/agronomy10081149
Ludemann, C. I., Wanner, N., Chivenge, P., Dobermann, A., Einarsson, R., Grassini, P., et al. (2024). A global FAOSTAT reference database of cropland nutrient budgets and nutrient use efficiency (1961–2020): nitrogen, phosphorus and potassium. Earth Syst. Sci. Data 16, 525–541. doi: 10.5194/essd-16-525-2024
Menegat, S., Ledo, A., and Tirado, R. (2022). Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 12:14490. doi: 10.1038/s41598-022-18773-w
Mughunth, R. J., Velmurugan, S., Mohanalakshmi, M., and Vanitha, K. (2024). A review of seaweed extract's potential as a biostimulant to enhance growth and mitigate stress in horticulture crops. Sci. Hortic. 334:113312. doi: 10.1016/j.scienta.2024.113312
Neumann, G., Nawaz, F., Weinmann, M., Arbona, V., Balestrini, R., Pagliarani, C., et al. (2024). Editorial: enhancing sustainable crop production: biostimulants and biotechnological approaches in challenging climates. Front. Plant Sci. 15:1534774. doi: 10.3389/fpls.2024.1534774
Nollendorfs, V., Osvalde, A., Čekstere, G., Karlsons, A., and Āboliņa, L. (2023). Diagnosis of mineral nutrition of plants and optimization of fertilization. Riga: Academic Publishing House of the University of Latvia, 240.
Nur, M. M. A., Mahreni,, Murni, S. W., Setyoningrum, T. M., Hadi, F., Widayati, T. W., et al. (2024). Innovative strategies for utilizing microalgae as dual-purpose biofertilizers and phycoremediators in agroecosystems. Biotechnol. Rep. (Amst.) 45:e00870. doi: 10.1016/j.btre.2024.e00870
Pascoalino, L. A., Pires, T. C., Pinela, J., Rodrigues, M. Â., Ferreira, I. C., Barros, L., et al. (2025). Foliar application of biostimulants improves nutritional and bioactive quality of walnuts. J. Sci. Food Agric. 105, 1138–1146. doi: 10.1002/jsfa.13904
Renganathan, P., Puente, E. O. R., Sukhanova, N. V., and Gaysina, L. A. (2024). Hydroponics with microalgae and cyanobacteria: emerging trends and opportunities in modern agriculture. Biotech 13:27. doi: 10.3390/biotech13030027
Ricci, M., Tilbury, L., Daridon, B., and Sukalac, K. (2019). General principles to justify plant biostimulant claims. Front. Plant Sci. 10:494. doi: 10.3389/fpls.2019.00494
Ronga, D., Biazzi, E., Parati, K., Carminati, D., Carminati, E., and Tava, A. (2019). Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy, 9, 192. doi: 10.3390/agronomy9040192
Rouphael, Y., Giordano, M., Cardarelli, M., Cozzolino, E., Mori, M., Kyriacou, M. C., et al. (2018). Plant- and Seaweed-Based Extracts Increase Yield but Differentially Modulate Nutritional Quality of Greenhouse Spinach through Biostimulant Action. Agronomy, 8, 126. doi: 10.3390/agronomy8070126
Ruzzi, M., Colla, G., and Rouphael, Y. (2024). Editorial: biostimulants in agriculture II: towards a sustainable future. Front. Plant Sci. 15:1427283. doi: 10.3389/fpls.2024.1427283
Salvo, A., Masciulli, F., Ambroselli, D., Romano, E., Ingallina, C., Spano, M., et al. (2024). Hydrolysates from cauliflower and artichoke industrial wastes as biostimulants on seed germination and seedling growth: a chemical and biological characterization. J. Sci. Food Agric. 105, 151–161. doi: 10.1002/jsfa.13813
Samantaray, A., Chattaraj, S., Mitra, D., Ganguly, A., Kumar, R., Gaur, A., et al. (2024). Advances in microbial based bio-inoculum for amelioration of soil health and sustainable crop production. Curr. Res. Microb. Sci. 7:100251. doi: 10.1016/j.crmicr.2024.100251
Sathya, B., Indu, H., Seenivasan, R., and Geetha, S. (2010). Influence of seaweed liquid fertilizer on the growth and biochemical composition of legume crop, Cajanus cajan (L.) mill sp. J. Phytology 2, 50–63.
Shahrajabian, M. H., Chaski, C., Polyzos, N., and Petropoulos, S. A. (2021). Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules, 11, 698. doi: 10.3390/biom11050698
Sharma, A., Chauhan, P., and Singh, R. (2024). Methane-derived microbial biostimulant reduces greenhouse gas emissions and improves rice yield. Front. Plant Sci. 15:1432460. doi: 10.3389/fpls.2024.1432460
Silva, L. D., Bahcevandziev, K., and Pereira, L. (2019). Production of bio-fertilizer from Ascophyllum nodosum and Sargassum muticum (Phaeophyceae). J. Ocean. Limnol. 37, 918–927. doi: 10.1007/s00343-019-8109-x
Sirbu, C., Cioroianu, T.-M., Marin, N., and Bireescu, G. (2023). The biostimulatory effect of brown algae Ascophyllum nodosum on grapevine. Ann. Univ. Craiova Biol. Horticult. Food Prod. Process. Technol. Environ. Eng. 28:77–84. doi: 10.52846/bihpt.v28i64.74
Skapste, I., Grinberga-Zalite, G., and Žaimis, U. (2025). Economic potential of algae biostimulant for sustainable agriculture in the Baltic Sea region: impact of Furcellaria lumbricalis digestate extract on basil growth promotion. Sustainability 17:3268. doi: 10.3390/su17073268
Vangenechten, B., De Coninck, B., and Ceusters, J. (2025). How to improve the potential of microalgal biostimulants for abiotic stress mitigation in plants? Front. Plant Sci. 16:1568423. doi: 10.3389/fpls.2025.1568423
Keywords: sustainable agriculture, soil health, sustainable food system, leafy and root vegetables, productivity, algae
Citation: Skapste I, Vircava I, Skutele K, Žaimis U, Grinberga-Zalite G and Zvirbule A (2025) Algae digestate biostimulants as an innovative solution for food system sustainability and productivity improvement. Front. Sustain. Food Syst. 9:1656867. doi: 10.3389/fsufs.2025.1656867
Edited by:
Narashans Alok Sagar, Chandigarh University, IndiaReviewed by:
Khadija El Moustaqim, Ibn Tofail University, MoroccoMuhamad Maulana Azimatun Nur, Universitas Pembangunan Nasional Veteran Yogyakarta, Indonesia
Ravi Shankar Yadav, Silesian University of Technology, Poland
Copyright © 2025 Skapste, Vircava, Skutele, Žaimis, Grinberga-Zalite and Zvirbule. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inese Skapste, aW5lc2Uuc2thcHN0ZUBsYnR1Lmx2
 Inese Skapste
Inese Skapste Ilze Vircava
Ilze Vircava Kristiana Skutele2
Kristiana Skutele2