- 1National Agency for Food and Drug Administration, Abuja, Nigeria
- 2World Bank Africa Centre of Excellence, Centre for Oilfield Chemical Research, University of Port Harcourt, Port Harcourt, Nigeria
- 3Centre for Competence in Environmental Biotechnology, College of Animal and Environmental Science, University of South Africa, Florida Science Campus, Johannesburg, South Africa
Biopesticides have emerged as a central focus in contemporary policy and scientific discourse due to their strong alignment with green chemistry, One Health initiatives, the Sustainable Development Goals (SDGs), and sustainable agriculture. Beyond their established role in integrated pest management (IPM), they serve as a pivotal driver in building resilient agricultural systems. However, their wider adoption is constrained by technical limitations and the high costs associated with refined formulations. This review aims to identify types and strategies of biopesticides that are both cost-effective and suitable for sustainable agriculture. Drawing on multiple case studies across diverse agroecological zones, the analysis reveals that cost-effective biopesticides are critical for advancing IPM in traditional and smallholder farming systems, while branded formulations predominantly benefit large-scale farms with greater economic capacity. Emphasis is placed on the utilization of readily accessible biopesticides, including pesticidal plants, natural enemies, entomopathogenic nematodes, and botanical extracts and seed/seedling treatments, which collectively mitigate pest pressure, reduce reliance on chemical pesticides, and enhance crop yields within a structured progression described as the IPY trend. This trend underscores the interlinked dynamics of infestation levels, pesticide consumption, and crop productivity under cost-effective, biopesticide-driven IPM. Within this low-tech and high-tech classification framework, persistent controversies and the misconception that developing nations, long reliant on traditional agricultural technologies, lack awareness of biopesticides can be systematically examined and addressed, thereby facilitating informed policy decisions and optimized implementation strategies.
1 Introduction
Biopesticides have emerged as a leading research focus due to their profound intersections with green chemistry, One Health, Sustainable Development Goals (SDGs), sustainable agriculture, and organic farming, each underscoring the shift toward ecological resilience and environmentally responsible pest control. Unlike synthetic pesticides, which often pose risks of bioaccumulation, toxicity, and resistance development, biopesticides harness naturally derived active compounds such as microbial pathogens, plant-based metabolites, and insect pheromones, minimizing unintended ecological consequences while optimizing targeted pest suppression. As a component of green chemistry, biopesticides operate based on principles that emphasize biodegradability, selective toxicity, and reduced environmental persistence (Fenibo et al., 2022). They eliminate the reliance on hazardous synthetic compounds while enhancing agricultural sustainability, ensuring pest control strategies align with ecological safety and pollution reduction initiatives. The One Health framework recognizes the interconnectedness of human health, animal welfare, and environmental stability, an approach directly applicable to biopesticide implementation. Unlike conventional pesticides that introduce chemical residues into food chains and drinking water sources, biopesticides minimize toxicity risks, thereby protecting livestock, pollinators, and human populations from long-term exposure. Their adoption mitigates concerns over antimicrobial resistance (AMR), endocrine disruption, and neurotoxic effects associated with synthetic pesticide exposure. Biopesticides actively contribute to multiple SDGs, particularly SDG 2 (zero hunger), SDG 3 (good health and wellbeing), and SDG 15 (life on land). By promoting safer pest control methods, they drive food production systems, enhance biodiversity conservation, and ensure agricultural sustainability while protecting humans from harmful pesticide residues (Deeksha et al., 2025). Their role in achieving climate-resilient farming is increasingly recognized as a required alternative to chemical-intensive pest control strategies for sustainable agriculture.
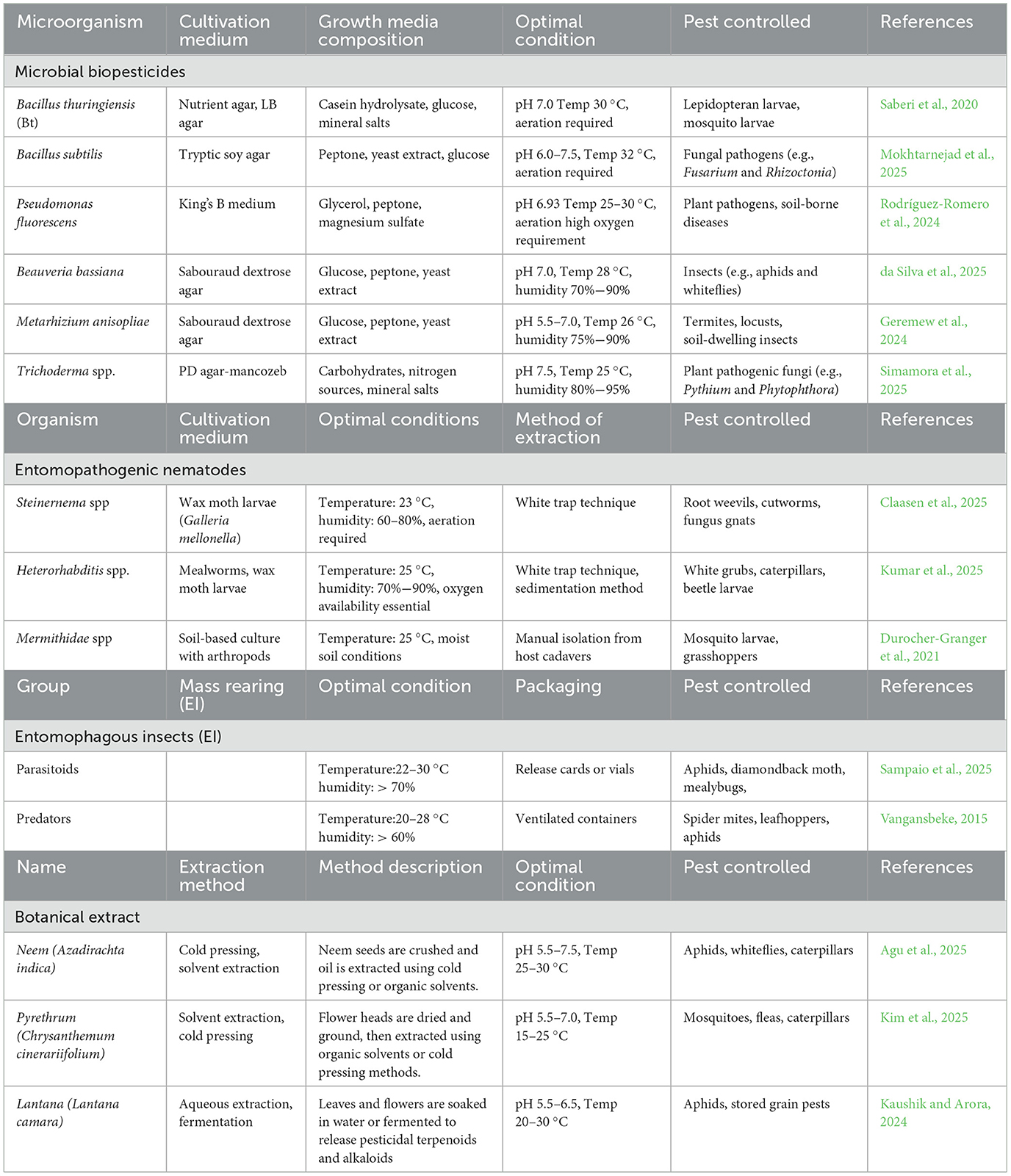
As sustainable agriculture pivots toward regenerative practices, biopesticides offer a viable alternative to synthetic agrochemicals, fostering resilient cropping systems. Their inclusion in organic farming aligns with regulatory mandates that prohibit synthetic pesticide use while preserving soil microbial diversity, reducing residue contamination, and enhancing crop resilience against biotic stress factors. Emerging innovations in biotechnological formulations, bio-control agents, and precision application techniques further reinforce their efficacy in modern agricultural frameworks (Karuppiah et al., 2025). The increasing emphasis on biopesticides signals a fundamental shift away from conventional pesticide dependency, advancing eco-conscious pest management strategies that prioritize food security, environmental integrity, and public health. As governments and research institutions accelerate investments in biotechnology-driven biological control agents, biopesticides continue to redefine the landscape of pesticide regulation, agricultural sustainability, and global food safety standards. This expanded perspective highlights the multifaceted benefits of biopesticides in shaping future-ready, sustainable pest control solutions (Sidahmed et al., 2025). Biopesticides have proven to be an effective and environmentally friendly alternative to synthetic pesticides in controlling pests and diseases that threaten agricultural productivity. Table 1 provides an overview of the ten most significant pests and diseases along with their effective biopesticide treatments. These include microbial biopesticides (bacteria, fungi, viruses), plant-based pesticides, biochemical pesticides (pheromones, enzymes), and other microbial biopesticides (Mawcha et al., 2025b). Their efficacy varies depending on the type of pest or disease, formulation, and application method. Agboola et al. (2022) previously stated that biopesticides can be as effective as synthetic pesticides while reducing toxicity to non-target organisms and promoting biodiversity and biodegradability, although they may require more time to achieve the desired effects. They are particularly useful in integrated pest management (IPM) strategies, helping to mitigate resistance issues that arise with chemical pesticides.
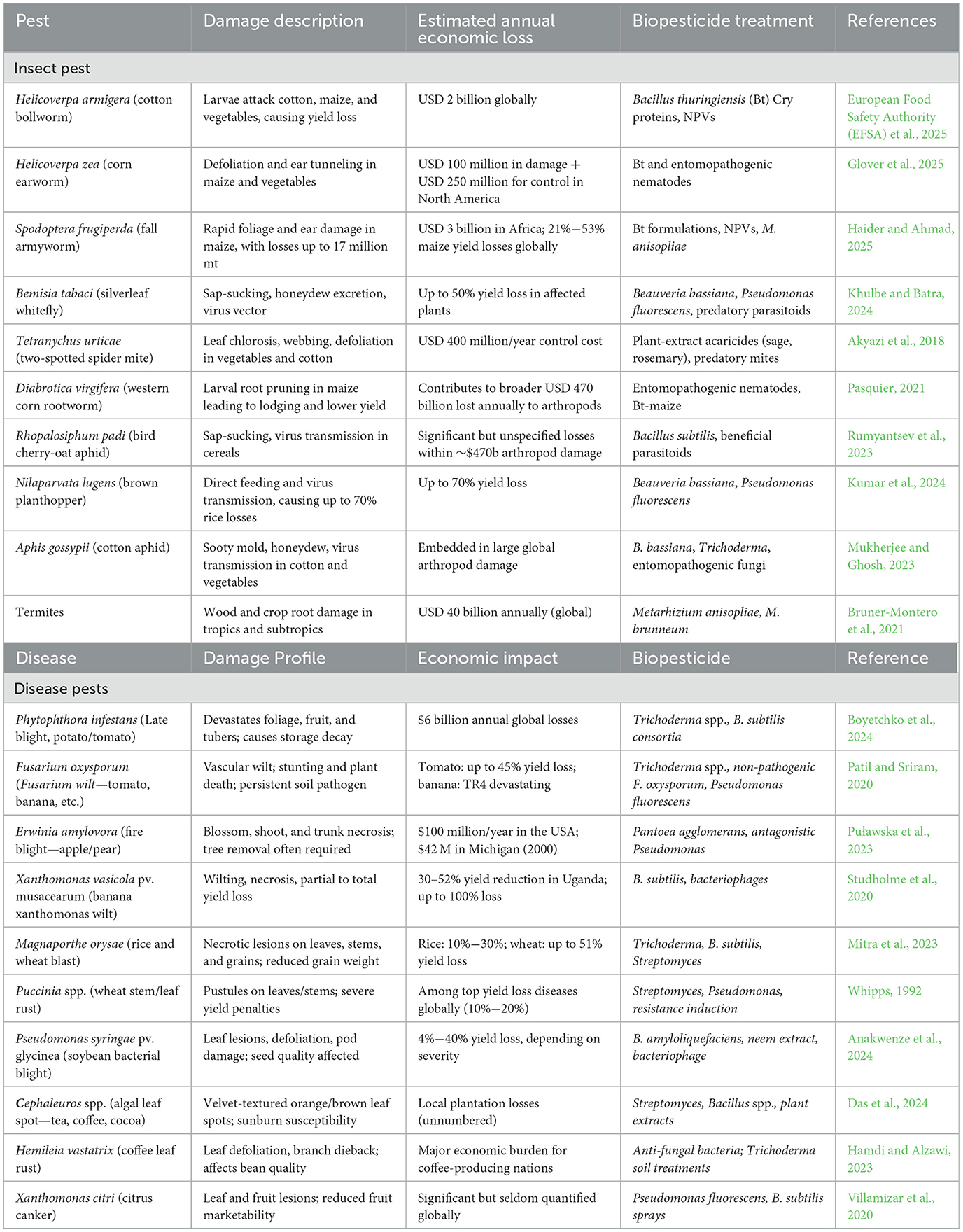
Table 1. Evidence-based summary of biopesticides being used against the 10 most notorious pests and diseases of plants.
IPM is a sustainable approach to pest control that combines cultural, physical, biological, and chemical methods in a pyramidal structure to minimize damage while preserving ecosystem balance. It emphasizes prevention through techniques such as crop rotation, habitat manipulation, and the use of resistant plant varieties as its foundational components (Zhou et al., 2024). Biological control plays a key role by promoting natural predators and beneficial microorganisms that suppress pest populations. When necessary, pesticides are applied selectively, focusing on minimal environmental disruption and reducing the likelihood of resistance. IPM also incorporates regular monitoring and economic threshold analysis to ensure interventions are only made when pest levels threaten significant economic loss.
By integrating multiple strategies, IPM enhances agricultural productivity while reducing reliance on harmful chemical pesticides. Biopesticide-centric IPM is widely regarded as a catalyst for sustainable agriculture, though challenges such as slow action, instability, regulatory hurdles, higher production costs, public acceptance, and limited market penetration remain areas for improvement. Agriculture, humanity's earliest vocation, originated with pest management practices that were natural, indigenous, and non-toxic. In this context, biopesticides are expected to be both abundant and economically viable compared to conventional chemical pesticides. Nevertheless, in practice, their deployment remains constrained by persistent challenges, including regulatory bottlenecks, high production costs, limited market penetration, and consumer reluctance, despite the increasing need for broader biopesticide adoption in light of recent scientific advances. Reconciling these contradictions forms the central thrust of this review. Accordingly, our study critically examines the broader conceptualization of biopesticides, their integration indices within pest management strategies, and their current applications in promoting sustainable agriculture, illustrated through case studies aligned with the principles of the IPM framework.
2 Attributes of biopesticides
2.1 Biopesticides: definition and types
Biopesticides are naturally derived inorganic materials, organic compounds, or living organisms and their byproducts that control, mitigate, or eliminate pests harmful to plants and animals. These controls can function through physical, physiological, behavioral, biochemical, or ecological mechanisms (Archana et al., 2022). Biopesticides fulfill essential criteria relevant to efficacy, environmental impact, non-target organisms, and human health. According to the U.S. Environmental Protection Agency (EPA), biopesticides are classified into three main groups: microbial biopesticides, biochemical biopesticides, and plant-incorporated protectants (Deeksha et al., 2025). However, certain pest-controlling organisms do not fit neatly into these categories, such as live plants and natural enemies that naturally suppress pests. These pesticidal plants and predatory or parasitoid insects can be categorized as macrobial biopesticides. While some literature classifies pesticidal plants as medicinal plants and beneficial insects as natural enemies, they still meet the essential criteria for biopesticides. Thus, biopesticides can be broadly grouped into four categories, as illustrated in Figure 1. It is important to note that biopesticides include natural inorganic materials and synthetic analogs. These inclusions may dilute or compromise the meaning of the “bio” prefix in the term. However, to justify this broader definition, biopesticides should be viewed from a technical perspective as nature-traceable compounds, organisms, or their parts with pesticidal effects that maintain environmentally friendly attributes, such as biodegradability, specificity, low toxicity to non-target organisms, a benign mode of action, and minimal induction of pest resistance.
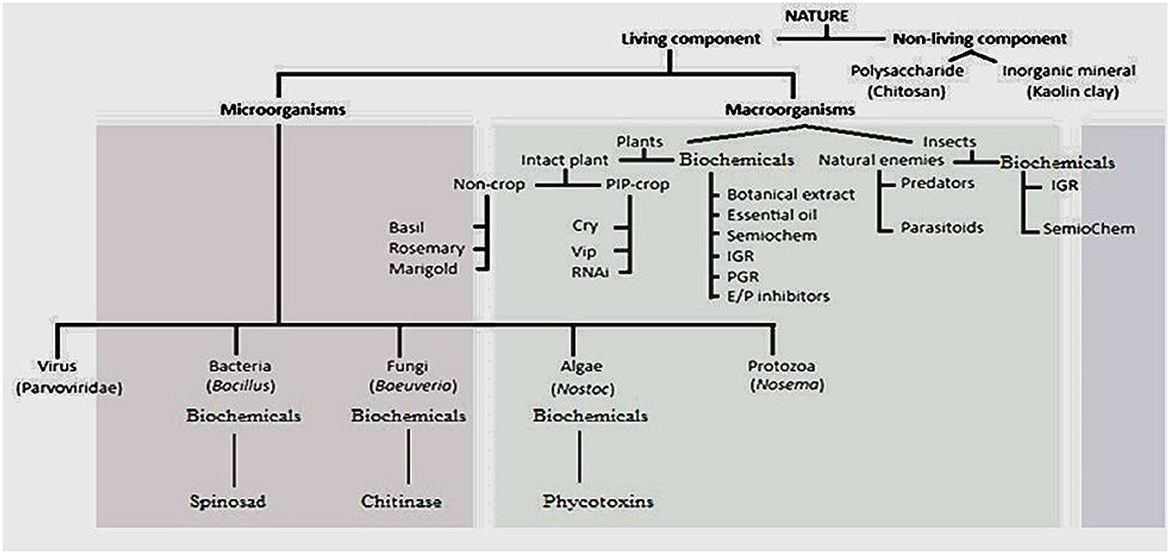
Figure 1. Classification of biopesticides with subcategories and examples. IGR, insect growth regulators; PGR, plant growth regulators; SemioChem, semiochemical; E/P, enzyme/protein inhibitors; Cry, crystal protein; Vip, vegetative insecticidal protein; RNAi, RNA interference.
Microbial biopesticides are pesticides that use microorganisms such as bacteria, fungi, algae, viruses, and protozoa as active ingredients to control pests. These microorganisms define different subcategories of biopesticides, including bacterial biopesticides, fungal biopesticides, viral biopesticides, algal biopesticides, and protozoan biopesticides. While microbial biopesticides exhibit the unique properties of biopesticides, they also possess advantages such as stealthiness and rapid replication, allowing them to overcome the frequent reapplication required by many other biopesticides. Macrobial biopesticides, on the other hand, rely on live insects and plants to control pests. This category includes natural enemies such as predators (e.g., ladybugs and praying mantises) and parasitoids (e.g., wasps), as well as pesticidal live plants that naturally repel pests, such as rosemary (Rosmarinus officinalis) and marigold (Tagetes spp.). Biochemical biopesticides are chemical compounds derived from non-living matter, microorganisms, insects, plants, and other living organisms that control pests through natural mechanisms. Examples include diatomaceous earth, chitosan, essential oils, botanical extracts, semiochemicals, insect growth regulators, plant growth regulators, and enzyme/protein inhibitors (Joshi and Chaudhuri, 2025). Plant-incorporated protectants (PIPs) are genetically engineered crops designed to resist pests or diseases. The most commonly used compounds in PIP technology include crystal toxins (Cry proteins), vegetative insecticidal proteins (Vip proteins), and RNA interference (RNAi) mechanisms. While transgenic PIPs represent an innovation in sustainable agriculture, there are growing reports of harm to non-target organisms, pest resistance, threats to biodiversity, genetic contamination, allergic reactions, and disruption of ecosystem services (Odelade et al., 2024). These concerns highlight the need for checks and balances in scientific advancements, particularly regarding the mode of action of PIPs.
2.2 Biopesticides' modes of action
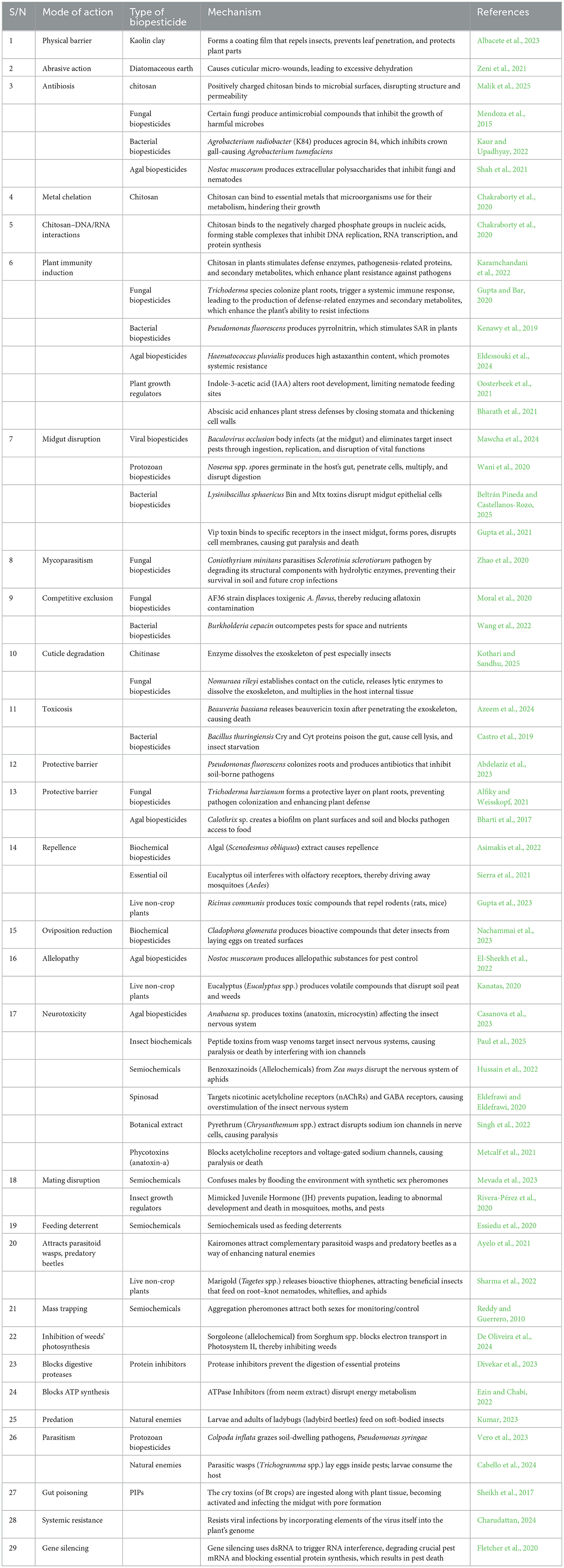
Biopesticides are recognized for their diverse modes of action, which significantly reduce the risk of resistance development, a common challenge associated with chemical pesticides. Non-living biopesticide compounds exhibit multiple modes of action. For example, kaolin clay forms a physical barrier by coating plant surfaces, creating an inhospitable environment that disrupts pest movement, host recognition, feeding, and egg-laying, thereby minimizing damage (Chuskit et al., 2024). Diatomaceous earth (DE) works by creating cuticular micro-wounds on the exoskeletons of pests, leading to excessive moisture loss, dehydration, and death (Alkan et al., 2023). Similarly, chitosan (a positively charged biopolymer) employs various biochemical strategies. Its antibiosis mode of action disrupts microbial cell membranes (partly through electrostatic interactions), affecting bacteria, fungi, and viruses by increasing permeability and allowing the leakage of vital cellular components, which ultimately leads to cell death (Alkhalil, 2025). Chitosan's metal chelation mechanism sequesters calcium, magnesium, and iron, interfering with microbial growth, enzyme activity, and cellular signaling, effectively inhibiting pathogen development. The ‘Chitosan-DNA/RNA interactions' mode of action forms complexes that inhibit DNA replication, RNA transcription, and protein synthesis, culminating in cell death (Xing et al., 2015). Immunity induction through the production of defense enzymes, pathogenesis-related proteins, and secondary metabolites such as phenolic compounds and lignin enhances plant resistance against pathogens (Rosales-Castillo et al., 2025). All of these non-living biopesticide compounds provide a protective barrier for plants; however, chitosan exhibits more modes of action than others due to its complex biochemical properties and its ability to interact directly with biological membranes, molecules, and systems. This implies that organic materials are more relevant to biopesticides compared to inorganic materials.
One common mode of action shared by viral, protozoan, and bacterial biopesticides is gut disruption, a parasitic mechanism by which these agents invade their hosts and attach to insect gut receptors using toxins and other metabolites. Viral biopesticides exhibit obligate parasitism that begins when insects ingest baculoviruses, specifically nucleopolyhedroviruses (NPV) and granuloviruses (GV). Once inside the alkaline midgut, the occlusion bodies dissolve, triggering an infection that leads to viral replication, systemic spread, larval death, host liquefaction, and subsequent virus transmission within the insect population (Mehrotra et al., 2017). Protozoan biopesticides operate similarly; however, their infection begins with the ingestion of spores (cysts or oospores), and reinfection occurs via fecal contamination. The sensitivity of the insect gut to baculovirus occlusion bodies, protozoan spores, and Bt crystal proteins is likely due to active enzymes, a nutrient-rich environment, and specialized receptors (Aware and Jadhav, 2022; Rao and Jurat-Fuentes, 2020). Beyond parasitism, protozoan biopesticides have also been shown to exhibit a predatory mode of action, targeting the larvae or pupae of fungus gnats, microfauna, and nematodes, a capability facilitated by their small size, overlapping ecological niches in soil, and similar nutrient profiles (Vaselek, 2024). Fungal biopesticides act predominantly through cuticle degradation with the aid of enzymes. Once these agents penetrate the insect tissue, they produce toxins and other harmful biochemicals. For instance, Beauveria bassiana germinates on insects, breaches the cuticle, and secretes beauvericin toxin, ultimately leading to the host's death. Other modes of action exhibited by fungal biopesticides include outcompetition, as seen in Aspergillus flavus AF36, which eliminates toxigenic strains; a bioprotective barrier against disease pathogens, as demonstrated by Trichoderma asperellum against Rhizoctonia and Pythium (Kipngeno et al., 2015); mycotoxicity of weed biomass, as demonstrated by Myrothecium verrucaria; and the enhancement of plant immunity (Elkhateeb and Daba, 2019). This broader range of modes of action corresponds with a wider spectrum of target pest control, including insects, pathogens, nematodes, mites, weeds, and plant growth promotion.
Bacterial biopesticides demonstrate similar mechanisms of action as fungal biopesticides but are often richer in toxins and secondary metabolites that exhibit high bioactivity against pests and promote plant growth (Beltrán Pineda and Castellanos-Rozo, 2025). For example, Bacillus thuringiensis (Bt) produces crystal (Cry) toxins, cytolytic (Cyt) toxins, vegetative insecticidal proteins (Vip toxins), and secreted insecticidal proteins (Sip toxins), each with a distinct gut receptor binding site yet complementary (Mendoza-Almanza et al., 2020). Bt toxins, activated in the insect midgut, bind to receptors, form pores, disrupt cellular integrity, and cause fatal septicemia and subsequent insect death. Multiple toxins offer enhanced efficacy through synergistic action, broaden the pest spectrum, and significantly reduce resistance development (Aswathi et al., 2024). Binary toxins from Bacillus sphaericus, thermostable exotoxins from Burkholderia rinojensis, Mtx toxins from Brevibacillus laterosporus, Pir toxins from Photorhabdus luminescens, and toxin complex (Tc) proteins from Photorhabdus and Xenorhabdus species all exhibit significant insecticidal activity similar to the Bt mechanism of action (Mohamed et al., 2023; Nascimento et al., 2020). Ben Khedher et al. (2020), Crouzet et al. (2020), and Nalini et al. (2023) elaborated on the potential of biosurfactants produced primarily by bacteria. Notably, Bacillus subtilis synthesizes surfactin and iturin lipopeptides that effectively inhibit fungal pathogens (Markelova and Chumak, 2025). Their main mechanism of action is the disruption of cellular membranes, particularly in fungi, although these compounds also target bacteria and viruses. Additionally, some biosurfactants trigger systemic resistance in plants. Species such as Bacillus subtilis display a remarkable range of actions, including parasitism, antibiosis, competition, bioprotective barrier formation, and immune induction (Nayak, 2021). Furthermore, Bacillus subtilis excels not only in pest control but also functions as a biofertilizer, stress alleviator, and plant growth promoter (Riaz et al., 2021), showcasing its multifaceted mechanisms. This versatility positions Bacillus subtilis as an exceptionally promising biopesticide. Due to their inherent biological and ecological characteristics, bacteria present several advantages over fungi in pest management applications. These include the synthesis of potent and species-specific toxins, adaptability across diverse ecological conditions, cost-efficient scalability in production, and sustained efficacy over time.
Algal biopesticides exhibit multiple modes of action, including the formation of a bioprotective barrier, with Calothrix spp. colonizing the rhizosphere to deter Fusarium and nematodes (Babu et al., 2015); antibiosis, as demonstrated by Tolypothrix tenuis, which controls root–knot nematodes (Holajjer et al., 2013), and the stimulation of plant immunity, evidenced by Haematococcus pluvialis producing astaxanthin, which confers systemic resistance in plants against blight pathogens such as Fusarium (Eissa et al., 2025). Additionally, the biochemical bioactivity of algal biopesticides exerts a broad inhibitory effect on pest growth and feeding. For instance, Spirulina platensis limits pest feeding (Al-Qahtani, 2021), Scenedesmus obliquus causes digestive inhibition and pest repellence (Tufan-Cetin and Cetin, 2023), Chlorella vulgaris reduces insect feeding and oviposition (Cavalcanti et al., 2021), Sargassum wightii lowers insect fecundity (Petchidurai et al., 2023), Gracilaria edulis suppresses larval development (Gowthish and Kannan, 2019), and Nostoc muscorum exhibits allelopathic effects that protect plants. Algal extracts, like botanical extracts, are examples of biochemical biopesticides that control pests in diverse ways. Their modes of action include repellence and antifeedant effects (behavioral manipulation) as well as inhibition of key physiological processes such as hormonal disruption, interference with the mitochondrial electron transport chain, nerve overstimulation, disruption of calcium homeostasis, and inhibition of ATP synthesis (Casanova et al., 2023; Mevada et al., 2023; Nachammai et al., 2023). Essential oils (e.g., clove oil, thyme oil, and cedarwood oil), botanical extracts, biochemicals from insects, protein inhibitors, semiochemicals, insect growth regulators, and plant growth regulators all exert these effects to varying degrees. As indicated in Table 2, some live plants (such as marigold) utilize attractants, repellents, and allelopathy to control pests (Gupta et al., 2023), while plant-incorporated protectants (PIPs) act through gut poisoning, induction of systemic resistance, and targeted gene silencing. PIPs are genetically engineered crops with built-in defense mechanisms: Bt crops produce Bacillus thuringiensis (Bt) Cry proteins (and other toxins) that bind to insect gut receptors, causing cell rupture and starvation; virus-based crops express viral coat proteins to prevent virus replication or employ RNA interference (RNAi) to silence viral genes and essential pest genes upon ingestion, leading to developmental failure or death.
Other biological control methods include predators and parasitoids. Predators such as lady beetles, lacewings, and praying mantises actively hunt a range of pests, including aphids, thrips, and caterpillars, resulting in a significant reduction in pest populations that can damage crops. Meanwhile, parasitoids like parasitic wasps (Trichogramma spp.), tachinid flies (family Tachinidae), and braconid wasps (family Braconidae) provide targeted pest control by laying their eggs in or on specific hosts, ultimately killing them as the larvae develop (Heraty, 2017). These methods strengthen ecological balance and enhance IPM systems, making them a cornerstone of sustainable agriculture. Beyond their ecological benefits, the advantages of biopesticides are also evident in their application within proven pest management systems. Such systems promote sustainable agriculture by balancing the need for maximum turnover and yield with the preservation of natural resources that support agricultural development. Moreover, biopesticides help limit the development of pest resistance, address the shortcomings of synthetic pesticides, offer promising technological solutions to correct their limitations, and feature prominently in the stages of the IPM pyramid. The exploration of biopesticides in IPM-driven sustainable agriculture remains an active pursuit for achieving global sustainability goals.
2.3 Biopesticides advantages: solutions to synthetic pesticide risks
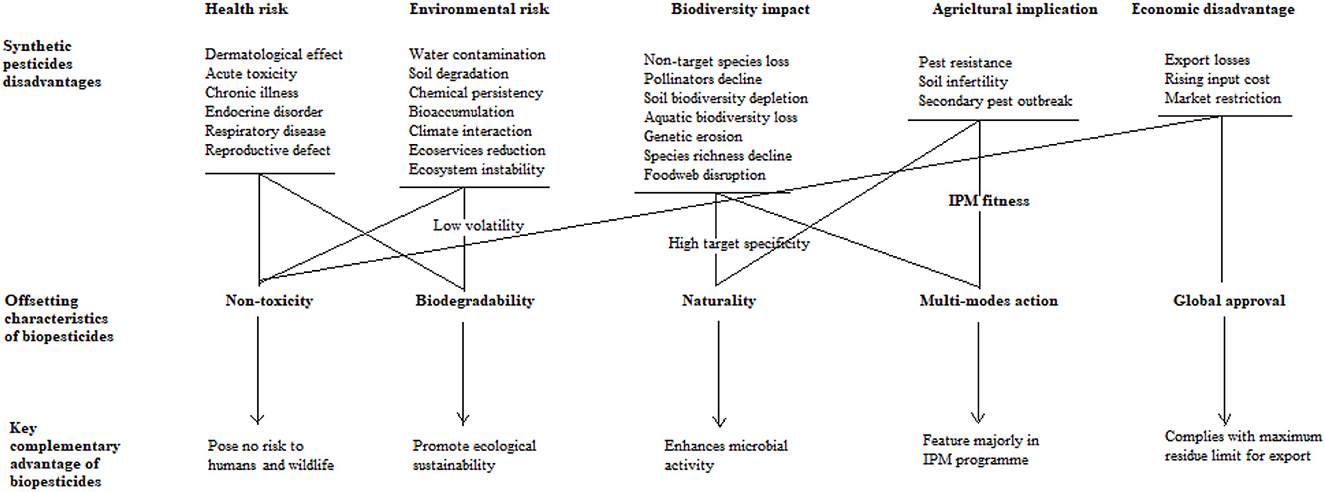
Synthetic pesticides are persistent organic chemicals that pose serious threats to the environment, human and animal health, biodiversity, and essential ecological functions. Their presence in agricultural produce results in food contamination and economic losses due to toxicity concerns, presenting a significant challenge to agribusiness. Prolonged use fosters pest resistance and inadvertently harms beneficial, non-target organisms, further destabilizing ecosystems. Biopesticides provide a safer and environmentally friendly alternative, effectively mitigating these harmful effects while promoting agricultural sustainability, as summarized in Figure 2. By degrading rapidly, they minimize environmental pollution, lower toxicity risks to wildlife, and reduce harmful residues in soil, water, and air, promoting ecological sustainability. In contemporary agricultural systems, fast-degrading biopesticides have emerged as vital tools, delivering notable ecological and economic advantages across diverse environments. While fulfilling their primary role of pest eradication and enhancing crop yields, a renowned vineyard in California employed these biopesticides to preserve soil microbiome integrity, prevent chemical residues on grapes, and safeguard nearby water sources from runoff (Wilson and Daane, 2017). In Vietnam's Mekong Delta, rice farmers utilized them to maintain clean aquatic ecosystems, thereby protecting local fish and amphibian populations (Stadlinger et al., 2018). Awudzi et al. (2022) reported reduced residual toxicity in nutrient-sensitive soils among cocoa growers in Ghana. Similarly, Mawcha et al. (2024) documented achieving safer working environments and preserving beneficial organisms in pest management protocols in high-tech greenhouse facilities.
Their selective targeting of pests preserves pollinators and natural predators, ensuring ecological balance and supporting biodiversity. Entomopathogenic fungi-based biopesticides were employed to target aphid infestations among almond growers in Southeastern Spain, leading to a 30% rise in pollinator populations over three growing seasons and a 40% decrease in chemical pesticide use (del Valle, 2020). A similar result was observed in an apple orchard in the Pacific Northwest, where natural predators were preserved, contributing to overall orchard biodiversity (Fenibo et al., 2022). Microbial biopesticides enhance soil fertility by stimulating beneficial microorganisms and strengthening sustainable farming practices. With Bacillus subtilis-based biopesticides, smallholder vegetable farmers in Kenya were able to control Fusarium and Pythium pathogens, which resulted in increased beneficial rhizobacteria, higher yields in tomatoes and onions, and a 25% reduction in chemical use (Wafula, 2022). Novara et al. (2020) reported a pilot project in a Mediterranean organic vineyard where microbial biopesticides were introduced to stimulate beneficial soil microorganisms. Unlike synthetic pesticides, which accelerate resistance development, biopesticides utilize natural pathogens and biochemicals with diverse modes of action, making it more difficult for pests to develop resistance. In Iowa, large-scale maize producers incorporated biochemical insect growth regulators (IGRs) derived from neem and Spinosad to eradicate resistance in Western corn rootworm over 5 years (Revilla et al., 2021). Their safer profile decreases toxic exposure for farmworkers and consumers, reducing health risks (cancer risk, allergy, gastrointestinal disorders, neurological effects, etc.) associated with pesticide residues in food (Chikte et al., 2024). By preventing resistance buildup and curbing excessive pesticide application, biopesticides ensure effective long-term pest management and healthier agricultural systems. Despite their advantages, biopesticides face barriers such as short shelf life, slow action, high production costs, limited availability, regulatory constraints, and low farmer awareness, which must be addressed through targeted improvements in stability, efficiency, and adoption strategies.
3 Biopesticides limitations and improvement opportunities
3.1 Limitations of biopesticides
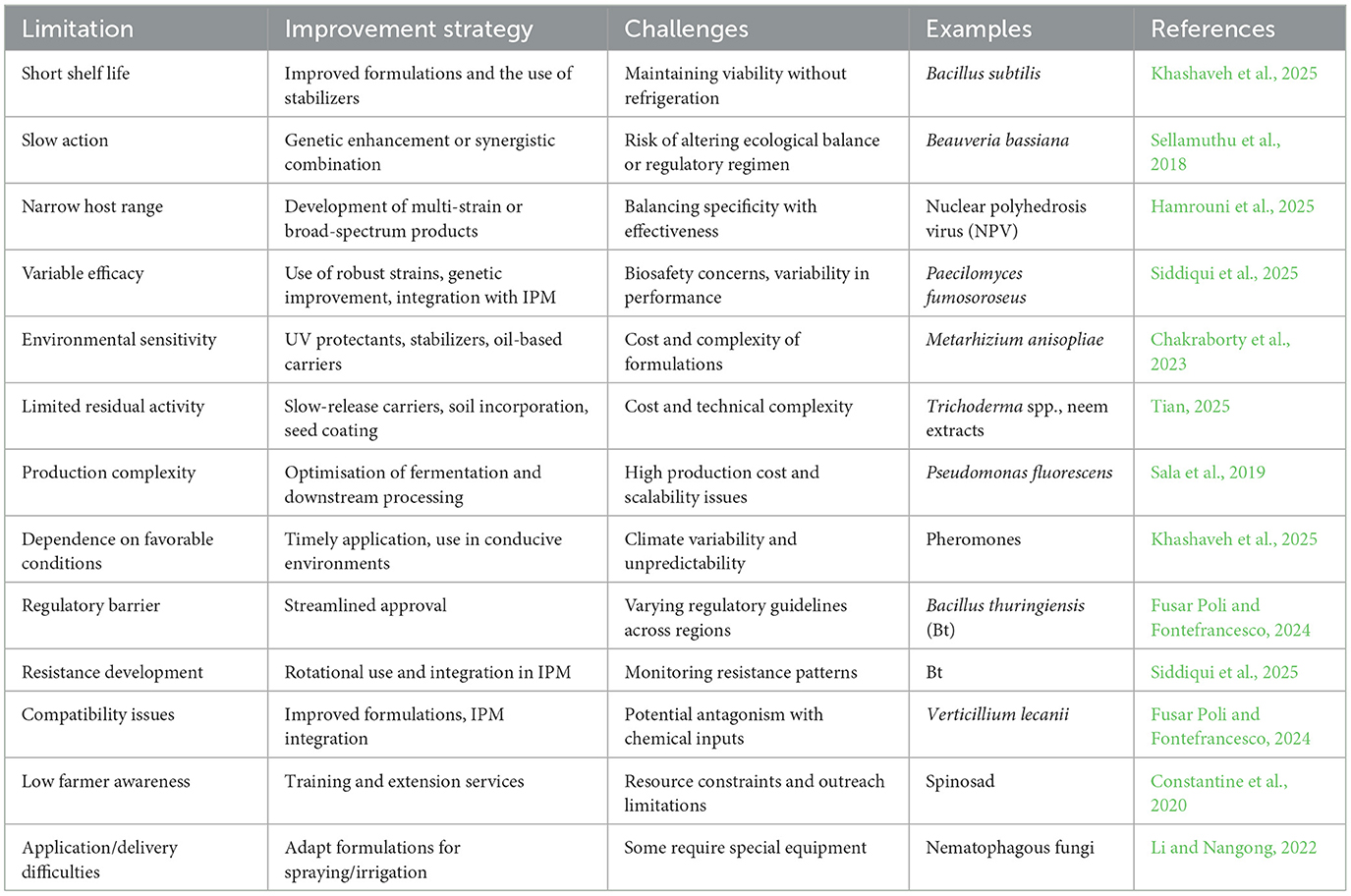
Biopesticides generally exhibit shorter shelf lives than synthetic pesticides due to their biological origin, derived from living organisms (bacteria, fungi, viruses, protozoa) or natural metabolites. These components are inherently sensitive to environmental factors such as heat, light, and moisture, which hasten their degradation. Unlike synthetic pesticides, they usually lack chemical stabilizers that extend longevity, since such additives may interfere with their biological activity. Their effectiveness often depends on the viability of microbial propagules (e.g., spores or cells), which can lose activity or die over time if not stored under optimal conditions. Consequently, biopesticides typically require cool, dry, and dark environments to remain effective; any deviation reduces their performance in the field.
Shelf life varies across different categories of biopesticides. Semiochemicals, such as pheromones and plant volatiles, are the least stable due to their high volatility and susceptibility to degradation by light, air, and heat, often requiring encapsulation to extend usability (Khashaveh et al., 2025). Biochemical biopesticides, including plant extracts (e.g., neem oil), hormones, and enzymes, are moderately stable but still prone to deterioration from UV exposure, microbial contamination, or elevated temperatures, generally lasting only a few months under ideal storage conditions. Microbial biopesticides differ in stability depending on their formulation: spore-forming microbes such as Bacillus thuringiensis tend to persist longer, especially in dry formulations, while non-spore-forming microbes like many gram-negative bacteria are highly sensitive and may survive only weeks without refrigeration (Arora, 2015). By contrast, plant-incorporated protectants (PIPs), such as genetically engineered crops expressing pesticidal proteins, remain stable as long as seed viability is preserved, representing the longest effective shelf life among biopesticides. Storage requirements also vary significantly: semiochemicals need airtight, cool, and dark storage, often under refrigeration; plant extracts require sealed, opaque containers in cool and dry environments; microbial products range from room-temperature stability for spore-formers to refrigeration needs for sensitive non-spore-formers; beneficial insects require temporary holding under controlled conditions that mimic their natural habitat; while PIPs, stored as seeds, remain the most stable and practical. The short shelf life of most biopesticides has important practical implications for their distribution and adoption. Farmers and suppliers must manage strict supply chains to avoid product expiration, which can be costly and logistically challenging in rural or resource-limited areas. Improperly stored or expired products lead to reduced efficacy, inadequate pest control, and economic losses. In some cases, frequent reapplication is needed compared to synthetic alternatives, further increasing labor and operational costs (Melo et al., 2025). The limited shelf life and delayed efficacy of biopesticides underpin many of the constraints highlighted in Table 3.
One of the most cited limitations of biopesticides is their relatively slow mode of action compared to synthetic pesticides, primarily due to their reliance on biological processes. However, this limitation is not uniform across all biopesticide types; it varies significantly depending on the biological mechanism involved. Many microbial and botanical agents require time to infect, colonize, or disrupt pest physiology, which can delay visible effects for several days or even weeks. This lag reduces their suitability for emergency pest control, where rapid suppression is critical. Bacterial toxins and botanicals are at the fast-acting end of the spectrum. Bacillus thuringiensis (Bt), for instance, delivers crystalline δ-endotoxins that perforate larval midgut cells within hours of ingestion, often killing susceptible caterpillars in 2–3 days (Sellamuthu et al., 2018). This makes Bt one of the few microbial products that can be used reactively against outbreaks. Similarly, neem (Azadirachta indica), through asadirachtin, disrupts molting hormones and feeding almost immediately, as shown in cotton and rice trials where Helicoverpa armigera larval activity dropped within a few days (European Food Safety Authority (EFSA) et al., 2025). Yet even here, the “speed” is relative. Unlike synthetic pyrethroids that produce near-instant knockdown, Bt and neem still allow a window during which crop damage may occur. Fungal and viral biopesticides illustrate the biological trade-offs more sharply. Beauveria bassiana and Metarhizium anisopliae require adhesion, cuticle penetration, and systemic colonization before killing the host, a process that typically spans 5–10 days (Petlamul and Prasertsan, 2012). This delay can frustrate growers facing acute infestations, but the same infection cycle enables horizontal transmission and long-term epizootics under humid conditions. Viruses such as nucleopolyhedroviruses (NPVs) are even slower, often taking 7–14 days for larval death, yet they uniquely self-replicate, persisting in the environment for successive pest generations (Wennmann et al., 2015). Semiochemicals push this spectrum to its slowest and most indirect form. Mating disruption, mass trapping, or repellency does not kill pests at all, but instead shifts population dynamics over weeks or seasons. Codling moth pheromone dispensers in apple orchards, for example, gradually suppressed pest pressure to non-detectable levels, but only after repeated seasonal deployment (Cardé, 2021). Their value lies not in crisis response but in reshaping population ecology, reducing reliance on chemicals and extending the durability of other controls. A gradient of action speed is evident: Bt and botanicals (relatively fast, but not instant) → fungi and viruses (slow, infection-based) → semiochemicals (indirect, behavioral, long-term). Each occupies a unique ecological niche. Rather than judging “slow action” as a universal weakness, it is more constructive to see it as a continuum that aligns with different pest pressures and farming goals.
Fast-acting agents suit outbreaks; slow-acting ones build resilience and suppress pest resurgence; semiochemicals restructure pest populations at the landscape scale. Case evidence increasingly shows that blending these categories, or combining them with reduced-dose synthetics, helps bridge speed gaps (Latifian, 2025; NarandŽić et al., 2025). For example, Bt applied with Beauveria bassiana against Helicoverpa armigera provided early mortality from Bt, while fungal infections enhanced control over time (Malinga and Laing, 2024). Similarly, NPVs applied alongside sublethal insecticides improved both knockdown and persistence (Hou et al., 2024). These integrations suggest that “slow action” is not merely a limitation, but a design parameter that, when understood and managed, can expand the versatility of biopesticides within integrated pest management (IPM).
Another major constraint limiting large-scale adoption of biopesticides is their relatively high cost compared to synthetic pesticides. This cost arises from several interrelated factors spanning production, formulation, storage, and application. Production costs are typically higher because biopesticides are based on living organisms or biologically active compounds that require controlled fermentation, culturing, and downstream processing. Unlike chemical pesticides that can be mass-produced through relatively inexpensive synthesis, microbial agents demand sterile facilities, nutrient-rich media, and continuous quality control systems to maintain viability and potency. For instance, producing Beauveria bassiana or nucleopolyhedroviruses (NPVs) often involves labor-intensive processes with lower yields compared to industrial chemical synthesis (Gopalkrishna et al., 2022). Formulation and stabilization costs further drive up prices. Since microbes and natural compounds are sensitive to heat, light, and desiccation, advanced formulation technologies are needed to extend shelf life and improve field stability. These technologies add significant costs but are essential to overcome rapid degradation under field conditions. Packaging innovations. Storage and distribution costs can be substantial because many biopesticides require cold-chain logistics to preserve microbial viability. This is particularly challenging in tropical or developing regions where infrastructure is limited. At the field level, biopesticides may also appear more expensive due to the need for repeated applications and specific dosage requirements, which translates to higher labor and input costs. For example, semiochemicals such as pheromone dispensers provide excellent population-level control but require multiple units per hectare, raising the overall expense. The high cost of biopesticides varies widely across categories depending on production and formulation requirements. Microbial biopesticides such as Bacillus thuringiensis (Bt) are among the least expensive because spore-forming bacteria can be mass-produced through fermentation and remain relatively stable, making them competitive with synthetic insecticides in some regions (Kumar et al., 2019). In contrast, fungal products like Beauveria bassiana and Metarhizium anisopliae are more expensive than Bt, since they require longer culture cycles, specialized substrates, and careful drying to maintain viability (Chandwani et al., 2023). Viral biopesticides such as nucleopolyhedroviruses (NPVs) are the costliest, as they depend on labor-intensive insect rearing and complex purification. Botanical products such as neem (Azadirachta indica) fall in the mid-range but are costlier than Beauveria bassiana production (Lavoir et al., 2022). Semiochemicals, such as pheromones, tend to be relatively expensive, though generally less so than botanical pesticides (Bourguet and Guillemaud, 2016). Their cost is influenced by the precision required in chemical synthesis and the extensive use of field dispensers. For example, controlling codling moths in European orchards using semiochemicals can cost nearly twice as much per hectare compared to conventional pesticide sprays (Kovanci, 2017). Among biopesticides, Bt and Beauveria are the most cost-competitive with synthetic pesticides, whereas semiochemicals, botanicals, and viral agents remain considerably more expensive, with costs increasing in that order.
Regulatory and legal hurdles also present a major bottleneck to the wider adoption of biopesticides, often making their registration slower, costlier, and more complex compared to that of synthetic pesticides. Although biopesticides are generally safer for humans and the environment, they are subject to the same rigorous frameworks designed primarily for chemicals, which demand extensive toxicological, ecotoxicological, and environmental fate data (Soetopo and Alouw, 2023). For small-scale innovators and local producers, especially in developing countries, meeting these data requirements can be prohibitively expensive. In the United States, the EPA's Biopesticide Division has streamlined some processes, allowing faster registration for products like Bacillus thuringiensis, but even there, dossiers require detailed molecular characterization and efficacy trials. In the European Union, the regulatory landscape is particularly challenging: Directive 91/414/EEC and its successors impose strict standards, and dossiers can take 5–7 years for approval, delaying market entry and discouraging investment (Marchand, 2024). In countries such as India and Nigeria, while registration systems exist under pesticide control authorities, the lack of harmonized guidelines for microbial, botanical, and semiochemical products creates uncertainty and inconsistent approvals. Moreover, because biopesticides are living organisms or complex natural mixtures, regulators struggle to apply conventional criteria, such as defining active ingredients in botanicals or stability in viral formulations, leading to prolonged negotiations and repeated dossier revisions. Legal frameworks also differ by region, making international commercialization difficult. For instance, a product approved in the UK or USA may require a completely new dossier in South Africa (Mawcha et al., 2025a). These hurdles not only raise costs but also reduce farmer access to innovative biopesticides, ultimately slowing their contribution to sustainable pest management. Biopesticides face significant regulatory and legal hurdles that slow their commercialization. Sundh and Eilenberg (2021) maintained that in the US, the EPA provides a relatively supportive framework with reduced data requirements, allowing approvals within 1–2 years, though small firms still struggle with efficacy trial costs. In contrast, the EU applies the same strict regulations as chemical pesticides, often taking 3–8 years and demanding extensive toxicological and environmental data, which discourages SMEs. In India, registration costs are lower, but weak enforcement allows substandard products that reduce farmer confidence, while in much of Africa, unclear and fragmented regulations delay both local and international products (Reddy et al., 2024). Globally, the lack of harmonized standards means the same biopesticide must undergo costly, duplicate evaluations in different regions. These hurdles disproportionately affect smaller developers, limiting innovation and slowing the adoption of safer, sustainable alternatives.
3.2 Biopesticide improvement strategies and slow release
Addressing the limitations of biopesticides requires achieving a balance between efficacy, environmental stability, and cost-effectiveness. The concept of slow release, which involves the controlled and sustained delivery of active substances over time, aligns closely with this objective. Effective slow-release systems rely on formulation technologies, protective additives, environmental stabilizers, biological enhancements, and optimized delivery methods. These strategies ensure that active ingredients are protected from degradation, consistently available to target pests at effective concentrations, and reduce the need for frequent reapplication. Accordingly, the discussion will now explore how each of these technologies facilitates slow release and serves its intended functions.
3.2.1 Advances formulation technologies
One of the major limitations of biopesticides is their short shelf life, which restricts large-scale use and consistent field performance. Advances in formulation technologies have been pivotal in overcoming this constraint by improving microbial stability, maintaining viability, and protecting active agents from abiotic stress. Techniques such as microencapsulation and nanocoating shield spores and metabolites against heat, UV radiation, desiccation, and microbial competition, thereby extending storage periods without significant loss of efficacy. Protective additives, including cryoprotectants, antioxidants, and humectants, further stabilize formulations by reducing oxidative stress and preserving moisture balance. Cutting-edge nanocarrier systems, including nanocapsules, nanogels, and polymeric nanoparticles, enable controlled release of actives at the target site, reducing the need for repeated applications (Anjaneyulu et al., 2024). Biodegradable polymers such as chitosan, cellulose, and polylactide provide encapsulation matrices that not only extend viability but also regulate discharge kinetics. For instance, chitosan-based nanogels have demonstrated superior encapsulation efficiency and prolonged microbial survival under field conditions (Pan et al., 2023). Likewise, the nanocoating of microbial cells enhances protection against environmental stress while ensuring gradual release and activity over time. Commercial carriers and dispersants, such as DV 066 and AgRHEA™ OD-EASY, enhance microbial shelf life by maintaining formulation integrity and preventing premature degradation (AgroPages, 2023). Oil-based products like BotaniGard® ES (Beauveria bassiana) and Met52® EC (Metarhizium anisopliae) now retain viability for 12–18 months, compared to under 3 months for unformulated spores (Swedaan and Al-Zurfi, 2023). Encapsulated nematodes in NemaGel®/NemaGlob® show 6–9 months stability vs. only weeks in suspension. Bacillus thuringiensis encapsulated by Valent BioSciences maintains >80% activity after 1 year, doubling conventional shelf life (Vemmer and Patel, 2013). In the field, chitosan nanogels extend the activity of Trichoderma and Bacillus from 7–10 days to 3–4 weeks, while nanoformulated NeemAzal® T/S increases residual activity from < 24 h to 5–7 days (Prasad et al., 2020). Stabilized Spinosad (Entrust®) formulations achieve up to 2 years of shelf life and 10–14 days persistence, reducing application frequency. These approaches collectively address the fragility of biopesticides, transforming them into more durable and market-ready products (Tamez-Guerra et al., 2018). Beyond extending shelf stability, the next challenge is achieving slow and sustained release under field conditions to ensure long-lasting pest suppression. Industry innovations, such as biodegradable encapsulation systems incorporated into seed coatings and foliar sprays (Padhan et al., 2024), further illustrate how controlled-release technologies translate into practical IPM solutions.
3.2.2 Advanced drying technologies
Advanced drying technologies have significantly enhanced the stability of biopesticides such as microbial pesticides. Techniques such as spray drying, freeze drying, spray-freeze drying, and electrospraying have transformed biopesticide formulation by producing concentrated, resilient powders with high microbial viability (Pattnaik and Mishra, 2022). Spray drying is particularly suited for large-scale production of heat-tolerant spore-formers like Bacillus thuringiensis, Beauveria bassiana, and Metarhizium anisopliae, while freeze drying effectively preserves delicate, heat-sensitive strains such as Pseudomonas fluorescens and Azospirillum spp. Spray-freeze drying generates porous particles that rehydrate easily, making them ideal for fragile microbes like Collimonas arenae, whereas electrospraying offers precise, low-temperature stabilization for sensitive fungi (e.g., Trichoderma spp.) and microbial metabolites like Bacillus subtilis lipopeptides (Pattnaik and Mishra, 2022). These drying methods not only extend shelf life but also facilitate the gradual release of active ingredients, reinforcing the efficacy and reliability of biopesticides in integrated pest management systems. Complementary packaging innovations, such as moisture barriers, UV shields, oxygen scavengers, and smart systems, extend shelf life and support slow release by maintaining formulation integrity during storage and transport (Janjarasskul and Suppakul, 2018). Functional additives like antioxidants (e.g., tea polyphenols and feruloylated soy glycerides), UV protectants (e.g., titanium dioxide, lignin biopolymers, and soyscreen), and stabilizers (e.g., Arabic gum, chitosan, and maltodextrin) enhance bioactivity and persistence (Chen K. et al., 2021). These components work synergistically to reduce oxidative degradation, shield against photodegradation, and ensure consistent release under variable environmental conditions. These technologies enhance the slow-release capabilities of biopesticides by stabilizing microbial agents and enabling controlled, sustained delivery in agricultural environments. In field applications, formulation type influences release dynamics and efficacy. Spray-dried formulations are suited for mechanized deployment, while liquid-fermented cultures integrate well with drip irrigation and foliar sprays. Emulsion-based systems, including Pickering emulsions, improve adhesion and spread on plant surfaces, enhancing effectiveness against pests like whiteflies and aphids (Ding et al., 2025). Advanced drying technologies, along with formulation advances, improve the stability limitations of biopesticides.
3.2.3 Smart delivery systems
Slow release is increasingly associated with smart delivery systems that employ stimulus-responsive carriers, such as pH-sensitive polymers, moisture-swellable hydrogels, enzyme-labile shells, or composite granules, to safeguard biopesticide agents until environmental conditions favor their efficacy, after which the payload is released in a controlled manner (Ma et al., 2025). For example, moisture-responsive hydrogels and oil-dispersion matrices have been developed to protect entomopathogenic fungi such as Beauveria bassiana and Metarhizium anisopliae from desiccation and UV stress, with conidia released and germinating under post-rain leaf wetness (Ma et al., 2025). pH-sensitive coatings have also been explored for baculoviruses, such as Helicoverpa armigera nucleopolyhedrovirus (HaNPV), where polymer films dissolve in the alkaline insect midgut to enable targeted infection and improved potency (Luo et al., 2022). Similarly, biodegradable soil granules incorporating hydrogel components have been used to deliver entomopathogenic nematodes like Steinernema carpocapsae, which remain dormant under dry conditions but are activated by soil moisture following irrigation or rainfall (Sharghi et al., 2025). Building on these principles, oil-dispersion carriers combining vegetable oils, emulsifiers, rheology modifiers, and humectants have been tested for fungal biopesticides in orchard crops, where they provide extended shelf life and rain-activated release (Hegde et al., 2023). Collectively, such responsive polymer-based systems exemplify how smart formulations can synchronize biopesticide activation with pest-favorable conditions, thereby enhancing field reliability, improving on-target activity, and reducing non-target exposure. Smart delivery systems are proving effective in sustainable agriculture by improving the stability and precision of biological control agents. Moisture-responsive granules improve nematode persistence, oil-dispersions protect fungal spores for rain-triggered activation, and pH-sensitive coatings boost baculovirus infection (De Waal et al., 2013; Lei et al., 2022; Schaly et al., 2022). Enzyme-labile carriers aid root colonization, nanocarriers stabilize essential oils, and controlled-release pheromone matrices enable codling moth disruption, collectively improving efficacy and reducing pesticide reliance. Despite these successes, the deployment of smart delivery systems requires careful formulation to preserve microbial viability, achieve predictable trigger thresholds, and meet regulatory requirements for both the biological active and the carrier material.
3.2.4 Biological optimisation strategy
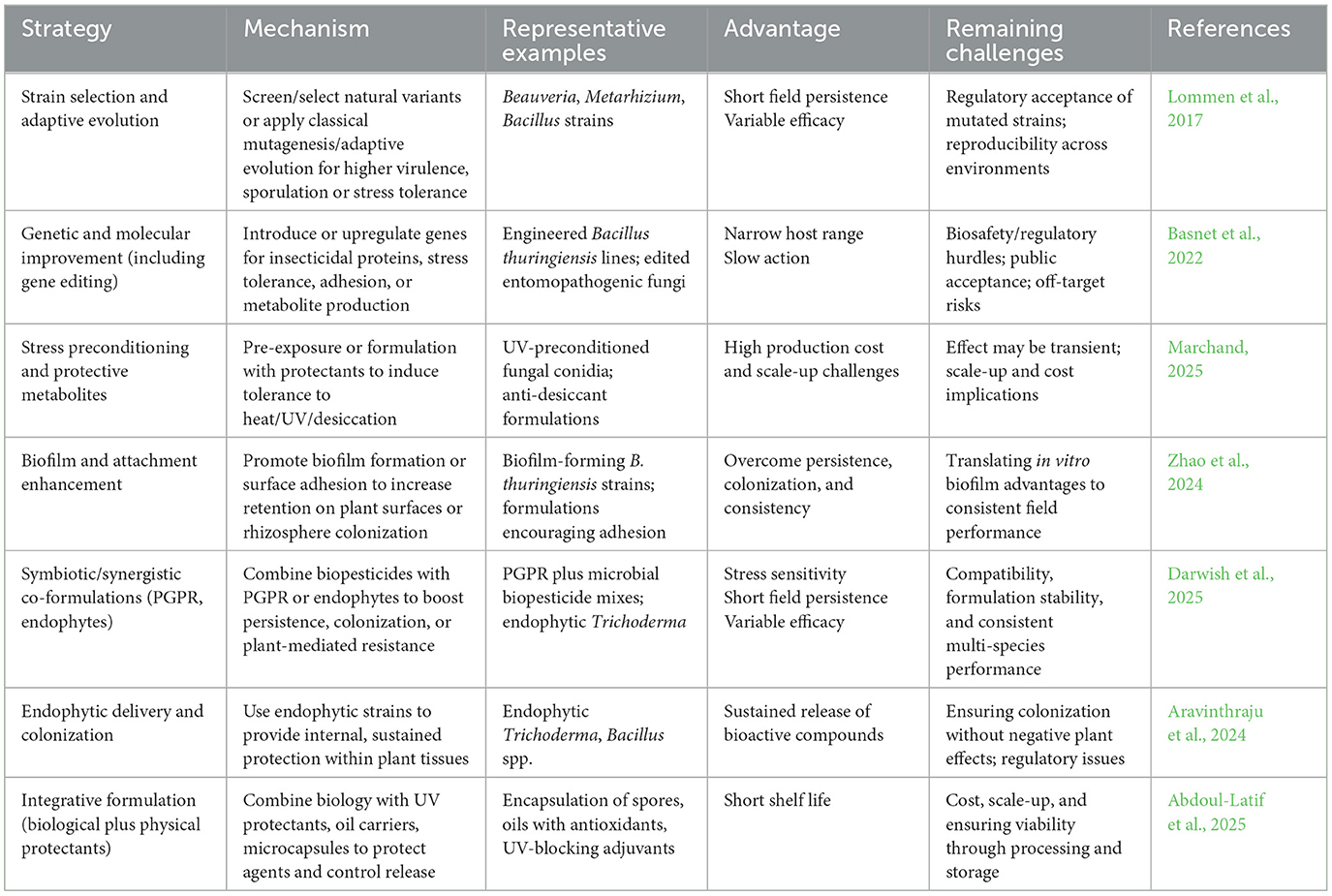
Besides advanced formulation technologies, strategic application and biological enhancement also contribute to the enhancement of slow-release system. Biological optimisation strategy enhances slow-release performance through the selection and genetic engineering of more virulent microbial strains. Recent advances, particularly through CRISPR-Cas9, have enabled precise modifications that significantly enhance the performance and reliability of biopesticides. These modifications include upregulating toxin-producing genes, suppressing traits that limit persistence, and introducing pest-specific toxins to broaden the host range while minimizing non-target effects. In Bacillus thuringiensis, CRISPR has been used to generate strains expressing multiple insecticidal proteins such as Cry and Vip toxins, thereby expanding pest-control spectra and extending activity (Gupta et al., 2021). Similarly, Bacillus subtilis has been optimized for increased lipopeptide production, Pseudomonas fluorescens for improved antibiotic synthesis and root colonization, and Trichoderma harzianum for the overexpression of cell wall–degrading enzymes that strengthen antifungal activity (Jha et al., 2016). Furthermore, entomopathogenic fungi can be modified for improved adhesion, penetration, and tolerance to UV radiation. Regulatory elements such as promoters have also been edited to modulate toxin expression for slow and sustained release, while stress-tolerance genes conferring UV resistance and desiccation survival have been introduced to improve field viability (Etemadifar et al., 2016). Beyond genetic approaches, biological optimization through strain selection and adaptive evolution has produced robust microbial variants with enhanced virulence and abiotic stress tolerance. Stress preconditioning and protective metabolites further increase conidial resilience, though these effects are often transient and costly to scale. Additional strategies such as promoting biofilm formation, leveraging plant growth-promoting rhizobacteria, and employing endophytic delivery systems improve persistence and plant-mediated resistance (Mmotla et al., 2025). These innovations are driving the development of next-generation biopesticides that are more potent, resilient, and sustainable, though challenges remain in achieving consistent field performance, ensuring large-scale viability, and navigating biosafety, regulatory, and cost constraints. Another aspect of biological optimization is summarized in Table 4. Molecular studies advance biological optimization by leveraging bioinformatics for precise pest identification, resistance profiling, and predictive modeling, while integrated genomics, transcriptomics, and computational modeling reveal resistance mechanisms, host preferences, and pest responses, thereby guiding the design of strains with enhanced traits such as faster infection and higher toxin yield.
3.2.5 Timely application of biopesticides
Timely application of biopesticides is essential for maximizing their effectiveness and supporting sustainable pest management. Because biopesticides often have short residual activity and are sensitive to environmental conditions, they are most effective when applied during stages when pests are most vulnerable, such as early larval, nymphal, or pre-infestation phases. For example, Bacillus thuringiensis (Bt) applied early in maize targets fall armyworm (Spodoptera frugiperda) larvae before they burrow into the whorl, while Trichoderma spp. or Pseudomonas fluorescens applied during early flowering in tomato helps suppress soil-borne pathogens like Fusarium wilt and early blight (Horikoshi et al., 2021). In cassava, early application of entomopathogenic fungi controls cassava mealybug and whiteflies, which are vectors of viral diseases, and in cowpea and rice, Beauveria bassiana and Metarhizium anisopliae manage Maruca vitrata and stem borers, respectively (Joelle et al., 2020; Sani et al., 2020). Applying biopesticides at the right time also ensures favorable environmental conditions for microbial survival, reduces the need for repeated treatments, lowers input costs, minimizes crop losses during critical growth stages, improves integration with other pest management strategies, supports resistance management, and promotes eco-friendly, sustainable agriculture. Effective integration of biopesticides with synthetic pesticides plays a crucial role in achieving slow-release pest control strategies that balance immediate suppression with long-term sustainability. Compatibility factors such as formulation composition, application timing, and environmental conditions must be carefully managed to preserve microbial viability and ensure gradual release. Inert ingredients like xylene, commonly found in chemical formulations, can impair microbial survival, while residual toxicity from synthetic pesticides may inhibit biopesticide performance if not timed appropriately. For instance, Beauveria bassiana is compatible with spinosad but negatively affected by chlorpyrifos, underscoring the importance of selective pairing (Mohsin et al., 2020).
Environmental parameters, particularly water pH, strongly influence microbial stability; many biopesticides degrade rapidly under alkaline conditions, compromising their slow-release potential. Species-specific interactions also matter: while Beauveria is harmless to predatory mites, it can adversely affect beneficial insects like Orius bugs, highlighting the need for ecological selectivity in IPM (Ambethgar et al., 2024). Field evidence supports the synergistic potential of combined applications. For example, Beauveria bassiana used alongside sublethal doses of imidacloprid significantly reduced Empoasca vitis populations in tea plantations (Pu et al., 2005). Similarly, co-application of Bacillus subtilis (Batistar WP®) and Beauveria bassiana (BotaniGard WP®) suppressed greenhouse whitefly and tomato powdery mildew without antagonism (Komagata et al., 2024). Bacillus amyloliquefaciens mixed with copper formulations improved fungal disease control, while hybrid products like Regev, which combine Tea Tree extract with difenoconazole, demonstrate how botanical and chemical components can deliver dual modes of action and support resistance management (Prakash and Shivakumar, 2025; Reuveni et al., 2023). Despite these successes, many biopesticides remain incompatible with synthetic pesticides, requiring careful tank mixing or sequential application strategies. A common approach involves applying a fast-acting synthetic pesticide first to achieve immediate knockdown, followed by a biopesticide for residual, slow-release activity. This complementary method enhances overall efficacy, delays resistance development, conserves beneficial organisms, and maintains ecological balance. These integration strategies address the slower action of biopesticides while preserving their environmental advantages. When combined with advancements in formulation, precise application, genetic optimization, and storage technologies, they contribute to more consistent, reliable, and sustained pest control.
Addressing the challenges of higher production costs, limited market penetration, and lengthy approval processes for biopesticides requires an integrated strategy that streamlines production, builds market confidence, and accelerates regulatory reviews. First, production costs can be reduced by optimizing resource inputs and processes, utilizing agricultural waste as a sustainable feedstock for microbial fermentation, adopting advanced fermentation techniques (such as solid-state, continuous, and process intensification methods), and improving downstream processing and formulation, all supported by public-private partnerships and government incentives. Strategies for reducing production costs of biopesticides have been documented by Sala et al. (2019). Simultaneously, expanding market penetration involves forming robust partnerships among researchers, regulators, producers, and farmers to harmonize quality standards and streamline registration (Marrone, 2023). This effort is further reinforced by targeted extension services, field demonstrations, and strategic marketing initiatives that build trust and lower adoption barriers within IPM programs (Diaz et al., 2020). Finally, shortening approval timelines requires the development of tailored regulatory frameworks that recognize the lower risk profiles of biopesticides, using risk-based approaches like predictive modeling and tiered testing (Levine et al., 2019), while enhancing coordination among national and international agencies through streamlined electronic submissions and clear communication (Deeksha et al., 2025). Together, these measures work cohesively to make biopesticides more competitive, widely accepted, and quickly accessible in the market. These approaches will ultimately address the lack of awareness surrounding the grassroots adoption of biopesticides.
4 Global trend of biopesticides awareness
The use of biopesticides is expanding and they are now integrated into sustainable agricultural practices. Sustainable agriculture is a holistic farming strategy designed to meet our current food, feed, and fiber needs while protecting the environment, enhancing economic viability, and advancing social equity for future generations. This approach embraces practices such as crop rotation, organic fertilization, water conservation, pest management, and the preservation of biodiversity, all of which nurture healthy soils and balanced ecosystems (Chhibber and Ravichandran, 2024). By focusing on efficient resource use and minimizing ecological damage, sustainable agriculture prioritizes reducing chemical inputs, especially synthetic pesticides. Consequently, as traditional chemical pesticide use declines, there is an increasing reliance on biopesticides as viable alternatives to combat more than 70,000 pests and diseases, with phytopathogenic microbes contributing to more than half (Tomar et al., 2024). With sustainable agriculture gaining momentum globally, the demand for biopesticides is rising: they currently represent about 5% of the $60B pesticide market (Basnet et al., 2022) and are projected to grow by approximately 15%−20% annually (Jiang and Wang, 2023), fuelled by stricter regulations on synthetic pesticides and heightened environmental and health concerns. The EPA has listed 430 active ingredients for more than 1,320 commercial biopesticide products (Rajput et al., 2021). Microbial pesticides account for 45%−63% of the biopesticides market, with bacterial-based products leading at 75%, while fungal-derived solutions hold approximately 10% of the share (Mnif and Ghribi, 2015). The key active ingredients in these categories are Bacillus thuringiensis (Bt) for bacterial biopesticides and Trichoderma spp. for fungal biopesticides. As of March 17, 2025, the U.S. Environmental Protection Agency (https://www.epa.gov/ingredients-used-pesticide-products/biopesticide-active-ingredients, accessed 11/05/2025) has a record of 136 microbial active ingredients, representing four viral strains, 14 bacterial species with 28 strains, and 20 fungal species with 26 strains, excluding five species from microalgae. In total, commercial bacterial and fungal biopesticides are primarily applied as biofungicides and bioinsecticides. Bacterial biopesticides (mostly as bioinsecticides) exhibit greater diversity than fungal biopesticides (mostly employed as fungicides). In turn, fungal biopesticides show more diversity compared to viral biopesticides, which are predominantly used as bioinsecticides.
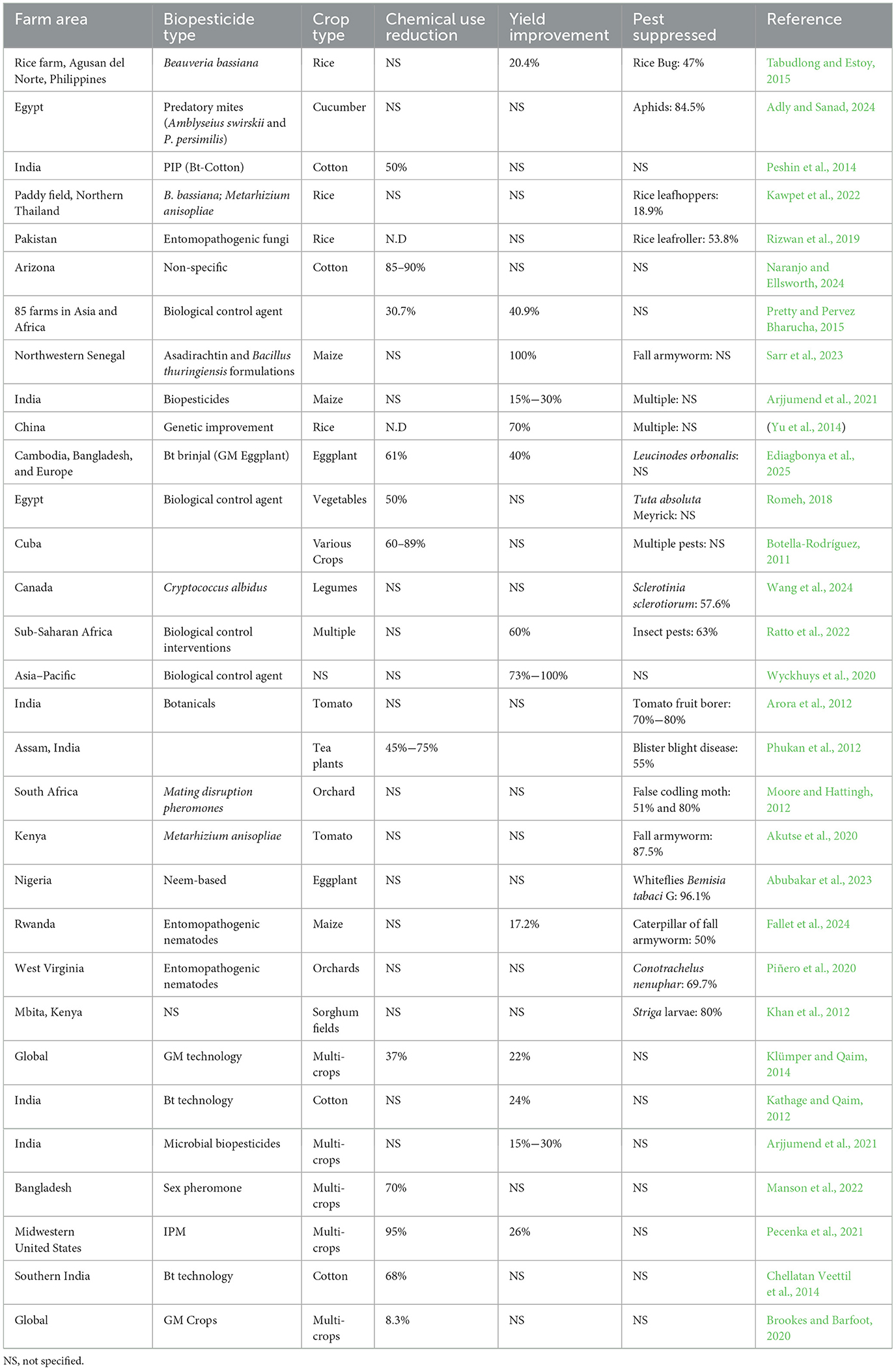
A comprehensive synthesis of publicly accessible data was conducted to elucidate key trends in global market demand, research and development, production indices, the number of allied companies, market revenue, adoption rates, and academic publications on biopesticides. This analysis drew on several public documents, including works by Ayilara et al. (2024), Chakraborty et al. (2023), Colmenarez and Vasquez (2024), Fusar Poli and Fontefrancesco (2024), Gc et al. (2022), Isman (2023), Marrone (2024), Srinivasan et al. (2019), cognitivemarketresearch.com,1 and databridgemarketresearch.com.2 The analysis indicates that from 2010, the beginning of significant biopesticide recognition, to 2024, global biopesticide demand surged by more than 80%. Over the period examined, shown in Figure 3, North America was the dominant player, accounting for 43% of global market demand, with Asia-Pacific ranking as the second-largest consumer, followed by Europe, the Middle East and Africa, and Latin America. North America also led in other investigated areas. Europe outperformed the other regions in terms of biopesticide commercialization, as evidenced by higher market revenue and adoption rates. The leading countries, ranked in decreasing order of publication output, are the USA (North America), India (Asia-Pacific), South Africa (Middle East and Africa), Germany (Europe), and Brazil (Latin America). With an increased focus on academia-industry partnerships, Asia-Pacific and the Middle East and Africa have the potential to lead in biopesticide production and commercialization, a strategy that has proven successful for developed economies. Although global awareness of sustainable practices has improved, the annual decline in synthetic pesticide use between 1990 and 2020 remained moderate at approximately 1.5%−2% (Cech et al., 2022; Kumar, 2023; Sharma et al., 2019). This modest decline is primarily driven by a shift in production toward synthetic pesticides with fewer regulatory restrictions, coupled with the inherent challenges of biopesticides (Assadpour et al., 2024) and inertia effects in the Middle East and Africa, Latin America, and some parts of Asia-Pacific. Globally, biopesticide awareness and adoption are hindered by economic barriers, limited knowledge exchange, weak extension services, and immature market infrastructure, with these constraints being more pronounced in developing regions, whereas North America continues to lead the market.
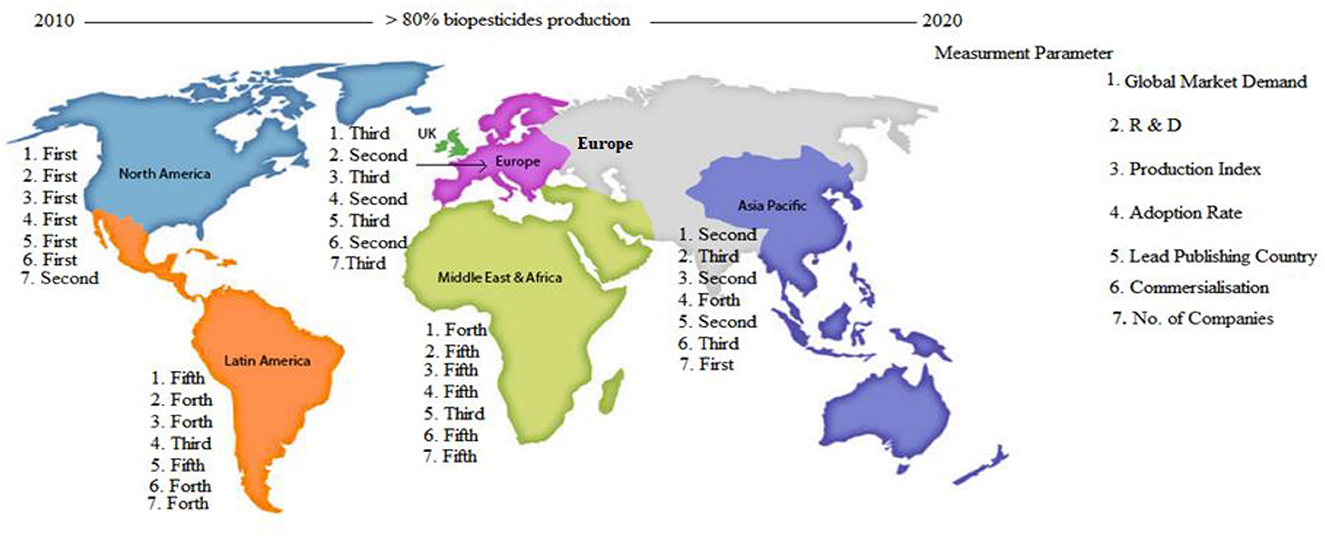
Figure 3. Regional distribution mapping of biopesticides across seven key parameters related to biopesticides between 2010 and 2014. The diagram illustrates a five-region trade structure, highlighting a global increase in biopesticide activity exceeding 80% between 2010 and 2020. It uses seven key parameters to assess regional performance: (1) global market demand for biopesticides, (2) research and development efforts, (3) biopesticide production index, (4) adoption rate, (5) volume of scientific publications, (6) commercialization level, and (7) number of active companies. Each region is ranked according to its position in these parameters. For instance, North America ranks first in global market demand (represented as “1. First”) and second in the number of biopesticide companies (represented as 7. Second). These rankings provide a comparative overview of how each region contributes to and leads in different aspects of biopesticide development and deployment.
5 Application and adoption of biopesticides
5.1 Methods of biopesticide application
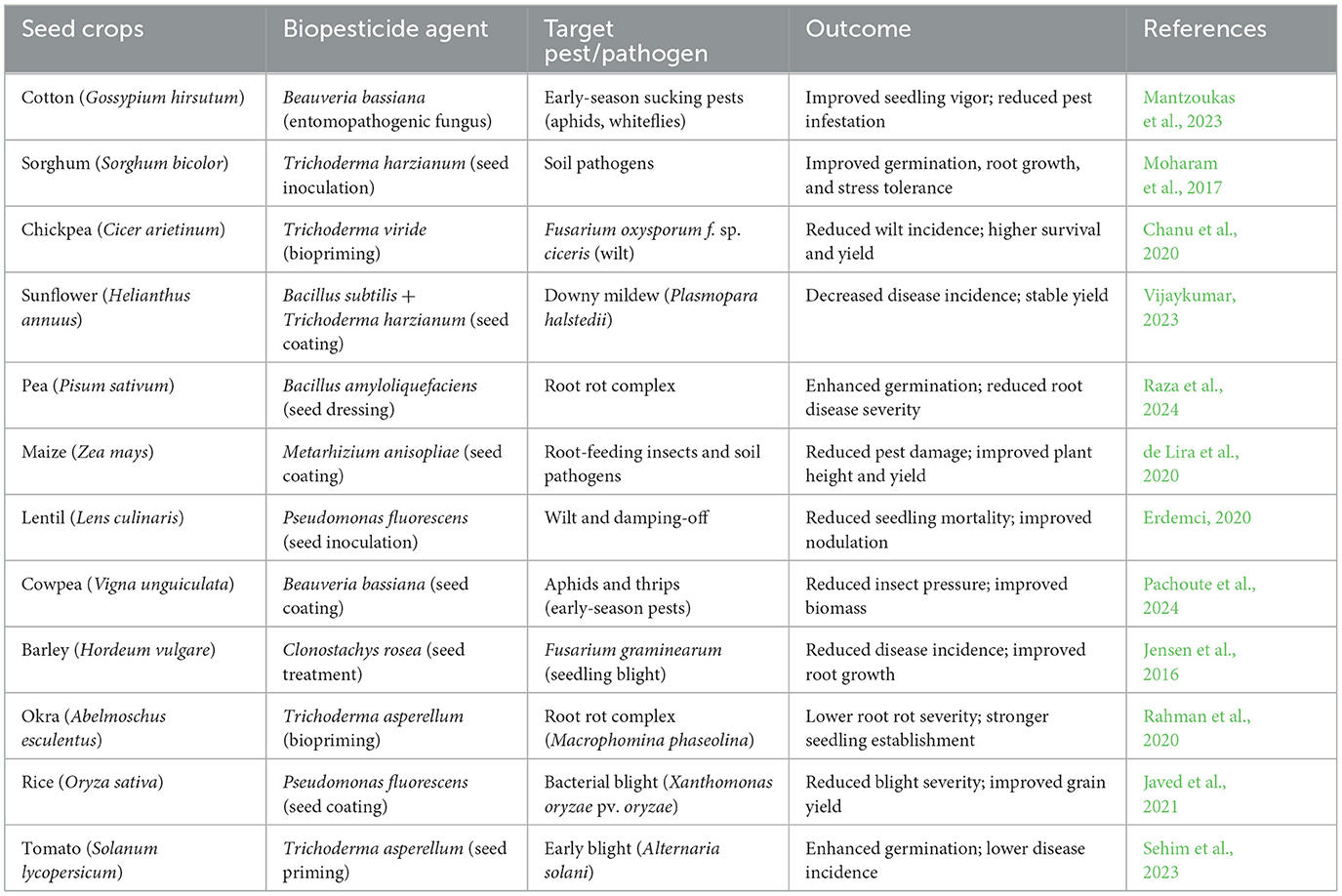
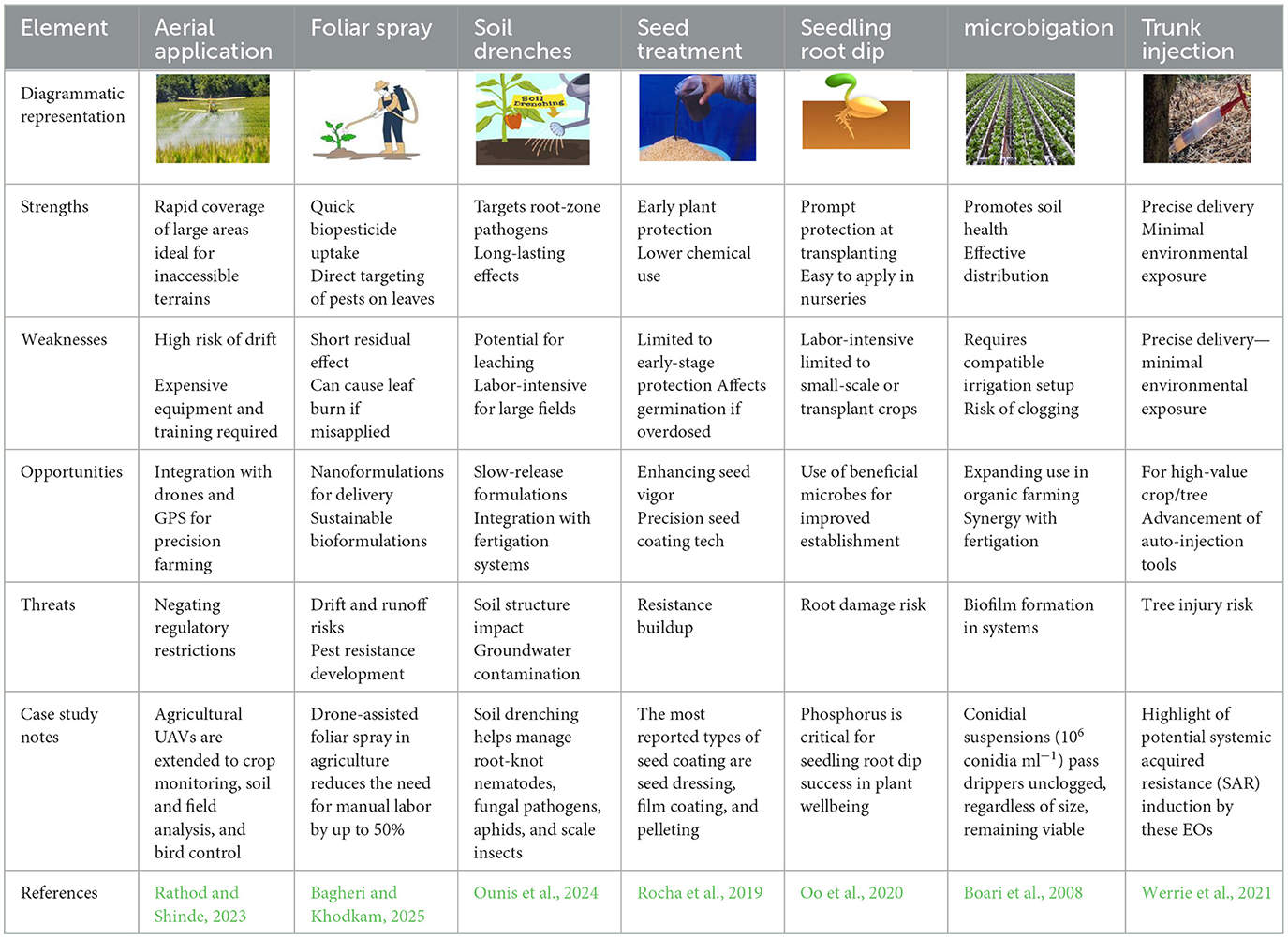
The effectiveness of biopesticides depends, in part, on their potency, formulation, and method of application. There are several application methods, which will be discussed here, starting with seed treatment. Seed treatment application of biopesticides involves coating seeds with biological agents, such as beneficial bacteria, fungi, or viruses, before planting. These biopesticides create a protective barrier, preventing soil-borne pathogens and insect pests from attacking young seedlings during germination and early growth stages. There are different types of seed treatments, including seed bio-priming, seed encapsulation, and gel seeding. Bio-priming integrates biological seed treatment with hydration, coating seeds with beneficial microorganisms like Trichoderma spp., Bacillus subtilis, or Pseudomonas fluorescens to suppress soil-borne pathogens and promote plant growth (Shil et al., 2025). This method improves seed vigor, accelerates germination, and strengthens plant defense mechanisms while reducing reliance on chemical pesticides. Seed encapsulation, on the other hand, encloses seeds within a protective coating, often using biodegradable polymers, hydrogels, or nutrient-rich materials, to enhance viability, storage, and controlled germination (Sarma et al., 2023). Widely used in precision agriculture, encapsulation ensures uniform seed distribution and facilitates microbial delivery for improved crop establishment. Gel seeding involves suspending seeds in a moisture-retaining gel to improve germination and early seedling development, providing a controlled environment that maintains hydration and prevents desiccation (Western, 2012). Particularly beneficial for small or delicate seeds like carrots and lettuce, gel seeding enhances seed-to-soil contact, reduces transplant shock, and improves seedling survival rates, making it a valuable tool for optimizing crop performance. Despite its simplicity, this application method has demonstrated consistent efficacy across diverse agricultural systems, as evidenced by a global meta-analysis by Lamichhane et al. (2022). The analysis reported significant improvements, including a 7% increase in seed germination, a 91% improvement in seedling emergence, a 53% enhancement in plant biomass, a 55% reduction in disease incidence, and a 21% increase in crop yield. Representative success stories of seed treatment are summarized in Table 5.
Some benefits of seed treatments include a reduced need for foliar pesticide applications and a lower quantity of active ingredients compared to traditional spraying methods. Choudhary et al. (2021) has shown that biopesticide seed treatments can effectively control soil-dwelling insects, plant-parasitic nematodes, fungal pathogens, root rot, and damping-off diseases. Seedling root dip is a valuable technique that enhances plant establishment, early growth, and resistance to soil-borne diseases by treating seedling roots with a nutrient-rich or biologically active solution before transplanting. The process involves trimming damaged or excessively long roots and then immersing them in the prepared dip solution for several hours, ideally up to 24 h, to allow absorption of essential nutrients and beneficial microbes (Gregorio et al., 2010). This coating helps seedlings adapt to new soil conditions, reducing transplant shock and improving survival rates, making root dips particularly useful for bare-root plants. The root dip mixture typically includes organic materials such as compost, manure, or microbial inoculants like Trichoderma spp. and Pseudomonas fluorescens, which promote beneficial soil interactions while suppressing harmful pathogens. By optimizing root health and microbial activity, this method ensures stronger plant development and resilience in various growing conditions. Seedling root dip treatment is especially effective for crops that require early protection against nutrient deficiencies and environmental stress (Ronga et al., 2021). Research highlights its benefits for different plant types, particularly those vulnerable to soil-related challenges. For instance, Ronga et al. (2021) demonstrated that pepper seedlings treated with phosphorus-enriched root dips exhibit improved nutrient uptake and resistance to adverse soil conditions. Similarly, rice seedlings benefit from phosphorus and biofertilizer slurry treatments, leading to stronger root development and increased resilience against acidic soils (Goswami and Kalidas-Singh, 2023). By integrating seedling root dips into cultivation practices, farmers can enhance plant vigor, improve crop survival rates, and establish more sustainable agricultural systems.
Soil drenching is an effective technique for applying biopesticides directly to the root zone through water-based solutions, ensuring beneficial microbes or bioactive compounds penetrate the soil to target pests, pathogens, and nematodes while promoting plant health (Sri et al., 2025). Unlike foliar sprays, this method allows biopesticides to establish themselves in the rhizosphere, forming a protective barrier against harmful organisms. Common biopesticides used in soil drenching include microbial agents such as Trichoderma spp., Bacillus subtilis, and Pseudomonas fluorescens, which suppress fungal infections and enhance root development. Additionally, entomopathogenic fungi like Beauveria bassiana and Metarhizium anisopliae effectively control soil-dwelling insect pests. The success of soil drenching depends on maintaining optimal conditions, including soil moisture, pH balance, and proper dilution ratios, to ensure microbial activity and pest suppression. For best results, soil moisture should be at field capacity, meaning adequately moist but not waterlogged, allowing biopesticides to reach the root zone without excessive runoff. The ideal pH range is 5.5–7.0, as extreme acidity or alkalinity can diminish microbial viability (Adeniji et al., 2024). Dilution ratios vary depending on the formulation, but a common recommendation is 1:100 to 1:500, ensuring proper dispersion while maintaining potency for effective pest control (Aremu et al., 2012). Soil drenching helps manage root-knot nematodes (Meloidogyne spp.), fungal pathogens such as Phytophthora spp., Fusarium spp., and Pythium spp., as well as aphids and scale insects (Aphis spp., Coccidae family), using moist, organic-rich soils that are ideal for the activity and persistence of these BCAs. Soil drenching has shown notable success across crops: Trichoderma harzianum reduced damping-off in tomato and improved seedling vigor in India (Bhardwaj, 2019); Pseudomonas fluorescens suppressed sheath blight and enhanced yield in rice (Nur Mawaddah et al., 2023); and Beauveria bassiana controlled root-feeding insects while promoting growth in cucumber under greenhouse conditions (Spescha et al., 2023). These examples demonstrate that soil drenching can combine disease suppression, pest reduction, and plant growth promotion, making it a versatile strategy in sustainable agriculture.
Agricultural pest control mechanisms brought about through irrigation systems are known as microbigation. This technique focuses on pest control and soil health improvement by introducing beneficial microbes and entomopathogenic nematodes that suppress harmful pathogens, enhance plant resilience, and promote sustainable farming practices. This method integrates pest management with plant nutrition delivery, ensuring that biopesticides reach the root zone and foliage effectively. Thus, microbigation is particularly useful for managing soil-borne diseases and improving microbial diversity in agricultural ecosystems (Bonaterra et al., 2022). A mix of microbigation and fertigation reduces labor costs, minimizes environmental contamination, and enhances the absorption of biopesticides, and maintains soil moisture levels, promoting microbial activity and thus leading to improved pest control and crop health. The controlled delivery through irrigation systems ensures that biopesticides remain active for longer periods, reducing the need for frequent applications. The active period of biopesticides can further be enhanced through liquid formulations (emulsion and suspension), encapsulation formulations (microencapsulation), and oil-based formulations. The liquid formulation ensures uniform distribution, microencapsulation enhances stability, and the oil-based formulation promotes adherence to the root system (Hegde and Vijaykumar, 2022). To avoid clogging in irrigation lines and enhance absorption by plant roots, microbial biopesticides should be formulated with stabilizers and surfactants. For optimal operation, the water pH should be around 5.5–7.0 to avoid clogging, the temperature should be around 18–25 °C to support microbial activity, and using mesh filters (100–200 microns) helps remove debris and prevent blockages (Wolcott et al., 2022). Bacillus thuringiensis and Trichoderma spp. are better suited for this application method because they target soil-borne pathogens and insect pests. Microbigation has shown notable successes: in Italy, Trichoderma harzianum and Paecilomyces lilacinus remained viable and suppressed soil-borne pathogens (Boari et al., 2008); in Brazil, drip-applied Azospirillum brasilense in maize enhanced root growth, nitrogen uptake, and yield (Galindo et al., 2022); and in Spain, Trichoderma asperellum fertigation in tomato reduced Fusarium oxysporum while improving vigor and fruit production (Leuratti et al., 2025), confirming the potential of irrigation-based microbial delivery for the sustainable intensification of agriculture.
Trunk injection is a precise biopesticide application method that delivers pest control agents directly into the stem or trunk of woody plants. By injecting biopesticides into the xylem, active ingredients are transported throughout the plant via the transpiration stream, ensuring systemic protection against pests such as borers and fungal pathogens (Li and Nangong, 2022). This technique is especially valuable for managing diseases in trees and high-value crops where foliar sprays or soil drenches may be ineffective or environmentally hazardous. One of its key advantages is the efficient delivery of biopesticides while minimizing environmental impact. Unlike aerial or soil applications, trunk injection eliminates spray drift, reduces worker exposure, and prevents contamination of non-target organisms. It is widely used in forestry, urban landscaping, and commercial agriculture, particularly for crops like avocados and citrus trees (Archer, 2022; Li and Nangong, 2022). Biopesticides used in trunk injection typically consist of microbial agents, botanical extracts, and carrier substances that facilitate systemic movement within the plant. Microbial biopesticides, such as Beauveria bassiana and Metarhizium anisopliae, target insect pests by colonizing their bodies and disrupting physiological functions. Botanical extracts, including essential oils like mint and cinnamon, have been studied for their ability to move through the vascular system, providing pest control benefits (Werrie et al., 2021). Carrier substances, such as water-based or oil-based solutions, improve absorption and stability, ensuring effective distribution of active ingredients. This method is used to treat various plant diseases, particularly those affecting trees and woody plants. Common diseases managed through trunk injection include oak wilt, Dutch elm disease, and sudden oak death (Phytophthora infection). According to Passey et al. (2019) trunk injection can aid combat apple scab and fire blight in apple trees, root rot and thrips in avocado trees, and powdery mildew and downy mildew in grapevines. In Florida, injection of oxytetracycline and microbial antagonists into citrus trees significantly suppressed Candidatus Liberibacter asiaticus (Hu et al., 2018), the causal agent of huanglongbing, prolonging tree productivity. In California vineyards, trunk injection of Trichoderma harzianum reduced grapevine trunk diseases caused by Phaeomoniella chlamydospora and Neofusicoccum spp., improving vine vigor and yield (Wallis et al., 2025). Similarly, in Central America, avocado orchards injected with Bacillus subtilis formulations experienced reduced wilt symptoms associated with Ralstonia solanacearum and Phytophthora cinnamomi, contributing to healthier root systems and an extended orchard lifespan (Hyakumachi et al., 2013). These examples demonstrate trunk injection's potential as a precise delivery method that enhances disease suppression while minimizing off-target effects.
Aerial application involves the dispersal of agricultural treatments using aircraft or drones, enabling extensive coverage of large farms while optimizing labor efficiency and ensuring uniform distribution of the applied substances. This method is designed for speed, precision, and effectiveness, helping to control pests, enhance crop productivity, and reduce soil compaction. Technological advancements, such as GPS-guided spraying and precision targeting, have further improved aerial application, making it more effective and environmentally sustainable (Rathod and Shinde, 2023). Its effectiveness depends on low wind speeds (below 10 mph) to minimize drift, moderate temperatures to prevent rapid evaporation, and humidity levels above 50% to ensure proper adhesion of spray droplets to crops (Awais et al., 2022). The best time for aerial spraying under these conditions is early morning or late afternoon when weather factors are most favorable. The efficiency of this method is further enhanced by factors such as large land area, uniform crop height, and even terrain, ensuring optimal coverage and penetration (Chen H. et al., 2021). To address environmental challenges that may impact the effectiveness of the application, GPS-guided spraying and precision targeting may be required to minimize waste and maximize efficiency. Environmental sustainability is maximized when the applied substances are biopesticides or other biological control agents, reducing chemical exposure and promoting eco-friendly pest management. As technological advancements continue, drones may be equipped with sophisticated sensors and data analytics capabilities to enable real-time monitoring of pest populations, leading to more strategic and environmentally friendly spraying techniques, defining precision agriculture. In Brazil, aerial spraying of Bacillus thuringiensis (Bt) formulations on soybean successfully controlled caterpillars such as Anticarsia gemmatalis, reducing chemical insecticide use across millions of hectares (Cai and Dimopoulos, 2024). In North America, aerial release of baculoviruses against the gypsy moth (Lymantria dispar) achieved significant population suppression while preserving natural enemies. Likewise, in Australia, aerial application of entomopathogenic fungi such as Metarhizium anisopliae has been employed to manage locust swarms, providing environmentally safer alternatives to broad-spectrum chemical insecticides (Scanlan et al., 2001). These case studies highlight the potential of aerial biopesticide applications to deliver scalable and effective solutions within integrated pest management programmes.
The most suitable biopesticides for aerial application in agriculture are microbial biopesticides due to their effectiveness, environmental safety, and scalability. Among these, Bacillus thuringiensis (Bt) is widely used for controlling caterpillar pests in crops like corn and cotton because of its stability as a spore-forming bacterium and its tolerance to UV exposure (Khan et al., 2016). Similarly, entomopathogenic fungi such as Beauveria bassiana and Metarhizium anisopliae have proven highly effective in oil-in-water formulations that withstand aerial dispersal conditions, targeting a broad spectrum of insect pests (De Oliveira et al., 2018). Viral biopesticides like Helicoverpa armigera nucleopolyhedrovirus (HaNPV) are used in cotton pest management and benefit from microencapsulation techniques that enhance their persistence in open environments (Pobożniak and Olczyk, 2025). These microbial biopesticides, their formulations, and the optimal environmental conditions are also suitable for foliar spray, a less sophisticated method of biopesticide application. It involves applying biologically derived substances directly to plant leaves (using sprayers like handheld, boom, backpack etc.), allowing for rapid absorption and enabling plants to utilize the active compounds quickly to combat pests and diseases. Foliar spray aids the active ingredient target insect pests, fungal infections, and bacterial pathogens that reside on the plant surface, reducing their population before they can cause significant damage. One unique advantage of foliar application is its ability to deliver biopesticides precisely to areas where pests are most prevalent. Modern advancements such as drone-assisted spraying and electrostatic technology improve droplet adhesion and coverage, maximizing pest control efficiency (Wu et al., 2024). Foliar spray remains one of the most widely adopted methods of biopesticide application, with demonstrated success across diverse crops. In India, foliar spraying of Beauveria bassiana effectively reduced whitefly (Bemisia tabaci) infestations in cotton, contributing to lower reliance on synthetic insecticides (Ghosal, 2018). In Kenya, neem-based foliar formulations controlled diamondback moth (Plutella xylostella) in cabbage, improving marketable yield and farmer income (Akol et al., 2002). Similarly, in the United States, foliar applications of Bacillus subtilis biocontrol products suppressed powdery mildew in strawberries, enhancing fruit quality and reducing disease-related losses (Awan and Shoaib, 2019). These instances highlight foliar spraying as a reliable and effective approach for administering biopesticides within integrated crop protection frameworks. Table 6 presents a consolidated overview of the seven application methods, outlining their respective strengths, weaknesses, opportunities, and threats, along with supporting diagrams and cited references.
Once the active ingredients are delivered to specific parts of plants, they produce three primary effects: antimicrobial activity, growth promotion, and/or induction of systemic resistance (ISR). Microbial biopesticides employ various mechanisms to directly inhibit pathogen growth and insect herbivores. These beneficial microbes secrete a diverse array of secondary metabolites, including toxins, chitinases, glucanases, lipopeptides, siderophores (iron-chelating molecules), hydrogen cyanide, and phenazine derivatives, all exhibiting broad-spectrum antimicrobial activity. Some of these microorganisms (mostly bacteria) also act as plant growth-promoting microorganisms (PGPMs), enhancing crop growth through intricate biochemical processes that improve nutrient acquisition and bolster plant defenses (Liu et al., 2025). For example, PGPMs synthesize phytohormones such as auxins, gibberellins, and cytokinins, which stimulate cell division, elongation, and differentiation, thus enhancing overall plant development. Moreover, certain PGPMs, like rhizobia and free-living nitrogen fixers, produce nitrogenase, converting atmospheric nitrogen into nitrate essential for plant proteins and other cellular components. Many PGPMs additionally release organic acids that solubilize otherwise inaccessible phosphorus in the soil, making this critical nutrient available for uptake. These processes support vital metabolic functions ranging from chlorophyll synthesis to DNA replication, contributing to both immediate growth and long-term plant health. Furthermore, when biological control agents (BCAs) interact with plant cells, their components are recognized as microbe-associated molecular patterns by pattern recognition receptors (Bhardwaj et al., 2024). This recognition triggers signaling cascades, such as mitogen-activated protein kinase pathways, which lead to the upregulation of defense-related genes and the production of protective compounds, including phytoalexins and pathogenesis-related proteins. The stability of the biopesticides in these processes is enhanced by appropriate formulation and delivery techniques, as extensively elaborated in Kala et al. (2020). Given the wide array of biopesticides and their complementary advantages over synthetic chemical pesticides it will be of interest to understand their adoption and use in sustainable agriculture.
5.2 Application of major types of biopesticides in case study scenarios
The application and implementation of biopesticides in large-scale farming is gaining traction in defining sustainable agriculture. Almost all types of biopesticides types are used, but microbial biopesticides are the most popular. Microbial biopesticides have emerged as powerful tools in sustainable pest management, offering effective alternatives to synthetic pesticides amid growing concerns over resistance development and environmental impact. Raghuvanshi (2015) documented the effective field deployment of entomopathogenic fungal consortia comprising Beauveria bassiana and Metarhizium anisopliae, similar to commercial formulations such as Bio-Magic Plus®, Eco-Bio Insecticide®, and Myco-Jet®, in Maharashtra, India. When administered to crops including aubergine, sugarcane, tomato, and chili, these biocontrol agents achieved pest suppression efficiencies of up to 85%, with concomitant yield enhancements reaching approximately 25%. Mollah and Hassan (2023) reported that the sequential deployment of Trichoderma harzianum, comparable to commercial formulations such as TRICHODEX®, Foster®, and Bactogang-24®, in aubergine (Solanum melongena) cultivation in Bangladesh markedly suppressed Leucinodes orbonalis infestations. This intervention resulted in a yield increase from 14.8 to 21.7 tons per hectare, representing a 46% enhancement relative to untreated control plots. Bacillus thuringiensis (Bt), as demonstrated by Ortiz and Sansinenea (2022), was formulated in products analogous to Dipel®, Thuricide®, and Ecotech Pro® and applied to maize and various horticultural crops in Mexico and Colombia. These biopesticidal applications achieved pest suppression rates between 70% and 90%, accompanied by yield increases of 15% to 20%, thereby validating the compatibility of Bt-based interventions within both organic and conventional agricultural paradigms. Similarly, Fenibo et al. (2023) documented a report in which smallholders employing Bt in maize and cabbage farming in Ghana and Kenya experienced pest reductions of 65%−85%, yield increases of up to 28%, and a 60% decrease in synthetic pesticide use. A study conducted in commercial vegetable greenhouses in the Netherlands assessed the efficacy of Pseudomonas fluorescens, comparable to Bio-Foray® and Pseudostar®, and Trichoderma harzianum in suppressing soil-borne phytopathogens (Mihajlović et al., 2017). The treatments reduced disease incidence by 60%, improved plant resilience, and led to 10%−15% higher yields compared to chemically managed controls. The observed trend (as shown in Table 7) of using microbial biopesticides suggests that the percentage of pest reduction is higher than the percentage of reduction in synthetic pesticide use, which, in turn, is higher than the percentage of yield increase.
The trend observed in microbial biopesticides also appears in the use of botanical biopesticides in sustainable agriculture. For example, neem-based extracts from Azadirachta indica, widely applied in Indian cropping systems, achieved up to a 75% reduction in pest populations, a 50% decrease in synthetic pesticide use, and a 20% increase in crop yields (Ngegba et al., 2022). Some examples of commercial neem extract products are NeemBaan®, Margosom®, Anosom®, and Derisom®, while Pyrethrum 5EC®, EverGreen®, and Monterey Bug Buster-O® are examples of pyrethrum-based formulations. Similarly, pyrethrum-based formulations derived from Chrysanthemum species, utilized in African fruit orchards, resulted in an 85% pest reduction, a 60% decline in chemical pesticide application, and a 25% yield boost (Riyaz et al., 2022). The case studies reported by Munyore and Rioba (2020), Lengai et al. (2020), and Malinga and Laing (2022) follow the same trend. Munyore and Rioba (2020) reported a 65% decline in pest incidence, a 45% reduction in supplementary pesticide usage, and an 18% improvement in marketable yields where garlic (Allium sativum) extracts were used in greenhouse vegetable production. Lengai et al. (2020) recorded a 70% decrease in pest pressure, a 55% reduction in conventional pesticide reliance, and a 20% increase in yield where chili pepper (Capsicum spp.) extracts were used in integrated pest management strategies for tomatoes and cucumbers across Southeast Asia and Africa. As expected, Malinga and Laing (2022) achieved a 75% reduction in bollworm infestation, a 65% decrease in synthetic pesticide use, and a 22% improvement in yield while treating a cotton plantation with eucalyptus oil extracted from Eucalyptus species. The trend can be expressed as ↓I% > ↓P% > ↑Y%, where ↓I% is the percentage reduction of pest infestation, ↓P% represents the percentage reduction of synthetic pesticide use, while ↑Y% stands for the percentage increase in yield. For simplicity, we term this expression the IPY trend, representing the common direction of tiered performance indicators for the successful application of biopesticides.
The most significant parameter that drives the trend is the pest loss percentage (↓I%), which can be used to indicate the success of biocontrol measures, as shown by the use of entomopathogenic nematodes (EPNs) in different major cropping systems. In Poland, Steinernema feltiae (e.g., Entonem® and NemaShield®) and S. carpocapsae (e.g., Entonem®) were successfully applied as foliar treatments in wheat fields to combat the cereal leaf beetle (Oulema melanopus), achieving up to 70% larval suppression and improving plant vigor (Mazurkiewicz et al., 2021). In Egypt, large-scale integration of Heterorhabditis bacteriophora (NemaSeek®) and S. glaseri (comparable to Galleria-Pro®) with organic soil amendments in wheat and barley fields led to 60%−85% suppression of cereal cyst nematodes and yield gains under sustainable cultivation practices (Abd-Elgawad, 2025). A European case study in organic horticulture demonstrated that EPNs like H. bacteriophora and S. feltiae, applied via drip irrigation in vegetable systems, achieved up to 90% pest control, confirming their compatibility with large-scale organic systems (Furmanczyk and Malusà, 2023). Additionally, in North African tomato production, cadaver-based applications of Heterorhabditis indica provided 65–75% reduction in root–knot nematodes and sustained crop protection for up to 2 months while improving fruit yield and quality (Abd-Elgawad, 2024). Koppenhöfer et al. (2020) reported that globally, mechanized application of S. feltiae and H. bacteriophora in strawberries and turfgrass systems yielded 50%−90% pest reduction, confirming the adaptability of EPNs to high-value crops and scalable operations. These studies affirm the scalability, pest specificity, and value of EPNs in ecologically intensive farming models.
Recent large-scale field studies have underscored the robust potential of natural enemies to deliver significant pest suppression and crop protection benefits in commercial agriculture. In Belgian cereal farms, the implementation of within-field wildflower strips led to a 60%−85% increase in aphid predation by natural enemies such as lady beetles and hoverflies, demonstrating the ecological intensification value of conservation biological control (Hatt et al., 2017). Beyond pest management, the integration of within-field wildflower strips confers a range of ancillary agronomic benefits. These include enhanced biodiversity, improved soil structure, attenuated soil erosion, optimized water infiltration, bolstered pollination efficacy, and elevated aesthetic quality of agricultural landscapes. Similarly, a meta-analysis across Sub-Saharan African vegetable systems revealed that augmentative releases of predators like Phytoseiulus spp. achieved 35%−80% pest suppression while preserving the diversity of native beneficial insects (Ratto et al., 2022). In Chinese wheat systems, large-scale applications of Aphidius gifuensis and Chrysoperla sinica led to up to 80% reduction in aphid populations and 15%−18% yield increases, reinforcing the yield benefits of natural enemy-based interventions (Ali et al., 2018). Mass releases of Trichogramma spp. in Southeast European maize fields effectively suppressed Ostrinia nubilalis by over 70%, drastically reducing pesticide reliance and representing one of the most successful parasitoid-based controls in monoculture grain systems (Ivezić et al., 2022). Furthermore, in organic strawberry systems in California, the strategic use of banker plants in combination with Orius insidiosus reduced thrips and whitefly damage by 60%, contributing to improved fruit quality without synthetic inputs (Van Lenteren, 2012). Collectively, these studies affirm that natural enemies provide ecologically grounded alternatives to chemical pest control that can be effectively applied across diverse crops and regions.
Advances in agroecological pest control have demonstrated the effectiveness of live pesticidal plants, used as repellent, banker, and trap crops (RBT plants), in large-scale farming systems, particularly within push-pull frameworks. Push–pull frameworks constitute an integrated pest management (IPM) system that employs dual tactical interventions: intercropped repellent species that deter pest colonization within the field (push), alongside perimeter-planted attractant trap crops that lure pests away from the target crop (pull), thereby optimizing pest suppression and safeguarding crop yield. In East African cereal systems, the integration of Desmodium (push) and Brachiaria (pull) across over 500,000 hectares has consistently achieved over 80% suppression of stemborers and 90% control of Striga weed, leading to substantial maize yield recovery (Khan et al., 2023). Similarly, a novel combination of nighttime light traps with push–pull intercropping in Ethiopia reduced fall armyworm (Spodoptera frugiperda) egg mass infestations by up to 90%, enhancing farmer-led maize protection efforts (Gebreziher and Gebreazgaabher, 2024). The later authors also reported that in China, maize used as a trap crop in vegetable systems attracted up to 80% of fall armyworm (Spodoptera frugiperda) infestations to field margins, significantly limiting pest pressure on main crops and reducing pesticide use.
Cabbage farms in Tanzania that deployed an integrated pest management approach, utilizing African nightshade as a repellent and mustard as a trap crop, achieved up to an 80% suppression of diamondback moth populations, resulting in yield increases of 20%−30% without the use of synthetic pesticides (Okoma et al., 2025). In crucifer vegetable production, push–pull systems integrating marigold and mustard achieved a 70%−85% reduction in pest populations and enhanced beneficial parasitoid activity, as demonstrated in Brazil (da Silva et al., 2022). Likewise, Kenyan tomato systems that utilized Ocimum spp. as repellent intercrops and decoy trap plants saw up to 78% control of Tuta absoluta, alongside increased biodiversity of natural enemies (Chidawanyika et al., 2025). Finally, UK strawberry farms using synthetic semiochemical-enhanced trap crops around fields reported 60%−70% suppression of the European tarnished plant bug (Lygus rugulipennis), a significant outcome achieved without synthetic pesticides (Fountain et al., 2021). These case studies jointly substantiate the strategic transferability of botanical intercropping and trap-cropping systems as sustainable pest management interventions across diverse agroecosystems. Beyond using non-crop live plants, crop plants also have been fortified with protectants, producing RNAi- and Bt-crops.
RNA interference (RNAi) is a post-transcriptional gene silencing mechanism that utilizes double-stranded RNA (dsRNA) to initiate a cascade resulting in the degradation of complementary messenger RNA (mRNA) sequences. The dsRNA is initially processed into short interfering RNAs (siRNAs) by the RNase III enzyme Dicer (Zhang et al., 2025). These siRNAs are then incorporated into the RNA-induced silencing complex (RISC), where the strand complementary to the target mRNA is selectively retained. The RISC, with Argonaute proteins as central components, directs sequence-specific cleavage of the target mRNA, thereby effectively downregulating gene expression. RNAi presents a highly promising strategy for pest control through its inherent target specificity (Chen et al., 2024). This molecular approach allows for the silencing of genes crucial for pest reproduction, survival, or pathogenicity, without adversely affecting non-target or beneficial organisms. The specificity arises from the requirement for near-perfect sequence complementarity between the siRNAs and the pest's mRNA, ensuring that off-target gene suppression is minimized. Deployment strategies for RNAi in agroecosystems include both transgenic and exogenous methods. In host-induced gene silencing (HIGS), crops are genetically engineered to express dsRNA constructs that target pest genes, thereby conferring systemic resistance through intracellular uptake and processing. Alternatively, spray-induced gene silencing (SIGS) involves the topical application of formulated dsRNA, often enhanced by nanoparticle carriers or other stabilizing agents to improve environmental persistence and uptake efficiency. Current research focuses on optimizing dsRNA delivery, enhancing stability under field-like conditions, and broadening the spectrum of target pests through refined bioinformatic analyses to avoid off-target effects (Basso et al., 2025). As advances in molecular biology and nanotechnology continue to evolve, RNAi is poised to become a cornerstone of species-specific, environmentally conscious pest management strategies in modern agroecosystems.
Recent field-scale studies have shown RNA interference (RNAi) to be a highly promising strategy for pest control in large-scale agriculture, with several applications achieving remarkable suppression rates. In the United States, MON 87411 transgenic maize expressing dsRNA targeting the Snf7 gene in Diabrotica virgifera (Western Corn Rootworm) recorded over 95% suppression of pests, significantly reducing yield losses and setting a benchmark for RNAi-based pest control (Mendoza-Alatorre et al., 2025). A follow-up regulatory trial also confirmed >90% WCR reduction with minimal off-target impacts, bolstering its environmental safety (Christiaens et al., 2022). In Germany, gene target screening via dsRNA in Leptinotarsa decemlineata (Colorado potato beetle) achieved >80% pest mortality by silencing critical genes involved in development and metabolism (Mehlhorn et al., 2021).
Similarly, in Brazil, the topical application of non-transformative dsRNA targeting Spodoptera frugiperda ATPase genes led to up to 80% larval mortality under field conditions, highlighting the feasibility of spray-based RNAi (Cagliari et al., 2019). In India, dsRNA foliar sprays on chickpea crops targeting Helicoverpa armigera protease genes resulted in over 75% larval suppression and increased pod yields in open-field evaluations (Mamta and Rajam, 2017). Field-scale RNAi trials in Belgium tested formulations for the Colorado potato beetle and Tuta absoluta, achieving 65%−85% pest reduction while emphasizing product scalability and readiness for regulatory clearance (De Schutter et al., 2022). Lastly, field trials in southern China applied siRNA sprays against Plutella xylostella on crucifer crops, achieving up to 70% larval mortality, thereby reducing reliance on conventional insecticides (Gong et al., 2013). These case studies demonstrate that RNAi can provide species-specific, effective, and scalable pest control solutions across diverse agroecosystems, as is also expected from Bt-crops.
The large-scale deployment of Bt maize (Zea mays) expressing the Cry1Ab protein in the United States since the mid-1990s has proven highly effective in controlling populations of the European corn borer (Ostrinia nubilalis). This genetic modification has contributed to increased yield stability through consistent pest suppression, significantly reducing the dependency on chemical insecticides. The implementation of integrated refuge strategies (planting non-Bt crops alongside Bt crops) further supports the durability of Bt maize by mitigating the risk of field-level resistance development, thereby ensuring the sustainability of pest management practices (Ostlie et al., 1997). Low refuge compliance in Bt maize has been reported to accelerate pest resistance development (Kang et al., 2025). In China, extensive cultivation of Bt cotton (Gossypium hirsutum) has led to a marked decline in populations of the major pest cotton bollworm (Helicoverpa armigera). The targeted expression of Bt toxins improves pest suppression while simultaneously enhancing crop productivity, decreasing pesticide application rates, and contributing to improved environmental conditions and farmworker safety (Lü et al., 2018). In South Africa, empirical studies validate the field performance of Bt maize, particularly in mitigating infestations from key pests such as Busseola fusca. The expression of specific Cry proteins significantly reduces pest pressure, leading to improved crop yields and a notable decrease in pesticide reliance (Kotey et al., 2017). Just recently, Nigeria approved four TELA maize varieties in January 2024. These are stacked for insect resistance and drought tolerance, with reported yield potentials reaching up to 10 t/ha under optimal management, significantly higher than the national average of approximately 6 t/ha for comparable hybrids (Oyekunle et al., 2023). However, not all experiences have been positive. Burkina Faso, once an early adopter of Bt cotton, phased out its use in 2016 due to concerns over lint quality (Luna and Dowd-Uribe, 2020). This case serves as a cautionary example, highlighting the importance of pairing biotech traits with high-performing cultivars and robust seed systems. In North America, genetically modified Bt maize (Zea mays) and Bt cotton (Gossypium hirsutum) predominate, owing to their enhanced resistance to lepidopteran pests and the resultant reduction in synthetic insecticide applications (Yang et al., 2022). In Asia, nations such as India and China have widely adopted Bt cotton, while the Philippines has recently sanctioned the commercialization of Bt brinjal (Herring, 2023). While Bt crop biotechnology is predominantly utilized in corn and cotton, it has also expanded into other economically significant crops.
The global adoption of Bt crops has similarly influenced agricultural sectors beyond maize and cotton. In India, Bt brinjal (Solanum melongena) has shown efficacy in controlling the fruit and shoot borer, although concerns remain regarding genetic diversity and potential biopiracy implications. China's Bt rice (Oryza sativa) demonstrates strong potential for yield enhancement and pesticide reduction, but its commercialization has faced regulatory delays linked to economic and policy concerns, with an opportunity cost of $12 billion per year (Jin et al., 2019). Since 1995, Bt potatoes (Solanum tuberosum) have been used in the United States to manage Colorado potato beetle (Leptinotarsa decemlineata) infestations, reducing dependence on certain chemical insecticides. Their integration into integrated pest management (IPM) strategies has improved environmental sustainability and agricultural productivity, benefiting growers, consumers, and the U.S. economy (Anon, 2000). Further research in the Philippines highlights that adoption patterns of Bt tomato (Solanum lycopersicum) and Bt eggplant, particularly in small-scale farming systems, have demonstrated reductions in insecticide use while increasing marketable fruit yields (Ruane et al., 2023). Brazil's Bt soybean (Glycine max), widely cultivated for over a decade, has played a critical role in insect pest suppression, with adoption rates exceeding 80% by 2020/2021 and reaching 94% in the 2023/2024 crop season (Bueno et al., 2025). Meanwhile, Ghana has transitioned from confined trials to full regulatory approval, culminating in the commercial release of a pod-borer-resistant (PBR/Bt) cowpea (event 709A) between 2022 and 2024 (Addae et al., 2020). This variety targets yield losses caused by Maruca and other pod borers. The Philippines became the first Asian country to approve Golden Rice for commercial propagation in July 2021. This biofortified rice aims to combat vitamin A deficiency, particularly among children, though its approval has faced legal and civil society challenges. These case studies underscore the varied benefits, challenges, and regulatory frameworks shaping Bt crop technologies across different regions, emphasizing their significance in sustainable agriculture and integrated pest management (IPM). Their adoption strengthens local food security while demonstrating the seamless integration of Bt innovations within IPM-driven agricultural systems. A summary of Bt crops adoption among countries is shown in Table 8.
6 Choice for cost-effective biopesticides
The biopesticide categories evaluated in the case studies, including botanical extracts, pesticidal plants (comprising functional repellent, banker, and trap plants, collectively referred to as RBT plants), and entomopathogenic nematodes (EPNs), demonstrate significant potential for integration into low-input agricultural systems. These biological control agents are well-recognized by both professional practitioners and local farmers and are particularly suited to traditional and smallholder farming contexts due to their compatibility with existing agronomic practices, low infrastructural requirements, and reliance on locally sourced biological materials. Their uptake is frequently influenced by economic factors, offering cost-effective alternatives to synthetic agrochemicals within Integrated Pest Management (IPM) frameworks. Botanical extracts enriched with phytochemicals (e.g., alkaloids, terpenoids, and flavonoids) sourced from indigenous flora are increasingly employed for their antimicrobial and insecticidal properties. These bioactive substances are typically obtained through processes such as maceration, decoction, or fermentation, using tools and methodologies accessible to resource-limited growers (Wanderley et al., 2024). The deployment of RBT plants enhances in situ conservation and proliferation of natural enemies, thereby strengthening the effectiveness of biological control strategies within cropping systems. While RBT plants and botanical extracts are generally accessible and easy to adopt, the application of EPNs necessitates a propagation phase facilitated through Natural Enemies Field Reservoirs (NEFRs). NEFRs are low-cost, on-farm structures engineered to conserve and augment populations of beneficial arthropods (Othim et al., 2024). Constructed using readily available materials such as bamboo and thatch, these reservoirs contain pest-laden substrates that support organisms like Trichogramma spp. and coccinellids. Nectar-producing flora, such as marigold, are cultivated in proximity to supply supplementary nourishment to adult beneficials. Routine management practices, including substrate replacement and humidity regulation, are essential for maintaining their efficacy (Farooq and Zada, 2022). These reservoirs are self-regenerating, environmentally benign, and ideally suited to smallholder agroecological systems, offering an ecologically sound alternative to chemical pest control. EPNs, particularly species within the genera Steinernema and Heterorhabditis, are effective against soil-dwelling insect pests and can be administered using low-tech aqueous suspension methods (Pérez-Campos et al., 2019). Collectively, these biologically based approaches advance the objectives of sustainable agriculture by reducing dependency on synthetic inputs, increasing on-farm biodiversity, and enhancing agroecosystem resilience, particularly in settings constrained by economic and technological limitations.
Alternatively, locally producing nematode biopesticides involves cultivating entomopathogenic nematodes (EPNs) like Steinernema and Heterorhabditis, which parasitize pest insects with the aid of symbiotic bacteria Xenorhabdus and Photorhabdus. These nematodes are mass-produced using insect hosts such as Galleria mellonella (wax moth larvae) under controlled conditions, where infective juveniles penetrate the host, release bacterial symbionts, and induce septicemia, leading to insect death (Hussein et al., 2022). The nematodes multiply within the cadaver, emerging as new infective juveniles (IJs) ready for extraction using techniques like White traps. The white trap technique is a nematode harvesting method that exploits the natural behavior of IJs as they emerge from insect cadavers and migrate toward moisture (Bakr et al., 2021).
In this setup, infected insect cadavers are placed on an elevated platform within a petri dish or shallow container, with a small amount of water added to the base. As nematodes complete their reproductive cycle within the host, the newly formed IJs exit the cadaver and instinctively move downward into the water, where they accumulate and can be collected for use. This technique is widely used for efficiently separating nematodes from host debris while maintaining their viability for biopesticide applications in agricultural pest control. According to Abongile (2021), nematode formulation involves suspending the harvested nematodes in carriers such as clay granules, alginate beads, water or gel formulations to enhance viability and application efficiency. Stored under cool, dark conditions, they remain viable until deployed in agricultural fields, where they actively seek out and infect target pests via chemical cues (kairomones), ensuring sustained pest control.
At an intermediate production scale, farmers may establish collaborative linkages with regional research institutions or universities to access ecologically adapted microbial strains tailored for biological pest management. These institutional partnerships facilitate the acquisition of pure cultures of beneficial microorganisms for subsequent downstream applications. Following procurement, farmers can initiate community-based microbial propagation, a decentralized model of biopesticide production operating at the village or local level. This model typically engages farmer cooperatives, rural enterprises, or agricultural self-help groups and relies on local bioresources and indigenous technical knowledge. It promotes the development of pest control agents that are environmentally sustainable, economically viable, and socially inclusive. From a technical perspective, these grassroots production systems employ cost-effective fermentation technologies for the large-scale multiplication of microbial agents. Fermentation outputs are standardized using colony-forming units (CFU/ml or CFU/g) to ensure consistency, efficacy, and quality assurance. Solid-state fermentation is generally preferred for microbial biopesticides, whereas submerged fermentation is more suitable for botanical-based formulations (Mattedi et al., 2023). For large-scale local formulation, the process commences with the selection of high-yielding microbial strains from academic or research institutions, such as Bacillus thuringiensis (Bt), Bacillus subtilis, Pseudomonas fluorescens, Beauveria bassiana, Metarhizium anisopliae, and Trichoderma spp., selected for their genetic stability and industrial scalability. The nutrient medium formulation integrates carbon sources (e.g., glucose or starch), nitrogen substrates (e.g., peptone or ammonium salts), essential trace elements, and growth-enhancing factors, maintaining an optimal pH range of 6.0–7.5 to support microbial homeostasis (Liang et al., 2023). Bioreactors equipped with mechanical stirring and aeration systems ensure efficient oxygen transfer, supporting aerobic metabolic activity. Growth conditions are monitored through continuous assessment of dissolved oxygen concentrations and regulation of temperature (typically 25–37 °C) and pH via buffering agents (Wei et al., 2019). Scale-up operations progress from seed cultures in laboratory-scale flasks to pilot and industrial-scale bioreactors, maximizing microbial biomass yield. Biomass recovery is achieved through downstream processing techniques such as filtration or centrifugation. Ultimately, the optimization of bioprocess parameters, including nutrient composition, aeration dynamics, and fermentation conditions, is fundamental to the economic sustainability of microbial biopesticide production.
The development of botanical pesticides in a local setting involves sourcing plant-based compounds with pesticidal properties, processing them into usable formulations, and applying them effectively for agricultural pest control. Locally available plants with bioactive pesticidal properties serve as the foundation for botanical pesticides. Commonly used plants include Azadirachta indica (neem), Capsicum spp. (chili peppers), Lantana camara (lantana), Tagetes spp. (marigold), and Euphorbia tirucalli (African milk bush) (Tavares et al., 2021). These plants contain phytochemicals such as alkaloids, flavonoids, terpenoids, and phenolics, which exhibit insecticidal, antifungal, and antimicrobial activities. Collection typically involves harvesting leaves, seeds, bark, or roots, ensuring that resources are gathered sustainably without harming local biodiversity. Once sourced, plant materials undergo processing to extract their active pesticidal compounds through various techniques. Drying and grinding involve air-drying plant parts in the shade to preserve bioactive compounds before pulverizing them into a fine powder for extraction (Ray, 2023). Aqueous extraction is carried out by soaking or boiling plant material in water, allowing the release of bioactive components suitable for liquid formulations (Htay et al., 2023). In solvent extraction, ethanol or methanol dissolves and isolates active ingredients, enhancing chemical stability and overall efficacy (Hikmawanti et al., 2021).
Some botanical pesticides, such as neem and chili extracts, undergo fermentation, where microbial action improves potency and extends shelf life (Teshome et al., 2022). After extraction, the raw liquid is meticulously filtered to remove plant debris, then diluted to an optimal concentration for application, ensuring efficacy while preventing phytotoxicity. This systematic approach maximizes the pesticidal potential of botanical compounds in sustainable pest management. Botanical pesticides can be formulated in different ways based on their intended application. Liquid formulations are prepared as emulsions or suspensions using water or oils, making them ideal for foliar spray applications. Powder and granule formulations involve drying and grinding plant materials before mixing them with inert carriers, allowing for efficient seed treatment and soil application. Local production of natural enemies, classified within macrobial biopesticides, involves cultivating beneficial organisms that provide biological pest control through established ecological interactions. The process encompasses augmentative rearing of predators, parasitoid insect cultivation, and predatory mite propagation, each requiring specific environmental and physiological conditions to optimize efficacy. Predatory insects such as Coccinellidae (lady beetles) and Chrysopidae (lacewings) are mass-reared in controlled insectary conditions, ensuring adequate prey availability and environmental stability (Pathrose et al., 2023). Aphids or other soft-bodied Hemiptera serve as prey for lady beetles, while lacewing larvae exhibit voracious predation on moth eggs (Sitotroga cerealella), fostering rapid population growth. Optimal rearing parameters include a temperature range of 22–28 °C, relative humidity of 60%−80%, and regulated photoperiods to simulate natural diurnal cycles, ensuring enhanced predator fitness and survival rates (Al-Azzazy and Alhewairini, 2020). Parasitoid wasps such as Trichogramma spp. are cultivated using oviposition hosts, primarily moth eggs. Host eggs are exposed to adult parasitoids in controlled rearing chambers with regulated aeration and light conditions. Upon parasitization, developing larvae consume internal egg contents before emerging as new parasitoid generations, ensuring sustainable augmentation (Cherif et al., 2021). Key environmental parameters include controlled temperatures (25–30 °C), relative humidity exceeding 70%, and sequential host exposure to maintain production cycles (Sampaio et al., 2025). Predatory mites, particularly Phytoseiulus persimilis, serve as biological control agents against Tetranychus spp. (spider mites). They are propagated under pest-rich conditions, where abundant target populations ensure stable reproduction. Eggs and juvenile stages are systematically collected and introduced into infested plant ecosystems, facilitating rapid establishment and sustained suppression of pest populations. Vangansbeke (2015) demonstrated that precise environmental regulation, including temperature stabilization between 20 and 28 °C and maintaining ambient humidity above 60%, is crucial for optimal propagation and efficacy post-release. Table 9 summarizes the cultivation conditions for locally accessible pesticidal natural resources.
With enhanced economic capacity and advancing biotechnological infrastructure, farmers are increasingly positioned to capitalize on commercial microbial biopesticides, biochemical biopesticides, including semiochemicals such as pheromones, and plant-incorporated protectants (PIPs) as critical components of sustainable crop protection frameworks. These biologically based inputs offer selective modes of action, reduced ecotoxicological risks, and favorable environmental persistence profiles, aligning with the principles of Integrated Pest Management (IPM) and agroecological intensification. However, in contrast to traditional pest control practices and low-tech botanical formulations, branded biochemical biopesticides and genetically engineered PIPs remain relatively underutilized, particularly in economically disadvantaged regions. Limited market penetration, lack of farmer awareness, and higher acquisition costs collectively constrain widespread adoption. Moreover, regulatory bottlenecks further compound these barriers by delaying product registration and increasing compliance overheads for developers and distributors. Nevertheless, the economic and administrative burden associated with commercial biopesticide registration can be alleviated through proportionate and science-based regulatory frameworks. Given the intrinsically lower hazard profiles, species specificity, and limited off-target effects of microbial and biochemical biopesticides, many national regulatory agencies now adopt streamlined authorization procedures under tiered or risk-based regulatory models. These frameworks, which often include simplified dossier requirements and reduced data submission for well-characterized actives, accelerate approval timelines and reduce the cost of market entry. Such regulatory flexibility not only incentivizes innovation and commercialization within the biopesticide sector but also supports the accessibility of safer and more sustainable crop protection solutions, particularly among resource-constrained farming communities. Ensuring regulatory harmonization and enhanced awareness through targeted extension services and public-private partnerships will be pivotal to scaling the adoption of these next-generation biocontrol technologies.
Establishing biopesticide manufacturing facilities in close proximity to farming localities can significantly alleviate market entry barriers by reducing distribution costs, enhancing supply chain efficiency, and ensuring the timely availability of formulations, particularly those containing sensitive microbial agents with limited shelf life. Localized production supports the development of regionally adapted biopesticide strains tailored to prevailing agroecological conditions while enabling adaptive formulations and rapid feedback integration. Moreover, the presence of nearby manufacturing hubs fosters stronger farmer engagement, on-site technical support, and practical training initiatives, thereby promoting confidence and correct usage among end-users. These facilities can serve as innovation nodes for participatory research and public–private collaborations, driving context-appropriate product development and accelerating technology transfer. In addition, decentralized production stimulates rural economic growth by creating employment opportunities, strengthening local input markets, and advancing inclusive agro-industrial development.
7 Biopesticides in integrated pest management (IPM)
The case studies presented collectively illustrate that nature-based solutions can effectively suppress pest and disease pressures, are adaptable to diverse agroecological contexts and farm-scale budgets, transcend geopolitical boundaries, and offer viable alternatives to synthetic agrochemicals. These approaches reinforce and operationalize the principles of IPM, promoting sustainable crop protection through ecological intensification. Integrated Pest Management (IPM) is a holistic, ecosystem-based approach to pest control that emphasizes the use of preventive and non-chemical methods, such as cultural practices, mechanical and physical exclusion techniques, and biological control agents, prior to the application of chemical pesticides, which are used only as a last resort. As expected, IPM strategies are guided by continuous monitoring and informed decision-making based on pest population dynamics, economic injury levels (EIL), and economic threshold levels (ETL), with the objective of maintaining pest populations below levels that cause economic harm while minimizing risks to human health, non-target organisms, and the environment (Wolff, 2023). Monitoring and decision-making constitute the second and third of the eight IPM principles outlined in the study by Fenibo et al. (2022). Monitoring, including pest identification, entails systematic entomological surveillance and diagnostic evaluations aimed at detecting, quantifying, and classifying pest populations based on their ecological significance and economic impact. The first principle pertains to the prevention and suppression of pest outbreaks through cultural practices such as the use of resistant cultivars, disease-free seeds or seedlings, field sanitation, and crop rotation. The fourth principle advocates for the application of non-chemical control methods, encompassing mechanical, physical, and biological interventions. The fifth principle, pesticide selection, emphasizes the use of targeted pesticides with minimal adverse effects on non-target organisms and the environment. The sixth principle addresses pesticide reduction, promoting the judicious use of effective synthetic chemicals at the lowest effective dose. The seventh principle focuses on anti-resistance strategies to mitigate the development of pest resistance to control measures, while the eighth and final principle underscores the importance of evaluation, involving continuous assessment of the effectiveness and sustainability of implemented IPM strategies.
The prevention and suppression phase within Integrated Pest Management (IPM) encompasses multiple sub-phases, beginning with the pre-planting stage, which is centered on prophylactic cultural interventions aimed at minimizing initial pest inoculum and optimizing agroecosystem conditions. A key strategy under this phase involves the strategic spatial integration of pesticidal flora, notably through the use of repellent intercrops and perimeter-planted trap crops, which collectively define the push–pull approach (Zhang et al., 2020). For instance, the Desmodium–Pennisetum purpureum (Napier grass) system has demonstrated efficacy in protecting maize (Zea mays) from lepidopteran stemborers by repelling adult moths from the crop while attracting oviposition to trap grasses. Another cultural tactic involves the use of companion planting, wherein botanically complementary species such as Tagetes spp. (marigold) and Ocimum basilicum (basil) are co-cultivated with crops like radish (Raphanus sativus) or cotton (Gossypium spp.) to deter insect pests and enhance crop performance. Another example of companion planting is the “Three Sisters system,” involving a maize–bean–squash association, in which maize provides structural support for climbing beans, beans fix atmospheric nitrogen through symbiosis with Rhizobium spp., and squash suppresses weed growth through its broad, ground-covering foliage (Cryan et al., 2025). A third preventive measure is the establishment of within-field wildflower strips, which serve as ecological infrastructure by attracting pollinators, augmenting natural enemy populations, and promoting functional biodiversity, thereby reinforcing pest regulation and ecological resilience (Hatt and Döring, 2025). This approach can be reinforced with methyl salicylate (semiochemicals), which function as kairomones or synomones to attract natural enemies like coccinellids (ladybirds) and parasitoid wasps (Xu et al., 2024). This form of indirect control enhances biological regulation of pest populations, particularly aphids and mites, through natural predation and parasitism. The planting phase encompasses agronomic and phytosanitary practices designed to ensure vigorous crop establishment while reducing the likelihood of early pest colonization. This phase often incorporates biopesticidal interventions, including seed treatments, root dips for seedlings, and soil drenching, utilizing a range of biological control agents such as entomopathogenic fungi (e.g., Beauveria bassiana and Metarhizium anisopliae) and beneficial bacteria (e.g., Bacillus thuringiensis and Pseudomonas fluorescens). The deployment of pest-resistant cultivars, including both conventionally bred varieties and transgenic lines, also contributes to a crop's innate defense capacity. During the growth and maintenance phase, the practices of microbigation, the targeted delivery of beneficial microbial inoculants, can further enhance plant resilience and suppress pest activity.
When these cultural strategies are integrated within a standard IPM framework (Figure 4), they constitute a proactive, ecologically sound line of defense, thereby reducing dependency on synthetic chemical controls and promoting sustainable pest regulation. In the monitoring phase of IPM, both biological control agents (BCAs) and semiochemicals are integral to the surveillance of pest populations, aiding in the detection, quantification, and evaluation of infestation levels relative to economic threshold levels (ETLs). Semiochemicals, encompassing pheromones, kairomones, and allomones, function as chemical mediators of interspecies interactions and are widely employed in pest detection strategies. Pheromone-baited traps, particularly those using sex pheromones, are effective in attracting and capturing specific pest taxa (e.g., Helicoverpa armigera and Spodoptera spp.), thereby enabling precise monitoring of population density, phenology, spatial distribution, and migration patterns (Alam et al., 2023). These traps contribute to threshold-based decision-making, where trap counts inform whether pest populations have reached levels that warrant intervention. Additionally, they serve as early warning systems, allowing for the pre-symptomatic detection of pests and timely deployment of control measures. Biological control agents, while traditionally employed for curative or suppressive action, also play a vital role in ecological monitoring. For instance, parasitism and predation rates, such as the proportion of pest larvae parasitized by Trichogramma spp. or Cotesia spp., act as bioindicators of pest activity and natural control efficacy (Cherif et al., 2021). The presence and abundance of these beneficial organisms, observed through sweep netting, visual scouting, or sticky traps, provide insight into the level of natural pest regulation already in effect, potentially obviating the need for chemical treatment. Sentinel systems, such as artificial pest egg cards deployed to track parasitoid activity, and mycosed cadaver assessments (e.g., pest bodies colonized by Beauveria bassiana), further contribute to understanding BCA dynamics in situ (Murchie et al., 2023). When semiochemical-based monitoring is integrated with data on BCA activity, it facilitates more accurate forecasting, enhances understanding of trophic interactions, and supports decisions that minimize unnecessary pesticide use. For example, Sisay et al. (2024) demonstrated that in a maize cropping system, pheromone traps baited with Chilo partellus sex pheromones can be used to monitor moth flight activity, while simultaneous assessments of parasitism by Cotesia flavipes provide a measure of natural enemy efficacy, together forming a robust, biologically informed surveillance framework within an IPM programme. Table 10 provides a summary of the roles played by biological control agents (BCAs) and semiochemicals in the monitoring of plant pests.

Figure 4. The eight principles of the integrated pest management (IPM). EIA, environmental impact assessment; IPM, integrated pest management; EIL, economic injury levels; ETL, economic threshold levels. *Biopesticide implicated.
In IPM, biopesticides play multifaceted roles across physical, mechanical, and biological control strategies, contributing to effective and sustainable pest suppression with minimal environmental impact, as demanded by principle four. From a mechanical control perspective, certain biopesticides create direct mechanical effects on pests. For example, diatomaceous earth functions as an abrasive desiccant. When applied to crop surfaces, it disrupts the insect's protective cuticle, causing rapid dehydration and mortality. Similarly, kaolin clay is employed as a fine particle film that forms a mechanical barrier on plant surfaces. This coating inhibits pest colonization by interfering with insect settling, feeding, and oviposition behavior, thereby reducing infestation levels through purely mechanical means. Regarding physical control, biopesticides complement direct pest removal or exclusion techniques. For instance, sticky traps baited with semiochemical lures attract target pest species and physically capture them, lowering pest populations without the need for chemical inputs. Additionally, barrier crops or mulches, although primarily physical in their pest suppression, can also harbor beneficial microbial communities that alter pest microhabitats, reducing pest establishment and reproduction (Smith et al., 2023). The core function of biopesticides within IPM lies in their biological control capabilities.
This includes the use of microbial agents such as entomopathogenic bacteria, fungi, and viruses that specifically target pest organisms. Notably, Bacillus thuringiensis produces insecticidal crystalline proteins that, upon ingestion, disrupt the midgut epithelium of susceptible larvae, leading to mortality. Entomopathogenic fungi, including Beauveria bassiana and Metarhizium anisopliae, infect insects by penetrating their cuticle, proliferating internally, and causing death through systemic infection. Similarly, insect-specific viruses, such as nucleopolyhedroviruses, induce lethal infections that suppress pest populations without affecting non-target fauna. Augmentation of natural enemies like parasitoids and predators further complements these microbial biopesticides, enhancing overall biological regulation within the agroecosystem. The integration of these biopesticidal methods, mechanical abrasion and barriers, physical trapping, and biological pathogenicity forms a robust pest management toolkit within IPM.
The fifth principle of Integrated Pest Management (IPM) stipulates that the application of pesticides should be a corrective measure, employed only when preventive, cultural, or biological strategies fail to suppress pest populations below the economic injury threshold. Within this framework, pesticides remain indispensable for managing severe pest and disease outbreaks, with their selection governed by criteria such as target specificity, environmental and human safety, resistance management, economic thresholds, and compatibility with other IPM tactics (Yarahmadi and Rajabpour, 2024). This approach prioritizes the use of selective and reduced-risk pesticides that minimize harm to natural enemies and ecosystems. For example, diamides such as chlorantraniliprole and cyantraniliprole are increasingly used in maize and rice systems to control lepidopteran pests, while neonicotinoids like imidacloprid and thiamethoxam are applied in seed treatments to manage early-season sap-sucking insects. Similarly, triazole fungicides (e.g., tebuconazole and propiconazole) and strobilurins (e.g., azoxystrobin and pyraclostrobin) are integrated into disease management programmes, often in combination with crop rotation and resistant varieties, to avoid excessive reliance on synthetic pesticides. This aligns with the sixth component of IPM, which promotes the integration of botanical pesticides, such as neem (Azadirachta indica) extracts, pyrethrins from chrysanthemum, and essential oil formulations alongside synthetics. These botanicals, while not directly lethal, disrupt insect behavior and are particularly valuable in low-residue and organic production systems (Chaudhary et al., 2024). Insect Growth Regulators (IGRs), including pyriproxyfen and methoprene, mimic juvenile hormones and interfere with metamorphosis and reproduction, effectively reducing populations of whiteflies, scales, and mosquitoes without harming adult insects, thereby conserving beneficial organisms (Ahmed et al., 2020). At the upper end of the severity scale is Spinosad, a macrocyclic lactone derived from the bacterium Saccharopolyspora spinosa, which exerts neurotoxic effects by persistently activating insect nicotinic acetylcholine receptors, leading to rapid excitation, paralysis, and death (Taillebois and Thany, 2022). In cotton IPM systems, neem-based biopesticides and the conservation of natural enemies are complemented by the rotational use of pyrethroids (e.g., deltamethrin and lambda-cyhalothrin) and organophosphates (e.g., dimethoate) to delay resistance development, in accordance with the seventh IPM component. To maximize efficacy and sustainability, synthetic pesticides are rotated with biopesticides such as Spinosad, ensuring a balanced and adaptive pest management strategy.
Critical examination reveals that biopesticides constitute approximately 83.3% of the hierarchical structure, demonstrating their pivotal role in sustainable pest management. The pyramidal structure of the Integrated Pest Management (IPM) framework demonstrates that six out of its eight foundational principles involve the direct application of pest mitigation strategies. Of these, five components, excluding synthetic chemical pesticide use, are compatible with biopesticide integration, representing 83.3% of the actionable interventions. Importantly, three of these five biopesticide-relevant components, excluding advanced microbial formulations and pheromone-based technologies, are accessible to local farmers through existing agronomic practices and indigenous knowledge systems. This suggests that approximately 52.1% of biopesticide applications within the operational domain of IPM can be implemented to cost-effectively and adapted locally. The IPM, while ecologically sound and strategically holistic, faces several implementation challenges. Chief among these is the limited awareness and technical proficiency among smallholder farmers, often compounded by inadequate extension services and insufficient training on IPM principles. Economically, the upfront costs for IPM tools, such as pheromone traps or microbial biopesticides, can deter adoption, especially in regions lacking subsidy schemes or access to premium markets. Additionally, inconsistencies in national policies and underdeveloped regulatory frameworks for biocontrol products hinder innovation and widespread application. The multifaceted nature of IPM itself, requiring pest identification, threshold-based interventions, and integration of diverse control strategies, can overwhelm users, particularly where literacy or labor constraints exist. Weak supply chains for quality-assured inputs and entrenched habits favoring synthetic pesticides further complicate the transition. Moreover, environmental variability, such as shifts in temperature or humidity, can affect the reliability of biological agents, leading to unpredictable outcomes. Addressing these barriers demands coordinated policy reform, capacity building, robust input supply systems, and sustained knowledge exchange among stakeholders.
8 Recommendation and conclusion
8.1 Recommendation for future research
A reflection on the resourcefulness and permeability of biopesticides within the framework of Integrated Pest Management (IPM) underscores their pivotal role in sustainable pest suppression. As explained in previous sections, specific biochemical pesticides, including semiochemicals (e.g., sex pheromones, kairomones, and allomones) and plant-incorporated protectants (PIPs), pass through sophisticated technological process that remain largely beyond the reach of traditional technologies. Agriculture was central to the identity of early human civilizations, and traditional agro-technologies, such as the use of biopesticides, formed a core part of that culture. However, with current realities, societies that once celebrated biodiversity, farming, and food sufficiency are now re-evaluating the role and meaning of biopesticides. Ironically, biopesticides are now often perceived as novel or even foreign, prompting renewed efforts to raise awareness and promote their adoption. At the same time, agricultural technology (agrotech) is rapidly evolving, emphasizing both economic gain and ecological sustainability. This contrast suggests a historical disconnect, the nature of which remains open to investigation. It would be particularly interesting to determine whether this gap predates the Green Revolution, a pivotal era of agricultural change in the mid-20th century. While the Green Revolution significantly boosted global food production and addressed hunger in regions like India, Mexico, and Southeast Asia, it also introduced long-term environmental challenges such as soil degradation, water pollution, and pest resistance due to excessive chemical input use. Although the Green Revolution benefited many economically disadvantaged countries by enhancing food security, it also marked the beginning of unsustainable farming practices. Interestingly, prior to this shift, many of these regions seemed to employ eco-friendly subsistence farming methods. India, for example, has long been recognized for its abundance of neem, a plant celebrated for its pest control properties and medicinal value. In fact, disputes over biopiracy surrounding neem formulations highlight its deep-rooted significance in traditional agriculture. Given this context, claims such as that from Praneetvatakul et al. (2024), stating that “biopesticides are relatively new in agriculture, and lack of awareness may hinder their adoption, especially in developing and underdeveloped countries,” appear both controversial and debatable. These narratives often overlook the historical use of natural pest control methods in these regions. Rather than unfamiliarity with biopesticides themselves, it may be the modern, high-tech versions of agro-inputs that remain relatively unknown or inaccessible in these areas.
The paradox of biopesticide affordability vs. market dynamics stems from the contrast between their natural accessibility and the commercial dominance of advanced synthetic formulations. Biopesticides, derived from biological sources, have inherently low production and procurement costs, as many can be directly harvested, minimally processed, and applied effectively. Some biopesticidal agents, such as wild pesticidal plants, play a crucial role in strategies like the push-pull pest management system, where their natural repellency and attractant properties are leveraged without significant processing. Additionally, biopesticides can be used in crude or semi-formulated states, often demonstrating efficacy comparable to fully developed synthetic alternatives. Their extensive presence in Integrated Pest Management (IPM) frameworks, constituting over 50% of control strategies, highlights their compatibility and sustainability within eco-friendly agricultural practices. These advantages position biopesticides as a more cost-effective option than synthetic pesticides. However, despite these benefits, biopesticides remain at a pricing disadvantage due to factors such as limited production scale, shorter shelf life, and weaker distribution networks (Constantine et al., 2020), which hinder their accessibility and cost-effectiveness compared to synthetic pesticides. Bridging this gap requires strategic investment in infrastructure, policy incentives, and farmer education to enhance biopesticide affordability and competitive market positioning. While this gap persists, it would be constructive to classify biopesticides into two categories: “trado-tech” products, which are more affordable than synthetic pesticides, and high-tech products, which tend to be more expensive than their synthetic counterparts.
Even high-tech biopesticide products have the potential to be significantly cheaper than synthetic pesticides due to their simpler production processes. Unlike synthetic pesticides, which require complex chemical synthesis and petroleum-based raw materials, biopesticides are typically derived from naturally occurring microorganisms, plant extracts, or beneficial insect-derived compounds. Their reliance on renewable natural resources reduces both manufacturing costs and environmental impact. Additionally, biopesticides tend to be less toxic, leading to lower regulatory costs, simpler approval processes, and reduced safety requirements for handling and application (Mawcha et al., 2025a). These advantages contribute to their theoretical affordability. However, despite their cost-saving potential, biopesticides are frequently more expensive in practice. Limited production scale is one of the major reasons; many biopesticide manufacturers operate on smaller scales compared to large agrochemical companies producing synthetic pesticides, leading to higher per-unit costs. Furthermore, market penetration remains low due to various factors, including farmer hesitancy to adopt newer pest management strategies and a lack of awareness or education about their benefits. A shorter shelf life is another drawback; because biopesticides rely on living or naturally derived components, they often degrade more quickly, making storage and transportation more challenging and costly. In developing countries, weak distribution networks further hinder accessibility and affordability, as logistical inefficiencies drive up prices.
On the other hand, synthetic pesticides have dominated the market for decades, benefiting from well-established manufacturing infrastructure and economies of scale. Mass production allows synthetic pesticides to be produced in bulk, lowering costs. Their longer shelf life makes storage and transportation easier, ensuring availability over extended periods. Established trust within the agricultural sector also plays a role; farmers and agribusinesses are more likely to choose synthetic pesticides due to their familiarity and predictable results. Additionally, synthetic pesticides often receive substantial government and industry support, whether through subsidies, research funding, or distribution partnerships, further strengthening their cost competitiveness (López de Mesa, 2020). This situation creates a market paradox where a product (biopesticides) that is theoretically cheaper to produce ends up being more expensive to purchase and use. To address this gap, several measures are needed. Expanding production infrastructure and scaling up manufacturing will help reduce costs by improving efficiency. Greater investment in distribution networks and supply chains can enhance accessibility, especially in regions where biopesticides remain underutilized. Farmer education and awareness campaigns can encourage adoption by demonstrating their effectiveness and long-term benefits, including improved soil health and reduced environmental contamination. Finally, supportive policies, such as subsidies for biopesticide production or incentives for farmers to transition to eco-friendly pest management, could create a more level playing field. Ultimately, bridging the cost gap between biopesticides and synthetic pesticides requires coordinated efforts across research, industry, policy, and education. With increased adoption and investment, biopesticides could become more cost-effective, making sustainable and environmentally friendly pest control solutions a viable option for farmers worldwide. Research efforts should be strategically aligned to address these knowledge gaps until the cost of biopesticide formulations becomes economically competitive with that of synthetic chemical pesticides.
8.2 Conclusion
Biopesticides occupy a critical yet underutilized niche in modern agriculture, especially within Integrated Pest Management frameworks. Although rooted in traditional agro-technological practices, their contemporary iterations are often misconstrued as novel, overshadowed by the industrial dominance of synthetic pesticides. The paradox of their theoretical affordability and practical inaccessibility reflects systemic challenges, including limited production scale, short shelf life, and weak distribution networks, that inhibit their widespread adoption, particularly in developing countries. Addressing these barriers will require a multipronged approach: expanding manufacturing capabilities, strengthening supply chains, implementing policy incentives, and promoting farmer education. While efforts are underway to address the limitations of biopesticides, low-tech technologies such as botanical extracts, pesticidal plants (RBT plants), and entomopathogenic nematodes (EPNs) can be relied upon by farmers to achieve significant success. This transformation is essential not only for enhancing agricultural productivity but also for ensuring long-term environmental resilience and food security. By bridging historical knowledge with modern agrotechnology and aligning efforts across research, industry, and policy, the full potential of biopesticides as sustainable, cost-effective pest control agents can be realized.
Author contributions
EF: Conceptualization, Project administration, Data curation, Writing – review & editing, Writing – original draft, Visualization. TM: Writing – review & editing, Supervision, Writing – original draft, Project administration, Validation, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^https://www.cognitivemarketresearch.com/regional-analysis/europe-biopesticides-market-report (accessed 10/05/2025).
2. ^https://www.databridgemarketresearch.com/reports/global-biopesticides-market (accessed 11/05/2025).
References
Abbas, S. S., Shahzad, M. F., Iqbal, J., Ullah, A., Batool, A., Nadeem, M., et al. (2020). Trichogramma chilonis as parasitoid: an eco-friendly approach against tomato fruit borer, Helicoverpa armigera. J. Agric. Sci. 12:167. doi: 10.5539/jas.v12n2p167
Abdelaziz, A. M., Hashem, A. H., El-Sayyad, G. S., El-Wakil, D. A., Selim, S., Alkhalifah, D. H. M., et al. (2023). Biocontrol of soil borne diseases by plant growth promoting rhizobacteria. Trop. Plant Pathol. 48, 105–127. doi: 10.1007/s40858-022-00544-7
Abd-Elgawad, M. M. (2024). Upgrading strategies for managing nematode pests on profitable crops. Plants 13:1558. doi: 10.3390/plants13111558
Abd-Elgawad, M. M. (2025). Integrated nematode management strategies: optimization of combined nematicidal and multi-functional inputs. Plants 14:1004. doi: 10.3390/plants14071004
Abdoul-Latif, F. M., Ainane, A., Mohamed, H., Ali, A. M., Cacciatore, S., and Ainane, T. (2025). Advanced formulation of ecological bioinsecticides based on citrus limonum in clayey matrices: optimization of diffusive dynamics. Sustainability 17:785. doi: 10.3390/su17020785
Abongile, N. (2021). Formulation of Steinernema yirgalemense by entrapment in alginate beads. Russ. J. Nematol. 29, 49–58. doi: 10.24411/0869-6918-2021-10005
Abubakar, M., Yadav, D., Koul, B., and Song, M. (2023). Efficacy of eco-friendly bio-pesticides against the whitefly Bemisia tabaci (Gennadius) for sustainable eggplant cultivation in Kebbi State, Nigeria. Agronomy 13:3083. doi: 10.3390/agronomy13123083
Addae, P. C., Ishiyaku, M. F., Tignegre, J.-B., Ba, M. N., Bationo, J. B., Atokple, I. D., et al. (2020). Efficacy of a cry1Ab gene for control of Maruca vitrata (Lepidoptera: Crambidae) in cowpea (Fabales: Fabaceae). J. Econ. Entomol. 113, 974–979. doi: 10.1093/jee/toz367
Adeniji, A., Fadiji, A. E., Li, S., and Guo, R. (2024). From lab bench to farmers' fields: co-creating microbial inoculants with farmers input. Rhizosphere 31:100920. doi: 10.1016/j.rhisph.2024.100920
Adly, D., and Sanad, A. S. (2024). Comparative evaluation of biological control programs and chemical pesticides for managing insect and mite pests in cucumber greenhouses: a sustainable approach for enhanced pest control and yield. Egypt. J. Biol. Pest Control 34:42. doi: 10.1186/s41938-024-00806-3
Agboola, A. R., Okonkwo, C. O., Agwupuye, E. I., and Mbeh, G. (2022). Biopesticides and conventional pesticides: comparative review of mechanism of action and future perspectives. AROC Agric. 1, 14–32. doi: 10.53858/arocagr01011432
AgroPages (2023). A Robust Ready-to-Use Solution for Fungal Biopesticides Oil Dispersions. AgroPages. Available online at: https://news.agropages.com/News/Detail-43577.htm (accessed May 15, 2025).
Agu, C. M., Orakwue, C. C., Ani, O. N., and Chinedu, M. P. (2025). Kinetics, thermodynamics and characterization of neem seeds (Azadirachta indica) oil extraction: extensive study of the processes. Green Technol. Sustain. 3:100126. doi: 10.1016/j.grets.2024.100126
Ahmed, T. H., Saunders, T. R., Mullins, D., Rahman, M. Z., and Zhu, J. (2020). Molecular action of pyriproxyfen: role of the methoprene-tolerant protein in the pyriproxyfen-induced sterilization of adult female mosquitoes. PLoS Negl. Trop. Dis. 14:e0008669. doi: 10.1371/journal.pntd.0008669
Akhtar, Z. R., Tariq, K., Handler, A. M., Ali, A., Ullah, F., Ali, F., et al. (2021). Toxicological risk assessment of some commonly used insecticides on Cotesia flavipes, a larval parasitoid of the spotted stem borer Chilo partellus. Ecotoxicology 30, 448–458. doi: 10.1007/s10646-021-02372-y
Akol, A. M., Sithanantham, S., Njagi, P. G. N., Varela, A., and Mueke, J. M. (2002). Relative safety of sprays of two neem insecticides to Diadegma mollipla (Holmgren), a parasitoid of the diamondback moth: effects on adult longevity and foraging behaviour. Crop Prot. 21, 853–859. doi: 10.1016/S0261-2194(02)00052-2
Akutse, K. S., Subramanian, S., Maniania, N. K., Dubois, T., and Ekesi, S. (2020). Biopesticide research and product development in Africa for sustainable agriculture and food security–experiences from the International Centre of Insect Physiology and Ecology (ICIPE). Front. Sustain. Food Syst. 4:563016. doi: 10.3389/fsufs.2020.563016
Akyazi, R., Soysal, M., Altunç, E. Y., Lisle, A., Hassan, E., and Akyol, D. (2018). Acaricidal and sublethal effects of tobacco leaf and garlic bulb extract and soft soap on Tetranychus urticae Koch. (Acari: Trombidiformes: Tetranychidae) 1. Syst. Appl. Acarol. 23, 2054–2069. doi: 10.11158/saa.23.10.13
Alam, A., Abbas, S., Abbas, A., Abbas, M., Hafeez, F., Shakeel, M., et al. (2023). Emerging trends in insect sex pheromones and traps for sustainable management of key agricultural pests in Asia: beyond insecticides—a comprehensive review. Int. J. Trop. Insect Sci. 43, 1867–1882. doi: 10.1007/s42690-023-01100-9
Al-Azzazy, M. M., and Alhewairini, S. S. (2020). Effect of temperature and humidity on development, reproduction, and predation rate of Amblyseius swirskii (Phytoseiidae) fed on Phyllocoptruta oleivora (Eriophyidae) and Eutetranychus orientalis (Tetranychidae). Int. J. Acarol. 46, 304–312. doi: 10.1080/01647954.2020.1773922
Albacete, S. C., Amalin, D. M., Carvajal, T. M., and Wise, J. C. (2023). Evaluation of Philippine-sourced clay particles as coating agents of cacao pods and carrier of entomopathogen against cacao pest, Helopeltis bakeri Poppius. Front. Agron. 5:1213131. doi: 10.3389/fagro.2023.1213131
Alfiky, A., and Weisskopf, L. (2021). Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi 7:61. doi: 10.3390/jof7010061
Ali, A., Desneux, N., Lu, Y., and Wu, K. (2018). Key aphid natural enemies showing positive effects on wheat yield through biocontrol services in northern China. Agric. Ecosyst. Environ. 266, 1–9. doi: 10.1016/j.agee.2018.07.012
Alkan, M., Atay, T., Tarhanaci, B., and Ertürk, S. (2023). Efficacy of surface applications of Diaterra® against Sitophilus granarius L. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Research Square. Available online at: https://www.researchsquare.com/article/rs-3184996/latest (accessed June 16, 2025).
Alkhalil, S. S. (2025). “Chitosan-based nanoparticles to treat highly resistant microorganisms and pathogenic parasites,” in Chitosan-Based Nanoparticles for Biomedical Applications (Amsterdam: Elsevier), 217–243. doi: 10.1016/B978-0-443-13997-0.00010-2
Al-Qahtani, W. H. (2021). The value of blue-green algae (Spirulina platensis) as a nutritive supplement and toxicant against almond moth [Cadra cautella (Lepidoptera: Pyralidae)]. PLoS ONE 16:e0259115. doi: 10.1371/journal.pone.0259115
Ambethgar, A. S., Rameshkumar, A., Krishna, K. R., and Sundaresan, S. (2024). Biological control of pests in major tropical vegetable crops: a review. Agric. Rev. 35, 515–530. doi: 10.18805/ag.R-2635
Anakwenze, V. N., Enukorah, E. C., Adewale, K. S., Nnadi, E. N., Isiaka, A. B., Anaukwu, C. G., et al. (2024). Harnessing phages as biocontrol agents against common bacterial blight diseases in plants. World J. Adv. Res. Rev. 23, 962–978. doi: 10.30574/wjarr.2024.23.2.2142
Anjaneyulu, B., Chauhan, V., Mittal, C., and Afshari, M. (2024). Innovative nanocarrier systems: a comprehensive exploration of recent developments in nano-biopesticide formulations. J. Environ. Chem. Eng. 12:113693. doi: 10.1016/j.jece.2024.113693
Anon, U. (2000). Bt Plant-Pesticides Biopesticides Registration Action Document. Washington, DC: United States Environmental Protection Agency.
Aravinthraju, K., Shanthi, M., Murugan, M., Srinivasan, R., Maxwell, L. A., Manikanda Boopathi, N., et al. (2024). Endophytic entomopathogenic fungi: their role in enhancing plant resistance, managing insect pests, and synergy with management routines. J. Fungi 10:865. doi: 10.3390/jof10120865
Archana, H. R., Darshan, K., Lakshmi, M. A., Ghoshal, T., Bashayal, B. M., and Aggarwal, R. (2022). “Biopesticides: a key player in agro-environmental sustainability,” in Trends of Applied Microbiology for Sustainable Economy (Amsterdam: Elsevier), 613–653. doi: 10.1016/B978-0-323-91595-3.00021-5
Archer, L. (2022). Trunk Injection for Delivery of Huanglongbing Therapeutics to Citrus Trees: Efficacy, Effects on Tree Physiology, and Risks [PhD Thesis]. University of Florida. Available online at: https://search.proquest.com/openview/235f88fbc4ff82a75843a58b62f1e298/1?pq-origsite=gscholarandcbl=18750anddiss=y (accessed May 18, 2025).
Aremu, A. O., Kulkarni, M. G., Bairu, M. W., Finnie, J. F., and Van Staden, J. (2012). Growth stimulation effects of smoke-water and vermicompost leachate on greenhouse grown-tissue-cultured ‘Williams' bananas. Plant Growth Regul. 66, 111–118. doi: 10.1007/s10725-011-9634-6
Arjjumend, H., Koutouki, K., and Neufeld, S. (2021). Comparative advantage of using bio-pesticides in Indian agro-ecosystems. Ecosystems 7, 1–15. doi: 10.21694/2378-9018.21001
Arora, R. (2015). “Microbial control in insect pest management: achievements and challenges,” in Biological and Molecular Approaches in Pest Management, eds. B. Singh, R. Arora, and S. S. Gosal (Delhi: SEAISCIENTIFICPUBLISHER) https://www.google.com/search?hl=en&gbpv=1&dq=BiologicalandMolecularApproachesinPestManagement&printsec=frontcover&q=inpublisher:%22SCIENTIFICPUBLISHERIND.%22&tbm=bks&sa=X&ved=2ahUKEwjm3b6HloOQAxXZGVkFHY1fGiEQmxN6BAg, 97–152.
Arora, S., Kanojia, A. K., Kumar, A., Mogha, N., and Sahu, V. (2012). Biopesticide formulation to control tomato lepidopteran pest menace. Curr. Sci. 102, 1051–1057.
Asimakis, E., Shehata, A. A., Eisenreich, W., Acheuk, F., Lasram, S., Basiouni, S., et al. (2022). Algae and their metabolites as potential bio-pesticides. Microorganisms 10:307. doi: 10.3390/microorganisms10020307
Assadpour, E., Can Karaça, A., Fasamanesh, M., Mahdavi, S. A., Shariat-Alavi, M., Feng, J., et al. (2024). Application of essential oils as natural biopesticides; recent advances. Crit. Rev. Food Sci. Nutr. 64, 6477–6497. doi: 10.1080/10408398.2023.2170317
Aswathi, N., Balakrishnan, N., Srinivasan, T., Kokiladevi, E., and Raghu, R. (2024). Diversity of Bt toxins and their utility in pest management. Egypt. J. Biol. Pest Control 34:40. doi: 10.1186/s41938-024-00803-6
Awais, M., Li, W., Cheema, M. J. M., Hussain, S., Shaheen, A., Aslam, B., et al. (2022). Assessment of optimal flying height and timing using high-resolution unmanned aerial vehicle images in precision agriculture. Int. J. Environ. Sci. Technol. 19, 2703–2720. doi: 10.1007/s13762-021-03195-4
Awan, Z. A., and Shoaib, A. (2019). Combating early blight infection by employing Bacillus subtilis in combination with plant fertilizers. Curr. Plant Biol. 20:100125. doi: 10.1016/j.cpb.2019.100125
Aware, C., and Jadhav, J. (2022). “Bioprospecting potential of microbes for the therapeutic application,” in Bioprospecting of Microbial Diversity (Amsterdam: Elsevier), 223–255. doi: 10.1016/B978-0-323-90958-7.00023-6
Awudzi, G. K., Adu-Acheampong, R., Avicor, S. W., Bukari, Y., Boateng, E. K. O., Ahadzi, S. K., et al. (2022). Pattern of insecticide usage in organic cocoa production in Ghana. Org. Agric. 12, 125–136. doi: 10.1007/s13165-022-00386-2
Ayelo, P. M., Pirk, C. W., Yusuf, A. A., Chailleux, A., Mohamed, S. A., and Deletre, E. (2021). Exploring the kairomone-based foraging behaviour of natural enemies to enhance biological control: a review. Front. Ecol. Evol. 9:641974. doi: 10.3389/fevo.2021.641974
Ayilara, M. S., Akinola, S. A., Adeleke, B. S., Gbadegesin, L. A., Adejumo, G. D., Glick, B. R., et al. (2024). “Biopesticides versus synthetic pesticides usage in Africa,” in Microbiome-Based Decontamination of Environmental Pollutants, eds. A. Kumar, J. Singh, and L. C. Basilio De Azevedo (Cambridge, MA: Academic Press), 417–450. doi: 10.1016/B978-0-443-21781-4.00016-5
Azeem, U., Dhingra, G. S., and Shri, R. (2024). “Metabolites, enzymes, and toxins in entomopathogenic fungi,” in Entomopathogenic Fungi, eds. S. K. Deshmukh, and K. R. Sridhar (Cham: Springer Nature Singapore), 409–431. doi: 10.1007/978-981-97-5991-0_16
Babu, S., Bidyarani, N., Chopra, P., Monga, D., Kumar, R., Prasanna, R., et al. (2015). Evaluating microbe-plant interactions and varietal differences for enhancing biocontrol efficacy in root rot disease challenged cotton crop. Eur. J. Plant Pathol. 142, 345–362. doi: 10.1007/s10658-015-0619-6
Bagheri, E., and Khodkam, H. (2025). Smart farming: how drones are transforming the future of food production? Biosyst. Eng. Sustain. Technol. 1, 49–67. doi: 10.22084/best.2025.30490.1004
Bakr, R. A., Mahdy, M. E., and Mousa, M. E. (2021). Pakistan Journal of Nematology: Abstracts (2011-2020)-Efficacy of soil solarization and post-planting mulch on control of root-knot nematodes. Pak. J. Nematol. 31, 65–70.
Barclay, H. J., and Vreysen, M. J. B. (2011). A dynamic population model for tsetse (Diptera: Glossinidae) area-wide integrated pest management. Popul. Ecol. 53, 89–110. doi: 10.1007/s10144-010-0224-7
Basnet, P., Dhital, R., and Rakshit, A. (2022). “Biopesticides: a genetics, genomics, and molecular biology perspective,” in Biopesticides, eds. A. Rakshit, V. S. Meena, P. C. Abhilash, B. K. Sarma, H. B. Singh, L. Fraceto (Cambridge: Woodhead Publishing), 107–116. doi: 10.1016/B978-0-12-823355-9.00019-5
Basso, M. F., Vásquez, D. D. N., Campos-Pinto, E. R., Pinheiro, D. H., Cruz, B., Maktura, G. C., et al. (2025). Progress and opportunities of in planta and topical RNAi for the biotechnological control of agricultural pests. Agronomy 15:859. doi: 10.3390/agronomy15040859
Beltrán Pineda, M. E., and Castellanos-Rozo, J. (2025). Bacterial insecticides beyond Bacillus thuringiensis. Phytopathol. Res. 7:19. doi: 10.1186/s42483-024-00306-0
Ben Khedher, S., Boukedi, H., Laarif, A., and Tounsi, S. (2020). Biosurfactant produced by Bacillus subtilis V26: a potential biological control approach for sustainable agriculture development. Org. Agric. 10(S1), 117–124. doi: 10.1007/s13165-020-00316-0
Betz, F. S., Hammond, B. G., and Fuchs, R. L. (2000). Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharmacol. 32, 156–173. doi: 10.1006/rtph.2000.1426
Bharath, P., Gahir, S., and Raghavendra, A. S. (2021). Abscisic acid-induced stomatal closure: an important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 12:615114. doi: 10.3389/fpls.2021.615114
Bhardwaj, S. (2019). Bio-intensive management of damping–off of tomato (Solanum lycopersicum L.) [PhD Thesis]. Dr. Yashwant Singh Parmar University of Horticulture and Forestry. Available online at: https://krishikosh.egranth.ac.in/server/api/core/bitstreams/5d38938e-c30b-4fde-b719-3c32e1aa5c02/content (accessed May 16, 2025).
Bhardwaj, T., Kumar, D., Parkirti Vikram, V., Singh, A., Kapoor, N., et al. (2024). “Plant-microbe interactions, signalling events, and cellular networks against harmful pathogens,” in Plant Microbiome and Biological Control, Vol. 20, eds. P. Mathur and S. Roy (Cham: Springer Nature Switzerland), 37–57. doi: 10.1007/978-3-031-75845-4_3
Bharti, A., Velmourougane, K., and Prasanna, R. (2017). Phototrophic biofilms: diversity, ecology and applications. J. Appl. Phycol. 29, 2729–2744. doi: 10.1007/s10811-017-1172-9
Boari, A., Zuccari, D., and Vurro, M. (2008). ‘Microbigation': delivery of biological control agents through drip irrigation systems. Irrig. Sci. 26, 101–107. doi: 10.1007/s00271-007-0076-x
Bonaterra, A., Badosa, E., Daranas, N., Francés, J., Roselló, G., and Montesinos, E. (2022). Bacteria as biological control agents of plant diseases. Microorganisms 10:1759. doi: 10.3390/microorganisms10091759
Botella-Rodríguez, E. (2011). Cuba's alternative/inward-looking development policies. Changing production patterns and land decentralisation: towards sustainable small farming (1990-2008). Hist. Agrar. 55, 135–171.
Bourguet, D., and Guillemaud, T. (2016). “The hidden and external costs of pesticide use,” in Sustainable Agriculture Reviews, Vol. 19, ed. E. Lichtfouse (Cham: Springer International Publishing), 35–120. doi: 10.1007/978-3-319-26777-7_2
Boyetchko, S. M., Dumonceaux, T. J., Kirby, C., and Audy, P. (2024). “Phytophthora infestans (Montagne) de Bary, Potato Late Blight/Mildiou de la pomme de terre (Pythiaceae),” in Biological Control Programmes in Canada, 2013-2023, eds. M. A. Vankosky and V. Martel (Boston, MA: CABI), 587–592. doi: 10.1079/9781800623279.0062
Brookes, G., and Barfoot, P. (2020). Environmental impacts of genetically modified (GM) crop use 1996–2018: impacts on pesticide use and carbon emissions. GM Crops Food 11, 215–241. doi: 10.1080/21645698.2020.1773198
Bruner-Montero, G., Wood, M., Horn, H. A., Gemperline, E., Li, L., and Currie, C. R. (2021). Symbiont-mediated protection of Acromyrmex leaf-cutter ants from the entomopathogenic fungus Metarhizium anisopliae. mBio 1:e01885-21. doi: 10.1128/mBio.01885-21
Bueno, A. D. F., Braz-Zini, E. C., Horikoshi, R. J., Bernardi, O., Andrade, G., and Sutil, W. P. (2025). Over 10 years of Bt soybean in Brazil: lessons, benefits, and challenges for its use in integrated pest management (IPM). Neotrop. Entomol. 54:61. doi: 10.1007/s13744-025-01275-5
Cabello, T., Gallego, J. R., Lopez, I., Gamez, M., and Garay, J. (2024). Importance of host feeding in the biological control of insect pests: case study of egg parasitoid species (Hymenoptera: Chalcidoidea: Trichogrammatidae). Insects 15:496. doi: 10.3390/insects15070496
Cagliari, D., Dias, N. P., Galdeano, D. M., Dos Santos, E. Á., Smagghe, G., and Zotti, M. J. (2019). Management of pest insects and plant diseases by non-transformative RNAi. Front. Plant Sci. 10:1319. doi: 10.3389/fpls.2019.01319
Cai, P., and Dimopoulos, G. (2024). Microbial biopesticides: a one health perspective on benefits and risks. One Health 20:100962. doi: 10.1016/j.onehlt.2024.100962
Cardé, R. T. (2021). “Mating disruption with pheromones for control of moth pests in area-wide management programmes,” in Area-Wide Integrated Pest Management (Boca Raton, FL: CRC Press), 779–794. doi: 10.1201/9781003169239-45
Casanova, L. M., Macrae, A., de Souza, J. E., Neves Junior, A., and Vermelho, A. B. (2023). The potential of allelochemicals from microalgae for biopesticides. Plants 12:1896. doi: 10.3390/plants12091896
Castro, B. M. C., Martinez, L. C., Barbosa, S. G., Serrão, J. E., Wilcken, C. F., Soares, M. A., et al. (2019). Toxicity and cytopathology mediated by Bacillus thuringiensis in the midgut of Anticarsia gemmatalis (Lepidoptera: Noctuidae). Sci. Rep. 9:6667. doi: 10.1038/s41598-019-43074-0
Cavalcanti, V. L. R., Brandão-Costa, R. M. P., Pontual, E. V., de Andrade, A. F., Alves, L. C., Porto, A. L. F., et al. (2021). Chlorella vulgaris lectin kills Aedes aegypti larvae. Algal Res. 56:102290. doi: 10.1016/j.algal.2021.102290
Cech, R., Leisch, F., and Zaller, J. G. (2022). Pesticide use and associated greenhouse gas emissions in sugar beet, apples, and viticulture in Austria from 2000 to 2019. Agriculture 12:879. doi: 10.3390/agriculture12060879
Chakraborty, M., Hasanuzzaman, M., Rahman, M., Khan, M. A. R., Bhowmik, P., Mahmud, N. U., et al. (2020). Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agriculture 10:624. doi: 10.3390/agriculture10120624
Chakraborty, N., Mitra, R., Pal, S., Ganguly, R., Acharya, K., Minkina, T., et al. (2023). Biopesticide consumption in India: insights into the current trends. Agriculture 13:557. doi: 10.3390/agriculture13030557
Chandwani, S., Naik, H. Y., Gamit, H. A., Chandarana, K. A., and Amaresan, N. (2023). “Mass multiplication, production cost analysis, and marketing of beauveria,” in Agricultural Microbiology Based Entrepreneurship, Vol. 39, eds. N. Amaresan, D. Dharumadurai, and O. O. Babalola (Cham: Springer Nature Singapore), 251–266. doi: 10.1007/978-981-19-5747-5_16
Chanu, W. T., Sinha, B., Chakrapani, K., Devi, K. S., Devi, H. C., Chakma, T., et al. (2020). Biopriming of pea seeds with native Trichoderma species for enhanced seedling vigour. Indian J. Plant Protect. 48, 438–440.
Charudattan, R. (2024). Use of plant viruses as bioherbicides: the first virus-based bioherbicide and future opportunities. Pest Manag. Sci. 80, 103–114. doi: 10.1002/ps.7760
Chaudhary, S., Yadav, S. K., and Verma, P. (2024). “Botanical insecticides for crop protection: major classes and possible mechanisms of action,” in Insecticides in Pest Control-Impact, Challenges and Strategies: Impact, Challenges and Strategies, ed. S. Kumar (London: InTechOpen), 161. doi: 10.5772/intechopen.1006743
Chellatan Veettil, P., Krishna, V. V., and Qaim, M. (2014). Bt Cotton and Ecosystem Impacts of Pesticide Reductions. GlobalFood Discussion Papers. Available online at: https://www.econstor.eu/handle/10419/103991 (accessed May 18, 2025).
Chen, H., Lan, Y., Fritz, B. K., Clint Hoffmann, W., and Liu, S. (2021). Review of agricultural spraying technologies for plant protection using unmanned aerial vehicle (UAV). Int. J. Agric. Biol. Eng. 14, 38–49. doi: 10.25165/j.ijabe.20211401.5714
Chen, K., Yuan, S., Wang, D., Liu, Y., Chen, F., and Qi, D. (2021). Basic amino acid-modified lignin-based biomass adjuvants: synthesis, emulsifying activity, ultraviolet protection, and controlled release of avermectin. Langmuir 37, 12179–12187. doi: 10.1021/acs.langmuir.1c02113
Chen, X., Li, R.-T., Chen, R.-Y., Shi, P.-D., Liu, Z.-X., Lou, Y.-N., et al. (2024). The subgenomic flaviviral RNA suppresses RNA interference through competing with siRNAs for binding RISC components. J. Virol. 98:e01954-23. doi: 10.1128/jvi.01954-23
Cherif, A., Mansour, R., and Grissa-Lebdi, K. (2021). The egg parasitoids Trichogramma: from laboratory mass rearing to biological control of lepidopteran pests. Biocontrol Sci. Technol. 31, 661–693. doi: 10.1080/09583157.2020.1871469
Chhibber, A., and Ravichandran, S. (2024). Eco friendly agriculture. Int. J. Anal. Appl. Chem. 10, 21–25.
Chidawanyika, F., Omuse, E. R., Agutu, L. O., Pittchar, J. O., Nyagol, D., and Khan, Z. R. (2025). An intensified cereal push-pull system reduces pest infestation and confers yield advantages in high-value vegetables. J. Crop Health 77:40. doi: 10.1007/s10343-024-01107-3
Chikte, T., Psota, V., Chwil, M., Kopta, T., and Arizmendi, J. (2024). A comprehensive review of low-and zero-residue pesticide methods in vegetable production. Agronomy 14:2745. doi: 10.3390/agronomy14112745
Choudhary, V., Kumar, K. K., Sahu, B., Muthappa, S.-K., and Das, A. D. A. (2021). Agronomic innovations in biotic stress management and its combined effect with abiotic stresses in crop production. Indian J. Agron. 66, 237–257.
Christiaens, O., Sweet, J., Dzhambazova, T., Urru, I., Smagghe, G., Kostov, K., et al. (2022). Implementation of RNAi-based arthropod pest control: environmental risks, potential for resistance and regulatory considerations. J. Pest Sci. 95, 1–15. doi: 10.1007/s10340-021-01439-3
Chuskit, R., Rana, A., Gupta, I., Ojha, S., Singh, R., Kaur, S., et al. (2024). “Alternative strategies for reducing neonicotinoids use,” in Neonicotinoids in the Environment, eds. R. Singh, V. K. Singh, A. Kumar, S. Tripathi, and R. Bhadouria (Cham: Springer Nature Switzerland), 199–213. doi: 10.1007/978-3-031-45343-4_15
Claasen, N., Dunn, M. D., and Malan, A. P. (2025). Mass-culture technique of a South African Heterorhabditis bacteriophora isolate, using in vitro liquid culture. Afr. Entomol. 33:e20609. doi: 10.17159/2254-8854/2025/a20609
Colmenarez, Y. C., and Vasquez, C. (2024). Benefits associated with the implementation of biological control programmes in Latin America. Biocontrol 69, 303–320. doi: 10.1007/s10526-024-10260-7
Constantine, K. L., Kansiime, M. K., Mugambi, I., Nunda, W., Chacha, D., Rware, H., et al. (2020). Why don't smallholder farmers in Kenya use more biopesticides? Pest Manag. Sci. 76, 3615–3625. doi: 10.1002/ps.5896
Crouzet, J., Arguelles-Arias, A., Dhondt-Cordelier, S., Cordelier, S., Pršić, J., Hoff, G., et al. (2020). Biosurfactants in plant protection against diseases: rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 8:1014. doi: 10.3389/fbioe.2020.01014
Cryan, T., Musselman, O., Baumgardner, A. W., Osborn, S., Beuscher, C. J., Stehn, C., et al. (2025). Yield, growth, and labor demands of growing maize, beans, and squash in monoculture versus the Three Sisters. Plants People Planet 7, 204–214. doi: 10.1002/ppp3.10576
da Silva, P. F., dos Santos, M. S. N., Araújo, B. de A., Kerber, B. D., de Oliveira, H. A. P., Guedes, J. V. C., et al. (2025). Co-cultivations of Beauveria bassiana, Metarhizium anisopliae, and Trichoderma harzianum to produce bioactive compounds for application in agriculture. Fermentation 11:30. doi: 10.3390/fermentation11010030
da Silva, V. F., dos Santos, A., Silveira, L. C. P., Tomazella, V. B., and Ferraz, R. M. (2022). Push-pull cropping system reduces pests and promotes the abundance and richness of natural enemies in brassica vegetable crops. Biol. Control 166:104832. doi: 10.1016/j.biocontrol.2021.104832
Darwish, I. A. A., Martins, D. P., Ryan, D., and Kakouli-Duarte, T. (2025). State of the art on the interaction of entomopathogenic nematodes and plant growth-promoting rhizobacteria to innovate a sustainable plant health product. Crops 5:52. doi: 10.3390/crops5040052
Das, R., Bharadwaj, P., and Thakur, D. (2024). Insights into the functional role of Actinomycetia in promoting plant growth and biocontrol in tea (Camellia sinensis) plants. Arch. Microbiol. 206:65. doi: 10.1007/s00203-023-03789-1
de Lira, A. C., Mascarin, G. M., and Júnior, Í. D. (2020). Microsclerotia production of Metarhizium spp. for dual role as plant biostimulant and control of Spodoptera frugiperda through corn seed coating. Fungal Biol. 124, 689–699. doi: 10.1016/j.funbio.2020.03.011
De Oliveira, D. G. P., Lopes, R. B., Rezende, J. M., and Delalibera, I. Jr (2018). Increased tolerance of Beauveria bassiana and Metarhizium anisopliae conidia to high temperature provided by oil-based formulations. J. Invertebr. Pathol. 151, 151–157. doi: 10.1016/j.jip.2017.11.012
De Oliveira, I. F., Gomes, T. C., Simeone, M. L. F., Karam, D., and De Sousa Tinoco, S. M. (2024). Sorgoleone unveiled: exploring its biosynthesis, functional perspectives and applications. Braz. J. Botany 47, 723–733. doi: 10.1007/s40415-024-01026-7
De Schutter, K., Taning, C. N. T., Van Daele, L., Van Damme, E. J., Dubruel, P., and Smagghe, G. (2022). RNAi-based biocontrol products: market status, regulatory aspects, and risk assessment. Front. Insect Sci. 1:818037. doi: 10.3389/finsc.2021.818037
De Steur, H., Stein, A. J., and Demont, M. (2022). From golden rice to golden diets: how to turn its recent approval into practice. Glob. Food Secur. 32:100596. doi: 10.1016/j.gfs.2021.100596
De Waal, J. Y., Malan, A. P., and Addison, M. F. (2013). Effect of humidity and a superabsorbent polymer formulation on the efficacy of Heterorhabditis zealandica (Rhabditida: Heterorhabditidae) to control codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biocontrol Sci. Technol. 23, 62–78. doi: 10.1080/09583157.2012.736472
Deeksha, M. G., Jadhav, M. M., Guleria, N., Harish, M. N., Chaitra, M., and Barman, M. (2025). “Legal framework for the development and application of biopesticides worldwide,” in Bio-control Agents for Sustainable Agriculture: Diversity, Mechanisms and Applications (Cham: Springer), 479–510. doi: 10.1007/978-981-96-3232-9_20
del Valle, E. R. (2020). How Does Landscape Restoration in La Junquera Farm Affect Pollination and Biological Control Services by Insects on Almond Cultivation. Wagningen: University and Research. Available online at: https://edepot.wur.nl/536194 (accessed June 16, 2025).
Diaz, J. M., Warner, L. A., and Oi, F. M. (2020). Identifying factors to increase the adoption of integrated pest management practices: an audience segmentation study. J. Int. Agric. Ext. Educ. 27, 7–23. doi: 10.4148/2831-5960.1114
Ding, J., Zhou, E., Wang, X., Jiang, H., Su, H., Gao, Q., et al. (2025). Cellulose nanocrystals-based pickering emulsion with enhanced foliar adhesion and pH responsiveness for intelligent delivery of pesticides. Int. J. Biol. Macromol. 286:138192. doi: 10.1016/j.ijbiomac.2024.138192
Divekar, P. A., Rani, V., Majumder, S., Karkute, S. G., Molla, K. A., Pandey, K. K., et al. (2023). Protease inhibitors: an induced plant defense mechanism against herbivores. J. Plant Growth Regul. 42, 6057–6073. doi: 10.1007/s00344-022-10767-2
Durocher-Granger, L., Mfune, T., Musesha, M., Lowry, A., Reynolds, K., Buddie, A., et al. (2021). Factors influencing the occurrence of fall armyworm parasitoids in Zambia. J. Pest Sci. 94, 1133–1146. doi: 10.1007/s10340-020-01320-9
Ediagbonya, T. F., Areo, I. O., Mupenzi, C., Mind'je, R., Kamuhanda, J. K., and Kabano, S. (2025). Reduced pesticide dependency through crop management. Discov. Appl. Sci. 7:776. doi: 10.1007/s42452-025-07248-y
Eissa, E.-S. H., Hendam, B. M., Dighiesh, H. S., Abd Elnabi, H. E., Sakr, S. E.-S., Kabary, H., et al. (2025). The benefits of astaxanthin-rich microalgal powder on growth, health, and disease resistance against Fusarium solani in Pacific white shrimp. Fish Shellfish Immunol. 156:110059. doi: 10.1016/j.fsi.2024.110059
Eldefrawi, M. E., and Eldefrawi, A. T. (2020). “Nervous-system-based insecticides,” in Safer Insecticides (CRC Press), 155–207. Availabel online at: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003065975-5/nervous-system-based-insecticides-mohyee-eldefrawi-amira-eldefrawi (accessed May 15, 2025).
Eldessouki, E. A. A., Elshopakey, G. E., Elbahnaswy, S., Shakweer, M. S., Abdelwarith, A. A., Younis, E. M., et al. (2024). Influence of astaxanthin-enriched Haematococcus pluvialis microalgae on the growth efficacy, immune response, antioxidant capacity, proinflammatory cytokines, and tissue histomorphology of hybrid red tilapia. Aquac. Int. 32, 7447–7468. doi: 10.1007/s10499-024-01524-1
Elkhateeb, W. A., and Daba, G. M. (2019). Myrothecium as promising model for biotechnological applications, potentials and challenges. J. Sci. Res. 16, 12126–12131. doi: 10.26717/BJSTR.2019.16.002869
El-Sheekh, M. M., Deyab, M. A., Hasan, R. S. A., Abu Ahmed, S. E., and Elsadany, A. Y. (2022). Biological control of Fusarium tomato-wilt disease by cyanobacteria Nostoc spp. Arch. Microbiol. 204:116. doi: 10.1007/s00203-021-02673-0
Erdemci, I. (2020). Effect of Pseudomonas fluorescent rhizobacteria on growth and seed quality in lentil (Lens Culinaris Medik.). Commun. Soil Sci. Plant Anal. 51, 1852–1858. doi: 10.1080/00103624.2020.1798987
Essiedu, J. A., Adepoju, F. O., and Ivantsova, M. N. (2020). “Benefits and limitations in using biopesticides: a review,” in AIP Conference Proceedings, 2313. Available onlin at: https://pubs.aip.org/aip/acp/article-abstract/2313/1/080002/963419 (accessed June 16, 2025).
Etemadifar, Z., Gholami, M., and Derikvand, P. (2016). UV-resistant bacteria with multiple-stress tolerance isolated from desert areas in Iran. Geomicrobiol. J. 33, 1–7. doi: 10.1080/01490451.2015.1063025
European Food Safety Authority (EFSA), Nougadère, A., Mastin, A., Scala, M., Sánchez, B., Baldassarre, F., et al. (2025). Helicoverpa zea Pest Report to support the ranking of EU candidate priority pests. EFSA Support. Publ. 22, 1–67. doi: 10.2903/sp.efsa.2025.EN-9448
Ezin, V., and Chabi, I. B. (2022). “Azadirachta indica: its biological, pharmacological, antidiabetic potential, and omics applications,” in Antidiabetic Plants for Drug Discovery (Apple Academic Press), 1–22. Available online at: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003282938-1/azadirachta-indica-biological-pharmacological-antidiabetic-potential-omics-applications-vincent-ezin-ifagbemi-bienvenue-chabi (accessed June 18, 2025).
Fallet, P., Bazagwira, D., Ruzzante, L., Ingabire, G., Levivier, S., Bustos-Segura, C., et al. (2024). Entomopathogenic nematodes as an effective and sustainable alternative to control the fall armyworm in Africa. PNAS Nexus 3:pgae122. doi: 10.1093/pnasnexus/pgae122
Farooq, M., and Zada, N. (2022). Parasitism potential of biological control agents of fruit fly under natural enemy field reservoirs (NEFR) technology. Bulg. J. Agric. Sci. 28, 876–881.
Fenibo, E. O., Christian, R., and Matambo, T. S. (2023). “Biopesticide commercialization in African countries: successful case studies,” in Development and Commercialization of Biopesticides, ed. O. Koul (Amsterdam: Elsevier), 297–328. doi: 10.1016/B978-0-323-95290-3.00006-6
Fenibo, E. O., Ijoma, G. N., Nurmahomed, W., and Matambo, T. (2022). The potential and green chemistry attributes of biopesticides for sustainable agriculture. Sustainability 14:14417. doi: 10.3390/su142114417
Fletcher, S. J., Reeves, P. T., Hoang, B. T., and Mitter, N. (2020). A perspective on RNAi-based biopesticides. Front. Plant Sci. 11:51. doi: 10.3389/fpls.2020.00051
Fountain, M. T., Deakin, G., Farman, D., Hall, D., Jay, C., Shaw, B., et al. (2021). An effective ‘push–pull' control strategy for European tarnished plant bug, Lygus rugulipennis (Heteroptera: Miridae), in strawberry using synthetic semiochemicals. Pest Manag. Sci. 77, 2747–2755. doi: 10.1002/ps.6303
Furmanczyk, E. M., and Malusà, E. (2023). Control of nematodes in organic horticulture exploiting the multifunctional capacity of microorganisms. Horticulturae 9:920. doi: 10.3390/horticulturae9080920
Fusar Poli, E., and Fontefrancesco, M. F. (2024). Trends in the implementation of biopesticides in the Euro-Mediterranean region: a narrative literary review. Sustain. Earth Rev. 7:14. doi: 10.1186/s42055-024-00085-8
Galindo, F. S., Rodrigues, W. L., Fernandes, G. C., Boleta, E. H. M., Jalal, A., Rosa, P. A. L., et al. (2022). Enhancing agronomic efficiency and maize grain yield with Azospirillum brasilense inoculation under Brazilian savannah conditions. Eur. J. Agron. 134:126471. doi: 10.1016/j.eja.2022.126471
Gc, Y. D., Hadi, B. A. R., and Wyckhuys, K. A. G. (2022). Contrasting national plant protection needs, perceptions and techno-scientific capabilities in the Asia-Pacific region. Front. Sustain. Food Syst. 6:853359. doi: 10.3389/fsufs.2022.853359
Gebreziher, H. G., and Gebreazgaabher, F. G. (2024). Night-time light-traps and push–pull integrated system enhanced the control of different life stages of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). CABI Agric. Biosci. 5:104. doi: 10.1186/s43170-024-00307-1
Geremew, D., Shiberu, T., and Leta, A. (2024). Isolation, morphological characterization, and screening virulence of Beauveria bassiana and Metarhizium robertsii fungal isolates on Galleria mellonella. F1000Res. 12:827. doi: 10.12688/f1000research.134020.5
Ghosal, A. (2018). Studies on the potency of some biopesticides against whitefly in cotton and tomato. J. Biopesticides 11, 60–68. doi: 10.57182/jbiopestic.11.1.60-68
Glover, J. P., Spaulding, N., George, J., Portilla, M., Reddy, G. V. P., and Dillman, A. (2025). Efficacy of the newly discovered entomopathogenic nematode Steinernema adamsi against Helicoverpa zea: life stage susceptibility, UV tolerance, and field performance. J. Nematol. 57:20250012. doi: 10.2478/jofnem-2025-0012
Gong, L., Chen, Y., Hu, Z., and Hu, M. (2013). Testing insecticidal activity of novel chemically synthesized siRNA against Plutella xylostella under laboratory and field conditions. PLoS ONE 8:e62990. doi: 10.1371/journal.pone.0062990
Gopalkrishna, H. R., Chakravarthy, A. K., and Prasad, H. N. N. (2022). “Enhancing genetic efficiency of natural enemies of crop pests,” in Genetic Methods and Tools for Managing Crop Pests, ed. A. K. Chakravarthy (Cham: Springer Nature Singapore), 211–249. doi: 10.1007/978-981-19-0264-2_7
Goswami, S., and Kalidas-Singh, S. (2023). Localized phosphorus application for onion cultivation (Allium cepa L.): seedling root dip in P-enriched soil slurry. J. Soil Sci. Plant Nutr. 23, 5962–5974. doi: 10.1007/s42729-023-01454-6
Gowthish, K., and Kannan, R. (2019). Insecticidal and Insect Growth Regulator Activity of a Red Algal Seaweed Gracilaria edulis (SG Gmelin) PC Silva Against Tobacco Caterpillar Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Available online at: https://www.cabidigitallibrary.org/doi/full/10.5555/20203540153 (accessed June 20, 2025).
Gregorio, N. O., Herbohn, J. L., and Harrison, S. R. (2010). Guide to Quality Seedling Production in Smallholder Nurseries. Leyte: Visayas State University, 1–43.
Gujar, G. T., Trisyono, Y. A., and Chen, M. (2021). “Biotech/genetically modified crops in Asia Pacific: a way forward,” in Genetically Modified Crops in Asia Pacific, eds. G. T. Gujar, Y. A. Trisyono, and M. Chen (Clayton, VIC: CSIRO). doi: 10.1071/9781486310913
Gupta, M., Kumar, H., and Kaur, S. (2021). Vegetative insecticidal protein (Vip): a potential contender from Bacillus thuringiensis for efficient management of various detrimental agricultural pests. Front. Microbiol. 1:659736. doi: 10.3389/fmicb.2021.659736
Gupta, R., and Bar, M. (2020). “Plant immunity, priming, and systemic resistance as mechanisms for Trichoderma spp. Biocontrol,” in Trichoderma, eds. A. K. Sharma, and P. Sharma (Singapore: Springer Singapore), 81–110. doi: 10.1007/978-981-15-3321-1_5
Gupta, R., Chaudhary, A. K., and Sharma, R. (2023). Analgesic and anti-inflammatory potential of Ricinus communis Linn.: evidence from pharmacology to clinical studies. Curr. Pharmacol. Rep. 10, 27–67. doi: 10.1007/s40495-023-00347-7
Haider, M. U., and Ahmad, S. (2025). Virulence of different entomopathogenic fungal strains against different life stages of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Wildl. Biodivers. 9, 183–199.
Hamdi, R. F., and Alzawi, K. S. (2023). Using fungi and bacteria as biological control agents of fungal plant diseases an article. J. Univ. Anbar Pure Sci. 17:178865. doi: 10.37652/juaps.2023.178865
Hamrouni, R., Regus, F., Farnet Da Silva, A.-M., Orsiere, T., Boudenne, J.-L., Laffont-Schwob, I., et al. (2025). Current status and future trends of microbial and nematode-based biopesticides for biocontrol of crop pathogens. Crit. Rev. Biotechnol. 45, 333–352. doi: 10.1080/07388551.2024.2370370
Hatt, S., and Döring, T. F. (2025). The interplay of intercropping, wildflower strips and weeds in conservation biological control and productivity. J. Pest Sci. 98, 159–174. doi: 10.1007/s10340-024-01801-1
Hatt, S., Lopes, T., Boeraeve, F., Chen, J., and Francis, F. (2017). Pest regulation and support of natural enemies in agriculture: experimental evidence of within field wildflower strips. Ecol. Eng. 98, 240–245. doi: 10.1016/j.ecoleng.2016.10.080
Hegde, G. M., Dobhal, A., Vijaykumar, K. N., and Jahagirdar, S. (2023). “Advances in formulations and efficacy of mycopesticides for plant disease management and sustainable yields,” in Fungal Resources for Sustainable Economy, eds. I. Singh, V. R. Rajpal, and S. S. Navi (Singapore: Springer Nature), 373–408. doi: 10.1007/978-981-19-9103-5_14
Hegde, G. M., and Vijaykumar, K. N. (2022). “Formulation, application and commercialization of biopesticides in India,” in Asia Pacific Biofertilizers and Biopesticides Information Platform. Available online at: https://apbb.fftc.org.tw/article/306 (accessed May 27, 2025).
Heraty, J. (2017). “Parasitoid biodiversity and insect pest management,” in Insect Biodiversity, 1st Edn., eds. R. G. Foottit, and P. H. Adler (Hoboken, NJ: Wiley), 603–625. doi: 10.1002/9781118945568.ch19
Herring, R. J. (2015). State science, risk and agricultural biotechnology: Bt cotton to Bt Brinjal in India. J. Peasant Stud. 42, 159–186. doi: 10.1080/03066150.2014.951835
Herring, R. J. (2023). Genetically modified democracy: transgenic crops in contemporary India. J. Agrar. Change 23:893.
Hikmawanti, N. P. E., Ramadon, D., Jantan, I., and Mun'im, A. (2021). Natural deep eutectic solvents (NADES): phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 10:2091. doi: 10.3390/plants10102091
Hodek, I., Van Emden, H. F., and Honěk, A. (Eds). (2012). Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), 1st Edn. Hoboken, NJ: Wiley. doi: 10.1002/9781118223208
Holajjer, P., Kamra, A., Gaur, H. S., and Manjunath, M. (2013). Potential of cyanobacteria for biorational management of plant parasitic nematodes: a review. Crop Prot. 53, 147–151. doi: 10.1016/j.cropro.2013.07.005
Horikoshi, R. J., Vertuan, H., De Castro, A. A., Morrell, K., Griffith, C., Evans, A., et al. (2021). A new generation of Bt maize for control of fall armyworm (Spodoptera frugiperda). Pest Manag. Sci. 77, 3727–3736. doi: 10.1002/ps.6334
Hou, Y., Zang, Z., Lü, W., Xu, W., Desneux, N., and Zang, L. (2024). Transgenerational hormesis and sublethal effects of five key insecticides for controlling Spodoptera frugiperda on its endoparasitoid Cotesia marginiventris. Pest Manag. Sci. 80, 1681–1691. doi: 10.1002/ps.7899
Htay, T. M., Sann, K. K., and Haini, H. (2023). Comparative study on phytochemical screening and antioxidant activity of aqueous extract from various parts of Bauhinia purpurea. Bioactivities 1, 24–31. doi: 10.47352/bioactivities.2963-654X.183
Hu, J., Jiang, J., and Wang, N. (2018). Control of citrus huanglongbing via trunk injection of plant defense activators and antibiotics. Phytopathology 108, 186–195. doi: 10.1094/PHYTO-05-17-0175-R
Huang, J., Mi, J., Lin, H., Wang, Z., Chen, R., Hu, R., et al. (2010). A decade of Bt cotton in Chinese fields: assessing the direct effects and indirect externalities of Bt cotton adoption in China. Sci. China Life Sci. 53, 981–991. doi: 10.1007/s11427-010-4036-y
Hussain, M. I., Araniti, F., Schulz, M., Baerson, S., Vieites-Álvarez, Y., Rempelos, L., et al. (2022). Benzoxazinoids in wheat allelopathy–from discovery to application for sustainable weed management. Environ. Exp. Bot. 202:104997. doi: 10.1016/j.envexpbot.2022.104997
Hussein, M. A., Salem, H. A., Hala, S., and Mahmoud, S. (2022). Effects of the nutrition of different diets and lipid content of the insect host larvae, Galleria mellonella on the efficacy of indigenous entomopathogenic nematodes. J. Plant Protect. Res. 62, 265–271. doi: 10.24425/jppr.2022.142133
Hyakumachi, M., Nishimura, M., Arakawa, T., Asano, S., Yoshida, S., Tsushima, S., et al. (2013). Bacillus thuringiensis suppresses bacterial wilt disease caused by Ralstonia solanacearum with systemic induction of defense-related gene expression in tomato. Microbes Environ. 28, 128–134. doi: 10.1264/jsme2.ME12162
Isman, M. B. (2023). “Commercialization and regulation of botanical biopesticides: a global perspective,” in Development and Commercialization of Biopesticides, ed. O. Koul (Amsterdam: Elsevier) 25–36. doi: 10.1016/B978-0-323-95290-3.00001-7
Ivezić, A., Trudić, B., and Draškić, G. (2022). The usage of beneficial insects as a biological control measure in large-scale farming-a case study review on Trichogramma spp. Acta Agric. Slov. 118, 1–13. doi: 10.14720/aas.2022.118.2.2402
Janjarasskul, T., and Suppakul, P. (2018). Active and intelligent packaging: the indication of quality and safety. Crit. Rev. Food Sci. Nutr. 58, 808–831. doi: 10.1080/10408398.2016.1225278
Javed, T., Afzal, I., and Mauro, R. P. (2021). Seed coating in direct seeded rice: an innovative and sustainable approach to enhance grain yield and weed management under submerged conditions. Sustainability 13:2190. doi: 10.3390/su13042190
Jensen, B., Lübeck, P. S., and Jørgensen, H. J. (2016). Clonostachys rosea reduces spot blotch in barley by inhibiting prepenetration growth and sporulation of Bipolaris sorokiniana without inducing resistance: the ability of C. rosea to control barley leaf pathogens. Pest Manag. Sci. 72, 2231–2239. doi: 10.1002/ps.4260
Jha, S. S., Joshi, S. J., and SJ, G. (2016). Lipopeptide production by Bacillus subtilis R1 and its possible applications. Braz. J. Microbiol. 47, 955–964. doi: 10.1016/j.bjm.2016.07.006
Jiang, Y., and Wang, J. (2023). The registration situation and use of mycopesticides in the world. J. Fungi 9:940. doi: 10.3390/jof9090940
Jin, Y., Drabik, D., Heerink, N., and Wesseler, J. (2019). The cost of postponement of Bt rice commercialization in China. Front. Plant Sci. 10:1226. doi: 10.3389/fpls.2019.01226
Joelle, T. M., Yeyinou, L. E. L., Ouorou, K. D. K., Elie, A. D., Karimou, Z., Parfait, K., et al. (2020). Management of the legume pod borer Maruca vitrata Fabricius (Lepidoptera: Crambidae) with field applications of the entomopathogenic fungus, Beauveria bassiana and a mixed formulation of the baculovirus MaviMNPV with emulsifiable neem oil. Afr. J. Agric. Res. 15, 113–121. doi: 10.5897/AJAR2019.14391
Joshi, G., and Chaudhuri, S. (2025). Biopesticides from Agricultural and Forest Biomass. Available online at: https://books.rsc.org/books/edited-volume/2280/chapter/8407014 (accessed June 28, 2025).
Kala, S., Sogan, N., Agarwal, A., Naik, S. N., Patanjali, P. K., and Kumar, J. (2020). “Biopesticides: formulations and delivery techniques,” in Natural Remedies for Pest, Disease and Weed Control, eds. C. Egbuna, and B. Sawicka (Amsterdam: Elsevier), 209–220. doi: 10.1016/B978-0-12-819304-4.00018-X
Kanatas, P. (2020). Potential role of Eucalyptus spp. and Acacia spp. allelochemicals in weed management. Chil. J. Agric. Res. 80, 452–458. doi: 10.4067/S0718-58392020000300452
Kang, G., Yang, X., Zhang, H., Huang, Y., Sun, Y., Liang, G., et al. (2025). Refuge strategies for managing resistance to Bt maize in fall armyworm in smallholder farming systems: a case study from China. J. Pest Sci. 98, 1551–1566. doi: 10.1007/s10340-025-01896-0
Karamchandani, B. M., Chakraborty, S., Dalvi, S. G., and Satpute, S. K. (2022). Chitosan and its derivatives: promising biomaterial in averting fungal diseases of sugarcane and other crops. J. Basic Microbiol. 62, 533–554. doi: 10.1002/jobm.202100613
Karuppiah, P., Thayalan, K. J., Thangam, G. S., and Eswaranpillai, U. (2025). “Bio-control agents: a sustainable approach for enhancing soil nutrients use efficiency in farming,” in Bio-control Agents for Sustainable Agriculture, eds. D. Mitra, S. De Los Santos Villalobos, A. Rani, B. Elena Guerra Sierra, and S. Andjelković (Cham: Springer Nature Singapore), 209–260. doi: 10.1007/978-981-96-3232-9_10
Kathage, J., and Qaim, M. (2012). Economic impacts and impact dynamics of Bt (Bacillus thuringiensis) cotton in India. Proc. Nat. Acad. Sci. 109, 11652–11656. doi: 10.1073/pnas.1203647109
Kaur, A., and Upadhyay, S. K. (2022). “Agricultural applications of engineered microbes,” in Current Developments in Biotechnology and Bioengineering, eds. S. Joshi, A. Pandey, A. Sirohi, and S. H. Park (Amsterdam: Elsevier), 363–375. doi: 10.1016/B978-0-323-88504-1.00008-X
Kaushik, Y., and Arora, P. (2024). Investigating the sustainable energy generation potential of an invasive weed: Lantana camara. Environ. Sci. Pollut. Res. 31, 62493–62509. doi: 10.1007/s11356-024-35322-2
Kawpet, R., Thancharoen, A., and Sridokchan, W. (2022). Potential of Entomopathogenic Fungi for Controlling Rice Leafhoppers and Lepidopterous LARVAE in northern Thailand. Available online at: https://www.thaiscience.info/Journals/Article/IJAT/10996771.pdf (accessed May 20, 2025).
Kenawy, A., Dailin, D. J., Abo-Zaid, G. A., Malek, R. A., Ambehabati, K. K., Zakaria, K. H. N., et al. (2019). “Biosynthesis of antibiotics by PGPR and their roles in biocontrol of plant diseases,” in Plant Growth Promoting Rhizobacteria for Sustainable Stress Management, Vol. 13, ed. R. Z. Sayyed (Singapore: Springer), 1–35. doi: 10.1007/978-981-13-6986-5_1
Khan, Md. A., Paul, B., Ahmad, W., Paul, S., Aggarwal, C., Khan, Z., et al. (2016). “Potential of Bacillus thuringiensis in the management of pernicious lepidopteran pests,” in Plant, Soil and Microbes, eds. K. R. Hakeem, and M. S. Akhtar (Cham: Springer International Publishing), 277–301. doi: 10.1007/978-3-319-29573-2_13
Khan, Z., Midega, C., Pittchar, J., Pickett, J., and Bruce, T. (2012). “Push–pull technology: a conservation agriculture approach for integrated management of insect pests, weeds and soil health in Africa: UK Government's foresight food and farming futures project,” in Sustainable Intensification (London: Routledge), 162–170. doi: 10.3763/ijas.2010.0558
Khan, Z. R., Pittchar, J. O., Drinkwater, L. E., and Pickett, J. A. (2023). “Enhancing soil system productivity and controlling pests with push-pull polycropping in East Africa,” in Biological Approaches to Regenerative Soil Systems (Cambridge, MA: CRC Press), 407–420. doi: 10.1201/9781003093718-39
Khashaveh, A., Yi, C., Tang, H., Song, X., Zhang, G., Xie, J., et al. (2025). Biological roles of pyrazines in insect chemical communication. Chem. Biol. Technol. Agric. 12:109. doi: 10.1186/s40538-025-00830-x
Khulbe, A., and Batra, P. (2024). “Insect-pests and diseases in greenhouse cultivation and their biological control,” in Protected Cultivation (Apple Academic Press), 217–254. Available online at: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003402596-9/insect-pests-diseases-greenhouse-cultivation-biological-control-anjani-khulbe-poonam-batra (accessed June 5, 2025).
Kim, H.-J., Cho, K. H., Kim, D.-I., and Kim, Y. C. (2025). Pyrethrum cultivation techniques in korea for pest management. Korean J. Org. Agric. 33, 65–75. doi: 10.11625/KJOA.2025.33.1.65
Kipngeno, P., Losenge, T., Maina, N., Kahangi, E., and Juma, P. (2015). Efficacy of Bacillus subtilis and Trichoderma asperellum against Pythium aphanidermatum in tomatoes. Biol. Control 90, 92–95. doi: 10.1016/j.biocontrol.2015.05.017
Klümper, W., and Qaim, M. (2014). A meta-analysis of the impacts of genetically modified crops. PLoS ONE 9:e111629. doi: 10.1371/journal.pone.0111629
Komagata, Y., Sekine, T., Oe, T., Kakui, S., and Yamanaka, S. (2024). Simultaneous use of Beauveria bassiana and Bacillus subtilis-based biopesticides contributed to dual control of Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) and tomato powdery mildew without antagonistic interactions. Egypt. J. Biol. Pest Control 34:18. doi: 10.1186/s41938-024-00782-8
Koppenhöfer, A. M., Shapiro-Ilan, D. I., and Hiltpold, I. (2020). Entomopathogenic nematodes in sustainable food production. Front. Sustain. Food Syst. 4:125. doi: 10.3389/fsufs.2020.00125
Kotey, D. A., Kotey, D. A., Obi, A., Assefa, Y., Assefa, Y., Erasmus, A., et al. (2017). Monitoring resistance to Bt maize in field populations of Busseola fusca (Fuller) (Lepidoptera: Noctuidae) from smallholder farms in the Eastern Cape Province of South Africa. Afr. Entomol. 25:200. doi: 10.4001/003.025.0200
Kothari, M., and Sandhu, S. S. (2025). “Entomopathogenic perspectives of Nomuraea rileyi,” in The Role of Entomopathogenic Fungi in Agriculture, eds. S. K. Deshmukh, and K. Sridhar (Cambridge, MA: CRC Press), 140–161. doi: 10.1201/9781003505228-8
Kovanci, O. B. (2017). Comparison of the costs of mating disruption with traditional insecticide applications for control of codling moth in apple orchards in Turkey. Sci. Pap. Ser. B. Hortic. 61, 455–460.
Kruger, M., Van Rensburg, J. B. J., and Van Den Berg, J. (2012). Transgenic Bt maize: farmers' perceptions, refuge compliance and reports of stem borer resistance in South Africa. J. Appl. Entomol. 136, 38–50. doi: 10.1111/j.1439-0418.2011.01616.x
Kumar, B. (2023). “Ladybird beetles (Coleoptera: Coccinellidae),” in Insect Predators in Pest Management (Cambridge, MA: CRC Press), 187–227. doi: 10.1201/9781003370864-8
Kumar, D., Kumar, A., and Kumar, V. (2025). In-vitro mass multiplication of native isolates of Heterorhabditis indica on different artificial growth media in Haryana, India. Environ. Conserv. J. 26, 9–14. doi: 10.36953/ECJ.28482882
Kumar, L. R., Ndao, A., Valéro, J., and Tyagi, R. D. (2019). Production of Bacillus thuringiensis based biopesticide formulation using starch industry wastewater (SIW) as substrate: a techno-economic evaluation. Bioresour. Technol. 294:122144. doi: 10.1016/j.biortech.2019.122144
Kumar, M. P. S., Keerthana, A., Priya Singh, S. K., Rai, D., Jaiswal, A., and Reddy, M. S. S. (2024). Exploration of culturable bacterial associates of aphids and their interactions with entomopathogens. Arch. Microbiol. 206:96. doi: 10.1007/s00203-024-03830-x
Lamichhane, J. R., Corrales, D. C., and Soltani, E. (2022). Biological seed treatments promote crop establishment and yield: a global meta-analysis. Agron. Sustain. Dev. 42:45. doi: 10.1007/s13593-022-00761-z
Latifian, M. (2025). “Biopesticides in organic farming,” in Advances in Organic Farming, ed. L. P. Awasthi (Boston, MA: CABI), 373–379. doi: 10.1079/9781800626850.0039
Lavoir, A.-V., Michel, T., Poëssel, J.-L., and Siegwart, M. (2022). “Challenges in developing botanical biopesticides for pest control,” in Extended Biocontrol, eds. X. Fauvergue, A. Rusch, M. Barret, M. Bardin, E. Jacquin-Joly, T. Malausa, and C. Lannou (Cham: Springer), 161–170. doi: 10.1007/978-94-024-2150-7_14
Lei, C. J., Halim, N. A., Asib, N., Zakaria, A., and Azmi, W. A. (2022). Conidial emulsion formulation and thermal storability of Metarhizium anisopliae against red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Microorganisms 10:1460. doi: 10.3390/microorganisms10071460
Lengai, G. M., Muthomi, J. W., and Mbega, E. R. (2020). Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 7:e00239. doi: 10.1016/j.sciaf.2019.e00239
Leuratti, T., Fellin, L., Michelon, N., Palacios Tario, J. B., Gutiérrez, J. E. S., Gianquinto, G., et al. (2025). Optimizing tomato seedling production in the tropics: effects of Trichoderma, Arbuscular Mycorrhizal Fungi, and key agronomical factors. Agronomy 15, 1–20. doi: 10.3390/agronomy15020392
Levine, S. L., Giddings, J., Valenti, T., Cobb, G. P., Carley, D. S., and McConnell, L. L. (2019). Overcoming challenges of incorporating higher tier data in ecological risk assessments and risk management of pesticides in the United States: findings and recommendations from the 2017 workshop on regulation and innovation in agriculture. Integr. Environ. Assess. Manag. 15, 714–725. doi: 10.1002/ieam.4173
Li, M., and Nangong, Z. (2022). Precision trunk injection technology for treatment of huanglongbing (HLB)-affected citrus trees—a review. J. Plant Dis. Protect. 129, 15–34. doi: 10.1007/s41348-021-00510-6
Li, Y., Hallerman, E. M., Liu, Q., Wu, K., and Peng, Y. (2016). The development and status of Bt rice in China. Plant Biotechnol. J. 14, 839–848. doi: 10.1111/pbi.12464
Liang, Z., Yan, Y., Zhang, W., Luo, H., Yao, B., Huang, H., et al. (2023). Review of glucose oxidase as a feed additive: production, engineering, applications, growth-promoting mechanisms, and outlook. Crit. Rev. Biotechnol. 43, 698–715. doi: 10.1080/07388551.2022.2057275
Liu, Y., Shi, A., Chen, Y., Xu, Z., Liu, Y., Yao, Y., et al. (2025). Beneficial microorganisms: regulating growth and defense for plant welfare. Plant Biotechnol. J. 23, 986–998. doi: 10.1111/pbi.14554
Lommen, S. T., de Jong, P. W., and Pannebakker, B. A. (2017). It is time to bridge the gap between exploring and exploiting: prospects for utilizing intraspecific genetic variation to optimize arthropods for augmentative pest control–a review. Entomol. Exp. Appl. 162, 108–123. doi: 10.1111/eea.12510
López de Mesa, Y. P. (2020). The decision-making process of synthetic pesticide use in agricultural communities in Colombia: a grounded theory approach. Rev. Fac. Nacional Salud Pública 38:e331277. doi: 10.17533/udea.rfnsp.e331277
Lü, L., Luo, J., Zhang, S., Yu, Q., Ma, L., Liu, X., et al. (2018). Efficiency of cotton bollworm (Helicoverpa armigera Hübner) control of different Bt cotton varieties in North China. J. Cotton Res. 1, 1–8. doi: 10.1186/s42397-018-0003-0
Luna, J. K., and Dowd-Uribe, B. (2020). Knowledge politics and the Bt cotton success narrative in Burkina Faso. World Dev. 136:105127. doi: 10.1016/j.worlddev.2020.105127
Luo, M., Lin, J., Zhou, X., and Pu, X. (2022). Study on physical properties of four pH responsive Spodoptera exigua multiple nucleopolyhedrovirus (SeMNPV) microcapsules as controlled release carriers. Sci. Rep. 12:21873. doi: 10.1038/s41598-022-26317-5
Luttrell, R. G., and Jackson, R. E. (2012). Helicoverpa zea and Bt Cotton in the United States. GM Crops Food 3, 213–227. doi: 10.4161/gmcr.20742
Ma, G., Zou, Y., Wang, S., Guo, X., Li, Z., Li, H., et al. (2025). Advancing sustainable agriculture with mesoporous nanomaterials for smart pesticide delivery. J. Agric. Food Chem. 73, 18480–18496. doi: 10.1021/acs.jafc.5c04278
Malik, S. I., Chimbekujwo, K. I., Maku, A. M., Oyewole, O. A., Omoregie, I. P., Adetunji, C. O., et al. (2025). “The application of nanochitosan biopesticides as a replacement to synthetic pesticides,” in Nanochitosan Applications for Enhanced Crop Production and Food Security, 1st Edn., eds. C. O. Adetunji, M. P. Shah, Y. M. Bello, D. Hefft, J. Singh, S. S. Pandey, and R. P. Singh (Hoboken, NJ: Wiley), 89–110. doi: 10.1002/9781394214945.ch4
Malinga, L. N., and Laing, M. D. (2022). Efficacy of biopesticides in the management of the cotton bollworm, Helicoverpa armigera (Noctuidae), under field conditions. Insects 13:673. doi: 10.3390/insects13080673
Malinga, L. N., and Laing, M. D. (2024). Efficacy of Bacillus thuringiensis and Beauveria bassiana in controlling Helicoverpa armigera. Entomol. Appl. Sci. Lett. 11, 16–23. doi: 10.51847/HiDiennNyk
Mamta, B., and Rajam, M. V. (2017). RNAi technology: a new platform for crop pest control. Physiol. Mol. Biol. Plants 23, 487–501. doi: 10.1007/s12298-017-0443-x
Manson, S., Campera, M., Hedger, K., Ahmad, N., Adinda, E., Nijman, V., et al. (2022). The effectiveness of a biopesticide in the reduction of coffee berry borers in coffee plants. Crop Prot. 1:106075. doi: 10.1016/j.cropro.2022.106075
Mantzoukas, S., Papantzikos, V., Katsogiannou, S., Papanikou, A., Koukidis, C., Servis, D., et al. (2023). Biostimulant and bioinsecticidal effect of coating cotton seeds with endophytic Beauveria bassiana in semi-field conditions. Microorganisms 11:2050. doi: 10.3390/microorganisms11082050
Marchand, P. (2024). The regulatory obstacles to bio control agents from Directive (EC) No 128/2009. J. Regul. Sci. 12:121279. doi: 10.21423/JRS.REGSCI.121279
Marchand, P. A. (2025). Bio-based strategies for biotic and abiotic stress management in sustainable agriculture in EU. Biocontrol Sci. Technol. 35, 357–373. doi: 10.1080/09583157.2024.2433544
Markelova, N., and Chumak, A. (2025). Antimicrobial activity of bacillus cyclic lipopeptides and their role in the host adaptive response to changes in environmental conditions. Int. J. Mol. Sci. 26:336. doi: 10.3390/ijms26010336
Marrone, P. G. (2023). “Biopesticide commercialization in North America: state of the art and future opportunities,” in Development and Commercialization of Biopesticides, ed. O. Koul (Cambridge, MA: Academic Press), 173–202. doi: 10.1016/B978-0-323-95290-3.00017-0
Marrone, P. G. (2024). Status of the biopesticide market and prospects for new bioherbicides. Pest Manag. Sci. 80, 81–86. doi: 10.1002/ps.7403
Mattedi, A., Sabbi, E., Farda, B., Djebaili, R., Mitra, D., Ercole, C., et al. (2023). Solid-state fermentation: applications and future perspectives for biostimulant and biopesticides production. Microorganisms 11:1408. doi: 10.3390/microorganisms11061408
Mawcha, K. T., Kyampaire, D., Marciale, C., Simiyu-Wafukho, S., Chinyama, C., Babalola, O. O., et al. (2025a). An overview of biopesticide regulatory frameworks in selected countries in Southern Africa. Front. Sustain. Food Syst. 9:1522526. doi: 10.3389/fsufs.2025.1522526
Mawcha, K. T., Malinga, L., Muir, D., Ge, J., and Ndolo, D. (2024). Recent advances in biopesticide research and development with a focus on microbials. F1000Res. 13:1071. doi: 10.12688/f1000research.154392.2
Mawcha, K. T., Malinga, L., Muir, D., Ge, J., and Ndolo, D. (2025b). Recent advances in biopesticide research and development with a focus on microbials. F1000Res. 13:1071. doi: 10.12688/f1000research.154392.4
Mazurkiewicz, A., Jakubowska, M., Tumialis, D., Bocianowski, J., and Roik, K. (2021). Foliar application of entomopathogenic nematodes against cereal leaf beetle Oulema melanopus L. (Coleoptera: Chrysomelidae) on wheat. Agronomy 11:1662. doi: 10.3390/agronomy11081662
Mehlhorn, S., Hunnekuhl, V. S., Geibel, S., Nauen, R., and Bucher, G. (2021). Establishing RNAi for basic research and pest control and identification of the most efficient target genes for pest control: a brief guide. Front. Zool. 18:60. doi: 10.1186/s12983-021-00444-7
Mehrotra, S., Kumar, S., Zahid, M., and Garg, M. (2017). “Biopesticides,” in Principles and Applications of Environmental Biotechnology for a Sustainable Future, ed. R. L. Singh (Cham: Springer Singapore), 273–292. doi: 10.1007/978-981-10-1866-4_8
Melo, A. A., Batista, D. J. D. O., Polanczyk, R. A., Lopes, L. L., Lenz, M., Zinelli, M. P., et al. (2025). Persistence and efficacy of Bacillus thuringiensis-based biopesticide against Chrysodeixis includens in Soybeans: a 3-year field study. ACS Agric. Sci. Technol. 5, 1510–1515. doi: 10.1021/acsagscitech.5c00303
Mendoza, J. L. H., Pérez, M. I. S., Prieto, J. M. G., Velásquez, J. D. Q., Olivares, J. G. G., and Langarica, H. R. G. (2015). Antibiosis of Trichoderma spp strains native to northeastern Mexico against the pathogenic fungus Macrophomina phaseolina. Braz. J. Microbiol. 46, 1093–1101. doi: 10.1590/S1517-838246420120177
Mendoza-Alatorre, M., Julian-Chávez, B., Solano-Ornelas, S., Siqueiros-Cendón, T. S., Torres-Castillo, J. A., Sinagawa-García, S. R., et al. (2025). RNAi in pest control: critical factors affecting dsRNA efficacy. Insects 16:737. doi: 10.3390/insects16070737
Mendoza-Almanza, G., Esparza-Ibarra, E. L., Ayala-Luján, J. L., Mercado-Reyes, M., Godina-González, S., Hernández-Barrales, M., et al. (2020). The cytocidal spectrum of Bacillus thuringiensis toxins: from insects to human cancer cells. Toxins 1:301. doi: 10.3390/toxins12050301
Metcalf, J. S., Tischbein, M., Cox, P. A., and Stommel, E. W. (2021). Cyanotoxins and the nervous system. Toxins 13:660. doi: 10.3390/toxins13090660
Mevada, R. R., Sisodiya, D. B., Parmarand, R. G., and Prajapati, D. R. (2023). Mating Disruption: An Ecological Step Towards Sustainable Pest Management. Available online at: https://www.indianjournals.com/ijor.aspx?target=ijor:jefaandvolume=18andissue=1andarticle=025 (accessed May 12, 2025).
Mihajlović, M., Rekanović, E., Hrustić, J., Grahovac, M., and Tanović, B. (2017). Methods for management of soilborne plant pathogens. Pestic. Phytomed. 32, 9–24. doi: 10.2298/PIF1701009M
Mitra, D., De Los Santos-Villalobos, S., Parra-Cota, F. I., Montelongo, A. M. G., Blanco, E. L., Olatunbosun, A. N., et al. (2023). Rice (Oryza sativa L.) plant protection using dual biological control and plant growth-promoting agents: current scenarios and future prospects. Pedosphere 33, 268–286. doi: 10.1016/j.pedsph.2022.06.034
Mmotla, K., Sibanyoni, N. R., Allie, F., Sitole, L., Mafuna, T., Mashabela, M. D., et al. (2025). Exploring the intricacies of plant growth promoting rhizobacteria interactions: an omics review. Ann. Microbiol. 75:5. doi: 10.1186/s13213-025-01793-y
Mnif, I., and Ghribi, D. (2015). Potential of bacterial derived biopesticides in pest management. Crop Prot. 77, 52–64. doi: 10.1016/j.cropro.2015.07.017
Mohamed, D. M., Wassim, N., Habib, T., and Khaled, H. (2023). characterization of antibacterial peptides in Culex pipiens (Diptera: Culicidae) in response to Bacillus sphaericus infection. Catrina 28, 33–42. doi: 10.21608/cat.2023.221522.1181
Moharam, M. H. A., Stephan, D., and Koch, E. (2017). Evaluation of plant-derived preparations and microorganisms as seed treatments for control of covered kernel smut of sorghum (Sporisorium sorghi). J. Plant Dis. Protect. 125, 159–166. doi: 10.1007/s41348-017-0123-7
Mohsin, A. K., Hamad, B. S., and Hanawi, M. J. (2020). Interaction of thiamethoxam, spinosad and Beauveria Bassiana isolates for control whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) on cucumber. Plant Arch. 20, 929–938.
Mokhtarnejad, L., Shameli, S., and Farzaneh, M. (2025). Optimizations of commercial culture medium of Bacillus subtilis isolate B2 and its formulation for controlling powdery mildew of cucumber at greenhouse. Appl. Entomol. Phytopathol.
Mollah, M. M. I., and Hassan, N. (2023). Efficacy of Trichoderma harzianum, as a biological fungicide against fungal diseases of potato, late blight and early blight. J. Nat. Pesticide Res. 5:100047. doi: 10.1016/j.napere.2023.100047
Moore, S., and Hattingh, V. (2012). A review of current pre-harvest control options for false codling moth in citrus in southern Africa. SA Fruit J. 11, 82–85.
Moral, J., Garcia-Lopez, M. T., Camiletti, B. X., Jaime, R., Michailides, T. J., Bandyopadhyay, R., et al. (2020). Present status and perspective on the future use of aflatoxin biocontrol products. Agronomy 1:491. doi: 10.3390/agronomy10040491
Mukherjee, A., and Ghosh, S. K. (2023). An eco-friendly approach of biocontrol of aphid (Aphis gossypii Glover) by Trichoderma harzianum. Environ. Monit. Assess. 195:102. doi: 10.1007/s10661-022-10726-0
Munyore, M., and Rioba, N. B. (2020). Evaluation of Garlic (Allium sativum) and Onion (Allium cepa) Extracts for the Management of Fall Armyworm (Spodoptera frugiperda) on Baby Corn (Zea mays L) Under Greenhouse Conditions. Available online at: http://197.136.17.126/handle/123456789/240 (accessed May 14, 2025).
Murchie, A. K., Hodgson, E. W., Dean, A. N., Huseth, A., Hutchison, W. D., Manoukis, N. C., et al. (2023). Advances in Monitoring of Native and Invasive Insect Pests of Crops, Vol. 128. Burleigh Dodds Science Publishing. Available online at: https://books.google.com/books?hl=enandlr=andid=GiUzEQAAQBAJandoi=fndandpg=PT9anddq=Sentinelsystems,suchasartificialpesteggcardsdeployedtotrackparasitoidactivity,andmycosedcadaverassessmentsandots=_FqGB7Yxx2andsig=_utRliuIvB7HUaYjwCRQEq9clI0 (accessed May 8, 2025).
Nachammai, K. T., Amaradeepa, S., Raageshwari, S., Swathilakshmi, A. V., Poonkothai, M., and Langeswaran, K. (2023). Unraveling the interaction mechanism of the compounds from Cladophora sp to recognize prospective larvicidal and bactericidal activities: in vitro and in silico approaches. Mol. Biotechnol. 67, 3154–3174. doi: 10.1007/s12033-023-00902-z
Nalini, S., Sathiyamurthi, S., Dhas, T. S., and Revathi, M. (2023). “Lipopeptide and rhamnolipid biosurfactant as biopesticides,” in Multifunctional Microbial Biosurfactants, eds. P. Kumar and R. C. Dubey (Cham: Springer Nature Switzerland), 171–187. doi: 10.1007/978-3-031-31230-4_8
NarandŽić, T., Šarac, V., Rodić, V., Vukelić, N., Lukač-Bulatović, M., Bijelić, S., et al. (2025). Exploring the known and mapping future directions in biopesticides research: a bibliometric analysis. Horticulturae 11:97. doi: 10.3390/horticulturae11010097
Naranjo, S. E., and Ellsworth, P. C. (2024). “Landscape considerations in pest management: case study of the arizona cotton IPM system,” in Arthropod Management and Landscape Considerations in Large-scale Agroecosystems, eds. M. J. Brewer and G. L. Hein (Boston, MA: CABI), 44–77. doi: 10.1079/97818006227777.0003
Nascimento, N. A., Torres-Quintero, M. C., Molina, S. L., Pacheco, S., Romão, T. P., Pereira-Neves, A., et al. (2020). Functional Bacillus thuringiensis Cyt1Aa Is necessary to synergize Lysinibacillus sphaericus binary toxin (Bin) against bin-resistant and -refractory mosquito species. Appl. Environ. Microbiol. 86:e02770-19. doi: 10.1128/AEM.02770-19
Navasero, M. V., Candano, R. N., Hautea, D. M., Hautea, R. A., Shotkoski, F. A., and Shelton, A. M. (2016). Assessing potential impact of Bt eggplants on non-target arthropods in the Philippines. PLoS ONE 11:e0165190. doi: 10.1371/journal.pone.0165190
Nayak, S. K. (2021). Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquacult. 13, 862–906. doi: 10.1111/raq.12503
Ngegba, P. M., Cui, G., Khalid, M. Z., and Zhong, G. (2022). Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 1:, 600. doi: 10.3390/agriculture12050600
Novara, A., Catania, V., Tolone, M., Gristina, L., Laudicina, V. A., and Quatrini, P. (2020). Cover crop impact on soil organic carbon, nitrogen dynamics and microbial diversity in a Mediterranean semiarid vineyard. Sustainability 12:3256. doi: 10.3390/su12083256
Nur Mawaddah, S., AW, M. Z., and Sapak, Z. (2023). The potential of Pseudomonas fluorescens as biological control agent against sheath blight disease in rice: a systematic review. Food Res. 7, 46–56. doi: 10.26656/fr.2017.7(S2).11
Odelade, K. A., Adetuyi, B. O., Omowumi, O. S., Moshood, N. A., Adeleke, D. A., Odine, G. O., et al. (2024). “Application of plant-based nanobiopesticides that could be applied as fumigants,” in Handbook of Agricultural Biotechnology, 1st Edn., eds. C. O. Adetunji, C. Egbuna, A. Ficai, and O. A. Ijabadeniyi (Hoboken, NJ: Wiley), 7–36. doi: 10.1002/9781394234769.ch2
Okoma, R. N., Omuse, E. R., Mutyambai, D. M., Beesigamukama, D., Murongo, M. F., Subramanian, S., et al. (2025). An assessment of vegetable production constraints, trait preferences and willingness to adopt sustainable intensification options in Kenya and Uganda. Front. Sustain. Food Syst. 9:1471333. doi: 10.3389/fsufs.2025.1471333
Oo, A. Z., Tsujimoto, Y., and Rakotoarisoa, N. M. (2020). Optimizing the phosphorus concentration and duration of seedling dipping in soil slurry for accelerating the initial growth of transplanted rice. Agronomy 10:240. doi: 10.3390/agronomy10020240
Oosterbeek, M., Lozano-Torres, J. L., Bakker, J., and Goverse, A. (2021). Sedentary plant-parasitic nematodes alter auxin homeostasis via multiple strategies. Front. Plant Sci. 12:668548. doi: 10.3389/fpls.2021.668548
Ortiz, A., and Sansinenea, E. (2022). “Bacillus thuringiensis based biopesticides for integrated crop management,” in Biopesticides, eds. A. Rakshit, V. S. Meena, P. C. Abhilash, B. K. Sarma, H. B. Singh, L. Fraceto, et al. (Amsterdam: Elsevier), 1–6. doi: 10.1016/B978-0-12-823355-9.00015-8
Ostlie, K. R., Hutchison, W. D., and Hellmich, R. L. (1997). Bt corn and European Corn Borer. Available online at: https://digitalcommons.unl.edu/entomologyfacpub/597/ (accessed June 10, 2025).
Othim, S. T., Opisa, S., Rwomushana, I., and Luke, B. (2024). Unleashing nature's defenders: farmer-managed natural enemies field reservoirs (NEFRs) enhance management of the invasive papaya mealybug (Paracoccus marginatus) in coastal Kenya. Biol. Control 193:105528. doi: 10.1016/j.biocontrol.2024.105528
Ounis, S., Turóczi, G., and Kiss, J. (2024). arthropod pests, nematodes, and microbial pathogens of okra (Abelmoschus esculentus) and their management—a review. Agronomy 14:2841. doi: 10.3390/agronomy14122841
Oyekunle, M., Adamu, R. S., Ndou, E., Beyene, Y., Abdulmalik, M. M., and Oikeh, S. O. (2023). Efficacy of drought-tolerant and insect-protected transgenic TELA® maize traits in Nigeria. Transgenic Res. 32, 169–178. doi: 10.1007/s11248-023-00345-x
Pachoute, J., Dos Santos, G. R., and De Souza, D. J. (2024). Antagonistic effects of Beauveria bassiana on seed-borne fungi of cowpea (Vigna unguiculata). Biologia 79, 1487–1495. doi: 10.1007/s11756-024-01615-7
Padhan, S. R., Kar, I., Mohanty, A., and Panigrahi, K. K. (2024). “Nanofertilizers: types, synthesis, methods, and mechanisms,” in Nanofertilizers for Sustainable, eds. K. A. Abd-Elsalam and M. A. Alghuthaymi (Cham: Springer Nature Switzerland), 61–98. doi: 10.1007/978-3-031-41329-2_3
Pan, X., Guo, X., Zhai, T., Zhang, D., Rao, W., Cao, F., et al. (2023). Nanobiopesticides in sustainable agriculture: developments, challenges, and perspectives. Environ. Sci. Nano 10, 41–61. doi: 10.1039/D2EN00605G
Pasquier, A. (2021). From Proof of Concept to Optimization-the Use of a Predatory Mite Species to Control the Population of the Western Corn Rootworm (Diabrotica virgifera virgifera) (LeConte, 1868) [PhD Thesis]. Université Côte d'Azur. Available online at: https://theses.hal.science/tel-03607797/ (accessed June 10, 2025).
Passey, T., Amiri, A., Gañán, L., Mao, Z., Wang, Y., Saville, R., et al. (2019). Integrated Management of Diseases and Insect Pests of Tree Fruit, Vol. 68. Burleigh Dodds Science Publishing. Available online at: https://books.google.com/books?hl=enandlr=andid=SiQzEQAAQBAJandoi=fndandpg=PT11anddq=trunkinjectionblightinappletrees,rootrotandthripsinavocadotrees,andpowderymildewanddownymildewingrapevinesandots=UDt4liu5Gaandsig=y7V_1elNu2NzYQdhWhZpraUEOY8 (accessed May 20, 2025).
Pathrose, B., Chellappan, M., and Ranjith, M. T. (2023). “Mass production of insect predators,” in Commercial Insects (Cambridge, MA: CRC Press), 270–299. doi: 10.1201/9781003454960-12
Patil, S., and Sriram, S. (2020). Biological control of Fusarium wilt in crop plants using non-pathogenic isolates of Fusarium species. Indian Phytopathol. 73, 11–19. doi: 10.1007/s42360-020-00202-5
Pattnaik, M., and Mishra, H. N. (2022). Amelioration of the stability of polyunsaturated fatty acids and bioactive enriched vegetable oil: blending, encapsulation, and its application. Crit. Rev. Food Sci. Nutr. 62, 6253–6276. doi: 10.1080/10408398.2021.1899127
Paul, M. R., Nair, S. S., Thomas, N. P., Shamshudeen, R., Nizar, S., and Sebastian, A. (2025). “Insect toxins: selective interactions in biological regime,” in Biotoxins, eds. M. N. V. Prasad, S. D. Tetali, and C. Bennetau-Pelissero (Cham: Springer Nature Switzerland), 197–215. doi: 10.1007/978-3-031-75309-1_9
Pecenka, J. R., Ingwell, L. L., Foster, R. E., Krupke, C. H., and Kaplan, I. (2021). IPM reduces insecticide applications by 95% while maintaining or enhancing crop yields through wild pollinator conservation. Proc. Nat. Acad. Sci. 118:e2108429118. doi: 10.1073/pnas.2108429118
Pérez-Campos, S.-J., Rodríguez-Hernández, A.-I., Del Rocío López-Cuellar, Ma., Martínez-Juárez, V.-M., Sanjuan-Galindo, R., Álvarez-Navarro, A., et al. (2019). Liquid culture of the entomopathogenic nematode, Steinernema colombiense: evolution of the nematode concentration, particles volume fraction, rheological properties and surface tension. Biocontrol Sci. Technol. 29, 1129–1145. doi: 10.1080/09583157.2019.1658181
Peshin, R., Kranthi, K. R., and Sharma, R. (2014). “Pesticide use and experiences with integrated pest management programs and Bt cotton in India,” in Integrated Pest Management, eds. R. Peshin and D. Pimentel (Cham: Springer), 269–306. doi: 10.1007/978-94-007-7802-3_11
Petchidurai, G., Sahayaraj, K., Al-Shuraym, L. A., Albogami, B. Z., and Sayed, S. M. (2023). Insecticidal activity of tannins from selected brown macroalgae against the cotton leafhopper Amrasca devastans. Plants 12:3188. doi: 10.3390/plants12183188
Petlamul, W., and Prasertsan, P. (2012). Evaluation of strains of Metarhizium anisopliae and Beauveria bassiana against Spodoptera litura on the basis of their virulence, germination rate, conidia production, radial growth and enzyme activity. Mycobiology 40, 111–116. doi: 10.5941/MYCO.2012.40.2.111
Phukan, I., Madhab, M., Bordoloi, M., Sarmah, S. R., Dutta, P., Begum, R., et al. (2012). Exploitation of PGP microbes of tea for improvement of plant growth and pest suppression: a novel approach. Two Bud. 59, 69–74.
Phuong, T. T. T., Fujii, T., Ishikawa, Y., Tung, N. D., and Giang, H. T. T. (2024). Female sex pheromone of Spodoptera frugiperda Vietnam population and a selected lure for adult monitoring in maize fields. J. Asia Pac. Entomol. 27:102343. doi: 10.1016/j.aspen.2024.102343
Piñero, J. C., Shapiro-Ilan, D., Cooley, D. R., Tuttle, A. F., Eaton, A., Drohan, P., et al. (2020). Toward the integration of an attract-and-kill approach with entomopathogenic nematodes to control multiple life stages of plum curculio (Coleoptera: Curculionidae). Insects 11:375. doi: 10.3390/insects11060375
Pobożniak, M., and Olczyk, M. (2025). Biocontrol in integrated pest management in fruit and vegetable field production. Horticulturae 11:522. doi: 10.3390/horticulturae11050522
Prakash, R., and Shivakumar, N. (2025). Development and evaluation of microbial formulations containing Bacillus amyloliquefaciens RP4 and Streptomyces rochei RP7 for the sustainable management of downy mildew in grapes. J. Plant Dis. Protect. 132:146. doi: 10.1007/s41348-025-01138-6
Praneetvatakul, S., Schreinemachers, P., Vijitsrikamol, K., and Potchanasin, C. (2024). Policy options for promoting wider use of biopesticides in Thai agriculture. Heliyon 10:e24486. doi: 10.1016/j.heliyon.2024.e24486
Prasad, R. D., Chandrika, K. P., and Godbole, V. (2020). A novel chitosan biopolymer based Trichoderma delivery system: storage stability, persistence and bio efficacy against seed and soil borne diseases of oilseed crops. Microbiol. Res. 237:126487. doi: 10.1016/j.micres.2020.126487
Pretty, J., and Pervez Bharucha, Z. (2015). Integrated pest management for sustainable intensification of agriculture in Asia and Africa. Insects 6, 152–182. doi: 10.3390/insects6010152
Pu, X.-Y., Feng, M.-G., and Shi, C.-H. (2005). Impact of three application methods on the field efficacy of a Beauveria bassiana-based mycoinsecticide against the false-eye leafhopper, Empoasca vitis (Homoptera: Cicadellidae) in the tea canopy. Crop Prot. 24, 167–175. doi: 10.1016/j.cropro.2004.07.006
Puławska, J., Mikiciński, A., and Sobiczewski, P. (2023). The history of fire blight biocontrol with Gram-negative bacteria and bacteriophages. J. Plant Pathol. 106, 839–851. doi: 10.1007/s42161-023-01554-3
Qaim, M., Subramanian, A., Naik, G., and Zilberman, D. (2006). Adoption of Bt cotton and impact variability: insights from India. Rev. Agric. Econ. 28, 48–58. doi: 10.1111/j.1467-9353.2006.00272.x
Raghuvanshi, N. (2015). Comparative Bio-Efficacy of Metarhizium Anisopliaebased Bio-Insecticide Met 52 OD in Chilli (Capsicumannuum L.) for the Control of Sucking Insect Pests [PhD Thesis]. Rajmata Vijayaraje Scindia Krishi Vishwa Vidyalaya. Available online at: https://krishikosh.egranth.ac.in/server/api/core/bitstreams/972f4c2a-7b6a-44c0-95a5-7f283f7d5553/content (accessed June 7, 2025).
Rahman, M. N., Tumpa, F. H., Islam, A. S., and Khokon, M. A. R. (2020). Bio-priming of cucurbits and okra seeds with culture filtrates of Trichoderma harzianum for controlling seed-borne fungi. J. Bangladesh Agric. Univ. 18, 12–16. doi: 10.5455/JBAU.94724
Rajput, M., Vivekanand, V., and Pareek, N. (2021). “Biofertilizers and biopesticides: a whole new dimension for ameliorating soil fertility and organic agriculture practice,” in Plant, Soil and Microbes in Tropical Ecosystems, eds. S. K. Dubey and S. K. Verma (Singapore: Springer Singapore), 369–389. doi: 10.1007/978-981-16-3364-5_17
Rao, T., and Jurat-Fuentes, J. L. (2020). “Advances in the use of entomopathogenic bacteria/microbial control agents (MCAs) as biopesticides in suppressing crop insect pests,” in Biopesticides for Sustainable Agriculture (Burleigh Dodds Science Publishing), 99–134. doi: 10.19103/AS.2020.0073.06
Rathod, P. D., and Shinde, G. U. (2023). Autonomous aerial system (UAV) for sustainable agriculture: a review. Int. J. Environ. Clim. Change 13, 1343–1355. doi: 10.9734/ijecc/2023/v13i82080
Ratto, F., Bruce, T., Chipabika, G., Mwamakamba, S., Mkandawire, R., Khan, Z., et al. (2022). Biological control interventions reduce pest abundance and crop damage while maintaining natural enemies: a meta-analysis. Authorea [Preprints]. doi: 10.22541/au.166082943.36348290/v1
Ray, A. K. (2023). “Drying,” in Coulson and Richardson's Chemical Engineering, ed. A. K. Ray (Amsterdam: Elsevier), 457–533. doi: 10.1016/B978-0-08-101097-6.00007-9
Raza, A., Hassan, A., Akram, W., Anjum, T., Aftab, Z.-H., and Ali, B. (2024). Seed coating with the synthetic consortium of beneficial Bacillus microbes improves seedling growth and manages Fusarium wilt disease. Sci. Hortic. 325:112645. doi: 10.1016/j.scienta.2023.112645
Reddy, A. A., Reddy, M., and Mathur, V. (2024). Pesticide use, regulation, and policies in Indian agriculture. Sustainability 16:7839. doi: 10.3390/su16177839
Reddy, G. V., and Guerrero, A. (2010). New pheromones and insect control strategies. Vitam. Horm. 83, 493–519. doi: 10.1016/S0083-6729(10)83020-1
Reuveni, M., Arroyo, C. J., and Ovadia, S. (2023). An effective hybrid fungicide containing tea tree oil and difenoconazole for grape powdery mildew management. Agriculture 13:979. doi: 10.3390/agriculture13050979
Revilla, P., Anibas, C. M., and Tracy, W. F. (2021). Sweet corn research around the world 2015–2020. Agronomy 11:534. doi: 10.3390/agronomy11030534
Riaz, U., Murtaza, G., Anum, W., Samreen, T., Sarfraz, M., and Nazir, M. Z. (2021). “Plant growth-promoting rhizobacteria (PGPR) as biofertilizers and biopesticides,” in Microbiota and Biofertilizers, eds. K. R. Hakeem, G. H. Dar, M. A. Mehmood, and R. A. Bhat (Cham: Springer International Publishing), 181–196. doi: 10.1007/978-3-030-48771-3_11
Rivera-Pérez, C., Clifton, M. E., Noriega, F. G., and Jindra, M. (2020). “Juvenile hormone regulation and action,” in Advances in Invertebrate (Neuro) Endocrinology (Apple Academic Press), 1–76. Available onine at: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003029861-1/juvenile-hormone-regulation-action-rivera-p%C3%A9rez-clifton-noriega-jindra (accessed May 21, 2025).
Riyaz, M., Mathew, P., Zuber, S. M., and Rather, G. A. (2022). “Botanical pesticides for an eco-friendly and sustainable agriculture: new challenges and prospects,” in Sustainable Agriculture, ed. S. A. Bandh (Cham: Springer International Publishing), 69–96. doi: 10.1007/978-3-030-83066-3_5
Rizwan, M., Atta, B., Sabir, A. M., Yaqub, M., and Qadir, A. (2019). Evaluation of the entomopathogenic fungi as a non-traditional control of the rice leaf roller, Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) under controlled conditions. Egypt. J. Biol. Pest Control 29:10. doi: 10.1186/s41938-019-0111-2
Rocha, I., Ma, Y., Souza-Alonso, P., Vosátka, M., Freitas, H., and Oliveira, R. S. (2019). Seed coating: a tool for delivering beneficial microbes to agricultural crops. Front. Plant Sci. 10:1357. doi: 10.3389/fpls.2019.01357
Rodríguez-Romero, V. M., Villanueva-Arce, R., and Durán-Páramo, E. (2024). “Nejayote” valorization as a culture medium for Pseudomonas fluorescens and production of antifungal extracts. Rev. Mexicana Fitopatol. 42:28. doi: 10.18781/R.MEX.FIT.2405-3
Romeh, A. A. (2018). “Integrated pest management for sustainable agriculture,” in Sustainability of Agricultural Environment in Egypt: Part II, Vol. 77, eds. A. M. Negm and M. Abu-hashim (Cham: Springer International Publishing), 215–234. doi: 10.1007/698_2018_267
Ronga, D., Vitti, A., Zaccardelli, M., Pane, C., Caradonia, F., Cardarelli, M., et al. (2021). Root zone management for improving seedling quality of organically produced horticultural crops. Agronomy 11:630. doi: 10.3390/agronomy11040630
Rosales-Castillo, R., Velázquez-de Lucio, B. S., Hernández-Domínguez, E. M., and Álvarez-Cervantes, J. (2025). Bio-inputs: an alternative to achieve sustainable agriculture. Rev. Terra Latinoam. 43:2405. doi: 10.28940/terra.v43i.2045
Ruane, J., Mba, C., Boettcher, P., Koskela, J., Mair, G., and Ramasamy, S. (2023). Case Studies of the Use of Agricultural Biotechnologies to Meet the Needs of smallholders in Developing Countries. Food and Agriculture Org. Available online at: https://books.google.com/books?hl=enandlr=andid=lV75EAAAQBAJandoi=fndandpg=PR5anddq=CaseStudiesoftheUseofAgriculturalBiotechnologiestoMeettheNeedsofSmallholdersinDevelopingCountriesandots=_ezOLbNHirandsig=EH2lNPCmM9Fb76WwJcqvwsFdJTQ (accessed June 1, 2025).
Rumyantsev, S. D., Alekseev, V. Y., Sorokan, A. V., Burkhanova, G. F., Cherepanova, E. A., Garafutdinov, R. R., et al. (2023). Additive effect of the composition of endophytic bacteria Bacillus subtilis on systemic resistance of wheat against greenbug aphid Schizaphis graminum due to lipopeptides. Life 13:214. doi: 10.3390/life13010214
Saberi, F., Marzban, R., Ardjmand, M., Shariati, F. P., and Tavakoli, O. (2020). Optimization of culture media to enhance the ability of local Bacillus thuringiensis var. Tenebrionis. J. Saudi Soc. Agric. Sci. 19, 468–475. doi: 10.1016/j.jssas.2020.08.004
Sala, A., Barrena, R., Artola, A., and Sánchez, A. (2019). Current developments in the production of fungal biological control agents by solid-state fermentation using organic solid waste. Crit. Rev. Environ. Sci. Technol. 49, 655–694. doi: 10.1080/10643389.2018.1557497
Sampaio, F., Marchioro, C. A., and Foerster, L. A. (2025). Modeling parasitoid development: climate change impacts on Telenomus remus (NIXON) and Trichogramma foersteri (Takahashi) in southern BRAZIL. Pest Manag. Sci. 81:8888. doi: 10.1002/ps.8888
Sampson, C., Turner, R., and Ali, A. (2021). Monitoring and trapping with sticky traps, what's new. Int. Pest Control 63, 166–169.
Sani, I., Ismail, S. I., Abdullah, S., Jalinas, J., Jamian, S., and Saad, N. (2020). A review of the biology and control of whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), with special reference to biological control using entomopathogenic fungi. Insects 11:619. doi: 10.3390/insects11090619
Sarma, H. H., Paul, A., Kakoti, M., Goswami, A., Talukdar, N., Borkotoky, B., et al. (2023). The aerial genesis: seed bombing for nurturing earth and ecosystem renaissance-a review. Pharma. Innov. J. 12:1719.
Sarr, O. M., Bal, A. B., and Gauthier, N. (2023). Biological management options against the fall armyworm Spodoptera frugiperda causing damage to maize in Senegal. Phytoparasitica 51, 975–992. doi: 10.1007/s12600-023-01109-3
Scanlan, J. C., Grant, W. E., Hunter, D. M., and Milner, R. J. (2001). Habitat and environmental factors influencing the control of migratory locusts (Locusta migratoria) with an entomopathogenic fungus (Metarhizium anisopliae). Ecol. Modell. 136, 223–236. doi: 10.1016/S0304-3800(00)00424-5
Schaly, S., Islam, P., Abosalha, A., Boyajian, J. L., Shum-Tim, D., and Prakash, S. (2022). Alginate–chitosan hydrogel formulations sustain baculovirus delivery and VEGFA expression which promotes angiogenesis for wound dressing applications. Pharmaceuticals 15:1382. doi: 10.3390/ph15111382
Scolari, F., Valerio, F., Benelli, G., Papadopoulos, N. T., and Vaníčková, L. (2021). Tephritid fruit fly semiochemicals: current knowledge and future perspectives. Insects 12:408. doi: 10.3390/insects12050408
Sehim, A. E., Hewedy, O. A., Altammar, K. A., Alhumaidi, M. S., and Abd Elghaffar, R. Y. (2023). Trichoderma asperellum empowers tomato plants and suppresses Fusarium oxysporum through priming responses. Front. Microbiol. 14:1140378. doi: 10.3389/fmicb.2023.1140378
Sellamuthu, G., Narayanasamy, P., and Padaria, J. C. (2018). “Bacillus thuringiensis: genetic engineering for insect pest management,” in Microbes for Climate Resilient Agriculture, 1st Edn., eds. P. L. Kashyap, A. K. Srivastava, S. P. Tiwari, and S. Kumar (Hoboken, NJ: Wiley), 255–278. doi: 10.1002/9781119276050.ch12
Shah, S. T., Basit, A., Ullah, I., and Mohamed, H. I. (2021). “Cyanobacteria and algae as biocontrol agents against fungal and bacterial plant pathogens,” in Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management, eds. H. I. Mohamed, H. E.-D. S. El-Beltagi, and K. A. Abd-Elsalam (Cham: Springer International Publishing), 1–23. doi: 10.1007/978-3-030-66587-6_1
Sharghi, H., Kary, N. E., and Mohammadi, D. (2025). Enhancing the shelf life of entomopathogenic nematodes formulation: the impact of super absorbent polymer and infective juveniles concentration. Crop Prot. 190:107091. doi: 10.1016/j.cropro.2024.107091
Sharma, A., Kumar, V., Shahzad, B., Tanveer, M., Sidhu, G. P. S., Handa, N., et al. (2019). Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1:1446. doi: 10.1007/s42452-019-1485-1
Sharma, G., Rajhansa, K. C., Sharma, P., Singh, A., Sharma, A., Sahu, M. K., et al. (2022). Marigold (Tagetes spp.): a diverse crop with multipurpose value for health and environment: a review. Agric. Rev. 45, 618–626. doi: 10.18805/ag.R-2475
Sheikh, A. A., Wani, M. A., Bano, P., Un, S., Nabi, T. A. B., Bhat, M. A., et al. (2017). An overview on resistance of insect pests against Bt crops. J. Entomol. Zool. Stud. 5, 941–948.
Shil, S., Ashwath, M. N., Das, S., Vats, P., Raj, A. K., Dash, U., et al. (2025). “Seed biopriming: a sustainable solution for enhancing seed vigor and crop productivity,” in Advances in Seed Quality Evaluation and Improvement, eds. B. Roy, G. Shukla, V. Dunna, P. Sharma, and P. S. Shukla (Cham: Springer Nature Singapore), 127–168. doi: 10.1007/978-981-96-5178-8_6
Sidahmed, H., Vad, A., and Nagy, J. (2025). Advances in sweet corn (Zea mays L. saccharata) research from 2010 to 2025: genetics, agronomy, and sustainable production. Agronomy 15:1260. doi: 10.3390/agronomy15051260
Siddiqui, A., Ahmad, S., and Afaq, U. (2025). Innovations in biopesticides: the role of plant-based biopesticides in sustainable agriculture. J. Biopesticides 18, 18–35. doi: 10.57182/jbiopestic.18.1.18-35
Sierra, I., Latorre-Estivalis, J. M., Traverso, L., Gonzalez, P. V., Aptekmann, A., Nadra, A. D., et al. (2021). Transcriptomic analysis and molecular docking reveal genes involved in the response of Aedes aegypti larvae to an essential oil extracted from Eucalyptus. PLoS Negl. Trop. Dis. 15:e0009587. doi: 10.1371/journal.pntd.0009587
Simamora, A. V., Nenotek, P. S., Hahuly, M. V., Mahayasa, I. N. W., Kasim, M., Widinugraheni, S., et al. (2025). In vitro screening of Trichoderma harzianum as bioremediator of mancozeb fungicide. IOP Conf. Ser. Earth Environ. Sci. 1482:012024. doi: 10.1088/1755-1315/1482/1/012024
Singh, A., Singh, A., Singh, P., Chakravarty, A., Singh, A., Singh, P., et al. (2022). Insecticidal activity, toxicity, resistance and metabolism of pyrethroids: a review. Sci. Technol. Indonesia 7, 238–250. doi: 10.26554/sti.2022.7.2.238-250
Sisay, B., Subramanian, S., Weldon, C. W., Krüger, K., Khamis, F., Tefera, T., et al. (2024). Evaluation of pheromone lures, trap designs and placement heights for monitoring the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize fields of Kenya. Crop Prot. 176:106523. doi: 10.1016/j.cropro.2023.106523
Smith, R. G., Lounsbury, N. P., and Palmer, S. A. (2023). “Mechanisms of weed suppression by cover crops, intercrops, and mulches,” in Ecologically-Based Weed Management, 1st Edn., eds. N. E. Korres, I. S. Travlos, and T. K. Gitsopoulos (Hoboken, NJ: Wiley), 172–195). doi: 10.1002/9781119709763.ch10
Soetopo, D., and Alouw, J. C. (2023). Biopesticide development and registration: challenges and strategies. IOP Conf. Ser. Earth Environ. Sci. 1179:012003. doi: 10.1088/1755-1315/1179/1/012003
Spescha, A., Zwyssig, M., Hess Hermida, M., Moix, A., Bruno, P., Enkerli, J., et al. (2023). When competitors join forces: consortia of entomopathogenic microorganisms increase killing speed and mortality in leaf- and root-feeding insect hosts. Microb. Ecol. 86, 1947–1960. doi: 10.1007/s00248-023-02191-0
Sri, M. S., Kumar, S., and Shricharan, S. (2025). Soil Drenching and Foliar Application of Nano Fertilizers Improved Quality Traits and Vase Life of Asparagus densiflorus cv.‘Sprengeri'. Available online at: https://www.researchgate.net/profile/Shricharan-Senthilkumar/publication/392896743_Soil_Drenching_and_Foliar_Application_of_Nano_Fertilizers_Improved_Quality_Traits_and_Vase_Life_of_Asparagus_densiflorus_cv_'Sprengeri'/links/6856bf6a99d2ce32c1ca0653/Soil-Drenching-and-Foliar-Application-of-Nano-Fertilizers-Improved-Quality-Traits-and-Vase-Life-of-Asparagus-densiflorus-cv-Sprengeri.pdf (accessed June 2, 2025).
Srinivasan, R., Sevgan, S., Ekesi, S., and Tamò, M. (2019). Biopesticide based sustainable pest management for safer production of vegetable legumes and brassicas in Asia and Africa. Pest Manag. Sci. 75, 2446–2454. doi: 10.1002/ps.5480
Stadlinger, N., Berg, H., Van Den Brink, P. J., Tam, Nguyen. T., and Gunnarsson, J. S. (2018). Comparison of predicted aquatic risks of pesticides used under different rice-farming strategies in the Mekong Delta, Vietnam. Environ. Sci. Pollut. Res., 25, 13322–13334. doi: 10.1007/s11356-016-7991-4
Studholme, D. J., Wicker, E., Abrare, S. M., Aspin, A., Bogdanove, A., Broders, K., et al. (2020). Transfer of Xanthomonas campestris pv. Arecae and X. campestris pv. Musacearum to X. vasicola (Vauterin) as X. vasicola pv. Arecae comb. Nov. And X. vasicola pv. Musacearum comb. Nov. and description of X. vasicola pv. Vasculorum pv. Nov. Phytopathology 110, 1153–1160. doi: 10.1094/PHYTO-03-19-0098-LE
Sundh, I., and Eilenberg, J. (2021). Why has the authorization of microbial biological control agents been slower in the EU than in comparable jurisdictions? Pest Manag. Sci. 77, 2170–2178. doi: 10.1002/ps.6177
Swedaan, S. S., and Al-Zurfi, S. M. (2023). Evaluation of the efficacy of commercial and local entomopathogenic fungi Metarhizium anisopliae against Ephestia cautella (Lepidoptera: Pyralida) under Lab Conditions. IOP Conf. Ser. Earth Environ. Sci. 1259:012119. doi: 10.1088/1755-1315/1259/1/012119
Tabudlong, B. M., and Estoy, G. F. Jr. (2015). Field evaluation of entomopathogenic fungus, Beauveria bassiana (Balsamo) Vuillimin for the control of rice bug, Leptocorisa oratorius Fabricius. Philippine Entomol. 29:227.
Taillebois, E., and Thany, S. H. (2022). The use of insecticide mixtures containing neonicotinoids as a strategy to limit insect pests: efficiency and mode of action. Pestic. Biochem. Physiol. 184:105126. doi: 10.1016/j.pestbp.2022.105126
Tamez-Guerra, P., Tamayo-Mejía, F., Gomez-Flores, R., Rodríguez-Padilla, C., Damas, G., Tamez-Guerra, R. S., et al. (2018). Increased efficacy and extended shelf life of spinosad formulated in phagostimulant granules against Spodoptera frugiperda. Pest Manag. Sci. 74, 100–110. doi: 10.1002/ps.4656
Tavares, W. R., Barreto, M. C. B., and Seca, A. M. L. (2021). Aqueous and ethanolic plant extracts as bio-insecticides—establishing a bridge between raw scientific data and practical reality. Plants 10, 1–30. doi: 10.3390/plants10050920
Teshome, E., Forsido, S. F., Rupasinghe, H. P. V., and Olika Keyata, E. (2022). Potentials of natural preservatives to enhance food safety and shelf life: a review. Sci. World J. 2022, 1–11. doi: 10.1155/2022/9901018
Tian, B. (2025). Research on the application potential and promotion strategies of biopesticides in green agriculture. J. Technol. Innov. Eng. 1:6. doi: 10.63887/jtie.2025.1.3.7
Tiwari, S., and Youngman, R. R. (2011). “Transgenic Bt corn hybrids and pest management in the USA,” in Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation, ed. E. Lichtfouse (Cham: Springer), 15–37. doi: 10.1007/978-94-007-0186-1_2
Tomar, P., Thakur, N., Jhamta, S., Chowdhury, S., Kapoor, M., Singh, S., et al. (2024). Bacterial biopesticides: biodiversity, role in pest management and beneficial impact on agricultural and environmental sustainability. Heliyon 10:e31550. doi: 10.1016/j.heliyon.2024.e31550
Tufan-Cetin, O., and Cetin, H. (2023). Use of micro and macroalgae extracts for the control of vector mosquitoes. PeerJ 11:e16187. doi: 10.7717/peerj.16187
Van Lenteren, J. C. (2012). The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57, 1–20. doi: 10.1007/s10526-011-9395-1
Vangansbeke, D. (2015). Effects of Temperature Regime and Food Supplementation on the Performance of Phytoseiid Mites as Biological Control Agents [PhD Thesis]. Ghent University. Available online at: https://core.ac.uk/download/pdf/55815473.pdf (accessed May 15, 2025).
Vaselek, S. (2024). The role of protists, nematodes and mites as natural control agents of sandfly populations. Front. Trop. Dis. 5:1369007. doi: 10.3389/fitd.2024.1369007
Vemmer, M., and Patel, A. V. (2013). Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 67, 380–389. doi: 10.1016/j.biocontrol.2013.09.003
Vero, S., Garmendia, G., Allori, E., Sanz, J. M., Gonda, M., Alconada, T., et al. (2023). Microbial biopesticides: diversity, scope, and mechanisms involved in plant disease control. Diversity 15:457. doi: 10.3390/d15030457
Vijaykumar, S. (2023). Biopolymer-based Multilayer Seed Coatings with Trichoderma, Rhizobium, or Bacillus and Compatible Fungicides Against Seed and Soil-borne Diseases in Sesamum, Groundnut, and Soybean [PhD Thesis]. Doctoral dissertation, Professor Jayashankar Telangana State Agricultural. Available online at: https://krishikosh.egranth.ac.in/bitstreams/64cdaaa0-c15c-42b9-a006-e62d7750aeb3/download (accessed June 4, 2025).
Villamizar, S., Ferro, J. A., Caicedo, J. C., and Alves, L. M. C. (2020). Bactericidal effect of entomopathogenic bacterium Pseudomonas entomophila against Xanthomonas citri reduces citrus canker disease severity. Front. Microbiol. 11:1431. doi: 10.3389/fmicb.2020.01431
Wafula, G. O. (2022). Integration of Thymol, Soil Improvers and Biostimulants for Management of Tomato Pests, Diseases, Nematodes and Rhizosphere Microbial Dynamics Kirinyaga County, Kenya (Doctoral dissertation). Kenyatta University.
Wallis, C. M., Shinde, R., Ellis, M. L., Gorman, Z., and Mekdara, N. (2025). Assessment of the potential of novel Californian grapevine Trichoderma isolates reduce colonization of fungal trunk canker pathogens and Xylella fastidiosa. Front. Plant Sci. 16:1609693. doi: 10.3389/fpls.2025.1609693
Wanderley, B. R. S. M., de Lima, N. D., Deolindo, C. T. P., Kempka, A. P., Moroni, L. S., Gomes, V. V., et al. (2024). Impact of pre-fermentative maceration techniques on the chemical characteristics, phenolic composition, in vitro bioaccessibility, and biological activities of alcoholic and acetic fermented products from jaboticaba (Plinia trunciflora). Food Res. Int. 197:115246. doi: 10.1016/j.foodres.2024.115246
Wang, S.-Y., Zhang, Y.-J., Chen, X., Shi, X.-C., Herrera-Balandrano, D. D., Liu, F.-Q., et al. (2024). Biocontrol methods for the management of Sclerotinia sclerotiorum in legumes: a review. Phytopathology 114, 1447–1457. doi: 10.1094/PHYTO-01-24-0006-RVW
Wang, X., Yang, R., Zafar, J., Peng, C., Zhang, X., Hong, Y., et al. (2022). Symbiotic and antagonistic functions of the bacterium Burkholderia cepacia BsNLG8, from the Nilaparvata lugens (Stal). Agriculture 12:2106. doi: 10.3390/agriculture12122106
Wani, J. A., Wali, A. F., Majid, S., Rasool, S., Rehman, M. U., Rashid, S. M., et al. (2020). “Bio-pesticides: application and possible mechanism of action,” in Bioremediation and Biotechnology, Vol 2, eds. R. A. Bhat, K. R. Hakeem, and M. A. Dervash (Cham: Springer International Publishing), 97–119. doi: 10.1007/978-3-030-40333-1_6
Wei, Y., Jiao, Y., An, D., Li, D., Li, W., and Wei, Q. (2019). Review of dissolved oxygen detection technology: from laboratory analysis to online intelligent detection. Sensors 19:3995. doi: 10.3390/s19183995
Wennmann, J. T., Köhler, T., Gueli Alletti, G., and Jehle, J. A. (2015). Mortality of cutworm larvae is not enhanced by agrotis segetum granulovirus and agrotis segetum nucleopolyhedrovirus B coinfection relative to single infection by either virus. Appl. Environ. Microbiol. 81, 2893–2899. doi: 10.1128/AEM.03726-14
Werrie, P.-Y., Burgeon, C., Le Goff, G. J., Hance, T., and Fauconnier, M.-L. (2021). Biopesticide trunk injection into apple trees: a proof of concept for the systemic movement of mint and cinnamon essential oils. Front. Plant Sci. 12:650132. doi: 10.3389/fpls.2021.650132
Western, T. L. (2012). The sticky tale of seed coat mucilages: production, genetics, and role in seed germination and dispersal. Seed Sci. Res. 22, 1–25. doi: 10.1017/S0960258511000249
Whipps, J. M. (1992). Status of biological disease control in horticulture. Biocontrol Sci. Technol. 2, 3–24. doi: 10.1080/09583159209355213
Wilson, H., and Daane, K. M. (2017). Review of ecologically-based pest management in California vineyards. Insects 8:108. doi: 10.3390/insects8040108
Wolcott, S., Hatwar, M., Endreny, T. A., and Newman, L. A. (2022). Suitability of select media for use in a novel green wall system used to treat brewery wastewater. Environ. Technol. 43, 2656–2670. doi: 10.1080/21622515.2021.1893829
Wolff, L. (2023). Regulating pests—material politics and calculation in integrated pest management. Environ. Plann. E: Nat. Space 6, 455–472. doi: 10.1177/25148486221076138
Wu, P., Lei, X., Zeng, J., Qi, Y., Yuan, Q., Huang, W., et al. (2024). Research progress in mechanized and intelligentized pollination technologies for fruit and vegetable crops. Int. J. Agric. Biol. Eng. 17, 11–21. doi: 10.25165/j.ijabe.20241706.9403
Wyckhuys, K. A., Lu, Y., Zhou, W., Cock, M. J., Naranjo, S. E., Fereti, A., et al. (2020). Ecological pest control fortifies agricultural growth in Asia–Pacific economies. Nat. Ecol. Evol. 4, 1522–1530. doi: 10.1038/s41559-020-01294-y
Xing, K., Zhu, X., Peng, X., and Qin, S. (2015). Chitosan antimicrobial and eliciting properties for pest control in agriculture: a review. Agron. Sustain. Dev. 35, 569–588. doi: 10.1007/s13593-014-0252-3
Xu, Y., Zhou, X., Yan, B., Yue, Y., Zhang, M., Yuan, H., et al. (2024). Methyl salicylate reduces aphid abundance in maize through multiple modes of action. J. Integr. Agric. 24, 3966–3977.
Yang, F., Wang, Z., and Kerns, D. L. (2022). Resistance of Spodoptera frugiperda to Cry1, Cry2, and Vip3Aa proteins in Bt corn and cotton in the Americas: implications for the rest of the world. J. Econ. Entomol. 115, 1752–1760. doi: 10.1093/jee/toac099
Yarahmadi, F., and Rajabpour, A. (2024). “Insecticides and natural enemies: applications in integrated pest management programs–challenges, criteria, and evaluation for recommendations,” in Insecticides in Pest Control-impact, chalLenges and Strategies (London: InTechOpen), 1–19. doi: 10.5772/intechopen.1005830
Yu, R., Xu, X., Liang, Y., Tian, H., Pan, Z., Jin, S., et al. (2014). The insect ecdysone receptor is a good potential target for RNAi-based pest control. Int. J. Biol. Sci. 10:1171. doi: 10.7150/ijbs.9598
Zeni, V., Baliota, G. V., Benelli, G., Canale, A., and Athanassiou, C. G. (2021). Diatomaceous earth for arthropod pest control: back to the future. Molecules 26:24. doi: 10.3390/molecules26247487
Zhang, X., Lövei, G. L., Ferrante, M., Yang, N., and Wan, F. (2020). The potential of trap and barrier cropping to decrease densities of the whitefly Bemisia tabaci MED on cotton in China. Pest Manag. Sci. 76, 366–374. doi: 10.1002/ps.5524
Zhang, Z., Li, H., Han, H., Qin, L., Lu, W., Yue, L., et al. (2025). Degradation of anthracene and phenanthrene by strain Streptomyces sp. M-1 and its application in the treatment of PAHs-contaminated water. J. Environ. Manage. 375:124298. doi: 10.1016/j.jenvman.2025.124298
Zhao, H., Zhou, T., Xie, J., Cheng, J., Chen, T., Jiang, D., et al. (2020). Mycoparasitism illuminated by genome and transcriptome sequencing of Coniothyrium minitans, an important biocontrol fungus of the plant pathogen Sclerotinia sclerotiorum. Microbial Genom. 6, 1–19. doi: 10.1099/mgen.0.000345
Zhao, X., Höfte, M., Spanoghe, P., Rajkovic, A., and Uyttendaele, M. (2024). Biofilm-forming ability of Bacillus thuringiensis strains from biopesticides on polystyrene and their attachment on spinach. J. Food Prot. 87:100321. doi: 10.1016/j.jfp.2024.100321
Keywords: bioresource, cost-effective options, IPM, macrobial pesticides, pesticidal plants, slow release, sustainable development
Citation: Fenibo EO and Matambo T (2025) Biopesticides for sustainable agriculture: feasible options for adopting cost-effective strategies. Front. Sustain. Food Syst. 9:1657000. doi: 10.3389/fsufs.2025.1657000
Received: 30 June 2025; Accepted: 23 September 2025;
Published: 31 October 2025.
Edited by:
Orlando Calcetas, Regional Crop Protection-IVA, PhilippinesReviewed by:
Wenjie Shangguan, Chinese Academy of Agricultural Sciences, ChinaFarouk A. Abdel-Galil, Assiut University, Egypt
Copyright © 2025 Fenibo and Matambo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanuel O. Fenibo, ZmVuaWJvZTE0NzhAZ21haWwuY29t
 Emmanuel O. Fenibo
Emmanuel O. Fenibo Tonderayi Matambo
Tonderayi Matambo