- 1School of Basic Sciences, Department of Botany, Central University of Punjab, Bathinda, Punjab, India
- 2Laboratory of Phytopathology and Microbial Biotechnology, Department of Botany, Mohanlal Sukhadia University, Udaipur, Rajasthan, India
Artificial preservatives such as nitrates, benzoates, sulphites, sorbates, parabens, formaldehyde, butylated hydroxytoluene (BHT), and butylated hydroxyanisole (BHA) have been used for ages to extend the shelf life of food items. However, increasing scientific evidence links their excessive intake to severe health hazards like cancer, endocrine disruption, allergies, and neurotoxicity. As people become more aware and prefer natural clean-label foods, the demand for safer options from the industry is growing. In this situation microalgae can be a strong natural source of preservatives. They are rich in active compounds that show both antioxidant and antimicrobial effects. Microalgal extracts give a green way to improve food safety and shelf life. This review discusses major antioxidant constituents of microalgae, including carotenoids (e.g., astaxanthin, β-carotene), phenolics, and vitamins that reduce oxidative degradation of food matrices. Mechanisms of action, delivery modes, and incorporation into active packaging and food coatings are covered. Despite efficiency challenges associated with extraction, compound stability, and large-scale industrial production, breakthroughs in bioprocessing and biotechnology are rapidly expanding the boundaries of commercial application. In summary, microalgal bioactives offer a promising and sustainable approach to natural food preservation and safety, while also addressing consumer demand for cleaner and safer food products.
1 Introduction
Throughout human evolution, civilizations have developed a wide array of food preservation techniques to ensure availability across seasons, prevent contamination, and reduce the risk of foodborne diseases. Today's food preservation techniques include a range of physical methods, including freezing, refrigeration, thermal processing, and air-drying, as well as chemical techniques that employ nitrates, benzoates, and BHT, along with other chemical agents, organic acids and salts (Mafe et al., 2024). Although they are useful for extending shelf life and inhibiting microbial spoilage, they may also have negative effects on the quality of food. This includes alterations in nutritional value, such as the loss of heat-sensitive vitamins and essential nutrients, as well as changes in organoleptic properties, including texture, flavor, color, and aroma (Wong et al., 2023). Growing consumer awareness and the shift toward green consumerism are prompting a reassessment of the validity and continued use of some commercially available synthetic preservatives. Consequently, there is a need to develop preservation strategies using natural products that maintain bioactivity and enhance shelf life. Although the search for natural food preservatives is a hot topic, most studies are focused on plant extracts.
Microalgae are photosynthetic microorganisms that are found everywhere in nature, thriving in a wide variety of aquatic environments from freshwater lakes and rivers to saline oceans and even in terrestrial environments like soil. They are known to be a rich source of bioactive compounds, such as proteins, lipids, pigments, polysaccharides, vitamins, and antioxidants (Gauthier et al., 2020). Secondary metabolites produced by microalgae include polyunsaturated fatty acids (PUFAs), carotenoids like β-carotene, astaxanthin, and lutein, and certain stress-induced enzymes- all of which have strong antioxidant properties (Cezare-Gomes et al., 2019). Along with high antioxidant properties, these metabolites exhibited strong broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacterial strains. Chlorellin, which is a growth inhibitor of both Gram-positive and Gram-negative bacteria can be extracted from Chlorella sp. likewise, eicosapentaenoic acid (EPA), hexadecatrienoic acid, and palmitoleic acid from Phaeodactylum tricornutum have been found to exhibit antimicrobial activity against methicillin-resistant Gram-positive Staphylococcus aureus (Alsenani et al., 2020; Smith et al., 2010).
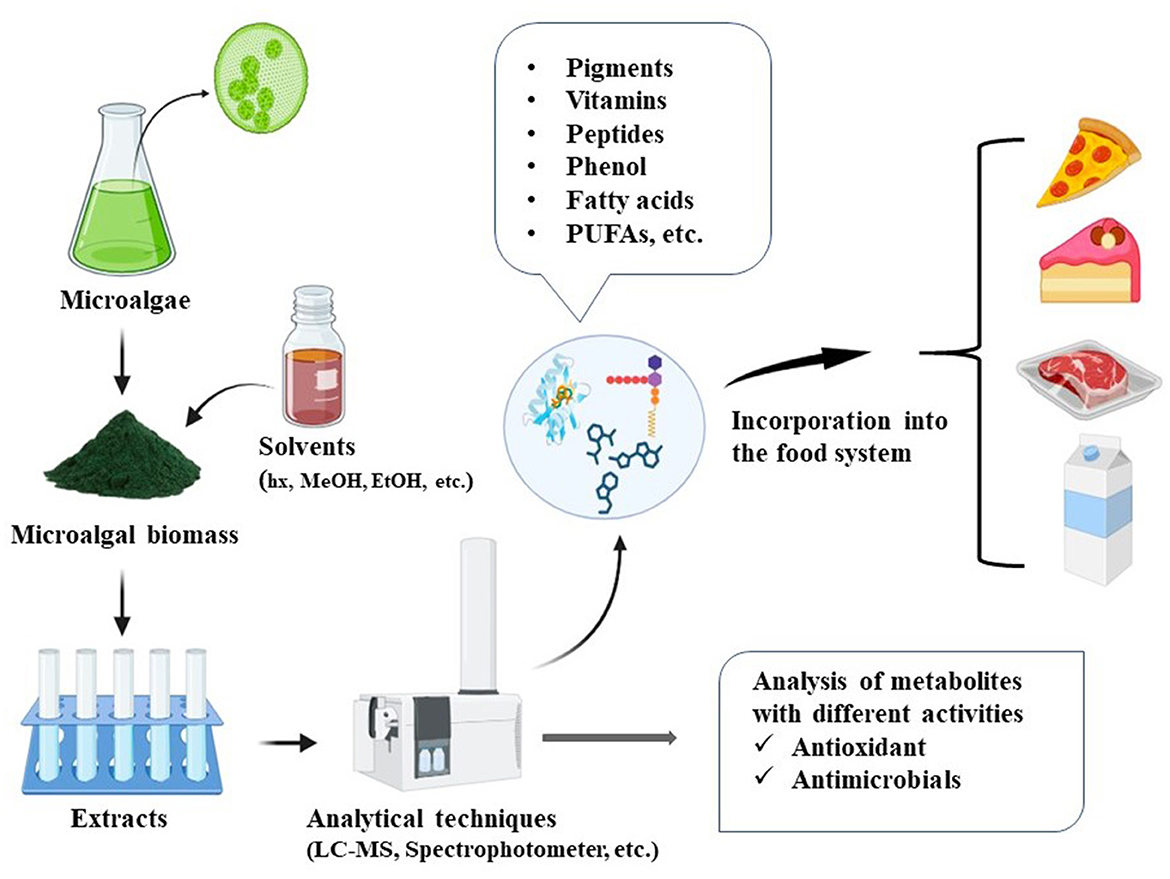
The incorporation of microalgae antioxidant and antimicrobial extracts in food preservation systems provides an attractive solution to address increasing consumer demand for natural and greener products (Figure 1). Microalgal extracts hold significant market potential given their functional diversity, sustainability, and capacity for year-round production. The global market for microalgal products is currently valued at approximately USD 4.96 billion and is projected to grow to between USD 8.9 and 9.1 billion by 2032, reflecting a robust compound annual growth rate (CAGR) in the coming years (Martínez-Ruiz et al., 2025).
This review highlights key antioxidant compounds from microalgae such as carotenoids, phenolics, and vitamins that help reduce oxidative degradation in foods as well as antimicrobial agents like fatty acids, peptides, and polysaccharides effective against various foodborne pathogens. It also covers their mechanisms of action, delivery systems, integration into active packaging and edible coatings, and addresses regulatory, safety, and consumer acceptance considerations for food industry applications.
2 Bioactive compounds in microalgae
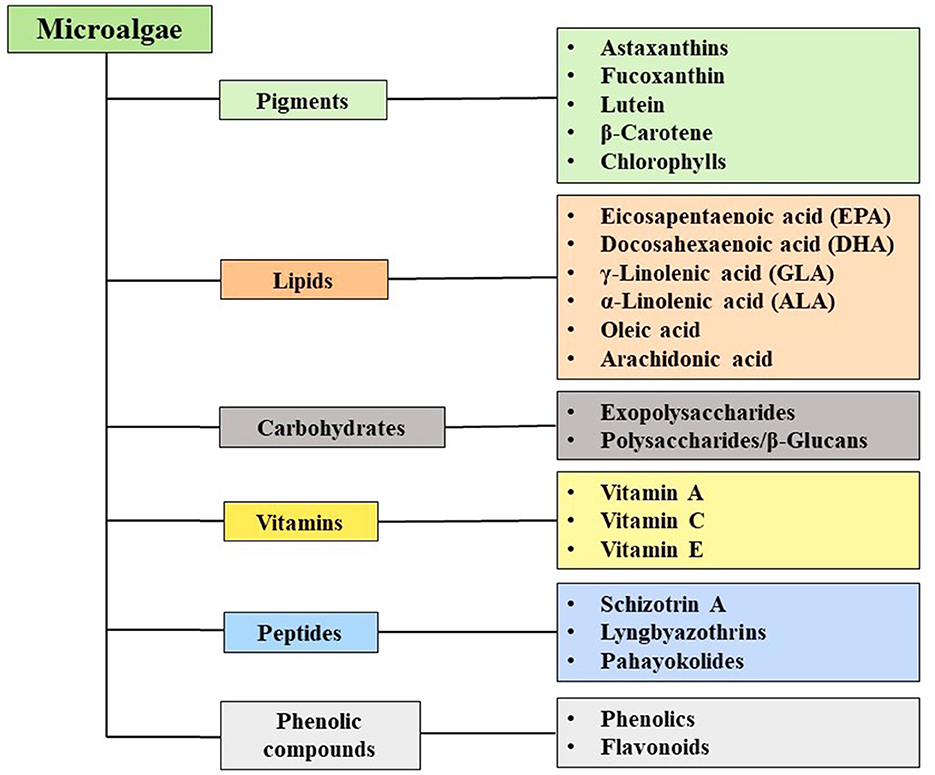
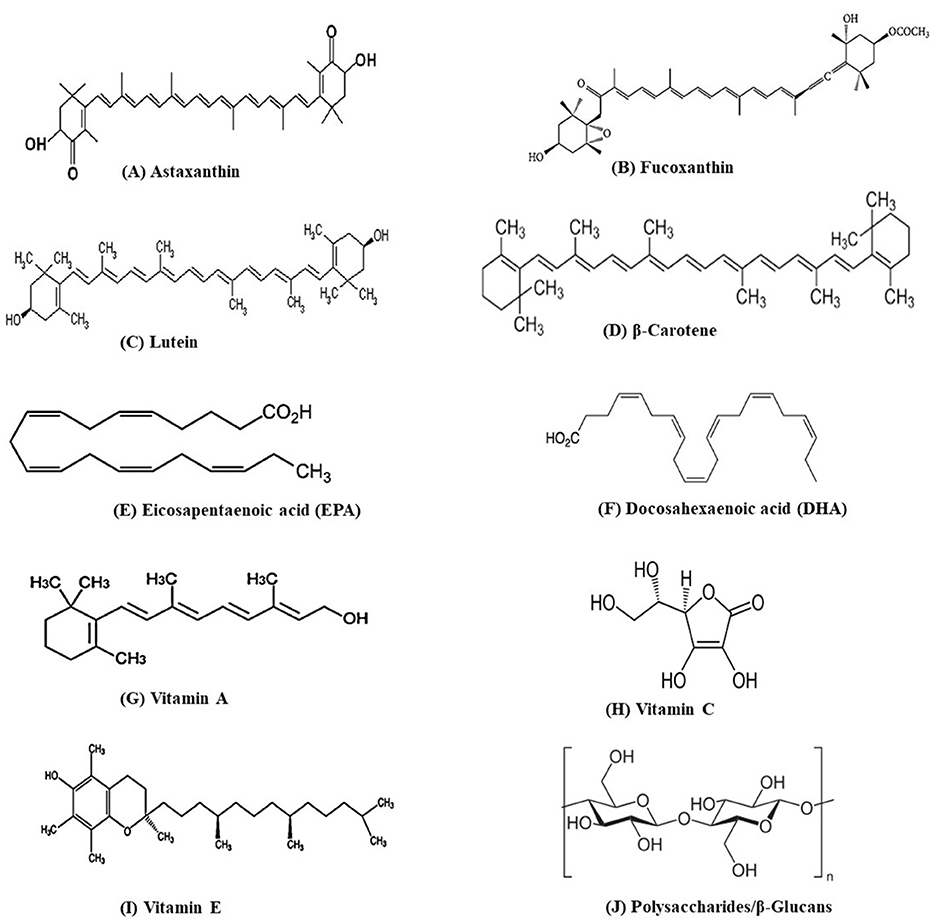
Microalgae constitute a promising and sustainable source of bioactive compounds with significant potential for applications in the food industry (Bhardwaj et al., 2025). Bioactive compounds like carotenoids and PUFAs have multiple applications in food and pharmaceuticals (Sun et al., 2023). Microalgae such as Chlorella vulgaris, Euglena gracilis, and Nannochloropsis have gained recognition as edible algae across numerous countries, primarily due to their rich content of proteins, lipids, and various bioactive metabolites (Maurício et al., 2023; Xie et al., 2023; Ragini and Arumugam, 2023). This global approval has led to a surge in the development of edible products based on microalgal biomass. For instance, a German company has developed a natural product called Green Trio Tantellen, which utilizes the biomass of Spirulina sp. and Chlorella sp. (Maehle and Skjeret, 2022). Furthermore, microalgal species including Haematococcus sp., Chlorella sp., Dunaliella sp., Scenedesmus sp., Chlamydomonas sp., and Phaeodactylum sp. are widely employed in biotechnological processes owing to their ability to produce a variety of valuable compounds such as proteins, carbohydrates, pigments, phenolic compounds, and vitamins (Figure 2) (Katiyar et al., 2017). The structure of bioactive compounds such as astaxanthin, fucoxanthin, lutein, β-carotene, eicosapentaenoic acid, docosahexanoic acid, vitamin A, C, and E, and polysaccharides/beta-glucans is shown in Figure 3.
Metabolites, such as phenolics, flavonoids, and tocopherols, can effectively mitigate oxidative stress and lower the risk of chronic diseases (Torres-Tiji et al., 2020). Spirulina phenolic extract has shown a strong radical scavenging activity of 79.95% at a concentration of 449 mg/ml, making it a promising supplement for antiaging and heart health (Bellahcen et al., 2020). Under specific growth conditions, Dunaliella salina can produce lipids, carbohydrates, and proteins at levels reaching up to 70%, 60%, and 20%, respectively (Roy et al., 2021). Additionally, Haematococcus pluvialis is capable of synthesizing astaxanthin up to 5% of its dry weight (DW) (Mularczyk et al., 2020), while Spirulina platensis can produce phycocyanin at concentrations as high as 17.5% (Khandual et al., 2021). Microalgae-based food products start entering the market, but some challenges need to be addressed to make them functional, new-era food. Efforts like screening of nutrient-rich microalgae species, development of nutrient-rich food products, and their promotion and marketing (Chen et al., 2022). Table 1 provides some important microalgal extracts with antioxidant and antimicrobial activity that are used in food system.
3 Antioxidant activities of microalgal extracts
Reactive oxygen species (ROS) like singlet oxygen (1O2), superoxide radicals (), hydrogen peroxide (H2O2), and hydroxyl radicals (OH) are reactive molecules with the potential to spoil food components (Scaglioni and Badiale-Furlong, 2017). ROS are responsible for lipid peroxidation, protein degradation, and vitamin losses, ultimately reducing food quality and accelerating spoilage. Figure 4 illustrates the steps of lipid and protein oxidation and the role of antioxidants in preventing it. Antioxidants are essential in neutralizing ROS, which thereby ensures the nutritional value, safety, and shelf life of food items. Microalgal extracts contain a variety of antioxidants, including vitamins A, C, and E, as well as polyphenols, carotenoids, and bioflavonoids (Vignaud et al., 2023).
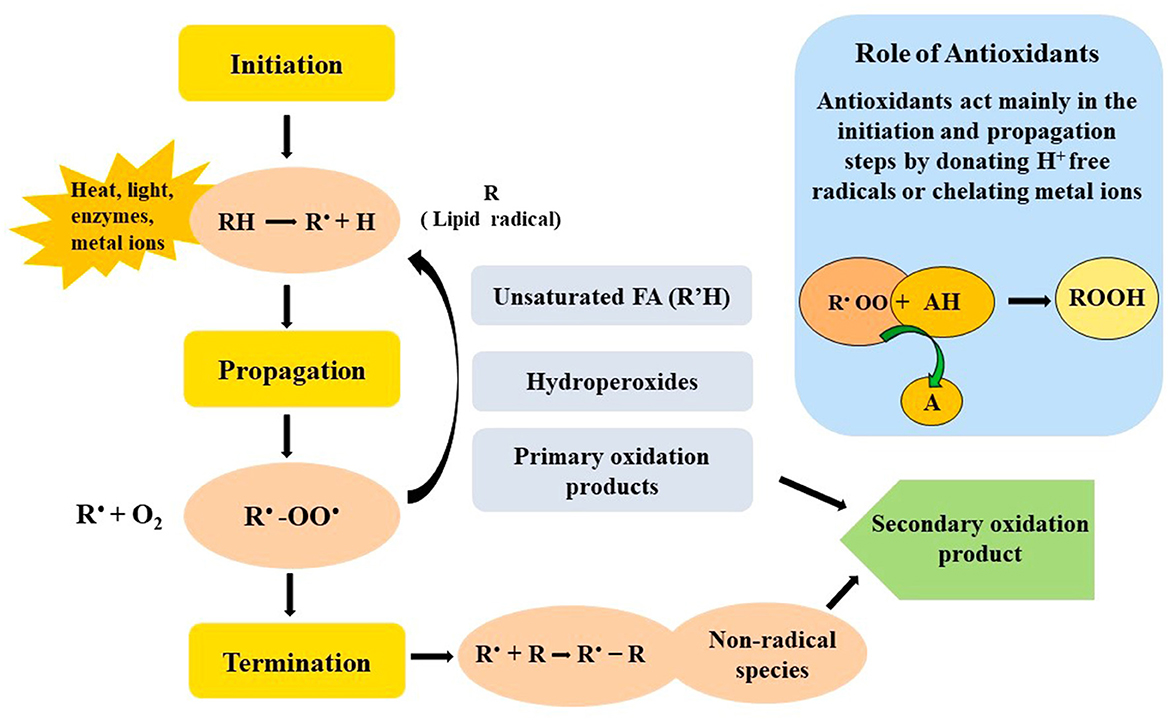
Figure 4. Steps involved in the oxidation of food matrices and the role of antioxidant in preventing it.
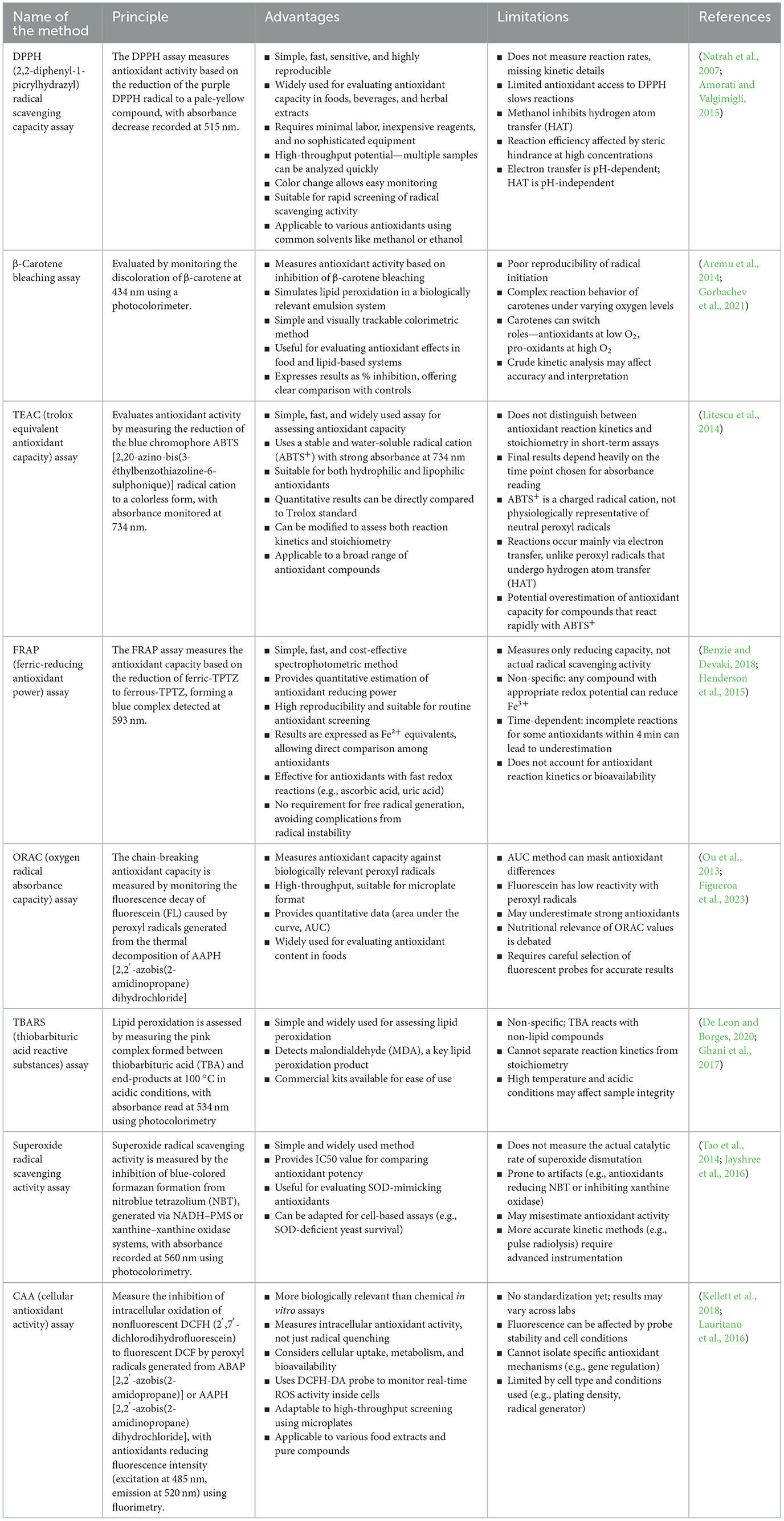
Among the most widely used analytical techniques for determining antioxidant activity are the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) assay, and the Folin–Ciocalteu antioxidant (FCA) assay. Each of these tests measures the ability of microalgal extracts to scavenge free radicals or reduce oxidative agents. For instance, in the DPPH assay, antioxidant activity can be quantified as the percentage of radical inhibition at specific extract concentrations, or expressed as IC50 values (the concentration needed to inhibit 50% of radicals), Trolox equivalents, or ascorbic acid equivalents per unit extract or dry weight (Martinez-Morales et al., 2020). Similarly, ABTS and FCA assays offer complementary insights by measuring total antioxidant capacity and phenolic content, respectively (Danet, 2021). Table 2 provides a summary of the most commonly used and recent antioxidant assays employed in microalgae-based studies, highlighting their relevance and application in evaluating natural antioxidant sources for potential use in food preservation and nutraceuticals.
3.1 Food oxidation processes and the role of antioxidants in preventing food oxidation
Reactive oxygen species (ROS) such as hydroxyl radicals (•OH) and superoxide anions (), react with molecules like PUFAs in food lipids. The removal of a hydrogen atom from a PUFA generates a lipid radical (R•), which reacts with oxygen to form unstable lipid peroxyl radicals (ROO•). These unstable lipid peroxyl radicals react with adjacent lipids, creating lipid hydroperoxides (ROOH). They are decomposed into aldehydes, ketones, and malondialdehyde which are responsible for rancidity, off-flavors, and cytotoxicity (Prisacaru, 2016; Martemucci et al., 2022). Similarly, proteins undergo oxidative modifications at specific amino acid residues (e.g., cysteine, methionine, tryptophan), leading to carbonylation, fragmentation, and aggregation. These molecular events disrupt food structure, color, taste, and nutritional integrity (Hellwig, 2019).
Antioxidants act at distinct molecular targets to prevent or delay these oxidative processes. Radical scavengers such as phenolic compounds, carotenoids, and tocopherols donate a hydrogen atom or an electron to lipid peroxyl radicals (ROO•). This converts the ROO• into stable, non-propagating molecules and terminates the chain reaction (Chaudhary et al., 2023). For instance, tocopherol reacts with ROO• to yield a stable, less reactive tocopheroxyl radical (Gulcin, 2025). Carotenoids and phycobiliproteins quench singlet oxygen (1O2) through physical energy transfer, dissipating its energy as heat instead of initiating oxidation (Ramel et al., 2012). By targeting radicals and excited oxygen species at the molecular level, natural antioxidants maintain food quality, slow nutrient degradation, and extend shelf life (Figure 4).
3.2 Pigments
Carotenoids like β-carotene, astaxanthin, lutein, and fucoxanthin derived from microalgae hold significant potential as natural food preservatives due to their strong antioxidant and health-promoting properties (Sathasivam and Ki, 2018; Aditi et al., 2025). β-Carotene, primarily produced by Dunaliella salina and Tetradesmus almeriensis and is widely used as a natural antioxidant and food colorant (Molino et al., 2018; Khaw et al., 2022; Seth et al., 2021). Astaxanthin, especially from Haematococcus pluvialis (up to 5% of its dry weight, DW) (Mularczyk et al., 2020) and Chlorella zofingiensis, exhibits antioxidant activity up to ten times stronger than other carotenoids, making it highly potential (Zhang et al., 2021). Lutein, sourced from Spirulina platensis and Chlorella species, enhances antioxidant content in food products, such as fish burgers, improving their shelf life and nutritional quality (Saeed et al., 2025). Fucoxanthin, abundant in marine algae can contribute to food preservation through its anti-inflammatory and antioxidant effects, offering a natural alternative to synthetic additives (Khaw et al., 2022).
The total carotenoid content in microalgae can vary depending on growth conditions, with stress conditions often leading to higher carotenoid production. For example, high light intensity exposure (240 μE m−2 s−1 for 20 days) in Chlamydomonas acidophila raised carotenoid content from < 40 to >50 mg L−1 culture. At 40 °C for 20 days, the levels went up from < 40 to >40 mg L−1 culture, whereas UV-A radiation (10 μE m−2 s−1 for 15 days) raised it from < 50 to >50 mg L−1 culture (Garbayo et al., 2008). Exposure to UV-B radiation (15 W m−2 for 1 h), Chlorella vulgaris increased from 0.98 to 1.18 mg g−1 FW, and Chlorococcum humicola from 1.02 to 1.36 mg g−1 FW (Singh et al., 2019). These findings indicate that light, temperature, and UV stress enhance carotenoid accumulation in microalgae. Applying such stresses can markedly boost metabolite production, which can subsequently be utilized in microalgae-based food systems.
3.3 Vitamin C, E, and glutathione
Microalgae are a rich natural source of vitamins such as vitamin C (ascorbic acid) and vitamin E (tocopherols and tocotrienols). These bioactive compounds are highly valued for their capacity to counteract oxidative stress, rendering them great prospects as natural food preservatives. Vitamin C is a water-soluble antioxidant primarily located in the cytosol and chloroplasts of microalgal cells. Scavenging ROS and regenerating other antioxidants like vitamin E and glutathione are some of its main functions (Rezayian et al., 2019). Vitamin E has the function of preventing lipid peroxidation by donating a hydrogen atom to lipid radicals and thereby halting oxidative chain reactions (Gulcin, 2020).
Vitamin C concentration differs greatly among microalgal species. Genus Skeletonema has been found to contain as low as 0.06 mg/g DW, while the genus Chaetoceros has a range of 0.12–18.79 mg/g DW (Del Mondo et al., 2020). Microalgae under stress conditions are often found to synthesize more ascorbic acid. Chlorella vulgaris under phosphorus limitation (0.01 mM, 5 days) increased from < 1.0 to >1.0 mg g−1 DW. Phaeodactylum tricornutum showed increases from < 1.0 to >1.5 mg g−1 DW under phosphorus limitation and from < 1.0 to >1.0 mg g−1 DW under nitrogen limitation (0.2 mM, 5 days). Tetraselmis suecica exhibited a stronger response, rising from < 2.0 to >5.0 mg g−1 DW under phosphorus limitation and from < 2.0 to >3.0 mg g−1 DW under nitrogen limitation (Goiris et al., 2015). This highlight species-specific enhancements in ascorbic acid production under nutrient stress. After oxidation, tocopherols and tocotrienols can be restored by ascorbate and glutathione or coenzyme Q, supplementing the antioxidant system in microalgal extracts (Coulombier et al., 2021).
Another critical antioxidant found in microalgae is glutathione, which is an aqueous-soluble tripeptide made up of glutamate, cysteine, and glycine. Glutathione is found in all cell compartments and is pivotal in the detoxification of ROS (Swapnil et al., 2017, 2021). Glutathione is a cofactor for glutathione peroxidase, facilitating the reduction of hydrogen peroxide to water, and also assists in the recycling of ascorbate and tocopherol to their active reduced forms (Sharma et al., 2012). In addition, glutathione can directly scavenge harmful species like superoxide radicals, hydroxyl radicals, and singlet oxygen (Cassier-Chauvat et al., 2023), all of which are responsible for food spoilage. Together, the antioxidant systems found in microalgae, glutathione and vitamins C and E act synergistically to counteract oxidative damage in food matrices (Pruteanu et al., 2023). Their ability to inhibit lipid peroxidation and stabilize sensitive food components makes microalgal extracts promising natural alternatives to synthetic antioxidants used in food preservation. The utilization of microalgae in this regard parallels the increasing interest in clean-label, sustainable, and health-benefiting food ingredients.
3.4 Phenols
Phenolic compounds are a diverse group of natural antioxidants widely distributed in higher plants, macroalgae, and increasingly recognized in microalgae. They can inhibit oxidative deterioration of lipids and proteins, thereby increasing shelf life and preserving food quality. A recent study by Almendinger et al. (2021) tested 13 microalgal species and revealed that Neochloris oleoabundans and Wilmottia murrayi had very high concentrations of phenolics, higher than 20 mg gallic acid equivalents per gram. León-Vaz et al. (2023) investigated 19 Nordic microalgal species under control and stress conditions (high light and cold exposure). They indicated that species such as Chlorococcum sp. and Scenedesmus sp. accumulated more polyphenols under stress, demonstrating the possibility of induced antioxidant accumulation through environmental control. Chlamydomonas reinhardtii was also found to accumulate more polyphenols when grown at high light, further indicating that stress conditions may be leveraged to maximize antioxidant production in microalgal cultures (Vignaud et al., 2023). The phenolic compounds extracted from Spirulina sp., Dunaliella salina, Fischerella ambigua, Oocystis pusilla, and Scenedesmus rubescens (Anwer et al., 2022; Faraloni et al., 2021) have significant antioxidant activity.
4 Antimicrobial activities of microalgal extracts
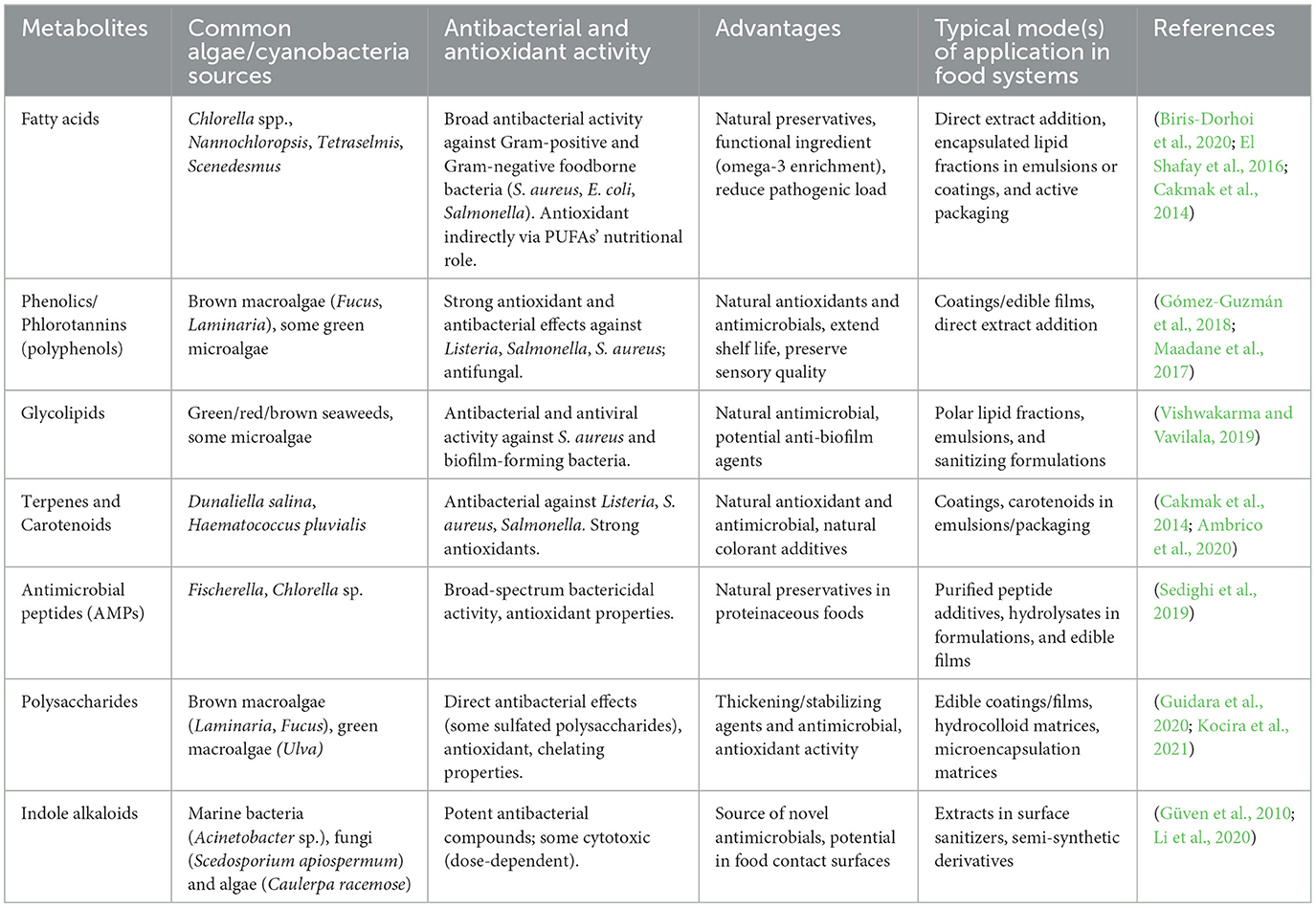
Multidrug-resistant (MDR) bacteria have been recognized by the World Health Organization (WHO) as a major public health threat (Salam et al., 2023). This has motivated the search for new antimicrobial agents, especially from natural sources. Microalgae have been of particular interest because they contain a rich variety of bioactive compounds with antibacterial activity. Microalgal natural products are also a few steps ahead of synthetic antibiotics. Microalgal derived antimicrobial compounds are safer, more biocompatible, and ecologically sustainable compared to traditional synthetic antimicrobial compounds (Pratap et al., 2020; Kumawat et al., 2024). They have fewer side effects, such as allergic reactions, immunosuppression, and hypersensitivity. These antimicrobials are also less likely to cause resistance in microbes and are suitable for long-term use, such as in food preservation. Recent research has indicated that extracts of microalgal species including Chlorella vulgaris, Dunaliella salina, Fischerella ambigua, Nostoc muscorum, Oocystis pusilla, and Scenedesmus rubescens exhibit strong antibacterial activity (Amaro et al., 2011; Jena and Subudhi, 2019; Dantas et al., 2019). The bioactive peptide extracts have been found to inhibit some of the most common foodborne bacteria, such as Staphylococcus aureus and Escherichia coli (Corrêa et al., 2023). Extracts from seaweed algae Enteromorpha intestinalis and Ulva reticulata were discovered to show potent inhibition against S. aureus and even Methicillin-resistant S. aureus (MRSA) (Uddin et al., 2020). Seaweed-derived laminarin and essential oils have been discovered to be active against Listeria monocytogenes, a severe food safety issue in milk and meat foods (Patra and Baek, 2016). Likewise, various extracts of Ulva lactuca, Chaetomorpha linum, and Turbinaria triquatra have been discovered to show potent bactericidal activity against Bacillus cereus, a common food spoilage microorganism (Silva et al., 2020; Shannon and Abu-Ghannam, 2016). Against Gram-negative bacteria, Cystoseira barbata, Padina gymnospora, and microalgae Tetraselmis spp. (Zerrifi et al., 2018) and Nannochloropsis oculata extracts showed significant inhibition zones against Salmonella spp. and E. coli (Wali et al., 2020). Furthermore, recent evidence on the activity of brown algal phlorotannins showed not only highly significant anti-Salmonella activity but also longer food shelf life when added to alginate-based nanofiber packaging (Surendhiran et al., 2019).
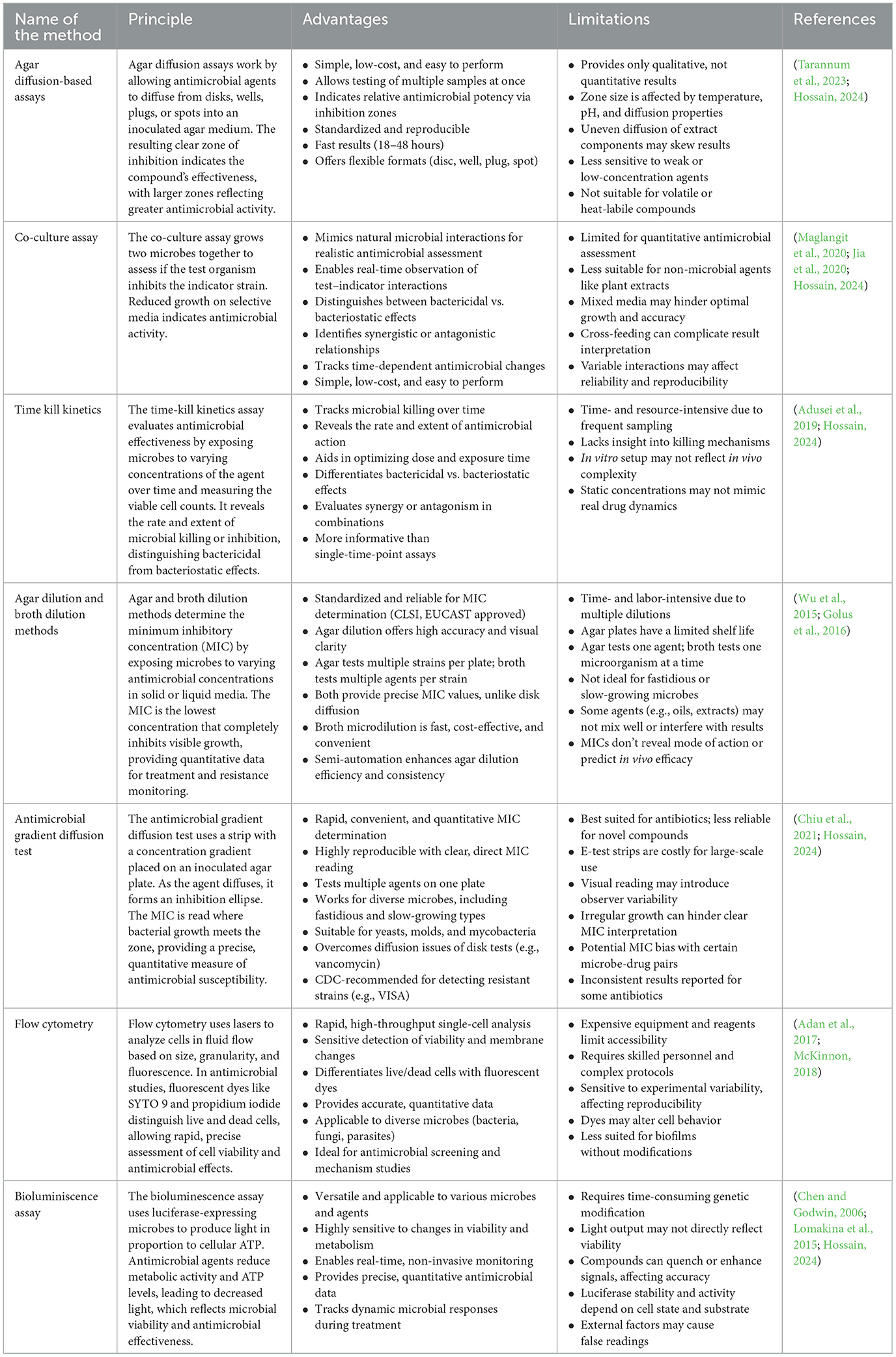
Methanolic and acetone extracts have shown strong antibacterial activity against foodborne and human pathogens (Ibrahim and Kebede, 2020; Ullah et al., 2020). Techniques such as supercritical CO2 extraction, pressurized liquid extraction (PLE), and subcritical water extraction (SWE) are gaining attention as these methods minimize solvent use, are faster, and offer better selectivity for target compounds. For example, supercritical CO2 successfully extracted lipid fractions from Chaetoceros muelleri with antibacterial effects against Staphylococcus aureus and Escherichia coli (Mendiola et al., 2007; Jena and Subudhi, 2019), even when conventional solvents showed no such activity. Likewise, PLE and SWE have been effective in extracting antimicrobial agents from Haematococcus pluvialis, especially during its red phase (Bueno et al., 2020). The antimicrobial efficacy of the extracts can be analyzed by techniques such as agar diffusion method, Time kill kinetics, Flow Cytometry, Agar dilution and broth dilution methods, etc. (Table 3).
4.1 Fatty acids and polyunsaturated fatty acids (PUFAs)
Microalgae-derived fatty acids (FAs) are of interest as naturally occurring antimicrobial compounds with possible applications in food preservation. Fatty acids such as 10-undecylenic acid are employed clinically for the treatment of fungal infections, thus demonstrating their effectiveness (Day et al., 2022). Microalgae synthesize Free fatty acids (FFAs) as a protection against environmental stress or microbial infection (Lauritano et al., 2020). Such naturally occurring FFAs are chemical deterrents that provide ecological security and are of potential application for biotechnological uses.
Microalgae are a rich source of PUFAs like eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), γ-linolenic acid (GLA), α-linolenic acid (ALA), oleic acid, and arachidonic acid (Maltsev and Maltseva, 2021). The PUFAs have shown wide-spectrum antimicrobial activity against foodborne pathogens like Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Bacillus species (Biris-Dorhoi et al., 2020; Marrez et al., 2019; Falaise et al., 2016). Apart from their antibacterial activity, PUFAs, more so ω-3 fatty acids such as EPA, DHA, and ALA, have demonstrated potent antibiofilm, antifungal, and antiparasitic activities (Table 4). Studies in animal models have revealed that oral or intraperitoneal supplementation with ω-3 PUFAs prevents infection with Plasmodium, Toxoplasma, and Trypanosoma species (Choi et al., 2019; Ilieva et al., 2024a,b). EPA and DHA-supplemented transgenic zebrafish survived by 70% following infection with Vibrio vulnificus, whereas wild-type fish survived by only 20% (Cheng et al., 2015). Similar advantages were demonstrated in mouse and caterpillar models. Such effects are not replicated by oral antimicrobial activity per se but also by improved immune modulation by increased production of anti-inflammatory cytokines.
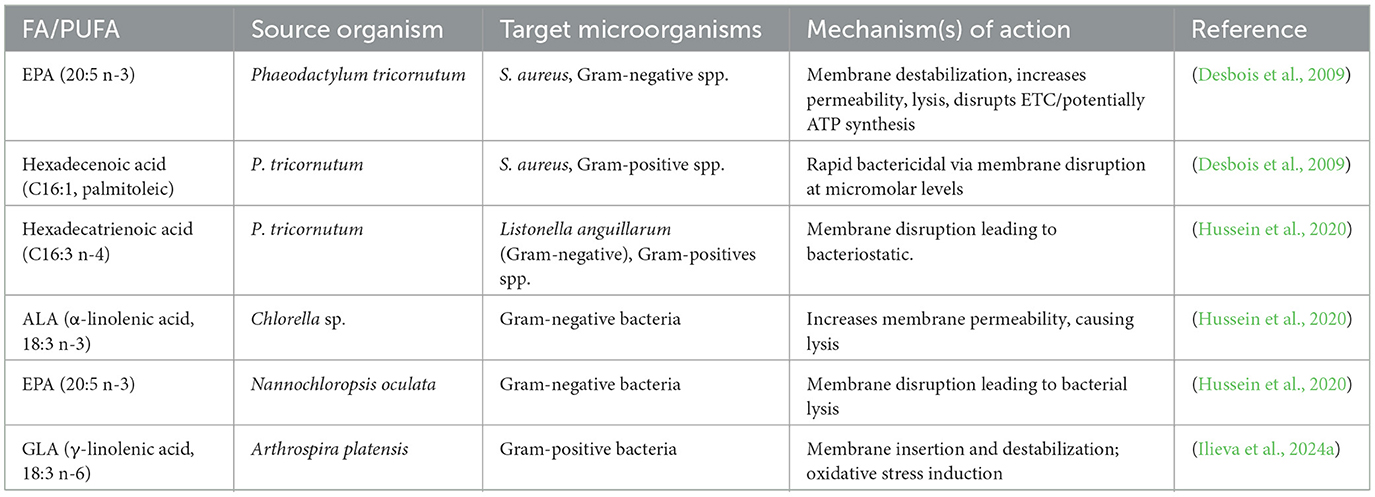
Table 4. Antibacterial fatty acids derived from microalgae and cyanobacteria, sources, and mechanisms of action.
Fatty acid structure–activity relation indicates that highly unsaturated and long-chain fatty acids are most effective against microbes. This makes microalgal PUFAs potential candidates for application as food, especially to substitute chemical preservatives with safer, chemical-free, and eco-friendly options. Their application as edible coatings, biodegradable packaging, or as natural food additives can prevent microbial contamination, improve shelf life, and comply with clean-label regulations.
4.1.1 Mechanism of action of antibacterial FAs and PUFAs
The amphipathic nature of FAs and PUFAs allows them to insert into bacterial cell destabilizing the membrane bilayers. This leads to increased permeability, pore formation, and eventual cell lysis. This membrane disruption can lead to either bacteriostatic effects (growth inhibition) or bactericidal effects (cell death). By compromising membrane integrity, FAs and PUFAs interfere with essential bacterial processes such as nutrient transport and osmotic balance, ultimately impairing cell survival (Obukhova and Murzina, 2024; Douglas et al., 2025).
FAs and PUFAs disrupt vital metabolic functions linked to the bacterial cell membrane, including the electron transport chain and oxidative phosphorylation. They may bind to electron carriers, alter membrane potential, and collapse the proton gradient, thereby reducing energy generation (Yoon et al., 2018). Moreover, they can inhibit key membrane-associated enzymes, such as glucosyltransferases, and impair nutrient uptake systems.
4.2 Antimicrobial peptides (AMPs)
Microalgae were found to be an effective source of AMPs which are gaining attention as natural alternatives to synthetic preservatives and conventional antibiotics (Vasquez-Moscoso et al., 2025). The peptides show strong activity against MDR bacteria. The increasing demand for clean-label, green food preservation also supports the investigation of AMPs of microalgal origin. AMPs are typically produced via enzymatic hydrolysis of algal proteins with proteolytic enzymes. Some of the most widely used species to produce peptide-rich protein hydrolysates are Chlorella vulgaris, Chlorella ellipsoidea, Tetradesmus obliquus, Navicula incerta, and Nannochloropsis oculata (Sathya et al., 2021; Yang et al., 2024). These bioactive peptides provide a range of desirable characteristics, including antioxidant, anticancer, antihypertensive, and strong antimicrobial effects. Notably, their antibacterial activity might be enhanced by structural modification, e.g., by the addition of essential amino acids such as lysine or alanine analogs, without increasing cytotoxicity (Ayswaria et al., 2023).
Peptides isolated from microalgae were shown to have direct antibacterial activity. An example is a 62 kDa peptide isolated from Chlorella vulgaris that inhibits Escherichia coli by interfering with bacterial cell wall formation (Sedighi et al., 2019). Similarly, peptides from Chlorella sorokiniana have been shown to inhibit E. coli and Staphylococcus aureus, as indicated by agar diffusion assays (Tejano et al., 2019). A heptapeptide (LWFYTMWH) known as AQ-1766, isolated from Tetraselmis suecica, exhibited broad-spectrum activity toward major foodborne and clinical pathogens such as Salmonella typhimurium, Bacillus cereus, Pseudomonas aeruginosa, and methicillin-resistant S. aureus (MRSA) (Sivakumar and Santhanam, 2011). Some of the antimicrobial peptides derived from microalgae and cyanobacteria along with their target microorganism are given in Table 5.
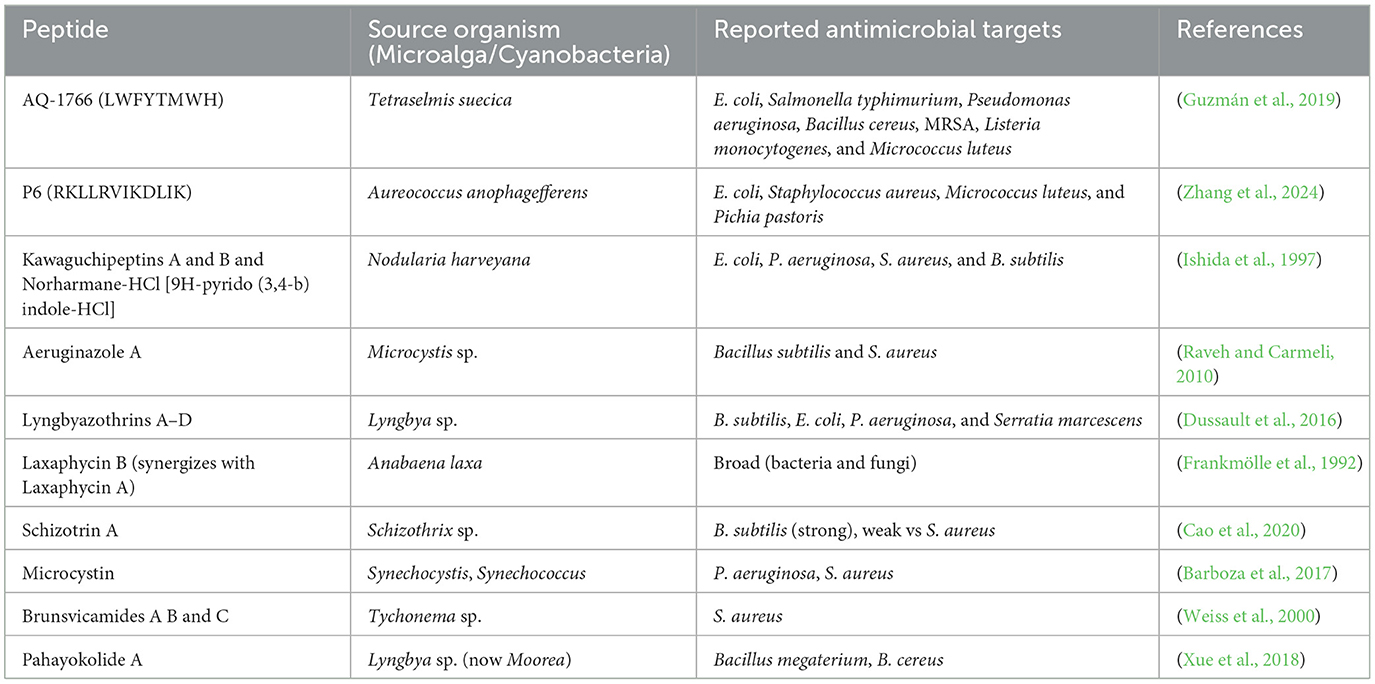
Table 5. Some peptide derived from microalgae and cyanobacteria along with their antimicrobial activity.
4.2.1 Mechanism of action of antimicrobial peptides
The mechanism of action for most of the microalgal AMPs, while not yet completely understood, is believed to be analogous to that of eukaryotic AMPs. It has been suggested that microalgal AMPs are induced or expressed due to environmental stress or pathogen-induced stress (Zehra et al., 2021; Meena et al., 2022; Tsintzou and Madesis, 2024). The peptides can be disruptive to microbial metabolism or cell integrity. Microalgal fatty acids have also been reported to lyse bacterial membranes, inhibit nutrient uptake, and inhibit respiration, providing a secondary mode of antibacterial action. Cationic peptides target the anionic bacterial membranes and induce disruption and cell lysis (Benfield and Henriques, 2020). Certain peptides exert non-membranous mechanisms such as inhibition of intracellular enzymes essential for bacterial survival. Brunsvicamides of Tychonema sp. inhibit phosphatase B of Mycobacterium tuberculosis and scyptolin A of Scytonema hofmanni inhibits bacterial transpeptidases for cell wall biosynthesis (Rojas et al., 2020).
4.3 Polysaccharides
Polysaccharides are natural polymers of 10 or more monosaccharide units in branched and linear chains. Polysaccharides occur in plants, animals, and microorganisms like microalgae. Polysaccharides in microalgae are of three general types: structural polysaccharides like cellulose in the cell wall, storage polysaccharides like starch and glycogen in the chloroplast, and extracellular polysaccharides secreted outside the cell to facilitate intercellular communication.
Microalgal polysaccharides have attracted significant attention due to their multi-functional uses in the food industry. Microalgal polysaccharides possess superior gelling, stabilizing, and emulsifying properties, and therefore are highly sought after as natural food additives (de Jesus Raposo et al., 2015). Apart from their functional applications, microalgal polysaccharides also possess potential antibacterial activity. Their antibacterial activities against a broad spectrum of Gram-positive and Gram-negative bacteria, including Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, and Salmonella Typhimurium have been reported. For instance, sulfated polysaccharides including alginates, fucoidans, and laminarin, isolated from algae like Chaetomorpha aerea, Ascophyllum nodosum, and Laminaria hyperborea, exhibit high antibacterial activities against these bacteria with MICs of about 50 mg/mL (Ilieva et al., 2024a,b; Parsaeimehr and Lutzu, 2016; Moreira J. B. et al., 2023; Mohan and Thirupathi, 2022; McGurrin et al., 2025).
4.3.1 Mechanism of action of antibacterial polysaccharides
The antibacterial effect of polysaccharides is multifaceted and intricate. They primarily disrupt the integrity of bacterial cell walls and membranes, causing leakage of cellular material and resultant cell death. Polysaccharides disrupt the formation of bacterial biofilms, which are involved in chronic food surface contamination (Singh et al., 2021). They disrupt bacterial metabolism and protein synthesis, thus preventing cell growth and reproduction. A second vital mode of action is direct interaction with bacterial DNA and plasmids. Some polysaccharides can interact with genetic material, inhibiting essential processes such as replication, transcription, and translation. Furthermore, most polysaccharides possess an anionic nature, which allows them to chelate metal ions such as iron. Since iron plays a vital role in bacterial metabolism and multiplication, the removal of iron from the environment may trigger a cut-off effect on bacterial viability (Zhao et al., 2023; Shankar and Akhter, 2024). The hydroxyl and carboxyl functional groups of the polysaccharides enhance the metal-binding ability, further inhibiting bacterial growth. Figure 5 illustrates the antibacterial mechanisms of action of polysaccharides.
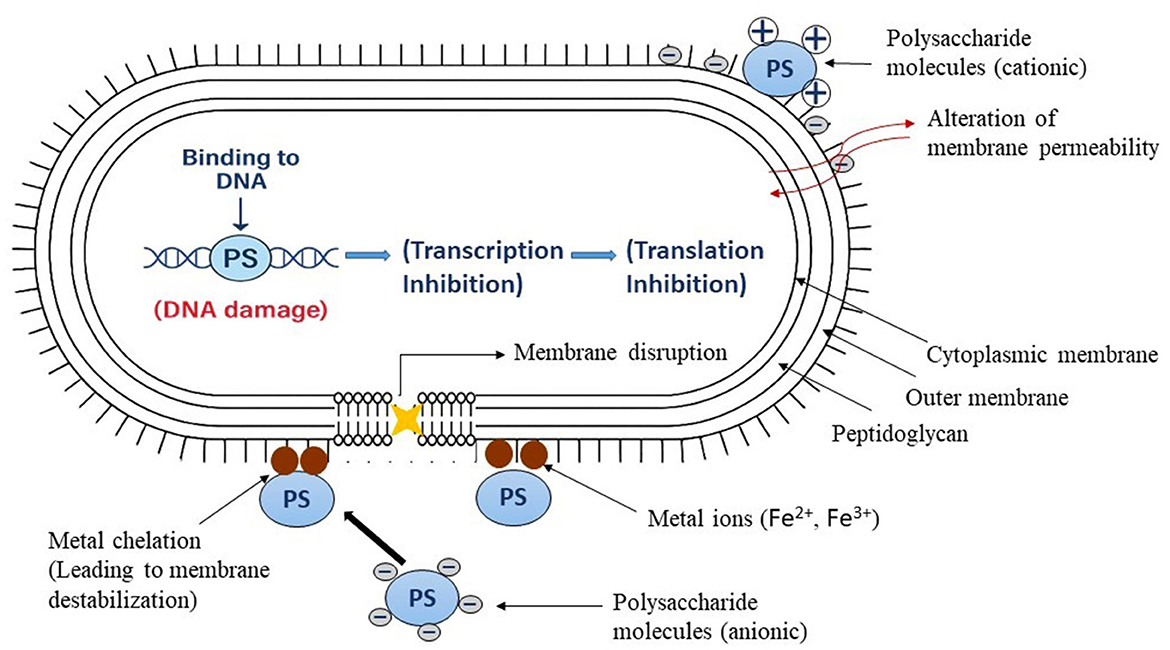
Figure 5. The antibacterial action of polysaccharides in bacterial cells resulting in cell death (Teixeira-Santos et al., 2021).
For food preservation, these bioactive polysaccharides are of immense promise. Microalgal polysaccharide-fortified edible films and coatings, as physical barriers and also with antimicrobial protection, enhance shelf life and food safety (Fan et al., 2025). Due to their natural origin, biodegradability, and wide-spectrum activity, they are the most suitable alternatives to substitute synthetic preservatives in the food system. More studies on their extraction, modification, and uses will result in more applications in food packaging and food safety technology.
5 Other applications of microalgal extracts in the food system
5.1 Microalgae-based films and coatings in food packaging
Microalgae-based films and coatings have emerged as eco-friendly and multifunctional materials with significant potential in the food industry. These biopolymer coatings contribute to preserving food quality, enhancing nutritional value, and extending the shelf life of a wide variety of products such as cereals, fruits, vegetables, meat, and seafood. One of the key advantages of these coatings is their ability to minimize moisture loss, maintain fruit firmness, and delay senescence, thereby ensuring longer freshness during storage (Morales-Jiménez et al., 2020; Shirai et al., 2025; Moreira A. S. et al., 2023).
Recent studies have confirmed the efficacy of microalgae-enriched coatings. A recent study by Onias et al. assessed the postharvest quality of Tommy Atkins mangoes with cassava and corn starch films enriched with Spirulina platensis. Mangoes were treated with six different coatings and stored for 12 days at 10 °C and 63% relative humidity. The B5 coating (3% cassava starch + 3% Spirulina) maximally enhanced the soluble solid content up to the eighth day, firmness was maintained, and weight loss was minimal. The B6 coating (3% corn starch + 3% Spirulina) was the best, enhancing the vitamin C content to 25 mg/100 g on day 11, maintaining firmness at 15 N, and inhibiting weight loss of < 4% (Onias et al., 2016).
De Medeiros Teodosio et al. examined the use of microalgae-based coatings for Spondias tuberosa fruit with Chlorella sp. and pomegranate seed oil (PSO) in another study. The fruits were stored at 14 ± 2 °C and 85 ± 5% RH for 12 days. The 2% Chlorella sp. coating was the most effective in slowing down ripening, firmness, and weight being preserved, and the color being greener compared to uncoated control fruits (de Medeiros Teodosio et al., 2021). This demonstrates the potential of Chlorella-based coatings in ensuring postharvest shelf life under cold storage. In a similar context, de Oliveira et al. studied the application of Chlorella sp. coatings for keeping Tommy Atkins mangoes stored at room temperature (23 °C). Peel and pulp color parameters showed that ripening was slowed with higher Chlorella concentrations. Mangoes treated with 2% Chlorella sp. contained more organic acids, and they were firmer, hence maintaining their quality for 10 days under 42% RH conditions (Oliveira et al., 2018; Alves et al., 2025). These results justify the industrial application of Chlorella-derived biofilms for keeping fruit quality under non-refrigerated storage.
5.2 Nanotechnology in microalgal-based food preservatives
Nanotechnology is another cutting-edge field in which microalgae are revolutionizing. Because of their size-dependent optical characteristics, nanodots (NDs) have drawn interest for use in food and biological science applications (Pyne et al., 2022). By altering their size and structure, they can be made to better absorb and emit light, providing special possibilities for creating biosensors and smart packaging materials (De Vries et al., 2015). The biogenic synthesis of nanoparticles using microalgae is a green chemistry technology that stands out among other synthesis methods. This method can create non-toxic and sustainable nanoparticles with a variety of compositions and physicochemical properties appropriate for use in the food system (Das et al., 2016). Microalgae-derived nanoparticles exhibit advantageous characteristics for applications in antimicrobial coatings, active packaging, and as carriers for bioactive chemicals. These nanoparticles improve oxidative stability and microbial suppression when added to edible films or sprayed on food surfaces. These applications provide a sustainable answer to contemporary food preservation issues by substituting naturally derived components for artificial additives.
6 Comparison with plant-based extracts
While both microalgal and plant-based extracts are highly valued for their natural preservative properties, their biochemical composition is not always the same. Terrestrial plants such as rosemary, oregano, and green tea are rich in well-known antioxidants like phenolic acids, flavonoids, and essential oils (Calderón-Oliver and Ponce-Alquicira, 2021). Whereas microalgae offer unique compounds such as astaxanthin, fucoxanthin, and phycobiliproteins metabolites that are rarely found in plants. Astaxanthin is reported to be significantly more effective than vitamin C and vitamin E in neutralizing reactive oxygen species (Chini Zittelli et al., 2023; Yadav et al., 2025).
Moreover, microalgae can be cultivated under controlled photobioreactor or open-pond conditions without the use of agricultural lands, ensuring consistent quality and bioactive content. Plants are more prone to seasonal and environmental fluctuations. On the other hand, plant-based extracts benefit from centuries of safe use in food systems, well-established regulatory approval, and lower production costs. This historical familiarity gives plant extracts a head start in consumer trust and acceptance, while microalgae still need to overcome perception barriers and production scalability challenges.
7 Consumer acceptance
Consumer perception plays an important role in the market success of a product. While plant extracts enjoy high familiarity and acceptance, microalgal products are often perceived as novel or unconventional. The unfamiliarity of microalgal food leads to skepticism regarding their taste, safety, and overall appeal. Consumer perception of microalgae varies greatly across regions. In East Asia (Japan, China, Korea), people are already familiar with algal food, therefore, consumer acceptance is high in comparison to Western countries (Wassmann et al., 2024). Blending microalgal extracts with plant-based ingredients can enhance familiarity while retaining functional benefits. Educational campaigns, transparent labeling, and the promotion of health benefits such as high antioxidant content, omega-3 enrichment, and sustainability can help shift consumer perception. Products targeting health-conscious demographics, including athletes, vegetarians, and individuals seeking functional foods may experience higher adoption rates.
8 Safety considerations and potential toxicity
While microalgal antioxidant-rich extracts are already finding applications in the food, cosmetic, and nutraceutical sectors, the precise assessment of antioxidant activity remains challenging. Some bioactive compounds such as free fatty acids (FFAs) and polyunsaturated fatty acids (PUFAs) are chemically unstable (Kiani et al., 2022). These can be overcome with the use of advanced formulation technologies like nanoencapsulation, emulsification, or co-formulation with synergistic agents.
Microalgal extracts require a thorough safety evaluation before widespread adoption in the food industry. Certain species among cyanobacteria can produce harmful metabolites such as microcystins, anatoxins, or saxitoxins, which are toxic to humans and animals (Nowruzi and Porzani, 2021; Chittora et al., 2020). Therefore, strain selection and cultivation under controlled, monitored conditions are critical to ensure food-grade safety. Another consideration is the high nucleic acid content in some microalgal biomass, which may increase uric acid levels when consumed in excess, potentially contributing to gout or kidney problems (Martínez-Ruiz et al., 2025). Ensuring the use of food-safe solvents and complete removal of extraction residues is essential for safe application of microalgal bioactive. Before industry-wide adoption, it is required to conduct systematic studies including in-product challenges, stability assessments in real matrices, toxicology and allergenicity evaluation, and compliance with food safety regulations (e.g., FSSAI, GRAS).
9 Conclusion and future prospects
Microalgae have been found to be an extremely promising source of natural bioactive compounds consisting of antioxidants (e.g., carotenoids, phenolics), antimicrobials (e.g., fatty acids, polysaccharides, etc.), and other functional biomolecules (Sangela et al., 2022). Considering recent advances in large-scale microalgal cultivation, eco-friendly extraction technologies, it is possible that microalgal-derived preservatives could progressively replace certain synthetic preservatives within the next decade. However, achieving full market penetration will require overcoming challenges related to production costs and batch-to-batch variability in bioactive content. Establishing comprehensive safety and efficacy profiles through regulatory approval processes would increase consumer acceptance.
Future studies should focus on optimizing the delivery systems in addition to searching for innovative strains and stress-induced cultivation protocols to enhance the yield and activity of microalgal bioactive compounds. If these challenges are addressed through integrated biorefinery approaches and optimized supply chains, microalgal preservatives could become competitive natural alternatives in food industries.
Author contributions
LS: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. PKumari: Conceptualization, Data curation, Methodology, Validation, Visualization, Writing – review & editing. PKumar: Formal analysis, Investigation, Methodology, Writing – review & editing. AY: Formal analysis, Investigation, Methodology, Writing – review & editing. RB: Investigation, Methodology, Project administration, Resources, Writing – review & editing. PS: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MM: Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to extend their sincere appreciation to the funding agency Anusandhan National Research Foundation (ANRF), Science and Engineering Research Board (SERB), State University Research Excellence (SURE) & Empowerment and Equity Opportunities for Excellence in Science, New Delhi, India. The author MM is also highly thankful to the Ministry of Education and SPD-RUSA Rajasthan for the financial support received under the RUSA-2.0 project. All the authors acknowledge their host institute for infrastructure support. The authors are also grateful to their respective universities for providing support during the work. All the authors read and approve the content of the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adan, A., Alizada, G., Kiraz, Y., Baran, Y., and Nalbant, A. (2017). Flow cytometry: basic principles and applications. Crit. Rev. Biotechnol. 37, 163–176. doi: 10.3109/07388551.2015.1128876
Aditi, B. R., Yadav, A., Swapnil, P., and Meena, M. (2025). Characterization of microalgal β-carotene and astaxanthin: exploring their health-promoting properties under the effect of salinity and light intensity. Biotechnol. Biofuels Bioprod. 18:18. doi: 10.1186/s13068-025-02612-x
Adusei, E. B., Adosraku, R. K., Oppong-Kyekyeku, J., Amengor, C. D., and Jibira, Y. (2019). Resistance modulation action, time-kill kinetics assay, and inhibition of biofilm formation effects of plumbagin from Plumbago zeylanica Linn. J. Trop. Med. 2019:1250645. doi: 10.1155/2019/1250645
Almendinger, M., Saalfrank, F., Rohn, S., Kurth, E., Springer, M., and Pleissner, D. (2021). Characterization of selected microalgae and cyanobacteria as sources of compounds with antioxidant capacity. Algal Res. 53:102168. doi: 10.1016/j.algal.2020.102168
Alsenani, F., Tupally, K. R., Chua, E. T., Eltanahy, E., Alsufyani, H., Parekh, H. S., et al. (2020). Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm. J. 28, 1834–1841. doi: 10.1016/j.jsps.2020.11.010
Alves, K. A., Araújo, R. H., Silva, A. S., Almeida, E. S., Oliveira, Á. M., Rocha, N. S., et al. (2025). Biodegradable film is enriched with pomegranate seed oil and microalgae for preservation of Cajarana (Spondias dulcis). Polymers 17:367. doi: 10.3390/polym17030367
Amaro, H. M., Guedes, A. C., and Malcata, F. X. (2011). “Antimicrobial activities of microalgae: An invited review,” in Science Against Microbial Pathogens: Communicating Current Research and Technological Advances (Badajoz: Formatex Research Center), 1272–1284.
Ambrico, A., Trupo, M., Magarelli, R., Balducchi, R., Ferraro, A., Hristoforou, E., et al. (2020). Effectiveness of Dunaliella salina extracts against Bacillus subtilis and bacterial plant pathogens. Pathogens 9:613. doi: 10.3390/pathogens9080613
Amorati, R., and Valgimigli, L. (2015). Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 49, 633–649. doi: 10.3109/10715762.2014.996146
Anwer, S. S., Sdiq, K. H., Muhammad, K. R., and Aladdin, L. M. (2022). Phenolic compound and fatty acid properties of some microalgae species isolated from Erbil city. Braz. J. Biol. 82:e256927. doi: 10.1590/1519-6984.256927
Aremu, A. O., Masondo, N. A., Stirk, W. A., Ördög, V., and Van Staden, J. (2014). Influence of culture age on the phytochemical content and pharmacological activities of five Scenedesmus strains. J. Appl. Phycol. 26, 407–415. doi: 10.1007/s10811-013-0144-y
Ayswaria, R., Vijayan, J., and Nathan, V. K. (2023). Antimicrobial peptides derived from microalgae for combating antibiotic resistance: current status and prospects. Cell Biochem. Funct. 41, 142–151. doi: 10.1002/cbf.3779
Barboza, G. F., Gorlach-Lira, K., Sassi, C. F., and Sassi, R. (2017). Microcystins production and antibacterial activity of cyanobacterial strains of Synechocystis, Synechococcus and Romeria from water and coral reef organisms (Brazil). Rev. Biol. Trop. 65, 890–899. doi: 10.15517/rbt.v65i3.29437
Bellahcen, T. O., Aamiri, A., Touam, I., Hmimid, F., Amrani, A. E., Cherif, A., et al. (2020). Evaluation of Moroccan microalgae: Spirulina platensis as a potential source of natural antioxidants. J. Complement. Integr. Med. 17:20190036. doi: 10.1515/jcim-2019-0036
Benfield, A. H., and Henriques, S. T. (2020). Mode-of-action of antimicrobial peptides: membrane disruption vs. intracellular mechanisms. Front. Med. Technol. 2:610997. doi: 10.3389/fmedt.2020.610997
Benzie, I. F., and Devaki, M. (2018). “The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: concepts, procedures, limitations and applications,” in Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications (Hoboken, NJ: John Wiley & Sons), 77–106. doi: 10.1002/9781119135388.ch5
Bhardwaj, R., Yadav, A., Sahoo, A., Kumari, P., Singh, L. A., Swapnil, P., et al. (2025). Microalgal-based sustainable bio-fungicides: a promising solution to enhance crop yield. Dis. Sustain. 6:39. doi: 10.1007/s43621-025-00795-9
Biris-Dorhoi, E. S., Michiu, D., Pop, C. R., Rotar, A. M., Tofana, M., Pop, O. L., et al. (2020). Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 12:3085. doi: 10.3390/nu12103085
Bueno, M., Gallego, R., Chourio, A. M., Ibáñez, E., Herrero, M., and Saldaña, M. D. (2020). Green ultra-high pressure extraction of bioactive compounds from Haematococcus pluvialis and Porphyridium cruentum microalgae. Innov. Food Sci. Emerg. Technol. 66:102532. doi: 10.1016/j.ifset.2020.102532
Cakmak, Y. S., Kaya, M., and Asan-Ozusaglam, M. (2014). Biochemical composition and bioactivity screening of various extracts from Dunaliella salina, a green microalga. EXCLI J. 13, 679–690.
Calderón-Oliver, M., and Ponce-Alquicira, E. (2021). Environmentally friendly techniques and their comparison in the extraction of natural antioxidants from green tea, rosemary, clove, and oregano. Molecules 26:1869. doi: 10.3390/molecules26071869
Cao, S., Xue, J., Chen, X., An, X., and Zhang, X. (2020). Magnetic nanoparticles mediate the transformation of antimicrobial peptides HeM into Chlorella ellipsoidea. J. Appl. Phycol. 32, 3913–3921. doi: 10.1007/s10811-020-02101-8
Cassier-Chauvat, C., Marceau, F., Farci, S., Ouchane, S., and Chauvat, F. (2023). The glutathione system: a journey from cyanobacteria to higher eukaryotes. Antioxidants 12:1199. doi: 10.3390/antiox12061199
Cezare-Gomes, E. A., Mejia-da-Silva, L. D. C., Pérez-Mora, L. S., Matsudo, M. C., Ferreira-Camargo, L. S., Singh, A. K., et al. (2019). Potential of microalgae carotenoids for industrial application. Appl. Biochem. Biotechnol. 188, 602–634. doi: 10.1007/s12010-018-02945-4
Chaudhary, P., Janmeda, P., Docea, A. O., Yeskaliyeva, B., Abdull Razis, A. F., Modu, B., et al. (2023). Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front. Chem. 11:1158198. doi: 10.3389/fchem.2023.1158198
Chen, C., Tang, T., Shi, Q., Zhou, Z., and Fan, J. (2022). The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 126, 99–112. doi: 10.1016/j.tifs.2022.06.016
Chen, F. C., and Godwin, S. L. (2006). Comparison of a rapid ATP bioluminescence assay and standard plate count methods for assessing microbial contamination of consumers' refrigerators. J. Food Prot. 69, 2534–2538. doi: 10.4315/0362-028X-69.10.2534
Cheng, C. L., Huang, S. J., Wu, C. L., Gong, H. Y., Ken, C. F., Hu, S. Y., et al. (2015). Transgenic expression of omega-3 PUFA synthesis genes improves zebrafish survival during Vibrio vulnificus infection. J. Biomed. Sci. 22:103. doi: 10.1186/s12929-015-0208-1
Chini Zittelli, G., Lauceri, R., Faraloni, C., Silva Benavides, A. M., and Torzillo, G. (2023). Valuable pigments from microalgae: phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 22, 1733–1789. doi: 10.1007/s43630-023-00407-3
Chittora, D., Meena, M., Barupal, T., Swapnil, P., and Sharma, K. (2020). Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem. Biophys. Rep. 22:100737. doi: 10.1016/j.bbrep.2020.100737
Chiu, C. T., Lai, C. H., Huang, Y. H., Yang, C. H., and Lin, J. N. (2021). Comparative analysis of gradient diffusion and disk diffusion with agar dilution for susceptibility testing of Elizabethkingia anophelis. Antibiotics 10:450. doi: 10.3390/antibiotics10040450
Choi, J. W., Lee, J., Lee, J. H., Park, B. J., Lee, E. J., Shin, S., et al. (2019). Omega-3 polyunsaturated fatty acids prevent Toxoplasma gondii infection by inducing autophagy via AMPK activation. Nutrients 11:2137. doi: 10.3390/nu11092137
Corrêa, J. A. F., de Melo Nazareth, T., Rocha, G. F. D., and Luciano, F. B. (2023). Bioactive antimicrobial peptides from food proteins: perspectives and challenges for controlling foodborne pathogens. Pathogens 12:477. doi: 10.3390/pathogens12030477
Coulombier, N., Jauffrais, T., and Lebouvier, N. (2021). Antioxidant compounds from microalgae: a review. Mar. Drugs 19:549. doi: 10.3390/md19100549
Danet, A. F. (2021). “Recent advances in antioxidant capacity assays,” in Antioxidants - Benefits, Sources, Mechanisms of Action (London: IntechOpen).
Dantas, D. M. D. M., Oliveira, C. Y. B. D., Costa, R. M. P. B., Carneiro-da-Cunha, M. D. G., Gálvez, A. O., and Bezerra, R. D. S. (2019). Evaluation of antioxidant and antibacterial capacity of green microalgae Scenedesmus subspicatus. Food Sci. Technol. Int. 25, 318–326. doi: 10.1177/1082013218825024
Das, M., Senapati, K., Panda, S. S., Bhattacharya, P., Jana, S., Mandal, S. M., et al. (2016). π-Stacking assisted redox active peptide–gallol conjugate: synthesis of a new generation of low-toxicity antimicrobial silver nanoparticles. RSC Adv. 6, 85254–85260. doi: 10.1039/C6RA13075E
Day, Z. I., Mayfosh, A. J., Giel, M. C., Hong, Y., Williams, S. A., Santavanond, J. P., et al. (2022). Novel formulation of undecylenic acid induces tumor cell apoptosis. Int. J. Mol. Sci. 23:14170. doi: 10.3390/ijms232214170
de Jesus Raposo, M. F., de Morais, A. M. M. B., and de Morais, R. M. S. C. (2015). “Bioactivity and applications of polysaccharides from marine microalgae,” in Polysaccharides (Cham: Springer), 1683–1727. doi: 10.1007/978-3-319-16298-0_47
De Leon, J. A. D., and Borges, C. R. (2020). Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. J. Vis. Exp. 12:159. 10.3791/61122.
de Medeiros Teodosio, A. E. M., Santos, B. G. F. L., Linné, J. A., de Lima Cruz, J. M. F., Onias, E. A., de Lima, J. F., et al. (2021). Preservation of Spondias tuberosa fruit with edible coatings based on Chlorella sp. enriched with pomegranate seed oil during storage. Food Bioprocess Technol. 14, 2020–2031. doi: 10.1007/s11947-021-02704-0
De Vries, R., Andrade, C. A., Bakuzis, A. F., Mandal, S. M., and Franco, O. L. (2015). Next-generation nanoantibacterial tools developed from peptides. Nanomedicine 10, 1643–1661. doi: 10.2217/nnm.15.9
Del Mondo, A., Smerilli, A., Sané, E., Sansone, C., and Brunet, C. (2020). Challenging microalgal vitamins for human health. Microb. Cell Factories 19:201. doi: 10.1186/s12934-020-01459-1
Desbois, A. P., Mearns-Spragg, A., and Smith, V. J. (2009). A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar. Biotechnol. 11, 45–52. doi: 10.1007/s10126-008-9118-5
Douglas, E. J., Palk, N., Rudolph, E. R., and Laabei, M. (2025). Anti-staphylococcal fatty acids: mode of action, bacterial resistance and implications for therapeutic application. Microbiology 171:001563. doi: 10.1099/mic.0.001563
Dussault, D., Vu, K. D., Vansach, T., Horgen, F. D., and Lacroix, M. (2016). Antimicrobial effects of marine algal extracts and cyanobacterial pure compounds against five foodborne pathogens. Food Chem. 199, 114–118. doi: 10.1016/j.foodchem.2015.11.119
El Shafay, S. M., Ali, S. S., and El-Sheekh, M. M. (2016). Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt. J. Aquat. Res. 42, 65–74. doi: 10.1016/j.ejar.2015.11.006
Falaise, C., François, C., Travers, M. A., Morga, B., Haure, J., Tremblay, R., et al. (2016). Antimicrobial compounds from eukaryotic microalgae against human pathogens and diseases in aquaculture. Mar. Drugs 14:159. doi: 10.3390/md14090159
Fan, Y., Ren, J., Xiao, X., Cao, Y., Zou, Y., Qi, B., et al. (2025). Recent advances in polysaccharide-based edible films/coatings for food preservation: fabrication, characterization, and applications in packaging. Carbohydr. Polym. 364:123779. doi: 10.1016/j.carbpol.2025.123779
Faraloni, C., Di Lorenzo, T., and Bonetti, A. (2021). Impact of light stress on the synthesis of both antioxidants polyphenols and carotenoids, as a fast photoprotective response in Chlamydomonas reinhardtii: new prospective for biotechnological potential of this microalga. Symmetry 13:2220. doi: 10.3390/sym13112220
Figueroa, J. D., Barroso-Torres, N., Morales, M., Herrera, B., Aranda, M., Dorta, E., et al. (2023). Antioxidant capacity of free and peptide tryptophan residues determined by the ORAC (oxygen radical absorbance capacity) assay is modulated by radical-radical reactions and oxidation products. Foods 12:4360. doi: 10.3390/foods12234360
Frankmölle, W. P., Larsen, L. K., Caplan, F. R., Patterson, G. M., Knübel, G., Levine, I. A., et al. (1992). Antifungal cyclic peptides from the terrestrial blue-green alga Anabaena laxa I. isolation and biological properties. J. Antibiot. 45, 1451–1457. doi: 10.7164/antibiotics.45.1451
Garbayo, I., Cuaresma, M., Vílchez, C., and Vega, J. M. (2008). Effect of abiotic stress on the production of lutein and β-carotene by Chlamydomonas acidophila. Process Biochem. 43, 1158–1161. doi: 10.1016/j.procbio.2008.06.012
Gauthier, M. R., Senhorinho, G. N. A., and Scott, J. A. (2020). Microalgae under environmental stress as a source of antioxidants. Algal Res. 52:102104. doi: 10.1016/j.algal.2020.102104
Ghani, M. A., Barril, C., Bedgood Jr, D. R., and Prenzler, P. D. (2017). Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 230, 195–207. doi: 10.1016/j.foodchem.2017.02.127
Goiris, K., Van Colen, W., Wilches, I., León-Tamariz, F., De Cooman, L., and Muylaert, K. (2015). Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 7, 51–57. doi: 10.1016/j.algal.2014.12.002
Golus, J., Sawicki, R., Widelski, J., and Ginalska, G. (2016). The agar microdilution method–a new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 121, 1291–1299. doi: 10.1111/jam.13253
Gómez-Guzmán, M., Rodríguez-Nogales, A., Algieri, F., and Gálvez, J. (2018). Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 16:250. doi: 10.3390/md16080250
Gorbachev, V., Klokonos, M., Orlovtseva, O., Tefikova, S., and Nikitin, I. (2021). Analysis of anti-radical activity of some food suitable algae of the sea of Okhotsk. EDP Sci. 279:03007. doi: 10.1051/e3sconf/202127903007
Guidara, M., Yaich, H., Benelhadj, S., Adjouman, Y. D., Richel, A., Blecker, C., et al. (2020). Smart ulvan films responsive to stimuli of plasticizer and extraction condition in physico-chemical, optical, barrier and mechanical properties. Int. J. Biol. Macromol. 150, 714–726. doi: 10.1016/j.ijbiomac.2020.02.111
Gulcin, I. (2020). Antioxidants and antioxidant methods: an updated overview. Arch. Toxicol. 94, 651–715. doi: 10.1007/s00204-020-02689-3
Gulcin, I. (2025). Antioxidants: a comprehensive review. Arch. Toxicol. 99, 1893–1997. doi: 10.1007/s00204-025-03997-2
Güven, K. C., Percot, A., and Sezik, E. (2010). Alkaloids in marine algae. Mar. Drugs 8, 269–284. doi: 10.3390/md8020269
Guzmán, F., Wong, G., Román, T., Cárdenas, C., Alvárez, C., Schmitt, P., et al. (2019). Identification of antimicrobial peptides from the microalgae Tetraselmis suecica (Kylin) Butcher and bactericidal activity improvement. Mar. Drugs 17:453. doi: 10.3390/md17080453
Hellwig, M. (2019). The chemistry of protein oxidation in food. Ange. Chem. Int. Ed. Engl. 58, 16742–16763. doi: 10.1002/anie.201814144
Henderson, T., Nigam, P. S., and Owusu-Apenten, R. K. (2015). A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 174, 119–123. doi: 10.1016/j.foodchem.2014.11.009
Hossain, T. J. (2024). Methods for screening and evaluation of antimicrobial activity: a review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 14, 97–115. doi: 10.1556/1886.2024.00035
Hussein, H. A., Syamsumir, D. F., Radzi, S. A. M., Siong, J. Y. F., Zin, N. A. M., and Abdullah, M. A. (2020). Phytochemical screening, metabolite profiling and enhanced antimicrobial activities of microalgal crude extracts in co-application with silver nanoparticle. Bioresour. Bioprocessing 7:39. doi: 10.1186/s40643-020-00322-w
Ibrahim, N., and Kebede, A. (2020). In vitro antibacterial activities of methanol and aqueous leaf extracts of selected medicinal plants against human pathogenic bacteria. Saudi J. Biol. Sci. 27, 2261–2268. doi: 10.1016/j.sjbs.2020.06.047
Ilieva, Y., Zaharieva, M. M., Kroumov, A. D., and Najdenski, H. (2024a). Antimicrobial and ecological potential of Chlorellaceae and Scenedesmaceae with a focus on wastewater treatment and industry. Fermentation 10:341. doi: 10.3390/fermentation10070341
Ilieva, Y., Zaharieva, M. M., Najdenski, H., and Kroumov, A. D. (2024b). Antimicrobial activity of Arthrospira (former Spirulina) and Dunaliella related to recognized antimicrobial bioactive compounds. Int. J. Mol. Sci. 25:5548. doi: 10.3390/ijms25105548
Ishida, K., Matsuda, H., Murakami, M., and Yamaguchi, K. (1997). Kawaguchipeptin B, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 60, 724–726. doi: 10.1021/np970146k
Jayshree, A., Jayashree, S., and Thangaraju, N. (2016). Chlorella vulgaris and Chlamydomonas reinhardtii: effective antioxidant, antibacterial, and anticancer mediators. Indian J. Pharm. Sci. 78, 575–581. doi: 10.4172/pharmaceutical-sciences.1000155
Jena, J., and Subudhi, E. (2019). “Microalgae: an untapped resource for natural antimicrobials,” in The Role of Microalgae in Wastewater Treatment (Singapore: Springer), 99–114. doi: 10.1007/978-981-13-1586-2_8
Jia, L., Kosgey, J. C., Wang, J., Yang, J., Nyamao, R. M., Zhao, Y., et al. (2020). Antimicrobial and mechanism of antagonistic activity of Bacillus sp. A2 against pathogenic fungus and bacteria: the implication on honey's regulatory mechanism on host's microbiota. Food Sci. Nutr. 8, 4857–4867. doi: 10.1002/fsn3.1770
Katiyar, R., Gurjar, B. R., Biswas, S., Pruthi, V., Kumar, N., and Kumar, P. (2017). Microalgae: an emerging source of energy-based bio-products and a solution for environmental issues. Renew. Sustain. Energ. Rev. 72, 1083–1093. doi: 10.1016/j.rser.2016.10.028
Kellett, M. E., Greenspan, P., and Pegg, R. B. (2018). Modification of the cellular antioxidant activity (CAA) assay to study phenolic antioxidants in a Caco-2 cell line. Food Chem. 244, 359–363. doi: 10.1016/j.foodchem.2017.10.035
Khandual, S., Sanchez, E. O. L., Andrews, H. E., and De la Rosa, J. D. P. (2021). Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: efficient extraction process and stability evaluation of phycocyanin. BMC Chem. 15:24. doi: 10.1186/s13065-021-00746-1
Khaw, Y. S., Yusoff, F. M., Tan, H. T., Noor Mazli, N. A. I., Nazarudin, M. F., Shaharuddin, N. A., et al. (2022). Fucoxanthin production of microalgae under different culture factors: a systematic review. Mar. Drugs 20:592. doi: 10.3390/md20100592
Kiani, H., Aznar, R., Poojary, M. M., Tiwari, B. K., and Halim, R. (2022). Chromatographic techniques to separate and identify bioactive compounds in microalgae. Front. Energy Res. 10:904014. doi: 10.3389/fenrg.2022.904014
Kocira, A., Kozłowicz, K., Panasiewicz, K., Staniak, M., Szpunar-Krok, E., and Hortyńska, P. (2021). Polysaccharides as edible films and coatings: characteristics and influence on fruit and vegetable quality—a review. Agronomy 11:813. doi: 10.3390/agronomy11050813
Kumawat, G., Vyas, P., Choudhary, S., Meena, M., and Harish. (2024). Microalgal biodiesel as a sustainable and green energy alternative: a metabolomic approach. Biomass Bioenergy 186:107257. doi: 10.1016/j.biombioe.2024.107257
Lauritano, C., Andersen, J. H., Hansen, E., Albrigtsen, M., Escalera, L., Esposito, F., et al. (2016). Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 3:68. doi: 10.3389/fmars.2016.00068
Lauritano, C., Rizzo, C., Lo Giudice, A., and Saggiomo, M. (2020). Physiological and molecular responses to the main environmental stressors of microalgae and bacteria in polar marine environments. Microorganisms 8:1957. doi: 10.3390/microorganisms8121957
León-Vaz, A., León, R., Vigara, J., and Funk, C. (2023). Exploring Nordic microalgae as a potential novel source of antioxidant and bioactive compounds. N. Biotechnol. 73, 1–8. doi: 10.1016/j.nbt.2022.12.001
Li, C. J., Chen, P. N., Li, H. J., Mahmud, T., Wu, D. L., Xu, J., et al. (2020). Potential antidiabetic fumiquinazoline alkaloids from the marine-derived fungus Scedosporium apiospermum F41-1. J. Nat. Prod. 83, 1082–1091. doi: 10.1021/acs.jnatprod.9b01096
Litescu, S. C., Eremia, S. A., Tache, A., Vasilescu, I., and Radu, G. L. (2014). “The use of oxygen radical absorbance capacity (ORAC) and Trolox equivalent antioxidant capacity (TEAC) assays in the assessment of beverages' antioxidant properties,” in Processing and Impact on Antioxidants in Beverages (Lonodn: Elsevier Academic Press). 245–251. doi: 10.1016/B978-0-12-404738-9.00025-8
Lomakina, G. Y., Modestova, Y. A., and Ugarova, N. N. (2015). Bioluminescence assay for cell viability. Biochemistry 80, 701–713. doi: 10.1134/S0006297915060061
Maadane, A., Merghoub, N., El Mernissi, N., Ainane, T., Amzazi, S., Wahby, I., et al. (2017). Antimicrobial activity of marine microalgae isolated from Moroccan coastlines. J. Microbiol. Biotechnol. Food Sci. 6:1257. doi: 10.15414/jmbfs.2017.6.6.1257-1260
Maehle, N., and Skjeret, F. (2022). Microalgae-based food: purchase intentions and willingness to pay. Future Foods 6:100205. doi: 10.1016/j.fufo.2022.100205
Mafe, A. N., Edo, G. I., Makia, R. S., Joshua, O. A., Akpoghelie, P. O., Gaaz, T. S., et al. (2024). A review on food spoilage mechanisms, food borne diseases and commercial aspects of food preservation and processing. Food Chem. Adv. 5:100852. doi: 10.1016/j.focha.2024.100852
Maglangit, F., Fang, Q., Kyeremeh, K., Sternberg, J. M., Ebel, R., and Deng, H. (2020). A co-culturing approach enables discovery and biosynthesis of a bioactive indole alkaloid metabolite. Molecules 25, 256. doi: 10.3390/molecules25020256
Maltsev, Y., and Maltseva, K. (2021). Fatty acids of microalgae: diversity and applications. Rev. Environ. Sci. Bio/Technol. 20, 515–547. doi: 10.1007/s11157-021-09571-3
Marrez, D. A., Naguib, M. M., Sultan, Y. Y., and Higazy, A. M. (2019). Antimicrobial and anticancer activities of Scenedesmus obliquus metabolites. Heliyon 5:e01404. doi: 10.1016/j.heliyon.2019.e01404
Martemucci, G., Costagliola, C., Mariano, M., D'andrea, L., Napolitano, P., and D'Alessandro, A. G. (2022). Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2, 48–78. doi: 10.3390/oxygen2020006
Martinez-Morales, F., Alonso-Castro, A. J., Zapata-Morales, J. R., Carranza-Álvarez, C., and Aragon-Martinez, O. H. (2020). Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem. Pap. 74, 3325–3334. doi: 10.1007/s11696-020-01161-x
Martínez-Ruiz, F. E., Andrade-Bustamante, G., Holguín-Peña, R. J., Renganathan, P., Gaysina, L. A., Sukhanova, N. V., et al. (2025). Microalgae as functional food ingredients: nutritional benefits, challenges, and regulatory considerations for safe consumption. Biomass 5:25. doi: 10.3390/biomass5020025
Maurício, T., Couto, D., Lopes, D., Conde, T., Pais, R., Batista, J., et al. (2023). Differences and similarities in lipid composition, nutritional value, and bioactive potential of four edible Chlorella vulgaris strains. Foods 12:1625. doi: 10.3390/foods12081625
McGurrin, A., Suchintita Das, R., Soro, A. B., Maguire, J., Flórez Fernández, N., Dominguez, H., et al. (2025). Antimicrobial activities of polysaccharide-rich extracts from the Irish seaweed Alaria esculenta, generated using green and conventional extraction technologies, against foodborne pathogens. Mar. Drugs 23:46. doi: 10.3390/md23010046
McKinnon, K. M. (2018). Flow cytometry: an overview. Curr. Protoc. Immunol. 120, 5.1.1–5.1.11. doi: 10.1002/cpim.40
Meena, M., Yadav, G., Sonigra, P., Nagda, A., Mehta, T., Swapnil, P., et al. (2022). Role of elicitors to initiate the induction of systemic resistance in plants to biotic stress. Plant Stress 5:100103. doi: 10.1016/j.stress.2022.100103
Mendiola, J. A., Torres, C. F., Toré, A., Martín-Álvarez, P. J., Santoyo, S., Arredondo, B. O., et al. (2007). Use of supercritical CO2 to obtain extracts with antimicrobial activity from Chaetoceros muelleri microalga. A correlation with their lipidic content. Eur. Food Res. Technol. 224, 505–510. doi: 10.1007/s00217-006-0353-6
Mohan, S. C., and Thirupathi, A. (2022). “Antioxidant and antibacterial activities of polysaccharides,” in Polysaccharides of Microbial Origin: Biomedical Applications (Cham: Springer International Publishing), 553–578. doi: 10.1007/978-3-030-42215-8_32
Molino, A., Iovine, A., Casella, P., Mehariya, S., Chianese, S., Cerbone, A., et al. (2018). Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 15:2436. doi: 10.3390/ijerph15112436
Morales-Jiménez, M., Gouveia, L., Yáñez-Fernández, J., Castro-Muñoz, R., and Barragán-Huerta, B. E. (2020). Production, preparation and characterization of microalgae-based biopolymer as a potential bioactive film. Coatings 10:120. doi: 10.3390/coatings10020120
Moreira, A. S., Gonçalves, J., Sousa, F., Maia, I., Pereira, H., Silva, J., et al. (2023). Potential of coccolithophore microalgae as fillers in starch-based films for active and sustainable food packaging. Foods 12:513. doi: 10.3390/foods12030513
Moreira, J. B., Santos, T. D., Cruz, C. G., Silveira, J. T. D., Carvalho, L. F. D., Morais, M. G. D., et al. (2023). Algal polysaccharides-based nanomaterials: general aspects and potential applications in food and biomedical fields. Polysaccharides 4, 371–389. doi: 10.3390/polysaccharides4040022
Mularczyk, M., Michalak, I., and Marycz, K. (2020). Astaxanthin and other nutrients from Haematococcus pluvialis—multifunctional applications. Mar. Drugs 18:459. doi: 10.3390/md18090459
Natrah, F. M. I., Yusoff, F. M., Shariff, M., Abas, F., and Mariana, N. S. (2007). Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J. Appl. Phycol. 19, 711–718. doi: 10.1007/s10811-007-9192-5
Nowruzi, B., and Porzani, S. J. (2021). Toxic compounds produced by cyanobacteria belonging to several species of the order Nostocales: a review. J. Appl. Toxicol. 41, 510–548. doi: 10.1002/jat.4088
Obukhova, E. S., and Murzina, S. A. (2024). Mechanisms of the antimicrobial action of fatty acids: a review. Appl. Biochem. Microbiol. 60, 1035–1043. doi: 10.1134/S0003683824605158
Oliveira, Á. M. D., Rocha, R. H. C., Guedes, W. A., Furtunato, T. D. S., and Lima, J. D. (2018). Postharvest conservation of ‘Tommy Atkins' mango with bio-organic coating of Chlorella sp. Científica 46, 8–16. doi: 10.15361/1984-5529.2018v46n1p08-16
Onias, E. A., Rocha, R. H. C., Lima, J. D., Onias, E. A., and Furtunato, T. D. S. (2016). Organic Tommy Atkins' mango postharvest quality when treated with biofilms enriched by Spirulina platensis. Científica 44, 286–293. doi: 10.15361/1984-5529.2016v44n3p286-293
Ou, B., Chang, T., Huang, D., and Prior, R. L. (2013). Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: first action 2012.23. J. AOAC Int. 96, 1372–1376. doi: 10.5740/jaoacint.13-175
Parsaeimehr, A., and Lutzu, G. A. (2016). “Algae as a novel source of antimicrobial compounds: current and future perspectives,” in Antibiotic Resistance: Mechanisms and New Antimicrobial Approaches (Academic Press. Elsevier) 377–396. doi: 10.1016/B978-0-12-803642-6.00018-6
Patra, J. K., and Baek, K. H. (2016). Anti-listerial activity of four seaweed essential oils against Listeria monocytogenes. Jundishapur J. Microbiol. 9:e31784. doi: 10.5812/jjm.31784
Pratap, K., Taki, A. C., Johnston, E. B., Lopata, A. L., and Kamath, S. D. (2020). A comprehensive review on natural bioactive compounds and probiotics as potential therapeutics in food allergy treatment. Front. Immunol. 11:996. doi: 10.3389/fimmu.2020.00996
Prisacaru, A. E. (2016). Effect of antioxidants on polyunsaturated fatty acids–review. Acta Sci. Pol. Technol. Aliment. 15, 121–129. doi: 10.17306/J.AFS.2016.2.12
Pruteanu, L. L., Bailey, D. S., Grădinaru, A. C., and Jäntschi, L. (2023). The biochemistry and effectiveness of antioxidants in food, fruits, and marine algae. Antioxidants 12:860. doi: 10.3390/antiox12040860
Pyne, S., Paria, K., Mandal, S. M., Srivastav, P. P., Bhattacharjee, P., and Barik, T. K. (2022). Green microalgae-derived organic nanodots used as food preservatives. Curr. Res. Green Sustain. Chem. 5:100276. doi: 10.1016/j.crgsc.2022.100276
Ragini, R., and Arumugam, M. (2023). In vivo studies on bioavailability, toxicity, and antioxidant defense of organic selenium-enriched microalga biomass in Wistar rats. J. Appl. Phycol. 35:1699–1713. doi: 10.1007/s10811-023-03007-x
Ramel, F., Birtic, S., Cuiné, S., Triantaphylides, C., Ravanat, J. L., and Havaux, M. (2012). Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 158, 1267–1278. doi: 10.1104/pp.111.182394
Raveh, A., and Carmeli, S. (2010). Aeruginazole A, a novel thiazole-containing cyclopeptide from the cyanobacterium Microcystis sp. Org. Lett. 12, 3536–3539. doi: 10.1021/ol1014015
Rezayian, M., Niknam, V., and Ebrahimzadeh, H. (2019). Oxidative damage and antioxidative system in algae. Toxicol. Rep. 6, 1309–1313. doi: 10.1016/j.toxrep.2019.10.001
Rojas, V., Rivas, L., Cárdenas, C., and Guzmán, F. (2020). Cyanobacteria and eukaryotic microalgae as emerging sources of antibacterial peptides. Molecules 25:5804. doi: 10.3390/molecules25245804
Roy, U. K., Nielsen, B. V., and Milledge, J. J. (2021). Antioxidant production in Dunaliella. Appl. Sci. 11:3959. doi: 10.3390/app11093959
Saeed, F., Tul-Zohra, K., Naveed, K., Zia, A., Khaliq, M., Noor, Z., et al. (2025). Algal proteins for sustainable nutrition and functional food innovation. Appl. Food Res. 5:100752. doi: 10.1016/j.afres.2025.100752
Salam, M. A., Al-Amin, M. Y., Salam, M. T., Pawar, J. S., Akhter, N., Rabaan, A. A., et al. (2023). Antimicrobial resistance: a growing serious threat for global public health. Healthcare 11:1946. doi: 10.3390/healthcare11131946
Sangela, V., Kumar, M., Choudhary, S., Gour, V. S., Meena, M., Vinayak, V., et al. (2022). Effect of nitrogen, phosphorus and sodium bicarbonate on lipid production and fatty acid profile in Coelastrella terrestris. Biocatal. Agric. Biotechnol. 45:102518. doi: 10.1016/j.bcab.2022.102518
Sathasivam, R., and Ki, J. S. (2018). A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 16:26. doi: 10.3390/md16010026
Sathya, R., MubarakAli, D., MohamedSaalis, J., and Kim, J. W. (2021). A systemic review on microalgal peptides: bioprocess and sustainable applications. Sustainability 13:3262. doi: 10.3390/su13063262
Scaglioni, P. T., and Badiale-Furlong, E. (2017). Can microalgae act as source of preservatives in food chain. J. Food Sci. Eng. 7, 283–296. doi: 10.17265/2159-5828/2017.06.001
Sedighi, M., Jalili, H., Darvish, M., Sadeghi, S., and Ranaei-Siadat, S. O. (2019). Enzymatic hydrolysis of microalgae proteins using serine proteases: a study to characterize kinetic parameters. Food Chem. 284, 334–339. doi: 10.1016/j.foodchem.2019.01.111
Seth, K., Kumar, A., Rastogi, R. P., Meena, M., Vinayak, V., and Harish. (2021). Bioprospecting of fucoxanthin from diatoms - challenges and perspectives. Algal Res. 60:102475. doi: 10.1016/j.algal.2021.102475
Shankar, G., and Akhter, Y. (2024). Stealing survival: iron acquisition strategies of Mycobacterium tuberculosis. Biochimie 227, 37–60. doi: 10.1016/j.biochi.2024.06.006
Shannon, E., and Abu-Ghannam, N. (2016). Antibacterial derivatives of marine algae: an overview of pharmacological mechanisms and applications. Mar. Drugs 14:81. doi: 10.3390/md14040081
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:217037. doi: 10.1155/2012/217037
Shirai, M. A., Baú, T. R., Zanela, J., and Pimentel, T. C. (2025). Microalgae as an innovative active ingredient for edible films and coatings for food applications. Algal Res. 86:103959. doi: 10.1016/j.algal.2025.103959
Silva, A., Silva, S. A., Lourenço-Lopes, C., Jimenez-Lopez, C., Carpena, M., Gullón, P., et al. (2020). Antibacterial use of macroalgae compounds against foodborne pathogens. Antibiotics 9:712. doi: 10.3390/antibiotics9100712
Singh, R., Upadhyay, A. K., Singh, D. V., Singh, J. S., and Singh, D. P. (2019). Photosynthetic performance, nutrient status and lipid yield of microalgae Chlorella vulgaris and Chlorococcum humicola under UV-B exposure. Curr. Res. Biotechnol. 1, 65–77. doi: 10.1016/j.crbiot.2019.10.001
Singh, S., Datta, S., Narayanan, K. B., and Rajnish, K. N. (2021). Bacterial exopolysaccharides in biofilms: role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 19:140. doi: 10.1186/s43141-021-00242-y
Sivakumar, J., and Santhanam, P. (2011). Antipathogenic activity of Spirulina powder. Recent Res. Sci. Technol. 3, 158−161.
Smith, V. J., Desbois, A. P., and Dyrynda, E. A. (2010). Conventional and unconventional antimicrobials from fish, marine invertebrates and micro-algae. Mar. Drugs 8, 1213–1262. doi: 10.3390/md8041213
Sun, H., Yang, S., Zhao, W., Kong, Q., Zhu, C., Fu, X., et al. (2023). Fucoxanthin from marine microalgae: a promising bioactive compound for industrial production and food application. Crit. Rev. Food Sci. Nutr. 63, 7996–8012. doi: 10.1080/10408398.2022.2054932
Surendhiran, D., Cui, H., and Lin, L. (2019). Encapsulation of Phlorotannin in Alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life 21:100346. doi: 10.1016/j.fpsl.2019.100346
Swapnil, P., Meena, M., and Rai, A. K. (2021). Molecular interaction of nitrate transporter proteins with recombinant glycinebetaine results in efficient nitrate uptake in the cyanobacterium Anabaena PCC 7120. PLoS ONE 16:e0257870. doi: 10.1371/journal.pone.0257870
Swapnil, P., Yadav, A. K., Srivastav, S., Sharma, N. K., Srikrishna, S., and Rai, A. K. (2017). Biphasic ROS accumulation and programmed cell death in a cyanobacterium exposed to salinity (NaCl and Na2SO4). Algal Res. 23, 88–95. doi: 10.1016/j.algal.2017.01.014
Tao, H., Zhou, J., Wu, T., and Cheng, Z. (2014). High-throughput superoxide anion radical scavenging capacity assay. J. Agric. Food Chem. 62, 9266–9272. doi: 10.1021/jf502160d
Tarannum, N., Hossain, T. J., Ali, F., Das, T., Dhar, K., and Nafiz, I. H. (2023). Antioxidant, antimicrobial and emulsification properties of exopolysaccharides from lactic acid bacteria of bovine milk: Insights from biochemical and genomic analysis. LWT 186:115263. doi: 10.1016/j.lwt.2023.115263
Teixeira-Santos, R., Lima, M., Gomes, L. C., and Mergulhao, F. J. (2021). Antimicrobial coatings based on chitosan to prevent implant-associated infections: a systematic review. iScience 24:103480. doi: 10.1016/j.isci.2021.103480
Tejano, L. A., Peralta, J. P., Yap, E. E. S., and Chang, Y. W. (2019). Bioactivities of enzymatic protein hydrolysates derived from Chlorella sorokiniana. Food Sci. Nutr. 7, 2381–2390. doi: 10.1002/fsn3.1097
Torres-Tiji, Y., Fields, F. J., and Mayfield, S. P. (2020). Microalgae as a future food source. Biotechnol. Adv. 41:107536. doi: 10.1016/j.biotechadv.2020.107536
Tsintzou, G., and Madesis, P. (2024). “Peptide elicitors for defense against abiotic stress,” in Plant elicitor Peptides: New Tool for Sustainable Agriculture (Singapore: Springer Nature Singapore), 19–47. doi: 10.1007/978-981-97-6374-0_2
Uddin, S. A., Akter, S., Hossen, S., and Rahman, M. A. (2020). Antioxidant, antibacterial and cytotoxic activity of Caulerpa racemosa (Forsskål) J. Agardh and Ulva (Enteromorpha) intestinalis L. Bangladesh J. Sci. Ind. Res. 55, 237–244. doi: 10.3329/bjsir.v55i4.50959
Ullah, F., Ayaz, M., Sadiq, A., Ullah, F., Hussain, I., Shahid, M., et al. (2020). Potential role of plant extracts and phytochemicals against foodborne pathogens. Appl. Sci. 10:4597. doi: 10.3390/app10134597
Vasquez-Moscoso, C. A., Merlano, J. A. R., Olivera Gálvez, A., and Volcan Almeida, D. (2025). Antimicrobial peptides (AMPs) from microalgae as an alternative to conventional antibiotics in aquaculture. Prep. Biochem. Biotechnol. 55, 26–35. doi: 10.1080/10826068.2024.2365357
Vignaud, J., Loiseau, C., Hérault, J., Mayer, C., Côme, M., Martin, I., et al. (2023). Microalgae produce antioxidant molecules with potential preventive effects on mitochondrial functions and skeletal muscular oxidative stress. Antioxidants 12:1050. doi: 10.3390/antiox12051050
Vishwakarma, J., and Vavilala, S. L. (2019). Evaluating the antibacterial and antibiofilm potential of sulphated polysaccharides extracted from green algae Chlamydomonas reinhardtii. J. Appl. Microbiol. 127, 1004–1017. doi: 10.1111/jam.14364
Wali, A. F., Al Dhaheri, Y., Ramakrishna Pillai, J., Mushtaq, A., Rao, P. G., Rabbani, S. A., et al. (2020). LC-MS phytochemical screening, in vitro antioxidant, antimicrobial and anticancer activity of microalgae Nannochloropsis oculata extract. Separations 7:54. doi: 10.3390/separations7040054
Wassmann, B., Hartmann, C., and Siegrist, M. (2024). Novel microalgae-based foods: what influences Singaporean consumers' acceptance? Food Qual. Prefer. 113:105068. doi: 10.1016/j.foodqual.2023.105068
Weiss, G. A., Watanabe, C. K., Zhong, A., Goddard, A., and Sidhu, S. S. (2000). Rapid mapping of protein functional epitopes by combinatorial alanine scanning. Proc. Nat. Acad. Sci. 97, 8950–8954. doi: 10.1073/pnas.160252097
Wong, S. X. E., Kiew, S. F., Lau, S. Y., and Pottas, P. W. (2023). Procedures to investigate potential of plants as natural food preservatives: extraction technology, phytochemical characterisation, and antimicrobial bioassays. Food Chem. Adv. 3:100435. doi: 10.1016/j.focha.2023.100435
Wu, G., Yang, Q., Long, M., Guo, L., Li, B., Meng, Y., et al. (2015). Evaluation of agar dilution and broth microdilution methods to determine the disinfectant susceptibility. J. Antibiot. 68, 661–665. doi: 10.1038/ja.2015.51
Xie, W., Li, X., Xu, H., Chen, F., Cheng, K. W., Liu, H., et al. (2023). Optimization of heterotrophic culture conditions for the microalgae Euglena gracilis to produce proteins. Mar. Drugs 21:519. doi: 10.3390/md21100519
Xue, Y., Zhao, P., Quan, C., Zhao, Z., Gao, W., Li, J., et al. (2018). Cyanobacteria-derived peptide antibiotics discovered since 2000. Peptides 107, 17–24. doi: 10.1016/j.peptides.2018.08.002
Yadav, A., Sharma, S., Nitesh, M. R., Bhardwaj, R., Swapnil, P., and Meena, M. (2025). Tapping the microalgal potential: genetic precision and stress-induction for enhanced astaxanthin and biofuel production. Biotechnol. Biofuels Bioprod. 18:92. doi: 10.1186/s13068-025-02656-z
Yang, S., Wang, Y., Wang, J., Cheng, K., Liu, J., He, Y., et al. (2024). Microalgal protein for sustainable and nutritious foods: a joint analysis of environmental impacts, health benefits and consumer's acceptance. Trends Food Sci. Technol. 143:104278. doi: 10.1016/j.tifs.2023.104278
Yoon, B. K., Jackman, J. A., Valle-González, E. R., and Cho, N. J. (2018). Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 19:1114. doi: 10.3390/ijms19041114
Zehra, A., Raytekar, N. A., Meena, M., and Swapnil, P. (2021). Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: a review. Curr. Res. Microb. Sci. 2:100054. doi: 10.1016/j.crmicr.2021.100054
Zerrifi, S. E. A., El Khalloufi, F., Oudra, B., and Vasconcelos, V. (2018). Seaweed bioactive compounds against pathogens and microalgae: potential uses on pharmacology and harmful algae bloom control. Mar. Drugs 16:55. doi: 10.3390/md16020055
Zhang, K., Yin, X., Huang, Y., Liu, C., Zhang, Q., Liu, Q., et al. (2024). A potent antibacterial peptide (P6) from the de novo transcriptome of the microalga Aureococcus anophagefferens. Int. J. Mol. Sci. 25:13736. doi: 10.3390/ijms252413736
Zhang, Y., Ye, Y., Bai, F., and Liu, J. (2021). The oleaginous astaxanthin-producing alga Chromochloris zofingiensis: potential from production to an emerging model for studying lipid metabolism and carotenogenesis. Biotechnol. Biofuels Bioprod. 14:119. doi: 10.1186/s13068-021-01969-z
Keywords: antioxidants, antimicrobials, food preservatives, microalgae, bioactive compound
Citation: Singh LA, Kumari P, Kumar P, Yadav A, Bhardwaj R, Swapnil P and Meena M (2025) Microalgae-derived antioxidants and antimicrobials: a sustainable approach for natural food preservatives. Front. Sustain. Food Syst. 9:1669731. doi: 10.3389/fsufs.2025.1669731
Received: 20 July 2025; Accepted: 27 August 2025;
Published: 12 September 2025.
Edited by:
Lourdes Maria Correa Cabral, Brazilian Agricultural Research Corporation (EMBRAPA), BrazilReviewed by:
Abdel Moneim Elhadi Sulieman, University of Hail, Saudi ArabiaMiguel Anchundia, Universidad Politécnica Estatal del Carchi, Ecuador
Copyright © 2025 Singh, Kumari, Kumar, Yadav, Bhardwaj, Swapnil and Meena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mukesh Meena, bXVrZXNobWVlbmFtbHN1QGdtYWlsLmNvbQ==; ZHJtdWtlc2htZWVuYTMyMUBtbHN1LmFjLmlu; bXVrZXNobWVlbmFiaHVAZ21haWwuY29t; Prashant Swapnil, cHJhc2hhbnQuc3dhcG5pbEBjdXAuZWR1Lmlu
†ORCID: Prashant Swapnil orcid.org/0000-0002-7361-4199
Mukesh Meena orcid.org/0000-0002-6336-1140
 Laishram Amarjit Singh
Laishram Amarjit Singh Pritee Kumari1
Pritee Kumari1 Ankush Yadav
Ankush Yadav Prashant Swapnil
Prashant Swapnil Mukesh Meena
Mukesh Meena